- ARGX Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
-
Insider
- Institutional
- Shorts
-
6-K Filing
argenx SE (ARGX) 6-KCurrent report (foreign)
Filed: 24 Apr 18, 12:00am
Phase 2 Study of Efgartigimod Patients with Generalized Myasthenia Gravis in James F. Howard Jr., MD Tim Van Hauwermeiren, CEO April 24, 2018 American Academy of Neurology, LA
Forward Looking Statements THIS PRESENTATION HAS BEEN PREPARED BY ARGENX SE (“ARGENX” OR THE “COMPANY”) FOR INFORMATIONAL PURPOSES ONLY AND NOT FOR ANY OTHER PURPOSE. NOTHING CONTAINED IN THIS PRESENTATION IS, OR SHOULD BE CONSTRUED AS, A RECOMMENDATION, PROMISE OR REPRESENTATION BY THE PRESENTER OR THE COMPANY OR ANY DIRECTOR, EMPLOYEE, AGENT, OR ADVISER OF THE COMPANY. THIS PRESENTATION DOES NOT PURPORT TO BE ALL-INCLUSIVE OR TO CONTAIN ALL OF THE INFORMATION YOU MAY DESIRE. THIS PRESENTATION ALSO CONTAINS ESTIMATES AND OTHER STATISTICAL DATA MADE BY INDEPENDENT PARTIES AND BY US RELATING TO MARKET SIZE AND GROWTH AND OTHER DATA ABOUT OUR INDUSTRY. THIS DATA INVOLVES A NUMBER OF ASSUMPTIONS AND LIMITATIONS, AND YOU ARE CAUTIONED NOT TO GIVE UNDUE WEIGHT TO SUCH ESTIMATES. Safe Harbor: Certain statements contained in this presentation, other than present and historical facts and conditions independently verifiable at the date hereof, may constitute forward-looking statements. Examples of such forward-looking statements include those regarding our investigational product candidates and preclinical and clinical trials and the status, plans, timing of expected data readouts and related presentations and related results thereto, future results of operations and financial positions, business strategy, plans and our objectives for future operations. When used in this presentation, the words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “will,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions identify forward-looking statements. Such statements, based as they are on the current analysis and expectations of management, inherently involve numerous risks and uncertainties, known and unknown, many of which are beyond the Company’s control. Such