As filed with the Securities and Exchange Commission on August 5, 2021
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
STEALTH BIOTHERAPEUTICS CORP
(Exact name of registrant as specified in its charter)
N/A
(Translation of Registrant’s Name into English)
Cayman Islands | 2834 | Not Applicable |
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
Stealth BioTherapeutics Corp
c/o Intertrust Corporate Services (Cayman) Limited
One Nexus Way, Camana Bay
Grand Cayman
KY1-9005 Cayman Islands
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
Stealth BioTherapeutics Inc.
140 Kendrick Street
Needham, MA 02494
(617) 600-6888
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
Rosemary G. Reilly, Esq.
Wilmer Cutler Pickering Hale and Dorr LLP
60 State Street
Boston, MA 02109
Telephone: (617) 526-6000
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging Growth Company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
TITLE OF EACH CLASS OF SECURITIES TO BE REGISTERED (1) | PROPOSED MAXIMUM
AGGREGATE
OFFERING PRICE (2) | AMOUNT OF
REGISTRATION
FEE (3) |
Ordinary shares, $0.0003 nominal or par value per share | $12,000,000 | $1,309 |
|
| (1) | American depositary shares issuable upon deposit of the ordinary shares registered hereby have been registered under a separate registration statement on Form F-6 (Registration No. 333-229509). Each American depositary share represents 12 ordinary shares. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (3) | Calculated pursuant to Rule 457(o) under the Securities Act of 1933, as amended, based on an estimate of the proposed maximum aggregate offering price. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED , 2021
PRELIMINARY PROSPECTUS
American Depositary Shares

Representing Ordinary Shares
We are offering American Depositary Shares, or ADSs, each representing 12 ordinary shares, $0.0003 nominal or par value per share, of Stealth BioTherapeutics Corp. Our ADSs are listed on The Nasdaq Global Market under the symbol “MITO.” We have assumed a public offering price of $ per ADS, the last sale price of our ADSs reported on The Nasdaq Global Market on , 2021. The public offering price will be determined through negotiation between us and the investors, in consultation with the placement agent, and may be at a discount to the then current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price.
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended, and have elected to comply with certain reduced public company reporting requirements. See “Prospectus Summary—Implications of Being an Emerging Growth Company.”
Investing in the ADSs involves a high degree of risk. See the “Risk Factors” section beginning on page 13 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We have engaged H.C. Wainwright & Co., LLC, or the placement agent or Wainwright, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent has no obligation to buy any securities from us or to arrange for the purchase or sale of any specific number or dollar amount of our securities. We have agreed to pay the placement agent the placement agent fees set forth in the table below. There is no arrangement for funds to be received in escrow, trust or similar arrangement. The placement agent may engage one or more sub-placement agents or selected dealers in connection with the offering. See “Plan of Distribution” beginning on page 144 of this prospectus for more information regarding this arrangement.
There is no required minimum number of ADSs or amount of proceeds that must be sold as a condition to completion of this offering.
| | PER ADS | | | TOTAL | |
Public Offering Price | | $ | | | | $ | | |
Placement agent fees (1) | | $ | | | | $ | | |
Proceeds to us before expenses | | $ | | | | $ | | |
| (1) | We have also agreed to pay the placement agent a management fee equal to 1.0% of the gross proceeds in this offering and to pay a the placement agent a non-accountable expense allowance equal to $35,000 and to reimburse legal fees and expenses and other out-of-pocket expenses of the placement agent in this offering up to $100,000. See “Plan of Distribution” beginning on page 144 for additional information regarding placement agent compensation. |
Delivery of the ADSs is expected to be made on or about , 2021
H.C. Wainwright & Co.
Prospectus dated , 2021
TABLE OF CONTENTS
Neither we nor the placement agent have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses we have prepared. We take no responsibility for, and can provide no assurance as to, the reliability of any other information that others may give you. We are offering to sell, and seeking offers to buy, ADSs only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of ADSs. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we nor the placement agent have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the ADSs and the distribution of this prospectus outside of the United States.
We are incorporated under the laws of the Cayman Islands as an exempted company with limited liability, and a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the U.S. Securities and Exchange Commission, or the SEC, we are currently eligible for treatment as a “foreign private issuer.” As a foreign private issuer, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic registrants whose securities are registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements incorporated by reference or included elsewhere in this prospectus. It does not contain all of the information that you should consider in making your investment decision. Before investing in the ADSs, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes incorporated by reference or included elsewhere in this prospectus. You should also consider, among other things, the matters described under “Risk Factors” beginning on page 13. Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Stealth,” “the Company,” “we,” “us” and “our” refer to Stealth BioTherapeutics Corp and its consolidated subsidiaries, or any one or more of them as the context may require.
Overview
We are a clinical-stage biotechnology company focused on the discovery, development and commercialization of novel therapies for diseases involving mitochondrial dysfunction. Mitochondria, found in nearly every cell in the body, are the body’s main source of energy production and are critical for normal organ function. Dysfunctional mitochondria characterize a number of rare genetic diseases and many common age-related diseases, leading to devastating cardiac, ophthalmic and neurological symptoms. Our mission is to be the leader in mitochondrial medicine, and we have assembled a highly experienced management team, board of directors and group of scientific advisors to help us achieve this mission.
We believe our product candidates have significant potential to treat the cardiac, ophthalmic and neurological symptoms of both rare genetic and common age-related mitochondrial diseases. We are focusing our development efforts on rare cardiomyopathies, ophthalmic diseases and rare neurological diseases.
Rare cardiomyopathies
Barth. Barth syndrome, or Barth, is characterized by heart muscle weakness, or cardiomyopathy; neutropenia, or low white blood cell count (which may lead to an increased risk for infections); skeletal muscle weakness; delayed growth; fatigue; and varying degrees of physical disability. Barth is estimated to affect between one in 300,000 to one in 400,000 births in the United States, and there are estimated to be less than 300 known living patients worldwide with Barth. There are no therapies approved by the United States Food & Drug Administration, or FDA, or the European Medicines Agency, or the EMA, for the treatment of Barth. We have received Fast Track designation from the FDA and Orphan Drug designation from the FDA and the EMA for the development of our clinical product candidate elamipretide in this indication. In February 2020, the FDA granted rare pediatric disease designation for elamipretide for the treatment of Barth, and we may therefore be eligible for a voucher that can be used to obtain priority review for a subsequent human drug application if our Barth product candidate meets relevant statutory requirements associated with the program, including FDA approval of the drug for the treatment of Barth.
In December 2018, we completed the placebo-controlled portion of a Phase 2/3 clinical trial in patients with Barth. While the trial did not reach its primary endpoints, we observed trends toward improvement in cardiac function as well as trends toward improvement in other endpoints in a prespecified subset of patients with higher levels of normal cardiolipin, who we believe are the patients most likely to respond to therapy, and trend toward improvement in cardiac function. We have observed significant improvements from baseline to week 72 across several functional endpoints, as well as improvement in heart function, in eight patients enrolled in an open-label extension trial. In February 2020, we completed a Phase 3 retrospective natural history comparative control study, comparing data from the open-label extension trial with matched natural history controls. The natural history control study met its primary efficacy endpoint of change in the six-minute walk test, or 6MWT, between the patients treated with elamipretide through week 36 of open-label extension and the prognostically matched natural history controls, as well as several secondary efficacy endpoints. We believe that the results of the natural history control study indicate that the results on the 6MWT for patients treated with elamipretide in open-label extension would not be predicted by the natural history of the disease.
We met with the Division of Cardiology and Nephrology at the FDA in November 2020, in February 2021 and April 2021 to discuss the clinical evidence to support a potential new drug application, or NDA, submission for Barth. We have had multiple recent communications with senior FDA officials in the Office of Cardiology, Hematology, Endocrinology and Nephrology and at the Division of Cardiology and Nephrology regarding our Barth syndrome program following the April 2021 meeting. We also received a petition signed by over 4,250 members of the Barth community requesting us to submit our NDA on the basis of our existing clinical data. The FDA expressed its view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review. The FDA recommended that we collect additional controlled clinical data in this indication prior
1
to an NDA submission. In May 2021, we submitted a randomized withdrawal clinical trial protocol to FDA. This design was previously suggested by multiple FDA review divisions, including most recently the Division of Cardiology and Nephrology. After reviewing our submission, FDA concluded that neither the proposed randomized withdrawal trial nor any new clinical trial data from the patients remaining on OLE would be likely to add meaningfully to the evidence to support an NDA. Due to the ultra-rare nature of Barth syndrome, neither the Company nor the FDA to date has been able to identify a feasible trial design to generate additional data. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
Duchenne cardiomyopathy. Cardiomyopathy is also a leading cause of death in Duchenne’s muscular dystrophy, or DMD, an inherited muscle wasting disease affecting an estimated one in 3,500 to 5,000 male births in the United States. There are no therapies approved by the FDA or EMA for the treatment of cardiac manifestations of DMD, or Duchenne cardiomyopathy. Most DMD patients develop Duchenne cardiomyopathy, and heart failure and sudden cardiac death are the most commonly reported causes of early mortality among these patients. The expert consensus from a clinical advisory board meeting we hosted in June 2020 is that mitochondrial dysfunction is central to the pathology of Duchenne cardiomyopathy and that elamipretide may be beneficial to treat this devastating and life-limiting sequela of the disease. We plan to launch early development efforts to commence a Phase 2/3 clinical trial in Duchenne cardiomyopathy in early 2022, subject to discussions with FDA, continued planning efforts, and financing plans.
FRDA. Friedreich’s ataxia, or FRDA, is the most common form of hereditary ataxia, or loss of coordination, affecting an estimated one in 40,000 people in the United States. There are no therapies approved by the FDA or EMA for the treatment of FRDA. Many FRDA patients experience progressive vision loss, and most FRDA patients develop cardiomyopathy. We plan to support the initiation of a Phase 2a open-label clinical trial assessing elamipretide in a cohort of patients affected by cardiomyopathy and/or visual decline associated with FRDA, which is anticipated to commence enrollment in second half of 2021.
We are also continuing to assess other orphan cardiac indications for future development.
Ophthalmic diseases
Geographic Atrophy. Geographic atrophy, or GA, an advanced form of dry age-related macular degeneration, or dry AMD, is estimated to affect approximately one million individuals in the United States. Dry AMD, a common ophthalmic disease associated with aging, is characterized by symptoms such as distorted vision, reduction in low light visual acuity, reduced overall visual acuity and blurred vision and is the leading cause of blindness among older adults in the developed world. There are no therapies approved by the FDA or the EMA for the treatment of dry AMD at any stage, including GA. We completed a Phase 1 clinical trial in dry AMD patients with both GA and high risk drusen, an early form of dry AMD, in which we observed statistically significant improvement over baseline in various parameters of visual function in both the drusen and GA cohorts. A recently completed post-hoc analysis of that data demonstrated that patients with better mitochondrial health at baseline were more likely to have greater improvement in visual function with elamipretide, which we believe provides further support for our therapeutic approach. We received Fast Track designation from the FDA for the development of elamipretide for patients with GA in November 2018. Our Phase 2b trial was fully enrolled in February 2021, and we expect data from this trial during the first half of 2022.
LHON. Leber’s hereditary optic neuropathy, or LHON, is characterized by central vision loss. We estimate that LHON affects approximately 10,000 individuals in the United States, of whom an estimated 70% have the genetic mutation, G11778A, that we studied in our Phase 2 clinical trial. There are no therapies approved by the FDA for the treatment of LHON, and there is only one EMA-approved therapy. We have received Fast Track and Orphan Drug designations from the FDA for the development of elamipretide in this indication. We may initiate a Phase 3 global clinical trial for elamipretide in LHON, subject to ongoing formulation studies expected to read out in early 2022, continued planning efforts and financing plans.
Rare neuromuscular and neurological diseases
nPMD. We have observed preclinical and clinical signals of efficacy with elamipretide in neuromuscular dysfunction associated with primary mitochondrial disease arising due to nuclear DNA, or nDNA, mutations, or nPMD. We observed improvement in this prespecified subgroup of patients in our primary mitochondrial myopathy, or PMM program, particularly in patients with nDNA mutations affecting mitochondrial DNA replication, or replisome-related mutations. We have reached agreement with the FDA on our trial design for a Phase 3 clinical trial for elamipretide in patients with PMM due to nPMD, which we plan to initiate during the second half of 2021,
2
subject to continued planning efforts and financing plans. We have received Fast Track and Orphan Drug Designation from the FDA for the development of elamipretide in patients with PMM.
SBT-272. In addition to our clinical development programs for elamipretide, we are developing SBT-272 for rare neurodegenerative diseases. Preliminary results from a Phase 1 clinical trial in healthy human volunteers completed during 2020 did not reach desired drug exposure levels. We are conducting subcutaneous dosing studies and plan to commence longer term toxicology studies in 2021 to support the initiation of a Phase 1 clinical trial in early 2022 and the potential initiation of a Phase 2 clinical trial in patients in late 2022. We observed an improvement in survival in a mouse model of amyotrophic lateral sclerosis, or ALS, a progressive neurodegenerative disease characterized by motor neuron deterioration and muscle atrophy estimated to affect one in 50,000 people in the United States. We have conducted and continue to conduct preclinical studies in neurological disease models to inform our decisions regarding our first Phase 2 indication. These preclinical studies are being conducted in a second ALS model and in a model of multiple system atrophy, or MSA, a neurological disorder characterized by a combination of symptoms affecting both the autonomic nervous system and movement that is estimated to affect one in 20,000 to 50,000 people in the United States.
Other pipeline compounds. We also plan to evaluate compounds in our SBT-550 family for rare neurological indications such as Leigh’s syndrome, a severe neurological condition affecting an estimated one in 40,000 newborns.
In addition, our internal discovery platform has generated a library of over 100 differentiated proprietary compounds which could have clinical benefit for diseases related to mitochondrial dysfunction and from which we plan to designate potential product candidates. We may also utilize certain of these compounds as part of our carrier platform, in which they could serve as mitochondria-targeted vectors to deliver other beneficial compounds to the mitochondria.
Our Pipeline
The following table summarizes our development pipeline, including preclinical studies and ongoing and planned clinical trials of our product candidates.
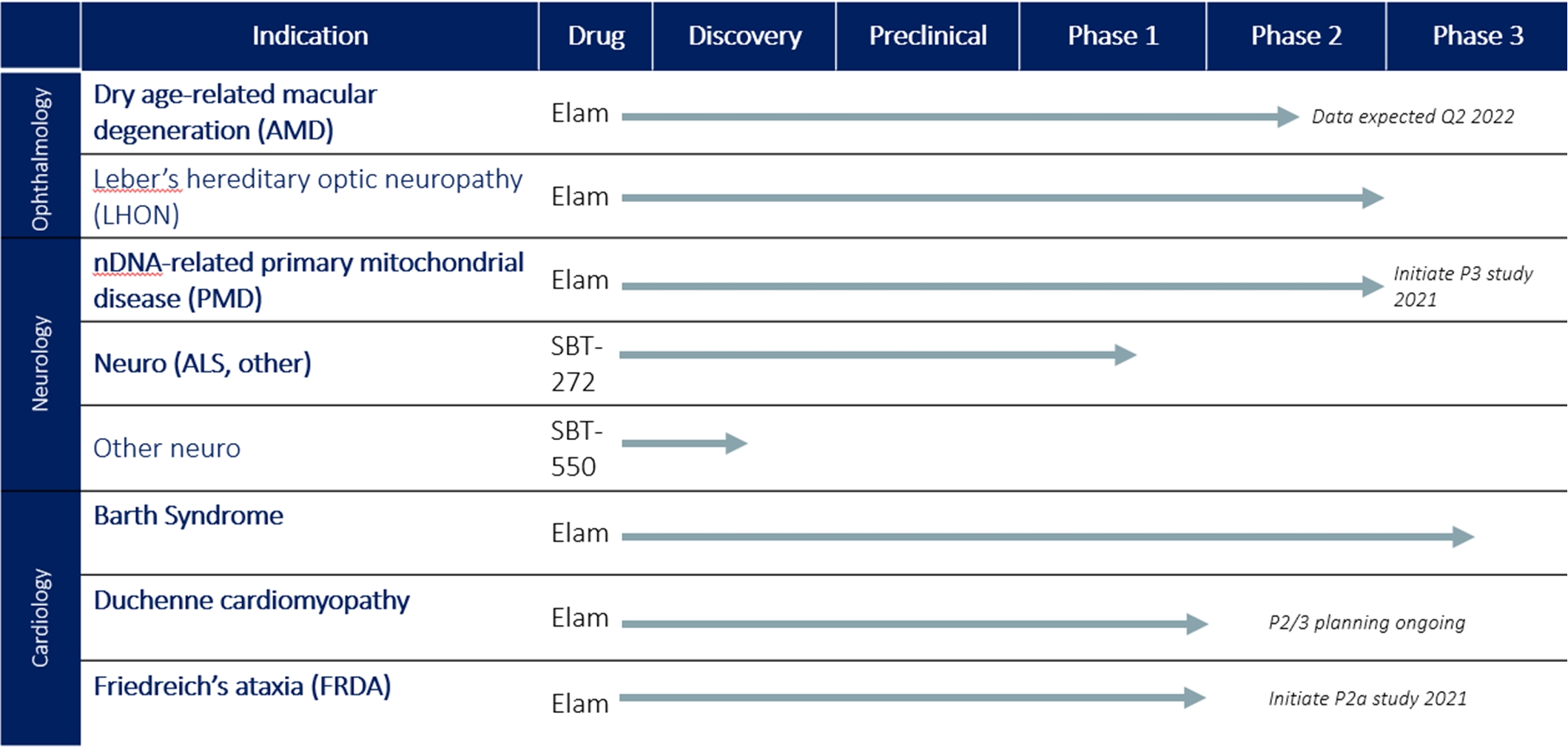
Our Clinical Product Candidates
Elamipretide
Elamipretide is a mitochondria-protecting peptide that targets and binds reversibly to cardiolipin, stabilizing mitochondrial structure and function under conditions of oxidative stress. Elamipretide has been reported to be well tolerated in clinical trials in over 1,000 subjects systemically exposed to it to date.
We are evaluating elamipretide in diseases involving devastating ophthalmic and rare cardiomyopathies, where there is a genetic basis for the underlying mitochondrial dysfunction and where we have the potential for expedited
3
regulatory review. These include Barth and FRDA, as well as LHON. We also believe that elamipretide and our pipeline compounds may be able to address the significant unmet medical needs of larger populations affected by common diseases associated with aging. We are progressing our development of elamipretide for GA, and we may in the future evaluate clinical trials for other common age-related disease indications in conjunction with our pipeline compounds.
SBT-272
SBT-272, our second clinical-stage pipeline compound, is a novel peptidomimetic that has been shown to increase adenosine triphosphate, or ATP, production and decrease levels of reactive oxygen species, or ROS, in dysfunctional mitochondria in preclinical studies. We are evaluating SBT-272 for rare neurodegenerative diseases, such as ALS and MSA. Based on preclinical studies, we believe that SBT-272 readily penetrates cell membranes and potentially improves mitochondrial function, with more than ten times greater exposure in the brain relative to elamipretide. We observed promising early signals in a preclinical SOD-1 model, the gold standard preclinical ALS model, and are conducting additional studies in a second preclinical ALS model as well as in a preclinical MSA model. Preliminary results from a Phase 1 clinical trial in healthy human volunteers completed during 2020 did not reach desired drug exposure levels. We are currently conducting subcutaneous dosing studies and plan to commence longer term toxicology studies in 2021 to support the initiation of a Phase 1 clinical trial in early 2022 and subsequent potential initiation of a Phase 2 clinical trial in patients during late 2022, subject to continued planning efforts, and financing plans.
Background on Mitochondrial Diseases
Mitochondria, found in almost all human cells, are the “powerhouse of the cell.” Normal mitochondrial function is essential for human life and for the proper functioning of many systems in our bodies. Mitochondria have their own DNA, called mitochondrial DNA, or mtDNA, which is inherited only from our mothers and is separate and distinct from nDNA. Mitochondrial diseases arising from inherited genetic defects, called primary mitochondrial diseases, are typically rare diseases which can impact multiple organ systems within the body and may lead to reduced lifespan. Symptoms of primary mitochondrial disease, including chronic pain, vision problems, cardiovascular problems and kidney problems, may be compared to “accelerated aging” as described by individuals with the disease and their caregivers.
Although mtDNA is originally inherited from our mothers, it is replicated within our mitochondria as mitochondria reproduce and is highly susceptible to mutation within specific cells and organ systems as we age. Mitochondrial diseases arising from these spontaneous mutations in our mtDNA, called secondary mitochondrial diseases, include heart disease (such as heart failure and atherosclerosis), diabetes, ophthalmic conditions (such as age-related macular degeneration, glaucoma, diabetic retinopathy and diabetic macular edema), neurodegenerative diseases (such as Alzheimer’s, Parkinson’s and ALS), senescence, cancer, diabetes, skeletal muscle dysfunction (such as sarcopenia) and kidney diseases.
Mitochondrial dysfunction, whether inherited or acquired, often impacts high energy-demanding organs such as those of the cardiac, renal, visual, neurological, central nervous, skeletal muscle, circulatory or endocrine systems.
Our approach
We have focused our development efforts on diseases and conditions that affect the organs in the body that generate significant energy because of the high mitochondrial content found in the cells comprising these organs. The activity of our compounds has been studied in several disease models, including heart failure, kidney disease, skeletal muscle weakness, diabetic retinopathy and neurodegenerative diseases. We believe that our product candidates may be most relevant for the visual system, the cardiorenal system and the brain, all of which are innately highly dependent on mitochondrial bioenergetics.
Our clinical-stage product candidates target cardiolipin, a conically shaped phospholipid that plays an important role in mitochondrial energy production. Reduced and damaged cardiolipin content has been observed in many diseases, and a deficiency of normal cardiolipin is thought to be centrally involved in mitochondrial dysfunction. Elamipretide, as well as SBT-272 and several of our other pipeline compounds, targets and binds reversibly to cardiolipin, stabilizing the inner mitochondrial membrane under conditions of oxidative stress.
We are also developing products to address other aspects of mitochondrial dysfunction beyond cardiolipin. We believe that our SBT-550 series of compounds acts upon the ferroptosis pathway, which has been implicated in many neurological diseases, including Huntington’s disease, FRDA, Alzheimer’s disease and Leigh’s syndrome. We are also progressing our carrier platform in which we utilize our proprietary compounds as mitochondria-targeted vectors to deliver other beneficial compounds to the mitochondria.
4
Our Strategy
We aspire to lead the development of mitochondrial medicine to improve the lives of patients with severe unmet medical needs. Our strategy is to focus on near-term rare disease opportunities in ophthalmic, cardiac and neurological indications, while continuing to progress the potential of our approach to treat diseases associated with aging in which mitochondrial dysfunction has been implicated. Particularly for larger common disease indications associated with aging, we plan to assess development collaborations with industry leaders. To achieve our goals, we intend to:
Progress toward approval of elamipretide in Barth
We have conducted a pivotal Phase 3 retrospective natural history control trial and a Phase 2/3 double-blind placebo-controlled trial in Barth. We observed improvements in cardiac and clinical endpoints in our pivotal Phase 3 clinical trial and during the open-label extension portion of our Phase 2/3 trial. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
We have received Fast Track in the United States and Orphan Drug designations for Barth in the United States and EMA. In addition to our planned NDA submission, we are evaluating regulatory pathways in Europe.
Advance the development of our mitochondrial medicines in cardiomyopathies
We are encouraged by the improvement in cardiac function observed in Barth patients, and plan to expand our efforts to develop elamipretide for other rare mitochondrial diseases affecting cardiac function. We are evaluating the potential for clinical trials in Duchenne cardiomyopathy, and FRDA, where a Phase 2a investigator-initiated trial is expected to begin by the end of 2021. We also plan to explore our second-generation and pipeline mitochondrial medicines in preclinical models of cardiac dysfunction.
Progress the clinical and preclinical development of our mitochondrial medicines in ophthalmology
We are developing elamipretide for ophthalmic conditions associated with mitochondrial dysfunction. We intend to continue to rapidly advance elamipretide through the completion of our Phase 2b clinical trial in GA, which was fully enrolled in the first quarter of 2021, with data expected in first half of 2022. We have received Fast Track designation for this indication in the United States. We believe there is a strong potential for elamipretide to treat rare diseases where mitochondrial dysfunction leads to visual dysfunction, including FRDA and LHON, for which we have received Fast Track and Orphan Drug designations in the United States. We are also exploring our second-generation and pipeline mitochondrial medicines in preclinical models of ophthalmic disease.
Advance the development of our mitochondrial medicines for rare neuromuscular and neurological diseases
We hope to initiate a pivotal trial for elamipretide in patients with PMM, due to nPMD during 2021. We have reached agreement with the FDA regarding the trial design for this study. We have received Fast Track and Orphan Drug Designation for this indication in the United States. We are also developing our second-generation and pipeline mitochondrial medicines for rare neurological diseases involving mitochondrial dysfunction.
Deliver on the promise of our carrier program
We have extensive experience in optimizing delivery of our compounds to the mitochondria, which has been a challenge for other drug delivery technologies. We have demonstrated capability to deliver beneficial payloads to mitochondria by conjugating them with our proprietary compounds, which serve as vectors or carriers to mitochondria. This approach has the potential to confer mitochondrial specificity to promising therapies that do not otherwise localize to mitochondria, potentially increasing the efficacy of a payload by targeting it to the part of the cell where it is needed most. These payloads might include small molecules, proteins, oligonucleotides, nanoparticles and liposomes. This delivery strategy, which we call our carrier program, has the potential to create new pipeline assets from known delivery of small molecules, enzymes, proteins or therapeutic genes to address inherited mitochondrial disorders.
Explore potential strategic partnerships
We may explore select strategic partnerships and alliances to support our drug development programs, while preserving significant development and commercialization rights, if we believe that such alliances will enable us to leverage the financial support and therapeutic area expertise and resources of a strategic partner to accelerate the development and commercialization of our product candidates.
5
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks include the following:
| ▪ | We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our research and drug development programs or commercialization efforts. If we do raise additional capital, it may cause dilution to our shareholders. |
| ▪ | Based on our cash balances, recurring losses and our projected spending in 2021 and 2022, and without giving effect to additional potential funding or milestone payments under the Development Funding Agreement, there is a substantial doubt about our ability to continue as a going concern. |
| ▪ | We have incurred significant losses since inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability. As of June 30, 2021, we had an accumulated deficit of $581.5 million. |
| ��� | We depend heavily on the success of our clinical product candidates, and we cannot be certain that we will receive regulatory approval for any of our product candidates or if we will successfully commercialize any of our product candidates even if we receive such regulatory approval. If the FDA does not accept or approve our NDAs for our most advanced product candidates, including our planned NDA for Barth, it may require that we conduct additional clinical, nonclinical or manufacturing validation studies and submit that data before it will reconsider our applications. |
| ▪ | Our approach to the discovery and development of product candidates that target mitochondria is unproven, and we do not know whether we will be able to develop any products of commercial value. |
| ▪ | If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates. |
| ▪ | We expect to depend on third parties for the development, marketing and/or commercialization of our product candidates in some cases. If those collaborations are not successful, we may not be able to capitalize on the market potential of our product candidates. |
| ▪ | We hold exclusive licenses from Cornell Research Foundation and the Institut de recherches cliniques de Montréal for our lead clinical-stage product candidate elamipretide. If these third parties terminate their agreements with us, our competitive position and our market share will be harmed. For example, our license agreement with Cornell required us to commercialize a product by December 31, 2020, subject to specified exceptions for causes due to scientific and regulatory events that are common in drug development, and Cornell has the right to terminate the license if we do not comply. We believe that our noncompliance is subject to the named exceptions, and to date we have not received any notice of termination from Cornell. |
| ▪ | Following completion of this offering, MVIL will beneficially own approximately % of our ordinary shares, without giving effect to any ADSs that may be purchased by it in this offering, assuming that we sell the number of ADSs set forth on the cover page of this prospectus, and will therefore continue to have substantial control over us after this offering, which could limit your ability to influence the outcome of key decisions and transactions involving us, including a change of control. |
| ▪ | As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and Nasdaq Stock Market, and corporate governance rules and are permitted to file less information with the Securities and Exchange Commission, than U.S. companies. This may limit the information available to holders of our securities. |
Corporate Information
Our registered office is located at c/o Intertrust Corporate Services (Cayman) Limited, One Nexus Way, Camana Bay, Grand Cayman, KY1-9005 Cayman Islands. Our agent for service of process in the United States is Stealth Delaware, and the executive offices of Stealth Delaware are located at 140 Kendrick Street, Needham, MA 02494, and the telephone number there is (617) 600-6888. Our website address is www.stealthbt.com. We have included our website address in this prospectus as an inactive textual reference only. The information contained in, or accessible through, our website does not constitute part of this prospectus.
6
Stealth BioTherapeutics Corp is a Cayman Islands exempted company and we conduct our operations in the United States through Stealth BioTherapeutics, Inc., a Delaware company, which we refer to as Stealth Delaware. All of our employees are employed by Stealth Delaware.
“Stealth BioTherapeutics,” the Stealth BioTherapeutics logo and other trademarks or service marks of Stealth BioTherapeutics Corp appearing in this prospectus are the property of Stealth BioTherapeutics or our subsidiaries. This prospectus contains additional trade names, trademarks and service marks of others, which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols.
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| ▪ | only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| ▪ | reduced disclosure about our executive compensation arrangements; |
| ▪ | exemption from the non-binding advisory votes on executive compensation, including golden parachute arrangements; and |
| ▪ | exemption from the auditor attestation requirement in the assessment of our internal controls over financial reporting. |
Generally, we may take advantage of these exemptions for up to five years from our initial public offering or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.07 billion in annual revenue, we have more than $700.0 million in market value of our shares held by non-affiliates or we issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold securities.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will not be subject to the same new or revised accounting standards as other public companies.
Implications of Being a Foreign Private Issuer
Our status as a foreign private issuer also exempts us from compliance with certain laws and regulations of the SEC and certain regulations of Nasdaq. Consequently, we are not subject to all of the disclosure requirements applicable to companies organized within the United States. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, our senior management and supervisory board members are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies. Accordingly, there may be less publicly available information concerning our company than there is for U.S. public companies.
In addition, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information.
We may take advantage of these exemptions until such time as we no longer qualify as a foreign private issuer. In order to maintain our current status as a foreign private issuer, either a majority of our shares must be directly or indirectly owned of record by non-residents of the United States, or a majority of our executive officers or directors may not be United States citizens or residents, more than 50% of our assets cannot be located in the United States and our business must be administered principally outside the United States.
7
The Offering
| |
ADSs offered by us | ADSs |
| |
ADSs to be outstanding following this offering | ADSs (with each ADS representing 12 ordinary shares) |
Ordinary shares to be outstanding following this offering | ordinary shares |
| |
The ADSs | Each ADS represents 12 ordinary shares, each with a nominal or par value of $0.0003 per share. You will have the rights of an ADS holder or beneficial owner (as applicable) as provided in the deposit agreement among us, the depositary and holders and beneficial owners of ADSs from time to time. To better understand the terms of our ADSs, see “Description of American Depositary Shares.” We also encourage you to read the deposit agreement, which is included as an exhibit to the registration statement of which this prospectus forms a part. |
| |
Depositary | Citibank, N.A. |
| |
Use of proceeds | We intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, to fund the continued clinical development of elamipretide and payments under our term loan facility, as well as for working capital and other general corporate purposes. See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. Because this is a best efforts offering with no minimum amount of ADSs or offering proceeds as a condition to closing, we may not sell all or any of the ADSs offered hereby. As a result, we may receive significantly less in net proceeds than we currently estimate. |
| |
Risk factors | You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in the ADSs. |
| |
Concentration of Ownership | Following the completion of this offering and without giving effect to any ADSs that may be purchased by it in the offering, Morningside Venture (I) Investments Limited will own % of our ordinary shares, assuming that we sell the number of ADSs set forth on the cover page of this prospectus. |
| |
Nasdaq Global Market symbol | MITO |
The number of ordinary shares to be outstanding after this offering is based on 690,993,790 ordinary shares issued and outstanding as of June 30, 2021, which number excludes 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans, and excluding:
| ▪ | 64,213,310 ordinary shares issuable upon exercise of share options outstanding, at a weighted-average exercise price of $0.51 per share; |
| ▪ | 13,913,257 ordinary shares reserved for issuance under our 2019 share incentive plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 13,734,600 ordinary shares (equivalent to 1,144,550 ADSs) issuable upon vesting of grants outstanding under our 2020 ADS plan; |
| ▪ | 9,162,420 ordinary shares (equivalent to 763,535 ADSs) reserved for issuance under our 2020 ADS plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
8
| ▪ | 14,691,541 ordinary shares reserved for issuance under our 2019 employee share purchase plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 83,865,384 ordinary shares issuable upon exercise of outstanding warrants with a weighted average exercise price equal to $0.14 per share; |
Unless otherwise indicated, this prospectus reflects and assumes the following:
| ▪ | no exercise of the outstanding options or warrants described above; and |
| ▪ | no purchases of ADSs by our existing shareholders in this offering. |
In addition, the discussion above assumes no additional sales of our ordinary shares or ADSs (i) under the sales agreement with Wainwright, pursuant to which we may offer and sell ADSs through Wainwright as our agent pursuant to an “at the market” offering with an aggregate offering price of up to approximately $4.7 million and (ii) under the purchase agreement, dated June 2, 2020, between Lincoln Park Capital Fund LLC, or Lincoln Park, and us, or the Purchase Agreement, pursuant to which we may sell, at our discretion, up to $20.0 million of our ordinary shares from time to time during the 36-month term of the Purchase Agreement. We suspended the sales agreement with Wainwright in November 2020.
Unless otherwise indicated, all information in this prospectus gives effect to a three-for-one reverse split of our ordinary shares that became effective on December 28, 2018.
9
Summary Consolidated Financial Data
We have derived the following summary of consolidated statement of operations data for the years ended December 31, 2018, 2019 and 2020 from our audited consolidated financial statements incorporated by reference in this prospectus. We have derived the following consolidated statements of operations data for the six months ended June 30, 2020, and 2021 and the consolidated balance sheet data as of June 30, 2021, from our unaudited condensed consolidated financial statements incorporated by reference in this prospectus. In the opinion of management, the unaudited condensed consolidated financial statements reflect all adjustments, which include normal recurring adjustments, necessary for a fair presentation of the consolidated financial statements. Historical results are not necessarily indicative of the results that may be expected in the future, and the results for the six months ended June 30, 2021, are not necessarily indicative of the results that may be expected for the full fiscal year or any other period. The summary consolidated financial data set forth below should be read together with our consolidated financial statements and the related notes to those statements as well as the sections of our Annual Report on Form 20-F for the fiscal year ended December 31, 2020, captioned “Item 3.A. Selected Financial Data” and “Item 5. Operating and Financial Review and Prospects” incorporated by reference in this prospectus.
| | YEAR ENDED DECEMBER 31, | | | SIX MONTHS ENDED JUNE 30, | |
| | 2018 | | | 2019 | | | 2020 | | | 2020 | | | 2021 | |
| | (in thousands, except share and per share data) | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenue | | $ | — | | | $ | 21,087 | | | $ | — | | | $ | — | | | $ | — | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 53,062 | | | $ | 44,604 | | | $ | 29,305 | | | $ | 17,252 | | | $ | 12,012 | |
General and administrative | | | 22,217 | | | | 22,315 | | | | 19,366 | | | | 9,703 | | | | 10,062 | |
Total operating expenses | | | 75,279 | | | | 66,919 | | | | 48,671 | | | | 26,955 | | | | 22,074 | |
Loss from operations | | | (75,279 | ) | | | (45,832 | ) | | | (48,671 | ) | | | (26,955 | ) | | | (22,074 | ) |
Other expense, net | | | (21,433 | ) | | | (25,896 | ) | | | (8,786 | ) | | | (954 | ) | | | (4,021 | ) |
Net loss attributable to ordinary shareholders | | $ | (96,712 | ) | | $ | (71,728 | ) | | $ | (57,457 | ) | | $ | (27,909 | ) | | $ | (26,095 | ) |
Net loss per share attributable to ordinary shareholders—basic and diluted | | $ | (1.41 | ) | | $ | (0.19 | ) | | $ | (0.10 | ) | | $ | (0.06 | ) | | $ | (0.04 | ) |
Weighted average ordinary shares used in net loss per share attributable to ordinary shareholders—basic and diluted | | | 68,476,149 | | | | 375,669,759 | | | | 556,169,255 | | | | 506,055,526 | | | | 663,833,037 | |
The following table sets forth summary consolidated balance sheet data as of June 30, 2021:
| ▪ | on an as adjusted basis to give further effect to our issuance and sale of ADSs in this offering at an assumed public offering price of $ per ADS, which is the closing price of the ADSs on Nasdaq on , 2021, after deducting placement agent fees and estimated offering expenses payable by us. |
10
| | AS OF JUNE 30, 2021 |
| | ACTUAL | | | ACTUAL AS ADJUSTED |
| | (in thousands) |
Consolidated Balance Sheet Data: | | | | | | |
Cash and cash equivalents | | $ | 30,766 | | | |
Working capital | | | 18,195 | | | |
Total assets | | | 32,258 | | | |
Total accumulated deficit | | | (581,549 | ) | | |
Total shareholders’ deficit | | | (26,315 | ) | | |
11
RISK FACTORS
Investing in the ADSs involves a high degree of risk. Before you decide to invest in the ADSs, you should consider carefully the risks described below, together with the other information contained in this prospectus, including our consolidated financial statements and the related notes appearing at the end of this prospectus. We believe the risks described below are the risks that are material to us as of the date of this prospectus. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of the ADSs could decline, and you may lose all or part of your investment.
Risks Related to Our Financial Position and Need for Additional Capital
We will need substantial additional funding. If we are unable to raise capital when needed, we may be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. We expect our expenses to increase in connection with our ongoing activities, particularly as we initiate new clinical trials of, initiate new research and preclinical development efforts for and seek marketing approval for our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we may incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of a future collaborator. Furthermore, we have incurred, and expect to continue to incur, significant additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed and on attractive terms, we may be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
As of June 30, 2021, we had cash and cash equivalents of $30.8 million. We believe that the net proceeds from this offering, together with our existing cash and cash equivalents and the $22.0 million committed to be funded by Morningside Venture (I) Investments Limited, or MVIL, in October and December 2021, under our Development Funding Agreement with MVIL, as amended, but exclusive of any other milestone payments under such Development Funding Agreement, will enable us to fund our operating expenses and capital expenditure requirements through .
We plan to use the net proceeds of this offering primarily to fund our ongoing research and development efforts, including our clinical development of elamipretide for rare and common ophthalmic indications, rare cardiomyopathies and rare neuromuscular and neurological indications. The proceeds from this offering will not be sufficient to support our planned Phase 3 clinical trial for Leber’s hereditary optic neuropathy, or LHON, our planned trials for primary mitochondrial disease, or PMD, arising due to nuclear DNA, or nDNA, mutations, or nPMD and Duchenne cardiomyopathy, any Phase 2 clinical trials for SBT-272 or any clinical development for SBT-550 or any other product candidates we may develop in the future. We will be required to expend significant funds in order to advance the development of elamipretide, SBT-272 and SBT-550, as well as any other product candidates we may develop in the future. In addition, while we may seek one or more collaborators for future development of our product candidates, and, in particular, may conduct any large Phase 3 clinical trials of elamipretide, such as those we would likely be required to conduct for common age-related diseases such as dry AMD, in collaboration with one or more partners that would finance most of the associated costs, we may not be able to enter into a collaboration for any of our product candidates on suitable terms, or at all. In any event, the net proceeds of this offering and our existing cash and cash equivalents will not be sufficient to fund all of the efforts that we plan to undertake or to fund the completion of development of any of our product candidates. Accordingly, we will be required to obtain further funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
Our estimate as to how long we expect our existing cash and cash equivalents to be able to fund our operations is based on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Further, changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we currently anticipate, and we may need to seek additional funds sooner than planned. Our future funding requirements, both short-term and long-term, will depend on many factors, including:
| ▪ | the scope, progress, timing, costs and results of our current and future clinical trials; |
12
| ▪ | research and preclinical development efforts for any future product candidates that we may develop; |
| ▪ | our ability to enter into and the terms and timing of any collaborations, licensing agreements or other arrangements; |
| ▪ | the number of future product candidates that we pursue and their development requirements; |
| ▪ | the outcome, timing and costs of seeking regulatory approvals; |
| ▪ | costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities; |
| ▪ | subject to receipt of marketing approval, revenue, if any, received from commercial sales of our current and future product candidates; |
| ▪ | our headcount growth and associated costs if and as we expand our research and development and establish a commercial infrastructure; |
| ▪ | costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| ▪ | costs of operating as a public company. |
Our recurring losses and negative cash flows raise substantial doubt regarding our ability to continue as a going concern.
Based on our cash balances, recurring losses and projected spending, there is doubt about our ability to continue as a going concern. Given our planned expenditures for the next several years, including, without limitation, expenditures in connection with our clinical trials of elamipretide, SBT-272, SBT-550 and other new compounds, we have concluded, in connection with the issuance of our consolidated financial statements for the year ended December 31, 2020 that there is a substantial doubt regarding our ability to continue as a going concern. Our independent registered public accounting firm has issued a going concern opinion in connection with the audit of our annual financial statements for the fiscal year ended December 31, 2020. A going concern opinion means that there is substantial doubt that the company can continue as an ongoing business for the next 12 months. If we are unable to continue as a going concern, we might have to liquidate our assets and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements. In addition, the inclusion of an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern and our lack of cash resources may materially adversely affect the price of the ADSs and our ability to raise new capital or to enter into critical contractual relations with third parties. There is no assurance that we will be able to adequately fund our operations in the future.
Raising additional capital may cause dilution to our shareholders, including purchasers of ADSs in this offering, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent that we raise additional capital through the sale of ordinary shares, ADSs, convertible securities or other equity securities, our existing shareholders’ ownership interest may be substantially diluted, and the terms of these securities could include liquidation or other preferences and anti-dilution protections that could adversely affect your rights as a holder of ADSs. Additional debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures, creating liens, redeeming shares or declaring dividends, that could adversely impact our ability to conduct our business. For example, in connection with our term loan facility with Hercules, we granted a security interest on all of our assets, excluding our intellectual property, and agreed to a negative pledge on our intellectual property. The term loan facility also contains restrictive covenants including, subject to certain exceptions, covenants that prohibit us from incurring additional indebtedness, creating any lien on our property, making investments, paying dividends or redeeming shares, transferring any material portion of our assets, merging with or acquiring another entity, entering into a transaction that will result in a change of control and making certain other corporate changes. Future debt securities or other financing arrangements could contain similar or more restrictive negative covenants. In addition, securing financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development of our product candidates.
13
If we raise additional funds through collaborations or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. In addition, under our Development Funding Agreement with MVIL, we are required to issue a warrant in connection with each additional funding by MVIL.
If we receive regulatory approval for the use of elamipretide for certain indications, we will be required to make substantial payments pursuant to our Development Funding Agreement.
If we receive regulatory approval for the use of elamipretide as a treatment for Barth, geographic atrophy an advanced form of dry age-related macular degeneration, or dry AMD, Friedreich’s ataxia, Duchenne cardiomyopathy, nPMD and LHON, we will be required to make substantial payments pursuant to our Development Funding Agreement. Our ability to make these required payments depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may generate cash flow from operations in the future sufficient to meet our obligations under the Development Funding Agreement. If we are unable to generate such cash flow or to obtain additional funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources on acceptable terms or at all, we could default on our payment obligations under the Development Funding Agreement.
We have incurred significant losses since inception and expect to incur significant and increasing losses for at least the next several years. We may never achieve or maintain profitability.
We have incurred significant annual net operating losses in every year since our inception. Our net losses were $57.5 million, $71.7 million, and $96.7 million for the years ended December 31, 2020, 2019 and 2018, respectively. As of June 30, 2021, we had an accumulated deficit of $581.5 million. We expect to continue to incur significant and increasing operating losses for the foreseeable future, and we do not know whether or when we will become profitable. We have not generated any revenues from product sales, have not completed the development of any product candidates and may never have a product candidate approved for commercialization. We have financed our operations to date through the issuance of our ADSs, ordinary shares, Series A convertible preferred shares, debt financings, a payment under an option agreement and a payment under our Development Funding Agreement, and have devoted substantially all of our financial resources and efforts to research and development, including preclinical studies and clinical development programs. Our net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital.
We anticipate that our expenses will increase substantially if and as we
| ▪ | continue to develop and conduct clinical trials with respect to, elamipretide, including our ongoing Phase 2b clinical trial for the treatment of geographic atrophy, or GA, any additional protocols or studies we may conduct in Barth to support NDA submission, our planned Phase 3 clinical trial in nPMD, our anticipated Phase 2 clinical trial for the treatment of Duchenne cardiomyopathy, our planned Phase 3 clinical trial for the treatment of LHON and any future clinical trials; |
| ▪ | initiate and continue research and preclinical and clinical development efforts for our other product candidates, including SBT-272 and compounds in the SBT-550 series; |
| ▪ | seek to identify and develop additional product candidates; |
| ▪ | seek regulatory and marketing approvals for our product candidates that successfully complete clinical trials, if any; |
| ▪ | establish sales, marketing, distribution and other commercial infrastructure in the future to commercialize various products for which we may obtain marketing approval, if any; |
| ▪ | require the manufacture of larger quantities of product candidates for clinical development and potentially commercialization; |
| ▪ | maintain, expand and protect our intellectual property portfolio; |
| ▪ | hire and retain additional personnel, such as clinical, quality control and scientific personnel; |
| ▪ | add operational, financial, management information systems and commercial personnel, including personnel to support our product development and help us comply with our obligations as a public company; and |
14
| ▪ | add property, equipment and physical infrastructure to support our research and development programs in the United States and Europe. |
Our ability to become and remain profitable depends on our ability to generate revenue. We do not expect to generate significant revenue unless and until we are, or any future collaborator is, able to obtain marketing approval for, and successfully commercialize, one or more of our product candidates. This will require our, or any of our future collaborators’, success in a range of challenging activities, including completing clinical trials of our product candidates; obtaining marketing approval for these product candidates; manufacturing, marketing and selling those products for which we, or any of our future collaborators, may obtain marketing approval; satisfying any post-marketing requirements; and obtaining reimbursement for our products from private insurance or government payors. Because of the uncertainties and risks associated with these activities, we are unable to accurately predict the timing and amount of increased expenses, and if or when we might achieve profitability. We and any future collaborators may never succeed in these activities and, even if we do, or any future collaborators do, we may never generate revenues that are large enough for us to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our pipeline of product candidates or continue our operations. A decline in the value of our company could cause you to lose all or part of your investment.
We have no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
We began operations in 2006 and initiated our first clinical trial in 2010. Our operations have been limited to financing and staffing our company and developing our technology and conducting preclinical research and clinical trials for our product candidates. We have not demonstrated an ability to obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially clinical-stage biopharmaceutical companies such as ours. Predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future.
As of June 30, 2021, we had $5.5 million of outstanding principal under our term loan facility with Hercules. In April 2021, we entered into an amendment to the term loan facility with Hercules, pursuant to which the maturity date was extended from July 1, 2021 to January 1, 2022, and all principal payments were deferred until the maturity date. Subject to the restrictions in this existing facility, we could incur additional indebtedness beyond our borrowings from Hercules.
Our outstanding indebtedness, including any additional indebtedness beyond our borrowings from Hercules, combined with our other financial obligations and contractual commitments, could have significant adverse consequences, including:
| ▪ | requiring us to dedicate a portion of our cash resources to the payment of interest and principal, reducing money available to fund working capital, capital expenditures, product development and other general corporate purposes; |
| ▪ | increasing our vulnerability to adverse changes in general economic, industry and market conditions; |
| ▪ | subjecting us to restrictive covenants that may reduce our ability to take certain corporate actions or obtain further debt or equity financing; |
| ▪ | limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we compete; and |
| ▪ | placing us at a competitive disadvantage compared to our competitors that have less debt or better debt servicing options. |
We may not have sufficient funds and may be unable to arrange for additional financing to pay the amounts due under our term loan facility. Failure to make payments or comply with other covenants under our term loan facility could result in an event of default and acceleration of amounts due. Additionally, under our loan and security agreement with Hercules, an occurrence that has a material adverse effect on our business, operations, properties, assets or financial condition; on the collateral, liens or priority of such liens; or on our ability to perform under the terms of the loan or associated agreements could be considered an event of default. If an event of default occurs and the lenders accelerate the amounts due, we may not be able to make accelerated payments, and the lenders
15
could seek to enforce security interests in the collateral securing such indebtedness, which includes substantially all of our assets other than our intellectual property. In addition, the covenants under our credit facility, the pledge of our assets as collateral and the negative pledge with respect to our intellectual property could limit our ability to obtain additional debt financing.
Risks Related to the Discovery, Development and Commercialization of Our Product Candidates
Our approach to the discovery and development of product candidates and the development of therapies targeting mitochondria generally are unproven, and we do not know whether we will be able to develop any products of commercial value.
We are focused on discovering and developing therapies for diseases involving mitochondrial dysfunction, particularly by developing therapies that target mitochondria in order to normalize the function of dysfunctional mitochondria. While we believe that our approach may ultimately enable drug research and clinical development for mitochondrial diseases across a wide range of therapeutic areas, this approach is unproven. We have not yet succeeded and may never succeed in demonstrating efficacy and safety for any of our product candidates in later stage clinical trials or in obtaining marketing approval thereafter. For example, we announced in December 2019 that our Phase 3 clinical trial in primary mitochondrial myopathy, or PMM, did not meet its primary efficacy endpoints. Furthermore, no products or therapies targeting mitochondrial dysfunction have ever obtained marketing approval from the U.S. Food and Drug Administration, or the FDA, and the European Medicines Agency, or the EMA, has approved one therapy to treat LHON (Raxone, or idebenone, made by Santhera Pharmaceuticals Holding), which is the only approved therapy to treat any primary mitochondrial disease.
If we are unable to successfully discover and develop product candidates, our business prospects will be substantially harmed.
We are dependent on the success of our clinical product candidates. If we are unable to complete the clinical development of, obtain marketing approval for or successfully commercialize any of our product candidates, either alone or with a collaborator, or if we experience significant delays in doing so, our business could be substantially harmed.
We have no products approved for sale and have invested a significant portion of our efforts and financial resources in the development of elamipretide for the treatment of rare primary mitochondrial diseases. Our prospects are substantially dependent on our ability, or the ability of any future collaborator, to develop, obtain marketing approval for and successfully commercialize elamipretide, SBT-272 or any of our other product candidates.
The success of elamipretide will depend on several factors, including the following:
| ▪ | successful recruitment of subjects, enrollment in and completion of our ongoing clinical trials; |
| ▪ | initiation and successful recruitment of subjects, enrollment in and completion of additional clinical trials; |
| ▪ | safety, tolerability and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority for marketing approval; |
| ▪ | our ability to identify success criteria and endpoints for our clinical trials such that the FDA and other regulatory authorities will be able to determine the clinical efficacy and safety profile of any product candidates we may develop; |
| ▪ | timely receipt of marketing approvals from applicable regulatory authorities; |
| ▪ | the performance of our future collaborators, if any; |
| ▪ | the extent of any required post-marketing approval commitments to applicable regulatory authorities; |
| ▪ | establishment of supply arrangements with third-party raw materials suppliers and manufacturers; |
| ▪ | establishment of arrangements with third-party manufacturers to obtain finished drug products that are appropriately packaged for sale; |
| ▪ | obtaining and maintaining patent, trade secret protection and regulatory exclusivity, both in the United States and internationally; |
| ▪ | protection of our rights in our intellectual property portfolio; |
| ▪ | successful launch of commercial sales following any marketing approval; |
| ▪ | a continued acceptable safety profile following any marketing approval; |
16
| ▪ | accuracy of the estimates of the current and future number of patients with mitochondrial associated or inherited mitochondrial diseases; |
| ▪ | commercial acceptance by patients, the medical community and third-party payors following any marketing approval; and |
| ▪ | our ability to compete with other therapies targeting diseases involving mitochondrial dysfunction. |
Many of these factors— including with respect to clinical development, the regulatory submission process, potential threats to our intellectual property rights and the manufacturing, marketing and sales efforts of any future collaborator—are beyond our control, and clinical development of product candidates is inherently risky and uncertain. For example, although we observed trends towards improvement in a certain subset of patients, our Phase 2/3 clinical trial in Barth failed to reach its primary efficacy endpoints during the double-blind, placebo-controlled portion of the trial. Our Phase 3 clinical trial in PMM also failed to meet its primary endpoints. If we are unable to develop, receive marketing approval for and successfully commercialize elamipretide, on our own or with any future collaborator, or experience delays as a result of any of these factors or otherwise, our business could be substantially harmed.
We are developing elamipretide for certain indications of the eye, including GA and LHON. Our clinical trial for the treatment of LHON involved administration of elamipretide by use of topical drops, and our clinical trial for the treatment of GA involves administration of elamipretide by subcutaneous injection. We are working to develop methods for intravitreal injection, or direct injection of drug into the eye, but we cannot predict whether those development efforts will be successful.
We may not be successful in our efforts to identify or discover and develop additional potential product candidates.
A significant portion of the research that we are conducting involves the development of new therapeutic compounds targeting the mitochondria. The drug discovery that we are conducting may not be successful in identifying compounds that have commercial value or therapeutic utility. Our discovery platform may initially show promise in identifying potential product candidates, yet fail to yield viable product candidates for clinical development or commercialization for a number of reasons, including the following:
| ▪ | compounds we develop may not demonstrate improved efficacy, safety or tolerability; |
| ▪ | potential product candidates may, on further study, be shown to have harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market acceptance; |
| ▪ | competitors may develop alternative therapies that render our potential product candidates non-competitive or less attractive; or |
| ▪ | a potential product candidate may not be capable of being produced at an acceptable cost. |
Our research programs to identify new product candidates will require substantial technical, financial and human resources, and we may be unsuccessful in our efforts to identify new product candidates. Further, the results we obtain in preclinical testing and early clinical trials may not be predictive of results that are obtained in later studies, and we may suffer significant setbacks in advanced clinical trials, even after seeing promising results in earlier studies. If we are unable to identify suitable additional compounds for preclinical and clinical development, our ability to develop product candidates and obtain product revenues in future periods could be compromised, which could result in significant harm to our financial position and adversely impact the price of the ADSs.
We have never obtained marketing approval for a product candidate and we may be unable to obtain, or may be delayed in obtaining, marketing approval for any of our product candidates.
We have never obtained marketing approval for a product candidate. It is possible that the FDA may refuse to accept for substantive review any NDAs we submit for our product candidates or may conclude after review of our data that our application is insufficient to obtain marketing approval of our product candidates. For example, we have met with the FDA to discuss the clinical evidence to support a potential NDA submission for Barth, and the FDA expressed its view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review. The FDA recommended that we collect additional controlled clinical data in this indication prior to an NDA submission. In May 2021, we submitted a randomized withdrawal clinical trial protocol to FDA. This design was previously suggested by multiple FDA review divisions, including most recently the Division of Cardiology and Nephrology. After reviewing our submission, FDA concluded that neither the proposed randomized withdrawal trial nor any new clinical trial data from the patients remaining on OLE would be likely to add meaningfully to the evidence to support an NDA. Due to the ultra-rare nature of Barth syndrome, neither the Company nor the FDA to date has been able to identify a feasible trial design to generate additional
17
data. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
If the FDA does not accept or approve our NDAs for our most advanced product candidates, it may require that we conduct additional clinical, nonclinical or manufacturing validation studies and submit that data before it will reconsider our applications.
Depending on the extent of these or any other FDA-required studies, approval of any NDA or application that we submit may be delayed by several years or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve our NDAs.
Any delay in obtaining, or an inability to obtain, any marketing approvals would prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability. If any of these outcomes occur, we may be forced to abandon our development efforts for our product candidates, which could significantly and materially harm our business.
Results of preclinical studies and early clinical trials may not be predictive of results of future clinical trials.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of clinical trials do not necessarily predict success in future clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development. We faced this type of setback when our Phase 3 clinical trial in PMM did not meet its primary efficacy endpoints despite encouraging signals in early clinical trials, and we cannot be certain that we will not face similar setbacks in other trials. The design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. If our trial designs are not sufficient, our clinical programs may be delayed, or we may decide to terminate one or more of such programs.
We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support marketing approval. In addition, preclinical and clinical data are often susceptible to varying interpretations and analyses. During the regulatory review process, we will need to identify success criteria and endpoints at the time of the initiation of the trial such that the FDA or other regulatory authorities will be able to determine the clinical efficacy and safety profile of any product candidates we may develop, and the resulting clinical data and results may be difficult to analyze. Even if the FDA or other regulatory authorities were to find our success criteria to be sufficiently validated and clinically meaningful, we may not achieve the pre-specified endpoints to a degree of statistical significance. Many companies that believed that their product candidates had performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain marketing approval of their product candidates. Even if we, or any future collaborators, believe that the results of clinical trials for our product candidates warrant marketing approval, the FDA or comparable foreign regulatory authorities may disagree and may not grant marketing approval of our product candidates.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. Specifically, the clinical trials we have completed to date have enrolled only small numbers of subjects, we have experienced dropout among participants, and we have not always successfully achieved our pre-specified clinical trial endpoints to a degree of statistical significance.
To date, other than our ongoing Phase 2b clinical trial in GA, our Phase 3 clinical trial in PMM, our Phase 3 retrospective natural history control trial in Barth, and our Phase 2/3 clinical trial in Barth, we have only conducted small Phase 1 and Phase 2 clinical trials, many of which have been undertaken to help inform our clinical strategy and develop later stage clinical trials intended to assess efficacy. While the endpoints and populations for these later stage clinical trials, including our Phase 2b clinical trial for GA, our planned Phase 3 clinical trial for LHON, our anticipated Phase 3 clinical trial for nPMD and our anticipated Phase 2 clinical trial for Duchenne cardiomyopathy, are or will be derived from results of our earlier trials and medical literature, in some cases we did not demonstrate a statistically significant effect in the population and on the efficacy endpoints in our prior clinical trials prospectively described in the clinical trial protocol. The lack of statistical significance could be attributed to various factors, including the lack of power to demonstrate significance, the design of the studies or the lack of a treatment benefit from our product candidate. In some cases, we conducted post hoc, retrospective analyses of data subsets and have designed, and expect to design later stage clinical trials based on the results of such post hoc analyses. For
18
example, the improvements in stroke volume and other parameters of cardiac function as well as in functional endpoints observed in our Barth Phase 2/3 trial were not statistically significant during the placebo-controlled portion of the trial, which we believe was due to the duration of therapy being too short to derive benefit. Although we plan to design our future trials in rare cardiomyopathies with a longer duration of dosing, we cannot predict the successfulness of that approach. Additionally, despite improvements observed in similar endpoints during a Phase 2 clinical trial, our Phase 3 clinical trial in PMM failed to reach its primary efficacy endpoints and, we observed that subjective, effort-dependent endpoints such as the six-minute walk test, or 6MWT, may be influenced by a placebo effect, such that patients randomized to placebo may experience meaningful improvements. Although we have incorporated and plan to incorporate objective endpoints including disease biomarkers such as echocardiographic parameters of cardiac function for Barth and other rare cardiomyopathies, and optical coherence tomography and fundus autofluorescence imaging of geographic atrophy progression for GA, we have also assessed and expect to assess functional endpoints including 6MWT, for Barth, and visual function, for GA.
