Exhibit 99.1

INMB non - confidential3Q19.v5

This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . Forward - Looking Statements 2 INMB non - confidential3Q19.v4
3 A Next Generation immunology company • Two product platforms targeting innate immune dysfunction • Programs in cancer, AD and NASH • Resistance to cancer immunotherapy in the TME • Neuroinflammation as a cause of neurodegenerative disease • Chronic inflammation as a cause of NASH • Platform technologies provide multiple strategic opportunities • Experienced management team • Multiple programs provide sum - of - parts value INMB non - confidential3Q19.v4

4 Financial Highlights • Nasdaq: INMB • Cash 6/30/19 $9.4 million • Debt $0 • Common Shares O/S 10.8 million Insider ownership: ~50% Notable Shareholder: Xencor (XNCR) XNCR licensing fee: Option to buy 10% of INMB for $10m at $100m valuation INMB non - confidential3Q19.v4

5 INmune Bio = Innate Immunity Our Vision: Become the Leader in Reprograming the Innate Immune System for the Treatment of Diseases INMB non - confidential3Q19.v4
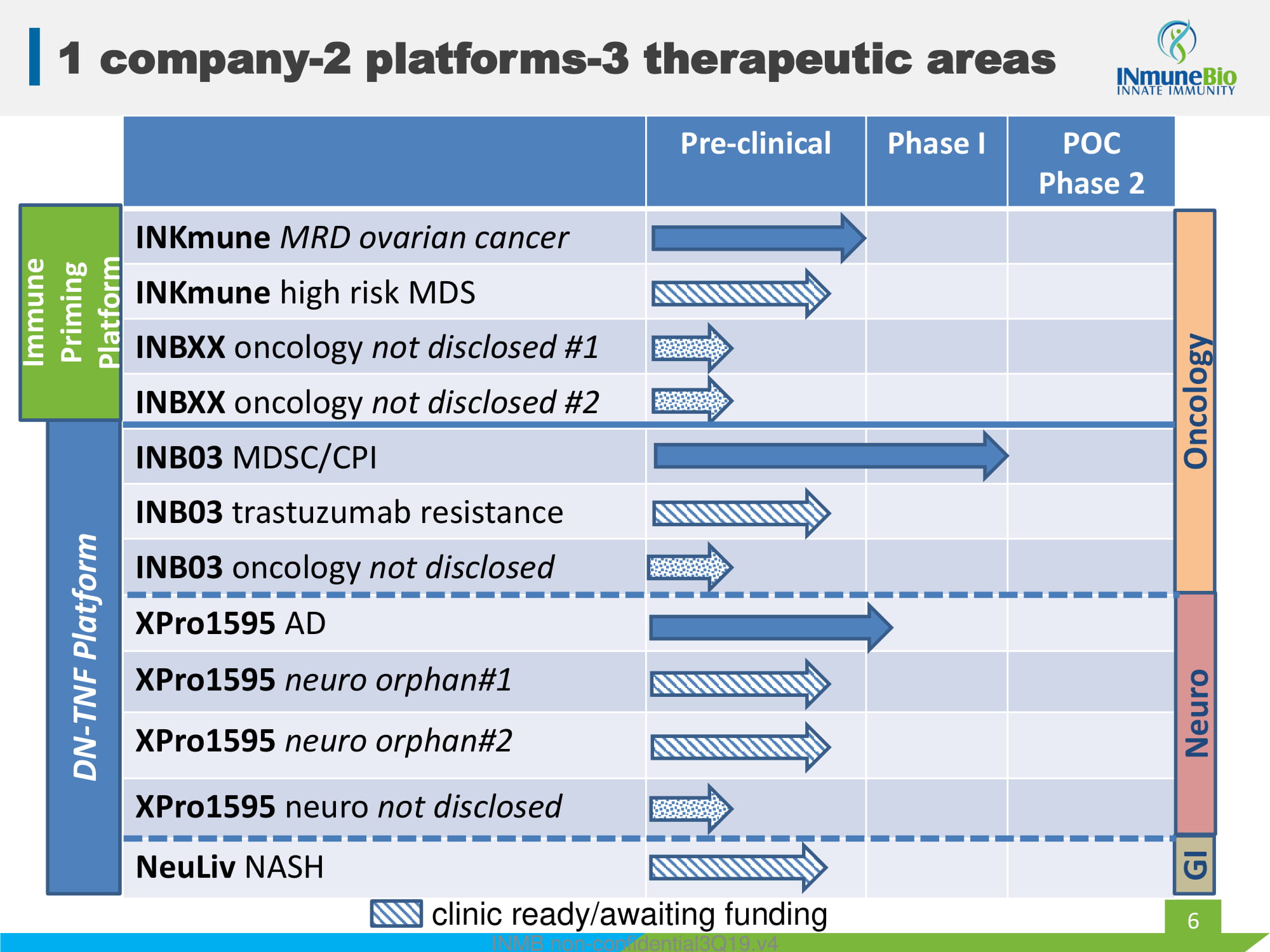
6 1 company - 2 platforms - 3 therapeutic areas Pre - clinical Phase I POC Phase 2 INKmune MRD ovarian cancer INKmune high risk MDS INBXX oncology not disclosed #1 INBXX oncology not disclosed #2 INB03 MDSC/CPI INB03 trastuzumab resistance INB03 oncology not disclosed XPro1595 AD XPro1595 neuro orphan#1 XPro1595 neuro orphan#2 XPro1595 neuro not disclosed NeuLiv NASH INMB non - confidential3Q19.v4 DN - TNF Platform Immune Priming Platform Oncology Neuro GI clinic ready/awaiting funding

Market Opportunity: announced programs 7 • XPro1595 - Alzheimer’s/dementia market* – 2019 est cost to care for US AD patients $280B • INKmune - Cancer residual disease market ** – 1,735,350 new cases and 609,640 people will die • INB03 - Resistance to Cancer Immunotherapy market*** – Cancer immunotherapy market est. >$119B in 2021 • NeuLiv – NASH market † – Market size >$7B * Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures. https://www.alz.org/alzheimers - dementia/facts - figures ** National Cancer Institute. https://www.cancer.gov/about - cancer/understanding/statistics *** MarketandMarkets . https://www.marketsandmarkets.com/Market - Reports/cancer - immunotherapy - market - 197577894.html? † BioPharmDive : https://www.biopharmadive.com/news/no - one - knows - the - size - of - the - nash - market/554240/ INMB non - confidential3Q19.v4

