
Exhibit 99.2 January 2020

Forward-Looking Statements This presentation contains forward-looking statements and information within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “will,” “could”, “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “seeks,” “endeavor,” “potential,” “continue” or the negative of such words or other similar expressions can be used to identify forward-looking statements. The express or implied forward-looking statements included in this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: uncertainties inherent in clinical studies and in the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of the trial; whether results from preclinical studies or earlier clinical studies will be predictive of the results of future trials; the expected timing of submissions for regulatory approval or review by governmental authorities, including review under accelerated approval processes; orphan drug designation eligibility; regulatory approvals to conduct trials or to market products; whether Magenta's cash resources will be sufficient to fund Magenta's foreseeable and unforeseeable operating expenses and capital expenditure requirements; and other risks set forth under the caption “Risk Factors” in Magenta’s Form 10-K filed with the Securities and Exchange Commission (the “SEC”) as updated by Quarterly Reports on Form 10-Q and its other filings made with the SEC from time to time. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although Magenta believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither Magenta nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements included in this presentation. Any forward-looking statement included in this presentation speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.

Multiple Sclerosis The Patient Journey

The Promise of a New Immune System: Jennie’s Transplant Journey
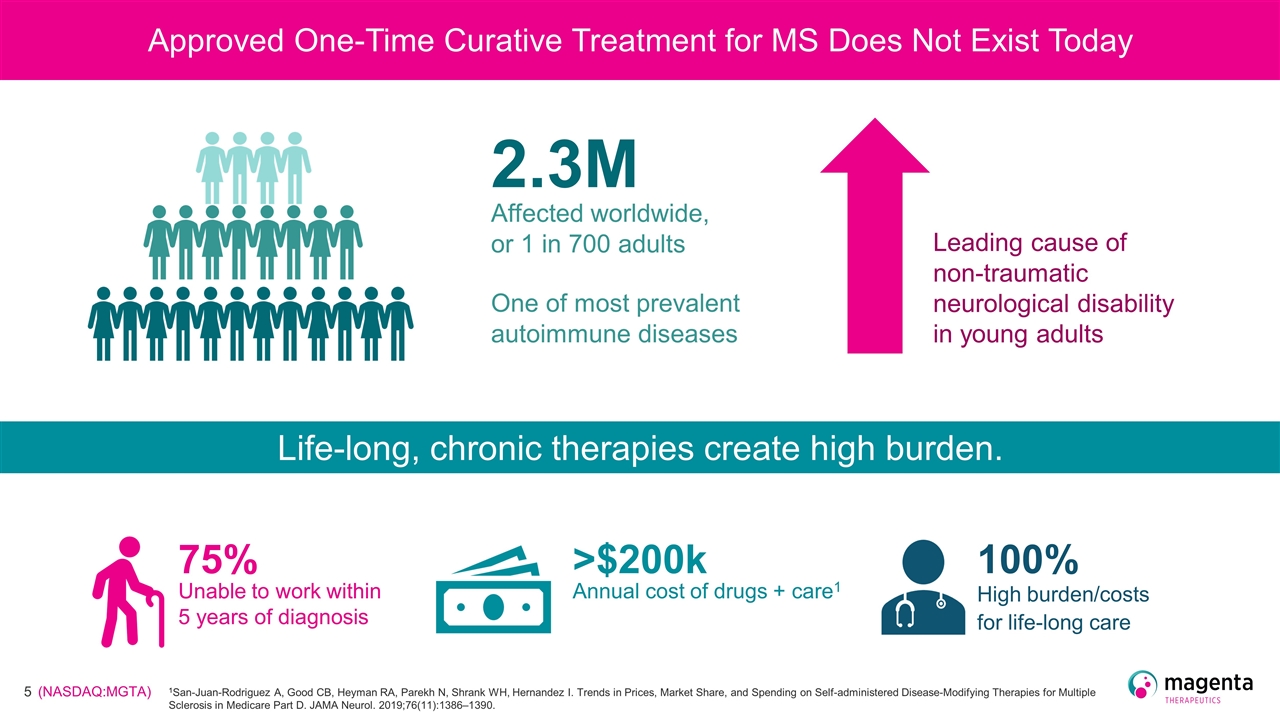
Approved One-Time Curative Treatment for MS Does Not Exist Today 2.3M Affected worldwide, or 1 in 700 adults One of most prevalent autoimmune diseases Leading cause of non-traumatic neurological disability in young adults Life-long, chronic therapies create high burden. 75% Unable to work within 5 years of diagnosis Annual cost of drugs + care1 >$200k High burden/costs for life-long care 100% 1San-Juan-Rodriguez A, Good CB, Heyman RA, Parekh N, Shrank WH, Hernandez I. Trends in Prices, Market Share, and Spending on Self-administered Disease-Modifying Therapies for Multiple Sclerosis in Medicare Part D. JAMA Neurol. 2019;76(11):1386–1390.
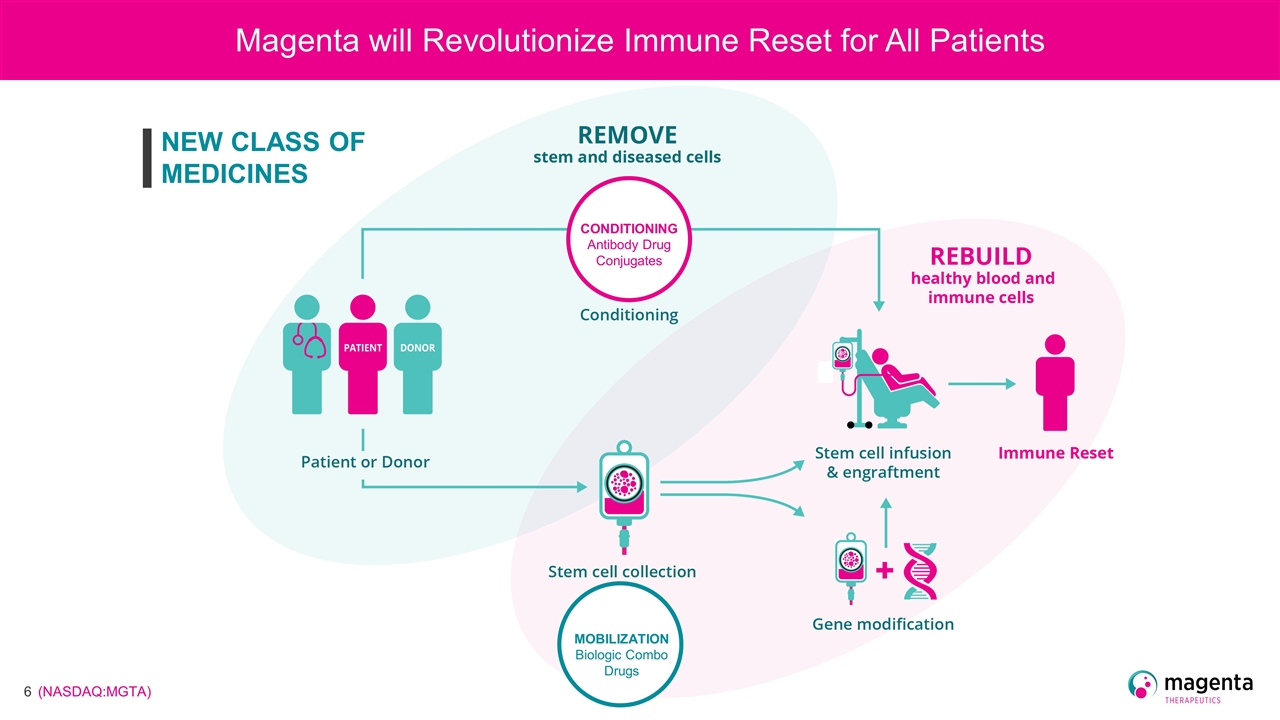
Magenta will Revolutionize Immune Reset for All Patients CONDITIONING Antibody Drug Conjugates MOBILIZATION Biologic Combo Drugs NEW CLASS OF MEDICINES
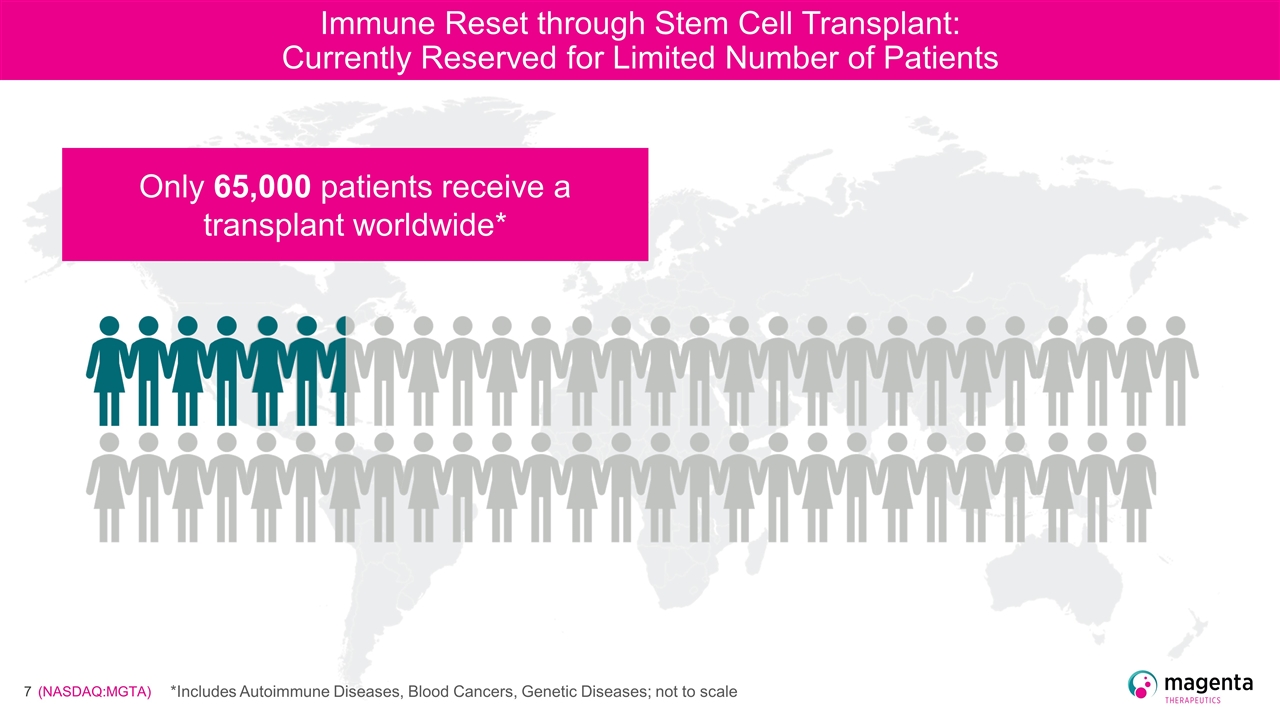
Immune Reset through Stem Cell Transplant: Currently Reserved for Limited Number of Patients Only 65,000 patients receive a transplant worldwide* *Includes Autoimmune Diseases, Blood Cancers, Genetic Diseases; not to scale
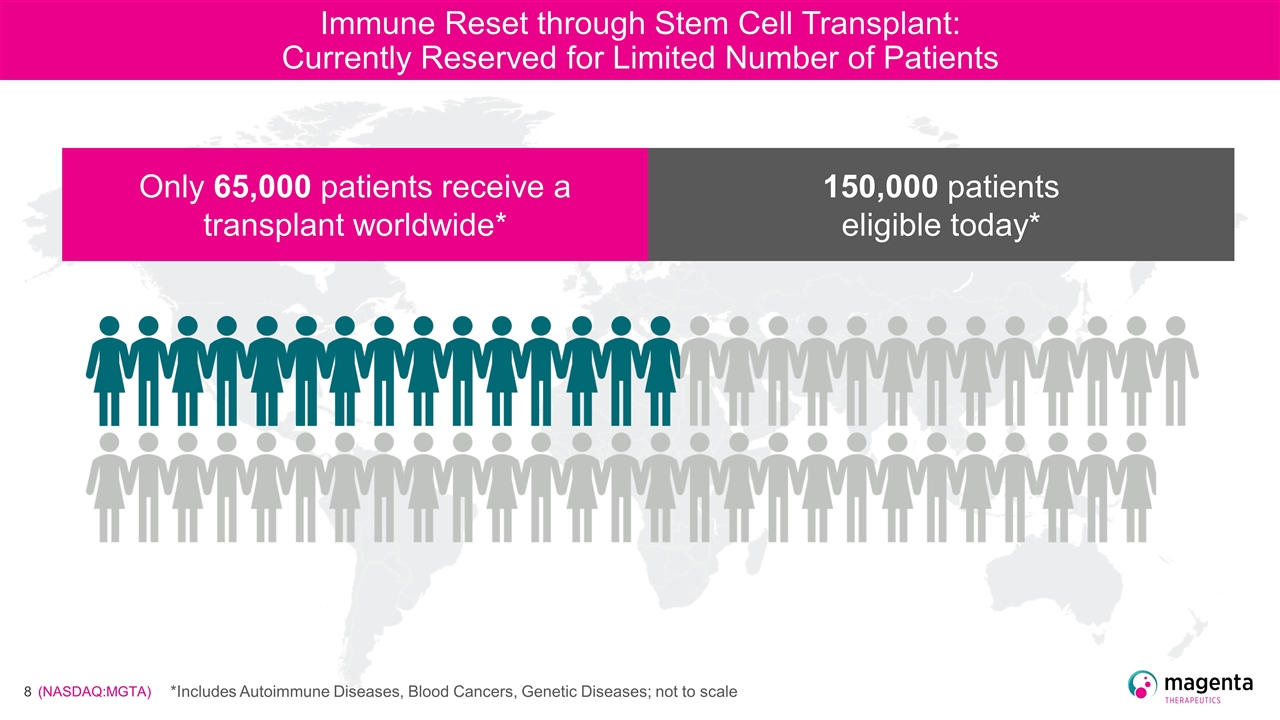
Immune Reset through Stem Cell Transplant: Currently Reserved for Limited Number of Patients Only 65,000 patients receive a transplant worldwide* 150,000 patients eligible today* *Includes Autoimmune Diseases, Blood Cancers, Genetic Diseases; not to scale
