UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1-K
ANNUAL REPORT PURSUANT TO REGULATION A |
|
|
For the fiscal year ended: March 31, 2018 | |

|
True Leaf Medicine International Ltd. |
(Exact name of issuer as specified in its charter) |
|
|
British Columbia | 00-0000000 |
State or other jurisdiction of incorporation or organization | (I.R.S. Employer Identification No.) |
|
|
100 Kalamalka Lake Road, Unit 32, Vernon, British Columbia V1T 9G1 |
(Full mailing address of principal executive of?ces) |
|
778-475-5323 |
(Issuer’s telephone number, including area code) |
| | |
1
Contents
2
Annual Report
In this Form 1-A - Annual Report Pursuant To Regulation A (“Annual Report” or “Form 1-A”), unless otherwise noted or the context indicates otherwise, the “we”, “us”, “our”, “True Leaf”, and “Company” refers to True Leaf Medicine International Ltd. and its subsidiaries, True Leaf Investments Corp. (“TL Investments”), True Leaf Medicine Inc. (“TL Medicine”), True Leaf Pet Inc. (“TL Pet”), True Leaf Pet Europe LLC Sàrl (“TL Europe”), and True Leaf USA Inc. (“TL USA”).
This Annual Report contains company names, product names, trade names, trademarks and service marks of the Company and other organizations, all of which are property of their respective owners.
All financial information in this Annual Information Form is reported in Canadian dollars and using International Financial Reporting Standards as issued by the International Accounting Standards Board.
The information contained herein is dated as of March 31, 2018 unless otherwise stated.
Forward Looking Statements
This Annual Report contains certain statements related to industry scope and state, production, revenue, expenses, plans, development schedules and similar items that represent forward-looking statements. Such statements are based on assumptions and estimates related to future economic and market conditions. Such statements include declarations regarding management’s intent, belief or current expectations. Certain statements contained herein may contain words such as “could”, “should”, “expect”, “believe”, “will” and similar expressions and statements relating to matters that are not historical facts but are forward-looking statements. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve a number of risks and uncertainties; actual results may differ materially from those indicated by such forward-looking statements. Some of the important factors, but certainly not all, that could cause actual results to differ materially from those indicated by such forward-looking statements are: (i) that the information is of a preliminary nature and may be subject to further adjustment, (ii) the possible unavailability of financing, (iii) start-up risks, (iv) general operating risks, (v) dependence on third parties, (vi) changes in government regulation, (vii) the effects of competition, (viii) dependence on senior management, (ix) impact of Canadian economic conditions, and (x) fluctuations in currency exchange rates and interest rates.
Item 1. Business
Corporate Structure
True Leaf Medicine International Ltd. was incorporated under the laws of British Columbia on June 9, 2014.
The Company’s head office is located at 100 Kalamalka Lake Road, Unit 32, Vernon, British Columbia V1T 9G1.
The Company’s registered and records office is located at 1055 West Hastings Street, Suite 1700, Vancouver, BC V6E 2E9.
3
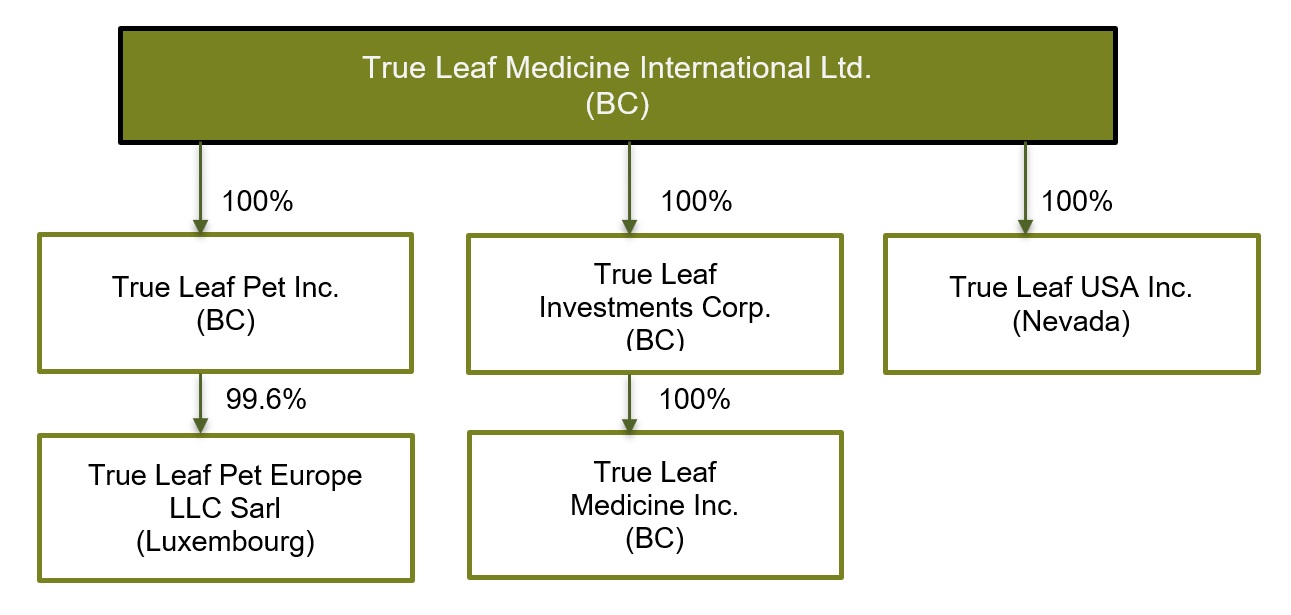
Intercorporate Relationships
We have five subsidiaries: True Leaf Investments Corp. (“TL Investments”), True Leaf Medicine Inc. (“TL Medicine”). True Leaf Pet Inc. (“TL Pet”), True Leaf Pet Europe LLC Sàrl (“TL Europe”), and True Leaf USA Inc. (“TL USA”).
TL Investments, TL Medicine, and TL Pet were formed in British Columbia on March 26, 2014, July 4, 2013, and November 18, 2015 respectively. TL Europe was formed in Luxemburg on July 18, 2016, and TL USA was formed in Nevada on September 11, 2017.
The following chart illustrates the Company’s corporate structure, the percentage of voting securities of each subsidiary owned by the Company, and the governing jurisdiction of each entity.
General Development of the Business
Overview
True Leaf is a plant-forward wellness brand for people and their pets.
We are a federally incorporated Canadian company with its common shares publicly traded on the Canadian Securities Exchange under the trading symbol: “MJ”, on the Frankfurt stock exchange under the trading symbol: “TLA”, and on the OTC Market Group's OTCQB Venture Market under the trading symbol "TRLFF". The Company is a reporting issuer in British Columbia, Alberta, and Ontario.
Founded in 2013, we have two main divisions: True Leaf Pet (“TL Pet”) and True Leaf Medicine (“TL Medicine”). TL Pet is focused on developing and selling supplements and treats for pets and TL Medicine is focused on becoming a licensed producer of cannabis developing cannabinoid related products for medicinal purposes. Our goal is to be a global cannabis-for-pets brand leader by embracing natural alternatives to help pets live healthier and longer lives. We believe that both the cannabis and pet industries represent high-growth industries. We plan to develop legally compliant medicinal cannabis products that can be sold across Canada, United States and other countries around the world.
4
True Leaf Pet

TL Pet launched its hemp-seed based pet supplement and treat product line in the fall of 2015. We share the commitment of our customers to improve the overall health of their pets with natural ingredients. We believe that consumers are looking for higher quality products that address the nutritional needs of their pets, without worrying about food safety or harmful side effects. Products containing hemp, including hemp seed oil, hemp protein and hemp extracts are gaining significant acceptance as evidence of their nutritional effectiveness becomes recognized. Our products are developed and marketed for the purpose of improving the health, comfort, enjoyment and safety of our customers' pets. We love animals and believe they deserve quality food and care.
Our current products are primarily sold through a combination of direct sales, distributors and brokers to veterinarians, food retailers, food wholesalers, drug stores, club stores, mass merchandisers, discount stores, natural foods stores and pet specialty stores. We currently sell our pet products in Canada, the United States and Europe.
Our Pet Products
In 2015, we began manufacturing, marketing and selling three dog chew products containing hemp which were created with veterinarian support and include other natural holistic ingredients. The hemp-based pet products we sell include additional quality ingredients such as green lipped mussel, curcuminoids from turmeric, L-theanine, lemon balm, chamomile, polyphenols from pomegranate, and DHA (an omega fatty acid) to create a high-value product that aligns with current consumer demands which focus on calming their pets, hip & joint pain relief and a daily omega supplement. The following are our hemp-based dog chew products:

5
True Hemp Chews - Calming
Support for Dogs | True Hemp Chews - Hip + Joint
Support for Dogs | True Hemp Chews- Immune + Heart Support for Dogs (Omega) |
Active Ingredients | Active Ingredients | Active Ingredients |
•Ground Hemp Seed - 500 mg •Hemp Seed Oil - 100 mg •L-Theanine - 25 mg •Chamomile - 12.5 mg •Lemon Balm - 12.5 mg | •Ground Hemp Seed - 500 mg •Hemp Seed Oil - 100 mg •Green Lipped Mussel - 150 mg •Turmeric Root Extract (95% Curcuminoids) - 35 mg | • Ground Hemp Seed - 500 mg • Hemp Seed Oil - 100mg • DHA - 75 mg • Polyphenols from Pomegranate - 25 mg |
| | |
Each of these products is natural, grain-free, and non-GMO certified hemp with no artificial colors or flavors. The inactive ingredients in all our chews include: peas, chickpeas, sweet potato, honey, cane molasses, gelatin, coconut oil, sea salt, calcium lactate, distilled vinegar, natural flavor, lactic acid, citric acid and natural preservatives.
Hemp seed oil, a major component of hemp seed itself and of TL Pet’s product line- has a variety of beneficial properties and may be used as a supplement. Because hemp oil is extracted from the industrial hemp plant, it contains no psychoactive reactors. Hemp contains known antioxidants from tocopherols and hosts a variety of other beneficial properties including anti-inflammatory compounds from terpenes, plant sterols and methyl salicylate - a relative of acetylsalicylic acid or ‘aspirin’. Furthermore, hemp seed oil supports enhanced blood circulation and stimulates cognitive thinking. TL Pet has created a hemp seed oil line of pet products which it sells that also support calming, hip & joint pain relief and the provision of a daily omega supplement, similar to the pet chew line of products.

Hemp and marijuana are different varieties of the same plant species of ‘Cannabis Sativa’. Marijuana plants contain high levels of tetrahydrocannabinol (“THC”). Hemp, on the other hand, is non-psychoactive and contains very little THC (less than 0.3% by law), but certain cultivars contain cannabidiol (“CBD”) located primarily in the hemp leaf and the hemp flower. TL Pet uses whole hemp seed and cold-pressed hemp seed oil in the True Hemp™ product line, which does not contain detectable levels of THC or CBD.
Hemp is rich in essential fatty acids and other polyunsaturated fatty acids. It has almost as much protein as soybean and is also rich in Vitamin E and minerals such as phosphorus, potassium, sodium, magnesium, sulphur, calcium, iron and zinc.[1] Dietary hempseed is also particularly rich in the omega-6 fatty acid, linoleic acid and also contains high concentrations of the omega-3 fatty acid, alpha-linolenic acid. The linoleic acid: alpha-linolenic acid ratio normally exists in hempseed at between 2:1 and 3:1 levels; therefore, we include hemp in all of our pet chews.
_____________________
[1] Callaway, J.C. “Hempseed as a nutritional resource: An overview” Euphytica (2004) 140: 65. doi:10.1007/s10681-004-4811-6
6
Hemp is also legally refined in industrial factories for textile and nutritional use. It is often consumed and mixed into other products including cereal and granola bars. Hemp for dogs is increasing in popularity because of its significant potential health benefits that may include joint support and antioxidant support.
We are currently developing other hemp-based pet products which we intend to test and - if suitable - add to our product line. In particular, we have expanded the functional line with chew stick supplements for dogs and we are planning to add functional cat chews and supplements. The launch of these and other products is dependent upon favorable market conditions, successful research and development and raising additional capital.
On December 30, 2016, we acquired the assets and intellectual property of OregaPet®, a Canadian brand of plant-forward natural supplement products for pets. The OregaPet®, product line includes: oregano first aid drops, oregano first aid gel, dental health mini treats, dental health treats, dental spray, pet toothpaste, ear drops, bed and body spray, shampoo therapy and oil of oregano for pets.
True Leaf Medicine

TL Medicine was launched in July 2013 to become a licensed producer of medical cannabis for the Canadian market under Canada’s Access to Cannabis for Medical Purposes Regulations (“ACMPR”) program administered by Health Canada. TL Medicine will be subject to a Health Canada inspection upon completion of the construction of its facility to allow for the cultivation, manufacturing and distribution of cannabis products. We anticipate completing our facility in the fall of 2018 and anticipate receiving our license to grow cannabis in 2019.
Cannabis for Medical Purposes. TL Medicine began as a “licensed producer” applicant in Canada’s Marijuana for Medical Purposes Regulations (“MMPR”) program. Our original submission was submitted in July of 2013. A “ready to build” approval was granted for the first application in January 2014, but issues arose regarding the facility location and local zoning. In March 2014, we secured a new location and submitted another application on April 8, 2014.
In July 2015, we received a notice from Health Canada stating that our application had passed through the preliminary screening process and was undergoing enhanced screening. Enhanced screening is one of the necessary steps in the process of becoming a licensed producer of cannabis for medical purposes under the MMPR. Shortly thereafter the federal government set the election date for October 19, 2015, the application process for all applicants under the MMPR stalled.
On August 24, 2016, the federal government adopted the ACMPR program to replace the MMPR program.
On November 27, 2017, the House of Commons passed Bill C-45, an Act respecting cannabis and to amend the Controlled Drugs and Substances Act, the Criminal Code and other Acts (the “Cannabis Act”), and on June 21, 2018, the Government of Canada announced that the Cannabis Act received royal assent. On July 11, 2018, the regulations issued pursuant to the Cannabis Act (the “Cannabis Regulations”) were released by the Government of Canada and broadened the scope of individuals required to hold security clearances. The Cannabis Act came into force on October 17, 2018.
7
As of the date of this Annual Report we do not have a license under the Cannabis Act, and no cannabis products are in commercial production or use. We will be required to satisfy additional obligations under the Cannabis Act in order to qualify for a license including the completion of a compliant facility on a parcel of purchased land in Lumby, British Columbia (the "Lumby Property"). We continue to work diligently to comply with all of the requirements of Health Canada.
Product development
Our focus for TL Medicine is to support the creation, research and development of cannabis products for medicinal purposes. Led by its strategic partners, TL Medicine aims to build market recognition with the goal of becoming a premium niche brand of medicinal cannabis in Canada.
Significant Acquisitions
During the most recently completed financial year, the Company has not completed any significant acquisitions.
The Business
General
Revenue for the year ended March 31, 2018 increased 280% to $1,400,511 from $368,536 for the same period in the previous fiscal year. All of the Company’s revenues from inception to date are from the sale of its hemp-based nutrition for pets, the majority of which have occurred in North America and Europe. Revenue growth was primarily fuelled by us expanding the commercial reach of our TL Pet division into new geographies both in-store and online.
| Year ended March 31 |
Description | 2018 | 2017 | 2016 |
Revenues | $ 1,400,511 | $ 368,536 | $ 37,330 |
Cost of sales | (779,182) | (248,909) | (26,117) |
Gross profit ($) | $ 621,329 | $ 119,627 | $ 11,213 |
Gross profit (%) | 44% | 32% | 30% |
Total operating expenditures | (4,809,855) | (1,857,834) | (1,034,170) |
| | | |
8
Strategic Outlook
Our objectives for the next 12 months are:
- Continue to build worldwide market share, distribution networks, secure new customers, and launch new items in the natural pet product space, growing our line of innovative supplements and natural remedy products for pets. Sales will be through traditional distribution channels, direct-to-store and direct-to-consumer online sales channels;
- Successfully complete additional capital financings in order to fund the objectives of our business plan;
- Complete build-out of a portion of the first phase of our 25,000 square foot medicinal cannabis production facility located in Lumby, British Columbia, in order to comply with Health Canada’s requirements to become a licensed producer under the Cannabis Act;
- Receive approval to sell medicinal cannabis under the Cannabis Act by mid-2019 in order to commence the research & development and sale of cannabis based products; and
- Review potential joint ventures or strategic acquisition targets in the pet, wellness and medicinal cannabis space.
Pet Support Supplements and Treats - TL Pet
Established in 2015, TL Pet markets hemp-seed based products for the pet industry. We launched the True Hemp™ pet supplement line in Canada, the United States, and Europe, becoming one of the first hemp-seed based pet product lines to be marketed worldwide. The hemp-seed based formula meets US and Canadian guidelines allowing TL Pet to establish a distribution network that includes more than 2,000 stores globally, with retail partners including PetSmart Canada, Pets Supplies Plus USA, PetsCorner UK and Amazon.
TL Pet’s formulations were created with veterinarian support and include other natural plant-based holistic ingredients. The hemp-seed based products are legally compliant in both the US and Canada and are part of a broader strategy to position us as a global, cannabis-for-pets brand market leader. We are currently working with a Vancouver branding firm to solidify this strategy across both TL Pet and TL Medicine and plan to bring more products to market in the future.
Our long-term business objectives for TL Pet are:
- Create a global cannabis-for-pets brand, with the mission to improve the quality of life for companion animals;
- Increase sales, distribution and store count within the pet specialty, mass-pet, veterinary and food/mass/drug market segments and assess and implement other non-traditional distribution channels to market and sell our pet products;
- Launch additional product lines and secure additional distribution partners in the European markets;
- Seek out key distribution partners for alternative market regions including Asia and South America;
- Continue to perform R&D work and, if and when permitted by the applicable legislation in the jurisdiction, launch new ‘CBD’ pet formulations for the North American and European markets; and
- Seek out potential long-term strategic partners to support the business.
9
Production and Services
We outsource the manufacturing of our products to third parties whose facilities are GMP compliant (good manufacturing practice). GMP is a system for ensuring that products are consistently produced and controlled according to quality standards. Our products are certified by the National Animal Supplement Council (“NASC”). The Company received the NASC Seal after successfully passing a thorough quality audit and documentation review. As part of the certification, NASC ensures stringent ingredient qualification, quality production processes, adverse event reporting procedures, continuous product and data monitoring, and allowable product claims. NASC is one of the highest-level certifications in the pet industry. We have an oral contractual relationship with Okanagan Naturals in British Columbia to manufacture our products and three additional manufacturers located in the United States.
Specialized Skill and Knowledge
The global pet business is complex and requires a specialized knowledge of industry distribution channels, formula and product creation, and specific pet food manufacturing expertise. Darcy Bomford, our founder and President has more than 25 years of pet industry experience.
Prior to founding us, Mr. Bomford was the founder, President, Chief Executive Officer and director of Darford International Inc., a manufacturer and marketer of branded and private pet food products with three federally inspected production plants in the United States and Canada, whose common shares formerly traded on the TSX Venture Exchange under the symbol “WUF”.
Mr. Bomford has extensive expertise with professional manufacturing systems, including comprehensive third party audited food safety systems, product development, marketing and sales within a highly regulated and competitive industry. Darcy is able to leverage his extensive contacts and experience in the North American and European pet products industry to expand sales and markets and to create unique formulations for the Company’s pet product division.
Our team also includes talent and experience from the pet industry who have worked at leading pet companies including Mars Petcare, IAMS and Petcurean.
Competitive Conditions
The cannabis-for-pets industry is a new industry that is growing very rapidly. While this industry is subject to strict regulation, many of our competitors are manufacturing and selling pet products that are not fully legally compliant in their jurisdictions. As of the date of this AIF, CBD for pets is not legal in either Canada or US.
We believe that we will face competition from the following sources:
(i) Illegal / “Grey” businesses:
There are illegal cannabis-for-pet companies operating in the grey and black markets. Even though they are operating in the grey/black market, they still act as competitors to us by either diverting customers away due to product choice including CBD for pets products. We believe that we can reduce the impact of competition from illegal businesses by focusing on education of the importance of our safe, legal and effective hemp-seed based solutions for pets.
10
(ii) Licensed producers:
A few licensed producers in Canada have publicly declared their interest or launched early initiatives in the cannabis-for-pets space. These companies have larger operations and more financial resources than we do. We believe that we can reduce the impact of competition from Licensed Producers by virtue of our focus as a cannabis-for-pets pure play. We believe that our growing distribution network, brand and team, who are all authentically passionate about dogs and pets, will better connect with pet owners than the cannabis conglomerates can. We intend to distinguish ourselves from our competitors by being a global cannabis-for-pets pure play with legally compliant products and established worldwide distribution in more than 2,000 stores.
We believe that our leadership team, brand strategy, commitment to high quality, ability to formulate unique leading pet products, outstanding client service, adherence to the law and properly capitalized operations will enable us to establish and retain a leadership position in the cannabis-for-pets market.
We have applied for a “licensed producer status” under the ACMPR in Canada (now the Cannabis Act). As of September 28, 2018, Health Canada had granted licenses under the ACMPR to a total of 120 producers (“Licensed Producers”) of which 64 are fully authorized to produce and sell cannabis, 49 have a license restricted to the cultivation of medical cannabis, four have a license just to sell medical cannabis, two have a license just to label, test or package medical cannabis and one has a license only to produce cannabis oils. These licenses issued under the ACMPR are now deemed to be licenses under the Cannabis Act.
See below under the heading “Risk Factors - Competition” for further information.
New Products
TL Pet’s current formulations were created with veterinarian support and include other plant-based holistic ingredients. TL Pet introduced hemp-based treats specially formulated for cats at a trade show in May of 2018, in Nuremberg, Germany. The functional treats provide calming support for cats, promote skin and coat health, help prevent hairballs and support urinary tract function. We plan for these to be made available to our customers worldwide.
To support future product development, we created a Veterinary Advisory Board (“VAB”) led by Dr. Katherine Kramer, an internationally recognized opinion leader in the area of cannabis-based healing for pets. Dr. Kramer has been practicing veterinary medicine for 16 years and is an advocate for the research and therapeutic use of cannabis for animals. Dr. Kramer is currently the Medical Director at the VCA-Canada Vancouver Animal Wellness Hospital. As chair of our VAB, Dr. Kramer’s role includes recruiting veterinarians from around the world to join our VAB, to support our development of legal and safe medicinal cannabis products for pets. She and her colleagues will be instrumental in leading trials for our existing hemp-seed based products and the research and development of a future CBD pet product line for us (including the design and execution of supportive trials and the development of education programs for veterinarians and pet owners on the best use of cannabis products for pets). The VAB’s mandate is to provide strategic direction and oversight to our pipeline of new products (both hemp-seed based and, if and when legal, those containing CBD) and ensure that we remain on the leading edge of plant-based holistic solutions to pet ailments.
Components
We provide our contract manufacturers with our formula and manufacturing specifications for each of our products. We purchase all of our hemp seed powder, hemp seed oil and all of our active ingredients (chamomile, L-Theanine, green lipped mussel, etc.) from suppliers vetted by T.L. Pet. We use only the
11
highest quality ingredients in all of our pet products. Our manufacturers source and purchase all inactive raw ingredients and manufacture their products to our specifications.
We utilize hemp processors in Canada, the US and Europe who are able to meet our quality and quantity requirements at a competitive price. T.L. Pet’s North American operations source all of our hemp from Canadian processors. True Leaf Pet’s European operations source all of our hemp from processors in Europe.
Our products are made with natural and fresh ingredients sourced from North America and Europe. We have not entered into any long-term supply contracts. We believe all of our current suppliers have the ability to scale to support our growth in the future. We have identified multiple alternative sources for a majority of our product ingredients that meet our quality and safety standards in order to not be dependant on a single source or supply of our component inputs.
Economic Dependence
TL Pet earns revenue from a mix of online, retail and distributor customers. We are not economically dependent on a single customer. For the year ended March 31, 2018, 39% of revenue was earned from three customers (March 31, 2017 - two customers 27%). As at March 31, 2018, these three customers amounted to 29% (March 31, 2017 - two customers amounted to 16%) of total trade receivables.
Changes to Contracts
The Company has not entered into any contracts which, if terminated or re-negotiated in the current financial year, would have a material impact on the Company’s operations.
Environmental Protection
There are no specific environmental requirements to the Company’s TL Pet business. TL Pet outsources its manufacturing process to third parties.
