Exhibit
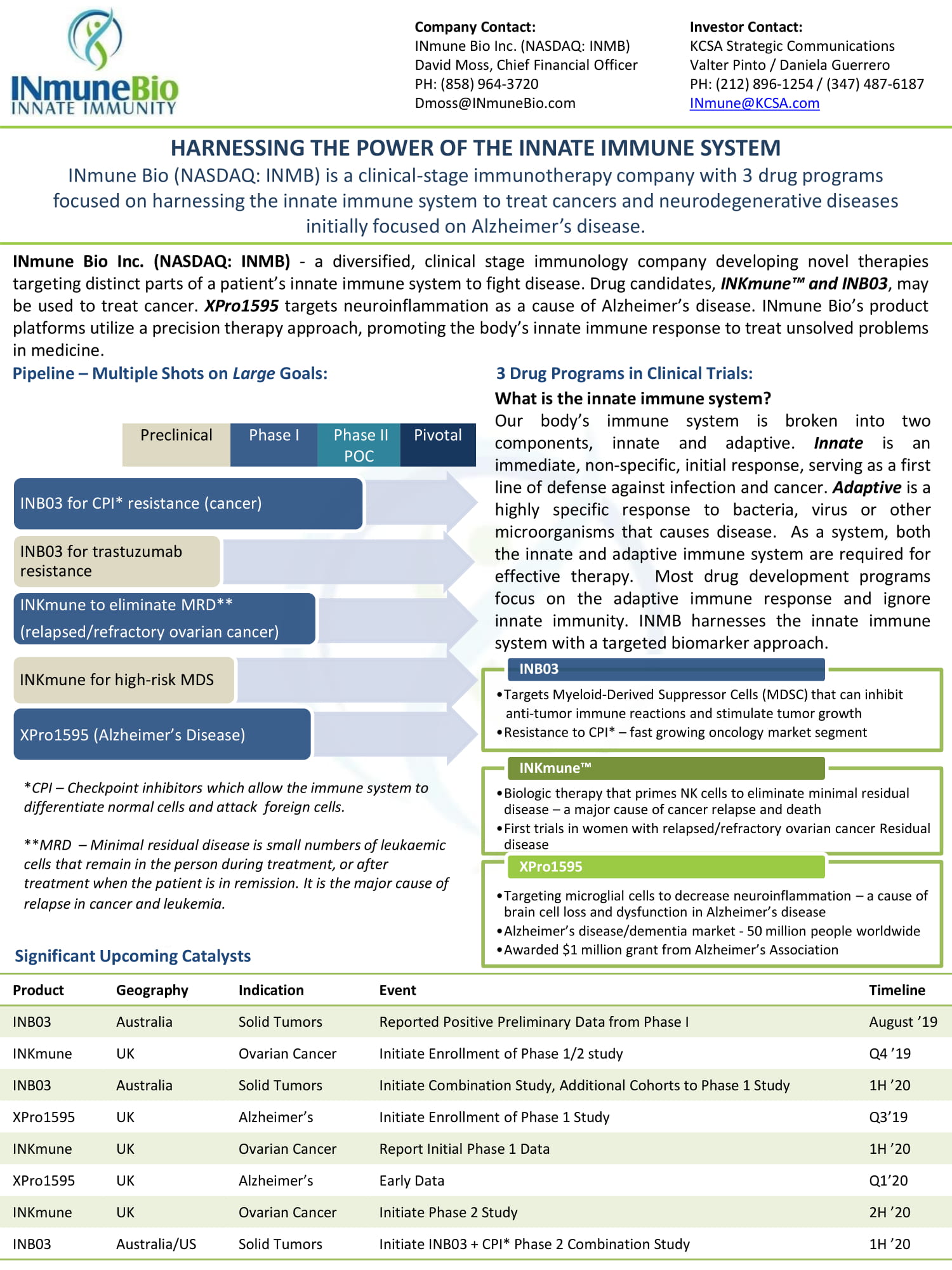
Company Contact: INmune Bio Inc. (NASDAQ: INMB) David Moss, Chief Financial Officer PH: (858) 964 - 3720 Dmoss@INmuneBio.com Investor Contact: KCSA Strategic Communications Valter Pinto / Daniela Guerrero PH: (212) 896 - 1254 / (347) 487 - 6187 INmune@KCSA.com INmune Bio Inc . (NASDAQ : INMB) - a diversified, clinical stage immunology company developing novel therapies targeting distinct parts of a patient’s innate immune system to fight disease . Drug candidates, INKmune™ and INB 03 , may be used to treat cancer . XPro 1595 targets neuroinflammation as a cause of Alzheimer’s disease . INmune Bio’s product platforms utilize a precision therapy approach, promoting the body’s innate immune response to treat unsolved problems in medicine . Pipeline – Multiple Shots on Large Goals: 3 Drug Programs in Clinical Trials: Significant Upcoming Catalysts Product Geography Indication Event Timeline INB03 Australia Solid Tumors Reported Positive Preliminary Data from Phase I August ’19 INKmune UK Ovarian Cancer Initiate Enrollment of Phase 1/2 study Q4 ’ 19 INB03 Australia Solid Tumors Initiate Combination Study, Additional Cohorts to Phase 1 Study 1H ’20 XPro1595 UK Alzheimer’s Initiate Enrollment of Phase 1 Study Q3’19 INKmune UK Ovarian Cancer Report Initial Phase 1 Data 1H ’ 20 XPro1595 UK Alzheimer’s Early Data Q1 ’ 20 INKmune UK Ovarian Cancer Initiate Phase 2 Study 2H ’ 20 INB03 Australia/US Solid Tumors Initiate INB03 + CPI* Phase 2 Combination Study 1H ’20 What is the innate immune system? Our body’s immune system is broken into two components, innate and adaptive . Innate is an immediate, non - specific, initial response, serving as a first line of defense against infection and cancer . Adaptive is a highly specific response to bacteria, virus or other microorganisms that causes disease . As a system, both the innate and adaptive immune system are required for effective therapy . Most drug development programs focus on the adaptive immune response and ignore innate immunity . INMB harnesses the innate immune system with a targeted biomarker approach . INB03 for CPI* resistance (cancer) INB03 for trastuzumab resistance INKmune to eliminate MRD** (relapsed/refractory ovarian cancer) INKmune for high - risk MDS XPro1595 (Alzheimer’s Disease) Preclinical Phase I Phase II POC Pivotal * CPI – Checkpoint inhibitors which allow the immune system to differentiate normal cells and attack foreign cells. ** MRD – Minimal residual disease is small numbers of leukaemic cells that remain in the person during treatment, or after treatment when the patient is in remission. It is the major cause of relapse in cancer and leukemia. HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM INmune Bio (NASDAQ: INMB) is a clinical - stage immunotherapy company with 3 drug programs focused on harnessing the innate immune system to treat cancers and neurodegenerative diseases initially focused on Alzheimer’s disease. • Targets Myeloid - Derived Suppressor Cells (MDSC) that can inhibit anti - tumor immune reactions and stimulate tumor growth • Resistance to CPI* – fast growing oncology market segment INB03 • Biologic therapy that primes NK cells to eliminate minimal residual disease – a major cause of cancer relapse and death • First trials in women with relapsed/refractory ovarian cancer Residual disease INKmune™ • Targeting microglial cells to decrease neuroinflammation – a cause of brain cell loss and dysfunction in Alzheimer’s disease • Alzheimer’s disease/dementia market - 50 million people worldwide • Awarded $1 million grant from Alzheimer’s Association XPro1595

