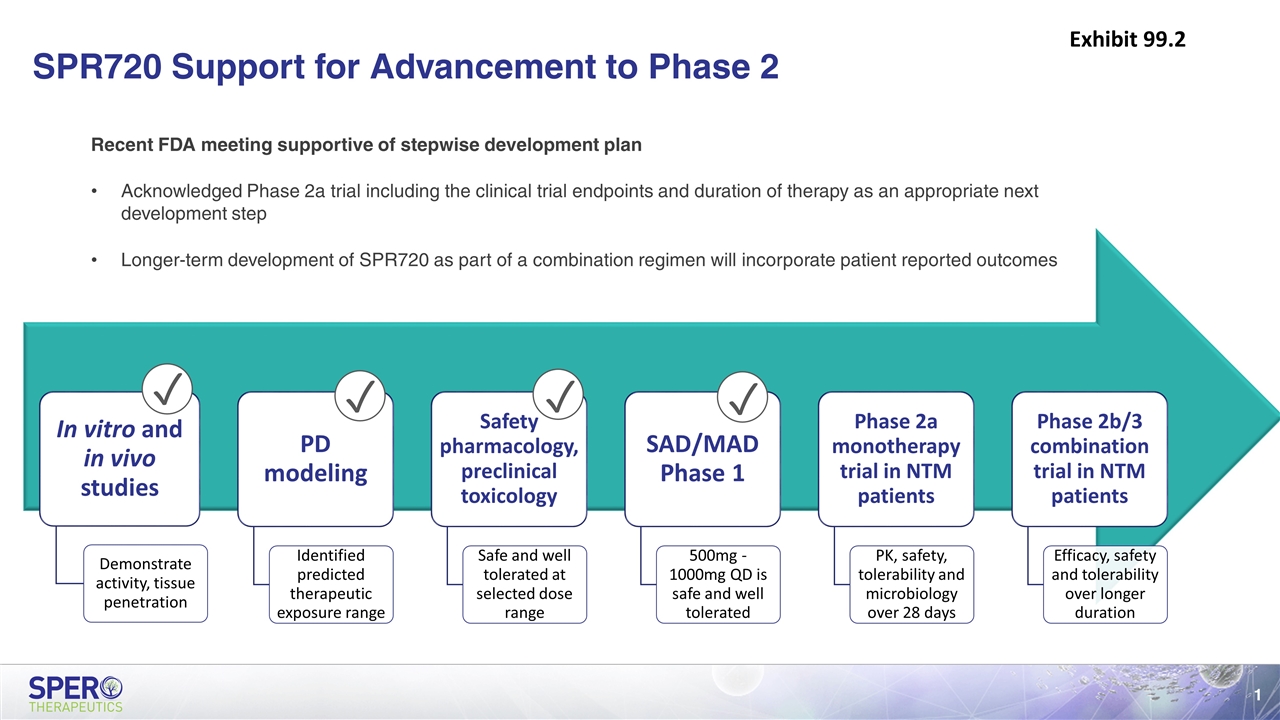
SPR720 Support for Advancement to Phase 2 ✓ ✓ ✓ ✓ Recent FDA meeting supportive of stepwise development plan Acknowledged Phase 2a trial including the clinical trial endpoints and duration of therapy as an appropriate next development step Longer-term development of SPR720 as part of a combination regimen will incorporate patient reported outcomes Exhibit 99.2 In vitro and in vivo studies PD modeling Phase 2a monotherapy trial in NTM patients Demonstrate activity, tissue penetration Identified predicted therapeutic exposure range Safety pharmacology, preclinical toxicology Safe and well tolerated at selected dose range SAD/MAD Phase 1 500mg - 1000mg QD is safe and well tolerated Phase 2b/3 combination trial in NTM patients Efficacy, safety and tolerability over longer duration PK, safety, tolerability and microbiology over 28 days
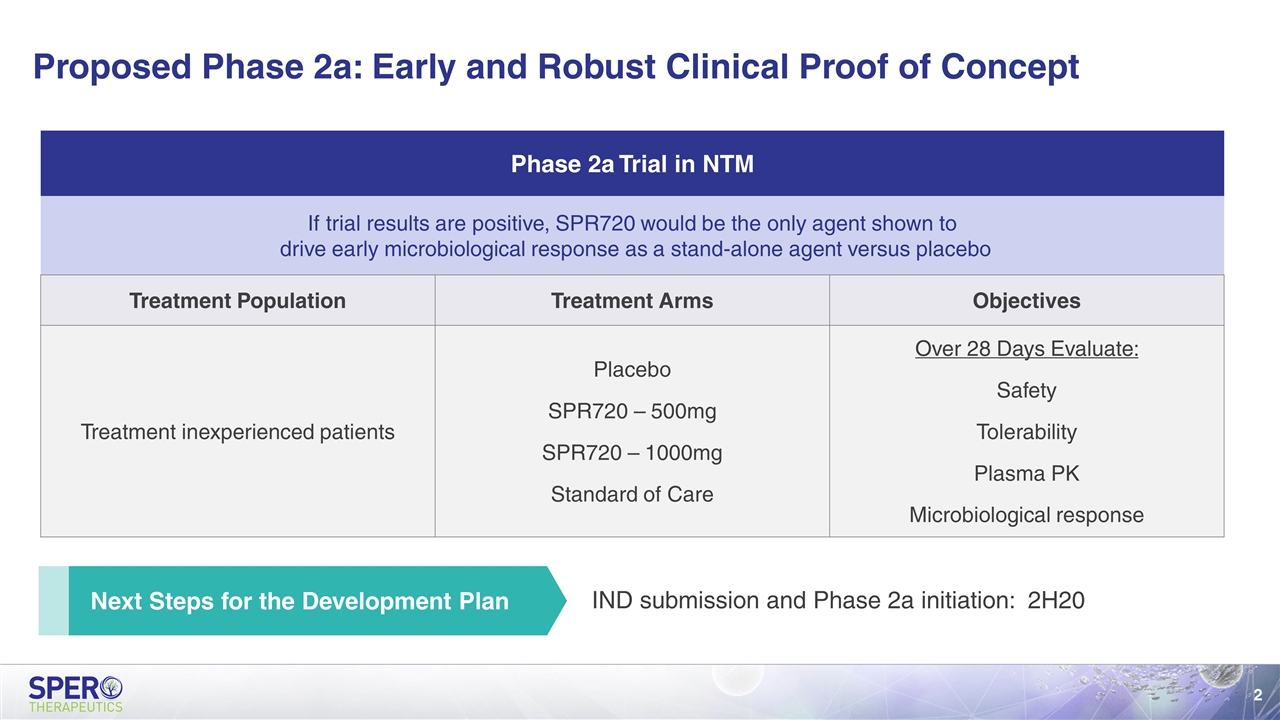
Proposed Phase 2a: Early and Robust Clinical Proof of Concept Next Steps for the Development Plan Phase 2a Trial in NTM If trial results are positive, SPR720 would be the only agent shown to drive early microbiological response as a stand-alone agent versus placebo Treatment Population Treatment Arms Objectives