Exhibit 99.2

J.P. Morgan Healthcare Conference January 2023

2 Forward Looking Statements This presentation has been prepared by argenx se (“ argenx ” or the “company”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or the company or any director, employee, agent, or adviser of the company. This presentation does not purport to be all - inclusive or to contain all of the information you may desire. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third - party sources and our own internal estimates and r esearch. While we believe these third - party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently v eri fied, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this p res entation relating to or based on such internal estimates and research. Certain statements contained in this presentation, other than present and historical facts and conditions independently verifiable at the date hereof, may constitute forward - looking statements . Examples of such forward - looking statements include those regarding preliminary financial results for the full year 2022 ; expectations of future profitability ; plans for geographic expansion ; the anticipated launch of argenx’s subcutaneous (“SC”) product in the U . S .; the initiation, timing, progress and results of our anticipated clinical development, data readouts and regulatory milestones and plans ; strategic priorities, including the timing and outcome of regulatory filings and regulatory approvals, and anticipated expansion in generalized myasthenia gravis ( gMG ) and IgG - mediated autoimmune diseases ; the potential of argenx’s innovative clinical programs ; and the nomination of new development candidates . A further list and description of these risks, uncertainties and other risks can be found in argenx’s U . S . Securities and Exchange Commission (“SEC”) filings and reports, including in argenx’s most recent annual report on Form 20 - F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC . Given these uncertainties, the reader is advised not to place any undue reliance on such forward - looking statements . These forward - looking statements speak only as of the date of publication of this presentation . argenx undertakes no obligation to publicly update or revise the information in this press release, including any forward - looking statements, except as may be required by law . This presentation contains trademarks, trade names and service marks of other companies, which are the property of their resp ect ive owners.

We are on a bold mission Transforming the lives of patients with severe autoimmune disease through immunology i nnovation

