Exhibit 99.1

HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM Modulating an Innate Immune Response Against Diseases IN MB CORPORATE PRESENTATION Nov 2020

FORWARD LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . There is no guarantee that any specific outcome will be achieved . Investment results are speculative and there is a risk of loss, potentially all loss of investments . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . 2
INMUNE BIO HIGHLIGHTS 3 Clinical - stage biopharmaceutical company developing a new class of therapeutics that target dysfunction of the innate immune system Two platforms : ▪ DN - TNF: First selective TNF inhibitor targeting inflammation without demyelination or immunosuppression ▪ NK Cell Priming: Primes patient’s NK cells to eliminate residual Highlight of upcoming catalysts: ▪ P1b AD data January ▪ P1 HR - MDS Start 1H 2021 ▪ P2 AD Initiation Mid - 2021 ▪ P2 TRD Initiation Mid - 2021 ▪ P2 CV - 19 data 2021 Third party validation: ▪ Over 65 publications from multiple universities worldwide on both platforms with extensive in vivo data ▪ More than $4M in grants from AA, ALS and NIH

DN - TNF PLATFORM DISEASE FIELD PRE - CLINICAL PHASE I PHASE II (POC) PIVOTAL EST. NEXT MILESTONE COVID - 19 Cytokine Storm Treatment Resistant Depression Alzheimer’s Disease CNS P2 Mid - 2021 P2 underway – data 2021 NK PRIMING PLATFORM DISEASE FIELD PRE - CLINICAL PHASE I PHASE II (POC) PIVOTAL NEXT MILESTONE Myelodysplastic Syndrome ONCOLOGY 1H 21 initiate P1 ACTIVE PIPELINE Therapies that Target the INNATE IMMUNE Response 4 Jan 2021 P1b data P2 start mid - 2021 INB03 for treatment of MUC4 positive cancer and LIVNate for NASH Phase II trials begin after resolution of pandemic INKmune for treatment of ovarian cancer Phase I trial begins after resolution of pandemic
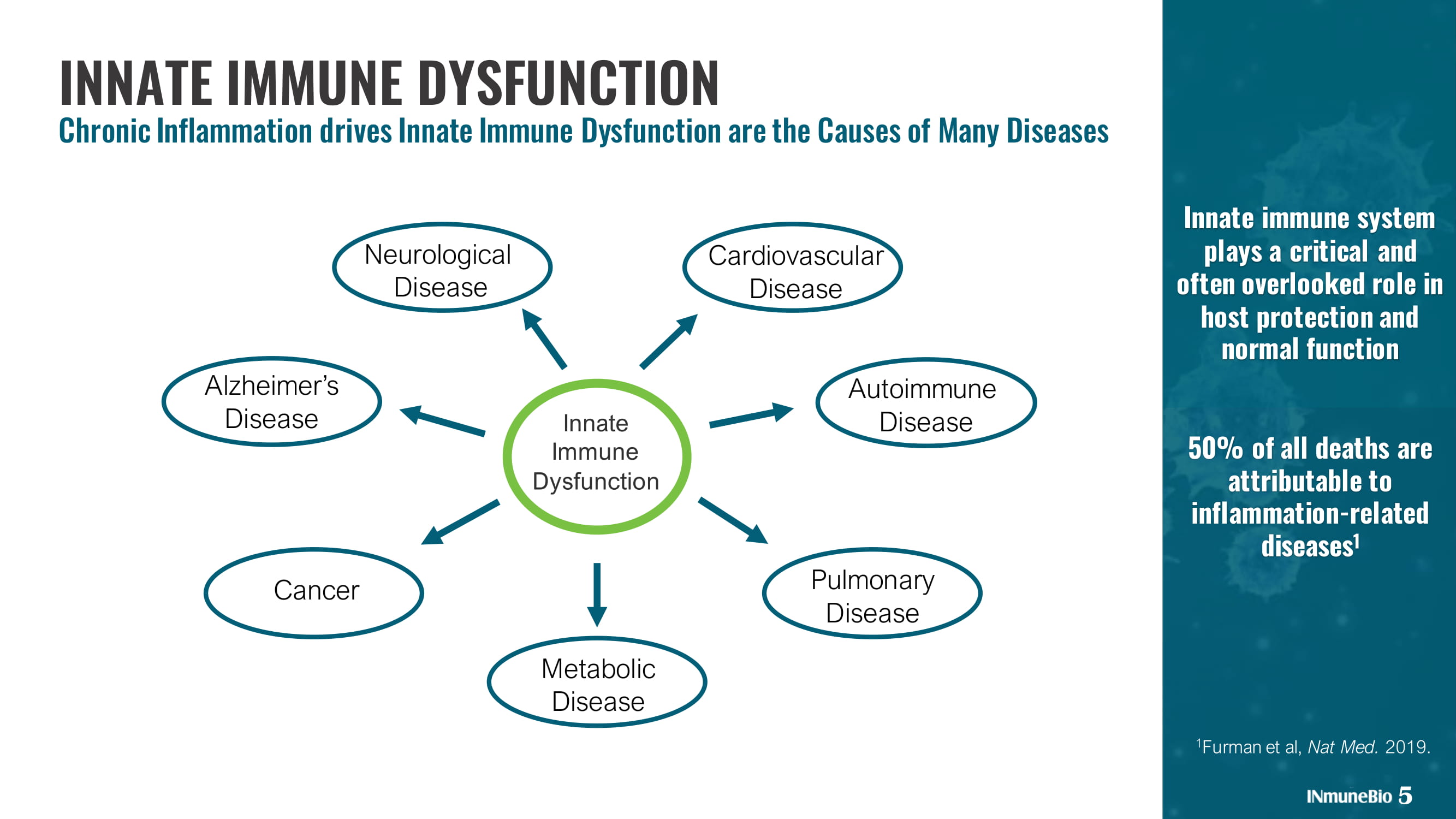
Innate immune system plays a critical and often overlooked role in host protection and normal function 50% of all deaths are attributable to inflammation - related diseases 1 5 INNATE IMMUNE DYSFUNCTION Chronic Inflammation drives Innate Immune Dysfunction are the Causes of Many Diseases 1 Furman et al, Nat Med. 2019. Innate Immune Dysfunction Cancer Pulmonary Disease Autoimmune Disease Metabolic Disease Neurological Disease Cardiovascular Disease Alzheimer’s Disease
Selective sTNF Neutralizer for the Treatment of Neuroinflammation in ALZHEIMER’S DISEASE 6

7 ALZHEIMER’S DISEASE (AD) Driven by Neuroinflammation ▪ Characterized by chronic inflammation which drives synaptic loss and neurodegeneration ▪ As of today, there is no treatment for the cognitive decline of AD Adapted from Alzheimer’s Association “Changing the Trajectory of Alzheimer's Disease: How a Treatment by 2025 Saves Lives and Dollars.” 1 Alzheimer’s Association 1250 1000 Projected cost to US per year ( billions ) 750 500 250 2020 2030 Number of Americans affected by AD (millions) 2050 2040
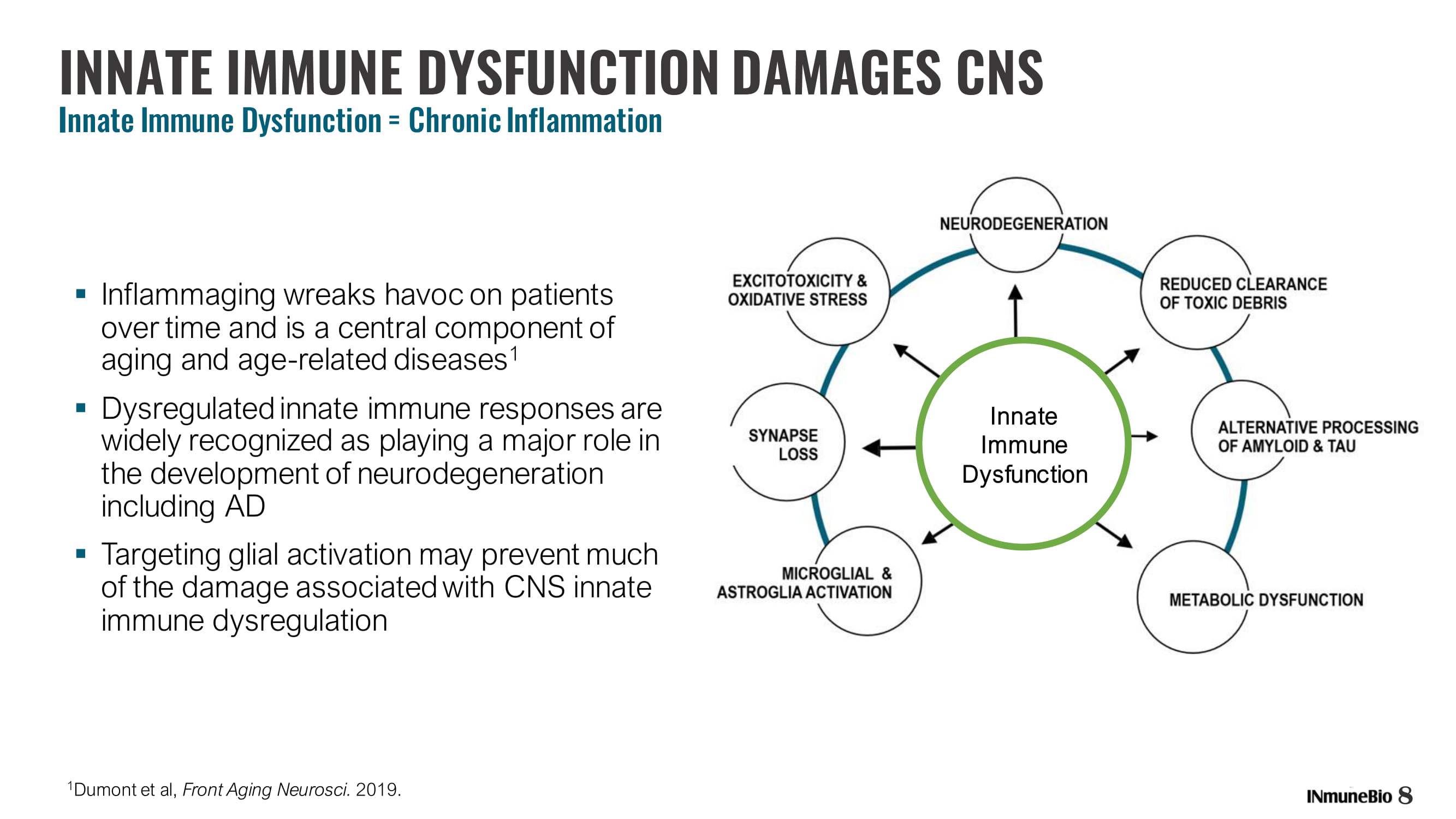
▪ Inflammaging wreaks havoc on patients over time and is a central component of aging and age - related diseases 1 ▪ Dysregulated innate immune responses are widely recognized as playing a major role in the development of neurodegeneration including AD ▪ Targeting glial activation may prevent much of the damage associated with CNS innate immune dysregulation INNATE IMMUNE DYSFUNCTION DAMAGES CNS I nnate Immune Dysfunction = Chronic Inflammation 1 Dumont et al, Front Aging Neurosci . 2019. 8 Innate Immune Dysfunction
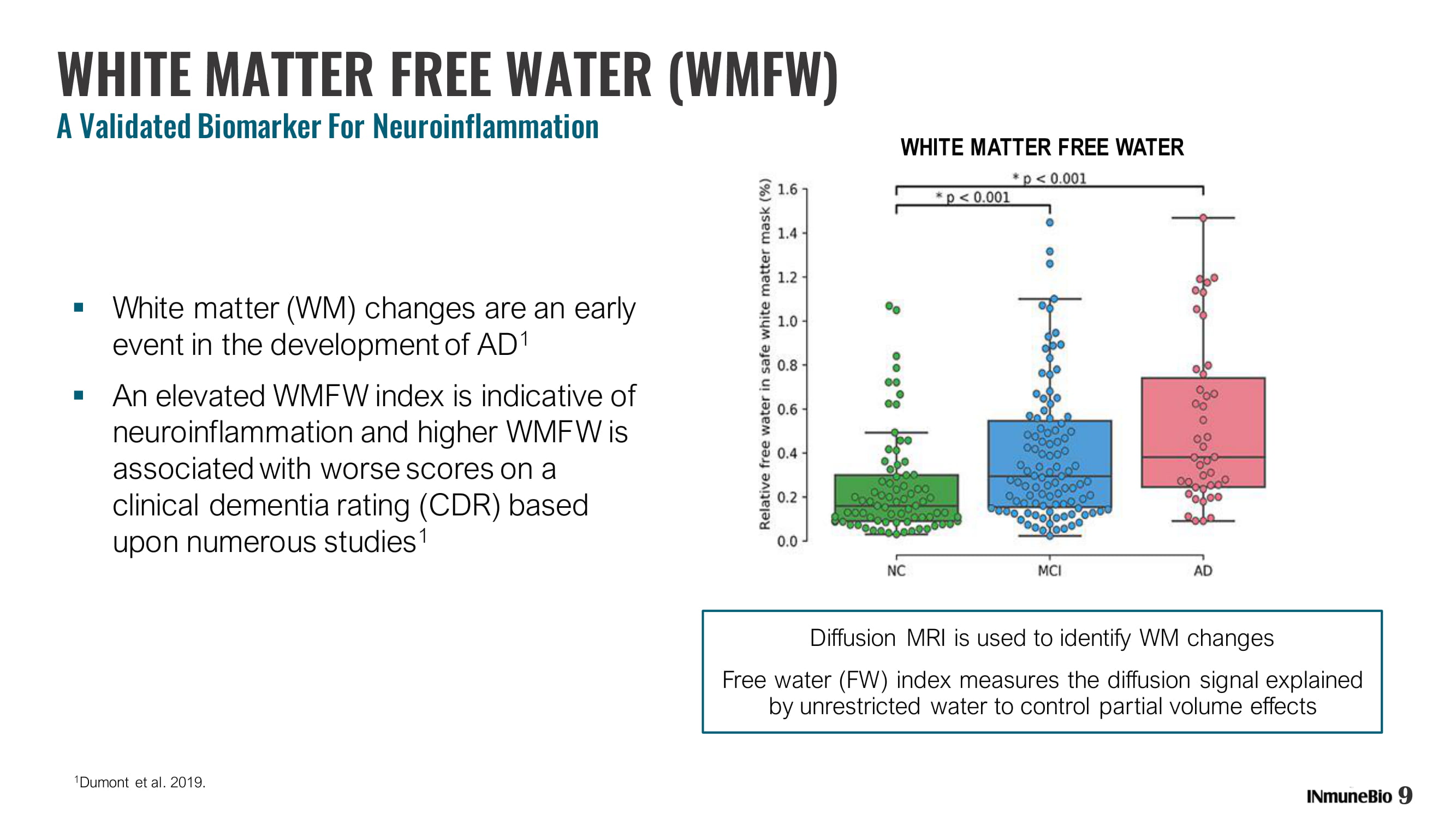
9 WHITE MATTER FREE WATER (WMFW) A Validated Biomarker For Neuroinflammation ▪ White matter (WM) changes are an early event in the development of AD 1 ▪ An elevated WMFW index is indicative of neuroinflammation and higher WMFW is associated with worse scores on a clinical dementia rating (CDR) based upon numerous studies 1 1 Dumont et al. 2019. Diffusion MRI is used to identify WM changes Free water (FW) index measures the diffusion signal explained by unrestricted water to control partial volume effects WHITE MATTER FREE WATER

