
STS101 for the acute treatment of migraine SUMMIT Phase 3 efficacy trial topline results November 14, 2022 conference call Exhibit 99.2

Disclaimer Important Notice This Presentation contains forward-looking statements concerning the business, operations and financial performance and condition of Satsuma Pharmaceuticals, Inc. (the “Company”), as well as the Company’s plans, objectives and expectations for its business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about the Company’s expectations regarding the potential safety and efficacy of STS101; the Company’s clinical and regulatory development plans; the Company’s expectations with regard to the ASCEND and SUMMIT trials; the Company’s expectations regarding the potential market size and size of the potential patient populations for STS101, if approved for commercial use; the Company’s commercialization, marketing and manufacturing plans and expectations; the pricing and reimbursement of STS101, if approved; the implementation of the Company’s business model and strategic plans for its business and STS101; the scope of protection the Company is able to establish and maintain for intellectual property rights covering STS101, including the projected terms of patent protection; estimates of the Company’s expenses, future revenue, capital requirements, its need for additional financing and its ability to obtain additional capital; the Company’s future financial performance; and developments and projections relating to the Company’s competitors and the Company’s industry, including competing therapies and procedures. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, filed with the Securities and Exchange Commission, as well as other documents that may be filed by the Company from time to time. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the accuracy of the Company’s estimates relating to its ability to initiate and/or complete clinical trials; the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of STS101; the Company’s ability to select suitable dosing regimens; the results of preclinical and clinical studies may not be predictive of future results; the unpredictability of the regulatory process; regulatory developments in the United States and foreign countries; the costs of clinical trials may exceed expectations; the Company’s ability to raise additional capital; and the risk that costs of clinical trials and preclinical activities will exceed expectations. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. This Presentation discusses STS101, a product candidate that is under clinical study, and which has not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of STS101 for the therapeutic use for which STS101 is being studied.
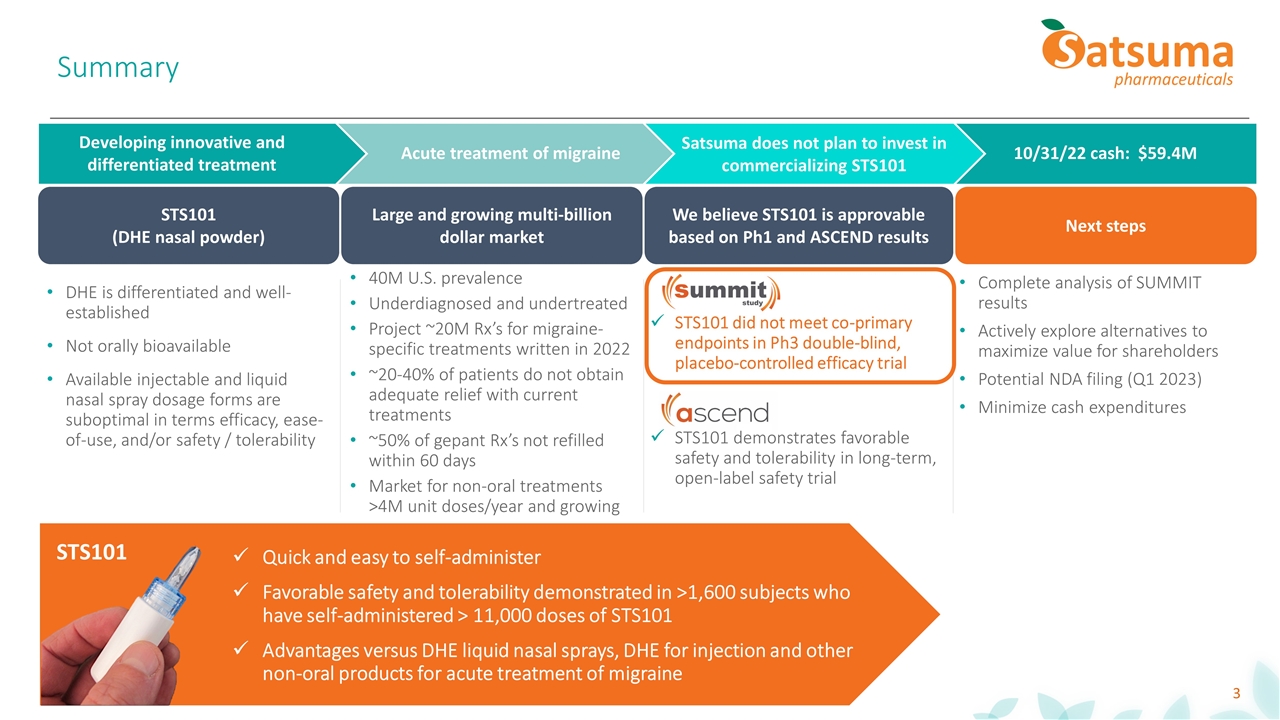
Large and growing multi-billion dollar market Summary STS101 (DHE nasal powder) Next steps 10/31/22 cash: $59.4M Developing innovative and differentiated treatment Satsuma does not plan to invest in commercializing STS101 We believe STS101 is approvable based on Ph1 and ASCEND results 40M U.S. prevalence Underdiagnosed and undertreated Project ~20M Rx’s for migraine-specific treatments written in 2022 ~20-40% of patients do not obtain adequate relief with current treatments ~50% of gepant Rx’s not refilled within 60 days Market for non-oral treatments >4M unit doses/year and growing Complete analysis of SUMMIT results Actively explore alternatives to maximize value for shareholders Potential NDA filing (Q1 2023) Minimize cash expenditures Quick and easy to self-administer Favorable safety and tolerability demonstrated in >1,600 subjects who have self-administered > 11,000 doses of STS101 Advantages versus DHE liquid nasal sprays, DHE for