If we fail to receive positive results in clinical trials of our product candidates and do not achieve statistical significance for the prospectively specified primary endpoints in our planned Phase 3 clinical trials, the development timeline and regulatory approval and commercialization prospects for our most advanced product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Reenie McCarthy, our Chief Executive Officer and a Director, as well as the other principal members of our management and scientific teams. Ms. McCarthy is employed “at will,” meaning we or she may terminate the employment relationship at any time. In the future, we may be dependent on other members of our management, scientific and development team. Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. Our industry has experienced a high rate of turnover of management personnel in recent years. If we lose one or more of our executive officers or other key employees, our ability to implement our business strategy successfully could be seriously harmed. Furthermore, replacing executive officers or other key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain marketing approval of and commercialize products successfully. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key employees on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions.
We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by other entities and may have commitments under consulting or advisory contracts with those entities that may limit their availability to us. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize our product candidates will be limited.
Because we are developing elamipretide for the treatment of several indications for which regulatory authorities have not issued definitive guidance as to how to measure and demonstrate efficacy, there is substantial risk that the design or outcomes of our clinical trials will not be satisfactory to support marketing approval.
We are developing elamipretide for several indications for which there are currently no approved therapies in the United States or the European Union, including Barth, dry AMD and nPMD, and for symptoms of diseases including Duchenne cardiomyopathy and FRDA, for which there are currently no approved therapies in the United States or the European Union (although there are therapies approved generally for the treatment of Duchenne which do not address the cardiomyopathic symptoms). We are developing elamipretide for LHON, for which there are no currently approved therapies in the United States and only one therapy approved in Europe. Furthermore, there has been limited historical clinical trial experience for the development of drugs to treat many of these indications. As a result, the design and conduct of clinical trials for these indications is subject to substantial risk. In particular, regulatory authorities in the United States and in other jurisdictions, including Europe, have not issued definitive guidance as to how to measure and demonstrate efficacy for Barth, LHON, dry AMD, nPMD, Duchenne cardiomyopathy or FRDA, and, as a result, there is substantial risk that the design or outcomes of our clinical trials will not be satisfactory to support marketing approval. For example, the endpoints in our Phase 2/3 clinical trial of elamipretide for the treatment of Barth included change in six-minute walk distance and change in a total fatigue scale, or BTHS-SA Total Fatigue, from the Barth symptom assessment, or BTHS-SA, a newly developed patient reported outcome measure, which has not been utilized in prior trials and may not be accepted by regulators as a
19
basis for approval. Even if this type of novel endpoint is accepted as a basis for approval in the United States, we cannot be certain that regulators outside of the United States will accept such endpoints or will not require us to conduct additional validation studies to support the suitability of such endpoints for approval in these jurisdictions.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we intend to focus on developing product candidates for specific indications that we identify as most likely to succeed, in terms of both their potential for marketing approval and commercialization. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may prove to have greater commercial potential.
Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to the product candidate.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome.
Clinical testing is expensive, time consuming and uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, or at all. The clinical development of our product candidates is susceptible to the risk of failure at any stage of drug development, including failure to demonstrate efficacy in a clinical trial or across a broad population of patients, the occurrence of adverse events that are severe or medically or commercially unacceptable, failure to comply with protocols or applicable regulatory requirements and determination by the FDA or any comparable foreign regulatory authority that a product candidate may not continue development or is not approvable. For example, Phase 3 clinical trials for common diseases associated with aging, such as dry AMD, would likely require a large number of subjects to be enrolled, which would cause any such trial to be very expensive. Moreover, it is possible that even if one or more of our product candidates has a beneficial effect, that effect will not be detected during clinical evaluation as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. Conversely, as a result of the same factors, our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any. Similarly, in our clinical trials we may fail to detect toxicity of or intolerability caused by our product candidates, or mistakenly believe that our product candidates are toxic or not well tolerated when that is not in fact the case. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we cannot be certain that we will not face additional setbacks. It is possible that any of our development programs may be placed on full or partial clinical hold by regulatory authorities at any point, which would delay and possibly prevent further development of our product candidates.
If clinical trials of our product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other comparable foreign regulators, we, or any future collaborators, may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of these product candidates.
We, and any future collaborators, are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval from the FDA. Comparable foreign regulatory authorities impose similar restrictions. We, and any future collaborators, may never receive such approvals. We, and any future collaborators, must complete extensive preclinical development and clinical trials to demonstrate the safety and efficacy of our product candidates in humans before we, or they, will be able to obtain these approvals.
Clinical testing is expensive, difficult to design and implement, can take many years to complete and is inherently uncertain as to outcome. We have not previously submitted an NDA to the FDA or similar drug approval filings to comparable foreign regulatory authorities for any of our product candidates. Any inability to complete preclinical and clinical development successfully could result in additional costs to us, or any future collaborators, and impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. Moreover, if (i) we, or any future collaborators, are required to conduct additional clinical trials or other testing of our product candidates beyond the trials and testing that we or they contemplate, (ii) we, or any future collaborators, are unable to successfully complete clinical trials of our product candidates or other testing, (iii) the results of these
20
trials or tests are unfavorable, uncertain or are only modestly favorable, or (iv) there are unacceptable safety concerns associated with our product candidates, we, or any future collaborators, may:
| ▪ | be delayed in obtaining marketing approval for our product candidates; |
| ▪ | not obtain marketing approval at all; |
| ▪ | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| ▪ | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| ▪ | be subject to additional post-marketing testing or other requirements; or |
| ▪ | be required to remove the product from the market after obtaining marketing approval. |
Adverse events or undesirable side effects caused by, or other unexpected properties of, any of our product candidates may be identified during development that could delay or prevent their marketing approval or limit their use.
Adverse events or undesirable side effects caused by, or other unexpected properties of, our product candidates could cause us, any future collaborators, an institutional review board or regulatory authorities to interrupt, delay or halt clinical trials of one or more of our product candidates and could result in a more restrictive label or the delay or denial of marketing approval by the FDA or comparable regulatory authorities. If any of our product candidates is associated with adverse events or undesirable side effects or has properties that are unexpected, we, or any future collaborators, may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. For example, subjects in certain of our clinical trials have reported adverse events arising from reaction at the injection site and some subjects have withdrawn as a result. Moreover, laboratory findings have demonstrated mild to moderate elevations in eosinophils, a variety of white blood cells that combats parasites and infections and controls mechanisms associated with allergy and asthma, beginning at approximately three to four weeks after initiation of elamipretide treatment, although these have not been reported to be associated with any systemic clinical manifestations of eosinophilia and in general were demonstrated to have returned to within normal range or to baseline levels after withdrawal of elamipretide therapy and, in most subjects, to decrease to within normal range after approximately 16 weeks of elamipretide therapy (and without withdrawal of therapy). Many compounds that initially showed promise in clinical or earlier stage testing have later been found to cause undesirable or unexpected side effects that prevented further development of the compound. If we, or any future collaborators, experience any of a number of possible unforeseen events in connection with clinical trials of our product candidates, potential marketing approval or commercialization of our product candidates could be delayed or prevented.
We, or any future collaborators, may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent marketing approval or commercialization of our product candidates, including the following:
| ▪ | clinical trials of our product candidates may produce unfavorable or inconclusive results; |
| ▪ | we, or any future collaborators, may decide, or regulators may require us, or them, to conduct additional clinical trials or abandon product development programs; |
| ▪ | the number of subjects required for clinical trials of our product candidates may be larger than we, or any future collaborators, anticipate, subject enrollment in these clinical trials may be slower than we, or any future collaborators, anticipate or participants may drop out of these clinical trials at a higher rate than we, or any future collaborators, anticipate; |
| ▪ | the cost of planned clinical trials of our product candidates may be greater than we anticipate; |
| ▪ | our third-party contractors or those of any future collaborators, including those manufacturing our product candidates or components or ingredients thereof or conducting clinical trials on our behalf or on behalf of any future collaborators, may fail to comply with regulatory requirements or meet their contractual obligations to us or any future collaborators in a timely manner, or at all; |
| ▪ | regulators or institutional review boards may not authorize us, any future collaborators or our or their investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| ▪ | we, or any future collaborators, may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
21
| ▪ | subjects that enroll in a clinical trial may misrepresent their eligibility to do so or may otherwise not comply with the clinical trial protocol, resulting in the need to drop the subjects from the clinical trial, increase the needed enrollment size for the clinical trial or extend the clinical trial’s duration; |
| ▪ | we, or any future collaborators, may have to delay, suspend or terminate clinical trials of our product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate; |
| ▪ | regulators or institutional review boards may require that we, or any future collaborators, or our or their investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or their standards of conduct, a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate or findings of undesirable effects caused by a chemically or mechanistically similar product or product candidate; |
| ▪ | the FDA or comparable regulatory authorities may disagree with our, or any future collaborators’, clinical trial designs or our or their interpretation of data from preclinical studies and clinical trials; |
| ▪ | the FDA or comparable regulatory authorities may fail to approve or subsequently find fault with the manufacturing processes or facilities of third-party manufacturers with which we, or any future collaborators, enter into agreements for clinical and commercial supplies; |
| ▪ | the supply or quality of raw materials or manufactured product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient, inadequate or not available at an acceptable cost, or we may experience interruptions in supply; and |
| ▪ | the approval policies or regulations of the FDA or comparable regulatory authorities may significantly change in a manner rendering our clinical data insufficient to obtain marketing approval. |
Product development costs for us, or any future collaborators, will increase if we, or they, experience delays in testing or pursuing marketing approvals and we, or they, may be required to obtain additional funds to complete clinical trials and prepare for possible commercialization of our product candidates. We do not know whether any preclinical tests or clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, or at all. Significant preclinical or clinical trial delays also could shorten any periods during which we, or any future collaborators, may have the exclusive right to commercialize our product candidates or allow our competitors, or the competitors of any future collaborators, to bring products to market before we, or any future collaborators, do and impair our ability, or the ability of any future collaborators, to successfully commercialize our product candidates and may harm our business and results of operations. In addition, many of the factors that lead to clinical trial delays may ultimately lead to the denial of marketing approval of any of our product candidates.
If we, or any future collaborators, experience delays or difficulties in the enrollment of subjects in clinical trials, our or their receipt of necessary regulatory approvals could be delayed or prevented.
We, or any future collaborators, may not be able to initiate or continue clinical trials for any of our product candidates if we, or they, are unable to locate and enroll a sufficient number of eligible subjects to participate in clinical trials as required by the FDA or comparable regulatory authorities. For example, we are developing elamipretide for the treatment of several rare diseases with small patient populations, such as Barth. Enrollment is a significant factor in the timing of clinical trials, and is affected by many factors, including:
| ▪ | the size and nature of the patient population; |
| ▪ | the severity of the disease under investigation; |
| ▪ | the proximity of subjects to clinical sites; |
| ▪ | the eligibility criteria for the trial; |
| ▪ | the design of the clinical trial; |
| ▪ | efforts to facilitate timely enrollment; |
| ▪ | competing clinical trials; |
| ▪ | COVID-19 related safety considerations; and |
22
| ▪ | clinician and patient perception as to the potential advantages and risks of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. |
Our inability, or the inability of any future collaborators, to enroll a sufficient number of subjects for our, or their, clinical trials could result in significant delays or may require us or them to abandon one or more clinical trials altogether. For example, our Phase 2a clinical trial of elamipretide in subjects pre-treated prior to a renal angioplasty was terminated early due to recruitment challenges after enrolling only 14 subjects of the 28 originally planned, and we have experienced COVID-19 related delays in enrolling the ReCLAIM 2 trial. Enrollment delays in clinical trials may result in increased development costs for our product candidates, delay or halt the development of and approval processes for our product candidates and jeopardize our, or any future collaborators’, ability to commence sales of and generate revenues from our product candidates, which could cause the value of our company to decline.
If any of our product candidates receives marketing approval and we, or others, later discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, our ability, or that of any future collaborators, to market the drug could be compromised.
Clinical trials of our product candidates are conducted in carefully defined subsets of subjects who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials, or those of any future collaborator, may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. In particular, because our product candidates will require chronic dosing over the lifetime of the patient, there may be undesirable side effects as a result of long-term exposure to the drug that were not observed in our clinical trials. If, following approval of a product candidate, we, or others, discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following adverse events could occur:
| ▪ | regulatory authorities may withdraw their approval of the drug or seize the drug; |
| ▪ | we, or any future collaborators, may be required to recall the drug, change the way the drug is administered or conduct additional clinical trials; |
| ▪ | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular drug; |
| ▪ | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ▪ | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| ▪ | we, or any future collaborators, may be required to create a medication guide outlining the risks of the previously unidentified side effects for distribution to patients; |
| ▪ | we, or any future collaborators, could be sued and held liable for harm caused to patients; |
| ▪ | the drug may become less competitive; and |
| ▪ | our reputation may suffer. |
Any of these events could have a material and adverse effect on our operations and business and could adversely impact the price of the ADSs.
Even if one of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success and the market opportunity for the product candidate may be smaller than we estimate.
We have never commercialized a product. Even if one of our product candidates is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. For example, physicians are often reluctant to switch their patients from existing therapies even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to the therapy that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch therapies due to lack of reimbursement for existing therapies.
23
Efforts to inform the medical community and third-party payors of the benefits of our product candidates may require significant resources and may not be successful. If any of our product candidates is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ▪ | the efficacy and safety of the product; |
| ▪ | the potential advantages of the product compared to alternative treatments; |
| ▪ | the prevalence and severity of any side effects; |
| ▪ | the clinical indications for which the product is approved; |
| ▪ | whether the product is designated under physician treatment guidelines as a first-line therapy or as a second- or third-line therapy; |
| ▪ | limitations or warnings, including distribution or use restrictions, contained in the product’s approved labeling; |
| ▪ | our ability, or the ability of any future collaborators, to offer the product for sale at competitive prices; |
| ▪ | the product’s convenience and ease of administration compared to alternative treatments; |
| ▪ | the willingness of the target patient population to try, and of physicians to prescribe, the product; |
| ▪ | the strength of sales, marketing and distribution support; |
| ▪ | the approval of other new products for the same indications; |
| ▪ | changes in the standard of care for the targeted indications for the product; |
| ▪ | the timing of market introduction of our approved products as well as competitive products; |
| ▪ | availability of coverage and the adequacy of reimbursement from government payors, managed care plans and other third-party payors; |
| ▪ | adverse publicity about the product or favorable publicity about competitive products; and |
| ▪ | potential product liability claims. |
The potential market opportunities for our product candidates are difficult to estimate precisely. Our estimates of the potential market opportunities are predicated on many assumptions, including industry knowledge and publications, third-party research reports and other surveys. While we believe that our internal assumptions are reasonable, these assumptions involve the exercise of significant judgment on the part of our management, are inherently uncertain and the reasonableness of these assumptions has not been assessed by an independent source. If any of the assumptions proves to be inaccurate, the actual markets for our product candidates could be smaller than our estimates of the potential market opportunities.
If we are unable to establish sales, marketing and distribution capabilities or enter into sales, marketing and distribution arrangements with third parties, we may not be successful in commercializing any product candidates that we develop if and when those product candidates are approved.
We do not have a sales, marketing or distribution infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We plan to use a combination of focused in-house sales and marketing capabilities and third-party collaboration, licensing and distribution arrangements to sell any of our products that receive marketing approval.
We generally plan to retain rights to participate in commercialization in the United States, particularly for products that we can commercialize with a specialized sales force and by building a focused sales and marketing organization in the United States to sell our products. Any efforts related to sales, marketing and distribution may be costly, and our investment could be lost if we cannot retain or reposition our sales and marketing personnel. In addition, we may not be able to hire or retain a sales force that is sufficient in size or has adequate expertise in the medical markets that we plan to target. If we are unable to establish or retain a sales force and marketing and distribution capabilities, our operating results may be adversely affected. If a potential partner has development or commercialization expertise that we believe is particularly relevant to one of our products, then we may seek to collaborate with that potential partner even if we believe we could otherwise develop and commercialize the product independently.
24
We hope to collaborate with third parties for commercialization in the United States of any products that require larger sales, marketing and product distribution infrastructure. We plan to commercialize our products outside the United States through collaboration, licensing and distribution arrangements with third parties. As a result of entering into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues may be lower, perhaps substantially lower, than if we were to directly market and sell products in those markets. Furthermore, we may be unsuccessful in entering into the necessary arrangements with third parties or may be unable to do so on terms that are favorable to us. In addition, we may have little or no control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively.
If we do not establish sales, marketing and distribution capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing any of our product candidates that receive marketing approval.
We have established commercial manufacturing processes for both the elamipretide drug substance and drug product and have begun validation of our manufacturing processes at a scale that exceeds our expected quantities of elamipretide required for commercial launch and beyond. The successful validation of manufacturing processes is a necessary part of pre-approval inspections by regulatory authorities. An unfavorable pre-approval inspection could delay anticipated approval of marketing authorization applications.
Our transition to a solution-phase process for making the active ingredient, elamipretide, was relatively recent. A change in the contract manufacturer for elamipretide was necessary late in the development timeline to meet the goals for scale and product quality. In addition to any findings that could result from a pre-approval inspection, regulatory authorities could require that additional batches be produced to demonstrate the suitability of the process at the current contract manufacturing sites and/or at the current commercial scale. Such additional batches could cause a delay in granting approval of marketing authorization applications, including NDAs.
We face substantial competition from other pharmaceutical and biotechnology companies, and our operating results may suffer if we fail to compete effectively.
The development and commercialization of new drug products is highly competitive. We expect that we, and any future collaborators, will face significant competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to any of our product candidates that we, or they, may seek to develop or commercialize in the future. Specifically, there are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of product candidates for the treatment of the key indications of our most advanced programs.
We are initially developing elamipretide for the treatment of rare primary mitochondrial diseases and common diseases of aging in which mitochondrial function is impaired. There are several companies developing treatments that target mitochondria or mitochondria-associated diseases. The majority of these efforts are in preclinical or early clinical development are focused on gene therapy or are proposing the use of generic compounds. To our knowledge, none of these is focused on cardiolipin remodeling. Our competitors include: Abliva AB, Reata Pharmaceuticals, Inc., LumiThera, Inc., Reneo Pharmaceuticals, Inc. and Santhera Pharmaceuticals Holding. In addition to competition from competitors who are developing treatments that seek to improve mitochondrial function or otherwise target the mitochondria, we also face competition from therapies that target the indications we are studying, particularly for diseases of aging such as GA. Such competitors who are developing or who have developed competing therapies include Apellis Pharmaceuticals Inc., Astellas Pharma Inc., Hemera Biosciences Inc., Ionis Pharmaceuticals, Inc. and IVERIC bio, Inc.
Our competitors may succeed in developing, acquiring or licensing technologies and drug products that are more effective, have fewer or more tolerable side effects or are less costly than any product candidates that we are currently developing or that we may develop, which could render our product candidates obsolete and noncompetitive.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we, or any future collaborators, may develop. Our competitors also may obtain FDA or other marketing approval for their products before we, or any future collaborators, are able to obtain approval for ours, which could result in our competitors establishing a strong market position before we, or any future collaborators, are able to enter the market.
Many of our existing and potential future competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing
25
approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
If the FDA or comparable regulatory authorities approve generic versions of any of our products that receive marketing approval, or such authorities do not grant our products appropriate periods of data exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
Once an NDA is approved, the product covered thereby becomes a “reference-listed drug” in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations.” Manufacturers may seek approval of generic versions of reference-listed drugs through submission of abbreviated new drug applications, or ANDAs, in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical studies. Rather, the applicant generally must show that its product has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as the reference-listed drug and that the generic version is bioequivalent to the reference-listed drug, meaning it is absorbed in the body at the same rate and to the same extent. Generic products may be significantly less costly to bring to market than the reference-listed drug and companies that produce generic products are generally able to offer them at lower prices. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference-listed drug may be typically lost to the generic product.
The FDA may not approve an ANDA for a generic product until any applicable period of non-patent exclusivity for the reference-listed drug has expired. The Federal Food, Drug, and Cosmetic Act, or FDCA, provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity, or NCE. Specifically, in cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification that a patent covering the reference-listed drug is either invalid or will not be infringed by the generic product, in which case the applicant may submit its application four years following approval of the reference-listed drug. We have an issued composition of matter patent on elamipretide. As such, the active ingredient will be treated as an NCE and any products containing elamipretide will be granted exclusivity based on that patent expiry date and other contributing factors. It is unclear whether the FDA will treat the active ingredients in our other product candidates as NCEs and, therefore, afford them five years of NCE data exclusivity if they are approved. If any product we develop does not receive five years of NCE exclusivity, the FDA may approve generic versions of such product three years after its date of approval. Manufacturers may seek to launch these generic products following the expiration of the applicable marketing exclusivity period, even if we still have patent protection for our product.
Competition that our products, if any, may face from generic versions of our products could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
Even if we, or any future collaborators, are able to commercialize any product candidate that we, or they, develop, the product may become subject to unfavorable pricing regulations, third-party payor reimbursement practices or healthcare reform initiatives that could harm our business.
The commercial success of our product candidates in key potential markets will depend substantially on the extent to which the costs of our product candidates will be paid by third-party payors, including government health administration authorities and private health coverage insurers. If coverage and reimbursement is not available, or reimbursement is available only to limited levels, we, or any future collaborators, may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us, or any future collaborators, to establish or maintain pricing sufficient to realize a sufficient return on our or their investments.
There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved drugs. Marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we, or any future collaborators, might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods,
26
which may negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability or the ability of any future collaborators to recoup our or their investment in one or more product candidates, even if our product candidates obtain marketing approval. Moreover, in the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their reimbursement rates, but also have their own methods and approval process apart from Medicare determinations. Therefore, coverage and reimbursement for products in the United States can differ significantly from payor to payor.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Therefore, our ability, and the ability of any future collaborators, to commercialize any of our product candidates will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from third-party payors. Third-party payors decide which medications they will cover and establish reimbursement levels. The healthcare industry is acutely focused on cost containment, both in the United States and worldwide. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability or that of any future collaborators to sell our product candidates profitably. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or those of any future collaborators, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives could cause us, or any future collaborators, to decrease the price we, or they, might establish for products, which could result in lower than anticipated product revenues. If the prices for our products, if any, decrease or if governmental and other third-party payors do not provide coverage or adequate reimbursement, our prospects for revenue and profitability will suffer.
There may also be delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the indications for which the drug is approved by the FDA or comparable regulatory authorities. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the drug and the clinical setting in which it is used. Reimbursement rates may also be based on reimbursement levels already set for lower cost drugs or may be incorporated into existing payments for other services.
In addition, increasingly, third-party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. We cannot be sure that coverage will be available for any product candidate that we, or any future collaborator, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any of our product candidates for which we, or any future collaborator, obtain marketing approval could significantly harm our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
The COVID-19 pandemic has and may continue to affect our ability to recruit or retain patients for our clinical trials, and may disrupt regulatory activities, disrupt preclinical studies or have other adverse effects on our business and operations.
The COVID-19 pandemic has caused many governments to implement measures to slow the spread of the pandemic through quarantines, travel restrictions, heightened border scrutiny and other measures. The pandemic and government measures taken in response have also had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, has spiked, while demand for other goods and services, such as travel, has fallen. The future progression of the pandemic and its effects on our business and operations are uncertain. We seek to enroll patients for our clinical trials at sites located in the United States and may be unable to continue trials as scheduled. We have and may continue to face difficulties recruiting or retaining patients in our ongoing and planned clinical trials if patients are affected by the virus or are fearful of traveling to our clinical trial sites because of the pandemic. For example, we experienced COVID-19 related delays in enrolling the ReCLAIM 2 trial. In response, we added several additional trial sites, and implemented best practices measures, including availability of night and weekend visits and visiting nurses, to alleviate COVID-19 related challenges. We are closely monitoring any COVID-19 related discontinuations in light of increased reported incidence in the United States. We and our third-party contract manufacturers, contract research organizations, academic collaborators and clinical sites may also face disruptions in accessing laboratory or clinical trial sites or procuring items that are essential for our research and development
27
activities, including, for example, raw materials used in the manufacture of our product candidates, medical and laboratory supplies used in our clinical trials or preclinical studies or animals that are used for preclinical testing, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the pandemic. These factors may increase our cost for future studies and may further delay timelines to start new studies. For example, we manufacture our products outside the United States and could be subject to disruptions in due to responses of other governments to outbreaks of COVID-19. Additionally, during various periods in 2020, the principal investigator for the TAZPOWER trial suspended all ongoing regular clinic visits in accordance with pandemic safety guidelines published by the Barth Syndrome Foundation, compromising the practicability of collecting additional controlled clinical data; similar disruptions in regularly scheduled visits also impacted our ReCLAIM 2 trial. Pandemic-related shutdowns could also impact our ability to initiate our Duchenne cardiomyopathy or nPMD trials. In addition, we may face impediments to regulatory meetings and approvals due to measures intended to limit in-person interactions. We cannot be certain what the overall impact of the COVID-19 pandemic will be on our business and it has the potential to adversely affect our business, operations and financial condition.
Product liability lawsuits against us could divert our resources, cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We face an inherent risk of product liability claims as a result of the clinical testing of our product candidates despite obtaining appropriate informed consents from our clinical trial participants. We will face an even greater risk if we or any future collaborators commercially sell any product that we or they may develop. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Regardless of the merits or eventual outcome, liability claims may result in:
| ▪ | decreased demand for our product candidates or products that we may develop; |
| ▪ | injury to our reputation and significant negative media attention; |
| ▪ | withdrawal of clinical trial subjects; |
| ▪ | significant costs to defend resulting litigation; |
| ▪ | substantial monetary awards to trial subjects or patients; |
| ▪ | reduced resources of our management to pursue our business strategy; and |
| ▪ | the inability to commercialize any products that we may develop. |
Although we believe we maintain adequate general and clinical trial liability insurance for a company at our stage, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if and when we begin selling any product candidate that receives marketing approval. In addition, insurance coverage is becoming increasingly expensive. If we are unable to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of our product candidates, which could adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Our Dependence on Third Parties
We expect to seek to establish collaborations and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. We expect to seek collaborators for the development and commercialization of one or more of our product candidates. For example, we hold worldwide rights for elamipretide and we own our new pipeline compounds, including SBT-272. We may explore partnerships for development of elamipretide or SBT-272, as well as one or more of our pipeline compounds, in selected other indications and territories. Likely future collaborators may include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. In addition, if we are able to obtain marketing
28
approval for any of our product candidates from foreign regulatory authorities, we intend to enter into strategic relationships with international biotechnology or pharmaceutical companies for the commercialization of such product candidates outside of the United States.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the potential differentiation of our product candidate from competing product candidates, design or results of clinical trials, the likelihood of approval by the FDA or comparable foreign regulatory authorities and the regulatory pathway for any such approval, the potential market for the product candidate, the costs and complexities of manufacturing and delivering the product to patients and the potential of competing products. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available for collaboration and whether such collaboration could be more attractive than the one with us for our product candidate.
Collaborations are complex and time-consuming to negotiate and document. Further, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
If we enter into collaborations with third parties for the development and commercialization of our product candidates, our prospects with respect to those product candidates will depend in significant part on the success of those collaborations.
We may enter into collaborations for the development and commercialization of certain of our product candidates. If we enter into such collaborations, we will have limited control over the amount and timing of resources that our collaborators will dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on any future collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. In addition, any future collaborators may have the right to abandon research or development projects and terminate applicable agreements, including funding obligations, prior to or upon the expiration of the agreed upon terms.
Collaborations involving our product candidates pose a number of risks, including the following:
| ▪ | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ▪ | collaborators may not perform their obligations as expected; |
| ▪ | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs, based on clinical trial results, changes in the collaborators’ strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities; |
| ▪ | collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ▪ | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| ▪ | disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time-consuming and expensive; |
29
| ▪ | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
| ▪ | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and |
| ▪ | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. For example, in October 2019, we granted Alexion Pharmaceuticals, Inc or Alexion, an exclusive option to co-develop and commercialize elamipretide. Alexion terminated the option agreement in January 2020. If any future collaborator of ours is involved in a business combination, it could decide to delay, diminish or terminate the development or commercialization of any product candidate licensed to it by us.
We rely on third parties to conduct our clinical trials. If they do not perform satisfactorily, our business could be significantly harmed.
We do not independently conduct clinical trials of any of our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to conduct these clinical trials and expect to rely on these third parties to conduct clinical trials of any other product candidate that we develop. Any of these third parties may terminate their engagements with us under certain circumstances. We may not be able to enter into alternative arrangements or do so on commercially reasonable terms. In addition, there is a natural transition period when a new contract research organization begins work. As a result, delays would likely occur, which could materially impact our ability to meet our expected clinical development timelines and harm our business, financial condition and prospects.
Further, our reliance on these third parties for clinical development activities limits our control over these activities, but we remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards. For example, notwithstanding the obligations of a contract research organization for a trial of one of our product candidates, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as current Good Clinical Practices, or cGCPs, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The FDA enforces these cGCPs through periodic inspections of trial sponsors, principal investigators, clinical trial sites and institutional review boards. If we or our third-party contractors fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our product candidates, which would delay the marketing approval process. We cannot be certain that, upon inspection, the FDA will determine that any of our clinical trials comply with cGCPs. Similar regulatory requirements apply outside the United States, including the International Council for Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use, or ICH. We are also required to register clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, the third parties conducting clinical trials on our behalf are not our employees, and except for remedies available to us under our agreements with such contractors, we cannot control whether or not they devote sufficient time, skill and resources to our ongoing development programs. These contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could impede their ability to devote appropriate time to our clinical programs. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates. If that occurs, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. In such an event, our financial results and the commercial prospects for any product candidates that we seek to develop could be harmed, our costs could increase and our ability to generate revenues could be impaired.
We also rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of any resulting products, producing additional losses and depriving us of potential product revenue.
30
We contract with third parties for the manufacture and distribution of our product candidates for clinical trials and expect to continue to do so in connection with our future development and commercialization efforts. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on contract manufacturers to produce both drug substance and drug product required for our clinical trials. We plan to continue to rely upon contract manufacturers, and potentially collaboration partners, to manufacture commercial quantities of our product candidates and, if approved, products. Reliance on such third-party contractors entails risks, including:
| ▪ | manufacturing delays if our third-party contractors give greater priority to the supply of other products over our product candidates or otherwise do not satisfactorily perform according to the terms of the agreements between us and them; |
| ▪ | disruptions in supply from manufacturers outside of the United States due to import/export limitations or responses of other governments to outbreaks of COVID-19; |
| ▪ | the possible termination or nonrenewal of agreements by our third-party contractors at a time that is costly or inconvenient for us; |
| ▪ | the possible breach by the third-party contractors of our agreements with them; |
| ▪ | the failure of third-party contractors to comply with applicable regulatory requirements; |
| ▪ | the possible mislabeling of clinical supplies, potentially resulting in the wrong dose amounts being supplied or active drug or placebo not being properly identified; |
| ▪ | the possibility of clinical supplies not being delivered to clinical sites on time, leading to clinical trial interruptions, or of drug supplies not being distributed to commercial vendors in a timely manner, resulting in lost sales; and |
| ▪ | the possible misappropriation of our proprietary information, including our trade secrets and know-how. |
We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical studies and clinical trials, as well as for commercial manufacture if our product candidates receive marketing approval. To date, we, or our partners on our behalf, have obtained materials for elamipretide and SBT-272 from third-party manufacturers. If any of our existing manufacturers should become unavailable to us for any reason, we may incur some delay in identifying or qualifying replacements.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations, delay our clinical trials and, if our products are approved for sale, result in lost sales. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to future contract manufacture caused by problems at suppliers could delay shipment of our product candidates, increase our cost of goods sold and result in lost sales.
If any of our product candidates are approved by any regulatory agency, we plan to enter into agreements with third-party contract manufacturers for the commercial production and distribution of those products. It may be difficult for us to reach agreement with a contract manufacturer on satisfactory terms or in a timely manner. In addition, we may face competition for access to manufacturing facilities as there are a limited number of contract manufacturers operating under current good manufacturing practices, or cGMPs, that are capable of manufacturing our product candidates. Consequently, we may not be able to reach agreement with third-party manufacturers on satisfactory terms, which could delay our commercialization efforts.
Third-party manufacturers are required to comply with cGMPs and similar regulatory requirements outside the United States, such as the ICH. Facilities used by our third-party manufacturers must be approved by the FDA after we submit an NDA and before potential approval of the product candidate. Similar regulations apply to manufacturers of our product candidates for use or sale in foreign countries. We do not control the manufacturing process and are completely dependent on our third-party manufacturers for compliance with the applicable regulatory requirements for the manufacture of our product candidates. If our manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA, and of any applicable foreign regulatory authority, we will not be able to secure the applicable approval for their manufacturing facilities. If these facilities are not approved for commercial manufacture, we may need to find
31
alternative manufacturing facilities, which could result in delays in obtaining approval for the applicable product candidate.
In addition, our manufacturers are subject to ongoing periodic inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements both prior to and following the receipt of marketing approval for any of our product candidates. Some of these inspections may be unannounced. Failure by any of our manufacturers to comply with applicable cGMPs or other regulatory requirements could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspensions or withdrawals of approvals, operating restrictions, interruptions in supply and criminal prosecutions, any of which could adversely affect supplies of our product candidates and significantly harm our business, financial condition and results of operations.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain sufficient patent protection for our product candidates, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to commercialize our product candidates successfully may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary product candidates. If we do not adequately protect our intellectual property, competitors may be able to erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. To protect our proprietary position, we file patent applications in the United States and abroad related to our novel product candidates that are important to our business. The patent application and approval process is expensive and time consuming. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain. No consistent policy regarding the breadth of claims allowed in biotechnology and pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. In addition, the determination of patent rights with respect to pharmaceutical compounds commonly involves complex legal and factual questions, which has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability, term and commercial value of our patent rights are highly uncertain.
Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent. However, prior to March 16, 2013, in the United States, the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
Moreover, because the issuance of a patent is not conclusive as to its inventorship, scope, validity, term or enforceability, our patents or pending patent applications may be challenged in the courts or patent offices in the United States and abroad. For example, we may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office, or USPTO, or become involved in post-grant review procedures, oppositions, derivations, reexaminations, inter partes review or interference proceedings, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Moreover, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. In addition, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. Furthermore, while it is our policy to require agreements with selected contractors, consultants, scientific advisors and collaborators requiring assignment of inventions or, in limited cases, the grant of an exclusive, worldwide license or option to license intellectual property rights developed in the
32
course of their work with or for us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. As a result, the inventorship or ownership of our intellectual property may be challenged in the future.
Our pending and future patent applications may not result in patents being issued which protect our product candidates, in whole or in part, or which effectively prevent others from commercializing competitive products. Our issued patents or any patents that may issue in the future may be invalidated or interpreted narrowly, such that they fail to provide us with any significant competitive advantage. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, the laws of foreign countries may not protect our rights to the same extent or in the same manner as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does.
Even if our patent applications have issued or do issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. Alternatively, our competitors may seek to market generic versions of any approved products by submitting ANDAs to the FDA in which they claim that patents owned or licensed by us are invalid, unenforceable or not infringed. In these circumstances, we may need to defend or assert our patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, and our business would be harmed.
While we have obtained composition of matter patents with respect to elamipretide and certain methods of use through an application family in-licensed from Cornell Research Foundation, Inc., a subsidiary of Cornell University, or Cornell, and Institut de recherches cliniques de Montréal, or the IRCM, we also rely on trade secret protection for certain aspects of our discovery platform. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, consultants, independent contractors, advisors, contract manufacturers, suppliers and other third parties. We also enter into confidentiality and invention or patent assignment agreements with employees, certain consultants, contractors and collaborators. To our knowledge, such agreements have been entered into with all relevant parties; however we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be misappropriated or disclosed to, or independently developed by, a competitor, our business and competitive position could be harmed.
Certain aspects of our product candidates and technology are protected by patents exclusively licensed from academic institutions. If these third parties terminate their agreements with us or fail to maintain or enforce the underlying patents, or we otherwise lose our rights to these patents, our competitive position and our market share in the markets for any of our approved products will be harmed.
We are a party to license agreements and certain aspects of our business depend on patents and/or patent applications owned by third parties. In particular, we hold exclusive licenses from Cornell and the IRCM for elamipretide as well as for other compounds and certain methods. We may enter into additional license agreements as part of the development of our business in the future. If we are unable to maintain these patent rights or our license to these patent rights for any reason, or if we are unable to maintain any future material license we may enter into, our ability to develop and commercialize our product candidates could be materially harmed.
Our licensors may not successfully prosecute certain patent applications under which we are licensed and on which our business depends. Even if patents issue from these applications, our licensors may fail to maintain these patents, may decide not to pursue litigation against third-party infringers, may fail to prove infringement, or may fail to defend against counterclaims of patent invalidity or unenforceability. For example, under our license agreement
33
with Cornell, we have the first right to enforce the licensed patents against third-party infringement. However, our first right to enforce is subject to Cornell’s consent.
Risks with respect to parties from whom we have obtained intellectual property rights may also arise out of circumstances beyond our control. Despite our best efforts, our licensors might conclude that we have materially breached our license agreements and might therefore terminate the license agreements, thereby removing our ability to obtain regulatory approval and to market products covered by these license agreements. For example, our license agreement with Cornell required us to commercialize a product by December 31, 2020, subject to specified exceptions for causes due to scientific and regulatory events that are common in drug development, such as institutional review board delays, clinical trial recruitment, clinical trial results and regulatory delays, and other events over which we cannot exert direct control, and Cornell has the right to terminate the license if we do not comply. We believe that our noncompliance is subject to the named exceptions, and to date we have not received any notice of termination from Cornell. Any actual termination of the license would be subject to cure periods and appeals before taking effect. If our license agreements are terminated, or if the underlying patents fail to provide the intended market exclusivity, competitors would have the freedom to seek regulatory approval of, and to market, products similar or identical to ours. Moreover, if our license agreements are terminated, our former licensors and/or assignors may be able to prevent us from utilizing the technology covered by the licensed or assigned patents and patent applications. This could have a material adverse effect on our competitive business position and our business prospects.
Our license agreements with Cornell and the IRCM impose, and future license agreements we may enter into may impose, various diligence, milestone payment, royalty and other obligations on us. For example, our license agreements with Cornell and the IRCM include an obligation to pay royalties on the net sales of product candidates or related technologies to the extent they are covered by the agreement. If we fail to comply with our obligations under our license agreement with Cornell and the IRCM or future license agreements, and if no such exceptions apply, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any product that is covered by the agreement or face other penalties under the agreement, such as loss of exclusivity. Such an occurrence could materially adversely affect the value of the product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
| ▪ | the scope of rights granted under the license agreement and other interpretation-related issues; |
| ▪ | the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| ▪ | the sublicensing of patent and other rights under our collaborative development relationships; |
| ▪ | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| ▪ | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| ▪ | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations and prospects.
Some of our intellectual property that was discovered through government-funded programs may be subject to federal regulation such as “march-in” rights, certain reporting requirements and a preference for United States industry. Compliance with such regulations may limit our exclusive rights, subject us to
34
expenditure of resources with respect to reporting requirements and limit our ability to contract with foreign manufacturers.
Some of our intellectual property with respect to our product candidates has been funded, at least in part, by the U.S. government and, therefore, would be subject to certain federal regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future product candidates pursuant to the Bayh-Dole Act of 1980, or Bayh-Dole Act. For example, under the “march-in” provisions of the Bayh-Dole Act, the government may have the right under limited circumstances to require the patent owners to grant exclusive, partially exclusive or non-exclusive rights to third parties for intellectual property discovered through the government-funded program. The government can exercise its march-in rights if it determines that action is necessary because the patent owner fails to achieve practical application of the new invention or because action is necessary to alleviate health concerns or address the safety needs of the public. Intellectual property discovered under the government-funded program is also subject to certain reporting requirements, compliance with which may require us or our licensors to expend substantial resources. Such intellectual property is also subject to a preference for U.S. industry, which may limit our ability to contract with foreign product manufacturers for products covered by such intellectual property. We may apply for additional U.S. government funding, and it is possible that we may discover additional compounds or product candidates as a result of such funding. Intellectual property under such discoveries would be subject to the applicable provisions of the Bayh-Dole Act. Similarly, intellectual property that we license in the future may have been made using government funding and may be subject to the provisions of the Bayh-Dole Act.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. We may be required to file infringement claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both. In any patent infringement proceeding, there is a risk that a court will decide that a patent of ours is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patent claims do not cover the invention. An adverse outcome in a litigation or proceeding involving our patents could limit our ability to assert our patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products. Any of these occurrences could adversely affect our competitive business position, business prospects and financial condition. Similarly, if we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
Even if we establish infringement, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential or trade-secret information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of the ADSs. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings. In addition, we may from time to time become involved in disputes, including litigation, with respect to intellectual property.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time-consuming and could prevent or delay us from developing or commercializing our product candidates.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell our product candidates without infringing the intellectual property and other proprietary rights of third parties. Third parties have U.S. and non-U.S. issued patents and pending patent applications relating to compounds and methods of use for the treatment of key indications for our priority programs, and we may be subject to claims that our research,
35
development and commercialization activities infringe or otherwise violate patents or other intellectual property rights owned or controlled by third parties. If any third-party patents or patent applications are found to cover our product candidates or their methods of use, we may not be free to manufacture or market our product candidates as planned without obtaining a license, which may not be available on commercially reasonable terms, or at all.
There is a substantial amount of intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to our products candidates, including derivation or interference proceedings, post grant and inter partes reviews, opposition proceedings, and the like in the United States and in other jurisdictions. Third parties may assert infringement claims against us based on existing or future intellectual property rights. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The pharmaceutical and biotechnology industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that our product candidates, products, or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, we may not have sufficient resources to bring these actions to a successful conclusion. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects.
If we are found to infringe a third party’s intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing, or commercializing the infringing product candidate or product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product candidate. However, we may not be able to obtain any required license on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Changes to the patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is therefore costly, time consuming and inherently uncertain. Patent reform legislation in the United States and other countries, including the Leahy-Smith America Invents Act, or the Leahy-Smith Act, signed into law in September 2011, could increase those uncertainties and costs. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art and provide more efficient and cost-effective avenues for competitors to challenge the validity of patents. In addition, the Leahy-Smith Act has transformed the U.S. patent system into a “first to file” system. The first-to-file provisions, however, only became effective in March 2013. Accordingly, it is not yet clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could make it more difficult to obtain patent protection for our inventions and increase the uncertainties and costs surrounding the prosecution of our or our collaboration partners’ patent applications and the enforcement or defense of our or our collaboration partners’ issued patents, all of which could harm our business, results of operations and financial condition.
The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. Additionally, there have been recent proposals for additional changes to the patent laws of the United States and other countries that, if adopted, could impact our ability to enforce our proprietary technology. Depending on future actions by the U.S. Congress, the U.S. courts, the USPTO and the relevant law-making bodies in other countries,
36
the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submissions, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our product candidates, our competitive position would be adversely affected.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly in developing countries. Competitors may use our technologies in jurisdictions where we have not pursued and obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents and any future patent claims, or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws. Additionally, laws of some countries outside of the United States and Europe do not afford intellectual property protection to the same extent as the laws of the United States and Europe. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, including China, India and other developing countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and Europe. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, if our ability to enforce our patents to stop infringing activities is inadequate. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business. Furthermore, while we intend to protect our intellectual property rights in major markets for our products, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our products. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
37
If we do not obtain patent term extension and data exclusivity for any product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration, and specifics of any FDA marketing approval of any product candidates we may develop, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Action of 1984, or Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent term extension of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and the extension only applies to those claims covering the approved drug, a method for using it or a method for manufacturing it. However, we may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations and prospects could be materially harmed. Furthermore, in the United States, only a single patent can be extended for each qualifying FDA approval, and any patent can be extended only once and only for a single product. Laws governing analogous patent term extensions in foreign jurisdictions vary widely, as do laws governing the ability to obtain multiple patents from a single patent family. Because both elamipretide and SBT-20 compositions-of-matter are protected by a single family of patents and applications, we may not be able to secure patent term extensions for both of these product candidates in all jurisdictions where these product candidates are or may be approved, including the United States.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Many of our employees and our licensors’ employees, including our senior management, were previously employed by others, including universities and other biotechnology and pharmaceutical companies, some of which are our competitors or potential competitors. Some of these employees, including each member of our senior management, executed proprietary rights, non-disclosure and non-competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms, or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
Risks Related to Regulatory Approval and Other Legal Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of any product candidates. If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize any product candidates, and our ability to generate revenue will be materially impaired.
Elamipretide, our other product candidates and any future product candidates we may identify and pursue and the activities associated with their development and commercialization, including design, development, testing, manufacture, packaging, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, export, import and adverse event reporting, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by the EMA and similar regulatory authorities outside of the United States. In addition, even if we receive approval, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of any such product candidates.
38
Marketing approval of drugs in the United States requires the submission of a new drug application, or NDA, to the FDA and we are not permitted to market any product candidate in the United States until we obtain approval from the FDA of the NDA for that product. An NDA must be supported by extensive clinical and preclinical data, as well as extensive information regarding pharmacology, toxicology, and chemistry, manufacturing and controls. We have not submitted an application for or received marketing approval for elamipretide or any other product candidates we may develop in the United States or in any other jurisdiction.
We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party clinical research organizations or other third-party consultants or vendors to assist us in this process. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing processes to, and inspection of manufacturing facilities by, the regulatory authorities. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use. If our manufacturing facilities cannot pass FDA pre-approval inspection, our application could be delayed or rejected. If any of any product candidates receives marketing approval, the accompanying label may limit the approved use of our drug, which could limit sales of the product.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
Finally, disruptions at the FDA and other agencies may prolong the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. The Trump Administration also took several executive actions that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engage in routine regulatory and oversight activities. Finally, the COVID-19 pandemic has significantly impacted FDA’s inspectional ability and timeframe in which inspections are completed. It is possible that we could experience significant delays in scheduling and completing a pre-approval inspection of the manufacturing facilities which could delay the ultimate approval of the NDA.
If we experience delays in obtaining approval or if we fail to obtain approval of elamipretide, or our other product candidates and any other product candidates we may develop, the commercial prospects for any product candidates may be harmed and our ability to generate revenues will be materially impaired.
Failure to obtain marketing approval in foreign jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our products in the European Union and many other foreign jurisdictions, we or our potential third-party collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside of the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside of the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or our potential third-party collaborators may not obtain approvals from regulatory authorities outside of the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside of the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries. We may not be
39
able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
Additionally, we could face heightened risks with respect to seeking marketing approval in the United Kingdom as a result of the recent withdrawal of the United Kingdom from the European Union, commonly referred to as Brexit which was effective December 31, 2020. On December 24, 2020, the United Kingdom and European Union entered into a Trade and Cooperation Agreement. The agreement sets out certain procedures for approval and recognition of medical products in each jurisdiction. Any delay in obtaining, or an inability to obtain, any marketing approvals, as a result of Brexit or otherwise, would prevent us from commercializing any product candidates in the United Kingdom and/or the European Union and restrict our ability to generate revenue and achieve and sustain profitability. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the United Kingdom and/or European Union for any product candidates, which could significantly and materially harm our business.
We expect that we will be subject to additional risks in commercializing any of our product candidates that receive marketing approval outside the United States, including tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; and workforce uncertainty in countries where labor unrest is more common than in the United States.
We have obtained Fast Track designation from the FDA for elamipretide for the treatment of Barth, LHON and GA. However, Fast Track designation may not actually lead to a faster development, regulatory review or approval process.
We have received Fast Track designation for elamipretide for the treatment of Barth, PMM and LHON, and for the treatment of patients with geographic atrophy, an advanced form of dry AMD. We may seek Fast Track designation for other product candidates we may develop. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA Fast Track designation. The FDA has broad discretion whether or not to grant this designation, so even if we believe a particular product candidate is eligible for this designation, we cannot be certain that the FDA would decide to grant it. Fast Track designation does not ensure that we will experience a faster development, regulatory review or approval process compared to conventional FDA procedures or that we will ultimately obtain regulatory approval of elamipretide. Additionally, the FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program.
We may not be able to obtain or maintain orphan drug designation or exclusivity for any product candidates and, even if we do, that exclusivity may not prevent the FDA or the EMA from approving other competing products.
We have obtained orphan drug designation from the FDA and the EMA for elamipretide for the treatment of Barth and from the FDA for the treatment of LHON. We may seek orphan drug designation in other indications or for any other future product candidates. Regulatory authorities in some jurisdictions, including the United States and the European Union, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA or the EMA from approving another marketing application for the same drug for that time period. The applicable period is seven years in the United States and ten years in the European Union. The exclusivity period in the European Union can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because competing drugs containing a different active ingredient can be approved for the same condition. In addition, even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
40
On August 3, 2017, the U.S. Congress passed the FDA Reauthorization Act of 2017, or FDARA. which, among other things, codified the FDA’s pre-existing regulatory interpretation to require that a drug sponsor demonstrate the clinical superiority of an orphan drug that is otherwise the same as a previously approved drug for the same rare disease in order to receive orphan drug exclusivity. The new legislation reverses prior precedent holding that the Orphan Drug Act unambiguously requires that the FDA recognize the orphan exclusivity period regardless of a showing of clinical superiority. The FDA may further reevaluate the Orphan Drug Act and its regulations and policies. We do not know if, when or how the FDA may change the orphan drug regulations and policies in the future, and it is uncertain how any changes might affect our business. Depending on what changes the FDA may make to its orphan drug regulations and policies, our business could be adversely impacted.
Although we have obtained Rare Pediatric Disease Designation, or RPDD, for elamipretide for the treatment of Barth, we may not be eligible to receive a priority review voucher in the event that FDA approval does not occur prior to October 1, 2022.
The Rare Pediatric Disease Priority Review Voucher Program, or PRV Program, is intended to incentivize pharmaceutical sponsors to develop drugs for rare pediatric diseases. A sponsor who obtains approval of an NDA or BLA for a rare pediatric disease may be eligible for a Priority Review Voucher, or PRV, under this program, which may be redeemed by the owner of such PRV to obtain priority review for a marketing application. A PRV is fully transferrable and can be sold to any sponsor, who in turn can redeem the PRV for priority review of a marketing application in six months, compared to the standard timeframe of approximately 10 months.
In December 2016, Congress extended the Rare Pediatric Disease Priority Review Voucher Program, authorizing the FDA to award vouchers through September 30, 2022, limited to drugs with rare pediatric disease designation granted by September 30, 2020. On September 30, 2020, Congress provided a short-term extension of the Priority Review Voucher Program. On December 27, 2020, the Rare Pediatric Disease Priority Review Voucher Program was further extended. Under the current statutory sunset provisions, after September 30, 2024, FDA may only award a voucher for an approved rare pediatric disease product application if the sponsor has rare pediatric disease designation for the drug, and that designation was granted by September 30, 2024. After September 30, 2026, FDA may not award any rare pediatric disease priority review vouchers. If we do not obtain approval of an NDA for elamipretide for the treatment of Barth by these dates, and if the PRV Program is not further extended by congressional action, we may not receive a PRV.
Even if we, or any future collaborators, obtain marketing approvals for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we, or they, manufacture and market our products, which could materially impair our ability to generate revenue.
Once marketing approval has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. We, and any future collaborators, must therefore comply with requirements concerning advertising and promotion for any of our product candidates for which we or they obtain marketing approval. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. Thus, we and any future collaborators will not be able to promote any products we develop for indications or uses for which they are not approved.
In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, our contract manufacturers, any future collaborators and their contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with cGMPs.
Accordingly, assuming we, or any future collaborators, receive marketing approval for one or more of our product candidates, we, and any future collaborators, and our and their contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control.
If we, or any of our future collaborators, are not able to comply with post-approval regulatory requirements, we, and any such future collaborators, could have the marketing approvals for our products withdrawn by regulatory authorities and our, or any future collaborators’, ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Further, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
Any product candidate for which we obtain marketing approval could be subject to post-marketing restrictions or withdrawal from the market and we may be subject to substantial penalties if we fail to
41
comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, including the requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS. If any product candidate receives marketing approval, the accompanying label may limit the approved use of our drug, which could limit sales of the product.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product, including the adoption and implementation of REMS. The FDA and other agencies, including the Department of Justice, or the DOJ, closely regulate and monitor the post-approval marketing and promotion of drugs to ensure, among other things, that they are marketed and distributed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA and other agencies impose and enforce stringent restrictions on manufacturers’ communications regarding off-label use, and if we promote our products beyond their approved indications, we may be subject to enforcement action or prosecution arising from off-label promotion. Violations of the FDCA and other statutes relating to the promotion and advertising of prescription drugs may lead to investigations and enforcement actions alleging violations of federal and state healthcare fraud and abuse laws, including the False Claims Act, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may have various consequences, including:
| ▪ | suspension of or restrictions on such products, manufacturers or manufacturing processes; |
| ▪ | restrictions and warnings on the labeling or marketing of a product; |
| ▪ | restrictions on product distribution or use; |
| ▪ | requirements to conduct post-marketing studies or clinical trials; |
| ▪ | warning letters or untitled letters; |
| ▪ | withdrawal of the products from the market; |
| ▪ | refusal to approve pending applications or supplements to approved applications that we submit; |
| ▪ | fines, restitution or disgorgement of profits or revenues; |
| ▪ | suspension of any ongoing clinical trials; |
| ▪ | suspension or withdrawal of marketing approvals; |
| ▪ | damage to relationships with any potential collaborators; |
| ▪ | unfavorable press coverage and damage to our reputation; |
| ▪ | refusal to permit the import or export of our products; |
| ▪ | product seizure or detention; |
| ▪ | injunctions or the imposition of civil or criminal penalties; or |
| ▪ | litigation involving patients using our products. |
Non-compliance with European Union requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with the European Union’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
42
In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs applicable to drug manufacturers or quality assurance standards applicable to medical device manufacturers, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, any contract manufacturers we may engage in the future, our future collaborators and their contract manufacturers will also be subject to other regulatory requirements, including submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements regarding the distribution of samples to clinicians, recordkeeping, and costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product such as the requirement to implement a REMS.
Current and future legislation may increase the difficulty and cost for us and any future collaborators to obtain reimbursement for any of our candidate products that do receive marketing approval and our ability to generate revenue will be materially impaired.
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or any collaborators, may receive for any approved products. If reimbursement of our products is unavailable or limited in scope, our business could be materially harmed.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the ACA. In addition, other legislative changes have been proposed and adopted since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2030 under the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for any of our product candidates for which we may obtain regulatory approval or the frequency with which any such product candidate is prescribed or used.
Since enactment of the ACA, there have been, and continue to be, numerous legal challenges and Congressional actions to repeal and replace provisions of the law. For example, with enactment of the Tax Cuts and Jobs Act of 2017, which was signed by President Trump on December 22, 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, became effective in 2019. Further, on December 14, 2018, a U.S. District Court judge in the Northern District of Texas ruled that the individual mandate portion of the ACA is an essential and inseverable feature of the ACA, and therefore because the mandate was repealed as part of the Tax Cuts and Jobs Act, the remaining provisions of the ACA are invalid as well. On December 18, 2019, the Court of Appeals for the Fifth Circuit court affirmed the lower court’s ruling that the individual mandate portion of the ACA is unconstitutional and it remanded the case to the district court for reconsideration of the severability question and additional analysis of the provisions of the ACA. Thereafter, the U.S. Supreme Court agreed to hear this case. On June 17, 2021, the Supreme Court dismissed this case after finding that plaintiffs do not have standing to challenge the constitutionality of the ACA. Litigation and legislation over the ACA are likely to continue, with unpredictable and uncertain results.
The Trump Administration also took executive actions to undermine or delay implementation of the ACA, including directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. On January 28, 2021, however, President Biden issued a new Executive Order which directs federal agencies to reconsider rules and other policies that limit Americans’ access to health care and consider actions that will protect and strengthen that access. Under this Executive Order, federal agencies are directed to re-examine: policies that undermine protections for people with pre-existing conditions, including complications related to COVID-19; demonstrations and waivers under Medicaid and the ACA that may reduce coverage or undermine the programs, including work requirements; policies that undermine the Health Insurance Marketplace or other markets for health
43
insurance; policies that make it more difficult to enroll in Medicaid and the ACA; and policies that reduce affordability of coverage or financial assistance, including for dependents.
Current and future legislative efforts may limit the costs for our products, if and when they are licensed for marketing, and that could materially impact our ability to generate revenues.
The costs of prescription pharmaceuticals have also been the subject of considerable discussion in the United States. To date, there have been several recent U.S. congressional inquiries, as well as proposed and enacted state and federal legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare and reform government program reimbursement methodologies for products. To those ends, President Trump issued five executive orders intended to lower the costs of prescription drug products. Several of these orders are reflected in recently promulgated regulations, and one of these regulations is currently subject to a nationwide preliminary injunction. It remains to be seen whether these orders and resulting regulations will remain in force during the Biden Administration. Further, on September 24, 2020, the Trump Administration finalized a rulemaking allowing states or certain other non-federal government entities to submit importation program proposals to the FDA for review and approval. Applicants are required to demonstrate that their importation plans pose no additional risk to public health and safety and will result in significant cost savings for consumers. The FDA has issued draft guidance that would allow manufacturers to import their own FDA-approved drugs that are authorized for sale in other countries (multi-market approved products).
At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Finally, outside the United States, in some nations, including those of the EU, the pricing of prescription pharmaceuticals is subject to governmental control and access. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we or our collaborators may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
We may be subject to certain healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, fines, disgorgement, exclusion from participation in government healthcare programs, curtailment or restricting of our operations, and diminished profits and future earnings.
Healthcare providers, third-party payors and others will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with healthcare providers and third-party payors will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. Potentially applicable U.S. federal and state healthcare laws and regulations include the following:
Anti-Kickback Statute. The federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid;
False Claims Laws. The federal false claims laws, including the civil False Claims Act, impose criminal and civil penalties, including those from civil whistleblower or qui tam actions against individuals or entities for knowingly presenting, or causing to be presented to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
44
HIPAA. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing or attempting to execute a scheme to defraud any healthcare benefit program;
HIPAA and HITECH. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, also imposes obligations on certain types of individuals and entities, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
False Statements Statute. The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
Transparency Requirements. The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children's Health Insurance Program, with specific exceptions, to report annually to the U.S. Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and
Analogous State and Foreign Laws. Analogous state laws and regulations, such as state anti-kickback and false claims laws, and transparency laws, may apply to sales or marketing arrangements, and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. Many state laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. Foreign laws also govern the privacy and security of health information in many circumstances.
