Exhibit 99.4
CONFIDENTIAL TREATMENT REQUESTED UNDER FREEDOM OF INFORMATION ACT
PORTIONS OF THE EXHIBIT MARKED BY HAVE BEEN OMITTED PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION
Valuation
Report
Xynomic Pharmaceuticals
Commissioned by Bison Capital Acquisition Corp.
Bison Capital Acquisition Corp. 609-610 21st Century Tower No. 40 Liangmaqiao Road Chaoyang District, Beijing 100016, China | Phone: +86(10) 8444-6968 Email: jim.li@bisonholding.com |
© Copyright 2018 Venture Valuation. All Rights Reserved
EXECUTIVE SUMMARY |
INTRODUCTION |
| | Venture Valuation was retained byBison Capital Acquisition Corp. to establish a fair value ofXynomic Pharmaceuticals, Incand its assets. For the purpose of this valuation, we have calculated the value ofXynomic’s lead product, namelyXP-101 (Abexinostat, an HDAC inhibitor) for use in cancers on the rNPV (risk adjusted Net Present Value) calculation, and have considered the value of follow on products and indications, includingXP-102, in the DCF (discounted cash flow) calculation. A further productXP-105 is also discussed in the appendix. |
COMPANY DESCRIPTION |
| | Xynomic Pharmaceuticals is a drug development company headquartered in Shanghai, China. The company is focused on development of therapies targeting cancers. The company’s most advanced product, XP-101 (Abexinostat), is in Phase II/III clinical trials for follicular lymphoma and renal cell carcinoma. Xynomic was established in 2017, is privately funded and currently employs 11.5 full time equivalents (FTEs) and is projecting to expand to 60 employees by the end of 2018. The company aims to generate revenues by development and marketing of the products in its pipeline including the most advanced drug, XP-101 (Abexinostat), and its other products including XP-102 BI 882370, a RAF inhibitor) for treatment of cancers. At present, Xynomic and Bison are in discussion about a potential business combination. |
| PRODUCTS - ANTINEOPLASTIC THERAPUTICS |
| |
| | Xynomic has a pipeline of development products, aimed for treatment of cancers. The two key assets are described, in summary, below: HDAC inhibitor: XP-101 (Abexinostat, Abex) ● Abexinostat (XP-101) is an orally bioavailable broad spectrum HDAC inhibitor, which inhibits Class I HDACs including HDAC1,2,3,6,10 and 11. Xynomic in-licensed this drug from Pharmacyclics LLC, currently a wholly owned subsidiary of AbbVie Inc., in Q1 2017, catalyzing the establishment of Xynomic. To date, a total of 18 Phase I and II clinical trials of Abexinostat have been completed, demonstrating the efficacy and safety profile of this compound in multiple types of cancers. Xynomic is now in the process of completing a number of Phase I-III clinical trials using XP-101, as listed below. RAF inhibitor: XP-102 (BI 882370) ● XP-102 (BI 882370) is a B-RAF inhibitor, that inhibits B-RAF in an ‘inactive’ mode (DFG-out confirmation) and has potential use in the treatment of cancers associated with B-RAF mutations, including malignant melanoma and colorectal cancer. Xynomic acquired exclusive global rights to XP-102 (BI 882370) from Boehringer Ingelheim in Q3 2017. To date XP-102 has demonstrated efficacy and safety in animal models of colorectal cancer (CRC) and melanoma. Xynomic aim to proceed toward Phase I clinical studies examining the utility of XP-102 in melanoma and CRC. |
© Copyright 2018 Venture Valuation. All Rights Reserved
2
| | A summary of the product development and pipeline is provided in the table below. |
| | Product Development and Pipeline |
| | Indication | | Develop. Stage | | Start Date | | Sites | | Co-Therapy | | Partner |
| | | | | | | | | | | | |
| | HDAC inhibitor: XP-101 (Abexinostat, Abex) | | | | |
| | Renal cell carcinoma | | Ph III# | | Q3 2018 | | Global | | Pazopanib | | Novartis, Parexel |
| | Follicular Lymphoma | | Ph II# | | Q3 2018 | | Global | | Monotherapy | | PPD |
| | Follicular Lymphoma | | Ph II# | | Q1 2019 | | China | | Monotherapy | | Quintiles |
| | DLBCL/MCL | | Ph I/II | | Q3 2018 | | US | | Imbruvica | | JNJ, Kettering |
| | DLBCL | | Ph II# | | Q1 2019 | | China | | Monotherapy | | Quintiles |
| | Multiple Solid Tumors | | Ph Ib/II | | Q3 2018 | | US | | Keytruda | | Merck, UCSF |
| | Breast cancer | | Ph Ib/II | | Q4 2018 | | US | | Kisqali | | Novartis, UCSF |
| | RAF inhibitor: XP-102 (BI 882370) | | | | | | |
| | Colorectal cancer | | Ph Ia | | Q1 2019 | | US | | Monotherapy | | n/a |
| | Melanoma | | Ph Ia | | Q1 2019 | | US | | Monotherapy | | n/a |
| | # | Pivotal study; Diffuse Large-B-Cell lymphoma (DLBCL); Mantle cell lymphoma (MCL) |
INDICATION - ONCOLOGY
| Disease Overview | Xynomicis focused on development of novel therapies for the treatment of a variety ofcancers. The company is running a number of clinical trials examining the efficacy of its lead drug XP-101 (Abexinostat) in renal cell carcinoma, follicular lymphoma, diffuse large-B-cell lymphoma and mantle cell lymphoma, multiple solid tumors and breast cancer, as well as its drug XP-102 (BI 882370) in colorectal cancer and melanoma. |
| | |
| Epidemiology | The epidemiology for differing cancers, in particular the prevalence, and the estimated number of patients is listed in the table below. |
| | |
| Target Patient Population | The target patient population in the US, EU and China is described below, with an upside arising from distribution in other geographies and worldwide. |
| | Disease Prevalence |
| | | US | EU | China |
| | Renal Cell Carcinoma (RCC) | 0.03% | 0.04% | n.a. |
| | Follicular Lymphoma (FL) | 0.004% | 0.002% | 0.0004% |
| | Diffuse Large-B-Cell lymphoma (DLBCL | 0.008% | 0.005% | 0.001% |
| | Mantle cell lymphoma (MCL) | 0.002% | 0.001% | n.a. |
| | Non–small cell Lung cancer (NSCLC) | 0.067% | 0.058% | 0.032% |
| | ER+ Breast cancer | 0.379% | 0.338% | 0.066% |
| COMPETITION |
| |
| Current Practice | The current therapies for the cancer indications, for which XP-101 and XP-102 are being developed, include surgery, monotherapies, and combination treatments. These approaches tend to represent first, second, and third line treatment methods, with palliative care measures instigated with terminal illness diagnosis. |
© Copyright 2018 Venture Valuation. All Rights Reserved
3
| Advantages over Current Practice | There are a number of advantages XP-101 has over current products. These primarily come from XP-101 being an oral HDAC inhibitor which has benefits over the injectable competitors. XP-101 assists in prolonging drug resistance of other cancer therapies, and works by modifying DNA. As such the levels of XP-101 in the body can be maintained at a constant low level, potentially offering better safety profile. The oral availability, meeting of unmet clinical needs and safety properties of XP-101 represent clear advantages. XP-102 is a 2nd generation pan-RAF kinase inhibitor that can block multiple RAF kinases and signaling pathways, which may provide benefits over 1st generation RAF kinase inhibitors that develop drug resistance. XP-102 has a unique binding mode, insofar as it binds the RAF kinase in a DFG-out ‘inactive’ confirmation providing high selectivity. For CRC and melanoma this property might help in providing synergistic efficacy with currently used MEK inhibitors. In toxicity studies, XP-102 appears to have a large safety/therapeutic window, which may be better than current 1stgeneration RAF kinase inhibitors. |
| Competing Players | There are a number of direct as well as mechanistic-based competitors. With regard to direct competitors, there are first, second and third line therapies for many of the cancer indications for which XP-101 and XP-102 are being developed. Please see main body of this report. Mechanistically, a number of pan-HDAC and selective HDAC inhibitors are marketed or in development that may compete with XP-101. Similarly, RAF inhibitor marketed drugs or those in development could represent competition to XP-102 (BI 882370) (please see below). |
| Competitor Products |
| Drug | | Company | | Approved |
| HDAC inhibitor: XP-101 (Abexinostat, Abex) | | | | |
| Vorinostat (Zolinza) | | Merck | | 2006 |
| Romidepsin (Romidespin) | | Celgene | | 2011 |
| Belinostat (Beleodaq) | | Spectrum | | 2014 |
| Chidamide (Epidaza) | | Chipscreen | | 2014 |
| Panobinostat (Farydak) | | Novartis | | 2015 |
| RAF inhibitor: XP-102 (BI 882370) |
| Vemurafenib (Zelboraf, PLX4032) | | Genentech (Roche) | | 2011 |
| Dabrafenib (Tafinlar) | | Novartis | | 2013 |
| Encorafenib (LGX818) | | Array Biopharma | | Phase III |
| MLN-2480 | | Millennium/Takeda | | Phase I |
| LY3009120 | | Eli Lilly | | Phase I |
| Strengths: | Below is a brief summary of the strengths as we see them: ● Strong evidence of safety and efficacy from clinical studies ● Significant market size and unmet medical need ● Opportunities for indication and territory extension ● First mover advantage ● Knowledge to penetrate China market ● Positive demographics, including aging population, rising disposable incomes, and healthcare awareness ● Significant expertise of company management as an asset to the company ● Well defined portfolio strategy and pipeline for sustained product development |
© Copyright 2018 Venture Valuation. All Rights Reserved
4
| Weaknesses: | Below is a brief summary of the weaknesses as we see them: ● Need for financial investment for clinical studies and running costs (approximately USD 50-60m). ● Uncertainties surrounding M&A and/or IPO ● High dependence on success of Abexinostat ● Lack of sufficient support staff to assist Senior Management ● Challenges of staff as company expands ● Need for the development of sales force teams in targeted territories ● Uncertainties surrounding future partnership plan with co-developers and distributors |
| | |
| RISK PROFILE |
| | Included in the probability of success: |
| | ● Product Development Risk | Medium |
| | ● Regulatory/Approval Risk | Medium |
| | ● Manufacturing Risk | Low |
| | Included in the discount rate: |
| | ● Partnering Risk | Low-Medium |
| | ● Financing Risk | Medium |
| | ● Management Risk | Low-Medium |
| | ● IP Risk | Medium |
| | ● Market Uptake Risk | Medium |
| | ● Competitive Risk | Medium-High |
| | ● Pricing Risk | Medium-High |
| VALUATION |
| |
| Methodology | To provide a balanced assessment of the value of Xynomic the following valuation methods were used: The risk-adjusted net present value (rNPV) method, the discounted cash flow (DCF) method, the market comparable method and the comparable transactions method. |
| Key Assumptions | The figures used for the calculation of the value range are based on information provided by the management of Xynomic, adjusted by Venture Valuation. Key value inflection points in the product development timeline include: ● Market entry of Abex targeting FL and targeting DLBCL in China in 2021; ● Launch of Abex targeting RCC in the US and Europe in 2022; ● Launch of Abex in the US and Europe targeting FL in 2022; ● Launch of Abex in the US and Europe targeting DLBCL and targeting MCL in 2025. Market penetration rates are based on the Tufts Center for Drug Development uptake curve, adjusted by Venture Valuation. For a full list of assumptions used please see Appendices. |
| Value Range | Our calculations, based on the XP-101 and XP-102 assets, result is a pre-money equity value range of USD 282m to USD 441m, with an average value of USD 349m. Additional value comes from the XP-105 product, which is discussed in the appendix. |
| | | | value spectrum | |
| | in USD millions | | weighting | | | low | | | > | | | Average | | | > | | | high | |
| | Sum of Parts Valuation | | | 33 | % | | | 241.4 | | | | 290.6 | | | | 351.5 | | | | 427.3 | | | | 522.5 | |
| | Market Comparables | | | 33 | % | | | 279.4 | | | | 300.3 | | | | 321.3 | | | | 345.4 | | | | 369.5 | |
| | Comp. Transactions | | | 33 | % | | | 325.0 | | | | 349.3 | | | | 373.7 | | | | 401.7 | | | | 429.8 | |
| | Average | | | | | | | 281.9 | | | | 313.4 | | | | 348.8 | | | | 391.5 | | | | 440.6 | |
© Copyright 2018 Venture Valuation. All Rights Reserved
5
| CONTENT | | |
| | EXECUTIVE SUMMARY | 2 |
| | INTRODUCTION | 7 |
| | COMPANY DESCRIPTION | 7 |
| | HISTORICAL PERSPECTIVE | 7 |
| | REASON FOR THE VALUATION | 7 |
| | STRATEGY AND BUSINESS MODEL | 8 |
| | PARTNERING APPROACH | 8 |
| | CAPITAL STRUCTURE AND FINANCING STAGE | 11 |
| | MANAGEMENT / ORGANISATION | 12 |
| | ORGANISATION | 12 |
| | EXPERIENCE | 14 |
| | MOTIVATION | 16 |
| | PRODUCTS – ANTINEOPLASTIC THERAPEUTICS: ABEXINOSTAT, XP-102 | 16 |
| | PRODUCT DEVELOPMENT | 17 |
| | PRODUCT PRICING | 19 |
| | REGULATORY AFFAIRS | 21 |
| | MANUFACTURING | 22 |
| | INTELLECTUAL PROPERTY (IP) | 22 |
| | MARKET OVERVIEW | 24 |
| | COMPETITIVE LANDSCAPE | 27 |
| | ASSESSMENT | 29 |
| | STRENGTHS | 29 |
| | WEAKNESSES | 29 |
| | RISK ANALYSIS | 30 |
| | RISK ASSESSMENT - SUMMARY | 30 |
| | VALUATION | 31 |
| | OVERVIEW | 31 |
| | DISCOUNT RATE | 32 |
| | rNPV/RISK-ADJUSTED NPV | 33 |
| | DISCOUNTED CASH FLOW | 35 |
| | SUM OF PARTS VALUATION | 36 |
| | MARKET COMPARABLES | 37 |
| | COMPARABLE TRANSACTIONS | 38 |
| | SUMMARY | 39 |
| | REFERENCES | 41 |
| | APPENDIX I: ABEXINOSTAT FOR RCC VALUATION ASSUMPTIONS | 43 |
| | APPENDIX II: ABEXINOSTAT FOR FL VALUATION ASSUMPTIONS | 44 |
| | APPENDIX III: ABEX FOR DLBCL/MCL VALUATION ASSUMPTIONS | 45 |
| | APPENDIX IV: COMPARABLE TRANSACTIONS - COMPANIES | 46 |
| | APPENDIX V: MARKET COMPARABLES - COMPANIES | 46 |
| | APPENDIX VI: DISCOUNTED CASH FLOW FORECAST | 47 |
| | APPENDIX VII: VALUATION OF XP-105 | 47 |
| | ABOUT VENTURE VALUATION | 58 |
© Copyright 2018 Venture Valuation. All Rights Reserved
6
| INTRODUCTION |
| |
| Professional background | This report was prepared by Venture Valuation VV AG (Venture Valuation), a company that specializes in the independent assessment and valuation of life sciences (biotech, pharma, medtech) companies and products. Additional information on Venture Valuation is available at the back of this document. |
| | |
| Assignment | Venture Valuation was asked byBison Capital Acquisition Corp. (Bison) to consult on the valuation ofXynomic Pharmaceuticals Inc. (Xynomic). For this valuation, we have considered the value of the lead product, abexinostat, for the treatment of a range of cancers, in the rNPV (risk adjusted Net Present Value) calculation. We have considered the value of the Xynomic pipeline of follow-on products and indications in the DCF (Discounted Cash Flow) calculation. We note that the present is a valuation of Xynomic current assets, and, as such, it represents a snapshot of the company’s value today. |
| Assumptions | All of the assumptions that were made are set out in the body of this report. If any of these assumptions change, the valuation represented herein may change accordingly. |
| Information | In certain instances, we have relied on information provided byBison andXynomicand have not been able to verify or audit the information received. Wherever possible, we have tried to base the opinion set out in the report on our own research and independent sources. |
| Statement of independence | This report is based on the assessment by Venture Valuation. Our fee in this case is not dependent on the outcome of the value. |
| COMPANY DESCRIPTION |
| |
HISTORICAL PERSPECTIVE |
| |
| | Xynomic Pharmaceuticals is a drug development company headquartered in Shanghai, China. The company is focused on development of therapies targeting cancers. The company’s most advanced product, XP-101 (Abexinostat), is in Phase II/III clinical trials for follicular lymphoma and renal cell carcinoma. Xynomic was established in 2017, by the three co-founders Y. Mark Xu, W. Jason Wu and Yong Cui. Xynomic is privately funded and currently employs 11.5 full time equivalents (FTEs) and is projecting to expand to 60 employees by the end of 2018. The company aims to generate revenues by development and marketing of the products in its pipeline including the most advanced drug, XP-101 (Abexinostat), and its other products including XP-102 BI 882370, a RAF inhibitor) for treatment of cancers. At present, Xynomic and Bison are in discussion about a potential business combination. |
| REASON FOR THE VALUATION |
| |
| | The valuation of Xynomic was conducted to obtain a fair company value, which can be used as a basis for negotiations with potential investors and industry partners. As an independent party specializing in valuations of high growth companies, Venture Valuation’s aim is to consult Bison on the assessment of Xynomic, based on figures and information presented by Xynomic’s management. The calculated figures should always be interpreted as the equity value of the company. The liabilities, if any, have already been considered; therefore, no deduction is necessary. The resulting company worth represents the pre-money value. |
© Copyright 2018 Venture Valuation. All Rights Reserved
7
| STRATEGY AND BUSINESS MODEL |
| |
| | Xynomic is focused on developing and commercializing its oncology products. The company strategy is to build a pipeline through the in-licencing of clinical stage oncology products. In addition, it aims to develop a small internal pre-clinical R&D activity. The company aims to generate revenues by the sale of these drugs. At present, Xynomic seeks a partner who will provide financial support for the R&D costs and clinical development of their products. In addition, the company are considering either an M&A and/or an IPO. |
PARTNERING APPROACH |
| Research and Development (R&D) Partners | A summary of the key early R&D partners are indicated below: |
| | R&D Partners/Development |
| | Activity | Partner & Comments |
| | Abexinostat (XP-101) Research Collaborators | ● Prof. Pamela Master (UCSF):Xynomic has a research collaboration with Prof. Pamela Master (University of California, San Francisco, UCSF) whose group is focused on investigating the molecular pathways of HDAC inhibitors. The group works to identify cancer indications in which HDAC inhibitors have the ability to synergize effects of existing marketed cancer therapies. Prof. Master’s group has also identified biomarkers that may stratify patients with renal cell carcinoma (RCC) into those who are prone to developing resistance against VEGF inhibitor therapies. Xynomic hope this collaboration will allow identification of additional cancers for which XP-101 may have utility. The company foresees the biomarker studies will lead to a diagnostic that may identifying patients prone to VEGF inhibitor resistance and thus suitable for early-line XP-101 therapy. We note, UCSF and Prof Master will not receive any revenues for XP-101 for this collaboration. |
