
OPTIMIZING DELIVERY AND EFFICACY OF NON - ADDICTIVE PAIN MEDICINES JANUARY 2021 Investor Presentation BUPIVACAINE Injectable Hydrogel for the extended release of local anesthetic DICLOFENAC Spray Film A topical anti - inflammatory for localized musculoskeletal pain ( Image is for illustration purposes only) ENKEPHALIN Nasal Spray a nano - peptide for the non - addictive management of acute and chronic pain, including pain associated with cancer 1 Filed Pursuant to Rule 433 of the Securities Act of 1933 Issuer Free Writing Prospectus dated January 8, 2021 Relating to the Preliminary Prospectus dated January 8, 2021 Registration Statement File No. 333 - 249417

CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS AND DISCLAIMERS All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward - looking statements . The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements . We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short - term and long - term business operations and objectives, and financial needs . These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those described in the “Risk Factors” section of the prospectus . Moreover, we operate in a very competitive and rapidly changing environment . New risks emerge from time to time . It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make . In light of these risks, uncertainties and assumptions, the future events and trends discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements . All references to dollar amounts in the offering summary or to use of proceeds are subject to change pending a final prospectus . This presentation highlights basic information about us and the offering . Because it is a summary, it does not contain all of the information that you should consider before investing . This offering may only be made by means of a prospectus . PROPRIETARY INFORMATION This document contains proprietary information that is the property of the company . Neither this document, nor the proprietary information contained herein, shall be published, reproduced, copied, disclosed or used for any other purpose, other than the review and consideration of this document . 2 Virpax Pharmaceuticals, Inc . (“we” or “us”) has filed a registration statement (including a preliminary prospectus) (the “Registration Statement”) with the Securities and Exchange Commission (the “SEC”) on Form S - 1 (SEC File No . 333 - 249417 ) for the offering to which this presentation relates . Such registration statement has not yet become effective . Shares of our common stock may not be sold, nor may offers to buy be accepted, prior to the time the registration statement becomes effective . Before you invest, you should read the preliminary prospectus and other documents we file with the SEC for more complete information about our company and this offering . You should read the prospectus in the Registration Statement and other documents that we have filed with the SEC for more complete information about us . You may access these documents for free by visiting EDGAR on the SEC website at www . sec . gov or by contacting ThinkEquity, a Division of Fordham Financial Mgmt . , Inc . , 17 State Street, 22 nd Flr, New York, NY 1004 , by telephone at ( 877 - 436 - 3673 or by e - mail at prospectus @thinkequity . com . FREE WRITING PROSPECTUS
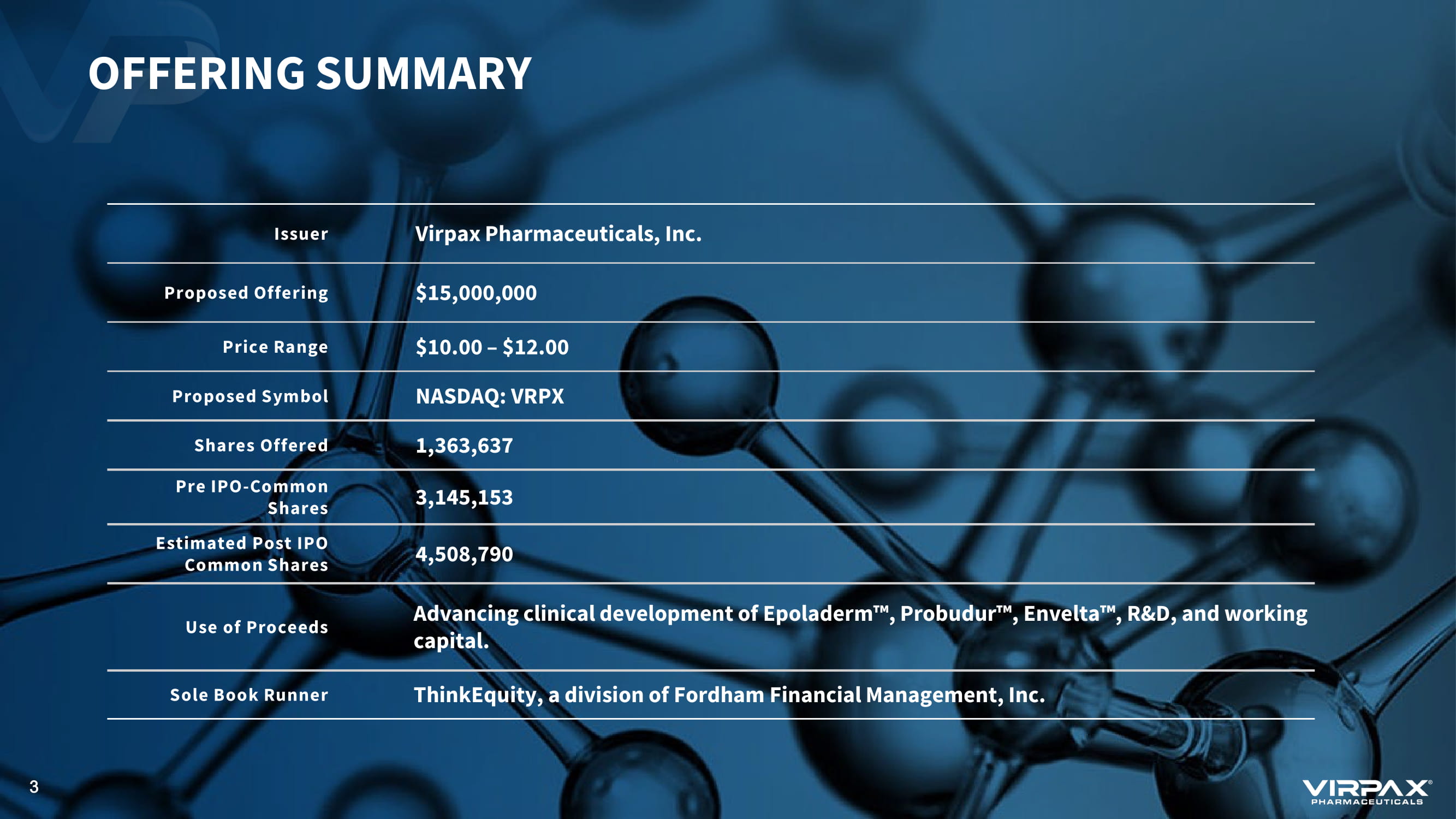
Issuer Virpax Pharmaceuticals, Inc. Proposed Offering $15,000,000 Price Range $10.00 – $12.00 Proposed Symbol NASDAQ: VRPX Shares Offered 1,363,637 Pre IPO - Common Shares 3,145,153 Estimated Post IPO Common Shares 4,508,790 Use of Proceeds Advancing clinical development of Epoladerm ҹ , Probudur ҹ , Envelta ҹ , R&D, and working capital. Sole Book Runner ThinkEquity, a division of Fordham Financial Management, Inc. OFFERING SUMMARY 3

MISSION STATEMENT Our mission is to improve widely - used and newly - developed non - addictive pain medicines by optimizing their delivery systems. 4

“A critical part of our efforts for 2019 is new steps to promote the development of drugs to treat pain that are not addictive.” Scott Gottlieb, MD - Statement by then - FDA Commissioner on the agency’s ongoing work to forcefully address the opioid crisis, February 2019 TRENDS IN PAIN TREATMENT 5

