Exhibit 99.1

HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM Modulating an Innate Immune Response Against Diseases IN MB INVESTOR PRESENTATION 2Q 20 V1

FORWARD LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . There is no guarantee that any specific outcome will be achieved . Investment results are speculative and there is a risk of loss, potentially all loss of investments . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . 2

▪ Immunology company, advancing through Phase I and Phase II clinical trials ▪ Two platforms with novel approaches to inflammation, neurodegenerative disease and oncology INB03™ & INKmune™ - Resistance to cancer immunotherapy by altering the TME and triggering NK cells XPro1595™ - Neuroinflammation as a cause of neurodegenerative disease LIVNate™ - Pleiotropic therapy for NASH ▪ 2020: Two Phase II programs, one Phase I readout and two Phase I program planned ▪ Over 65 publications from multiple universities worldwide on both platforms with extensive in vivo data ▪ Efficient use of capital including non - dilutive sources ▪ Experienced management team Dominant Negative Tumor Necrosis Factor (DN - TNF) PLATFORM NK Priming PLATFORM INVESTMENT SUMMARY Nasdaq INMB Cash (12/31/19) $7.0 Million Debt $0 Common Shares ~10.8 Million Inside Ownership ~50% Notable Shareholder Xencor (XNCR) Xencor Option $10m @ 100m Strike price for 10% of INMB Exp. ~ 4 years 3

MANAGEMENT TEAM Raymond J . Tesi, MD, CEO/CMO & Chairman of the Board Dr . Tesi has been our President, Chief Executive Officer and acting Chief Medical Officer since the formation of the Company in September 2015 . From November 2011 to May 2015 , Dr . Tesi was CEO, President and Acting Chief Medical Officer of FPRT Bio Inc . , a development - stage biotech company formed to develop XPro 1595 for the treatment of neurodegenerative disease and other inflammatory diseases . From November 2010 to October 2011 , Dr . Tesi was Chief Medical Officer of Adienne SRL, an emerging biotech company in Bergamo, Italy focused on products to treat patients with hematologic malignancy . From June 2007 to September 2010 , Dr . Tesi was CEO and President of Coronado Biosciences, a company he founded . Dr . Tesi received his MD degree from Washington University School of Medicine in 1982 . Dr . Tesi has been a licensed physician since 1982 and Fellow of the American College of Surgery since 1991 . David J . Moss, CFO Mr . Moss has been CFO since the formation of the Company in September 2015 . Mr . Moss has founded, funded and taken public various companies in a variety of industries since 1995 . Mr . Moss sits on the board of CareSpan International, Inc . and China Xiangtai Food Co . Ltd (Nasdaq : PLIN) and also serves as chair of their Audit Committee . Mr . Moss was a founding investor in Reliant Service Group LLC, which was acquired in 2015 by a leading private equity firm . Mr . Moss previously served as Managing Director, Corporate Finance for a New York - based securities firm, where he advised companies on corporate strategy, financings and business development . Prior to that, he served as Managing Partner at a Seattle - based venture capital firm . Mr . Moss holds an MBA from Rice University and a BA in Economics from the University of California, San Diego . Christopher J . Barnum, PhD, Director of Neuroscience Dr . Barnum is the Director of Neuroscience at INmune Bio, Inc . Dr . Barnum is a neuroimmunologist with broad expertise across neurodegenerative and psychiatric diseases holding multiple positions in academia and industry, including Emory University, FPRT Bio . , SonosBio, and most recently Takeda Pharmaceuticals . His focus has been on translating inflammatory therapies into clinical treatments for neurologic diseases using a biomarker - directed approach . Dr . Barnum has been working with XPro 1595 for more than a decade, first at Emory University with Dr . Malú Tansey and subsequently as a consultant for FPRT Bio . , Inc . and INmune Bio, before joining INmune Bio full time in 2018 . Dr . Barnum’s research has been supported by NIH, The Michael J Fox Foundation, and the Alzheimer’s Association . He received his PhD in Neuroscience from Binghamton University . Joshua S . Schoonover, Esq . , Assoc . General Counsel Mr . Schoonover is our in - house counsel, primarily focused on developing intellectual property rights for the Company, as well as managing various licenses and other agreements . Mr . Schoonover has over ten years of experience building and monetizing intellectual property portfolios . His experience also includes multiple transactions, including a recent nine - figure acquisition of a portfolio containing over 230 patent matters which Mr . Schoonover drafted and prosecuted . He received a JD from Western Sierra Law School and a BS in Chemical Physics from San Diego State University . Mr . Schoonover is a member of the State Bar of California and is licensed to practice before the United States Patent & Trademark Office, the Southern District of California and the 9 th Circuit Court of Appeals . The Phoenix Partners TEAM PEDEGREE Mark W . Lowdell, PhD, FRCPath, FRSB, CSO Prof . Lowdell has been a Chief Scientific Officer and Chief Manufacturing Officer since the formation of the Company in September 2015 . Prof . Lowdell is Professor of Cell and Tissue Therapy at University College London, where he has led a translational immunotherapy group since 1994 . Since February 2009 , Prof . Lowdell has also been Director of Cellular Therapy at the Royal Free London NHS Foundation Trust . He received his PhD in clinical immunology from London Hospital Medical College, University of London in 1992 and is a qualified immunopathologist . 4

NON - EMPLOYEE BOARD OF DIRECTORS David Szymkowski, PhD David E . Szymkowski leads the immunology group as Vice President of Cell Biology at Xencor Inc where he is focused on translational development of Fc - engineered and bispecific antibodies for the treatment of autoimmune diseases, allergic diseases, and cancer . Prior to joining Xencor in 2002 , Dr . Szymkowski was a principal scientist in the respiratory group at Roche Bioscience in Palo Alto, CA . With 25 years of big pharma and biotech R&D experience at Roche and at Xencor, Dr . Szymkowski has been instrumental in 10 IND submissions, coauthored over forty papers and reviews, is an inventor on over a dozen patents, and speaks frequently on the development of antibody therapeutics and other biologics . He received his B . A . at Johns Hopkins University and his Ph . D . in molecular and cell biology from Penn State, and completed a postdoc at the Imperial Cancer Research Fund (U . K . ) . J . Kelly Ganjei Mr . Ganjei has been a director since September 2016 . He is Chief Executive Officer of Cognate BioServices, Inc . , a position he has held since 2011 . Mr . Ganjei has over 20 years of experience within the life science, venture capital and IT sectors and has lead companies through various stages of development . Prior to joining Cognate, Mr . Ganjei was Principal at an SBA venture capital firm where he instrumental in supporting deal flow with a specific focus on regenerative medicine, immunotherapy and cell therapy investment opportunities .. He began his career at the National Institutes of Health . Mr . Ganjei has published numerous scientific, peer - reviewed papers and has been a speaker and presenter at various business forums . Mr . Ganjei received his B . S . in Microbiology from the University of Maryland College Park in 1995 . Timothy Schroeder Mr . Schroeder has been a director since December 2016 Mr . Schroeder has more than 35 years of clinical and academic industry experience in global drug and device development programs . He is CEO of CTI Clinical Trial and Consulting Services, a multi - national research firm with locations in North America, Europe, Latin America and Asia - Pacific . Prior to founding CTI, Mr . Schroeder was a faculty member of the University of Cincinnati College of Medicine . He previously served as Executive Vice President of Clinical Development at SangStat Medical Corporation, a firm he co - founded . Mr . Schroeder is a board member for more than a dozen corporate and non - profit organizations . He was named as EY Entrepreneur of the Year in 2015 and was recognized as Top Leader by the Enquirer Media in 2016 . Edgardo (Ed) Baracchini, PhD Mr . Eduardo (“Ed”) Bracchini has been a director since July 2019 . He is also current a member of the board of directors of 4 D Pharma PLC . Prior to providing biotech consulting services since September 2018 , Ed was chief business officer of Xencor, Inc . Ed was associated with Metabasis Therapeutics, initially as vice president of business development, and later as SVP of business development . Ed holds over 25 years of experience in structuring and negotiating research and development partnerships, mergers and acquisitions, and licensing agreements . He has personally, negotiated more than 80 business transactions with multinational and Asian pharmaceutical firms, biotechnology companies, and prominent universities, leading to transactions valued in excess of $ 5 . 3 billion .. Additionally, have been a key member of executive teams that have raised over $ 850 million in private and public financing, and that have successfully completed two IPOs . Ed received his MBA from the University of California, Irvine, his PhD in molecular and cell biology from the University of Texas at Dallas, and his B . S . in microbiology from the University of Notre Dame . BOARD PEDEGREE Marcia Allen Marcia Allen has been a director since November 2019 . Ms . Allen was a founder and served as CFO and Director of The Movie Group, (AMX) the originating company which is today Lionsgate Entertainment (NYSE) . She has more than 25 years with mergers and acquisitions, corporate finance and CFO and CEO experience . Ms . Allen was a Chief Financial Officer and Corporate Development Officer for W . R . Grace & Co . (NYSE) and was based both in Newport Beach, CA with the Restaurant Division and in its New York headquarters . Ms . Allen moved to California from Tennessee where she was part of the founding group of Ruby Tuesday, Inc . , (NYSE) a national restaurant chain . She relocated to join Taco Bell, Inc . as the Company's Chief Financial Officer where she structured and facilitated the acquisition of Taco Bell, Inc . by PepsiCo, Inc . She currently serves as the chairperson of the audit committee for Ark Restaurants Corp (Nasdaq) . Ms . Allen received a Bachelors, Finance and Accounting degree from the University of Tennessee . 5 Scott Juda, JD Mr . Juda has been a director since March 2018 . Mr . Juda is Manager and co - founder of Fossick Capital, a technology - focused hedge fund . Mr . Juda co - founded The Juda Group, Inc . , an institutional capital markets focused broker - dealer division of CCM, where he served as Chief Executive Officer from 2012 to 2016 . Before that, Mr . Juda was at SMH Capital from 2002 to 2011 , serving as a Managing Director in the Investment Banking Group and Chief Operating Officer of The Juda Group subsidiary . From 2000 to 2002 , Mr . Juda was an institutional sales - trader for Sutro & Co . From 1997 to 2000 , Mr . Juda practiced corporate and securities law at Buchalter Nemer LLP . Mr . Juda received his bachelor degree from the University of Southern California and his juris doctor from the University of Pepperdine School Of Law . Mr . Juda is a member of the State Bar of California .
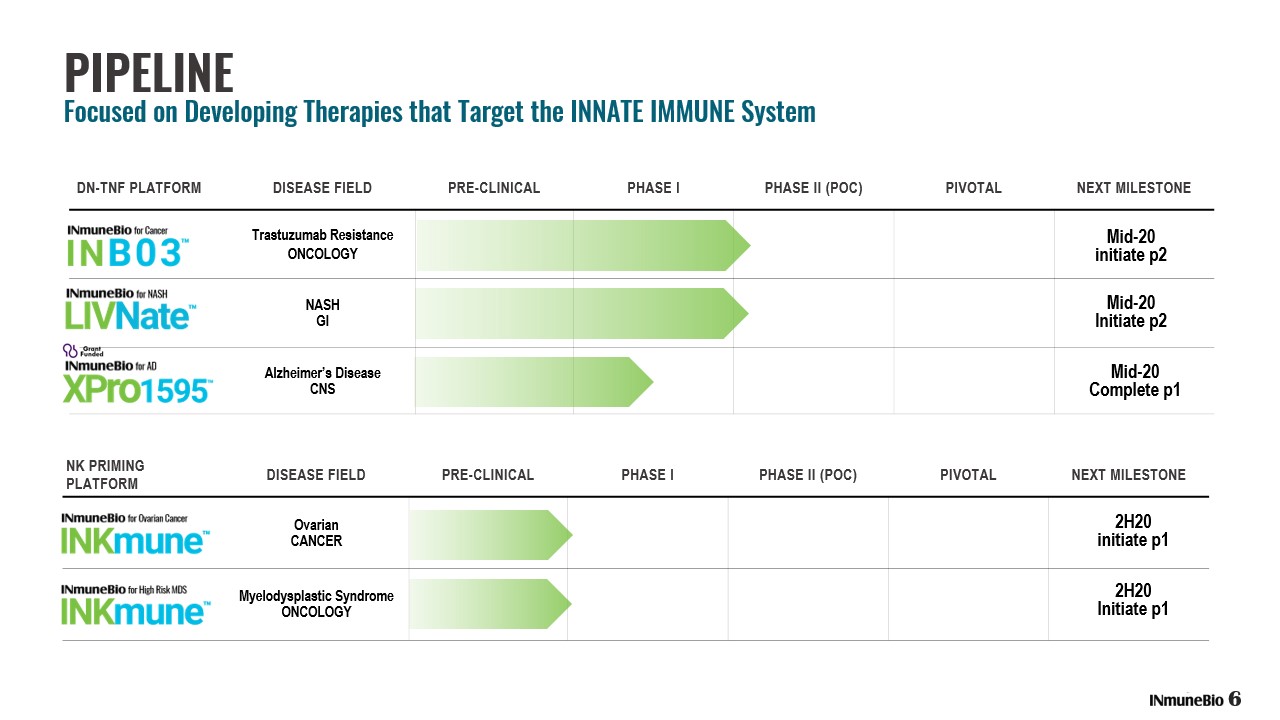
DN - TNF PLATFORM DISEASE FIELD PRE - CLINICAL PHASE I PHASE II (POC) PIVOTAL NEXT MILESTONE Trastuzumab Resistance ONCOLOGY NASH GI Alzheimer’s Disease CNS Mid - 20 Initiate p2 Mid - 20 initiate p2 NK PRIMING PLATFORM DISEASE FIELD PRE - CLINICAL PHASE I PHASE II (POC) PIVOTAL NEXT MILESTONE Ovarian CANCER Myelodysplastic Syndrome ONCOLOGY 2H20 Initiate p1 2H20 initiate p1 PIPELINE Focused on Developing Therapies that Target the INNATE IMMUNE System 6 Mid - 20 Complete p1

