
Exhibit 99.1 pharmaceuticals Satsuma Pharmaceuticals and STS101 Update 5th Annual Evercore ISI HealthCONx Conference November 30, 2022
Disclaimer Important Notice This Presentation contains forward-looking statements concerning the business, operations and financial performance and condition of Satsuma Pharmaceuticals, Inc. (the “Company”), as well as the Company’s plans, objectives and expectations for its business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about the Company’s expectations regarding the potential safety and efficacy of STS101; the Company’s clinical and regulatory development plans; the Company’s expectations with regard to the ASCEND and SUMMIT trials; the Company’s expectations regarding the potential market size and size of the potential patient populations for STS101, if approved for commercial use; the Company’s commercialization, marketing and manufacturing plans and expectations; the pricing and reimbursement of STS101, if approved; the implementation of the Company’s business model and strategic plans for its business and STS101; the scope of protection the Company is able to establish and maintain for intellectual property rights covering STS101, including the projected terms of patent protection; estimates of the Company’s expenses, future revenue, capital requirements, its need for additional financing and its ability to obtain additional capital; the Company’s future financial performance; and developments and projections relating to the Company’s competitors and the Company’s industry, including competing therapies and procedures. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, filed with the Securities and Exchange Commission, as well as other documents that may be filed by the Company from time to time. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the accuracy of the Company’s estimates relating to its ability to initiate and/or complete clinical trials; the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of STS101; the Company’s ability to select suitable dosing regimens; the results of preclinical and