20% of the US Population Suffers from Chronic Pain OPIOIDS are an effective treatment option but can cause harm to patients There has been a 3X INCREASE in opioid prescriptions in the last 20 years INVESTOR OVERVIEW ▪ Developing treatments for the management of acute musculoskeletal pain, chronic pain, cancer pain, neuropathic pain and post - operative pain. ▪ Advancing research on non - addictive New Chemical Entities, New Molecular Entities and accelerated 505(b)(2) New Drug Applications. ▪ Executives, R&D team, partners and consultants have extensive experience in product approvals and brand - recognized product launches for Pain, CNS, and Inflammatory Diseases. ▪ Experienced commercial management team with proven track record developing, launching or marketing multiple brand - recognized pain products. ▪ NIH NCATS Grant awarded in August 2020 plus additional federal funding identified including: BARDA, the NIH and the DoD. ▪ Novel delivery systems can also serve as Platform Biotechnologies to potentially enhance the delivery of other drugs. . 1 2 1 Centers for Disease Control and Prevention (CDC), Prevalence of Chronic Pain and High - Impact Chronic Pain Among Adults – United States, 2016; September 14, 2018 2 National Institutes of Health (NIH), The Role of Opioids in the Treatment of Chronic Pain; June 5, 2020 6
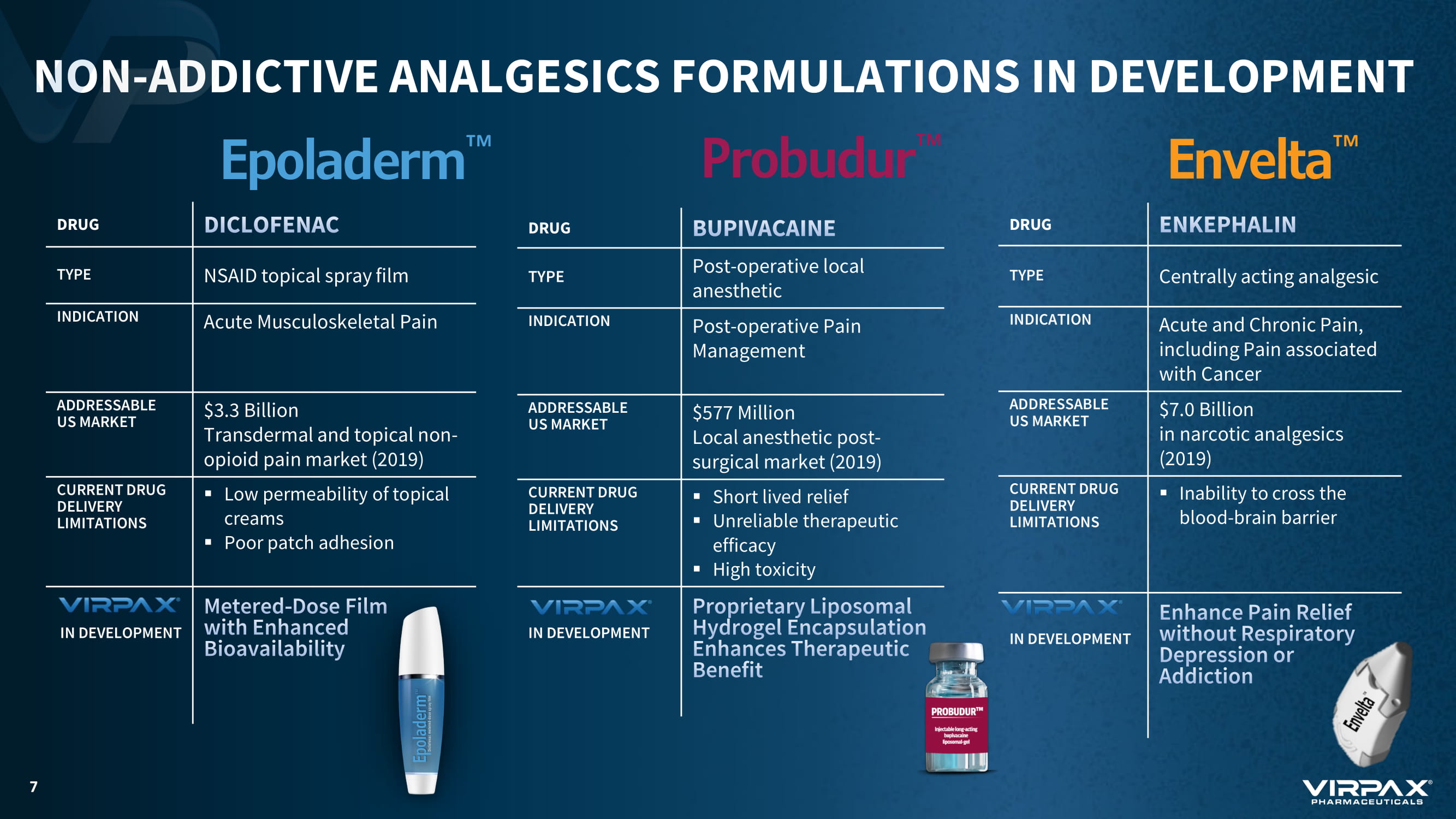
NON - ADDICTIVE ANALGESICS FORMULATIONS IN DEVELOPMENT DRUG TYPE NSAID topical spray film INDICATION Acute Musculoskeletal Pain ADDRESSABLE US MARKET $3.3 Billion Transdermal and topical non - opioid pain market (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Low permeability of topical creams ▪ Poor patch adhesion IN DEVELOPMENT DRUG TYPE Post - operative local anesthetic INDICATION Post - operative Pain Management ADDRESSABLE US MARKET $577 Million Local anesthetic post - surgical market (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Short lived relief ▪ Unreliable therapeutic efficacy ▪ High toxicity IN DEVELOPMENT DRUG TYPE Centrally acting analgesic INDICATION Acute and Chronic Pain, including Pain associated with Cancer ADDRESSABLE US MARKET $7.0 Billion in narcotic analgesics (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Inability to cross the blood - brain barrier IN DEVELOPMENT 7 Probudur Ρ Envelta Ρ Epoladerm Ρ
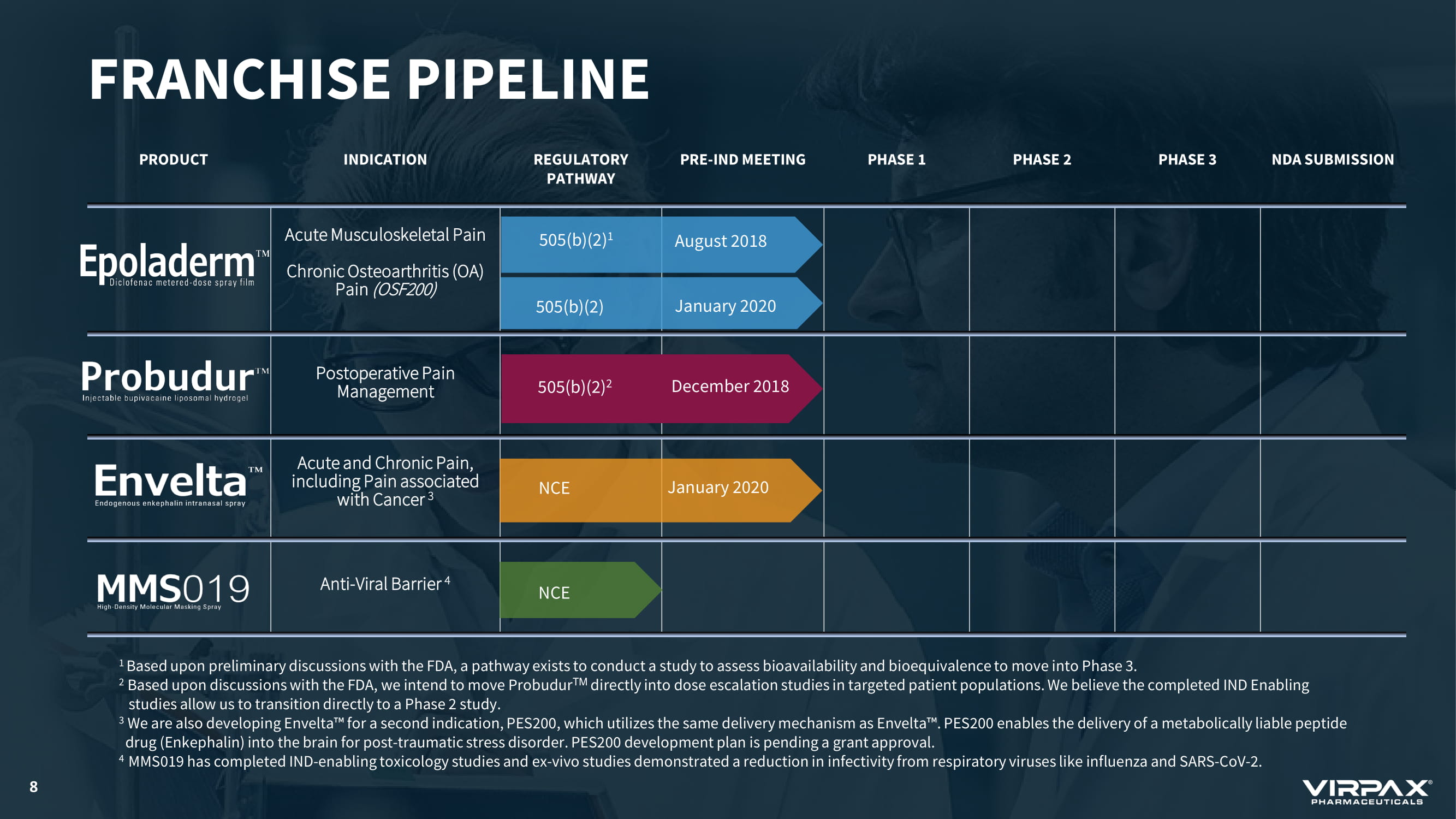
PRODUCT INDICATION REGULATORY PATHWAY PRE - IND MEETING PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Acute Musculoskeletal Pain Chronic Osteoarthritis (OA) Pain (OSF200) Postoperative Pain Management Acute and Chronic Pain, including Pain associated with Cancer 3 Anti - Viral Barrier 4 FRANCHISE PIPELINE 505(b)(2) 1 NCE 505(b)(2) 2 August 2018 December 2018 January 2020 8 505(b)(2) January 2020 NCE 1 Based upon preliminary discussions with the FDA, a pathway exists to conduct a study to assess bioavailability and bioequival enc e to move into Phase 3. 2 Based upon discussions with the FDA, we intend to move Probudur TM directly into dose escalation studies in targeted patient populations. We believe the completed IND Enabling studies allow us to transition directly to a Phase 2 study. 3 We are also developing Envelta ҹ for a second indication, PES200, which utilizes the same delivery mechanism as Envelta ҹ . PES200 enables the delivery of a metabolically liable peptide drug (Enkephalin) into the brain for post - traumatic stress disorder. PES200 development plan is pending a grant approval. 4 MMS019 has completed IND - enabling toxicology studies and ex - vivo studies demonstrated a reduction in infectivity from respirator y viruses like influenza and SARS - CoV - 2.

