Exhibit 99.1

H A R N E SS I N G T H E P O W E R O F T H E I NN A T E I MM U N E S Y S T E M Targeting Innate Immune Dysfunction I N M B INVESTOR PRESENTATION September 2021

F O R W A R D L OO K I N G S T A T E M E N T S This presentation contains “forward - looking statements” Forward - looking statements reflect our current view about future events . When used in this presentation, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward - looking statements . Such statements, include, but are not limited to, statements contained in this presentation relating to our business strategy, our future operating results and liquidity and capital resources outlook . Forward - looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions . Because forward – looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict . Our actual results may differ materially from those contemplated by the forward - looking statements . They are neither statements of historical fact nor guarantees of assurance of future performance . We caution you therefore against relying on any of these forward - looking statements . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, without limitation, our ability to raise capital to fund continuing operations ; our ability to protect our intellectual property rights ; the impact of any infringement actions or other litigation brought against us ; competition from other providers and products ; our ability to develop and commercialize products and services ; changes in government regulation ; our ability to complete capital raising transactions ; and other factors relating to our industry, our operations and results of operations . There is no guarantee that any specific outcome will be achieved . Investment results are speculative and there is a risk of loss, potentially all loss of investments . Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . 2 2
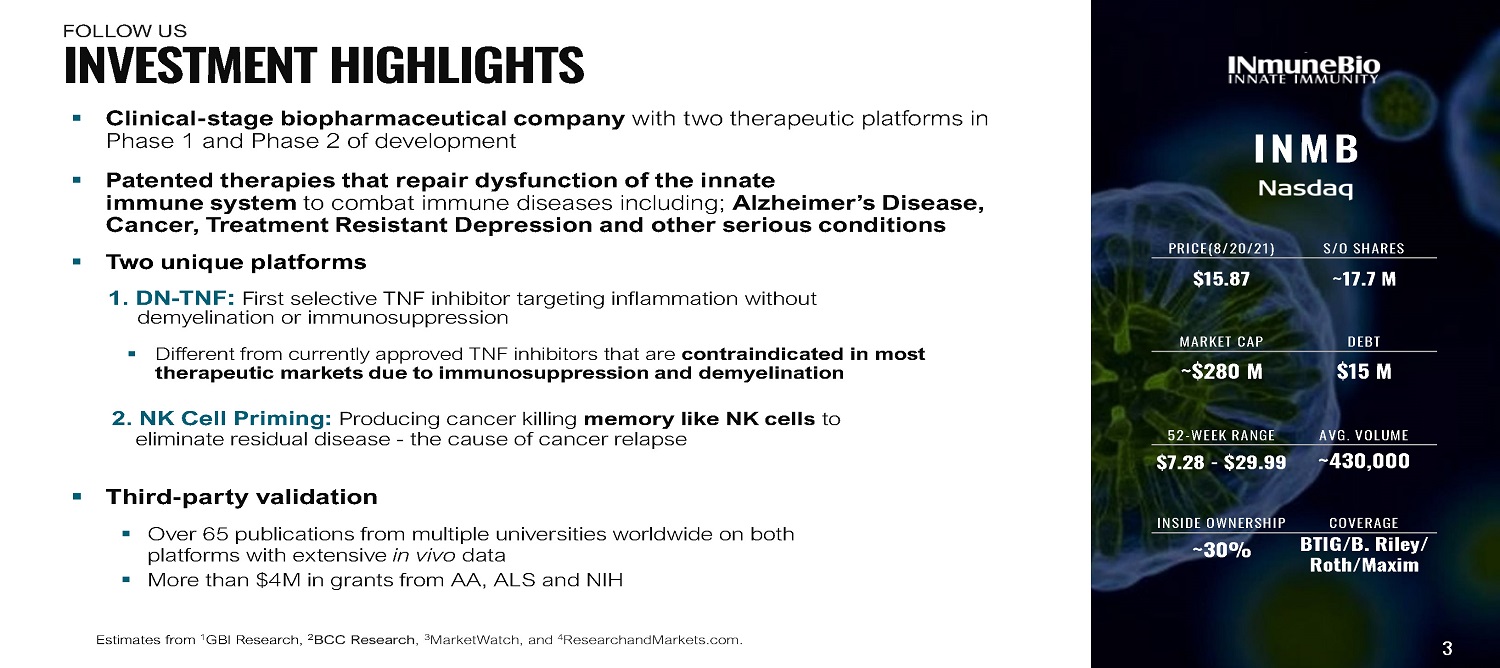
▪ Clinical - stage biopharmaceutical company with two therapeutic platforms in Phase 1 and Phase 2 of development ▪ Patented therapies that repair dysfunction of the innate immune system to combat immune diseases including; Alzheimer’s Disease, Cancer, Treatment Resistant Depression and other serious conditions ▪ T w o uniq u e p l a t f or ms 1. DN - TNF : First selective TNF inhibitor targeting inflammation without demyelination or immunosuppression ▪ Different from currently approved TNF inhibitors that are contraindicated in most therapeutic markets due to immunosuppression and