risks include, but are not limited to: the impact of general economic conditions, general conditions in the biopharmaceutical industries, changes in the global and regional regulatory environments in the jurisdictions in which the Company does or plans to do business, market volatility, fluctuations in costs and changes to the competitive environment. Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements. In the case of forward-looking statements regarding investigational product candidates and continuing further development efforts, specific risks which could cause actual results to differ materially from the Company’s current analysis and expectations include: failure to demonstrate the safety, tolerability and efficacy of our product candidates; final and quality controlled verification of data and the related analyses; the expense and uncertainty of obtaining regulatory approval, including from the U.S. Food and Drug Administration and European Medicines Agency; the possibility of having to conduct additional clinical trials and our reliance on third parties such as our licensors and collaboration partners regarding our suite of technologies and product candidates. Further, even if regulatory approval is obtained, biopharmaceutical products are generally subject to stringent on-going governmental regulation, challenges in gaining market acceptance and competition. These statements are also subject to a number of material risks and uncertainties that are described in the Company’s filings with the U.S. Securities and Exchange Commission (“SEC”), including in argenx’s most recent annual report on Form 20-F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC. The reader should not place undue reliance on any forward-looking statements included in this presentation. These statements speak only as of the date made and the Company is under no obligation and disavows any obligation to update or revise such statements as a result of any event, circumstances or otherwise, unless required by applicable legislation or regulation. 2
Introduction

