
Spearheading Immunotherapies PIPELINE UPDATE June 4, 2021 Exhibit 99.3

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “target,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward looking statements are based on Harpoon Therapeutics’ expectations and assumptions as of the date of this presentation. Each of these forward-looking statements involves risks and uncertainties that could cause Harpoon Therapeutics’ clinical development programs, future results or performance to differ significantly from those expressed or implied by the forward-looking statements. Forward-looking statements contained in this presentation include, but are not limited to, statements about the progress, timing, scope, design and anticipated results of clinical trials, the timing of the presentation of data, the association of data with potential treatment outcomes, the development and advancement of product candidates, and the timing of development milestones for product candidates. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data observed during clinical studies, clinical trial site activation or enrollment rates that are lower than expected, unanticipated or greater than anticipated impacts or delays due to COVID-19, changes in expected or existing competition, changes in the regulatory environment, the uncertainties and timing of the regulatory approval process, the risk that initial or interim results from a clinical trial may not be predictive of the final results of the trial or the results of future trials, the risk that trials may be delayed and may not have satisfactory outcomes, and unexpected litigation or other disputes that impede clinical trial progress. Other factors that may cause Harpoon Therapeutics’ actual results to differ from those expressed or implied in the forward-looking statements in this presentation are discussed in Harpoon Therapeutics’ filings with the U.S. Securities and Exchange Commission, including the “Risk Factors” sections contained therein. Except as required by law, Harpoon Therapeutics assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. Forward Looking Statements

Presentation Agenda Corporate Overview Highlights from HPN424 ASCO Presentation Corporate Pipeline Update HPN424 HPN536 HPN217 HPN328 ProTriTAC Scientific Update Program Status Review and 2021 Milestones

Corporate Overview
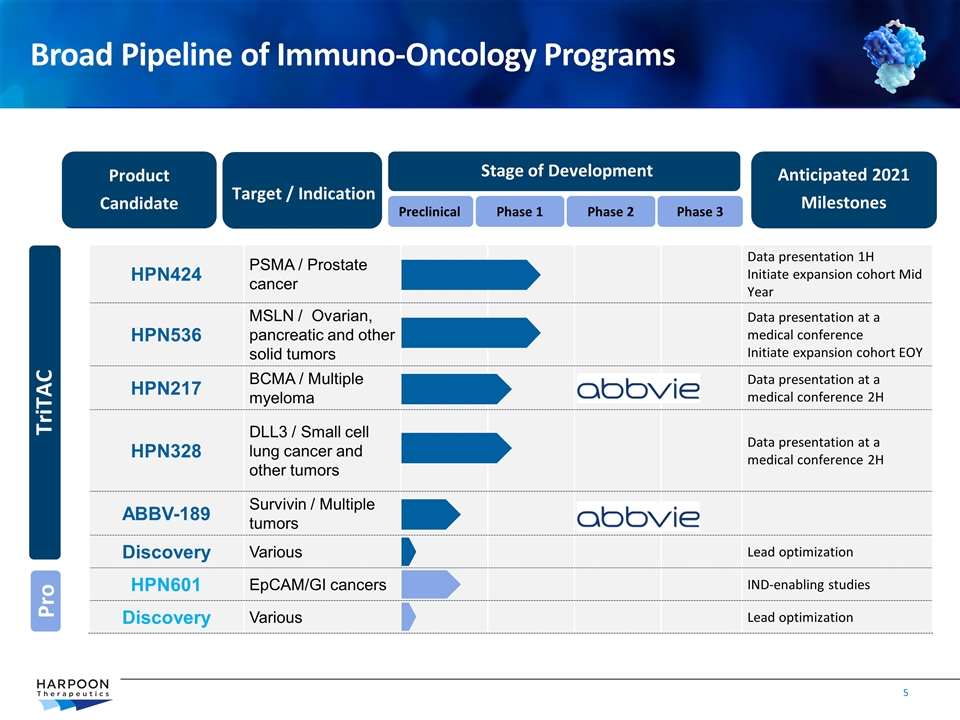
Product Candidate Target / Indication Stage of Development Anticipated 2021 Milestones Preclinical Phase 1 Phase 2 HPN424 PSMA / Prostate cancer Data presentation 1H Initiate expansion cohort Mid Year HPN536 MSLN / Ovarian, pancreatic and other solid tumors Data presentation at a medical conference Initiate expansion cohort EOY HPN217 BCMA / Multiple myeloma Data presentation at a medical conference 2H HPN328 DLL3 / Small cell lung cancer and other tumors Data presentation at a medical conference 2H ABBV-189 Survivin / Multiple tumors Discovery Various Lead optimization HPN601 EpCAM/GI cancers IND-enabling studies Discovery Various Lead optimization Phase 3 TriTAC Broad Pipeline of Immuno-Oncology Programs Pro