The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medicinal products is prohibited in the European Union. Payments made to physicians in certain European Union Member States must be publicly disclosed. Moreover, agreements with physicians often must be the subject of prior notification and approval by the physician’s employer, his or her competent professional organization and/or the regulatory authorities of the individual European Union Member States. These requirements are provided in the national laws, industry codes or professional codes of conduct applicable in the European Union Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Efforts to ensure that our business arrangements with third parties, and our business generally, will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages, and reputational harm, any of which could substantially disrupt our operations. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Regulatory or legislative developments regarding privacy and data security matters could adversely affect our ability to conduct our business.
We are subject to data privacy and security regulation in the jurisdictions in which we conduct our business, particularly in light of increased regulatory scrutiny of and user expectations regarding the processing, collection, use, storage, dissemination, transfer and disposal of user data. The regulatory frameworks regarding privacy issues in many jurisdictions are constantly evolving and can be subject to significant changes from time to time, and therefore we may not be able to comprehensively assess the scope and extent of our compliance responsibility at a global level. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, thus complicating compliance efforts. Data privacy concerns may result in increased costs of operations and threats of lawsuits, enforcement actions and related liabilities, including financial penalties.
45
The New Economic Substance Law in the Cayman Islands may have an adverse effect on our business.
The Cayman Islands is a member of the Organisation for Economic Co-operation and Development, or OECD, Inclusive Framework on Base Erosion and Profit Shifting, and, along with other OECD-compliant jurisdictions, enacted economic substance legislation in January 2019. Pursuant to the legislation, namely the International Tax Cooperation (Economic Substance) Law (as amended) together with related regulations and guidance, referred to as the ES Law, we may need to incur additional costs in order to comply with filing or other requirements. While we are taking steps to comply with the ES Law, there is a risk of inadvertent non-compliance and the payment of associated penalties. International standards are continuing to develop, and it is anticipated that the ES Law will evolve and be subject to further clarification. Hence, it is not possible to determine with certainty the extent to which the ES Law may affect us.
Our internal computer systems, or those of our collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Our internal computer systems and those of any collaborators, contractors or consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such systems are also vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees, third-party vendors and/or business partners, or from cyberattacks by malicious third parties. Cyber incidents are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. For example, we have experienced attempts at phishing and e-mail fraud with the goal of causing payments to be transmitted to an unintended recipient. Cyber incidents could also include the deployment of harmful malware, ransomware, denial-of-service attacks, unauthorized access to or deletion of files, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information.
In the event any cyberattack security breach or system failure were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other similar disruptions. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, our competitive position and reputation could be harmed and the further development and commercialization of IMR-687 and any other product candidates we may develop could be delayed.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anticorruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, or FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties to sell our products outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
If we or any third-party manufacturers we engage now or in the future fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs or liabilities that could harm our business.
46
We and third-party manufacturers we engage now are, and any third-party manufacturers we may engage in the future will be, subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. Liability under certain environmental laws governing the release and cleanup of hazardous materials is joint and several and could be imposed without regard to fault. We also could incur significant costs associated with civil or criminal fines and penalties or become subject to injunctions limiting or prohibiting our activities for failure to comply with such laws and regulations.
Although we maintain general liability insurance as well as workers’ compensation insurance to cover us for costs and expenses, we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Further, with respect to the operations of our current and any future third-party contract manufacturers, it is possible that if they fail to operate in compliance with applicable environmental, health and safety laws and regulations or properly dispose of wastes associated with our products, we could be held liable for any resulting damages, suffer reputational harm or experience a disruption in the manufacture and supply of any product candidates or products. In addition, our supply chain may be adversely impacted if any of our third-party contract manufacturers become subject to injunctions or other sanctions as a result of their non-compliance with environmental, health and safety laws and regulations.
In the future, if we decide to market our products outside of the United States, such as in the European Union or the United Kingdom, we would need to obtain additional approvals and comply with additional regulatory requirements.
Our primary regulatory strategy has been to apply first for approvals in the United States for our rare disease programs. We may in the future apply for approvals in Europe and the United Kingdom, which may be following receipt of marketing authorization in the United States or, in the case of Barth, be concurrent with or even prior to United States regulatory interactions. As we also plan to consider collaboration for commercialization efforts in Europe and the United Kingdom, we anticipate that potential commercialization partners may have input into regulatory strategies in those jurisdictions. To date, we have focused our regulatory efforts primarily on achieving approvals and marketing authorization in the United States, but we are now undertaking preliminary efforts in Europe in the context of Barth. In order to market any product outside of the United States, we will need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not we obtain FDA approval for a product, we or our collaborators would need to obtain the necessary approvals by the comparable foreign regulatory authorities before marketing the product in those countries or jurisdictions. We cannot be sure whether and when we would be able to obtain the necessary approvals, which could adversely affect our business and prospects.
Governments outside the United States may impose strict price controls, which may adversely affect our revenues, if any.
In some countries, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we, or any future collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
47
Our employees, independent contractors, consultants and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, consultants and vendors. Misconduct by these partners could include intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or similar foreign regulatory authorities, comply with manufacturing standards, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. This could include violations of HIPAA, other U.S. federal and state law, and requirements of non-U.S. jurisdictions, including the European Union Data Protection Directive. We are also exposed to risks in connection with any insider trading violations by employees or others affiliated with us. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards, regulations, guidance or codes of conduct. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Risks Related to this Offering and Ownership of ADSs
MVIL has a controlling ownership interest in our ordinary shares and the ability to substantially control all matters submitted to shareholders for approval.
As of June 30, 2021, MVIL beneficially owns 64.9% of our ordinary shares. In addition, certain entities associated within MVIL beneficially own an additional 8.5% of our ordinary shares. As a result, MVIL and such entities will be able to control all matters submitted to our shareholders for approval that require an ordinary resolution or special resolution, as well as our management and affairs. For example, MVIL would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of ownership control may:
| ▪ | delay, defer or prevent a change in control; |
| ▪ | entrench our management or the board of directors; or |
| ▪ | impede a merger, consolidation, takeover or other business combination involving us that other shareholders may desire. |
MVIL owns a controlling portion of our ordinary shares and may have conflicts of interest with us and other shareholders in the future.
The interests of MVIL may not always be consistent with the interests of our company or of our other shareholders. Accordingly, MVIL could cause us to enter into transactions or agreements of which other holders of our ordinary shares would not approve or make decisions with which such holders would disagree. Gerald L. Chan, one of our directors, is a co-founder of the Morningside group, a private investment group with venture, private equity and property investments. In addition, Reenie McCarthy, our Chief Executive Officer and a director, served as a member of the investment team at Morningside Technology Advisory, LLC (and affiliates) from 1993 through 2016, and remains a director of Morningside Technology Advisory, LLC, which provides advisory services to entities associated with the Morningside group.
Although Dr. Chan is not an officer, director or employee of MVIL and has neither voting nor dispositive control over the ordinary shares held by MVIL and does not otherwise beneficially own such shares, as a result of his ongoing relationship with the Morningside group, transactions between us and MVIL may present an actual or perceived conflict of interest. Although Ms. McCarthy is not an officer, director or employee of MVIL, and has neither voting nor dispositive control over our ordinary shares held by MVIL and does not otherwise beneficially own such shares, as a result of her historic relationship with the Morningside group and her ongoing relationship with Morningside Technology Advisory, LLC, transactions between us and MVIL may present an actual or perceived conflict of interest. Any actual or perceived conflicts of interest may lead Dr. Chan and Ms. McCarthy to recuse themselves from actions of our board of directors with respect to transactions involving MVIL and its affiliates. For example, in a situation in which MVIL is adverse to us, such as if it breaches an agreement with us, a conflict could arise. We may not be able to resolve any potential conflicts, and even if we do, the resolution may be less favorable than if we were dealing with an unaffiliated party.
48
MVIL is in the business of making investments in companies and could from time to time acquire and hold interests in businesses that compete with us. MVIL may also pursue acquisition opportunities that may be complementary to our business, and as a result, desirable acquisitions may not be available to us. As long as MVIL continues to own a significant amount of our equity, it will continue to be able to strongly influence or effectively control our decisions.
If you purchase ADSs in this offering, you will suffer immediate dilution in the net tangible book value of your investment.
The public offering price of the ADSs will be substantially higher than the net tangible book value per ADS. Therefore, if you purchase ADSs in this offering, you will pay a price per ADS that substantially exceeds our net tangible book value per ADS after this offering. Based on the public offering price of $ per ADS, you will experience immediate dilution of $ per ADS, representing the difference between our as adjusted net tangible book value per ADS after giving effect to this offering and the assumed public offering price. For a further description of the dilution that you will experience immediately after this offering, see “Dilution.”
The price of the ADSs has been, and is likely to continue to be, highly volatile, which could result in substantial losses for purchasers of the ADSs in this offering.
The price of the ADSs has been, and is likely to continue to be, highly volatile. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your ADSs at or above the public offering price and you may lose some or all of your investment. The market price for the ADSs may be influenced by many factors, including:
| ▪ | our ability to commercialize or obtain regulatory approval for our product candidates, or delays in commercializing or obtaining regulatory approval; |
| ▪ | announcements relating to our clinical trials, including any periodic updates relating to enrollment of trial subjects, adverse events, site initiation, and timing of release of interim analyses and final trial results; |
| ▪ | commencement or termination of collaborations for our development programs; |
| ▪ | failure or discontinuation of any of our development programs; |
| ▪ | results from, or any delays in, clinical trials relating to our product candidates, including our clinical trials for elamipretide; |
| ▪ | any need to suspend or discontinue clinical trials due to side effects or other safety risks, or any need to conduct studies on the long-term effects associated with the use of our product candidates; |
| ▪ | manufacturing issues related to our product candidates for clinical trials or future products for commercialization; |
| ▪ | commercial success and market acceptance of our product candidates following regulatory approval; |
| ▪ | undesirable side effects caused by product candidates after they have entered the market; |
| ▪ | ability to discover, develop and commercialize additional product candidates; |
| ▪ | announcements relating to collaborations that we may enter into with respect to the development or commercialization of our product candidates; |
| ▪ | success of our competitors in discovering, developing or commercializing products; |
| ▪ | strategic transactions undertaken by us; |
| ▪ | additions or departures of key personnel; |
| ▪ | product liability claims related to our clinical trials or product candidates; |
| ▪ | business disruptions caused by earthquakes or other natural disasters or a public health crisis (for example, an outbreak of a contagious disease such as COVID-19); |
| ▪ | disputes concerning our intellectual property or other proprietary rights; |
| ▪ | FDA or EMA or other regulatory actions affecting us or our industry; |
| ▪ | healthcare reform measures in the United States; |
| ▪ | future sales or issuances of equity or debt securities by us; |
49
| ▪ | fluctuations in our semi-annual operating results; |
| ▪ | announcement or expectation of additional financing efforts; |
| ▪ | sales of our ordinary shares by us, our insiders or other shareholders; |
| ▪ | actual and anticipated variations in our results of operations; |
| ▪ | changes in securities analysts’ estimates or market perception of our financial performance; |
| ▪ | announcements by us of significant acquisitions, disposals, strategic alliances or joint ventures; |
| ▪ | market developments affecting us or the markets in which we operate; |
| ▪ | regulatory or legal developments, including litigation; |
| ▪ | the operating and share price performance of companies that investors consider to be comparable to us; |
| ▪ | the depth and liquidity of the market for the ADSs; |
| ▪ | the release or expiry of lock-up or other transfer restrictions on our ordinary shares and ADSs; |
| ▪ | general economic, political and stock market conditions in the United States and the countries in which we operate and elsewhere in the world; and |
| ▪ | the other factors described in this “Risk Factors” section. |
Additionally, in the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us in light of the significant stock price volatility we and other pharmaceutical companies have experienced in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of the ADSs. The failure by our management to apply these funds effectively could result in financial losses that could significantly harm our business, cause the price of the ADSs to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the Securities and Exchange Commission than U.S. companies. This may limit the information available to holders of the ADSs.
We are a “foreign private issuer,” as defined in the rules and regulations of the Securities and Exchange Commission, or the SEC, and, consequently, we are not subject to all of the disclosure requirements applicable to companies organized within the United States. For example, we are exempt from certain rules under the Exchange Act, that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, our senior management and supervisory board members are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies. Accordingly, there may be less publicly available information concerning our company than there is for U.S. public companies.
In addition, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
If we are, or another entity in our group is, a controlled foreign corporation, or CFC, there could be adverse U.S. federal income tax consequences to certain U.S. Holders.
50
If a U.S. Holder, as defined in the section of this prospectus titled “Taxation - Material U.S. Federal Income Tax Considerations for U.S. Holders,” is treated as owning, directly, indirectly or constructively, at least 10% of the value or voting power of our ordinary shares (including ADSs), such U.S. Holder may be treated under the Internal Revenue Code of 1986, as amended, or the Code, as a “United States shareholder,” or a Ten-Percent Shareholder, with respect to each CFC in our group, if any. We do not believe that we were a CFC for our taxable year ended December 31, 2020, though we have not made a determination regarding our CFC status in the current taxable year, and we may become a CFC in a subsequent taxable year. In addition, because Stealth BioTherapeutics Corp has a U.S. subsidiary, any future newly formed or acquired non-U.S. subsidiaries of Stealth BioTherapeutics Corp may be treated as CFCs, regardless of whether we are treated as a CFC.
A Ten-Percent Shareholder of a CFC may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by the CFC, regardless of whether it makes any distributions. In addition, a Ten-Percent Shareholder that realizes gain from the sale or exchange of shares in a CFC may be required to classify a portion of such gain as dividend income rather than capital gain. An individual that is a Ten-Percent Shareholder with respect to a CFC generally is not be allowed certain tax deductions or foreign tax credits that would be allowed to a Ten-Percent Shareholder that is a U.S. corporation.
Failure to comply with CFC reporting obligations may subject a Ten-Percent Shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any Ten-Percent Shareholder information that may be necessary to comply with the reporting and tax-paying obligations applicable under the CFC rules of the Code.
Our status, or the status of any subsidiary, as a CFC will generally not have any U.S. federal income tax consequences for U.S. Holders holding our ordinary shares or ADSs who are not Ten-Percent Shareholders. It is possible that, following this offering, a shareholder treated as a U.S. person for U.S. federal income tax purposes will own or acquire, directly or indirectly, enough shares to be treated as a Ten-Percent Shareholder.
U.S. Holders should consult their own tax advisors with respect to the potential adverse tax consequences of becoming a Ten-Percent Shareholder in a CFC.
For further discussion of the CFC rules and the adverse U.S. federal income tax consequences in the event we are classified as a CFC, see the section of this prospectus titled “Taxation - Material U.S. Federal Income Tax Considerations for U.S. Holders.”
If we are a passive foreign investment company, or PFIC, for any taxable year, there could be adverse U.S. federal income tax consequences to U.S. Holders.
Under the Code, we will be a PFIC for any taxable year in which (1) 75% or more of our gross income consists of passive income or (2) 50% or more of the value of our assets (generally determined on the basis of a weighted quarterly average) consists of assets that produce, or are held for the production of, passive income (including cash and cash-equivalents). For purposes of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property and certain rents and royalties. In addition, for purposes of the above calculations, a non-U.S. corporation that directly or indirectly owns at least 25% by value of the shares of another corporation is treated as holding and receiving directly its proportionate share of assets and income of such corporation. If we are a PFIC for any taxable year during which a U.S. Holder holds our shares, the U.S. Holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC in future years, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, the imposition of interest charges on certain taxes treated as deferred and additional reporting requirements.
Based on our analysis of our activities and current estimates (and not fully audited financials) of the composition of our income and assets, and taking into account the public offering price of our ADSs in this offering and the expected price of the ADSs following this offering, we do not believe that we were a PFIC in our taxable year ended December 31, 2020 and we do not expect to be a PFIC in our current taxable year. The determination of whether we are a PFIC is a fact-intensive determination made on an annual basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation. In addition, for our current and future taxable years, the total value of our assets (including goodwill) for PFIC testing purposes may be determined in part by reference to the market price of our ordinary shares or ADSs from time to time, which may fluctuate considerably. Accordingly, if our market capitalization declines while we hold a substantial amount of cash and cash-equivalents for any taxable year, we may be a PFIC for that taxable year. Under the income test, our status as a PFIC depends on the composition of our income for the relevant taxable year which will depend on the transactions we enter into in the future and our corporate structure. The composition of our income and assets is also affected by how we spend the cash we raise in any offering, including this offering. We may be a PFIC for any
51
taxable year in which we do not generate sufficient amounts of active income to offset our passive financing income. Therefore, we cannot express an expectation regarding our PFIC status for the current or any future taxable year. Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the U.S. Internal Revenue Service, or IRS, will agree with our conclusion and that the IRS would not successfully challenge our position.
If we are a PFIC, a U.S. Holder will generally be subject to similar rules with respect to distributions we receive from, and our dispositions of the stock of, any of our direct or indirect subsidiaries or any other entities in which we hold equity interests that also are PFICs, or lower-tier PFICs, as if such distributions were indirectly received by, and/or dispositions were indirectly carried out by, such U.S. Holder.
If we are classified as both a CFC (as discussed above) and a PFIC, we generally will not be treated as a PFIC with respect to those U.S. Holders that meet the definition of a Ten-Percent Shareholder during the period in which we are a CFC.
U.S. Holders should consult their own tax advisors with respect to the potential adverse tax consequences of acquiring, holding and disposing of shares in a PFIC.
For further discussion of the PFIC rules and the adverse U.S. federal income tax consequences in the event we are classified as a PFIC, as well as certain elections that may be available to U.S. Holders, see the section of this prospectus titled “Taxation - Material U.S. Federal Income Tax Considerations for U.S. Holders.”
We intend to continue to rely on Nasdaq Stock Market rules that permit us to comply with applicable Cayman Islands corporate governance practices, rather than the corresponding domestic U.S. corporate governance practices, and therefore your rights as a shareholder will differ from the rights you would have as a shareholder of a domestic U.S. issuer.
As a foreign private issuer whose ADSs are listed on The Nasdaq Global Market, we are permitted in certain cases to follow Cayman Islands corporate governance practices instead of the corresponding requirements of the Nasdaq Stock Market rules. A foreign private issuer that elects to follow a home country practice instead of Nasdaq requirements must submit to Nasdaq in advance a written statement from an independent counsel in such issuer’s home country certifying that the issuer’s practices are not prohibited by the home country’s laws. In addition, a foreign private issuer must disclose in its annual reports filed with the SEC each such requirement that it does not follow and describe the home country practice followed instead of any such requirement. In accordance with Cayman Islands law:
| ▪ | we do not require a remuneration committee to have entirely independent directors; |
| ▪ | we do not require an independent director oversight of director nominations; and |
| ▪ | we do not require the board of directors to have regularly scheduled meetings at which only independent directors are present. |
For further information upon the differences between Delaware law and Cayman Islands law, please see “Description of Share Capital and Articles of Association—Differences in Corporate Law.”
We may lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our ordinary shares are directly or indirectly held by residents of the United States and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing rules. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we do not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors and more expensive to procure director and officer liability insurance.
52
We incur increased costs as a result of operating as a public company, and our management is now required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Global Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel devote a substantial amount of time towards maintaining compliance with these requirements. Moreover, these requirements have increased our legal and financial compliance costs and make some activities more time consuming and costly.
Pursuant to SOX Section 404, we will be required to furnish a report by our management on our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with SOX Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe, or at all, that our internal control over financial reporting is effective as required by SOX Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
A significant portion of our total outstanding shares may be sold into the market, which could cause the market price of the ADSs to decline significantly, even if our business is doing well.
Sales of a substantial number of ADSs in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of ordinary shares intend to sell ADSs, could reduce the market price of the ADSs. After this offering, we will have ADSs outstanding based on the ordinary shares represented by ADSs outstanding as of , 2021.
All ADSs we are selling in this offering may be resold in the public market immediately, unless purchased by our affiliates. For additional information on applicable restrictions on sales of ordinary shares and ADSs after this offering, see the “Shares and ADSs Eligible For Future Sale” section of this prospectus.
On June 2, 2020, we entered into the Purchase Agreement with Lincoln Park pursuant to which Lincoln Park has committed to purchase up to $20.0 million of our ordinary shares. On August 6, 2020, we entered into the At The Market Offering Agreement with Wainwright, pursuant to which we may offer and sell, from time to time through Wainwright, ADSs. We generally have the right to control the timing and amount of any future sales of ordinary shares to Lincoln Park or ADSs through Wainwright; however, we suspended the facility with Wainwright in November 2020. Sales of ordinary shares or ADSs, if any, to Lincoln Park or, in the event we reactivate the facility with Wainwright, through Wainwright, respectively, will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park or through Wainwright all, some or none of the additional ordinary shares or ADSs that may be available for us to sell pursuant to the Purchase Agreement or the At The Market Offering Agreement, respectively. Therefore, sales to Lincoln Park or through Wainwright by us could result in substantial dilution to the interests of other holders of our ordinary shares and ADSs. Additionally, the sale of a substantial number of ordinary shares to Lincoln Park or ADSs through Wainwright, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales. If and when we do sell ordinary shares to Lincoln Park, after Lincoln Park has acquired the ordinary shares, Lincoln Park may resell all, some or none of those ordinary shares at any time or from time to time in its discretion. If and when we do sell ADSs through Wainwright, after the purchaser has acquired the ADSs, such purchaser may resell all, some or none of those ADSs at any time or from time to time in its discretion.
We have also reserved 118.7 million ordinary shares that we may issue under our equity compensation plans. The ADSs representing these ordinary shares can be freely sold in the public market upon issuance and once vested, subject to volume limitations applicable to affiliates.
53
We have issued warrants to purchase an aggregate of 83,365,384 ordinary shares to MVIL in connection with funding under our Development Funding Agreement. Subject to compliance with applicable securities laws, and volume limitations, ADSs representing the ordinary shares issued upon exercise of these warrants may be sold in the public market.
We do not anticipate paying any cash dividends on the ADSs in the foreseeable future. Accordingly, holders of ADSs must rely on capital appreciation, if any, for any return on their investment.
We have never declared nor paid cash dividends on our share capital. We currently plan to retain all of our future earnings, if any, to finance the operation, development and growth of our business. In addition, the terms of our existing loan and security agreement preclude us from paying cash dividends without the consent of our lender. As a result, capital appreciation, if any, of the ADSs will be your sole source of gain for the foreseeable future. However, if we do pay a cash dividend on our ordinary shares in the future, we may only pay such dividend out of our profits or share premium (subject to applicable solvency requirements) under Cayman Islands law.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for the ADSs will likely depend, in part, on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will continue to cover us or provide favorable coverage. If one or more analysts downgrade the ADSs or change their opinion of the ADSs, our share price would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the price of the ADSs or trading volume to decline.
Holders of our ADSs have fewer rights than our shareholders and must act through the depositary to exercise their rights.
Holders of our ADSs do not have the same rights as our shareholders and may only exercise their voting rights with respect to the underlying ordinary shares in accordance with the provisions of the deposit agreement. Holders of the ADSs have appointed the depositary or its nominee as their representative to exercise the voting rights attaching to the ordinary shares represented by the ADSs. When a general meeting is convened, if you hold ADSs, you may not receive sufficient notice of a shareholders’ meeting to permit you to withdraw the ordinary shares underlying your ADSs to allow you to vote directly with respect to any specific matter. We cannot assure you that you will receive voting materials in time to instruct the depositary to vote, and it is possible that you, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote. Furthermore, the depositary will not be liable for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, you may not be able to exercise your right to vote and you may lack recourse if your ADSs are not voted as you request. In addition, in your capacity as an ADS holder, you will not be able to call a shareholders’ meeting. See “Description of American Depositary Shares.”
Holders of our ADSs may face limitations on transfer and withdrawal of underlying ordinary shares.
Our ADSs, which may be evidenced by American Depositary Receipts, or ADRs, are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of your ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason subject to your right to cancel your ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of your ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, you may not be able to cancel your ADSs and withdraw the underlying ordinary shares when you owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. See “Description of American Depositary Shares.”
ADS holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiff(s) in any such action.
The deposit agreement governing the ADSs representing our ordinary shares provides that holders and beneficial owners of ADSs irrevocably waive the right to a trial by jury in any legal proceeding arising out of or relating to the deposit agreement or the ADSs, including in respect of claims under federal securities laws, against us or the depositary to the fullest extent permitted by applicable law. If this jury trial waiver provision is prohibited by
54
applicable law, an action could nevertheless proceed under the terms of the deposit agreement with a jury trial. To our knowledge, the enforceability of a jury trial waiver under the federal securities laws has not been finally adjudicated by a federal court. However, we believe that a jury trial waiver provision is generally enforceable under the laws of the State of New York, which govern the deposit agreement, by a court of the State of New York or a federal court, which have non-exclusive jurisdiction over matters arising under the deposit agreement, applying such law. In determining whether to enforce a jury trial waiver provision, New York courts and federal courts will consider whether the visibility of the jury trial waiver provision within the agreement is sufficiently prominent such that a party has knowingly waived any right to trial by jury. We believe that this is the case with respect to the deposit agreement and the ADSs. In addition, New York courts will not enforce a jury trial waiver provision in order to bar a viable setoff or counterclaim sounding in fraud or one which is based upon a creditor’s negligence in failing to liquidate collateral upon a guarantor’s demand, or in the case of an intentional tort claim (as opposed to a contract dispute), none of which we believe are applicable in the case of the deposit agreement or the ADSs. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with any provision of the federal securities laws. If you or any other holder or beneficial owner of ADSs brings a claim against us or the depositary in connection with such matters, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and/or the depositary. If a lawsuit is brought against us and/or the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in any such action, depending on, among other things, the nature of the claims, the judge or justice hearing such claims, and the venue of the hearing.
You may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts may be limited, because we are incorporated under Cayman Islands law and many of our directors reside outside of the United States.
We are an exempted company incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our Amended and Restated Memorandum and Articles of Association, referred to as our Articles of Association, the Companies Law (2020 Revision) (as amended) of the Cayman Islands, referred to as the Companies Law, and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary duties of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England and Wales, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. Similarly, the rights of our shareholders and the fiduciary duties of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States, and some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. As a Cayman Islands exempted company, we may not have standing to initiate a derivative action in a federal court of the United States. As a result, you may be limited in your ability to protect your interests if you are harmed in a manner that would otherwise enable you to sue in a United States federal court. In addition, shareholders of Cayman Islands companies may not have standing to initiate a shareholder derivative action in U.S. federal courts.
Shareholders of Cayman Islands exempted companies like us have very limited statutory rights under Cayman Islands law to inspect the corporate records of Cayman Islands exempted companies into which they are invested and have no statutory rights to obtain copies of registers of shareholders of Cayman Islands exempted companies. Although our shareholders may request access to our books and records, our directors have discretion under our Articles of Association to determine whether or not, and under what conditions, certain of our corporate records may be inspected by our shareholders. Under the Companies Law, shareholders are entitled to view our Articles of Association. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Certain corporate governance practices in the Cayman Islands, which is the jurisdiction of our incorporation, differ significantly from requirements for companies incorporated in other jurisdictions such as the United States. To the extent we choose to follow practice in the Cayman Islands with respect to corporate governance matters, our shareholders may be afforded less protection than they otherwise would under rules and regulations applicable to U.S. domestic issuers.
The Cayman Islands has no legislation specifically dedicated to the rights of investors in securities or statutorily defined private causes of action to investors in securities such as those found under the Securities Act of 1933, or
55
the Securities Act, or the Exchange Act. Subject to limited exceptions, under Cayman Islands law, a shareholder is not entitled to bring a derivative action against the board of directors. U.S.-style class action lawsuits are not recognized in the Cayman Islands, but groups of shareholders with identical interests may bring representative proceedings in a similar fashion.
As a result of all of the above, our shareholders may have more difficulty in protecting their interests in the face of actions taken by management, or members of the board of directors than they would as public shareholders of a company incorporated in the United States. For a discussion of significant differences between the provisions of the Companies Law of the Cayman Islands and the laws applicable to companies incorporated in the United States and their shareholders, see “Description of Share Capital and Articles of Association—Material Differences in Corporate Law.”
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
Our corporate affairs and the rights of holders of ordinary shares are governed by our Articles of Association, the Companies Law, and the common law of the Cayman Islands. Certain rights and responsibilities of our shareholders, ADS holders and members of our board of directors under Cayman Islands law are different from those that apply to a Delaware corporation.
Directors of Cayman Islands exempted companies are required to observe certain fiduciary duties. These fiduciary duties are owed to the Cayman Islands company and include the duty to act in the best interests of the company and the shareholders as a whole. However, the fiduciary duties of a director of a Cayman Islands exempted company may not be the same as the fiduciary duty of a director of a U.S. corporation.
In addition, controlling shareholders of U.S. corporations owe fiduciary duties to minority shareholders, while shareholders (including controlling shareholders) of Cayman Islands companies generally owe no fiduciary duties to the company or other shareholders.
The rights of our shareholders to bring shareholders’ suits against us or our board of directors under Cayman Islands law are much more limited than those of shareholders of a U.S. corporation. For example, under Cayman Islands law, a shareholder who wishes to bring a claim against a director would generally need to obtain permission from the Grand Court of the Cayman Islands, or Cayman Islands Court, to bring a derivative action, in the name of the company, against the director. This is because the director of a Cayman Islands exempted company owes duties to the company and not to individual shareholders. As a result, our shareholders, including holders of ADSs, may have more difficulty protecting their rights in connection with actions taken by our directors than they would as shareholders of a U.S. corporation.
Minority shareholders in a Cayman Islands exempted company have more limited rights than minority shareholders in a U.S. corporation in relation to mergers and similar transactions that the company may carry out. For example, if a merger under the Companies Law involving a Cayman Islands exempted company is approved by the requisite majority of shareholders, a dissenting minority shareholder would have the right to be paid the fair value of their shares (which, if not agreed between the parties, will, following the course of legal proceedings, be determined by the Cayman Islands Court) if the shareholders follow the statutorily prescribed procedure for initiating such proceedings, subject to certain exceptions. Such dissenter rights differ substantially from the appraisal rights, which would ordinarily be available to dissenting shareholders of Delaware corporations. Further, if a takeover offer is made to the shareholders of a Cayman Islands exempted company and accepted by holders of 90% of the shares affected, the offeror may require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Cayman Islands Court, but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion. A minority shareholder in this scenario would have no rights comparable to the appraisal rights which would generally be available to a dissenting shareholder of a U.S. corporation in similar circumstances. See the section of this prospectus titled “Description of Share Capital and Articles of Association” for a description of the principal differences between the provisions of Cayman Islands law applicable to us and the U.S. Delaware General Corporation Law relating to shareholders’ rights and protections.
This is a best efforts offering, no minimum number of ADSs or amount of proceeds is required to be sold, and we may not raise the amount of capital we believe is required for our business plans.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the ADSs in this offering. The placement agent has no obligation to buy any of the ADSs from us or to arrange for the purchase or sale of any specific number or dollar amount of the ADSs. There is no required minimum number of ADSs or amount of proceeds that must be sold as a condition to completion of this offering. Because there is no minimum
56
offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the ADSs offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to fund our business plans. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable to us.
57
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. These statements include all matters that are not related to present facts or current conditions or that are not historical facts, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate,” “expect,” “hope,” “plan,” “potential,” “possible,” “will,” “believe,” “estimate,” “intend,” “may,” “predict,” “project,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements regarding:
| ▪ | our plans to develop and commercialize elamipretide, SBT-272, SBT-550, and our other product candidates, and to identify additional product candidates; |
| ▪ | ongoing and planned clinical trials and preclinical studies for our product candidates, including the timing of initiation of these trials and studies and the timing of the anticipated results; |
| ▪ | our plan to submit a new drug application, or NDA, for Barth Syndrome; |
| ▪ | our plans to possibly enter into collaborations for the development of product candidates and the potential benefits of any collaboration; |
| ▪ | the timing of anticipated regulatory filings, meetings with regulatory agencies or regulatory approvals and plans and expectations for expedited regulatory review for our product candidates; |
| ▪ | the potential advantages and clinical utility of our product candidates; |
| ▪ | our commercialization, marketing and manufacturing capabilities and strategy; |
| ▪ | our intellectual property position and strategy; |
| ▪ | our estimates regarding the potential market opportunity for our product candidates; |
| ▪ | our expectations related to the use of proceeds from this offering; and |
| ▪ | our estimates regarding expenses, future revenue, capital requirements, sufficiency of our current cash and cash equivalents, expected proceeds from this offering and our need for and ability to obtain additional funding. |
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the “Risk Factors” section, that could cause actual results or events to differ materially from the forward-looking statements that we make. Except as context otherwise requires, our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make.
You should read this prospectus, the documents that we reference in this prospectus and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus are made as of the date of this prospectus and we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
This prospectus includes statistical and other industry and market data, which we obtained from our own internal estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that each of these studies, reports and publications is reliable, we have not independently verified market and industry data from third-party sources. While we believe our internal company research is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source.
58
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $ , based on an assumed public offering price of $ per ADS, which is the closing price of the ADSs on Nasdaq on , 2021, after deducting placement agent fees and estimated offering expenses payable by us. This is a best efforts offering with no minimum number of ADSs or amount of proceeds as a condition of closing and we may not sell all or any of the ADSs offered pursuant to this prospectus. As a result, we may receive significantly less in net proceeds. A $ increase or decrease in the assumed public offering price of $ per ADS, the last sale price of our ADSs reported on The Nasdaq Global Market on , 2021, would increase or decrease the net proceeds to us by approximately $, after deducting the placement agent fees and estimated offering expenses payable by us
As of June 30, 2021, we had cash and cash equivalents of $30.8 million. In May 2021, we entered into an amendment to the Development Funding Agreement with MVIL. Under the amendment, MVIL paid $8.0 million to us in May 2021 and has agreed to pay us additional funding of, (i) $11.0 million on or about October 1, 2021 and (ii) $11.0 million on or about December 1, 2021. We currently estimate that we will use the net proceeds from this offering, together with our existing cash and cash equivalents, and additional funding from the Development Funding Agreement, as follows:
| ▪ | approximately $ million to fund the continued clinical development of elamipretide; |
| ▪ | approximately $ million for principal, interest and other payments that we are obligated to make over the remaining life of our term loan facility with Hercules; and |
| ▪ | the balance for working capital and other general corporate purposes, including advancement of our pipeline of preclinical and discovery compounds. |
As of June 30, 2021, we had $5.5 million of outstanding principal under our term loan facility with Hercules. Borrowings under the term loan facility bear interest at a floating per annum rate equal to the greater of (i) the Wall Street Journal prime rate plus 5.50% or (ii) 9.50%. In April 2021, we entered into an amendment to the term loan facility with Hercules, pursuant to which the maturity date was extended from July 1, 2021 to January 1, 2022, and all principal payments were deferred until the maturity date.
This expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the impact of the COVID-19 pandemic on our business operations, the progress of our development of product candidates, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties, and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
We anticipate that, assuming our current operating plan, the net proceeds from this offering, together with our existing cash and cash equivalents of $30.8 million and the $22.0 million committed to be funded by MVIL in October and December 2021, under our Development Funding Agreement with MVIL, as amended, but excluding any additional milestone payments under the Development Funding Agreement, will enable us to fund our operating expenses and capital expenditure requirements through . Based on our current operating plan, we will require additional capital to advance elamipretide through pivotal trials for indications other than Barth and to advance our other product candidates through later-stage clinical development, as well as to commercialize any of our product candidates if we receive regulatory approval. We have based these estimates on assumptions that may prove to be wrong, including assumptions regarding the clinical trials necessary for FDA approval of our product candidates, and we could use our capital resources sooner than we currently expect. Due to the numerous risks and uncertainties associated with product development, including risks and uncertainties with respect to successful enrollment and completion of clinical trials, at this time we cannot reasonably estimate the amount of additional funding that will be necessary to complete the clinical development of any of our product candidates. If we receive regulatory approval for elamipretide or any of our other product candidates, we expect to incur significant commercialization expenses related to product manufacturing, sales, marketing and distribution. Accordingly, we will be required to obtain further funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources.
Pending use of the proceeds as described above, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities.
59
DIVIDEND POLICY
We have never declared or paid any cash dividends on our share capital. We intend to retain all of our future earnings, if any, to finance the growth and development of our business, and we do not intend to pay cash dividends to the holders of our ordinary shares for the foreseeable future. In addition, the terms of our existing loan and security agreement preclude us from paying cash dividends without the consent of our lender. However, if we do pay a cash dividend on our ordinary shares in the future, we may only pay such dividend out of our profits or share premium (subject to applicable solvency requirements) under Cayman Islands law.
60
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2021:
| ▪ | on an as adjusted basis giving additional effect to (i) the increase to our authorized share capital from 1,200,000,000 ordinary shares of a nominal or par value of US$0.0003 each to 1,600,000,000 ordinary shares of a nominal or par value of US$0.0003 each and (ii) the sale of ADSs offered in this offering, with an assumed public offering price of $ per ADS, which is the closing price of the ADSs on Nasdaq on , 2021, after deducting placement agent fees and estimated offering expenses payable by us. |
You should read this information in conjunction with our consolidated financial statements and the related notes incorporated by reference in this prospectus, the unaudited condensed consolidated financial statements and the related notes included elsewhere in this prospectus, the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section, the “Selected Consolidated Financial Data” section and other financial information contained or incorporated by reference in this prospectus. The as adjusted information set forth below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
| | AS OF JUNE 30, 2021 | |
| | ACTUAL | | | ACTUAL AS ADJUSTED | |
| | (in thousands, except share data) | |
Cash and cash equivalents | | $ | 30,766 | | | $ | | |
Long-term debt, less current portion(1) | | | — | | | | | |
Shareholders’ equity: | | | | | | | | |
Ordinary Shares, $0.0003 nominal or par value; 1,200,000,000 shares authorized,690,993,790 shares issued and outstanding, actual; 1,600,000,000 shares authorized, shares issued and outstanding, as adjusted | | | 207 | | | | | |
Additional paid-in capital | | | 555,027 | | | | | |
Accumulated deficit | | | (581,549 | ) | | | | |
Total shareholders’ equity (deficit) | | | (26,315 | ) | | | | |
Total capitalization | | $ | 4,451 | | | $ | | |
(1) | This excludes $5.5M of the debt that is classified as current portion of the term debt on the Balance Sheet as of June 30, 2021 |
The table above is based on 690,993,790 ordinary shares issued and outstanding as of June 30, 2021, which number excludes 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans as of that date, and does not include the following as of June 30, 2021:
| ▪ | 64,213,310 ordinary shares issuable upon exercise of share options outstanding, at a weighted-average exercise price of $ 0.51 per share; |
| ▪ | 13,913,257 ordinary shares reserved for issuance under our 2019 share incentive plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 13,734,600 ordinary shares (equivalent to 1,144,550 ADSs) issuable upon vesting of grants outstanding under our 2020 ADS plan; |
| ▪ | 9,162,420 ordinary shares (equivalent to 763,535 ADSs) reserved for issuance under our 2020 ADS plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 14,691,541 ordinary shares reserved for issuance under our 2019 employee share purchase plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 83,865,384 ordinary shares issuable upon exercise of outstanding warrants with a weighted average exercise price equal of $0.14 per share |
61
DILUTION
If you invest in the ADSs in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per ADS and the as adjusted net tangible book value per ADS after this offering.
Our net tangible book value as June 30, 2021 was approximately ($26.3) million, or ($0.038) per ordinary share and ($0.46) per ADS. Each ADS represents 12 ordinary shares. Our net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the number of ordinary shares outstanding as of June 30, 2021.
After giving effect to the sale of ADSs in this offering at a public offering price of $ per ADS, which is the closing price of the ADSs on Nasdaq on , 2021, after deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2021 would have been $ , or $ per ordinary share and $ per ADS. This amount represents an immediate increase in net tangible book value of $ per ordinary share and $ per ADS to our existing shareholders and an immediate dilution in net tangible book value of $ per ADS to new investors purchasing ADSs in this offering.
The following table illustrates dilution on a per ADS basis:
Assumed public offering price per ADS | | | | | | $ | | |
Net tangible book value per ADS as of June 30, 2021 | | $ | (0.46 | ) | | | | |
Increase in net tangible book value per ADS attributable to investors purchasing ADSs in this offering | | | | | | | | |
As adjusted net tangible book value per ADS after this offering | | | | | | | | |
Dilution per ADS to new investors | | | | | | $ | | |
The dilution information set forth in the table above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The table above is based on 690,993,790 ordinary shares issued and outstanding as of June 30, 2021, which number excludes 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans as of that date, and does not include the following as of June 30, 2021:
| ▪ | 64,213,310 ordinary shares issuable upon exercise of share options outstanding, at a weighted-average exercise price of $0.51 per share; |
| ▪ | 13,913,257 ordinary shares reserved for issuance under our 2019 share incentive plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 13,734,600 ordinary shares (equivalent to 1,144,550 ADSs) issuable upon vesting of grants outstanding under our 2020 ADS plan; |
| ▪ | 9,162,420 ordinary shares (equivalent to 763,535 ADSs) reserved for issuance under our 2020 ADS plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 14,691,541 ordinary shares reserved for issuance under our 2019 employee share purchase plan, which plan includes provisions that automatically increase the number of ordinary shares reserved for issuance thereunder each year; |
| ▪ | 83,865,384 ordinary shares issuable upon exercise of outstanding warrants with a weighted average exercise price equal of $0.14 per share. |
62
SELECTED CONSOLIDATED FINANCIAL DATA
We have derived the following selected consolidated statement of operations data for the fiscal years ended December 31, 2018, 2019 and 2020 and the selected consolidated balance sheet data as of December 31, 2019, and 2020 from our audited consolidated financial statements incorporated by reference in this prospectus. The consolidated statements of operations data for the six months ended June 30, 2020, and 2021 and the consolidated balance sheet data as of June 30, 2021 have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus and have been prepared on the same basis as the audited consolidated financial statements. In the opinion of management, the unaudited data reflects all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the financial information contained in those statements. Our historical results are not necessarily indicative of the results that should be expected for any future period. The selected consolidated financial data set forth below should be read together with our consolidated financial statements, as well as the sections of our Annual Report on Form 20-F for the fiscal year ended December 31, 2020, captioned “Item 5. Operating and Financial Review and Prospects” incorporated by reference in this prospectus.
| | YEAR ENDED DECEMBER 31, | | | SIX MONTHS ENDED JUNE 30, | |
| | 2018 | | | 2019 | | | 2020 | | | 2020 | | | 2021 | |
| | (in thousands, except share and per share data) | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenue | | $ | — | | | $ | 21,087 | | | $ | — | | | $ | — | | | $ | — | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 53,062 | | | $ | 44,604 | | | $ | 29,305 | | | $ | 17,252 | | | $ | 12,012 | |
General and administrative | | | 22,217 | | | | 22,315 | | | | 19,366 | | | | 9,703 | | | | 10,062 | |
Total operating expenses | | | 75,279 | | | | 66,919 | | | | 48,671 | | | | 26,955 | | | | 22,074 | |
Loss from operations | | | (75,279 | ) | | | (45,832 | ) | | | (48,671 | ) | | | (26,955 | ) | | | (22,074 | ) |
Other expense, net | | | (21,433 | ) | | | (25,896 | ) | | | (8,786 | ) | | | (954 | ) | | | (4,021 | ) |
Net loss attributable to ordinary shareholders | | $ | (96,712 | ) | | $ | (71,728 | ) | | $ | (57,457 | ) | | $ | (27,909 | ) | | $ | (26,095 | ) |
Net loss per share attributable to ordinary shareholders—basic and diluted | | $ | (1.41 | ) | | $ | (0.19 | ) | | $ | (0.10 | ) | | $ | (0.06 | ) | | $ | (0.04 | ) |
Weighted average ordinary shares used in net loss per share attributable to ordinary shareholders—basic and diluted | | | 68,476,149 | | | | 375,669,759 | | | | 556,169,255 | | | | 506,055,526 | | | | 663,833,037 | |
| | AS OF DECEMBER 31, | | | AS OF JUNE 30, | |
| | 2019 | | | 2020 | | | 2021 | |
| | (in thousands) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 50,768 | | | $ | 32,787 | | | $ | 30,766 | |
Working capital | | | 18,448 | | | | 13,991 | | | | 18,195 | |
Total assets | | | 52,743 | | | | 35,848 | | | | 32,258 | |
Total accumulated deficit | | | (497,997 | ) | | | (555,454 | ) | | | (581,549 | ) |
Total shareholders’ equity (deficit) | | | 17,267 | | | | (10,372 | ) | | | (26,315 | ) |
63
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our consolidated financial statements and related notes and other financial information incorporated by reference. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a clinical-stage biotechnology company focused on the discovery, development and commercialization of novel therapies for diseases involving mitochondrial dysfunction. Mitochondria, found in nearly every cell in the body, are the body’s main source of energy production and are critical for normal organ function. Dysfunctional mitochondria characterize a number of rare genetic diseases and many common age-related diseases, leading to devastating cardiac, ophthalmic and neurological symptoms. We believe our product candidates have significant potential to treat the cardiac, ophthalmic and neurological symptoms of both rare genetic and common age-related mitochondrial diseases. Our mission is to be the leader in mitochondrial medicine, and we have assembled a highly experienced management team, board of directors and group of scientific advisors to help us achieve this mission. Our leadership team has decades of experience leading drug discovery and development programs, including at GlaxoSmithKline, Novo Nordisk and Pfizer.
Our first clinical product candidate, elamipretide, is a small peptide that targets and binds reversibly to cardiolipin, an essential structural element of mitochondria, stabilizing the inner mitochondrial membrane under conditions of oxidative stress. This novel mechanism of action has shown potential clinical benefit in both rare genetic and common age-related ophthalmic and cardiac diseases entailing mitochondrial dysfunction. Elamipretide has been generally well tolerated in clinical trials with over 1,000 subjects systemically exposed to it to date.
We are studying elamipretide in the following indications:
| ▪ | Barth Syndrome, or Barth, an inherited cardiomyopathic disease, for which we have conducted a Phase 3 retrospective natural history-controlled trial and a Phase 2/3 clinical trial in the United States; and |
| ▪ | Geographic atrophy or GA, an advanced form of dry age-related macular degeneration, for which we conducted a Phase 1 clinical trial in the United States and are currently conducting a Phase 2b clinical trial in the United States. Our Phase 2b trial was fully enrolled in February 2021, and we expect data from this trial during the first half of 2022. |
We met with the Division of Cardiology and Nephrology at the U.S. Food and Drug Administration, or FDA, in November 2020, in February 2021 and April 2021 to discuss the clinical evidence to support a potential new drug application, or NDA, submission for Barth. We have had multiple recent communications with senior FDA officials in the Office of Cardiology, Hematology, Endocrinology and Nephrology and at the Division of Cardiology and Nephrology regarding our Barth syndrome program following the April 2021 meeting. We also received a petition signed by over 4,250 members of the Barth community requesting us to submit our NDA on the basis of our existing clinical data. The FDA expressed its view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review. The FDA recommended that we collect additional controlled clinical data in this indication prior to an NDA submission. In May 2021, we submitted a randomized withdrawal clinical trial protocol to FDA. This design was previously suggested by multiple FDA review divisions, including most recently the Division of Cardiology and Nephrology. After reviewing our submission, FDA concluded that neither the proposed randomized withdrawal trial nor any new clinical trial data from the patients remaining on OLE would be likely to add meaningfully to the evidence to support an NDA. Due to the ultra-rare nature of Barth syndrome, neither the Company nor the FDA to date has been able to identify a feasible trial design to generate additional data. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
64
We are evaluating the potential for additional clinical trials of elamipretide in the following cardiac, ophthalmic and neurological diseases in which mitochondrial dysfunction is implicated:
| ▪ | Duchenne cardiomyopathy, which is the heart muscle weakness associated with Duchenne’s muscular dystrophy, or DMD, is the leading cause of early mortality in this disease; |
| ▪ | Friedreich’s ataxia, or FRDA, which is associated with both cardiomyopathy and progressive decline in visual function; |
| ▪ | Leber’s hereditary optic neuropathy, or LHON, an inherited disease of central blindness, for which we have conducted a Phase 2 clinical trial in the United States; and |
| ▪ | Primary mitochondrial disease caused by mutations in nuclear genes that encode for mitochondrial proteins, or nPMD. |
Subject to discussions with the FDA, continued planning efforts and financing plans, we hope to initiate a clinical development program for elamipretide in DMD patients with cardiomyopathy during the first half of 2022, focusing primarily on cardiac endpoints. We plan to support an investigator-initiated Phase 2a open-label clinical trial of elamipretide assessing both visual and cardiac endpoints in FRDA, which is anticipated to commence enrollment in 2021, and we hope that results from this trial will help inform a pivotal trial design. We also plan to initiate a pivotal trial for elamipretide in patients with nPMD during the second half of 2021, subject to continued planning efforts and financing plans. Patients with nPMD comprised a prespecified subgroup of patients with nuclear DNA mutations in whom improvements were observed in our Phase 3 primary mitochondrial myopathy trial. Although we plan to initiate a Phase 3 global clinical trial for elamipretide in LHON, the initiation of this trial is subject to ongoing formulation studies expected to read out in early 2022, continued planning efforts, and financing plans.
Our second clinical product candidate, SBT-272, is a novel peptidomimetic that has been shown to increase adenosine triphosphate, or ATP, production and decrease levels of reactive oxygen species, or ROS, in dysfunctional mitochondria in preclinical studies. In early experiments, SBT-272 demonstrated higher mitochondrial uptake and greater concentrations in the brain relative to elamipretide. We are developing SBT-272 for rare neurological diseases involving mitochondrial dysfunction. Preliminary results from a Phase 1 clinical trial in healthy human volunteers completed during 2020 did not reach desired drug exposure levels. We are conducting subcutaneous dosing studies and plan to commence longer term toxicology studies in 2021 to support the initiation of a Phase 1 clinical trial in early 2022 and the potential initiation of a Phase 2 clinical trial in patients in late 2022. We have conducted and continue to conduct preclinical studies in neurological disease models to inform our decisions regarding our first Phase 2 indication.
We have discovered and own over 100 compounds, including SBT-272 and the SBT-550 family, that also target the mitochondria and form the basis of our broad proprietary pipeline of mitochondrial-targeted product candidates. We are evaluating compounds in the SBT-550 family for rare neurological indications. In addition, our internal discovery platform has generated a library of over 100 differentiated proprietary compounds which could have clinical benefit for diseases related to mitochondrial dysfunction and from which we plan to designate potential product candidates. We may also utilize certain of these compounds as part of our carrier program, in which they could potentially serve as scaffolds to deliver other beneficial compounds to the mitochondria.
As of December 31, 2020, we held exclusive worldwide rights or an option for exclusive worldwide rights under 393 issued patents and 188 patent applications to protect our platform and product candidates. We have exclusive worldwide rights to elamipretide and a second product candidate, SBT-20, both of which we licensed from Cornell Research Foundation, Inc., a subsidiary of Cornell University , or Cornell, and Institut de recherches cliniques de Montréal, or the IRCM, in 2006. The unique mitochondrial activity of elamipretide was first published in The Journal of Biological Chemistry in August 2004. Since licensing elamipretide and SBT-20, we and our collaborators have published approximately 100 peer-reviewed articles highlighting the activity of our compounds in several disease models, including heart failure, kidney disease, skeletal muscle weakness, diabetic retinopathy and neurodegenerative diseases. Our compounds have been evaluated in preclinical and clinical studies at academic and clinical institutions, including Boston Children’s Hospital, Charité Berlin, Children’s Hospital of Philadelphia, Columbia University, Cornell University, Duke University, Johns Hopkins University, Massachusetts General Hospital, Mayo Clinic, Stanford University, University of California Los Angeles, University of California San Diego, University of Colorado and University of Washington.
As of June 30, 2021, we had an accumulated deficit of $581.5 million. Our net loss was $96.7 million, $71.7 million and $57.5 million for the years ended December 31, 2018, 2019 and 2020, respectively, and $26.1 million for the six months ended June 30, 2021. We have incurred significant net operating losses in every year since our
65
inception and expect to continue to incur increasing net operating losses and significant expenses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase significantly as we:
| ▪ | continue to advance our clinical programs and initiate additional clinical programs; |
| ▪ | continue our current research programs and development activities; |
| ▪ | seek to identify additional research programs and additional product candidates; |
| ▪ | initiate preclinical testing and clinical trials for any product candidates we identify; |
| ▪ | develop, maintain, expand and protect our intellectual property portfolio; |
| ▪ | hire additional research, clinical and scientific personnel; and |
| ▪ | incur additional costs associated with operating as a public company, including expanding our operational, finance and management teams. |
We do not expect to generate revenues from product sales unless and until we successfully complete development and obtain regulatory approval for a product candidate, which is subject to significant uncertainty. We currently use contract research organizations, or CROs, and contract manufacturing organizations, or CMOs, to carry out our preclinical and clinical development activities, and we do not yet have a commercial organization. If we obtain regulatory approval for our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution. Accordingly, we may seek to fund our operations through public or private equity or debt financings or other sources, including strategic collaborations. We may, however, be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, if at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop our current product candidates, or any additional product candidates, if developed. As of June 30, 2021, we had cash and cash equivalents of $30.8 million. We believe that the net proceeds from this offering, together with our existing cash and cash equivalents and the $22.0 million committed to be funded by Morningside Venture (I) Investments Limited, or MVIL, in October and December 2021 under our Development Funding Agreement with MVIL, as amended, but exclusive of any other milestone payments under such Development Funding Agreement, will enable us to fund our operating expenses and capital expenditure requirements through .
Beyond that point, we will need to raise additional capital to finance our operations, which cannot be assured. We have concluded that this circumstance raises substantial doubt about our ability to continue as a going concern within one year after the issuance date of our consolidated financial statements for the six months ended June 30, 2021. See Note 1 to our unaudited interim condensed consolidated financial statements appearing at the end of this prospectus for additional information on our assessment.
Financial Overview
Revenue
We have not generated any revenue from product sales and do not expect to do so in the near future. We expect that any revenue will be less than our expenses for the foreseeable future and that we will experience increasing losses as we continue our development of, and seek regulatory approvals for, our product candidates and begin to commercialize any approved products. Our ability to generate revenues for any product candidate for which we receive regulatory approval will depend on numerous factors, including competition, commercial manufacturing capability and market acceptance of our products.
In 2019 we received a non-refundable upfront payment of $15.0 million under the terms of the option agreement and $15.0 million under an equity agreement with Alexion Pharmaceutical, Inc or Alexion. We recognized revenue as it relates to the Alexion arrangement under Accounting Standards Codification Topic 606, Revenue from Contracts with Customers, or ASC 606. In accordance with ASC 606, the option agreement and the equity agreement were deemed to be one arrangement, and any premium paid on the equity agreement was deemed to be included in the transaction price and allocated to the performance obligation identified.
In 2019, revenue represents non-refundable upfront payments under the Alexion arrangement that were recognized in full in accordance with ASC 606 as we completed our performance obligation in 2019. Alexion terminated the option agreement in January 2020 and, as such, no additional revenue was recognized under the Alexion arrangement.
66
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including development of our preclinical and clinical product candidates, which include:
| ▪ | employee-related expenses, including salaries, benefits and share-based compensation expense; |
| ▪ | expenses incurred under agreements with CROs, CMOs and independent contractors that conduct research and development, preclinical and clinical activities on our behalf; |
| ▪ | costs of purchasing lab supplies and non-capital equipment used in our preclinical activities and in manufacturing preclinical study and clinical trial materials; |
| ▪ | consulting, licensing and professional fees related to research and development activities; and |
| ▪ | facility costs, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other supplies. |
We expense research and development costs as incurred. We recognize costs for certain development activities, such as preclinical studies and clinical trials, based on an evaluation of the progress to completion of specific tasks using information provided to us by our vendors such as patient enrollment or clinical site activations for services received and efforts expended.
Research and development activities are central to our business model. We expect research and development costs to increase significantly for the foreseeable future as our current development programs progress and new programs are added.
We track certain external research and development expenses for our lead product candidates. We manage certain activities, such as contract research and manufacturing of our product candidates and our discovery programs, through our third-party vendors and have captured the costs of these activities on an individual product basis from our financial records. We use our employee, consultant and infrastructure resources across our development programs and do not track and do not allocate the cost of these activities on a program-by-program basis. The following summarizes our research and development expenses:
| | YEAR ENDED DECEMBER 31, | | | SIX MONTHS ENDED JUNE 30, | |
| | 2019 | | | 2020 | | | 2020 | | | 2021 | |
Product candidate expenses: | | | | | | | | | | | | | | | | |
Elamipretide | | $ | 20,633 | | | $ | 15,919 | | | $ | 9,324 | | | $ | 4,915 | |
SBT-20 | | | 2 | | | | — | | | | — | | | | — | |
SBT-272 | | | 2,143 | | | | 1,851 | | | | 828 | | | | 1,136 | |
SBT-550 | | | — | | | | — | | | | — | | | | 203 | |
Total costs directly allocated to product candidates | | | 22,778 | | | | 17,770 | | | | 10,152 | | | | 6,254 | |
Expenses not directly allocated to product candidates: | | | | | | | | | | | | | | | | |
Research and development programs | | | 1,615 | | | | 1,026 | | | | 765 | | | | 660 | |
Consultants and professional expenses | | | 6,547 | | | | 1,965 | | | | 986 | | | | 1,315 | |
Employee expenses including cash compensation, benefits and share-based compensation | | | 13,664 | | | | 8,544 | | | | 5,349 | | | | 3,783 | |
Total expenses not directly allocated to product candidates | | | 21,826 | | | | 11,535 | | | | 7,100 | | | | 5,758 | |
Total research and development expenses | | $ | 44,604 | | | $ | 29,305 | | | $ | 17,252 | | | $ | 12,012 | |
Because of the numerous risks and uncertainties associated with product development, we cannot determine with certainty the duration and completion costs of the current or future preclinical studies and clinical trials or if, when, or to what extent we will generate revenues from the commercialization and sale of our product candidates. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs and timing of preclinical studies and clinical trials and development of our product candidates will depend on a variety of factors, including:
| ▪ | successful completion of preclinical studies and investigational new drug-enabling studies; |
| ▪ | successful enrollment in and completion of clinical trials; |
67
| ▪ | receipt of marketing approvals from applicable regulatory authorities; |
| ▪ | establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
| ▪ | obtaining and maintaining patent and trade secret protection and non-patent exclusivity; |
| ▪ | launching commercial sales of the product, if and when approved, whether alone or in collaboration with others; |
| ▪ | acceptance of the product, if and when approved, by patients, the medical community and third-party payors; |
| ▪ | effectively competing with other therapies and treatment options; |
| ▪ | continued acceptable safety profile following approval; |
| ▪ | enforcing and defending intellectual property and proprietary rights and claims; and |
| ▪ | achieving desirable therapeutic properties for the intended indications. |
A change in the outcome of any of these factors could mean a significant change in the costs and timing associated with the development of our current and future preclinical and clinical product candidates. For example, if the FDA or other regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of clinical development, or if we experience significant delays in execution of or enrollment in any of our preclinical studies or clinical trials, we could be required to expend significant additional financial resources and time on the completion of preclinical and clinical development.
