
Magenta Therapeutics January 2019 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements and information. The use of words such as "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: uncertainties inherent in clinical studies and in the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of the trial; whether results from preclinical studies or earlier clinical studies will be predictive of the results of future trials; submissions for regulatory approval or review by governmental authorities, including review under accelerated approval processes; orphan drug designation eligibility; regulatory approvals to conduct trials or to market products; whether Magenta's cash resources will be sufficient to fund Magenta's foreseeable and unforeseeable operating expenses and capital expenditure requirements; other matters that could affect the availability or commercial potential of Magenta's therapeutic candidates; and other factors that could affect Magenta’s ability to secure future funding to its operating expenses and capital expenditure requirements. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

The Promise of a One-Time Curative Therapy
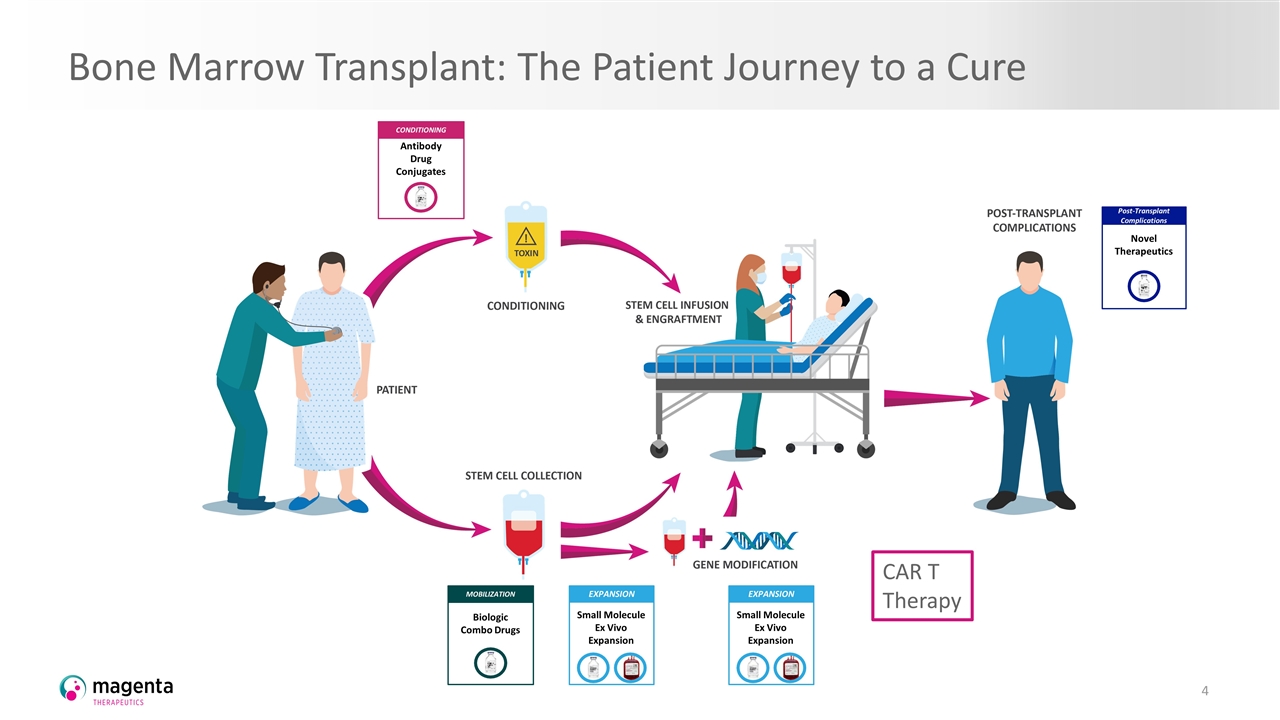
Bone Marrow Transplant: The Patient Journey to a Cure CONDITIONING Antibody Drug Conjugates EXPANSION Small Molecule Ex Vivo Expansion MOBILIZATION Biologic Combo Drugs Post-Transplant Complications Novel Therapeutics EXPANSION Small Molecule Ex Vivo Expansion CAR T Therapy
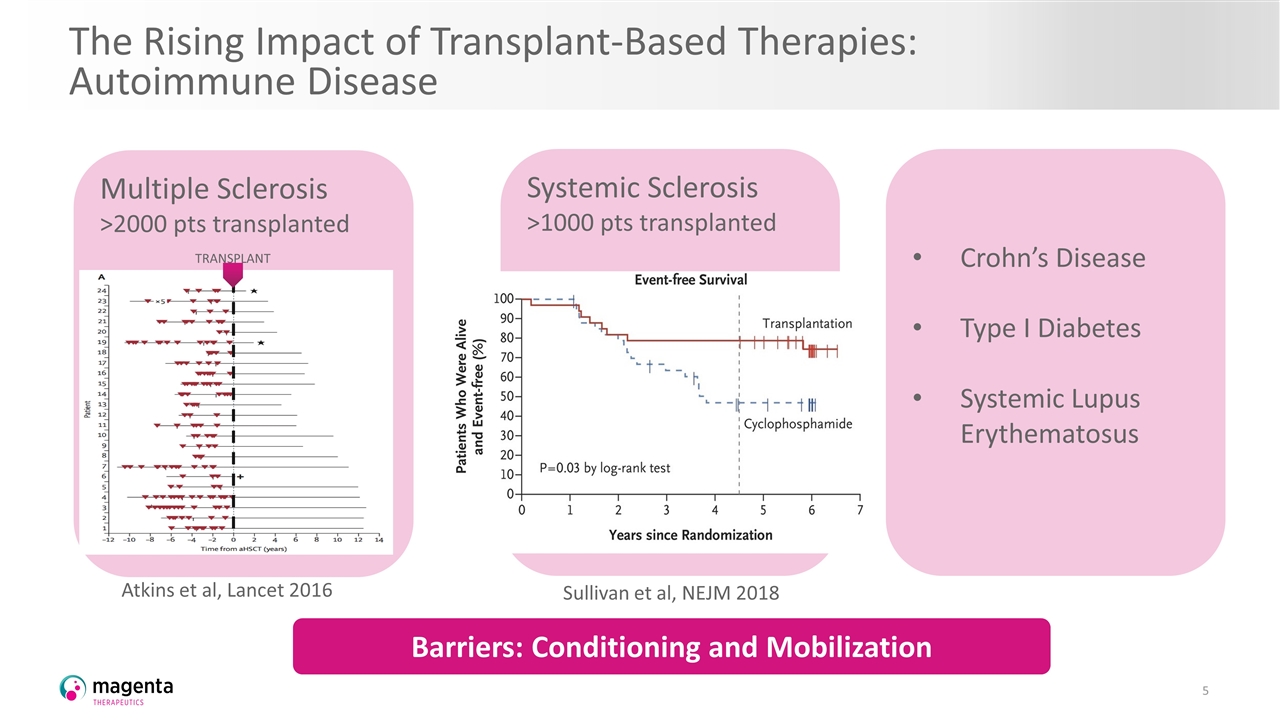
The Rising Impact of Transplant-Based Therapies: Autoimmune Disease Sullivan et al, New England Journal Medicine 2019 Multiple Sclerosis >2000 pts transplanted Crohn’s Disease Type I Diabetes Systemic Lupus Erythematosus Atkins et al, Lancet 2016 TRANSPLANT Systemic Sclerosis >1000 pts transplanted Barriers: Conditioning and Mobilization Sullivan et al, NEJM 2018
