
OPTIMIZING DELIVERY OF NON - ADDICTIVE PAIN MEDICINES SEPTEMBER 2021 Investor Presentation BUPIVACAINE Injectable Hydrogel for the extended release of local anesthetic DICLOFENAC Spray Film A topical anti - inflammatory for localized musculoskeletal pain ( Image is for illustration purposes only) ENKEPHALIN Nasal Spray a nano - peptide for the non - addictive management of acute and chronic pain, including pain associated with cancer 1 Filed Pursuant to Rule 433 of the Securities Act of 1933 Issuer Free Writing Prospectus dated September 9, 2021 Relating to the Preliminary Prospectus dated September 9, 2021 Registration Statement File No. 333 - 259421 NASDAQ:VRPX

CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS AND DISCLAIMERS All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward - looking statements . The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements . We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short - term and long - term business operations and objectives, and financial needs . These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those described in the “Risk Factors” section of the prospectus . Moreover, we operate in a very competitive and rapidly changing environment . New risks emerge from time to time . It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make . In light of these risks, uncertainties and assumptions, the future events and trends discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements . All references to dollar amounts in the offering summary or to use of proceeds are subject to change pending a final prospectus . This presentation highlights basic information about us and the offering . Because it is a summary, it does not contain all of the information that you should consider before investing . This offering may only be made by means of a prospectus . PROPRIETARY INFORMATION This document contains proprietary information that is the property of the company . Neither this document, nor the proprietary information contained herein, shall be published, reproduced, copied, disclosed or used for any other purpose, other than the review and consideration of this document . 2 Virpax Pharmaceuticals, Inc . (“we” or “us”) has filed a registration statement (including a preliminary prospectus) (the “Registration Statement”) with the Securities and Exchange Commission (the “SEC”) on Form S - 1 (SEC File No . 333 - 259421 ) for the offering to which this presentation relates . Such registration statement has not yet become effective . Shares of our common stock may not be sold, nor may offers to buy be accepted, prior to the time the registration statement becomes effective . Before you invest, you should read the preliminary prospectus and other documents we file with the SEC for more complete information about our company and this offering . You should read the prospectus in the Registration Statement and other documents that we have filed with the SEC for more complete information about us . You may access these documents for free by visiting EDGAR on the SEC website at www . sec . gov or by contacting ThinkEquity , LLC, 17 State Street, 22 nd Flr, New York, NY 1004 , by telephone at ( 877 - 436 - 3673 or by e - mail at prospectus @thinkequity . com . FREE WRITING PROSPECTUS NASDAQ:VRPX

