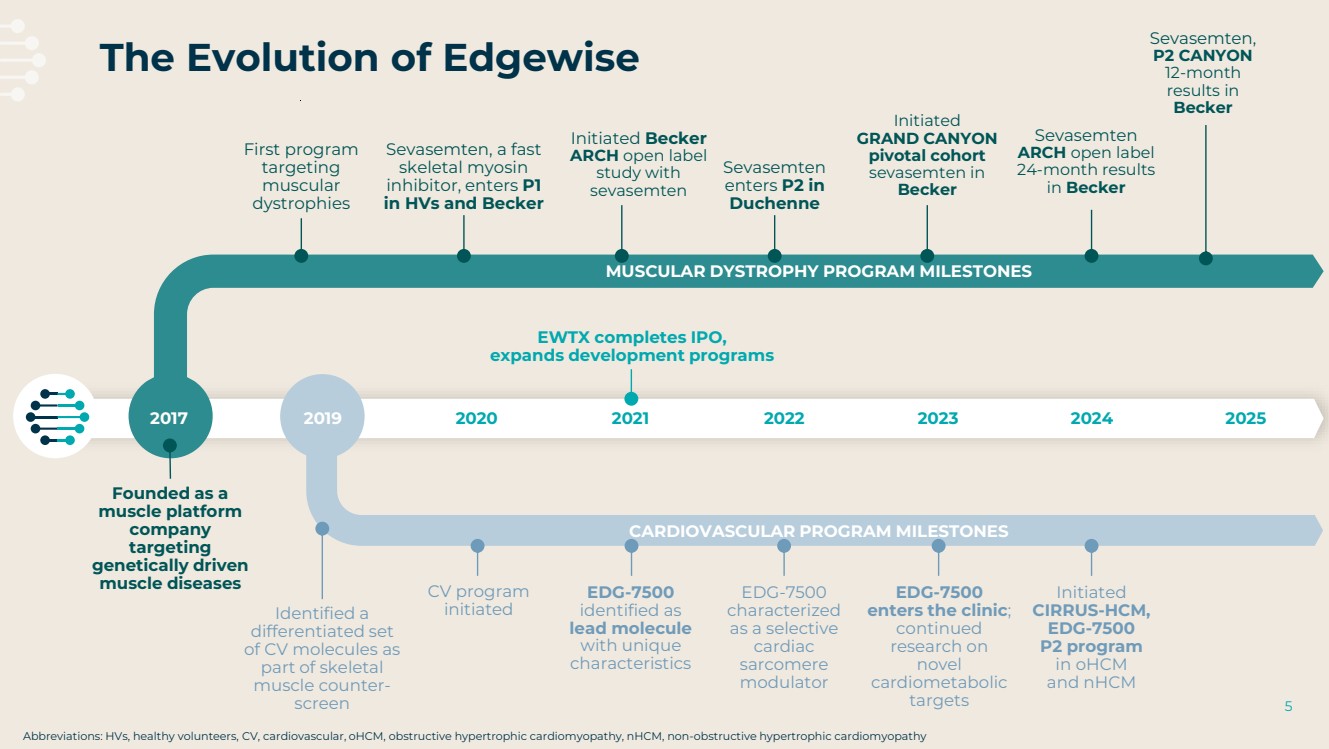
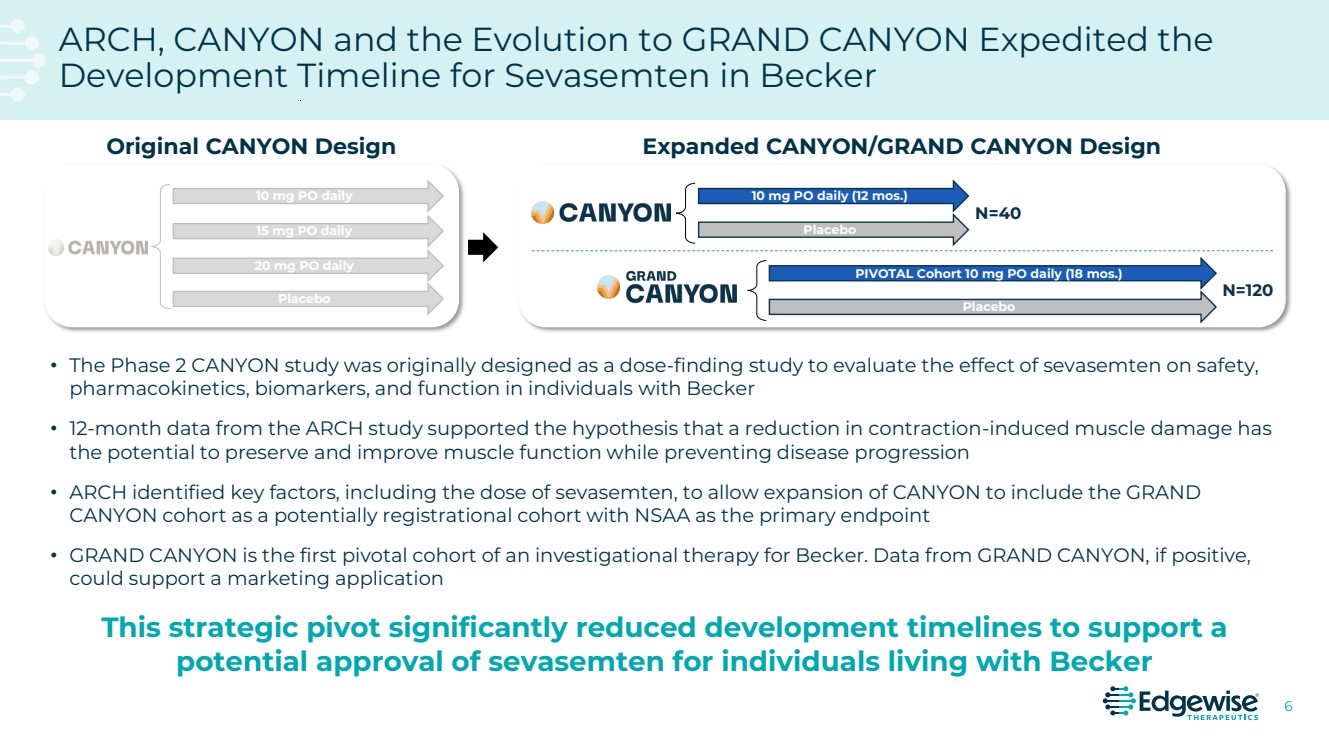
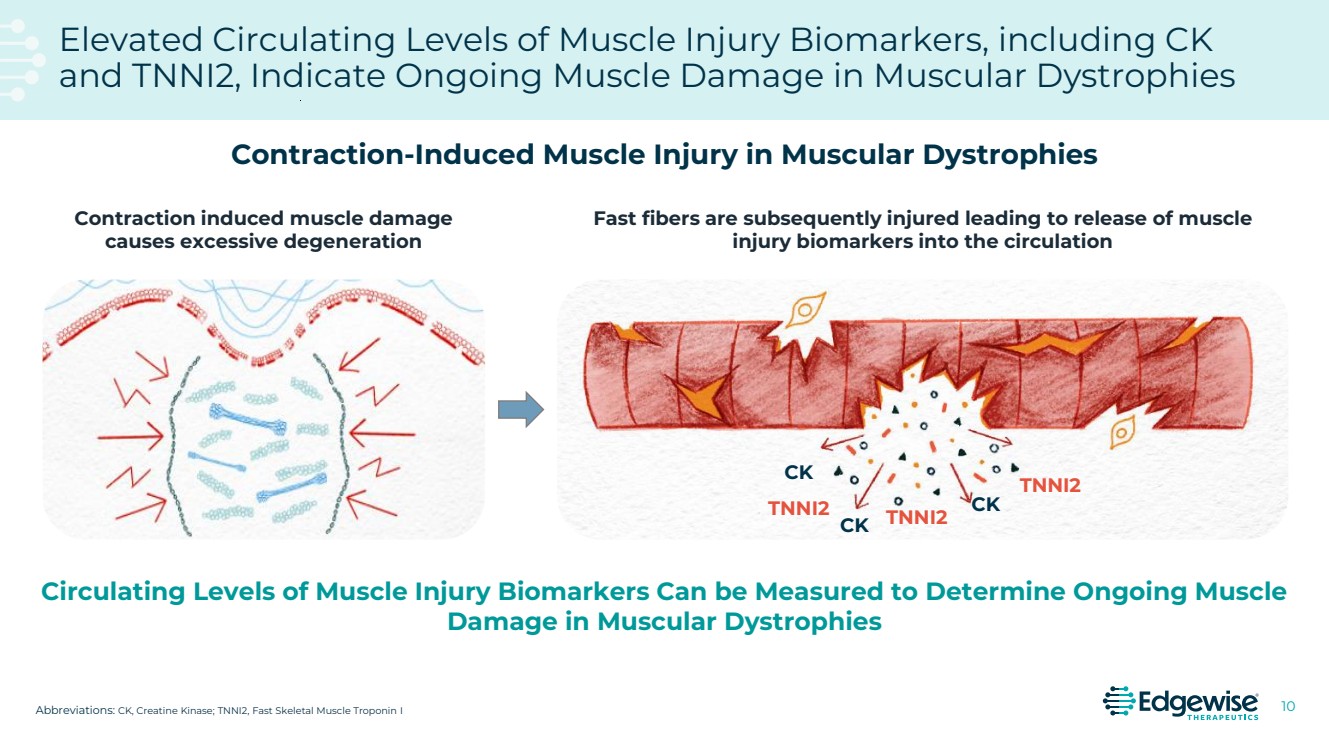
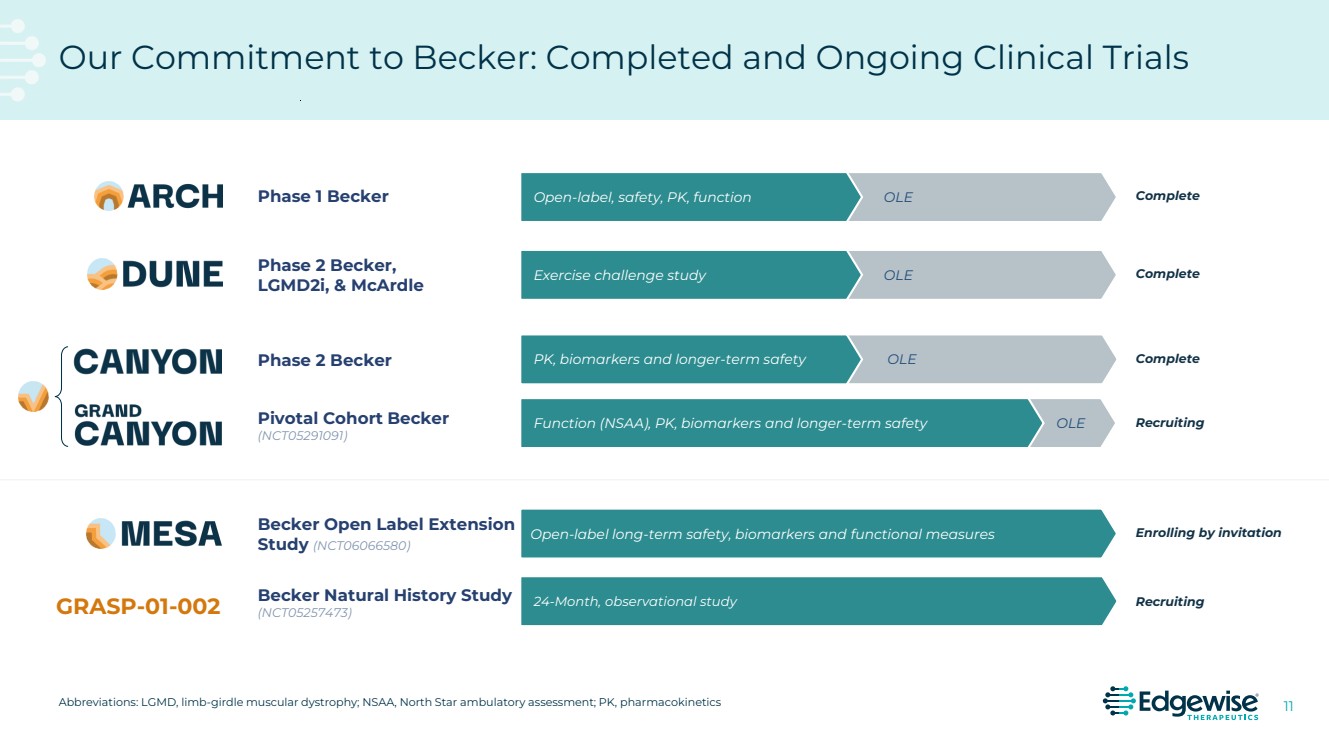
| 2 This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this presentation that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential of, and expectations regarding Edgewise’s cash runway, Edgewise’s expectations relating to its clinical trials and clinical development of sevasemten (EDG-5506) and EDG-7500, including statements regarding the number of individuals to be recruited, timing of completion of recruitment and over-enrollment of the GRAND CANYON trial; statements regarding the potential of, and expectations regarding, Edgewise’s product candidates and programs, including sevasemten and EDG-7500; statements regarding Edgewise’s milestones; statements regarding whether data from the GRAND CANYON trial could support a marketing