Regulatory Framework
Pet Food-Related Regulation - Canada
In Canada, the labeling and advertising of pet food is regulated by the Consumer Packaging and Labelling Act and the Competition Act, administered by Industry Canada. The Consumer Packaging and Labelling Act sets out certain items that are required to be included on pet food labels.
The Pet Food Association of Canada (“PFAC”) with input from members from the Competition Bureau has also established pet food labeling guidelines. These guidelines are voluntary. The PFAC guidelines recommends pet food labels should include the following items: (1) a list of ingredients in descending order by percentage of weight; (2) feeding instructions; and (3) guaranteed analysis, being information on the minimum and maximum nutritional quantities. For example, the analysis will include the maximum or minimum percentage of protein, fat, fibre, and moisture as well as the nutritional adequacy or intended life stage for which the food is suitable. They also recommend that ingredients be listed and identified by their common name. When an ingredient or combination of ingredients makes up 90% or more of the total weight of all ingredients, these ingredients should also form a part of the product name. For example, if the product contains 90% or more beef, it may be called “my brand beef dog food”.
In Canada, products that pass the Canadian Veterinary Medical Association (“CVMA”) Pet Food Certification Program, which involves a feeding trial, carry a CVMA label on their packaging. Participation in the program is voluntary.
12
Pet Supplement Regulation - Canada
Products sold and marketed as ‘pet supplements’ in Canada are currently administered by the Canadian Low Risk Veterinary Health Products (“LVRHP”) Interim Notification Program. Health Canada considers the current INP (Interim Notification Program) structure to be a temporary measure pending the new veterinary drug framework to improve the regulation of LRVHPs.
The INP allows for LRVHPs to obtain a notification number if certain conditions have been met, the significant ones being:
- The product is for use only in dogs, cats, or horses that are not intended for food.
- All ingredients are listed in and meet the conditions of admissible substances in the ‘List of Substances’ established by Health Canada.
- There is objective and credible evidence demonstrating that the product is safe and can support a reasonable expectation of effectiveness when the product is used as intended.
- Product labeling information and any other information supplied to the users will match the information provided on the notification form (e.g. health claims) and comply with the conditions of admissible substances (e.g. contraindications, cautions and warnings).
Participation in the INP is voluntary and industry members may instead prefer to obtain a Notice of Compliance and Drug Identification Number through the normal regulatory process.
The company is participating in the program, has submitted all product information for registration and is currently waiting to be assigned its notification numbers for each product.
Pet Food-Related Regulation - United States
In the United States, the Food and Drug Administration’s (“FDA”) Center for Veterinary Medicine regulates animal feed, including pet food, under the Federal Food, Drug and Cosmetic Act (“FFDCA”) and its implementing regulations. Although pet foods are not required to obtain premarket approval from the FDA, any substance that is added to or is expected to become a component of a pet food must be used in accordance with a food additive regulation unless it is generally recognized as safe (“GRAS”) under the conditions of its intended use.
The labeling of pet foods is regulated by both the FDA and individual state regulatory authorities. FDA regulations require proper identification of the product, a net quantity statement, a statement of the name and place of business of the manufacturer or distributor and proper listing of all the ingredients in order of predominance by weight. The FDA also considers certain specific claims on pet food labels to be medical claims and therefore subject to prior review and approval by the FDA. In addition, the Food and Drug Administration Amendments Act of 2007 requires the FDA to establish ingredient standards and definitions for pet food, processing standards for pet food and updated labeling standards for pet food that include nutritional and ingredient information. The FDA is currently working to implement these requirements.
The FDA recently noted an increase in the number of dog and cat foods labeled as being intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease and noted that animal health may suffer when such products are not subject to pre-market FDA approval and are provided in the absence of a valid veterinarian-client-patient relationship. The FDA recently issued guidance containing a list of specific factors it will consider in determining whether to initiate enforcement action against products that satisfy the definitions of both an animal food and an animal drug, but which do not comply with the regulatory requirements applicable to animal drugs. These include, among other things, whether the product is only made available through or under the direction of a veterinarian and does not present a known safety risk when used as labeled. We believe that we market our products in compliance with the policy articulated in FDAs guidance and in other claim-specific guidance, but
13
the FDA may disagree or may classify some of our products differently than we do and may impose more stringent regulations applicable to animal drugs, such as requirements for pre-market approval and compliance with GMPs for the manufacturing of pharmaceutical products.
Under Section 423 of the FFDCA, the FDA may require the recall of a pet food product if there is a reasonable probability that the product is adulterated or misbranded and the use of or exposure to the product will cause serious adverse health consequences or death. In addition, pet food manufacturers may voluntarily recall or withdraw their products from the market.
Most states also enforce their own labeling regulations, many of which are based on model definitions and guidelines developed by Association of American Feed Control Officials (“AAFCO”). AAFCO is a voluntary, non-governmental membership association of local, state and federal agencies that are charged with regulation of the sale and distribution of animal feed, including pet foods. The degree of oversight of the implementation of these regulations varies by state, but typically includes a state review and approval of each product label as a condition of sale in that state.
Most states require that pet foods distributed in the state be registered or licensed with the appropriate state regulatory agency.
Facilities that manufacture, process, pack, or hold foods, including pet foods, must register with the FDA and must renew their registration every two years. This includes most foreign facilities as well as domestic facilities. Registration must occur before the facility begins its pet food manufacturing, processing, packing, or holding operations.
We are also subject to the Food Safety Modernization Act (“FSMA”). Under the FSMA, the FDA implemented the Current Good Manufacturing Practice, Hazard Analysis and Risk-Based Preventive Controls for Food. Our manufacturing facilities must comply with the Foreign Supplier Verification Program on or before July 2017.
Pet Supplement Regulation - United States
Some of the company’s product line is marketed as dietary supplements for animals, not feed or treats. According to the FDA, dietary supplements for animals are not recognized as a class of products. Under the Federal Food, Drug, and Cosmetic Act, products marketed as dietary supplements for use in animals are classified as either foods or drugs, depending on their intended use.
In order to find a pathway to market for its supplement products, the company is a member and complies with the product guidelines of the National Animal Supplement Council (“NASC”). NASC was formed in 2001 when the animal health supplement industry was threatened to be shut down from a complicated and erratic regulatory environment under the AAFCO and FDA regulatory bodies.
The NASC put together a framework under which companies could market and distribute products, as long as they were ‘non-food’ and didn’t make nutritional claims or references anywhere on the label, website or promotional material. Product claims could only involve how the ingredients impacted the structure or function of the animal, ‘joint support’ or ‘cardiovascular health’ are common examples.
Since 2002, AAFCO, the FDA and the NASC have worked together and supported this product category, thus allowing the marketing and sale of animal supplements.
The company follows the NASC member requirements, including implementing standards for good manufacturing practices, participating in the NASC Adverse Event Reporting System and complying with all supplement labeling and claims guidelines.
14
Medical Marijuana - TL Medicine
TL Medicine was launched in July 2013 to become a licensed producer of medical cannabis for the Canadian market under Canada’s (now repealed) ACMPR program administered by Health Canada. Pursuant to the Cannabis Act and Cannabis Regulations, TL Medicine will be subject to a Health Canada inspection upon completion of the construction of its facility to allow for the cultivation, manufacturing and distribution of cannabis products. The Company anticipates completing its facility in the fall of 2018 and anticipates receiving its license to grow cannabis in 2019.
The Company’s long-term business objectives for TL Medicine are:
- Complete construction of the medicinal cannabis facility and be approved as a grower and seller of medicinal cannabis under the Cannabis Act in Canada;
- Build brand recognition with the goal of becoming the premium niche brand of over-the-counter hemp-based supplements and medicinal cannabis in Canada;
- Implement in-house lab and build out lab services business model;
- Implement extraction, fractionation and remediation equipment;
- Assess the creation and sale/lease of space within the Lumby facility, as well as offering value-added services, to support the micro-cultivator/craft cannabis community;
- Assess ongoing demand and implement plans for the build out and implementation of phase two, expanding production capacity to a total of 10,000 kilograms of dried flower per year;
- Assign capital towards research and development in order to build a base of intellectual property from proprietary formulations, cultivars, delivery mechanisms, with a focus on unique pet product formulations and supplements; and
- Assess and explore opportunities to develop a base of wholesale supply contracts for the recreational or medicinal markets.
Products and Services
We plan to be a cultivation-focused Licensed Producer with a specific focus on premium cannabis flower. Premium cannabis flower is produced through a combination of high quality genetics, facilities and standard operating procedures (“SOPs”).
If we receive a license to grow and sell cannabis from Health Canada, we plan to create a variety of premium CBD strains and offerings to support the research and development of a full line of unique and proprietary CBD pet supplements and products. Currently, CBD products for pets are not approved or legal, however, our expectation is these pet product formulations will become a legal, mainstream offering for the pet market in the future. We expect to have a competitive advantage to market and sell our CBD line for pets given our strong branding and global distribution network already in place.
We expect that any cannabis grown and not used for our CBD pet products and supplements line will be sold to the wholesale market in Canada.
We also intend to assess the merits of creating space to sell and/or lease, as well as offer value-added services, to support micro-cultivators. The Cannabis Regulations allow micro-cultivator licenses to small companies and individuals that would permit them to grow and sell cannabis to other licensed producers, licensed retailers and storefront dispensaries. These changes by Health Canada have created opportunity not only for the large commercial growers but also for independent growers.
15
Specialized Skill and Knowledge
The primary specialized skill and knowledge requirement for success as a Licensed Producer of cannabis for medical purposes is with respect to cultivating and producing cannabis. We believe our experienced growing team can produce premium, high quality cannabis to support the creation of industry leading cannabis products for pets.
Health Canada, pursuant to the Cannabis Regulations, sets the standard required for production and sale of medical cannabis. Our team of growers and quality assurance personnel will work to ensure a premium, consistent product is produced, meeting or exceeding Health Canada standards.
Competitive Conditions
We have applied for “licensed producer status” under the ACMPR in Canada (now transitioning to the Cannabis Act). As of September 28, 2018, Health Canada has granted licenses to a total of 120 Licensed Producers of which 64 are fully authorized to produce and sell cannabis, 49 have a license restricted to the cultivation of medical cannabis, four have a license just to sell medical cannabis, two have a license just to label, test or package medical cannabis and one has a license only to produce cannabis oils. A number of other entities have applications pending or will seek to obtain licensed producer status under the new Cannabis Act.
The differentiators of cannabis between competitors are expected to be price, quality (smell/taste/appearance), organic purity (zero additive, pesticide, mould treatment or anti-biological) and production process. The cost of growing an inexpensive strain (i.e. mass market) is identical to growing premium strains and the crop risks are identical (disease, pests and infrastructure failure). The majority of firms with listed product often overlap in strains and strengths (THC/CBD). We believe we will successfully compete with other Licensed Producers as our cannabis will be used to create unique, value added products to sell into the large, growing pet supplement market.
The Lumby Property
During the year ended March 31, 2018, we acquired 40 acres located in Lumby B.C. for total consideration of $3,380,387 to build our cannabis cultivation facility, the True Leaf Campus. As at March 31, 2018, construction costs incurred of $726,955 are capitalized and depreciation will commence when the facility is put into use. The total budget for the cannabis cultivation facility is estimated at $7.4 million. We have retained Colliers Project Leaders to provide professional project management services and assist us in keeping the project on time and on budget. As at September 30, 2018, we have spent $5.2 million on the facility, and anticipate spending an additional $2.2 million to complete the facility.
The True Leaf Campus and cultivation facility has provided a number of jobs to residents of the City of Lumby during the construction phase and is expected to support additional permanent jobs upon completion. The City of Lumby supports True Leaf’s presence and positive economic impact on its community.
The first phase of the project includes a two-storey 9,000 square foot central hub for the initial grow area, laboratory services, whole-plant extraction, and the research, development and production of cannabis products, plus a 16,000 square foot wing for cannabis cultivation. Ownership of the 40-acre site means we are well positioned to expand to meet future market demands. We are assessing best options and business models to fully realize optimal economic benefits of the Lumby property and believe we are well positioned for significant growth and expansion of our cannabis growing facilities if demand warrants.
16
True Leaf Campus (Final), Phase 1, Lumby, B.C

True Leaf Campus (as of Oct 19, 2018), Phase 1, Lumby, B.C
We expect to finish the construction of our Lumby facility in the fall of 2018. Our application to become a licensed producer or grower of cannabis under the ACMPR will be migrated to the CTLS under the Cannabis Act for review and approval. We also plan to apply for a license to sell cannabis products pursuant to standard processing techniques. We anticipate securing both licenses by the end of 2019.
Regulatory Framework
Legal Developments
On November 27, 2017, the House of Commons passed the Cannabis Act and on June 21, 2018, the Government of Canada announced that the Cannabis Act received Royal Assent. The Cannabis Act came into force on October 17, 2018. On July 11, 2018, the Cannabis Regulations were released by the government and these regulations also came into force on October 17, 2018. The Cannabis Regulations, among other things, set forth the following:
17
- Licenses, Permits and Authorizations;
- Security Clearances;
- Cannabis Tracking System;
- Cannabis Products;
- Packaging and Labelling;
- Cannabis for Medical Purposes; and
- Drugs Containing Cannabis.
Licenses, Permits and Authorizations
The Cannabis Regulations establish six classes of licenses: (1) cultivation licenses; (2) processing licenses; (3) analytical testing licenses; (4) sales for medical purposes licenses; (5) research licenses; and (6) cannabis drug licenses. The Cannabis Regulations also create subclasses for cultivation licenses (standard cultivation, micro-cultivation and nursery) and processing licenses (standard processing and micro-processing). Different licenses and each sub-class therein, carry differing rules and requirements that are intended to be proportional to the public health and safety risks posed by each license category and each sub-class.
The Cannabis Regulations provide that all licenses issued under the Cannabis Act are valid for a period of no more than five years and that no licensed activity may be conducted in a dwelling-house. The Cannabis Regulations also permit both outdoor and indoor cultivation of cannabis. The implications of the proposal to allow outdoor cultivation are not yet known, but such a development could be significant as it may reduce start-up capital required for new entrants in the cannabis industry. It may also ultimately lower prices as capital expenditure requirements related to growing outside are typically much lower than those associated with indoor growing.
Generally, the Cannabis Act provides that licenses issued under the ACMPR that were in force immediately before the Cannabis Act coming into force on October 17, 2018 are deemed to be licenses issued under the corresponding provisions of the Cannabis Act for the applicable activity and any such licenses will continue in force so long as they are renewed and are not revoked or expired. For example, under the ACMPR authorizing the production of fresh or dried cannabis, or cannabis plants or seeds is deemed to be a cultivation license under the Cannabis Act, a licence under the ACMPR authorizing the production of cannabis oil or cannabis resins deemed to be a processing license under the Cannabis Act, and a license under the ACMPR authorizing sale of cannabis plants, seeds, fresh or dried cannabis or cannabis oil to medical users is deemed to be a license for sale for medical purposes, provided that the license holder meets certain requirements.
Similarly, the Cannabis Act generally provides that licenses pertaining to cannabis or its derivatives issued under the Narcotic Control Regulations that are in force immediately before the Cannabis Act came into force are deemed to be licenses issued under the corresponding provisions of the Cannabis Act and any such license continues in force until revoked or it expires. For example, a license issued under the NCR authorizing cultivation of cannabis for scientific purposes shall be a research license under the Cannabis Act.
Security Clearances
Under the Cannabis Regulations, certain people associated with cannabis licensees, including individuals occupying a key position, directors, officers, large shareholders and individuals identified by the Minister of Health, must hold a valid security clearance issued by the Minister. This includes individuals that have direct control over a license holder, as well as the officers and directors of any corporation having direct control over a license holder (e.g., officers and directors of a parent corporation). The Cannabis Regulations
18
provide a three-month grace period for current license holders to identify those individuals that require security clearances and to apply for such security clearances (i.e., until January 17, 2019). Security clearances issued under the ACMPR are considered to be security clearances for the purposes of the Cannabis Act and Cannabis Regulations.
Under the Cannabis Regulations, the Minister of Health may refuse to grant security clearances to individuals with associations to organized crime or with past convictions for, or an association with drug trafficking, corruption or violent offences. Individuals who have histories of nonviolent, lower-risk criminal activity (for example, simple possession of cannabis, or small-scale cultivation of cannabis plants) are not precluded from participating in the legal cannabis industry, and the grant of security clearance to such individuals is at the discretion of the Minister and such applications will be reviewed on a case-by-case basis.
Cannabis Tracking System
Under the Cannabis Act, the Minister of Health is authorized to establish and maintain a national cannabis tracking system (the “Cannabis Tracking System”). The Cannabis Regulations provide the Minister of Health with the authority to make a ministerial order that would require certain persons named in such order to report specific information about their authorized activities with cannabis, in the form and manner specified by the Minister.
The Ministerial Order regarding the Cannabis Tracking System was published in the Canada Gazette, Part II, on September 5th, 2018 and came into effect on October 17, 2018. The purpose of this system is to track the flow of cannabis throughout the supply chain as a means of preventing the illegal inversion and diversion of cannabis into and out of the regulated system. Under the Cannabis Tracking System, a holder of a licence for cultivation, licence for processing, or a licence for sale for medical purposes is required to submit monthly reports to Health Canada. The first monthly reports from licence holders and provinces and territories under the Cannabis Tracking System are due no later than November 15, 2018.
Cannabis Products
The Cannabis Regulations permit the sale to the public of dried cannabis, cannabis oil, fresh cannabis, cannabis plants, and cannabis seeds, including in such forms as “pre-rolled” and in capsules. The THC content or and size of certain cannabis products is limited by the Cannabis Regulations. The sale of edible cannabis products and concentrates (such as hashish, wax and vaping products) are currently prohibited but are expected to be permitted within one year following the Cannabis Act coming into force.
Packaging and Labelling
The Cannabis Regulations set out requirements pertaining to the packaging and labelling of cannabis products. Such requirements are intended to promote informed consumer choice and allow for the safe handling and transportation of cannabis. All cannabis products are required to be packaged in a manner that is tamper-proof and child-resistant in accordance with the Cannabis Regulations.
Health Canada is proposing strict limits on the use of colours, graphics, and other special characteristics of packaging. Cannabis package labels must include specific information, such as: (i) product source information, including the class of cannabis and the name, phone number and email of the cultivator; (ii) a mandatory health warning, rotating between Health Canada’s list of standard health warnings; (iii) the Health Canada standardized cannabis symbol; and (iv) information specifying THC and CBD content.
A cannabis product brand name may only be displayed once on the principal display panel or, if there are separate principal display panels for English and French, only once on each principal display panel. It can be in any font style and any size, so long as it is equal to or smaller than the health-warning message.
19
The font must not be in metallic or fluorescent colour. In addition to the brand name, only one other brand element can be displayed.
The Cannabis Regulations provide a six-month transitional period to allow licensed holders under the ACMPRs to sell cannabis products labelled in accordance with the ACMPRs.
Advertising
The Cannabis Act provides for prohibitions regarding the promotion of cannabis products. Subject to a few exceptions, all promotion of cannabis products is prohibited unless authorized by the Cannabis Act. The prohibitions apply to anyone who may be involved in promotion cannabis, cannabis accessories and services related to cannabis, including: (1) persons who produce, sell or distribute cannabis; (2) persons who sell or distribute cannabis accessories; (3) persons who provide cannabis-related services; or (4) media organizations.
Limited promotion of cannabis, cannabis accessories and cannabis-related services is permitted under the Cannabis Act in specific circumstances including (1) informational promotion or brand-preference promotion; (2) point of sale; and (3) brand elements on things that are not cannabis or a cannabis accessory subject to restrictions.
Cannabis for Medical Purposes
On October 17, 2018, the medical cannabis regime under the ACMPRs was repealed and substantively reinstituted into the Cannabis Regulations under the Cannabis Act. As a result, the medical cannabis regulatory framework under the Cannabis Act and the Cannabis Regulations will remain substantively the same as previously existing under the Controlled Drugs and Substances Act and the ACMPR, with adjustments to create consistency with rules for non-medical use, improve patient access, and reduce the risk of abuse within the medical access system (see Part 14 of the Cannabis Regulations entitled “Access to Cannabis for Medical Purposes”). The sale of medical cannabis will remain federally regulated and in each case, sales can only be made by an entity that holds a licence to sell under the Cannabis Regulations to clients that have a medical document and that have registered with the licensed entity. Just as with the previous medical cannabis regime under the ACMPRs, under the Cannabis Regulations, clients (patients) will need to obtain a medical document (a document similar to a prescription) from their doctor and then register as a client with a cannabis company that has a licence to sell for medical purposes (the registration is only good for up to a year).
Provincial Regulatory Framework
While the Cannabis Act provides for the regulation of the commercial production of cannabis for recreational purposes and related matters by the federal government, the Cannabis Act proposes that affords the provinces and territories of Canada with the authority to regulate other aspects of cannabis for recreational purposes (similar to what is currently the case for liquor and tobacco products) such as sale and distribution, minimum age requirements that are greater than the minimum of 18 included in the Cannabis Act, places where cannabis can be consumed, and a range of other matters.
With respect to sale and distribution, there are essentially three general frameworks that the provinces and territories have implemented: (i) privately operated cannabis retailers licensed by the province; (ii) government run retail stores; or (iii) a combination of both frameworks (e.g. allowing for privately-operated and government-operated brick and mortar retail stores, while online retail stores, in most jurisdictions, are operated by the applicable provincial or territorial government). Regardless of the framework, the recreational cannabis market will ultimately be supplied by federally licensed cultivators and processors. Most jurisdictions have implemented a government-run wholesale model. Brick and mortar retail stores are required to obtain their cannabis products from the wholesalers, while the wholesalers, in turn, acquire the cannabis products from the federally licensed cultivators and processors.
20
General
Intangible Properties
The ownership and protection of our intellectual property is integral to our future success. Currently, we protect our intangible assets through trade secrets, technical know-how and proprietary information (the “Intellectual Property”). We protect our Intellectual Property by seeking and obtaining registered trademark protection where possible, developing and implementing standard operating procedures and entering into non-disclosure agreements with parties that have access to our Intellectual Property to protect our confidentiality and ownership of its Intellectual Property. We also seek to preserve the integrity and confidentiality of our Intellectual Property by maintaining physical security of our premises and physical and electronic security of our information technology systems.
Employees
We have thirteen (13) employees and engaged eight (8) consultants.
Foreign Operations
Our TL Pet division sells a similar range of pet treats in Europe.
Lending
The Company does not lend funds as part of its regular operations.
Bankruptcy and Similar Procedures
There have been no bankruptcy, receivership or similar proceedings against the Company or any of its subsidiaries, or any voluntary bankruptcy, receivership or similar proceedings by the Company or any of its subsidiaries, within the three most recently completed financial years or during or proposed for the current financial year.
Social or Environmental Policies
Construction of the Company’s cannabis production facility in Lumby, BC is in compliance with all applicable environmental requirements.
Risk Factors
General Business Risks
The existence of material uncertainties may cast significant doubt on our ability to continue as a going concern.
Our auditor has issued an opinion on our consolidated financial statements which states that the consolidated financial statements were prepared assuming we will continue as a going concern. Our auditor concurred with management’s assessment that our continued operations are dependent on our ability to generate future cash flows from operations and obtain additional funding through external financing to deliver on its business plan. There is a risk that financing will not be available on a timely basis or on terms acceptable to us.
21
Our success depends in part on our ability to attract and retain senior management and key skilled professionals which we may or may not be able to do. Our failure to do so could prevent us from achieving our goals or becoming profitable.
Our success is dependent on the ability of our directors and officers to develop our business and manage our operations. It is also dependent on our ability to attract and retain key quality assurance, scientific, sales, public relations, and marketing staff. The loss of any key person or the inability to find and retain new key persons could have a material adverse effect on our business. Competition for sales and marketing staff as well as officers and directors - can be intense. While competitive compensation packages are provided as a primary method of retaining the services of key individuals, no assurance can be provided that we will be able to attract or retain key personnel in the future. This may adversely impact our operations.
We will need a significant amount of capital to execute our business plan. Unless we are able to raise sufficient funds, we may be forced to discontinue our operations.
We are in the development stage and will likely operate at a loss until our business becomes established. We will require additional financing in order to fund future operations. Our ability to secure any required financing in order to commence and sustain our operations will depend, in part, upon prevailing capital market conditions, as well as our business success. There can be no assurance that we will be successful in our efforts to secure any additional financing or additional financing on terms satisfactory to our management. If additional financing is raised by issuing common shares, control may change and shareholders may suffer additional dilution. If adequate funds are not available or they are unavailable on acceptable terms, we may be required to scale back our business plan or cease operating.
We have a limited operating history, and accordingly, we are subject to many of the risks of early stage enterprises.
