- ARGX Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
-
Insider
- Institutional
- Shorts
-
DRS Filing
argenx SE (ARGX) DRSDraft registration statement
Filed: 1 Mar 17, 12:00am
Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO FINANCIAL STATEMENTS
As confidentially submitted to the United States Securities and Exchange Commission on March 1, 2017.
This draft registration statement has not been publicly filed with the United States Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ARGENX N.V.
(Exact name of registrant as specified in its charter)
| The Netherlands (State or other jurisdiction of incorporation or organization) | 2836 (Primary Standard Industrial Classification Code Number) | Not applicable (I.R.S. Employer Identification Number) |
Willemstraat 5
4811 AH, Breda, the Netherlands
+31 763 030 488
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
C T Corporation System
111 8th Avenue
New York, New York 10011
(212) 894-8940
(Name, address, including zip code, and telephone number, including
area code, of agent for service)
| Copies to: | ||||||
Tim Van Hauwermeiren argenx BVBA Industriepark Zwijnaarde 7, Building C 9052 Zwijnaarde (Gent) Belgium +32 9 310 34 00 | ||||||
Michael H. Bison Edwin M. O'Connor Goodwin Procter LLP 100 Northern Avenue Boston, MA 02210 (617) 570-1000 | Geert Verhoeven Freshfields Bruckhaus Deringer LLP Bastion Tower Place du Champ de Mars/ Marsveldplein 5 B-1050 Brussels, Belgium +32 2 504 7000 | Petra Zijp NautaDutilh N.V. Beethovenstraat 400 1082 PR Amsterdam The Netherlands +31 20 717 1000 | Divakar Gupta Brent B. Siler Richard C. Segal Cooley LLP 1114 Avenue of the Americas New York, NY 10036 (212) 479-6000 | |||
Approximate date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee | ||
|---|---|---|---|---|
Ordinary Shares, nominal value of €0.10 per share | $ | $ | ||
| ||||
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), shall determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PROSPECTUS (Subject to completion) | Dated , 2017 |
Shares

Ordinary Shares
This is our initial public offering in the United States. We are offering ordinary shares.
Our ordinary shares are listed on Euronext Brussels under the symbol "ARGX." On , 2017, the last reported sale price of our ordinary shares on Euronext Brussels was € per share, equivalent to a price of $ per share, based on an exchange rate of $ to €1.00.
We intend to apply to list our ordinary shares on The NASDAQ Global Select Market under the symbol "ARGX."
We are an "emerging growth company" under the applicable Securities and Exchange Commission rules and will be subject to reduced public company reporting requirements for this prospectus and future filings. See "Prospectus Summary—Implications of Being an Emerging Growth Company."
Our business and investment in our ordinary shares involves risks that are described in the "Risk Factors" section beginning on page 13 of this prospectus.
Neither the Securities and Exchange Commission nor any U.S. state or other securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| | Per Ordinary Share | Total | |||||
|---|---|---|---|---|---|---|---|
Initial public offering price | $ | $ | |||||
Underwriting discounts and commissions(1) | $ | $ | |||||
Proceeds, before expenses, to argenx N.V. | $ | $ | |||||
The underwriters may also purchase up to an additional ordinary shares from us at the initial public offering price, less the underwriting discounts and commissions, within 30 days from the date of this prospectus.
The underwriters expect to deliver the ordinary shares against payment in New York, New York on or about , 2017.
| Cowen and Company | Piper Jaffray | |
JMP Securities | Wedbush PacGrow |
, 2017
TABLE OF CONTENTS
| | Page | |||
|---|---|---|---|---|
Presentation of Financial Information | iii | |||
Market, Industry and Other Data | iv | |||
Summary | 1 | |||
Risk Factors | 13 | |||
Special Note Regarding Forward-Looking Statements | 69 | |||
Currency Exchange Rates | 70 | |||
Market Information | 71 | |||
Use of Proceeds | 72 | |||
Dividend Policy | 74 | |||
Capitalization | 75 | |||
Dilution | 76 | |||
Selected Consolidated Financial Data | 78 | |||
Management's Discussion and Analysis of Financial Condition and Results of Operations | 79 | |||
Business | 98 | |||
Management | 154 | |||
Related-Party Transactions | 168 | |||
Principal Shareholders | 170 | |||
Description of Share Capital | 173 | |||
Shares Eligible for Future Sale | 199 | |||
Material United States and Dutch Income Tax Considerations | 201 | |||
Enforcement of Civil Liabilities | 213 | |||
Underwriting | 214 | |||
Expenses of the Offering | 222 | |||
Legal Matters | 223 | |||
Experts | 223 | |||
Where You Can Find Additional Information | 224 | |||
Index to Financial Statements | F-1 | |||
We and the underwriters have not authorized anyone to provide any information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we may have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither we nor the underwriters are making an offer to sell the ordinary shares in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of the ordinary shares. Our business, financial condition, results of operations and any such prospects may have changed since the date on the front cover of this prospectus.
No action is being taken in any jurisdiction outside the United States to permit a public offering of our ordinary shares or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of the prospectus applicable to that jurisdiction.
We are incorporated in the Netherlands, and to the best of our knowledge, a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the U.S. Securities and Exchange Commission, or SEC, we are currently eligible for treatment as a "foreign private issuer." As a foreign private issuer, we will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic registrants whose securities are registered under the Securities Exchange Act of 1934, as amended.
We own various trademark registrations and applications, and unregistered trademarks, including SIMPLE Antibody™, NHance®, ABDEG™ and our corporate logo and are authorized to use POTELLIGENT® by Kyowa Hakko Kirin Co. Ltd. We have a European Community Trademark and have a U.S. trademark registration for the arGEN-X name and a European Community Trademark for the argenx name. The name is also the subject of a number of domain name registrations. All other trade names, trademarks and service marks of other companies appearing in this prospectus are the property of their respective holders. Solely for convenience, the trademarks and trade names in this prospectus may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend to use or display other companies' trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
ii
PRESENTATION OF FINANCIAL INFORMATION
This prospectus includes our audited consolidated financial statements as of and for the year ended December 31, 2015 prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. This prospectus also includes our unaudited interim condensed financial statements as of June 30, 2016 and for the six-month periods ended June 30, 2016 and 2015, prepared in accordance with International Accounting Standard 34Interim Financial Reporting.
This prospectus also includes summary unaudited and unreviewed results of operations for the nine-month periods ended September 30, 2015 and 2016 and balance sheet data as of September 30, 2016. These data have been prepared solely on the basis of currently available information by, and are the responsibility of, management. Our independent public accounting firm, Deloitte Accountants B.V., has not audited or reviewed, and does not express an opinion with respect to, these data. These data are not a comprehensive statement of our financial results for these periods. The interim data are not necessarily indicative of the data to be expected for the annual period. Pursuant to SEC rules, we are including these data because we otherwise make it publicly available.
Pursuant to the applicable provisions of the Fixing America's Surface Transportation Act, we are not required to file our financial information for the historical 2014 annual period because we expect to file our financial information for the year ended December 31, 2016 in an amendment to our registration statement before it is declared effective. While the 2014 financial information is otherwise required by Regulation S-X, we reasonably believe that it will not be required to be included in the Form F-1 filing at the time of the contemplated offering.
None of our financial statements were prepared in accordance with generally accepted accounting principles in the United States.
Our financial statements are presented in euros. For the convenience of the reader, some euro amounts have been translated into U.S. dollars at the rate of $1.00 to € , the official exchange rate quoted as of , 2017 by the U.S. Federal Reserve Bank, unless otherwise noted. These translations should not be considered representations that any such amounts have been, could have been or could be converted into U.S. dollars at that or any other exchange rate as at that or any other date.
All references in this prospectus to "$," "US$," "U.S. dollars," "dollars" and "USD" mean U.S. dollars and all references to "€," "EUR" and "euros" mean euros, unless otherwise noted.
iii
MARKET, INDUSTRY AND OTHER DATA
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to this information. Based on our industry experience, we believe that the third-party sources are reliable and that the conclusions contained in the publications are reasonable. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in "Risk Factors."
iv
The following summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider before investing in our ordinary shares. You should read the entire prospectus carefully, including "Risk Factors" and our consolidated financial statements and the related notes appearing elsewhere in this prospectus. You should carefully consider, among other things, the matters discussed in "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business" before making an investment decision. Unless otherwise indicated, "argenx," "the company," "our company," "we," "us" and "our" refer to argenx N.V. and its consolidated subsidiaries.
Overview
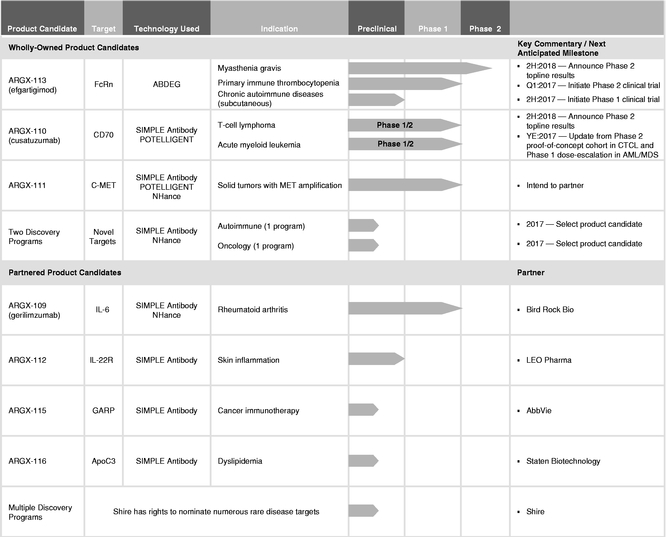
We are a clinical-stage biotechnology company developing a deep pipeline of differentiated antibody-based therapies for the treatment of severe autoimmune diseases and cancer. Utilizing our suite of technologies, we are focused on developing product candidates with the potential to be either first-in-class against novel targets or best-in-class against known, but complex, targets in order to treat diseases with a significant unmet medical need. Our SIMPLE Antibody Platform, based on the powerful llama immune system, allows us to exploit novel and complex targets, and our three antibody Fc engineering technologies are designed to enable us to expand the therapeutic index of our product candidates. Together with our antibody discovery and development expertise, this suite of technologies has enabled our pipeline of seven product candidates. Two of our product candidates will be in clinical proof-of-concept trials for three indications within the first half of 2017.
Our most advanced product candidate, ARGX-113, is in a Phase 2 clinical trial for the treatment of the rare autoimmune disease myasthenia gravis, or MG, and, in March 2017, we plan to initiate a Phase 2 clinical trial of ARGX-113 for the treatment of another rare autoimmune disease, primary immune thrombocytopenia, or ITP. We are currently developing our second lead product candidate, ARGX-110, for rare and aggressive hematological cancers, initially for T-cell lymphoma, or TCL, and acute myeloid leukemia, or AML, as well as high-risk myelodysplastic syndrome, or MDS. In December 2016, we commenced a Phase 1/2 clinical trial of ARGX-110 in combination with azacitidine for the treatment of newly diagnosed AML or high-risk MDS patients, and in March 2017, we expect to initiate the Phase 2 part of a Phase 1/2 clinical trial of ARGX-110 for the treatment of cutaneous TCL, or CTCL.
We have a disciplined strategy to maximize the value of our pipeline whereby we plan to retain development and commercialization rights to those product candidates that we believe we can ultimately commercialize successfully on our own if they are approved. We plan to collaborate on product candidates that we believe have promising utility in disease areas or patient populations that are better served by the resources of larger biopharmaceutical companies. We have entered into collaborations with a number of biopharmaceutical companies, including our collaboration with AbbVie S.Á.R.L., or AbbVie, for ARGX-115, a cancer immunotherapy-focused product candidate, against the novel target glycoprotein A repetitions predominant, or GARP. We received a $40.0 million (€35.1 million as of the date the payment was received) upfront payment in connection with this collaboration.
Our product candidates are focused on indications for which there is a solid biological rationale and for which we believe there is an advantage to utilizing our suite of technologies outlined below:
1
produces highly diverse panels of antibodies with a high human homology, or similarity, in their V-regions when immunized with targets of human disease. By contrast, most antibody platforms start with antibodies generated in inbred mice or synthetic antibody library systems, approaches that are limited by insufficient antibody repertoires and limited diversity, respectively. Our SIMPLE Antibody Platform allows us to access and explore a broad target universe, including novel and complex targets, while minimizing the long timelines associated with generating antibody candidates using traditional methods.
Our product candidate pipeline includes both wholly-owned and partnered programs. We refer to programs for which we retain the exclusive right to develop and commercialize the product candidate on a worldwide basis as our wholly-owned programs. We refer to programs for which we have entered into collaboration agreements with third parties for the development and commercialization of the product candidate as our partnered programs.
Our product candidate pipeline enabled by our suite of technologies is set forth below:
2
We believe that our clinical expertise and execution capabilities position us well to advance our product pipeline and enter into collaborations designed to maximize the value of our portfolio. We have assembled a team of over 60 employees with experience across the spectrum of antibody drug discovery and development and business development. Members of our board of directors and management team have extensive experience in the life sciences industry and have previously served at companies including Cambridge Antibody Technology Group Plc; Celgene Corporation; Galapagos NV; GlaxoSmithKline plc; Janssen Pharmaceuticals, Inc.; Micromet, Inc.; Nicox S.A.; The Procter & Gamble Company; Quintiles IMS Holdings, Inc. and Unilever NV.
Our Competitive Strengths
We believe that the combination of our technologies, expertise and disciplined focus will enable us to overcome many of the challenges associated with antibody drug development and positions us to be a leader in delivering therapies to patients suffering from severe autoimmune disease and cancers for which the current treatment paradigm is inadequate. Our competitive strengths include:
Our Suite of Technologies
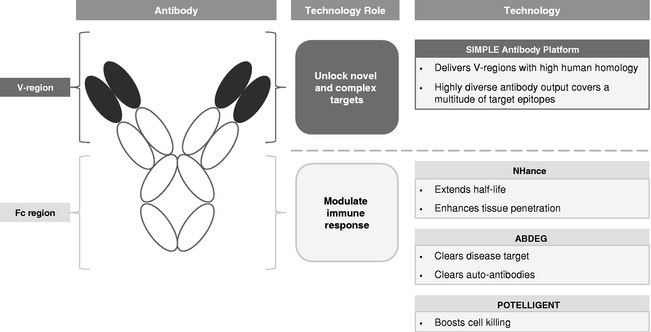
We employ a suite of technologies to optimize antibodies for the discovery and development of our product candidates. Used alone or in combination, we believe that our technologies enable us to create product candidates with potential first-in-class or best-in-class therapeutic activity against a wide range of targets. Our technologies specifically focus on enhancements around both the V-region and Fc region of an antibody.
Antibodies are Y-shaped proteins used by the immune system to target and clear foreign bodies, including pathogens and tumor cells. Antibodies are composed of two structurally independent parts, the variable region, or V-region, and the constant, or Fc, region. The V-region is responsible for targeting a specific antibody to an antigen, which is a substance that induces an immune response, and is different for every type of antibody. The Fc region does not interact with antigens, but rather interacts with components of the immune system through a variety of receptors on immune and other cells. These interactions allow antibodies to regulate the immune response and levels of cell-killing ability, or cytotoxicity, as well as their persistence in circulation and tissues. Fc regions are the same and interchangeable from antibody to antibody.
Our proprietary SIMPLE Antibody Platform sources V-regions from the immune system of outbred llamas, each of which has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology in their V-regions when immunized with targets of human disease. Our SIMPLE Antibody Platform allows us to access and explore a broad target universe, including novel and complex targets, while minimizing the long timelines associated with generating antibody candidates using traditional methods.
By focusing on the Fc region of antibodies, our Fc technologies—NHance, ABDEG and POTELLIGENT—augment the interactions with immune system components, thereby potentially
3
improving the therapeutic potential of our product candidates. Specifically, these technologies allow us to modify antibody half-life, tissue penetration, rate of disease target clearance and potency.
The figure below illustrates the role of each of our individual technologies:
Our Two Lead Wholly-Owned Product Candidates
ARGX-113. We are currently developing our lead product candidate, ARGX-113, for the treatment of patients with MG and ITP, both of which are rare and severe autoimmune diseases associated with high levels of pathogenic immunoglobulin G, or IgG, antibodies for which few innovative biologic treatments have been approved and severe unmet medical need exists. ARGX-113 utilizes our ABDEG engineering technology and is designed to block the recycling of IgG antibodies, which results in their removal from circulation. We believe that our approach presents potential benefits relative to the current standard of care for MG and ITP: corticosteroids and immunosuppressants in the early stages, followed by intravenous IgG, or IVIg, and plasma exchange, or plasmapheresis, as the disease progresses. These potential benefits include improved time of onset, increased magnitude and duration of therapeutic benefit, a more favorable safety and tolerability profile and reduced cost burden to the healthcare system.
We have completed single and multiple ascending dose parts of a double-blind, placebo-controlled Phase 1 clinical trial of ARGX-113 in 62 healthy volunteers. In the single ascending dose part of our clinical trial, we observed that a single two-hour infusion of 10 mg/kg of ARGX-113 was associated with an approximate 50% reduction of circulating IgG antibody levels. In the multiple ascending dose part of the Phase 1 clinical trial, repeat administration of ARGX-113 every seven days, with four doses in total, was associated with a gradual reduction in levels of four classes of IgG antibodies of 60% to 85%. We observed the reduction in circulating IgG antibody levels to persist for more than four weeks after the last dose. We believe that a reduction of pathogenic IgG antibody levels, which are a subset of circulating IgG antibodies in people with autoimmune disease, of at least 30% would be clinically meaningful. ARGX-113 was reported to be well-tolerated in both parts of the Phase 1 clinical trial, except for 50 mg/kg, the highest dose in the single ascending dose part, which was moderately tolerated. One serious adverse event, hyperventilation, was observed in
4
the multiple ascending dose part. This event, which occurred six days after drug administration, was considered by the clinical investigator as unlikely to be related to ARGX-113.
As a result of these promising data, we launched a Phase 2 clinical trial of ARGX-113 in patients with MG in January 2017. In parallel, we plan to launch a second Phase 2 clinical trial of ARGX-113 in patients with ITP in March 2017. We expect topline data from these clinical trials in the second half of 2018. Depending on the outcome of these clinical trials and subject to discussions with regulatory agencies, we intend to structure our pivotal program for ARGX-113 in one or both of these indications. In addition to the intravenous formulation of ARGX-113 that we are using in our current clinical trials, we are also developing a subcutaneous formulation designed to make ARGX-113 accessible to larger patient populations, including patients requiring chronic therapy, potentially outside of the hospital setting. We plan to initiate a Phase 1 clinical trial in healthy volunteers for a subcutaneous formulation of ARGX-113 in the second half of 2017.
ARGX-110. We are developing ARGX-110 in cancer indications, initially for TCL and AML, as well as high-risk MDS. TCL and AML are rare and aggressive hematological cancers for which significant unmet medical needs exist. MDS, a rare bone marrow disorder, is often a precursor to AML. ARGX-110 is a SIMPLE Antibody that blocks the cell surface protein CD70, which is overexpressed in B-cell and T-cell lymphomas and leukemias and is involved in the proliferation and survival of these cells. ARGX-110 is designed to kill CD70-positive cells via its potent antibody effector functions through the use of POTELLIGENT technology.
ARGX-110 is currently being evaluated in an open-label, multi-site Phase 1/2 clinical trial in patients with advanced malignancies expressing CD70. To date, as part of a step-wise adaptive trial design, we have enrolled a total of 94 patients in the Phase 1 part of the clinical trial. This clinical trial design is adaptive in that it allows us to make data driven decisions and open up new cohorts in indications where we have seen the most promising early signals of biological activity.
While the primary goal of this Phase 1 clinical trial is to investigate safety and pharmacokinetics, we have also observed evidence of biological activity in several of the patients treated. In the completed dose-escalation part of the Phase 1 clinical trial, in which ARGX-110 was administered to 26 patients, no dose-limiting toxicities were observed. The most frequent drug-related adverse events were fatigue and infusion-related reactions. There were 20 serious adverse events seen in these heavily pre-treated patients, but no significant trends in terms of safety were observed between the dose groups. To date, we have observed promising signs of biological activity in patients with a range of cancers, including platinum-refractory ovarian cancer, head-and-neck cancer, myoepithelial carcinoma, mesothelioma, renal cell carcinoma and TCL. We are currently concluding the Phase 1 safety-expansion cohort of this clinical trial in relapsed or refractory CD70-positive TCL patients and have seen promising preliminary results in some of the first 10 evaluable CTCL patients. At doses of 1 mg/kg every three weeks, we observed three patients to have a partial response and two patients to have stable disease.
Based on the preliminary results from the Phase 1 part of the clinical trial, we plan to transition into the Phase 2 part of the clinical trial in adult relapsed or refractory CD70-positive CTCL patients in March 2017, with interim results expected to be available by the end of 2017. We expect to report topline results from this clinical trial in the second half of 2018. In December 2016, we initiated a Phase 1/2 clinical trial of ARGX-110 in combination with azacitidine in newly diagnosed AML or high-risk MDS patients. We expect the majority of patient enrollment in this clinical trial to be AML patients. We expect to report interim results from the dose-escalation part of this clinical trial by the end of 2017.
5
Our Partnered Programs
In addition to our wholly-owned product candidates, we are developing a pipeline of partnered programs—those we believe have promising utility in disease areas or patient populations that are better served by the resources of larger biopharmaceutical companies. We have entered into collaborations with a number of companies, including our collaboration with AbbVie for ARGX-115, a cancer immunotherapy-focused product candidate. Additional partnered programs include our research collaboration with Bird Rock Bio, Inc. for ARGX-109, advancing into a Phase 2 clinical trial for rheumatoid arthritis, our collaboration with LEO Pharma A/S for ARGX-112 and our collaboration with Staten Biotechnology B.V. for ARGX-116. We are also party to a collaboration agreement with Shire AG to discover, develop and commercialize novel human therapeutic antibodies against up to three targets implicated in diverse rare and unmet diseases. For more information on our relationships with our collaboration partners, see "Business—Collaborations." In addition to collaborating on our product candidates, we may also elect to enter into collaborations for third-party product candidates for which we believe that our technologies and expertise may be valuable.
Our Strategy
Our goal is to deliver therapies that are either first-in-class or best-in-class to patients suffering from severe autoimmune diseases and various cancers for which there exists a significant unmet medical need. We are also focused on attaining this goal in a manner that is disciplined for a company of our size. We plan to:
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk Factors" section of this prospectus. These risks include, but are not limited to, the following:
6
We were incorporated under the laws of the Netherlands on April 25, 2008 as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid). On May 28, 2014 we converted to a Dutch public company with limited liability (naamloze vennootschap). Our official seat is in Rotterdam, the Netherlands, and our registered office is at Willemstraat 5, 4811 AH, Breda, the Netherlands. We are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our telephone number is +31 (0) 10 70 38 441. Our website address is http://www.argen-x.com. The information contained on, or that can be accessed through,
7
our website is not a part of, and shall not be incorporated by reference into, this prospectus. We have included our website address as an inactive textual reference only.
Implications of Being an Emerging Growth Company
We qualify as an "emerging growth company" as defined in the U.S. Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of (1) the last day of the fiscal year in which we have more than $1.0 billion in annual revenue; (2) the date we qualify as a "large accelerated filer," with at least $700 million of equity securities held by non-affiliates; (3) the issuance, in any three-year period, by our company of more than $1.0 billion in non-convertible debt securities; and (4) the last day of the fiscal year ending after the fifth anniversary of this offering. We may choose to take advantage of some but not all of these exemptions. For example, Section 107 of the JOBS Act provides that an emerging growth company can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. Given that we currently report and expect to continue to report under International Financial Reporting Standards as issued by the International Accounting Standards Board, or IASB, we have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
Implications of Being a Foreign Private Issuer
We are also considered a "foreign private issuer." In our capacity as a foreign private issuer, we are exempt from certain rules under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and "short-swing" profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose
8
securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (1) the majority of our executive officers or directors are U.S. citizens or residents, (2) more than 50% of our assets are located in the United States or (3) our business is administered principally in the United States.
We have taken advantage of certain reduced reporting and other requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
9
Issuer | argenx N.V. | |
Ordinary shares offered by us | shares | |
Underwriters' option to purchase additional ordinary shares | shares | |
Ordinary shares to be outstanding immediately after this offering | shares (or shares if the underwriters exercise their option to purchase additional ordinary shares from us in full) | |
Use of proceeds | We estimate that our net proceeds from this offering will be approximately $ million (or approximately $ million if the underwriters exercise their option to purchase additional shares in full), assuming an initial public offering price of $ per ordinary share, based on the closing price of our ordinary shares on Euronext Brussels and the exchange rate on , 2017, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds from this offering, together with cash, cash equivalents and current financial assets on hand, to fund research and development efforts for our product candidates, for our other current and future research and development activities, to progress technology development and for working capital and other general corporate purposes. See "Use of Proceeds" for a more complete description of the intended use of proceeds from this offering. | |
Risk factors | See "Risk Factors" and the other information included in this prospectus for a discussion of factors you should consider before deciding to invest in our ordinary shares. | |
Proposed NASDAQ symbol | "ARGX" | |
Euronext Brussels trading symbol | "ARGX" |
The number of our ordinary shares to be outstanding after this offering is based on ordinary shares outstanding as of June 30, 2016, but excludes ordinary shares issuable upon the exercise of share options outstanding as of June 30, 2016 at a weighted average exercise price of € per share.
Unless otherwise indicated, all information contained in this prospectus assumes no exercise of the underwriters' option to purchase up to additional ordinary shares.
10
Summary Consolidated Financial Data
The following table sets forth a summary of our consolidated financial data for the periods indicated. The summary consolidated financial data for the year ended December 31, 2015 have been derived from our audited financial statements included elsewhere in this prospectus. The summary consolidated financial data as of June 30, 2016 and for the six months ended June 30, 2015 and 2016 have been derived from our unaudited interim condensed financial statements included elsewhere in this prospectus. The unaudited interim condensed financial statements have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly our financial position as of June 30, 2016 and the results of operations for the six months ended June 30, 2015 and 2016.
Our historical results for any prior period are not necessarily indicative of results to be expected in any future period and our interim results are not necessarily indicative of results to be expected for the full year ending December 31, 2016, or any other period. The information set forth below should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and with our consolidated financial statements and notes thereto included elsewhere in this prospectus.
We present our financial data in euros and prepare our financial statements in accordance with IFRS as issued by the IASB.
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands, except share and per share data) | |||||||||
Statement of profit and loss and other | ||||||||||
Revenue | € | 6,854 | € | 2,708 | € | 5,656 | ||||
Other operating income | 3,101 | 1,640 | 1,317 | |||||||
Research and development expenses | (20,635 | ) | (9,284 | ) | (11,263 | ) | ||||
General and administrative expenses | (4,925 | ) | (2,314 | ) | (3,063 | ) | ||||
| | | | | | | | | | | |
Operating loss | (15,605 | ) | (7,250 | ) | (7,353 | ) | ||||
| | | | | | | | | | | |
Financial income | 112 | 100 | 39 | |||||||
Exchange gains (losses) | 181 | 130 | (42 | ) | ||||||
| | | | | | | | | | | |
Total comprehensive loss | € | (15,312 | ) | € | (7,020 | ) | € | (7,356 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Weighted average number of shares outstanding | 15,734,007 | 15,705,112 | 17,356,799 | |||||||
Basic and diluted loss per share | € | (0.97 | ) | € | (0.45 | ) | € | (0.42 | ) | |
11
The following table sets forth our summary consolidated statement of financial position data as of June 30, 2016 on:
| | As of June 30, 2016 | ||||||
|---|---|---|---|---|---|---|---|
| | Actual | As adjusted(1) | |||||
| | (In thousands) | ||||||
Statement of financial position data: | |||||||
Cash, cash equivalents and current financial assets | € | 108,744 | |||||
Total assets | 117,334 | ||||||
Deferred revenue | 36,786 | ||||||
Total liabilities | 41,934 | ||||||
Total equity | 75,400 | ||||||
12
You should carefully consider the risks and uncertainties described below and the other information in this prospectus before making an investment in our ordinary shares. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occurs, and as a result, the market price of our ordinary shares could decline and you could lose all or part of your investment. This prospectus also contains forward-looking statements that involve risks and uncertainties. See "Special Note Regarding Forward-Looking Statements." Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors.
Risks Related to Our Financial Position and Need for Additional Capital
We are a clinical-stage biopharmaceutical company and have incurred significant losses since our inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.
We are a clinical-stage biopharmaceutical company with a limited operating history. Since our inception, we have incurred significant operating losses. We incurred total comprehensive losses of €15.3 million for the year ended December 31, 2015 and €7.4 million for the six months ended June 30, 2016. As of June 30, 2016, we had an accumulated loss of €58.5 million. Our losses resulted principally from costs incurred in research and development, preclinical testing, clinical development of our product candidates as well as costs incurred for research programs and from general and administrative costs associated with our operations. In the future, we intend to continue to conduct research and development, preclinical testing, clinical trials and regulatory compliance activities that, together with anticipated general and administrative expenses, will result in incurring further significant losses for the next several years. Our losses, among other things, will continue to cause our working capital and shareholders' equity to decrease. We anticipate that our expenses will increase substantially if and as we:
13
Since our inception in 2008, we have invested most of our resources to developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations. We do not currently have any approved products and have never generated any revenue from product sales. To date, we have funded our operations through public and private placements of equity securities, upfront, milestone and expense reimbursement payments received from our collaborators, funding from governmental bodies and interest income from the investment of our cash, cash equivalents and financial assets.
To become and remain profitable, we must succeed in developing and eventually commercializing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing preclinical testing and clinical trials of our product candidates, discovering and developing additional product candidates, obtaining regulatory approval for any product candidates that successfully complete clinical trials, establishing manufacturing and marketing capabilities and ultimately selling any products for which we may obtain regulatory approval. We are only in the preliminary stages of most of these activities. We may never succeed in these activities and, even if we do, may never generate revenue that is significant enough to achieve profitability.
Because of the numerous risks and uncertainties associated with pharmaceutical and biological product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. If we are required by the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, or other comparable foreign authorities to perform studies in addition to those we currently anticipate, or if there are any delays in completing our clinical trials or the development of any of our product candidates, our expenses could increase and revenue could be further delayed.
Even if we do generate product royalties or product sales, we may never achieve or sustain profitability on a quarterly or annual basis. Our failure to sustain profitability would depress the market price of our ordinary shares and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. A decline in the market price of our ordinary shares also could cause you to lose all or a part of your investment.
We may need substantial additional funding in order to complete the development and commercialization of our product candidates. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our product development or research operations.
To date, we have funded our operations through public and private placements of equity securities, upfront, milestone and expense reimbursement payments received from our collaborators, funding from governmental bodies and interest income from the investment of our cash, cash equivalents and financial assets. We expect to require additional funding in the future to sufficiently finance our operations and advance development of our product candidates.
We expect that our existing cash, cash equivalents and investments, together with anticipated net proceeds from this offering, will enable us to fund our operating expenses and capital expenditure requirements through at least the next months. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than
14
we currently expect. Our future capital requirements for ARGX-113, ARGX-110 or our preclinical programs will depend on many factors, including:
Our ability to raise additional funds will depend on financial, economic and market conditions and other factors, over which we may have no or limited control. If adequate funds are not available on commercially acceptable terms when needed, we may be forced to delay, reduce or terminate the development or commercialization of all or part of our research programs or product candidates or we may be unable to take advantage of future business opportunities.
Raising additional capital may cause dilution to our holders, including purchasers of ordinary shares in this offering, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our operations with our existing cash, cash equivalents and financial assets, the net proceeds from this offering, revenue from our collaborations, funding from governmental bodies and interest income from the investment of our cash, cash equivalents and financial assets. In order to further advance development of our product candidates, discover additional product candidates and pursue our other business objectives, however, we will need to seek additional funds.
We cannot guarantee that future financing will be available in sufficient amounts or on commercially reasonable terms acceptable, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of holders of our ordinary shares and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares to decline. The sale of additional equity or convertible securities would dilute all of our existing shareholders and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our ordinary shares. The incurrence of indebtedness could result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional
15
debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborators or others at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Further, any additional fundraising efforts may divert our management from its day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any of our product candidates, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Since our inception in 2008, we have invested most of our resources to developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations. Our most advanced candidate, ARGX-113, is in a Phase 2 clinical trial for the treatment of myasthenia gravis and, in March 2017, we plan to initiate a Phase 2 clinical trial of ARGX-113 for the treatment primary immune thrombocytopenia. We have not yet demonstrated an ability to obtain regulatory approvals, manufacture a commercial-scale product or conduct sales and marketing activities necessary for successful product commercialization. In addition, given our limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors in achieving our business objectives. If we are successful at completing the approval process for one of our product candidates, we may consider transitioning from our current research and development focus to focusing on commercializing our products. We may not be successful in such a transition or may incur greater costs than expected, which would materially adversely affect our business, prospects, financial condition and results of operation. Additionally, we expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history or more experience developing antibody-based drugs.
Risks Related to the Development and Clinical Testing of Our Product Candidates
All of our product candidates are in preclinical or early-stage clinical development. Clinical drug development is a lengthy and expensive process with uncertain timelines and uncertain outcomes. If clinical trials of our product candidates, particularly ARGX-113 and ARGX-110, are prolonged or delayed, we or our collaborators may be unable to obtain required regulatory approvals, and therefore will be unable to commercialize our product candidates on a timely basis or at all, which will adversely affect our business.
To obtain the requisite regulatory approvals to market and sell any of our product candidates, we or our collaborator for such candidates must demonstrate through extensive preclinical studies and clinical trials that our products are safe, pure and potent or effective in humans. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process and our future clinical trial results may not be successful.
16
We may experience delays in our ongoing clinical trials and we do not know whether planned clinical trials will begin on time, need to be redesigned, enroll patients on time or be completed on schedule, if at all.
Clinical trials can be delayed, suspended, or terminated for a variety of reasons, including the following:
We could encounter delays if a clinical trial is suspended or terminated by us, by the IRBs of the institutions in which such trials are being conducted or ethics committees, by the Data Review Committee, or DRC, or Data Safety Monitoring Board, or DSMB, for such trial or by the EMA, the FDA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the EMA, the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, including those relating to the class to which our product
17
candidates belong, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our product candidates and impair our ability to commercialize our product candidates and may harm our business and results of operations.
Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Clinical trials must be conducted in accordance with the FDA, the EMA and other applicable regulatory authorities' legal requirements and regulations, and are subject to oversight by these governmental agencies and IRBs at the medical institutions where the clinical trials are conducted or ethics committees. In addition, clinical trials must be conducted with supplies of our product candidates produced under current good manufacturing practices, or cGMP, requirements and other regulations. Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and while we have agreements governing their committed activities, we have limited influence over their actual performance. We depend on our collaborators and on medical institutions and CROs to conduct our clinical trials in compliance with Good Clinical Practice, or GCP, requirements. To the extent our collaborators or the CROs or investigators fail to enroll participants for our clinical trials, fail to conduct the study to GCP standards or are delayed for a significant time in the execution of trials, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, clinical trials that are conducted in countries outside the EU and the United States may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-EU and non-U.S. CROs, as well as expose us to risks associated with clinical investigators who are unknown to the FDA or the EMA, and different standards of diagnosis, screening and medical care.
Preclinical drug development is uncertain. Some or all of our preclinical programs, such as ARGX-115, ARGX-112 and ARGX-116, may experience delays or may never advance to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these product candidates on a timely basis or at all, which would have an adverse effect on our business.
In order to obtain FDA or EMA approval to market a new biological product we must demonstrate proof of safety, purity and potency or efficacy in humans. To meet these requirements we will have to conduct adequate and well-controlled clinical trials. Before we can commence clinical trials for a product candidate, we must complete extensive preclinical testing and studies that support our planned Investigational New Drug application, or IND, in the United States, or a Clinical Trial Authorization Application, or CTA, in Europe. While we have had Pre-IND discussions with the FDA on certain of our product candidates, including for an indication we are no longer pursuing, we do not have any active INDs for our proposed indications at this time nor have we conducted any of our clinical development to date in the United States. We cannot be certain of the timely completion
18
or outcome of our preclinical testing and studies and cannot predict if the FDA or EMA will accept our proposed clinical programs or if the outcome of our preclinical testing and studies will ultimately support the further development of these product candidates. Thus, we cannot be sure that we will be able to submit INDs or CTAs for our preclinical programs on the timelines we expect, if at all, and we cannot be sure that submission of INDs or CTAs will result in the FDA or EMA allowing clinical trials to begin.
Conducting preclinical testing is a lengthy, time-consuming and expensive process. The length of time may vary substantially according to the type, complexity, novelty and intended use of the product candidate, and often can be several years or more per product candidate. Delays associated with product candidates for which we are directly conducting preclinical testing and studies may cause us to incur additional operating expenses. Moreover, we may continue to be affected by delays associated with the preclinical testing and studies of certain product candidates conducted by our potential partners over which we have no control. The commencement and rate of completion of preclinical studies and studies for a product candidate may be delayed by many factors, including, for example:
Moreover, even if clinical trials do begin for these preclinical programs, our development efforts may not be successful, and clinical trials that we conduct or that third parties conduct on our behalf may not demonstrate sufficient safety, purity and potency or efficacy to obtain the requisite regulatory approvals for any of our product candidates or product candidates employing our technology. Even if we obtain positive results from preclinical studies or initial clinical trials, we may not achieve the same success in future trials.
The results of preclinical studies and early-stage clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. Initial success in our ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials.
Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. Furthermore, there can be no assurance that any of our clinical trials will ultimately be successful or support further clinical development of any of our product candidates. There is a high failure rate for drugs and biologics proceeding through clinical trials. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development could have a material adverse effect on our business and operating results.
Preliminary and interim data from our clinical studies may change as more patient data become available. Preliminary or interim data from our clinical studies are not necessarily predictive of final results. Preliminary and interim data are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues, more patient data become available and we issue our final clinical study report. As a result, preliminary and interim data should be viewed with
19
caution until the final data are available. Material adverse changes in the final data compared to the interim data could significantly harm our business prospects.
Interim, topline and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, topline or preliminary data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Interim, topline and preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim, topline and preliminary data should be viewed with caution until the final data are available.
Our product candidates may have serious adverse, undesirable or unacceptable side effects which may delay or prevent marketing approval. If such side effects are identified during the development of our product candidates or following approval, if any, we may need to abandon our development of such product candidates, the commercial profile of any approved label may be limited, or we may be subject to other significant negative consequences following marketing approval, if any.
Undesirable side effects that may be caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. While our preclinical and clinical studies for our product candidates to date have generally been well tolerated from a risk-benefit perspective, the results from future trials may not support this conclusion.
In the dose-escalation part of our Phase 1 clinical trial for ARGX-110 in patients with advanced malignancies expressing CD70, we observed serious adverse events in some patients, including seven patient deaths, of which five deaths were attributed to disease progression, one death was attributed to sepsis and one death was attributed to respiratory failure. None of these deaths were deemed to be drug-related according to the investigator. In the two safety-expansions cohorts that have been completed, there was one drug-related patient death attributed sepsis. In the dose-escalation part, anti-drug antibodies were observed in all doses except the 10 mg dose and appeared to be inversely related to the administered dose. In our two completed safety-expansion cohorts, anti-drug antibodies were detected. In a preclinical mouse efficacy model of acute lymphocytic leukemia, or ALL, the administration of an ARGX-110 variant at higher doses led to the acute death of some animals with high tumor load. The cause of death in this preclinical mouse study has not been determined, although a literature search conducted on our behalf revealed some similarities of this symptomatology with anecdotal reports in ALL patients treated with compounds having antibody-dependent cell-mediated cytotoxicity enhanced, or ADCC-enhanced, Fc regions who experienced a cytokine storm, a potentially fatal immune reaction to immunotherapy. We are not currently evaluating ARGX-110 for patients with ALL and have no intention of doing so. However, we cannot guarantee that we will not see evidence of cytokine storm or similar adverse events in patients with other forms of cancer, such as those being evaluated in our current Phase 1/2 clinical trial in patients with either AML or high-risk MDS.
The results of future clinical studies may show that our product candidates cause undesirable or unacceptable side effects or even death. In such an event, our trials could be suspended or terminated and the FDA, the EMA or comparable foreign regulatory authorities could order us to
20
cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly. Further, because all of our product candidates and preclinical programs, other than ARGX-113, are based on our SIMPLE Antibody platform, any adverse safety or efficacy findings related to any product candidate or preclinical program may adversely impact the viability of our other product candidates or preclinical programs.
Additionally, if any of our product candidates receives marketing approval and we or others later identify undesirable or unacceptable side effects caused by such products, a number of potentially significant negative consequences could result, including:
Any of these events could prevent us, our collaborators or our potential future partners from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenue from the sale of our products.
We face significant competition for our drug discovery and development efforts, and if we do not compete effectively, our commercial opportunities will be reduced or eliminated. We may not be successful in our efforts to use and expand our SIMPLE Antibody platform, our NHance and ABDEG technologies, or the licensed POTELLIGENT technology, to build a pipeline of product candidates and develop marketable products due to significant competition and technological change, which could limit or eliminate the market opportunity for our product candidates and technology platforms.
The market for pharmaceutical products is highly competitive. Our competitors include many established pharmaceutical companies, biotechnology companies, universities and other research or commercial institutions, many of which have substantially greater financial, research and development resources than us. Large pharmaceutical companies, in particular, have extensive experience in clinical testing, obtaining regulatory approvals, recruiting patients and in manufacturing pharmaceutical products. Smaller and early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management
21
personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our products. The fields in which we operate are characterized by rapid technological change and innovation. There can be no assurance that our competitors are not currently developing, or will not in the future develop, technologies and products that are equally or more effective or are more economically attractive as any of our current or future technology or product. Competing products or technology platforms may gain faster or greater market acceptance than our products or technology platforms and medical advances or rapid technological development by competitors may result in our product candidates or technology platforms becoming non-competitive or obsolete before we are able to recover our research and development and commercialization expenses. If we, our product candidates or our technology platforms do not compete effectively, it may have a material adverse effect on our business, prospects, financial condition and results of operation.
Competition in the autoimmune space is intense and involves multiple monoclonal antibodies, other biologics and small molecules either already marketed or in development by many different companies including large pharmaceutical companies such as AbbVie Inc. (Humira/rheumatoid arthritis); Amgen Inc. (Enbrel/rheumatoid arthritis); Biogen, Inc. (Tysabri/multiple sclerosis); GlaxoSmithKline plc (Benlysta/lupus); F. Hoffman-La Roche AG, or Roche (Rituxan/often used off label) and Janssen Pharmaceuticals Inc., or Janssen (Remicade/rheumatoid arthritis and Stelara/psoriasis). In addition, these and other pharmaceutical companies have monoclonal antibodies or other biologics in clinical development for the treatment of autoimmune diseases. Furthermore, we are aware of competing products specifically targeting FcRn and being developed by UCB S.A.; Momenta, Inc.; Shire plc; Syntimmune Inc. and Hannal Biotech.
Competition in the leukemia and lymphoma space is intense, with many compounds in clinical trials by large multinational pharmaceutical companies and specialized biotech companies. Rituxan (Roche), Adcetris (Seattle Genetics Inc./Takeda Pharmaceutical Company Ltd), Darzalex (Janssen) and Poteligeo (Kyowa Hakko Kirin Co., Ltd.) are some examples of monoclonal antibodies approved for the treatment of Hodgkin's lymphoma, non-Hodgkin's lymphoma, multiple myeloma or other blood cancers. In addition, we are aware of a number of other companies with development stage programs that may compete with ARGX-110 in the future. We anticipate that we will face intense and increasing competition as new treatments enter the market and advanced technologies become available.
Similarly, other companies have monoclonal antibody drug discovery platforms that may compete with us in the search for novel therapeutic antibody targets, including Adimab LLC; Merus N.V.; Regeneron Pharmaceuticals Inc.; Xencor Inc. and MorphoSys AG. We are aware that a product candidate in development by Scholar Rock, Inc. may compete with ARGX-115 and a product candidate in development by Ionis Pharmaceuticals, Inc. may compete with ARGX-116, if they are approved.
We depend on enrollment of patients in our clinical trials for our product candidates. If we are unable to enroll patients in our clinical trials, our research and development efforts and business, financial condition and results of operations could be materially adversely affected.
Identifying and qualifying patients to participate in our clinical trials is critical to our success. Patient enrollment depends on many factors, including the size and nature of the patient population, eligibility criteria for the trial, the proximity of patients to clinical sites, the design of the clinical protocol, the availability of competing clinical trials, the availability of new drugs approved for the indication the clinical trial is investigating, and clinicians' and patients' perceptions as to the potential advantages of the drug being studied in relation to other available therapies. Since some of our
22
product candidates are focused on addressing rare diseases and conditions, there are limited patient pools from which to draw in order to complete our clinical trials in a timely and cost-effective manner. For example, the number of patients suffering from each of myasthenia gravis, or MG; primary immune thrombocytopenia, or ITP; T-cell lymphoma, or TCL; and acute myeloid leukemia, or AML, is small and has not been established with precision. If the actual number of patients with these disorders is smaller than we anticipate, we may encounter difficulties in enrolling patients in our clinical trials, thereby delaying or preventing development and approval of our drug candidates. Even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials.
Furthermore, our efforts to build relationships with patient communities may not succeed, which could result in delays in patient enrollment in our clinical trials. In addition, any negative results we may report in clinical trials of our drug candidate may make it difficult or impossible to recruit and retain patients in other clinical trials of that same drug candidate. Delays in the completion of any clinical trial of our product candidates will increase our costs, slow down our product candidate development and approval process and delay or potentially jeopardize our ability to commence product sales and generate revenue. In addition, some of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
We may become exposed to costly and damaging liability claims, either when testing our product candidates in the clinic or at the commercial stage; and our product liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Currently, we have no products that have been approved for commercial sale; however, the current and future use of product candidates by us and our corporate collaborators in clinical trials, and the sale of any approved products in the future, may expose us to liability claims. These claims might be made by patients that use the product, healthcare providers, pharmaceutical companies, our corporate collaborators or others selling such products. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our product candidates or any prospects for commercialization of our product candidates. Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates. Regardless of the merits or eventual outcome, liability claims may result in:
23
Although we maintain adequate product liability insurance for our product candidates, it is possible that our liabilities could exceed our insurance coverage. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for any of our product candidates. However, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Should any of the events described above occur, this could have a material adverse effect on our business, financial condition and results of operations.
The regulatory approval processes of the FDA, the EMA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA, the EMA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate's clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
24
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, results of operations and prospects. The FDA, the EMA and other comparable foreign authorities have substantial discretion in the approval process, and determining when or whether regulatory approval will be obtained for any of our product candidates. Even if we believe the data collected from clinical trials of our product candidates are promising, such data may not be sufficient to support approval by the FDA, the EMA or any other regulatory authority.
In addition, even if we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
Even if our product candidates obtain regulatory approval, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products.
If the FDA, the EMA or a comparable foreign regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and GCPs for any clinical trials that we conduct post-approval, all of which may result in significant expense and limit our ability to commercialize such products. In addition, any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate.
Our product candidates are classified as biologics in the United States and, therefore, can only be sold if we obtain a BLA from the FDA. The holder of a BLA is obligated to monitor and report adverse events and any failure of a product to meet the specifications in the BLA. The holder of a BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Failure to comply with a BLA or any other ongoing regulatory obligation may result in suspension of approval to manufacture or distribute the relevant product, as well as fines or imprisonment for violations.
25
If there are changes in the application of legislation, regulations or regulatory policies, or if problems are discovered with a product or our manufacture of a product, or if we or one of our distributors, licensees or co-marketers fails to comply with regulatory requirements, the regulators could take various actions. These include imposing fines on us, imposing restrictions on the product or its manufacture and requiring us to recall or remove the product from the market. The regulators could also suspend or withdraw our marketing authorizations, requiring us to conduct additional clinical trials, change our product labeling or submit additional applications for marketing authorization. If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations.
Due to our limited resources and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates over other potential candidates. These decisions may prove to have been wrong and may adversely affect our revenues.
Because we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount of resources to allocate to each. Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular compounds, product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources away from better opportunities. Similarly, our decisions to delay, terminate or collaborate with third parties in respect of certain product development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the biopharmaceutical industry, in particular for our lead product candidates, our business, financial condition and results of operations could be materially adversely affected.
Risks Related to Commercialization of Our Product Candidates
Enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and may affect the prices we may set.
In the United States, the EU and other foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, became law. The ACA is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Among the provisions of the ACA of importance to our potential product candidates are the following:
26
federal poverty level, thereby potentially increasing a manufacturer's Medicaid rebate liability;
The current administration supports a repeal of the ACA and an Executive Order has been signed commanding federal agencies to try to waive or delay requirements of the ACA that impose economic or regulatory burdens on states, families, the health-care industry and others. The Executive Order also declares that the administration will seek the "prompt repeal" of the law and that the government should prepare to "afford the States more flexibility and control to create a more free and open healthcare market." At this time, the immediate impact of the Executive Order is not clear. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed. We cannot predict how the ACA, its possible repeal, or any legislation that may be proposed to replace the ACA will impact our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These new laws may result in additional reductions in Medicare and other healthcare funding. For example, on August 2, 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, including without limitation the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law,
27
which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other health care funding, which could have a material adverse effect on our customers and accordingly, our financial operations.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. The U.S. Department of Health and Human Services, or HHS, has set a goal of moving 30% of Medicare payments to alternative payment models by 2016 and 50% of Medicare payments into these alternative payment models by the end of 2018. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Further, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our product candidates, may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing conditions and other requirements.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
In the EU, similar political, economic and regulatory developments may affect our ability to profitably commercialize our current or any future products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the EU or member state level may result in significant additional requirements or obstacles that may increase our operating costs. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have
28
resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval. In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we or our collaborators are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or our collaborators are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
We may be subject to healthcare laws, regulation and enforcement; our failure to comply with these laws could harm our results of operations and financial conditions.
Although we do not currently have any products on the market, our current and future operations may be directly, or indirectly through our customers and third-party payors, subject to various U.S. federal and state healthcare laws and regulations, including, without limitation, the U.S. federal Anti-Kickback Statute. Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. These laws impact, among other things, our proposed sales, marketing and education programs and constrain our business and financial arrangements and relationships with third-party payors, healthcare professionals who participate in our clinical research program, healthcare professionals and others who recommend, purchase, or provide our approved products, and other parties through which we market, sell and distribute our products for which we obtain marketing approval. In addition, we may be subject to patient data privacy and security regulation by both the U.S. federal government and the states in which we conduct our business. Finally, our current and future operations are subject to additional healthcare-related statutory and regulatory requirements and enforcement by foreign regulatory authorities in jurisdictions in which we conduct our business. The laws that may affect our ability to operate include:
29
It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm and the curtailment or restructuring of our operations.
30
The risk of us being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. For example, the definition of the "remuneration" under the federal Anti-Kickback Statute has been interpreted to include anything of value. Further, courts have found that if "one purpose" of remuneration is to induce referrals, the federal Anti-Kickback Statute is violated.
Additionally, recent healthcare reform legislation has strengthened federal and state healthcare fraud and abuse laws. For example, the ACA amends the intent requirement of the federal Anti-Kickback Statute and criminal healthcare fraud statutes to clarify that liability under these statutes does not require a person or entity to have actual knowledge of the statutes or a specific intent to violate them. Moreover, the ACA provides that the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
If we fail to obtain orphan drug designation or obtain or maintain orphan drug exclusivity for our products, our competitors may sell products to treat the same conditions and our revenue will be reduced.
Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is intended to treat a rare disease or condition, defined as a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. In the EU, after a recommendation from the EMA's Committee for Orphan Medicinal Products, or COMP, the European Commission grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the EU. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition when, without incentives, it is unlikely that sales of the drug in the EU would be sufficient to justify the necessary investment in developing the drug or biological product or where there is no satisfactory method of diagnosis, prevention or treatment, or, if such a method exists, the medicine must be of significant benefit to those affected by the condition.
In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. In addition, if a product receives the first FDA approval for the indication for which it has orphan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity or where the manufacturer is unable to assure sufficient product quantity. In the
31
EU, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity following drug or biological product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
We may from time to time seek orphan drug designation in the United States or Europe for certain indications addressed by our product candidates. Even if we are able to obtain orphan designation, we may not be the first to obtain marketing approval for such indication due to the uncertainties associated with developing pharmaceutical products. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan drug is approved, the FDA or the EMA can subsequently approve the same drug with the same active moiety for the same condition if the FDA or the EMA concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process.
The successful commercialization of our product candidates will depend in part on the extent to which governmental authorities and health insurers establish adequate reimbursement levels and pricing policies. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue.
The availability and adequacy of coverage and reimbursement by governmental healthcare programs such as Medicare and Medicaid, private health insurers and other third-party payors are essential for most patients to be able to afford products such as our product candidates, assuming approval. Our ability to achieve acceptable levels of coverage and reimbursement for products by governmental authorities, private health insurers and other organizations will have an effect on our ability to successfully commercialize, and attract additional collaboration partners to invest in the development of our product candidates. Assuming we obtain coverage for a given product by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. We cannot be sure that coverage and reimbursement in the United States, the EU or elsewhere will be available for any product that we may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs when an equivalent generic drug or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidate and other therapies as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidate, pricing of existing drugs may limit the amount we will be able to charge for our product candidate. These payors may deny or revoke the reimbursement status of a given drug product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in product development. If reimbursement is not available or is available
32
only at limited levels, we may not be able to successfully commercialize our product candidates, and may not be able to obtain a satisfactory financial return on products that we may develop.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse health care providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
Obtaining and maintaining reimbursement status is time-consuming and costly. No uniform policy for coverage and reimbursement for drug products exists among third-party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of healthcare and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval.
Moreover, increasing efforts by governmental and third-party payors in the EU, the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional
33
legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
The future commercial success our product candidates will depend on the degree of market acceptance of our products among physicians, patients, healthcare payers and the medical community.
Our product candidates are at varying stages of development and we may never have a product that is commercially successful. To date, we have no product authorized for marketing. Our lead product candidates are in early stages of clinical development. Our lead product candidates will require further clinical investigation, regulatory review, significant marketing efforts and substantial investment before they can provide us with any significant revenues. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many other companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain regulatory approval for the marketing of their product. Due to the inherent risk in the development of pharmaceutical products, it is probable that not all or none of the product candidates in our portfolio will successfully complete development and be commercialized. We do not expect to be able to commercialize any of our products for a number of years. Furthermore, when available on the market, our products may not achieve an adequate level of acceptance by physicians, patients and the medical community, and we may not become profitable. In addition, efforts to educate the medical community and third-party payers on the benefits of our products may require significant resources and may never be successful which would prevent us from generating significant revenues or becoming profitable. Market acceptance of our future products by physicians, patients and healthcare payers will depend on a number of factors, many of which are beyond our control, including, but not limited to:
If our product candidates fail to gain market acceptance, this will have a material adverse impact on our ability to generate revenues to provide a satisfactory, or any, return on our investments. Even
34
if some products achieve market acceptance, the market may prove not to be large enough to allow us to generate significant revenues.
We have never commercialized a product candidate before and may lack the necessary expertise, personnel and resources to successfully commercialize our products on our own or together with suitable partners.
We do not have a sales or marketing infrastructure and have no experience in the sale or marketing of pharmaceutical products. To achieve commercial success for any approved product, we must develop or acquire a sales and marketing organization, outsource these functions to third parties or enter into partnerships.
We may decide to establish our own sales and marketing capabilities and promote our product candidates if and when regulatory approval has been obtained in the major EU countries and the United States. There are risks involved should we decide to establish our own sales and marketing capabilities or enter into arrangements with third parties to perform these services. Even if we establish sales and marketing capabilities, we may fail to launch our products effectively or to market our products effectively since we have no experience in the sales and marketing of pharmaceutical products. In addition, recruiting and training a sales force is expensive and time consuming and could delay any product launch. In the event that any such launch is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. Factors that may inhibit our efforts to commercialize our products on our own include:
If we enter into arrangements with third parties to perform sales and marketing services, our product revenues or the profitability of these product revenues to us could be lower than if we were to market and sell any products that we develop ourselves. Such collaborative arrangements with partners may place the commercialization of our products outside of our control and would make us subject to a number of risks including that we may not be able to control the amount or timing of resources that our collaborative partner devotes to our products or that our collaborator's willingness or ability to complete its obligations, and our obligations under our arrangements may be adversely affected by business combinations or significant changes in our collaborator's business strategy. In addition, we may not be successful in entering into arrangements with third parties to sell and market our products or may be unable to do so on terms that are favorable to us. Acceptable third parties may fail to devote the necessary resources and attention to sell and market our products effectively.
35
If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we may not be successful in commercializing our products, which in turn would have a material adverse effect on our business, prospects, financial condition and results of operations.
Our product candidates for which we intend to seek approval as biologic products may face competition sooner than anticipated.
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor's own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning are subject to uncertainty.
We believe that any of our product candidates approved as a biological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
Risks Related to Our Business and Industry
Nearly all aspects of our activities are subject to substantial regulation. No assurance can be given that any of our product candidates will fulfill regulatory compliance. Failure to comply with such regulations could result in delays, suspension, refusals and withdrawal of approvals as well as fines.
The international biopharmaceutical and medical technology industry is highly regulated by the FDA, the EMA and other comparable foreign authorities and by other national or supra-national regulatory authorities that impose substantial requirements covering nearly all aspects of our activities notably on research and development, manufacturing, preclinical tests, clinical trials, labeling, marketing, sales, storage, record keeping, promotion and pricing of our product candidates. Such regulation is further subject to regular review by the FDA, the EMA and other comparable foreign authorities which may result in changes in applicable regulation. If we do not comply with one or more of these requirements in a timely manner, or at all, our product development could experience significant delays as a result of the FDA, the EMA or other comparable regulatory authorities recommending non-approval or restrictions on approval of a product candidate, leading to an inability to successfully commercialize any of our product candidates, which would materially harm our business. Any failure of any of our product candidates in clinical studies or to receive regulatory approval could have a material adverse effect on our business, results of operations and financial condition. If any of our product candidates fails to obtain approval on the basis of any applicable condensed regulatory approval process, this will prevent such product candidate from
36
obtaining approval in a shortened time frame, or at all, resulting in increased expenses which would materially harm our business.
Compliance with requirements laid down by local regulatory authorities is necessary in each country where we, or any of our partners or licensees, conduct said activities in whole or in part. Local regulatory authorities notably include the EMA and the FDA. In order to market our future products in regions such as the European Economic Area, United States of America, Asia Pacific and many other foreign jurisdictions, we must obtain separate regulatory approvals. The approval procedures vary among countries and can require additional clinical testing, and the time required to obtain approval may differ from that required to obtain for example FDA or EMA approval. Moreover, clinical studies conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA or EMA does not ensure approval by the comparable foreign authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA or EMA.
There can be no assurance that our product candidates will fulfil the criteria required to obtain necessary regulatory approval to access the market. Also, at this time, we cannot guarantee or know the exact nature, precise timing and detailed costs of the efforts that will be necessary to complete the remainder of the development of our research programs and products candidates. Each of the FDA, the EMA and other comparable foreign authorities may impose its own requirements, may discontinue an approval or revoke a license, may refuse to grant approval, or may require additional data before granting approval, notwithstanding that approval may have been granted by the FDA, the EMA or one or more other comparable foreign authority. The FDA, the EMA or other comparable foreign authorities may also approve a product candidate for fewer or more limited indications or patient sub-segments than requested or may grant approval subject to the performance of post-marketing studies. The EMA's, the FDA's or other regulatory authority's approval may be delayed, limited or denied for a number of reasons, most of which are beyond our control. Such reasons could include, among others, the production process or site not meeting the applicable requirements for the manufacture of regulated products, or the products not meeting applicable requirements for safety, purity or potency, or efficacy, during the clinical development stage or after marketing. No assurance can be given that clinical trials will be approved the FDA, the EMA or other comparable foreign authorities or that products will be approved for marketing by such regulatory authorities in any pre-determined indication or intended use. Any of the FDA, the EMA and other comparable foreign authorities may disagree with our interpretation of data submitted for their review.
We and our collaborative partners are, or may become subject to, numerous ongoing other regulatory obligations, such as data protection, environmental, health and safety laws and restrictions on the experimental use of animals. The costs of compliance with such applicable regulations, requirements or guidelines could be substantial, and failure to comply could result in sanctions, including fines, injunctions, civil penalties, denial of applications for marketing authorization of our products, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly increase our or our collaborative partners' costs or delay the development and commercialization of our product candidates.
37
Because we are subject to environmental, health and safety laws and regulations, we may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities which may adversely affect our business and financial condition.
Our operations, including our research, development, testing and manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions.
As with other companies engaged in activities similar to ours, we face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of or exposure to hazardous or biological materials. Environmental, health and safety laws and regulations are becoming more stringent. We may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, our production and development efforts may be interrupted or delayed and our financial condition and results of operations may be materially adversely affected.
Our employees, independent contractors, principal investigators, CROs, consultants, vendors and collaboration partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk that our employees, independent contractors, principal investigators, CROs, consultants, vendors and collaboration partners may engage in fraudulent conduct or other illegal activities. Misconduct by these parties could include intentional, reckless and negligent conduct or unauthorized activities that violate: (i) the regulations of the FDA, the EMA and other comparable foreign authorities, including those laws that require the reporting of true, complete and accurate information to such authorities; (ii) manufacturing standards; (iii) federal and state data privacy, security, fraud and abuse and other healthcare laws and regulations in the United States and abroad; or (iv) laws that require the reporting of true, complete and accurate financial information and data. Specifically, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws could also involve the improper use or misrepresentation of information obtained in the course of clinical trials or creating fraudulent data in our preclinical studies or clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgements, possible exclusion from participation in Medicare, Medicaid and other U.S. federal healthcare programs, individual imprisonment, additional reporting requirements and oversight if we become
38
subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, other sanctions, contractual damages, reputational harm, diminished profits and future earnings and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Our high dependency on public perception of our products may negatively influence the success of these products.
If any of our product candidates are approved for commercial sale, we will be highly dependent upon consumer perceptions of the safety and quality of our products. We could be adversely affected if we were subject to negative publicity or if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to patients. Because of our dependence upon consumer perception, any adverse publicity associated with illness or other adverse effects resulting from patients' use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our business, prospects, financial condition and results of operations.
Future adverse events in research into the cancer, inflammation and severe autoimmune diseases that we focus our research efforts on, or the biopharmaceutical industry more generally, could also result in greater governmental regulation, stricter labeling requirements and potential regulatory delays in the testing or approvals of our products. Any increased scrutiny could delay or increase the costs of obtaining regulatory approval for our product candidates.
Failure to successfully identify, develop and commercialize additional products or product candidates could impair our ability to grow.
Although a substantial amount of our efforts will focus on the continued preclinical and clinical testing and potential approval of our product candidates in our current pipeline, a key element of our long-term growth strategy is to develop and market additional products and product candidates. Because we have limited financial and managerial resources, research programs to identify product candidates will require substantial additional technical, financial and human resources, whether or not any product candidates are ultimately identified. The success of this strategy depends partly upon our ability to identify, select and develop promising product candidates and products. Our technology platforms may fail to discover and to generate additional product candidates that are suitable for further development. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate may not be suitable for clinical development as a result of its harmful side effects, limited efficacy or other characteristics that indicate that it is unlikely to be a product that will receive approval by the FDA, the EMA and other comparable foreign regulatory authorities and achieve market acceptance. If we do not successfully develop and commercialize product candidates based upon our technological approach, we may not be able to obtain product or collaboration revenues in future periods, which would adversely affect our business, prospects, financial condition and results of operations.
Our long-term growth strategy to develop and market additional products and product candidates is heavily dependent on precise, accurate and reliable scientific data to identify, select and develop promising pharmaceutical product candidates and products. Our business decisions may therefore be adversely influenced by improper or fraudulent scientific data sourced from third parties. Any irregularities in the scientific data used by us to determine our focus in research and development of product candidates and products could have a material adverse effect on our business, prospects, financial condition and results of operations.
39
Service or supply failures, or other failures, business interruptions, or other disasters affecting the manufacturing facilities of any party participating in the supply chain, would adversely affect our ability to supply our products.