SKIN INCISION PRE - FILLED DEVICE WITH DICLOFENAC SOLUTION Specially formulated for metered - dosing CONCENTRATED DICLOFENAC SPRAY FILM With a high - level of adhesiveness, accessibility and skin - drying DRYING TIMES Between 60 and 90 seconds NOSE LONG - ACTING BUPIVACAINE Hydrogel encapsulated formulation for fast onset and long duration SATURATED DRUG SOLUTION ADMINISTRATION Surgeon injects therapy at site of wound CONCEPT Multi - use device and pressurized single use cartridge ENKEPHALIN NANOPARTICLES Protected by Molecular Envelope Technology DELIVERY Facilitated drug delivery by transport along the olfactory neurons VIRPAX THERAPIES’ DRUG DELIVERY SYSTEMS THE IMPORTANT ROLE OF DRUG DELIVERY AND THE BENEFITS OF PROPRIETARY FORMULATIONS CONVENIENT, EASY - TO - APPLY IMPROVED ONSET AND EXTENDED DURATION OF ACTION ACUTE AND CHRONIC PAIN, INCLUDING CANCER PAIN 9 ..REMAINS ON THE SKIN AS A SPRAY FILM

INDICATION REGULATORY PATHWAY PRE - IND MEETING PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Acute Musculoskeletal Pain Chronic Osteoarthritis (OA) Pain (OSF200) INDICATION MARKET SIZE DELIVERY SYSTEM Acute Musculoskeletal Pain $ 3.3 BILLION 505(b)(2) August 2018 A NOVEL, TOPICAL ANALGESIC FORMULATION 10 505(b)(2) January 2020
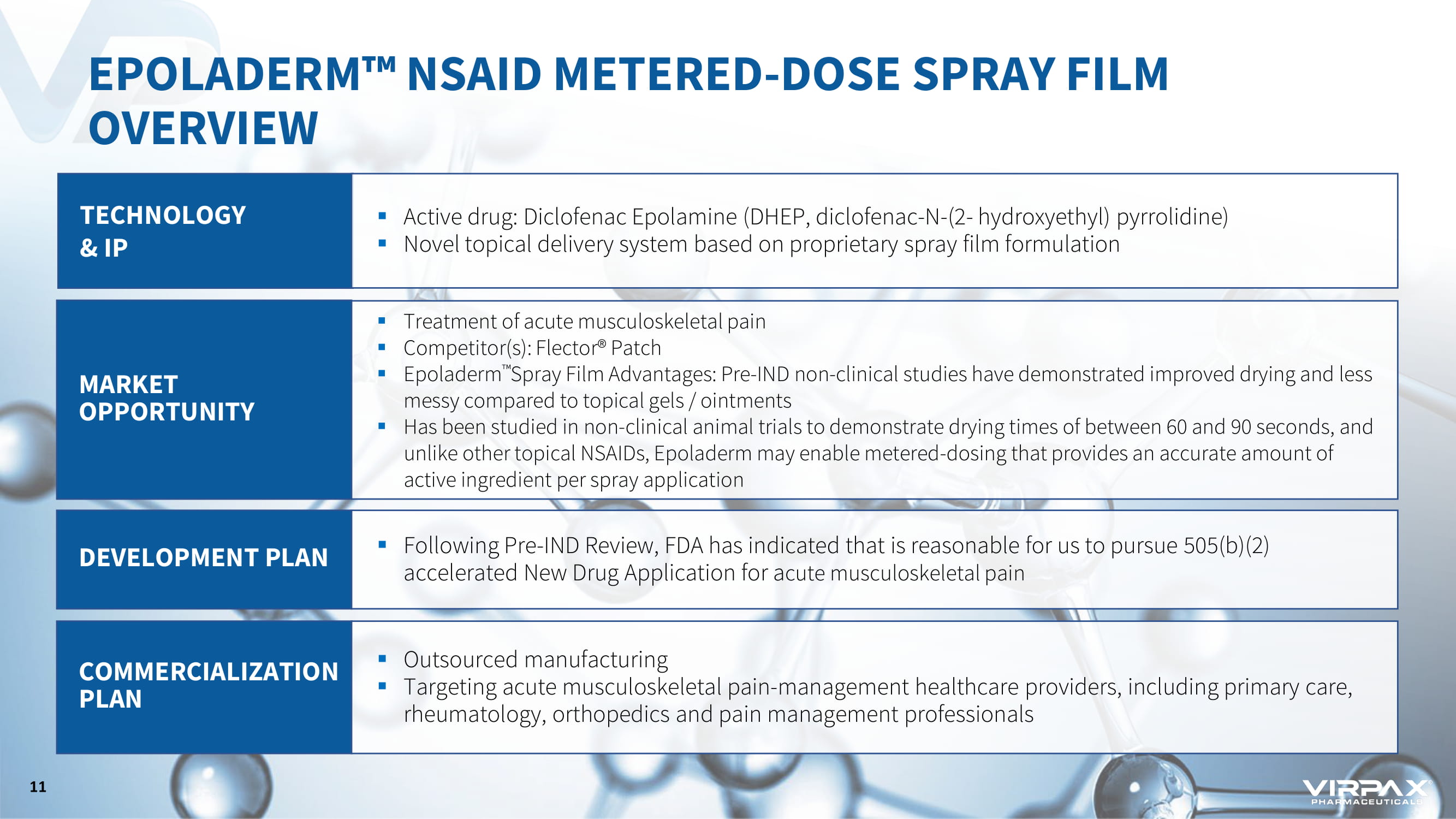
EPOLADERM ҹ NSAID METERED - DOSE SPRAY FILM OVERVIEW TECHNOLOGY & IP ▪ Active drug: Diclofenac Epolamine (DHEP, diclofenac - N - (2 - hydroxyethyl) pyrrolidine) ▪ Novel topical delivery system based on proprietary spray film formulation MARKET OPPORTUNITY ▪ Treatment of acute musculoskeletal pain ▪ Competitor(s): Flector® Patch ▪ Epoladerm ҹ Spray Film Advantages: Pre - IND non - clinical studies have demonstrated improved drying and less messy compared to topical gels / ointments ▪ Has