DN - TNF Platform INMB non - confidential3Q19.v4
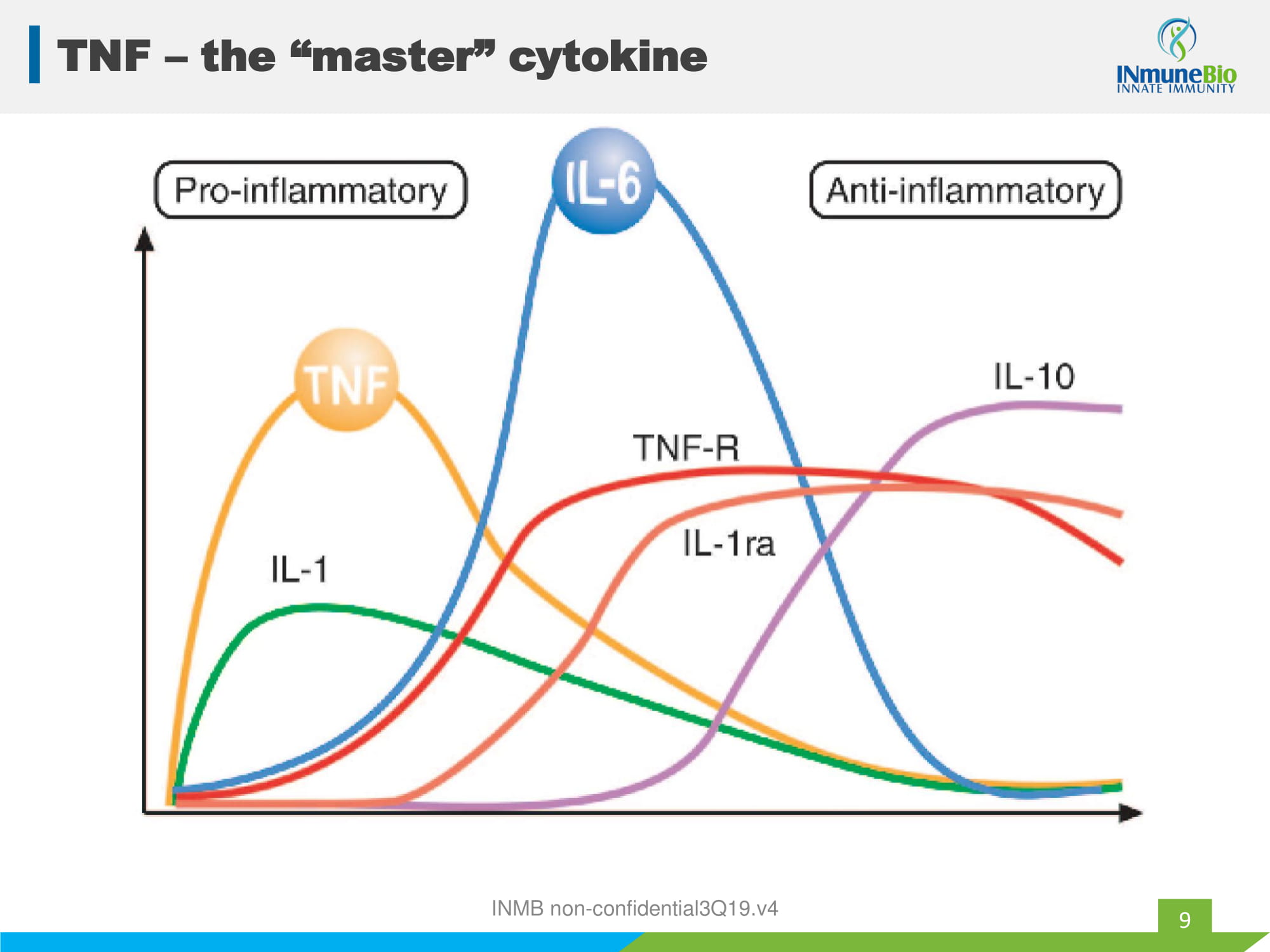
INMB non - confidential3Q19.v4 TNF – the “master” cytokine 9
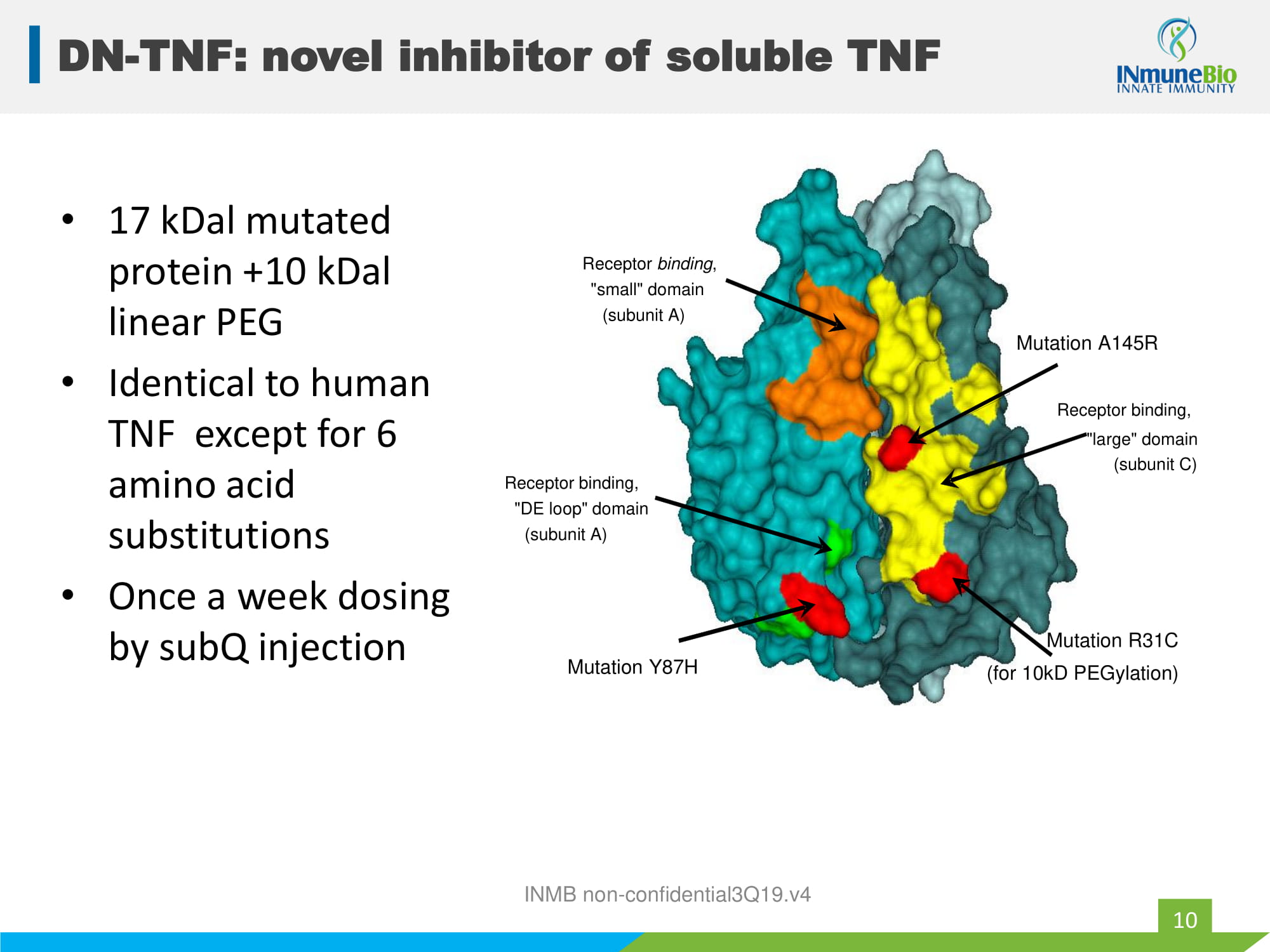
• 17 kDal mutated protein +10 kDal linear PEG • Identical to human TNF except for 6 amino acid substitutions • Once a week dosing by subQ injection INMB non - confidential3Q19.v4 Receptor binding, "DE loop" domain (subunit A) Receptor binding , "small" domain (subunit A) Mutation A145R Receptor binding, "large" domain (subunit C) Mutation R31C (for 10kD PEGylation) Mutation Y87H DN - TNF: novel inhibitor of soluble TNF 10
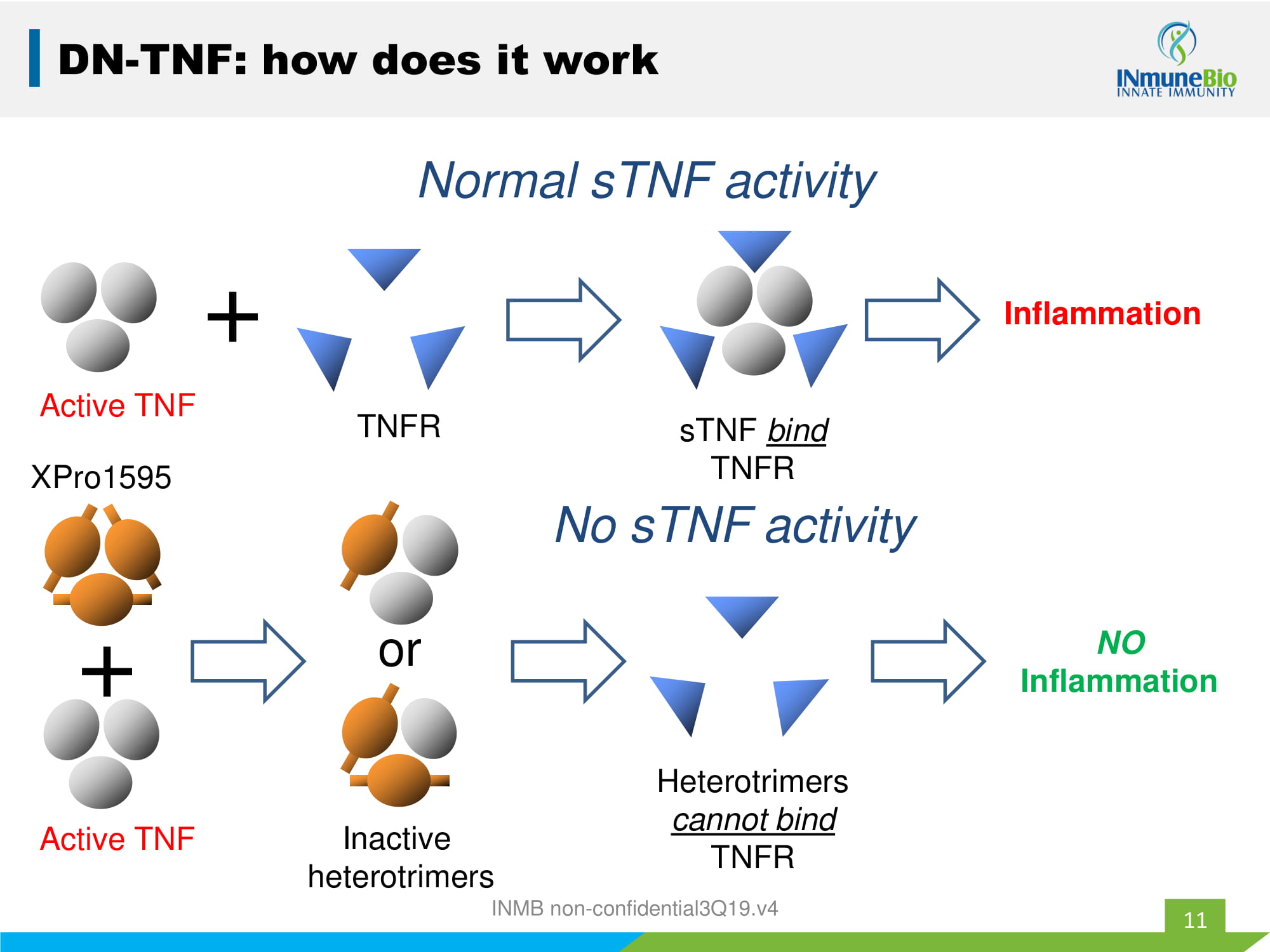
Active TNF + Inflammation Normal sTNF activity TNFR sTNF bind TNFR XPro1595 Active TNF + Inactive heterotrimers or NO Inflammation Heterotrimers cannot bind TNFR No sTNF activity DN - TNF: how does it work 11 INMB non - confidential3Q19.v4
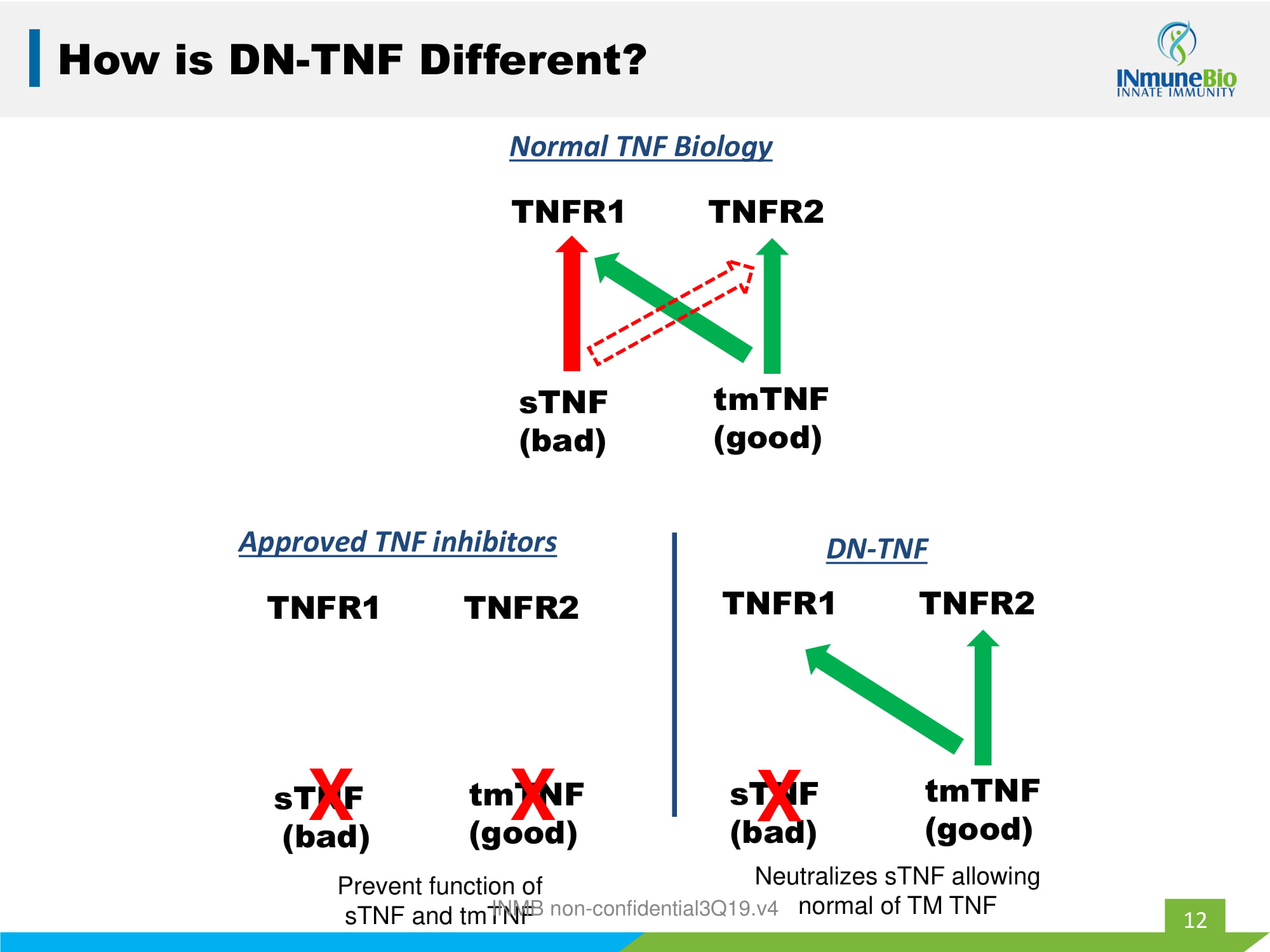
12 How is DN - TNF Different? TNFR1 TNFR2 tmTNF (good) sTNF (bad) Normal TNF Biology 12 TNFR1 TNFR2 tmTNF (good) sTNF (bad) Approved TNF inhibitors X X TNFR1 TNFR2 tmTNF (good) sTNF (bad) DN - TNF X Prevent function of sTNF and tmTNF Neutralizes sTNF allowing normal of TM TNF INMB non - confidential3Q19.v4
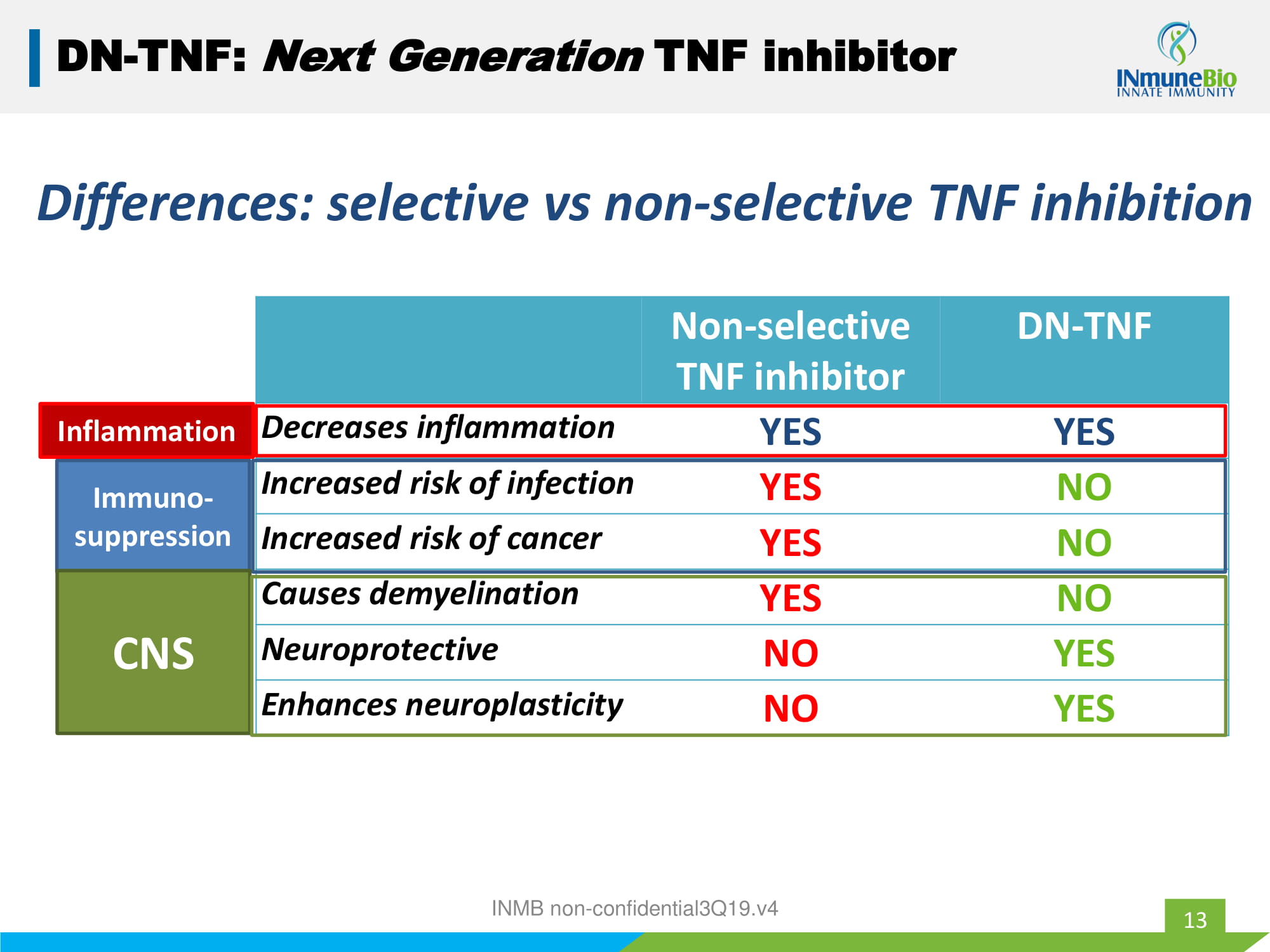
13 DN - TNF: Next Generation TNF inhibitor Non - selective TNF inhibitor DN - TNF Decreases inflammation YES YES Increased risk of infection YES NO Increased risk of cancer YES NO Causes demyelination YES NO Neuroprotective NO YES Enhances neuroplasticity NO YES Differences: selective vs non - selective TNF inhibition INMB non - confidential3Q19.v4 Immuno - suppression CNS Inflammation