application; statements regarding the Company’s plans to engage the FDA and European Medicines Agency; statements about sevasemten being the potential first treatment for Becker; and statements by Edgewise’s chief executive officer and chief medical officer and Craig M. McDonald, M.D. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon Edgewise’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical stage company, including the potential for Edgewise’s product candidates to cause serious adverse events; Edgewise’s ability to develop, initiate or complete clinical trials for, obtain approvals for and commercialize any of its product candidates; Edgewise’s ability to take advantage of potential benefits associated with designations granted by FDA and/or to maintain qualifications for applicable designations over time; the timing, progress and results of clinical trials for sevasemten and EDG-7500; Edgewise’s ability to enroll and maintain patients in clinical trials; Edgewise’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; the timing, scope and likelihood of regulatory filings and approvals; the potential for any clinical trial results to differ from preclinical, interim, preliminary, topline or expected results, including that the primary endpoint of the GRAND CANYON trial (change from baseline in NSAA) will be met even though it was not met as a secondary endpoint in the CANYON trial; the potential that the outcome of preclinical testing and early clinical trials, including the results from the CANYON trial, may not be predictive of the success of later clinical trials, including that the trends from the CANYON trial will also be seen, and will be statistically significant, in the GRAND CANYON trial; Edgewise may gain further insights from its analysis of the CANYON trial results over time, including Edgewise’s ongoing evaluation of additional secondary and exploratory endpoints; Edgewise’s ability to develop a proprietary drug discovery platform to build a pipeline of product candidates; Edgewise’s manufacturing, commercialization and marketing capabilities and strategy; the size of the market opportunity for Edgewise’s product candidates; the loss of key scientific or management personnel; competition in the industry in which Edgewise operates; Edgewise’s reliance on third parties; Edgewise’s ability to obtain and maintain intellectual property protection for its product candidates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that Edgewise files from time to time with the U.S. Securities and Exchange Commission. These forward-looking statements are made as of the date of this presentation, and Edgewise assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Forward Looking Statement |