| | XP-102 Research Collaborators | ● Evol Science and Shanghai Kechow Pharma Inc:Xynomic are exploring an opportunity with research companies to explore the synergistic effects of XP-102 (Evol Science) and MEK inhibitors (Shanghai Kechow Pharma) in cellular and animal models of cancer. These studies aim to identify additional cancer indications for which XP-102 can be utilised. |
© Copyright 2018 Venture Valuation. All Rights Reserved
8
| | Own Research Capacity | ● Central South University (CSU)/School of Pharmaceutical Sciences:Xynomic have been collaborating with CSU in China to support its R&D activities. The company has a service agreement and pays a fixed monthly fee for the student and professor services as well as overheads. Xynomic estimates a cost of approximately USD 50,000 per year. Xynomic is also in the process of establishing its own research facility in the outskirts of Shanghai. At present a university professor has been appointed as head of the centre. The company aims to establish 3 functions, namely biology, chemistry and analytics. Xynomic aim to appoint 10 staff, raising to a maximum of 30 staff over 3 years. We note a recent press release, with mention of the first two molecules being focused on at this center as XP-103, a TRK/Fra-1 inhibitor, and XP-104, a RET inhibitor. |
| Clinical Partners | Xynomic are running a number of clinical studies with XP-101, in collaboration with pharma partners. A list of these clinical partners is summarised below. |
| | Clinical Development Partners |
| | Activity | Partner(s) & Comments |
| | Renal cell carcinoma (RCC) (Ph III Pivotal Study; US, EU, Global). | ● Novartis and Pazopanib (Votrient): XP-101 will be tested in combination with Pazopanib. This Novartis drug is a receptor tyrosine kinase inhibitor approved in 2009 for advanced renal cell carcinoma. Novartis and Xynomics will share the costs of the clinical study. Xynomics may share marketing costs and activities, keeping revenue from their own products. Alternatively Xynomics may give marketing rights to Novartis for Renal cell carcinoma for e.g. 20% share of revenues. ● Parexel, MA, USA: Parexel (www.parexel.com) is a leading global company with strong presence in the US and China. Xynomic aim to use the multi-centre global trial for US (FDA) and China (CDA) registrations. The strong presence of Parexel in China will be helpful in this regard. |
| | Follicular Lymphoma (FL) (Ph II Pivotal Study; US, EU, Global Study) | ● PPD, NC, USA: Xynomic engaged Pharmaceutical Product Development, LLC (PPD) (www.ppdi.com) to assist in the clinical study as well and in US (FDA) discussions. Xynomic expect to market XP-101 as 4th line treatment for FL in the US/EU, where PPD could assist in the education of KOL’s in this regard. ● We note that PPD have also assisted in the technology transfer of XP-102 during in-licencing from Boehringer Ingelheim. PPD have provided a gap analysis (i.e. toxicology, CMC, pharmacology) of IND requirements for XP-102 and will assist in the IND filing in the US. |
| | DLBCL/MCL (Ph I/II; US) | ● JNJ (Janssen) and Imbruvica (Ibrutinib): XP-101 will be tested in combination with Imbruvica. This JNJ drug is an inhibitor of Bruton’s tyrosine kinase (BTK) which plays a role in B cells and approved to treat B-cell cancers such as mantle cell lymphoma (MCL) in 2013, chronic lymphocytic leukaemia (CLL) in 2014, Waldenström’s macroglobulinemia in 2015 and graft vs host disease in 2017. Xynomic will pay for this trial with JNJ cost sharing and also providing the drug (Imbruvica). The marketing deal terms are likely to be similar to those described for Pazopanib. We note, AbbVie/PCYC own the US rights for Imbruvica while JNJ own ex-US rights; a discussion between AbbVie, JNJ and Xynomic will likely be required to further explore marketing terms of XP-102. |
© Copyright 2018 Venture Valuation. All Rights Reserved
9
| | | ● Memorial Sloan Kettering Cancer Centre: The study will be conducted at Memorial Sloan Kettering Cancer Centre (www.mskcc.org) |
| | | |
| | Follicular Lymphoma (FL) & DLBCL (Ph II Pivotal Studies; China) | ● Quintiles/IQVIA, USA: Xynomic will conduct two Phase II Pivotal Studies in China (FL and DLBCL) and have employed the CRO Quintiles (www.iqvia.com) for this purpose. IMS Health and Quintiles are now IQVIA and provide a number of clinical development services globally. ● Cancer Hospital Chinese Academy of Medical Sciences:This site is working with Xynomic to assist in the XP-101 clinical studies. |
| | Multiple Solid Tumors (Ph Ib/II; US) | ● Merck and Keytruda (Pembrolizumab): XP-101 will be tested in combination with Keytruda. This Merck drug is a humanized antibody that targets the programmed cell death 1 (PD-1) receptor of lymphocytes. The drug was approved in 2015 for treatment of metastatic non-small cell lung cancer and in 2017 for metastatic solid tumor. Xynomic are not in specific collaboration with Merck regarding this clinical study. Patients in this trial will be taking Keytruda as part of their treatment (covered by insurance). ● Prof. Pamela Master (UCSF):The trial will be conducted in collaboration with UCSF (lead by Prof. Pamela Munster). We note, UCSF will have no commercial rights. |
| | Breast Cancer (Phase Ib/II; US) | ● Novartis and Kisqali (Ribociclib): XP-101 will be tested in combination with Kisqali. This Novartis drug is a cyclin D1/CDK4 and CDK6 inhibitor approved in 2017 for breast cancers. CDK4 and 6 promote cell division in normal and cancer cells, with many cancer cells increased CDK activity, which inactivates tumor suppressor genes. As above, Novartis and Xynomic will share the costs of the clinical study. The marketing deal terms are likely to be similar to those described for pazopanib. ● Prof. Pamela Master (UCSF):The trial will be conducted in collaboration with UCSF (lead by Prof. Pamela Munster). We assume, UCSF will have no commercial rights. |
| Manufacturing Partners | The manufacturing partners for XP-101 were pre-established by the licensor Pharmacyclics. Xynomic have audited these partners and will continue to use them. Details are provided in the table below. For XP-102, the company will seek manufacturing partners in China, two possibilities are also listed below. |
| | Manufacturing Partners (CMOs) |
| | Partner | Comments |
| | XP-101 Manufacturers and Related Partners | ● Oril Industrie:Oril Industrie (wholly owned subsidiary of Servicer) (www. servier.com). Bolbec, France, provides Xynomic the API Drug. ● Rottendorf Pharma: Rottendorf Pharma (www.rottendorf.com). Ennigerloh, Germany assists Xynomic with drug product, placebo, formulation supplies. ● PCI Pharma Services: PCI Pharma Services (Formerly Penn Pharma) (www.pciservices.com). Tredegar, Gwent, Wales provides packaging, clinical supply support. |
© Copyright 2018 Venture Valuation. All Rights Reserved
10
| | XP-102 Manufacturers and Related Partners | ● Bioduro Ltd: This is a Chinese company based in San Diego, US. This is a specialised pharmaceutical provider that can provide API and formulation services. ● JOINN Laboratories: This company will provide GLP level XP-102 product for toxicology studies, under guidance of PPD. These IND-related studies are expected to be completed by Q4/2018 (please see below for development details). |
| Distributors & Marketing Partners | At present the distribution and marketing partners are not identified. The company have 2-4 years before product launch and thus have significant time to establish a distribution and marketing strategy. At present, Xynomic consider establishing a marketing team in China and US. The company may also consider co-marketing or providing marketing rights to a co-developing large pharma partner. For EU, Xynomic note this market is fragmented with country and region specific nuances, and will seek partners to assist with distribution and marketing. |
| Investors and Shareholders | The investors and shareholders of Xynomic are provided in the table below. Future investors may come from IPO or M&A activities. |
| Partnering Risk | Xynomic has a well-established set of collaborators and partnerships in place for development of its products, whom also include large pharma such as Novartis and JNJ. We consider this risk as LOW-MEDIUM. |
| CAPITAL STRUCTURE AND FINANCING STAGE |
| |
| Financing to date | Xynomic has received funds from Series A funding (USD 4.3m) and Series B funding (USD 17m), with the founder Y. Mark Xu providing an additional USD 1.9m. Xynomic have approximately USD 2.5m cash in the bank with the Series B funds of USD 17m to arrive end Q2 2018. |
| Shareholder Capital | Investors and Shareholders are indicated in the table below. |
| | Shareholders’ capitalization table |
| | Name | | Number of Shares* | | | % Share | | | Total Purchase Price (USD)* | |
| | Yinglin Mark XU | | | 23,870,293 | | | | 44.75 | % | | | 1,900,000 | |
| | Grand Ascent Group Limited | | | 6,610,500 | | | | 12.39 | % | | | 661 | |
| | Yong CUI | | | 6,611 | | | | 0.01 | % | | | 1 | |
| | Bridge Pharm International Inc. | | | 3,000,010 | | | | 5.62 | % | | | 300 | |
| | Infinite Fortune Limited | | | 13,079,459 | | | | 24.52 | % | | | 7,300,000 | |
| | Northern Light Venture Capital | | | 1,553,265 | | | | 2.91 | % | | | 5,000,000 | |
| | Bison Healthcare Venture | | | 1,553,265 | | | | 2.91 | % | | | 5,000,000 | |
| | Other Series B Investors | | | 1,739,656 | | | | 3.26 | % | | | 5,600,000 | |
| | ESOP 1 | | | 1,397,938 | | | | 2.62 | % | | | | |
| | ESOP 2 | | | 533,444 | | | | 1.00 | % | | | | |
| | TOTAL | | | 53,344,441 | | | | 100 | % | | | 24,800,962 | |
| | * Total of Series A and Series B is shown; figures rounded up. |
© Copyright 2018 Venture Valuation. All Rights Reserved
11
| Future Financing | Grant Agency Funding ● Xynomic indicate they are not in receipt of any governmental finance or grant funding. The company aims not to engage in this type of grant funding in order to keep its IP rights and company ownership within the private sector. Future Financing ● Xynomic seek funds for the clinical development of XP-101 and pipeline products XP-102 and others. In order to raise funds, Xynomic expect to conduct an IPO or M&A. The company indicates the lead asset XP-101 as being advanced enough to allow the company to aim for NASDAQ or Taiwan IPO. Alternatively, Xynomic may seek a partner for its acquisition by a large pharma. Estimates of costs for clinical development are provided in the sections below. Term Sheets ● We note that no term sheets currently exist between Xynomic and grant agencies, additional investors, potential out-licensors, or collaborative partners that would assist the company with its financing. |
| Financing Risk | Xynomic has raised approximately USD 25m equity to date in private investments. The company aims to raise funds for the clinical development of its lead and pipeline products, either by IPO or M&A. We are not aware of any future financial incomes secured or term sheets signed. We note however that XP-101 is an attractive asset and we deem this risk as MEDIUM. |
| MANAGEMENT / ORGANISATION |
| ORGANISATION |
| |
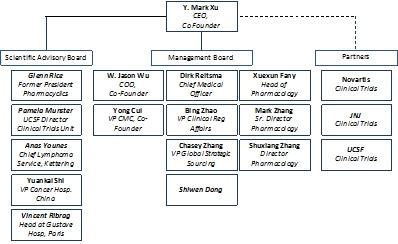
| Company Structure & Organogram | Xynomic currently employs 11.5 full time equivalents (FTEs) and is projecting to expand to 60 employees by the end of 2018. For illustrative purposes, an organogram of the company management, relevant boards, employees and partners is shown below: |
| | 
|
© Copyright 2018 Venture Valuation. All Rights Reserved
12
| Sites & Locations | Xynomic operates from four main locations as outlined below: ● Headquarters (China).The headquarter office of Xynomic is located in Shanghai, China. This office employs 6 FTEs. ● Research Center (China).Xynomic are in the process of building a research facility in the outskirts of Shanghai. At present, this facility is being headed by a university professor of which Xynomic have collaborations. The company sees this center employing 10 research staff in the near-mid future, with a maximum number of 30 FTEs as a longer term strategy. ● Clinical Team (China).Xynomic have 3 FTEs employed in Beijing, China, with 2 persons managing manufacturing and CMO partners and 1 person managing the two clinical pivotal studies (i.e. Follicular Lymphoma and DLBCL) in China. ● Financial Team (US).Working with Mark Xu, the company has staff (primarily as part time) at a location in San Francisco, US. This team is primarily assisting Mark Xu with finance raising activities (i.e. IPO, fund raising). There are 4 staff in the US (1 FTE, 1/2 FTE, and two 1/8 FTEs). |
| Future Hires and Expansion | Xynomic are planning expansion in FTEs over the coming months. They also aim to appoint a CMO to the management board. The company has plans to develop clinical teams in US and EU as summarized below. These teams will be established by Jason Wu, who has hired a Project Manager to assist in US and will do so similarly for EU. Both US and EU Project Managers will have a reporting line to Jason Xu. ● Clinical Team (US). The company aim to establish a clinical team in North Carolina, US composed of 10 FTEs to oversee the clinical trials and sites (approximately 40-50 clinical sites). These personnel will also include safety monitoring, regulatory and CMC functions. ● Clinical Team (EU). Xynomic will likely set-up a clinical team of 7 FTEs to oversee the clinical trials and sites in EU (approximately 40-50 clinical sites). These personnel will also include safety monitoring, regulatory and CMC functions. Regarding sales and marketing, Xynomic aims to build its own sales teams in US and China, while seeking a partner for marketing in EU countries. ● Sales Team (China). Xynomic aspires to build a sales team of 70-100 reps to cover sale of XP-101 in Follicular Lymphoma and DLBCL indications across China. The company expects to earn in sales approximately USD 5-10m per rep. ● Sales Team (US). In the US, Xynomic will target the top KOLs for each indication as well as marketing via key conferences as a strategy for marketing. The company aims to employ 30 sales reps, with 10 FTEs dedicated to renal cell carcinoma and expected sales of USD 20-30m per rep. Xynomic may utilise a distributor in the US, with terms of offering to the distributor, for example. For breast cancer Xynomic may co-market with ● Sales Team (EU). Xynomic note the EU market as fragmented with country and region specific nuances. Xynomic will seek partners to assist with distribution and marketing; likely collaborating with mid-size companies and offering to local partners. A similar approach will likely be adopted for Japan. |
© Copyright 2018 Venture Valuation. All Rights Reserved
13
| EXPERIENCE |
| | The management and relevant company boards of Xynomic have considerable seniority and expertise, which represents a significant asset to the company. A list of the management and relevant company boards is provided in the tables below. |
| | Management Board |
| | Name | Function | Notes (brief) |
| | Y. Mark Xu | Co-Founder, Chairman, CEO & President | ● Co-founded Bridge Labs and Pacific Biopharma, which merged with Pharmacyclics. ● General Manager of Trout Group, investment bank ● Progressive positions in Schering, McKesson, Stanford Research Institute International, BAS, and UL. ● MBA (Stanford Uni), MSc Chemistry (Purdue Uni), BA Chemistry (Hanover College). |
| | W. Jason Wu | Co-Founder, Chief Operating Officer | ● Over 20 years’ experience in pharma in US and China. ● Senior management in Lilly, Merck, Hutchison Medi Pharma, and Institute of Materia Medica. ● PhD (Purdue Uni) and Adjunct Professor at Inst. Materia Medica (IMM) Chinese Acad. of Med. Sci. (CAMS). ● Consultant for life sciences companies, advising clients on tech transfer, licensing, and M&A. |
| | Yong Cui | Co-Founder, VP CMC | ● Has 18 yr experience in pharm in US and China. ● Obtained his BSc and MSc (China Pharm Uni) and PhD (Ohio State Uni) ● Formulation scientist (Vertex Pharma and Genentech), Director of Formulation Development (Hisun-Pfizer Pharma) and Vice President R&D (Qilu Pharma). ● Lead scientist for two NDAs in US, and involved in multiple interactions with the US FDA. |
| | Dirk Reitsma | Chief Medical Officer | ● VP and Head Oncology Global Product Develop. at PPD. ● VP Clinical Develop. AstraZenica’s MedImmune subsidiary. ● Associate Director and Senior Clinical Research Physician in Oncology at Novartis. ● MD from Erasmus University in The Netherlands. |
| | Bing Zhao | VP Clinical & Regulatory Affairs (Greater China) | ● Received BSc, MSc, and MD (all majored in Oncology) from Xi’an Jiaotong University Health Science Center. ● Practicing oncologist in Xi’An, China. ● Joined BMS in clinical affairs department. ● Clinical trial expert at Beijing H&J CRO Int. (VP & Director Clinical Center), Shanghai Rundo CRO (Director Clinical Center), 3D-HTS (VP), Osmunda (Executive Vice General Manager and Director of Clinical Center). |
© Copyright 2018 Venture Valuation. All Rights Reserved
14
| | Chasey Zhang | VP Global Strategic Sourcing | ● Over 20 yr experience in pharma in US, Singapore, China and other APAC countries. ● Senior management positions in BMS, Clearstone Central Lab, World Courier, and an international CRO. ● Pharmacist with BSc in Pharmacy & Pharmacol. (Second Military Medical Uni China), MBA (Drexel Uni). Post MBA certification (Rutgers Uni) in pharmaceutical management. |
| | Xuexun Fang | Head of Pharmacology | ● Professor and PhD Advisor at Key Laboratory for Molecular Enzymology & Engineering at Jilin University. ● Formerly Vice General Manager of Dual Pharma China. ● Post-doctoral at Sanford Burnham Prebys Medical Discovery Institute in La Jolla, California, USA. ● BSc Biochemistry (Jilin Univ.), PhD Molecular Biology and Biochemistry (Univ. West Virginia). |
