- ARGX Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
-
Insider
- Institutional
- Shorts
-
6-K Filing
argenx SE (ARGX) 6-KCurrent report (foreign)
Filed: 1 Feb 21, 8:58am
Exhibit 99.2

Reaching Patients Through Immunology Innovation FEBRUARY 2021 “GO” Decision from ADHERE Trial in CIDP 1

Forward Looking Statements Safe Harbor: Certain statements contained in this presentation, other than present and historical facts and conditions independently verifiable at the date hereof, may constitute forward - looking statements. Examples of such forward - looking state - ments include those regarding our business and financial outlook and related plans; the therapeutic and commercial potential of our product candidates; the intended results of our strategy; the expected benefits of our collaborations, including with respect to our collaboration with Zai Lab; our and our collaboration partners’ clinical development and regulatory plans, including the timing, design and outcome of ongoing and planned clinical trials and preclinical activities and the timing and outcome of regulatory filings and approvals; the timing, progress and benefits of marketing and commercialization activities; and the expected size of the markets for our product candidates. When used in this presenta - tion, the words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “will,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions identify forward - looking statements. Such statements, based as they are on the current analysis and expectations of ma - nagement, inherently involve numerous risks and uncertainties, known and unknown, many of which are beyond the Company’s control. Such risks include, but are not limited to: the impact of COVID - 19 pandemic on our business, the