Broad Pipeline of T cell Engagers Advancing to Treat Multiple Cancers Four TriTACs in clinical development poised to advance into Phase 2 expansion studies Dose-escalations reveal target engagement and T cell activation in all programs All studies are implementing step dosing to maximize high drug exposure and tolerability Clinical sites in US and EU fully-engaged and delivering patients Evidence of clinical benefit observed 3 of 4 programs (HPN424, HPN536 and HPN328) together have shown stable disease, tumor size reductions, meaningful treatment duration CRS observed and manageable; severe CRS low incidence in all programs to date Clinical validation of TriTAC platform properties Antibody-like half life has been achieved across four targets in six indications Clinical biomarkers support T cell activation across all four clinical programs ProTriTAC pipeline (targeting tumor microenvironment) in development for new cancer targets and tumor indications HPN601 targeting EpCAM in IND-enabling studies in 2021 Discovery efforts underway for new products using both TriTAC and ProTriTAC platforms

HPN424 ASCO Data Highlights
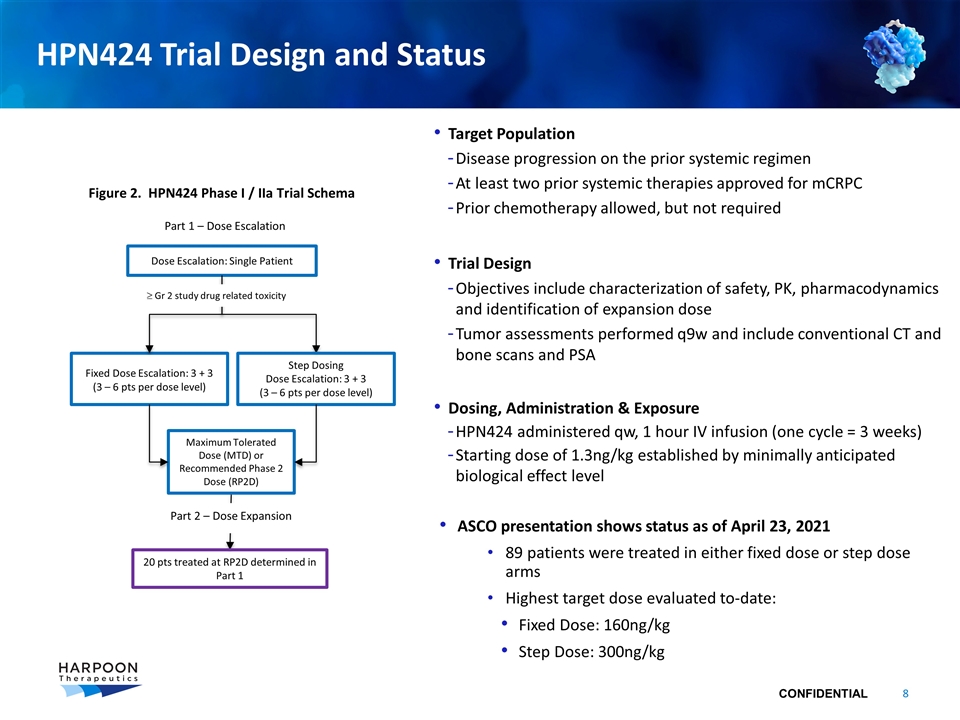
HPN424 Trial Design and Status Dose Escalation: Single Patient Fixed Dose Escalation: 3 + 3 (3 – 6 pts per dose level) Part 1 – Dose Escalation 20 pts treated at RP2D determined in Part 1 Target Population Disease progression on the prior systemic regimen At least two prior systemic therapies approved for mCRPC Prior chemotherapy allowed, but not required Trial Design Objectives include characterization of safety, PK, pharmacodynamics and identification of expansion dose Tumor assessments performed q9w and include conventional CT and bone scans and PSA Dosing, Administration & Exposure HPN424 administered qw, 1 hour IV infusion (one cycle = 3 weeks) Starting dose of 1.3ng/kg established by minimally anticipated biological effect level ASCO presentation shows status as of April 23, 2021 89 patients were treated in either fixed dose or step dose arms Highest target dose evaluated to-date: Fixed Dose: 160ng/kg Step Dose: 300ng/kg Maximum Tolerated Dose (MTD) or Recommended Phase 2 Dose (RP2D) Step Dosing Dose Escalation: 3 + 3 (3 – 6 pts per dose level) ³ Gr 2 study drug related toxicity Part 2 – Dose Expansion Figure 2. HPN424 Phase I / IIa Trial Schema
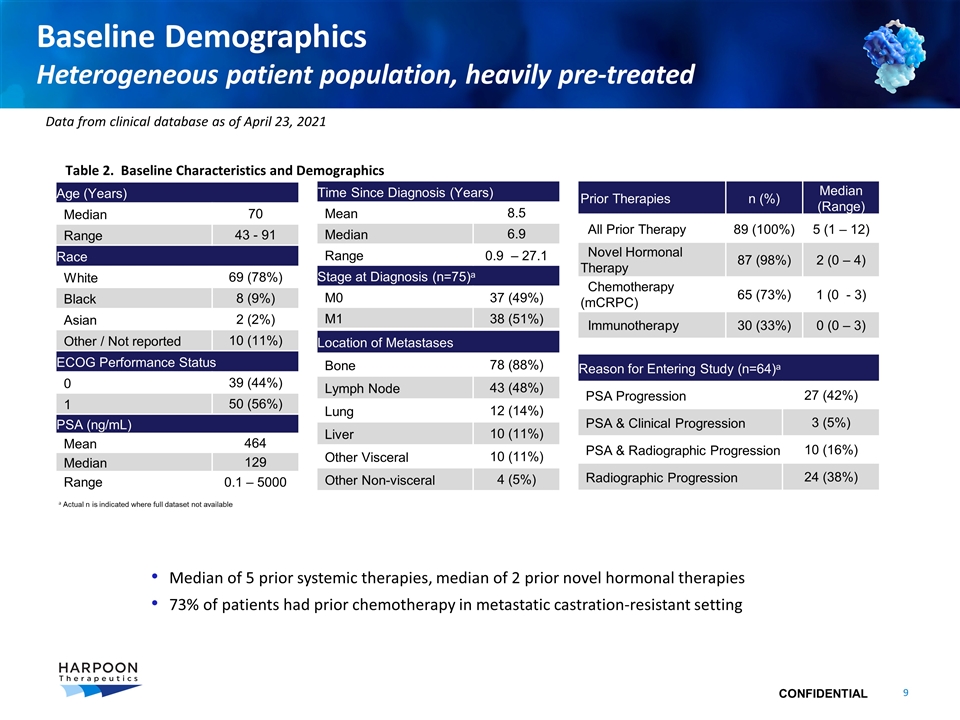
Baseline Demographics Heterogeneous patient population, heavily pre-treated Prior Therapies n (%) Median (Range) All Prior Therapy 89 (100%) 5 (1 – 12) Novel Hormonal Therapy 87 (98%) 2 (0 – 4) Chemotherapy (mCRPC) 65 (73%) 1 (0 - 3) Immunotherapy 30 (33%) 0 (0 – 3) Age (Years) Median 70 Range 43 - 91 Race White 69 (78%) Black 8 (9%) Asian 2 (2%) Other / Not reported 10 (11%) ECOG Performance Status 0 39 (44%) 1 50 (56%) Time Since Diagnosis (Years) Mean 8.5 Median 6.9 Range 0.9 – 27.1 Stage at Diagnosis (n=75)a M0 37 (49%) M1 38 (51%) Median of 5 prior systemic therapies, median of 2 prior novel hormonal therapies 73% of patients had prior chemotherapy in metastatic castration-resistant setting a Actual n is indicated where full dataset not available Table 2. Baseline Characteristics and Demographics PSA (ng/mL) Mean 464 Median 129 Range 0.1 – 5000 Reason for Entering Study (n=64)a PSA Progression 27 (42%) PSA & Clinical Progression 3 (5%) PSA & Radiographic Progression 10 (16%) Radiographic Progression 24 (38%) Location of Metastases Bone 78 (88%) Lymph Node 43 (48%) Lung 12 (14%) Liver 10 (11%) Other Visceral 10 (11%) Other Non-visceral 4 (5%) Data from clinical database as of April 23, 2021