MISSION STATEMENT Our mission is to improve widely - used and newly - developed non - addictive pain medicines, as well as neurological product candidates, by optimizing our proprietary delivery systems. 3 NASDAQ:VRPX

INVESTMENT HIGHLIGHTS 4 ▪ Addressing an unmet global need in pain management with non - addictive, non - opioid pain product candidates ▪ 20% of the US population suffers from chronic pain 1 ▪ 3X INCREASE in opioid prescriptions in the last 20 years 2 ▪ Attempting to enhance the effectiveness of approved pain drugs through novel patented delivery platforms ▪ Potential to utilize these delivery platforms to expand our portfolio of indications ▪ Demonstrating 3 rd party validation through the NIH non - dilutive in - kind support program ▪ NIH NCATS In - Kind Award in 2020 ▪ Other grant applications pending ▪ Developing high density molecular masking spray, AnQlar ҹ (MMS019), as an anti - viral barrier ▪ Proven management team with experience in drug development, product approval and commercialization 1 Centers for Disease Control and Prevention (CDC), Prevalence of Chronic Pain and High - Impact Chronic Pain Among Adults – United States, 2016; September 14, 2018 2 National Institutes of Health (NIH), The Role of Opioids in the Treatment of Chronic Pain; June 5, 2020 NASDAQ:VRPX
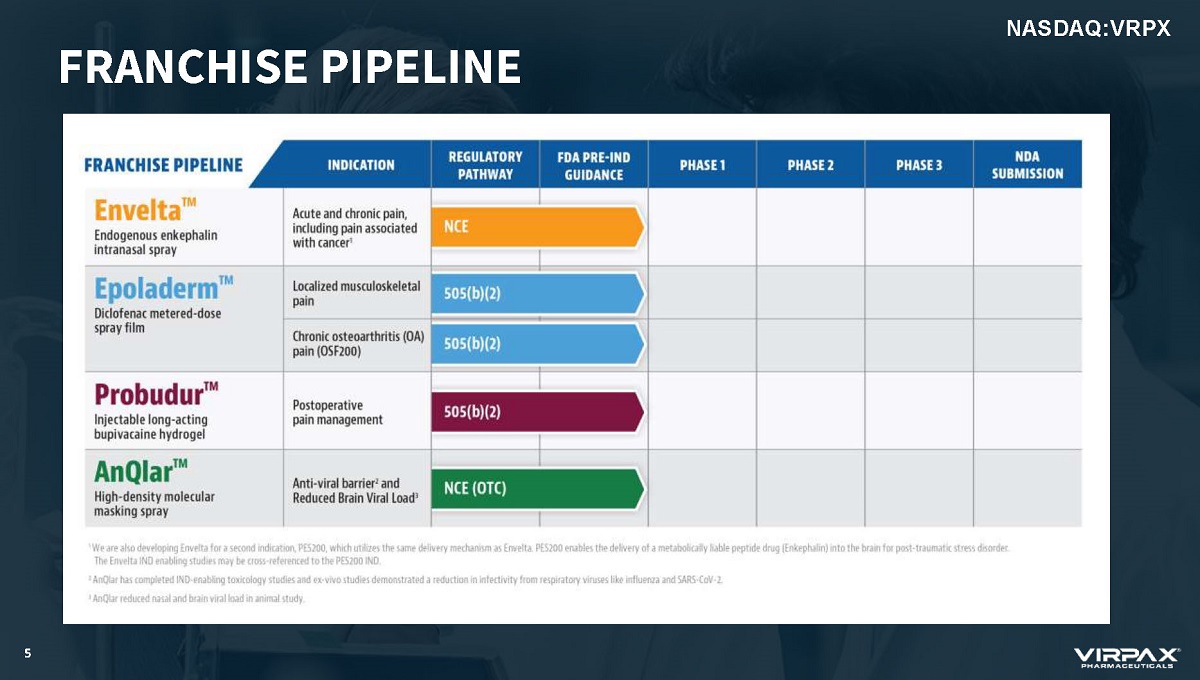
5 NASDAQ:VRPX FRANCHISE PIPELINE
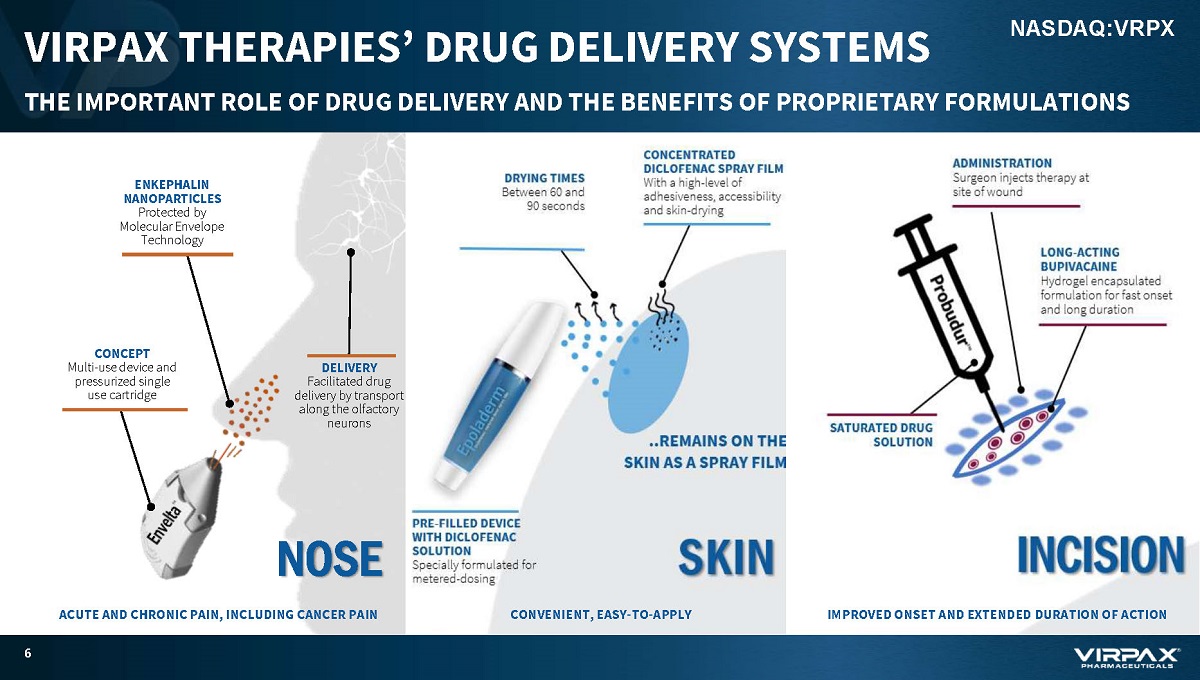
VIRPAX THERAPIES’ DRUG DELIVERY SYSTEMS THE IMPORTANT ROLE OF DRUG DELIVERY AND THE BENEFITS OF PROPRIETARY FORMULATIONS 6 NASDAQ:VRPX DELIVERY Facilitated drug delivery by transport along the olfactory neurons NOSE ACUTE AND CHRONIC PAIN, INCLUDING CANCER PAIN CONCEPT Multi - use device and pressurized single use cartridge ENKEPHALIN NANOPARTICLES Protected by Molecular Envelope Technology IMPROVED ONSET AND EXTENDED DURATION OF ACTION CONVENIENT, EASY - TO - APPLY

NON - ADDICTIVE ANALGESICS FORMULATIONS IN DEVELOPMENT DRUG TYPE NSAID topical spray film INDICATION Localized Musculoskeletal Pain ADDRESSABLE US MARKET $3.3 Billion Topical musculoskeletal pain market (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Low permeability of topical creams ▪ Poor patch adhesion IN DEVELOPMENT DRUG TYPE Post - operative local anesthetic INDICATION Post - operative Pain Management ADDRESSABLE US MARKET $577 Million Local anesthetic post - surgical pain market (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Short lived relief ▪ Unreliable therapeutic efficacy ▪ High toxicity IN DEVELOPMENT DRUG TYPE Centrally acting analgesic INDICATION Acute and Chronic Pain, including Pain associated with Cancer ADDRESSABLE US MARKET $7.0 Billion in moderate to severe analgesic market (2019) CURRENT DRUG DELIVERY LIMITATIONS ▪ Inability to cross the blood - brain barrier IN DEVELOPMENT 7 Probudur Ρ Envelta Ρ Epoladerm Ρ NASDAQ:VRPX

