Exhibit 99.1

INMB non - confidential3Q19.v2

This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . Forward - Looking Statements 2 INMB non - confidential3Q19.v2
3 Building a Next Generation immunology company • Immunology focused - platforms in immuno - oncology and neurodegenerative diseases • Target “ignored” elements of immune system • Innate immune system • TME (tumor microenvironment) • Neuroinflammation is a cause of neurodegenerative disease • Multiple clinical programs allows for robust news flow • Platform technologies provide opportunities for therapeutic expansion as resources become available • Experienced management team • Multiple programs provide sum - of - parts value INMB non - confidential3Q19.v2

4 Financial Highlights • Nasdaq: INMB • Pro Forma Cash 6/30/19 ~$9.4 million • Debt $0 • Common Shares outstanding 10.8 million Insider ownership: ~50% Notable Shareholder: Xencor (XNCR) XNCR outstanding licensing fee: buy 10% of INMB at $100m pre INMB non - confidential3Q19.v2

5 INmune Bio = Innate Immunity Our Vision: Become the Leader in Reprograming the Innate Immune System for the Treatment of Diseases INMB non - confidential3Q19.v2
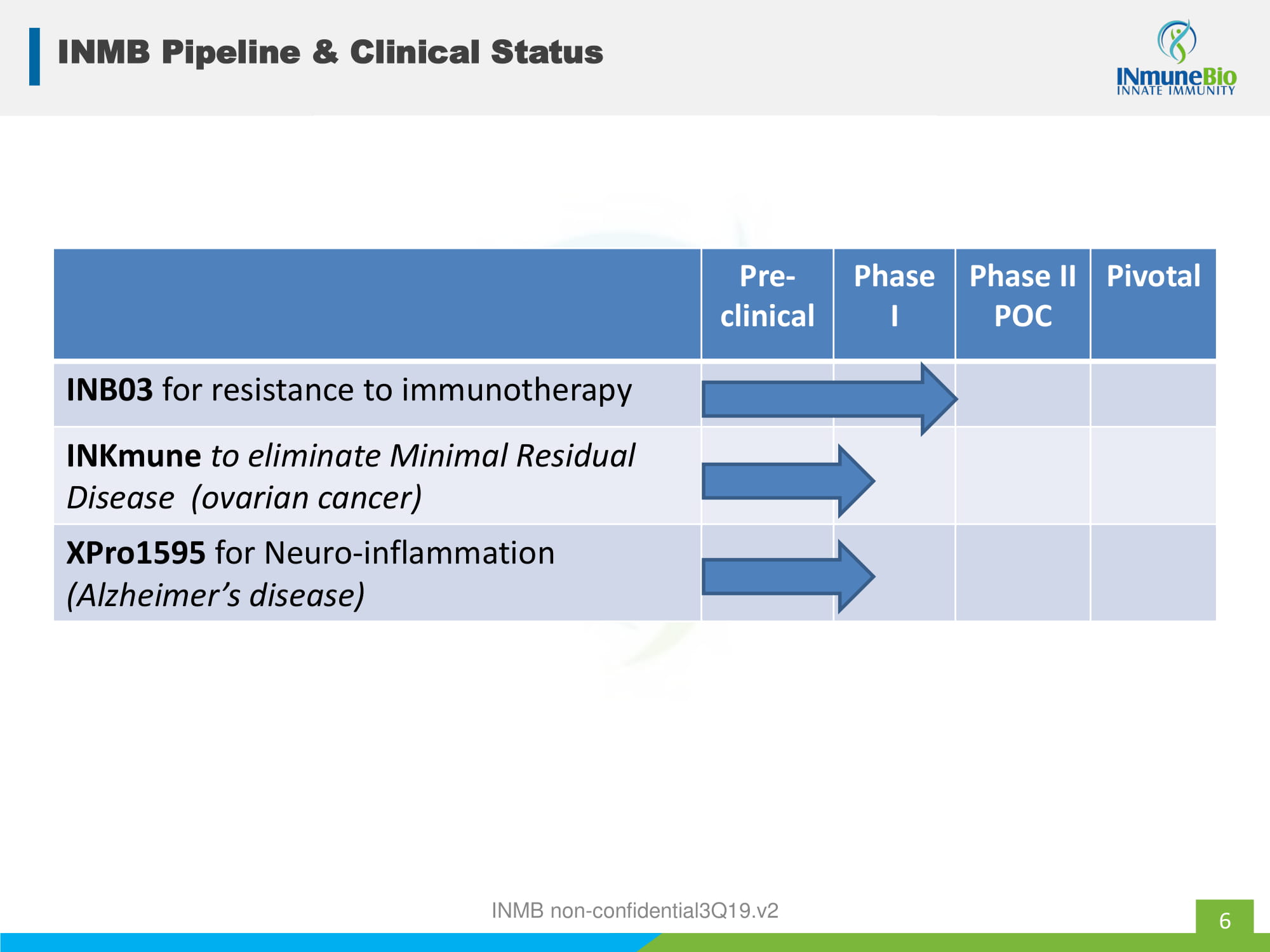
6 INMB Pipeline & Clinical Status Pre - clinical Phase I Phase II POC Pivotal INB03 for resistance to immunotherapy INKmune to eliminate Minimal Residual Disease (ovarian cancer) XPro1595 for Neuro - inflammation (Alzheimer’s disease) INMB non - confidential3Q19.v2

Pipeline Market Opportunity 7 • XPro1595 - Alzheimer’s/dementia market* – 5.8 M patients in US – 2019 est cost to care for US AD patients $280B • INKmune - Cancer residual disease market ** – 1,735,350 new cases and 609,640 people will die – Initial targets: Ovarian Cancer and High Risk MDS • INB03 - Resistance to Cancer Immunotherapy market*** – Cancer immunotherapy market est. >$119B in 2021 – Primary and secondary resistance to cancer immunotherapy common – Combination therapy with INB03 should decrease the number of resistant patients. – Initial targets: Breast or Renal Cell Cancer * Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. https://www.alz.org/alzheimers - dementia/facts - figures ** National Cancer Institute. https://www.cancer.gov/about - cancer/understanding/statistics *** MarketandMarkets . https://www.marketsandmarkets.com/Market - Reports/cancer - immunotherapy - market - 197577894.html? INMB non - confidential3Q19.v2

INB03 for treatment of cancers resistance to immunotherapy INMB non - confidential3Q19.v2

• Small cell Lung • Melanoma • RCC • Bladder • Ureothelial • Merkel Cell • NSCLC • Gastric • HNC • HCC • Microsatellite instability INMB non - confidential3Q19.v2 Resistance to immunotherapy case study: CPI 9 Eligible Benefit 10% 30% 20% 40% 60% JAMA Hasim2019 Eligibility vs Benefit CPI US patients 2018
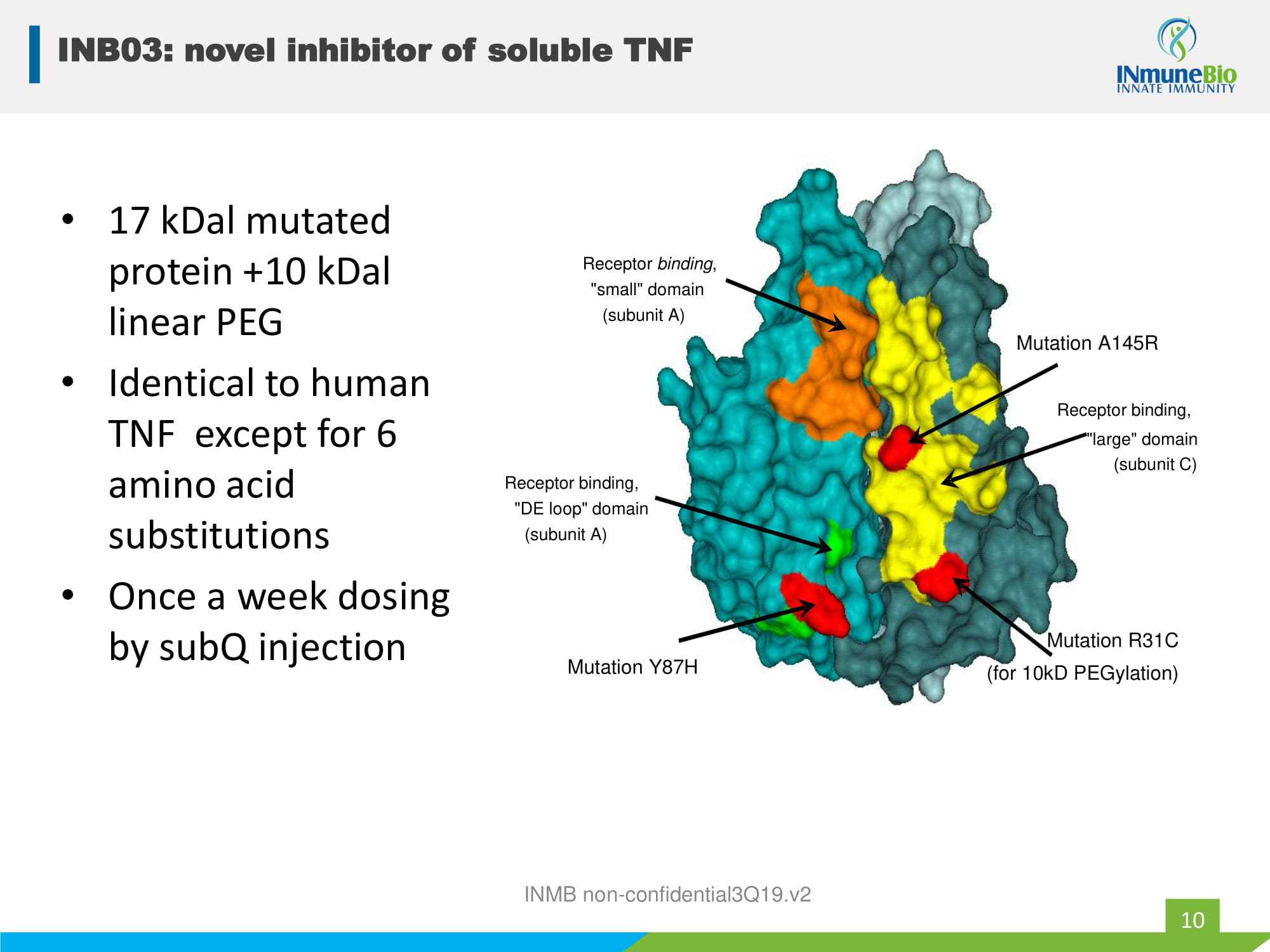
• 17 kDal mutated protein +10 kDal linear PEG • Identical to human TNF except for 6 amino acid substitutions • Once a week dosing by subQ injection INMB non - confidential3Q19.v2 Receptor binding, "DE loop" domain (subunit A) Receptor binding , "small" domain (subunit A) Mutation A145R Receptor binding, "large" domain (subunit C) Mutation R31C (for 10kD PEGylation) Mutation Y87H INB03: novel inhibitor of soluble TNF 10
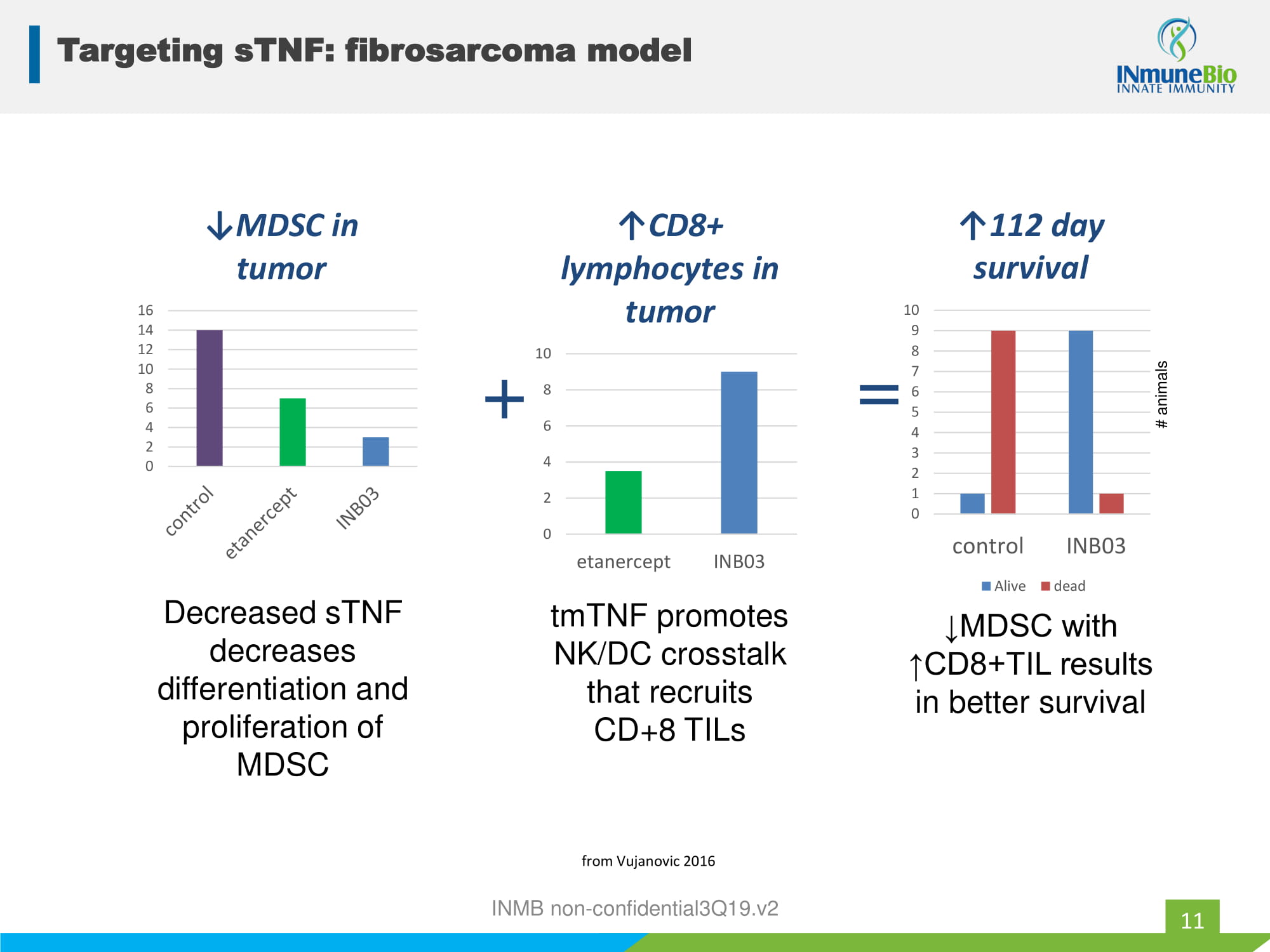
INMB non - confidential3Q19.v2 0 2 4 6 8 10 12 14 16 ↓MDSC in tumor Decreased sTNF decreases differentiation and proliferation of MDSC 0 2 4 6 8 10 etanercept INB03 ↑CD8+ lymphocytes in tumor + tmTNF promotes NK/DC crosstalk that recruits CD+8 TILs 0 1 2 3 4 5 6 7 8 9 10 control INB03 ↑112 day survival Alive dead = ↓MDSC with ↑CD8+TIL results in better survival # animals from Vujanovic 2016 Targeting sTNF: fibrosarcoma model 11
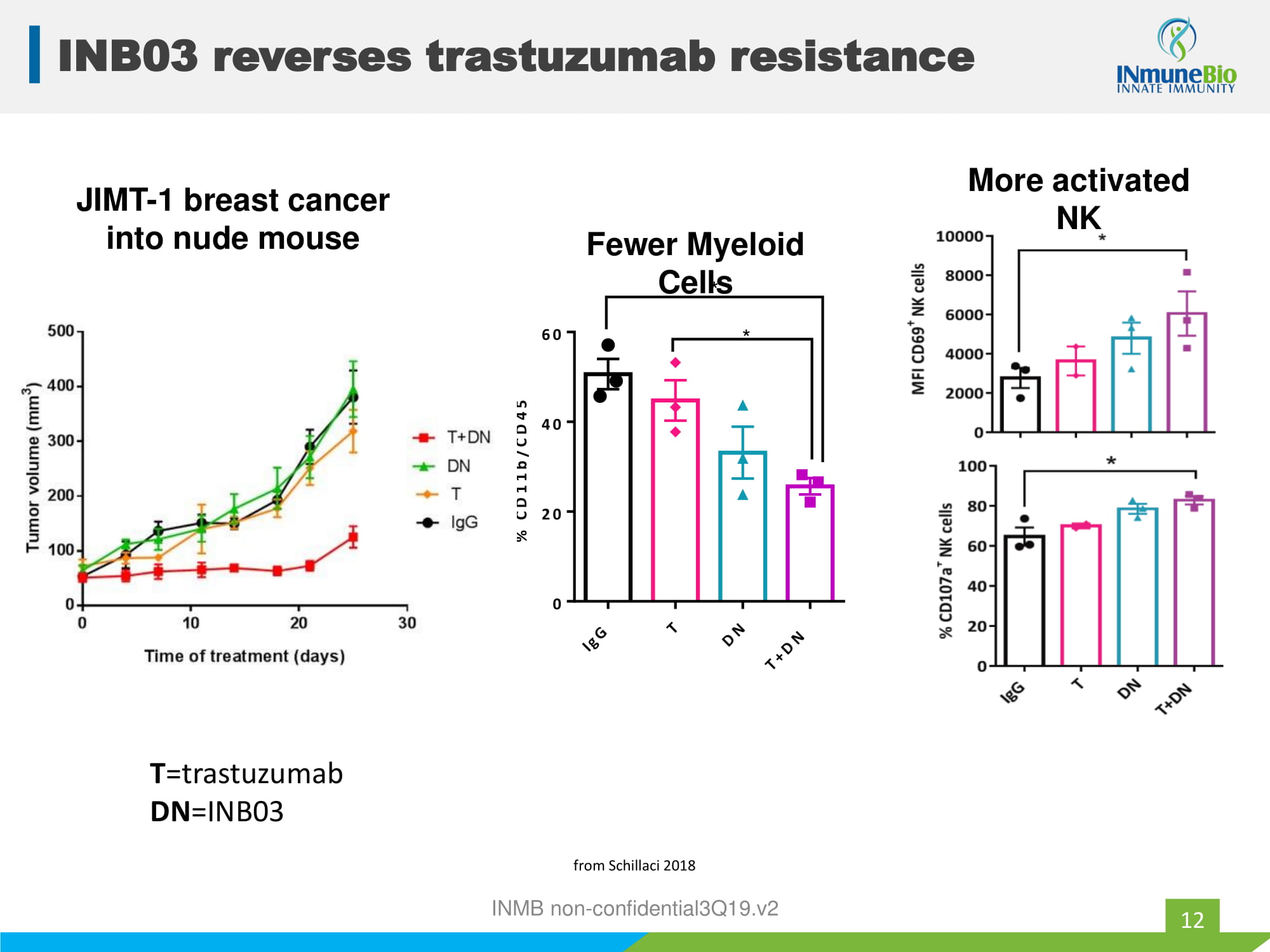
JIMT - 1 breast cancer into nude mouse I g G T D N T + D N 0 20 40 60 % C D 1 1 b / C D 4 5 + * * Fewer Myeloid Cells from Schillaci 2018 T =trastuzumab DN =INB03 INMB non - confidential3Q19.v2 More activated NK INB03 reverses trastuzumab resistance 12
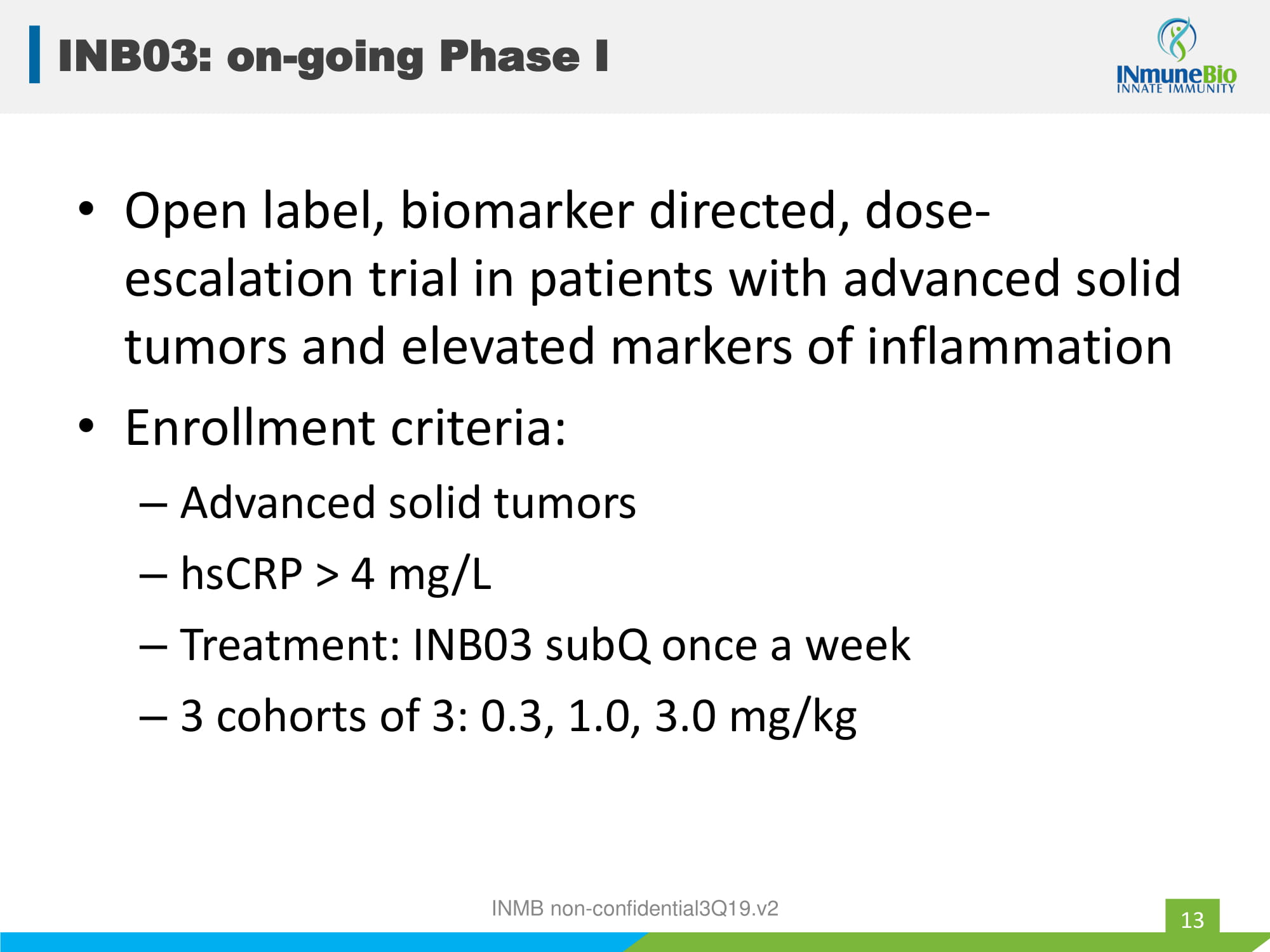
• Open label, biomarker directed, dose - escalation trial in patients with advanced solid tumors and elevated markers of inflammation • Enrollment criteria: – Advanced solid tumors – hsCRP > 4 mg/L – Treatment: INB03 subQ once a week – 3 cohorts of 3: 0.3, 1.0, 3.0 mg/kg INMB non - confidential3Q19.v2 INB03: on - going Phase I 13
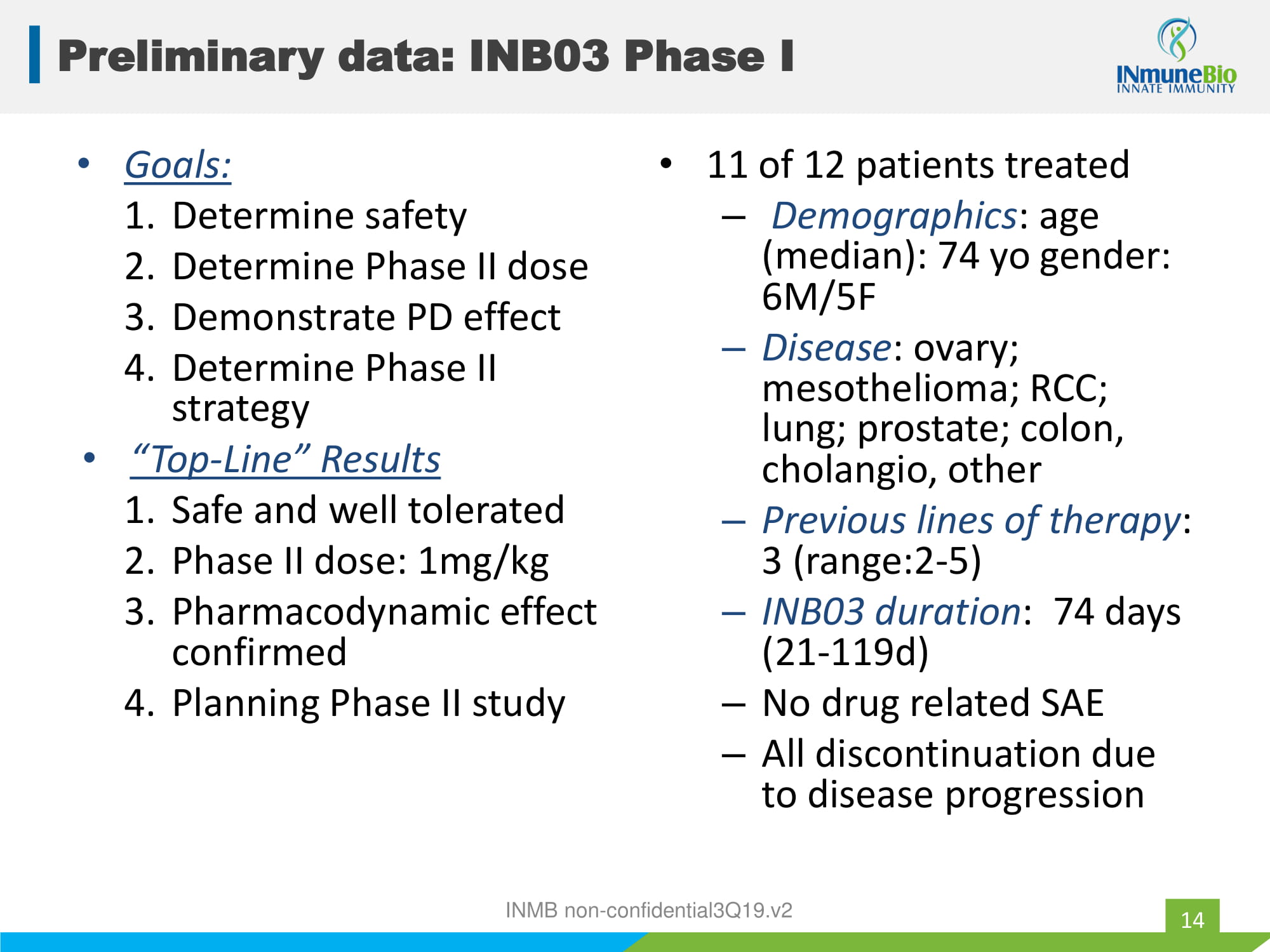
• Goals: 1. Determine safety 2. Determine Phase II dose 3. Demonstrate PD effect 4. Determine Phase II strategy • “Top - Line” Results 1. Safe and well tolerated 2. Phase II dose: 1mg/kg 3. Pharmacodynamic effect confirmed 4. Planning Phase II study • 11 of 12 patients treated – Demographics : age (median): 74 yo gender: 6M/5F – Disease : ovary; mesothelioma; RCC; lung; prostate; colon, cholangio , other – Previous lines of therapy : 3 (range:2 - 5) – INB03 duration : 74 days (21 - 119d) – No drug related SAE – All discontinuation due to disease progression INMB non - confidential3Q19.v2 Preliminary data: INB03 Phase I 14
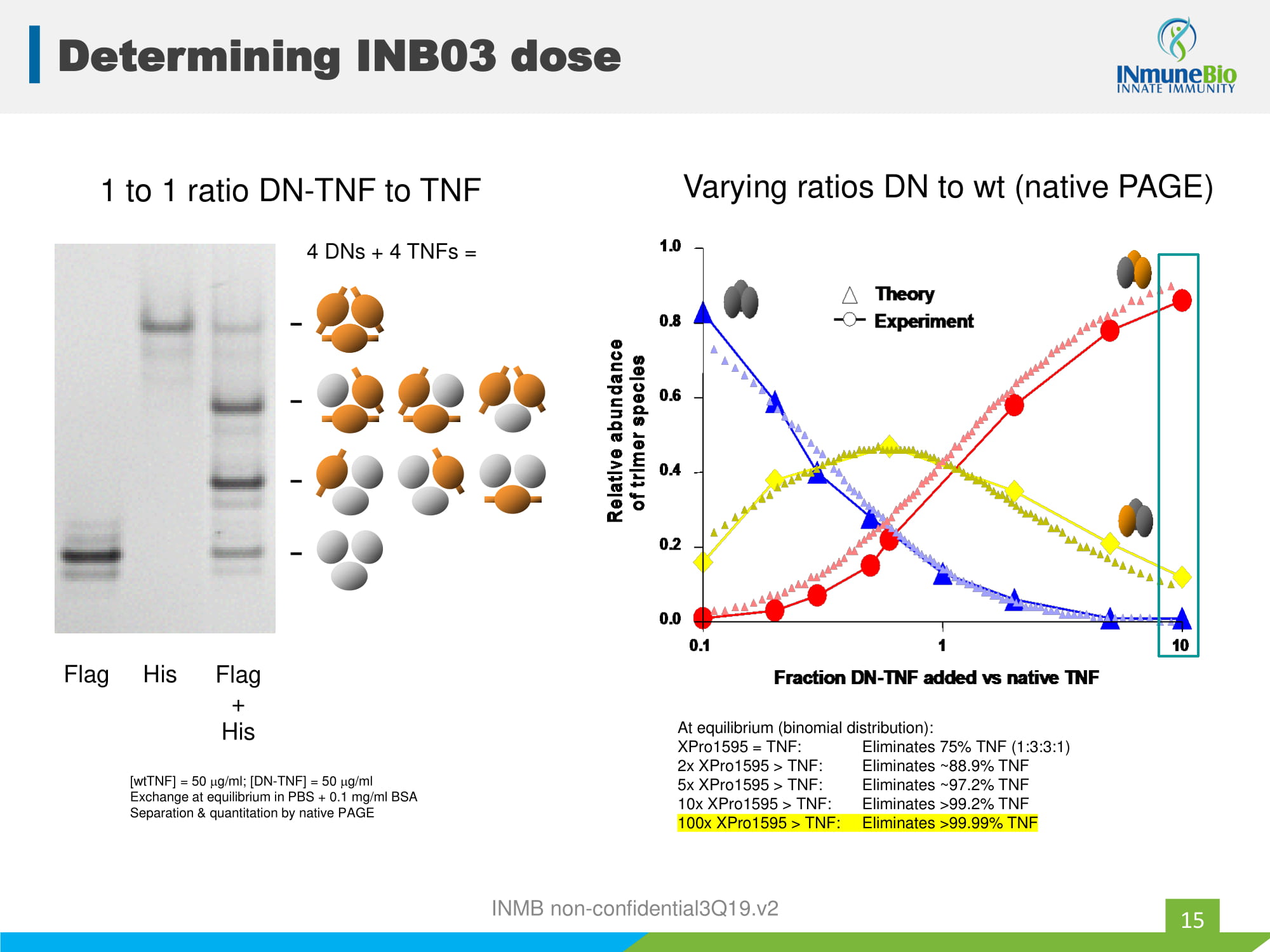
[ wtTNF ] = 50 g/ml; [DN - TNF] = 50 g/ml Exchange at equilibrium in PBS + 0.1 mg/ml BSA Separation & quantitation by native PAGE Flag His Flag + His 1 to 1 ratio DN - TNF to TNF 4 DNs + 4 TNFs = Theory Experiment 0.1 1 10 0.0 0.2 0.4 0.6 0.8 1.0 Fraction DN-TNF added vs native TNF Relative abundance of trimer species Theory Experiment Theory Experiment 0.1 1 10 0.0 0.2 0.4 0.6 0.8 1.0 Fraction DN-TNF added vs native TNF Relative abundance of trimer species Relative abundance of trimer species Varying ratios DN to wt (native PAGE) At equilibrium (binomial distribution): XPro1595 = TNF: Eliminates 75% TNF (1:3:3:1) 2x XPro1595 > TNF: Eliminates ~88.9% TNF 5x XPro1595 > TNF: Eliminates ~97.2% TNF 10x XPro1595 > TNF: Eliminates >99.2% TNF 100x XPro1595 > TNF: Eliminates >99.99% TNF Determining INB03 dose 15 INMB non - confidential3Q19.v2
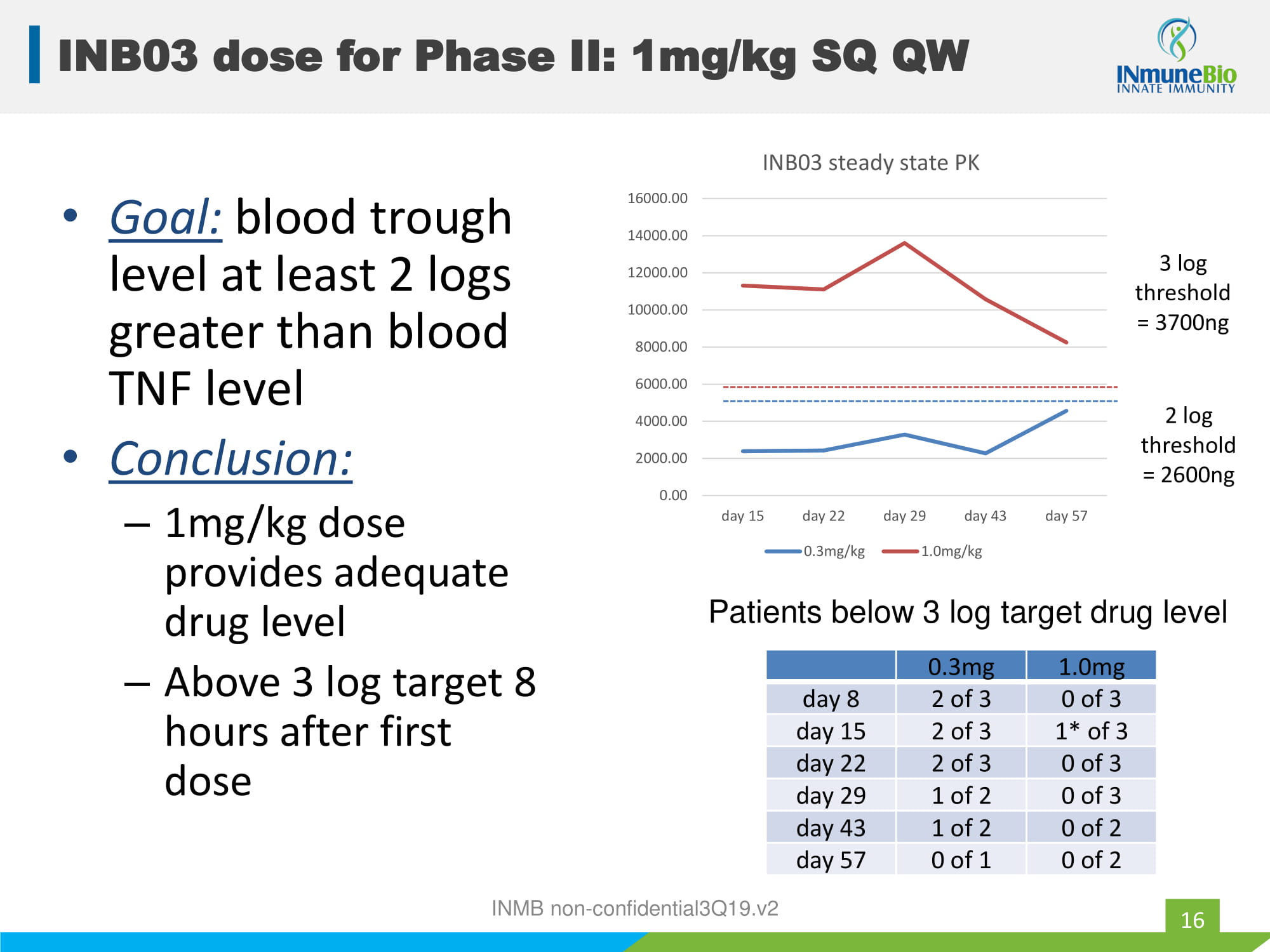
• Goal: blood trough level at least 2 logs greater than blood TNF level • Conclusion: – 1mg/kg dose provides adequate drug level – Above 3 log target 8 hours after first dose 0.3mg 1.0mg day 8 2 of 3 0 of 3 day 15 2 of 3 1* of 3 day 22 2 of 3 0 of 3 day 29 1 of 2 0 of 3 day 43 1 of 2 0 of 2 day 57 0 of 1 0 of 2 Patients below 3 log target drug level 0.00 2000.00 4000.00 6000.00 8000.00 10000.00 12000.00 14000.00 16000.00 day 15 day 22 day 29 day 43 day 57 INB03 steady state PK 0.3mg/kg 1.0mg/kg 2 log threshold = 2600ng 3 log threshold = 3700ng INMB non - confidential3Q19.v2 INB03 dose for Phase II: 1mg/kg SQ QW 16
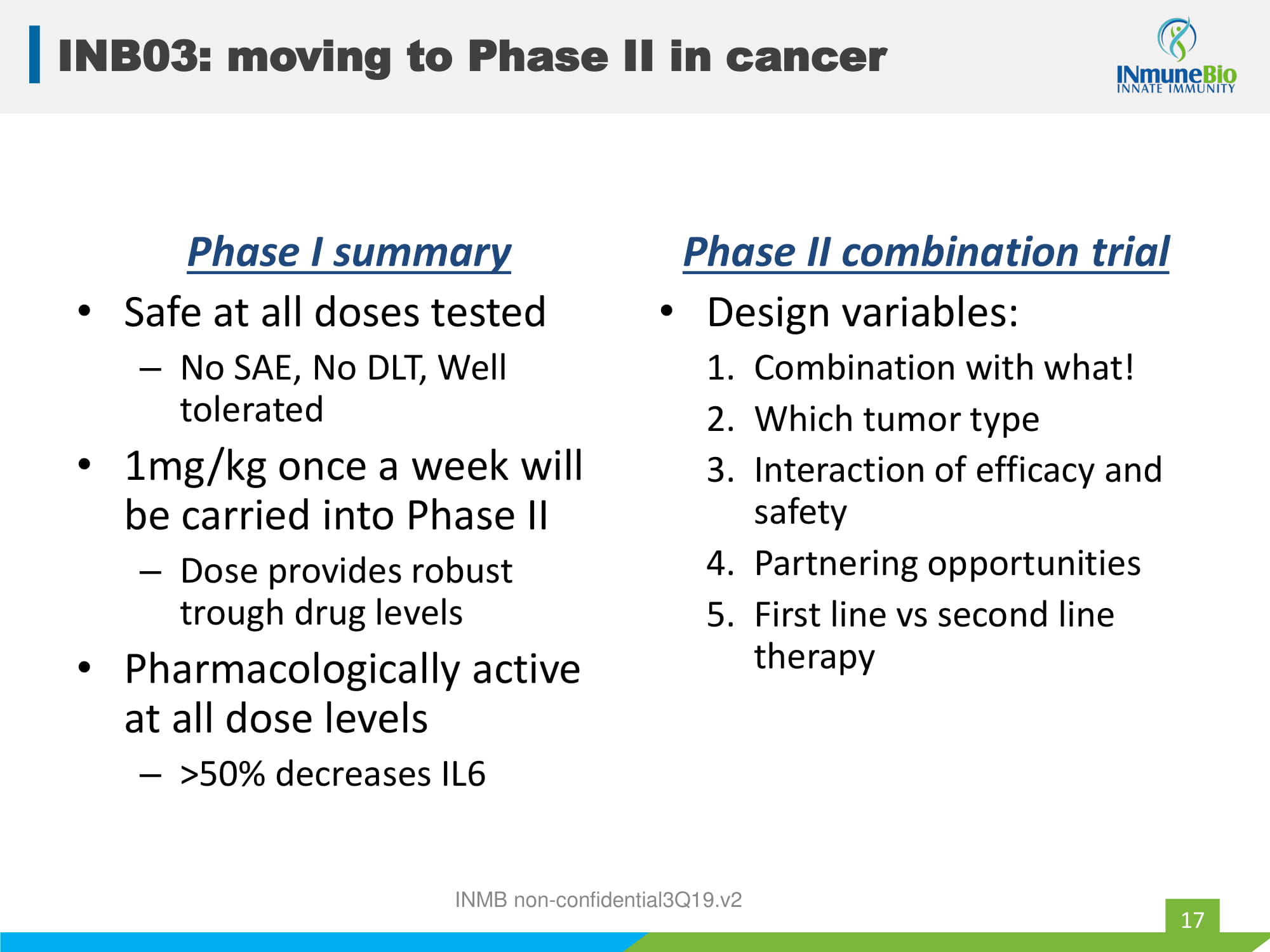
Phase I summary • Safe at all doses tested – No SAE, No DLT, Well tolerated • 1mg/kg once a week will be carried into Phase II – Dose provides robust trough drug levels • Pharmacologically active at all dose levels – >50% decreases IL6 Phase II combination trial • Design variables: 1. Combination with what! 2. Which tumor type 3. Interaction of efficacy and safety 4. Partnering opportunities 5. First line vs second line therapy INMB non - confidential3Q19.v2 INB03: moving to Phase II in cancer 17