Our product candidates are biologics and require processing steps that are more difficult than those required for most chemical pharmaceuticals. Accordingly, multiple steps are needed to control the manufacturing processes. Problems with these manufacturing processes, even minor deviations from the normal process or from the materials used in the manufacturing process, which may not be detectable by us in a timely manner, could lead to product defects or manufacturing failures, resulting in lot failures, product recalls, product liability claims and insufficient inventory.
Also, certain raw materials or other products necessary for the manufacture and formulation of our product candidates, some of which are difficult to source, are provided by single-source unaffiliated third-party suppliers. In addition, we rely on certain third parties to perform filling, finishing, distribution, laboratory testing and other services related to the manufacture of our product candidates, and to supply various raw materials and other products. We would be unable to obtain these raw materials, other products, or services for an indeterminate period of time if any of these third parties were to cease or interrupt production or otherwise fail to supply these materials, products, or services to us for any reason, including due to regulatory requirements or actions (including recalls), adverse financial developments at or affecting the supplier, failure by the supplier to comply with cGMPs, contamination, business interruptions, or labor shortages or disputes. In any such circumstances, we may not be able to engage a backup or alternative supplier or service provider in a timely manner or at all. This, in turn, could materially and adversely affect our ability to supply product candidates, which could materially and adversely affect our business and future prospects.
Certain of the raw materials required in the manufacture and the formulation of our product candidates may be derived from biological sources, including mammalian tissues, bovine serum and human serum albumin. There are certain European regulatory restrictions on using these biological source materials. If we are required to substitute for these sources to comply with European regulatory requirements, our clinical development or commercial activities may be delayed or interrupted.
Our business may be adversely affected as a result of computer system failures.
Any of the internal computer systems belonging to us or our third-party service providers are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failure. Any system failure, accident or security breach that causes interruptions in our own or in third-party service vendors' operations could result in a material disruption of our product development programs. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our or our partners' regulatory approval efforts and significantly increase our costs in order to recover or reproduce the lost data. To the extent that any disruption or security breach results in a loss or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we may incur liability, our product development programs and competitive position may be adversely affected and the further development of our product candidates may be delayed. Furthermore, we may incur additional costs to remedy the damage caused by these disruptions or security breaches.
40
Risks Related to Our Dependence on Third Parties
We rely, and expect to continue to rely, on third parties, including independent clinical investigators and CROs, to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third parties, including independent clinical investigators and third-party CROs, to conduct our preclinical studies and clinical trials and to monitor and manage data for our ongoing preclinical and clinical programs. We rely on these parties for execution of our preclinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies and trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. We and our third party contractors and CROs are required to comply with GCP requirements, which are regulations and guidelines enforced by the FDA, the EMA and comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we, our investigators or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
Further, these investigators and CROs are not our employees and we will not be able to control, other than by contract, the amount of resources, including time, which they devote to our product candidates and clinical trials. If independent investigators or CROs fail to devote sufficient resources to the development of our product candidates, or if their performance is substandard, it may delay or compromise the prospects for approval and commercialization of any product candidates that we develop. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated.
Our CROs have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the subjects participating in our clinical trials warrants such termination, if we make a general assignment for the benefit of our creditors or if we are liquidated.
There is a limited number of third-party service providers that specialize or have the expertise required to achieve our business objectives. If any of our relationships with these third-party CROs or clinical investigators terminate, we may not be able to enter into arrangements with alternative CROs or investigators or to do so on commercially reasonable terms. If CROs or clinical investigators do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our
41
results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Switching or adding additional CROs (or investigators) involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We rely and will continue to rely on collaborative partners regarding the development of our research programs and product candidates. If we fail to enter into new strategic relationships our business, financial condition, commercialization prospects and results of operations may be materially adversely affected.
We are, and expect to continue to be, dependent on partnerships with partners relating to the development and commercialization of our existing and future research programs and product candidates. We currently have collaborative research relationships with various pharmaceutical companies such as Abbvie, Bird Rock Bio Inc. and Shire and with various academic and research institutions worldwide, for the development of product candidates resulting from such collaborations. We had, have and will continue to have discussions on potential partnering opportunities with various pharmaceutical companies. If we fail to enter into or maintain collaborations on reasonable terms or at all, our ability to develop our existing or future research programs and product candidates could be delayed, the commercial potential of our products could change and our costs of development and commercialization could increase.
Our dependence on collaborative partners subjects us to a number of risks, including, but not limited to, the following:
42
We face significant competition in seeking appropriate collaborative partners. Our ability to reach a definitive agreement for a partnership will depend, among other things, upon an assessment of the collaborator's resources and expertise, the terms and conditions of the proposed partnership and the proposed collaborator's evaluation of a number of factors. These factors may include the design or results of clinical trials, the likelihood of regulatory approval, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership regardless of the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a partnership could be more attractive than the one with us.
We rely on third parties to supply and manufacture our product candidates, and we expect to continue to rely on third parties to manufacture our products, if approved. The development of such product candidates and the commercialization of any products, if approved, could be stopped, delayed or made less profitable if any such third party fails to provide us with sufficient quantities of product candidates or products or fails to do so at acceptable quality levels or prices or fails to maintain or achieve satisfactory regulatory compliance
We do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our product candidates for use in the conduct of our clinical studies or for commercial supply, if our products are approved. Instead, we rely on, and expect to continue to rely on contract
43
manufacturing organizations, or CMOs. We currently rely mainly on Lonza Sales AG, or Lonza, based in Slough, UK for the manufacturing of the drug substance of all our products and the production cell line POTELLIGENT CHOK1SV jointly owned by Lonza and Biowa, Inc. for clinical and commercial scale production of ADCC enhanced antibody products. Reliance on third-party providers may expose us to more risk than if we were to manufacture our product candidates ourselves. We do not control the manufacturing processes of the CMOs we contract with and are dependent on those third parties for the production of our product candidates in accordance with relevant regulations (such as cGMP), which includes, among other things, quality control, quality assurance and the maintenance of records and documentation.
If we were to experience an unexpected loss of supply of or if any supplier were unable to meet our demand for any of our product candidates, we could experience delays in our research or planned clinical studies or commercialization. We could be unable to find alternative suppliers of acceptable quality, in the appropriate volumes and at an acceptable cost. Moreover, our suppliers are often subject to strict manufacturing requirements and rigorous testing requirements, which could limit or delay production. The long transition periods necessary to switch manufacturers and suppliers, if necessary, would significantly delay our clinical studies and the commercialization of our products, if approved, which would materially adversely affect our business, prospects, financial condition and results of operation.
In complying with the manufacturing regulations of the FDA, the EMA and other comparable foreign authorities, we and our third-party suppliers must spend significant time, money and effort in the areas of design and development, testing, production, record-keeping and quality control to assure that the products meet applicable specifications and other regulatory requirements. The failure to comply with these requirements could result in an enforcement action against us, including the seizure of products and shutting down of production. We and any of these third-party suppliers may also be subject to audits by the FDA, the EMA or other comparable foreign authorities. If any of our third-party suppliers fails to comply with cGMP or other applicable manufacturing regulations, our ability to develop and commercialize the products could suffer significant interruptions. We face risks inherent in relying on a single CMO, as any disruption, such as a fire, natural hazards or vandalism at the CMO could significantly interrupt our manufacturing capability. We currently do not have alternative production plans in place or disaster-recovery facilities available. In case of a disruption, we will have to establish alternative manufacturing sources. This would require substantial capital on our part, which we may not be able to obtain on commercially acceptable terms or at all. Additionally, we would likely experience months or years of manufacturing delays as we build or locate replacement facilities and seek and obtain necessary regulatory approvals. If this occurs, we will be unable to satisfy manufacturing needs on a timely basis, if at all. Also, operating any new facilities may be more expensive than operating our current facility. Further, business interruption insurance may not adequately compensate us for any losses that may occur and we would have to bear the additional cost of any disruption. For these reasons, a significant disruptive event of the manufacturing facility could have drastic consequences, including placing our financial stability at risk.
The manufacturing of all of our product candidates requires using cells which are stored in a cell bank. We have one master cell bank for each product manufactured in accordance with cGMP. Working cell banks have not yet been manufactured. Half of each master cell bank is stored at a separate site so that in case of a catastrophic event at one site we believe sufficient vials of the master cell banks are left at the alternative storage site to continue manufacturing. We believe sufficient working cell banks could be produced from the vials of the master cell bank stored at a given site to assure product supply for the future. However, it is possible that we could lose multiple cell banks and have our manufacturing significantly impacted by the need to replace these cell
44
banks, which could materially adversely affect our business, prospects, financial condition and results of operations.
Risks Related to Intellectual Property
We rely on patents and other intellectual property rights to protect our product candidates and the SIMPLE Antibody, NHance and ABDEG platform technologies, the enforcement, defense and maintenance of which may be challenging and costly. Failure to enforce or protect these rights adequately could harm our ability to compete and impair our business.
Our commercial success depends in part on obtaining and maintaining patents and other forms of intellectual property rights for our product candidates, methods used to manufacture those products and the methods for treating patients using those products, or on licensing in such rights. Failure to protect or to obtain, maintain or extend adequate patent and other intellectual property rights could materially adversely affect our ability to develop and market our products and product candidates.
We cannot be certain that patents will be issued or granted with respect to applications that are currently pending, or that issued or granted patents will not later be found to be invalid or enforceable. The patent position of biopharmaceutical companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the European Patent Office the United States Patent and Trademark Office, or USPTO, and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biopharmaceutical patents. Consequently, patents may not issue from our pending patent applications. As such, we do not know the degree of future protection that we will have on our proprietary products and technology. The scope of patent protection that the European Patent Office the USPTO will grant with respect to the antibodies in our antibodies product pipeline is uncertain. It is possible that the European Patent Office the USPTO will not allow broad antibody claims that cover antibodies closely related to our product candidates as well as the specific antibody. As a result, upon receipt of EMA or FDA approval, competitors may be free to market antibodies almost identical to ours, including biosimilar antibodies, thereby decreasing our market share. However, a competitor cannot submit to the FDA an application for a biosimilar product based on one of our products until four years following the date of approval of our "reference product," and the FDA may not approve such a biosimilar product until 12 years from the date on which the reference product was approved. See the section of this prospectus entitled "Biosimilars and Exclusivity" for more details regarding biosimilar regulatory exclusivities.
The patent prosecution process is expensive and time-consuming, and we and our current or future licensors, licensees or collaboration partners may not be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or our licensors, licensees or collaboration partners will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Further, the issuance, scope, validity, enforceability and commercial value of our and our current or future licensors', licensees' or collaboration partners' patent rights are highly uncertain. Our and our licensors' pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Moreover, in some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from or license to third parties and are reliant on our licensors, licensees or collaboration partners. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. If our current or future licensors, licensees or
45
collaboration partners fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our licensors, licensees or collaboration partners are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. The patent examination process may require us or our licensors, licensees or collaboration partners to narrow the scope of the claims of our or our licensors', licensees' or collaboration partners' pending and future patent applications, which may limit the scope of patent protection that may be obtained. We cannot assure you that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may an initiate opposition, interference, re-examination, post-grant review,inter partes review, nullification or derivation action in court or before patent offices, or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated. Our and our licensors', licensees' or collaboration partners' patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications, and then only to the extent the issued claims cover the technology.
Because patent applications are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications on or before March 15, 2013, an interference proceeding can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such applications after March 15, 2013, a derivation proceeding can be initiated by such third parties to determine whether our invention was derived from theirs. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license.
Issued patents covering one or more of our products or the SIMPLE Antibody, NHance and ABDEG platform technologies could be found invalid or unenforceable if challenged in court.
To protect our competitive position, we may from time to time need to resort to litigation in order to enforce or defend any patents or other intellectual property rights owned by or licensed to us, or to determine or challenge the scope or validity of patents or other intellectual property rights of third parties. As enforcement of intellectual property rights is difficult, unpredictable and expensive, and many of our or our licensors' or collaboration partners' adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we or our licensors or collaboration partners can. Accordingly, despite our or our licensors' or collaboration partners' efforts, we or our licensors or collaboration partners may not prevent third parties from infringing upon or misappropriating intellectual property rights we own or control, particularly in countries where the laws may not protect those rights as fully as in the EU and the United States. We may fail in enforcing our rights—in which case our competitors may be permitted to use our technology without being required to pay us any license fees. In addition, however, litigation involving our patents carries the risk that one or more of our patents will be held invalid (in whole or in part, on a claim-by-claim basis) or held unenforceable. Such an adverse court ruling could allow third parties to commercialize our products or use our SIMPLE Antibody, NHance and ABDEG platform technologies, and then compete directly with us, without payment to us.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the United States or in Europe, defendant counterclaims alleging invalidity or
46
unenforceability are commonplace. A claim for a validity challenge may be based on failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. A claim for unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the European Patent Office or the USPTO or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our SIMPLE Antibody, NHance and ABDEG platform technologies. Such a loss of patent protection could have a material adverse impact on our business. Further, litigation could result in substantial costs and diversion of management resources, regardless of the outcome, and this could harm our business and financial results. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without infringing our patents or other intellectual property rights.
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates, such that we could be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
Our competitive position may suffer if patents issued to third parties or other third-party intellectual property rights cover our products or elements thereof, our manufacture or uses relevant to our development plans, the targets of our product candidates, or other attributes of our product candidates or our technology. In such cases, we may not be in a position to develop or commercialize products or product candidates unless we successfully pursue litigation to nullify or invalidate the third-party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. We are aware of certain issued patents held by third parties that some may argue cover certain aspects of our product candidates, including ARGX-110 and ARGX-111. In the event that a patent has not expired at the time of approval of such product candidate and the patent owner were to bring an infringement action against us, we may have to argue that our product, its manufacture or use does not infringe a valid claim of the patent in question. Alternatively, if we were to challenge the validity of any issued U.S. patent in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. patent. This means that in order to prevail, we would need to present clear and convincing evidence as to the invalidity of the patent's claims. There is no assurance that a court would find in our favor on questions of infringement or validity. In the event that a patent is successfully asserted against us such that the patent is found to be valid and enforceable and infringed by our product, unless we obtain a license to such a patent, which may not be available on commercially reasonable terms or at all, we could be prevented from continuing to develop or commercialize our product. Similarly, the targets for certain of our product candidates have also been the subject of research by other companies, which have filed patent applications or have patents on aspects of the targets or their uses. There can be no assurance any such patents will not be asserted against us or that we will not need to seek licenses from such third parties. We may not be able to secure such licenses on acceptable terms, if at all, and any such litigation would be costly and time-consuming.
It is also possible that we failed to identify relevant patents or applications. For example, certain U.S. applications filed after November 29, 2000 that will not be filed outside the United States may remain confidential until patents issue. In general, patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is
47
claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our products or platform technology could have been filed by others without our knowledge. Furthermore, we operate in a highly competitive field, and given our limited resources, it is unreasonable to monitor all patent applications purporting to gain broad coverage in the areas in which we are active. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our products or the use of our products.
Third-party intellectual property right holders, including our competitors, may actively bring infringement claims against us. The granting of orphan drug status in respect of any of our product candidates does not guarantee our freedom to operate and is separate from our risk of possible infringement of third parties' intellectual property rights. We may not be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in marketing our products.
If we fail in any such dispute, in addition to being forced to pay damages, we or our licensees may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might, if possible, also be forced to redesign product candidates so that we no longer infringe the third-party intellectual property rights. Or, we may be required to seek a license to any such technology that we are found to infringe, which license may not be available on commercially reasonable terms, or at all. Even if we or our licensors or collaboration partners obtain a license, it may be non-exclusive (for example, the POTELLIGENT platform), thereby giving our competitors access to the same technologies licensed to us or our licensors or collaboration partners. In addition, we could be found liable for monetary damages, including treble damages and attorneys' fees, if we are found to have willfully infringed a patent. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
In addition, if the breadth or strength of protection provided by our or our licensors' or collaboration partners' patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Our ability to compete may be adversely affected if we are unsuccessful in defending against any claims by competitors or others that we are infringing upon their intellectual property rights.
The various markets in which we plan to operate are subject to frequent and extensive litigation regarding patents and other intellectual property rights. In addition, companies producing therapeutics to treat and potentially cure cancer, have employed intellectual property litigation as a means to gain an advantage over their competitors. As a result, we may be required to defend against claims of intellectual property infringement that may be asserted by our competitors against us and, if the outcome of any such litigation is adverse to us, it may affect our ability to compete effectively.
Our involvement in litigation, and in,e.g., any interference, derivation, reexamination,inter partes review opposition or post-grant proceedings or other intellectual property proceedings inside and outside of the EU or the United States may divert management time from focusing on business operations, could cause us to spend significant amounts of money and may have no guarantee of success. Any current and potential intellectual property litigation also could force us to do one or more of the following:
48
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, we could have a substantial adverse effect on the price of our ordinary shares. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We may not be successful in obtaining or maintaining necessary rights to our product candidates through acquisitions and in-licenses.
Because our programs may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license, maintain or use these proprietary rights. We may be unable to acquire or in-license any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that we identify as necessary for our product candidates. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and non-U.S. academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution's rights in technology resulting from the collaboration. Regardless of such option, we may be unable to negotiate a license within the specified timeframe or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our applicable product candidate or program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain a license to third-party intellectual property rights necessary for the development
49
of a product candidate or program, we may have to abandon development of that product candidate or program and our business and financial condition could suffer.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. If other entities use trademarks similar to ours in different jurisdictions, or have senior rights to ours, it could interfere with our use of our current trademarks throughout the world.
If we do not obtain protection under the Hatch-Waxman Amendments and similar non-U.S. legislation for extending the term of patents covering each of our product candidates, our business may be materially harmed.
Patents have a limited duration. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates, their manufacture, or use are obtained, once the patent life has expired, we may be open to competition from competitive medications, including biosimilar medications. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Depending upon the timing, duration and conditions of FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act and similar legislation in the EU. The Hatch-Waxman Act permits a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. The patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent applicable to an approved drug may be extended. However, we may not receive an extension if we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner than we expect. As a result, our revenue from applicable products could be reduced, possibly materially.
50
We enjoy only limited geographical protection with respect to certain patents and may face difficulties in certain jurisdictions, which may diminish the value of intellectual property rights in those jurisdictions.
We often file our first patent application (i.e., priority filing) at the European Patent Office. International applications under the Patent Cooperation Treaty, or PCT, are usually filed within twelve months after the priority filing. Based on the PCT filing, national and regional patent applications may be filed in additional jurisdictions where we believe our product candidates may be marketed. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant. Finally, the grant proceeding of each national/regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant patent offices, while granted by others. It is also quite common that depending on the country, the scope of patent protection may vary for the same product candidate or technology.
Competitors may use our and our licensors' or collaboration partners' technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we and our licensors or collaboration partners have patent protection, but enforcement is not as strong as that in the United States and the EU. These products may compete with our product candidates, and our and our licensors' or collaboration partners' patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the United States and the EU, and companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions.
Some countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, some countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired and our business and results of operations may be adversely affected.
Proceedings to enforce our and our licensors' or collaboration partners' patent rights in foreign jurisdictions could result in substantial costs and divert our and our licensors' or collaboration partners' efforts and attention from other aspects of our business, could put our and our licensors' or collaboration partners' patents at risk of being invalidated or interpreted narrowly and our and our licensors' or collaboration partners' patent applications at risk of not issuing and could provoke third parties to assert claims against us or our licensors or collaboration partners. We or our licensors or collaboration partners may not prevail in any lawsuits that we or our licensors or collaboration partners initiate and the damages or other remedies awarded, if any, may not be commercially meaningful.
51
If we fail to comply with our obligations under the agreements pursuant to which we license intellectual property rights from third parties, or otherwise experience disruptions to our business relationships with our licensors, we could lose the rights to intellectual property that are important to our business.
We are a party to license agreements under which we are granted rights to intellectual property that are important to our business and we expect that we may need to enter into additional license agreements in the future. Existing license agreements impose, and we expect that future license agreements will impose, various development obligations, payment of royalties and fees based on achieving certain milestones, as well as other obligations. If we fail to comply with our obligations under these agreements, the licensor may have the right to terminate the license. The termination of any license agreements or failure to adequately protect such license agreements could prevent us from commercializing product candidates covered by the licensed intellectual property. Several of our existing license agreements are sublicenses from third parties which are not the original licensor of the intellectual property at issue. Under these agreements, we must rely on our licensor to comply with its obligations under the primary license agreements under which such third party obtained rights in the applicable intellectual property, where we may have no relationship with the original licensor of such rights. If the licensors fail to comply with their obligations under these upstream license agreements, the original third-party licensor may have the right to terminate the original license, which may terminate the sublicense. If this were to occur, we would no longer have rights to the applicable intellectual property and, in the case of a sublicense, if we were not able to secure our own direct license with the owner of the relevant rights, which it may not be able to do at a reasonable cost or on reasonable terms, it may adversely affect our ability to continue to develop and commercialize the product candidates incorporating the relevant intellectual property.
Disputes may arise regarding intellectual property subject to a licensing agreement, including:
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
52
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological complexity and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the America Invents Act, or the AIA, has been recently enacted in the United States, resulting in significant changes to the U.S. patent system.
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a "first-to-file" system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded
53
a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application, but circumstances could prevent us from promptly filing patent applications on our inventions.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. The AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Additionally, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and protect other proprietary information.
We consider proprietary trade secrets, confidential know-how and unpatented know-how to be important to our business. We may rely on trade secrets or confidential know-how to protect our technology, especially where patent protection is believed to be of limited value. However, trade secrets and confidential know-how are difficult to maintain as confidential.
To protect this type of information against disclosure or appropriation by competitors, our policy is to require our employees, consultants, contractors and advisors to enter into confidentiality agreements with us. However, current or former employees, consultants, contractors and advisers may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third party obtained illegally and is using trade secrets or confidential know-how is expensive, time consuming and unpredictable. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction. Furthermore, if a competitor lawfully obtained or independently developed any of our trade secrets, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
Failure to obtain or maintain trade secrets or confidential know-how trade protection could adversely affect our competitive position. Moreover, our competitors may independently develop
54
substantially equivalent proprietary information and may even apply for patent protection in respect of the same. If successful in obtaining such patent protection, our competitors could limit our use of our trade secrets or confidential know-how.
Under certain circumstances, we may also decide to publish some know-how to attempt to prevent others from obtaining patent rights covering such know-how.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees, including our senior management, were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these employees executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed confidential information or intellectual property, including trade secrets or other proprietary information, of any such employee's former employer. Litigation may be necessary to defend against these claims.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we successfully prosecute or defend against such claims, litigation could result in substantial costs and distract management.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance and annuity fees on any issued patent are due to be paid to the USPTO, the European Patent Office and foreign patent agencies in several stages over the lifetime of the patent. The USPTO, the European Patent Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors or collaboration partners fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market, which would have an adverse effect on our business.
Risks Related to Our Organization and Operations
Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel.
Our success depends upon the continued contributions of our key management, scientific and technical personnel, many of whom have been instrumental for us and have substantial experience
55
with our therapies and related technologies. These key management individuals include the members of our board of directors, including Tim Van Hauwermeiren, our co-founder and Chief Executive Officer, Prof. Hans de Haard, our Chief Scientific Officer, and Dr. Nicolas Leupin, our Chief Medical Officer.
The loss of key managers and senior scientists could delay our research and development activities. In addition, our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon our ability to attract and retain highly qualified management, scientific and medical personnel. Many other biotechnology and pharmaceutical companies and academic institutions that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. Therefore, we might not be able to attract or retain these key persons on conditions that are economically acceptable. Furthermore, we will need to recruit new managers and qualified scientific personnel to develop our business if we expand into fields that will require additional skills. Our inability to attract and retain these key persons could prevent us from achieving our objectives and implementing our business strategy, which could have a material adverse effect on our business and prospects.
We expect to expand our development, regulatory and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We may not be able to integrate efficiently or achieve the expected benefits of any acquisitions of complementary businesses, product candidates or technologies.
Since our inception in 2008, we have grown organically without any acquisitions. Should we in the future contemplate to acquire any complementary business, product candidates or technologies, our ability to integrate and manage acquired businesses, product candidates or technologies effectively will depend upon a number of factors including the size of the acquired business, the complexity of any product candidate or technology and the resulting difficulty of integrating the acquired business's operations, if any. Our relationship with current employees or employees of any acquired business may become impaired. We may also be subject to unexpected claims and liabilities arising from such acquisitions. These claims and liabilities could be costly to defend, could be material to our financial position and might exceed either the limitations of any applicable indemnification provisions or the financial resources of the indemnifying parties. There can also be no assurance that we will be able to assess ongoing profitability and identify all actual or potential liabilities of a business, product candidate or technology prior to its acquisition. If we acquire businesses, product candidates or technologies that result in assuming unforeseen liabilities in respect of which it has not obtained contractual protections or for which protection is not available, this could materially adversely affect our business, prospects, financial condition and results of operations.
56
Our business is subject to economic, political, regulatory and other risks associated with international operations.
Our business is subject to risks associated with conducting business internationally. Accordingly, our future results could be harmed by a variety of factors, including:
We have obtained significant funding from agencies of the government of the Flemish region of Belgium and have benefited from certain research and development incentives. The tax authorities may challenge our eligibility for or our calculation of such incentives.
We have contracted over the past year numerous funding agreements with agencies of the Flemish government to partially finance our research and development programs. These funding agreements are subject to various criteria linked to employment and investment in the Flemish region of Belgium. We have committed to establish our operational site in the Flemish region, which must remain our major effective operational site, and to maintain our site and all our existing
57
activities, including research and development in the Flemish region. Similarly, our funding agreement with one such agency of the Flemish government requires us to maintain substantial research and development activities in the Flemish region. Such undertakings restrict our ability to choose the most convenient or cost-effective location of our premises.
If we were to breach these contractual obligations, we may be held liable by the agencies of the Flemish government with which we have funding agreements for any damage incurred by the such agencies resulting from the breach of contract and we could be required to reimburse in full the subsidies granted by such agencies.
Further, pursuant to the general terms of each grant, certain Flemish agencies are entitled to re-evaluate the subsidies granted to us in case of a fundamental change in our shareholding base, which could negatively impact the funding that we receive.
The research and development incentives from which we have benefited as a company active in research and development in Belgium can be offset against Belgian corporate income tax due. The excess portion may be refunded at the end of a five-year fiscal period for the Belgian research and development incentive. The research and development incentives are both calculated based on the amount of eligible research and development expenditure. The Belgian tax authorities may audit each research and development program in respect of which a tax credit has been claimed and assess whether it qualifies for the tax credit regime. The tax authorities may challenge our eligibility for, or our calculation of, certain tax reductions or deductions in respect of our research and development activities and, should the Belgian be successful, we may be liable for additional corporate income tax, and penalties and interest related thereto, which could have a significant impact on our results of operations and future cash flows. Furthermore, if the Belgian government decide to eliminate, or reduce the scope or the rate of, the research and development incentive benefit, either of which it could decide to do at any time, our results of operations could be adversely affected.
Exchange rate fluctuations or abandonment of the euro currency may materially affect our results of operations and financial condition.
Due to the international scope of our operations, our assets, earnings and cash flows are influenced by movements in exchange rates of several currencies, particularly the U.S. dollar, British pound and Swiss francs. Our functional currency is the euro and the majority of our operating expenses are paid in euros, but we also receive payments from our main business partners AbbVie and Shire in U.S. dollars and we regularly acquire services, consumables and materials in U.S. dollars, Swiss francs and British pounds. Further, potential future revenue may be derived from abroad, particularly from the United States. As a result, our business and share price may be affected by fluctuations in foreign exchange rates between the euro and these other currencies, which may also have a significant impact on our reported results of operations and cash flows from period to period. Currently, we do not have any exchange rate hedging arrangements in place.
In addition, the possible abandonment of the euro by one or more members of the European Union, or the EU, could materially affect our business in the future. Despite measures taken by the EU to provide funding to certain EU member states in financial difficulties and by a number of European countries to stabilize their economies and reduce their debt burdens, it is possible that the euro could be abandoned in the future as a currency by countries that have adopted its use. This could lead to the re-introduction of individual currencies in one or more EU member states, or in more extreme circumstances, the dissolution of the EU. The effects on our business of a potential dissolution of the EU, the exit of one or more EU member states from the EU or the abandonment
58
of the euro as a currency, are impossible to predict with certainty, and any such events could have a material adverse effect on our business, financial condition and results of operations
Recent developments relating to the United Kingdom's referendum vote in favor of withdrawal from the EU could adversely affect us.
The United Kingdom held a referendum on June 23, 2016 in which a majority voted for the United Kingdom's withdrawal from the EU, or Brexit. As a result of this vote, negotiations are expected to commence to determine the terms of the United Kingdom's withdrawal from the EU as well as its relationship with the EU going forward, including the terms of trade between the United Kingdom and the EU. The effects of Brexit have been and are expected to continue to be far-reaching. Brexit and the perceptions as to its impact may adversely affect business activity and economic conditions in Europe and globally and could continue to contribute to instability in global financial and foreign exchange markets. Brexit could also have the effect of disrupting the free movement of goods, services and people between the United Kingdom and the EU; however, the full effects of Brexit are uncertain and will depend on any agreements the United Kingdom may make to retain access to EU markets.
In addition, we expect that Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the United Kingdom determines which EU laws to replicate or replace. If the United Kingdom were to significantly alter its regulations affecting the pharmaceutical industry, we could face significant new costs. It may also be time-consuming and expensive for us to alter our internal operations in order to comply with new regulations. Altered regulations could also add time and expense to the process by which our product candidates receive regulatory approval in the United Kingdom and EU. Similarly, it is unclear at this time what Brexit's impact will have on our intellectual property rights and the process for obtaining, maintaining and defending such rights. It is possible that certain intellectual property rights, such as trademarks, granted by the EU will cease being enforceable in the United Kingdom absent special arrangements to the contrary, and we may be required to refile our trademarks and other intellectual property applications domestically in the United Kingdom. As a result of the Brexit, other European countries may seek to conduct referenda with respect to their continuing membership in the EU. Given these possibilities and others we may not anticipate, as well as the lack of comparable precedent, we cannot be certain of the full extent to which Brexit could adversely affect our business, results of operations and financial condition.
We are exposed to unanticipated changes in tax laws and regulations, adjustments to our tax provisions, exposure to additional tax liabilities, or forfeiture of our tax assets.
The determination of our provision for income taxes and other tax liabilities requires significant judgment, including the adoption of certain accounting policies and our determination of whether our deferred tax assets are, and will remain, tax effective. Although management believes its estimates and judgments are reasonable, they remain subject to review by the relevant tax authorities. We cannot guarantee that our interpretation or structure will not be questioned by the relevant tax authorities, or that the relevant tax laws and regulations, or the interpretation thereof, including through tax rulings, by the relevant tax authorities, will not be subject to change. Any adverse outcome of such a review may lead to adjustments in the amounts recorded in our financial statements, and could have a materially adverse effect on our operating results and financial condition.
We are subject to laws and regulations on tax levies and other charges or contributions in different countries, including transfer pricing and tax regulations for the compensation of personnel and third parties. Our tax structure involves a number of transfers and transfer price determinations between our parent company and our subsidiaries.
59
Our effective tax rates could be adversely affected by changes in tax laws, treaties and regulations, both internationally and domestically, or the interpretation thereof by the relevant tax authorities, including changes to the patent income deduction, possible changes to the corporate income tax base, wage withholding tax incentive for qualified research and development personnel in Belgium and other tax incentives and the implementation of new tax incentives such as the innovation deduction. An increase of the effective tax rates could have an adverse effect on our business, financial position, results of operations and cash flows.
In addition, we may not be able to use, or changes in tax regulations may affect the use of, certain tax assets or credits that we have built over the years. For instance, as of December 31, 2015, we had approximately €60.4 million of tax loss carry forwards. Some of these tax loss carry forwards may be forfeited in whole, or in part, as a result of transactions, or their utilization may be restricted by statutory law in the relevant jurisdiction. Any corporate reorganization by us or relating to our shareholding structure may result in partial or complete forfeiture of tax loss carry forwards. The tax burden would increase if profits, if any, could not be offset against tax loss carry forwards.
Risks Related to the Offering and Our Ordinary Shares
The price of our ordinary shares may be volatile and may fluctuate due to factors beyond our control.
The share price of publicly-traded emerging biopharmaceutical and drug discovery and development companies has been highly volatile and is likely to remain highly volatile in the future. The market price of our ordinary shares may fluctuate significantly due to a variety of factors, including:
These and other market and industry factors may cause the market price and demand for our securities to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ordinary shares and may otherwise negatively affect the liquidity of our ordinary shares. In addition, the stock market in general, and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
60
We will incur increased costs as a result of operating as a U.S.-listed public company, and our board of directors will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we no longer qualify as an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur as a public company listed on Euronext Brussels. We are a Dutch public company with limited liability (naamloze vennootschap). The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Stock Market, or NASDAQ, and other applicable securities rules and regulations impose various requirements on non-U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our board of directors and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors.
However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we will be required to furnish a report by our board of directors on our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Our ordinary shares will be traded on more than one market and this may result in price variations.
Our ordinary shares have been traded on Euronext Brussels since 2014 and we have applied to have our ordinary shares listed on NASDAQ. Trading in our ordinary shares on these markets will take place in different currencies (dollars on NASDAQ and euros on Euronext Brussels), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Belgium). The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on one of these markets could cause a decrease in the trading price of our ordinary shares on the other market.
61
There has been no active public market for our ordinary shares in the United States prior to this offering, and an active market in the shares may not develop in which investors can resell our ordinary shares.
Prior to this offering, while our ordinary shares have been traded on Euronext Brussels since 2014, there has been no active public market for our ordinary shares in the United States. We cannot predict the extent to which an active market for our ordinary shares in the United States will develop or be sustained after this offering, or how the development of such a market might affect the market price for our ordinary shares. The initial public offering price of our ordinary shares in this offering will be agreed upon between us and the underwriters based on a number of factors, including market conditions in effect at the time of the offering, which may not be indicative of the price at which our shares will trade following completion of the offering. Investors may not be able to sell their shares at or above the initial public offering price.
Certain significant shareholders will continue to own a substantial number of our ordinary shares and as a result, may be able to exercise control over us, including the outcome of shareholder votes. These shareholders may have different interests from us or your interests.
We have a number of significant shareholders. For an overview of our current significant shareholders, please see "Principal Shareholders." Following the completion of this offering, these significant shareholders and their affiliates, in the aggregate, will own approximately % of our ordinary shares.
Currently, we are not aware that any of our existing shareholders have entered or will enter into a shareholders' agreement with respect to the exercise of their voting rights. Nevertheless, depending on the level of attendance at our general meetings of shareholders, or the General Meeting, these significant shareholders could, alone or together, have the ability to determine the outcome of decisions taken at any such General Meeting. Any such voting by these shareholders may not be in accordance with our interests or those of our shareholders. Among other consequences, this concentration of ownership may have the effect of delaying or preventing a change in control and might therefore negatively affect the market price of our ordinary shares.
Future sales, or the possibility of future sales, of a substantial number of our ordinary shares could adversely affect the price of the shares and dilute shareholders.
If our existing shareholders sell, or indicate an intent to sell, substantial amounts of ordinary shares in the public market, the trading price of the ordinary shares could decline significantly and could decline below the public offering price in this offering. Upon completion of this offering, we will have outstanding ordinary shares, approximately of which are subject to a 90-day contractual lock-up. The representatives of the underwriters may permit us and the holders of the lock-up shares to sell shares prior to the expiration of the lock-up agreements. See "Underwriting." After the lock-up agreements pertaining to this offering expire, and based on the number of ordinary shares outstanding upon completion of this offering, additional shares will be eligible for sale in the public market, all of which shares are held by directors and certain key employees and will be subject to volume limitations under Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, for sales in the United States. In addition, ordinary shares subject to outstanding options under our equity incentive plans and the shares reserved for future issuance under our equity incentive plan will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. We also intend to enter into a registration rights agreement upon the closing of this offering pursuant to which we will agree under certain circumstances to file a registration statement to register the resale of the shares held by certain of our existing shareholders, as well as to cooperate in certain public offerings of such shares. In addition, we
62
intend to register all ordinary shares that we may issue under our equity compensation plans. Once we register these ordinary shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Shares Eligible for Future Sale" section of this prospectus.
Provisions of our Articles of Association or Dutch corporate law might deter acquisition bids for us that might be considered favorable and prevent or frustrate any attempt to replace or remove the then board of directors.
Provisions of our Articles of Association may make it more difficult for a third party to acquire control of us or effect a change in our board of directors. These provisions include:
If you purchase ordinary shares in this offering, you will suffer immediate dilution of your investment.
The initial public offering price of our ordinary shares is substantially higher than the as adjusted net tangible book value per share. Therefore, if you purchase ordinary shares in this offering, you will pay a price per share that substantially exceeds our as adjusted net tangible book value per share after this offering. To the extent outstanding options are exercised, you will incur further dilution. Based on the assumed initial public offering price of $ per share, you will experience immediate dilution of $ per share, representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the initial public offering price. See "Dilution."
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our board of directors will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our ordinary shares. The failure by our board of directors to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our ordinary shares to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We do not expect to pay cash dividends in the foreseeable future.
We have not paid any cash dividends since our incorporation. Even if future operations lead to significant levels of distributable profits, we currently intend that any earnings will be reinvested in our business and that cash dividends will not be paid until we have an established revenue stream to support continuing cash dividends. In addition, payment of any future dividends to shareholders would be subject to shareholder approval at our general meeting, upon proposal of the board of directors, which proposal would be subject to the approval of the majority of the non-executive directors after taking into account various factors including our business prospects, cash requirements, financial performance and new product development. In addition, payment of future cash dividends may be made only if our shareholders' equity exceeds the sum of our paid-in and called-up share capital plus the reserves required to be maintained by Dutch law or by our Articles of Association. Accordingly, investors cannot rely on cash dividend income from our ordinary shares and any returns on an investment in our ordinary shares will likely depend entirely upon any future appreciation in the price of our ordinary shares.
63
Holders of our ordinary shares outside the Netherlands may not be able to exercise preemptive rights.
In the event of an increase in our share capital, holders of our ordinary shares are generally entitled under Dutch law to full preemptive rights, unless these rights are excluded either by a resolution of the General Meeting, or by a resolution of the board of directors (if the board of directors has been designated by the General Meeting for this purpose). See "Description of Share Capital—Preemptive Rights." Certain holders of our ordinary shares outside the Netherlands, in particular U.S. holders of our ordinary shares, may not be able to exercise preemptive rights unless a registration statement under the Securities Act is declared effective with respect to our ordinary shares issuable upon exercise of such rights or an exemption from the registration requirements is available.
Investors with a reference currency other than euros or U.S. dollars will become subject to foreign exchange rate risk when investing in our ordinary shares
Our ordinary shares are denominated in euros (for ordinary shares listed on Euronext Brussels) and in U.S. dollars (for ordinary shares listed on NASDAQ), and any dividends to be announced in respect of such shares would be denominated in euros. An investment in our ordinary shares by an investor whose principal currency is not the euro or the U.S. dollar, as applicable, exposes the investor to currency exchange rate risk that may impact the value of the investment in such shares or any dividends.
We are a Dutch public company with limited liability (naamloze vennootschap). The rights of our shareholders may be different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions.
We are a Dutch public company with limited liability (naamloze vennootschap). Our corporate affairs are governed by our Articles of Association and by the laws governing companies incorporated in the Netherlands. The rights of shareholders and the responsibilities of members of our board of directors may be different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our board of directors is required by Dutch law to consider the interests of our company, our shareholders, our employees and other stakeholders, in all cases with due observation of the principles of reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a shareholder. See "Description of Share Capital—Comparison of Dutch Corporate Law, our Articles of Association and Board By-Laws and U.S. Corporate Law—Corporate Governance."
We are not obligated to and do not comply with all the best practice provisions of the Dutch Corporate Governance Code, which may affect your rights as a shareholder.
As a Dutch public company with limited liability (naamloze vennootschap), we are subject to the Dutch Corporate Governance Code dated December 8, 2016 which is in force as of the financial year starting on or after January 1, 2017, or the DCGC. The DCGC contains both principles and best practice provisions for board of directors, management boards, supervisory boards, shareholders and general meetings of shareholders, financial reporting, auditors, disclosure, compliance and enforcement standards. The DCGC applies to all Dutch companies listed on a regulated market, such as Euronext Brussels. The principles and best practice provisions apply to our board of directors (in relation to role and composition, conflicts of interest and independency requirements, board committees and remuneration), shareholders and the General Meeting (for example, regarding anti-takeover protection and our obligations to provide information to our shareholders) and financial reporting (such as external auditor and internal audit requirements). We
64
do not comply with all the best practice provisions of the DCGC. As a Dutch company, we are required to disclose in our annual report, filed in the Netherlands, whether we comply with the provisions of the DCGC. If we do not comply with the provisions of the DCGC (for example, because of a conflicting NASDAQ requirement or otherwise), we must list the reasons for any deviation from the DCGC in our annual report. See "Description of Share Capital—Comparison of Dutch Corporate Law, our Articles of Association and Board By-Laws and U.S. Corporate Law—Dutch Corporate Governance Code." This may affect your rights as a shareholder and you may not have the same level of protection as a shareholder in another Dutch public company with limited liability (naamloze vennootschap) listed in the Netherlands that fully complies with the DCGC.
Claims of U.S. civil liabilities may not be enforceable against us.
We are incorporated under the laws of the Netherlands. Substantially all of our assets are located outside the United States. The majority of the members of our board of directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws.
The United States and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to the Dutch court the final judgment rendered by the U.S. court. If and to the extent that the Dutch court finds that the jurisdiction of the U.S. court has been based on grounds which are internationally acceptable and that proper legal procedures have been observed, the court of the Netherlands will, in principle, give binding effect to the judgment of the U.S. court, unless such judgment contravenes principles of public policy of the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Code of Civil Procedure (Wetboek van Burgerlijke Rechtsvordering).
Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against us or members of our board of directors or certain experts named herein who are residents of the Netherlands or countries other than the United States any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws.
We are a foreign private issuer and, as a result, we will not be subject to U.S. proxy rules and will be subject to Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those of a U.S. domestic public company.
Upon the closing of this offering, we will report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for
65
insiders who profit from trades made in a short period of time; and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. In addition, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers. However, we are subject to Dutch laws and regulations with regard to such matters and intend to furnish quarterly unaudited financial information to the SEC on Form 6-K.
As a foreign private issuer and as permitted by the listing requirements of NASDAQ, we will rely on certain home country governance practices rather than the corporate governance requirements of NASDAQ.
We qualify as a foreign private issuer. As a result, in accordance with the listing requirements of NASDAQ, we will rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of NASDAQ. In accordance with Dutch law and generally accepted business practices, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from the requirement of NASDAQ Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Although we must provide shareholders with an agenda and other relevant documents for the General Meeting, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands, thus our practice will vary from the requirement of NASDAQ Listing Rule 5620(b). In addition, we have opted out of certain Dutch shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice varies from the requirements of NASDAQ Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. For an overview of our corporate governance principles, see "Description of Share Capital—Comparison of Dutch Corporate Law, our Articles of Association and Board By-Laws and U.S. Corporate Law—Corporate Governance." Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to these NASDAQ requirements.
We may lose our foreign private issuer status which would then require us to comply with the Exchange Act's domestic reporting regime and cause us to incur significant legal, accounting and other expenses.
We are a foreign private issuer and therefore we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We may no longer be a foreign private issuer as of June 30, 2018 (the end of our second fiscal quarter in the fiscal year after this offering), which would require us to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers as of January 1, 2018. In order to maintain our current status as a foreign private issuer, either (a) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the United States or (b)(i) a majority of our executive officers or directors may not be U.S. citizens or residents, (ii) more than 50 percent of our assets cannot be located in the United States and (iii) our business must be administered principally outside the United States. If
66
we lost this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC and NASDAQ rules. The regulatory and compliance costs to us under U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time consuming and costly. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors.
We are an "emerging growth company," and we cannot be certain if the reduced reporting requirements applicable to "emerging growth companies" will make our ordinary shares less attractive to investors.
We are an "emerging growth company," as defined in the U.S. Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an "emerging growth company," we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies," including not being required to comply with the auditor attestation requirements of Section 404, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. As an "emerging growth company," we are required to report only two years of financial results and selected financial data compared to three and five years, respectively, for comparable data reported by other public companies. We may take advantage of these exemptions until we are no longer an "emerging growth company." We could be an "emerging growth company" for up to five years, although circumstances could cause us to lose that status earlier, including if the aggregate market value of our ordinary shares held by non-affiliates exceeds $700 million as of any June 30 (the end of our second fiscal quarter) before that time, in which case we would no longer be an "emerging growth company" as of the following December 31 (our fiscal year-end). We cannot predict if investors will find our ordinary shares less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and the price of our ordinary shares may be more volatile.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our ordinary shares.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our ordinary shares.
67
We will be required to disclose changes made in our internal controls and procedures on a quarterly basis and our management will be required to assess the effectiveness of these controls annually. However, for as long as we are an "emerging growth company" under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an "emerging growth company" for up to five years. An independent assessment of the effectiveness of our internal controls could detect problems that our management's assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, the price of our ordinary shares and our trading volume could decline.
The trading market for our ordinary shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on us. If no or too few securities or industry analysts commence coverage on us, the trading price for our ordinary shares would likely be negatively affected. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
We do not anticipate being treated as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes for the current taxable year, but this conclusion is a factual determination that is made annually and thus may be subject to change. If we were to qualify as a PFIC, this could result in adverse U.S. tax consequences to certain U.S. holders.
Generally, if, for any taxable year, at least 75% of our gross income is passive income, or at least 50% of the value of our assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest, and gains from the sale or exchange of investment property and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. Our status as a PFIC depends on the composition of our income and the composition and value of our assets (for which purpose the total value of our assets may be determined in part by the market value of our ordinary shares, which are subject to change) from time to time. If we are characterized as a PFIC, U.S. holders of our ordinary shares may suffer adverse tax consequences, including having gains realized on the sale of our ordinary shares treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares by individuals who are U.S. holders, and having interest charges apply to distributions by us and the proceeds of sales of our ordinary shares. See "Material United States Income Tax and Dutch Tax Considerations—Certain Material U.S. Federal Income Tax Considerations to U.S. Holders—Passive Foreign Investment Company Considerations."
Based upon the expected value of our assets, including any goodwill, and the expected composition of our income and assets, we do not anticipate being treated as a PFIC with respect to the current taxable year, and should not be treated as such for subsequent taxable years. However, our status as a PFIC is a fact-intensive determination made on an annual basis, and we cannot provide any assurances regarding our PFIC status for the current, prior or future taxable years.
68
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, particularly the sections of this prospectus titled "Summary," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business," contains forward-looking statements. All statements other than present and historical facts and conditions contained in this prospectus, including statements regarding our future results of operations and financial positions, business strategy, plans and our objectives for future operations, are forward-looking statements. When used in this prospectus, the words "anticipate," "believe," "can," "could," "estimate," "expect," "intend," "is designed to," "may," "might," "will," "plan," "potential," "predict," "objective," "should," or the negative of these and similar expressions identify forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
You should refer to the section of this prospectus titled "Risk Factors" for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
69
The euro is our functional currency and the currency in which we report our financial results. The following table sets forth, for each period indicated, the low and high exchange rates of U.S. dollars per euro, the exchange rate at the end of such period and the average of such exchange rates on the last day of each month during such period, based on the noon buying rate of the Federal Reserve Bank of New York for the euro. As used in this document, the term "noon buying rate" refers to the rate of exchange for the euro, expressed in U.S. dollars per euro, as certified by the Federal Reserve Bank of New York for customs purposes. The exchange rates set forth below demonstrate trends in exchange rates, but the actual exchange rates used throughout this prospectus may vary.
| | 2012 | 2013 | 2014 | 2015 | 2016 | 2017* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
High | 1.3463 | 1.3816 | 1.3927 | 1.2015 | 1.1516 | 1.0802 | |||||||||||||
Low | 1.2062 | 1.2774 | 1.2101 | 1.0524 | 1.0375 | 1.0416 | |||||||||||||
Rate at end of period | 1.3186 | 1.3779 | 1.2101 | 1.0859 | 1.0552 | 1.0580 | |||||||||||||
Average rate per period | 1.2859 | 1.3281 | 1.3297 | 1.1096 | 1.1072 | 1.0644 | |||||||||||||
The following table sets forth, for each of the last six months, the low and high exchange rates of U.S. dollars per euro and the exchange rate at the end of the month based on the noon buying rate as described above.
| | August 2016 | September 2016 | October 2016 | November 2016 | December 2016 | January 2017 | February 2017* | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
High | 1.1334 | 1.1271 | 1.1212 | 1.1121 | 1.0758 | 1.0794 | 1.0802 | |||||||||||||||
Low | 1.1078 | 1.1158 | 1.0866 | 1.0560 | 1.0375 | 1.0416 | 1.0551 | |||||||||||||||
Rate at end of period | 1.1146 | 1.1238 | 1.0962 | 1.0578 | 1.0552 | 1.0794 | 1.0580 | |||||||||||||||
On , 2017, the noon buying rate of the Federal Reserve Bank of New York for the euro was €1.00 = $ . Unless otherwise indicated, currency translations in this prospectus reflect the , 2017 exchange rate.
70
Our ordinary shares have been trading on Euronext Brussels under the symbol "ARGX" since July 2014.
The following table sets forth for the periods indicated the reported high and low sale prices per ordinary share on Euronext Brussels in euros.
Period | High | Low | |||||
|---|---|---|---|---|---|---|---|
Annual: | |||||||
2014 (beginning July 10, 2014) | € | 8.75 | € | 6.23 | |||
2015 | € | 14.27 | € | 7.40 | |||
2016 | € | 15.99 | € | 9.23 | |||
2017 (through February 17, 2017) | € | 16.80 | € | 14.75 | |||
Quarterly: | |||||||
First Quarter 2015 | € | 10.15 | € | 7.40 | |||
Second Quarter 2015 | € | 14.27 | € | 8.60 | |||
Third Quarter 2015 | € | 11.75 | € | 8.46 | |||
Fourth Quarter 2015 | € | 11.35 | € | 8.71 | |||
First Quarter 2016 | € | 11.58 | € | 9.23 | |||
Second Quarter 2016 | € | 12.34 | € | 10.15 | |||
Third Quarter 2016 | € | 15.38 | € | 11.56 | |||
Fourth Quarter 2016 | € | 15.99 | € | 12.50 | |||
First Quarter 2017 (through February 24, 2017) | € | 16.80 | € | 14.75 | |||
Month ended: | |||||||
August 2016 | € | 14.20 | € | 12.28 | |||
September 2016 | € | 15.38 | € | 13.75 | |||
October 2016 | € | 15.40 | € | 13.40 | |||
November 2016 | € | 14.65 | € | 12.50 | |||
December 2016 | € | 15.99 | € | 14.40 | |||
January 2017 | € | 16.80 | € | 15.62 | |||
February 2017 (through February 24, 2017) | € | 16.48 | € | 14.75 | |||
On February 27, 2017, the last reported sale price of our ordinary shares on Euronext Brussels was €15.20 per share.
71
We estimate that we will receive net proceeds from this offering of approximately $ million (or approximately $ million if the underwriters exercise their option to purchase additional ordinary shares in full), assuming a public offering price of $ per share, based on the closing price of our ordinary shares on Euronext Brussels and the exchange rate on , 2017, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase or decrease in the assumed public offering price of $ per share would increase or decrease our net proceeds by $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus remains the same. Each increase or decrease of 1,000,000 shares in the aggregate number of shares offered by us would increase or decrease the net proceeds to us by $ million, assuming that the assumed public offering price remains the same. The actual net proceeds we receive will depend on the actual number of shares offered by us, the actual public offering price and other terms of this offering determined at pricing.
We intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and current financial assets, as follows:
This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. We may also use a portion of the net proceeds to in-license, acquire, or invest in additional businesses, technologies, products or assets. We cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering or the amounts that we will actually spend on the uses set forth above. Predicting the costs necessary to develop antibody candidates can be difficult. The amounts and timing of our actual expenditures and the extent of clinical development may vary significantly depending on numerous factors, including the progress, timing and completion of our development efforts, the status of and results from preclinical studies and any ongoing clinical trials or clinical trials we may commence in the future, the time and costs involved in obtaining regulatory approval for our product candidates as well as maintaining our existing collaborations and any collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
72
Based on our planned use of the net proceeds of this offering and our current cash, cash equivalents and current financial assets, we estimate that such funds will be sufficient to enable us to fund our operating expenses and capital expenditure requirements through at least the next months. We have based this estimate on assumptions that may prove to be incorrect, and we could use our available capital resources sooner than we currently expect.
Pending their use, we plan to invest the net proceeds from this offering in short- and intermediate-term interest-bearing obligations and certificates of deposit.
73
We have never paid or declared any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business.
Under Dutch law, a Dutch public company with limited liability (naamloze vennootschap) may only pay dividends if the shareholders' equity (eigen vermogen) exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law or our Articles of Association. Subject to such restrictions, any future determination to pay dividends would be at the discretion of our general meeting upon the proposal of our board of directors, with the consent of the majority of the non-executive directors.
74
The following table sets forth our cash, cash equivalents and current financial assets and our capitalization as of June 30, 2016 on:
You should read this table together with our consolidated financial statements and related notes include elsewhere in this prospectus, as well as "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations."
| | June 30, 2016 | ||||||
|---|---|---|---|---|---|---|---|
| | Actual | As adjusted(1) | |||||
| | (In thousands) | ||||||
Cash, cash equivalents and current financial assets | € | 108,744 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Equity: | |||||||
Share capital | € | 2,004 | |||||
Share premiums | 126,088 | ||||||
Accumulated deficit | (58,474 | ) | |||||
Other reserves | 5,782 | ||||||
Total equity | 75,400 | ||||||
| | | | | | | | |
Total capitalization | € | 75,400 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
75
If you invest in the ordinary shares in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share and the as adjusted net tangible book value per share after this offering. Our net tangible book value as of June 30, 2016 was € million ($ million), or € ($ ) per share. Net tangible book value per share is determined by dividing our total assets less our intangible assets and our total liabilities by the number of ordinary shares outstanding as of June 30, 2016.
After giving effect to our sale of ordinary shares in this offering at an assumed public offering price of $ per share, based on the closing price of our ordinary shares on Euronext Brussels and the exchange rate on , 2017, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2016 would have been € million ($ million), or € ($ ) per share. This amount represents an immediate increase in net tangible book value of $ per share to our existing shareholders and an immediate dilution in net tangible book value of $ per share to new investors.
The following table illustrates this dilution on a per share basis:
Assumed initial public offering price per share | $ | ||||||
Historical net tangible book value per share as of June 30, 2016 | $ | ||||||
Increase in net tangible book value per share attributable to new investors participating in this offering | |||||||
| | | | | | | | |
As adjusted net tangible book value per share after this offering | |||||||
| | | | | | | | |
Dilution per share to new investors participating in this offering | $ | ||||||
| | | | | | | | |
If the underwriters exercise their option to purchase additional shares in full, the as adjusted net tangible book value per share after this offering as of June 30, 2016 would have been € ($ ) per share, the increase in the as adjusted net tangible book value to existing shareholders would be $ per share, and the dilution to new investors participating in this offering would be $ per share.
Each $1.00 increase or decrease in the assumed public offering price would increase or decrease our as adjusted net tangible book value by € million ($ million), or $ per share, and would increase or decrease dilution to new investors participating in this offering by $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. An increase of 1,000,000 shares offered by us would increase the as adjusted net tangible book value by € million ($ million), or $ per share, and the dilution to new investors participating in this offering would be $ per share, assuming that the assumed public offering price remains the same. A decrease of 1,000,000 shares offered by us would decrease the as adjusted net tangible book value by € million ($ million), or $ per share, and the dilution to new investors participating in this offering would be $ per share, assuming that the assumed public offering price remains the same.
The following table sets forth, as of June 30, 2016, on the as adjusted basis described above, the consideration paid to us for ordinary shares purchased from us by our existing shareholders and by new investors participating in this offering, assuming a public offering price of $ per share,
76
based on the closing price of our ordinary shares on Euronext Brussels and the exchange rate on , 2017, before deducting underwriting discounts and commissions and estimated offering expenses payable by us:
| | Ordinary shares purchased from us | Total consideration | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Average price per ordinary share | ||||||||||||||
| | Number | Percent | Amount | Percent | |||||||||||
Existing shareholders | % | $ | % | $ | |||||||||||
New investors | |||||||||||||||
| | | | | | | | | | | | | | | | |
Total | 100.0 | % | $ | 100.0 | % | ||||||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Each $1.00 increase or decrease in the assumed public offering price would increase or decrease the total consideration paid by new investors participating in this offering by $ million and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage points, assuming that the number of shares offered by us, as set forth on the cover page of the prospectus, remains the same and before deducting underwriting discounts and commissions. An increase or decrease in the aggregate number of shares offered by us by 1,000,000 shares would increase or decrease the total consideration paid by new investors participating in this offering by $ million and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage points, assuming that the assumed public offering price remains the same and before deducting underwriting discounts and commissions.
If the underwriters exercise their option to purchase additional ordinary shares in full, the number of shares held by the existing shareholders after this offering would be reduced to % of the total number of ordinary shares outstanding after this offering, and the number of ordinary shares held by new investors participating in this offering would increase to , or % of the total number of ordinary shares outstanding after this offering.
The table above excludes ordinary shares issuable upon the exercise of share options outstanding as of June 30, 2016 at a weighted average exercise price of € ($ ) per share.
77
SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected consolidated financial data for the periods indicated. The selected consolidated financial data as of and for the year ended December 31, 2015 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. The selected consolidated financial data as of and for the six months ended June 30, 2015 and 2016 have been derived from our unaudited interim condensed financial statements included elsewhere in this prospectus. The unaudited interim condensed consolidated financial statements have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly our financial position as of June 30, 2015 and 2016 and the results of operations for the six months ended June 30, 2015 and 2016.
Our historical results for any prior period are not necessarily indicative of results to be expected in any future period and our interim results are not necessarily indicative of results to be expected for the full year ending December 31, 2016, or any other period. The information set forth below should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and with our consolidated financial statements and notes thereto included elsewhere in this prospectus.
We present our financial data in euros and prepare our financial statements in accordance with IFRS as issued by the IASB.
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands, except share and per share data) | |||||||||
Statement of profit and loss and other comprehensive income data: | ||||||||||
Revenue | € | 6,854 | € | 2,708 | € | 5,656 | ||||
Other operating income | 3,101 | 1,640 | 1,317 | |||||||
Research and development expenses | (20,635 | ) | (9,284 | ) | (11,263 | ) | ||||
General and administrative expenses | (4,925 | ) | (2,314 | ) | (3,063 | ) | ||||
| | | | | | | | | | | |
Operating loss | (15,605 | ) | (7,250 | ) | (7,353 | ) | ||||
| | | | | | | | | | | |
Financial income | 112 | 100 | 39 | |||||||
Exchange gains (losses) | 181 | 130 | (42 | ) | ||||||
| | | | | | | | | | | |
Total comprehensive loss | € | (15,312 | ) | € | (7,020 | ) | € | (7,356 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Weighted average number of shares outstanding | 15,734,007 | 15,705,112 | 17,356,799 | |||||||
Basic and diluted loss per share | € | (0.97 | ) | € | (0.45 | ) | € | (0.42 | ) | |
| | | As of June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Statement of financial position data: | ||||||||||
Cash, cash equivalents and current financial assets | € | 42,327 | € | 50,548 | € | 108,744 | ||||
Total assets | 45,962 | 54,523 | 117,334 | |||||||
Deferred revenue | 4,141 | 5,502 | 36,786 | |||||||
Total liabilities | 8,684 | 10,339 | 41,934 | |||||||
Total equity | 37,278 | 44,184 | 75,400 | |||||||
78
MANAGEMENT'S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion of our financial condition and results of operations in conjunction with "Selected Consolidated Financial Data" and our audited consolidated financial statements and unaudited interim condensed financial statements, including the notes thereto, included elsewhere in this prospectus. The following discussion includes forward-looking statements that involve certain risks and uncertainties. Our actual results could differ materially from those discussed in these statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in "Risk Factors."
Overview
We are a clinical-stage biotechnology company developing a deep pipeline of differentiated antibody-based therapies for the treatment of severe autoimmune diseases and cancer. Utilizing our suite of technologies, we are focused on developing product candidates with the potential to be either first-in-class against novel targets or best-in-class against known, but complex, targets in order to treat diseases with a significant unmet medical need. Our SIMPLE Antibody Platform, based on the powerful llama immune system, allows us to exploit novel and complex targets, and our three antibody Fc engineering technologies are designed to enable us to expand the therapeutic index of our product candidates. Together with our antibody discovery and development expertise, this suite of technologies has enabled our pipeline of seven product candidates. Two of our product candidates will be in clinical proof-of-concept trials for three indications within the first half of 2017.
Our most advanced product candidate, ARGX-113, is in a Phase 2 clinical trial for the treatment of the rare autoimmune disease myasthenia gravis, or MG, and, in March 2017, we plan to initiate a Phase 2 clinical trial of ARGX-113 for the treatment of another rare autoimmune disease, primary immune thrombocytopenia, or ITP. We are currently developing our second lead product candidate, ARGX-110, for rare and aggressive hematological cancers, initially for T-cell lymphoma, or TCL, and acute myeloid leukemia, or AML, as well as high-risk myelodysplastic syndrome, or MDS. In December 2016, we commenced a Phase 1/2 clinical trial of ARGX-110 in combination with azacitidine for the treatment of newly diagnosed AML or high-risk MDS patients, and in March 2017, we expect to initiate the Phase 2 part of a Phase 1/2 clinical trial of ARGX-110 for the treatment of cutaneous TCL, or CTCL.
We have a disciplined strategy to maximize the value of our pipeline whereby we plan to retain development and commercialization rights to those product candidates that we believe we can ultimately commercialize successfully on our own if they are approved. We plan to collaborate on product candidates that we believe have promising utility in disease areas or patient populations that are better served by the resources of larger biopharmaceutical companies. We have entered into collaborations with a number of biopharmaceutical companies, including our collaboration with AbbVie S.Á.R.L., or AbbVie, for ARGX-115, a cancer immunotherapy-focused product candidate, against the novel target glycoprotein A repetitions predominant. We received a $40.0 million (€35.1 million as of the date the payment was received) upfront payment in connection with this collaboration.
Since our inception in 2008, we have focused most of our financial resources and efforts towards developing our SIMPLE Antibody Platform and antibody engineering technologies, identifying potential product candidates, establishing process, development and manufacturing capabilities for our product candidates and advancing multiple discovery programs into the clinic. We have advanced four internally developed product candidates into clinical development—ARGX-113,
79
ARGX-110, ARGX-111 and ARGX-109—three into the preclinical stage—ARGX-115, ARGX-112 and ARGX-116—and currently have multiple programs in the discovery stages. Through June 30, 2016, we have raised an aggregate of €144.5 million, including (i) an aggregate of €46.0 million from the private placement of equity securities in 2008, 2009 and 2011, (ii) €41.8 million from our initial public offering on the Euronext Brussels in 2014, (iii) €46.0 million from the private placement of equity securities, primarily to U.S.-based institutional investors, in 2016 and (iv) €10.7 million from governmental bodies. In addition, we have received upfront payments, milestone payments and research and development service fees from our collaborators totaling €58.3 million as of June 30, 2016. As of June 30, 2016, we had cash, cash equivalents and current financial assets of €108.7 million.
Since our inception, we have incurred significant operating losses. We do not currently have any approved products and have never generated any revenue from product sales. Our ability to generate revenue sufficient to achieve profitability will depend significantly upon the successful development and eventual commercialization of one or more of our product candidates, which may never occur. For the year ended December 31, 2015, we incurred total comprehensive losses of €15.3 million and for the six months ended June 30, 2015 and 2016, we incurred total comprehensive losses of €7.0 million and €7.4 million, respectively. As of June 30, 2016, we had an accumulated loss of €58.5 million.