General and Administrative Expenses
General and administrative expenses consist primarily of employee-related expenses, including salaries, benefits and share-based compensation for personnel in executive, finance, pre-commercial, facility operations and administrative functions. Significant costs are incurred in our pre-commercial activities including market research, public relations, patient advocacy, advisory boards and conferences and professional consulting. Other significant costs include facility costs not otherwise included in research and development expenses, legal fees relating to intellectual property and patent prosecution and maintenance, other legal fees, insurance for directors and officers and fees for accounting, tax and consulting services.
We anticipate that our general and administrative expenses will increase in the future to support continued research and development activities, potential commercialization of our product candidates and increased costs of operating as a public company. These increases will likely include costs related to the hiring of additional personnel and fees to outside consultants, attorneys and accountants, among other expenses. We expect the increased costs associated with being a public company to include expenses related to services associated with maintaining compliance with the requirements of Nasdaq and the SEC, director and officer insurance and investor and public relations costs.
Other Expense, Net
Other income (expense), net, primarily consists of amortization of debt discount and interest expense incurred on convertible notes payable and incurred on our term loan facility, interest income earned on cash and cash equivalents and changes in the fair value of our derivative liability.
68
Results of Operations
Comparison of the Six Months Ended June 30, 2020 and 2021
The following table summarizes our results of operations for the six months ended June 30, 2020, and 2021, together with the dollar change in those items:
| | SIX MONTHS ENDED JUNE 30, | | | DOLLAR | |
| | 2020 | | | 2021 | | | CHANGE | |
| | (in thousands) | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | $ | 17,252 | | | $ | 12,012 | | | $ | (5,240 | ) |
General and administrative | | | 9,703 | | | | 10,062 | | | | 359 | |
Total operating expenses | | | 26,955 | | | | 22,074 | | | | (4,881 | ) |
Loss from operations | | | (26,955 | ) | | | (22,074 | ) | | | 4,881 | |
Other income (expense): | | | | | | | | | | | | |
Interest income | | | 137 | | | | 2 | | | | (135 | ) |
Interest expense | | | (1,091 | ) | | | (488 | ) | | | 603 | |
Gain (loss) from remeasurement of derivative liability | | | — | | | | (3,535 | ) | | | (3,535 | ) |
Total other expenses, net | | | (954 | ) | | | (4,021 | ) | | | (3,067 | ) |
Net loss | | $ | (27,909 | ) | | $ | (26,095 | ) | | $ | 1,814 | |
Research and Development Expenses
R&D expenses were $12.0 million for the six months ended June 30, 2021, compared to $17.3 million for the same period in 2020. This decrease was due to a net decrease of $4.0 million in clinical costs primarily driven by the closeout of our Primary Mitochondrial Myopathy development efforts, a decrease of $1.5 million in employee related costs due to the strategic repositioning in 2020 offset in part by a $0.3 million increase in consultant costs and a decrease of $0.9 million in manufacturing cost offset in part by a $0.6 million increase in preclinical costs and a $0.2 million net increase in regulatory costs.
General and Administrative Expenses
G&A expenses were $10.1 million for the six months ended June 30, 2021, compared to $9.7 million for the same period in 2020. The increase was primarily attributable a $0.4 million increase in costs of insurance and $0.3 million increase in precommercial activities offset in part by a net decrease of $0.3 million in employee and consultant related costs.
Other Expense
Other expense was $4.0 million for the six months ended June 30, 2021, compared to other expense of $1.0 million for the same period in 2020. Other expense in 2021 consisted of a $3.5 million loss due to the change in fair value of the derivative liability and $0.5 million in interest expense. Other expense in 2020 consisted of a $1.1 million in interest expense offset by $0.1 million in interest income.
Comparison of the Years Ended December 31, 2019 and 2020
A discussion of our results of operations for the years ended December 31, 2019, and 2020 may be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations—Comparison of the Years Ended December 31, 2020 and 2019” in our Annual Report on Form 20-F for the fiscal year ended December 31, 2020 incorporated by reference in this prospectus.
Liquidity and Capital Resources
Overview
We have funded our operations from inception through June 30, 2021, primarily through aggregate gross proceeds from the sale of Series A convertible preferred shares, the issuance of convertible promissory notes, a term loan, the sale of ordinary shares, the sale and issuance of ADSs, proceeds from the Development Funding Agreement, as well as gross proceeds received from Alexion. As of June 30, 2021, we had cash and cash equivalents of $30.8 million.
69
Indebtedness
Term Loan Facility. On June 30, 2017, we entered into a loan and security agreement with Hercules Capital, Inc., or Hercules, which we refer to as the Term Loan Facility. The Term Loan Facility was amended in March, July and October of 2018 and March and October of 2019, July 2020, and April 2021. We have borrowed an aggregated principal amount of $20.0 million and have an outstanding principal balance of $5.5 million as of June 30, 2021.
Borrowings under the Term Loan Facility bear interest at a floating per annum rate equal to the greater of (i) the Wall Street Journal prime rate plus 5.50% or (ii) 9.50%. In an event of default, as defined in the loan and security agreement, the interest rate applicable to borrowings under such agreement will be increased by 4.0%. Interest payments are due monthly in arrears. Under the Term Loan Facility, as amended, we make interest only payments through December 31, 2021, the total principal and final end of term charge is payable on the maturity date of January 1, 2022.
We may voluntarily prepay all, but not less than all, of the outstanding principal at any time prior to the maturity date, subject to a prepayment fee, of 0.5% of the outstanding principal at the time the prepayment is made. On January 1, 2021, we paid the end of term charge of $1.3 million. An additional end of term charge of $0.2 million is due upon the earlier to occur of July 1, 2021, the acceleration or prepayment of all outstanding principal, or the termination of the Term Loan Facility. An additional end of term charge of $0.1 million is due upon the earlier to occur of January 1, 2022, the acceleration or prepayment of all outstanding principal, or the termination of the Term Loan Facility.
Borrowings under the Term Loan Facility are secured by a first priority lien on all of our assets, excluding our intellectual property. We have agreed to a negative pledge on our intellectual property. The Term Loan Facility contains customary events of default and affirmative and negative covenants, including restrictions on our ability to pay dividends and incur additional debt, but does not contain any financial covenants. An event of default had not occurred as of June 30, 2021.
In connection with our entry into the Term Loan Facility, we issued to Hercules a warrant to purchase our ordinary shares. See “Description of Share Capital and Articles of Association—Warrant” for a description of the warrant.
Lincoln Park Agreement. In June 2020, we entered into a $20.0 million purchase agreement, or the Purchase Agreement, together with a registration rights agreement, with Lincoln Park Capital Fund, LLC, or Lincoln Park. Under the terms and subject to the conditions of the Purchase Agreement, we have the right to sell to Lincoln Park, and Lincoln Park is obligated to purchase, up to $20.0 million of our ordinary shares, subject to certain limitations, from time to time, over the 36-month period commencing on June 22, 2020. As of June 30, 2021, pursuant to the Purchase Agreement a total of 25,380,000 ordinary shares were sold to Lincoln Park for aggregate gross proceeds totaling $3.2 million.
ATM Offering Agreement. In August 2020, we and Wainwright, entered into an At The Market Offering Agreement pursuant to which we may offer and sell, from time to time, through Wainwright, ADSs, each representing 12 ordinary shares, with a nominal or par value of $0.0003 per share. We have no obligation to sell any ADSs pursuant to the agreement and may at any time suspend sales pursuant to the agreement. Each party may terminate the agreement at any time without liability. As of June 30, 2021, we have not sold any shares under the ATM Offering Agreement, and we suspended our ability to sell under the facility as of November 19, 2020.
Development Funding Agreement. In October 2020, we entered into the Development Agreement, under which MVIL agreed to provide funding to support our efforts to secure regulatory approval for elamipretide and to develop elamipretide for the treatment of Barth, dry AMD, FRDA, DMD, nPMD and LHON. We received initial cash proceeds of $20.0 million pursuant to the Development Funding Agreement and in February 2021 we received a milestone payment of $10.0 million. We may receive up to an additional $5.0 million upon the completion of a final milestone. We may agree to add additional investors to the Development Funding Agreement, subject to the prior written consent of MVIL, on the same terms and subject to the same conditions as MVIL’s initial commitments, prior to the completion of the final milestone. We are obligated to make success payments to MVIL upon receipt of certain regulatory approvals of elamipretide in the designated indications. In May, 2021, we amended the Development Funding Agreement, and under the amended Development Funding Agreement MVIL paid $8.0 million to us in May 2021 and has agreed to pay us additional funding of (i) $11.0 million on or about October 1, 2021 and (ii) $11.0 million on or about December 1, 2021. The amended Development Funding Agreement is subject to the same terms and condition as MVIL’s initial commitment.
Registered Direct Offerings. In November 2020, we entered into a Securities Purchase Agreement with certain institutional investors for a registered public offering, or the 2020 Public Offering, of an aggregate of 2,844,446
70
ADSs at a public offering price of $1.125 per ADS for net proceeds of approximately $2.6 million. In February 2021, we entered into a Securities Purchase Agreement with certain institutional investors for a registered direct offering, or the 2021 Public Offering, of an aggregate of 2,339,000 ADSs at a public offering price of $2.00 per ADS for net proceeds of approximately $4.1 million.
Cash Flows
The following table provides information regarding our cash flows for each of the periods presented:
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Net cash (used in) provided by: | | | | | | | | |
Operating activities | | $ | (32,900 | ) | | $ | (23,280 | ) |
Investing activities | | | (14 | ) | | | 245 | |
Financing activities | | | 13,980 | | | | 21,014 | |
Net increase (decrease) in cash and cash equivalents | | $ | (18,934 | ) | | $ | (2,021 | ) |
Net Cash Used in Operating Activities
The use of cash for operating activities in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital.
Net cash used in operating activities decreased by $9.7 million to $23.3 million during the six months ended June 30, 2021 from $32.9 million during the six months ended June 30, 2020. Cash used in operating activities during the six months ended June 30, 2021, consisted of our net loss of $26.1 million, partially offset by non-cash charges of $5.9 million which includes, $3.5 million loss driven by change in fair value of the derivative liability, $2.2 million in shared-based compensation expense, and $0.2 million in other non-cash items. Changes in operating assets and liabilities included $4.3 million decrease in accounts payable, accrued expenses and other current liabilities offset in part by a $1.2 million decrease in prepaid expenses and other current assets. Cash used in operating activities during the six months ended June 30, 2020, consisted of our net loss of $27.9 million, partially offset by non-cash charges of $2.5 million which includes $2.0 million in share-based compensation, $0.2 million in amortization of the debt discount, $0.2 million in non-cash interest expense and $0.1 million in in depreciation and amortization. Changes in operating assets and liabilities included $8.0 million in decreases in accounts payable, accrued expenses and other current liabilities offset in part by a $0.5 million decrease in prepaid expenses and other current assets.
Net Cash Used in Investing Activities
Net cash used in investing activities increased by $0.2 million during the six months ended June 30, 2021 from $14,000 during the six months ended June 30, 2020. The increase was primarily driven by refund of a security deposit from the previous facility lease.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $21.0 million during the six months ended June 30, 2021, compared to $14.0 million during the six months ended June 30, 2020. Cash provided by financing activities during the six months ended June 30, 2021, was primarily attributable to the receipt of $18.0 million from the development funding agreement milestone payment and additional funding under the amended development funding agreement and $6.9 million in connection with the issuance of ordinary shares as part of our financing activities, offset in part by $3.6 million of principal payments made on the Term Loan Facility and $0.3 million of payment of offering cost.
Comparison of the Years Ended December 31, 2019 and 2020
A discussion of our results of cash flows for the years ended December 31, 2019 and 2020 may be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and capital resources—Cash Flows—Comparison of the Years Ended December 31, 2020 and 2019” in our Annual Report on Form 20-F for the fiscal year ended December 31, 2020 incorporated by reference in this prospectus.
Funding Requirements
We expect our expenses to increase in connection with our ongoing clinical activities, particularly as we continue to develop and conduct clinical trials with respect to elamipretide and new compounds, including our ongoing and planned clinical trials; advance the development of pipeline programs; initiate new research and preclinical
71
development efforts; and seek marketing approval for any product candidates that we successfully develop. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to establishing sales, marketing, distribution and other commercial infrastructure to commercialize such products. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
As of June 30, 2021, we had cash and cash equivalents of $30.8 million. We believe that the net proceeds from this offering, together with our existing cash and cash equivalents and the $22.0 million committed to be funded by MVIL in October and December 2021, under our Development Funding Agreement with MVIL, as amended, but exclusive of any other milestone payments under such Development Funding Agreement, will enable us to fund our operating expenses and capital expenditure requirements through . Our capital expenditures for the six months ended June 30, 2021 and for the years ended December 31, 2020 and 2019 amounted to $0.01 million, $0.1 million and $0.13 million, respectively.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect, and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with the research, development and commercialization of our product candidates, we are unable to estimate the exact amount of our operating capital requirements. Our future capital requirements will depend on many factors, including:
| ▪ | the scope, progress, timing, costs and results of our current and future clinical trials; |
| ▪ | research and preclinical development efforts for any future product candidates that we may develop; |
| ▪ | our ability to enter into and the terms and timing of any collaborations, licensing agreements or other arrangements; |
| ▪ | the number of future product candidates that we pursue and their development requirements; |
| ▪ | the outcome, timing and costs of seeking regulatory approvals; |
| ▪ | costs of commercialization activities for any of our product candidates that receive marketing approval to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities; |
| ▪ | subject to receipt of marketing approval, revenue, if any, received from commercial sales of our current and future product candidates; |
| ▪ | our headcount growth and associated costs if and as we expand our research and development and establish a commercial infrastructure; |
| ▪ | costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| ▪ | costs of operating as a public company. |
Identifying potential product candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes many years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
Until such time, if ever, that we can generate substantial revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing investors will be diluted, and the terms of the securities we issue may include liquidation or other preferences that adversely affect the rights of holders of ADSs. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through future collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds
72
through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which we have prepared in accordance with GAAP. We believe that several accounting policies are important to understanding our historical and future financial performance. We refer to these policies as critical because these specific areas generally require us to make judgments and estimates about matters that are uncertain at the time we make the estimate, and we could have used different estimates which also would have been reasonable. On an ongoing basis, we evaluate our estimates and judgments, including those described in greater detail below. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements included in our Annual Report on Form 20-F for the fiscal year ended December 31, 2020 and incorporated by reference or appearing elsewhere in this prospectus, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Accrued Research and Development Expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed for us and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our consolidated financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments, if necessary. Examples of estimated accrued research and development expenses include fees paid to:
| ▪ | CROs in connection with clinical trials; |
| ▪ | CMOs with respect to clinical materials, intermediates, drug substance and drug product; |
| ▪ | vendors in connection with research and preclinical development activities; and |
| ▪ | vendors related to manufacturing, development and distribution of clinical supplies. |
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with CROs that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the clinical expense. Payments under some of these contracts depend on factors such as the successful enrollment of subjects and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed, enrollment of subjects and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid expense accordingly. To date, there have been no material differences from our estimates to the amounts actually incurred.
Development Derivative Liability
Under the Development Funding Agreement, MVIL paid us $20.0 million upon execution of the Agreement, and in February 2021, paid us $10.0 million upon completing enrollment of our ReCLAIM 2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD, or Tranche 2 Milestone Event. MVIL has also agreed to pay us $5.0 million within 15 days of our submission of a new drug application to the FDA for elamipretide for the treatment of Barth, or Tranche 3 Milestone Event. Upon receipt of funding for each Tranche 2 Milestone Event and Tranche 3 Milestone Event, we are required to issue a warrant, or the Future Warrants, exercisable for ordinary shares at an exercise price that is 115% of the implied price of our ordinary shares on the date of issuance, with such number of ordinary shares being equal to the quotient of 30% of the amount of each funding received divided by the exercise price. Upon execution of the Development Funding Agreement, we issued a warrant to MVIL exercisable for 46,153,846 ordinary shares or the Initial Warrant, at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The Initial Warrant was immediately exercisable and has a term of three years.
73
The Development Funding Agreement is presented as a derivative liability on our consolidated balance sheet as of December 31, 2020 and June 30, 2021. The success payments feature in the Development Funding Agreement meets the criteria for derivative accounting as it has multiple underlyings, payment provisions, nominal initial net investment and a net settlement provision. The Development Funding Agreement also includes provisions that allow for the issuance of Future Warrants upon receipt of additional funding. At inception, the Future Warrants were not considered “fixed-for-fixed” as the exercise price and number of ordinary shares are dependent on the date and share price at the date of issuance. As such the Future Warrants were deemed to be liability classified. The Development Funding Agreement and the Future Warrants are considered to be a hybrid instrument recorded as the development derivative liability on our consolidated balance sheets.
At the inception of the arrangement, we identified two units of account (i) the Initial Warrant and (ii) derivative liability, which included the success payments feature and the Future Warrants. The derivative liability was initially recorded at the value of $18.1 million, the estimated fair value at inception of the arrangement, and is remeasured at fair value at each reporting date. The remaining amount of $1.9 million of the initial cash received of $20.0 million was attributed to the Initial Warrant, which met the criteria for equity classification.
In February 2021, we received a milestone payment of $10.0 million from MVIL, in accordance with Development Agreement, upon completion of enrollment for the ReCLAIM-2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD. We issued a warrant to MVIL exercisable for 18,750,000 ordinary shares at an exercise price of $0.16 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The warrant was immediately exercisable and has a term of three years. Upon the receipt of the milestone payment, the warrant met the criteria for equity classification as it was considered “fixed-for-fixed” and was recognized as a component of additional paid in capital and was not remeasured.
On May 17, 2021, we and MVIL amended the Development Agreement. Under the amended Development Agreement, we received $8 million in May 2021 and will receive an additional (i) $11.0 million on or about October 1, 2021 and (ii) $11.0 million on or about December 1, 2021. We are required to issue a warrant to MVIL, in connection with each such additional funding, under the same terms and conditions as the initial commitment. In connection with the funding received in May 2021, we issued a warrant to MVIL exercisable for 18,461,538 ordinary shares at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The warrant was immediately exercisable and has a term of three years. Upon the receipt of the funding tranche, the warrant met the criteria for equity classification as it was considered “fixed-for-fixed” and was recognized as a component of additional paid in capital and was not remeasured.
The development derivative liability is considered a level 3 fair value measurement, as it is dependent upon significant unobservable inputs. The derivative is valued using a scenario-based discounted cash flow method, whereby each scenario makes assumptions regarding the probability and timing of cash flows, and the present value of such cash flows is determined valued using a risk-adjusted discount rate. See Note 3 in our consolidated financial statements included in our Annual Report on Form 20-F for the fiscal year ended December 31, 2020 and our unaudited interim condensed consolidated financial statements appearing at the end of this prospectus for additional information.
If actual results or events differ materially from the estimates, judgments and assumptions used by us in applying these policies, our reported financial condition and results of operations could be materially affected.
Contractual Obligations
The following table summarizes our outstanding contractual obligations as of payment due date by period at December 31, 2020:
| | TOTAL | | | LESS THAN 1 YEAR | | | 1 TO 3 YEARS | | | 3 TO 5 YEARS | | | MORE THAN 5 YEARS | |
| | (in thousands) | |
Operating leases | | $ | 265 | | | $ | 139 | | | $ | 126 | | | $ | — | | | $ | — | |
Term Loan Facility (1) | | | 10,565 | | | | 10,565 | | | | — | | | | — | | | | — | |
Total | | $ | 10,830 | | | $ | 10,704 | | | $ | 126 | | | $ | — | | | $ | — | |
(1) Represents principal amount of the outstanding term loan as of December 31, 2020 as well as an end of term charge of $1.5 million due under the Term Loan Facility, of which $1.3 million was paid on January 1, 2021. The loan is subject to variable interest that will be calculated as payments become due.
74
We enter into contracts in the normal course of business with CROs and clinical sites for the conduct of clinical trials, professional consultants and other vendors for clinical supply, manufacturing or other services. These contracts are not included in the table above as they provide for termination on notice and, therefore, are cancelable contracts and do not include any minimum purchase commitments.
We have entered into several license agreements with Cornell Research Foundation, Inc., a subsidiary of Cornell University, or Cornell, and Institut de recherches cliniques de Montréal, or the IRCM, pursuant to which Cornell and IRCM granted us an exclusive, worldwide rights under patents related to elamipretide, SBT-20 and other technology. In connection with the licenses granted under the original Cornell agreement, we issued Cornell 666,667 ordinary shares. With respect to the other Cornell license agreements, we paid Cornell upfront license fees of $60,000, annual fees of approximately $60,000 and are obligated to pay Cornell royalties on net sales, if any, by us and our sublicensees of any licensed product. Subject to specified reductions and royalty offsets, such royalties are calculated as a tiered, low-to-mid single digit percentage of net sales of licensed products under each of the Cornell license agreements, except that for licensed products under the original Cornell agreement, such royalties are calculated as a tiered, low single-digit to sub-teen double-digit percentage of net sales, depending on patent coverage, amount of net sales and type of licensed product. Our obligation to pay royalties as to any licensed product extends until the later of the expiration of the last-to-expire valid claim of any licensed patent covering such licensed product or 15 years after the date of our first commercial sale of such licensed product. If a licensed product is covered by licenses granted under the original Cornell agreement and another Cornell license agreement, then, for each unit of product, royalties will only be due under the original Cornell agreement.
We are obligated to pay Cornell a low double-digit percentage of specified payments we receive in connection with granting a sublicense under the Cornell license agreements. We have also agreed to reimburse Cornell for its out-of-pocket expenses incurred in preparing, filing, prosecuting and maintaining the licensed patents, except for any licensed patents as to which we elect to waive our licensed rights. We also have agreed to pay Cornell annual license maintenance fees in dollars in the mid-five-digits for the original Cornell agreement, and mid-four-digits for each of the other Cornell license agreements starting on the date specified in each such agreement, in all cases until the first commercial sale of a specified type of licensed product under such agreement.
Off-balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under the applicable regulations of the SEC.
Recent Accounting Pronouncements
Note 2, “Summary of Significant Accounting Policies,” in the accompanying notes to the interim condensed consolidated financial statements includes a discussion of recent accounting pronouncements. There were no new accounting pronouncements adopted during 2020 that had a material effect on our consolidated financial statements.
Emerging Growth Company Status
The Jumpstart Our Business Startups Act of 2012 permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will not be subject to the same new or revised accounting standards as other public companies. As a result, our financial statements may not be comparable to the financial statements of reporting companies that are required to comply with the effective dates for new or revised accounting standards that are otherwise applicable to public companies.
Qualitative and Quantitative Disclosures about Market Risk
We are minimally exposed to market risk related to changes in interest rates. As of June 30, 2021, we had cash and cash equivalents of $30.8 million, consisting primarily of U.S. Treasury funds. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our cash equivalents are held in short-term treasury funds. We do not believe we are materially at risk to sudden drops in interest rates based on the amounts subject to these potential changes.
Our Term Loan Facility has a floating per annum rate equal to the greater of (i) the Wall Street Journal prime rate plus 5.50% or (ii) 9.50%, which exposes us to market interest rate risk when we have outstanding borrowings. As of June 30, 2021, we had $5.5 million of outstanding borrowings under the Term Loan Facility. Assuming our outstanding debt remains constant for an entire year and the applicable annual interest rate increases by 1%, our annual interest expense would increase by $0.1 million.
75
BUSINESS
Overview
We are a clinical-stage biotechnology company focused on the discovery, development and commercialization of novel therapies for diseases involving mitochondrial dysfunction. Mitochondria, found in nearly every cell in the body, are the body’s main source of energy production and are critical for normal organ function. Dysfunctional mitochondria characterize a number of rare genetic diseases and many common age-related diseases, leading to devastating cardiac, ophthalmic and neurological symptoms. We believe our product candidates have significant potential to treat the cardiac, ophthalmic and neurological symptoms of both rare genetic and common age-related mitochondrial diseases. Our mission is to be the leader in mitochondrial medicine, and we have assembled a highly experienced management team, board of directors and group of scientific advisors to help us achieve this mission. Our leadership team has decades of experience leading drug discovery and development programs, including at GlaxoSmithKline, Novo Nordisk and Pfizer.
Our first clinical product candidate, elamipretide, is a small peptide that targets and binds reversibly to cardiolipin, an essential structural element of mitochondria, stabilizing the inner mitochondrial membrane under conditions of oxidative stress. This novel mechanism of action has shown potential clinical benefit in both rare genetic and common age-related ophthalmic and cardiac diseases entailing mitochondrial dysfunction. Elamipretide has been generally well tolerated in clinical trials with over 1,000 subjects systemically exposed to it to date.
We are studying elamipretide in the following indications:
| ▪ | Barth Syndrome, or Barth, an inherited cardiomyopathic disease, for which we have conducted a Phase 3 retrospective natural history-controlled trial and a Phase 2/3 clinical trial in the United States; and |
| ▪ | Geographic atrophy or GA, an advanced form of dry age-related macular degeneration, for which we conducted a Phase 1 clinical trial in the United States and are currently conducting a Phase 2b clinical trial in the United States. Our Phase 2b trial was fully enrolled in February 2021, and we expect data from this trial during the first half of 2022. |
We met with the Division of Cardiology and Nephrology at the U.S. Food and Drug Administration, or FDA, in November 2020, in February 2021 and April 2021 to discuss a potential new drug application, or NDA, submission for Barth. We have had multiple recent communications with senior FDA officials at the Office of Cardiology, Hematology, Endocrinology and Nephrology and at the Division of Cardiology and Nephrology regarding our Barth syndrome program following the April 2021 meeting. We also received a petition signed by over 4,250 members of the Barth community requesting us to submit our NDA on the basis of our existing data. The FDA expressed its view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review. The FDA recommended that we collect additional controlled clinical data in this indication prior to an NDA submission. In May 2021, we submitted a randomized withdrawal clinical trial protocol to FDA. This design was previously suggested by multiple FDA review divisions, including most recently the Division of Cardiology and Nephrology. After reviewing our submission, FDA concluded that neither the proposed randomized withdrawal trial nor any new clinical trial data from the patients remaining on OLE would be likely to add meaningfully to the evidence to support an NDA. Due to the ultra-rare nature of Barth syndrome, neither the Company nor the FDA to date has been able to identify a feasible trial design to generate additional data. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
We are evaluating the potential for additional clinical trials of elamipretide in the following cardiac, ophthalmic and neurological diseases in which mitochondrial dysfunction is implicated:
| ▪ | Duchenne cardiomyopathy, which is the heart muscle weakness associated with Duchenne’s muscular dystrophy, or DMD, is the leading cause of early mortality in this disease; |
| ▪ | Friedreich’s ataxia, or FRDA, which is associated with both cardiomyopathy and progressive decline in visual function; |
| ▪ | Leber’s hereditary optic neuropathy, or LHON, an inherited disease of central blindness, for which we have conducted a Phase 2 clinical trial in the United States; and |
| ▪ | Primary mitochondrial disease caused by mutations in nuclear genes that encode for mitochondrial proteins, or nPMD. |
76
Subject to discussions with the FDA, continued planning efforts and financing plans, we hope to initiate a clinical development program for elamipretide in DMD patients with cardiomyopathy during the first half of 2022, focusing primarily on cardiac endpoints. We plan to support an investigator-initiated Phase 2a open-label clinical trial of elamipretide assessing both visual and cardiac endpoints in FRDA, which is anticipated to commence enrollment in 2021, and we hope that results from this trial will help inform a pivotal trial design. We also plan to initiate a pivotal trial for elamipretide in patients with nPMD during the second half of 2021, subject to continued planning efforts and financing plans. Patients with nPMD comprised a prespecified subgroup of patients with nuclear DNA mutations in whom improvements were observed in our Phase 3 primary mitochondrial myopathy trial. Although we plan to initiate a Phase 3 global clinical trial for elamipretide in LHON, the initiation of this trial is subject to ongoing formulation studies expected to read out in early 2022, continued planning efforts, and financing plans.
Our second clinical product candidate, SBT-272, is a novel peptidomimetic that has been shown to increase adenosine triphosphate, or ATP, production and decrease levels of reactive oxygen species, or ROS, in dysfunctional mitochondria in preclinical studies. In early experiments, SBT-272 demonstrated higher mitochondrial uptake and greater concentrations in the brain relative to elamipretide. We are developing SBT-272 for rare neurological diseases involving mitochondrial dysfunction. Preliminary results from a Phase 1 clinical trial in healthy human volunteers completed during 2020 did not reach desired drug exposure levels. We are conducting subcutaneous dosing studies and plan to commence longer term toxicology studies in 2021 to support the initiation of a Phase 1 clinical trial in early 2022 and the potential initiation of a Phase 2 clinical trial in patients in late 2022. We have conducted and continue to conduct preclinical studies in neurological disease models to inform our decisions regarding our first Phase 2 indication.
We have discovered and own over 100 compounds, including SBT-272 and the SBT-550 family, that also target the mitochondria and form the basis of our broad proprietary pipeline of mitochondrial-targeted product candidates. We are evaluating compounds in the SBT-550 family for rare neurological indications. In addition, our internal discovery platform has generated a library of over 100 differentiated proprietary compounds which could have clinical benefit for diseases related to mitochondrial dysfunction and from which we plan to designate potential product candidates. We may also utilize certain of these compounds as part of our carrier program, in which they could potentially serve as scaffolds to deliver other beneficial compounds to the mitochondria.
As of December 31, 2020, we held exclusive worldwide rights or an option for exclusive worldwide rights under 393 issued patents and 188 patent applications to protect our platform and product candidates. We have exclusive worldwide rights to elamipretide and a second product candidate, SBT-20, both of which we licensed from Cornell Research Foundation, Inc., a subsidiary of Cornell University , or Cornell, and Institut de recherches cliniques de Montréal, or the IRCM, in 2006. The unique mitochondrial activity of elamipretide was first published in The Journal of Biological Chemistry in August 2004. Since licensing elamipretide and SBT-20, we and our collaborators have published approximately 100 peer-reviewed articles highlighting the activity of our compounds in several disease models, including heart failure, kidney disease, skeletal muscle weakness, diabetic retinopathy and neurodegenerative diseases. Our compounds have been evaluated in preclinical and clinical studies at academic and clinical institutions, including Boston Children’s Hospital, Charité Berlin, Children’s Hospital of Philadelphia, Columbia University, Cornell University, Duke University, Johns Hopkins University, Massachusetts General Hospital, Mayo Clinic, Stanford University, University of California Los Angeles, University of California San Diego, University of Colorado and University of Washington.
77
Our Pipeline
The following table summarizes our development pipeline, including preclinical studies and ongoing and planned clinical trials of our product candidates.

Our Strategy
We aspire to lead the development of mitochondrial medicine to improve the lives of patients with severe unmet medical needs. Our strategy is to focus on near-term rare disease opportunities in ophthalmic, cardiac and neurological indications, while continuing to progress the potential of our approach to treat diseases associated with aging in which mitochondrial dysfunction has been implicated. Particularly for larger common disease indications associated with aging, we plan to assess development collaborations with industry leaders. To achieve our goals, we intend to:
Progress toward approval of elamipretide in Barth
We have conducted a pivotal Phase 3 retrospective natural history control trial and a Phase 2/3 double-blind placebo-controlled trial in Barth. We observed improvements in cardiac and clinical endpoints in our pivotal Phase 3 clinical trial and during the open-label extension portion of our Phase 2/3 trial. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
We have received Fast Track in the United States and Orphan Drug designations for Barth in the United States and EMA. In addition to our planned NDA submission, we are evaluating regulatory pathways in Europe.
Advance the development of our mitochondrial medicines in cardiomyopathies
We are encouraged by the improvement in cardiac function observed in Barth patients, and plan to expand our efforts to develop elamipretide for other rare mitochondrial diseases affecting cardiac function. We are evaluating the potential for clinical trials in Duchenne cardiomyopathy, and plan to initiate a Phase 2a clinical trial in FRDA in the second half of 2022. We also plan to explore our second-generation and pipeline mitochondrial medicines in preclinical models of cardiac dysfunction.
Progress the clinical and preclinical development of our mitochondrial medicines in ophthalmology
We are developing elamipretide for ophthalmic conditions associated with mitochondrial dysfunction. We intend to continue to rapidly advance elamipretide through the completion of our Phase 2b clinical trial in GA, which was fully enrolled in the first quarter of 2021, with data expected in 2022. We have received Fast Track designation for this indication in the United States. We believe there is a strong potential for elamipretide to treat rare diseases where mitochondrial dysfunction leads to visual dysfunction, including FRDA and LHON, for which we have received Fast
78
Track and Orphan Drug designations in the United States. We are also exploring our second-generation and pipeline mitochondrial medicines in preclinical models of ophthalmic disease.
Advance the development of our mitochondrial medicines for rare neuromuscular and neurological diseases
We hope to initiate a pivotal trial for elamipretide in patients with primary mitochondrial myopathy due to nuclear DNA mutations, or nPMD, during 2021. We have reached alignment with the FDA regarding the trial design for this trial. We have received Fast Track and Orphan Drug designations for this indication in the United States. We are also developing our second-generation and pipeline mitochondrial medicines for rare neurological diseases involving mitochondrial dysfunction.
Deliver on the promise of our carrier program
We have extensive experience in optimizing delivery of our compounds to the mitochondria, which has been a challenge for other drug delivery technologies. We have demonstrated capability to deliver beneficial payloads to mitochondria by conjugating them with our proprietary compounds, which serve as vectors or carriers to mitochondria. This approach has the potential to confer mitochondrial specificity to promising therapies that do not otherwise localize to mitochondria, potentially increasing the efficacy of a payload by targeting it to the part of the cell where it is needed most. These payloads might include small molecules, proteins, oligonucleotides, nanoparticles and liposomes. This delivery strategy, which we call our carrier program, has the potential to create new pipeline assets from known delivery of small molecules, enzymes, proteins or therapeutic genes to address inherited mitochondrial disorders.
Explore potential strategic partnerships
We may explore select strategic partnerships and alliances to support our drug development programs, while preserving significant development and commercialization rights, if we believe that such alliances will enable us to leverage the financial support and therapeutic area expertise and resources of a strategic partner to accelerate the development and commercialization of our product candidates.
Background
Mitochondria
Mitochondria, found in almost all human cells, are the “powerhouse of the cell.” Mitochondria produce 90% of our energy by converting food into ATP, a molecule that carries energy within cells. Mitochondria produce approximately our body weight in ATP daily, providing the energy that allows cardiac muscles, for example, to beat an estimated 100,000 times every 24 hours, or 2.5 billion times by age 70, without stopping. Our heart, kidney, eyes, brain and skeletal muscle are among the highest producers and users of mitochondrial ATP in our bodies, as ATP is required for their critical functions such as the contraction of skeletal, cardiac, vasculature and lung muscle, maintenance of cell membrane potential, cellular transport and secretion of hormones and neurotransmitters. Normal mitochondrial function is essential for human life and for the proper functioning of many systems in our bodies.
Mitochondria are highly specialized structures. They have their own DNA, called mitochondrial DNA, or mtDNA, which is inherited only from our mothers and is separate and distinct from nuclear DNA, or nDNA. In addition, they are under dual genetic control with nDNA, which encodes for over 90% of the mitochondrial proteome. Mitochondria are located within the cell, which is protected by the cell membrane, and they also have their own inner and outer membrane, which create further barriers to the effective delivery of therapeutics to these specialized organelles. In normal mitochondria, cardiolipin, which is a phospholipid unique to the mitochondria, is responsible for creating folds, called cristae, in the inner mitochondrial membrane, or IMM. The cristae house the electron transport chain, or ETC, which is composed of five protein complexes responsible for mitochondrial ATP production through a process known as oxidative phosphorylation. The curved architecture of the cristae in the IMM is essential to keep the electron transport chain complexes in optimal close configuration for normal oxidative phosphorylation.
79
An illustration of a healthy mitochondria and its curved cristae structure is shown below.

Mitochondrial Dysfunction, Aging and Human Disease
Mitochondrial dysfunction most often arises from mutations in mtDNA or nDNA, that can either be inherited or, in the case of mtDNA mutations, can occur as we age. Dysfunctional mitochondria not only produce less ATP, which impairs the normal functioning of our major organ systems, but they also generate unhealthy levels of ROS, which damages cardiolipin. ROS-mediated damage of cardiolipin can lead to pathological oxidative stress, causing the inflammation, fibrosis and cell death which are causal or contributory to the process of human aging.
Mitochondrial dysfunction, whether inherited or acquired, often impacts high energy-demanding organs such as those of the cardiac, renal, visual, neurological, central nervous, skeletal muscle, circulatory or endocrine systems. Mitochondrial diseases arising from inherited genetic defects, called primary mitochondrial diseases, are typically rare diseases which can impact multiple organ systems within the body and may lead to reduced lifespan. Symptoms of primary mitochondrial disease include cardiovascular and kidney problems, vision problems and chronic pain.
Although mtDNA is originally inherited from our mothers, it is replicated within our mitochondria as mitochondria reproduce and is highly susceptible to mutation within specific cells and organ systems as we age. Mitochondrial diseases arising from these spontaneous mutations in our mtDNA, called secondary mitochondrial diseases, include heart disease (such as heart failure and atherosclerosis), diabetes, ophthalmic conditions (such as age-related macular degeneration, glaucoma, diabetic retinopathy and diabetic macular edema), neurodegenerative diseases (such as Alzheimer’s, Parkinson’s and ALS), senescence, cancer, diabetes, skeletal muscle dysfunction (such as sarcopenia) and kidney diseases.
Targeting Mitochondrial Dysfunction: Role of Cardiolipin
Several of our product candidates, including elamipretide and SBT-272, target cardiolipin in the IMM, stabilizing the IMM under conditions of oxidative stress.
Cardiolipin is a conically shaped phospholipid that plays an important role in establishing the cristae architecture within the IMM and optimizing the function of the ETC. Reduced and damaged cardiolipin content has been observed in many diseases, and a deficiency of normal cardiolipin is thought to be centrally involved in mitochondrial dysfunction.
80
Cardiolipin is essential for normal oxidative phosphorylation, the process by which most ATP is made. Cardiolipin congregates in and around the cristae of the IMM. Cardiolipin’s conical shape is responsible for creating the curved architecture of the cristae. This curvature helps to keep the electron transport chain complexes in close association with one another, increasing the efficiency of ATP production and minimizing the electron leakage that leads to oxidative stress, as illustrated below.
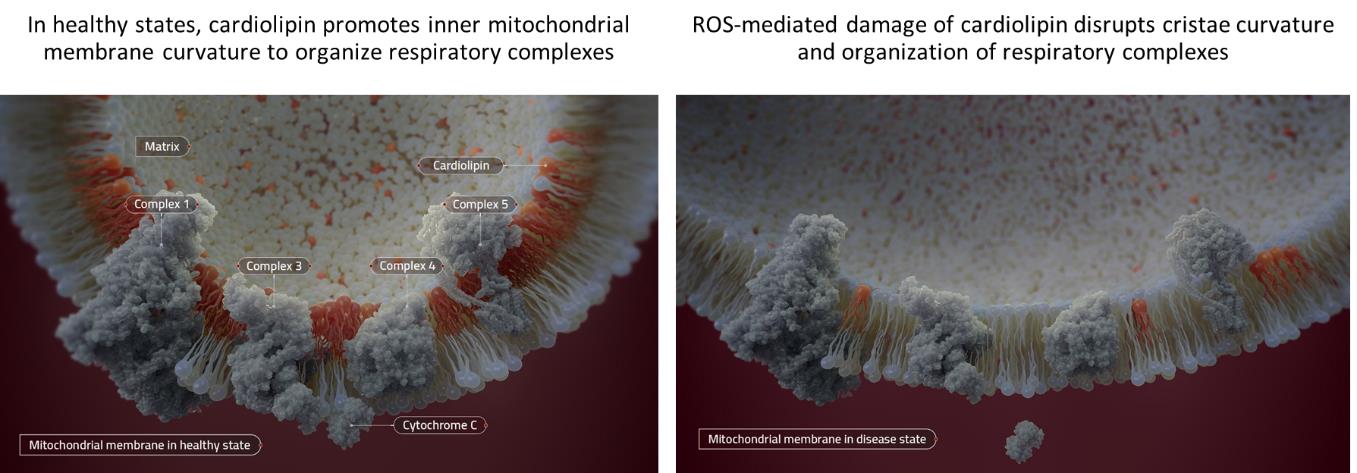
Cardiolipin is embedded with the complexes of the ETC, as can be seen above, and its interaction with the ETC complexes facilitates super-complex association, a process by which electron transport chain complexes selectively associate with, or merge with, one another, to optimize the efficiency of the oxidative phosphorylation process.
Correct mitochondrial morphology is also essential for mitochondrial network connectivity and function. Mitochondrial networks exhibit coordination of inner mitochondrial membrane cristae at inter-mitochondrial junctions, as illustrated below.
This mitochondrial network connectivity is associated with cellular signaling pathways, including:
| ▪ | fusion, in which mitochondria join to spread metabolites, enzymes and mitochondrial gene products through the mitochondrial network, optimizing mitochondrial function and counteracting the accumulation of mitochondrial mutations during aging; |
| ▪ | fission, or the division of mitochondria, which plays an important role in the removal of damaged organelles; |
| ▪ | mitophagy, a mechanism to remove damaged mitochondria; |
| ▪ | ROS-mediated pathways, including the PI3K/Akt pathway, an intracellular signaling pathway important in regulating the cell cycle, and the tumor necrosis factor alpha (TNFα) signaling pathway, a proinflammatory pathway involved in various biological processes including regulation of cell proliferation, differentiation, apoptosis and immune response; |
81
| ▪ | calcium regulation, entailing the transfer of calcium from the endoplasmic reticulum to the mitochondria to facilitate mitochondrial respiration (disrupted calcium regulation is thought to be implicated in cardiomyopathy associated with DMD and FDRA, as well as in heart failure with preserved ejection fraction, or HFpEF); |
| ▪ | various transcription factors, which are proteins that control the rate of transcription of genetic information from DNA to messenger RNA; and |
| ▪ | certain protein kinase C (PKC) signaling pathways that can affect cardiomyocyte function and are involved in the induction of mitophagy. |
Cardiolipin is also required for the structural integrity of the translocase of outer membrane, or TOM, which serves as a central entry gate for almost all mitochondrial proteins including tafazzin, which is deficient in Barth, and frataxin, which is deficient in FRDA.
Cardiolipin is susceptible to peroxidation, or degradation, by oxidative stress produced by dysfunctional mitochondria. When cardiolipin is degraded, it can lose its conical shape, compromising the structural integrity of the IMM by leading to a relaxation of the cristae and a drifting apart of the electron transport chain complexes. Shuttling of electrons through the electron transport chain becomes less efficient with the complexes further apart from one another, resulting in lower ATP production and higher ROS generation. Disruption of mitochondrial morphology also impairs fission and fusion, impacting signaling pathways including mitophagy. This can trigger the cellular and extra-cellular cascades involving inflammation, fibrosis and cell death that underlie many diseases. The images below show healthy mitochondria, on the left, with normal cardiolipin content and cristae structure, and unhealthy mitochondria, on the right, with reduced cardiolipin content and collapsed cristae.
Various diseases alter cardiolipin composition and reduce cardiolipin content within the mitochondria. In Barth, which entails a cardiolipin deficiency, experiments in patient-derived lymphoblastoid cell lines showed 50%-60% less cardiolipin than control cell lines, and work done in Barth patient-derived cardiomyocytes, or heart cells, showed up to 75% less cardiolipin than control cardiomyocytes. Cardiolipin and lipid peroxidation have also been implicated in FRDA, and cardiolipin decrements have been observed in both pediatric and adult patients with heart failure. Aging has also been shown to decrease cardiolipin content in high energy-demanding organs, such as the heart, brain, liver and kidney, as well as the epidermis. Studies suggest that oxidative stress and peroxidation of cardiolipin may contribute to the overall loss of cardiolipin content in these diseases.
Our Approach to Mitochondrial Medicine
We have focused our development efforts on diseases and conditions that affect the organs in the body that generate significant energy because of the high mitochondrial content found in the cells comprising these organs. The activity of our compounds has been studied in several disease models, including heart failure, kidney disease, skeletal muscle weakness, diabetic retinopathy and neurodegenerative diseases. We believe that our product candidates may be most relevant for the visual system, the cardiorenal system and the brain, all of which are innately highly dependent on mitochondrial bioenergetics.
Elamipretide is known to compensate for cardiolipin deficit by improving lipid packing, membrane curvature and membrane surface area. When brought into close proximity with the inner mitochondrial membrane, elamipretide’s positively charged residues interact electrostatically with the anionic headgroups of cardiolipin, increasing local concentration levels. Elamipretide’s nonpolar side chains subsequently penetrate the IMM at gaps created by cardiolipin and interact hydrophobically with the acyl chains, depicted in the graphic below. This electrostatic/
82
hydrophobic binding modulates the surface electrostatics of the inner membrane to facilitate increases in lipid packing, membrane curvature and membrane surface area integral to cristae formation, supercomplex association and efficient oxidative phosphorylation.

In preclinical studies or clinical trials, we have observed that elamipretide normalized function in dysfunctional mitochondria, including by reducing peroxidation of cardiolipin, increasing mitochondrial respiration (the process by which mitochondria produce energy), improving ATP levels, reducing formation of ROS and reducing inflammation, fibrosis and cell death. Importantly, we have not observed any effect of elamipretide on healthy or normal mitochondria.
Following treatment with elamipretide and other pipeline candidates, we observed normalization of mitochondrial morphology across various disease models, including models of diabetic retinopathy, as illustrated by the electron microscopic images below, and kidney reperfusion injury, each of which were published in Clinical Pharmacology & Therapeutics in December 2014.

We are also developing products to address other aspects of mitochondrial dysfunction beyond cardiolipin. We believe that our SBT-550 series of compounds acts upon the ferroptosis pathway, a recently recognized pathway for regulated cell death characterized morphologically by the presence of smaller than normal mitochondria with condensed mitochondrial membrane densities, reduction or vanishing of mitochondria cristae, and outer mitochondrial membrane rupture. The ferroptosis pathway has been implicated in many neurological diseases, including Huntington’s disease, FRDA, Alzheimer’s disease and Leigh’s syndrome. We are also progressing our carrier program in which we utilize our proprietary compounds as mitochondria-targeted vectors to deliver other beneficial compounds to the mitochondria.
Our Product Candidates
We believe that our product candidates have significant potential to address the ophthalmic, cardiac and neurological symptoms of various diseases associated with mitochondrial dysfunction. In addition to our clinical and
83
preclinical focus on rare and common age-related ophthalmic diseases, rare cardiomyopathies and rare neurological diseases, we have conducted preclinical studies and Phase 1 and Phase 2 clinical trials in common diseases and conditions that affect the organs in the body that have significant mitochondrial content to meet their high energy needs; these include the heart, the kidney, the brain (inclusive of the visual system) and skeletal muscle. We believe that our product candidates may be most relevant for the visual system, the cardiorenal system and the brain, which are innately highly dependent on mitochondrial bioenergetics, and we expect these to continue to be key focus areas with respect to some of our pipeline compounds.
We believe that there is significant potential for mitochondrial medicine beyond the indications we are currently studying, including with respect to common diseases associated with aging. In addition to our clinical-stage product candidates, we have a growing pipeline of over 100 compounds that have been screened for mitochondrial activity, including in some cases preferential mitochondria-targeting characteristics; improved tissue distribution in targeted tissues, such as the heart and brain; and differentiated mechanistic targets, including the ferroptosis pathway of cell death. Some of these compounds may be suitable for oral formulations, which we believe may be more appropriate for development for common diseases associated with aging. We have also designed proprietary compounds, which benefit from our peptide carriers, that can potentially deliver beneficial payloads to mitochondria; for example, if genetic mutations impact the production of certain proteins necessary for proper mitochondrial function, this proprietary technology might help us deliver those missing proteins to the mitochondria.
Elamipretide
Elamipretide is a mitochondria-protecting peptide that is known to compensate for cardiolipin deficit by improving lipid packing, membrane curvature and membrane surface area. Elamipretide has been reported to be well tolerated in clinical trials in over 1,000 subjects systemically exposed to it to date. See “—Elamipretide Safety Data” below. We are evaluating or plan to evaluate elamipretide in rare cardiomyopathies where we have the potential for expedited regulatory review, including Barth, for which we have received Fast Track designation from the FDA and Orphan Drug designation from the FDA and EMA. We hope to initiate a clinical development program for elamipretide in Duchenne cardiomyopathy during 2022, subject to continued planning efforts, regulatory interactions, and financing plans. We plan to support an investigator-initiated Phase 2a open-label clinical trial assessing both visual and cardiac endpoints in FRDA, which is anticipated to commence enrollment in 2021, and we hope that results from this trial will help inform a pivotal trial design. We are evaluating elamipretide in ophthalmic indications, including GA, for which we have received Fast Track designation from the FDA. We expect to announce data from our ongoing Phase 2b clinical trial in GA in 2022. We may initiate a Phase 3 global clinical trial for LHON, subject to ongoing formulation studies expected to read out in early 2022, continued planning efforts, and financing plans. We have received Fast Track and Orphan Drug designations for this indication in the United States. We plan to initiate a Phase 3 clinical trial for elamipretide in nPMD during 2021, subject to regulatory interactions and financing plans.
Rare Cardiomyopathies
Background on Elamipretide in Cardiac Settings. We have extensive preclinical and early clinical support for the use of elamipretide in the setting of heart failure, which can arise due to dysfunction of either the contractile or filling mechanisms of the heart. In a study published in JACC: Basic to Translational Science in April 2019, elamipretide was shown to rapidly (within four hours) improve multiple parameters of mitochondrial function in freshly explanted subsarcomal tissue from heart failure transplant subjects, including samples taken from pediatric and adult patients across a broad range of phenotypes, including dilated cardiomyopathy, hypertrophic cardiomyopathy, ischemic cardiomyopathy and muscular dystrophy. Data published on bioRxiv in January 2021 demonstrated that elamipretide remediated respiratory chain and mitochondrial quality control abnormalities caused by cardiolipin deficiency within days to weeks. Data published in medRxiv in November 2021 demonstrated that in patients with Barth, elamipretide improved cardiac substrate metabolism within months during the double-blind portion of the TAZPOWER trial. Collectively these data, together with the long-term data showing improvement in parameters of cardiac structure and function during the open-label extension portion of the TAZPOWER trial, suggest a time-course to elamipretide-mediated cardiac improvements that starts within hours at the cellular level and may result within months or years in cardiac reverse remodeling at the organ system level.
For rare cardiomyopathies associated with diseases entailing mitochondrial dysfunction, including Barth, DMD, and FRDA, typical phenotypes include hypertrophic cardiomyopathies, dilated cardiomyopathies and conduction disorders. Hypertrophic cardiomyopathy entails a thickening of the heart muscle and resulting reduced left ventricular cavity size, so that the heart cannot fill adequately with blood. With less blood entering the heart, less blood is available to expel from the heart, leading to lack of adequate perfusion throughout the body. Hypertrophic cardiomyopathy in metabolic diseases such as Barth, DMD or FRDA may ensue in response to metabolic challenges as the heart switches from fatty acid to glucose metabolism in response to physiological stress. In some
84
cases, there appears to be a progression from a hypertrophic to a dilated phenotype, in which the heart muscle becomes stiff and is unable to relax properly. Dilated cardiomyopathy may lead eventually to congestive heart failure and death.
In a study of a mouse model of hypertrophic cardiomyopathy published in Circulation: Heart Failure in September 2013, treatment with elamipretide attenuated heart failure induced by transverse aortic constriction, or TAC. As shown in the images below of a healthy mouse heart, a mouse heart with TAC-induced hypertrophic cardiomyopathy treated with placebo, and a mouse heart with TAC-induced hypertrophic cardiomyopathy treated with elamipretide, elamipretide-treated mice retained normal cardiac structure despite the TAC intervention.
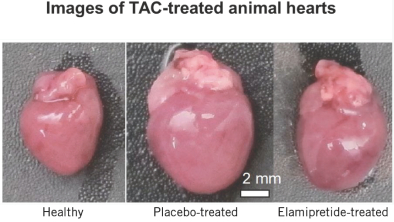
Dilated cardiomyopathy with ataxia syndrome, or DCMA, also known as 3-methylglutaconic aciduria type V, is a rare autosomal recessive disorder which is phenotypically related to Barth. DCMA is characterized by 3-methylglutaconic aciduria, dilated cardiomyopathy, developmental delay, neuromotor abnormalities, growth failure and prolongation of the QT interval. End-stage heart failure leading to death in early childhood is common. In a preclinical study published in Frontiers in Cardiovascular Medicine in November 2019, in which primary dermal fibroblasts isolated from pediatric DCMA patients were treated with elamipretide, the high fragmentation and significant increased ROS production observed in DCMA fibroblasts was reversed.
Heart failure with preserved ejection fraction, or HFpEF, is characterized by the inability of the heart to relax properly. In a porcine model of HFpEF, in which percutaneous renal angioplasty and stenting, or PTRS, of animals with renovascular hypertension resulted in myocardial damage in placebo-treated animals, treatment with elamipretide attenuated that damage across several parameters of cardiac function, as reported in the Journal of Hypertension in January 2014. The effect of elamipretide in patients with HFpEF was assessed in a double-blind, placebo-controlled, Phase 2 clinical trial enrolling 47 subjects with HFpEF who were randomized on a one-to-one basis to receive 28 days of 40 mg subcutaneous elamipretide injections or placebo. Trends favoring elamipretide were observed across various endpoints, particularly on assessments conducted during maximal exercise when clinical symptoms most commonly present in this patient population. Notably, left ventricular end diastolic volume, an important functional parameter in this disease in which the heart is not filling to its full potential, also improved, although the changes were not significant overall. Although the trial did not meet its primary endpoint of change in filling pressure at rest, overall, most endpoints favored elamipretide, as illustrated below in a comparison of matched baseline and end-of-treatment echocardiographic parameters from participating subjects.

85
Overall, the improvements in cardiac function observed across multiple clinical and preclinical hypertrophic heart failure phenotypes lead us to believe that elamipretide may be a promising therapeutic treatment for the cardiac dysfunction presenting in Barth, DMD and other rare cardiomyopathies.
Barth Syndrome. Barth is estimated to affect between one in 300,000 to one in 400,000 births in the United States, and there are estimated to be less than 300 known living patients worldwide with Barth. There are no therapies approved by the FDA or the European Medicines Agency, or EMA for the treatment of Barth. We have received Fast Track designation from the FDA and Orphan Drug designation from the FDA and EMA for the development of elamipretide in Barth. In February 2020, the FDA granted rare pediatric disease designation for elamipretide for the treatment of Barth, and we may therefore be eligible for a voucher that can be used to obtain priority review for a subsequent human drug application if our Barth product candidate meets relevant statutory requirements associated with the program, including FDA approval of the drug in this indication.
Barth typically presents in infancy or early childhood. The disease is characterized by cardiomyopathy, which makes it harder for the heart to pump blood to the rest of the body; reduced muscle tone and muscle weakness; delayed growth; fatigue; low white blood cell count, or neutropenia, which can compromise the body’s ability to fight off infections; and varying degrees of physical disability. Some individuals with Barth require one or more heart transplants, including during infancy. Implantable cardioverter defibrillators may be used to prevent sudden death due to life-threatening ventricular arrhythmias, and other heart failure medications including ACE-inhibitors and beta blockers may also be used to help manage cardiac dysfunction. In addition to medical and surgical intervention, individuals with Barth may require physiotherapists and occupational therapists, speech and language therapists, psychologists and educational support workers. Barth can be a lethal infantile and early childhood disease, and mortality is highest in the first four years of life. Although improvements in the management of the disease have increased survival for some patients, with reports of individuals with Barth living into their late 40’s and a single individual with Barth reported as surviving to age 51, the disease nevertheless is associated with premature death, most often due to cardiac problems.
Barth is caused by a genetic mutation in the TAZ gene that leads to decreased production of tafazzin, an enzyme required to produce cardiolipin; as a result, there is an abnormal composition of cardiolipin in individuals with Barth, particularly in the heart and skeletal muscle mitochondria. Barth patients have less tetralinoleylcardiolipin, or L4-CL, and increased amounts of monolysocardiolipin, or MLCL, than healthy subjects, and the disease can be diagnosed by the ratio of MLCL to L4-CL, called the MLCL:CL ratio, or by genetic testing. MLCL, a phospholipid found in the inner mitochondrial membrane, is considered to be an immature form of cardiolipin. MLCL is structurally differentiated from L4-CL due to its lack of a fourth acyl chain, which alters the typical conical structure of the lipid, causing alterations to mitochondrial morphology. These morphological alterations result in destabilization of respiratory chain supercomplexes and increased oxidative stress. Studies have shown increased susceptibility of cardiolipin to peroxidation in Barth patient-derived pluripotent stem cells, leading to increased accumulation of MLCL. Analyses of cardiolipin levels in Barth patient-derived lymphoblasts have shown up to 60% lower levels of cardiolipin than in healthy control cells; this cardiolipin deficit has been found to range to up to 95% in other Barth cell lines or animal models.
86
The images of lymphoblast mitochondria below indicate that, compared to normal mitochondria, the mitochondria of individuals with Barth have unhealthy morphology, including a lack of inner membranes, a poor alignment of cristae, which are the curves of the IMM, and swollen or collapsed segments of cristae.
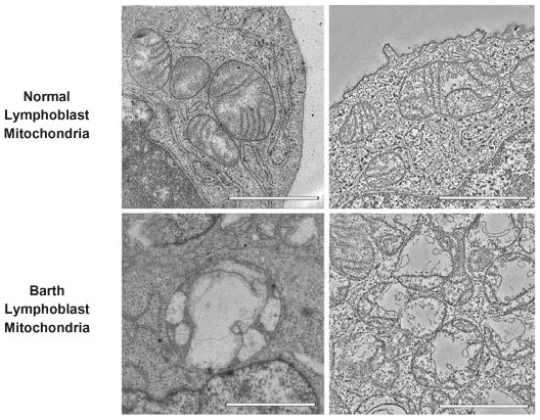
The Barth Syndrome Foundation, an advocacy group for Barth awareness and research, asked us to conduct a clinical trial of elamipretide for Barth. As the mechanism of elamipretide is to bind reversibly to cardiolipin, which is deficient in individuals with Barth, we undertook preclinical work to better characterize the safety profile of elamipretide for Barth as well as to gain insight into whether there would be adequate target engagement for elamipretide given the severe depletion of cardiolipin that characterizes this disease.