INB03 for treatment of cancers resistance to immunotherapy INMB non - confidential3Q19.v4
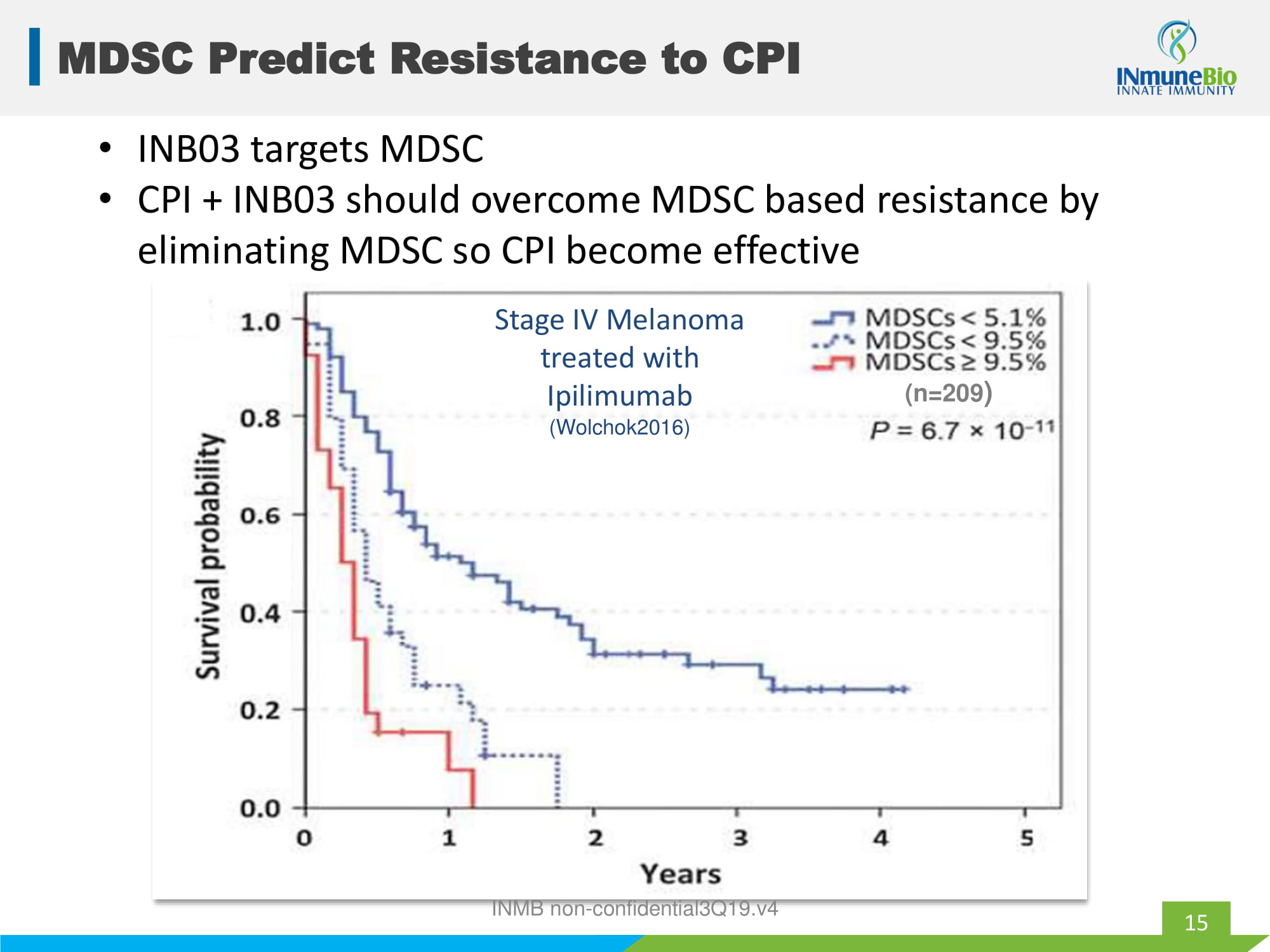
Stage IV Melanoma treated with Ipilimumab (Wolchok2016) MDSC Predict Resistance to CPI 15 (n=209 ) • INB03 targets MDSC • CPI + INB03 should overcome MDSC based resistance by eliminating MDSC so CPI become effective INMB non - confidential3Q19.v4
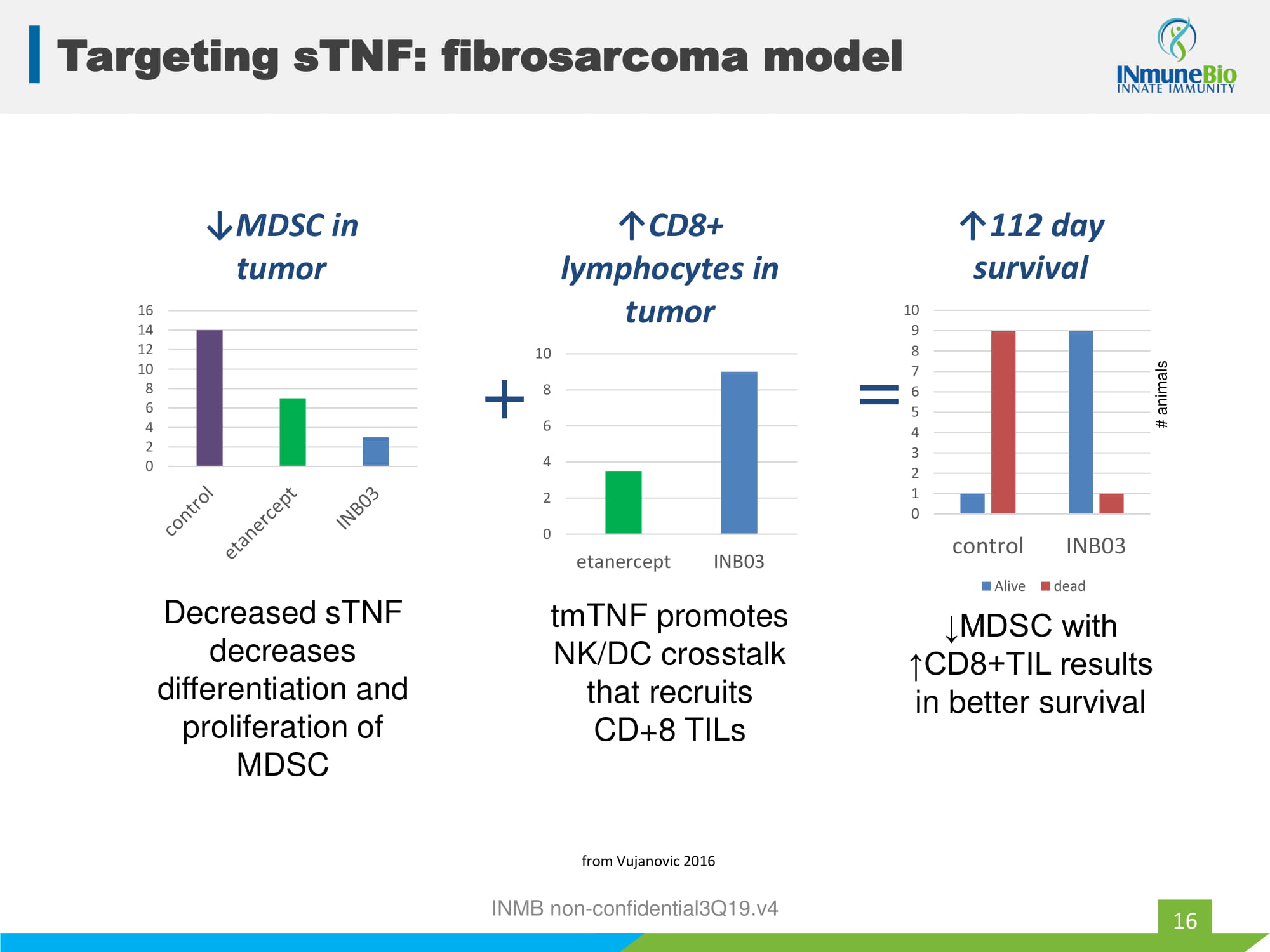
INMB non - confidential3Q19.v4 0 2 4 6 8 10 12 14 16 ↓MDSC in tumor Decreased sTNF decreases differentiation and proliferation of MDSC 0 2 4 6 8 10 etanercept INB03 ↑CD8+ lymphocytes in tumor + tmTNF promotes NK/DC crosstalk that recruits CD+8 TILs 0 1 2 3 4 5 6 7 8 9 10 control INB03 ↑112 day survival Alive dead = ↓MDSC with ↑CD8+TIL results in better survival # animals from Vujanovic 2016 Targeting sTNF: fibrosarcoma model 16
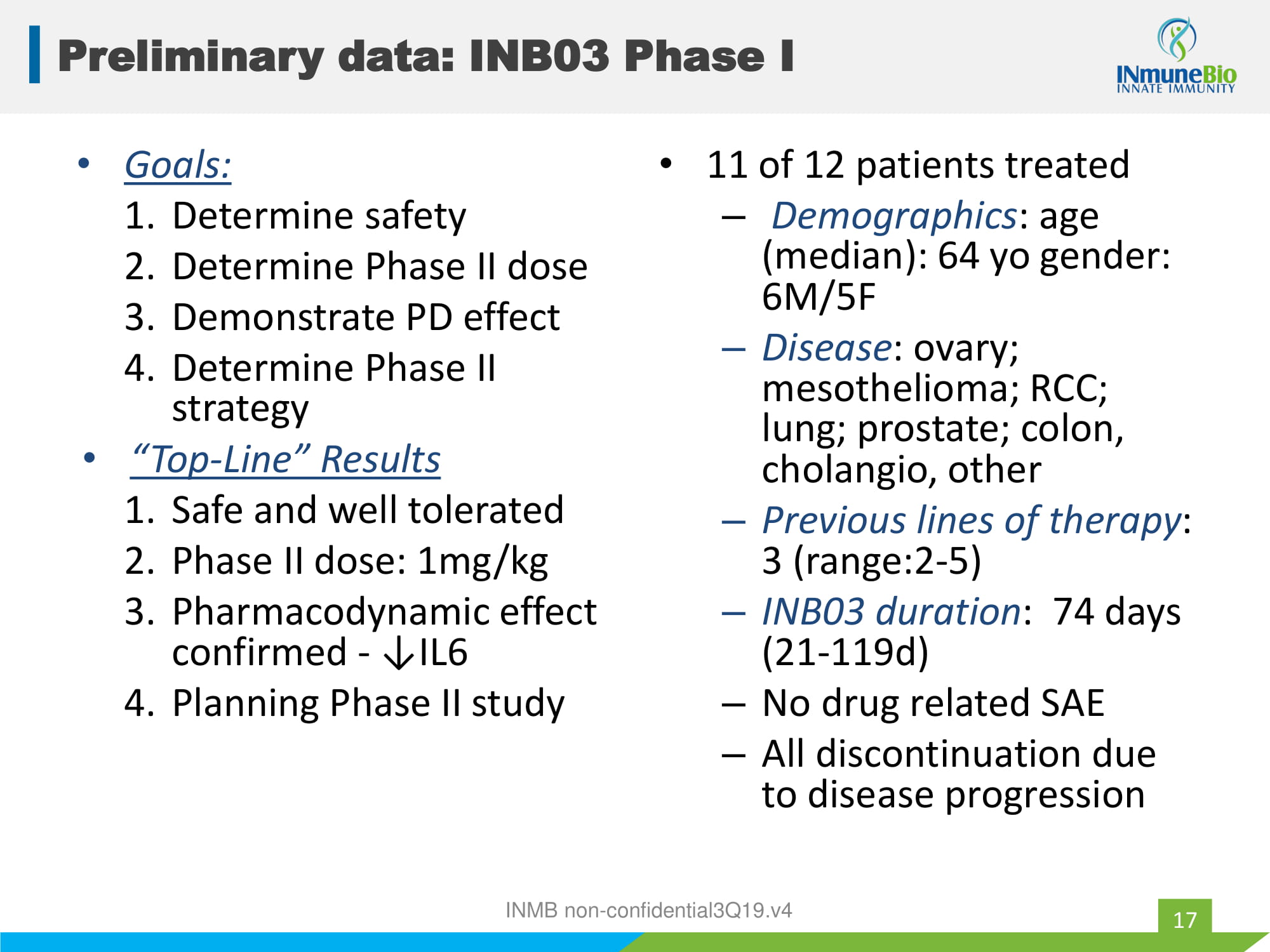
• Goals: 1. Determine safety 2. Determine Phase II dose 3. Demonstrate PD effect 4. Determine Phase II strategy • “Top - Line” Results 1. Safe and well tolerated 2. Phase II dose: 1mg/kg 3. Pharmacodynamic effect confirmed - ↓IL6 4. Planning Phase II study • 11 of 12 patients treated – Demographics : age (median): 64 yo gender: 6M/5F – Disease : ovary; mesothelioma; RCC; lung; prostate; colon, cholangio , other – Previous lines of therapy : 3 (range:2 - 5) – INB03 duration : 74 days (21 - 119d) – No drug related SAE – All discontinuation due to disease progression INMB non - confidential3Q19.v4 Preliminary data: INB03 Phase I 17