been studied in non - clinical animal trials to demonstrate drying times of between 60 and 90 seconds, and u nlike other topical NSAIDs, Epoladerm may enable metered - dosing that provides an accurate amount of active ingredient per spray application DEVELOPMENT PLAN ▪ Following Pre - IND Review, FDA has indicated that is reasonable for us to pursue 505(b)(2) accelerated New Drug Application for a cute musculoskeletal pain COMMERCIALIZATION PLAN ▪ Outsourced manufacturing ▪ Targeting acute musculoskeletal pain - management healthcare providers, including primary care, rheumatology, orthopedics and pain management professionals 11
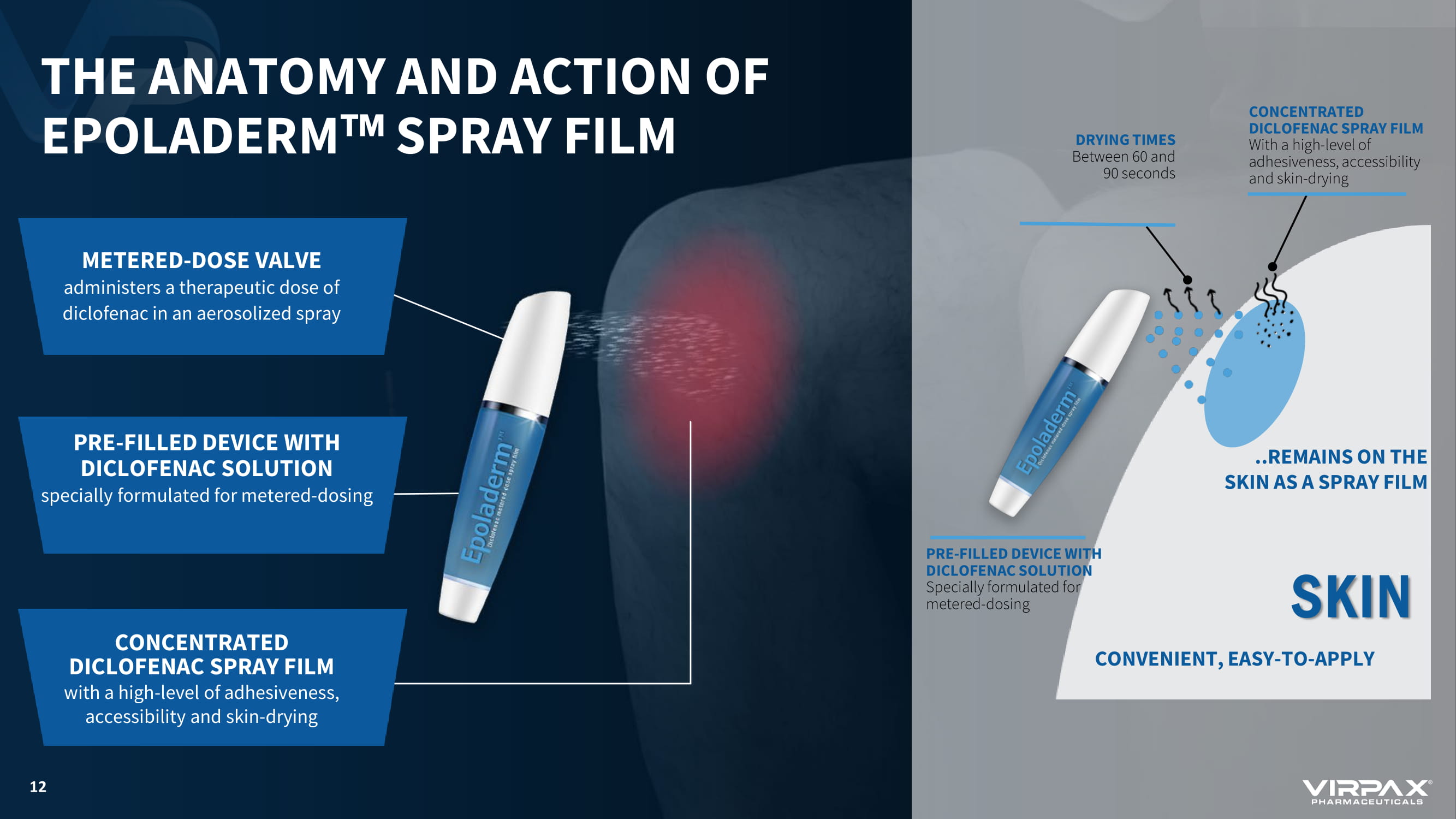
THE ANATOMY AND ACTION OF EPOLADERM w SPRAY FILM METERED - DOSE VALVE administers a therapeutic dose of diclofenac in an aerosolized spray CONCENTRATED DICLOFENAC SPRAY FILM with a high - level of adhesiveness, accessibility and skin - drying PRE - FILLED DEVICE WITH DICLOFENAC SOLUTION specially formulated for metered - dosing 12 SKIN PRE - FILLED DEVICE WITH DICLOFENAC SOLUTION Specially formulated for metered - dosing CONCENTRATED DICLOFENAC SPRAY FILM With a high - level of adhesiveness, accessibility and skin - drying DRYING TIMES Between 60 and 90 seconds CONVENIENT, EASY - TO - APPLY ..REMAINS ON THE SKIN AS A SPRAY FILM
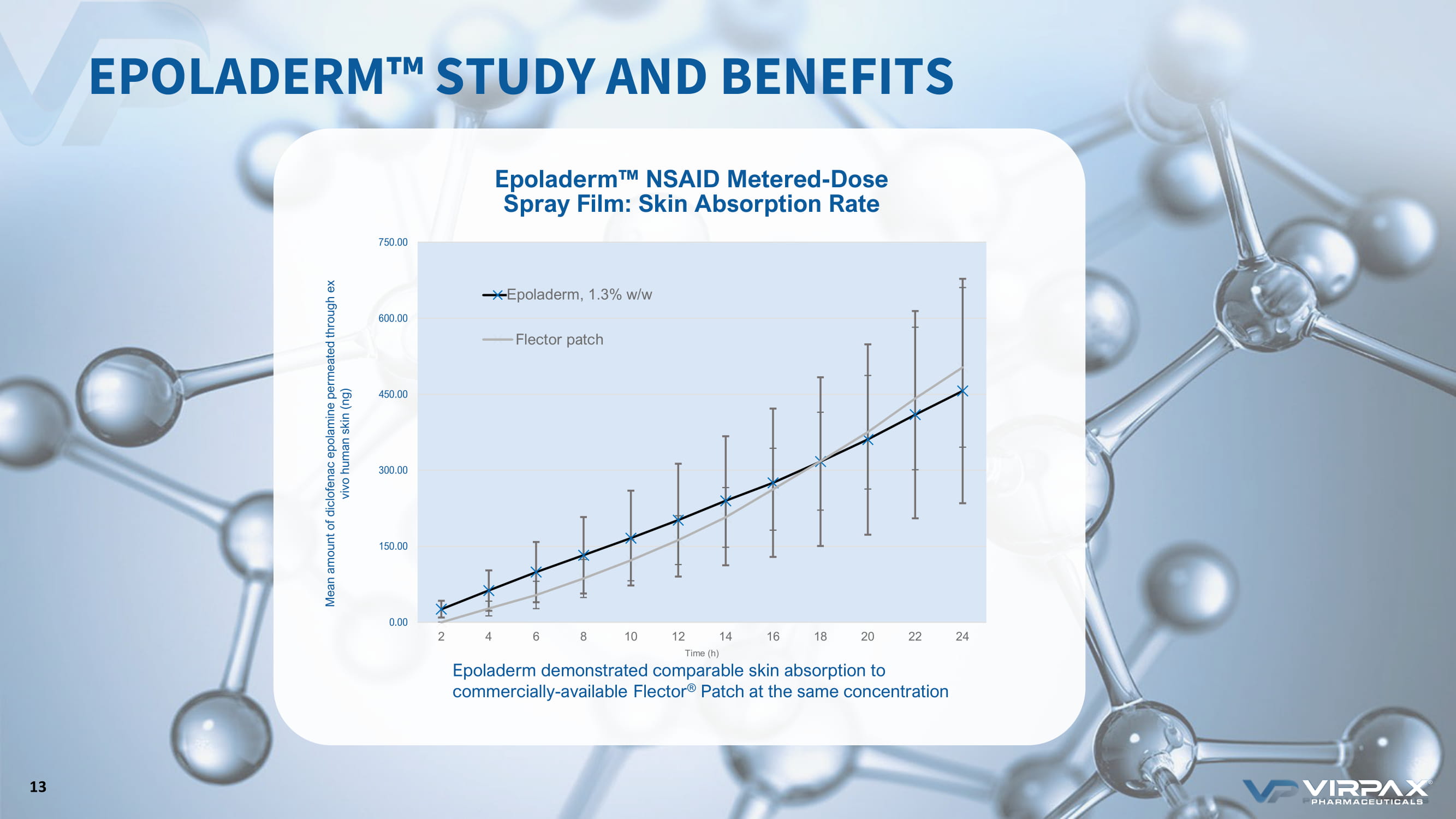
0.00 150.00 300.00 450.00 600.00 750.00 2 4 6 8 10 12 14 16 18 20 22 24 Mean amount of diclofenac epolamine permeated through ex vivo human skin (ng) Time (h) MS77, 1.3% w/w Flector patch Epoladerm, 1.3% w/w Epoladerm w NSAID Metered - Dose Spray Film: Skin Absorption Rate Epoladerm demonstrated comparable skin absorption to commercially - available Flector ® Patch at the same concentration EPOLADERM ҹ STUDY AND BENEFITS 13