demyelination 2. NK Cell Priming: Producing cancer killing memory like NK cells to eliminate residual disease - the cause of cancer relapse ▪ Third - party validation ▪ Over 65 publications from multiple universities worldwide on both platforms with extensive in vivo data ▪ More than $4M in grants from AA, ALS and NIH Estimates from 1 GBI Research, 2 BCC Research , 3 MarketWatch, and 4 ResearchandMarkets.com. I N M B PRICE(8/20/21) S/O SHARES $15.87 ~17.7 M MARKET CAP DEBT ~$280 M $15 M 52 - WEEK RANGE AVG. VOLUME $7.28 - $29.99 ~430,000 INSIDE OWNERSHIP COVERAGE ~30% BTIG/B. Riley/ Roth/Maxim 3 I N V E S T M E N T H I G H L I G H T S F OL L O W US
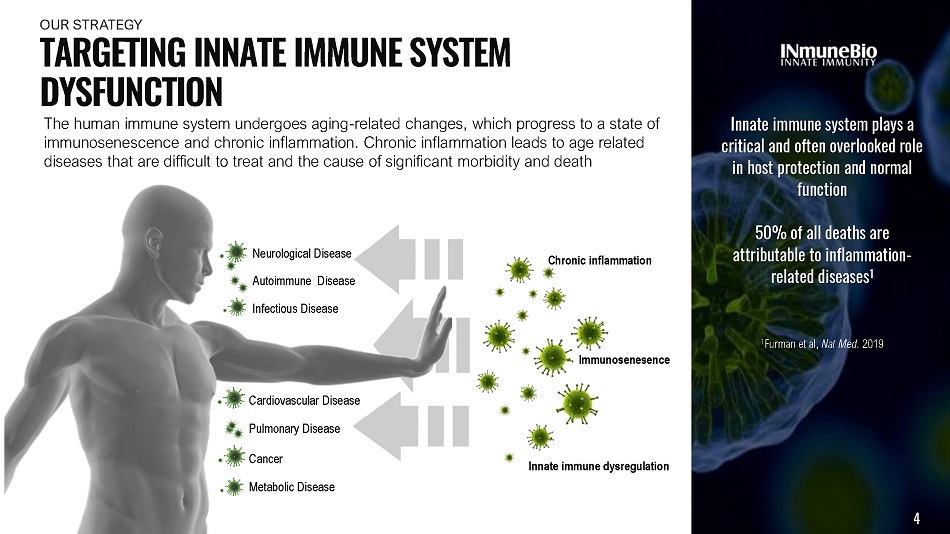
Metabolic Disease Neurological Disease Autoimmune Disease Cardiovascular Disease Pulmonary Disease Infectious Disease Innate immune system plays a critical and often overlooked role in host protection and normal function 50% of all deaths are attributable to inflammation - related diseases 1 1 Furman et al, Nat Med. 2019 The human immune system undergoes aging - related changes, which progress to a state of immunosenescence and chronic inflammation. Chronic inflammation leads to age related diseases that are difficult to treat and the cause of significant morbidity and death Cancer Innate immune dysregulation 4 Immunosenesence Chronic inflammation T A R G E T I N G I NN A T E I MM U N E S Y S T E M DYSFUNCTION OUR S T R A TE G Y
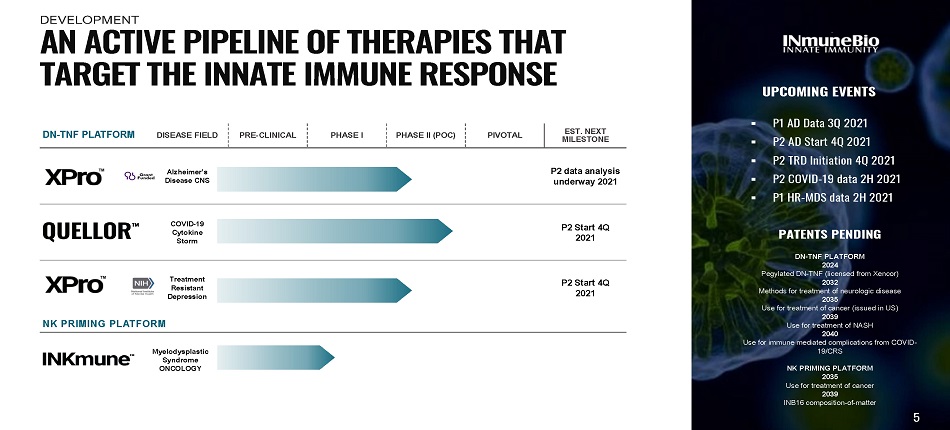
DN - TNF PLATFORM DISEASE FIELD PRE - CLINICAL PHASE I PHASE II (POC) PIVOTAL EST. NEXT MI L ES T ONE Alzheimer’s Disease CNS P2 data analysis underway 2021 COVID - 19 C y t ok i ne Storm P2 Start 4Q 2021 Treatment Resistant D epre s s i on P2 Start 4Q 2021 NK PRIMING PLATFORM M y e lody s pl a s t ic Syndrome ONCOLOGY UPCOMING EVENTS ▪ P1 AD Data 3 Q 2 0 2 1 ▪ P2 AD S t art 4Q 2 0 2 1 ▪ P2 T RD I n itiatio n 4Q 2 0 2 1 ▪ P2 COVID - 19 data 2H 2021 ▪ P1 H R - MDS data 2 H 2 0 2 1 PATENTS PENDING DN - TNF PLATFORM 2024 Pegylated DN - TNF (licensed from Xencor) 2032 Methods for treatment of neurologic disease 2035 Use for treatment of cancer (issued in US) 2039 Use for treatment of NASH 2040 Use for immune mediated complications from COVID - 19/CRS NK PRIMING PLATFORM 2035 Use for treatment of cancer 2039 INB16 composition - of - matter A N A C T I V E P I P E L I N E O F T H E R A P I E S T H A T T A R G E T T H E I NN A T E I MM U N E R E S P O N S E 5 DEVELOPMENT