IMMUNOLOGIC BIOMARKERS BEHAVIORAL BIOMARKERS OF INFLAMMATION SLEEP DISORDERS DEPRESSION PSYCHOSIS APATHY AGGRESSION Inflammation & Neurodegeneration (Roche NeuroTool kit) BIOLOGICAL BIOMARKERS OF INFLAMMATION DESIGN ▪ 18 patients in 3 cohorts ▪ Weekly XPro1595 w SubQ injections for 3 months ▪ Biomarkers of inflammation at 0, 6, 12 weeks INCLUSION Must have 1 inflammatory biomarker (local lab): ▪ hsCRP > 1.5 ▪ ESR > 10 ▪ HbA1C > 6% ▪ APOE4 positive ENDPOINTS ▪ Safety ▪ Change in inflammation (blood, CSF, Brain, breath, behavior) ▪ Change in neuropsychiatric symptoms, cognition, QOL PHASE 1 AD TRIAL Phase IB trial in Alzheimer’s patients with biomarkers of inflammation 10 Inflammation & Neurodegeneration (Roche NeuroTool kit) Volatile Organic Compounds FreeWater content of white matter MRI BLOOD CSF BREATH

XPRO1595 REDUCES NEUROINFLAMMATION Premotor cortex Broca’s area Wernicke's area The arcuate plays a key role in language processing – the ability to understand or express speech – critical to AD XPro1595 Reduces Neuroinflammation Within the Arcuate Fasciculus 11 XPro1595 Reduces Whole Brain Neuroinflammation XPro1595 Reduces Neuroinflammation within the AF Anticipating Phase 2 clinical study (U.S.) in AD to launch in 2H - 2021