We expect our expenses to increase substantially in connection with our ongoing development activities related to our preclinical and clinical programs. In addition, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company in the United States. We anticipate that our expenses will increase substantially if and as we:
80
As a result, we will need additional financing to support our continuing operations. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
Collaboration Agreements
We have a disciplined strategy to maximize the value of our pipeline whereby we plan to retain all development and commercialization rights to those product candidates that we believe we can ultimately commercialize successfully, if approved. We have partnered, and plan to continue to partner, product candidates that we believe have promising utility in disease areas or patient populations that are better served by resources of larger biopharmaceutical companies. Below are summaries of our key collaborations. See "Business—Collaborations" for a more detailed description of these agreements.
AbbVie. In April 2016, we entered into a collaboration agreement with AbbVie to develop and commercialize ARGX-115. Under the terms of the collaboration agreement, we will be responsible for conducting and funding all ARGX-115 research and development activities up to completion of investigational new drug, or IND, -enabling studies.
We have granted AbbVie an exclusive option, for a specified period following completion of IND-enabling studies, to obtain a worldwide, exclusive license to the ARGX-115 program to develop and commercialize products. Following the exercise of the option, AbbVie will assume certain development obligations, and will be solely responsible for all research, development and regulatory costs relating to the products. We received an upfront, non-refundable, non-creditable payment of $40 million (€35.1 million as of the date the payment was received) from AbbVie for the exclusive option to license ARGX-115 and are eligible to receive two near-term preclinical milestones of $10 million each. We are also eligible, if AbbVie exercises its option, to receive additional development, regulatory and commercial milestone payments in an aggregate of up to $625 million as well as tiered royalties on sales at percentages ranging from the mid-single digits to the lower teens, subject to customary reductions. In addition to the ARGX-115 program, and upon reaching a predetermined preclinical stage milestone, AbbVie will fund further GARP-related research by us for an initial period of two years. AbbVie will have the right to license additional therapeutic programs emerging from this research, for which we could receive associated milestone and royalty payments.
If AbbVie does not exercise its option to license ARGX-115, we have the right to pursue development and commercialization of ARGX-115 by ourselves or with another partner.
Bird Rock Bio. In October 2012, we entered into an exclusive license agreement with Bird Rock Bio, Inc., or Bird Rock Bio, under which we granted Bird Rock Bio an exclusive, worldwide, royalty-bearing license to develop and commercialize ARGX-109. We received a non-refundable, non-creditable upfront payment from Bird Rock Bio of €0.5 million in cash plus shares of Bird Rock Bio stock, and we are eligible to receive additional development, regulatory and commercial milestone payments of up to approximately €32.5 million in cash and additional shares of Bird Rock Bio stock. We are eligible to receive tiered royalties on Bird Rock Bio's commercial sales of ARGX-109 at percentages ranging from the low to high single digits and a tiered percentage of Bird Rock Bio's sublicensing income in the low double digits, subject to customary reductions.
LEO Pharma. In May 2015, we entered into a collaboration agreement with LEO Pharma A/S, or LEO Pharma, to develop and commercialize ARGX-112. We are eligible to receive a total of
81
€4.5 million in pre-IND payments from LEO Pharma, including an upfront payment that we received in 2015 and milestone payments. We are also eligible to receive clinical, regulatory and commercial milestone payments in an aggregate of approximately €100 million, as well as tiered royalties on product sales at percentages ranging from the low single digits to the low teens, subject to customary reductions. Under the terms of the collaboration, LEO Pharma will fund more than half of all product development costs up to approval of a Clinical Trial Authorization Application, or CTA, in Europe for a first product in a Phase 1 clinical trial, with our share of such costs capped. After CTA approval of a first product in a Phase 1 clinical trial, LEO Pharma will be solely responsible to fund the clinical development of the program.
Shire. In February 2012, we entered into a collaboration agreement with Shire AG (now known as Shire International Gmbh), or Shire, to discover, develop and commercialize novel human therapeutic antibodies against up to three targets to address diverse rare and unmet diseases. In May 2014, we expanded the collaboration agreement to accommodate research and development on several novel targets implicated in multiple disease areas.
Through June 30 2016, Shire has paid us an aggregate total of (i) €3.4 million in upfront payments, (ii) €0.3 million in milestone payments and (iii) $8.3 million in research and development fees. In addition, Shire purchased €12.0 million of our common stock in July 2014 by participating in our initial public offering on Euronext Brussels.
Shire has the option to license antibodies discovered under the agreement for further development and commercialization worldwide, in return for milestone payments and single-digit percentage royalties on product sales.
Bayer. In May 2014, we entered into a research collaboration and exclusive product license option agreement with Bayer AG, focused on the creation of novel human therapeutic antibodies against complex targets in various therapeutic indications using our SIMPLE Antibody technology. We received technology access fees and research funding totaling €3.3 million. We concluded all research under this collaboration in 2016 and we have no further commitment pursuant to this agreement.
Basis of Presentation
Revenue
To date, our revenue has consisted principally of collaboration revenue consisting of (i) upfront payments, including upfront licensing fees, (ii) milestone payments based on achievement of research and development goals and (iii) research and development service fees related to charges for full time equivalents, or FTEs, at contracted rates and reimbursement of research and development expenses. We currently have no products approved for sale. Other than the sources of revenue described above, we do not expect to receive any revenue from any product candidates that we develop, including ARGX-113, ARGX-110 and our preclinical product candidates, until we obtain regulatory approval and commercialize such products, or until we potentially enter into collaborative agreements with third parties for the development and commercialization of such candidates.
Collaborations typically contain license fees, non-refundable upfront fees, research and development service fees and milestone payments and may involve multiple elements. We evaluate whether the elements under these arrangements have value to our collaboration partner on a standalone basis. If we determine that multiple deliverables exist, the consideration is allocated to one or more units of accounting based upon the best estimate of the selling price of each
82
deliverable. Deliverables that do not meet these criteria are not evaluated separately for the purpose of revenue recognition.
Other Operating Income
As a company that carries extensive research and development activities, we benefit from various grants, research and development incentives and payroll tax rebates from certain governmental agencies. These grants and research and development incentives generally aim to partly reimburse approved expenditures incurred in our research and development efforts. The primary grants, research and development incentives and payroll tax rebates are as follows:
Government Grants
Research and Development Incentives
Payroll Tax Rebates
The government grants and research and development incentives generally aim to partly reimburse approved expenditures incurred in our research and development efforts and are credited to the income statement, under other operating income, when the relevant expenditure has been incurred and there is reasonable assurance that the grant or research and development incentive is receivable.
Research and Development Expenses
Research and development expenses consist principally of:
83
We incur various external expenses under our collaboration agreements for material and services consumed in the discovery and development of our partnered product candidates. Under our agreements with Shire, LEO Pharma and Bayer, our collaboration partner reimburses us for part or all of these external expenses and compensates us for time spent on the project by our employees. Under our agreement with AbbVie, our own research and development expenses are not reimbursed. Research and development expenses are recognized in the period in which they are incurred.
We typically utilize our employee, consultant and infrastructure resources across all of our development programs. We separately track external development costs with respect to ARGX-113 and ARGX-110, our most advanced product candidates.
Our research and development expenses may vary substantially from period to period based on the timing of our research and development activities, including the timing of the initiation of clinical trials, production of product batches and enrolment of patients in clinical trials. Research and development expenses are expected to increase as we advance the clinical development of ARGX-113 and ARGX-110 and the preclinical development of ARGX-115 and further advance the research and development of our other preclinical and discovery stage product candidates. The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from, any of our product candidates. This is due to numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
Any of these variables with respect to the development of ARGX-113, ARGX-110 or any other product candidate that we may develop could result in a significant change in the costs and timing associated with, and the viability of, the development of such product candidates. For example, if the FDA, the EMA or other regulatory authority were to require us to conduct preclinical studies or clinical trials beyond those we currently anticipate will be required for the completion of clinical development or if we experience significant delays in enrolment in any clinical trials, we could be required to expend significant additional financial resources and time on the completion of our clinical development programs and the viability of the product candidate in question could be adversely affected.
84
General and Administrative Expenses
General and administrative expenses consist primarily of (i) personnel expenses relating to salaries and related costs for personnel, including share-based compensation, of our employees in executive, finance, business development and support functions, (ii) consulting fees relating to professional fees for accounting, business development, IT, audit and legal services and investor relations costs, (iii) supervisory board expenses consisting of directors' fees, (iv) allocated facilities costs and (v) other general and administrative expenses, including leasing costs, office expenses, travel costs.
We expect our general and administrative expenses to increase as we continue to support our growth and as we prepare to become and operate as a public company in the United States. Such costs include increases in our finance and legal personnel, additional external legal and audit fees, and expenses and costs associated with compliance with the regulations governing public companies. We also expect to incur increased costs for directors' and officers' liability insurance and an enhanced investor relations function.
Financial Income (Expense)
Financial income reflects interest earned on the financial investments of our cash and cash equivalents and financial assets. Financial expense corresponds to interest expenses.
Exchange Gains (Losses)
Our exchange gains (losses) relate to our transactions denominated in foreign currencies, mainly in U.S. dollar and British pounds, which generate exchange gains or losses.
Income tax
We have a history of losses. We expect to continue incurring losses as we continue to invest in our clinical and pre-clinical development programs and our discovery platform. Consequently, we do not have any deferred tax asset on our statement of financial position.
Results of Operation
Presentation of Year Ended December 31, 2015 and Comparison of Six-Month Periods Ended June 30, 2015 and 2016
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended, December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Revenue | € | 6,854 | € | 2,708 | € | 5,656 | ||||
Other operating income | 3,101 | 1,640 | 1,317 | |||||||
| | | | | | | | | | | |
Total operating income | 9,955 | 4,348 | 6,973 | |||||||
| | | | | | | | | | | |
Research and development expenses | (20,635 | ) | (9,284 | ) | (11,263 | ) | ||||
General and administrative expenses | (4,925 | ) | (2,314 | ) | (3,063 | ) | ||||
| | | | | | | | | | | |
Operating loss | (15,605 | ) | (7,250 | ) | (7,353 | ) | ||||
| | | | | | | | | | | |
Financial income | 112 | 100 | 39 | |||||||
Exchange gains (losses) | 181 | 130 | (42 | ) | ||||||
| | | | | | | | | | | |
Total comprehensive loss | € | (15,312 | ) | € | (7,020 | ) | € | (7,356 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
85
Revenue
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Upfront payments | € | 2,194 | € | 858 | € | 2,499 | ||||
Milestone payments | 343 | — | 500 | |||||||
Research and development service fees | 4,317 | 1,850 | 2,657 | |||||||
| | | | | | | | | | | |
Total revenue | € | 6,854 | € | 2,708 | € | 5,656 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Our revenue increased by €3.0 million in the six-month period ended June 30, 2016 to €5.7 million, compared to €2.7 million in the same period in 2015.
The increase of €1.6 million in upfront payments in the first half of 2016 compared to the prior period corresponds principally to the payments received in connection with entering into the collaboration agreements with LEO Pharma in May 2015 and with AbbVie in April 2016. These upfront payments were recognized in revenue based on the progress of the research and development programs that are the subject of both collaborations.
The milestone payment recognized in the first half of 2016 related to a payment received under the LEO Pharma collaboration.
The increase of €0.8 million in research and development service fees in the first six months of 2016 compared to the prior period related to payments under the collaboration agreements with LEO Pharma and Shire.
For the year ended December 31, 2015, our revenue was €6.9 million. The upfront payments of €2.2 million principally relates to the partial recognition in revenue over the period of the upfront payments received in connection with the collaborations with Bayer and Shire in May and June 2014, respectively, and with LEO Pharma in May 2015. These upfront payments are recognized in revenue over the period based on the progress of the respective research and development programs. The €4.3 million of research and development service fees for the year ended December 31, 2015 correspond to payments related to the collaboration agreements with Bayer, Shire and LEO Pharma.
Other Operating Income
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Government grants | € | 1,598 | € | 848 | € | 515 | ||||
Research and development incentives | 608 | 230 | 265 | |||||||
Payroll tax rebates | 895 | 562 | 537 | |||||||
| | | | | | | | | | | |
Total | € | 3,101 | € | 1,640 | € | 1,317 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Other operating income decreased by €0.3 million in the six-month period ended June 30, 2016 to €1.3 million, compared to €1.6 million for the same period in 2015, as a result of a decrease in grants received from the Flemish government. For the six-month period ended June 30, 2016, we accrued research and development incentives income of €0.3 million compared to €0.2 million for
86
the same period in 2015, corresponding to Belgian research and development incentives with regard to incurred research and development expenses which will be paid to us in cash after a five-year period, if not offset against the taxable basis over the respective period. We received €0.5 million of payroll tax rebates in the first half of 2016, compared to €0.6 million in the first half of 2015, for employing certain research and development personnel.
For the year ended December 31, 2015, we recorded research and development incentives income of €0.6 million for the Belgian research and development incentive scheme. We also received €1.6 million in grants from the Flemish government and €0.9 million of payroll tax rebates for certain personnel employed in our research and development department.
Research and Development Expenses
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Personnel expense | € | 6,665 | € | 2,950 | € | 4,224 | ||||
External research and development expenses | 11,653 | 5,359 | 5,320 | |||||||
Materials and consumables | 1,050 | 522 | 561 | |||||||
Depreciation and amortization | 196 | 88 | 150 | |||||||
Other expenses | 1,071 | 365 | 1,008 | |||||||
| | | | | | | | | | | |
Total | € | 20,635 | € | 9,284 | € | 11,263 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Our research and development expenses totaled €9.3 million and €11.3 million for the six-month periods ended June 30, 2015 and 2016, respectively. The increase of €1.3 million in personnel expense in the first six months of 2016 corresponded principally to (i) costs associated with additional research and development personnel and (ii) increased share-based compensation expense related to the grant of stock options to our research and development employees.
Our external research and development expenses in the first half of 2016 totalled €5.3 million compared to €5.4 million in the first six months of 2015, reflecting higher clinical trial costs related to the development of our product candidate portfolio but lower manufacturing expenses compared to the same period in 2015. The increase of €0.6 million in other expenses in the first half of 2016 corresponded to (i) patent expenses of €0.2 million related to the growth of our product portfolio, (ii) license fees of €0.1 million we paid to one of our licensors as a result of the signing of the AbbVie agreement, and (iii) €0.3 million of expenses corresponding principally to travel expenses, clinical trial insurance premiums and recruitment for research and development employees. The table below provides additional detail on our external research and development expenses by program:
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
ARGX-113 | € | 4,148 | € | 2,184 | € | 1,194 | ||||
ARGX-110 | 3,816 | 1,636 | 1,385 | |||||||
Other programs | 3,689 | 1,539 | 2,741 | |||||||
| | | | | | | | | | | |
Total | € | 11,653 | € | 5,359 | € | 5,320 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
87
For the year ended December 31, 2015, our research and development expenses amounted to €20.6 million. Our research and development personnel expenses were €6.7 million. For the year ended December 31, 2015, our external research and development expenses amounted to €11.7 million, attributable primarily to the clinical development of ARGX-113, ARGX-110 and ARGX-111. These studies required the production of drug material in large scale production batches during 2015.
General and Administrative Expenses
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Personnel expense | € | 1,607 | € | 684 | € | 999 | ||||
Consulting fees | 2,395 | 1,125 | 1,555 | |||||||
Supervisory board | 165 | 73 | 111 | |||||||
Office costs | 758 | 432 | 398 | |||||||
| | | | | | | | | | | |
Total | € | 4,925 | € | 2,314 | € | 3,063 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Our general and administrative expenses totaled €3.1 million and €2.3 million for the six-month periods ended June 30, 2016 and 2015, respectively. The increase in our general and administrative expenses in the first half of 2016 was principally due to (i) an increase of €0.3 million of personnel expenses related to employees recruited to strengthen our general and administrative activities, including the share based compensation expenses related to the grant of stock options to our general and administrative employees and (ii) an increase of €0.4 million of consulting fees related to investor relations, business development, IT, legal and audit activities in the first half of 2016.
For the year ended December 31, 2015, our general and administrative personnel expenses amounted to €1.6 million. The amount of consulting fees of €2.4 million in the year ended December 31, 2015 resulted from (i) expenses incurred for supporting activities as a public company such as investor relations, legal and audit fees following our initial public offering on Euronext Brussels in July 2014 and business development and IT consulting activities (ii) the share based compensation expenses related to the grant of stock options to certain of our consultants.
Financial Income (Expense)
For the six-month period ended June 30, 2016, financial income amounted to €0.04 million compared to €0.1 million during the same period in 2015 and €0.1 million for the year ended December 31, 2015.
Exchange Gains (Losses)
The exchange gains of €0.2 million recorded for the year ended December 31, 2015 and the exchange losses of €0.04 million for the six months ended June 30, 2016 were realized by converting U.S. dollars into euros.
Recent Developments
The following table presents our summary unaudited and unreviewed statement of profit and loss and other income data for the nine-month periods ended September 30, 2015 and 2016, and statement of financial position data as of September 30, 2015 and 2016. These data have been prepared solely on the basis of currently available information by, and are the responsibility of, management. Our independent registered public accounting firm, Deloitte Accountants B.V., has not
88
audited or reviewed, and does not express an opinion with respect to, these data. This summary is not a comprehensive statement of our financial results for these periods. The interim data below are not necessarily indicative of the data to be expected for the annual period. In accordance with SEC rules, we are providing this information because we otherwise make it publicly available.
| | Nine months ended | | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Sept 30, 2015 | Sept 30, 2016 | Variance | |||||||
| | (In thousands) | |||||||||
Revenue | € | 4,981 | € | 10,515 | € | 5,535 | ||||
Other operating income | 2,320 | 2,010 | (310 | ) | ||||||
| | | | | | | | | | | |
Total operating income | 7,300 | 12,525 | 5,225 | |||||||
| | | | | | | | | | | |
Research and development expenses | (14,200 | ) | (20,170 | ) | (5,970 | ) | ||||
General and administrative expenses | (3,345 | ) | (4,927 | ) | (1,581 | ) | ||||
| | | | | | | | | | | |
Operating profit/(loss) | (10,245 | ) | (12,572 | ) | (2,327 | ) | ||||
| | | | | | | | | | | |
Financial income/(expense) | 51 | 55 | 4 | |||||||
Exchange gains/(losses) | 119 | (51 | ) | (170 | ) | |||||
| | | | | | | | | | | |
Total comprehensive loss the period | € | (10,075 | ) | € | (12,568 | ) | € | (2,494 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net increase (decrease) in cash, cash-equivalents and current financial assets (compared to year end 2015 and 2014, as applicable) | € | (9,336 | ) | € | 60,740 | |||||
Cash, cash-equivalents and current financial assets at the end of the period | € | 46,637 | € | 103,067 | ||||||
Liquidity and Capital Resources
Sources of Funds
Since our inception in 2008, we have invested most of our resources to developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations. We do not currently have any approved products and have never generated any revenue from product sales. To date, we have funded our operations through public and private placements of equity securities, upfront, milestone and expense reimbursement payments received from our collaborators, funding from governmental bodies and interest income from the investment of our cash, cash equivalents and financial assets. Through June 30, 2016, we have raised gross proceeds of €133.8 million from private and public offerings of equity securities, received aggregate gross proceeds of €58.3 million from our collaborators, and received €10.7 million in grants and incentives from governmental bodies.
Our cash flows may fluctuate and are difficult to forecast and will depend on many factors. On June 30, 2016, we had cash, cash equivalents and current financial assets of €108.7 million.
We have no ongoing material financing commitments, such as lines of credit or guarantees, that are expected to affect our liquidity over the next five years, other than operating leases.
89
Cash Flows
The table below summarizes our cash flows for the year ended December 31, 2015 and the six-month periods ended June 30, 2015 and 2016:
| | | Six months ended June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Year ended December 31, 2015 | |||||||||
| | 2015 | 2016 | ||||||||
| | (In thousands) | |||||||||
Net cash flows (used in) provided by operating activities | €(13,897 | ) | €(5,446 | ) | €22,487 | |||||
Net cash flows provided by (used in) investing activities | 16,812 | (658 | ) | (623 | ) | |||||
Net cash flows provided by financing activities | 238 | 0 | 44,582 | |||||||
Net increase (decrease) in cash and cash equivalents | 3,153 | (6,104 | ) | 66,446 | ||||||
Net Cash (Used in) Provided by Operating Activities
Cash used for operating activities for the six-month period ended June 30, 2016 was a net inflow of €22.5 million, compared to a net outflow of €5.4 million for the six-month period ended June 30, 2015 and a net outflow of €13.9 million for the year ended December 31, 2015. The net cash inflow in the six-month period ended June 30, 2016 relates to the upfront payment of $40 million (€35.1 million) received from AbbVie in April 2016. The net cash outflow for the year ended December 31, 2015 relates to increased operating losses due to increased clinical trial and product candidate manufacturing activities in 2015.
Net Cash Provided by (Used in) Investing Activities
Investing activities consist primarily of purchase of laboratory equipment and interest received from the placements of our cash and cash equivalents and current financial assets. Cash flow from investing activities represented a net outflow of €0.6 million and €0.7 million for the six-month periods ended June 30, 2016 and 2015, respectively, compared to a net inflow of €16.8 million for the year ended December 31, 2015. The net inflow in 2015 corresponded to the sale of a money market fund previously classified in current financial assets.
Net Cash Provided by Financing Activities
Financing activities consist of net proceeds from our private placements of our ordinary shares and exercise of stock options. The net cash inflow from financing activities reached €44.6 million in the six month period ended June 30, 2016 compared to €0.2 million for the year ended December 31, 2015. There was no cash flow from financing activities in the six month period ended June 30, 2015. The net cash inflow in the first six months of 2016 is attributed to two private placements of our ordinary shares issued to institutional investors in January and June 2016 for total gross proceeds of €46 million.
Operating and Capital Expenditure Requirements
We have never achieved profitability and, as of June 30, 2016, we had an accumulated loss of €58.5 million. We expect to continue to incur significant operating losses for the foreseeable future as we continue our research and development efforts and seek to obtain regulatory approval and commercialization of our product candidates.
We expect that our existing cash, cash equivalents and current financial assets, together with anticipated net proceeds from this offering, will enable us to fund our operating expenses and capital expenditure requirements through at least the next months. We have based this estimate on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the
90
development of ARGX-113, ARGX-110 and our other product candidates and discovery stage programs and because the extent to which we may enter into collaborations with third parties for development of these product candidates is unknown, we are unable to estimate the amounts of increased capital outlays and operating expenses associated with completing the research and development of our product candidates. Our future capital requirements for ARGX-113, ARGX-110 and our other product candidates and discovery stage programs will depend on many factors, including:
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if ever. Accordingly, we will need to obtain substantial additional funds to achieve our business objectives.
Adequate additional funds may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Additional debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends and may require the issuance of warrants, which could potentially dilute your ownership interest.
If we raise additional funds through collaborations or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favourable to us. If we
91
are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development programs or any future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Contractual Obligations and Commitments
The table below summarizes our contractual obligations at December 31, 2015.
| | Payments due by period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Total | Less than 1 year | 1–3 years | 3–5 years | More than 5 years | |||||||||||
| | (In thousands) | |||||||||||||||
Operating lease commitments | € | 1,902 | € | 630 | € | 1,130 | € | 142 | €— | |||||||
We signed a lease agreement effective April 2016 for new laboratory and office space in Zwijnaarde, Belgium. This lease agreement is for a period of nine years starting from April 1, 2016, with the possibility to terminate the lease by giving a notice of at least 12 months in advance at the occasion of the third and sixth anniversary of the agreement. Our operating lease commitments include a lease plan for company cars with maturity dates up to four years.
For our offices in the Netherlands we have a lease agreement renewable on an annual base.
The commitment amounts in the table above are associated with contracts that are enforceable and legally binding and that specify all significant terms, including fixed or minimum services to be used, fixed, minimum or variable price provisions and the approximate timing of the actions under the contracts. The table does not include obligations under agreements that we can cancel without a significant penalty.
We have received various governmental grants that may need to be repaid if certain conditions related to these grants are not met. We believe that it is uncertain whether we will be required to repay these grants and, accordingly, have not included them in the table above.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Quantitative and Qualitative Disclosure about Market Risks
We are exposed to a variety of financial risks, including interest rate risk and foreign exchange risk.
Interest Rate Risk
We are currently not exposed to significant interest rate risk. Our only variable interest-bearing financial assets are cash at banks and our investments in money market funds. Given the short-term nature of these investments, the sensitivity towards interest rate fluctuations is deemed not to be significant. Therefore, the effect of an increase or decrease in interest rates would only have an immaterial effect on our financial results.
Foreign Exchange Risk
We undertake transactions denominated in foreign currencies; consequently, exposures to exchange rate fluctuations arise. Our functional currency is the euro and the majority of our
92
operating expenses are paid in euros, but we also receive payments from our main business partners, AbbVie and Shire, in U.S. dollars and we regularly acquire services, consumables and materials in U.S. dollars, Swiss Francs and British Pounds.
Critical Accounting Policies and Significant Judgments and Estimates
In the application of our accounting policies, we are required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
The following elements are areas where key assumptions concerning the future, and other key sources of estimation uncertainty at the end of the reporting period, have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year:
Revenue Recognition
Evaluating the criteria for revenue recognition with respect to our collaboration agreements requires management's judgment to ensure that all criteria have been fulfilled prior to recognizing any amount of revenue in accordance to International Accounting Standard 18. In particular, such judgments are made with respect to determination of the nature of transactions, whether simultaneous transactions shall be considered as one or more revenue-generating transactions, allocation of the contractual price (upfront and milestone payments in connection with a collaboration agreement) to several elements included in an agreement. All of our revenue-generating transactions have been subject to such evaluation by management.
We generate revenue under our collaboration agreements and recognize this revenue as follows:
Upfront Payments
Upfront payments for which there are subsequent deliverables are initially reported as deferred income and are recognized as revenue when earned over the period of the development collaboration or the manufacturing obligation. Upfront payments also include license fees received upfront.
Deferred revenue reflects the part of upfront payments that has not been recognized as revenue immediately on receipt of payment and which relates to agreements with multiple components that cannot be separated. Deferred revenue is measured at nominal value.
Milestone Payments
Revenue associated with performance milestones is recognized based upon the achievement of the milestone event if the event is substantive, objectively determinable and represents an important point in the development life cycle of the product candidate.
93
Research and Development Services Fees
Research and development service fees are recognized as revenue over the life of the research agreement as the required services are provided and costs are incurred. These services are usually in the form of a defined number of FTEs at a specified rate per FTE.
Commercial collaborations resulting in a reimbursement of research and development costs are recognized as revenue as the related costs are incurred. The corresponding research and development expenses are included in research and development expenses in the consolidated financial statements.
With respect to the allocation of value to the separate elements, we use the stand alone selling prices or management's best estimates of selling prices to estimate the fair value of the elements and account for them separately allocated to each deliverable based on the fair value of each individual element and is recognized when the revenue recognition criteria described above are met. Upfront fees under collaboration or licensing agreements are recognized over the expected duration of the performance obligations, unless there is no continuous involvement required. Management estimates this period at the start of the collaboration and validates the remaining estimated collaboration term at each closing date. The recognition of revenue is linked (i) to the period during which we are continuously involved in the development of the product candidates subject to the collaboration and (ii) in relation to the expenses incurred over the period, which defines a percentage of achievement compared to the original budget.
Measurement of Share-Based Payments
We determine the costs of the share-based payment plan (i.e., our stock option plan) on the basis of the fair value of the equity instrument at grant date in accordance with IFRS 2. For the determination of the fair value we are using the Black Scholes pricing model. This requires the input into the valuation model of amounts that require judgment, like the estimated useful life of the stock options and the volatility of our stock. Once calculated, the fair value of the stock options granted is recognized as an expense in our statement of comprehensive income and not re-measured subsequently.
In accordance with the terms of our stock option plan, as approved by our shareholders, our employees, certain of our consultants and our directors may be granted options to purchase ordinary shares at an exercise price per ordinary share equal to the average of the closing share prices of the last 30 calendar days preceding the date of the grant by the board of directors. Each stock option converts into one ordinary share upon exercise. No amounts are paid or payable by the beneficiary upon receipt of the option. The stock options carry neither rights to dividends nor voting rights. Stock option may be exercised at any time from the date of vesting to the date of their expiry.
The stock options generally vest as follows:
No other conditions are attached to the stock options.
On June 30, 2016, the total number of stock options outstanding totaled 2,027,668 compared to 1,752,926 on December 31, 2015. On June 30, 2016, no stock options had expired, a total of 55,292 stock options had been exercised and 18,917 stock options had been forfeited.
94
The expected volatility used in the Black Scholes model is based, for the periods before 2016, on the historical volatility of peer companies. The peer companies are publicly traded entities active in the business of developing antibody-based therapeutics, treatments and drugs and are selected taking into consideration the availability of meaningful trading data history and market capitalization. For grants beginning in 2016, we only considered the historical volatility of our stock price calculated since our initial public offering.
Below is an overview of the parameters used in relation to the options granted in the six-month period ended June 30, 2016:
| | Stock options granted in | ||||||
|---|---|---|---|---|---|---|---|
| | May 2016 | June 2016 | |||||
Number of options granted | 288,950 | 60,000 | |||||
Average fair value of options | € | 5.32 | € | 5.46 | |||
Share price | € | 11.10 | € | 11.36 | |||
Exercise price | € | 11.47 | € | 11.38 | |||
Expected volatility | 40.2 | % | 39.6 | % | |||
Average expected option life (in years) | 10 | 10 | |||||
Risk-free interest rate | 0.52 | % | 0.46 | % | |||
Expected dividends | 0 | % | 0 | % | |||
The grant date fair value of the options in the above table is estimated using the following assumptions:
The total share-based payment expense recognized in the consolidated statement of profit and loss and other comprehensive income was €1.1 million for each of the six months periods ended June 30, 2015 and 2016 and €2.3 million for the year ended December 31, 2015.
Recognition of Deferred Tax Assets
We are subject to income taxes in the Netherlands and in Belgium. Significant judgment is required in determining the use of net operating loss carry-forwards and taxation of upfront and milestone payments for income tax purposes. There are many transactions and calculations for which the ultimate tax determination is uncertain. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the current and deferred income tax assets and liabilities in the period in which such determination is made.
No tax charge or income was recognized during the reporting periods since we are in a loss-making position and have a history of losses. We had tax loss carry-forwards of €60.4 million as of December 31, 2015.
95
Deferred income tax assets are recognized for tax losses and other temporary differences to the extent that the realization of the related tax benefit through future taxable profits is probable. We recognize deferred tax assets arising from unused tax losses or tax credits only to the extent the relevant fiscal unity has sufficient taxable temporary differences or if there is convincing other evidence that sufficient taxable profit will be available against which the unused tax losses or unused tax credits can be utilized by the fiscal unity. Our judgment is that sufficient convincing other evidence is not available and therefore, a deferred tax asset is not recognized.
Changes in Accounting Firm Relationships
At the general meeting of our shareholders held on May 13, 2015, Deloitte Accountants BV was appointed as our new external audit firm for the 2015 reporting year (replacing PriceWaterhouseCoopers NV). The appointment of Deloitte Accountants BV was the result of a tender process completed in March 2015 and the recommendation of Deloitte Accountants BV by our audit committee. The change in auditors was made to comply with the Dutch Audit Profession Act for audit firm rotation.
During the two years prior to December 31, 2014, (1) PriceWaterhouseCoopers NV had not issued any reports on our financial statements that contained an adverse opinion or a disclaimer of opinion, nor were the auditors' reports of PriceWaterhouseCoopers NV qualified or modified as to uncertainty, audit scope or accounting principles and (2) there has not been any disagreement over any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures, which disagreements if not resolved to PriceWaterhouseCoopers NV's satisfaction would have caused it to make reference to the subject matter of the disagreement in connection with its auditors' reports.
Furthermore, in the two years prior to December 31, 2014, we have not consulted with Deloitte Accountants BV regarding either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered with respect to our consolidated financial statements or (ii) any matter that was the subject of a disagreement or a reportable event.
JOBS Act Transition Period
In April 2012, the U.S. Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an "emerging growth company" can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Given that we currently report and expect to continue to report under IFRS as issued by the IASB, we have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We intend to rely on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we may rely on certain of these exemptions, including without limitation, (1) providing an auditor's attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (2) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We would cease to be an emerging growth company upon the earliest to
96
occur of (1) the last day of the fiscal year in which we have more than $1.0 billion in annual revenue; (2) the date we qualify as a "large accelerated filer," with at least $700.0 million of equity securities held by non-affiliates; (3) the issuance, in any three-year period, by our company of more than $1.0 billion in non-convertible debt securities held by non-affiliates; and (4) the last day of the fiscal year ending after the fifth anniversary of the global offering. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
Recent Accounting Pronouncements
For information on recent accounting pronouncements, see our consolidated financial statements and the related notes appearing elsewhere in this prospectus. There are no IFRS standards as issued by the IASB or interpretations issued by the IFRS interpretations committee that are effective for the first time for the financial year beginning on or after January 1, 2015 that would be expected to have a material impact on our financial position.
97
Overview
We are a clinical-stage biotechnology company developing a deep pipeline of differentiated antibody-based therapies for the treatment of severe autoimmune diseases and cancer. Utilizing our suite of technologies, we are focused on developing product candidates with the potential to be either first-in-class against novel targets or best-in-class against known, but complex, targets in order to treat diseases with a significant unmet medical need. Our SIMPLE Antibody Platform, based on the powerful llama immune system, allows us to exploit novel and complex targets, and our three antibody Fc engineering technologies are designed to enable us to expand the therapeutic index of our product candidates. Together with our antibody discovery and development expertise, this suite of technologies has enabled our pipeline of seven product candidates. Two of our product candidates will be in clinical proof-of-concept trials for three indications within the first half of 2017.
Our most advanced product candidate, ARGX-113, is in a Phase 2 clinical trial for the treatment of the rare autoimmune disease myasthenia gravis, or MG, and, in March 2017, we plan to initiate a Phase 2 clinical trial of ARGX-113 for the treatment of another rare autoimmune disease, primary immune thrombocytopenia, or ITP. We are currently developing our second lead product candidate, ARGX-110, for rare and aggressive hematological cancers, initially for T-cell lymphoma, or TCL, and acute myeloid leukemia, or AML, as well as high-risk myelodysplastic syndrome, or MDS. In December 2016, we commenced a Phase 1/2 clinical trial of ARGX-110 in combination with azacitidine for the treatment of newly diagnosed AML or high-risk MDS patients, and in March 2017, we expect to initiate the Phase 2 part of a Phase 1/2 clinical trial of ARGX-110 for the treatment of cutaneous TCL, or CTCL.
We have a disciplined strategy to maximize the value of our pipeline whereby we plan to retain development and commercialization rights to those product candidates that we believe we can ultimately commercialize successfully on our own if they are approved. We plan to collaborate on product candidates that we believe have promising utility in disease areas or patient populations that are better served by the resources of larger biopharmaceutical companies. We have entered into collaborations with a number of biopharmaceutical companies, including our collaboration with AbbVie S.À.R.L, or AbbVie, for ARGX-115, a cancer immunotherapy-focused product candidate against the novel target glycoprotein A repetitions predominant, or GARP. We received a $40.0 million (€35.1 million as of the date the payment was received) upfront payment in connection with this collaboration.
Our product candidates are focused on indications for which there is a solid biological rationale and for which we believe there is an advantage to utilizing our suite of technologies outlined below:
98
Our product candidate pipeline includes both wholly-owned and partnered programs. We refer to programs for which we retain the exclusive right to develop and commercialize the product candidate on a worldwide basis as our wholly-owned programs. We refer to programs for which we have entered into collaboration agreements with third parties for the development and commercialization of the product candidate as our partnered programs.
Our product candidate pipeline enabled by our suite of technologies is set forth below:
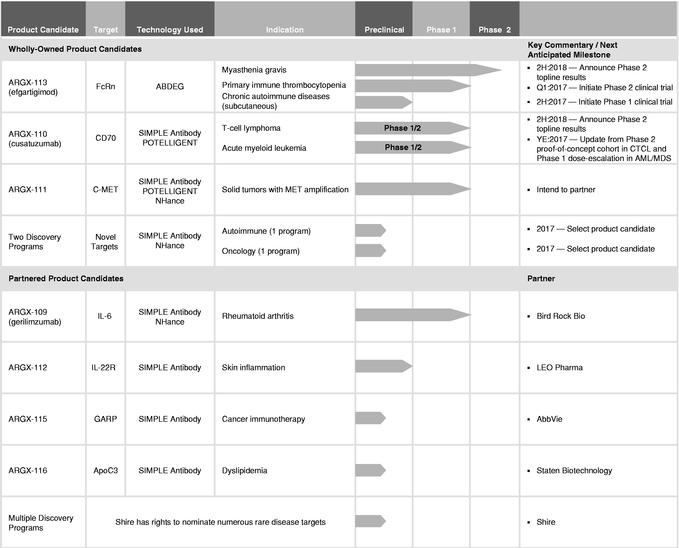
We believe that our clinical expertise and execution capabilities position us well to advance our product pipeline and enter into collaborations designed to maximize the value of our portfolio. We have assembled a team of over 60 employees with experience across the spectrum of antibody drug discovery and development and business development. Members of our board of directors and management team have extensive experience in the life sciences industry and have previously
99
served at companies including Cambridge Antibody Technology Group Plc; Celgene Corporation; Galapagos NV; GlaxoSmithKline plc; Janssen Pharmaceuticals, Inc.; Micromet, Inc.; Nicox S.A.; The Procter & Gamble Company; Quintiles IMS Holdings, Inc. and Unilever NV.
Our Competitive Strengths
We believe that the combination of our technologies, expertise and disciplined focus will enable us to overcome many of the challenges associated with antibody drug development and positions us to be a leader in delivering therapies to patients suffering from severe autoimmune disease and cancers for which the current treatment paradigm is inadequate. Our competitive strengths include:
100
Our Strategy
Our goal is to deliver therapies that are either first-in-class or best-in-class to patients suffering from severe autoimmune diseases and various cancers for which there exists a significant unmet medical need. We are also focused on attaining this goal in a manner that is disciplined for a company of our size. We plan to:
101
Our Suite of Technologies
Harnessing the Therapeutic Potential of Antibodies
Antibodies are Y-shaped proteins used by the immune system to target and clear foreign bodies, including pathogens, such as bacteria and viruses, and tumor cells. Antibodies are composed of two structurally independent parts, the variable region, or V-region, and the constant, or Fc, region. The V-region is responsible for targeting a specific antibody to an antigen and is different for every type of antibody. The Fc region does not interact with antigens, but rather interacts with components of the immune system through a variety of receptors on immune and other cells. These interactions allow antibodies to regulate the immune response and levels of cell-killing ability, or cytotoxicity, as well as their persistence in circulation and tissues. Fc regions are the same and interchangeable from antibody to antibody.
102
As shown inFigure 1, we apply a unique suite of technologies to create antibodies with optimized V-regions and an enhanced Fc region. Used alone or in combination, we believe that our suite of technologies enable us to create product candidates with potential first-in-class and best-in-class therapeutic activity against a wide range of targets.
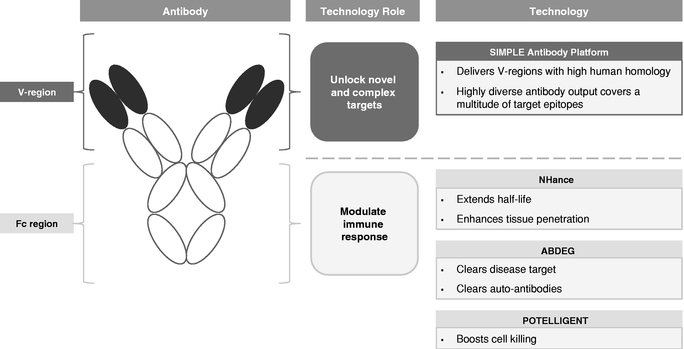
Figure 1: Overview of our suite of technologies
Our Proprietary SIMPLE Antibody Platform
Our proprietary SIMPLE Antibody Platform sources V-regions from conventional antibodies existing in the immune system of outbred llamas. Outbred llamas are those that have been bred from genetically diverse parents, and each has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology in their V-regions when immunized with human disease targets. We then combine these llama V-regions with Fc regions of fully human antibodies, resulting in antibodies that we then produce in industry-validated production cell lines. The resulting antibodies are diverse and, due to their similarity to human antibodies, we believe they are well suited to human therapeutic use. With this breadth of antibodies, we are able to test many different epitopes. Being able to test many different epitopes with our antibodies enables us to search for an optimized combination of safety, potency and species cross-reactivity with the potential for maximum therapeutic effect on disease. These antibodies are often cross-reactive with the rodent version of chosen disease targets. This rodent cross-reactivity enables more efficient preclinical development of our product candidates because most animal efficacy models are rodent-based. By contrast, most other antibody discovery platforms start with antibodies generated in inbred mice or synthetic antibody libraries, approaches that are limited by insufficient antibody repertoires and limited diversity, respectively. Our SIMPLE Antibody Platform allows us to access and explore a broad target universe, including novel and complex targets, while minimizing the long timelines associated with generating antibody candidates using traditional methods.
Our Fc Engineering Technologies
Our antibody engineering technologies—NHance, ABDEG and POTELLIGENT—focus on engineering the Fc region of antibodies in order to augment their interactions with components of the immune system, thereby potentially expanding the therapeutic index of our product candidates by modifying their half-life, tissue penetration, rate of disease target clearance and potency. For
103
example, our NHance and ABDEG engineering technologies enable us to modulate the interaction of the Fc region with FcRn, which is responsible for regulating half-life, tissue distribution and pharmacodynamic properties of IgG antibodies. Similarly, our POTELLIGENT engineering technology modulates the interaction of the antibody Fc region with receptors located on specialized immune cells known as natural killer, or NK, cells. These NK cells can destroy the target cell, resulting in enhanced antibody-dependent cell-mediated cytotoxicity, or ADCC.
NHance and ABDEG: Modulation of Fc Interaction with FcRn
An illustration of the FcRn-mediated antibody recycling mechanism is shown inFigure 2.![]() Serum proteins, including IgG antibodies, are routinely removed from the circulation by cell uptake.
Serum proteins, including IgG antibodies, are routinely removed from the circulation by cell uptake.![]() Antibodies can bind to FcRn, which serves as a dedicated recycling receptor in the endosomes, which have an acidic environment, and then
Antibodies can bind to FcRn, which serves as a dedicated recycling receptor in the endosomes, which have an acidic environment, and then![]() return to the circulation by binding with their Fc region to FcRn.
return to the circulation by binding with their Fc region to FcRn.![]() Unbound antibodies end up in the lysosomes and are degraded by enzymes. Because this Fc/FcRn interaction is highly pH-dependent, antibodies tightly bind to FcRn at acidic pH (pH 6.0) in the endosomes, but release again at neutral pH (pH 7.4) in the circulation.
Unbound antibodies end up in the lysosomes and are degraded by enzymes. Because this Fc/FcRn interaction is highly pH-dependent, antibodies tightly bind to FcRn at acidic pH (pH 6.0) in the endosomes, but release again at neutral pH (pH 7.4) in the circulation.
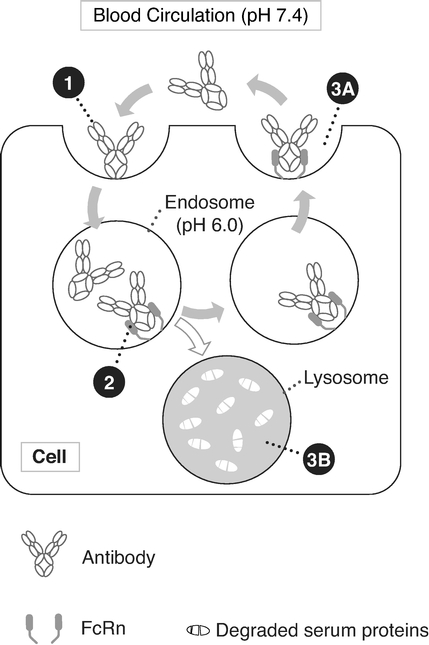
Figure 2: The FcRn-mediated recycling mechanism
104
NHance
NHance refers to two mutations that we introduce into the Fc region of an IgG antibody. NHance is designed to extend antibody serum half-life and increase tissue penetration. In certain cases, it is advantageous to engineer antibodies that remain in the circulation longer, allowing them to potentially exert a greater therapeutic effect or be dosed less frequently. As shown inFigure 3,![]() NHance antibodies bind to FcRn with higher affinity, specifically under acidic pH conditions.
NHance antibodies bind to FcRn with higher affinity, specifically under acidic pH conditions.![]() Due to these tighter bonds, NHance FcRn-mediated antibody recycling is strongly favored over lysosomal degradation, although some degradation does occur.
Due to these tighter bonds, NHance FcRn-mediated antibody recycling is strongly favored over lysosomal degradation, although some degradation does occur.![]() NHance allows a greater proportion of antibodies to return to the circulation potentially resulting in increased bioavailability and reduced dosing frequency. ARGX-111, ARGX-109 and a number of our discovery-stage programs utilize NHance.
NHance allows a greater proportion of antibodies to return to the circulation potentially resulting in increased bioavailability and reduced dosing frequency. ARGX-111, ARGX-109 and a number of our discovery-stage programs utilize NHance.
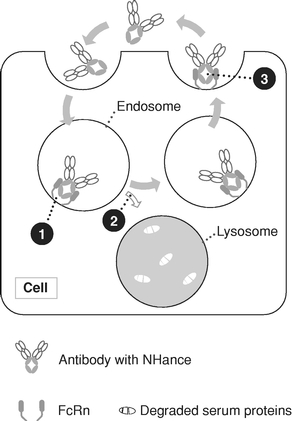 | ||
Figure 3: NHance mutations favor the FcRn-mediated recycling of IgG antibodies | ||
ABDEG
ABDEG refers to five mutations that we introduce in the Fc region that increase its affinity for FcRn at both neutral and acidic pH. In contrast to NHance, ABDEG-modified Fc regions remain bound to FcRn if the pH changes, occupying FcRn with such high affinity that they deprive endogenous IgG antibodies of their recycling mechanism, leading to enhanced clearance of such antibodies by the lysosomes. Some diseases mediated by IgG antibodies are directed against self-antigens. These self-directed antibodies are referred to as auto-antibodies. We use our ABDEG technology to reduce the level of these pathogenic auto-antibodies in the circulation by increasing the rate at which they are cleared by the lysosomes. ABDEG is a component in a number of our product candidates, including ARGX-113.
105
As shown inFigure 4, our ABDEG technology can also be used with our pH-dependent SIMPLE Antibodies in a mechanism referred to as sweeping. Certain SIMPLE Antibodies bind to their target in a pH-dependent manner. These antibodies![]() bind tightly to a target at neutral pH while in circulation, and
bind tightly to a target at neutral pH while in circulation, and![]() release the target at acidic pH in the endosome.
release the target at acidic pH in the endosome.![]() The unbound target is degraded in the lysosome.
The unbound target is degraded in the lysosome.![]() However, when equipped with our ABDEG technology, the therapeutic antibodies remain tightly bound to FcRn at all pH levels and are not degraded themselves. Instead, they are returned to the circulation where they can bind new targets. We believe this is especially useful in situations where high levels of the target are circulating or where the target needs to be cleared very quickly from the system.
However, when equipped with our ABDEG technology, the therapeutic antibodies remain tightly bound to FcRn at all pH levels and are not degraded themselves. Instead, they are returned to the circulation where they can bind new targets. We believe this is especially useful in situations where high levels of the target are circulating or where the target needs to be cleared very quickly from the system.
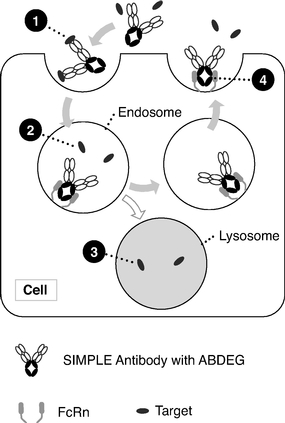
Figure 4: SIMPLE Antibody and ABDEG
technologies work in concert to sweep
disease targets
POTELLIGENT: Modulation of Fc Interaction with NK Cells
POTELLIGENT modulates the interaction of the Fc region with the Fc gamma receptor IIIa located on specialized immune cells, known as NK cells. These NK cells can destroy the target cell, resulting in enhanced ADCC. POTELLIGENT changes the Fc structure by excluding a particular sugar unit such that it enables a tighter fit with the Fc gamma receptor IIIa. The strength of this interaction is a key factor in determining the killing potential of NK cells. An independent publication reported that the exclusion of this sugar unit of the Fc region increases the ADCC-mediated cell-killing potential of antibodies by 10- to 1000-fold. ARGX-110 and ARGX-111 utilize POTELLIGENT.
106
Our Wholly-Owned Programs
The following is the pipeline of our wholly-owned product candidates and discovery programs:
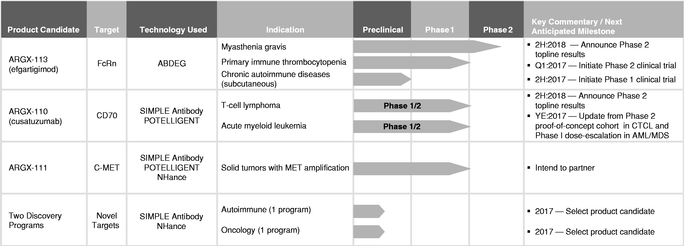
ARGX-113
We are currently developing our lead product candidate, ARGX-113, for the treatment of patients with MG and ITP, both of which are rare and severe autoimmune diseases associated with high levels of circulating pathogenic IgG antibodies for which there are few innovative biologic treatments and a severe unmet medical need exists. ARGX-113 utilizes our ABDEG engineering technology and is designed to block the recycling of IgG antibodies, which results in their removal from circulation. We believe that our approach presents potential benefits relative to the current standard of care for MG and ITP: corticosteroids and immunosuppressants in the early stages, followed by intravenous IgG, or IVIg, and plasma exchange, or plasmapheresis, as the disease progresses. We believe these potential benefits include improved time of onset, increased magnitude and duration of therapeutic benefit, a more favorable safety and tolerability profile and a reduced cost burden to the healthcare system.
We have completed the single and multiple ascending dose parts of a double-blind, placebo-controlled Phase 1 clinical trial of ARGX-113 in 62 healthy volunteers. This clinical trial was conducted at one site in Belgium. We launched a Phase 2 clinical trial of ARGX-113 in patients with MG in January 2017. This clinical trial has been initiated in Europe and, subject to submission of an investigational new drug, or IND, application to the U.S. Food and Drug Administration, or FDA, that goes into effect, will include U.S. sites. In parallel, we plan to launch a second Phase 2 clinical trial of ARGX-113 in patients with ITP in March 2017 in Europe. We expect to report topline data from these clinical trials in the second half of 2018. Depending on the outcome of these clinical trials and subject to discussions with regulatory agencies, we intend to structure our pivotal program for ARGX-113 in one or both of these indications. In addition to the intravenous formulation of ARGX-113 that we are using in our current clinical trials, we are also developing a subcutaneous formulation designed to make ARGX-113 accessible to larger patient populations, including patients requiring chronic therapy, potentially outside of the hospital setting. We plan to initiate a Phase 1 clinical trial in healthy volunteers for a subcutaneous formulation of ARGX-113 in the second half of 2017.
Overview of Myasthenia Gravis
MG is an autoimmune disorder associated with muscle weakness that is triggered by IgG auto-antibodies. These antibodies attack critical signaling proteins at the junction between nerve and muscle cells, thereby impairing their communication signals. As shown inFigure 5, in MG these auto-antibodies either bind and occupy or cross-link and internalize the receptor on the muscle cells,
107
thereby preventing the binding of acetylcholine, the signal sent by the nerve cell. In addition, these auto-antibodies can cause destruction of the neuromuscular junction by recruiting complement, a potent cell-destroying mechanism of the human immune system.
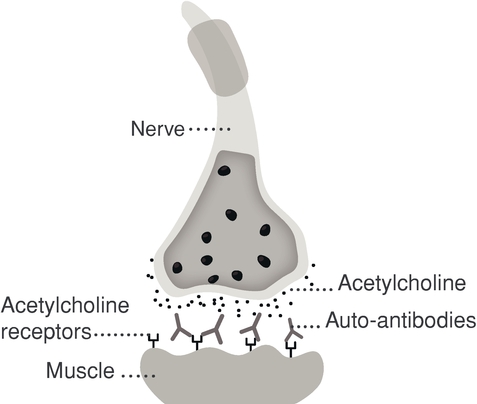
Figure 5: MG is caused by auto-antibodies attacking the transmission of nerve impulses to muscles
The muscle weakness associated with MG usually presents initially in ocular muscles and can then spread into a generalized form affecting multiple muscles. MG initially causes droopy eyelids and blurred or double vision due to partial paralysis of eye movements. As MG becomes generalized it affects muscles in the neck and jaw, causing problems in speaking, chewing and swallowing. MG can also cause weakness in skeletal muscles leading to problems in limb function. In the most severe cases, respiratory function can be weakened to the point where it becomes life-threatening. These respiratory crises occur at least once in the lives of approximately 15% to 20% of MG patients.
The U.S. prevalence of MG is estimated at approximately 20 cases per 100,000. Currently, there are an estimated 64,000 MG patients in the United States, of which an estimated 55,000 patients are suffering from generalized MG. We believe that the prevalence in Europe is at a similar level. Our initial focus is on generalized MG patients whose disease is not well-controlled with corticosteroids and immunosuppressants, which we believe represents a majority of generalized MG patients.
Limitations of Current MG Treatments
Early in their disease, patients are treated with cholinesterase inhibitors, such as pyridostigmine, followed by corticosteroids and immunosuppressants. The majority of patients with MG require some form of immunotherapy at some point during their illness. Corticosteroids are associated with a number of significant side effects, including bone thinning, weight gain, diabetes, hypertension, osteoporosis and depression. The side effects of immunosuppressants, depending on the particular immunosuppressant, include weakness, sweating, transaminase elevations, neutropenia, including severe neutropenia with infection, acute deep venous thrombosis, nausea, vomiting and the incidence of cancer. As MG becomes more advanced, patients can be treated with IVIg and plasmapheresis. Both of these approaches are associated with significant side effects.
108
Treatment with IVIg is based on the principle of altering the balance between synthesis and degradation of antibodies in the body. IVIg treatment results in a large increase in the quantity of IgG antibodies in circulation. This excess of exogenously added IgG antibodies competes with the endogenous autoimmune antibodies for various pathways including the FcRn antibody recycling pathway. Saturation of this pathway with exogenous IgG antibodies promotes antibody destruction, which in turn leads to a decrease in the level of autoimmune antibodies. IVIg treatment is associated with a number of adverse events including fever, myalgia, headache, nausea and impaired kidney function or kidney disease, and IVIg can lead to life-threatening complications such as pulmonary edema, acute kidney dysfunction or stroke in elderly patients.
Plasmapheresis involves collecting blood from a patient and physically removing the IgG antibodies and other serum proteins from the plasma before returning it to the patient. Plasmapheresis is also associated with known limitations and drawbacks. Potential complications include thrombotic events, bleeding, catheter occlusion, infection, nausea, hypotension and arrhythmias. In most cases, these symptoms are mild and transient, but in some cases they can be severe and life-threatening.
Both of these approaches place a heavy cost burden on the healthcare system. In addition to the costs of the IVIg or plasmapheresis treatment itself, hospitalization of patients receiving these treatments further adds to this cost burden. According to a 2011 study, the average short-term cost for utilizing IVIg or plasmapheresis for MG crisis was $78,814 and $101,140 per patient, respectively. In addition to patients experiencing an MG crisis, we believe a substantial number of MG patients receive chronic IVIg or plasmapheresis for which they require frequent hospitalization
Finally, a minority of MG patients undergo thymectomy, the surgical removal of the thymus, an immune organ which is believed to play a role in the pathogenesis of the disease.
For MG patients who have advanced to the point where they are not well-controlled with corticosteroids and immunosuppressants, we believe ARGX-113 may offer advantages over IVIg and plasmapheresis, including the potential to deliver a faster onset of action, a larger and longer lasting therapeutic effect and an improved safety and tolerability profile. In addition, a subcutaneous formulation of ARGX-113 could further expand its use to patients requiring chronic therapy, potentially outside of the hospital setting.
Overview of Primary Immune Thrombocytopenia
ITP is a bleeding disease caused by an autoimmune reaction in which a patient develops antibodies that attack and destroy their own platelets, which are blood cells that help blood to clot, or their own platelet-forming cells. ITP, which develops for no known reason, is differentiated from secondary immune thrombocytopeania, which is associated with other illnesses, such as infections or autoimmune diseases, or which occurs after transfusion or taking other drugs, such as cancer drugs. Platelet deficiency, or thrombocytopenia, can cause bleeding in tissues, bruising and slow blood clotting after injury. ITP affects approximately 72,000 patients in the United States.
Limitations of Current ITP Treatments
Treatment for ITP is focused on either reducing the autoimmune activity that is causing accelerated platelet destruction and allowing the platelets to recover on their own, or directly stimulating platelet production with specific growth factors. Patients with less severe ITP are treated with corticosteroids and immunosuppressants, which are associated with significant side effects also seen with such treatment of other autoimmune diseases, such as MG. For more severe ITP, splenectomy is sometimes used as treatment, although its use is rapidly declining. The use of thrombopoietin receptor agonists, which stimulate the production and differentiation of platelets and
109
are approved for last-line therapy, is increasing. Patients diagnosed with severe ITP are primarily offered IVIg or, to a lesser extent, plasmapheresis.
IVIg can raise the platelet count within days in most patients, but the effect is usually transient. IVIg introduces high levels of exogenously added IgG antibodies to the blood stream that compete with the patient's auto-antibodies for various pathways including the FcRn-dependent antibody recycling pathway, thereby lowering the impact of the auto-antibodies. IVIg treatment for ITP requires intravenous dosing of up to 2 g/kg per day of IVIg and is associated with many of the adverse events seen with IVIg treatment of other autoimmune diseases, such as MG as described above. Both IVIg and plasmapheresis when used to treat ITP carry a high cost burden on the healthcare system as they do when used to treat MG.
The production of platelets in patients refractory to other treatments can be stimulated by drugs such as romiplostim (Nplate) or eltrombopag (Promacta) that mimic thrombopoietin. While these therapies lead to increases in blood platelet counts, they do not address the underlying cause of the disease, which is the destruction of platelets by the immune system. Romiplostim (Nplate) is an approved last-line therapy for ITP and generated global revenues of $525 million in 2015.
Our Solution: ARGX-113
Our lead product candidate, ARGX-113, is an antibody that we believe has the potential to overcome many of the limitations of the current standard of care for MG and ITP, including with respect to time of onset, magnitude and duration of therapeutic benefit and safety profile. We developed ARGX-113 using our ABDEG Fc engineering technology.
ARGX-113 targets FcRn with high affinity, thereby reducing levels of all four classes of IgG antibodies, which are referred to as IgG1, IgG2, IgG3 and IgG4. In the case of MG, the large majority of patients have auto-antibodies of the IgG1 and IgG3 classes, while in the case of ITP these auto-antibodies consist mainly of the IgG1 class.
As shown inFigure 6, ARGX-113's mechanism of action is to block the recycling of IgG antibodies and remove them from circulation. Antibodies are routinely removed from circulation by being internalized into cells, where they can either become destined for degradation in the lysosomes or recycled back into circulation. IgG antibodies not bound to FcRn are degraded, while those bound to FcRn are recycled back into circulation.![]() As a result of our ABDEG technology and the modifications we made to the Fc region, ARGX-113 binds to FcRn with high affinity making this receptor unavailable to circulating IgG antibodies.
As a result of our ABDEG technology and the modifications we made to the Fc region, ARGX-113 binds to FcRn with high affinity making this receptor unavailable to circulating IgG antibodies.![]() The IgG antibodies can then no longer effectively be rescued and end up in the lysosomes where they are degraded. Compared to alternative immunosuppressive approaches, such as B-lymphocyte, or B-cell, depleting agents, ARGX-113 acts in a highly selective manner by reducing IgG antibody levels, while leaving levels of
The IgG antibodies can then no longer effectively be rescued and end up in the lysosomes where they are degraded. Compared to alternative immunosuppressive approaches, such as B-lymphocyte, or B-cell, depleting agents, ARGX-113 acts in a highly selective manner by reducing IgG antibody levels, while leaving levels of
110
antibodies of the immunoglobulin A, or IgA, immunoglobulin M, or IgM, and immunoglobulin D, or IgD, types as well as all components of the innate immune system intact.
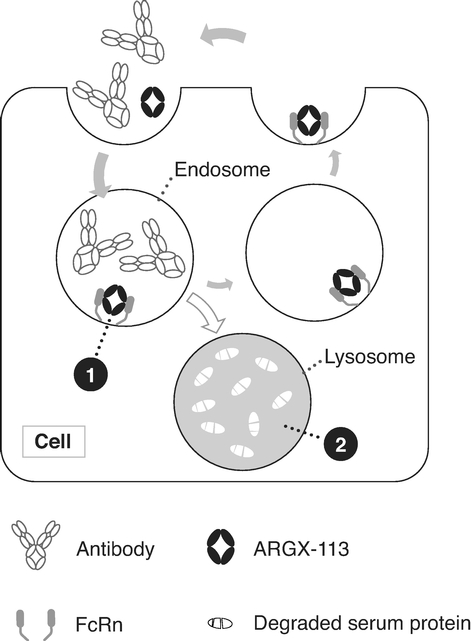
Figure 6: ARGX-113's mechanism of action blocks the recycling of IgG antibodies and removes them from circulation
Based on our preclinical studies and early clinical trial results, we believe that ARGX-113 has the potential to reduce levels of pathogenic IgG antibodies. Our clinical data suggest that ARGX-113 reduces circulating IgG antibodies more rapidly than current therapies, which we believe could translate into faster therapeutic benefit if replicated with respect to pathogenic IgG antibodies. Our clinical data also suggest that the quantity of ARGX-113 required to achieve and maintain suppression of circulating antibodies is lower than the levels of IVIg required for therapeutic benefit, which could translate into fewer infusions, shorter infusion time and a more favorable safety and tolerability profile.
111
In addition to MG and ITP, we believe there are other autoimmune diseases that may benefit from the mechanism of action of ARGX-113 therapy. We intend to pursue initial approval for one or both of MG and ITP because these diseases have significant unmet medical needs. We then intend to expand our clinical development efforts for ARGX-113 into additional indications also mediated by pathogenic IgG antibodies. Pathogenic auto-antibodies have been shown to be associated with skin blistering diseases—such as pemphigus, bullous pemphigoid and epidermolysis bullosa acquisita, which are all rare, severe and chronic conditions—and systemic lupus erythematosus and multiple sclerosis, or MS, which affect larger numbers of patients.
Clinical Development Plan
We are currently evaluating ARGX-113 in a Phase 2 clinical trial in patients with MG, and we plan to initiate a Phase 2 clinical trial in patients with ITP in March 2017. We expect to report topline data from these clinical trials in the second half of 2018. Following the conclusion of these Phase 2 clinical trials, we plan to prioritize MG and ITP and prepare to conduct a pivotal clinical trial in one or both of the indications. In addition to the current intravenous formulation of ARGX-113, we are also developing a subcutaneous formulation designed to make ARGX-113 accessible to larger patient populations including patients requiring chronic therapy, potentially outside of the hospital setting. We plan to initiate a Phase 1 clinical trial in healthy volunteers for a subcutaneous formulation of ARGX-113 in the second half of 2017.
Phase 2 Clinical Trial in MG
We are conducting a randomized, double-blind, placebo-controlled Phase 2 clinical trial to evaluate the safety, efficacy and pharmacokinetics of ARGX-113 in 24 generalized MG patients who are stable on cholinesterase inhibitors, steroids and immunosuppressants which make up the typical first- and second-line standard-of-care therapies. We intend to conduct the clinical trial at approximately 25 sites across Europe and the United States, subject to submission of an IND application to the FDA that goes into effect. Patients will be randomly assigned to two arms of 12 patients each. Patients in one treatment arm will receive 10 mg/kg of ARGX-113, and the other treatment arm will receive placebo. All patients will continue to receive the standard of care. Dosing will take place during a three-week period with four weekly doses of ARGX-113 or placebo. Patient follow-up will continue for eight weeks after treatment.