A SELECTIVE, SAFER, NEXT GENERATION TNF INHIBITOR TARGETING MARKETS EXISTING TNF INHIBITORS MUST AVOID DN - TNF PLATFORM TECNOLOGY Pro - inflammatory cytokines are positively associated with age. XPro1595™ can be used in markets such as cancer and neurodegenerative diseases where existing TNF inhibitors are prohibited because of their side effects of immunosuppression. Parker et al. 2008 7
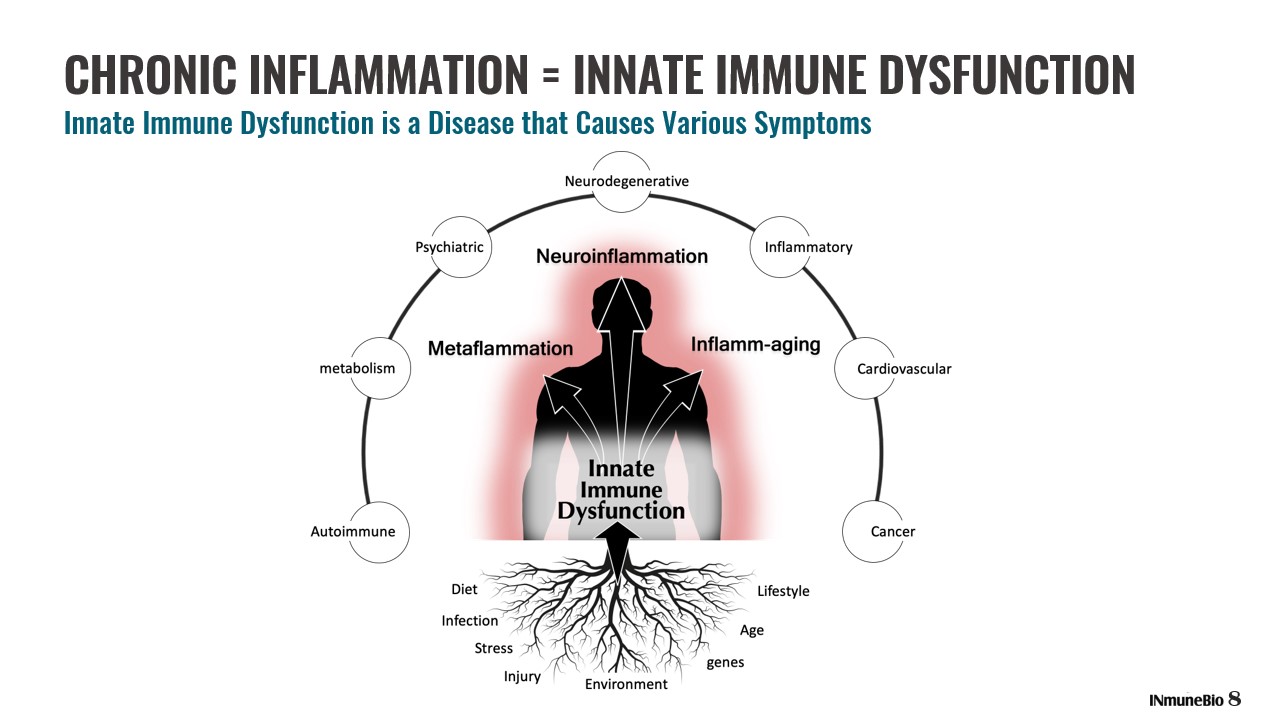
CHRONIC INFLAMMATION = INNATE IMMUNE DYSFUNCTION Innate Immune Dysfunction is a Disease that Causes Various Symptoms 8
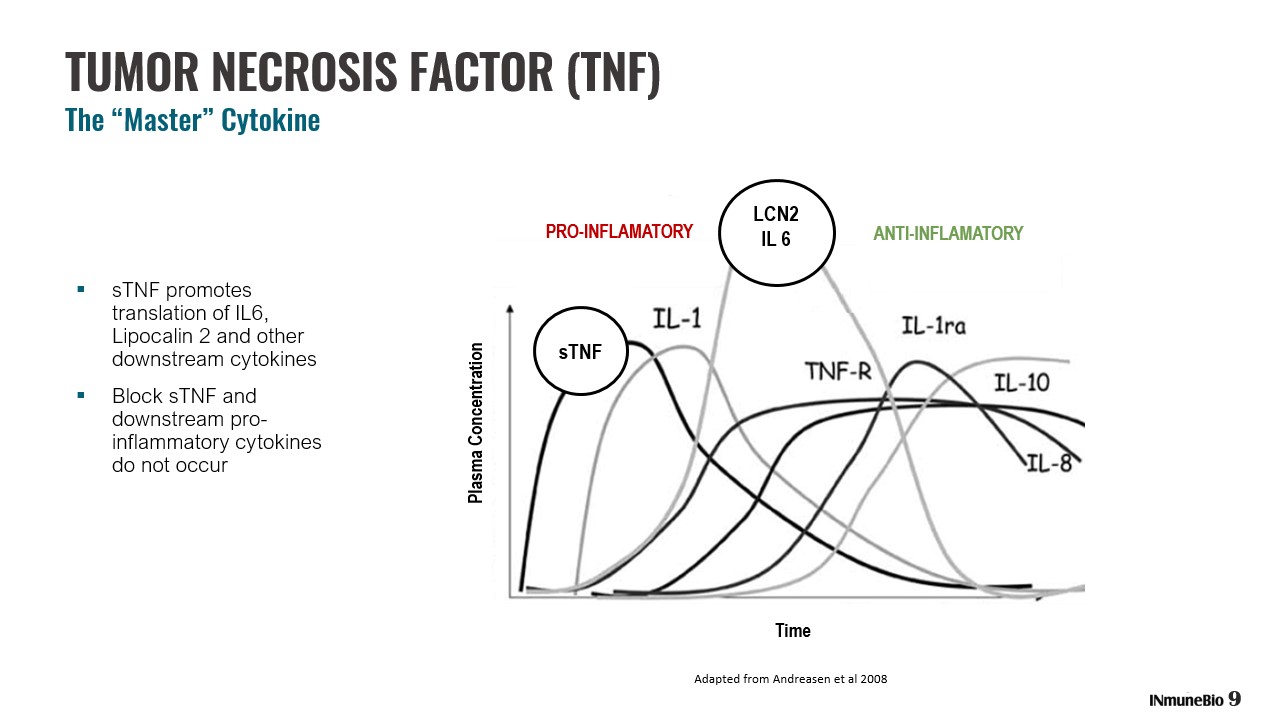
TUMOR NECROSIS FACTOR (TNF) The “Master” Cytokine ▪ sTNF promotes translation of IL6, Lipocalin 2 and other downstream cytokines ▪ Block sTNF and downstream pro - inflammatory cytokines do not occur LCN2 IL 6 ANTI - INFLAMATORY Time Plasma Concentration sTNF 9 PRO - INFLAMATORY Adapted from Andreasen et al 2008
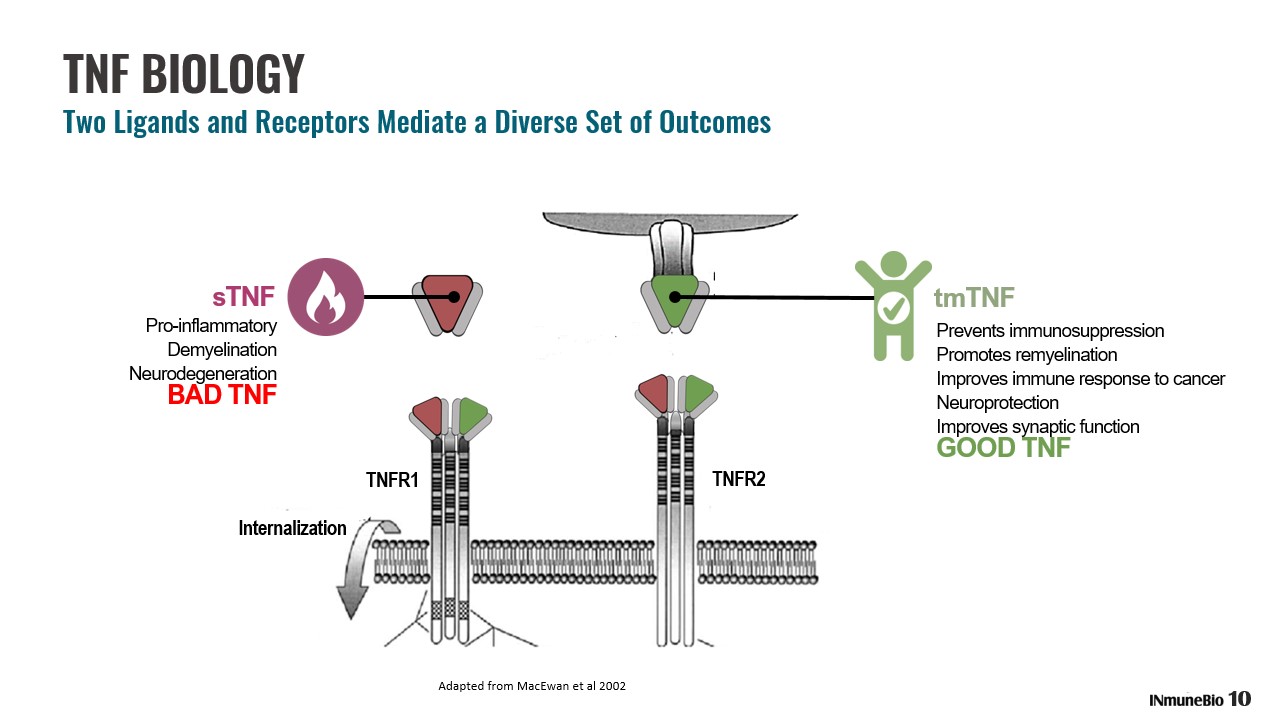
TNF BIOLOGY Two Ligands and Receptors Mediate a Diverse Set of Outcomes tmTNF sTNF Pro - inflammatory Demyelination Neurodegeneration BAD TNF Prevents immunosuppression Promotes rem yelination Improves immune response to cancer Neuroprotection Improves synaptic function GOOD TNF 10 TNFR1 TNFR2 Internalization Adapted from MacEwan et al 2002

Negative effects of TNF are a result of sTNF WHEREAS tmTNF PROVIDES IMMUNOSUPPORTIVE AND NEUROPROTECTIVE FUNCTION IMPORTANT TO HOST SURVIVAL THE BIOLOGY OF CURRENT INHIBITORS Current Inhibitors Are Not Selective and Block Both Forms of TNF Transmembrane - bound (‘tmTNF’) GOOD Soluble TNF (‘sTNF’) BAD TNFR1 TNFR2 Internalization 11 X X X Adapted from MacEwan et al 2002
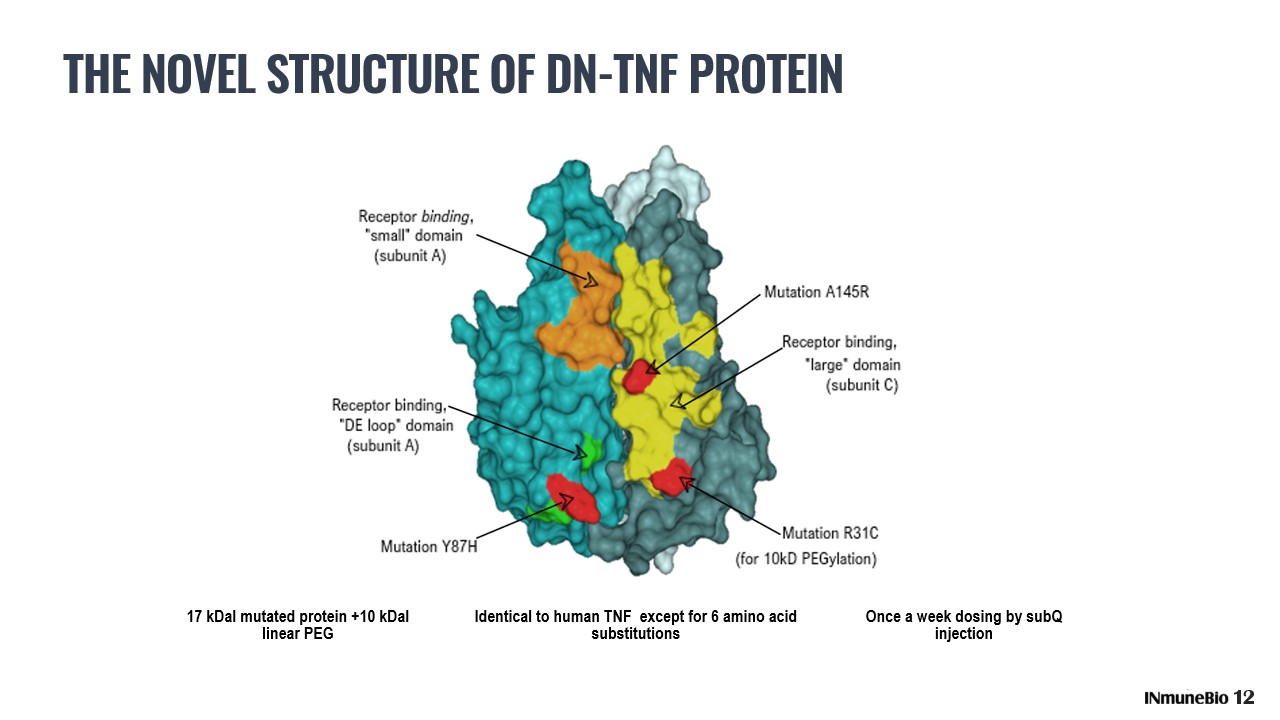
THE NOVEL STRUCTURE OF DN - TNF PROTEIN 17 kDal mutated protein +10 kDal linear PEG Identical to human TNF except for 6 amino acid substitutions Once a week dosing by subQ injection 12