We have earned revenues from TL Pet and TL Europe since they began operations in 2015 and 2016 respectively; however, these two operations have not yet achieved profitability.
TL Medicine was launched in July 2013 to become a licensed producer of medicinal cannabis for the Canadian market under the ACMPR program administered by Health Canada. TL Medicine will be subject to a Health Canada inspection upon completion of the construction of its facility to allow for the cultivation, manufacturing, and distribution of cannabis products. As at March 31, 2018 we have completed the security clearance stage, but we do not have a license to produce cannabis and no products are in commercial production or use. We were not granted an ACMPR license prior to October 17, 2018 and will be required to satisfy additional obligations in order to qualify, including the completion of a compliant medical cannabis cultivation facility at the parcel of land owned by us in Lumby, British Columbia. Moving forward, TL Medicine will be complying with the licensing requirements pursuant to the Cannabis Act and Cannabis Regulations. We continue to work diligently to comply with all of the requirements of Health Canada in order to be successful at receiving a license to sell cannabis for medical purposes under the Cannabis Act. There is no guarantee that we will receive a license to produce cannabis under these new regulations. The Company is exploring alternative business models for TL Medicine in the event that it is unsuccessful in obtaining its license.
We are therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that our future operations will result in profitability. If we cannot generate sufficient revenues to operate profitably, we may suspend or cease our operations. There is no assurance that we will be successful in achieving a return on shareholders’ investment and the likelihood of success must be considered in light of the early stage of operations.
22
We have a history of operating losses.
We have a history of net losses, may incur significant net losses in the future and may not achieve or maintain profitability. We have incurred losses in recent periods. We may not be able to achieve or maintain profitability and may continue to incur significant losses in the future. In addition, we expect to continue to increase operating expenses as we implement initiatives to continue to grow our business and deliver on our business plan. If our revenues do not increase to offset these expected increases in costs and operating expenses, we will not be profitable. There is no assurance that future revenues will be sufficient to generate the funds required to continue operations without external funding.
Risks Related to Our Common Shares
If we issue additional common shares, shareholders may experience further dilution in their ownership of us.
We are authorized to issue an unlimited number of common shares without par value. We have the right to raise additional capital or incur borrowings from third parties to finance our business. Our board of directors has the authority, without the consent of any of our shareholders, to cause us to issue more common shares. Consequently, shareholders may experience more dilution in their ownership of us in the future. Our board of directors and majority shareholders have the power to amend our constating documents in order to affect forward and reverse stock splits, and recapitalizations of the company. The issuance of additional common shares by us would dilute all existing shareholders' ownership in us.
We cannot assure that we will ever pay dividends.
We do not currently anticipate declaring and paying dividends to our shareholders in the near future. It is our current intention to apply net earnings, if any, in the foreseeable future to increase our capital base and marketing. Prospective investors seeking or needing dividend income or liquidity should therefore not purchase our common shares. We cannot assure that we will ever have sufficient earnings to declare and pay dividends to the holders of our common shares, and in any event, a decision to declare and pay dividends is at the sole discretion of our board of directors.
We are controlled by our principal shareholder, Darcy Bomford, whose interests may differ from those of the other shareholders.
Mr. Darcy Bomford is our CEO, founder, principal shareholder and a director of the Company. As of the date of this AIF he owns directly and indirectly a total of 23,713,752 common shares or 24.89% of the total issued and outstanding common shares of our company.
Mr. Bomford, as our principal shareholder, is able to exercise significant control over all matters requiring shareholder approval including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common shares. This concentration of ownership may not be in the best interests of all of our shareholders.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
23
Our articles provide that we will indemnify our directors and officers in each case to the fullest extent permitted by the Business Corporations Act (British Columbia) (the “BCA”). We must indemnify our officers and directors against all reasonable fees, expenses, charges and other costs of any type or nature whatsoever. This includes any and all expenses and obligations paid or incurred in connection with investigating, defending, being a witness in, participating in (including on appeal), or preparing to defend against any completed, actual, pending or threatened action, suit, claim or proceeding, whether civil, criminal, administrative or investigative, or establishing or enforcing a right to indemnification under the indemnification agreement.
Risks Related to the Trading of Our Common Shares
Future sales of our common shares, or the perception that such sales may occur could depress our common share price.
Our notice of articles authorizes us to issue up to an unlimited number of common shares. In the future, we may issue additional common shares or other securities if we need to raise additional capital. The number of new common shares issued in connection with raising additional capital could constitute a material portion of those current outstanding common shares. Any future sales of our common shares, or the perception that such sales may occur, could negatively impact the price of our common shares.
Our common shares are thinly traded and you may be unable to sell at or near asking price, or at all.
We do not have a liquid market for our common shares, and we cannot predict the extent to which an active public market for trading our common shares will be achieved or sustained. We can be thinly traded given we are a small company that is relatively unknown to stock analysts, stockbrokers, institutional investors and others in the investment community who generate or influence sales volume. Even if we came to the attention of such persons, those persons tend to be risk-averse and may be reluctant to follow, purchase, or recommend the purchase of shares of an unproven company such as ours until such time as we become more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common shares will develop or be sustained, or that current trading levels will be sustained.
The market price for our common shares may be volatile, which may result in a decline in value of your investment.
The trading price of our common shares has been and may continue to be volatile. Securities markets worldwide experience significant price and volume fluctuations. This market volatility, as well as general economic, market or political conditions could reduce the market price of our common shares in spite of our operating performance. In addition, our results of operations could fail to meet the expectations of investors due to a number of potential factors, including variations in our quarterly results of operations, additions or departures of key management personnel, failure to meet our projected operational milestones, litigation and government investigations. Other factors which may affect the value of our common shares include: changes or proposed changes in laws, new regulations, or differing interpretations or enforcement of the law, adverse market reaction to any indebtedness we may incur or securities we may issue in the future, changes in market valuations of similar companies or speculation in the press or investment community, announcements by our competitors of significant contracts, acquisitions, dispositions, strategic partnerships, joint ventures or capital commitments, adverse publicity about our industry or individual scandals. All of these events could result in a decrease of the market price of our common shares and as a result, you may be unable to resell your common shares at or above the price you acquired our securities.
24
Risks Relating to Our Pet Business
We are subject to significant risks associated with introducing new products including the risk that our new product developments will not produce sufficient sales to recoup our investment.
Our pet support supplements and chews include ingredients not traditionally found in such products. Our success will depend on our ability to build a following for our products. We cannot assure you that we will be successful in achieving market acceptance of our products. Our failure to successfully market and build a customer base for our products could harm our ability to grow our business and could have a material adverse effect on our business, results of operations and financial condition.
We may not be able to successfully implement our growth strategy on a timely basis or at all.
Our future success depends, in large part, on our ability to implement our growth strategy, including expanding distribution in Canada, United States, and Europe, and generating sales in other key markets such as Asia, South Africa, Australia and New Zealand, attracting new consumers to our brand, introducing new products and product line extensions, and expanding into new markets. Our ability to implement this growth strategy depends, among other things, on our ability to:
- enter into distribution and other strategic arrangements with retailers and other potential distributors of our products;
- expand and maintain brand loyalty;
- effectively compete with specialty pet products;
- secure shelf space in stores;
- increase our brand recognition by effectively implementing our marketing strategy and advertising initiatives;
- develop new products and product line extensions that appeal to consumers;
- maintain sources for the required supply of quality raw ingredients to meet our growing demand; and
- identify and successfully enter and market our products in new geographic markets and market segments.
If we fail to implement our growth strategy or if we invest resources in a growth strategy that ultimately proves unsuccessful, our business, financial condition and results of operations may be materially adversely affected.
We rely on co-packers to provide our supply of pet supplement and treat products. Any failure by co-packers to fulfill their obligations or any termination or renegotiation of our co-packing agreements could adversely affect our results of operations.
We have supply agreements with co-packers that require them to provide us with specific finished products. We rely on co-packers as our sole-source for our products. The failure for any reason of a co-packer to fulfill its obligations under the applicable agreements with us or the termination or renegotiation of any such co-packing agreement could result in disruptions to our supply of finished goods and have an adverse effect on our results of operations. Additionally, from time to time, a co-packer may experience financial difficulties, bankruptcy, or other business disruptions which could disrupt our supply of finished goods. We may also be required to incur additional expenses from the need to provide financial accommodations to the co-packer or taking other steps to minimize or avoid supply disruption, such as establishing a new co-packing arrangement with another provider. During an economic downturn, our co-packers may be more susceptible to experiencing such financial difficulties, bankruptcies or other business disruptions. We mitigate this risk by working with co-packers who have an established track record. In the event we need to hire a new co-packer, the new co-packing arrangement may not be available on terms as favorable to us as the existing co-packing arrangement, if at all.
25
If we do not manage our supply chain effectively, including inventory levels, our business, financial condition and results of operations may be adversely affected.
The inability of any supplier, co-packer, third-party distributor or transportation provider to deliver or perform for us in a timely or cost-effective manner could cause our operating costs to increase and our profit margins to decrease. We must continuously monitor our inventory and product mix against forecasted demand or risk having inadequate supplies to meet consumer demand as well as having too much inventory on hand that may reach its expiration date and become unsalable. If we are unable to manage our supply chain effectively and ensure that our products are available to meet consumer demand, our operating costs could increase, and our profit margins could decrease.
Failure by our transportation providers to deliver our products on time or at all could result in lost sales.
We use third-party transportation providers for our product shipments. We rely on a number of different providers for our shipments based on cost efficiency and availability at the time of shipping. Transportation services include scheduling and coordinating transportation of finished products to our customers, shipment tracking and freight dispatch services. Risks include higher costs as a result of increases in fuel prices, potential employee strikes, inclement weather or other factors which could delay or cancel the transportation of our products. In the future we may not be able to obtain terms as favorable as those we receive from the third-party transportation providers that we currently use which, in turn, would increase our costs and thereby adversely affect our business, financial condition, and results of operations.
We may face difficulties as we expand into countries in which we have no prior operating experience.
We have recently launched sales of our products in the United States, Europe, Asia, South Africa, Australia and New Zealand. We intend to continue to expand in and into countries in which we have no prior operating experience. From time to time, we expect to encounter economic, political, regulatory, personnel, technological, and language barriers and other risks that may increase our expenses or delay our ability to become profitable in such countries. These risks include:
- the risk that we must spend significant amounts of time and money to build brand recognition without certainty that we will be successful;
- currency fluctuations;
- enforcing agreements and collecting receivables through some foreign legal systems;
- potentially longer payment cycles and greater difficulty in collecting accounts;
- changes in local tax laws, and tax rates that may exceed those of the United States or Canada;
- changes in economic conditions, consumer preferences, or demand for our products in these foreign markets;
- the credit risk of local customers and distributors;
- differences in culture and trends in foreign countries with respect to pets and pet care;
- government regulations that would have a direct or indirect adverse impact on our business and market opportunities, including nationalization of private enterprise; and
- our expansion into new countries may require significant resources and the efforts and attention of our management and other personnel, which could divert resources from our existing business operations.
26
As we expand globally, our success will depend on our ability to anticipate and effectively manage these and other risks associated with our foreign operations. We mitigate this risk through the use of local, on-ground employees and contractors.
Competition in the markets in which we operate, including internet-based competition, is strong. If we are unable to compete effectively, our ability to generate sales may suffer and our operating income and net income could decline.
We are one of many companies in the consumable pet products market competing for a significant market share. Our competition in the healthy feeding systems and healthy consumable products markets are both domestic and foreign companies, many of whom manufacture their products in low cost areas such as India, East Asia, Southeast Asia, and Mexico. Many of these companies also have more brand awareness. We are still building our market presence. Any reputation that we may successfully gain with retailers for quality products does not necessarily translate into name recognition or increased market share with the end consumer.
We compete with a significant number of companies of varying sizes, including divisions or subsidiaries of larger companies who may have greater financial resources and larger customer bases than we have. As a result, these competitors may be able to identify and adapt to changes in consumer preferences more rapidly than we can due to their larger resource base and scale. They may also be more successful in marketing and selling their products, better able to increase prices to reflect cost pressures, and more capable in increasing their promotional activity, which may impact us and the entire pet food industry.
We also compete with other smaller niche market companies focused on the same area of the consumable pet food markets we have entered. These companies may be more innovative and/or able to bring products to market faster and move more quickly to exploit and serve niche markets than we are. If these competitive pressures cause our products to lose or unable to gain market share, our business, financial conditions and results of operations may be materially adversely affected.
The loss of any of our key suppliers, or distribution arrangements with key vendors, would negatively impact our business.
We purchase significant amounts of products from vendors with differing supply capabilities. There can be no assurance that the vendors who currently supply us with the ingredients necessary to create our pet products will be able to accommodate the anticipated growth and expansion of our locations and e-commerce. An inability of our existing vendors to provide products in a timely or cost-effective manner may impair our business, financial condition and results of operations.
We maintain no long-term supply contracts with any of our distributors. As a consequence, any distributor may discontinue selling our pet products at any time which would result in the inability to sell our products in certain retail locations. The loss of any of our vendors would, therefore, have a negative impact on our business and financial condition.
If we are unable to identify or enter into supply or distribution relationships with new vendors or to replace the loss of any of our existing vendors, we may experience a competitive disadvantage, our business may be disrupted, and our results of operations may be adversely affected. We are working to expand our online presence to mitigate the risk of any such losses.
We may be exposed to significant product liability claims which our insurance may not cover, and which could harm our reputation.
In the ordinary course of our business, we may be named as a defendant in lawsuits involving product liability claims. In any such proceeding, plaintiffs may seek to recover large and sometimes unspecified amounts of damages and the matters may remain unresolved for several years.
27
Any such matters could have a material adverse effect on our business, results of operations and financial condition if we are unable to successfully defend against or settle these matters or if our insurance coverage is insufficient to satisfy any judgments against us or settlements relating to these matters. Although we have product liability insurance coverage and an excess umbrella policy, our insurance policies may not provide coverage for certain claims against us or may not be sufficient to cover all possible liabilities. Additionally, we do not maintain product recall insurance. We may not be able to maintain such insurance on acceptable terms, if at all, in the future. Moreover, any adverse publicity arising from claims made against us, even if the claims are not successful, could adversely affect the reputation and sales of our products. In particular, product recalls or product liability claims challenging the safety of our products may result in a decline in sales for a particular product and could damage the reputation or the value of the related brand. This could be true even if the claims themselves are ultimately settled for immaterial amounts. This type of adverse publicity could occur, and product liability claims could be made in the future.
We face various risks as an ecommerce retailer, if we do not manage these risks effectively, our ability to generate sales may suffer and our operating income and net income may decline.
Although ecommerce represents a growing segment of the pet industry, ecommerce operations are still in the early stages of development. We may require additional capital in the future to sustain or grow our ecommerce business. Business risks related to our ecommerce business include our ability to keep pace with rapid technological change, failure in our security procedures and operational controls, failure or inadequacy in our systems or ability to process customer orders, government regulation and legal uncertainties with respect to ecommerce, and collection of sales or other taxes by one or more states or foreign jurisdictions. If any of these risks materialize, it could have an adverse effect on our business.
Increased transactions through our website may result in a reduction in sales at store locations that sell our products. There is a risk that vendors who sell our products may decide to discontinue the sale of our products due to a reduction in sales. If vendors decide to discontinue the sale of our products, this could reduce our exposure to new or potential customers, therefore having an adverse effect on our business.
In addition, we face competition from established companies who sell their products online and have a large customer base. A failure to positively differentiate our product and service offerings from other Internet retailers could have a materially adverse effect on our business, results of operations, or financial condition.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our products and services could be harmed significantly.
We rely on trade secrets, know-how and other proprietary information in operating our business. If this information is not adequately protected, then it may be disclosed or used in an unauthorized manner. To the extent that consultants, key employees or other third parties apply technological information independently developed by them or by others to our proposed products, disputes may arise as to the proprietary rights to such information, which may not be resolved in our favor. The risk that other parties may breach confidentiality agreements or that our trade secrets may become known or independently discovered by competitors could harm us by enabling our competitors, who may have greater experience and financial resources, to copy or use our trade secrets and other proprietary information in the advancement of their products, methods or technologies. The disclosure of our trade secrets would impair our competitive position, thereby weakening demand for our products or services and harming our ability to maintain or increase our customer base.
Risks Relating to Our Proposed Cannabis for Medical Purposes Business
We have not commenced operations and are currently seeking to lay the foundation to commence our business.
28
We have not received a cannabis production license from Health Canada and there can be no assurance that we will receive a production license. Until we receive a production license, we cannot begin the production, sale and distribution of cannabis for medical purposes. It is currently not known when or if we will be granted a production license. The key milestones to obtaining a production license include filing an application, receiving a pre-licensing approval notice, completion of the upgrades as per the application, approval to produce upon inspection of the facility, and approval to distribute and sell the product.
We are subject to all of the business risks and uncertainties associated with any new business enterprise, including the risks that we will be unable to acquire the necessary production license, successfully produce the product, or establish a market for our product. There can be no assurance that consumer demand for our product will be as anticipated, or that we will become profitable. As a result, an investment in our common shares involves a high degree of risk and should only be acquired if you can afford to lose your entire investment.
Our proposed cannabis business is subject to significant regulation by the Canadian Federal Government. There is no assurance that we will be granted licensed producer status by Health Canada. Any continued delay or failure in obtaining such status would materially and adversely affect our operations.
The success of our subsidiary, TL Medicine, depends heavily on acquiring a production license from Health Canada so that it can grow, store and distribute cannabis for medical purposes in Canada. There is no assurance that we will be approved by Health Canada or will be granted licensed producer status. Should we be unable to obtain all required licenses, or if the regulations in Canada continue to change, our proposed cannabis production business would not be able to operate or there could be a significant cost to change our operations to remain compliant with the laws and regulations.
Once a production license is obtained, any failure to comply with the terms of the production license, or any failure to renew the production license after its expiry date would have a materially adverse impact on the financial condition and operations of our business.
Our operations are subject to regulations promulgated by government regulatory agencies from time to time. The cost of compliance with changes in governmental regulations has the potential to reduce the profitability of operations. However, there can be no guarantee that we will be able to obtain and maintain, at all times, all necessary licenses and permits required to carry out our business.
There are many regulations and governmental agencies that regulate the cannabis for medical purposes industry and there will likely be increased and/or changing regulations as the industry becomes more mainstream with more participants.
Our proposed cannabis production operations are subject to a variety of laws, regulations and guidelines relating to the manufacture, management, transportation, storage and disposal of cannabis for medical purposes but also including laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. While, to the knowledge of management, we are currently in compliance with all such laws, changes to such laws, regulations and guidelines due to matters beyond our control may cause adverse effects to our operations.
There are sales risks associated with the cannabis and cannabis for medical purposes industries because cannabis is subject to regulation under the Cannabis Act.
The Cannabis Act provides for prohibitions regarding the promotion of cannabis products. Subject to a few exceptions, all promotion of cannabis products is prohibited unless authorized by the Cannabis Act. The prohibitions apply to anyone who may be involved in promotion cannabis accessories and services
29
related to cannabis, including: (1) persons who produce, sell or distribute cannabis; (2) persons who sell or distribute cannabis accessories; (3) persons who provide cannabis-related services; or (4) media organizations. Limited promotion of cannabis, cannabis accessories and cannabis-related services is permitted under the Cannabis Act in specific circumstances including (1) informational promotion or brand-preference promotion; (2) point of sale; and (3) brand elements on things that are not cannabis or a cannabis accessory subject to the restrictions as described in greater detail in the section “Legal Developments- Advertising”. If we are unable to properly conduct sales in a regulated environment or target the appropriate audiences for our cannabis for medical purposes, our results of operations and business prospects could be substantially impaired.
We may not be able to use the facilities as planned and will, therefore, not be able to commence operations on the timetable or the scale that we have planned.
To date, our proposed cannabis production activities and resources have been primarily focused on our proposed facility in Lumby, BC and we will continue to be focused on this facility for the foreseeable future. Adverse changes or developments affecting the facility, including but not limited to a breach of security, could have a material and adverse effect on our business, financial condition and prospects. Any breach of the security measures and other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by Health Canada, could also have an impact on our ability to continue to operate under any license we may receive.
We may not acquire market share or achieve profits due to competition in the medical cannabis for medical purposes industry.
We will face intense competition from other companies, some of which can be expected to have longer operating histories, more financial resources, and greater manufacturing and marketing experience than us. Increased competition by larger and better-financed competitors could materially and adversely affect our business, financial condition, and results of operations.
Because of the early stage of the industry in which we plan to operate, we expect to face additional competition from new entrants. If the number of users of cannabis for medical purposes in Canada increases, the demand for products will increase, and we expect that competition will become more intense as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, we will require a continued high level of investment in research and development, marketing, sales, and client support. We may not have sufficient resources to maintain research and development, marketing, sales, and client support efforts on a competitive basis which could materially and adversely affect our proposed cannabis production business, financial condition and results of operations.
Legal Proceedings and Regulatory Actions
Legal Proceedings
To the best of our knowledge, there have been no legal proceedings the Company is or was a party to, or that any of our property is or was the subject of, during the Company's financial year, or any such legal proceedings your company knows to be contemplated.
Regulatory Actions
To the best of our knowledge, there have been no penalties or sanctions imposed against the Company by a court relating to securities legislation or by a securities regulatory authority during the financial year, any other penalties or sanctions imposed by a court or regulatory body against the Company that would likely be considered important to a reasonable investor in making an investment decision, or any settlement agreements the Company entered into before a court relating to securities legislation or with a securities regulatory authority during the financial year.
30
This Management's Discussion & Analysis (this “MD&A”) has been prepared by management and should be read in conjunction with the annual consolidated financial statements of the Company together with the related notes thereto for the year ended March 31, 2018. The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and interpretations of the International Financial Reporting Interpretations Committee (“IFRIC”). All amounts are stated in Canadian dollars unless otherwise indicated.
Forward Looking Statements
This MD&A contains certain statements related to industry scope and state, production, revenue, expenses, plans, development schedules and similar items that represent forward-looking statements. Such statements are based on assumptions and estimates related to future economic and market conditions. Such statements include declarations regarding management's intent, belief or current expectations. Certain statements contained herein may contain words such as “could”, “should”, “expect”, “believe”, “will” and similar expressions and statements relating to matters that are not historical facts but are forward-looking statements. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve a number of risks and uncertainties; actual results may differ materially from those indicated by such forward-looking statements. Some of the important factors, but certainly not all, that could cause actual results to differ materially from those indicated by such forward-looking statements are: (i) that the information is of a preliminary nature and may be subject to further adjustment, (ii) the possible unavailability of financing, (iii) start-up risks, (iv) general operating risks, (v) dependence on third parties, (vi) changes in government regulation, (vii) the effects of competition, (viii) dependence on senior management, (ix) impact of Canadian economic conditions, and (x) fluctuations in currency exchange rates and interest rates.
Management's Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of the annual consolidated financial statements of the Company together with the related notes thereto for the year ended March 31, 2018 in accordance with International Financial Reporting Standards, and for such internal control as management determines is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error.
Results of Operations
Selected Annual Financial Information
| Year Ended March 31 |
Description | | 2018 | | 2017 | | 2016 |
Revenues | $ | 1,400,511 | $ | 368,536 | $ | 37,330 |
Cost of Sales | | (779,192) | | (248,909) | | (26,117) |
Gross Profit ($) | $ | 621,329 | $ | 119,627 | $ | 11,213 |
Gross Profit (%) | | 44% | | 32% | | 30% |
Total Operating Expenditures | | (4,809,855) | | (1,857,834) | | (1,034,170) |
Loss and comprehensive loss for the year | $ | (3,967,936) | $ | (1,743,050) | $ | (1,039,320) |
Basis and diluted loss per share | $ | (0.05) | $ | (0.03) | $ | (0.03) |
|
31
Revenue for the year ended March 31, 2018 increased 280% from $368,536 for the same period in the previous fiscal year. All of the Company's revenues from inception to date are from the sale of its hemp-based nutrition for pets, mostly in North America and Europe. Revenue growth was primarily fueled by True Leaf expanding the commercial reach of its True Leaf Pet division into new geographies both in-store and online. True Hemp™ dog chews, dental sticks and supplement oils are now sold in more than 1,800 stores worldwide, including PetSmart Canada and Pets Corner UK, and online on Amazon.
Revenue is recorded net of customer discounts, promotional allowances and includes freight collected in connection with online sales. Cost of sales may include different costs compared to other manufacturing and distribution companies. Our cost of sales includes inventory, product related costs and costs to ship products to customers. The growth in fiscal 2018 revenue created efficiencies, in particular in our shipping costs, contributing to a 38% improvement in gross profit to 44% for the year ended March 31, 2018 from 32% for fiscal 2017.
