Exhibit 99.1 Progress in CNS Disorders

SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations, business strategy, current and prospective product candidates, results of ongoing clinical trials, planned clinical trials and preclinical activities, product approvals, timing and likelihood of success, plans and objectives of management for future operations, future results of anticipated product candidates, current or planned collaborations, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risks and uncertainties may emerge from time to time, and it is not possible for management to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. 2

Management On Today’s Call David Giljohann, PhD David Snyder, MBA Matthias Schroff, PhD Chief Executive Officer Chief Financial Officer Chief Operating Officer 3
Introduction to Exicure • Clinical-stage biotechnology company developing nucleic acid therapeutics based on our proprietary Spherical Nucleic Acid, or SNA, technology • SNA technology is intended to provided differentiated therapeutic benefits: better uptake, greater stability ➢ Demonstrated local topical delivery in CNS, eye, GI tract, liver, lung, and skin ➢ Architecture protects against degradation of nucleic acid ➢ Applicable for antisense, siRNA, miRNA, TLR9 modulators, splice-switching oligonucleotides • Ongoing clinical progress ➢ AST-008 Phase1b/2 preliminary results in cancer patients in late 2019 ➢ Nominate neurology therapeutic candidate in late 2019 ➢ In partnership with Dermelix, pursuing therapeutic candidate for Netherton syndrome • Platform showing preclinical promise ➢ Ophthalmology, gastroenterology, pulmonology 4
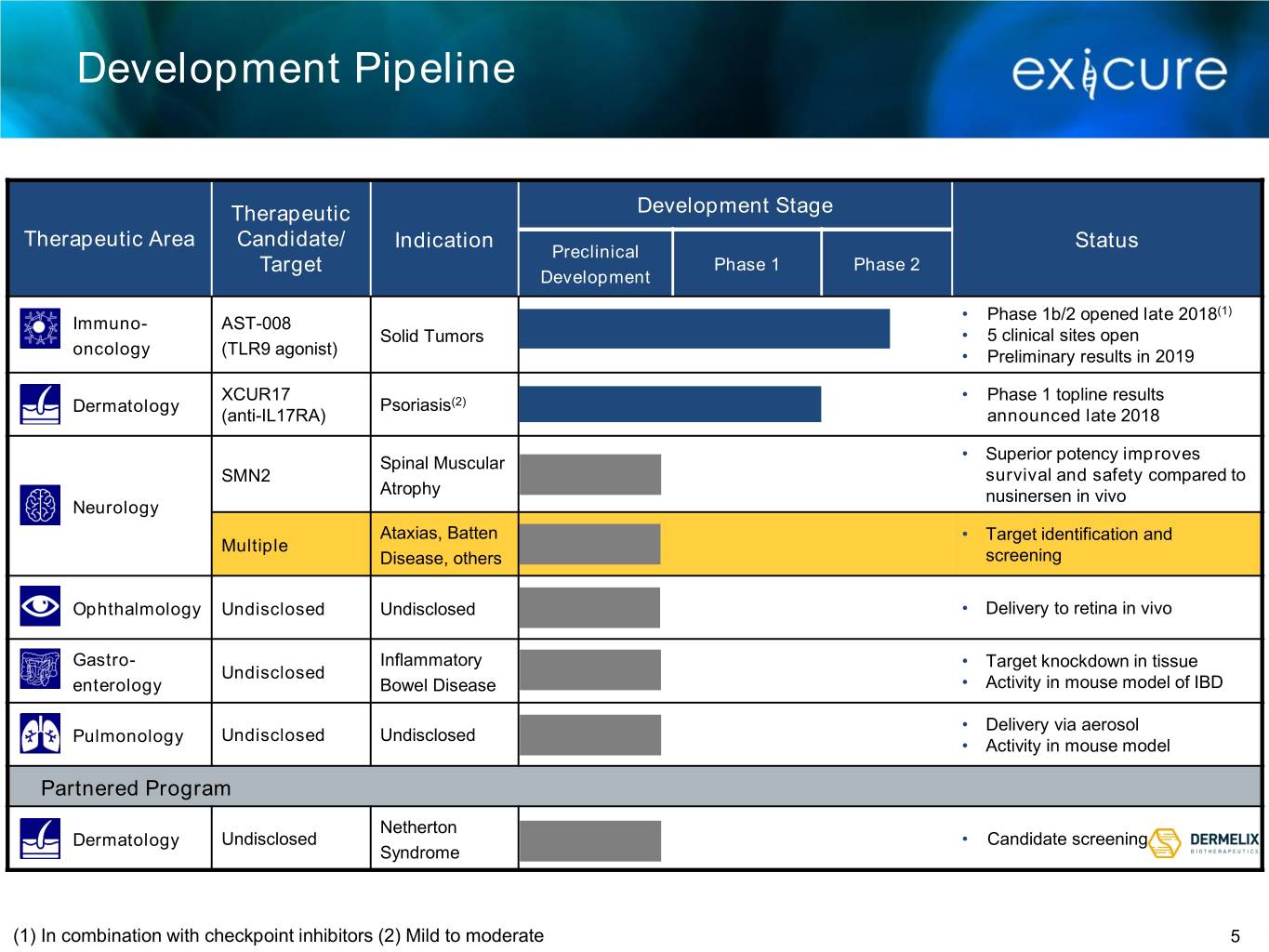
Development Pipeline Therapeutic Development Stage Therapeutic Area Candidate/ Indication Status Preclinical Target Phase 1 Phase 2 Development • Phase 1b/2 opened late 2018(1) Immuno- AST-008 Solid Tumors • 5 clinical sites open oncology (TLR9 agonist) • Preliminary results in 2019 XCUR17 • Phase 1 topline results Dermatology Psoriasis(2) (anti-IL17RA) announced late 2018 • Superior potency improves Spinal Muscular SMN2 survival and safety compared to Atrophy nusinersen in vivo Neurology Ataxias, Batten • Target identification and Multiple Disease, others screening Ophthalmology Undisclosed Undisclosed • Delivery to retina in vivo Gastro- Inflammatory • Target knockdown in tissue Undisclosed enterology Bowel Disease • Activity in mouse model of IBD • Delivery via aerosol Pulmonology Undisclosed Undisclosed • Activity in mouse model Partnered Program Netherton Dermatology Undisclosed • Candidate screening Syndrome (1) In combination with checkpoint inhibitors (2) Mild to moderate 5
Oligonucleotide Therapeutics Provide New Treatment Options in Neurological Diseases • Large number of neurological diseases can potentially be addressed by oligonucleotide therapeutics • Approval of nusinersen (Spinraza™) shows potential of oligonucleotide therapeutics in neurological diseases • SNA technology potentially improves efficacy of commonly used oligonucleotides • Nusinersen was used as benchmark for the SNA technology in a comprehensive preclinical evaluation ➢ In vitro activity ➢ In vivo activity ➢ Biodistribution studies in rodents and non-human primates Note regarding trademark: SPINRAZA is a trademark of Biogen 6
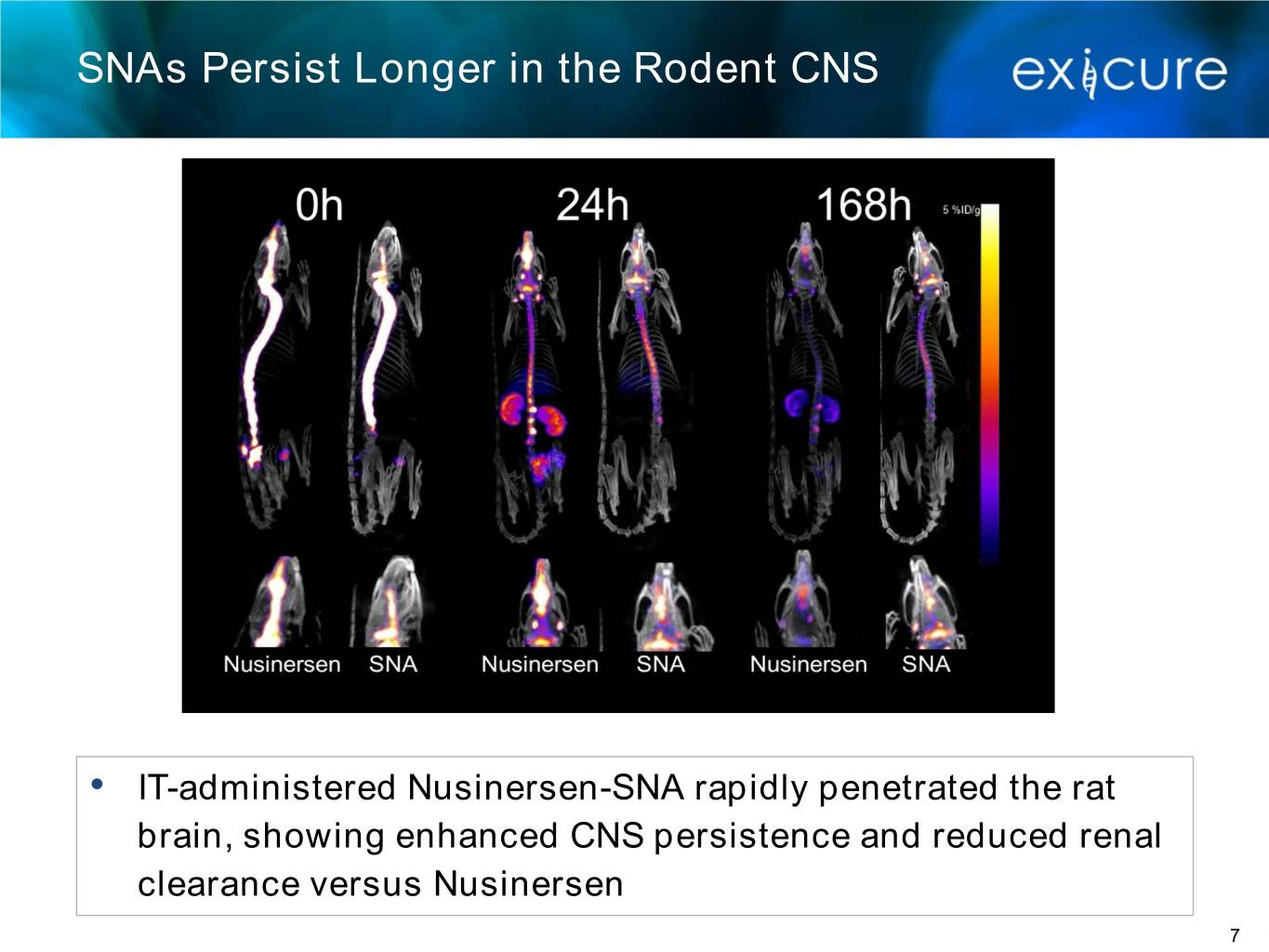