Decreases inflammation Y Y Increased risk of infection Y N Increased risk of cancer Y N Causes demyelination Y N Neuroprotective N Y Enhances neuroplasticity N Y DIFFERENCES: Selective vs. Non - Selective TNF Inhibition Non - Selective DN TNF TNF BIOLOGY. HOW DN - TNF WORKS DN - TNF Neutralizes ONLY Soluble TNF and Leaves Transmembrane TNF and the Receptors Functional TNFR1 “BAD” s TNF with DN - TNF does not bind to receptors Only “GOOD” tmTNF continues to bind to TNFR1 and TNFR2 Internalization TNFR1 TNFR2 13 Adapted from MacEwan et al 2002
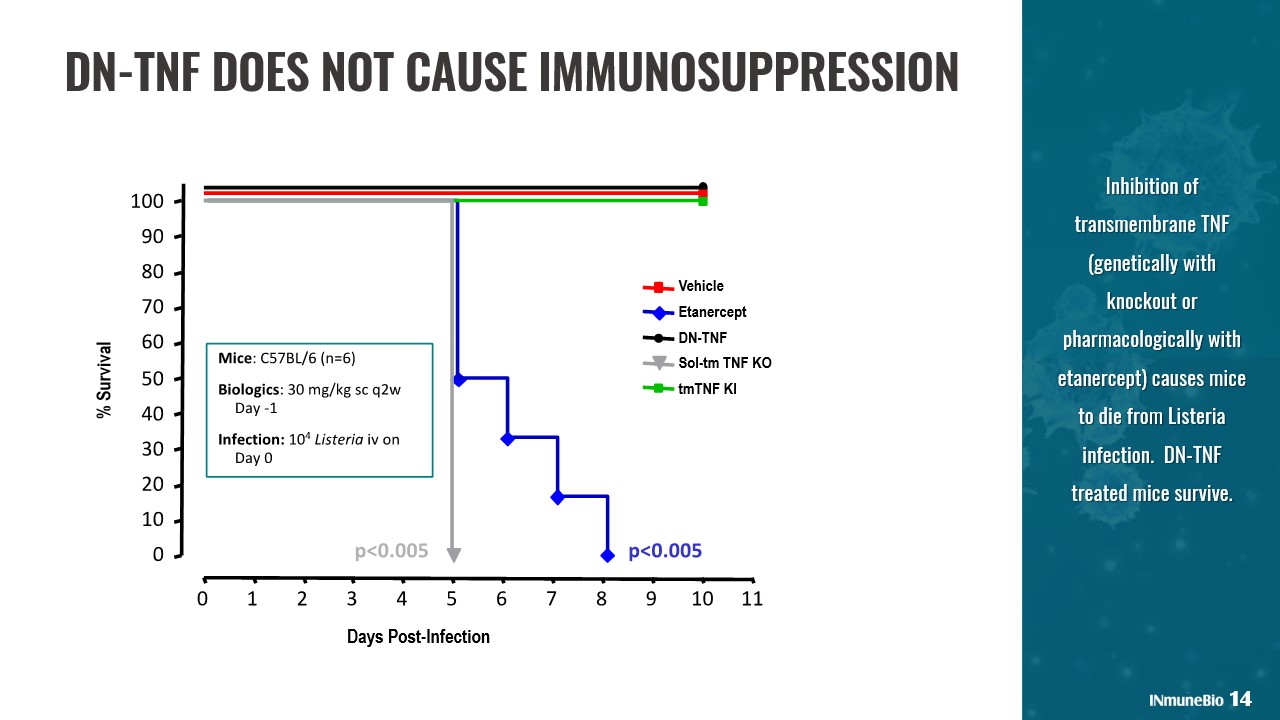
DN - TNF DOES NOT CAUSE IMMUNOSUPPRESSION Inhibition of transmembrane TNF (genetically with knockout or pharmacologically with etanercept) causes mice to die from Listeria infection. DN - TNF treated mice survive. 14 Vehicle Etanercept DN - TNF Sol - tm TNF KO tmTNF KI Days Post - Infection % Survival

SELECTIVE TARGETING OF TNF PREVENTS DEMYLINATION Non - selective TNF Inhibition Causes Demyelination, DN - TNF Promotes Remyelination EXACERBATED DEMYELINATION REMYELINATION ETANERCEPT Anti - inflammatory AND immunosuppressive DN - TNF Anti - inflammatory NOT immunosuppressive CUPRIZONE Model of Multiple Sclerosis NORMAL Cuprizone model of demyelination in mice: Prober 2017 https://inmunebio.com/index.php/en/science/xpro1595/references 15