Autoimmunity is Prime for REVOLUTION A pipeline of precision tools Redefining Autoimmune Diseases With Precision Medicine Approach Redefine our pipeline of autoimmune diseases as IgG - mediated or complement - driven Offer new targeted treatment modality in diseases where innovation is most needed

Redefining What ‘Well - Controlled’ Means for the Patient Achieve minimal symptom expression Reduce reliance on broad immunosuppressants Regain control of their lives, including professionally and socially Minimize treatment burden We want to transform gMG treatment for patients
Lead with Compassion for our Patients Drive Impact Through Innovation Pioneer with Our Science Build the Company We Want to Work For argenx 2025: A Leading, Sustainable Immunology Company …and gMG is just the beginning
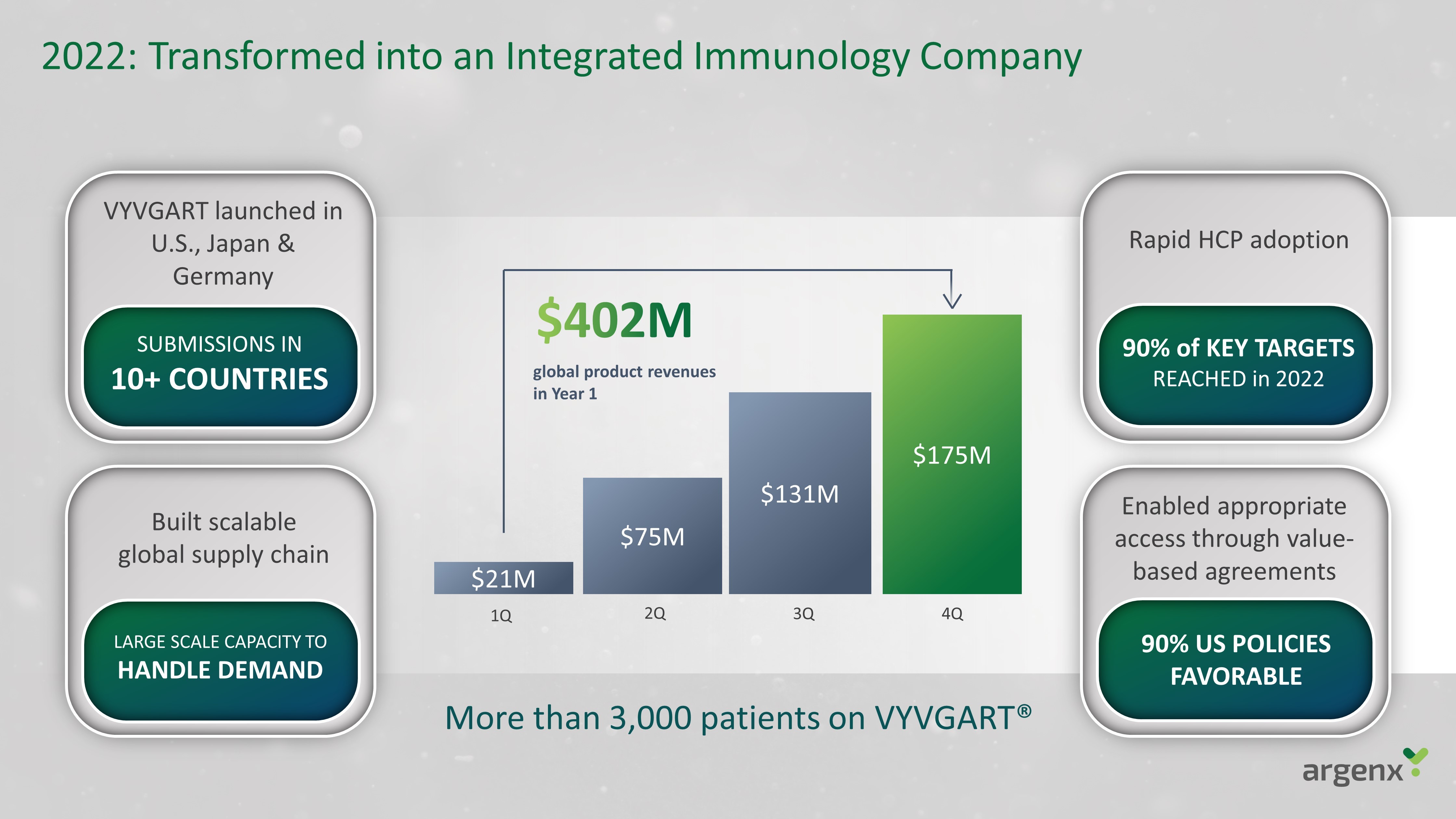
2022: Transformed into an Integrated Immunology Company VYVGART launched in U.S., Japan & Germany Enabled appropriate access through value - based agreements 90% US POLICIES FAVORABLE Built scalable global supply chain LARGE SCALE CAPACITY TO HANDLE DEMAND Rapid HCP adoption 90% of KEY TARGETS REACHED in 2022 SUBMISSIONS IN 10+ COUNTRIES $21M $75M $131M $ 175M 1Q 2Q 3Q 4Q global product revenues in Year 1 More than 3,000 patients on VYVGART®