RECENT EVENTS 8 ▪ IND Enabling Studies of Envelta ҹ underway in partnership with the NIH ▪ Charles River Laboratories initiated preclinical IND enabling studies of Epoladerm ҹ as of August 24, 2021 ▪ Entered into a commercial manufacturing and clinical supply agreement with Seqens for AnQlar ҹ (MMS019) ▪ AnQlar ҹ (MMS019) demonstrated 24 - hour duration, inhibition of viral replication of SARS and influenza, and reduced brain viral load in a recent animal study ▪ Engaged Syneos Health to assist with regulatory strategy and product development for AnQlar ҹ ▪ If we successfully complete the required clinical trials for this product candidate, we intend to move forward and pursue an NDA for AnQlar ҹ (MMS019) as a once daily intranasal treatment ▪ The FDA has indicated that, upon successful completion of studies, we may pursue an NDA drug approval with the Office of Non - Prescription Drugs ▪ We are optimizing the Liposomal Bupivacaine formulation for Probudur ҹ , which may allow the filing of provisional patents ▪ Manufacturing pre - clinical batches for stability batches, animal and toxicology ▪ Engaged Torreya Capital to advise on global partnering and sublicensing efforts NASDAQ:VRPX

INDICATION REGULATORY PATHWAY FDA PRE - IND GUIDANCE PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Acute and Chronic Pain, including Pain associated with Cancer INDICATION MARKET SIZE DELIVERY SYSTEM Intranasal Enkephalin For Acute and Chronic Pain, including Pain associated with Cancer $ 7.0 BILLION NCE ENKEPHALIN NANOPARTICLES WITH MOLECULAR ENVELOPE TECHNOLOGY (MET) 9 NASDAQ:VRPX
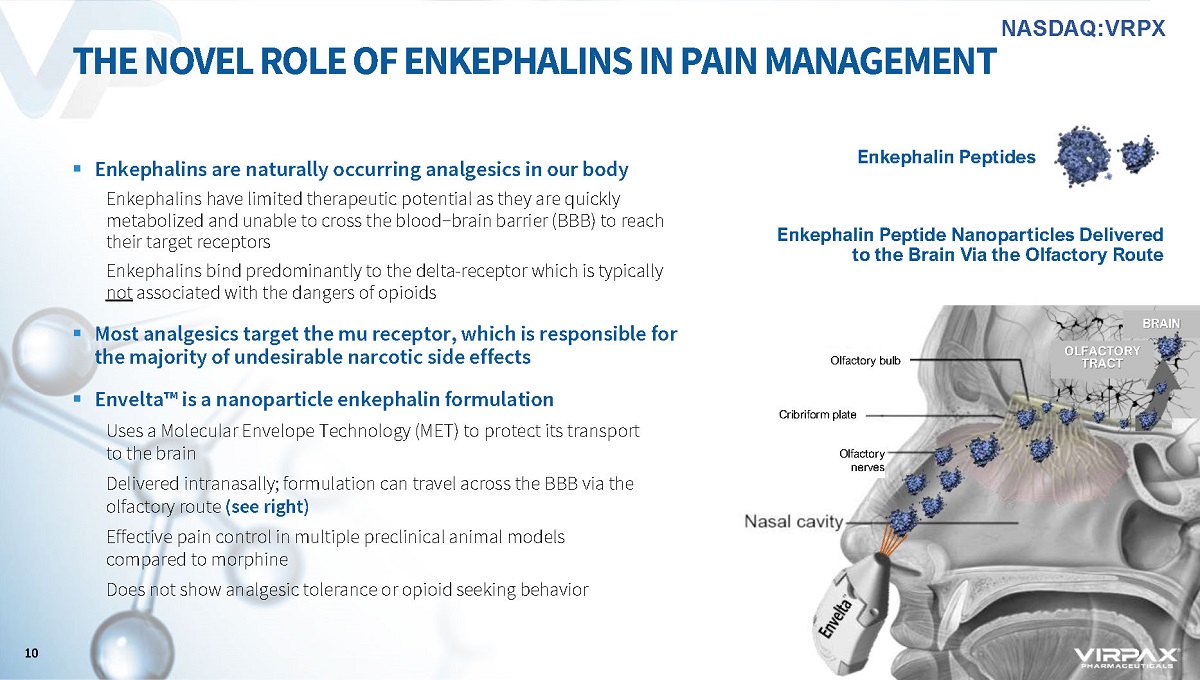
▪ Enkephalins are naturally occurring analgesics in our body Enkephalins have limited therapeutic potential as they are quickly metabolized and unable to cross the blood−brain barrier (BBB) to reach their target receptors Enkephalins bind predominantly to the delta - receptor which is typically not associated with the dangers of opioids ▪ Most analgesics target the mu receptor, which is responsible for the majority of undesirable narcotic side effects ▪ Envelta ҹ is a nanoparticle enkephalin formulation Uses a Molecular Envelope Technology (MET) to protect its transport to the brain Delivered intranasally; formulation can travel across the BBB via the olfactory route (see right) Effective pain control in multiple preclinical animal models compared to morphine Does not show analgesic tolerance or opioid seeking behavior THE NOVEL ROLE OF ENKEPHALINS IN PAIN MANAGEMENT Nasal cavity Enkephalin Peptides Olfactory bulb Olfactory nerves Cribriform plate OLFACTORY TRACT BRAIN Enkephalin Peptide Nanoparticles Delivered to the Brain Via the Olfactory Route 10 NASDAQ:VRPX
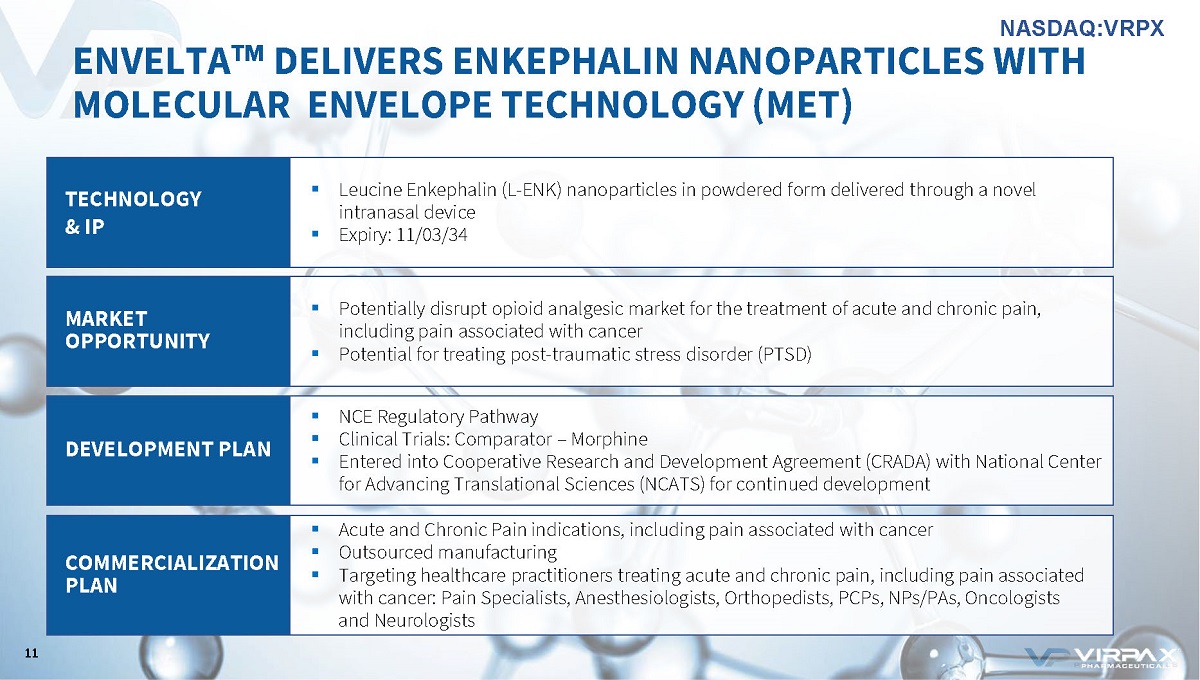
ENVELTA ҹ DELIVERS ENKEPHALIN NANOPARTICLES WITH MOLECULAR ENVELOPE TECHNOLOGY (MET) TECHNOLOGY & IP ▪ Leucine Enkephalin (L - ENK) nanoparticles in powdered form delivered through a novel intranasal device ▪ Expiry: 11/03/34 MARKET OPPORTUNITY ▪ Potentially disrupt opioid analgesic market for the treatment of acute and chronic pain, including pain associated with cancer ▪ Potential for treating post - traumatic stress disorder (PTSD) DEVELOPMENT PLAN ▪ NCE Regulatory