Exhibit 99.1

HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM Modulating an Innate Immune Response Against Diseases IN MB XPro1595 Alzheimer’s KOL event Jan 21, 2021

FORWARD LOOKING STATEMENTS This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . There is no guarantee that any specific outcome will be achieved . Investment results are speculative and there is a risk of loss, potentially all loss of investments . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . INMB KOL AD January 21, 2021

DESIGN ▪ Multiple dosing cohorts ▪ Weekly XPro1595 Œ SubQ injections for 3 months ▪ Biomarkers of neuro inflammation at 0 and 12 weeks Inclusion ▪ AD Diagnosis ▪ Biomarkers of inflammation (CRP, ESR, HgbA1C, APOE4) STUDY GOAL Demonstrate peripheral administration of XPro1595 reduces biomarkers of neuroinflammation P atients with biomarkers of inflammation Neuroinflammation measures: ▪ Imaging (MRI) ▪ Cerebral spinal fluid (CSF)* PHASE 1 b TRIAL IN A LZHEIMER’S INMB KOL AD January 21, 2021
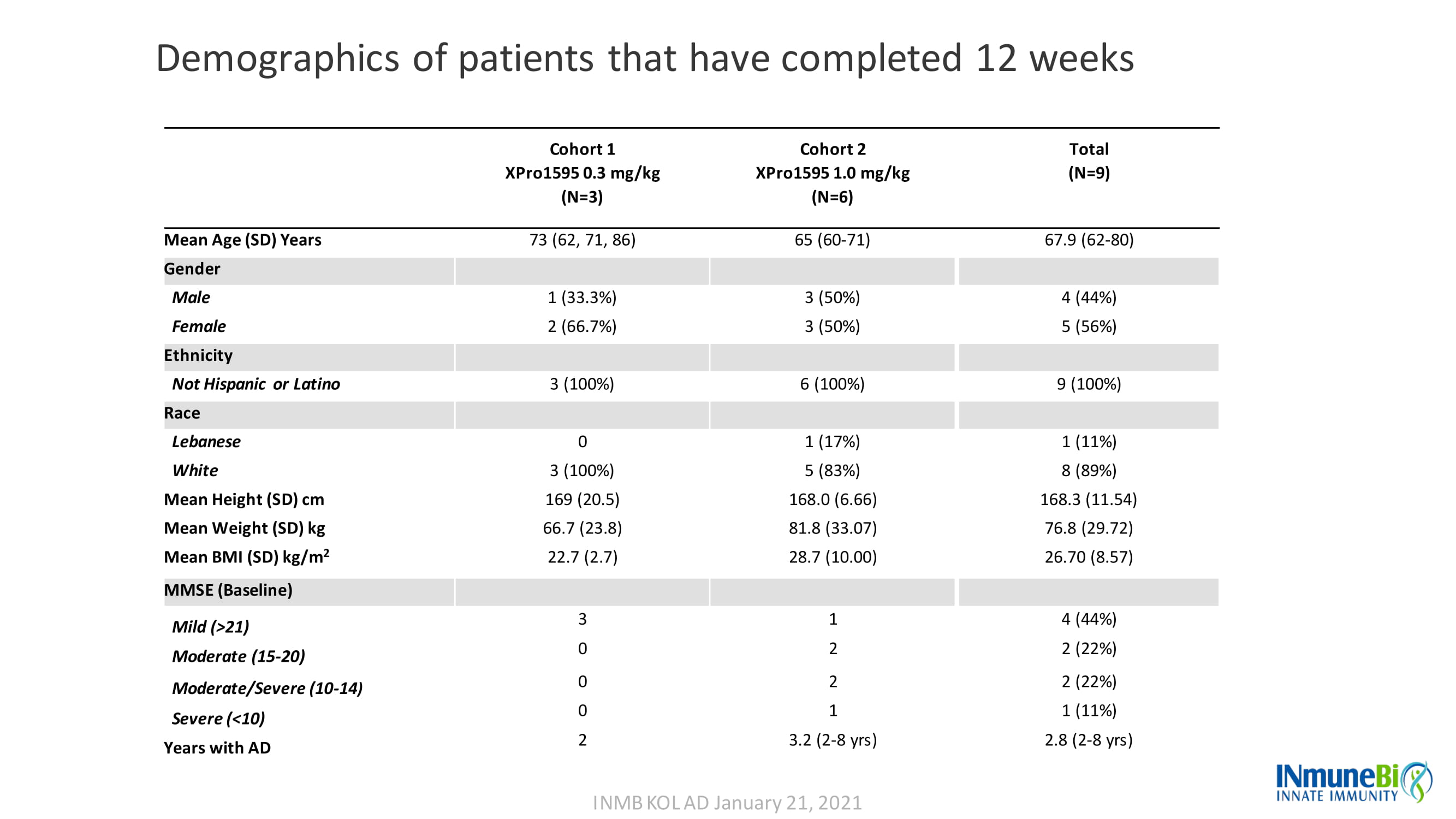
Demographics of patients that have completed 12 weeks Cohort 1 XPro1595 0.3 mg/kg (N=3) Cohort 2 XPro1595 1.0 mg/kg (N=6) Total (N=9) Mean Age (SD) Years 73 (62, 71, 86) 65 (60 - 71) 67.9 (62 - 80) Gender Male 1 (33.3%) 3 (50%) 4 (44%) Female 2 (66.7%) 3 (50%) 5 (56%) Ethnicity Not Hispanic or Latino 3 (100%) 6 (100%) 9 (100%) Race Lebanese 0 1 (17%) 1 (11%) White 3 (100%) 5 (83%) 8 (89%) Mean Height (SD) cm 169 (20.5) 168.0 (6.66) 168.3 (11.54) Mean Weight (SD) kg 66.7 (23.8) 81.8 (33.07) 76.8 (29.72) Mean BMI (SD) kg/m 2 22.7 (2.7) 28.7 (10.00) 26.70 (8.57) MMSE (Baseline) Mild (>21) 3 1 4 (44%) Moderate (15 - 20) Moderate/Severe (10 - 14 ) 0 0 2 2 2 (22%) 2 (22%) Severe (<10) 0 1 1 (11%) Years with AD 2 3.2 (2 - 8 yrs ) 2.8 (2 - 8 yrs ) INMB KOL AD January 21, 2021
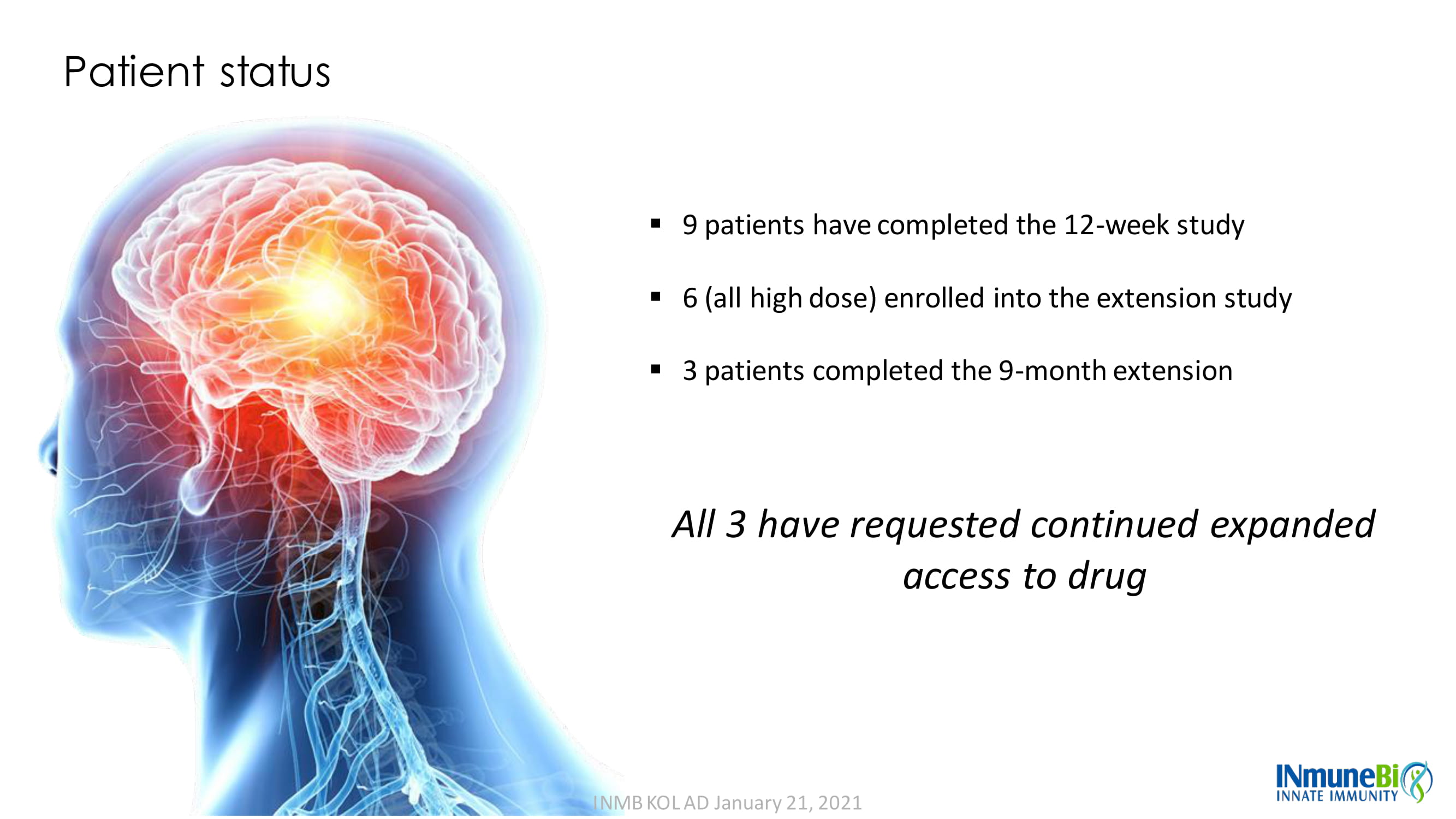