2022: Strengthened Efgartigimod Data Story 0 - 30 - 60 Baseline 1 2 3 4 5 6 7 8 10 Mean I gG reduction % IgG reduction (%) in all ADAPT - SC and ADAPT participants LS - Mean Platelet Count Change From Baseline (x10 9 /L) - 20 0 20 40 0 12 24 Week VYVGART PLACEBO Clear Clinical Benefit in ITP Disease - Modifying Potential of FcRn Blockade SC Noninferiority to IV
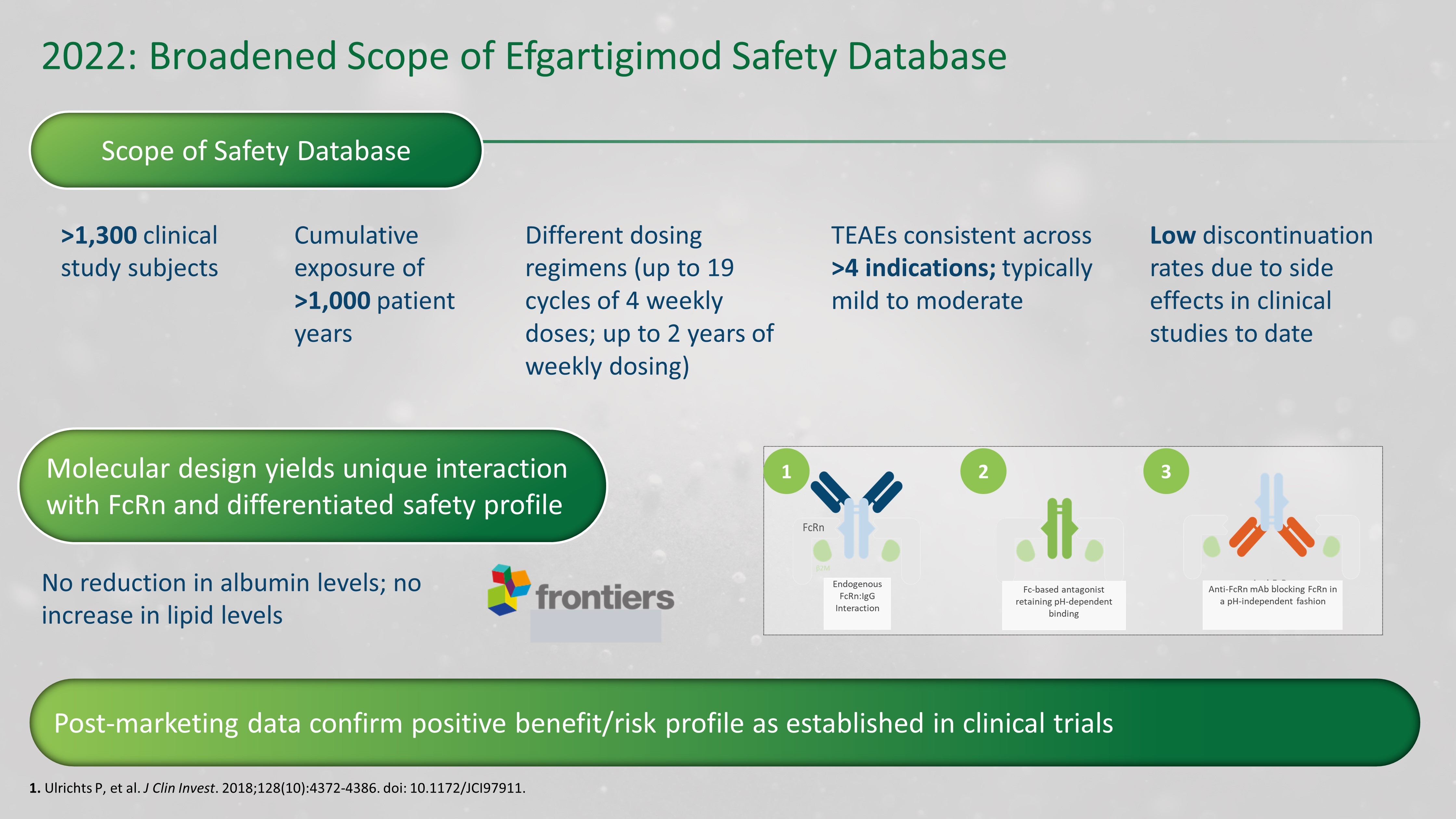
2022 : Broadened Scope of Efgartigimod Safety Database 1. Ulrichts P, et al. J Clin Invest . 2018;128(10):4372 - 4386. doi : 10.1172/JCI97911. Scope of Safety Database >1,300 clinical study subjects Molecular design yields unique interaction with FcRn and differentiated safety profile Post - marketing data confirm positive benefit/risk profile as established in clinical trials No reduction in albumin levels; no increase in lipid levels Cumulative exposure of >1,000 patient years Different dosing regimens (up to 19 cycles of 4 weekly doses; up to 2 years of weekly dosing) TEAEs consistent across >4 indications; typically mild to moderate Low discontinuation rates due to side effects in clinical studies to date 1 2 3
Reach more patients with VYVGART globally Pioneer FcRn class of medicines Broaden immunology pipeline Build out innovation ecosystem commercial clinical clinical discovery 2023: Key Drivers of our Path to Profitability