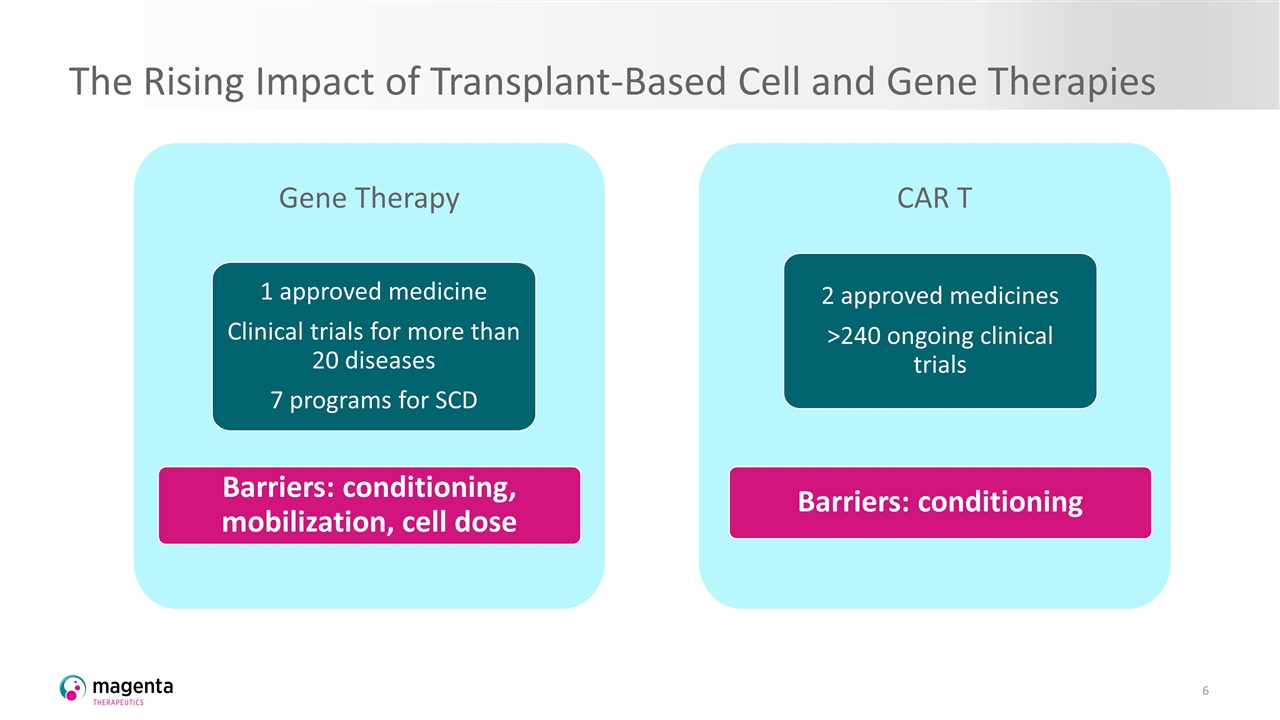
The Rising Impact of Transplant-Based Cell and Gene Therapies Gene Therapy 1 approved medicine Clinical trials for more than 20 diseases 7 programs for SCD Barriers: conditioning, mobilization, cell dose CAR T 2 approved medicines >240 ongoing clinical trials Barriers: conditioning
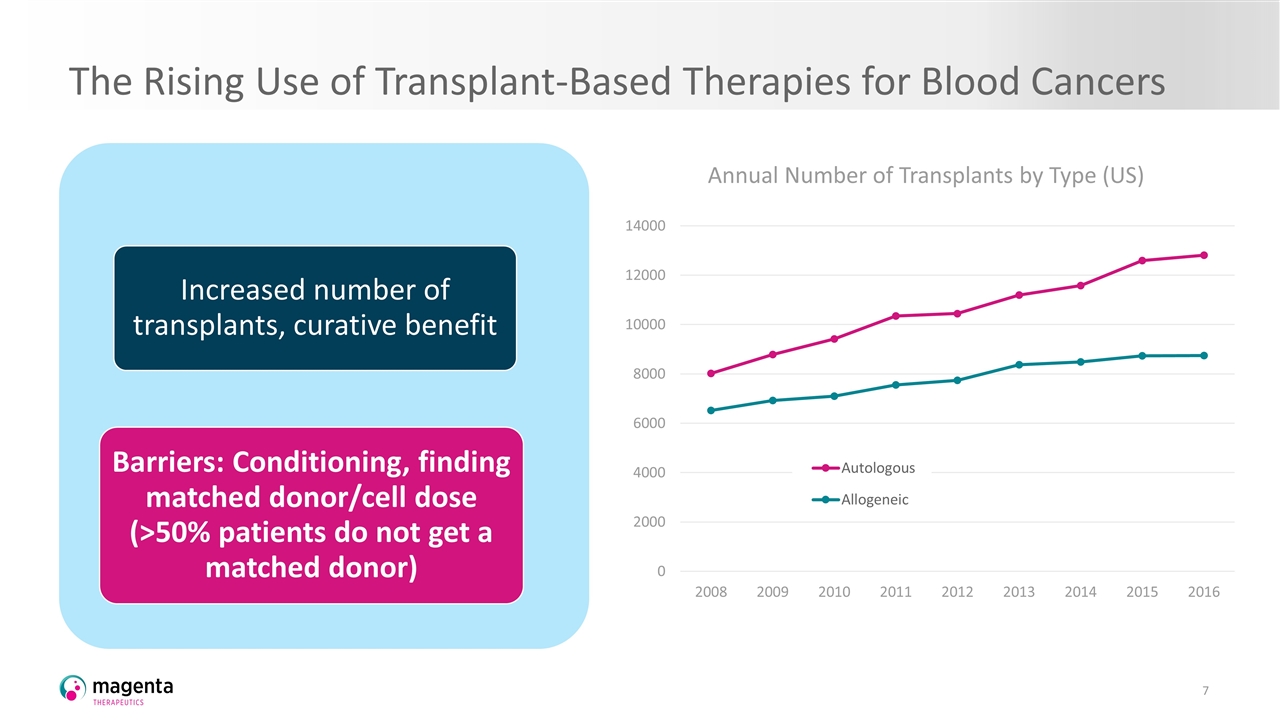
The Rising Use of Transplant-Based Therapies for Blood Cancers Annual Number of Transplants by Type (US) Increased number of transplants, curative benefit Barriers: Conditioning, finding matched donor/cell dose (>50% patients do not get a matched donor)
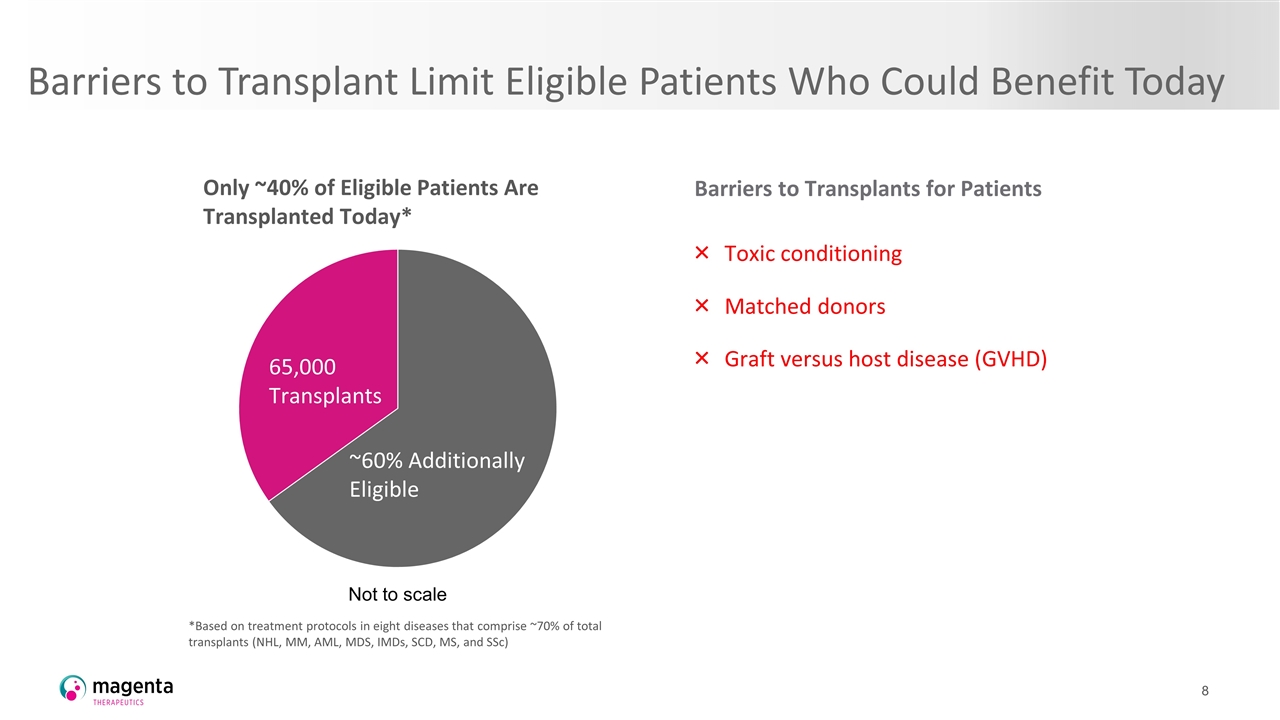
Barriers to Transplant Limit Eligible Patients Who Could Benefit Today Only ~40% of Eligible Patients Are Transplanted Today* *Based on treatment protocols in eight diseases that comprise ~70% of total transplants (NHL, MM, AML, MDS, IMDs, SCD, MS, and SSc) Malignant Non-malignant 65,000 Transplants ~60% Additionally Eligible Barriers to Transplants for Patients Toxic conditioning Matched donors Graft versus host disease (GVHD) Not to scale
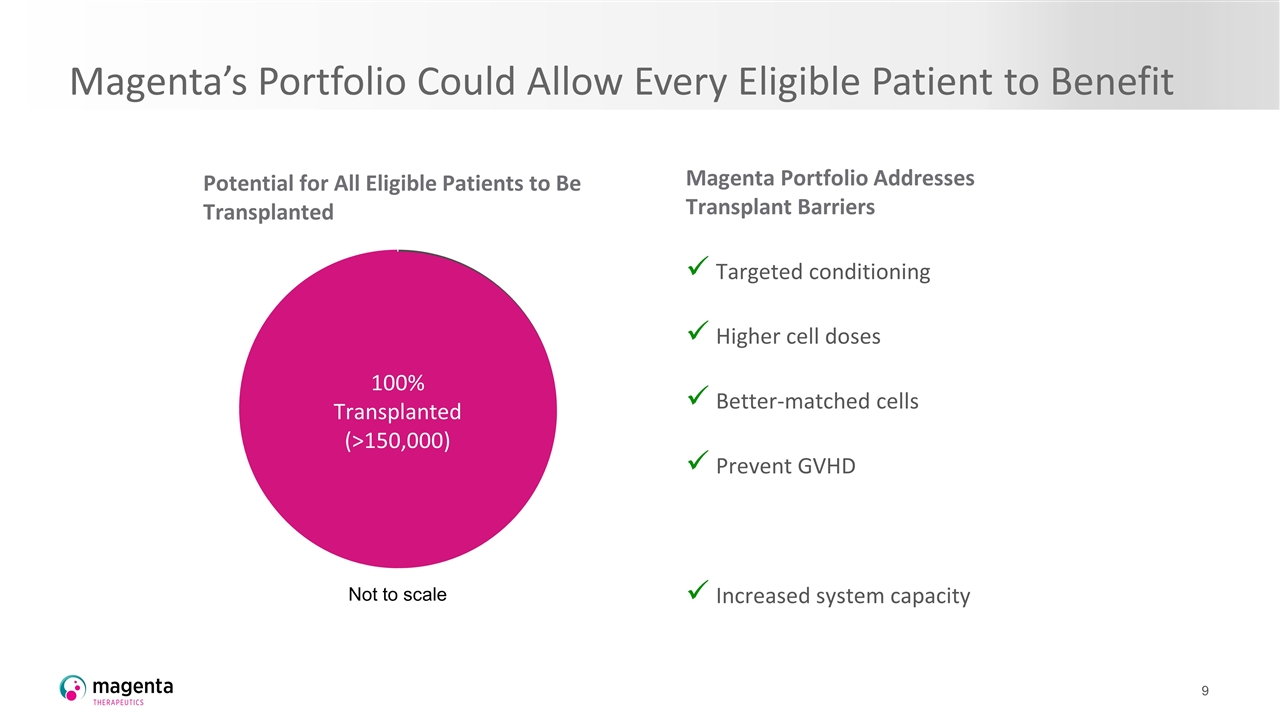
Magenta’s Portfolio Could Allow Every Eligible Patient to Benefit Potential for All Eligible Patients to Be Transplanted Magenta Portfolio Addresses Transplant Barriers Targeted conditioning Higher cell doses Better-matched cells Prevent GVHD Increased system capacity 65,000 Transplants Malignant Non-malignant 100% Transplanted (>150,000) Not to scale
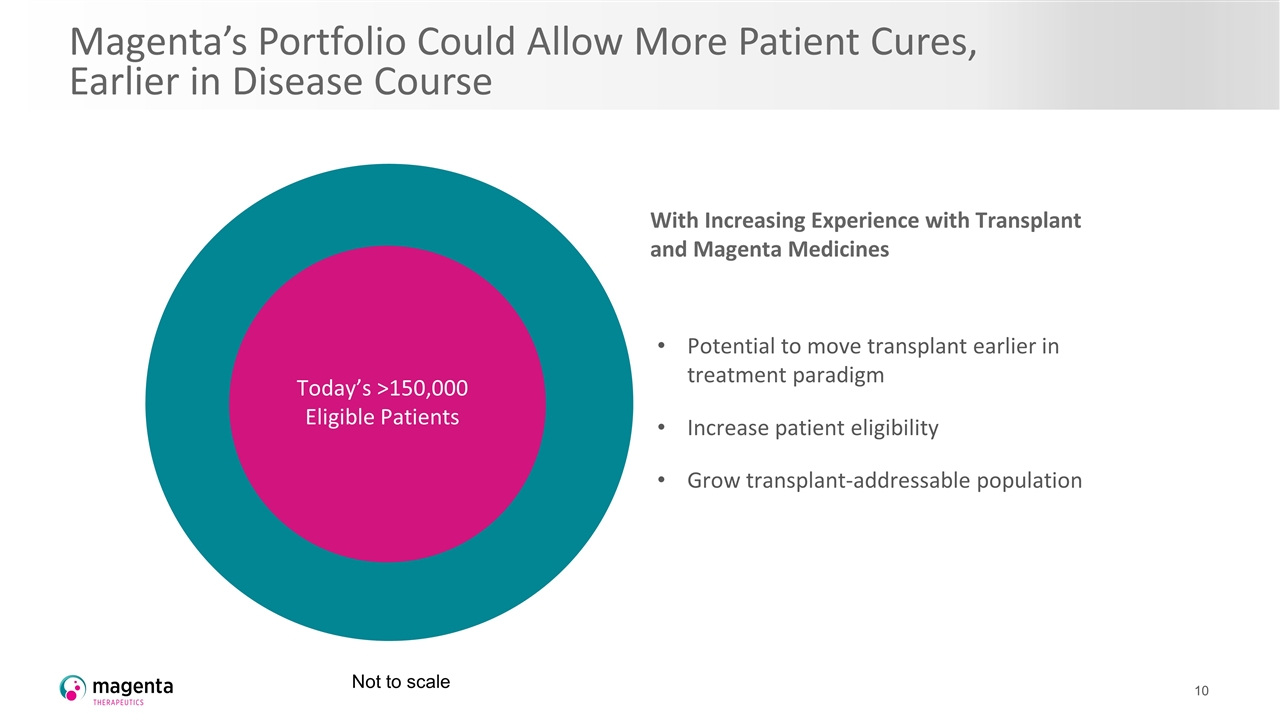