clinical studies may not be predictive of future results; the unpredictability of the regulatory process; regulatory developments in the United States and foreign countries; the costs of clinical trials may exceed expectations; the Company’s ability to raise additional capital; and the risk that costs of clinical trials and preclinical activities will exceed expectations. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. This Presentation discusses STS101, a product candidate that is under clinical study, and which has not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of STS101 for the therapeutic use for which STS101 is being studied. 2
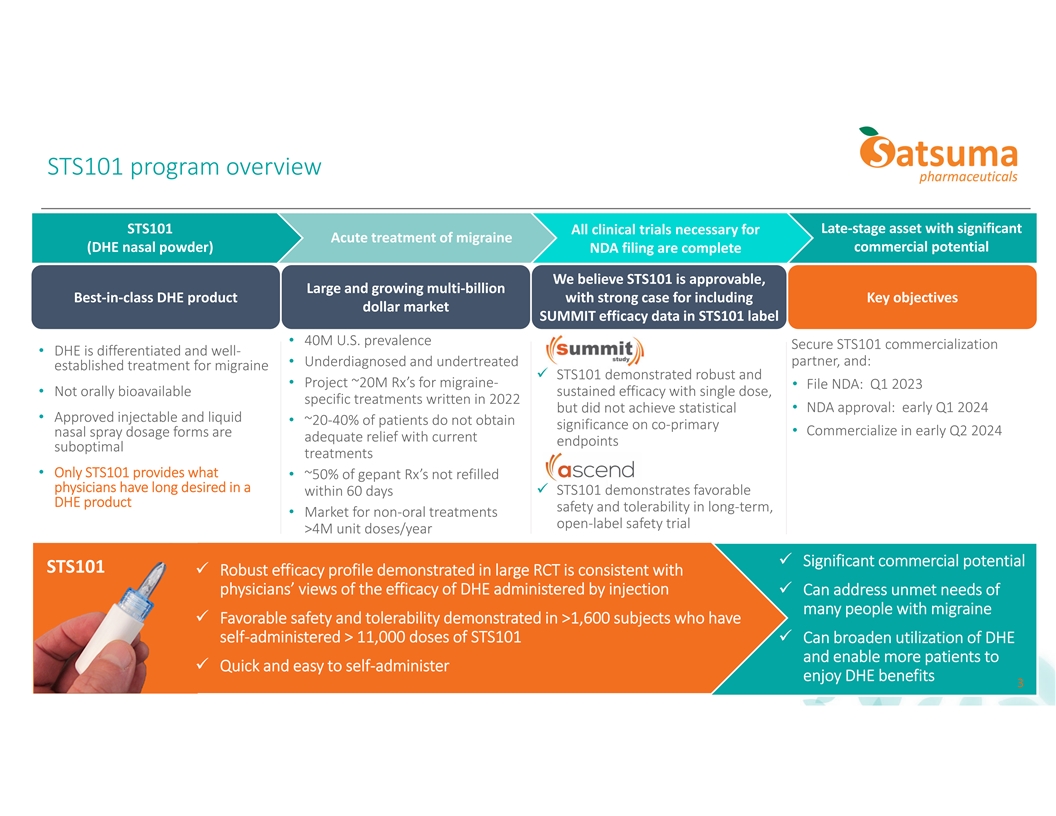
STS101 program overview STS101 Late-stage asset with significant All clinical trials necessary for Acute treatment of migraine (DHE nasal powder) commercial potential NDA filing are complete We believe STS101 is approvable, Large and growing multi-billion Best-in-class DHE product with strong case for including Key objectives dollar market SUMMIT efficacy data in STS101 label • 40M U.S. prevalence Secure STS101 commercialization • DHE is differentiated and well- partner, and: • Underdiagnosed and undertreated established treatment for migraine ü STS101 demonstrated robust and • Project ~20M Rx’s for migraine- • File NDA: Q1 2023 • Not orally bioavailable sustained efficacy with single dose, specific treatments written in 2022 • NDA approval: early Q1 2024 but did not achieve statistical • Approved injectable and liquid • ~20-40% of patients do not obtain significance on co-primary • Commercialize in early Q2 2024 nasal spray dosage forms are adequate relief with current endpoints suboptimal treatments • Only STS101 provides what • ~50% of gepant Rx’s not refilled physicians have long desired in a ü STS101 demonstrates favorable within 60 days DHE product safety and tolerability in long-term, • Market for non-oral treatments open-label safety trial >4M unit doses/year ü Significant commercial potential STS101 ü Robust efficacy profile demonstrated in large RCT is consistent with