XPro1595 (for the treatment of Alzheimer's Disease/dementia) INMB non - confidential3Q19.v4
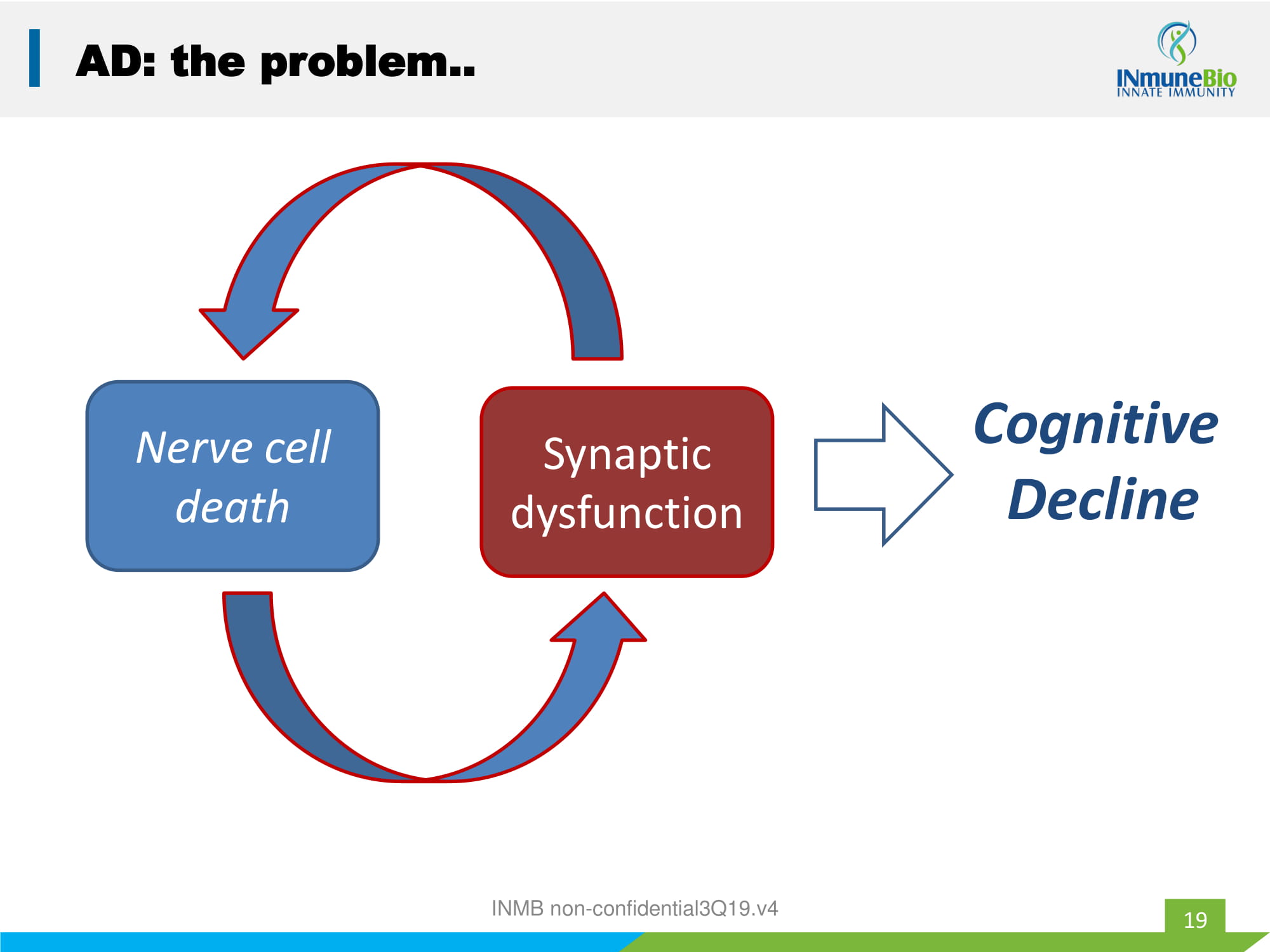
19 AD: the problem.. Nerve cell death Synaptic dysfunction Cognitive Decline INMB non - confidential3Q19.v4