injection and other non-oral products for acute treatment of migraine STS101 STS101 did not meet co-primary endpoints in Ph3 double-blind, placebo-controlled efficacy trial STS101 demonstrates favorable safety and tolerability in long-term, open-label safety trial DHE is differentiated and well-established Not orally bioavailable Available injectable and liquid nasal spray dosage forms are suboptimal in terms efficacy, ease-of-use, and/or safety / tolerability Acute treatment of migraine

STS101 SUMMIT Phase 3 trial results
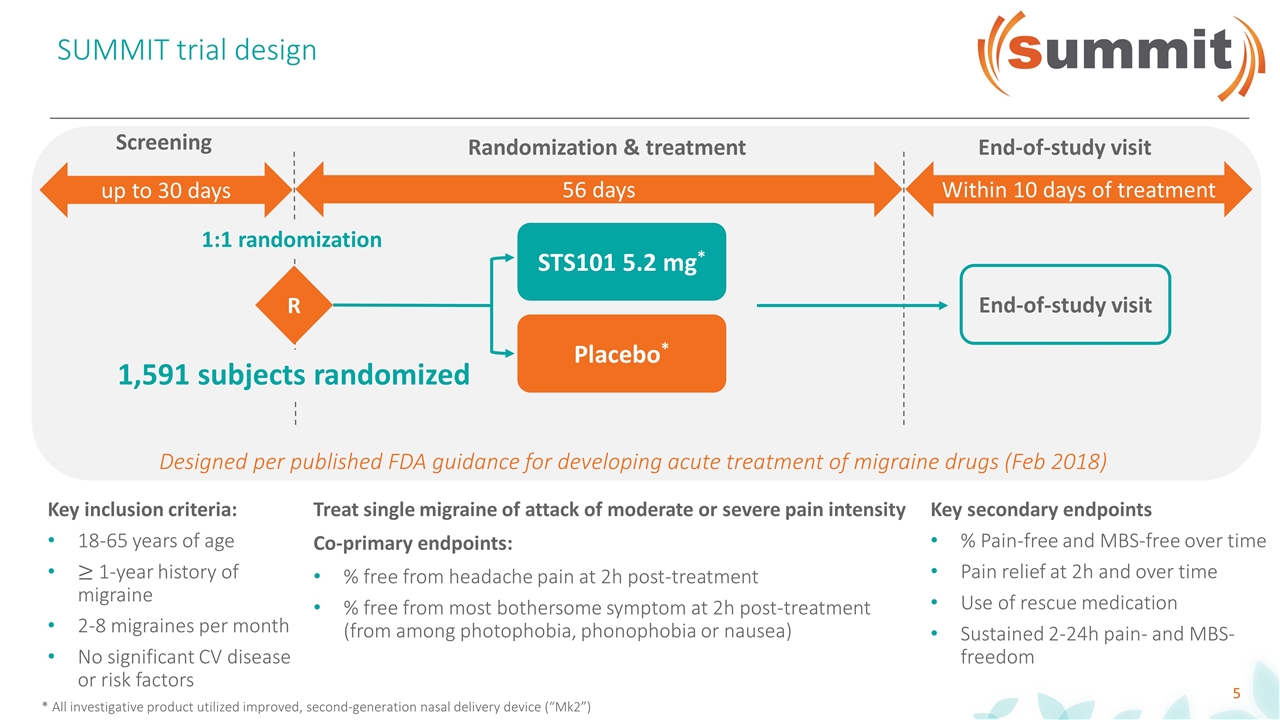
SUMMIT trial design R STS101 5.2 mg* Placebo* up to 30 days 56 days Within 10 days of treatment Screening Randomization & treatment End-of-study visit * All investigative product utilized improved, second-generation nasal delivery device (“Mk2”) End-of-study visit 1,591 subjects randomized Treat single migraine of attack of moderate or severe pain intensity Co-primary endpoints: % free from headache pain at 2h post-treatment % free from most bothersome symptom at 2h post-treatment (from among photophobia, phonophobia or nausea) Key inclusion criteria: 18-65 years of age 1-year history of migraine 2-8 migraines per month No significant CV disease or risk factors 1:1 randomization Designed per published FDA guidance for developing acute treatment of migraine drugs (Feb 2018) Key secondary endpoints % Pain-free and MBS-free over time Pain relief at 2h and over time Use of rescue medication Sustained 2-24h pain- and MBS- freedom
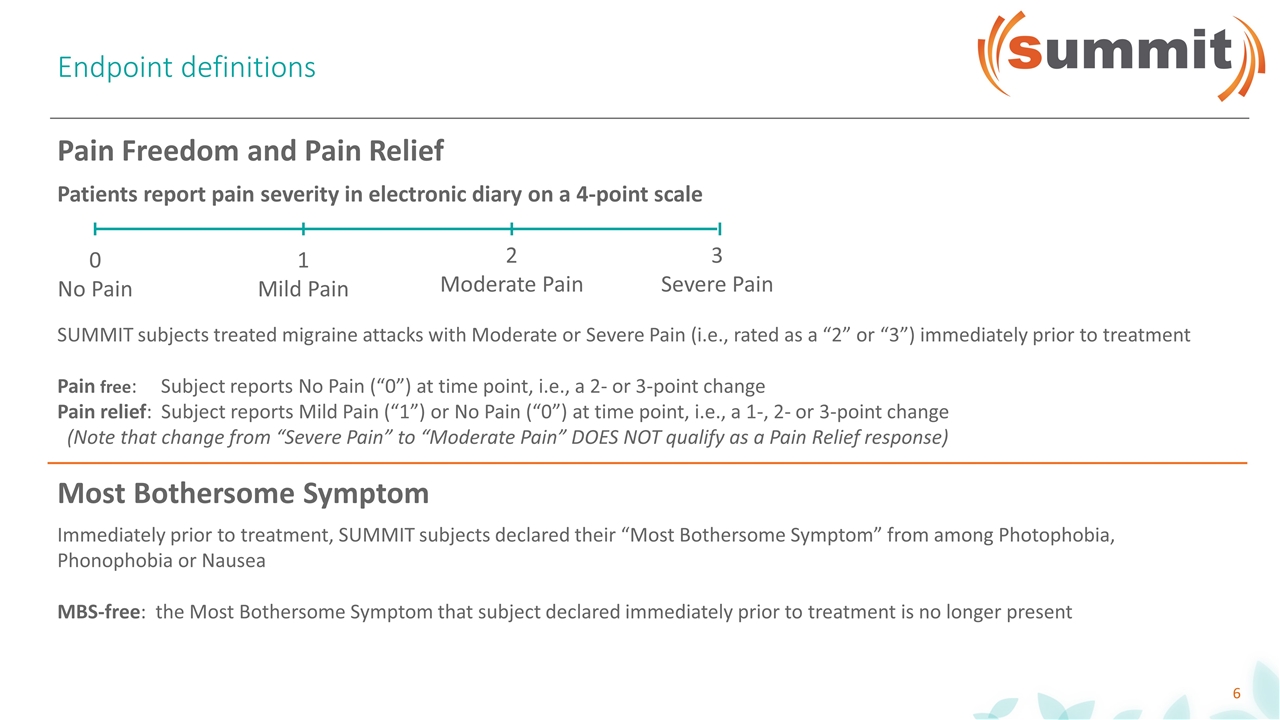
Endpoint definitions Patients report pain severity in electronic diary on a 4-point scale 0 No Pain 1 Mild Pain 2 Moderate Pain 3 Severe Pain SUMMIT subjects treated migraine attacks with Moderate or Severe Pain (i.e., rated as a “2” or “3”) immediately prior to treatment Pain free: Subject reports No Pain (“0”) at time