Company Highlights Differentiated therapeutic antibodies pioneering in severe autoimmune diseases & cancer • ARGX-113: first-in-class FcRn antagonist targeting array of IgG mediated AI diseases Novel concept in autoimmunity • • Phase 1: favorable tolerability profile; IgG reduction up to 85% Phase 2: achieved proof of concept in myasthenia gravis, ongoing in immune thrombocytopenia and pemphigus vulgaris Deep pipeline with multiple shots on goal • • ARGX-110: first-in-class CD70 antagonist in Phase 1/2 in CTCL and AML 4 clinical stage programs; 3 preclinical programs; Innovative Access Program Powerful technology suite • • SIMPLE Antibody™: Human V-regions sourced from llama unlock novel & complex targets NHance®, ABDEG™, POTELLIGENT®: Fc engineering to augment natural properties of antibodies • : ARGX-115 (Immuno-oncology-focused novel target GARP) $40mm upfront and up to $625mm in potential milestone payments Validating selective partnerships • • Additional partnerships designed to maximize value of platform in non-core areas • • • Strong cash position: €360 mm December 31, 2017 Blue chip investor base: more than 60% U.S. shareholders 32.29 mm shares outstanding Well financed to execute plan $ 4
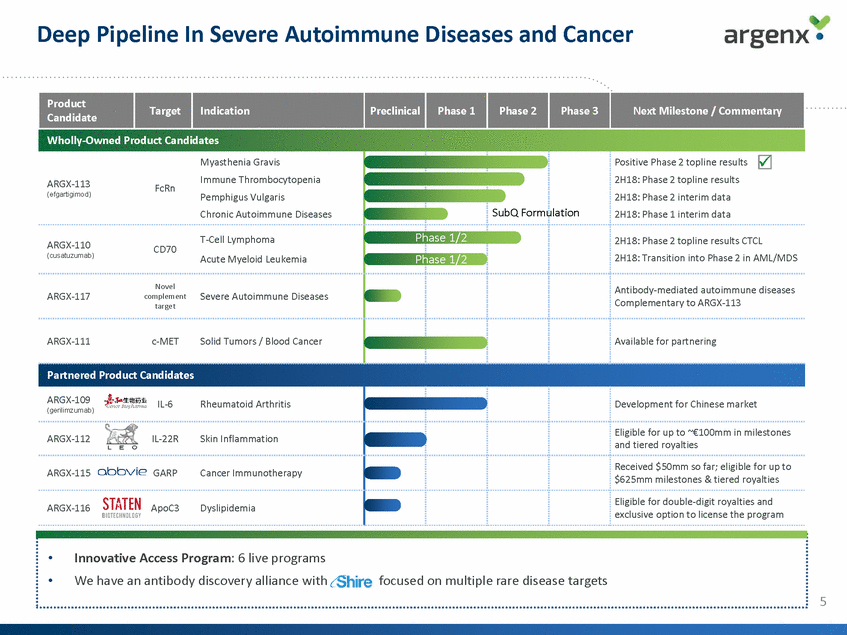
Deep Pipeline In Severe Autoimmune Diseases and Cancer Wholly-Owned Product Candidates 2H18: Phase 2 interim data Formulation Phase 1/2 Phase 1/2 Complementary to ARGX-113 Partnered Product Candidates (gerilimzumab) and tiered royalties $625mm milestones & tiered royalties exclusive option to license the program 5 •Innovative Access Program: 6 live programs •We have an antibody discovery alliance withfocused on multiple rare disease targets ARGX-109IL-6Rheumatoid Arthritis Development for Chinese market ARGX-112IL-22RSkin Inflammation Eligible for up to ~€100mm in milestones ARGX-115GARPCancer Immunotherapy Received $50mm so far; eligible for up to ARGX-116ApoC3Dyslipidemia Eligible for double-digit royalties and Myasthenia Gravis ARGX-113FcRnImmune Thrombocytopenia (efgartigimod)Pemphigus Vulgaris Chronic Autoimmune Diseases SubQ Positive Phase 2 topline results 2H18: Phase 2 topline results 2H18: Phase 1 interim data ARGX-110CD70T-Cell Lymphoma (cusatuzumab)Acute Myeloid Leukemia 2H18: Phase 2 topline results CTCL 2H18: Transition into Phase 2 in AML/MDS Novel ARGX-117complementSevere Autoimmune Diseases target Antibody-mediated autoimmune diseases ARGX-111c-METSolid Tumors / Blood Cancer Available for partnering Product Candidate Target Indication Preclinical Phase 1 Phase 2 Phase 3 Next Milestone / Commentary
Phase 2 Study of Efgartigimod in Patients with Generalized Myasthenia Gravis
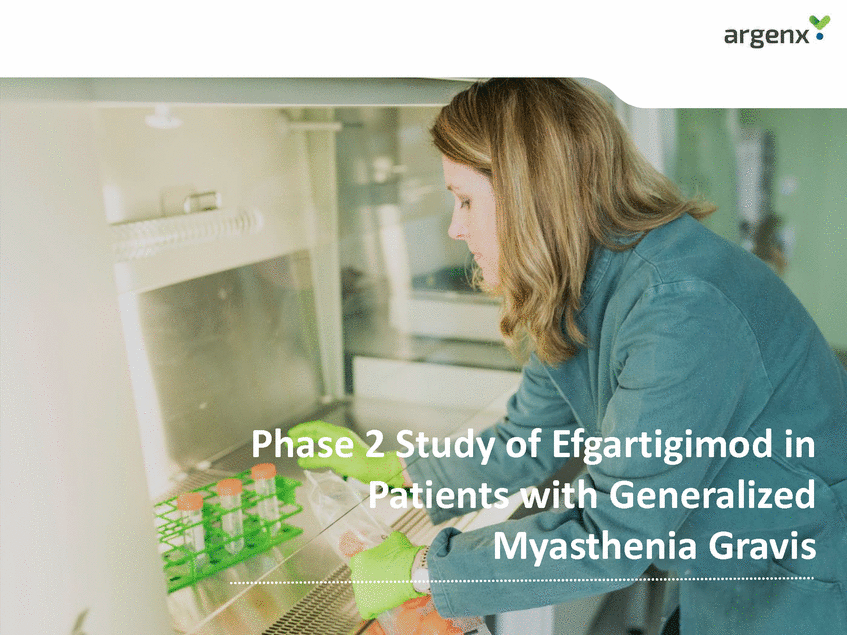
Pathogenic IgGs Play Key Role in MG Clearance by efgartigimod un.i.q.cuaueunIsmgeGedtcbmlyeaeprdaitnchacolegnmeeneich.I.ag. Gnissm Antibody FcRn Efgartigimod • • ‘ISgnGo-mwefldaikaet’eddisdeisaesease WAuatxoi-nagnatingdenwsaAnCinhgR, MuSK, LRP4
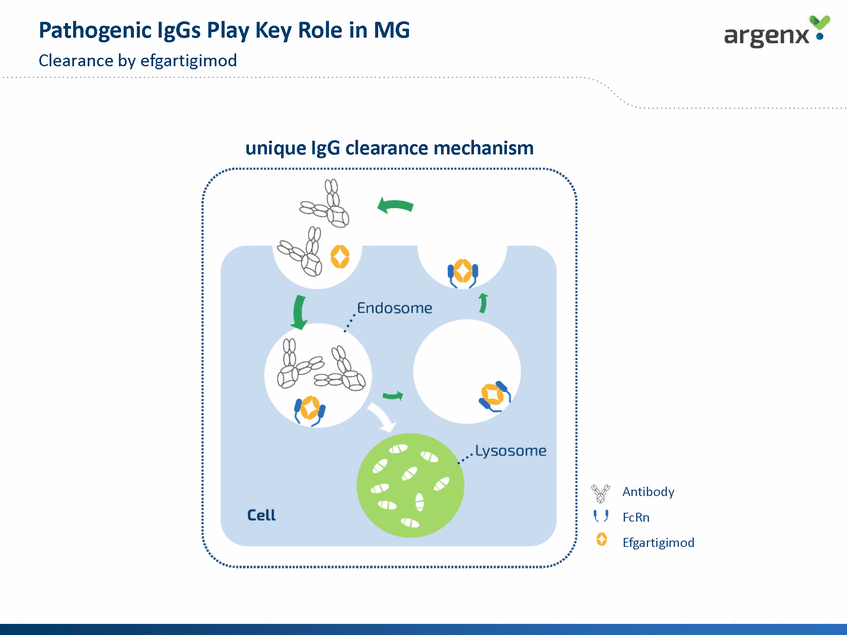
Pathogenic IgGs Play Key Role in MG Clearance by efgartigimod unmet medical need... ...caused by pathogenic IgGs • • ‘Snowflake’ disease Waxing and waning • • IgG-mediated disease Auto-antigens AChR, MuSK, LRP4 unique IgG clearance mechanism Antibody FcRn Efgartigimod 8
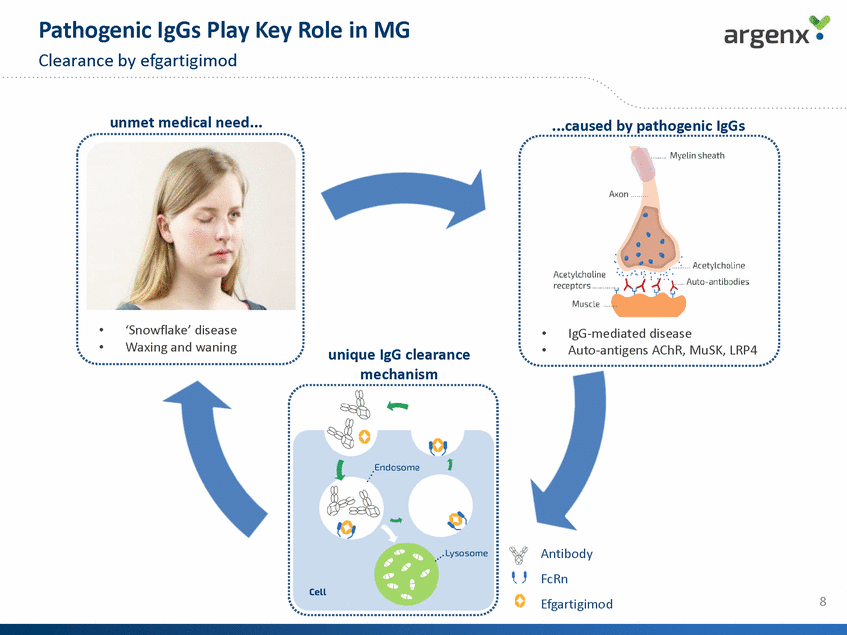
Phase 2 Trial Design N=12 Primary endpoint Secondary endpoints Efficacy MG-ADL; QMG; MGC; MG-QoL PD total IgG; pathogenic IgG Immuno-genicity PK Safety & tolerability (*) >50% of the score attributed to non-ocular items 9 Clinicaltrials.gov: NCT02965573, argenx data Screening/Randomization Treatment Phase Follow-up Phase Key inclusion criteria • Generalized MG patients • MGFA Class II, III, or IVa • Positive for anti-AChR antibodies • MG-ADL score of > 5 at screening (*) • On a stable dose of SoC <2 weeks SoC + efgartigimod (10mg/kg) SoC + Placebo N=12 4 doses; N= 24 3 weeks 8 weeks
Baseline Population and Disease Characteristics Age (mean ± SD) 43.5 ± 19.3 55.3 ± 13.6 Race • • • Asian Black / African White - 1 (8.3%) 11 (91.7%) 1 (8.3%) - 11 (91.7%) American Baseline QMG score (mean ± SD) (min, median, max score) 11.8 ± 5.4 (3, 12.5, 24) 14.5 ± 6.3 (6, 14, 30) Baseline MGC score (mean ± SD) 14.5 ± 4.5 16.7 ± 8.7 SoC • • • Acetylcholinesterase inhibitors N (%) Corticosteroids N (%) Immunosuppressants N (%) 11 (91.7%) 5 (41.7%) 2 (16.7%) 12 (100.0%) 8 (66.7%) 9 (75.0%) 10 argenx data
