
Corporate Presentation October 2024 Exhibit 99.2

Forward-looking Statement This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, the future development and commercialization of SPR720 and SPR206; the potential number of patients who could be treated by SPR 720 and tebipenem HBr and market demand for SPR720 and tebipenem HBr generally; the potential regulatory path forward for tebipenem HBr, the potential approval of tebipenem HBr by the U.S. Food and Drug Administration (FDA) and the timing thereof; the potential commercialization of tebipenem HBr and its future value, the potential receipt of milestone payments and royalties on future sales of tebipenem HBr under the GlaxoSmithKline Intellectual Property (No. 3) Limited (GSK) license agreement; the effectiveness of tebipenem HBr and its potential impact on healthcare resource utilizations; the anticipated shift in treating patients from intravenous to oral administration; the initiation, timing, progress and results of the Company’s preclinical studies and clinical trials and its research and development programs, including management’s assessment of such results; the timing of the availability of data from the Company’s clinical trials; the timing of the Company’s filings with regulatory agencies; product candidate benefits; competitive position; business strategies; potential growth opportunities; potential market size; projected costs and the availability of additional non-dilutive funding from governmental agencies beyond any initially funded awards. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the FDA will require any additional clinical data or place labeling restrictions on the use of tebipenem HBr that would add costs for the Company, delay approval and/or reduce the commercial prospects of tebipenem HBr; the Company’s need for additional funding; the lengthy, expensive, and uncertain process of clinical drug development; the Company’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved; the ability to develop and commercialize the Company’s product candidates, if approved; the Company’s ability to retain key personnel; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials and whether preliminary data from the Company’s clinical trials will be predictive of final results from such trials; the Company’s dependence on raising capital and whether the Company’s product candidates will advance through the preclinical development and clinical trial process on a timely basis, or at all, taking into account such factors as the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial design, clinical data requirements and clinical outcomes; whether the results of such clinical trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; decisions made by the FDA and equivalent foreign regulatory agencies with respect to the development and commercialization of the Company’s product candidates; the commercial potential of the Company’s product candidates; the Company’s ability to obtain adequate third-party reimbursement for its product candidates; whether the Company will satisfy all of the pre-conditions to receipt of the milestone payments under its various license and collaboration agreements; the Company’s ability to implement its strategic plans; the Company’s ability to obtain, maintain and enforce intellectual property and other proprietary rights for its product candidates; the risks and uncertainties related to market conditions; whether the Company’s cash resources will be sufficient to fund its continuing operations for the periods and/or trials anticipated; and other factors discussed in the “Risk Factors” section of the Company’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC), and risks described in other filings the Company may make with the SEC in the future. The forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.
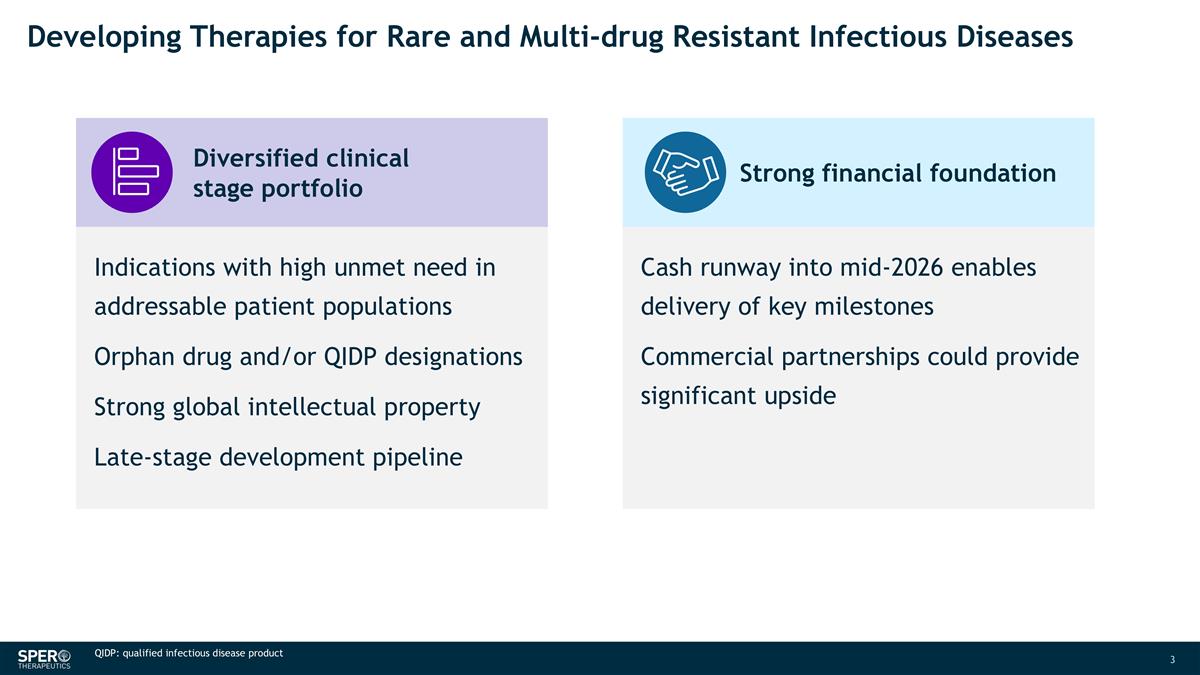
Indications with high unmet need in addressable patient populations Orphan drug and/or QIDP designations Strong global intellectual property Late-stage development pipeline Diversified clinical stage portfolio Developing Therapies for Rare and Multi-drug Resistant Infectious Diseases QIDP: qualified infectious disease product Cash runway into mid-2026 enables delivery of key milestones Commercial partnerships could provide significant upside Strong financial foundation
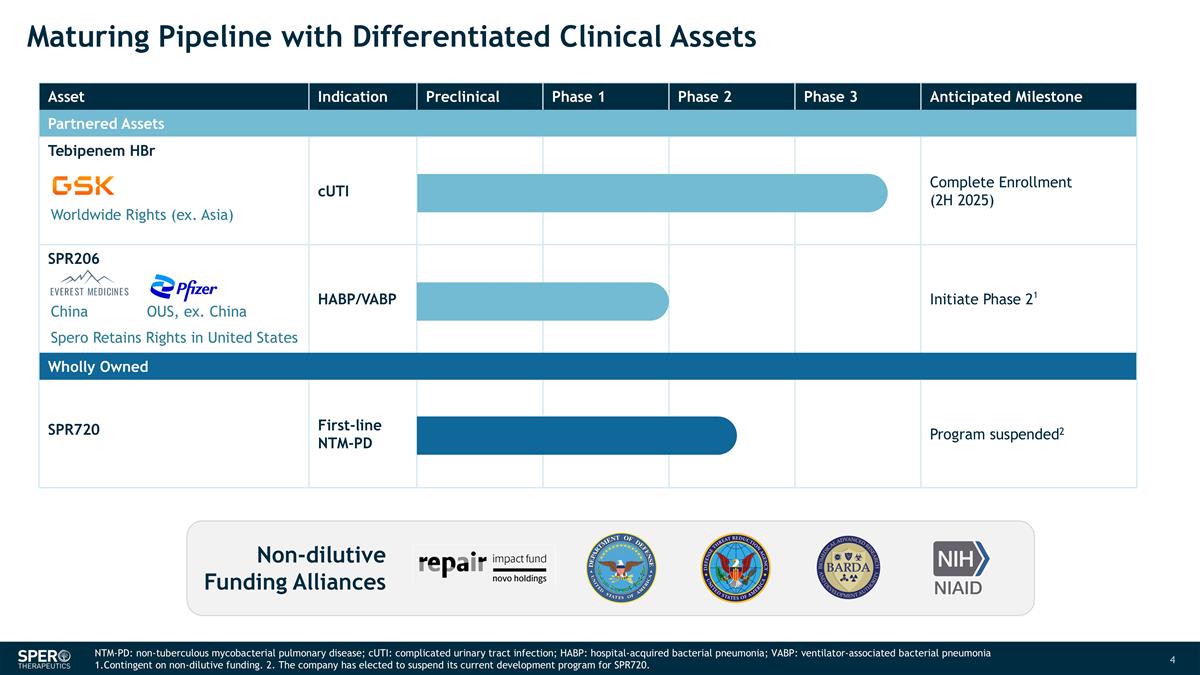
Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone Partnered Assets Tebipenem HBr cUTI Complete Enrollment (2H 2025) SPR206 HABP/VABP Initiate Phase 21 Wholly Owned SPR720 First-line NTM-PD Program suspended2 Maturing Pipeline with Differentiated Clinical Assets Non-dilutive Funding Alliances Worldwide Rights (ex. Asia) Spero Retains Rights in United States China OUS, ex. China NTM-PD: non-tuberculous mycobacterial pulmonary disease; cUTI: complicated urinary tract infection; HABP: hospital-acquired bacterial pneumonia; VABP: ventilator-associated bacterial pneumonia 1.Contingent on non-dilutive funding. 2. The company has elected to suspend its current development program for SPR720.