The primary objectives of this Phase 2 clinical trial are to evaluate the safety and tolerability of ARGX-113 with primary endpoints evaluating the incidence and severity of adverse events and serious adverse events, and evaluating vital signs, electrocardiogram and laboratory assessments. As a secondary objective, efficacy will be assessed. Efficacy measures include the quantitative MG score, a clinically validated quantitative test of disease severity in MG, as well as changes in other measures of MG disease severity. In addition, immunogenicity, pharmacokinetics and pharmacodynamics will be measured.
We expect to announce topline data from this Phase 2 clinical trial in the second half of 2018.
Phase 2 Clinical Trial in ITP
In March 2017, we plan to initiate a randomized, double-blind, placebo-controlled Phase 2 clinical trial to evaluate the safety, efficacy and pharmacokinetics of ARGX-113 in 36 ITP patients, who have platelet counts lower than 30 × 109/L and who are stable on standard-of-care treatment, consisting of corticosteroids, permitted immunosuppressants and/or thrombopoietin receptor agonists. We intend to conduct the clinical trial at approximately 35 sites across Europe. Patients will be randomly assigned to three arms of 12 patients each. All patients in this clinical trial will continue to receive standard-of-care treatment. One treatment arm will receive 5 mg/kg ARGX-113, the second arm will receive 10 mg/kg ARGX-113 and the third arm will receive placebo. Dosing will take place
112
in a three-week period with four weekly doses of ARGX-113 or placebo. Patient follow-up will continue for eight weeks after treatment.
The primary objectives of this Phase 2 clinical trial are to evaluate safety and tolerability of ARGX-113 with primary endpoints evaluating the incidence and severity of adverse events and serious adverse events and evaluating vital signs, electrocardiogram and laboratory assessments. Secondary objectives include evaluation of efficacy, based on platelet count, use of rescue treatment and bleeding events; pharmacokinetics; pharmacodynamics; and immunogenicity.
Planned Phase 1 Clinical Trial for Subcutaneous Formulation of ARGX-113
In addition to the intravenous product formulation of ARGX-113 that we are currently using in our clinical trials, we are also developing a subcutaneous product formulation designed to enable administration of ARGX-113 to larger patient populations, including patients requiring chronic therapy, potentially outside the hospital setting.
We evaluated the intravenous and subcutaneous formulations of ARGX-113 head-to-head in a preclinical cynomolgus monkey model. The results suggest that both formulations result in comparable half-life in circulation of ARGX-113, a favorable bioavailability of 75% of the subcutaneous formulation and a comparable pharmacodynamic effect shown by reduction of total IgG antibodies. We believe these results suggest subcutaneous dosing of ARGX-113 in humans may be feasible. We plan to initiate a Phase 1 clinical trial in healthy volunteers for a subcutaneous formulation in the second half of 2017.
Phase 1 Clinical Data
We have completed enrollment in a double-blind, placebo-controlled Phase 1 clinical trial in healthy volunteers to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of single and multiple doses of ARGX-113. In the first part of the clinical trial, 30 subjects were randomized to receive a single dose of ARGX-113 or placebo ranging from 0.2 mg/kg to 50 mg/kg. In the second part of the clinical trial, 32 subjects were randomized to receive multiple ascending doses of ARGX-113 or placebo up to a maximum of 25 mg/kg.
We announced interim data from this Phase 1 clinical trial in June 2016 and at a workshop we sponsored in conjunction with the American Society of Hematology annual meeting in December 2016. We expect that the full results from this clinical trial will be published in a peer-reviewed journal during the first half of 2017.
Single Ascending Dose
We observed that a single two-hour infusion of 10 mg/kg ARGX-113 was associated with an approximate 50% reduction of circulating IgG antibody levels. We observed that a reduction of circulating IgG antibody levels persisted for more than four weeks after the last dose, as shown inFigure 7. We believe this sustained reduction would be clinically meaningful if replicated with respect
113
to pathogenic IgG antibodies because IVIg and plasmapheresis typically result in a 30% to 60% reduction in pathogenic IgG antibody levels.
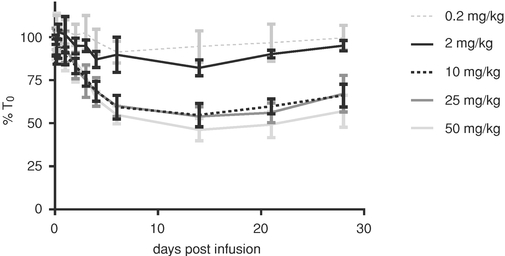
Figure 7. Selective reduction of IgG by administration of ARGX-113 to healthy volunteers in the single ascending dose part of our Phase 1 clinical trial
Administration of ARGX-113 at single doses up to 25 mg/kg was reported to be well-tolerated and administration of a single dose of 50 mg/kg was reported to be moderately tolerated. There were no drug- or infusion-related serious adverse events associated with doses up to 50 mg/kg. The most frequently reported drug-related adverse events included abnormal white blood cell count, increased C-reactive protein levels, headache, dizziness and chills. All of these adverse events were mild or moderate and reported only in the two highest dose groups (25 mg/kg and 50 mg/kg). While ARGX-113 was associated with a decrease in the levels of IgG antibodies, there were no observed changes in IgM or IgA levels or serum albumin observed in the clinical trial, suggesting that ARGX-113 has the potential to be a highly selective immunosuppressant.
Multiple Ascending Dose
In the multiple ascending dose part of the Phase 1 clinical trial, repeat administration of both 10 mg/kg and 25 mg/kg of ARGX-113 every seven days, four doses in total, was associated with a gradual reduction in levels of all four classes of IgG antibodies by 60% to 85%, with 10 mg/kg dose results shown inFigure 8. For both doses, we observed the reduction in circulating IgG antibody levels to persist for more than four weeks after the last dose with levels below 50% at approximately three weeks, and did not return to baseline levels for more than one month. Pharmacokinetic analysis of serum baseline levels of ARGX-113 indicates that it has a half-life of approximately three to four days with no drug accumulation following subsequent weekly dosing. The prolonged activity on the levels of IgG antibodies is consistent with the mechanism of action of ARGX-113 and the
114
effect of the ABDEG technology on increasing the intracellular recycling of ARGX-113. Similar to the single ascending dose part, no significant reductions in IgM, IgA or serum albumin were observed.
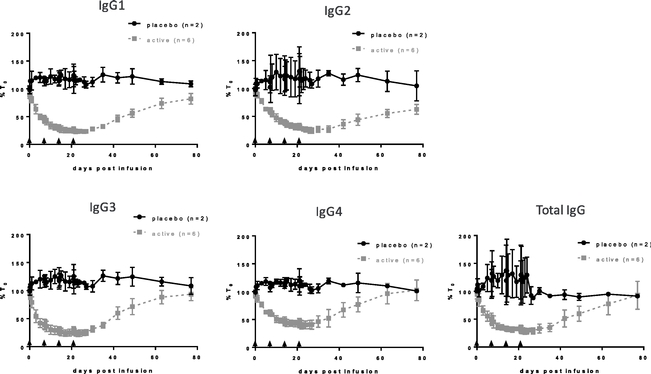
Figure 8. Reduction in the levels of four IgG antibody classes and total IgG levels in the multiple ascending dose part of our Phase 1 clinical trial of ARGX-113 in healthy volunteers at a dose of 10 mg/kg every seven days
Administration of multiple ARGX-113 doses of 10 mg/kg was reported to be well-tolerated. One serious adverse event, hyperventilation, was observed in the multiple ascending dose part. This event, which occurred six days after drug administration, was considered by the clinical investigator as unlikely to be related to ARGX-113. Some patients had changes C-reactive protein levels that were considered clinically significant. The most frequently reported drug-related adverse events included headache, feeling cold, chills and fatigue, all of which were mild or moderate and reported only in the highest dose group of 25 mg/kg.
Preclinical Data
We conducted several preclinical studies of ARGX-113. The role of FcRn in maintaining IgG homeostasis was observed in knockout mice lacking functional FcRn. In preclinicalin vitro studies, ARGX-113 bound to human FcRn with an affinity that was 30 to 500 times higher than the naturally occurring Fc region of human IgG1. In preclinical testing in cynomolgus monkeys, ARGX-113 specifically blocked IgG antibody recycling and did not lead to reductions in IgA, IgM or serum albumin levels. In preclinical animal efficacy models of MG, ITP, rheumatoid arthritis and MS, different prototypes of ARGX-113 showed the potential to reduce pathogenic IgG antibodies, thereby reducing disease symptoms.
ARGX-110
We are developing ARGX-110 in cancer indications, initially for TCL and AML, as well as high-risk MDS. TCL and AML are rare and aggressive hematological cancers for which significant unmet medical needs exist. MDS, a rare bone marrow disorder, is often a precursor to AML. ARGX-110 is a SIMPLE Antibody that blocks the cell surface protein CD70, which is overexpressed
115
in B-cell and T-lymphocyte, or T-cell, lymphomas and leukemias and is involved in the proliferation and survival of these cells. ARGX-110 is designed to kill CD70-positive cells via its potent antibody effector functions through the use of POTELLIGENT technology.
ARGX-110 is currently being evaluated in an open-label, multi-site Phase 1/2 clinical trial in Europe in patients with advanced malignancies expressing CD70. To date, we have enrolled a total of 94 patients in the Phase 1 part of the clinical trial. In this clinical trial, we have observed promising signs of biological activity in patients with a range of cancers including platinum-refractory ovarian cancer, head-and-neck cancer, myoepithelial carcinoma, mesothelioma, renal cell carcinoma and TCL, including in five out of 10 cutaneous TCL, or CTCL, patients. We are currently concluding the Phase 1 safety-expansion cohort of this clinical trial in relapsed or refractory, CD70-positive TCL patients. Based on the preliminary results from the Phase 1 part of the clinical trial, we plan to transition into the Phase 2 part of the clinical trial in adult relapsed or refractory CD70-positive CTCL patients in March 2017, with interim results expected to be available by the end of 2017. We expect to report topline results from this clinical trial in the second half of 2018. In December 2016, we initiated a Phase 1/2 clinical trial of ARGX-110 in combination with azacitidine in newly diagnosed AML or high-risk MDS patients. We expect the majority of patient enrollment in this clinical trial to be AML patients. We expect to report interim results from the dose-escalation part of this clinical trial by the end of 2017. This clinical trial is currently being conducted at a single site in Switzerland.
Overview of T-Cell Lymphoma
Lymphoma is the most common type of blood cancer. The two main forms of lymphoma are Hodgkin's lymphoma and non-Hodgkin's lymphoma. Lymphoma occurs when lymphocytes, a type of white blood cell, grow and multiply uncontrollably. Cancerous lymphocytes can travel to many parts of the body, including the blood and bone marrow, giving rise to leukemias, and to lymph nodes, spleen, skin or other organs, forming a mass known as a tumor. The body has two main types of lymphocytes that can develop into lymphomas: B-cells and T-cells. Hodgkin's lymphoma involves B-cells, while non-Hodgkin's lymphoma may involve either B-cells or T-cells.
TCL accounts for 6% of all cases of lymphoma and can be divided into subtypes such as peripheral TCL, or PTCL, angioimmunoblastic TCL, anaplastic large cell lymphoma, or ALCL, and CTCL. These subtypes differ by location, distribution and aggressiveness of the primary tumor as well as by specific changes to the affected lymphocytes. Overall, there are approximately 7,900 new cases of TCL in the United States each year. According to the Cutaneous Lymphoma Foundation, the incidence of CTCL in the United States is approximately 3,000 new cases per year.
The two most common types of CTCL are mycosis fungoides, representing approximately 50% of CTCL patients, and a more advanced form known as Sézary syndrome, representing approximately 15% of CTCL patients. In both mycosis fungoides and Sézary syndrome, visible skin lesions offer an ongoing means with which to monitor both the progression of disease and the impact of treatment. Sézary syndrome is distinguished by the presence of malignant lymphocytes in the blood, an extensive rash covering over 80% of the body and tumors visible on the skin.
TCL is generally very aggressive and is typically treated with standard anticancer chemotherapy agents used in combination such as cyclophosphamide, doxorubicin, vincristine and prednisone, with or without the addition of biologics. Mogamulizumab, an antibody targeting the chemokine receptor CCR4, is approved in Japan for the treatment of adult TCL, and brentuximab, an anti-CD30 antibody-drug conjugate, is approved by the U.S. Food and Drug Administration, or FDA, for the treatment of ALCL. The five-year survival for all non-Hodgkin's TCL patients is 65%, with poor prognosis for subtypes such as Sézary syndrome and several PTCL types. Recently, two compounds have been approved for the treatment of TCL by the FDA: romidepsin (ISTODAX) and pralatrexate (Folotyn). TCL patients treated with either of these agents had response rates of up to 35% (romidepsin) and 27% (pralatrexate). However, the duration of response for these therapies is between nine and 15 months, underscoring the unmet need for effective, long-lasting TCL treatments.
116
Overview of Acute Myeloid Leukemia and Myelodysplastic Syndrome
AML is a hematologic cancer characterized by excessive proliferation of myeloid stem cells and their failure to properly differentiate into mature white blood cells. AML is the second most common subtype of leukemia in adults. In the United States, AML has an incidence of approximately 22,000 new cases annually. AML is generally a disease of elderly people, with more than 60% of diagnosed patients being older than 60 years, and AML is uncommon before the age of 45. The average age of an AML patient is 67. The average five-year survival rate for patients with AML is 27%, but there are significant differences in prognosis depending on the age of the patient at diagnosis. For patients under the age of 45, the five-year survival rate is approximately 57%, while for those over the age of 65 it is only 6%. There are likely multiple reasons for this discrepancy, including the ability of younger patients to tolerate more aggressive therapy.
Current first-line treatments in AML typically involve aggressive chemotherapy for younger patients to induce remission. This therapy is not recommended for older patients or patients with comorbidities, who are often treated with hypomethylating agents. We believe there is a significant need for safer, more effective AML treatments that can also be used in elderly patients. Because relapse is often due to leukemic stem cells present next to the bulk of malignant AML cells, or blasts, therapies targeting both blasts and leukemic stem cells may be more efficacious than chemotherapy only and could increase survival rates.
MDS also affects bone marrow cells, reducing their ability to produce red and white blood cells or platelets. In the United States, MDS has an incidence of approximately 13,000 new cases annually. There are currently an estimated 60,000 MDS patients in the United States. Approximately 75% of MDS patients are older than 60 years of age when diagnosed, and, like with AML, as the population ages the disease prevalence is expected to rise. Some MDS patients are at high risk to develop AML and are treated in a similar way as AML patients.
Our Solution: ARGX-110
Our product candidate ARGX-110 is an antibody that we believe has the potential to add to the treatment paradigm for lymphomas and leukemias by both increasing the response rates and extending the duration of response for patients with CD70-positive advanced-stage cancers. We developed ARGX-110 using our SIMPLE Antibody Platform and the POTELLIGENT Fc engineering technology.
ARGX-110 binds to the cell surface protein CD70 with high affinity, blocking the interaction between CD70 and its receptor CD27 and targeting CD70 expressing cells for destruction by multiple immune pathways. CD70 is a cell surface protein that is highly expressed in cancer, including in T-cell and B-cell lymphomas, leukemias and certain solid tumors. In normal tissues, CD70 expression is either low or absent. Binding of CD70 to its receptor, CD27, initiates a cascade of intracellular events leading to cell proliferation and survival. As a byproduct of CD70 binding to CD27, the extracellular portion of CD27 is cleaved, creating a soluble form of CD27 known as sCD27, which can easily be measured. The presence of sCD27 is thought to be correlated with CD70 activity and potentially tumor load. Because sCD27 can easily be measured, it may serve as a biomarker for CD70 activity, potentially allowing us to identify target patients based on the likelihood of response to treatment, monitor disease progression and measure the impact of anti-CD70 therapy.
ARGX-110 exhibits potent ADCC through the use of POTELLIGENT technology as well as complement-dependent cytotoxicity and antibody-dependent cellular phagocytosis leading to the killing of cells expressing CD70.
117
Based on the broad overexpression of CD70 in hematological cancers, we may decide to study ARGX-110 in additional hematological cancer indications beyond TCL and AML.
In addition to ARGX-110's potential as a monotherapy, we believe that it may be suited for combination therapy given its reported tolerability to date; the fact that certain cancer treatments, such as histone deacetylase inhibitors, hypomethylating agents and irradiation, may upregulate CD70; and resistance to certain treatment with tyrosine kinase inhibitors may be effected through CD70 overexpression.
Clinical Development Plan
We are currently evaluating ARGX-110 in an open-label, multi-site Phase 1/2 clinical trial in Europe in patients with advanced malignancies expressing CD70. We announced interim data from the Phase 1 dose-escalation and dose-expansion part of this clinical trial in September 2016 and at a workshop we sponsored in conjunction with the American Society of Hematology annual meeting in December 2016. We expect that this clinical trial will transition into the open-label Phase 2 part in 10 adult, relapsed or refractory CD70-positive CTCL patients in March 2017, with interim data expected to be available by the end of 2017. We expect to report topline results from this clinical trial in the second half of 2018.
In December 2016, we initiated an open-label Phase 1/2 clinical trial of ARGX-110 at a single site in Switzerland for the treatment of newly diagnosed AML or high-risk MDS patients. We expect the majority of patient enrollment in this clinical trial to be AML patients. We expect to report interim results from the dose-escalation phase of this clinical trial by the end of 2017. Patient recruitment is currently ongoing, and we have recruited one AML patient to date.
Phase 1/2 Clinical Trial in Patients with Advanced Malignancies Expressing CD70
We followed a step-wise adaptive clinical trial design for ARGX-110, in which 93 patients have been treated to date. We initially completed a Phase 1 dose-escalation part in 26 patients overexpressing CD70. Subsequently, we completed two Phase 1 safety-expansion cohorts in 20 patients with solid tumors and 19 patients with hematological cancers overexpressing CD70, respectively. In addition, we have a safety-expansion cohort in nasopharyngeal cancer with 10 patients. This clinical trial design is adaptive in that it allows us to make data driven decisions and open-up new cohorts in indications where we have seen the most promising early signals of biological activity. While the primary goal of this Phase 1 clinical trial is to investigate safety and pharmacokinetics, we have also observed evidence of biological activity in several of the patients treated. These results led us to pursue the further evaluation of ARGX-110 in additional Phase 1 safety-expansion cohorts aiming to recruit 10 CTCL and 10 PTCL patients. Patient recruitment is currently ongoing, and we have recruited 12 CTCL patients and six PTCL patients to date.
Phase 1 Dose-Escalation
In the dose-escalation part of the Phase 1/2 clinical trial, ARGX-110 doses ranging between 0.1 mg/kg and 10 mg/kg were administered to patients with CD70-positive tumors who had failed all available therapies. In total, 127 treatment cycles, with dosing every three weeks, of ARGX-110 were administered to 26 patients.
No dose-limiting toxicities were observed. The most frequent drug-related adverse events were fatigue in 27% of patients and infusion-related reactions in 43% of patients. Other monoclonal antibodies engineered using POTELLIGENT or similar third-party products that augment ADCC such as mogamulizumab, obinutuzumab and imgatuzumab also have infusion-related reaction rates of 24% to 77%. Premedication with acetaminophen, antihistamines and/or corticosteroids are used to reduce the impact of infusion-related reactions. Administration of ARGX-110 was associated with
118
inflammatory responses such as swelling and redness in skin lesions followed by reductions in the size, or necrosis, of these skin lesions, and overall improvement in the clinical appearance of the skin.
There were 20 serious adverse events seen in some of these heavily pre-treated patients, but no significant trends in terms of safety were observed between the dose groups. Grade 3 and 4 drug-related adverse events from the dose-escalation part of the clinical trial are summarized inTable 1.
Table 1. Grade 3 and 4 drug-related adverse events in ARGX-110 in open-label, Phase 1 dose-escalation part
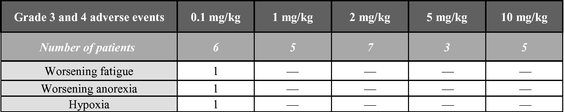
Seven patient deaths were reported in the dose-escalation part of the clinical trial, of which five deaths were attributed to disease progression, one death was attributed to sepsis and one death was attributed to respiratory failure. None of these deaths were deemed to be drug-related according to the investigator.
In this clinical trial, the half-life of ARGX-110 was observed to be approximately 13 days. Anti-drug antibodies were detected in 50% of all patients, the majority of which were seen at the 0.1 mg/kg and 1mg/kg doses. In two relapsed or refractory CD70-positive patients with Sézary syndrome, we observed that CD70-positive tumor cells were reduced in the blood after dosing of ARGX-110, one patient at a dose level of 0.1 mg/kg and the other at a dose level of 10 mg/kg.
Phase 1 Safety-Expansion
Two safety-expansion cohorts have been completed using a 5 mg/kg dose of ARGX-110, one in 20 patients with CD70-positive solid tumors, and one in 19 patients with CD70-positive hematological tumors. In these cohorts, there was one drug-related patient death attributed to sepsis. Anti-drug antibodies were detected in 13% of the patients.
Two additional safety-expansion cohorts are ongoing, one in up to 10 heavily pretreated patients with CD70-positive CTCL and one in up to 10 heavily pretreated patients with CD70-positive PTCL. Currently we have recruited 12 CTCL patients and six PTCL patients. We expect to announce topline results from this expansion phase of the Phase 1 clinical trial before the end of 2017.
While the safety-expansion part is still ongoing, we have seen promising preliminary results in some of the first 10 evaluable CTCL patients. At doses of 1 mg/kg every three weeks, we observed three patients to have a partial response and two patients to have stable disease. The preliminary responses observed in the first 10 evaluable CTCL patients can be seen inTable 2.
119
Table 2. Overview of 10 CTCL patients treated with ARGX-110 in the TCL safety-expansion cohort
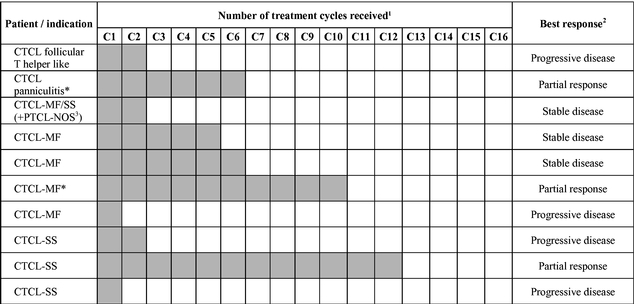
Note: MF = mycosis fungoides; SS = Sézary syndrome.
Phase 2 Clinical Trial in CTCL
We expect the ongoing Phase 1 safety-expansion cohort for ARGX-110 to transition into the open-label Phase 2 part in 10 adult, relapsed or refractory CD70-positive CTCL patients in March 2017. We plan to conduct this clinical trial at multiple centers in Europe. All patients in this clinical trial will receive ARGX-110 monotherapy at a dose of 5 mg/kg. Patients will cease treatment if necessary for either safety reasons or disease progression.
Phase 1/2 Clinical Trial in Combination with Azacitidine in Patients with AML or High-Risk MDS
We are evaluating ARGX-110 in an open-label, dose-escalating Phase 1/2 clinical trial to evaluate its safety, tolerability and efficacy in combination with azacitidine in newly diagnosed AML or high-risk MDS patients. The clinical trial was initiated in December 2016. All patients in this clinical trial will receive ARGX-110 in combination with 75 mg/m2 azacitidine, which is the standard of care for AML. During the dose-escalation part of the clinical trial, three doses of ARGX-110, 1 mg/kg, 3 mg/kg and 10 mg/kg administered bi-weekly, will be evaluated in up to 18 patients. Patients will be dosed every two weeks until disease progression. The primary objective of the Phase 1 part of the clinical trial is to determine the maximum tolerated dose of ARGX-110 and/or the recommended Phase 2 dose in combination with azacitidine. Once the dose for the combination therapy is selected, efficacy will be evaluated in the Phase 2 proof-of-concept part involving up to 18
120
patients. We expect this clinical trial will be a multi-center trial conducted in Europe. We expect to report interim results from the dose-escalation phase of this clinical trial by the end of 2017.
Preclinical Data
We conducted preclinical studies of ARGX-110 in support of our clinical program. In preclinical testing in cynomolgus monkeys, ARGX-110 was well-tolerated. In preclinical mouse efficacy models, ARGX-110 variants showed the potential to prolong survival in Burkitt's lymphoma, overcome tyrosine kinase inhibitor resistance thereby prolonging survival in chronic myeloid leukemia, or reduce blast and leukemic stem cell burden thereby prolonging survival in AML. In a preclinical mouse efficacy model of acute lymphocytic leukemia, the administration of an ARGX-110 variant led to the acute death of some animals with high tumor load.
ARGX-111
We are developing ARGX-111 for the treatment of patients with certain solid tumors that overexpress c-Met, a receptor associated with tumor growth and metastasis, or tumors that are mesenchymal-epithelial transition factor, or MET, amplified. MET-amplified tumors possess multiple copies of the MET gene, resulting in elevated c-Met levels. While c-Met overexpression and MET amplification both result in elevated c-Met levels, clinical and preclinical evidence suggests c-Met from MET-amplified tumors is a disease driver in some cancers. ARGX-111 employs our SIMPLE Antibody, NHance and POTELLIGENT technologies to drive tissue penetration in the body and to increase its ability to enhance ADCC. ARGX-111 binds to c-Met with high affinity and does not cause dimerization of the c-Met receptor, which differentiates it from other, earlier attempts to direct antibodies against c-Met. Dimerization is a process which can result in receptor activation, undermining the intended therapeutic effect of antibodies blocking hepatocyte growth factor, or HGF, binding to c-Met. By blocking both HGF-dependent and independent c-Met activation, ARGX-111 is able to block c-Met receptor activation which could trigger survival, proliferation and metastasis of tumor cells. Thus, we believe ARGX-111 may have a differentiated clinical profile.
Clinical Development Plan
We conducted a Phase 1 clinical trial in Europe consisting of a dose-escalation part in 19 treatment-refractory patients whose tumors overexpress c-Met and a safety-expansion part in five treatment-refractory patients whose tumors were MET-amplified. We chose to focus the safety-expansion part on MET-amplified tumors, rather than c-Met overexpressing tumors, because of the accumulating preclinical and clinical evidence suggesting MET amplification is an oncogenic driver. The primary objective of this Phase 1 clinical trial was to determine the recommended Phase 2 dose of ARGX-111, with the primary endpoint evaluating the incidence of dose-limiting toxicity. As a secondary objective, safety, immunogenicity, pharmacokinetics and pharmacodynamics were characterized, with secondary endpoints being the pharmacokinetics and pharmacodynamics profile of ARGX-111, as well as tumor response.
In the dose-escalation part, we evaluated doses ranging from 0.3 mg/kg to 10 mg/kg and observed dose-limiting infusion-related reactions at doses above 3 mg/kg. Accordingly, we determined that 3 mg/kg would be the maximum dose tested in future clinical trials. No other drug-related serious adverse events were observed in either part of the clinical trial. In both parts, we observed signs of biological activity. Although neither part was designed to evaluate the efficacy of ARGX-111, we anecdotally observed reduced tumor burden at various sites and stable disease in a gastric cancer patient with bone metastases who was refractory to multiple rounds of prior treatment and in a MET-amplified renal cancer patient with metastases and progressive disease. We observed signs of biological activity for ARGX-111 in seven out of 19 patients in the dose-escalation part, including one partial response, and we expect to report our initial results of the safety-expansion cohort during the first half of 2017.
121
In preclinical orthotopic breast cancer models in mice, ARGX-111 was observed to reduce circulating tumor cells and cancer metastasis both in the adjuvant and the neo-adjuvant setting.
Given the size of the potential patient populations and the costs of clinical development for ARGX-111, we intend to begin Phase 2 development only if and when we have entered into a collaboration with an appropriate partner.
Our Partnered Programs
The following is the pipeline for our partnered product candidates and discovery programs. For more information on our collaborations, see "—Collaborations."
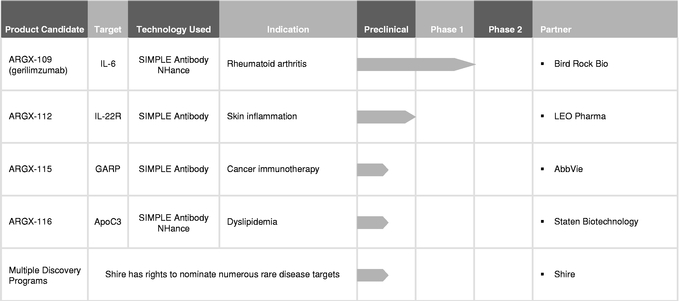
ARGX-115 (partnered with AbbVie)
We are developing ARGX-115 as a cancer immunotherapy against the novel target GARP, a protein present on the surface of activated regulatory T-cells, or Tregs. We are developing ARGX-115 with our collaboration partner AbbVie. See "—Collaborations."
ARGX-115 employs our SIMPLE Antibody technology and works by stimulating a patient's immune system after a tumor has suppressed the immune system by co-opting immunosuppressive cells such as Tregs. While the normal function of Tregs is to suppress portions of the immune system to prevent a self-directed immune response through the release of active transforming growth factor beta, or TGF-b, Tregs can also prevent the immune system from recognizing and suppressing pathogenic cells including cancer cells. By binding to GARP, which plays a key role in the regulation of production and release of active TGF-b, AGRX-115 works to limit the immunosuppressive activity of Tregs and thereby stimulate the immune system to attack cancer cells. We believe this specific inhibition of TGF-b release by Tregs is potentially superior as a therapy to systemic inhibition of TGF-b activity or the depletion of Tregs, the presumed mode of action of ipilimumab (Yervoy), and that its specificity has the potential to provide an improved safety profile.
ARGX-115 was observed to be active in a mouse model of graft-versus-host disease, or GVHD, where it was able to completely block the activity of Tregs, suggesting its potential to re-activate the immune system against cancer cells. In this model, human peripheral blood lymphocytes, or PBMCs, are introduced into mice leading to a rapid onset of disease, caused by these PBMCs attacking the
122
mouse host. When human Tregs are added to the human PBMCs, they can significantly delay disease onset and reduce disease severity. However, the addition of ARGX-115 completely neutralized the effect of human Tregs, resulting in a rapid onset of the disease again. The purpose of the experiment was to show that when ARGX-115 binds to GARP on Tregs, the normal immune suppressive function of Tregs is itself suppressed so that the immune system is free to act. In this experiment, the PBMCs represent the human immune system. The Tregs suppress the PBMCs when they are added (illustrated by lower PBMC activity—in this case represented by less activity against the mouse host). ARGX-115 suppresses the Tregs, allowing the immune system to act (as represented by the PBMCs once again attacking the mouse host). A prototype of ARGX-115 devoid of cell-killing ability was as effective as ARGX-115 with cell-killing ability as shown inFigure 9, leading us to believe the effect of ARGX-115 is mainly due to blocking Treg activity.
Figure 9. Preclinical data of ARGX-115 in a graft-versus-host disease model
We are currently advancing ARGX-115 through preclinical studies up to completion of IND-enabling studies, at which point AbbVie has the right to exercise an option to obtain a worldwide, exclusive license to ARGX-115.
ARGX-109 (partnered with Bird Rock Bio)
ARGX-109 (gerilimzumab) is being developed for the treatment of rheumatoid arthritis, or RA, by our collaboration partner Bird Rock Bio. See "—Collaborations."
ARGX-109 employs our SIMPLE Antibody and NHance technologies and blocks interleukin 6, or IL-6, a cell-signaling protein that is an important driver of inflammatory response implicated in the transition from acute to chronic inflammation. Chronic inflammation is a notable feature of several diseases, including RA, psoriatic arthritis and chronic kidney disease. In particular, IL-6 has been shown to stimulate the immune system to increase tissue destruction and joint damage in RA patients. By targeting a unique epitope, ARGX-109 potentially enables blocking of IL-6 with high potency, with the goal of mitigating inflammatory responses at lower and less frequent doses than current therapies directed at IL-6.
Bird Rock Bio has completed two Phase 1 clinical trials of ARGX-109 in 50 healthy volunteers to assess the safety and tolerability of the compound in single and multiple ascending doses compared to placebo. The clinical trials also explored the pharmacokinetics of ARGX-109. In these clinical trials, ARGX-109 was reported to be well-tolerated with no serious adverse events. Further, ARGX-109 was observed to have a prolonged half-life in circulation. In January 2017, Bird Rock Bio announced that it had received approval for the initiation of a Phase 2 clinical trial in Brazil in approximately 200 patients with RA.
123
ARGX-112 (partnered with LEO Pharma)
We are developing ARGX-112 for the treatment of dermatologic indications involving inflammation, together with our collaboration partner LEO Pharma. See "—Collaborations."
ARGX-112 employs our SIMPLE Antibody technology and blocks the interleukin-22 receptor, or IL-22R, in order to neutralize the signaling of interleukin-22, or IL-22, and interleukin-20, or IL-20, both of which are cytokines involved in the proliferation and differentiation of skin cells. When overexpressed, IL-22 and IL-20 are implicated in autoimmune diseases of the skin, including atopic dermatitis, psoriasis and pustular psoriasis. In preclinical studies, ARGX-112 was observed to have high neutralization potency for IL-22R and favorablein vivo pharmacokinetics and distribution to the skin.
Under the collaboration, LEO Pharma will fund more than half of all product development costs up approval of a clinical trial application, or CTA, in Europe for a first product in a Phase 1 clinical trial. After CTA approval of a first product in a Phase 1 clinical trial, LEO Pharma will be solely responsible to fund the clinical development of the program.
ARGX-116 (partnered with Staten Biotechnology)
We are developing ARGX-116 for the treatment of dyslipidemia, together with our collaboration partner Staten Biotechnology. See "—Collaborations."
ARGX-116 employs our SIMPLE Antibody technology and blocks APOC3, a metabolic target involved in triglyceride metabolism. APOC3 is supported as a therapeutic target by human genetic evidence suggesting that deactivating mutations in the APOC3 gene results in a favorable lipoprotein profile, lower insulin sensitivity, longevity and protection from cardiovascular disease.
ARGX-116 is the first of up to three research programs under the collaboration. Under the terms of the collaboration, the parties are jointly responsible for conducting research under a mutually agreed research program, with Staten reimbursing us for all costs of carrying out our research responsibilities under each research program.
Innovative Access Program
We have developed a program designed to secure access to early, cutting edge targets, which we call our Innovative Access Program. Through our Innovative Access Program, we are able to serially collaborate with leading academic labs by providing them access to our SIMPLE Antibody Platform technology with the goal of expediting the validation of new targets and accelerating the addition of new product candidates to our pipeline. In return, we receive early access to these targets and provide academic groups a simple path to clinical validation and future commercialization of promising ideas in which we and the academic lab both share in the upside potential.
One example of the value of the Innovative Access Program is ARGX-115, which was developed in collaboration with the de Duve Institute / Université Catholique de Louvain. We provided antibodies to the academic groups to help validate the target. This in turn, allowed the groups to advance their work successfully, including the facilitation of supportive publications. Subsequently, this program formed the basis of our collaboration with AbbVie. ARGX-115 exemplifies how our Innovative Access Program enables us to generate product candidates against novel targets that may be of high interest for collaboration with biopharmaceutical partners. Another example is ARGX-116, which was discovered in close collaboration with disease biology experts from Staten Biotechnology, an emerging biotechnology company specialized in the field of dyslipidemia.
124
Manufacturing and Supply
We utilize third-party contract manufacturers who act in accordance with the FDA's good laboratory practices, or GLP, and current good manufacturing practices, cGMP, for the manufacture of drug substance and product. Currently, we contract with Lonza Sales AG, or Lonza, based in Slough, UK, for all activities relating to the development of our cell banks, development of our manufacturing processes and the production of all drug substance, thereby using validated and scalable systems broadly accepted in our industry. We use additional contract manufacturers to fill, label, package, store and distribute investigational drug products.
ARGX-113, ARGX-110, ARGX-111 and ARGX-112 are each manufactured using an industry-standard mammalian cell culture of a Chinese hamster ovary cell line that expresses the product, followed by multiple purification and filtration steps typically used in producing monoclonal antibodies.
All of our antibodies are manufactured by starting with cells, which are stored in a cell bank. We have one master cell bank for each product manufactured in accordance with cGMP. Half of each master cell bank is stored at a separate site with the goal that, in case of a catastrophic event at one site, sufficient vials of the master cell bank would remain at the alternative storage site to continue manufacturing.
Competition
We participate in a highly innovative industry characterized by a rapidly growing understanding of disease biology, quickly changing technologies, strong intellectual property barriers to entry, a multitude of companies involved in the creation, development and commercialization of novel therapeutics. These companies are highly sophisticated and often strategically collaborate with each other.
We compete with a wide range of pharmaceutical companies, biotechnology companies, academic institutions and other research organizations for novel therapeutic antibody targets, new technologies for optimizing antibodies, talent, financial resources, intellectual property rights and collaboration opportunities. Many of our competitors and potential competitors have substantially greater scientific, research and product development capabilities as well as greater financial, manufacturing, marketing and sales and human resources than we do. In addition, there is intense competition for establishing clinical trial sites and registering patients for clinical trials. Many specialized biotechnology firms have formed collaborations with large, established companies to support the research, development and commercialization of products that may be competitive with ours. Accordingly, our competitors may be more successful than we may be in developing, commercializing and achieving widespread market acceptance.
Competition in the autoimmune field is intense and involves multiple monoclonal antibodies, other biologics and small molecules either already marketed or in development by many different companies including large pharmaceutical companies such as AbbVie Inc. (Humira/rheumatoid arthritis); Amgen Inc. (Enbrel/rheumatoid arthritis); Biogen, Inc. (Tysabri/multiple sclerosis); GlaxoSmithKline plc, or GSK, (Benlysta/lupus); F. Hoffman-La Roche AG, or Roche, (Rituxan/often used off label); and Janssen Pharmaceuticals, Inc., or Janssen (Remicade/rheumatoid arthritis and Stelara/psoriasis). In some cases, these competitors are also our collaborators. In addition, these and other pharmaceutical companies have monoclonal antibodies or other biologics in clinical development for the treatment of autoimmune diseases. We are aware that Alexion Pharmaceuticals, Inc.; GSK; Roche; Novartis AG; CSL Behring; Grifols, S.A.; BioMarin Pharmaceutical Inc.; Curayac and Millenium Pharmaceuticals, Inc., among others, are developing drugs that may have utility for the treatment of MG. We are aware that Rigel Pharmaceuticals, Inc.;
125
Eisai Inc.; Bristol-Myers Squibb; Shire Immunomedics; Protalex Inc. and others are developing drugs that may have utility for the treatment of ITP. Furthermore, we are aware of competing products specifically targeting FcRn and being developed by UCB S.A.; Momenta, Inc.; Shire Plc; Syntimmune, Inc. and Hannal Biotech.
Competition in the leukemia and lymphoma space is intense, with many compounds in clinical trials by large multinational pharmaceutical companies and specialized biotech companies. Rituxan (Roche), Adcetris (Seattle Genetics, Inc./Takeda Pharmaceutical Company Ltd), Darzalex (Janssen), Poteligeo (Kyowa Hakko Kirin Co., Ltd.) are some examples of monoclonal antibodies approved for the treatment of Hodgkin's lymphoma, non-Hodgkin's lymphoma, multiple myeloma or other blood cancers. In addition, we are aware of a number of other companies with development stage programs that may compete with ARGX-110 in the future if it is approved. We anticipate that we will face intense and increasing competition as new treatments enter the market and advanced technologies become available.
There are several monoclonal antibody drug discovery companies that may compete with us in the search for novel therapeutic antibody targets, including Adimab LLC; Merus N.V.; Regeneron Pharmaceuticals, Inc.; Xencor Inc. and MorphoSys AG. We are aware that a product candidate in development by Scholar Rock, Inc. may compete with ARGX-115 and a product candidate in development by Ionis Pharmaceuticals, Inc. may compete with ARGX-116, if they are approved.
Our commercial opportunity could be reduced or eliminated if our competitors' products prove to be safer and more tolerable, more effective, more convenient to dose, less expensive, faster to approve, or more effectively marketed and reimbursed than any of our product candidates that may gain regulatory approval. In addition, the level of generic competition and the availability of reimbursement from government and other third-party payors will impact the commercial viability of our programs.
Collaborations
We have entered into multiple collaboration agreements with pharmaceutical partners. We expect to continue to collaborate selectively with pharmaceutical and biotechnology companies to leverage our discovery platform and accelerate product candidate development.
Our Strategic Partnership with AbbVie (for ARGX-115)
In April 2016, we entered into a collaboration agreement with AbbVie S.À.R.L., or AbbVie, to develop and commercialize ARGX-115. Under the terms of the collaboration agreement, we will be responsible for conducting and funding all ARGX-115 research and development activities up to completion of IND-enabling studies.
We have granted AbbVie an exclusive option, for a specified period following completion of IND-enabling studies, to obtain a worldwide, exclusive license to the ARGX-115 program to develop and commercialize products. Following the exercise of the option, AbbVie will assume certain development obligations, and will be solely responsible for all research, development and regulatory costs relating to the products. We received an upfront, non-refundable, non-creditable payment of $40 million (€35.1 million as of the date the payment was received) from AbbVie for the exclusive option to license ARGX-115 and are eligible to receive two near-term preclinical milestones of $10 million each. We are also eligible, if AbbVie exercises its option, to receive additional development, regulatory and commercial milestone payments in an aggregate of up to $625 million as well as tiered royalties on sales at percentages ranging from the mid-single digits to the lower teens, subject to customary reductions.
126
We have the right, on a product-by-product basis to co-promote ARGX-115-based products in the European Economic Area and Switzerland and combine the product with our own future immuno-oncology programs. The co-promotion effort would be governed by a co-promotion agreement negotiated in good faith by the parties. In addition to the ARGX-115 program, and upon reaching a predetermined preclinical stage milestone, AbbVie will fund further GARP-related research by us for an initial period of two years. AbbVie will have the right to license additional therapeutic programs emerging from this research, for which we could receive associated milestone and royalty payments.
If AbbVie does not exercise its option to license ARGX-115, we have the right to pursue development and commercialization of ARGX-115 by ourselves or with another partner.
Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the agreement, the term of the option and license agreement ends, with respect to the ARGX-115 program, upon the earliest of (i) a technical failure of the IND-enabling studies which is outside of our control, (ii) AbbVie's election to not exercise its option, or (iii) following AbbVie's exercise of the option, fulfilment of all payment obligations under the agreement. AbbVie may terminate the agreement for any reason upon prior written notice to us.
Our Collaboration with Bird Rock Bio (for ARGX-109)
In October 2012, we entered into an exclusive license agreement with Bird Rock Bio, Inc. (formerly known as RuiYi, Inc. and Anaphore, Inc.), or Bird Rock Bio, to develop and commercialize ARGX-109. Under the terms of the collaboration, Bird Rock Bio is solely responsible for and bears all costs incurred in the research, development and commercialization of ARGX-109.
We have granted Bird Rock Bio an exclusive, worldwide, royalty-bearing license to develop and commercialize ARGX-109. Bird Rock Bio has certain diligence obligations with regard to development and commercialization of ARGX-109 and must report their progress in achieving these milestones on an annual basis. We received a non-refundable, non-creditable upfront payment from Bird Rock Bio of €468,750 in cash plus shares of Bird Rock Bio stock, and we are eligible to receive additional development, regulatory and commercial milestone payments of up to approximately €32.5 million in cash and additional shares of Bird Rock Bio stock. We are eligible to receive tiered royalties on Bird Rock Bio's commercial sales of ARGX-109 at percentages ranging from the low to high single digits and a tiered percentage of Bird Rock Bio's sublicensing income in the low double digits, subject to customary reductions. In connection with the collaboration, we also granted Bird Rock Bio a sublicense under our license agreement with the University of Texas with respect to our NHance Fc engineering technology, which is incorporated into ARGX-109.
In the event that Bird Rock Bio fails to achieve a certain performance milestone within a designated period after entering the agreement, we have the right to terminate the agreement, unless Bird Rock Bio pays us an amount equal to the milestone payment that would have been payable had the milestone event occurred.
Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the agreement, the collaboration term ends with the expiry of the last royalty term under the agreement. Each royalty term expires, on a product-by-product and country-by-country basis, on the date that is the later of (i) 10 years after the first commercial sale of such product sold in that country under the agreement or (ii) such time as there are no valid claims covering such product. Bird Rock Bio may terminate the agreement upon prior written notice to us in the event of a technical failure in product development.
127
Our Strategic Partnership with LEO Pharma (for ARGX-112)
In May 2015, we entered into a collaboration agreement with LEO Pharma A/S, or LEO Pharma, to develop and commercialize ARGX-112. Under the terms of the collaboration, LEO Pharma will fund more than half of all product development costs up to CTA approval of a first product in a Phase 1 clinical trial, with our share of such costs capped. After CTA approval of a first product in a Phase 1 clinical trial, LEO Pharma will be solely responsible to fund the clinical development of the program.
Up through specified periods following the latest to occur of (i) submission of an application to commence a Phase 2b dose finding trial (or Phase 3 clinical trial if a Phase 2b is not conducted) or (ii) the availability of an International Preliminary Examination report for ARGX-112 patent rights after completion of a Phase 2a clinical trial, LEO Pharma may exercise an option to obtain an exclusive, worldwide license to further develop and commercialize products. Following the exercise of the option, LEO Pharma would assume full responsibility for the continued development, manufacture and commercialization of such product, subject to certain diligence obligations. If LEO Pharma elects to exercise this option, it must pay us an option fee. We are eligible to receive a total of €4.5 million in pre-IND payments from LEO Pharma, including an upfront payment that we received in 2015 and milestone payments. We are also eligible to receive additional clinical, regulatory and commercial milestone payments in an aggregate of approximately €100 million, as well as tiered royalties on product sales at percentages ranging from the low single digits to the low teens, subject to customary reductions.
If LEO Pharma does not exercise its option prior to expiration of the applicable option period, if it does not meet certain diligence obligations within a specified time, or if the agreement is terminated other than for reasons of our breach or insolvency, then we have the right to develop and commercialize ARGX-112 alone, subject to our obligation to pay LEO Pharma low-single digit percentage royalties on net sales of any product covered by any LEO Pharma patents, know-how or rights in research results generated under the collaboration. If the agreement is terminated for reasons of our breach or insolvency, rights to product candidates in development at the time of such termination will be allocated to the parties through a mechanism specified in the agreement.
Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the agreement, the term of the agreement ends upon the later of (i) the expiration of the option period, (ii) the expiration of the last license which has been granted under the agreement, and (iii) the fulfilment of all payment obligations which may arise under the agreement. LEO Pharma may terminate the agreement for any reason upon prior written notice to us.
Our Research Collaboration with Staten (for ARGX-116)
In January 2015, we entered into a collaboration agreement with Staten Biotechnology B.V., or Staten, to develop and commercialize products in the area of dyslipidemia therapy. Under the collaboration agreement, the parties will seek to discover and characterize antibodies against at least one and up to three different human gene targets that have therapeutic relevance in the field of dyslipidemia and/or cardiovascular disease. Each research program will last no more than 24 months from commencement unless the parties agree otherwise. The first research program under this agreement has commenced and been extended to December 2017. ARGX-116 will be the initial product candidate under the collaboration. Under the terms of the collaboration, the parties are jointly responsible for conducting research under a mutually agreed research program, with Staten reimbursing us for all costs of carrying out our research responsibilities under each research program.
128
On a research program-by-research program basis, up through a specified period within such research program, we have granted Staten an option to obtain an exclusive, worldwide, permanent license to research, develop and commercialize products identified under the collaboration. If Staten elects to exercise this option for a product, it would be obligated to pay us a percentage of any payments payable to or on behalf of Staten's shareholders in the event of (i) a change of control of Staten, (ii) any licensing, sale, disposition or similar transaction relating to any such product, or (iii) otherwise from the research, development or commercialization of that product. This percentage varies by stage of development for an applicable product and ranges up to the low-twenties, subject to downward proportional adjustment in the event a portion of the proceeds from the applicable transaction does not include payment for the product candidate we developed with Staten. Staten has certain diligence obligations to develop and commercialize at least one product during the term of the agreement and must report on their progress in doing so on an annual basis.
If Staten does not exercise its option with respect to a research program prior to expiration of the applicable option period, then we have the right to research, develop and commercialize product candidates in relation to the relevant target at our sole cost and expense.
Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the agreement, the collaboration term ends on the later of (i) January 2020, (ii) expiration of the last license granted by us under the agreement, (iii) expiration of last option period for Staten and (iv) fulfilment of all payment obligations which have arisen or may arise pursuant to the agreement. In addition, we may terminate the agreement in whole or with respect to a research program if no targets have been selected within 24 months of the effective date of the agreement, other than the target selected for the research program already under way.
Our Strategic Collaboration with Shire
In February 2012, we entered into a collaboration agreement with Shire AG (now known as Shire International Gmbh), or Shire, to discover, develop and commercialize novel human therapeutic antibodies against up to three targets to address diverse, rare and unmet diseases. Under the terms of the collaboration, for any target selected for study under the collaboration, the parties worked together to conduct research and development through discovery of antibodies with certain specificity for and functional activity against those targets.
Up through a specified period following completion of each study for a target, we have granted Shire an exclusive option to obtain all right, title and interest in any antibodies discovered under a study and to obtain an exclusive, worldwide license under our intellectual property which is necessary to further develop and commercialize products incorporating such antibodies. Following exercise of its exclusive option, Shire has certain diligence obligations to develop and commercialize at least one product. To exercise this option with respect to antibodies discovered against any of the three initial targets named in the agreement, Shire must pay us a one-time option fee.
In May 2014, we expanded the collaboration agreement to accommodate research and development of additional novel targets implicated in multiple disease areas to provide Shire with a sublicense under our license agreement with the University of Texas with respect to our NHance and ABDEG engineering technologies and to provide an option to a sublicense to the POTELLIGENT technology of BioWa, Inc. The initial three year term of this expanded agreement expires on May 30, 2017, and Shire has opted to extend the collaboration term for a further year until May 30, 2018.
Shire may exercise options to develop and commercialize programs arising under our expanded agreement, in which case an option fee is due on a per program basis.
129
In addition to option fees, Shire would also be obligated to pay us on a per-product basis upon achievement of specified development, regulatory and commercial milestones and a percentage of net sales as a royalty. Milestones are paid on a first product per indication per study target basis. The royalties payable to us are tiered, single digit and are subject to customary reductions. Through June 30 2016, pursuant to the agreement Shire has paid us an aggregate total of (i) €3.4 million in upfront payments, (ii) €0.3 million in milestone payments and (iii) $8.3 million in research and development fees. In addition, Shire purchased €12.0 million of our ordinary shares in July 2014 by participating in our initial public offering on Euronext Brussels.
If Shire does not exercise its option with respect to any discovered antibody within the specified period, then we are free to research, develop and commercialize antibodies in relation the applicable study target, subject to negotiation of a license from Shire for the use of any antibodies that were discovered during the applicable study, or any Shire confidential information, Shire intellectual property or Shire's interest in any joint intellectual property. If (a) Shire (i) does not exercise its option with respect to any discovered antibody, or (ii) exercises its option but later abandons development of such antibody or (iii) the agreement is terminated other than for our breach or insolvency, and (b) Shire is no longer pursuing a development program with respect to the applicable study target, then we may elect to continue the development of such antibody at our sole cost and expense, subject to negotiation of a license from Shire under which Shire will receive either specified royalties, if we commercialize the program ourselves, or a percentage of sublicensing revenues, if the program is subsequently sublicensed to a third party.
Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the agreement, the collaboration term ends with the expiry of the last royalty term under the agreement. Each royalty term expires, on a product-by-product and country-by-country basis, on the date that is the later of (i) such time as there are no valid claims covering such product or (ii) 10 years after the first commercial sale of such product sold in that country under the agreement. Shire may terminate the agreement for any reason upon prior written notice to us.
License Agreements
We are a party to a number of license agreements under which we license patents, patent applications and other intellectual property from third parties. We enter into these agreements to augment our proprietary intellectual property portfolio. The licensed intellectual property covers some of our product candidates and some of the Fc engineering technologies that we use. These licenses impose various diligence and financial payment obligations on us. We expect to continue to enter into these types of license agreements in the future.
Our Exclusive License with the University of Texas (NHance and ABDEG)
In February 2012, we entered into an exclusive license with The Board of Regents of The University of Texas System, or UoT, for use of certain patents rights relating to the NHance platform, for any use worldwide. The agreement was amended on December 23, 2014 to also include certain patent rights relating to the ABDEG platform.
Upon commercialization of any of our products that use the in-licensed patent rights, we will be obligated to pay UoT a percentage of net sales as a royalty until the expiration of any patents covering the product. This royalty varies with net sales volume and is subject to an adjustment for royalties we receive from a sublicensee of our rights under this agreement, but in any event does not exceed 1%. In addition, we must make annual license maintenance payments to UoT until termination of the agreement. We have assumed certain development, regulatory and commercial milestone payment obligations and must report on our progress in achieving these milestones on
130
quarterly basis. We also have certain diligence requirements with respect to development and commercialization of products which use the in-licensed patent rights.
Under the terms of the license, we have the right to grant sublicenses to third parties, subject to certain restrictions. If we receive any non-royalty income in connection with such sublicenses we must pay UoT a percentage of such income varying from low-middle single digits to middle-upper single digits depending on the nature of the sublicense. Such fees are waived if a sublicensee agrees to pay the milestone payments as set forth in our agreement with UoT.
We may unilaterally terminate the license agreement for convenience upon prior written notice. Absent early termination, the agreement will automatically expire upon the expiration of all issued patents and filed patent applications within the patent rights covered by the agreement.
Our Non-Exclusive License with BioWa (POTELLIGENT)
In October 2010, we entered into a non-exclusive license agreement with BioWa, Inc., or BioWa, for use of certain patents and know-how owned by BioWa and relating to its POTELLIGENT® Technology, for use in the field of prevention and treatment of human diseases. POTELLIGENT® Technology is referred to herein as POTELLIGENT. Under the terms of the license, we are granted a non-exclusive right to use POTELLIGENT to research, develop and commercialize antibodies and products containing such antibodies. BioWa retains a right of first negotiation for the exclusive right to develop and commercialize, in certain countries only, any product we develop using POTELLIGENT. We successfully applied POTELLIGENT to ARGX-110, an anti-CD70 mAb, and ARGX-111, an anti-c-Met mAb, under this license.
Upon commercialization of our products developed using POTELLIGENT, which we expect would include at least our ARGX-110 and ARGX-111 product candidates, we will be obligated to pay BioWa a percentage of net sales of a licensed product as a royalty. This royalty varies with net sales volume, ranging in the low single digits, and it is reduced by half if during the following 10 years from the first commercial sale of the product in a country the last valid claim within the licensed patent(s) that covers the product expires or ends. In addition, we must make annual license maintenance payments which cease with commencement of our royalty payments to BioWa. We have assumed certain development, regulatory and commercial milestone payment obligations and must report on our progress toward achieving these milestones on an annual basis. We also have certain diligence requirements with respect to development and commercialization of products.
Under the terms of the license, we have the right to grant sublicenses to third parties, subject to certain restrictions.
We may terminate the license agreement at any time by sending BioWa prior written notice. Absent early termination, the agreement will automatically expire upon the expiry of our royalty obligations under the agreement. In the event the agreement is terminated for any reason, the license grant to us would cease but BioWa would grant our sublicensees a direct license following such termination. In the event the agreement is terminated other than for our breach or insolvency, we would retain the right to sell licensed products then on hand for a certain period of time post-termination.
Our Non-Exclusive Licenses with BioWa and Lonza (POTELLIGENT CHOK1SV)
To scale up production of our product candidates ARGX-110 and ARGX-111 for clinical trial supply, we required a license to a GMP cell line in which POTELLIGENT antibodies could be expressed. This cell line, POTELLIGENT CHOK1SV, was jointly developed by BioWa and Lonza. In
131
December 2013 and August 2014, respectively, we entered non-exclusive commercial license agreements for ARGX-110 and ARGX-111 with BioWa and Lonza Sales AG, or Lonza, for use of certain patents and know-how relating to the POTELLIGENT® CHOK1SV Technology, which is a combination of Lonza's GS System and BioWa's POTELLIGENT® Technology, for use in the field of prevention and treatment of human diseases. Under the terms of each commercial license, we received a non-exclusive right to research, develop and commercialize products containing an antibody generated specifically against a specific target using POTELLIGENT® CHOK1SV, namely the target CD70 in the case of ARGX-110 and c-Met in the case of ARGX-111. Both targets are designated as reserved targets under our 2010 license agreement with BioWa, which continues to govern our research, development and commercialization of products utilizing BioWa's POTELLIGENT® Technology. Under the terms of each commercial license, BioWa retains a right of first negotiation for the exclusive right to develop and commercialize, in certain countries only, any product we develop using POTELLIGENT® CHOK1SV.
Upon commercialization of our products developed using POTELLIGENT® CHOK1SV, we will be obligated to pay both BioWa and Lonza a percentage of net sales as a royalty. We are required to pay a royalty to BioWa on net sales for any specific licensed product under only one license—either POTELLIGENT® or POTELLIGENT® CHOK1SV, but not both. The BioWa royalty is tiered, ranging in the low single digits and is reduced by half if during the following 10 years from the first commercial sale of the product in a country the last valid claim within the licensed BioWa patent(s) that covers the product expires or ends. The Lonza royalty varies based on whether the product is manufactured by Lonza, us or a third party, but in any event is in the low single digits and is reduced by half if during the following 10 years from the first commercial sale of the product in a country the last valid claim within the licensed Lonza patent(s) that covers the product expires or ends. In addition, we must make annual license maintenance payments to BioWa which cease with commencement of payment of the BioWa royalty, and annual payments to Lonza in the event that any product is manufactured by a party other than Lonza, us or one of our affiliates or strategic partners named in the agreement.
Under the terms of both commercial licenses, we have the right to grant sublicenses to certain pre-approved third parties, but otherwise must obtain BioWa and Lonza's prior written consent.
We have assumed certain development, regulatory and commercial milestone payment obligations to both BioWa and Lonza and must report on our progress toward achieving these milestones on an annual basis.
We may terminate the agreement at any time by sending BioWa and Lonza prior written notice. Absent early termination, the agreement will automatically expire upon the expiry of our royalty obligations under the agreement. In the event the agreement is terminated for any reason, the license grant to us would cease but BioWa and Lonza would grant our sublicensees a direct license following such termination. In the event the agreement is terminated other than for our failure to make milestone or royalty payments, we would retain the right to sell products then on hand for a certain period of time post-termination.
Our Collaboration with UCL (GARP)
In January 2013, we entered into a collaboration and exclusive product license agreement with Université Catholique de Louvain, or UCL, and Sopartec S.A., or Sopartec, to discover and develop novel human therapeutic antibodies against GARP. Under the terms of the collaboration, each party was responsible for all of its own costs and in connection with the activities assigned to it under a mutually agreed research plan.
132
In January 2015, we exercised the option we had been granted to enter into an exclusive, worldwide commercial license for use of certain GARP-related intellectual property rights owned by UCL and the Ludwig Institute for Cancer Research to further develop and commercialize licensed products. Upon the expiration of the agreement, this license became a fully paid up, perpetual worldwide exclusive license under the GARP intellectual property for any purpose, subject to UCL's retention of non-commercial research rights.
Under the terms of the license, we have the right to grant sublicenses to third parties, subject to certain restrictions. If we receive any income in connection with such sublicenses, we must pay Sopartec a percentage of that income varying from mid-single digit to lower teen digit depending on the stage of development of the licensed products at the time the sublicense was entered into. In the event that we have not granted a sublicense, we are required to pay a percentage of net sales as a royalty. This royalty varies with net sales volume, but does not exceed 1% in all tiers, and the royalty is subject to customary reductions. In the event that we have not granted a sublicense, we have certain development and commercial milestone payment obligations. We also have certain diligence obligations with respect to development and commercialization of products.
Intellectual Property
We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover the platform technologies incorporated into, or used to produce, our product candidates, the compositions of matter of our product candidates and their methods of use, as well as other inventions that are important to our business. In addition to patent protection, we also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of our llama immunization and antibody affinity maturation approaches.
Our commercial success depends in part upon our ability to obtain and maintain patent and other proprietary protection for commercially important technologies, inventions and know-how related to our business, defend and enforce our intellectual property rights, particularly our patent rights, preserve the confidentiality of our trade secrets and operate without infringing valid and enforceable intellectual property rights of others.
The patent positions for biotechnology companies like us are generally uncertain and can involve complex legal, scientific and factual issues. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued, and its scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our platform technologies and product candidates will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
Platform Technologies
With regard to our platform technologies, we own or have rights in patents and patent applications directed to our SIMPLE Antibody discovery platform, the ABDEG and NHance platforms and the POTELLIGENT platform.
With regard to our SIMPLE Antibody discovery platform, we own a patent family containing six issued U.S. patents with composition of matter claims directed to chimeric antibodies containing variable domains comprising CDRs obtained from conventional heterotetrameric llama antibodies
133
fused to one or more domains of a human antibody, polynucleotides encoding such chimeric antibodies, libraries of expression vectors comprising cDNA sequences encoding camelid antibodies, method claims directed to the preparation of such chimeric antibodies, and methods of modulating the binding of a human target antigen to its ligand or receptor by administering such a chimeric antibody. The U.S. patents are expected to expire in 2029 to 2033. In addition, the patent family contains patents that have been granted in Australia, Europe and Israel, and at least five patent applications pending in various other countries and regions in North America, Europe and Asia. In addition, we have a second patent family containing patents granted in the United States and Australia, and eight patent applications pending in the United States and other countries in North America, Europe and Asia, with composition of matter claims directed to a chimeric antibody containing variable regions with CDRs derived from a llama antibody and certain amino acid substitutions corresponding to amino acids present in a human germline variable region. The granted U.S. patent and the pending U.S. patent application, if issued as a patent, are expected to expire in 2029.
With regard to the ABDEG platform, we co-own with, and exclusively license from, the University of Texas, a patent family containing a pending U.S. patent application with composition of matter claims directed to an isolated FcRn-antagonist comprising an variant immunoglobulin Fc region having an increased affinity for an Fc gamma receptor relative to a wild-type IgG1 Fc region, and method of use claims directed to a method of using such an FcRn-antagonist to treat certain antibody mediated disorders. The U.S. patent application, if issued as a U.S. patent, is expected to expire in 2034. In addition, we have at least 10 patent applications pending in various other countries and regions in North America, South America, Europe and Asia. In addition, own a second patent family containing a pending U.S. patent application and an international application filed under the Patent Cooperation Treaty with claims directed to methods of reducing the serum levels of an Fc-containing agent in a subject by administering to the subject an FcRn-antagonist containing a variant immunoglobulin Fc region containing certain amino acid substitutions. A U.S. patent, if issued from the U.S. patent application, is expected to expire in 2036.
With regard to the NHance platform, we have exclusively licensed from the University of Texas two U.S. patents with composition of matter claims directed to an IgG molecule comprising a variant human Fc domain, and method of use claims directed to a method of blocking FcRn function in a subject by providing to the subject such an IgG molecule. The U.S. patents are expected to expire in 2027 to 2028. The patent family also includes a granted European patent.
With regard to the POTELLIGENT platform, which is currently used in the production of our ARGX-110 and ARGX-111 product candidates, we have non-exclusively licensed from BioWa certain patent rights that relate to different aspects of the POTELLIGENT platform.
Product Candidates: Wholly-Owned Programs
With regard to the ARGX-113 product candidate, ARGX-113 incorporates the ABDEG technology platform, the coverage of which is discussed above under "Platform Technologies." It is expected that U.S. patents, if they were to issue from the two patent families directed to the ABDEG technology platform are expected to expire in 2034 or 2036, without taking a potential patent term extension into account.
With regard to the ARGX-110 product candidate, we have one issued U.S. patent with composition of matter claims directed to the ARGX-110 antibody, and two U.S. patent applications with composition of matter claims directed to polynucleotides that encode the ARGX-110 antibody and method of use claims directed to the treatment of cancer with the ARGX-110 antibody. The issued U.S. patent and the U.S. patent applications, if issued as U.S. patents, are expected to expire
134
in 2032, without taking a potential patent term extension into account. In addition, we have a patent that has been granted in Japan and at least nine patent applications pending in various other countries and regions in North America, South America, Europe and Asia. Furthermore, ARGX-110 incorporates or employs the SIMPLE Antibody and POTELLIGENT technology platforms, which are covered by one or more of the patents and patent applications discussed above under "Platform Technologies."