These experiments suggested that elamipretide may improve mitochondrial respiration in cardiomyocytes derived from individuals with Barth. In lipid model systems intended to simulate a cardiolipin deficiency in the IMM, although elamipretide ameliorated the reduced membrane-surface area attributable to the cardiolipin deficiency, elamipretide’s effect was more pronounced with less severe cardiolipin loss, suggesting that therapeutic benefit may be more pronounced or more rapidly observed in subjects with more moderate cardiolipin loss.
While Barth patients have some normal cardiolipin, the ratio of abnormal MLCL to normal cardiolipin may vary from patient to patient. The MLCL:CL ratio has been observed to correlate with functional impairment; patients with a lower MLCL:CL ratio are typically less impaired than those with a higher MLCL:CL ratio. For example, a prior observational study of 34 Barth patients suggests that the MLCL:CL ratio is inversely correlated with performance on the six-minute walk test, or 6MWT (p=0.00014). Accordingly, if the interaction of elamipretide with normal cardiolipin is critical to therapeutic effect, such therapeutic effect may also vary among patients, and as a result may be more rapidly observed in a subset of patients.
We initiated TAZPOWER, a clinical trial of elamipretide for individuals diagnosed with Barth, in the third quarter of 2017 at Johns Hopkins. TAZPOWER was a double-blind, placebo-controlled cross-over trial to evaluate the efficacy of once daily subcutaneous administration of elamipretide in 12 individuals who were 12 years of age or older and had been diagnosed with Barth. During the controlled portion of the trial, or Part 1, subjects were randomized in a one-to-one ratio to either 40 mg elamipretide or placebo administered daily by subcutaneous injection for an initial 12-week treatment period, or Treatment Period 1. After an initial treatment period, on either the 40 mg elamipretide treatment arm or the placebo treatment arm, treatment was discontinued for a four-week wash-out period, following
87
which the subjects crossed over to the other treatment arm for a second 12-week treatment period, or Treatment Period 2. Subjects enrolled in TAZPOWER were eligible for participation in an optional open-label extension trial, or Part 2, that is contributing to our safety database and includes periodic efficacy assessments to support the durability of any effects observed in the placebo-controlled phase of the trial.
The objectives of the trial were to evaluate the safety, tolerability and efficacy of once daily subcutaneous elamipretide injections in individuals with Barth. During each of Treatment Periods 1 and 2, subjects completed assessments including the 6MWT at the beginning of each treatment period, four weeks into each treatment period and at the end of each treatment period. Certain assessments were also conducted at initial screening, and at a follow-up visit, four weeks following the end of the second treatment period. In addition, the Barth Syndrome Symptom Assessment, or BTHS-SA, a patient-reported outcome questionnaire developed based upon interviews of individuals with Barth to measure the fatigue and muscle weakness associated with the disease, was completed by subjects daily and assessed based on the average of seven days of daily values preceding the assessment date.
Elamipretide was reported to be well tolerated by patients with Barth. Other than injection site reactions, which were experienced in both groups but with higher frequency in the elamipretide treatment group, there were overall less events reported during the elamipretide treatment periods of the trial, as compared to the placebo treatment periods.
TAZPOWER did not meet its primary efficacy endpoints of (i) change in the 6MWT or (ii) change in Total Fatigue on the BTHS-SA, which is composed of three questions from the BTHS-SA, assessing tiredness at rest and during activities and muscle weakness during activities, between end of treatment on elamipretide and end of treatment on placebo. Improvements were observed in the subgroup of patients with relatively more cardiolipin at baseline, which we believe suggests that elamipretide therapy may more rapidly affect subjects with relatively more normal cardiolipin. Improvements in surrogate echocardiographic measures of cardiac function were observed in 10 of the 12 subjects following exposure to elamipretide. Significant changes in metabolites associated with cellular bioenergetics were observed in elamipretide-treated subjects relative to placebo-treated subjects which we believe suggest that elamipretide improved cellular metabolism; these included improvements in plasma medium-chain acylcarnitines (p=0.007) which are known to be elevated in Barth and other cardiac diseases. We believe the data suggest that although improvements in disease markers were starting to occur during the 12-week treatment period, we did not treat for long enough to see significant changes overall in the primary endpoints.
An important assumption for a cross-over trial is that any effect of the therapeutic intervention will not carry over into, or will washout by, the second treatment period. Although statistically significant evidence of a carryover effect was not observed during Part 1, with so few patients enrolled, the trial may have been underpowered to detect such an effect. Subjects randomized to elamipretide therapy during the first three-month treatment period in Part 1 demonstrated a greater overall improvement in end diastolic volume during Part 1, with a median increase of 18ml versus a median increase of 6ml for those randomized to elamipretide therapy during the second treatment period. The subjects who were randomized to elamipretide in the first treatment period also appeared to continue to experience improvement in left ventricular end diastolic volume during the second treatment period, while they were receiving placebo, which may be indicative of a carryover effect and support the premise that improvements began upon exposure to elamipretide therapy and gradually increased over time. Part 2 of the trial, which is an open label extension, was intended to assess longitudinal trends in efficacy with longer duration of therapy. Eight subjects remain enrolled in the open label extension and have completed the week 72 visit.
With longer duration of therapy during open-label extension, adaptive myocardial changes in both cardiac proportion and function may indicate the occurrence of physiologic cardiac remodeling. At baseline, all subjects demonstrated impaired left ventricular, or LV, cardiac function as assessed by 3-D echocardiogram measurements of LV end systolic volumes, which is the volume of blood in the left ventricle at the end of contraction and the beginning of filling, LV end diastolic volumes, which is the volume of blood in the left ventricle at the end of filling, before contraction, and LV stroke volume, which is the amount of blood pumped by the heart’s left ventricle per contraction, in each case indexed to body surface area, or BSA. LV stroke volume is one of the primary determinants of cardiac output, or the volume of blood pumped by the heart, and an important indicator of how efficiently the heart can meet the body’s demands for perfusion to various organs. During Part 1, improvement in left ventricular volumes were observed in 10 of the 12 individual subjects following randomization to elamipretide; during open-label extension, an overall increase from baseline up to week 72 was observed in LV end diastolic volume, LV end systolic volume, and LV stroke volume, in each case indexed to baseline body surface area, demonstrating a statistically significant slope of change for each parameter (indexed LV end diastolic volume overall slope = 0.020; p = 0.0001; indexed LV end systolic volume overall slope = 0.007; p = 0.0002; indexed LV stroke volume overall slope = 0.012; p = 0.0001). There were no meaningful changes to heart rate, blood pressure or ejection fraction observed. Together, these changes may be suggestive of a durable reversal of disease pathology.
88
LV stroke volume has been reported to be a major determinant of peak exercise capacity in patients presenting with this cardiac phenotype. With longer duration of therapy during open label extension, we are observing continued improvement in functional assessments of exercise capacity, including distance walked on the 6MWT (change of 95.9, 97.4 and 106.8 meters from study baseline to week 36, 48 and 72 of open-label extension, respectively), which are increasingly correlated with improvements in LV stroke volume (week 36, Rs0.21, p=0.29; week 48, Rs0.36, p=0.39; week 72, Rs 0.52, p=0.18). The referenced correlations are to the Spearman’s Rank Correlation Coefficient, or Rs, which is a is a statistical measure of the strength of a link or relationship between two sets of data; Rs of 1.0 indicates a perfect positive correlation, -1.0 indicates a perfect negative correlation, 0 indicates no association between the sets of data.
At week 72 of open label extension, in addition to the 106.8 meter improvement from baseline in 6MWT distance (p=0.02), improvements from baseline were observed on BTHS-SA Total Fatigue (-1.7 decrease in fatigue from baseline, p=0.07); muscle strength as measured by hand-held dynamometry, or Muscle Strength by HHD (43 newton increase from baseline, p=0.008); the SWAY Balance Score, a measure of postural sway that is an important indicator of possible balance deficits (13 point improvement from baseline, p=0.01); five times sit to stand, or 5XSST, in which patients are required to sit and stand five times in succession (2.1 second improvement from baseline, p=0.10); CGI symptoms (0.88 improvement from baseline, p=0.0006)(investigator rated 4 out of 8 subjects at week 72 as having no signs or symptoms of Barth); and Patient Global Impression, or PGI, symptoms (0.5 improvement from baseline, p=0.10). The results of these assessments at baseline, week 36 of open label extension, and week 72 of open label extension are depicted graphically below.
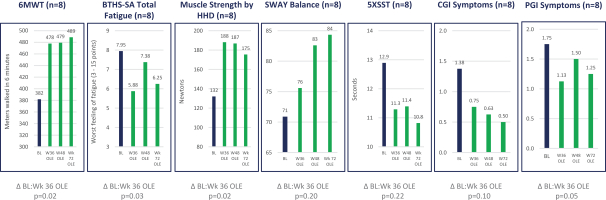
The MLCL:CL ratio, which measures the relative level of abnormal cardiolipin to normal cardiolipin and is diagnostic for the disease, has also improved for all patients. For each of the 12 subjects who enrolled in TAZPOWER, their MLCL:CL ratio was lower at their last visit than at baseline. For the eight patients who completed week 72 of open label extension, the mean MLCL:CL ratio is improving over time, with a mean change of -5.6 at week 36, -7.1 and week 48 (p=0.04) and -17.9 at week 72 (p=0.03).
Overall, for the 8 subjects still enrolled at week 72 of open label extension, the ratio was significantly improved (-17.9, p=0.03). Although there are some inherent limitations regarding the degree of precision with which this diagnostic biomarker is assayed, these changes may suggest improvement at the cellular level.
In February 2020, we completed a Phase 3 retrospective natural history comparative control study to establish the efficacy of elamipretide as a treatment for subjects with Barth. The treatment arm for this pivotal trial derived from the long term, open-label extension arm of TAZPOWER, and the control arm derived from a natural history database maintained by the same team at Johns Hopkins involved in the TAZPOWER trial, ensuring consistency of data collection. The FDA published guidance in 2019 recognizing the utility of natural history controls as a possible control group for single-arm or open-label trials, noting that while the inability to control for certain biases could limit the ability of externally controlled trials to demonstrate substantial evidence of effectiveness, this bias may be mitigated in certain situations where the disease course is predictable and the treatment effect is dramatic.
The natural history study met the primary efficacy endpoint of change in the 6MWT between the eight patients treated with elamipretide through week 36 of open-label extension and 19 prognostically matched natural history controls, with a least square means improvement of 81.26 meters on elamipretide versus 0.59 meters in the natural history control cohort (p=0.0005). A similar finding at later timepoints, corresponding to weeks 48 and 72 (which was a post-hoc sensitivity analysis) of open-label extension, suggests the durability of this response, with a least square means improvement of 93.08 meters on elamipretide versus 0.88 meters in the natural history cohort
89
(p=0.0006) at the timepoint corresponding to week 48 and a least square means improvement of 116.92 meters on elamipretide versus 1.73 meters in the natural history cohort (p=0.0003) at the timepoint corresponding to week 72.
The natural history study also met several secondary efficacy endpoints:
| ▪ | Statistically significant differences in muscle strength as measured by hand-held dynamometry, or Muscle Strength by HHD, were observed for eight patients treated with elamipretide as compared to 19 prognostically matched natural history controls across a cumulative time period corresponding to both week 36 of open-label extension (difference of 41.8 newtons; p=0.0002), week 48 of open-label extension (difference of 47.9 newtons; p=0.0004) and week 72 of open-label extension (difference of 58.2 newtons; p=0.0002). |
| ▪ | Statistically significant differences in 5XSST were observed for eight patients treated with elamipretide as compared to 15 prognostically matched natural history controls across a cumulative time period corresponding to both week 36 of open-label extension (difference of -2.3 seconds; p=0.047), week 48 of open-label extension (difference of -2.8 seconds; p=0.039) and week 72 of open-label extension (difference of -3.237 seconds; p=0.008; n=14 natural history controls). |
| ▪ | Improvements in the SWAY Balance Score were observed for eight patients treated with elamipretide as compared to 12 prognostically matched natural history controls across a cumulative time period corresponding to week 36 of open-label extension (improvement of 6.46 out of 100; p=0.1275), week 48 of open-label extension (improvement of 7.6 out of 100; p=0.1232) and week 72 of open-label extension (improvement of 11.92 out of 100; p=0.0258). |
| ▪ | A multi-domain responder index was included to inform as to the clinical meaningfulness of any changes observed. For this index, a 10% improvement on any of the 6MWT, Muscle Strength by HHD, 5XSST, and SWAY Balance Score by the eight patients treated with elamipretide as compared to 12 prognostically matched natural history controls was considered clinically meaningful and scored as +1, and a 10% decline on any endpoint was considered clinically meaningful and scored as -1, with any other changes scored as 0. This endpoint demonstrated statistically significant differences across a cumulative time period corresponding to both week 36 (2.4; p=0.0001), week 48 (2.4; p=0.0001) and week 72 (2.53; p=0.0006). |
In addition, FDA requested that we analyze whether the improvements observed in cardiac function at week 72 of the TAZPOWER open label extension would be expected in the natural course of the disease. In the natural history study, an analysis of natural history age-matched controls demonstrates that left ventricular stroke volume would be expected to decline in the natural course of the disease. A similar decline in stroke volume for patients greater than 12 years old affected by Barth was also observed in another longitudinal cardiac natural history database.
90
These pivotal data are depicted graphically below.

In July 2020, we had a Type C interaction with Division of Rare Diseases and Medical Genetics, or DRDMG, of the FDA regarding our data in Barth syndrome. DRDMG did not agree that the current data package is sufficient to support an NDA submission and recommended that we collect additional controlled clinical data in this indication prior to an NDA submission.
More than 4,250 members of the Barth syndrome community subsequently signed a petition asking the FDA and us to work together to provide Barth syndrome patients access to elamipretide, asking us to submit an NDA on the basis of the existing data, and asking the FDA to review and approve an NDA for elamipretide to treat this ultra-rare disease. In their petition, patients expressed serious concern regarding delays anticipated by the FDA’s recommendation that we conduct additional controlled clinical trials prior to NDA submission.
We met with the Division of Cardiology and Nephrology at FDA in November 2020, in February 2021 and April 2021 to discuss the clinical evidence to support a potential NDA submission for Barth. We have had multiple recent communications with senior FDA officials in the Office of Cardiology, Hematology, Endocrinology and Nephrology and at the Division of Cardiology and Nephrology regarding our Barth syndrome program following the April 2021 meeting. The FDA expressed its view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review. The FDA recommended that we collect additional controlled clinical data in this indication prior to an NDA submission. In May 2021, we submitted a randomized withdrawal clinical trial protocol to FDA. This design was previously suggested by multiple FDA review divisions, including most recently the Division of Cardiology and Nephrology. After reviewing our submission, FDA concluded that neither the proposed randomized withdrawal trial nor any new clinical trial data from the patients remaining on OLE would be likely to add meaningfully to the evidence to support an NDA. Due to the ultra-rare nature of Barth syndrome, neither the Company nor the FDA to date has been able to identify a feasible trial design to generate additional data. Despite FDA’s view that the existing clinical data are insufficient to demonstrate substantial evidence of effectiveness and would not support NDA review, we believe that the data could support an NDA review and plan to submit our NDA to the FDA in August 2021, although there is no assurance that the FDA will file the NDA.
The NDA will include data from the Phase 3 Retrospective Natural History Control Trial, which met its primary and most secondary endpoints. The NDA will also include data from the TAZPOWER Phase 2/3 Clinical Trial, in which short-term therapy during the double-blind placebo-controlled crossover portion of the trial, despite not meeting the primary endpoints, resulted in improvements in metabolites that have been associated with clinical outcomes in chronic heart failure, and in which long-term therapy during OLE showed significant improvements in echocardiographic measures of cardiac function. We consider these data to reasonably likely predict improvements in morbidity or mortality. Long term therapy also improved clinical endpoints including exercise tolerance, strength, and physician- and patient- reported outcomes, as well as biomarker endpoints diagnostic of the disease. We believe the data could support NDA review and approval on either an accelerated or full basis, although there can be no assurance that the FDA will file the NDA.
91
Duchenne Cardiomyopathy. In addition to Barth, cardiomyopathy is a leading cause of death in diseases including Duchenne’s muscular dystrophy, or DMD, an inherited muscle wasting disease affecting an estimated one in 3,500 to 5,000 male births in the United States. There are no therapies approved by the FDA or EMA for the treatment of cardiac manifestations of DMD, or Duchenne cardiomyopathy. Most DMD patients develop Duchenne cardiomyopathy, and heart failure and sudden cardiac death are the most commonly reported causes of early mortality among these patients. We plan to explore the potential of elamipretide as a treatment for Duchenne cardiomyopathy.
DMD patients often suffer from a hypertrophic cardiomyopathy, a similar phenotype as observed in our TAZPOWER trial. Also similar to what is believed to occur with progression of cardiac symptoms in Barth, their hearts typically maintain adequate systolic function until shortly before death. We believe based on the improvements in echocardiographic parameters of heart function observed in TAZPOWER as well as in a compassionate use case in Senger’s syndrome, elamipretide therapy may help ameliorate the cardiac manifestations of DMD. Elamipretide has been shown to improve mitochondrial respiration in failing cardiac tissue from a patient with Becker’s muscular dystrophy, which is phenotypically similar to Duchenne cardiomyopathy.
In June 2020, we hosted a clinical advisory board meeting involving 16 external thought leaders with expertise in DMD, Duchenne cardiomyopathy and/or heart failure to discuss our planned development efforts in Duchenne cardiomyopathy. The expert consensus from the meeting is that mitochondrial dysfunction is central to the pathology of Duchenne cardiomyopathy and that elamipretide may be beneficial to treat this devastating and life-limiting sequalae of the disease. We plan to launch early development efforts to commence a Phase 2/3 clinical trial in Duchenne cardiomyopathy in the second half of 2021, subject to discussions with FDA, continued planning efforts and financing plans.
FRDA. Friedreich’s ataxia, or FRDA, the most common form of hereditary ataxia, or loss of coordination, affects an estimated one in 40,000 people in the United States. There are no therapies approved by the FDA or EMA for the treatment of FRDA. Many FRDA patients experience progressive vision loss, and most FRDA patients develop cardiomyopathy, and heart failure and sudden cardiac death are the most commonly reported causes of early mortality among these patients. We plan to explore the potential of elamipretide as a treatment for the cardiac and visual manifestations of FRDA.
In preclinical studies, elamipretide has been shown to improve mitochondria structure and function as well as to increase mature frataxin levels in FRDA patient lymphoblasts.
We plan to support the initiation of a Phase 2a open-label clinical trial assessing elamipretide in a cohort of patients affected by visual decline and/or cardiomyopathy associated with FRDA, which is expected to commence in 2021. We anticipate that data from this trial will help inform pivotal trial design.
Ophthalmic Diseases
Normal mitochondria play a critical role for ocular function, and dysfunctional mitochondria are implicated in several rare and common diseases of the eye. Ophthalmologic diseases that have not traditionally been considered to have obvious mitochondrial origins are increasingly recognized to result in part from impaired mitochondrial function, increased oxidative stress and increased apoptosis. As a high energy-demand organ, the eye is particularly susceptible to the consequences of mitochondrial damage. Oxidative damage that results over time from inherited mtDNA mutations or prolonged oxidative stress instability leads to cumulative mitochondrial damage, which is recognized to be an important pathogenic factor in inherited ophthalmologic disorders such as LHON as well as age-related ophthalmologic diseases such as diabetic retinopathy, glaucoma and dry AMD.
We have observed beneficial effects of treatment with elamipretide in preclinical models of diabetic retinopathy, dry AMD and glaucoma. We have dosed elamipretide both topically, instilled as a topical ophthalmic solution, and systemically, by subcutaneous injection, in different animal models and in early clinical trials. We observed improvement from baseline in visual function in subjects enrolled in a Phase 1 clinical study of elamipretide in dry AMD who were treated with 40 mg subcutaneous systemic elamipretide injections once daily for six months. We also observed signs of clinical benefit in a Phase 1/2 clinical trial of elamipretide topical ophthalmic solution in patients with Fuchs’ corneal endothelial dystrophy, or Fuchs, and in the open-label extension portion of a Phase 2 clinical trial of elamipretide topical ophthalmic solution in patients with LHON. Based on our studies in animals, we believe that higher concentrations of elamipretide may be found in the retina following subcutaneous administration than topical ophthalmic administration.
Geographic Atrophy. We are advancing development of elamipretide for GA, an advanced form of dry AMD. Dry AMD is a common ophthalmic disease associated with aging and the leading cause of blindness among older adults in the developed world. GA is estimated to impact approximately one million individuals in the United States. There are no treatments approved by the FDA or EMA for the disease.
92
The earliest clinical manifestation of dry AMD is often a reduction in low luminance, or low light, visual acuity, which can make it challenging to conduct normal daily activities such as reading in artificial light, driving at dusk or at night and navigating indoors in low light. The disease may progress to the GA stage, which includes blurred vision and loss of central vision, which can impair facial recognition, mobility, watching television and computer use, and can eventually lead to blindness. These limitations may impair the independence of older adults and have been associated with increased depression.
The pathophysiology of GA involves the gradual deterioration, or geographic atrophy, of the central part of the retina, known as the macula. The retinal pigment epithelium provides nutrition to the retina, which has a very active metabolism and rids the eye of waste by phagocytosis of photoreceptor outer segments, protects against photooxidation and enables perception of light through retinal recycling. The eye is the highest consumer of mitochondrial ATP in the central nervous system, due to the intensive bioenergetics required to support visual function. Preclinical studies suggest that diseases of the retinal pigment epithelium, such as dry AMD, may be exacerbated by light- induced mitochondrial dysfunction, and that mitochondrial DNA mutations appear to accumulate over time in diseased retinal pigment epithelium as a consequence of chronic and ongoing oxidative stress. Cigarette smoking and high fat diets, both of which contribute to mitochondrially deleterious oxidative stress, are known to be environmental risk factors for dry AMD onset and progression. These findings suggest a key role for mitochondrial dysfunction in the pathology of the disease.
Elamipretide was evaluated in several preclinical models of dry AMD, with data suggesting that treatment with elamipretide improved mitochondrial morphology. In a preclinical model, 24-month old atherosclerotic mice (roughly equivalent to human octogenarians) accumulated drusen-like deposits when fed a high fat diet, and, after one month of subcutaneous administration of elamipretide, showed normal mitochondrial morphology and ultrastructure of the retinal pigment endothelium cells. Additionally, the animals treated with elamipretide were observed to have normalization of b-wave amplitudes on electroretinograms, which suggests an improvement in photoreceptor function reflecting improved visual acuity.
The table below provides a summary of our completed and ongoing trials for dry AMD and GA.
Trial | | Indication | | Stage; Status | | Trial Design |
ReCLAIM | | dry AMD | | Phase 1; completed in March 2018 | | Open-label, single-center clinical trial involving 19 subjects with non-central geographic atrophy, which occurs when the photoreceptors no longer work and the patients develop a blind spot or spot of poor vision in the macula, and 21 subjects with high risk drusen, which are large deposits of debris located between the retina and the Bruch’s membrane, that can interfere with waste products getting removed from the macula. Subjects received once daily subcutaneous injections of elamipretide for 24 weeks. |
| | | | | | |
ReCLAIM-2 | | GA | | Phase 2b; fully enrolled in February 2021 | | Double-blind, placebo-controlled, multi-center clinical trial involving 176 subjects with non-central geographic atrophy, receiving once daily subcutaneous injections of either elamipretide or placebo for approximately 48 weeks. |
93
We conducted our ReCLAIM Phase 1 open-label clinical trial at Duke Eye Center to evaluate the safety, tolerability and efficacy of daily subcutaneous injections of 40 mg elamipretide given over 24 weeks to 40 individuals with intermediate characteristics of dry AMD, including 21 individuals with high-risk drusen, the most common early sign of dry AMD, and19 individuals with non-central geographic atrophy, or areas of dysfunctional macula. All subjects had a five-letter or greater deficit in low luminance visual acuity, or LLVA, at baseline. For the 19 subjects with high-risk drusen and 15 subjects with non-central geographic atrophy who completed 24 weeks of therapy, we observed significant improvements in both cohorts, as summarized below.
ENDPOINT | | DRUSEN COHORT (N=19) | | | GEOGRAPHIC ATROPHY COHORT (N=15) | |
Best corrected visual acuity (regular light) mean letters gained/p value | | | 3.58 | | | | 4.60 | |
| | (p=0.0253) | | | (p=0.0034) | |
Low luminance visual acuity mean letters gained/p value | | | 5.63 | | | | 5.40 | |
| | (p=0.0055) | | | (p=0.0186) | |
Reading speed (regular light) mean reduction in time/p value | | | -0.11 | | | | -0.02 | |
| | (p=0.0054) | | | (p=0.5501) | |
Low luminance reading speed mean reduction in time/p value | | | -0.28 | | | | -0.52 | |
| | (p<0.0001) | | | p=0.0172 | |
Visual function questionnaire composite score | | | 9.25 | | | | 6.59 | |
| | (p=0.0004) | | | (p=0.0125) | |
Low luminance questionnaire general dim light vision score | | | 20.75 | | | | 10.32 | |
| | (p=0.0003) | | | (p=0.027) | |
Since we did not have a placebo, or control group, in this study, we evaluated natural history data and prior placebo-controlled trials of subjects with similar disease burden to understand the likelihood that we would observe a learning or placebo effect in this study. In a number of other reported interventional studies conducted by others, including Chroma, Spectri and Filly (combined n>700), as well as in several natural history studies conducted by others, including Proxima, Holz and Ladd (combined n>250), BCVA was observed to decline in similar patient groups by four to six letters over an up to one-year period, and LLVA was observed to decline in similar patient groups by approximately two letters over a six-month to one-year period. In the once-monthly interventional and sham arms of the Filly trial, LLVA was observed to decline by 1 letter over a six-month period. This supports our belief that the improvements observed in the ReCLAIM trial are unlikely to be due to the natural variability of the disease.
While each subject in ReCLAIM had one eye designated as a study eye, which met the inclusion criteria for the trial, the other eye was not required to meet inclusion criteria. Fourteen subjects in the trial had neovascular age-related macular degeneration, or wet AMD, that was at the quiescent stage, meaning that it was stable on standard-of-care anti-vascular endothelial growth factor, or anti-VEGF, therapy. While there is an improvement in visual acuity when some subjects are first dosed with anti-VEGF therapy, improvement typically plateaus and even declines slightly when the disease reaches the quiescent state. We observed that subjects with wet AMD experienced similar improvements in vision as was observed in the study eyes, with a 5.6 letter mean gain from baseline in BCVA, which was statistically significant at p=0.0027, and a 6.1 letter mean gain from baseline in LLVA, which was statistically significant at p=0.0012.
94
We also assessed the rate of progression of geographic atrophy in the non-central geographic atrophy cohort relative to what has been observed in other studies. The typical rate of geographic atrophy progression in dry AMD is well understood from prior studies and the natural history, and we believe slowing of geographic atrophy progression could be a meaningful endpoint as we pursue approval by the FDA. This analysis was conducted using several types of imaging technologies including fundus auto-fluorescence, or FAF, an advanced imaging technique for observing the fundus, which is the interior surface of the eye opposite the lens including the retina, optic disk, macula, fovea and posterior pole, FAF squared, or FAF SQRT, a calculation performed to eliminate dependence of growth rates on lesion measurements, and optical coherence tomography, or OCT, a non-invasive imaging test which uses light waves to take cross-sectional pictures of the retina, also squared, or OCT SQRT, to eliminate dependence of growth rates on lesion measurements. Each of these imaging technologies showed that six months’ treatment with elamipretide was associated with slower progression of geographic atrophy than was observed in prior published studies conducted by others (assuming, for prior studies which were completed over a longer time period, a linear progression of geographic atrophy enabling calculation at the 6-month time point). FAF demonstrated mean growth of 0.50 mm2, versus 0.91 mm2 mean observation from ten prior studies over a similar time period (assuming linear progression), FAF SQRT demonstrated mean growth of 0.136 mm, versus 0.19 mm mean observation from five prior studies over a similar time period (assuming linear progression), and OCT SQRT demonstrated mean growth of 0.11 mm, versus 0.18 mm mean observation from five prior published studies over a similar time period (assuming linear progression). By comparison, in the once monthly interventional and sham arms of the FILLY trial, FAF SQRT demonstrated mean growth of 0.156 and 0.17 respectively, and in the 2 mg interventional and sham arms of the GATHER-1 trial, FAF SQRT demonstrated mean growth of 0.14 and 0.195 respectively. Although the patient populations in these trials do not entirely overlap since we are studying non-central GA and smaller lesion sizes, we believe reported growth rates would be similar utilizing this measurement.
We recently conducted a post hoc analysis of the ReCLAIM trial data, to assess whether there was any relationship between baseline mitochondrial dysfunction and improvement in visual function which could help inform inclusion criteria for any Phase 3 trial we may initiate in the future. Most of the mitochondria in the retina are located in the ellipsoid zone (EZ), which is a layer of the retina located proximate to the photoreceptors and near the retinal pigment endothelium, as shown below in an OCT image of a normal eye. It has been previously reported that patients with the early stages of AMD exhibit reduced relative intensity of the EZ when compared with healthy controls, and that in eyes with GA, retinal areas with disrupted EZ have a greater risk for progressing to dense areas of atrophy compared with areas with intact EZ.

95
The data, presented at the 2021 Association for Research in Vision and Ophthalmology (ARVO) Virtual Annual Meeting, showed that for patients with GA treated with elamipretide for 24-weeks, improvements from baseline in LLVA were significantly correlated to both baseline macular percentage of total EZ attenuation (r = -0.72; P = 0.002) and baseline EZ-RPE volume (r = 0.62; P = 0.01), with eyes gaining 2 or more lines of LLVA having significantly less macular total EZ attenuation at baseline (9.0% vs 27%; P = 0.03) and significantly less percentage area of macular GA (4.7% vs 15.6%; P = 0.004), as shown below in select OCT images. We believe that these data support the mitochondrial pathophysiology of vision loss in dry AMD, provide further support for our Phase 2b study inclusion criteria, which was modelled on our Phase 1 inclusion criteria, and offer potentially valuable enrichment strategies for any future Phase 3 trials we may initiate.

We initiated ReCLAIM 2, a Phase 2b placebo-controlled clinical trial with once daily subcutaneous dosing in subjects with non-central geographic atrophy in March 2019. ReCLAIM 2 is designed to enroll up to 180 subjects, of whom 120 will be treated with an elamipretide 40 mg once daily subcutaneous injection, and the remainder will receive placebo for a 48-week period. Eligible subjects are required to have a geographic atrophy area greater than or equal to 0.05mm2 and less than 10.16 mm2, BCVA greater than or equal to 55 letters and greater than 5 letters low luminance deficit. Efficacy endpoints in ReCLAIM 2 include BCVA, FAF, OCT, low-luminance best-corrected visual acuity, low-luminance reading acuity, National Eye Institute Visual Function Questionnaire-39 score, visual function by the Low-luminance Questionnaire and conversion to choroidal neovascularization.
We completed enrollment in the ReCLAIM 2 clinical trial in February 2021, and we expect to have data during the second quarter of 2022.
Although we believe that individuals experiencing a progressive decline in visual activity will be compliant with daily subcutaneous injections, we are evaluating the feasibility of developing a sustained-release formulation for intravitreal injection for this indication. We expect to have data to inform a decision on this by the time we receive the ReCLAIM-2 data in early 2022 so that we can make a determination regarding Phase 3 formulation.
LHON. LHON is a maternally inherited genetic disorder caused by mtDNA mutations in genes encoding for subunits of Complex I of the electron transport chain. LHON is characterized by retinal ganglion cell dysfunction and degeneration of the optic nerve in the back of the eye leading to bilateral blindness. LHON primarily affects young men between the ages of 18 and 30, although it can affect women as well as younger children. The initial clinical expression of LHON is often a sudden, painless, acute or sub-acute central vision loss, frequently accompanied by loss of color vision and reduced visual acuity. We have received Fast Track and Orphan Drug designations from the FDA for the development of elamipretide for this indication.
We estimate that approximately 10,000 individuals in the United States have LHON, of whom an estimated 70% have the genetic mutation, G11778A, that we studied in our Phase 2 clinical trial. Currently, there are no treatments approved by the FDA for the treatment of LHON. Raxone (idebenone), a synthetic form of Coenzyme Q10, has been approved by the EMA for the treatment of LHON, although actual availability varies by country. Raxone has orphan designation, and its marketing authorization was granted by the EMA under its authority to grant marketing authorization under “exceptional circumstances” due to the lack of comprehensive data on efficacy and safety.
We believe based on preclinical and early clinical findings that systemic elamipretide may be beneficial for subjects with LHON. Preclinically, elamipretide has been observed to improve mitochondrial function under oxidative stress conditions in mouse-derived retinal ganglion cells, the type of cells most affected by LHON, by dose-dependently reducing ROS production, mitochondrial depolarization, cytochrome c release, morphological change, apoptosis and cell death. Experiments in a mouse model of acute traumatic optic neuropathy also suggest that systemic administration of elamipretide post-trauma may improve retinal ganglion cell survival and visual function, supporting
96
the plausibility of therapeutic benefit in the presence of LHON-associated, oxidative-stress mediated damage of the optic nerve.
We observed trends that we believe are suggestive of potential clinical benefit in our ReSIGHT Phase 2 clinical trial, which was a 52-week, randomized, double-masked, vehicle-controlled Phase 2 clinical trial of elamipretide topical ophthalmic drops in 12 subjects with the G11778A mutation of LHON followed by an open label extension. In the double-masked portion of the trial, subjects were randomized to a single drop of elamipretide 1.0% ophthalmic solution or placebo, twice daily, with four subjects receiving elamipretide dosed in both eyes and eight subjects receiving elamipretide in one eye and placebo in the other eye. The endpoints were safety, tolerability and efficacy. Although the trial did not meet its primary endpoint of change in best corrected visual acuity, or BCVA, measured as the average change over week 20 to 52 from baseline, we believe this was largely due to unexpected variability in the placebo group, in which two subjects experienced gains in BCVA of more than 20 letters on a standard eye chart and one subject experienced a loss of more than 20 letters, resulting in no change overall between elamipretide- and placebo-treated groups. Improvement in elamipretide-treated eyes was observed across several other endpoints over the treatment period. In particular, improvement in Humphrey’s visual field, which measures the expanse of space visible at a given instance without moving the eye, was observed (mean improvement of 0.8, p=0.02, when averaged over the entire treatment period). A post hoc analysis of the Humphrey’s visual field data demonstrated that most of the change was attributable to improvement in the central visual field (mean improvement of 1.96, p=0.00003), which is expected to be most compromised in LHON, which is a disease of central blindness. This analysis is shown below.
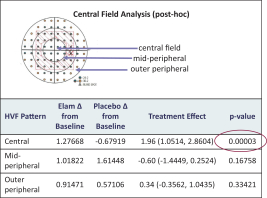
After six months of open-label extension, we observed continued improvement from study baseline in multiple parameters of visual function, including BCVA, color sensitivity, contrast sensitivity and visual field, particularly central visual field, as illustrated below, in which the black line depicts the average between each subject’s eyes during the double-masked portion of the trial and the gray line depicts the average between each subject’s eyes during the open-label portion of the trial.
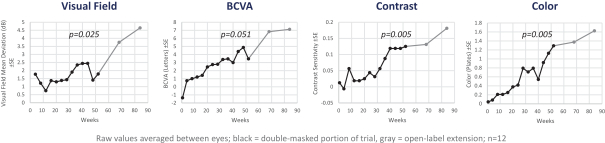
We met with the FDA in June 2019 to review the data from the ReSIGHT trial. FDA concurred with our proposal to conduct a pivotal Phase 3 clinical trial, which we proposed to conduct utilizing our subcutaneous formulation of elamipretide. We are alternatively evaluating the feasibility of developing a sustained-release formulation for intravitreal injection for this indication. We expect to have data to inform this decision in early 2022 and will make a final decision regarding formulation and Phase 3 commencement at that time.
97
Rare neurological diseases
Increasing evidence suggests that mitochondria are involved in both inherited and age-related neurological diseases. Although we have designed several of our pipeline compounds, including SBT-272 and the SBT-550 series of compounds, to optimize their suitability for neurological diseases of mitochondrial dysfunction, we have also observed preclinical and clinical signals of efficacy with elamipretide in certain neuromuscular diseases during the course of our development of elamipretide for primary mitochondrial myopathy. We plan to continue to develop elamipretide for these specific neuromuscular diseases, while exploring broader rare neurological diseases for development using our pipeline compounds.
nPMD. We are planning to initiate a Phase 3 clinical trial of elamipretide for primary mitochondrial disease arising due to nDNA mutations (nPMD). We observed improvement in this subgroup of patients in our previous primary mitochondrial myopathy development program, which enrolled participants irrespective of their genetic diagnosis (i.e., including both nDNA and mtDNA mutations). We have received Fast Track and Orphan Drug designations from the FDA for the development of elamipretide for this indication.
We studied elamipretide in three clinical trials for the treatment of primary mitochondrial myopathy, a disease characterized by debilitating skeletal muscle weakness, exercise intolerance and fatigue accompanied by a confirmed molecular genetic diagnosis with mutations in one or more of an estimated 250 different nDNA or mtDNA genes. Although we did not observe improvement overall in a Phase 3 clinical trial in primary mitochondrial myopathy, we did observe improvement in a prespecified subgroup of patients with nuclear genetic mutations, most of whom had mutations in nuclear genes encoding for proteins necessary for mtDNA replication, or replisome-related mutations. Scientifically, this may be due to the fact that cardiolipin, which is the target for elamipretide, is involved in mitochondrial protein and metabolite transporters and machinery associated with mtDNA packaging and replication.
A post hoc analysis of the prespecified subgroup of subjects with nDNA mutations (n=59) enrolled in MMPOWER-3 trail demonstrated a mean increase of 25.5 meters from baseline in distance walked after 24 weeks (which was the end of treatment) for subjects receiving elamipretide compared to only a 0.3-meter increase for subjects receiving placebo, a 25.2-meter difference between the two groups favoring elamipretide (p= 0.03), as shown below. Additionally, an exposure-response analysis, which is an analysis conducted to establish the relationship of blood concentrations of elamipretide to its pharmacologic effect on the distance walked on the 6MWT, showed that the increase in distance walked on the 6MWT for subjects with an nDNA mutation was related to blood concentrations of elamipretide (p = 0.03).
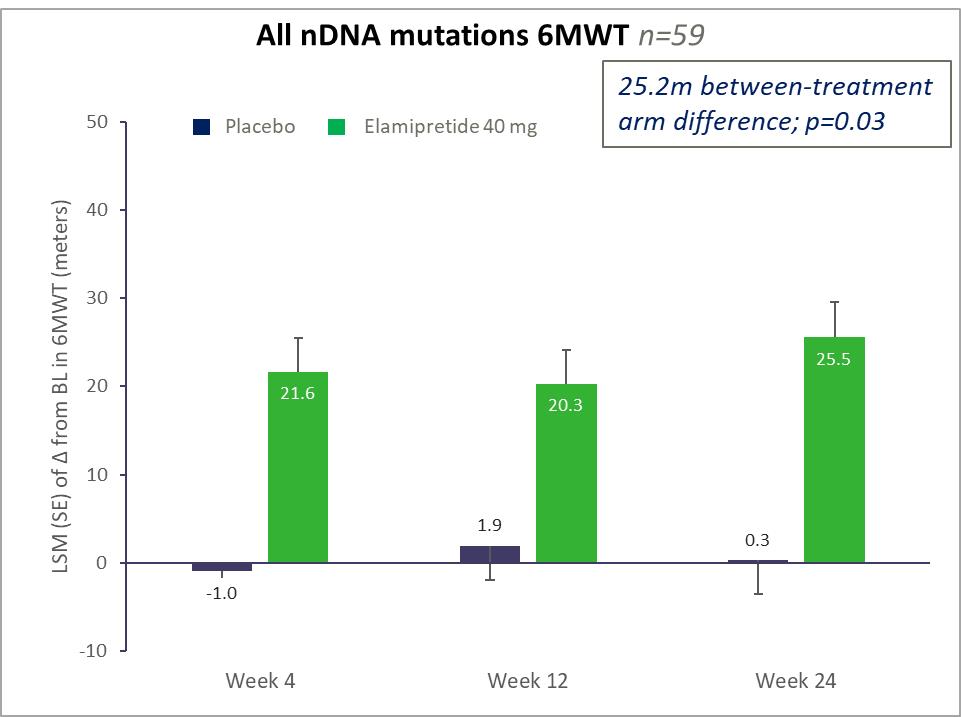
Most of the subjects in the prespecified nDNA subgroup had nDNA mutations affecting the nuclear expression of components of the mitochondrial replisome, or replisome-related mutations. The mitochondrial replisome is
98
responsible for transcribing mtDNA within the mitochondria. Most common among these replisome-related mutations were mutations in polymerase gamma, or POLG, which is the most common cause of primary mitochondrial disease. POLG-related disorders typically affect tissues with high energy demand, such as the nervous system, muscle and liver, and are progressive conditions that show direct correlation between age of onset and severity. Other replisome-related mutations included mutations in the TWINKLE, MPV17, DGUOK, TK2 and RRM2B genes. Although we plan to enroll patients with nPMD in our proposed Phase 3 clinical trial irrespective of their nDNA mutation, we plan to conduct our primary efficacy analysis only on the patients with replisome-related mutations.
As with most primary mitochondrial diseases, symptoms of nPMD can affect multiple organ systems including the brain and the skeletal muscle. To ensure a more homogenous and less variable cohort of patients in our Phase 3 clinical trial, our key opinion leaders encouraged us to also require that patients have a diagnosis of progressive external ophthalmoplegia, or PEO, which is the progressive weakening of the eye muscles thought to be almost always observed with skeletal muscle involvement in this disease. A post hoc analysis of those nPMD patients with replisome-related mutations and PEO, on whom we proposed to conduct our primary efficacy analysis in our proposed Phase 3 clinical trial, showed that patients who met these criteria and were randomized to elamipretide had a greater increase in 6MWT, as shown below.
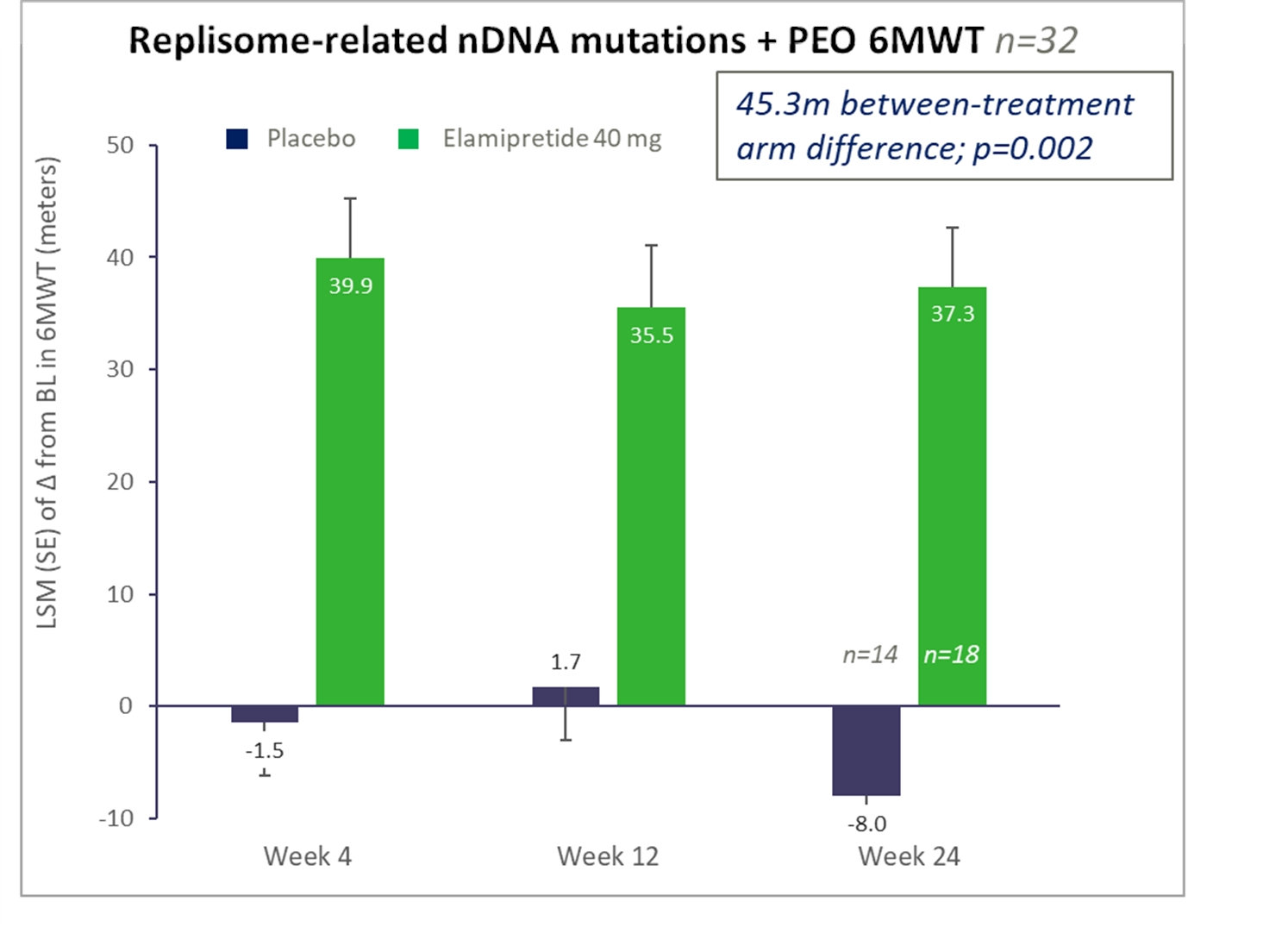
99
Our proposed Phase 3 clinical trial is a double-blind, placebo controlled, global trial enrolling 90 patients with nPMD due to replisome-related mutations (primary analysis population) and up to 40 additional patients with other nDNA mutations. Patients will be randomized 1:1 to elamipretide 60 mg SC daily or placebo for 48-weeks, as shown below. The primary efficacy endpoint will be distance walked on the 6MWT; secondary endpoints will include other assessments of axial muscle strength and patient reported outcomes.
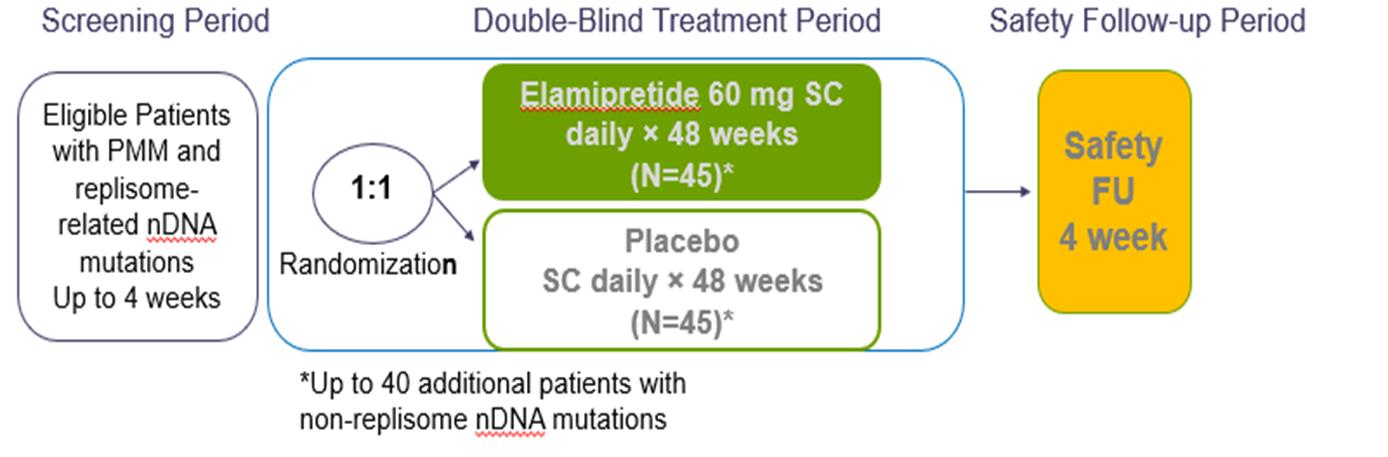
We have reached alignment with the FDA regarding this trial design and plan to commence enrollment during the second half of 2021.
Elamipretide Safety Data
We have a significant amount of clinical trial data indicating that elamipretide is generally well tolerated. As of December 31, 2020, 27 clinical trials had been completed with single and multiple intravenous and subcutaneous administrations of elamipretide at dose levels ranging from approximately 0.7 mg/day to 300 mg/day. These included 15 clinical pharmacology studies enrolling approximately 312 healthy subjects in which the primary objective was to assess safety rather than to treat a disease state, and 12 clinical trials enrolling approximately 618 subjects across multiple patient populations, including subjects with primary mitochondrial myopathy, skeletal muscle mitochondrial dysfunction, stable chronic heart failure, acute coronary syndrome and acute kidney injury and dry AMD.
The most commonly reported systemic treatment-emergent adverse events, or TEAEs, that were reported in greater frequency among elamipretide-treated subjects as compared to placebo-treated subjects included headache and dizziness in both single dose and repeat dose cohorts. TEAEs observed exclusively in repeat-dosed elamipretide-treated patients included incidences of increased blood immunoglobulin E (though no associated clinical signs or symptoms were present), urinary tract infections and viral gastroenteritis, as well as upper respiratory tract infections in an open-label trial in an elderly population where there was no placebo-control group. A mild to moderate increase in eosinophils, a variety of white blood cells that combat parasites and infections and control mechanisms associated with allergy and asthma, were observed in a significant percentage of patients treated with longer-term dosing regimens, with no associated clinical signs and symptoms. These appear to decrease to within normal limits with longer duration of elamipretide administration and return to pre-treatment levels after the end of elamipretide treatment. In addition, injection site reactions were reported in the majority of subjects receiving elamipretide by subcutaneous injection; most commonly these entailed mild redness, swelling and itchiness which usually resolved within four hours of dosing.
Earlier Clinical Trials of Elamipretide
We have studied elamipretide in clinical trials in several diseases associated with aging, including studies enrolling subjects with reduced skeletal muscle mitochondrial function, subjects with heart failure with reduced ejection fraction, or HFrEF, subjects with HFpEF, subjects undergoing percutaneous transluminal renal angioplasty, subjects with acute coronary syndrome and subjects with Fuchs. These trials were designed as small proof-of-concept studies to inform our decision whether to progress later stage development in these indications, and as such were generally not well powered to achieve statistical significance. Although we have decided not to independently progress development of elamipretide for these common disease indications, we saw signs of clinical benefit from treatment with elamipretide in several of these indications, which may help inform our future development of pipeline compounds for age-related diseases.
100
SBT-272
SBT-272, our second clinical-stage pipeline compound, is a second-generation novel peptidomimetic that targets the mitochondria, stabilizing mitochondrial function under conditions of oxidative stress. SBT-272 has been shown to increase ATP production and decrease levels of ROS in dysfunctional mitochondria in preclinical studies. Our primary objective in designing this compound was to increase brain exposure relative to elamipretide, as we believe that mitochondrial therapeutics may be beneficial in various neurological disorders. We also sought to improve the potency and stability of the compound relative to elamipretide.
An important differentiating aspect of SBT-272 from elamipretide is that the compound demonstrates higher mitochondrial uptake and greater concentrations in the brain than elamipretide. In early experiments, SBT-272 demonstrated approximately three times greater maximum concentration in the brain of rats relative to elamipretide, in each case dosed 10 mg/kg subcutaneously. SBT-272 has demonstrated more than 25 times greater area under the drug concentration-time curve in the brains of rats relative to elamipretide, in each case dosed 10 mg/kg subcutaneously, suggesting significantly higher brain exposure and residence time. In addition, the compound has shown greater than six times higher mitochondrial uptake relative to elamipretide in cell-based assays of isolated mitochondria, suggesting improved potency. In a murine stroke model, SBT-272 demonstrated improved respiratory control ratio in brain mitochondria after ischemia reperfusion injury relative to placebo (p=0.006), suggesting neuroprotective benefit.
We have conducted a Phase 1 clinical trial of SBT-272 in healthy volunteers. We plan to conduct toxicity studies in 2021 to support a proposed Phase 1 trial in early 2022 to assess the safety and tolerability of SC dosing and different doses. We hope to commence Phase 2 studies by the end of 2022. We have conducted and are continuing to conduct studies in preclinical models of neurological diseases to help inform selection of indication for Phase 2. We are also evaluating SBT-272 in preclinical ophthalmic disease models.
SBT-272 Safety
In January 2020, we initiated a double-blind, placebo-controlled, single-ascending dose study enrolling up to 40 healthy subjects across multiple cohorts. Based on SBT-272’s improved oral bioavailability relative to elamipretide in early animal studies, SBT-272 was administered orally in the study. As a primary objective, the study evaluated the safety and tolerability of SBT-272. Secondary objectives included an analysis of the pharmacokinetic profile and appropriate dose range. Preliminary results from a Phase 1 clinical trial in healthy human volunteers completed during 2020 did not reach desired drug exposure levels. We expect to conduct additional toxicology studies in 2021 to enable a Phase 1 trial to assess safety, tolerability and different doses of subcutaneous SBT-272 in early 2022, after which we hope to progress into a Phase 2 clinical trial in patients during the second half of 2022.
Preclinical Data of SBT-272 for Rare Neurological Diseases
We have conducted preclinical studies in several neurological disease models to help inform selection of Phase 2 indications.
Superoxide dismutase 1 pathology. Mutations in superoxide dismutase 1, or SOD-1, have been associated with ALS, a progressive neurodegenerative disease characterized by motor neuron deterioration and muscle atrophy. In a preclinical SOD-1 mouse model, 60 model mice were randomized to daily intraperitoneal injections of placebo, 0.5 mg/kg of SBT-272 or 5.0 mg/kg of SBT-272 for up to 10 weeks. The 10 male mice treated with the higher dose of SBT-272 demonstrated a statistically significant delay in the onset of neurological symptoms and increase in lifespan compared with male mice treated with placebo. Statistically significant reductions in circulating plasma levels of neurofilament light chain—a biomarker of nerve damage—were also noted with the 5.0 mg dose versus placebo. Significant differences were not seen with SBT-272 versus placebo in the female mice, which are known to present with a milder phenotype and lower levels of neurofilament light chain.
Transitive response DNA/RNA-binding protein 43 kDa pathology. Transitive response DNA/RNA-binding protein 43 kDa, or TDP43, is a nDNA encoded protein that has been identified as the major component of the pathological hallmark, ubiqui-tin-positive protein inclusions, in patients with ALS and frontotemporal lobar degeneration, or FTLD, Lewy Body Dementia, or LBD, Progressive Supranuclear Palsy, or PSP, and Alzheimer’s Disease, or AD. These and other characteristic TDP-43-related pathological features are usually referred to as TDP-43 proteinopathy. In mutant TDP43 primary upper motor neurons, SBT-272 improved neurite length and branching.
Alpha-synucleinopathy. Alpha-synuclein, or aSyn, is a protein expressed in the presynaptic terminals of the brain. Pathologic aggregation of aSyn has been demonstrated across several neurological diseases, called alpha-synucleinopathies, including LBD, Parkinson’s disease, or PD, and multiple system atrophy, or MSA. We evaluated SBT-272 in a preclinical model of alpha-synucleinopathy, which showed neuroprotective benefit.
101
Discovery Compounds
We have an active discovery and development program focused on novel compounds targeting mitochondria. Mitochondria have been an extremely challenging therapeutic target, due in part to difficulty in targeting delivery of drugs to mitochondria. Successful delivery requires traversing not only the cell membrane, but also achieving intracellular diffusion/transport through the outer membrane of the mitochondria to act on processes in the inner membrane space or the matrix. We believe the differentiated mitochondrial targeting characteristics of our compounds, our development of proprietary assays to screen new compounds for mitochondrial targeting capability and pharmacologic activity, and our experience working with various models of mitochondrial dysfunction position us to lead the next generation of development of mitochondrial product candidates, including SBT-272, that are improved relative to elamipretide.
We have developed multiple series of novel compounds with improved pharmacokinetic properties. These include over 100 different compounds, including peptidomimetics, small molecules and novel peptides, that we have screened to broaden our existing mitochondrial product candidate portfolio. We are focused on producing agents with mitochondrial therapeutic potential and improved properties over our first-generation compounds, by altering the rate and extent of absorption, the bio-distribution and/or the routes of metabolism and excretion. We are evaluating certain of these compounds in models of ischemic reperfusion injury, including burn, ophthalmic diseases and cardiac diseases.
Compounds within the SBT-550 family of compounds, which are small molecules that may be suitable for oral formulations, appear to be mechanistically differentiated from elamipretide, SBT-272 and SBT-20. Preliminary in vitro studies in primary fibroblast cells from FRDA patients stressed by eliminating the glutathione defense mechanism (typically leading to cell death) show dose-dependent improvements in cell viability (survival) with SBT-550 family compounds. We plan to evaluate compounds in the SBT-550 family for rare neurological indications such as FRDA and Leigh’s syndrome, a severe neurological condition affecting an estimated one in 40,000 newborns.
Carrier program
We have also conducted experiments in our carrier program in which we observed that we can use our proprietary compounds as vectors or carriers to selectively deliver various therapeutic payloads to mitochondria, conferring organelle specificity to promising therapies. Many individuals diagnosed with primary mitochondrial disease, for which there are no therapies approved by the FDA, take a so-called “mito cocktail” of vitamins and supplements, usually in high doses and comprising up to 50 pills per day if not compounded. These may typically include co-enzyme Q-10, or Co Q-10, or its analogs, L-carnitine, B vitamins and antioxidants. The reason these are taken in such high doses is because delivery to the mitochondria is likely confounded by permeability challenges traversing the cell and outer mitochondrial membranes. By contrast, we have observed mitochondria-targeting capabilities in our proprietary compounds and have also observed that we can conjugate payloads to our compounds and direct the conjoined carrier/payload to the mitochondria.
For example, idebenone is a Co Q-10 analog that introduces electrons into the electron transport chain downstream of complexes I and II, a promising mechanism for bypassing defective complexes in genetic diseases. Because idebenone is poorly absorbed and does not specifically target mitochondria, it has demonstrated limited pharmacologic activity even at high doses. Preliminary preclinical data show that our idebenone-conjugated peptide was effective at stimulating complex III enzyme activity at a concentration of approximately 100 times lower than the dose achieved with systemically administered idebenone. We believe this is promising support for the potential of our carrier program, and we are actively evaluating other mitochondrial beneficial payloads for evaluation in this program.
Development Funding Agreement
On October 30, 2020, we entered into a development funding agreement, or the Development Funding Agreement, with Morningside Venture (I) Investments Limited, or MVIL, under which MVIL agreed to provide funding to us to support our efforts to secure regulatory approval for elamipretide and to develop elamipretide for the treatment of Barth, dry AMD, FRDA, Duchenne cardiomyopathy, nPMD and LHON, which we collectively refer to as the Designated Indications.