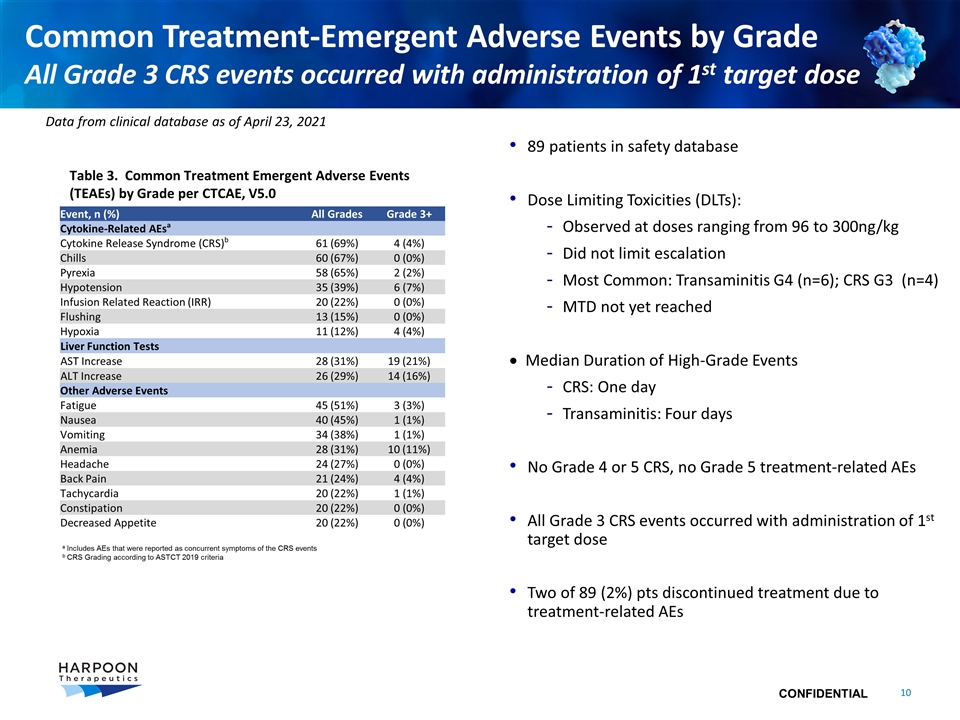
Common Treatment-Emergent Adverse Events by Grade All Grade 3 CRS events occurred with administration of 1st target dose Event, n (%) All Grades Grade 3+ Cytokine-Related AEsa Cytokine Release Syndrome (CRS)b 61 (69%) 4 (4%) Chills 60 (67%) 0 (0%) Pyrexia 58 (65%) 2 (2%) Hypotension 35 (39%) 6 (7%) Infusion Related Reaction (IRR) 20 (22%) 0 (0%) Flushing 13 (15%) 0 (0%) Hypoxia 11 (12%) 4 (4%) Liver Function Tests AST Increase 28 (31%) 19 (21%) ALT Increase 26 (29%) 14 (16%) Other Adverse Events Fatigue 45 (51%) 3 (3%) Nausea 40 (45%) 1 (1%) Vomiting 34 (38%) 1 (1%) Anemia 28 (31%) 10 (11%) Headache 24 (27%) 0 (0%) Back Pain 21 (24%) 4 (4%) Tachycardia 20 (22%) 1 (1%) Constipation 20 (22%) 0 (0%) Decreased Appetite 20 (22%) 0 (0%) 89 patients in safety database Dose Limiting Toxicities (DLTs): Observed at doses ranging from 96 to 300ng/kg Did not limit escalation Most Common: Transaminitis G4 (n=6); CRS G3 (n=4) MTD not yet reached · Median Duration of High-Grade Events CRS: One day Transaminitis: Four days No Grade 4 or 5 CRS, no Grade 5 treatment-related AEs All Grade 3 CRS events occurred with administration of 1st target dose Two of 89 (2%) pts discontinued treatment due to treatment-related AEs a Includes AEs that were reported as concurrent symptoms of the CRS events b CRS Grading according to ASTCT 2019 criteria Table 3. Common Treatment Emergent Adverse Events (TEAEs) by Grade per CTCAE, V5.0 Data from clinical database as of April 23, 2021
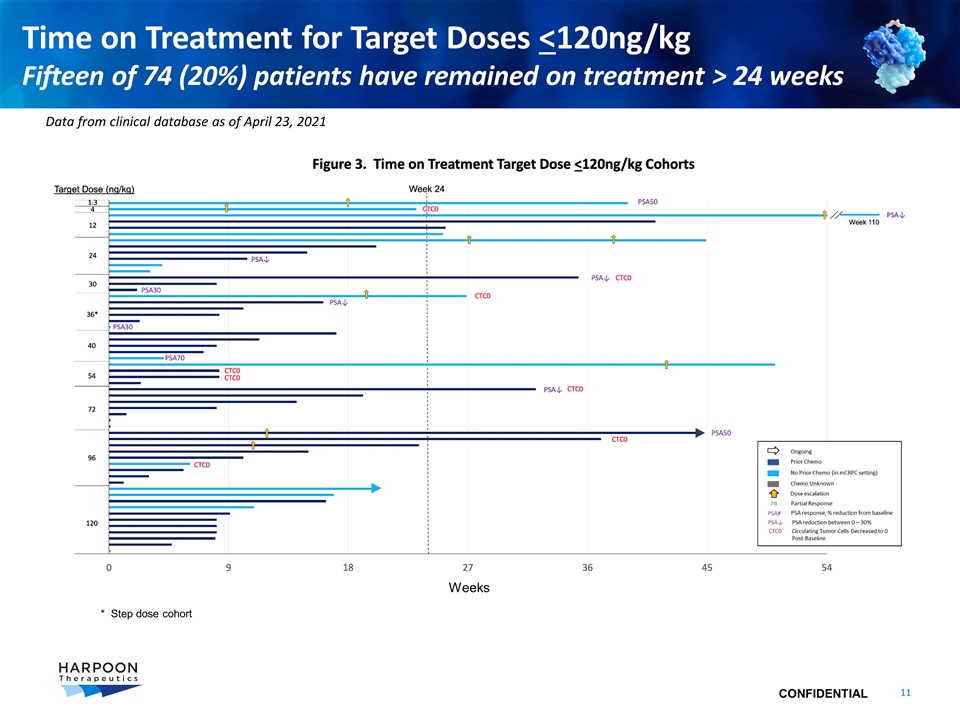
Time on Treatment for Target Doses <120ng/kg Fifteen of 74 (20%) patients have remained on treatment > 24 weeks Data from clinical database as of April 23, 2021 Weeks * Step dose cohort
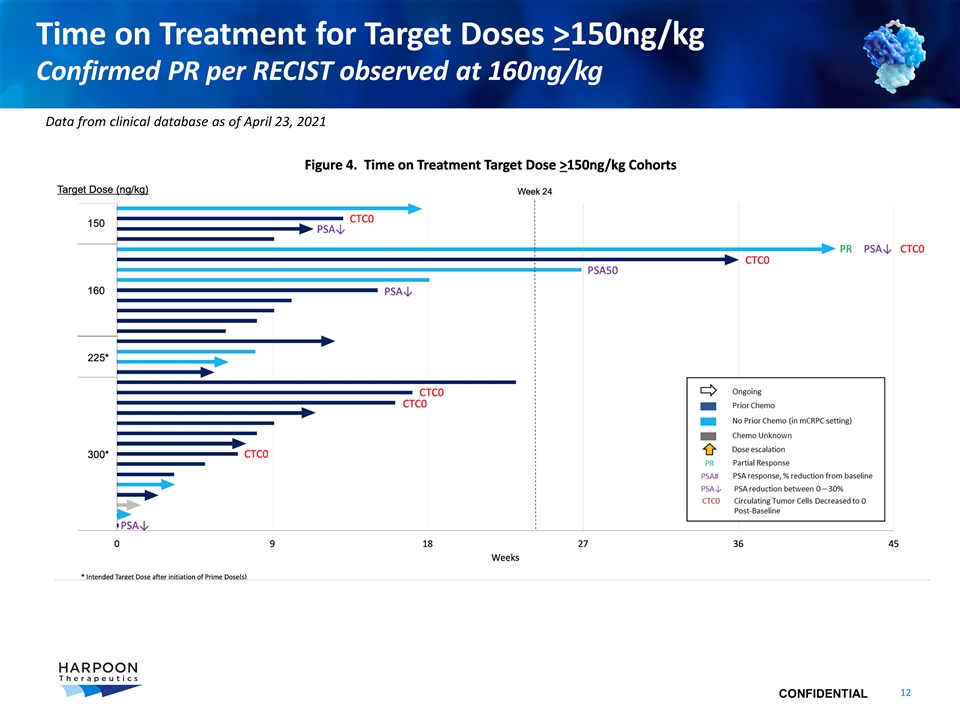
Time on Treatment for Target Doses >150ng/kg Confirmed PR per RECIST observed at 160ng/kg Data from clinical database as of April 23, 2021
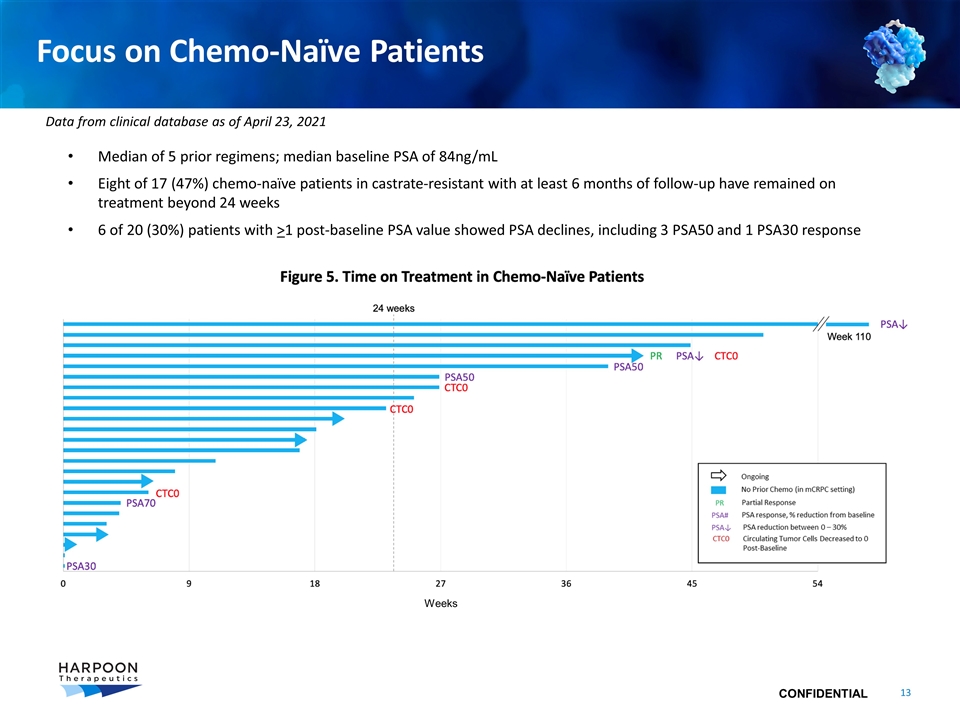
Focus on Chemo-Naïve Patients Median of 5 prior regimens; median baseline PSA of 84ng/mL Eight of 17 (47%) chemo-naïve patients in castrate-resistant with at least 6 months of follow-up have remained on treatment beyond 24 weeks 6 of 20 (30%) patients with >1 post-baseline PSA value showed PSA declines, including 3 PSA50 and 1 PSA30 response Weeks Data from clinical database as of April 23, 2021
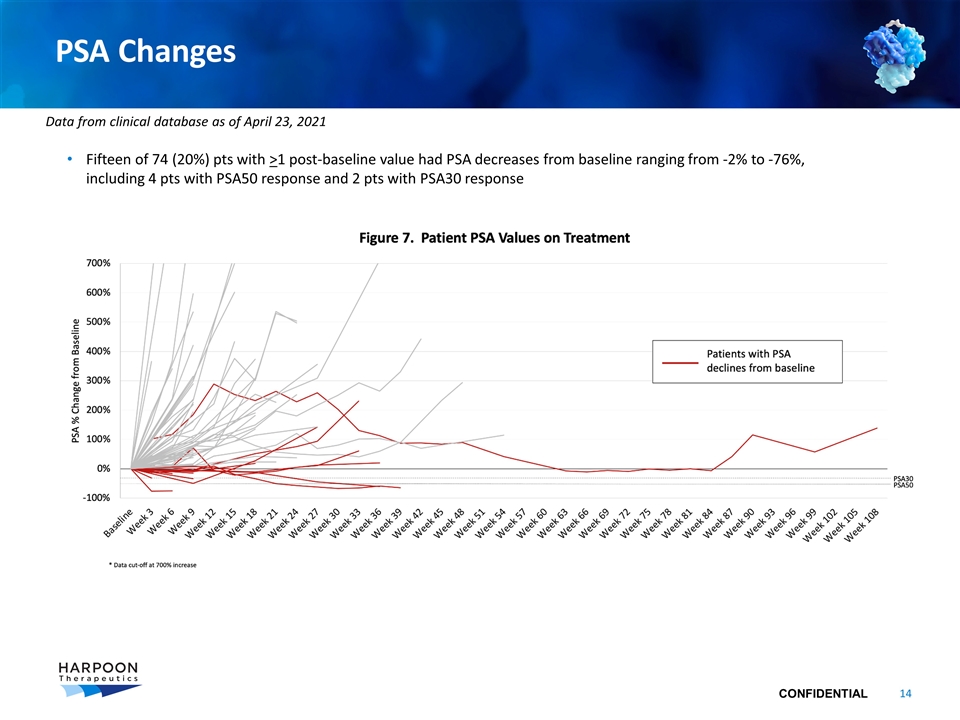
PSA Changes Fifteen of 74 (20%) pts with >1 post-baseline value had PSA decreases from baseline ranging from -2% to -76%, including 4 pts with PSA50 response and 2 pts with PSA30 response Data from clinical database as of April 23, 2021
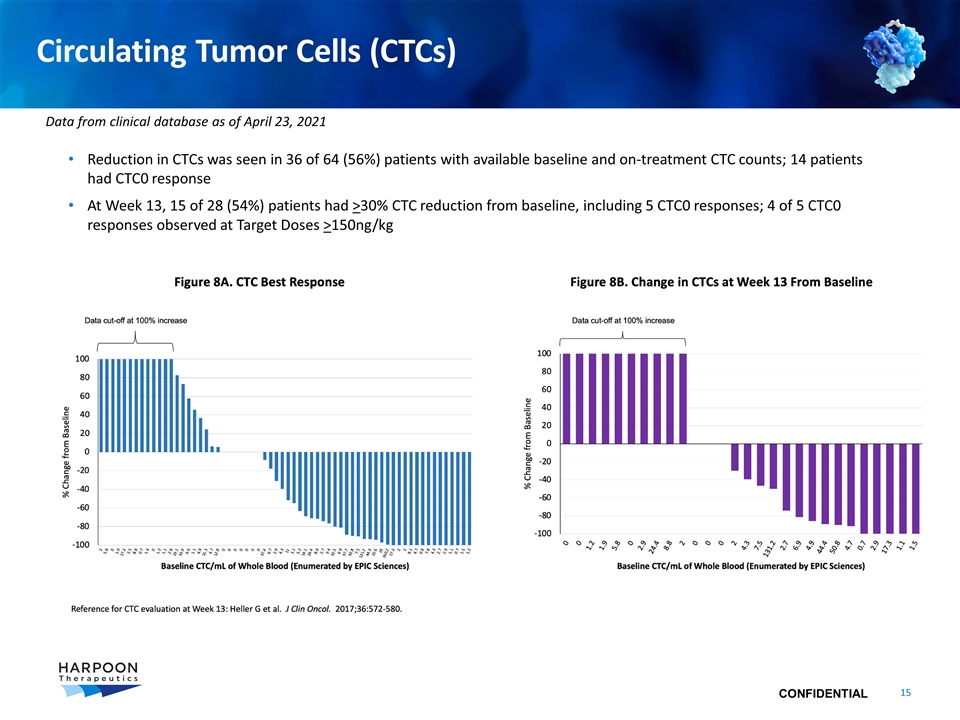
Circulating Tumor Cells (CTCs) Reduction in CTCs was seen in 36 of 64 (56%) patients with available baseline and on-treatment CTC counts; 14 patients had CTC0 response At Week 13, 15 of 28 (54%) patients had >30% CTC reduction from baseline, including 5 CTC0 responses; 4 of 5 CTC0 responses observed at Target Doses >150ng/kg Data from clinical database as of April 23, 2021
