Japan. In each case, we determine the strategy and territories required after discussion with our patent attorneys so that we obtain relevant coverage in territories that are commercially important to us and our product candidates. We additionally rely on data exclusivity, market exclusivity and patent term extensions when available. We also rely on trade secrets andknow-how relating to our underlying platform technology and product candidates. Prior to making any decision on filing any patent application, we consider with our patent attorneys whether patent protection is the most sensible strategy for protecting the invention concerned or whether the invention should be maintained as confidential.
As of October 11, 2018, we owned 318 granted patents (of which eight are U.S.-issued patents) and 258 pending patent applications (of which 14 are U.S. pending patent applications). Commercially or strategically importantnon-U.S. jurisdictions in which we hold issued or pending patent applications include: Australia, Canada, China, Eurasia (in the form of a regional patent), Europe (in the form of a regional patent), Hong Kong, India, Israel, Japan, South Korea, Malaysia, Mexico, Philippines, Singapore and South Africa. Not included in the patent count is one U.S. patent that is currently in reissue proceedings which were initiated by us for purposes of narrowing the claims covered by such patent.
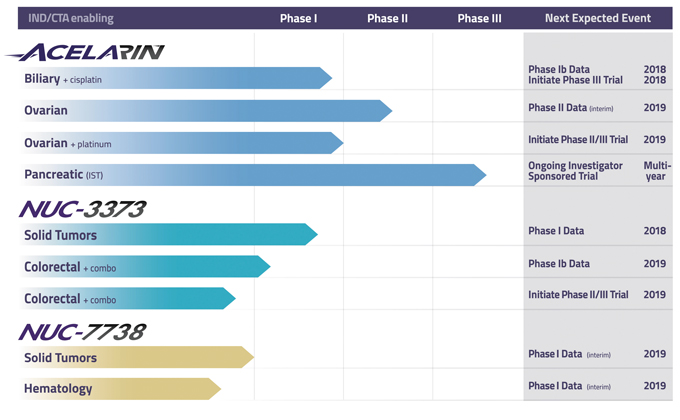
Acelarin
We own 63 granted patents covering the composition of matter of our Acelarin product candidate. The patent claims are directed to the Acelarin product candidate and to a genus around that candidate. Acelarin was originally formed as a mixture of two diastereoisomers, both of which are biologically active, and these composition of matter patents cover Acelarin either as a single diastereoisomer or as a mixture of diastereoisomers. The patent has been granted in major territories, including Europe and Japan. These granted patents are expected to expire in 2024, excluding any patent term adjustments and any patent term extensions. As disclosed above, there is also one patent in the United States for Acelarin that is currently under reissue.
We own 45 granted patents, as well as 33 pending patent applications, directed towards Acelarin in single diastereoisomer form. The more soluble single diastereoisomer is being used for clinical development and is the form which we expect to use in our planned upcoming clinical trials. A patent claiming the more soluble single diastereoisomer of Acelarin has been granted in Europe and the United States, and corresponding patent applications are pending in other major territories, including Japan. These granted patents and patents arising from the pending applications, if issued, are expected to expire in 2033 and 2035, excluding any patent term adjustments and any patent term extensions.
We own granted patents and patent applications covering formulations of Acelarin (including those used in the clinical trials), methods of making Acelarin (including as a single diastereoisomer), and specific uses of Acelarin, including the use of Acelarin in combination with carboplatin and Acelarin in combination with cisplatin. A patent claiming the clinical formulation of Acelarin has been granted in Europe and indicated to be allowable in the United States. Patents arising from these pending applications have been filed in all major territories, including the United States, Europe and Japan and are expected to expire in 2035, 2036 and 2038 excluding any patent term adjustments and any patent term extensions.