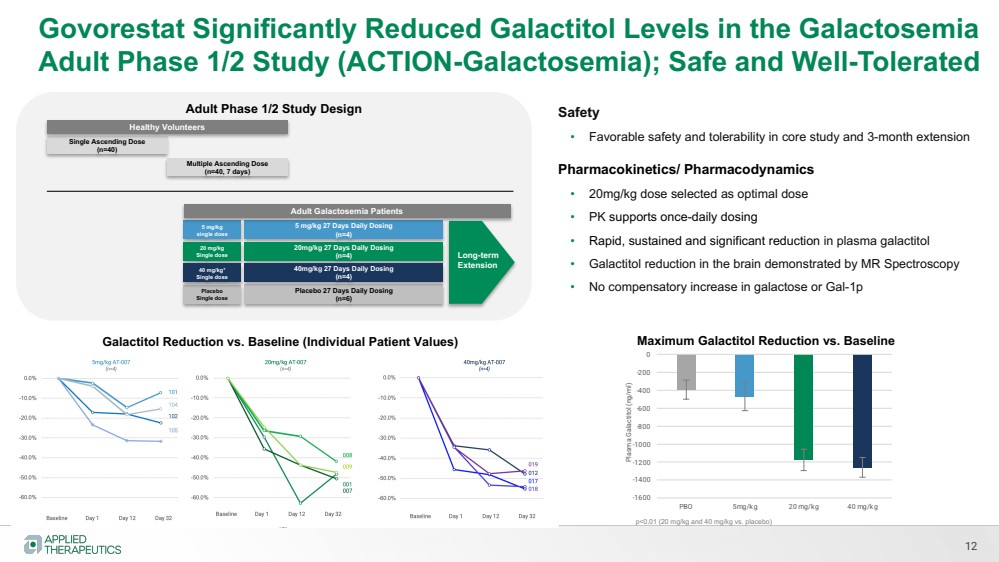
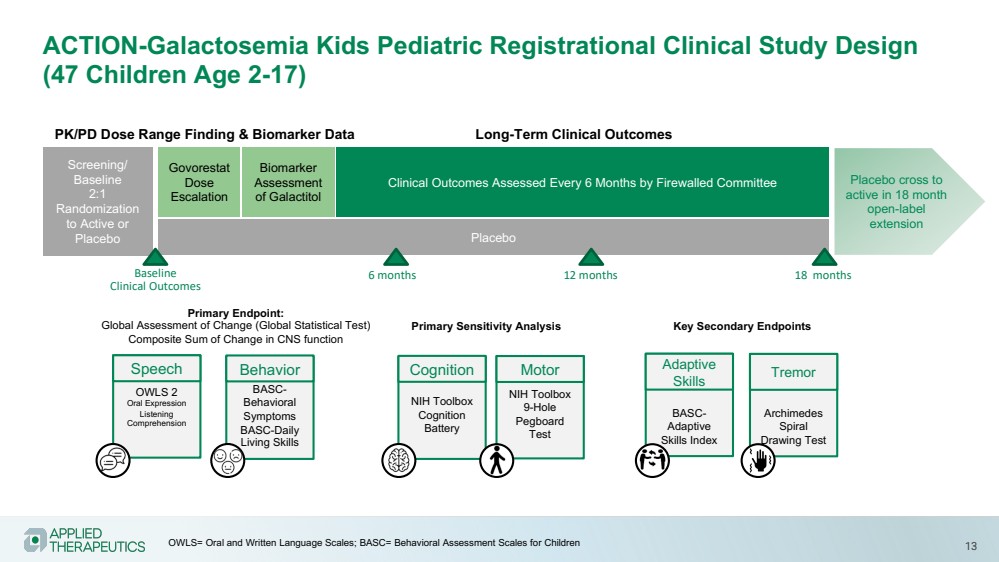
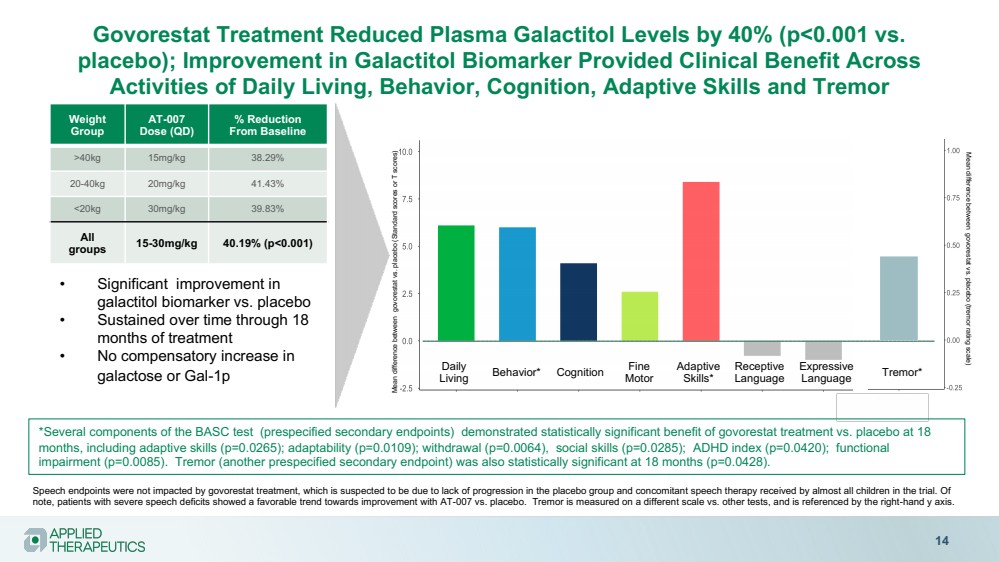
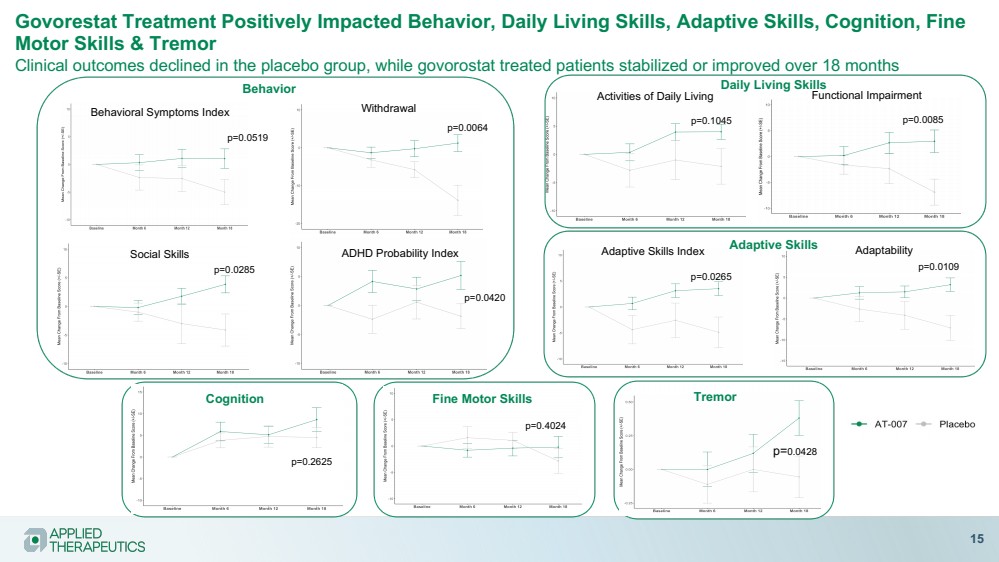
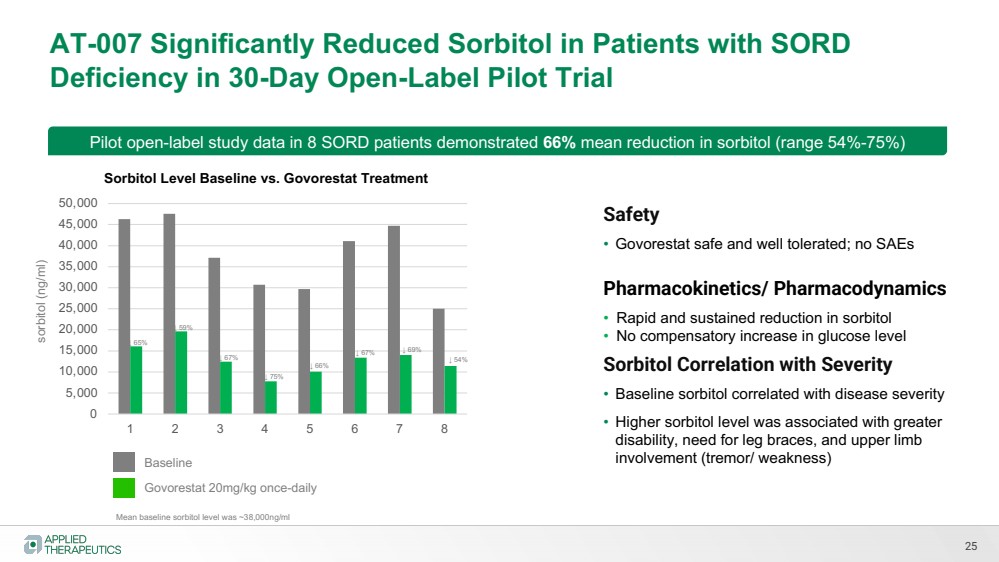
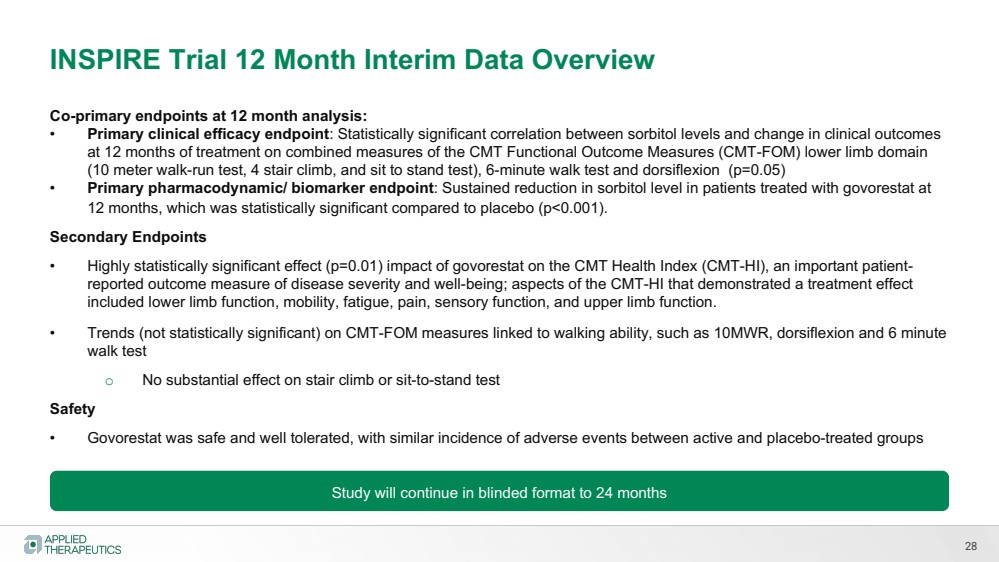
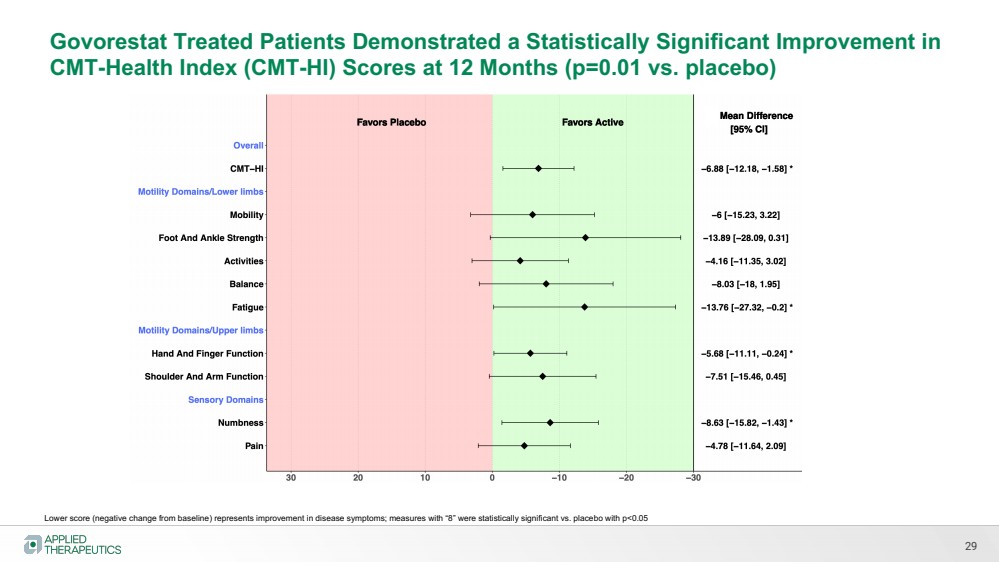
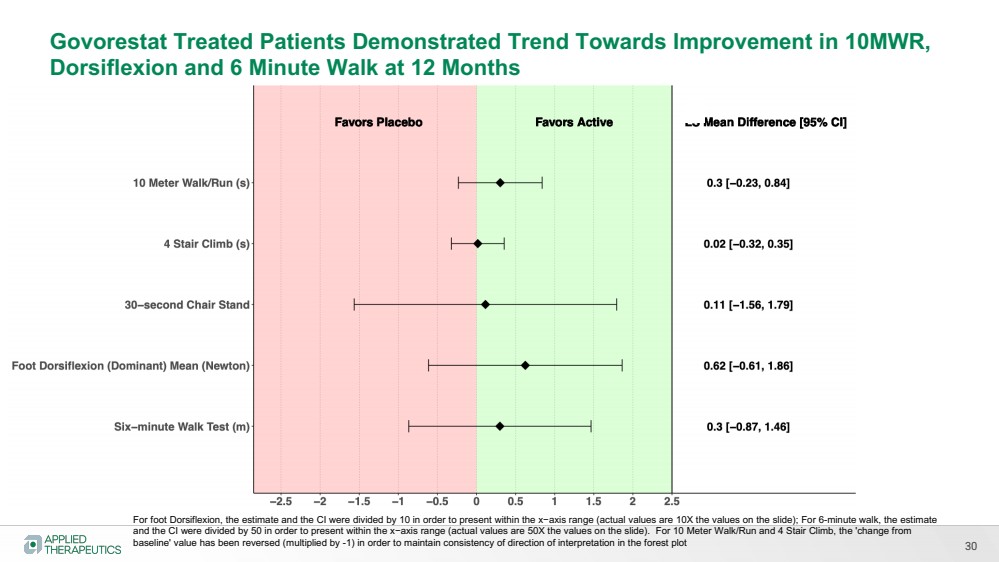
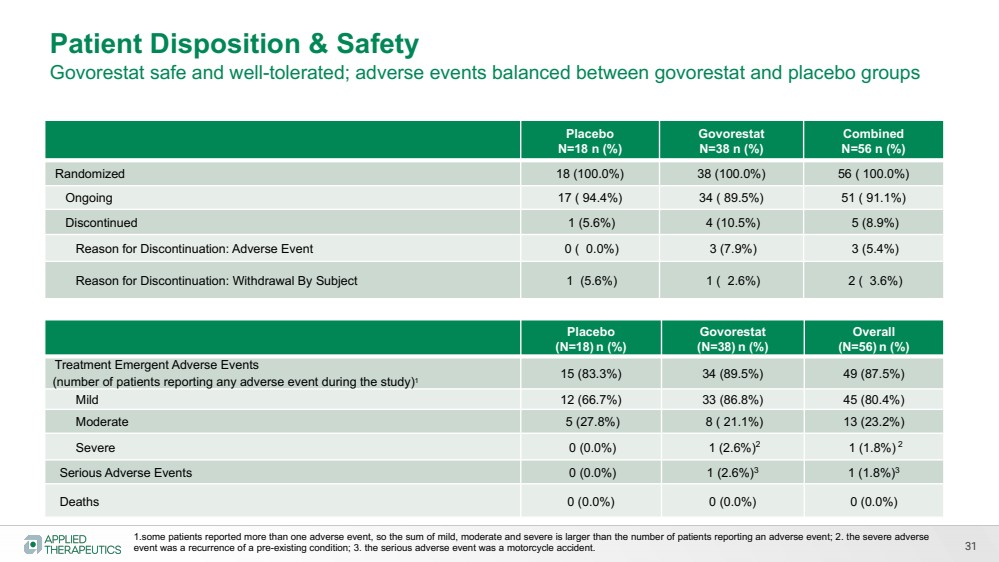
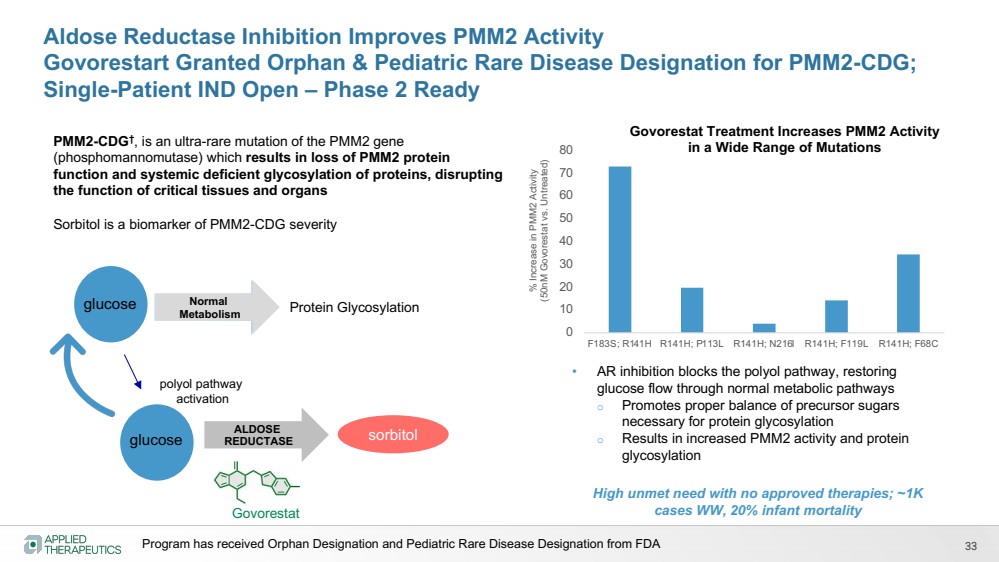
| Various statements in this presentation concerning the Company’s future expectations, plans and prospects constitute forward-looking statements. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” the negative of these and other similar expressions are intended to identify such forward looking statements. Such