Under the Development Funding Agreement, MVIL paid us $20 million upon execution of the Development Funding Agreement, and in February 2021 paid us $10 million upon our completing enrollment of our RECLAIM-2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD. MVIL has also agreed to pay us $5 million upon the submission of an NDA to the FDA for elamipretide for the treatment of Barth, the Tranche 3 Milestone Event.
102
Prior to the occurrence of the Tranche 3 Milestone Event, we may agree to add additional investors to the Development Funding Agreement (each, an Additional Investor, and any such Additional Investors together with MVIL, are referred to as the Investors), subject to the prior written consent of MVIL. The commitment from each such Additional Investor will be on the same terms and subject to the same conditions as the initial commitments, and, together with the commitment from MVIL, the aggregate commitments of the Investors will not exceed $70 million without the consent of MVIL. Prior to any Investor having an obligation to provide the funding due upon the occurrence of the Tranche 3 Milestone Event, we must satisfy certain customary conditions.
In addition, upon the mutual agreement, at any time after we receive positive data from a clinical trial in a Designated Indication, we may request that the Investors make additional commitments of up to an additional $35 million in the aggregate, or the Additional Funding. Each Investor may agree to fund such commitment or not in its sole discretion. In May 2021, MVIL agreed to fund an additional $30 million in Additional Funding during 2021 based on the positive post hoc analysis of data from our ReCLAIM clinical trial, showing the relationship between mitochondrial health and elamipretide’s potential to improve visual function, and the positive post hoc analysis from our primary mitochondrial myopathy trial, showing that patients with nPMD appeared to respond to elamipretide therapy and supporting further development efforts.
During the term of the Development Funding Agreement, we agreed to use commercially reasonable efforts to (i) seek and maintain regulatory approval of elamipretide for the treatment of Barth in the United States and (ii) initiate clinical trials in two of the Designated Indications other than Barth, which are referred to together as the Development Efforts.
We are required to make success payments to the Investors, or Success Payments, upon receipt of an approval of elamipretide, or a Regulatory Approval of a NDA by the FDA or a marketing authorization application by the EMA for the treatment of (i) dry AMD, or a Common Approval, and (ii) Barth, FRDA, Duchenne cardiomyopathy, nPMD or LHON (each, an Orphan Approval) as follows, subject to certain adjustments:
| ▪ | If the first Regulatory Approval is an Orphan Approval, we will pay Success Payments of $2 million and then an additional $158 million in the aggregate in seven additional annual payments; and |
| ▪ | If the first Regulatory Approval is a Common Approval, or upon a second regulatory approval (whether a Common Approval or an Orphan Approval), we will make total Success Payments reflecting a 27% internal rate of return over a seven-year term following such approval. |
All Success Payments will be proportionately adjusted in the event that the actual funding received by us from Investors is lower or greater than $70 million (including as a result of the payment of the Additional Funding).
If our board of directors determines to seek a Regulatory Approval from both the FDA and EMA, then 66% of each applicable Success Payment will be due upon Regulatory Approval by the FDA and each applicable anniversary thereof and 34% of each applicable Success Payment will be due upon Regulatory Approval by the EMA and each applicable anniversary thereof.
At any time within 60 days of a receipt of (a) a Common Approval, if such approval is the first Regulatory Approval or (b) the second Regulatory Approval, if the first Regulatory Approval is an Orphan Approval, we have the right, at our option, to make one-time cash payments to the Investors to buy out all or a portion of the future unpaid Success Payments for a price that reflects a discount rate of 5%.
In addition, we have agreed that our obligations to the Investors under the Development Funding Agreement will be subordinated to its existing indebtedness owed to Hercules Capital, Inc., or Hercules, under our Loan and Security Agreement, as amended. We, Hercules and the Investors have entered in a customary subordination agreement.
Upon execution of the Development Funding Agreement, we issued a warrant to MVIL exercisable for 46,153,846 ordinary shares at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. Upon MVIL’s payment to us in February 2021, we issued a warrant to MVIL exercisable for 18,750,000 ordinary shares at an exercise price of $0.16 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. Upon MVIL’s payment to us in May 2021, we issued a warrant to MVIL exercisable for 18,461,538 ordinary shares at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The warrants have three-year terms. We agreed to issue to substantially identical warrants to any Additional Investors.
The Development Funding Agreement terminates upon the payment of all Success Payments or the final Buyout Payment owed to the Investors, unless earlier terminated. The Development Funding Agreement may be terminated by us or a majority of the Investors following failure to receive Regulatory Approval which would be deemed to occur upon (a) the failure to receive Regulatory Approval in at least one of the Designated Indications
103
within five years after the occurrence of the Tranche 3 Milestone Event, despite exercises of commercially reasonable efforts or (b) the reasonable determination of a majority of the Investors that the research results do not support Regulatory Approval due to failure of the clinical trials to achieve their primary endpoint. A majority of the Investors may terminate the Development Funding Agreement in the event of a (i) breach by us of our obligations with respect to the Development Efforts or its payment obligations to the Investors, (ii) material breach by us of certain representations, warranties or covenants in the development funding agreement, (iii) a change of control of us or (iv) a majority of the Investors reasonably determine that we will likely be prevented from further developing elamipretide for the Designated Indications and its future value may be adversely affected in a material way due to third-party patents. We may terminate the Development Funding Agreement (i) for convenience for any reason or no reason at any time prior to the receipt of the first Regulatory Approval or (ii) in the event of a product safety concern.
In certain instances, upon the termination of the Development Funding Agreement, we will be obligated to pay the Investors a multiple of the amounts paid to us under the Development Funding Agreement, including specifically:
| (i) | 300% of such amounts, less any Success Payments actually made, in the event that (i) the Investors terminate the agreement due to specified fundamental breaches of the agreement by us or (iii) we terminate for convenience; |
| (ii) | 150% in the event the agreement is upon a change of control of us; |
| (iii) | 100% in the event of a termination due to a breach of a representation, warranty or covenant, plus simple interest; and |
| (iv) | 100% in the event of a termination due to third party patents. |
In addition, if following a termination for any reason other than due to a breach of representation, warrant or covenant, due to third party patents or change in control, we continue to develop elamipretide and obtain a Regulatory Approval, we will make the Success Payments to the Investors as if the Development Funding Agreement had not been terminated less any payments made upon termination.
Manufacturing
We do not have any manufacturing facilities. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical studies and clinical trials, as well as for commercial manufacture if our product candidates receive marketing approval. We have also obtained key raw materials for elamipretide from third-party manufacturers. For elamipretide, we intend to identify and qualify a single manufacturer to provide the active pharmaceutical ingredient and other manufacturers for fill-and-finish services for each of our elamipretide-containing drug products prior to submission of an NDA to the FDA. This approach allows us to reduce the risk to NDA approval by focusing our resources on preparing only one manufacturing site for active pharmaceutical ingredient and one for each drug product for pre-approval inspections. We can sufficiently reduce the supply risk usually associated with a single source of product based on our capability to build pre-launch inventory and the relatively small demand for material projected for our rare disease indications.
All of our product candidates are small molecules and are manufactured in reliable and reproducible synthetic processes from readily available starting materials. The chemistry is amenable to scale up and does not require unusual equipment in the manufacturing process. Elamipretide has been produced historically by a solid-phase manufacturing process that has been commonly used to produce commercial peptides. Due to a lack of scalability, we deemed this process undesirable for production of commercial quantities of elamipretide. A solution-phase process for producing elamipretide as a hydrochloride salt has been developed and implemented at a contract manufacturing site at a scale sufficient to meet the projected commercial demand. The solution-phase process for manufacturing is proprietary to us, and the equipment and the unit operations used in the process are not unique to any particular contract manufacturer. We have transferred this process to contract manufacturing sites capable of using such processes to manufacture large quantities of similar drug substances, and we have completed the drug supply for pivotal clinical trials and have progressed into commercial production. Manufacturing at a higher production scale has led to a significant reduction in our cost-of-goods and provided us with the ability to respond to any need to supply large clinical trials or unanticipated commercial demand in the future. Following FDA review of test results demonstrating the same/similar identity, quality, purity and strength of elamipretide from early and commercial-scale processes, the FDA has stated that non-clinical and clinical trials with drug substance from the former processes can be used to support further development and registration of elamipretide made by the commercial process.
We have active clinical programs for which our contract manufacturing organizations, or CMOs, are routinely manufacturing a sterile solution product for subcutaneous injection. Our CMOs have successfully produced our
104
product on a scale of tens of thousands of units and shown, using validated stability-indicating methods, that the product would meet specifications over a shelf life typical of commercial products. We believe we are well positioned to complete validation of our manufacturing processes at commercial-ready CMOs and support a commercial launch of elamipretide-containing products.
Intellectual Property
We strive to protect the proprietary technologies that we believe are important to our business, including seeking and maintaining patent protection intended to cover our lead product candidates, elamipretide and SBT-272, and related compositions, our core clinical applications and other know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary or intellectual property rights worldwide. Our patent portfolio, which includes patents and patent applications that we own, as well as those that we have exclusively in-licensed, is structured to provide layers of protection for the proprietary technologies central to our business. Our portfolio includes claims to the elamipretide and SBT-20 peptides, the SBT-272 peptidomimetic, the 550 family of small molecules, compositions comprising the same, and use of the peptides and other therapeutically active molecules for our core clinical applications. As of December 31, 2020, the patent portfolio included 393 granted patents (58 U.S., 335 foreign, which include individual national patents based on granted European patents) and 188 pending applications, including provisional applications (57 U.S., 125 foreign, 6 Patent Cooperation Treaty).
We also rely on trade secret protection, technical knowledge, and continuing technological innovation to develop and maintain our proprietary and intellectual property position. We seek to protect our proprietary information, in part, using confidentiality agreements with our collaborators, scientific advisors, employees, consultants and select contractors, and invention assignment agreements with our employees.
We also have agreements with selected contractors, consultants, scientific advisors and collaborators requiring assignment of inventions or, in limited cases, the grant of an exclusive, worldwide license or option to license intellectual property rights developed in the course of their work with or for us. As with other biotechnology and pharmaceutical companies, our capacity to obtain, maintain and protect our proprietary and intellectual property positions for our products and technologies depends on our continued ability to obtain relevant patent rights and to enforce those patent rights, if necessary. However, patent applications that we may file or license from third parties may not necessarily result in the grant of rights. We also cannot predict the scope of rights that may be granted to us in the future, our desire or ability to seek enforcement of any granted rights, or the willingness of courts or other administrative bodies to uphold or enforce our rights.
In addition, any currently issued patents or any future patents, should they issue, may be challenged, invalidated, or circumvented, such as through district court proceedings or inter partes review. For example, we cannot be certain of the priority of inventions covered by pending third-party patent applications, and proceedings to establish our rights could result in substantial costs, even if the eventual outcome is favorable. Due to the extensive time required for clinical development and regulatory review, it is possible that, before any of our product candidates can be commercialized, any related patent right may expire or its term may have substantially run, leaving its remaining term in force for only a short period following commercialization. To the extent that occurs, the possible commercial advantage conferred by such patents would be reduced. Accordingly, we have attempted to design a patent portfolio with both breadth and depth of potential protection, with the goal of maximizing coverage for elamipretide and related peptides and their uses in commercially relevant countries.
Elamipretide
Patent rights relating to elamipretide peptide and compositions comprising elamipretide have been granted in Australia, Canada, China, Europe, Hong Kong, Japan and the United States. The U.S. patent claiming elamipretide has an adjusted statutory expiration date in 2026, which includes 717 days of patent term adjustment, or PTA, granted by the USPTO upon issue of the patent. The foreign patents have a statutory expiration date in 2024. We hold an exclusive license to these rights from Cornell and the IRCM.
Patent rights to the use of elamipretide as a carrier for the transport of therapeutic molecules into a cell as well as related compositions have been granted or allowed in Australia, Canada, China, Europe, Hong Kong, Japan and the United States. The first of four issued U.S. patents in this family has an adjusted statutory expiration date in 2027, which includes 1,215 days of PTA granted by the USPTO upon issue of the patent. The remaining three issued U.S. patents and the foreign patents have a statutory expiration date in 2024. We hold an exclusive license to these patent rights from Cornell.
Patent rights related to compositions including elamipretide and a second therapeutic compound have also been granted. For example, claims directed to elamipretide-cyclosporine conjugates have been granted in the United States. The U.S. patent has a statutory expiration date in 2031. The USPTO recently granted a patent claims to a
105
conjugate of a portfolio peptide conjugated to frataxin, a therapeutic biological molecule associated with mitochondrial disorders. This patent has a statutory expiration date in 2035. These patent rights are owned exclusively by us.
Patents directed to methods of treating or preventing various diseases and medical conditions by administering elamipretide have been granted to us, or have been in-licensed by us, in a number of countries. Where possible, the scope of granted claims has been tailored to provide broad generic support encompassing a wide range of conditions as well as specific disease states. By way of example, there are granted patents related to the use of elamipretide to treat basic, adverse cellular events that contribute to disease, such as mitochondrial permeability transition, or MPT.
Patents related to MPT have been granted in Australia, Canada, China, Europe, Hong Kong, Japan and the United States. Two of the granted U.S. patents in this family have an adjusted statutory expiration date in 2026, and one of them is the same patent referred to above as covering the composition of elamipretide. The other two issued patents and all related foreign patents have statutory expiration dates in 2024. We hold exclusive rights to these patents by way of a license agreement with Cornell and the IRCM.
Our patent portfolio also protects or aims to protect the use of elamipretide to treat or prevent specific clinical indications. By way of example, our portfolio includes granted claims drawn to the use of elamipretide to treat diabetes, metabolic syndrome, renal diseases, certain cardiovascular diseases, ocular diseases, and neurodegenerative diseases (including Alzheimer’s disease, Huntington’s disease and ALS) that are in patents owned by us or in-licensed to us. Claims relating to the use of elamipretide to treat Barth were recently granted in the United States, Canada, Europe and Japan, and applications related to treating this clinical indication remain pending in the United States, Europe, China, Japan and Hong Kong. Claims related to using elamipretide to treat Friedreich’s ataxia were recently granted in the United States and applications related to treating this clinical indication remain pending in the United States and Canada. We have granted patents for treating Alport Syndrome in the United States, Europe and Japan, and applications related to treating this clinical indication remain pending in the United States, Europe, Canada, China and Hong Kong. We have granted patents in the United States, Canada, Europe and Hong Kong for the use of elamipretide in the treatment of Leigh syndrome, Alpers’ disease, ataxia-neuropathy disorders or progressive external ophthalmoplegia, with pending applications remaining in the United States, Europe, China, and Japan. We have patents granted in the United States, Europe, Australia, Canada, Hong Kong and Japan with respect to using elamipretide in the treatment of neuropathic pain, such as in pain induced by treatments with a chemotherapeutic agent like vincristine, with pending applications remaining in China and the United States. Other clinical indications covered by pending claims in our patent portfolio include LHON, primary mitochondrial myopathy, traumatic optic neuropathy, Senger’s syndrome and mitochondrial diseases associated with certain gene mutations such as POLG are pending in applications owned by us. Furthermore, our portfolio includes granted and pending claims drawn to the process we use to produce elamipretide, as well as certain intermediates and processes that produce crystalline drug substance. Our portfolio also includes granted and pending claims that disclose similar processes that we have conceived that could be competitive with our preferred process to produce commercial quantities of elamipretide.
SBT-272
Claims drawn to the SBT-272 peptidomimetic are currently pending in the United States, Europe, Australia, Canada, China, India, Israel, South Korea, and Japan. If granted (and not subject to any terminal disclaimer, statutory adjustment or extension of time), any patent claiming priority to these applications will have a statutory expiration date in 2038. These patent rights are owned exclusively by us. Claims drawn to using SBT-272 for the treatment of ALS and other neurodegenerative conditions such as α-synucleinopathies or TDP-43 proteinopathies, including Frontotemporal Lobar Degeneration (FTLD), Parkinson’s disease (PD), PD with dementia, dementia with Lewy bodies, and Multiple System Atrophy were filed in a United States and a Patent Cooperation Treaty, or PCT, application that claims priority to a now expired United States provisional patent application. If granted (and not subject to any terminal disclaimer, statutory adjustment or extension of time), any patent claiming priority to this U.S. provisional patent application is expected to have a statutory expiration date in 2040. These patent rights are owned exclusively by us.
Claims drawn to the use of the SBT-272 peptidomimetic and compositions for the treatment of ophthalmic indications is currently pending as a United States provisional patent application. If granted (and not subject to any statutory adjustment or extension of time), any patent claiming priority to this U.S. provisional patent application is expected to have a statutory expiration date in 2041. These patent rights are owned exclusively by us.
550 Family
Claims drawn to one class of compounds in the 550 family are currently pending in United States and PCT applications that claim priority to a now expired United States provisional patent application. If granted (and not
106
subject to any terminal disclaimer, statutory adjustment or extension of time), any patent claiming priority to these non-provisional applications will have a statutory expiration date in 2040. These patent rights are owned exclusively by us.
Claims drawn to a separate class of compounds in the 550 family are currently pending in United States and PCT applications that claim priority to a now expired United States provisional patent application. . If granted (and not subject to any terminal disclaimer, statutory adjustment or extension of time), any patent claiming priority to this provisional application will have a statutory expiration date in 2041. These patent rights are owned exclusively by us.
SBT-20
Claims drawn to the SBT-20 peptide and compositions comprising the SBT-20 peptide have been granted in Australia, Canada, Japan and the United States, and are pending in Europe. The U.S. patent has an adjusted statutory expiration date in 2026, which includes 717 days of PTA granted by the USPTO upon issue of the patent, and is the same patent described above as related to elamipretide peptide. The foreign patents have statutory expiration dates in 2024. We hold exclusive rights to the patents and applications by way of an exclusive agreement with either Cornell alone or Cornell in conjunction with the IRCM.
Our patent portfolio also protects or aims to protect the use of SBT-20 to treat or prevent specific clinical indications. By way of example, our portfolio includes granted claims drawn to the use of SBT-20 to treat complications of diabetes, renal diseases, ocular diseases, and neurodegenerative diseases that are owned by us or in-licensed. Claims relating to the use of SBT-20 to treat Parkinson’s disease, Alzheimer’s disease, Huntington’s, and ALS are granted in Australia, in a patent in-licensed to us with a statutory expiration date in 2024. Furthermore, our portfolio includes pending claims drawn to the SBT-20 peptide produced in crystalline salt forms.
We hold patent rights to additional pipeline compounds in the portfolio and are continuing to expand coverage in the United States and commercially relevant foreign jurisdictions. Subject matter for new filings is expected to include, but will not necessarily be limited to, the use of peptides or other therapeutically active molecules to treat additional disease indications, new combination therapies, new peptide formulations, new compositions and uses of the same.
The term of a patent depends upon the legal length of the term of patents in the jurisdiction in which it is issued. In most countries in which we file, the patent term is 20 years from the earliest date of filing of a non-provisional patent application. Patent term adjustment is a process of extending the term of a United States patent beyond the 20-year statutory patent term to accommodate for delays caused by the USPTO during prosecution. By contrast, a patentee or applicant may file a terminal disclaimer which disclaims or dedicates to the public the entire term or any terminal part of the term of a patent or patent to be granted.
In the United States, the term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Amendments permits a patent term extension of up to five years beyond the regularly scheduled expiration of a patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval by the FDA, only one patent applicable to an approved drug may be extended, and a given patent can only be extended based on one approved drug. Similar provisions are available in Europe and certain other jurisdictions to extend the term of a patent that covers an approved drug. We anticipate that we will apply for patent term extensions for relevant U.S. patents, if and when our pharmaceutical products receive FDA approval. We also anticipate seeking patent term extensions for issued patents in any jurisdiction where patent term extension is available, however, there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. Unless specifically indicated, the above statutory patent terms refer to the 20-year base statutory term and do not include any patent term adjustment or extension that may be available in any jurisdiction.
Cornell License Agreements
We have entered into several license agreements with Cornell and the IRCM, pursuant to which Cornell granted us specified exclusive, worldwide rights under patents related to elamipretide, SBT-20, and other technology described below, which we refer to collectively as the licensed patents. The original Cornell agreement was entered into with Cornell and the IRCM in April 2006 and subsequently amended in October 2010. Concurrent with our execution of the original Cornell agreement, we entered into a sponsored research agreement with Cornell in which we agreed to fund specified research at Cornell for three years. We retained the right to license inventions arising under such sponsored research agreement, as well as certain material transfer agreements entered into between us and
107
Cornell, through entry into license agreements on substantially the same terms as the original Cornell agreement. Such subsequent agreements under which we obtained rights under additional patent families, which we refer to as other Cornell license agreements, and collectively with the original Cornell agreement as the Cornell license agreements, were entered into in November 2010 and November 2011. In each of the Cornell license agreements, Cornell granted us an exclusive, worldwide license under specified patents and patent application families claiming certain inventions, including inventions related to elamipretide, SBT-20, certain other peptides and/or specified uses of the foregoing, which we refer to collectively as the licensed patents, to make, use, sell, lease, import, export or otherwise dispose of products or services that incorporate, utilize or are otherwise described and claimed in the licensed patents, which we refer to as the licensed products, in any and all fields. Our rights under the Cornell license agreements are subject to the rights of the United States government and other applicable restrictions imposed by the Bayh-Dole Act and its implementing regulations, and the rights of Cornell, and in some cases certain other specified institutions, to practice the inventions claimed in the licensed patents for educational and research purposes.
We have agreed to use best efforts (as defined in each of the Cornell license agreements) to commercialize licensed products and to achieve specified diligence milestones by specified target dates. We are also required to periodically set forth additional milestones until first commercial sale of a specified licensed product. We believe that to date we have met each diligence milestone with respect to our licensed products and the specific licensed indications and/or formulations which we are developing. If however we fail in the future to meet any diligence milestone within a specified period after the corresponding target date, our exclusive license under the applicable Cornell license agreement will convert to a non-exclusive license and, in the case of the original Cornell agreement, such conversion will occur only with respect to the peptide, indication and/or formulation that is subject of the unachieved milestone.
In connection with the licenses granted under the original Cornell agreement, we issued Cornell 666,667 ordinary shares. With respect to the other Cornell license agreements, we paid Cornell upfront license fees of $60,000, annual fees of approximately $60,000 and royalties on net sales, if any, by us and our sublicensees of any licensed product, on a product-by-product and country-by-country basis. Subject to specified reductions and royalty offsets, such royalties are calculated as a tiered, low-to-mid single digit percentage of net sales of licensed products under each of the Cornell license agreements, except that for licensed products under the original Cornell agreement, such royalties are calculated as a tiered, low single digit to sub-teen percentage of net sales, depending on patent coverage, amount of net sales and type of licensed product. Our obligation to pay royalties as to any licensed product extends until the later of the expiration of the last-to-expire valid claim of any licensed patent covering such licensed product or 15 years after the date of our first commercial sale of such licensed product. If a licensed product is covered by licenses granted under the original Cornell agreement and another Cornell license agreement, then, for each unit of product, royalties will only be due under the original Cornell agreement.
We are obligated to pay Cornell a low double-digit percentage of specified payments we receive in connection with granting a sublicense under the Cornell license agreements. We have also agreed to reimburse Cornell for its out-of-pocket expenses incurred in preparing, filing, prosecuting and maintaining the licensed patents, except for any licensed patents as to which we elect to waive our licensed rights. We also have agreed to pay Cornell annual license maintenance fees in the mid-five digits for the original Cornell agreement, and mid-four digits for each of the other Cornell license agreements starting on a date specified in each such agreement, in all cases until the first commercial sale of a specified type of licensed product under such agreement.
If Cornell identifies any licensed product that we are not actively developing or commercializing and we do not elect within a specified period to develop or commercialize such licensed product ourselves or through a sublicensee, or, if we do so elect, we do not then agree on reasonable diligence goals with Cornell or enter into an agreement with such a sublicensee within specified periods as to such licensed product, then Cornell may terminate our rights under the applicable Cornell license agreement for such licensed product.
Unless earlier terminated, each of the Cornell license agreements will remain in effect until the expiration or invalidation of the last of all licensed patents and as long as no licensed patent applications remain pending. Cornell (together with the IRCM in the case of the original Cornell agreement) can terminate a Cornell license agreement if we are in material breach of such license agreement, if we intentionally provide false reports, or if we are in default in our payment obligations, and we fail to cure such breach, false report or default within a specified period. In addition, Cornell can terminate the original Cornell agreement and certain of the other Cornell license agreements if we fail to achieve first commercial sale of a therapeutic licensed product by the date specified in the respective agreement (which, with respect to the original Cornell agreement, was December 31, 2020); however, there are a number of exceptions to Cornell’s termination right, including:
| ▪ | delays due to clinical development, including clinical trial enrollment challenges or data read-outs; |
108
| ▪ | delays due to regulatory matters; or |
| ▪ | delays due to other events over which we cannot exert direct control. |
We believe that our noncompliance is subject to the named exceptions, and to date we have not received any notice of termination from Cornell. If we receive a notice of termination from Cornell, we will have a 60-day period in which to cure the breach before any actual termination would occur. We can terminate any of the Cornell license agreements in its entirety or on a patent-by-patent, licensed product-by-licensed product or country-by-country basis if we have a reasonable basis for doing so by giving Cornell a specified number of days’ prior notice. We can transfer each of the Cornell license agreements with Cornell’s prior written approval (not to be unreasonably withheld) in the event of a sale of the Company, sale of assets or sale of shares, provided that such sale is not primarily for the benefit of creditors. If we fail to obtain Cornell’s prior written approval for such transfer, Cornell can terminate the respective agreement and require that the transfer of such agreement be voided. We cannot assign the Cornell license agreements without Cornell’s (and in the case of the original Cornell agreement, IRCM’s) written consent.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies; academic institutions and governmental agencies; and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
We are initially developing elamipretide for the treatment of rare primary mitochondrial diseases and common diseases of aging in which mitochondrial function is impaired. There are several companies developing treatments that target mitochondria or mitochondria-associated diseases. The majority of these efforts are in preclinical or early clinical development, are focused on gene therapy or are proposing the use of generic compounds. To our knowledge, none of these are focused on cardiolipin remodeling. Our competitors include NeuroVive Pharmaceutical AB, Reata Pharmaceuticals, Inc., LumiThera, Inc., Reneo Pharmaceuticals, Inc. and Santhera Pharmaceuticals Holding. In addition to competition from competitors who are developing treatments that seek to improve mitochondrial function or otherwise target the mitochondria, we also face competition from therapies that target the indications we are studying, particularly for diseases of aging such as GA. Such competitors who are developing or who have developed competing therapies include Apellis Pharmaceuticals Inc., Astellas Pharma Inc., Hemera Biosciences Inc., Ionis Pharmaceuticals, Inc. and IVERIC bio, Inc.
Many of the companies against which we are competing or against which we may compete in the future may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
The key competitive factors affecting the success of all of our therapeutic product candidates, if approved, are likely to be their efficacy, safety, tolerability, convenience and price and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Facilities
Our operations are conducted at Stealth Delaware, which is located in Needham, Massachusetts, where it occupies 6,051 square feet of office space. The lease expires October 31, 2022.
109
Employees
As of June 30, 2021, we had 34 full-time employees, 20 of whom were primarily engaged in research and development activities and 9 of whom had a Ph.D. or Pharm.D. degree. All of our full-time employees are based in the United States.
Our employees are not represented by any collective bargaining agreements.
Legal Proceedings
We are not currently a party to any legal proceedings.
110
PRINCIPAL SHAREHOLDERS
The following table sets forth information with respect to the beneficial ownership of the ordinary shares, as of June 30, 2021 by:
| ▪ | each of our executive officers; |
| ▪ | all of our directors and executive officers as a group; and |
| ▪ | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of the ordinary shares. |
The percentage ownership calculations for beneficial ownership prior to the offering are based on a total of 690,993,790 ordinary shares issued and outstanding as of June 30, 2021, which number excludes 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans. The column entitled “Percentage of Shares Beneficially Owned—After Offering” is based on ordinary shares to be outstanding after this offering, including the ordinary shares represented by the ADSs that we are selling in this offering.
The number of shares beneficially owned by each shareholder is determined under rules issued by the Securities and Exchange Commission and includes voting or investment power with respect to securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, ordinary shares subject to options or other rights held by such person that are currently exercisable or will become exercisable within 60 days of June 30, 2021, are considered outstanding, although such shares subject to options or other rights are not considered outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the address of all listed shareholders is c/o Stealth BioTherapeutics Inc.,140 Kendrick Street, Needham, Massachusetts 02494. Each of the shareholders listed has sole voting and investment power with respect to the shares beneficially owned by the shareholder unless noted otherwise, subject to community property laws where applicable.
| | SHARES | | | PERCENTAGE OF SHARES BENEFICIALLY OWNED |
NAME OF BENEFICIAL OWNER | | BENEFICIALLY OWNED | | | BEFORE OFFERING | | | AFTER OFFERING |
5% Shareholders | | | | | | | | | | |
Morningside Venture (I) Investments Limited (1) | | | 561,525,395 | | | 72.5% | | | |
Executive Officers and Directors | | | | | | | | | | |
Reenie McCarthy (2) | | | 11,387,174 | | | 1.6% | | | |
Robert Weiskopf (3) | | | 835,989 | | | * % | | | |
Brian D. Blakey, Pharm.D. (4) | | | 2,595,763 | | | * % | | | |
James R. Carr, Pharm.D. (5) | | | 2,131,443 | | | * % | | | |
Martin Redmon (6) | | | 1,866,372 | | | * % | | | |
Gerald L. Chan, Sc.D. (7) | | | 2,853,704 | | | * % | | | |
Francis W. Chen, Ph.D. (8) | | | 403,704 | | | * % | | | |
Louis Lange (9) | | | 416,100 | | | * % | | | |
Kevin F. McLaughlin (10) | | | 403,704 | | | * % | | | |
Edward P. Owens (11) | | | 893,424 | | | * % | | | |
Eve Slater (12) | | | 193,267 | | | * % | | | |
All executive officers and directors as a group (11 persons) (13) | | | 23,980,644 | | | 3.4% | | | |
(1) | Based on information set forth in Schedule 13 D/A filed with the SEC on February 17, 2021. Includes (1) 465,713,861 ordinary shares beneficially owned by Morningside Venture (I) Investments Limited or MVIL et al, consisting of (i) 418,960,015 ordinary shares (ii) 83,365,384 ordinary shares upon exercise of warrants and (iii) 600,000 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021; (2) 3,255,523 ADSs, representing 39,066,276 ordinary shares, beneficially owned by Season Pioneer |
111
| Investments Limited, or SPIL; and (3) 1,627,810 ADSs, representing 19,533,720 ordinary shares, beneficially owned by Equal Talent Investments Limited, or ETIL. Francis Ann Elizabeth Richards, Jill Marie Franklin, Peter Stuart Allenby Edwards and Raymond Long Sing Tang, the directors of MVIL, share voting and dispositive control over the shares held by MVIL. As a result, Ms. Richards, Ms. Franklin, Mr. Edwards and Mr. Tang may be deemed to possess voting and investment control over and may be deemed to have indirect beneficial ownership with respect to, all shares held by MVIL. MVIL is ultimately beneficially owned by a family trust established by Madam Chan Tan Ching Fen. Each of Ms. Richard, Ms. Franklin, Mr. Edwards and Mr. Tang disclaims beneficial ownership of such shares, except to the extent of their respective pecuniary interests therein. Tracy Gia Yunn Tsoi is the sole director of SPIL and ETIL and has sole voting and dispositive power with respect to securities held by SPIL and ETIL. SPIL is ultimately wholly beneficially owned by a trust over which Mr. Edwards has sole authority to remove the trustee. ETIL is ultimately wholly beneficially owned by a trust over which Mr. Edwards has sole authority to remove the trustee. Ms. Tsoi disclaims beneficial ownership of the securities owned directly by SPIL and ETIL, except to the extent of her pecuniary interest therein. MVIL, SPIL and ETIL may act together with respect to the voting and disposition of the securities held by such entities. The principal business address for MVIL, SPIL and ETIL is 2nd Floor, Le Prince de Galles, 3-5 Avenue des Citronniers, MC 98000, Monaco. |
(2) | Consists of 10,166,402 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(3) | Consists of 639,717 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(4) | Consists of 2,208,643 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(5) | Consists of 1,736,811 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(6) | Consists of 1,463,100 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(7) | Consists of 2,853,704 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(8) | Consists of 403,704 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(9) | Consists of 416,100 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(10) | Consists of 403,704 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(11) | Consists of 403,704 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(12) | Consists of 193,267 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
(13) | Consists of 20,888,856 ordinary shares issuable upon the exercise of options exercisable within 60 days after June 30, 2021. |
Holdings by U.S. Shareholders
Citibank N.A., or Citibank, is the holder of record for the company’s American Depositary Receipt program, pursuant to which each ADS represents 12 ordinary shares. As of June 30, 2021, Citibank held 231,487,164 ordinary shares representing 33.5 % of the issued share capital held at that date. As of June 30, 2021, we had 14 holders of record with addresses in the United States, and such holders held 2.4% of our issued share capital held at that date. As a result, the number of holders of record or registered holders in the United States is not representative of the number of beneficial holders or of the residence of beneficial holders.
DESCRIPTION OF SHARE CAPITAL AND ARTICLES OF ASSOCIATION
The following describes our issued share capital, summarizes the material provisions of our Articles of Association and highlights certain differences in corporate law in the Cayman Islands and the United States.
General
We were incorporated in April 2006 under the name of Stealth Peptides International Inc as an exempted company incorporated with limited liability under the laws of the Cayman Islands, and we changed our name in June 2015 to
112
Stealth BioTherapeutics Corp. Our affairs are governed by our Articles of Association, the Companies Law and the common law of the Cayman Islands. The objects for which we are established are unrestricted and, pursuant to our Articles of Association, we have full power and authority to carry out any object not prohibited by any law as provided by Section 7(4) of the Companies Law. The following description of our share capital and provisions of our Articles of Association are summaries and are qualified by reference to the Articles of Association filed as an exhibit to our registration statement of which this prospectus forms a part.
Issued Share Capital
Our authorized share capital is $480,000 divided into 1,600,000,000 ordinary shares.
As of December 31, 2019, there were 436,720,810 ordinary shares outstanding, each with a nominal or par value of $0.0003 per share, and as of December 31, 2020, there were 635,092,150 ordinary shares issued and outstanding, which number excludes 84,684 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans, each with a nominal or par value of $0.0003 per share.
As of June 30, 2021, there were 690,993,790 ordinary shares issued and outstanding, which number excludes 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans, each with a nominal or par value of $0.0003 per share.
Articles of Association
Subject to other provisions in our Articles of Association, our shareholders may by ordinary resolution increase our authorized share capital or by special resolution reduce the share capital and amend our Articles of Association.
General
All of our outstanding ordinary shares are fully paid and non-assessable. No share certificates will be issued in connection with the offering.
The ordinary shares issued upon the completion of the offering are not entitled to any preemptive conversion or redemption rights at the sole option of the holder of ordinary shares. Our shareholders may freely hold and vote their shares (subject to certain restrictions contained in our Articles of Association, such as the process for validly appointing a proxy).
Our board of directors may provide for other classes of shares, including classes of preferred shares, out of our authorized but unissued share capital, which could be utilized for a variety of corporate purposes, including future offerings to raise capital for corporate purposes or for use in employee benefit plans. Such additional classes of shares shall have such rights, restrictions, preferences, privileges and payment obligations as determined by our board of directors. If we issue any preferred shares, the rights, preferences and privileges of holders of our ordinary shares will be subject to, and may be adversely affected by, the rights of the holders of such preferred shares. See “—Variation of Rights of Shares.”
Repurchase Rights
Any repurchase of our own shares by us as may be agreed with the relevant shareholders shall be approved by our board of directors in compliance with the Companies Law and our Articles of Association, and we may make a payment in respect of such repurchase in any manner authorized by the Companies Law and our Articles of Association, including out of our capital. A payment out of capital by a Cayman Islands company is not lawful unless immediately following the date on which the payment out of capital is proposed to be made the company shall be able to pay its debts as they fall due in the ordinary course of business. Only shares that are fully paid may be repurchased, and there must be at least one share remaining in issue following the repurchase.
Voting Rights
Voting at any meeting of shareholders is by a poll. Each ordinary share is entitled to one vote.
A quorum required for a meeting of shareholders consists of at least one or more of shareholders present in person or by proxy and entitled to vote representing the holders of at least a majority of all of our issued voting share capital. Shareholders’ meetings are held annually and may otherwise be convened by our board of directors on its own initiative, with at least 10 days’ advance notice to the shareholders. Shareholders’ meetings shall also be convened on the requisition in writing of any shareholder or shareholders holding at least a majority of the issued voting share capital, subject to certain procedural requirements. Advance notice of at least 21 days is required for convening extraordinary general meetings.
Any ordinary resolution to be made by our shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast in person or by proxy at a meeting of our shareholders. A special resolution requires the affirmative vote of not less than two-thirds of the votes cast in person or by proxy at a
113
meeting of our shareholders. A special resolution is required for certain matters specified in the Companies Law as requiring approval by special resolution, including, without limitation, amending our Articles of Association, reducing our authorized share capital, changing our name and appointing a voluntary liquidator.
An ordinary resolution or a special resolution may also be adopted by way of unanimous written resolution signed by or on behalf of each shareholder who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Dividends
With the exception of section 34 of the Companies Law, there are no statutory provisions relating to the payment of dividends in the Cayman Islands. Based upon English case law, which is regarded as persuasive in the Cayman Islands, dividends may be paid only out of profits. Section 34 of the Companies Law permits, subject to a solvency test and the provisions, if any, of the company’s memorandum and articles of association, the payment of dividends and distributions out of the share premium account.
Any dividends will be paid to the custodian of the ADSs being issued in this offering and shall be subject to further distribution to you as a beneficial owner of the underlying ordinary shares by the custodian. See “Description of American Depositary Shares—Dividends and Other Distributions.”
Liquidation
On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of ordinary shares), assets available for distribution among the holders of ordinary shares shall be distributed among the holders of the ordinary shares on a pro rata basis.
Transfer of Shares
Subject to the restrictions of our Articles of Association and the Nasdaq Listing Rules or any relevant securities law, any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form prescribed by Nasdaq or any other form approved by our board of directors.
Subject to the Nasdaq Listing Rules and any rights or restrictions for time being attached to any share, our board of directors may in their absolute discretion decline to register any transfer of shares.
Subject to our Articles of Association and the Nasdaq Listing Rules and any rights or restrictions for time being attached to any share, the registration of transfers of shares may be suspended and our register of members closed at such times and for such periods as our board of directors may from time to time determine.
Variation of Rights of Shares
The rights attached to any class of shares may, subject to any rights or restrictions attached to any class, be materially adversely varied or abrogated only with written consent of the holders of not less than two-thirds of the issued shares of the relevant class or with the sanction of a resolution passed at a separate meeting of the holders of the shares of such class by a majority of two-thirds of the votes cast at such a meeting.
The rights conferred upon the shareholders of any class (including, without limitation and for the avoidance of doubt, the ordinary shares) shall not, subject to any rights or restrictions attached to the shares of that class, be deemed to be materially adversely varied or abrogated by, among other things, the creation, allotment or issuance of further shares ranking pari passu with or subsequent to such class or the redemption or purchase of any shares of any class by us.
Inspection of Books and Records
Holders of our ordinary shares have no general right under the Companies Law to inspect or obtain copies of our register of members or our corporate records other than our Articles of Association.
Borrowing Power
Our board of directors may exercise all powers to borrow money and to mortgage or charge our undertaking, property and uncalled capital, to provide for a security interest to be taken in such undertaking, property or uncalled capital, to issue debentures, debenture stock and other securities whenever money is borrowed or as security for any debt, liability or obligation of us or of any third party.
Share Options
As of December 31, 2020, options to purchase 44,959,938 ordinary shares at a weighted-average exercise price of $0.69 per share were outstanding, of which options to purchase 22,098,025 ordinary shares were exercisable, at a weighted-average exercise price of $0.88 per share. The options lapse after ten years from the date of grant.
114
Warrants
In June 2017, we issued a warrant to Hercules Capital, Inc., which is exercisable for 500,000 ordinary shares at an exercise price of $1.00 per share. The warrant expires in June 2027.
In October 2020, we issued a warrant to Morningside Venture (I) Investments Limited, which is exercisable for 46,153,849 ordinary shares at an exercise price of $0.13 per share. The warrant expires in October 2023.
In February 2021, we issued a warrant to Morningside Venture (I) Investments Limited, which is exercisable for 18,750,000 ordinary shares at an exercise price of $0.16 per share. The warrant expires in February 2024.
In May 2021, we issued a warrant to Morningside Venture (I) Investments Limited, which is exercisable for 18,461,538 ordinary shares at an exercise price of $0.13 per share. The warrant expires in May 2024.
Differences in Corporate Law
| | Delaware | | Cayman Islands |
Title of Organizational Documents | | Certificate of Incorporation and Bylaws | | Memorandum and Articles of Association |
| | | | |
Duties of Directors | | Under Delaware law, the business and affairs of a corporation are managed by or under the direction of its board of directors. In exercising their powers, directors are charged with a fiduciary duty of care to protect the interests of the corporation and a fiduciary duty of loyalty to act in the best interests of its shareholders. The duty of care requires that directors act in an informed and deliberative manner and inform themselves, prior to making a business decision, of all material information reasonably available to them. The duty of care also requires that directors exercise care in overseeing and investigating the conduct of the corporation’s employees. The duty of loyalty may be summarized as the duty to act in good faith, not out of self-interest, and in a manner which the director reasonably believes to be in the best interests of the shareholders. | | As a matter of Cayman Islands law, directors of Cayman Islands companies owe fiduciary duties to the company which include, amongst other things, a duty to act in good faith in their dealings with or on behalf of the company and exercise their powers and fulfill the duties of their office honestly. Five core duties are: ▪ a duty to act in good faith in what the directors bona fide consider to be the best interests of the company (and in this regard, it should be noted that the duty is owed to the company and not to associate companies, subsidiaries or holding companies); ▪ a duty not to personally profit from opportunities that arise from the office of director (unless the company permits him to do so); ▪ a duty of trusteeship of the company’s assets; ▪ a duty to avoid conflicts of interest; and |
115
| | Delaware | | Cayman Islands |
| | | | ▪ a duty to exercise powers for the purpose for which such powers were conferred. |
| | | | A director of a Cayman Islands company also owes the company a duty to act with skill, care and diligence. A director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. |
| | | | |
Limitations on Personal Liability of Directors | | Subject to the limitations described below, a certificate of incorporation may provide for the elimination or limitation of the personal liability of a director to the corporation or its shareholders for monetary damages for a breach of fiduciary duty as a director. Such provision cannot limit liability for breach of loyalty, acts or omissions not in good faith, intentional misconduct, unlawful payment of dividends or unlawful share purchase or redemption. In addition, the certificate of incorporation cannot limit liability for any act or omission occurring prior to the date when such provision becomes effective. | | The Companies Law has no equivalent provision to Delaware law regarding the limitation of director’s liability. However, as a matter of public policy, Cayman Islands law will not allow the limitation of a director’s liability to the extent that the liability is a consequence of the director committing a crime or of the director’s own fraud, dishonesty or willful default. |
| | | | |
Indemnification of Directors, Officers, Agents, and Others | | A corporation has the power to indemnify any director, officer, employee or agent of the corporation who was, is or is threatened to be made a party who acted in good faith and in a manner they believed to be in the best interests of the corporation, and if with respect to a criminal proceeding, had no reasonable cause to believe his conduct would be unlawful, against amounts actually and reasonably incurred. | | Cayman Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of directors and officers, except to the extent any such provision may be held by the Cayman Islands Court to be contrary to public policy, such as to provide indemnification against the consequences of committing a crime, or against the indemnified person’s own fraud, dishonesty or willful default. |
| | | | |
116
| | Delaware | | Cayman Islands |
Interested Directors | | Under Delaware law, a transaction in which a director who has an interest is not void or voidable solely because such interested director is present at or participates in the meeting that authorizes the transaction if: (i) the material facts as to such interested director’s relationship or interests are disclosed or are known to the board of directors and the board in good faith authorizes the transaction by the affirmative vote of a majority of the disinterested directors, even though the disinterested directors are less than a quorum; (ii) such material facts are disclosed or are known to the shareholders entitled to vote on such transaction and the transaction is specifically approved in good faith by vote of the shareholders; or (iii) the transaction is | | Under our Articles of Association, directors who are in any way, whether directly or indirectly, interested in a contract or proposed contract with our company must declare the nature of their interest at a meeting of the board of directors. Following such declaration, a director may vote in respect of any contract or proposed contract notwithstanding his interest; provided that, in exercising any such vote, such director’s duties remain as described above. |
| | | | |
| | fair as to the corporation as of the time it is authorized, approved or ratified. Under Delaware law, a director could be held liable for any transaction in which such director derived an improper personal benefit. | | |
| | | | |
Voting Requirements | | The certificate of incorporation may include a provision requiring supermajority approval by the directors or shareholders for any corporate action. In addition, under Delaware law, certain business combinations involving interested shareholders require approval by a supermajority of the non-interested shareholders. | | As a matter of Cayman Islands law, certain matters must be approved by special resolution of the shareholders, including amending or adopting memorandum or articles of association of a Cayman Islands company, appointment of inspectors to examine company affairs, reduction of share capital (subject, in relevant circumstances, to court approval), change of name, authorization of a plan of merger or transfer by way of continuation to another jurisdiction or consolidation, voluntary winding up of the company or the recalling of the liquidation of the company. The Companies Law requires that a special resolution be passed by a majority of at least two-thirds or such higher percentage as set forth in the articles of association, of shareholders being entitled to vote and do vote in person or by proxy at a general meeting, or by unanimous written consent of shareholders entitled to vote at a general meeting. Our Articles of Association does not provide for a higher threshold. |
117
| | Delaware | | Cayman Islands |
| | | | The Companies Law defines “special resolutions” only. A company’s articles of association can therefore tailor the definition of “ordinary resolutions” as a whole, or with respect to specific provisions. Our Articles of Association provide that an ordinary resolution is a resolution (i) passed by a simple majority of such shareholders as, being entitled to do so, vote in person (or, where proxies are allowed, by proxy) at a general meeting and regard shall be had in computing a majority to the number of votes to which each shareholder is entitled or (ii) approved in writing by all of the shareholders entitled to vote at a general meeting in one or more instruments each signed by one or more of the shareholders and the effective date of the resolution so adopted shall be the date on which the instrument (or the last of such instruments, if more than one) is executed. |
| | | | |
Voting for Directors | | Under Delaware law, unless otherwise specified in the certificate of incorporation or bylaws of the corporation, directors shall be elected by a plurality of the votes of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. | | Our Articles of Association provide that at an annual general meeting where a resolution for the election of directors is proposed in accordance with our Articles of Association, a plurality of the votes cast shall be sufficient to elect a director. |
| | | | |
Cumulative Voting | | No cumulative voting for the election of directors unless so provided in the certificate of incorporation. | | No cumulative voting for the election of directors unless so provided in the articles of association. Our Articles of Association do not provide for cumulative voting on the election of the directors as described above. |
| | | | |
Directors’ Powers Regarding Bylaws | | The certificate of incorporation may grant the directors the power to adopt, amend or repeal bylaws. | | Our Articles of Association may only be amended by a special resolution of the shareholders. |
| | | | |
118
| | Delaware | | Cayman Islands |
Nomination and Removal of Directors and Filling Vacancies on Board | | Shareholders may generally nominate directors if they comply with advance notice provisions and other procedural requirements in company bylaws. Holders of a majority of the shares may remove a director with or without cause, except in certain cases involving a classified board or if the company uses cumulative voting. Unless otherwise provided for in the certificate of incorporation or bylaws, directorship vacancies are filled by a majority of the directors elected or then in office. | | Nomination and removal of directors and filling of board vacancies are governed by the terms of the articles of association. Our Articles of Association provide that a director shall hold office until such time they resign upon the expiry of a full term of three years, if they are removed from office by ordinary resolution of the shareholders or otherwise in accordance with our Articles of Association. |
| | | | |
Mergers and Similar Arrangements | | Under Delaware law, with certain exceptions, a merger, consolidation, exchange or sale of all or substantially all the assets of a corporation must be approved by the board of directors and a majority of the outstanding shares entitled to vote thereon. Under Delaware law, a shareholder of a corporation participating in certain major corporate transactions may, under certain circumstances, be entitled to appraisal rights pursuant to which such shareholder may receive cash in the amount of the fair value of the shares held by such shareholder (as determined by a court) in lieu of the consideration such shareholder would otherwise receive in the transaction. Delaware law also provides that a parent corporation, by resolution of its board of directors, may merge with any subsidiary, of which it owns at least 90% of each class of | | The Companies Law provides for the merger or consolidation of two or more companies into a single entity. The legislation makes a distinction between a “consolidation” and a “merger.” In a consolidation, a new entity is formed from the combination of each participating company, and the separate consolidating parties, as a consequence, cease to exist and are each stricken by the Registrar of Companies in the Cayman Islands, referred to as the Registrar of Companies. In a merger, one company remains as the surviving entity, having in effect absorbed the other merging parties that then cease to exist. Two or more Cayman Islands-registered companies may merge or consolidate. Cayman Islands-registered companies may also merge or consolidate with overseas companies provided that the laws of the |
119
| | Delaware | | Cayman Islands |
| | | | |
| | capital stock without a vote by shareholders of such subsidiary. Upon any such merger, dissenting shareholders of the subsidiary would have appraisal rights. | | foreign jurisdiction permit such merger or consolidation. A plan of merger or consolidation shall be authorized by each constituent company by way of (i) a special resolution of the members of each such constituent company and (ii) such other authorization, if any, as may be specified in such constituent company’s articles of association. If one of the constituent companies is an overseas company, a declaration from a director of the overseas company is required to confirm that the merger or consolidation is permitted or not prohibited by the constituent overseas company and by the laws of the jurisdiction in which the overseas company is existing, and that those constitutional documents have been or will be complied with. Shareholder approval is not required where a parent company registered in the Cayman Islands seeks to merge with one or more of its subsidiaries registered in the Cayman Islands and a copy of the plan of merger is given to every member of each subsidiary company to be merged unless that member agrees otherwise. Secured creditors must consent to the merger although application can be made to the Cayman Islands Court for such requirement to be waived if such secured creditor does not grant its consent to the merger. Where an overseas company wishes to merge with a Cayman Islands company, consent or approval to the transfer of any security interest granted by the overseas company to the resulting Cayman Islands entity in the transaction is required, unless otherwise released or waived by the secured party. If the merger plan is approved, it is then filed with the Cayman Islands General Registry along with a declaration |
120
| | Delaware | | Cayman Islands |
| | | | by a director of each company. The Registrar of Companies will then issue a certificate of merger which shall be prima facie evidence of compliance with all requirements of the Companies Law in respect of the merger or consolidation. The surviving or consolidated entity remains or becomes active while the other company or companies are automatically dissolved. Unless the shares of such shareholder are |
| | | | publicly listed or quoted, dissenting shareholders in a merger or consolidation of this type are entitled to payment of the fair value of their shares if such shareholder provides a written objection before the vote on such merger or consolidation. With respect to shares that are listed or quoted, a shareholder shall have similar rights only if it is required by the terms of the merger or consolidation to accept for such shares property other than: (i) shares (or depositary receipts in respect thereof) in the surviving or consolidated company; (ii) listed or quoted shares (or depositary receipts in respect thereof) of another company; (iii) cash in lieu of any fractions of shares or depositary receipts described at (i) and (ii); or (iv) any combination of shares, depositary receipts or cash described in (i)-(iii). Cayman Islands companies may also be restructured or amalgamated under supervision of the Cayman Islands Court by way of a court-sanctioned “scheme of arrangement.” A scheme of arrangement is one of several transactional mechanisms available in the Cayman Islands for achieving a restructuring. Others include share capital exchange, merger (as described above), asset acquisition or control, through contractual arrangements, of an operating business. A Cayman Islands |
121
| | Delaware | | Cayman Islands |
| | | | Court scheme of arrangement requires the approval of a majority in number, of each class of shareholders and creditors with whom the arrangement is to be made and who must in addition represent three-fourths in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at the meeting summoned for that purpose. The convening of the meetings and subsequently the terms of the arrangement must be sanctioned by the Cayman Islands Court. While a dissenting shareholder would have the right to express to the Cayman Islands Court its view that the transaction ought not be approved, the Cayman Islands Court can be expected to approve the scheme of arrangement if it is satisfied that: ▪ the classes which are required to approve the scheme of arrangement |
| | | | |
| | | | have been properly constituted, so that the members of such classes are properly represented; ▪ the meetings held by the company in relation to the approval of the scheme of arrangement by such classes have been convened and held in accordance with any directions given by the Cayman Islands Court; ▪ the scheme of arrangement has been properly explained to the shareholders or creditors so that they have been able to exercise an informed vote in respect of the scheme; the scheme of arrangement is one which an intelligent and honest man, who is a member of the relevant class and properly acting, might approve. When a takeover offer is made and accepted by holders of 90% of the shares within four months, the offeror may, within a two-month period following the expiration of the said four month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection may be made to the Cayman Islands Court but is unlikely to succeed unless there is evidence of fraud, bad faith or collusion. If the arrangement and reconstruction are thus approved, any dissenting shareholders would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of U.S. corporations, providing rights to receive payment in cash for the judicially determined value of the shares. Our Articles of Association provide that we may by special resolution resolve to merge or consolidate in accordance with the Companies Law. |
122
| | Delaware | | Cayman Islands |
| | | | |
Shareholder Suits | | Class actions and derivative actions generally are available to shareholders under Delaware law for, among other things, breach of fiduciary duty, corporate waste and actions not taken in accordance with applicable law. In such actions, the court generally has discretion to permit the winning party to | | The rights of shareholders under Cayman Islands law are not as extensive as those under Delaware law. Class actions are generally not available to shareholders under Cayman Islands laws; historically, there have not been any reported instances of such class actions having been successfully brought before the Cayman Islands Court. In |
| | | | |
123
| | Delaware | | Cayman Islands |
| | recover attorneys’ fees incurred in connection with such action. | | principle, we will normally be the proper plaintiff and a derivative action may be brought by a minority shareholder in only limited circumstances. In this regard, the Cayman Islands Court would ordinarily be expected to follow English case law precedent, which would permit a shareholder to commence an action in the company’s name to remedy a wrong done to the company where the act complained of cannot be ratified by the shareholders and where control of the company by the wrongdoer results in the company not pursuing a remedy itself. The case law shows that derivative actions have been permitted in respect of acts that are beyond the company’s corporate power, illegal, where the individual rights of the plaintiff shareholder have been infringed or are about to be infringed and acts that are alleged to constitute a “fraud on the minority.” The winning party in such an action generally would be able to recover a portion of attorney’s fees incurred in connection with such action. |
| | | | |
Inspection of Corporate Records | | Under Delaware law, shareholders of a Delaware corporation have the right during normal business hours to inspect for any proper purpose, and to obtain copies of list(s) of shareholders and other books and records of the corporation and its subsidiaries, if any, to the extent the books and records of such subsidiaries are available to the corporation. | | Shareholders of a Cayman Islands exempted company have no general right under Cayman Islands law to inspect or obtain copies of the register of members or other corporate records (other than the register of mortgages or charges) of the company. However, these rights may be provided in the company’s articles of association. Our Articles of Association provide that the holders of our ordinary shares will have no general right to inspect or obtain copies of our register of members or our corporate records other than our Articles of Association. |
124
| | Delaware | | Cayman Islands |
| | | | |
Shareholder Proposals | | Unless provided in the corporation’s certificate of incorporation or bylaws, Delaware law does not include a provision restricting the manner in which shareholders may bring business before a meeting. | | The Companies Law does not provide shareholders any right to bring business before a meeting or requisition a general meeting. However, these rights may be provided in the company’s articles of association. Our Articles of Association provide for extraordinary general meeting to be convened on the requisition in writing of any shareholder(s) entitled to attend and vote at our extraordinary general meetings and to |
| | | | |
| | | | exercise at least a majority of the votes permitted to be exercised at any such meeting subject to certain procedural requirements. |
| | | | |
Approval of Corporate Matters by Written Consent | | Delaware law permits shareholders to take action by written consent signed by the holders of outstanding shares having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting of shareholders. | | The Companies Law allows a special resolution to be passed in writing only if signed by all the voting shareholders (if authorized by the articles of association). |
| | | | |
Calling of Special Shareholders Meetings | | Delaware law permits the board of directors or any person who is authorized under a corporation’s certificate of incorporation or bylaws to call a special meeting of shareholders. | | The Companies Law does not have provisions governing the proceedings of shareholders meetings which are usually provided in the articles of association. Our Articles of Association provide for an extraordinary general meeting to be convened on the requisition in writing of any shareholder(s) entitled to attend and vote at our extraordinary general meetings and to exercise at least a majority of the votes permitted to be exercised at any such meeting subject to certain procedural requirements. |
Listing on The Nasdaq Global Market
Our ADSs are listed on The Nasdaq Global Market under the trading symbol “MITO.”
Registrar
Our register of members, or share register, reflects only record owners of our ordinary shares. Holders of our ADSs are not treated as one of our shareholders and their names will therefore not be entered in our share register. The depositary, the custodian or their nominees will be the holder of the shares underlying our ADSs. For further discussion on our ADSs and ADS holder rights, see “Description of American Depositary Shares” in this prospectus.
125
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
Description of American Depositary Shares
Citibank, N.A., or Citibank, is acting as the depositary for the ADSs. Citibank’s depositary offices are located at 388 Greenwich Street, New York, New York 10013. ADSs represent ownership interests in securities that are on deposit with the depositary. ADSs may be represented by certificates that are commonly known as American Depositary Receipts, or ADRs. The depositary typically appoints a custodian to safekeep the securities on deposit. In this case, the custodian is Citibank, N.A.—Hong Kong, located at 9/F, Citi Tower, One Bay East, 83 Hoi Bun Road, Kwun Tong, Kowloon, Hong Kong.
We have appointed Citibank as depositary pursuant to a deposit agreement. A copy of the deposit agreement is on file with the SEC under cover of a report on Form 6-K. You may obtain a copy of the deposit agreement from the SEC’s website (www.sec.gov). Please refer to File No. 001-38810 when retrieving such copy.
We are providing you with a summary description of the material terms of the ADSs and of your material rights as an owner of ADSs. Please note that summaries by their nature lack the precision of the information summarized and the rights and obligations of an owner of ADSs will be determined by reference to the terms of the deposit agreement and not by this summary. We urge you to review the deposit agreement in its entirety. The portions of this summary that are italicized describe matters that may be relevant to ownership of ADSs but may not be contained in the deposit agreement.