Magenta’s Portfolio Could Allow More Patient Cures, Earlier in Disease Course With Increasing Experience with Transplant and Magenta Medicines Potential to move transplant earlier in treatment paradigm Increase patient eligibility Grow transplant-addressable population Today’s >150,000 Eligible Patients Not to scale
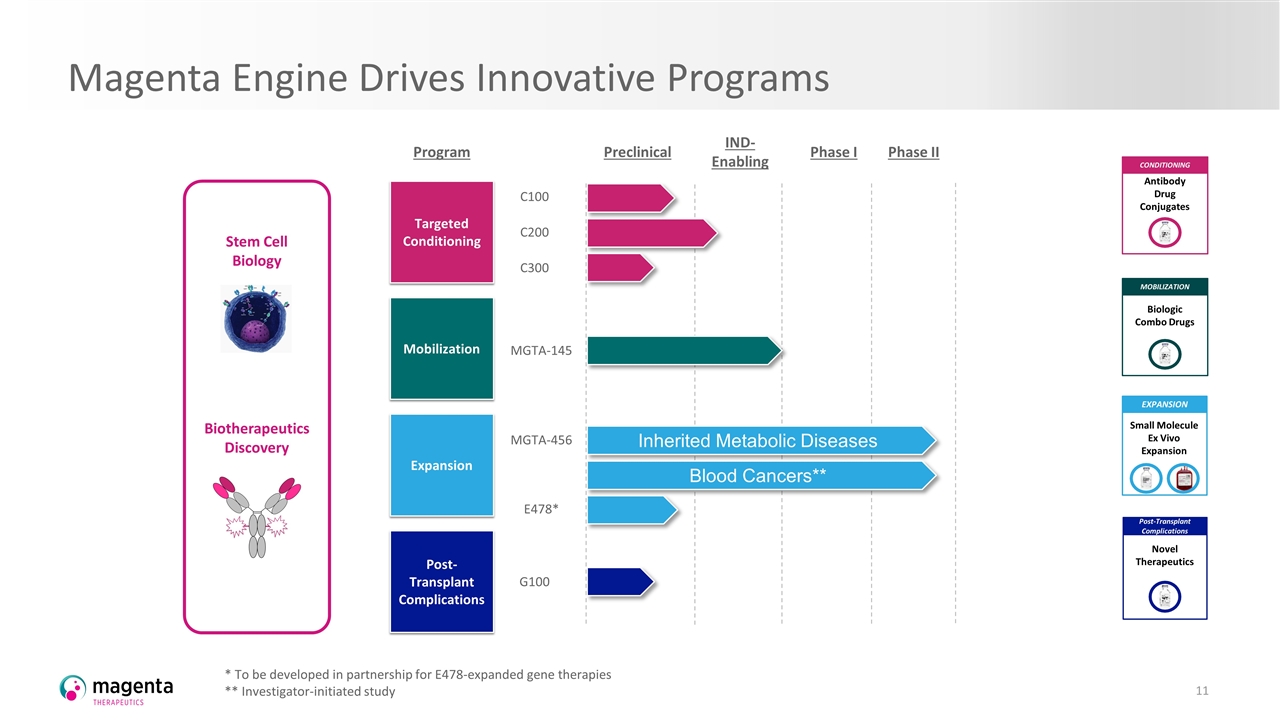
Magenta Engine Drives Innovative Programs Discovery Biology * To be developed in partnership for E478-expanded gene therapies ** Investigator-initiated study Biotherapeutics Discovery Stem Cell Biology Targeted Conditioning Mobilization Expansion Post- Transplant Complications Program C100 C200 C300 Preclinical Phase I Phase II MGTA-145 E478* G100 MGTA-456 Inherited Metabolic Diseases IND-Enabling Post-Transplant Complications Novel Therapeutics EXPANSION Small Molecule Ex Vivo Expansion MOBILIZATION Biologic Combo Drugs CONDITIONING Antibody Drug Conjugates Blood Cancers**
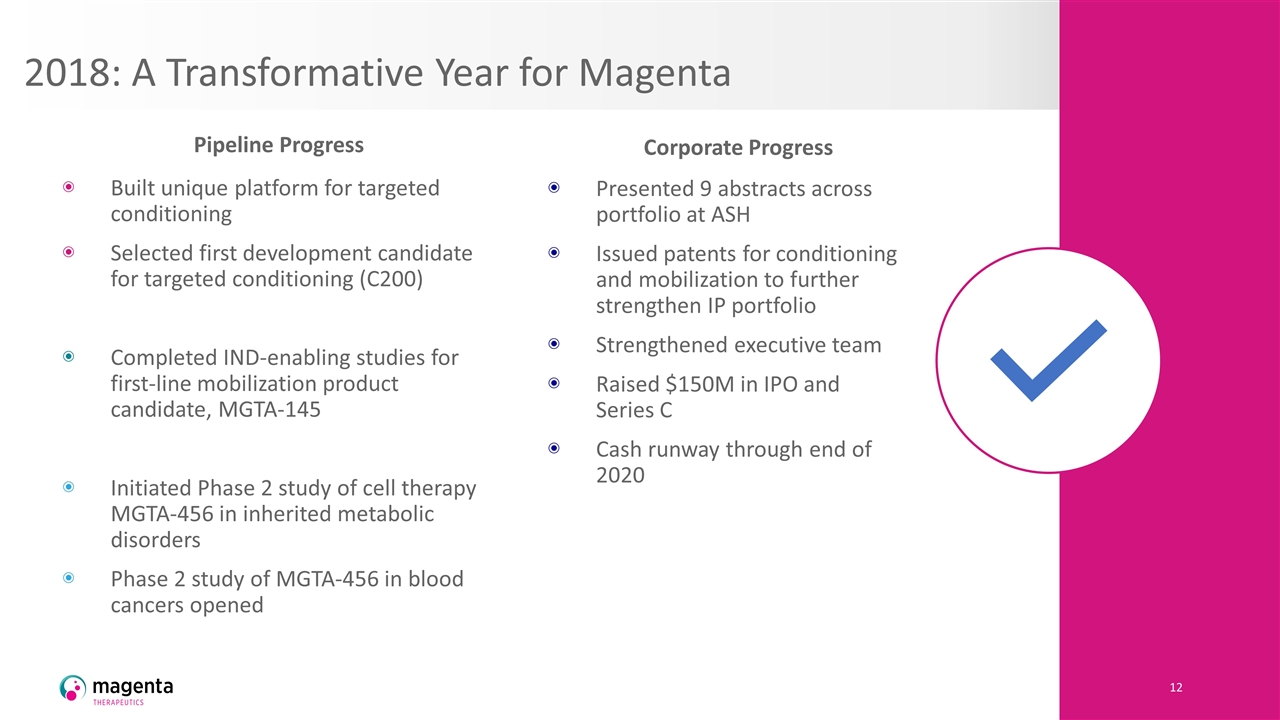
2018: A Transformative Year for Magenta Built unique platform for targeted conditioning Selected first development candidate for targeted conditioning (C200) Completed IND-enabling studies for first-line mobilization product candidate, MGTA-145 Initiated Phase 2 study of cell therapy MGTA-456 in inherited metabolic disorders Phase 2 study of MGTA-456 in blood cancers opened Presented 9 abstracts across portfolio at ASH Issued patents for conditioning and mobilization to further strengthen IP portfolio Strengthened executive team Raised $150M in IPO and Series C Cash runway through end of 2020 Pipeline Progress Corporate Progress

Key Takeaways Today Potential patient impact Next steps for each program Near-term milestones and long-term path
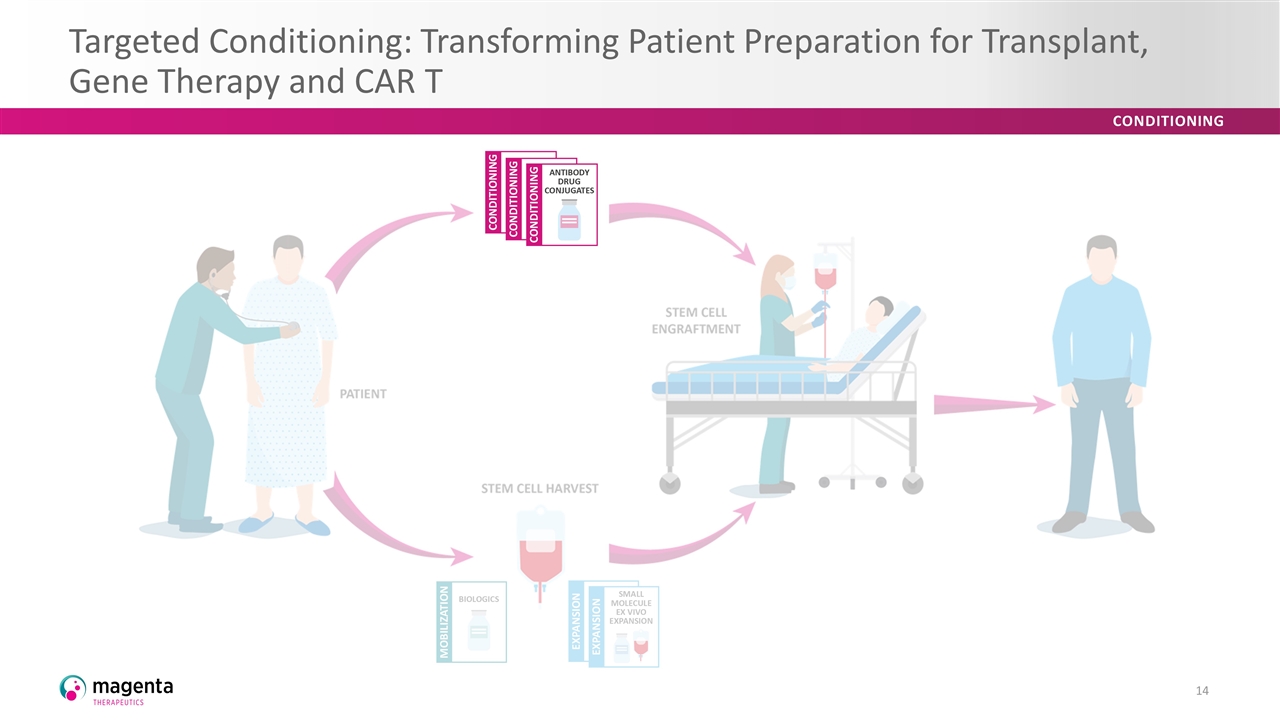
Targeted Conditioning: Transforming Patient Preparation for Transplant, Gene Therapy and CAR T