Efgartigimod Safety And Tolerability Profile 2 hour infusion enabling out-patient administration 11 argenx data
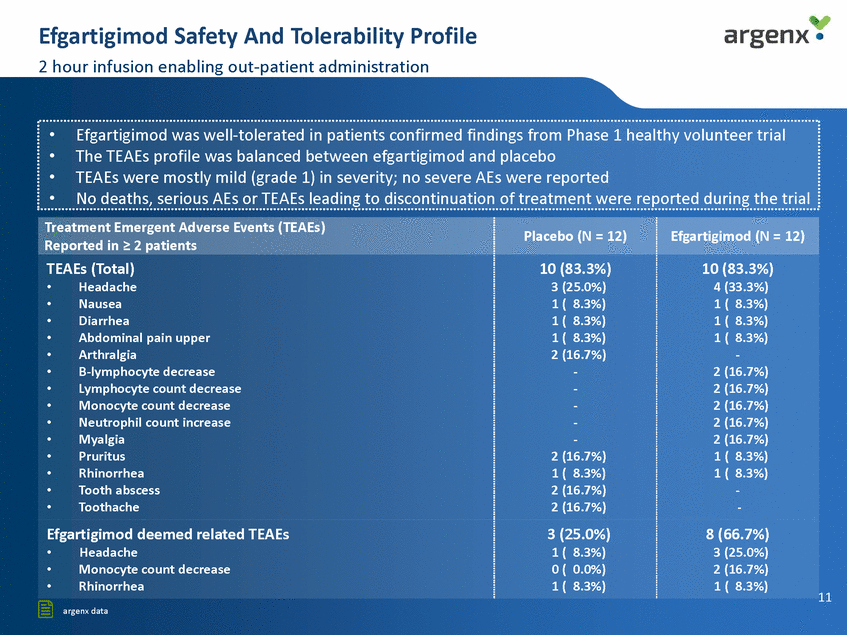
Lasting lgG Reduction Potent lgG reduction across isotypes argenx • Total lgG LEVELS lgGl lgG3 150 l 150 .... Vl +I 0 1-'* .... 100 100 Vl .... 100 0 LU Vl +I 0 1-"<!< 1-'* so so 50 20 20 40 Study d a y 60 80 40 St u dy d ay 60 80 0 20 40 60 80 Stu dy d a y lgG2 lgG4 150 150 100 100 w VI +I t-"' '* UJ "' ... Efgartigimod (MG study) t-"' 50 50 ---Placebo (MG study) 20 4 0 Study day 60 80 20 40 Study day 60 80 ......... Efgartigimod (HV study) • • PO effect of efgartigimod in the Phase 2 clinical trial very similar to the Phase 1trial in healthy volunteers Significant lgG reduction across lgG subtypes (AChR autoantibodies are lgGl/3; MuSK autoantibodies are lgG4) • lgM, lgA and albumin levels not affected (data not shown) ···························································································································································································································' 12 [§ argenx data
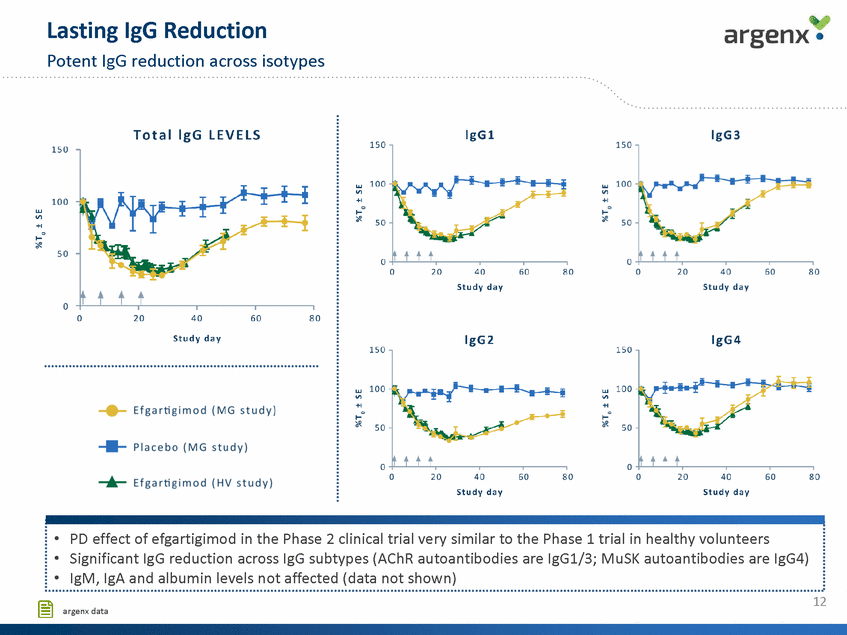
argenx . Clinically Meaningful and QMG and MG-ADL scores Long-lasting Reduction of Efficacy Scores QMG MG-ADL w V) +I c "Q'l E w V) +I c 0 0 "Q'l -2 E -2 Q) c Ql Ill .".0' Ql c Ql Ill -4 .".0' -4 -0 E E -0 -6 t t t Ql Ql ti.O ti.O c c -8 -6 .".c' .z":'. 0 20 40 Study day 60 80 0 20 40 Study day 60 80 u u ··························································································································································································································· * p < 0.05 - Efgartigimod - Placebo • Clinically meaningful and statistically significant improvement reached in small patient population (N=24) • Clear consistency between QMG and MG-ADL scores ···························································································································································································································' 13 [§ argenx data