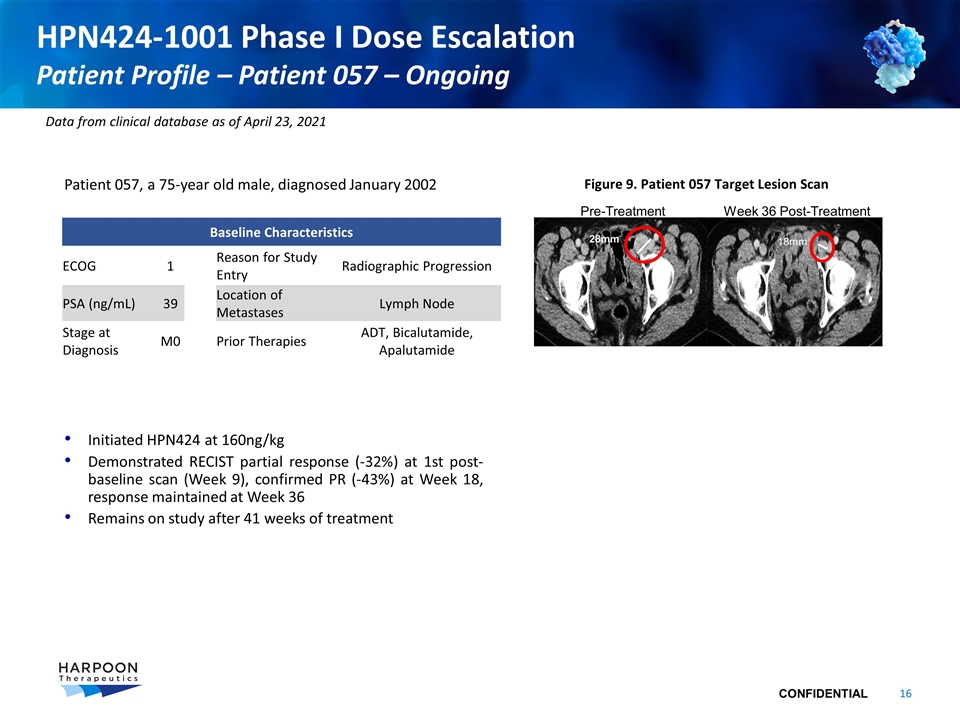
Patient 057, a 75-year old male, diagnosed January 2002 Initiated HPN424 at 160ng/kg Demonstrated RECIST partial response (-32%) at 1st post-baseline scan (Week 9), confirmed PR (-43%) at Week 18, response maintained at Week 36 Remains on study after 41 weeks of treatment HPN424-1001 Phase I Dose Escalation Patient Profile – Patient 057 – Ongoing Baseline Characteristics ECOG 1 Reason for Study Entry Radiographic Progression PSA (ng/mL) 39 Location of Metastases Lymph Node Stage at Diagnosis M0 Prior Therapies ADT, Bicalutamide, Apalutamide Figure 9. Patient 057 Target Lesion Scan Pre-Treatment Week 36 Post-Treatment 28mm 18mm Data from clinical database as of April 23, 2021
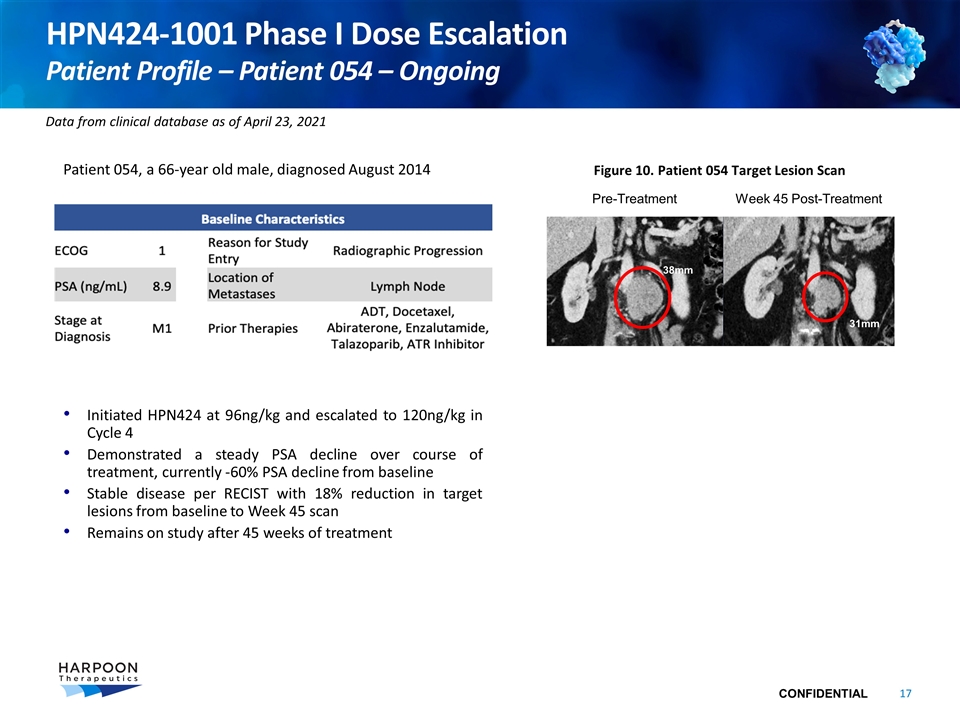
HPN424-1001 Phase I Dose Escalation Patient Profile – Patient 054 – Ongoing Patient 054, a 66-year old male, diagnosed August 2014 Initiated HPN424 at 96ng/kg and escalated to 120ng/kg in Cycle 4 Demonstrated a steady PSA decline over course of treatment, currently -60% PSA decline from baseline Stable disease per RECIST with 18% reduction in target lesions from baseline to Week 45 scan Remains on study after 45 weeks of treatment Figure 10. Patient 054 Target Lesion Scan Pre-Treatment Week 45 Post-Treatment 38mm 31mm Data from clinical database as of April 23, 2021
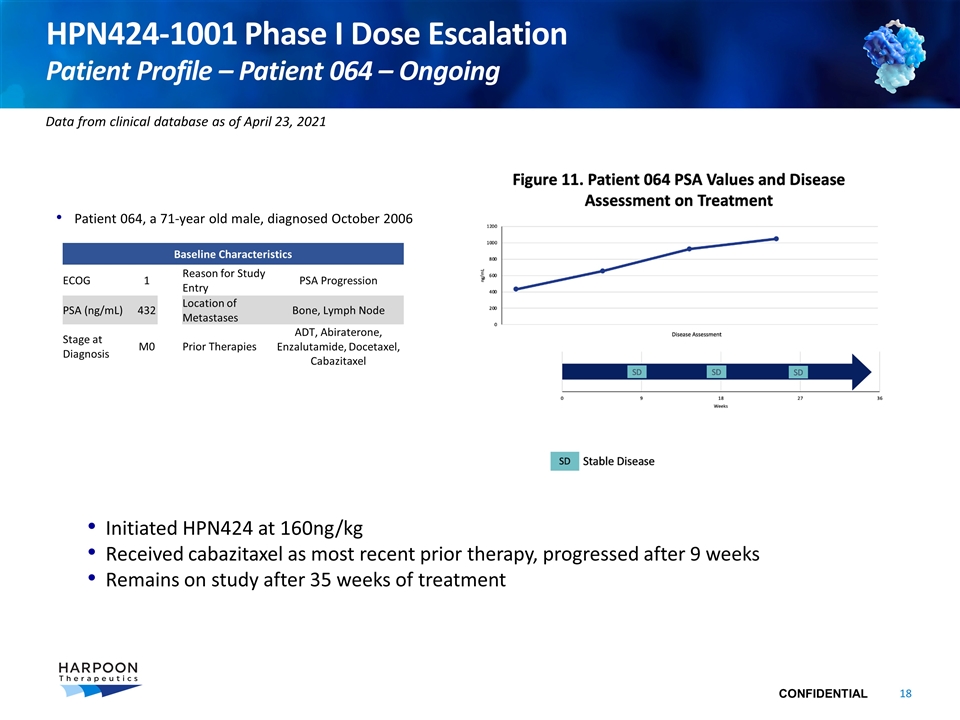
HPN424-1001 Phase I Dose Escalation Patient Profile – Patient 064 – Ongoing Patient 064, a 71-year old male, diagnosed October 2006 Initiated HPN424 at 160ng/kg Received cabazitaxel as most recent prior therapy, progressed after 9 weeks Remains on study after 35 weeks of treatment Baseline Characteristics ECOG 1 Reason for Study Entry PSA Progression PSA (ng/mL) 432 Location of Metastases Bone, Lymph Node Stage at Diagnosis M0 Prior Therapies ADT, Abiraterone, Enzalutamide, Docetaxel, Cabazitaxel Data from clinical database as of April 23, 2021

Pipeline Update

HPN424
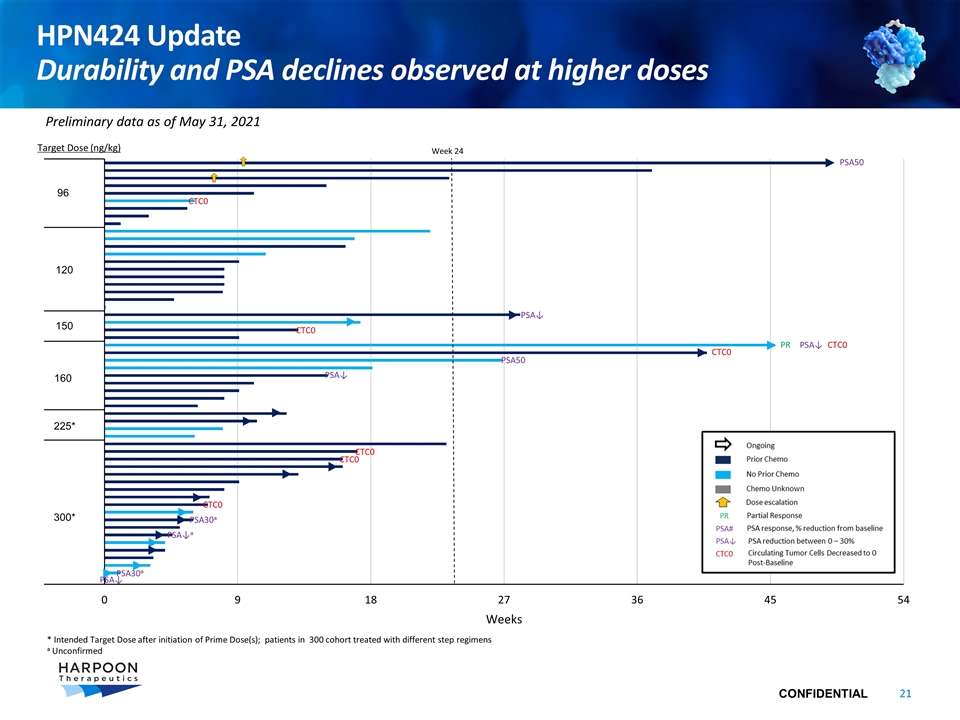
HPN424 Update Durability and PSA declines observed at higher doses Target Dose (ng/kg) 225* 300* PSA↓ CTC0 150 160 * Intended Target Dose after initiation of Prime Dose(s); patients in 300 cohort treated with different step regimens a Unconfirmed Week 24 PR Preliminary data as of May 31, 2021 120 96 CTC0 PSA50 PSA50 PSA↓ CTC0 CTC0 PSA↓ PSA↓ CTC0 CTC0 CTC0 PSA30a PSA30a PSA↓a
HPN424-1001 Phase I Dose Escalation Summary HPN424, a novel half-life extended PSMA-targeting T cell engager, is active and generally well tolerated CRS has been transient and manageable with 4% of patients experiencing Grade 3 CRS, no Grade 4 or 5 CRS and transaminitis events observed most often in Cycle 1, with diminished frequency and severity in subsequent cycles HPN424 has antitumor activity including a confirmed PR per RECIST, PSA declines and CTC reductions Treatment duration > 24 weeks observed in 15 of 74 (20%) pts, including 8 of 17 (47%) chemo-naïve patients Durability of 24 weeks or greater observed in patients at higher fixed dose cohorts (96 – 160ng/kg) Introduction of step dose regimens has allowed for the administration of higher target doses, currently at 300ng/kg Data remains early for patients in the most recent step dose cohort at target dose of 300ng/kg with a positive trend noted: PSA declines observed early in treatment course