14 EPOLADERM ҹ SPRAY FILM Competitive Advantages SPRAY FILM versus TOPICAL PATCHES and GELS METERED - DOSE SPRAY FILM MARKET POSITION The AAFP r eleased a new clinical guideline recommending that physicians treat acute pain from non - low back musculoskeletal injuries with topical NSAIDs as first - line therapy. ▪ Poor adhesion is the number one problem with patches ▪ Spray film may provide better adhesion to the skin (does not require tape reinforcement) ▪ Pliable, optimized for curved joints and contoured body parts ▪ Invisible, therefore more aesthetically appealing ▪ Less messy to apply, faster drying (Does not require physical handling of the drug) ▪ Delivers a measured dose ▪ Does not require physical handling to deliver active ingredient ▪ Future indications may include estrogen levels, Alzheimer’s disease, dementia, Parkinson’s disease, neuropathic issues, and acute and chronic pain ▪ 505(b)(2) abbreviated FDA regulatory development pathway ▪ Potential for osteoarthritis indication ▪ Exclusive worldwide rights 14

INDICATION MARKET SIZE DELIVERY SYSTEM Postoperative Pain Management $ 577 MILLION LONG - ACTING LOCAL ANESTHETICS: Liposomal Encapsulated Bupivacaine INDICATION REGULATORY PATHWAY PRE - IND MEETING PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Postoperative Pain Management 505(b)(2) December 2018 15

PROBUDUR ҹ LONG - ACTING LOCAL ANESTHETIC LIPOSOMAL HYDROGEL OVERVIEW TECHNOLOGY & IP ▪ Large Multi - Vesicular Vesicle (LMVV) product delivery system, consisting of encapsulated bupivacaine in hydrogel and other “GRAS” lipids MARKET OPPORTUNITY ▪ Improved onset and longer - lasting post - operative pain control ▪ Post - surgical treatment of pain via local injection ▪ Competitor: Exparel® 1.3% bupivacaine liposome injectable suspension DEVELOPMENT PLAN ▪ US FDA - 505(b)(2) abbreviated regulatory pathway ▪ Clinical Trials: Comparator products – Exparel® COMMERCIALIZATION PLAN ▪ Outsourced manufacturing ▪ Targeting anesthesiologists and all surgeons with a focus on general and orthopedic surgeries 16

PIG WOUND MODEL: Probudur w Superior to Free Bupivacaine and Exparel ® at 96 Hours Formulation Vehicle Probudur w 30mg/kg Probudur w 50mg/kg Free Bupivacaine 9mg/kg Exparel ® 30mg/kg ▪ Probudur ҹ demonstrated longer duration and higher peak activity compared to Exparel ® ▪ Persistent analgesia noted at 96 hours; 24 hours longer than competitor claims ▪ Peak achieved around 6 hours ▪ Considering successful Phase 3 Trials, Probudur ҹ may be the first long - acting local anesthetic with an opioid sparing label PROBUDUR w STUDY AND BENEFITS 17 Given the expanding role of ambulatory surgery and the need to facilitate an earlier hospital discharge, improving postoperative pain control has become an increasingly important issue for all anesthesiologists. Paul White, Researcher, 2005 The best approach is to start with a non - opioid. The Joint Commission, 2018