Patient status ▪ 9 patients have completed the 12 - week study ▪ 6 (all high dose) enrolled into the extension study ▪ 3 patients completed the 9 - month extension All 3 have requested continued expanded access to drug INMB KOL AD January 21, 2021

Biomarker Approach to measuring Neuroinflammation Proteomics Olink® Target 48 Cytokine Free Water White Matter CSF MRI INMB KOL AD January 21, 2021

-50% -45% -40% -35% -30% -25% -20% -15% -10% -5% 0% MRI CSF XPro1595 (1 mg/kg) reduced whole brain neuroinflammation (12 weeks) WMFW - 4.47% Olink® Target 48 Cytokine
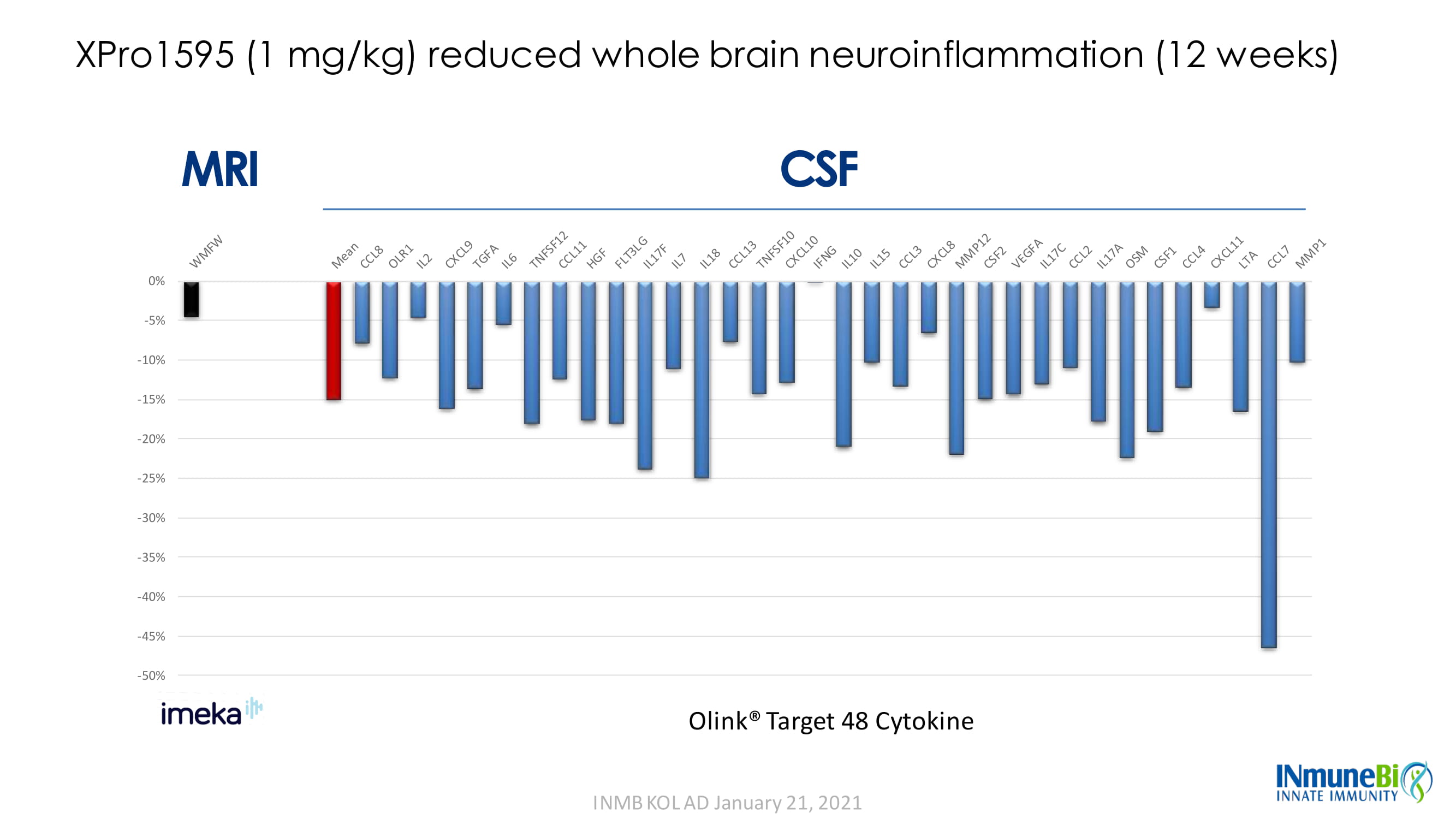
XPro1595 (1 mg/kg) reduced whole brain neuroinflammation (12 weeks) -50% -45% -40% -35% -30% -25% -20% -15% -10% -5% 0% MRI CSF Olink® Target 48 Cytokine INMB KOL AD January 21, 2021
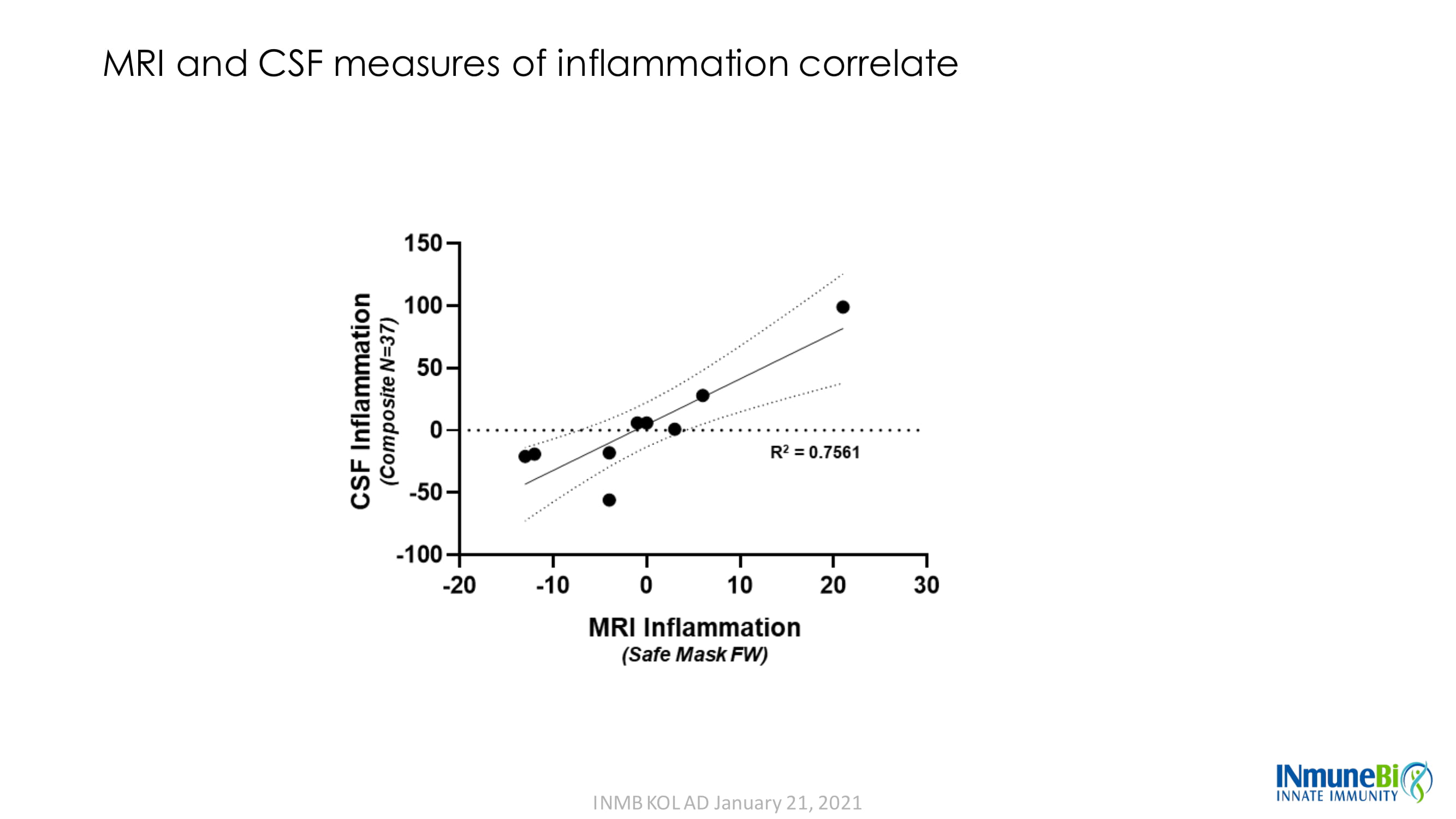
MRI and CSF measures of inflammation correlate INMB KOL AD January 21, 2021
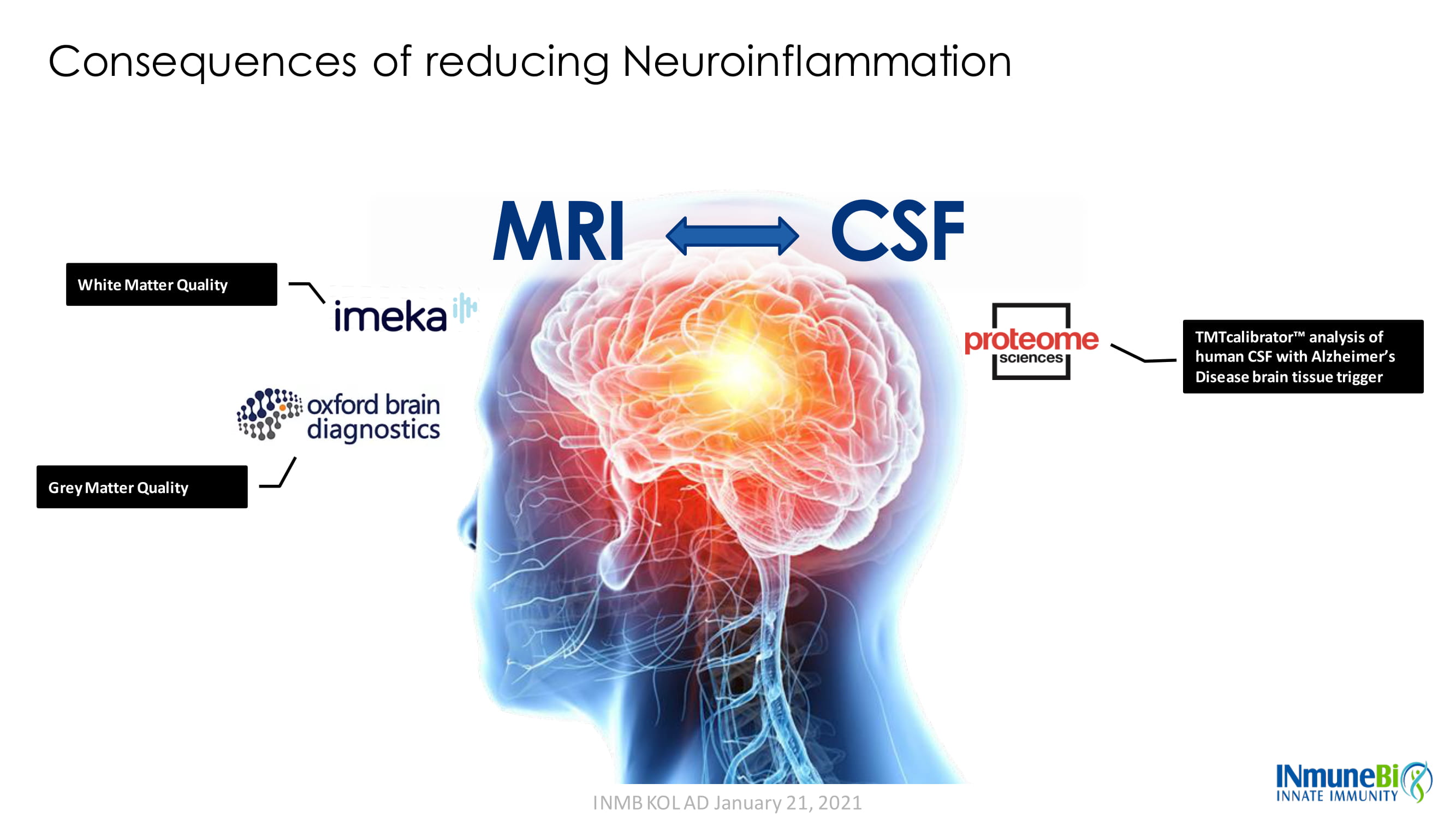