Dumont et al Descoteaux Frontiers in Aging Neuroscience 2019 TNF and NEUROINFLAMMATION ARE VALIDED TARGETS IN AD Correlation with Changes to Amyloid 12 RISK OF ALZHEIMER’S DISEASE IN MAN Risk of general population Adapted from Chou et al. 2016 8.5M claims
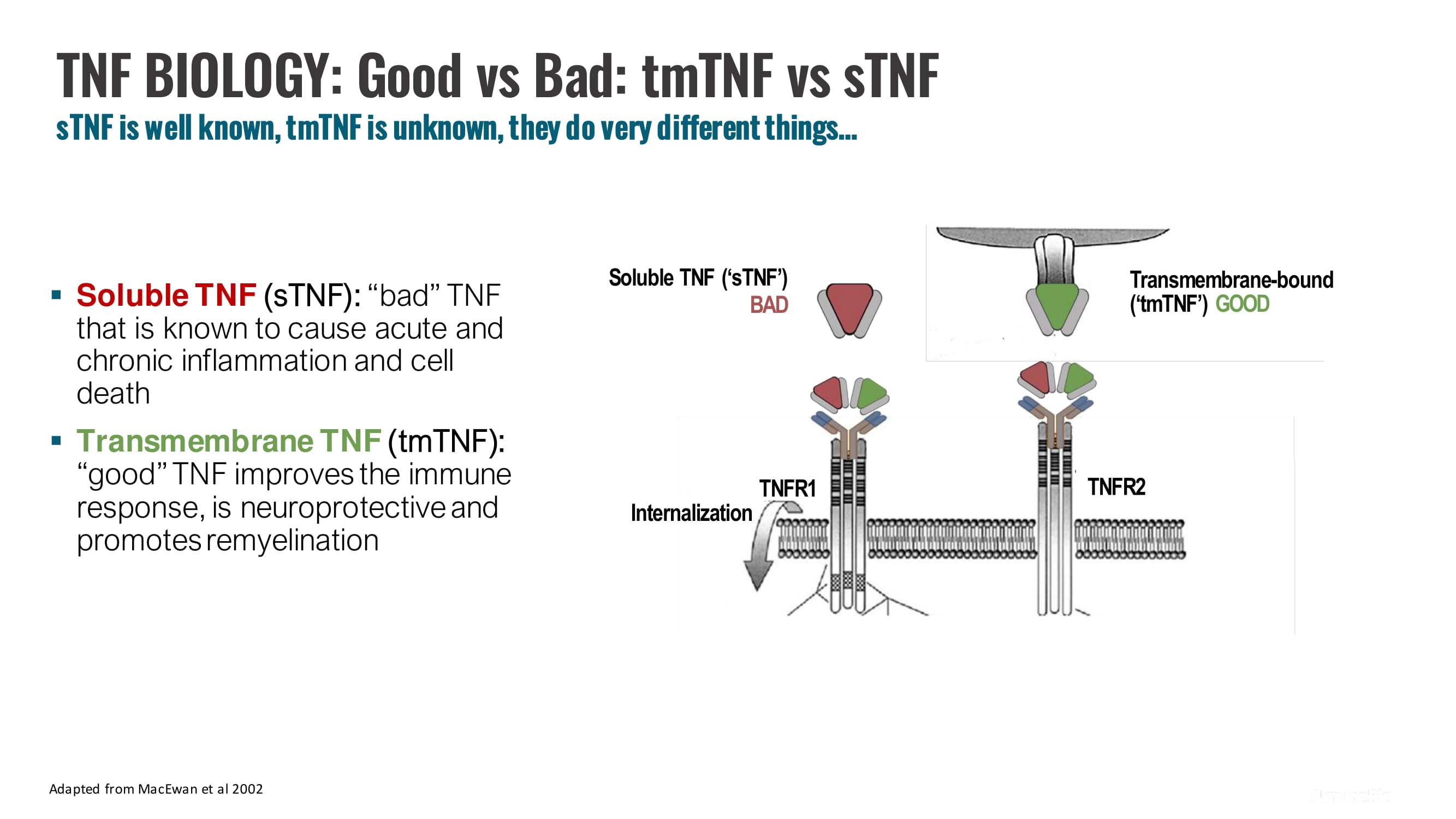
13 Adapted from MacEwan et al 2002 TNF BIOLOGY : Good vs Bad: tmTNF vs sTNF sTNF is well known, tmTNF is unknown, they do very different things… ▪ Soluble TNF ( sTNF ): “bad” TNF that is known to cause acute and chronic inflammation and cell death ▪ Transmembrane TNF ( tmTNF ): “good” TNF improves the immune response, is neuroprotective and promotes remyelination Soluble TNF (‘sTNF’) BAD TNFR1 TNFR2 Internalization Transmembrane - bound (‘tmTNF’) GOOD
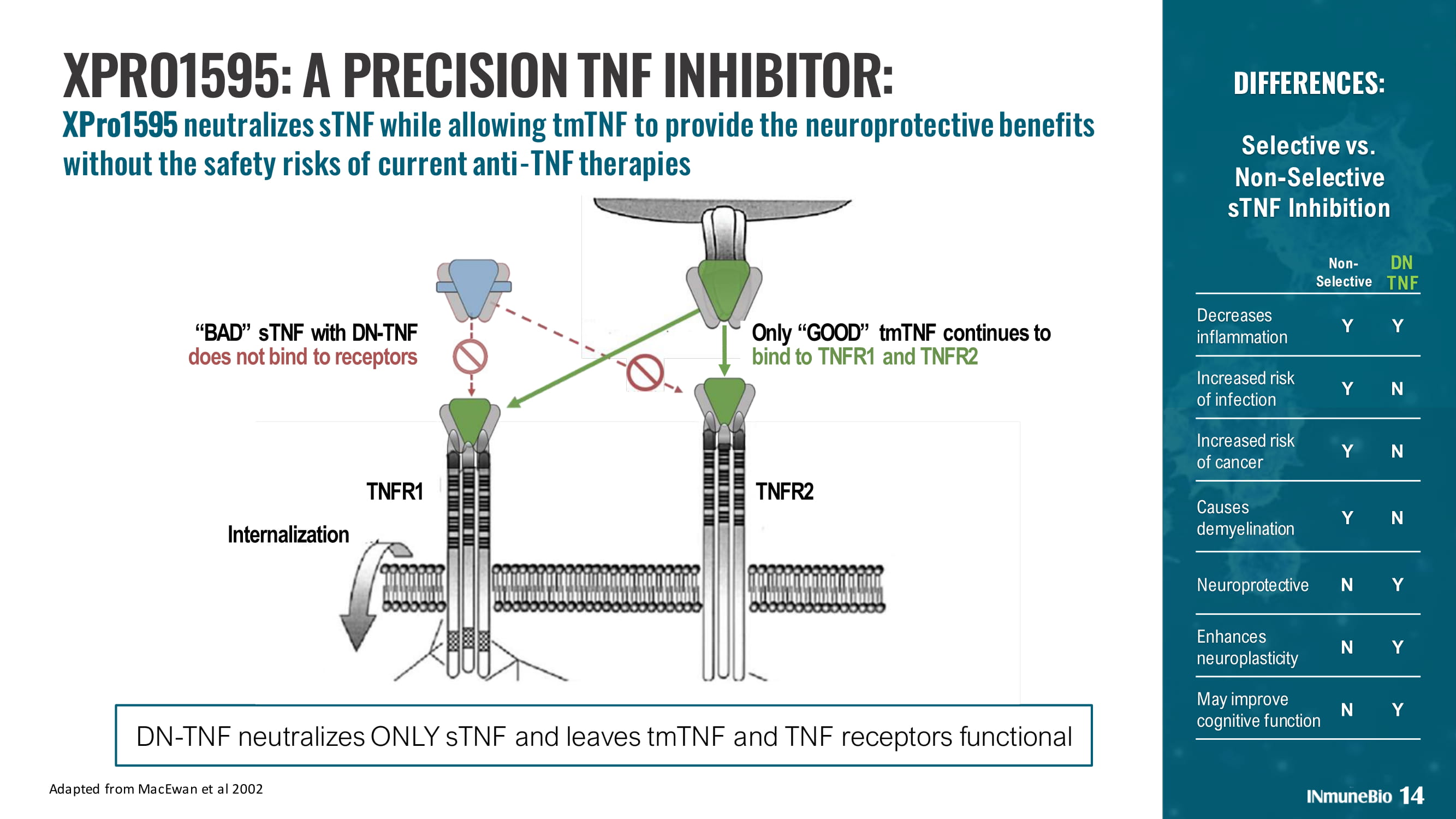
Decreases inflammation Y Y Increased risk of infection Y N Increased risk of cancer Y N Causes demyelination Y N Neuroprotective N Y Enhances neuroplasticity N Y May improve cognitive function N Y DIFFERENCES: Selective vs. Non - Selective sTNF Inhibition Non - Selective DN TNF 14 Adapted from MacEwan et al 2002 XPRO1595: A PRECISION TNF INHIBITOR: XPro1595 neutralizes sTNF while allowing tmTNF to provide the neuroprotective benefits without the safety risks of current anti - TNF therapies DN - TNF neutralizes ONLY sTNF and leaves tmTNF and TNF receptors functional “BAD” s TNF with DN - TNF does not bind to receptors Only “GOOD” tmTNF continues to bind to TNFR1 and TNFR2 Internalization TNFR1 TNFR2
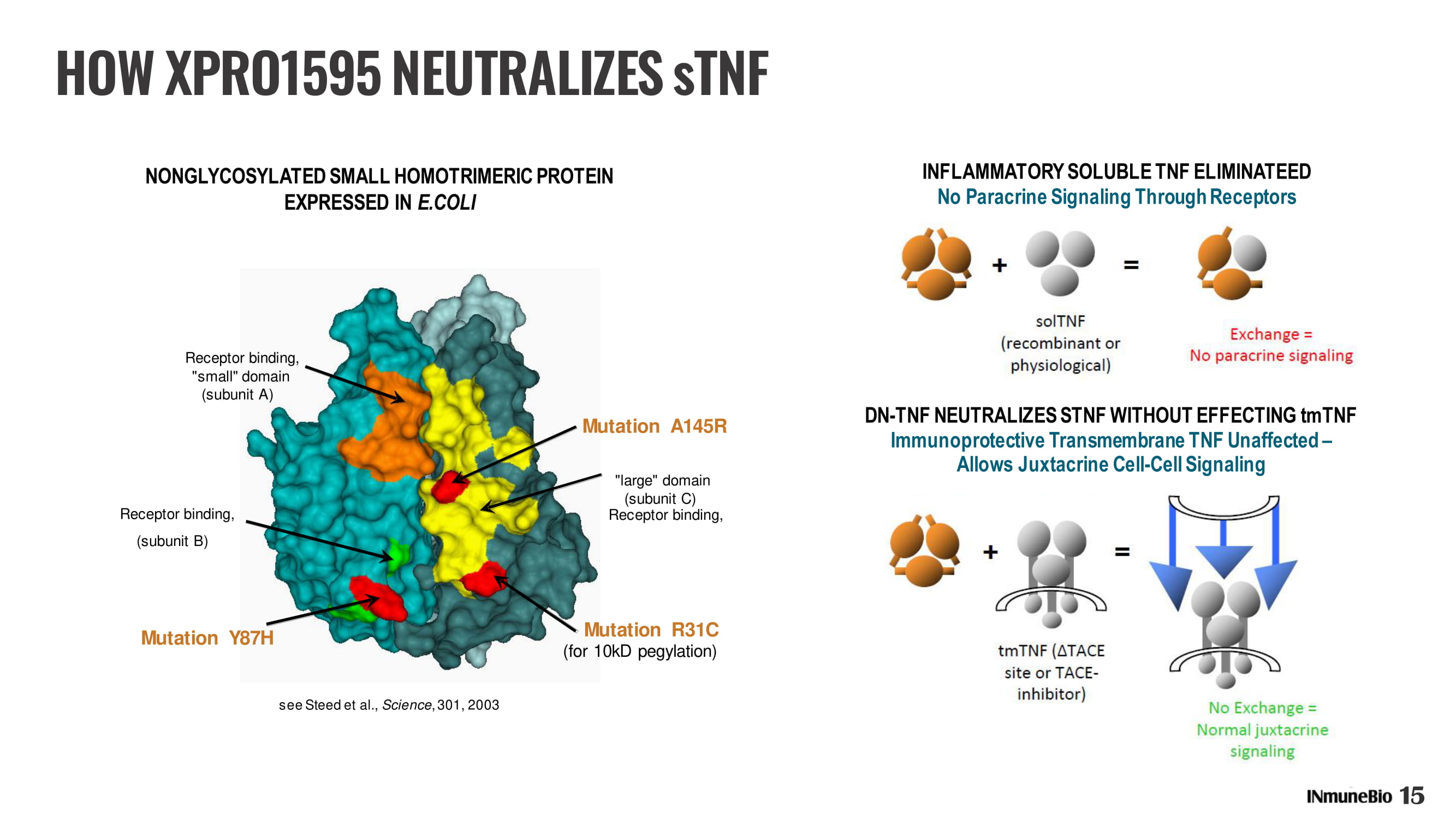
see Steed et al., Science , 301, 2003 Receptor binding, Receptor binding, "small" domain (subunit A) Receptor binding, (subunit B) Mutation Y87H (subunit C) Mutation R31C (for 10kD pegylation) "large" domain Mutation A145R NONGLYCOSYLATED SMALL HOMOTRIMERIC PROTEIN EXPRESSED IN E.COLI DN - TNF NEUTRALIZES STNF WITHOUT EFFECTING tmTNF Immunoprotective Transmembrane TNF Unaffected – Allows Juxtacrine Cell - Cell Signaling HOW XPRO1595 NEUTRALIZES sTNF 15 INFLAMMATORY SOLUBLE TNF ELIMINATEED No Paracrine Signaling Through Receptors
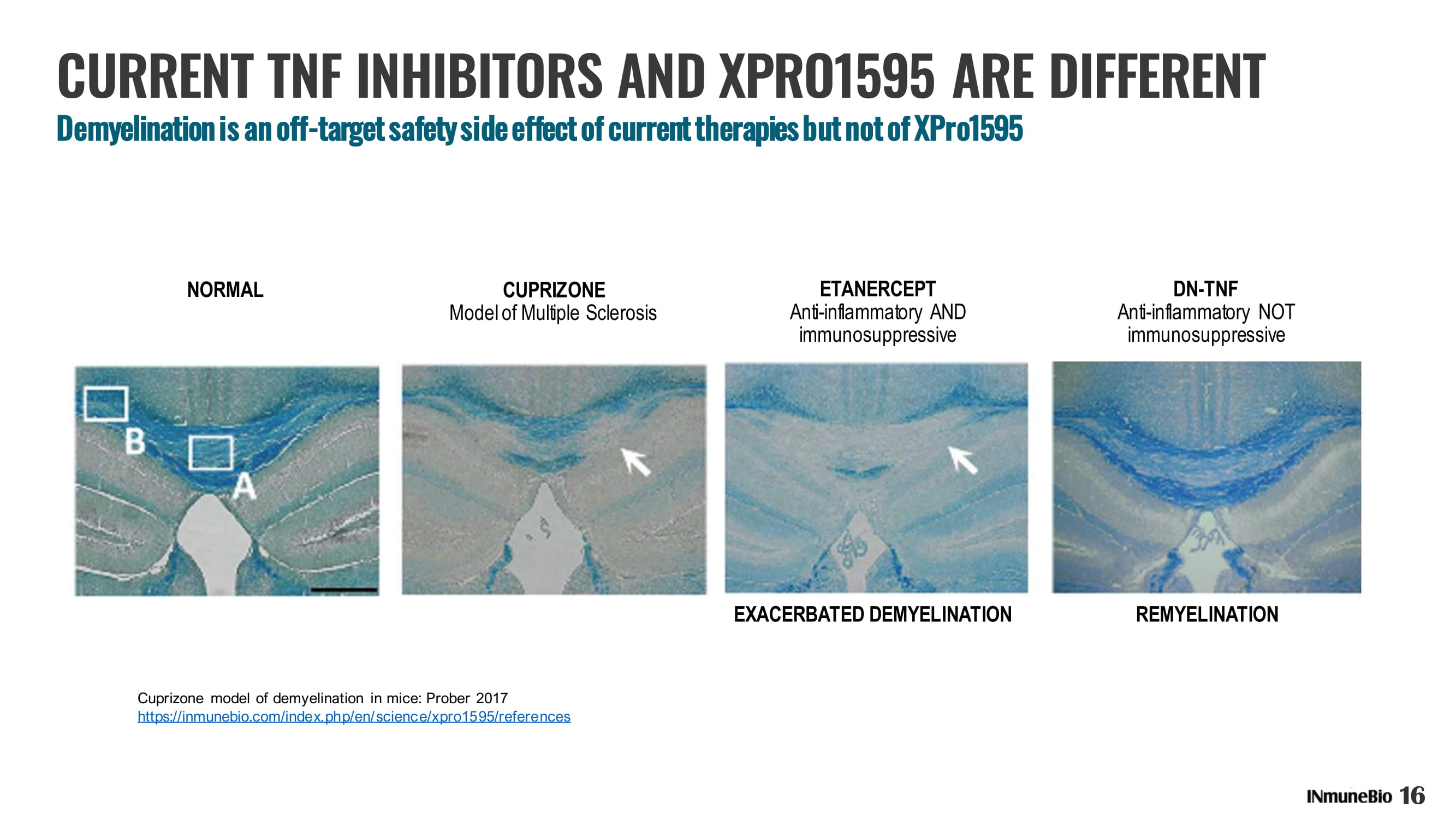
CURRENT TNF INHIBITORS AND XPRO1595 ARE DIFFERENT Demyelination is an off - target safety side effect of current therapies but not of XPro1595 EXACERBATED DEMYELINATION REMYELINATION ETANERCEPT Anti - inflammatory AND immunosuppressive DN - TNF Anti - inflammatory NOT immunosuppressive CUPRIZONE Model of Multiple Sclerosis NORMAL Cuprizone model of demyelination in mice: Prober 2017 https://inmunebio.com/index.php/en/science/xpro1595/references 16

Global TNF inhibitor drug market is estimated to reach $42 billion by 2026 1 17 UNIQUE MOA MEANS EXPANDED MARKET OPPORTUNITY Current TNF inhibitors contraindicated in most markets Estimates from 1 GBI Research, 2 BCC Research , 3 MarketWatch, and 4 ResearchandMarkets.com. Innate Immune Dysfunction Cancer Pulmonary Disease Autoimmune Disease Metabolic Disease Neurological Disease Cardiovascular Disease Alzheimer’s Disease

Selective sTNF Neutralization for the Treatment of Neuroinflammation in TREATMENT RESISTANT DEPRESSION (TRD) 18