PROBUDUR vs. FREE BUPIVACAINE and EXPAREL® HYDROGEL ENCAPSULATION MARKET POSITION ▪ Anticipated, post - operative pain control for up to 96 hours, nearly 24 hours longer than Exparel ® ▪ Proprietary formulation for immediate and sustained pain control ▪ Developed by researchers at Hebrew University ▪ Encapsulation allows for a larger dose of bupivacaine per volume – 3.0% vs. 1.3% ▪ Hydrogel encapsulation concentrates drug at wound site, potentially reducing systemic exposure and risk to toxicity. ▪ 505(b)(2) abbreviated FDA regulatory development pathway ▪ Potential for opioid sparing label claim as well as comparator claim versus Exparel® ▪ Exclusive worldwide rights PROBUDUR w LONG - ACTING HYDROGEL Competitive Advantages 18 ADMINISTRATION Surgeon injects therapy at site of wound INCISION IMPROVED ONSET AND EXTENDED DURATION OF ACTION SATURATED DRUG SOLUTION LONG - ACTING BUPIVACAINE Hydrogel encapsulated formulation for fast onset and long duration

INDICATION REGULATORY PATHWAY PRE - IND MEETING PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Acute and Chronic Pain, including Pain associated with Cancer INDICATION MARKET SIZE DELIVERY SYSTEM Intranasal Enkephalin For Acute and Chronic Pain, including Pain associated with Cancer $ 7.0 BILLION NCE January 2020 ENKEPHALIN NANOPARTICLES WITH MOLECULAR ENVELOPE TECHNOLOGY (MET) 19
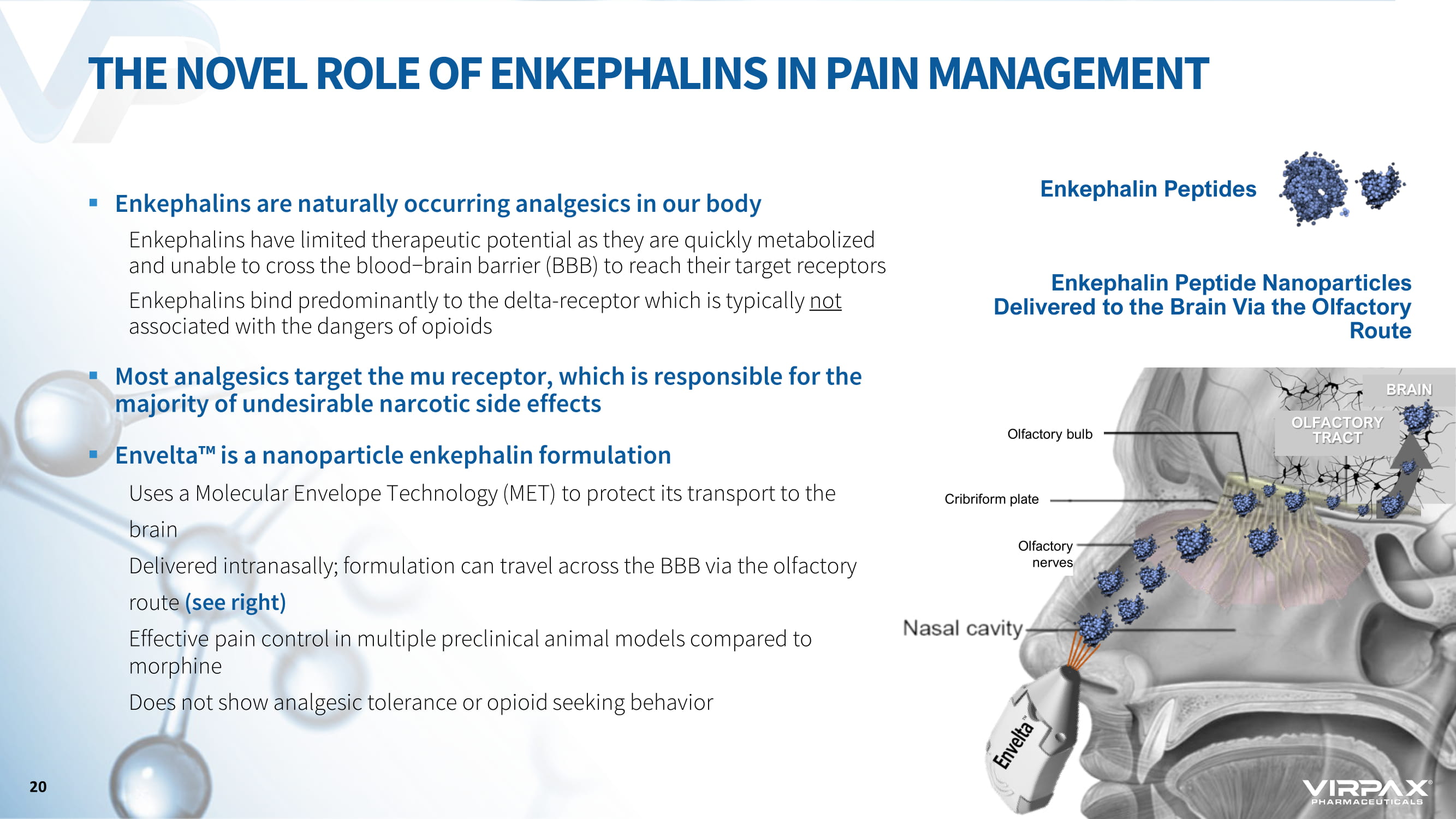
▪ Enkephalins are naturally occurring analgesics in our body Enkephalins have limited therapeutic potential as they are quickly metabolized and unable to cross the blood−brain barrier (BBB) to reach their target receptors Enkephalins bind predominantly to the delta - receptor which is typically not associated with the dangers of opioids ▪ Most analgesics target the mu receptor, which is responsible for the majority of undesirable narcotic side effects ▪ Envelta ҹ is a nanoparticle enkephalin formulation Uses a Molecular Envelope Technology (MET) to protect its transport to the brain Delivered intranasally; formulation can travel across the BBB via the olfactory route (see right) Effective pain control in multiple preclinical animal models compared to morphine Does not show analgesic tolerance or opioid seeking behavior THE NOVEL ROLE OF ENKEPHALINS IN PAIN MANAGEMENT Nasal cavity Enkephalin Peptides Olfactory bulb Olfactory nerves Cribriform plate OLFACTORY TRACT BRAIN Enkephalin Peptide Nanoparticles Delivered to the Brain Via the Olfactory Route 20
ENVELTA ҹ DELIVERS ENKEPHALIN NANOPARTICLES WITH MOLECULAR ENVELOPE TECHNOLOGY (MET) TECHNOLOGY & IP ▪ Leucine Enkephalin (L - ENK) nanoparticles in powdered form delivered through a novel intranasal device ▪ Expiry: 11/03/34 MARKET OPPORTUNITY ▪ Potentially disrupt opioid analgesic market for the treatment of acute and chronic pain, including pain associated with cancer ▪ Potential for treating post - traumatic stress disorder (PTSD) DEVELOPMENT PLAN ▪ NCE Regulatory Pathway ▪ Clinical Trials: Comparator – Morphine ▪ Entered into Cooperative Research and Development Agreement (CRADA) with National Center for Advancing Translational Sciences (NCATS) for continued development COMMERCIALIZATION PLAN ▪ Acute and Chronic Pain indications, including pain associated with cancer ▪ Outsourced manufacturing ▪ Targeting healthcare practitioners treating acute and chronic pain, including pain associated with cancer: Pain Specialists, Anesthesiologists, Orthopedists, PCPs, NPs/PAs, Oncologists and Neurologists 21
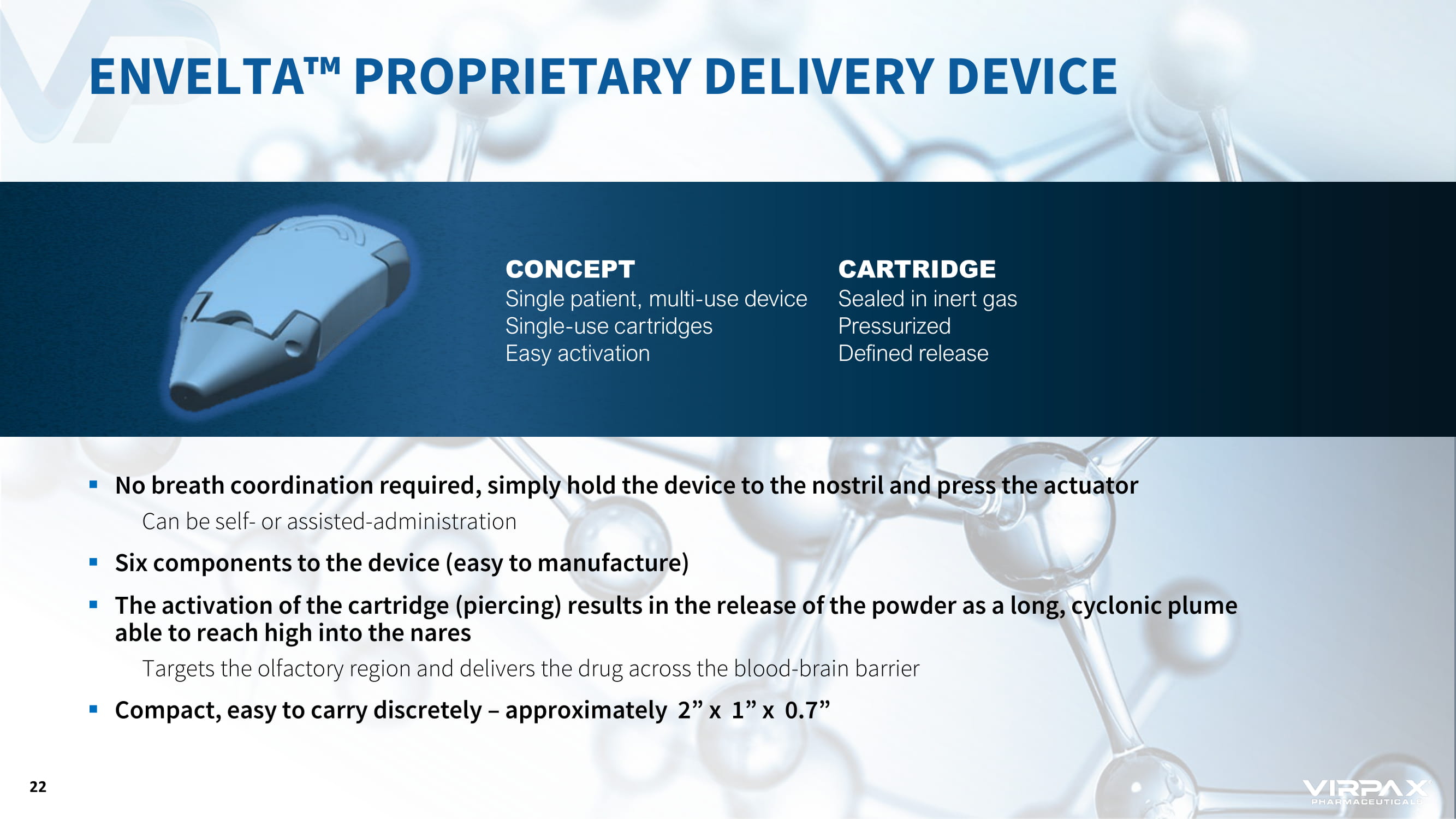
CONCEPT Single patient, multi - use device Single - use cartridges Easy activation CARTRIDGE Sealed in inert gas Pressurized Defined release ▪ No breath coordination required, simply hold the device to the nostril and press the actuator Can be self - or assisted - administration ▪ Six components to the device (easy to manufacture) ▪ The activation of the cartridge (piercing) results in the release of the powder as a long, cyclonic plume able to reach high into the nares Targets the olfactory region and delivers the drug across the blood - brain barrier ▪ Compact, easy to carry discretely – approximately 2” x 1” x 0.7” ENVELTA ҹ PROPRIETARY DELIVERY DEVICE 22