TMTcalibrator Ρ analysis of human CSF with Alzheimer’s Disease brain tissue trigger White Matter Quality CSF MRI Grey Matter Quality Consequences of reducing Neuroinflammation INMB KOL AD January 21, 2021
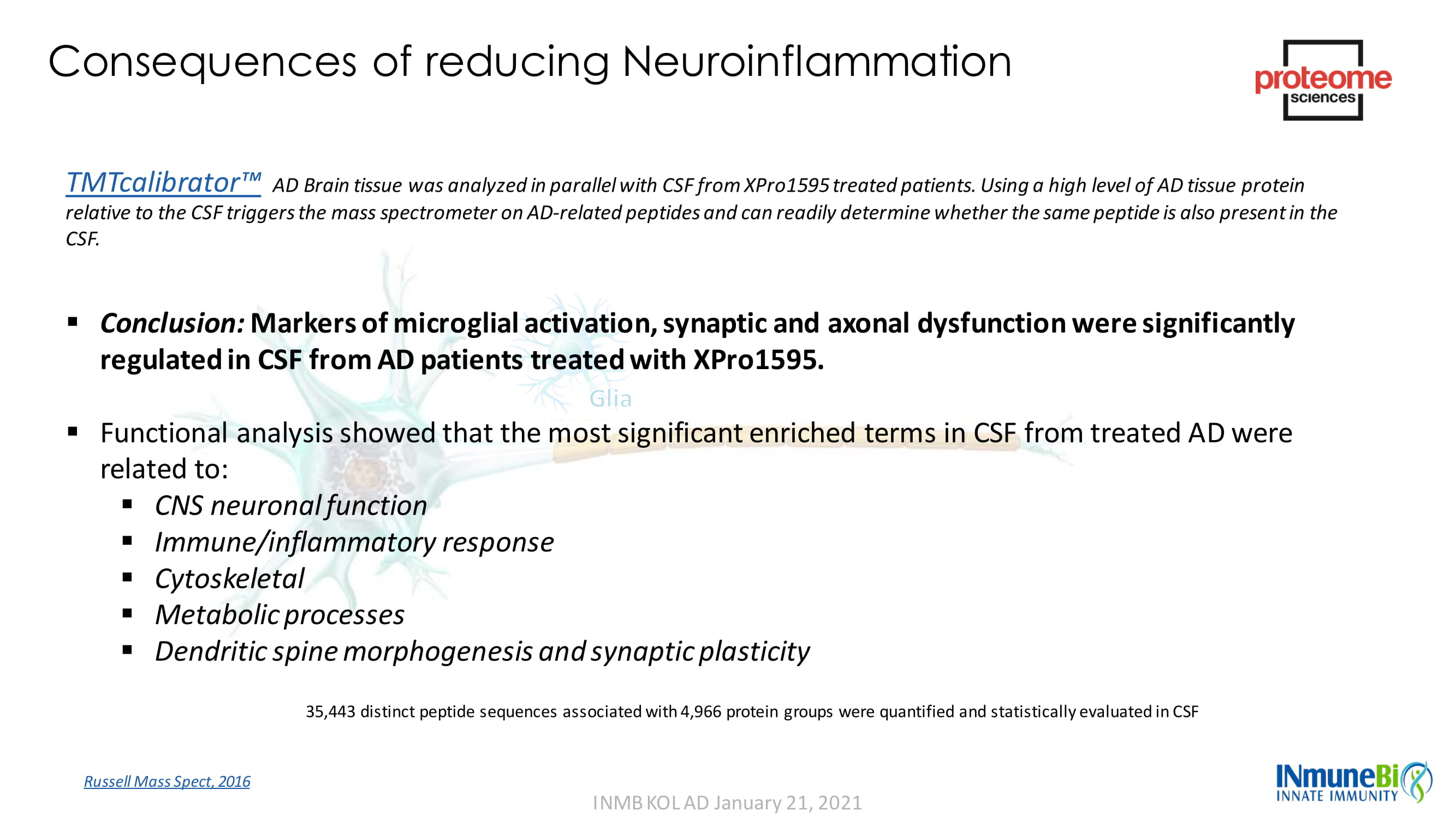
Consequences of reducing Neuroinflammation TMTcalibrator Ρ AD Brain tissue was analyzed in parallel with CSF from XPro1595 treated patients. Using a high level of AD tissue protein relative to the CSF triggers the mass spectrometer on AD - related peptides and can readily determine whether the same peptide is also present in the CSF. ▪ Conclusion: Markers of microglial activation, synaptic and axonal dysfunction were significantly regulated in CSF from AD patients treated with XPro1595. ▪ Functional analysis showed that the most significant enriched terms in CSF from treated AD were related to: ▪ CNS neuronal function ▪ Immune/inflammatory response ▪ Cytoskeletal ▪ Metabolic processes ▪ Dendritic spine morphogenesis and synaptic