With regard to the ARGX-111 product candidate, we have one issued U.S. patent with composition of matter claims directed to the ARGX-111 antibody, and three U.S. patent applications with composition of matter claims directed to ARGX-111 or directed to polynucleotides that encode the ARGX-111 antibody and method of use claims directed to the treatment of cancer with the ARGX-111 antibody. The issued U.S. patent and the U.S. patent applications, if issued as U.S. patents, are expected to expire in 2031, without taking a potential patent term extension into account. In addition, we have patents that have been granted in Australia, Europe and Japan, and at least nine patent applications pending in various other countries and regions in North America, South America, Europe and Asia. Furthermore, ARGX-111 also incorporates or employs the SIMPLE Antibody, POTELLIGENT and NHance technology platforms, which are covered by one or more of the patents and patent applications discussed above under "Platform Technologies." In addition, we have one pending U.S. patent application, patents granted in Australia and Europe, and eight patent applications pending in various other countries and regions in North America, South America, Europe and Asia with composition of matter claims directed to a combination of antibodies or a multi-specific antibody, where one of the antigen binding regions in the combination of antibodies or the multi-specific antibody binds the epitope bound by the ARGX-111 antibody. A U.S. patent, if issued from the U.S. patent application, is expected to expire in 2031.
Product Candidates: Partnered Programs
With regard to the ARGX-115 product candidate, we co-own with, and exclusively license from, the Ludwig Institute for Cancer Research and Université Catholique de Louvain, a pending U.S. patent application with composition of matter claims directed to an antibody that binds GARP the presence of TGF-b and method of use claims directed to the use of such an antibody in the treatment of cancer. A U.S. patent, if issued from the U.S. patent application, is expected to expire in 2034, without taking a potential patent term extension into account. In addition, the patent family contains at least 10 patent applications pending in various other countries and regions in North America, South America, Europe and Asia. In addition, we co-own with, and exclusively license from, the Université Catholique de Louvain a pending U.S. patent application and an international application with composition of matter claims directed to an antibody that binds an epitope of a complex formed by human GARP and TGF-b and method of use claims directed to the use of such an antibody in the treatment of cancer. A U.S. patent, if issued from the U.S. patent application, is expected to expire in 2034. Furthermore, ARGX-115 incorporates or employs the SIMPLE Antibody technology platform, which is covered by one or more of the patents and patent applications discussed above under "Platform Technologies."
With regard to the ARGX-109 product candidate, we have a pending U.S. patent application with composition of matter claims directed to ARGX-109. A U.S. patent, if it were to issue, would be expected to expire in 2033, without taking a potential patent term extension into account. We also have counterpart patents and pending patent applications in various jurisdictions, including North America, Europe and Asia. Furthermore, ARGX-109 incorporates or employs the SIMPLE Antibody technology and the NHance technology, which is covered by one or more of the patents and patent applications discussed above under "Platform Technologies."
135
With regard to the ARGX-112 product candidate, we have a pending United Kingdom application with composition of matter claims directed to an antibody that binds human IL-22R. A U.S. patent, if it were to issue, that claims priority to the United Kingdom application would be expected to expire in 2037, without taking a potential patent term extension into account. Furthermore, ARGX-112 incorporates the SIMPLE Antibody technology, which is covered by one or more of the patents and patent applications discussed above under "Platform Technologies."
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application.
In the United States, the term of a patent covering an FDA-approved drug may be eligible for a patent term extension under the Hatch-Waxman Act as compensation for the loss of patent term during the FDA regulatory review process. The period of extension may be up to five years beyond the expiration of the patent, but cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent among those eligible for an extension may be extended. Similar provisions are available in Europe and in certain other jurisdictions to extend the term of a patent that covers an approved drug. It is possible that issued U.S. patents covering each of our product candidates may be entitled to patent term extensions. If our product candidates receive FDA approval, we intend to apply for patent term extensions, if available, to extend the term of patents that cover the approved product candidates. We also intend to seek patent term extensions in any jurisdictions where they are available, however, there is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such extensions.
In addition to patent protection, we also rely on trade secret protection for our proprietary information that is not amenable to, or that we do not consider appropriate for, patent protection, including, for example, certain aspects of our llama immunization and antibody affinity maturation approaches. However, trade secrets can be difficult to protect. Although we take steps to protect our proprietary information, including restricting access to our premises and our confidential information, as well as entering into agreements with our employees, consultants, advisors and potential collaborators, third parties may independently develop the same or similar proprietary information or may otherwise gain access to our proprietary information. As a result, we may be unable to meaningfully protect our trade secrets and proprietary information.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in the European Union and other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products, including biological products. In addition, some jurisdictions regulate the pricing of pharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Licensure and Regulation of Biologics in the United States
In the United States, our product candidates are regulated as biological products, or biologics, under the Public Health Service Act, or PHSA, and the Federal Food, Drug, and Cosmetic Act, or FDCA, and their implementing regulations. The failure to comply with the applicable U.S.
136
requirements at any time during the product development process, including nonclinical testing, clinical testing, the approval process or post-approval process, may subject an applicant to delays in the conduct of a study, regulatory review and approval, and/or administrative or judicial sanctions. These sanctions may include, but are not limited to, the FDA's refusal to allow an applicant to proceed with clinical testing, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning or untitled letters, adverse publicity, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines and civil or criminal investigations and penalties brought by the FDA or the Department of Justice or other governmental entities.
An applicant seeking approval to market and distribute a new biologic in the United States generally must satisfactorily complete each of the following steps:
Nonclinical Studies and Investigational New Drug Application
Before testing any biologic product candidate in humans, the product candidate must undergo nonclinical testing. Nonclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as animal studies to evaluate the potential for activity and toxicity. The conduct of the nonclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the nonclinical tests, together with manufacturing
137
information and analytical data, are submitted to the FDA as part of an Investigational New Drug, or IND, application. The IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about the product or conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trial can begin.
As a result, submission of the IND may result in the FDA not allowing the trial to commence or on the terms originally specified by the sponsor in the IND. If the FDA raises concerns or questions either during this initial 30-day period, or at any time during the IND process, it may choose to impose a partial or complete clinical hold. This order issued by the FDA would delay either a proposed clinical study or cause suspension of an ongoing study, until all outstanding concerns have been adequately addressed and the FDA has notified the company that investigation may proceed. This could cause significant delays or difficulties in completing planned clinical trials in a timely manner. The FDA may impose clinical holds on a biologic product candidate at any time before or during clinical trials due to safety concerns or non-compliance. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA.
Human Clinical Trials in Support of a BLA
Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator in accordance with GCP requirements. Clinical trials are conducted under study protocols detailing, among other things, the objectives of the study, inclusion and exclusion criteria, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND.
A sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of the BLA so long as the clinical trial is well-designed and well-conducted in accordance with GCP, including review and approval by an independent ethics committee, and the FDA is able to validate the study data through an onsite inspection, if necessary.
Further, each clinical trial must be reviewed and approved by an institutional review board, or IRB, either centrally or individually at each institution at which the clinical trial will be conducted. The IRB will consider, among other things, clinical trial design, patient informed consent, ethical factors and the safety of human subjects. An IRB must operate in compliance with FDA regulations. The FDA, IRB, or the clinical trial sponsor may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP rules and the requirements for informed consent. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group may recommend continuation of the study as planned, changes in study conduct, or cessation of the study at designated check points based on access to certain data from the study.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Additional studies may be required after approval.
138
In some cases, the FDA may approve a BLA for a product candidate but require the sponsor to conduct additional clinical trials to further assess the product candidate's safety and effectiveness after approval. Such post-approval trials are typically referred to as Phase 4 clinical trials. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of biologics approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement or to request a change in the product labeling. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in withdrawal of approval for products.
Compliance with cGMP Requirements
Before approving a BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in full compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. The PHSA emphasizes the importance of manufacturing control for products like biologics whose attributes cannot be precisely defined.
Manufacturers and others involved in the manufacture and distribution of products must also register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process. Any product manufactured by or imported from a facility that has not registered, whether foreign or domestic, is deemed misbranded under the FDCA. Establishments may be subject to periodic unannounced inspections by government authorities to ensure compliance with cGMPs and other laws. Manufacturers may have to provide, on request, electronic or physical records regarding their establishments. Delaying, denying, limiting, or refusing inspection by the FDA may lead to a product being deemed to be adulterated.
Review and Approval of a BLA
The results of product candidate development, nonclinical testing and clinical trials, including negative or ambiguous results as well as positive findings, are submitted to the FDA as part of a BLA requesting a license to market the product. The BLA must contain extensive manufacturing
139
information and detailed information on the composition of the product and proposed labeling as well as payment of a user fee.
The FDA has 60 days after submission of the application to conduct an initial review to determine whether the BLA is sufficient to accept for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission has been accepted for filing, the FDA begins an in-depth review of the application. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or the PDUFA, the FDA has ten months in which to complete its initial review of a standard application and respond to the applicant, and six months for a priority review of an application. The FDA does not always meet its PDUFA goal dates for standard and priority BLAs. The review process may often be significantly extended by FDA requests for additional information or clarification. The review process and the PDUFA goal date may be extended by three months if the FDA requests or if the applicant otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
On the basis of the FDA's evaluation of the application and accompanying information, including the results of the inspection of the manufacturing facilities and any FDA audits of clinical trial sites to assure compliance with GCPs, the FDA may issue an approval letter, denial letter, or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. Under the PHSA, the FDA may approve a BLA if it determines that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure and potent. If the application is not approved, the FDA may issue a complete response letter, which will contain the conditions that must be met in order to secure final approval of the application, and when possible will outline recommended actions the sponsor might take to obtain approval of the application. Sponsors that receive a complete response letter may submit to the FDA information that represents a complete response to the issues identified by the FDA. Such resubmissions are classified under PDUFA as either Class 1 or Class 2, based on the information submitted by an applicant in response to an action letter. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has two months to review a Class 1 resubmission and six months to review a Class 2 resubmission. The FDA will not approve an application until issues identified in the complete response letter have been addressed. The FDA issues a denial letter if it determines that the establishment or product does not meet the agency's requirements.
The FDA may also refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. In particular, the FDA may refer applications for novel biologic products or biologic products that present difficult questions of safety or efficacy to an advisory committee. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
If the FDA approves a new product, it may limit the approved indications for use of the product. It may also require that contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may call for post-approval studies, including Phase 4 clinical trials, to further assess the product's safety after approval. The agency may also require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, to help ensure that the benefits of the product outweigh the potential risks. REMS can include medication
140
guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patent registries. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Fast Track, Breakthrough Therapy and Priority Review Designations
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are referred to as fast track designation, breakthrough therapy designation and priority review designation.
The FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For fast track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product's application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA's time period goal for reviewing a fast track application does not begin until the last section of the application is submitted. Fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In 2012, Congress enacted the Food and Drug Administration Safety and Innovation Act, or FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as "breakthrough therapies." A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
The FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA's goal for taking action on a marketing application from 10 months to six months.
141
Accelerated Approval Pathway
The FDA may grant accelerated approval to a product for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. The FDA may also grant accelerated approval for such a condition when the product has an effect on an intermediate clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, or IMM, and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Products granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a product, such as an effect on IMM. The FDA has limited experience with accelerated approvals based on intermediate clinical endpoints, but has indicated that such endpoints generally may support accelerated approval where the therapeutic effect measured by the endpoint is not itself a clinical benefit and basis for traditional approval, if there is a basis for concluding that the therapeutic effect is reasonably likely to predict the ultimate clinical benefit of a product.
The accelerated approval pathway is most often used in settings in which the course of a disease is long and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. Thus, accelerated approval has been used extensively in the development and approval of products for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large trials to demonstrate a clinical or survival benefit.
The accelerated approval pathway is usually contingent on a sponsor's agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the product's clinical benefit. As a result, a product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, would allow the FDA to withdraw the product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
Post-Approval Regulation
If regulatory approval for marketing of a product or new indication for an existing product is obtained, the sponsor will be required to comply with all regular post-approval regulatory requirements as well as any post-approval requirements that the FDA has imposed as part of the approval process. The sponsor will be required to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP regulations, which impose certain procedural and documentation requirements upon manufacturers. Accordingly, the sponsor and its third-party
142
manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMP regulations and other regulatory requirements.
A biologic product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official lot release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer's tests performed on the lot, to the FDA. The FDA may in addition perform certain confirmatory tests on lots of some products before releasing the lots for distribution. Finally, the FDA will conduct laboratory research related to the safety, purity, potency and effectiveness of pharmaceutical products.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Pharmaceutical products may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Orphan Drug Designation
Orphan drug designation in the United States is designed to encourage sponsors to develop products intended for rare diseases or conditions. In the United States, a rare disease or condition is statutorily defined as a condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available the product for the disease or condition will be recovered from sales of the product in the United States.
Orphan drug designation qualifies a company for tax credits and market exclusivity for seven years following the date of the product's marketing approval if granted by the FDA. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. A product becomes an orphan when it receives orphan drug designation from the Office of Orphan Products Development, or OOPD, at the FDA based on
143
acceptable confidential requests made under the regulatory provisions. The product must then go through the review and approval process like any other product.
A sponsor may request orphan drug designation of a previously unapproved product or new orphan indication for an already marketed product. In addition, a sponsor of a product that is otherwise the same product as an already approved orphan drug may seek and obtain orphan drug designation for the subsequent product for the same rare disease or condition if it can present a plausible hypothesis that its product may be clinically superior to the first drug. More than one sponsor may receive orphan drug designation for the same product for the same rare disease or condition, but each sponsor seeking orphan drug designation must file a complete request for designation.
The period of exclusivity begins on the date that the marketing application is approved by the FDA and applies only to the indication for which the product has been designated. The FDA may approve a second application for the same product for a different use or a second application for a clinically superior version of the product for the same use. The FDA cannot, however, approve the same product made by another manufacturer for the same indication during the market exclusivity period unless it has the consent of the sponsor or the sponsor is unable to provide sufficient quantities.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, a BLA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests and other information required by regulation. The applicant, the FDA, and the FDA's internal review committee must then review the information submitted, consult with each other and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity. This six-month exclusivity may be granted if a BLA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA's request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application.
144
Biosimilars and Exclusivity
The Biologics Price Competition and Innovation Act, or BPCIA, established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable biosimilars. To date, while biosimilar products have been approved by the FDA for use in the United States, no interchangeable biosimilars have been approved.
Under the BPCIA, a manufacturer may submit an application for licensure of a biologic product that is "biosimilar to" or "interchangeable with" a previously approved biological product or "reference product." In order for the FDA to approve a biosimilar product, it must find that there are no clinically meaningful differences between the reference product and proposed biosimilar product in terms of safety, purity and potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must find that the biosimilar product can be expected to produce the same clinical results as the reference product, and (for products administered multiple times) that the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved. Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version of that product if the FDA approves a full BLA for such product containing the sponsor's own nonclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. However, to rely on such exclusivities for establishing or protecting our market position is not without risk, as such laws are subject to changes by the legislature. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed "interchangeable" by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
Regulation and Procedures Governing Approval of Medicinal Products in the European Union
In order to market any product outside of the United States, a company also must comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can initiate clinical trials or marketing of the product in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the European Union, or EU, generally follows the same lines as in the United States. It entails satisfactory completion of pharmaceutical development, nonclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. It also requires the submission to relevant competent authorities for clinical trials authorization and to the European Medicines Authority, or EMA, for a marketing authorization application, or MAA, and granting of a marketing authorization by these authorities before the product can be marketed and sold in the EU.
Clinical Trial Approval
Pursuant to the currently applicable Clinical Trials Directive 2001/20/EC and the Directive 2005/28/EC on GCP, a system for the approval of clinical trials in the EU has been implemented through national legislation of the member states. Under this system, an applicant must
145
obtain approval from the competent national authority of an EU member state in which the clinical trial is to be conducted or in multiple member states if the clinical trial is to be conducted in a number of member states. Furthermore, the applicant may only start a clinical trial at a specific study site after the independent ethics committee has issued a favorable opinion. The clinical trial application, or CTA, must be accompanied by an investigational medicinal product dossier with supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and corresponding national laws of the member states and further detailed in applicable guidance documents.
In April 2014, the EU adopted a new Clinical Trials Regulation (EU) No 536/2014, which is set to replace the current Clinical Trials Directive 2001/20/EC. It is expected that the new Clinical Trials Regulation will apply by October 2018 with a three-year transition period. It will overhaul the current system of approvals for clinical trials in the EU. Specifically, the new regulation, which will be directly applicable in all member states, aims at simplifying and streamlining the approval of clinical trials in the EU. For instance, the new Clinical Trials Regulation provides for a streamlined application procedure via a single entry point and strictly defined deadlines for the assessment of clinical trial applications.
Marketing Authorization
To obtain a marketing authorization for a product under the EU regulatory system, an applicant must submit an MAA, either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in EU Member States (decentralized procedure, national procedure, or mutual recognition procedure). A marketing authorization may be granted only to an applicant established in the EU. Regulation (EC) No 1901/2006 provides that prior to obtaining a marketing authorization in the EU, an applicant must demonstrate compliance with all measures included in an EMA-approved Pediatric Investigation Plan, or PIP, covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, class waiver, or a deferral for one or more of the measures included in the PIP.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all EU member states. Pursuant to Regulation (EC) No. 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or the CHMP, established at the EMA is responsible for conducting the assessment of a product to define its risk/benefit profile. Under the centralized procedure, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation may be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts such a request, the time limit of 210 days will be reduced to 150 days, but it is possible that the CHMP may revert to the standard time limit for the centralized procedure if it determines that it is no longer appropriate to conduct an accelerated assessment.
146
Periods of Authorization and Renewals
A marketing authorization is valid for five years, in principle, and it may be renewed after five years on the basis of a reevaluation of the risk benefit balance by the EMA or by the competent authority of the authorizing member state. To that end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal period. Any authorization that is not followed by the placement of the drug on the EU market (in the case of the centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid.
Regulatory Requirements after Marketing Authorization
Following approval, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of the medicinal product. These include compliance with the EU's stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. In addition, the manufacturing of authorized products, for which a separate manufacturer's license is mandatory, must also be conducted in strict compliance with the EMA's GMP requirements and comparable requirements of other regulatory bodies in the EU, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. Finally, the marketing and promotion of authorized products, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Union under Directive 2001/83EC, as amended.
Orphan Drug Designation and Exclusivity
Regulation (EC) No 141/2000 and Regulation (EC) No. 847/2000 provide that a product can be designated as an orphan drug by the European Commission if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of (1) a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the EU when the application is made, or (2) a life-threatening, seriously debilitating or serious and chronic condition in the EU and that without incentives it is unlikely that the marketing of the drug in the EU would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention, or treatment of the condition in question that has been authorized in the EU or, if such method exists, the drug has to be of significant benefit compared to products available for the condition.
An orphan drug designation provides a number of benefits, including fee reductions, regulatory assistance and the possibility to apply for a centralized EU marketing authorization. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. During this market exclusivity period, neither the EMA nor the European Commission or the member states can accept an application or grant a marketing authorization for a "similar medicinal product." A "similar medicinal product" is defined as a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation because, for example, the product is sufficiently profitable not to justify market exclusivity.
147
Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we may obtain regulatory approval. Even if our product candidates are approved for marketing, sales of such product candidates will depend, in part, on the extent to which third-party payors, including government health programs in the United States (such as Medicare and Medicaid), commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such product candidates. In the United States and markets in other countries, patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use any product candidates we may develop unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of such product candidates. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, and the cost of these studies would be in addition to the costs required to obtain FDA or other comparable marketing approvals. Even after pharmacogenomic studies are conducted, product candidates may not be considered medically necessary or cost effective. A decision by a third-party payor not to cover any product candidates we may develop could reduce physician utilization of such product candidates once approved and have a material adverse effect on our sales, results of operations and financial condition. Additionally, a payor's decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. For example, the payor may require co-payments that patients find unacceptably high. Further, one payor's determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor. Third-party reimbursement and coverage may not be adequate to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. The insurance coverage and reimbursement status of newly-approved products for orphan diseases is particularly uncertain, and failure to obtain or maintain adequate coverage and reimbursement for any such product candidates could limit a company's ability to generate revenue.
The containment of healthcare costs also has become a priority of federal, state and foreign governments and the prices of pharmaceuticals have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company's revenue from the sale of any approved products. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Outside the United States, we will face challenges in ensuring obtaining adequate coverage and payment for any product candidates we may develop. Pricing of prescription pharmaceuticals is
148
subject to governmental control in many countries. Pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory marketing approval for a product and may require us to conduct a clinical trial that compares the cost effectiveness of any product candidates we may develop to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in our commercialization efforts.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost effectiveness of a particular product candidate to currently available therapies (so called health technology assessments, or HTAs) in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its member states to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other member states allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the European Union have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the European Union. The downward pressure on health care costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and parallel trade (arbitrage between low-priced and high-priced member states) can further reduce prices. Special pricing and reimbursement rules may apply to orphan drugs. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any drug. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results based rules of reimbursement may apply. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries.
Healthcare Law and Regulation
Healthcare providers and third-party payors play a primary role in the recommendation and prescription of pharmaceutical products that are granted marketing approval. Our current and future arrangements with providers, researchers, consultants, third-party payors and customers are subject to broadly applicable federal and state fraud and abuse, anti-kickback, false claims, transparency and patient privacy laws and regulations and other healthcare laws and regulations that may constrain our business and/or financial arrangements. Restrictions under applicable federal and state healthcare laws and regulations include, without limitation, the following:
149
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring pharmaceutical manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
We will be required to spend substantial time and money to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations. Recent healthcare reform legislation has strengthened these federal and state healthcare laws. For example, the ACA amends the intent requirement of the federal Anti-Kickback Statute and criminal healthcare fraud statutes to clarify that liability under these statutes does not require a person or entity to have actual knowledge of the statutes or a specific intent to violate them. Moreover, the ACA provides that the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws.
Violations of these laws can subject us to criminal, civil and administrative sanctions including monetary penalties, damages, fines, disgorgement, individual imprisonment and exclusion from
150
participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm, and we may be required to curtail or restructure our operations. Moreover, we expect that there will continue to be federal and state laws and regulations, proposed and implemented, that could impact our future operations and business.
Healthcare Reform
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. There have been a number of federal and state proposals during the last few years to reduce the cost of care through changes in the healthcare system, including limits on the pricing, coverage and reimbursement of pharmaceutical and biopharmaceutical products, especially under government-funded health care programs, and increased governmental control of drug pricing.
By way of example, in March 2010, the United States Congress enacted the ACA, which, among other things, includes changes to the coverage and payment for products under governmental and private insurance plans. Among the provisions of the ACA of importance to our potential product candidates are:
151
Since its enactment, there have been judicial and Congressional challenges to numerous aspects of the ACA. In January, Congress voted to adopt a budget resolution for fiscal year 2017 that, while not a law, is widely viewed as the first step toward the passage of legislation to repeal the ACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed. We cannot predict how the ACA, its possible repeal, or any legislation that may be proposed to replace the ACA will impact our business.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, in August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2012 through 2021, was unable to reach required goals, thereby triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2024 unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. Such reforms could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain marketing approval and may affect our overall financial condition and ability to develop product candidates.
Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
152
Employees
As of February 15, 2017, we had 62 employees. At each date shown below, we had the following number of full-time equivalent employees, broken out by department and geography:
| | At December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2016 | 2015 | 2014 | |||||||
Function: | ||||||||||
Research and development | 48 | 35 | 28 | |||||||
General and administrative | 10 | 6 | 3 | |||||||
Total | 58 | 41 | 31 | |||||||
Geography: | ||||||||||
Zwijnaarde, Belgium | 58 | 41 | 31 | |||||||
Breda, the Netherlands | 0 | 0 | 0 | |||||||
Total | 58 | 41 | 31 | |||||||
Collective bargaining agreements, or CBAs, can be entered into in Belgium at the national, industry, or company levels. These CBAs are binding on both employers and employees. We have no trade union representation or CBAs at the company level, but we are subject to the national and industry level CBAs that relate to the chemical industry. The CBAs currently applicable to us relate to employment conditions such as wages, working time, job security, innovation and supplementary pensions. We have not had, and do not anticipate having, disputes on any of these subjects. CBAs may, however, change the employment conditions of our employees in the future and hence adversely affect our employment relationships.
Facilities
We lease our operational offices and laboratory space, which consists of approximately 1,500 square meters, located in Zwijnaarde, Belgium. The lease for this facility expires in 2026. We believe our current facility is sufficient to meet our needs for the foreseeable future. We also lease an office in Breda, the Netherlands.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceeding. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
153
Our Board of Directors
We have a one-tier board structure consisting of executive directors who are responsible for our day-to-day management and non-executive directors who are responsible for the supervision of the executive directors. Our executive directors and our non-executive directors are collectively responsible for our general affairs. We may be represented by our board of directors or by two executive directors acting jointly. Our board of directors is currently comprised of two executive directors and six non-executive directors, who we refer to individually as a director. Less than a majority of the directors of our board of directors are citizens or residents of the United States.
The following table sets forth certain information with respect to the current members of our board of directors, including their ages, as of February 16, 2017:
Name | Age | Position | Nationality | Date of appointment | Term expiration | |||||
|---|---|---|---|---|---|---|---|---|---|---|
Tim Van Hauwermeiren | 44 | Executive Director (CEO) | BE | July 9, 2014 | 2018 | |||||
Eric Castaldi | 52 | Executive Director (CFO) | F | July 9, 2014 | 2018 | |||||
Peter K.M. Verhaeghe | 58 | Non-Executive Director (chairperson) | BE | July 9, 2014 | 2018 | |||||
John Paul de Koning | 48 | Non-Executive Director | NL | July 9, 2014 | 2018 | |||||
David L. Lacey | 64 | Non-Executive Director | U.S. | July 9, 2014 | 2018 | |||||
Werner Lanthaler | 49 | Non-Executive Director | AT | July 9, 2014 | 2018 | |||||
J. Donald deBethizy | 66 | Non-Executive Director | U.S. | May 13, 2015 | 2019 | |||||
Pamela Klein | 55 | Non-Executive Director | U.S. | April 28, 2016 | 2020 |
The address for our directors is our registered office, Willemstraat 5, 4811 AH, Breda, the Netherlands.
Our board of directors has determined that members of the board are independent under the NASDAQ's listing requirements and that of the members of the board of directors are independent under the Dutch Corporate Governance Code.
The following is the biographical information of the members of our board of directors:
Tim Van Hauwermeiren co-founded our company in 2008 and has served as our Chief Executive Officer since April 2008. He has served as a member of our board of directors since July 2014. Mr. Van Hauwermeiren has more than 20 years of general management and business development experience across the life sciences and consumer goods sectors. Mr. Van Hauwermeiren holds a B.Sc. and M.Sc. in bioengineering from Ghent University (Belgium) and an Executive MBA from The Vlerick School of Management.
Eric Castaldi has served as our Chief Financial Officer since April 2014 and as a member of our board of directors since July 2014. Mr. Castaldi has 28 years of international financial executive management experience, including 19 years in the biopharmaceutical industry. From 1998 to 2014, Mr. Castaldi served as chief financial officer and a member of the executive committee of Nicox SA, a Euronext-listed biotechnology company. From 2008 to 2012, he served as a member of the board of directors and as chairman of the audit committee of Hybrigenics Services SAS, a Euronext-listed French biopharmaceutical company specializing in oncology. Mr. Castaldi graduated with a degree in finance, accountancy and administration from the University of Nice.
Peter K.M. Verhaeghe has served as a member and chairman of our board of directors since July 2014. Mr. Verhaeghe is the managing partner of VVGB Advocaten—Avocats, a corporate
154
finance law and tax law firm, a position he has held since July 1999. He is currently lead counsel to a number of Belgian, Dutch and Swiss biotechnology and diagnostics companies. Mr. Verhaeghe currently serves as the president of the board of directors of Merisant France SAS, as a member of the management board of Merisant Company 2 sàrl and as a member of the board of directors of CzechPak Manufacturing s.r.o. He previously served as the chairman of the board of directors of PharmaNeuroBoost NV from December 2006 to January 2013 and as liquidator in charge of KBC Private Equity Fund Biotech NV from April 2009 to December 2012. Mr. Verhaeghe holds a degree in law from the University of Leuven and an LLM degree from Harvard Law School.
Dr. John Paul de Koning has served as a member of our board of directors since July 2014. Dr. de Koning is a partner at Life Sciences Partners, a European investment firm in the healthcare sector, a position he has held since 2009. Dr. de Koning currently serves on the supervisory boards of Merus B.V., G-Therapeutics SA, and eTheRNA immunotherapies NV. Previously, he also served on the supervisory boards of BMEYE B.V., Prosensa Holding N.V., Skyline Diagnostics B.V., Pronota N.V. and Innovative Biosensors Inc. Dr. de Koning holds an M.Sc. in medical biology from the University of Utrecht and a Ph.D. in oncology and hematology from the Erasmus University Medical Center. After obtaining his Ph.D., he received a fellowship from the Dutch Cancer Society to work at the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
Dr. David L. Lacey has served as a member of our board of directors since July 2014. Dr. Lacey is a biopharmaceutical consultant at David L. Lacey LLC, where he advises academic institutions, biotechnology companies and venture capital firms, a position he has held since July 2011. He currently serves as a director of Inbiomotion SL, Atreca, Inc. and Nurix, Inc. From 1994 until his retirement in 2011, he held various positions, including head of discovery research, at Amgen Inc., where he played a fundamental scientific role in the discovery of the OPG/RANKL/RANK pathway, which led to the development of the anti-RANKL human mAb denosumab, for both osteoporosis (Prolia) and cancer-related bone diseases (XGEVA). He holds a Bachelor's degree in biology and an M.D. from the University of Colorado, and has his board certification in anatomic pathology.
Dr. Werner Lanthaler has served as a member of our board of directors since July 2014. Dr. Lanthaler is the chief executive officer of Evotec AG, a global drug discovery research organization, a position he has held since March 2009. Dr. Lanthaler previously served on the supervisory boards of Bioxell SpA and Pantec Biosolutions AG. Dr. Lanthaler holds a degree in psychology, a Ph.D. in business administration from Vienna University of Economics and Business and a Master's degree in public administration from Harvard University.
Dr. J. Donald deBethizy has served as a member of our board of directors since May 2015. Mr. deBethizy has 30 years of experience in research and development and financial, business and operating management in the biotechnology and consumer products industry. He is the president of White City Consulting ApS. Previously, Mr. deBethizy served as president and chief executive officer of Santaris Pharma A/S until October 2014, when the company was sold to Roche. From August 2000 to June 2012, Mr. deBethizy was co-founder and chief executive officer of Targacept, Inc., a U.S. biotechnology company listed on NASDAQ. He currently serves on the supervisory boards of Albumedix A/S, Newron Pharmaceuticals SpA, Noxxon Pharma NV and AG, Rigontec GmbH and Proterris, Inc. From May 2013 to November 2014, he served as executive chairman of Contera Pharma ApS. He previously served on the boards of Asceneuron SA, Serendex Pharmaceuticals A/S, Enbiotix Inc., Targacept Inc. and Biosource Inc. Mr. deBethizy has held adjunct appointments at Wake Forest University Babcock School of Management, Wake Forest University School of Medicine
155
and Duke University. Mr. deBethizy holds a B.Sc. in biology from the University of Maryland, and an M.Sc. and a Ph.D. in toxicology from Utah State University.
Dr. Pamela Klein has served as a member of our board of directors since April 2016. Dr. Klein is a principal and founder of PMK BioResearch, which offers strategic consulting in oncology drug development to corporate boards, management teams and the investment community, a position she has held since 2008. She currently serves as a member of various scientific advisor boards. Previously, Dr. Klein spent seven years at the National Cancer Institute as Research Director of the NCI-Navy Breast Center, after which she joined Genentech and was VP, Development until 2001. She served as Chief Medical Officer for Intellikine which was acquired by Takeda. She was previously Vice President, Development for Genentech. Dr. Klein holds a Bachelor's degree in biology from California State University and an M.D. from Stritch School of Medicine, Loyola University Chicago and is trained in internal medicine and medical oncology.
Key Employees
The following table sets forth certain information with respect to our key employees, including their ages, as of February 16, 2017:
Name | Age | Position | Nationality | Date of appointment | ||||
|---|---|---|---|---|---|---|---|---|
Hans de Haard | 57 | Chief Scientific Officer | NL | July 1, 2008 | ||||
Nicolas Leupin | 43 | Chief Medical Officer | CH | February 1, 2016 |
The address for our key employees is Industriepark Zwijnaarde 7, Building C, 9052 Zwijnaarde (Gent), Belgium.
The following is a brief summary of the biographical information of our key employees.
Prof. Hans de Haard has served as our chief scientific officer since July 2008. Prof. de Haard has been active in the antibody engineering field since 1989. He also serves as a Professor of Immunology at University of Franche Comté (France). Prof. de Haard holds an M.Sc. in biochemistry from the Higher Professional Education for Laboratory Technicians (Oss, the Netherlands) and a M.Sc. in chemistry from the Institute of Technology (Rotterdam, the Netherlands) and a Ph.D. in molecular immunology from Maastricht University.
Dr. Nicolas Leupin has served as our chief medical officer since February 2016. Dr. Leupin has clinical and industry expertise in medical oncology as well as experience in drug development. He currently lectures at the University of Bern. From 2008 to 2015, Dr. Leupin served in different positions in clinical development at Celgene, including Director of Clinical Development of EMEA Celgene, where he contributed to building the clinical development department in Europe and then led the European lymphoma and myeloma teams, served as clinical lead for several compounds up to phase III clinical trials, and was responsible for running and managing hematology and oncology clinical trials, including both industry-sponsored trials and academic cooperative groups, several of them through to registration. Among other activities, he was responsible for specific clinical documents of registration dossiers that lead to European and American registrations. Dr. Leupin holds an MBA from Jones International University and an M.D. from the University of Bern and was board certified in medical oncology (Switzerland).
Director Independence
As a foreign private issuer, under the listing requirements and rules of NASDAQ, we are not required to have independent directors on our board of directors, except that our audit committee is
156
required to consist fully of independent directors, subject to certain phase-in schedules. However, our board of directors has determined that, under current listing requirements and rules of NASDAQ and taking into account any applicable committee independence standards, , and are "independent directors." In making such determination, our board of directors considered the relationships that each non-executive director has with us and all other facts and circumstances our board of directors deemed relevant in determining the director's independence, including the number of ordinary shares beneficially owned by the director and his or her affiliated entities (if any).
The Dutch Corporate Governance Code requires that the composition of the non-executive directors is such that the members are able to operate independently and critically vis-à-vis one another, the executive directors, and any particular interests involved. At the date of this prospectus, non-executive director does not meet the independence criteria contained in the Dutch Corporate Governance Code.
Role of the Board in Risk Oversight
Our board of directors is responsible for the oversight of our risk management activities and has delegated to the audit committee the responsibility to assist our board in this task. While our board oversees our risk management, our management is responsible for day-to-day risk management processes. Our board of directors expects our management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face.
Composition, Appointment and Dismissal
Our directors are appointed at the general meeting of shareholders, or the General Meeting. The board of directors is required to make one or more proposals for each seat on our board of directors to be filled. A resolution to nominate a director by our board of directors (with support from the remuneration and nomination committee) may be adopted by a simple majority of the votes cast. A nomination for appointment of an executive director must state the candidate's age and the positions he or she holds, or has held, insofar as these are relevant for the performance of the duties of an executive director. The nomination must state the reasons for the nomination of the relevant person. A nomination for appointment of a non-executive director must state the candidate's age, his or her profession, the number of shares he or she holds and the employment positions he or she holds, or has held, insofar as these are relevant for the performance of the duties of a non-executive director. Furthermore, the names of the legal entities of which he or she is already a supervisory board member or a non-executive member of the board shall be indicated; if those include legal entities which belong to the same group, a reference to that group will be sufficient. The nomination must state the reasons for the nomination of the relevant person.
A resolution of the General Meeting to appoint a director other than in accordance with a nomination of our board of directors shall require a majority of at least two-thirds of the votes cast if less than one-half of the company's issued capital is represented at the meeting.
Our directors are appointed as either an executive director or as a non-executive director at the General Meeting. Our board of directors designates one of the executive directors as chief executive officer and one of the executive directors as chief financial officer. In addition, the board of directors may grant other titles to executive directors. Our board of directors designates a non-executive director as chairperson of the board of directors. The legal relationship between a member of the board of directors and the company will not be considered as an employment agreement. Employment agreements between an executive director and a group company (other than us) are
157
permitted. In the absence of an employment agreement, members of a board of directors generally do not enjoy the same protection as employees under Dutch labor law.
Directors may be suspended or removed at the General Meeting at any time, with or without cause, by means of a resolution passed by a simple majority of the votes cast. Executive directors may also be suspended by the board of directors. A suspension of an executive director by the board of directors may be discontinued at any time at the General Meeting. Any suspension may be extended one or more times but may not last longer than three months in the aggregate.
Committees
In accordance with the Dutch Corporate Governance Code, our board of directors can set up specialized committees to analyze specific issues and advise the board of directors on those issues.
The committees are advisory bodies only, and the decision-making remains within the collegial responsibility of the board of directors. The board of directors determines the terms of reference of each committee with respect to the organization, procedures, policies and activities of the committee.
Our board of directors has established and appointed an audit committee, a remuneration and nomination committee and a research and development committee. The composition and function of all of our committees will comply with all applicable requirements of the Exchange Act, the exchanges on which the ordinary shares are listed, SEC rules and regulations and the Dutch Corporate Governance Code.
Only non-executive directors qualify for membership of the committees. The audit committee and the remuneration and nomination committee may not be chaired by the chairperson of the board of directors or by a former executive director of the company.
Audit Committee
Our audit committee consists of three members: Werner Lanthaler (chairman), John Paul de Koning and Peter K.M. Verhaeghe.
Our board of directors has determined that members of our audit committee are independent under Rule 10A-3 of the Exchange Act and the applicable rules of the NASDAQ Stock Market and members of our audit committee are independent under the applicable rules of the Dutch Corporate Governance Code, and that qualifies as an "audit committee financial expert" as defined under the Exchange Act.
Our audit committee assists our board of directors in overseeing the accuracy and integrity of our accounting and financial reporting processes and audits of our consolidated financial statements, the implementation and effectiveness of an internal control system and our compliance with legal and regulatory requirements, the independent auditors' qualifications and independence and the performance of the independent auditors.
The audit committee will be governed by a charter that complies with NASDAQ listing rules. Our audit committee is responsible for, among other things:
158
Our audit committee meets as often as is required for its proper functioning, but at least four times a year. Our audit committee must meet at least once a year with our statutory auditor.
Our audit committee reports regularly to our board of directors on the exercise of its functions. It informs our board of directors about all areas in which action or improvement is necessary in its opinion and produces recommendations concerning the necessary steps that need to be taken. The audit review and the reporting on that review cover us and our subsidiaries as a whole. The members of the audit committee are entitled to receive all information which they need for the performance of their function, from our board of directors and employees. Every member of the audit committee shall exercise this right in consultation with the chairman of the audit committee.
Remuneration and Nomination Committee
Our remuneration and nomination committee consists of three members: J. Donald deBethizy (chairman), Peter K.M. Verhaeghe and Werner Lanthaler.
Our board of directors has determined that members of our remuneration and nomination committee are independent under the applicable rules of the NASDAQ Stock Market and members of our remuneration and nomination committee are independent under the applicable rules of the Dutch Corporate Governance Code.
Our remuneration and nomination committee is responsible for, among other things:
159
The remuneration and nomination committee consists of at least three members. The remuneration and nomination committee meets as often as is required for its proper functioning, but at least once per year to evaluate its functioning.
Research and Development Committee
Our research and development committee consists of three members: David L. Lacey (chairman), J. Donald deBethizy and Pamela Klein.
Our board of directors has determined that members of our research and development committee are independent under the applicable rules of the NASDAQ Stock Market and members of our research and development committee are independent under the applicable rules of the Dutch Corporate Governance Code.
The research and development committee is responsible for, among other things:
All members of the research and development committee shall have adequate industrial, academic and/or practical experience with the research and development of biopharmaceuticals.
One purpose of our research and development committee is to engage in discussion with our research and development management, and the committee's responsibilities to carry out this purpose include, among others: monitoring the research and development activities, performing strategic reviews of the key research and development programs; and reviewing the scientific publication plan.
Our research and development committee meets as often as is required for its proper functioning, but at least prior to each meeting of our board of directors, and reports regularly to our board of directors on the outcome of the strategic reviews. Our research and development committee consists of at least three members with adequate industrial experience with the research and development of biopharmaceuticals. The chairman of our research and development committee shall report formally to our board of directors on the research and development committee's deliberations, findings and proceedings after each meeting on all matters within its duties and responsibilities.
160
General Information About Our Directors
As of the date of this prospectus and except as set out below, none of the directors for at least the previous five years:
Peter K.M. Verhaeghe—PharmaNeuroBoost NV
Mr. Verhaeghe was chairman of the board of directors of PharmaNeuroBoost NV, which voluntarily filed for bankruptcy in 2013 after its Phase 3 trial failed and no additional funding was found to continue its operations.
Peter K.M. Verhaeghe—KBC Private Equity Fund Biotech NV
Mr. Verhaeghe was a member of the board of directors of KBC Private Equity Fund Biotech NV, a Euronext-listed fund, when it voluntarily liquidated pursuant to a decision of its shareholders. Mr. Verhaeghe was appointed as liquidator in charge and closed the liquidation by the end of 2012 with net proceeds for the shareholders of over €6 per share.
John Paul de Koning—Skyline Diagnostics B.V.
Dr. de Koning is partner at Life Sciences Partners, a venture capital investment firm that invests in private life sciences companies, often in a very early stage. Dr. de Koning served as a member of the supervisory board of one of those companies, Skyline Diagnostics B.V., which filed for bankruptcy in 2013.
Corporate Governance Practices
Our board of directors has adopted rules, or the Board By-Laws, that describe the procedure for holding meetings of the board of directors, for the decision-making by the board of directors and the board of directors' operating procedures.
Under the Board By-Laws, the members of our board of directors must endeavour, insofar as is possible, to ensure that resolutions are adopted unanimously. Where unanimity cannot be achieved and Dutch law, the Articles of Association or the Board By-Laws do not prescribe a larger majority, all resolutions of our board of directors must be adopted by a simple majority of the votes cast in a meeting at which at least a majority of the members of our board of directors then in office are present or represented.
Resolutions of our board of directors can also be adopted without holding a meeting, provided that the relevant proposal has been submitted to the members of our board of directors then in office and none of them has objected to the manner of adopting resolutions.
161
Differences between Our Corporate Governance Practices and the Listing Rules of the NASDAQ Stock Market
We are considered a foreign private issuer. As a result, in accordance with the listing requirements of NASDAQ, we may rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of NASDAQ. In accordance with Dutch law and generally accepted business practices in the Netherlands, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from the requirement of NASDAQ Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Although we must provide shareholders with an agenda and other relevant documents for the General Meeting, Dutch law does not have a regulatory regime for the solicitation of proxies, and the solicitation of proxies is not a generally accepted business practice in the Netherlands; thus, our practice will vary from the requirement of NASDAQ Listing Rule 5620(b). In addition, we have opted out of certain Dutch shareholder approval requirements for the issuance of securities in connection with certain events, such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees and a change of control of us and certain private placements. To this extent, our practice varies from the requirements of NASDAQ Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. For an overview of our corporate governance principles, see "Description of Share Capital—Comparison of Dutch Corporate Law, our Articles of Association and Board By-Laws and U.S. Corporate Law—Corporate Governance."
Code of Business Conduct and Ethics
Prior to the completion of this offering, we expect to adopt a Code of Business Conduct and Ethics, or the Code of Conduct, that is applicable to all of our employees and directors. Upon adoption, the Code of Conduct will be available on our website atwww.argenx.com. The audit committee of our board of directors will be responsible for overseeing the Code of Conduct and will be required to approve any waivers of the Code of Conduct for employees and directors. We expect that any amendments to the Code of Conduct, and any waivers of its requirements, will be disclosed on our website.
Compensation of Directors
Our shareholders have adopted a policy governing the remuneration of our board of directors, which is aimed to attract, reward and retain highly qualified executive and non-executive directors and to provide and motivate the members of our board of directors with a balanced and competitive remuneration that is focused on sustainable results and is aligned with the long-term strategy of the company as set out in its business plan.
At the General Meeting on April 28, 2016, the shareholders approved an amended remuneration policy, or the Renumeration Policy, which allows for the granting of compensation packages to our directors in line with a benchmarking analysis performed by an independent consulting firm engaged by our remuneration and nomination committee and an assessment of the duties of the directors, and includes competitive severance arrangements intended to attract and retain highly qualified personnel. In line with the Remuneration Policy, our board of directors have approved amendments to the employment and management agreements with our executive directors. For a discussion of our employment arrangements with our executive directors, see the section of this prospectus titled "Related Party Transactions—Agreements with Our Executive Directors."
162
Except the arrangements described in the section of this prospectus titled "Related-Party Transactions—Agreements with Our Executive Directors," there are no arrangements or understanding between us and any of the executive directors providing for benefits upon termination of their employment, other than as required by applicable law.
Compensation of Executive Directors
Pursuant to the remuneration policy, the remuneration of the executive directors consists of the following fixed and variable components:
Fixed base salary. The base salary of the executive directors was determined on the basis of a benchmarking analysis completed by an independent consulting firm. In accordance with this benchmarking analysis, our board of directors has resolved to aim for a compensation of executive directors in the 50th percentile of the compensation offered by the European peer group identified by the independent consulting firm used in this analysis. In line with the amended remuneration policy discussed above, our board of directors has amended the current contracts between us and our executive directors to be brought in line with the new remuneration policy.
Variable annual cash bonus. The objective of this short-term annual cash incentive is to ensure that the executive directors are incentivized to achieve performance targets in the shorter term. An executive director is eligible for an annual cash incentive up to a maximum percentage of his/her annual base salary. On September 3, 2015, the maximum percentage for this purpose was set at 40% of base salary of the chief executive officer, and at 35% of base salary of the chief financial officer. Performance conditions are established by our board of directors before or at the beginning of the relevant calendar year and shall include criteria concerning the Company's financial performance, qualitative criteria representing Company performance and/or individual qualitative performance.
Long-term incentive awards. Our board of directors intends to incentivize the executive directors by issuing Options from time to time to be able to attract and retain well-qualified executive directors in connection with the argenx Employee Stock Option Plan, as set out below.
Severance arrangements. Pursuant to the Remuneration Policy, in case of a dismissal, executive directors will not be entitled to a severance payment of a maximum of one year's base salary unless the board of directors decides otherwise based on a recommendation of the remuneration and nomination committee. However, we have entered into a management contract and employment agreement with our chief executive officer and chief financial officer, respectively, each of which provides for certain minimum notice periods if his service or employment with us is terminated in certain circumstances as described below in "Related Party Transactions—Agreements with our Executive Directors and Non-Executive Directors."
Pension and fringe benefits. The executive directors participate in a defined contribution pension scheme operated by a third party pension insurance organization. The executive directors are entitled to customary fringe benefits, such as a company car and a hospitalization plan.
163
The following tables set forth information regarding compensation earned by our executive directors during the year ended December 31, 2016:
Tim Van Hauwermeiren
| | Compensation (€) | |||
|---|---|---|---|---|
Base salary | 253,284 | |||
Option awards (1) | 488,020 | |||
Non-equity incentive plan compensation | 101,314 | |||
All other compensation (2) | 22,213 | |||
| | | | | |
Total | 864,831 | |||
Eric Castaldi
| | Compensation (€) | |||
|---|---|---|---|---|
Base salary | 235,952 | |||
Option awards (1) | 354,577 | |||
Non-equity incentive plan compensation | 82,583 | |||
All other compensation (2) | 221,096 | |||
| | | | | |
Total | 894,208 | |||
The following table sets forth information regarding option awards granted to the executive directors during the year ended December 31, 2016:
Name | Stock options | Expiration date | Exercise price | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Tim Van Hauwermeiren | 50,000 | 5/25/2026 | € | 11.47 | ||||||
Tim Van Hauwermeiren | 30,600 | 12/13/2026 | € | 14.13 | ||||||
Eric Castaldi | 28,200 | 5/25/2026 | € | 11.47 | ||||||
Eric Castaldi | 28,200 | 12/13/2026 | € | 14.13 | ||||||
Compensation of Our Non-Executive Directors
The remuneration of the individual members of the board of directors is determined by the non-executive directors, at the recommendation of the remuneration and nomination committee, within the limits of the Remuneration Policy adopted by the shareholders at the General Meeting.
164
Pursuant to the Remuneration Policy, the remuneration of the non-executive directors consists of the following fixed and variable components:
Fixed fee. The board of directors has set the base remuneration for non-executive directors at €35,000, additional remuneration for the chairman of the board of directors at €20,000, additional remuneration for the chairman of the audit committee of the board of directors at €10,000 and additional remuneration for the chairman of the remuneration and nomination committee of the board of directors at €8,000.
Long-term incentive plan. The board of directors intends to incentivize the non-executive directors by issuing options from time to time to be able to attract and retain well-qualified non-executive directors in connection with the argenx Employee Stock Option Plan. The board of directors grants options to the non-executive directors on the recommendation of the remuneration and nomination committee. Such option grants are based on an option allocation scheme established by the board of directors pursuant to our Employee Stock Option Plan. The conditions of our option plan apply to our non-executive directors, as set forth in "—argenx Employee Stock Option Plan."
Success payment. In exceptional circumstances, the board of directors may decide to reward a non-executive director with a success payment relating to the occurrence of specific events achieved through the exceptional efforts of that person (such as a platform licensing or product licensing deal brokered by that non-executive director).
The following table sets forth the information regarding the compensation earned by our non-executive directors during the year ended December 31, 2016:
Name | Fees earned or paid in cash (€) | Option awards (€) (1) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Peter K.M. Verhaeghe | 55,000 | 54,579 | 109,579 | |||||||
John Paul de Koning (2) | — | — | — | |||||||
David L. Lacey | 45,930 | 54,579 | 100,509 | |||||||
Werner Lanthaler | 45,000 | 54,579 | 99,579 | |||||||
Pamela Klein (3) | 35,000 | 54,579 | 89,579 | |||||||
J. Donald deBethizy | 43,000 | 54,579 | 97,579 | |||||||
165
argenx Employee Stock Option Plan
On December 18, 2014, our board of directors adopted an employee stock option plan, or the Option Plan, which was approved by the shareholders at the General Meeting on May 13, 2015 and amended by the General Meeting on April 28, 2016. The aim of the Option Plan is to encourage key employees, directors and key outside consultants and advisors to acquire an economic and beneficial ownership interest in the growth and performance of the company, to increase their incentive to contribute to our value and to attract and retain individuals who are key to our company.
In connection with the Option Plan, our board of directors has also established an option allocation scheme. The option allocation scheme contains (i) the date on which options are granted each year, which shall be the same date each year and (ii) the number of options granted to each person or to each group of persons, which shall be based on objective criteria only.
Our board of directors, in each case subject to the approval of the majority of the non-executive directors, may grant options to the key employees, directors or key outside consultants or advisors and in accordance with the option allocation scheme. Our board of directors may also grant options at its discretion outside of the option allocation scheme, but only in a period when no inside information (as specified our insider trading policy) is available. Persons to whom options are granted cannot refuse to accept such options.
The aggregate number of shares that may be available for the issuance of options is equal to 14.5% of our fully-diluted share capital. Shares issued pursuant to the exercise of an option are counted towards the share capital, and options that cease to exist (whether through exercise, termination or otherwise) are restored to the foregoing limit and shall again be available for issuance under the Option Plan. Shares shall be charged against the forgoing limit upon the grant of each option, but if such shares are thereafter forfeited or such option otherwise terminates without the issuance of such shares or of other consideration in lieu of such shares, the shares so forfeited or related to the terminated portion of such option shall be restored to the foregoing limit and shall again be available for options under the Option Plan.
Options granted pursuant to the Option Plan shall vest with respect to one third of the shares upon the first anniversary of the date of grant, with the remaining two thirds vesting in twenty-four equal monthly installments with the option fully vesting upon the third anniversary of the date of grant, subject, in each case, to the optionee's continued status.
Each option shall be granted with an exercise price equal to the fair market value upon the date of grant and shall have a term equal to ten years from the date of grant. In the case of a (i) sale, merger, consolidation, tender offer or similar acquisition of shares or other transaction or series of related transactions as a result of which a change in control occurs, (ii) sale or other disposition of all or substantially all of the company's assets or (iii) dissolution and/or liquidation of the company, then 100% of any unvested options shall vest.
Our board of directors, upon approval of a majority of the non-executive directors may amend or terminate the Option Plan or may amend the terms of any outstanding options, provided that no
166
amendment or termination may affect any existing rights without the consent of the affected optionees.
On April 28, 2016, the shareholders at the General Meeting designated our board of directors to issue shares under the Option Plan and to limit or exclude preemption rights of shareholders for such shares and option rights to subscribe for shares with the prior consent of the majority of the non-executive directors for a period of eighteen months. It is expected that prior to completion of this offering at the recommendation of the board of directors the shareholders at the General Meeting will prolong and renew such designation for another period of eighteen months following the day of that meeting.
167
Since January 1, 2014, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party in which any of the members of our board of directors or senior management, holders of more than 10% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the compensation and shareholding arrangements we describe in "Management" and "Principal Shareholders," and the transactions we describe below.
Agreements with Our Executive Directors
Employment and Management Agreements
We have entered into a management agreement with Tim Van Hauwermeiren as our chief executive officer and executive director. We have also entered into an employment agreement with Eric Castaldi, our chief financial officer. The key terms of these agreements are as follows:
Tim Van Hauwermeiren | Eric Castaldi | |||
Base salary |
|
| ||
Cash bonus |
|
| ||
Pension contributions |
|
| ||
Duration |
|
|
We may terminate Mr. Van Hauwermeiren's services upon 18 months' notice, or payment of 18 months' pro-rated base salary in lieu of notice. Mr. Van Hauwermeiren would be entitled to the same payment in lieu of notice in the event he terminated his services with us in circumstances in which it cannot reasonably be expected for him to continue providing services to us (and after our failure to remedy such conditions after being provided at least 14 days' notice). Mr. Van Hauwermeiren would also be entitled to payment in lieu of notice in the event he terminated his services with us in certain cases of our failure to comply with obligations under applicable law or his agreement (and after our failure to remedy such non-compliance, if non-deliberate, after being provided at least 14 days' notice). In these cases, there will be a full acceleration of the vesting of any outstanding stock options held by Mr. Van Hauwermeiren. There will be no notice period or payment in lieu of notice in certain cases of Mr. Van Hauwermeiren's failure to comply with obligations under applicable law or his agreement. In the case of Mr. Castaldi, if we terminate his employment without taking into account the statutory notice period (other than a termination for serious cause), or Mr. Castaldi terminates his employment with us in circumstances in which it cannot reasonably be expected for him to continue employment with us (and provided we have failed to remedy the condition after a period of 14 days from being given notice of such condition) then Mr. Castaldi shall be entitled to receive the higher of (i) 12 months' gross annual salary or (ii) salary and benefits as defined under Belgian law for the statutory notice period (or, if the termination took into account all or part of the statutory notice period, for the remainder of the statutory notice period). In each such case (other than a termination for serious cause), there will be a full acceleration of the vesting of any outstanding stock options held by Mr. Castaldi. Each of
168
Messrs. Van Hauwermeiren and Castaldi may be dismissed immediately as executive directors of the Company.
Transactions with Related Companies
Agreement with FairJourney LDA
FairJourney LDA, or FairJourney, is a fee-for-service company focused on antibody discovery and engineering services. FairJourney was founded in 2012 and, as compensation for their support with the formation of FairJourney, our chief executive officer and executive director Tim Van Hauwermeiren acquired shares representing 5% of the equity securities of FairJourney, and our chief scientific officer, Hans de Haard, acquired shares representing 20% of the equity securities of FairJourney. In July 2012, we entered into a license and exclusive option agreement with FairJourney, pursuant to which we granted FairJourney a worldwide, non-exclusive license to our SIMPLE Antibody Platform to develop, manufacture and commercialize SIMPLE Antibodies to certain targets selected by FairJourney. Under the terms of the agreement, once FairJourney has advanced a product candidate discovered under the agreement to near proof-of-concept stage, we have the option to acquire patent rights generated by FairJourney specific to such product candidate along with a non-exclusive license to additional FairJourney intellectual property useful for further development, manufacture, or commercialization of the product candidate. Upon exercising this option, we must pay FairJourney an option fee equal to two times the expenses incurred by FairJourney for advancing such product candidate through the option exercise date, and we are required to pay a specified royalty in the mid-single digits on any sub-licensing revenue received by us for such product candidate. Alternatively, if we elect not to exercise the option, FairJourney is required to pay us a specified royalty in the mid-single digits on any sub-licensing revenue received by FairJourney for such product candidate. In connection with the agreement, we acquired shares of FairJourney representing 15% of the fully-diluted equity securities of FairJourney at the time of issuance.
Services Provided by VVGB Advocaten-Avocats
In relation to the initial public offering of our shares on Euronext Brussels in July 2014, VVGB Advocaten-Avocats provided legal services to us. Peter K.M. Verhaeghe, one of our non-executive directors, is the managing partner of VVGB Advocaten-Avocats.
Related Party Transactions Policy
Prior to the closing of this offering, we intend to enter into a related party transaction policy.
169
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of February 16, 2017 for:
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include ordinary shares that can be acquired within 60 days of February 16, 2017. The percentage ownership information shown in the table prior to this offering is based upon 20,126,479 ordinary shares outstanding as of February 16, 2017. The percentage ownership information shown in the table after this offering is based upon ordinary shares outstanding, assuming the sale of shares by us in this offering and no exercise of the underwriters' options to purchase additional shares. The percentage ownership information shown in the table after this offering if the underwriters' options to purchase additional shares are exercised in full is based upon ordinary shares outstanding, assuming the sale of shares by us in this offering and assuming the exercise in full of the underwriters' options to purchase additional shares.
Except as otherwise indicated, all of the shares reflected in the table are ordinary shares and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
In computing the number of ordinary shares beneficially owned by a person and the percentage ownership of that person, we deemed outstanding ordinary shares subject to warrants held by that person that are immediately exercisable or exercisable within 60 days of February 16, 2017. We did not deem these shares outstanding, however, for the purpose of computing the percentage
170
ownership of any other person. The information in the table below is based on information known to us or ascertained by us from public filings made by the shareholders.
| | Shares beneficially owned before this offering | Shares beneficially owned after this offering | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name and address of beneficial owner | Number | Percent | Number | Percent | |||||||||
5% or Greater Shareholders: | |||||||||||||
LSP IV Management B.V.(1)(2) | 1,715,215 | 8.52 | % | ||||||||||
Forbion Capital Fund II Coöperatief U.A.(1)(3) | 2,126,243 | 10.56 | |||||||||||
Shire plc(1)(4) | 1,411,764 | 7.01 | |||||||||||
R.W. Wong(1)(5) | 1,841,731 | 9.15 | |||||||||||
Federated Equity Management Company of Pennsylvania(1)(6) | 1,480,420 | 7.36 | |||||||||||
Perceptive Advisors LLC(1)(7) | 1,124,478 | 5.59 | |||||||||||
Directors: | |||||||||||||
Tim Van Hauwermeiren(8) | 190,703 | * | |||||||||||
Eric Castaldi(9) | 139,256 | * | |||||||||||
Peter Verhaeghe(10) | 23,335 | * | |||||||||||
John de Koning | — | — | |||||||||||
David Lacey(11) | 16,243 | * | |||||||||||
Werner Lanthaler(12) | 18,766 | * | |||||||||||
Donald deBethizy(13) | 8,750 | * | |||||||||||
Pamela Klein(14) | 8,750 | * | |||||||||||
All board members (8 persons)(15) | 405,803 | 2.00 | |||||||||||
171
Each of our shareholders is entitled to one vote per ordinary share. None of the holders of our shares will have different voting rights from other holders of shares after the closing of this offering. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
172
General
We were incorporated on April 25, 2008, as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) under Dutch law. On May 28, 2014, we converted into a public company with limited liability (naamloze vennootschap) pursuant to a notarial deed of conversion and amendment.
We are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our corporate seat is in Rotterdam, the Netherlands, and our registered office is at Willemstraat 5, 4811 AH, Breda, the Netherlands.
We intend to apply to list our ordinary shares on The NASDAQ Global Select Market, or NASDAQ, under the symbol "ARGX."
Initial settlement of the ordinary shares issued in this offering will take place on the closing of this offering through The Depository Trust Company, or DTC, in accordance with its customary settlement procedures for equity securities. Each person owning ordinary shares held through DTC must rely on the procedures thereof and on institutions that have accounts therewith to exercise any rights of a holder of the ordinary shares.
Issue of Shares
The Articles of Association provide that shares may be issued or rights to subscribe for our shares may be granted pursuant to a resolution of the shareholders at the General Meeting, or alternatively, by our board of directors if so designated by the General Meeting. A resolution of the shareholders at the General Meeting to issue shares, to grant rights to subscribe for shares or to designate our board of directors as the corporate body of the company authorized to do so can only take place at the proposal of our board of directors with the consent of the majority of the non-executive directors. Shares may be issued or rights to subscribe for shares may be granted by resolution of our board of directors, if and insofar as our board of directors is designated to do so by the shareholders at the General Meeting. Designation by resolution of the shareholders at the General Meeting cannot be withdrawn unless determined otherwise at the time of designation. The scope and duration of our board of directors' authority to issue shares or grant rights to subscribe for shares (such as granting stock options or issuing convertible bonds) is determined by a resolution of the shareholders at the General Meeting and relates, at the most, to all unissued shares in the company's authorized capital at the relevant time. The duration of this authority may not exceed a period of five years. Designation of our board of directors as the body authorized to issue shares or grant rights to subscribe for shares may be extended by a resolution of the shareholders at the General Meeting for a period not exceeding five years in each case. The number of shares that may be issued is determined at the time of designation.
No shareholders' resolution or board of directors resolution is required to issue shares pursuant to the exercise of a previously granted right to subscribe for shares. A resolution of our board of directors to issue shares and to grant rights to subscribe for shares can only be taken with the consent of the majority of the non-executive directors.
On April 28, 2016, the shareholders at the General Meeting authorized our board of directors to issue shares and grant rights to subscribe for shares and to limit or exclude preemption rights of shareholders for such shares with the prior consent of the majority of the non-executive directors for a period of eighteen months. In its resolution, the shareholders at the General Meeting restricted the
173
competency of our board of directors as regards the issue of shares and the grant of rights to subscribe for shares to a maximum of 20% of the company's total issued and outstanding share capital as at April 28, 2016.
Preemptive Rights
Dutch law and the Articles of Association give shareholders preemptive rights to subscribe on apro rata basis for any issue of new shares or, upon a grant of rights, to subscribe for shares. Holders of shares have no preemptive rights upon (1) the issue of shares against a payment in kind (being a contribution other than in cash); (2) the issue of shares to our employees or the employees of a member of our group; and (3) the issue of shares to persons exercising a previously granted right to subscribe for shares.
A shareholder may exercise preemptive rights during a period of at least two weeks from the date of the announcement of the issue of shares. Pursuant to the Articles, the shareholders at the General Meeting may restrict or exclude the preemptive rights of shareholders. A resolution of the shareholders at the General Meeting to restrict or exclude the preemptive rights or to designate our board of directors as our body authorized to do so, may only be adopted on the proposal of our board of directors with the consent of the majority of the non-executive directors. A resolution of the shareholders at the General Meeting to exclude or restrict preemptive rights, or to authorize our board of directors to exclude or restrict preemptive rights, requires a majority of at least two-thirds of the votes cast, if less than 50% of our issued and outstanding share capital is present or represented at the General Meeting.