Tebipenem HBr Oral Carbapenem
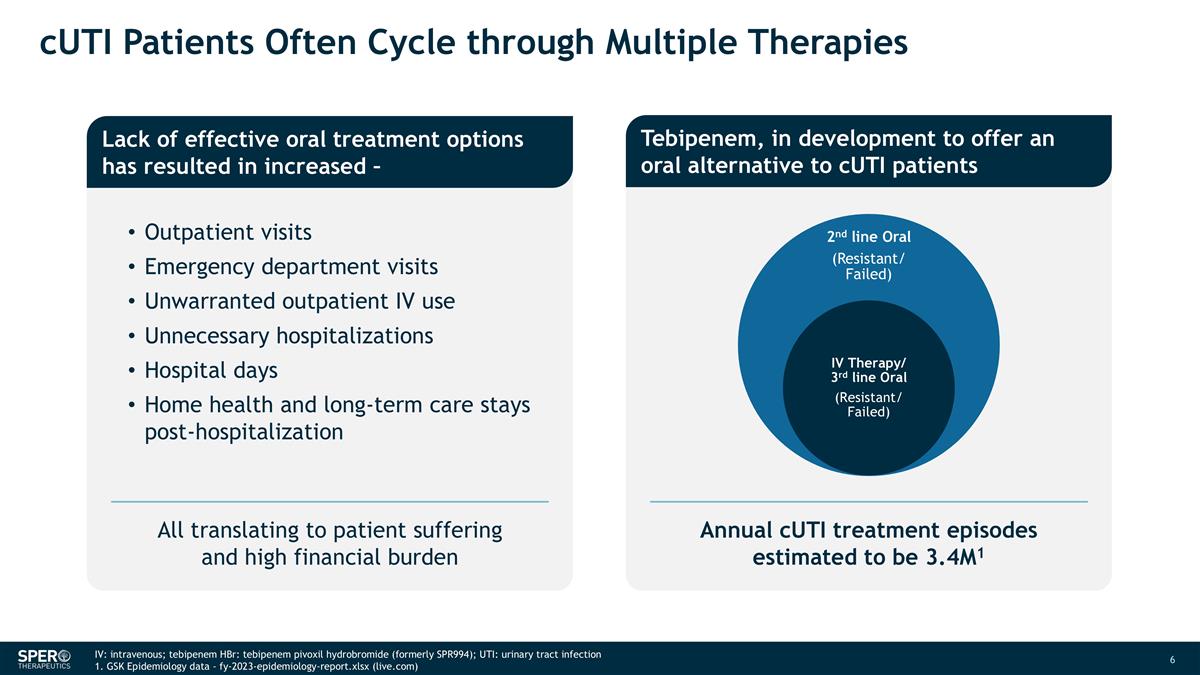
cUTI Patients Often Cycle through Multiple Therapies Lack of effective oral treatment options has resulted in increased – Outpatient visits Emergency department visits Unwarranted outpatient IV use Unnecessary hospitalizations Hospital days Home health and long-term care stays post-hospitalization IV: intravenous; tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994); UTI: urinary tract infection 1. GSK Epidemiology data - fy-2023-epidemiology-report.xlsx (live.com) Annual cUTI treatment episodes estimated to be 3.4M1 Tebipenem, in development to offer an oral alternative to cUTI patients All translating to patient suffering and high financial burden
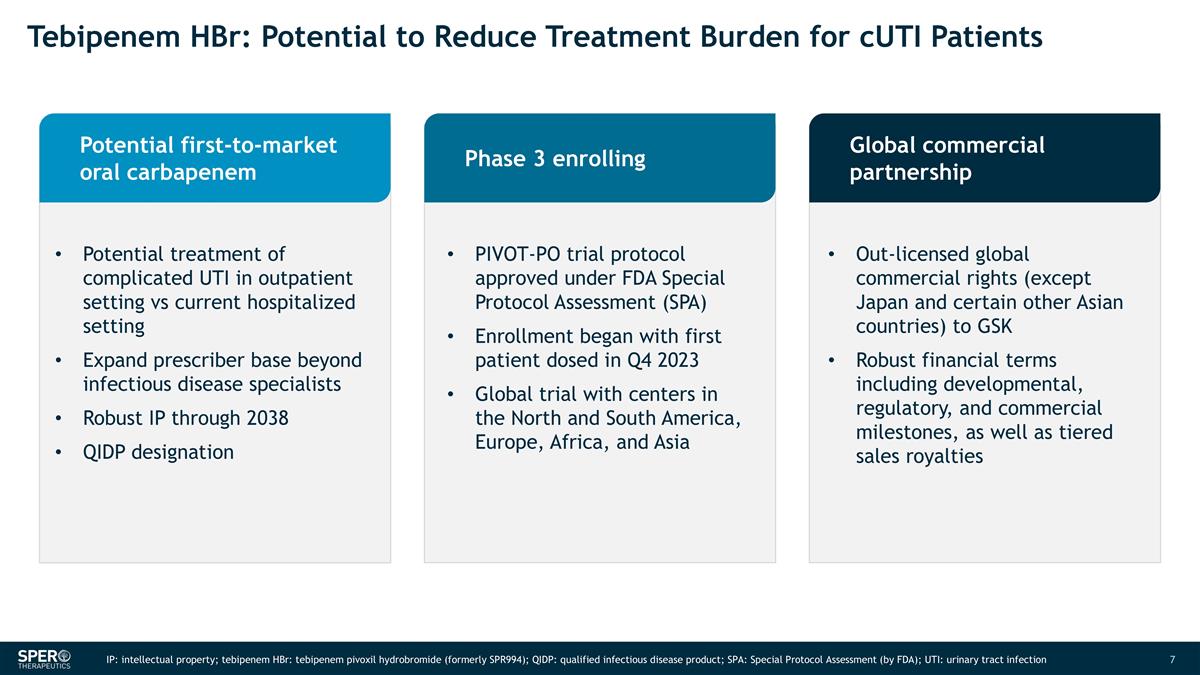
Tebipenem HBr: Potential to Reduce Treatment Burden for cUTI Patients Potential first-to-market oral carbapenem Phase 3 enrolling Global commercial partnership Potential treatment of complicated UTI in outpatient setting vs current hospitalized setting Expand prescriber base beyond infectious disease specialists Robust IP through 2038 QIDP designation PIVOT-PO trial protocol approved under FDA Special Protocol Assessment (SPA) Enrollment began with first patient dosed in Q4 2023 Global trial with centers in the North and South America, Europe, Africa, and Asia Out-licensed global commercial rights (except Japan and certain other Asian countries) to GSK Robust financial terms including developmental, regulatory, and commercial milestones, as well as tiered sales royalties IP: intellectual property; tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994); QIDP: qualified infectious disease product; SPA: Special Protocol Assessment (by FDA); UTI: urinary tract infection
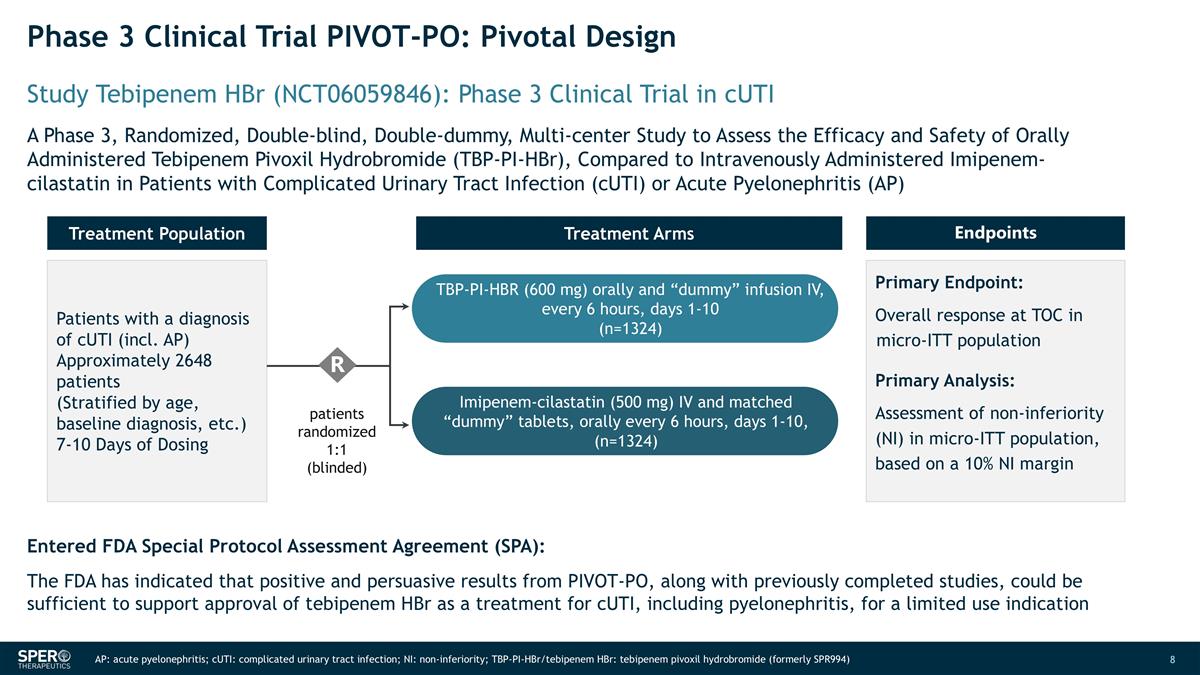
Phase 3 Clinical Trial PIVOT-PO: Pivotal Design Entered FDA Special Protocol Assessment Agreement (SPA): The FDA has indicated that positive and persuasive results from PIVOT-PO, along with previously completed studies, could be sufficient to support approval of tebipenem HBr as a treatment for cUTI, including pyelonephritis, for a limited use indication AP: acute pyelonephritis; cUTI: complicated urinary tract infection; NI: non-inferiority; TBP-PI-HBr/tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994) Study Tebipenem HBr (NCT06059846): Phase 3 Clinical Trial in cUTI A Phase 3, Randomized, Double-blind, Double-dummy, Multi-center Study to Assess the Efficacy and Safety of Orally Administered Tebipenem Pivoxil Hydrobromide (TBP-PI-HBr), Compared to Intravenously Administered Imipenem-cilastatin in Patients with Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) Patients with a diagnosis of cUTI (incl. AP) Approximately 2648 patients (Stratified by age, baseline diagnosis, etc.) 7-10 Days of Dosing Treatment Population Endpoints Primary Endpoint: Overall response at TOC in micro-ITT population Primary Analysis: Assessment of non-inferiority (NI) in micro-ITT population, based on a 10% NI margin Treatment Arms patients randomized 1:1 (blinded) R TBP-PI-HBR (600 mg) orally and “dummy” infusion IV, every 6 hours, days 1-10 (n=1324) Imipenem-cilastatin (500 mg) IV and matched “dummy” tablets, orally every 6 hours, days 1-10, (n=1324)