XPro1595 (for the treatment of Alzheimer's Disease/dementia) INMB non - confidential3Q19.v2
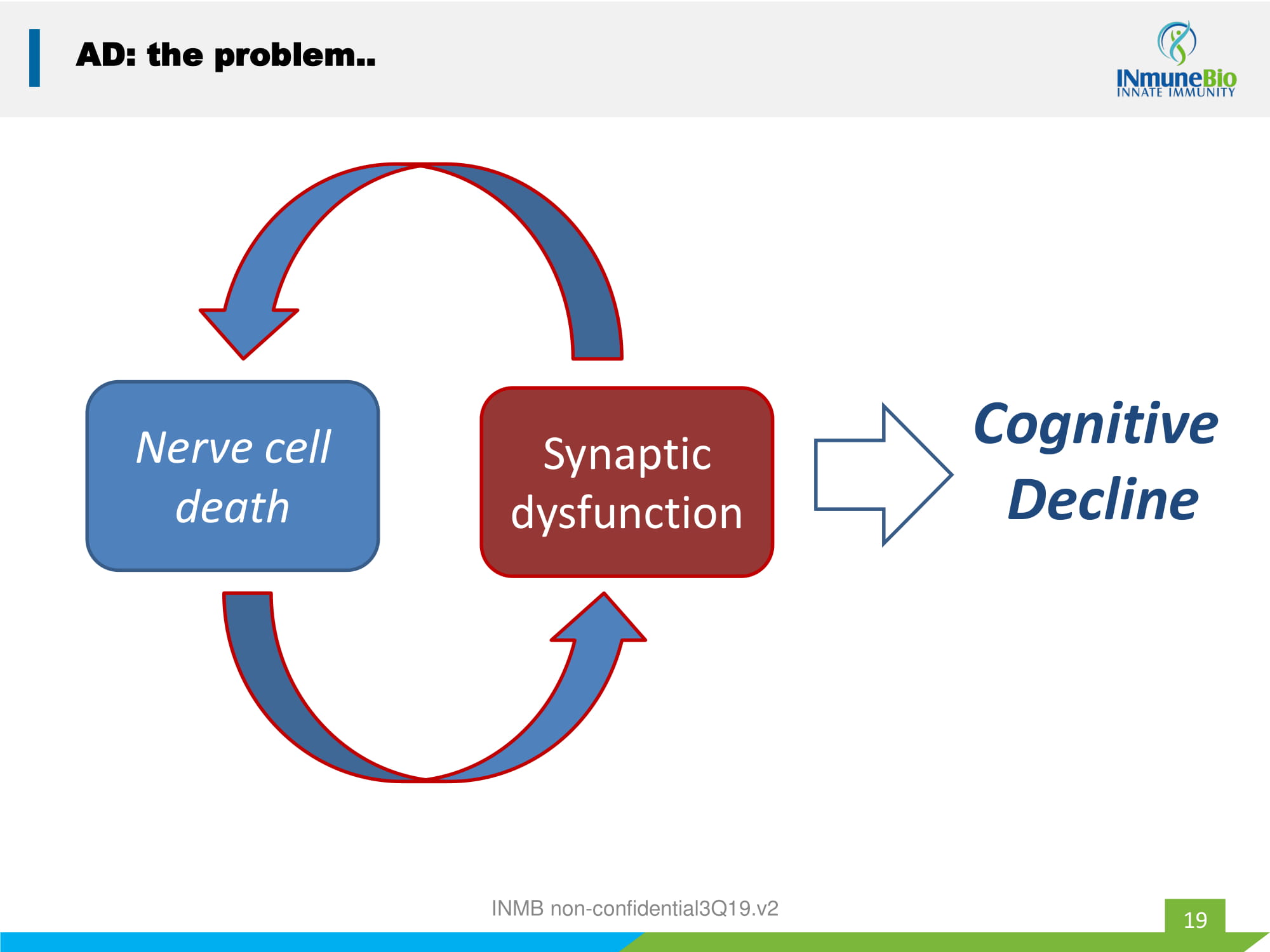
19 AD: the problem.. Nerve cell death Synaptic dysfunction Cognitive Decline INMB non - confidential3Q19.v2
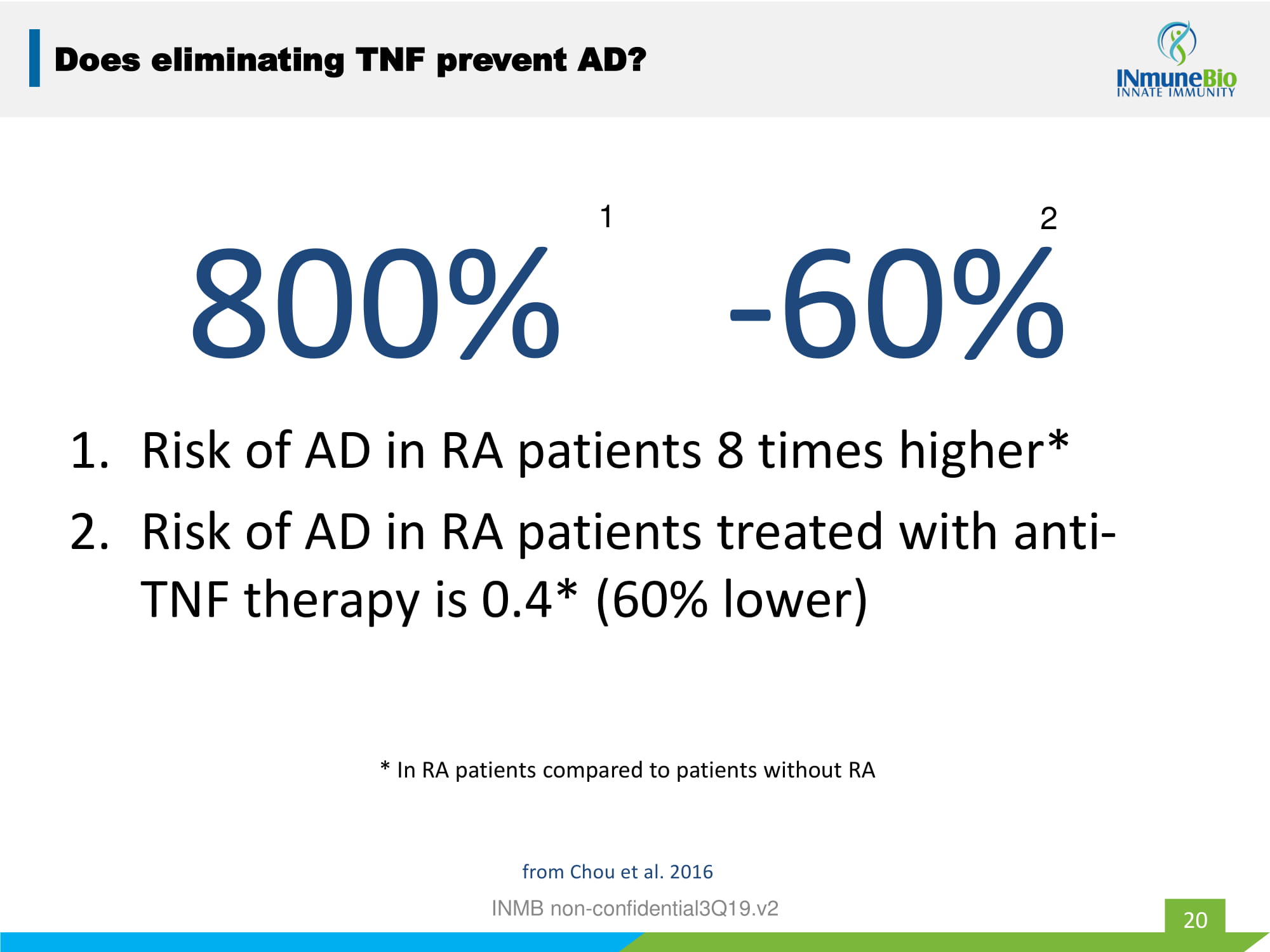
800% - 60% 1. Risk of AD in RA patients 8 times higher* 2. Risk of AD in RA patients treated with anti - TNF therapy is 0.4* (60% lower) * In RA patients compared to patients without RA 1 2 20 Does eliminating TNF prevent AD? from Chou et al. 2016 INMB non - confidential3Q19.v2
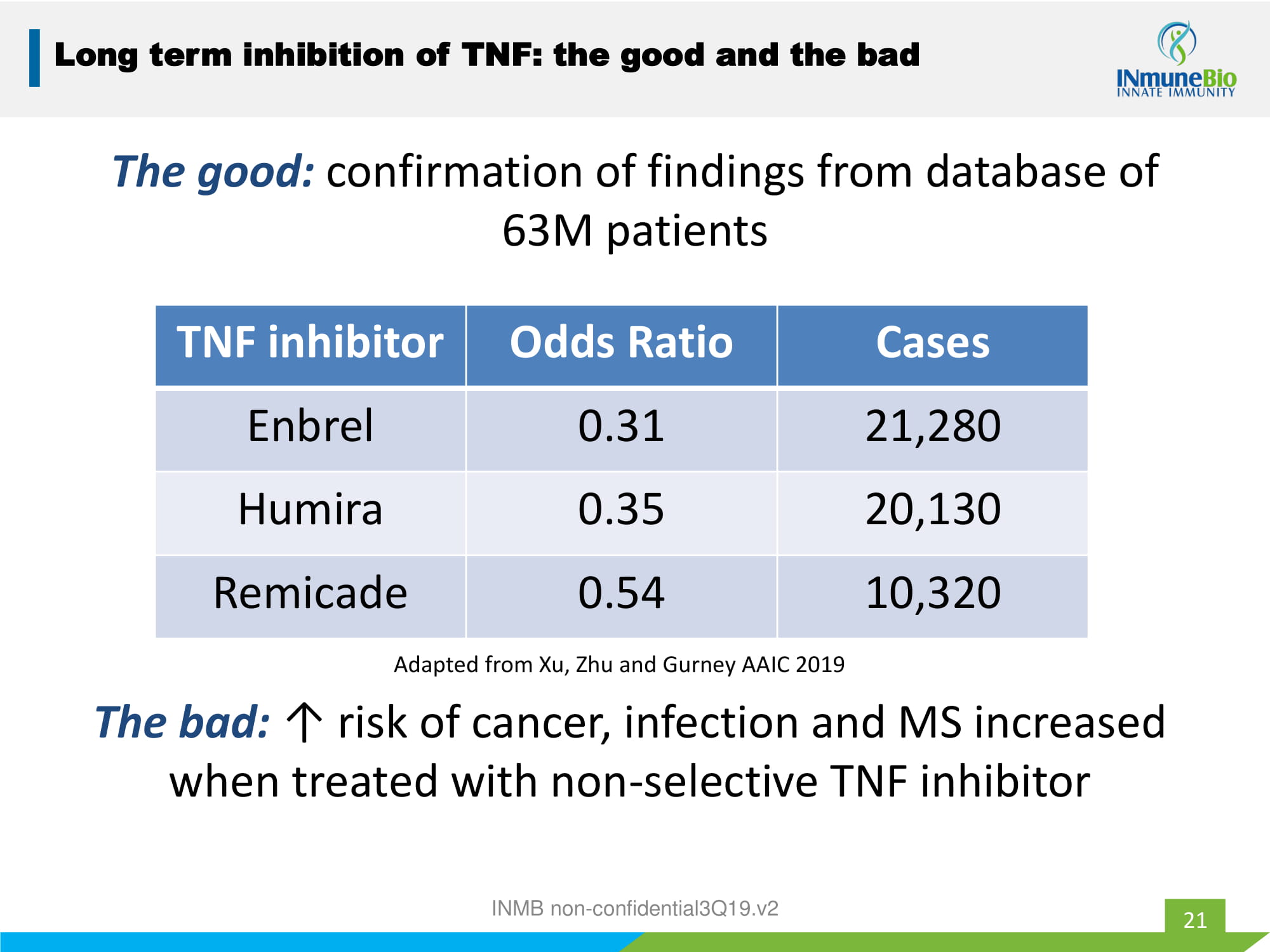
The good: confirmation of findings from database of 63M patients INMB non - confidential3Q19.v2 21 TNF inhibitor Odds Ratio Cases Enbrel 0.31 21,280 Humira 0.35 20,130 Remicade 0.54 10,320 Adapted from Xu, Zhu and Gurney AAIC 2019 Long term inhibition of TNF: the good and the bad The bad: ↑ risk of cancer, infection and MS increased when treated with non - selective TNF inhibitor