▪ Visible programs AD, TRD, ALS ▪ “Out - of - Sight” PD, MS, FTD, LBD, TBI PTSD, autism, bi - polar disease Much, much, more…. ▪ Visible programs High risk MDS; Ovarian Cancer ▪ “Out - of - Sight” AML, MM, lymphoma RCC, Lung, prostate and breast cancer DN - T N F A N D I N K M U N E TIP OF THE ICEBERG 6

f o r A D Treating Alzheimer’s Disease as an Immunologic Disease … not A Neurologic Disease 7
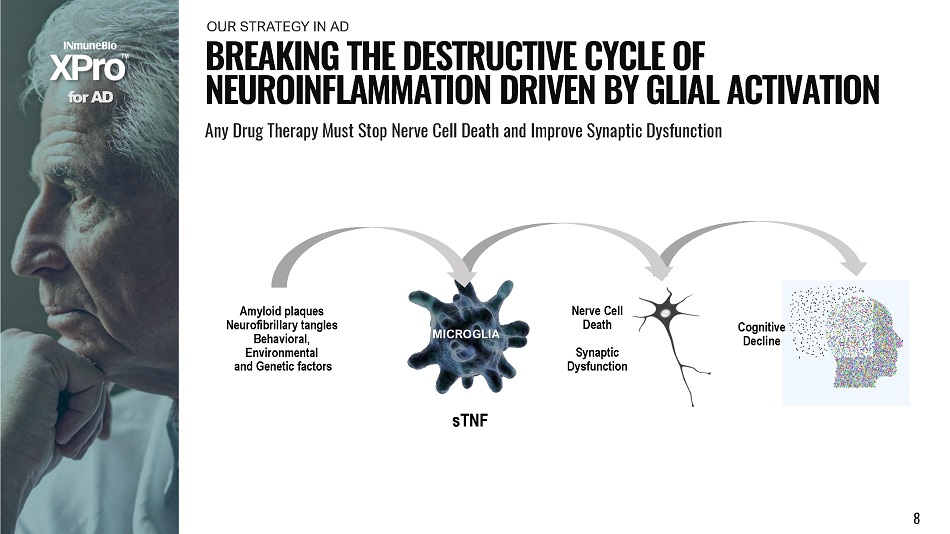
f o r A D B R E A K I N G T H E D E S T R U C T I V E C Y C L E O F N E U R O I N F L A MM A T I O N D R I V E N BY G L I A L A C T I V A T I O N Any Drug Therapy Must Stop Nerve Cell Death and Improve Synaptic Dysfunction OU R S T R A TE G Y I N AD s T NF Amyloid plaques Neurofibrillary tangles Behavioral, Environmental and Genetic factors MICROGLIA Nerve Cell Death Synaptic Dy s f un ct ion Co g ni t i v e Decline 8
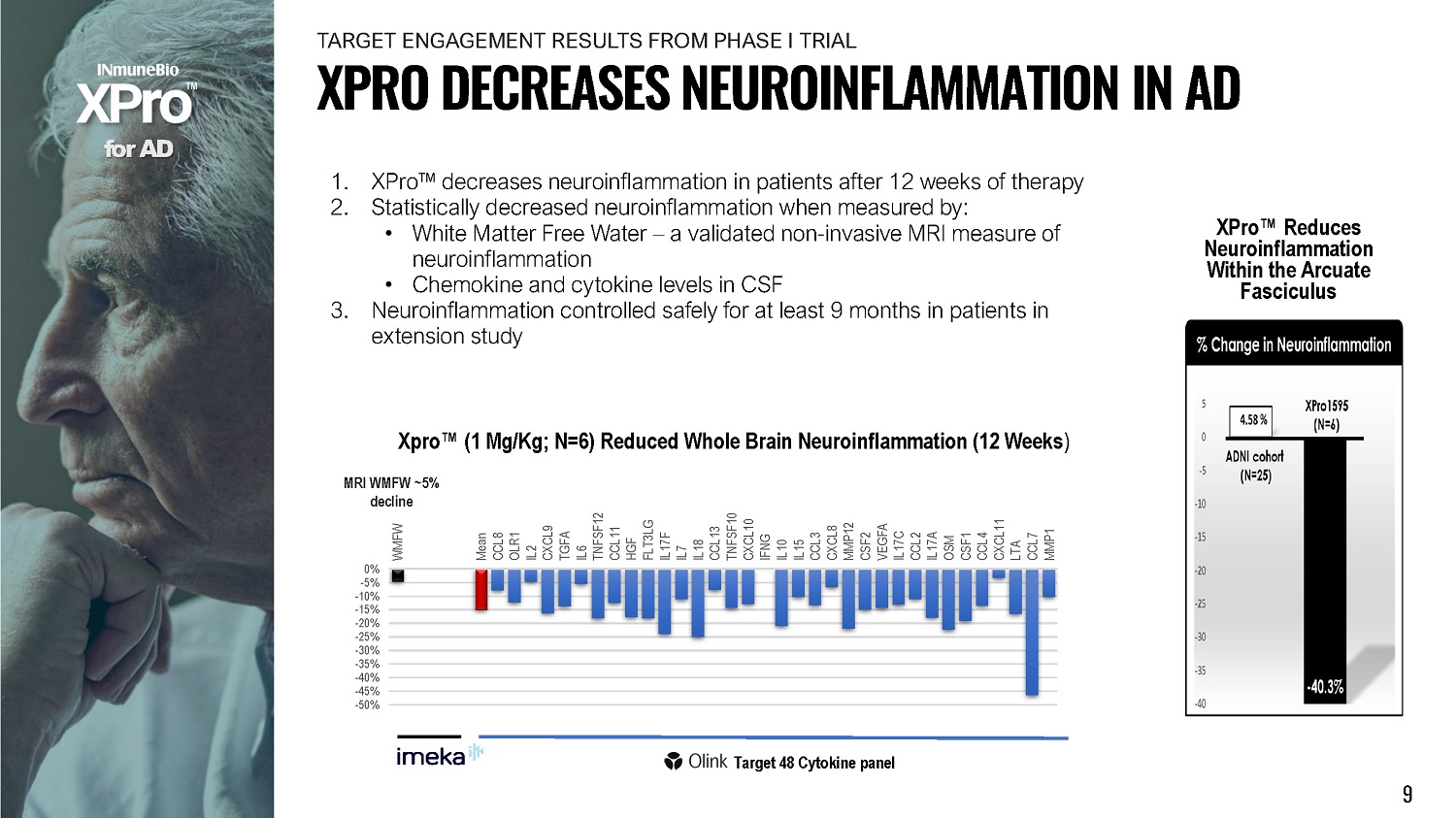
1. XPro Œ decreases neuroinflammation in patients after 12 weeks of therapy 2. Statistically decreased neuroinflammation when measured by: • White Matter Free Water – a validated non - invasive MRI measure of neuroinflammation • Chemokine and cytokine levels in CSF 3. Neuroinflammation controlled safely for at least 9 months in patients in extension study 0% - 5% - 1 0 % - 1 5 % - 2 0 % - 2 5 % - 3 0 % - 3 5 % - 4 0 % - 4 5 % - 5 0 % W M FW Mean CCL8 OLR1 IL2 C X C L9 TGFA IL6 T N FS F 1 2 CCL11 HGF FL T 3 LG IL17F IL7 IL18 CCL13 T N FS F 10 CXCL10 IFNG IL10 IL15 CCL3 CXCL8 MMP12 CSF2 VEGFA IL17C CCL2 IL17A OSM CSF1 CCL4 C X C L 1 1 LTA CCL7 MMP1 Xpro Œ (1 Mg/Kg; N=6) Reduced Whole Brain Neuroinflammation (12 Weeks ) MRI WMFW ~5% decline f o r A D Target 48 Cytokine panel XPro Œ Reduces Neuroinfl am m at ion Within the Arcuate Fasciculus 9 X P R O D E C R E A S E S N E U R O I N F L A MM A T I O N I N A D T A R G E T ENG A G E MENT RE S U L T S F R OM P H A S E I TR I AL
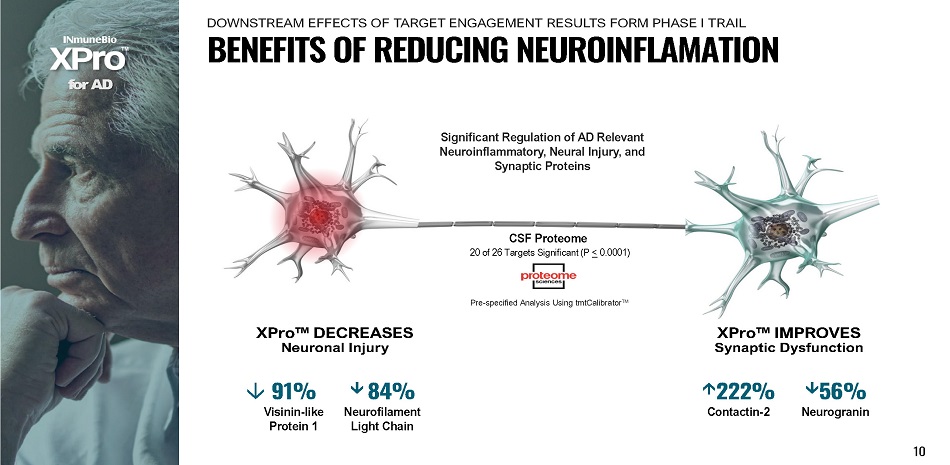
XPro ΠDECREASES Neuronal Injury f o r A D CSF Proteome 20 of 26 Targets Significant (P < 0.0001) 10 XPro ΠIMPROVES Synaptic Dysfunction 222% Contactin - 2 56% Neurogranin 91% V isi n i n - l i k e Protein 1 8 4 % N e urofilam e nt Light Chain Pre - specified Analysis Using tmtCalibrator ΠSignificant Regulation of AD Relevant Neuroinflammatory, Neural Injury, and Synaptic Proteins B E N E F I T S O F R E D U C I N G N E U R O I N F L A M A T I O N DOWNSTREAM EFFECTS OF TARGET ENGAGEMENT RESULTS FORM PHASE I TRAIL
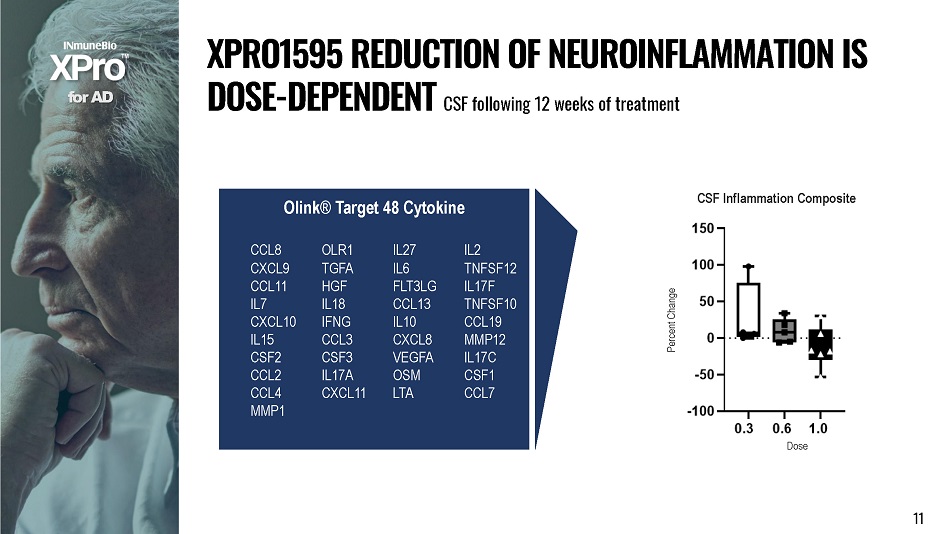