Operating expenditures for the year ended March 31, 2018 increased $2,952,021 compared to the same period in the previous fiscal year. The increase in expenditures is consistent with the Company's business plan of building worldwide market share and revenue for TL Pet products, development of the TL Medicine business, and costs incurred in connection with the successful raise of $14 million through a public offering.
Selling and marketing expenses include salaries, commissions, promotional activities and other costs in connection with the sale of pet products as well as to raise awareness of the True Leaf brand to consumers and investors. The Company invested $658,834 in selling and marketing expenses for the year ended March 31, 2018, an increase of $231,014 over fiscal 2017. The Company hired dedicated sales staff to grow revenue in North America, enhanced our European sales team to increase our European reach and appointed a chief marketing officer and branding partner.
Administrative and office expenses of $1,564,926 for the year ended March 31, 2018 increased $801,428 over fiscal 2017. Public company costs of $220,257 such as regulatory filing fees, transfer agent fees and insurance increased $185,026 compared to the year ended March 31, 2017 due to the high volume of share capital activity and expanded investor base created in fiscal year 2018. Additional employees and contractors were hired during the year ended March 31, 2018 consistent with the Company's focused effort to assemble a world-class leadership team who will deliver on the Company's growth plans, execute on the design and build of the cultivation and production facility and lead the development of its medicinal cannabis products. Salaries, payroll expenses and consultant fees accounted for $896,362 of total administrative and office expense, an increase of $390,599 over the year ended March 31, 2017. The Company retained Hill+Knowlton, Canada's top-rated government relations firm, to provide insight into the cannabis regulatory environment as we develop our medicinal cannabis product line and bring it to market.
Operating expenditures include an inventory write-down of $217,436 (2017 - $36,000), primarily associated with supplies and packaging materials that will not be used for current product lines. We also wrote off a small amount of finished goods; a portion which used our old product formulation and some private label products. Led by the Chief Marketing Officer, the Company is defining a strategy and rebrand for the Orega Pet product line, acquired by the Company in December 2016. The rebrand will align these products with the Company's objective of creating a plant-focused wellness brand for people and their pets.
32
Research and development costs of $57,808 for the year ended March 31, 2018 include costs to develop the Company's hemp-based cat treats which were introduced at a trade show in Germany subsequent to March 31, 2018.
Share-based compensation costs of $1,836,441 (March 31, 2017 - $265,917) were the largest component of the increase in operating expenditures and represent the fair value of stock options granted to employees and others. The fair value is determined using the Black-Scholes option pricing model.
Investors who participated in the Company's Regulation A public offering in January 2018 funded their investment in US dollars, although the offering was priced in Canadian dollars, providing the Company with US proceeds. As a result, the Company recorded a foreign exchange gain of $202,425 for the year ended March 31, 2018 arising from a weak Canadian dollar translating the Company's US dollar bank account, as well as translation of the financial results of its European subsidiary.
In January 2018, the TL Medicine team formed a Medical Advisory Board (“MAB”), consisting of independent medical experts and Dr. Chris Spooner, the Company's Chief Medical Officer. The MAB will provide strategic direction to the Company in designing the Company's medical cannabis product offerings.
The Company incurred a net and comprehensive loss of $1,251,577 for the three-month period ended March 31, 2018 (2017 - $376,349). The main components of the loss for the three-month period ended March 31, 2018 were selling, marketing and administrative costs of $425,878, an inventory write-down of $217,436 and share-based compensation of $967,081 in connection with options granted during the period.
Additional employees, contractors and advisors were hired during the three-months ended March 31, 2018 consistent with the Company's focused effort to assemble a world-class leadership team who will deliver on the Company's growth plans, execute on the design and build of the cultivation and production facility.
Quarterly Results
The following table presents selected financial information for the most eight quarters from March 31, 2018:
| Three Months Ended |
| March 31, 2018 | December 31, 2017 | September 30, 2017 | June 30, 2017 |
| | | | |
Revenues | $ 383,844 | $ 265,555 | $ 461,923 | $ 289,189 |
Total Operating Expenditures | (1,655,130) | (764,569) | (743,946) | (1,646,210) |
Net loss and comprehensive loss for period | (1,251,577) | (670,705) | (530,919) | (1,514,735) |
Basic and diluted loss per share | (0.01) | (0.04) | (0.01) | (0.03) |
| | | | |
| Three Months Ended |
| March 31, 2017 | December 31, 2016 | September 30, 2016 | June 30, 2016 |
| | | | |
Revenues | $ 99,331 | $ 143,135 | $ 8,9652 | $ 36,418 |
Total Operating Expenditures | (390,901) | (684,868) | (420,269) | (361,796) |
Net loss and comprehensive loss for period | (376,349) | (623,661) | (387,771) | (355,260) |
Basic and diluted loss per share | (0.01) | (0.01) | (0.01) | (0.01) |
| | | | |
Three-months ended March 31, 2018 and three-months ended March 31, 2017
Revenue for the three-months ended March 31, 2018 increased 286% to $383,844, compared to $99,331 for the same period in the prior year. During the three-months ended March 31, 2018, the Company hired additional experienced sales personnel in order to provide aggressive sales and marketing support and grow revenue for its North American and European markets. This focus resulted in a 45% increase in revenue for the three-months ended March 31, 2018 compared to revenue for the three-months ended December 31, 2017, from $265,555 to $383,844. Revenues have been increasing steadily since TL Pet began operations in early 2016 with a relatively small sales team.
During the three-months ended March 31, 2018 the Company's strategic focus continued to be on positioning the Company for growth. Additional expertise was added to the TL Medicine team to assist the Company in obtaining its license to produce medicinal cannabis from Health Canada.
33
In January 2018, the TL Medicine team formed a Medical Advisory Board ("MAB"), consisting of independent medical experts and Dr. Chris Spooner, the Company's Chief Medical Officer. The MAB will provide strategic direction to the Company in designing the Company's medical cannabis product offerings.
The Company incurred a net and comprehensive loss of $1,251,577 for the three-month period ended March 31, 2018 (2017 - $376,349). The main components of the loss for the three-month period ended March 31, 2018 were selling, marketing and administrative costs of $425,878, an inventory write-down of $217,436 and share-based compensation of $967,081 in connection with options granted during the period.
Additional employees, contractors and advisors were hired during the three-months ended March 31, 2018 consistent with the Company's focused effort to assemble a world-class leadership team who will deliver on the Company's growth plans, execute on the design and build of the cultivation and production facility and lead the development of its medicinal cannabis products. Salaries, payroll expenses and contractor fees accounted for $325,759 of administrative expenses for the three-month period ended March 31, 2018 (2017 - $108,640) as the number of employees and contractors increased from nine at March 2017 to 21 at March 31, 2018. The balance of administrative expenses of approximately $184,000 relate to public company costs such as regulatory filing fees, transfer agent fees and insurance for the three-months ended March 31, 2018 (2017 - $23,300). These higher costs are due to the expanded investor base created in fiscal year 2018, leading to a higher volume of share capital activity, predominantly during the three-months ended March 31, 2018.
Liquidity and Capital Resources
Working capital
Our financial position and liquidity were strong at March 31, 2018. Proceeds from the issuance of share capital through two private placements, the Regulation A crowdfunding and Canadian side car, as well as various warrant and stock option exercises during the year ended March 31, 2018 increased the ending cash position to $10,812,815 (March 31, 2017 - $159,575).
As at March 31, 2018, the Company had working capital of $10,868,317. Receivables of $385,671 (2017 - $66,179) include trade receivables of $202,683 (2017- $62,098). As at March 31, 2018, 29% of the Company's trade receivable balance was concentrated with its three largest customers, based on revenue for the year ended March 31, 2018. Trade receivables are non-interest bearing and due within 30 days. As at March 31, 2018 the Company did not have any significant trade receivables that were past due and consequently, the Company's allowance for doubtful accounts was $nil.
Inventory balances as at March 31 were as follows:
Description | | | | March 31,
2018 | | March 31,
2017 |
Finished goods | | | $ | 432,729 | $ | 92,207 |
Supplies | | | | 137,865 | | 300,701 |
| | | $ | 570,594 | $ | 392,908 |
|
Product inventory increased to $570,594 at March 31, 2018 (2017 - $392,908) to service immediate sales to US, Canadian and European customers. During the year ended March 31, 2018, the Company wrote off $217,436 (2017 - $36,000) associated with supplies and packaging materials, primarily associated with the Orega Pet product line. We also wrote off a small amount of finished goods; a portion which used our old product formulation and some private label product.
34
Led by the Chief Marketing Officer, the Company is defining a strategy and rebrand for the Orega Pet product line to align these products with the Company's objective of creating a plant-focused wellness brand for people and their pets.
As at March 31, 2018, prepaid expenses and deposits includes a deposit of $25,827, refundable upon completion of the construction project, subject to approval by the Village of Lumby that the Company has complied with conditions set out in its landscaping permit.
As at March 31, 2018, the Company has an accrued liability of $98,661 as holdbacks against construction in progress. Construction is on schedule for completion in Fall 2018 and payment of the holdback is expected to occur by March 31, 2019.
Investing activities
During the year ended March 31, 2018 the Company acquired a property of 40 acres located in Lumby B.C. for total consideration of $3,380,387 to build its cannabis cultivation facility. As at March 31, 2018, construction costs incurred of $726,955 are capitalized and depreciation will commence when the facility is put into use. The total budget for the cannabis cultivation facility is estimated at $6.5 million. The Company has retained Colliers Project Leaders to provide professional project management services and assist management in keeping the project on time and on budget.
The first phase of the project includes a two-story 9,000 square foot central hub for the initial grow area, laboratory services, whole-plant extraction, and the production of therapeutic cannabis products, plus a 16,000 square foot wing for cannabis cultivation. Ownership of the 40-acre site means the company is well-positioned to expand to meet future market demands. The Company's capital assets consist of office furniture and equipment, leasehold improvements and the corporate website and had a net book value of $132,420 at March 31, 2018. Capital asset additions for the year ended March 31, 2018 totaled $138,474 for the purchase of office furniture and equipment and leasehold improvements to the Company's Vernon, BC head office to accommodate growth in the Company's management and staff, consistent with the growth activities of the Company. The Company's intangible assets consist of its websites, trademarks and related costs, and intellectual property which had a net book value of $142,690 at March 31, 2018. Intangible asset additions for the year ended March 31, 2018 totaled $37,124 for the protection of trademarks used in the TL Pet business.
Financing activities
The Company's operations during the year ended March 31, 2018 and execution on its business plan, were funded primarily through the issuance of share capital providing gross proceeds of $18,464,265 as described below.
Date of Issuance | Type of Security | Number of Securities | Price per Security | Aggregate Proceeds | Nature of Issuance |
April 24, 2017 | Common Shares | 328,570 | $0.185 | $60,784 | Shares issued pursuant to an exercise of stock options |
April 25, 2017 | Common Shares | 328,570 | $0.185 | $60,784 | Shares issued pursuant to an exercise of stock options |
May 5, 2017 | Common Shares | 328,570 | $0.185 | $60,784 | Shares issued pursuant to an exercise of stock options |
May 29, 2017 | Units | 3,099,829 | $0.30 | $929,950 | Units issued pursuant to a private placement. Each Unit consists of one common share and one warrant exercisable into one common share at a price of $0.45 for a period of 24 months. |
May 29, 2017 | Stock Options | 3,900,000 | $0.395 | Not Applicable | Stock options granted to various directors, officers, consultants and employees of the Company |
May 30, 2017 | Common Shares | 74,479 | $0.185 | $8,937 | Shares issued pursuant to an exercise of stock options |
35
Date of Issuance | Type of Security | Number of Securities | Price per Security | Aggregate Proceeds | Nature of Issuance |
June 13, 2017 | Units | 4,641,816 | $0.30 | $1,392,545 | Units issued pursuant to a private placement. Each Unit consists of one common share and one warrant exercisable into one common share at a price of $0.45 for a period of 24 months. |
June 29, 2017 | Common Shares | 328,570 | $0.185 | $60,784 | Shares issued pursuant to an exercise of stock options |
July 12, 2017 | Common Shares | 100,000 | $0.185 | $18,500 | Shares issued pursuant to an exercise of stock options |
July 18, 2017 | Stock Options | 300,000 | $0.445 | Not Applicable | Stock options granted to an employee of the Company |
July 31, 2017 | Common Shares | 187,500 | $0.15 | $28,125 | Shares issued pursuant to an exercise of warrants |
August 11, 2017 | Common Shares | 187,500 | $0.15 | $28,125 | Shares issued pursuant to an exercise of warrants |
October 11, 2017 | Common Shares | 125,000 | $0.45 | $56,250 | Shares issued pursuant to an exercise of warrants |
October 11, 2017 | Common Shares | 100,000 | $0.185 | $18,500 | Shares issued pursuant to an exercise of stock options |
November 1, 2017 | Common Shares | 239,500 | $0.45 | $107,775 | Shares issued pursuant to an exercise of warrants |
November 1, 2017 | Common Shares | 20,000 | $0.45 | $9,000 | Shares issued pursuant to an exercise of warrants |
November 9, 2017 | Common Shares | 35,000 | $0.45 | $15,750 | Shares issued pursuant to an exercise of warrants |
November 9, 2017 | Common Shares | 7,749 | $0.45 | $3,487 | Shares issued pursuant to an exercise of warrants |
November 20, 2017 | Common Shares | 1,100 | $0.15 | $165 | Shares issued pursuant to an exercise of warrants |
November 20, 2017 | Common Shares | 95,000 | $0.45 | $42,750 | Shares issued pursuant to an exercise of warrants |
November 29, 2017 | Common Shares | 107,166 | $0.45 | $48,224.70 | Shares issued pursuant to an exercise of warrants |
December 5, 2017 | Common Shares | 25,000 | $0.45 | $11,250 | Shares issued pursuant to an exercise of warrants |
December 5, 2017 | Common Shares | 20,000 | $0.45 | $9,000 | Shares issued pursuant to an exercise of warrants |
December 5, 2017 | Common Shares | 10,500 | $0.45 | $4,725 | Shares issued pursuant to an exercise of warrants |
December 5, 2017 | Common Shares | 40,000 | $0.45 | $18,000 | Shares issued pursuant to an exercise of warrants |
December 21, 2017 | Common Shares | 7,000 | $0.45 | $3,150 | Shares issued pursuant to an exercise of warrants |
December 21, 2017 | Common Shares | 120,000 | $0.395 | $47,400 | Shares issued pursuant to an exercise of stock options |
36
Date of Issuance | Type of Security | Number of Securities | Price per Security | Aggregate Proceeds | Nature of Issuance |
December 21, 2017 | Common Shares | 10,000 | $0.45 | $4,500 | Shares issued pursuant to an exercise of warrants |
December 21, 2017 | Common Shares | 10,000 | $0.45 | $4,500 | Shares issued pursuant to an exercise of warrants |
December 21, 2017 | Common Shares | 500,000 | $0.15 | $75,000 | Shares issued pursuant to an exercise of warrants |
January 4, 2018 | Common Shares | 8,833,590 | $0.70 | $6,183,513 | Shares issued pursuant to the first tranche closing of the Regulation A+ Offering |
January 8, 2018 | Common Shares | 36,300 | $0.45 | $16,335 | Shares issued pursuant to an exercise of warrants |
January 8, 2018 | Common Shares | 73,500 | $0.45 | $33,075 | Shares issued pursuant to an exercise of warrants |
January 8, 2018 | Common Shares | 10,000 | $0.45 | $4,500 | Shares issued pursuant to an exercise of warrants |
January 8, 2018 | Common Shares | 35,000 | $0.45 | $15,750 | Shares issued pursuant to an exercise of warrants |
January 8, 2018 | Common Shares | 328,570 | $0.185 | $60,785 | Shares issued pursuant to an exercise of stock options |
January 12, 2018 | Common Shares | 30,000 | $0.45 | $13,500 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 20,000 | $0.45 | $9,000 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 30,000 | $0.45 | $13,500 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 100,000 | $0.45 | $45,000 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 100,000 | $0.45 | $45,000 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 50,000 | $0.45 | $22,500 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 15,000 | $0.45 | $6,750 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 40,000 | $0.45 | $18,000 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 24,000 | $0.45 | $10,800 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 63,700 | $0.45 | $28,665 | Shares issued pursuant to an exercise of warrants |
January 12, 2018 | Common Shares | 40,000 | $0.45 | $18,000 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 31,660 | $0.45 | $14,247 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 80,000 | $0.45 | $36,000 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 10,000 | $0.45 | $4,500 | Shares issued pursuant to an exercise of warrants |
37
Date of Issuance | Type of Security | Number of Securities | Price per Security | Aggregate Proceeds | Nature of Issuance |
January 18, 2018 | Common Shares | 50,000 | $0.45 | $22,500 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 200,000 | $0.45 | $90,000 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 228,571 | $0.15 | $34,285 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 200,000 | $0.45 | $90,000 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 33,333 | $0.45 | $15,000 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 30,000 | $0.45 | $13,500 | Shares issued pursuant to an exercise of warrants |
January 18, 2018 | Common Shares | 20,000 | $0.45 | $9,000 | Shares issued pursuant to an exercise of warrants |
January 22, 2018 | Common Shares | 5,452,125 | $0.45 | $3,816,487.50 | Shares issued pursuant to an exercise of warrants |
January 24, 2018 | Common Shares | 5,864,636 | $0.70 | $4,105,245.20 | Shares issued pursuant to an exercise of warrants |
January 25, 2018 | Common Shares | 15,000 | $0.45 | $6,750 | Shares issued pursuant to an exercise of warrants |
January 25, 2018 | Common Shares | 100,000 | $0.45 | $45,000 | Shares issued pursuant to an exercise of warrants |
January 25 2018 | Common Shares | 25,000 | $0.45 | $11,250 | Shares issued pursuant to an exercise of warrants |
January 25, 2018 | Common Shares | 100,000 | $0.395 | $39,500 | Shares issued pursuant to an exercise of warrants |
February 1, 2018 | Common Shares | 397,921 | Various
$0.15,
$0.185, and
$0.45 | $75,538 | Shares issued pursuant to an exercise of warrants |
February 6, 2018 | Common Shares | 2,000,000 | $0.94 | Not
Applicable | Stock options granted to various directors, consultants and advisory board members of the Company |
February 7, 2018 | Common Shares | 40,000 | $0.45 | $18,000 | Shares issued pursuant to an exercise of warrants |
February 14, 2018 | Common Shares | 50,000 | $0.185 | $9,250 | Shares issued pursuant to an exercise of warrants |
February 16, 2018 | Common Shares | 175,251 | $0.12 | $21.030.12 | Shares issued pursuant to an exercise of warrants |
February 19, 2018 | Common Shares | 50,000 | $0.395 | $19,750 | Shares issued pursuant to an exercise of warrants |
March 7, 2018 | Common Shares | 100,000 | $0.395 | $39,500 | Shares issued pursuant to an exercise of warrants |
38
Note:
a) Shares issued at a deemed price of $0.60 per share for an aggregate total of $60,000.
On January 22, 2018 the Company completed a Regulation A+ crowdfunding campaign approved by the United States Securities and Exchange Commission (the “SEC”) raising $10,000,000 in gross proceeds, consisting of 14,285,715 common shares of the Company at a purchase price of $0.70 per share (the “Offering”). True Leaf was the first Canadian-listed company to conduct a successful Regulation A+ Offering. The use of Regulation A+ allowed the Company to offer and sell its common shares to public retail investors as well as traditional accredited and institutional investors. The Company engaged Boustead Securities, LLC Member: FINRA/SIPC (“Boustead”), as lead underwriter in connection with the Regulation A+ crowdfunding campaign, which commenced November 2017 and closed for non-Canadian investors on January 22, 2018.
The Company paid Boustead $800,000 representing 8% of the gross proceeds of the aggregate Offering amount and issued 857,103 agent's warrants representing 6% of the aggregate number of the securities sold in the offering. Each agent warrant is exercisable into one common share of the Company at an exercise price of $1.05, expiring November 21, 2020. The Company also paid Boustead an advisory fee of US$25,000 and offering expenses. The warrants were valued at $789,767 ($0.92 per warrant) using the Black-Scholes option pricing model.
On the same terms as the Offering, the Company closed a concurrent Canadian private placement on January 24, 2018 of 5,788,078 common shares raising an aggregate total of $4,051,655. The Canadian offering was non-brokered and no commissions or fees were paid in connection with the shares issued. An additional 76,658 common shares, valued at $53,661, were issued in connection with the Canadian offering to compensate certain Canadian investors for foreign currency transaction costs incurred as a consequence of the Company collecting a portion of subscription receipts in U.S. dollar funds for a Canadian dollar denominated share offering.
Based on our strong cash position in the fourth quarter, we repaid early the outstanding obligation of $85,117 under a promissory note in March 2018.
Subsequent Sales
Subsequent to the year ended March 31, 2018 and up to December 7, 2018 the Company has issued the following securities
Date of Issuance | Type of Security | Number of Securities | Price per Security | Aggregate Proceeds | Nature of Issuance |
October 11, 2018 | Common Shares | 60,000 | $0.45 | $27,000 | Shares issued pursuant to various exercises of warrants |
October 17, 2018 | Common Shares | 100,000 | $0.60 | $60,000(1) | Shares issued pursuant to the terms of the CFO Employment Agreement entered into between the Company and Kerry Biggs |
| | | | | |
Going Concern
The consolidated financial statements were prepared on a going concern basis, which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business.
On January 24, 2018 the Company closed two financings which raised gross proceeds of $14,051,655 in two offerings and put the Company in a strong cash position at March 31, 2018. The proceeds of the financing are being used to execute on the Company's business plan, including construction of its cannabis cultivation facility.
39
Management estimates the total cost of this project will be approximately $6.5 million, of which $726,955 has been incurred and capitalized as at March 31, 2018. For the year ended March 31, 2018, the Company incurred a loss of $3,967,936 and had an accumulated deficit of $8,961,872. The Company earned revenues of $1,400,511 (2017 - $368,536) from TL Pet and TL Pet Europe; although, these two operations have not yet achieved profitability.
Management is of the opinion the continued operations of the Company are dependent on its ability to generate future cash flows from operations and obtain additional funding through external financing to deliver on its business plan. There is a risk that financing will not be available on a timely basis or on terms acceptable to the Company.
The consolidated financial statements do not give effect to any adjustments which would be necessary should the Company be unable to continue operations. Management has assessed that it has sufficient working capital for the Company to continue operations for the next fiscal year.
Related Party Transactions
Key management compensation
The Company's key management personnel have the authority and responsibility for planning, directing and controlling the activities of the Company and consists of the Company's Directors, Chief Executive Officer and Chief Financial Officer. The total paid as salaries, management fees, accounting fees and share-based compensation for the year ended March 31, 2018 was $833,156. The Company paid:
- Mr. Bomford, the CEO and a director of the Company, $70,000 in cash compensation and $151,749 in share-based compensation in the form of stock options;
- Mr. Austin, the CFO of the Company, $24,000 in cash compensation and $40,688 in share-based compensation in the form of stock options;
- Mr. Spooner, the Chief Medical Officer and a director of the Company, $23,500 in cash compensation and $151,749 in share-based compensation in the form of stock options;
- Mr. Bottomley, the Vice President and a director of the Company, $65,500 in cash compensation and $151,749 in share-based compensation in the form of stock options;
Related Party Transaction
The Company had the following transactions with related parties during the year ended March 31, 2018:
a) Goods and services
- Paid or accrued a total of $30,000 (2017 - $30,000) to its Chief Executive Officer for rent;
- Paid or accrued $92,266 (2017 - $14,271) to First Pacific Enterprises, a company controlled by its Chief Executive Officer, for costs associated with supplies inventory; and
- Paid or accrued $21,000 (2017- $nil) to Paradigm Medical Services, a company controlled by Chris Spooner, a Director, for medical advisory services provided.
b) Included in accounts receivable at March 31, 2018 is an amount due from director, Chris Spooner, of $119,770.
c) The following amounts were due to related parties as at March 31, 2018 and are unsecured, non-interest bearing with no scheduled terms of repayment:
- First Pacific Enterprises $16,531 (2017 - $23,771)
- Paradigm Medical Services $6,000 (2017 - $nil)
40
d) Share-based compensation
- On February 6, 2018 the Company granted a total of 1,900,000 stock options, 800,000 of which were to directors and officers of the Company, having a fair value of $549,707.