impact of general economic conditions, general conditions in the biopharmaceutical industries, changes in the global and regional regulatory environments in the jurisdictions in which the Company does or plans to do business, market volatility, fluctuations in costs and ! 2 changes to the competitive environment. Consequently, actual future results may differ materially from the anticipated results expressed in the forward - looking state ments. In the case of forward - looking statements regarding investigational product candidates and continuing further development efforts, specific risks which could cause actual results to differ materially from the Company’s current analysis and expectations include: failure to demonstrate the safety, tolerability and efficacy of our product candidates; final and quality controlled verification of data and the related analyses; the expense and uncertainty of obtaining regulatory approval, including from the U.S. Food and Drug Administration and European Medicines Agency; the possibility of having to conduct additional clinical trials; our ability to obtain and main tain intellectual property protection for our product candidates; and our reliance on third parties such as our licensors and collaboration partners regarding our suite of technologies and product candidates. Further, even if regulatory approval is obtain ed, biopharmaceutical products are generally subject to stringent on - going govern - mental regulation, challenges in gaining market acceptance and competition. These statements are also subject to a number of material risks and uncertainties that are described in the Company’s filings with the U.S. Securities and Exchange Commission (“SEC”), including in argenx’s most recent annual report on Form 20 - F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC. The reader should not place undue reliance on any forward - looking statements included in this presentation. These statements speak only as of the date made and the Company is under no obligation and disavows any obligation to update or revise such statements as a result of any event, circumstances or otherwise, unless required by applicable legislation.
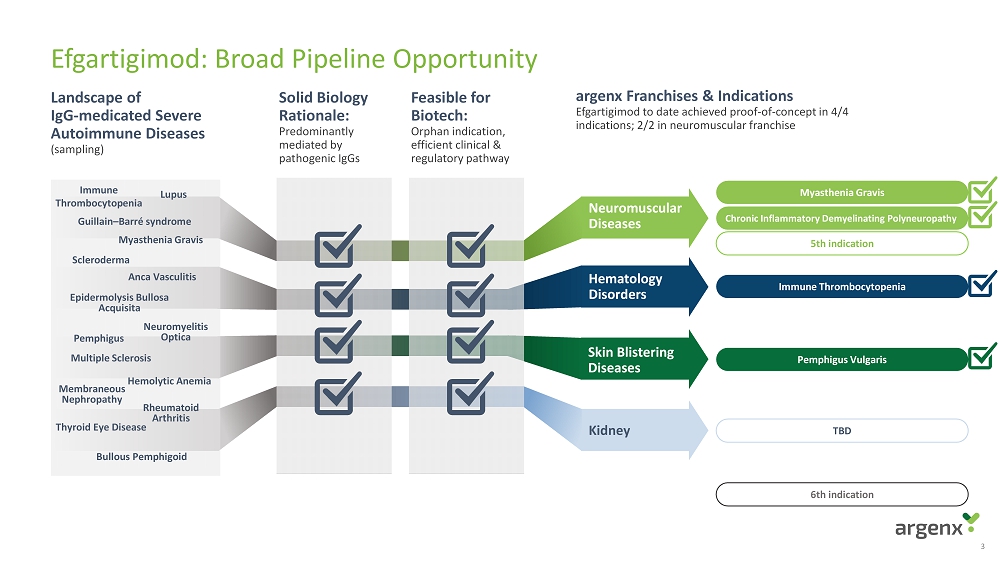
Efgartigimod: Broad Pipeline Opportunity 3 Landscape of IgG - medicated Severe Autoimmune Diseases (sampling) Solid Biology Rationale: Predominantly mediated by pathogenic IgGs Feasible for Biotech: Orphan indication , efficient clinical & regulatory pathway argenx Franchises & Indications Efgartigimod to date achieved proof - of - concept in 4/4 indications; 2/2 in neuromuscular franchise Myasthenia Gravis Chronic Inflammatory Demyelinating Polyneuropathy 5th indication Immune Thrombocytopenia Pemphigus Vulgaris 6th indication Hematology Disorders Kidney Neuromuscular Diseases Skin Blistering Diseases TBD Epidermolysis Bullosa Acquisita Immune Thrombocytopenia Pemphigus Lupus Rheumatoid Arthritis Scleroderma Myasthenia Gravis Bullous Pemphigoid Multiple Sclerosis Anca Vasculitis Thyroid Eye Disease Neuromyelitis Optica Hemolytic Anemia Membraneous Nephropathy Guillain – Barré syndrome
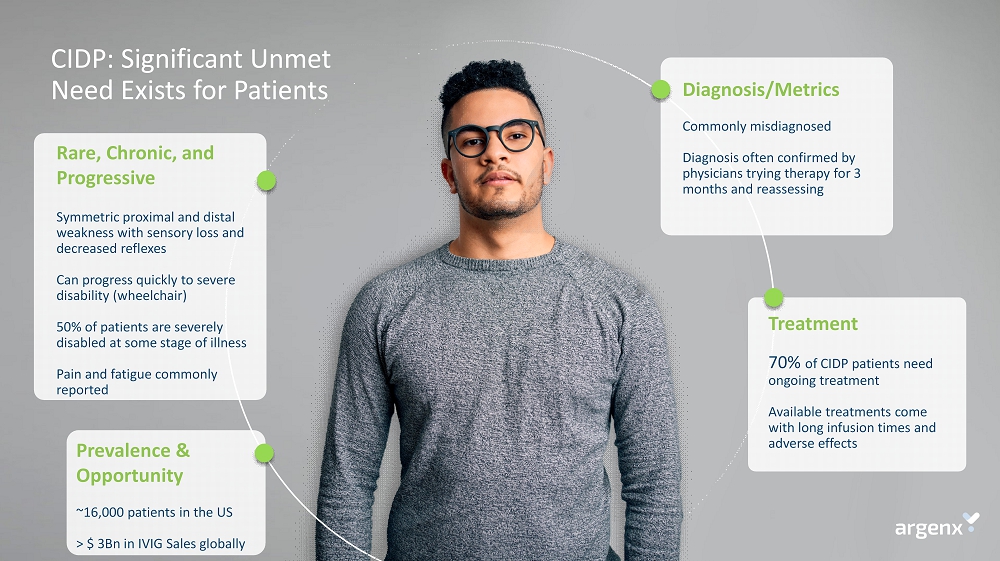
Rare, Chronic, and Progressive Symmetric proximal and distal weakness with sensory loss and decreased reflexes Can progress quickly to severe disability (wheelchair) 50% of patients are severely disabled at some stage of illness Pain and fatigue commonly reported Diagnosis/Metrics Commonly misdiagnosed Diagnosis often confirmed by physicians trying therapy for 3 months and reassessing Treatment 70% of CIDP patients need ongoing treatment Available treatments come with long infusion times and adverse effects CIDP: Significant Unmet Need Exists for Patients Prevalence & Opportunity ~16,000 patients in the US > $ 3Bn in IVIG Sales globally