f o r A D X P R O 1 5 9 5 R E D U C T I O N O F N E U R O I N F L A MM A T I O N I S DOSE - DEPENDENT CSF following 12 weeks of treatment Olink® Target 48 Cytokine CCL8 OLR1 IL27 IL2 CXCL9 TGFA IL6 TNFSF12 CCL11 HGF FLT3LG IL17F IL7 IL18 CCL13 TNFSF10 CXCL10 IFNG IL10 CCL19 IL15 CCL3 CXCL8 MMP12 CSF2 CSF3 VEGFA IL17C CCL2 IL17A OSM CSF1 CCL4 CXCL11 LTA CCL7 MMP1 CSF Inflammation Composite 11 D o se Perce nt C hange
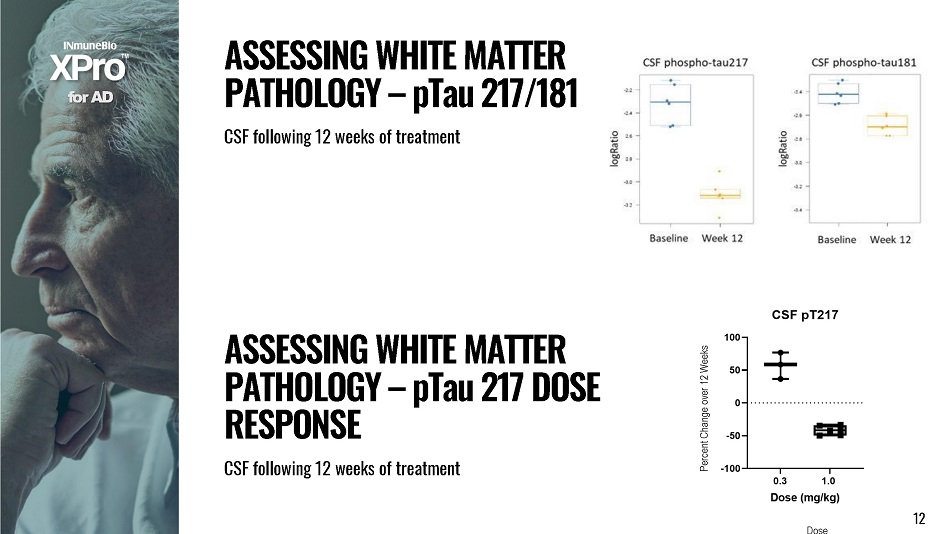
1 2 f o r A D 12 A SS E SS I N G W H I T E M A TT E R P A T H O L O G Y – p T a u 2 1 7 D O S E RESPONSE CSF fol l ow i ng 12 w e e ks of t rea t ment A SS E SS I N G W H I T E M A TT E R P A T H O L O G Y – p T a u 2 1 7 / 1 8 1 CSF fol l owing 12 w e e k s of t r eatment D o se Percent Change over 12 Weeks
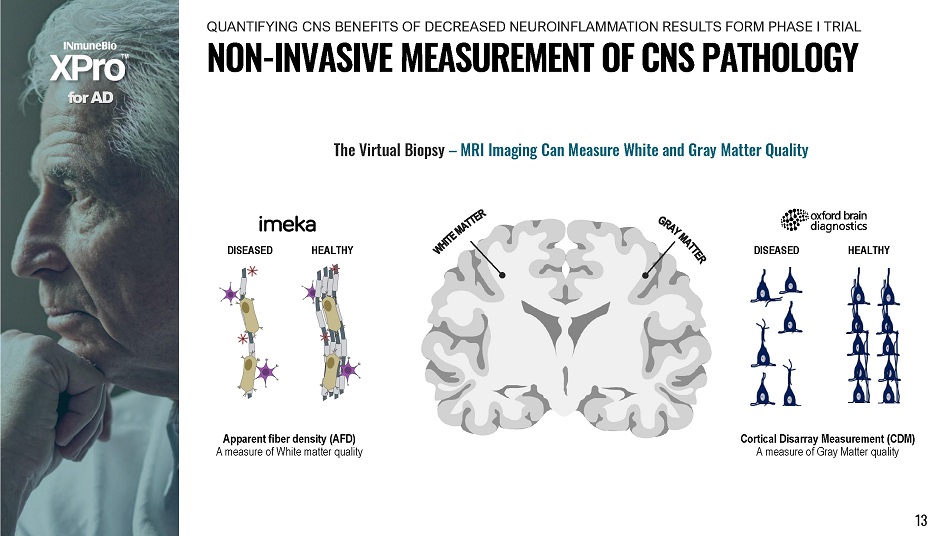
D I SE A SE D Apparent fiber density (AFD) A measure of White matter quality H E A LT H Y D I SE A SE D H E A LT H Y Cortical Disarray Measurement (CDM) A measure of Gray Matter quality f o r A D The Virtual Biopsy – MRI Imaging Can Measure White and Gray Matter Quality 13 N O N - I N V A S I V E M E A S U R E M E N T O F C N S P A T H O L O G Y QUANTIFYING CNS BENEFITS OF DECREASED NEUROINFLAMMATION RESULTS FORM PHASE I TRIAL
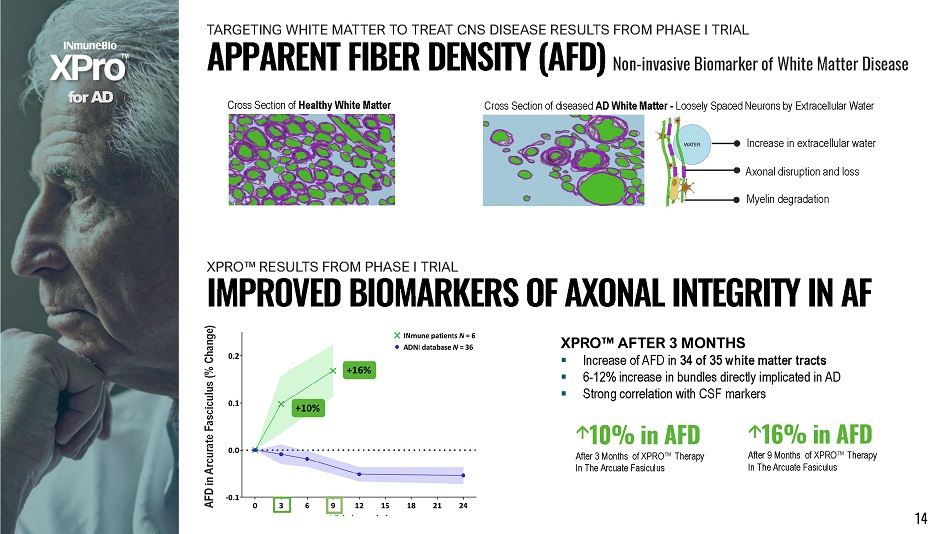
f o r A D AFD in Arcurate Fasciculus (% Change) ▪ Increase of AFD in 34 of 35 white matter tracts ▪ 6 - 12% increase in bundles directly implicated in AD ▪ Strong correlation with CSF markers I M P R O V E D B I O M A R K E R S O F A X O N A L I N T E G R I T Y I N A F XPRO Œ AFTER 3 MONTHS Cross Section of Healthy White Matter Cross Section of diseased AD White Matter - Loosely Spaced Neurons by Extracellular Water Increase in extracellular water Axonal disruption and loss Myelin degradation 10% in AFD After 3 Months of XPRO Œ Therapy In The Arcuate Fasiculus 14 16% in AFD After 9 Months of XPRO Œ Therapy In The Arcuate Fasiculus APPARENT FIBER DENSITY (AFD) Non - invasive Biomarker of White Matter Disease TARGETING WHITE MATTER TO TREAT CNS DISEASE RESULTS FROM PHASE I TRIAL X P R O Œ RE S U L T S F R OM P H A S E I TRIAL

0 3 6 9 12 15 18 21 24 - 10 0 - 5 5 15 10 20 Fiber Density in AD bundles (% change) XPro1595 1 mg/kg ADNI database N = 36 N = 6 N = 5 N = 5 N = 3 +17% f o r A D LONGITUDINAL APPARENT FIBER DENSITY Xpro ΠRESULTS Visit (months) 15
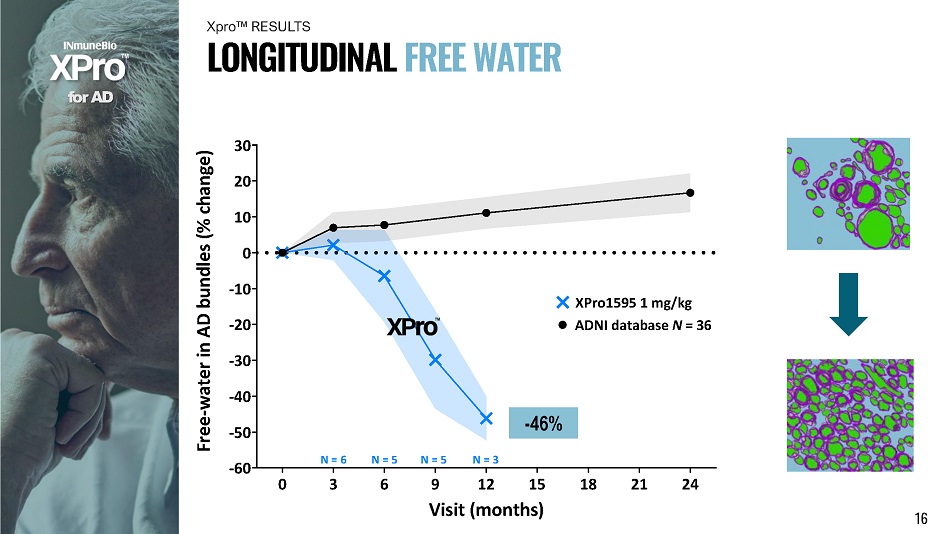
0 3 6 9 12 15 18 21 24 - 60 - 50 - 30 - 40 - 20 - 10 30 20 10 0 Free - water in AD bundles (% change) XPro1595 1 mg/kg ADNI database N = 36 N = 6 N = 5 N = 5 N = 3 f o r A D L O N G I T U D I N A L F R E E W A T E R Xpro ΠRESULTS - 46% Visit (months) 16