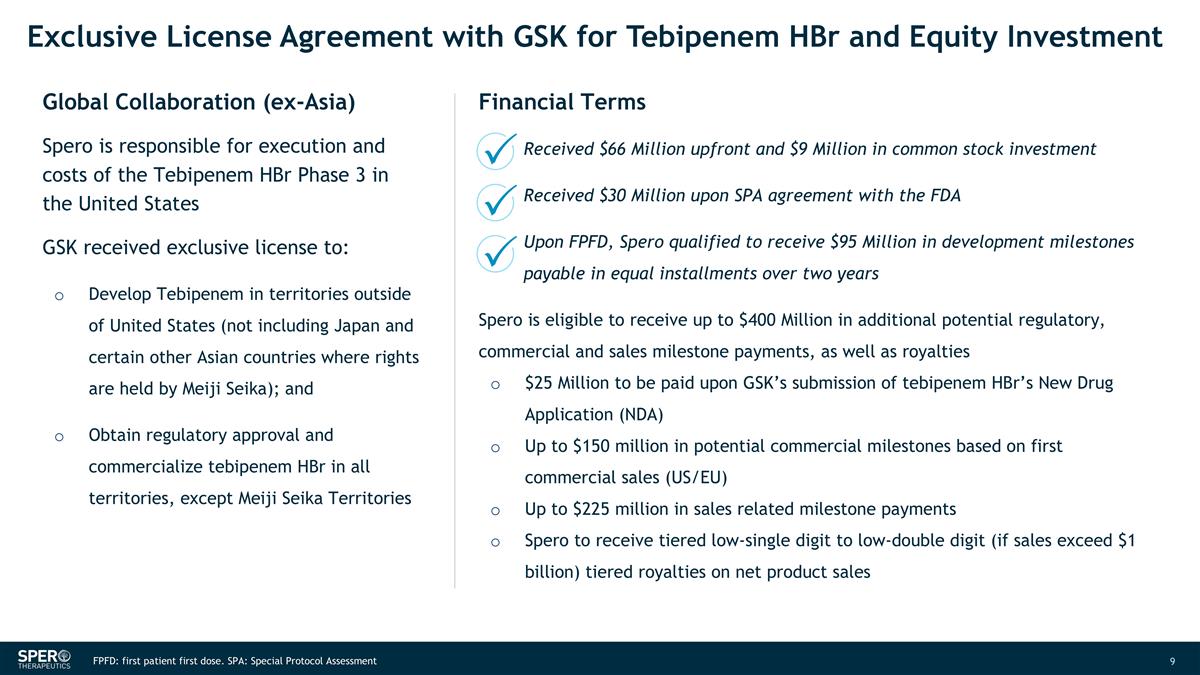
Exclusive License Agreement with GSK for Tebipenem HBr and Equity Investment Global Collaboration (ex-Asia) Financial Terms Spero is responsible for execution and costs of the Tebipenem HBr Phase 3 in the United States GSK received exclusive license to: Develop Tebipenem in territories outside of United States (not including Japan and certain other Asian countries where rights are held by Meiji Seika); and Obtain regulatory approval and commercialize tebipenem HBr in all territories, except Meiji Seika Territories Received $66 Million upfront and $9 Million in common stock investment Received $30 Million upon SPA agreement with the FDA Upon FPFD, Spero qualified to receive $95 Million in development milestones payable in equal installments over two years Spero is eligible to receive up to $400 Million in additional potential regulatory, commercial and sales milestone payments, as well as royalties $25 Million to be paid upon GSK’s submission of tebipenem HBr’s New Drug Application (NDA) Up to $150 million in potential commercial milestones based on first commercial sales (US/EU) Up to $225 million in sales related milestone payments Spero to receive tiered low-single digit to low-double digit (if sales exceed $1 billion) tiered royalties on net product sales FPFD: first patient first dose. SPA: Special Protocol Assessment
SPR206 Direct Acting IV Potentiator
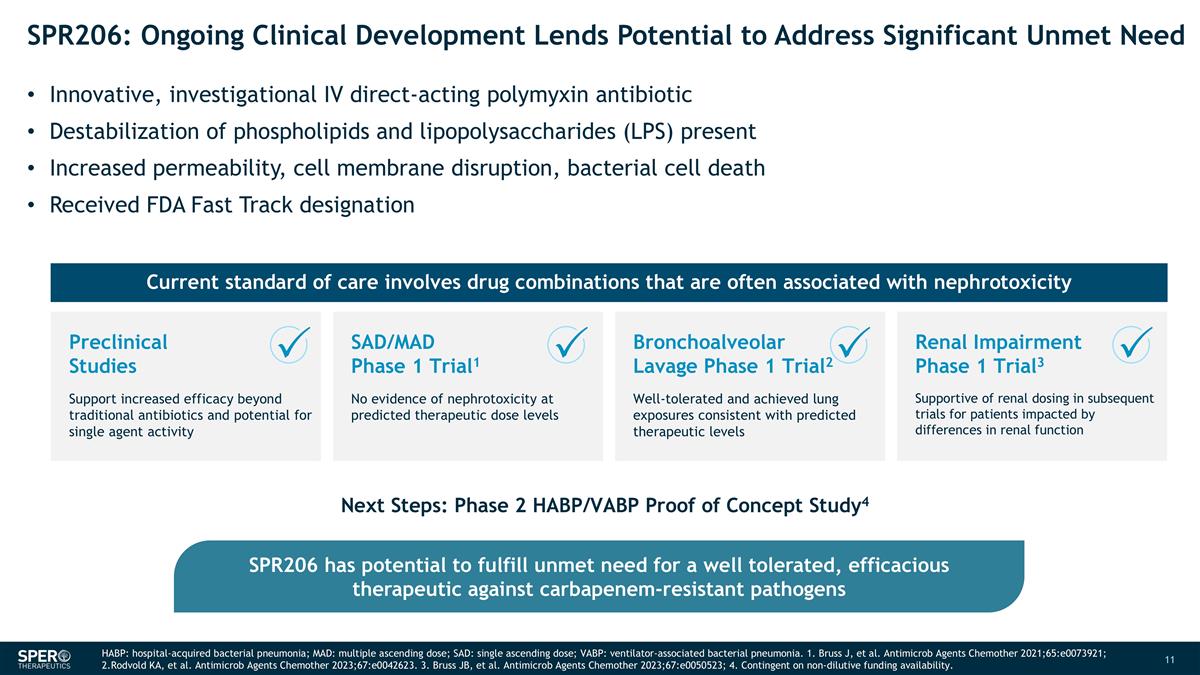
SPR206: Ongoing Clinical Development Lends Potential to Address Significant Unmet Need Innovative, investigational IV direct-acting polymyxin antibiotic Destabilization of phospholipids and lipopolysaccharides (LPS) present Increased permeability, cell membrane disruption, bacterial cell death Received FDA Fast Track designation Next Steps: Phase 2 HABP/VABP Proof of Concept Study4 SPR206 has potential to fulfill unmet need for a well tolerated, efficacious therapeutic against carbapenem-resistant pathogens Current standard of care involves drug combinations that are often associated with nephrotoxicity HABP: hospital-acquired bacterial pneumonia; MAD: multiple ascending dose; SAD: single ascending dose; VABP: ventilator-associated bacterial pneumonia. 1. Bruss J, et al. Antimicrob Agents Chemother 2021;65:e0073921; 2.Rodvold KA, et al. Antimicrob Agents Chemother 2023;67:e0042623. 