Pathway ▪ Clinical Trials: Comparator – Morphine ▪ Entered into Cooperative Research and Development Agreement (CRADA) with National Center for Advancing Translational Sciences (NCATS) for continued development COMMERCIALIZATION PLAN ▪ Acute and Chronic Pain indications, including pain associated with cancer ▪ Outsourced manufacturing ▪ Targeting healthcare practitioners treating acute and chronic pain, including pain associated with cancer: Pain Specialists, Anesthesiologists, Orthopedists, PCPs, NPs/PAs, Oncologists and Neurologists 11 NASDAQ:VRPX
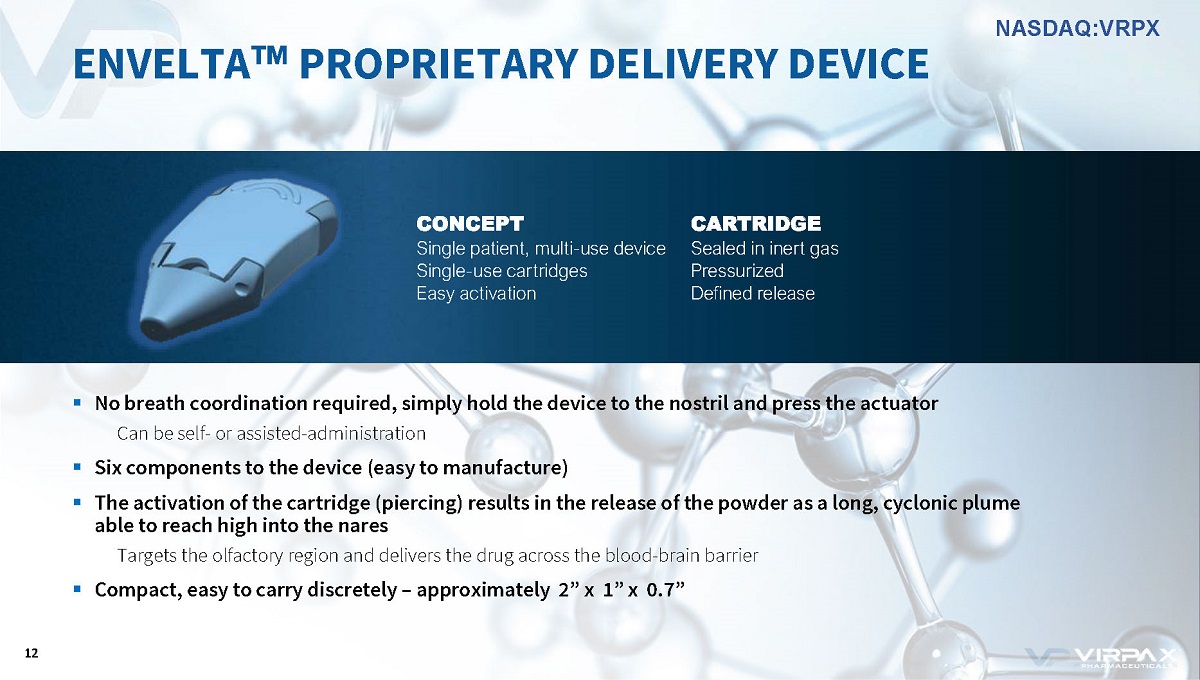
CONCEPT Single patient, multi - use device Single - use cartridges Easy activation CARTRIDGE Sealed in inert gas Pressurized Defined release ▪ No breath coordination required, simply hold the device to the nostril and press the actuator Can be self - or assisted - administration ▪ Six components to the device (easy to manufacture) ▪ The activation of the cartridge (piercing) results in the release of the powder as a long, cyclonic plume able to reach high into the nares Targets the olfactory region and delivers the drug across the blood - brain barrier ▪ Compact, easy to carry discretely – approximately 2” x 1” x 0.7” ENVELTA ҹ PROPRIETARY DELIVERY DEVICE 12 NASDAQ:VRPX

FORMULATION BENEFIT MARKET POSITION ▪ Novel enkephalin formulation ▪ Potential for comparable efficacy to mu opioid without dangerous side effects ▪ No tolerance, dependence or re ward seeking behavior in animal models ▪ MET encapsulation protects enkephalins from degradation ▪ Intranasal delivery device facilitates transport and delivery ▪ In development for acute and chronic pain, including pain associated with cancer ▪ Potential for treating post - traumatic stress disorder (PTSD) ▪ Outsourced manufacturing ▪ Exclusive worldwide rights ▪ Targeting oncologists, opioid prescribers and pain centers ENVELTA ҹ Competitive Advantages Delta opioid receptors have a built - in mechanism for pain relief and can be precisely targeted with drug - delivering nanoparticles, making them a promising target for treating chronic inflammatory pain with fewer side effects. Journal of Controlled Release. Volume 270, 28 Jan 2018, P135 - 144 13 NASDAQ:VRPX

INDICATION REGULATORY PATHWAY FDA PRE - IND GUIDANCE PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Anti - viral barrier and Reduced Brain Viral Load INDICATION MARKET SIZE DELIVERY SYSTEM Intranasal Mucosal Anti - Viral Barrier and Reduced Brain Viral Load $ 13.16 BILLION NCE (OTC) INTRANASAL MOLECULAR ENVELOPE TECHNOLOGY (MET) NANOPARTICLE DELIVERY 14 NASDAQ:VRPX
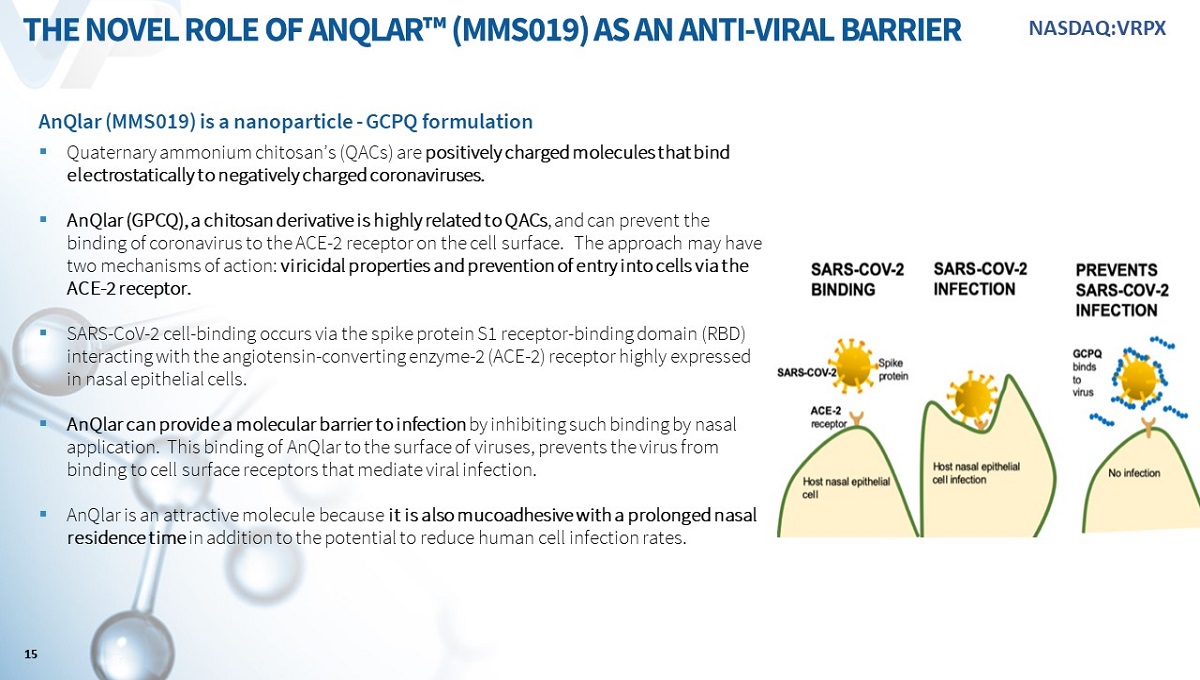
AnQlar (“MMS019”) is a nanoparticle - GCPQ formulation ▪ Quaternary ammonium chitosan’s (QACs) are positively charged molecules that bind electrostatically to negatively charged coronaviruses. ▪ AnQlar (GPCQ), a chitosan derivative is highly related to QACs , and can prevent the binding of coronavirus to the ACE - 2 receptor on the cell surface. The approach may have two mechanisms of action: viricidal properties and prevention of entry into cells via the ACE - 2 receptor. ▪ SARS - CoV - 2 