physicians’ views of the efficacy of DHE administered by injectionü Can address unmet needs of many people with migraine ü Favorable safety and tolerability demonstrated in >1,600 subjects who have self-administered > 11,000 doses of STS101ü Can broaden utilization of DHE and enable more patients to ü Quick and easy to self-administer enjoy DHE benefits 3

Key points 1. Although SUMMIT trial did not show statistical significance vs placebo on co-primary endpoints at 2h, results provide strong, evidence-based support for STS101 as a differentiated acute treatment for migraine • SUMMIT efficacy results are robust, compare favorably with results from controlled trials of approved DHE products and are consistent with physicians’ perceptions of efficacy of DHE for injection • STS101 has a best-in-class profile among DHE products 2. Based on input from our expert legal-regulatory consultants, we are confident the STS101 data package can support NDA filing and potential approval and believe there is strong case for including SUMMIT efficacy results in the STS101 prescribing information 3. We believe STS101 has large commercial potential, can address the unmet needs of many people with migraine and will enjoy broader utilization than current DHE products

STS101 profile is a highly differentiated and best-in-class DHE product • STS101 has best-in-class profile with key differentiating features long desired by physicians in a DHE product: ü Proven robust and long-lasting efficacy with single dose ü Quick and easy self-administration ü Favorable safety and tolerability, with low rates of local nasal irritative symptoms and iatrogenic nausea/vomiting • SUMMIT efficacy results are consistent with physicians’ views of the efficacy and therapeutic utility of DHE for injection (but not DHE liquid nasal sprays) ü Robust and long-lasting efficacy, with ability to “break” and abort attacks ü Beneficial for difficult-to-treat and long-duration attacks ü Beneficial for refractory attacks and as a rescue medication • STS101 SUMMIT trial efficacy results are superior to efficacy results demonstrated with approved DHE products in placebo-controlled trials • STS101 ease-of-use and favorable safety / tolerability are differentiating features that we believe will increase utilization of STS101 beyond current DHE products 5