| | Mark Zhang | Senior Director Pharmaceutical Development | ● Over 6 yr’ experience in pharma. ● Worked in R&D in Pfizer and PPD. Led generic drug projects (from inception to ANDA). ● Also an expert in API process development to cGMP. ● Obtained MSc from East China University of Science and Technology and PhD in Nanyang Technological Univ. |
| | Shuziang Zhang | Director of Pharmaceutical | ● Director of R&D of Beijing Sunho Pharma. ● Deputy General Manager of Research Institute (Dr. Jason Wu). ● Visiting Scholar: School of Medicine, Univ. Toyama, Japan. ● BSc and MSc, Inner Mongolia Medical University; PhD, Peking Univ. |
| | Shiwen Dong | Project Manager for XP-102 | ● Over 10 years’ experience in pharma. ● Expertise in R&D and registration to patent filing. ● Led successful efforts for registration of several CTMs. ● Completed many patent filings. Received MD and BSc in medicinal chemistry from Peking University School of Pharma Sci, majoring. |
| | Scientific Advisory Board |
| | Name | Notes (brief) |
| | Glenn Rice | ● Founder of several biotech and internet tech companies in US, China, India, Taiwan and Singapore. ● Companies have led to multiple FDA approved drugs in cancer, autoimmune diseases, cardiovascular, ocular and dermatology. ● Former COO of Pharmacyclics, Board Director of ILEX Oncology, Founder of Convergence Pharma, Founder of EmergingMed, Co-founder of C-PATH Institute. |
| | Pamela Munster | ● Professor of Medicine UCSF. ● Director of the Early Phase Clinical Trials Unit at UCSF. ● Collaborator in mechanistic, biomarker and clinical studies with Xynomics on XP-101. |
© Copyright 2018 Venture Valuation. All Rights Reserved
15
| | Anas Younes | ● Chief, Lymphoma Service, Memorial Sloan Kettering Cancer Center ● Professor, Weill-Cornell Medical College, New York. ● Led trials that resulted in FDA approval of brentuximab, vedotin and nivolumab for Hodgkin lymphoma. ● Served as principal investigator of more than 60 clinical trials. |
| | Yuankai Shi | ● Vice President Cancer Hospital, Chinese Academy of Medical Sciences. ● Director cancer center, professor, doctoral advisor. ● Vice President Chinese Society of Hematology, Chinese Medical Association. ● Director Chinese anti-cancer association, Beijing Association for Medical Education, the CFDA reviewer. |
| | Vincent Ribrag | ● Obtained Maitrise of Science et Biologie Médicale in Molecular Biology. ● Received Diplôme d’Études Approfondies in molecular and cellular pharmacology at the Pierre et Marie Curie (Paris Vl) Univeristy. ● Obtained Diplôme d’Études Secondaires in Medical Oncology at the Paris Xl Faculty of Medicine. ● Assistant at oncology centers of Gustave-Roussy lnstitute and Head of early drug program (DITEP) in Gustave Roussy. |
| MOTIVATION |
| Management and Employee Company Shares | The company Chairman, CEO & President, Mark Xu, owns almost 45% of the company. Other members of the management currently have few shares. We note the company has set aside Employee Stock Ownership Plan (ESOP) that comprises approximately 4% of the company. |
| Management Risk | The management at Xynomic are a key asset to the company and together have extensive expertise to manage the business. We consider this underlying management risk to be LOW. We note; however, that a certain level of challenge exists if the company were to establish a large sales force and if the company were to entry an M&A. Thus, we see this risk as LOW-MEDIUM. |
| PRODUCTS – ANTINEOPLASTIC THERAPEUTICS: ABEXINOSTAT, XP-102 |
| |
| Overview | Xynomic has a pipeline of development products, aimed for treatment of cancers. The two key assets are described, in summary, below: HDAC inhibitor: XP-101 (Abexinostat, Abex) ● Abexinostat (XP-101) is an orally bioavailable broad spectrum HDAC inhibitor, which inhibits Class I HDACs including HDAC1,2,3,6,10 and 11.Xynomic in-licensed this drug from Pharmacyclics LLC (currenly a fully owned subsidiary of AbbVie Inc.) in Q1 2017, catalyzing the establishment of Xynomic. To date, a total of 18 Phase I and II clinical trials of Abexinostat have been completed, demonstrating the efficacy and safety profile of this compound in multiple types of cancers. Xynomic is now in the process of completing a number of Phase I-III clinical trials using XP-101, as listed below.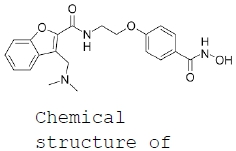 ● In simple terms, XP-101 (as an HDAC inhibitor) modifies DNA allowing cancer drugs to which tumors have become resistant to continue working. XP-101 is thus being developed as a drug used in combination with existing cancer therapies. XP-101 also has potential as a monotherapy in some cancer indications. Please see below for an outline of indications in which XP-101 is being developed. |
© Copyright 2018 Venture Valuation. All Rights Reserved
16
| RAF inhibitor: XP-102 (BI 882370) ● XP-102 (BI 882370) is a B-RAF inhibitor, that inhibits B-RAF in an ‘inactive’ mode (DFG-out confirmation) and has potential use in the treatment of cancers associated with B-RAF mutations, including malignant melanoma and colorectal cancer. Xynomic acquired exclusive global rights to XP-102 (BI 882370) from Boehringer Ingelheim in Q3 2017. To date XP-102 has demonstrated efficacy and safety in animal models of colorectal cancer (CRC) and melanoma. Xynomic aim to proceed toward Phase I clinical studies examining the utility of XP-102 in melanoma and colorectal cancer.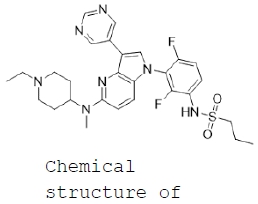 |
| Pipeline | A summary of the product development and pipeline is provided in the table below. |
| | Product Development and Pipeline |
| | Indication | Develop. Stage | Start Date | Sites | Co-Therapy | Partner |
| | HDAC inhibitor: XP-101 (Abexinostat, Abex) |
| | Renal cell carcinoma | Ph III# | Q3 2018 | Global | Pazopanib | Novartis, Parexel |
| | Follicular Lymphoma | Ph II# | Q3 2018 | Global | Monotherapy | PPD |
| | Follicular Lymphoma | Ph II# | Q1 2019 | China | Monotherapy | Quintiles |
| | DLBCL/MCL* | Ph I/II | Q3 2018 | US | Imbruvica | JNJ, Kettering |
| | DLBCL | Ph II# | Q1 2019 | China | Monotherapy | Quintiles |
| | Multiple Solid Tumors | Ph Ib/II | Q3 2018 | US | Keytruda | Merck, UCSF |
| | Breast cancer | Ph Ib/II | Q4 2018 | US | Kisqali | Novartis, UCSF |
| | RAF inhibitor: XP-102 (BI 882370) |
| | Colorectal cancer | Ph Ia | Q1 2019 | US | Monotherapy | n/a |
| | Melanoma | Ph Ia | Q1 2019 | US | Monotherapy | n/a |
| | #Pivotal study; Diffuse Large-B-Cell lymphoma (DLBCL); Mantle cell lymphoma (MCL) |
| PRODUCT DEVELOPMENT |
| |
Preclinical Research and Development | We consider, that early studies for XP-101 and XP-102 to investigate the mechanism of action, preclinical efficacy, and toxicity/safety/ADME have been conducted. Thus, we assume preclinical R&D is not a limiting factor in the development of XP-101 and XP-102. We note Xynomic have two in-house products in early development, namely, XP-103, a TRK/Fra-1 inhibitor, and XP-104, a RET inhibitor for which pre-clinical studies will likely be required. |
Clinical Studies | Phase I: Safety Study ● Phase Ia/b studies utilize typically healthy volunteers of less than 100. These studies aim to determine the safety, tolerability, pharmacokinetics and pharmacodynamics of the drug. ● For these early studies, Xynomic assume a cost of USD 20,000 per patient, with 40 patients per study. |
© Copyright 2018 Venture Valuation. All Rights Reserved
17
| Phase II: Efficacy & Dose Finding Study ● Phase II studies generally involve 100-500 patients to assess efficacy, dose effect and short-term side effects of the drug. ● For the two studies being conducted in China employing Quintiles/IQVIA as the CRO managing this study. Xynomic assume a cost of USD 3m for each study with 70 patients per study. Phase III: Clinical Efficacy Studies ● Phase III studies may enroll 1,000-5,000 patients across several clinical trial sites. ● Xynomic assume a cost of USD 40m for the renal cell carcinoma study (pivotal trial, fast track designation) with an upfront payment of 10% to Parexel as the CRO managing this study. For the Follicular Lymphoma study (pivitol trial, Accelerated approval) they assume a cost of USD 18m, with a similar 10% upfront payment to PPD as the CRO managing this study. A summary of the product development timeline and costs is provided below and in the Appendices I-III: |
| Clinical Studies – Summary of Details |
| Indication | Treatment | Sites | Patients | Trial Type | Notes | Cost (USD) |
| HDAC inhibitor: XP-101 (Abexinostat, Abex) |
| Renal cell carcinoma (RCC) | +pazopanib, NVS | Global | 390 | Ph III, Pivotal, Fast track | 4 yr study. Control arm study. Parexel as managing CRO 260 Patients take XP-101+pazopanib and 130 will take placebo+pazopanib. | 40m (4m upfront) |
| Follicular Lymphoma (FL) | Monotherapy 4th line | Global | 120 | Ph II, Pivotal, Accelerated | 2.5 yr study. Open arm study. PPD as managing CRO Run Ph IV under accelerated approval. | 18m (2m upfront) |
| Follicular Lymphoma (FL) | Monotherapy 3rd line | China | 68 | Ph II, Pivotal | 1.5yr study. Open arm study. Quintiles as managing CRO, employed for China study. | 3m |
| DLBCL/MCL | +Imbruvica, JNJ | US | 40 | Ph I/II | 2 yr study. Kettering Cancer Centre as study site. We assume a follow-on Ph III Pivotal Control arm study of 4 yr with 20m estimated costs. | 1m (+20m Ph III) |
| DLBCL | Monotherapy | China | 88 | Ph II, Pivotal | 1.5 yr study. Quintiles as managing CRO, employed for China study. | 1.5m |
| Multiple Solid Tumors | +Keytruda, MSK | US | 40 | Ph Ib/II | 1.5yr study. UCSF as collaborator. Delay of Keytruda resistance. Possible focus on lung cancer in Ph III Pivotal Control arm 4 yr study of 20m costs. | 1m (+20m Ph III) |
| Breast cancer | +Kisqali, NVS | US | 40 | Ph Ib/II | 2 yr study UCSF as collaborator. Delay of Kisqali resistance. We assume a follow-on Ph III Pivotal Control arm 4 yr study of 20m costs. | 1m (+20m Ph III) |
© Copyright 2018 Venture Valuation. All Rights Reserved
18
| RAF inhibitor: XP-102 (BI 882370) |
| Colorectal cancer (CRC) | Monotherapy | US | 15 | Ph Ia | 6 mth study (each) Then Ph Ib/IIa study (40 patients) of 2 yr in either CRC or Melanoma in combination with e.g. Cetuximab. | 0.4m |
| Melanoma | Monotherapy | US | 15 | Ph Ia | Thereafter a Ph III Pivotal Control arm study of 4 yr with USD 20m estimated costs. | 0.4m |
| Product Development – Estimated Gantt Chart & Timelines |
| Indication | Treatment | Sites | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 |
| HDAC inhibitor: XP-101 (Abexinostat, Abex) |
| Renal cell carcinoma | +Pazopanib | Global | Ph III | | | | NDA | Launch | | |
| Follicular Lymphoma | Monotherapy | Global | Ph II | | | NDA | Launch | | | |
| Follicular Lymphoma | Monotherapy | China | | Ph II | NDA | Launch | | | | |
| DLBCL/MCL | +Imbruvica | US | Ph I/II | | Ph III | | | | NDA | Launch |
| DLBCL (China) | Monotherapy | China | | Ph II | NDA | Launch | | | | |
| Multiple Solid Tumors | +Keytruda | US | Ph I/II | | Ph III | | | | NDA | Launch |
| ER+ Breast cancer | +Kisqali | US | Ph I/II | | Ph III | | | | NDA | Launch |
| RAF inhibitor: XP-102 (BI 882370) |
| Colorectal cancer | Monotherapy | US | IND | Ph Ia | P1b/II | | Ph II | | | |
| Melanoma | Monotherapy | US | IND | Ph Ia | | | | | | |
| Project Development Risk | Xynomic have a number of clinical studies planned and ongoing. Failure in one or more clinical trial could be terminal for the company or leave it in a vulnerable position. We note, however, this would be the case for other companies in a similar position and do not consider a specific development risk posed by Xynomic. We deem this risk as MEDIUM. |
| PRODUCT PRICING |
| Unit Pricing | Xynomic suggest a product price USD (with also available as required per indication and patient). Treatment regime for renal cell carcinoma is expected to be 4 day on / 3 day off. For follicular lymphoma the treatment protocol will likely be 1 week on / 1 week off. Xynomic are projecting revenues of USD 140,000 per patient per year in the US at the point of peak sales, with an expected 20% discount in China and Europe, representative of oncology based treatments. We note that, in cases of where XP-101 is provided as a combination therapy, the total price of XP-101 and the partner drug may fall out with the affordability of a certain population and perhaps subject to regulatory scrutiny. This may apply to, for example, DLBCL/MCL with Imbruvica, multiple solid tumors (e.g. non–small cell lung cancer) with Keytruda and ER+ breast cancer with Kisqali. |
© Copyright 2018 Venture Valuation. All Rights Reserved
19
| | For DLBCL and MCL in Europe and the US, where XP-101 will be given in combination with Imbruvica, itself a highly priced drug, Xynomic expect to undergo discounts in the reimbursement level and project average revenues of USD 100’000 per patient. We note that for RCC, where the treatment will be a combination treatment with pazopanib, pazopanib will be out of patent by 2021, which is the earliest the combination will be approved. Thus, it is expected that the treatment cost with pazopanib will undergo significant discounts and allow for the higher reimbursement level of XP-101. |
| | |
| Reimbursement | Regarding reimbursement, in China there are three principle systems: 1. Commercial and state owned company insurance provided by the employer, which applies to approximately 30-40 million people in China. Xynomic will aim to target initially this population; 2. State and provincial reimbursement systems that list established therapies, which enjoy a 80-90% reimbursement and can be afforded by 40-50% of the population. In this case, Xynomic will have to negotiate the listing of XP-101 and XP-102 once established and will likely be required to reduce their price by 40-50% if listed; 3. Governmental and army personnel at medium-high ranking for which there are approximately 1 million people and whom receive 100% reimbursement for medical care. These persons will also immediately benefit from Xynomic drugs coming to market. |
| PRICES OF MARKETED PRODUCTS – CO-THERAPIES AND KEY COMPETITORS |
| Indication | | Drug | | Company | | Annual Price USD | | | Dose Regime |
| Renal cell carcinoma | | Votrient (Pazopanib) | | Novartis | | | 140,000 | | | Once daily (od) |
| Renal cell carcinoma | | Sutent (Sunitinib) | | Pfizer | | | 20,000 | | | Once daily (od) (4 wk on, 2 wk off) |
| Follicular Lymphoma | | Rituximab (Rituxan) | | Biogen | | | 25,000 | | | For two infusions |
| DLBCL/MCL | | Imbruvica (Ibrutinib) | | AbbVie/JNJ | | | 130,000 | | | Four times daily (qds) |
| Multiple Solid Tumors | | Keytruda (Pembrolizumab) | | Merck | | | 150,000 | | | For i.v. over 30 min every 3 weeks |
| ER+ Breast cancer | | Kisqali (Ribociclib) | | Novartis | | | 110,000 | | | Once daily (od) (3 wk on, 1 wk off) |
| ER+ Breast cancer | | Ibrance (Palbociclib) | | Pfizer | | | 120,000 | | | Once daily (od) (3 wk on, 1 wk off) |
| Pricing Risk | We consider the pricing strategy to be as per current therapeutics on the market. However some regulatory scrutiny may occur when summed with the cost of partner drugs. We note, a risk associated with reimbursement listing. Thus, we deem the pricing risk to be MEDIUM-HIGH. |
© Copyright 2018 Venture Valuation. All Rights Reserved
20
| REGULATORY AFFAIRS |
| Current Knowledge on Safety | We note the following matters related to safety: Supporting Evidence ● XP-101 (Abexinostat) has been tested on approximately 600 patients worldwide in 18 clinical trials. These include solid tumors, hematological tumors, lymphoma, leukemia, Hodgkin’s disease, non-Hodgkin lymphoma, digestive cancer, nasopharyngeal carcinoma, breast cancer, ovarian cancer, metastatic sarcoma. No safety concerns precluding from further development of XP-101 has been noted. Xynomic notes that the PK profile, tolerability and efficacy data for XP-101 indicate it as best-in-class HDAC inhibitor; safer than 1st generation and similar to 2nd generation HDAC inhibitors. ● XP-102 is an early stage compound with safety studies ongoing. Xynomic report no relevant findings in exploratory toxicology studies, with high efficacy and good tolerability in combination therapy (chemotherapy, targeted agents). Required Data ● We consider the safety data for XP-101 not a limiting factor in its further development. ● For XP-102, PPD have provided a gap analysis (i.e. toxicology, CMC, pharmacology) of IND requirements and will assist in the IND filing in the US. |
| Evidence of Clinical Efficacy | XP-101 (Abexinostat) ● The further development of XP-101 is based on earlier clinical studies. XP-101 has been tested in a Ph Ib clinical study, showing efficacy in treating VEGF inhibitor refractory solid tumour in combination with Pazopanib, including renal cell carcinoma. In Follicular Lymphoma XP-101 has been shown to give an Objective Response Rate (ORR) of 44% in Ph I and 56% in Ph II clinical studies. In DLBCL, XP-101 shows an ORR of 23% in Ph I and 31% in Ph II clinical studies. In PTCL, XP1-101 gave an ORR of 22% in Ph I and 40% in Ph II studies. XP-102 ● XP-102 is at pre-IND stage. Animal studies have demonstrated pre-clinical efficacy of XP-102. In a model of CRC, XP-102 has demonstrated efficacy compared to Vermurafenib (Roche). XP-102 has also demonstrated efficacy in combination with Cetuximab. In Melanoma animal studies, XP-102 is efficacious compared to Vermurafenib, Dabrafenib and Trametinib (Novartis). XP-102 has also been shown to be efficacious after the development of drug resistance to Vermurafenib and Trametinib. |
Regulatory Classification and Ongoing Discussions with
Regulators | FDA (US) ● Xynomic has gained Fast Track designation for XP-101 in renal cell carcinoma (RCC) and an Accelerated Approval Program for XP-101 in Follicular Lymphoma, requiring demonstration of clinical benefit in Ph IV confirmatory trials and subject to this data may be withdrawn or approved. Regarding Follicular Lymphoma, we note FDA are unable to allow head-to-head trial with the 3rd line (3L) drugs copanlisib (Aliqopa, Bayer) and idelalisab (Zydelig, Gilead) given both were approved via fast track and require a Phase IV post-market trial. As such, XP-101 is proposed initially as a 4thline (4L) treatment in US and 3rd line in China, with eventual 3L classification worldwide. ● Xynomic has received indication from the FDA that XP-101 will be approved for Follicular Lymphoma if the drug obtains a clinical efficacy measured by ORR of 45% or more. We note that this will likely increase the overall approval rate and have reflected this in the probability of success outlined below. |