SNAs Persist Longer in the Rodent CNS • IT-administered Nusinersen-SNA rapidly penetrated the rat brain, showing enhanced CNS persistence and reduced renal clearance versus Nusinersen 7
Non-human Primate (NHP) Biodistribution
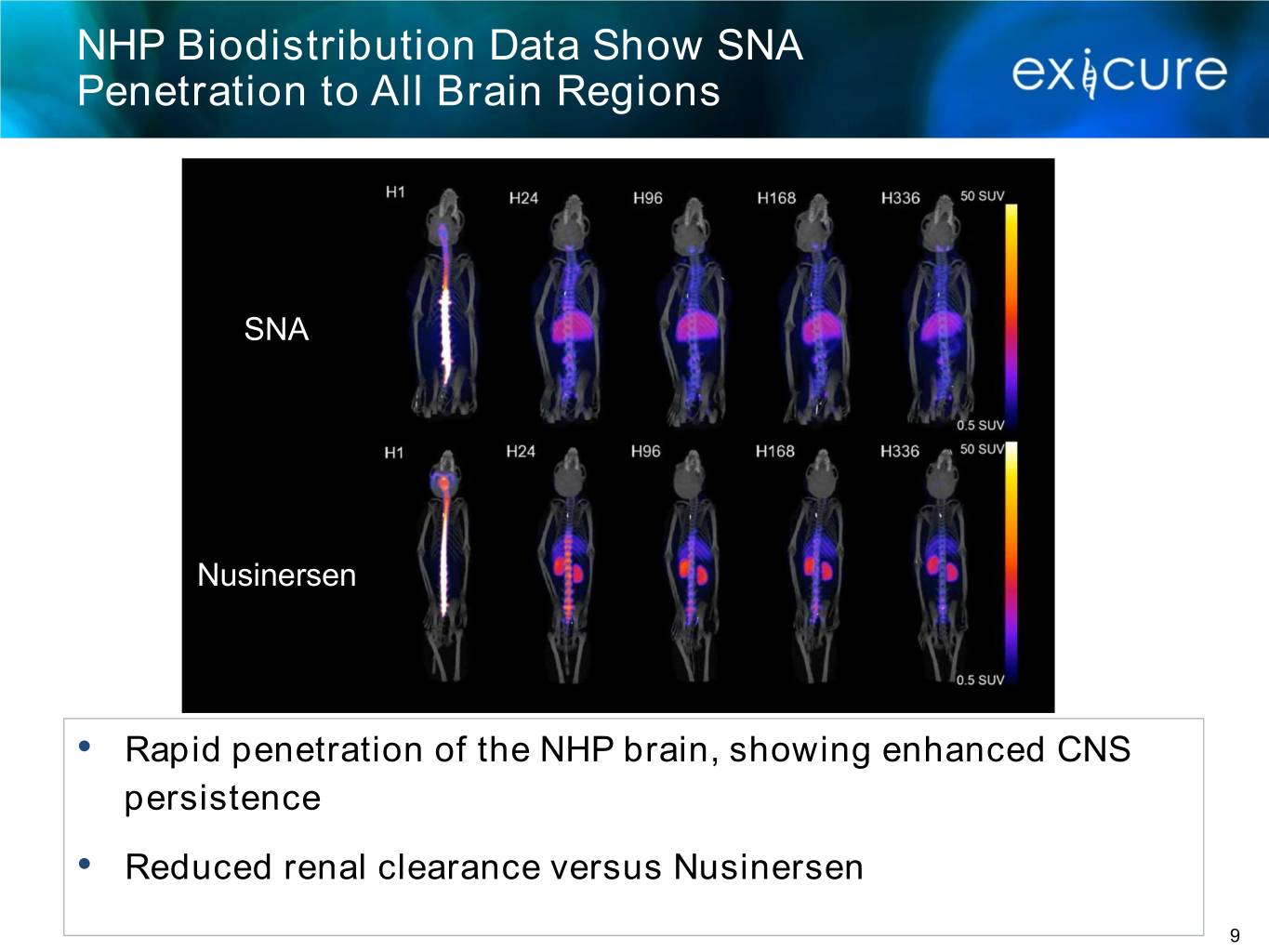
NHP Biodistribution Data Show SNA Penetration to All Brain Regions SNA Nusinersen • Rapid penetration of the NHP brain, showing enhanced CNS persistence • Reduced renal clearance versus Nusinersen 9
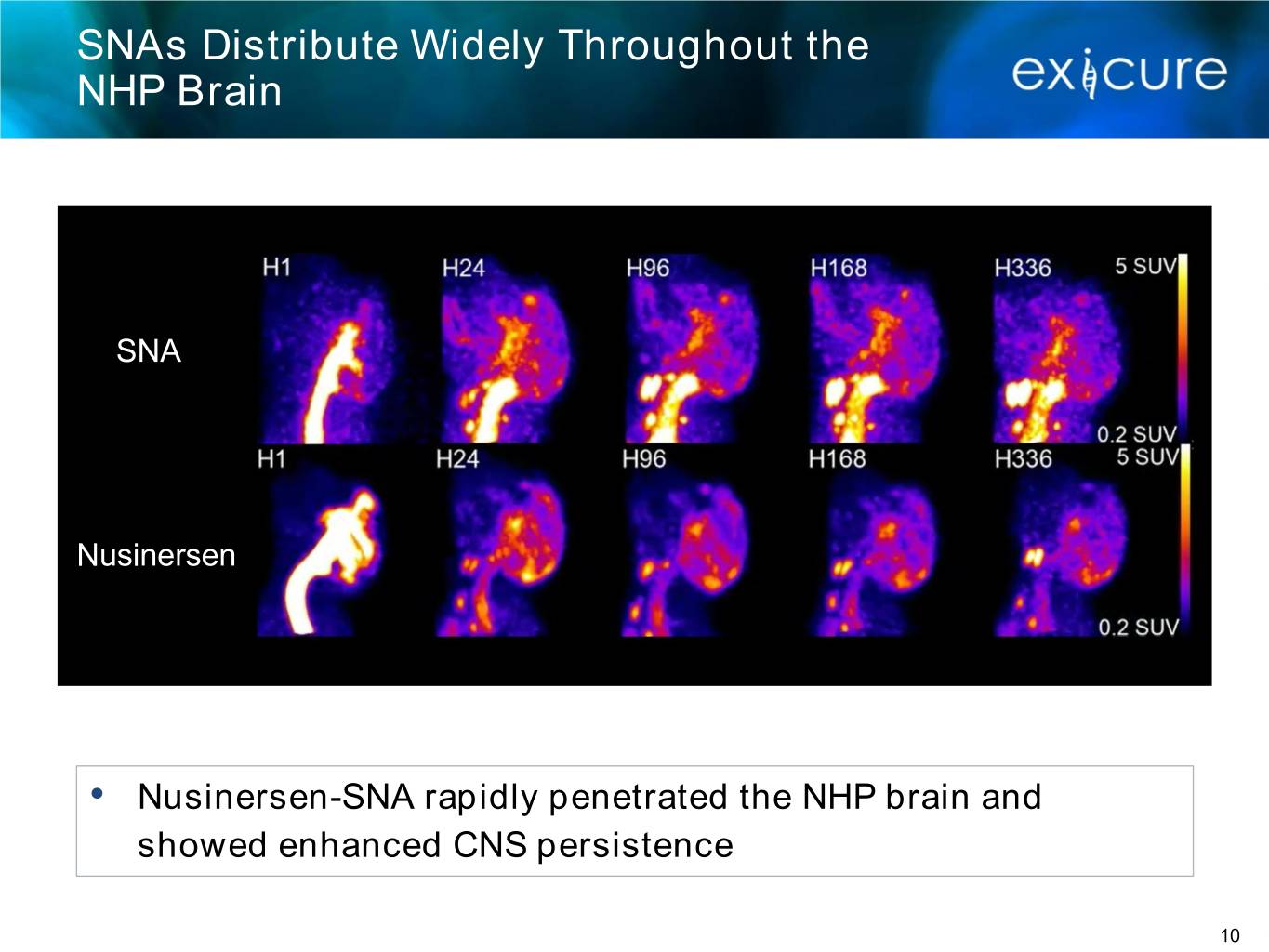
SNAs Distribute Widely Throughout the NHP Brain SNA Nusinersen • Nusinersen-SNA rapidly penetrated the NHP brain and showed enhanced CNS persistence 10
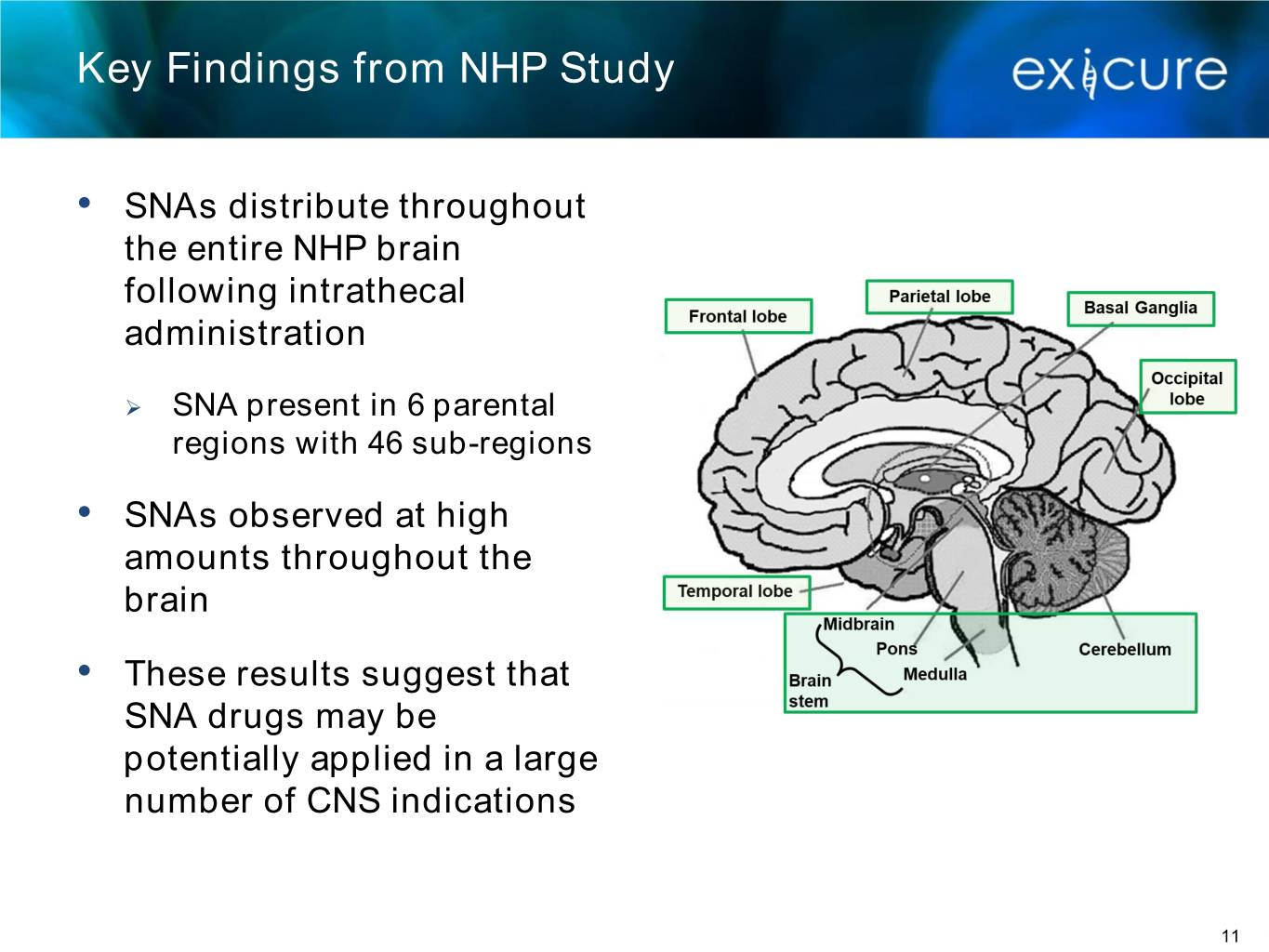