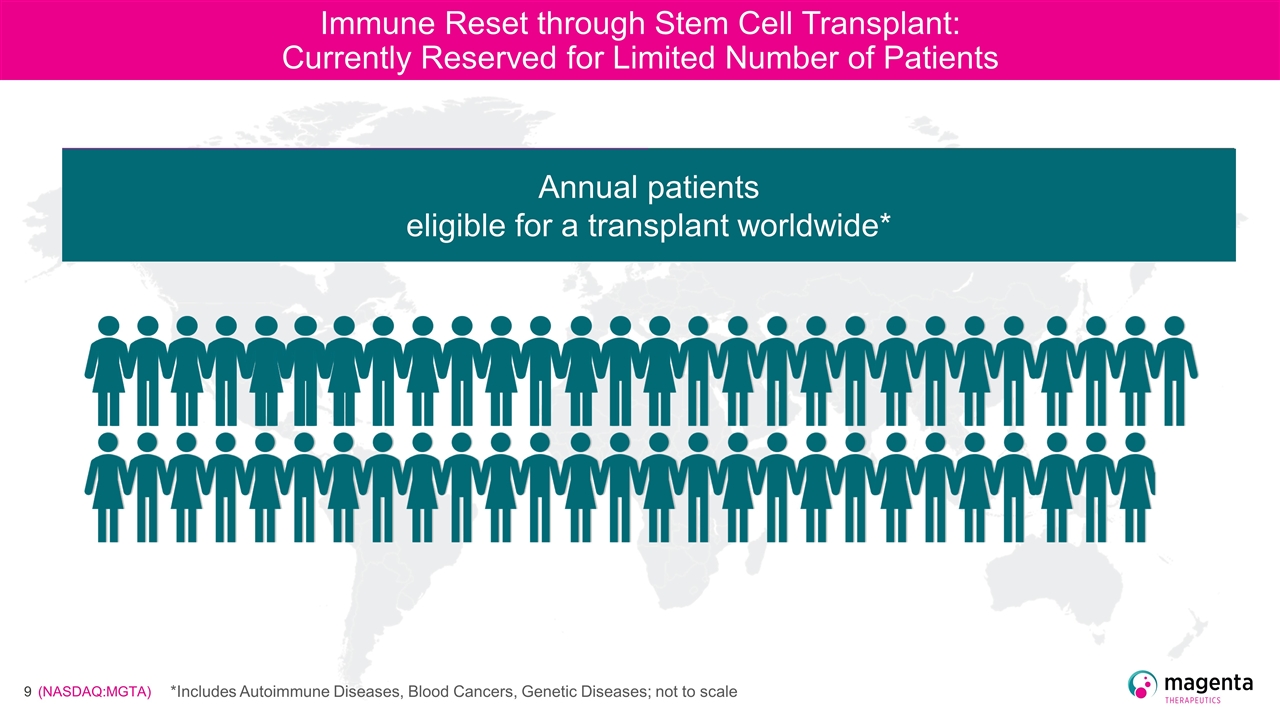
Immune Reset through Stem Cell Transplant: Currently Reserved for Limited Number of Patients Only 65,000 patients receive a transplant worldwide* 150,000 patients eligible today* Annual patients eligible for a transplant worldwide* *Includes Autoimmune Diseases, Blood Cancers, Genetic Diseases; not to scale
Platform Pipeline Vision From Platform to Medicines and One-time Cures Discovery Engine Differentiated first-in-class medicines Total patient care in outpatient setting

We Are Magenta

Delivering Immune System Reset to Make Cures Possible for More Patients Only company comprehensively transforming transplant across autoimmune diseases, genetic diseases and blood cancers People, platforms and product engine to change practice of transplant medicine Portfolio of innovative, first-in-class therapies, including two clinical programs Large market opportunity Strong cash position of approximately $146M to fund operations into 4Q 2021
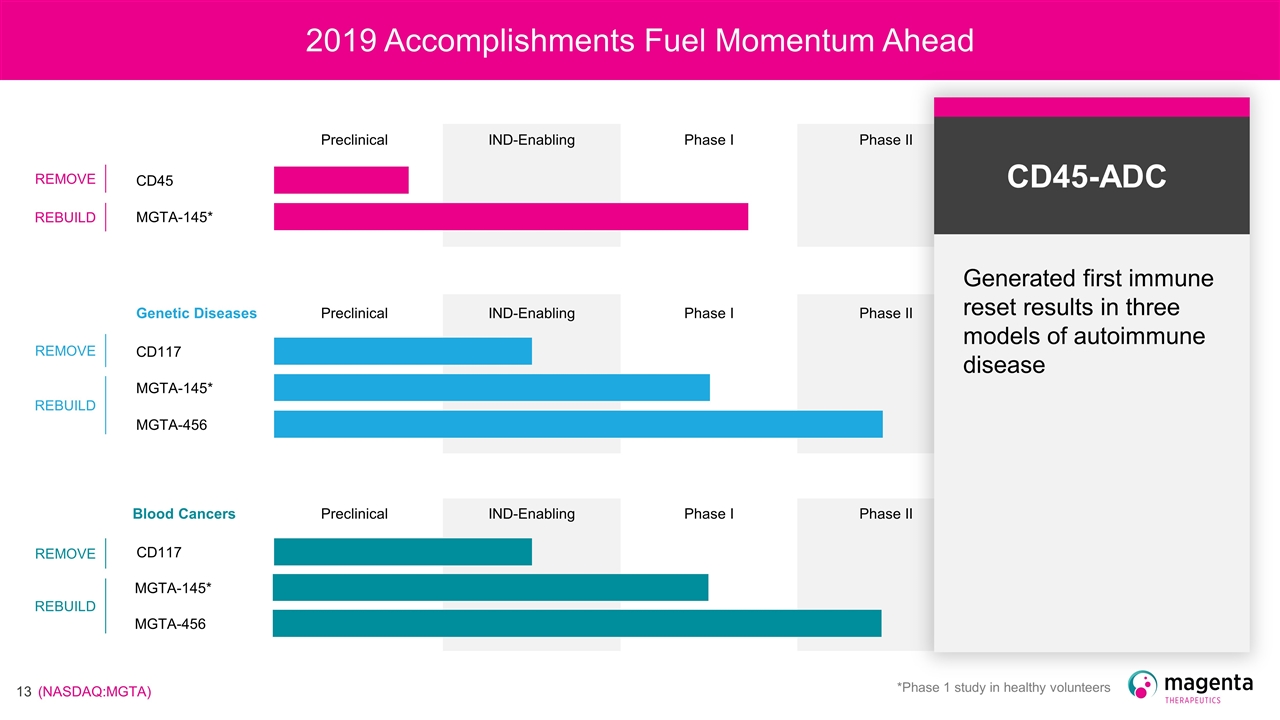
2019 Accomplishments Fuel Momentum Ahead Preclinical IND-Enabling Phase I Phase II CD45 MGTA-145* Genetic Diseases Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 Blood Cancers Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 REMOVE REBUILD REMOVE REBUILD REMOVE REBUILD *Phase 1 study in healthy volunteers CD45-ADC Generated first immune reset results in three models of autoimmune disease

