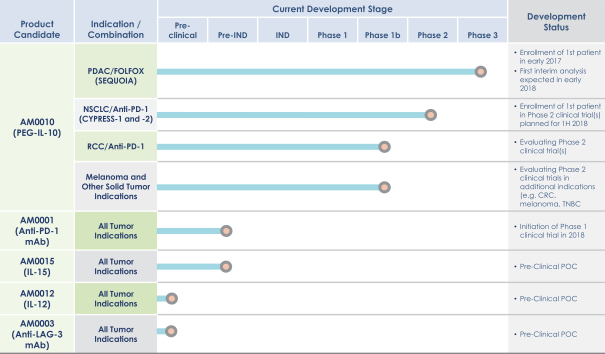
ARMO-003 family include Australia, Austria, Belgium, Canada, China, The Czech Republic, Denmark, European Patent Office, Finland, France, Germany, Greece, Hong Kong, Hungary, India, Ireland, Italy, Japan, The Netherlands, Norway, Poland, Portugal, Russia, Sweden, Switzerland and the United Kingdom. The type of protection provided by the patents and pending patent applications includes protection related to methods of treatment (use), composition of matter, and methods of manufacturing. In general, the patent terms run 20 years from the earliestnon-provisional priority application to which priority is claimed in each patent family. The normal statutory term of the United States and foreign patents and applications will expire on September 28, 2021 for theARMO-001 patent family, September 27, 2027 for theARMO-002 patent family, and December 15, 2029 for theARMO-003 patent family. Certain U.S. patents in these patent families are entitled today-by-day extensions of term and may have a later expiration date. For more information about the Merck Agreement, see the section below entitled “Merck Agreement.”
As of December 21, 2017, we also own pending patent applications in the United States and foreign jurisdictions with claims directed to compositions of matter ofIL-10 variant polypeptides, methods of usingIL-10 in combination therapies, including PEGylated forms ofIL-10, as well as toIL-10 variant polypeptides that retainIL-10 activity, for treating various diseases and disorders. The foreign jurisdictions in which we own such pending patent applications include Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, Japan, Mexico, New Zealand, Philippines, Russia, South Africa, and South Korea. Several of these pending patent applications are in the international phase as Patent Cooperation Treaty (PCT) applications, which preserve the ability to pursue foreign rights in the countries listed above (in addition to otherPCT-member countries). In the event that any patents issue based on these patent applications, such patents would be expected to expire between 2034 and 2036, absent any patent term adjustments or extensions.
As of December 21, 2017, we have one issued U.S. patent, one pending U.S. application and five pending foreign patent applications with claims directed to methods for characterizing IL-10 species. The foreign jurisdictions in which we own such pending patent applications include Australia, Canada, Europe, Hong Kong and Japan. As of December 21, 2017, we own one pending U.S. and five foreign patent applications relating to processes for refolding IL-10 from inclusion bodies arising from recombinant expression in bacterial cells, respectively. The jurisdictions in which we own such pending patent applications include Australia, Canada, China, Europe, and Japan. In the event that patents issue based on these patent applications, such patents would be expected to expire between 2034 and 2035, absent any patent term adjustments or extensions.
Additionally, as of December 21, 2017, we have one U.S. patent application and eight foreign applications with composition of matter claims directed to IL-15 variant peptides and methods of using such variant peptides for treating various diseases, disorders or conditions. The foreign jurisdictions in which we own such pending patent applications include Australia, Canada, China Europe, Japan, South Korea, Mexico, and South Africa. We own another patent application with claims directed to PEGylated forms of IL-15 and methods of using such variant peptides for treating various diseases, disorders or conditions. As of December 21, 2017, this application is in the PCT international stage of prosecution. In the event that patents issue based on these patent applications, such patents would be expected to expire in 2035 and 2036, respectively, absent any patent term adjustments or extensions.
Additionally, as of December 21, 2017, we own a PCT patent application directed to clinical applications ofIL-12 in combination withIL-10, including PEGylated forms ofIL-10. In the event that patents issue based on this patent application, such patents would be expected to expire in 2036, absent any patent term adjustments or extensions.
Patent Term Adjustments Arising From Patent Office Administrative Delays
Patents are granted on a territorial basis and the term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the date of filing of the firstnon-provisional application to which priority is claimed. In the United States, the term of a patent may be extended due to patent term adjustment in the United States Patent and Trademark Office pursuant to 35 USC 154, which law is intended to compensate a patentee for administrative delays in the patent prosecution process caused by the U.S. Patent and Trademark Office. Alternatively, the term of a patent may be shortened if a patent is terminally disclaimed over an earlier-filed patent. When applicable, we expect to apply for patent term extensions for patents owned, controlled or exclusively licensed to us.
93