With respect to an issuance of shares pursuant to a resolution of our board of directors, the preemptive rights of shareholders may be restricted or excluded by resolution of our board of directors if and insofar as our board of directors is designated to do so by the shareholders at the General Meeting. A resolution of our board of directors to restrict or exclude preemptive rights can only be taken with the consent of the majority of the non-executive directors.
On April 28, 2016, the shareholders at the General Meeting resolved to authorize our board of directors to restrict or exclude preemptive rights with regard to such issuance. The designation of our board of directors as the body competent to restrict or exclude the preemptive rights may be extended by a resolution of the shareholders at the General Meeting for a period not exceeding five years in each case. Designation by resolution of the shareholders at the General Meeting cannot be withdrawn unless determined otherwise at the time of designation.
On April 28, 2016, the shareholders at the General Meeting designated our board of directors to issue shares and grant rights to subscribe for shares and to limit or exclude preemption rights of shareholders for such shares with the prior consent of the majority of the non-executive directors for a period of eighteen months from the day of that meeting. In its resolution, the shareholders at the General Meeting restricted the competency of our board of directors as regards the issue of shares and the grant of rights to subscribe for shares to a maximum of 20% of our total issued and outstanding share capital as at the day of that meeting.
Acquisition of Shares by the Company
We may not subscribe for our own shares on issue. We may acquire fully paid-up shares at any time for no consideration or, if:
174
As part of the authorization, the General Meeting must specify the number of shares that may be repurchased, the manner in which the shares may be acquired and the price range within which the shares may be acquired. A resolution of our board of directors to repurchase shares can only be taken with the consent of the majority of the non-executive directors.
Shares held by us in our own share capital do not carry a right to any distribution. Furthermore, no voting rights may be exercised for any of the shares held by us or our subsidiaries unless such shares a are subject to the right of usufruct or to a pledge in favor of a person other than us or its subsidiaries and the voting rights were vested in the pledgee or usufructuary before us or its subsidiaries acquired such shares. Neither we nor our subsidiaries may exercise voting rights in respect of shares for which we or our subsidiaries have a right of usufruct or a pledge.
Reduction of Share Capital
The shareholders at General Meeting may, upon a proposal of our board of directors with the consent of the majority of the non-executive directors, resolve to reduce the issued share capital by cancelling shares or by amending the Articles of Association to reduce the nominal value of the shares. Only shares held by us or shares for which we hold the depositary receipts may be cancelled. A resolution of the shareholders at the General Meeting to reduce the number of shares must designate the shares to which the resolution applies and must lay down rules for the implementation of the resolution. A resolution to reduce the issued share capital requires a majority of at least two-thirds of the votes cast, if less than 50% of our issued and outstanding share capital is present or represented at the General Meeting.
Articles of Association and Dutch Law
When we refer to our Articles of Association in this prospectus, we refer to our Articles of Association as they will be in force after the expected execution of an amendment to our Articles of Association prior to the closing of this offering.
Set forth below is a summary of relevant information concerning our share capital and material provisions of our Articles of Association and applicable Dutch law. This summary does not constitute legal advice regarding those matters and should not be regarded as such.
Amendment of Articles of Association
The shareholders at the General Meeting may resolve to amend the Articles of Association, at the proposal of our board of directors, with the consent of the majority of the non-executive directors. A resolution by the shareholders at the General Meeting to amend the Articles of Association requires a simple majority of the votes cast in a meeting in which at least half of our issued and outstanding capital is present or represented, or at least two-thirds of the votes cast, if less than half of our issued and outstanding capital is present or represented at that meeting.
Company's Shareholders' Register
Subject to Dutch law and the Articles of Association, we must keep our shareholders' register accurate and up-to-date. Our board of directors keeps our shareholders' register and records names and addresses of all holders of shares, showing the date on which the shares were acquired, the
175
date of the acknowledgement by or notification of us as well as the amount paid on each share. The register also includes the names and addresses of those with a right of usufruct (vruchtgebruik) in shares belonging to another or a pledge in respect of such shares.
Corporate Objectives
Our corporate objectives are: (a) to exploit, including all activities relating to research, development, production, marketing and commercial exploitation; biological, chemical or other products, processes and technologies in the life sciences sector in general, and more specifically in the diagnostic, pharmaceutical, medical, cosmetic, chemical and agricultural sector; (b) to design and develop instruments which may be used in medical diagnosis and affiliated areas; (c) the worldwide distribution of, sale of and rendering services relating to our products and subsidiaries directly to customers as well as through third parties; (d) to incorporate, to participate in any way whatsoever, to manage, to supervise, to operate and to promote enterprises, businesses and companies; (e) to render advice and services to businesses and companies with which we form a group and to third parties; (f) to finance businesses and companies; (g) to borrow, to lend and to raise funds, including the issue of bonds, promissory notes or other securities or evidence of indebtedness as well as to enter into agreements in connection with the aforementioned; (h) to render guarantees, to bind us and to pledge our assets for obligations of the companies and enterprises with which we form a group and on behalf of third parties; (i) to obtain, alienate, manage and exploit registered property and items of property in general; (j) to trade in currencies, securities and items of property in general; (k) to develop and trade in patents, trademarks, licenses, know-how and other industrial property rights; and (l) to perform any and all activities of industrial, financial or commercial nature, as well as everything pertaining the foregoing, relating thereto or conductive thereto, all in the widest sense of the word.
Limitation on Liability and Indemnification Matters
Under Dutch law, our board of directors and certain other officers may be held liable for damages in the event of improper or negligent performance of their duties. They may be held jointly and severally liable for damages to our company and to third parties for infringement of the Articles of Association or of certain provisions of the Dutch Civil Code. In certain circumstances, they may also incur additional specific civil and criminal liabilities. Directors and certain other officers are insured under an insurance policy taken out by us against damages resulting from their conduct when acting in the capacities as such directors or officers. In addition, our Articles of Association provide for indemnification of our directors, including reimbursement for reasonable legal fees and damages or fines based on acts or failures to act in their duties. No indemnification shall be given to a member of our board of directors if a Dutch court has established, without possibility for appeal, that the acts or omissions of such indemnified person that led to the financial losses, damages, suit, claim, action or legal proceedings resulted from either an improper performance of his or her duties as a director or an officer of our company or an unlawful or illegal act, and only to the extent that his or her financial losses, damages and expenses are covered by an insurance and the insurer has settled these financial losses, damages and expenses (or has indicated that it would do so). Furthermore, such indemnification will generally not be available in instances of willful (opzettelijk), intentionally reckless (bewust roekeloos) or seriously culpable (ernstig verwijtbaar) conduct unless Dutch law provides otherwise.
Shareholders' Meetings and Consents
General Meeting
General meetings of shareholders are held in Rotterdam, Breda, Den Haag, Maastricht, Amsterdam, Utrecht and Schiphol Airport, municipality of Haarlemmermeer, the Netherlands. The annual General Meeting must be held within six months of the end of each financial year. Additional
176
extraordinary General Meetings may also be held whenever considered appropriate by our board of directors. Pursuant to Dutch law, one or more shareholders and others entitled to attend a General Meeting, who jointly represent at least one-tenth of the issued capital, may request our board of directors to convene a General Meeting. If our board of directors has not taken the steps necessary to ensure that a General Meeting will be held within the relevant statutory period after the request, the requesting persons may, at his/her/their request, be authorized by court in preliminary relief proceedings to convene a General Meeting. The court shall disallow the application if it does not appear that the applicants have previously requested our board of directors to convene a General Meeting and our board of directors has not taken the necessary steps so that the General Meeting could be held within six weeks after the request.
General meetings of shareholders can be convened by a notice, which shall include an agenda stating the items to be discussed, including for the annual General Meeting, among other things, the adoption of the annual accounts, appropriation of our profits and proposals relating to the composition of our board of directors, including the filling of any vacancies in our board of directors. In addition, the agenda shall include such items as have been included therein by our board. The agenda shall also include such items requested by one or more shareholders, and others entitled to attend General Meetings, representing at least 3% of the issued share capital. Requests must be made in writing and received by our board of directors at least 60 days before the day of the convocation of the meeting. No resolutions shall be adopted on items other than those which have been included in the agenda. In accordance with the DCGC, a shareholder may include an item on the agenda only after consulting our board of directors in that respect. If one or more shareholders intends to request that an item be put on the agenda that may result in a change in the company's strategy, our board of directors may invoke a response time of a maximum of 180 days until the day of the General Meeting.
The General Meeting is presided over by the chairperson. However, the chairperson may charge another person to preside over the General Meeting in his place even if he himself is present at the meeting. If the chairperson is absent and he has not charged another person to preside over the meeting in his place, the board members present at the meeting shall appoint one of the non-executive directors to be chairperson. Board members may attend a General Meeting. In these meetings, they have an advisory vote. The chairperson of the meeting may decide at its discretion to admit other persons to the meeting.
Admission and Registration
All shareholders, and each usufructuary and pledgee to whom the right to vote on our shares accrues, are entitled, in person or represented by a proxy authorized in writing, to attend and address the General Meeting and exercise voting rights pro rata to their shareholding. Shareholders may exercise their rights if they are the holders of our shares on the record date as required by Dutch law, which is currently the 28th day before the day of the General Meeting, and they or their proxy have notified us of their intention to attend the General Meeting in writing or by any other electronic means that can be reproduced on paper ultimately at a date set for that purpose by our board of directors which date may not be earlier than the seventh day prior to the General Meeting, specifying such person's name and the number of shares for which such person may exercise the voting rights and/or meeting rights at such General Meeting. The convocation notice shall state the record date and the manner in which the persons entitled to attend the General Meeting may register and exercise their rights.
177
Quorum and Voting Requirements
Each ordinary share confers the right on the holder to cast one vote at the General Meeting. Shareholders may vote by proxy. The voting rights attached to any shares held by us are suspended as long as they are held in treasury. Nonetheless, the holders of a right of usufruct (vruchtgebruik) in shares belonging to another and the holders of a right of pledge in respect of ordinary shares held by us are not excluded from any right they may have to vote on such ordinary shares, if the right of usufruct (vruchtgebruik) or the right of pledge was granted prior to the time such ordinary share was acquired by us. We may not cast votes in respect of a share in respect of which there is a right of usufruct (vruchtgebruik) or a right of pledge. Shares which are not entitled to voting rights pursuant to the preceding sentences will not be taken into account for the purpose of determining the number of shareholders that vote and that are present or represented, or the amount of the share capital that is provided or that is represented at a General Meeting.
In accordance with Dutch law and generally accepted business practices, our Articles of Association do not provide quorum requirements generally applicable to General Meeting. To this extent, our practice varies from the requirement of NASDAQ Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Decisions of the General Meeting are taken by an absolute majority of votes cast, except where Dutch law or the Articles of Association provide for a qualified majority or unanimity.
Board Members
Election of Board Members
Under our Articles of Association, our directors are appointed by the shareholders at the General Meeting upon nomination by our board of directors. However, the shareholders at the General Meeting may at all times overrule the binding nomination by a resolution adopted by at least a two-thirds majority of the votes cast, provided such majority represents more than half of the issued share capital.
Duties and Liabilities of Directors
Under Dutch law, our board of directors is collectively responsible for our general affairs. Pursuant to our Articles of Association, our board of directors shall divide its duties among its members, with our day-to-day management entrusted to the executive directors. The non-executive directors supervise the management of the executive directors and the general affairs of our company and the business connected with it and provide the executive directors with advice. In addition, both the executive directors and the non-executive directors must perform such duties as are assigned to them pursuant to the Articles of Association. The division of tasks within our board of directors is determined (and amended, if necessary) by our board of directors. Each director has a duty to properly perform the duties assigned to him or her and to act in our corporate interest. Under Dutch law, the corporate interest extends to the interests of all corporate stakeholders, such as shareholders, creditors, employees and other stakeholders.
Dividends and Other Distributions
Amount Available for Distribution
Pursuant to Dutch law and the Articles of Association, the distribution of profits will take place following the adoption of our annual accounts, from which we will determine whether such distribution is permitted. We may only make distributions to the shareholders, whether from profits or from its freely distributable reserves, only insofar as its shareholders' equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law.
178
Our board of directors, with the consent of the majority of the non-executive directors, may determine which part of our profits will be added to the reserves in consideration of our reserves and dividends policy. The remaining part of the profits after the addition to the reserves will be at the disposal of the General Meeting. Distributions of dividends will be made pro rata to the nominal value of each share.
Subject to Dutch law and the Articles of Association, our board of directors, with the consent of the majority of the non-executive directors, may resolve to distribute an interim dividend if it determines such interim dividend to be justified by our profits. For this purpose, our board of directors must prepare an interim statement of assets and liabilities. Such interim statement shall show our financial position not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. An interim dividend can only be paid if (a) an interim statement of assets and liabilities is drawn up showing that the funds available for distribution are sufficient, and (b) our shareholders' equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law.
Our board of directors, with the consent of the majority of the non-executive directors, may resolve that we make distributions to shareholders from one or more of its freely distributable reserves, other than by way of profit distribution, subject to the due observance of our policy on reserves and dividends. Any such distributions will be made pro rata to the nominal value of each share.
Dividends and other distributions shall be made payable not later than the date determined by our board. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable, will lapse and any such amounts will be considered to have been forfeited to us (verjaring).
We do not anticipate paying any cash dividends for the foreseeable future.
Exchange Controls
Pursuant to Dutch law, there are no exchange controls applicable to the transfer to persons outside of the Netherlands of dividends or other distributions with respect to, or of the proceeds from the sale of, shares of a Dutch company.
Public Offer
In accordance with Directive 2004/25/EC, each EU member state should ensure the protection of minority shareholders by obliging any person that acquires control of a company to make an offer to all the holders of that company's voting securities for all their holdings at an equitable price.
The Directive 2004/25/EC applies to all companies governed by the laws of a EU member state of which all or some voting securities are admitted to trading on a regulated market in one or more EU member states. The laws of the EU member state in which a company has its registered office will determine the percentage of voting rights that is regarded as conferring control over that company.
In accordance with Section 5:70 of the Dutch Financial Supervision Act (Wet op het financieel toezicht), or the DFSA, any person—whether acting alone or in concert with others—who, directly or indirectly, acquires a controlling interest in a company will be obliged to launch a mandatory public offer for all our outstanding shares. A controlling interest is deemed to exist if a (legal) person is able to exercise, alone or acting in concert, at least 30% of the voting rights in the General Meeting. An
179
exception is made for, amongst others, shareholders who—whether alone or acting in concert with others—(i) have an interest of at least 30% of our voting rights before our shares are first admitted to trading on Euronext Brussels and NASDAQ and who still have such an interest after such first admittance to trading, and (ii) reduce their holding to below 30% of the voting rights within 30 days of the acquisition of the controlling interest provided that (a) the reduction of their holding was not effected by a transfer of shares to an exempted party and (b) during such period such shareholders or group of shareholders did not exercise their voting rights.
The rules under the DFSA regarding mandatory public offers apply to us because the company has its statutory seat in the Netherlands. However, as the shares are not admitted to trading on a regulated market in the Netherlands but are admitted to trading on Euronext Brussels and will be admitted to trading on NASDAQ, the Dutch Decree on public offers (Besluit openbare biedingen Wft) will only apply in relation to matters relating to information to be provided to trade unions and employees and company law matters, including the convocation of a General Meeting in the event of a public offer and a position statement by our board of directors. In case of a mandatory public offer, the provisions regarding the offered consideration and the bid procedure will be governed by Belgian law pursuant to article 4§1, 3° of the Belgian law dated April 1, 2007 on public takeover bids. Pursuant to article 53 of the implementing Royal Decree, a mandatory public offer on our shares must be launched at a price equal to the higher of (i) the highest price paid by the offeror or persons acting in concert with it for the acquisition of shares during the last 12 months and (ii) the weighted average trading prices during the last 30 days before the obligation to launch a mandatory public offer was triggered. The price can be in cash or in securities. However, if the securities that are offered as consideration are not liquid securities that are traded on a regulated market or if the offeror or persons acting in concert with it have acquired shares for cash in the last 12 months, a cash alternative has to be offered.
No takeover bid has been instigated by third parties in respect of our equity during the previous financial year and the current financial year.
Squeeze Out Procedures
Pursuant to Section 92a, Book 2, Dutch Civil Code, a shareholder who for his own account contributes at least 95% of our issued share capital may initiate proceedings against our minority shareholders jointly for the transfer of their shares to the claimant. The proceedings are held before the Dutch Enterprise Chamber of the Amsterdam Court of Appeal (Ondernemingskamer van het Gerechtshof te Amsterdam), or the Enterprise Chamber, and can be instituted by means of a writ of summons served upon each of the minority shareholders in accordance with the provisions of the Dutch Code of Civil Procedure (Wetboek van Burgerlijke Rechtsvordering). The Enterprise Chamber may grant the claim for squeeze out in relation to all minority shareholders and will determine the price to be paid for the shares, if necessary after appointment of one or three experts who will offer an opinion to the Enterprise Chamber on the value to be paid for the shares of the minority shareholders. Once the order to transfer becomes final before the Enterprise Chamber, the person acquiring the shares shall give written notice of the date and place of payment and the price to the holders of the shares to be acquired whose addresses are known to him. Unless the addresses of all of them are known to the acquiring person, such person is required to publish the same in a daily newspaper with a national circulation.
In addition, pursuant to Section 359c, Book 2 of the Dutch Civil Code, following a public offer, a holder of at least 95% of our issued share capital and voting rights has the right to require the minority shareholders to sell their shares to it. Any such request must be filed with the Enterprise Chamber within three months after the end of the acceptance period of the public offer. Conversely, pursuant to article 2:359d of the Dutch Civil Code each minority shareholder has the right to require
180
the holder of at least 95% of our issued share capital and voting rights to purchase its shares in such case. The minority shareholder must file such claim with the Enterprise Chamber within three months after the end of the acceptance period of the public offer.
Market Abuse Rules
As of July 3, 2016, setting aside previously applicable national legislation in the EU member states, the Market Abuse Regulation (Regulation (EU) No 596/2014), or MAR, provides for specific rules intended to prevent market abuse, such as prohibitions on inside trading, divulging inside information and tipping and market manipulation. The company, the members of our board of directors and other insiders and persons performing or conducting transactions in the company's financial instruments, as applicable, will be subject to the insider trading prohibition, the prohibition on divulging inside information and tipping and the prohibition on market manipulation. In certain circumstances, the company's investors may also be subject to market abuse rules.
Inside information is any information of a precise nature relating (directly or indirectly) to us, or to our shares or other financial instruments, which information has not been made public and which, if it were made public, would be likely to have a significant effect on the price of the shares or the other financial instruments or on the price of related derivative financial instruments.
Pursuant to the MAR, a person is prohibited to possess inside information and use that information by acquiring or disposing of, for its own account or for the account of a third party, directly or indirectly, our shares and other financial instruments to which that information relates (which is considered to be insider dealing). The use of inside information by cancelling or amending an order concerning our shares or other financial instruments to which the information relates where the order was placed before the person concerned possessed the inside information, is also prohibited. In addition, a person is also prohibited to recommend another person to engage in insider dealing, or induce another person to engage in insider dealing, which arises where the person possesses inside information and (a) recommends, on the basis of that information, that another person acquires or disposes of our shares or other financial instruments to which that information relates, or induces that person to make such an acquisition or disposal or (b) recommends, on the basis of that information, that another person cancels or amends an order concerning our shares or other financial instruments to which that information relates, or induces that person to make such a cancellation or amendment.
The company will be under an obligation to make any inside information immediately public. However, the company may, on its own responsibility, delay the publication of inside information if it can ensure the confidentiality of the information. Such deferral is only possible if the publication thereof could damage the company's legitimate interests and if the deferral does not risk misleading the market. If the company wishes to use this deferral right it needs to inform the Belgian Financial Services and Markets Authority thereof and provide a written explanation of how the conditions for deferral were met, immediately after the information is disclosed to the public. The company will be subject to Belgian law and MAR regarding the publication of inside information.
Directors, other persons discharging managerial responsibilities and persons closely associated with them are covered by the MAR notification obligations. Directors and other persons discharging managerial responsibilities as well as persons closely associated with them, must notify the AFM of every transaction conducted on their own account relating to the shares or debt instruments of the company, or to derivatives or other financial instruments linked to those shares or debt instruments. Notification must be made within three working days after the date of the transaction. Under MAR, no notification of a transaction needs to be made until transactions in a calendar year by that director, persons discharging managerial responsibilities or persons closely associated with them
181
exceed a threshold of €5,000 (without netting). Once the threshold has been reached, all transactions will need to be notified, regardless of amount and wherever concluded.
Non-compliance with these reporting obligations could lead to criminal penalties, administrative fines and cease-and-desist orders (and the publication thereof), imprisonment or other sanctions.
Transparency Directive
We are a public company with limited liability (naamloze vennootschap) incorporated and existing under the laws of the Netherlands. The Netherlands is our home EU member state (lidstaat van herkomst) for the purposes of Directive 2004/109/EC, or the Transparency Directive as amended by Directive 2010/73/EU, as a consequence of which we will be subject to the DFSA in respect of certain ongoing transparency and disclosure obligations. In addition, as long as our shares are listed on Euronext Brussels and NASDAQ, we are required to disclose any regulated information which has been disclosed pursuant to the DFSA as well in accordance with the Belgian Act of May 2, 2007, the Belgian Royal Decree of November 14, 2007 and NASDAQ listing rules.
We must publish our annual accounts within four months after the end of each financial year and our half-yearly figures within two months after the end of the first six months of each financial year. Within five calendar days after adoption of our annual accounts, we must file our adopted annual accounts with the AFM.
Pursuant to the DFSA, we will be required to make public without delay any change in the rights attaching to our shares or any rights to subscribe our shares.
Dutch Financial Reporting Supervision Act
Pursuant to the Dutch Financial Reporting Supervision Act (Wet toezicht financiële verslaggeving), the DFRSA, the AFM supervises the application of financial reporting standards by companies whose official seat is in the Netherlands and whose securities are listed on a regulated Dutch or foreign stock exchange.
Pursuant to the DFRSA, the AFM has an independent right to (i) request an explanation from us regarding its application of the applicable financial reporting standards if, based on publicly known facts or circumstances, it has reason to doubt the our financial reporting meets such standards and (ii) recommend to us that we make available further explanations and files these with the AFM. If we do not comply with such a request or recommendation, the AFM may request that the Enterprise Chamber order us to (a) provide an explanation of the way we have applied the applicable financial reporting standards to our financial reports or (b) prepare our financial reports in accordance with the Enterprise Chamber's instructions.
Our Obligations and Obligations of our Shareholders and Directors to Notify Holders of Shares and Voting Rights
Pursuant to chapter 5.3 of the DFSA, any person who, directly or indirectly, acquires or disposes of an actual or potential capital interest or voting rights in the company must immediately give written notice to the AFM of such acquisition or disposal if, as a result of such acquisition or disposal, the percentage of capital interest and/or voting rights held by such person reaches, exceeds or falls below the following thresholds: 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 75% and 95%.
For the purpose of calculating the percentage of capital interest or voting rights, the following interests must be taken into account: (i) shares and/or voting rights directly held (or acquired or
182
disposed of) by any person; (ii) shares or voting rights held (or acquired or disposed of) by such person's controlled entities or by a third party for such person's account; (iii) voting rights held (or acquired or disposed of) by a third party with whom such person has concluded an oral or written voting agreement; (iv) voting rights acquired pursuant to an agreement providing for a temporary transfer of voting rights in consideration for a payment; (v) shares which such person, or any controlled entity or third party referred to above, may acquire pursuant to any option or other right to acquire shares; (vi) shares which determine the value of certain cash settled financial instruments such as contracts for difference and total return swaps; (vii) shares that must be acquired upon exercise of a put option by a counterparty; and (viii) shares which are the subject of another contract creating an economic position similar to a direct or indirect holding in those shares.
Controlled entities (gecontroleerde ondernemingen) within the meaning of the DFSA do not themselves have notification obligations under the DFSA as their direct and indirect interests are attributed to their (ultimate) parent. If a person who has a 3% or larger interest in the company's share capital or voting rights ceases to be a controlled entity it must immediately notify the AFM and all notification obligations under the DFSA will become applicable to such former controlled entity.
Special rules apply to the attribution of shares and/or voting rights which are part of the property of a partnership or other form of joint ownership. A holder of a pledge or right of usufruct in respect of shares can also be subject to notification obligations, if such person has, or can acquire, the right to vote on the shares. The acquisition of (conditional) voting rights by a pledgee or beneficial owner may also trigger notification obligations as if the pledgee or beneficial owner were the legal holder of the shares and/or voting rights.
Furthermore, when calculating the percentage of capital interest a person is also considered to be in possession of shares if (i) such person holds a financial instrument the value of which is (in part) determined by the value of the shares or any distributions associated therewith and which does not entitle such person to acquire any shares, (ii) such person may be obliged to purchase shares on the basis of an option, or (iii) such person has concluded another contract whereby such person acquires an economic interest comparable to that of holding a share.
Under the DFSA, we are required to notify the AFM promptly of any change of 1% or more in our issued and outstanding share capital or voting rights since the previous notification. Other changes in our issued and outstanding share capital or voting rights must be notified to the AFM within eight days after the end of the quarter in which the change occurred. If a person's capital interest or voting rights reaches, exceeds or falls below the above-mentioned thresholds as a result of a change in our issued and outstanding share capital or voting rights, such person is required to make a notification not later than on the fourth trading day after the AFM has published our notification as described above.
Every holder of 3% or more of our share capital or voting rights who, in relation to its previous notification, reaches, exceeds or falls below any of the above mentioned thresholds as a consequence of a different composition by means of an exchange or conversion into shares or the exercise of rights pursuant to an agreement to acquire voting rights, must notify the AFM at the latest within four trading days.
Furthermore, each director must notify the AFM of each change in the number of shares he or she holds and of each change in the number of votes he or she is entitled to cast in respect of our issued and outstanding share capital, immediately after the relevant change.
183
The AFM does not issue separate public announcements of the notifications. It does, however, keep a public register of and publishes all notifications made pursuant to the DFSA at its website (www.afm.nl). Third parties can request to be notified automatically by email of changes to the public register in relation to a particular company's shares or a particular notifying party.
Non-compliance with these notification obligations is an economic offence and may lead to criminal prosecution. The AFM may impose administrative penalties for non-compliance, and the publication thereof. In addition, a civil court can impose measures against any person who fails to notify or incorrectly notifies the AFM of matters required to be notified. A claim requiring that such measures be imposed may be instituted by us, or by one or more of our shareholders who alone or together with others represent at least 3% of our issued and outstanding share capital of or voting rights. The measures that the civil court may impose include:
Shareholders are advised to consult with their own legal advisors to determine whether the notification obligations apply to them.
Pursuant to the DCGC and in accordance with the rules intended to prevent market abuse, prior to the closing of this offering we intend to adopt an insider trading policy in respect of the holding of and carrying out of transactions by board members and employees in our shares or in financial instruments the value of which is determined by the value of our shares. Furthermore, we have drawn up a list of those persons working for us who could have access to inside information on a regular or incidental basis and have informed such persons of the rules on insider trading and market manipulation, including the sanctions which can be imposed in the event of a violation of those rules.
Short Positions
Net Short Position
Pursuant to EU regulation No 236/2012, each person holding a net short position attaining 0.2% of our issued share capital of must report it to the AFM. Each subsequent increase of this position by 0.1% above 0.2% will also have to be reported. Each net short position equal to 0.5% of our issued share capital and any subsequent increase of that position by 0.1% will be made public via the AFM short selling register. To calculate whether a natural person or legal person has a net short position, their short positions and long positions must be set off. A short transaction in a share can only be contracted if a reasonable case can be made that the shares sold can actually be delivered, which requires confirmation of a third party that the shares have been located. The notification shall be made no later than 15:30 CET on the following trading day.
184
Gross Short Position
Furthermore, each person holding a gross short position in relation to our issued share capital that reaches, exceeds or falls below one of the following thresholds: 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 75% and 95%, must immediately give written notice to the AFM.
If a person's gross short position reaches, exceeds or falls below one of the abovementioned thresholds as a result of a change in our issued share capital, such person is required to make a notification not later than on the fourth trading day after the AFM has published our notification in the public register of the AFM.
The AFM keeps a public register of the short selling notifications. Shareholders are advised to consult with their own legal advisors to determine whether any of the above short selling notification obligations apply to them.
Comparison of Dutch Corporate Law, our Articles of Association and Board By-Laws and U.S. Corporate Law
The following comparison between Dutch corporation law, which applies to us, and Delaware corporation law, the law under which many publicly listed corporations in the United States are incorporated, discusses additional matters not otherwise described in this prospectus. Although we believe this summary is materially accurate, the summary is subject to Dutch law, including Book 2 of the Dutch Civil Code and Delaware corporation law, including the Delaware General Corporation Law.
Corporate Governance
Duties of Board Members
The Netherlands. We have a one-tier board structure consisting of our executive directors and non-executive directors.
Under Dutch law, our board of directors is collectively responsible for our general affairs. Pursuant to our Articles of Association, our board of directors shall divide its duties among its members, with our day-to-day management entrusted to the executive directors. The non-executive directors supervise the management of the executive directors and the general affairs in the company and the business connected with it and provide the executive directors with advice. In addition, both the executive directors and the non-executive directors must perform such duties as are assigned to them pursuant to the Articles of Association. The division of tasks within our board of directors is determined (and amended, if necessary) by our board of directors. Each director has a duty to properly perform the duties assigned to him or her and to act in our corporate interest. Under Dutch law, the corporate interest extends to the interests of all corporate stakeholders, such as shareholders, creditors, employees and other stakeholders.
An executive director may not be allocated the tasks of: (i) serving as chairperson of our board of directors; (ii) determining the remuneration of the executive directors; or (iii) nominating directors for appointment. An executive director may not participate in the adoption of resolutions (including any deliberations in respect of such resolutions) relating to the remuneration of executive directors. Certain resolutions of our board can only be adopted with the consent of a majority of the non-executive directors.
185
Board of Directors Resolutions Requiring a Special Majority
Under the Board By-Laws, the following actions require the consent of the majority of the non-executive directors:
186
Our board of directors may designate further resolutions which also require the consenting vote of a majority of the non-executive directors. These further resolutions must be clearly specified and in writing.
Resolutions of the board of directors entailing a significant change in the identity or character of the company or its business require the approval of the shareholders at the General Meeting. This includes in any case: (i) the transfer to a third party of the business of the company or practically the entire business of the company; (ii) the entry into or breaking off of any long-term cooperation of the company or a subsidiary with another legal entity or company or as a fully liable partner of a general partnership or limited partnership, where such entry or breaking off is of far-reaching importance to the company; or (iii) the acquisition or disposal by the company or a subsidiary of an interest in the capital of a company with a value of at least one-third of the company's assets according to the consolidated balance sheet with explanatory notes included in the last adopted annual accounts of the company. Failure to obtain the approval of the General Meeting for these resolutions of the board of directors does not affect the power of representation of the board of directors.
The board of directors as a whole is authorized to represent the company. In addition, two executive directors acting jointly are also authorized to represent the company.
Tasks that have not been specifically allocated fall within the power of our board of directors as a whole. All directors remain collectively responsible for proper management regardless of the allocation of tasks.
The executive directors and the non-executive directors may adopt legally valid resolutions with regard to matters that fall within the scope of their respective duties. Our board of directors may only adopt resolutions when the majority of the relevant directors in office shall be present or represented, with a simple voting majority of the votes cast, which is 50% plus one.
187
Delaware. The board of directors bears the ultimate responsibility for managing the business and affairs of a corporation. In discharging this function, directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its stockholders. Delaware courts have decided that the directors of a Delaware corporation are required to exercise informed business judgment in the performance of their duties. Informed business judgment means that the directors have informed themselves of all material information reasonably available to them. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation. In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the stockholders.
Board Member Terms
The Netherlands. Pursuant to the Articles of Association, a member of our board of directors shall retire not later than on the day on which the first General Meeting is held following lapse of four years since his appointment. A retiring member of our board of directors may be re-appointed. non-executive directors may be appointed for no more than three four-year terms.
The General Meeting has the authority to suspend or remove members of our board of directors at any time, with or without cause, by means of a resolution passed by a simple majority of the votes cast. Executive directors may also be suspended by our board of directors. A suspension by our board of directors may be discontinued by the General Meeting at any time. Any suspension may be extended one or more times but may not last longer than three months in the aggregate.
Delaware. The Delaware General Corporation Law generally provides for a one-year term for directors, but permits directorships to be divided into up to three classes with up to three-year terms, with the years for each class expiring in different years, if permitted by the certificate of incorporation, an initial bylaw or a bylaw adopted by the stockholders. A director elected to serve a term on a "classified" board may not be removed by stockholders without cause. There is no limit in the number of terms a director may serve, unless stated otherwise in the certificate of incorporation or bylaws.
Board Member Vacancies
The Netherlands. Under Dutch law, the General Meeting appoints the members of our board of directors. For each seat on our board of directors to be filled, our board of directors shall make one or more proposals. A resolution to appoint a member of our board of directors nominated by our board of directors may be adopted by a simple majority of the votes cast. A nomination for appointment of an executive director must state the candidate's age and the positions he or she holds, or has held, insofar as these are relevant for the performance of the duties of a member of our board of directors. The nomination must state the reasons for the nomination of the relevant person. A nomination for appointment of a non-executive director must state the candidate's age, his or her profession, the number of shares he or she holds and the positions he or she holds, or has held, insofar as these are relevant for the performance of the duties of a member of our board of directors. Furthermore, the names of the legal entities of which he or she is already a supervisory board member or a non-executive member of the board shall be indicated; if those include legal entities which belong to the same group, a reference to that group will be sufficient. The nomination must state the reasons for the nomination of the relevant person.
A resolution of the General Meeting to appoint a member of our board of directors other than in accordance with a nomination of the board of directors shall require a majority of at least two-thirds of the votes cast if less than one-half of the company's issued capital is represented at the meeting.
188
Delaware. The Delaware General Corporation Law provides that vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) unless (i) otherwise provided in the certificate of incorporation or bylaws of the corporation or (ii) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy.
Conflict-of-Interest Transactions
The Netherlands. The non-executive directors shall decide, without the director concerned being present, whether there is a conflict of interest. A conflict of interest in relation to a director in any event exists if we intend to enter into a transaction with a legal entity (i) in which such director personally has a material financial interest, (ii) which has an executive director or a member of the management board who is related under family law to such director or (iii) in which such director has an executive or non-executive position. An executive director shall not participate in any discussions and decision making if he has a conflict of interest in the matter being discussed. If for this reason no resolution can be taken by the executive directors, the non-executive directors will resolve on the matter. A non-executive director shall not participate in any discussions and decision making if he has a conflict of interest in the matter being discussed. If for this reason no resolution can be taken by the non-executive directors or our board of directors as a whole, a General Meeting will resolve on the matter. A director shall not participate in any discussions and decision making if he has a conflict of interest in the matter being discussed. If for this reason no resolution can be taken by our board of directors a whole, a General Meeting will resolve on the matter. All transactions in which there are conflicts of interest with directors shall be agreed on terms that are customary in the sector concerned. Decisions to enter into transactions in which there are conflicts of interest with directors that are of material significance to us or to the relevant director require the approval of the non-executive directors. All transactions between us and legal or natural persons who hold at least one tenth of our shares shall be agreed on terms that are customary in the sector in which we and our combined businesses are active. The non-executive directors are required to approve such transactions that are of a material significance to us or to such persons.
Delaware. The Delaware General Corporation Law generally permits transactions involving a Delaware corporation and an interested director of that corporation if:
Proxy Voting by Board Members
The Netherlands. A non-executive director may issue a proxy for a specific board meeting but only to another non-executive directors in writing. An executive director may issue a proxy for a specific board meeting but only to another executive directors in writing.
Delaware. A director of a Delaware corporation may not issue a proxy representing the director's voting rights as a director.
189
Shareholder Rights
Voting Rights
The Netherlands. In accordance with Dutch law and our Articles of Association, each issued ordinary share confers the right to cast one vote at the General Meeting. Each holder of ordinary shares may cast as many votes as it holds shares. Shares that are held by us or our direct or indirect subsidiaries do not confer the right to vote.
Shareholders may exercise their rights at a General Meeting if they are the holders of our shares on the record date as required by Dutch law, which is currently the 28th day before the day of the General Meeting, and they or their proxy have notified us of their intention to attend the General Meeting in writing or by any other electronic means that can be reproduced on paper ultimately at a date set for that purpose by our board of directors (which date was for the previous General Meetings set on the seventh day prior to the relevant General Meeting), specifying such person's name and the number of shares for which such person may exercise the voting rights and/or meeting rights at such General Meeting. The record date and the manner in which shareholders can register and exercise their rights will be set out in the notice of the meeting.
Delaware. Under the Delaware General Corporation Law, each stockholder is entitled to one vote per share of stock, unless the certificate of incorporation provides otherwise. In addition, the certificate of incorporation may provide for cumulative voting at all elections of directors of the corporation, or at elections held under specified circumstances. Either the certificate of incorporation or the bylaws may specify the number of shares or the amount of other securities that must be represented at a meeting in order to constitute a quorum, but in no event will a quorum consist of less than one third of the shares entitled to vote at a meeting.
Stockholders as of the record date for the meeting are entitled to vote at the meeting, and the board of directors may fix a record date that is no more than 60 nor less than 10 days before the date of the meeting, and if no record date is set then the record date is the close of business on the day next preceding the day on which notice is given, or if notice is waived then the record date is the close of business on the day next preceding the day on which the meeting is held. The determination of the stockholders of record entitled to notice or to vote at a meeting of stockholders shall apply to any adjournment of the meeting, but the board of directors may fix a new record date for the adjourned meeting.
Shareholder Proposals
The Netherlands. Pursuant to our Articles of Association, extraordinary General Meetings will be held whenever our board of directors deems such to be necessary. Pursuant to Dutch law, one or more shareholders, who jointly represent at least one-tenth of the issued capital may request our board of directors to convene a General Meeting. If our board of directors has not taken the steps necessary to ensure that a General Meeting could be held within the relevant statutory period after the request, the requesting persons may, at his/her/their request, be authorized by Court in preliminary relief proceedings to convene a General Meeting. The court shall disallow the application if it does not appear that the applicants have previously requested our board of directors to convene a General Meeting and our board of directors has not taken the necessary steps so that the General Meeting could be held within six weeks after the request.
Also, the agenda for a General Meeting shall include such items requested by one or more shareholders, and others entitled to attend General Meetings, representing at least 3% of the issued share capital, except where the articles of association state a lower percentage. Our Articles of Association do not state such lower percentage. Requests must be made in writing and received by our board of directors at least 60 days before the day of the convocation of the meeting. In
190
accordance with the DCGC, a shareholder shall exercise the right of putting an item on the agenda only after consulting our board of directors in that respect. If one or more shareholders intends to request that an item be put on the agenda that may result in a change in the company's strategy, our board of directors may invoke a response time of a maximum of 180 days until the day of the General Meeting.
Delaware. Delaware law does not specifically grant stockholders the right to bring business before an annual or special meeting. However, if a Delaware corporation is subject to the SEC's proxy rules, a stockholder who owns at least $2,000 in market value, or 1% of the corporation's securities entitled to vote, may propose a matter for a vote at an annual or special meeting in accordance with those rules.
Action by Written Consent
The Netherlands. Our Articles of Association do not provide for the possibility that shareholders' resolutions can also be adopted in writing without holding a meeting of shareholders. Although permitted by Dutch law, for a listed company, this method of adopting resolutions is not feasible.
Delaware. Although permitted by Delaware law, publicly listed companies do not typically permit stockholders of a corporation to take action by written consent.
Appraisal Rights
The Netherlands. The concept of appraisal rights is not known as such under Dutch law.
However, pursuant to Dutch law a shareholder who for his own account contributes at least 95% of our issued share capital may initiate proceedings against our minority shareholders jointly for the transfer of their shares to the claimant. The proceedings are held before the Enterprise Chamber. The Enterprise Chamber may grant the claim for squeeze out in relation to all minority shareholders and will determine the price to be paid for the shares, if necessary after appointment of one or three experts who will offer an opinion to the Enterprise Chamber on the value to be paid for the shares of the minority shareholders. Once the order to transfer becomes final before the Enterprise Chamber, the person acquiring the shares shall give written notice of the date and place of payment and the price to the holders of the shares to be acquired whose addresses are known to him. Unless the addresses of all of them are known to the acquiring person, such person is required to publish the same in a daily newspaper with a national circulation.
Furthermore, in accordance with the Directive 2005/56/EC of the European Parliament and the Council of October 26, 2005 on cross-border mergers of limited liability companies, Dutch law provides that, to the extent that the acquiring company in a cross-border merger is organized under the laws of another EU member state, a shareholder of a Dutch disappearing company who has voted against the cross-border merger may file a claim with the Dutch company for compensation. Such compensation to be determined by one or more independent experts. The shares of such shareholder that are subject to such claim will cease to exist as of the moment of entry into effect of the cross-border merger.
Payment by the acquiring company is only possible if the resolution to approve the cross-border merger by the corporate body of the other company or companies involved in the cross-border merger includes the acceptance of the rights of the shareholders of the Dutch company to oppose the cross-border merger.
191
Delaware. The Delaware General Corporation Law provides for stockholder appraisal rights, or the right to demand payment in cash of the judicially determined fair value of the stockholder's shares, in connection with certain mergers and consolidations.
Shareholder Suits
The Netherlands. In the event a third party is liable to a Dutch company, only the company itself can bring a civil action against that party. The individual shareholders do not have the right to bring an action on behalf of the company. Only in case cause for the liability of a third party to the company also constitutes a tortious act directly against a shareholder such shareholder has an individual right of action against such third party in its own name. The Dutch Civil Code provides for the possibility to initiate such actions collectively. A foundation or an association whose objective is to protect the rights of a group of persons having similar interests can institute a collective action. The collective action itself cannot result in an order for payment of monetary damages but may only result in a declaratory judgment (verklaring voor recht). In order to obtain compensation for damages, the foundation or association and the defendant may reach—often on the basis of such declaratory judgment—a settlement. A Dutch court may declare the settlement agreement binding upon all the injured parties with an opt-out choice for an individual injured party. An individual injured party may also itself institute a civil claim for damages.
Delaware. Under the Delaware General Corporation Law, a stockholder may bring a derivative action on behalf of the corporation to enforce the rights of the corporation. An individual also may commence a class action suit on behalf of himself and other similarly situated stockholders where the requirements for maintaining a class action under Delaware law have been met. A person may institute and maintain such a suit only if that person was a stockholder at the time of the transaction which is the subject of the suit. In addition, under Delaware case law, the plaintiff normally must be a stockholder at the time of the transaction that is the subject of the suit and throughout the duration of the derivative suit. Delaware law also requires that the derivative plaintiff make a demand on the directors of the corporation to assert the corporate claim before the suit may be prosecuted by the derivative plaintiff in court, unless such a demand would be futile.
Repurchase of Shares
The Netherlands. Under Dutch law, a company such as ours may not subscribe for newly issued shares in its own capital. Such company may, however, subject to certain restrictions of Dutch law and its Articles of Association, acquire shares in its own capital. We may acquire fully paid shares in our own capital at any time for no valuable consideration. Furthermore, we may repurchase fully paid shares in our own capital if (i) such repurchase would not cause our shareholders' equity to fall below an amount equal to the sum of the paid-up and called-up part of the issued share capital and the reserves we are required to maintain pursuant to applicable law, (ii) we (including our subsidiaries) would thereafter not hold shares or hold a pledge over shares with an aggregate nominal value exceeding 50% of our issued share capital and (iii) our board of directors has been authorized thereto by the General Meeting.
An authorization by the General Meeting to our board of directors for the repurchase of shares can be granted for a maximum period of 18 months. Such authorization must specify the number and class of shares that may be acquired, the manner in which these shares may be acquired and the price range within which the shares may be acquired.
No authorization of the General Meeting is required if ordinary shares are acquired by us with the intention of transferring such ordinary shares to our employees under an applicable employee stock purchase plan.
192
Delaware. Under the Delaware General Corporation Law, a corporation may purchase or redeem its own shares unless the capital of the corporation is impaired or the purchase or redemption would cause an impairment of the capital of the corporation. A Delaware corporation may, however, purchase or redeem out of capital any of its preferred shares or, if no preferred shares are outstanding, any of its own shares if such shares will be retired upon acquisition and the capital of the corporation will be reduced in accordance with specified limitations.
Anti-takeover Provisions
The Netherlands. Under Dutch law, various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law. We have adopted several provisions that may have the effect of making a takeover of our company more difficult or less attractive, including requirements that certain matters, including an amendment of our Articles of Association, may only be brought to our shareholders for a vote upon a proposal by our board of directors.
Delaware. In addition to other aspects of Delaware law governing fiduciary duties of directors during a potential takeover, the Delaware General Corporation Law also contains a business combination statute that protects Delaware companies from hostile takeovers and from actions following the takeover by prohibiting some transactions once an acquirer has gained a significant holding in the corporation.
Section 203 of the Delaware General Corporation Law prohibits "business combinations," including mergers, sales and leases of assets, issuances of securities and similar transactions by a corporation or a subsidiary with an interested stockholder that beneficially owns 15% or more of a corporation's voting stock, within three years after the person becomes an interested stockholder, unless:
A Delaware corporation may elect not to be governed by Section 203 by a provision contained in the original certificate of incorporation of the corporation or an amendment to the original certificate of incorporation or to the bylaws of the company, which amendment must be approved by a majority of the shares entitled to vote and may not be further amended by the board of directors of the corporation. Such an amendment is not effective until twelve months following its adoption.
Inspection of Books and Records
The Netherlands. The board of directors provides the General Meeting in good time with all information that the shareholders require for the exercise of their powers, unless this would be contrary to an overriding interest of us. If the board of directors invokes an overriding interest, it must give reasons.
Delaware. Under the Delaware General Corporation Law, any stockholder may inspect for any proper purpose certain of the corporation's books and records during the corporation's usual hours of business.
193
Removal of Board Member
The Netherlands. A General Meeting has the authority to suspend or remove members of our board of directors at any time, with or without cause, by means of a resolution passed by a simple majority of the votes cast. Executive directors may also be suspended by our board of directors. A suspension by our board of directors may be discontinued by a General Meeting at any time. Any suspension may be extended one or more times but may not last longer than three months in the aggregate.
Delaware. Under the Delaware General Corporation Law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (i) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board is classified, stockholders may effect such removal only for cause or (ii) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part.
Preemptive Rights
The Netherlands. Under Dutch law, in the event of an issuance of ordinary shares or upon a grant of rights to subscribe for ordinary shares, each shareholder will have a pro rata preemptive right in proportion to the aggregate nominal value of the ordinary shares held by such holder (with the exception of ordinary shares to be issued to employees or ordinary shares issued against a contribution other than in cash or the issue of shares to persons exercising a previously granted right to subscribe for shares). A shareholder may exercise preemptive rights during a period of at least two weeks from the date of the announcement of the issue of shares. Under our Articles of Association, the preemptive rights in respect of newly issued ordinary shares may be restricted or excluded by a resolution of the General Meeting upon proposal of our board of directors with the consent of the majority of the non-executive directors.
Our board of directors, with the consent of the majority of the non-executive directors, may restrict or exclude the preemptive rights in respect of newly issued ordinary shares if it has been designated as the authorized body to do so by the General Meeting. Such designation can be granted for a period not exceeding five years. A resolution of the General Meeting to restrict or exclude the preemptive rights or to designate our board of directions as the authorized body to do so requires a two-thirds majority of the votes cast, if less than one half of our issued share capital is represented at the meeting.
Delaware. Under the Delaware General Corporation Law, stockholders have no preemptive rights to subscribe for additional issues of stock or to any security convertible into such stock unless, and to the extent that, such rights are expressly provided for in the certificate of incorporation.
Dividends
The Netherlands. Pursuant to Dutch law and the Articles of Association, the distribution of profits will take place following the adoption of our annual accounts, from which we will determine whether such distribution is permitted. We may only make distributions to the shareholders, whether from profits or from its freely distributable reserves, only insofar as its shareholders' equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law.
194
Our board of directors, with the consent of the majority of the non-executive directors, may determine which part of our profits will be added to the reserves in consideration of our reserves and dividends policy. The remaining part of the profits after the addition to the reserves will be at the disposal of the General Meeting. Distributions of dividends will be made pro rata to the nominal value of each share.
Subject to Dutch law and the Articles of Association, our board of directors, with the consent of the majority of the non-executive directors, may resolve to distribute an interim dividend if it determines such interim dividend to be justified by our profits. For this purpose, our board of directors must prepare an interim statement of assets and liabilities. Such interim statement shall show our financial position not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. An interim dividend can only be paid if (a) an interim statement of assets and liabilities is drawn up showing that the funds available for distribution are sufficient, and (b) our shareholders' equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law.
Our board of directors, with the consent of the majority of the non-executive directors, may resolve that we make distributions to shareholders from one or more of its freely distributable reserves, other than by way of profit distribution, subject to the due observance of our policy on reserves and dividends. Any such distributions will be made pro rata to the nominal value of each share.
Dividends and other distributions shall be made payable not later than the date determined by our board of directors. Claims to dividends and other distribution not made within five years from the date that such dividends or distributions became payable, will lapse and any such amounts will be considered to have been forfeited to us (verjaring).
Delaware. Under the Delaware General Corporation Law, a Delaware corporation may pay dividends out of its surplus (the excess of net assets over capital), or in case there is no surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year (provided that the amount of the capital of the corporation is not less than the aggregate amount of the capital represented by the issued and outstanding stock of all classes having a preference upon the distribution of assets). In determining the amount of surplus of a Delaware corporation, the assets of the corporation, including stock of subsidiaries owned by the corporation, must be valued at their fair market value as determined by the board of directors, without regard to their historical book value. Dividends may be paid in the form of ordinary shares, property or cash.
Shareholder Vote on Certain Reorganizations
The Netherlands. Under Dutch law, the General Meeting must approve resolutions of our board of directors relating to a significant change in the identity or the character of the company or the business of the company, which includes:
195
statement of financial position and explanatory notes in the last adopted annual accounts of the company.
Under Dutch law, a shareholder who, for its own account, owns shares representing at least 95% of the nominal value of a company's issued share capital may institute proceedings against the company's other shareholders jointly for the transfer of their shares to that shareholder. The proceedings are held before the Enterprise Chamber, which may grant the claim for squeeze-out in relation to all minority shareholders and will determine the price to be paid for the shares, if necessary after appointment of experts who will offer an opinion to the Enterprise Chamber on the value of the shares.
Delaware. Under the Delaware General Corporation Law, the vote of a majority of the outstanding shares of capital stock entitled to vote thereon generally is necessary to approve a merger or consolidation or the sale of all or substantially all of the assets of a corporation. The Delaware General Corporation Law permits a corporation to include in its certificate of incorporation a provision requiring for any corporate action the vote of a larger portion of the stock or of any class or series of stock than would otherwise be required.
Under the Delaware General Corporation Law, no vote of the stockholders of a surviving corporation to a merger is needed, however, unless required by the certificate of incorporation, if (i) the agreement of merger does not amend in any respect the certificate of incorporation of the surviving corporation, (ii) the shares of stock of the surviving corporation are not changed in the merger and (iii) the number of shares of common stock of the surviving corporation into which any other shares, securities or obligations to be issued in the merger may be converted does not exceed 20% of the surviving corporation's common stock outstanding immediately prior to the effective date of the merger. In addition, stockholders may not be entitled to vote in certain mergers with other corporations that own 90% or more of the outstanding shares of each class of stock of such corporation, but the stockholders will be entitled to appraisal rights.
Remuneration of Board Members
The Netherlands. Under Dutch law and our Articles of Association, we must adopt a remuneration policy for our board members. Such remuneration policy shall be adopted by the General Meeting upon the proposal of the non-executive directors. The remuneration of the individual members of the board of directors shall be determined by the non-executive directors, at the recommendation of the remunerations and nominations committee, within the limits of the remuneration policy adopted by the General Meeting. With respect to remuneration schemes in the form of shares or rights to shares is submitted by the board to the General Meeting for its approval. This proposal must set out at least the maximum number of shares or rights to shares to be granted to our board of directors and the criteria for granting or amendment.
Delaware. Under the Delaware General Corporation Law, the stockholders do not generally have the right to approve the compensation policy for directors or the senior management of the corporation, although certain aspects of executive compensation may be subject to stockholder vote due to the provisions of U.S. federal securities and tax law, as well as exchange requirements.
Dutch Corporate Governance Code
As a Dutch company we are subject to the DCGC. On December 8, 2016, the monitoring committee for the DCGC has published the revised DCGC, which is in force as of the financial year starting on or after January 1, 2017 and replaces the DCGC dated December 10, 2008 which was in force as of January 1, 2009 until December 31, 2016. It is expected that the revised DCGC will be enshrined in Dutch law by the Dutch legislator in the first half of 2017.
196
The DCGC contains both principles and best practice provisions for management boards, supervisory boards, shareholders and general meetings of shareholders, financial reporting, auditors, disclosure, compliance and enforcement standards. A copy of the DCGC can be found onwww.corpgov.nl. As a Dutch company, we are subject to the DCGC and are required to disclose in our annual report, filed in the Netherlands, whether we comply with the provisions of the DCGC. If we do not comply with the provisions of the DCGC (for example, because of a conflicting NASDAQ requirement or otherwise), we must list the reasons for any deviation from the DCGC in our annual report.
We acknowledge the importance of good corporate governance. However, at this stage, we do not comply with all the provisions of the DCGC, to a large extent because such provisions conflict with or are inconsistent with the corporate governance rules of NASDAQ and U.S. securities laws that apply to us, or because such provisions do not reflect best practices of global companies listed on NASDAQ.
The discussions below summarize the most important differences between our expected governance structure following this offering and the principles and best practices of the DCGC that has come into force as of the financial year starting on or after January 1, 2017:
197
Listing
We intend to apply to list our ordinary shares on The NASDAQ Global Select Market under the symbol "ARGX."
Transfer Agent and Registrar
The U.S. transfer agent and registrar for the ordinary shares is American Stock Transfer & Trust Company, LLC.
198
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, no public market existed in the United States for our ordinary shares. Future sales of ordinary shares in the public market after this offering, and the availability of ordinary shares for future sale, could adversely affect the market price of our ordinary shares prevailing from time to time. As described below, most of our currently outstanding ordinary shares will be available for sale immediately after this offering, and the remainder will be available for sale 90 days after the expiration of contractual restrictions on transfers of ordinary shares. Accordingly, sales of substantial amounts of the ordinary shares, or the perception that these sales could occur, could adversely affect prevailing market prices for our ordinary shares and could impair our future ability to raise equity capital.
Based on the number of ordinary shares outstanding as of , 2017, upon completion of this offering, ordinary shares will be outstanding, assuming no outstanding options are exercised. All of the ordinary shares sold in this offering will be freely transferable without restriction or further registration under the Securities Act, except for any ordinary shares sold to our "affiliates." In addition, all of our ordinary shares outstanding before this offering will be freely transferable and may be resold without restriction or further registration under the Securities Act. Under Rule 144 of the Securities Act, an "affiliate" of a company is a person that directly or indirectly controls, is controlled by or is under common control with that company. Affiliates may sell only the volume of shares described below and their sales are subject to additional restrictions described below.
Rule 144
In general, persons who have beneficially owned restricted ordinary shares for at least six months, and any affiliate of our company who owns either restricted or unrestricted ordinary shares, are entitled to sell their securities without registration with the SEC under an exemption from registration provided by Rule 144 under the Securities Act. A non-affiliated person who has beneficially owned restricted securities within the meaning of Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
In general, a person who has beneficially owned restricted ordinary shares for at least six months would be entitled to sell their securities pursuant to Rule 144 under the Securities Act provided that (1) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (2) we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sale by non-affiliates must also comply with the current public information provision of Rule 144. Persons who have beneficially owned restricted ordinary shares for at least six months, but who are our affiliates at the time of, or at any time during the 90 days preceding a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
199
provided, in each case, that we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sales by affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus-delivery requirements of the Securities Act. Accordingly, ordinary shares held by our affiliates may be sold in offshore transactions in compliance with Regulation S.
Lock-Up Agreements
We, the members of our board of directors and certain key employees have agreed that, without the prior written consent of Cowen and Company, LLC and Piper Jaffray & Co., we and they will not, subject to limited exceptions, during the period ending 90 days after the date of this prospectus, directly or indirectly, offer, pledge, sell, contract to sell, pledge or otherwise dispose of any ordinary shares or other shares of our capital stock or any securities convertible into, exerciseable or exchangeable for such capital stock. See "Underwriting" for additional information.
Cowen and Company, LLC and Piper Jaffray & Co. on behalf of the underwriters will have discretion in determining if, and when, to release any shares subject to lock-up agreements.
200
MATERIAL UNITED STATES INCOME TAX AND DUTCH TAX CONSIDERATIONS
The information presented under the caption "Certain Material U.S. Federal Income Tax Considerations to U.S. Holders" below is a discussion of certain material U.S. federal income tax considerations to a U.S. holder (as defined below) of investing in our ordinary shares. The information presented under the caption "Dutch Tax Consequences" is a discussion of the material Dutch tax consequences of investing in our ordinary shares.
You should consult your tax advisor regarding the applicable tax consequences to you of investing in our ordinary shares under the laws of the United States (federal, state and local), the Netherlands and any other applicable foreign jurisdiction.
Certain Material U.S. Federal Income Tax Considerations to U.S. Holders
The following is a summary of certain material U.S. federal income tax considerations relating to the acquisition, ownership and disposition of our ordinary shares by a U.S. holder (as defined below). This summary addresses only the U.S. federal income tax considerations for U.S. holders that are initial purchasers of our ordinary shares pursuant to this offering and that will hold such ordinary shares as capital assets for U.S. federal income tax purposes. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder. This summary does not address tax considerations applicable to a holder of our ordinary shares that may be subject to special tax rules including, without limitation, the following:
Further, this summary does not address the U.S. federal estate, gift, or alternative minimum tax considerations, or any U.S. state, local, or non-U.S. tax considerations of the acquisition, ownership and disposition of our ordinary shares.
This description is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code; existing, proposed and temporary U.S. Treasury Regulations promulgated thereunder, administrative and judicial interpretations thereof; and the income tax treaty between the Netherlands and the United States in each case as in effect and available on the date hereof. All the foregoing is
201
subject to change, which change could apply retroactively, and to differing interpretations, all of which could affect the tax considerations described below. There can be no assurances that the U.S. Internal Revenue Service, or the IRS, will not take a contrary or different position concerning the tax consequences of the acquisition, ownership and disposition of our ordinary shares or that such a position would not be sustained. Holders should consult their own tax advisors concerning the U.S. federal, state, local and non-U.S. tax consequences of owning and disposing of our ordinary shares in their particular circumstances.
For the purposes of this summary, a "U.S. holder" is a beneficial owner of our ordinary shares that is (or is treated as), for U.S. federal income tax purposes:
If a partnership (or any other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds our ordinary shares, the U.S. federal income tax consequences relating to an investment in those ordinary shares will depend in part upon the status of the partner and the activities of the partnership. Such a partner or partnership should consult its tax advisor regarding the U.S. federal income tax considerations of owning and disposing of our ordinary shares in its particular circumstances.
In general, a U.S. holder who owns our ordinary shares will be treated as the beneficial owner of the underlying shares represented by those ordinary shares for U.S. federal income tax purposes. Accordingly, no gain or loss will generally be recognized if a U.S. holder exchanges our ordinary shares for the underlying shares represented by those ordinary shares.
As indicated below, this discussion is subject to U.S. federal income tax rules applicable to a "passive foreign investment company," or a PFIC.
Persons considering an investment in our ordinary shares should consult their own tax advisors as to the particular tax consequences applicable to them relating to the acquisition, ownership and disposition of our ordinary shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Distributions. Although we do not currently plan to pay dividends, and subject to the discussion under "—Passive Foreign Investment Company Considerations" below, the gross amount of any distribution (before reduction for any amounts withheld in respect of Dutch withholding tax) actually or constructively received by a U.S. holder with respect to our ordinary shares will be taxable to the U.S. holder as a dividend to the extent of the U.S. holder's pro rata share of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of earnings and profits will be non-taxable to the U.S. holder to the extent of, and will be applied against and reduce, the U.S. holder's adjusted tax basis in our ordinary shares. Distributions in excess of earnings and profits and such adjusted tax basis will generally be
202
taxable to the U.S. holder as either long-term or short-term capital gain depending upon whether the U.S. holder has held our ordinary shares for more than one year as of the time such distribution is received. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. Non-corporate U.S. holders may qualify for the preferential rates of taxation with respect to dividends on our ordinary shares applicable to long-term capital gains (i.e., gains from the sale of capital assets held for more than one year) applicable to qualified dividend income (as discussed below) if we are a "qualified foreign corporation" and certain other requirements (discussed below) are met. A non-U.S. corporation (other than a corporation that is a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation with respect to any dividend it pays on shares which are readily tradable on an established securities market in the United States. We intend to list our ordinary shares on The NASDAQ Global Select Market, or NASDAQ, which is an established securities market in the United States, and we expect our ordinary shares to be readily tradable on NASDAQ. However, there can be no assurance that our ordinary shares will be considered readily tradable on an established securities market in the United States in later years. Therefore, subject to the discussion under "—Passive Foreign Investment Company Considerations" below, such dividends will generally be "qualified dividend income" in the hands of non-corporate U.S. holders, provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are met. The dividends will not be eligible for the dividends-received deduction generally allowed to corporate U.S. holders.
A U.S. holder generally may claim the amount of any Dutch withholding tax as either a deduction from gross income or a credit against U.S. federal income tax liability. However, the foreign tax credit is subject to numerous complex limitations that must be determined and applied on an individual basis. Generally, the credit cannot exceed the proportionate share of a U.S. holder's U.S. federal income tax liability that such U.S. holder's taxable income bears to such U.S. holder's worldwide taxable income. In applying this limitation, a U.S. holder's various items of income and deduction must be classified, under complex rules, as either "foreign source" or "U.S. source." In addition, this limitation is calculated separately with respect to specific categories of income. The amount of a distribution with respect to our ordinary shares that is treated as a "dividend" may be lower for U.S. federal income tax purposes than it is for Dutch income tax purposes, potentially resulting in a reduced foreign tax credit for the U.S. holder. Furthermore, Dutch income taxes withheld in excess of the rate applicable under the income tax treaty between the Netherlands and the United States will not be eligible for credit against U.S. holders' federal income tax liability. Each U.S. holder should consult its own tax advisors regarding the foreign tax credit rules.
Sale, Exchange or Other Taxable Disposition of our Ordinary Shares. A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale, exchange or other taxable disposition of our ordinary shares in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. holder's tax basis for those ordinary shares. Subject to the discussion under "—Passive Foreign Investment Company Considerations" below, this gain or loss will generally be a capital gain or loss. The adjusted tax basis in our ordinary shares generally will be equal to the cost of such ordinary shares. Capital gain from the sale, exchange or other taxable disposition of our ordinary shares of a non-corporate U.S. holder is generally eligible for a preferential rate of taxation applicable to capital gains, if the non-corporate U.S. holder's holding period determined at the time of such sale, exchange or other taxable disposition for such ordinary shares exceeds one year (i.e., such gain is long-term taxable gain). The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations.
203
Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
Medicare Tax. Certain U.S. holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their "net investment income," which may include all or a portion of their dividend income and net gains from the disposition of our ordinary shares. Each U.S. holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in our ordinary shares.
Passive Foreign Investment Company Considerations. If we are a PFIC for any taxable year, a U.S. holder would be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
A corporation organized outside the United States generally will be a PFIC for U.S. federal income tax purposes for any taxable year in which either: (i) at least 75% of its gross income is "passive income" or (ii) at least 50% of the average quarterly value of its total gross assets (for which purpose the total value of our assets may be determined in part by reference to the market value of our ordinary shares, which is subject to change) is attributable to assets that produce "passive income" or are held for the production of "passive income."
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income, and includes amounts derived by reason of the temporary investment of cash, including the funds raised in offerings of our ordinary shares. If a non-U.S. corporation owns directly or indirectly at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation's income for purposes of the PFIC tests. If we are a PFIC for any year with respect to which a U.S. holder owns our ordinary shares, we will continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns our ordinary shares, regardless of whether we continue to meet the tests described above.