Treatment inexperienced patients Placebo SPR720 – 500mg SPR720 – 1000mg Standard of Care Over 28 Days Evaluate: Safety Tolerability Plasma PK Microbiological response IND submission and Phase 2a initiation: 2H20
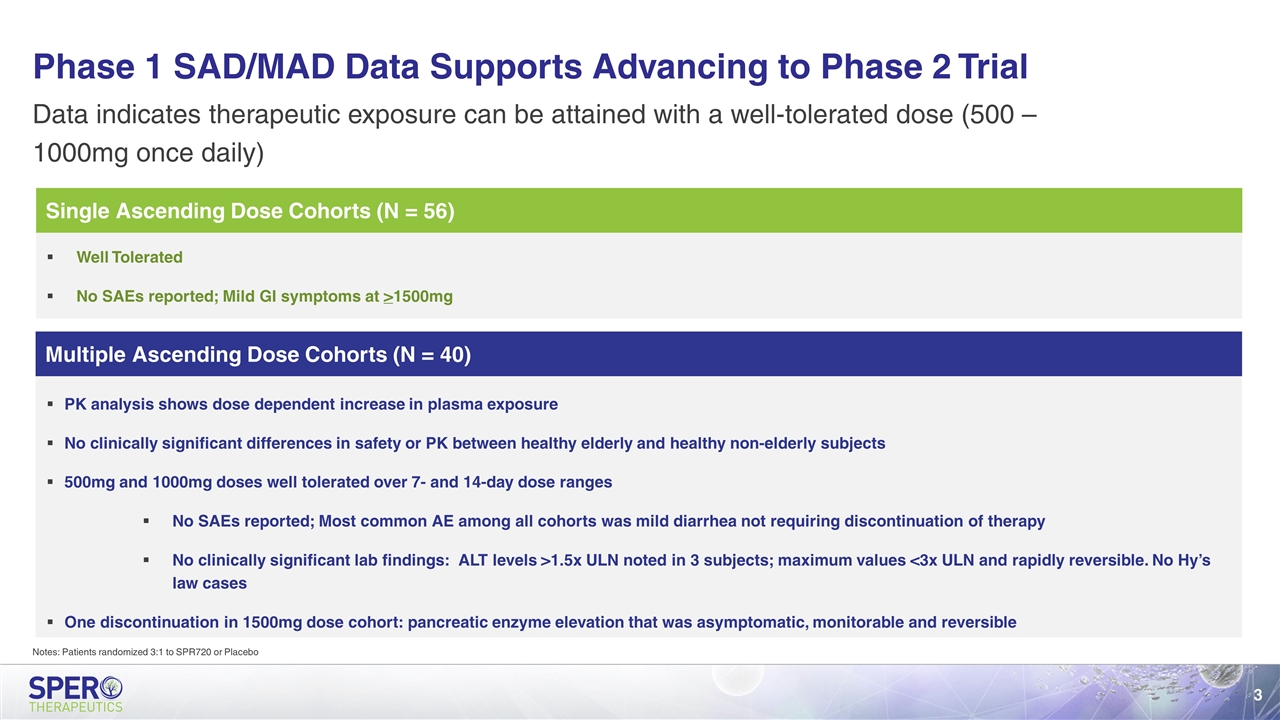
Multiple Ascending Dose Cohorts (N = 40) Single Ascending Dose Cohorts (N = 56) Phase 1 SAD/MAD Data Supports Advancing to Phase 2 Trial Data indicates therapeutic exposure can be attained with a well-tolerated dose (500 – 1000mg once daily) Well Tolerated No SAEs reported; Mild GI symptoms at >1500mg PK analysis shows dose dependent increase in plasma exposure No clinically significant differences in safety or PK between healthy elderly and healthy non-elderly subjects 500mg and 1000mg doses well tolerated over 7- and 14-day dose ranges No SAEs reported; Most common AE among all cohorts was mild diarrhea not requiring discontinuation of therapy No clinically significant lab findings: ALT levels >1.5x ULN noted in 3 subjects; maximum values <3x ULN and rapidly reversible. No Hy’s law cases One discontinuation in 1500mg dose cohort: pancreatic enzyme elevation that was asymptomatic, monitorable and reversible Notes: Patients randomized 3:1 to SPR720 or Placebo