22 XPro1595 Clinical Development Supported by the Alzheimer’s Association Alzheimer’s Association awards INmune Bio Part the Cloud Grant - $1M USD translational funding to study XPro1595 in Alzheimer's disease INMB non - confidential3Q19.v2
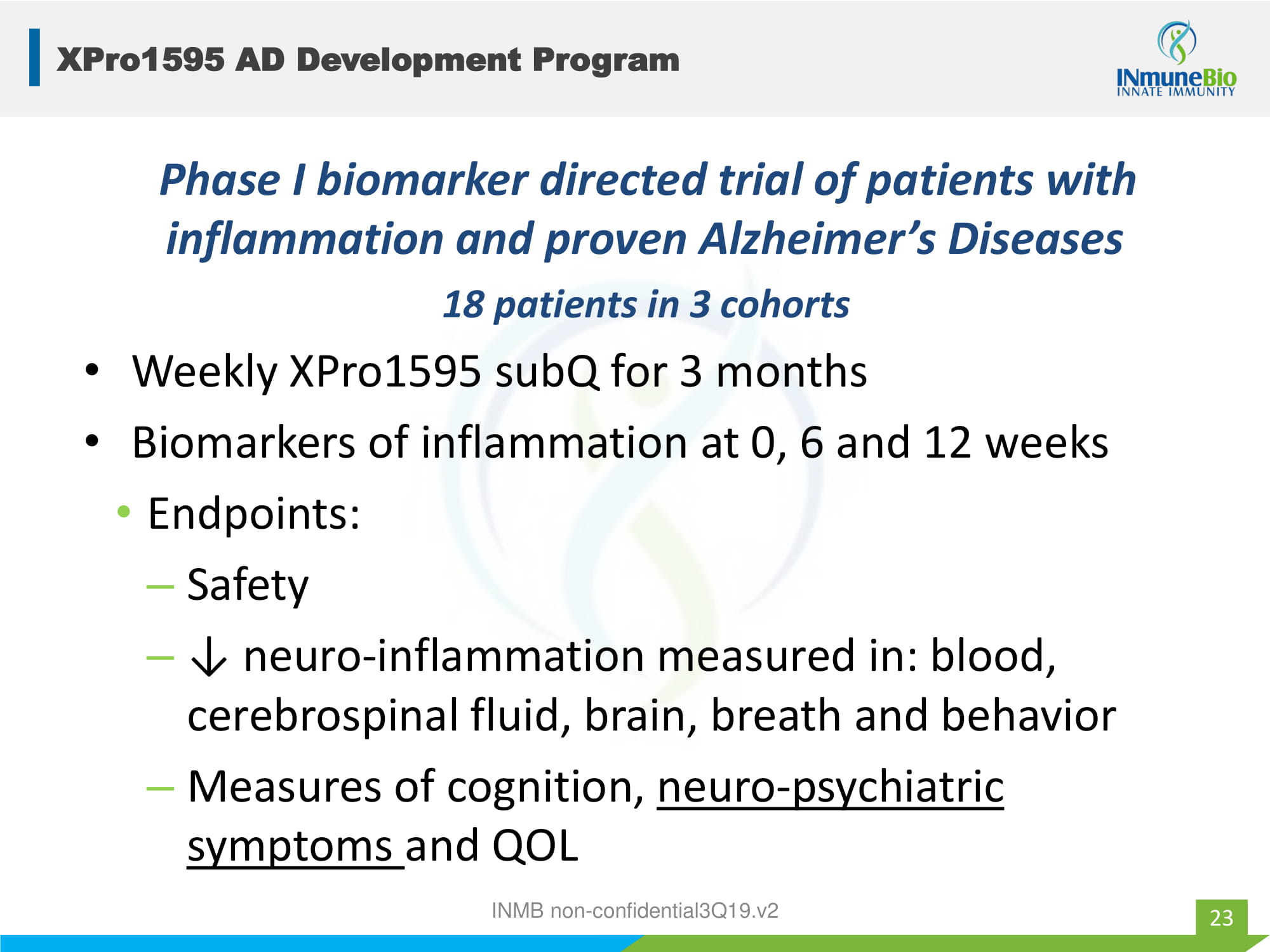
XPro1595 AD Development Program 18 patients in 3 cohorts • Weekly XPro1595 subQ for 3 months • Biomarkers of inflammation at 0, 6 and 12 weeks • Endpoints: – Safety – ↓ neuro - inflammation measured in: blood, cerebrospinal fluid, brain, breath and behavior – Measures of cognition, neuro - psychiatric symptoms and QOL 23 Phase I biomarker directed trial of patients with inflammation and proven Alzheimer’s Diseases INMB non - confidential3Q19.v2
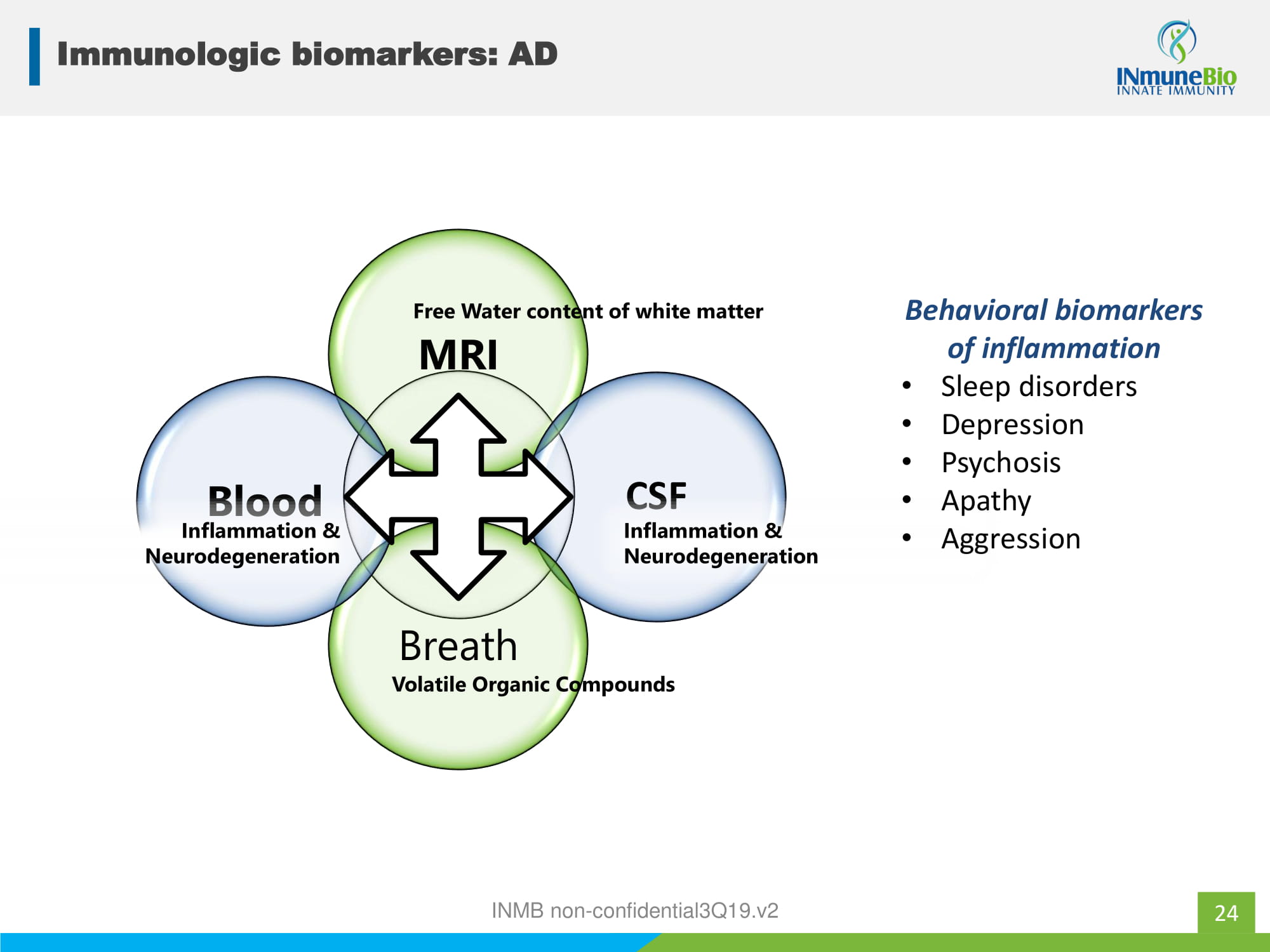
24 Immunologic biomarkers: AD MRI CSF Breath Blood Free Water content of white matter Volatile Organic Compounds Inflammation & Neurodegeneration Inflammation & Neurodegeneration Behavioral biomarkers of inflammation • Sleep disorders • Depression • Psychosis • Apathy • Aggression INMB non - confidential3Q19.v2

INKmune (for the treatment of Ovarian Cancer and high risk MDS) INMB non - confidential3Q19.v2
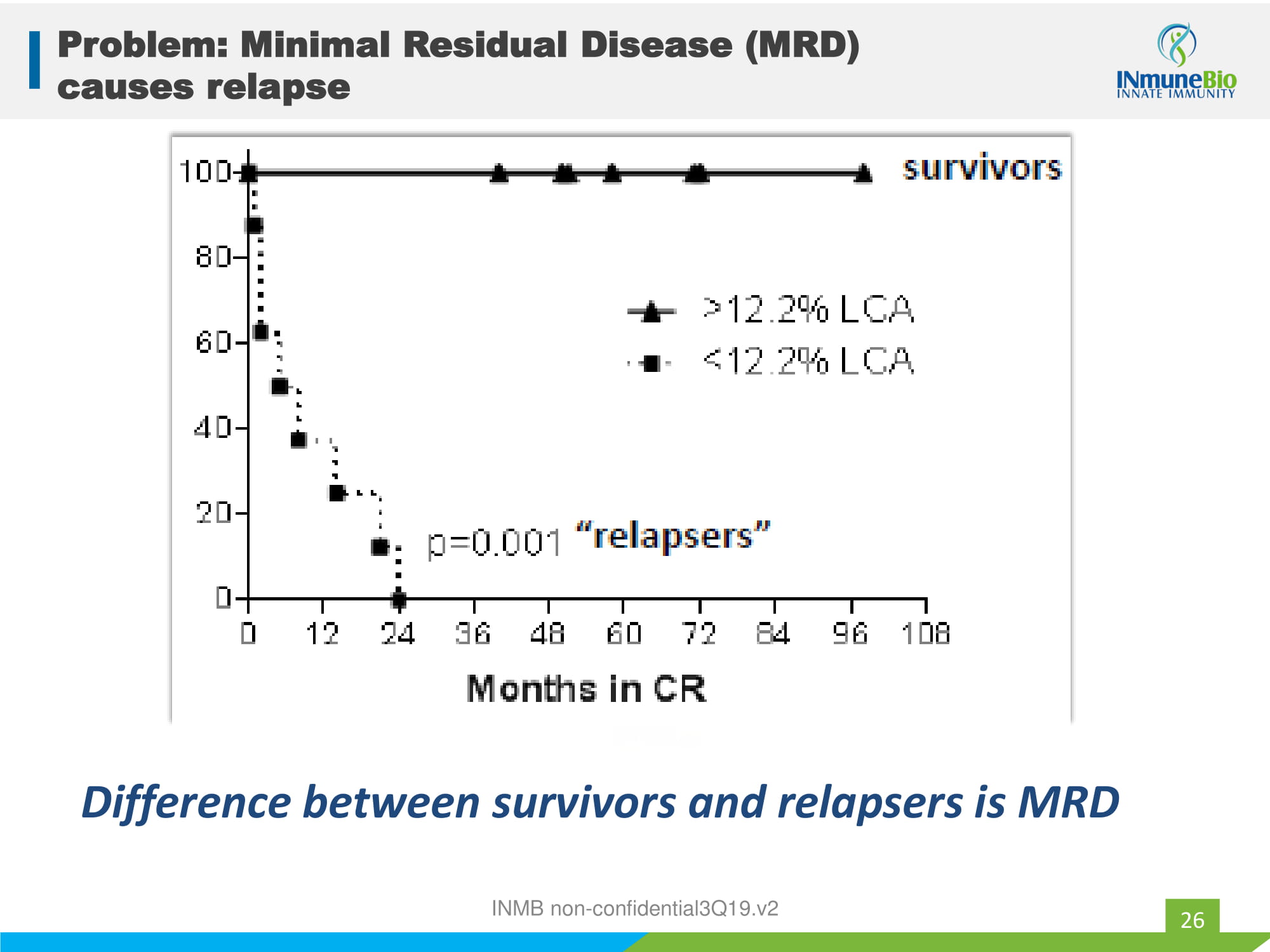
Problem: Minimal Residual Disease (MRD) causes relapse 26 Difference between survivors and relapsers is MRD INMB non - confidential3Q19.v2
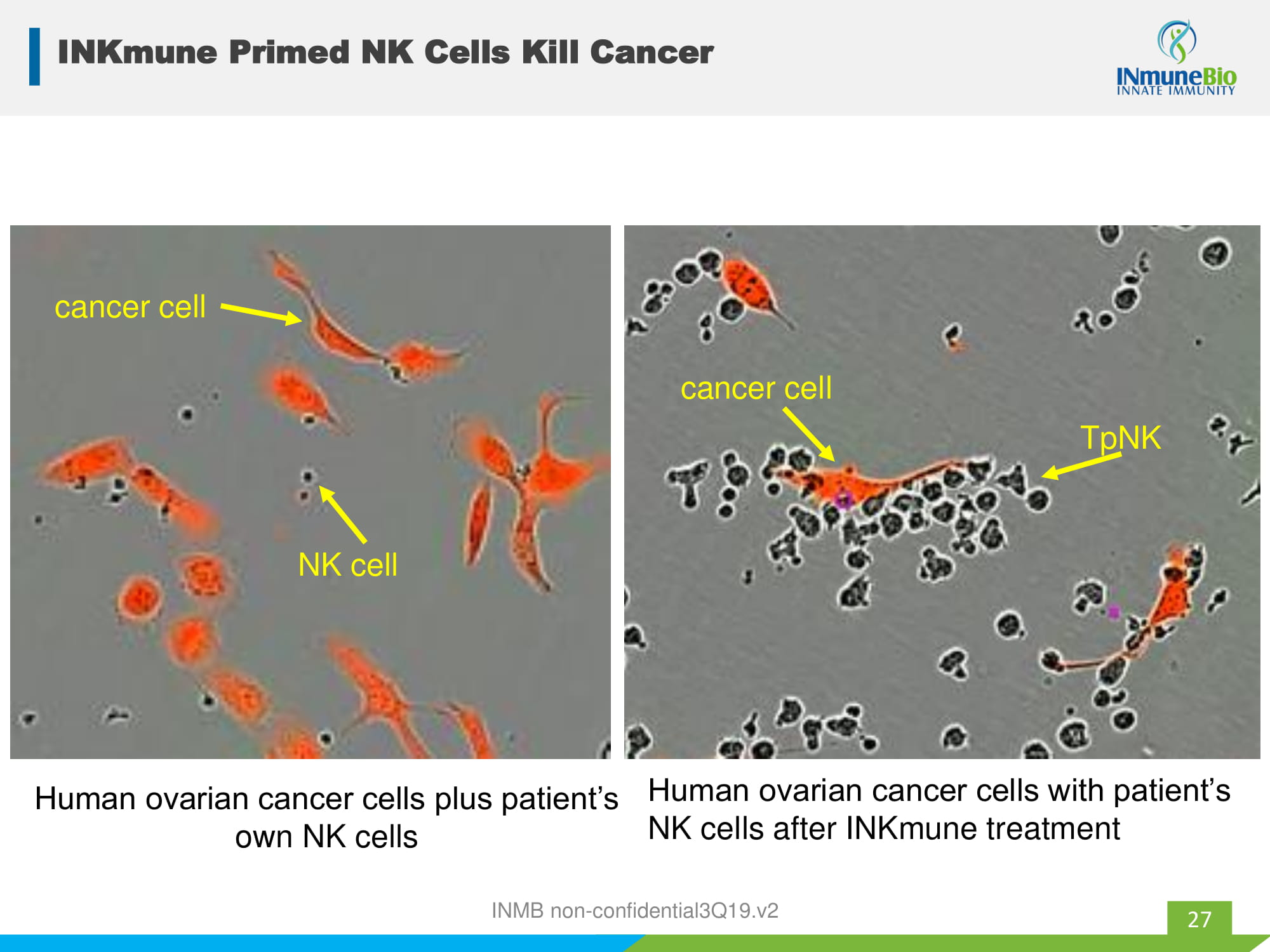
INKmune Primed NK Cells Kill Cancer 27 rN Human ovarian cancer cells plus patient’s own NK cells cancer cell NK cell Human ovarian cancer cells with patient’s NK cells after INKmune treatment TpNK cancer cell INMB non - confidential3Q19.v2

INKmune Clinical Trial Phase 1/2 study in relapsed/ refractory CaOva Platinum resistant/refractory patients with residual disease • Treatment: INKmune - 6 doses • Endpoints: – Safety – ↑ NK activation and tumor killing – ↓ tumor burden* 28 INKmune causes in vivo priming so patient’s NK cells can kill their cancer by targeting naturally occurring tumor antigens INKmune therapy weekly times three then monthly * when in Phase II INMB non - confidential3Q19.v2

INmune Bio = Innate Immunity INMB non - confidential3Q19.v2

Milestones 30 February 1, 2019 – IPO closed Feb19 – Part the Cloud award – Alzheimer’ Assoc 3Q19 – initial data on INB03 Phase I 4Q19 – first patient enrolled INKmune CaOva 1H20 – initiate INB03 combination Phase II trial 3Q19 – first patient enrolled XPro1595 AD trial 1H20 – initial data from INKmune Phase I 2H20 – Start INKmune Phase II CaOVA 2H20 – XPro1595 AD Phase II trial February 4, 2019 – NASDAQ Capital Markets listing INMB non - confidential3Q19.v2
Experienced Leadership 31 RJ Tesi, MD Chief Executive Officer & Chief Medical Officer Mark Lowdell, PhD Chief Scientific Officer David Moss Chief Financial Officer Management Board of Directors J. Kelly Ganjei CEO of Cognate Timothy Schroeder CEO and Founder of CTI David Szymkowski, PhD VP of Cell Biology Scott Juda Founder and Managing Member, Fossick Capital The picture can't be displayed. For complete biographies, visit www.inmunebio.com INMB non - confidential3Q19.v2
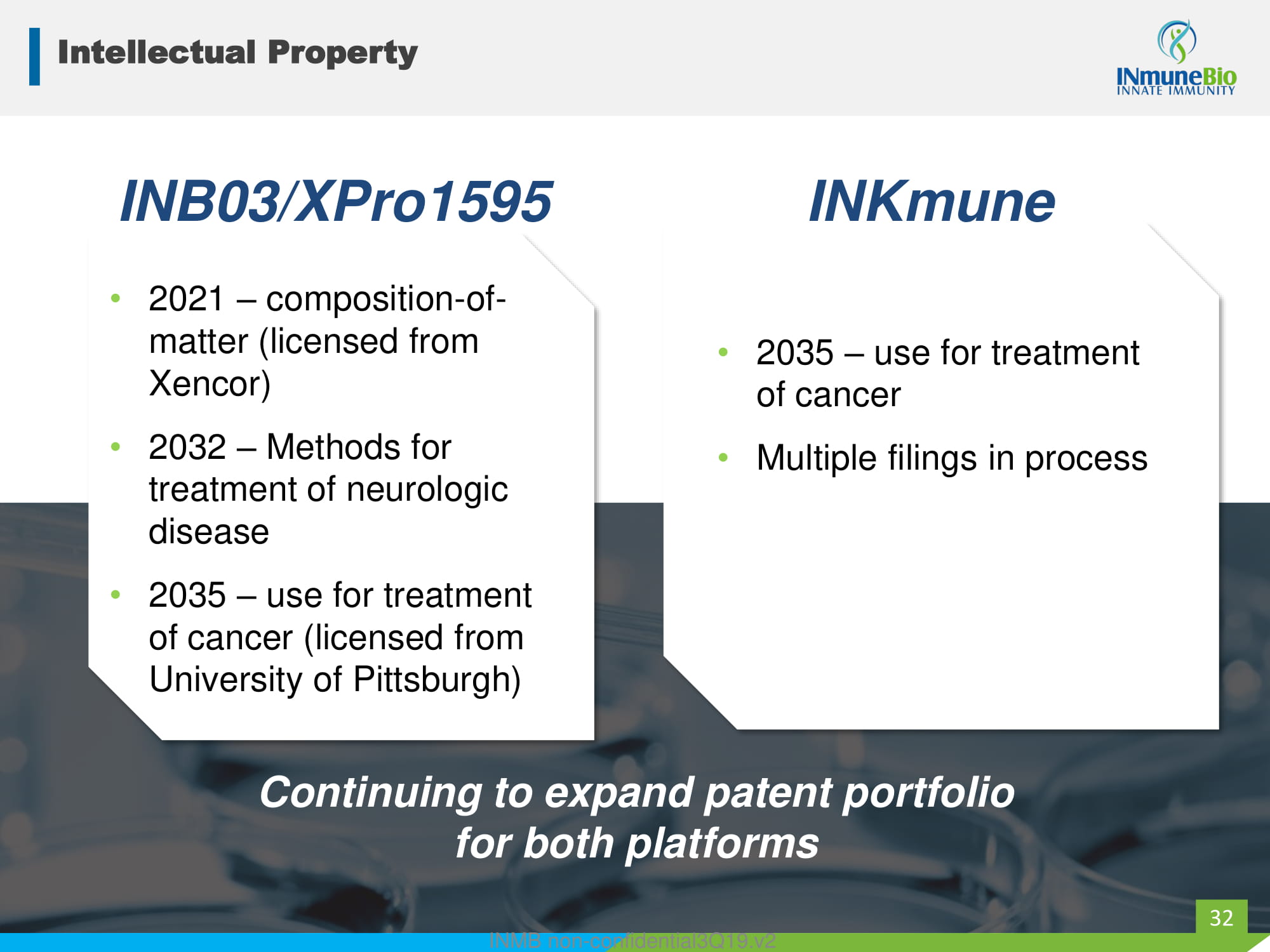
Intellectual Property • 2021 – composition - of - matter (licensed from Xencor) • 2032 – Methods for treatment of neurologic disease • 2035 – use for treatment of cancer (licensed from University of Pittsburgh) INB03/XPro1595 INKmune Continuing to expand patent portfolio for both platforms 32 • 2035 – use for treatment of cancer • Multiple filings in process INMB non - confidential3Q19.v2