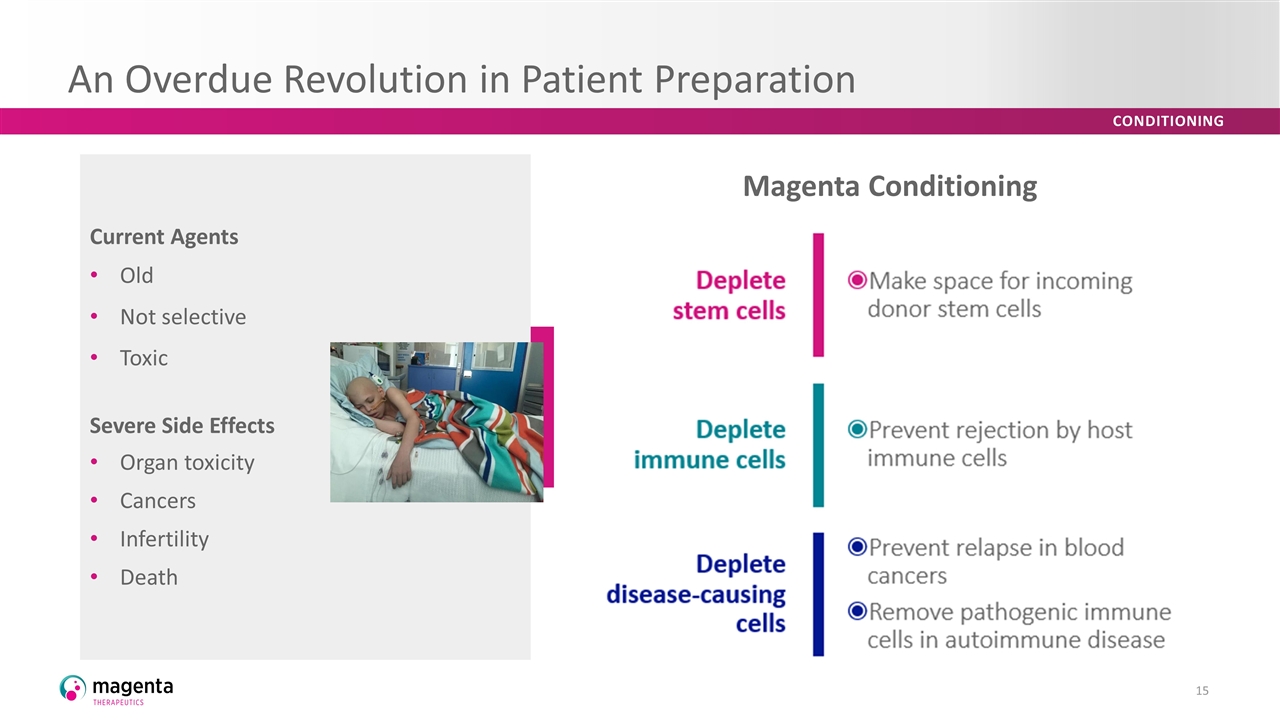
An Overdue Revolution in Patient Preparation Deplete stem cells Make space for incoming donor stem cells Prevent rejection by host immune cells Prevent relapse in blood cancers Remove pathogenic immune cells in autoimmune disease Current Agents Old Not selective Toxic Severe Side Effects Organ toxicity Cancers Infertility Death Magenta Conditioning
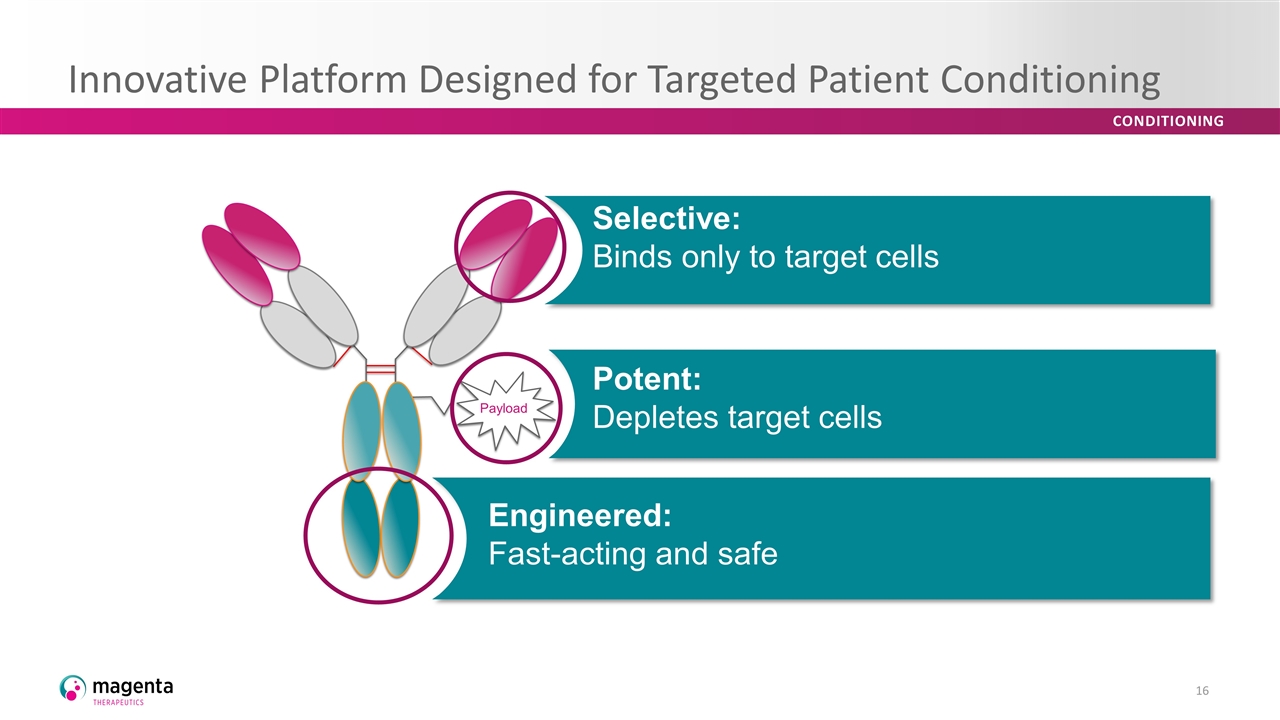
Innovative Platform Designed for Targeted Patient Conditioning Payload Potent: Depletes target cells Selective: Binds only to target cells Engineered: Fast-acting and safe
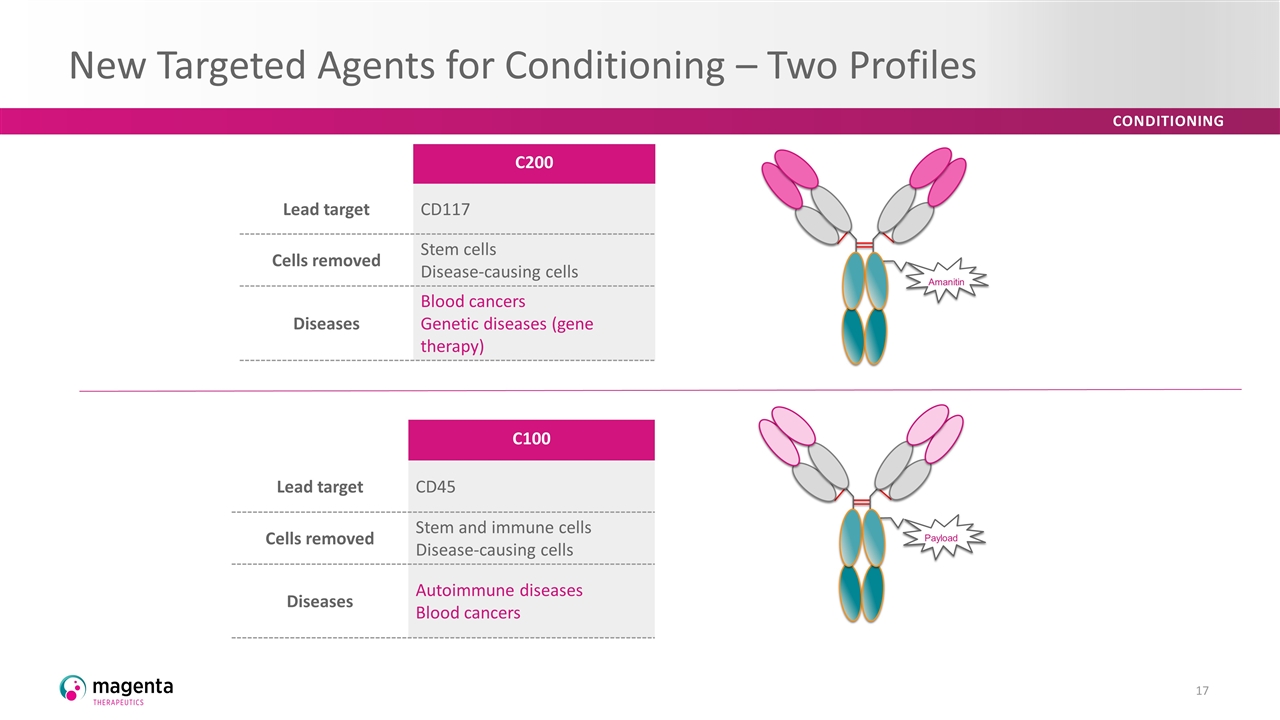
New Targeted Agents for Conditioning – Two Profiles C200 Lead target CD117 Cells removed Stem cells Disease-causing cells Diseases Blood cancers Genetic diseases (gene therapy) C100 Lead target CD45 Cells removed Stem and immune cells Disease-causing cells Diseases Autoimmune diseases Blood cancers Amanitin Payload
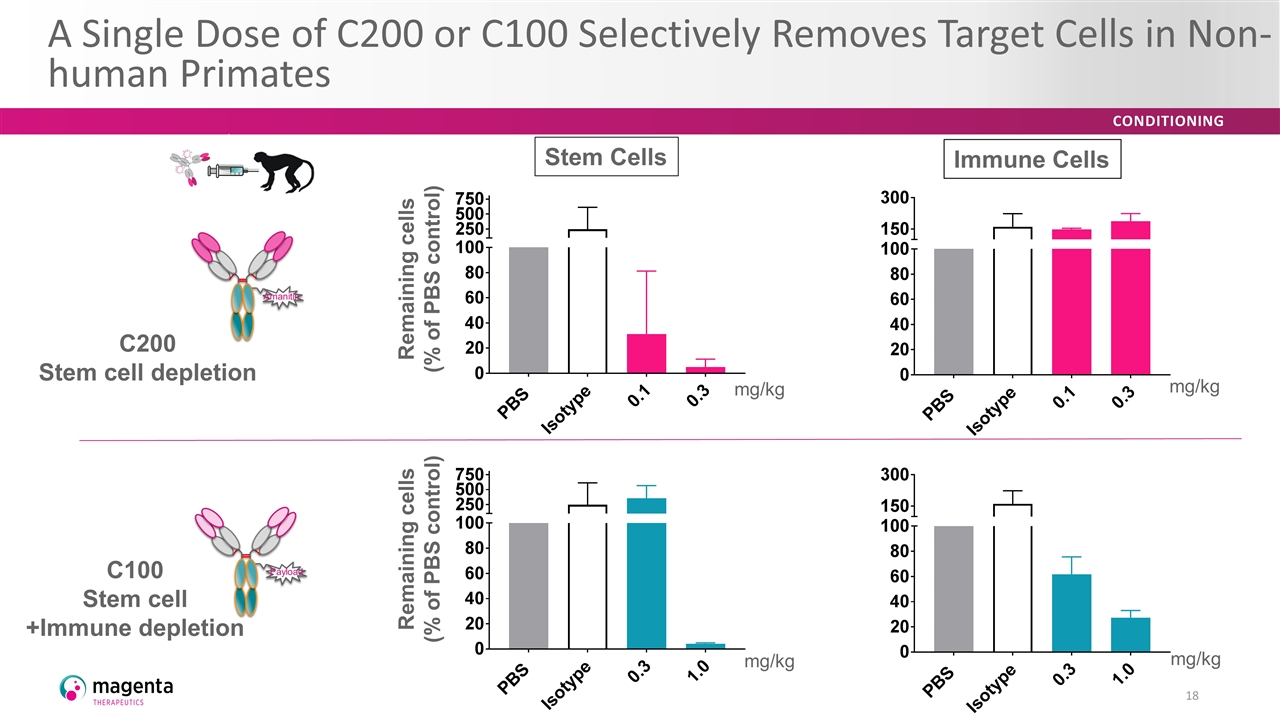
A single dose of fast-half life anti-CD117–Amanitin shows greater than 95% depletion of HSC and CFU in the bone marrow of NHPs 7 days after treatment Well tolerated in non-human primates A Single Dose of C200 or C100 Selectively Removes Target Cells in Non-human Primates 18 Swap out for Brad’s graphs Stem Cells Immune Cells Remaining cells (% of PBS control) Remaining cells (% of PBS control) mg/kg mg/kg mg/kg mg/kg C200 Stem cell depletion C100 Stem cell +Immune depletion Amanitin Payload
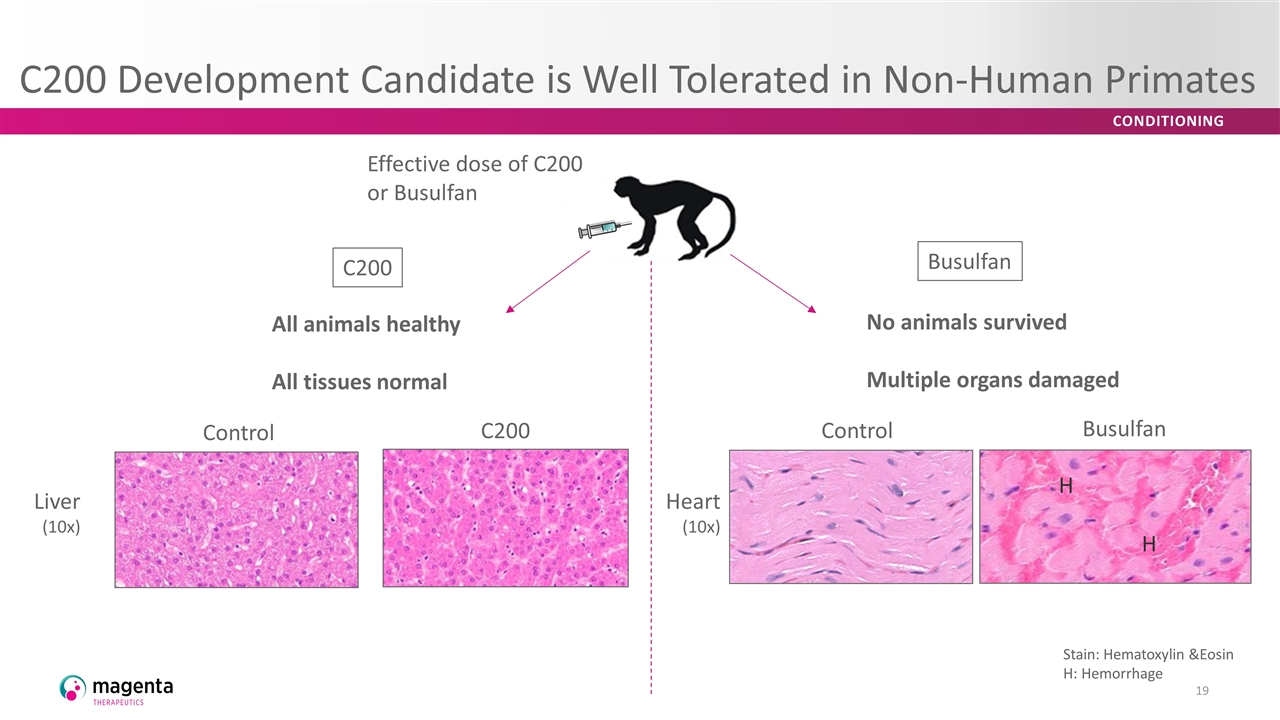
C200 Development Candidate is Well Tolerated in Non-Human Primates C200 Control Liver (10x) Heart (10x) Stain: Hematoxylin &Eosin H: Hemorrhage H H All animals healthy All tissues normal Effective dose of C200 or Busulfan C200 Busulfan No animals survived Multiple organs damaged Control Busulfan