cell - binding occurs via the spike protein S1 receptor - binding domain (RBD) interacting with the angiotensin - converting enzyme - 2 (ACE - 2) receptor highly expressed in nasal epithelial cells. ▪ AnQlar can provide a molecular barrier to infection by inhibiting such binding by nasal application. This binding of AnQlar to the surface of viruses, prevents the virus from binding to cell surface receptors that mediate viral infection. ▪ AnQlar is an attractive molecule because it is also mucoadhesive with a prolonged nasal residence time in addition to the potential to reduce human cell infection rates. THE NOVEL ROLE OF ANQLAR ҹ (MMS019) AS AN ANTI - VIRAL BARRIER 15 NASDAQ:VRPX
ANQLAR ҹ (MMS019) MOLECULAR ENVELOPE TECHNOLOGY (MET)IS A LIQUID NANOPARTICLE DISPERSION TECHNOLOGY & IP ▪ AnQlar ҹ ( MMS019) consists of GCPQ ( N - palmitoyl - N - monomethyl - N,N - dimethyl - N,N,N - trimethyl - 6 - O - glycolchitosan) , a low molecular weight chitosan derivative that confers anti - viral activity apparently based on electrostatic interactions between GCPQ and coro naviruses Expiry: 08/24/41 MARKET OPPORTUNITY ▪ First Responders, HEALTHCARE WORKERS, Clinics, ANTI - VAXERS”, TRANSPLANT AND OTHER IMMUNOCOMPROMISED OR AT RISK PATIENTS ▪ DOD/Military Forces, VA, and Federal Agencies ▪ Defense against disease spread in close settings: workplace, public transportation, etc. DEVELOPMENT PLAN ▪ NDA OTC Regulatory Pathway ▪ Complete second toxicology STUDY in Second Species ▪ Plan to complete all studies required for NDA submission with FDA Non - Prescription Division COMMERCIALIZATION Opportunity ▪ White Label Distribution with major Pharmacy Chains ▪ Contract Commercial Organization ▪ Joint Venture / Co - promote ▪ Sublicense / Divest 16 NASDAQ:VRPX

17 NASDAQ:VRPX ANQLAR ҹ (MMS019) Competitive Advantages FORMULATION ▪ Novel Liquid Dispersion GCPQ Nanoparticle formulation ▪ Nasal (+/ - Oral) spray for daily application ▪ Acts as a MOLECULAR MASK BENEFIT ▪ AnQlar (MMS019) is muco - adhesive and can be expected to stay at the application site at least 24 hours ▪ Preliminary in vitro, ex - vivo and in - vivo data demonstrated that AnQlar inhibits replication of SARS - CoV - 2 and may inhibit viral spread as well as viral brain load ▪ MMS019 shown to inhibit the ability of the virus to replicate at non - toxic concentrations MARKET POSITION ▪ Outsourced manufacturing ▪ Over - the - Counter (OTC) NDA ▪ Strategy Partner Opportunity
INDICATION REGULATORY PATHWAY FDA PRE - IND GUIDANCE PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Localized Musculoskeletal Pain Chronic Osteoarthritis (OA) Pain (OSF200) INDICATION MARKET SIZE DELIVERY SYSTEM Localized Musculoskeletal Pain $ 3.3 BILLION 505(b)(2) A NOVEL, TOPICAL ANALGESIC FORMULATION 18 505(b)(2) NASDAQ:VRPX