INB03/XPro1595 MOA Appendix INMB non - confidential3Q19.v2
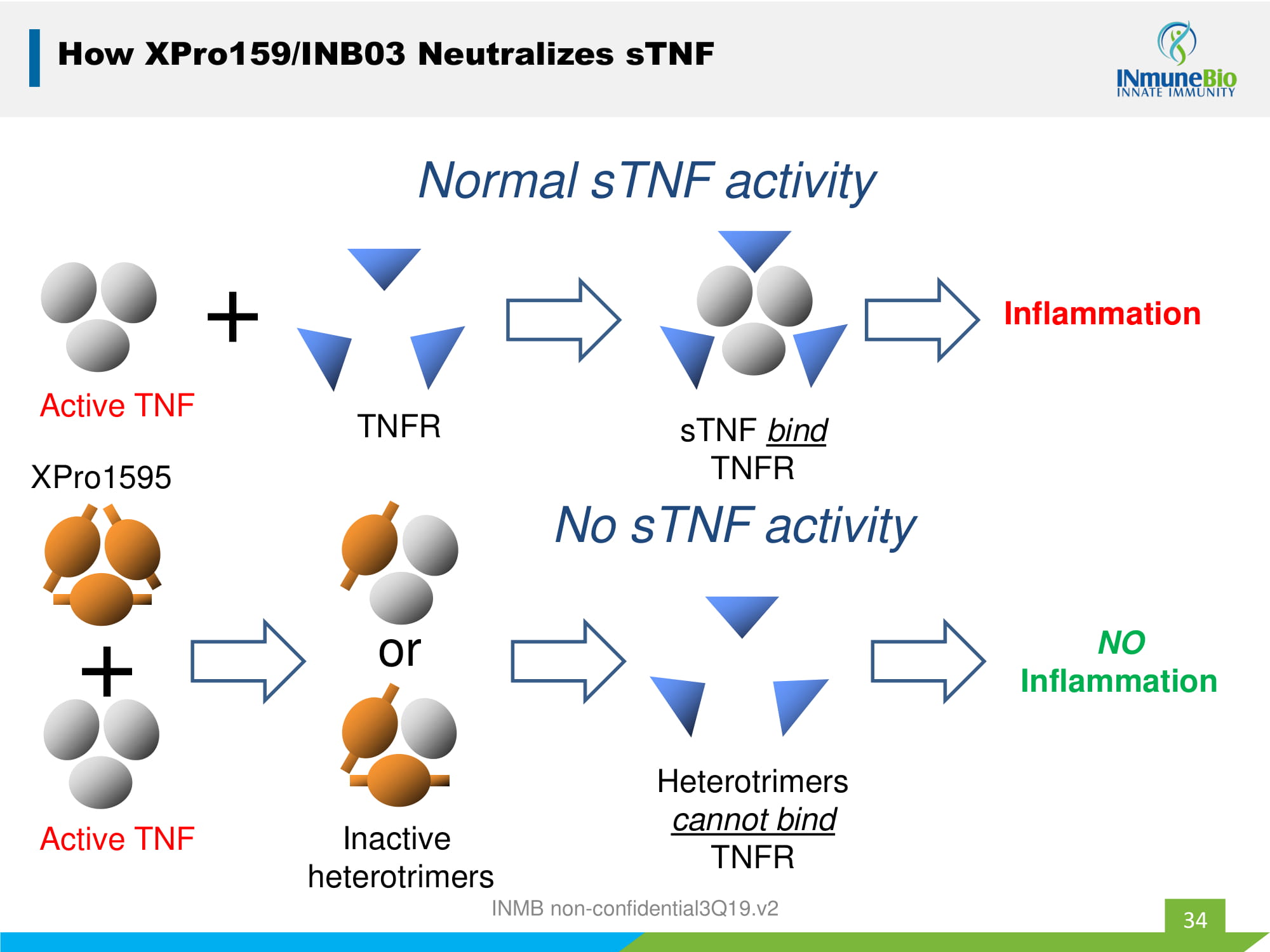
Active TNF + Inflammation Normal sTNF activity TNFR sTNF bind TNFR XPro1595 Active TNF + Inactive heterotrimers or NO Inflammation Heterotrimers cannot bind TNFR No sTNF activity How XPro159/INB03 Neutralizes sTNF 34 INMB non - confidential3Q19.v2
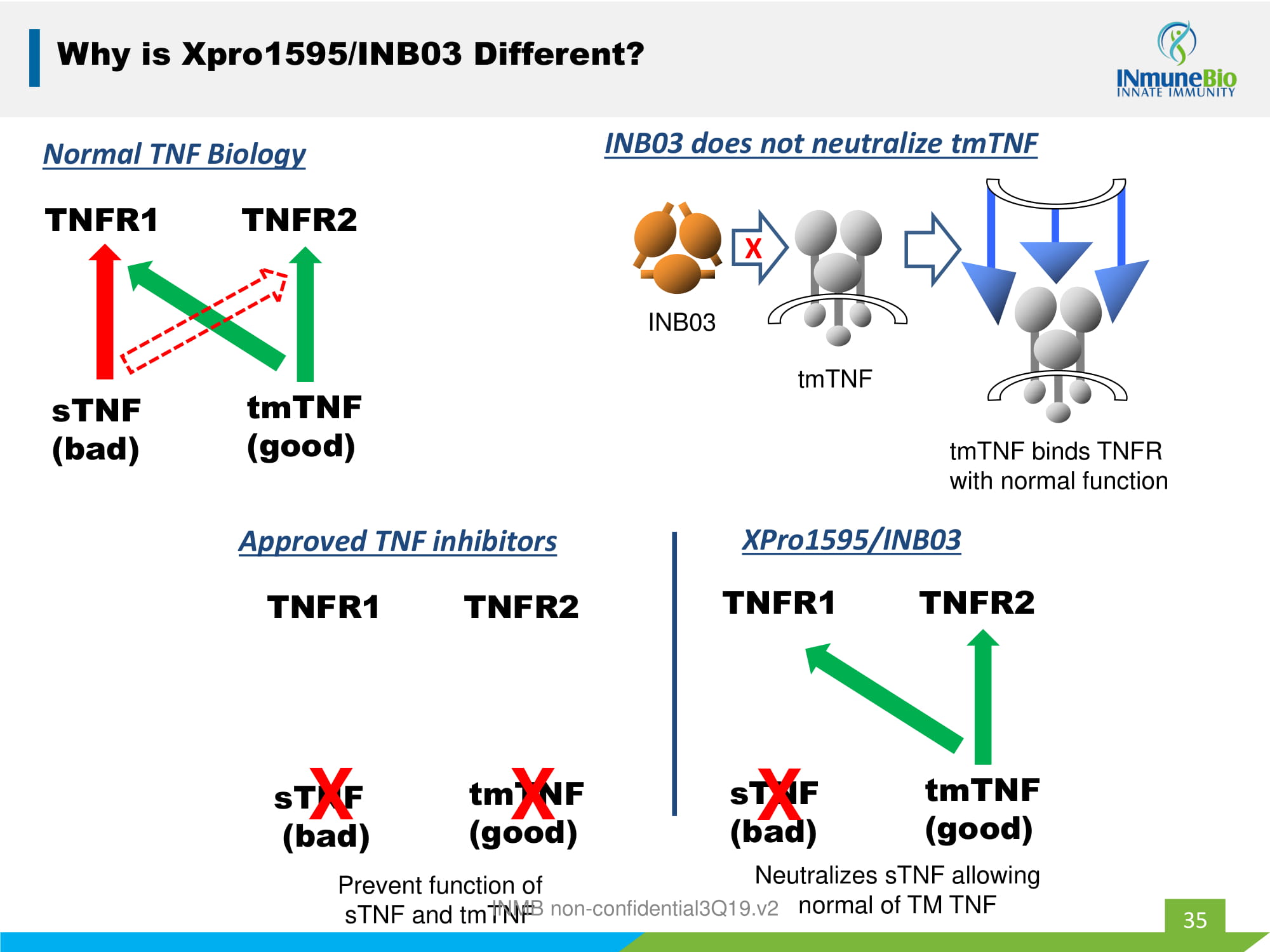
35 Why is Xpro1595/INB03 Different? TNFR1 TNFR2 tmTNF (good) sTNF (bad) Normal TNF Biology 35 tmTNF INB03 INB03 does not neutralize tmTNF X tmTNF binds TNFR with normal function TNFR1 TNFR2 tmTNF (good) sTNF (bad) Approved TNF inhibitors X X TNFR1 TNFR2 tmTNF (good) sTNF (bad) XPro1595/INB03 X Prevent function of sTNF and tmTNF Neutralizes sTNF allowing normal of TM TNF INMB non - confidential3Q19.v2
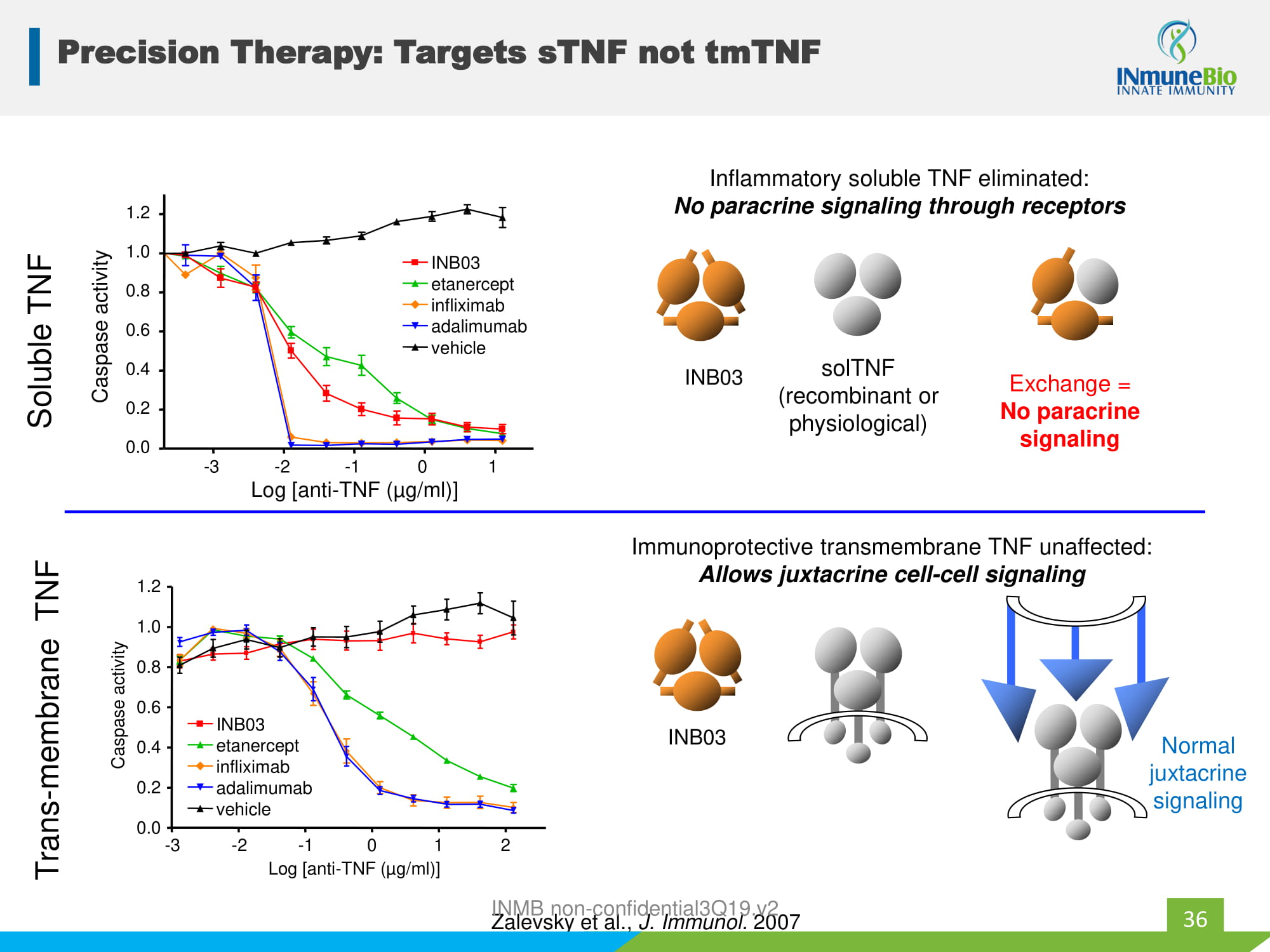
Precision Therapy: Targets sTNF not tmTNF Inflammatory soluble TNF eliminated: No paracrine signaling through receptors Immunoprotective transmembrane TNF unaffected: Allows juxtacrine cell - cell signaling solTNF (recombinant or physiological) Exchange = No paracrine signaling Normal juxtacrine signaling - 3 - 2 - 1 0 1 0.0 0.2 0.4 0.6 0.8 1.0 1.2 etanercept infliximab adalimumab INB03 vehicle Log [anti - TNF ( µ g/ml)] Caspase activity Zalevsky et al., J. Immunol. 2007 Soluble TNF - 3 - 2 - 1 0 1 2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Log [anti - TNF ( µ g/ml)] Caspase activity etanercept infliximab adalimumab INB03 vehicle Trans - membrane TNF 36 INB03 INB03 INMB non - confidential3Q19.v2
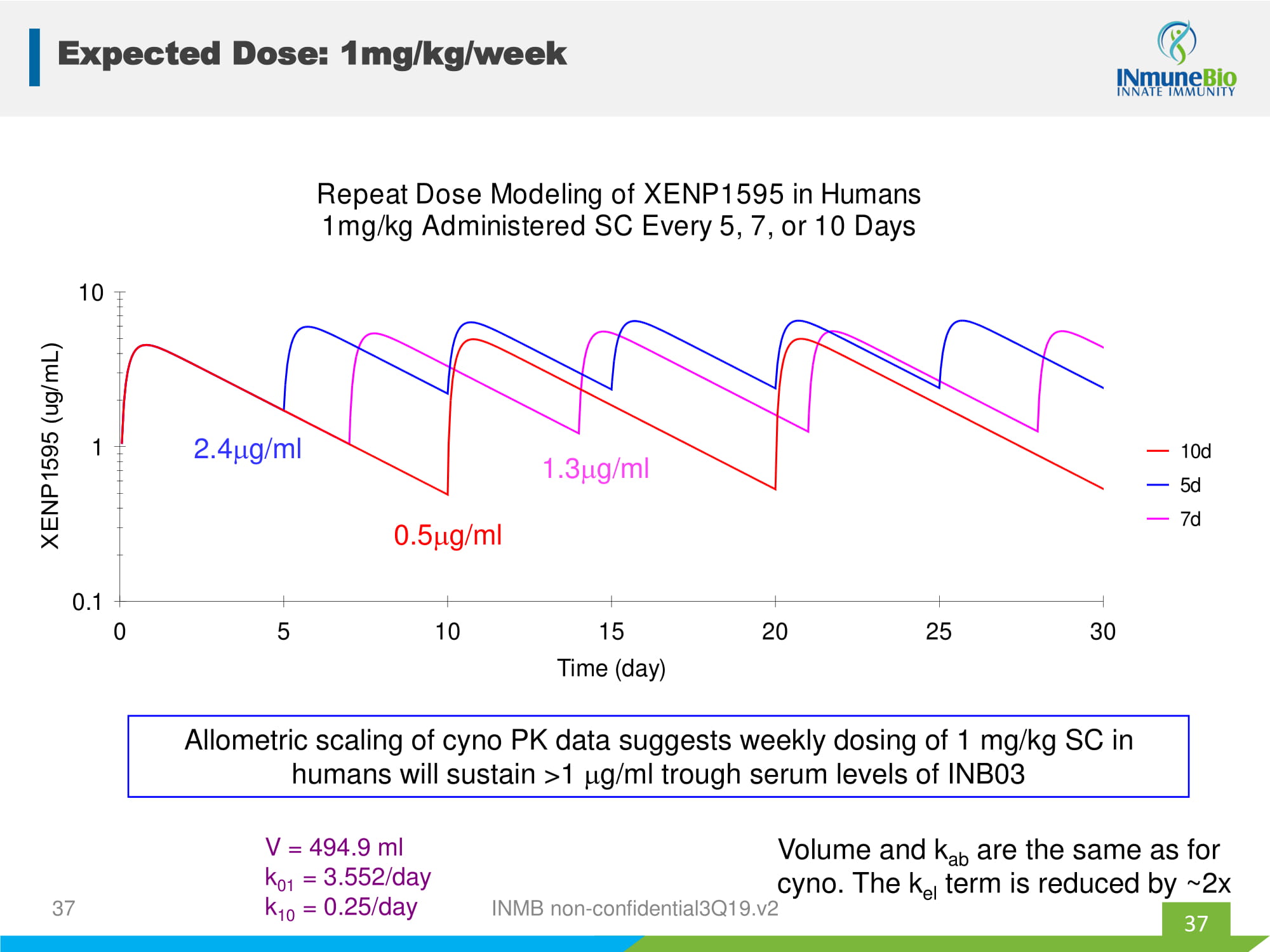
0.1 1 10 0 5 10 15 20 25 30 Time (day) 10d 5d 7d Repeat Dose Modeling of XENP1595 in Humans 1mg/kg Administered SC Every 5, 7, or 10 Days 0.5 g/ml 2.4 g/ml 1.3 g/ml V = 494.9 ml k 01 = 3.552/day k 10 = 0.25/day Allometric scaling of cyno PK data suggests weekly dosing of 1 mg/kg SC in humans will sustain >1 g/ml trough serum levels of INB03 Volume and k ab are the same as for cyno. The k el term is reduced by ~2x 37 Expected Dose: 1mg/kg/week 37 INMB non - confidential3Q19.v2
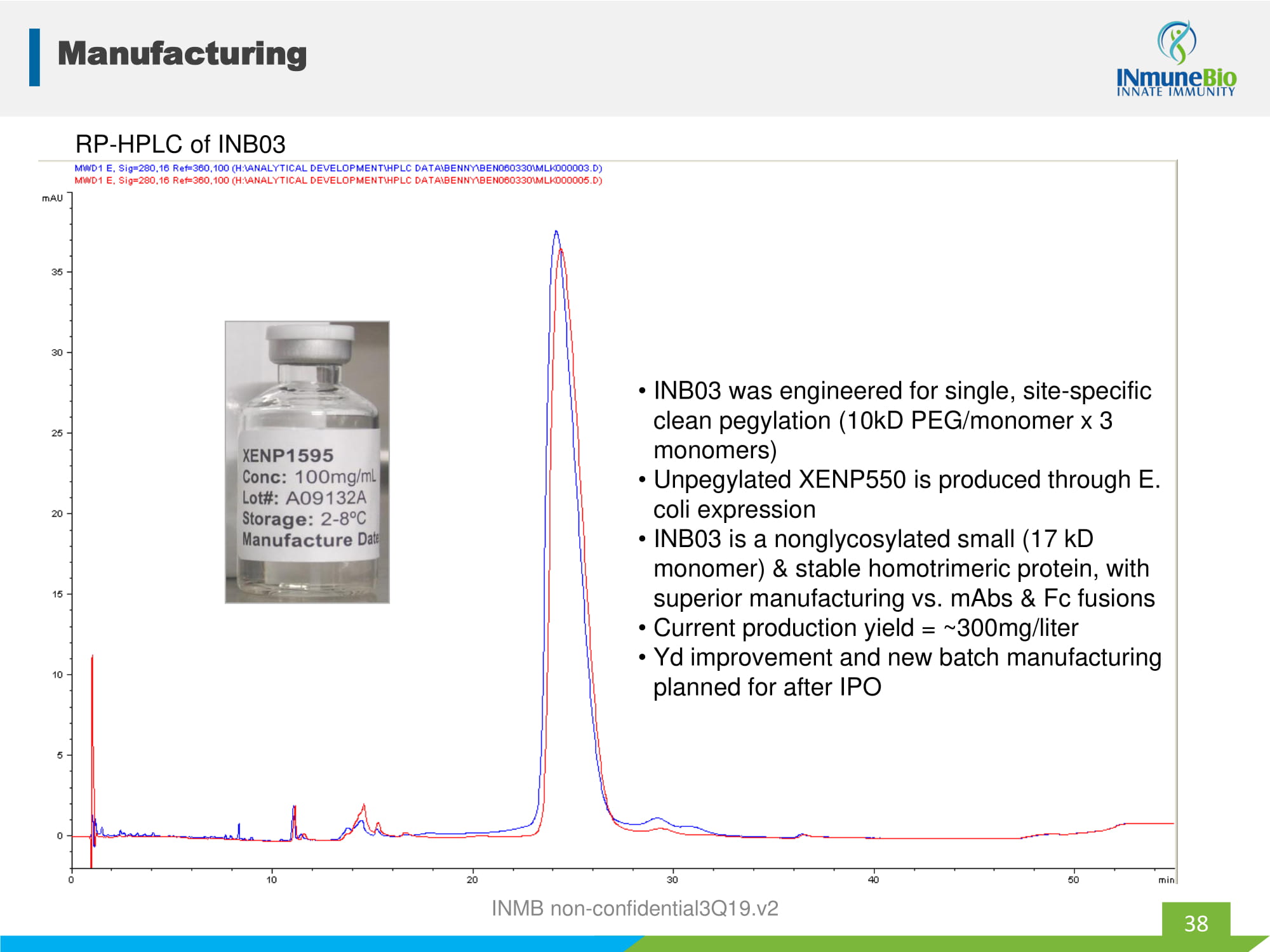
Pegylated [92 - 94% main peak] unpegylated Receptor binding, Subunit C Subunit B Receptor binding, "DE loop" domain (subunit A) "large" domain (subunit C) Receptor binding, "small" domain (subunit A) Subunit C Subunit B Mutation R31C (for 10kD pegylation) Subunit A RP - HPLC of INB03 • INB03 was engineered for single, site - specific clean pegylation (10kD PEG/monomer x 3 monomers) • Unpegylated XENP550 is produced through E. coli expression • INB03 is a nonglycosylated small (17 kD monomer) & stable homotrimeric protein, with superior manufacturing vs. mAbs & Fc fusions • Current production yield = ~300mg/liter • Yd improvement and new batch manufacturing planned for after IPO Manufacturing 38 INMB non - confidential3Q19.v2