- On May 29, 2017 the Company granted a total of 3,900,000 stock options, 1,400,000 of which were to directors and officers of the Company, having a fair value of $284,675.
- On December 12, 2016, the Company granted a total of 2,799,995 stock options, 1,642,875 of which were to directors and officers of the Company having a fair value of $156,025.
Share Capital
The Company's authorized share capital consists of an unlimited number of common shares without par value and an unlimited number of preferred non-voting shares without par value. As of March 31, 2018, the total number of issued and outstanding common shares was 95,369,059 (95,529,059 - November 30, 2018) and there were no preferred shares outstanding.
Warrants
As at November 30, 2018 the following warrants are outstanding and exercisable:
| Number | Exercise | | | |
| of Warrants | Price ($) | | Expiry Date | |
Warrants | 2,037,954 | 0.45 | | May 29, 2019 | |
Warrants | 3,342,283 | 0.45 | | June 12, 2019 | |
Warrants | 857,143 | 1.05 | | November 21, 2020 | |
| 6,237,380 | | | | |
| | | | | |
Stock Options
As at November 30, 2018 the following stock options are outstanding and exercisable:
| Number of Options | | |
Outstanding | Exercisable | Exercise Price ($) | Expiry Date |
857,145(1) | 857,145 | 0.19 | December 12, 2018 |
2,750,000(2) | 2,750,000 | 0.40 | May 29, 2019 |
300,000 | 300,000 | 0.45 | July 18, 2019 |
100,000 | 100,000 | 0.94 | February 6, 2019 |
1,800,000 | 1,200,000 | 0.94 | February 6, 2019 |
| 935,000 | 450,000 | 0.50 | July 31, 2023 |
1,050,000 | - | 0.56 | September 10, 2023 |
| | | | |
7,792,145 | 5,657,145 | | |
| | | |
Note: (1) All of these stock options were exercised on December 12, 2018.
(2) 300,000 stock options expired on December 12, 2018. 90 days after the resignation of an officer of the Company.
Financial Instruments and Risk
Fair Value
Financial instruments recorded at fair value on the consolidated statement of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements.
41
Financial Instruments and Risk
Fair Value
Financial instruments recorded at fair value on the consolidated statement of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements.
The fair value hierarchy has the following levels:
Level 1 - Unadjusted quoted prices in active markets for identical assets or liabilities;
Level 2 - Inputs other than quoted prices that are observable for assets or liabilities, either directly or indirectly; and
Level 3 - Inputs for assets and liabilities that are not based on observable market data.
The fair value hierarchy requires the use of observable market inputs whenever such inputs exist. A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value. The carrying value of accounts payable, accrued liabilities and due to related parties approximates their fair value because of the short-term nature of these instruments.
The fair values of cash and marketable securities are measured based on Level 1 inputs of the fair value hierarchy.
Risk
The Company is exposed to various risks through its financial instruments and has a risk management framework to monitor, evaluate and manage these risks. The following analysis provides information about the Company's risk exposure and concentration as of March 31, 2018:
Credit Risk
Credit risk refers to the risk that another entity will default on its contractual obligations which will result in a loss for the Company. At March 31, 2018, the Company's exposure to credit risk consists of the carrying value of cash and accounts receivable. The Company limits its credit exposure on cash by holding its deposits with established financial institutions. Accounts receivable consists of trade accounts receivable and other receivables. The Company mitigates the risk of default of accounts receivable by assessing the credit worthiness of customers prior to sale and shipment of inventory and by putting a security agreement in place for the related party receivable at March 31, 2018.
Liquidity Risk
Liquidity risk arises from our general and capital financing needs with respect to future growth and the Company's construction of its cannabis cultivation facility which has an estimated budget of $6.5 million. Liquidity risk could arise if the Company encounters difficulty in meeting future obligations with financial liabilities. As at March 31, 2018, the Company had cash and cash equivalents of $10,812,815 to settle current liabilities of $1,049,962. The Company has planning, budgeting and forecasting processes to help determine its funding requirements to meet various contractual and other obligations and to manage liquidity risk. Additionally, the Company has retained Colliers Project Leaders to provide professional project management services and assist management to keep the project on time and on budget.
Currency Risk
The operating results and financial position of the Company are reported in Canadian dollars. The Company is exposed to currency risk arising from the translation of its European subsidiary's operations. The Company is exposed to currency transaction risk as some of the Company's financial instruments are denominated in U.S. dollars. The results of the Company's operations are subject to currency translation and transaction risks.
The Company's main risk is associated with fluctuations in Canadian dollars and euros. Assets and liabilities are translated based on the Company's foreign currency translation policy.
42
The Company has determined that, with other variables unchanged, the effect of a 10% increase in the Canadian dollar as at March 31, 2018:
- against the euro on its net European operations, and
- against the U.S. dollar on financial assets and liabilities, including cash, accounts receivable, accounts payables and accrued liabilities denominated in U.S. dollars
would result in an increase of approximately $705,000 to the net loss and comprehensive loss for the year ended March 31, 2018 (March 31, 2017- reduction to net loss of $3,000). The inverse effect would result if the Canadian dollar weakened by 10% against the Euro and U.S. dollar.
At March 31, 2018, the Company had no hedging agreements in place with respect to foreign exchange rates. Certain operational costs are denominated in U.S. dollars and funded directly from the Company's U.S. funds. The Company's European operation provides further natural hedging as U.S. dollars are used to periodically fund operations. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks at this time. Management monitors fluctuations in foreign currency rates to maximize conversion of U.S. dollars to Canadian dollars.
Interest Rate Risk
Interest rate risk is the risk that the value of a financial instrument might be adversely affected by a change in the interest rates. As at March 31, 2018, the Company did not have any liabilities that bear interest at rates fluctuating with the prime rate.
Changes in Accounting Policies
During the year ended March 31, 2018, there were no new IFRS or IAS accounting standards that became effective that had a material impact on the Company's consolidated financial statements. There are however a number of new standards and amendments to existing standards effective in future periods. The following may impact the reporting and disclosures of the Company:
New standard IFRS 9 “Financial Instruments” - This new standard will replace IAS 39 Financial Instruments: Recognition and Measurement in its entirety. IFRS 9 is intended to reduce the complexity in the classification and measurement of financial instruments. IFRS 9 is effective for periods beginning on or after January 1, 2018. The Company has reviewed the implications of the adoption of IFRS 9 and the final standard will not have a material impact on the Company's consolidated financial statements other than increased levels of note disclosure.
New standard IFRS 15 “Revenues from contracts with Customers” - This new standard establishes principles for reporting the nature, amount, timing, and uncertainty of revenue and cash flows arising from an entity's contracts with customers. It provides a single model in order to depict the transfer of promised goods or services to customers. IFRS 15 supersedes IAS 11, Construction Contracts, IAS 18, Revenue, IFRIC 13, Customer Loyalty Programs, IFRIC 15, Agreements for the Construction of Real Estate, IFRIC 18, Transfers of Assets from Customers, and SIC-31, Revenue- Barter Transactions involving Advertising Service. IFRS 15 establishes a single fivestep model framework for determining the nature, amount, timing and uncertainty of revenue and cash flows arising from a contract with a customer. The standard is effective for annual periods beginning on or after January 1, 2018, with early adoption permitted. The Company has reviewed the implications of the adoption of IFRS 15 and concluded the final standard will not have a material impact on the Company's consolidated financial statements.
IFRS 16 Leases was issued in January 2016 and is effective for periods beginning on or after January 1, 2019. The new standard eliminates the classification of leases as either operating or finance leases.
43
It provides a single lessee accounting model, requiring lessees to recognize assets and liabilities for all leases unless the lease term is 12 months or less or the underlying asset has a low value. Management is currently reviewing the impact that adoption of the new standard will have on the Company's consolidated financial statements.
Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet arrangements.
Proposed Transactions
The Company does not have any proposed transactions.
Summary of Executive Officers, Directors and Significant Employees
The following table sets out the company's officers and directors. All but Mr. Bomford work with the company on a part-time basis.
Name | Position | Age | Term of Office | Approximate
hours per week |
Executive Officers: |
Darcy Bomford | C.E.O. and President | 51 | Since June 9, 2014 | 40 |
Chuck Austin | Former C.F.O. | 80 | June 9, 2014- Sept.10, 2018 | 20 |
Kerry Biggs | C.F.O. | 49 | Since Sept. 10, 2018 | 20 |
Directors: |
Kevin Bottomley | Director and Corporate Communication | 39 | Since June 9, 2014 | 20 |
Christopher Spooner | Director | 48 | June 9, 2014- July 10, 2018 | 20 |
Michael Harcourt | Director | 74 | Since June 9, 2014 | 2 |
Sylvian Toutant | Director | 55 | Since July 10, 2018 | 5 |
| | | | |
44
Business Experience
 Darcy Bomford, Chief Executive Officer and President
Darcy Bomford, Chief Executive Officer and President
Mr. Bomford is the founder and President of True Leaf Medicine Inc., the Company's wholly owned subsidiary and an applicant under Canada's MMPR (now the ACMPR) to become a licensed producer and marketer of medical marijuana. Prior to this, Mr. Bomford was the founder, President, Chief Executive Officer and director of Darford International Inc., a manufacturer and marketer of branded and private pet food products with three federally inspected production plants in the United States and Canada, and whose common shares formerly traded on the TSX Venture Exchange under the symbol “WUF”. Mr. Bomford has extensive expertise with professional manufacturing systems, including comprehensive third party audited food safety systems, product development, marketing and sales within a highly regulated and competitive industry.
 Michael Harcourt, Chairman and Director
Michael Harcourt, Chairman and Director
Mr. Harcourt is the former Premier of British Columbia and former Mayor of Vancouver. He serves as Honorary Chair of the International Centre for Sustainable Cities and is Co-Chair of the International Panel of Advisers. Mr. Harcourt also serves as an advisor to Translink BC and is an Associate Director at the Centre for Sustainability, Continuing Studies at UBC. He is the honorary co-chair at the University of British Columbia's Advisory Council on Sustainability, as well is on the Canadian Electricity Association's Sustainable Electricity Program Advisory Panel.
Notably, in 2005 Mr. Harcourt was awarded the Woodrow Wilson Award for Public Service, in 2008 he was awarded the Alumni Achievement Award for Distinction for contributions to BC, Canada and the world from the University of British Columbia, and in 2012 he was named an Officer of the Order of Canada.
In February 2017, Mr. Harcourt received the Freedom of the City Award from the City of Vancouver.
 Kevin Bottomley, Corporate Communications and Director
Kevin Bottomley, Corporate Communications and Director
Mr. Bottomley has spent the last nine years working on corporate communications with two publicly traded companies, Zimtu Capital Corp. and Commerce Resources Corp. He has been involved with successful capital raises in excess of $70 million and has strong working relationships with contacts in Canada, the United States, Europe and Asia.
45
 Sylvain Toutant, Director
Sylvain Toutant, Director
Mr. Toutant has more than 17 years of experience as an executive in the beverage and consumer packaged goods industries and is a recognized specialist in retailing. He most recently served as the Chief Executive Officer and President of DAVIDsTEA from May 2014 to January 2017, Canada's largest specialty tea boutique, where he was responsible for the company's growth in Canada, the United States, and around the world. He also led the company's successful IPO to NASDAQ.
Since leaving DAVIDsTEA, Mr. Toutant has served as a director on various boards he continues to serve today including: GelPac, Les Chocolat Favoris Inc., Angelcare, and YUZU Sushi among others.
Previously he served as President of Keurig Canada from 2008 to 2014, where he accelerated growth through a strategic alliance with Keurig Green Mountain in the United States. He also headed Keurig's operations in the United Kingdom.
 Kerry Biggs, Chief Financial Officer
Kerry Biggs, Chief Financial Officer
Mr. Biggs has more than 20 years of finance and business experience, most recently with lululemon athletica Inc. from June 2016 to September 2018, where he was Vice President, Treasurer looking after capital markets, liquidity, treasury, insurance and risk activities for the NASDAQ-listed company.
Previously, he served as Vice President, Finance at Global Container Terminals from March 2008 to May 2016, where he was responsible for capital markets, risk management, accounting, tax planning, and corporate M&A activities.
He also worked in senior finance roles for Finning International and Enbridge, both large publicly traded companies.
 Chris Spooner, Former Director
Chris Spooner, Former Director
Mr. Spooner is a Naturopathic Doctor practicing in Vernon, British Columbia, and a graduate of the University of Victoria and the Canadian College of Naturopathic Medicine. An adjunct faculty member at UBC Okanagan, the University of Bridgeport, the Boucher Institute of Naturopathic Medicine and the Canadian College of Naturopathic Medicine, Mr. Spooner is regularly invited to lecture at colleges and medical conferences.
46
 Chuck Austin, Former Chief Financial Officer
Chuck Austin, Former Chief Financial Officer
Mr. Austin was formerly a senior audit partner for Ernst & Young and a member of Ernst & Young International, which has worldwide operations and more than 600 associated offices. Mr. Austin has more than 35 years of experience providing audit, accounting, taxation and general business advice to a wide range of clients. Mr. Austin brings his significant experience auditing public companies and assisting them to obtain initial public financing to his position as Chief Financial Officer of True Leaf.
Board of Directors
Our board of directors currently consists of four directors. Three of our directors are “independent” as defined by Rule 4200 of FINRA's listing standards. In the future, we may appoint additional independent directors to our board of directors to serve on our planned committees.
Term of Office
Our directors are appointed for a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our articles. Our officers are appointed by our board of directors and hold office until removed by the board.
Family Relationships
There are no family relationships between or among the directors, executive officers, or persons nominated or chosen by us to become directors or executive officers.
Cease Trade Orders, Bankruptcies, Penalties or Sanctions
Cease Trade Orders
Darcy Bomford, the President, CEO and a director of the Company, was the CEO and a director of Darford International Inc. (“Darford”), a corporation that was the subject of a cease trade order issued by the British Columbia Securities Commission on December 6, 2012 for failure to file its interim financial statements and management's discussion and analysis for the period ended September 30, 2012. Mr. Bomford resigned from his position as the CEO of Darford on October 12, 2012.
Darford was also the subject of a cease trade order issued by the Alberta Securities Commission on April 14, 2014 for failure to file its annual financial statements and management's discussion and analysis for the year ended March 31, 2013, and interim financial statements and management's discussion and analysis for the periods ended September 30, 2012, December 31, 2012, June 30, 2013, September 30, 2013 and December 31, 2013.
Other than as described above, no proposed director of the Company is, or within the 10 years before the date of this Annual Information Form has been, a director, chief executive officer or chief financial officer of any company that:
47
was subject to an order that was issued while the proposed director was acting in the capacity as director, chief executive officer or chief financial officer; or
was subject to an order that was issued after the proposed director ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer.
Bankruptcies
On October 22, 2012, The Bowra Group Inc. was appointed as receiver and manager of all assets, undertakings and properties of Darford and its wholly-owned subsidiaries Darford USA Inc., Darford Industries Ltd, and Darford USA Holding Co. Darcy Bomford, the President, CEO and a director of the Company, was the CEO and a director of Darford but resigned from his position as the CEO of Darford on October 12, 2012. Following its appointment, the receiver initiated a sale process to sell Darford's assets on a going-concern basis.
Other than as described above, no proposed director of the Company is, or within 10 years before the date of this Annual Information Form has been, a director or executive officer of any company that, while the person was acting in that capacity, or within a year of that person ceasing to act in the capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold its assets.
Personal Bankruptcies
No proposed director of the Company has, within 10 years before the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the proposed director.
Securities Related Penalties and Sanctions
No proposed director has been subject to, or entered into a settlement agreement resulting from:
- any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or
- any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable securityholder in deciding whether to vote for a proposed director.
Conflicts of Interest
Our directors are required by law to act honestly and in good faith with a view to our best interests and to disclose any interests which they may have in any project or opportunity of ours. If a conflict of interest arises, any director in a conflict will disclose his interest and abstain from voting on such matter at a meeting of the Board of Directors.
To the best of our knowledge, there are no known existing or potential conflicts of interest among the Company, directors and officers, any of our subsidiaries or other members of management of ours or any proposed promoter, director, officer, or other member of management as a result of their outside business
48
interests, except that certain of the directors and officers serve as directors and officers of other companies, and therefore it is possible that a conflict may arise between their duties to us and their duties as a director or officer of such other companies.
Committees of the Board
Until further determination by the board, the full board of directors will undertake the duties of the audit committee, compensation committee and nominating committee.
Audit Committee
On February 6, 2015, we adopted an audit committee charter and appointed members of the audit committee.
As of this Annual Report, the following directors are the members of the audit committee:
Name | Independence | Financial Literacy |
Darcy Bomford (1) | Not Independent | Financially literate |
Kevin Bottomley | Independent | Financially literate |
Michael Harcourt | Independent | Financially literate |
| | |
Note:
(1) Chair of the audit committee.
Our audit committee approves the selection of, meets with, and interacts with our independent accountants to discuss issues related to financial reporting. In addition, our audit committee reviews the scope and results of the audit with the independent accountants, reviews with management and the independent accountants our annual operating results, considers the adequacy of our internal accounting procedures, and considers other auditing and accounting matters including fees to be paid to the independent auditor and the performance of the independent auditor.
Nomination Committee
Our board of directors does not maintain a nominating committee. As a result, no written charter governs the director nomination process. Our size and the size of our board, at this time, do not require a separate nominating committee.
When evaluating director nominees, our directors consider the following factors:
- The appropriate size of our board of directors;
- Our needs with respect to the particular talents and experience of our directors;
- The knowledge, skills and experience of nominees, including experience in finance, administration or public service, in light of prevailing business conditions and the knowledge, skills and experience already possessed by other members of the board;
- Experience in political affairs;
- Experience with accounting rules and practices; and
- The desire to balance the benefit of continuity with the periodic injection of the fresh perspective provided by new board members.
49
Our goal is to assemble a board that brings together a variety of perspectives and skills derived from high quality business and professional experience. In doing so, the board will also consider candidates with appropriate non-business backgrounds.
Other than the foregoing, there are no stated minimum criteria for director nominees, although the board may also consider such other factors as it may deem are in our best interests as well as our shareholders. In addition, the board identifies nominees by first evaluating the current members of the board willing to continue in service. Current members of the board with skills and experience that are relevant to our business and who are willing to continue in service are considered for re-nomination. If any member of the board does not wish to continue in service or if the board decides not to re-nominate a member for re-election, the board then identifies the desired skills and experience of a new nominee in light of the criteria above. Current members of the board are polled for suggestions as to individuals meeting the criteria described above. The board may also engage in research to identify qualified individuals. To date, we have not engaged third parties to identify, evaluate, or assist in identifying potential nominees (although we reserve the right in the future to retain a third-party search firm, if necessary). The board does not typically consider shareholder nominees because it believes that its current nomination process is sufficient to identify directors who serve our best interests.
Code of Ethics
We currently have not adopted a code of ethics for the board or executives. The Board of Directors has found that the fiduciary duties placed on individual directors by the Company's governing corporate legislation and the common law and the restrictions placed by applicable corporate legislation on an individual director's participation in decisions of the Board in which the director has an interest have been sufficient to ensure that the Board operates independently of management and in the best interests of the Company
Promoters
On December 18, 2017, the Company entered into an agreement with Edison Advisors (“Edison”), a global strategic advisory firm, as the Company's investor relations advisor. Under the terms of the agreement, the Company is required to pay Edison $8,000 USD per month. The agreement term is 12 months and may be cancelled on 90 days written notice.
Executive Compensation
The table below summarizes the annual compensation of each of our executive officers and directors for our last fiscal year, ended March 31, 2018:
Name | Capacities in Which
Compensation was Received | Cash
Compensation
($) | Other
Compensation
($) | Total
Compensation
($) |
Darcy Bomford (1) | Chief Executive Officer, President and Director | 70,000 | 151,749 | 221,749 |
Chuck Austin (2) | Chief Financial Officer | 24,000 | 40,660 | 64,660 |
Michael Harcourt | Chairman and Director | 2,500 | 151,749 | 154,249 |
Chris Spooner | Director | 23,500 | 151,749 | 175,249 |
Kevin Bottomley | Director | 65,500 | 151,749 | 217,249 |
| | | | |
50
Notes:
On May 1, 2014, we entered into an executive management agreement with our Chief Executive Officer, Darcy Bomford. See “Management Agreements”.
On June 20, 2014, we entered into an executive consulting agreement with our Chief Financial Officer, Chuck Austin. See “Management Agreements”. Mr. Austin resigned effective September 10, 2018.
Our directors and executive officers are also reimbursed for their business expenses. The employment compensation for certain executive officers may include automobile and housing allowances.
Management Agreements
Chief Executive Officer
On May 1, 2014, we entered into an executive management agreement with our Chief Executive Officer Mr. Bomford. Under the terms of the agreement Mr. Bomford will serve as the company's CEO for a one-year term. This is subject to earlier termination as provided in the agreement, commencing on May 1, 2014. The agreement automatically renews annually. Mr. Bomford's annual base salary is $60,000. The actual amount of the bonus earned will be based on the achievement of certain financial performance goals established by the board of directors of the Company.
Mr. Bomford's management agreement does not provide any severance payment on termination or as a result of a change of control event.
Chief Financial Officer
On July 18, 2018, we entered into a CFO employment agreement with Kerry Biggs for an indefinite term with four (4) weeks written notice required by Mr. Biggs to the Company to terminate the agreement. Pursuant to the terms of the CFO employment agreement, Mr. Biggs will receive an annual salary of $195,000. In addition to the annual salary, Mr. Biggs has also been granted 750,000 incentive stock options with an exercise price of $0.56 per common share for a period of 5 years. The incentive stock options vest over a three-year schedule. Mr. Biggs has also been issued 100,000 common shares in the capital of the Company at a deemed price of $0.60 per common share for an aggregate deemed value of $60,000 on October 17, 2018. Pursuant to the compensation structure of the CFO employment agreement, Mr. Biggs is eligible to earn an annual cash bonus equal to up to 55% of his annual base salary. The actual amount of the bonus earned will be based on milestone achievements set by the Board.
In the event the Company wishes to terminate the agreement, notice provisions of the agreement including written notice, salary in lieu of, or a combination of both will apply.
Former Chief Financial Officer
On June 20, 2014, we entered into an executive consulting agreement with our Chief Financial Officer, Chuck Austin. Under the terms of the agreement, Mr. Austin will serve as our Chief Financial Officer for a one-year term. This is subject to earlier termination as provided in the agreement, commencing on June 20, 2014. The agreement automatically renews annually. Mr. Austin' annual base salary is $24,000. He will have the opportunity to earn an annual cash bonus equal to up to 20% of his annual base salary. The actual amount of the bonus earned will be based on the achievement of certain financial performance goals to be established by the board of directors of the company. No bonuses have been paid to date.
51
Mr. Austin's consulting agreement does not provide any severance payment on termination or as a result of a change of control event.
Termination and Change of Control Benefits
Other than as disclosed in this Annual Report, here are no compensatory plans or arrangements with respect to the named executive officers resulting from the resignation, retirement, or any other termination of the officers' employment or change of named executive officers' responsibilities following a change of control. We have not granted any termination or change of control benefits. In case of termination of named executive officers, common law and statutory law applies.
Stock Incentive Plan
On March 19, 2015, our board of directors adopted an equity incentive stock option plan (the “2015 Plan”). The 2015 Plan provides for the issuance of stock options to acquire up to 10% of our issued and outstanding common shares as of the date of the grant. The exercise price of each stock option is based on the market price of our common shares on the CSE at the date of the grant, subject to a minimum price of $0.10. The 2015 Plan contains limits with respect to how many stock options individuals and consultants can receive as well as limits on the amounts of stock options that may be granted for investor relations activities. Our board of directors is responsible for administering the 2015 Plan until such time as such authority has been delegated to a committee of the board of directors. The 2015 Plan was ratified by our shareholders in 2015. As of November 30, 2018, there were 7,192,145 outstanding options to purchase common shares.
Pension Plan Benefits
We do not currently provide any pension plan benefits to our executive officers, directors, or employees.
Director Compensation
Following are the amounts of compensation provided to our directors for the most recently completed financial year March 31, 2018 (other than our directors who are also NEO's as their compensation is fully reflected in the tables above):
Name | Fees earned ($) | Share- based awards ($) | Option
based
awards
($) | Non-equity
incentive
plan
compensation
($) | Pension
value
($) | All
other
compensation
($) | Total |
Kevin Bottomley | 2,500 | Nil | Nil | Nil | Nil | Nil | 2,500 |
Christopher Spooner | 2,500 | Nil | Nil | Nil | Nil | Nil | 2,500 |
Mike Harcourt | 2,500 | Nil | Nil | Nil | Nil | Nil | 2,500 |
| | | | | | | |
On June 6, 2018, we entered into a director's consulting agreement with Sylvain Toutant for an indefinite term with thirty (30) days' notice required by either party to terminate the agreement. Pursuant to the terms of the agreement, Mr. Sylvain will receive $2,000 plus reasonable expenses for all Board meetings he participates in.