3. Bruss JB, et al. Antimicrob Agents Chemother 2023;67:e0050523; 4. Contingent on non-dilutive funding availability. SAD/MAD Phase 1 Trial1 No evidence of nephrotoxicity at predicted therapeutic dose levels Bronchoalveolar Lavage Phase 1 Trial2 Well-tolerated and achieved lung exposures consistent with predicted therapeutic levels Preclinical Studies Support increased efficacy beyond traditional antibiotics and potential for single agent activity Renal Impairment Phase 1 Trial3 Supportive of renal dosing in subsequent trials for patients impacted by differences in renal function
SPR720 Oral Antibiotic for Non-Tuberculosis Mycobacterium Pulmonary Disease (NTM-PD) The company is suspending its current development for SPR720
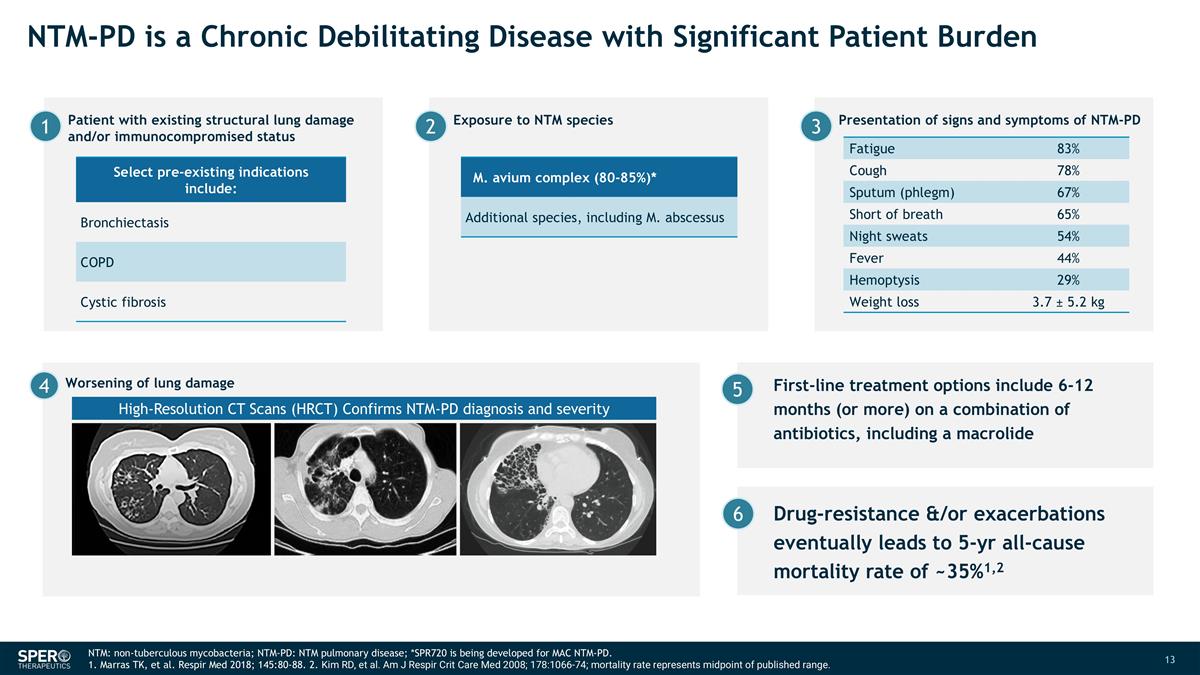
First-line treatment options include 6-12 months (or more) on a combination of antibiotics, including a macrolide NTM-PD is a Chronic Debilitating Disease with Significant Patient Burden NTM: non-tuberculous mycobacteria; NTM-PD: NTM pulmonary disease; *SPR720 is being developed for MAC NTM-PD. 1. Marras TK, et al. Respir Med 2018; 145:80-88. 2. Kim RD, et al. Am J Respir Crit Care Med 2008; 178:1066-74; mortality rate represents midpoint of published range. Fatigue 83% Cough 78% Sputum (phlegm) 67% Short of breath 65% Night sweats 54% Fever 44% Hemoptysis 29% Weight loss 3.7 ± 5.2 kg 5 High-Resolution CT Scans (HRCT) Confirms NTM-PD diagnosis and severity 4 Worsening of lung damage Select pre-existing indications include: Bronchiectasis COPD Cystic fibrosis M. avium complex (80-85%)* Additional species, including M. abscessus Patient with existing structural lung damage and/or immunocompromised status 1 Exposure to NTM species 2 Presentation of signs and symptoms of NTM-PD 3 Drug-resistance &/or exacerbations eventually leads to 5-yr all-cause mortality rate of ~35%1,2 6
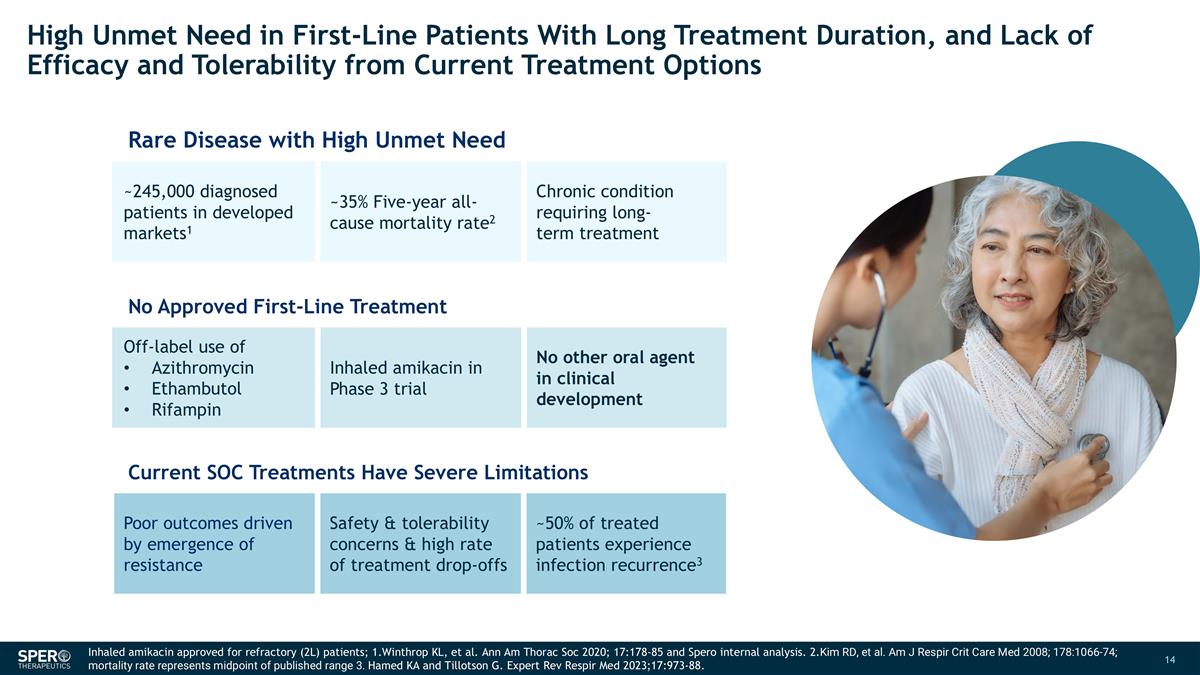
High Unmet Need in First-Line Patients With Long Treatment Duration, and Lack of Efficacy and Tolerability from Current Treatment Options Rare Disease with High Unmet Need ~245,000 diagnosed patients in developed markets1 ~35% Five-year all-cause mortality rate2 Chronic condition requiring long-term treatment No Approved First-Line Treatment Off-label use of Azithromycin Ethambutol Rifampin Inhaled amikacin in Phase 3 trial No other oral agent in clinical development Current SOC Treatments Have Severe Limitations Poor outcomes driven by emergence of resistance Safety & tolerability concerns & high rate of treatment drop-offs ~50% of treated patients experience infection recurrence3 Inhaled amikacin approved for refractory (2L) patients; 1.Winthrop KL, et al. Ann Am Thorac Soc 2020; 17:178-85 and Spero internal analysis. 2.Kim RD, et al. Am J Respir Crit Care Med 2008; 178:1066-74; mortality rate represents midpoint of published range 3. Hamed KA and Tillotson G. Expert Rev Respir Med 2023;17:973-88.