2019 Accomplishments Fuel Momentum Ahead Preclinical IND-Enabling Phase I Phase II CD45 MGTA-145* Genetic Diseases Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 Blood Cancers Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 REMOVE REBUILD REMOVE REBUILD REMOVE REBUILD *Phase 1 study in healthy volunteers CD117-ADC Generated proof of concept results in transplant gene therapy – validates ADC as single dose, single agent for transplant or gene therapy; advances conditioning portfolio

2019 Accomplishments Fuel Momentum Ahead Preclinical IND-Enabling Phase I Phase II CD45 MGTA-145* Genetic Diseases Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 Blood Cancers Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 REMOVE REBUILD REMOVE REBUILD REMOVE REBUILD *Phase 1 study in healthy volunteers MGTA-145 Clinically validated through Phase I data in healthy volunteers – readthrough for donors and patients

2019 Accomplishments Fuel Momentum Ahead Preclinical IND-Enabling Phase I Phase II CD45 MGTA-145* Genetic Diseases Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 Blood Cancers Preclinical IND-Enabling Phase I Phase II CD117 MGTA-145* MGTA-456 REMOVE REBUILD REMOVE REBUILD REMOVE REBUILD *Phase 1 study in healthy volunteers MGTA-456 New Phase II data deliver rapid and durable resolution of disease in patients with IMD – beyond benefit seen with gene therapies

Delivering Immune Reset to Patients
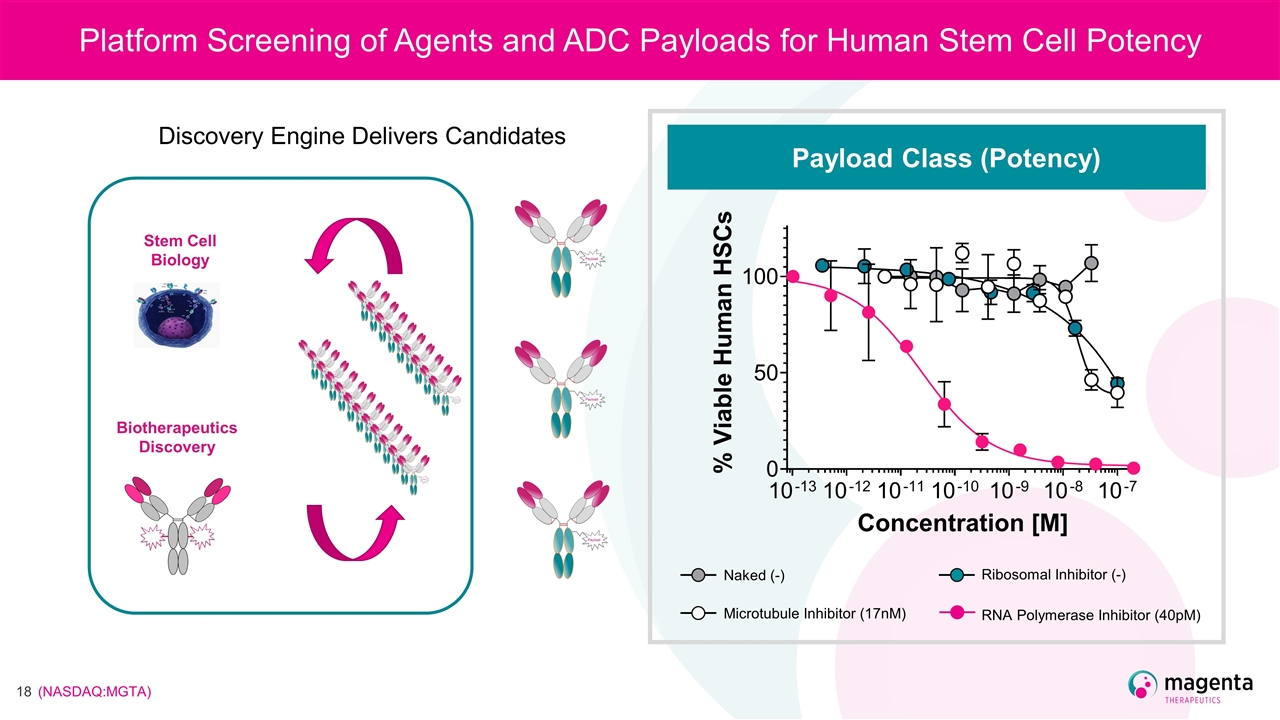
Platform Screening of Agents and ADC Payloads for Human Stem Cell Potency Discovery Biology Biotherapeutics Discovery Stem Cell Biology Payload Class (Potency) RNA Polymerase Inhibitor (40pM) Microtubule Inhibitor (17nM) Ribosomal Inhibitor (-) Naked (-) Discovery Engine Delivers Candidates
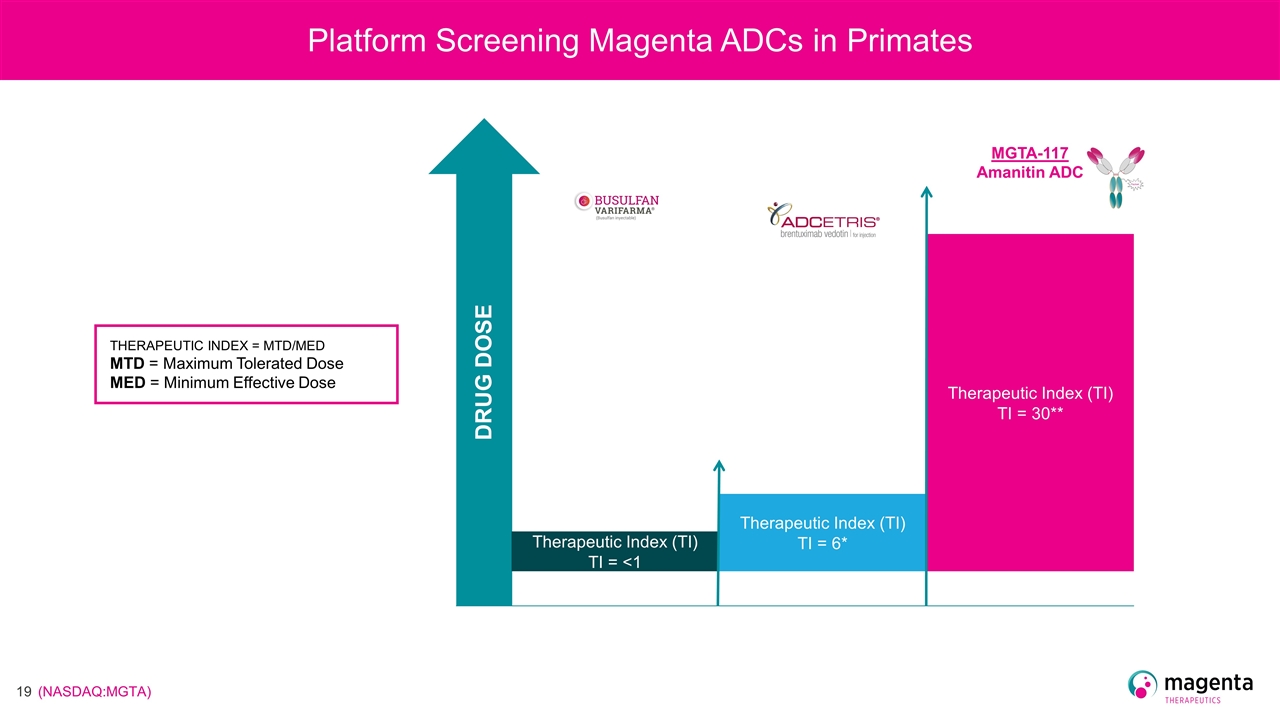