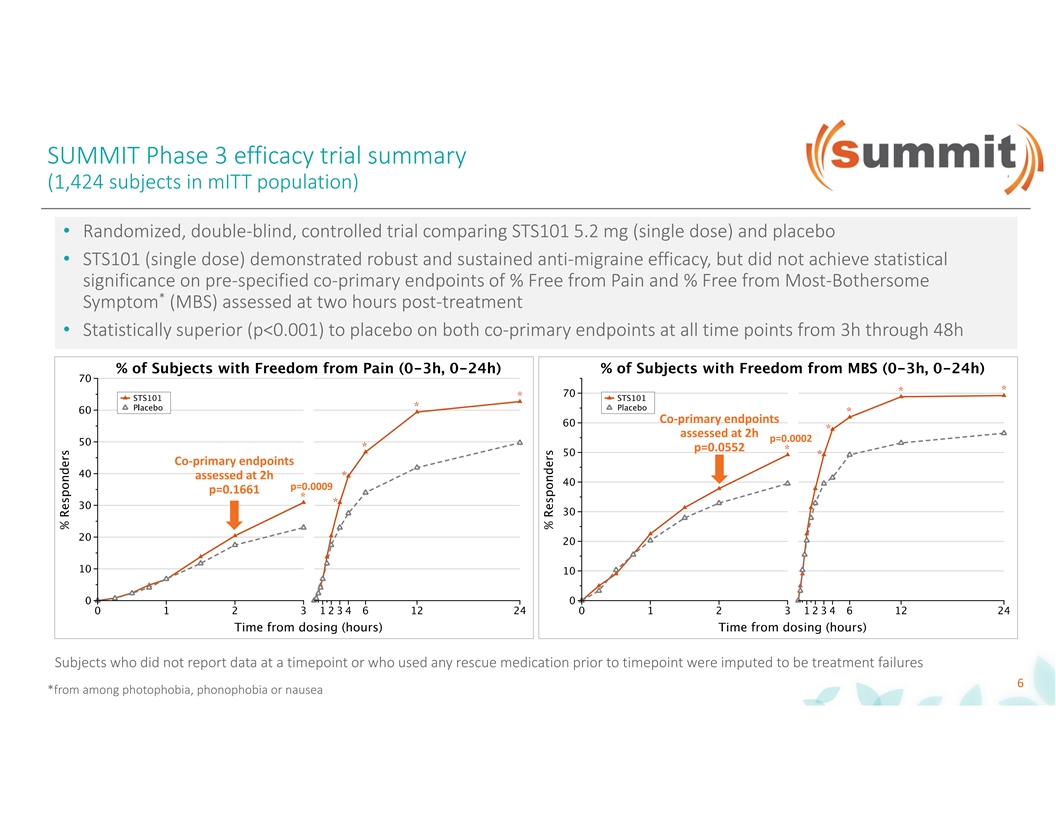
SUMMIT Phase 3 efficacy trial summary (1,424 subjects in mITT population) • Randomized, double-blind, controlled trial comparing STS101 5.2 mg (single dose) and placebo • STS101 (single dose) demonstrated robust and sustained anti-migraine efficacy, but did not achieve statistical significance on pre-specified co-primary endpoints of % Free from Pain and % Free from Most-Bothersome * Symptom (MBS) assessed at two hours post-treatment • Statistically superior (p<0.001) to placebo on both co-primary endpoints at all time points from 3h through 48h Co-primary endpoints assessed at 2h p=0.0002 p=0.0552 Co-primary endpoints assessed at 2h p=0.0009 p=0.1661 Subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures 6 *from among photophobia, phonophobia or nausea
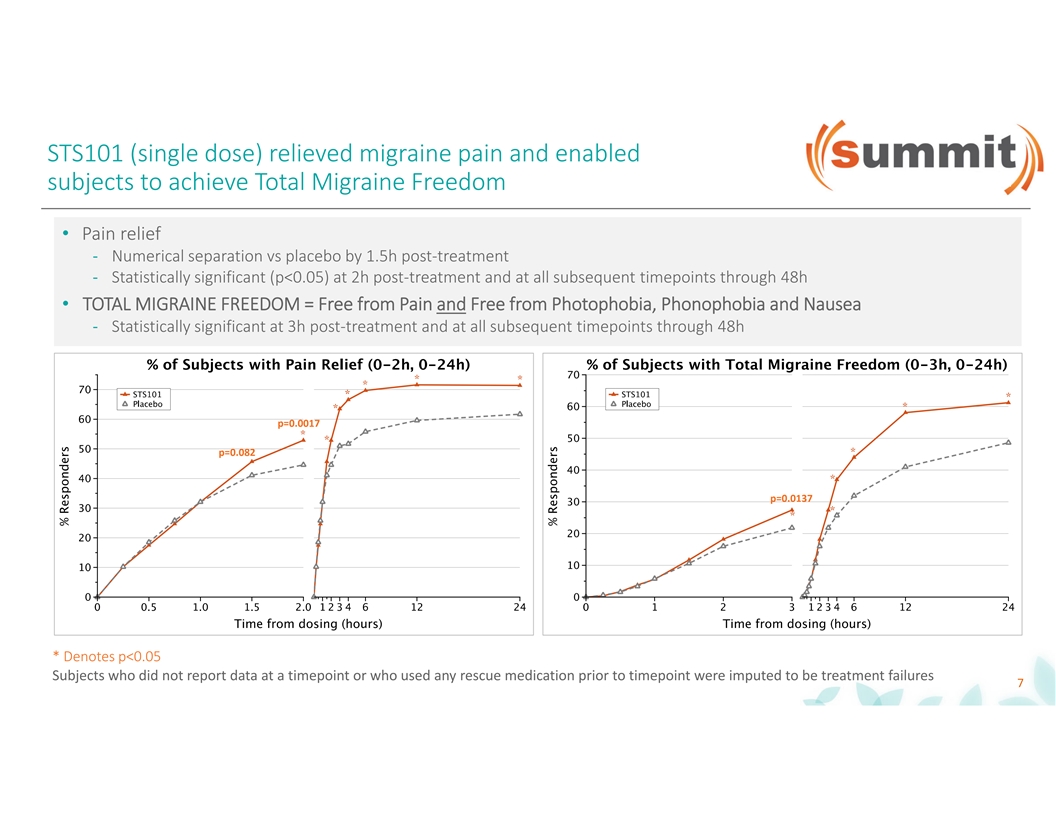
STS101 (single dose) relieved migraine pain and enabled subjects to achieve Total Migraine Freedom • Pain relief - Numerical separation vs placebo by 1.5h post-treatment - Statistically significant (p<0.05) at 2h post-treatment and at all subsequent timepoints through 48h • TOTAL MIGRAINE FREEDOM = Free from Pain and Free from Photophobia, Phonophobia and Nausea - Statistically significant at 3h post-treatment and at all subsequent timepoints through 48h p=0.0017 p=0.082 p=0.0137 * Denotes p<0.05 Subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures 7
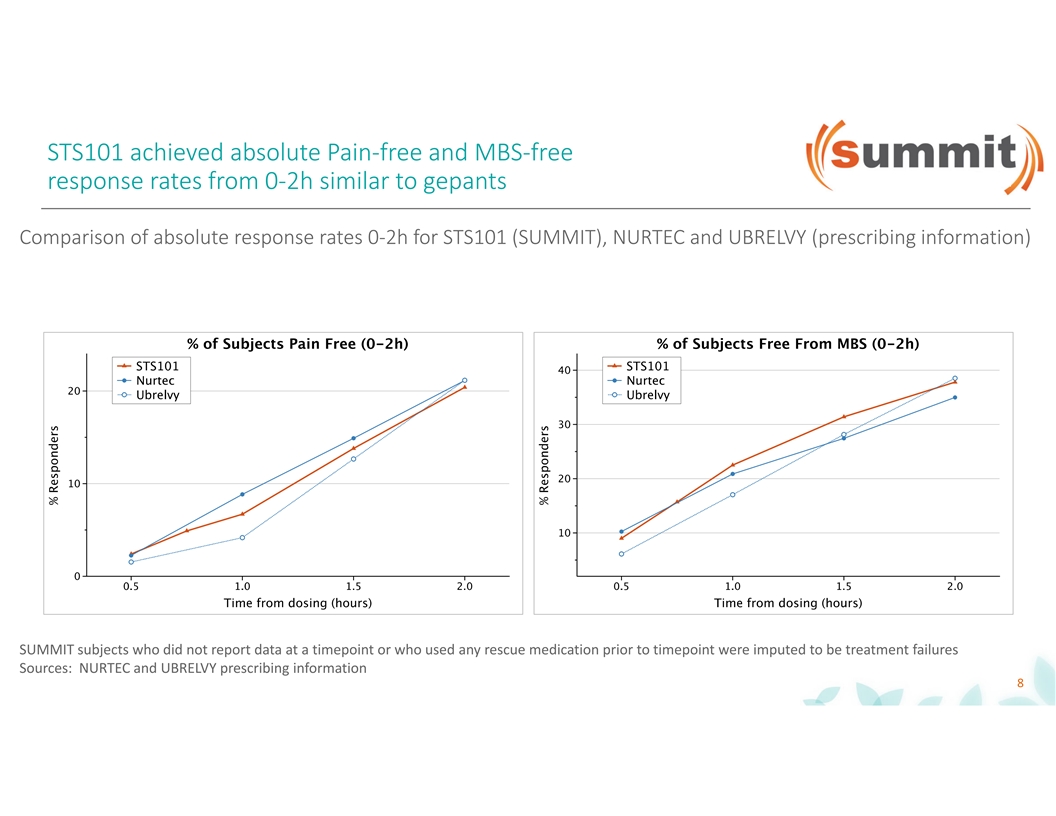
STS101 achieved absolute Pain-free and MBS-free response rates from 0-2h similar to gepants Comparison of absolute response rates 0-2h for STS101 (SUMMIT), NURTEC and UBRELVY (prescribing information) SUMMIT subjects who did not report data at a timepoint or who used any rescue medication prior to timepoint were imputed to be treatment failures Sources: NURTEC and UBRELVY prescribing information 8