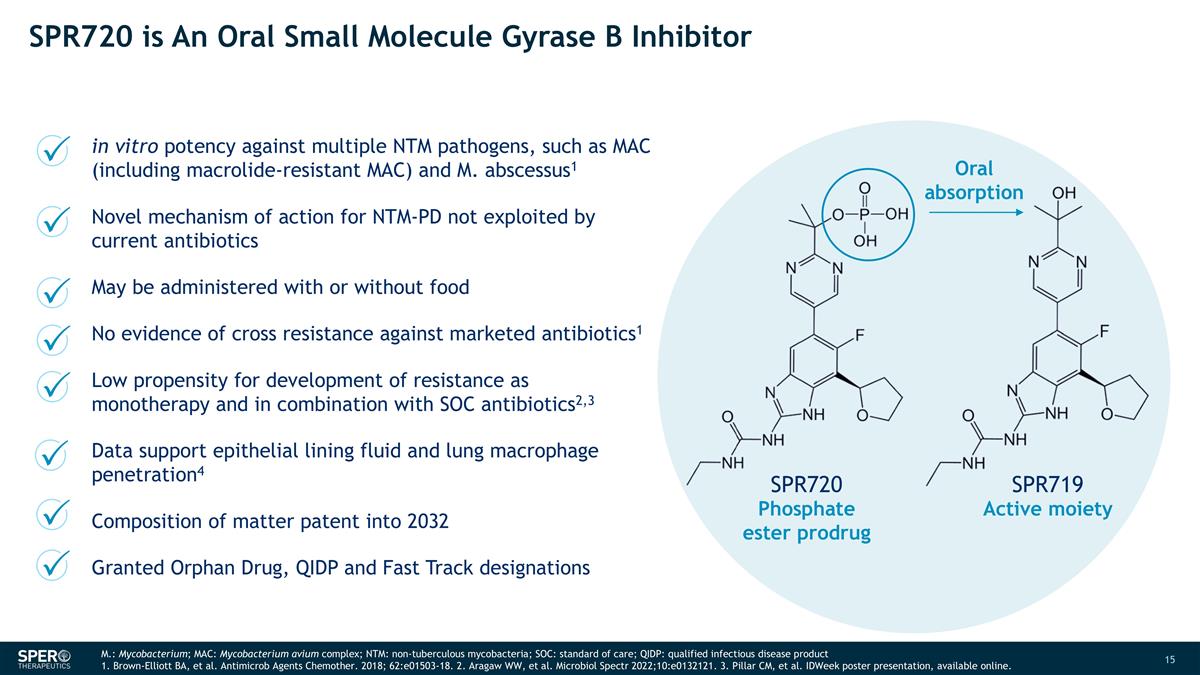
SPR720 is An Oral Small Molecule Gyrase B Inhibitor M.: Mycobacterium; MAC: Mycobacterium avium complex; NTM: non-tuberculous mycobacteria; SOC: standard of care; QIDP: qualified infectious disease product 1. Brown-Elliott BA, et al. Antimicrob Agents Chemother. 2018; 62:e01503-18. 2. Aragaw WW, et al. Microbiol Spectr 2022;10:e0132121. 3. Pillar CM, et al. IDWeek poster presentation, available online. in vitro potency against multiple NTM pathogens, such as MAC (including macrolide-resistant MAC) and M. abscessus1 Novel mechanism of action for NTM-PD not exploited by current antibiotics May be administered with or without food No evidence of cross resistance against marketed antibiotics1 Low propensity for development of resistance as monotherapy and in combination with SOC antibiotics2,3 Data support epithelial lining fluid and lung macrophage penetration4 Composition of matter patent into 2032 Granted Orphan Drug, QIDP and Fast Track designations Phosphate ester prodrug Active moiety Oral absorption SPR720 SPR719
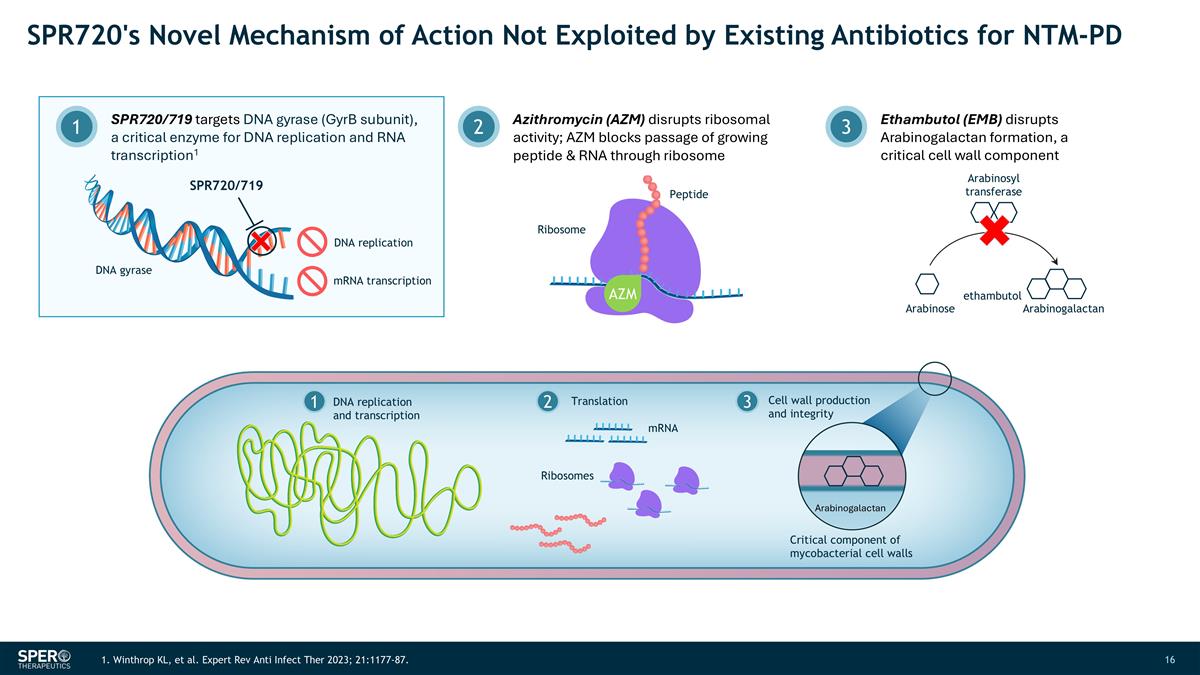
SPR720's Novel Mechanism of Action Not Exploited by Existing Antibiotics for NTM-PD SPR720/719 targets DNA gyrase (GyrB subunit), a critical enzyme for DNA replication and RNA transcription1 1 Azithromycin (AZM) disrupts ribosomal activity; AZM blocks passage of growing peptide & RNA through ribosome 2 Ethambutol (EMB) disrupts Arabinogalactan formation, a critical cell wall component 3 Arabinosyl transferase Arabinose ethambutol Arabinogalactan DNA replication and transcription mRNA Ribosomes Translation Ribosome Peptide AZM 1 2 3 SPR720/719 mRNA transcription DNA replication DNA gyrase Cell wall production and integrity Arabinogalactan Critical component of mycobacterial cell walls 1. Winthrop KL, et al. Expert Rev Anti Infect Ther 2023; 21:1177-87.