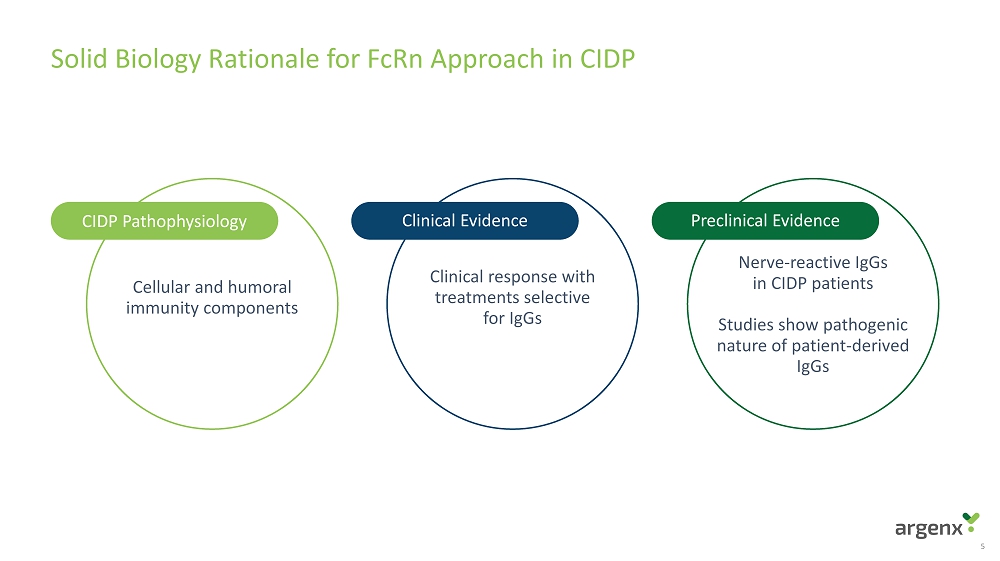
Solid Biology Rationale for FcRn Approach in CIDP 5 Cellular and humoral immunity components Clinical response with treatments selective for IgGs Nerve - reactive IgGs in CIDP patients Studies show pathogenic nature of patient - derived IgGs CIDP Pathophysiology Clinical Evidence Preclinical Evidence

CIDP: Therapeutic Activity Shown With Increasing Selectivity For IgG Reductions Response rate active 54% vs placebo 21% after 24 weeks (N=117) S e l e c tivity for IgG R esponse R a t e Therapeutic ICE Trial 50 - 70% 80 - 100% IA with protein A matrix 33 - 80% Comparable t o P L E X IA with t r y p t o p h a n matrix I V I g P L E X I mmun o a d sorpt i o n Oaklander et al (2017), Cochrane Database Syst Rev Lieker et al (2017), J Clin Apher , 32(6): 486 - 493 Zinman et al (2005 ) Transfus Apher Sci . 2005 Nov;33(3):317 - 24. Cli n i c a l e vidence for the r ole of p athogenic autoa nt i bodies in CIDP 6
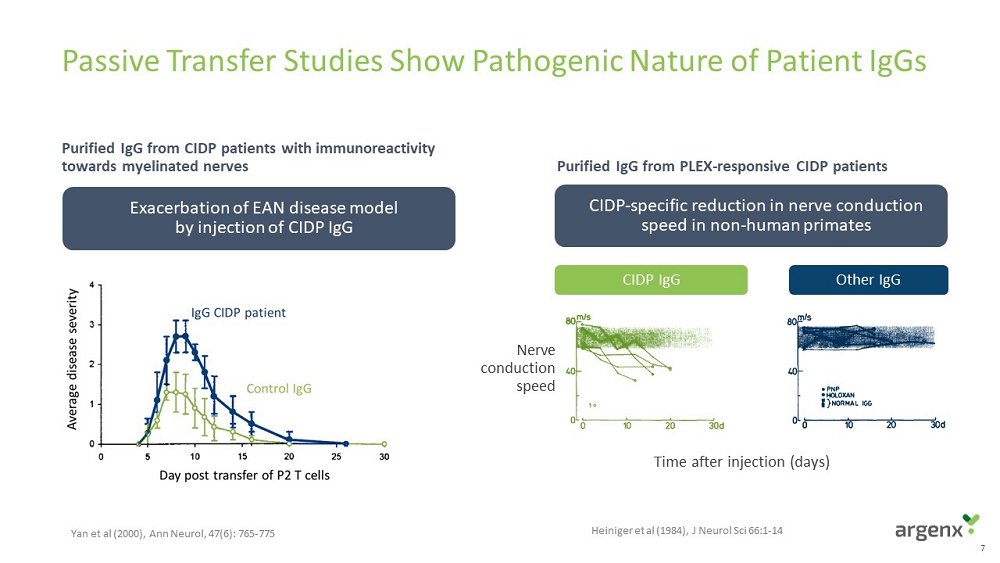
7 Passive Transfer Studies Show Pathogenic Nature of Patient IgGs Ex a cerbation of EAN disease model b y injection of CIDP IgG CIDP Ig G O t h e r I gG N e r v e c o n d u c t i o n s p e e d A D Purified IgG from PLEX - responsive CIDP patients Purified IgG from CIDP patients with immunoreactivity towards myelinated nerves CIDP - specific reduction in nerve conduction spee d in non - human primates Yan et al (2000), Ann Neurol , 47(6): 765 - 775 Heiniger et al (1984), J Neurol Sci 66:1 - 14 T i m e a f t er in j e c t i on (days)