SUMMIT Phase 3 efficacy trial summary ü STS101 demonstrated robust and sustained anti-migraine effects across numerous secondary endpoints Endpoint p-value (*indicates p<0.05) * Pain relief at 2h 0.0017 (2h - 48h* all timepoints) 1 Sustained pain-free 2-24h 0.0479 No use of rescue medication within 24h and 48h≤0.0001 1 1 Sustained freedom from MBS from 2-24h and 2-48h 0.0018 / 0.0082 1 1 Sustained relief from pain from 2-24h and 2-48h 0.0001 / 0.0006 Total freedom from migraine 0.0137 at 3h and stat sig at all timepoints thru 48h (no pain, no photophobia, no phonophobia and no nausea) Freedom from nausea 0.0039 at 3h and stat sig at all timepoints thru 48h Freedom from photophobia 0.0109 at 3h and stat sig at all timepoints thru 48h Freedom from phonophobia 0.0089 at 3h and stat sig at all timepoints thru 48h * Time to pain freedom Median time to pain freedom reduced by ~48% ü STS101 showed effects in difficult-to-treat attacks, e.g,. attacks with allodynia and menstrually associated attacks ü STS101 exhibited a favorable safety and tolerability profile, with low rates of local nasal adverse effects and minimal nausea or vomiting 9 1 - With no relapse or use of rescue medications
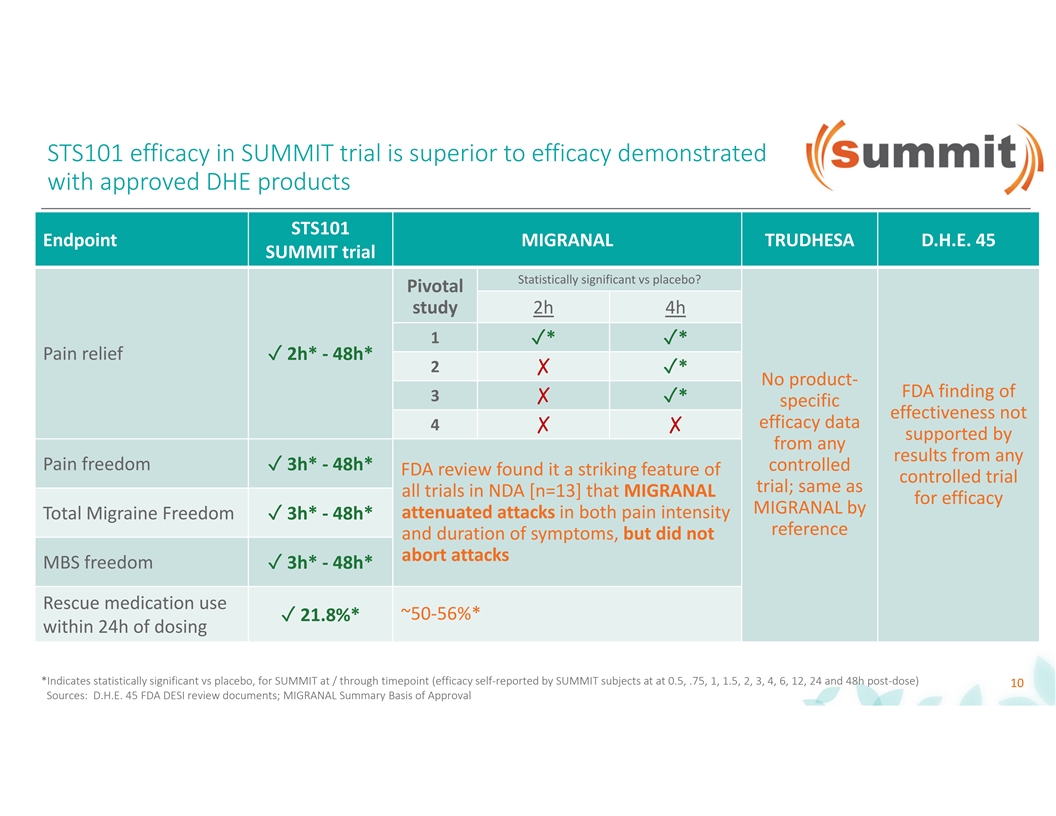
STS101 efficacy in SUMMIT trial is superior to efficacy demonstrated with approved DHE products STS101 Endpoint MIGRANAL TRUDHESA D.H.E. 45 SUMMIT trial Statistically significant vs placebo? Pivotal study 2h 4h 1✓*✓* Pain relief✓ 2h* - 48h* 2 ✗✓* No product- FDA finding of 3 ✗✓* specific effectiveness not efficacy data 4 ✗✗ supported by from any results from any Pain freedom✓ 3h* - 48h* controlled FDA review found it a striking feature of controlled trial trial; same as all trials in NDA [n=13] that MIGRANAL for efficacy MIGRANAL by attenuated attacks in both pain intensity Total Migraine Freedom✓ 3h* - 48h* reference and duration of symptoms, but did not abort attacks MBS freedom✓ 3h* - 48h* Rescue medication use ~50-56%* ✓ 21.8%* within 24h of dosing *Indicates statistically significant vs placebo, for SUMMIT at / through timepoint (efficacy self-reported by SUMMIT subjects at at 0.5, .75, 1, 1.5, 2, 3, 4, 6, 12, 24 and 48h post-dose) 10 Sources: D.H.E. 45 FDA DESI review documents; MIGRANAL Summary Basis of Approval