HPN536
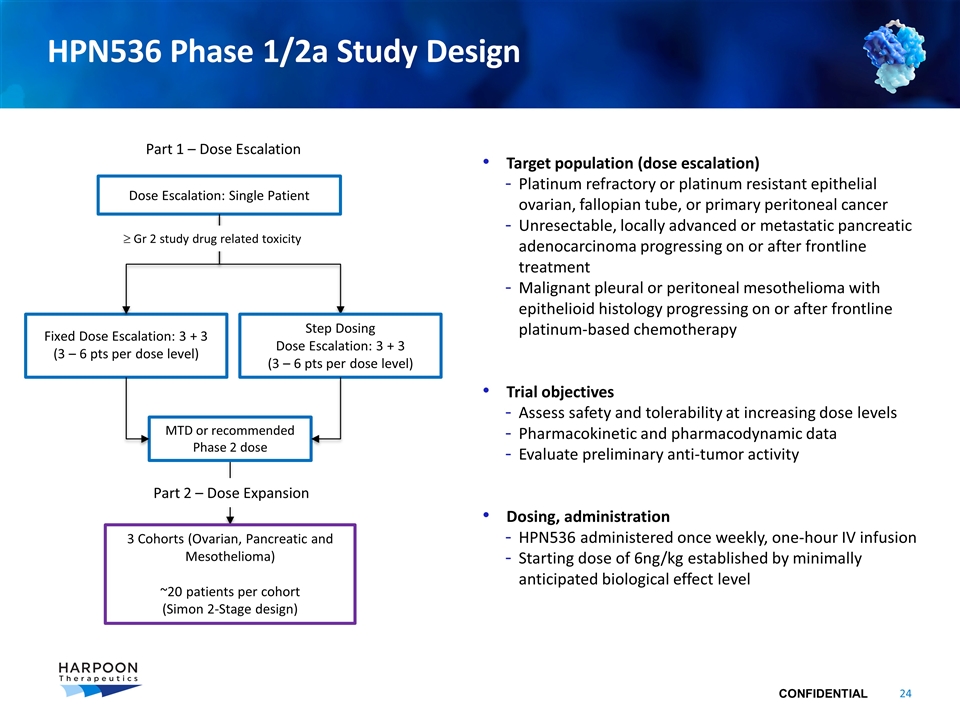
HPN536 Phase 1/2a Study Design Target population (dose escalation) Platinum refractory or platinum resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer Unresectable, locally advanced or metastatic pancreatic adenocarcinoma progressing on or after frontline treatment Malignant pleural or peritoneal mesothelioma with epithelioid histology progressing on or after frontline platinum-based chemotherapy Trial objectives Assess safety and tolerability at increasing dose levels Pharmacokinetic and pharmacodynamic data Evaluate preliminary anti-tumor activity Dosing, administration HPN536 administered once weekly, one-hour IV infusion Starting dose of 6ng/kg established by minimally anticipated biological effect level Dose Escalation: Single Patient Fixed Dose Escalation: 3 + 3 (3 – 6 pts per dose level) Part 1 – Dose Escalation 3 Cohorts (Ovarian, Pancreatic and Mesothelioma) ~20 patients per cohort (Simon 2-Stage design) MTD or recommended Phase 2 dose Step Dosing Dose Escalation: 3 + 3 (3 – 6 pts per dose level) ³ Gr 2 study drug related toxicity Part 2 – Dose Expansion
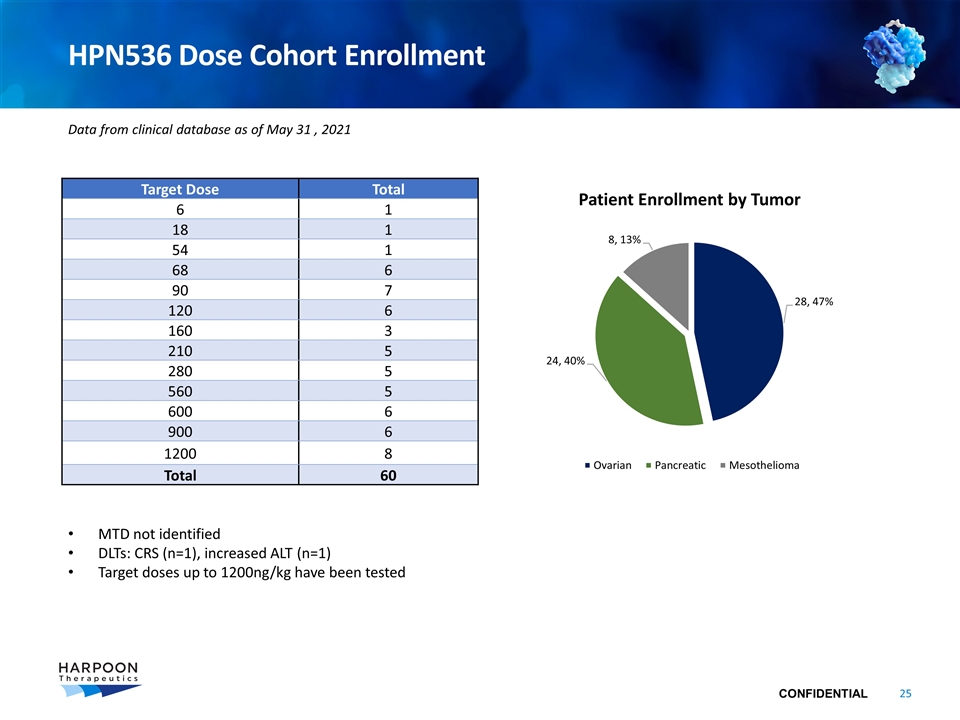
HPN536 Dose Cohort Enrollment Target Dose Total 6 1 18 1 54 1 68 6 90 7 120 6 160 3 210 5 280 5 560 5 600 6 900 6 1200 8 Total 60 MTD not identified DLTs: CRS (n=1), increased ALT (n=1) Target doses up to 1200ng/kg have been tested Data from clinical database as of May 31 , 2021
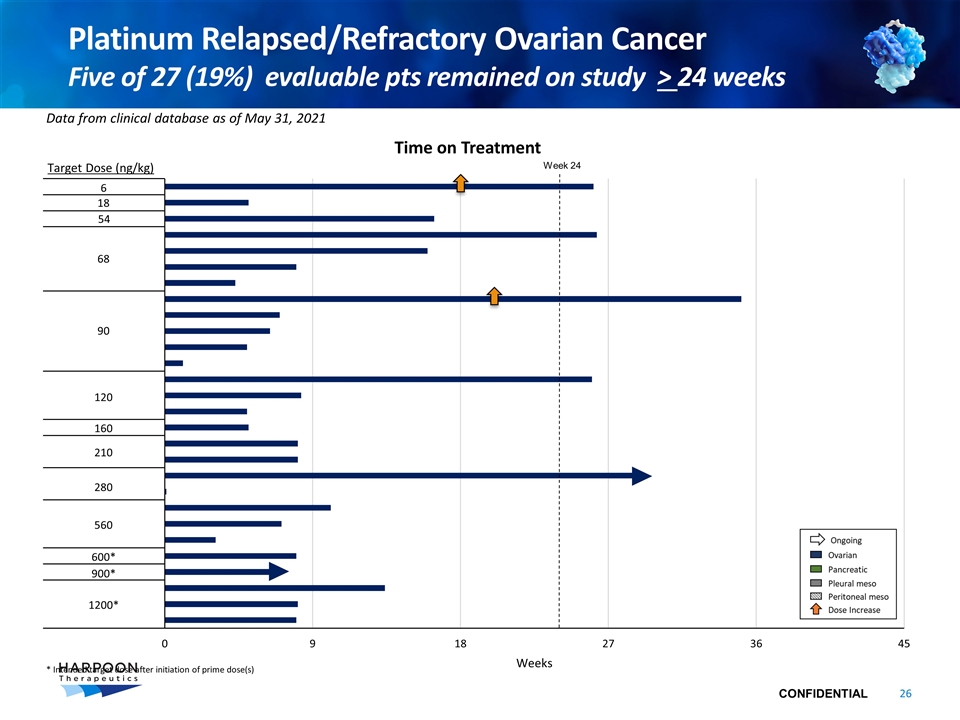
Platinum Relapsed/Refractory Ovarian Cancer Five of 27 (19%) evaluable pts remained on study > 24 weeks Target Dose (ng/kg) Data from clinical database as of May 31, 2021 Time on Treatment Week 24 6 18 54 68 90 120 160 210 280 560 600* 900* 1200* * Intended target dose after initiation of prime dose(s)
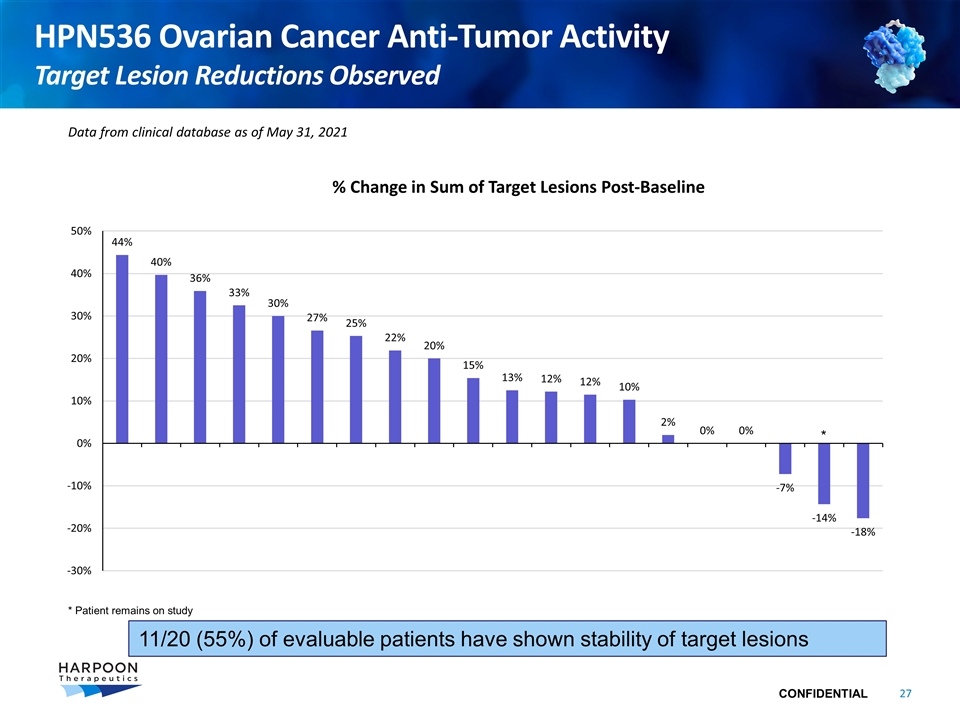
HPN536 Ovarian Cancer Anti-Tumor Activity Target Lesion Reductions Observed Data from clinical database as of May 31, 2021 % Change in Sum of Target Lesions Post-Baseline * Patient remains on study * 11/20 (55%) of evaluable patients have shown stability of target lesions
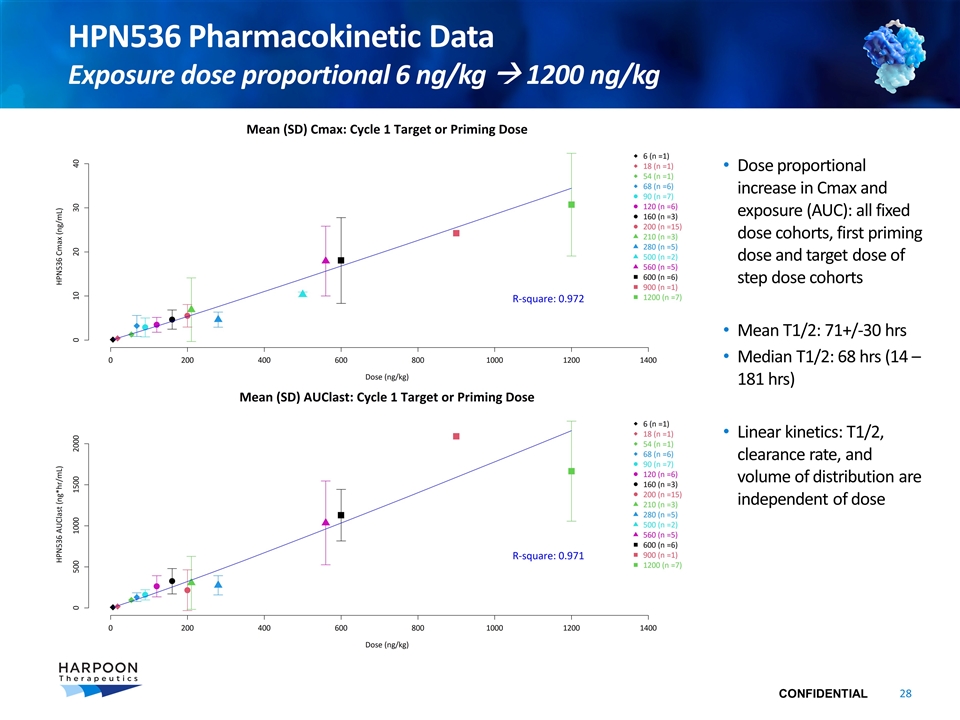
Dose proportional increase in Cmax and exposure (AUC): all fixed dose cohorts, first priming dose and target dose of step dose cohorts Mean T1/2: 71+/-30 hrs Median T1/2: 68 hrs (14 – 181 hrs) Linear kinetics: T1/2, clearance rate, and volume of distribution are independent of dose HPN536 Pharmacokinetic Data Exposure dose proportional 6 ng/kg à 1200 ng/kg

HPN217
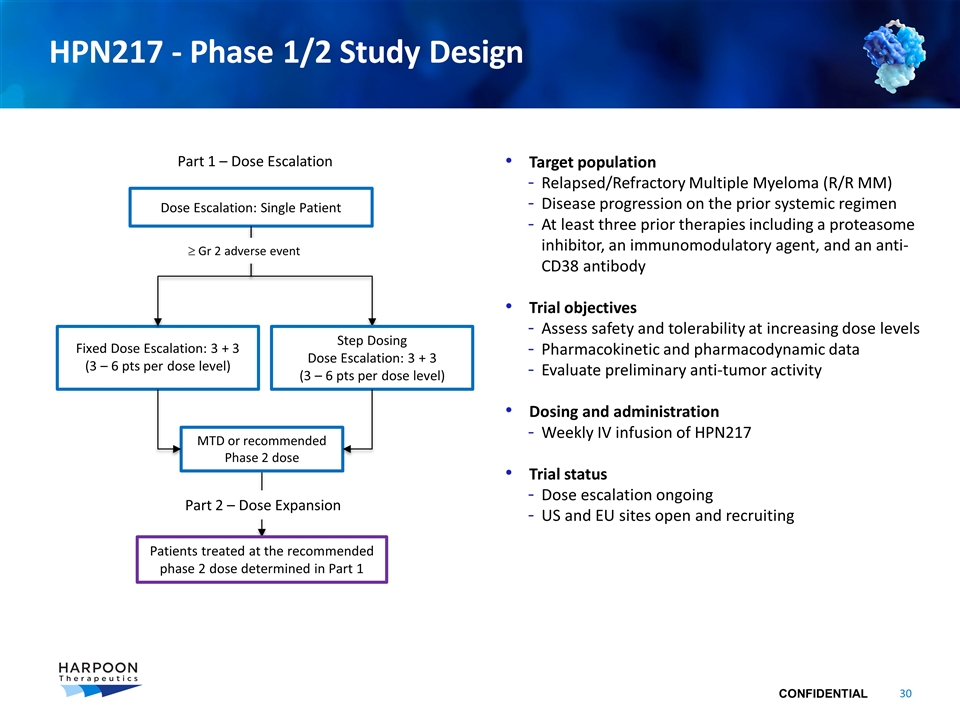