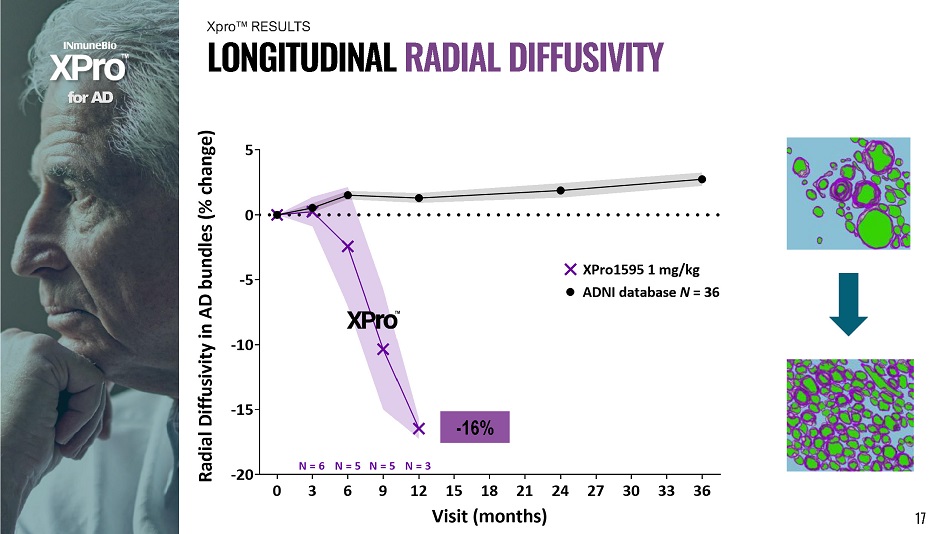
f o r A D L O N G I T U D I N A L R A D I A L D I FF U S I V I T Y Xpro ΠRESULTS - 16% 17

f o r A D I N C R E A S E I N W H I T E M A TT E R V O L U M E (MACROSTRUCTURE) Xpro ΠRESULTS 18 0 12 3 6000 0 4 4000 0 4 2000 0 4 0000 0 3 8000 0 4 6000 0 3 6 9 V isit ( m o nt h s ) Total White Matter volume (mm 3 ) 0 . 146 9 0 12 5 00 0 5 50 0 6 00 0 6 50 0 3 6 9 Visit (months) Left Inferior Temporal volume (mm 3 ) 0. 038 1 0.1226 0. 083 9
In Alzheimer’s Disease bundles over a 12 - month period: 1) XPro1595 reduces FreeWater by 46% (neuroinflammation) 2) XPro1595 increases Apparent Fiber Density by 17% (axon integrity) 3) XPro1595 reduces tissue Radial Diffusivity by 16% (remyelination) x Confirmed by CSF inflammatory & tau markers f o r A D x White matter microstructure degenerates with Alzheimer’s Disease x Neuroinflammation as well as axon & myelin disruption x Non - invasive advanced MRI & Imeka tools quantify where & how white matter is disrupted and rescued S U MM A R Y 19
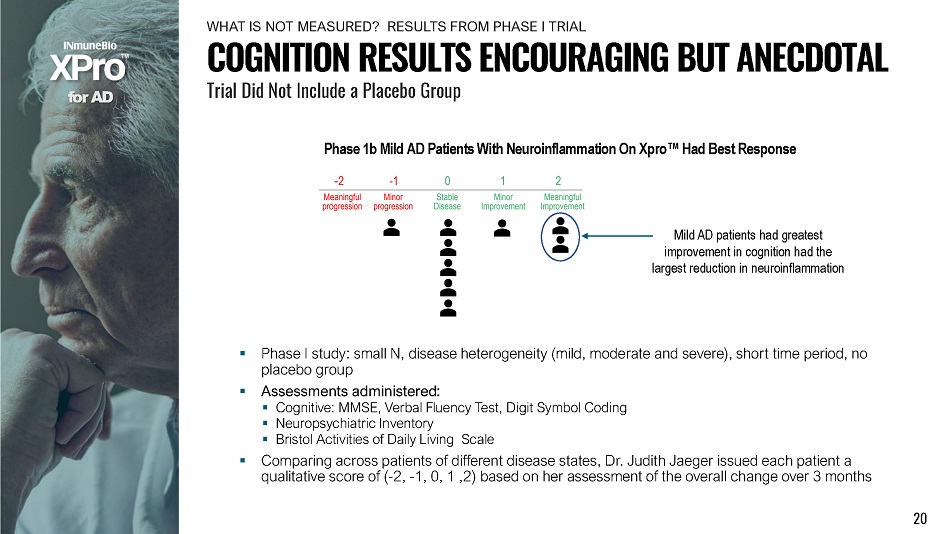
f o r A D Phase 1b Mild AD Patients With Neuroinflammation On Xpro Œ Had Best Response ▪ Phase I study: small N, disease heterogeneity (mild, moderate and severe), short time period, no placebo group ▪ Assessments administered: ▪ Cognitive: MMSE, Verbal Fluency Test, Digit Symbol Coding ▪ Neuropsychiatric Inventory ▪ Bristol Activities of Daily Living Scale ▪ Comparing across patients of different disease states, Dr. Judith Jaeger issued each patient a qualitative score of ( - 2, - 1, 0, 1 ,2) based on her assessment of the overall change over 3 months Mild AD patients had greatest improvement in cognition had the largest reduction in neuroinflammation COGNITION RESULTS ENCOURAGING BUT ANECDOTAL Trial Did Not Include a Placebo Group 20 WH A T I S N O T ME A S U R E D ? RE S U L T S F R OM P H A S E I TR I AL
▪ INCLUSION Mild AD with biomarkers of inflammation ▪ SIZE - 200 patients 2:1 randomization (134 XPro Œ vs 66 placebo) ▪ DOSE 1 mg/kg/week subQ ▪ DU R A TION – 6 m o n t h s ▪ COGNITION END - POINT - EMACC Phase I inclusion criteria selected for patients with neuroinflammation Patients with mild AD and neuroinflammation progress rapidly safe/effective for 50 weeks Low variability in neuroinflammation EMACC sensitive to improved cognition f o r A D ▪ Enrichment: matching patient’s disease (neuroinflammation) with drug MOA de - risks trial ▪ Placebo group: essential in CNS studies. Placebo effects are frequent in patient can care giver ▪ Cognition: choice of cognitive measure must consider patient stage (MIC, mild, mod, etc) and sensitivity to change. ADA Cog is not sensitive and can not measure improvements in disease ▪ Variability: variable response rates drive size of study. Low variability allows for smaller trials. 