EPOLADERM ҹ NSAID METERED - DOSE SPRAY FILM OVERVIEW TECHNOLOGY & IP ▪ Active drug: Diclofenac Epolamine (DHEP, diclofenac - N - (2 - hydroxyethyl) pyrrolidine) ▪ Novel topical delivery system based on proprietary spray film formulation MARKET OPPORTUNITY ▪ Treatment of localized musculoskeletal pain ▪ Competitor(s): Flector® Patch; Pennsaid® ▪ Epoladerm ҹ Spray Film Advantages: Pre - IND non - clinical studies have demonstrated improved drying and less messy compared to topical gels / ointments ▪ Has been studied in non - clinical animal trials to demonstrate drying times of between 60 and 90 seconds, and u nlike other topical NSAIDs, Epoladerm may enable metered - dosing that provides an accurate amount of active ingredient per spray application DEVELOPMENT PLAN ▪ Following Pre - IND Review, FDA has indicated that is reasonable for us to pursue 505(b)(2) accelerated New Drug Application for localized musculoskeletal pain COMMERCIALIZATION PLAN ▪ Outsourced manufacturing ▪ Targeting localized musculoskeletal pain - management healthcare providers, including primary care, rheumatology, orthopedics and pain management professionals 19 NASDAQ:VRPX
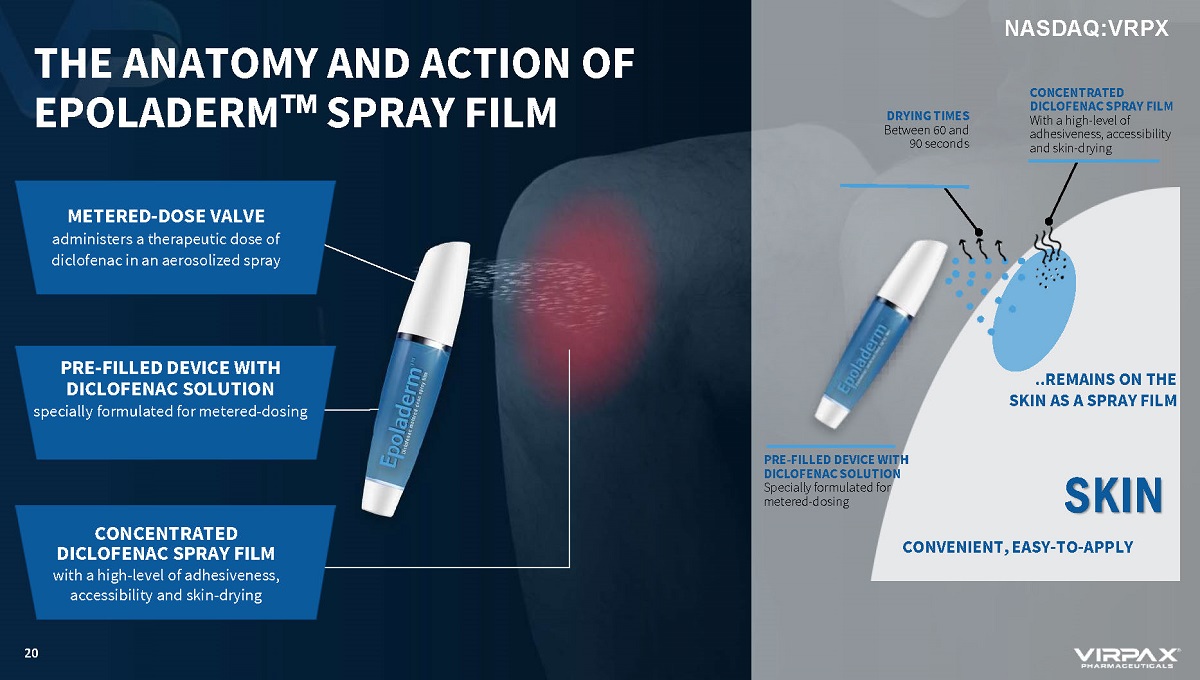
THE ANATOMY AND ACTION OF EPOLADERM w SPRAY FILM METERED - DOSE VALVE administers a therapeutic dose of diclofenac in an aerosolized spray CONCENTRATED DICLOFENAC SPRAY FILM with a high - level of adhesiveness, accessibility and skin - drying PRE - FILLED DEVICE WITH DICLOFENAC SOLUTION specially formulated for metered - dosing 20 SKIN PRE - FILLED DEVICE WITH DICLOFENAC SOLUTION Specially formulated for metered - dosing CONCENTRATED DICLOFENAC SPRAY FILM With a high - level of adhesiveness, accessibility and skin - drying DRYING TIMES Between 60 and 90 seconds CONVENIENT, EASY - TO - APPLY ..REMAINS ON THE SKIN AS A SPRAY FILM NASDAQ:VRPX
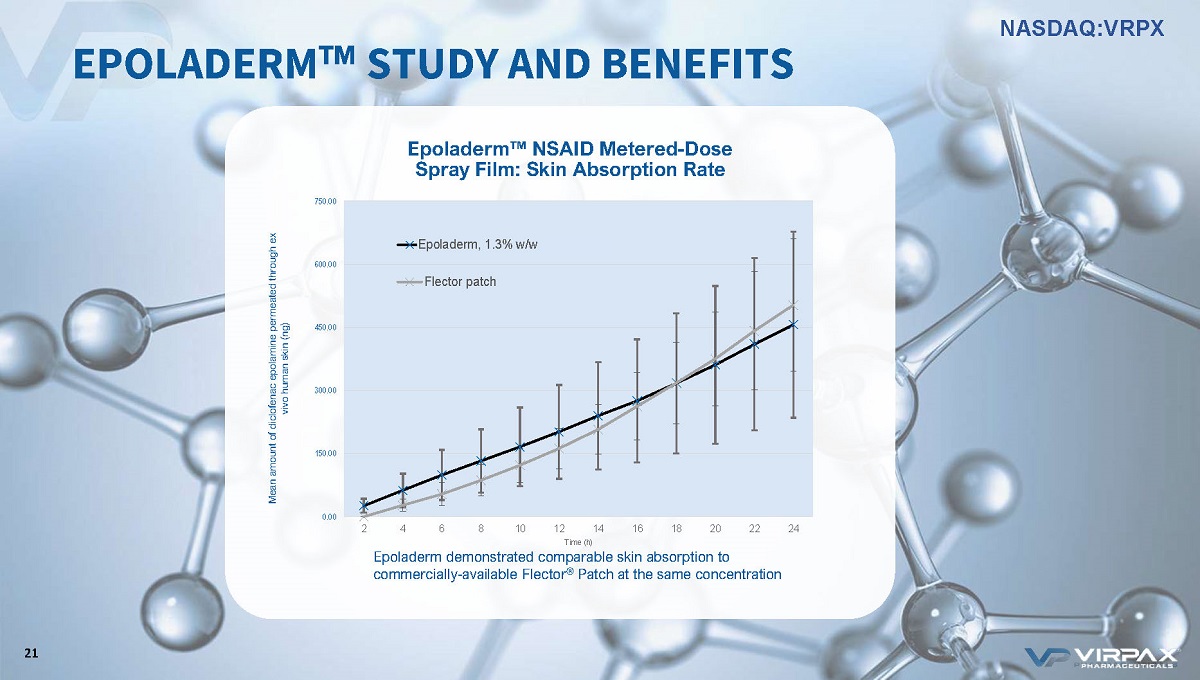
0.00 150.00 300.00 450.00 600.00 750.00 2 4 6 8 10 12 14 16 18 20 22 24 Mean amount of diclofenac epolamine permeated through ex vivo human skin (ng) Time (h) MS77, 1.3% w/w Flector patch Epoladerm, 1.3% w/w Epoladerm w NSAID Metered - Dose Spray Film: Skin Absorption Rate Epoladerm demonstrated comparable skin absorption to commercially - available Flector ® Patch at the same concentration EPOLADERM ҹ STUDY AND BENEFITS 21 NASDAQ:VRPX

22 EPOLADERM ҹ SPRAY FILM Competitive Advantages SPRAY FILM versus TOPICAL PATCHES and GELS METERED - DOSE SPRAY FILM MARKET POSITION The AAFP r eleased a new clinical guideline recommending that physicians treat acute pain from non - low back musculoskeletal injuries with topical NSAIDs as first - line therapy. ▪ Poor adhesion is the number one problem with patches ▪ Spray film may provide better adhesion to the skin (does not require tape reinforcement) ▪ Pliable, optimized for curved joints and contoured body parts ▪ Invisible, therefore more aesthetically appealing ▪ Less messy to apply, faster drying (does not require physical handling of the drug) ▪ Delivers a measured dose ▪ Does not require physical handling to deliver active ingredient ▪ Future indications may include estrogen levels, Alzheimer’s disease, dementia, and Parkinson’s disease ▪ 505(b)(2) abbreviated FDA regulatory development pathway ▪ Potential for osteoarthritis indication ▪ Exclusive worldwide rights 22 NASDAQ:VRPX