HPN217 - Phase 1/2 Study Design Target population Relapsed/Refractory Multiple Myeloma (R/R MM) Disease progression on the prior systemic regimen At least three prior therapies including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody Trial objectives Assess safety and tolerability at increasing dose levels Pharmacokinetic and pharmacodynamic data Evaluate preliminary anti-tumor activity Dosing and administration Weekly IV infusion of HPN217 Trial status Dose escalation ongoing US and EU sites open and recruiting Dose Escalation: Single Patient Fixed Dose Escalation: 3 + 3 (3 – 6 pts per dose level) Part 1 – Dose Escalation Patients treated at the recommended phase 2 dose determined in Part 1 MTD or recommended Phase 2 dose Step Dosing Dose Escalation: 3 + 3 (3 – 6 pts per dose level) ³ Gr 2 adverse event Part 2 – Dose Expansion
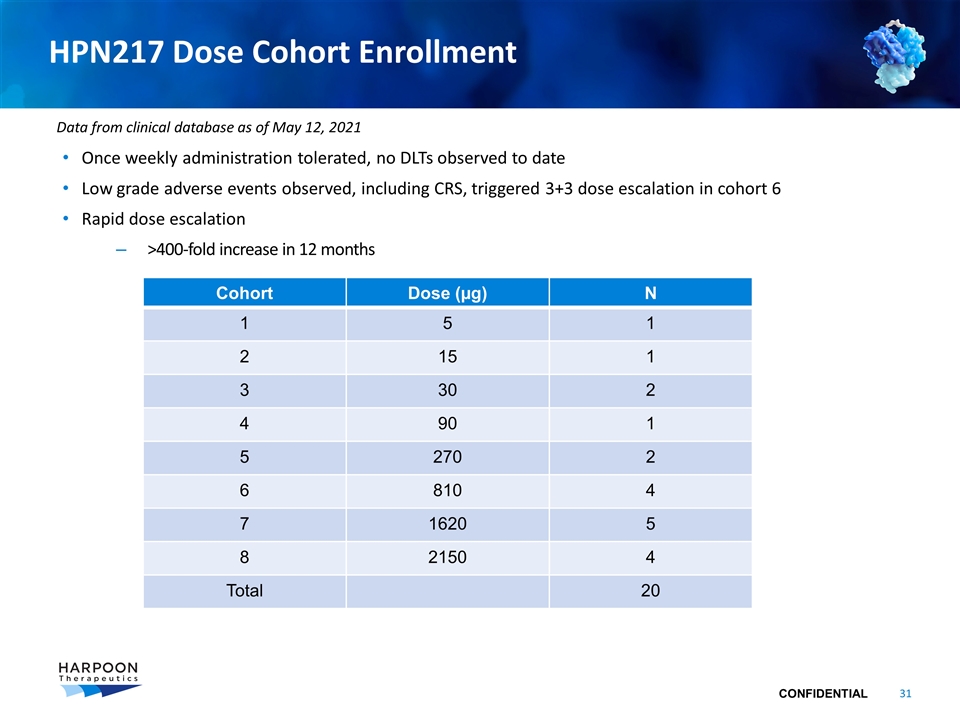
HPN217 Dose Cohort Enrollment Once weekly administration tolerated, no DLTs observed to date Low grade adverse events observed, including CRS, triggered 3+3 dose escalation in cohort 6 Rapid dose escalation >400-fold increase in 12 months Cohort Dose (µg) N 1 5 1 2 15 1 3 30 2 4 90 1 5 270 2 6 810 4 7 1620 5 8 2150 4 Total 20 Data from clinical database as of May 12, 2021
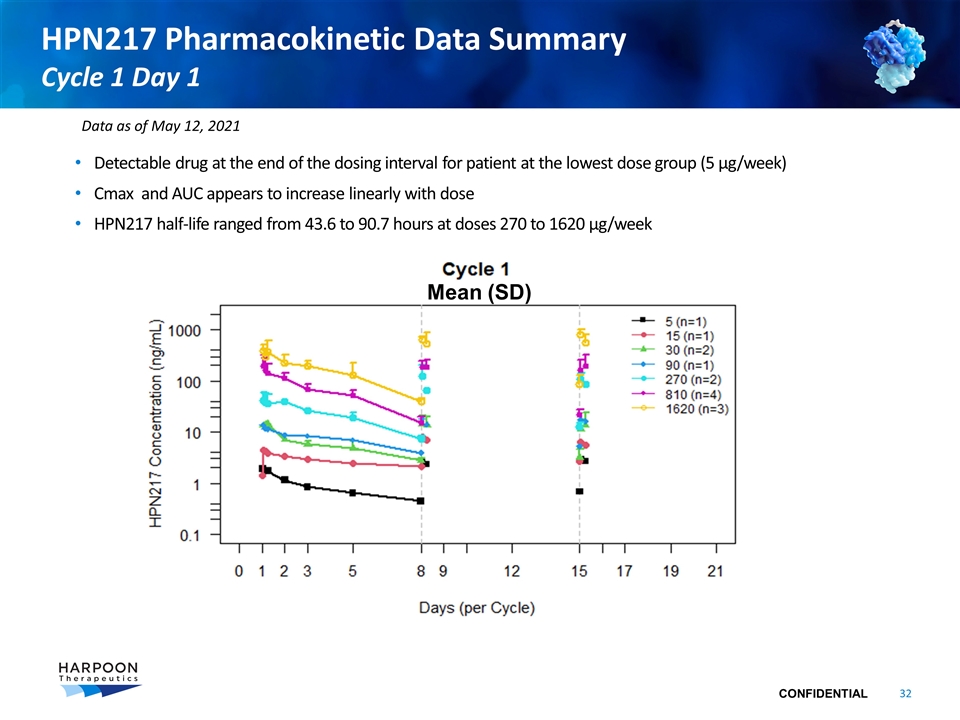
HPN217 Pharmacokinetic Data Summary Cycle 1 Day 1 Detectable drug at the end of the dosing interval for patient at the lowest dose group (5 µg/week) Cmax and AUC appears to increase linearly with dose HPN217 half-life ranged from 43.6 to 90.7 hours at doses 270 to 1620 µg/week Mean (SD) Data as of May 12, 2021

HPN328
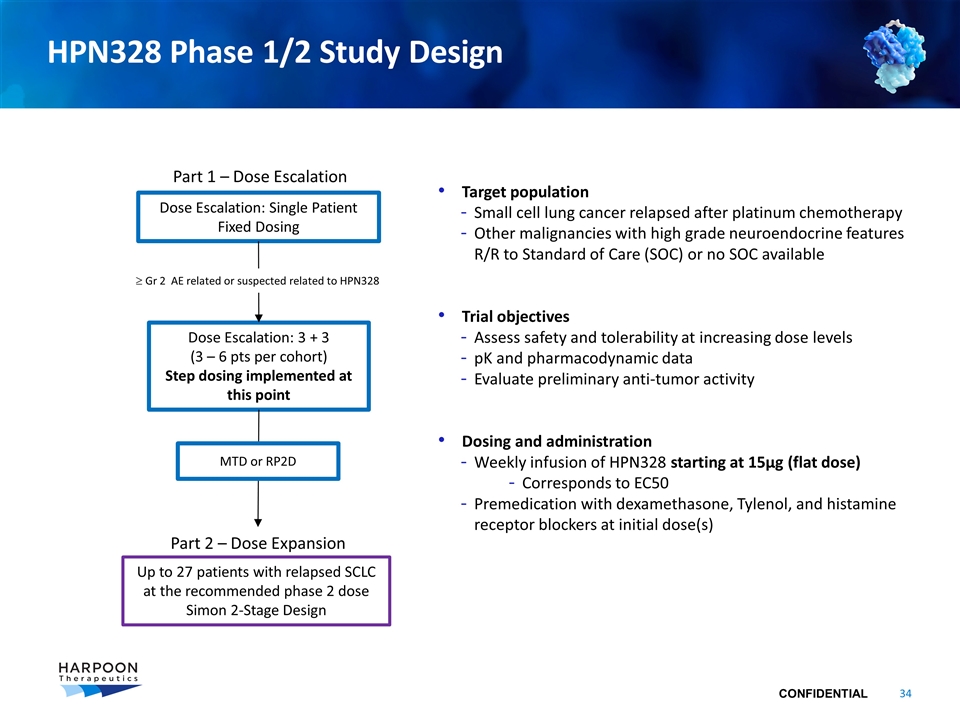
HPN328 Phase 1/2 Study Design Dose Escalation: Single Patient Fixed Dosing Dose Escalation: 3 + 3 (3 – 6 pts per cohort) Step dosing implemented at this point Part 1 – Dose Escalation Part 2 – Dose Expansion Up to 27 patients with relapsed SCLC at the recommended phase 2 dose Simon 2-Stage Design ³ Gr 2 AE related or suspected related to HPN328 Target population Small cell lung cancer relapsed after platinum chemotherapy Other malignancies with high grade neuroendocrine features R/R to Standard of Care (SOC) or no SOC available Trial objectives Assess safety and tolerability at increasing dose levels pK and pharmacodynamic data Evaluate preliminary anti-tumor activity Dosing and administration Weekly infusion of HPN328 starting at 15µg (flat dose) Corresponds to EC50 Premedication with dexamethasone, Tylenol, and histamine receptor blockers at initial dose(s) MTD or RP2D
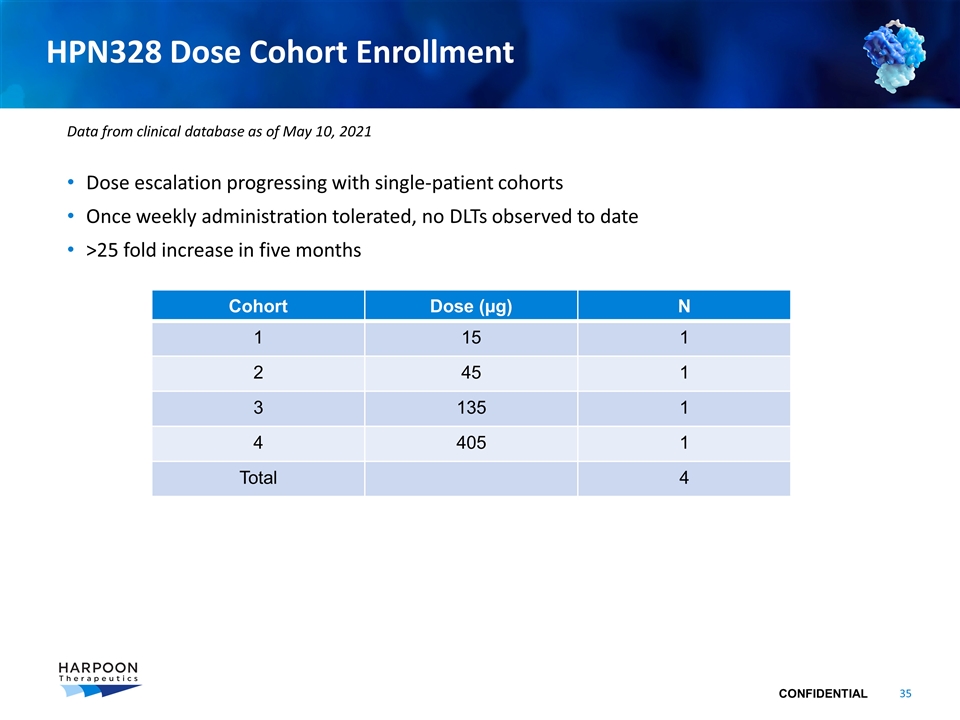
HPN328 Dose Cohort Enrollment Dose escalation progressing with single-patient cohorts Once weekly administration tolerated, no DLTs observed to date >25 fold increase in five months Cohort Dose (µg) N 1 15 1 2 45 1 3 135 1 4 405 1 Total 4 Data from clinical database as of May 10, 2021
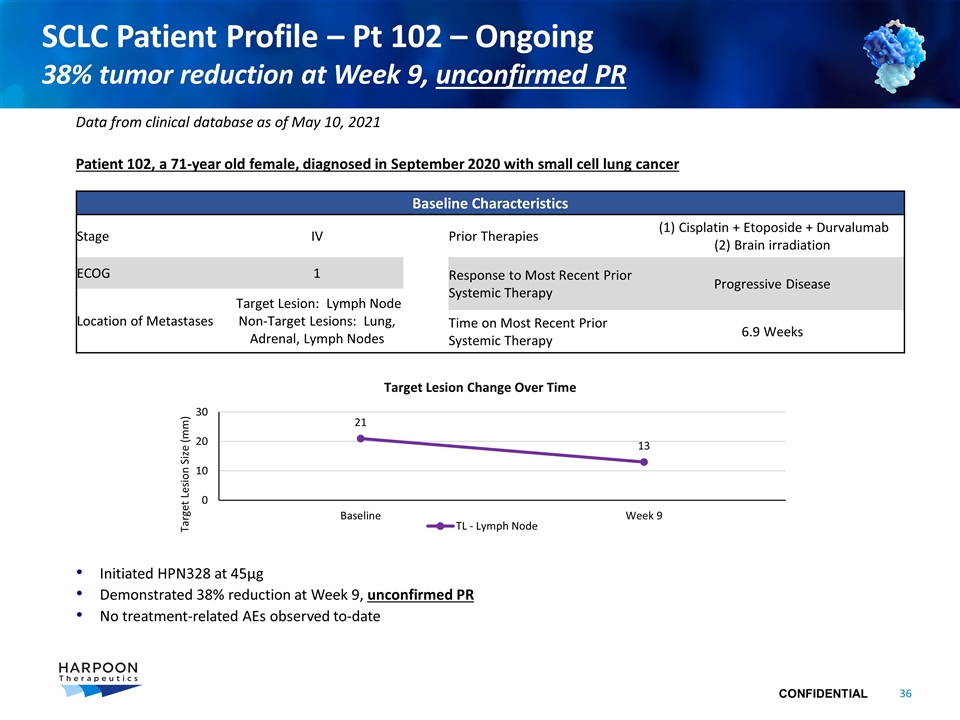
SCLC Patient Profile – Pt 102 – Ongoing 38% tumor reduction at Week 9, unconfirmed PR Patient 102, a 71-year old female, diagnosed in September 2020 with small cell lung cancer Initiated HPN328 at 45µg Demonstrated 38% reduction at Week 9, unconfirmed PR No treatment-related AEs observed to-date Data from clinical database as of May 10, 2021 Baseline Characteristics Stage IV Prior Therapies (1) Cisplatin + Etoposide + Durvalumab (2) Brain irradiation ECOG 1 Response to Most Recent Prior Systemic Therapy Progressive Disease Location of Metastases Target Lesion: Lymph Node Non-Target Lesions: Lung, Adrenal, Lymph Nodes Time on Most Recent Prior Systemic Therapy 6.9 Weeks