point, i.e., a 2- or 3-point change Pain relief: Subject reports Mild Pain (“1”) or No Pain (“0”) at time point, i.e., a 1-, 2- or 3-point change (Note that change from “Severe Pain” to “Moderate Pain” DOES NOT qualify as a Pain Relief response) Pain Freedom and Pain Relief Most Bothersome Symptom Immediately prior to treatment, SUMMIT subjects declared their “Most Bothersome Symptom” from among Photophobia, Phonophobia or Nausea MBS-free: the Most Bothersome Symptom that subject declared immediately prior to treatment is no longer present
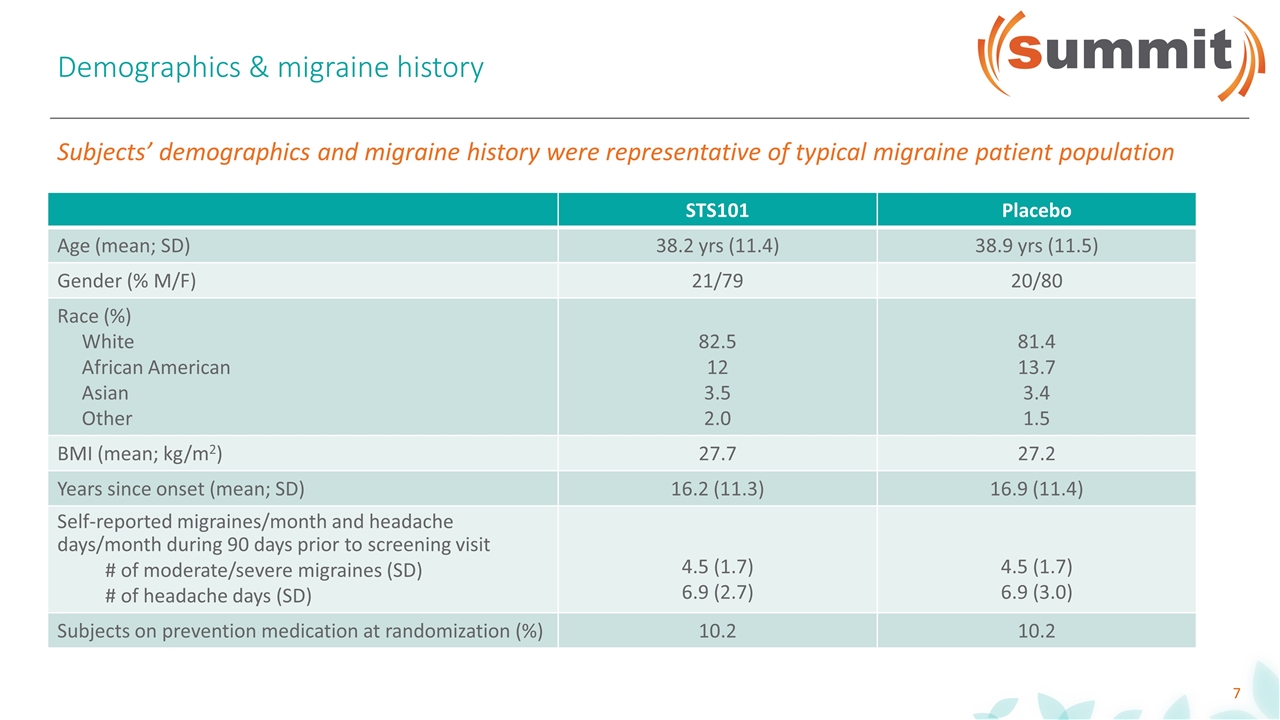
Demographics & migraine history Subjects’ demographics and migraine history were representative of typical migraine patient population STS101 Placebo Age (mean; SD) 38.2 yrs (11.4) 38.9 yrs (11.5) Gender (% M/F) 21/79 20/80 Race (%) White African American Asian Other 82.5 12 3.5 2.0 81.4 13.7 3.4 1.5 BMI (mean; kg/m2) 27.7 27.2 Years since onset (mean; SD) 16.2 (11.3) 16.9 (11.4) Self-reported migraines/month and headache days/month during 90 days prior to screening visit # of moderate/severe migraines (SD) # of headache days (SD) 4.5 (1.7) 6.9 (2.7) 4.5 (1.7) 6.9 (3.0) Subjects on prevention medication at randomization (%) 10.2 10.2
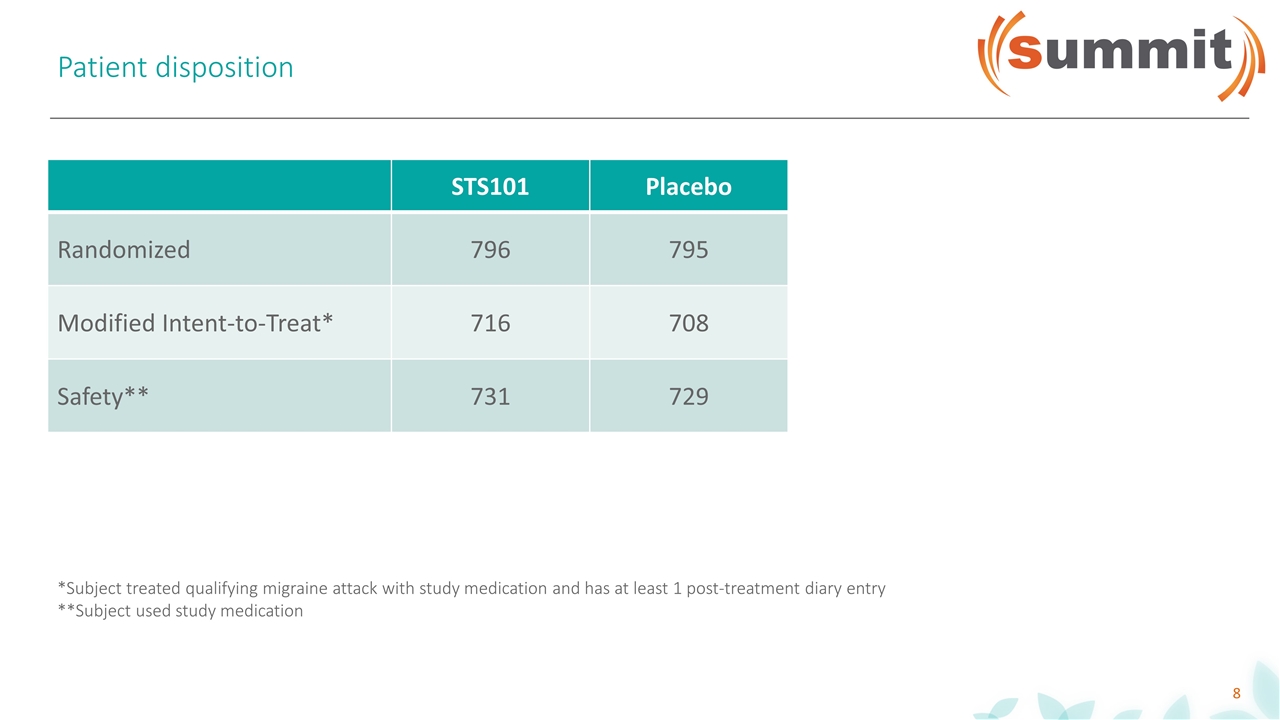
Patient disposition STS101 Placebo Randomized 796 795 Modified Intent-to-Treat* 716 708 Safety** 731 729 *Subject treated qualifying migraine attack with study medication and has at least 1 post-treatment diary entry **Subject used study medication
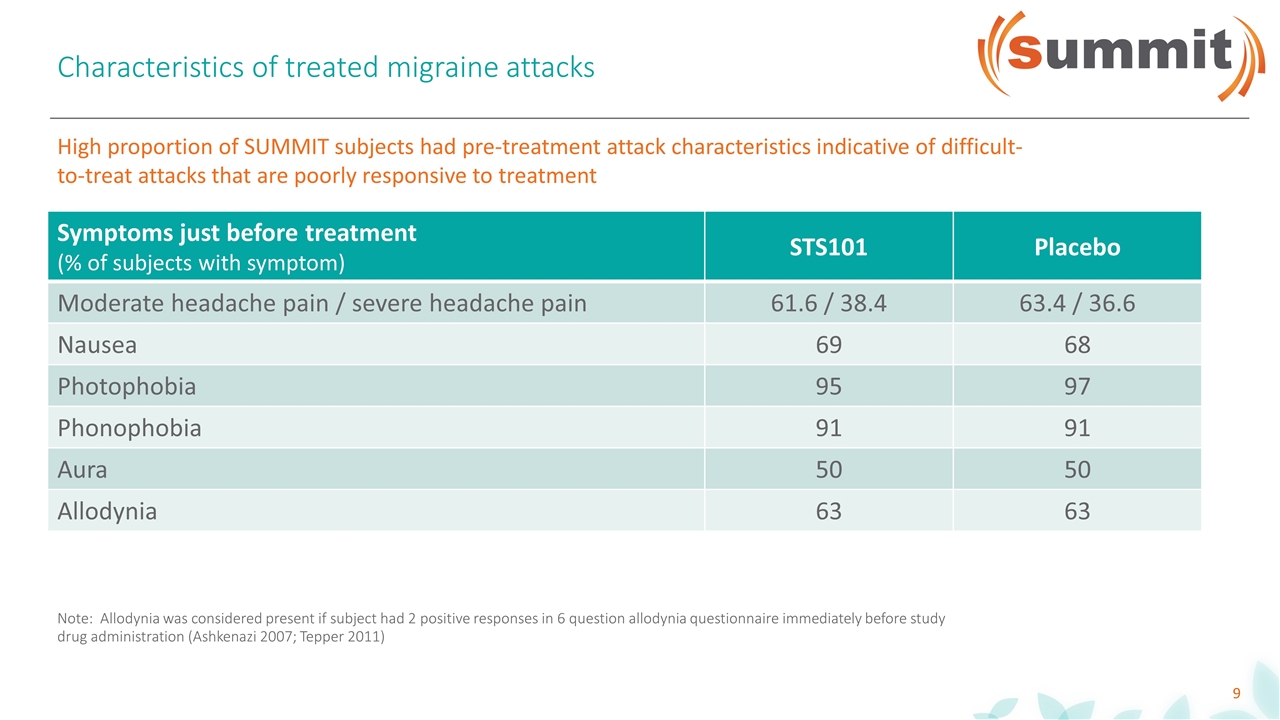
Characteristics of treated migraine attacks Note: Allodynia was considered present if subject had 2 positive responses in 6 question allodynia questionnaire immediately before study drug administration (Ashkenazi 2007; Tepper 2011) Symptoms just before treatment (% of subjects with symptom) STS101 Placebo