INmune Bio Inc. (NASDAQ: INMB) www.INmuneBio.com Information about Forward - Looking Statements This fact sheet may contain forward - looking statements made pursuant to the Private Securities Litigation Reform Act of 1995 . Words such as “anticipate,” “estimate,” “expect,” “intend,” “plan,” “project” and other similar words and expressions are intended to signify forward - looking statements . Forward - looking statements are not guarantees of future results and conditions but rather are subject to various risks and uncertainties . Some of these risks and uncertainties are identified in the company’s filings with the SEC . The occurrence of any of these risks and uncertainties could have a material adverse effect on the company’s business, financial condition, and results of operations . For additional disclosure regarding risks faced by INmune Bio, Inc . , please see our public filings with the Securities and Exchange Commission, available on the Investors section of our website at www . INmuneBio . com and on the SEC's website at www . SEC . gov . Management Raymond J. Tesi, MD – CEO, CMO, Chairman David J. Moss – CFO Professor Mark Lowdell, PhD – CSO/CMO Directors Raymond J. Tesi, MD (Chairman) David Szymkowski , PhD (VP of Cell Biology, Xencor ) J. Kelly Ganjei (CEO of Cognate) Scott Juda (Founder and Managing Member, Fossick Capital) Timothy Schroeder (CEO and Founder of CTI) Edgardo (Ed) Baracchini (Board Member of 4D Pharma PLC) Experienced Leadership Financial Snapshot (as of August 19 , 2019 ) NASDAQ (closed IPO in February 2019): INMB Share Price: $5.80 Market Cap: 56.4M Shares Outstanding (common/ fully diluted, as of 8/09/19): 10.8M / 13.9M Insider Ownership: 50.0% Debt: $0 Analyst Coverage : Maxim (BUY, $9 PT) H.C. Wainwright (BUY, $11.50 PT) INB03 Improves Checkpoint Inhibitor Function MDSC “force field” before INB03 MDSC “force field” after INB03 Cancer killing T cell after CPI* can not kill cancer cells due to MDSC Cancer cells live and grow Cancer killing T cell after CPI* kills cancer cells when MDSC is eliminated by INB03 Cancer cells killed by CPI* powered T cells INB03 Development Program Completed Phase 1 Open - Label, Dose - Escalation Trial 12 patients with advanced solid tumors with biomarkers of chronic inflammation including increased MDSC • Treatment: INB03 sub - cutaneous once a week • Endpoints: Safety and decreased MDSC Patients resistant to CPI* with increased MDSC • Treatment: Combination therapy INB03 + - CPI* • Endpoints: Decreased MDSC and no resistance to CPI* with improved progression free/overall survival *subject to modification pending results of Phase 1 Xpro1595: Neuroinflammation, the “Ignored” Element of Alzheimer’s Disease Phase 1: Alzheimer’s Disease Biomarker directed trial of patients with inflammation and proven Alzheimer’s diseases 18 patients in 3 cohorts • Weekly XPro1595 subQ for 3 months • Biomarkers of inflammation at 0, 6 and 12 weeks • Endpoints: • Safety • Decreased inflammation blood, cerebrospinal fluid (CSF), brain and breath • Measures of cogitation, psychiatric symptoms and quality of life (QOL) INKmune: Treat Minimal Residual Disease (MRD**) Residual disease is the cancer that is left behind after treatment; two types – overt (visible by imaging studies) and minimal (MRD** – NOT visible by imaging studies) NK cells are responsible for eliminating MRD**, problem is patient’s NK cells are inactive, however INKmune primes the NK cells to kill the cancer Phase 1/2 study in relapsed/refractory CaOva • Platinum resistant/refractory patients with minimal residual disease • Treatment: INKmune - 6 doses • Endpoints: Safety, increased NK activation and tumor killing and decreased tumor burden (when in Phase 2) Phase 2 Trial in Stage IV Melanoma* + - CPI