Reach More Patients with VYVGART Globally Geographic expansion Launch SC product offering Drive usage in earlier line patients Reach more patients with VYVGART globally commercial File for ITP in Japan Ongoing studies in new indications DRIVE GROWTH IN NEW PATIENT SEGMENTS DRIVE MULTI - DIMENSIONAL EXPANSION IN gMG
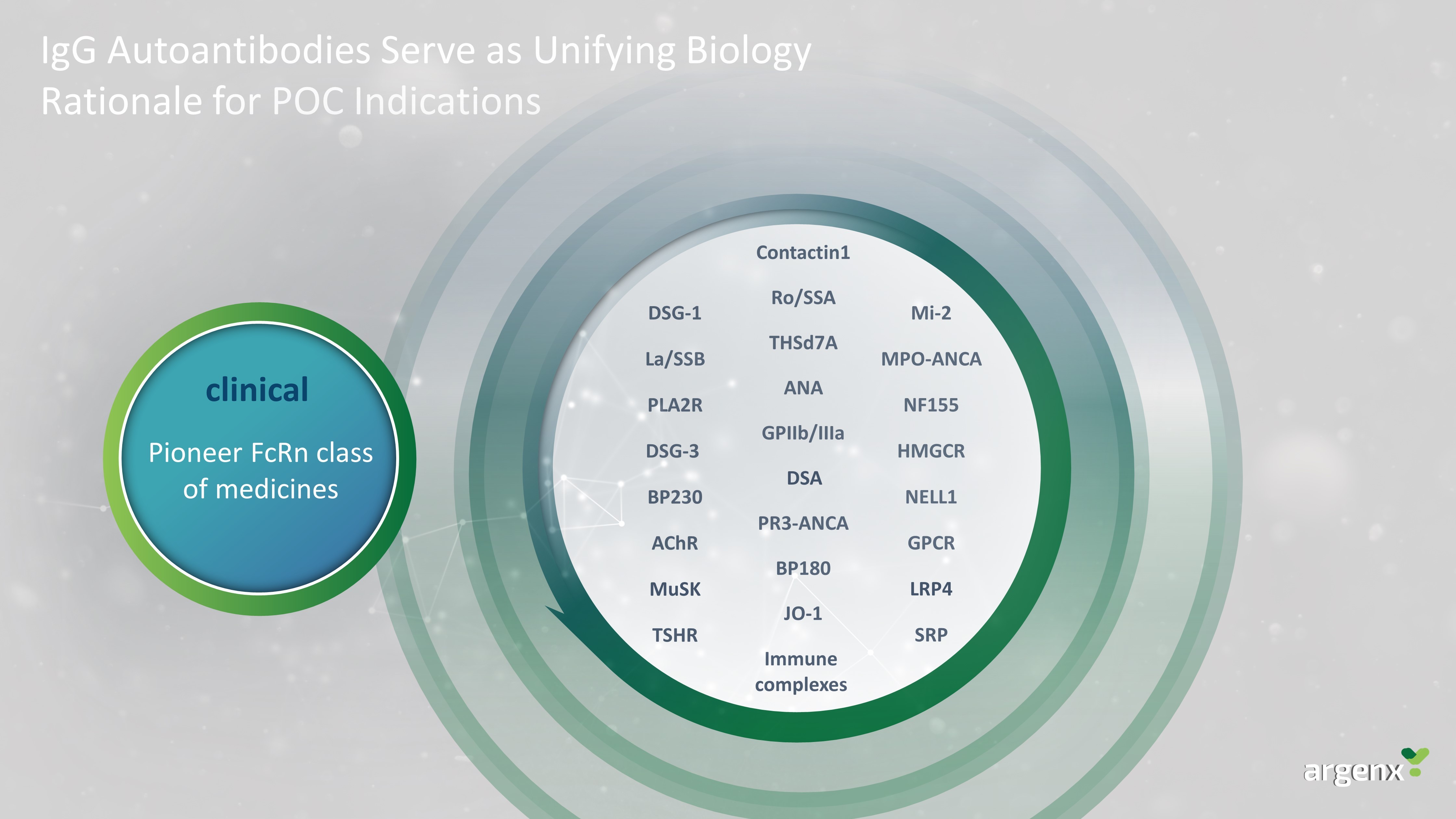
IgG Autoantibodies Serve as Unifying Biology Rationale for POC Indications DSG - 1 DSG - 3 AChR GPIIb /IIIa NF155 Contactin1 BP180 BP230 JO - 1 SRP HMGCR Mi - 2 MPO - ANCA PR3 - ANCA PLA2R GPCR Immune complexes TSHR THSd7A NELL1 ANA Ro/SSA La/SSB Pioneer FcRn class of medicines clinical MuSK LRP4 DSA
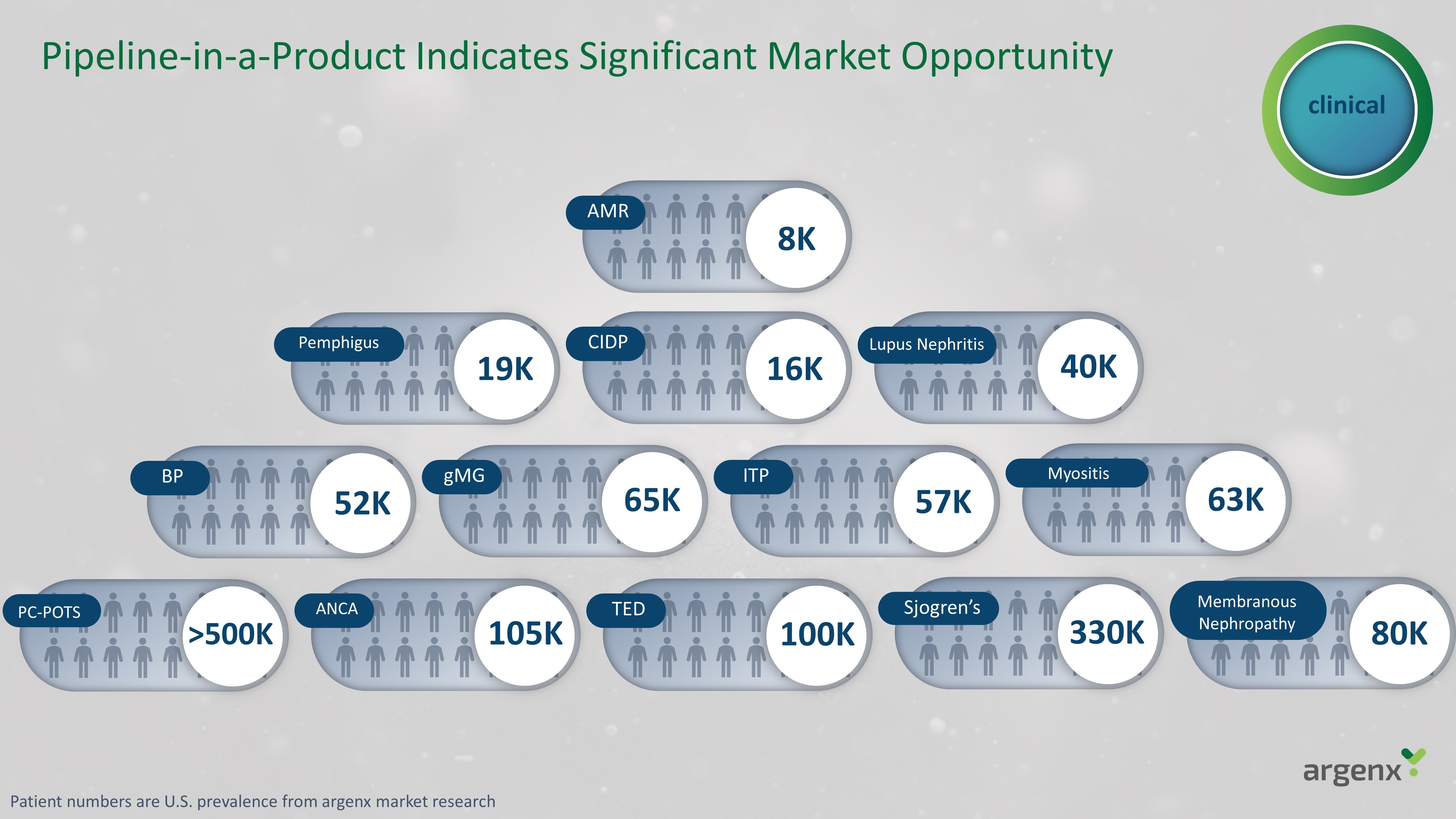
Pipeline - in - a - Product Indicates Significant Market Opportunity PC - POTS > 500K ANCA 105K TED 10 0K Sjogren’s 330K Membranous Nephropathy 8 0K BP 52K gMG 65K ITP 57K Myositis 63K Pemphigus 19K CIDP 16K Lupus Nephritis 40 K AMR 8K clinical Patient numbers are U.S. prevalence from argenx market research

gMG 65K CIDP 16K BP* 52K Membranous Nephropathy 80K TED 100K ANCA 105K AMR 8K PC - POTS >500K Sjogren’s 330K Lupus Nephritis 40K Steady Cadence of Data Readouts Over Next Several Years APPROVED APPROVED/IN REVIEW 2023 DATA READOUTS 2023 DATA READOUTS 2024 DATA READOUTS 2024 DATA READOUTS 2025+ DATA READOUTS 2025+ DATA READOUTS Myositis* 63K Pemphigus 19K ITP 57K Late Stage Proof - of - Concept clinical Patient numbers are U.S. prevalence from argenx market research *GO/NO GO Decisions.