Moderate headache pain / severe headache pain 61.6 / 38.4 63.4 / 36.6 Nausea 69 68 Photophobia 95 97 Phonophobia 91 91 Aura 50 50 Allodynia 63 63 High proportion of SUMMIT subjects had pre-treatment attack characteristics indicative of difficult-to-treat attacks that are poorly responsive to treatment
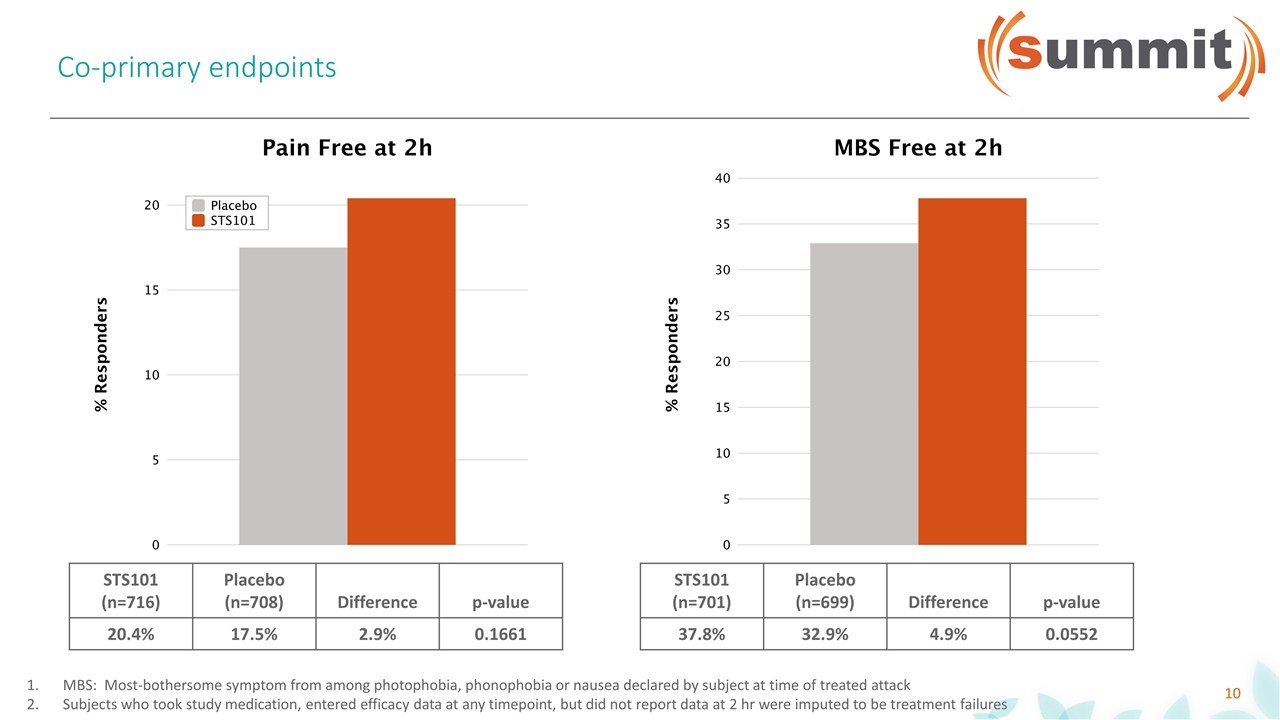
Co-primary endpoints MBS: Most-bothersome symptom from among photophobia, phonophobia or nausea declared by subject at time of treated attack Subjects who took study medication, entered efficacy data at any timepoint, but did not report data at 2 hr were imputed to be treatment failures STS101 (n=716) Placebo (n=708) Difference p-value 20.4% 17.5% 2.9% 0.1661 STS101 (n=701) Placebo (n=699) Difference p-value 37.8% 32.9% 4.9% 0.0552
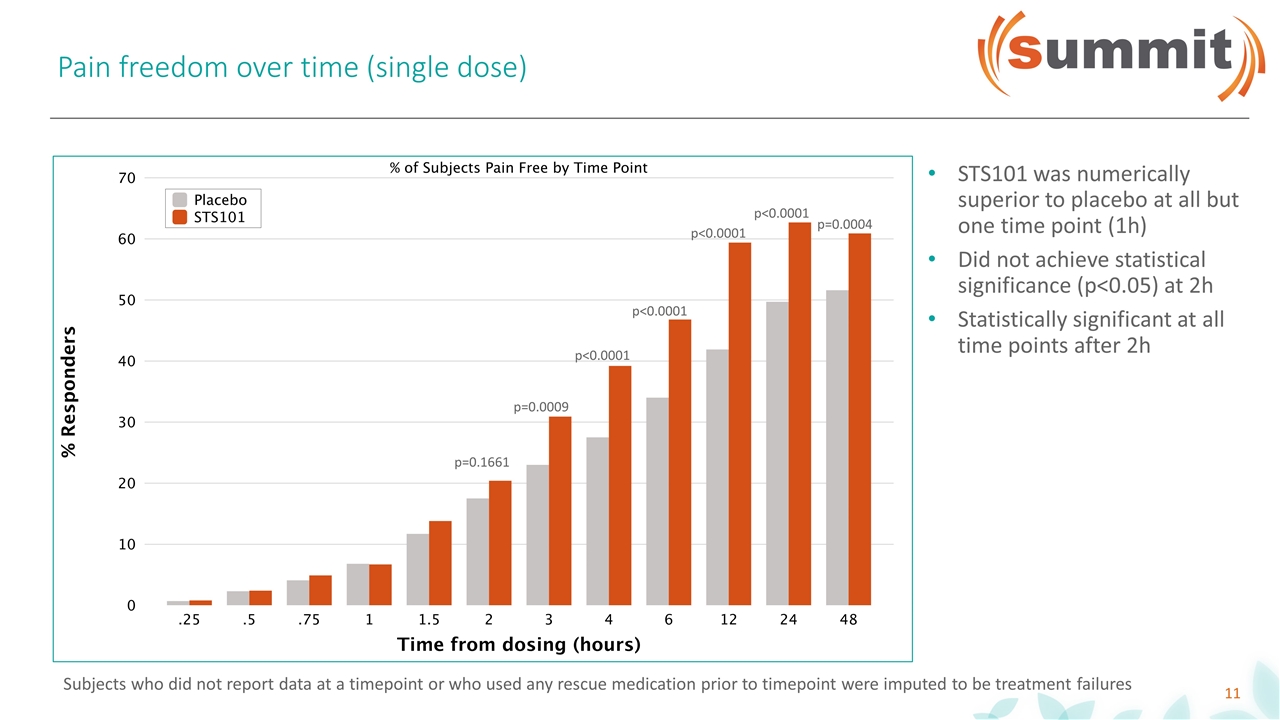
Pain freedom over time (single dose) p=0.1661 p=0.0009 p<0.0001 p<0.0001 p<0.0001 p<0.0001 p=0.0004 STS101 was numerically superior to placebo at all but one time point (1h) Did not achieve statistical significance (p<0.05) at 2h Statistically significant at all time points after 2h Subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures
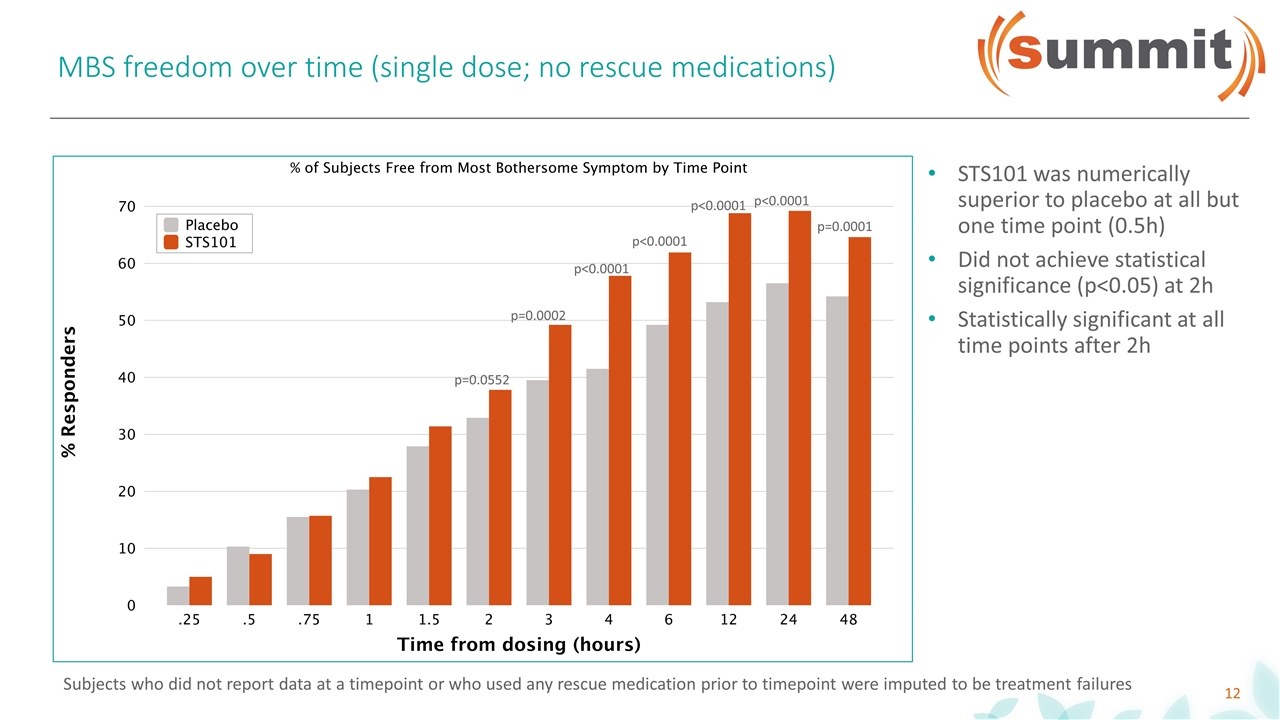
MBS freedom over time (single dose; no rescue medications) p=0.0552 p=0.0002 p<0.0001 p<0.0001 p<0.0001 p<0.0001 p=0.0001 STS101 was numerically superior to placebo at all but one time point (0.5h) Did not achieve statistical significance (p<0.05) at 2h Statistically significant at all time points after 2h Subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures
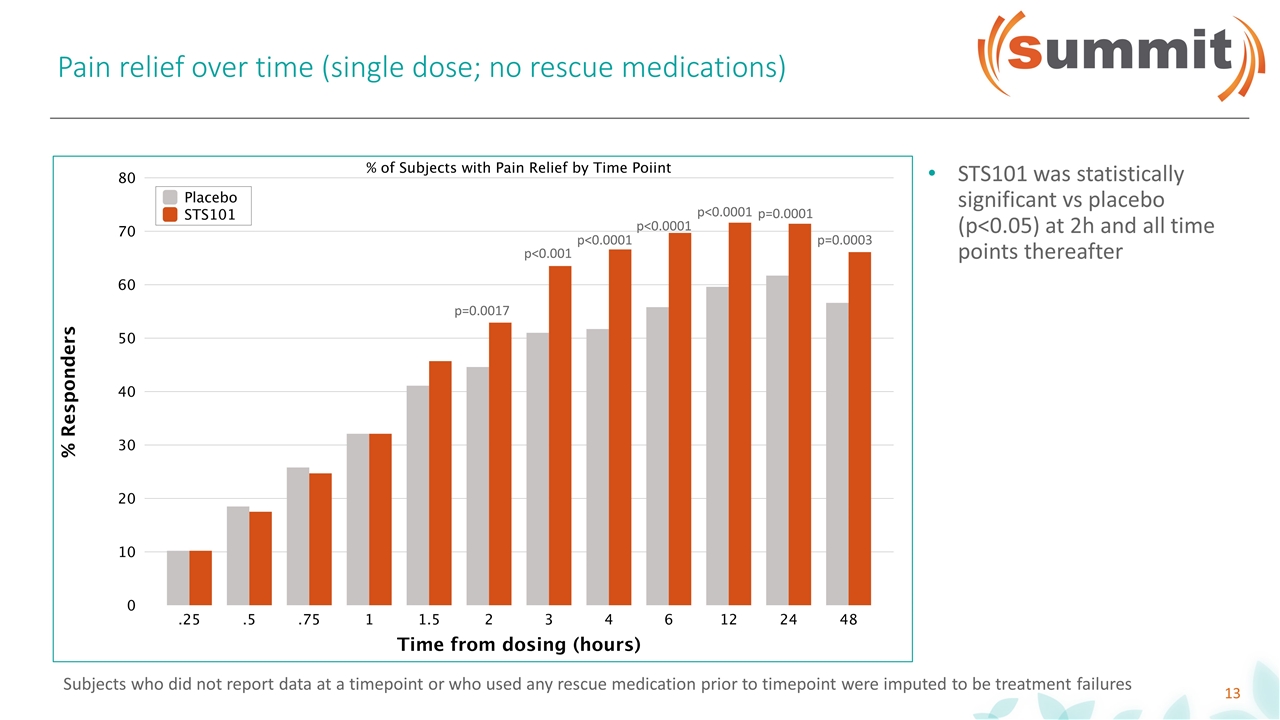
Pain relief over time (single dose; no rescue medications) p=0.0017 p<0.001 p<0.0001 p=0.0001 p<0.0001 p<0.0001 p=0.0003 STS101 was statistically significant vs placebo (p<0.05) at 2h and all time points thereafter Subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures