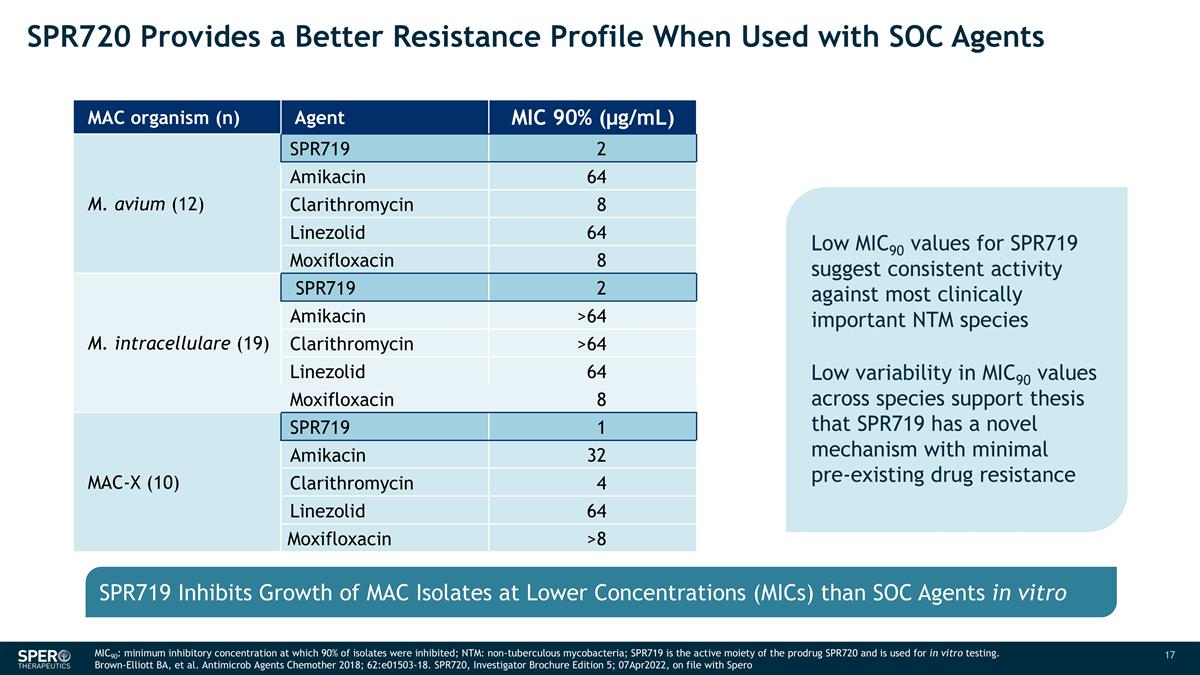
SPR720 Provides a Better Resistance Profile When Used with SOC Agents MIC90: minimum inhibitory concentration at which 90% of isolates were inhibited; NTM: non-tuberculous mycobacteria; SPR719 is the active moiety of the prodrug SPR720 and is used for in vitro testing. Brown-Elliott BA, et al. Antimicrob Agents Chemother 2018; 62:e01503-18. SPR720, Investigator Brochure Edition 5; 07Apr2022, on file with Spero Low MIC90 values for SPR719 suggest consistent activity against most clinically important NTM species Low variability in MIC90 values across species support thesis that SPR719 has a novel mechanism with minimal pre-existing drug resistance MAC organism (n) Agent MIC 90% (µg/mL) M. avium (12) SPR719 2 Amikacin 64 Clarithromycin 8 Linezolid 64 Moxifloxacin 8 M. intracellulare (19) SPR719 2 Amikacin >64 Clarithromycin >64 Linezolid 64 Moxifloxacin 8 MAC-X (10) SPR719 1 Amikacin 32 Clarithromycin 4 Linezolid 64 Moxifloxacin >8 SPR719 Inhibits Growth of MAC Isolates at Lower Concentrations (MICs) than SOC Agents in vitro
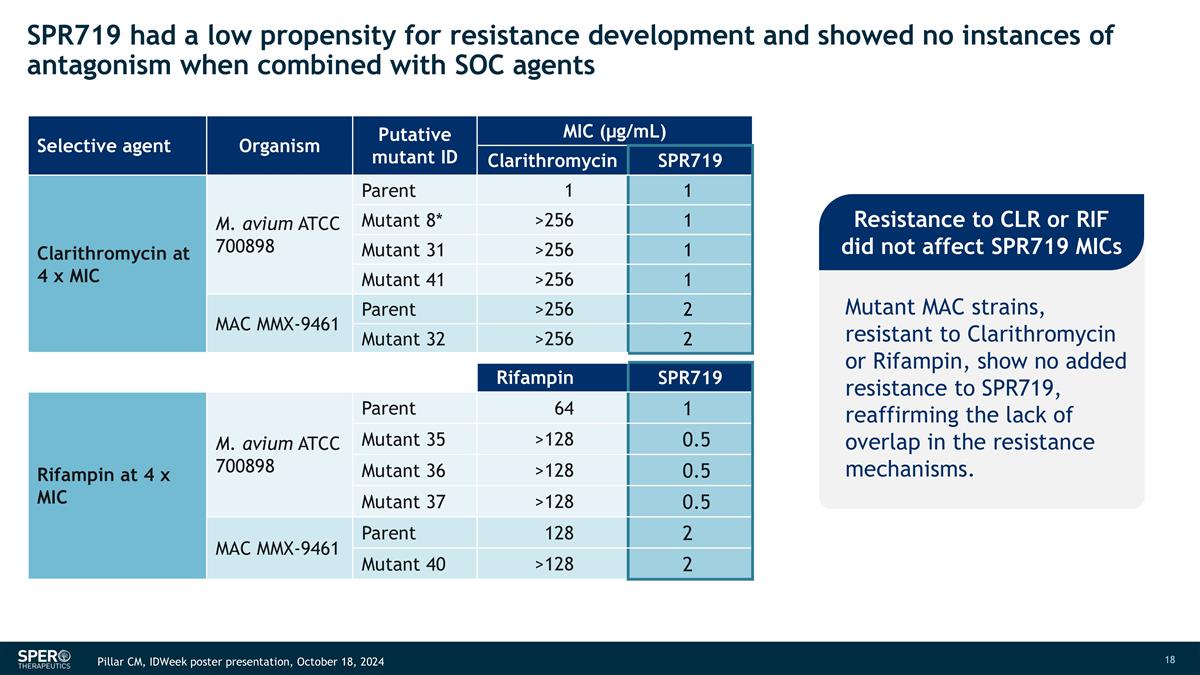
SPR719 had a low propensity for resistance development and showed no instances of antagonism when combined with SOC agents Selective agent Organism Putative mutant ID MIC (µg/mL) Clarithromycin SPR719 Clarithromycin at 4 x MIC M. avium ATCC 700898 Parent 1 1 Mutant 8* >256 1 Mutant 31 >256 1 Mutant 41 >256 1 MAC MMX-9461 Parent >256 2 Mutant 32 >256 2 Rifampin SPR719 Rifampin at 4 x MIC M. avium ATCC 700898 Parent 64 1 Mutant 35 >128 0.5 Mutant 36 >128 0.5 Mutant 37 >128 0.5 MAC MMX-9461 Parent 128 2 Mutant 40 >128 2 Resistance to CLR or RIF did not affect SPR719 MICs Mutant MAC strains, resistant to Clarithromycin or Rifampin, show no added resistance to SPR719, reaffirming the lack of overlap in the resistance mechanisms. Pillar CM, IDWeek poster presentation, October 18, 2024
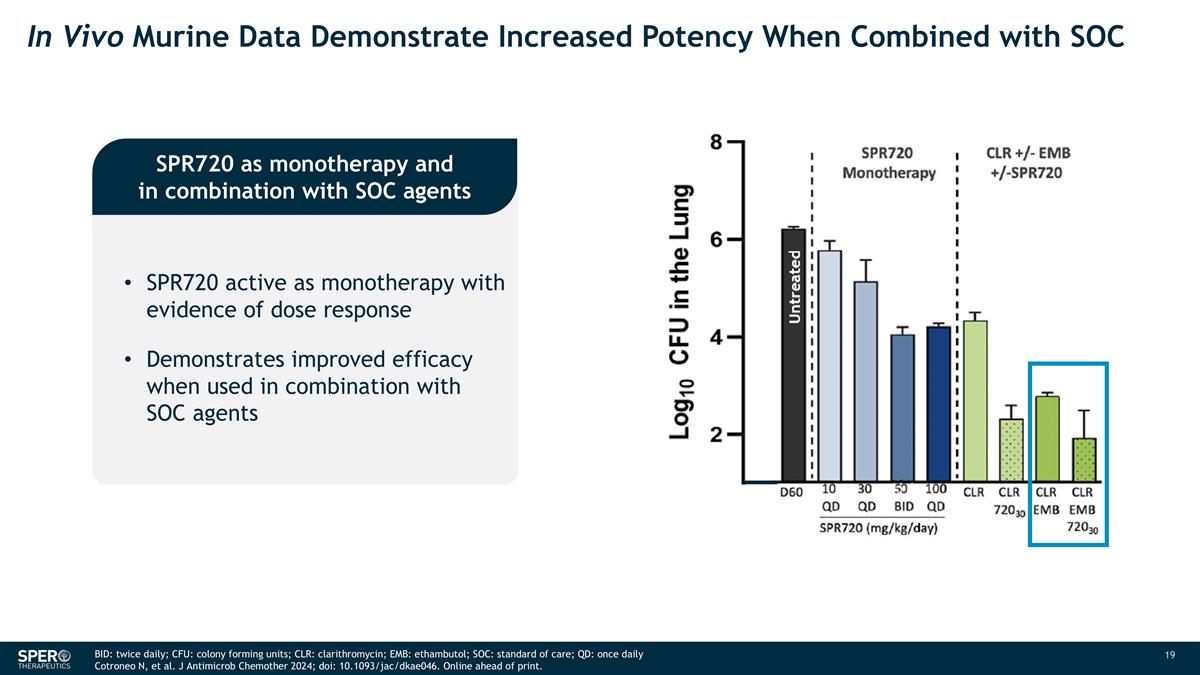
In Vivo Murine Data Demonstrate Increased Potency When Combined with SOC SPR720 as monotherapy and in combination with SOC agents SPR720 active as monotherapy with evidence of dose response Demonstrates improved efficacy when used in combination with SOC agents BID: twice daily; CFU: colony forming units; CLR: clarithromycin; EMB: ethambutol; SOC: standard of care; QD: once daily Cotroneo N, et al. J Antimicrob Chemother 2024; doi: 10.1093/jac/dkae046. Online ahead of print.