20 XPro1595 for AD receives external validation Alzheimer’s Association awards INmune Bio Part the Cloud Grant - $1M USD translational funding to study XPro1595 in Alzheimer's disease INMB non - confidential3Q19.v4
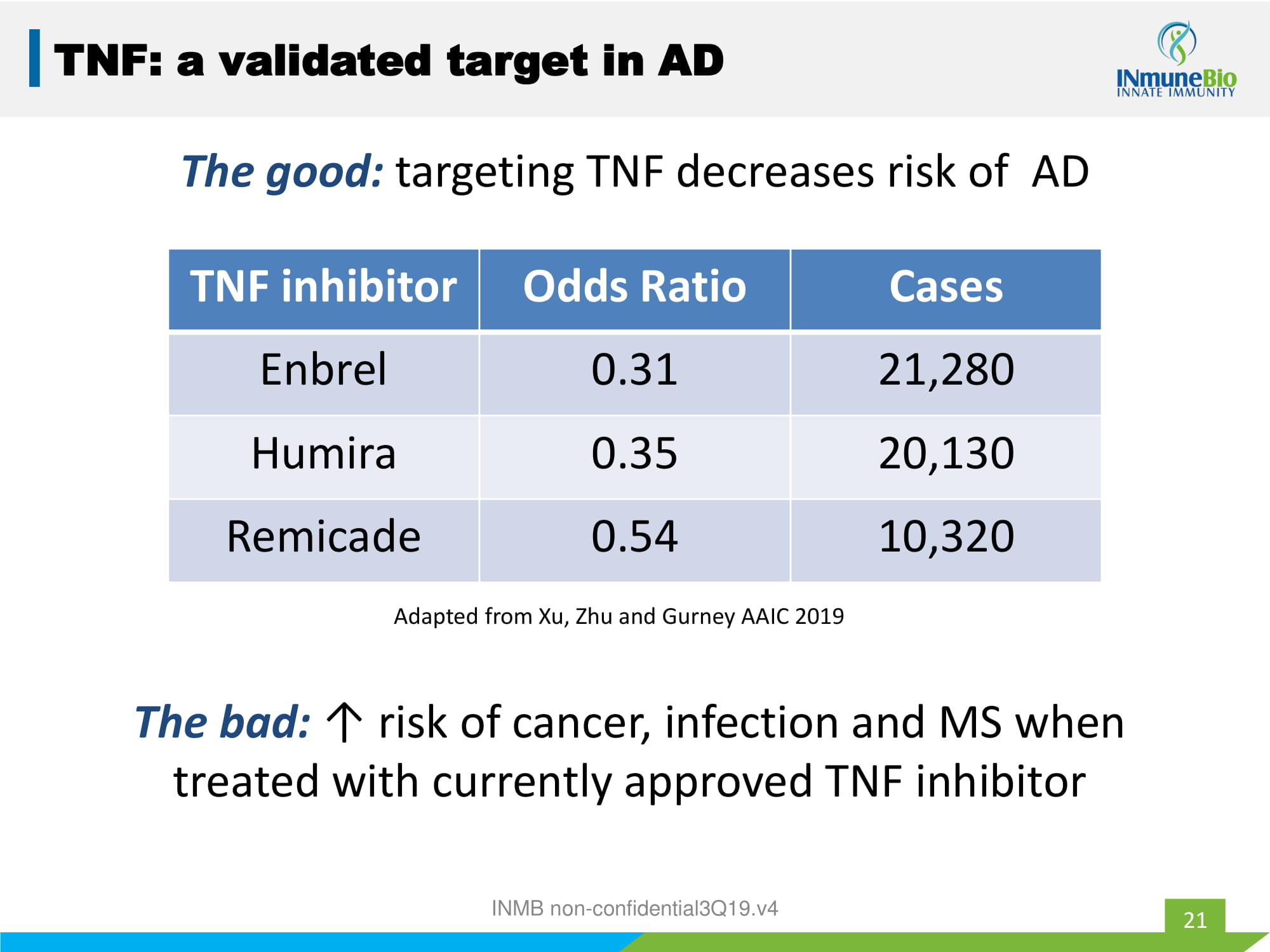
The good: targeting TNF decreases risk of AD INMB non - confidential3Q19.v4 21 TNF inhibitor Odds Ratio Cases Enbrel 0.31 21,280 Humira 0.35 20,130 Remicade 0.54 10,320 Adapted from Xu, Zhu and Gurney AAIC 2019 TNF: a validated target in AD The bad: ↑ risk of cancer, infection and MS when treated with currently approved TNF inhibitor

XPro1595 AD Development Program 18 patients in 3 cohorts • Weekly XPro1595 subQ for 3 months • Biomarkers of inflammation at 0, 6 and 12 weeks • Endpoints: – Safety – ↓ neuro - inflammation measured in: blood, cerebrospinal fluid, brain, breath and behavior – Measures of cognition, neuro - psychiatric symptoms and QOL 22 Phase I biomarker directed trial of patients with inflammation and proven Alzheimer’s Diseases INMB non - confidential3Q19.v4
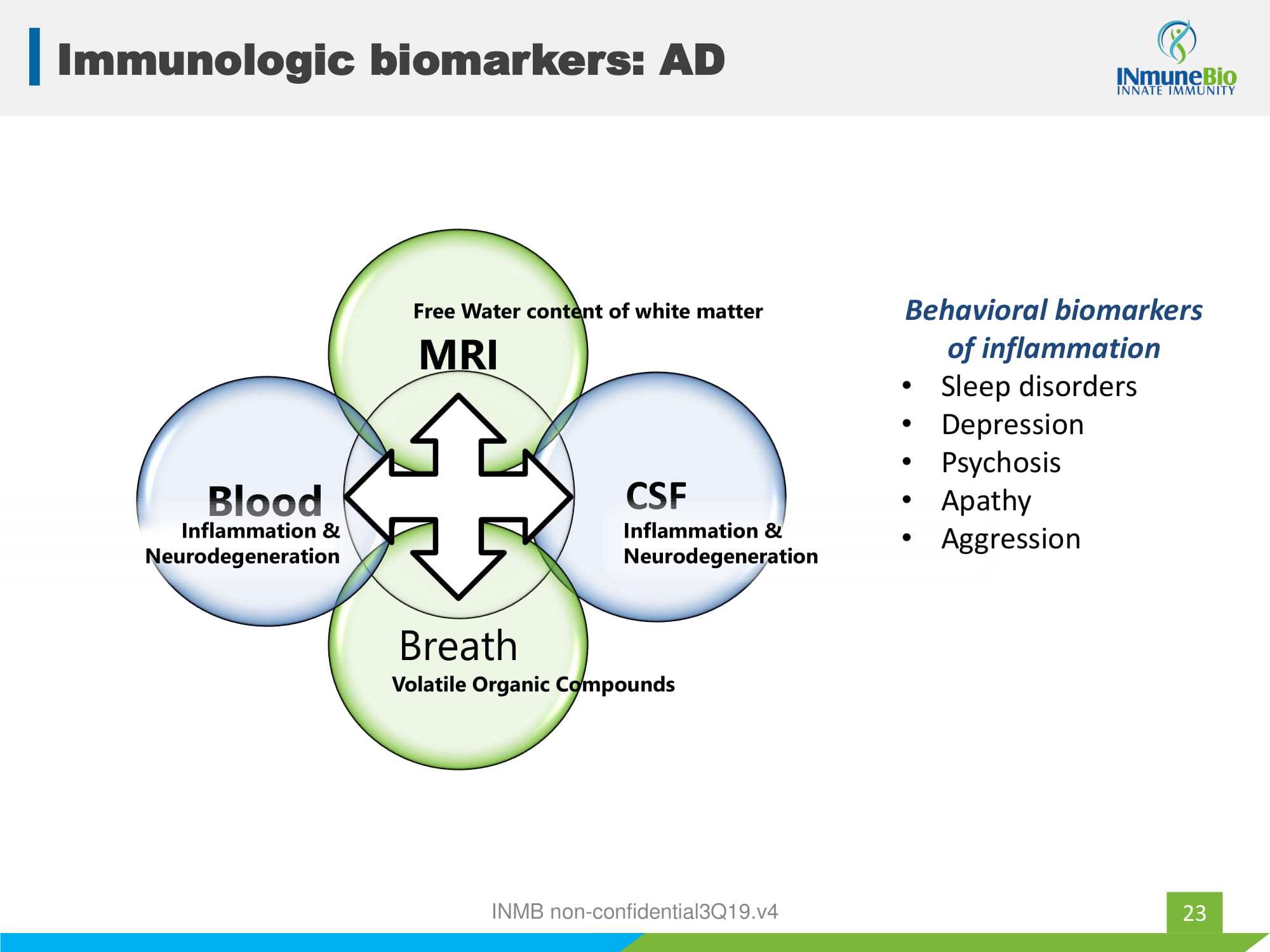
23 Immunologic biomarkers: AD MRI CSF Breath Blood Free Water content of white matter Volatile Organic Compounds Inflammation & Neurodegeneration Inflammation & Neurodegeneration Behavioral biomarkers of inflammation • Sleep disorders • Depression • Psychosis • Apathy • Aggression INMB non - confidential3Q19.v4