52
Outstanding Share-Based Awards and Option-Based Awards
The following are all outstanding share-based and option-based awards granted or issued to each of our directors and executive officers as of March 31, 2018.
| Option-based award | Share-based Awards |
Name | Number of securities underlying unexer-
cised options | Option exercise price
($) | Option expira-
tion date
($) | Value of unexer-
cised in-the- money Options(1) ($) | Number of shares or units of shares that have not vested ($) | Market or payout value of share- based awards that have not vested
($) | Market or payout value of vested share- based awards not paid out or distributed ($) |
Darcy
Bomford | 328,575 | $0.185 | 12/12/2018 | $ 188,931 | Nil | Nil | Nil |
300,000 | $0.395 | 05/29/2019 | $ 109,500 | Nil | Nil | Nil |
200,000 (2) | $0.940 | 02/06/2023 | $ Nil | Nil | Nil | Nil |
Kevin Bottomley | 300,000 | $0.395 | 05/29/2019 | $ 109,500 | Nil | Nil | Nil |
200,000 (2) | $0.940 | 02/06/2023 | Nil | Nil | Nil | Nil |
Christopher Spooner | 300,000 | $0.395 | 05/29/2019 | $ 109,500 | Nil | Nil | Nil |
200,000 (2) | $0.940 | 02/06/2023 | $ Nil | Nil | Nil | Nil |
Chuck Austin | 300,000 | $0.395 | 05/29/2019 | $ 109,500 | Nil | Nil | Nil |
Mike Harcourt | 328,570 | $0.185 | 12/12/2018 | $ 188,928 | Nil | Nil | Nil |
300,000 | $0.395 | 05/29/2019 | $ 109,500 | Nil | Nil | Nil |
200,000 (2) | $0.940 | 02/06/2023 | $ Nil | Nil | Nil | Nil |
| | | | | | | |
Notes:
Based on the closing price of the Company's stock on March 29, 2018 of $0.76.
100,000 stock options vest immediately, 25,000 vest every 6 months thereafter.
Mr. Spooner's stock options have been cancelled: 300,000 stock options at $0.395 were cancelled on October 8, 2018, 100,000 stock options at $0.94 were cancelled July 10, 2018, and 100,000 stock options exercisable at $0.94 were cancelled on October 8, 2018.
Mr. Austin's 300,000 stock options were cancelled December 10, 2018.
Limitation of Liability and Indemnification of Officers and Directors.
Our articles provide that we will indemnify our directors, officers, employees and other agents to the fullest extent permitted by law. We believe that indemnification under our articles covers at least negligence and gross negligence on the part of indemnified parties. Our articles also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our articles permit such indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable.
53
There is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
Indebtedness of Directors and Executive Officers
No director or executive officer, or any associate or affiliate of any such director or senior officer, is or has been indebted to us since the date of incorporation. No director or executive officer, or associate or affiliate of any such director or senior officer, is or has been indebted to us since the beginning of the last completed financial year.
The Company is authorized to issue an unlimited number of common shares without par value. As of November 30, 2018, a total of 95,529,059 common shares were issued and outstanding (March 31, 2018 - 95,369,059). Each common share carries the right to one vote at meetings of the common shareholders.
The following table sets out, as of November 30, 2018, the voting securities of the Company that are owned by executive officers, directors, and other persons holding more than 10% of the Company's voting securities or having the right to acquire those securities.
Title of
class | Name and address
of beneficial owner (1) | Amount and nature of beneficial
ownership (2) | Amount and nature of
beneficial ownership
acquirable | Percent
of class (3) |
Common shares | Darcy Bomford | 22,049,084 (4)
directly owned
1,664,668 (5)
indirectly owned | 828,575 shares available from issued stock options (6) and 666,666 shares may be issued on exercise of warrants (7) | 24.82% |
Common shares | Kevin Bottomley | 828,570
directly owned | 500,000 shares available from issued stock options (8) and 85,000 shares may be issued on exercise of warrants (9) | 0.87% |
55
Title of
class | Name and address
of beneficial owner (1) | Amount and nature of beneficial
ownership (2) | Amount and nature of
beneficial ownership
acquirable | Percent
of class (3) |
Common shares | Michael Harcourt | 1,500,000
directly owned | 828,570 shares available from issued stock options (10) | 1.57% |
Common shares | Sylvain Toutant | 0 | 100,000 shares available from issued stock options (11) | 0% |
Common shares | Kerry Biggs | 100,000
directly owned | 750,000 shares available from issued stock options (12) | 0.11% |
Common shares | Chuck Austin | 364,570
directly owned | 300,000 shares available from issued stock options (13) | 0.38% |
Common shares | Christopher Spooner | 428,570
directly owned | 500,000 shares available from issued stock options (8) (14) | 0.45% |
Common shares | CDS & Co. | 63,113,566 (13) | N/A | 66.07% |
| | | | |
Notes:
The business address for each of our directors and officers is: 100 Kalamalka Lake Road, Unit 32, Vernon, British Columbia V1T 9G1.
The number of common shares beneficially owned, or controlled or directed, directly or indirectly, at the date of this Annual Report is based upon information furnished to us by the individual directors.
As of November 30, 2018, there were 95,529,059 issued and outstanding.
Mr. Bomford's securities are subject to a stock restriction agreement dated February 2, 2015, entered into at the time we listed on the CSE. As of November 13, 2017, 2,434,134 common shares remain in escrow. On February 15, 2018, these common shares will be released from escrow.
The 1,664,668 common shares held indirectly by Mr. Bomford are held in the name of First Pacific Enterprises Inc.
328,575 of these stock options were exercised on December 12, 2018, 200,000 expire on May 29, 2019 and 300,000 expire on February 6, 2023. The 328,575 stock options that expire in 2018 can be exercised to acquire one additional common share at $0.185 per share. The 200,000 stock options that expire in 2019 can be exercised to acquire one additional common share at $0.395 per share. The 300,000 stock options that expire in 2023 can be exercised to acquire one additional common share at $0.94 per share.
666,666 warrants can be exercised to acquire one additional common share at an exercise price of $0.45 prior to May 29, 2019 and are held directly by Mr. Bomford.
200,000 of these stock options of expire on May 29, 2019, 200,000 expire on May 29, 2019 and 300,000 expire on February 6, 2018. The 200,000 stock options that expire in 2019 can be exercised to acquire one additional common share at $0.395 per share. The 300,000 stock options that expire in 2023 can be exercised to acquire one additional common share at $0.94 per share.
The warrants can be exercised to acquire one additional common share at an exercise price of $0.45 prior to June 12, 2019.
328,570 of these stock options were exercised on December 12, 2018, 300,000 of these stock options of expire on May 29, 2019 and 300,000 expire on February 6, 2023. The 328,575 stock options that expire in 2018 can be exercised to acquire one additional common share at $0.185 per share. The 200,000 stock options that expire in 2019 can be exercised to acquire one additional common share at $0.395 per share. The 300,000 stock options that expire in 2023 can be exercised to acquire one additional common share at $0.94 per share.
The 100,000 stock options issued to Mr. Toutant expire on July 31, 2023 and can be exercised to acquire one additional common share at $0.50 per share.
The 750,000 stock options issued to Mr. Biggs expire on September 10, 2023 and can be exercised to acquire one additional common share at $0.56 per share.
Effective September 10, 2018, Mr. Austin resigned as CFO of the Company. His stock options were cancelled on December 10, 2018.
Effective July 10, 2018, Mr. Spooner resigned as a director of the Company. Mr. Spooner's stock options as a result have been cancelled: 300,000 stock options exerciseable at $0.395 were cancelled on October 8, 2018, 100,000 stock options exerciseable at $0.94 were cancelled July 10, 2018, and 100,000 stock options exercisable at $0.94 were cancelled on October 8, 2018 .
Includes 10,069918 common shares held by Darcy Bomford. Other than Mr. Bomford, management is unaware of the beneficial holders of the shares registered in the name of CDS & Co., a depositary trust company. CDS& Co has several offices in Canada including: 650 West Georgia Street, Suite 2700, Vancouver, BC, V6B 4N9.
56
Collectively, as at March 31, 2018 the directors and officers of the Company own, directly or indirectly, or exercise control or direction over 27,143,662 common shares, representing 28.46% of the Company's then issued and outstanding common shares at that date (95,369,059).
As at November 30, 2018, the current directors and officers of the Company own, directly or indirectly, or exercise control or direction over 26,570,892 common shares, representing 27.81% of the Company's issued and outstanding common shares.
To the best of the Company's knowledge other than as set forth in this Annual Report, there were no material transactions or series of similar transactions nor were there any currently proposed transactions or series of similar transactions to which the Company was or are to be a party to in which the amount involved exceeds the lesser of $120,000 or one percent of the average of the Company's assets at year-end for the last two completed fiscal years. To the best of the Company's knowledge other than as set forth in this Annual Report, there have been no transactions in which any director, executive officer, or security holder is known to own of record or beneficially more than 5% of any class of the Company's common shares nor does any member of the immediate family of any of the foregoing persons have an interest (other than compensation to the Company's officers and directors in the ordinary course of business).
The Company has closed its Regulation A financing having raised the full $10 million under the offering. As of the date of this Annual Report the Company has 546 shareholders of record.
57
True Leaf Medicine International Ltd.
Consolidated Financial Statements
March 31, 2018
(Expressed in Canadian dollars)
58
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
True Leaf Medicine International Ltd.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated financial statements of True Leaf Medicine International Ltd. (the “Entity”), which comprise the consolidated statements of financial position as of March 31, 2018 and 2017, the consolidated statements of loss and comprehensive loss, changes in shareholders' equity (deficit), and cash flows for the years ended March 31, 2018 and 2017 and the related notes, comprising a summary of significant accounting policies and other explanatory information (collectively referred to as the consolidated financial statements).
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Entity as at March 31, 2018 and 2017 and its financial performance and its cash flows for the years ended March 31, 2018 and 2017 in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
Management's Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors' Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement, whether due to error or fraud. Those standards also require that we comply with ethical requirements, including independence. We are required to be independent with respect to the Entity in accordance with the ethical requirements that are relevant to our audit of the consolidated financial statements in Canada, the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We are a public accounting firm registered with the PCAOB.
An audit includes performing procedures to assess the risks of material misstatements of the consolidated financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included obtaining and examining, on a test basis, audit evidence regarding the amounts and disclosures in the consolidated financial statements. The procedures selected depend on our judgment, including the assessment of the risks of material misstatement of the consolidated financial statements,
59
whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the entity's preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity's internal control. The Entity is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Accordingly, we express no such opinion.
An audit also includes evaluating the appropriateness of accounting policies and principles used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a reasonable basis for our audit opinion.
Other Matters
We have served as the Entity's auditor since 2014.
“DAVIDSON & COMPANY LLP”
Chartered Professional Accountants
Vancouver, Canada
June 26, 2018
60
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Consolidated Statements of Financial Position (Expressed in Canadian dollars) |
| |
| | | | | | March 31, 2018 | | March 31, 2017 |
Assets | | | | | | | |
| | | | | | | | |
Current assets | | | | | | | |
Cash and cash equivalents | | | | $ | 10,812,815 | $ | 159,575 |
Receivables (Notes 6, 13) | | | | | 385,671 | | 66,179 |
Inventories (Note 7) | | | | | 570,594 | | 392,908 |
Prepaid expenses and deposits (Note 9) | | | | | 149,199 | | 15,920 |
Total current assets | | | | | 11,918,279 | | 634,582 |
| | | | | | | | |
Non-current assets Marketable securities (Note 8) | | | | | - | | 50 |
Land (Note 9) | | | | | 3,380,387 | | - |
Construction in progress (Note 9) | | | | | 726,955 | | - |
Capital assets (Note 10) | | | | | 132,420 | | 8,811 |
Intangible assets (Note 11) | | | | | 142,690 | | 128,180 |
Total assets | | | | $ | 16,300,731 | $ | 771,623 |
Liabilities and shareholders' equity | | | | | |
| | | | | | | | |
Current liabilities | | | | | | | |
Accounts payable and accrued liabilities | | | $ | 927,987 | $ | 211,452 |
Construction holdback payable (Note 9) | | | | | 98,661 | | - |
Due to related parties (Note 13) | | | | | 23,314 | | 23,771 |
Promissory note payable (Note 12) | | | | | - | | 46,428 |
Total current liabilities | | | | | 1,049,962 | | 281,651 |
| | | | | | | |
Non-current liabilities | | | | | | | |
Promissory note payable (Note 12) | | | | - | | 63,169 |
Total liabilities | | | | 1,049,962 | | 344,820 |
Shareholders' equity | | | | | | | |
Share capital (Note 14) | | | | | 21,693,918 | | 5,088,454 |
Reserves | | | | | 2,518,723 | | 339,802 |
Deficit | | | | | (8,961,872) | | (5,001,453) |
Total shareholders' equity | | | | | 15,250,769 | | 426,803 |
Total liabilities and shareholders' equity | | $ | 16,300,731 | $ | 771,623 |
Nature of Operations and Going Concern (Note 1) |
Commitments (Note 17) |
| |
Approved on behalf of the Board of Directors on June 26, 2018 |
| | | | | | | | |
“Darcy Bomford” | Director | | “Michael Harcourt” | | Director |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
61
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Consolidated Statements of Loss and Comprehensive Loss |
(Expressed in Canadian dollars) |
| |
| | | Year Ended
March 31,
2018 | | Year Ended
March 31,
2017 |
Sales | $ | 1,400,511 | $ | 368,536 |
Cost of sales | | (779,182) | | (248,909) |
Gross profit | | 621,329 | | 119,627 |
| | | | |
Operating expenditures | | | | |
Accounting and legal (Note 13) | | 411,352 | | 288,039 |
Accretion (Note 12) | | 18,079 | | 3,192 |
Administrative and office (Note 15) | | 1,564,926 | | 763,498 |
Amortization - intangible assets (Note 11) | | 22,614 | | 14,742 |
Depreciation - capital assets (Note 10) | | 14,865 | | 1,990 |
Directors' fees (Note 13) | | 7,500 | | 7,500 |
Inventory write-down (Note 7) | | 217,436 | | 36,000 |
Research and development | | 57,808 | | 49,136 |
Selling and marketing | | 658,834 | | 427,820 |
Share-based compensation (Notes 13 and 14) | | 1,836,441 | | 265,917 |
Total operating expenditures | | (4,809,855) | | (1,857,834) |
| | | | |
Loss from operations | | (4,188,526) | | (1,738,207) |
Bargain purchase on acquisition of OregaPet assets (Note 5) | | - | | 5,338 |
Foreign exchange gain (loss) | | 202,425 | | (3,223) |
Loss on debt settlement (Note 14) | | - | | (6,958) |
Rental income | | 18,215 | | - |
Write-down marketable securities (Note 8) | | (50) | | - |
Loss and comprehensive loss for the year | $ | (3,967,936) | $ | (1,743,050) |
| | | | | |
Loss per common share- basic and diluted | $ | (0.05) | $ | (0.03) |
Weighted average number of common shares outstanding- basic and diluted | | 78,314,081 | | 54,039,396 |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
62
TRUE LEAF MEDICINE INTERNATIONAL LTD.
Consolidated Statements of Changes in Shareholders' Equity (Deficit)
(Expressed in Canadian dollars)
| Number of Shares | Share Capital | Convertible Debt - Equity | Reserves | Deficit | Total Shareholders' Equity (Deficit) |
| | | | | | |
Balance, March 31, 2016 | 41,971,949 | $ 2,436,675 | $ 4,373 | $ 312,795 | $ (3,283,342) | $ (529,499) |
Shares issued for debt settlement, net of share issuance costs | 2,461,785 | 289,800 | - | - | - | 289,800 |
Shares issued on conversion of debt | 601,843 | 63,193 | (4,373) | - | - | 58,820 |
Private placements, net of share issuance costs | 9,012,453 | 1,141,340 | - | - | - | 1,141,340 |
Shares issued on exercise of warrants | 2,813,163 | 554,675 | - | (25,200) | - | 529,475 |
Shares issued on exercise of stock options | 3,090,000 | 502,771 | - | (188,771) | - | 314,000 |
Fair value adjustment on expiry of stock options | - | - | - | (24,939) | 24,939 | - |
Shares issued for acquisition of OregaPet assets | 476,190 | 100,000 | - | - | - | 100,000 |
Share-based compensation | - | - | - | 265,917 | - | 265,917 |
Loss for the year | - | - | - | - | (1,743,050) | (1,743,050) |
Balance, March 31, 2017 | 60,427,383 | 5,088,454 | - | 339,802 | (5,001,453) | 426,803 |
Private placements, net of share issuance costs | 7,741,645 | 2,289,573 | - | - | - | 2,289,573 |
Regulation A public offering, net of share issuance costs | 14,285,715 | 7,764,545 | - | 789,767 | - | 8,554,312 |
Private placement, Canadian side car, net of share issuance costs | 5,864,736 | 4,021,459 | - | - | - | 4,021,459 |
Shares issued on exercise of warrants | 3,707,000 | 1,246,472 | - | - | - | 1,246,472 |
Shares issued on exercise of stock options | 3,342,580 | 1,283,415 | - | (439,770) | | 843,645 |
Fair value adjustment on expiry of stock options | - | - | - | (7,517) | 7,517 | - |
Share-based compensation | - | - | - | 1,836,441 | - | 1,836,441 |
Loss for the year | - | - | - | - | (3,967,936) | (3,967,936) |
Balance, March 31, 2018 | 95,369,059 | $ 21,693,918 | $ - | $ 2,518,723 | $ (8,961,872) | $ 15,250,769 |
The accompanying notes are an integral part of these consolidated financial statements.
63
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Consolidated Statements of Cash Flows |
(Expressed in Canadian dollars) |
| | | Year
Ended
March 31,
2018 | | Year
Ended
March 31,
2017 |
| | | | | |
Operating activities Loss for the year | $ | (3,967,936) | $ | (1,743,050) |
Items not affecting cash: | | | | |
Depreciation- capital assets | | 14,865 | | 14,742 |
Depreciation- intangible assets | | 22,614 | | 1,990 |
Bargain purchase on acquisition of OregaPet assets | | - | | 5,338 |
Accretion | | 18,079 | | 3,192 |
Share-based compensation | | 1,836,441 | | 265,917 |
Loss on debt settlement | | - | | 6,958 |
Inventory write-down | | 217,436 | | 36,000 |
Write-down marketable securities | | 50 | | - |
| | | | | |
Changes in non-cash working capital items: | | | | |
Prepaid expenses and deposits | | (133,279) | | (8,378) |
Accounts payable and accrued liabilities | | 509,534 | | (61,023) |
Due to related parties | | (2,717) | | (10,096) |
Inventories | | (351,318) | | (141,085) |
Receivables | | (319,492) | | (53,254) |
Net cash used in operating activities | | (2,155,723) | | (1,682,749) |
Investing activities Purchase of capital assets | | (135,656) | | (9,231) |
Intangible asset costs | | (47,185) | | (33,949) |
Development of cannabis cultivation facility | | (3,835,981) | | - |
Acquisition of OregaPet assets | | - | | (100,000) |
Net cash used in investing activities | | (4,018,822) | | (143,180) |
Financing activities Proceeds from issuance of share capital | | 18,464,265 | | 1,998,107 |
Repayment of promissory note | (127,676) | | - |
| | | - |
Share issue costs | (1,508,804) | | (16,340) |
| Net cash provided by financing activities | 16,827,785 | | 1,981,767 |
| Change in cash and cash equivalents for the year | 10,653,240 | | 155,838 |
| Cash and cash equivalents, beginning of the year | 159,575 | | 3,737 |
Cash and cash equivalents, end of the year | $ | 10,812,815 | $ | 159,575 |
| | | | | | | | | | | | |
Supplemental disclosure with respect to cash flows (Note 18)
The accompanying notes are an integral part of these consolidated financial statements.
64
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- NATURE OF OPERATIONS AND GOING CONCERN
True Leaf Medicine International Ltd. (the “Company”) was incorporated under the Business Corporations Act of the Province of British Columbia on June 9, 2014 and is the legal parent of True Leaf Investments Corp. (“TL Investments”), True Leaf Medicine Inc. (“TL Medicine”), True Leaf Pet Inc. (“TL Pet”) and True Leaf Pet Europe LLC Sàrl (”TL Pet Europe”). TL Investments, TL Medicine and TL Pet were all incorporated under the Business Corporations Act of the Province of British Columbia on March 26, 2014, July 4, 2013 and November 18, 2015 respectively and TL Pet Europe was incorporated under the Business Corporation Act in Luxembourg on July 18, 2016. The Company's head office and registered office is located at 200, 1238 Homer Street, Vancouver, BC, V6B 2Y5. On February 9, 2015, the Company began trading on the Canadian Securities Exchange (the “CSE”) under the symbol “MJ” and on July 20, 2017 the Company began trading on the OTC under the ticker symbol “TRLFF”.
The Company, through TL Medicine, is seeking to become a licensed producer of medicinal cannabis for the Canadian market under Canada's Access to Cannabis for Medical Purposes Regulations (“ACMPR”) program administered by Health Canada. As at March 31, 2018, the Company does not have a license with the ACMPR and no products are in commercial production or use. The Company has not been granted an ACMPR license and will be required to satisfy additional obligations in order to qualify, including the completion of a compliant facility on a parcel land owned by the Company in Lumby, British Columbia (Note 9). There is some risk that the Company will not receive an ACMPR license, thus rendering the Company unable to proceed with its business model. The Company continues to work diligently to comply with all of the requirements of Health Canada.
The Company also manufactures and distributes hemp-based nutrition for pets. TL Pet and TL Pet Europe have entered the Canadian, US and European natural pet product market with a product line consisting of hemp functional chews and supplemental products for pets.
Going Concern
These consolidated financial statements have been prepared on a going concern basis, which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business.
On January 24, 2018 the Company closed a financing which raised an aggregate total of $14,051,655 in two offerings (Note 14), the proceeds of which are being used to execute on the Company's business plan, including construction of its cannabis cultivation facility (Note 9). For the year ended March 31, 2018, the Company incurred a loss of $3,967,936 and had an accumulated deficit of $8,961,872. The Company earned revenues of $1,400,511 (2017 - $368,536) from TL Pet and TL Pet Europe; although, these two operations have not yet achieved profitability.
Management is of the opinion the continued operations of the Company are dependent on its ability to generate future cash flows from operations and obtain additional funding through external financing to deliver on its business plan. There is a risk that financing will not be available on a timely basis or on terms acceptable to the Company.
These consolidated financial statements do not give effect to any adjustments which would be necessary should the Company be unable to continue operations. Management has assessed that it has sufficient working capital for the Company to continue operations for the next fiscal year.
65
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- BASIS OF PREPARATION
(a) Statement of compliance
These consolidated financial statements have been prepared using accounting policies consistent with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and interpretations of the International Financial Reporting Interpretations Committee (“IFRIC”). These consolidated financial statements were authorized for issue by the Company's directors on June 26, 2018.
(b) Principles of consolidation
These consolidated financial statements incorporate the financial statements of the Company and its controlled subsidiaries. Control exists when the Company has the power, directly or indirectly, to govern the financial and operating policies of an entity so as to obtain benefits from its activities. The consolidated financial statements include the accounts of the Company and its direct wholly-owned subsidiaries: TL Investments, TL Medicine, TL Pet and TL Pet Europe. All significant intercompany transactions and balances have been eliminated on consolidation.
(c) Basis of measurement
The consolidated financial statements have been prepared on a historical cost basis, except for certain financial instruments which are measured at fair value. All amounts on the consolidated financial statements are presented in Canadian dollars which is the functional currency of the Company and its subsidiaries.
(d) Foreign currency translation
The functional currency is the currency of the primary economic environment in which the entity operates. The functional currency of each of the entities in the group is the Canadian dollar. The functional currency determinations were conducted through an analysis of the factors identified in International Accounting Standard (“IAS”) 21, The Effects of Changes in Foreign Exchange Rates.
The presentation currency of the Company is the Canadian dollar. Transactions in currencies other than the Canadian dollar are recorded at exchange rates prevailing on the dates of the transactions. At the end of each reporting period, the monetary assets and liabilities of the Company that are denominated in foreign currencies are translated at the rate of exchange at the reporting date while non-monetary assets and liabilities are translated at historical rates. Revenues and expenses are translated at the exchange rates approximating those in effect on the date of the transactions. Exchange gains and losses arising on translation are included in the consolidated statement of loss and comprehensive loss.