INDICATION MARKET SIZE DELIVERY SYSTEM Postoperative Pain Management $ 577 MILLION LONG - ACTING LOCAL ANESTHETICS: Liposomal Encapsulated Bupivacaine INDICATION REGULATORY PATHWAY FDA PRE - IND GUIDANCE PHASE 1 PHASE 2 PHASE 3 NDA SUBMISSION Postoperative Pain Management 505(b)(2) 23 NASDAQ:VRPX
PROBUDUR ҹ LONG - ACTING LOCAL ANESTHETIC LIPOSOMAL HYDROGEL OVERVIEW TECHNOLOGY & IP ▪ Large Multi - Vesicular Vesicle (LMVV) product delivery system, consisting of encapsulated bupivacaine in hydrogel and other “GRAS” lipids MARKET OPPORTUNITY ▪ Improved onset and longer - lasting post - operative pain control ▪ Post - surgical treatment of pain via local injection ▪ Competitor: Exparel® 1.3% bupivacaine liposome injectable suspension DEVELOPMENT PLAN ▪ US FDA - 505(b)(2) abbreviated regulatory pathway ▪ Clinical Trials: Comparator products – Exparel® COMMERCIALIZATION PLAN ▪ Outsourced manufacturing ▪ Targeting anesthesiologists and all surgeons with a focus on general and orthopedic surgeries 24 NASDAQ:VRPX
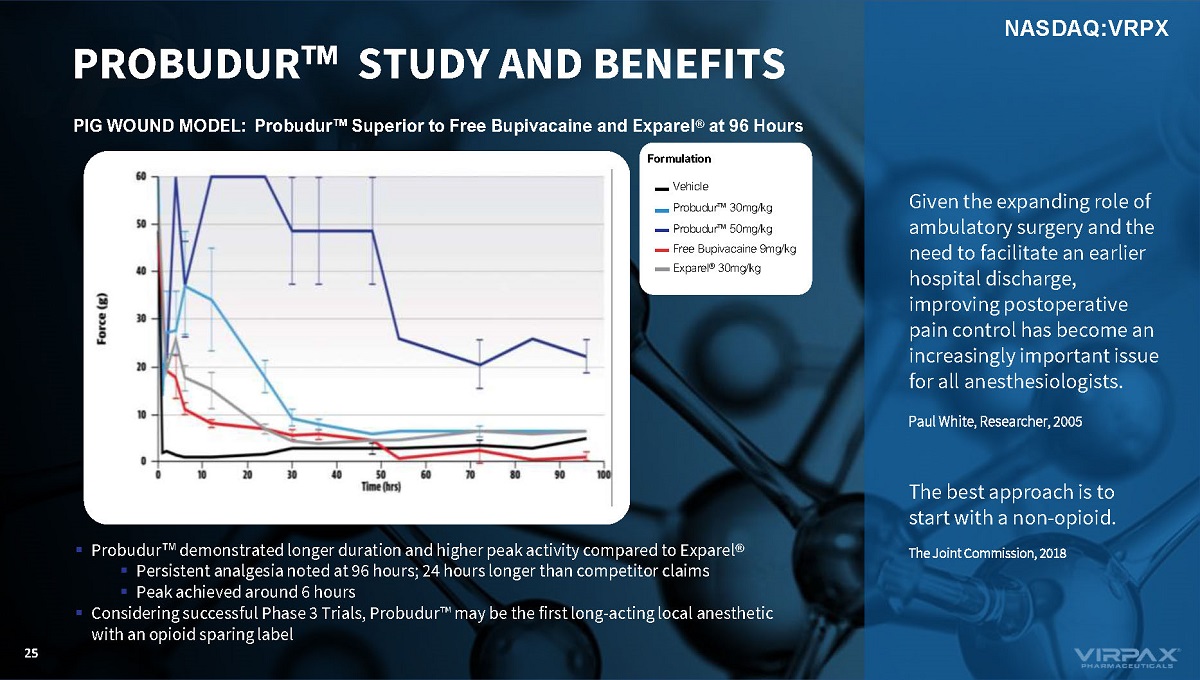
PIG WOUND MODEL: Probudur w Superior to Free Bupivacaine and Exparel ® at 96 Hours Formulation Vehicle Probudur w 30mg/kg Probudur w 50mg/kg Free Bupivacaine 9mg/kg Exparel ® 30mg/kg ▪ Probudur ҹ demonstrated longer duration and higher peak activity compared to Exparel ® ▪ Persistent analgesia noted at 96 hours; 24 hours longer than competitor claims ▪ Peak achieved around 6 hours ▪ Considering successful Phase 3 Trials, Probudur ҹ may be the first long - acting local anesthetic with an opioid sparing label PROBUDUR w STUDY AND BENEFITS 25 Given the expanding role of ambulatory surgery and the need to facilitate an earlier hospital discharge, improving postoperative pain control has become an increasingly important issue for all anesthesiologists. Paul White, Researcher, 2005 The best approach is to start with a non - opioid. The Joint Commission, 2018 NASDAQ:VRPX
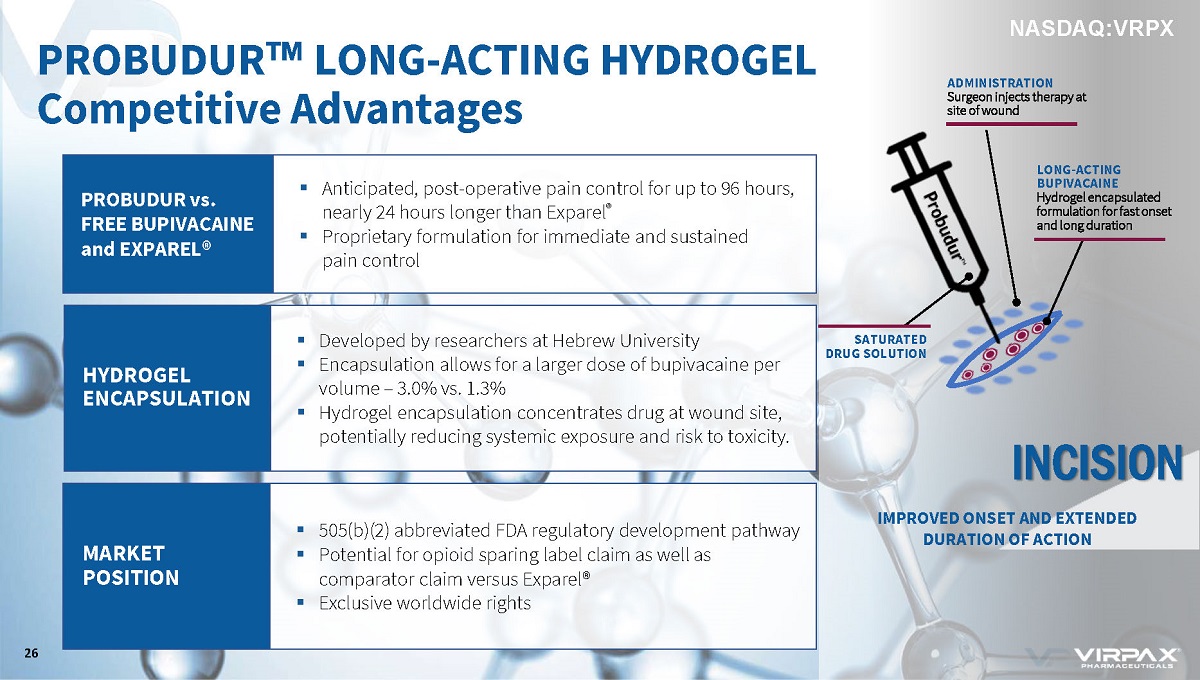
PROBUDUR vs. FREE BUPIVACAINE and EXPAREL® HYDROGEL ENCAPSULATION MARKET POSITION ▪ Anticipated, post - operative pain control for up to 96 hours, nearly 24 hours longer than Exparel ® ▪ Proprietary formulation for immediate and sustained pain control ▪ Developed by researchers at Hebrew University ▪ Encapsulation allows for a larger dose of bupivacaine per volume – 3.0% vs. 1.3% ▪ Hydrogel encapsulation concentrates drug at wound site, potentially reducing systemic exposure and risk to toxicity. ▪ 505(b)(2) abbreviated FDA regulatory development pathway ▪ Potential for opioid sparing label claim as well as comparator claim versus Exparel® ▪ Exclusive worldwide rights PROBUDUR w LONG - ACTING HYDROGEL Competitive Advantages 26 ADMINISTRATION Surgeon injects therapy at site of wound INCISION IMPROVED ONSET AND EXTENDED DURATION OF ACTION SATURATED DRUG SOLUTION LONG - ACTING BUPIVACAINE Hydrogel encapsulated formulation for fast onset and long duration NASDAQ:VRPX

