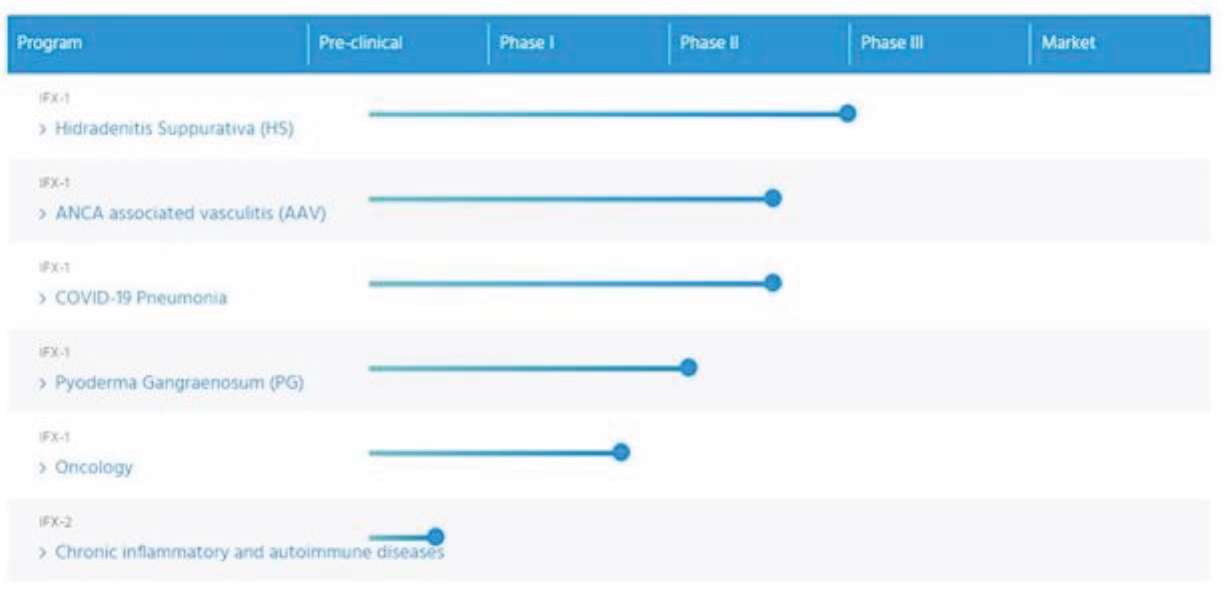
each validated in 37 EPC member states, one European patent validated in 3 countries, four pending European applications, 36 pending foreign patent applications and two pending applications filed under the Patent Cooperation Treaty (PCT). These patents include claims relating to C5a inhibitors and associated methods of use.
Our patent portfolio relating to IFX-1 and IFX-2, as of June 1, 2020, is summarized below.
As of June 1, 2020, we owned three issued U.S. patents and one pending U.S. non-provisional patent application covering the composition of matter of antibodies that block C5a and their use in blocking C5a-induced biological effects in patients with diseases that involve acute or chronic inflammation, which would include in their scope HS and AAV. In addition, we owned 13 issued foreign patents, one European patent application, six pending foreign patent applications, one issued Eurasian Patent validated in nine countries, two European patent each validated in 37 EPC member states covering the composition of matter of antibodies that block C5a and their use in the treatment of various diseases that involve acute or chronic inflammation, which would include in their scope HS and AAV, and, depending on the jurisdiction of the applicable patent, specifically cover the use of such antibodies in treating diseases such as ischemia and reperfusion related injuries, acute lung injury and pneumonia.
The issued U.S. and foreign patents are expected to expire in 2030, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending U.S. and foreign patent applications would be expected to expire in 2030, excluding any additional term for patent term adjustments or patent term extensions.
As of June 1, 2020, we owned one issued US patent and one pending U.S. non-provisional patent application, one issued EP patent validated in three EPC member states and 6 pending foreign patent applications covering the use of certain binding moieties, such as antibodies, that inhibit C5a for the treatment of viral pneumonia. If granted, the pending U.S. and foreign patent applications would be expected to expire in 2035, excluding any additional term for patent term adjustments or patent term extensions.