plasticity Russell Mass Spect , 2016 INMB KOL AD January 21, 2021 35,443 distinct peptide sequences associated with 4,966 protein groups were quantified and statistically evaluated in CSF
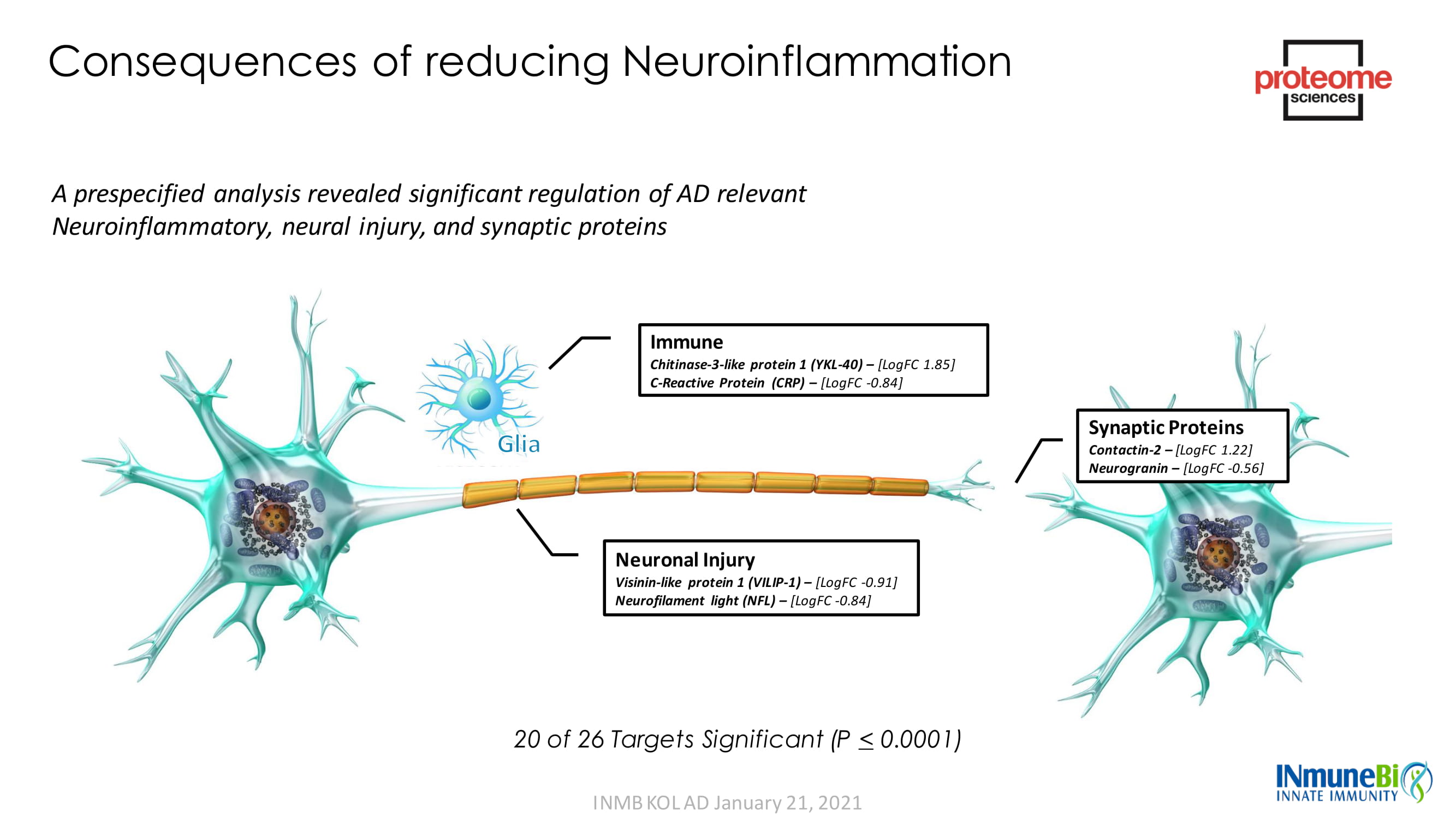
20 of 26 Targets Significant (P < 0.0001) Synaptic Proteins Contactin - 2 – [ LogFC 1.22] Neurogranin – [ LogFC - 0.56] Neuronal Injury Visinin - like protein 1 (VILIP - 1) – [ LogFC - 0.91] Neurofilament light (NFL) – [ LogFC - 0.84] Immune Chitinase - 3 - like protein 1 (YKL - 40) – [ LogFC 1.85] C - Reactive Protein (CRP) – [ LogFC - 0.84] Consequences of reducing Neuroinflammation A prespecified analysis revealed significant regulation of AD relevant Neuroinflammatory, neural injury, and synaptic proteins INMB KOL AD January 21, 2021
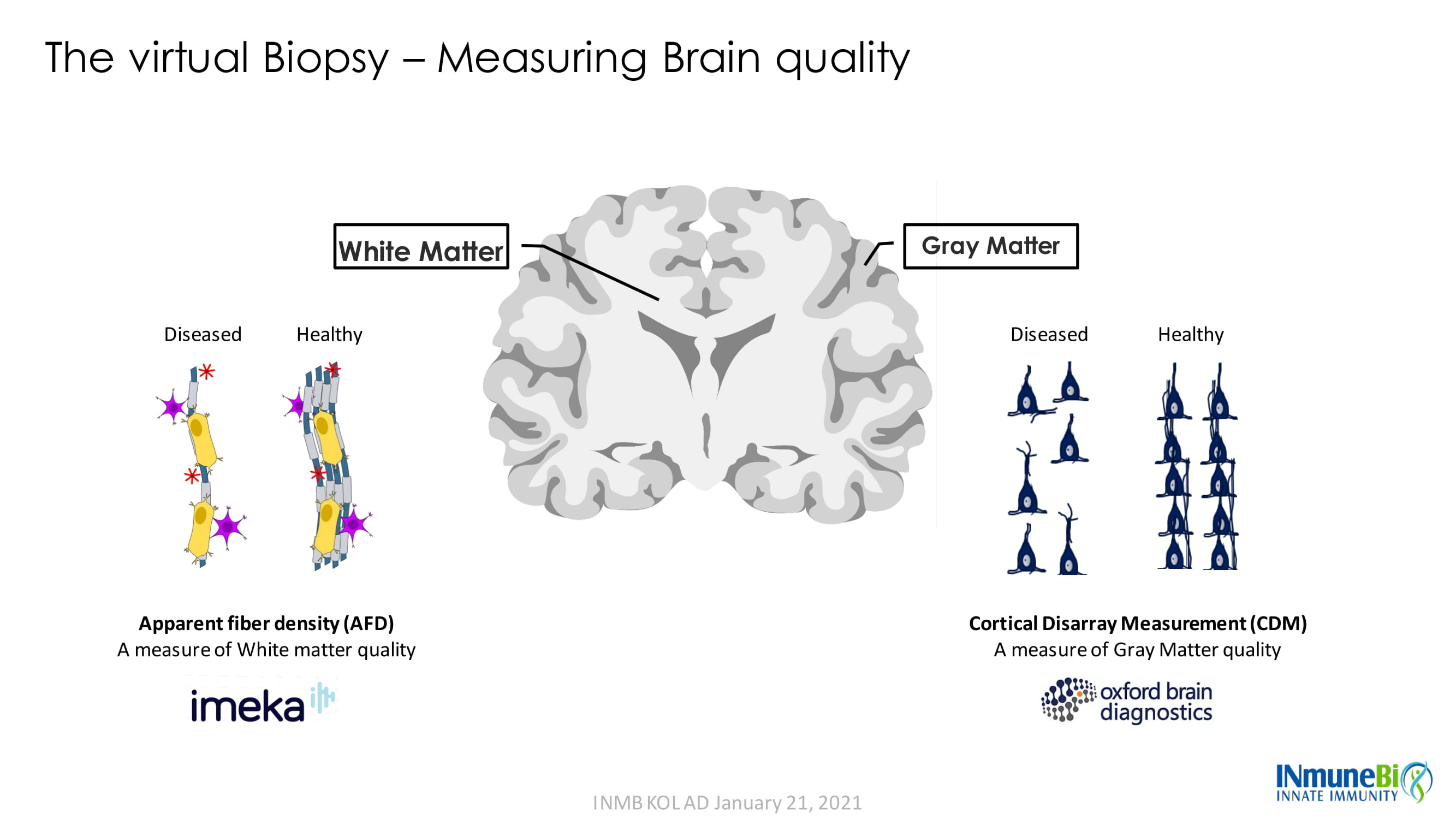
The virtual Biopsy – Measuring Brain quality White Matter Gray Matter Diseased Apparent fiber density (AFD) A measure of White matter quality Healthy Diseased Healthy Cortical Disarray Measurement (CDM) A measure of Gray Matter quality INMB KOL AD January 21, 2021