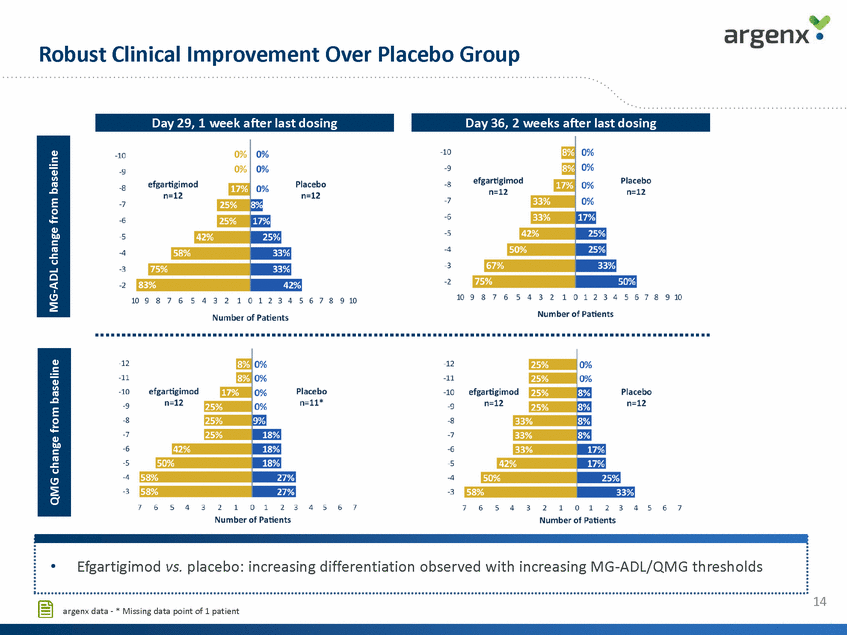
Robust Clinical Improvement Over Placebo Group 14 argenx data - * Missing data point of 1 patient MG-ADL change from baseline QMG change from baseline •Efgartigimod vs. placebo: increasing differentiation observed with increasing MG-ADL/QMG thresholds Day 36, 2 weeks after last dosing Day 29, 1 week after last dosing
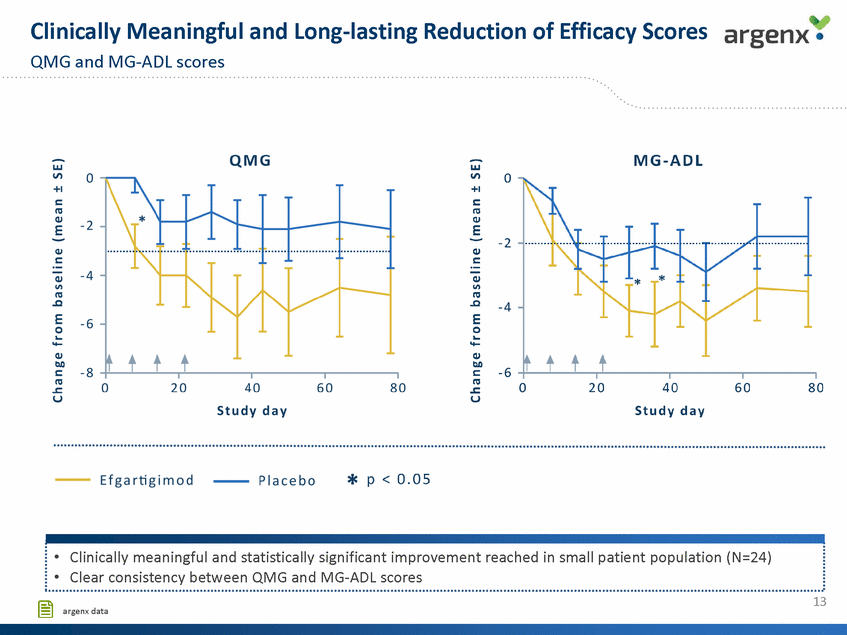
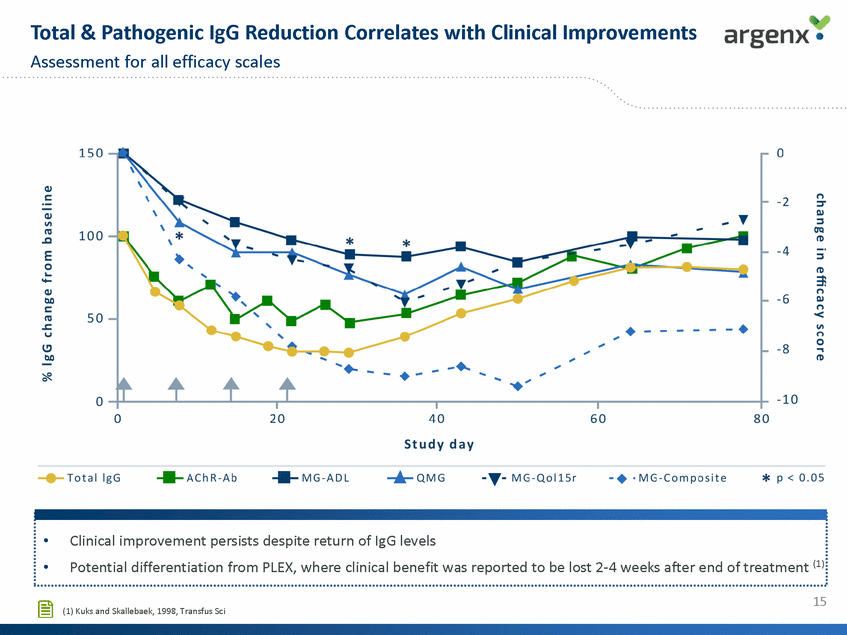
Total & Pathogenic lgG Reduction Correlates with Clinical Improvements Assessment for all efficacy scales argenx • 150 0 -2 Q.l Ill nJ 100 -4 E :::s tD 0... ..... Q.l b.O s:: 3i n OJ - 6 ·------· n < 50 nJ .s::. ·-Ill , n u 0 -8 ... ""' ....., tD , ... 0 20 40 Study day 60 80 * p < 0.05 -+ ·MG - Com pos it e -T-MG-QollSr -+-Total lgG .....AChR - Ab .....MG - ADL ...._ QMG . 1 • Clinical improvement persists despite return of lgG levels •Potential differentiation from PLEX, where clinical benefit was reported to be lost 2-4 weeks after end of treatment (l) l ............................................................................................................................................................................................................................... 15 [§ (1) Kuks and Skallebaek, 1998,Transfus Sci
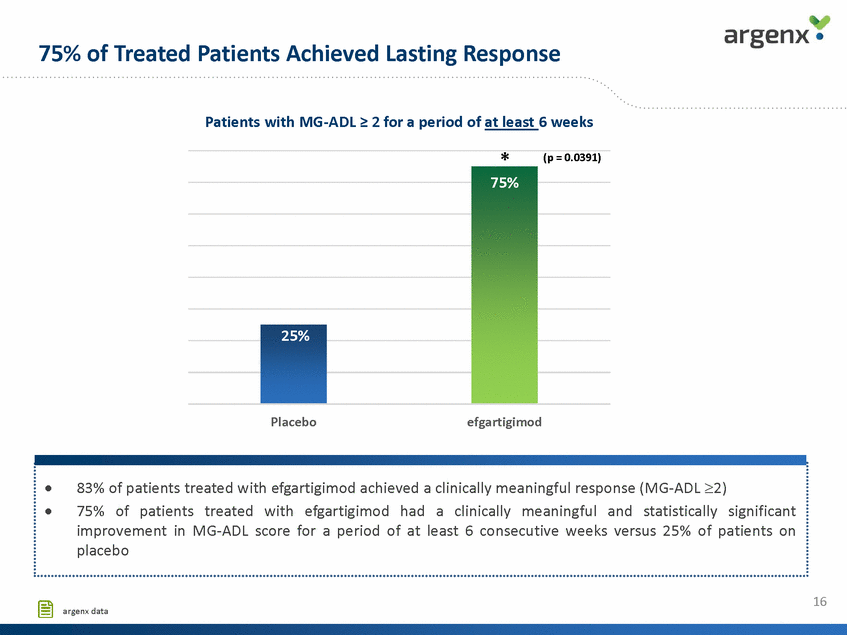
75% of Treated Patients Achieved Lasting Response Patients with MG-ADL > 2 for a period of at least 6 weeks * (p = 0.0391) 75% 25% Placebo efgartigimod 16 argenx data 83% of patients treated with efgartigimod achieved a clinically meaningful response (MG-ADL 2) 75% of patients treated with efgartigimod had a clinically meaningful and statistically significant improvement in MG-ADL score for a period of at least 6 consecutive weeks versus 25% of patients on placebo
Conclusions Phase 2 Trial of Efgartigimod in Patients with gMG 17 Consistent and compelling safety & tolerability Fast, long-lasting and sustained benefit; clinically meaningful and statistically significant Strong correlation between IgG level reduction and disease improvement; validating focus on IgG-mediated diseases Significant reduction of AChR autoantibodies Phase 2 execution accelerates efgartigimod towards Phase 3