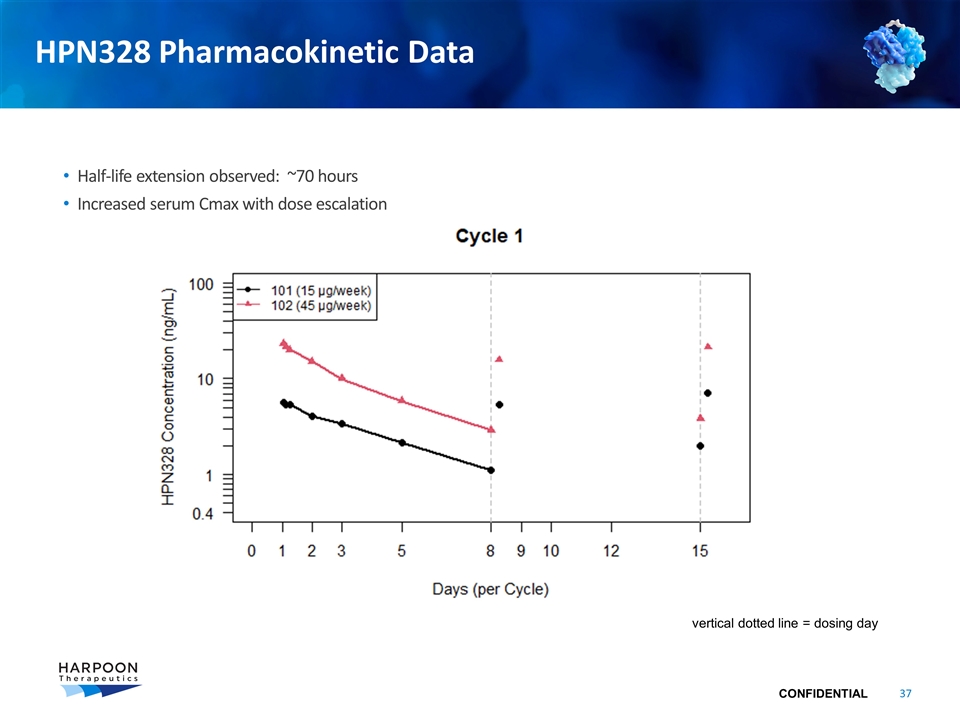
HPN328 Pharmacokinetic Data Half-life extension observed: ~70 hours Increased serum Cmax with dose escalation vertical dotted line = dosing day
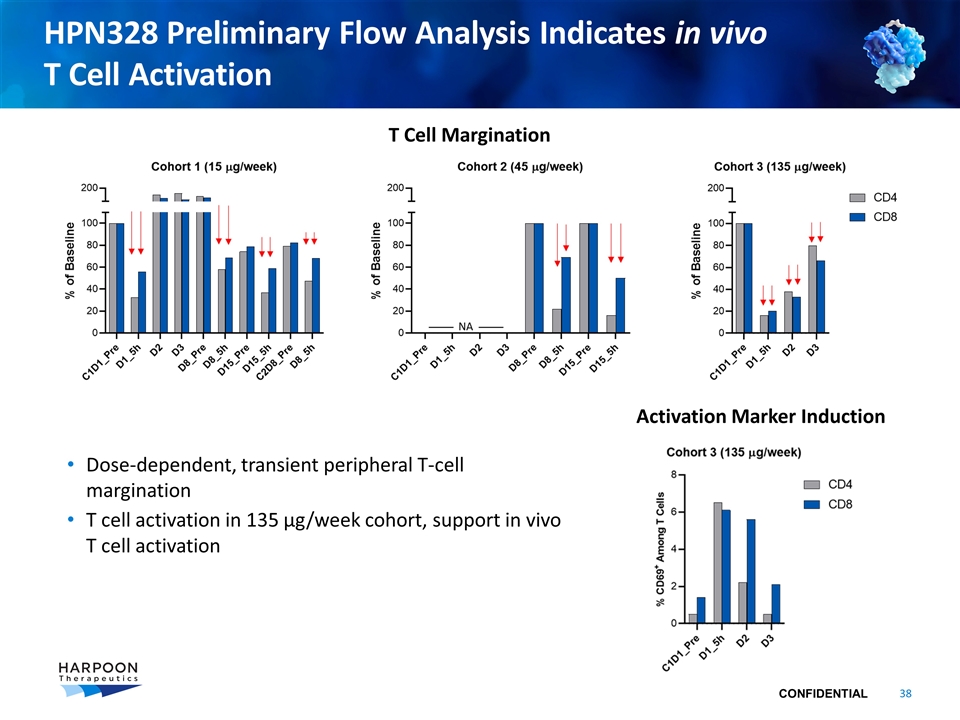
HPN328 Preliminary Flow Analysis Indicates in vivo T Cell Activation Dose-dependent, transient peripheral T-cell margination T cell activation in 135 µg/week cohort, support in vivo T cell activation T Cell Margination Activation Marker Induction
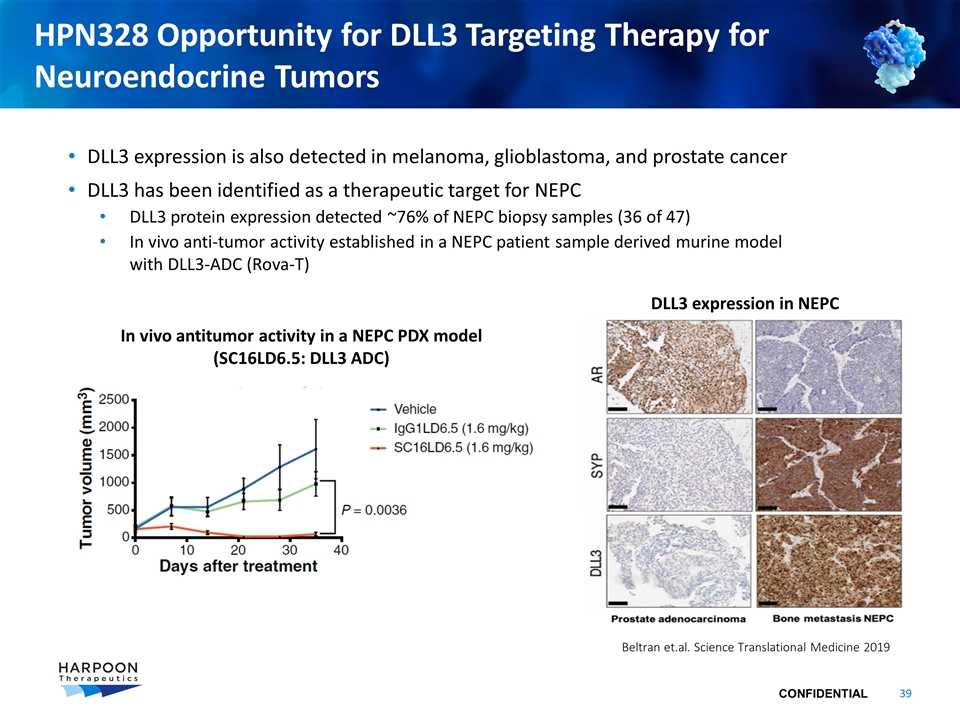
HPN328 Opportunity for DLL3 Targeting Therapy for Neuroendocrine Tumors DLL3 expression is also detected in melanoma, glioblastoma, and prostate cancer DLL3 has been identified as a therapeutic target for NEPC DLL3 protein expression detected ~76% of NEPC biopsy samples (36 of 47) In vivo anti-tumor activity established in a NEPC patient sample derived murine model with DLL3-ADC (Rova-T) In vivo antitumor activity in a NEPC PDX model (SC16LD6.5: DLL3 ADC) DLL3 expression in NEPC Beltran et.al. Science Translational Medicine 2019
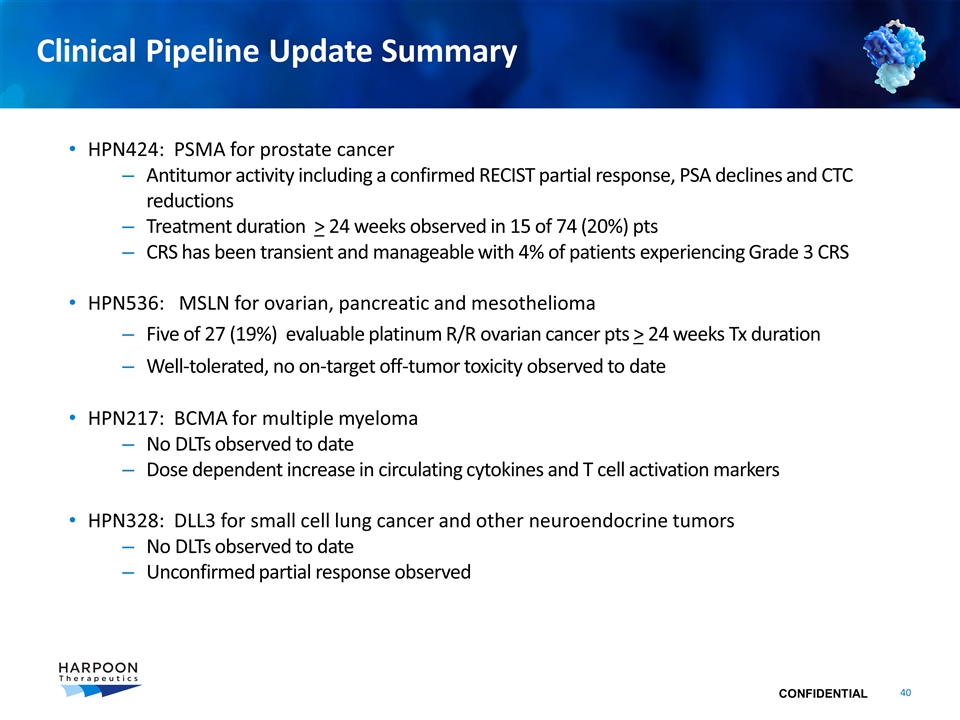
Clinical Pipeline Update Summary HPN424: PSMA for prostate cancer Antitumor activity including a confirmed RECIST partial response, PSA declines and CTC reductions Treatment duration > 24 weeks observed in 15 of 74 (20%) pts CRS has been transient and manageable with 4% of patients experiencing Grade 3 CRS HPN536: MSLN for ovarian, pancreatic and mesothelioma Five of 27 (19%) evaluable platinum R/R ovarian cancer pts > 24 weeks Tx duration Well-tolerated, no on-target off-tumor toxicity observed to date HPN217: BCMA for multiple myeloma No DLTs observed to date Dose dependent increase in circulating cytokines and T cell activation markers HPN328: DLL3 for small cell lung cancer and other neuroendocrine tumors No DLTs observed to date Unconfirmed partial response observed

ProTriTAC Scientific Update
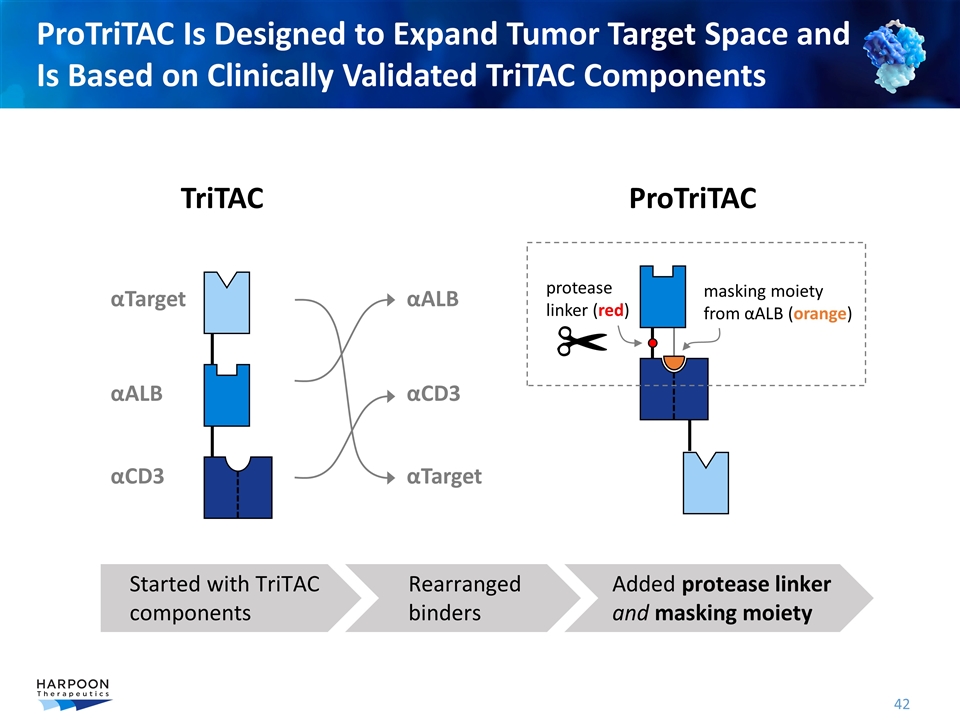
ProTriTAC Is Designed to Expand Tumor Target Space and Is Based on Clinically Validated TriTAC Components αALB αTarget αCD3 TriTAC ProTriTAC protease linker (red) masking moiety from αALB (orange) αALB αTarget αCD3 Started with TriTAC components Rearranged binders Added protease linker and masking moiety
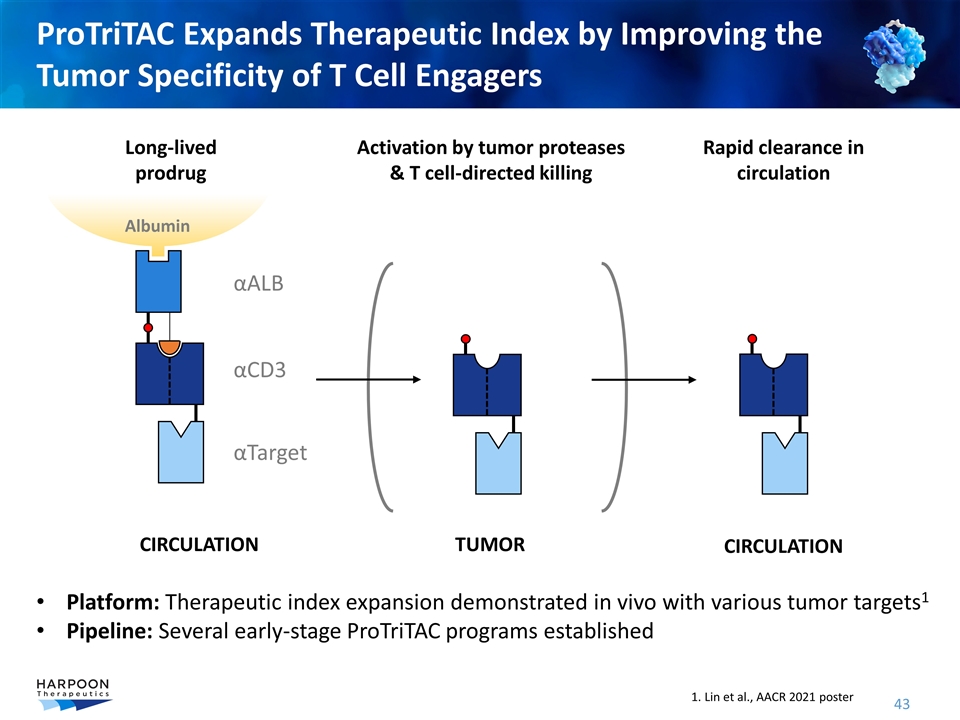
ProTriTAC Expands Therapeutic Index by Improving the Tumor Specificity of T Cell Engagers αALB αTarget αCD3 Long-lived prodrug Rapid clearance in circulation TUMOR CIRCULATION CIRCULATION Activation by tumor proteases & T cell-directed killing Albumin Platform: Therapeutic index expansion demonstrated in vivo with various tumor targets1 Pipeline: Several early-stage ProTriTAC programs established 1. Lin et al., AACR 2021 poster
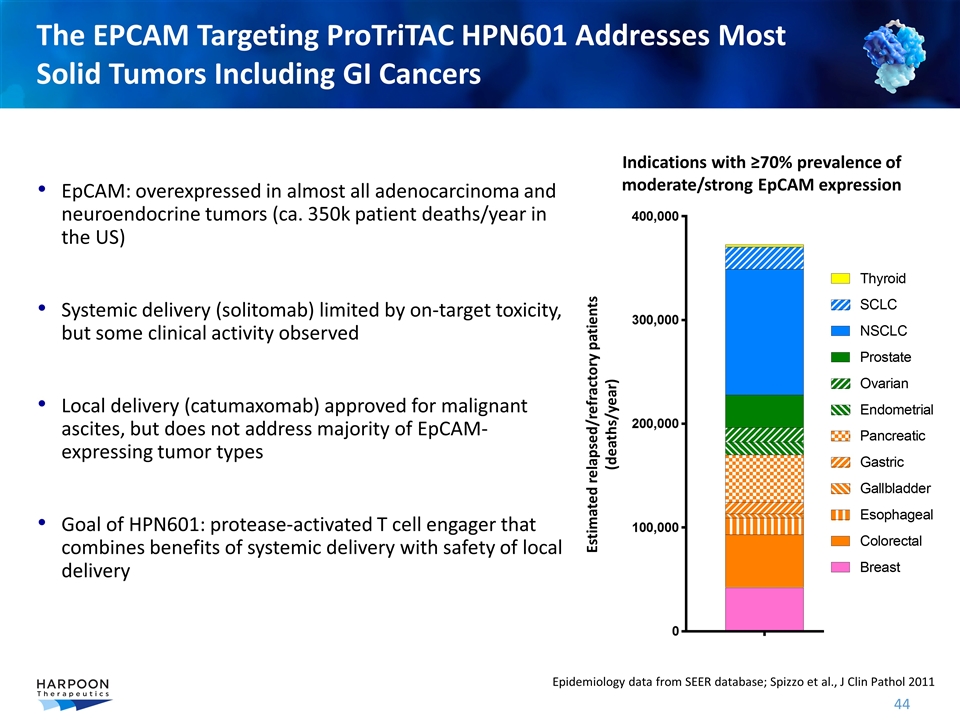
EpCAM: overexpressed in almost all adenocarcinoma and neuroendocrine tumors (ca. 350k patient deaths/year in the US) Systemic delivery (solitomab) limited by on-target toxicity, but some clinical activity observed Local delivery (catumaxomab) approved for malignant ascites, but does not address majority of EpCAM-expressing tumor types Goal of HPN601: protease-activated T cell engager that combines benefits of systemic delivery with safety of local delivery The EPCAM Targeting ProTriTAC HPN601 Addresses Most Solid Tumors Including GI Cancers Epidemiology data from SEER database; Spizzo et al., J Clin Pathol 2011 Estimated relapsed/refractory patients (deaths/year) Indications with ≥70% prevalence of moderate/strong EpCAM expression