© Copyright 2018 Venture Valuation. All Rights Reserved
21
| | CDA (China) ● A recent change in policy has allowed in parallel drug approval in US and China. While there are drugs in US used for 3rd line (3L) treatments of Follicular Lymphoma, according to Xynomic, no 3L therapies exist for this illness in China. Thus, in China, Xynomic will be allowed to conduct a pivotal study for 3L treatment in Follicular Lymphoma. The same is held true for DLBCL. This provides a significant competitive advantage for Xynomic in the Chinese market. |
| Regulatory Risk | Xynomic and their representative CRO partners are in discussion with regulatory authorities and have ongoing clinical studies. Given the significant experience within the company we consider this risk as MEDIUM. |
| MANUFACTURING |
| COGs | Xynomic estimate a COG as USD per tablet. |
| Chemistry, manufacture and control (CMC) & Manufacturers | The manufacturing partners for XP-101 were pre-established by the licensor Pharmacyclics. Xynomic have audited these partners and will continue to use them. For XP-102, the company will seek manufacturing partners in China. Details are provided in the section and table above (please see manufacturing partners). |
| Status today & Scale Up Plan | We note that Xynomic have 6,000 bottles of XP-101 tablets ready for the renal cell carcinoma study. |
| Shelf Life/Formulations | Xynomic have of XP-101. According to Xynomic, the shelf life/stability of XP-101 in tablet form is years. |
| Manufacturing Risk | Xynomic have in place the manufacturing partners for XP-101 and XP-102. We see no particular hurdles with this activity. We deem the manufacturing risk as LOW. |
| INTELLECTUAL PROPERTY (IP) |
| License Ownership | The patents related to XP-101 and XP-102 are outlined below. XP-101 ● The original inventors of XP-101 were acquisition of Axys Pharmaceuticals who were acquired by Celera Genomics in 2001. Celera Genomics then out-licensed XP-101 to Pharmacyclics in 2005. In 2009 Pharmacyclics entered into an alliance with Servier to develop XP-101; this alliance was terminated in 2014. In 2015, Pharmacyclics was acquired by AbbVie. Xynomic in-licensed this drug from Pharmacyclics LLC (now a company wholly owned by AbbVie) in Q1 2017, catalyzing the establishment of Xynomic.. The details of the license payments from Xynomic to AbbVie are indicated in the table below. XP-102 ● Regarding XP-102, Xynomic acquired exclusive global rights to XP-102 (BI 882370) from Boehringer Ingelheim in Q3/2017. The details of the license payments from Xynomic to Boehringer are indicated in the table below. Xynomic employ the patent attorneys, Foley Hoag LLP and Fish & Richardson to maintain the XP-101 patent estate. For XP-102, we note that PPD have assisted in the technology transfer during in-licencing from Boehringer Ingelheim. Details of the royalties and conditions associated with this license transfer are listed below. |
| Details of License Payments to AbbVie: XP-101 |
| Development & Milestone Payments | | USD (m) |
| Upfront Milestone Payment | | 3.5 |
| Initiation of first pivotal clinical trial | | 3.5 |
| Regulatory approval for 1st indication (China or US) | | 6.5 |
| Regulatory approval for 2nd indication (China or US) | | 4.0 |
| | | |
Royalty Payments | | Royalty Rate (%) |
| Annual Net Sales | | |
| Details of License Payments to Boehringer: XP-102 |
| Development & Milestone Payments | | USD (m) |
| Upfront Milestone Payment | | 0.3 |
| Initiation of Phase I | | 1.7 |
| Initiation of pivotal Phase III in 1st indication (US or China) | | 4.0 |
| Initiation of pivotal Phase III in 2nd indication (US or China) | | 2.0 |
| Regulatory approval for first indication in US | | 7.0 |
| Regulatory approval for first indication in China | | 3.0 |
| | | |
Royalty Payments | | Royalty Rate (%) |
| Annual Net Sales (USD 0-50m) | | |
| Annual Net Sales (USD 50-200m) | | |
| Annual Net Sales (USD over 200m) | | |
| Patent Territories, Claims, Priority Dates, Projected Expiration Dates and Potential for Extension of Exclusivity | The patents pertaining to XP-101 provide protection in states that have adhered to the PCT, essentially worldwide. The original patent has as expiration date of 2024. A further patent exists based on the composition matter, solving the hydroscopic properties of XP-101 allowing a switch to capsule to table. This extends the patent to 2034. For XP-102, there are two patent applications covering substance and crystalline salts, with provisional application in the USA. The patent filing was in.2012 and we assume an expiration date of 2033. A list of the patents is provided below: |
© Copyright 2018 Venture Valuation. All Rights Reserved
22
| | Details of Highest Value Patent |
| | Title | NOVEL HYDROXAMATES AS THERAPEUTIC AGENTS |
| | Applicant | AXYS PHARMACEUTICALS INC [US] |
| | Inventors | Eric J. Verner [US/US], Martin Sendzik [DE/US], Chitra Baskaran [IN/US], Joseph J. Buggy [US/US], James Robinson [US/US] |
| | International Publication No. | 28.10.2004 | WO 2004/092115 |
| | International Application No. | 06.04.2004 | PCT/US2004/010549 |
| | Priority Date | 07.04.2003 | US 10/818755 |
| | | | CN 2011/10221277 |
| | | | EP 2004/0749791 |
| | International Filing Date | 06.04.2004 | Priority Date +1 year |
| | Current Expiration Date | 2024 | Priority Date +20 years |
| | Projected Expiration Date | 2033 | See PCT/FR2014/050455 |
| | Projected Marketing Date | 2021 | e.g. Follicular Lymphoma in China |
| | No Yrs. Patent Protected | 13 years |
| | Note | Covers Abexinostat. Highest Value. Almost granted in all perusing countries, the following are some patent number: CN102391222, EP1611088B1, US7276612, US7420089, US7482466 |
| | Details of other Relevant Patents |
| | Patent No. | Filing Date | Notes |
| | XP101-Related | | |
| | PCT/FR2014/050455 | 03.03.2014 | HIGH VALUE: New salt of Abexinostat |
| | US12/761588 (US only) | 31.10.2013 | HIGH VALUE: Formulations of Abexinostat |
| | PCT/US2015/048243 | 03.09.2015 | Methods for preparing novel salts |
| | PCT/US2011/051470 | 13.09.2011 | Formulations with bendamustine |
| | PCT/EP2014/056780 | 04.04.2014 | Biomarkers related |
| | PCT/US2008/052540 | 30.01.2008 | Method determining drug resistance |
| | PCT/US2007/086874 | 07.12.2007 | Biomarkers related |
| | PCT/FR2012/052004 | 07.09.2012 | Administration regime |
| | XP102-Related | | |
| | US13/359555 | 02.02.2012 | Granted US, JP, Germany, UK |
| | US62/577313 | 26.10.2017 | Salts of B-RAF kinase inhib. (Provisional) |
| Company Knowhow | Xynomic knowhow comes from its knowledge of the Chinese market and networks as well as the from the management knowhow in business & development. Xynomic note that their strategy is to in-licence later staged compounds to reduce attrition rates. Xynomic being one of the front runner pharma companies in China, will benefit from the change in attitudes in China to further open the market to foreign medicines. |
| Barriers Placed | Xynomic consider they have a 4-5 year advantage on any future competitor aiming to replicate a XP-101-like product and that their first mover advantage will dissuade competitors. The company considers a further key advantage is their first mover position in China. Regarding XP-101, Xynomic consider the safety and efficacy profile of this compound to be superior that current HDAC inhibitors. HDAC is a relatively old drug target and Xynomic consider the likelihood of new upcoming drugs to be minimal. |
© Copyright 2018 Venture Valuation. All Rights Reserved
23
| | For XP-102, while large pharma worked on RAF as a drug target in 2009-2011, this seemed to lose priority with the advent of immunotherapy in 2014, with most large pharma stopping RAF inhibitor projects. While, there is a number of drugs used for melanoma, Xynomic consider CRC or non-small-cell lung carcinoma (NSCLC) may provide a relatively large market for XP-102. Xynomic note also that CRC or NSCLC is relatively highly prevalent in China. |
| | |
| Enforceability and Limitations | In general terms, ownership risks can come from former employers and employees, contractors and consultants, who (while not named on the patent) may claim a right over the IP. The freedom-to-operate and third-party infringement may also be pursued by competitors, even if low, to instigate patent litigation and derail growth. If the IP of the products listed herein has been derived from an academic setting, where research scientist contracts may be casual, the company may have some risk in ownership claims, freedom-to-operate and third-party infringement. In this regard, additional authors listed on publications related to the products in comparison to those listed on the patents may be present. Given that Xynomic has collaborations with universities they may seek to confirm any potential ownership claims, freedom-to-operate and third-party infringement. |
| IP Risk | The existing of patents related to XP101 appears well covered with an expiration date of 2034 and 13 years of patent protection. We deem this risk as MEDIUM. |
| MARKET OVERVIEW |
| |
| Disease Overview | Xynomic is focused on development of novel therapies for the treatment of a variety of cancers. The company is running a number of clinical trials examining the efficacy of its lead drug XP-101 (Abexinostat) in renal cell carcinoma, follicular lymphoma, diffuse large-B-cell lymphoma and mantle cell lymphoma, multiple solid tumors and breast cancer, as well as its drug XP-102 (BI 882370) in colorectal cancer and melanoma. |
| Target Market | A brief outline of Xynomic’s market environment is provided below: Market Size/Epidemiology ● The epidemiology for differing cancers, in particular the prevalence, and the estimated number of patients is listed in the table below. Territories ● The target patient population in the US, EU and Japan and China is described below, with an upside arising from distribution in other geographies and worldwide. Customer Segments and Indications ● We assume the population that receives a prescription in US as a 100% benchmark; with EU being typically 55-75% of this as the sales ratio reported by companies; and in China based on the population eligible for and that can afford expensive treatments. We assume, also a high (80%) compliance for life threatening cancer treatments supervised by specialists. Further assumptions for each of the disease indications for XP-101 are described below (those of XP-102 are not listed): ● Renal cell carcinoma (RCC): In this case the prevalence rate is based on a 3 year prevalence of kidney cancer, of which 90% is RCC. Sub-population of 30% is based on patient population taking Pazobanib (Votrient) with advanced stage IV RRC. Market share for XP-101 is based on half that of pazobanib estimated share. |
© Copyright 2018 Venture Valuation. All Rights Reserved
24
| | ● Follicular Lymphoma (FL): The prevalence rate is based on a 3 year prevalence of NHL, of which 92% being B-cell type and of this 12% being FL. Sub-population of 60% is based on patients having received treatment with rituximab and being in relapsing or refractory stage. Market share for XP-101 is expected to be higher in China based on its use as an earlier treatment line. We note FL patients can have long survival times, allowing for use of 4th line medication, where XP-101 may be useful. ● DLBCL/MCL: We base the prevalence rate is based on a 3 year prevalence of NHL, of which 92% being B-cell and of this 22% being DLBCL and 5% MC. Sub-population of 60% is based on patients having received treatment with rituximab, who are relapsing or refractory. Market share for XP-101 is expected to be higher in China based on its use as an earlier treatment line. ● Multiple Solid Tumors (non–small cell lung cancer): Xynomic have suggested a focus potentially toward lung cancer. A prevalence rate is based on a 3 year prevalence of lung cancer, of which 90% is non–small cell lung cancer (NSCLC). The sub-population of 28% is based on patient population testing positive with more than 50% tumor proportion score (TPS) for PDL1 (programmed death ligand 1), for which Keytruda can be used. Market share for XP-101 is based on half that of PDL1 positive NSCLC patients. ● Breast cancer: The prevalence rate is based on a 3 year prevalence of breast cancer, of which 80% being ER+ breast cancer. Sub-population, based on assumption that 50% of patient population is taking Kisqali by 2025 at launch of XP-101, as per Novartis estimating Kisqali having blockbuster growth. In 2017 sales for Kisqali was USD 26m compared to Ibrance of USD 643m. Market share of XP-101 based on half that of Kisqali estimated share in 2025. An illustrative projected target population is provided in the table below (please also see Appendix I-III).We note that,for the purpose of this valuation, we have focused on the lead indications in RCC, FL, DLBCL/MCL in the rNPV calculation, and other indications are considered in the pipeline (see Valuation section below). |
| | Target Population Calculation |
| | | | Prevalence rate (%) | | | Sub-pop. (%) | | | Pop.prescribed (%) | | | Market share (%) | | | Compliance rate (%) | |
| | Renal Cell Carcinoma |
| | US | | | 0.03 | % | | | 30 | % | | | 100 | % | | | 7.5 | % | | | 80 | % |
| | EU | | | 0.04 | % | | | 30 | % | | | 55 | % | | | 7.5 | % | | | 80 | % |
| | CN | | | n.a. | | | | n.a. | | | | n.a. | | | | n.a. | | | | n.a. | |
| | Follicular Lymphoma (FL) | | | | | | | | | | | | | | | | | | | | |
| | US | | | 0.004 | % | | | 60 | % | | | 100 | % | | | 30 | % | | | 80 | % |
| | EU | | | 0.002 | % | | | 60 | % | | | 75 | % | | | 30 | % | | | 80 | % |
| | CN | | | 0.0004 | % | | | 60 | % | | | 15 | % | | | 60 | % | | | 80 | % |
| | Diffuse Large-B-Cell lymphoma (DLBCL) | | | | | | | | | | | | | | | | | | | | |
| | US | | | 0.008 | % | | | 60 | % | | | 100 | % | | | 30 | % | | | 80 | % |
| | EU | | | 0.005 | % | | | 60 | % | | | 75 | % | | | 30 | % | | | 80 | % |
| | CN | | | 0.001 | % | | | 60 | % | | | 15 | % | | | 60 | % | | | 80 | % |
| | Mantle cell lymphoma (MCL) | | | | | | | | | | | | | | | | | | | | |
| | US | | | 0.002 | % | | | 60 | % | | | 100 | % | | | 50 | % | | | 80 | % |
| | EU | | | 0.001 | % | | | 60 | % | | | 75 | % | | | 50 | % | | | 80 | % |
| | CN | | | n.a. | | | | n.a. | | | | n.a. | | | | n.a. | | | | n.a. | |
| | Multiple Solid Tumors (Non–small cell Lung cancer) | | | | | | | | | | | | | | | | | | | | |
| | US | | | 0.067 | % | | | 28 | % | | | 100 | % | | | 14 | % | | | 80 | % |
| | EU | | | 0.058 | % | | | 28 | % | | | 75 | % | | | 14 | % | | | 80 | % |
| | CN | | | 0.032 | % | | | 28 | % | | | 15 | % | | | 14 | % | | | 80 | % |
| | ER+ Breast cancer | | | | | | | | | | | | | | | | | | | | |
| | US | | | 0.379 | % | | | 50 | % | | | 100 | % | | | 25 | % | | | 80 | % |
| | EU | | | 0.338 | % | | | 50 | % | | | 75 | % | | | 25 | % | | | 80 | % |
| | CN | | | 0.066 | % | | | 50 | % | | | 15 | % | | | 25 | % | | | 80 | % |
© Copyright 2018 Venture Valuation. All Rights Reserved
25
| Market Entry and Uptake | Market Entry ● Given the clinical development timelines outlined above, we assume XP-101 to be ready for market launch in Follicular Lymphoma and DLBCL by 2021 in China, in Follicular Lymphoma by 2022 in US/EU, in Renal cell carcinoma by 2023, and in DLBCL/ML, Multiple Solid Tumors and ER+ Breast Cancer by 2025. ● XP-102 is an early stage pre-clinical compound. We assume timelines of a 6 mth Ph Ia study (15 patients), then a Ph Ib/IIa study (40 patients) of 2 yr in either CRC or Melanoma in combination with e.g. Cetuximab and thereafter a Ph III Pivotal Control arm study of 4 yr, with 1 yr for NDA registration. Therefore, we estimate a market launch in 2027. Market Uptake and Market Campaign ● Regarding sales and marketing, Xynomic aims to build its own sales teams in US and China, while seeking a partner for marketing in EU countries. For details of sales teams, please see above (section on future hires and expansion). Product Distribution ● Country Level: Xynomic may utilise local distributors and/or co-market with collaborating large pharma (e.g. Novartis, JNJ) offering of 10-20% in sales to the distributor, local partners, and large pharma partners depending on marketing and distribution assistance. ● Patient Level: We assume, XP-101 and XP-102 will be administered orally (in tablet form) to patients either hospitalized or to out-patients attending regular clinics. Therefore, we assume these products will be distributed in a hospital or clinic setting under medical supervision. |
| Market drivers | Positive market drivers in our view include: ● Improved clinical benefits to existing therapies ● First mover advantage especially in China ● Significant market size and unmet medical need in indications being proposed for development ● Opportunity to extend into a number of indications ● Opportunity for global growth in various territories ● Generic versions of pazopanib, Imbruvica, Keytruda, Kisqali and other co-treatments with Abexinostat (XP-101) and XP-102 may drive market share ● Positive demographics, including aging population, rising disposable incomes and healthcare access and awareness |
© Copyright 2018 Venture Valuation. All Rights Reserved
26
| | Negative market drivers include: ● Possible threat from yet unknown novel technologies ● Competitive pressure from existing products already marketed ● High pricing strategies and regulatory/governmental body approval ● Limits in affordability by patients ● Lack of diagnostic biomarkers that might assist in identifying and better stratifying patient populations ● Need to develop distribution networks, sales and marketing ● Need for significant market investment to launch products |
| | |
| Market Uptake Risk | Xynomic have a sufficient plan in place and a reasonable timeline to develop a marketing strategy for XP-101 and XP-102. We see this activity on par with other drug development projects at a similar stage of development. We deem the market uptake risk as MEDIUM. |