66
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Financial instruments
Financial instruments consist of financial assets and financial liabilities and are initially recognized at fair value net of transaction costs, if applicable. Measurement in subsequent periods depends on whether the financial instrument has been classified as “fair value through profit or loss,” “loans and receivables,” “available-for-sale,” “held-to-maturity,” or “other financial liabilities” as follows:
(i) Financial assets
Financial assets classified as fair value through profit or loss are measured at fair value with unrealized gains and losses recognized in net loss for the period in which such gains or losses occur. The Company's cash and cash equivalents are classified as fair value through profit or loss.
Financial assets classified as loans and receivables and held-to-maturity are measured at amortized cost using the effective interest rate method. Under this method, all cash flows from these instruments are discounted, where material, to their present value. Over time, this present value is accreted to the future value of remaining cash flows, and this accretion is recorded as interest income. Certain of the Company's receivables are classified as loans and receivables and no financial assets have been classified as held-to-maturity.
Financial assets classified as available-for-sale are measured at fair value with unrealized gains and losses recognized in other comprehensive income except for losses in value that are considered other than temporary. Upon disposal of an available-for-sale financial asset, any accumulated other comprehensive income or loss at the time of disposal is recognized in profit or loss. The Company does not hold any financial assets that have been classified as available-for-sale.
Transaction costs associated with fair value through profit or loss financial assets are expensed as incurred, while transaction costs associated with all other financial assets are included in the initial carrying amount of the asset.
The Company assesses, at each reporting date, whether there is objective evidence that a financial asset or a group of financial assets is impaired. A financial asset or group of financial assets is deemed to be impaired if, and only if, there is objective evidence of impairment as a result of one or more events that has occurred after the initial recognition of the asset and that event has an impact on the estimated future cash flows of the financial asset or group of financial assets.
(ii) Financial liabilities
Financial liabilities classified as other financial liabilities are initially recognized at fair value less directly attributable transaction costs. After initial recognition, other financial liabilities are subsequently measured at amortized cost using the effective interest rate method. The effective interest rate method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that discounts estimated future cash payments through the expected life of the financial liability, or, where appropriate, a shorter period. The Company's accounts payable and accrued liabilities, construction holdback payable and amounts due to related parties are classified as other financial liabilities.
(b) Cash and cash equivalents
Cash and cash equivalents include cash on hand, bank deposits and short-term, highly liquid investments that are readily convertible to known amounts of cash and/or with original maturities of three months or less.
67
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont'd...)
(c) Inventories
Inventories include finished goods and supplies in respect of hemp-based nutrition for pets. The classification of inventories is determined by the stage in the manufacturing process. Finished goods inventories are valued based on the lower of actual production costs incurred or estimated net realizable value. Production costs include all direct manufacturing costs, freight, labour and other costs to deliver inventory to our distribution locations. Cost is determined using the weighted average cost basis. Supplies are valued at the lower of average cost or net realizable value. If carrying value exceeds the net realizable amount, a write-down is recognized. The write-down may be reversed in a subsequent period if the circumstances which caused it no longer exist.
(d) Construction in progress
Costs incurred toward the construction of the Company's cannabis cultivation facility have been deferred and capitalized until the facility is considered substantially complete and ready for use.
(e) Capital assets
Capital assets are carried at cost, less accumulated depreciation and accumulated impairment losses. Depreciation is recognized using the straight-line method at the following rates:
Office equipment and furniture- 5 years
Leasehold improvements- 5 years
The Company's capital assets are reviewed for an indication of impairment at the end of each reporting period. If an indication of impairment exists, the asset's recoverable amount is estimated. Impairment losses are recognized in profit or loss. An impairment loss is reversed if there is an indication that there has been a change in the estimates used to determine the recoverable amount. An impairment loss is reversed only to the extent that the asset's carrying amount does not exceed the carrying amount that would have been determined, net of depreciation, if no impairment loss had been recognized.
(f) Intangible assets
The Company owns intangible assets consisting of various direct costs associated with the acquisition of trademarks and intellectual property, as well as website costs. Intangible assets are measured on initial recognition at cost. Following initial recognition, intangible assets are carried at cost less any accumulated amortization and any accumulated impairment losses. Subsequent expenditures are capitalized only when they increase the future economic benefits embodied in the specific asset to which they relate. All other expenditures are recognized in profit or loss as incurred. The Company does not hold any intangible assets with indefinite lives.
Amortization is recognized using the straight-line method at the following rates:
Trademarks and related costs- 5-10 years
Website costs- 3 years
(g) Provisions
Provisions are recorded when a present legal, statutory or constructive obligation exists as a result of past events where it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation, and a reliable estimate of the amount of the obligation can be made. The amount recognized as a provision is the best estimate of the consideration required to settle the present obligation at the statement of financial position date, taking into account the risks and uncertainties surrounding the obligation. Where a
68
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont'd...)
(g) Provisions (continued)
provision is measured using the cash flows estimated to settle the present obligation, if the effect is material, its carrying amount is the present value of those cash flows.
(h) Share capital
Common shares are classified as equity. Transaction costs directly attributable to the issue of common shares, warrants and stock options are recognized as a deduction from equity, net of any tax effects. Common shares issued for consideration other than cash are valued based on their market value at the date the shares are issued.
The Company has adopted a residual value method with respect to the measurement of shares and warrants issued as private placement units. The residual value method first allocates value to the more easily measurable component based on fair value and then the residual value, if any, to the less easily measurable component.
The Company considers the fair value of common shares issued in the private placements to be the more easily measurable component and the common shares are valued at their estimated fair value. The balance, if any, is allocated to the attached warrants. Any fair value attributed to the warrants is recorded as reserves.
(i) Share-based payments
Options granted to employees and others providing similar services are measured at grant date at the fair value of the instruments issued. Fair value is determined using the Black-Scholes option pricing model taking into account the terms and conditions upon which the options were granted. The amount recognized as an expense is adjusted to reflect the actual number of options that are expected to vest. Each tranche in an award with graded vesting is considered a separate grant with a different vesting date and fair value. Each grant is accounted for on that basis.
Options granted to non-employees are measured at the fair value of the goods or services received, unless that fair value cannot be estimated reliably, in which case the fair value of the equity instruments issued is used. The value of the goods or services is recorded at the earlier of the vesting date, or the date the goods or services are received.
On vesting, share-based payments are recorded as an operating expense and as reserves. When options are exercised the consideration received is recorded as share capital. In addition, the related share-based payments originally recorded as reserves are transferred to share capital. When an option is cancelled or expires, the initial recorded value is reversed from reserves and charged against deficit.
(j) Earnings (loss) per share
The Company presents basic and diluted earnings (loss) per share (“EPS”) data for its common shares, calculated by dividing the profit or loss attributable to equity shareholders of the Company by the weighted average number of common shares issued and outstanding during the period. Diluted EPS is calculated by adjusting the profit or loss attributable to equity shareholders and the weighted average number of common shares outstanding for the effects of all potentially dilutive common shares. The calculation of diluted EPS assumes that the proceeds to be received on the exercise of dilutive stock options and warrants are used to repurchase common shares at the average market price during the period. For the periods presented, the calculation proved to be anti-dilutive as the Company was in a loss position.
69
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont'd...)
(k) Revenue recognition and related costs
Revenue is measured at the fair value of the consideration received or receivable. The Company recognizes revenue when the risks and rewards of ownership have been transferred to the buyer, the amount of revenue can be measured reliably and it is probable that future economic benefits will flow to the entity. For transactions initiated online and delivered directly to the customer, the Company recognizes revenue upon shipment of the order as the time between shipment and receipt is typically less than 5 days.
Revenue is reported net of sales returns and discounts granted to customers. Amounts related to shipping and handling that are billed to customers are recorded in net revenue.
The Company does not sell any of its current products on a consignment basis.
(l) Cost of sales
Cost of sales includes inventory, product-related costs and costs to ship products to customers.
(m) Selling and marketing
Selling and marketing expenses include costs attributable to the sale of pet products and include salaries, fees and commissions for the related staff. Marketing expenses also include costs associated with the True Leaf corporate brand.
(n) Income taxes
Income tax expense consists of current and deferred tax expense. Income tax expense is recognized in the statement of loss and comprehensive loss. Current tax expense is the expected tax payable on the taxable income for the period, using tax rates enacted or substantively enacted at period end, adjusted for amendments to tax payable with regards to previous periods.
Deferred tax assets and liabilities are recognized for deferred tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
Deferred tax assets and liabilities are measured using the enacted or substantively enacted tax rates expected to apply when the asset is realized or the liability settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that substantive enactment occurs. A deferred tax asset is recognized to the extent that it is probable that future taxable profits will be available against which the asset can be utilized. To the extent that the Company does not consider it probable that a deferred tax asset will be recovered, the deferred tax asset is reduced.
(o) Segmented information
An operating segment is a component of the company that engages in business activities from which it may earn revenues and incur expenses, including revenues and expenses that relate to transactions with any of the Company's other components. Operating segment results are reviewed regularly by the Company's President and Chief Executive Officer (“CEO”) to make decisions about resources to be allocated to the segment and assess performance, for which discrete financial information is available.
70
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont'd...)
(p) Comparative figures
Certain comparative figures have been restated to conform to the current periods' presentation.
(q) New standards not yet adopted
During the year ended March 31, 2018, there were no new IFRS or IAS accounting standards that became effective that had a material impact on the Company's consolidated financial statements. There are however a number of new standards and amendments to existing standards effective in future periods.
The following may impact the reporting and disclosures of the Company:
- New standard IFRS 9 “Financial Instruments” - This new standard will replace IAS 39 Financial Instruments: Recognition and Measurement in its entirety. IFRS 9 is intended to reduce the complexity in the classification and measurement of financial instruments. IFRS 9 is effective for periods beginning on or after January 1, 2018. The Company has reviewed the implications of the adoption of IFRS 9 and the final standard will not have a material impact on the Company's consolidated financial statements other than increased levels of note disclosure.
- New standard IFRS 15 “Revenues from contracts with Customers” - This new standard establishes principles for reporting the nature, amount, timing, and uncertainty of revenue and cash flows arising from an entity's contracts with customers. It provides a single model in order to depict the transfer of promised goods or services to customers. IFRS 15 supersedes IAS 11, Construction Contracts, IAS 18, Revenue, IFRIC 13, Customer Loyalty Programs, IFRIC 15, Agreements for the Construction of Real Estate, IFRIC 18, Transfers of Assets from Customers, and SIC-31, Revenue- Barter Transactions involving Advertising Service. IFRS 15 establishes a single five-step model framework for determining the nature, amount, timing and uncertainty of revenue and cash flows arising from a contract with a customer. The standard is effective for annual periods beginning on or after January 1, 2018, with early adoption permitted. The Company has reviewed the implications of the adoption of IFRS 15 and concluded the final standard will not have a material impact on the Company's consolidated financial statements.
- IFRS 16 Leases was issued in January 2016 and is effective for periods beginning on or after January 1, 2019. The new standard eliminates the classification of leases as either operating or finance leases. It provides a single lessee accounting model, requiring lessees to recognize assets and liabilities for all leases unless the lease term is 12 months or less or the underlying asset has a low value. Management is currently reviewing the impact that adoption of the new standard will have on the Company's consolidated financial statements.
- USE OF ESTIMATES
The preparation of these consolidated financial statements requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the period. These estimates are, by their nature, uncertain. The impacts of such estimates are pervasive throughout the consolidated financial statements, and may require accounting adjustments based on future occurrences. Revisions to accounting estimates are recognized in the period in which the estimate is revised and future periods if the revision affects both current and future periods. These estimates are based on current and future economic conditions and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
71
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- USE OF ESTIMATES (cont'd…)
(a) Estimates
Critical accounting estimates are estimates and assumptions made by management that may result in a material adjustment to the carrying amount of assets and liabilities within the next financial year and include, but are not limited to, the following:
(i) Share-based payments and compensation
The Company has applied estimates with respect to the valuation of shares issued for non-cash consideration and shares determined to have been issued at a discount. Shares are valued at the fair value of the equity instruments granted at the date the Company receives the goods or services.
The Company measures the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. Estimating fair value for share- based payment transactions requires determining the most appropriate valuation model, which is dependent on the terms and conditions of the grant.
(ii) Income taxes
The determination of income tax is inherently complex and requires making certain estimates and assumptions about future events. While income tax filings are subject to audits and reassessments, the Company has adequately provided for all income tax obligations. However, changes in facts and circumstances as a result of income tax audits, reassessments, jurisprudence and any new legislation may result in an increase or decrease in the Company's provision for income taxes.
(iii) Accounting for the business combination
The fair value of assets acquired and the resulting bargain purchase option required that management make estimates based on the information provided by the acquiree. Changes to the provisional values of assets acquired, including the associated deferred income taxes and resulting bargain purchase option, may be retrospectively adjusted when the final measurements are determined (within one year of acquisition date). The determination of fair value as of the acquisition date requires management to make certain estimates about future events, including, but not restricted to, profitability of assets acquired, useful lives and discount rates.
(iv) Amortization rates for intangible assets
Amortization expenses are calculated based on assumed intangible asset lives. Should the intangible asset life or amortization rates differ from the initial estimate, an adjustment would be made in the consolidated statement of loss and comprehensive loss.
72
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- USE OF ESTIMATES (cont'd…)
(b) Critical judgements
Information about critical judgments in applying accounting policies that have the most significant effect on the amounts recognized in the consolidated financial statements include, but are not limited to, the following:
(i) Functional currency
The functional currency of each of the Company's subsidiaries is the currency of the primary economic environment in which the entity operates. Determination of the functional currency may involve certain judgments to determine the primary economic environment and the Company reconsiders the functional currency of its entities if there is a change in events and conditions which determined the primary economic environment.
(ii) Business combination
Determination of whether the set of assets acquired constitute a business required the Company to make certain judgments, taking into account all facts and circumstances. A business is presumed to be an integrated set of activities and assets capable of being conducted and managed for the purpose of providing a return in the form of dividends, lower costs or economic benefits. The acquisition of the Oregapet assets (Note 5) was determined to constitute a business acquisition.
(iii) Financial instruments
Financial assets and liabilities are designated upon inception to various classifications. The designation determines the method by which the financial instruments are carried on the consolidated statements of financial position subsequent to inception and how changes in value are recorded. The designation may require the Company to make certain judgments, taking into account management's intention of the use of the financial instruments.
- ACQUISITION OF OREGAPET ASSETS
On December 22, 2016 the Company entered into an Asset Purchase Agreement (the “Agreement”) with T.L.M. Developments Ltd., a private British Columbia company (“TLM”), to purchase certain assets which make up the OregaPet pet product line (“OregaPet”). These assets consisted of trademarks, formulas, inventory and a customer list. No physical facilities, employees, market distribution systems or sales force were acquired. The Company acquired OregaPet with the intent to rebrand certain products under its TL Pet branding.
On November 3, 2016 the Company made a non-refundable deposit of $1,000 upon signing the binding term sheet and a further non-refundable deposit on December 9, 2016, of $99,000 to secure the Agreement. The Company also issued 476,190 common shares with a fair value of $100,000 and issued a Promissory Note (the “Note”) (Note 12) with a face value of $139,283. The Note was repayable in equal monthly instalments over a term of three years commencing January 31, 2017 and did not bear interest. However, in the case of a breach of the repayment terms for a period of more than 30 days, the entire balance of the Note was payable immediately with interest payable at a rate of 12% on the unpaid balance from the date of such non-payment.
73
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- ACQUISITION OF OREGAPET ASSETS (cont'd...)
The acquisition of OregaPet was considered a business combination and was accounted for using the acquisition method. The excess of the aggregate fair value of net assets acquired over the consideration paid is considered a gain on bargain purchase and was recognized in the consolidated statement of loss and comprehensive loss.
The purchase price allocation based on the fair value of OregaPet's assets acquired and liabilities assumed is summarized as follows:
| | December 31, 2016 |
Purchase Price: | | |
Cash | $ | 100,000 |
Common shares | | 100,000 |
Promissory Note- current | | 43,870 |
Promissory Note- long term | | 74,142 |
Total purchase price | $ | 318,012 |
Purchase Price Allocation: | | |
Intangible assets | $ | 55,500 |
Inventories | | 267,850 |
Net assets acquired | | 323,350 |
Gain on bargain purchase | $ | 5,338 |
- RECEIVABLES
| | March 31, 2018 | | March 31, 2017 |
Trade receivables (a) | $ | 202,683 | $ | 62,098 |
Miscellaneous receivable (b) | | 119,770 | | - |
Goods and services taxes receivable | | 63,218 | | 4,081 |
| $ | 385,671 | $ | 66,179 |
(a) Trade receivables
Trade receivables are non-interest bearing and are due within 30 days. As at March 31, 2018, the Company did not have any trade receivables that were past due.
For the year ended March 31, 2018, 39% of revenue was earned from three customers (March 31, 2017 - two customers 27%). As at March 31, 2018, these three customers amounted to 29% (March 31, 2017 - two customers amounted to 16%) of total trade receivables.
(b) Miscellaneous receivables
At March 31, 2018 a director was indebted to the Company for an amount of $119,770. The Company has remitted withholding tax on behalf of the director in connection with his exercise of stock options in January 2018. The balance is non-interest bearing and payable in full by March 15, 2019. A share purchase agreement has been put in place as security against the amount due.
74
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- INVENTORIES
| | March 31, 2018 | | March 31, 2017 |
Finished goods | $ | 432,729 | $ | 92,207 |
Supplies | | 137,865 | | 300,701 |
| $ | 570,594 | $ | 392,908 |
During the year ended March 31, 2018, the Company wrote off $217,436 (2017 - $36,000) associated with supplies and packaging materials that will not be used for current product lines, as well as some finished goods no longer saleable.
- MARKETABLE SECURITIES
In connection with a Plan of Arrangement entered into in June 2014, the Company received 5,000 common shares of Noor Energy Corporation (“Noor”) valued at $0.01 per share. During the year ended March 31, 2018 the shares in Noor were written down to $Nil.
- CONSTRUCTION IN PROGRESS
During the year ended March 31, 2018 the Company acquired a property of 40 acres located in Lumby B.C. for total consideration of $3,380,387 to build its cannabis cultivation facility. As at March 31, 2018, construction costs incurred of $726,955 are capitalized and depreciation will commence when the facility is put into use.
As at March 31, 2018, prepaid expenses and deposits includes a deposit of $25,827, refundable upon completion of the construction project, subject to approval by the Village of Lumby that the Company has complied with conditions set out in its landscaping permit. As at March 31, 2018, the Company has accrued a liability of $98,661 as holdbacks against construction in progress.
75
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- CAPITAL ASSETS
Cost: | | Office furniture | | Office equipment | | Leasehold improvements | | Total |
Balance, March 31, 2016 | $ | - | $ | 2,105 | $ | - | $ | 2,105 |
Additions | | - | | 5,537 | | 3,694 | | 9,231 |
Balance, March 31, 2017 | | - | | 7,642 | | 3,694 | | 11,336 |
Additions | | 20,165 | | 42,074 | | 76,235 | | 138,474 |
Balance, March 31, 2018 | $ | 20,165 | $ | 49,716 | $ | 79,929 | $ | 149,810 |
Accumulated depreciation: | | | | | | |
Balance, March 31, 2016 | $ | - | $ | 535 | $ | - | $ | 535 |
Depreciation for the year | | - | | 1,251 | | 739 | | 1,990 |
Balance, March 31, 2017 | | - | | 1,786 | | 739 | | 2,525 |
Depreciation for the year | | 2,150 | | 4,353 | | 8,362 | | 14,865 |
Balance, March 31, 2018 | $ | 2,150 | | 6,139 | $ | 9,101 | $ | 17,390 |
Carrying value | | | | | | | | |
As at March 31, 2017 | $ | - | $ | 5,856 | $ | 2,955 | $ | 8,811 |
As at March 31,2018 | $ | 18,015 | $ | 43,577 | $ | 70,828 | $ | 132,420 |
| | | | | | | | |
- INTANGIBLE ASSETS
Cost: | | Website | | Trademarks and related costs | | Intellectual property | | Total |
Balance - March 31, 2016 | $ | 10,801 | $ | 35,008 | $ | - | $ | 45,809 |
Additions | | - | | 45,268 | | 55,500 | | 100,768 |
Balance - March 31, 2017 | | 10,801 | | 80,276 | | 55,500 | | 146,577 |
Additions | | - | | 37,124 | | - | | 37,124 |
Balance- March 31, 2018 | $ | 10,801 | $ | 117,400 | $ | 55,500 | $ | 183,701 |
Accumulated amortization: | | | | | | | |
Balance - March 31, 2016 | $ | 3,655 | $ | - | $ | - | $ | 3,655 |
Amortization for the year | | 3,601 | | 5,591 | | 5,550 | | 14,742 |
Balance - March 31, 2017 | | 7,256 | | 5,591 | | 5,550 | | 18,397 |
Amortization for the year | | 2,700 | | 11,589 | | 8,325 | | 22,614 |
Balance- March 31, 2018 | $ | 9,956 | $ | 17,180 | $ | 13,875 | $ | 41,011 |
Carrying value | | | | | | | | |
As at March 31, 2017 | $ | 3,545 | $ | 74,685 | $ | 49,950 | $ | 128,180 |
As at March 31, 2018 | $ | 845 | $ | 100,220 | $ | 41,625 | $ | 142,690 |
- PROMISSORY NOTE
In March 2018, the Company repaid all outstanding obligations under a Promissory Note entered into on January 31, 2017 (Note 5), initially recorded at its present value of $118,012. The Company recorded $18,079 (2017 - $3,192) in accretion expense prior to repayment of the Note during the year ended March 31, 2018.
76
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- RELATED PARTY BALANCES AND TRANSACTIONS
(a) Goods and services
The Company had the following transactions with related parties during the year ended March 31, 2018:
- Paid or accrued a total of $30,000 (2017 - $30,000) to its Chief Executive Officer for rent. Subsequent to March 31, 2018, negotiations were in progress to increase the rent to a market-based rate;
- Paid or accrued $92,266 (2017 - $14,271) to a company controlled by its Chief Executive Officer for costs associated with supplies inventory;
- Paid or accrued $21,000 (2017- $nil) to a company controlled by a Director for services provided; and
- Paid or accrued amounts to Directors, including the CEO, totalling $747,496 (2017 - $227,424) recorded as director fees, salaries and share-based compensation.
(b) Compensation of key management personnel
The Company considers its key management personnel to be its Chief Executive Officer and its Chief Financial Officer. The comprehensive loss for the year ended March 31, 2018 includes $286,409 (2017 - $146,462) paid to key management personnel as salaries, management fees, consulting fees and amounts recorded as share-based compensation.
(c) Amount due from a director of $119,770 included in receivables at March 31, 2018 (2017 - $nil) (Note 6).
(d) Amounts payable to related parties as at March 31, 2018 of $23,314 (2017 - $23,771) are unsecured, non-interest bearing with no scheduled terms of repayment.
(e) Share-based compensation
On February 6, 2018, the Company granted a total of 1,900,000 stock options, 800,000 of which were to directors and officers of the Company, having an aggregate fair value of $549,707, of which $363,036 is included in operating expenditures for the year ended March 31, 2018.
On May 29, 2017 the Company granted a total of 3,900,000 stock options, 1,400,000 of which were to directors and officers of the Company, having a fair value of $284,675.
On December 12, 2016, the Company granted a total of 2,799,995 stock options, 1,642,875 of which were to directors and officers of the Company having a fair value of $156,025.
- SHARE CAPITAL
Authorized:
Unlimited Common voting shares with no par value
Unlimited Preferred non-voting shares with no par value
Issued:
The Company had the following share capital transactions during the year ended March 31, 2017:
77
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SHARE CAPITAL (cont'd…)
- The Company issued 2,813,163 common shares pursuant to the exercise of share purchase warrants for gross proceeds of $529,475.
- On May 11, 2016 the Company completed a private placement of 7,028,404 common shares at a price of $0.105 per share for gross proceeds of $737,982. The Company incurred share issue costs of $13,292 in association with the financing.
- On May 11, 2016 the Company issued 2,229,843 common shares at a value of $0.105 per share pursuant to debt settlement agreements with various vendors. The Company settled aggregate debt totaling $234,134 through issuance of these shares.
- On May 11, 2016 the Company issued 601,843 common shares and 300,921 share purchase warrants pursuant to the conversion of debt and interest (see below) owing on a loan from First Pacific Enterprises Inc. (“First Pacific”).