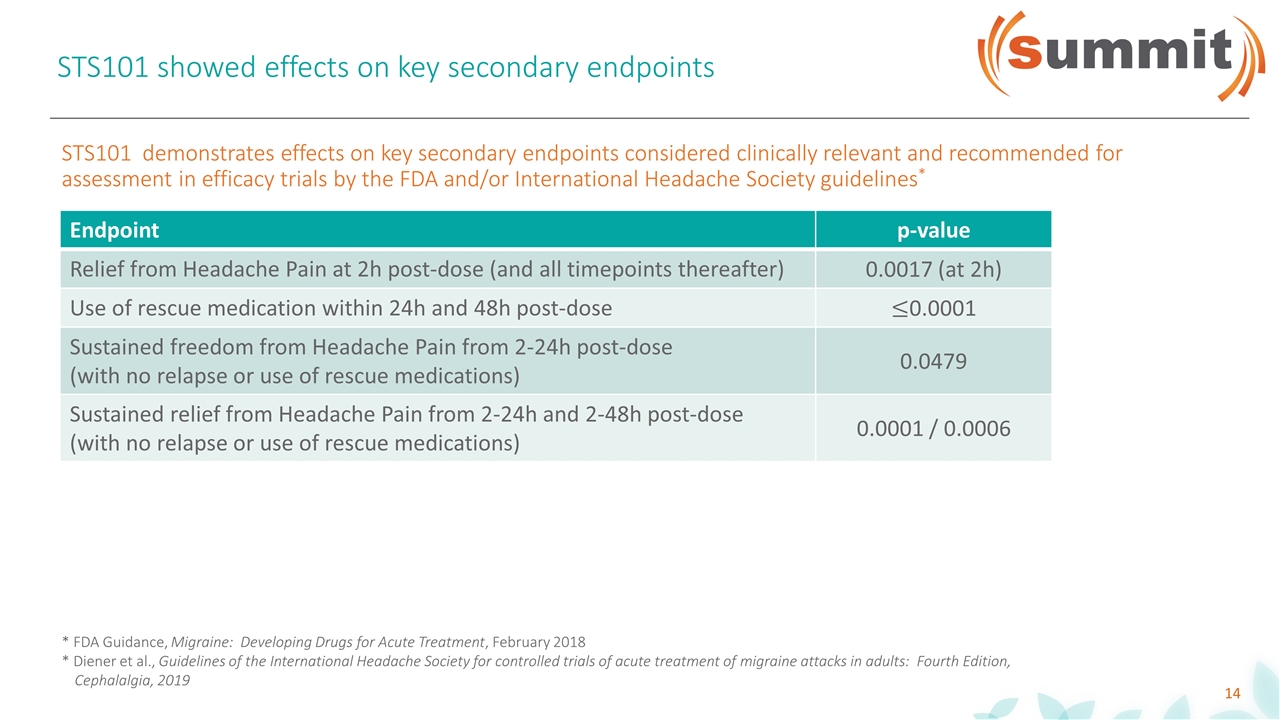
STS101 showed effects on key secondary endpoints Endpoint p-value Relief from Headache Pain at 2h post-dose (and all timepoints thereafter) 0.0017 (at 2h) Use of rescue medication within 24h and 48h post-dose 0.0001 Sustained freedom from Headache Pain from 2-24h post-dose (with no relapse or use of rescue medications) 0.0479 Sustained relief from Headache Pain from 2-24h and 2-48h post-dose (with no relapse or use of rescue medications) 0.0001 / 0.0006 Endpoint p-value Relief from Headache Pain at 2h post-dose (and all timepoints thereafter) 0.0017 (at 2h) Use of rescue medication within 24h and 48h post-dose Sustained freedom from Headache Pain from 2-24h post-dose (with no relapse or use of rescue medications) 0.0479 Sustained relief from Headache Pain from 2-24h and 2-48h post-dose (with no relapse or use of rescue medications) 0.0001 / 0.0006 STS101 demonstrates effects on key secondary endpoints considered clinically relevant and recommended for assessment in efficacy trials by the FDA and/or International Headache Society guidelines* * FDA Guidance, Migraine: Developing Drugs for Acute Treatment, February 2018 * Diener et al., Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth Edition, Cephalalgia, 2019
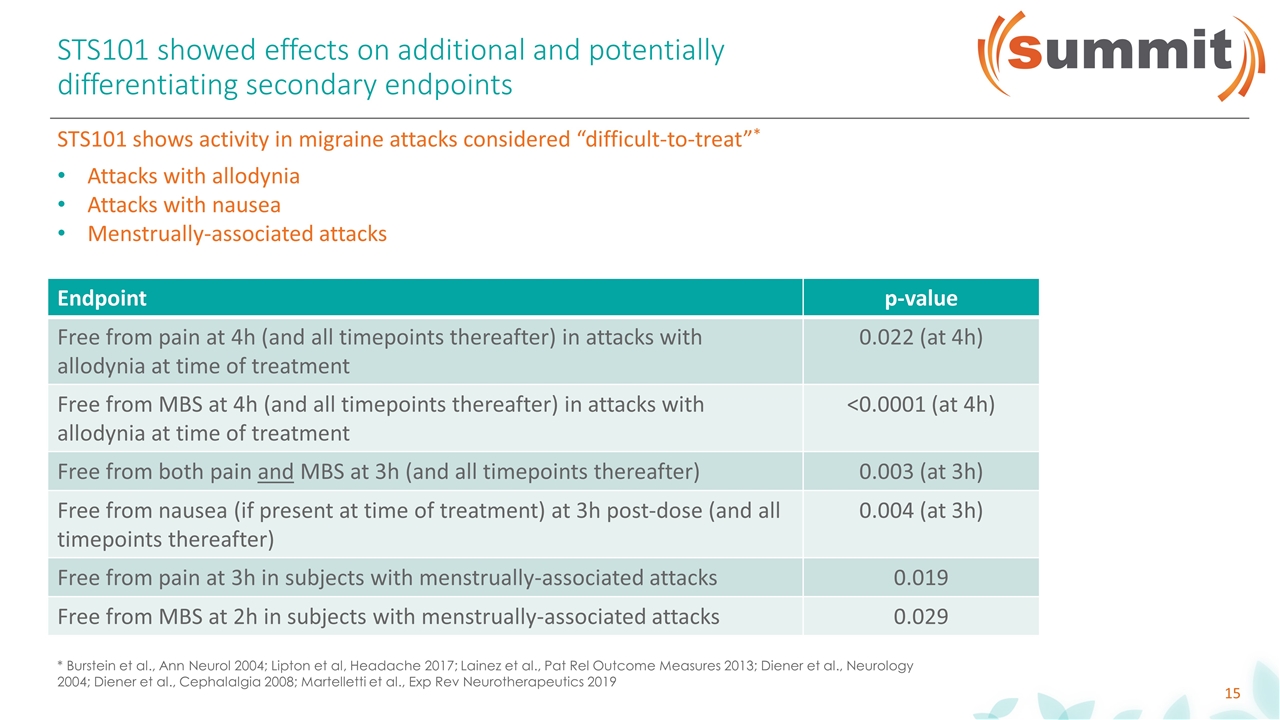
STS101 showed effects on additional and potentially differentiating secondary endpoints Endpoint p-value Free from pain at 4h (and all timepoints thereafter) in attacks with allodynia at time of treatment 0.022 (at 4h) Free from MBS at 4h (and all timepoints thereafter) in attacks with allodynia at time of treatment <0.0001 (at 4h) Free from both pain and MBS at 3h (and all timepoints thereafter) 0.003 (at 3h) Free from nausea (if present at time of treatment) at 3h post-dose (and all timepoints thereafter) 0.004 (at 3h) Free from pain