| COMPETITIVE LANDSCAPE |
| Current Practice | The current therapies for the cancer indications, for which XP-101 and XP-102 are being developed, include surgery, monotherapies, and combination treatments. These approaches tend to represent first, second, and third line treatment methods, with palliative care measures instigated with terminal illness diagnosis. |
| Advantages over Current Practice | XP-101 ● XP-101 is an oral HDAC inhibitor which has advantage over the injectable competitors. ● Given that XP-101 assists in prolonging drug resistance of other cancer therapies, and works by modifying DNA, its levels in the body can be maintained at a constant low level, potentially offering better safety profile. According to Xynomic, the c-max properties of XP-101 allow for a low level maintenance, compared to higher c-max levels of competitor HDAC inhibitors. The tablet form of XP-101 allows for further control, in this regard. ● XP-101 is a pan-HDAC inhibitor. Xynomic suggest its profile has advantage over other HDAC inhibitors in terms of safety, e.g. compared to those having HDAC8 inhibitor activity. XP-102 ● XP-102 is a 2nd generation pan-RAF kinase inhibitor that can block multiple RAF kinases and signaling pathways. Xynomic consider 1st generation RAF kinase inhibitors develop drug resistance, which will not be the case for the pan-RAF kinase inhibitor XP-102. ● XP-102 has a unique binding mode, insofar as it binds the RAF kinase in a DFG-out ‘inactive’ confirmation providing high selectivity. For CRC and melanoma this property might help in providing synergistic efficacy with currently used MEK inhibitors. |
| | ● In toxicity studies, Xynomic indicate they were unable to reach toxic dose of XP-102, remarking the drug would be expected to have a large safety/therapeutic window. |
| | |
| Competing Players | Direct Competitors ● With regard to direct competitors, there are first, second and third line therapies for many of the cancer indications for which XP-101 and XP-102 are being developed. Please see table below. Therapeutic & Mechanistic Based Competition ● Mechanistically, a number of pan-HDAC and selective HDAC inhibitors are marketed or in development that may compete with XP-101. Similarly, RAF inhibitor marketed drugs or those in development could represent competition to XP-102 (BI 882370) (please see below). Other & Future Competitors ● Other competitors, while likely in the distant future, may include novel technologies that target similar therapeutic pathways, as well as those derived from novel molecular approaches, gene therapy and/or cell-based therapies. In this regard, the upcoming CAR-T cell technology appears promising. Chimeric antigen receptor (CAR) T cells are immune cells genetically engineered to target an antigen present on tumor cells. Kite Pharma KTE-C19 (or axicabtagene ciloleucel), Novartis (CTL019, Kymriah tisagenlecleucel), and Juno Therapeutics (JCAR014 and JCAR017) have been leading the development of CAR T-cell products for the treatment of DLBCL or MCL. At present these therapies target CD19 and in the futures, other targeted antigens, e.g. CD30 or CD22, may allow expansion of CAR T-cell therapy possibly for other cancers. Xynomic consider a combination treatment with Imbruvica and XP-101 may provide similar efficacy as reported for CAR-T cell technology in DLBCL or MCL. |
| Market Share & Competitor Products | Based on our analysis, the number of therapeutic products in different stages of development, as well as marketed products, for the indications targeted by XP101 and XP102 are listed below. We can therefore assume an increasing competition in the future, with new products coming to the market. This risk has been taken into account in the market share. We note, however that competition may also represent a potential out-licensing and/or collaborative (co-development, distribution and/or marketing) opportunity, as the interest of large partners in the field is pertinent. The number of products in development as well as key marketed and upcoming competitors are outlined below. These lists are for example purposes and not exhaustive. |
© Copyright 2018 Venture Valuation. All Rights Reserved
27
| | KEY MARKETED COMPETITORS: MECHANISM COMPETITORS |
| | Drug | Company | Approved |
| | HDAC inhibitor: XP-101 (Abexinostat, Abex) |
| | Vorinostat (Zolinza) | Merck | 2006 |
| | Romidepsin (Romidespin) | Celgene | 2011 |
| | Belinostat (Beleodaq) | Spectrum | 2014 |
| | Chidamide (Epidaza) | Chipscreen | 2014 |
| | Panobinostat (Farydak) | Novartis | 2015 |
| | | | |
| | RAF inhibitor: XP-102 (BI 882370) |
| | Vemurafenib (Zelboraf, PLX4032) | Genentech (Roche) | 2011 |
| | Dabrafenib (Tafinlar) | Novartis | 2013 |
| | Encorafenib (LGX818) | Array Biopharma | Phase III |
| | MLN-2480 | Millennium/Takeda | Phase I |
| | LY3009120 | Eli Lilly | Phase I |
| KEY MARKETED COMPETITORS: INDICATION COMPETITORS |
| Drug Name | Company | Mechanism of Action | Marketed |
| RENAL CELL CARCINOMA (RCC) |
| Votrient (Pazopanib) | Novartis | Multikinase inhibitor of c-KIT, FGFR, PDGFR and VEGFR | 2009 |
| Afinitor (Everolimus) | Novartis | Inhibitor of mammalian target of rapamycin (mTOR) | 2009 |
| Proleukin (Aldesleukin) | Novartis | Interleukin-2 (IL-2) | 1992 |
| Avastin (Bevacizumab) | Roche/Genentech | Monoclonal antibody inhibiting VEGF, blocking angiogenesis | 2004 |
| Inlyta (Axitinib) | Pfizer | Tyrosine kinase inhibitor of VEGFR, c-KIT and PDGFR | 2012 |
| Sutent (Sunitinib) | Pfizer | Multi-targeted receptor tyrosine kinase (RTK) inhibitor | 2006 |
| Torisel (Temsirolimus) | Pfizer | Specific inhibitor of mTOR | 2007 |
| Nexavar (Sorafenib) | Bayer/Amgen | Tyrosine kinase inhibitor of VEGFR, PDGFR & Raf kinase | 2005 |
| Cometriq (Cabozantinib) | Exelixis | Inhibits receptor tyrosine kinases | 2011 |
| Lenvima (Lenvatinib) | Eisai Co. | Multiple kinase inhibitor | 2015 |
| Opdivo (Nivolumab) | BMS | IgG4 anti-PD-1 monoclonal antibody | 2015 |
| FOLLICULAR LYMPHOMA (FL) |
| Rituximab (Rituxan) | Roche | Monoclonal antibody against CD20 | 1997 |
| Obinutuzumab (Gazyva) | Roche | CD20 monoclonal antibody (2nd line treatment) | 2013 |
| Copanlisib (Aliqopa) | Bayer | Phosphoinositide 3-kinase inhibitor (3rd line treatment) | 2015 |
| Idelalisab (Zydelig) | Gilead | Phosphoinositide 3-kinase inhibitor (3rd line treatment) | 2014 |
| B-CELL LYMPHOMA (DLBCL) OR MANTLE CELL LYMPHOMA (MCL) |
| Imbruvica (Ibrutinib) | AbbVie/JNJ | Inhibits Bruton’s tyrosine kinase (BTK) in B cells | 2013 |
| Yescarta | Kite Pharma, Inc. | Engineered T-cells targeting cancer cells (CAR-T) | 2017 |
| Kymriah (tisagenlecleucel) | Novartis | Engineered T-cells targeting cancer cells (CAR-T) | 2018 |
| Multiple Solid Tumors (Non–small cell Lung cancer) |
| Keytruda (Pembrolizumab) | Merck | Monoclonal antibody targets PD-1 receptor | 2015 |
| Tarceva (Erlotinib) | Roche/Genentech | Tyrosine kinase inhibitor of EGFR | 2015 |
| ER+ BREAST CANCER |
| Kisqali (Ribociclib) | Novartis | Inhibitor of CDK4 and CDK6 | 2017 |
| Ibrance (Palbociclib) | Pfizer | Inhibitor of CDK4 and CDK6 | 2015 |
| Verzenio (Abemaciclib) | Eli Lilly | Inhibitor of CDK4 and CDK6 | 2015 |
| Tykerb (lapatinib) | GSK | Tyrosine kinase inhibitor of HER2 and EGFR pathways | 2007 |
| Perjeta (Pertuzumab) | Genentech | Monoclonal antibody inhibits HER receptor dimerization | 2012 |
| Herceptin (Trastuzumab) | Roche/Genentech | Monoclonal antibody targeting HER2 | 1998 |
© Copyright 2018 Venture Valuation. All Rights Reserved
28
| NUMBER OF PRODUCTS IN DEVELOPMENT | |
| Stage | Renal Cell Carcinoma (RCC) | Follicular Lymphoma (FL) | B-cell lymphoma (DLBCL) | Mantle cell lymphoma (MCL) | Non–small cell Lung cancer (NSCLC) | ER+ Breast cancer |
| Preclinical | 9 | 1 | 1 | 1 | 40 | n.a. |
| Phase I | 15 | 2 | 2 | 1 | 54 | 1 |
| Phase II | 10 | 6 | 2 | 4 | 58 | 2 |
| Phase III | 18 | 7 | 3 | 1 | 54 | 2 |
| NDA | 5 | n.a. | n.a. | n.a. | 8 | n.a. |
| Biotechgate, Search Filters: Type of product, Biotechnology - Therapeutics and Diagnostics; Main area, Neoplasms/cancer/oncology; Therapeutic sub-sector - Renal cell carcinoma, Follicular Lymphoma, Diffuse large-cell B-cell lymphoma, Mantle cell lymphoma, Non–small cell Lung cancer, or HER2+ Breast cancer. |
| | | | | | | | |
| Competitive Risk | There are a number of first, second and third line competitor products currently on the market. A competitive risk is also likely to come from novel products, of which many are in development. Given the current and future competition level, we deem this risk to be MEDIUM-HIGH. |
| ASSESSMENT |
| |
| STRENGTHS |
| | Below is a brief summary of the strengths as we see them: ● Strong evidence of safety and efficacy from clinical studies ● Significant market size and unmet medical need ● Opportunities for indication and territory extension ● First mover advantage ● Knowledge to penetrate China market ● Positive demographics, including aging population, rising disposable incomes, and healthcare awareness ● Significant expertise of company management as an asset to the company ● Well defined portfolio strategy and pipeline for sustained product development |
| WEAKNESSES |
| | Below is a brief summary of the weaknesses as we see them: ● Need for financial investment for clinical studies and running costs (approximately USD 50-60m) ● Uncertainties surrounding M&A and/or IPO ● High dependence on success of Abexinostat ● Lack of sufficient support staff to assist Senior Management ● Challenges of staff as company expands ● Need for the development of sales force teams in targeted territories ● Uncertainties surrounding future partnership plan with co-developers and distributors |
© Copyright 2018 Venture Valuation. All Rights Reserved
29
| RISK ANALYSIS |
| |
| RISK ASSESSMENT - SUMMARY |
| |
| Risk Factors | Below we summarize the risk factors as we see them associated with Xynomic and with the company’s lead products. Risks are presented as product-specific risks and non-product specific risks. We note that product-specific risks are reflected in a direct risk adjustment as part of the rNPV method, whereas non-product specific risks are reflected in the overall discount rate. The ratings used are designated aslow (lower than average as compared to comparable companies/products),medium (average), andhigh (higher than average). |
| Product-Specific Risks | We see the key product specific risks as follows: Product Development Risk: ● Xynomic have a number of clinical studies planned and ongoing. Failure in one or more clinical trial could be leave the company in a vulnerable position. We note, however, this would be the case for all companies in a similar stage. We deem this risk asMEDIUM. Regulatory/Approval Risk ● Xynomic and their representative CRO partners are in discussion with regulatory authorities and have ongoing clinical studies. Given the significant experience within the company we consider this risk asMEDIUM. Manufacturing Risk: ● Xynomic have in place the manufacturing partners for XP-101 and XP-102. We see no particular hurdles with this activity. We deem the manufacturing risk asLOW. |
| Non-Product Specific Risks | Non-product specific risks are as follows: Partnering Risk ● Xynomic has a well-established set of collaborators and partnerships in place for development of its products, which also include large Pharma such as Novartis and JnJ. We consider this risk asLOW-MEDIUM. Financing Risk ● Xynomic has raised approximately USD 25m equity to date in private investments. The company aims to raise funds for the clinical development of its lead and pipeline products, either by IPO or M&A. We are not aware of any future financial incomes secured or term sheets signed. We note however that XP-101 is an attractive asset and we deem this risk asMEDIUM. Management Risk ● The management at Xynomic are a key asset to the company and together have extensive expertise to manage the business. We consider this underlying management risk to be LOW. We note; however, that a certain level of challenge exists if the company were to establish a large sales force and if the company were to entry an M&A. Thus, we see this risk asLOW-MEDIUM. Intellectual Property (IP) Risk: ● The existing of patents related to XP-101 appears well covered with an expiration date of 2034 and 13 years of patent protection. We deem this risk asMEDIUM. Market Uptake Risk: ● Xynomic have a well-established plan with a reasonable timeline to develop a marketing strategy for XP-101 and XP-102. We see this activity on par with other drug development projects at a similar stage of development. We deem the market uptake risk asMEDIUM. Competitive Risk: ● There are a number of first, second and third line competitor products currently on the market. A competitive risk is also likely to come from novel products, of which many are in development. Given the current and future competition level, we deem this risk to beMEDIUM-HIGH. Pricing Risk: ● We consider the pricing strategy to be as per current therapeutics on the market. However some regulatory scrutiny may occur when summed with the cost of partner drugs. We note that there exists a risk associated with reimbursement listing. Thus, we deem the pricing risk to beMEDIUM-HIGH. |
© Copyright 2018 Venture Valuation. All Rights Reserved
30
| VALUATION |
| |
| OVERVIEW |
| |
| Company snapshot and future upside potential | We note that the present is a valuation of Xynomic’s current assets, and, as such it represents a snapshot of the company’s value today. We have considered the following value drivers for Xynomic: ● Abexinostat (XP-101) initially for the treatment of NHL and RCC; ● Abexinostat in follow-on oncology indications in solid tumors including breast cancer; ● XP-102 in oncology indications; ● Pipeline of in-house products including XP-103 and XP-104. An additional potential asset, XP-105 is discussed in Appendix VII. Market penetration rates are based on the Tufts Center for Drug Development uptake curve, adjusted by Venture Valuation. For a full list of assumptions used please see Appendices I, II, III, VI. |
| Methods used | To provide a balanced assessment of the value of Xynomic the following valuation methods were used: ● The sum of parts, including: o the risk-adjusted net present value (rNPV) method; o the discounted cash flow (DCF) method; ● The market comparable method; ● The comparable transactions method. |
| Sum of Parts | In the sum of parts, the value of the products determined with the rNPV method was summed to the net present value of potential revenues associated with Xynomic’s pipeline calculated with the DCF method to result in the final net present value of Xynomic. |
| rNPV/Risk-Adjusted NPV | The most appropriate method to value pharmaceutical products in development is the risk-adjusted net present value (rNPV). This method factors in the success rates of therapeutic products in pharmaceutical development and the probability of failure is then used to discount the yearly free cash flows over the entire life cycle of the product. The rNPV calculation, as well as key assumptions for Xynomic’s lead product in development, are described in the sections below and in Appendices I-III. The analysis takes into account a likely licensing scenario and the generation of future cash flows from potential upfront, milestone and royalty payments as a result of a deal with a pharmaceutical or a specialty pharmaceutical company. This method was used to value the development candidates, Abexinostat in RCC (worldwide) and NHL (FL worldwide, DLBCL worldwide, MCL in US and Europe). |
| Discounted Cash Flow (DCF) | The discounted cash Flow method uses Xynomic’s future positive and negative free cash flow projections discounted to the present value. The DCF valuation does not consider the lead programs that were valued separately using the rNPV method. |
| Market Comparables | The market comparable method utilizes data from public, peer-group companies to determine multiples which are used for calculating the value of Xynomic. Multiples are determined for key variables such as the ratio of number of employees to market capitalization and R&D investment to market capitalization. |
| Comparable Transactions | In the comparable transactions method the value of peer-group companies (determined following an investment or acquisition) is used to determine the value of Xynomic. Multiples are determined for key variables such as the ratio of number of employees to market capitalization and the ratio of raised capital to market capitalization. |
© Copyright 2018 Venture Valuation. All Rights Reserved
31
| DISCOUNT RATE |
| |
| Systematic risk at 5% | To calculate the discount rate used in the DCF method, a risk- free rate of return of 2.9% was used as a basis. The systematic risk was then determined using the market risk premium, which averages around 3.6%, and the beta, which measures the sensitivity of the company to the stock market. Using the betas of the comparable companies also used in the market comparables method, a beta of 1.3 is calculated for Xynomic. The systematic risk is subsequently calculated to be 4.5%. |
| Liquidity premium of 11%* | A liquidity premium is applied because, in contrast to publicly listed companies, it would be more difficult and would require more time to sell the equity of Xynomic at this stage. This premium was determined to be 11% to take into account that Xynomic is a private company. |
| Value added premium of 1%* | In this case a value added premium of 0.8% is applied. It is expected that a new investor would add value through their reputation, industry contacts and experience. |
| Cash flow adjustment of 15%* | The last premium added is the cash flow adjustment. Since only one scenario is calculated, we use this premium to take into account the risk of failure. On the one hand, Xynomic is based on established R&D programs. On the other hand, the company is still at an early stage with a pipeline consisting of early stage products in development. Therefore a cash flow adjustment premium of 14.6% is applied. |
| Applied discount rate of 34% | Summing up the premium surcharges results in a discount rate of 34%, which is used as the mean in further calculations. |
*Rounded number.