Platform Screening Magenta ADCs in Primates DRUG DOSE Therapeutic Index (TI) TI = <1 THERAPEUTIC INDEX = MTD/MED MTD = Maximum Tolerated Dose MED = Minimum Effective Dose Therapeutic Index (TI) TI = 6* MGTA-117 Amanitin ADC Therapeutic Index (TI) TI = 30**
MOBILIZATION Biologic Combo Drugs Magenta will Revolutionize Immune Reset for All Patients CONDITIONING Antibody Drug Conjugates NEW CLASS OF MEDICINES
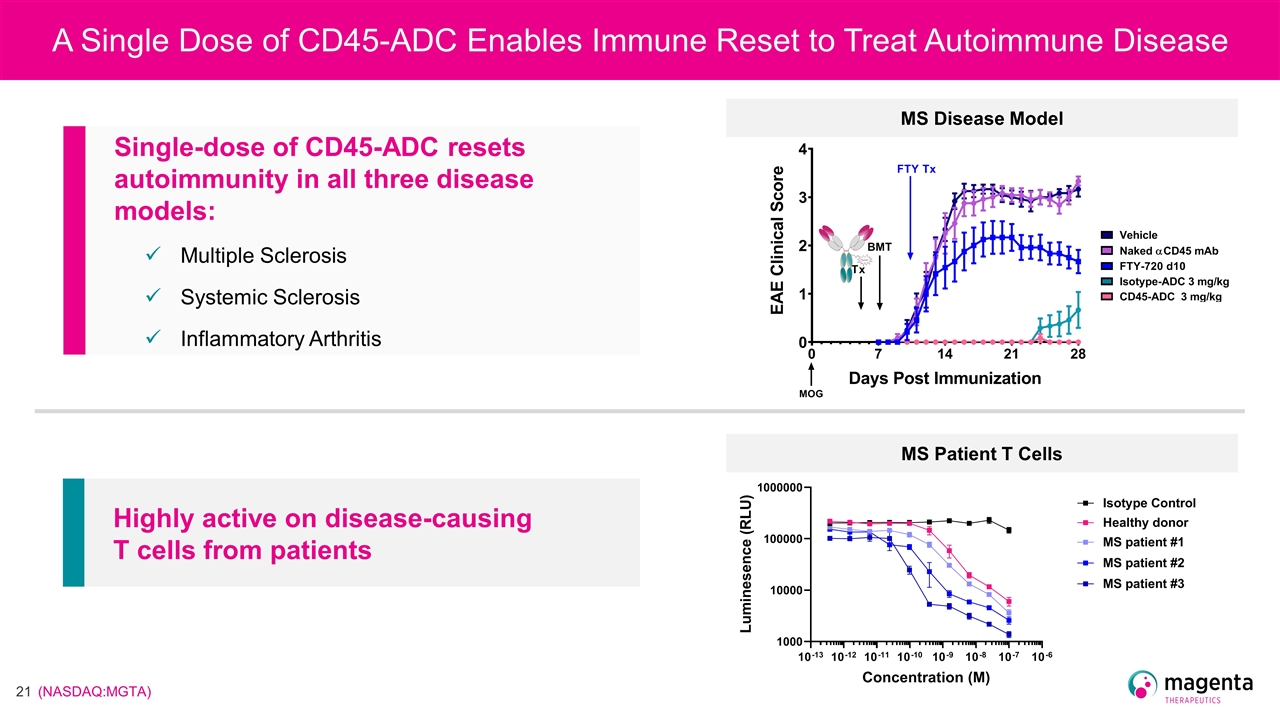
Highly active on disease-causing T cells from patients Single-dose of CD45-ADC resets autoimmunity in all three disease models: Multiple Sclerosis Systemic Sclerosis Inflammatory Arthritis MS Patient T Cells MS Disease Model A Single Dose of CD45-ADC Enables Immune Reset to Treat Autoimmune Disease
Potential opportunity Autoimmune Disease Future Next steps for CD45-ADC GMP manufacturing started Advance IND-enabling studies Initiate Phase I/II clinical trials in patients Transplants today * Not to scale
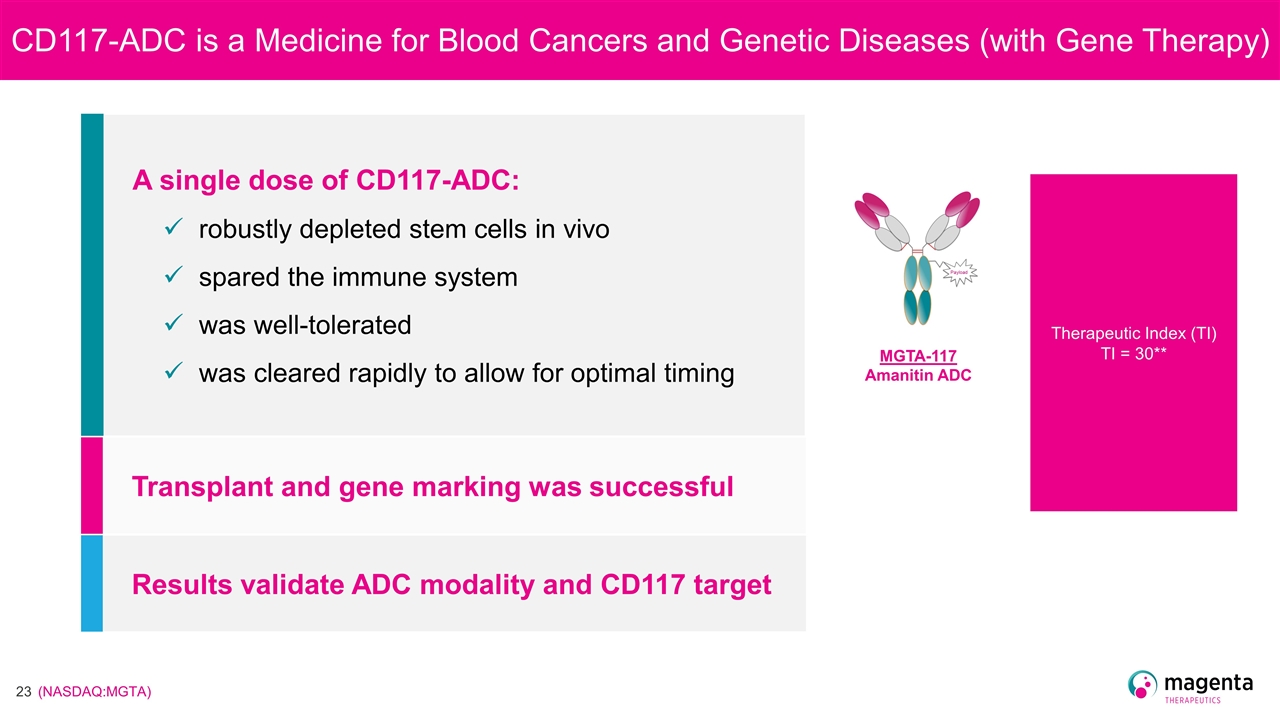
CD117-ADC is a Medicine for Blood Cancers and Genetic Diseases (with Gene Therapy) A single dose of CD117-ADC: robustly depleted stem cells in vivo spared the immune system was well-tolerated was cleared rapidly to allow for optimal timing Transplant and gene marking was successful Results validate ADC modality and CD117 target MGTA-117 Amanitin ADC Therapeutic Index (TI) TI = 30**
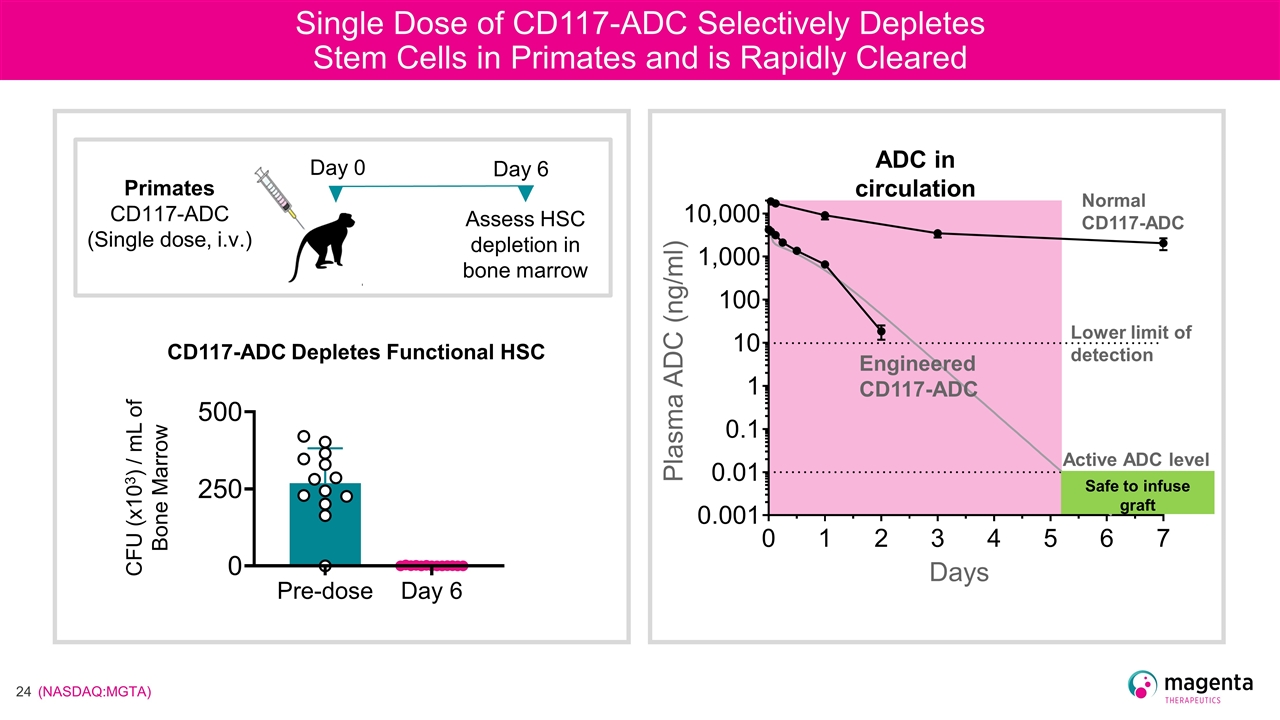
Single Dose of CD117-ADC Selectively Depletes Stem Cells in Primates and is Rapidly Cleared CD117-ADC Depletes Functional HSC CFU (x103) / mL of Bone Marrow Assess HSC depletion in bone marrow Primates CD117-ADC (Single dose, i.v.) Day 6 Day 0 Plasma ADC (ng/ml) Days Engineered CD117-ADC Lower limit of detection ADC in circulation Normal CD117-ADC Safe to infuse graft Active ADC level