Each ADS represents the right to receive, and to exercise the beneficial ownership interests in, 12 ordinary shares that are on deposit with the depositary and/or custodian. An ADS also represents the right to receive, and to exercise the beneficial interests in, any other property received by the depositary or the custodian on behalf of the owner of the ADS but that has not been distributed to the owners of ADSs because of legal restrictions or practical considerations. We and the depositary may agree to change the ADS-to-ordinary shares ratio by amending the deposit agreement. This amendment may give rise to, or change, the depositary fees payable by ADS owners. The custodian, the depositary and their respective nominees will hold all deposited property for the benefit of the holders and beneficial owners of ADSs. The deposited property does not constitute the proprietary assets of the depositary, the custodian or their nominees. Beneficial ownership in the deposited property will under the terms of the deposit agreement be vested in the beneficial owners of the ADSs. The depositary, the custodian and their respective nominees will be the record holders of the deposited property represented by the ADSs for the benefit of the holders and beneficial owners of the corresponding ADSs. A beneficial owner of ADSs may or may not be the holder of ADSs. Beneficial owners of ADSs will be able to receive, and to exercise beneficial ownership interests in, the deposited property only through the registered holders of the ADSs, the registered holders of the ADSs (on behalf of the applicable ADS owners) only through the depositary, and the depositary (on behalf of the owners of the corresponding ADSs) directly, or indirectly, through the custodian or their respective nominees, in each case upon the terms of the deposit agreement.
If you become an owner of ADSs, you will become a party to the deposit agreement and therefore will be bound to its terms and to the terms of any ADR that represents your ADSs. The deposit agreement and the ADR specify our rights and obligations as well as your rights and obligations as owner of ADSs and those of the depositary. As an ADS holder you appoint the depositary to act on your behalf in certain circumstances. The deposit agreement and the ADRs are governed by New York law. However, our obligations to the holders of ordinary shares will continue to be governed by the laws of the Cayman Islands, which may be different from the laws of the United States.
In addition, applicable laws and regulations may require you to satisfy reporting requirements and obtain regulatory approvals in certain circumstances. You are solely responsible for complying with such reporting requirements and obtaining such approvals. Neither the depositary, the custodian, us or any of their or our respective agents or affiliates shall be required to take any actions whatsoever on your behalf to satisfy such reporting requirements or obtain such regulatory approvals under applicable laws and regulations.
As an owner of ADSs, we will not treat you as one of our shareholders and you will not have direct shareholder rights. The depositary will hold on your behalf the shareholder rights attached to the ordinary shares underlying your ADSs. As an owner of ADSs you will be able to exercise the shareholders rights for the ordinary shares represented by your ADSs through the depositary only to the extent contemplated in the deposit agreement. To exercise any shareholder rights not contemplated in the deposit agreement you will, as an ADS owner, need to arrange for the cancellation of your ADSs and become a direct shareholder.
126
The manner in which you own the ADSs (e.g., in a brokerage account vs. as registered holder, or as holder of certificated vs. uncertified ADSs) may affect your rights and obligations, and the manner in which, and extent to which, the depositary’s services are made available to you. As an owner of ADSs, you may hold your ADSs either by means of an ADR registered in your name, through a brokerage or safekeeping account, or through an account established by the depositary in your name reflecting the registration of uncertificated ADSs directly on the books of the depositary (commonly referred to as the direct registration system or DRS). The direct registration system reflects the uncertificated (book-entry) registration of ownership of ADSs by the depositary. Under the direct registration system, ownership of ADSs is evidenced by periodic statements issued by the depositary to the holders of the ADSs. The direct registration system includes automated transfers between the depositary and The Depository Trust Company, or DTC, the central book-entry clearing and settlement system for equity securities in the United States. If you decide to hold your ADSs through your brokerage or safekeeping account, you must rely on the procedures of your broker or bank to assert your rights as ADS owner. Banks and brokers typically hold securities such as the ADSs through clearing and settlement systems such as DTC. The procedures of such clearing and settlement systems may limit your ability to exercise your rights as an owner of ADSs. Please consult with your broker or bank if you have any questions concerning these limitations and procedures. All ADSs held through DTC will be registered in the name of a nominee of DTC. This summary description assumes you have opted to own the ADSs directly by means of an ADS registered in your name and, as such, we will refer to you as the “holder.” When we refer to “you,” we assume the reader owns ADSs and will own ADSs at the relevant time.
The registration of the ordinary shares in the name of the depositary or the custodian shall, to the maximum extent permitted by applicable law, vest in the depositary or the custodian the record ownership in the applicable ordinary shares with the beneficial ownership rights and interests in such ordinary shares being at all times vested with the beneficial owners of the ADSs representing the ordinary shares. The depositary or the custodian shall at all times be entitled to exercise the beneficial ownership rights in all deposited property, in each case only on behalf of the holders and beneficial owners of the ADSs representing the deposited property.
Dividends and Distributions
As a holder of ADSs, you generally have the right to receive the distributions we make on the securities deposited with the custodian. Your receipt of these distributions may be limited, however, by practical considerations and legal limitations. Holders of ADSs will receive such distributions under the terms of the deposit agreement in proportion to the number of ADSs held as of the specified record date, after deduction of the applicable fees, taxes and expenses.
Distributions of Cash
Whenever we make a cash distribution for the securities on deposit with the custodian, we will deposit the funds with the custodian. Upon receipt of confirmation of the deposit of the requisite funds, the depositary will arrange for the funds received in a currency other than U.S. dollars to be converted into U.S. dollars and for the distribution of the U.S. dollars to the holders, subject to the laws and regulations of the Cayman Islands.
The conversion into U.S. dollars will take place only if practicable and if the U.S. dollars are transferable to the United States. The depositary will apply the same method for distributing the proceeds of the sale of any property (such as undistributed rights) held by the custodian in respect of securities on deposit.
The distribution of cash will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. The depositary will hold any cash amounts it is unable to distribute in a non-interest bearing account for the benefit of the applicable holders and beneficial owners of ADSs until the distribution can be effected or the funds that the depositary holds must be escheated as unclaimed property in accordance with the laws of the relevant states of the United States.
Distributions of Shares
Whenever we make a free distribution of ordinary shares for the securities on deposit with the custodian, we will deposit the applicable number of ordinary shares with the custodian. Upon receipt of confirmation of such deposit, the depositary will either distribute to holders new ADSs representing the ordinary shares deposited or modify the ADS-to-ordinary shares ratio, in which case each ADS you hold will represent rights and interests in the additional ordinary shares so deposited. Only whole new ADSs will be distributed. Fractional entitlements will be sold and the proceeds of such sale will be distributed as in the case of a cash distribution.
The distribution of new ADSs or the modification of the ADS-to-ordinary shares ratio upon a distribution of ordinary shares will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes or governmental charges, the depositary may sell all or a portion of the new ordinary shares so distributed.
127
No such distribution of new ADSs will be made if it would violate a law (e.g., the U.S. securities laws) or if it is not operationally practicable. If the depositary does not distribute new ADSs as described above, it may sell the ordinary shares received upon the terms described in the deposit agreement and will distribute the proceeds of the sale as in the case of a distribution of cash.
Distributions of Rights
Whenever we intend to distribute rights to purchase additional ordinary shares, we will give prior notice to the depositary and we will assist the depositary in determining whether it is lawful and reasonably practicable to distribute rights to purchase additional ADSs to holders.
The depositary will establish procedures to distribute rights to purchase additional ADSs to holders and to enable such holders to exercise such rights if it is lawful and reasonably practicable to make the rights available to holders of ADSs, and if we provide all of the documentation contemplated in the deposit agreement (such as opinions to address the lawfulness of the transaction). You may have to pay fees, expenses, taxes and other governmental charges to subscribe for the new ADSs upon the exercise of your rights. The depositary is not obligated to establish procedures to facilitate the distribution and exercise by holders of rights to purchase new ordinary shares other than in the form of ADSs.
The depositary will not distribute the rights to you if:
| ▪ | we do not timely request that the rights be distributed to you or we request that the rights not be distributed to you; or |
| ▪ | we fail to deliver satisfactory documents to the depositary; or |
| ▪ | it is not reasonably practicable to distribute the rights. |
The depositary will sell the rights that are not exercised or not distributed if such sale is lawful and reasonably practicable. The proceeds of such sale will be distributed to holders as in the case of a cash distribution. If the depositary is unable to sell the rights, it will allow the rights to lapse.
Elective Distributions
Whenever we intend to distribute a dividend payable at the election of shareholders either in cash or in additional shares, we will give prior notice thereof to the depositary and will indicate whether we wish the elective distribution to be made available to you. In such case, we will assist the depositary in determining whether such distribution is lawful and reasonably practicable.
The depositary will make the election available to you only if it is reasonably practicable and if we have provided all of the documentation contemplated in the deposit agreement. In such case, the depositary will establish procedures to enable you to elect to receive either cash or additional ADSs, in each case as described in the deposit agreement.
If the election is not made available to you, you will receive either cash or additional ADSs, depending on what a shareholder in the Cayman Islands would receive upon failing to make an election, as more fully described in the deposit agreement.
Other Distributions
Whenever we intend to distribute property other than cash, ordinary shares or rights to purchase additional ordinary shares, we will notify the depositary in advance and will indicate whether we wish such distribution to be made to you. If so, we will assist the depositary in determining whether such distribution to holders is lawful and reasonably practicable.
If it is reasonably practicable to distribute such property to you and if we provide to the depositary all of the documentation contemplated in the deposit agreement, the depositary will distribute the property to the holders in a manner it deems practicable.
The distribution will be made net of fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes and governmental charges, the depositary may sell all or a portion of the property received.
128
The depositary will not distribute the property to you and will sell the property if:
| ▪ | we do not request that the property be distributed to you or if we ask that the property not be distributed to you; or |
| ▪ | we do not deliver satisfactory documents to the depositary; or |
| ▪ | the depositary determines that all or a portion of the distribution to you is not reasonably practicable. |
The proceeds of such a sale will be distributed to holders as in the case of a cash distribution.
Redemption
Whenever we decide to redeem any of the securities on deposit with the custodian, we will notify the depositary in advance. If it is practicable and if we provide all of the documentation contemplated in the deposit agreement, the depositary will provide notice of the redemption to the holders.
The custodian will be instructed to surrender the shares being redeemed against payment of the applicable redemption price. The depositary will convert the redemption funds received into U.S. dollars upon the terms of the deposit agreement and will establish procedures to enable holders to receive the net proceeds from the redemption upon surrender of their ADSs to the depositary. You may have to pay fees, expenses, taxes and other governmental charges upon the redemption of your ADSs. If less than all ADSs are being redeemed, the ADSs to be retired will be selected by lot or on a pro rata basis, as the depositary may determine.
Changes Affecting Ordinary Shares
The ordinary shares held on deposit for your ADSs may change from time to time. For example, there may be a change in nominal or par value, split-up, cancellation, consolidation or any other reclassification of such ordinary shares or a recapitalization, reorganization, merger, consolidation or sale of assets of the Company.
If any such change were to occur, your ADSs would, to the extent permitted by law, represent the right to receive the property received or exchanged in respect of the ordinary shares held on deposit. The depositary may in such circumstances deliver new ADSs to you, amend the deposit agreement, the ADRs and the applicable Registration Statement(s) on Form F-6, call for the exchange of your existing ADSs for new ADSs and take any other actions that are appropriate to reflect as to the ADSs the change affecting the ordinary shares. If the depositary may not lawfully distribute such property to you, the depositary may sell such property and distribute the net proceeds to you as in the case of a cash distribution.
Issuance of ADSs upon Deposit of Ordinary Shares
Upon completion of this offering, the ordinary shares being offered pursuant to this prospectus will be deposited by us with the custodian. Upon receipt of confirmation of such deposit, the depositary will issue ADSs to the placement agent for delivery to the investors purchasing in this offering.
After the closing of this offering, the depositary may create ADSs on your behalf if you or your broker deposit ordinary shares with the custodian. The depositary will deliver these ADSs to the person you indicate only after you pay any applicable issuance fees and any charges and taxes payable for the transfer of the ordinary shares to the custodian. Your ability to deposit ordinary shares and receive ADSs may be limited by the legal considerations in the United States and Cayman Islands applicable at the time of deposit.
The issuance of ADSs may be delayed until the depositary or the custodian receives confirmation that all required approvals have been given and that the ordinary shares have been duly transferred to the custodian. The depositary will only issue ADSs in whole numbers.
When you make a deposit of ordinary shares, you will be responsible for transferring good and valid title to the depositary. As such, you will be deemed to represent and warrant that:
| ▪ | the ordinary shares are duly authorized, validly issued, fully paid, non-assessable and legally obtained; |
| ▪ | all preemptive (and similar) rights, if any, with respect to such ordinary shares have been validly waived or exercised; |
| ▪ | you are duly authorized to deposit the ordinary shares; |
| ▪ | the ordinary shares presented for deposit are free and clear of any lien, encumbrance, security interest, charge, mortgage or adverse claim, and are not, and the ADSs issuable upon such deposit will not be, “restricted securities” (as defined in the deposit agreement); |
| ▪ | the ordinary shares presented for deposit have not been stripped of any rights or entitlements; and |
| ▪ | the deposit of the ordinary shares does not violate any applicable provisions of the Cayman Islands. |
129
If any of the representations or warranties are incorrect in any way, we and the depositary may, at your cost and expense, take any and all actions necessary to correct the consequences of the misrepresentations.
Transfer, Combination and Split Up of ADRs
As an ADR holder, you will be entitled to transfer, combine or split up your ADRs and the ADSs evidenced thereby. For transfers of ADRs, you will have to surrender the ADRs to be transferred to the depositary and also must:
| ▪ | ensure that the surrendered ADR is properly endorsed or otherwise in proper form for transfer; |
| ▪ | provide such proof of identity and genuineness of signatures as the depositary deems appropriate; |
| ▪ | provide any transfer stamps required by the State of New York or the United States; and |
| ▪ | pay all applicable fees, charges, expenses, taxes and other government charges payable by ADR holders pursuant to the terms of the deposit agreement, upon the transfer of ADRs. |
To have your ADRs either combined or split up, you must surrender the ADRs in question to the depositary with your request to have them combined or split up, and you must pay all applicable fees, charges and expenses payable by ADR holders, pursuant to the terms of the deposit agreement, upon a combination or split up of ADRs.
Withdrawal of Ordinary Shares Upon Cancellation of ADSs
As a holder, you will be entitled to present your ADSs to the depositary for cancellation and then receive the corresponding number of underlying ordinary shares at the custodian’s offices. Your ability to withdraw the ordinary shares held in respect of the ADSs may be limited by the legal considerations in the United States and Cayman Islands applicable at the time of withdrawal. In order to withdraw the ordinary shares represented by your ADSs, you will be required to pay to the depositary the fees for cancellation of ADSs and any charges and taxes payable upon the transfer of the ordinary shares. You assume the risk for delivery of all funds and securities upon withdrawal. Once canceled, the ADSs will not have any rights under the deposit agreement.
If you hold ADSs registered in your name, the depositary may ask you to provide proof of identity and genuineness of any signature and such other documents as the depositary may deem appropriate before it will cancel your ADSs. The withdrawal of the ordinary shares represented by your ADSs may be delayed until the depositary receives satisfactory evidence of compliance with all applicable laws and regulations. Please keep in mind that the depositary will only accept ADSs for cancellation that represent a whole number of securities on deposit.
You will have the right to withdraw the securities represented by your ADSs at any time except for:
| ▪ | temporary delays that may arise because (i) the transfer books for the ordinary shares or ADSs are closed, or (ii) ordinary shares are immobilized on account of a shareholders’ meeting or a payment of dividends; |
| ▪ | obligations to pay fees, taxes and similar charges; |
| ▪ | restrictions imposed because of laws or regulations applicable to ADSs or the withdrawal of securities on deposit; and/or |
| ▪ | other circumstances specifically contemplated by Section I.A.(l) of the General Instructions to Form F-6 (as such General Instructions may be amended from time to time). |
The deposit agreement may not be modified to impair your right to withdraw the securities represented by your ADSs except to comply with mandatory provisions of law.
Voting Rights
As a holder, you generally have the right under the deposit agreement to instruct the depositary to exercise the voting rights for the ordinary shares represented by your ADSs. The voting rights of holders of ordinary shares are described in “Description of Share Capital and Articles of Association—Articles of Association” in this prospectus.
At our request, the depositary will distribute to you any notice of shareholders’ meeting received from us together with information explaining how to instruct the depositary to exercise the voting rights of the securities represented by ADSs. In lieu of distributing such materials, the depositary may distribute to holders of ADSs instructions on how to receive such materials upon request.
If the depositary timely receives voting instructions from a holder of ADSs, it will endeavor to vote the securities (in person or by proxy) represented by the holder’s ADSs as follows:
| ▪ | The depositary will vote (or cause the custodian to vote) the ordinary shares held on deposit in accordance with the voting instructions received from the holders of ADSs. |
130
| ▪ | Holders of ADSs in respect of which no timely voting instructions have been received shall be deemed to have instructed the depositary to give a discretionary proxy to a person designated by us to vote the ordinary shares represented by such holders’ ADSs; provided, however, that no such discretionary proxy shall be given with respect to any matter to be voted upon as to which we inform the depositary that (i) we do not wish such proxy to be given, (ii) substantial opposition exists, or (iii) the rights of holders of ordinary shares may be adversely affected. |
Securities for which no voting instructions have been received will not be voted (except as otherwise contemplated in the deposit agreement). Please note that the ability of the depositary to carry out voting instructions may be limited by practical and legal limitations and the terms of the securities on deposit. We cannot assure you that you will receive voting materials in time to enable you to return voting instructions to the depositary in a timely manner.
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
SERVICE | FEE |
Issuance of ADSs (e.g., an issuance of ADS upon a deposit of ordinary shares or upon a change in the ADS(s)-to-ordinary shares ratio), excluding ADS issuances as a result of distributions of ordinary shares | Up to $5.00 per 100 ADSs (or fraction thereof) issued |
| |
Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property or upon a change in the ADS(s)-to-ordinary shares ratio) | Up to $5.00 per 100 ADSs (or fraction thereof) cancelled |
| |
Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) | Up to $5.00 per 100 ADSs (or fraction thereof) held |
| |
Distribution of ADSs pursuant to (i) share dividends or other free share distributions, or (ii) exercise of rights to purchase additional ADSs | Up to $5.00 per 100 ADSs (or fraction thereof) held |
| |
Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) | Up to $5.00 per 100 ADSs (or fraction thereof) held |
| |
ADS Services | Up to $5.00 per 100 ADSs (or fraction thereof) held on the applicable record date(s) established by the depositary |
As an ADS holder you will also be responsible to pay certain charges such as:
| ▪ | taxes (including applicable interest and penalties) and other governmental charges; |
| ▪ | the registration fees as may from time to time be in effect for the registration of ordinary shares on the share register and applicable to transfers of ordinary shares to or from the name of the custodian, the depositary or any nominees upon the making of deposits and withdrawals, respectively; |
| ▪ | certain cable, telex and facsimile transmission and delivery expenses; |
| ▪ | the expenses and charges incurred by the depositary in the conversion of foreign currency; |
| ▪ | the fees and expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs and ADRs; and |
| ▪ | the fees and expenses incurred by the depositary, the custodian or any nominee in connection with the servicing or delivery of deposited property. |
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person to whom the ADSs are issued (in the case of ADS issuances) and to the person whose ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and
131
(ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder. Certain depositary fees and charges (such as the ADS services fee) may become payable shortly after the closing of the ADS offering. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary. You will receive prior notice of such changes. The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Amendments and Termination
We may agree with the depositary to modify the deposit agreement at any time without your consent. We undertake to give holders 30 days’ prior notice of any modifications that would materially prejudice any of their substantial rights under the deposit agreement. We will not consider to be materially prejudicial to your substantial rights any modifications or supplements that are reasonably necessary for the ADSs to be registered under the Securities Act or to be eligible for book-entry settlement, in each case without imposing or increasing the fees and charges you are required to pay. In addition, we may not be able to provide you with prior notice of any modifications or supplements that are required to accommodate compliance with applicable provisions of law.
You will be bound by the modifications to the deposit agreement if you continue to hold your ADSs after the modifications to the deposit agreement become effective. The deposit agreement cannot be amended to prevent you from withdrawing the ordinary shares represented by your ADSs (except as permitted by law).
We have the right to direct the depositary to terminate the deposit agreement. Similarly, the depositary may in certain circumstances on its own initiative terminate the deposit agreement. In either case, the depositary must give notice to the holders at least 30 days before termination. Until termination, your rights under the deposit agreement will be unaffected.
After termination, the depositary will continue to collect distributions received (but will not distribute any such property until you request the cancellation of your ADSs) and may sell the securities held on deposit. After the sale, the depositary will hold the proceeds from such sale and any other funds then held for the holders of ADSs in a non-interest bearing account. At that point, the depositary will have no further obligations to holders other than to account for the funds then held for the holders of ADSs still outstanding (after deduction of applicable fees, taxes and expenses).
In connection with any termination of the deposit agreement, the depositary may make available to owners of ADSs a means to withdraw the ordinary shares represented by ADSs and to direct the depositary of such ordinary shares into an unsponsored American depositary share program established by the depositary. The ability to receive unsponsored American depositary shares upon termination of the deposit agreement would be subject to satisfaction of certain U.S. regulatory requirements applicable to the creation of unsponsored American depositary shares and the payment of applicable depositary fees.
Books of Depositary
The depositary will maintain ADS holder records at its depositary office. You may inspect such records at such office during regular business hours but solely for the purpose of communicating with other holders in the interest of business matters relating to the ADSs and the deposit agreement.
The depositary will maintain in New York facilities to record and process the issuance, cancellation, combination, split-up and transfer of ADSs. These facilities may be closed from time to time, to the extent not prohibited by law.
Limitations on Obligations and Liabilities
The deposit agreement limits our obligations and the depositary’s obligations to you. Please note the following:
| ▪ | We and the depositary are obligated only to take the actions specifically stated in the deposit agreement without negligence or bad faith. |
132
| ▪ | The depositary disclaims any liability for any failure to carry out voting instructions, for any manner in which a vote is cast or for the effect of any vote, provided it acts in good faith and without negligence and in accordance with the terms of the deposit agreement. |
| ▪ | The depositary disclaims any liability for any failure to determine the lawfulness or practicality of any action, for the content of any document forwarded to you on our behalf or for the accuracy of any translation of such a document, for the investment risks associated with investing in ordinary shares, for the validity or worth of the ordinary shares, for any tax consequences that result from the ownership of ADSs, for the credit-worthiness of any third party, for allowing any rights to lapse under the terms of the deposit agreement, for the timeliness of any of our notices or for our failure to give notice. |
| ▪ | We and the depositary will not be obligated to perform any act that is inconsistent with the terms of the deposit agreement. |
| ▪ | We and the depositary disclaim any liability if we or the depositary are prevented or forbidden from or subject to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required by the terms of the deposit agreement, by reason of any provision, present or future of any law or regulation, or by reason of present or future provision of any provision of our Articles of Association, or any provision of or governing the securities on deposit, or by reason of any act of God or war or other circumstances beyond our control. |
| ▪ | We and the depositary disclaim any liability by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our Articles of Association or in any provisions of or governing the securities on deposit. |
| ▪ | We and the depositary further disclaim any liability for any action or inaction in reliance on the advice or information received from legal counsel, accountants, any person presenting ordinary shares for deposit, any holder of ADSs or authorized representatives thereof, or any other person believed by either of us in good faith to be competent to give such advice or information. |
| ▪ | We and the depositary also disclaim liability for the inability by a holder to benefit from any distribution, offering, right or other benefit that is made available to holders of ordinary shares but is not, under the terms of the deposit agreement, made available to you. |
| ▪ | We and the depositary may rely without any liability upon any written notice, request or other document believed to be genuine and to have been signed or presented by the proper parties. |
| ▪ | We and the depositary also disclaim liability for any consequential or punitive damages for any breach of the terms of the deposit agreement. |
| ▪ | No disclaimer of any Securities Act or Exchange Act liability is intended by any provision of the deposit agreement, in each case to the extent established under applicable U.S. laws. |
Nothing in the deposit agreement gives rise to a partnership or joint venture, or establishes a fiduciary relationship, among us, the depositary bank and you as ADS holder.
Nothing in the deposit agreement precludes Citibank (or its affiliates) from engaging in transactions in which parties adverse to us or the ADS owners have interests, and nothing in the deposit agreement obligates Citibank to disclose those transactions, or any information obtained in the course of those transactions, to us or to the ADS owners, or to account for any payment received as part of those transactions.
Taxes
You will be responsible for the taxes and other governmental charges payable on the ADSs and the securities represented by the ADSs. We, the depositary and the custodian may deduct from any distribution the taxes and governmental charges payable by holders and may sell any and all property on deposit to pay the taxes and governmental charges payable by holders. You will be liable for any deficiency if the sale proceeds do not cover the taxes that are due.
133
The depositary may refuse to issue ADSs; to deliver, transfer, split and combine ADRs; or to release securities on deposit until all taxes and charges are paid by the applicable holder. The depositary and the custodian may take reasonable administrative actions to obtain tax refunds and reduced tax withholding for any distributions on your behalf. However, you may be required to provide to the depositary and to the custodian proof of taxpayer status and residence and such other information as the depositary and the custodian may require to fulfill legal obligations. You are required to indemnify us, the depositary and the custodian for any claims with respect to taxes based on any tax benefit obtained for you.
Foreign Currency Conversion
The depositary will arrange for the conversion of all foreign currency received into U.S. dollars if such conversion is practical, and it will distribute the U.S. dollars in accordance with the terms of the deposit agreement. You may have to pay fees and expenses incurred in converting foreign currency, such as fees and expenses incurred in complying with currency exchange controls and other governmental requirements.
If the conversion of foreign currency is not practical or lawful, or if any required approvals are denied or not obtainable at a reasonable cost or within a reasonable period, the depositary may take the following actions in its discretion:
| ▪ | Convert the foreign currency to the extent practical and lawful and distribute the U.S. dollars to the holders for whom the conversion and distribution is lawful and practical. |
| ▪ | Distribute the foreign currency to holders for whom the distribution is lawful and practical. |
| ▪ | Hold the foreign currency (without liability for interest) for the applicable holders. |
Governing Law/Waiver of Jury Trial
The deposit agreement, the ADRs and the ADSs will be interpreted in accordance with the laws of the State of New York. The rights of holders of ordinary shares (including ordinary shares represented by ADSs) is governed by the laws of the Cayman Islands.
AS A PARTY TO THE DEPOSIT AGREEMENT, YOU IRREVOCABLY WAIVE YOUR RIGHT TO TRIAL BY JURY, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, IN ANY LEGAL PROCEEDING ARISING OUT OF THE DEPOSIT AGREEMENT OR THE ADRs AGAINST US AND/OR THE DEPOSITARY.
134
SHARES AND ADSs ELIGIBLE FOR FUTURE SALE
Based upon the 690,993,790 ordinary shares that were issued and outstanding on June 30, 2021 (excluding 861,660 ordinary shares held as ADSs in a custodial account for settlement of awards under our equity compensation plans), upon the closing of this offering, we will have outstanding ordinary shares, which include ordinary shares represented by ADSs, after giving effect to this offering.
Future sales of the ADSs in the public market, including those representing ordinary shares issued upon exercise of outstanding options, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity securities.
Of the ADSs to be outstanding immediately after the closing of this offering, ADSs are freely tradable and the ADSs sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining ADSs and any ordinary shares not represented by ADSs outstanding after this offering will be “restricted securities” under Rule 144. These restricted securities may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, under Rule 144, any person who is not our affiliate and has held their securities for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell securities without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not our affiliate and has not been our affiliate at any time during the preceding three months and has held their securities for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available.
A person who is our affiliate or who was our affiliate at any time during the preceding three months may sell any unrestricted securities, as well as restricted securities that the person has beneficially owned for at least six months, including the holding period of any prior owner other than one of our affiliates, under Rule 144. Affiliates selling restricted or unrestricted securities may sell a number of securities within any three-month period that does not exceed the greater of:
| ▪ | 1% of the number of ordinary shares then outstanding, which will equal approximately ordinary shares, or ADSs, immediately after this offering; and |
| ▪ | the average weekly trading volume of the ordinary shares in the form of ADSs on The Nasdaq Global Market during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities Pursuant to Rule 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701 of the Securities Act, any of our employees, consultants or advisors, other than our affiliates, who purchased shares from us in connection with a qualified compensatory stock plan or other written agreement is eligible to resell these shares in reliance on Rule 144, but without compliance with the holding period requirements of Rule 144 and without regard to the volume of such sales or the availability of public information about us.
Regulation S
Regulation S under the Securities Act provides that ordinary shares owned by any person may be sold without registration in the United States, provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our ordinary shares may be sold in some other manner outside the United States without requiring registration in the United States.
Share Options, Restricted ADS Awards and Warrants
As of June 30, 2021, we had outstanding options to purchase 64,213,310 ordinary shares, of which options to purchase 27,225,923 shares were vested, and we had 1,144,550 outstanding restricted ADS awards representing 13,734,600 ordinary shares subject to future issuance upon vesting.
In March 2019, we filed a registration statement on Form S-8 under the Securities Act to register all of our ordinary shares subject to outstanding options and reserved for future options and other awards under the 2006 Share Incentive Plan and 2019 Share Incentive Plan, in April 2020 we filed a registration statement on form S-8 under the
135
securities Act to register ADS awards under our 2020 ADS Incentive plan, in April 2021 we filed a registration statement on form S-8 under the securities Act to register our ordinary shares issuable under the 2019 Share Incentive Plan and ADSs issuable under the 2020 ADS Incentive Plan pursuant to plan’s evergreen provision. Accordingly, our ordinary shares registered under the registration statements on Form S-8 are available for sale in the open market, subject to Rule 144 volume limitations applicable to affiliates, and subject to any vesting restrictions and lock-up agreements applicable to these shares.
As of June 30, 2021, we had outstanding warrants to purchase 83,865,384 ordinary shares. Any shares acquired through the exercise of these warrants will be eligible for sale subject to securities laws applicable to these shares.
Lincoln Park Facility
As of June 30, 2021, there were a total of 90,332,040 ordinary shares available to be issued to Lincoln Park under a Purchase Agreement. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Lincoln Park Agreement” for additional information regarding this facility.
ATM Offering
As of June 30, 2021, we have the ability to issue up to $4.7 million of ADSs through our At The Market Offering Agreement with Wainwright; however, we suspended this facility on November 19, 2020. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—ATM Offering Agreement” for additional information regarding this facility.
136
TAXATION
The following is a summary of the material U.S. federal income tax consequences to U.S. Holders, as defined below, of owning and disposing of ordinary shares or ADSs. It does not set forth all tax considerations that may be relevant to a particular investor’s decision to acquire ordinary shares or ADSs.
This summary applies only to a U.S. Holder that acquires our ADSs in this offering and holds our ordinary shares or ADSs as capital assets for U.S. federal income tax purposes (generally, property held for investment). In addition, it does not set forth all of the U.S. federal income tax or other tax consequences that may be relevant in light of the U.S. Holder’s particular circumstances, including state, local and non-U.S. tax consequences, estate tax consequences, alternative minimum tax consequences, the potential application of the provisions of the Code known as the Medicare contribution tax and tax consequences applicable to U.S. Holders subject to special rules, such as:
| • | banks, insurance companies and certain other financial institutions; |
| • | dealers or traders in securities who use a mark-to-market method of tax accounting; |
| • | brokers, dealers or traders in securities, commodities or currencies; |
| • | persons holding ordinary shares or ADSs as part of a hedging transaction, straddle, wash sale, conversion transaction or other integrated transaction or persons entering into a constructive sale with respect to the ordinary shares or ADSs; |
| • | persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar; |
| • | persons who acquired our ordinary shares or ADSs pursuant to the exercise of any employee stock option or otherwise as compensation; |
| • | regulated investment companies or real estate investment trusts; |
| • | S corporations, partnerships, or other entities or arrangements classified as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt entities, including individual retirement accounts or Roth IRAs, or government organizations; |
| • | persons required for U.S. federal income tax purposes to conform the timing of income accruals with respect to the ordinary shares or ADSs to their financial statements under Section 451(b) of the Code; |
| • | except to the extent expressly discussed below, persons that own or are deemed to own 10% or more of our shares (including ADSs), by vote or value, or Ten-Percent Shareholders; |
| • | persons holding ordinary shares or ADSs in connection with a trade or business, permanent establishment or fixed base outside of the United States; or |
| • | U.S. expatriates and certain former citizens or long-term residents of the United States. |
If an entity that is classified as a partnership for U.S. federal income tax purposes holds ordinary shares or ADSs, the U.S. federal income tax treatment of a partner will depend on the status of the partner and the activities of the partnership.
This summary is based on the Code, administrative pronouncements, judicial decisions, final, temporary and proposed U.S. Treasury Regulations, all as of the date hereof, any of which is subject to change or differing interpretations, possibly with retroactive effect. Any such change or different interpretation could alter the tax consequences to U.S. Holders described in this summary. In addition, there can be no assurance that the IRS will not challenge one or more of the tax consequences described in this summary.
A “U.S. Holder” is a holder that, for U.S. federal income tax purposes, is a beneficial owner of ordinary shares or ADSs that is:
137
| • | a citizen or individual resident of the United States; |
| • | a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; |
| • | a trust if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) the trust has a valid election to be treated as a U.S. person under applicable U.S. Treasury Regulations. |
This summary assumes that the representations to be contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement will be complied with in accordance with their terms.
Generally, a holder of an ADS should be treated for U.S. federal income tax purposes as holding the ordinary shares represented by the ADS. Accordingly, no gain or loss will be recognized upon an exchange of ADSs for the underlying ordinary shares represented by such ADSs. The U.S. Treasury has expressed concerns that intermediaries in the chain of ownership between the holder of an ADS and the issuer of the security underlying the ADS may be taking actions that are inconsistent with the beneficial ownership of the underlying security. Accordingly, the creditability of foreign taxes, if any, as described below, could be affected by actions taken by intermediaries in the chain of ownership between the holders of ADSs and our company if as a result of such actions the holders of ADSs are not properly treated as beneficial owners of the underlying ordinary shares.
U.S. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS CONCERNING THE U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF OWNING AND DISPOSING OF ORDINARY SHARES OR ADSs IN THEIR PARTICULAR CIRCUMSTANCES.
Taxation of Distributions
We do not currently expect to make distributions on our ordinary shares or ADSs. In the event that we do make distributions of cash or other property, subject to the discussion below under - “Passive Foreign Investment Company Rules,” distributions paid on ordinary shares or ADSs, other than certain pro rata distributions of ordinary shares or ADSs, will generally be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because we may not calculate our earnings and profits under U.S. federal income tax principles, we expect that distributions generally will be reported to U.S. Holders as dividends.
Subject to certain holding-period requirements for so long as our ordinary shares or ADSs are listed on the Nasdaq Global Market or another established securities market in the United States, dividends paid to certain non-corporate U.S. Holders will be eligible for taxation as “qualified dividend income,” which is taxable at rates not in excess of the long-term capital gain rate applicable to such U.S. Holders. However, the qualified dividend income treatment will not apply if we are treated as a PFIC with respect to the U.S. Holder for our taxable year of the distribution or the preceding taxable year.
The amount of a dividend will be treated as foreign-source dividend income to U.S. Holders and will not be eligible for the dividends-received deduction available to U.S. corporations under the Code. Dividends will generally be included in a U.S. Holder’s income on the date of the U.S. Holder’s receipt of the dividend. The amount of any dividend income paid in foreign currency will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars at that time. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. Such gain or loss would generally be treated as U.S.-source ordinary income or loss.
The amount of any distribution of property other than cash (and other than certain pro rata distributions of ordinary shares or ADSs or rights to acquire ordinary shares or ADSs) will be the fair market value of such property on the date of distribution.
138
Exchange of ADSs for ordinary shares
In general, a U.S. Holder that owns ADSs will be treated as the owner of the underlying shares represented by those ADSs for U.S. federal income tax purposes. Accordingly, no gain or loss will be recognized if a U.S. Holder exchanges ADSs for the underlying shares represented by those ADSs.
Sale or Other Taxable Disposition of Ordinary Shares or ADSs
Subject to the discussion below under “- Passive Foreign Investment Company Rules,” gain or loss realized on the sale or other taxable disposition of ordinary shares or ADSs will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the ordinary shares or ADSs for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ordinary shares or ADSs disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes. The deductibility of capital losses is subject to limitations.
If the consideration received by a U.S. Holder is not paid in U.S. dollars, the amount realized will be the U.S. dollar value of the payment received determined by reference to the spot rate of exchange on the date of the sale or other disposition. However, if the ordinary shares or ADSs are treated as traded on an “established securities market” and the U.S. Holder is either a cash basis taxpayer or an accrual basis taxpayer that has made a special election (which must be applied consistently from year to year and cannot be changed without the consent of the IRS), the U.S. Holder will determine the U.S. dollar value of the amount realized in a non-U.S. dollar currency by translating the amount received at the spot rate of exchange on the settlement date of the sale. If the U.S. Holder is an accrual basis taxpayer that is not eligible to or does not elect to determine the amount realized using the spot rate on the settlement date, the U.S. Holder will recognize foreign currency gain or loss to the extent of any difference between the U.S. dollar amount realized on the date of sale or disposition and the U.S. dollar value of the currency received at the spot rate on the settlement date.
Passive Foreign Investment Company Rules
We are a “foreign corporation,” within the meaning of the Code. Under the Code, we will be a PFIC for any taxable year in which (1) 75% or more of our gross income consists of passive income or (2) 50% or more of the value of our assets (generally determined on the basis of a weighted quarterly average) consists of assets that produce, or are held for the production of, passive income (including cash and cash equivalents). For purposes of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property and certain rents and royalties. In addition, for purposes of the above calculations, a non-U.S. corporation that directly or indirectly owns at least 25% by value of the shares of another corporation is treated as holding and receiving directly its proportionate share of assets and income of such corporation. If we are a PFIC for any taxable year during which a U.S. Holder holds our shares, the U.S. Holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC in future years, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, the imposition of interest charges on certain taxes treated as deferred and additional reporting requirements.
Based on our analysis of our activities and current estimates (and not fully audited financials) of the composition of our income and assets, and taking into account the public offering price of the ADSs in this offering, the expected price of the ADSs following this offering and the nature of our business, we do not believe that we were a PFIC in our taxable year ended December 31, 2020 and we do not expect to be a PFIC in our current taxable year. The determination of whether we are a PFIC is a fact-intensive determination made on an annual basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation. Subject to certain exceptions, for purposes of the asset test, if we are treated as a non-publicly traded CFC for the period being tested for purposes of the PFIC rules, the value of our assets will be measured by the adjusted tax basis of our assets. If we are a publicly traded CFC or not a CFC for such period, the total value of our assets (including goodwill) for PFIC testing purposes may be determined in part by reference to the market price of our ordinary shares or ADSs from time to time, which may fluctuate considerably. Accordingly, if our total asset value is determined by reference to the market price of our ordinary shares or ADSs and our market capitalization declines while we hold a substantial amount of cash and cash-equivalents for any taxable year, we may be a PFIC for that taxable year as a result of such decline. Under the income test, our status as a PFIC depends on the composition of our income for the relevant taxable year which will depend on the transactions we enter into in the future and our corporate structure. The composition of our income and assets is also affected by how we spend the cash we raise in any offering, including this offering. We may be a PFIC for any taxable year in which we do not generate sufficient amounts of active income to offset our passive financing income. Therefore, we cannot express an expectation regarding our PFIC status for the current or any future taxable year. Even if we determine that we are
139
not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position.
If we are classified as a PFIC in any year with respect to which a U.S. Holder owns the ordinary shares or ADSs, we will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which the U.S. Holder owns the ordinary shares or ADSs, regardless of whether we continue to meet the tests described above, unless we cease to be a PFIC and the U.S. Holder has made a “deemed sale” election under the PFIC rules. If such a deemed sale election is made, a U.S. Holder will be deemed to have sold the ordinary shares or ADSs that the U.S. Holder holds at their fair market value and any gain from such deemed sale would be subject to the rules described below. After the deemed sale election, so long as we do not become a PFIC in a subsequent taxable year, the U.S. Holder’s ordinary shares or ADSs with respect to which such election was made will not be treated as shares in a PFIC and the U.S. Holder will not be subject to the rules described below with respect to any “excess distribution” the U.S. Holder receives from us or any gain from an actual sale or other disposition of the ordinary shares or ADSs.
For each taxable year that we are treated as a PFIC with respect to a U.S. Holder, the U.S. Holder will be subject to special tax rules with respect to any “excess distribution” such U.S. Holder receives and any gain such U.S. Holder recognizes from a sale or other disposition (including a pledge) of ordinary shares or ADSs, unless (i) such U.S. Holder timely makes a “qualified electing fund” election, or QEF Election, with respect to all taxable years during such U.S. Holder's holding period in which we are a PFIC, or (ii) our ordinary shares or ADSs constitute “marketable stock” and such U.S. Holder makes a mark-to-market election (as discussed below). Distributions a U.S. Holder receives in a taxable year that are greater than 125% of the average annual distributions a U.S. Holder received during the shorter of the three preceding taxable years or the U.S. Holder’s holding period for the ordinary shares or ADSs will be treated as an excess distribution. Under the special PFIC tax rules:
| • | the excess distribution in respect of, or gain recognized from a sale or other disposition of, ordinary shares or ADSs will be allocated ratably over a U.S. Holder’s holding period for the ordinary shares or ADSs; |
| • | the amount so allocated to the taxable year of the distribution or disposition (as applicable) and to any taxable year in the U.S. Holder’s holding period prior to the first taxable year during such holding period in which we became a PFIC will be subject to tax (as ordinary income) at the U.S. federal income tax rates applicable to the U.S. Holder for the year in which the distribution or disposition occurs; and |
| • | the amount so allocated to each other year in the U.S. Holder’s holding period will be subject to tax (as ordinary income) at the highest U.S. federal income tax rate in effect for individuals or corporations, as applicable, for the year to which the income is allocated and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of the “excess distribution” or disposition cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the disposition of the ordinary shares or ADSs cannot be treated as capital, even if the U.S. Holder holds the ordinary shares or ADSs as capital assets.
If we are a PFIC, a U.S. Holder will generally be subject to similar rules with respect to distributions we receive from, and our dispositions of the stock of, any of our direct or indirect subsidiaries or any other entities in which we hold equity interests that also are PFICs, or lower-tier PFICs, as if such distributions were indirectly received by, and/or dispositions were indirectly carried out by, such U.S. Holder.
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund, or QEF, election. As we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election, prospective investors should assume that a QEF election will not be available.
Alternatively, U.S. Holders can avoid the interest charge on excess distributions or gain relating to the sale or other disposition of ordinary shares or ADSs by making a valid “mark-to-market” election with respect to the ordinary shares or ADSs, provided that the ordinary shares or ADSs are “marketable stock.” Ordinary shares or ADSs will be marketable stock if they are “regularly traded” on certain U.S. stock exchanges or on a non-U.S. stock exchange that meets certain conditions. For these purposes, the ordinary shares or ADSs will be considered regularly traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Any trades that have as their principal purpose meeting this requirement will be
140
disregarded. Our ADSs (but not ordinary shares) will be listed on the Nasdaq Global Market, which is a qualified exchange for these purposes. Consequently, if our ADSs remain listed on a qualified exchange and are regularly traded, we expect the mark-to-market election would be available to U.S. Holders of our ADSs if we are a PFIC.
A U.S. Holder that makes a mark-to-market election must include in ordinary income for each year an amount equal to the excess, if any, of the fair market value of the ordinary shares or ADSs at the close of the taxable year over the U.S. Holder’s adjusted tax basis in the ordinary shares or ADSs. An electing U.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. Holder’s adjusted tax basis in the ordinary shares or ADSs over the fair market value of the ordinary shares or ADSs at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains for prior years. Gains from an actual sale or other disposition of the ordinary shares or ADSs in any year in which we are a PFIC will be treated as ordinary income, and any losses incurred on a sale or other disposition of the shares will be treated as an ordinary loss to the extent of any net mark-to-market gains for prior years. Once made, the election cannot be revoked without the consent of the IRS unless the ordinary shares or ADSs cease to be marketable stock.
A mark-to-market election generally cannot be made for equity interests in any lower-tier PFICs that we own, unless shares of such lower-tier PFIC are themselves “marketable stock.” As a result, even if a U.S. Holder validly makes a mark-to-market election with respect to our ordinary shares or ADSs, the U.S. Holder may continue to be subject to the PFIC rules (described above) with respect to its indirect interest in any of our investments that are treated as an equity interest in a PFIC for U.S. federal income tax purposes.
Unless otherwise provided by the U.S. Treasury, each U.S. Holder that holds stock of a PFIC is required to file an annual report containing such information as the U.S. Treasury may require. A U.S. Holder’s failure to file the annual report may result in substantial penalties and extend the statute of limitations with respect to the U.S. Holder’s U.S. federal income tax return.
Controlled Foreign Corporation Rules
Under the Code, each “Ten-Percent Shareholder” (as defined below) in a non-U.S. corporation that is classified as a CFC generally is required to annually report and include in its U.S. taxable income its pro rata share of certain types of income earned by the CFC, including “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by the CFC, even if the CFC has made no distributions to its shareholders. In addition, a Ten-Percent Shareholder that realizes gain from the sale or exchange of shares in a CFC may be required to classify a portion of such gain as dividend income rather than capital gain (see discussion above in “Taxation of Distributions” regarding the tax treatment of dividend income). An individual that is a Ten-Percent Shareholder with respect to a CFC generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a Ten-Percent Shareholder that is a U.S. corporation.
A non-U.S. corporation generally will be classified as a CFC if Ten-Percent Shareholders own, directly, indirectly or constructively, more than 50% of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock of such corporation. A “Ten-Percent Shareholder” is a United States person (as defined by the Code) who owns, directly, indirectly or constructively, 10% or more of either the total combined voting power of all classes of stock (including ADSs) of such corporation entitled to vote or of the total value of the stock (including ADSs) of such corporation.
We do not believe that we were a CFC in our taxable year ended March 31, 2021, though we have not made a determination regarding our CFC status in the current taxable year, and we may become a CFC in a subsequent taxable year. In addition, because Stealth BioTherapeutics Corp has a U.S. subsidiary, any future newly formed or acquired non-U.S. subsidiaries of Stealth BioTherapeutics Corp will be treated as CFCs, regardless of whether we are treated as a CFC. The determination of CFC status is complex and includes attribution rules, the application of which is not entirely certain. If we are classified as both a CFC and a PFIC, we generally will not be treated as a PFIC with respect to those U.S. Holders that meet the definition of a Ten-Percent Shareholder during the period in which we are a CFC.
Failure to comply with CFC reporting obligations may subject a Ten-Percent Shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any Ten-Percent Shareholder information that may be necessary to comply with the reporting and tax-paying obligations applicable under the CFC rules of the Internal Revenue Code of 1986, as amended, or the Code.
141
Our status, or the status of any subsidiary, as a CFC will generally not have any U.S. federal income tax consequences for U.S. Holders holding our ordinary shares or ADSs who are not Ten-Percent Shareholders. It is possible that, following this offering, a shareholder treated as a U.S. person for U.S. federal income tax purposes will own or acquire, directly or indirectly, enough shares to be treated as a Ten-Percent Shareholder.
Information Reporting and Backup Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in our ordinary shares or ADSs, including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment Company Rules,” each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than $100,000 for our ordinary shares or ADSs may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to timely comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of our ADSs may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (i) fails to provide an accurate U.S. taxpayer identification number or otherwise establish a basis for exemption or (ii) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
Reporting With Respect to Foreign Financial Assets
Certain U.S. Holders who are individuals and certain closely-held entities may be required to report information relating to an interest in our ordinary shares or ADSs by filing a Form 8398 (Statement of Specified Foreign Financial Assets) with their U.S. federal income tax return, subject to certain exceptions (including an exception for ordinary shares or ADSs held in accounts maintained by certain U.S. financial institutions, in which case the accounts themselves may have to be reported if maintained by non-U.S. financial institutions). Failure to file a Form 8398 where required can result in monetary penalties and the extension of the relevant statute of limitations with respect to all or a part of the relevant U.S. tax return.
Transfer Reporting Requirements
A U.S. Holder may be required to file an IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) if the amount paid by the U.S. Holder in connection with the purchase of ordinary shares or ADSs in this offering, when aggregated with all transfers of cash made by the U.S. Holder (or any related person) to us within the preceding twelve-month period, exceeds $100,000 (or its foreign currency equivalent). U.S. Holders that are required to file IRS Form 926, but fail to do so, could be subject to substantial penalties.
Cayman Islands Taxation
Prospective investors should consult their professional advisors on the possible tax consequences of buying, holding or selling any ADSs under the laws of their country of citizenship, residence or domicile.
The following is a discussion on certain Cayman Islands income tax consequences of an investment in the ADSs. The discussion is a general summary of present law, which is subject to prospective and retroactive change. It is not intended as tax advice, does not consider any investor’s particular circumstances and does not consider tax consequences other than those arising under Cayman Islands law.
No stamp duty, capital duty, registration or other issue or documentary taxes are payable in the Cayman Islands on the creation, issuance or delivery of the ADSs. The Cayman Islands currently have no form of income, corporate or capital gains tax and no estate duty, inheritance tax or gift tax. There are currently no Cayman Islands taxes or duties of any nature on gains realized on a sale, exchange, conversion, transfer or redemption of the ADSs. Payments of dividends and capital in respect of the ADSs or ordinary shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of interest and principal or a dividend or capital to any holder of the ADSs, nor will gains derived from the disposal of the ADSs be subject to Cayman Islands income or corporation tax as the Cayman Islands currently have no form of income or corporation taxes.
142
Pursuant to section 6 of the Tax Concessions Law (2018 Revision) of the Cayman Islands, we have obtained an undertaking from the Governor-in-Cabinet:
| ▪ | that no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciation shall apply to us or our operations; and |
| ▪ | that no such tax or any tax in the nature of estate duty or inheritance tax shall be payable on or in respect of the ADSs or ordinary shares, debentures or other obligations of ours. |
The undertaking for the Company is for a period of twenty years from April 11, 2006.
143
PLAN OF DISTRIBUTION
Pursuant to an engagement agreement, we have engaged H.C. Wainwright & Co., LLC (“Wainwright” or the “placement agent”), to act as our exclusive placement agent in connection with this offering of our ADSs pursuant to this prospectus. Wainwright is not purchasing or selling any ADSs, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of ADSs, but only to use its reasonable best efforts to arrange for the sale of the ADSs by us pursuant to this prospectus. Therefore, we may not sell the entire amount of the ADSs being offered hereby. We will enter into a securities purchase agreement directly with the institutional investors purchasing our ADSs in this offering, at such investor’s option, providing such investors with certain representations, warranties and covenants from us, which representations, warranties and covenants will not be available to other investors which do not enter into a securities purchase agreement. Investors which do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our ADSs in the offering. Wainwright may engage one or more sub-placement agents or selected dealers in connection with this offering.
There is no required minimum number of ADSs or amount of proceeds that must be sold as a condition to completion of this offering.
Fees and Expenses
The following table shows the per ADS and total placement agent fees we will pay in connection with the sale of the ADSs in this offering, assuming the purchase of all of the ADSs we are offering.
Per ADS placement agent cash fee | | |
Total placement agent cash fee | | |
We have agreed to pay the placement agent a total cash fee equal to 6.0% of the gross proceeds of this offering, reduced by $100,000. We will also pay to the placement agent a management fee equal to 1.0% of the gross proceeds of this offering and a non-accountable expense allowance of $35,000 and to reimburse the placement agent’s reasonable and documented expenses in connection with this offering, including fees and expenses of counsel, in the amount of up to $100,000 and the placement agent’s clearing expenses in the amount of up to $15,950. We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent fees and expenses, will be approximately $
Right of First Refusal
We have granted a right of first refusal to the placement agent to participate in any public offering (including an at-the-market facility), private placement or any other capital-raising financing of equity, equity-linked or debt securities using an underwriter or placement agent by us or any of our subsidiaries, subject to certain exclusions, with (at least 50% of the economics in connection with a financing with gross proceeds to us of $25 million or less or at least 35% of the economics in connection with a financing with gross proceeds to us of over $25 million, at any time prior to the six-month anniversary of the earlier of the date of the closing of the offering or the date of termination or expiration of our engagement of the placement agent.
Tail
In the event that any investor which the placement agent had contacted or introduced to us during the term of its engagement provides any capital to us in a public or private offering or capital-raising transaction, subject to certain exceptions, within the 6 months following the expiration of termination of the engagement of the placement agent, we shall pay the placement agent the cash compensation and expense reimbursement provided above, calculated in the same manner.
Lock-up Agreements
We have agreed with the placement agent, for a period of days following the date of closing of the offering, and each of our officers and directors have agreed, for a period of days following the date of closing of the offering, to be subject to a lock-up period, subject to certain customary exceptions. This means that, during the applicable lock-up period, we may not issue, enter into any agreement to issue or announce the issuance or proposed issuance of any shares of common stock or their equivalents, and our officers and directors may not transfer or sell any shares of common stock or their equivalents, subject to certain exceptions. The placement agent may waive the terms of these lock-up agreements in its sole discretion and without notice. In addition, subject to certain exceptions after days following the date of closing of the offering, we have
144
agreed to not issue any securities that are subject to a price reset based on the trading prices of our common stock or upon a specified or contingent event in the future or enter into any agreement to issue securities at a future determined price, for months following the date of closing of the offering pursuant to this prospectus. The placement agent may waive this prohibition in its sole discretion and without notice.
Determination of Offering Price
The actual offering price of the ADSs we are offering will be negotiated between us and the investors, in consultation with the placement agent, based on the trading of our ADSs prior to the offering, among other things. Other factors considered in determining the offering price of the securities we are offering include the history and prospects of our company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Nasdaq Listing
Our ADSs are listed on the Nasdaq Global Market under the symbol “MITO.”
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act of 1933, as amended, or the Securities Act, or to contribute to payments the placement agent may be required to make with respect to any of these liabilities.
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act and any fees received by it and any profit realized on the sale of the securities by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities Act and Securities Exchange Act of 1934, as amended, or the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
Other Relationships
The placement agent and its respective affiliates have engaged in, and may in the future engage in, investment banking, advisory and other commercial dealings in the ordinary course of business with us or our affiliates for which they have received and may continue to receive customary fees and commissions. The placement agent is acting as sales agent under our at the market offering and has been paid compensation in connection with that role.
145
EXPENSES OF THIS OFFERING
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, all of which will be paid by us. All amounts are estimated except the SEC registration fee and the FINRA filing fee.
| | AMOUNT | |
SEC registration fee | | $ | 1,309 | |
FINRA filing fee | | $ | 2,300 | |
Accountants’ fees and expenses | | * | |
Legal fees and expenses | | * | |
Transfer agent’s fees and expenses | | * | |
Printing and engraving expenses | | * | |
Miscellaneous | | * | |
Total expenses | | * | |
* To be filed by amendment.
146
LEGAL MATTERS
Legal matters with respect to U.S. federal and New York state law in connection with this offering will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York. Certain legal matters with respect to Cayman Islands law in connection with the validity of the ADSs being offered by this prospectus and other legal matters will be passed upon for us by Walkers, George Town, Cayman Islands.
EXPERTS
The financial statements, incorporated in this Prospectus by reference from the Company’s Annual Report on Form 20-F for the year ended December 31, 2020, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference, which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph referring to the Company’s ability to continue as a going concern. Such financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The registered business address of Deloitte & Touche LLP is 200 Berkeley Street, Boston, Massachusetts 02116.
147
ENFORCEMENT OF CIVIL LIABILITIES
Our agent for service of process in the United States is Stealth Delaware, and the executive offices of Stealth Delaware are located at 140 Kendrick Street, Needham, MA 02494, and the telephone number there is (617) 600-6888.
We are incorporated under the laws of the Cayman Islands. We have been advised that there is some doubt as to the enforceability in the Cayman Islands, in original actions or in actions for enforcement of judgments of the U.S. courts, of civil liabilities based solely on the federal securities laws of the United States. In addition, awards for punitive damages in actions brought in the United States, or elsewhere may be unenforceable in the Cayman Islands. An award for monetary damages under the U.S. securities laws would be considered punitive if it does not seek to compensate the claimant for loss or damage suffered and is intended to punish the defendant. The enforceability of any judgment in the Cayman Islands will depend on the particular facts of the case as well as the laws and treaties in effect at the time. The United States and the Cayman Islands do not currently have a treaty providing for recognition and enforcement of judgments (other than arbitration awards) in civil and commercial matters.
We have been advised by our Cayman Islands legal counsel that the Cayman Islands Court is unlikely (i) to recognize or enforce against us judgments of the courts of the United States predicated upon the civil liability provisions of the federal securities laws of the United States or any state; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against us predicated upon the civil liability provisions of the federal securities laws of the United States or any state, so far as the liabilities imposed by those provisions are penal in nature. In certain circumstances, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the Cayman Islands Court will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits and based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty , inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). The Cayman Islands Court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
148
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1, including relevant exhibits and schedules under the Securities Act with respect to underlying ordinary shares represented by the ADSs, to be sold in this offering. A related registration statement on F-6 has been filed with the SEC to register the ADSs. This prospectus, which constitutes a part of the registration statement, does not contain all of the information contained in the registration statement. You should read the registration statement and its exhibits and schedules for further information with respect to us and our ADSs.
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. The SEC maintains an internet site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Copies of certain information filed by us with the SEC are also available on our website at http://www.stealthbt.com. Our website is not a part of this prospectus and is not incorporated by reference in this prospectus.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend to furnish the depositary with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP, and all notices of shareholders’ meeting and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
INCORPORATION BY REFERENCE
The SEC allows us to incorporate by reference in this prospectus much of the information we have filed with the SEC, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part of this prospectus. This prospectus incorporates by reference the documents listed below (File No. 001-38810):
| • | Annual Report on Form 20-F for the fiscal year ended December 31, 2020, filed with the SEC on April 6, 2021; |
| • | Item 7.B, “Related Party Transactions,” from our Annual Report on Form 20-F for the fiscal year ended December 31, 2019, filed with the SEC on April 1, 2020; |
| • | Item 7.B, “Related Party Transactions,” from our Annual Report on Form 20-F for the fiscal year ended December 31, 2018, filed with the SEC on April 4, 2019; |
| • | The section under the heading “Related Party Transactions” from our prospectus dated February 14, 2019, filed with the SEC pursuant to Rule 424(b); |
| • | Our Reports of Foreign Private Issuer on Form 6-K, filed with the SEC on February 11, 2021, April 30, 2021, May 18, 2021, June 15, 2021, July 20, 2021, and August 5, 2021; and |
| • | The description of our Ordinary Shares and ADSs contained in our registration statement on Form 8-A, filed with the SEC under the Exchange Act on February 12, 2019, including any amendment or report filed for the purpose of updating such description. |
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
c/o Stealth BioTherapeutics Inc.