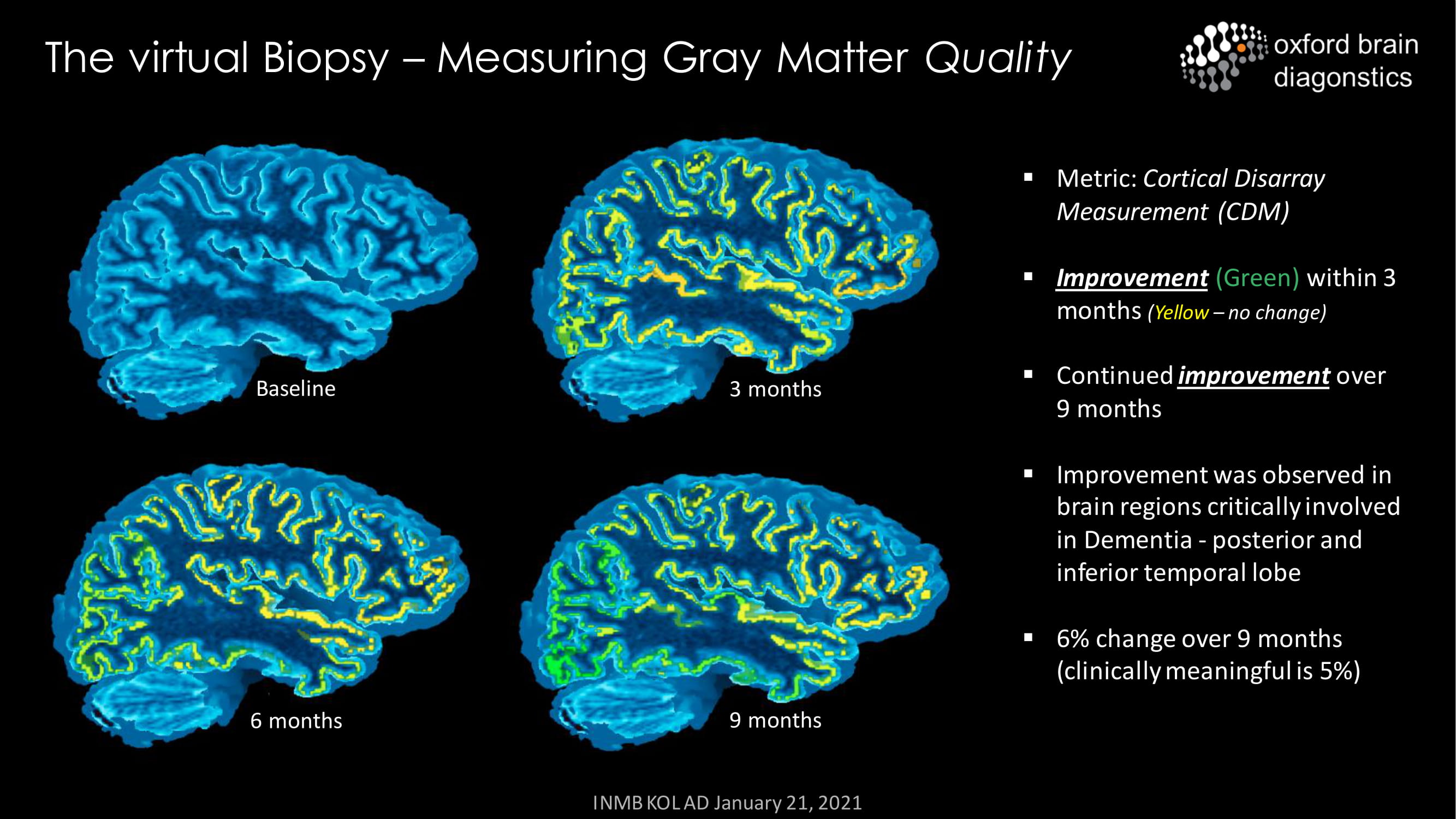
The virtual Biopsy – Measuring Gray Matter Quality Baseline 3 months 6 months 9 months ▪ Metric: Cortical Disarray Measurement (CDM) ▪ Improvement (Green) within 3 months ( Yellow – no change) ▪ Continued improvement over 9 months ▪ Improvement was observed in brain regions critically involved in Dementia - posterior and inferior temporal lobe ▪ 6% change over 9 months (clinically meaningful is 5%) INMB KOL AD January 21, 2021
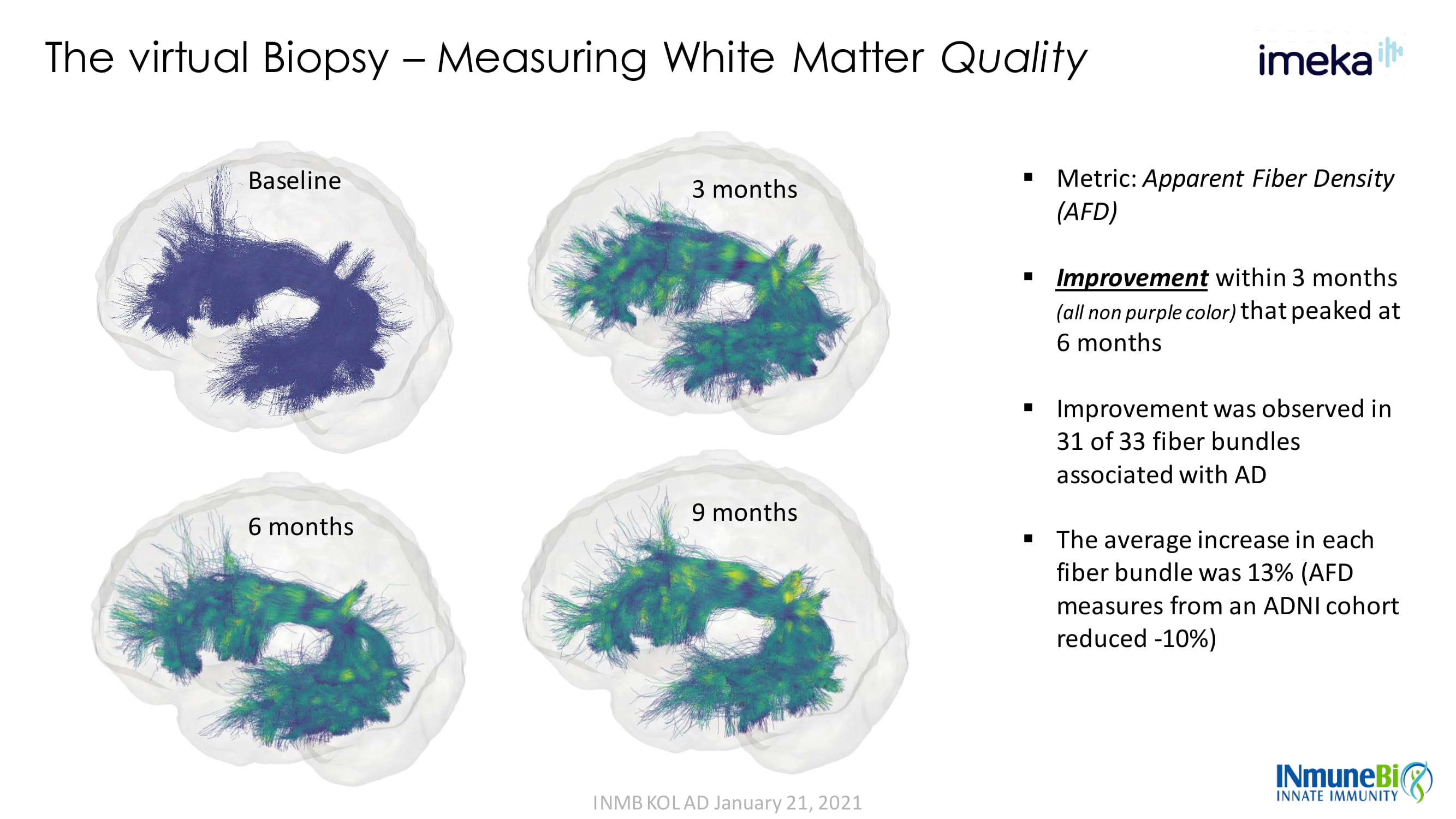
The virtual Biopsy – Measuring White Matter Quality INMB KOL AD January 21, 2021 Baseline 3 months 6 months 9 months ▪ Metric: Apparent Fiber Density (AFD) ▪ Improvement within 3 months (all non purple color) that peaked at 6 months ▪ Improvement was observed in 31 of 33 fiber bundles associated with AD ▪ The average increase in each fiber bundle was 13% (AFD measures from an ADNI cohort reduced - 10%)