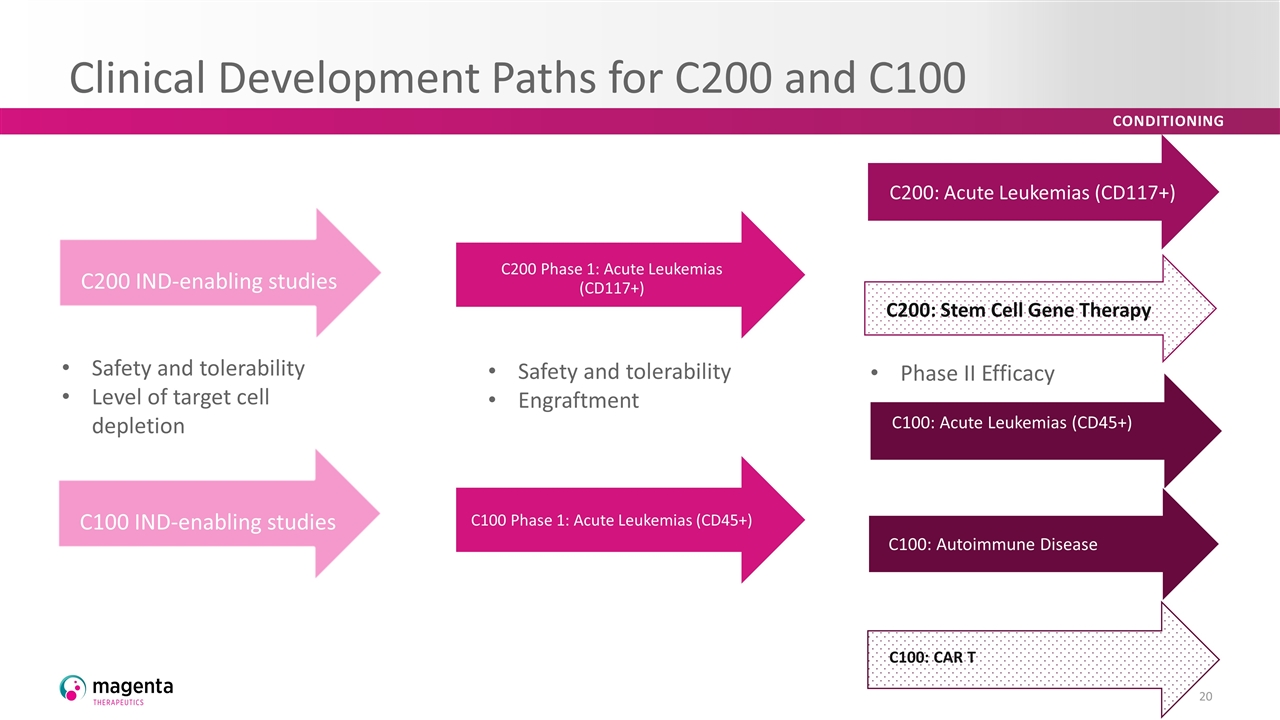
Clinical Development Paths for C200 and C100 C100: Acute Leukemias (CD45+) C100: Autoimmune Disease Safety and tolerability Level of target cell depletion Safety and tolerability Engraftment C200: Stem Cell Gene Therapy C200: Acute Leukemias (CD117+) C100: CAR T Phase II Efficacy C200 Phase 1: Acute Leukemias (CD117+) C100 IND-enabling studies C100 Phase 1: Acute Leukemias (CD45+) C200 IND-enabling studies
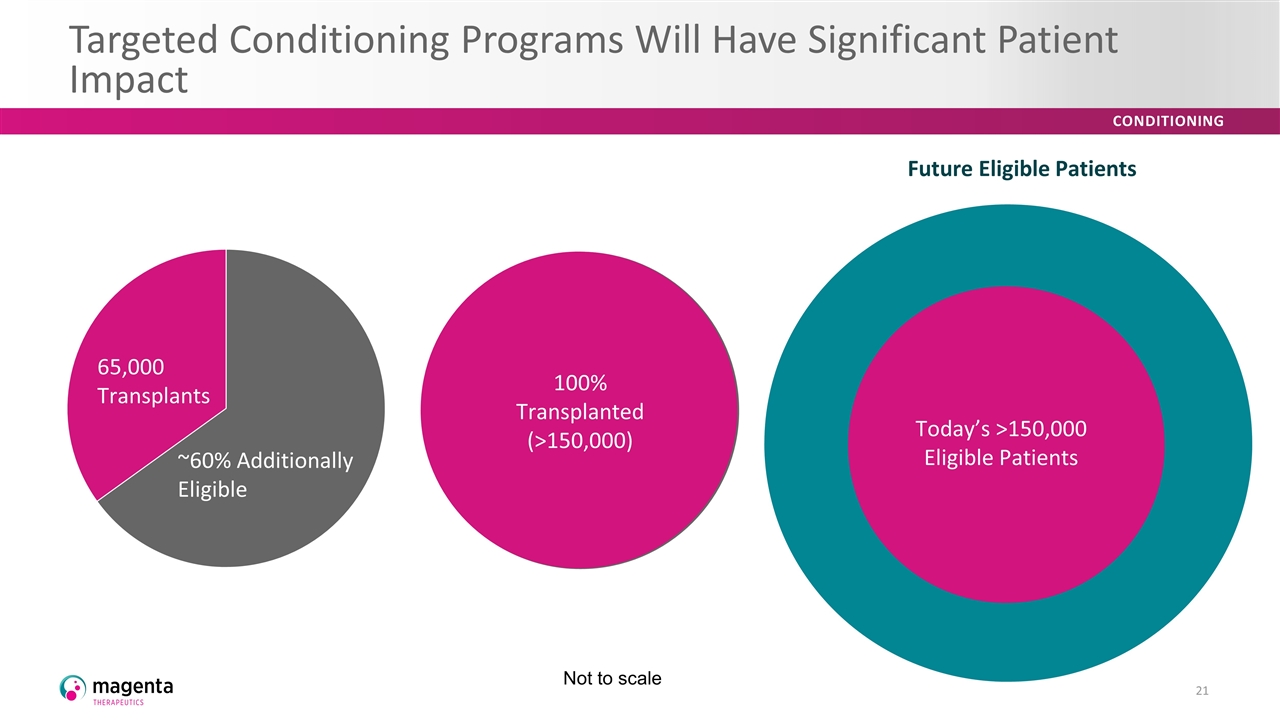
Targeted Conditioning Programs Will Have Significant Patient Impact 65,000 Transplants ~60% Additionally Eligible 100% Transplanted (>150,000) Future Eligible Patients Today’s >150,000 Eligible Patients Future Eligible Patients Today’s >150,000 Eligible Patients 65,000 Transplants ~60% Additionally Eligible Not to scale Future Eligible Patients Today’s >150,000 Eligible Patients Today’s >150,000 Eligible Patients
Magenta Will Optimize Stem Cell Collection
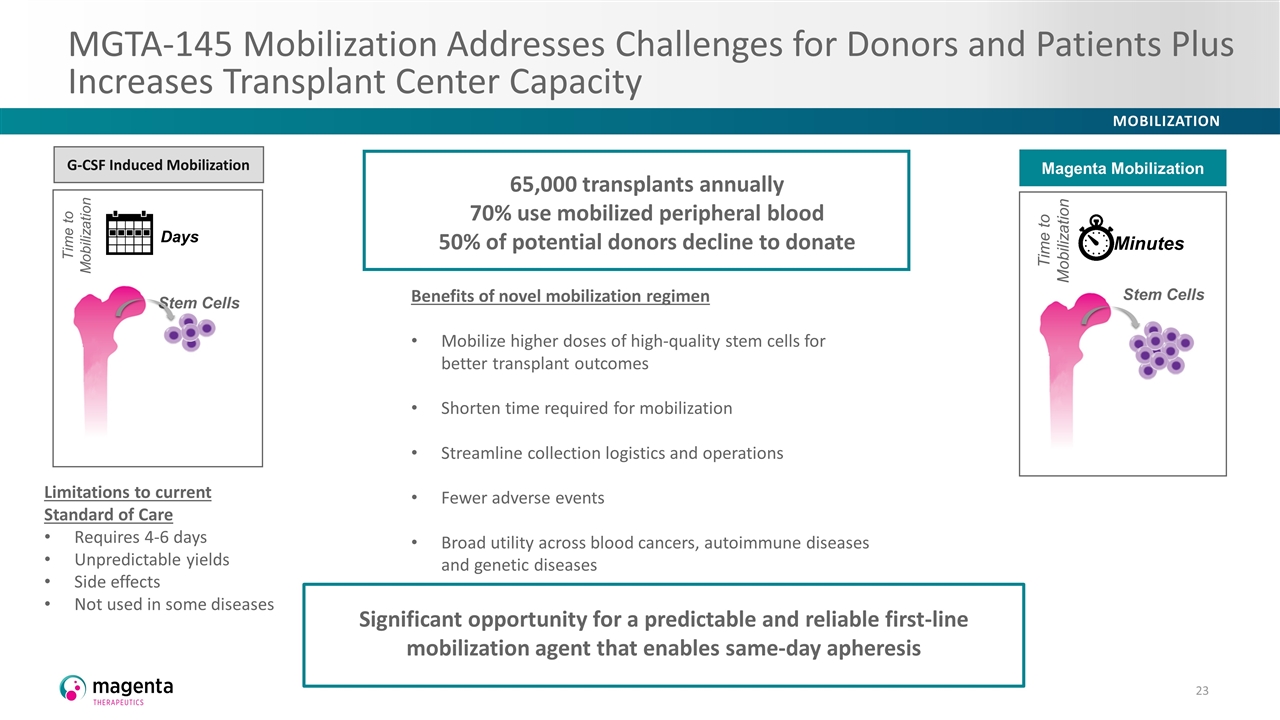
MGTA-145 Mobilization Addresses Challenges for Donors and Patients Plus Increases Transplant Center Capacity Limitations to current Standard of Care Requires 4-6 days Unpredictable yields Side effects Not used in some diseases Benefits of novel mobilization regimen Mobilize higher doses of high-quality stem cells for better transplant outcomes Shorten time required for mobilization Streamline collection logistics and operations Fewer adverse events Broad utility across blood cancers, autoimmune diseases and genetic diseases Days Stem Cells G-CSF Induced Mobilization Time to Mobilization Minutes Stem Cells Magenta Mobilization Time to Mobilization 65,000 transplants annually 70% use mobilized peripheral blood 50% of potential donors decline to donate Significant opportunity for a predictable and reliable first-line mobilization agent that enables same-day apheresis