TREATMENT RESISTANT DEPRESSION (TRD) The Subset of People Who Have Failed At Least Two Anti Depressants 1 out of 3 MDD patients or 33% of the estimated 7M patients Economic toll: $64B/year Higher comorbidity Chronic course of MDD TNF biology in TRD POC studies with anti - TNF blood CRP predicts response Symptoms affected anhedonia, psychomotor retardation, fatigue, cognition ▪ Drug development in psychiatry is a “one size fits all” approach ▪ 200+ ways to be diagnosed with depression ▪ There are 200+ symptom combinations that will lead to a depression diagnosis ▪ No Biomarkers ▪ High placebo response ▪ Drug development failure rate in psychiatry in Phase II: 35% in Phase III: 65% ▪ Effectiveness of approved drugs ▪ 50% do not achieve remission ▪ 33% have no response (treatment resistant) THE PROBLEM MDD, TRD and Inflammation Frustrations of psychiatric drug development 19
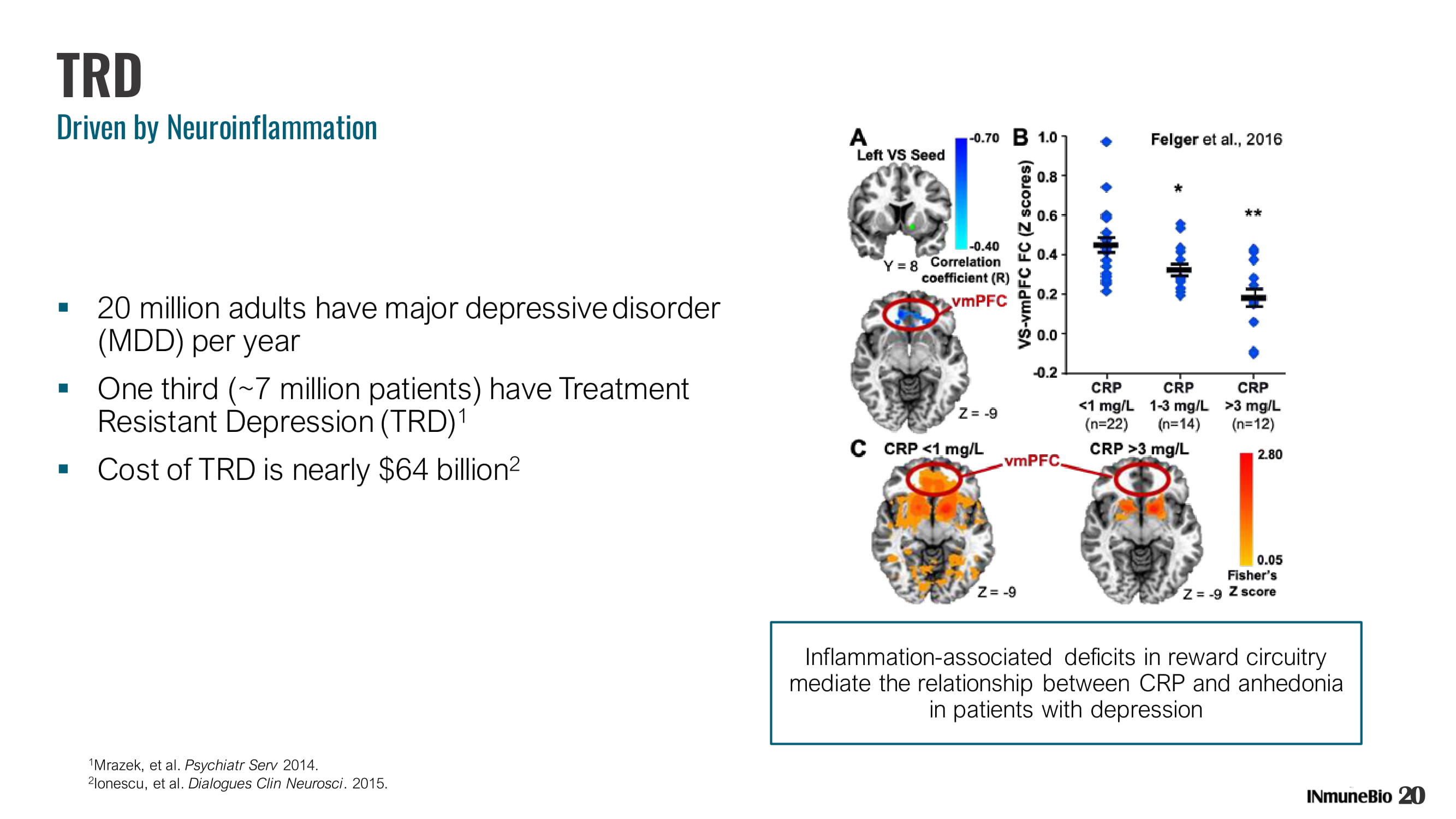
20 TRD Driven by Neuroinflammation ▪ 20 million adults have major depressive disorder (MDD) per year ▪ One third (~7 million patients) have Treatment Resistant Depression (TRD) 1 ▪ Cost of TRD is nearly $64 billion 2 1 Mrazek, et al. Psychiatr Serv 2014. 2 Ionescu, et al. Dialogues Clin Neurosci . 2015. Inflammation - associated deficits in reward circuitry mediate the relationship between CRP and anhedonia in patients with depression
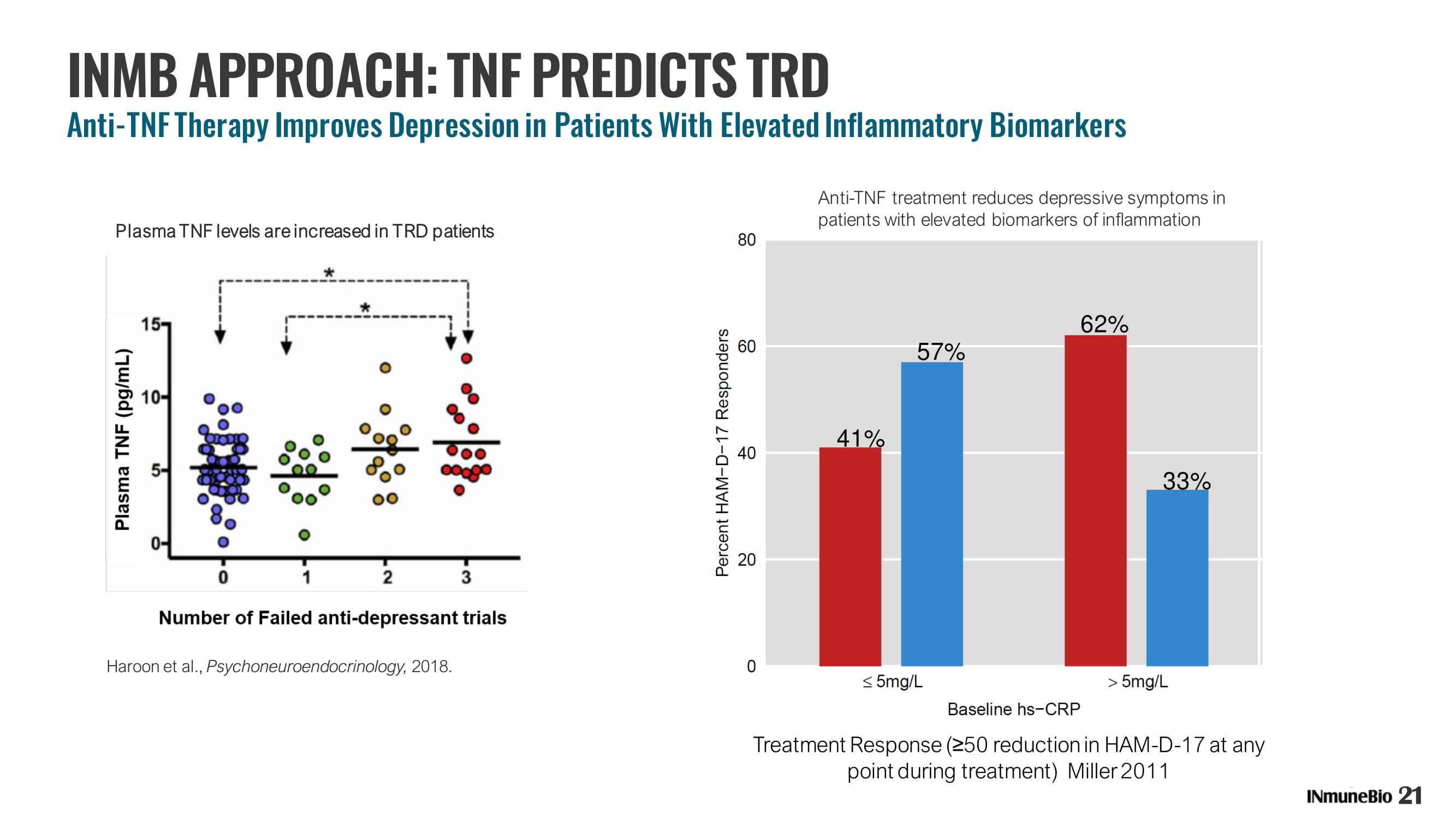
Haroon et al., Psychoneuroendocrinology , 2018. Plasma TNF levels are increased in TRD patients INMB APPR OACH: TNF PREDICTS TRD Anti - TNF Therapy Improves Depression in Patients With Elevated Inflammatory Biomarkers 21 Treatment Response ( ≥ 50 reduction in HAM - D - 17 at any point during treatment) Miller 2011 62% 33% 57% 41% Anti - TNF treatment reduces depressive symptoms in patients with elevated biomarkers of inflammation
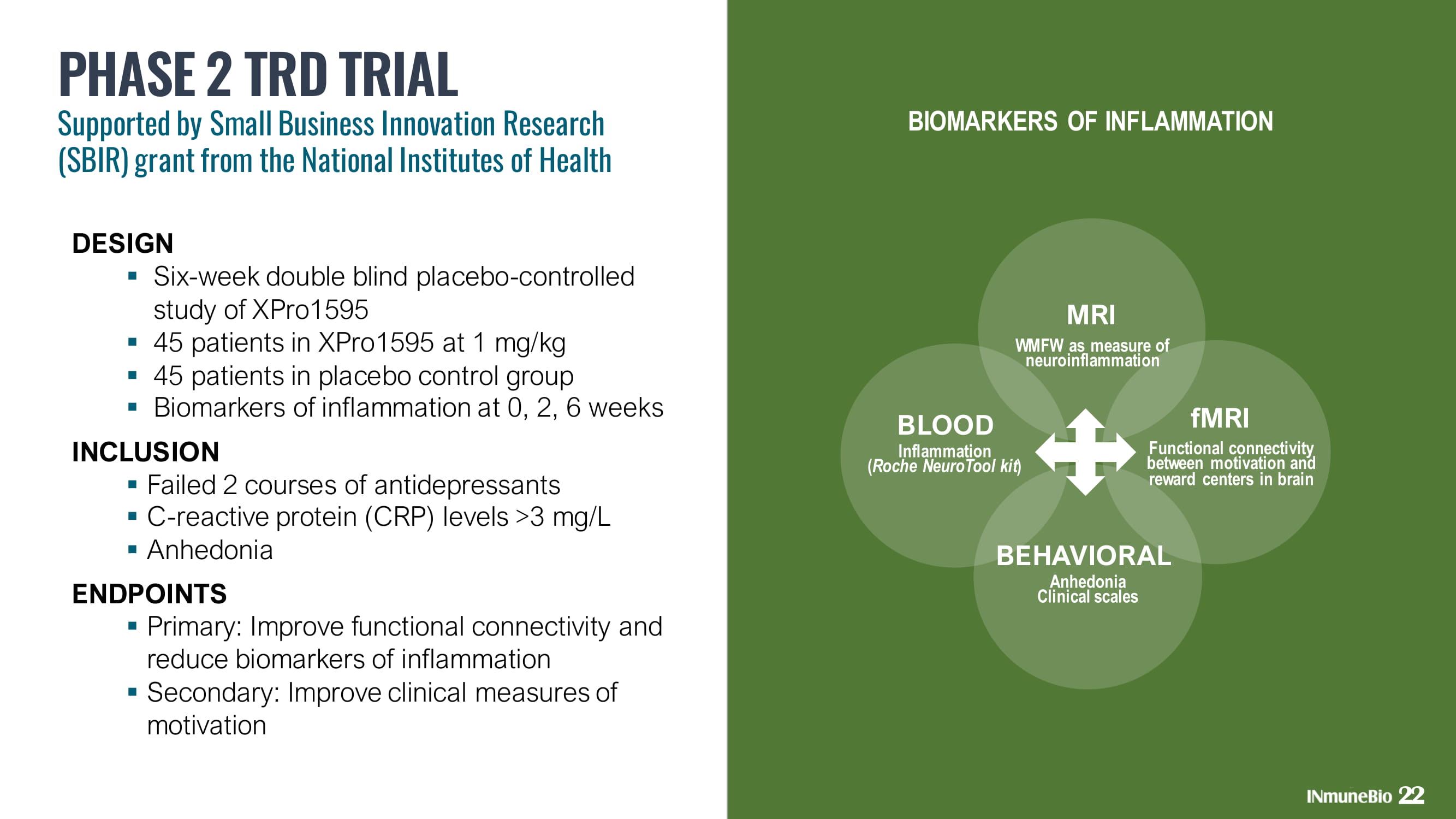
Functional connectivity between motivation and reward centers in brain BIOMARKERS OF INFLAMMATION DESIGN ▪ Six - week double blind placebo - controlled study of XPro1595 ▪ 45 patients in XPro1595 at 1 mg/kg ▪ 45 patients in placebo control group ▪ Biomarkers of inflammation at 0, 2, 6 weeks INCLUSION ▪ Failed 2 courses of antidepressants ▪ C - reactive protein (CRP) levels >3 mg/L ▪ Anhedonia ENDPOINTS ▪ Primary: Improve functional connectivity and reduce biomarkers of inflammation ▪ Secondary: Improve clinical measures of motivation PHASE 2 TRD TRIAL Supported by Small Business Innovation Research (SBIR) grant from the National Institutes of Health 22 Inflammation ( Roche NeuroTool kit ) Anhedonia Clinical scales WMFW as measure of neuroinflammation MRI BLOOD fMRI BEHAVIORAL

23 Clinical Trial to Determine if Targeting Soluble TNF (sTNF) may Prevent Catastrophic Complications of COVID - 19 due to Cytokine Storm