DN - TNF Options in Immuno - Oncology for Treatment of Resistance to Immunotherapies 16
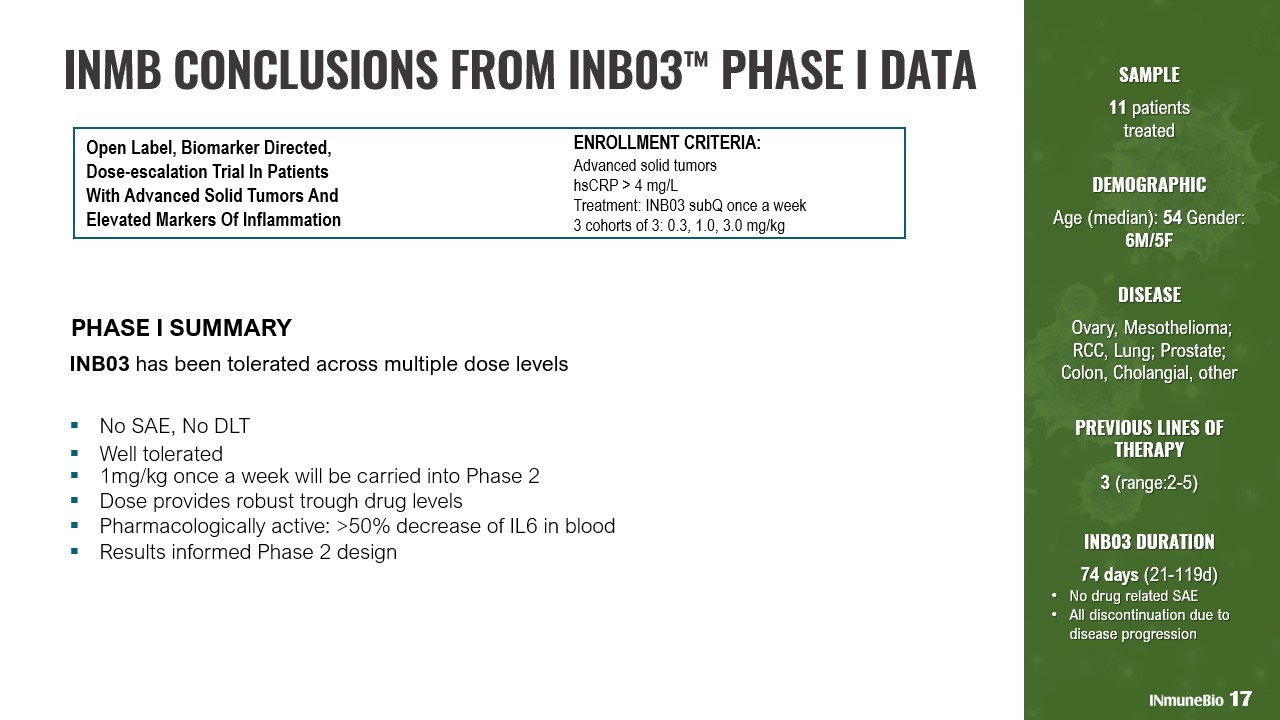
SAMPLE 11 patients treated DEMOGRAPHIC Age (median): 54 Gender: 6M/5F DISEASE Ovary, Mesothelioma; RCC, Lung; Prostate; Colon, Cholangial, other PREVIOUS LINES OF THERAPY 3 (range:2 - 5) INB03 DURATION 74 days (21 - 119d) • No drug related SAE • All discontinuation due to disease progression PHASE I SUMMARY INB03 has been tolerated across multiple dose levels ▪ No SAE, No DLT ▪ Well tolerated ▪ 1mg/kg once a week will be carried into Phase 2 ▪ Dose provides robust trough drug levels ▪ Pharmacologically active: >50% decrease of IL6 in blood ▪ Results informed Phase 2 design Open Label, Biomarker Directed, Dose - escalation Trial In Patients With Advanced Solid Tumors And Elevated Markers Of Inflammation ENROLLMENT CRITERIA: Advanced solid tumors hsCRP > 4 mg/L Treatment: INB03 subQ once a week 3 cohorts of 3: 0.3, 1.0, 3.0 mg/kg 17 INMB CONCLUSIONS FROM INB03 ™ PHASE I DATA
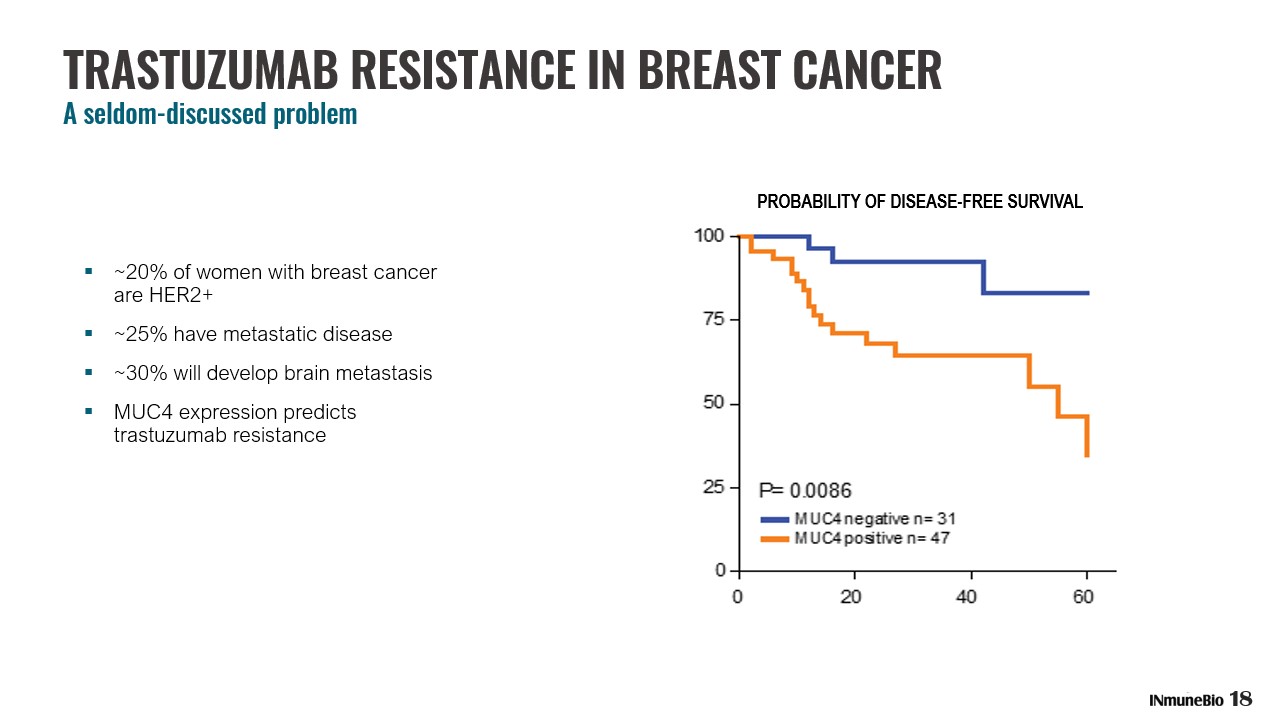
▪ ~20% of women with breast cancer are HER2+ ▪ ~25% have metastatic disease ▪ ~30% will develop brain metastasis ▪ MUC4 expression predicts trastuzumab resistance PROBABILITY OF DISEASE - FREE SURVIVAL 18 TRASTUZUMAB RESISTANCE IN BREAST CANCER A seldom - discussed problem
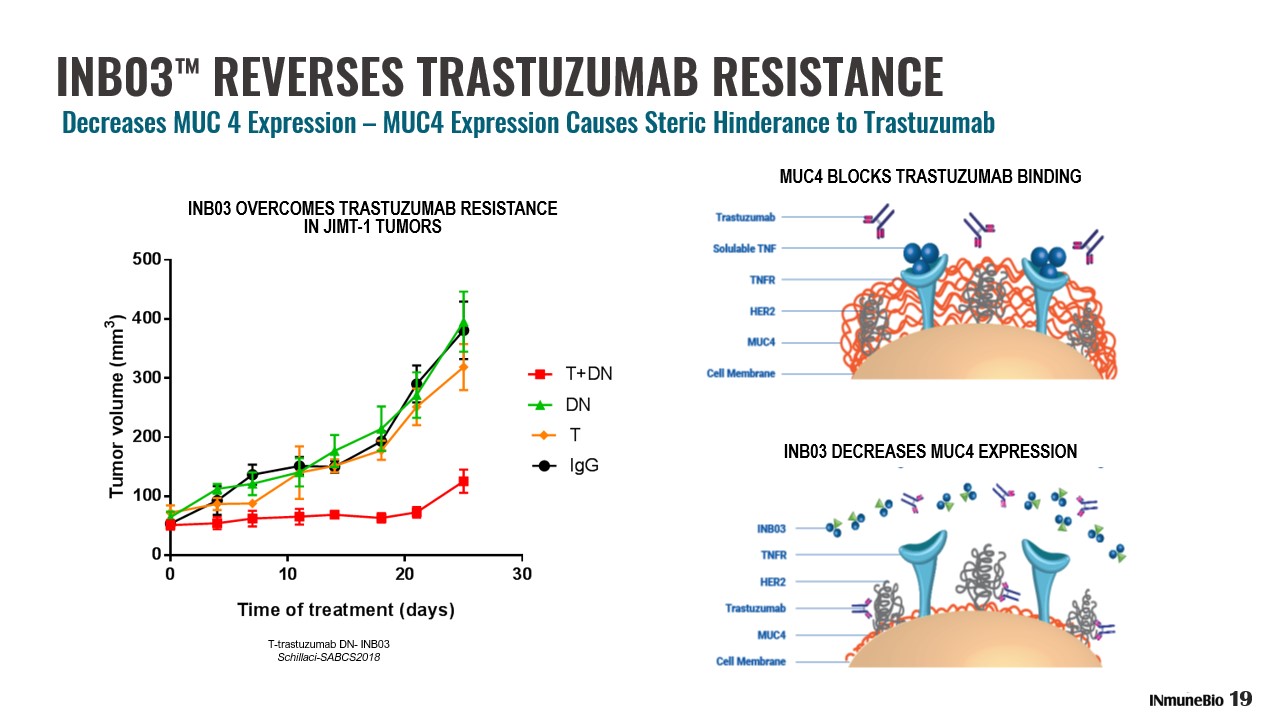
INB03 ™ REVERSES TRASTUZUMAB RESISTANCE Decreases MUC 4 Expression – MUC4 Expression Causes Steric Hinderance to Trastuzumab INB03 OVERCOMES TRASTUZUMAB RESISTANCE IN JIMT - 1 TUMORS T - trastuzumab DN - INB03 Schillaci - SABCS2018 MUC4 BLOCKS TRASTUZUMAB BINDING INB03 DECREASES MUC4 EXPRESSION 19
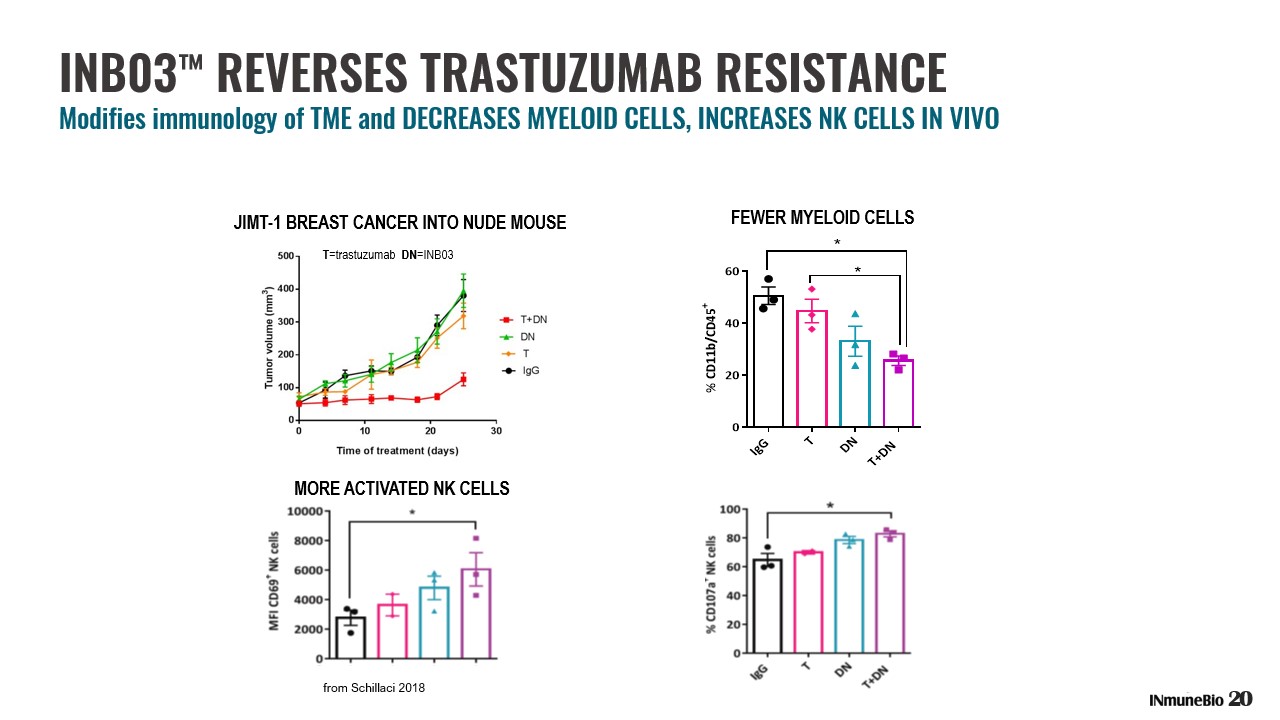
INB03 ™ REVERSES TRASTUZUMAB RESISTANCE Modifies immunology of TME and DECREASES MYELOID CELLS, INCREASES NK CELLS IN VIVO I g G T D N T + D N 0 20 40 60 % C D 1 1 b / C D 4 5 + * * FEWER MYELOID CELLS from Schillaci 2018 T =trastuzumab DN =INB03 JIMT - 1 BREAST CANCER INTO NUDE MOUSE MORE ACTIVATED NK CELLS 20

The Problem: HER2+ BC no effective therapy for brain metastasis The Goal : make “cold” tumors “hot!” ▪ Treatment with INB03/Drug X ▪ Both drugs cross BBB ▪ 12 patients ▪ Composite end - point ▪ If positive, petition FDA for Break - Through Designation ▪ If positive, consider adding CPI 21 INB03 ™ PHASE II CANCER Modifying the TME To Reverse Resistance To Immunotherapy
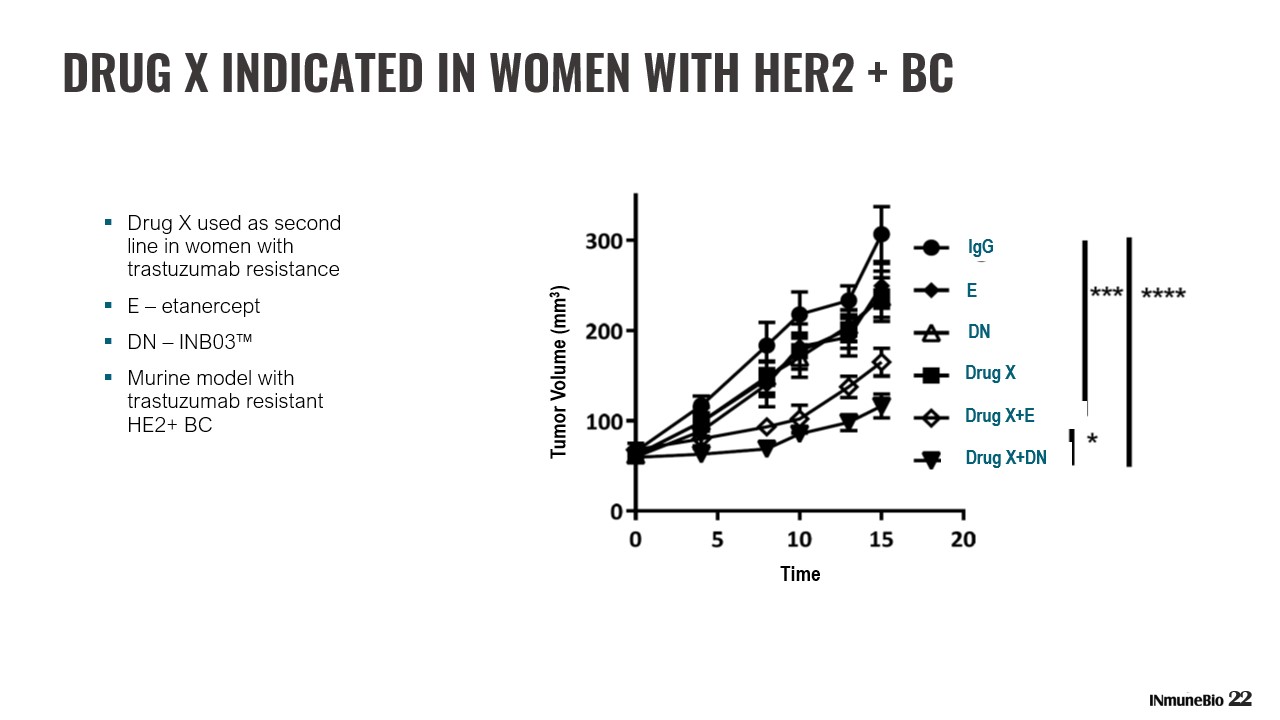
Drug X+DN Drug X+E Drug X ▪ Drug X used as second line in women with trastuzumab resistance ▪ E – etanercept ▪ DN – INB03™ ▪ Murine model with trastuzumab resistant HE2+ BC 22 DRUG X INDICATED IN WOMEN WITH HER2 + BC DN E IgG Time Tumor Volume (mm 3 )