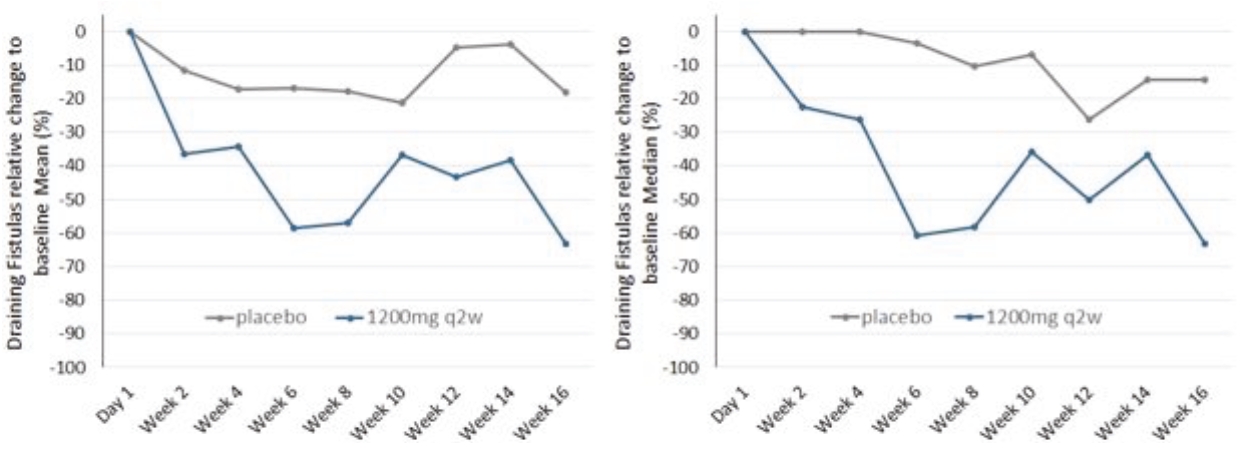
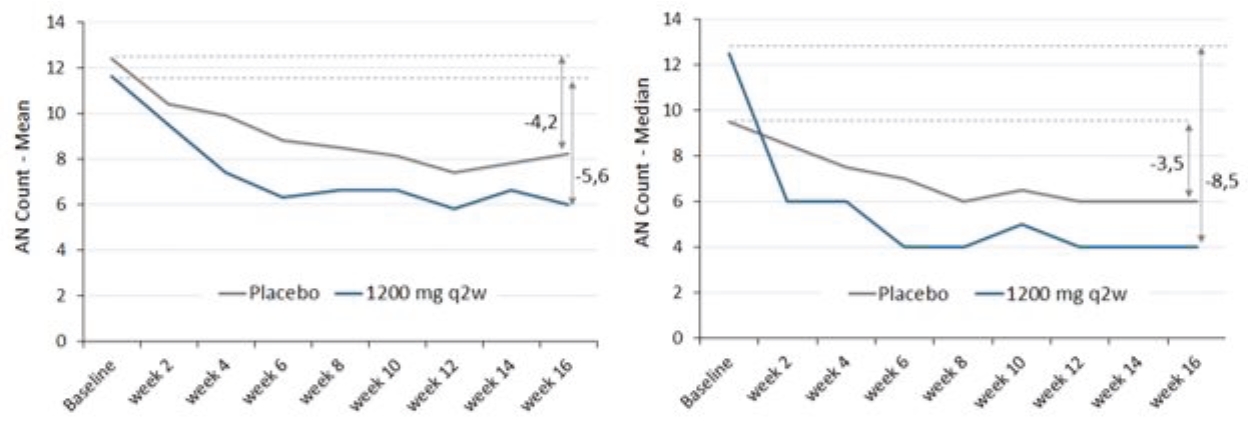
As of June 1, 2020, we owned one issued US patent and two pending U.S. non-provisional patent applications, 24 pending foreign patent applications, two pending European patent application and two granted foreign patents covering the use of an inhibitor of C5a activity, for example, IFX-1, for treating HS and other cutaneous, neutrophilic inflammatory diseases. We plan to file additional European and foreign patent applications on the basis of the two pending applications under the PCT which, if granted, would be expected to expire in 2038, excluding any additional term for patent term adjustments or patent term extensions.
As of June 1, 2020, we owned one pending patent application under the PCT covering the use of an inhibitor of C5a activity, for example, IFX-1, for treating COVID-19 which, if granted, would be expected to expire in 2040, excluding any additional term for patent term adjustments or patent term extensions.
As of June 1, 2020, we owned one pending European patent application covering the composition of matter of humanized antibodies, for example, IFX-2, that block C5a and their use in blocking C5a-induced biological effects in patients which, if granted, would be expected to expire in 2041, excluding any additional term for patent term adjustments or patent term extensions.
Corporate Information
The common shares covered by this prospectus refer to the common shares of InflaRx N.V. InflaRx was founded in 2007 as InflaRx GmbH by Professor Niels Riedemann and Professor Renfeng Guo in Jena, Germany. The offices of InflaRx N.V. are located at Winzerlaer Str. 2, 07745 Jena, Germany. Our telephone number is (+49) 3641 508 180. Investors should contact us for any inquiries at the address and telephone number of our principal executive office. Our principal website is www.inflarx.com. The information contained on our website is not a part of this prospectus.