24 WHAT BRINGS PATIENTS TO THE HOSPITAL WITH COVID - 19? Cytokine Storm is making patients sick. At time of admission viral titer is decreasing Blunting the effects of Cytokine Storm will make patients better Target the Master Cytokine of the Cytokine Storm with QUELLOR Problem Solution Hypothesis The dysregulated innate immune response to the virus causes Cytokine Storm
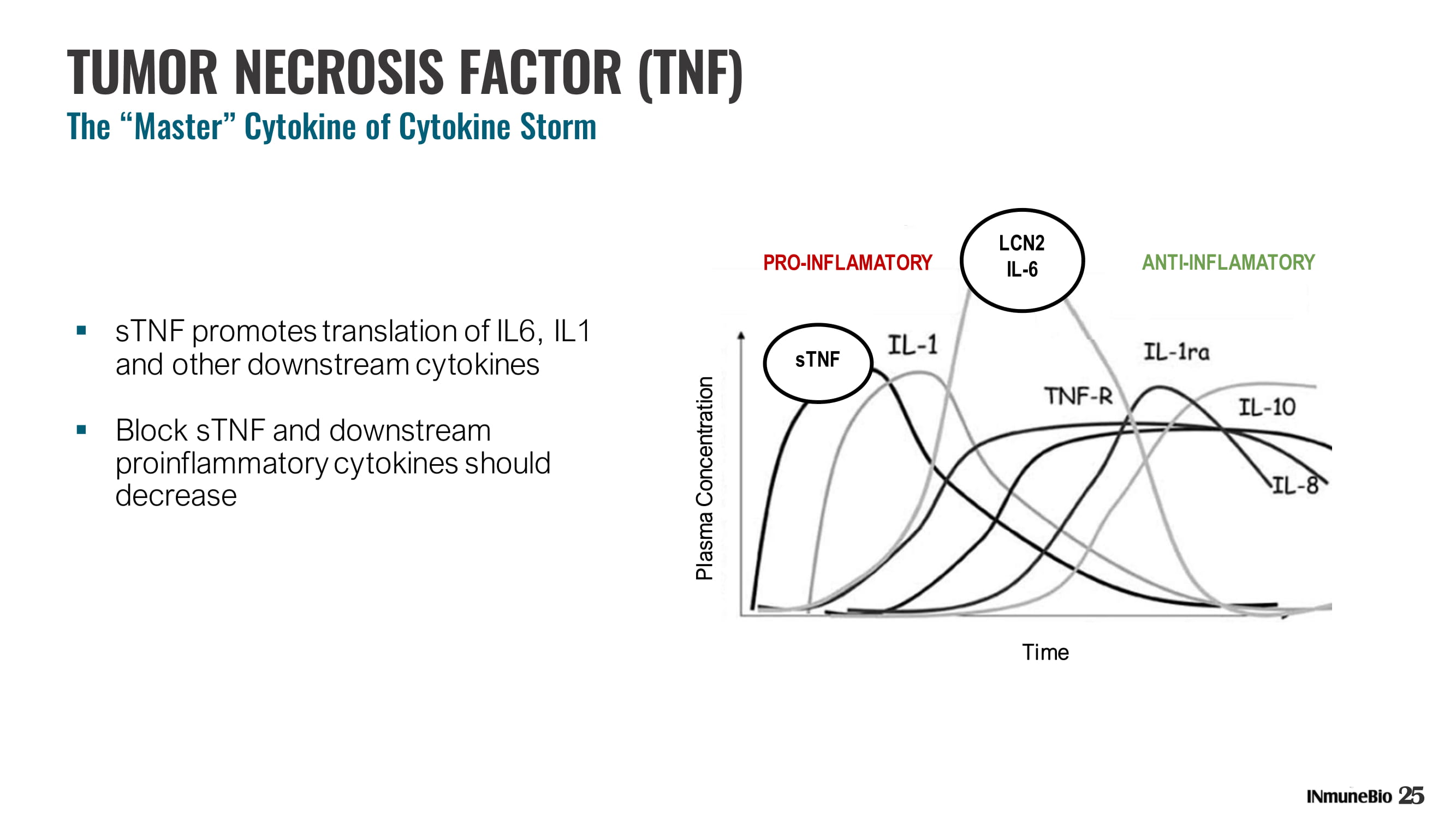
TUMOR NECROSIS FACTOR (TNF) The “Master” Cytokine of Cytokine Storm 25 ▪ sTNF promotes translation of IL6, IL1 and other downstream cytokines ▪ Block sTNF and downstream proinflammatory cytokines should decrease LCN2 IL - 6 ANTI - INFLAMATORY Time Plasma Concentration sTNF PRO - INFLAMATORY
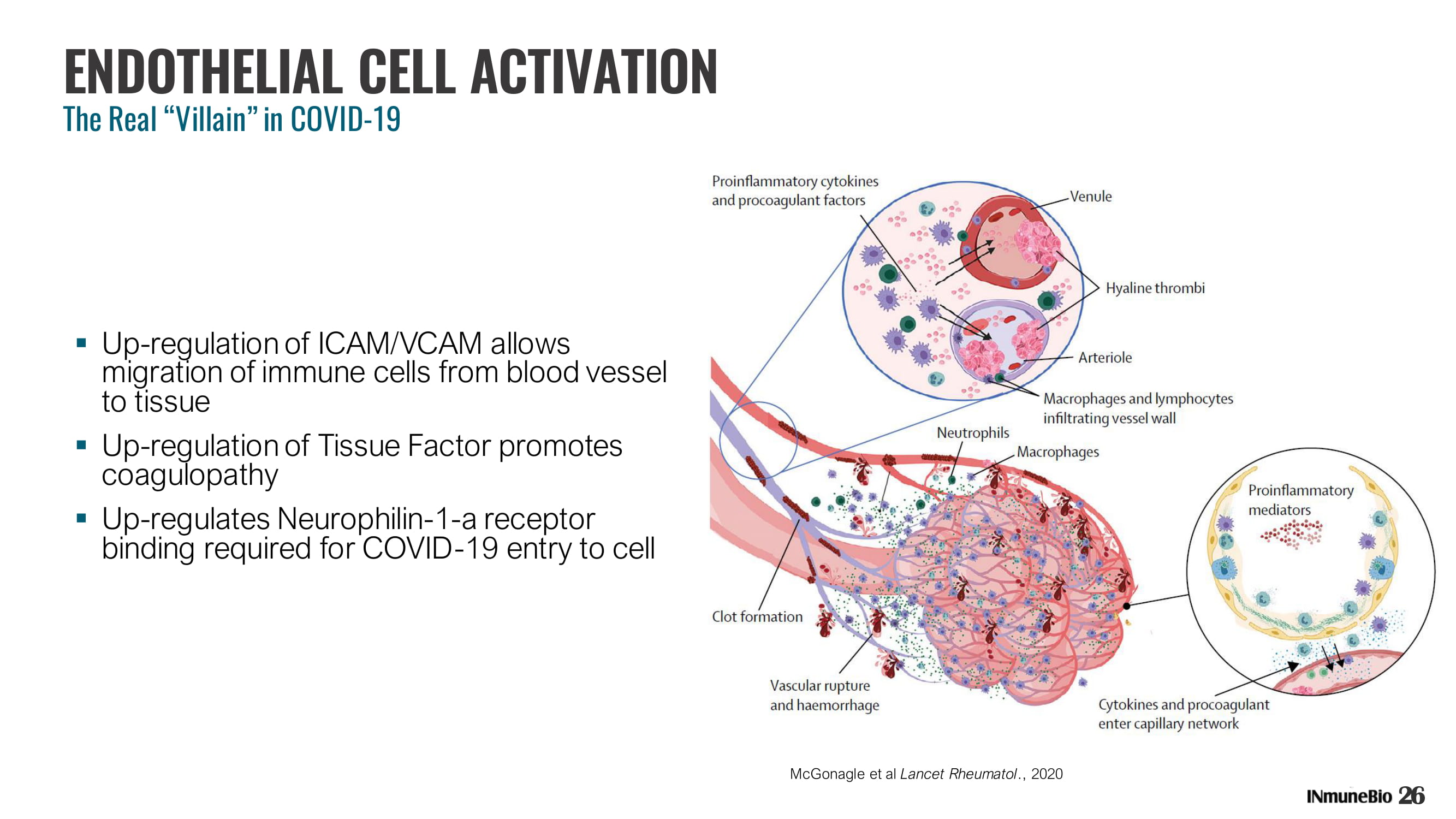
ENDOTHELIAL CELL ACTIVATION The Real “Villain” in COVID - 19 ▪ Up - regulation of ICAM/VCAM allows migration of immune cells from blood vessel to tissue ▪ Up - regulation of Tissue Factor promotes coagulopathy ▪ Up - regulates Neurophilin - 1 - a receptor binding required for COVID - 19 entry to cell McGonagle et al Lancet Rheumatol ., 2020 26

PHASE 2 TRIAL: Treating Pulmonary Complications of COVID - 19 27 27 DESIGN ▪ SOC vs SOC+Quellor w 1mg/kg subQ day 1 and 7 (if in hospital) ▪ Patient discharged based on clinical status or final study visit day 28 ▪ 360 patients randomized 1:1 INCLUSION ▪ COVID - 19 infection with room air SaO2<94% ▪ One or more medical/demographic comorbidities: age ≥ 60; hypertension, cardiovascular disease, BMI ≥ 30; diabetes, Black or Hispanic race ENDPOINTS ▪ Primary: Need for mechanical ventilation in 28 days ▪ Secondary: Transfer to ICU, new onset neurologic, cardiovascular or thromboembolic disease, development of renal failure or death GOAL: PREVENT PROGRESSION TO CATASTROPHIC COMPLICATIONS First 100 patients “proof - of - concept” to GO/NOGO decision If DSMB says “GO”, follow - on study 266 patients
Provides essential signals to patients’ NK cells to target and kill tumor cells. INKmune ʳ is not antigen - specific so there is no need to identify the specific tumor antigen 28
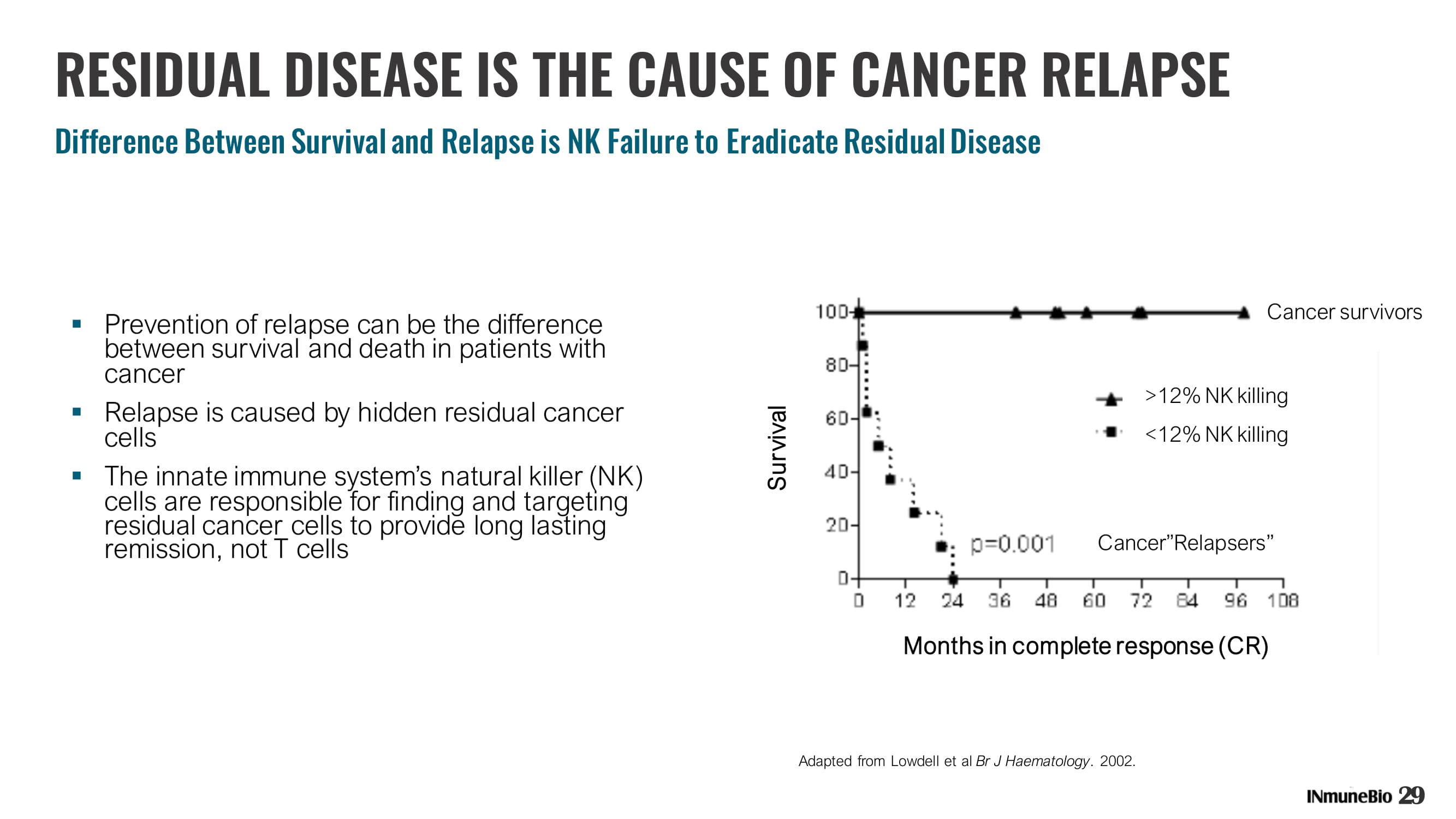
RESIDUAL DISEASE IS THE CAUSE OF CANCER RELAPSE Difference Between Survival and Relapse is NK Failure to Eradicate Residual Disease ▪ Prevention of relapse can be the difference between survival and death in patients with cancer ▪ Relapse is caused by hidden residual cancer cells ▪ The innate immune system’s natural killer (NK) cells are responsible for finding and targeting residual cancer cells to provide long lasting remission, not T cells 29 Adapted from Lowdell et al Br J Haematology . 2002. Months in complete response (CR) >12% NK killing <12% NK killing Cancer survivors Cancer”Relapsers ” Survival
• Cancer patient NK cells are terminally defective and must be replaced with NK from healthy donors • Nanktwest • Fate • Takeda • Glycostem • Cancer patient NK cells can be enhanced to mediate autologous tumor killing • Rituximab / Herceptin etc • IL15RA • Ex - vivo activated/expanded NK cells • INmuneBio INKmune TWO HYPOTHESIS BEHIND NK IMMUNOTHERAPY Difference Between Survival and Relapse is NK Failure to Eradicate Residual Disease