statements, based as they are on the current analysis and expectations of management, inherently involve numerous risks and uncertainties, known and unknown, many of which are beyond the Company’s control. Such risks include, but are not limited to: the impact of general economic conditions, general conditions in the biopharmaceutical industries, changes in the global and regional regulatory environments in the jurisdictions in which the Company does or plans to do business, market volatility, fluctuations in costs and changes to the competitive environment, the Company’s ability to fund its working capital requirements and expectations regarding the sufficiency of our capital resources and the Company’s ability to achieve the anticipated benefits from the agreements entered into in connection with our partnership with Advanz Pharma. Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements. In the case of forward-looking statements regarding investigational product candidates and continuing further development efforts, specific risks which could cause actual results to differ materially from the Company’s current analysis and expectations include: failure to demonstrate the safety, tolerability and efficacy of our product candidates; final and quality controlled verification of data and the related analyses; the expense and uncertainty of obtaining regulatory approval, including from the U.S. Food and Drug Administration and European Medicines Agency; the possibility of having to conduct additional clinical trials and our reliance on third parties such as our licensors and collaboration partners regarding our suite of technologies and product candidates; the Company’s ability to take advantage of expedited regulatory pathways for any of our product candidates; the Company’s intellectual property position and the duration of its patent rights; developments or disputes concerning the Company’s intellectual property or other proprietary rights. Further, even if regulatory approval is obtained, biopharmaceutical products are generally subject to stringent on-going governmental regulation, challenges in gaining market acceptance and competition. These risks and uncertainties are described more fully under the caption ”Risk Factors” in the Company’s filings with the Securities and Exchange Commission. Other risks and uncertainties of which the Company is not currently aware may also affect Company’s forward-looking statements. The reader should not place undue reliance on any forward-looking statements included in this presentation. These statements speak only as of the date made and the Company is under no obligation and disavows any obligation to update or revise such statements as a result of any event, circumstances or otherwise, unless required by applicable legislation or regulation. Forward Looking Statements |