Acknowledgements to the Teams of: Andrzej Szczudlik, Centrum Neurologii Klinicznej, Poland Maria Rosa Rottoli, Azienda Socio Sanitaria Territoriale Papa Giovanni XXIII, Italy Angela Genge, Montreal Neurological Institute and Hospital, Canada Philip Van Damme, UZ Leuven, Belgium Renato Mantegazza, Fondazione IRCCS Istituto Neurologico Carlo Besta, Italy Exuperio Diez Tejedor, Hospital Universitario La Paz, Spain Said R. Beydoun, University of Southern California, U.S. Frederik Piehl, Karolinska Universitetssjukhuset, Solna, Sweden Tuan Vu, University of South Florida, U.S. James F. Howard Jr., University of North Carolina at Chapel Hill, U.S. Vera Bril, Toronto General Hospital, Canada Jan Verschueren, Leids Universitair Medisch Centrum, the Netherlands Malgorzata M. Billenska, Uniwersyteckie Centrum Kliniczne, Poland 18 Thank you to the patients & their families
Efgartigimod: Pipeline-in-a-product opportunity & outlook 2018

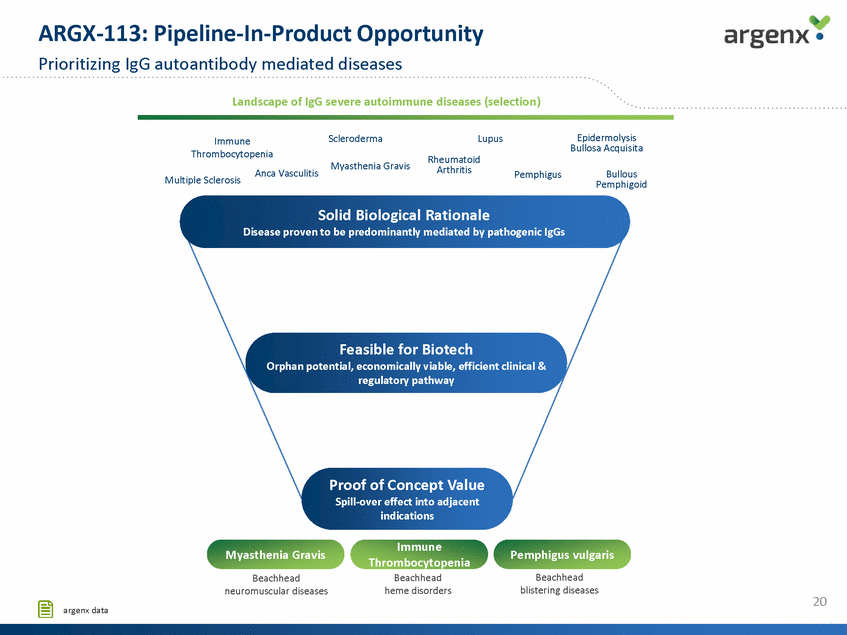
ARGX-113: Pipeline-In-Product Opportunity Prioritizing IgG autoantibody mediated diseases Landscape of IgG severe autoimmune diseases (selection) Epidermolysis Bullosa Acquisita Scleroderma Lupus Rheumatoid Arthritis Immune Thrombocytopenia Myasthenia Gravis Anca Vasculitis Bullous Pemphigoid Pemphigus Multiple Sclerosis Solid Biological Rationale Disease proven to be predominantly mediated by pathogenic IgGs Feasible for Biotech Orphan potential, economically viable, efficient clinical & regulatory pathway Proof of Concept Value Spill-over effect into adjacent indications Immune Thrombocytopenia Beachhead heme disorders Myasthenia Gravis Beachhead neuromuscular diseases Pemphigus vulgaris Beachhead blistering diseases 20 argenx data
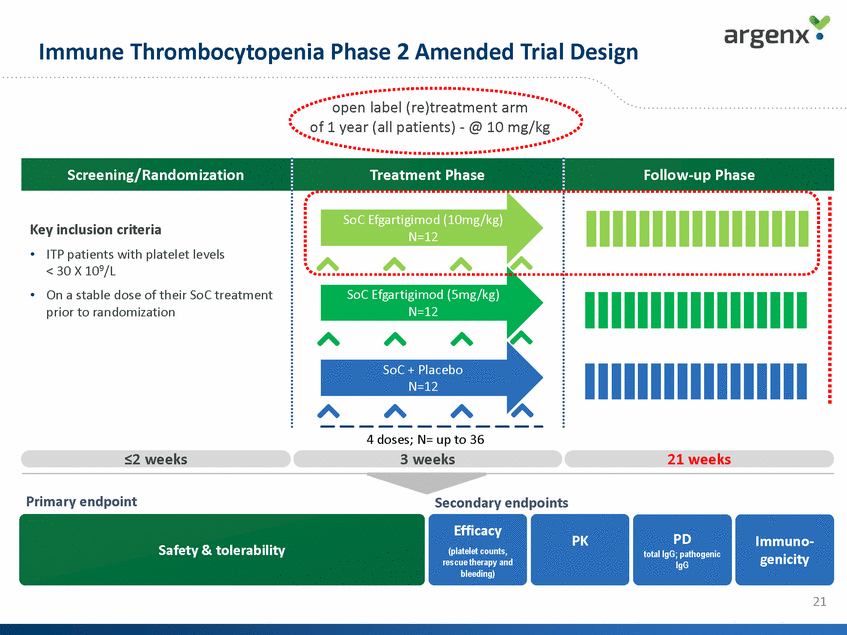