MDSC Appendix INMB non - confidential3Q19.v2
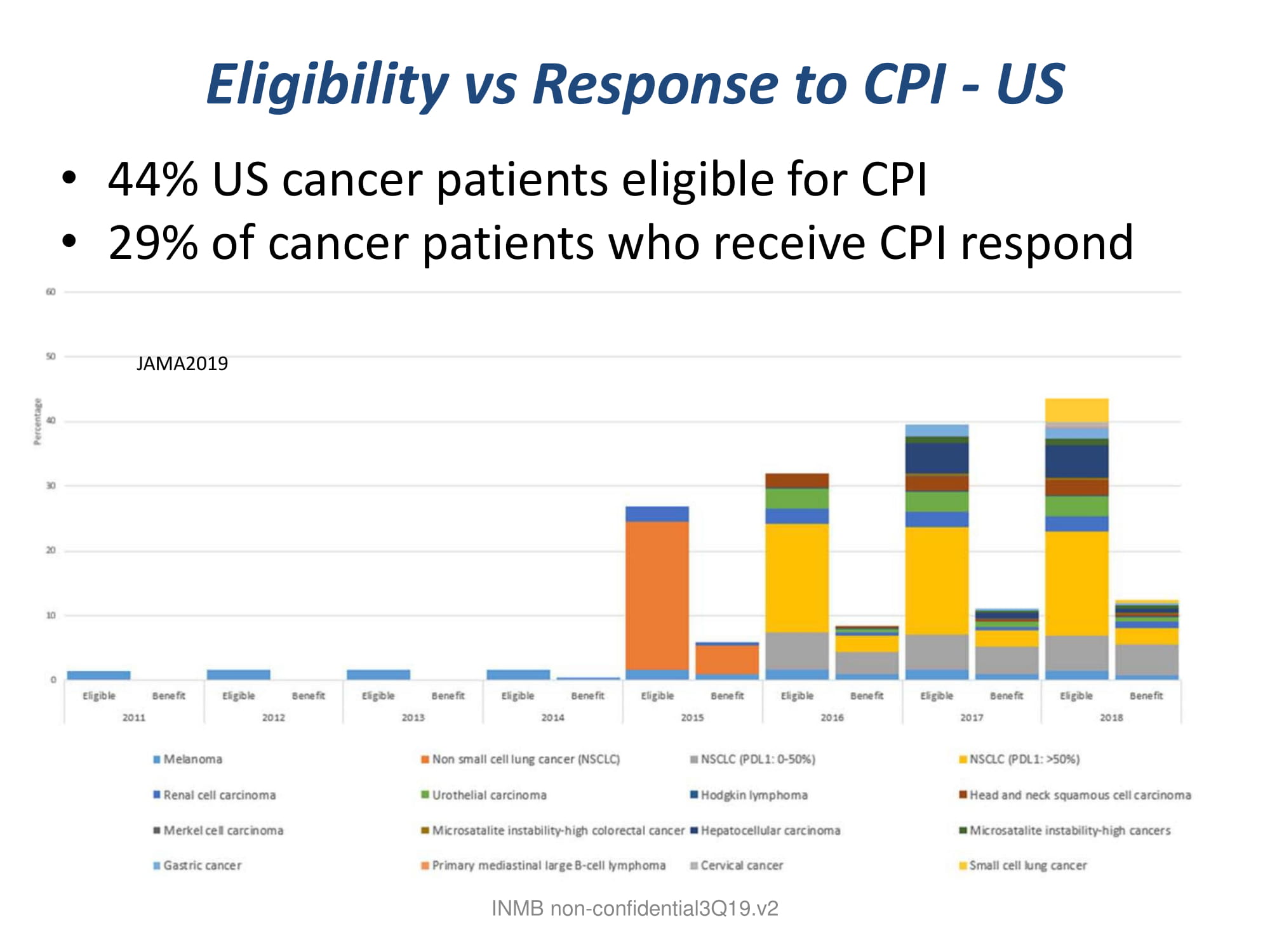
Eligibility vs Response to CPI - US • 44% US cancer patients eligible for CPI • 29% of cancer patients who receive CPI respond INMB non - confidential3Q19.v2 JAMA2019
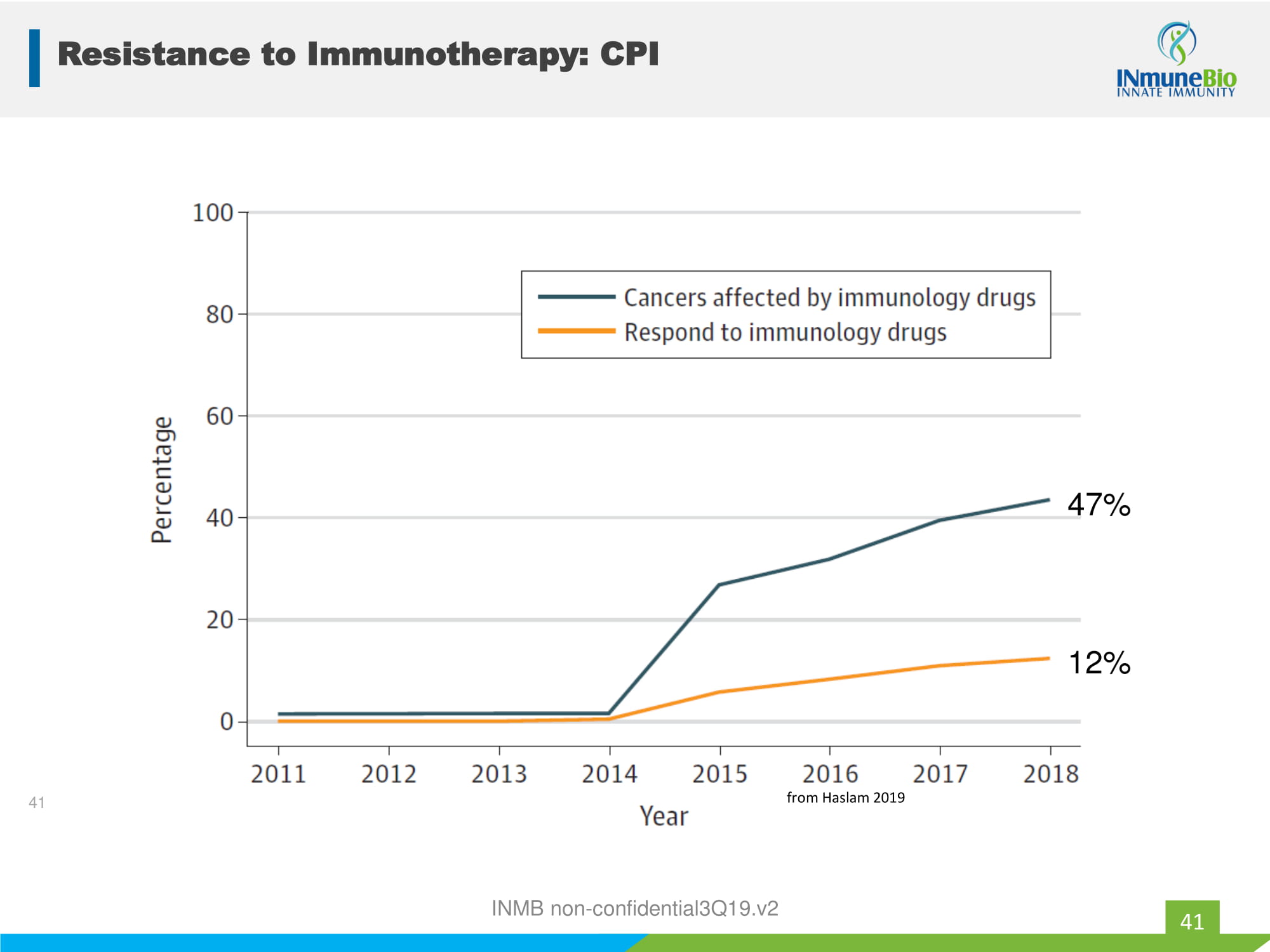
INMB non - confidential3Q19.v2 12% 47% 41 from Haslam 2019 Resistance to Immunotherapy: CPI 41
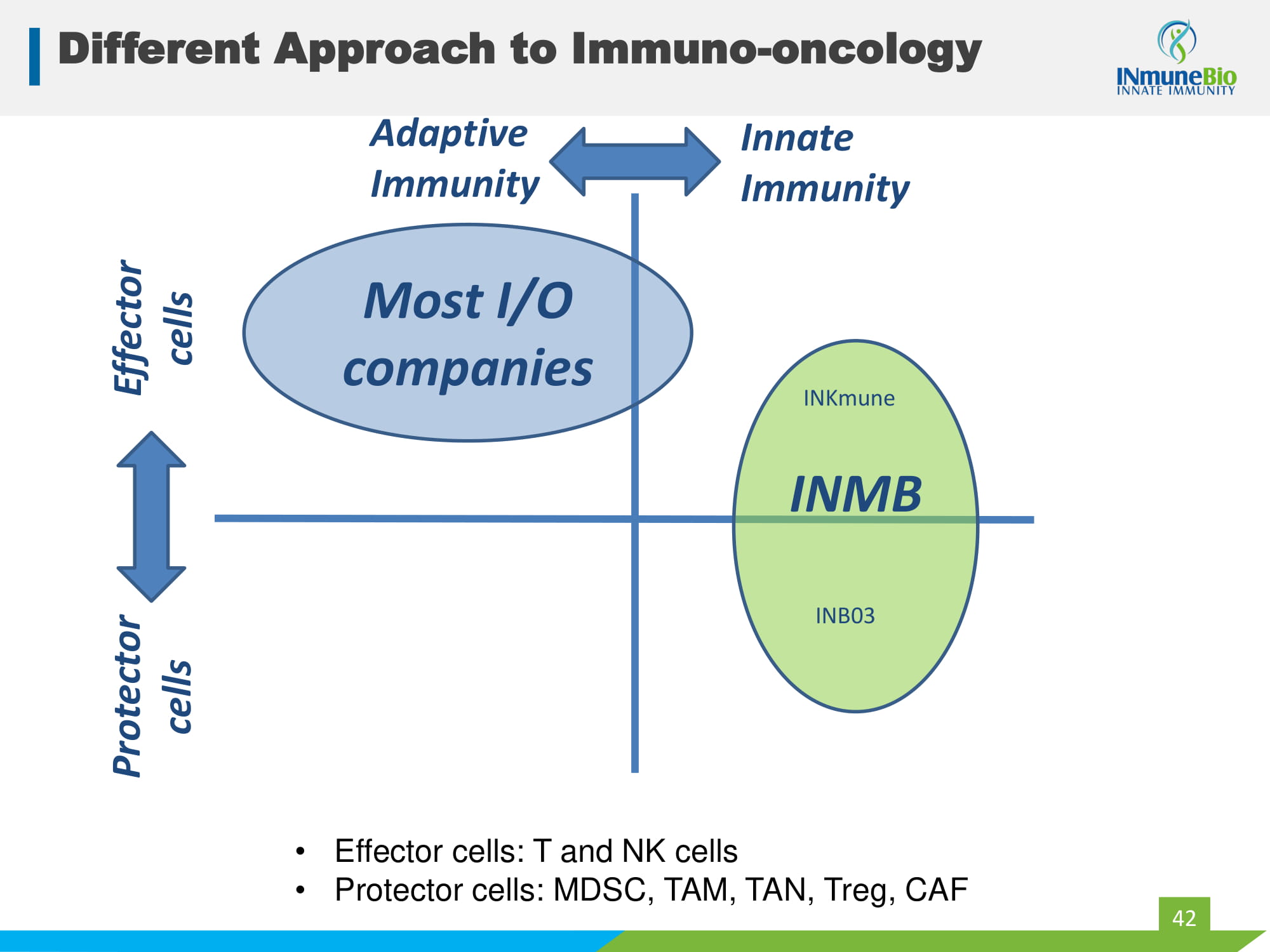
Different Approach to Immuno - oncology 42 MDSC NK cells T & NK cells Innate Immunity Protector cells Effector cells Adaptive Immunity Most I/O companies • Effector cells: T and NK cells • Protector cells: MDSC, TAM, TAN, Treg, CAF INMB INKmune INB03
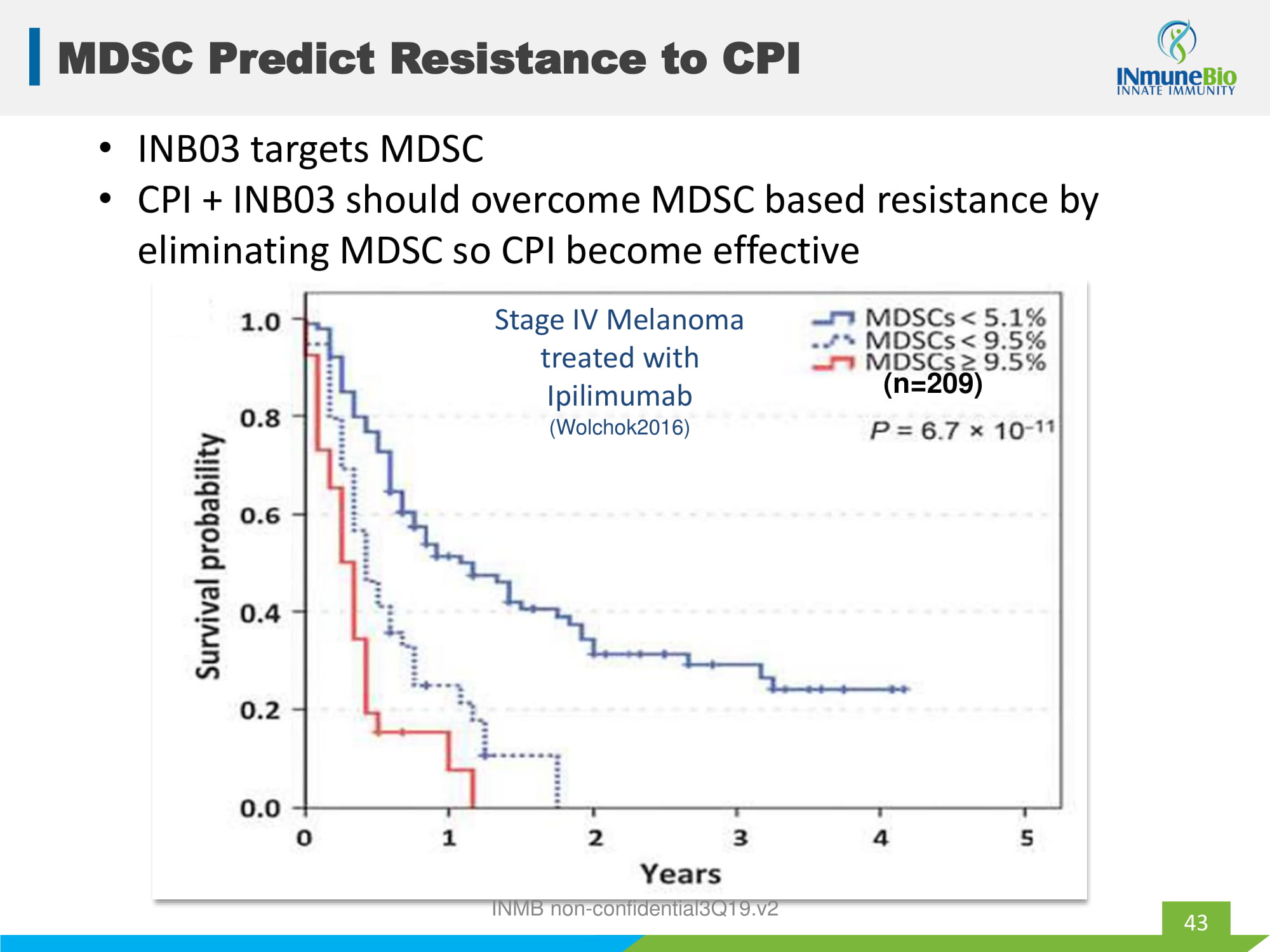
Stage IV Melanoma treated with Ipilimumab (Wolchok2016) MDSC Predict Resistance to CPI 43 (n=209) • INB03 targets MDSC • CPI + INB03 should overcome MDSC based resistance by eliminating MDSC so CPI become effective INMB non - confidential3Q19.v2
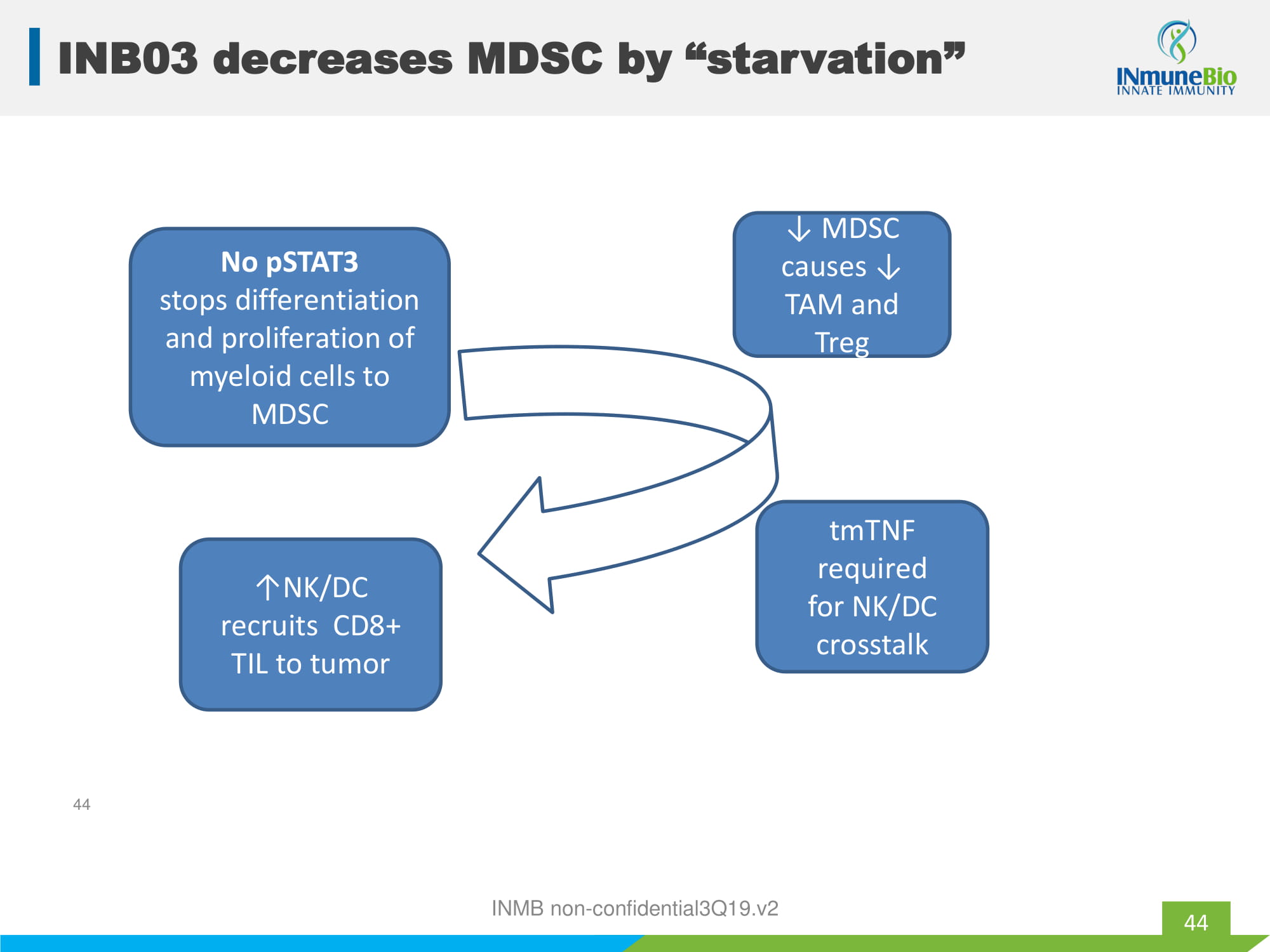
tmTNF required for NK/DC crosstalk ↓ MDSC causes ↓ TAM and Treg No pSTAT3 stops differentiation and proliferation of myeloid cells to MDSC ↑NK/DC recruits CD8+ TIL to tumor INMB non - confidential3Q19.v2 44 INB03 decreases MDSC by “starvation” 44

INB03 HER2/MUC4 Appendix INMB non - confidential3Q19.v2
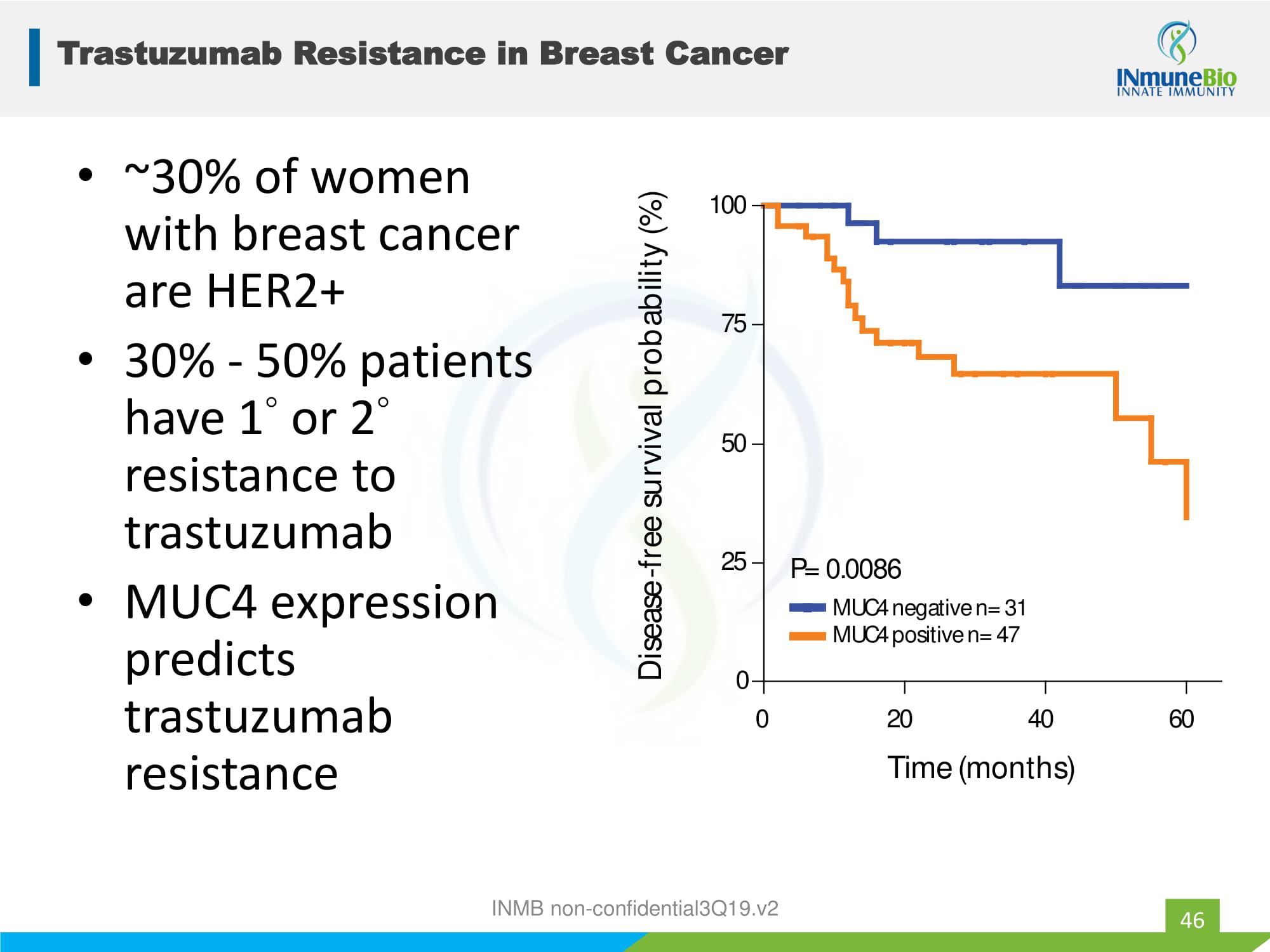
Trastuzumab Resistance in Breast Cancer 46 • ~30% of women with breast cancer are HER2+ • 30% - 50% patients have 1 ◦ or 2 ◦ resistance to trastuzumab • MUC4 expression predicts trastuzumab resistance 0 2 0 4 0 6 0 0 2 5 5 0 7 5 10 0 M U C 4 n e g a t i ve n = 3 1 M U C 4 po s i t i ve n = 4 7 P = 0 . 008 6 T i m e ( m on t h s ) D i sease -f r ee s u r v i va l p r ob a b ili t y ( % ) INMB non - confidential3Q19.v2
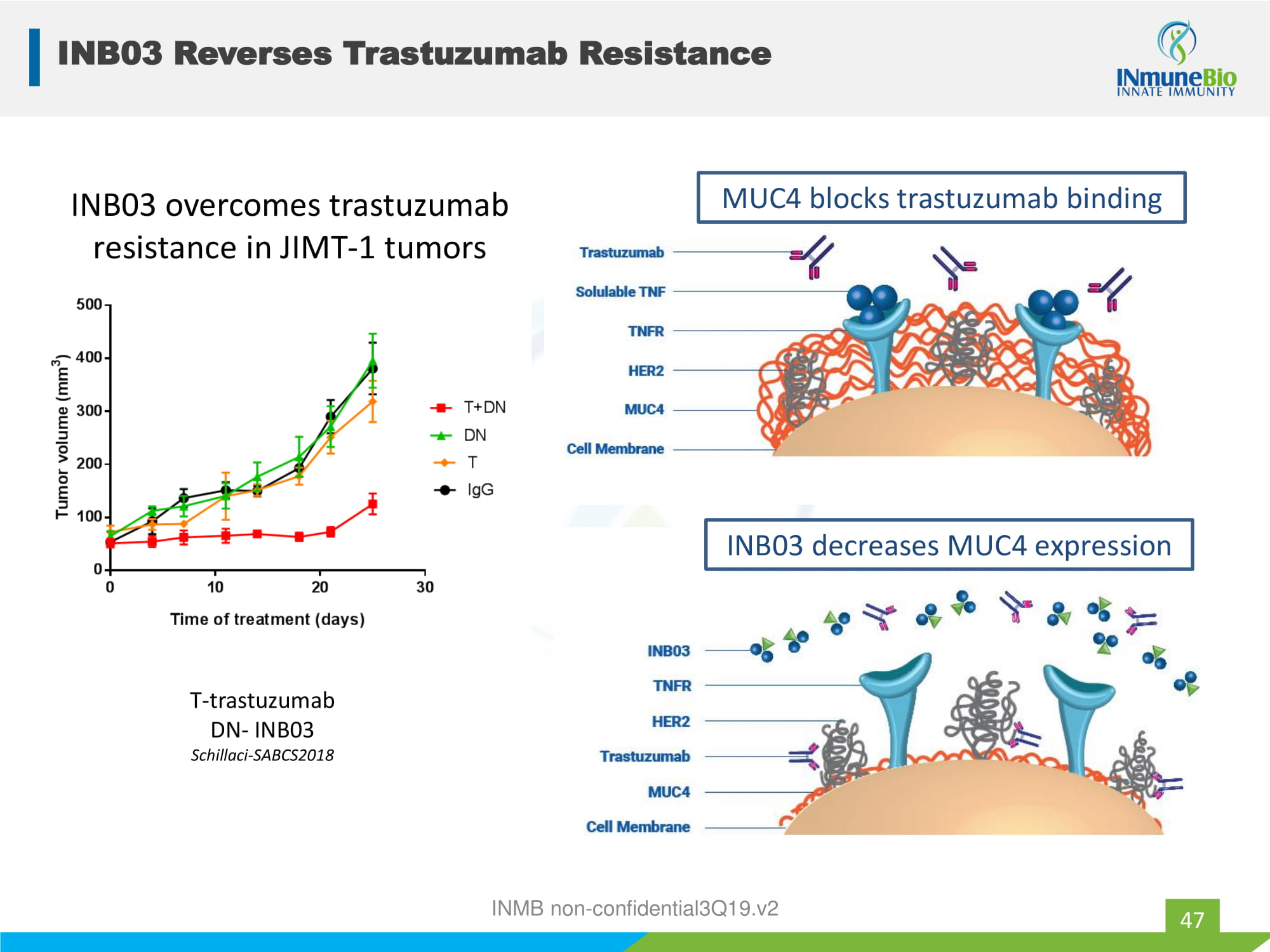
INB03 Reverses Trastuzumab Resistance 47 INB03 overcomes trastuzumab resistance in JIMT - 1 tumors T - trastuzumab DN - INB03 Schillaci - SABCS2018 MUC4 blocks trastuzumab binding INB03 decreases MUC4 expression INMB non - confidential3Q19.v2

