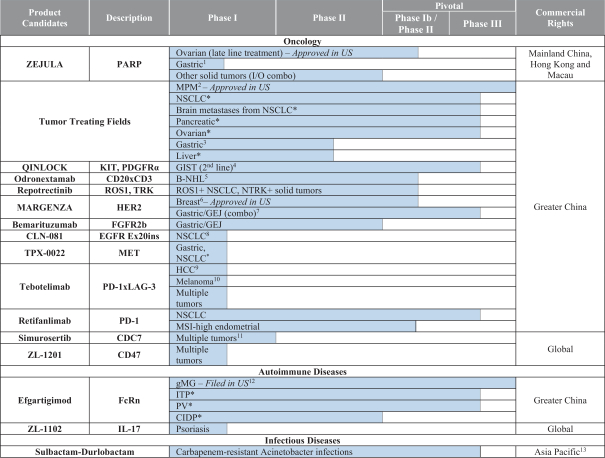
licenses or, as applicable, our rights are limited to Greater China. Also, in the case of our agreement with Entasis for durlobactam, our license is limited to China, Hong Kong, Macau, Taiwan, Korea, Vietnam, Thailand, Cambodia, Laos, Malaysia, Indonesia, the Philippines, Singapore, Australia, New Zealand and Japan. In the case of our agreement with Takeda for simurosertib
(TAK-931),
our license is worldwide except for Japan. As a result, we may not be able to prevent competitors from developing and commercializing competitive products in all such fields and territories.
Patents may be invalidated and patent applications relating to bemarituzumab (FPA144), Tumor Treating Fields, margetuximab, tebotelimab, durlobactam, a
pre-clinical
multi-specific TRIDENT molecule or retifanlimab (INCMGA0012
(PD-1))
as well as Regeneron’s patents relating to odronextamab (REGN1979), may not be granted for a number of reasons, including known or unknown prior art, deficiencies in the patent application or the lack of novelty of the underlying invention or technology. It is also possible that we will fail to identify patentable aspects of our research and development output in time to obtain patent protection. Although we enter into
non-disclosure
and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and any other third parties, any of these parties may breach such agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. In addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases, not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or
in-licensed
patents or pending patent applications or that we or our licensors were the first to file for patent protection of such inventions. Furthermore, China and the United States have adopted the
or the
“first-inventor-to
file” system under which whoever first files a patent application will be awarded the patent if all other patentability requirements are met. Under the
or the
first-inventor-to
file system third parties may be granted a patent relating to a technology, which we invented.
In addition, under Chinese Patent Law, any organization or individual that applies for a patent in a foreign country for an invention or utility model accomplished in China is required to report to the CNIPA for confidentiality examination. Otherwise, if an application is later filed in China, the patent right will not be granted. Moreover, even if patents do grant from any of the applications, the grant of a patent is not conclusive as to its scope, validity or enforceability.
The coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications we license or own currently or in the future issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. In addition, the patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in China, United States and abroad. We and our licensors and collaboration partners may be subject to a third-party preissuance submission of prior art to the United States Patent and Trademark Office, or USPTO, or become involved in opposition, derivation, revocation,
re-examination,
post-grant and
review, or interference proceedings or similar proceedings in foreign jurisdictions challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our owned or
in-licensed
patent rights, allow third parties to commercialize our technology, products or product candidates and compete directly with us without payment to us, or result in our inability to manufacture or commercialize products or product candidates without infringing, misappropriating or otherwise violating third-party patent rights. Moreover, we, or