Optimal recycling pH and Ca2 + switch No effector function ARGX - 117: Targets C2 at Junction of Classical and Lectin Pathways 2024 DATA READOUTS Three Indications Selected Based on Biology Rationale 2024 DATA READOUTS ARGX - 117: Sweeping Antibody Broaden immunology pipeline clinical Indication selection starts with autoimmune diseases driven by classical and/or lectin pathway Phase 2 proof - of - concept trial underway in multifocal motor neuropathy (MMN) with interim data expected in mid - 2023 Phase 2 trials to start in delayed graft function and dermatomyositis
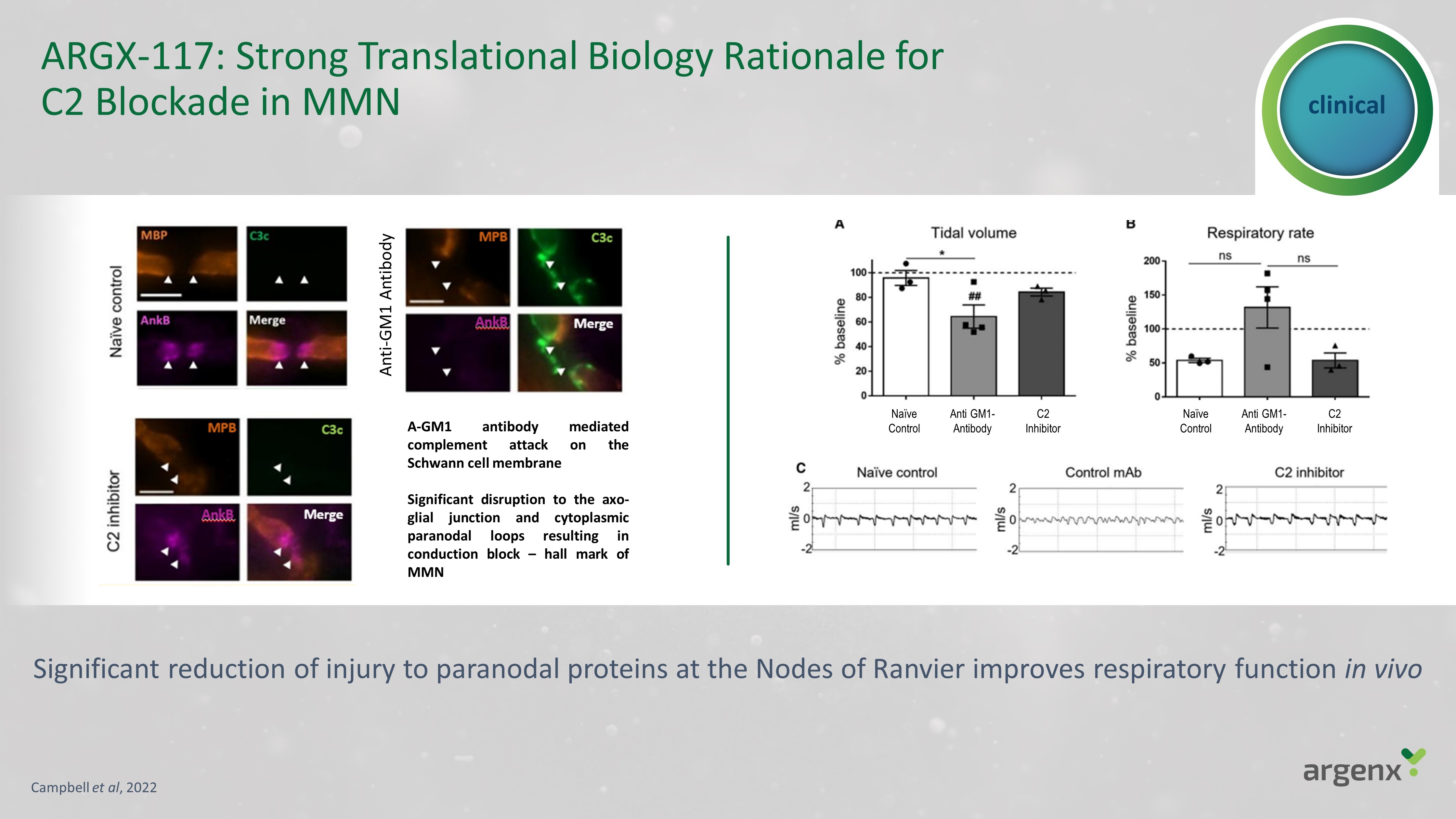
clinical ARGX - 117: Strong Translational Biology Rationale for C2 Blockade in MMN A - GM 1 antibody mediated complement attack on the Schwann cell membrane Significant disruption to the axo - glial junction and cytoplasmic paranodal loops resulting in conduction block – hall mark of MMN Campbell et al , 2022 Significant reduction of injury to paranodal proteins at the Nodes of Ranvier improves respiratory function in vivo Anti - GM1 Antibody Anti GM1 - Antibody Anti GM1 - Antibody Naïve Control C2 Inhibitor Anti GM1 - Antibody Naïve Control C2 Inhibitor