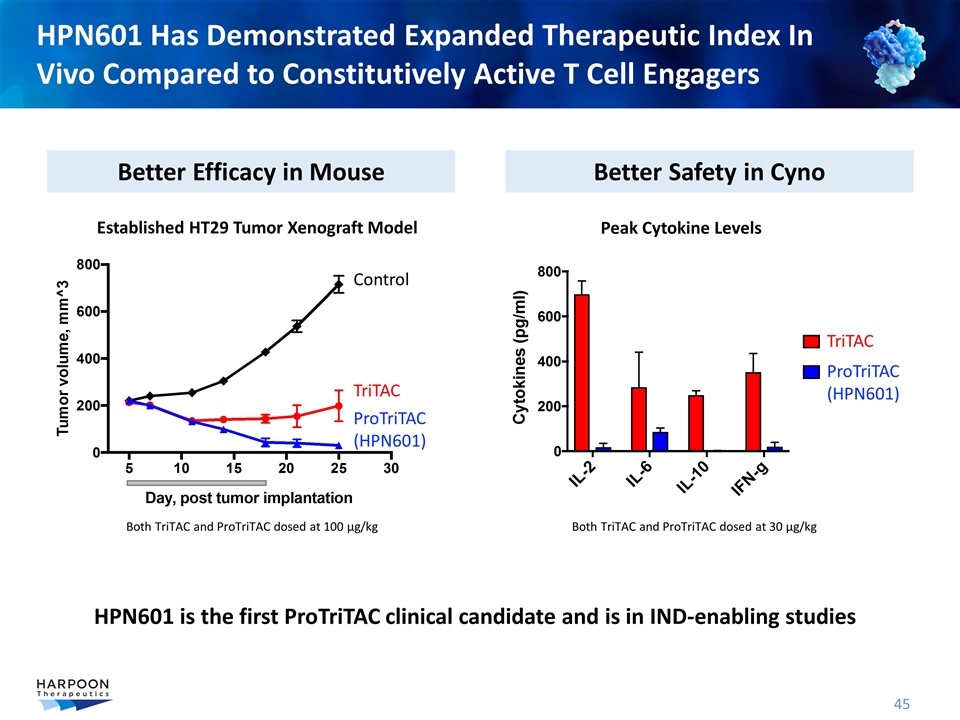
HPN601 Has Demonstrated Expanded Therapeutic Index In Vivo Compared to Constitutively Active T Cell Engagers Better Efficacy in Mouse Better Safety in Cyno Control TriTAC ProTriTAC (HPN601) Both TriTAC and ProTriTAC dosed at 100 µg/kg Peak Cytokine Levels Both TriTAC and ProTriTAC dosed at 30 µg/kg Established HT29 Tumor Xenograft Model TriTAC ProTriTAC (HPN601) HPN601 is the first ProTriTAC clinical candidate and is in IND-enabling studies

Program Status Review and 2021 Milestones

Pipeline Next Steps HPN424: PSMA for prostate cancer Complete dose selection and finalize target patient population for Phase 2 expansion cohort initiation HPN536: MSLN for ovarian, pancreatic and mesothelioma Continue dose escalation and further enrich study with mesothelioma patients HPN217: BCMA for multiple myeloma Continue dose escalation and identify dose and patients for Phase 2 expansion HPN328: DLL3 for small cell lung cancer and other neuroendocrine tumors Continue dose escalation and further enrich study to include prostate cancer patients HPN601: EPCAM or GI cancers and other solid tumors Continue IND enabling studies including clinical drug supply
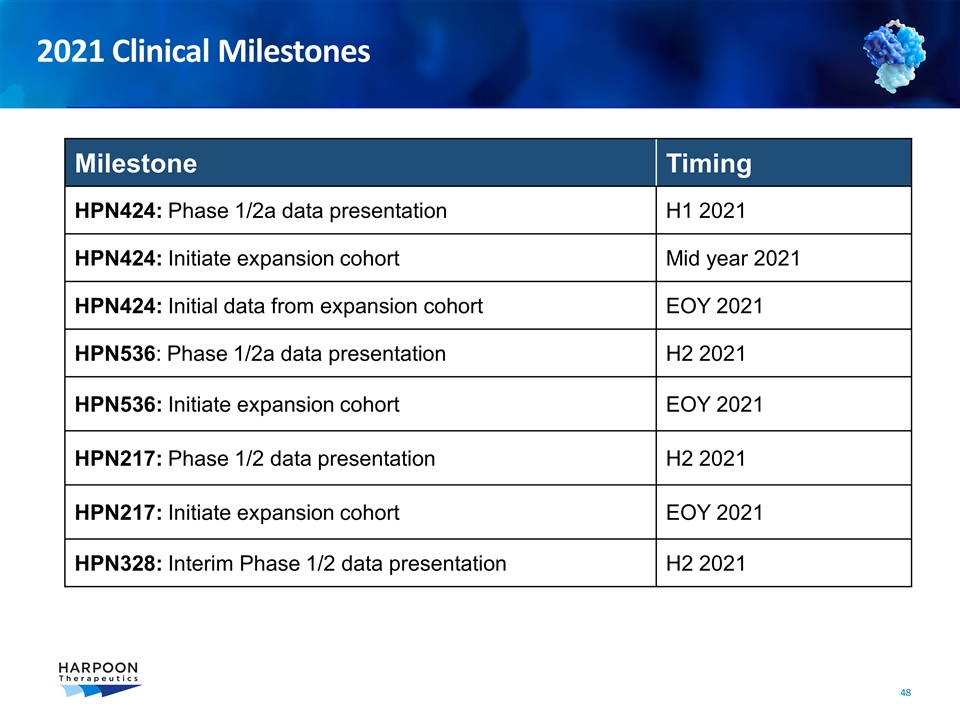
Milestone Timing HPN424: Phase 1/2a data presentation H1 2021 HPN424: Initiate expansion cohort Mid year 2021 HPN424: Initial data from expansion cohort EOY 2021 HPN536: Phase 1/2a data presentation H2 2021 HPN536: Initiate expansion cohort EOY 2021 HPN217: Phase 1/2 data presentation H2 2021 HPN217: Initiate expansion cohort EOY 2021 HPN328: Interim Phase 1/2 data presentation H2 2021 2021 Clinical Milestones
Broad Pipeline of T cell Engagers Advancing to Treat Multiple Cancers Four TriTACs in clinical development poised to advance into Phase 2 expansion studies Dose-escalations reveal target engagement and T cell activation in all programs All studies are implementing step dosing to maximize high drug exposure and tolerability Clinical sites in US and EU fully-engaged and delivering patients Evidence of clinical benefit observed 3 of 4 programs (HPN424, HPN536 and HPN328) together have shown stable disease, tumor size reductions, meaningful treatment duration CRS observed and manageable; severe CRS low incidence in all programs to date Clinical validation of TriTAC platform properties Antibody-like half life has been achieved across four targets in six indications Clinical biomarkers support T cell activation across all four clinical programs ProTriTAC pipeline (targeting tumor microenvironment) in development for new cancer targets and tumor indications HPN601 targeting EpCAM in IND-enabling studies in 2021 Discovery efforts underway for new products using both TriTAC and ProTriTAC platforms

Spearheading Immunotherapies PIPELINE UPDATE June 4, 2021

















