Chronic TNF Therapy Can Reduce Incidence of Alzheimer Disease (AD) 23

MULTIPLE SCLEROSIS ALZHEIMER’S DISEASE PARKINSON’S DISEASE Synaptic Dysfunction Nerve Cell Death Parkinson’s Affected Neuron Damaged Myelin NUMBER OF DN - TNF PUBLICATIONS FOR EACH DISEASE DEMONSTRATING DISEASE - MODIFYING ACTIVITY 6 5 5 24 CENTRAL ROLE OF INFLAMMATION IN NEURODEGENERATION Inflammation is Associated with Many Neurological Diseases
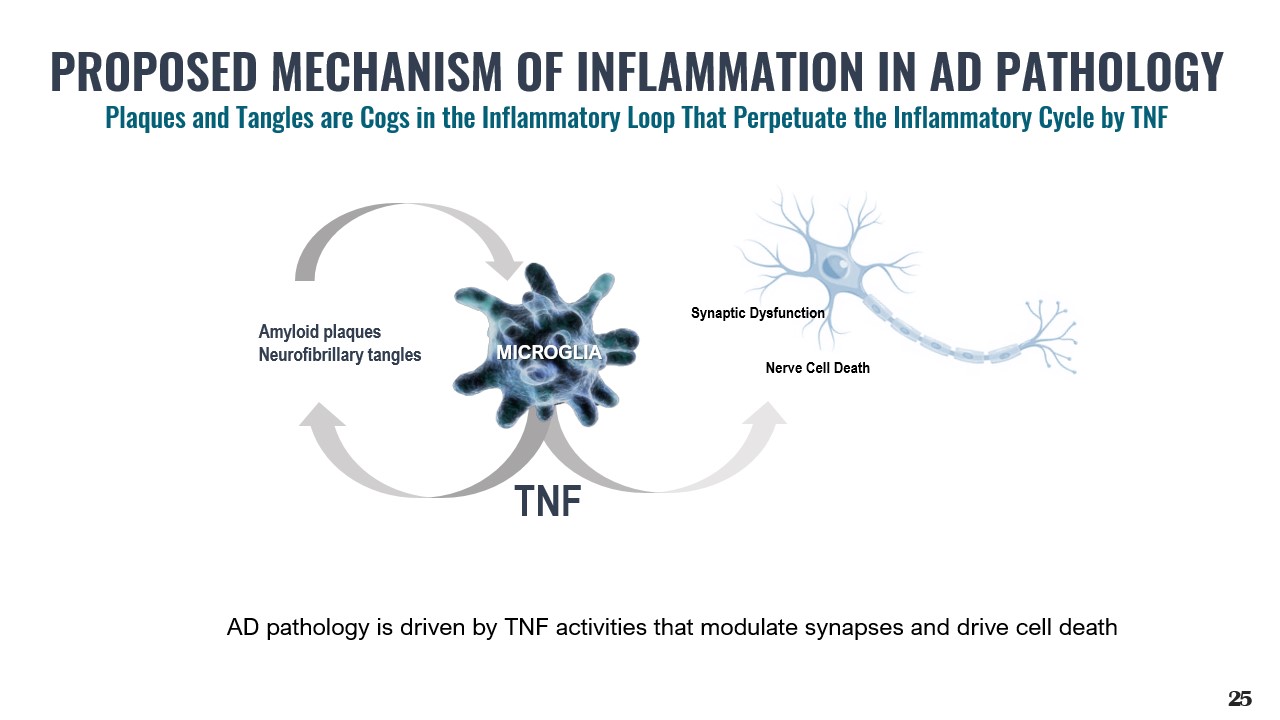
TNF Amyloid plaques Neurofibrillary tangles MICROGLIA Synaptic Dysfunction Nerve Cell Death AD pathology is driven by TNF activities that modulate synapses and drive cell death 25 PROPOSED MECHANISM OF INFLAMMATION IN AD PATHOLOGY Plaques and Tangles are Cogs in the Inflammatory Loop That Perpetuate the Inflammatory Cycle by TNF
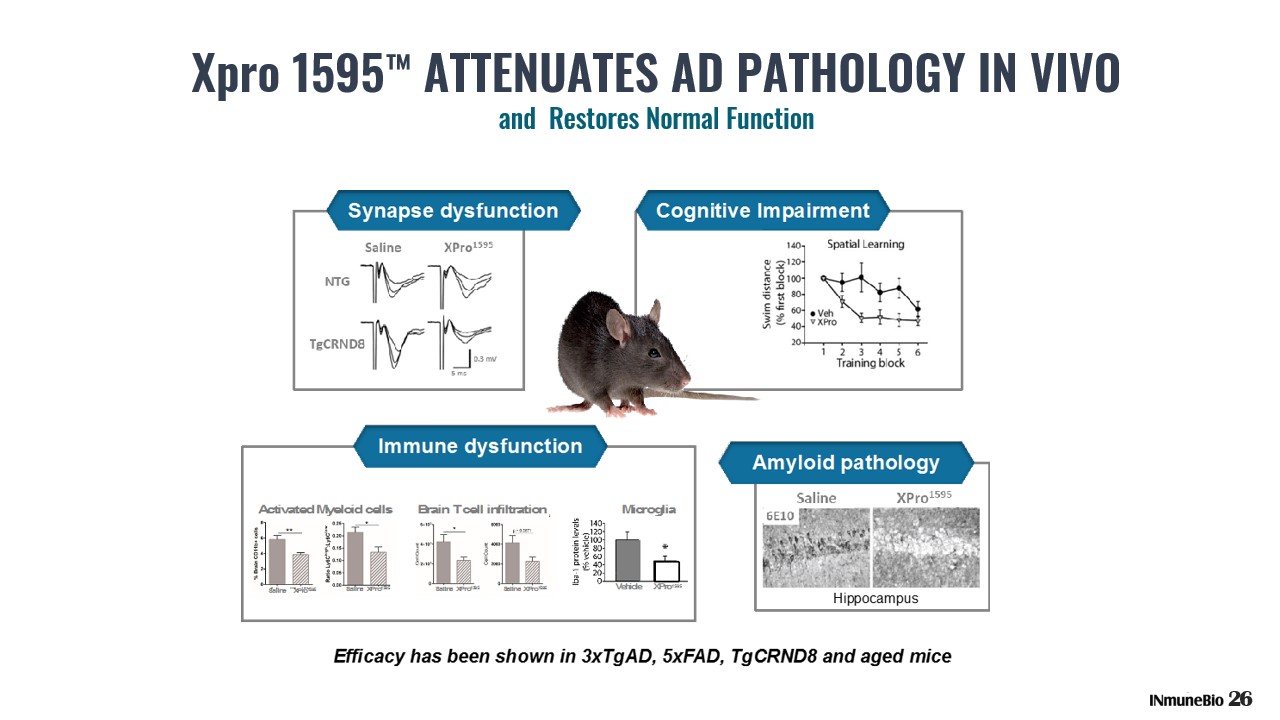
26 Xpro 1595 ™ ATTENUATES AD PATHOLOGY IN VIVO and Restores Normal Function

RISK OF ALZHEIMER’S DISEASE Risk of general population Adapted from Chou et al. 2016 8.5M claims ANTI - TNFS PROTECT AGAINST AD & DEMENTIA Adapted from Zhou et al. 2016 56M claims Despite positive AD activity, non - selective TNF inhibitors increase the risk of cancer, infection, and MS AD risk is not reduced by other anti - inflammatory treatments 27 TNF INHIBITORS HAVE A PROTECTIVE EFFECT AGAINST DEVELOPING ALZHEIMERS DISEASE Inflammation is Associated with the Following Conditions and Diseases
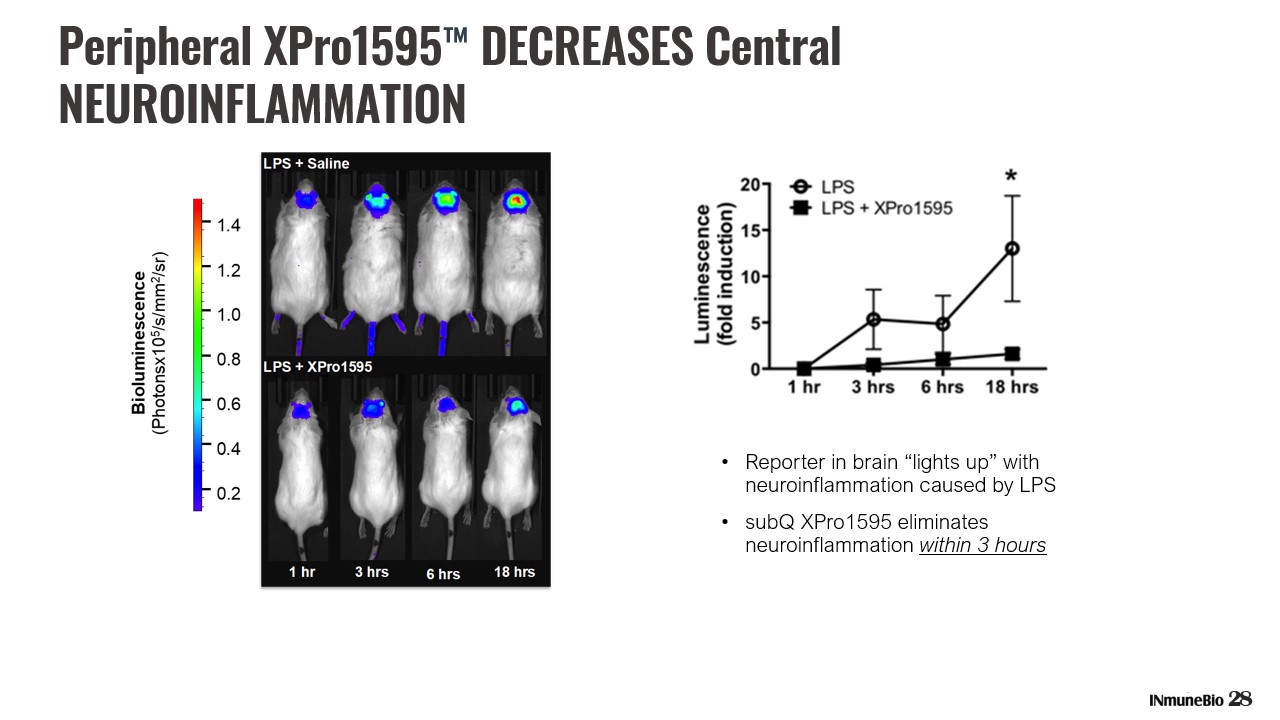
28 • Reporter in brain “lights up” with neuroinflammation caused by LPS • subQ XPro1595 eliminates neuroinflammation within 3 hours Peripheral XPro1595 ™ DECREASES Central NEUROINFLAMMATION

IMMUNOLOGIC BIOMARKERS BEHAVIORAL BIOMARKERS OF INFLAMMATION SLEEP DISORDERS DEPRESSION PSYCHOSIS APATHY AGGRESSION MRI CSF Breath Blood FreeWater content of white matter (Imeka) Inflammation & Neurodegeneration ( Roche NeuroTool kit ) Volatile Organic Compounds Inflammation & Neurodegeneration ( Roche NeuroTool kit ) BIOLOGICAL BIOMARKERS OF INFLAMMATION DESIGN ▪ 18 patients in 3 cohorts ▪ Weekly XPro1595™ SubQ injections for 3 months ▪ Biomarkers of inflammation at 0, 6, 12 weeks INCLUSION Must have 1 inflammatory biomarker (local lab): ▪ hsCRP > 1.5 ▪ ESR > 10 ▪ HbA1C > 6% ▪ APOE4 positive ENDPOINTS ▪ Safety ▪ Change in inflammation (blood, CSF, Brain, breath, behavior) ▪ Change in neuropsychiatric symptoms, cognition, QOL PHASE 1 AD TRIAL Phase IB trial in Alzheimer’s patients with biomarkers of inflammation 29
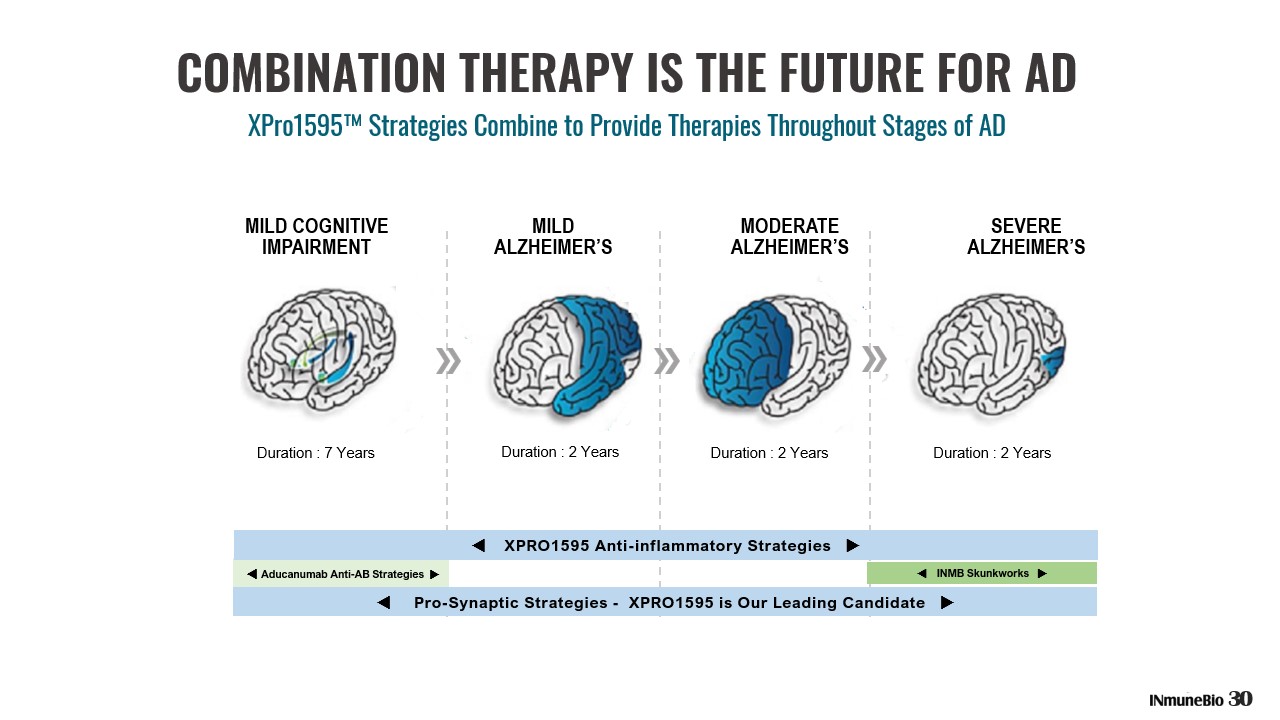
COMBINATION THERAPY IS THE FUTURE FOR AD MILD COGNITIVE IMPAIRMENT MILD ALZHEIMER’S MODERATE ALZHEIMER’S SEVERE ALZHEIMER’S Duration : 7 Years Duration : 2 Years Duration : 2 Years Duration : 2 Years 30 Aducanumab Anti - AB Strategies XPro1595™ Strategies Combine to Provide Therapies Throughout Stages of AD Pro - Synaptic Strategies - XPRO1595 is Our Leading Candidate INMB Skunkworks XPRO1595 Anti - inflammatory Strategies