21 Early/mild Alzheimer’s Cognitive Composite (EMACC)*: *Hassenstab, 2017 PHASE 1 TRIAL INFORMED DOSE, DURATION AND DESIGN DATA DRIVEN TRIAL DESIGN LEARNINGS FROM PHASE I TRIAL

INCLUSION ▪ 200 patients 2:1 randomization (134 XPro Œ patients vs 66 placebo patients) ▪ 1mg/kg XPro Œ once a week for 6 months or placebo ▪ Inclusion criteria Mild AD - CDR global: 0.5 or 1 MMSE>21 Two or more biomarkers of inflammation CRP>1.5 mg/L ESR>10 sec HgbA1c>6% ApoE4 positive EN D - P O I N T S ▪ Primary: EMACC* Internatonal Shopping List Test - Immediate recall Digit Span Forward and Backward Category fl uency Test (2 categories) Letter fl uency Test (3 letters) Trail making Test Part A & B Digit Symbol Coding Test ▪ Secondary: Goal Attainment Scale (GAS) ADCS - ADL A D A S - C o g 1 3 CDR - SB E - Cog NPI MRI: AFD, WMFW, CDM Blood in fl ammatory and neurodegeneration biomarkers f o r A D PHASE 2 BLINDED, RANDOMIZED, PLACEBO - CONTROLLED IN MILD AD WITH BIOMARKERS OF INFLAMMATION 22 Early/mild Alzheimer’s Cognitive Composite (EMACC)*: *Hassenstab, 2017
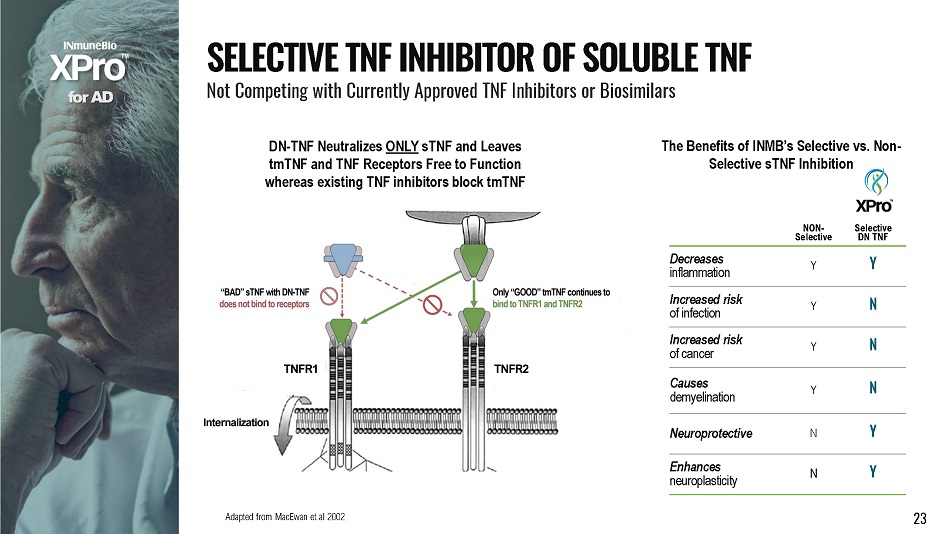
NON - Selective S e l ec t i v e DN TNF Decreases inflammation Y Y Increased risk o f i n f e c t i o n Y N Increased risk o f c an c e r Y N Causes demyelination Y N Neuroprotective N Y Enhances neuroplasticity N Y The Benefits of INMB’s Selective vs. Non - Selective sTNF Inhibition Adapted from MacEwan et al 2002 23 f o r A D DN - TNF Neutralizes ONLY sTNF and Leaves tmTNF and TNF Receptors Free to Function whereas existing TNF inhibitors block tmTNF S E L E C T I V E T N F I N H I B I T O R O F S O L U B L E T N F Not Competing with Currently Approved TNF Inhibitors or Biosimilars

see Steed et al., Science , 301, 2003 Soluble TNF Function Eliminated No inflammatory signaling f o r A D Nonglycosylated Protein Expressed in E. Coli DN - TNF Can Not Neutralize tmTNF receptors not blocked so tmTNF biology preserved H O W X P r o Œ N E U T R A L I ZE S s T N F And importantly leaves tmTNF and TNF receptors alone. sTNF and tmTMF Do Very Different Things… 24