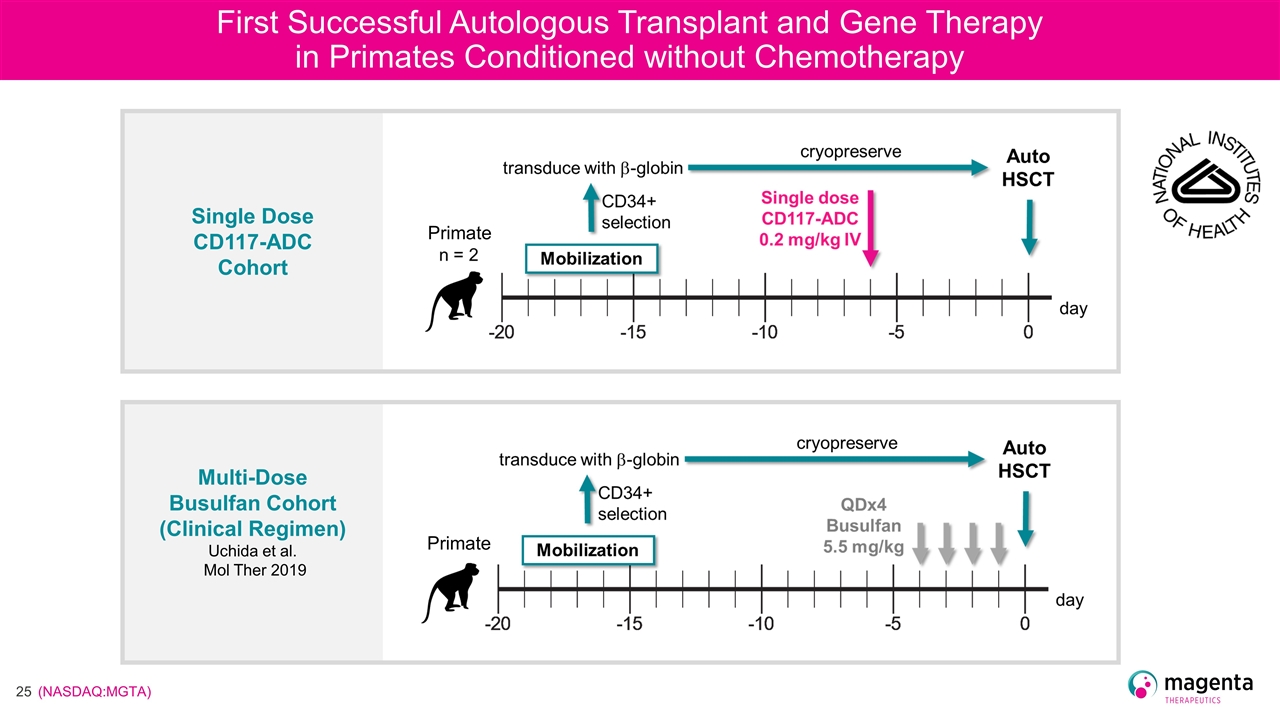
First Successful Autologous Transplant and Gene Therapy in Primates Conditioned without Chemotherapy Single Dose CD117-ADC Cohort Multi-Dose Busulfan Cohort (Clinical Regimen) Uchida et al. Mol Ther 2019 Primate Primate
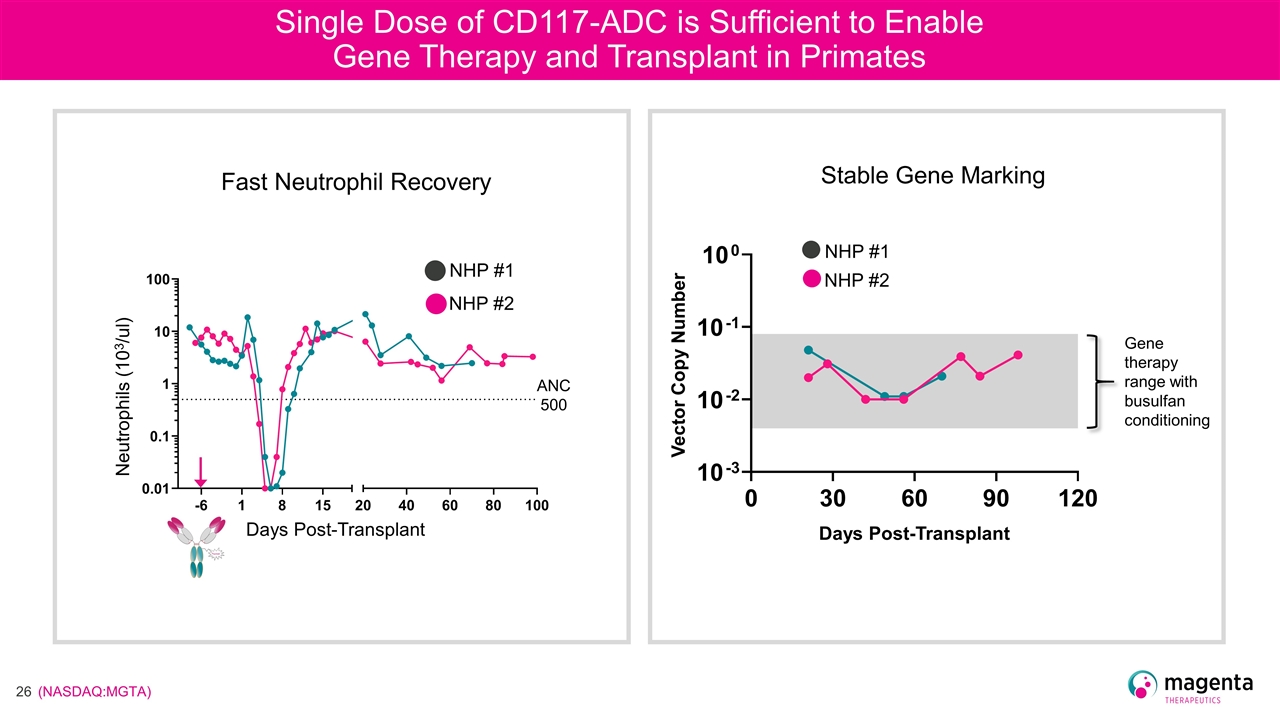
Single Dose of CD117-ADC is Sufficient to Enable Gene Therapy and Transplant in Primates Days Post-Transplant Neutrophils (103/ul) NHP #1 NHP #2 Fast Neutrophil Recovery ANC 500 Vector Copy Number Days Post-Transplant NHP #1 NHP #2 Gene therapy range with busulfan conditioning Stable Gene Marking
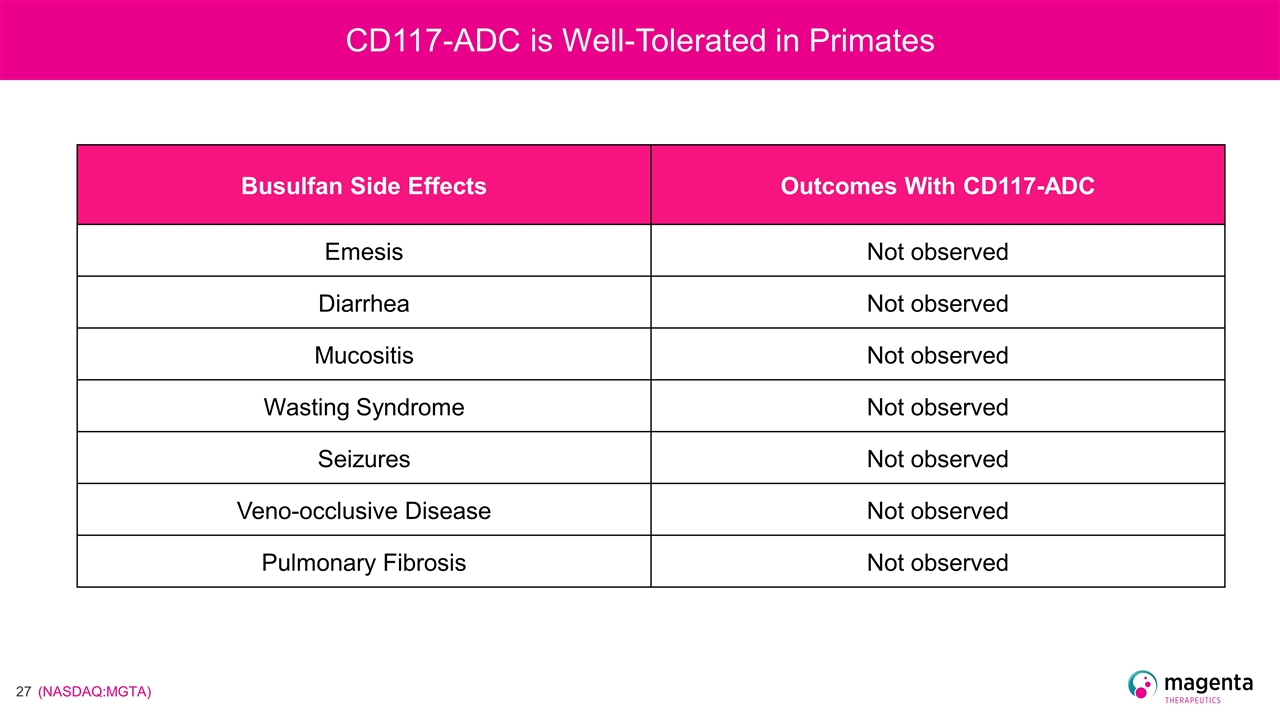
Busulfan Side Effects Outcomes With CD117-ADC Emesis Not observed Diarrhea Not observed Mucositis Not observed Wasting Syndrome Not observed Seizures Not observed Veno-occlusive Disease Not observed Pulmonary Fibrosis Not observed CD117-ADC is Well-Tolerated in Primates
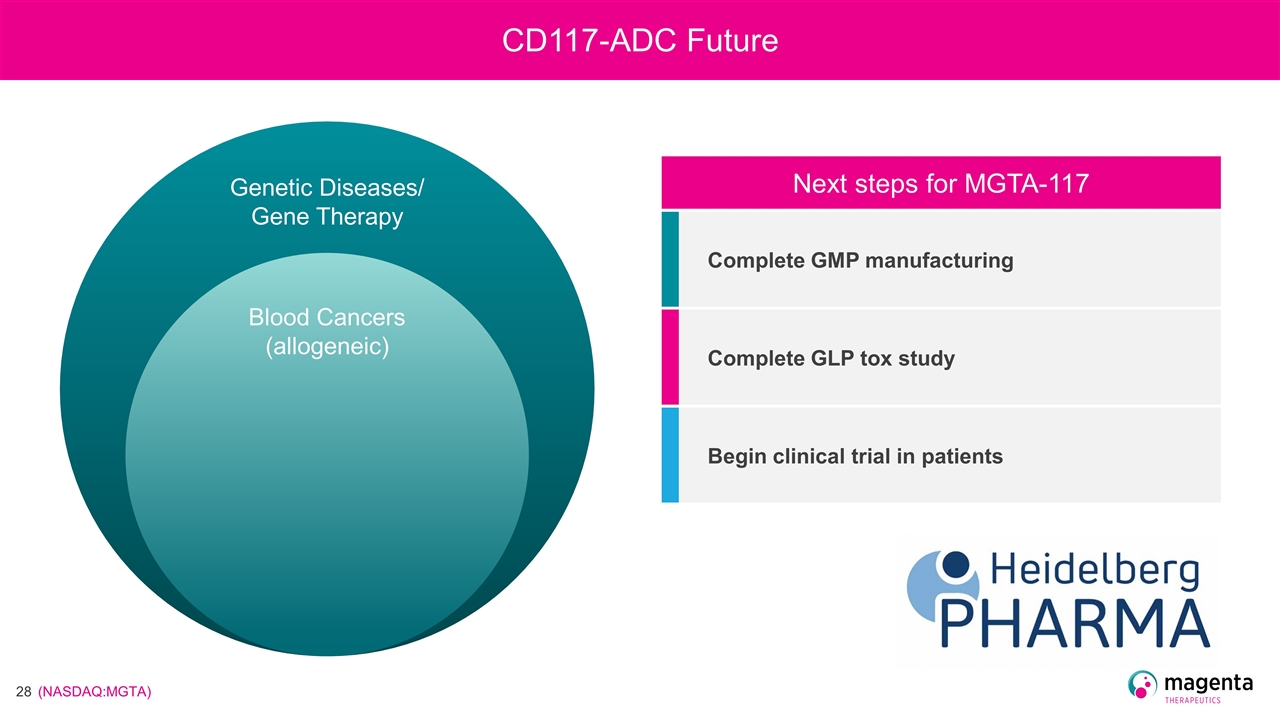
CD117-ADC Future Next steps for MGTA-117 Complete GMP manufacturing Complete GLP tox study Begin clinical trial in patients Blood Cancers (allogeneic) Genetic Diseases/ Gene Therapy
Magenta will Revolutionize Immune Reset for All Patients CONDITIONING Antibody Drug Conjugates NEW CLASS OF MEDICINES MOBILIZATION Biologic Combo Drugs
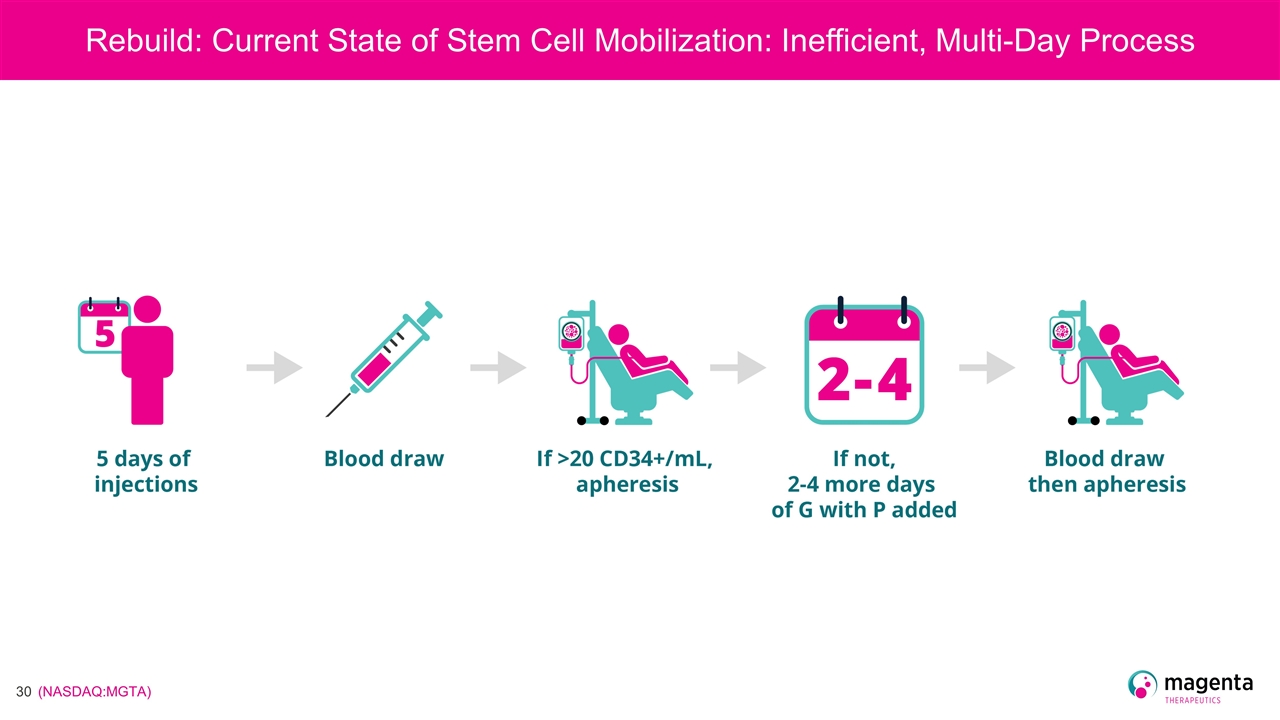
Rebuild: Current State of Stem Cell Mobilization: Inefficient, Multi-Day Process
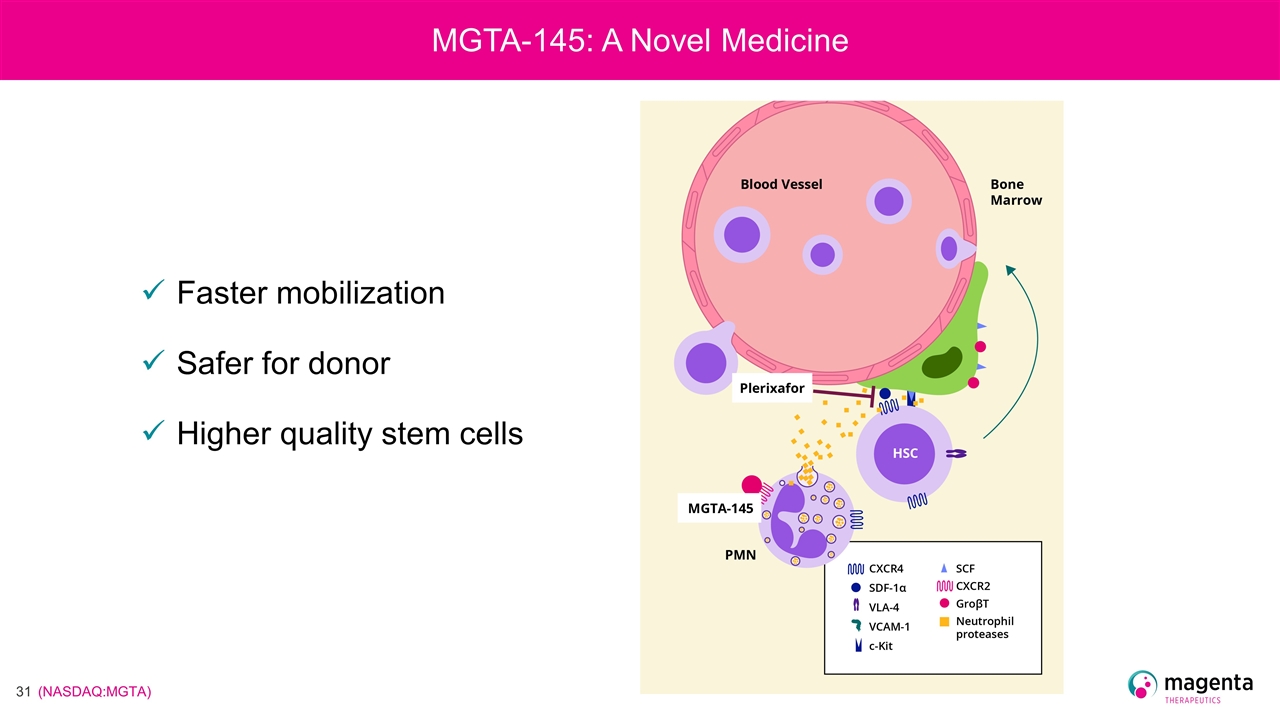
Faster mobilization Safer for donor Higher quality stem cells MGTA-145: A Novel Medicine
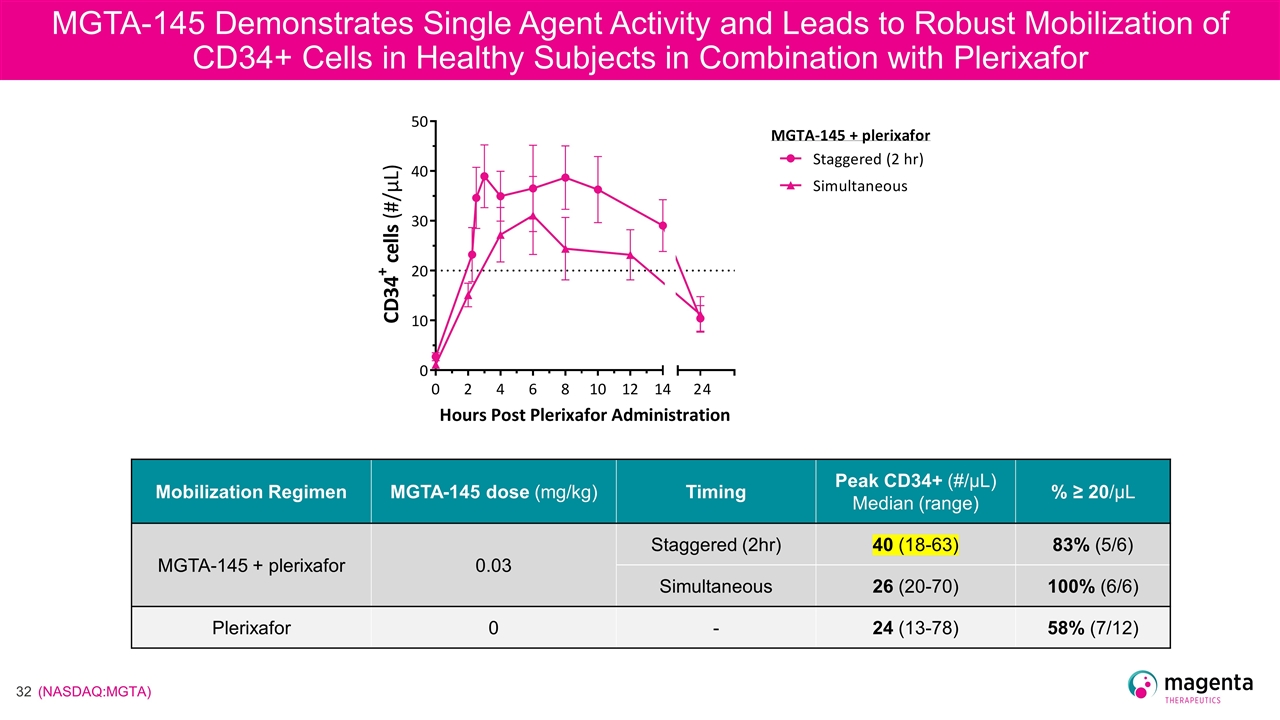