Addressing the Link Between Liver Fibrosis, the Innate Immune Cells of the Liver and Inflammation, a Pleiotropic Approach 31

THREE PRINCIPLES OF INMB’s NASH PROGRAM OBESITY IN THE U.S. Obesity increases the risk of dementia and NASH. XPro1595 has been shown to reverse both in vivo. Tansey, et. Al. 1. NASH is a complex pleiotropic disease with metabolic, biochemical, immunologic and intestinal elements that combine to cause hepatocyte death and fibrosis 2. Drugs with that target multiple pathologies may have an advantage over drugs that target a single pathology 3. Targeting the causes of progressive fibrosis may be a more efficient therapeutic strategy in many patients 32
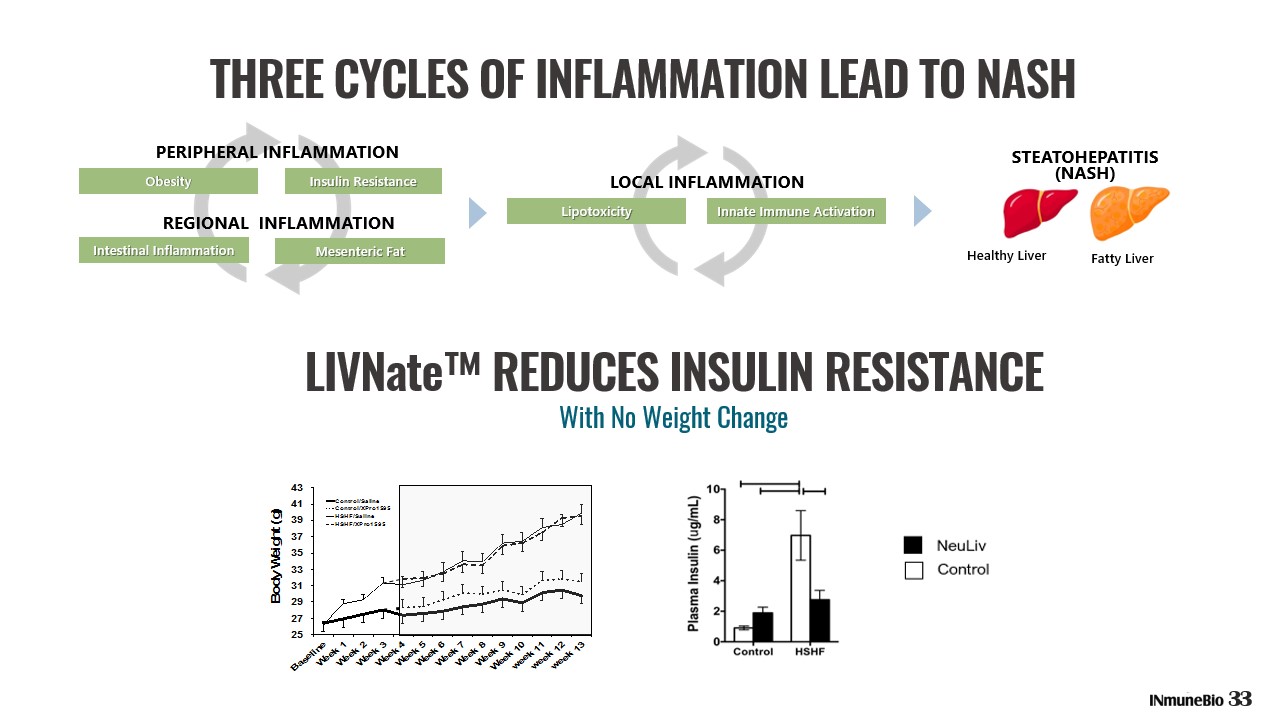
THREE CYCLES OF INFLAMMATION LEAD TO NASH LIVNate™ REDUCES INSULIN RESISTANCE 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 33 With No Weight Change REGIONAL INFLAMMATION PERIPHERAL INFLAMMATION Obesity Insulin Resistance Intestinal Inflammation Mesenteric Fat LOCAL INFLAMMATION Lipotoxicity Innate Immune Activation STEATOHEPATITIS (NASH) Healthy Liver Fatty Liver
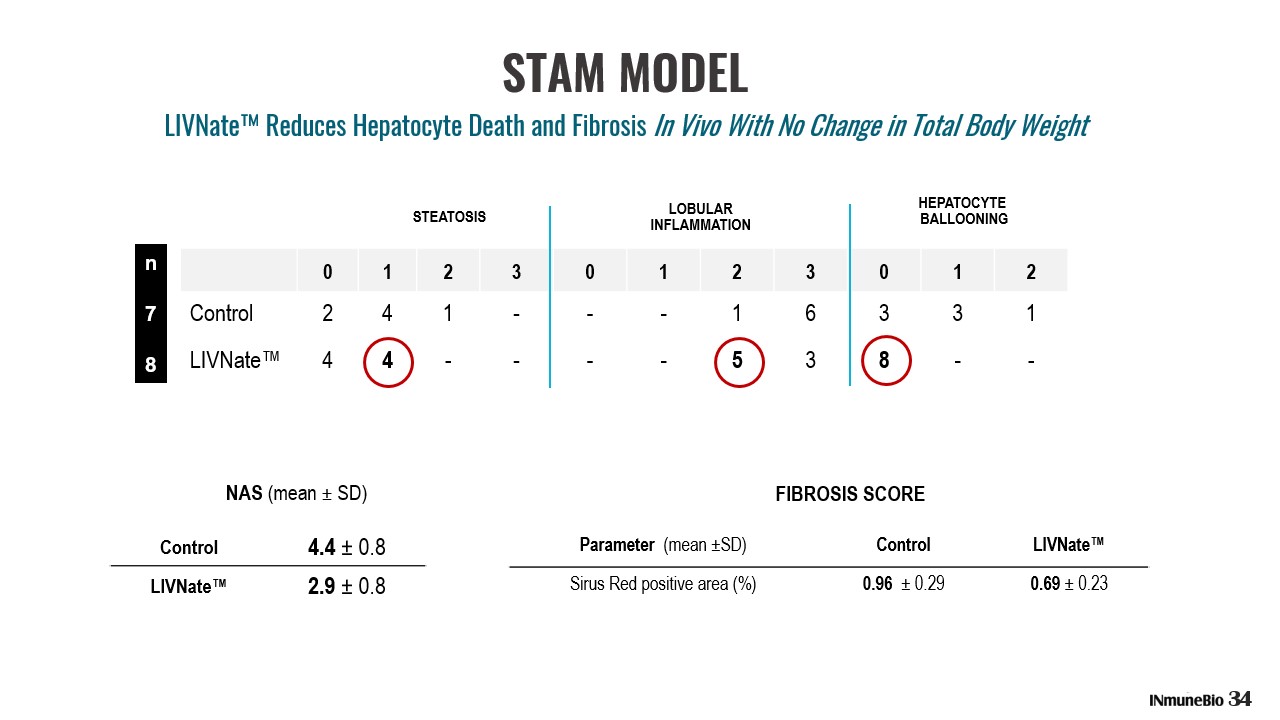
0 1 2 3 0 1 2 3 0 1 2 Control 2 4 1 - - - 1 6 3 3 1 LIVNate™ 4 4 - - - - 5 3 8 - - HEPATOCYTE BALLOONING LOBULAR INFLAMMATION STEATOSIS Control 4.4 ц 0.8 LIVNate™ 2.9 ц 0.8 NAS (mean ц SD) Parameter (mean ц SD) Control LIVNate™ Sirus Red positive area (%) 0.96 ц 0.29 0.69 ц 0.23 FIBROSIS SCORE n 7 8 STAM MODEL 34 LIVNate™ Reduces Hepatocyte Death and Fibrosis In Vivo With No Change in Total Body Weight
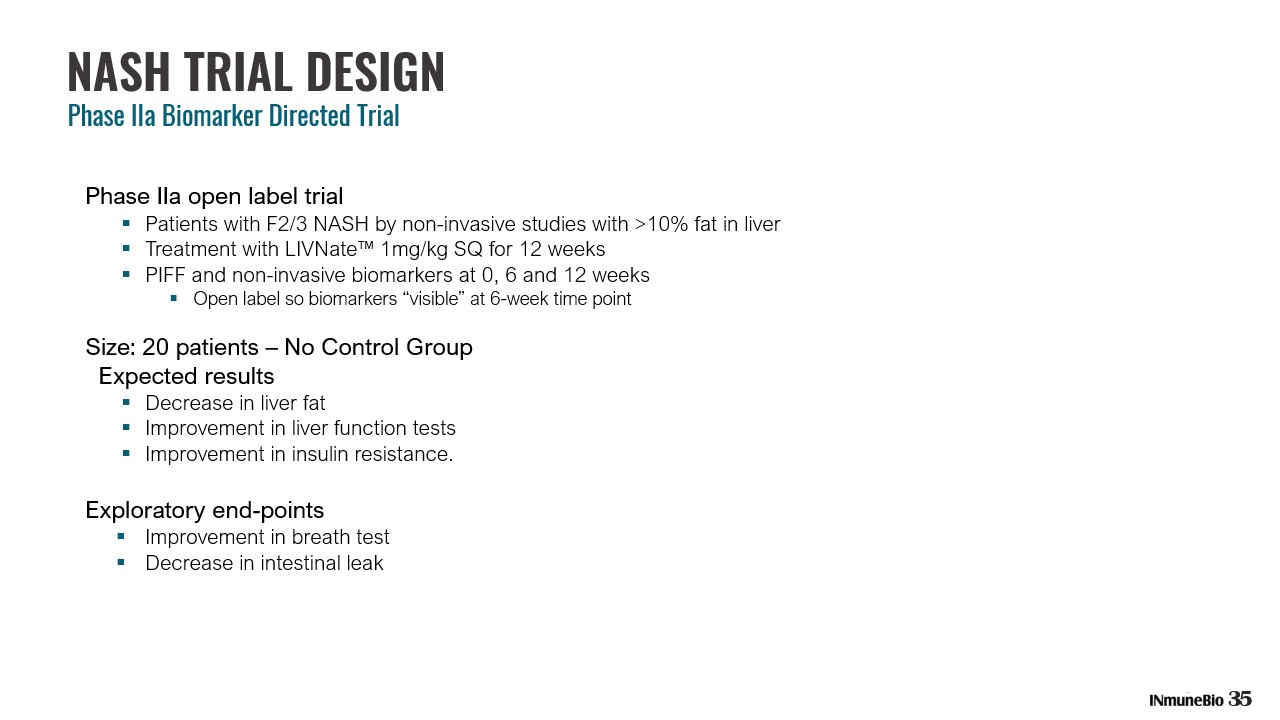
NASH TRIAL DESIGN Phase IIa Biomarker Directed Trial Phase IIa open label trial ▪ Patients with F2/3 NASH by non - invasive studies with >10% fat in liver ▪ Treatment with LIVNate™ 1mg/kg SQ for 12 weeks ▪ PIFF and non - invasive biomarkers at 0, 6 and 12 weeks ▪ Open label so biomarkers “visible” at 6 - week time point Size: 20 patients – No Control Group Expected results ▪ Decrease in liver fat ▪ Improvement in liver function tests ▪ Improvement in insulin resistance. Exploratory end - points ▪ Improvement in breath test ▪ Decrease in intestinal leak 35