at 3h in subjects with menstrually-associated attacks 0.019 Free from MBS at 2h in subjects with menstrually-associated attacks 0.029 STS101 shows activity in migraine attacks considered “difficult-to-treat”* Attacks with allodynia Attacks with nausea Menstrually-associated attacks * Burstein et al., Ann Neurol 2004; Lipton et al, Headache 2017; Lainez et al., Pat Rel Outcome Measures 2013; Diener et al., Neurology 2004; Diener et al., Cephalalgia 2008; Martelletti et al., Exp Rev Neurotherapeutics 2019
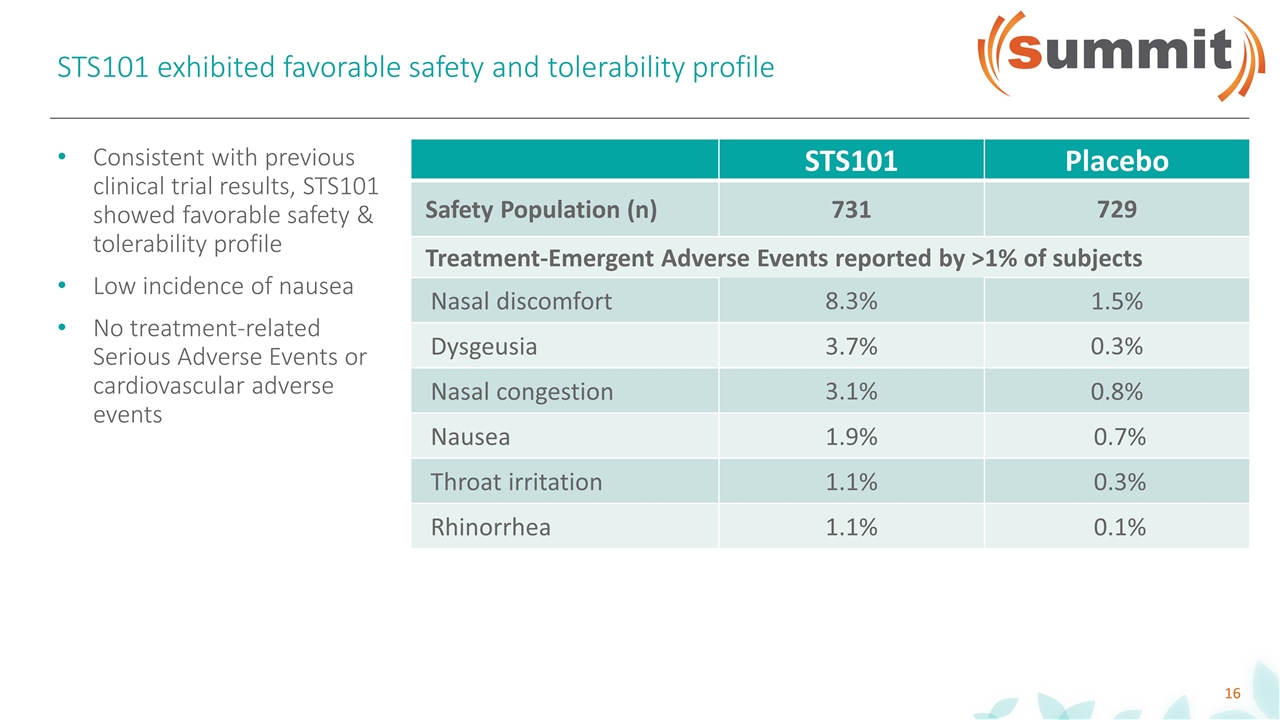
STS101 exhibited favorable safety and tolerability profile STS101 Placebo Safety Population (n) 731 729 Treatment-Emergent Adverse Events reported by >1% of subjects Nasal discomfort 8.3% 1.5% Dysgeusia 3.7% 0.3% Nasal congestion 3.1% 0.8% Nausea 1.9% 0.7% Throat irritation 1.1% 0.3% Rhinorrhea 1.1% 0.1% Consistent with previous clinical trial results, STS101 showed favorable safety & tolerability profile Low incidence of nausea No treatment-related Serious Adverse Events or cardiovascular adverse events
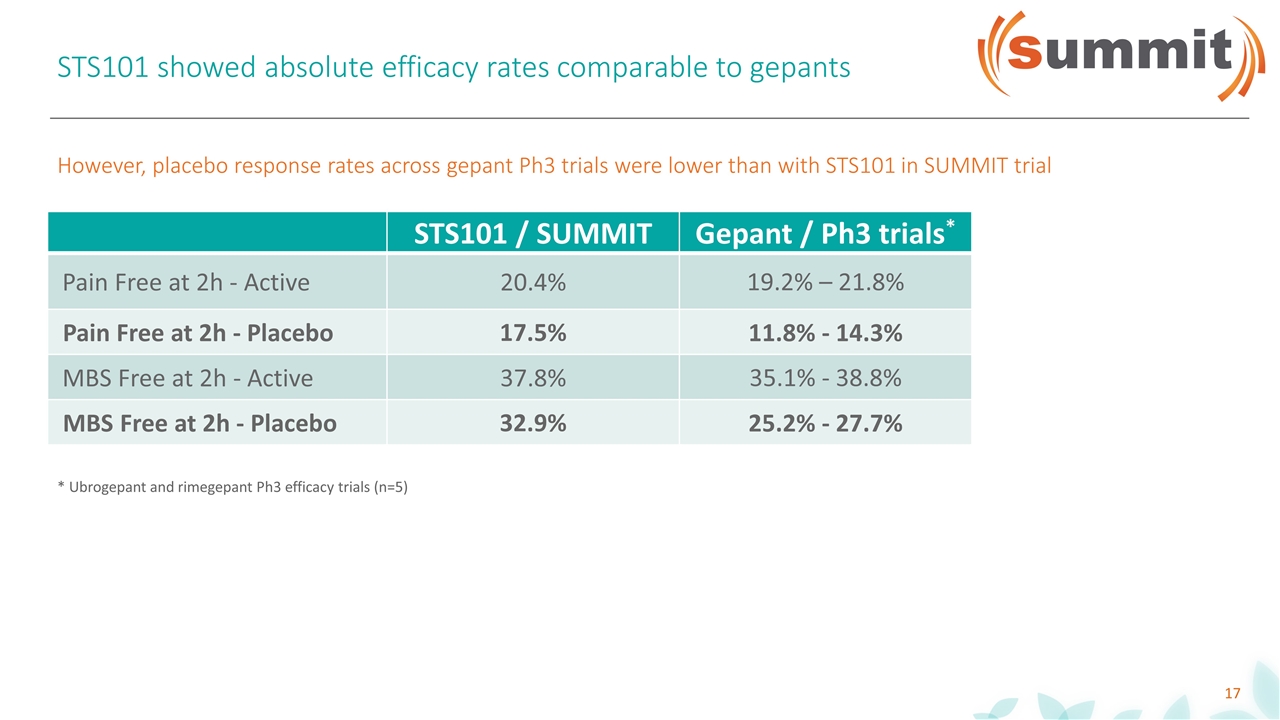
STS101 showed absolute efficacy rates comparable to gepants STS101 / SUMMIT Gepant / Ph3 trials* Pain Free at 2h - Active 20.4% 19.2% – 21.8% Pain Free at 2h - Placebo 17.5% 11.8% - 14.3% MBS Free at 2h - Active 37.8% 35.1% - 38.8% MBS Free at 2h - Placebo 32.9% 25.2% - 27.7% However, placebo response rates across gepant Ph3 trials were lower than with STS101 in SUMMIT trial * Ubrogepant and rimegepant Ph3 efficacy trials (n=5)

Summary and conclusions SUMMIT was a rigorously-conducted, large, placebo-controlled trial that addressed factors thought to contribute to previous EMERGE trial failing to achieve statistical significance on co-primary endpoints at 2h STS101 did not demonstrate statistical significance over placebo on co-primary endpoints at 2h STS101 