EXACERBATED DEMYELINATION REMYELINATION XPro ΠPROMOTES R E M Y E L I N A T I O N ETANERCEPT A n t i - i nf l amma t o r y AND immunosuppressive DN - TNF Anti - inflammatory NOT immunosuppressive CUPRIZONE Model of Multiple Sclerosis NORMAL Cuprizone model of demyelination in mice: Prober 2017 https://inmunebio.com/index.php/en/science/xpro1595/references f o r A D A PP R O V E D T N F I N H I B I T O R S S H O U L D N O T B E U S E D I N C N S Demyelination Is An Off - Target Safety Side Effect of Currently Approved TNF Therapie s 25
Off - the - Shelf NK Therapy Producing memory like NK cells from Patient’s Own NK cells in Their Circulation (ie: in vivo) f o r Oncolo g y 26
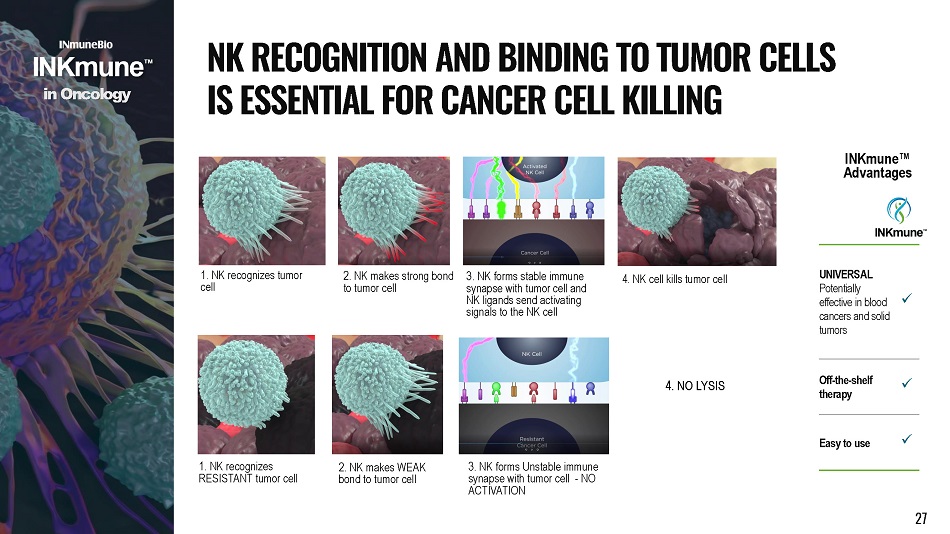
1. NK recognizes tumor cell 3. NK forms stable immune synapse with tumor cell and NK ligands send activating signals to the NK cell 2. NK makes strong bond to tumor cell 4. NK cell kills tumor cell 1. NK recognizes RESISTANT tumor cell 3 . NK forms Unstable immune synapse with tumor cell - NO ACTIVATION 2. NK makes WEAK bond to tumor cell i n Onco l o g y N K R E C O G N I T I O N A N D B I N D I N G T O T U M O R C E LL S I S E SS E N T I A L F O R C A N C E R C E L L K I LL I N G 4. NO LYSIS UNIVERSAL Potentially e f fe c t iv e i n b l oo d c an c e r s an d s o li d tumors x Off - the - shelf therapy x Eas y t o u s e x INKmune ΠAd va ntages 27

A M U L T I - D I M E N S I O N A L “ P S E U D O K I N E ” Moa #2 – InkMUNE Œ Differentiates Resting NK to “Memory - Like” NK Cells Cytokine cocktail (IL - 12/15/18) drives resting NK to memory - like NK (SciTranslMed 2016; Romee,Fenheger, et al) 0 5000 10000 15000 NK G 2 C M e F I 0 10000 20000 30000 40000 NK G 2 D M e F I 0 10000 20000 30000 N KG2 A M e F I 0 20000 40000 60000 80000 CD1 6 CD9 4 M e F I 0 5000 10000 15000 20000 M e F I Inkmune Generates Memory - like NK Cells with a Single Pseudokine Cytokine Induced Memory Like (CIML) NK cells and INKmune primed NK (TpNK) cells phenotypically identical Advantages of memory - like NK cells: ↑cytotoxicity, ↑persistence, ↑proliferation, active against solid tumors i n Onco l o g y 28
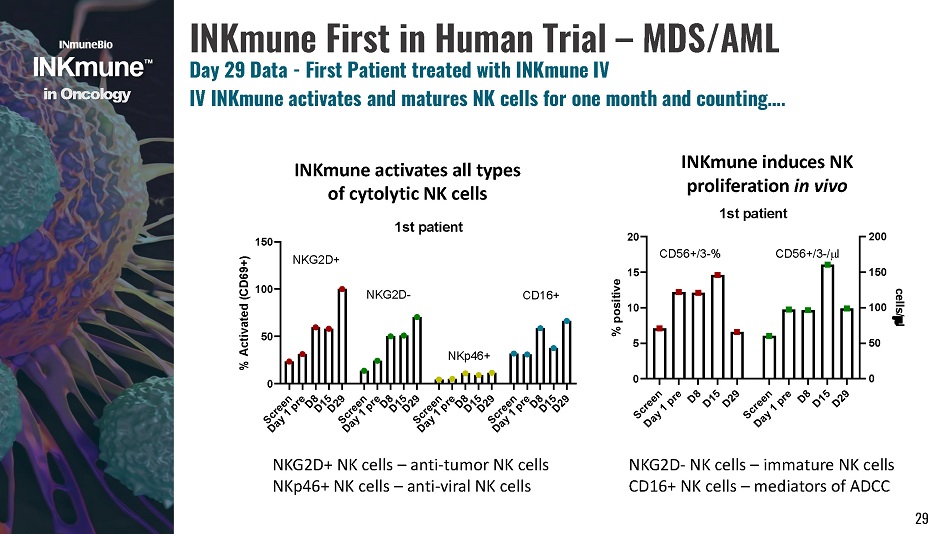
i n Onco l o g y INKmune First in Human Trial – MDS/AML Day 29 Data - First Patient treated with INKmune IV IV INKmune activates and matures NK cells for one month and counting…. INKmune activates all types of cytolytic NK cells INKmune induces NK proliferation in vivo 1st patient 0 5 1 0 1 5 2 0 0 5 0 100 150 200 % positive cells/ l CD56+/3 - % C D 56 + /3 - / l 29 0 5 0 100 150 % Activated (CD69+) N K G 2 D + N K G 2 D - 1st patient NKG2D+ NK cells – anti - tumor NK cells NKp46+ NK cells – anti - viral NK cells NKG2D - NK cells – immature NK cells CD16+ NK cells – mediators of ADCC