MGTA-145 Demonstrates Single Agent Activity and Leads to Robust Mobilization of CD34+ Cells in Healthy Subjects in Combination with Plerixafor Mobilization Regimen MGTA-145 dose (mg/kg) Timing Peak CD34+ (#/µL) Median (range) % ≥ 20/µL MGTA-145 + plerixafor 0.03 Staggered (2hr) 40 (18-63) 83% (5/6) Simultaneous 26 (20-70) 100% (6/6) Plerixafor 0 - 24 (13-78) 58% (7/12)
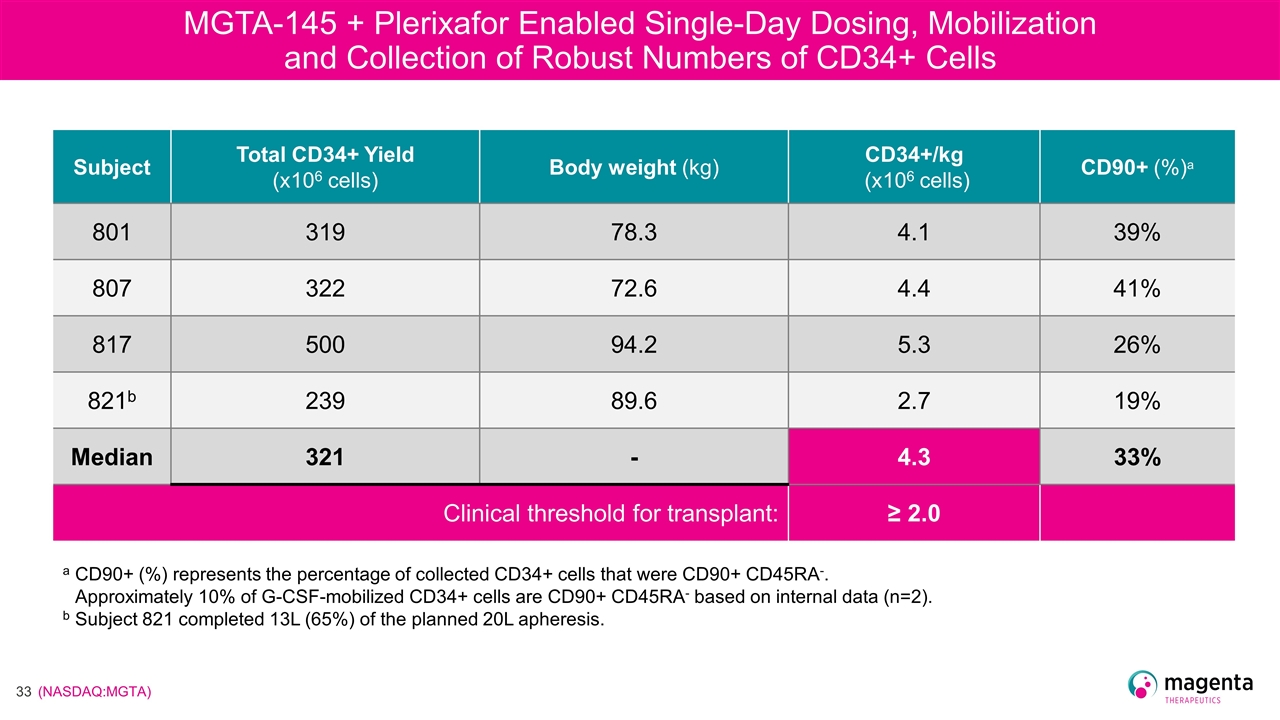
MGTA-145 + Plerixafor Enabled Single-Day Dosing, Mobilization and Collection of Robust Numbers of CD34+ Cells Subject Total CD34+ Yield (x106 cells) Body weight (kg) CD34+/kg (x106 cells) CD90+ (%)a 801 319 78.3 4.1 39% 807 322 72.6 4.4 41% 817 500 94.2 5.3 26% 821b 239 89.6 2.7 19% Median 321 - 4.3 33% Clinical threshold for transplant: ≥ 2.0 a CD90+ (%) represents the percentage of collected CD34+ cells that were CD90+ CD45RA-. Approximately 10% of G-CSF-mobilized CD34+ cells are CD90+ CD45RA- based on internal data (n=2). b Subject 821 completed 13L (65%) of the planned 20L apheresis.
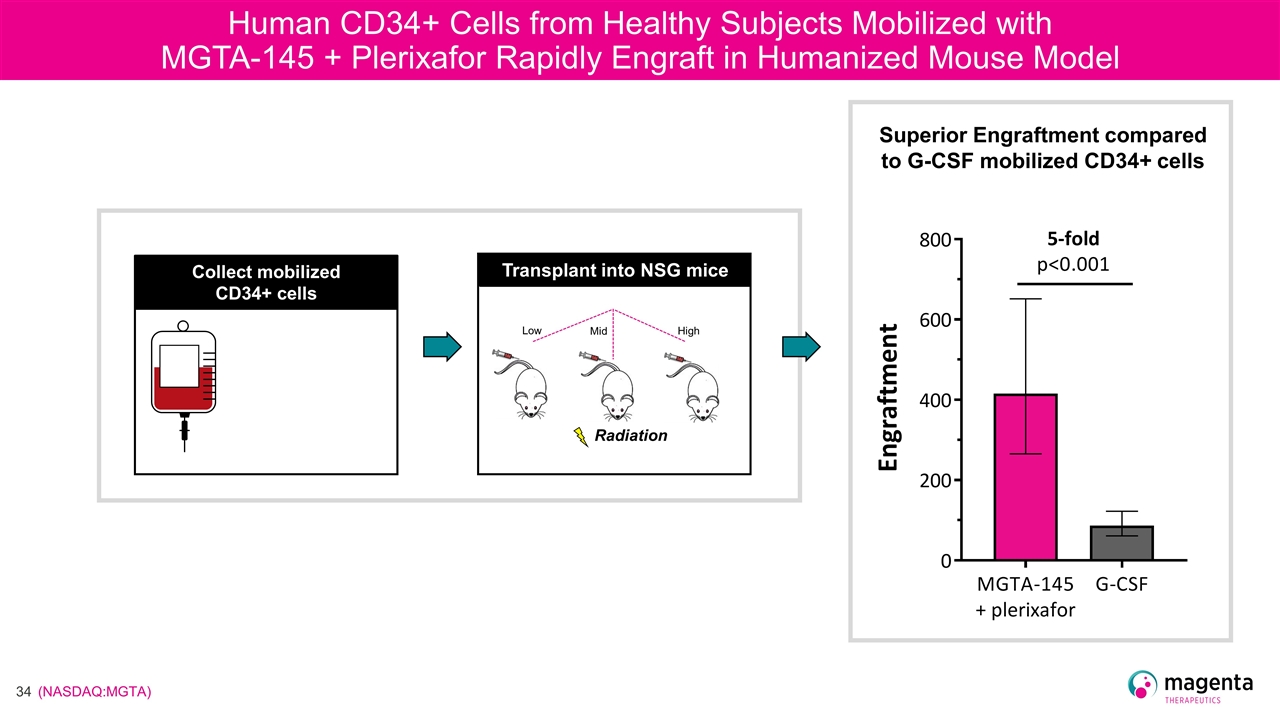
Human CD34+ Cells from Healthy Subjects Mobilized with MGTA-145 + Plerixafor Rapidly Engraft in Humanized Mouse Model Radiation Transplant into NSG mice Collect mobilized CD34+ cells Low Mid High Superior Engraftment compared to G-CSF mobilized CD34+ cells
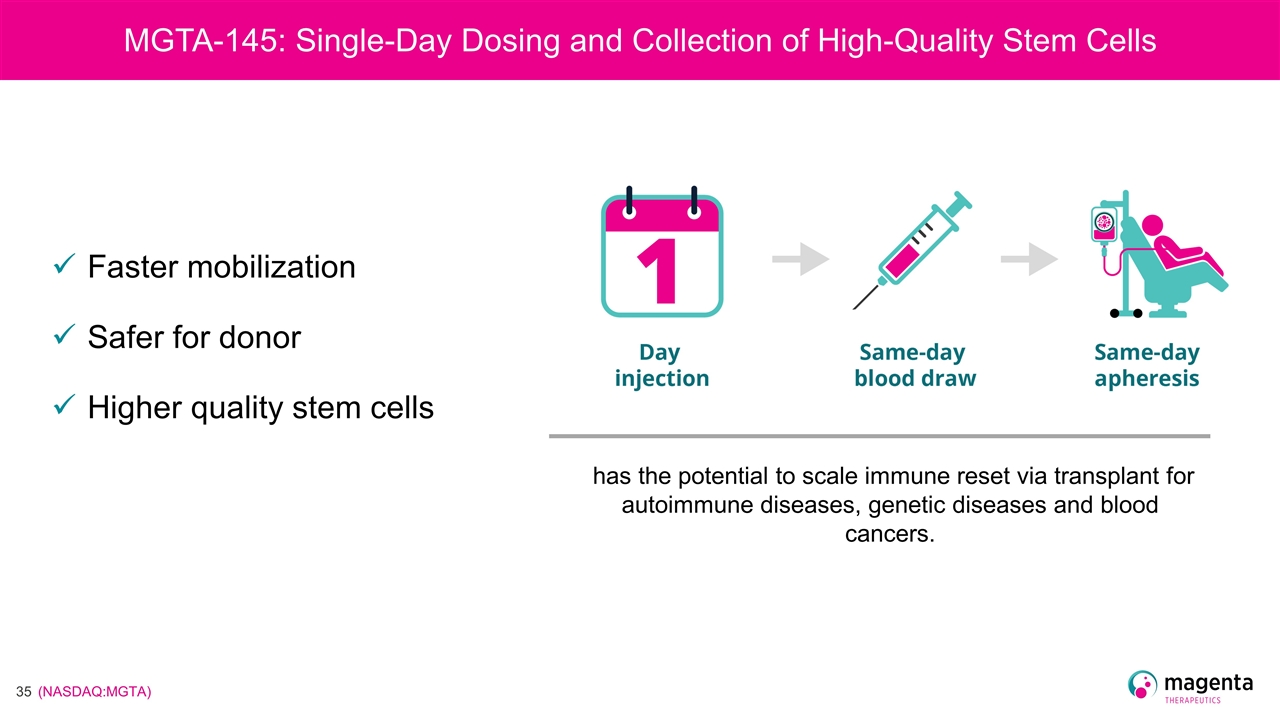
has the potential to scale immune reset via transplant for autoimmune diseases, genetic diseases and blood cancers. MGTA-145: Single-Day Dosing and Collection of High-Quality Stem Cells Faster mobilization Safer for donor Higher quality stem cells
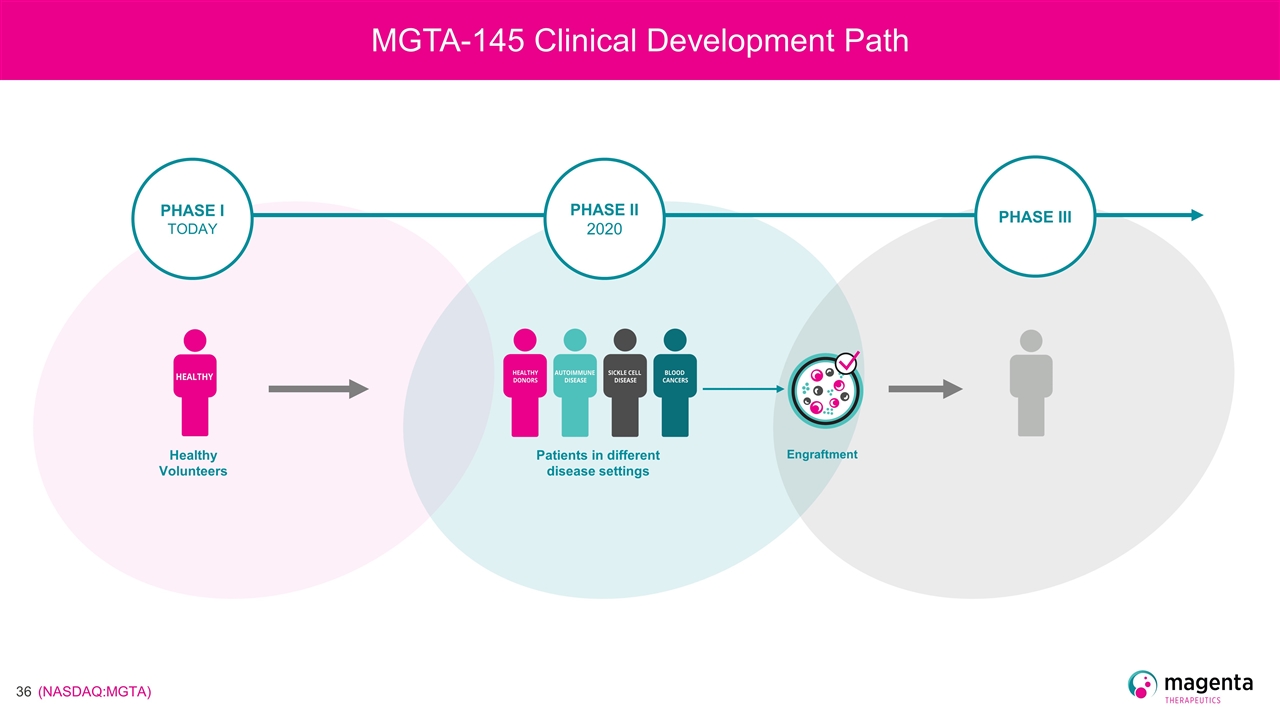