Provides the innate signals necessary to prime a patient’s resting NK cells, empowering the immune system to eliminate residual cancer cells INKmune Œ CELLS PRIME NK CELLS TO ELIMINATE RESIDUAL DISEASE Tumor cells down - regulate surface molecules which prime NK cell activity, thus evading NK cell killing allowing the cancer to grow RELAPSE DUE TO RESIDUAL DISEASE S1 S2 S2 Resting NK Cell Cancer Cell Priming Triggering S2 Tumor - primed NK Cell Dead - Cancer Cell Triggering S2 31 S2 31 INKmune ADVANTAGES: x Universal — Potentially effective in blood cancers and solid tumors x Off - the - shelf therapy x Easy to use INKmune ʳ IS A BIOLOGIC SYSTEM TO DELIVER ESSENTIAL PRIMING SIGNALS TO PATIENTS’ RESTING NK CELLS
32 HIGH - RISK MYELODYSPLASTIC SYNDROME (MDS) ▪ MDS is an incurable disease ▪ One - third of all MDS cases evolve to become acute myeloid leukemia (AML) ▪ Survival for patients with high - risk MDS is dismal ▪ Patients with high - risk MDS have functionally defective NK cells 1 ▪ Level of NK dysfunction is predictive of overall survival 2 ▪ By 2022, global MDS drug market is expected to reach $2.4 billion USD 2 1 Kiladjian et al 2006 2 Tsirogianni et al 2019 3 Grand View Research
INKmune ʳ PHASE 1 STUDY DESIGN ▪ Open - label dose escalation study of intravenous INKmune enrolling 9 patients INCLUSION ▪ Patients with MDS with excess blasts ENDPOINTS ▪ Primary: Evaluate the safety and tolerability of INKmune when given intravenously ▪ Secondary: ▪ Assess the change in [or effect upon] number and percentage of blasts in peripheral blood and bone marrow ▪ Assess the overall response rate in subjects administered INKmune w using WHO criteria ▪ Assess the duration of response First in human trial of INKmune 33 Measure primed NK cells and function Assess pharmacodynamics Phase I Trial in High - Risk MDS GOAL: EVALUATE SAFETY & TOLERABILITY OF INKmune w IN HIGH - RISK MDS

34 2019 Q1 Q2 Q3 Q4 NASDAQ Listing $1 Million Park the Cloud Award – Alzheimer’s Association Initial Data on INB03 Phase 1 $1 Million Park the Cloud Award – Alzheimer’s Association INB03 Phase 1 Readout XPro1595 First Patient Enrolled in Phase 1 AD Trial $500,000 ALS Association award Phase 2 Trial Initiation Interim readout showing decreases neuroinflammation First Patient high - risk MDS Trial MILESTONES AND FUTURE CATALYSTS First Patient Ovarian Cancer Phase 2 AD initiation 2020 Q1 Q2 Q3 Q4 2021 1H 2H $25 M Capital Raise IND for CV - 19 $2.9 5 M Grant from NIMH for TRD Phase II TRD Phase 2 Phase 2 Expanded P1 readout

DN - TNF PATENTS* 2024 pegylated DN - TNF (licensed from Xencor) 2032 Methods for treatment of neurologic disease 2035 use for treatment of cancer (issued in US) 2039 use for treatment of NASH 2040 use for immune mediated complications from COVID - 19/CRS 35 NK PATENTS* 2035 use for treatment of cancer 2039 INB16 composition - of - matter FUTURE Broad Platforms allow for continual R&D and new IP * Subject to issuance by patent granting authority

CONTACT US 36 HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM INMB INmune Bio Inc. 1200 Prospect Str. Suite 525 La Jolla, CA 92037 (858) 964 - 3720 www.inmunebio.com

APPENDIX 37

MANAGEMENT TEAM The Phoenix Partners TEAM PEDEGREE 38 Raymond J. Tesi, M.D. CEO/CMO & Chairman of the Board David J. Moss CFO Mark W. Lowdell , Ph.D., FRCPath , FRSB, CSO Christopher J. Barnum, Ph.D. Director of Neuroscience Joshua S. Schoonover, Esq. Assoc. General Counsel

Free water = edema/swelling = inflammation WHITE MATTER FREE WATER A Biomarker For Neuroinflammation
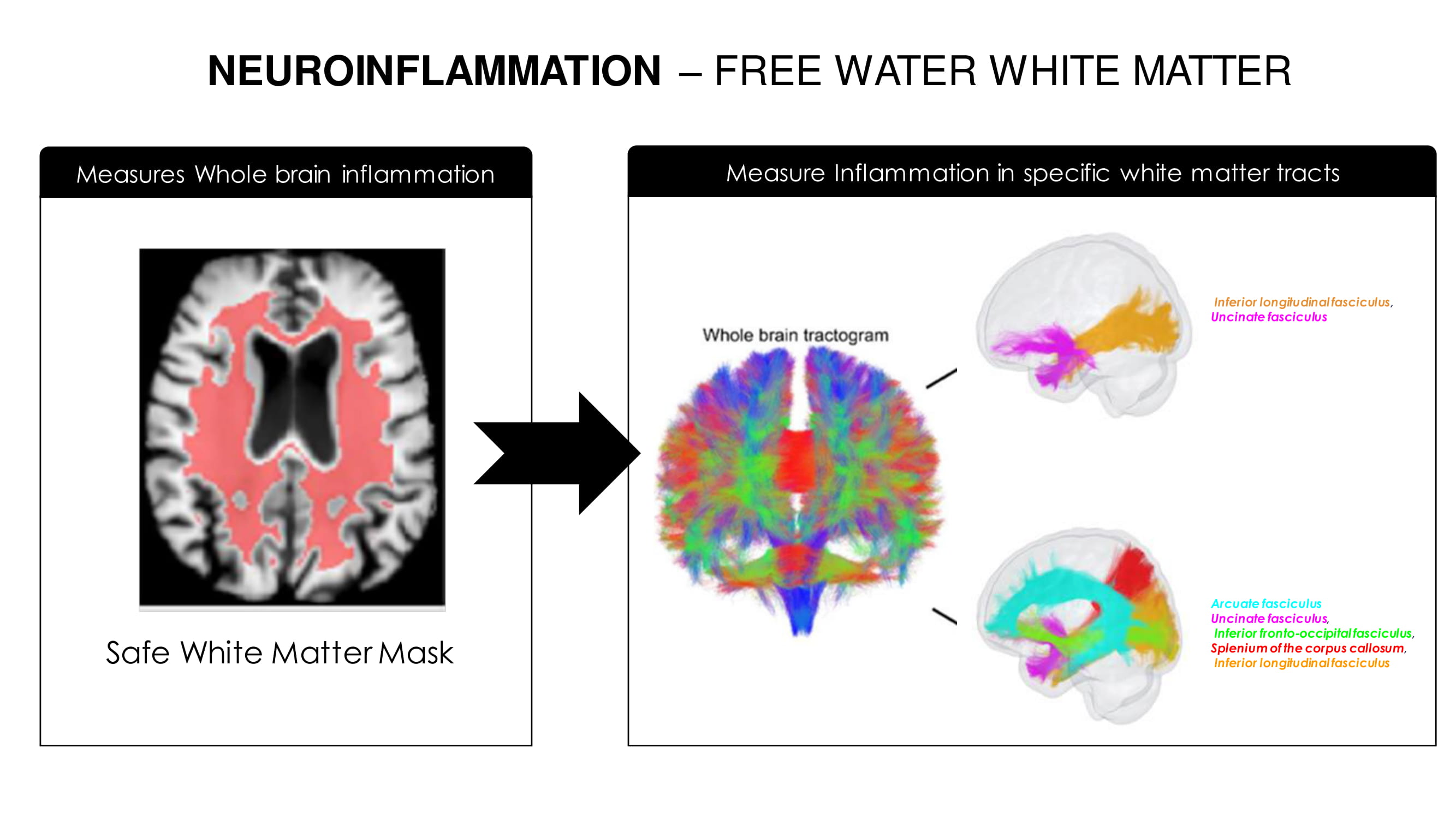
NEUROINFLAMMATION – FREE WATER WHITE MATTER Safe White Matter Mask Arcuate fasciculus Uncinate fasciculus , Inferior fronto - occipital fasciculus , Splenium of the corpus callosum , Inferior longitudinal fasciculus Inferior longitudinal fasciculus , Uncinate fasciculus Measures Whole brain inflammation Measure Inflammation in specific white matter tracts

DN - TNF Options in Immuno - Oncology for Treatment of Resistance to Immunotherapies 41
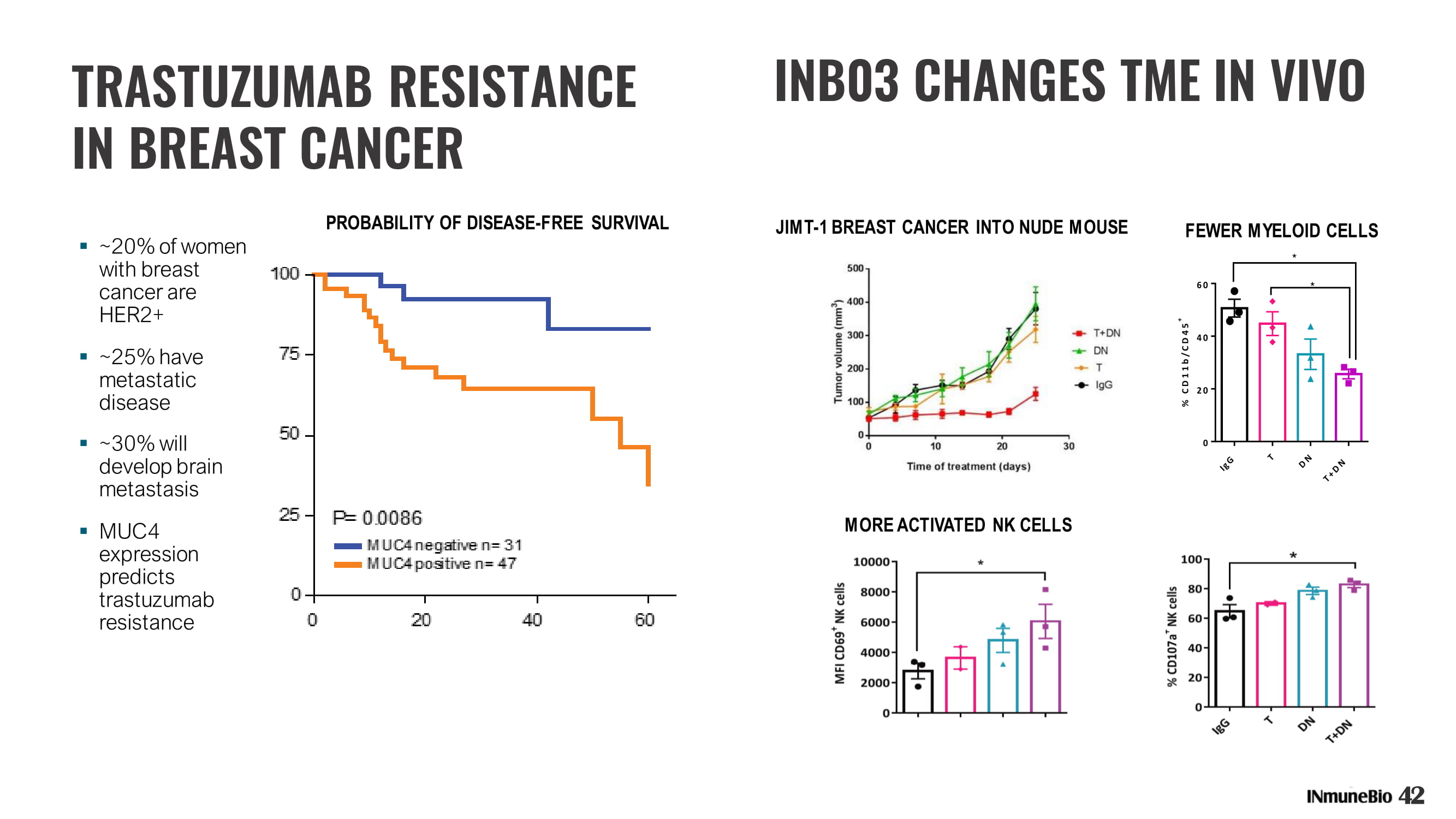
▪ ~20% of women with breast cancer are HER2+ ▪ ~25% have metastatic disease ▪ ~30% will develop brain metastasis ▪ MUC4 expression predicts trastuzumab resistance PROBABILITY OF DISEASE - FREE SURVIVAL 42 TRASTUZUMAB RESISTANCE IN BREAST CANCER FEWER MYELOID CELLS JIMT - 1 BREAST CANCER INTO NUDE MOUSE MORE ACTIVATED NK CELLS INB03 CHANGES TME IN VIVO