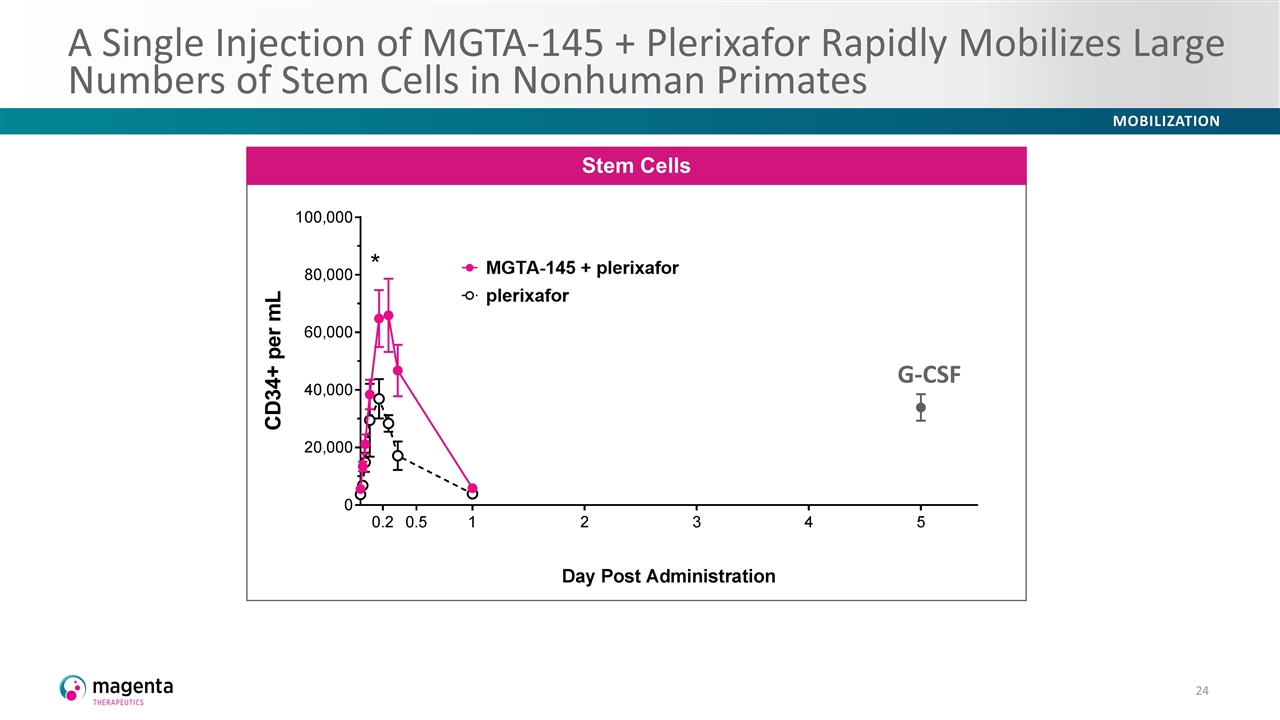
A Single Injection of MGTA-145 + Plerixafor Rapidly Mobilizes Large Numbers of Stem Cells in Nonhuman Primates Stem Cells G-CSF G-CSF
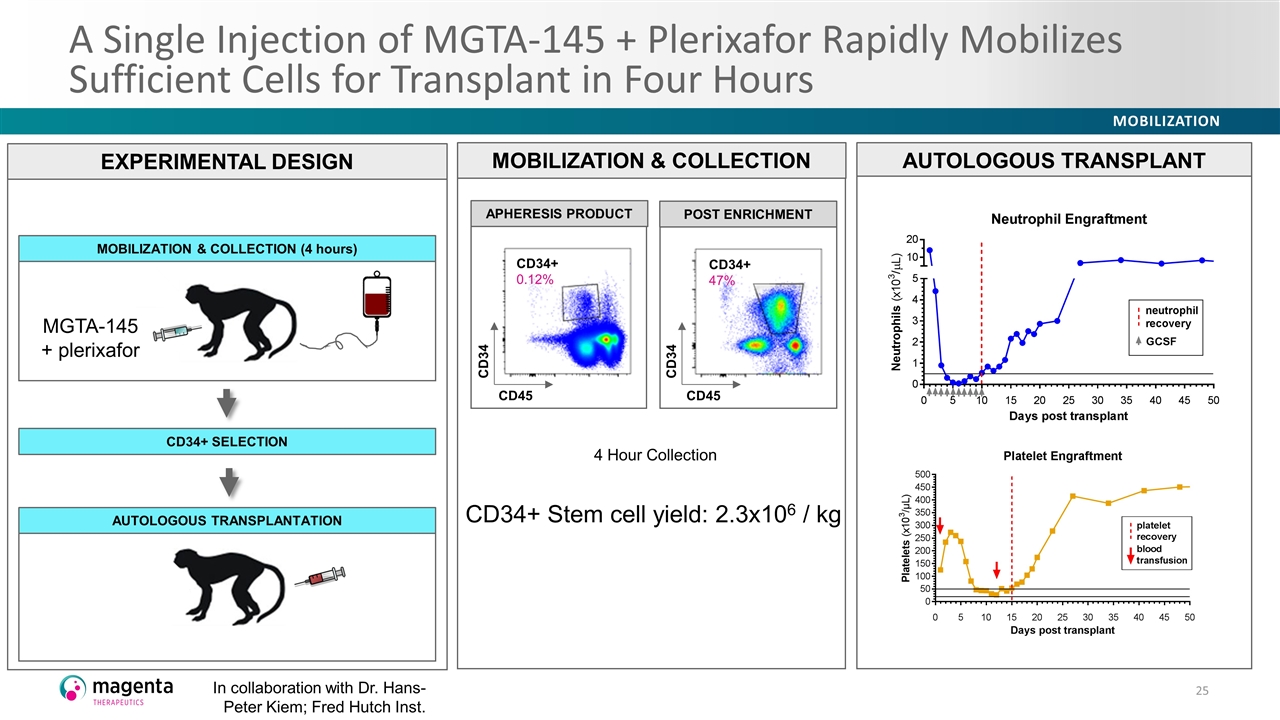
A Single Injection of MGTA-145 + Plerixafor Rapidly Mobilizes Sufficient Cells for Transplant in Four Hours EXPERIMENTAL DESIGN MOBILIZATION & COLLECTION (4 hours) AUTOLOGOUS TRANSPLANTATION CD34+ SELECTION MGTA-145 + plerixafor In collaboration with Dr. Hans-Peter Kiem; Fred Hutch Inst. MOBILIZATION & COLLECTION CD34+ 47% CD45 CD34 POST ENRICHMENT CD45 CD34 CD34+ 0.12% APHERESIS PRODUCT 4 Hour Collection CD34+ Stem cell yield: 2.3x106 / kg AUTOLOGOUS TRANSPLANT
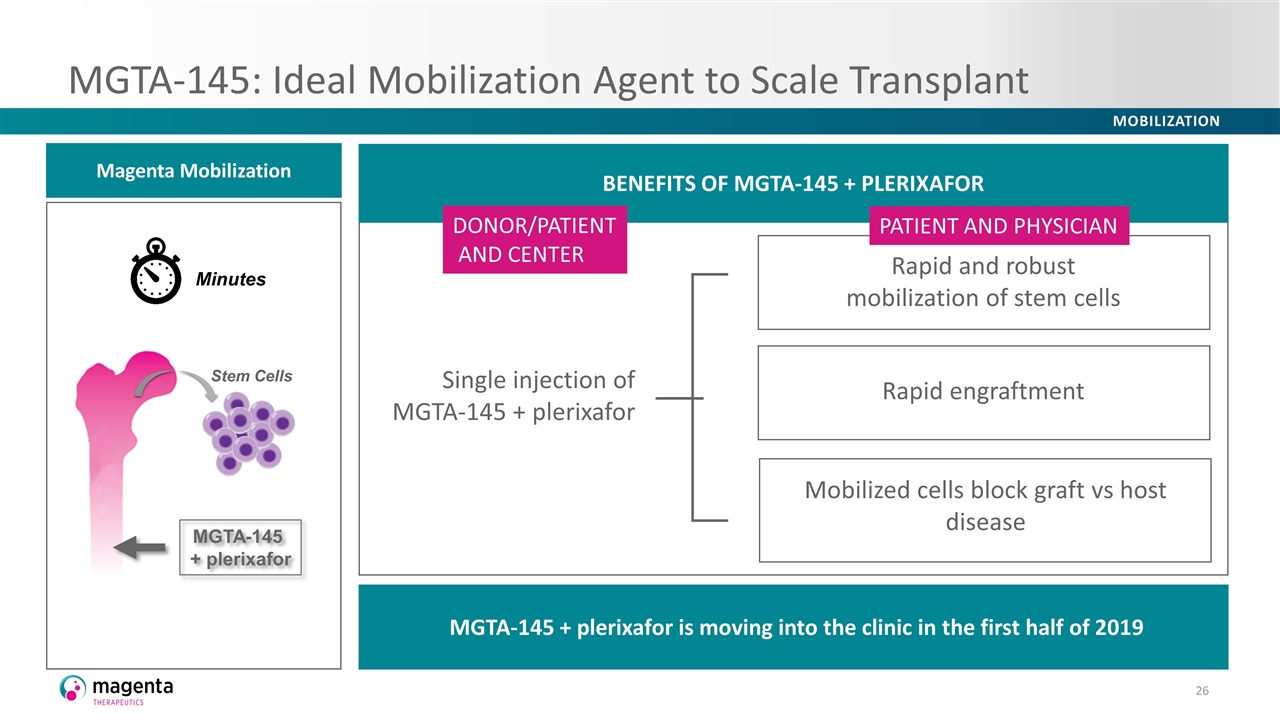
MGTA-145: Ideal Mobilization Agent to Scale Transplant BENEFITS OF MGTA-145 + PLERIXAFOR Minutes Stem Cells Magenta Mobilization MGTA-145 + plerixafor Single injection of MGTA-145 + plerixafor Rapid and robust mobilization of stem cells Mobilized cells block graft vs host disease Rapid engraftment MGTA-145 + plerixafor is moving into the clinic in the first half of 2019 DONOR/PATIENT AND CENTER PATIENT AND PHYSICIAN
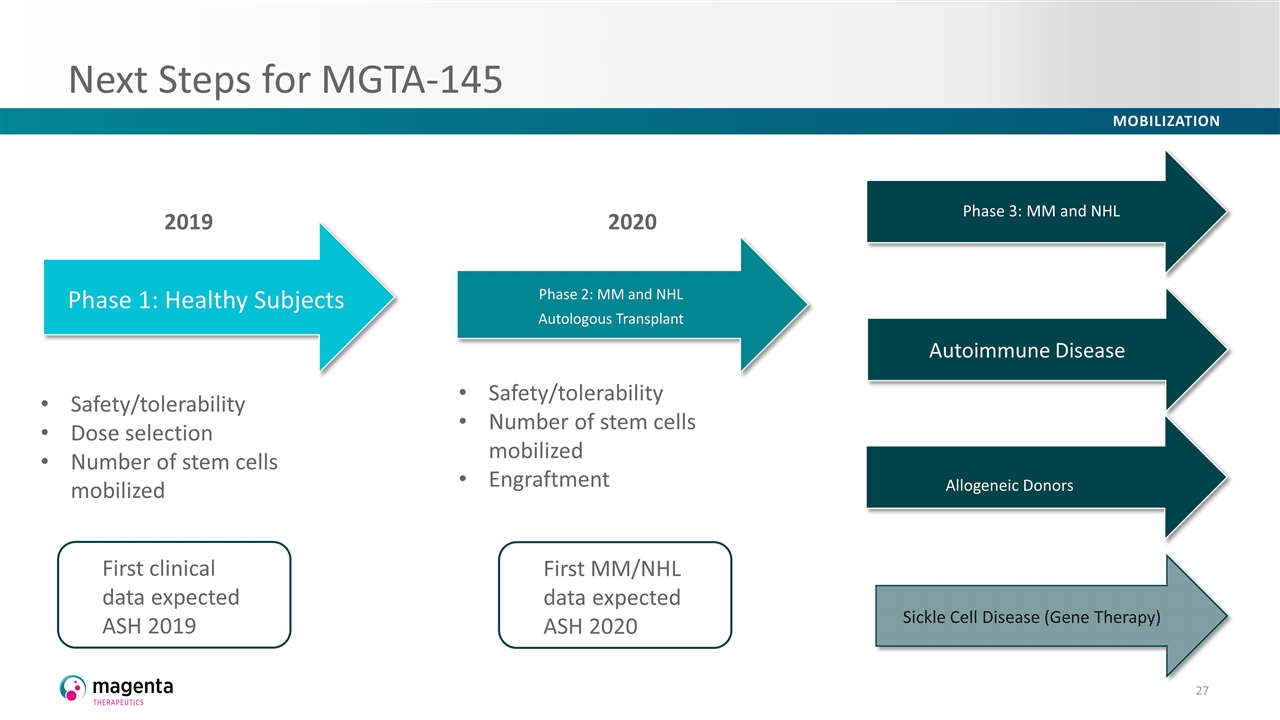
Next Steps for MGTA-145 2020 2019 Allogeneic Donors Safety/tolerability Dose selection Number of stem cells mobilized Safety/tolerability Number of stem cells mobilized Engraftment Phase 3: MM and NHL First clinical data expected ASH 2019 First MM/NHL data expected ASH 2020 Phase 2: MM and NHL Autologous Transplant Autoimmune Disease Sickle Cell Disease (Gene Therapy) Phase 1: Healthy Subjects
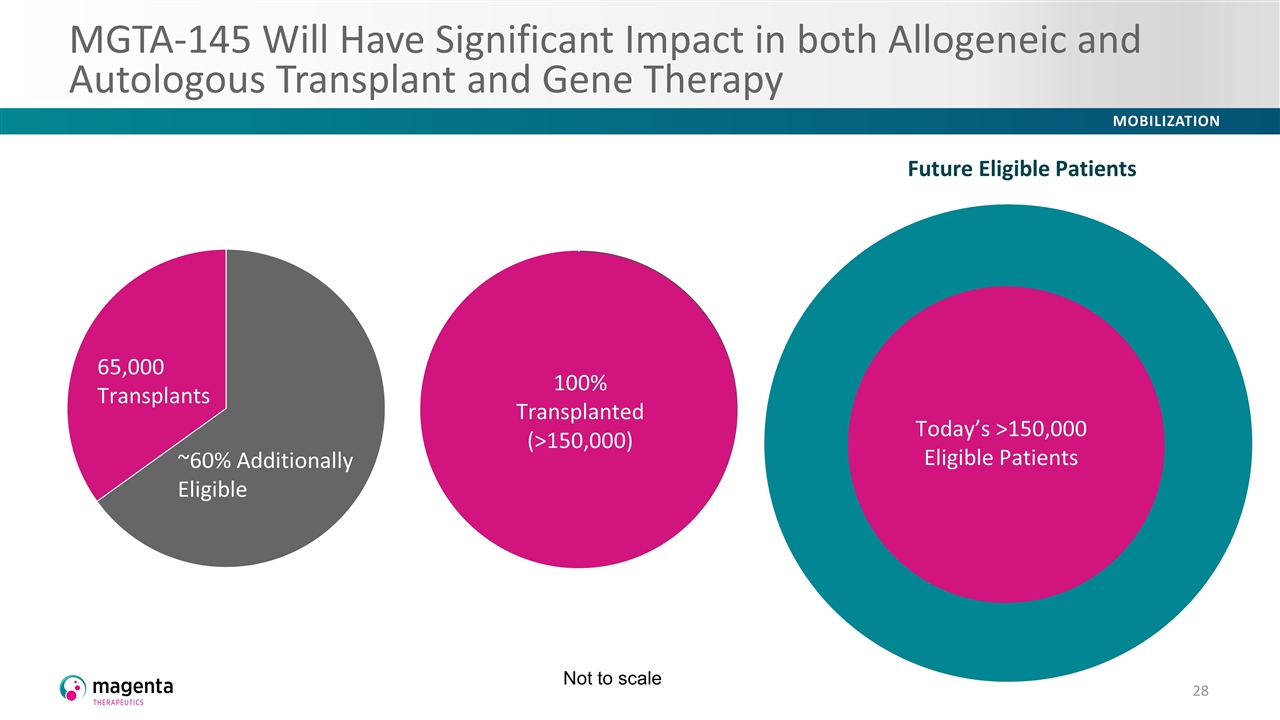