MGTA-145 Clinical Development Path Healthy Volunteers PHASE I TODAY PHASE II 2020 PHASE III Patients in different disease settings Engraftment
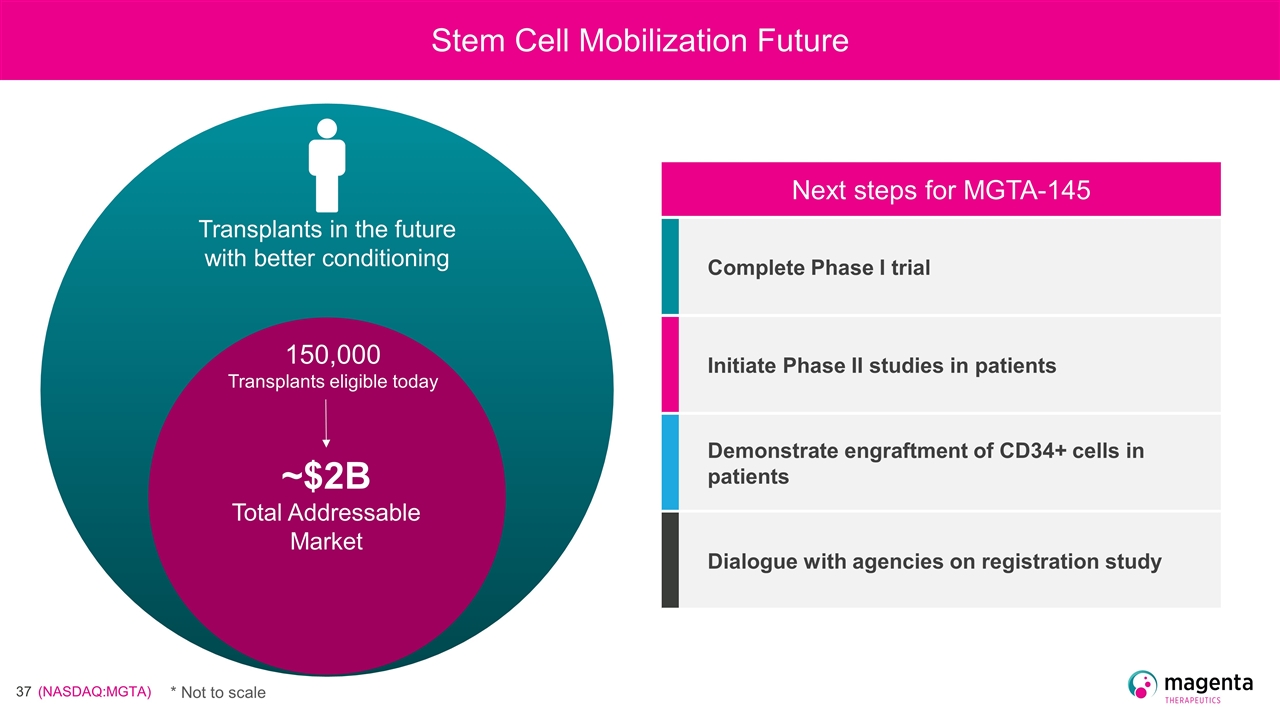
Transplants in the future with better conditioning 150,000 Transplants eligible today Stem Cell Mobilization Future ~$2B Total Addressable Market Next steps for MGTA-145 Complete Phase I trial Initiate Phase II studies in patients Demonstrate engraftment of CD34+ cells in patients Dialogue with agencies on registration study * Not to scale

Looking Ahead

Key Goals and Milestones Ahead in 2020 (NASDAQ: MGTA) CD45-ADC Advance GMP manufacturing MGTA-117 Complete manufacturing and prepare to enter clinical trials MGTA-145 Launch Phase II studies to show engraftment in patients MGTA-456 Complete Phase II trial and define registration path in US and Europe
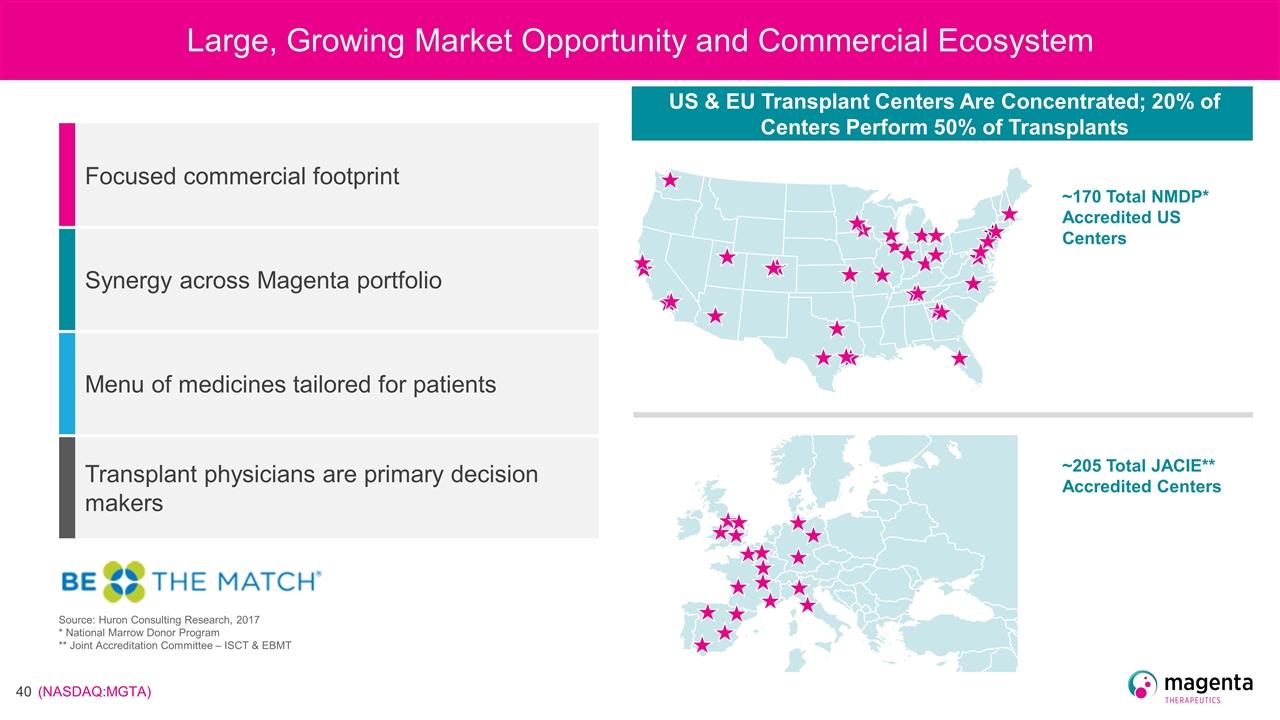
Large, Growing Market Opportunity and Commercial Ecosystem US & EU Transplant Centers Are Concentrated; 20% of Centers Perform 50% of Transplants ~170 Total NMDP* Accredited US Centers ~205 Total JACIE** Accredited Centers Source: Huron Consulting Research, 2017 * National Marrow Donor Program ** Joint Accreditation Committee – ISCT & EBMT Focused commercial footprint Synergy across Magenta portfolio Menu of medicines tailored for patients Transplant physicians are primary decision makers
1st Horizon 2nd Horizon 3rd Horizon Long-Term Vision for Total Patient Care and Cures First global commercial launch of medicine Multiple first-in-class therapies across programs Total patient care with outpatient setting for all transplants

The Promise of a One-Time Curative Therapy

THANK YOU










