Alzheimer’s Disease Appendix INMB non - confidential3Q19.v2
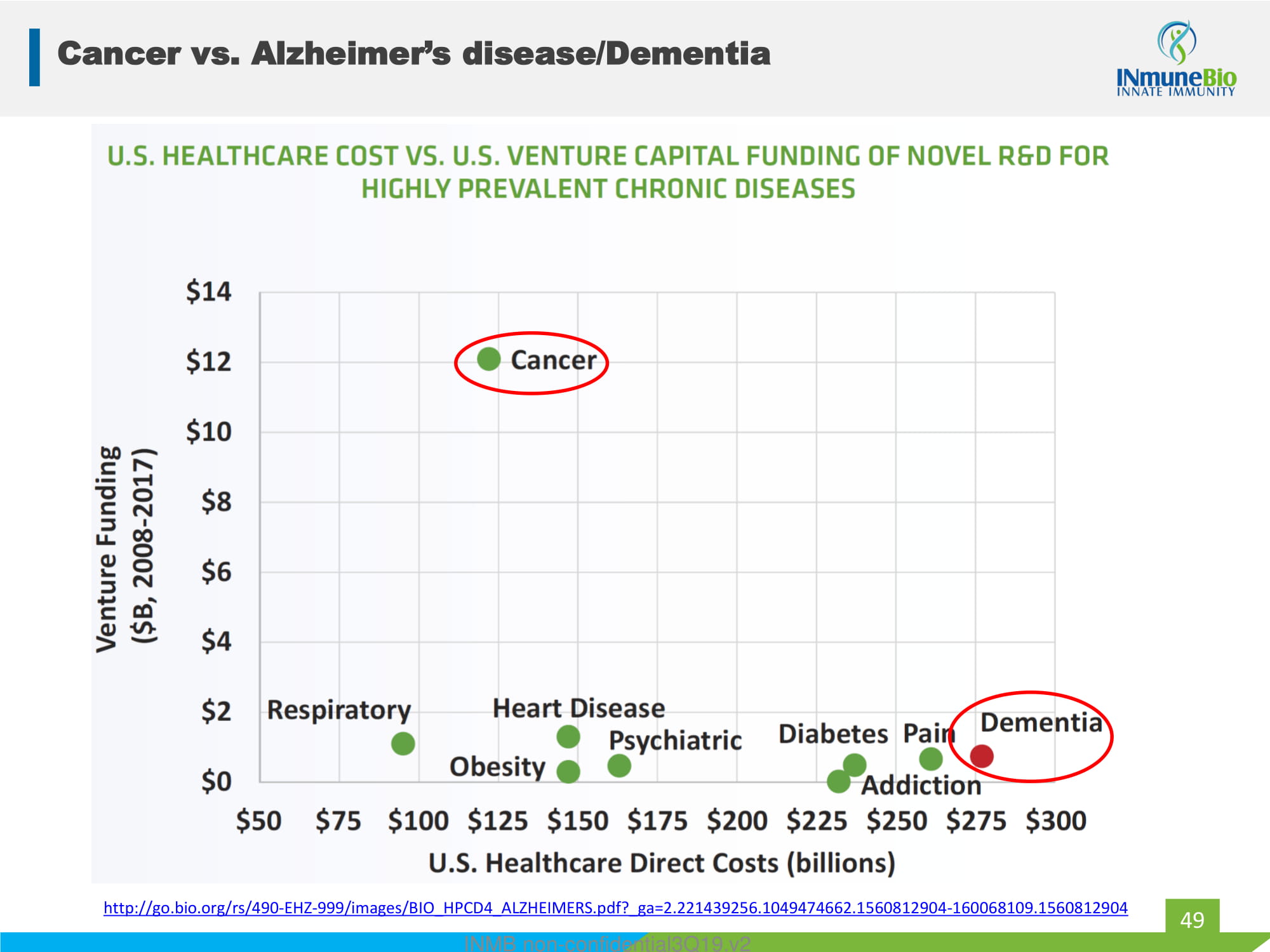
Cancer vs. Alzheimer’s disease/Dementia 49 http://go.bio.org/rs/490 - EHZ - 999/images/BIO_HPCD4_ALZHEIMERS.pdf?_ga=2.221439256.1049474662.1560812904 - 160068109.1560812904 INMB non - confidential3Q19.v2
XPro1595 to Treat Alzheimer’s Disease 50 AD is an expanding, expensive and intractable disease that defies a therapeutic solution • Problem: synaptic pruning and nerve cell death • Solution: reduce neuroinflammation • Drug: XPro1595 • Target: activated glial cells in the brain • Status: entering Phase I trial in patients with mild to moderate Alzheimer’s disease INMB non - confidential3Q19.v2
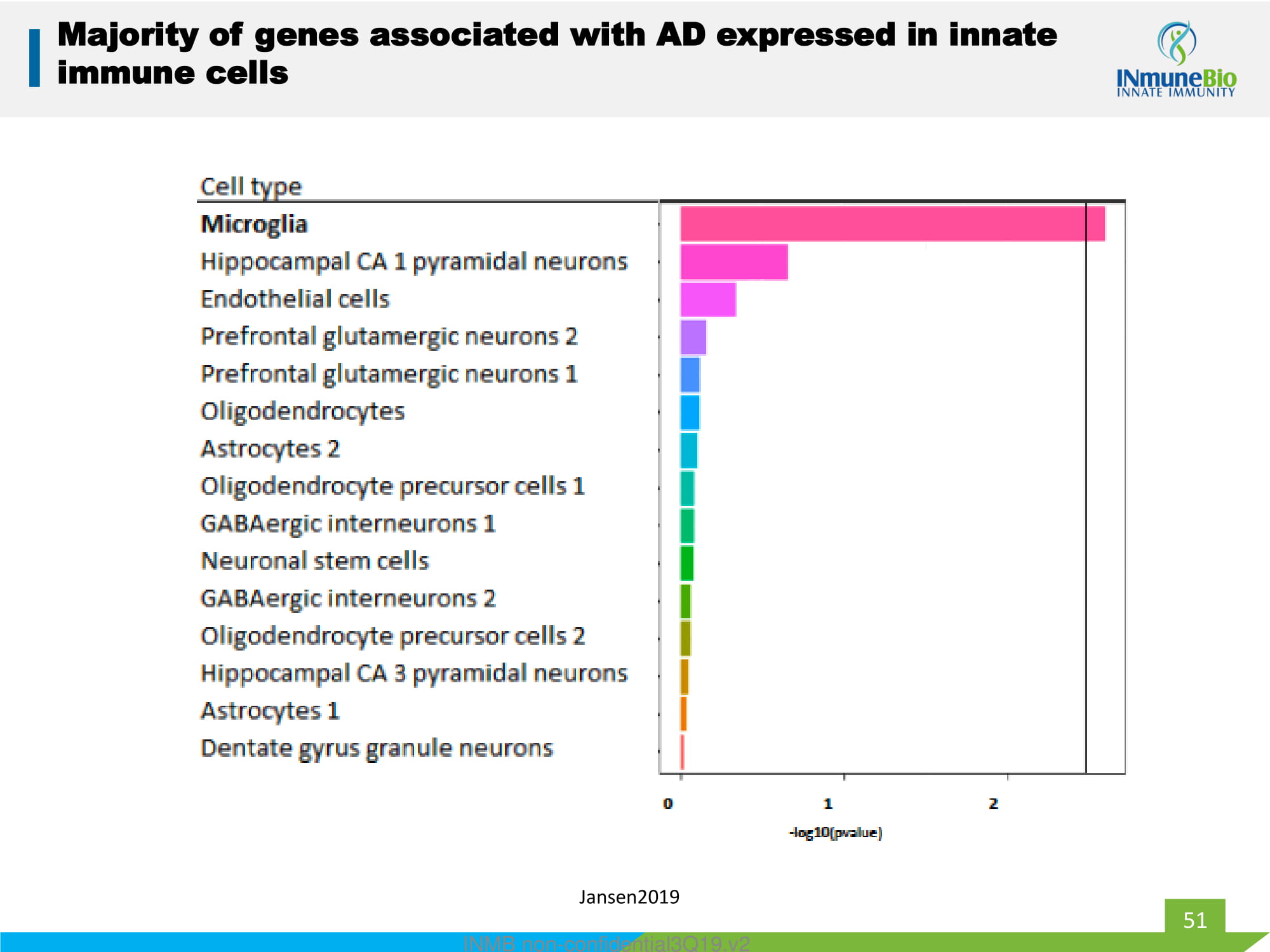
51 INMB non - confidential3Q19.v2 Jansen2019 Majority of genes associated with AD expressed in innate immune cells
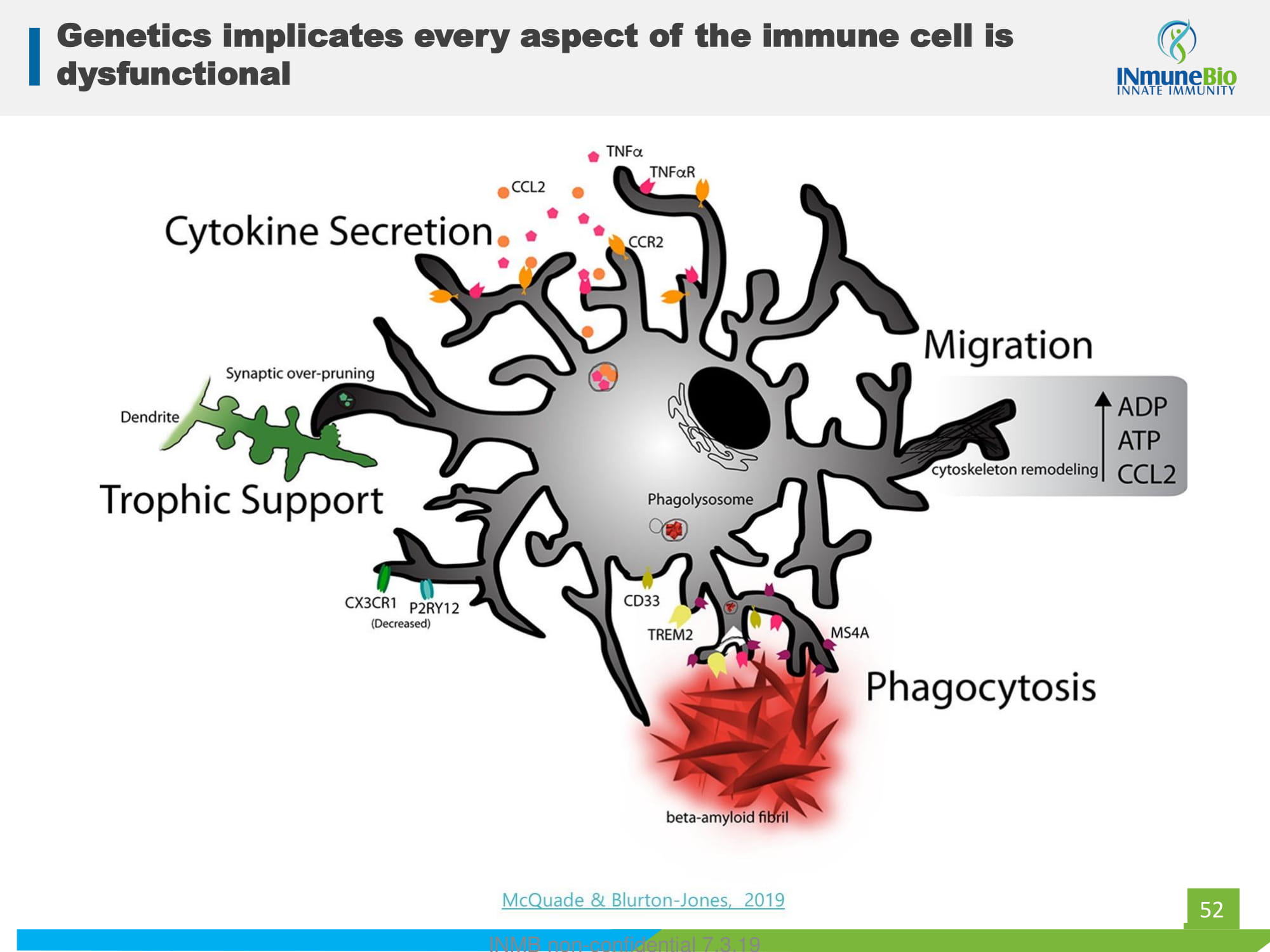
52 Genetics implicates every aspect of the immune cell is dysfunctional INMB non - confidential 7.3.19
53 Chronic inflammation: real disease….bad name Acute inflammation fast and furious, coming and going quickly for the benefit of the patient. Chronic inflammation low, slow inflammatory response that doesn’t end and harms the patient. INMB non - confidential3Q19.v2
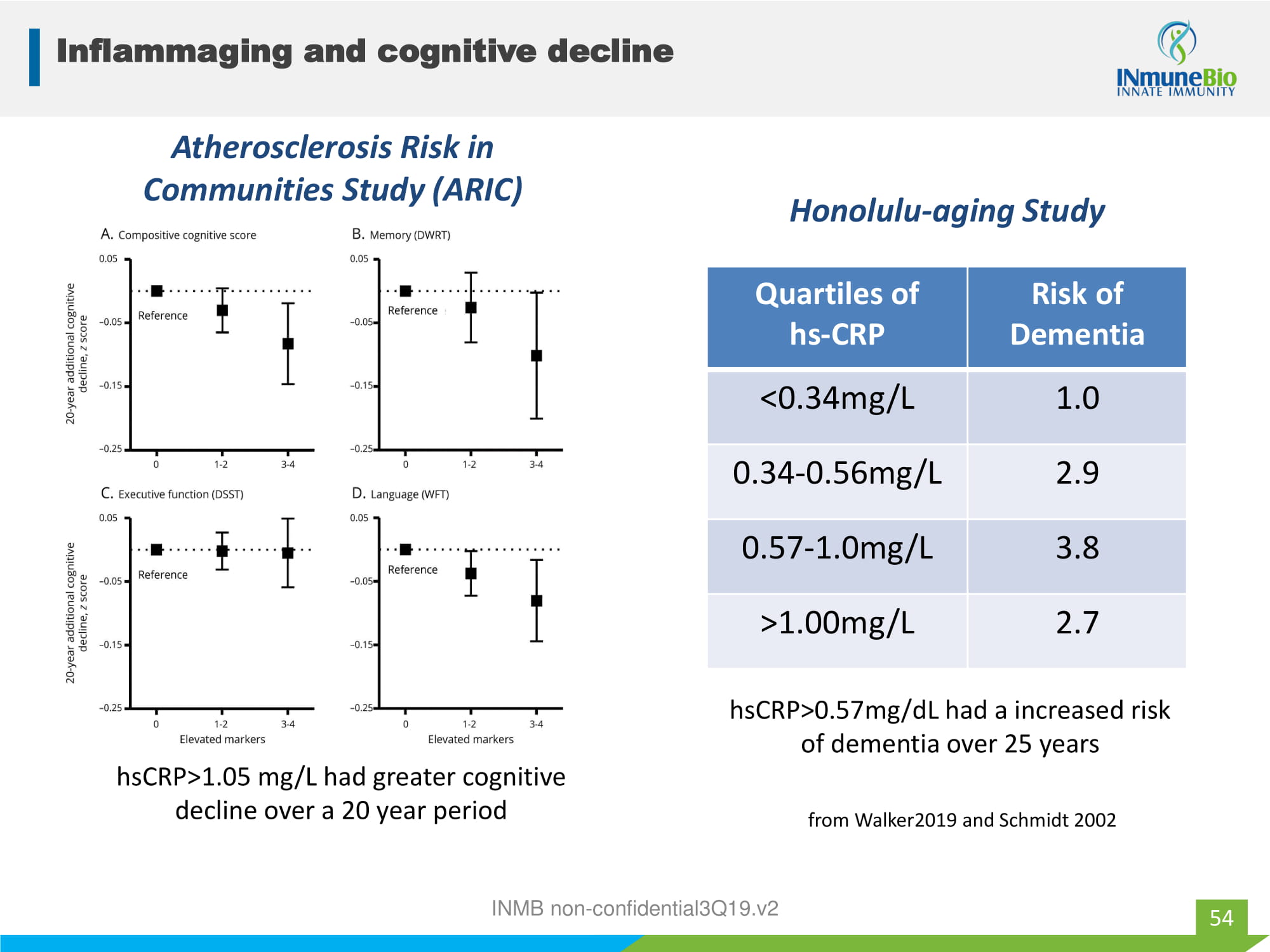
Inflammaging and cognitive decline 54 INMB non - confidential3Q19.v2 Quartiles of hs - CRP Risk of Dementia <0.34mg/L 1.0 0.34 - 0.56mg/L 2.9 0.57 - 1.0mg/L 3.8 >1.00mg/L 2.7 Atherosclerosis Risk in Communities Study (ARIC) hsCRP>1.05 mg/L had greater cognitive decline over a 20 year period Honolulu - aging Study hsCRP>0.57mg/dL had a increased risk of dementia over 25 years from Walker2019 and Schmidt 2002
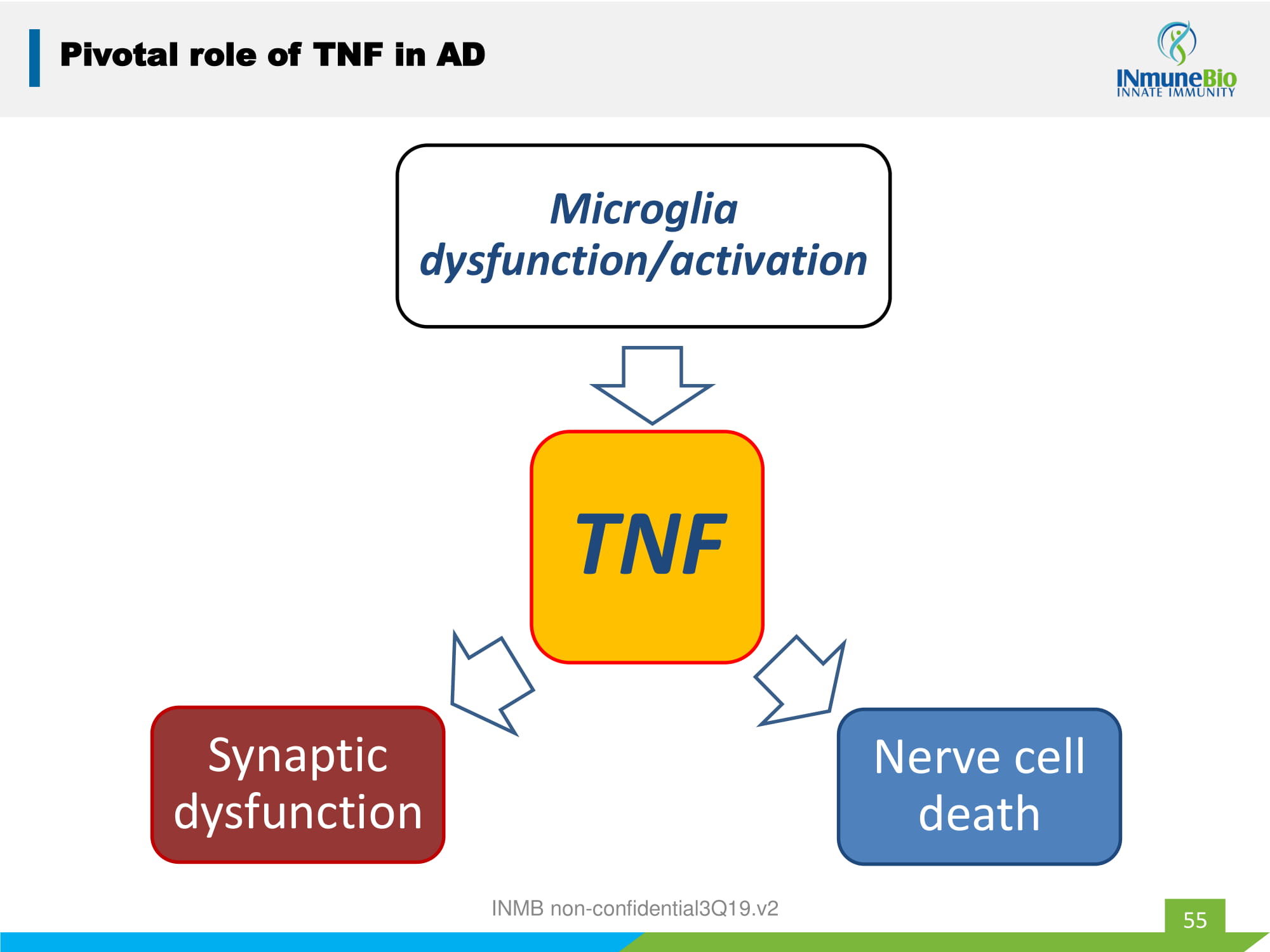
55 Pivotal role of TNF in AD TNF Microglia dysfunction/activation Nerve cell death Synaptic dysfunction INMB non - confidential3Q19.v2
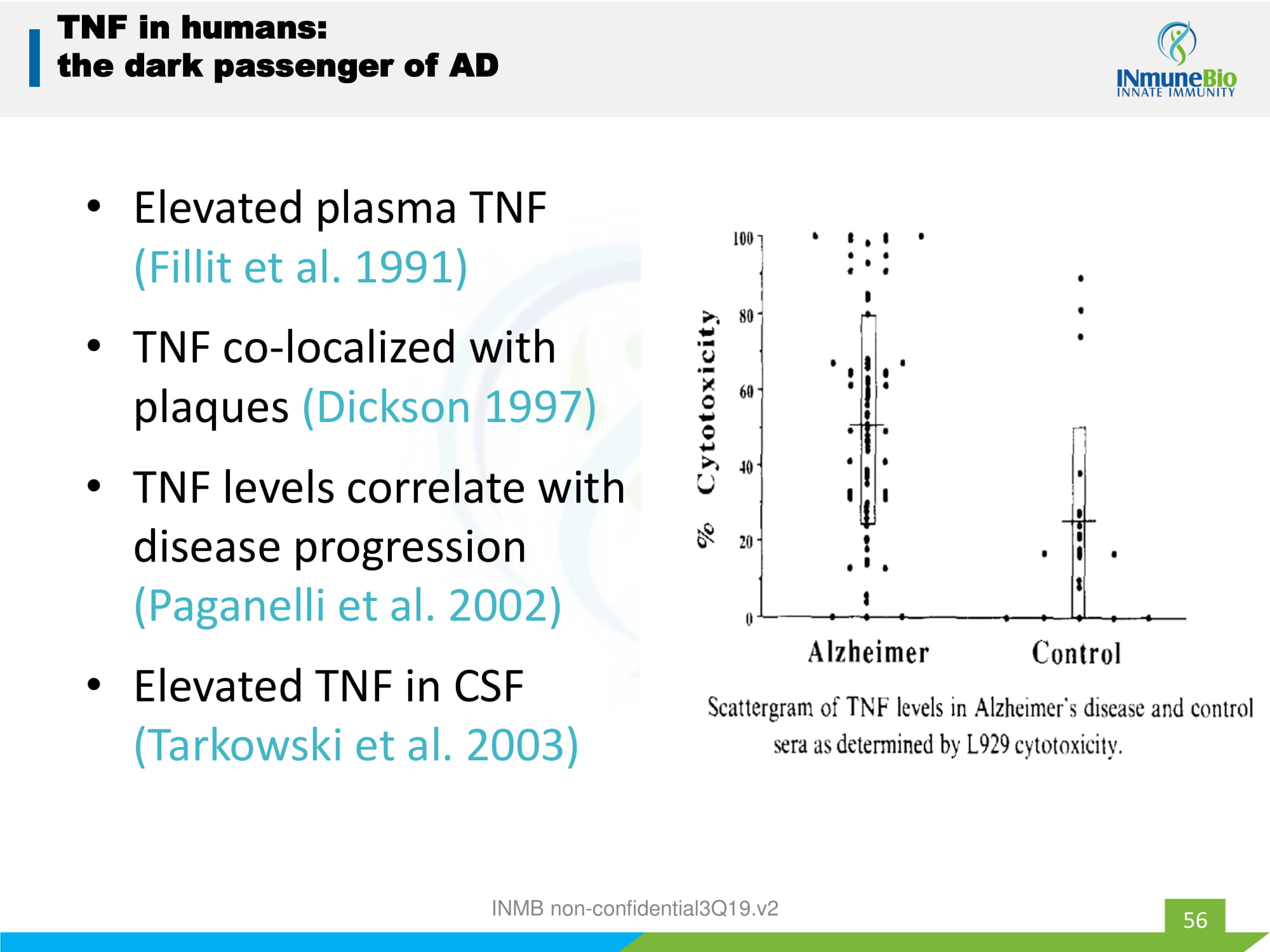
56 TNF in humans: the dark passenger of AD • Elevated plasma TNF (Fillit et al. 1991) • TNF co - localized with plaques (Dickson 1997) • TNF levels correlate with disease progression ( Paganelli et al. 2002) • Elevated TNF in CSF ( Tarkowski et al. 2003) INMB non - confidential3Q19.v2
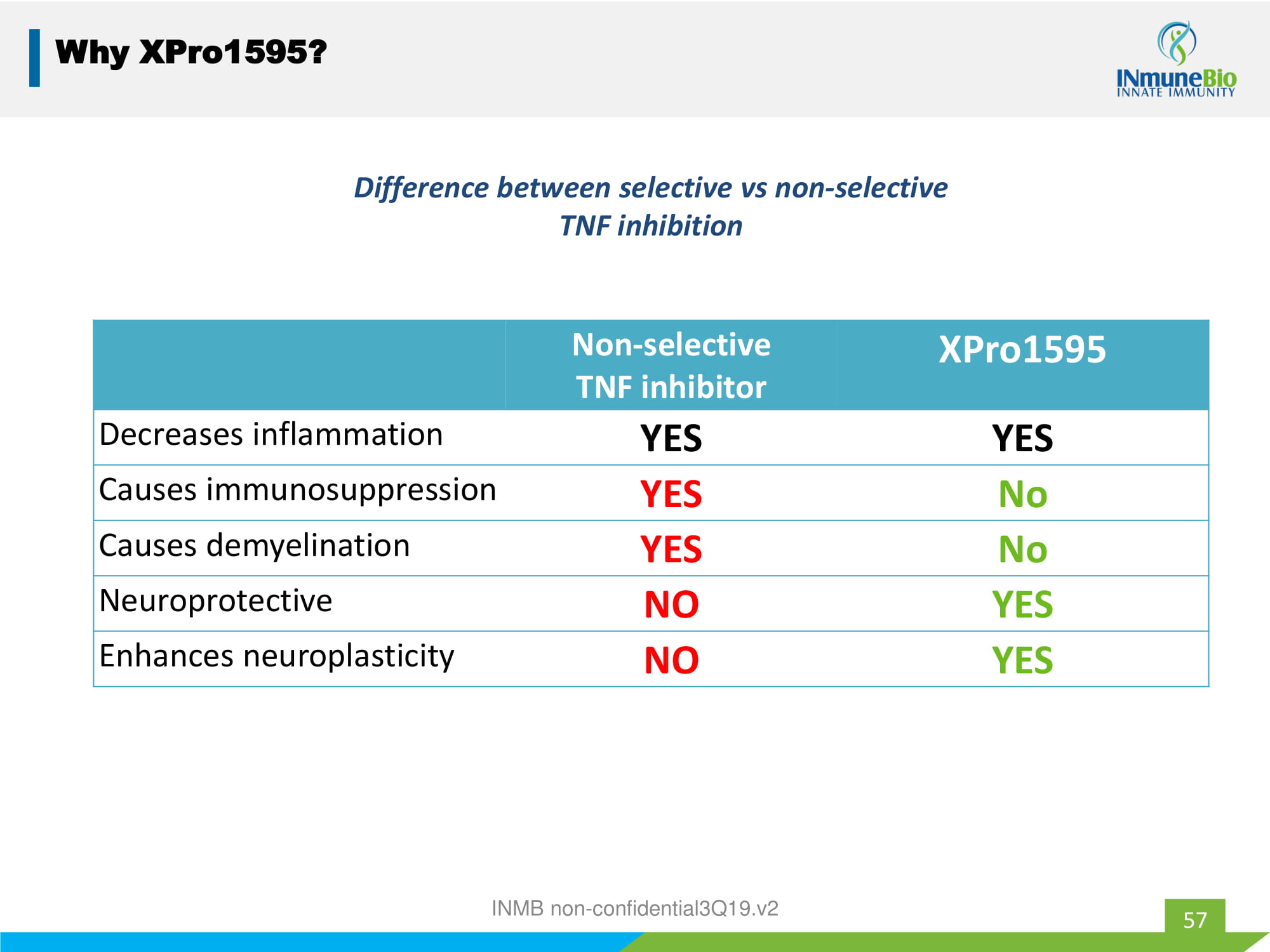
57 Why XPro1595? Non - selective TNF inhibitor XPro1595 Decreases inflammation YES YES Causes immunosuppression YES No Causes demyelination YES No Neuroprotective NO YES Enhances neuroplasticity NO YES Difference between selective vs non - selective TNF inhibition INMB non - confidential3Q19.v2
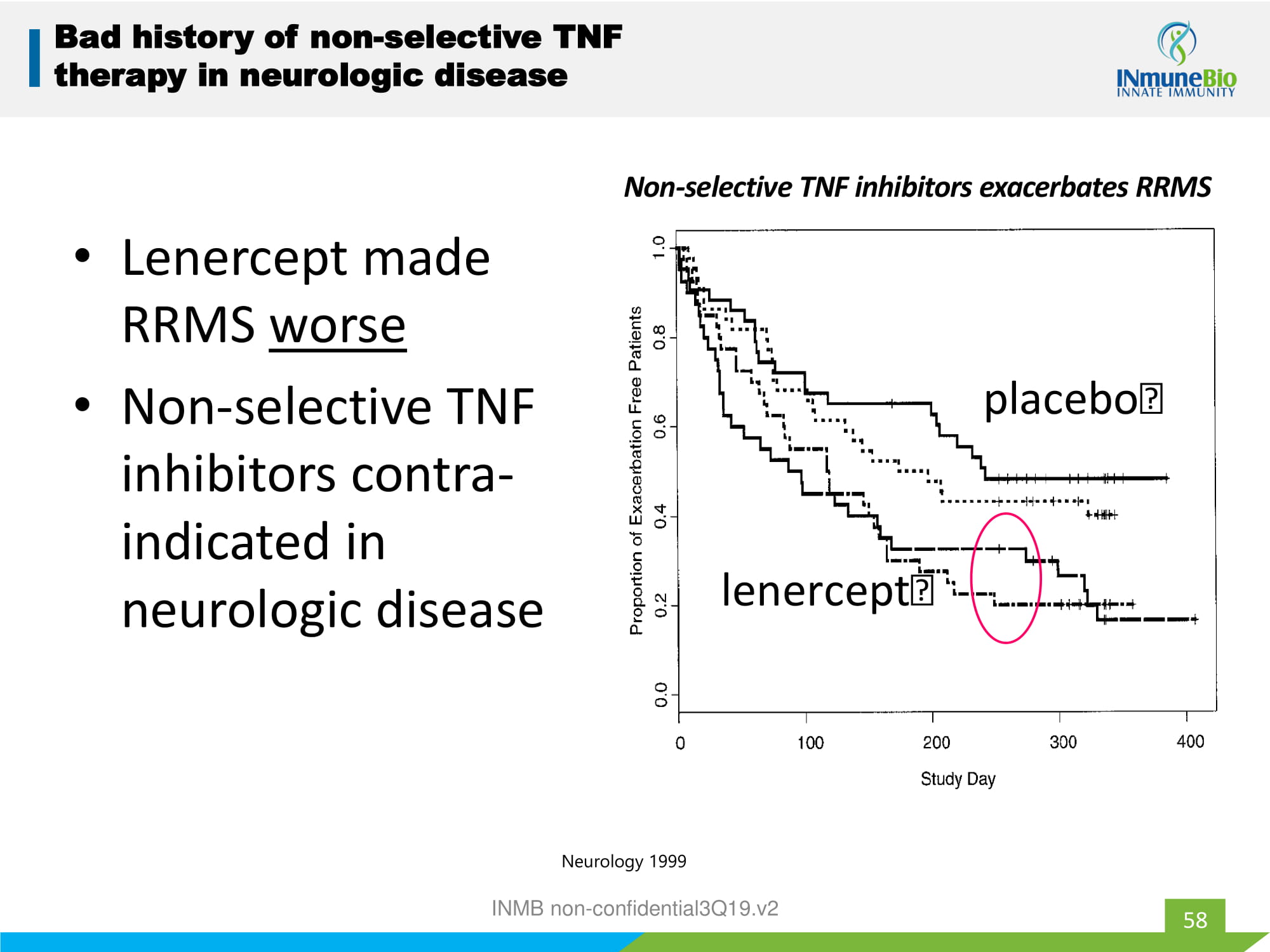
• Lenercept made RRMS worse • Non - selective TNF inhibitors contra - indicated in neurologic disease 58 Bad history of non - selective TNF therapy in neurologic disease placebo lenercept Non - selective TNF inhibitors exacerbates RRMS Neurology 1999 INMB non - confidential3Q19.v2
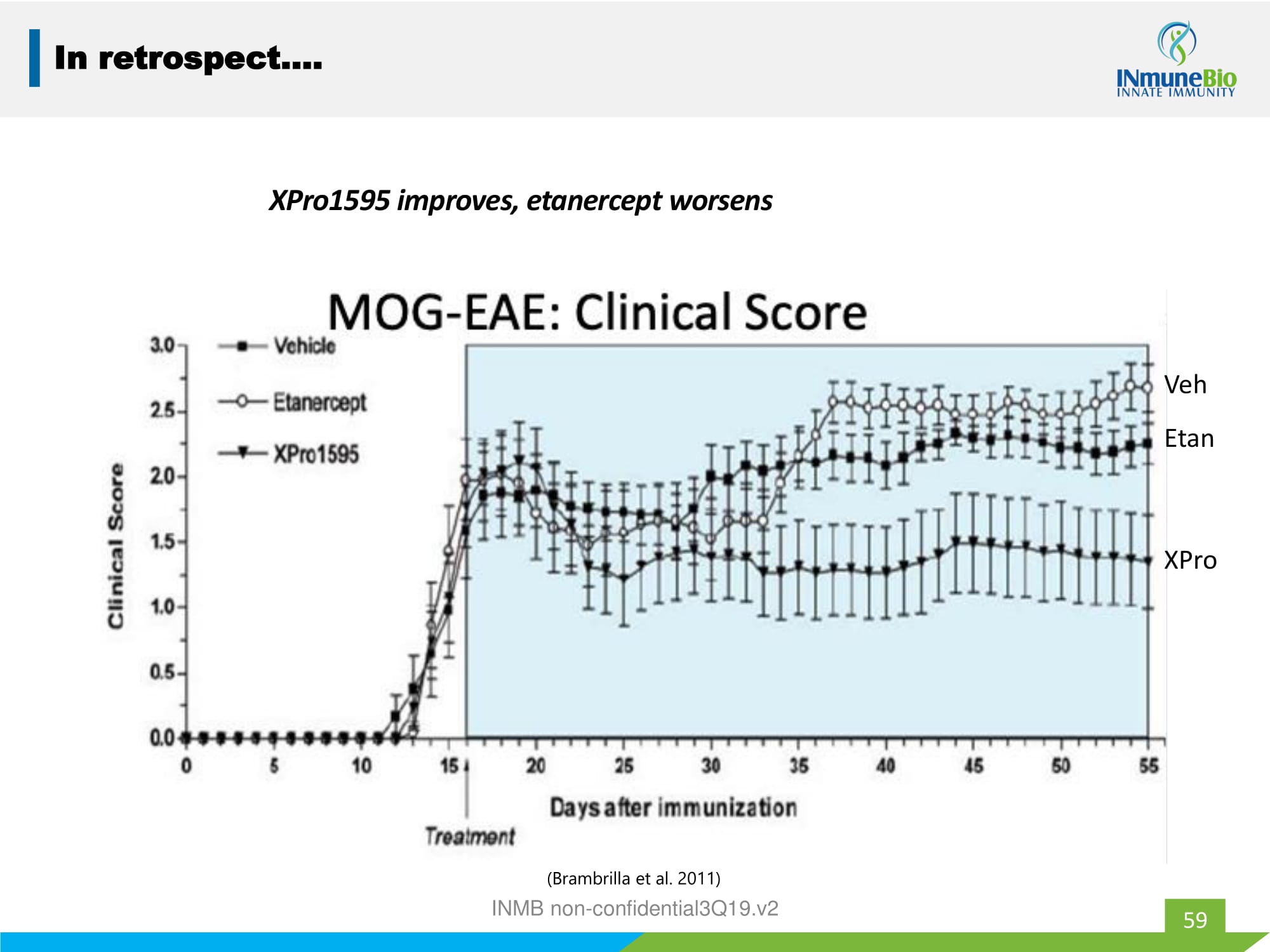
59 In retrospect…. XPro1595 improves, etanercept worsens ( Brambrilla et al. 2011) INMB non - confidential3Q19.v2 XPro Etan Veh
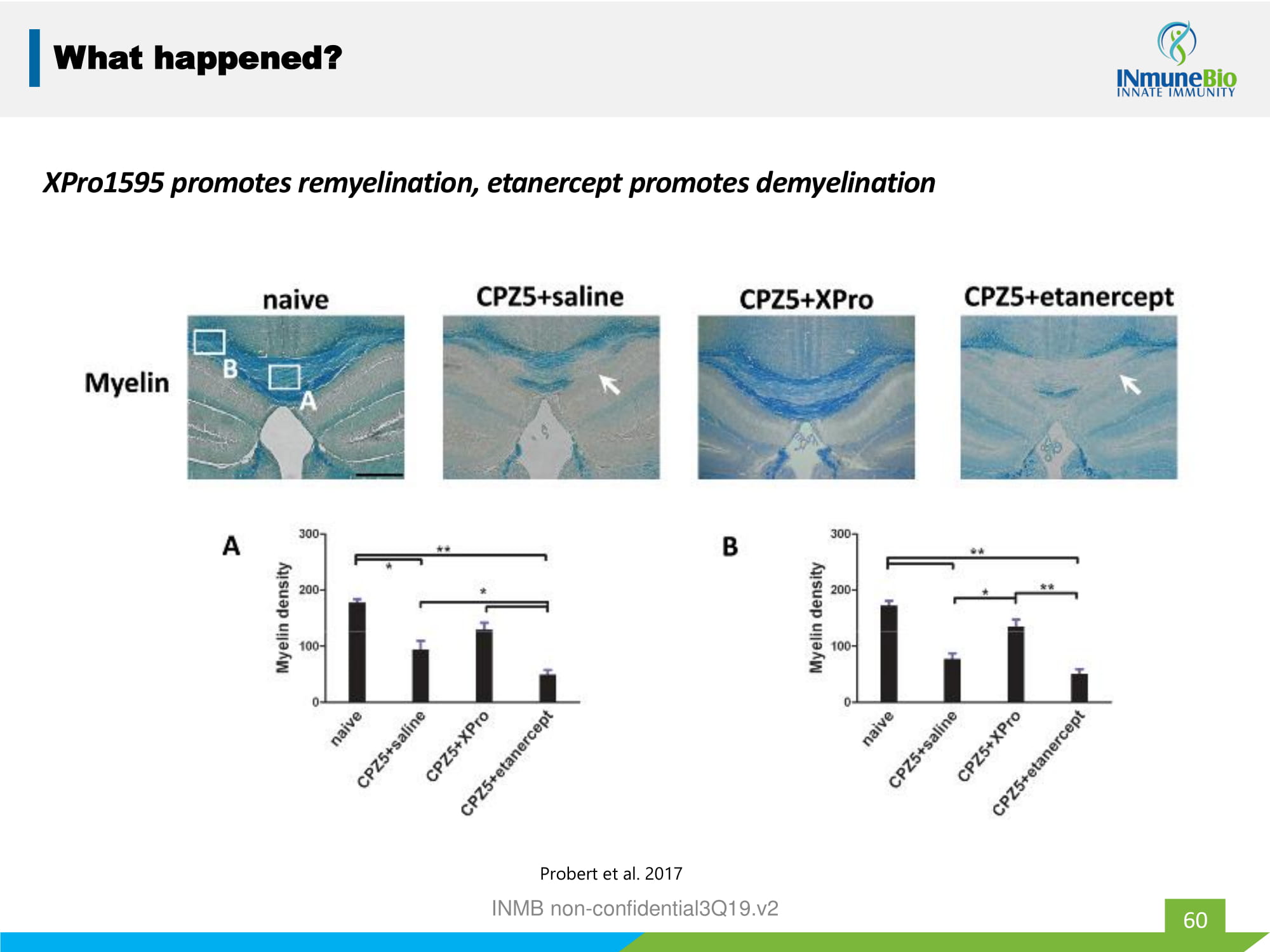
60 What happened? XPro1595 promotes remyelination, etanercept promotes demyelination Probert et al. 2017 INMB non - confidential3Q19.v2
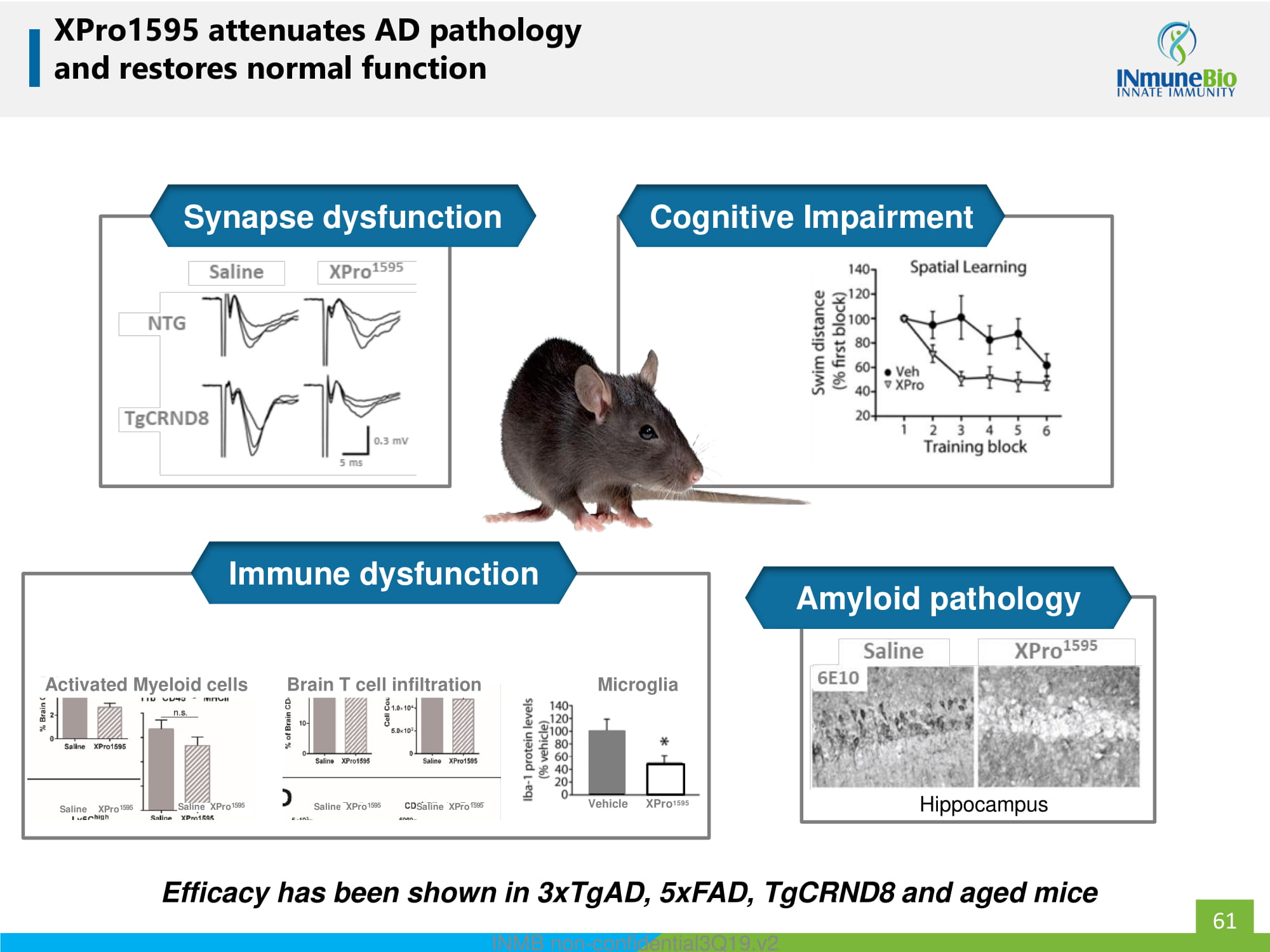
XPro1595 attenuates AD pathology and restores normal function Efficacy has been shown in 3xTgAD, 5xFAD, TgCRND8 and aged mice Hippocampus Saline XPro 1595 Activated Myeloid cells Saline XPro 1595 Brain T cell infiltration Saline XPro 1595 Saline XPro 1595 Vehicle XPro 1595 Microglia Cognitive Impairment Synapse dysfunction Immune dysfunction Amyloid pathology 61 INMB non - confidential3Q19.v2
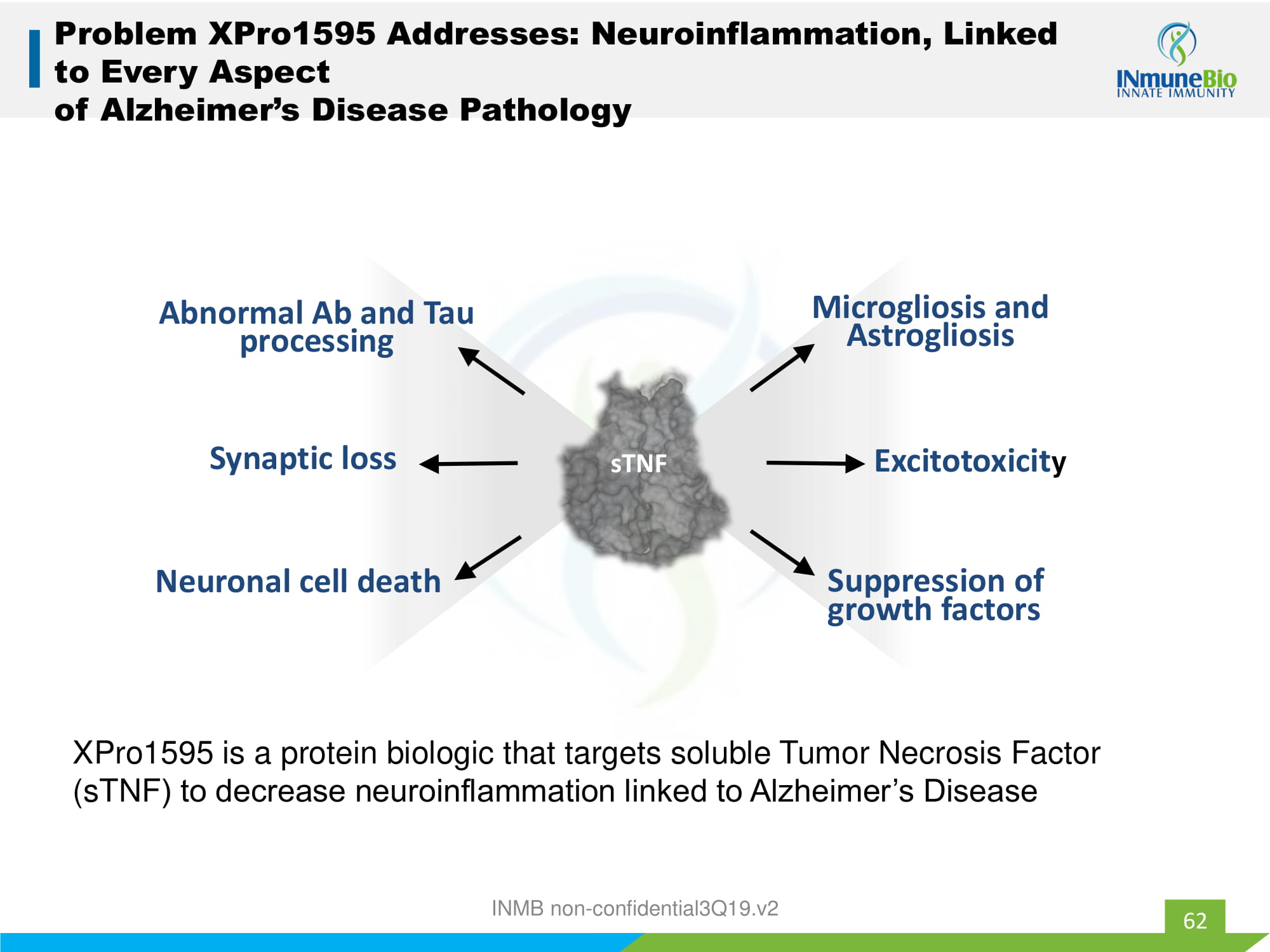
62 Problem XPro1595 Addresses: Neuroinflammation, Linked to Every Aspect of Alzheimer’s Disease Pathology Microgliosis and Astrogliosis Excitotoxicit y Suppression of growth factors Neuronal cell death Synaptic loss sTNF Abnormal Ab and Tau processing XPro1595 is a protein biologic that targets soluble Tumor Necrosis Factor ( sTNF ) to decrease neuroinflammation linked to Alzheimer’s Disease INMB non - confidential3Q19.v2