Natural Killer cells are responsible for clearing residual disease 36
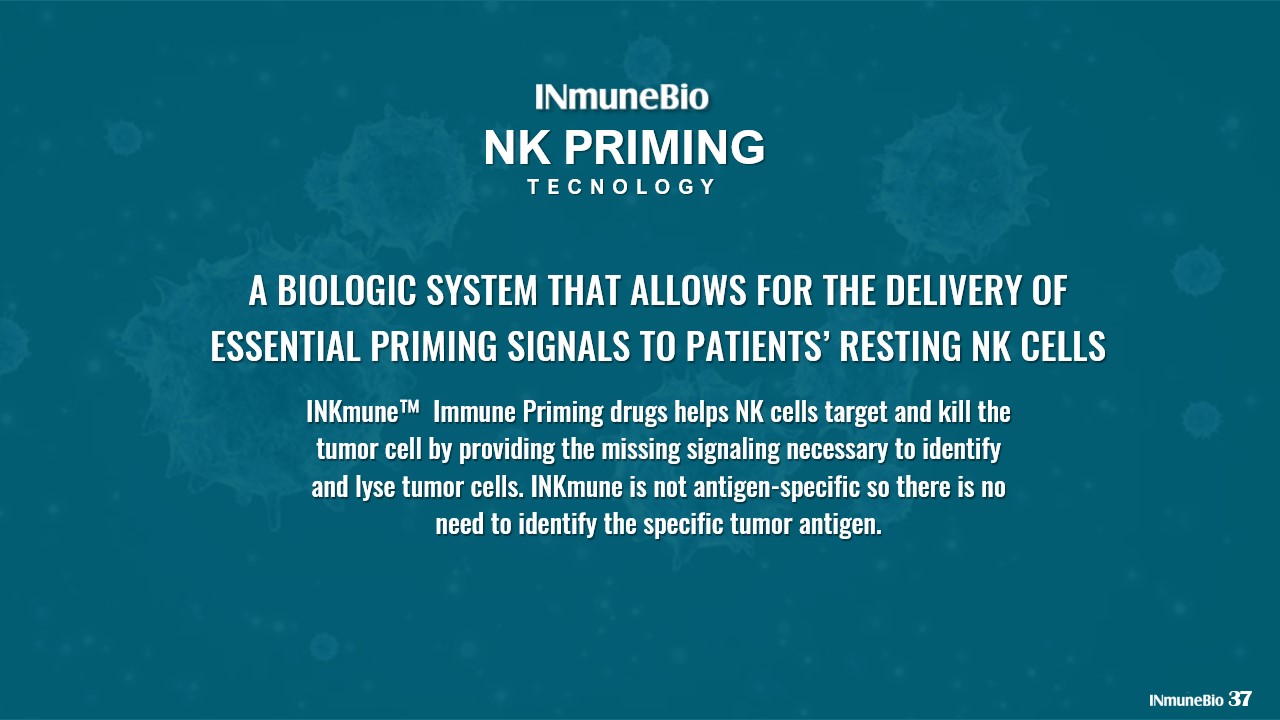
A BIOLOGIC SYSTEM THAT ALLOWS FOR THE DELIVERY OF ESSENTIAL PRIMING SIGNALS TO PATIENTS’ RESTING NK CELLS NK PRIMING TECNOLOGY INKmune™ Immune Priming drugs helps NK cells target and kill the tumor cell by providing the missing signaling necessary to identify and lyse tumor cells. INKmune is not antigen - specific so there is no need to identify the specific tumor antigen. 37

INKmune ™ TREATS RESIDUAL DISEASE Minimal Residual Disease (MRD) is the cause of relapse and death in the majority of cancer patients Problem Cancer remains after treatment Solution Eliminate residual disease to reduce relapse Drug INKmune Target Patient’s own NK cells Status Entering Phase I trial in high - risk Myelodysplastic Syndrome (MDS) and Ovarian Cancer 38
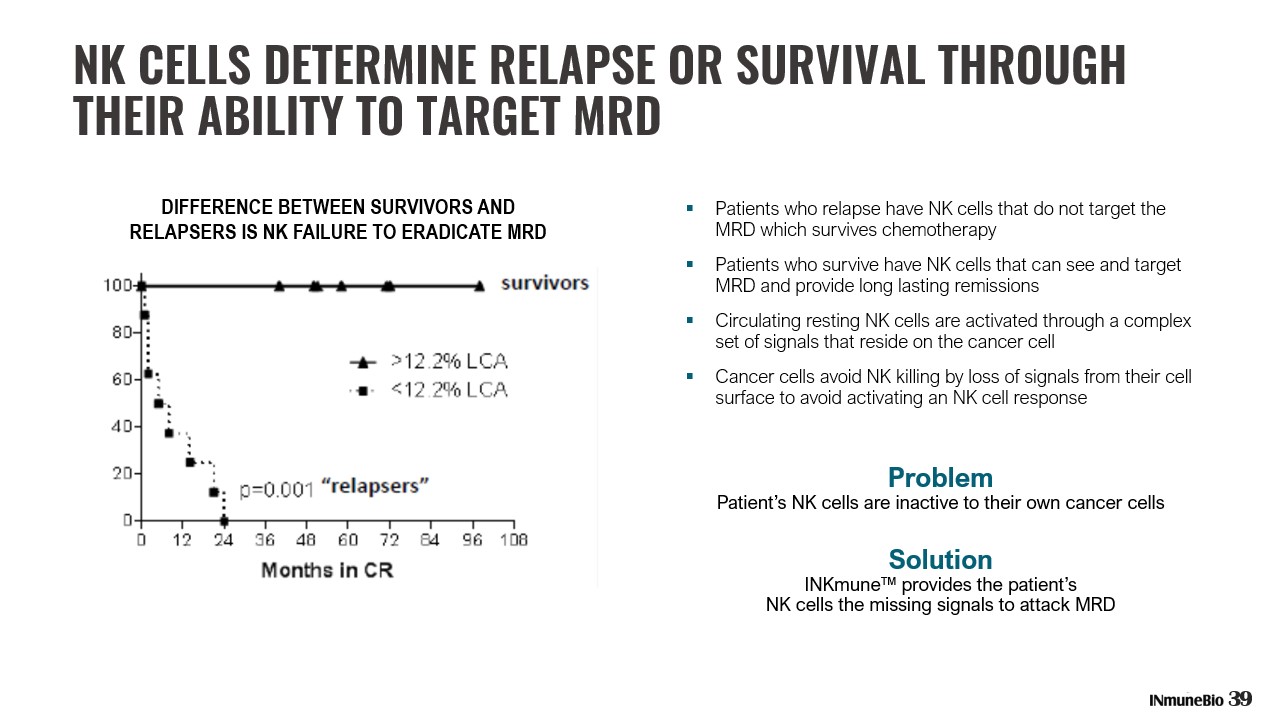
NK CELLS DETERMINE RELAPSE OR SURVIVAL THROUGH THEIR ABILITY TO TARGET MRD ▪ Patients who relapse have NK cells that do not target the MRD which survives chemotherapy ▪ Patients who survive have NK cells that can see and target MRD and provide long lasting remissions ▪ Circulating resting NK cells are activated through a complex set of signals that reside on the cancer cell ▪ Cancer cells avoid NK killing by loss of signals from their cell surface to avoid activating an NK cell response Problem Patient’s NK cells are inactive to their own cancer cells Solution INKmune™ provides the patient’s NK cells the missing signals to attack MRD DIFFERENCE BETWEEN SURVIVORS AND RELAPSERS IS NK FAILURE TO ERADICATE MRD 39
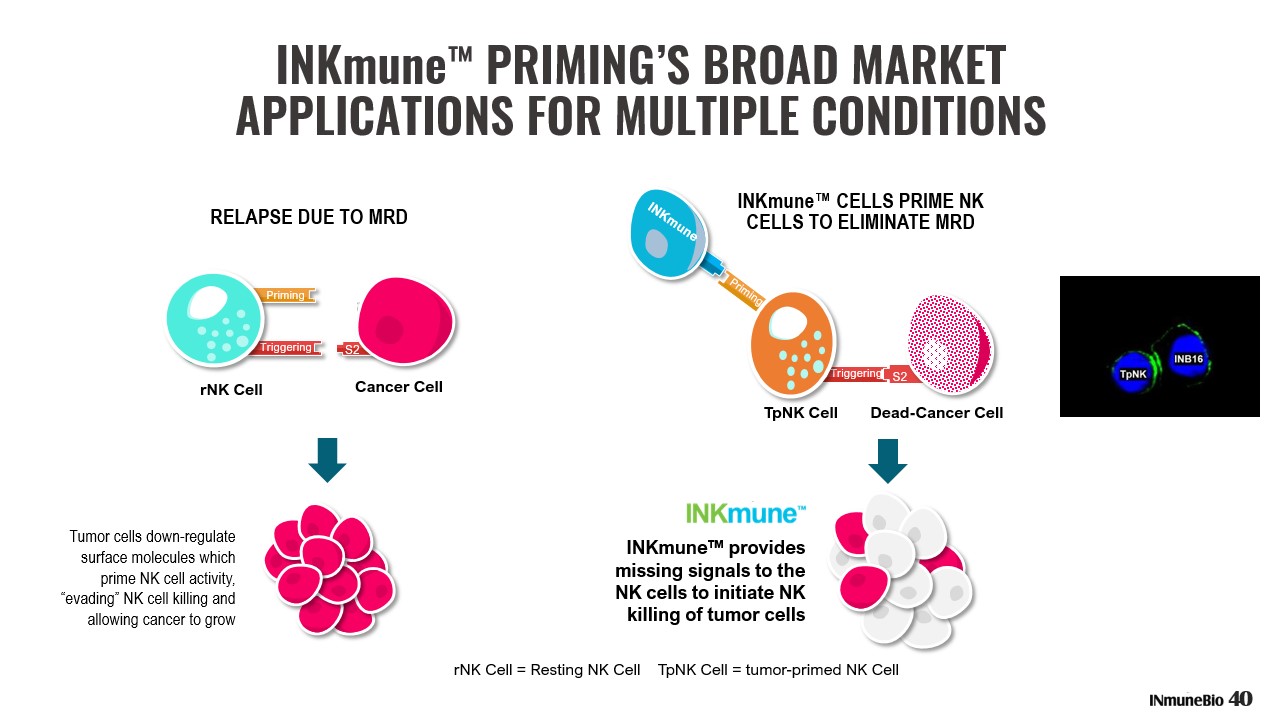
S1 S2 rNK Cell = Resting NK Cell TpNK Cell = tumor - primed NK Cell INKmune™ provides missing signals to the NK cells to initiate NK killing of tumor cells INKmune™ CELLS PRIME NK CELLS TO ELIMINATE MRD S2 Tumor cells down - regulate surface molecules which prime NK cell activity, “evading” NK cell killing and allowing cancer to grow rNK Cell Cancer Cell RELAPSE DUE TO MRD Priming Triggering INKmune ™ PRIMING’S BROAD MARKET APPLICATIONS FOR MULTIPLE CONDITIONS S2 TpNK Cell Dead - Cancer Cell Triggering S2 40
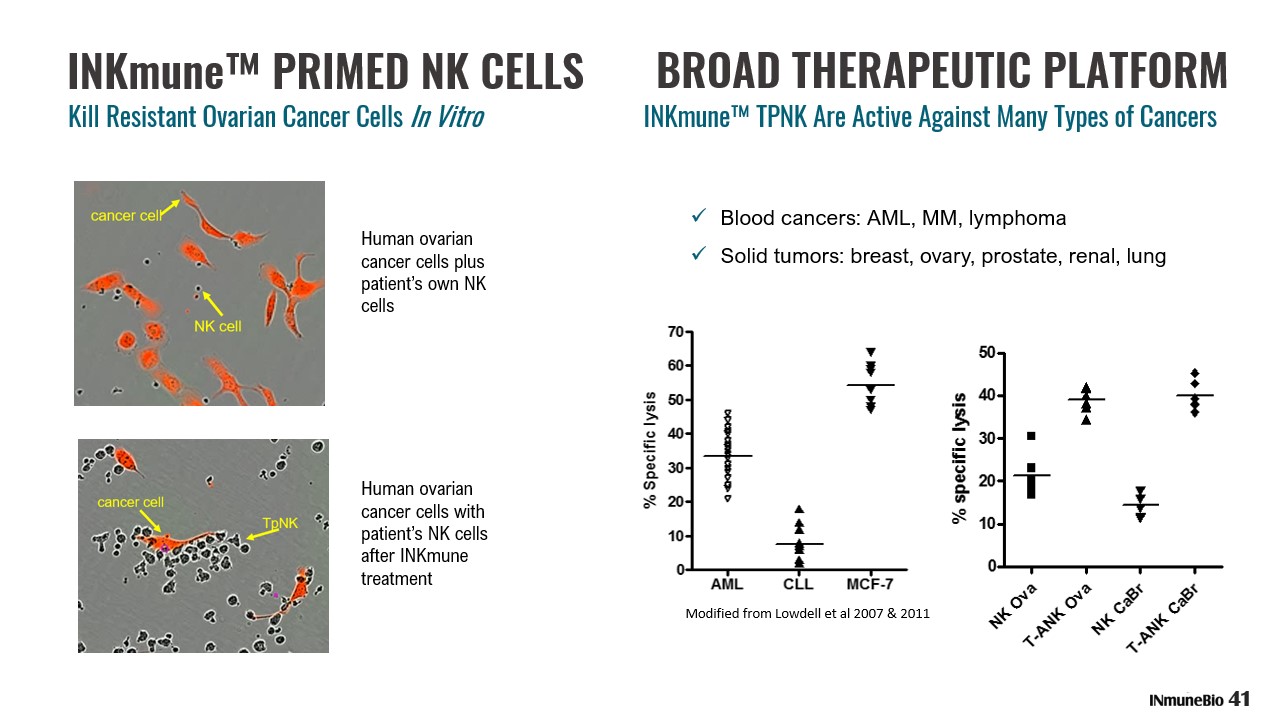
INKmune™ PRIMED NK CELLS Human ovarian cancer cells plus patient’s own NK cells Human ovarian cancer cells with patient’s NK cells after INKmune treatment Modified from Lowdell et al 2007 & 2011 BROAD THERAPEUTIC PLATFORM x Blood cancers: AML, MM, lymphoma x Solid tumors: breast, ovary, prostate, renal, lung 41 Kill Resistant Ovarian Cancer Cells In Vitro INKmune™ TPNK Are Active Against Many Types of Cancers