EXECUTIVE LEADERSHIP 27 NASDAQ:VRPX ▪ 25+ years of pharmaceutical experience ▪ Executive experience at EKR Therapeutics, Endo Pharmaceuticals, and Purdue Pharmaceuticals ▪ Founder of Virpax, Scilex Pharmaceuticals and Prosolus ANTHONY P. MACK, MBA Chairman & CEO ▪ 25+ years pain management, clinician, consultant & researcher ▪ Board certified in Anesthesiology, Pain Management, Addiction Medicine and Palliative Care ▪ Anesthesiologist, Pain Management Fellowship at Yale University School of Medicine ▪ Professor, University of Miami, Department of Anesthesiology and Pain Management JEFFREY GUDIN, MD EVP, Chief Medical Officer ▪ 25+ years of public and private accounting experience ▪ Managing Partner of Chipman & Chipman, LLC ▪ CFO, NYSE:AMEX listed Capital Gold Corporation ▪ Public accounting experience at Grant Thornton, LLP CHRISTOPHER M. CHIPMAN, CPA Chief Financial Officer ▪ 30+ years in pharmaceutical & medical nutrition industry ▪ Group Product Director, Analgesics at Johnson and Johnson ▪ VP, Sales at Bristol - Myers Squibb, $1.2B in Sales ▪ VP, Managed Markets Bristol - Myers Squibb, $7B US Sales GERALD W. BRUCE EVP, Commercial Operations ▪ 20+ years of pharmaceutical industry experience ▪ Expertise in global regulatory & drug development ▪ Senior Director Global Regulatory Affairs at Sun Pharma ▪ Leadership roles at Merck US and Aventis Pharmaceuticals SHEILA A. MATHIAS, PHD, JD, MBA Chief Scientific Officer

INDEPENDENT DIRECTORS 28 NASDAQ:VRPX ▪ Chairman, Scientific and Technology Committee; Compensation Committee ▪ 21+ years of regulatory experience within the pharmaceutical industry, including Neurogene, Axovant Sciences, Dohmen Life Science Services, and Lundbeck ERIC FLOYD, PHD ▪ Chairman, Audit Committee ▪ Certified Financial Planner since 1986 and maintains an active practice ▪ Public company management experience JERROLD SENDROW, CFP ▪ Chairman, Corporate Governance and Nominating Committee ▪ Pfizer Fellow and Professor in the Department of Pharmaceutical and Healthcare Marketing at St. Joseph’s University, Erivan K. Haub School of Business, Philadelphia, Pennsylvania THANI JAMBULINGAM, PHD ▪ 35+ years experience in the pharmaceutical and biotechnology industries ▪ Co - Founder and Former CEO, Synergy Pharmaceuticals ▪ Former CEO, Immuron, Inc. GARY S. JACOB, PHD ▪ Immediate past Chief Medical Officer in the US Department of Health and Human Services ▪ Board certified in Pain Medicine and Anesthesiology ▪ Clinical Associate Professor of Anesthesiology, Pain and Peri - operative Medicine at Stanford VANILA M. SINGH, MD, MACM ▪ Certified Public Account (CPA) ▪ Former Managing Partner, RSMUS LP (RSM) ▪ Independent consultant and advisor with 40+ years experience in manufacturing, distribution, financial services, and pharma MICHAEL F. DUBIN, CPA
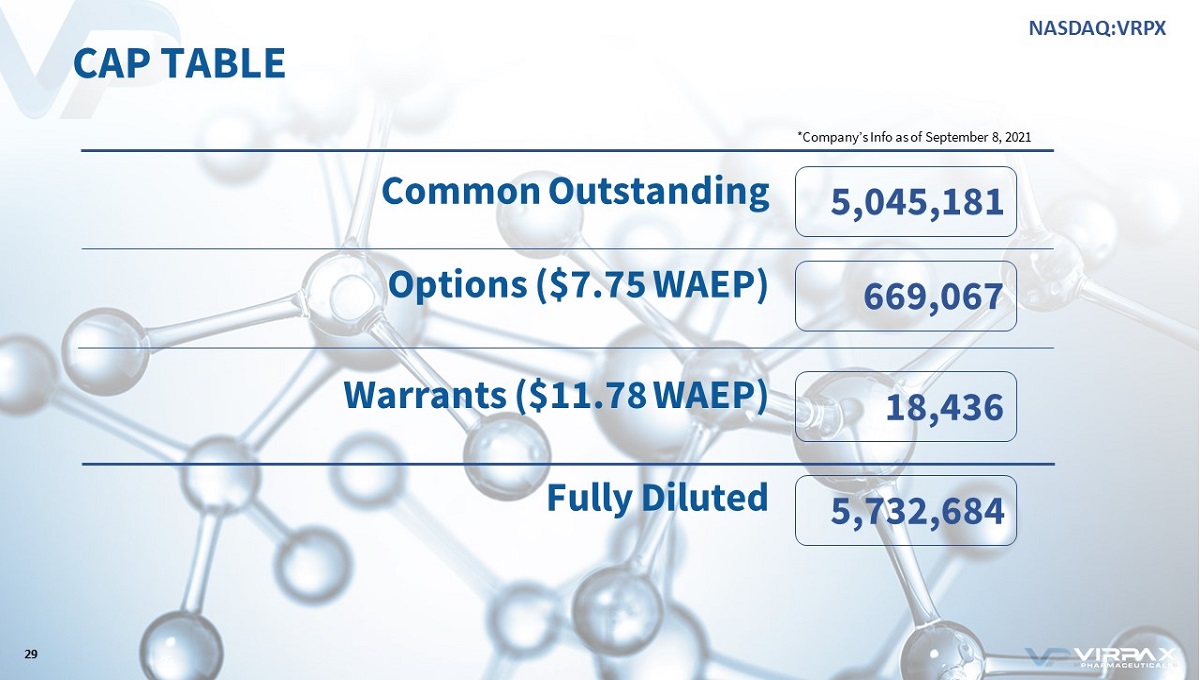
CAP TABLE 5,747,633 18,436 5,030,018 699,179 29 Common Outstanding Options ($7.84 WAEP) Warrants ($11.78 WAEP) Fully Diluted *Company’s Info as of August 31, 2021 NASDAQ:VRPX

Christopher Chipman, CPA Chief Financial Officer cchipman@virpaxpharma.com 610 - 727 - 4597 30 Investor Relations Betsy Brod Affinity Growth Advisors betsy.brod@affinitygrowth.com 212 - 661 - 2231 NASDAQ:VRPX
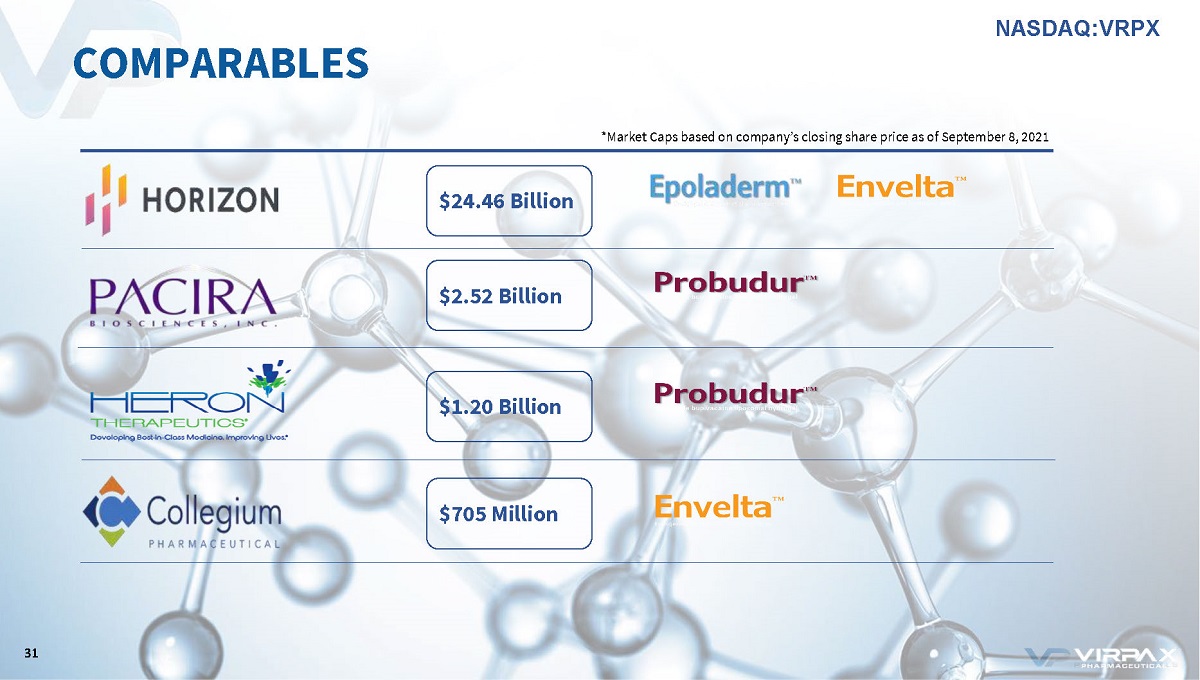
COMPARABLES *Market Caps based on company’s closing share price as of September 8, 2021 $705 Million $1.20 Billion $24.46 Billion $2.52 Billion 31 NASDAQ:VRPX






