NeuLiv for NASH INMB non - confidential3Q19.v4

25 Three Principles of INMB NASH program 1. Fibrosis is a highly conserved wound healing response and represents the final common pathway of virtually all chronic inflammatory injuries Prof. John Iresdale 2013 2. Chronic inflammation in the liver is caused by innate immune cells 3. Targeting chronic inflammation should prevent progressive fibrosis and allow liver to heal INMB non - confidential3Q19.v4
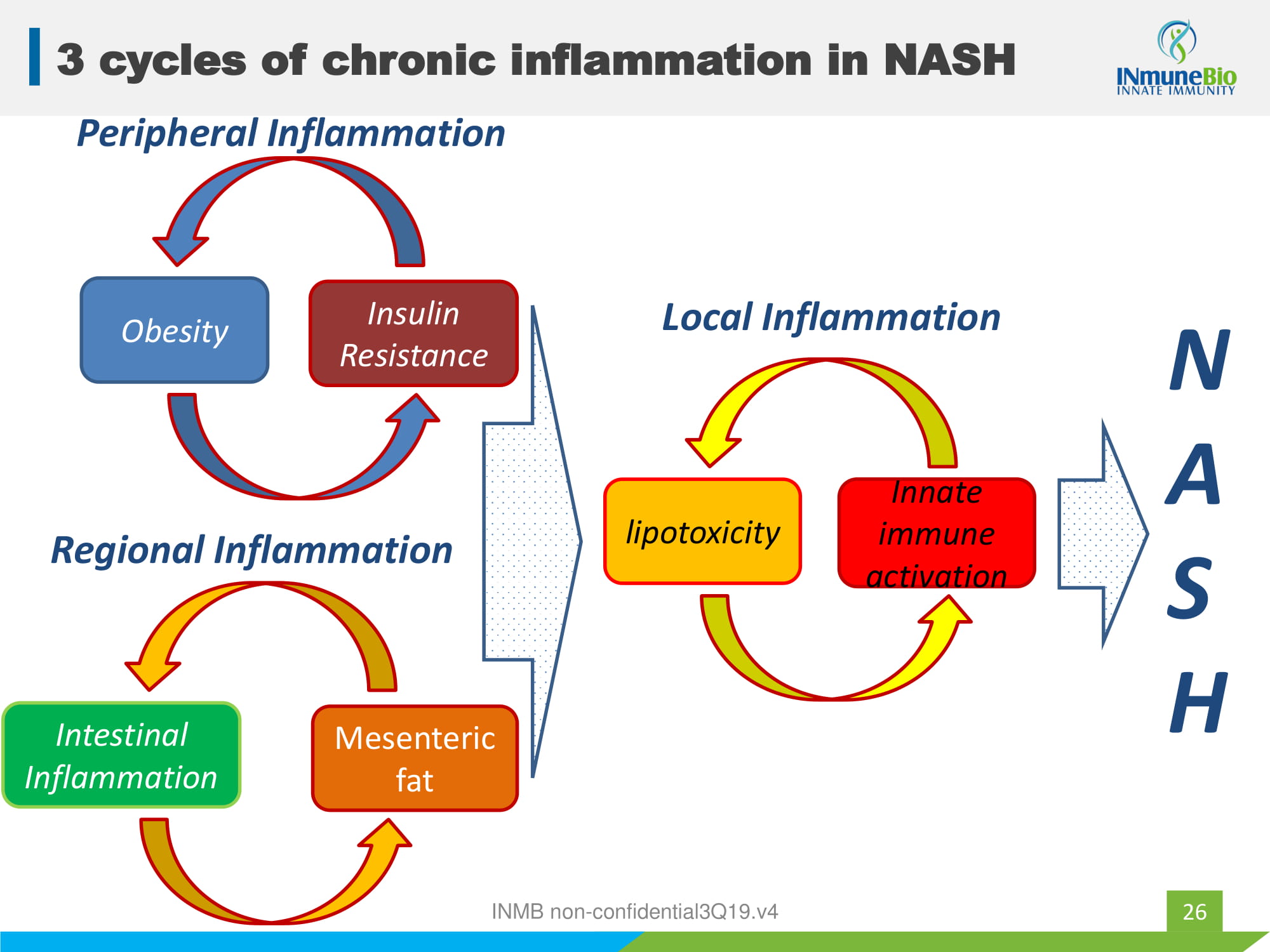
26 3 cycles of chronic inflammation in NASH Obesity Insulin Resistance Peripheral Inflammation Intestinal Inflammation Mesenteric fat Regional Inflammation lipotoxicity Innate immune activation Local Inflammation N A S H INMB non - confidential3Q19.v4
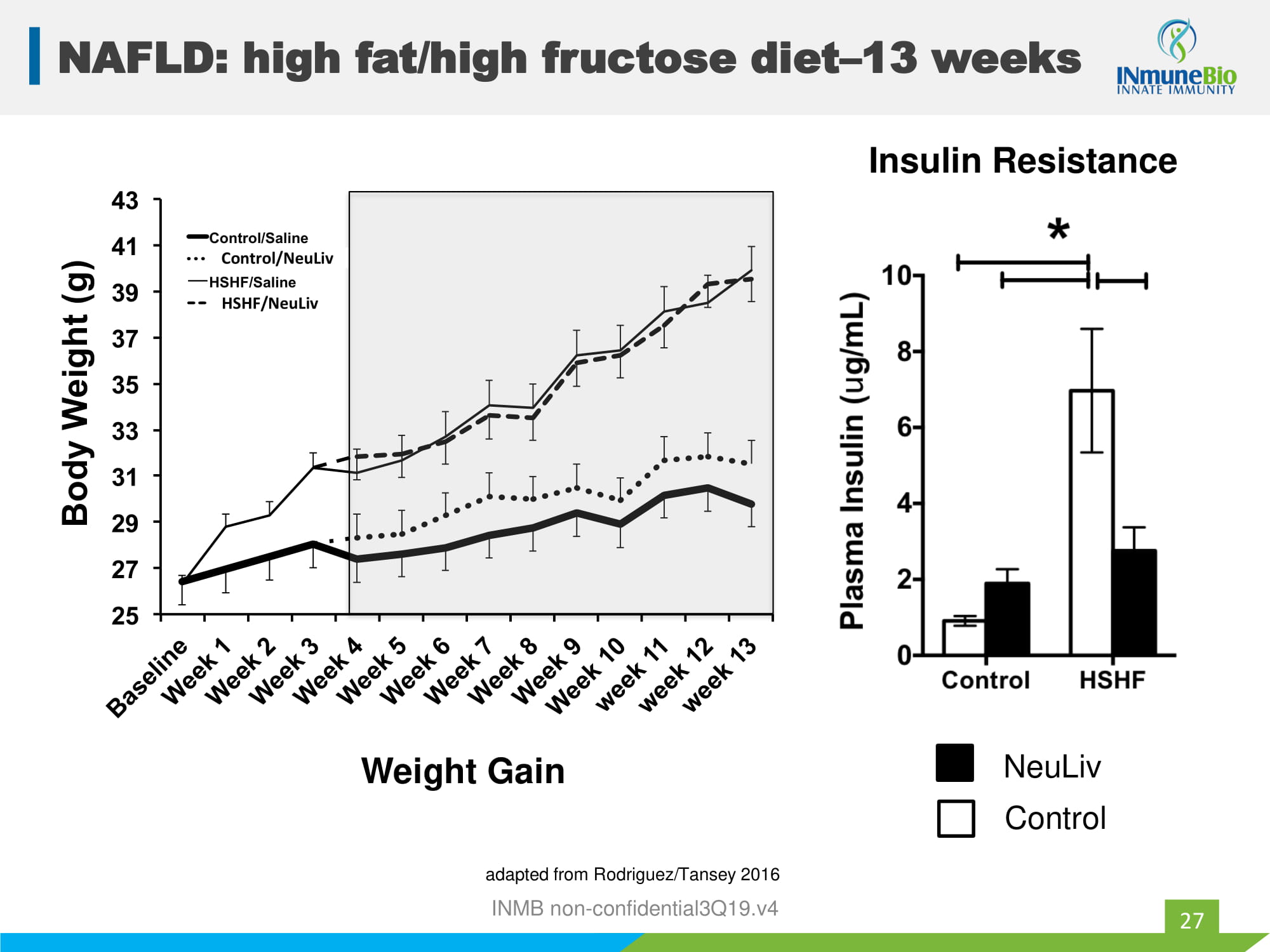
INMB non - confidential3Q19.v4 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 NeuLiv Control NAFLD: high fat/high fructose diet – 13 weeks 27 HSHF/NeuLiv Control/NeuLiv Weight Gain Insulin Resistance adapted from Rodriguez/Tansey 2016
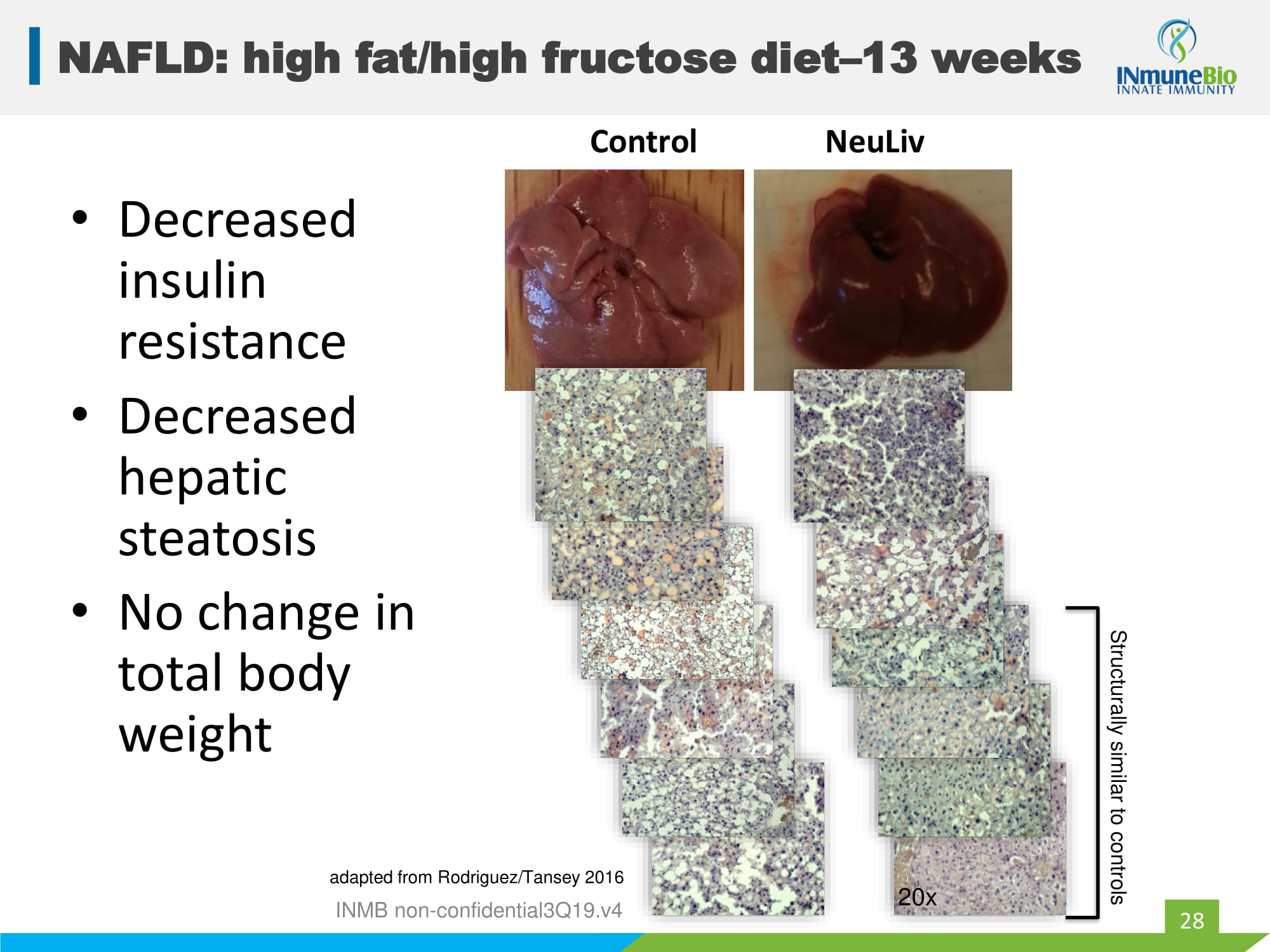
NAFLD: high fat/high fructose diet – 13 weeks 28 Structurally similar to controls 20x • Decreased insulin resistance • Decreased hepatic steatosis • No change in total body weight INMB non - confidential3Q19.v4 Control NeuLiv adapted from Rodriguez/Tansey 2016
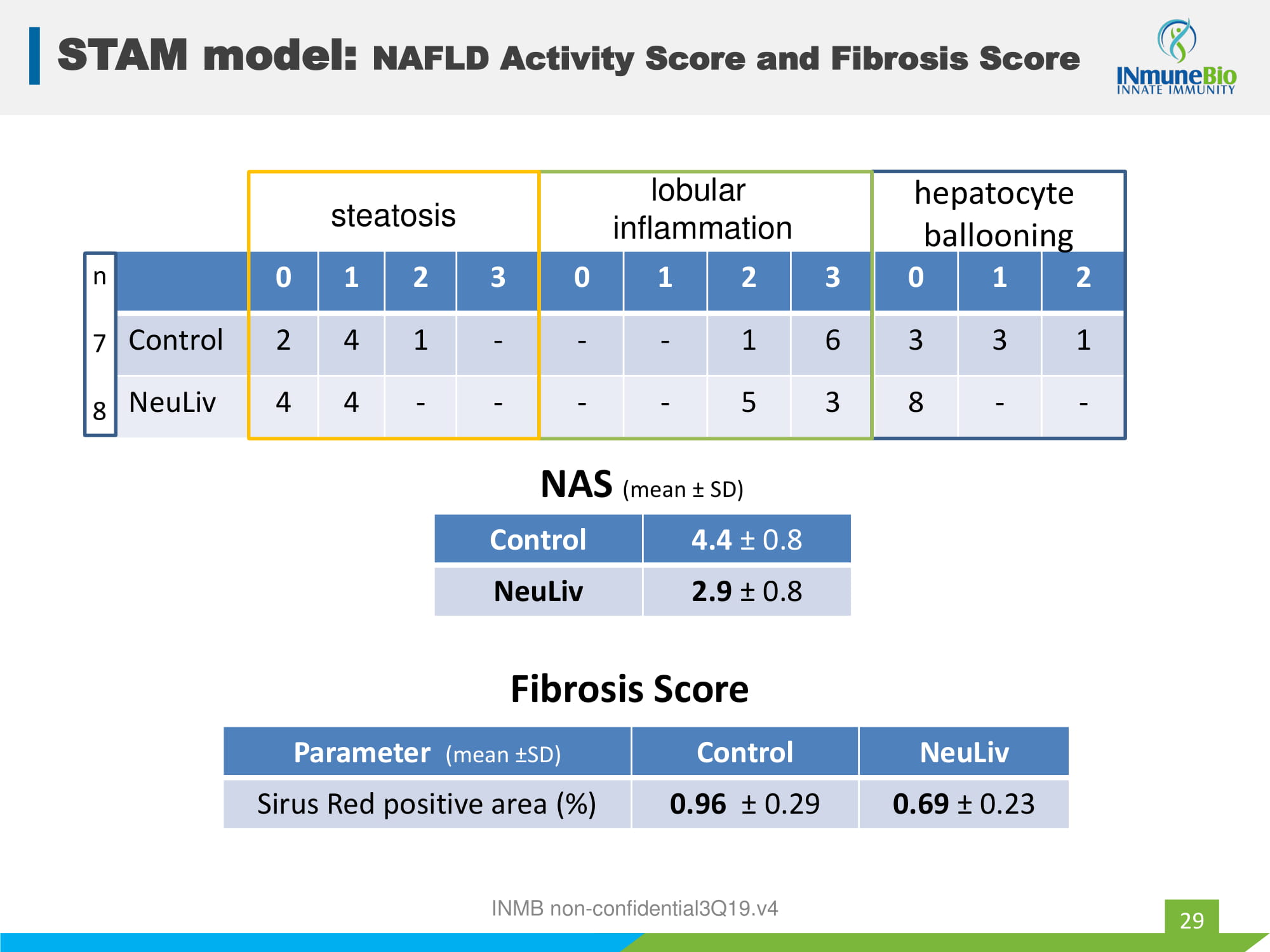
INMB non - confidential3Q19.v4 STAM model: NAFLD Activity Score and Fibrosis Score 29 0 1 2 3 0 1 2 3 0 1 2 Control 2 4 1 - - - 1 6 3 3 1 NeuLiv 4 4 - - - - 5 3 8 - - hepatocyte ballooning lobular inflammation steatosis Control 4.4 ц 0.8 NeuLiv 2.9 ц 0.8 NAS (mean ц SD) Parameter (mean ц SD) Control NeuLiv Sirus Red positive area (%) 0.96 ц 0.29 0.69 ц 0.23 Fibrosis Score n 7 8