Phase 1b Biomarker Directed, Proof of Biology Study in Mild to Moderate AD Patients 63

INKmune Appendix INMB non - confidential3Q19.v2

INKmune to Treat Residual Disease 65 Residual disease is the cause of relapse and death in a majority of patients • Problem: cancer remains after treatment • Solution: eliminate residual disease • Drug: INKmune • Target: patient’s NK cells • Status: entering Phase I/II trial in women with relapse/refractory ovarian cancer INMB non - confidential3Q19.v2
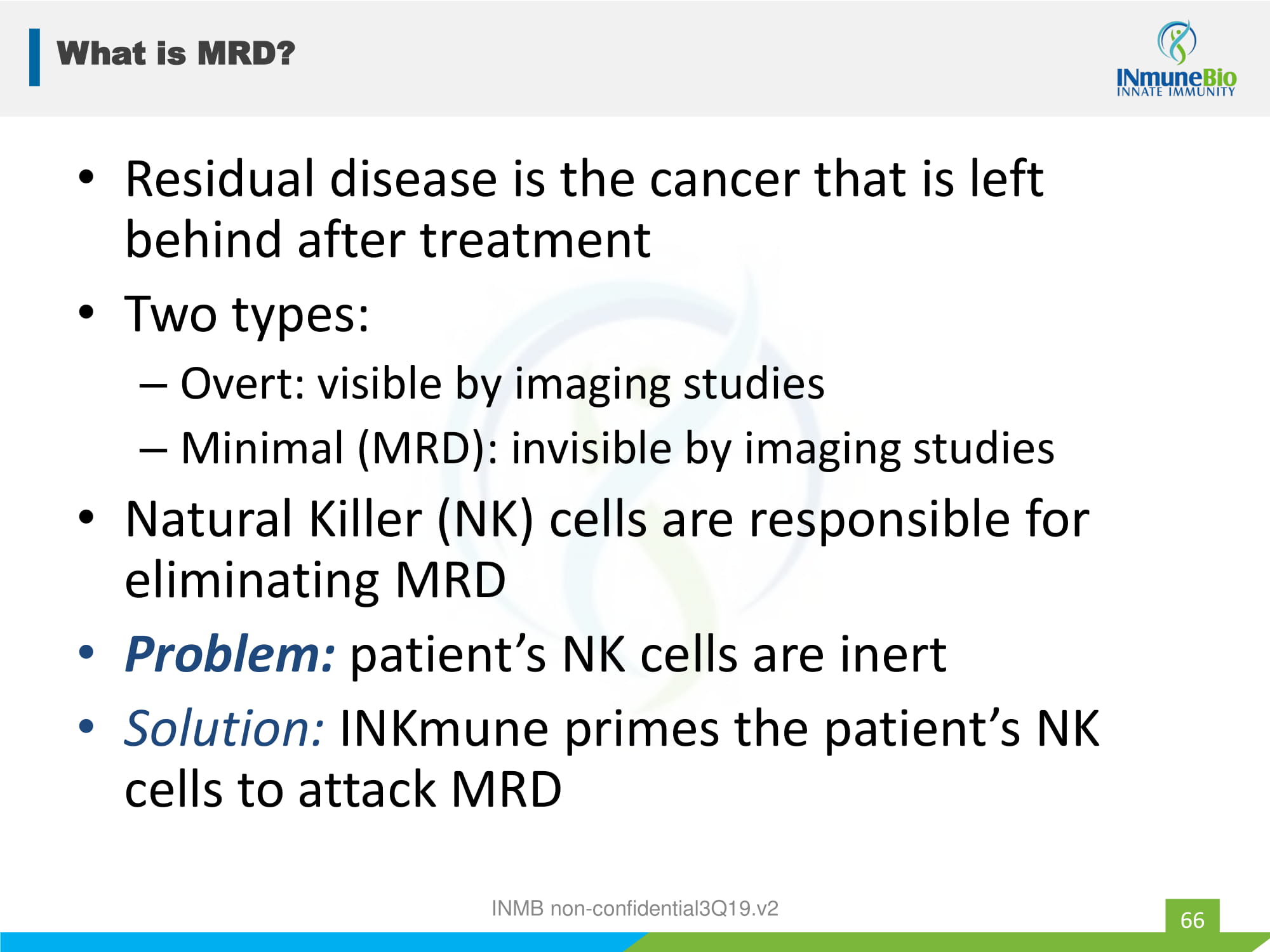
What is MRD? 66 • Residual disease is the cancer that is left behind after treatment • Two types: – Overt: visible by imaging studies – Minimal (MRD): invisible by imaging studies • Natural Killer (NK) cells are responsible for eliminating MRD • Problem: patient’s NK cells are inert • Solution: INKmune primes the patient’s NK cells to attack MRD INMB non - confidential3Q19.v2