| | Discount rate |
| | Risk free rate of return | | | 2.9 | % |
| | Systematic risk | | | 4.5 | % |
| | Liquidity premium | | | 11.0 | % |
| | Value added premium* | | | 1.0 | % |
| | Cash flow adjustment premium* | | | 15.0 | % |
| | Discount rate* | | | 34.0 | % |
| | *Rounded number. | | | | |
© Copyright 2018 Venture Valuation. All Rights Reserved
32
| rNPV/RISK-ADJUSTED NPV |
| |
| Calculation based on business plan | The rNPV calculation is based on figures provided by the management of Xynomic, adjusted by Venture Valuation. The calculated value of Abexinostat considers a licensing scenario, where Xynomic develops and markets the product worldwide. Considering that the product is in late stage of development, and that clinical trials protocols have already been agreed with the regulatory authority, we consider the risk of the development plan reduced. There remains however the risk of establishing marketing operations. A median discount rate of 22%, and a range from 18% to 26% for sensitivity analysis were used. The next section describes the breakdown of each of the calculation components. |
| Deal Value Split | The total value of a project in the event of a divestiture or out license is shared between the licensor and the licensee. For the current valuation we have assumed the licensing terms outlined previously (see “Details of License Payments to AbbVie: XP-101” in the “Intellectual Property” section above). |
| Sales forecast | The sales curve for Abexinostat, in the treatment of RCC and NHL (FL, DLBCL), has been forecasted using the Tufts Center for Drug Development uptake curve as a baseline. Other key assumptions are described in Appendices I-III |
| Sales forecast for Abexinostat, in the treatment of RCC , FL and DLBCL(USD 000s) |
| | 
|
| ABEXINOSTAT FOR THE TREATMENT OF ADVANCED RCC |
| |
| Probability of success | Taking into consideration the stage of development and the cumulative success rate of Abexinostat for the treatment of advanced RCC, the probability of product launch has been determined to be 70%. |
© Copyright 2018 Venture Valuation. All Rights Reserved
33
| | | Success rate |
| | | Pivotal Phase III | Approval |
| | Probability of Success | 70% | 100% |
| | Cumulative POS | | 70% |
| Product valuation for RCC (Xynomic share) between USD 60.4m and USD 132.3m | The share of the value of Abexinostat to Xynomic is between USD 140.5m and USD 307.7m. Using a discount rate of 22%, the average value is USD 205.8m. |
| | Product Valuation | Discount rate |
| | in USD 000’s | 26% | 24% | 22% | 20% | 18% |
| | rNPV Total product value | 200,966 | 242,675 | 294,369 | 358,875 | 439,949 |
| | rNPV licensor (AbbVie) | 60,432 | 72,975 | 88,520 | 107,917 | 132,297 |
| | rNPV licensee (Xynomic) | 140,534 | 169,700 | 205,849 | 250,958 | 307,652 |
| ABEXINOSTAT FOR THE TREATMENT OF FL |
| |
| Probability of success | Taking into consideration the stage of development and the cumulative success rate of Abexinostat for the treatment of FL, the probability of product launch has been determined to be 57%. |
| | | Success rate |
| | | Pivotal Phase II | Approval |
| | Probability of Success | 60% | 95% |
| | Cumulative POS | | 57% |
| Product valuation for FL (Xynomic share) between USD 59.8m and USD 132.5m | The share of the value of Abexinostat to Xynomic is between USD 59.8m and USD 132.5m. Using a discount rate of 22%, the average value is USD 88.3m. |
| | Product Valuation | Discount rate |
| | in USD 000’s | 26% | 24% | 22% | 20% | 18% |
| | rNPV Total product value | 89,660 | 108,777 | 132,441 | 161,931 | 198,944 |
| | rNPV licensor (AbbVie) | 29,829 | 36,244 | 44,177 | 54,053 | 66,434 |
| | rNPV licensee (Xynomic) | 59,831 | 72,533 | 88,264 | 107,878 | 132,510 |
© Copyright 2018 Venture Valuation. All Rights Reserved
34
| ABEXINOSTAT FOR THE TREATMENT OF DLBCL |
| |
| Probability of success | Taking into consideration the stage of development and the cumulative success rate of Abexinostat for the treatment of DLBCL in the US and Europe, the probability of product launch has been determined to be 15%. In China, where only a Pivotal Phase II is planned in order to obtain approval for Abexinostat for the treatment of DLBCL, the probability of product launch has been determined to be 37%. |
| | US, Europe | Success rate |
| | | Phase I/II | Pivotal Ph III | Approval |
| | Probability of Success | 40% | 44% | 83% |
| | Cumulative POS | | 18% | 15% |
| | China | Success rate |
| | | Pivotal Phase II | Approval |
| | Probability of Success | 44% | 83% |
| | Cumulative POS | | 37% |
| Product valuation for DLBCL (Xynomic share) between USD 18.1m and USD 45.1m | The share of the value of Abexinostat to Xynomic is between USD 18.1m and USD 45.1m. Using a discount rate of 22%, the average value is USD 28.3m. |
| | Product Valuation | Discount rate |
| | in USD 000’s | 26% | 24% | 22% | 20% | 18% |
| | rNPV Total product value | 25,406 | 31,731 | 39,760 | 50,014 | 63,195 |
| | rNPV licensor (AbbVie) | 7,322 | 9,128 | 11,417 | 14,337 | 18,087 |
| | rNPV licensee (Xynomic) | 18,084 | 22,604 | 28,343 | 35,677 | 45,108 |
| DISCOUNTED CASH FLOW |
| |
| Calculation based on business plan | The DCF calculation is based on projected upfront payments and future milestones for Xynomic’s development pipeline as well as its industrial biotech business resulting in the following free cash flows in the first five years excluding any cash flows associated with Abexinostat targeting RCC, DLBCL/MCL and FL (valued separately in the product valuations). |
| | USD 000’s | Year |
| | | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 |
| | Cash Flow | -5,308 | -7,456 | -8,544 | -10,373 | -12,616 | -15,374 | -18,778 | -13,828 | 94 | 22,709 |
© Copyright 2018 Venture Valuation. All Rights Reserved
35
| A continuing value is calculated | For the period after 2027, a growth rate (g1) of 20% has been determined until 2032. Afterwards a growth rate (g2) of 5% was used in calculations, representing the convergence of company growth with expected long-term increases in the market. The normalized free cash flow is USD 109m, which leads to a continuing value of USD 576m. |
| | DCF |
| | in USD 000’s | norm. FCF | g1 | g2 | continuing value |
| | Scenario BP | 109,000 | 20% | 5% | 576,012 |
| DCF-range between USD 3m and USD 18m | Next, these figures were discounted to the present value. The base discount rate of 33.8%, results in a value of around USD 9.5m. Depending on the discount rate used, the DCF value ranges between USD 3.4m and USD 17.7m. |
| | DCF value | Discount rate |
| | in USD 000’s | 36% | 35% | 34% | 33% | 32% |
| | Scenario BP | 3,426 | 6,252 | 9,524 | 13,316 | 17,718 |
| SUM OF PARTS VALUATION |
| |
| Abexinostat targeting RCC, Abexinostat targeting DLBCL/MCL and Abexinostat targeting FL and the R&D Pipeline | The company value is determined by summing the value of Abexinostat targeting RCC, Abexinostat targeting DLBCL/MCL , Abexinostat targeting FL and the R&D Pipeline. The sum of parts valuation of Xynomic Pharma is approximately USD 351m. |
| | Sum of parts | |
| | in USD ’000 | Low | > | Average | > | High |
| | Net cash position | 19,500 | 19,500 | 19,500 | 19,500 | 19,500 |
| | Abex targeting RCC | 140,534 | 169,700 | 205,849 | 250,958 | 307,652 |
| | Abex targeting DLBCL/MCL | 18,084 | 22,604 | 28,343 | 35,677 | 45,108 |
| | Abex targeting FL | 59,831 | 72,533 | 88,264 | 107,878 | 132,510 |
| | R&D Pipeline | 3,426 | 6,252 | 9,524 | 13,316 | 17,718 |
| | Total value* | 241,375 | 290,589 | 351,480 | 427,329 | 522,487 |
| | *Discount rate for the calculation of the product valuation ranges from 18% to 26% and the used discount rate for the R&D pipeline ranges from 32%-36%. |
© Copyright 2018 Venture Valuation. All Rights Reserved
36
| MARKET COMPARABLES |
| |
| Quoted competitors used | To calculate a market comparable value for Xynomic Pharma, the quoted comparable companies outlined in the following table were used. The selected companies are not necessarily direct competitors; however, they are viewed as peer group companies as some of their activities and operational variables match Xynomic’s business. |
| | in USD ’000s | Market Cap. (P) | Employees | R&D |
| | 3SBio | 6,330,509 | 4,051 | 35,478 |
| | Aslan | 230,229 | 38 | 30,445 |
| | Hutchison China MediTech | 1,981,851 | 560 | 75,523 |
| | Zai Lab | 1,028,205 | 88 | 39,341 |
| | BeiGene | 9,852,910 | 900 | 269,018 |
| | Average | 3,347,996 | 945 | 89,789 |
| | With this information available, comparable ratios were calculated, as shown in the following table. |
| | Market comparable ratios |
| | | P/Empl. | P/R&D |
| | 3SBio | 1,563 | 178 |
| | Aslan | 6,059 | 8 |
| | Hutchison China MediTech | 3,539 | 26 |
| | Zai Lab | 11,684 | 26 |
| | BeiGene | 10,948 | 37 |
| | Low1 | 7,906 | 41 |
| | Average | 9,092 | 47 |
| | High1 | 10,456 | 54 |
| | | | |
| | 1To determine the high and low value for the ratios, a +/- 15% spread from the average value was used to eliminate outliers. |
| | Using the above ratios, a value range of USD 279.4m to USD 369.5m can be estimated for Xynomic Pharma. When calculated using the market comparable method the average value is around USD 321.3m. |
| | Market comparable valuation |
| | | Xynomic | Ratio | in USD 000’s |
| | | | Low | Ave. | High | Low | Ave. | High |
| | Employees | 65 | 7,906 | 9,092 | 10,456 | 513,884 | 590,967 | 679,612 |
| | R&D | 6,646 | 41 | 47 | 54 | 272,053 | 312,861 | 359,791 |
| | Average (without liquidity adjustment) | 313,910 | 360,996 | 415,146 |
| | Less liquidity adjustment¹ (11%) | -34,530 | -39,710 | -45,666 |
| | Adjusted average | 279,380 | 321,287 | 369,480 |
| | 1The liquidity premium takes into consideration that Xynomic is still a private company and that it would be more difficult and require more time to sell its equity at this stage. |
© Copyright 2018 Venture Valuation. All Rights Reserved
37
| COMPARABLE TRANSACTIONS |
| |
| IPO and VC transactions used | To calculate a comparable transaction value for Xynomic the investments and IPOs outlined below were used. The selected transactions include companies that are not necessarily direct competitors, however, the companies are viewed as peer group companies as some of their activities, and operational variables match Xynomic Pharma’s activities. |
| Comparable transactions |
| | Type | Date | Index Acqui.1 | Value in USD (pre-money) | Empl. | Raised | |
| BeyondSpring | IPO | 02/2017 | 2,980 | 536.7m | 31 | n.a. | |
| Athenex | IPO | 06/2017 | 3,099 | 629.2m | n.a. | USD 137.6m | |
| Kura Oncology | 2PO, post reverse merger | 11/2015 | 3,467 | 125.8m | 25 | USD 64.8m | |
| Syndax Pharm. | Last VC round before IPO | 02/2014 | 2,676 | 114m | n.a. | 83.4m | |
| G1 Therapeutics | Last VC round before IPO | 07/2016 | 2,748 | 207.2m | 26 | 45.7m | |
| Zymeworks | Last VC round before IPO | 01/2016 | 3,160 | 102.5m | n.a. | 86.7m | |
| Jounce Ther. | Last VC round before IPO | 07/2016 | 2,822 | 397.7m | n.a. | 101m | |
| AnaptysBio | Last VC round before IPO | 07/2015 | 3,969 | 72.9m | n.a. | 38.4m | |
| AC Immune | Last VC round before IPO | 05/2016 | 2,739 | 155.3m | n.a. | 69.8m | |
| Syros Pharm. | Last VC round before IPO | 01/2016 | 3,097 | 172.7m | n.a. | 75.3m | |
| Merus N.V. | Last VC round before IPO | 08/2015 | 3,582 | 69.2m | n.a. | 57.7m | |
| Aeglea BioTher. | Last VC round before IPO | 03/2015 | 3,752 | 35.5m | n.a. | 10.3m | |
| Corvus Pharm. | Last VC round before IPO | 09/2015 | 3,419 | 162.7m | n.a. | 31.5m | |
| BeiGene | Last VC round before IPO | 05/2015 | 2,727 | 756.8m | n.a. | 68.1m | |
| CytomX Ther. | Last VC round before IPO | 06/2015 | 3,221 | 363.3m | 55 | 64.8m | |
| Mirna Ther. | Last VC round before IPO | 03/2015 | 3,169 | 105.3m | n.a. | 44.3m | |
| NantKwest | Last VC round before IPO | 07/2015 | 3,912.6 | 1561.3m | n.a. | 52.6m | |
| Sierra Oncology / ProNAi Ther. | Last VC round before IPO | 04/2014 | 4,086.5 | 297.6m | n.a. | 36.5m | |
| Adaptimmune Therapeutics | Last VC round before IPO | 09/2014 | 3,539.6 | 984.8m | n.a. | 3.4m | |
| Aduro BioTech | Last VC round before IPO | 03/2015 | 3,738.9 | 815.2m | n.a. | 132.1m | |
| Juno Therapeutics | Last VC round before IPO | 08/2014 | 3,280.1 | 1694m | n.a. | 153.5m | |
| Bellicum Pharm. | Last VC round before IPO | 09/2014 | 3,174.6 | 381.7m | 30 | 38.6m | |
| Neothetics | Last VC round before IPO | 09/2014 | 3,077.2 | 140.8m | n.a. | 53.8m | |
| Atara Biother. | Last VC round before IPO | 01/2014 | 2,683.4 | 203.8m | n.a. | 18m | |
| Calithera Biosc. | Last VC round before IPO | 07/2014 | 2,819.8 | 120.9m | n.a. | 55.6m | |
| VBL Ther./ Vascular Biogenics | Last VC round before IPO | 01/1900 | 2,692.6 | 101m | n.a. | n.a. | |
| Immune Design | Last VC round before IPO | 10/2013 | 2,709.9 | 164.6m | 20 | 40.5m | |
| Kite Pharma | Last VC round before IPO | 04/2014 | 2,617.9 | 658.2m | n.a. | 31.3m | |
| Argos Ther. | Last VC round before IPO | 11/2013 | 2,475.0 | 149.8m | 90 | 161.4m | |
1.Change of NASDAQ Biotech Index between investment date and June 2018, thus value is index corrected.
© Copyright 2018 Venture Valuation. All Rights Reserved
38
| | Using the comparable transaction method, the following ratios were calculated for the selected comparable transactions. |
| | Comparable transactions valuation |
| | USD 000 | Xynomic | Ratio* | value in USD '000 |
| | low | mean | high | low | mean | high |
| | Employ. | 45 | 7,396 | 8,505 | 9,781 | 332,813 | 382,735 | 440,146 |
| | Raised | 21,800 | 14.5 | 16.7 | 19.2 | 317,101 | 364,667 | 419,367 |
| | Average | | | | | 324,957 | 373,701 | 429,756 |
| Comparable transaction range from USD 325m to USD 430m | When the above ratios of the number of employees and raised capital are used, a value range for Xynomic Pharma can be calculated with the comparable transaction method. The value ranges from approximately USD 325m to USD 429.8m, with an average of USD 373.7m. |
| SUMMARY |
| |
| Value between USD 282m and USD 441m | The pre-money value range for Xynomic Pharma was calculated using a combination of four valuation approaches. This results in a pre-money value range of USD 281.9m to USD 440.6m. The average pre-money value is USD 348.8m when calculated with a discount rate of 34%, using the Sum of Parts method, the Market Comparables method, and the Comparable Transactions method. These calculations are based primarily on XP-101 and XP-102 assets. Additional value comes from the XP-105 asset, which is discussed in the Appendix VII. |
| | Value range | value spectrum |
| | in USD 000’s | Weighting | Low | > | Average | > | High |
| | Sum of Parts1 | 33% | 241,375 | 290,589 | 351,480 | 427,329 | 522,487 |
| | Market Comp. | 33% | 279,380 | 300,333 | 321,287 | 345,383 | 369,480 |
| | Comp. Trans. | 33% | 324,957 | 349,329 | 373,701 | 401,729 | 429,756 |
| | Average | | 281,904 | 313,417 | 348,823 | 391,480 | 440,574 |
| | 1Discount rate for the calculation of the rNPV (part of Sum of Parts) ranges from 18% to 26%. |
© Copyright 2018 Venture Valuation. All Rights Reserved
39
| | Overview of the value range |
| | in USDm 
|
| Copyright © 2018 Venture Valuation VV Ltd. All rights reserved. This report is provided for information purposes only. Under no circumstances is it to be used or considered as an offer, solicitation, or recommendation to sell, or a solicitation of any offer to or buy any security. While the information contained herein has been obtained from sources deemed reliable, Venture Valuation VV Ltd (i) makes no guarantees that it is accurate, complete, timely, or contains the correct sequencing of the information, or (ii) make any warranties with regard to the results to be obtained from its use, and (iii) shall have no liability for any claims, losses or damages arising from or occasioned by any inaccuracy, error, delay, or omission, or from use of the report or actions taken in reliance on the information contained in the report. Reproduction or redistribution of this report in any form is prohibited except with written permission. The providers of this report make it explicitly public in the report if a position in the securities discussed herein is held. |
© Copyright 2018 Venture Valuation. All Rights Reserved
40
| REFERENCES |
| | Meeting and Teleconference: ● Telephonic communication: Bison Capital Acquisition Corp., Xynomic Pharmaceuticals, Inc., 29 May 2018; 13 June 2018; 17 June 2018 ● Meeting, London UK: Xynomic Pharmaceuticals, Inc., 31 May 2018 ● Telephonic communication: Bison Capital Acquisition Corp., Early Bird Capital, Hunter Taubman Fischer & Li, Ogier, Marcum, Cassel Salpeter & Co., Xynomic Pharmaceuticals, Inc., Sidley Austin, KPMG. 04 Jun 2018 Company Information (Documents provided/considered): ● Business plan/Company presentation ● Patent information ● Market information ● R&D development plans ● Financials projection - outline Databases: ● Internal Company, Product and Deal database: Biotechgate, biotechgate.com ● External proprietary databases Reports: ● Annual reports for market comparable companies ● Lymphoma Research Foundation DLBCL FactSheet. May 2018 https://www.lymphoma.org/aboutlymphoma/nhl/dlbcl/ ● Lymphoma Research Foundation FL FactSheet. Feb 2018 https://www.lymphoma.org/aboutlymphoma/nhl/fl/ ● Lymphoma Research Foundation MCL FactSheet. Jun 2016 https://www.lymphoma.org/aboutlymphoma/nhl/fl/ ● GlobalData Jan 2016. OpportunityAnalyzer: B-Cell Non-Hodgkin’s Lymphoma (NHL) – Opportunity Analysis and Forecasts to 2024 ● Pfizer 2012 RCC Factsheet ● Scrip_3846 24 Mar 17 ● Pharmacy Prior Authorization Form PriorityHealth ● Breast cancer facts and figures American Cancer Society 2015-2016 |
© Copyright 2018 Venture Valuation. All Rights Reserved
41
| | Articles/Books: ● Cassidy S. and B.A. Syed. (2017) Colorectal cancer drugs market. Nature Reviews Drug Discovery. 16:525–526. ● Frei P. (2006) Assessment and Valuation of high growth companies, Haupt Verlag. ● Hay M et.al (2014) Clinical development success rates for investigational drugs, Nature Biotechnology, 32, 40–51. ● Mistry, R. et al. (2018) Cost-Effectiveness of Ribociclib plus Letrozole Versus Palbociclib plus Letrozole and Letrozole Monotherapy in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: A U.S. Payer Perspective. J Manag Care Spec Pharm, 24(6):514-523. “ ● Rovira J, Valera A, Colomo L, et al. (2015) Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Annals of Hematology. 94(5):803-812. ● Frei, P. and Peire, A (2016) What is the value of a deal? BioPharma Dealmakers, Nature Publishing Group, B27-28. ● Zacharakis M, et al. (2010) Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Research February. 30 (2): 653-660. URLs: ● www.nasdaq.com ● www.finance.yahoo.com ● www.oanda.com ● www.sec.gov ● www.ncbi.nlm.nih.gov/ ● www.drugs.com · www.clinicaltrials.gov ● www.chemoexperts.com ● www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm ● www.hematologyandoncology.net/archives/april-2017/the-use-of-car-t-cells-in-diffuse-large-b-cell-lymphoma-and-mantle-cell-lymphoma/ ● www.reuters.com/article/us-gilead-sciences-fda/fda-approves-gilead-cancer-gene-therapy-price-set-at-373000-idUSKBN1CN35H ● https://arstechnica.com/science/2018/04/doctors-tried-to-lower-148k-cancer-drug-cost-makers-triple-price-of-pill/ ● www.grandviewresearch.com/industry-analysis/kidney-cancer-drugs-market ● www.novartis.com/investors/financial-data/product-sales ● www.drugs.com/condition/breast-cancer.html?category_id=12116&include_rx=true&submitted=true ● www.healthline.com/health/rcc/stage-4-renal-cell-carcinoma ● https://emedicine.medscape.com/article/1690010-overview ● www.healthline.com/health/breast-cancer/understanding-managing/understanding-hr-breast-cancer-diagnosis#3 ● www.pmlive.com/pharma_news/novartis_bags_new_breakthrough_status_for_kisqali_in_breast_cancer_1215110 pmlive 03 Jan 2018 ● https://seekingalpha.com/article/4055345-kisqali-battles-ibrance-safety-cost ● www.cancer.net/cancer-types/colorectal-cancer/treatment-options |
© Copyright 2018 Venture Valuation. All Rights Reserved
42
| APPENDIX I: ABEXINOSTAT FOR THE TREATMENT OF RCC VALUATION ASSUMPTIONS |
| Criteria | Value | Reference |
| Patent expiration | 2033 | VV assumption/Xynomic |
| | | |
| Patient population | Advanced RCC patients, in combination with pazopanib | Xynomic |
| | | |
| | US | Europe | RoW | |
| Price in USD | | | n.a. | Xynomic’s assumption; Europe price estimated at 80% of US price |
| | | | | |
| Prevalence rate (%) | 0.03% | 0.04% | | Based on 3-year prevalence of kidney cancer, of which 90% is RCC.
Globocan; healthline.com |
| Advanced, Stage IV (%) | 30% | | | Based on pazopanib patient population FDA; Pfizer |
| Population that receives a prescription (%) | 100% benchmark | 55% | | Adjustment to account for sales in Europe vs US tipically at 45:55.
VV assumption |
| Market share (%) | 12% | | | Half Votrient’s estimated share of 24%.
VV assumption |
| Compliance rate (%) | 80% | | | High compliance for treatments supervised by specialists.
VV assumption |
| CAGR (%) | 2% | | | To account for expansion of use also to earlier treatment lines, driven by post-marketing studies.