INKmune for the treatment cancer INMB non - confidential3Q19.v4
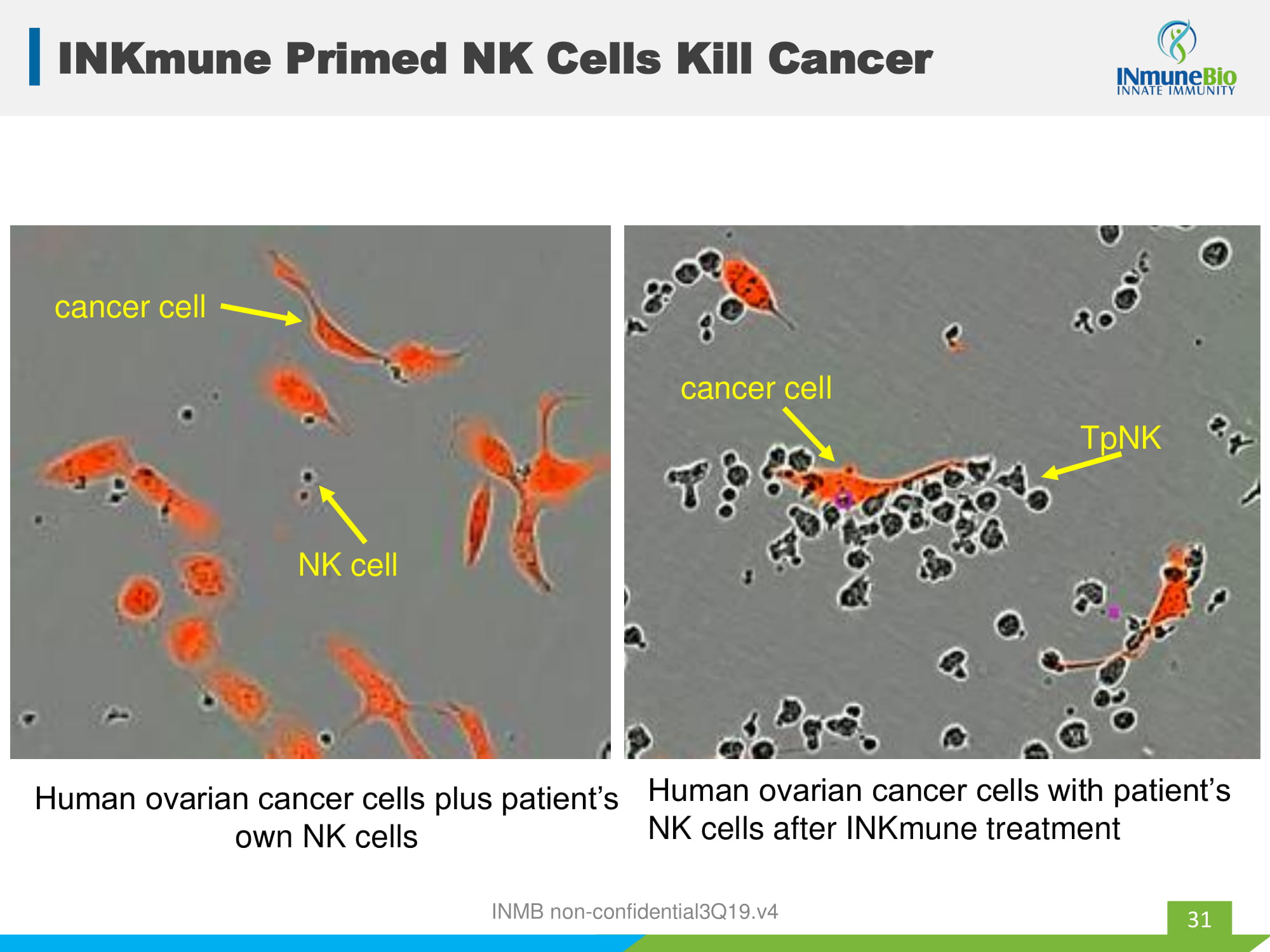
INKmune Primed NK Cells Kill Cancer 31 rN Human ovarian cancer cells plus patient’s own NK cells cancer cell NK cell Human ovarian cancer cells with patient’s NK cells after INKmune treatment TpNK cancer cell INMB non - confidential3Q19.v4
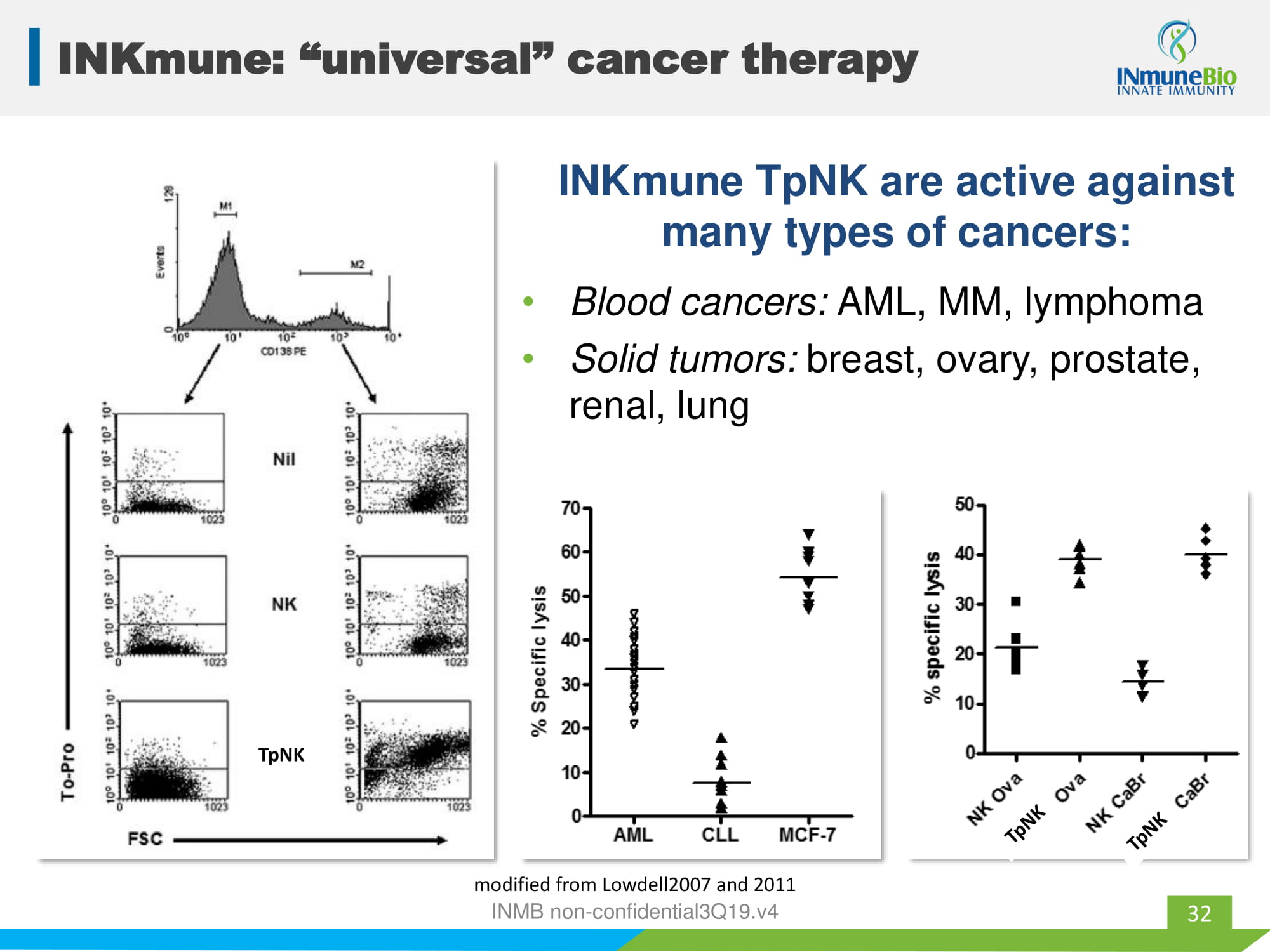
INKmune: “universal” cancer therapy INKmune TpNK are active against many types of cancers: • Blood cancers: AML, MM, lymphoma • Solid tumors: breast, ovary, prostate, renal, lung 32 TpNK modified from Lowdell2007 and 2011 INMB non - confidential3Q19.v4
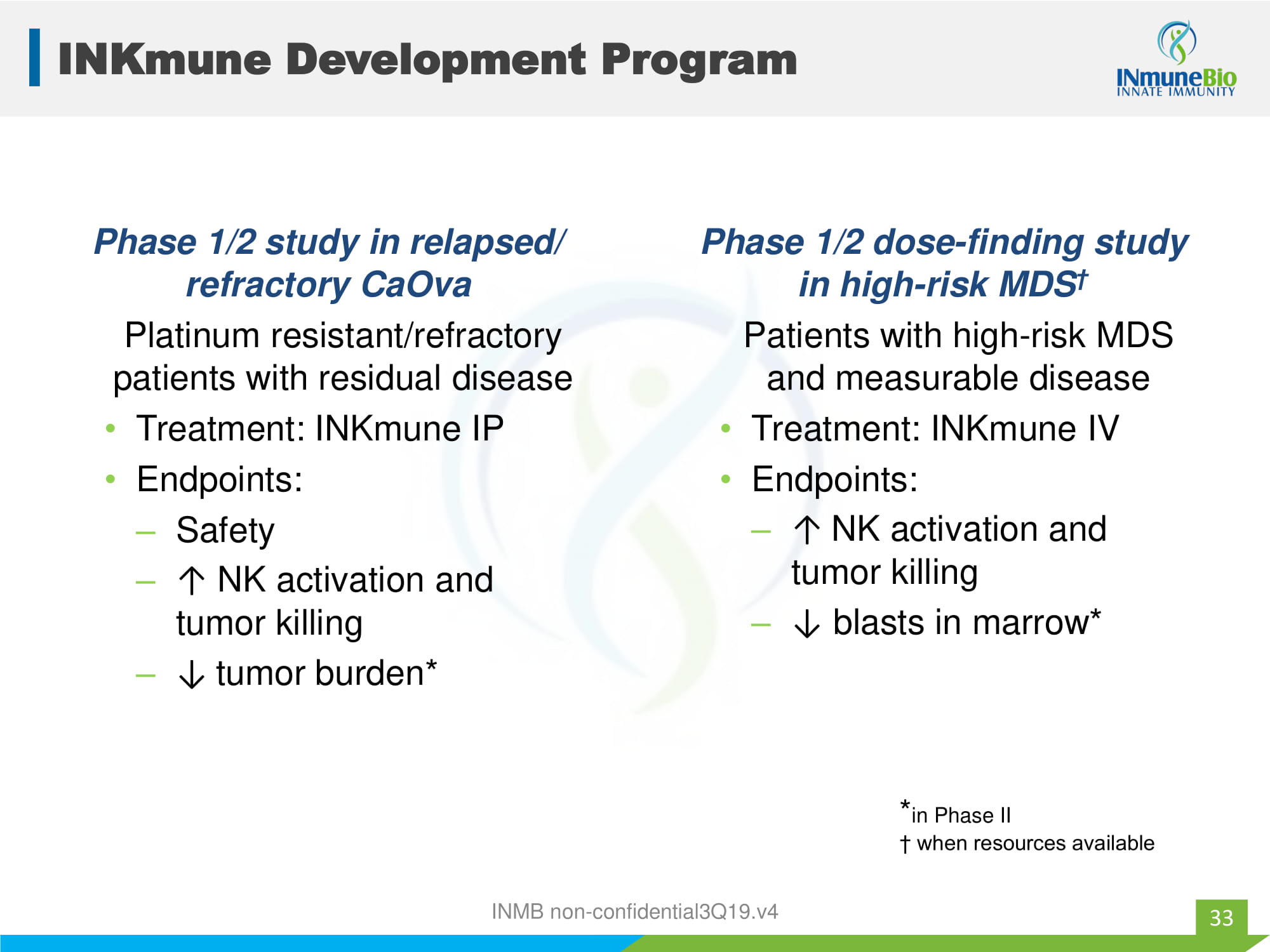
INKmune Development Program Phase 1/2 study in relapsed/ refractory CaOva Platinum resistant/refractory patients with residual disease • Treatment: INKmune IP • Endpoints: – Safety – ↑ NK activation and tumor killing – ↓ tumor burden* Phase 1/2 dose - finding study in high - risk MDS † Patients with high - risk MDS and measurable disease • Treatment: INKmune IV • Endpoints: – ↑ NK activation and tumor killing – ↓ blasts in marrow* 33 * in Phase II † when resources available INMB non - confidential3Q19.v4