- On November 11, 2016 the Company completed a private placement of 1,984,048 common shares at a price of $0.21 per share for gross proceeds of $416,650.
- On November 17, 2016 the Company issued 231,942 common shares at a value of $0.24 per share pursuant to debt settlement agreements with various vendors. The Company settled aggregate debt totaling $48,708 through the issuance of these shares, incurred share issue costs of $1,744 in association with the transaction and recognized a loss on debt settlement in the amount of $6,958.
- On December 30, 2016 the Company issued 476,190 common shares to satisfy $100,000 of the OregaPet purchase price at a deemed value of $0.21 per share (Note 5).
- The Company issued 3,090,000 common shares pursuant to the exercise of stock options for gross proceeds of $314,000.
78
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SHARE CAPITAL (cont'd…)
The Company had the following share capital transactions during the year ended March 31, 2018:
- On May 29, 2017, the Company completed a private placement by issuing 3,099,829 units at a price of $0.30 per unit for gross proceeds of $929,948. Each unit is comprised of one common share and one share purchase warrant. Each warrant is exercisable into one additional common share at a price of $0.45 per share for a period of two years. No value was assigned to the warrants issued as part of the unit offering. The Company incurred $12,474 in share issue costs associated with this financing.
- On June 12, 2017, the Company completed a private placement by issuing 4,641,816 units at a price of $0.30 per unit for gross proceeds of $1,392,545. Each unit is comprised of one common share and one share purchase warrant. Each warrant is exercisable into one additional common share at a price of $0.45 per share for a period of two years. No value was assigned to the warrants issued as part of the unit offering. The Company incurred $20,447 in share issue costs associated with this financing.
- The Company issued 3,342,580 common shares pursuant to the exercise of stock options for gross proceeds of $843,645.
- The Company issued 3,707,000 common shares pursuant to the exercise of share purchase warrants for gross proceeds of $1,246,472.
- In January 2018 the Company completed a Regulation A public offering (the “Offering”) raising $10,000,000 in gross proceeds. The Company closed its Offering of common shares, qualified by the U.S. Securities and Exchange Commission, with non-Canadian investors on January 22, 2018, consisting of 14,285,715 common shares at a purchase price of $0.70 per share.
Boustead Securities LLC (“Boustead”), a FINRA registered broker dealer, was the lead underwriter outside Canada. The Company paid Boustead a commission of $800,000 representing 8% of the gross proceeds of the aggregate Offering amount and issued 857,143 agent's warrants representing 6% of the aggregate number of the securities sold in the Offering. Each agent warrant is exercisable into one common share of the Company at an exercise price of $1.05, expiring November 21, 2020. The warrants were valued at $789,767 ($0.92 per warrant) using the Black-Scholes option pricing model with the following assumptions: term of 2.8 years, historical volatility of 96.66%, risk-free rate of 1.76% and expected dividends of $nil.
The Company incurred $645,689 in share issue costs associated with this financing, including an advisory fee paid to Boustead.
- On the same terms as the Offering, the Company closed a concurrent Canadian private placement on January 24, 2018 of 5,788,078 common shares raising an aggregate total of $4,051,655. The Canadian offering was non-brokered and no commissions or fees were paid in connection with the shares issued. An additional 76,658 common shares, valued at $53,661, were issued in connection with the Canadian offering to compensate certain Canadian investors for foreign currency transaction costs incurred as a consequence of the Company collecting a portion of subscription receipts in U.S. dollar funds for a Canadian dollar-denominated share offering. The Company incurred $83,856 in share issue costs associated with this financing.
79
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SHARE CAPITAL (cont'd…)
Convertible debt
On October 30, 2015, the Company entered into a convertible debt agreement with First Pacific, pursuant to which First Pacific loaned the Company a total of $60,000. First Pacific is a company controlled by the Company's Chief Executive Officer. First Pacific had the right to convert all or any portion of the loan and interest into units of the Company at a conversion price of $0.105 per unit. Each unit would consist of one common share of the Company and one half of one share purchase warrant, with each full warrant exercisable into one additional common share at a price of $0.15 for a period of two years. The loan was converted during the year ended March 31, 2017 which resulted in the issuance of 601,842 common shares and 300,921 share purchase warrants.
Share purchase warrants
Share purchase warrant transactions are summarized as follows:
| | Number of Warrants | | Weighted Average Exercise Price |
Balance, March 31, 2016 | 10,215,304 | $ | 0.19 |
| | | | |
| Warrants expired | (5,886,664) | | 0.21 |
| Warrants exercised | (2,813,163) | | 0.19 |
| Warrants issued | 300,921 | | 0.15 |
Balance, March 31, 2017 | 1,816,398 | | 0.15 |
| | | |
| Warrants expired | (410,806) | | 0.15 |
| Warrants exercised | (3,707,000) | | 0.34 |
| Warrants issued | 8,598,788 | | 0.51 |
Balance, March 31, 2018 | 6,297,380 | $ | 0.53 |
As at March 31, 2018 the following share purchase warrants are outstanding and exercisable:
Number | Exercise | | |
of Warrants | Price ($) | | Expiry Date |
2,097,954 | 0.45 | | May 29, 2019 |
3,342,283 | 0.45 | | June 12, 2019 |
857,143 | 1.05 | | November 21, 2020 |
6,297,380 | | | |
80
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SHARE CAPITAL (cont'd…)
Stock options
The Company has a stock option plan (the “Plan”) in place under which it is authorized to grant options to directors, executive officers, employees and consultants enabling them to acquire up to 10% of the issued and outstanding common shares of the Company in any 12-month period. Under the Plan, the exercise price of each stock option is subject to a minimum of $0.10 and may not be less than the closing market price of Company's common the shares on the trading day immediately preceding the date of grant of the options. The options can be granted for a maximum term of five years and vest at the discretion of the board of directors.
Stock option transactions are summarized as follows:
| | Number of Options | | Weighted Average Exercise Price |
Balance March 31, 2016 | 3,850,000 | $ | 0.10 |
| | | |
| Stock options exercised | (3,090,000) | | 0.10 |
| Stock options expired | (410,000) | | 0.10 |
| Stock options granted | 2,799,995 | | 0.19 |
Balance, March 31, 2017 | 3,149,995 | | 0.18 |
| | | | |
| Stock options exercised | (3,342,580) | | 0.25 |
| Stock options expired | (100,270) | | 0.12 |
| Stock options granted | 6,200,000 | | 0.57 |
Balance, March 31, 2018 | 5,907,145 | $ | 0.55 |
As at March 31, 2018 the following stock options are outstanding and exercisable:
Number of Options | | |
Outstanding | Exercisable | Exercise Price ($) | Expiry Date |
857,145 | 857,145 | 0.19 | December 12, 2018 |
2,750,000 | 2,750,000 | 0.40 | May 29, 2019 |
300,000 | 300,000 | 0.45 | July 18, 2019 |
100,000 | 100,000 | 0.94 | February 6, 2019 |
1,900,000 | 1,100,000 | 0.94 | February 6, 2023 |
5,907,145 | 5,107,145 | | |
On December 12, 2016, the Company granted a total of 2,799,995 stock options to directors, officers, employees and consultants that vested immediately. The stock options were valued at $265,917 ($0.095 per option) using the Black-Scholes option pricing model with the following assumptions: term of 2 years, historical volatility of 97.5%, risk-free rate of 0.76% and expected dividends of $nil.
On May 29, 2017, the Company granted a total of 3,900,000 stock options to directors, officers, employees and consultants that vested immediately. The stock options were valued at $793,020 ($0.203 per option) using the Black-Scholes option pricing model with the following assumptions: term of 2 years, historical volatility of 95.8%, risk-free rate of 0.71% and expected dividends of $nil.
81
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SHARE CAPITAL (cont'd…)
On July 18, 2017, the Company granted 300,000 stock options to an employee that vested immediately. The stock options were valued at $76,340 ($0.254 per option) using the Black-Scholes option pricing model with the following assumptions: term of 2 years, historical volatility of 94.76%, risk-free rate of 1.19% and expected dividends of $nil.
On February 6, 2018 the Company granted 100,000 stock options at an exercise price of $0.94 per option to a vendor which vested immediately. The stock options were valued at $34,870 ($0.349 per option) using the Black-Scholes option pricing model with the following assumptions: term of 1-year, historical volatility of 94.92%, risk-free rate of 1.90% and expected dividends of $nil.
On February 6, 2018 the Company granted 1,900,000 stock options at an exercise price of $0.94 per option to directors, officers, and consultants, of which 1,100,000 options vested immediately. The balance of 800,000 options vest evenly over two years, with 25% of the options vesting each six months by February 6, 2020. The stock options were valued at $1,305,554 in total ($0.687 per option), of which $932,211 is included in operating expenditures for the year ended March 31, 2018. The options were valued using the Black-Scholes option pricing model with the following assumptions: term of 5 years, historical volatility of 95.80%, risk-free rate of 2.17% and expected dividends of $nil.
- ADMINISTRATIVE AND OFFICE EXPENSE
| | For the year ended |
| | March 31, 2018 | | March 31, 2017 |
Consultants | $ | 362,851 | $ | 268,691 |
Filing fees | | 91,256 | | 10,655 |
Insurance | | 98,534 | | 16,456 |
Office | | 189,637 | | 72,901 |
Rent | | 51,600 | | 54,400 |
Transfer agent | | 30,467 | | 8,120 |
Travel and meals | | 207,070 | | 95,303 |
Wages and salaries | | 533,511 | | 237,072 |
| $ | 1,564,926 | $ | 763,498 |
82
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- FINANCIAL INSTRUMENTS, RISK AND CAPITAL MANAGEMENT
Fair Value
Financial instruments recorded at fair value on the consolidated statement of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The fair value hierarchy has the following levels:
(a) Level 1 - Unadjusted quoted prices in active markets for identical assets or liabilities;
(b) Level 2 - Inputs other than quoted prices that are observable for assets or liabilities, either directly or indirectly; and
(c) Level 3 - Inputs for assets and liabilities that are not based on observable market data.
The fair value hierarchy requires the use of observable market inputs whenever such inputs exist. A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value. The carrying value of accounts payable and accrued liabilities, construction holdback payable and due to related parties approximates their fair value because of the short-term nature of these instruments.
The fair values of cash and cash equivalents are measured based on level 1 inputs of the fair value hierarchy.
Risk
The Company is exposed to various risks through its financial instruments and has a risk management framework to monitor, evaluate and manage these risks. The following analysis provides information about the Company's risk exposure and concentration as of March 31, 2018:
Credit risk
Credit risk refers to the risk that another entity will default on its contractual obligations which will result in a loss for the Company. At March 31, 2018, the Company's exposure to credit risk consists of the carrying value of cash and cash equivalents, and receivables. The Company limits its credit exposure on cash by holding its deposits with established financial institutions. Accounts receivable consists of trade accounts receivable and miscellaneous receivables. The Company mitigates the risk of default of accounts receivable by assessing the credit worthiness of customers prior to sale and shipment of inventory.
Liquidity risk
Liquidity risk arises from our general and capital financing needs with respect to future growth and the Company's construction of its cannabis cultivation facility (Note 9). Liquidity risk could arise if the Company encounters difficulty in meeting future obligations with financial liabilities. As at March 31, 2018, the Company has cash and cash equivalents of $10,812,815 to settle current liabilities of $1,049,962. The Company has planning, budgeting and forecasting processes to help determine its funding requirements to meet various contractual and other obligations and to manage liquidity risk.
83
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- FINANCIAL INSTRUMENTS, RISK AND CAPITAL MANAGEMENT (cont'd…)
Currency risk
The operating results and financial position of the Company are reported in Canadian dollars. The Company is exposed to currency risk arising from the translation of its European subsidiary's operations and to currency transaction risk as some of the Company's financial instruments are denominated in U.S. dollars. The results of the Company's operations are subject to currency translation and transaction risks.
The Company's main risk is associated with fluctuations in Canadian and U.S. dollars and Euros. Assets and liabilities are translated based on the Company's foreign currency translation policy.
The Company has determined that, with other variables unchanged, the effect of a 10% increase in the Canadian dollar as at March 31, 2018:
- against the Euro on its net European operations, and
- against the U.S. dollar on financial assets and liabilities, including cash, accounts receivable, accounts payables and accrued liabilities denominated in U.S. dollars
would result in an increase of approximately $705,000 to the net loss and comprehensive loss for the year ended March 31, 2018 (March 31, 2017- reduction to net loss of $3,000). The inverse effect would result if the Canadian dollar weakened by 10% against the Euro and U.S. dollar.
At March 31, 2018, the Company had no hedging agreements in place with respect to foreign exchange rates as the Company's operations provide a natural hedge. Certain operational costs are denominated in U.S. dollars and funded directly from the Company's U.S. funds. The Company's European operation provides further natural hedging as U.S. dollars are used to periodically fund operations. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks at this time. Management monitors fluctuations in foreign currency rates to maximize conversion of U.S. dollars to Canadian dollars.
Interest rate risk
Interest rate risk is the risk that the value of a financial instrument might be adversely affected by a change in the interest rates. In seeking to minimize the risk from interest rate fluctuations, the Company manages exposure through its normal operating and financing activities. As at March 31, 2018, the Company does not have any liabilities that bear interest at rates fluctuating with the prime rate.
Capital Management
The Company's capital includes share capital and the accumulated deficit. The Company's objectives when managing capital are to safeguard the entity's ability to continue as a going concern, so that it can continue to provide returns for shareholders and benefits for other stakeholders. The Company manages the capital structure and adjusts it in the light of changes in economic conditions and the risk characteristics of the underlying assets. The Company may issue new shares in order to meet its financial obligations. The Company has not changed its approach to capital management during the year ended March 31, 2018.
84
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- COMMITMENTS
The Company has the following commitments as of March 31, 2018:
- On May 20, 2014, the Company entered into a contractual agreement with its Chief Financial Officer whereby the Company will pay $2,000 per month for accounting and financial reporting services rendered for an initial term of two years. At March 31, 2018, the Company is negotiating the terms of a new agreement with its Chief Financial Officer, and continues to pay $2,000 per month in line with the original agreement.
- On February 1, 2016, the Company (through TL Pet) entered into an agreement with Pet Horizons Ltd., UK (“Pet Horizons”) whereby Pet Horizons will develop strategic plans to launch the TL Pet products in Europe for an initial term ending June 2019. The sales territory includes the European Union, Switzerland and Norway, as well as central and eastern Europe including Russia, Ukraine and Belarus. At March 31, 2018 the Company is negotiating the terms of a new agreement with Pet Horizons and is paying an interim monthly fee of approximately $9,000 (EUR6,000).
- Effective December 1, 2017, the Company entered into an agreement with Edison Investment Research Inc. to provide the Company with investor relations services for US$8,000 per month. The agreement has a minimum initial one-year term ending December 1, 2018, cancellable with 90 days-notice by either party and payment of US$24,000 to cover the notice period.
- Effective December 15, 2017, the Company entered into an agreement with Paradigm Medical Inc., a company controlled by a Director, to provide strategic medical direction to the Company's cannabis operations for a fee of $6,000 per month. Subsequent to March 31, 2018, the fee was increased to $8,000 per month.
- Effective February 15, 2018 the Company entered into an agreement with a branding and market positioning expert to provide the Company with consulting services in connection with the Company's brand and marketing expenditures at a minimum cost of $15,000 per month. The agreement has an initial one-year term ending February 15, 2019 with two one-year renewable terms, cancellable with 60days-notice by either party and payment of the prorated portion of the fees due.
85
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SUPPLEMENTAL DISCLOSURE WITH RESPECT TO CASH FLOWS
The significant non-cash investing and financing activities during the years ended March 31, 2018 and 2017 consisted of the following:
| 2018 | 2017 |
Inventory costs included in accounts payable and accrued liabilities. | $48,554 | $7,010 |
Inventory costs included in due to related parties. | 16,531 | 14,271 |
Intangible asset costs included in accounts payable and accrued liabilities. | 1,258 | 11,319 |
Capital assets included in accounts payable and accrued liabilities | 2,818 | - |
Construction costs included in accounts payable and accrued liabilities | 172,700 | - |
Construction costs included in construction holdback payable | 98,661 | - |
Fair value relating to 100,270 (2017- 410,000) stock options expired recorded as a reduction from reserves with an offset to deficit | 7,517 | 24,939 |
Fair value relating to nil (2017 - 2,813,163) share purchase warrants exercised recorded as an increase to share capital and a reduction from reserves. | - | 25,200 |
Fair value relating to 3,292,580 (2017 - 3,090,000) stock options exercised was recorded as an increase to share capital and a reduction from reserves. | 439,770 | 188,771 |
Fair value relating to 857,143 (2017 - nil) share purchase warrants issued in connection with the Regulation A public offering recorded as a reduction to share capital and an increase to reserves. | 789,767 | - |
Fair value relating to 76,658 (2017- nil) shares issued as compensation for foreign exchange fluctuations in connection with the Canadian side car private placement. | 53,661 | - |
During the year ended March 31, 2017 there were the following additional significant non-cash investing and financing activities:
- The Company issued 2,461,785 common shares to settle debt totaling $289,900. Of this amount, 1,293,728 shares were issued to settle related party debt of $135,841.
- The Company issued 601,843 common shares and 300,921 share purchase warrants to settle convertible debt (Note 14) of $63,194 with a related company.
86
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- INCOME TAXES
A reconciliation of income taxes at statutory rates with the reported taxes is as follows:
| 2018 | 2017 |
| | |
Loss for the year | $ (3,967,936) | $ (1,743,050) |
| | |
Expected income tax recovery | $ (1,042,000) | $ (453,000) |
Change in statutory tax rates and other Permanent differences Share issue costs | (8,000) 478,000 (430,000) | 21,000 71,000 (3,000) |
Change in unrecognized deductible temporary differences | 1,002,000 | 364,000 |
| | |
Total income tax expense (recovery) | $ - | $ - |
The significant components of the Company's deferred tax assets that have not been included on the consolidated statement of financial position are as follows:
| 2018 | 2017 |
Deferred tax assets: | | |
Share issue costs | $ 349,000 | $ 8,000 |
Capital assets | 5,000 | 2,000 |
Intangible assets | 5,000 | 1,000 |
Non-capital losses available for future periods | 1,447,000 | 792,000 |
| | |
| 1,806,000 | 803,000 |
Unrecognized deferred tax assets | (1,806,000) | (803,000) |
| | |
Net deferred tax assets | $ - | $ - |
The significant components of the Company's temporary differences, unused tax credits and unused tax losses that have not been included on the consolidated statement of financial position are as follows:
| 2018 | Expiry Date Range | 2017 | Expiry Date Range |
Temporary differences: | | | | |
Share issue costs | $ 1,293,000 | 2039 to 2042 | $ 31,000 | 2038 to 2041 |
Capital assets | $ 17,500 | No expiry date | $ 10,000 | No expiry date |
Intangible assets | $ 17,500 | No expiry date | $ 4,000 | No expiry date |
| | | | |
Non-capital losses available for future periods | | | | |
Canadian | $ 5,177,000 | 2034 to 2038 | $ 2,999,000 | 2032 to 2037 |
Luxembourg | 329,000 | 2034 to 2035 | 79,000 | 2034 |
| $ 5,506,000 | | $ 3,078,000 | |
Tax attributes are subject to review, and potential adjustment, by tax authorities.
87
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SEGMENTED INFORMATION
Operating segmented information
As at March 31, 2018, the Company had three reportable segments, being the sale of hemp-based nutrition for pets (Pet), the planned sale of medical marijuana under Canada's ACMPR program (Medicine) and Corporate. The Corporate segment is responsible for treasury management, regulatory reporting, financial and legal administration and general corporate activities conducted in Canada, Europe and U.S. The Company has identified these reporting segments based on the internal reports reviewed and used by the Chief Executive Officer, its chief decision maker, in allocating resources and assessing performance. Operations whose revenue, earnings, losses or assets exceed 10% of the total consolidated revenue, earnings, losses or assets are reportable segments.
Operating segmented information is presented as follows:
| | | | |
As at March 31, 2018 | Pet | Medicine | Corporate | Total |
Current assets | $1,054,069 | $211,549 | $10,652,661 | $11,918,279 |
Non-current assets | 229,085 | 4,153,367 | - | 4,382,452 |
Total assets | 1,283,154 | 4,364,916 | 10,652,661 | 16,300,731 |
Liabilities | (227,118) | (488,808) | (334,036) | (1,049,962) |
Net assets | $1,056,036 | $3,876,108 | $10,318,625 | $15,250,769 |
| | | | |
For the year ended
March 31, 2018 | | | | |
Revenues | $1,400,511 | - | - | $1,400,511 |
Gross profit | 621,329 | - | - | 621,329 |
Operating expenses | (1,504,877) | (206,664) | (3,098,313) | (4,809,855) |
Loss from operations | $(883,548) | $(206,664) | $(3,098,313) | $(4,188,526) |
As at and for the year ended March 31, 2017, the Company had one operating segment, being the sale of hemp-based nutrition for pets in North America and Europe, which accounted for all of the Company's revenues from inception to date. Therefore, comparative information at 2017 is not presented.
88
TRUE LEAF MEDICINE INTERNATIONAL LTD. |
Notes to Consolidated Financial Statements |
| March 31, 2018 |
(Expressed in Canadian dollars) |
- SEGMENTED INFORMATION (cont'd…)
Geographic segmented information
The Company operates in two main geographic locations, North America and Europe, selling hemp-based nutrition for pets in North America and Europe, which has accounted for all of the Company's revenues since its inception.
As at March 31, 2018 | | North America | | Europe | | Total |
Current assets | $ | 11,753,046 | $ | 165,233 | $ | 11,918,279 |
Non-current assets | | 4,374,765 | | 7,687 | | 4,382,452 |
Liabilities | | (987,060) | | (62,902) | | (1,049,962) |
Total net assets | $ | 15,140,751 | $ | 110,018 | $ | 15,250,769 |
As at March 31, 2017 | | North America | | Europe | | Total |
Current assets | $ | 600,078 | $ | 34,504 | $ | 634,582 |
Non-current assets | | 137,041 | | - | | 137,041 |
Liabilities | | (325,733) | | (19,087) | | (344,820) |
Total net assets | $ | 411,386 | $ | 15,417 | $ | 426,803 |
89
Index to Exhibits
- Filed on February 17, 2017 with Form 1-A Offering Statement
- Filed on May 16, 2017 with Amendment No. 1 - Form 1-A Offering Statement
- Filed on July 10, 2017 with Amendment No. 2- Form 1-A Offering Statement
- Filed on August 25, 2017 with Amendment No. 3- Form 1-A Offering Statement
- Filed on October 26, 2017 with Amendment No. 4- Form 1-A Offering Statement
90
Pursuant to the requirements of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
True Leaf Medicine International Ltd. | |
| | | |
| | | |
By: | /s/ Darcy Bomford | |
| | Darcy Bomford | |
| | Chief Executive Officer | |
Pursuant to the requirements of Regulation A, this report has been signed below by the following persons on behalf of the issuer and in the capacities and on the dates indicated.
| | | |
| | /s/ Darcy Bomford | |
By: | | |
| | Darcy Bomford | |
| | Chief Executive Officer and Director | |
Date: | December 17, 2018 | |
| | | |
| | /s/ Kerry Biggs | |
By: | | |
| | Kerry Biggs | |
| | Chief Financial Officer, Principal Accounting Officer | |
Date: | December 17, 2018 | |
| | |
| | | |
| | /s/ Kevin Bottomley | |
By: | | |
| | Kevin Bottomley | |
| | Director | |
Date: | December 17, 2018 | |
| | |
| | | |
| | /s/ Sylvain Toutant | |
By: | | |
| | Sylvain Toutant | |
| | Director | |
Date: | December 17, 2018 | |
| | | |
| | /s/ Michael Harcourt | |
By: | | |
| | Michael Harcourt | |
| | Director | |
Date: | December 17, 2018 | |
END
91








 Darcy Bomford, Chief Executive Officer and President
Darcy Bomford, Chief Executive Officer and President Michael Harcourt, Chairman and Director
Michael Harcourt, Chairman and Director Kevin Bottomley, Corporate Communications and Director
Kevin Bottomley, Corporate Communications and Director Sylvain Toutant, Director
Sylvain Toutant, Director Kerry Biggs, Chief Financial Officer
Kerry Biggs, Chief Financial Officer Chris Spooner, Former Director
Chris Spooner, Former Director Chuck Austin, Former Chief Financial Officer
Chuck Austin, Former Chief Financial Officer