INB03 REVERSES TRASTUZUMAB RESISTANCE 1 st – It Modifies immunology of TME and DECREASES MYELOID CELLS, INCREASES NK CELLS IN VIVO 2 nd – It Decreases MUC4 Expression – MUC4 Expression resistance to Trastuzumab and promotes metastasis T - trastuzumab DN - INB03 Schillaci - NYAS2020 MUC4 BLOCKS TRASTUZUMAB BINDING INB03 DECREASES MUC4 EXPRESSION 43 Model of Metastasis: Downregulation of MUC4 prevents wound closure in in JIMT - 1 TUMORS 0 50 100 % Wound Closure ** ns n s MUC4 BLOCKS FUNCTION OF TRASTUZUMAB CONJUGATES Using MUC4 silencing siRNA confirms MUC4 causes resistance to trastuzumab based immunotherapy – naked or as part of conjugate (Schillaci 2017)

SAMPLE 11 patients treated DEMOGRAPHIC Age (median): 54 Gender: 6M/5F DISEASE Ovary, Mesothelioma; RCC, Lung; Prostate; Colon, Cholangial, other PREVIOUS LINES OF THERAPY 3 (range:2 - 5) INB03 DURATION 74 days (21 - 119d) • No drug related SAE • All discontinuation due to disease progression PHASE I SUMMARY INB03 has been tolerated across multiple dose levels ▪ No SAE, No DLT ▪ Well tolerated ▪ 1mg/kg once a week will be carried into Phase 2 ▪ Dose provides robust trough drug levels ▪ Pharmacologically active: >50% decrease of IL6 in blood ▪ Results informed Phase 2 design Open Label, Biomarker Directed, Dose - escalation Trial In Patients With Advanced Solid Tumors And Elevated Markers Of Inflammation ENROLLMENT CRITERIA: Advanced solid tumors hsCRP > 4 mg/L Treatment: INB03 subQ once a week 3 cohorts of 3: 0.3, 1.0, 3.0 mg/kg 44 CONCLUSIONS FROM INB03 ʳ PHASE I DATA
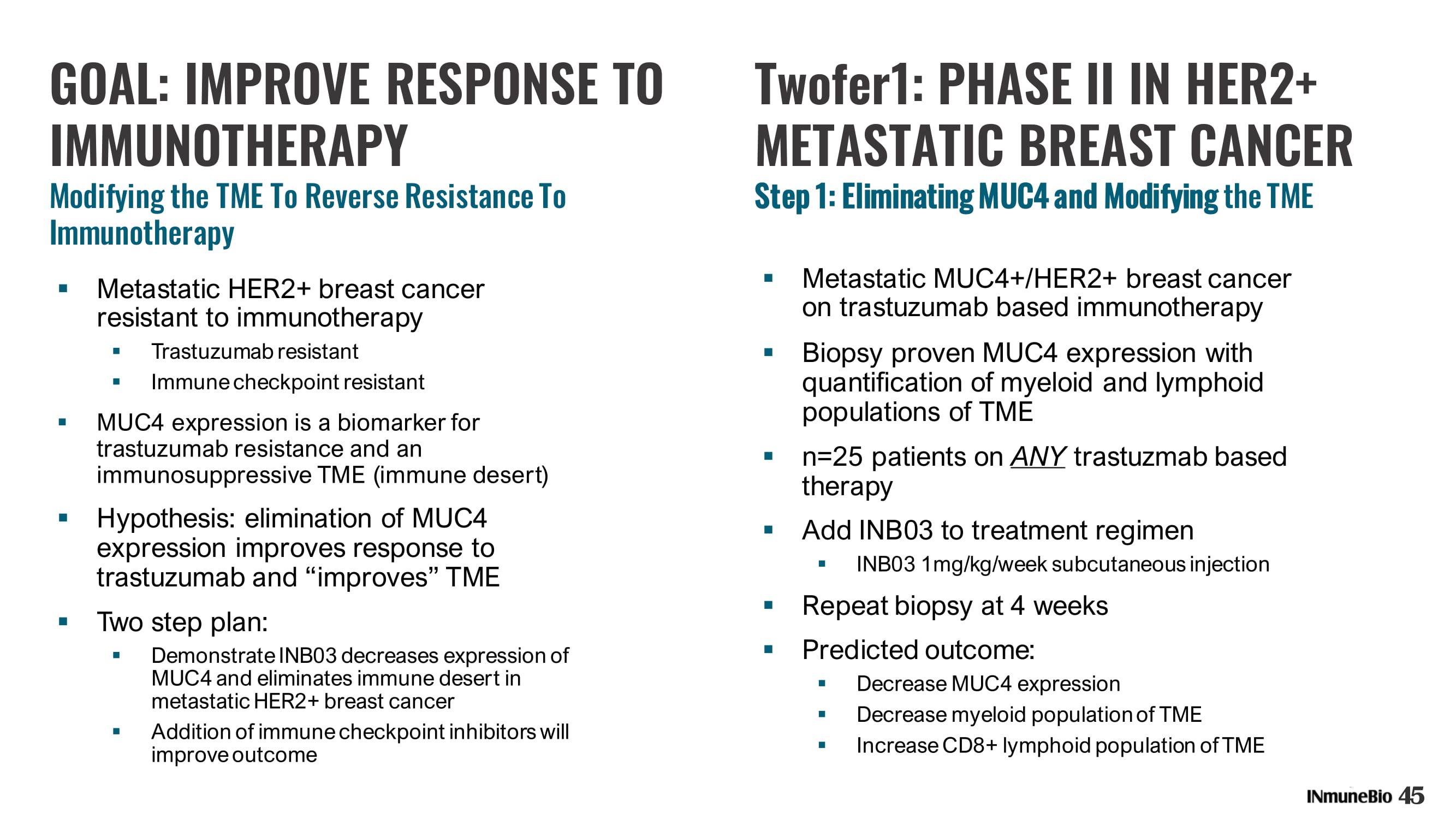
45 Twofer1: PHASE II IN HER2+ METASTATIC BREAST CANCER Step 1: Eliminating MUC4 and Modifying the TME GOAL: IMPROVE RESPONSE TO IMMUNOTHERAPY Modifying the TME To Reverse Resistance To Immunotherapy ▪ Metastatic HER2+ breast cancer resistant to immunotherapy ▪ Trastuzumab resistant ▪ Immune checkpoint resistant ▪ MUC4 expression is a biomarker for trastuzumab resistance and an immunosuppressive TME (immune desert) ▪ Hypothesis: elimination of MUC4 expression improves response to trastuzumab and “improves” TME ▪ Two step plan: ▪ Demonstrate INB03 decreases expression of MUC4 and eliminates immune desert in metastatic HER2+ breast cancer ▪ Addition of immune checkpoint inhibitors will improve outcome ▪ Metastatic MUC4+/HER2+ breast cancer on trastuzumab based immunotherapy ▪ Biopsy proven MUC4 expression with quantification of myeloid and lymphoid populations of TME ▪ n=25 patients on ANY trastuzmab based therapy ▪ Add INB03 to treatment regimen ▪ INB03 1mg/kg/week subcutaneous injection ▪ Repeat biopsy at 4 weeks ▪ Predicted outcome: ▪ Decrease MUC4 expression ▪ Decrease myeloid population of TME ▪ Increase CD8+ lymphoid population of TME