ARGX - 119: MuSK Agonist with Broad Potential in Neuromuscular Disease 10 dose cohorts 3 dose cohorts/4 weekly doses Congenital Myasthenic Syndrome (CMS) and MuSK MG First - in - Patient Single Ascending Dose (0.005mg/kg – 15mg/kg) Multiple Ascending Dose (0.3mg/kg – 2.5mg/kg) First - in - patient trial in CMS and MuSK MG to serve as proof of biology Translational work ongoing in amyotrophic lateral sclerosis First - in - Human Phase 1 Healthy Volunteer Study Broaden immunology pipeline clinical

Neuromuscular Neuromuscular Neuromuscular Neuromuscular Neuromuscular Neuromuscular Neuromuscular Neuromuscular Positioned for Long - term Franchise Growth Hematology and Rheumatology Neurology Dermatology Nephrology gMG , CIDP, Myositis, TED, MMN, CMS, Musk MG, ALS ITP, Sjogren’s, POTS, Anca Vasculitis Pemphigus, Bullous Pemphigoid, Dermatomyositis Membranous Nephropathy, Lupus Nephritis, AMR, DGF

Innovation Ecosystem Build out innovation ecosystem discovery

Positioned for a Catalyst - Rich 2023 VYVGART gMG Approval in China VYVGART gMG Approval in Canada VYVGART gMG Launch in France, UK, Italy SC efgartigimod gMG Approval in US SC efgartigimod gMG Approval in EU SC efgartigimod gMG Submission in Japan VYVGART ITP Submission in Japan 2Q 