Survivor AML Survivor NK cells Kills Relapser AML Relapser NK cells Does not kill • Relapser NK cells kill survivors AML. • Survivor NK cells can not kill relapser AML. Conclusion: AML mutates to evade NK cell killing. 67 67 Tumor not Immune System Cause of Relapse INMB non - confidential3Q19.v2
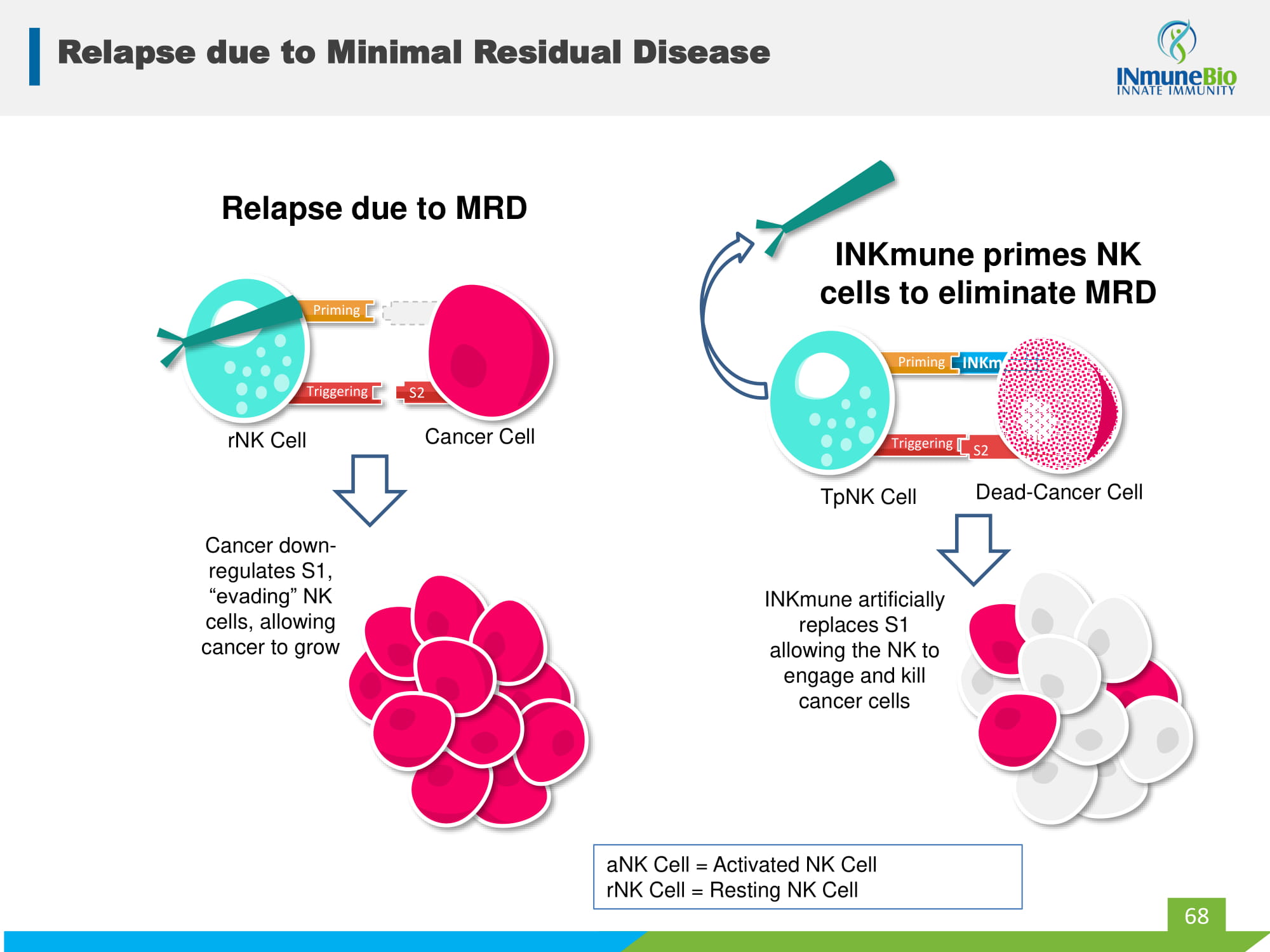
S1 Relapse due to Minimal Residual Disease 68 S2 S2 aNK Cell = Activated NK Cell rNK Cell = Resting NK Cell INKmune INKmune artificially replaces S1 allowing the NK to engage and kill cancer cells TpNK Cell Dead - Cancer Cell Priming Triggering S2 INKmune primes NK cells to eliminate MRD S2 Cancer down - regulates S1, “evading” NK cells, allowing cancer to grow rNK Cell Cancer Cell Relapse due to MRD Priming Triggering
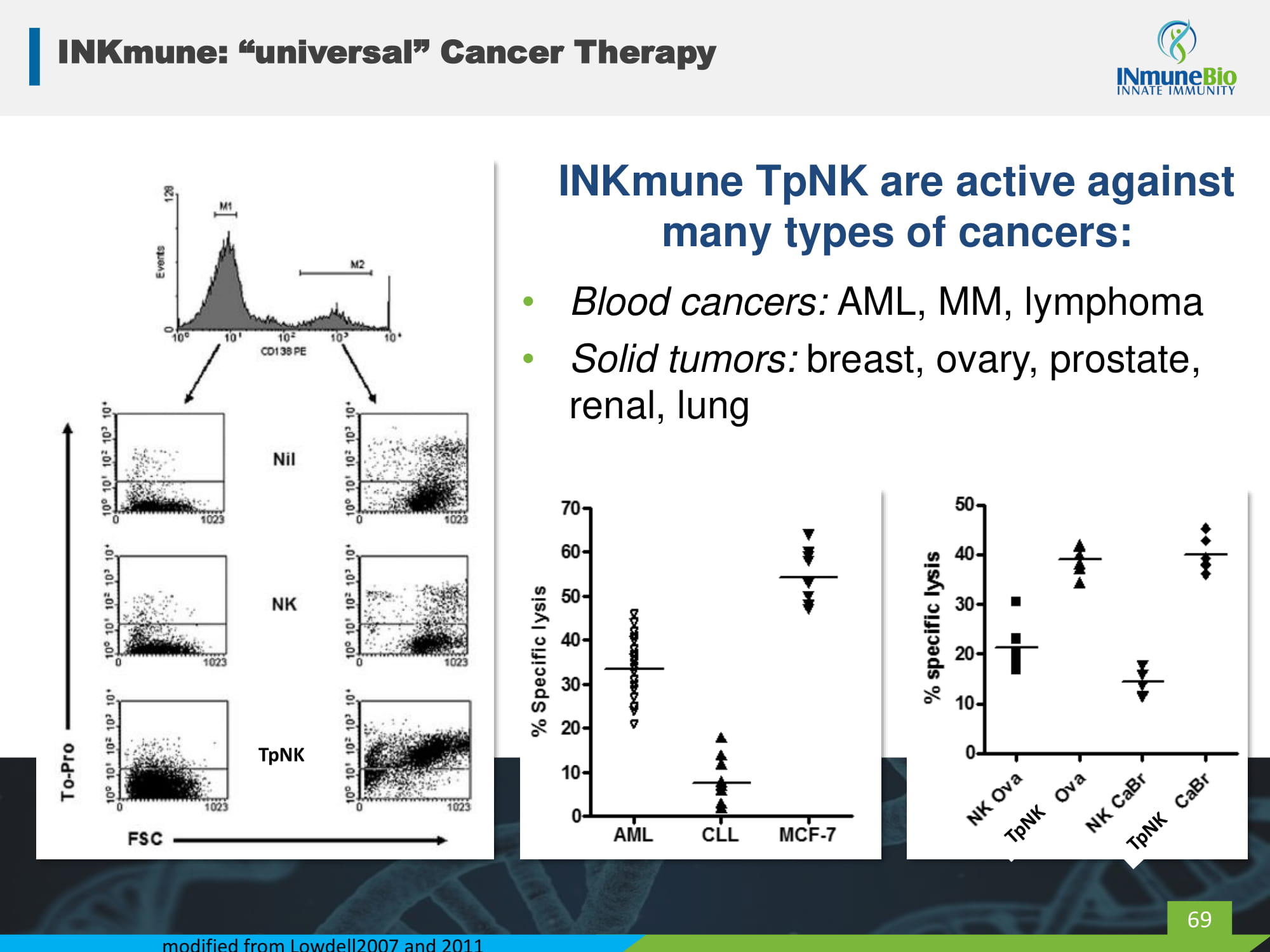
INKmune: “universal” Cancer Therapy INKmune TpNK are active against many types of cancers: • Blood cancers: AML, MM, lymphoma • Solid tumors: breast, ovary, prostate, renal, lung modified from Lowdell2007 and 2011 69 TpNK
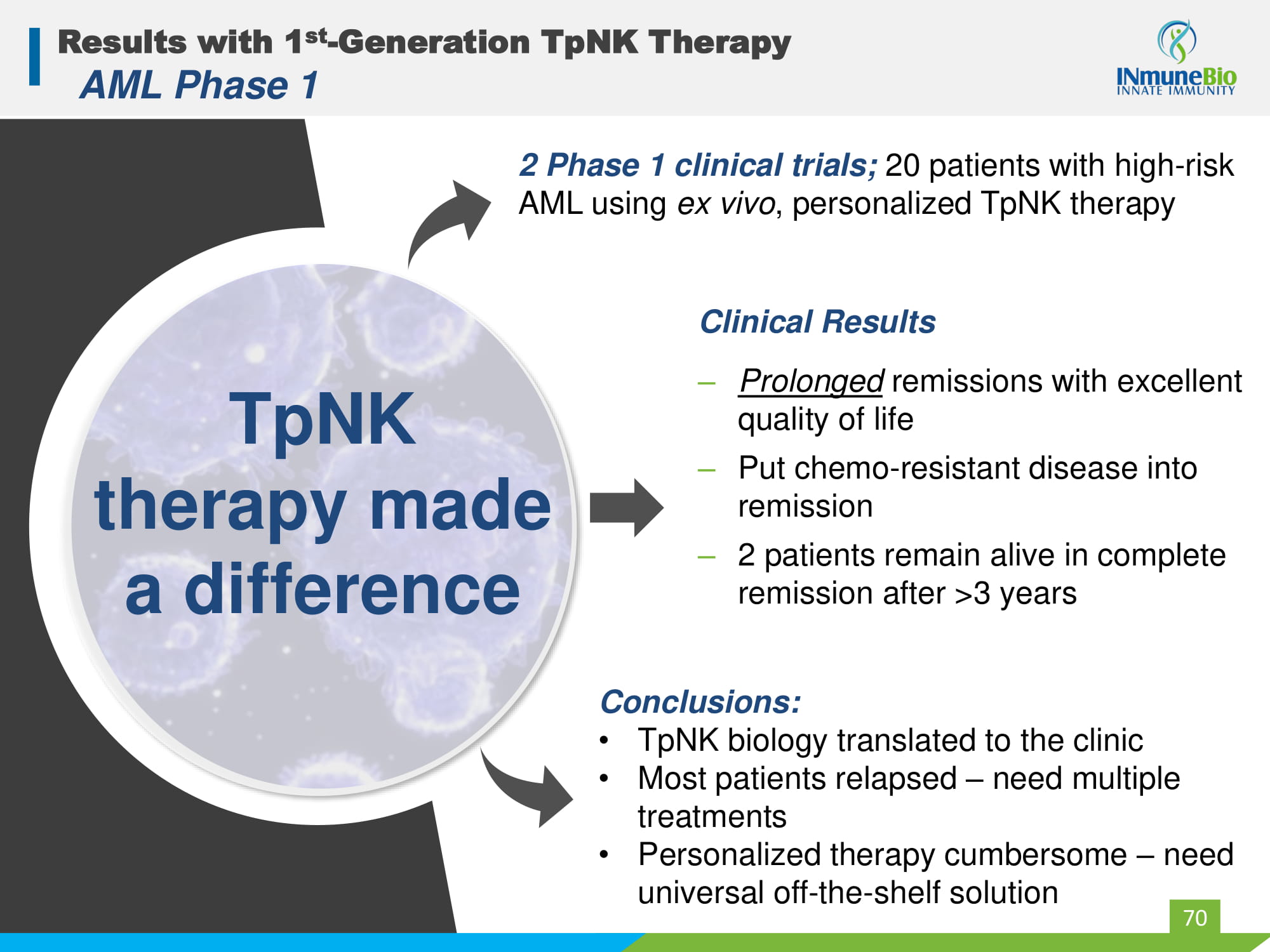
Results with 1 st - Generation TpNK Therapy AML Phase 1 TpNK therapy made a difference 70 2 Phase 1 clinical trials; 20 patients with high - risk AML using ex vivo , personalized TpNK therapy Clinical Results ‒ Prolonged remissions with excellent quality of life ‒ Put chemo - resistant disease into remission ‒ 2 patients remain alive in complete remission after >3 years Conclusions: • TpNK biology translated to the clinic • Most patients relapsed – need multiple treatments • Personalized therapy cumbersome – need universal off - the - shelf solution

AML CLL MCF-7 0 10 20 30 40 50 60 70 % Specific lysis Autologous INKmune pNK cells lyse primary MM cells irrespective of prior treatment INKmune Normal B cells Myeloma B cells INKmune pNK target tumor and NOT normal cells Am J Hematology; 86:967 - 973 71 71 Biomarker – Patient Selection INMB non - confidential3Q19.v2
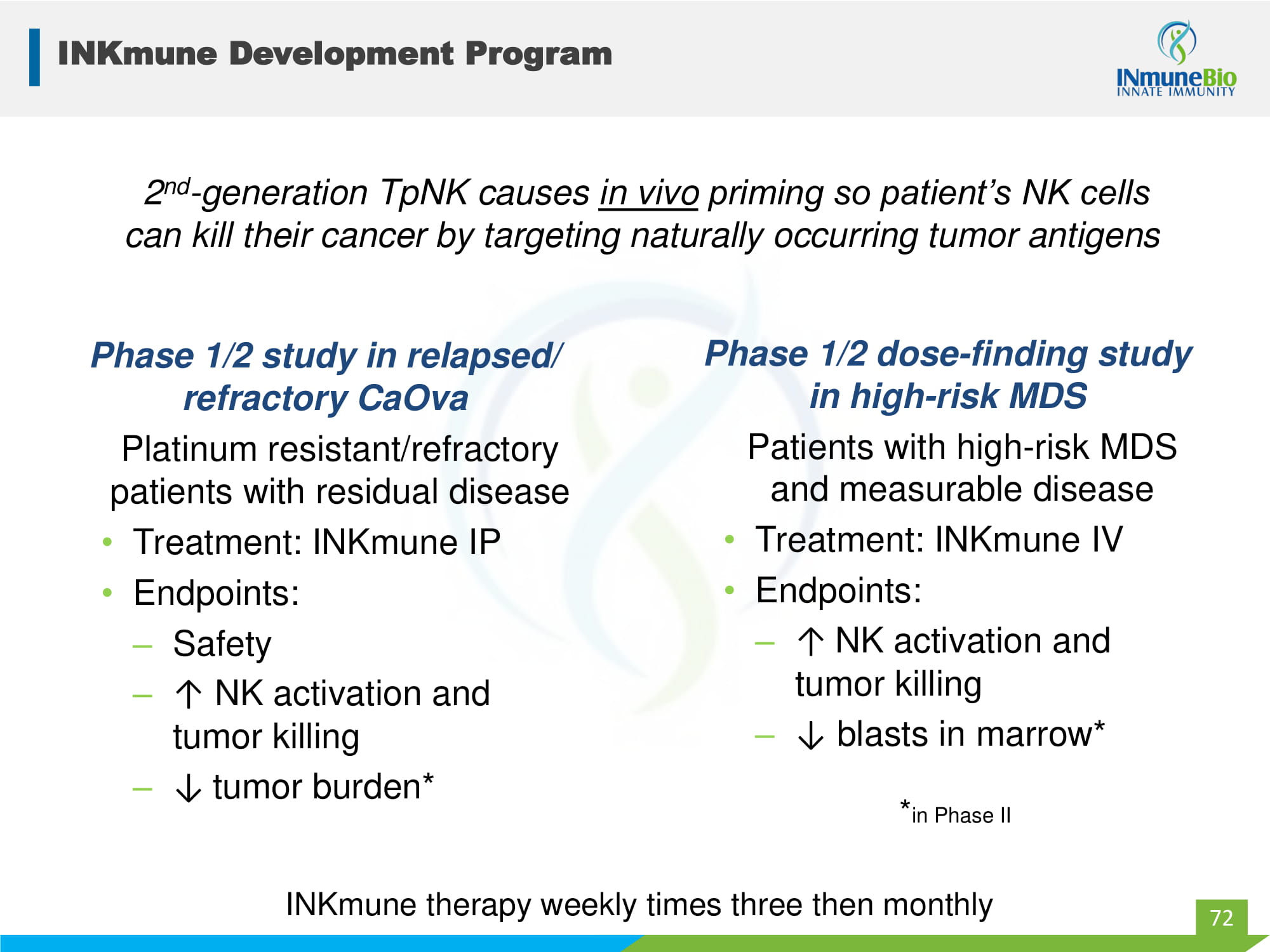
INKmune Development Program Phase 1/2 study in relapsed/ refractory CaOva Platinum resistant/refractory patients with residual disease • Treatment: INKmune IP • Endpoints: – Safety – ↑ NK activation and tumor killing – ↓ tumor burden* Phase 1/2 dose - finding study in high - risk MDS Patients with high - risk MDS and measurable disease • Treatment: INKmune IV • Endpoints: – ↑ NK activation and tumor killing – ↓ blasts in marrow* 72 2 nd - generation TpNK causes in vivo priming so patient’s NK cells can kill their cancer by targeting naturally occurring tumor antigens INKmune therapy weekly times three then monthly * in Phase II

SKOV3 killing by NK cells from healthy donor (HD) or CaOva patient ascites SKOV-3 ALONEHD NK+SKOV3HD TpNK+SKOV3HD LAK+SKOV3PT08 ASC NK+SKOV3PT08 ASC TpNK+SKOV3PT08 ASC LAK+SKOV3 0 20 40 60 80 100 % Viable SKOV3 Cells * * NK cells and INKmune in Ovarian Cancer 73 IL2 primed NK cells do not kill SKOV3 * Patient ascites NK cells kill SKOV3 INMB non - confidential3Q19.v2
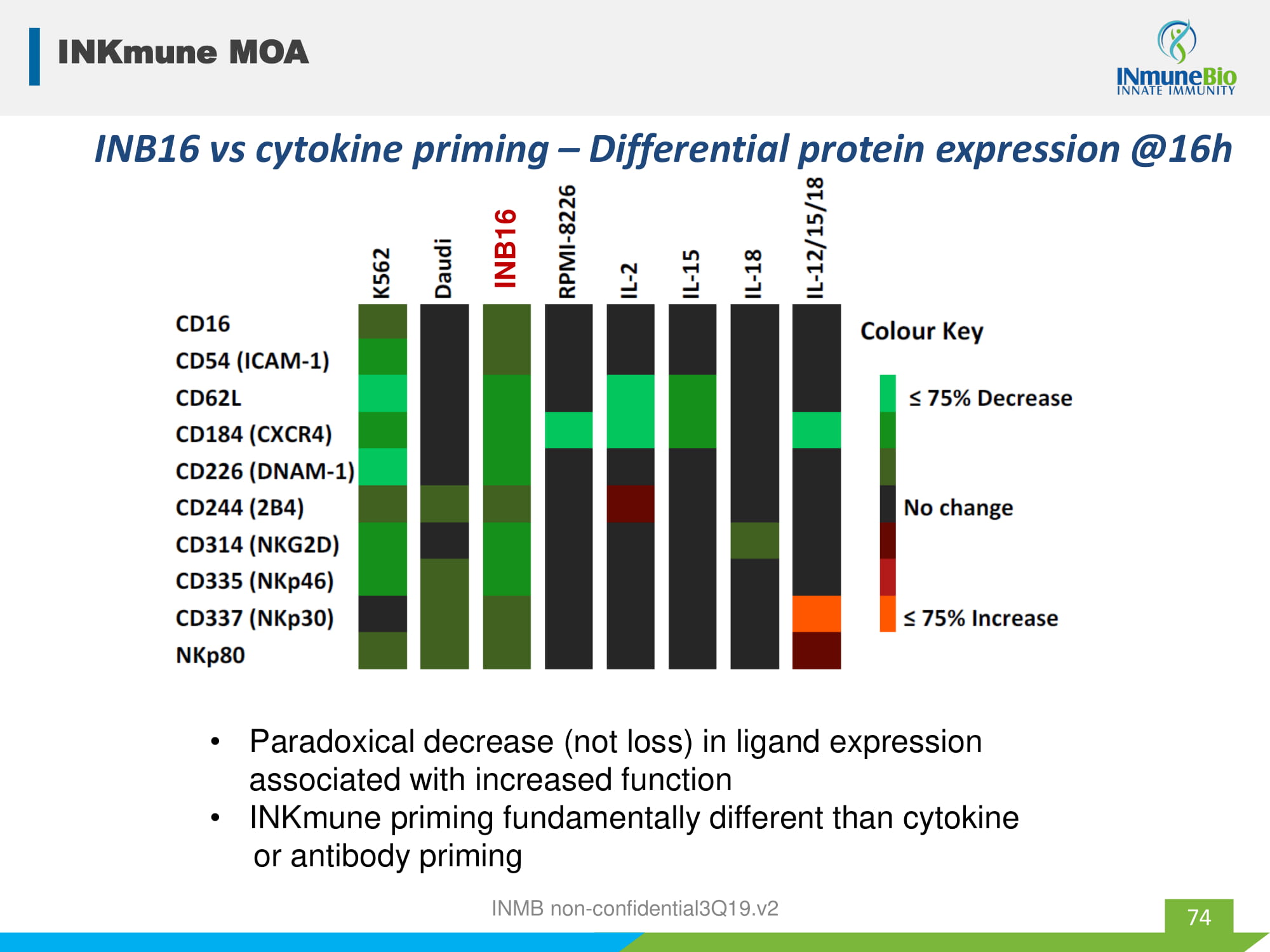
INB16 INB16 vs cytokine priming – Differential protein expression @16h • Paradoxical decrease (not loss) in ligand expression associated with increased function • INKmune priming fundamentally different than cytokine or antibody priming INKmune MOA 74 INMB non - confidential3Q19.v2
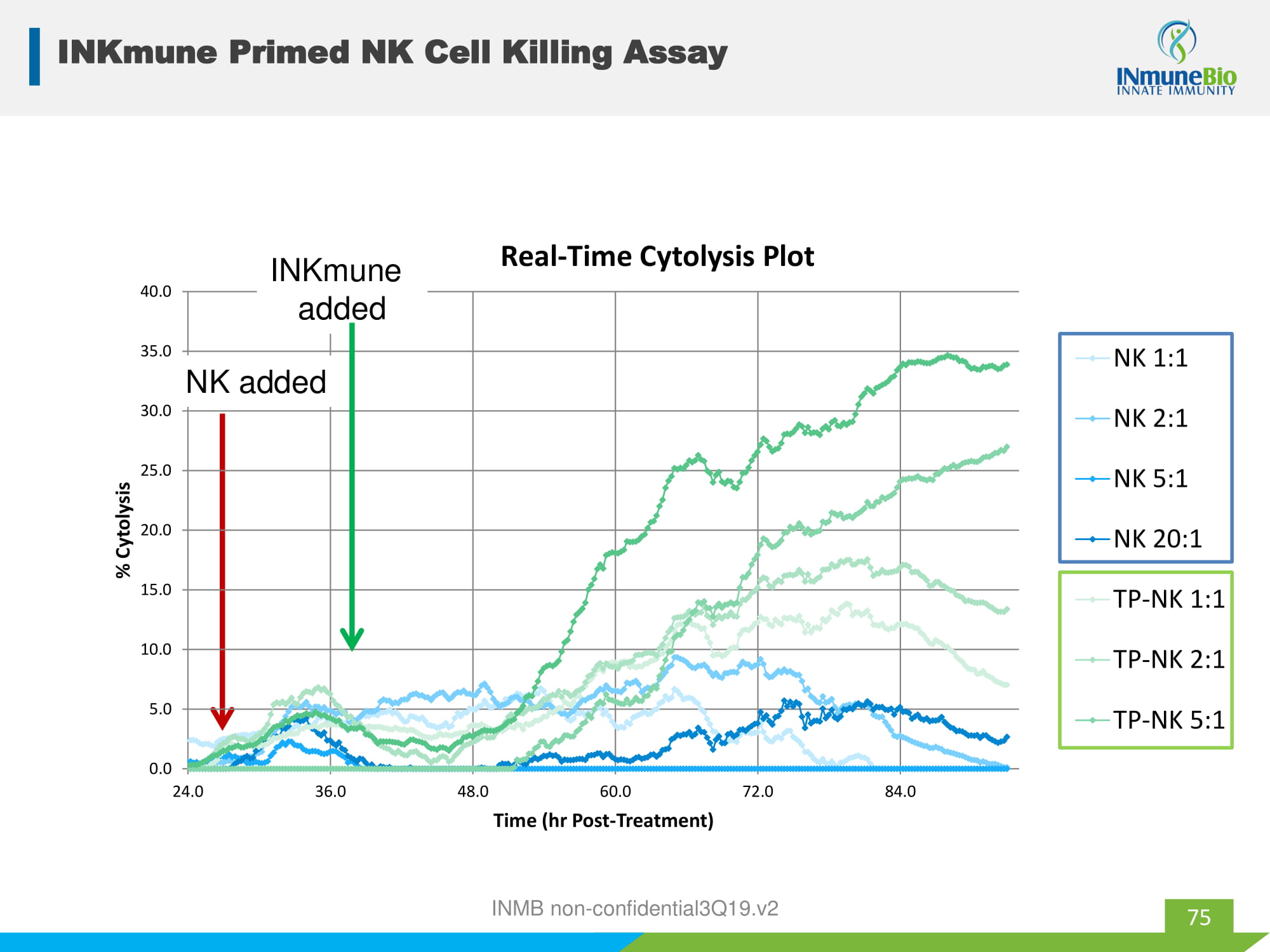
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 24.0 36.0 48.0 60.0 72.0 84.0 % Cytolysis Time (hr Post - Treatment) Real - Time Cytolysis Plot NK 1:1 NK 2:1 NK 5:1 NK 20:1 TP-NK 1:1 TP-NK 2:1 TP-NK 5:1 NK added INKmune added 75 INKmune Primed NK Cell Killing Assay INMB non - confidential3Q19.v2

TaNK alone TaNK +CD15TaNK+OKT11TaNK+CD15+OKT11TaNK+ W632 0 5 10 15 20 25 30 35 %CD25 Expression TaNK alone TaNK +CD15TaNK+OKT11TaNK+CD15+OKT11TaNK+ W632 0 10 20 30 %RAJI Lysis CD2 CD16 CD3 z SS CD2L CD15 epitope INB16 NK rNK TpNK 4h NK signalling upon co-culture with CTV-1 0 10 20 30 40 50 60 70 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 pLAT pZAP70 Min % positive INKmune MOA 76 Lowdell2007 INMB non - confidential3Q19.v2
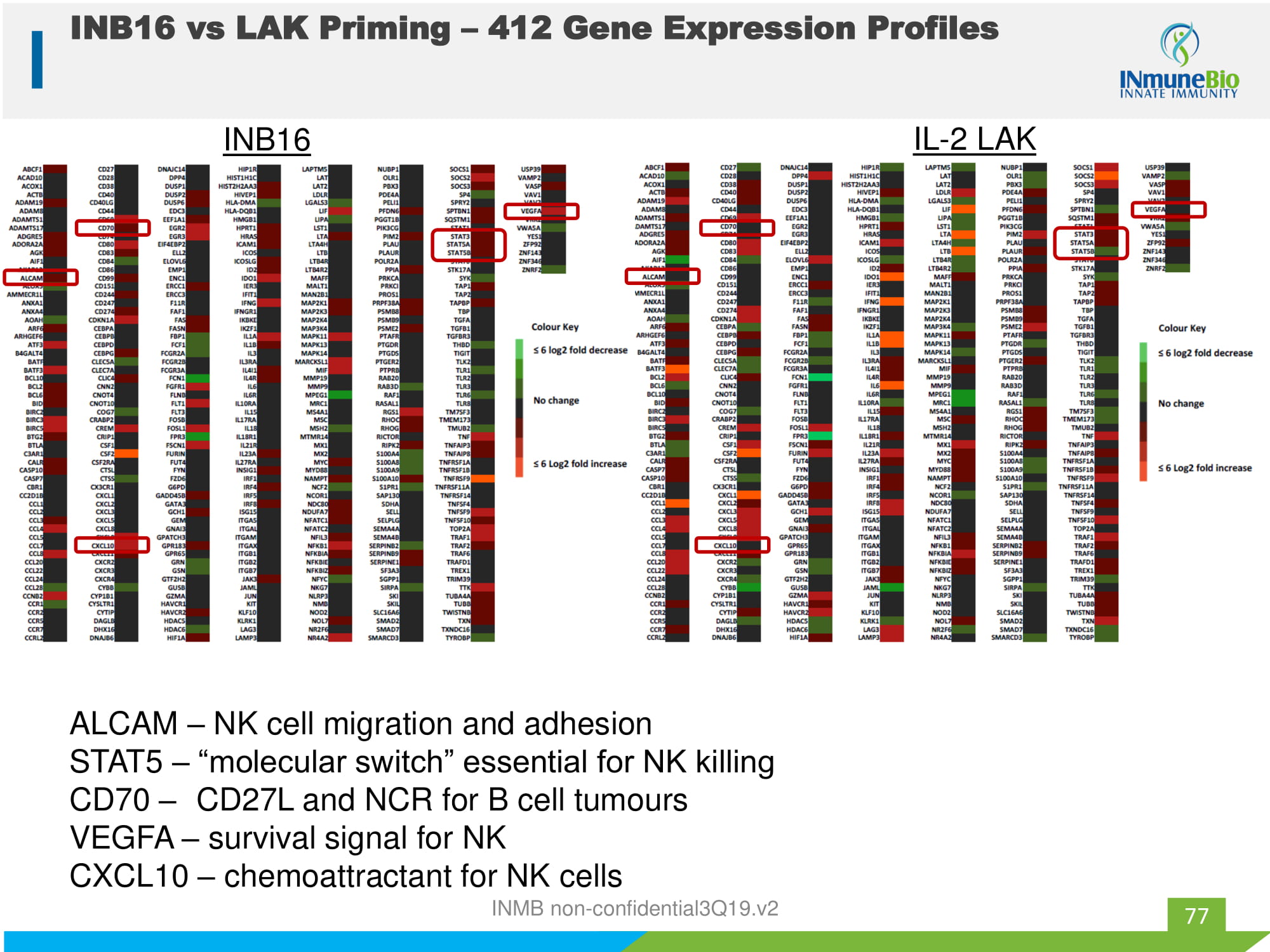
INB16 IL - 2 LAK ALCAM – NK cell migration and adhesion STAT5 – “molecular switch” essential for NK killing CD70 – CD27L and NCR for B cell tumours VEGFA – survival signal for NK CXCL10 – chemoattractant for NK cells 77 INB16 vs LAK Priming – 412 Gene Expression Profiles INMB non - confidential3Q19.v2












































