FORMULATION BENEFIT MARKET POSITION ▪ Novel enkephalin formulation ▪ Potential for comparable efficacy to mu opioid without dangerous side effects ▪ No tolerance, dependence or re ward seeking behavior in animal models ▪ MET encapsulation protects enkephalins from degradation ▪ Intranasal delivery device facilitates transport and delivery ▪ In development for acute and chronic pain, including pain associated with cancer ▪ Potential for treating post - traumatic stress disorder (PTSD) ▪ Outsourced manufacturing ▪ Exclusive worldwide rights ▪ Targeting oncologists, opioid prescribers and pain centers ENVELTA ҹ Competitive Advantages Delta opioid receptors have a built - in mechanism for pain relief and can be precisely targeted with drug - delivering nanoparticles, making them a promising target for treating chronic inflammatory pain with fewer side effects. Journal of Controlled Release. Volume 270, 28 Jan 2018, P135 - 144 23

24 Anthony P. Mack, MBA Chairman and Chief Executive Officer Gerald W. Bruce EVP, Commercial Operations EXECUTIVE LEADERSHIP • More than 25 years experience in pharmaceutical and finance industries • Founding Chairman & CEO of Virpax (led acquisition of three drug delivery platforms focused on non - addictive pain management) • Founding Chairman & CEO of SCILEX Pharmaceuticals • Founding President and Director of ProSolus Pharmaceuticals (Completed M&A of ProSolus to Mission Pharmacal) • Executive MBA in Pharmaceutical & Healthcare Marketing from Saint Joseph’s University • 30 years in the Pharmaceutical and Medical Nutrition industry • Started career at Johnson & Johnson where he was an award - winning sales representative and held leadership positions of increasing responsibility in sales & marketing ending his role as Group Product Director of Analgesics • VP, Sales at Bristol - Myers Squibb; led the Cardiovascular & Metabolic sales force • Senior VP, Commercial Operations at NitroMed; responsible for building commercial strategy; led team responsible for the development and implementation of the commercial plan for start - up of company’s first product for treatment of Heart Failure • Bachelor’s in Business Administration from Lincoln University; Master’s in Leadership from McDonough School of Business at Georgetown University • Nationally - renowned pain specialist with a career focused on research & new drug development • Co - founder, EVP, and CMO of Virpax Pharmaceuticals • Clinical Associate Professor in Anesthesiology at the Rutgers New Jersey Medical School • Board Certified in Pain Medicine, Anesthesiology, Addiction Medicine and Hospice and Palliative Medicine • Residency in anesthesiology completed at Yale University School of Medicine, New Haven, Connecticut • Postdoctoral fellowship in pain medicine at the Yale Center for Pain Management, where he was actively involved in research and teaching Jeffrey Gudin, MD EVP, Chief Medical Officer • Managing Member of Chipman & Chipman, LLC since November 2000, a consulting firm that assists public companies with preparation of periodic reports required to be filed with the SEC & compliance with Section 404 of the Sarbanes Oxley Act of 2002 • CFO & Secretary of Capital Gold Corp, a publicly - held gold production & exploration company until its acquisition by AuRico Gold, Inc. • B.A. in Economics from Ursinus College and is a Certified Public Accountant and a member of the American and Pennsylvania Institute of Certified Public Accountants Christopher M. Chipman, CPA Chief Financial Officer