2023 2H 2023 2H 2023 4Q 2023 Additional pipeline • ARGX - 117: ARDA MMN interim results • ARGX - 117: Initiate DGF POC study • ARGX - 119: Initiate Phase 1 study Efgartigimod • ADHERE data in CIDP • ADDRESS data in Pemphigus • ADVANCE (SC) data in ITP • POC data in Post - COVID POTS • Initiate registrational trial in TED • Initiate POC studies in AAV and AMR Commercial Clinical 4Q 2023 4Q 2023 Mid - 2023 1Q 2023 2H 2023 YE 2023 3Q 2023 YE 2023 Mid - 2023 March 20, 2023 1Q 2023 4Q 2023

Our mission continues… Humility Innovation Excellence Co - Creation Empowerment
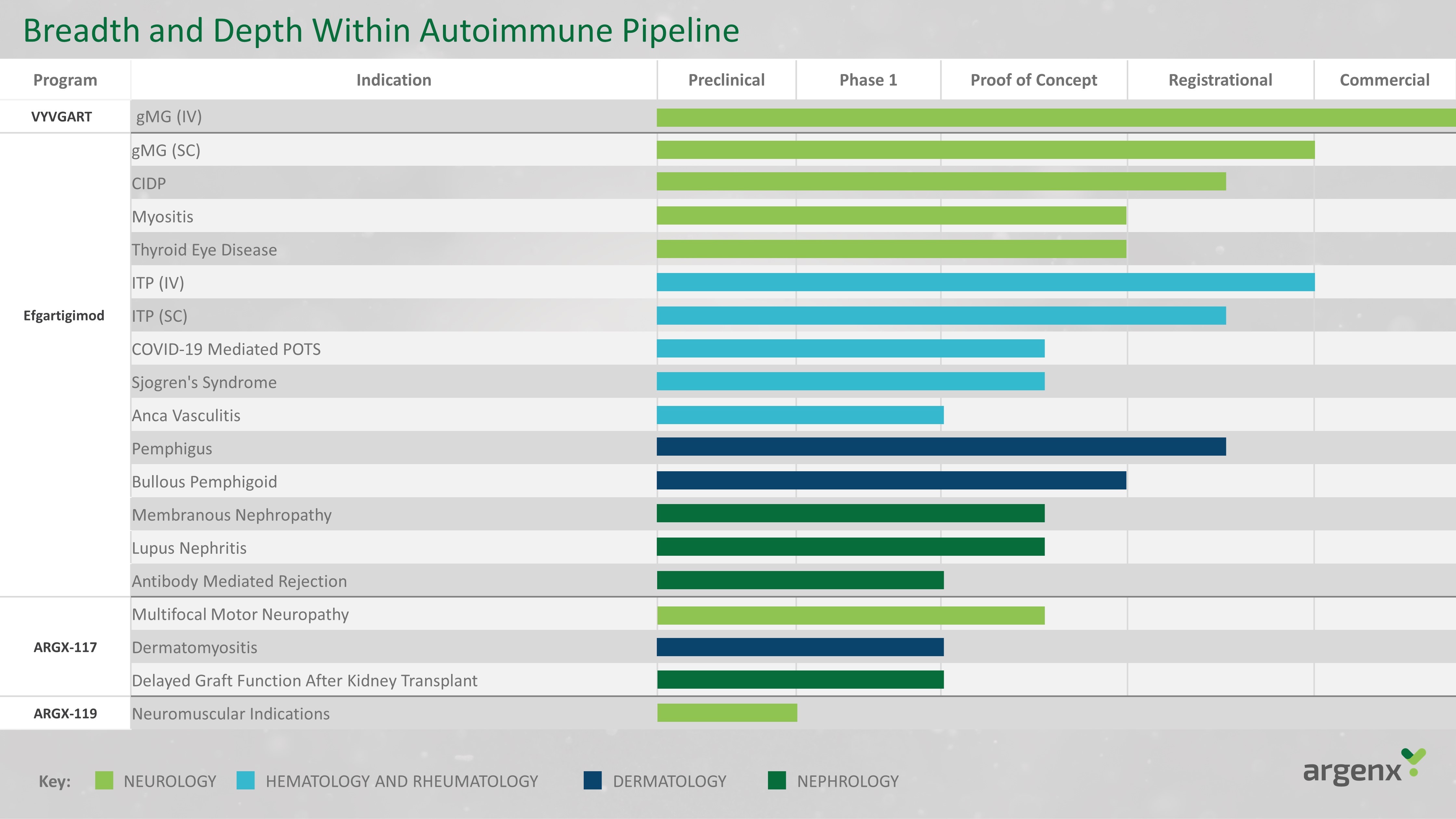
Program Indication Preclinical Phase 1 Proof of Concept Registrational Commercial VYVGART T gMG (IV) Efgartigimod gMG (SC) CIDP Myositis Thyroid Eye Disease ITP (IV) ITP (SC) COVID - 19 Mediated POTS Sjogren's Syndrome Anca Vasculitis Pemphigus Bullous Pemphigoid Membranous Nephropathy Lupus Nephritis Antibody Mediated Rejection ARGX - 117 Multifocal Motor Neuropathy Dermatomyositis Delayed Graft Function After Kidney Transplant ARGX - 119 Neuromuscular Indications NEUROLOGY HEMATOLOGY AND RHEUMATOLOGY DERMATOLOGY NEPHROLOGY Key: Breadth and Depth Within Autoimmune Pipeline





















