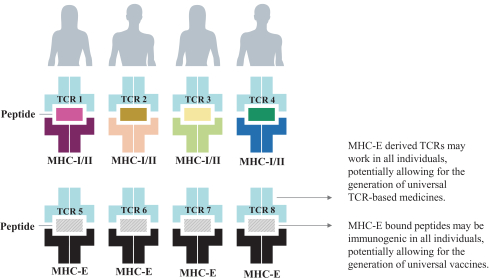
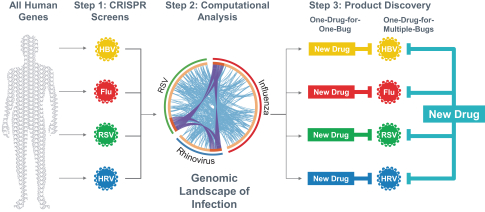
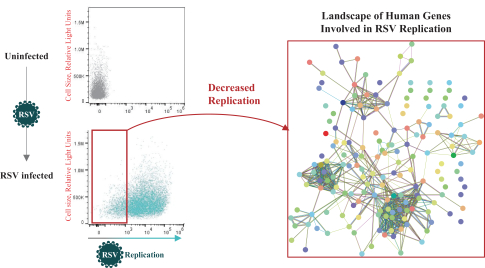
Two of these families collectively include, as of May 31, 2019, three issued patents in the United States directed to composition of matter claims and method of treatment claims. The20-year term of these patents is presently estimated to expire between 2031 and 2032, absent any available patent term adjustments or extensions. Additionally, the four patent families collectively include, as of May 31, 2019, 130 issued patents in Australia, Austria, Belgium, Bulgaria, Canada, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Japan, Latvia, Lithuania, Luxembourg, Monaco, Macedonia, Malta, New Zealand, Netherlands, Norway, Poland, Portugal, Romania, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey and the United Kingdom directed to composition of matter claims, pharmaceutical composition claims, method of treatment claims, composition for use in treatment claims and process (methods of producing) claims. The20-year term of these patents is presently estimated to expire between 2025 and 2035, absent any available patent term adjustments or extensions.
The four licensed families also collectively include, as of May 31, 2019, four patent applications in the United States and 28 patent applications in ARIPO (Africa), Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, Indonesia, Israel, India, Japan, Korea, Mexico, New Zealand, Singapore, South Africa, Thailand and the Ukraine directed to composition of matter claims, pharmaceutical composition claims, method of treatment claims, composition for use in treatment claims and process (methods of producing) claims. The20-year term of any patents issuing from patent applications in these families is presently estimated to expire between 2025 and 2035, absent any available patent term adjustments or extensions.
Patent Portfolio by Technology Platform
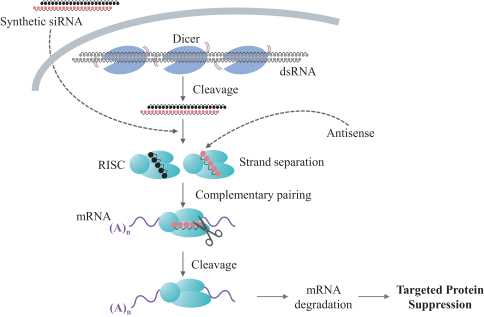
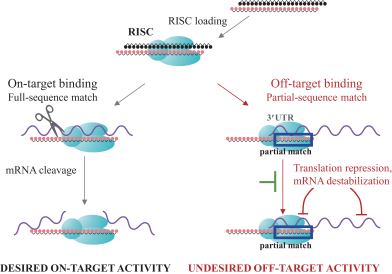
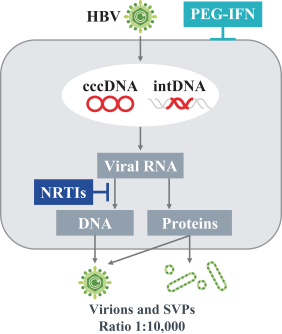
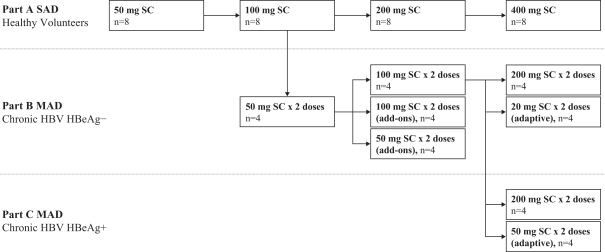
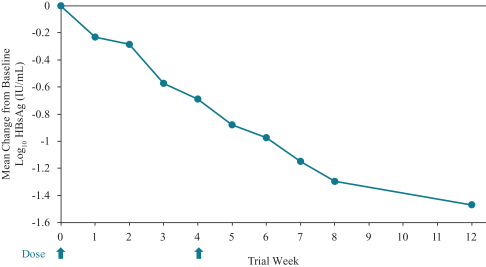
siRNA Platform
Licensed Patents
Our siRNA intellectual property portfolio includes three additional different patent families that we have exclusively licensed from Alnylam.
Two of the three families collectively include, as of May 31, 2019, seven issued patents in the United States directed to composition of matter claims, pharmaceutical composition claims and method of treatment claims. The20-year term of these patents is presently estimated to expire between 2024 and 2031, absent any available patent term adjustments or extensions. Additionally, the three patent families collectively include, as of May 31, 2019, 55 issued patents in Australia, Belgium, China, Croatia, Denmark, Finland, France, Germany, Hungary, Iceland, Ireland, Japan, Latvia, Lithuania, Luxembourg, Monaco, Macedonia, Macao, Netherlands, Norway, Russia, Singapore, Slovenia, Sweden, Switzerland and the United Kingdom directed to composition of matter claims, pharmaceutical composition claims, method of treatment claims and composition for use in treatment claims. The20-year term of these patents is presently estimated to expire between 2024 and 2031, absent any available patent term adjustments or extensions.
The three licensed families also collectively include, as of May 31, 2019, two patent applications in the United States and 17 patent applications in Australia, Canada, China, Europe, Hong Kong, Indonesia, India, Japan, Korea, Russia and Thailand directed to composition of matter claims, pharmaceutical composition claims, method of treatment claims and composition for use in treatment claims. The20-year term of the issued patent and any patents issuing from pending patent applications in these families is presently estimated to expire between 2024 and 2031, absent any available patent term adjustments or extensions.
We have also exclusively licensed from Alnylam, as of May 31, 2019, two issued patents in the United States directed to composition of matter claims, pharmaceutical composition claims and method of treatment claims. The20-year term of these patents is presently estimated to expire between 2022 and 2028, absent any available patent term adjustments or extensions.
We have also exclusively licensed from Alnylam, as of May 31, 2019, two patent applications in the United States directed to composition of matter claims and pharmaceutical composition claims. The20-year term of any
136