INMB = Innate Immunity INMB non - confidential3Q19.v4

Milestones 35 February 1, 2019 – IPO closed Feb19 – Part the Cloud award – Alzheimer’ Assoc 3Q19 – initial data on INB03 Phase I 4Q19 – first patient enrolled INKmune cancer trial 1H20 – initiate INB03 combination Phase II cancer trial 3Q19 – first patient enrolled XPro1595 AD trial 1H20 – initial data from INKmune Phase I 2H20 – XPro1595 AD Phase II trial and INKmune Phase II cancer February 4, 2019 – NASDAQ Capital Markets listing 3Q19 – announce NeuLiv NASH INMB non - confidential3Q19.v4
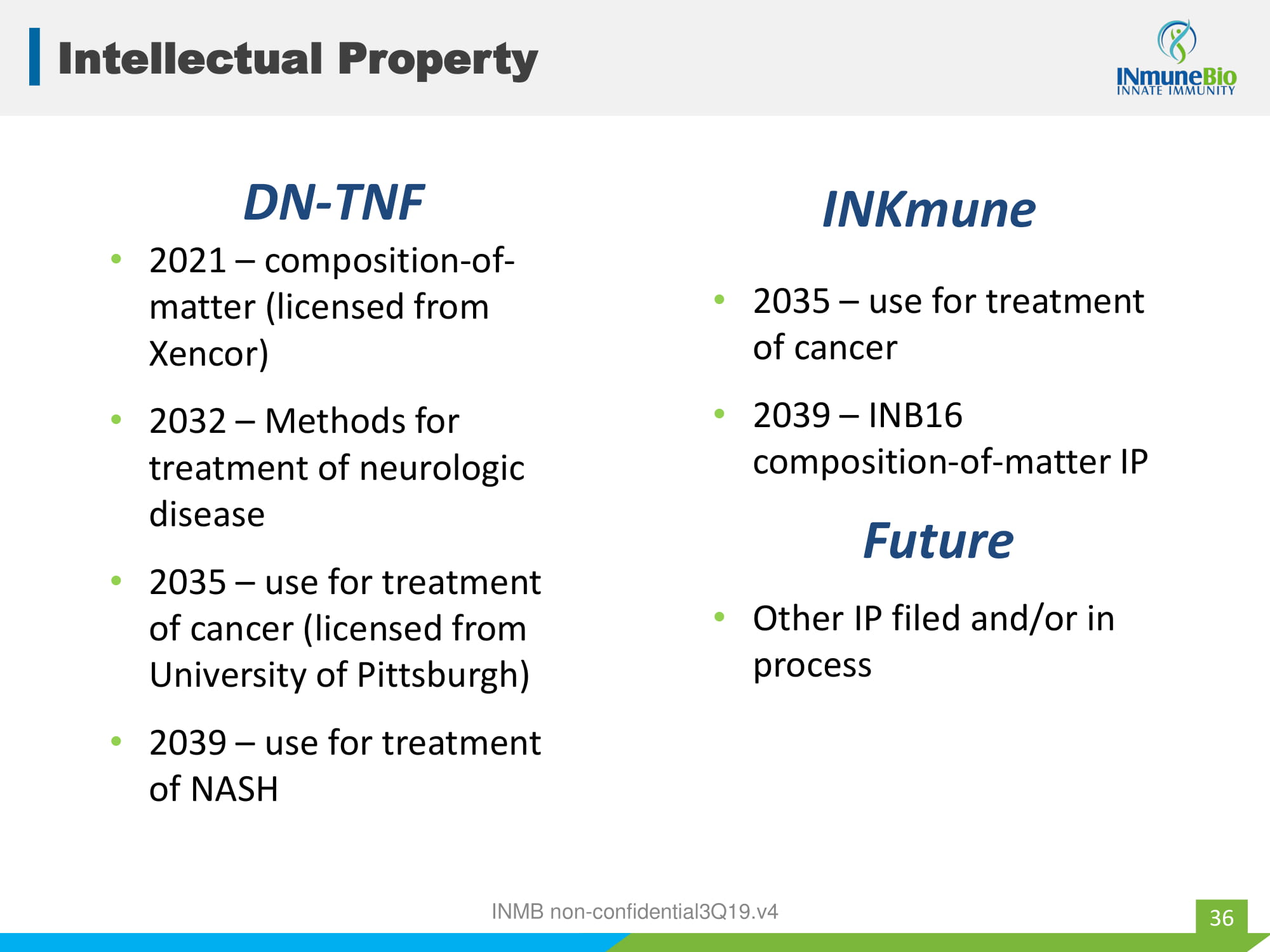
Intellectual Property • 2021 – composition - of - matter (licensed from Xencor) • 2032 – Methods for treatment of neurologic disease • 2035 – use for treatment of cancer (licensed from University of Pittsburgh) • 2039 – use for treatment of NASH DN - TNF INKmune 36 • 2035 – use for treatment of cancer • 2039 – INB16 composition - of - matter IP Future • Other IP filed and/or in process INMB non - confidential3Q19.v4

Experienced Leadership 37 RJ Tesi, MD Chief Executive Officer & Chief Medical Officer Mark Lowdell, PhD Chief Scientific Officer David Moss Chief Financial Officer Management Board of Directors J. Kelly Ganjei CEO of Cognate Timothy Schroeder CEO and Founder of CTI David Szymkowski, PhD VP of Cell Biology Scott Juda Founder and Managing Member, Fossick Capital Edgardo (Ed) Baracchini BD and licensing professional For complete biographies, visit www.inmunebio.com INMB non - confidential3Q19.v4
38 INMB: summary • Public clinical stage immunology company • Two product platforms with multiple clinical programs • Efficient use of capital and funding sources to maximize run - way • Opportunity to add clinical programs as resources become available INMB non - confidential3Q19.v4





