HIGH - RISK MDS CLINICAL STUDY OBJECTIVES A Phase I Open - Label Dose Escalation Study of Intravenous INKmune™ in Patients with Myelodysplastic Syndrome with Excess Blasts (MDS - EB - 1/2 - MDS - CMML 1/2) Primary 1. To evaluate the safety and tolerability of INKmune when given intravenously (i.v.) 42 Exploratory 1. To assess the pharmacodynamics (PD) (proof of biology) by analysing natural killer (NK) cells obtained from peripheral blood and bone marrow as available 2. To measure primed NK cells and their functional ability using the tumour killing assay Secondary 1. To assess the change in [or effect upon] number and percentage of blasts in peripheral blood and bone marrow 2. To assess the overall response rate (ORR, partial response [PR] or complete response [CR]) in subjects administered INKmune using WHO criteria 3. To assess the duration of response
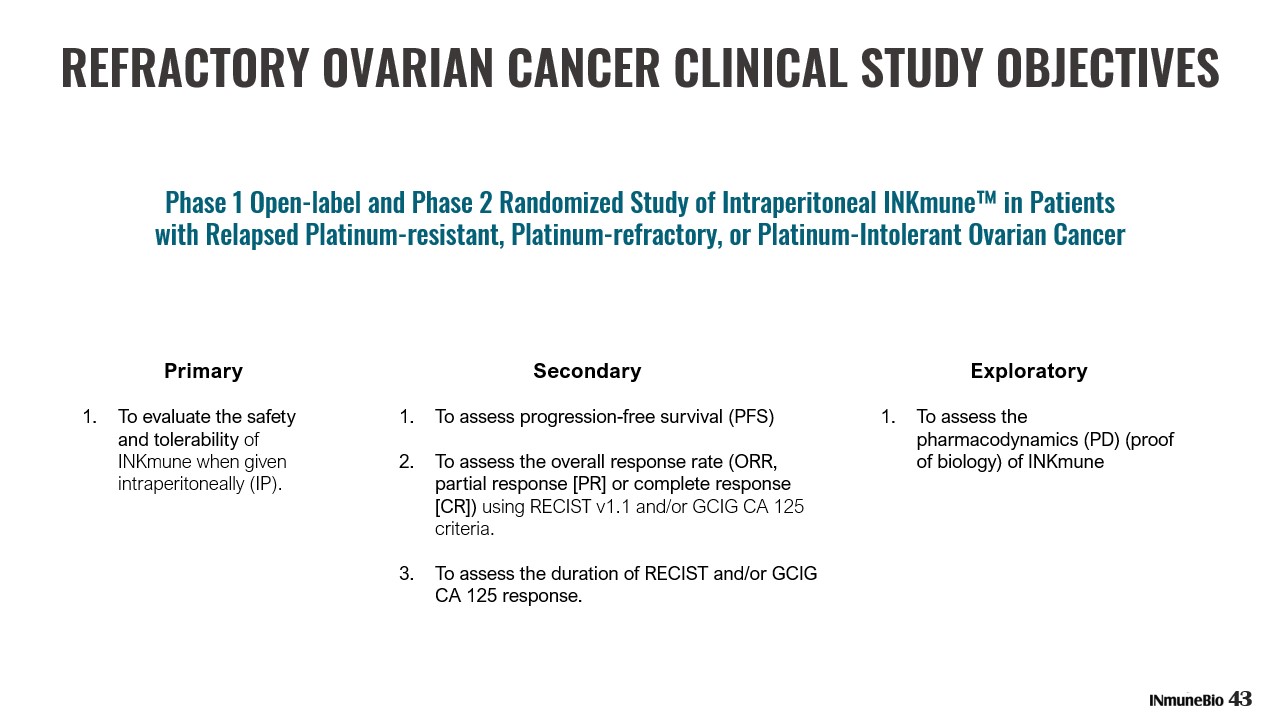
REFRACTORY OVARIAN CANCER CLINICAL STUDY OBJECTIVES Phase 1 Open - label and Phase 2 Randomized Study of Intraperitoneal INKmune™ in Patients with Relapsed Platinum - resistant, Platinum - refractory, or Platinum - Intolerant Ovarian Cancer Primary 1. To evaluate the safety and tolerability of INKmune when given intraperitoneally (IP). 43 Exploratory 1. To assess the pharmacodynamics (PD) (proof of biology) of INKmune Secondary 1. To assess progression - free survival (PFS) 2. To assess the overall response rate (ORR, partial response [PR] or complete response [CR]) using RECIST v1.1 and/or GCIG CA 125 criteria. 3. To assess the duration of RECIST and/or GCIG CA 125 response.
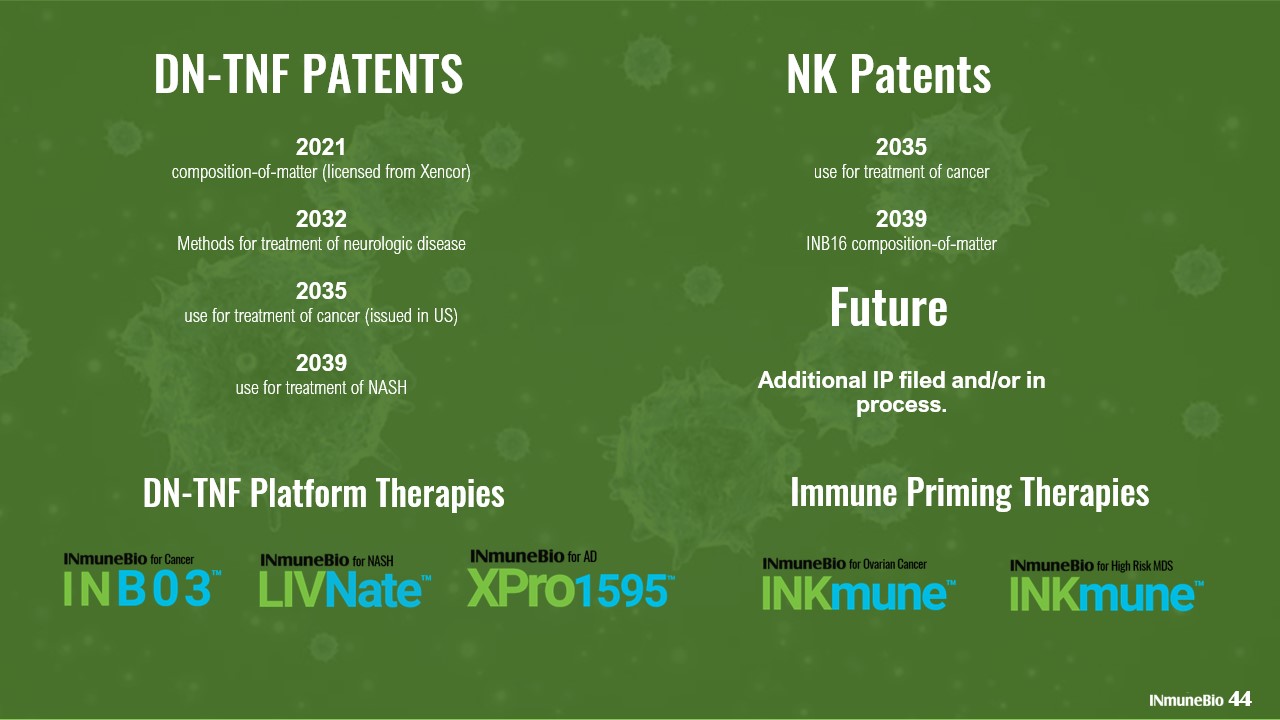
DN - TNF PATENTS 2021 composition - of - matter (licensed from Xencor) 2032 Methods for treatment of neurologic disease 2035 use for treatment of cancer (issued in US) 2039 use for treatment of NASH DN - TNF Platform Therapies 44 NK Patents 2035 use for treatment of cancer 2039 INB16 composition - of - matter Future Additional IP filed and/or in process. Immune Priming Therapies
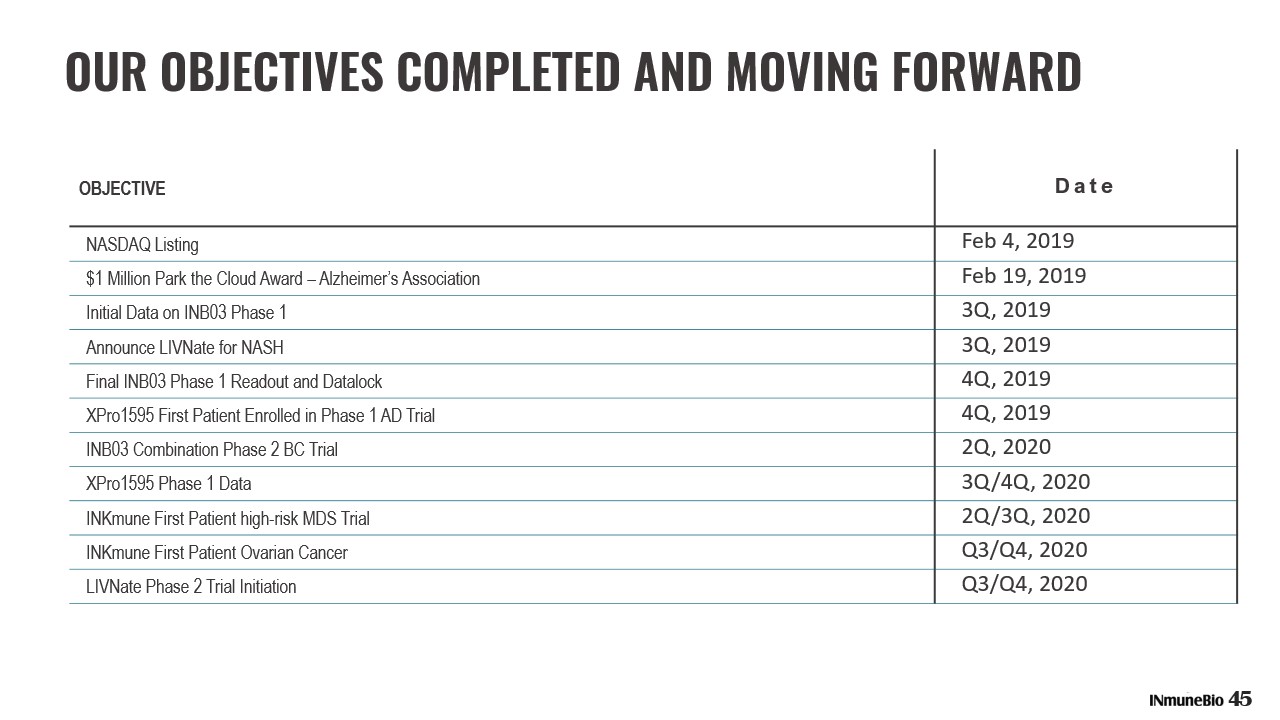
OUR OBJECTIVES COMPLETED AND MOVING FORWARD OBJECTIVE Date NASDAQ Listing Feb 4, 2019 $1 Million Park the Cloud Award – Alzheimer’s Association Feb 19, 2019 Initial Data on INB03 Phase 1 3Q, 2019 Announce LIVNate for NASH 3Q, 2019 Final INB03 Phase 1 Readout and Datalock 4Q, 2019 XPro1595 First Patient Enrolled in Phase 1 AD Trial 4Q, 2019 INB03 Combination Phase 2 BC Trial 2Q, 2020 XPro1595 Phase 1 Data 3Q/4Q, 2020 INKmune First Patient high - risk MDS Trial 2Q/3Q, 2020 INKmune First Patient Ovarian Cancer Q3/Q4, 2020 LIVNate Phase 2 Trial Initiation Q3/Q4, 2020 45

SUMMARY ▪ Two platforms target a wide variety of diseases in humans ▪ First dominant - negative TNF therapeutic drug targeting the master cytokine of inflammation TNF ▪ Novel approach in signaling the patients own NK cells to target residual disease ▪ Extensive data both in animals and humans on both drug platforms with 69 publications ▪ Programs in diseases without solutions: Immuno - oncology, Alzheimer’s Disease, NASH ▪ Multiple clinical catalysts in 2020: Phase 1 INB03™ data presented, Phase 2 expected mid 2020 Phase 2 LIVNate™ expected mid 2020 Phase 1 INKmune™ in MDS expected 2H 2020 Phase 1 INKmune ™ in Ovarian Cancer expected 2H 2020 Phase 1 XPro1595™ expected to complete 2H 2020, Phase 2 expected late 2020 or early 2021 ▪ Proven abilities of management and board to deliver data ▪ Simple capital structure and use of non - dilutive financing sources 46 INMB
CONTACT US 47 HARNESSING THE POWER OFTHE INNATE IMMUNE SYSTEM INMB INmune Bio Inc. 1200 Prospect Str. Suite 525 La Jolla, CA 92037 (858) 964 - 3720 dmoss@inmunebio.com www.inmunebio.com














