Assessing Cognition ▪ Challenge: Small N, disease status heterogeneity, short time period ▪ Assessments administered: ▪ Cognitive: MMSE, Verbal Fluency Test, Digit Symbol Coding ▪ Neuropsychiatric Inventory ▪ Bristol Activities of Daily Living Scale ▪ To compare across patients of different disease states, Dr. Judith Jaeger issued each patient a qualitative score of ( - 2, - 1, 0, 1 ,2) based on her assessment of the overall change over 3 months. - 2 - 1 0 1 2 Meaningful progression Minor progression Stable Disease Minor Improvement Meaningful Improvement INMB KOL AD January 21, 2021 Patients with the greatest improvement in cognition had the largest reduction in neuroinflammation
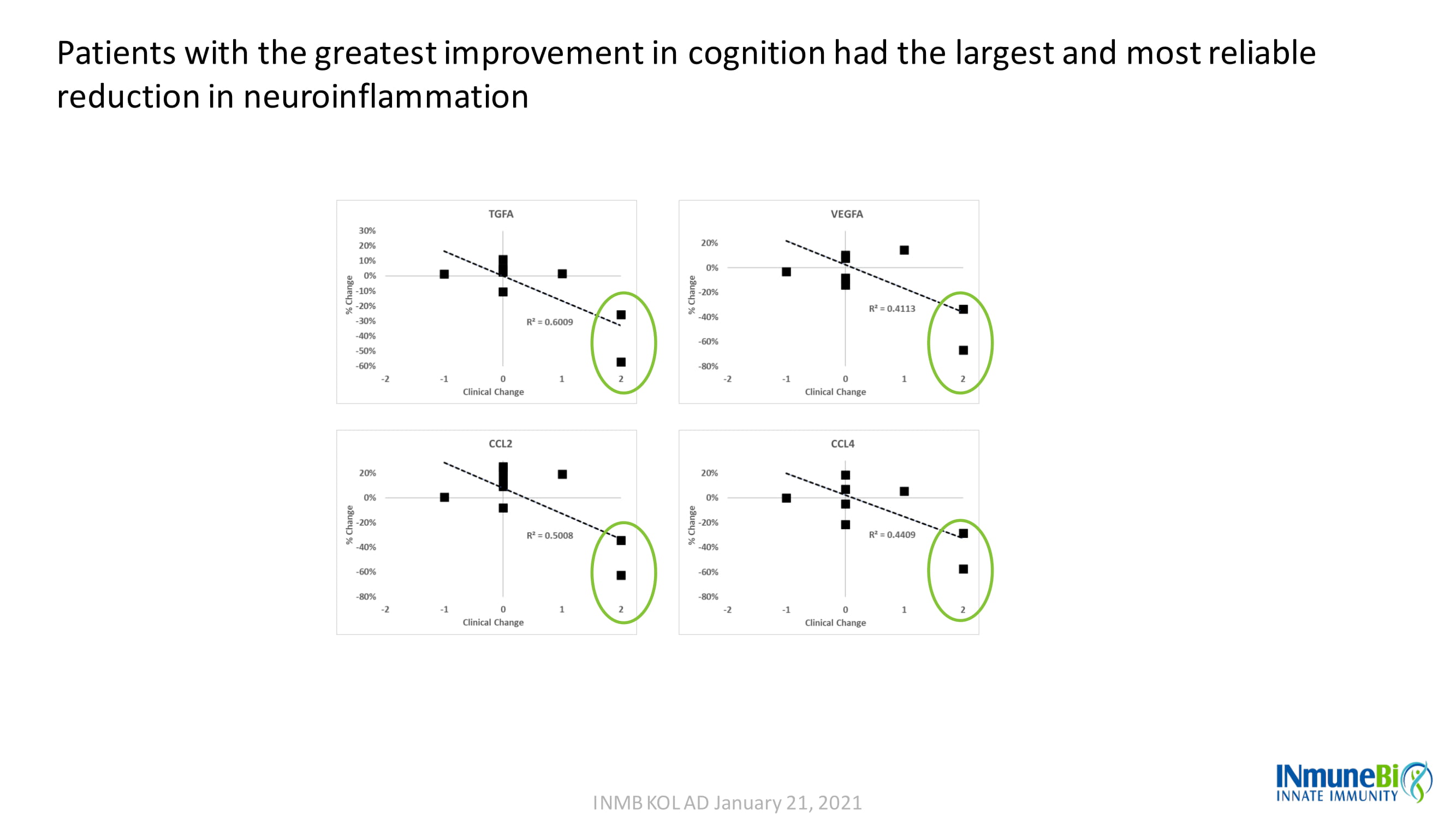
INMB KOL AD January 21, 2021 Patients with the greatest improvement in cognition had the largest and most reliable reduction in neuroinflammation