achieved statistical significance versus placebo on co-primary endpoints by 3h and all subsequent timepoints STS101 showed statistically significant effect on pain relief at 2h and all subsequent timepoints STS101 showed statistically significant effects on multiple clinically relevant and potentially differentiating secondary endpoints Consistent with clinical trial experience to date, STS101 showed favorable safety and tolerability

Summary and conclusions SUMMIT results suggest STS101 may have particular utility for people with migraine who experience: Attacks with allodynia Long-duration migraine attacks, including menstrually-associated migraine Nausea STS101 may be an attractive treatment option for patients who: Desire a long-lasting antimigraine treatment that mitigates need for re-dosing and/or rescue medications Desire an easy-to-use treatment Desire a well-tolerated treatment Would benefit from a non-oral treatment due to nausea, vomiting or gastroparesis secondary to migraine attack Dislike injectable treatments or liquid nasal sprays

STS101 program and plans

STS101 program and plans STS101 is a differentiated asset Satsuma has established automated, commercial-scale manufacturing capability for STS101 Based on feedback from three meetings with FDA, Satsuma believes Phase 1 PK and ASCEND long-term, open-label safety trials will support NDA filing and potential approval FDA has consistently communicated stated that an efficacy trial is not necessary for STS101 approval Assuming NDA filing in Q1 2023 and first-cycle FDA approval, STS101 commercial launch could occur in Q1/Q2 2024 We do not plan to invest in commercialization

Next steps Complete analysis of SUMMIT trial results Potential NDA filing (Q1 2023) Actively explore alternatives to maximize value for shareholders Minimize cash expenditures

Thank you






