Whether we are a PFIC for any taxable year will depend on the composition of our income and the projected composition and estimated fair market values of our assets in each year, and because this is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC for any taxable year. The market value of our assets may be determined in large part by reference to the market price of our ordinary shares, which is likely to fluctuate after this offering. Based on the foregoing, we do not anticipate that we will be a PFIC for the current taxable year based upon the expected value of our assets, including any goodwill, and the expected composition of our income and assets, however, as previously mentioned, we cannot provide any assurances regarding our PFIC status for the current or any prior or future taxable years.
If we are a PFIC, for any taxable year, then unless you make one of the elections described below, a special tax regime will apply to both (a) any "excess distribution" by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for our ordinary shares) and (b) any gain realized on the sale or other disposition of our ordinary shares. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the amount deemed realized in each year had been subject to tax in each year of
204
that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. holder's regular ordinary income rate for the current year and would not be subject to the interest charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. In addition, dividend distributions made to you will not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under "—Distributions."
Certain elections exist that would result in an alternative treatment (such as mark-to-market treatment) of our ordinary shares. If a U.S. holder makes the mark-to- market election, the U.S. holder generally will recognize as ordinary income any excess of the fair market value of our ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of our ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. holder makes the election, the U.S. holder's tax basis in our ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of our ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). The mark-to- market election is available only if we are a PFIC and our ordinary shares are "regularly traded" on a "qualified exchange." Our ordinary shares will be treated as "regularly traded" in any calendar year in which more than a de minimis quantity of our ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter (subject to the rule that trades that have as one of their principal purposes the meeting of the trading requirement as disregarded). NASDAQ is a qualified exchange for this purpose and, consequently, if our ordinary shares are regularly traded, the mark- to-market election will generally be available to a U.S. holder.
If we are a PFIC for any year during which a U.S. holder holds our ordinary shares, we must generally continue to be treated as a PFIC by that U.S. holder for all succeeding years during which the U.S. holder holds our ordinary shares, unless we cease to meet the requirements for PFIC status and the U.S. holder makes a "deemed sale" election with respect to our ordinary shares. If such election is made, the U.S. holder will be deemed to have sold our ordinary shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from such deemed sale would be subject to the consequences applicable to sales of PFIC shares described above. After the deemed sale election, the U.S. holder's ordinary shares with respect to which the deemed sale election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC.
The tax consequences that would apply if we were a PFIC would also be different from those described above if a U.S. holder were able to make a valid "qualified electing fund," or QEF, election. However, we do not currently intend to provide the information necessary for U.S. holders to make a QEF election if we were treated as a PFIC for any taxable year and prospective investors should assume that a QEF election will not be available. U.S. holders should consult their tax advisors to determine whether any of these above elections would be available and if so, what the consequences of the alternative treatments would be in their particular circumstances.
If we are determined to be a PFIC, the general tax treatment for U.S. holders described in this section would apply to indirect distributions and gains deemed to be realized by U.S. holders in respect of any of our subsidiaries that also may be determined to be PFICs.
205
If a U.S. holder owns our ordinary shares during any taxable year in which we are a PFIC, the U.S. holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company, generally with the U.S. holder's federal income tax return for that year. If our company were a PFIC for a given taxable year, then you should consult your tax advisor concerning your annual filing requirements.
The U.S. federal income tax rules relating to PFICs are complex. Prospective U.S. investors are urged to consult their own tax advisors with respect to the acquisition, ownership and disposition of our ordinary shares, the consequences to them of an investment in a PFIC, any elections available with respect to our ordinary shares and the IRS information reporting obligations with respect to the acquisition, ownership and disposition of our ordinary shares.
Backup Withholding and Information Reporting. U.S. holders generally will be subject to information reporting requirements with respect to dividends on our ordinary shares and on the proceeds from the sale, exchange or disposition of our ordinary shares that are paid within the United States or through U.S.-related financial intermediaries, unless the U.S. holder is an "exempt recipient." In addition, U.S. holders may be subject to backup withholding on such payments, unless the U.S. holder provides a correct taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. holder's U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting. Certain U.S. holders who are individuals and certain entities controlled by individuals may be required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for shares held in accounts maintained by U.S. financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their federal income tax return. U.S. holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their acquisition, ownership and disposition of our ordinary shares.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN OUR ORDINARY SHARES IN LIGHT OF THE INVESTOR'S OWN CIRCUMSTANCES.
Dutch Tax Consequences
The following summary outlines certain Dutch tax consequences in connection with the acquisition, ownership and disposal of our ordinary shares. All references in this summary to the Netherlands and Dutch law are to the European part of the Kingdom of the Netherlands and its law, respectively, only. The summary does not purport to present any comprehensive or complete picture of all Dutch tax aspects that could be of relevance to the acquisition, ownership and disposal of our ordinary shares by a (prospective) holder of our ordinary shares who may be subject to special tax treatment under applicable law. The summary is based on the tax laws and practice of the Netherlands as in effect on the date of this prospectus, which are subject to changes that could prospectively or retrospectively affect the Dutch tax consequences.
For purposes of Dutch income and corporate income tax, shares legally owned by a third party such as a trustee, foundation or similar entity or arrangement, or a Third Party, may under certain
206
circumstances have to be allocated to the (deemed) settlor, grantor or similar originator, or the Settlor, or, upon the death of the Settlor, his/her beneficiaries, or the Beneficiaries, in proportion to their entitlement to the estate of the Settlor of such trust or similar arrangement, or the Separated Private Assets.
The summary does not address the tax consequences of a holder of our ordinary shares who is an individual and who has a substantial interest (aanmerkelijk belang) in the company. Generally, a holder of our ordinary shares will have a substantial interest in the company if such holder of our ordinary shares, whether alone or together with his spouse or partner and/or certain other close relatives, holds directly or indirectly, or as Settlor or Beneficiary of Separated Private Assets (i) (x) the ownership of, (y) certain other rights, such as usufruct, over, or (z) rights to acquire (whether or not already issued), shares representing 5% or more of the total issued and outstanding capital (or the issued and outstanding capital of any class of shares) of the company or (ii) (x) the ownership of, or (y) certain other rights, such as usufruct over, profit participating certificates (winstbewijzen) that relate to 5% or more of the annual profit of the company or to 5% or more of the liquidation proceeds of the company.
In addition, a holder of our ordinary shares has a substantial interest in the company if he, whether alone or together with his spouse or partner and/or certain other close relatives, has the ownership of, or other rights over, shares in, or profit certificates issued by, the company that represent less than 5% of the relevant aggregate that either (a) qualified as part of a substantial interest as set forth above and where shares, profit certificates and/or rights there over have been, or are deemed to have been, partially disposed of, or (b) have been acquired as part of a transaction that qualified for non-recognition of gain treatment.
This summary does not address the tax consequences of a holder of our ordinary shares who:
PROSPECTIVE HOLDERS OF OUR ORDINARY SHARES SHOULD CONSULT THEIR OWN PROFESSIONAL ADVISER WITH RESPECT TO THE TAX CONSEQUENCES OF ANY ACQUISITION, OWNERSHIP OR DISPOSAL OF OUR ORDINARY SHARES IN THEIR INDIVIDUAL CIRCUMSTANCES.
Dividend Withholding Tax
General
The company is generally required to withhold dividend withholding tax imposed by the Netherlands at a rate of 15% on dividends distributed by the company in respect of our ordinary shares. The expression "dividends distributed by the company" as used herein includes, but is not limited to:
207
Holders of Our Ordinary Shares Resident in the Netherlands
A holder of our ordinary shares that is resident or deemed to be resident in the Netherlands is generally entitled, subject to the anti-dividend stripping rules described below, to a full credit against its (corporate) income tax liability, or a full refund, of the Dutch dividend withholding tax. The same generally applies to holders of our ordinary shares that are neither resident nor deemed to be resident in the Netherlands if our ordinary shares are attributable to a permanent establishment in the Netherlands of such non-resident holder.
Holders of Our Ordinary Shares Resident Outside the Netherlands
A holder of our ordinary shares that is resident in a country with which the Netherlands has a double taxation convention in effect, may, depending on the terms of such double taxation convention and subject to the anti-dividend stripping rules described below, be eligible for a full or partial exemption from, or full or partial refund of, Dutch dividend withholding tax on dividends received.
A holder of our ordinary shares, that is a legal entity (a) resident in (i) a Member State of the European Union, or (ii) Iceland, Norway or Liechtenstein, and (b) that is in its state of residence under the terms of a double taxation agreement concluded with a third state, not considered to be resident for tax purposes outside the European Union, Iceland, Norway and Liechtenstein, is generally entitled, subject to the anti-dividend stripping rules described below, to a full exemption from Dutch dividend withholding tax on dividends received if it holds an interest of at least 5% (in shares or, in certain cases, in voting rights) in the company or if it holds an interest of less than 5%, in either case where, had the holder of our ordinary shares been a Dutch resident, it would have had the benefit of the participation exemption (this may include a situation where another related party holds an interest of 5% or more in the company).
A holder of our ordinary shares, that is an entity resident in (i) a Member State of the European Union, or (ii) Iceland, Norway or Liechtenstein, or (iii) in a jurisdiction which has an arrangement for the exchange of tax information with the Netherlands (and such holder as described under (iii) holds our ordinary shares as a portfolio investment,i.e. such holding is not acquired with a view to the establishment or maintenance of lasting and direct economic links between the holder of our ordinary shares and the company and does not allow the holder of our ordinary shares to participate effectively in the management or control of the company), which is exempt from tax in its country of residence, and that would have been exempt from Dutch corporate income tax if it had been a resident of the Netherlands, is generally entitled, subject to the anti-dividend stripping rules described below, to a full refund of Dutch dividend withholding tax on dividends received. This full refund will in general benefit certain foreign pension funds, government agencies and certain government controlled commercial entities.
According to the anti-dividend stripping rules, no exemption, reduction, credit or refund of Dutch dividend withholding tax will be granted if the recipient of the dividend paid by the company is not
208
considered the beneficial owner (uiteindelijk gerechtigde) of the dividend as defined in these rules. A recipient of a dividend is not considered the beneficial owner of the dividend if, as a consequence of a combination of transactions, (i) a person (other than the holder of the dividend coupon), directly or indirectly, partly or wholly benefits from the dividend, (ii) such person directly or indirectly retains or acquires a comparable interest in our ordinary shares, and (iii) such person is entitled to a less favourable exemption, refund or credit of dividend withholding tax than the recipient of the dividend distribution. The term "combination of transactions" includes transactions that have been entered into in the anonymity of a regulated stock market, the sole acquisition of one or more dividend coupons and the establishment of short-term rights or enjoyment on our ordinary shares (e.g., usufruct).
Holders of Our Ordinary Shares Resident in the U.S.
Dividends distributed by the company to U.S. resident holders of our ordinary shares that are eligible for benefits under the Convention between the Kingdom of the Netherlands and the United States of America for the avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes and Income, dated December 18, 1992 as amended by the protocol of March 8, 2004, or the U.S. Tax Treaty, generally will be entitled to a reduced dividend withholding tax rate of 5% in case of certain U.S. corporate shareholders owning at least 10% of the company's total voting power. Certain U.S. pension funds and tax-exempt organisations may qualify for a complete exemption from Dutch dividend withholding tax.
Under the U.S. Tax Treaty such benefits are generally available to U.S. residents if such resident is the beneficial owner of the dividends, provided that such shareholder does not have an enterprise or an interest in an enterprise that is, in whole or in part, carried on through a permanent establishment or permanent representative in the Netherlands and to which enterprise or part of an enterprise our ordinary shares are attributable. A person may, however, not claim the benefits of the U.S. Tax Treaty if such person's entitlement to such benefits is limited by the provisions of Article 26 (the limitation on benefits provision) of the U.S. Tax Treaty. The reduced dividend withholding tax rate can generally be applied at source upon the distribution of the dividends, provided that the proper forms have been filed in advance of the distribution. In the case of certain tax-exempt organisations, as a general rule, the so-called refund method applies; only when certain administrative conditions have been fulfilled may such tax-exempt organisation use the exemption method.
Taxes on Income and Capital Gains
Holders of Our Ordinary Shares Resident in the Netherlands: Individuals
A holder of our ordinary shares, who is an individual resident or deemed to be resident in the Netherlands will be subject to regular Dutch income tax on the income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares by the holder thereof, if:
209
where such lucrative interest provides the holder thereof, economically speaking, with certain benefits that have a relation to the relevant work or services.
If either of the abovementioned conditions (a) or (b) applies, income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares will in general be subject to Dutch income tax at the progressive rates up to 52%.
If the abovementioned conditions (a) and (b) do not apply, a holder of our ordinary shares who is an individual, resident or deemed to be resident in the Netherlands will not be subject to taxes on income and capital gains in the Netherlands. Instead, such individual is generally taxed at a flat rate of 30% on deemed income from "savings and investments" ("sparen en beleggen"), which deemed income is determined on the basis of the amount included in the individual's "yield basis" ("rendementsgrondslag") at the beginning of the calendar year (minus a tax-free threshold). For the 2017 tax year, the deemed income derived from savings and investments will amount to 2.87% of the individual's yield basis up to €75,000, 4.6% of the individual's yield basis exceeding €75,000 up to and including €975,000 and 5.39% of the individual's yield basis in excess of €975,000. The percentages to determine the deemed income will be reassessed every year.
Holders of Our Ordinary Shares Resident in the Netherlands: Corporate Entities
A holder of our ordinary shares that is resident or deemed to be resident in the Netherlands for corporate income tax purposes, and that is:
but which is not:
will in general be subject to regular corporate income tax, generally levied at a rate of 25% (20% over profits up to €200,000) over income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares, unless, and to the extent that, the participation exemption (deelnemingsvrijstelling) applies.
Holders of Our Ordinary Shares Resident Outside the Netherlands: Individuals
A holder of our ordinary shares who is an individual, not resident or deemed to be resident in the Netherlands will not be subject to any Dutch taxes on income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares (other than the dividend withholding tax described above), unless:
210
representative (vaste vertegenwoordiger) in the Netherlands and to which enterprise or part of an enterprise, as the case may be, our ordinary shares are attributable; or
If either of the abovementioned conditions (a) or (b) applies, income or capital gains in respect of dividends distributed by the company or in respect of any gains realised upon the acquisition, redemption and/or disposal of our ordinary shares will in general be subject to Dutch income tax at the progressive rates up to 52%.
Holders of Our Ordinary Shares Resident outside the Netherlands: Legal and Other Entities
A holder of our ordinary shares, that is a legal entity, another entity with a capital divided into shares, an association, a foundation or a fund or trust, not resident or deemed to be resident in the Netherlands for corporate income tax purposes, will not be subject to any Dutch taxes on income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares (other than the dividend withholding tax described above), unless:
If one of the abovementioned conditions applies, income derived from our ordinary shares and the gains realised upon the acquisition, redemption and/or disposal of our ordinary shares will, in general, be subject to Dutch regular corporate income tax, levied at a rate of 25% (20% over profits up to €200,000), (x) unless, and to the extent that, with respect to a holder as described under (a), the participation exemption (deelnemingsvrijstelling) applies and (y) except that a holder as described under (b) will generally be subject to an effective corporate income tax rate of 15% if it holds the substantial interest in the company with the avoidance of Dutch dividend withholding tax (but not Dutch income tax) as (one of) the main purpose(s).
Gift, Estate and Inheritance Taxes
Holders of Our Ordinary Shares Resident in the Netherlands
Gift tax may be due in the Netherlands with respect to an acquisition of our ordinary shares by way of a gift by a holder of our ordinary shares who is resident or deemed to be resident of the Netherlands at the time of the gift.
211
Inheritance tax may be due in the Netherlands with respect to an acquisition or deemed acquisition of our ordinary shares by way of an inheritance or bequest on the death of a holder of our ordinary shares who is resident or deemed to be resident of the Netherlands, or by way of a gift within 180 days before his death by an individual who is resident or deemed to be resident in the Netherlands at the time of his death.
For purposes of Dutch gift and inheritance tax, an individual with the Dutch nationality will be deemed to be resident in the Netherlands if he has been resident in the Netherlands at any time during the ten years preceding the date of the gift or his death. For purposes of Dutch gift tax, an individual not holding the Dutch nationality will be deemed to be resident of the Netherlands if he has been resident in the Netherlands at any time during the twelve months preceding the date of the gift.
Holders of Our Ordinary Shares Resident Outside the Netherlands
No gift, estate or inheritance taxes will arise in the Netherlands with respect to an acquisition of our ordinary shares by way of a gift by, or on the death of, a holder of our ordinary shares who is neither resident nor deemed to be resident of the Netherlands, unless, in the case of a gift of our ordinary shares by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands.
Certain Special Situations
For purposes of Dutch gift, estate and inheritance tax, (i) a gift by a Third Party will be construed as a gift by the Settlor, and (ii) upon the death of the Settlor, as a rule his/her Beneficiaries will be deemed to have inherited directly from the Settlor. Subsequently, such Beneficiaries will be deemed the settlor, grantor or similar originator of the Separated Private Assets for purposes of Dutch gift, estate and inheritance tax in case of subsequent gifts or inheritances.
For the purposes of Dutch gift and inheritance tax, a gift that is made under a condition precedent is deemed to have been made at the moment such condition precedent is satisfied. If the condition precedent is fulfilled after the death of the donor, the gift is deemed to be made upon the death of the donor.
Value Added Tax
No Dutch value added tax will arise in respect of or in connection with the subscription, issue, placement, allotment or delivery of our ordinary shares.
Other Taxes and Duties
No Dutch registration tax, capital tax, custom duty, transfer tax, stamp duty or any other similar documentary tax or duty, other than court fees, will be payable in the Netherlands in respect of or in connection with the subscription, issue, placement, allotment or delivery of our ordinary shares.
Residency
A holder of our ordinary shares will not be treated as a resident, or a deemed resident, of the Netherlands by reason only of the acquisition, or the holding, of our ordinary shares or the performance by the company under our ordinary shares.
212
ENFORCEMENT OF CIVIL LIABILITIES
We are a public company with limited liability (naamloze vennootschap) incorporated under the laws of the Netherlands. Substantially all of our assets are located outside the United States. The majority of our directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the United States.
The United States and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to the Dutch court the final judgment rendered by the U.S. court. This court will have discretion to attach such weight to the judgment rendered by the relevant U.S. court as it deems The Dutch courts can be expected to give conclusive effect to a final and enforceable judgment of such court in respect of the contractual obligations thereunder without re-examination or re-litigation of the substantive matters adjudicated upon, provided that: (i) that the court involved accepted jurisdiction on the basis of internationally recognized grounds to accept jurisdiction, (ii) the proceedings before such court being in compliance with principles of proper procedure (behoorlijke rechtspleging), (iii) such judgment not being contrary to the public policy of the Netherlands and (iv) such judgment not being incompatible with a judgment given between the same parties by a Netherlands court or with a prior judgment given between the same parties by a foreign court in a dispute concerning the same subject matter and based on the same cause of action, provided such prior judgment is fulfils the conditions necessary for it to be given binding effect in the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Civil Procedure Code.
Dutch civil procedure differs substantially from U.S. civil procedure in a number of respects. Insofar as the production of evidence is concerned, U.S. law and the laws of several other jurisdictions based on common law provide for pre-trial discovery, a process by which parties to the proceedings may prior to trial compel the production of documents by adverse or third parties and the deposition of witnesses. Evidence obtained in this manner may be decisive in the outcome of any proceeding. No such pre-trial discovery process exists under Dutch law.
Subject to the foregoing and service of process in accordance with applicable treaties, investors may be able to enforce in the Netherlands judgments in civil and commercial matters obtained from U.S. federal or state courts. However, no assurance can be given that those judgments will be enforceable. In addition, it is doubtful whether a Dutch court would accept jurisdiction and impose civil liability in an original action commenced in the Netherlands and predicated solely upon U.S. federal securities laws.
213
We and the underwriters for this offering named below have entered into an underwriting agreement with respect to the ordinary shares being offered. Subject to the terms and conditions of the underwriting agreement, each underwriter has severally agreed to purchase from us the number of ordinary shares set forth opposite its name below. Cowen and Company, LLC and Piper Jaffray & Co. are the representatives of the underwriters.
Underwriter | Number of Shares | |||
|---|---|---|---|---|
Cowen and Company, LLC | ||||
Piper Jaffray & Co. | ||||
JMP Securities LLC | ||||
Wedbush Securities Inc. | ||||
| | | | | |
Total | ||||
| | | | | |
| | | | | |
| | | | | |
The underwriting agreement provides that the obligations of the underwriters are subject to certain conditions precedent and that the underwriters have agreed, severally and not jointly, to purchase all of the ordinary shares sold under the underwriting agreement if any of these shares are purchased, other than those shares covered by the overallotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the ordinary shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The address of Cowen and Company, LLC is 599 Lexington Avenue, New York, NY 10022, and the address of Piper Jaffray & Co. is 345 Park Avenue, New York, New York 10154.
Overallotment Option to Purchase Additional Shares
We have granted to the underwriters an option to purchase up to additional ordinary shares at the public offering price, less the underwriting discount, in this offering of ordinary shares. This option is exercisable for a period of 30 days. The underwriters may exercise this option solely for the purpose of covering overallotments, if any, made in connection with the sale of ordinary shares offered hereby. To the extent that the underwriters exercise this option, the underwriters will purchase additional shares from us in approximately the same proportion as shown in the table above.
Discounts and Commissions
The following table shows the public offering price, underwriting discount and proceeds, before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters' overallotment option.
214
We estimate that the total expenses of this offering of ordinary shares, excluding underwriting discounts and commissions, will be approximately $ and are payable by us. We have also agreed to reimburse the underwriters for certain of their expenses as set forth in the underwriting agreement, including legal fees incurred in the qualification of this offering and the concurrent offering of notes with the Financial Regulatory Authority, or FINRA, in an amount of up to $ , which amount is deemed to be underwriting compensation by FINRA.
| | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Per Share | Without Overallotment | With Overallotment | |||||||
Initial public offering price | ||||||||||
Underwriting discounts and commissions | ||||||||||
| | | | | | | | | | | |
Proceeds, before expenses, to argenx | ||||||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The underwriters propose to offer the ordinary shares to the public at the public offering price set forth on the cover of this prospectus. The underwriters may offer the ordinary shares to securities dealers at the public offering price less a concession not in excess of $ per share. If all of the shares are not sold at the public offering price, the underwriters may change the offering price and other selling terms. Sales of ordinary shares made outside of the United States may be made by affiliates of certain of the underwriters. Certain of the underwriters may sell shares to the public through one or more of their affiliates as selling agents.
Discretionary Accounts
The underwriters do not intend to confirm sales of the ordinary shares to any accounts over which they have discretionary authority.
Market Information
Prior to this offering, our ordinary shares have not been listed for trading on an exchange in the United States. However, our shares are listed on Euronext Brussels exchange under the symbol "ARGX." The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In addition to prevailing market conditions, the factors to be considered in these negotiations include:
An active trading market for our ordinary shares may not develop on The NASDAQ Global Select Market, or if such a market develops, may not be sustained. It is also possible that after this offering the shares will not trade in the public market at or above the initial public offering price.
215
We intend to apply to list our ordinary shares on The NASDAQ Global Select Market under the symbol "ARGX."
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering of ordinary shares, the underwriters may engage in stabilizing transactions, overallotment transactions, syndicate covering transactions, penalty bids and purchases to cover positions created by short sales.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our ordinary shares or preventing or retarding a decline in the market price of our ordinary shares. As a result, the price of our ordinary shares in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our ordinary shares. These transactions may be effected on The NASDAQ Global Select Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Lock-Up Agreements
Pursuant to certain "lock-up" agreements, we, the members of our board of directors and certain key employees, have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part,
216
the economic risk of ownership of, directly or indirectly, or make any demand or request or exercise any right with respect to the registration of, or file with the SEC a registration statement under the Securities Act relating to, any ordinary shares or securities convertible into or exchangeable or exercisable for any ordinary shares without the prior written consent of Cowen and Company, LLC and Piper Jaffray & Co., for a period of 90 days after the date of the underwriting agreement.
This lock-up provision applies to ordinary shares and to securities convertible into or exchangeable or exercisable for ordinary shares. It also applies to ordinary shares owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition. The lock-up agreements include customary exceptions.
Cowen and Company, LLC and Piper Jaffray & Co., in their sole discretion, may release our ordinary shares and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release our ordinary shares and other securities from lock-up agreements, Cowen and Company, LLC and Piper Jaffray & Co. will consider, among other factors, the holder's reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time of the request. In the event of such a release or waiver for one of our directors or officers, Cowen and Company, LLC and Piper Jaffray & Co. shall provide us with notice of the impending release or waiver at least three business days before the effective date of such release or waiver and we will announce the impending release or waiver by issuing a press release at least two business days before the effective date of the release or waiver.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other Relationships
Certain of the underwriters and their affiliates have provided, and may in the future provide, various investment banking, commercial banking and other financial services for us and our affiliates for which they have received, and may in the future receive, customary fees.
Kempen & Co N.V. is acting as issuer's advisor in connection with this offering. Kempen & Co N.V. is not acting as an underwriter and will not sell or offer to sell any securities and will not identify, solicit or engage directly with potential investors. In addition, Kempen & Co N.V. will not underwrite or purchase any of the offered securities or otherwise participate in any such undertaking.
Selling Restrictions
No action has been taken in any jurisdiction except the United States that would permit a public offering of our ordinary shares, or the possession, circulation or distribution of this prospectus or any other material relating to us or our ordinary shares in any jurisdiction where action for that purpose is
217
required. Accordingly, the shares may not be offered or sold, directly or indirectly, and neither this prospectus nor any other offering material or advertisements in connection with the shares may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Canada
The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106Prospectus Exemptions or subsection 73.3(1) of theSecurities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser's province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser's province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
United Kingdom
Each of the underwriters has, separately and not jointly, represented and agreed that:
Switzerland
The securities will not be offered, directly or indirectly, to the public in Switzerland and this prospectus does not constitute a public offering prospectus as that term is understood pursuant to article 652a or 1156 of the Swiss Federal Code of Obligations.
218
Israel
In the State of Israel this prospectus shall not be regarded as an offer to the public to purchase shares of ordinary shares under the Israeli Securities Law, 5728 - 1968, which requires a prospectus to be published and authorized by the Israel Securities Authority, if it complies with certain provisions of Section 15 of the Israeli Securities Law, 5728 - 1968, including,inter alia, if: (i) the offer is made, distributed or directed to not more than 35 investors, subject to certain conditions (the "Addressed Investors"); or (ii) the offer is made, distributed or directed to certain qualified investors defined in the First Addendum of the Israeli Securities Law, 5728 - 1968, subject to certain conditions (the "Qualified Investors"). The Qualified Investors shall not be taken into account in the count of the Addressed Investors and may be offered to purchase securities in addition to the 35 Addressed Investors. The company has not and will not take any action that would require it to publish a prospectus in accordance with and subject to the Israeli Securities Law, 5728 - 1968. We have not and will not distribute this prospectus or make, distribute or direct an offer to subscribe for our ordinary shares to any person within the State of Israel, other than to Qualified Investors and up to 35 Addressed Investors.
Qualified Investors may have to submit written evidence that they meet the definitions set out in of the First Addendum to the Israeli Securities Law, 5728 - 1968. In particular, we may request, as a condition to be offered ordinary shares, that Qualified Investors will each represent, warrant and certify to us and/or to anyone acting on our behalf: (i) that it is an investor falling within one of the categories listed in the First Addendum to the Israeli Securities Law, 5728 - 1968; (ii) which of the categories listed in the First Addendum to the Israeli Securities Law, 5728 - 1968 regarding Qualified Investors is applicable to it; (iii) that it will abide by all provisions set forth in the Israeli Securities Law, 5728 - 1968 and the regulations promulgated thereunder in connection with the offer to be issued ordinary shares; (iv) that the ordinary shares that it will be issued are, subject to exemptions available under the Israeli Securities Law, 5728 - 1968: (a) for its own account; (b) for investment purposes only; and (c) not issued with a view to resale within the State of Israel, other than in accordance with the provisions of the Israeli Securities Law, 5728 - 1968; and (v) that it is willing to provide further evidence of its Qualified Investor status. Addressed Investors may have to submit written evidence in respect of their identity and may have to sign and submit a declaration containing,inter alia, the Addressed Investor's name, address and passport number or Israeli identification number.
European Economic Area
In relation to each Member State of the European Economic Area, or the EEA, which has implemented the European Prospectus Directive (each, a "Relevant Member State"), an offer of our shares may not be made to the public in a Relevant Member State other than:
provided that no such offer of our shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the European Prospectus Directive or supplement prospectus pursuant to Article 16 of the European Prospectus Directive.
219
For the purposes of this description, the expression an "offer to the public" in relation to the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the expression may be varied in that Relevant Member State by any measure implementing the European Prospectus Directive in that member state, and the expression "European Prospectus Directive" means Directive 2003/71/EC (and amendments hereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State. The expression 2010 PD Amending Directive means Directive 2010/73/EU.
We have not authorized and do not authorize the making of any offer of securities through any financial intermediary on our behalf, other than offers made by the underwriters and their respective affiliates, with a view to the final placement of the securities as contemplated in this document. Accordingly, no purchaser of the shares, other than the underwriters, is authorized to make any further offer of shares on our behalf or on behalf of the underwriters.
Hong Kong
The contents of this document have not been reviewed or approved by any regulatory authority in Hong Kong. This document does not constitute an offer or invitation to the public in Hong Kong to acquire shares. Accordingly, unless permitted by the securities laws of Hong Kong, no person may issue or have in its possession for the purposes of issue, this document or any advertisement, invitation or document relating to the shares, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong other than in relation to shares which are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" (as such term is defined in the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) ("SFO") and the subsidiary legislation made thereunder); or in circumstances which do not result in this document being a "prospectus" as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32, Laws of Hong Kong) ("CO"); or which do not constitute an offer or an invitation to the public for the purposes of the SFO or the CO. The offer of the shares is personal to the person to whom this document has been delivered, and a subscription for shares will only be accepted from such person. No person to whom a copy of this document is issued may issue, circulate or distribute this document in Hong Kong, or make or give a copy of this document to any other person. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor pursuant to Section 274 of the Securities and Futures Act, Chapter 289 of Singapore ("SFA"), (ii) to a relevant person (as defined in Section 275(2) of the SFA), or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
220
Where the shares are subscribed or purchased pursuant to an offer made in reliance on Section 275 of the SFA by a relevant person which is:
shares, debentures and units of shares and debentures of that corporation, or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferable for six months after that corporation or that trust has acquired the shares under Section 275 except:
221
Set forth below is an itemization of the total expenses, excluding underwriting discounts and commissions, which are expected to be incurred in connection with our sale of ordinary shares in this offering. With the exception of the registration fee payable to the SEC and the filing fee payable to FINRA, all amounts are estimates.
Itemized expenses | Amount | |||
|---|---|---|---|---|
SEC registration fee | $ | * | ||
NASDAQ listing fee | * | |||
FINRA filing fee | * | |||
FSMA filing fee | * | |||
Euronext listing fee | * | |||
Printing expenses | * | |||
Legal fees and expenses | * | |||
Accounting fees and expenses | * | |||
Miscellaneous costs | * | |||
| | | | | |
Total | $ | * | ||
| | | | | |
| | | | | |
| | | | | |
222
Goodwin Procter LLP, Boston, Massachusetts, is representing the company in connection with this offering. Freshfields Bruckhaus Deringer LLP, will pass upon the validity of the ordinary shares offered hereby and other legal matters concerning this offering relating to Dutch law, including matters of Dutch income tax law. Legal counsel to the underwriters in connection with this offering are Cooley LLP, New York, New York, with respect to U.S. federal law, and NautaDutilh N.V. with respect to Dutch Law.
The financial statements included in this prospectus have been audited by Deloitte Accountants B.V., an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the registration statement. Such financial statements are included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The offices of Deloitte Accountants B.V. are located at Flight Forum 1, 5657 DA Eindhoven.
223
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the U.S. Securities and Exchange Commission, the SEC, a registration statement (including amendments and exhibits to the registration statement) on Form F-1 under the Securities Act. This prospectus, which is part of the registration statement, does not contain all of the information set forth in the registration statement and the exhibits and schedules to the registration statement. For further information, we refer you to the registration statement and the exhibits and schedules filed as part of the registration statement. If a document has been filed as an exhibit to the registration statement, we refer you to the copy of the document that has been filed. Each statement in this prospectus relating to a document filed as an exhibit is qualified in all respects by the filed exhibit.
You may review a copy of the registration statement, including exhibits and any schedule filed therewith, and obtain copies of such materials at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
Upon completion of this offering, we will become subject to the informational requirements of the Exchange Act. Accordingly, we will be required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. Those reports may be inspected without charge at the locations described above. As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act, although we intend to report our results of operations voluntarily on a quarterly basis.
We maintain a corporate website atwww.argenx.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
224
Unaudited Interim Condensed Financial Statements for the period ended June 30, 2016
Unaudited Interim Condensed Statement of Financial Position | F-2 | |
Unaudited Interim Condensed Statement of Profit and Loss and Other Comprehensive Income | F-3 | |
Unaudited Interim Condensed Statement of Cash Flows | F-4 | |
Unaudited Interim Condensed Statement of Changes in Equity | F-5 | |
Notes to the Unaudited Interim Condensed Financial Statements | F-6 |
Consolidated Financial Statements for the period ended December 31, 2015
Report of Independent Registered Public Accounting Firm | F-17 | |
Consolidated Statement of Financial Position | F-18 | |
Consolidated Statement of Profit and Loss and Other Comprehensive Income | F-19 | |
Consolidated Statement of Cash Flows | F-20 | |
Consolidated Statement of Changes in Equity | F-21 | |
Notes to the Consolidated Financial Statements | F-22 |
F-1
ARGENX N.V.
UNAUDITED INTERIM CONDENSED STATEMENT OF FINANCIAL POSITION
| (in thousands of euros) | Note | At December 31, 2015 | At June 30, 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| | | AUDITED | UNAUDITED | ||||||
ASSETS | |||||||||
Current assets | |||||||||
Cash and cash equivalents | 4.6 | 35,514 | 101,918 | ||||||
Current restricted cash | 4.5 | 0 | 632 | ||||||
Financial assets | 4.4 | 6,813 | 6,826 | ||||||
Prepaid expenses | 4.3 | 454 | 2,214 | ||||||
Trade and other receivables | 4.2 | 1,356 | 1,851 | ||||||
| | | | | | | | | | |
Total current assets | 44,137 | 113,441 | |||||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
Non-current assets |
| ||||||||
Restricted cash | 0 | 1,304 | |||||||
Research and development incentive receivables | 4.1 | 1,568 | 1,833 | ||||||
Financial assets | 1 | 1 | |||||||
Property, plant and equipment | 249 | 731 | |||||||
Intangible assets | 7 | 24 | |||||||
| | | | | | | | | | |
Total non-current assets | 1,825 | 3,893 | |||||||
| | | | | | | | | | |
TOTAL ASSETS | 45,962 | 117,334 | |||||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
EQUITY AND LIABILITIES | |||||||||
Equity | 3.1 | ||||||||
Equity attributable to owners of the parent | |||||||||
Share capital | 1,580 | 2,004 | |||||||
Share premium | 82,169 | 126,088 | |||||||
Accumulated deficits | (51,118 | ) | (58,474 | ) | |||||
Other reserves | 3.2 | 4,647 | 5,782 | ||||||
| | | | | | | | | | |
Total equity | 37,278 | 75,400 | |||||||
| | | | | | | | | | |
Non-current liabilities | 0 | 0 | |||||||
Current liabilities | 8,684 | 41,934 | |||||||
Trade and other payables | 4,543 | 5,148 | |||||||
Deferred revenue | 4.7 | 4,141 | 36,786 | ||||||
| | | | | | | | | | |
Total liabilities | 8,684 | 41,934 | |||||||
| | | | | | | | | | |
TOTAL EQUITY AND LIABILITIES | 45,962 | 117,334 | |||||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
The notes are an integral part of these unaudited interim condensed financial statements.
F-2
ARGENX N.V.
UNAUDITED INTERIM CONDENSED STATEMENT OF COMPREHENSIVE INCOME
| (in thousands of euros except for shares and EPS) | Note | Six months ended June 30, 2015 | Six months ended June 30, 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| | | UNAUDITED | UNAUDITED | ||||||
Revenue | 5.1 | 2,708 | 5,656 | ||||||
Other operating income | 5.2 | 1,640 | 1,317 | ||||||
Total operating income | 4,348 | 6,973 | |||||||
Research and development expenses | 5.4 | (9,284 | ) | (11,263 | ) | ||||
General and administrative expenses | 5.5 | (2,314 | ) | (3,063 | ) | ||||
| | | | | | | | | | |
Operating loss | (7,250 | ) | (7,353 | ) | |||||
| | | | | | | | | | |
Financial income/ (expense) | 100 | 39 | |||||||
Exchange gains/(losses) | 130 | (42 | ) | ||||||
| | | | | | | | | | |
Loss before taxes | (7,020 | ) | (7,356 | ) | |||||
| | | | | | | | | | |
Income tax (income/expense) | 0 | 0 | |||||||
| | | | | | | | | | |
TOTAL COMPREHENSIVE LOSS OF THE PERIOD | (7,020 | ) | (7,356 | ) | |||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
Weighted average number of shares outstanding | 15,705,112 | 17,356,799 | |||||||
Basic and diluted loss per share (in €) | (0.45 | ) | (0.42 | ) | |||||
There are no non-controlling interests in the Group.
The notes are an integral part of these unaudited interim condensed financial statements.
F-3
ARGENX N.V.
UNAUDITED INTERIM CONDENSED STATEMENT OF CASH FLOWS
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | UNAUDITED | UNAUDITED | |||||
CASH FLOWS FROM OPERATING ACTIVITIES | |||||||
Operating result | (7,250 | ) | (7,353 | ) | |||
Adjustments for non-cash items | |||||||
Amortization of intangible assets | 3 | 5 | |||||
Depreciation of property, plant and equipment | 85 | 145 | |||||
Expense recognized in respect of share-based payments | 1,120 | 1,135 | |||||
| (6,042 | ) | (6,068 | ) | ||||
Movements in assets/liabilities | |||||||
(Increase)/decrease in trade and other receivables | (968 | ) | (760 | ) | |||
(Increase)/decrease in other current assets | (347 | ) | (2,392 | ) | |||
(Increase)/decrease in other non-current assets | 0 | (1,304 | ) | ||||
(Increase)/decrease in trade and other payables | (139 | ) | 366 | ||||
Increase/(decrease) in deferred revenue | 2,050 | 32,645 | |||||
Cash generated (used in) operating activities | (5,446 | ) | 22,487 | ||||
| | | | | | | | |
NET CASH FLOWS FROM OPERATING ACTIVITIES | (5,446 | ) | 22,487 | ||||
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | |||||||
Purchase of intangible assets | (5 | ) | (21 | ) | |||
Purchase of property, plant and equipment | (204 | ) | (628 | ) | |||
Increase/decrease in current financial assets | (549 | ) | (13 | ) | |||
Interest received | 100 | 39 | |||||
| | | | | | | | |
NET CASH FLOWS FROM INVESTING ACTIVITIES | (658 | ) | (623 | ) | |||
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | |||||||
Proceeds from issue of ordinary shares | 0 | 44,366 | |||||
Proceeds from issue of ordinary shares under the stock option plan | 0 | 216 | |||||
| | | | | | | | |
NET CASH FLOWS FROM FINANCING ACTIVITIES | 0 | 44,582 | |||||
| | | | | | | | |
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | (6,104 | ) | 66,446 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Cash and cash equivalents at the beginning of the period | 32,180 | 35,514 | |||||
| | | | | | | | |
Exchange gains/(losses) on cash & cash equivalents | 130 | (42 | ) | ||||
| | | | | | | | |
Cash and cash equivalents at the end of the period | 26,206 | 101,918 | |||||
| | | | | | | | |
The notes are an integral part of these unaudited interim condensed financial statements.
F-4
ARGENX N.V.
UNAUDITED INTERIM CONDENSED STATEMENT OF CHANGES IN EQUITY
| | Attributable to owners of the parent | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Share capital | Share premium | Accumulated deficit | Other reserves Equity-settled share-based payment reserve | Total equity attributable to owners of the parent | | TOTAL EQUITY | ||||||||||||||
Balance at January 1, 2015 | 1,571 | 81,940 | (35,806 | ) | 2,378 | 50,082 | 50,082 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss of the period | (7,020 | ) | (7,020 | ) | (7,020 | ) | |||||||||||||||
Issue of share capital | 0 | 0 | |||||||||||||||||||
Transaction costs for equity issue | 0 | 0 | |||||||||||||||||||
Share-based payment | 1,120 | 1,120 | 1,120 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
Balance at June 30, 2015 | 1,571 | 81,940 | (42,825 | ) | 3,498 | 44,184 | 44,184 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2015 | 1,580 | 82,168 | (51,118 | ) | 4,647 | 37,277 | 37,277 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss of the period | (7,356 | ) | (7,356 | ) | (7,356 | ) | |||||||||||||||
Issue of ordinary shares | 418 | 45,559 | 45,977 | 45,977 | |||||||||||||||||
Issue of ordinary shares under the stock option plan | 6 | 210 | 216 | 216 | |||||||||||||||||
Transaction costs for equity issue | (1,849 | ) | (1,849 | ) | (1,849 | ) | |||||||||||||||
Share-based payment | 1,135 | 1,135 | 1,135 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
Balance at June 30, 2016 | 2,004 | 126,088 | (58,474 | ) | 5,782 | 75,400 | 75,400 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
The notes are an integral part of these unaudited interim condensed financial statements.
F-5
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016
1. General information
argenx N.V. (the Company) is a public company with limited liability incorporated under the laws of the Netherlands. The Company's official seat is in Rotterdam, the Netherlands, and its registered office is at Willemstraat 5, 4811 AH, Breda, the Netherlands. An overview of the Company and its subsidiaries (the Group) are described in note 7.3.
2. Summary of significant accounting policies
2.1. Statement of compliance and basis of preparation
These condensed interim financial statements for the six months ended June 30, 2016 have been prepared in accordance with IAS 34 'Interim financial reporting'. The condensed interim financial statements should be read in conjunction with the annual financial statements for the year-ended December 31, 2015, which have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB).
The condensed interim financial statements have been approved for issue by the Board of Directors on August 24, 2016.
The accounting policies adapted in the preparation of the condensed interim financial statements are consistent with those applied in the financial statements for the year ended December 31, 2015. New standards or interpretations applicable from January 1, 2016 do not have any significant impact on the condensed interim financial statements.
The principal accounting policies applied in the preparation of the above financial statements are set out below. All amounts are presented in thousands of euros, unless otherwise indicated, rounded to the nearest EUR '000. These unaudited interim condensed financial statements have been reviewed, not audited.
We anticipate that the new Standards and Interpretations as issued by the IASB but are not yet mandatory will not have a significant impact on the financial statements of the Company in the period of initial application except for IFRS 15 and IFRS 16 for which the impact is currently being investigated.
The financial statements have been established assuming the Company is in a state of going concern.
2.2. Segment reporting
The Group manages its activities and operates as one business unit which is reflected in its organizational structure and internal reporting. The Group does not distinguish in its internal reporting different segments, neither business nor geographical segments. The chief operating decision-maker is the Board of Directors.
F-6
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty
In the application of the Company's accounting policies, which are described above, the Company is required to make judgements, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
The following areas are areas where key assumptions concerning the future, and other key sources of estimation uncertainty at the end of the reporting period, have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year:
Going concern
The interim results for the six months ended June 30, 2016 show a negative result, and the balance sheet includes a loss carried forward. The Board has examined the statements and accounting standards. Taking into account the cash, cash equivalents and financial assets position, the Board is of the opinion that it can submit the interim financial statements on a going concern basis.
Revenue recognition
For revenue recognition, the significant estimates relate to allocation of value to the separate elements in multiple-element arrangements. With respect to the allocation of value to the separate elements, the Company is using the stand-alone selling prices or management's best estimates of selling prices to estimate the fair value of the elements and account for them separately. Revenue is allocated to each deliverable based on the fair value of each individual element and is recognized when the revenue recognition criteria described above are met.
Upfront fees under collaboration or licensing agreements are recognized over the expected duration of the performance obligations, unless there is no continuous involvement required. Management estimates this period at the start of the collaboration and validates the remaining estimated collaboration term at each closing date.
Measurement of share-based payments
In accordance with IFRS 2—Share-based Payment, the fair value of the options at grant date is recognized as an expense in the statement of comprehensive income over the vesting period, the period of delivery of work. Subsequently, the fair value equity-settled is not re-measured.
The fair value of each stock option granted during the year is calculated using the Black-Scholes pricing model. This pricing model requires the input of subjective assumptions, which are detailed in note 3.2.
F-7
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty (continued)
Recognition of deferred tax assets
Deferred tax assets are recognized only if management assesses that these tax assets can be offset against positive taxable income within a foreseeable future.
This judgment is made on an ongoing basis and is based on budgets and business plans for the coming years, including planned commercial initiatives.
Since inception, the Company has reported losses, and as a consequence, the Company have unused tax losses. Therefore, management has concluded that deferred tax assets should not be recognized as of June 30, 2016. The deferred tax assets are currently not deemed to meet the criteria for recognition as management is not able to provide any convincing positive evidence that deferred tax assets should be recognized.
3.1. Equity
Roll forward of number of shares outstanding:
Number of shares outstanding as per 12/31/2015 | 15,802,767 | |||
Investment by Federated Investors in January 2016 | 1,480,420 | |||
Exercise of stock options during first half of 2016 | 55,292 | |||
Investment by institutional Investors in June 2016 | 2,703,000 | |||
Number of shares outstanding as per 30/06/2016 | 20,041,479 |
In January 2016 the Company announced an investment of €16 million by Federated Advisors, resulting in the issuance of 1,480,420 new shares. In June 2016 the Company announced a private placement of €30 million with institutional investors, resulting in the issuance of 2,703,000 new shares.
3.2. Share-based payments
The Company has a stock option scheme for the employees of the Company and its subsidiaries. In accordance with the terms of the plan, as approved by shareholders, employees may be granted options to purchase ordinary shares at an exercise price as mentioned below per ordinary share.
The Group has granted on May 25, 2016 a total of 288,950 stock options and on June 18, 2016 a total of 60,000 stock options to employees and consultants. The total number of stock options outstanding at June 30, 2016 totals 2,027,668 (December 31, 2015: 1,752,927). No stock options are expired and a total of 55,292 stock options have been exercised as of June 30, 2016. A total of 18,917 share options have been forfeited as of June 30, 2016.
The stock options are granted to employees, consultants or directors of the Company and its subsidiaries. The stock options have been granted free of charge. Each employee stock option converts into one ordinary share of the Company on exercise. No amounts are paid or payable by the recipient on receipt of the option. The options carry neither rights to dividends nor voting rights. Options may be exercised at any time from the date of vesting to the date of their expiry.
F-8
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty (continued)
The stock options granted vest, in principle, as follows:
No other conditions are attached to the stock options.
The following share-based payment arrangements were in existence during the current and prior years and which are exercisable at closing of each period presented:
| | | Outstanding stock options for the period ended | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expiry date | Exercise price per stock options | At December 31, 2015 | At June 30, 2016 | |||||||
| | (in EUR) | AUDITED | UNAUDITED | |||||||
2019 | 3.95 | 103,370 | 50,278 | |||||||
2020 | 3.95 | 62,460 | 62,460 | |||||||
2021 | 3.95 | 3,800 | 3,800 | |||||||
2021 | 2.44 | 275,520 | 273,320 | |||||||
2021 | 2.44 | 157,530 | 157,530 | |||||||
2021 | 2.44 | 83,820 | 83,820 | |||||||
2021 | 3.95 | 55,747 | 55,747 | |||||||
2021 | 2.44 | 169,862 | 169,862 | |||||||
2024 | 7.17 | 537,918 | 537,500 | |||||||
2025 | 11.44 | 56,500 | 39,000 | |||||||
2025 | 10.34 | 3,000 | 3,000 | |||||||
2025 | 9.47 | 215,200 | 214,201 | |||||||
2026 | 9.47 | 28,200 | 28,200 | |||||||
2027 | 11.47 | — | 288,950 | |||||||
2027 | 11.38 | — | 60,000 | |||||||
| | | | | | | | | | | |
Total | 1,752,927 | 2,027,668 | ||||||||
| | | | | | | | | | | |
The fair market value of the stock options has been determined based on the Black and Scholes model. The expected volatility in the model is based on the historical volatility of peer companies and historical volatility of the Group since its initial public offering. For the new grants as from 2016, the Group will only consider historical volatility of the argenx stock price, since there are now relevant data available.
F-9
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty (continued)
Below is an overview of the parameters used in relation to the new grants:
stock options granted in | May 2016 | June 2016 | |||||
|---|---|---|---|---|---|---|---|
Number of options granted | 288,950 | 60,000 | |||||
Average fair value of options (in EUR) | 5.32 | 5.46 | |||||
Share price (in EUR) | 11.10 | 11.36 | |||||
Exercise price (in EUR) | 11.47 | 11.38 | |||||
Expected volatility | 40.2 | % | 39.6 | % | |||
Average expected option life (in years) | 10 | 10 | |||||
Risk-free interest rate | 0.52 | % | 0.46 | % | |||
Expected dividends | 0 | % | 0 | % | |||
The total share-based payment expense recognized in the consolidated statement of comprehensive income totalled €1.1 million for the six-month periods ended June 30, 2016 and 2015.
4. Notes to the condensed statement of financial position
4.1. Research and development incentive receivables
On June 30, 2016, the Group has recorded a research and development incentive receivable of €1.8 million, compared to €1.6 million on December 31, 2015, in relation with a research and development incentive tax scheme in Belgium under which the research and development incentives can be refunded after five years if not offset against future income tax expense. The research and development incentives are recorded in other operating income (see note 5.2) in the consolidated statement of profit and loss and other comprehensive income. These amounts are expected to be gradually reimbursed in cash as from 2017 onwards.
4.2. Trade and other receivables
The trade and other receivables are detailed below:
| (in thousands of euros) | At December 31, 2015 | At June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | AUDITED | UNAUDITED | |||||
VAT receivable | 175 | 319 | |||||
Trade receivables | 719 | 874 | |||||
Interest receivable | 17 | 11 | |||||
Flanders Innovation & Entrepreneurship (IWT) grants to receive | 445 | 647 | |||||
| | | | | | | | |
| 1,356 | 1,851 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The nominal amount of all trade and other receivables approximates their respective fair values.
The VAT receivable relates to VAT amounts to be recovered in the second half of 2016.
F-10
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
4. Notes to the condensed statement of financial position (continued)
Trade receivables correspond to amounts invoiced to the collaborators or strategic allies of the Group. No trade receivables were past due on June 30, 2016. The Flanders Innovation & Entrepreneurship grants to receive consists of earned income from government grants for which no payments have been received but for which the relating expenditures have been incurred. For more information on the government grants to receive from Flanders Innovation & Entrepreneurship Agency see note 5.2.
4.3. Prepaid expenses
The prepaid expenses on June 30, 2016 amount to €2.2 million (€0.5 million on December 31, 2015) and relate to (i) a success fee paid to a third party involved in the license agreement signed with LEO Pharma in 2015 and (ii) a license fee paid to a third party involved in the license agreement signed with Abbvie in April 2016. These amounts will be recognized as expenses in the income statement over the remaining period of the license agreements.
4.4. Current financial assets
On June 30, 2016, the current financial assets amounted to €6.8 million (same amount on December 31, 2015), and corresponded to financial instruments in the form of money market funds with a recommended maturity of 6 months. These funds are highly liquid investments and can be readily convertible into a known amount of cash. Because of their historical volatility these funds cannot be classified as cash and cash equivalents. Values recognized on the balance sheet are the fair values.
4.5. Restricted cash
On June 30, 2016 the company had a total amount of €1.9 million of restricted cash. This amount is split as follows:
4.6. Cash and Cash Equivalents
On June 30, 2016, cash and cash equivalents amounted to €101.9 compared to €35.5 million on December 31, 2015 and included (i) cash on hand, (ii) current and savings accounts in different banks and (iii) short term investment funds in the form of money market funds with a recommended maturity of less than 6 months and with a low historical volatility which allows such money market funds to be classified as cash equivalents. These money market funds are highly liquid investments, can be readily convertible into a known amount of cash and subject to an insignificant risk of changes in value.
F-11
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
4. Notes to the condensed statement of financial position (continued)
4.7. Deferred revenue
Deferred revenue relates to cash received from collaborations or strategic alliances prior to completion of the earnings process. For the six-month period ended on June 30, 2016, deferred revenue increased to €36.8 million compared to €4.1 million on December 31, 2015.
5. Notes to the condensed statement of comprehensive income
5.1. Revenue
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | UNAUDITED | UNAUDITED | |||||
Upfront payments | 858 | 2,499 | |||||
Milestone payments | 0 | 500 | |||||
Research and development service fees | 1,850 | 2,657 | |||||
| | | | | | | | |
Total | 2,708 | 5,656 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Upfront payments, milestone payments and research and development service fees are recognized according to the accounting principles set by the company.
The upfront payments in the first half of 2016 corresponds principally to the partial recognition in revenue over the period of the upfront payments received following the signatures of a strategic alliance with Shire in June 2014, an alliance with LEO Pharma in May 2015 and an alliance with Abbvie in April 2016. These payments are recognized as revenue over the estimated period of argenx' continuing involvement in the research and development activities provided for under the terms of these agreements.
The milestone payment recognized in the six-month period ended on June 30, 2016 relates to a payment under the LEO Pharma collaboration.
The research and development service fees (FTE) correspond to FTE-payments related to the collaboration agreement with LEO Pharma and a strategic alliance with Shire as indicated above.
F-12
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
5. Notes to the condensed statement of comprehensive income (continued)
5.2. Other operating income
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | UNAUDITED | UNAUDITED | |||||
Flanders Innovation & Entrepreneurship and Oncornet grants | 848 | 515 | |||||
Grants on employment | 562 | 537 | |||||
Research and development incentives | 230 | 265 | |||||
| | | | | | | | |
| 1,640 | 1,317 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
5.3. Segment reporting
The Group operates from Belgium and the Netherlands. Revenues are invoiced by the holding company in the Netherlands and are generated by clients geographically located as shown in the table below. In the table next to this, it is indicated where the non-current assets from the group are situated.
| | Revenue from external customers | Non-current assets | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | At December 31, 2015 | At June 30, 2016 | |||||||||
Netherlands | 167 | 269 | 1 | 1,324 | |||||||||
Belgium | 1,824 | 2,569 | |||||||||||
Germany | 934 | 311 | |||||||||||
Denmark | 83 | 1,716 | |||||||||||
Switzerland | 1,432 | 1,610 | |||||||||||
Luxemburg | 0 | 1,727 | |||||||||||
United States | 91 | 23 | |||||||||||
| | | | | | | | | | | | | | |
Total | 2,708 | 5,656 | 1,825 | 3,893 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
From the €5.7 million (€2.7 million in 2015) received from upfront payments, milestone payments and research and development fees, €1.7 million come from the Group's largest client, €1.7 million (€0.08 million in 2015) from its second largest client and €1.6 million (€1.4 million in 2015) from its third largest client.
F-13
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
5. Notes to the condensed statement of comprehensive income (continued)
5.4. Research and development expenses
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | UNAUDITED | UNAUDITED | |||||
Personnel expenses | 2,950 | 4,224 | |||||
Depreciation and amortization | 88 | 150 | |||||
External research and development expenses | 5,359 | 5,320 | |||||
Materials and consumables | 522 | 561 | |||||
Other expenses | 365 | 1,008 | |||||
| | | | | | | | |
| 9,284 | 11,263 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
5.5. General and Administrative expenses
| (in thousands of euros) | Six months ended June 30, 2015 | Six months ended June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
| | UNAUDITED | UNAUDITED | |||||
Personnel expenses | 684 | 999 | |||||
Consulting fees | 1,125 | 1,555 | |||||
Supervisory board | 73 | 111 | |||||
Office costs | 432 | 398 | |||||
| | | | | | | | |
| 2,314 | 3,063 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
6. Financial instruments and financial risk management
6.1. Overview of financial instruments
| | At December 31, 2015 | At June 30, 2016 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Carrying amount | Fair value | Carrying amount | Fair value | |||||||||
Non-current financial assets | 1 | 1 | 1 | 1 | |||||||||
| | | | | | | | | | | | | | |
Financial assets available for sale | 1 | 1 | 1 | 1 | |||||||||
Current financial assets | 6,813 | 6,813 | 6,826 | 6,826 | |||||||||
| | | | | | | | | | | | | | |
Financial assets at fair value through P/L | 6,813 | 6,813 | 6,826 | 6,826 | |||||||||
Trade and other receivables | 1,356 | 1,356 | 1,851 | 1,851 | |||||||||
Current and non-current restricted cash | 0 | 0 | 1,936 | 1,936 | |||||||||
Cash and bank balances | 35,514 | 35,514 | 101,918 | 101,918 | |||||||||
| | | | | | | | | | | | | | |
Loans and receivables | 36,870 | 36,870 | 105,705 | 105,705 | |||||||||
| | | | | | | | | | | | | | |
Total financial assets | 43,683 | 43,683 | 112,532 | 112,532 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Trade and other payables | 4,543 | 4,543 | 5,148 | 5,148 | |||||||||
| | | | | | | | | | | | | | |
Financial liabilities at amortized cost | 4,543 | 4,543 | 5,148 | 5,148 | |||||||||
| | | | | | | | | | | | | | |
Total financial liabilities | 4,543 | 4,543 | 5,148 | 5,148 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-14
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
6. Financial instruments and financial risk management (continued)
Financial assets at fair value through P/L:
Loans and receivables:
Financial liabilities:
Due to the current nature of the financial liabilities, the nominal value of all financial liabilities presented above approximates their fair value.
7. Other disclosures
7.1 Related party transactions
The shareholders of the Company are several minority investors and venture capitalists which individually do not hold a significant stake in the Company. Balances and transactions between the Company and its subsidiaries, which are related parties of the Company, have been eliminated on consolidation and are not disclosed in this note. There were no significant transactions with related parties during the period, other than compensation of the independent directors and the key management personnel.
F-15
ARGENX N.V.
NOTES TO THE UNAUDITED INTERIM CONDENSED FINANCIAL STATEMENTS FOR THE PERIOD ENDED JUNE 30, 2016 (Continued)
7. Other disclosures (continued)
7.2. Commitments
At closing date, there were no commitments signed for the acquisition of property, plant and equipment or intangible assets. The operating lease commitments are listed in the table below.
Operating Lease commitments (in thousands of euros) | At December 31, 2015 | At June 30, 2016 | |||||
|---|---|---|---|---|---|---|---|
Not later than 1 year | 630 | 968 | |||||
Later than 1 year and not later than 5 years | 1,272 | 1,591 | |||||
| | | | | | | | |
| 1,902 | 2,559 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The Group has a lease plan for the company's cars with maturity dates up to 4 years.
The Group has signed a lease agreement in March 2016 for new laboratory and office space in Zwijnaarde in Belgium. The lease agreement is for a period of 9 years starting from April 1, 2016, with the possibility to terminate the lease by giving a notice of at least twelve (12) months in advance at the occasion of the third and sixth anniversary of the agreement.
For its offices in the Netherlands the Company has a lease agreement renewable on an annual base.
No purchase options are in effect under the lease agreements described above.
7.3. Overview of consolidation scope
The parent company argenx NV is domiciled in the Netherlands.
Details of the Group's subsidiaries at the end of the reporting period are as follows:
Name | Registration number | Country | Participation | Main activity | |||||
|---|---|---|---|---|---|---|---|---|---|
argenx 110 BV | 853245496 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | ||||
argenx 111 BV | 853245332 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | ||||
argenx 113 BV | 854976954 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | ||||
argenx 115 BV | 855638059 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | ||||
argenx BVBA | 0818292196 | Belgium | 100.00 | % | Biotechnical research on drugs and pharma processes | ||||
7.4. Events after the balance sheet date
F-16
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
argenx N.V.
Breda
We have audited the accompanying consolidated statement of financial position of argenx N.V. and subsidiaries (the "Company") as of December 31, 2015, and the related consolidated statements of profit and loss and other comprehensive income, changes in equity and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
The Company omitted financial statements for a preceding comparative period. In our opinion, disclosures of comparative financial statements and related information is required by International Financial Reporting Standards as issued by the International Accounting Standards Board. The omission of comparative financial statements and related information results in an incomplete presentation of the Company's consolidated financial statements.
In our opinion, except for the omission of comparative financial statements and related information as discussed in the preceding paragraph, such consolidated financial statements present fairly, in all material respects, the financial position of argenx N.V. and subsidiaries as of December 31, 2015, and the results of their operations and their cash flows for the year then ended in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
/s/ Deloitte Accountants B.V.
Eindhoven, the Netherlands
February 28, 2017
F-17
ARGENX N.V.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
| (in thousands of euros) | Note | Year ended December 31, 2015 | ||||
|---|---|---|---|---|---|---|
ASSETS | ||||||
Current assets | ||||||
Cash and cash equivalents | 4.8 | 35,514 | ||||
Financial assets | 4.7 | 6,813 | ||||
Prepaid expenses | 4.6 | 454 | ||||
Trade and other receivables | 4.5 | 1,356 | ||||
Total Current assets | 44,137 | |||||
Non-current assets |
| |||||
Research and development incentive receivables | 4.4 | 1,568 | ||||
Financial assets | 4.3 | 1 | ||||
Property, plant and equipment | 4.2 | 249 | ||||
Intangible assets | 4.1 | 7 | ||||
Total Non-current assets | 1,825 | |||||
| | | | | | | |
TOTAL ASSETS | 45,962 | |||||
| | | | | | | |
| | | | | | | |
| | | | | | | |
| (in thousands of euros) | Note | Year ended December 31, 2015 | |||||
|---|---|---|---|---|---|---|---|
EQUITY AND LIABILITIES | |||||||
Equity | 4.9 | ||||||
Equity attributable to owners of the parent | |||||||
Share capital | 1,580 | ||||||
Share premium | 82,169 | ||||||
Accumulated deficits | (51,118 | ) | |||||
Other reserves | 4.12 | 4,647 | |||||
| | | | | | | | |
Total equity | 37,278 | ||||||
| | | | | | | | |
Non-current liabilities | 0 | ||||||
Current liabilities | 8,684 | ||||||
Trade and other payables | 4.10 | 4,543 | |||||
Deferred revenue | 4.11 | 4,141 | |||||
| | | | | | | | |
Total liabilities | 8,684 | ||||||
| | | | | | | | |
TOTAL EQUITY AND LIABILITIES | 45,962 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The notes are an integral part of these consolidated financial statements.
F-18
ARGENX N.V.
CONSOLIDATED STATEMENT OF PROFIT AND LOSS AND OTHER COMPREHENSIVE
INCOME
(in thousands of euros except for shares and EPS) | Note | Year ended December 31, 2015 | ||||
|---|---|---|---|---|---|---|
Revenue | 5.1 | 6,854 | ||||
Other operating income | 5.2 | 3,101 | ||||
Total operating income | 9,955 | |||||
Research and development expenses | 5.4 | (20,635 | ) | |||
General and administrative expenses | 5.5 | (4,925 | ) | |||
| | | | | | | |
Operating loss | (15,605 | ) | ||||
| | | | | | | |
Financial income | 5.8 | 112 | ||||
Financial expenses | 0 | |||||
Exchange gains/(losses) | 5.8 | 181 | ||||
| | | | | | | |
Loss before taxes | (15,312 | ) | ||||
| | | | | | | |
Income tax (income/expense) | 5.10 | 0 | ||||
| | | | | | | |
TOTAL COMPREHENSIVE LOSS OF THE PERIOD | (15,312 | ) | ||||
| | | | | | | |
| | | | | | | |
| | | | | | | |
Weighted average number of shares outstanding | 15,734,007 | |||||
Basic and diluted loss per share (in €) | 5.11 | (0.97 | ) | |||
The notes are an integral part of these consolidated financial statements.
F-19
ARGENX N.V.