MGTA-145 Will Have Significant Impact in both Allogeneic and Autologous Transplant and Gene Therapy 65,000 Transplants Malignant Non-malignant 100% Transplanted (>150,000) Future Eligible Patients Today’s >150,000 Eligible Patients Future Eligible Patients Today’s >150,000 Eligible Patients Not to scale Today’s >150,000 Eligible Patients Future Eligible Patients Today’s >150,000 Eligible Patients 65,000 Transplants ~60% Additionally Eligible
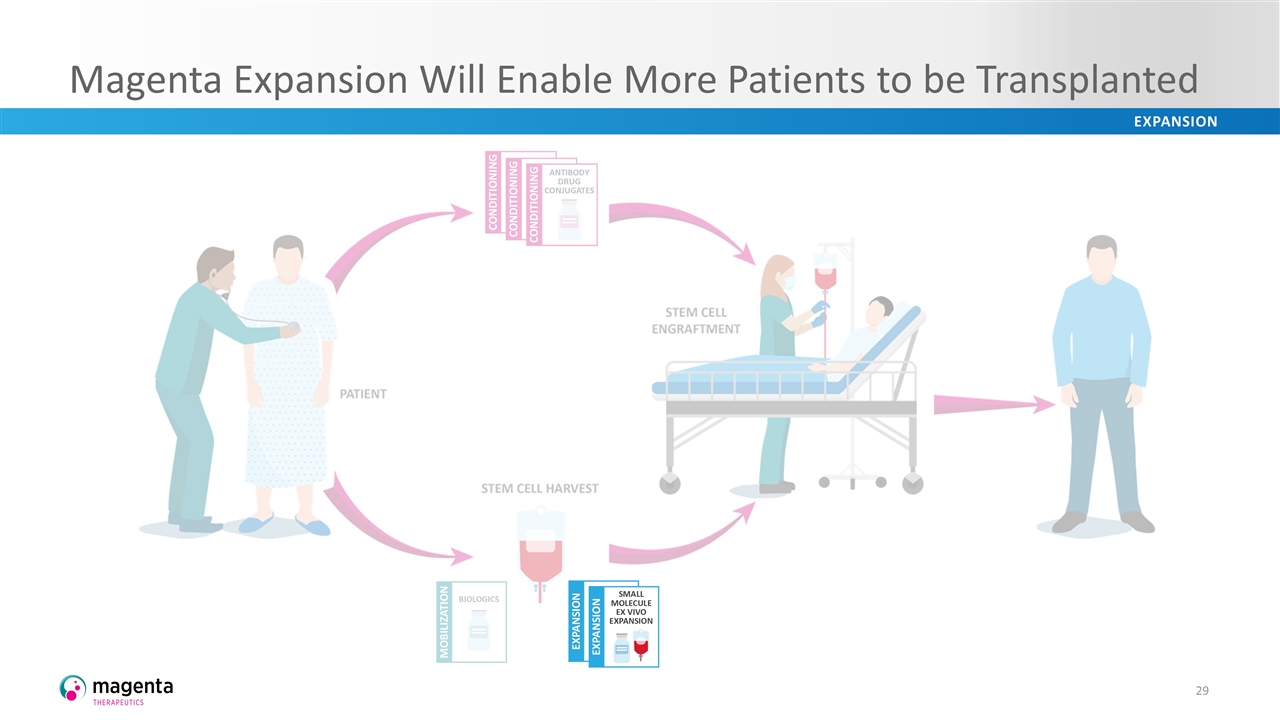
Magenta Expansion Will Enable More Patients to be Transplanted
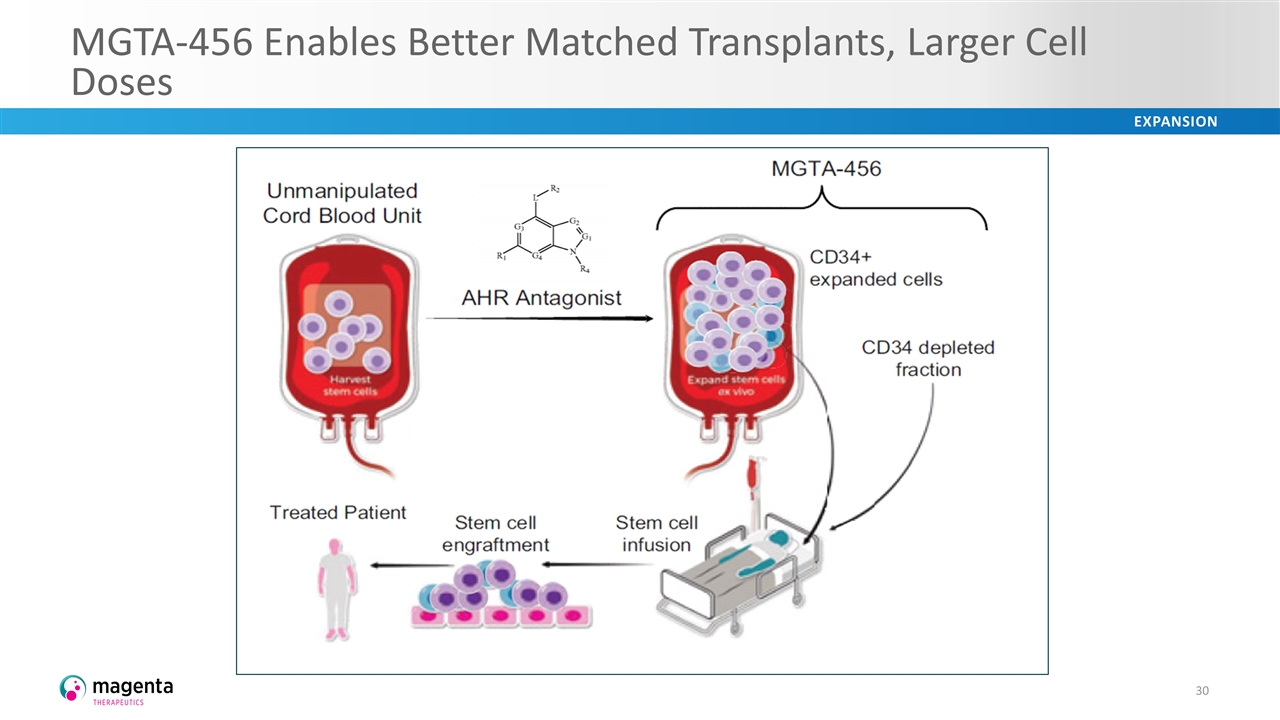
MGTA-456 Enables Better Matched Transplants, Larger Cell Doses
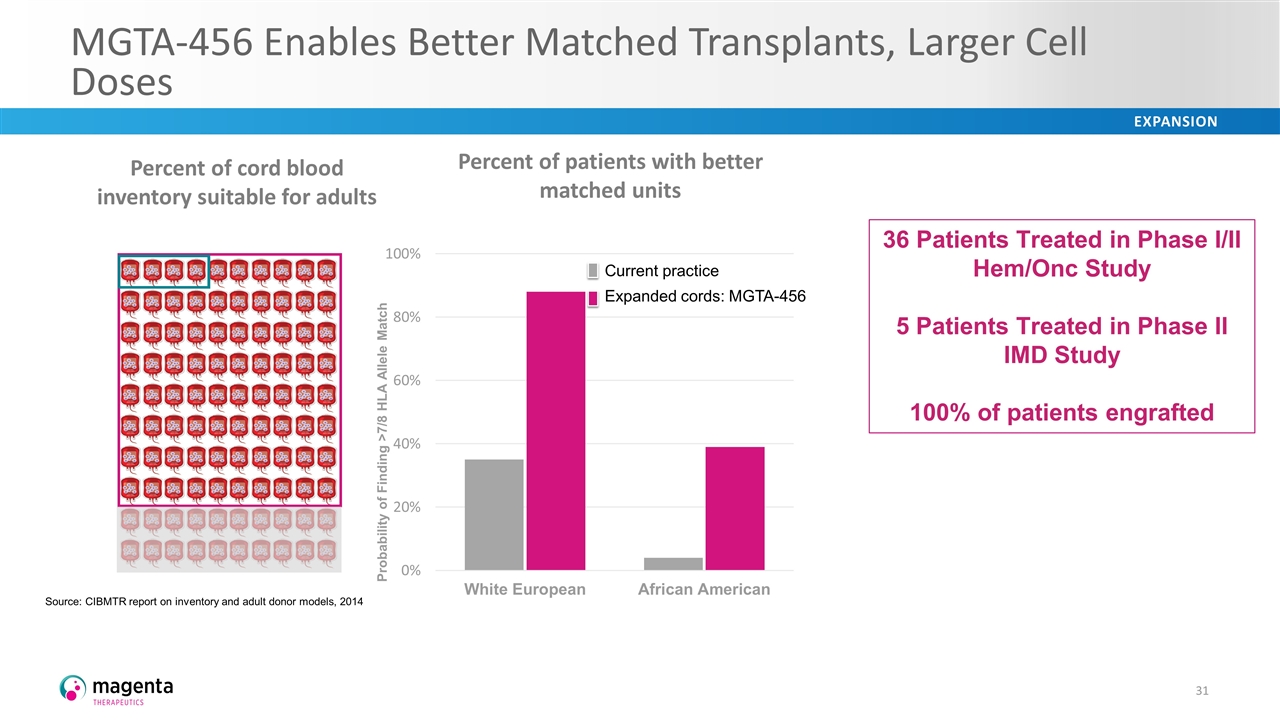
MGTA-456 Enables Better Matched Transplants, Larger Cell Doses 36 Patients Treated in Phase I/II Hem/Onc Study 5 Patients Treated in Phase II IMD Study 100% of patients engrafted Current practice Expanded cords: MGTA-456 Percent of patients with better matched units Source: CIBMTR report on inventory and adult donor models, 2014 Percent of cord blood inventory suitable for adults
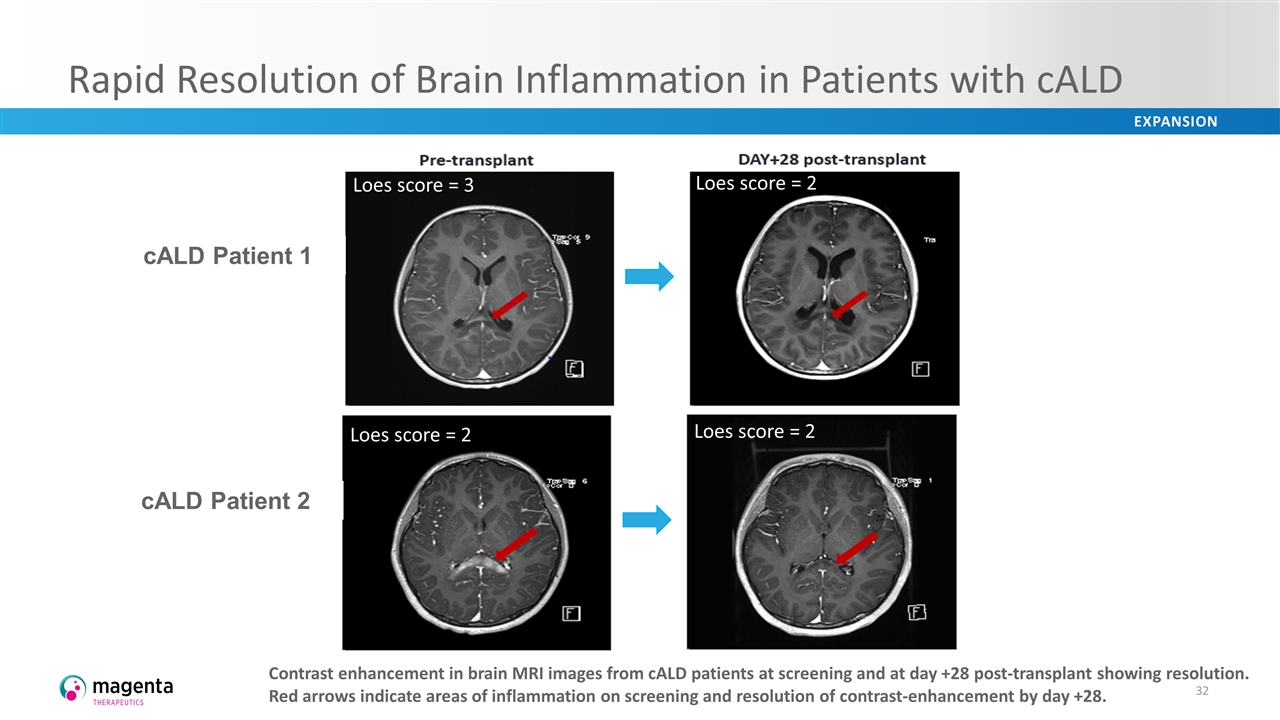
Rapid Resolution of Brain Inflammation in Patients with cALD Contrast enhancement in brain MRI images from cALD patients at screening and at day +28 post-transplant showing resolution. Red arrows indicate areas of inflammation on screening and resolution of contrast-enhancement by day +28. cALD Patient 1 cALD Patient 2 Loes score = 3 Loes score = 2 Loes score = 2 Loes score = 2
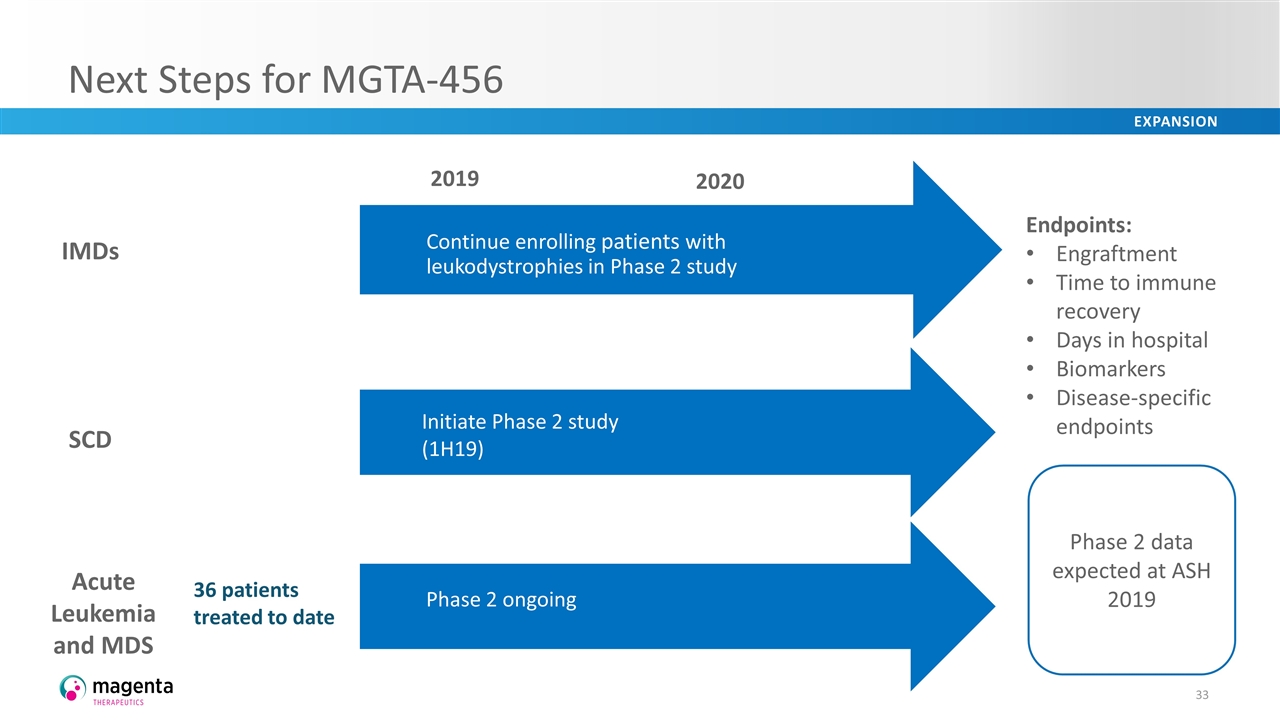
Next Steps for MGTA-456 Continue enrolling patients with leukodystrophies in Phase 2 study 2020 2019 IMDs SCD Initiate Phase 2 study (1H19) Acute Leukemia and MDS Phase 2 ongoing 36 patients treated to date Endpoints: Engraftment Time to immune recovery Days in hospital Biomarkers Disease-specific endpoints Phase 2 data expected at ASH 2019