Immune Thrombocytopenia Phase 2 Amended Trial Design open label (re)treatment arm of 1 year (all patients) - @ 10 mg/kg Screening/Randomization Treatment Phase Follow-up Phase SoC Efgartigimod (10mg/kg) N=12 Key inclusion criteria • ITP patients with platelet levels < 30 X 109/L On a stable dose of their SoC treatment prior to randomization • SoC Efgartigimod (5mg/kg) N=12 SoC + Placebo N=12 4 doses; N= up to 36 3 weeks <2 weeks 21 weeks Primary endpoint Secondary endpoints Efficacy (platelet counts, rescue therapy and bleeding) PD total IgG; pathogenic IgG PK Immuno-genicity Safety & tolerability 21
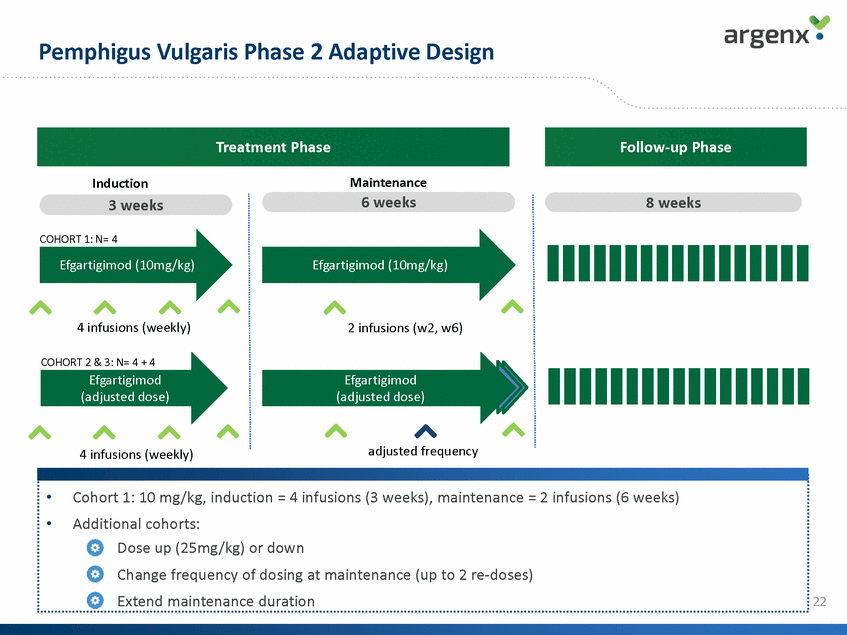
Pemphigus Vulgaris Phase 2 Adaptive Design Treatment Phase Follow-up Phase Maintenance 6 weeks Induction 3 weeks 8 weeks COHORT 1: N= 4 Efgartigimod (10mg/kg) Efgartigimod (10mg/kg) 4 infusions (weekly) 2 infusions (w2, w6) COHORT 2 & 3: N= 4 + 4 Efgartigimod (adjusted dose) Efgartigimod (adjusted dose) adjusted frequency 4 infusions (weekly) 22 •Cohort 1: 10 mg/kg, induction = 4 infusions (3 weeks), maintenance = 2 infusions (6 weeks) •Additional cohorts: •Dose up (25mg/kg) or down •Change frequency of dosing at maintenance (up to 2 re-doses) •Extend maintenance duration
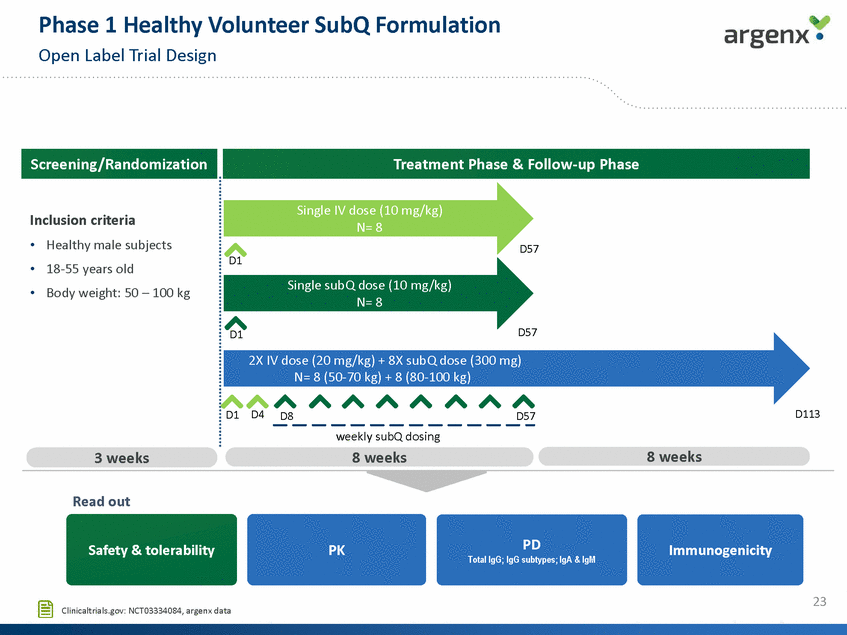
Phase 1 Healthy Volunteer SubQ Formulation Open Label Trial Design Screening/Randomization Treatment Phase & Follow-up Phase Single IV dose (10 mg/kg) N= 8 Inclusion criteria • • • Healthy male subjects 18-55 years old Body weight: 50 – 100 kg D57 D1 Single subQ dose (10 mg/kg) N= 8 D57 D1 2X IV dose (20 mg/kg) + 8X subQ dose (300 mg) N= 8 (50-70 kg) + 8 (80-100 kg) D1 D4 D113 D8 D57 weekly subQ dosing 8 weeks 8 weeks 3 weeks Read out PD Total IgG; IgG subtypes; IgA & IgM Safety & tolerability PK Immunogenicity 23 Clinicaltrials.gov: NCT03334084, argenx data
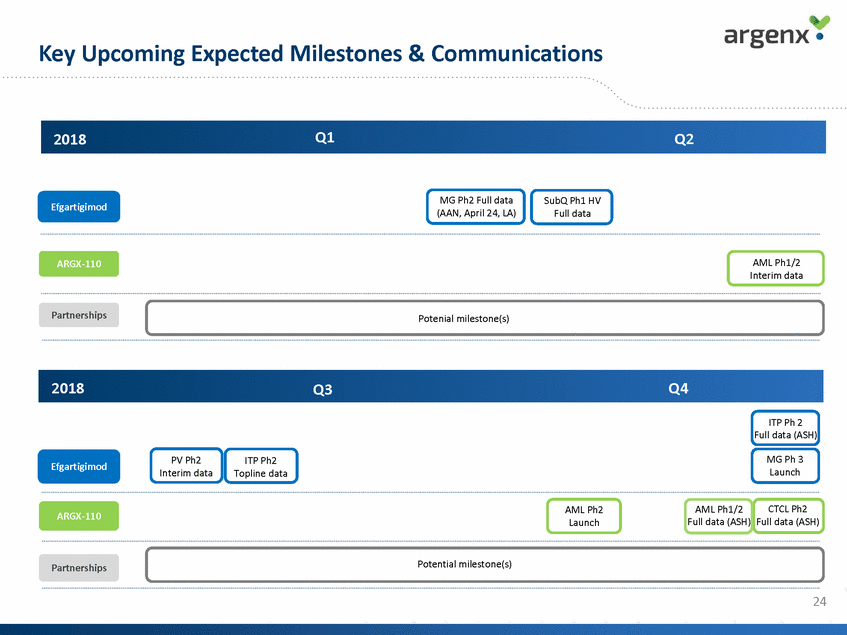
Key Upcoming Expected Milestones & Communications Q1 2018 Q2 MG Ph2 Full data (AAN, April 24, LA) SubQ Ph1 HV Full data Efgartigimod AML Ph1/2 Interim data ARGX-110 Partnerships Potenial milestone(s) 2018 Q4 Q3 ITP Ph 2 Full data (ASH) MG Ph 3 Launch PV Ph2 Interim data ITP Ph2 Topline data Efgartigimod AML Ph1/2 CTCL Ph2 AML Ph2 Launch ARGX-110 Full data (ASH) Full data (ASH) Potential milestone(s) Partnerships 24
Q&A