VV assumption |
| Peak sales (USDm) | 631 | 434 | 106 | RoW sales estimated as 10% of total. Model estimate |
| Total sales (2022-2039) | USD 15.4bn | Model estimate |
| Key expenses for the product valuation |
| Expense | Value | Reference |
| Clinical & Manufacturing expenses (USDm) | Pivotal Phase III (45 months): 40
Approval (6 months): 1.5 | Xynomic communication/ VV assumption; duration of regulatory approval based on RCC being line extension |
| | | |
| Launch date | US & Europe: Q4 2022
RoW: Q4 2024 | Xynomic/Venture Valuation estimate |
| | | |
| COGS (% of revenue) | 5% | Venture Valuation estimate |
| | | |
Sales & Marketing
(% of revenue) | 25% | Venture Valuation estimate |
Discounts, Returns and Allowances
(% of revenue) | 5% | Venture Valuation estimate |
| | | |
| G&A rate (% of revenue) | 5% | Venture Valuation estimate |
| | | |
| Tax rate | 23.5% | OECD average |
© Copyright 2018 Venture Valuation. All Rights Reserved
43
| APPENDIX II: ABEXINOSTAT FOR THE TREATMENT OF FL VALUATION ASSUMPTIONS |
| Criteria | Value | Reference |
| Patent expiration | 2033 | VV assumption/Xynomic |
| Patient population | FL patients, 2nd or 3rd line of treatment, after rituximab monotherapy | Xynomic |
| | US | Europe | China | |
| Price in USD | | | Xynomic’s assumption |
| Prevalence rate (%) | 0.004% | 0.002% | 0.0004% | Based on 3-year prevalence of NHL; B-cell: 92% of NHL; FL 12%.
Globocan; Lymphoma Research Foundation |
| Relapsing or refractory (%) | 60% | Including non-respondents; 5 years OS-PFS; relapse from CR .
Rovira J, Valera A, Colomo L, et al. 2015 |
| | | |
| Population that receives a prescription (%) | 100% benchmark | 75% | 15% | In Europe, based on reported sales ratio by companies; in China, based on population eligible for and that can afford expensive treatments.
VV assumption |
| Market share (%) | 30% | 30% | 60% | In China higher market share driven by earlier treatment line.
VV assumption |
| Compliance rate (%) | 80% | High compliance for treatments supervised by specialists.
VV assumption |
| CAGR (%) | 2% | Driven by post-marketing studies and use in earlier treatment lines. VV assumption |
| Peak sales (USDm) | 394 | 192 | 33
(RoW 29) | VV estimate. Sales in RoW estimated as 5% of US+Europe sales |
| Total sales (2021-2039) | USD 8.5bn | Model estimate |
| Key expenses for the product valuation |
| Expense | Value | Reference |
| Clinical & Manufacturing expenses (USDm) | US & Europe | Xynomic communication/ VV assumption |
| | Pivotal Phase II (30 months): 18 | |
| | Approval (12 months): 1.5 | |
| | | |
| | China | |
| | Pivotal Phase II (18 months): 3 | |
| | Approval (12 months): 1 | |
| | | |
| Launch date | US & Europe: Q1 2022 | Xynomic/Venture Valuation estimate |
| | China: Q3 2021 | |
| | RoW: Q1 2024 | |
| | | |
| COGS (% of revenue) | 5% | Venture Valuation estimate |
| | | |
Sales & Marketing
(% of revenue) | 25% | Venture Valuation estimate |
| | | |
Discounts, Returns and Allowances
(% of revenue) | 5% | Venture Valuation estimate |
| | | |
| G&A rate (% of revenue) | 5% | Venture Valuation estimate |
| | | |
| Tax rate | 23.5% | OECD average |
© Copyright 2018 Venture Valuation. All Rights Reserved
44
| APPENDIX III: ABEX FOR THE TREATMENT OF DLBCL/MCL VALUATION ASSUMPTIONS |
| Criteria | Value | Reference |
| Patent expiration | 2033 | VV assumption/Xynomic |
| | | |
| Patient population | relapsing or refractory DLBCL (worldwide) and MCL (US, Europe) | Xynomic |
| | | |
| | US | Europe | China | |
| Price in USD | | | | Xynomic’s assumption based on the cost of combination treatment with Imbruvica in the US and Europe |
| Prevalence rate | DLBCL | 0.008% | 0.005% | 0.001% | Based on 3-year prevalence of NHL; B-cell: 92% of NHL; DLBCL 22%; MC 5%. Globocan; Lymphoma Research Foundation |
| (%) | MCL | 0.001% | 0.002% | n.a. |
| Relapsing or refractory (%) | 60% | Including non-respondents; 5 years OS-PFS; relapse from CR. Rovira J, Valera A, Colomo L, et al. 2015 |
| Population that receives a prescription (%) | 100% benchmark | 75% | 15% | In Europe, based on reported sales ratio by companies; in China, based on population eligible for and that can afford expensive treatments.
VV assumption |
| Market share | DLBCL | 30% | 30% | 60% | In China higher market share driven by earlier treatment line.
VV assumption |
| (%) | MCL | 60% | 60% | n.a. |
| Compliance rate (%) | 80% | High compliance for treatments supervised by specialists. VV assumption |
| | | |
| CAGR (%) | 2% | Driven by post-marketing studies and use in earlier treatment lines
VV assumption |
| Peak sales (USDm) | 704 | 340 | 60
(RoW 50) | VV estimate |
| Total sales (2021-2042) | USD 13.9bn | Model estimate |
| Key expenses for the product valuation |
| Expense | Value | Reference |
| Clinical & Manufacturing expenses (USDm) | US & Europe Phase I/II (24 months): 1 Pivotal Phase III (48 months): 20 Approval (6 months): 1.5 China Pivotal Phase II (18 months): 1.5 Approval (12 months): 1 | Xynomic communication/ VV assumption; duration of regulatory approval in US & Europe based on DLBCL/MCL being line extensions |
| Launch date | US & Europe: Q1 2025 China: Q3 2021 RoW: Q1 2027 | Xynomic/Venture Valuation estimate |
| COGS (% of revenue) | 5% | Venture Valuation estimate |
Sales & Marketing (% of revenue) | 25% | Venture Valuation estimate |
Discounts, Returns and Allowances (% of revenue) | 5% | Venture Valuation estimate |
G&A rate (% of revenue) | 5% | Venture Valuation estimate |
| Tax rate | 23.5% | OECD average |
© Copyright 2018 Venture Valuation. All Rights Reserved
45
| APPENDIX IV: COMPARABLE TRANSACTIONS - COMPANIES |
| Company | Country | Website | Year founded | Ownership |
| BeyondSpring | BeyondSpring Pharmaceuticals Inc. is a global clinical-stage biopharmaceutical company. The company is focused on the development of cancer therapies. |
| USA | www.beyondspringpharma.com | 2013 | Public |
| Athenex | Athenex is an oncology pharmaceutical company focused on the development and commercialization of therapies for cancer diseases. Athenex is focused on generating clinical candidates focused on oral delivery, and currently have seven products including two currently in Phase III studies. |
| USA | http://www.athenex.com/ | 2003 | Public |
| Kura Oncology | Developer of precision drugs for the treatment of solid tumors and blood cancers designed to realize the promise of precision medicines for cancer. The company’s precision drugs for cancer consists of small molecules that target cancer signaling pathways where there is a strong scientific and clinical rationale to improve outcomes by identifying those patients most likely to benefit from treatment, providing them with treatments that are safer and more effective for patients with particular cancers. |
| USA | www.kuraoncology.com | 2014 | Public |
| | | | | | |
| APPENDIX V: MARKET COMPARABLES - COMPANIES |
| Company | Country | Website | Year founded | Ticker |
| 3SBio | 3SBio Inc. is a biotechnology company focused on researching, developing, manufacturing and marketing biopharmaceutical products primarily in China. |
| China | www.3sbio.com | 1993 | 1530.hk |
| Aslan | ASLAN Pharmaceuticals is an oncology-focused biotechnology company developing a portfolio of immuno-oncology agents and targeted therapies. ASLAN’s portfolio is comprised of four product candidates which target validated growth pathways applied to new patient segments, novel immune checkpoints and novel cancer metabolic pathways. |
| Singapore | www.aslanpharma.com | 2010 | 6497.TWO |
| Hutchison China MediTech | Hutchison China MediTech, known as Chi-Med, is an innovative biopharmaceutical company which researches, develops, manufactures and sells pharmaceuticals and healthcare products. Its Innovation Platform, Hutchison MediPharma, focuses on discovering and developing innovative therapeutics in oncology and autoimmune diseases for the global market. Its Commercial Platform manufactures, markets, and distributes prescription drugs and consumer health products in China. |
| Hong Kong | www.chi-med.com | 2000 | HCM |
| Zai Lab | Zai Lab is a biotech company based in China focused on discovering and developing innovative medicines for unmet medical needs globally. The company is building a strong portfolio of therapeutic programs aimed at transforming patients’ lives. Zai used in-licensings to become a clinical-stage company in short order. It has six candidates so far, including a clinical-stage PARP inhibitor, already approved for Phase III China trials in ovarian and breast cancer. The company has completed Phase II proof-of-concept trials on the PARP inhibitor and a novel antibiotic. |
| China | www.zailaboratory.com | 2013 | ZLAB |
| BeiGene | BeiGene is a clinical-stage biopharma company focusing on discovering and developing targeted and immuno-oncology drugs that address severe unmet medical needs in a variety of cancer indications. |
| China | www.beigene.com | 2010 | BGNE |
© Copyright 2018 Venture Valuation. All Rights Reserved
46
| APPENDIX VI: DISCOUNTED CASH FLOW FORECAST |
| Year | | 2018 | | | 2019 | | | 2020 | | | 2021 | | | 2022 | | | 2023 | | | 2024 | | | 2025 | | | 2026 | | | 2027 | |
| Pipeline Revenues | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 10,000 | | | | 30,000 | | | | 60,000 | |
| Total Revenues | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 10,000 | | | | 30,000 | | | | 60,000 | |
| R&D | | | 600 | | | | 1,100 | | | | 600 | | | | 840 | | | | 1,176 | | | | 1,646 | | | | 2,305 | | | | 3,227 | | | | 4,518 | | | | 6,325 | |
| G&A | | | 4,708 | | | | 6,356 | | | | 7,944 | | | | 9,533 | | | | 11,440 | | | | 13,728 | | | | 16,473 | | | | 19,768 | | | | 23,722 | | | | 28,466 | |
| Total Costs | | | 5,308 | | | | 7,456 | | | | 8,544 | | | | 10,373 | | | | 12,616 | | | | 15,374 | | | | 18,778 | | | | 22,995 | | | | 28,240 | | | | 34,791 | |
| EBITDA | | | -5,308 | | | | -7,456 | | | | -8,544 | | | | -10,373 | | | | -12,616 | | | | -15,374 | | | | -18,778 | | | | -12,995 | | | | 1,760 | | | | 25,209 | |
| FCF | | | -5,308 | | | | -7,456 | | | | -8,544 | | | | -10,373 | | | | -12,616 | | | | -15,374 | | | | -18,778 | | | | -13,828 | | | | 94 | | | | 22,709 | |
*R&D- and G&A expenses related to the lead product has been deducted from the Discounted Cash Flow Forecast.
| APPENDIX VII: VALUATION OF XP-105 |
| OVERVIEW |
| | Xynomic is in the process of in-licensing XP-105 (BI 860585) from Boehringer Ingelheim. This asset will be a significant part of Xynomic’s pipeline, and in particular represents a middle development stage compound that balances the late stage XP-101 and early stage XP-102 portfolio. |
| Product Value (Xynomic share) between USD 61.5m and USD 161.7m | The share of the value of XP-105 to Xynomic is between USD 61.5m and USD 161.7m. Using a discount rate of 22%, the average value is USD 99.3m. |
| Product Valuation | | Discount rate | |
| in USD 000’s | | | 26 | % | | | 24 | % | | | 22 | % | | | 20 | % | | | 18 | % |
| rNPV Total product value | | | 86,257 | | | | 109,751 | | | | 139,764 | | | | 178,318 | | | | 228,140 | |
| rNPV licensor (BI) | | | 24,708 | | | | 31,619 | | | | 40,444 | | | | 51,773 | | | | 66,403 | |
| rNPV licensee (Xynomic) | | | 61,550 | | | | 78,132 | | | | 99,320 | | | | 126,545 | | | | 161,737 | |
© Copyright 2018 Venture Valuation. All Rights Reserved
47
| Company Value – Xynomic, including XP-105 | Adding XP-105 to the existing pipeline (see body of this report), our calculations result is a pre-money equity value range of USD 302.4m to USD 494.5m, with an average value of USD 381.9m. |
| | | value spectrum | |
| in USD millions | | weighting | | | low | | | > | | | Average | | | > | | | high | |
| Sum of Parts Valuation | | | 33 | % | | | 302.9 | | | | 368.7 | | | | 450.8 | | | | 553.9 | | | | 684.2 | |
| Market Comparables | | | 33 | % | | | 279.4 | | | | 300.3 | | | | 321.3 | | | | 345.4 | | | | 369.5 | |
| Comp. Transactions | | | 33 | % | | | 325.0 | | | | 349.3 | | | | 373.7 | | | | 401.7 | | | | 429.8 | |
| Average | | | | | | | 302.4 | | | | 339.5 | | | | 381.9 | | | | 433.7 | | | | 494.5 | |
| PRODUCT – XP-105 (BI 860585) |
| Overview | XP-105 (BI 860585)is an orally bioavailable ATP-competitive inhibitor of mTOR kinase, specifically a dual inhibitor of mTORC1 and mTORC2 (mTORC1/2). The compound has high potency and inhibitory activity at low nanomolar concentration, with cellular activity comparable to other competitor mTORC1/2 inhibitors. XP-105 has anti-proliferative activity that is pronounced over more selective allosteric mTORC1 inhibitors. Boehringer Ingelheim note that XP-105 has over 100-fold selectivity toward mTORC1/2 compared to other kinases. Importantly, XP-105 has been tested in a number of preclinical tumor animal models, demonstrating efficacy across multiple cancer indications in these studies. These studies support the use of XP-105 in combination with established and novel therapies, as well as potential use of XP-105 possibly as a monotherapy in cases. Specifically, the XP-105 has been tested in animal models in combination with chemotherapies such as: doxil (ovarian cancer), cisplatin, etoposide, topotecan or paclitaxel (SCLC), letrozole or palbociclib (HR+ breast cancer), BI853520 (ovarian cancer) and BI882370 (colorectal cancer). Most importantly, XP-105 has completed a Phase I study that included a total of 83 patients with advanced or metastatic solid tumors. The study has demonstrated safety and efficacy, as described further below. |
| Pipeline | A summary of the product development and pipeline is provided in the table below. |
| Product Development and Pipeline |
| Indication | Develop. Stage | Start Date | Sites | Co-Therapy | Partner |
| Breast cancer | Ph III | 2019 | US, CN | Kisqali | Novartis1; PPD2 |
| | Ph III | 2021 | EU, JP | | |
| Colorectal cancer | Ph Ib1 | 2019 | US, China | XP-102 | PPD3 |
| | Ph III | 2020 | | | |
| | Ph III | 2022 | EU, JP | | |
1The specifics of a potential development partnership with Novartis are to be determined. However, Xynomic considers there may be possible cost-sharing with Novartis similar to that described for breast cancer development of Abex. For example, 50:50 split of clinical costs and full, free supply of Kisqali. 2Xynomics have engaged PPD for assistance in technology transfer of XP-105 and will likely engage PPD to support clinical development. Details of the PPD company can be found in the main body of the this report. |
© Copyright 2018 Venture Valuation. All Rights Reserved
48
| PRODUCT DEVELOPMENT |
| Preclinical Research and Development | XP-105 has demonstrated efficacy in a numbers of tumor animal models. The compound has shown efficacy in tumor cell line derived xenografts in immunodeficient mice of the following cancer models: breast, ovarian, endometrial, NSCLS, SCLC, CRC, gastric, leiomyosarcoma, Ewing’s sarcoma and Burkitt lymphoma. Studies also demonstrate efficacy in patient-derived tumor xenograft model of ovarian cancer. |
Clinical Studies | Below we provide an overview of the average number of patients and length of study for Ph I – III clinical trials of colorectal cancer and ER+/HER+ breast cancer is provided in the table below for comparative purposes. A summary of clinical studies planned by Xynomic for XP-105, as well as an estimated Gantt chart and timelines is also provided in the tables below. General information can be found in the main body of the text. |
| PRODUCTS IN CLINICAL STUDIES: CURRENTLY RECRUITING |
| Stage | Colorectal cancer | ER+, HER+ Breast cancer |
| | Patient
Numbers | Length of
Study | Number of
Studies | Patient
Numbers | Length of
Study | Number
of Studies |
| Phase I | 117 | 2.9 years | 79 | 92 | 2.7 years | 26 |
| Phase II | 97 | 3.1 years | 55 | 188 | 2.9 years | 26 |
| Phase III | 743 | 4.6 years | 20 | 470 | 2.6 years | 12 |
| Search filters of clinicaltrials.gov: Recruiting, Interventional, Funder Type Industry. (Updated, Sep 2018). Numbers listed are averages taken from the number of studies indicated. |
| Clinical Studies – Summary of Details |
| Indication | Treatment | Sites | Patients | Trial Type | Notes | Cost
(USD)* |
| Breast cancer (ER+ type) | +Kisqali, NVS | US, CN | 160 | Ph III | 4 years Ph III study (2019-2022) Approval (2023) We assume approximate costs of USD 100,000 per patient, with total costs of USD 16m in US and USD 3m in China, with a further 2.5m approval costs. | 21.5m |
| | | EU, JP | | Ph III | 4 years Ph III study (2021-2024) Approval (2025) We assume further Ph III clinical trial development costs of USD 24m total in territories of EU and Japan with similar USD 2.5m approval costs. | 26.5m |
© Copyright 2018 Venture Valuation. All Rights Reserved
49
Colorectal cancer (CRC) | +XP-102 | US, CN | 40 | Ph Ib Ph III | 1 years Ph Ib study (2019) 4 years Ph III study (2020-2023) Approval (2024) We assume a Ph1b study with a follow-on Ph III study (including 150 patients at an approximate cost of USD 100,000 per patient). We assume total costs of approximately USD 3m for Ph 1b and USD 17m for Ph III, with USD 3.5m approval costs. | 23.5m |
| | | EU, JP | | Ph III | 4 years study (2022-2025) Approval (2026) We assume further clinical trial Ph III development costs of approximately USD 20m total in territories of EU and Japan with additional USD 4m approval costs. | 24m |
| * all values rounded up to nearest 0.5m |
| Product Development – Estimated Gantt Chart & Timelines |
| Indication | Treatment | Sites | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 |
| ER+ Breast cancer | +Kisqali | US, China | Ph III | | | | NDA | Launch | | | |
| | | EU, Japan | | | Ph III | | | | NDA | Launch | |
| Colorectal cancer | +XP-102 | US, China | Ph Ib | Ph III | | | | NDA | Launch | | |
| | | EU, Japan | | | | Ph III | | | | NDA | Launch |
| PRODUCT PRICING |
| | General information on unit pricing and reimbursement is outlined in the main body of the text. For XP-105, Xynomic consider an annual price per patient in line with competitor and own products (see below “Valuation Assumptions”). |
| REGULATORY AFFAIRS |
| Current Knowledge on Safety | Supporting Evidence: ● XP-105 has been tested in a number of pre-clinical safety studies demonstrating an acceptable side effect profile in: genotoxicity, safety pharmacology, rat and dog toxicology, immunotoxicity and phototoxicity. Data investigating pharmacokinetic, liver enzyme metabolism (e.g. CYP interaction), transporter interaction (e.g. P-gp) and metabolic profiling also exists for XP-105. Required Data: ● We consider that the safety data for XP-105 is unlikely to constitute a limiting factor in its further development. |