25 Eric Floyd, PhD Chairman, Scientific and Regulatory Advice Committee Thani Jambulingam, PhD Chairman, Corporate Governance INDEPENDENT DIRECTORS • Dr. Floyd has over 21 years of regulatory experience within the pharmaceutical industry, including Axovant Sciences, Dohmen Life Science Services, Lundbeck Inc., and Hospira. • Dr. Jambulingam is a Pfizer Fellow and Professor in the Department of Pharmaceutical and Healthcare Marketing at St. Joseph’s University, Erivan K. Haub School of Business, Philadelphia, Pennsylvania • Mr. Sendrow has been a Certified Financial Planner since 1986 and maintains a moderate practice Jerrold Sendrow, CFP Chairman, Audit Committee • Dr. Jacob has over 35 years of extensive experience in the pharmaceutical and biotechnology industries across multiple disciplines, including research and development, operations, business development, capital financing activities and senior management expertise Gary S. Jacob, PhD Director Vanila M. Singh, MD, MACM Director • Dr. Singh was the immediate past Chief Medical Officer in the US Department of Health and Human Services (“HHS”)

OBJECTIVE CAPITAL $7.0 Million 1.7 Million (1) (2) 1.1 Million 1.0 Million Working Capital & General and Administrative Expenses (3) 2.4 Million TOTAL $13.2 Million USE OF NET PROCEEDS (1) In August 2020, Virpax received an NCATS in - kind grant to further develop Envelta Ρ through NDA filing (2) Includes the repayment of $0.5 Million in convertible notes (3) Includes the repayment of $0.1 Million in outstanding promissory notes 26
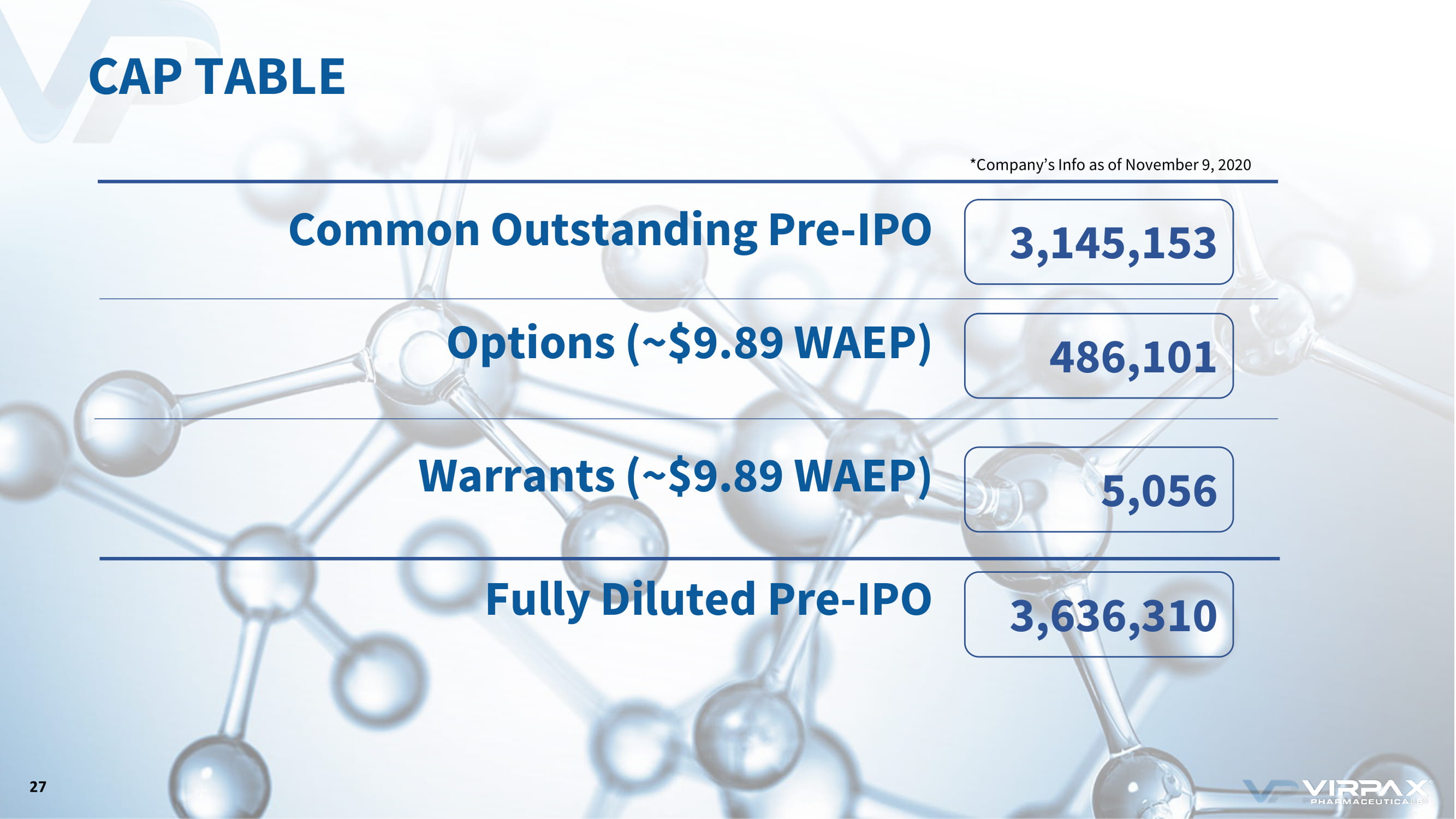
CAP TABLE 3,636,310 5,056 3,145,153 486,101 27 Common Outstanding Pre - IPO Options (~$9.89 WAEP) Warrants (~$9.89 WAEP) Fully Diluted Pre - IPO *Company’s Info as of November 9, 2020

COMPARABLES *Market Caps based on company’s closing share price as of January 7, 2021 $781 Million $1.77 Billion $17.57 Billion $2.94 Billion 28

INVESTOR HIGHLIGHTS 29 ▪ NCATS/NIH grant awarded for Envelta ҹ ▪ Envelta ҹ demonstrated effective pain control compared to morphine without analgesic tolerance ▪ Plan to file Pre - IND for Cancer Pain for Envelta ҹ in 2021 ▪ Probudur ҹ demonstrated longer duration and higher peak activity compared to Exparel® ▪ Submitted DoD grant applications for Probudur ҹ and PES200 ▪ Epoladerm ҹ demonstrated comparable skin absorption to Flector® Patch in ex - vivo model ▪ Viral inhibition was observed after infecting reconstituted epithelial cells with the SARS - CoV - 2 virus in the presence of MMS019




