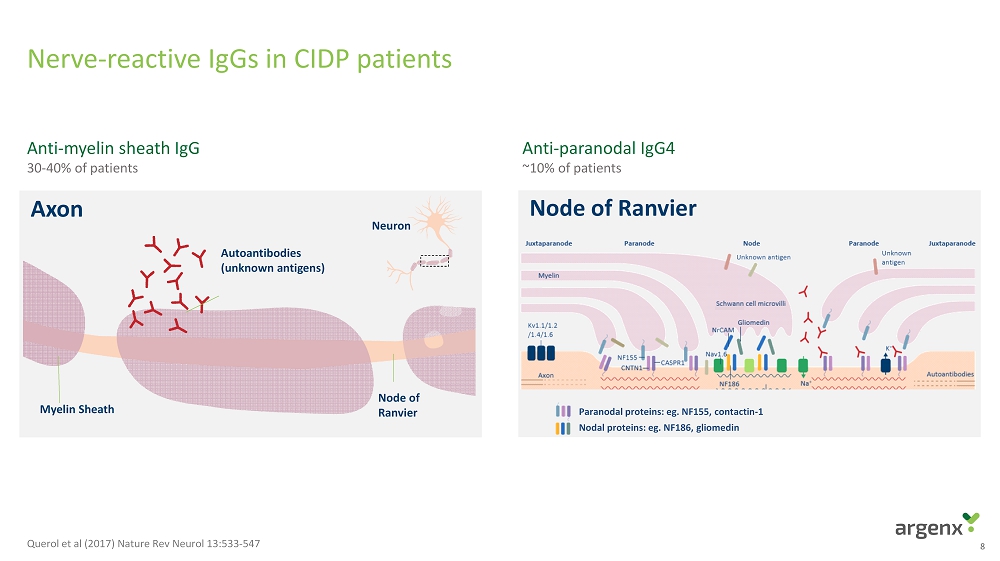
Nerve - reactive IgGs in CIDP patients 8 Anti - paranodal IgG4 ~ 10% of patients A n t i - m y e l i n sheath I g G 30 - 40% o f p a t i e n t s Myelin Sheath Node of Ranvier Autoantibodies (unknown antigens) Neuron Axon Paranodal proteins: eg. NF155, contactin - 1 Nodal proteins: eg. NF186, gliomedin Node of Ranvier Querol et al (2017) Nature Rev Neurol 13:533 - 547

• Worsening of disease within 12 weeks after drug withdrawal (INCAT, I - RODS, grip strength) • Newly diagnosed/ treatment naïve skip run - in period • Confirmation of diagnosis by independent committee 9 Treatment period Open - label Placebo - controlled Ide n t i f y patients with active CIDP Confirm IgG autoantibody involvement Assess e fficacy & safety efgartigimod vs placebo Run - in period Stage A Stage B (Stage A responders only) Screening Efficacy analysis based on relapse (adjusted INCAT) Study endpoint with 88 relapse events in stage B N= sample size estimation ~120 - 130 Followed by Open Label Extension study Go/No Go N=30 ≤13weeks ≤ 4 weeks E f g a r t i g i m o d w e e k l y SC Placebo w e e k l y SC E f g a r t i g i m o d w e e k l y SC Up to 12 weeks, until clinical improvement (ECI) Up to 48 weeks Chronic Inflammatory Demyelinating Polyneuropathy: Phase 2/3 ADHERE Trial Focus of Today
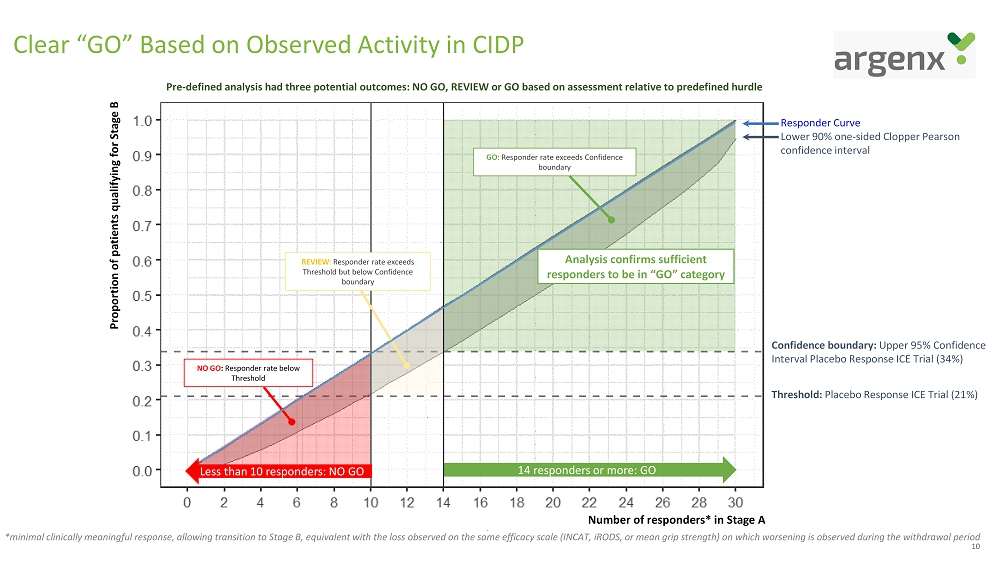
10 Clear “GO” Based on Observed Activity in CIDP 14 responders or more: GO Less than 10 responders: NO GO Proportion of patients qualifying for Stage B Number of responders* in Stage A Threshold: Placebo Response ICE Trial (21%) NO GO : Responder rate below Threshold GO: Responder rate exceeds Confidence boundary REVIEW : Responder rate exceeds Threshold but below Confidence boundary Pre - defined analysis had three potential outcomes: NO GO, REVIEW or GO based on assessment relative to predefined hurdle Analysis confirms sufficient responders to be in “GO” category Responder Curve Lower 90% one - sided Clopper Pearson confidence interval Confidence boundary: Upper 95% Confidence Interval Placebo Response ICE Trial (34%) * minimal clinically meaningful response, allowing transition to Stage B, equivalent with the loss observed on the same efficac y s cale (INCAT, iRODS , or mean grip strength) on which worsening is observed during the withdrawal period
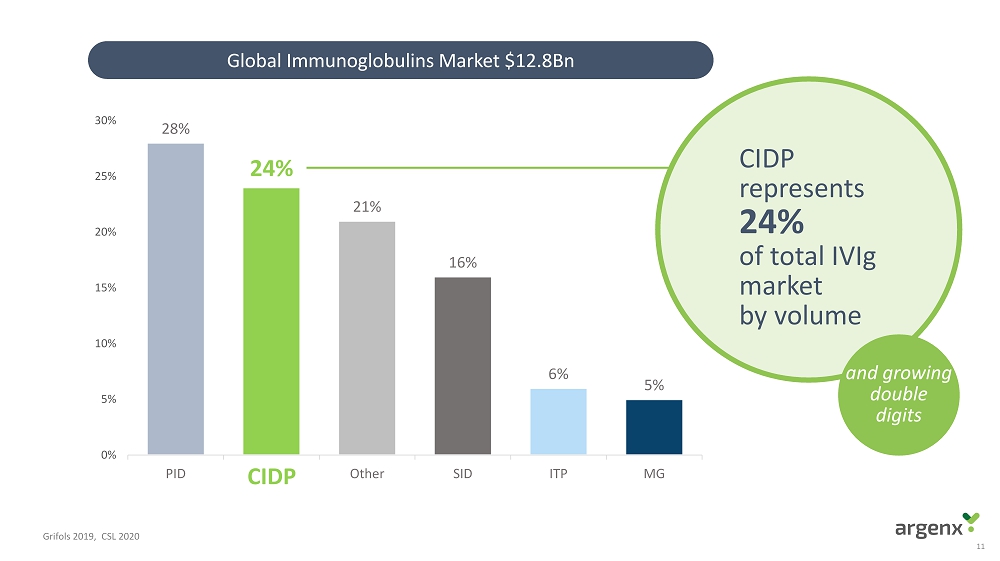
11 Grifols 2019, CSL 2020 28% 24% 21% 16% 6% 5% 0% 5% 10% 15% 20% 25% 30% PID CIDP Other SID ITP MG CIDP 24% CIDP represents 24% of total IVIg market by volume Global Immunoglobulins Market $12.8Bn and growing double digits
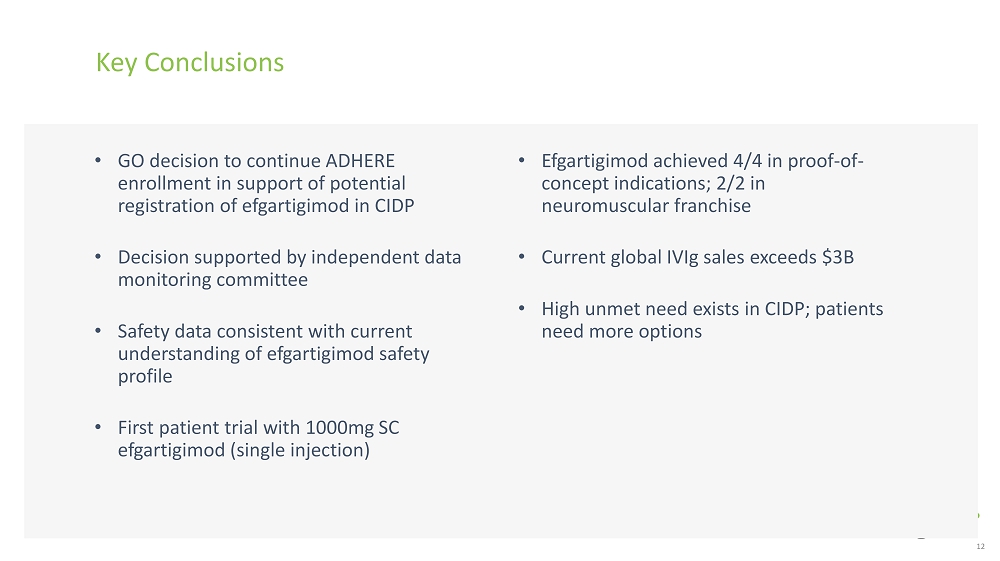
Key Conclusions • Efgartigimod achieved 4/4 in proof - of - concept indications; 2/2 in neuromuscular franchise • Current global IVIg sales exceeds $3B • High unmet need exists in CIDP; patients need more options 12 • GO decision to continue ADHERE enrollment in support of potential registration of efgartigimod in CIDP • Decision supported by independent data monitoring committee • Safety data consistent with current understanding of efgartigimod safety profile • First patient trial with 1000mg SC efgartigimod (single injection)

MG ITP PV CIDP 5 th 6 th Zai collaboration Neuro Heme Skin Kidney Uniquely Positioned For exponential expansion o efgartigimod indications o therapeutic franchises o global markets ROW China Europe Japan US 13 Indications Therapeutic Franchises Global Markets

Reaching Patients Through Immunology Innovation FEBRUARY 2021 14 “GO” Decision from ADHERE Trial in CIDP