Key Findings from NHP Study • SNAs distribute throughout the entire NHP brain following intrathecal administration ➢ SNA present in 6 parental regions with 46 sub-regions • SNAs observed at high amounts throughout the brain • These results suggest that SNA drugs may be potentially applied in a large number of CNS indications 11
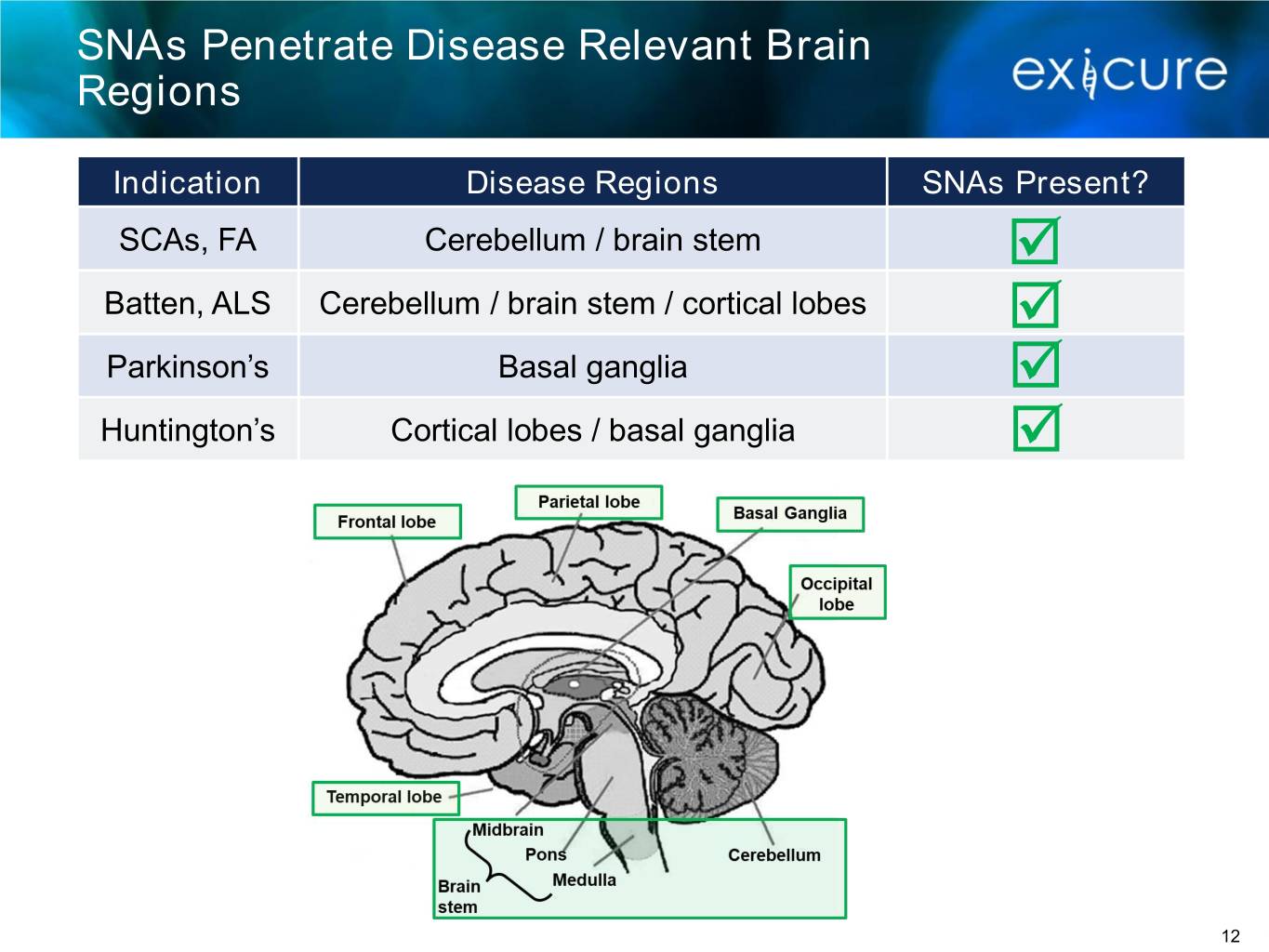
SNAs Penetrate Disease Relevant Brain Regions Indication Disease Regions SNAs Present? SCAs, FA Cerebellum / brain stem Batten, ALS Cerebellum / brain stem / cortical lobes Parkinson’s Basal ganglia Huntington’s Cortical lobes / basal ganglia 12

Contact David Giljohann, CEO davidg@exicuretx.com David Snyder, CFO dsnyder@exicuretx.com Matthias Schroff, COO mschroff@exicuretx.com