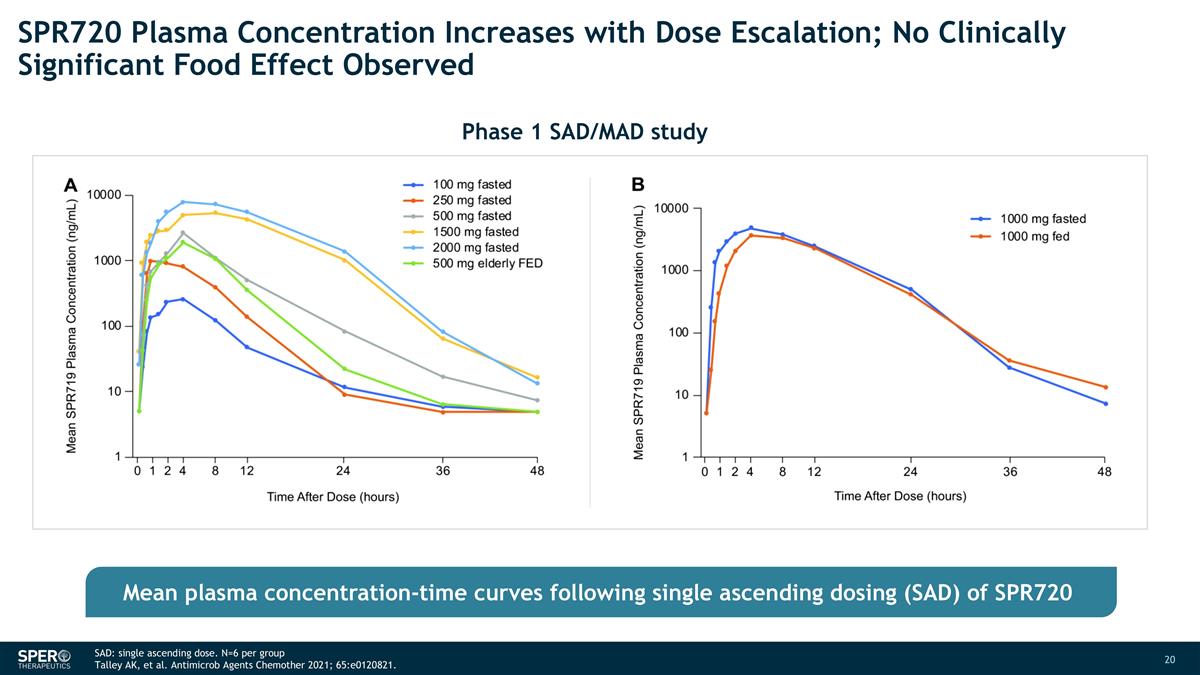
SAD: single ascending dose. N=6 per group Talley AK, et al. Antimicrob Agents Chemother 2021; 65:e0120821. SPR720 Plasma Concentration Increases with Dose Escalation; No Clinically Significant Food Effect Observed Phase 1 SAD/MAD study Mean plasma concentration-time curves following single ascending dosing (SAD) of SPR720
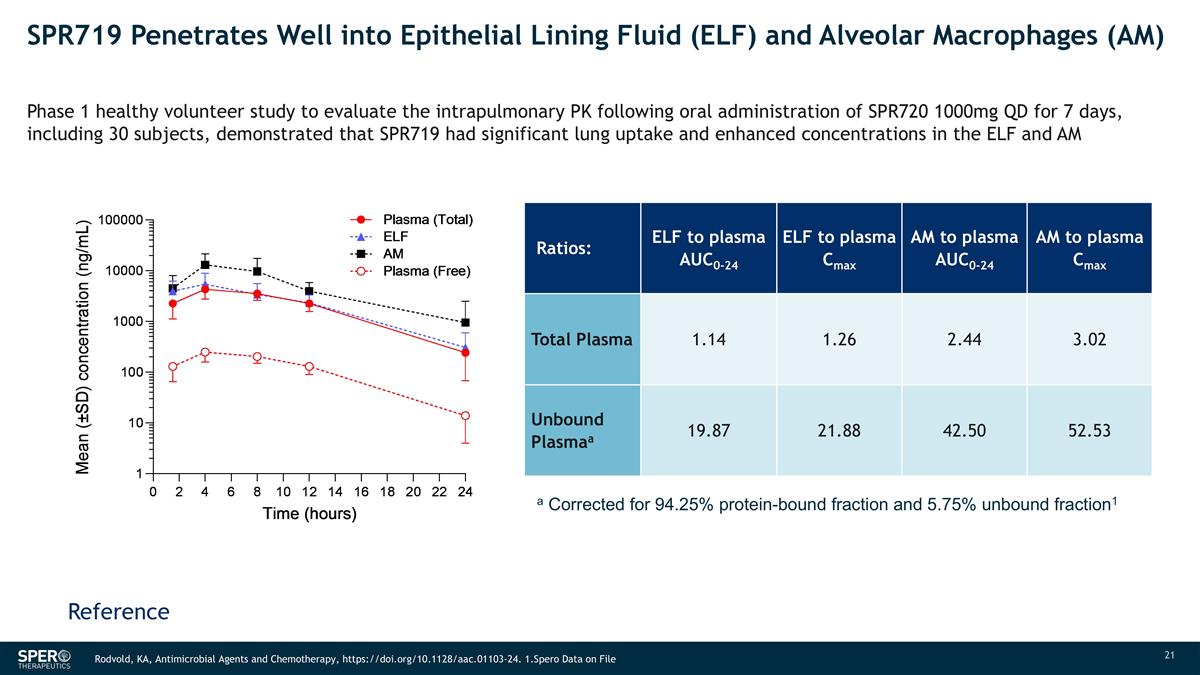
SPR719 Penetrates Well into Epithelial Lining Fluid (ELF) and Alveolar Macrophages (AM) Ratios: ELF to plasma AUC0-24 ELF to plasma Cmax AM to plasma AUC0-24 AM to plasma Cmax Total Plasma 1.14 1.26 2.44 3.02 Unbound Plasmaa 19.87 21.88 42.50 52.53 a Corrected for 94.25% protein-bound fraction and 5.75% unbound fraction1 Phase 1 healthy volunteer study to evaluate the intrapulmonary PK following oral administration of SPR720 1000mg QD for 7 days, including 30 subjects, demonstrated that SPR719 had significant lung uptake and enhanced concentrations in the ELF and AM Reference Rodvold, KA, Antimicrobial Agents and Chemotherapy, https://doi.org/10.1128/aac.01103-24. 1.Spero Data on File
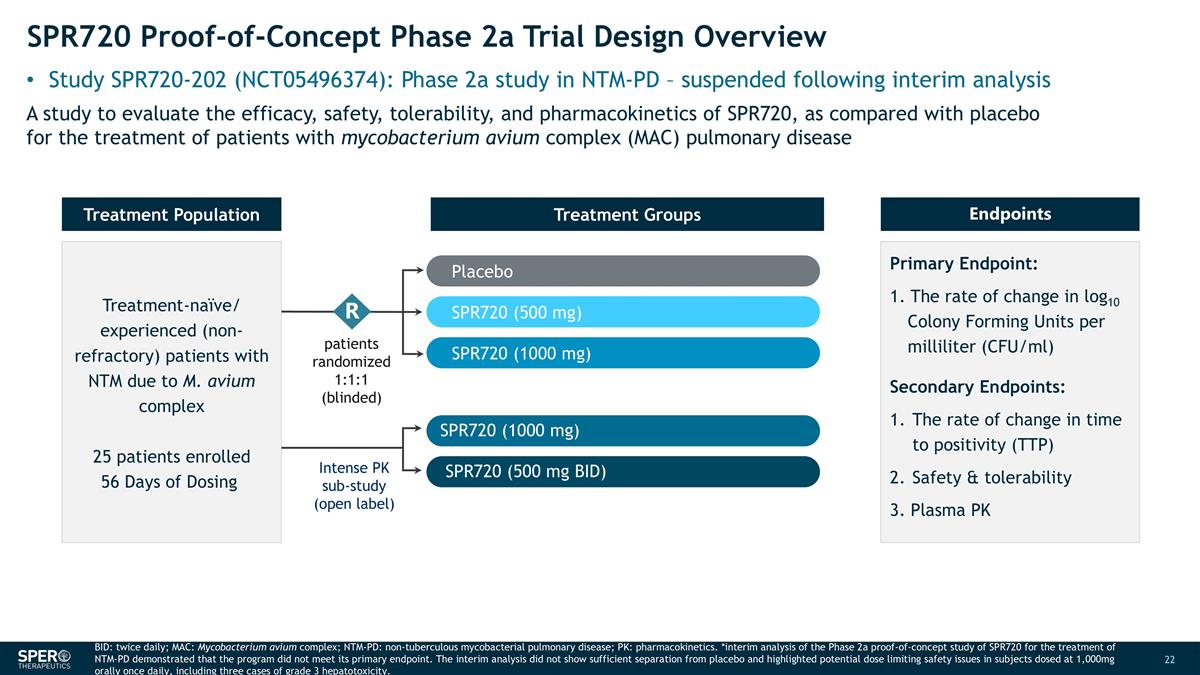
SPR720 Proof-of-Concept Phase 2a Trial Design Overview Study SPR720-202 (NCT05496374): Phase 2a study in NTM-PD – suspended following interim analysis Intense PK sub-study (open label) Treatment-naïve/ experienced (non-refractory) patients with NTM due to M. avium complex 25 patients enrolled 56 Days of Dosing Treatment Population Endpoints Primary Endpoint: 1. The rate of change in log10 Colony Forming Units per milliliter (CFU/ml) Secondary Endpoints: The rate of change in time to positivity (TTP) Safety & tolerability 3. Plasma PK Treatment Groups patients randomized 1:1:1 (blinded) R Placebo SPR720 (500 mg) A study to evaluate the efficacy, safety, tolerability, and pharmacokinetics of SPR720, as compared with placebo for the treatment of patients with mycobacterium avium complex (MAC) pulmonary disease SPR720 (1000 mg) SPR720 (1000 mg) SPR720 (500 mg BID) BID: twice daily; MAC: Mycobacterium avium complex; NTM-PD: non-tuberculous mycobacterial pulmonary disease; PK: pharmacokinetics. *interim