140 Kendrick Street
Needham, MA 02494
(617) 600-6888
149
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
STEALTH BIOTHERAPEUTICS CORP
Condensed Consolidated Balance Sheets
(unaudited)
(in thousands, except share and per share amounts)
| | DECEMBER 31, 2020 | | | JUNE 30, 2021 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 32,787 | | | $ | 30,766 | |
Prepaid expenses and other current assets | | | 2,253 | | | | 844 | |
Total current assets | | | 35,040 | | | | 31,610 | |
| | | | | | | | |
Property and equipment, net | | | 106 | | | | 72 | |
Deferred financing cost and other assets | | | 702 | | | | 576 | |
Total assets | | $ | 35,848 | | | $ | 32,258 | |
Liabilities and shareholders’ deficit | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 3,526 | | | $ | 2,566 | |
Accrued expenses and other current liabilities | | | 7,024 | | | | 5,134 | |
Accrued interest payable | | | 1,499 | | | | 263 | |
Current portion of debt | | | 9,000 | | | | 5,452 | |
Total current liabilities | | | 21,049 | | | | 13,415 | |
Long-term deferred rent, less current portion | | | 16 | | | | 6 | |
Development derivative liability -related party | | | 25,155 | | | | 45,152 | |
Total liabilities | | | 46,220 | | | | 58,573 | |
| | | | | | | | |
Shareholders’ deficit: | | | | | | | | |
Ordinary shares, $.0003 nominal or par value; 1,200,000,000 shares authorized and 635,092,150 shares issued and outstanding at December 31, 2020;1,200,000,000 shares authorized and 690,993,790 shares issued and outstanding at June 30, 2021 | | | 191 | | | | 207 | |
Additional paid-in capital | | | 544,891 | | | | 555,027 | |
Accumulated deficit | | | (555,454 | ) | | | (581,549 | ) |
Total shareholders’ deficit | | | (10,372 | ) | | | (26,315 | ) |
Total liabilities and shareholders’ deficit | | $ | 35,848 | | | $ | 32,258 | |
See the accompanying notes to these unaudited condensed consolidated financial statements.
F-1
STEALTH BIOTHERAPEUTICS CORP
Condensed Consolidated Statements of Operations
(unaudited)
(in thousands, except share and per share amounts)
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Operating expenses: | | | | | | | | |
Research and development | | $ | 17,252 | | | $ | 12,012 | |
General and administrative | | | 9,703 | | | | 10,062 | |
Total operating expenses | | | 26,955 | | | | 22,074 | |
Loss from operations | | | (26,955 | ) | | | (22,074 | ) |
Other income (expense): | | | | | | | | |
Interest income | | | 137 | | | | 2 | |
Interest expense | | | (1,091 | ) | | | (488 | ) |
Gain (loss) from remeasurement of derivative liability | | | — | | | | (3,535 | ) |
Total other expense, net | | | (954 | ) | | | (4,021 | ) |
Net loss attributable to ordinary shareholders | | $ | (27,909 | ) | | $ | (26,095 | ) |
Net loss per share attributable to ordinary shareholders—basic and diluted | | $ | (0.06 | ) | | $ | (0.04 | ) |
Weighted average ordinary shares used in net loss per share attributable to ordinary shareholders—basic and diluted | | | 506,055,526 | | | | 663,833,037 | |
See the accompanying notes to these unaudited condensed consolidated financial statements.
F-2
STEALTH BIOTHERAPEUTICS CORP
Condensed Consolidated Statements of Shareholders’ Deficit
(unaudited)
(in thousands, except share and per share amounts)
| | ORDINARY SHARES | | | ADDITIONAL PAID-IN | | | ACCUMULATED | | | TOTAL SHAREHOLDERS’ | |
| | SHARES | | | AMOUNT | | | CAPITAL | | | DEFICIT | | | EQUITY (DEFICIT) | |
Balance at December 31, 2019 | | | 436,720,810 | | | $ | 131 | | | $ | 515,133 | | | $ | (497,997 | ) | | $ | 17,267 | |
Share-based compensation expense | | | — | | | | — | | | | 2,000 | | | | — | | | | 2,000 | |
Issuance of ordinary shares, net of issuance cost of $0 | | | 152,858,460 | | | | 46 | | | | 19,954 | | | | — | | | | 20,000 | |
Ordinary share issued under share incentive plan upon vesting of restricted stock units | | | 2,290,440 | | | | 1 | | | | (1 | ) | | | — | | | | — | |
Issuance of commitment shares | | | 2,203,812 | | | | | | | | 227 | | | | — | | | | 227 | |
Net loss | | | — | | | | — | | | | — | | | | (27,909 | ) | | | (27,909 | ) |
Balance at June 30, 2020 | | | 594,073,522 | | | $ | 178 | | | $ | 537,313 | | | $ | (525,906 | ) | | $ | 11,585 | |
Balance at December 31, 2020 | | | 635,092,150 | | | $ | 191 | | | $ | 544,891 | | | $ | (555,454 | ) | | $ | (10,372 | ) |
Issuance of ordinary shares, net of issuance cost of $789 | | | 48,768,000 | | | | 14 | | | | 6,380 | | | | — | | | | 6,394 | |
Ordinary share issued under share incentive plan upon vesting of restricted stock units | | | 7,133,640 | | | | 2 | | | | (2 | ) | | | — | | | | — | |
Equity classified warrants | | | — | | | | — | | | | 1,538 | | | | — | | | | 1,538 | |
Share-based compensation expense | | | — | | | | — | | | | 2,220 | | | | — | | | | 2,220 | |
Net loss | | | — | | | | — | | | | — | | | | (26,095 | ) | | | (26,095 | ) |
Balance at June 30, 2021 | | | 690,993,790 | | | $ | 207 | | | $ | 555,027 | | | $ | (581,549 | ) | | $ | (26,315 | ) |
See the accompanying notes to these unaudited condensed consolidated financial statements.
F-3
STEALTH BIOTHERAPEUTICS CORP
Condensed Consolidated Statements of Cash Flows
(unaudited)
(in thousands)
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Cash flows from operating activities: | | | | | | | | |
Net loss | | $ | (27,909 | ) | | $ | (26,095 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 136 | | | | 39 | |
Change in fair value of derivative liability | | | — | | | | 3,535 | |
Amortization of debt discount | | | 188 | | | | 23 | |
Non-cash interest expense | | | 188 | | | | 130 | |
Share-based compensation | | | 2,000 | | | | 2,220 | |
Changes in operating assets and liabilities: | | | | | | | | |
Prepaid expenses and other current assets | | | 547 | | | | 1,157 | |
Accounts payable | | | (4,805 | ) | | | (1,020 | ) |
Accrued expenses, accrued interest payable and other current liabilities | | | (3,245 | ) | | | (3,269 | ) |
Net cash used in operating activities | | | (32,900 | ) | | | (23,280 | ) |
Cash flows from investing activities: | | | | | | | | |
Purchase of property and equipment | | | (14 | ) | | | (5 | ) |
Receipt for security deposit | | | — | | | | 250 | |
Net cash provided (used) in investing activities | | | (14 | ) | | | 245 | |
Cash flows from financing activities: | | | | | | | | |
Proceeds from issuance of common stock net of commissions and underwriters' fees | | | 20,000 | | | | 6,903 | |
Proceeds from development funding agreement | | | — | | | | 18,000 | |
Payment of offering costs | | | — | | | | (318 | ) |
Principal payments on term debt | | | (6,020 | ) | | | (3,571 | ) |
Net cash provided by financing activities | | | 13,980 | | | | 21,014 | |
Net decrease in cash and cash equivalents | | | (18,934 | ) | | | (2,021 | ) |
Cash and cash equivalents, beginning of period | | | 50,768 | | | | 32,787 | |
Cash and cash equivalents, end of period | | $ | 31,834 | | | $ | 30,766 | |
Supplemental cash flow information: | | | | | | | | |
Cash paid for end of term charge for the term debt | | $ | — | | | $ | 1,335 | |
Cash paid for interest | | $ | 747 | | | $ | 361 | |
Supplemental disclosure of noncash investing and financing activity: | | | | | | | | |
Offering costs included in accounts payable and accrued expenses | | $ | 307 | | | $ | 64 | |
Commitment shares issued to LPC | | $ | 227 | | | $ | — | |
Reclassification of deferred offering costs to additional paid-in capital | | $ | — | | | $ | 126 | |
See the accompanying notes to these unaudited condensed consolidated financial statements.
F-4
STEALTH BIOTHERAPEUTICS CORP
Notes to Unaudited Condensed Consolidated Financial Statements
Six months ended June 30, 2020 and 2021
1. Organization and Operations
The Company
Stealth BioTherapeutics Corp was incorporated in Grand Cayman, Cayman Islands as Stealth Peptides International, Inc. in April 2006. Its wholly owned subsidiary, Stealth BioTherapeutics Inc., was incorporated in Delaware as Stealth Peptides Inc. in October 2007. In addition, a wholly owned subsidiary, Stealth BioTherapeutics (HK) Limited, was incorporated in Hong Kong in September 2017. In May 2018, Stealth BioTherapeutics (Shanghai) Limited was formed as a wholly foreign owned enterprise in China. In 2020, Stealth BioTherapeutics (Shanghai) limited was dissolved. Hereinafter, Stealth BioTherapeutics Corp, Stealth BioTherapeutics Inc., and Stealth BioTherapeutics (HK) Limited are referred to as the “Company.” The Company is a clinical-stage biotechnology company focused on the discovery and development of novel pharmaceutical agents to treat patients suffering from diseases involving mitochondrial dysfunction through its mitochondrial medicine platform. The consolidated financial statements include the assets, liabilities and operating results of the Company and its wholly owned subsidiaries. Since inception, the Company has devoted substantially all of its efforts to research and development, business planning, acquiring operating assets, seeking intellectual property protection for its technology and product candidates, and raising capital.
The Company has entered into numerous debt and equity issuances with Morningside Venture Investments Limited (“MVIL” ). As of June 30, 2021, MVIL and certain entities associated with MVIL together beneficially owned approximately 72.5% of the Company’s outstanding shares.
The Company has incurred net losses and negative cash flows from operations and had an accumulated deficit of $581.5 million as of June 30, 2021. The Company has financed its operations to date through its issuance of preferred shares, initial public offering (“IPO”), issuance of ordinary shares, American depositary shares (“ADS”) offerings, convertible debt and long-term debt.
Liquidity and Going Concern
These condensed consolidated financial statements have been prepared on a going concern basis, which assumes the realization of assets and settlement of liabilities in the normal course of business. Since its inception, the Company has incurred recurring losses, including net losses of $26.1 million for the six months ended June 30, 2021. The Company expects to continue to incur operating losses in the foreseeable future.
Management believes that cash and cash equivalents of $30.8 million at June 30, 2021, together with the proceeds of $22.0 million committed to be funded by MVIL, will not be sufficient to fund operating expenses for twelve months from the date these interim condensed consolidated financial statements are issued. The Company may seek to obtain financing through equity and debt issuances, collaborative agreements, and grants from government and private sponsors. Because the ability to obtain additional financing is outside of the Company’s control, the foregoing conditions raise substantial doubt in regard to the Company’s ability to continue as a going concern. If the Company is unable to obtain additional funding when needed, or to the extent needed, it may be necessary to scale back operations or halt certain research and development activities, which could prevent the Company from successfully executing on its operating plan. The condensed consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded assets or liabilities that might be necessary should the Company be unable to continue its operations.
2. Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended December 31, 2020, incorporated by reference in this prospectus. Since the date of those financial statements, there have been no changes to its significant accounting policies. There were no new accounting pronouncements adopted during 2021 that had a material effect on our consolidated financial statements.
Unaudited Interim Financial Information
The accompanying unaudited condensed consolidated financial statements have been prepared by the Company in accordance with accounting principles generally accepted in the United States for interim financial reporting and as required by Regulation S-X, Rule 10-01. The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for a fair statement of the Company’s financial position as of June 30, 2021, and the results of its operations and its cash flows for the six months ended
F-5
June 30, 2020, and 2021. The financial data and other information disclosed in these notes related to the six months ended June 30, 2020, and 2021 are unaudited. The results for the six months ended June 30, 2021, are not necessarily indicative of results to be expected for the year ending December 31, 2021, any other interim periods, or any future year or period. Accordingly, these condensed consolidated financial statements should be read in conjunction with the financial statements and notes thereto incorporated by reference in this prospectus and included in the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2020.
3. Fair Value of Financial Assets and Liabilities
Fair Value Hierarchy
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value are performed in a manner to maximize the use of observable inputs and minimize the use of unobservable inputs. The accounting standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1—Quoted prices in active markets that are accessible at the market date for identical unrestricted assets or liabilities.
Level 2—lnputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs for which all significant inputs are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The following tables present information about the Company’s financial assets and liabilities measured at fair value on a recurring basis and indicate the level of the fair value hierarchy utilized to determine such fair values as of December 31, 2020, and June 30, 2021 (in thousands):
| | FAIR VALUE MEASUREMENTS AS OF DECEMBER 31, 2020 USING: | |
| | LEVEL 1 | | | LEVEL 2 | | | LEVEL 3 | | | TOTAL | |
Assets: | | | | | | | | | | | | | | | | |
Short-term money market funds | | $ | 32,643 | | | $ | — | | | $ | — | | | $ | 32,643 | |
Total financial assets | | $ | 32,643 | | | $ | — | | | $ | — | | | $ | 32,643 | |
Liabilities: | | | | | | | | | | | | | | | | |
Development Derivative liability | | $ | — | | | $ | — | | | $ | 25,155 | | | $ | 25,155 | |
Total financial liability | | $ | — | | | $ | — | | | $ | 25,155 | | | $ | 25,155 | |
| | FAIR VALUE MEASUREMENTS AS OF JUNE 30, 2021 USING: | |
| | LEVEL 1 | | | LEVEL 2 | | | LEVEL 3 | | | TOTAL | |
Assets: | | | | | | | | | | | | | | | | |
Short-term money market funds | | $ | 30,666 | | | $ | — | | | $ | — | | | $ | 30,666 | |
Total financial assets | | $ | 30,666 | | | $ | — | | | $ | — | | | $ | 30,666 | |
Liabilities: | | | | | | | | | | | | | | | | |
Development Derivative liability | | $ | — | | | $ | — | | | $ | 45,152 | | | $ | 45,152 | |
Total financial liability | | $ | — | | | $ | — | | | $ | 45,152 | | | $ | 45,152 | |
F-6
As of December 31, 2020, and June 30, 2021, the carrying amounts of cash, accounts payable and accrued expenses approximated their estimated fair values because of the short-term nature of these financial instruments. The Company’s cash equivalents, which are in money market funds, are classified within Level 1 of the fair value hierarchy because they are valued using quoted prices as of December 31, 2020, and June 30, 2021.
As of December 31, 2020, and June 30, 2021, the outstanding debt from Term Loan bears interest at a rate which approximate prevailing market rate for an instrument with similar characteristics and, accordingly, the carrying value for this instrument approximates fair value.
There have been no transfers between fair value measure levels during the years ended December 31, 2020, and 2021.
Development Derivative Liability
On October 30, 2020, the Company entered into a development funding agreement (“Development Agreement”) with MVIL under which MVIL agreed to provide funding to us to support our efforts to secure regulatory approval for elamipretide and to develop elamipretide for the treatment of Barth syndrome (“Barth”), geographic atrophy, an advanced form of dry-age related macular degeneration (“dry AMD”), Friedreich’s ataxia (“FRDA”), Duchenne cardiomyopathy (“DMDC”), Leber’s hereditary optic neuropathy (“LHON”) and mitochondrial replisome-related disorders, which we collectively refer to as the Designated Indications.
Under the Development Agreement, MVIL has paid $20.0 million to the Company upon execution of the Agreement, and in February 2021 paid $10.0 million to the Company upon completing enrollment of its ReCLAIM 2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD (the “Tranche 2 Milestone Event’). MVIL has also agreed to pay $5.0 million within 15 days of the submission by the Company of a new drug application to the U.S. Food and Drug Administration (the “FDA”) for elamipretide for the treatment of Barth (the “Tranche 3 Milestone Event”). Upon receipt of funding for each Tranche 2 Milestone Event and Tranche 3 Milestone Event, the Company is required to issue a warrant exercisable for ordinary shares at an exercise price that is 115% of the implied price of the Company’s ordinary shares on the date of issuance, with such number of ordinary shares being equal to the quotient of 30% of the amount of each funding received divided by the exercise price (“Future Warrants”).
Prior to the occurrence of the Tranche 3 Milestone Event, the Company may agree to add additional investors to the Agreement (each, an “Additional Investor”, and any such Additional Investors together with MVIL, the “Investors”), subject to the prior written consent of MVIL. The commitment from each such Additional Investor will be on the same terms and subject to the same conditions as the initial commitments, and, together with the commitment from MVIL, the aggregate commitments of the Investors will not exceed $70.0 million without the consent of MVIL.
In addition, upon the mutual agreement of the Company and the Investors, at any time after the Company receives positive data from a clinical trial in a Designated Indication, the Company may request that the Investors make additional commitments of up to an additional $35.0 million in the aggregate. Each Investor may agree to fund such commitment or not at its sole discretion.
The Company is required to make success payments to the Investors (“Success Payments”) upon receipt of an approval of elamipretide (a “Regulatory Approval”) of a NDA by the FDA or a marketing authorization application by the European Medicines Agency (the “EMA”) for the treatment of (i) dry AMD (a “Common Approval”) and (ii) Barth, FRDA, DMDC, replisome-related disorders or LHON (each, an “Orphan Approval”), subject to certain adjustments with most payments due in the 5th through 7th year following regulatory approval. No payments are owed should regulatory approval not be achieved for elamipretide in the designated indications.
If the first Regulatory Approval is an Orphan Approval, the Company will pay Success Payments of $2 million upon approval and then an additional $158 million in the aggregate in seven additional annual payments. All Success Payments will be proportionately adjusted in the event that the actual funding received by the Company from Investors is lower or greater than $70.0 million including as a result of the payment of the Additional Funding. If the first Regulatory Approval is a Common Approval, or upon a second regulatory approval (whether a Common Approval or an Orphan Approval), the Company will make total Success Payments reflecting a 27% internal rate of return over a seven-year term following such approval.
If the Company’s board of directors determines to seek a Regulatory Approval from both the FDA and EMA, then 66% of each applicable Success Payment will be due upon Regulatory Approval by the FDA and each applicable anniversary thereof and 34% of each applicable Success Payment will be due upon Regulatory Approval by the EMA and each applicable anniversary thereof.
F-7
In addition, the Company has agreed that its obligations to the Investors under the Development Agreement will be subordinated to its existing indebtedness owed to Hercules Capital, Inc. (“Hercules”) under the Company’s Loan and Security Agreement, as amended (Note 7). The Company, Hercules and the Investors have entered in a customary subordination agreement.
Upon execution of the Development Agreement, the Company issued a warrant to MVIL exercisable for 46,153,846 ordinary shares (“Initial Warrant”) at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The warrant was immediately exercisable and has a term of three years.
The Development Agreement is presented as a derivative liability on the consolidated balance sheet as of December 31, 2020. The Success Payments feature in the Development Agreement meets the criteria for derivative accounting as it has multiple underlying, payment provisions, nominal initial net investment and a net settlement provision. The Development Agreement also includes provisions that allow for the issuance of Future Warrants upon receipt of additional funding. At inception, the Future Warrants were not considered “fixed-for-fixed” as the exercise price and number of ordinary shares are dependent on the date and share price at the date of the issuance. As such the Future Warrants were deemed to be liability classified. The Development Agreement and the Future Warrants are considered to be a hybrid instrument recorded as the development derivative liability on our consolidated balance sheets.
At the inception of the arrangement, the Company identified two units of account (i) the Initial Warrant and (ii) derivative liability, which included the Success Payments feature and the Future Warrants. The development derivative liability was initially recorded at the value of the $18.1 million, the estimated fair value at inception of the arrangement, and is remeasured at fair value at each reporting date. The remaining amount of $1.9 million of the initial cash received of $20.0 million, was attributed to the Initial Warrant, which met the criteria for equity classification and was recognized as a component of additional paid in capital and was not remeasured.
In February 2021, the Company received a milestone payment of $10.0 million from MVIL, in accordance with Development Agreement, upon completion of enrollment for the ReCLAIM-2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD. The Company issued a warrant to MVIL exercisable for 18,750,000 ordinary shares at an exercise price of $0.16 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. The warrant was immediately exercisable and has a term of three years. Upon the receipt of the milestone payment, the warrant met the criteria for equity classification as it was considered “fixed-for-fixed” and was recognized as a component of additional paid in capital and was not remeasured.
On May 17, 2021, the Company and MVIL amended the Development Agreement. Pursuant to the amended Development Agreement the Company received $8.0 million in May 2021 and will receive an additional (i) $11.0 million on or about October 1, 2021 and (ii) $11.0 million on or about December 1, 2021. The Company is required to issue a warrant to MVIL, in connection with each such additional funding, under the same terms and conditions as Development Agreement. Upon the MVIL commitment of $30.0 million in additional funding the Company recognized a loss related to the development derivative liability of $3.3 million. In connection with the funding received in May 2021, the Company issued a warrant to MVIL exercisable for 18,461,538 ordinary shares at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s commitment divided by the exercise price. Upon the receipt of the funding tranche of $8.0 million, the related Future Warrant met the criteria for equity classification as it was considered “fixed-for-fixed” and was recognized as a component of additional paid in capital and was not remeasured. The warrant was immediately exercisable and has a term of three years.
During the six months ended June 30, 2021, the fair value of warrants recognized as a component of additional paid in capital was $1.5 million based on a Black Scholes Merton model with the following ranges of assumptions: volatility of 67.61%-78.25%, share price of $0.11-$0.14, risk-free rate of 0.3%-0.34%, a term of 3 years and 11.5%-15.5% discount for lack of marketability.
F-8
The development derivative liability is considered a level 3 fair value measurement, as it is dependent upon significant unobservable inputs and as such is recorded at fair value and remeasured at each reporting period. The change in valuation of the development derivative liability of $3.5 million was recorded as a loss for the six months ended June 30, 2021, on the condensed consolidated statement of operations. The development derivative liability has a remeasured fair value on June 30, 2021, of $45.2 million.
The following table presents the development derivative liability measured at fair value using unobservable inputs (Level 3) as of the six months ended June 30, 2021 (in thousands):
Fair value at January 1, 2021 | | $ | 25,155 | |
Amounts received under the Development Agreement | | | 10,000 | |
Amounts received under the Development Agreement as amended | | | 8,000 | |
Loss from remeasurement of development derivative liability during the reporting period | | | 3,535 | |
Reclassification of equity classified warrants | | | (1,538 | ) |
Fair value at June 30, 2021 | | $ | 45,152 | |
The derivative liability is valued using a scenario-based discounted cash flow method, whereby each scenario makes assumptions regarding the probability and timing of cash flows, and the present value of cash flows is determined using a risk-adjusted discount rate.
The fair value of the Future Warrants was estimated as of the inception of the agreement and as of each reporting period, using the Black Scholes Merton valuation model. As of June 30, 2021, the valuation used the ranges of assumptions: volatility of 69.9%, simulated share price of $0.08-$0.24 based on a Monte Carlo model, risk-free rate of 0.46%, a term of 3 years and a 15.4% discount for lack of marketability.
Key inputs to the level 3 fair value model at inception and as of the reporting date include (i) the probability and timing of achieving stated development milestones to receive the next tranches of funding and the related issuance of Future Warrants upon receipt of the respective tranches of funding (ii) the probability and timing of achieving FDA and EMA approval of the designated indications, and (iii) the Company’s implied cost of borrowing (17.1% as of reporting period).
4. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following (in thousands):
| | AS OF DECEMBER 31, 2020 | | | AS OF JUNE 30, 2021 | |
Research and development | | $ | 357 | | | $ | 199 | |
Prepaid insurance | | | 1,378 | | | | 421 | |
Other | | | 518 | | | | 224 | |
Total | | $ | 2,253 | | | $ | 844 | |
5. Property and Equipment, Net
Property and equipment, net consists of the following (in thousands):
| | AS OF DECEMBER 31, 2020 | | | AS OF JUNE 30, 2021 | |
Computer equipment and software | | $ | 116 | | | $ | 116 | |
Furniture, fixtures and other | | | 147 | | | | 153 | |
Laboratory equipment | | | 289 | | | | 289 | |
Leasehold improvements | | | 16 | | | | 16 | |
| | | 568 | | | | 574 | |
Accumulated depreciation | | | (462 | ) | | | (502 | ) |
Property and equipment, net | | $ | 106 | | | $ | 72 | |
F-9
Depreciation expense for the six months ended June 30 2020, and 2021 was $0.1 million and $39,000, respectively.
6. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| | AS OF DECEMBER 31, 2020 | | | AS OF JUNE 30, 2021 | |
Research and development | | $ | 3,098 | | | $ | 2,558 | |
Employee compensation costs | | | 2,983 | | | | 1,641 | |
Consulting and professional services | | | 647 | | | | 528 | |
Legal expenses | | | 245 | | | | 349 | |
Deferred rent | | | 6 | | | | 19 | |
Other | | | 45 | | | | 39 | |
Total | | $ | 7,024 | | | $ | 5,134 | |
7. Debt
Term Loan
In June 2017, the Company entered into a Loan and Security Agreement (the “LSA”) with a lender that permits the Company to borrow up to an aggregate principal amount of $40.0 million through a multiple tranche term loan (the “Term Loan”). The tranche advances are based on the Company achieving certain performance milestones as defined in the LSA. Upon closing of the Term Loan, the Company drew the first tranche less expenses, which resulted in net proceeds of $12.1 million. In September 2017, the Company drew the second tranche advance of $2.5 million upon achieving the first milestone. In March 2018, the Company drew the third tranche advance of $5.0 million upon achieving a second milestone, bringing the total gross amount borrowed to $20.0 million as of June 30, 2021. The Term Loan included a $0.2 million facility charge, which was paid to the lender on the closing date. The Company paid a $30,000 due diligence fee prior to the Term Loan closing, and the Company incurred additional cash expenses of $0.4 million related to the Term Loan. These three amounts were all recorded as a debt discount and are being amortized as interest expense using the effective interest method over the life of the Term Loan. The Term Loan also includes an end of term charge equal to 5% of the aggregate principal amount of all advances. The end of term charge is being accreted and recorded to interest expense over the life of the Term Loan using the effective interest method.
The Term Loan bears interest at the greater of (i) the prime rate plus 5.5% or (ii) 9.5%. As of June 30, 2021, the interest rate was 9.5%. Interest accrues from the closing date and interest payments are due monthly in arrears on the first of the month. Payments under the Term Loan were interest only for the first twelve months after closing followed by a 30-month amortization period of principal and interest payments that were scheduled to begin on August 1, 2018 and continue through the scheduled maturity date of January 1, 2021. During 2018, the Term Loan was amended to, among other things, postpone the principal payments to December 1, 2018. In March 2019, the Term Loan was amended to postpone principal payments to October 1, 2019. These amendments to the Term Loan were accounted for as a debt modification. For consideration of the amendments, the Company agreed to pay an additional end of term charge of $0.3 million at maturity which is being accrued and recorded to interest expense over the life of the loan using the effective interest method. In October 2019, subsequent to the October principal payment, the principal payments on the Term Loan were deferred to February 1, 2020, based on achievement of certain performance milestones. In July 2020, subsequent to the July principal payment, the Term loan was amended to defer the principal payments until March 1, 2021 and extend the maturity date from January 1, 2021 to July 1, 2021. In April 2021, subsequent to the April principal payment, the Term Loan was amended to extend the maturity date from July 1, 2021 to January 1, 2022 and all principal payments were deferred until the maturity date. For consideration of the amendments, the Company agreed to pay an additional end of term charge of $0.1 million at maturity which is being accrued and recorded to interest expense over the life of the loan using the effective interest method. On January 1, 2021, the Company paid an end of term charge of $1.3M. As of June 30, 2021, the total end of term charge was $0.3 million.
F-10
The Company may voluntarily prepay all, but not less than all, of the outstanding principal at any time prior to the maturity date, subject to a prepayment fee of 0.5% of the outstanding principal at the time the prepayment is made. The end of term charge of $0.2 million and $0.1 million is due on July 1, 2021 and January 1, 2022, respectively or earlier if the Term Loan Facility is terminated prior the maturity of the loan. The Company’s obligations to the lender are secured by a first priority security interest in substantially all of its assets, excluding intellectual property (“IP”). The lender maintains a negative pledge on IP with a security interest in the proceeds of the sale of the IP. The Term Loan contains certain covenants related to restrictions on payments for certain investments, additional debt, distributions and transfers. As consideration for the Term Loan, the Company and the lender entered into a warrant agreement, pursuant to which the lender, as Warrant holder, has the right to purchase a quantity of shares equal to the quotient derived by dividing (a) the Warrant coverage by (b) the exercise price. Warrant coverage means the greater of (a) $312,500 plus 2.5% of future tranche advances in the event all or part of the tranches are funded or (b) $375,000. The exercise price is (a) the purchase price of Series A preferred shares, $2.30769 per share, or (b) the price per share paid in the next equity round of financing of ordinary shares or preferred shares, which results in aggregate gross proceeds of at least $30.0 million. Upon the closing of the IPO, the Warrant became exercisable for 500,000 ordinary shares at an exercise price of $1.00 per ordinary share. The Warrant was exercisable beginning in June 2017, in whole or in part, and expires in ten years. The Warrant was originally recorded as a liability and the discount on the debt was being amortized through interest expense using the effective interest rate method over the remaining term of the Term Loan. Upon the completion of the IPO, the Warrant met the criteria for equity classification as it was indexed to the Company’s stock and as such was reclassified to an equity instrument and was included in additional paid-in capital. See Note 3 for fair value considerations and disclosures. In addition, the lender can declare a material adverse effect while monitoring our business, operations, properties, assets or financial condition. A material adverse effect is considered an event of default under the LSA. In the event of default, repayment of amounts due under the Term Loan may be accelerated by the lender.
Future principal payments under the Term Loan, as amended, as of June 30, 2021, are as follows:
2022 | | $ | 5,456 | |
Total future principal payments | | | 5,456 | |
Less unamortized debt discount | | | 4 | |
Total balance as depicted on the balance sheet | | $ | 5,452 | |
Term loan—current portion | | | 5,452 | |
Total balance as depicted on the balance sheet | | $ | 5,452 | |
Interest expense related to the Term Loan for the six months ended June 30, 2020, and 2021 was $1.1 million and $0.5 million, respectively. Accrued interest as of June 30, 2021 was $0.3 million.
8. Shareholders’ Deficit
Ordinary Shares
At December 31, 2020 and June 30, 2021, 1,200,000,000 ordinary shares, $0.0003 nominal or par value, were authorized for issuance and 635,092,150 and 690,993,790 ordinary shares were issued and outstanding, respectively.
In January 2017, the Company issued a warrant to purchase 231,989 ordinary shares to an affiliate of the interim chief financial officer of Stealth BioTherapeutics Inc. at an exercise price of $1.38 per share. The warrant was fully vested as of December 31, 2017 and expires in January 2022. In June 2018, the warrant was amended and restated to be treated as an option agreement under the Company’s 2006 Share Incentive Plan (the “2006 Plan”).
F-11
On April 10, 2020, the Company entered into an ordinary share purchase agreement (the “Purchase Agreement”), pursuant to which the Company issued and sold to MVIL 152,858,460 ordinary shares, nominal or par value $0.0003 per share (the “Shares”), at a price of $0.13084 per share, for an aggregate purchase price of $20.0 million.
In November 2020, the Company entered into a Securities Purchase Agreement with certain institutional investors for a registered public offering of an aggregate of 2,844,446 ADSs at a public offering price of $1.125 per ADS for net proceeds of approximately $2.6 million.
In February, 2021, the Company entered into a Securities Purchase Agreement with certain institutional investors for a registered public offering of an aggregate of 2,339,000 ADSs or 28,068,000 ordinary shares at a public offering price of $2.00 per ADS for net proceeds of approximately $4.1 million.
Lincoln Park Capital
On June 2, 2020, the Company entered into a $20.0 million purchase agreement (the “LPC Purchase Agreement”), together with a registration rights agreement, with Lincoln Park Capital Fund, LLC ( “LPC”). Under the terms and subject to the conditions of the Purchase Agreement, the Company has the right to sell to and LPC is obligated to purchase up to $20.0 million in shares of the Company’s ordinary shares, subject to certain limitations, from time to time, over the 36-month period commencing on June 22, 2020.
The purchase price of the Ordinary Shares purchased by LPC under the LPC Purchase Agreement will be derived from prevailing market prices of the Company’s ADSs immediately preceding the time of sale. The Company may direct LPC, at its sole discretion and subject to certain conditions, to purchase up to 900,000 ordinary shares on any business day on which the closing sale price of the Company’s ADSs is not below $1.00 per ADS (such purchases, a “Regular Purchase”). The maximum number of Ordinary Shares that the Company may direct LPC to purchase in any single Regular Purchase under the LPC Purchase Agreement increases, up to a maximum of 1,800,000 Ordinary Shares, if on the purchase date for such Regular Purchase the closing sale price of the Company’s ADSs is above certain threshold prices set forth in the LPC Purchase Agreement, provided that LPC’s total purchase obligation under any single Regular Purchase shall not exceed $2,000,000. Additionally, as consideration for entering into the LPC Purchase Agreement, the Company paid LPC a commitment fee of 2,203,812 ordinary shares.
Sales of shares of ordinary shares to LPC under the LPC Purchase Agreement are limited to no more than the number of shares that would result in the beneficial ownership by Lincoln Park and its affiliates, at any single point in time of more than 4.99% of the outstanding Ordinary Shares. Furthermore, under applicable rules of The Nasdaq Global Market, in no event may the Company issue or sell to LPC under the Purchase Agreement more than 19.99% of the Ordinary Shares outstanding immediately prior to the execution of the Purchase Agreement (the “Exchange Cap”), unless (i) the Company obtains shareholder approval to issue Ordinary Shares in excess of the Exchange Cap or (ii) the average price of ADSs that represent the equivalent of all applicable sales of Ordinary Shares to LPC under the Purchase Agreement equals or exceeds $1.9674 per share, such that the transactions contemplated by the LPC Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules.
The LPC Purchase Agreement contains customary representations, warranties, covenants, closing conditions and indemnification and termination provisions by, between and for the benefit of the parties. The Company agreed with LPC that it will not enter into any “variable rate” transactions with any third party for a period defined in the LPC Purchase Agreement. LPC has agreed not to cause or engage in any direct or indirect short selling or hedging of the Company’s ADSs. The LPC Purchase Agreement may be terminated by the Company at any time, at its sole discretion, without any cost or penalty.
Pursuant to the LPC Purchase Agreement, during the six months ended June 30, 2021, a total of 20,700,000 ordinary shares were sold to LPC for net proceeds totaling $2.3 million. No ordinary shares were sold during the six months ended June 30, 2020. To date the Company has sold 25,380,000 ordinary shares to LPC for gross proceeds totaling $3.2 million.
F-12
At the Market Offering
On August 6, 2020, the Company and H.C. Wainwright & Co., LLC ( “Wainwright” ) entered into an At The Market Offering (“ATM”) Agreement pursuant to which the Company may offer and sell, from time to time, through Wainwright, ADSs, each representing 12 ordinary shares, with a nominal or par value of $0.0003 per share. Any such sales would be effective pursuant to the Company’s registration statement on Form F-3 (File No. 333-237542), which was declared effective by the SEC on April 10, 2020. The Company has no obligation to sell any ADSs pursuant to the agreement and may at any time suspend sales pursuant to the agreement. Each party may terminate the agreement at any time without liability. The Company suspended the ATM as of November 19, 2020. As of June 30, 2021, the Company has not sold any shares under the ATM.
Development Agreement
On October 30, 2020, pursuant to the Development Agreement, the Company issued a warrant to MVIL exercisable for 46,153,846 ordinary shares at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s initial funding divided by the exercise price. The warrant was immediately exercisable and has a term of three years.
In February 2021, in accordance with the Development Agreement, the Company received a milestone payment of $10.0 million upon completion of enrollment for the ReCLAIM-2 Phase 2 clinical trial of elamipretide for the treatment of dry AMD. Pursuant to the Development Agreement, the Company issued warrants exercisable for 18,750,000 ordinary shares to MVIL, at an exercise price of $0.16 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s milestone funding divided by the exercise price. The warrant was immediately exercisable and has a term of three years.
In May 2021, in accordance with the Development Agreement, the Company received a payment of $8.0 million upon the Amendment. Pursuant to the Development Agreement, the Company issued warrants exercisable for 18,461,538 ordinary shares to MVIL, at an exercise price of $0.13 with such number of ordinary shares being equal to the quotient of 30% of the amount of MVIL’s milestone funding divided by the exercise price. The warrant was immediately exercisable and has a term of three years.
9. Share Incentive Plan
The Company’s 2006 Plan provided for the grant of share options or other awards to employees, directors, advisors and consultants for the purchase of up to 25,544,054 ordinary shares. Share options vest over varying schedules as determined by the Company’s board of directors and typically expire 10 years from the date of grant. Certain options provide for accelerated vesting if there is a change in control, as defined in the 2006 Plan. The 2006 Plan expired in 2019 and no additional awards can be made under it.
In January 2019, the Company adopted the 2019 Share Incentive Plan (“2019 Plan”) and as a result no further awards will be made under the 2006 Plan. In addition, any ordinary shares subject to awards under the 2006 Plan that expire, are forfeited, or are otherwise surrendered, without having been fully exercised or resulting in any ordinary shares being issued will become available for issuance under the 2019 Plan, up to an additional 15,794,199 shares, which is the number of shares issuable pursuant to outstanding awards granted under the 2006 Plan. On January 1, 2020, 17,468,832 ordinary shares were added to the 2019 Plan pursuant to the Evergreen Provision. In March 2020, upon shareholder approval the 2019 Plan was amended ( “Amended 2019 Plan”) and the number of shares reserved under the plan was reduced by 24,999,996 shares, as those shares are now reserved under the 2020 ADS incentive plan (“2020 ADS Plan”). The Amended 2019 Plan provides for the grant of shares or other awards to employees, directors, advisors and consultants for the purchase of up to 55,898,342 ordinary shares. Share options vest over varying schedules as determined by the Company’s board of directors and typically expire 10 years from the date of grant. Certain options provide for accelerated vesting if there is a change in control, as defined in the Amended 2019 Plan.
In March 2020, upon shareholder approval, the Company adopted the 2020 ADS Plan to provide for grants of restricted ADSs, restricted ADS units and other ADS-based awards. The 2020 ADS Plan provides for the grant of ADS-based awards to employees, directors, advisors and consultants of up to 24,999,996 ordinary shares. Pursuant to the Evergreen Provision, effective January 1, 2021, an additional 22,228,225 ordinary shares were added to the Amended 2019 Plan and an additional 9,526,380 ordinary shares or 793,865 ADSs were added to the 2020 ADS Plan.
F-13
The fair value of each share option granted to employees and directors was estimated on the date of grant using the following assumptions:
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Risk-free interest rate | | 0.5% - 1.38% | | | 0.51%-1.06% | |
Expected dividend yield | | | — | | | | — | |
Expected term (in years) | | 5.23 - 6.98 | | | 5.46 - 6.72 | |
Expected volatility | | 74% - 79% | | | 76% - 79% | |
The following table summarizes share option activity for the Amended 2019 Plan for the six months ended June 30, 2021:
| | NUMBER OF SHARES | | | WEIGHTED- AVERAGE EXERCISE PRICE | | | AVERAGE REMAINING CONTRACTUAL LIFE (IN YEARS) | | | AGGREGATE INTRINSIC VALUE | |
Outstanding at December 31, 2020 | | | 44,959,937 | | | $ | 0.69 | | | | 7.5 | | | $ | 11,650 | |
Granted | | | 21,103,728 | | | $ | 0.15 | | | | | | | | | |
Exercised | | | — | | | $ | — | | | | | | | | | |
Cancelled or forfeited | | | (1,850,355 | ) | | $ | 0.83 | | | | | | | | | |
Outstanding at June 30, 2021 | | | 64,213,310 | | | $ | 0.51 | | | | 7.9 | | | $ | 14,398 | |
Exercisable at June 30, 2021 | | | 27,225,923 | | | $ | 0.78 | | | | 6.3 | | | $ | 1,774 | |
Vested and expected to vest at June 30, 2021 | | | 59,336,318 | | | $ | 0.52 | | | | 7.8 | | | $ | 11,472 | |
The weighted average grant date fair value per share for awards granted during the six months ended June 30, 2021, was $0.10.
The following table summarizes restricted share unit activity for the 2020 ADS Plan for the six months ended June 30, 2021 (in ordinary shares):
| | NUMBER OF SHARES | | | WEIGHTED- AVERAGE GRANT-DATE FAIR VALUE | |
Non-vested at December 31, 2020 | | | 15,013,296 | | | $ | 0.11 | |
Granted | | | 6,054,492 | | | $ | 0.15 | |
Vested | | | (7,133,640 | ) | | $ | 0.11 | |
Cancelled or forfeited | | | (199,548 | ) | | $ | 0.11 | |
Non-vested as of June 30, 2021 | | | 13,734,600 | | | $ | 0.13 | |
The fair value of restricted share units is measured using the stock price on the date of grant and share-based compensation expense for the restricted stock units is recorded ratably over their vesting period.
Total share-based compensation expense as of June 30, 2020, and 2021 is as follows (in thousands):
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Research and development | | $ | 536 | | | $ | 646 | |
General and administrative | | | 1,464 | | | | 1,574 | |
Total | | $ | 2,000 | | | $ | 2,220 | |
As of June 30, 2021, total unrecognized compensation expense related to non-vested share options and restricted stock units, net of related forfeiture estimates, was $6.8 million. The Company expects to recognize its remaining unrecognized share-based compensation expense over a weighted-average period of approximately 2.2 years.
F-14
10. License Agreements
In 2006, the Company entered into a license agreement, as amended, with Cornell Research Foundation, Inc. (“Cornell”) and a research institute (collectively “licensor”) for certain intellectual property rights and, subsequently, entered into four additional license agreements with Cornell. Under the terms of the original license agreement, the Company issued an aggregate of 666,667 ordinary shares to Cornell between 2006 and 2009. The Company has also paid an upfront license fee of $60,000 and annual fees of approximately $60,000. The Company is also required to pay royalties on the commercial sale of products that result from the licensed intellectual property, as well as a percentage of any sublicensing revenue. Subject to specified reductions and royalty offset, such royalties are calculated as a tiered, low-to-mid single digit percentage of net sales of licensed products under each of the license agreements, except that for licensed products under the original agreement, such royalties are calculated as a tiered, low single-digit to sub-teen percentage of net sales, depending on patent coverage, amount of net sales and type of licensed product. Under this license agreement, the Company was required to commercialize a product by the date specified in the respective agreement, which with respect to the original Cornell agreement was December 31, 2020. The licensor may terminate the license, subject to specified exceptions for causes due to scientific, regulatory, and other events over which the Company cannot exert direct control. The Company believes that failure to commercialize is subject to the named exceptions, and to date has not received any notice of termination from the licensor. Any actual terminations of the license would be subject to cure periods and appeals before taking effect.
11. Income Taxes
During the year ended December 31, 2020 and six months ended June 30, 2021, the Company recorded a full valuation allowance on federal and state deferred tax assets since management does not forecast the Company to be in a profitable position in the near future.
12. Net Loss Per Share Attributable to Ordinary Shareholders
Basic and diluted net loss per ordinary share are calculated as follows (in thousands, other than share and per share data):
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Numerator: | | | | | | | | |
Net loss attributable to ordinary shareholders | | $ | (27,909 | ) | | $ | (26,095 | ) |
Denominator: | | | | | | | | |
Weighted average number of ordinary shares used in loss per share attributable to ordinary shareholders—basic and diluted | | | 506,055,526 | | | | 663,833,037 | |
Net loss per share attributable to ordinary shareholders—basic and diluted | | $ | (0.06 | ) | | $ | (0.04 | ) |
The following ordinary share equivalents, presented on an as converted basis, were excluded from the calculation of net loss per share for the periods presented, as their effect is anti-dilutive:
| | SIX MONTHS ENDED JUNE 30, | |
| | 2020 | | | 2021 | |
Ordinary share warrant | | | 500,000 | | | | 500,000 | |
Ordinary share warrants issued to MVIL - related party | | | - | | | | 83,365,384 | |
Outstanding ordinary share options | | | 45,704,574 | | | | 64,213,310 | |
Non-vested restricted stock units | | | 15,834,216 | | | | 13,734,600 | |
Total | | | 62,038,790 | | | | 161,813,294 | |
13. Related Party
Except as disclosed elsewhere in the notes to the accompanying consolidated financial statements, there were no other material transactions with related parties.
F-15
14. Subsequent Events
On July 14, 2021, the shareholders approved the increase to the Company’s authorized share capital from 1,200,000,000 ordinary shares of a nominal or par value of $0.0003 each to 1,600,000,000 ordinary shares of a nominal or par value of $0.0003 each.
Except as disclosed above and elsewhere in the notes to the accompanying consolidated financial statements, the Company has concluded that no further subsequent events have occurred that require disclosure.
F-16
American Depositary Shares

Representing Ordinary Shares
Preliminary Prospectus
H.C. Wainwright & Co.
, 2021
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers.
Every director and officer is indemnified and secured harmless out of the assets and funds of the Company against all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by such director or officer in or about the conduct of the Company’s affairs or in the execution of such director or officer’s duties, powers, authorities or discretions, including any costs, expenses, losses or liabilities incurred by such director or officer in defending (whether successfully or otherwise) any civil proceedings concerning the Company or its affairs in any court whether Cayman Islands or elsewhere.
Item 7. Recent Sales of Unregistered Securities.
Set forth below is information regarding ordinary shares, preferred shares, share options, and warrants to purchase shares issued by us within the past three years that were not registered under the Securities Act. Also included is the consideration received by us for such securities and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed. All of the securities described below are deemed restricted securities for purposes of the Securities Act.
Except as otherwise indicated herein or as the context otherwise requires, references below to “Stealth,” “the Company,” “we,” “us” and “our” refer to Stealth BioTherapeutics Corp and its consolidated subsidiaries, or any one or more of them as the context may require.
Since August 5, 2018, we have issued the following unregistered securities:
(a) Share option grants and option exercises
We granted options to purchase an aggregate of 7,165,139 ordinary shares, with exercise prices ranging from $1.20 to $2.22 per share, pursuant to our 2006 share incentive plan, as amended. We issued an aggregate of 71,777 ordinary shares upon the exercise of options for aggregate consideration of $68,903.
In October 2018, we reduced the exercise price per share of an aggregate of 385,833 options granted in June 2018 from $2.22 per share to $1.53 per share.
No underwriters were involved in the foregoing issuances of securities. The issuances of share options and the ordinary shares issued upon the exercise of the options described in this paragraph (a) of Item 7 were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption provided by Rule 701 promulgated under the Securities Act, or pursuant to Section 4(a)(2) under the Securities Act, relative to transactions by an issuer not involving any public offering, to the extent an exemption from such registration was required. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
(b) Convertible Promissory Note Issuances
Between August 2018 and January 2019, we issued convertible notes in an aggregate principal amount of $30.0 million. The notes were converted into ordinary shares upon the closing of our IPO.
No underwriters were involved in the issuance of these securities. The convertible securities described in this paragraph (b) of Item 7 were issued to investors in reliance upon the exemption from the registration requirements of the Securities Act provided under Regulation D promulgated under the Securities Act, or pursuant to Section 4(a)(2) under the Securities Act, relating to transactions by an issuer not involving any public offering. Each recipient of the security in the transaction described above represented that it was an accredited investor and was acquiring the security for its own account for investment purposes only and not with a view to the public resale or distribution thereof and that it could bear the risks of the investment and could hold the security for an indefinite period of time, and appropriate legends were affixed to the instrument representing the security issued in such transaction.
(c) Warrant Issuances
In October 2020, in connection with our entry into a development funding agreement, we issued a warrant to purchase 46,153,849 ordinary shares to Morningside Venture (I) Investments Limited at an exercise price of $0.13 per share.
In February 2021, we issued a warrant to Morningside Venture (I) Investments Limited, which is exercisable for 18,750,000 ordinary shares at an exercise price of $0.16 per share. The warrant expires in February 2024.
II-1
In May 2021, we issued a warrant to Morningside Venture (I) Investments Limited, which is exercisable for 18,461,538 ordinary shares at an exercise price of $0.13 per share. The warrant expires in May 2024.
No underwriters were involved in the issuance of these warrants. The issuance of the warrant described in this paragraph (c) of Item 7 were issued in reliance upon the exemption from the registration requirements of the Securities Act provided under Regulation D promulgated under the Securities Act, or pursuant to Section 4(a)(2) under the Securities Act, relating to transactions by an issuer not involving any public offering. The recipient of the security in the transaction described above represented that it was an accredited investor and was acquiring the security for its own account for investment purposes only and not with a view to the public resale or distribution thereof and that it could bear the risks of the investment and could hold the security for an indefinite period of time, and appropriate legends were affixed to the instrument representing the security issued in such transaction.
(d) Ordinary Share Issuance
In October 2019, in connection with our entry into an option agreement, we issued 16,304,347 ordinary shares for an aggregate amount of $15.0 million, pursuant to a Share Purchase Agreement.
In April 2020, we issued 152,858,460 ordinary shares to an investor for an aggregate purchase price of $20.0 million, pursuant to a share purchase agreement.
In June 2020, in connection with our entry into an equity agreement we issued a total of 2,203,812 shares to Lincoln Park Capital, LLC for no consideration. In July and August 2020, we issued a total of 4,680,000 ordinary shares to Lincoln Park under the equity agreement for an aggregate purchase price of $0.7 million. In Q2 2021, we issued a total of 20,700,000 ordinary shares to Lincoln Park under the equity agreement for an aggregate purchase price of $2.5 million
No underwriters were involved in the issuance of these ordinary shares. The issuance of the ordinary shares described in this paragraph (d) of Item 7 were issued to an investor in reliance upon the exemption from the registration requirements of the Securities Act provided under Regulation D promulgated under the Securities Act, or pursuant to Section 4(a)(2) under the Securities Act, relating to transactions by an issuer not involving any public offering. Each recipient of the securities in the transactions described above represented that it was an accredited investor and was acquiring the security for its own account for investment purposes only and not with a view to the public resale or distribution thereof and that it could bear the risks of the investment and could hold the security for an indefinite period of time.
Item 8. Exhibits and Financial Statement Schedules.
(a) Exhibits.
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
(b) Financial Statement Schedules.
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or notes.
Item 9. Undertakings.
(a) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer, or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(b) The undersigned hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
II-2
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
EXHIBIT INDEX
Exhibit Number | | Description of Exhibit |
| | |
3.1 | | Amended and Restated Memorandum and Articles of Association of the Registrant (incorporated by reference to Exhibit 1.1 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 1, 2020) |
| | |
4.1 | | Deposit Agreement among Registrant, Citibank, N.A., as depositary, and all Owners and Holders of ADSs issued thereunder (incorporated by reference to Exhibit 99.3 of our Report on Form 6-K (File No. 001-38810), filed on March 5, 2019) |
| | |
4.2 | | Form of American Depositary Receipt (included in Exhibit 4.1) |
| | |
4.3 | | Warrant Agreement, dated June 30, 2017, by and between the Company and Hercules Capital Inc., as amended and restated on June 7, 2018 (incorporated by reference to Exhibit 4.3 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
4.4 | | Ordinary Share Purchase Agreement dated as of October 10, 2019, by and between the Company and Alexion Pharmaceuticals, Inc. (incorporated by reference to Exhibit 99.2 of our Report on Form 6-K (File No. 001-38810), filed on October 10, 2019) |
| | |
4.5 | | Ordinary Share Purchase Warrant, dated October 30, 2020, between the Company and Morningside Venture (I) Investments Limited (filed as Exhibit 10.2 of our Report on Form 6-K (File No. 001-38810), filed November 4, 2020) |
| | |
5.1* | | Opinion of Walkers |
| | |
8.1* | | Tax Opinion of Wilmer Cutler Pickering Hale and Dorr LLP |
| | |
10.1 | | 2006 Share Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.2 | | Form of Incentive Option Agreement under 2006 Share Incentive Plan, as amended (incorporated by reference to Exhibit 10.2 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.3 | | Form of Nonstatutory Option Agreement under 2006 Share Incentive Plan, as amended (incorporated by reference to Exhibit 10.3 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.4 | | 2019 Share Incentive Plan, as amended (incorporated by reference to Exhibit 4.5 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 1, 2020) |
| | |
10.5 | | Form of Share Option Agreement under 2019 Share Incentive Plan (incorporated by reference to Exhibit 10.5 to Amendment No. 1 to our Registration Statement on Form F-1 (File No. 333-229097), filed on January 30, 2019) |
| | |
10.6 | | Form of Restricted Share Agreement under 2019 Share Incentive Plan (incorporated by reference to Exhibit 10.6 to Amendment No. 1 to our Registration Statement on Form F-1 (File No. 333-229097), filed on January 30, 2019) |
| | |
10.7 | | Form of Director and Officer Indemnification Agreement by and between the Registrant and each of its officers and directors (incorporated by reference to Exhibit 10.7 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.8† | | Exclusive License Agreement, dated April 20, 2006, among the Company, Cornell Research Foundation, Inc. and Institut de recherches cliniques de Montréal, as amended by First Amendment to Exclusive License Agreement dated October 7, 2010 (incorporated by reference to Exhibit 10.8 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.9† | | Exclusive License Agreement, dated November 22, 2010, between the Company and Cornell University
(incorporated by reference to Exhibit 10.9 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.10† | | Exclusive License Agreement, dated November 3, 2011, by and between the Company and Cornell University (incorporated by reference to Exhibit 10.10 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.11† | | Exclusive License Agreement, dated December 27, 2012, by and between the Company and Cornell University (incorporated by reference to Exhibit 10.11 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.12† | | Exclusive License Agreement, dated August 12, 2013, by and between the Company and Cornell University (incorporated by reference to Exhibit 10.12 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
II-4
Exhibit Number | | Description of Exhibit |
| | |
| | |
10.13 | | Loan and Security Agreement, dated June 30, 2017, by and between the Company and Hercules Capital Inc., as amended on March 12, 2018, July 26, 2018 and October 10, 2018 (incorporated by reference to Exhibit 10.15 of our Registration Statement on Form F-1 (File No. 333-229097), filed on December 28, 2018) |
| | |
10.14 | | Fourth Amendment to Loan and Security Agreement dated as of March 29, 2019, by and between Hercules Capital Inc. and the Company (incorporated by reference to Exhibit 4.19 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 4, 2019) |
| | |
10.15 | | Fifth Amendment to Loan and Security Agreement dated as of July 28, 2020, by and between Hercules Capital Inc. and the Company (incorporated by reference to Exhibit 10.1 to our Report on Form 6-K (File No. 001-38810), filed on August 7, 2020) |
| | |
10.16 | | 2019 Employee Share Purchase Plan (incorporated by reference to Exhibit 10.16 to Amendment No. 1 to our Registration Statement on Form F-1 (File No. 333-229097), filed on January 30, 2019) |
| | |
10.17 | | 2020 ADS Incentive Plan (incorporated by reference to Exhibit 4.20 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 1, 2020) |
| | |
10.18 | | Form of Restricted ADS Unit Award Agreement under 2020 ADS Incentive Plan (incorporated by reference to Exhibit 4.21 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 1, 2020) |
| | |
10.19 | | Purchase Agreement, between the Company and Lincoln Park Capital Fund, LLC, dated June 2, 2020 (incorporated by reference to Exhibit 1.1 of our Report on Form 6-K (File No. 001-38810), filed June 3, 2020) |
| | |
10.20 | | Registration Rights Agreement, dated June 2, 2020, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 1.2 our Report on Form 6-K (File No. 001-38810), filed on June 6, 2020) |
| | |
10.21 | | At the Market Offering Agreement, dated August 6, 2020, between the Company and H.C. Wainwright & Co., LLC (incorporated by reference to Exhibit 1.1 of our Report on Form 6-K (File No. 001-38810), filed August 7, 2020) |
| | |
10.22 | | Development Funding Agreement, dated October 30, 2020, between the Company and Morningside Venture (I) Investments Limited (incorporated by reference to Exhibit 10.1 of our Report on Form 6-K (File No. 001-38810), filed November 4, 2020) |
| | |
10.23 | | Sixth Amendment to Loan and Security Agreement dated as of April 29, 2021, by and between Hercules Capital Inc and the Company (incorporated by reference to Exhibit 10.1 of our Report on Form 6-K (File No. 001-38810), filed April 30, 2021) |
| | |
10.24 | | Amendment to Development Funding Agreement dated as of May 17, 2021, by and between the Company and Morningside Venture (I) Investments Limited (incorporated by reference to Exhibit 10.1 of our Report on Form 6-K (File No. 001-38810), filed May 18, 2021) |
| | |
10.25 | | Form of Warrant issued pursuant to Development Funding Agreement (incorporated by reference to Exhibit 10.1 of our Report on Form 6-K (File No. 001-38810), filed August 5, 2021) |
| | |
21.1 | | Subsidiaries of the Registrant (incorporated by reference to Exhibit 8.1 to our Annual Report on Form 20-F (File No. 001-38810), filed on April 1, 2020) |
| | |
23.1 | | Consent of Deloitte & Touche, LLP, independent registered public accounting firm |
| | |
23.2* | | Consent of Walkers (included in Exhibit 5.1) |
| | |
23.3* | | Consent of Wilmer Cutler Pickering Hale and Dorr LLP (included in Exhibit 8.1) |
| | |
24.1* | | Power of Attorney (included on signature page) |
| | |
* | To be filed by amendment. |
† | Confidential treatment granted as to certain portions, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the Town of Needham, Massachusetts on this 5th day of August 2021.
STEALTH BIOTHERAPEUTICS CORP |
| |
By: | /s/ Irene P. McCarthy |
| Irene P. McCarthy |
| Chief Executive Officer |
We, the undersigned officers and directors of Stealth BioTherapeutics Corp, hereby severally constitute and appoint Irene P. McCarthy and Henry Hess, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for him or her and in his or her name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Irene P. McCarthy | | | | |
Irene P. McCarthy | | Chief Executive Officer and Director (principal executive officer, principal financial officer and principal accounting officer) | | August 5, 2021 |
| | | | |
/s/ Francis W. Chen Francis W. Chen, Ph.D. | | Director | | August 5, 2021 |
| | | | |
/s/ Gerald L. Chan Gerald L. Chan, Sc.D. | | Director | | August 5, 2021 |
| | | | |
/s/ Kevin F. McLaughlin Kevin F. McLaughlin | | Director | | August 5, 2021 |
| | | | |
/s/ Louis Lange Louis Lange, M.D., Ph.D. | | Director | | August 5, 2021 |
| | | | |
/s/ Edward P. Owens Edward P. Owens | | Director | | August 5, 2021 |
| | | | |
/s/ Eve Slater Eve Slater, M.D. | | Director | | August 5, 2021 |
Stealth BioTherapeutics, Inc. Authorized Representative in the United States |
| | |
| By: | /s/ Irene P. McCarthy |
| Name: | Irene P. McCarthy |
| Title: | Chief Executive Officer |
II-6
II-7





