Based on input from expert legal-regulatory consultants, we are confident our STS101 regulatory strategy will support NDA filing and potential approval • Satsuma’s longstanding STS101 regulatory strategy has been to seek FDA approval via the 505(b)(2) pathway • In three meetings, including May 2022 pre-NDA meeting, FDA has agreed with our regulatory strategy and indicated STS101 approval requirements could be met by: 1. Establishment of a “pharmacokinetic bridge” to the listed drugs, D.H.E. 45 and MIGRANAL ü FDA agrees our Ph1 PK trial appears to establish the requisite PK bridge via a bracketing approach 2. Characterizing the long-term nasal toxicity of STS101 via the ASCEND long-term, open-label safety trial * ü FDA agrees results of the ASCEND trial appear to FDA has already found D.H.E. 45 and MIGRANAL “safe” support NDA filing and may support approval and “effective” and their PK curves bracket STS101; ergo, • FDA has consistently communicated that a Ph3 efficacy FDA should also consider STS101 “effective” and “safe” trial, such as SUMMIT, is NOT REQUIRED for STS101 with respect to its systemic adverse event profile approval, but could potentially be included in label * 6-month safety set with second-generation / Mk2 nasal delivery device 11

Based on input from expert legal-regulatory consultants, we believe there is a strong case for including SUMMIT trial efficacy results in the STS101 label Arguments for including SUMMIT efficacy trial results in the MIGRANAL (approved 1997) D.H.E. 45 STS101 label ü SUMMIT is a large, placebo-controlled trial conducted in Determined FDA review found multiple issues with design accordance with current migraine trial design, data analysis “effective” in early and conduct of trials and quality standards 1970s via DESI process ü SUMMIT co-primary endpoints are a high efficacy bar that no other DHE product has cleared Pivotal trials (n=4) utilized a “low-efficacy-bar” FDA finding of endpoints, Pain Relief and Reduction in Pain ü STS101 demonstrates robust anti-migraine effects that no effectiveness not Intensity, evaluated at both 2h and 4h post- other DHE product has shown in a placebo-controlled trial, supported by any treatment including Pain Freedom (3h*); MBS Freedom (3h*) and Total controlled trial for Migraine Freedom (3h*), i.e., the ability to abort attacks Showed inconsistent efficacy on pain and efficacy secondary symptom endpoints across trials ü Migraine trial construct (wait to treat until pain intensity is moderate or severe) is artificial; in the real world, patients There are no clinical MIGRANAL does not abort migraines are instructed to treat early, when pain is mild trial results in the FDA reviewer: “It is a striking feature of all D.H.E. 45 prescribing ü 2h timepoint for co-primary endpoint assessment is based studies in this Application [n=13] that the information on ethical considerations; postponing use of effective rescue use of [MIGRANAL] attenuated migraine medications beyond 2h deemed to subject trial participants attacks in both intensity of pain and duration to undue harm of symptoms, but did not abort the attacks.” ü STS101 SUMMIT trial results provide HCPs and patients with important and relevant information * Time point at which statistically significant effect vs placebo observed 12 Sources: D.H.E. 45 FDA DESI review documents; MIGRANAL Summary Basis of Approval, Diener et al., Cephalagia 2019
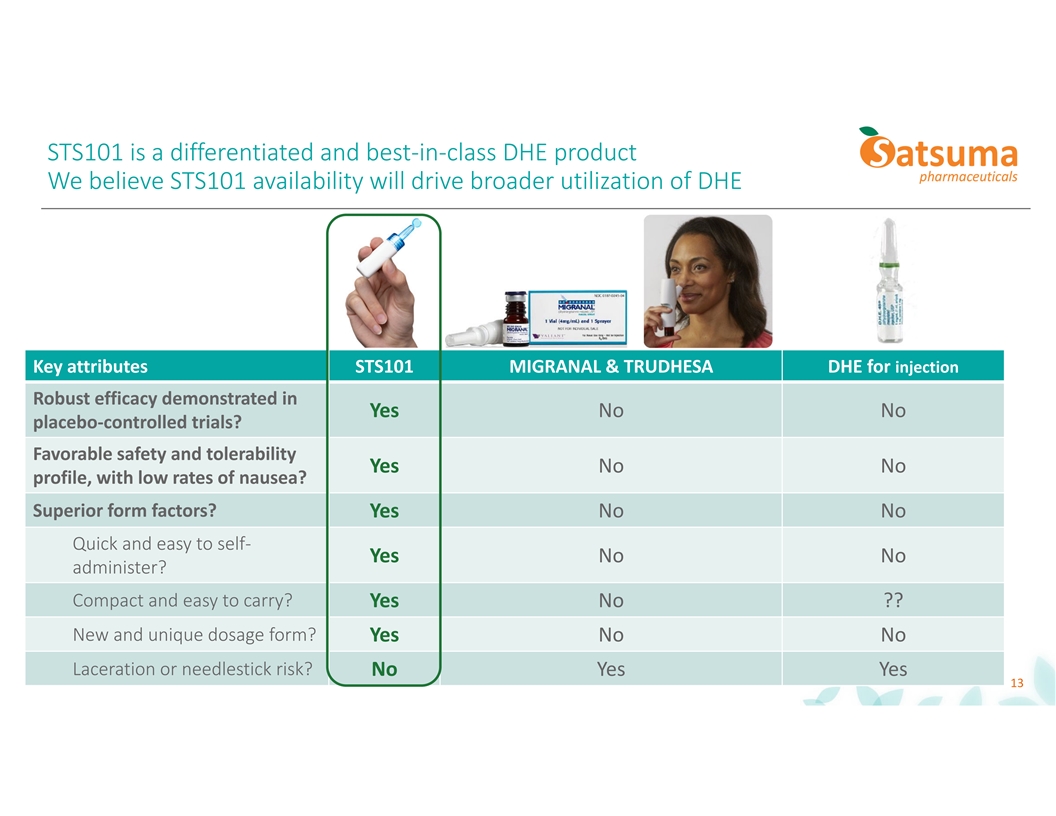
STS101 is a differentiated and best-in-class DHE product We believe STS101 availability will drive broader utilization of DHE Key attributes STS101 MIGRANAL & TRUDHESA DHE for injection Robust efficacy demonstrated in Yes No No placebo-controlled trials? Favorable safety and tolerability Yes No No profile, with low rates of nausea? Superior form factors? Yes No No Quick and easy to self- Yes No No administer? Compact and easy to carry? Yes No ?? New and unique dosage form? Yes No No Laceration or needlestick risk? No Yes Yes 13

Thank you