CONSOLIDATED STATEMENT OF CASH FLOWS
| (in thousands of euros) | Note | Year ended December 31, 2015 | |||||
|---|---|---|---|---|---|---|---|
CASH FLOWS FROM OPERATING ACTIVITIES | |||||||
Operating result | (15,605 | ) | |||||
Adjustments for non-cash items | |||||||
Amortization of intangible assets | 5 | ||||||
Depreciation of property, plant and equipment | 191 | ||||||
Expense recognized in respect of share-based payments | 2,270 | ||||||
| (13,139 | ) | ||||||
Movements in assets/liabilities | |||||||
(Increase)/decrease in trade and other receivables | 4.5 | (651 | ) | ||||
(Increase)/decrease in other current assets | 4.6 | (362 | ) | ||||
Increase/(decrease) in trade and other payables | 4.10 | (434 | ) | ||||
Increase/(decrease) in deferred revenue | 4.11 | 689 | |||||
Cash used in operating activities | (13,897 | ) | |||||
Interests paid | 0 | ||||||
| | | | | | | | |
NET CASH FLOWS USED IN OPERATING ACTIVITIES | (13,897 | ) | |||||
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | |||||||
Purchase of intangible assets | 4.1 | (5 | ) | ||||
Purchase of property, plant and equipment | 4.2 | (274 | ) | ||||
(Increase)/decrease in current financial assets | 4.7 | 16,979 | |||||
Interest received | 5.8 | 112 | |||||
| | | | | | | | |
NET CASH FLOWS FROM INVESTING ACTIVITIES | 16,812 | ||||||
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | |||||||
Proceeds from issue of shares | 4.9 | 238 | |||||
Transaction costs for equity issue | 4.9 | 0 | |||||
| | | | | | | | |
NET CASH FLOWS FROM FINANCING ACTIVITIES | 238 | ||||||
| | | | | | | | |
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 3,153 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Cash and cash equivalents at the beginning of the period | 32,180 | ||||||
| | | | | | | | |
Exchange gains/losses on cash & cash equivalents | 5.8 | 181 | |||||
Cash and cash equivalents at the end of the period | 4.8 | 35,514 | |||||
The notes are an integral part of these consolidated financial statements.
F-20
ARGENX N.V.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
| | Attributable to owners of the parent | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Share capital | Share premium | Accumulated deficit | Other reserves Equity-settled share-based payment reserve | Total equity attributable to owners of the parent | | Total Equity | ||||||||||||||
Balance Year started January 1, 2015 | 1,571 | 81,940 | (35,806 | ) | 2,378 | 50,082 | 50,082 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss of the period | (15,312 | ) | (15,312 | ) | (15,312 | ) | |||||||||||||||
Issue of share capital | 9 | 229 | 238 | 238 | |||||||||||||||||
Transaction costs for equity issue | 0 | 0 | |||||||||||||||||||
Share-based payment | 2,270 | 2,270 | 2,270 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
Balance Year ended December 31, 2015 | 1,580 | 82,169 | (51,118 | ) | 4,648 | 37,278 | 37,278 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
The notes are an integral part of these consolidated financial statements.
F-21
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. General information
argenx N.V. (the Company) is a public company with limited liability incorporated under the laws of the Netherlands. The Company's official seat is in Rotterdam, the Netherlands, and its registered office is at Willemstraat 5, 4811 AH, Breda, the Netherlands. The principal activities of the Company are described in the General Information section. An overview of the Company and its subsidiaries (the Group) are described in note 7.5.
The Company is listed on Euronext Brussel since July 2014.
2. Significant accounting policies
The principal Group accounting policies are summarized below.
2.1. Statement of compliance and basis of preparation
The consolidated financial statements are prepared in accordance with the International Financing Reporting Standards (IFRS), issued by the International Accounting Standard Board (IASB) and the interpretations issued by the IASB's International Financial Reporting Interpretation Committee. The consolidated financial statements provide a general overview of the Group's activities and the results achieved. They give a true and fair view of the entity's financial position, its financial performance and cash flows, on a going concern basis.
The preparation of consolidated financial statements in conformity with IFRS, issued by the IASB, requires the use of certain critical accounting estimates. It also requires management to exercise its judgment in the process of applying the Group's accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in Note 3.
The principal accounting policies applied in the preparation of the above financial statements are set out below. All amounts are presented in thousands of euros, unless otherwise indicated, rounded to the nearest EUR '000.
2.2. Basis of consolidation
The consolidated financial statements include the financial statements of the Company and entities controlled by the Company (its subsidiaries). Control is achieved where the Company is exposed, or has rights, to variable returns from its involvement with an entity and has the ability to affect those returns through its power over the entity.
Income and expenses of subsidiaries acquired or disposed of during the year are included in the consolidated statement of profit and loss and other comprehensive income from the effective date of acquisition and up to the effective date of disposal, as appropriate. Total comprehensive income of subsidiaries is attributed to the owners of the Company and to the non-controlling interests even if this results in the non-controlling interests having a deficit balance.
When necessary, adjustments are made to the financial statements of subsidiaries to bring their accounting policies into line with those used by other members of the Group.
All intra-group transactions, balances, income and expenses are eliminated in full on consolidation.
F-22
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
2.3. Foreign currency transactions
Functional and presentation currency
The financial statements are presented in euros, which is the Group's functional and presentation currency.
Transactions and balances
Transactions in foreign currencies are translated at the exchange rate ruling at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the exchange rate ruling at the reporting date. Foreign exchange differences arising on translation are recognized in the income statement part of the statement of profit and loss and other comprehensive income. Non-monetary assets and liabilities denominated in foreign currencies are translated at the foreign exchange rate ruling at the date of the transaction.
2.4. Intangible assets
Intangible assets with finite useful lives that are acquired separately are carried at cost less accumulated amortization and accumulated impairment losses. Amortization is recognized on a straight-line basis over their estimated useful lives. The estimated useful life and amortization method are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis. Intangible assets with indefinite useful lives that are acquired separately are carried at cost less accumulated impairment losses.
Intangible assets related to software are amortised over 3 years.
Expenditure on research activities is recognized as an expense in the period in which it is incurred.
An internally-generated intangible asset arising from development (or from the development phase of an internal project) is recognized if, and only if, all of the following have been demonstrated:
The amount initially recognized for internally-generated intangible assets is the sum of the expenditure incurred from the date when the intangible asset first meets the recognition criteria listed above. Where no internally-generated intangible asset can be recognized, research expenditures are
F-23
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
recognized in the statement of profit and loss and other comprehensive income in the period in which they are incurred.
Due to uncertainties inherent to the development and registration with the relevant healthcare authorities of its products, the Company estimates that the conditions for capitalisation are not met until the regulatory procedures required by such healthcare authorities have been finalised. The Company currently does not own products that have been approved by the relevant healthcare authorities. As such, research expenditures not satisfying the above criteria and expenditures in the research phase of internal projects are recognized in the statement of profit and loss and other comprehensive income as they are incurred.
Subsequent to initial recognition, internally-generated intangible assets are reported at cost less accumulated amortization and accumulated impairment losses, on the same basis as intangible assets that are acquired separately.
An intangible asset is derecognized either on disposal or when no future economic benefits are expected from its use. Gains or losses arising from derecognition of an intangible asset, measured as the difference between the net disposal proceeds and the carrying amount of the asset, are recognized in profit or loss when the asset is derecognized.
2.5. Property, plant and equipment
Items of property, plant and equipment held for use in the production or supply of goods or services, or for administrative purposes, are stated in the statement of financial position at their cost, less accumulated depreciation and accumulated impairment losses.
The cost comprises the initial purchase price plus other direct purchase costs (such as non-refundable tax and transport).
Depreciation is recognized at acquisition date (unless asset is not ready for use) so as to write off the cost or valuation of assets (other than freehold land and properties under construction) less their residual values over their useful lives, using the straight-line method. The estimated useful lives, residual values and depreciation method are reviewed at the end of each reporting period, with the effect of any changes in estimate accounted for on a prospective basis.
Unless revised due to specific changes in the estimated useful life, annual depreciation rates are as follows:
An item of property, plant and equipment is derecognized upon disposal or when no future economic benefits are expected to arise from the continued use of the asset. Any gain or loss arising on the disposal or retirement of an item of property, plant and equipment is determined as the difference between the sales proceeds and the carrying amount of the asset and is recognized in profit or loss.
F-24
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
2.6. Leases
Operating lease payments are recognized as an expense on a straight-line basis over the lease term, except where another systematic basis is more representative of the time pattern in which economic benefits from the leased asset are consumed. Contingent rentals arising under operating leases are recognized as an expense in the period in which they are incurred.
In the event that lease incentives are received to enter into operating leases, such incentives are recognized as a liability. The aggregate benefit of incentives is recognized as a reduction of rental expense on a straight-line basis, except where another systematic basis is more representative of the time pattern in which economic benefits from the leased asset are consumed.
2.7. Impairment of assets
At the end of each reporting period, the Company reviews the carrying amounts of its tangible and intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Where it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash-generating unit to which the asset belongs. Where a reasonable and consistent basis of allocation can be identified, corporate assets are also allocated to individual cash-generating units, or otherwise they are allocated to the smallest group of cash-generating units for which a reasonable and consistent allocation basis can be identified.
Intangible assets with indefinite useful lives and intangible assets not yet available for use are tested for impairment at least annually, and whenever there is an indication that the asset may be impaired.
Recoverable amount is the higher of fair value less costs to sell and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset for which the estimates of future cash flows have not been adjusted.
If the recoverable amount of an asset (or cash-generating unit) is estimated to be less than its carrying amount, the carrying amount of the asset (or cash-generating unit) is reduced to its recoverable amount. An impairment loss is recognized immediately in profit or loss.
Where an impairment loss subsequently reverses, the carrying amount of the asset (or a cash-generating unit) is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset (or cash-generating unit) in prior years. A reversal of an impairment loss is recognized immediately in profit or loss.
2.8. Financial assets
Investments in financial assets are divided into various categories. Classification of these investments depends on the purposes for which investments have been acquired. Management determines the classification at the time of the purchase and re-evaluates such designation at each subsequent balance sheet date.
F-25
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
Purchase and sale of financial assets are recognized on the settlement date, which is the date an asset is delivered to or by the Group. The cost of financial assets includes transaction costs.
The carrying amounts of all financial assets in this note are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount is impaired. If objective evidence exists that a financial asset or group of financial assets is impaired, the amount of the impairment loss is calculated as the difference between the carrying amount of the financial asset and the present value of estimated future cash flows, discounted at the original effective interest rate (i.e., the effective interest rate computed at initial recognition of these financial assets). The resulting impairment loss is immediately recognized in net finance costs.
An impairment loss on financial assets is revered if, in a subsequent period, the amount of the impairment loss decreased and this decrease can be related objectively to an event occurring after the impairment loss was recognized. Such reversal is immediately recognized in net finance costs.
2.9. Trade receivables
Trade receivables are initially recognized at fair value and are subsequently carried at amortised cost using the effective interest method. A provision for impairment of trade receivables is established when there is objective evidence that the Company will not be able to collect all amounts due according to the original terms of the receivables.
2.10. Cash and cash equivalents
Cash and cash equivalents includes cash in hand, deposits held at call with banks and other short term highly liquid investments with original maturities of three months or less and with an insignificant risk of changes in value. Bank overdrafts, if any, are shown within borrowings in current liabilities on the statement of financial position.
For the purpose of the statements of cash flows, cash and cash equivalents includes cash on hand and deposits held at call or short term maturity with banks (three months or less with insignificant risk of changes in value), net of bank overdrafts.
2.11. Shareholder's equity
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by the Company are recognized at the proceeds received, net of direct issue costs.
Where the Company purchases the Company's equity share capital (treasury shares), the consideration paid, including any directly attributable incremental costs (net of income taxes) is deducted from equity attributable to the Company's equity holders until the shares are cancelled, reissued or disposed of. Where such shares are subsequently sold or reissued, any consideration received, net of any directly attributable incremental transaction costs and the related income tax effects is included in equity attributable to the Company's equity holders.
F-26
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
2.12. Trade payables
Payables after and within one year are measured at amortised cost, i.e. at the net present value of the payable amount. Unless the impact of discounting is material, the nominal value is taken.
2.13. Provisions
Provisions are recognized when the Company has a present obligation (legal or constructive) as a result of a past event, it is probable that the Company will be required to settle the obligation, and a reliable estimate can be made of the amount of the obligation.
The amount recognized as a provision is the best estimate of the consideration required to settle the present obligation at the end of the reporting period, taking into account the risks and uncertainties surrounding the obligation. When a provision is measured using the cash flows estimated to settle the present obligation, its carrying amount is the present value of those cash flows (where the effect of the time value of money is material).
When some or all of the economic benefits required to settle a provision are expected to be recovered from a third party, a receivable is recognized as an asset if it is reasonably certain that reimbursement will be received and the amount of the receivable can be measured reliably.
2.14. Retirement benefits
The Company offers a post-employment, death, disability and healthcare benefit scheme. All employees have access to these schemes. The death, disability and healthcare benefits granted to employees of the Company are covered by an external insurance company, where premiums are paid annually and charged to the income statement as they were incurred.
The post-employment pension plan granted to employees of the Company is a defined contribution plan under Belgian Law.
Under defined contribution plans, the Company pays contributions based on salaries to organisations responsible for paying out pensions and social security benefits, in accordance with the laws and agreements applicable in each country.
The Belgian defined contribution pension plans are by law subject to minimum guaranteed rates of return, currently 3.25% on employer contributions and 3.75% on employee contributions. These rate have been modified by the law of December 18, 2015 and effective for contribution paid as from 2016 to a new variable minimum return based on the OLO ('Obligation Lineaire Obligaties'—Belgian Government Bond) rates, with a minimum of 1.75% and a maximum of 3.75%.
In theory these plans qualify as defined benefit plans. However, when taken into account the historical discussions on how to account for these specific type of plans where the contributions paid are subject to a minimum guaranteed return at the level of IFRIC, the Company believes the application of the projected unit credit method to these plans is troublesome and will not provide a faithful representation of the liability with respect to these promises.
The Group adopted a retrospective approach whereby the net liability recognized in the statement of financial position was based on the sum of the positive differences, determined by
F-27
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
individual plan participant, between the minimum guaranteed reserves and the accumulated contributions based on the actual rates of return at the closing date.
2.15. Short-term employee benefits
Short-term employee benefits include salaries and social security taxes, paid vacation and bonuses. They are recognized as expenses for the period in which employees perform the corresponding services. Outstanding payments at the end of the period are shown as other current liabilities.
2.16. Share-based payments
Equity-settled share-based payments to employees and others providing similar services are measured at the fair value of the equity instruments at the grant date. Details regarding the determination of the fair value of equity-settled share-based transactions are set out in note 4.12.
The fair value determined at the grant date of the equity-settled share-based payments is expensed on a straight-line basis over the vesting period, based on the Group's estimate of equity instruments that will eventually vest, with a corresponding increase in equity. At the end of each reporting period, the Group revises its estimate of the number of equity instruments expected to vest. The impact of the revision of the original estimates, if any, is recognized in profit or loss such that the cumulative expense reflects the revised estimate, with a corresponding adjustment to the equity-settled share-based payment reserve.
Where the terms of equity—settled share-based payments are modified, the minimum expense recognized is the expense that would have been recognized if the terms had not been modified. An additional expense is recognized for any modification that increases the total fair value of the share—based payments, or is otherwise beneficial to the employee as measured at the date of modification.
2.17. Financial liabilities
Debt and equity instruments issued by the Company are classified as either financial liabilities or as equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument.
Financial liabilities are classified as either "financial liabilities at fair value through profit or loss" or "other financial liabilities".
2.18. Government grants
Government grants are not recognized until there is reasonable assurance that the Company will comply with the conditions attaching to them and that the grants will be received.
Government grants are recognized in profit or loss on a systematic basis over the periods in which the Company recognises as expenses the related costs for which the grants are intended to compensate. Specifically, government grants whose primary condition is that the Company should purchase, construct or otherwise acquire non-current assets are recognized as deferred revenue in
F-28
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
the statement of financial position and transferred to profit or loss on a systematic and rational basis over the useful lives of the related assets.
The benefit of a government loan at a below-market rate of interest is treated as a government grant, measured as the difference between proceeds received and the fair value of the loan based on prevailing market interest rates.
Grants related to research projects received from governmental agencies are recognized at their fair value over the period necessary to match them with the costs that they are intended to compensate, and when there is reasonable assurance the Group will comply with the conditions attached to the grants, but not prior to the formal grant approval. These grants are presented in the income statement as a separate category of other operating income.
2.19. Income taxes
Income tax expense represents the sum of the tax currently payable and deferred tax.
The tax currently payable is based on taxable profit for the year. Taxable profit differs from profit as reported in the statement of profit and loss and other comprehensive income because of items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Company's liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the reporting period.
Deferred tax is recognized on temporary differences between the carrying amounts of assets and liabilities in the financial statements and the corresponding tax bases used in the computation of taxable profit (e.g. differences between carrying amounts under IFRS and the statutory tax bases). Deferred tax liabilities are generally recognized for all taxable temporary differences. Deferred tax assets are generally recognized for all deductible temporary differences to the extent that it is probable that taxable profits will be available against which those deductible temporary differences can be utilised. Such deferred tax assets and liabilities are not recognized if the temporary difference arises from goodwill or from the initial recognition (other than in a business combination) of other assets and liabilities in a transaction that affects neither the taxable profit nor the accounting profit.
The carrying amount of deferred tax assets is reviewed at the end of each reporting period and reduced to the extent that it is no longer probable that sufficient taxable profits will be available to allow all or part of the asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the period in which the liability is settled or the asset realised, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. The measurement of deferred tax liabilities and assets reflects the tax consequences that would follow from the manner in which the Company expects, at the end of the reporting period, to recover or settle the carrying amount of its assets and liabilities.
F-29
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
Deferred tax assets and deferred tax liabilities are offset if there is a legally enforceable right to offset current tax assets and liabilities and if they relate to income taxes imposed by the same authority on the same taxable entity or in different tax entities that intend to settle current tax assets and liabilities on a net basis or their tax assets and liabilities will be realised simultaneously.
2.20. Revenue recognition and other operating income
The Group generates revenue from collaborations and strategic alliances.
Revenue is recognized when it is probable that future economic benefits will flow to the group and these benefits can be measured reliably. Further, revenue recognition requires that all significant risks and rewards of ownership of the goods included in the transaction have been transferred to the buyer or when the related services are performed and specific criteria have been met for each of the Group's activities as described below.
Collaborations
Collaborations typically contain upfront payments, milestone payments, research and development service fees and may involve multiple elements. The Group evaluates whether the elements under these arrangements have value to its collaboration partner or client on a stand-alone basis. If the Group determines that multiple deliverables exist, the consideration is allocated to one or more units of accounting based upon the best estimate of the selling price of each deliverable. Deliverables that do not meet these criteria are not evaluated separately for the purpose of revenue recognition.
The Group receives from these collaborations and strategic alliances upfront, milestone and other similar payments related to the sale of services or out-licensing of products.
The revenue recognition policies can be summarized as follows:
Upfront payments
Upfront payments for which there are subsequent deliverables are initially reported as deferred revenue and are recognized as revenue when earned over the period of the development collaboration or the manufacturing obligation. Upfront payments also include license fees received upfront.
Deferred revenue reflects the part of revenue that has not been recognized as income immediately on receipt of payment and which concerns agreements with multiple components which cannot be separated. Deferred revenue is measured at nominal value.
Milestone payments
Revenue associated with performance milestones is recognized based upon the achievement of the milestone event if the event is substantive, objectively determinable and represents an important point in the development life cycle of the product.
F-30
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
Research and development services fees
Research and development service fees are recognized as revenue over the life of the research agreement as the required services are provided and costs are incurred. These services are usually in the form of a defined number of full-time equivalents (FTE) at a specified rate per FTE.
Commercial collaborations resulting in a reimbursement of research and development (research and development) costs are recognized as revenue as the related costs are incurred. The corresponding research and development expenses are included in research and development expenses in the consolidated financial statements.
Grants, research and development incentives and payroll tax rebates
As a company that carries extensive research and development activities, the Group benefits from various grants, research and development incentives and payroll tax rebates from certain governmental agencies. These grants, research and development incentives and payroll tax rebates generally aim to partly reimburse approved expenditures incurred in research and development efforts of the Group and are credited to the income statement, under other income, when the relevant expenditure has been incurred and there is reasonable assurance that the grants or research and development incentives are receivable.
2.21. Earnings per share
Basic net profit / (loss) per share is computed based on the weighted average number of ordinary shares outstanding during the period, excluding treasury shares.
Diluted net profit / (loss) per share is computed based on the weighted-average number of ordinary shares outstanding including the dilutive effect of options. Options should be treated as dilutive, when and only when their conversion to ordinary shares would decrease net profit per share from continuing operations.
2.22. Fair value measurement
Historical cost is generally based on the fair value of the consideration given in exchange for assets.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value measurement is based on the presumption that the transaction to sell the asset or transfer the liability takes place either in the principal market for the asset or liability or in the absence of a principal market, in the most advantageous market for the asset or liability. The principal or the most advantageous market must be accessible by the Company. The fair value of an asset or a liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest.
F-31
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
All assets and liabilities for which fair value is measured or disclosed in the financial statements are categorised within the fair value hierarchy, described as follows, based on the lowest level input that is significant to the fair value measurement as a whole:
| Level 1—Quoted (unadjusted) market prices in active markets for identical assets or liabilities |
| Level 2—Valuation techniques for which the lowest level input that is significant to the fair value measurement is directly or indirectly observable |
| Level 3—Valuation techniques for which the lowest level input that is significant to the fair value measurement is unobservable |
2.23. Adoption of new and revised standards
The following new standards and amendments to standards are mandatory for the first time for the financial year beginning 1 January 2015:
The above-mentioned Standards and Interpretations do not have a significant impact on the financial statements of the Company.
The following new interpretation and amendments to standards have been issued but are not mandatory for the first time for the financial year beginning 1 January 2015:
F-32
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
F-33
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Significant accounting policies (continued)
line in financial statements globally. Companies using IFRS will be required to apply the revenue standard for annual periods beginning on or after January 1, 2018.
The Group anticipates that the above-mentioned Standards and Interpretations will not have a significant impact on the financial statements of the Company in the period of initial application except for IFRS 15 and IFRS 16 for which the impact is currently being investigated.
2.24. Segment reporting
Segment results include revenue and expenses directly attributable to a segment and the relevant portion of revenue and expenses that can be allocated on a reasonable basis to a segment. Segment assets and liabilities comprise those operating assets and liabilities that are directly attributable to the segment or can be allocated to the segment on a reasonable basis. Segment assets and liabilities do not include income tax items. The Group manages its activities and operates as one business unit which is reflected in its organizational structure and internal reporting. The Group does not distinguish in its internal reporting different segments, neither business nor geographical segments. The chief operating decision-maker is the Board of Directors.
3. Critical accounting judgements and key sources of estimation uncertainty
In the application of the Company's accounting policies, which are described above, the Company is required to make judgements, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
F-34
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty (continued)
The following area are areas where key assumptions concerning the future, and other key sources of estimation uncertainty at the end of the reporting period, have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year:
Going concern
The Group has incurred net losses since its inception and on December 31, 2015, its consolidated statement of profit and loss and other comprehensive income reflects a net loss, and its consolidated statement of financial position includes a loss carried forward. On March 9, 2016, the Board has reviewed and approved the consolidated financial statements and accounting standards. Taking into account the cash position of €42.3 million on December 31, 2015 and the €16.0 million of proceeds from the subsequent increase of capital in January 2016, the Board is of the opinion that it can submit the annual accounts prepared for the Group on a going concern basis.
Whilst the current cash position is sufficient for the Group's immediate and mid-term needs, the Board pointed out that if the research and development activities continue to deliver added value, argenx may seek additional funding to support the continuing development of its portfolio of products or to be able to execute other business opportunities.
Revenue recognition
For revenue recognition, the significant estimates relate to allocation of value to the separate elements in multiple-element arrangements. With respect to the allocation of value to the separate elements, the Company is using the stand-alone selling prices or management's best estimates of selling prices to estimate the fair value of the elements and account for them separately. Revenue is allocated to each deliverable based on the fair value of each individual element and is recognized when the revenue recognition criteria described above are met.
Upfront fees under collaboration or licensing agreements are recognized over the expected duration of the performance obligations, unless there is no continuous involvement required. Management estimates this period at the start of the collaboration and validates the remaining estimated collaboration term at each closing date.
Measurement of share-based payments
In accordance with IFRS 2—Share-based Payment, the fair value of the options at grant date is recognized as an expense in the statement of profit and loss and other comprehensive income over the vesting period, the period of delivery of work. Subsequently, the fair value equity-settled is not re-measured.
The fair value of each warrant granted during the year is calculated using the Black-Scholes pricing model. This pricing model requires the input of subjective assumptions, which are detailed in note 4.12.
Recognition of deferred tax assets
Deferred tax assets are recognized only if management assesses that these tax assets can be offset against positive taxable income within a foreseeable future.
F-35
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Critical accounting judgements and key sources of estimation uncertainty (continued)
This judgment is made on an ongoing basis and is based on budgets and business plans for the coming years, including planned commercial initiatives.
Since inception, the Company has reported losses, and as a consequence, the Company have unused tax losses. Therefore, management has concluded that deferred tax assets should not be recognized as of December 31, 2015. The deferred tax assets are currently not deemed to meet the criteria for recognition as management is not able to provide any convincing positive evidence that deferred tax assets should be recognized.
4. Notes relating to the consolidated statement of financial position
4.1. Intangible assets
| (in thousands of euros) | | |||
|---|---|---|---|---|
Opening balance on January 1, 2015 | ||||
Purchase price | 67 | |||
Accumulated depreciation | (60 | ) | ||
Book value at the beginning of the year | 7 | |||
Movements | ||||
Investments | 5 | |||
Depreciation | (5 | ) | ||
Balance on December 31, 2015 | ||||
Purchase price | 72 | |||
Accumulated depreciation | (65 | ) | ||
Book value at year end | 7 | |||
The intangible assets correspond to software. There are no commitments to acquire additional intangible assets.
No intangible assets are pledged as security for liabilities nor are there any intangible assets whose title is restricted.
4.2. Property, plant and equipment
| (in thousands of euros) | IT equipment | Office and lab equipment | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Opening balance on January 1,2015 | ||||||||||
Purchase price | 63 | 935 | 998 | |||||||
Accumulated depreciation | (48 | ) | (784 | ) | (832 | ) | ||||
Book value at the beginning of the year | 15 | 151 | 166 | |||||||
Movements | ||||||||||
Investments | 30 | 244 | 274 | |||||||
Depreciation | (18 | ) | (173 | ) | (191 | ) | ||||
Closing balance on December 31, 2015 | ||||||||||
Purchase price | 92 | 1,179 | 1,272 | |||||||
Accumulated depreciation | (66 | ) | (957 | ) | (1,023 | ) | ||||
Bookvalue at year end | 27 | 222 | 249 | |||||||
F-36
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Notes relating to the consolidated statement of financial position (continued)
There are no commitments to acquire property, plant and equipment.
Furthermore, no items of property, plant and equipment are pledged.
4.3. Non-current financial assets
Non current financial assets consist of minority participations in Bird Rock Bio (formerly RuiYi Inc.) and Fair Journey LDA. The company has no significant influence over these investments. These investments are qualified as "fair value through other comprehensive income"—investments and if no reliable fair value measurements are available, valued at cost. At the end of 2015, both investments were recorded at cost as no reliable fair value information was available.
4.4. Research and development incentive receivables
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Research and development incentive related to research and development expenditure | 1,568 | |||
On December 31, 2015, the Group has recorded a tax receivable of €1.6 million, in relation with a research and development incentive tax scheme in Belgium under which the research and development incentives can be refunded after five years if not offset against future income tax expense. The research and development incentives are recorded in other operating income (see note 5.2) in the consolidated statement of profit and loss and other comprehensive income. These amounts are expected to be gradually reimbursed in cash as from 2017 onwards.
4.5. Trade and other receivables
The trade and other receivables are composed of receivables which are detailed below:
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
VAT receivable | 175 | |||
Trade receivables | 719 | |||
Interest receivable | 17 | |||
IWT grants to receive | 445 | |||
| | | | | |
| 1,356 | ||||
| | | | | |
| | | | | |
| | | | | |
The nominal amounts of all trade and other receivables approximate their respective fair values.
The VAT receivable related to VAT amounts to be recovered in the first quarter of 2016.
Trade receivables correspond to amounts invoiced to the collaborators or strategic allies of the Group. No trade receivables were past due on December 31, 2015. The IWT grant to receive
F-37
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Notes relating to the consolidated statement of financial position (continued)
consists of earned income from government grants for which no payments have been received but for which the relating expenditures have been incurred.
For more information on the government grants to receive from IWT see note 5.2.
4.6. Prepaid expenses
The prepaid expenses on December 31, 2015 amount to €0.5 million and relates primarily to a success fee paid to a third party involved in the license agreement signed with LEO Pharma. The amount will be recognized as expense in the profit and loss statement over the period of the agreement.
4.7. Current financial assets
On December 31, 2015, the current financial assets amounted to €6.8 million and corresponded to financial instruments in the form of money market funds with a recommended maturity of 6 months. These funds are highly liquid investments and can be readily convertible into a known amount of cash. Because of their historical volatility these funds cannot be classified as cash and cash equivalents. Values recognized on the balance sheet are the fair values.
Please also refer to note 6.1 for more information on the financial instruments.
4.8. Cash and cash equivalents
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Cash equivalents | 11,006 | |||
Cash and bank balances | 24,508 | |||
| | | | | |
| 35,514 | ||||
| | | | | |
| | | | | |
| | | | | |
On December 31, 2015, cash and cash equivalents amounted to €35.5 million and included (i) cash on hand and (ii) current and savings accounts in different banks.
4.9. Shareholders' Capital
Roll forward of number of shares outstanding:
Number of shares outstanding on January 1, 2015 | 15,705,112 | |||
Exercise of stock options on 01/09/15 | 97,655 | |||
Number of shares outstanding on 12/31/2015 | 15,802,767 |
New shares issued in 2015
As result of the exercise of stock options under the company's Employee Stock Option Plan 97,655 new shares were issued in September 2015.
F-38
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Notes relating to the consolidated statement of financial position (continued)
This results in a total of 15,802,767 ordinary shares with a nominal value of €0.1 per share on December 31, 2015.
The authorised unissued share capital of the Company amounts to €4.5 million divided into 45 million ordinary shares.
4.10. Trade and other payables
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Trade payables | 1,886 | |||
Accruals for invoices to be received | 825 | |||
Short-term employee benefits | 1,418 | |||
Accrued expenses | 414 | |||
| | | | | |
| 4,543 | ||||
| | | | | |
| | | | | |
| | | | | |
Trade payables correspond primarily to clinical and manufacturing activities. The fair value of trade payables approximate their carrying amount, no trade payables were overdue.
The accruals for invoices to be received correspond mainly to invoices not yet received from suppliers. The total amount of €0.8 million includes (i) an amount of €0.1 million related to invoices to be received from a clinical manufacturing organization for the manufacturing of drug products to be used in clinical trials (ii) an amount of €0.6 million related to invoices to be received from a clinical research organisation for the pass-through expenses incurred by clinical sites used in relation with the ongoing clinical trials of ARGX110 and ARGX113 and not yet recharged to the Group.
Short-term employee benefits include payables and accruals for salaries and bonuses to be paid to the employees of the Group.
4.11. Deferred revenue
Deferred revenue relates to cash received from collaborations and strategic alliances prior to completion of the earnings process. In 2015, deferred revenue amounted to €4.1 million and correspond principally to the payments received from the alliance signed with LEO Pharma in May 2015. These payments are recognized as revenue over the estimated duration of argenx' involvement in the research and development programs provided for under the terms of the agreements.
4.12. Share-based payments
The Company has a stock options scheme for the employees of the Company and its subsidiaries. In accordance with the terms of the plan, as approved by shareholders, employees may be granted options to purchase ordinary shares at an exercise price as mentioned below per ordinary share.
F-39
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Notes relating to the consolidated statement of financial position (continued)
The Group has granted on June 18, 2015 at total of 56,500 stock options, on September 3, 2015 a total of 3,000 stock options and on December 15, 2015 a total of 243,400 stock options to employees and consultants. The total number of stock options outstanding at December 31, 2015 totals 1,752,926. No stock options are expired and 97,656 stock options have been exercised as of December 31, 2015. A total of 47,333 stock options have been forfeited as of December 31, 2015.
The stock options are granted to employees, consultants or directors of the Company and its subsidiaries. The stock options have been granted free of charge. Each employee's stock option converts into one ordinary share of the Company upon exercise. No amounts are paid or payable by the recipient on receipt of the option. The options carry neither rights to dividends nor voting rights. Options may be exercised at any time from the date of vesting to the date of their expiry.
The stock options granted vest, in principle, as follows:
No other conditions are attached to the stock options.
The following share-based payment arrangements were in existence during the current and prior years and which are exercisable at closing of each period presented:
| | Outstanding stock options | ||||||
|---|---|---|---|---|---|---|---|
Expiry date | Exercise price per stock options (in EUR) | Year ended December 31, 2015 | |||||
2019 | 3.95 | 103,370 | |||||
2020 | 3.95 | 62,460 | |||||
2021 | 3.95 | 3,800 | |||||
2021 | 2.44 | 275,520 | |||||
2021 | 2.44 | 157,530 | |||||
2021 | 2.44 | 83,820 | |||||
2021 | 3.95 | 55,747 | |||||
2021 | 2.44 | 169,862 | |||||
2024 | 7.17 | 537,917 | |||||
2025 | 11.44 | 56,500 | |||||
2025 | 10.34 | 3,000 | |||||
2025 | 9.47 | 243,400 | |||||
| | | | | | | | |
| 1,752,926 | |||||||
| | | | | | | | |
F-40
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Notes relating to the consolidated statement of financial position (continued)
| | 2015 | ||||||
|---|---|---|---|---|---|---|---|
| | Number of stock options | Weighted average exercise price (in EUR) | |||||
Outstanding on January 1 | 1,595,015 | 4.39 | |||||
| | | | | | | | |
Granted | 302,900 | 9.84 | |||||
Exercised | (97,656 | ) | 2.44 | ||||
Forfeited | (47,333 | ) | 7.17 | ||||
| | | | | | | | |
Outstanding on December 31 | 1,752,926 | 5.37 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Exercisable on December 31 | 1,366,703 | 4.41 | |||||
The weighted average remaining contractual life of the stock options outstanding amounts to 7.28 years as of December 31, 2015. The table below shows the weighted average remaining contractual life for each range of exercise price:
Exercise price (in euros) | Outstanding options on December 31, 2015 | Weighted average remaining contractual life (in years) | |||||
|---|---|---|---|---|---|---|---|
2.44 - 3.95 | 912,109 | 5.43 | |||||
7.17 - 11.44 | 840,817 | 9.29 | |||||
The fair market value of the stock options has been determined based on the Black and Scholes model. The expected volatility in the model is based on the historical volatility of peer companies and historical volatility of the Group since its initial public offering.
Below is an overview of the parameters used in relation to the new grant during 2015:
Stock options granted in | June 2015 | Sept 2015 | Dec 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Number of options granted | 56,500 | 3,000 | 243,400 | |||||||
Average fair value of options (in EUR) | 7.79 | 6.79 | 6.25 | |||||||
Share price (in EUR) | 11.58 | 10.24 | 9.85 | |||||||
Exercise price (in EUR) | 11.44 | 10.34 | 9.47 | |||||||
Expected volatility | 59 | % | 59 | % | 58 | % | ||||
Average expected option life (in years) | 10 | 10 | 10 | |||||||
Risk-free interest rate | 1,21 | % | 1.08 | % | 0.98 | % | ||||
Expected dividends | 0 | % | 0 | % | 0 | % | ||||
The total share-based payment expense recognized in the consolidated statement of comprehensive income totalled €2.3 million for the year ended December 31, 2015.
F-41
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income
5.1. Revenue
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Upfront payments | 2,194 | |||
Milestone payments | 343 | |||
Research and development service fees | 4,317 | |||
| | | | | |
| 6,854 | ||||
| | | | | |
| | | | | |
| | | | | |
The upfront payments in 2015 corresponds principally to the partial recognition in revenue over the period of the upfront payments received following the signatures of the collaboration agreements with Bayer and Shire respectively in May and June 2014 and a with LEO Pharma in May 2015. These upfront payments are recognized in revenue over the period based on the progress of the research and development programs subjects of these collaborations.
The research and development service fees correspond principally to FTE-payments received under the collaboration agreements with Bayer, Shire and LEO Pharma as indicated above.
The Group leverages its suite of antibody technology platforms and know-how in strategic alliances with pharmaceutical companies, where the focus is on antibody drug discovery targeting complex and novel targets across multiple therapeutic areas. The most significant active collaborations are explained below:
Shire
In February 2012 the Group entered into a research collaboration and exclusive product license option agreement with Shire International GmbH (Shire). Pursuant to the agreement the Group is using its SIMPLE Antibody™ Technology to create novel human therapeutic antibodies addressing diverse rare and unmet diseases being pursued by Shire. Shire has the option to license the most promising antibody leads from each collaborative program for further developments and commercialization worldwide, in return for milestone and royalty payments. Under the terms of the license, the Group has already received technology access fees and research funding and is eligible to receive discovery milestone payments. In September 2013, the Group received a first technical success milestone payment from Shire, and in January 2014, the Group received two extra discovery milestone payments from Shire. In January 2013 the scope of the agreement was expanded by the parties with no change to the agreement structure.
On May 30, 2014 the collaboration between Shire and the Group was expanded to include in addition to the use of the Group's entire suite of human antibody discovery technologies for an expanded set of disease targets. Pursuant to the amended agreement (which is in addition to the existing collaboration), the Group shall apply during multiple years these technologies for the generation and development of human mAbs against multiple targets selected by Shire in line with its therapeutic focus.
F-42
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
Shire has the option to license the most promising antibody leads for further developments and commercialization worldwide, in return for fees, clinical, regulatory and sales milestones, as well as single digit royalties on therapeutic product sales. As of the reporting date, this is considered contingent revenue. Shire will be responsible for clinical development and commercialization of products, with the Group having the right to license any programs not pursued by Shire into its own development pipeline. Under the amended agreement, Shire made an upfront cash payment of €3 million. At the same time as expanding the collaboration, Shire made an equity investment during the Group's IPO in July 2014 of €12 million.
The upfront cash payment is recognized based on the principle of percentage of completion of the work plan. Research funding based on an agreed FTE-rate, is recognized on a monthly basis in the income statement.
Bayer
In May 2014 the Group entered into a research collaboration and exclusive product license option agreement with Bayer AG. Pursuant to the agreement the Group is using its SIMPLE Antibody Technology to create novel human therapeutic antibodies addressing complex targets from various therapeutic areas. Bayer has the option to license the most promising antibody leads from each collaborative program for further developments and commercialization worldwide, in return for milestone payments. Under the terms of the license, the Group has already received technology access fees and research funding and is eligible to receive preclinical success payments, which are, as of the reporting date, considered contingent revenue.
Technology access fees are recognized based on the principle of percentage of completion of the work plan. Research funding based on an agreed FTE-rate, is recognized on a monthly basis in the income statement.
Leo Pharma
In May 2015 the Group and LEO Pharma A/S, a global healthcare company dedicated to helping people achieve healthy skin, entered into an alliance in which they will collaborate to develop innovative antibody-based solutions for the treatment of chronic inflammation underlying many skin conditions.
Under the terms of the agreement, LEO Pharma received exclusive access to an existing argenx antibody currently in preclinical development for inflammation-related skin diseases. The Group receives pre-IND payments of €4.5 million, including an upfront payment. The companies will co-fund product development costs up to clinical trial application (CTA) filing.
The Group will also receive clinical, regulatory and sales milestone payments, as well as tiered, potentially double digit royalties on resulting products, which are, as of the reporting date, considered contingent revenue.
F-43
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
Access fee to the existing argenx antibody is recognized based on the principle of percentage of completion of the work plan. Development and management funding based on an agreed FTE-rate, is recognized on a monthly basis in the income statement.
5.2. Other operating income
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Government grants | 1,598 | |||
Research and development incentives | 608 | |||
Payroll tax rebates | 895 | |||
| | | | | |
| 3,101 | ||||
| | | | | |
| | | | | |
| | | | | |
Government grants
The agency for Flanders Innovation & Entrepreneurship Agency (IWT), provided argenx with several grants.
On December 31, 2015, the situation of the grants received by argenx reflects the expenses incurred by the Group in the various research and development projects sponsored by IWT and is as follows:
1) IWT—TGO
| | (in thousands of euros) | |||
|---|---|---|---|---|
Grantor: IWT | ||||
Start date: | 01/01/2013 | |||
End date: | 12/31/2016 | |||
Amount granted and approved by IWT: | 2,697 | |||
Amount received: | 2,429 | |||
2) IWT—Baekelandt
Grantor: IWT | ||||
Start date: | 01/01/2014 | |||
End date: | 12/31/2017 | |||
Amount granted and approved by IWT: | 277 | |||
Amount received: | 150 |
F-44
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
3) IWT 4
Grantor: IWT | ||||
Start date: | 01/01/2015 | |||
End date: | 12/31/2017 | |||
Amount granted and approved by IWT: | 1,568 | |||
Amount received: | 885 |
No conditions related to the above government grants are unfulfilled, nor are there any contingencies related thereon at the date of the approval of these financial statements, except for those described in note 7.2 of this report.
Research and developments incentives
argenx has accounted for a tax receivable of €0.6 million in 2015 following an research and development tax incentive scheme in Belgium according to which the incentive will be refunded after a 5 year period, if not offset against the taxable basis over the period. (see also note 4.4).
Payroll tax rebates
argenx received €0.9 million in 2015 as a reduction in withholding income taxes for its highly-qualified personnel employed in its research and development department.
5.3. Segment reporting
The Group operates from Belgium and the Netherlands. Revenues are invoiced by the holding company in the Netherlands and are generated by clients geographically located as shown in the table below. In the table next to this, it is indicated where the non-current assets from the group are situated.
| (in thousands of euros) | Revenue from external customers Year ended December 31, 2015 | Non-current assets Year ended December 31, 2015 | |||||
|---|---|---|---|---|---|---|---|
Netherlands | 275 | 1 | |||||
Belgium | 1,824 | ||||||
Germany | 2,190 | ||||||
Denmark | 827 | ||||||
Switzerland | 3,127 | ||||||
United States | 435 | ||||||
| | | | | | | | |
Total | 6,854 | 1,825 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-45
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
Information about major clients:
From the €6.9 million received from upfront payments, milestone payments and research and development fees, €3.1 million come from the Group's largest client, €2.2 million from its second largest client and €0.8 million from its third largest client.
5.4. Research and development expenses
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Personnel expenses | 6,665 | |||
External research and development expenses | 11,653 | |||
Materials and consumables | 1,050 | |||
Depreciation and amortization | 196 | |||
Other expenses | 1,071 | |||
| | | | | |
| 20,635 | ||||
| | | | | |
| | | | | |
| | | | | |
5.5. General and administrative expenses
(in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Personnel expenses | 1,607 | |||
Consulting fees | 2,395 | |||
Supervisory board | 165 | |||
Office costs | 758 | |||
| | | | | |
| 4,925 | ||||
| | | | | |
| | | | | |
| | | | | |
5.6. Personnel expenses
The personnel expenses, which excludes consultants mentioned above, are as follows:
(in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Short-term employee benefits—Salaries | 5,316 | |||
Short-term employee benefits—Social Security | 802 | |||
Post-employment benefits | 207 | |||
Share-based payment | 1,945 | |||
| | | | | |
| 8,270 | ||||
| | | | | |
| | | | | |
| | | | | |
The post-employment benefits relate to the pension plans the company has in place for its employees.
F-46
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
The share-based payment increase in 2015 is due to the additional stock options granted to employees, directors and consultants during the period.
The number of full-time equivalents (FTE) employees by department is presented below:
Number of FTE | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Research and development | 31.4 | |||
General and administrative | 5.8 | |||
| | | | | |
| 37.2 | ||||
| | | | | |
| | | | | |
| | | | | |
These FTE's are working outside the Netherlands.
5.7. Operating leases
Operating lease payments recognized as an expense in the statement of profit and loss and other comprehensive income amount to €0.2 million in 2015. The Group's future operating lease commitments are as follows:
Operating Lease commitments (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Not later than 1 year | 630 | |||
Later than 1 year and not later than 5 years | 1,272 | |||
Later than 5 years | 0 | |||
| | | | | |
| 1,902 | ||||
| | | | | |
| | | | | |
| | | | | |
The Group has a lease plan for the company's cars with maturity dates up to four years.
For the laboratory and office space, the Group has a lease agreement in Zwijnaarde Belgium with maturity date in 2016, for which a termination notice was given in 2014 and that will expire in April 2016.
In 2015 the Group has signed a binding term sheet for a new lease for new laboratory and office spaces in Ghent. The new lease agreement will be for a period of nine years starting from April 1, 2016, with the possibility to terminate the lease by giving a notice of at least twelve months in advance at the occasion of the third and sixth anniversary of the agreement.
For its offices in the Netherlands the Company has a lease agreement renewable on an annual base.
No purchase options are in effect under the lease agreements described above.
F-47
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
5.8. Financial result and exchange gains/(losses)
(in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Interest income on bank deposits | 76 | |||
Net gains on investments | 36 | |||
| | | | | |
Financial income | 112 | |||
Net losses on investments | ||||
Other financial expenses | 0 | |||
| | | | | |
Financial expenses | 0 | |||
Exchange gains/(losses) | 181 | |||
| | | | | |
| 293 | ||||
| | | | | |
| | | | | |
| | | | | |
Financial income corresponds to the return on the financial investments of the Group's cash and cash equivalents and financial instruments. Net gains on investments relate to the money market funds with a maturity more than 3 months.
The exchange gains of €0.2 million in December 2015 were realized by converting USD accounts into EUR at a favourable conversion rate.
5.9. Retirement benefit obligations
The post-employment benefits of the Belgian employees of the Group are defined contribution plans for which a minimum return is guaranteed until retirement. The Group funds the plan by paying a fixed percentage of the monthly salary of the employee to the external insurance company in addition to an employee contribution. There is a risk that the Company may have to pay additional contributions related to past service. Any such additional contributions will depend on the actual investment returns as well as the future evolution of the minimum guaranteed rates of return.
As a consequence of the law of December 18, 2015, minimum returns are guaranteed by the employer as follows:
In 2014, under the previous legal framework, the application of the Projected Unit Credit ('PUC') method was considered problematic, and there was uncertainty with respect to the future evolution of the minimum guaranteed rates of return. As a consequence, the Group adopted a retrospective
F-48
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
approach whereby the net liability recognized in the statement of financial position was based on the sum of the positive differences, determined by individual plan participant, between the minimum guaranteed reserves and the accumulated contributions based on the actual rates of return at the closing date.
On December 31, 2015 a liability of €0.01 million was recognized in the balance sheet as the sum of the positive difference per plan participant between the minimum guaranteed reserves of €0.3 million and the accumulated reserves of €0.3 million. The impact in the consolidated income statement is a past service cost recognized in personnel expenses. The total expense recognized in the consolidated income statement for contributions made under these defined contribution plans amount to €0.2 million in 2015.
The expected 2016 employer contributions amount to approximately €0.2 million. The weighted average age of the plan participants equals 46 years on December 31, 2015.
5.10. Income taxes
The income tax expense for the year can be reconciled to the accounting profit (loss) as follows:
(in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Current income taxes | 0 | |||
Total | 0 | |||
Loss of the year | (15,312 | ) | ||
Research and development capitalization | (676 | ) | ||
IWT Grants | (1,557 | ) | ||
Stock issuance costs | 0 | |||
Share-based payments | 2,270 | |||
Other | (15 | ) | ||
Total taxable result | (15,290 | ) | ||
Corporate tax is calculated at 25%, which is the tax rate applicable in the Netherlands, of the estimated assessable profit of the year. Current group result before tax is a loss before tax as well as last year. The applied tax rate for the other territorial jurisdiction (Belgium) is the tax rate applicable in that jurisdiction (33.99%). For the purposes of the above overview the effect of difference is tax rate between both jurisdictions in considered not to be material.
The unrecognized deferred tax asset on deductible temporary differences, unused tax losses and unused tax credits amount to €15.6 million on December 31, 2015.
The Group has unused tax losses carry forward. This, combined with other temporary differences, results in a net deferred tax asset position. The unused tax losses carry forward will expire between 2017 and 2025.
F-49
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
Due the uncertainty surrounding the Group's ability to realise taxable profits in the near future, the Company did not recognise any deferred tax assets.
5.11. Loss per share
(in thousands of euros except number of shares and EPS) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Loss of the year | (15,312 | ) | ||
Weighted average number of shares outstanding | 15,734,007 | |||
Basic and diluted loss per share (in €) | (0.97 | ) | ||
Earnings/losses per ordinary share are calculated by dividing the net result attributable to shareholders by the weighted average number of ordinary shares during the year.
As the Group is suffering operating losses, options have an anti-dilutive effect. As such, there is no difference between basic and diluted earnings/losses per ordinary share.
6.1. Overview of financial instruments
| | Year ended December 31, 2015 | ||||||
|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Carrying amount | Fair value | |||||
Non-current financial assets | 1 | 1 | |||||
| | | | | | | | |
Financial assets available for sale | 1 | 1 | |||||
Current financial assets | 6,813 | 6,813 | |||||
| | | | | | | | |
Financial assets at fair value through P/L | 6,813 | 6,813 | |||||
Trade and other receivables | 1,356 | 1,356 | |||||
Cash and bank balances | 35,514 | 35,514 | |||||
| | | | | | | | |
Loans and receivables | 36,869 | 36,869 | |||||
| | | | | | | | |
Total financial assets | 43,684 | 43,684 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Trade and other payables | 4,543 | 4,543 | |||||
| | | | | | | | |
Financial liabilities at amortized cost | 4,543 | 4,543 | |||||
| | | | | | | | |
Total financial liabilities | 4,543 | 4,543 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Financial assets at fair value through P/L:
F-50
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
value at reporting date. These investment funds are recognized at fair value in the Group's financial statements (level 1). The fair value corresponds to the quoted market price and can therefore be classified as a level 1 fair value measurement. The net (NAV asset value) of the funds is available on a daily basis. Any difference between amounts invested and fair value at reporting date is taken in P/L.
Loans and receivables:
Financial liabilities:
Due to the current nature of the financial liabilities, the nominal value of all financial liabilities presented above approximates their fair value.
Fair value hierarchy:
The Group carried the following assets at fair value on December 31, 2015 respectively:
| | Year ended December 31, 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (in thousands of euros) | Level 1 | Level 2 | Level 3 | ||||||
Non-current financial assets | 1 | ||||||||
Current financial assets | 6,813 | ||||||||
| | | | | | | | | | |
Assets carried at fair value | 6,813 | 1 | |||||||
| | | | | | | | | | |
All assets and liabilities for which fair value is measured or disclosed in the financial statements are categorised within the fair value hierarchy, described as follows, based on the lowest level input that is significant to the fair value measurement as a whole:
During the calendar year, no transfer occurred between the applicable categories. Given the insignificant value of the Group's assets categorised as Level 3, the additional Level 3 disclosures have been omitted.
F-51
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
6.2. Capital risk
The Group manages its capital to ensure that it will be able to continue as a going concern. The capital structure of the Group consists of limited or no financial debt and equity attributed to the holders of equity instruments of the Group, such as capital, reserves and retained earnings as mentioned in the consolidated statement of changes in equity. The Group makes the necessary adjustments in the light of changes in the economic circumstances, risks associated to the different assets and the projected cash needs of the current and projected research activities. The current cash situation of €42.3 million on December 31, 2015. The total capital amounts to €83.7 million on December 31, 2015. The Group's objective is to maintain the capital structure at a level to be able to finance its activities for at least twelve months. Cash income from existing and new partnerships is taken into account and, if needed and possible, the Group can issue new shares or enter into financing agreements.
6.3. Credit risk
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Group. The Group has adopted a policy of only dealing with creditworthy counterparties and obtaining sufficient collateral, where appropriate, as a means of mitigating the risk of financial loss from defaults. Concentrations in credit risk are determined based on an analysis of counterparties and their importance on the overall outstanding contractual obligations at year end.
The Group has a limited number of collaboration partners and therefore has a significant concentration of credit risk. However, it has policies in place to ensure that credit exposure is kept to a minimum and significant concentrations of credit exposure are only granted for short periods of time to high credit quality collaboration partners.
Credit exposure is controlled by counterparty limits that are reviewed and approved by management annually.
Cash and cash equivalents and short-term deposits are invested with highly reputable banks and financial institutions. The Group holds its cash and cash equivalents with different banks which are independently rated with a minimum rating of 'A'.
The Group also holds short term investment funds in the form of money market funds with a recommended maturity of 6 months maximum but with a low historical volatility. These money market funds are highly liquid investments, can be readily convertible into a known amount of cash. Since they are a basket of funds there is no individual credit risk involved.
The average credit rating of the underlying instruments for the investment fund with a recommended maturity period of 6 months is BBB+.
The maximum credit risk, to which the Group is theoretically exposed as at the balance sheet date, is the carrying amount of the financial assets.
F-52
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
At the end of the reporting period no financial assets were past due, consequently no financial assets were subject to impairment.
6.4. Liquidity risk
The Group manages liquidity risk by maintaining adequate reserves, by continuously monitoring forecast and actual cash flows, and by matching the maturity profiles of financial assets and liabilities.
The Group's main sources of cash inflows are obtained through capital increases and collaboration agreements. This cash is invested in savings accounts and short term investment funds in the form of money market funds. These money market funds represent the majority of the Group's available sources of liquidity however since all of these are immediately tradable and convertible in cash they have a limited impact on the liquidity risk.
All financial liabilities have a maturity within 3 months unless otherwise disclosed in these financial statements.
6.5. Interest rate risk
The Group is exposed to interest rate risk through its investments in money market funds as described in note 6.1.
Given the short-term nature of these investments the sensitivity towards interest rate fluctuations is deemed not to be significant. If applicable interest rates would increase/decrease with 25 basis points this would have a positive/negative impact of €0.06 million.
6.6. Foreign exchange risk
The Group undertakes transactions denominated in foreign currencies; consequently, exposures to exchange rate fluctuations arise.
The Group is mainly exposed to the US Dollar and GBP.
The net exposure to exchange differences of the monetary assets (being cash and cash equivalents) of the Group at the end of the reporting period are as follows:
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
USD | 345 | |||
GBP | 0 | |||
If the USD/EUR exchange rate would increase/decrease with 10%, this would have a negative/positive impact of €0.03 million. If the GBP/EUR exchange rate would increase/decrease with 10%, this would have no significant impact.
F-53
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Notes to consolidated statement of profit and loss and other comprehensive income (continued)
10% is the sensitivity rate used when reporting foreign currency risk internally to key management personnel and represents management's assessment of the reasonably possible change in foreign exchange rates. The sensitivity analysis includes only outstanding foreign currency denominated monetary items and adjusts their translation at the period end for a 10% change in foreign currency rates.
7. Other disclosures
7.1. Related party transactions
Amongst the shareholders of the Company, there are several minority investors and venture capitalist funds which individually do not hold a significant influence on the Company. Balances and transactions between the Company and its subsidiaries, which are related parties of the Company, have been eliminated on consolidation and are not disclosed in this note. There were no significant transactions with related parties during the period, other than compensation of key management personnel.
Compensation of key management personnel
Key management personnel of the Company is composed of the Chief Executive Officer, the Chief Financial Officer, the Chief Scientific Officer, the Chief Development Officer, the Chief Medical Officer, and the Vice President of Business Development.
The remuneration of the independent directors and other members of key management personnel during the year was as follows:
| (in thousands of euros) | Year ended December 31, 2015 | |||
|---|---|---|---|---|
Short term employee benefits | 1,482 | |||
Post employment benefits | 59 | |||
Termination benefits | 124 | |||
Share-based payment | 1,761 | |||
| | | | | |
| 3,426 | ||||
| | | | | |
| | | | | |
| | | | | |
7.2. Contingencies
The Group is currently not facing any outstanding claims or litigations that may have a significant adverse impact on the Group's financial position.
As described in note 5.2 the Group has received several types of government grants which are granted subject to a certain number of conditions that need to be met at grant date and in the future. The Group recognizes grant income from Belgian and Flemish, grant bodies when all contractual conditions are met. These government institutions may however subsequently perform an audit which may result in a (partial) claw back of the grant. The Group deems that the claw back risk is remote in view of the continuous monitoring of the contractual conditions. Currently the Group has fulfilled all the existing conditions relating to the recognition of its grant income. Contracts with these grant bodies also typically include clauses that define the need for future validation of the project
F-54
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Other disclosures (continued)
results after completion of the initial grant term during which the subsidised expenses or investments have been incurred and for which the grant was earned. Should this validation not occur or be deemed inadequate, the grant bodies have the right to reclaim funds previously granted.
7.3. Commitments
At closing date, there were no commitments signed for the acquisition of property, plant and equipment or intangible assets.
For information on the operating leases see note 5.7.
7.4. Audit Fees
The following auditors' fees were expensed in the income statement:
| | | | | | |||||
|---|---|---|---|---|---|---|---|---|---|
| | | | | | | | | | |
| | Fees in thousands of euros | | 2015 | | |||||
| | | | | | | | | | |
| Audit fees | 70 | |||||||
| | | | | | | | | | |
| Audit related fees | 35 | |||||||
| | | | | | | | | | |
| Tax and other services(1) | 3 | |||||||
| | | | | | | | | | |
| Total(2) | 108 | |||||||
| | | | | | | | | | |
7.5. Overview of consolidation scope
The parent company argenx N.V. is domiciled in the Netherlands.
Details of the Group's subsidiaries at the end of the reporting period are as follows.
F-55
ARGENX N.V.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Other disclosures (continued)
Overview of subsidiaries
Name | Registration number | Country | Participation | Main activity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
argenx 110 BV | 853245496 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | |||||
argenx 111 BV | 853245332 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | |||||
argenx 113 BV | 854976954 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | |||||
argenx 115 BV | 855638059 | Netherlands | 100.00 | % | Biotechnical research on drugs and pharma processes | |||||
argenx BVBA | 0818292196 | Belgium | 100.00 | % | Biotechnical research on drugs and pharma processes | |||||
7.6. Events after the balance sheet date
F-56
Shares

Ordinary Shares
PRELIMINARY PROSPECTUS
| | | |
|---|---|---|
Cowen and Company | Piper Jaffray | |
JMP Securities | Wedbush PacGrow |
, 2017
Through and including , 2017 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This requirement is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers.
Under Dutch law, members of the board of directors may be liable to the registrant for damages in the event of improper or negligent performance of their duties. They may be jointly and severally liable for damages to the registrant and third parties for infringement of our Articles of Association or certain provisions of the Dutch Civil Code. In certain circumstances, they may also incur additional specific civil and criminal liabilities.
The liability of members of the board of directors and other key employees is covered by a directors' and officers' liability insurance policy. This policy contains customary limitations and exclusions, such as willful misconduct or intentional recklessness (opzet of bewuste roekeloosheid).
Pursuant to our Articles of Association, the registrant shall indemnify any and all of its directors, officers, former directors and former officers against any and all liabilities, claims, judgments, fines and penalties incurred by them as a result of any threatened, pending or completed action, investigation or other proceeding, whether civil, criminal or administrative, brought by any party other than the registrant itself or its group companies, as defined in the Articles of Association, in relation to acts or omissions in or related to his or her capacity as our director or officer of the registrant, except in relation to claims insofar as they relate to the gaining in fact of personal profits, advantages or remuneration to which the relevant person was not legally entitled, or if the relevant person has been adjudged to be liable for willful misconduct or intentional recklessness. Such indemnification shall not be deemed exclusive of any other rights to which those indemnified may be entitled otherwise.
The underwriting agreement the registrant will enter into in connection with the offering of ordinary shares being registered hereby provides that the underwriters will indemnify, under certain conditions, the registrant's board of directors and its officers against certain liabilities arising in connection with this offering.
Item 7. Recent Sales of Unregistered Securities.
Set forth below are the sales of all securities sold by the registrant within the past three years (i.e., since January 1, 2014, up to the date of this registration statement) which were not registered under the Securities Act:
II-1
These sales were exempt from registration under Section 4(a)(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act.
Option Grants
The table below summarizes the share options we granted to the members of our board of directors and our employees within the past three years. The grant of the options and the issuance of common shares upon the exercise of options described in the table below were or will be made pursuant to Regulation S under the Securities Act or Section 4(a)(2) of the Securities Act.
Grant Date | Number of underlying options | Exercise price per share | |||||
|---|---|---|---|---|---|---|---|
June 30, 2014 | 109,820 | € | 2.44 | ||||
September 30, 2014 | 194,081 | € | 2.44 | ||||
September 30, 2014 | 55,746 | € | 3.95 | ||||
December 18, 2014 | 585,250 | € | 7.17 | ||||
June 18, 2015 | 60,000 | € | 11.38 | ||||
June 18, 2015 | 56,500 | € | 11.44 | ||||
September 3, 2015 | 3,000 | € | 10.34 | ||||
December 15, 2015 | 243,400 | € | 9.47 | ||||
May 25, 2016 | 288,950 | € | 11.47 | ||||
December 13, 2016 | 363,226 | € | 14.13 | ||||
Item 8. Exhibits and Financial Statement Schedules.
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
All information for which provision is made in the applicable accounting regulations of the Securities and Exchange Commission is either included in the financial statements or is not required under the related instructions or is inapplicable, and therefore has been omitted.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
II-2
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Breda, the Netherlands, on , 2017.
| ARGENX N.V. | ||||||
By: | | |||||
| Name: | Tim Van Hauwermeiren | |||||
| Title: | Chief Executive Officer | |||||
We, the undersigned directors, officers and/or authorized representative in the United States of argenx N.V., hereby severally constitute and appoint Tim Van Hauwermeiren and Eric Castaldi, and each of them singly, our true and lawful attorneys, with full power to any of them, and to each of them singly, to sign for us and in our names in the capacities indicated below the registration statement on Form F-1 filed herewith, and any and all pre-effective and post-effective amendments to said registration statement, and any registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, in connection with the registration under the Securities Act of 1933, as amended, of equity securities of argenx N.V., and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of them might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities indicated on , 2017.
Signature | Title | |||
|---|---|---|---|---|
| Tim Van Hauwermeiren | Chief Executive Officer and Director (Principal Executive Officer) | |||
Eric Castaldi | Chief Financial Officer and Director (Principal Financial and Accounting Officer) | |||
Peter K.M. Verhaeghe, Ph.D. | Chairman of the Board | |||
II-4
Signature | Title | |||
|---|---|---|---|---|
| John Paul de Koning, Ph.D. | Director | |||
David L. Lacey, M.D. | Director | |||
Werner Lanthaler, Ph.D. | Director | |||
J. Donald deBethizy, Ph.D. | Director | |||
Pamela Klein, M.D. | Director | |||
Puglisi & Associates | ||||
By: | Authorized Representative in the United States | |||
| Name: | ||||
| Title: | ||||
II-5
| Exhibit Number | Description of Exhibit | ||
|---|---|---|---|
| 1.1 | * | Form of Underwriting Agreement | |
| 3.1 | Articles of Association (English translation), as amended | ||
| 3.2 | Rules for the Board of Directors | ||
| 5.1 | * | Opinion of Freshfields Bruckhaus Deringer LLP | |
| 8.1 | * | Tax Opinion of Freshfields Bruckhaus Deringer LLP | |
| 10.1 | Leases dated April 1, 2016 between argenx BVBA and Bio-Incubator Gent 2 NV | ||
| 10.2 | * | Patent License Agreement, dated February 15, 2012, between the registrant and The Board of Regents of the University of Texas System, as amended | |
| 21.1 | List of Subsidiaries of the registrant | ||
| 23.1 | * | Consent of Deloitte Accountants B.V. | |
| 23.2 | * | Consent of Freshfields Bruckhaus Deringer LLP (included in Exhibits 5.1 and 8.1) | |
| 24.1 | * | Power of Attorney (included on signature page to the original filing of this Registration Statement on Form F-1) | |