Addressing the Link Between Liver Fibrosis, the Innate Immune Cells of the Liver and Inflammation, a Pleiotropic Approach 46
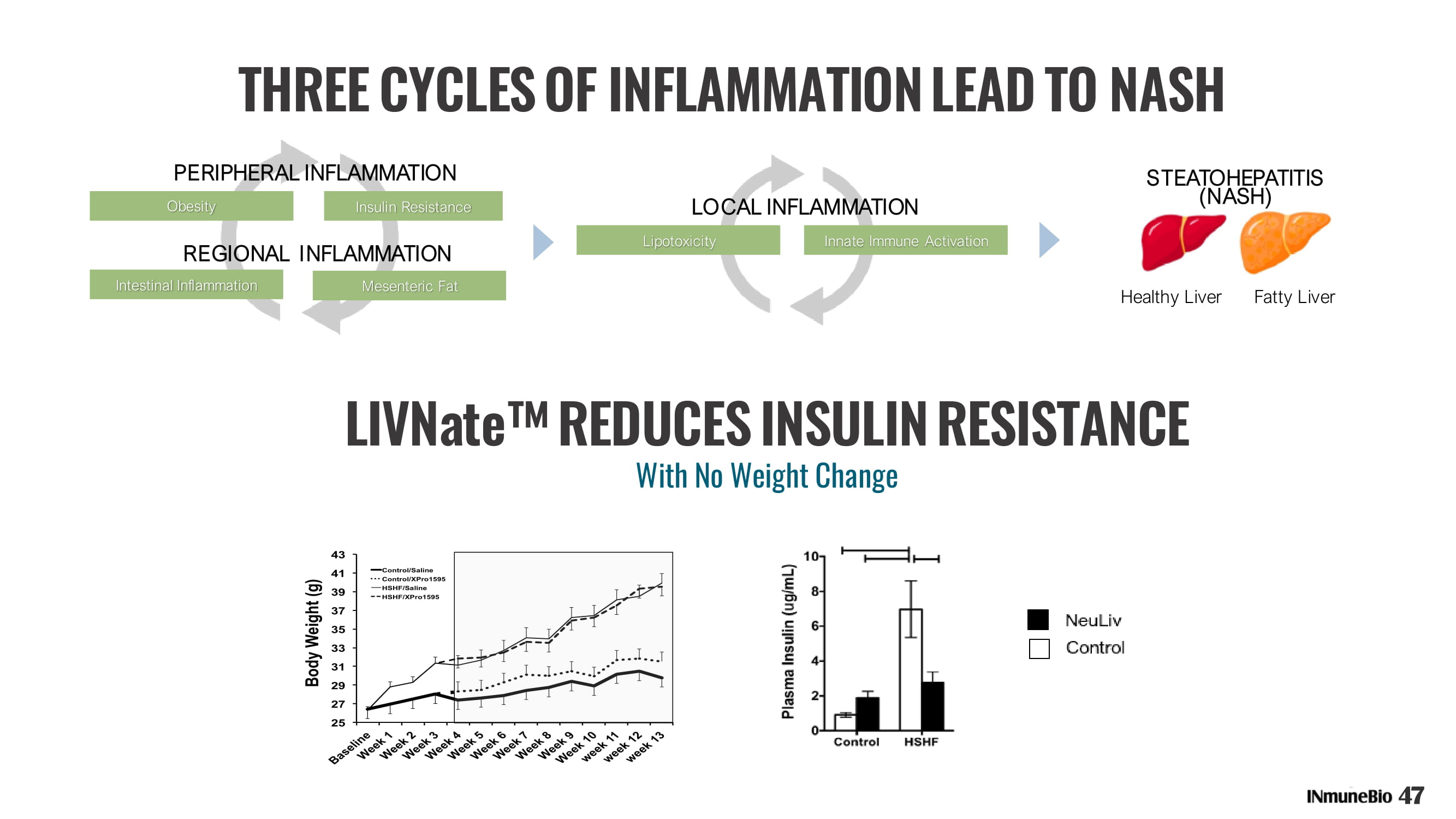
THREE CYCLES OF INFLAMMATION LEAD TO NASH LIVNate ʳ REDUCES INSULIN RESISTANCE 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 25 27 29 31 33 35 37 39 41 43 B a s e l i n e W e e k 1 W e e k 2 W e e k 3 W e e k 4 W e e k 5 W e e k 6 W e e k 7 W e e k 8 W e e k 9 W e e k 1 0 w e e k 1 1 w e e k 1 2 w e e k 1 3 B o d y W e i g h t ( g ) Control/Saline Control/XPro1595 HSHF/Saline HSHF/XPro1595 47 With No Weight Change REGIONAL INFLAMMATION PERIPHERAL INFLAMMATION Obesity Insulin Resistance Intestinal Inflammation Mesenteric Fat LOCAL INFLAMMATION Lipotoxicity Innate Immune Activation STEATOHEPATITIS (NASH) Healthy Liver Fatty Liver
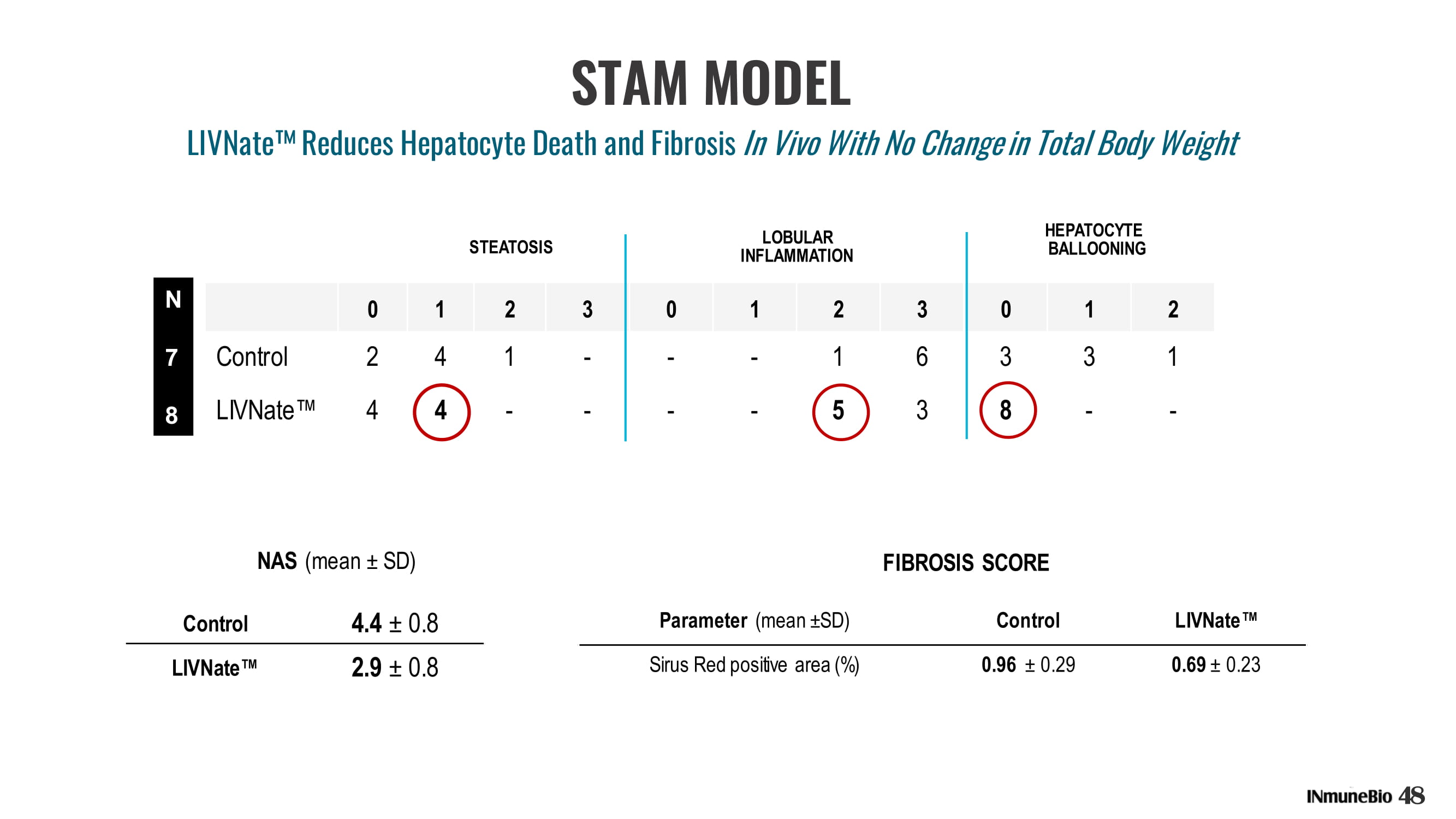
0 1 2 3 0 1 2 3 0 1 2 Control 2 4 1 - - - 1 6 3 3 1 LIVNate Œ 4 4 - - - - 5 3 8 - - HEPATOCYTE BALLOONING LOBULAR INFLAMMATION STEATOSIS Control 4.4 ц 0.8 LIVNate Œ 2.9 ц 0.8 NAS (mean ц SD) Parameter (mean ц SD) Control LIVNate Œ Sirus Red positive area (%) 0.96 ц 0.29 0.69 ц 0.23 FIBROSIS SCORE N 7 8 STAM MODEL 48 LIVNate ʳ Reduces Hepatocyte Death and Fibrosis In Vivo With No Change in Total Body Weight

NASH TRIAL DESIGN Phase IIa Biomarker Directed Trial Phase IIa open label trial ▪ Patients with F2/3 NASH by non - invasive studies with >10% fat in liver with LIVNate Œ 1mg/kg SQ treatment with for 12 weeks ▪ PIFF and non - invasive biomarkers at 0, 6 and 12 weeks ▪ Open label so biomarkers “visible” at 6 - week time point N = 20 patients Expected results 1. Decrease in liver fat 2. Improvement in liver function tests 3. Improvement in insulin resistance. Exploratory end - points ▪ Improvement in breath test ▪ Decrease in intestinal leak 49

NON - EMPLOYEE BOARD OF DIRECTORS David Szymkowski, PhD David E . Szymkowski leads the immunology group as Vice President of Cell Biology at Xencor Inc where he is focused on translational development of Fc - engineered and bispecific antibodies for the treatment of autoimmune diseases, allergic diseases, and cancer . Prior to joining Xencor in 2002 , Dr . Szymkowski was a principal scientist in the respiratory group at Roche Bioscience in Palo Alto, CA . With 25 years of big pharma and biotech R&D experience at Roche and at Xencor, Dr . Szymkowski has been instrumental in 10 IND submissions, coauthored over forty papers and reviews, is an inventor on over a dozen patents, and speaks frequently on the development of antibody therapeutics and other biologics . He received his B . A . at Johns Hopkins University and his Ph . D . in molecular and cell biology from Penn State, and completed a postdoc at the Imperial Cancer Research Fund (U . K . ) . J . Kelly Ganjei Mr . Ganjei has been a director since September 2016 . He is Chief Executive Officer of Cognate BioServices, Inc . , a position he has held since 2011 . Mr . Ganjei has over 20 years of experience within the life science, venture capital and IT sectors and has lead companies through various stages of development . Prior to joining Cognate, Mr . Ganjei was Principal at an SBA venture capital firm where he instrumental in supporting deal flow with a specific focus on regenerative medicine, immunotherapy and cell therapy investment opportunities .. He began his career at the National Institutes of Health . Mr . Ganjei has published numerous scientific, peer - reviewed papers and has been a speaker and presenter at various business forums . Mr . Ganjei received his B . S . in Microbiology from the University of Maryland College Park in 1995 . Timothy Schroeder Mr . Schroeder has been a director since December 2016 Mr . Schroeder has more than 35 years of clinical and academic industry experience in global drug and device development programs . He is CEO of CTI Clinical Trial and Consulting Services, a multi - national research firm with locations in North America, Europe, Latin America and Asia - Pacific . Prior to founding CTI, Mr . Schroeder was a faculty member of the University of Cincinnati College of Medicine . He previously served as Executive Vice President of Clinical Development at SangStat Medical Corporation, a firm he co - founded . Mr . Schroeder is a board member for more than a dozen corporate and non - profit organizations . He was named as EY Entrepreneur of the Year in 2015 and was recognized as Top Leader by the Enquirer Media in 2016 . Edgardo (Ed) Baracchini, PhD Mr . Eduardo (“Ed”) Baracchini has been a director since July 2019 . He is also current a member of the board of directors of 4 D Pharma PLC . Prior to providing biotech consulting services since September 2018 , Ed was chief business officer of Xencor, Inc . Ed was associated with Metabasis Therapeutics, initially as vice president of business development, and later as SVP of business development . Ed holds over 25 years of experience in structuring and negotiating research and development partnerships, mergers and acquisitions, and licensing agreements . He has personally, negotiated more than 80 business transactions with multinational and Asian pharmaceutical firms, biotechnology companies, and prominent universities, leading to transactions valued in excess of $ 5 . 3 billion .. Additionally, have been a key member of executive teams that have raised over $ 850 million in private and public financing, and that have successfully completed two IPOs . Ed received his MBA from the University of California, Irvine, his PhD in molecular and cell biology from the University of Texas at Dallas, and his B . S . in microbiology from the University of Notre Dame . BOARD PEDEGREE Marcia Allen Marcia Allen has been a director since November 2019 . Ms . Allen was a founder and served as CFO and Director of The Movie Group, (AMX) the originating company which is today Lionsgate Entertainment (NYSE) . She has more than 25 years with mergers and acquisitions, corporate finance and CFO and CEO experience . Ms . Allen was a Chief Financial Officer and Corporate Development Officer for W . R . Grace & Co . (NYSE) and was based both in Newport Beach, CA with the Restaurant Division and in its New York headquarters . Ms . Allen moved to California from Tennessee where she was part of the founding group of Ruby Tuesday, Inc . , (NYSE) a national restaurant chain . She relocated to join Taco Bell, Inc . as the Company's Chief Financial Officer where she structured and facilitated the acquisition of Taco Bell, Inc . by PepsiCo, Inc . She currently serves as the chairperson of the audit committee for Ark Restaurants Corp (Nasdaq) . Ms . Allen received a Bachelors, Finance and Accounting degree from the University of Tennessee . 50 Scott Juda, JD Mr . Juda has been a director since March 2018 . Mr . Juda is Manager and co - founder of Fossick Capital, a technology - focused hedge fund . Mr . Juda co - founded The Juda Group, Inc . , an institutional capital markets focused broker - dealer division of CCM, where he served as Chief Executive Officer from 2012 to 2016 . Before that, Mr . Juda was at SMH Capital from 2002 to 2011 , serving as a Managing Director in the Investment Banking Group and Chief Operating Officer of The Juda Group subsidiary . From 2000 to 2002 , Mr . Juda was an institutional sales - trader for Sutro & Co . From 1997 to 2000 , Mr . Juda practiced corporate and securities law at Buchalter Nemer LLP . Mr . Juda received his bachelor degree from the University of Southern California and his juris doctor from the University of Pepperdine School Of Law . Mr . Juda is a member of the State Bar of California .

REFRACTORY OVARIAN CANCER CLINICAL Phase 1 Open - label Study of Intraperitoneal INKmune ʳ in Patients with Relapsed Platinum - resistant, Platinum - refractory, or Platinum - Intolerant Ovarian Cancer Primary 1. To evaluate the safety and tolerability of INKmune when given intraperitoneally 51 Exploratory 1. To assess the pharmacodynamics of INKmune Secondary 1. To assess progression - free survival 2. To assess the overall response rate using RECIST v1.1 and/or GCIG CA 125 criteria 3. To assess the duration of RECIST and/or GCIG CA 125 response ▪ Refractory CaOva is an incurable disease ▪ Despite optimal surgery and paclitaxel – carboplatin chemotherapy approximately 70% of patients with primary ovarian cancer will relapse in the first 3 years ▪ Objective response rates to second - line therapies such as weekly paclitaxel, doxorubicin, topotecan and gemcitabine are in the r ange of 20 - 30% and median overall survival is <1 year (Gordon et al 2000, Markman et al 2002) ▪ The ascites of CaOva patients contains plentiful NK but few T cells; suggesting that the principal IR to CaOva is NK mediated (supported by failure of CPIs) ▪ Our in vitro data show that defective NK cells from CaOva ascites can be primed with INKmune to kill NK - resistant CaOva tumor cells


















