Concluding Remarks

Key Takeaways Today Potential patient impact Next steps for each program Near-term milestones and long-term path
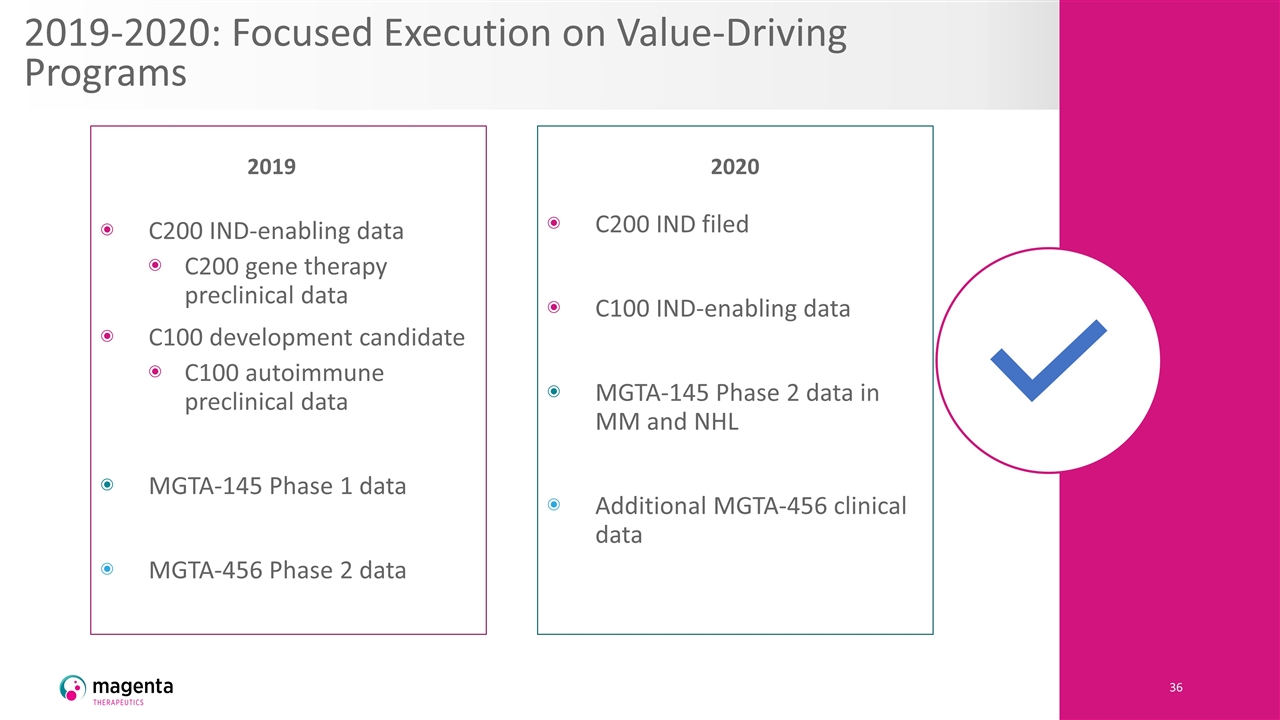
2019-2020: Focused Execution on Value-Driving Programs C200 IND-enabling data C200 gene therapy preclinical data C100 development candidate C100 autoimmune preclinical data MGTA-145 Phase 1 data MGTA-456 Phase 2 data C200 IND filed C100 IND-enabling data MGTA-145 Phase 2 data in MM and NHL Additional MGTA-456 clinical data 2019 2020
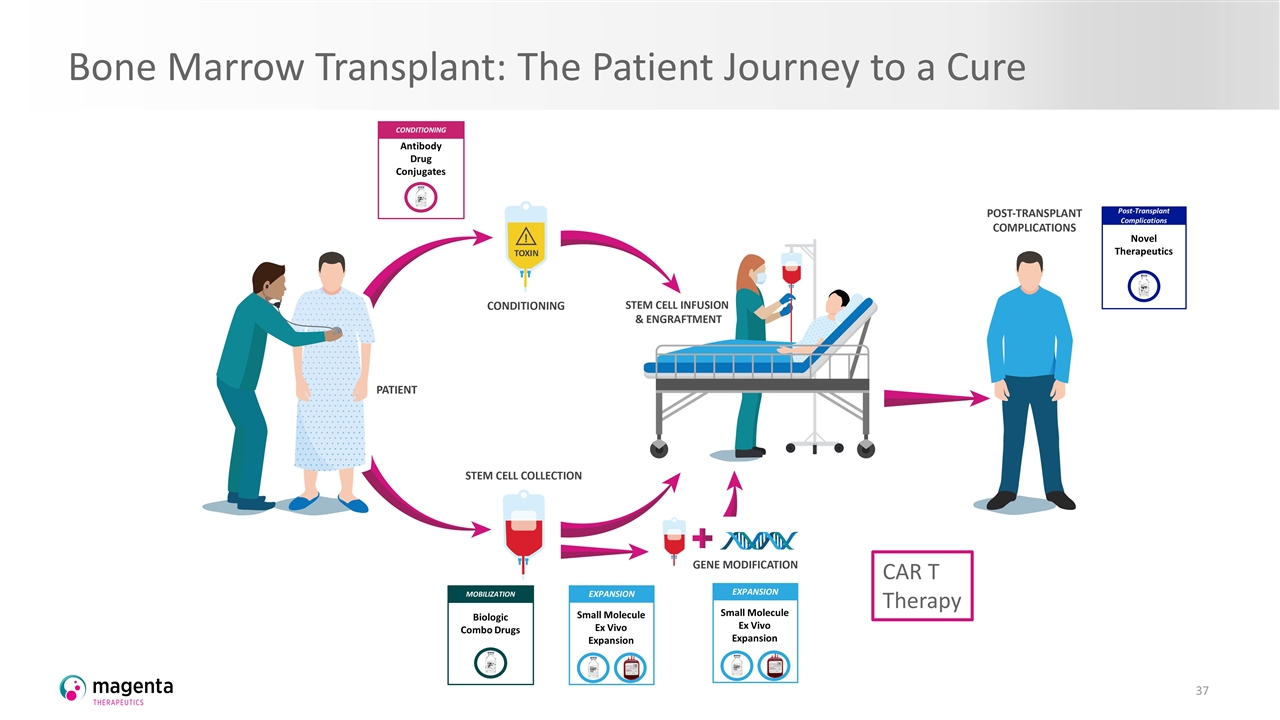
Bone Marrow Transplant: The Patient Journey to a Cure CONDITIONING Antibody Drug Conjugates EXPANSION Small Molecule Ex Vivo Expansion MOBILIZATION Biologic Combo Drugs Post-Transplant Complications Novel Therapeutics EXPANSION Small Molecule Ex Vivo Expansion CAR T Therapy
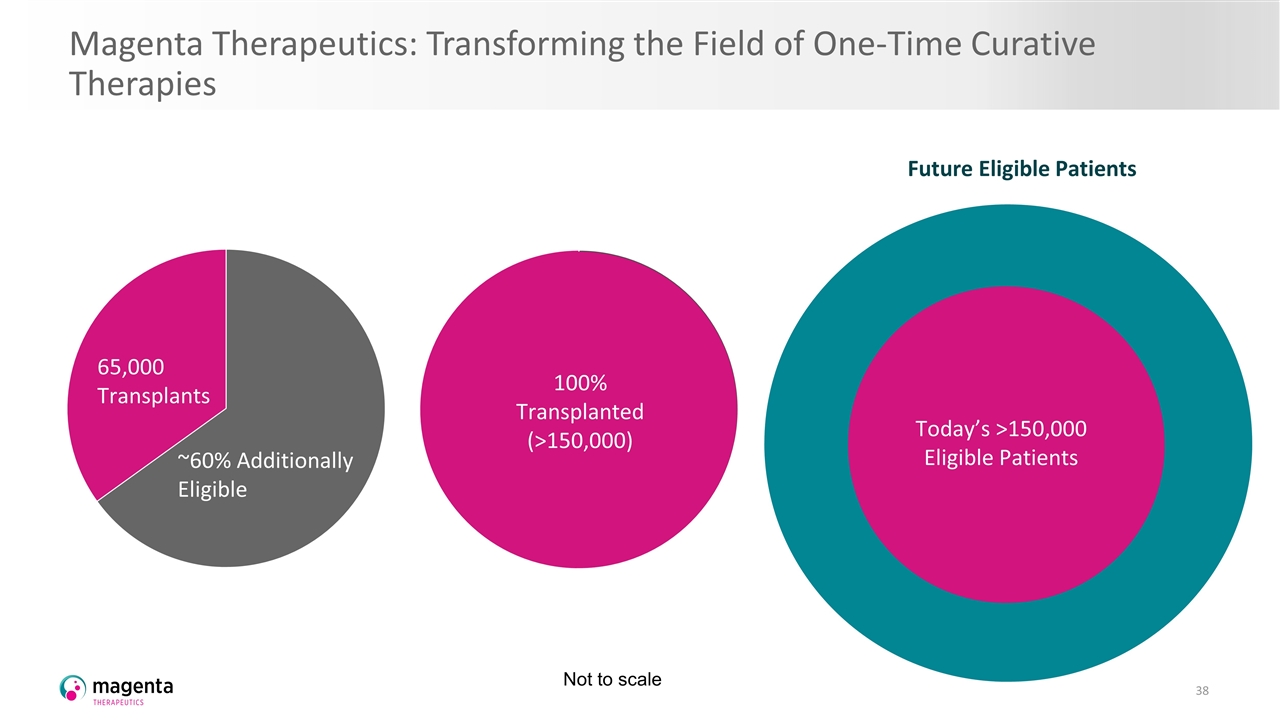
Magenta Therapeutics: Transforming the Field of One-Time Curative Therapies 65,000 Transplants Malignant Non-malignant 100% Transplanted (>150,000) Future Eligible Patients Today’s >150,000 Eligible Patients Future Eligible Patients Today’s >150,000 Eligible Patients Not to scale Today’s >150,000 Eligible Patients Future Eligible Patients Today’s >150,000 Eligible Patients 65,000 Transplants ~60% Additionally Eligible
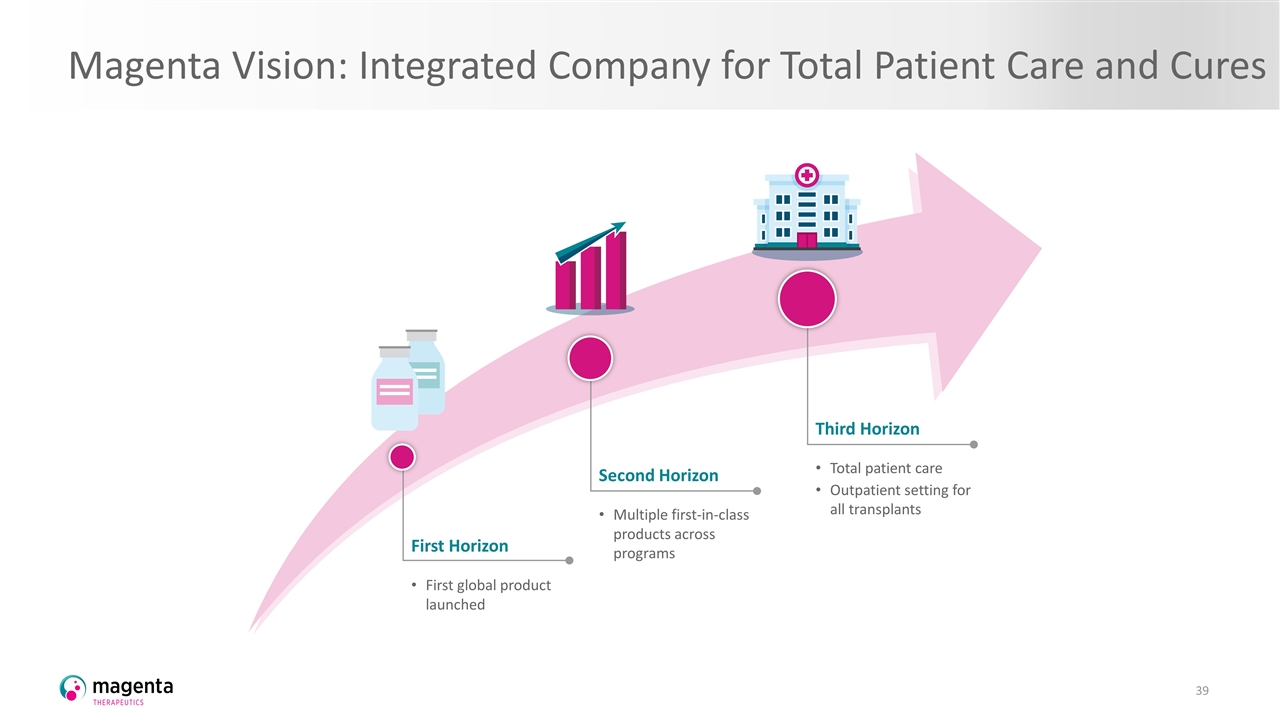
Magenta Vision: Integrated Company for Total Patient Care and Cures Third Horizon Total patient care Outpatient setting for all transplants Second Horizon Multiple first-in-class products across programs First Horizon First global product launched

The Promise of a One-Time Curative Therapy







