© Copyright 2018 Venture Valuation. All Rights Reserved
50
| Evidence of Clinical Efficacy | XP-105: ● XP-105 has been tested in Phase I study. ● The study included a total of 83 patients with advanced or metastatic solid tumors and progressive disease within the last 6 months before study entry. The treatment cycle was 28-days and the study contained three arms as follows: o Arm A: XP-105 (5-300 mg/day) (41 patients). This study reported no grade 5 adverse events. Efficacy of the treatment on disease was reported in six patients up to 6-20 months. o Arm B: XP-105 (40-220 mg/day) + Exemestane (25 mg/day) (25 patients). This study reported no grade 4/5 adverse events. Efficacy was reported in six patients up to 4-11 months. o Arm C: XP-105 (80-160 mg/day) + paclitaxel (60-80mg/m2/wk) (17 patients). This study reported no grade 5 adverse events. Efficacy on disease was reported in five patients up to 4-12 months. ● Overall the study reported favorable and manageable safety profile, in line with the safety profile of mTORC1 inhibitors (e.g. everolimus, rapamycin). The adverse events were reported as mostly mild or moderate, and reversible. The PK analysis showed a dose proportional PK profile up to 220 mg, with no food effect or drug interaction with exemestane or paclitaxel. Biomarker analysis also provided evidence that tolerable doses (i.e. 120-220 mg) of XP-105 lead to inhibition of both mTORC1 and mTORC2 signaling. ● Importantly, the study showed efficacy with both monotherapy and combinations. Reponses were observed in patients with ER+ breast cancer, uterine carcinoma and bladder cancer. Long disease stabilizations were observed in various tumor types. |
| Regulatory Classification and Ongoing Discussions with Regulators | Discussion with regulatory affairs authorities regarding the development route of XP-105 are to be defined and may alter clinical trial design. For example purposes, we note that, for colorectal cancer, Xynomic consider a Phase Ib trial to be followed by a Phase II or Phase III study depending on discussion with regulatory agencies. On completion of Phase Ib the company expects to enter a pivotal Phase II if approved for an accelerated route or Phase III via a traditional pathway. This trial will be a combination (XP-105+XP-102) with Phase Ib determining MTD, safety and PK profile and the Phase II (single arm) or Phase III (vs placebo) to access the efficacy and safety in CRC patients with KRAS mutation. For the purpose of this valuation, we consider a Phase Ib followed by a Phase III clinical trial. |
| MANUFACTURING |
| | Xynomic have manufacturing data available from Boehringer for XP-105. We see no particular hurdles hampering the manufacturing process. |
© Copyright 2018 Venture Valuation. All Rights Reserved
51
| INTELLECTUAL PROPERTY (IP) |
| License Ownership | Xynomic is due to acquire rights to XP-105 (BI 860585) from Boehringer Ingelheim in Q4/2018. We note that PPD will assist in the technology transfer during in-licensing from Boehringer Ingelheim. Details of the royalties and conditions associated with this license transfer are listed below. |
| Details of License Payments to Boehringer: XP-105 |
| Development & Milestone Payments | USD (m) |
| Upfront Milestone Payment | 1.0 |
| Initiation of pivotal Ph III trial | 8.0 |
| First approval | 10.0 |
Royalty Payments | Royalty Rate (%) |
| Annual Net Sales (USD 0-50m) | |
| Annual Net Sales (USD 50-200m) | |
| Annual Net Sales (USD over 200m) | |
| Projected Expiration Dates | Current patents for XP-105 covering composition of matter expire in January 2031. Xynomic will aim to extend this date by development of and estimate a time of approximately years before needing to do so. We assume an expiration date of 2031, with an additional 3-year extension to 2034. |
| MARKET OVERVIEW |
| Disease Overview | A brief outline of XP-105 market environment is provided below: Breast cancer: ● Of all breast cancer patients, an estimated 80% have ER+ mutations. For the purpose of this valuation, we are assuming that 50% of patient population will be treated with Kisqali by 2025, as per Novartis estimating Kisqali having blockbuster growth. In 2017 sales for Kisqali was USD 26m compared to Ibrance of USD 643m. For a more in-depth description of this indication, see “Market Overview” on the body of this report. |
© Copyright 2018 Venture Valuation. All Rights Reserved
52
| | Colorectal cancer: |
| | ● Approximately 30-50% of colorectal cancers have a mutated KRAS, with up to 50% of patients responding to anti-epidermal growth factor receptor (EGFR) antibody therapy and 40-60% of patients with wild-type KRAS tumors being unresponsive. In these patients, the mutated BRAF gene is present in 5-10% of tumors and can affect response to these agents. A subset of CRC patients whose cancer over express the EGFR receptor on their cell surfaces will exhibit a favourable clinical response to anti-EGFR antibody therapy. Further, the presence of a mutated (abnormal) KRAS is associated with poor outcomes of anti-EGFR antibody treatment. Thus, patients with wild-type (normal) KRAS CRC may derive some benefit from anti-EGFR therapy, whereas patients with mutated (abnormal) KRAS CRC are unlikely to respond. Data suggests that mutated BRAF may also confer further resistance to anti-EGFR therapy. In patients with wild-type KRAS CRC, the anti-EGFR antibodies cetuximab (Erbitux) and panitumumab (Vectibix) have a favorable survival outcome, with Cetuximab plus FOLFOX (fluorouracil + leucovorin + oxaliplatin) or Cetuximab plus FOLFIRI (fluorouracil + leucovorin + irinotecan) improving outcomes, although not in patients with mutated BRAF. Overall, patients with mutated KRAS are unlikely to benefit from anti-EGFR antibody therapy. Data also suggests that none of the KRAS wild-type patients with BRAF mutations responded to cetuximab or panitumumab. Thus, anti-EGFR antibody treatment may be best suited to patients with both KRAS wild-type and BRAF wild-type tumors, although such suggestions require further clinical evidence. |
| COMPETITIVE LANDSCAPE |
| Market Share and Competitor Products | The number of products in development as well as key marketed and upcoming competitors are outlined below. Considering development timelines of XP-105 and compared to competition, Xynomic consider themselves approximately 2 years behind competition. These lists below are for example purposes and not exhaustive. Other information regarding competitive landscape can be found in the main body of this report. |
| NUMBER OF PRODUCTS IN DEVELOPMENT: INDICATIONS COMPETITORS |
| Stage | Colorectal cancer | ER+ Breast cancer |
| Preclinical | 43 | n.a. |
| Phase I | 46 | 4 |
| Phase II | 29 | 2 |
| Phase III | 26 | 3 |
| Search Filters of Biotechgate: Type of product, Biotechnology - Therapeutics and Diagnostics; Main area, Neoplasms/cancer/oncology; Therapeutic sub-sector – Colorectal cancer or HER2+ Breast cancer. (Updated, Sep 2018) |
| KEY MARKETED COMPETITORS: MECHANISM COMPETITORS |
| Drug | Company | Expected launch |
| mTORC1/2 inhibitor: XP-105 (BI 860585) |
| TAK228 (=MLN0128) | Millenium /Takeda | 2020 |
| AZD-2014 | AstraZeneca | 2021 |
| CC-223 | Celgene | 2022 |
| DS-3078 | Daiichi Sankyo | 2023 |
© Copyright 2018 Venture Valuation. All Rights Reserved
53
| KEY MARKETED COMPETITORS: INDICATION COMPETITORS |
| Drug Name | Company | Mechanism of Action | Marketed |
| ER+ BREAST CANCER |
| Kisqali (Ribociclib) | Novartis | Inhibitor of CDK4 and CDK6 | 2017 |
| Ibrance (Palbociclib) | Pfizer | Inhibitor of CDK4 and CDK6 | 2015 |
| Verzenio (Abemaciclib) | Eli Lilly | Inhibitor of CDK4 and CDK6 | 2015 |
| Tykerb (lapatinib) | GSK | Tyrosine kinase inhibitor of HER2 and EGFR pathways | 2007 |
| Perjeta (Pertuzumab) | Genentech | Monoclonal antibody inhibits HER receptor dimerization | 2012 |
| Herceptin (Trastuzumab) | Roche/Genentech | Monoclonal antibody targeting HER2 | 1998 |
| CRC |
| FOLFOX | Miscellaneous | Oxaliplatin + Leucovorin + 5-flurouracil combo | n.a. |
| FOLFIRI | Miscellaneous | Irinotecan + Leucovorin + 5-flurouracil combo | n.a. |
| CapeOX | Sanofi, Genentech | Oxaliplatin (Eloxatin) + Capecitabine (Xeloda) combo | n.a. |
| Bevacizumab (Avastin) | Genentech | VEGF inhib anti-angiogenesis (+FOLFOX, FOLFIRI, CapeOX) | 2004 |
| Regorafenib (Stivarga) | Bayer | VEGF inhib. anti-angiogenesis. | 2012 |
| Ziv-aflibercept (Zaltrap) | Sanofi-Aventis | VEGF inhib. anti-angiogenesis (Combo with FOLFIRI) | 2012 |
| Ramucirumab (Cyramza | Eli Lilly | VEGF inhib. anti-angiogenesis (Combo with FOLFIRI) | 2014 |
| Panitumumab (Vectibix) | Amgen | EGFR inhib. (Combo with FOLFOX, FOLFIRI) | 2006 |
| Cetuximab (Erbitux) | BMS, Eli Lilly | EGFR inhib. (Combo with FOLFOX, FOLFIRI) | 2004 |
| Pembrolizumab (Keytruda) | Merck | Anti-PD1 antibody (receptor on tumor cells) | 2014 |
| Nivolumab (Opdivo) | BMS | Anti-PD1 antibody (receptor on tumor cells) | 2014 |
| Ipilimumab (Yervoy) | BMS | Anti-CTLA4 (Combo with Nivolumab) | 2011 |
| PRODUCT VALUATION |
XP-105 FOR THE TREATMENT OF BC |
| Probability of success | Taking into consideration the stage of development and the cumulative success rate of XP-105 for the treatment of BC, the probability of product launch has been determined to be 39%. |
| | | Success rate | |
| | | Pivotal Phase III | | | Approval | |
| Probability of Success | | | 56 | % | | | 70 | % |
| Cumulative POS | | | | | | | 39 | % |
| Product valuation of XP-105 (Xynomic share) between USD 60m and USD 155m | The share of the value of XP-105 for the treatment of breast cancer to Xynomic is between USD 59.9m and USD 154.6m. Using a discount rate of 22%, the average value is USD 95.6m. |
| Product Valuation | | Discount rate | |
| in USD 000’s | | | 26 | % | | | 24 | % | | | 22 | % | | | 20 | % | | | 18 | % |
| rNPV Total product value | | | 83,886 | | | | 105,823 | | | | 133,786 | | | | 169,634 | | | | 215,872 | |
| rNPV licensor (BI) | | | 24,024 | | | | 30,235 | | | | 38,137 | | | | 48,252 | | | | 61,277 | |
| rNPV licensee (Xynomic) | | | 59,861 | | | | 75,589 | | | | 95,649 | | | | 121,382 | | | | 154,594 | |
© Copyright 2018 Venture Valuation. All Rights Reserved
54
| XP-105 FOR THE TREATMENT OF CRC |
| Probability of success | Taking into consideration the stage of development and the cumulative success rate of XP-105 for the treatment of CRC, the probability of product launch has been determined to be 8%. |
| | | Success rate | |
| | | Phase Ib | | | Pivotal Phase III | | | Approval | |
| Probability of Success | | | 21 | % | | | 39 | % | | | 100 | % |
| Cumulative POS | | | | | | | 8 | % | | | 8 | % |
| Product valuation of XP-105 (Xynomic share) between USD 2m and USD 7m | The share of the value of XP-105 for the treatment of colorectal cancer to Xynomic is between USD 1.7m and USD 7.1m. Using a discount rate of 22%, the average value is USD 3.7m. |
| Product Valuation | | Discount rate | |
| in USD 000’s | | | 26 | % | | | 24 | % | | | 22 | % | | | 20 | % | | | 18 | % |
| rNPV Total product value | | | 2,372 | | | | 3,928 | | | | 5,977 | | | | 8,684 | | | | 12,268 | |
| rNPV licensor (BI) | | | 683 | | | | 1,385 | | | | 2,307 | | | | 3,521 | | | | 5,126 | |
| rNPV licensee (Xynomic) | | | 1,689 | | | | 2,543 | | | | 3,671 | | | | 5,162 | | | | 7,142 | |
© Copyright 2018 Venture Valuation. All Rights Reserved
55
| XP-105 IN THE TREATMENT OF BC – VALUATION ASSUMPTIONS |
| Criteria | Value | Reference |
| Patent expiration | 2034 | VV assumption/Xynomic |
| | | |
| Patient population | metastatic Breast Cancer patients, HR+/ HER2- | VV assumption/Xynomic |
| | | |
| | US | China | Europe | Japan | |
| Price in USD | | | | VV assumption/Xynomic |
| Prevalence rate (%) | 0.49% | Based on US prevalence; Novartis press release July 18, 2018. |
| HR+/HER2- | 74% | healthline.com |
| Population that receives a prescription (%) | 100% benchmark | 15% | 75% | In Europe and Japan, based on reported sales ratio by companies; in China, based on population eligible for and that can afford expensive treatments.
VV assumption |
| Market share (%) | 5% | Based on multiple competitors, VV assumption |
| Compliance rate (%) | 80% | High compliance for treatments supervised by specialists.
VV assumption |
| CAGR (%) | 0% | Growth will come from trials in other indications. VV assumption |
| Peak sales (USDm) | 661 | 223 | 565 | 130
(RoW 79) | VV estimate. Sales in RoW estimated as 5% of other sales |
| Total sales (2024-2043) | USD 22.3bn | Model estimate |
| Key expenses for the product valuation |
| Expense | Value | Reference |
| Clinical & Manufacturing expenses (USDm) | US & China Pivotal Phase III (48 months): 19 Approval (12 months): 2.5 Europe & Japan Pivotal Phase III (48 months): 19 Approval (12 months): 2.5 | Xynomic communication/ VV assumption |
| Launch date | US & China: Q1 2024 RoW (inc. Europe/ Japan): Q1 2026 | Xynomic/Venture Valuation estimate |
| COGS (% of revenue) | 5% | Venture Valuation estimate |
Sales & Marketing (% of revenue) | 25% | Venture Valuation estimate |
Discounts, Returns and Allowances (% of revenue) | 5% | Venture Valuation estimate |
G&A rate (% of revenue) | 5% | Venture Valuation estimate |
| Tax rate | 23.5% | OECD average |
© Copyright 2018 Venture Valuation. All Rights Reserved
56
| XP-105 IN THE TREATMENT OF CRC – VALUATION ASSUMPTIONS |
| Criteria | Value | Reference |
| Patent expiration | 2034 | VV assumption/Xynomic |
| | | |
| Patient population | stage IV CRC patients with KRAS+ mutation | VV assumption/Xynomic |
| | | |
| | US | China | Europe | Japan | |
| Price in USD | | | | VV assumption/Xynomic |
| Prevalence rate CRC (%) | 0.044% | 0.014% | 0.061% | 0.034% | Based on 3-year prevalence of CRC, of which 40% is stage IV |
| KRAS+ | 40% | emedicine.medscape.com |
| Population that receives a prescription (%) | 100% benchmark | 15% | 75% | In Europe and Japan, based on reported sales ratio by companies; in China, based on population eligible for and that can afford expensive treatments.
VV assumption |
| Market share (%) | 5% | Based on multiple competitors, VV assumption |
| Compliance rate (%) | 80% | High compliance for treatments supervised by specialists.
VV assumption |
| CAGR (%) | 0% | Growth will come from trials in other indications. VV assumption |
| Peak sales (USDm) | 319 | 34 | 375 | 48
(RoW 38) | VV estimate. Sales in RoW estimated as 5% of other sales |
| Total sales (2025-2044) | USD 11.0bn | Model estimate |
| Key expenses for the product valuation |
| Expense | Value | Reference |
| Clinical & Manufacturing expenses (USDm) | US & China Phase Ib (18 months): 4 Pivotal Phase III (48 months): 18 Approval (12 months): 2.5 Europe & Japan Pivotal Phase III (48 months): 23 Approval (12 months): 2.5 | Xynomic communication/ VV assumption |
| Launch date | US & China: Q1 2025 RoW (inc. Europe/ Japan): Q3 2027 | Xynomic/Venture Valuation estimate |
| COGS (% of revenue) | 5% | Venture Valuation estimate |
Sales & Marketing (% of revenue) | 25% | Venture Valuation estimate |
Discounts, Returns and Allowances (% of revenue) | 5% | Venture Valuation estimate |
G&A rate (% of revenue) | 5% | Venture Valuation estimate |
| Tax rate | 23.5% | OECD average |
© Copyright 2018 Venture Valuation. All Rights Reserved
57
| ABOUT VENTURE VALUATION |
Mission statement | Venture Valuation specializes in independent assessment and valuation of technology-driven companies in the Life Sciences (biotech, pharma, medtech) and other high growth industries (ICT, high-tech, nanotech, renewable energy) Besides valuation services, Venture Valuation offers high quality, focused information services based on the company’s unique positioning in the industry. Through focus and unique position, Venture Valuation strives to fulfil and surpass customers’ needs and add value in the partnership. Clients include investors, companies and development agencies. |
| Services | With access to scientific, product development, regulatory affairs, patenting and financial expertise, Venture Valuation is able to provide insightful valuation reports to customers. Company experts perform comprehensive analyses of financial and technical value while accounting for soft factors such as management experience and track record, assessment of scientific and technological quality, intellectual property and market developments and trends. A valuation report from Venture Valuation helps to highlight critical success factors and strategic elements that will drive a company’s value in the long-term. Venture Valuation also has developed a sophisticated system to follow a company’s progress, and provides valuation updates based on balanced scorecard measurements. Using Venture Valuation’s market expertise the company offers individual product valuations and tools for licensing deal negotiations. Venture Valuation also provides information services, including Biotechgate (www.biotechgate.com), a global database containing over 40’000 life science companies. As a supplier of unique data, venture valuation is able to fully complement its valuation services with in-depth market and industry knowledge in a growing international market. |
| Track Record | Since 1999, Venture Valuation has worked with over 400 high growth companies and several prominent venture funds, including Novartis Venture Fund and the European Investment Bank, providing evaluations of companies and products. The solidity of Venture Valuation’s approach has been validated by a number of publications, including articles in high-profile journals such as Nature Biotechnology. Venture Valuation experts are invited on a regular basis to provide seminars and courses to top-ranking business schools such as IMD and EPFL and have contributed to several books on the valuation of high growth companies. |
| Contact | Venture Valuation is headquartered in Switzerland, with offices in Canada, Germany, Ireland and Singapore. Venture Valuation AG Kasernenstrasse 11 CH-8004 Zurich, Switzerland Phone: +41 (43) 321 86 60 Fax: +41 (43) 321 86 61 E-mail: info@venturevaluation.com Website: www.venturevaluation.com |
© Copyright 2018 Venture Valuation. All Rights Reserved.
58