analysis of the Phase 2a proof-of-concept study of SPR720 for the treatment of NTM-PD demonstrated that the program did not meet its primary endpoint. The interim analysis did not show sufficient separation from placebo and highlighted potential dose limiting safety issues in subjects dosed at 1,000mg orally once daily, including three cases of grade 3 hepatotoxicity.
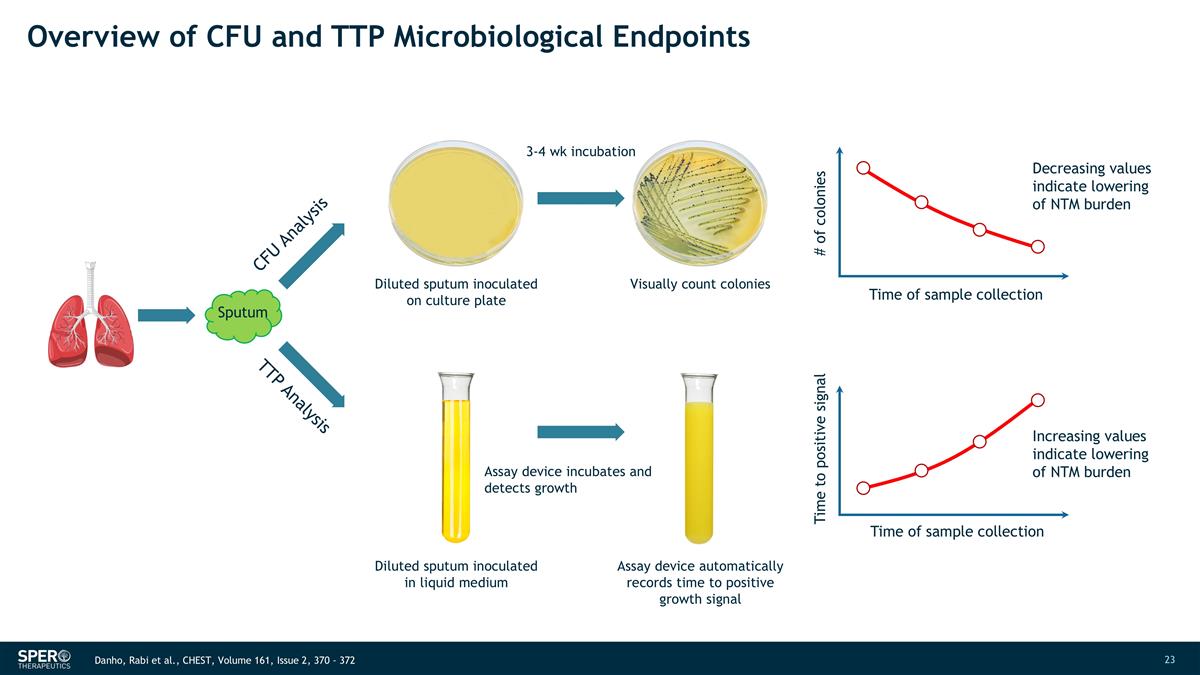
Overview of CFU and TTP Microbiological Endpoints Sputum CFU Analysis Diluted sputum inoculated on culture plate Visually count colonies TTP Analysis Diluted sputum inoculated in liquid medium Assay device incubates and detects growth Assay device automatically records time to positive growth signal # of colonies Time of sample collection Decreasing values indicate lowering of NTM burden Time to positive signal Time of sample collection Increasing values indicate lowering of NTM burden 3-4 wk incubation Danho, Rabi et al., CHEST, Volume 161, Issue 2, 370 - 372

Esther Rajavelu Chief Financial Officer and Chief Business Officer Over two decades of life science sector experience, combining equities research, investment banking, strategy consulting, and M&A Prior CFO at Fulcrum Therapeutics. Senior equity research analyst at UBS, Oppenheimer and Deutsche Bank. Healthcare Investment Banker at Bank of America Merrill Lynch Sath Shukla President and Chief Executive Officer Prior CFO at Spero Therapeutics; Prior CFO at Ziopharm Oncology; VP and Global Head of Corporate Finance at Vertex Over 20 years of financial leadership, executing within commercial and clinical companies Leadership Team Timothy Keutzer Chief Operating Officer Previously, Spero’s Chief Development Officer Prior VP Program and Portfolio Management, Cubist Extensive antibiotic development experience from pre-clinical to approval Over 30 years in the pharmaceutical industry James Brady Chief Human Resource Officer Prior CHRO at uniQure Therapeutics; Vice President, Human Resources at Intarcia Therapeutics Close to 30 years of senior human resources experience with over 17 years in the life science space

Thank You
