Summary ▪ XPro1595 reduces neuroinflammation across multiple measures and assays ▪ Pathway analyses show a significant effect of XPro1595 in AD relevant pathways and corroborated by novel MRI metrics ▪ XPro1595 significantly reduced markers of neuroinflammation, neural injury, improved synaptic proteins ▪ Changes in biomarkers all appear within 3 months and were sustained over the duration of the trial ▪ Safety - well tolerated (AEs - injection site reactions) ▪ These data support advancing to Phase 2 (2H2021) INMB KOL AD January 21, 2021

Q&A XPro1595 Alzheimer’s KOL event Jan 21, 2021
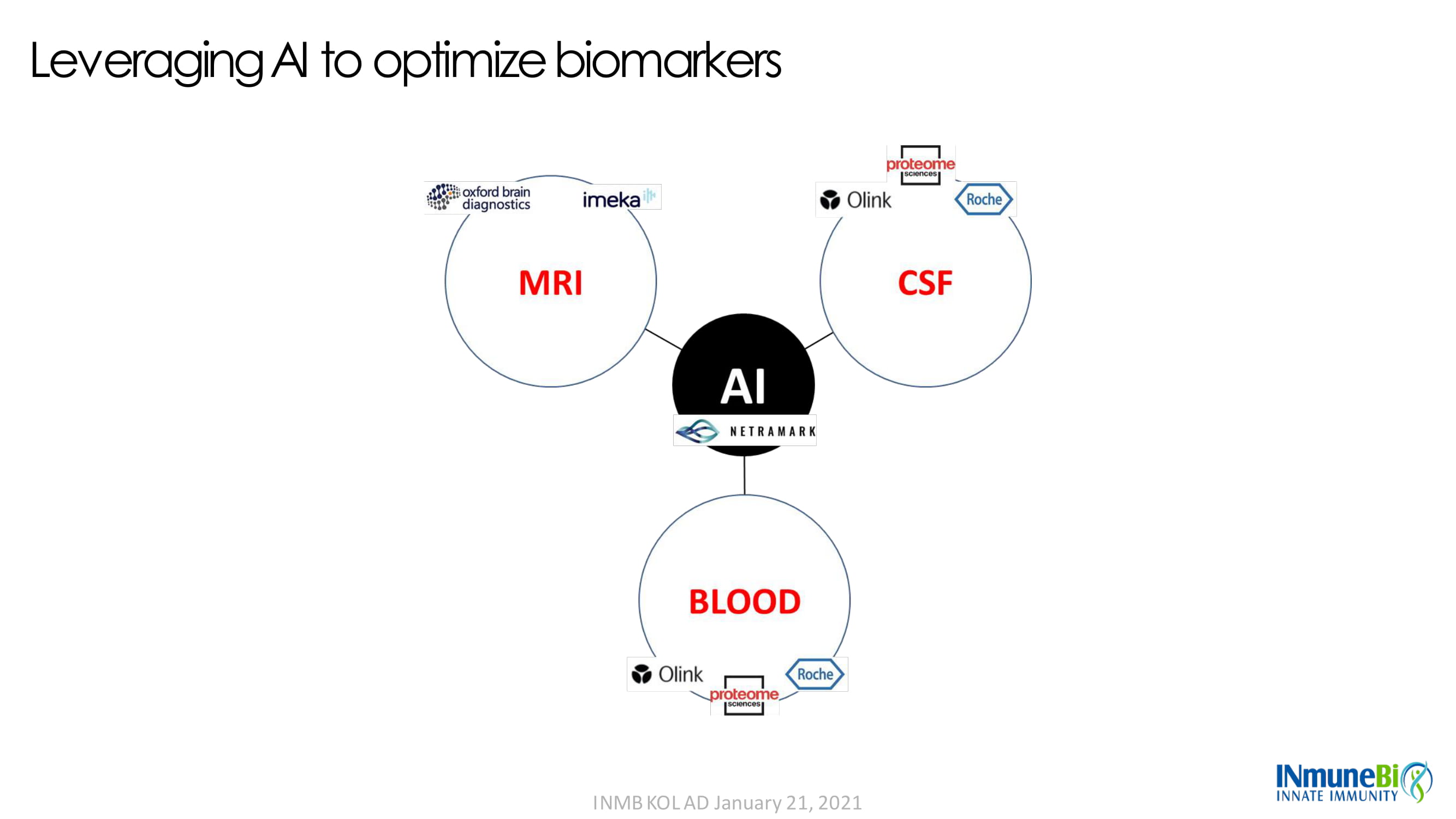
Leveraging AI to optimize biomarkers INMB KOL AD January 21, 2021
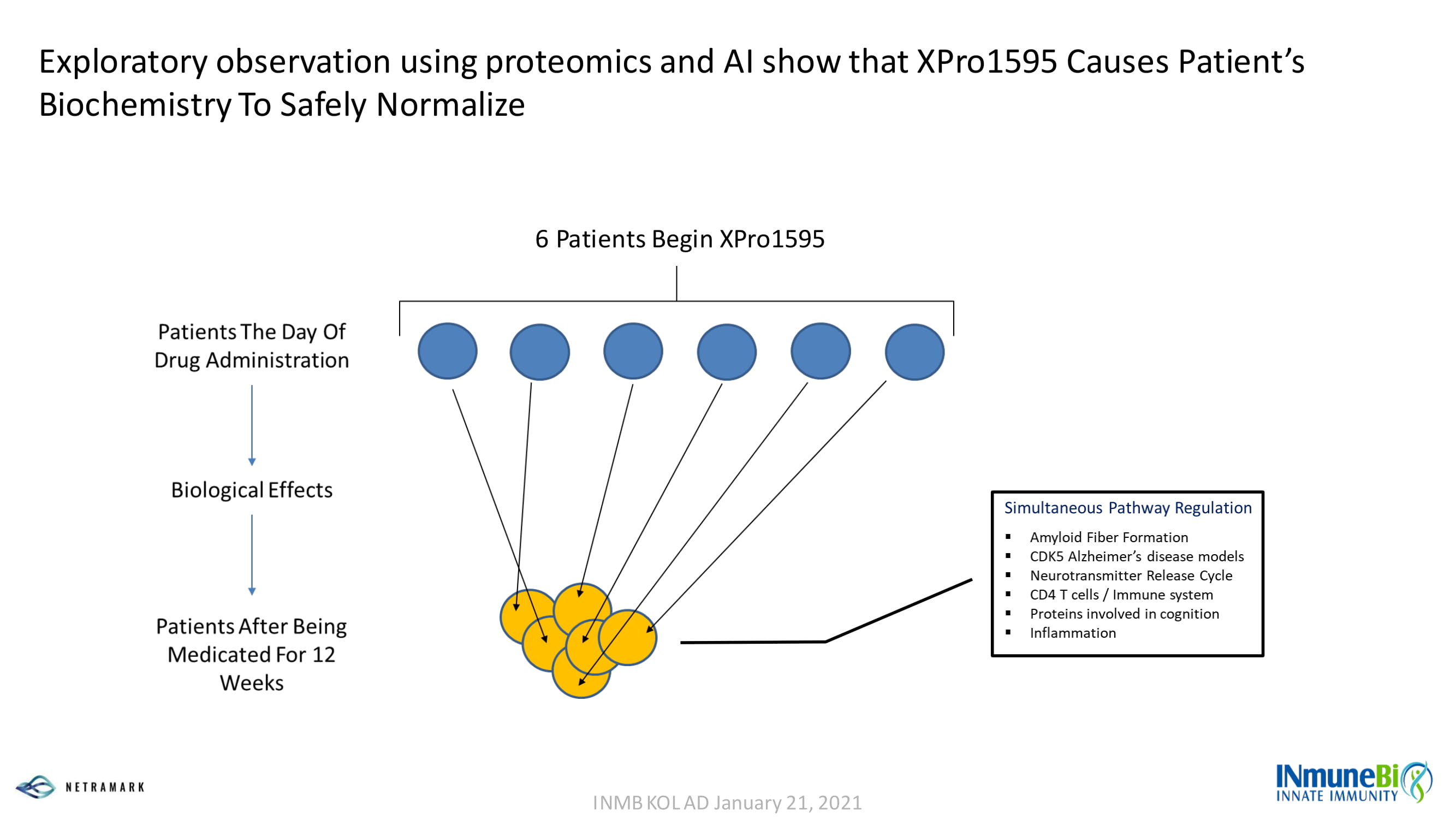
Exploratory observation using proteomics and AI show that XPro1595 Causes Patient’s Biochemistry To Safely Normalize 6 Patients Begin XPro1595 INMB KOL AD January 21, 2021




