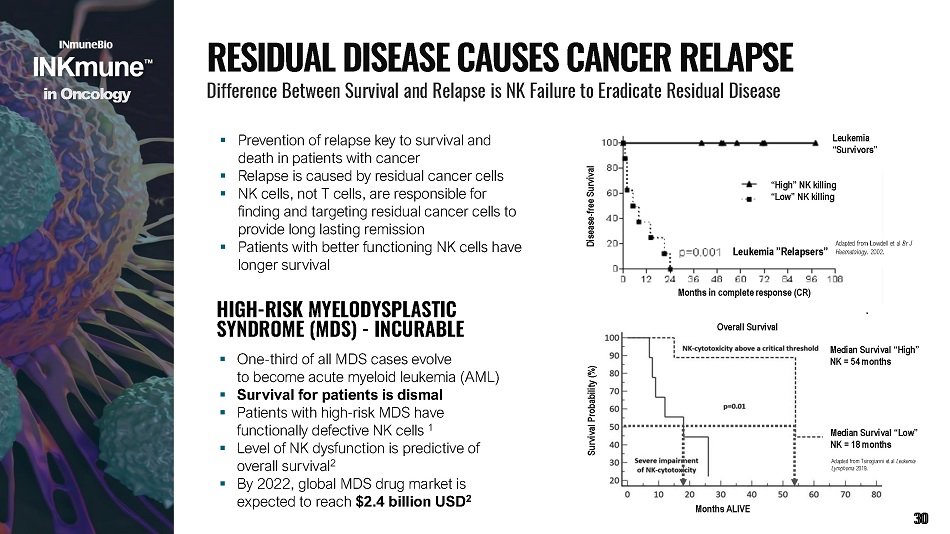
▪ Prevention of relapse key to survival and death in patients with cancer ▪ Relapse is caused by residual cancer cells ▪ NK cells, not T cells, are responsible for finding and targeting residual cancer cells to provide long lasting remission ▪ Patients with better functioning NK cells have longer survival Months in c om ple t e r e spon s e (C R ) “H igh” N K killi ng “Low” NK killing Leukemia ” R elapsers” Disea s e - f r e e S u r vi v al Leukemia “Survivors” Adapted from Lowdell et al Br J Haematology . 2002. Months ALI V E Median Survival “High” NK = 54 months Survival Probability (%) Overall Survival Median Survival “Low” NK = 18 months Adapted from Tsirogianni et al Leukemia Lymphoma 2019. overall survival 2 ▪ By 2022, global MDS drug market is expected to reach $2.4 billion USD 2 HIGH - RISK MYELODYSPLASTIC SYNDROME (MDS) - INCURABLE ▪ One - third of all MDS cases evolve to become acute myeloid leukemia (AML) ▪ Survival for patients is dismal ▪ Patients with high - risk MDS have functionally defective NK cells 1 ▪ Level of NK dysfunction is predictive of R E S I D U A L D I S E A S E C A U S E S C A N C E R R E L A P S E Difference Between Survival and Relapse is NK Failure to Eradicate Residual Disease i n Onco l o g y 30
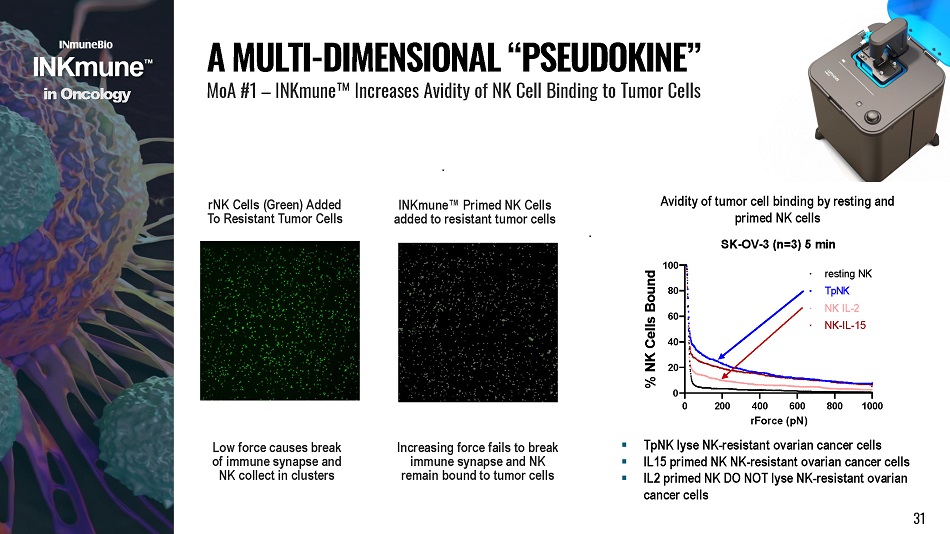
rNK Cells (Green) Added To Resistant Tumor Cells INKmune Œ Primed NK Cells added to resistant tumor cells Low force causes break of immune synapse and NK collect in clusters Increasing force fails to break immune synapse and NK remain bound to tumor cells 0 200 800 1000 400 600 rForce (pN) 4 0 2 0 0 6 0 8 0 % NK Cells Bound TpNK NK IL - 2 N K - I L - 1 5 Avidity of tumor cell binding by resting and primed NK cells SK - OV - 3 (n=3) 5 min 100 resting NK A MULTI - DIMENSIONAL “ PSEUDOKINE ” MoA #1 – INKmune Œ Increases Avidity of NK Cell Binding to Tumor Cells ▪ TpNK lyse NK - resistant ovarian cancer cells ▪ IL15 primed NK NK - resistant ovarian cancer cells ▪ IL2 primed NK DO NOT lyse NK - resistant ovarian cancer cells i n Onco l o g y 31
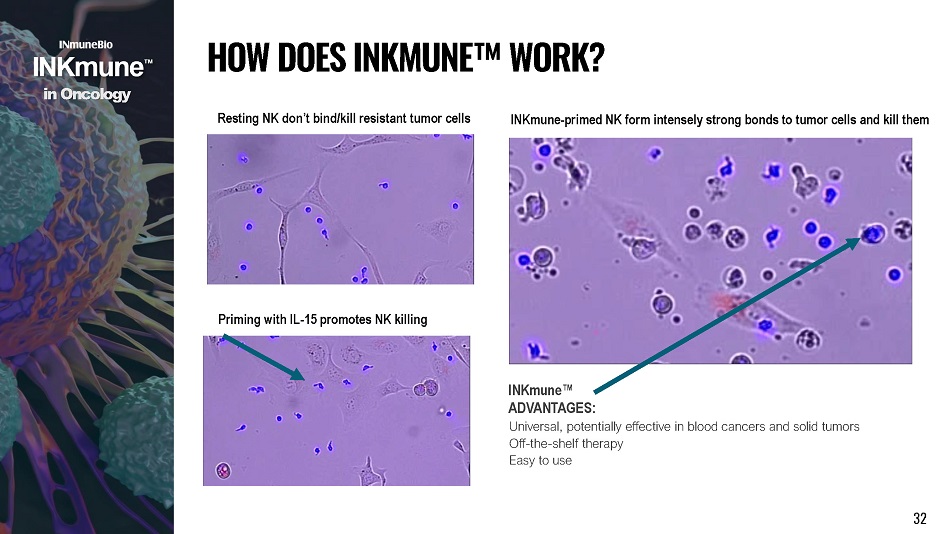
Resting NK don’t bind/kill resistant tumor cells Priming with IL - 15 promotes NK killing INKmune - primed NK form intensely strong bonds to tumor cells and kill them INKmune Œ AD V AN T AGE S : Universal, potentially effective in blood cancers and solid tumors Off - the - shelf therapy Easy to use H O W D O E S I NK M U N E Œ W O R K ? i n Onco l o g y 32
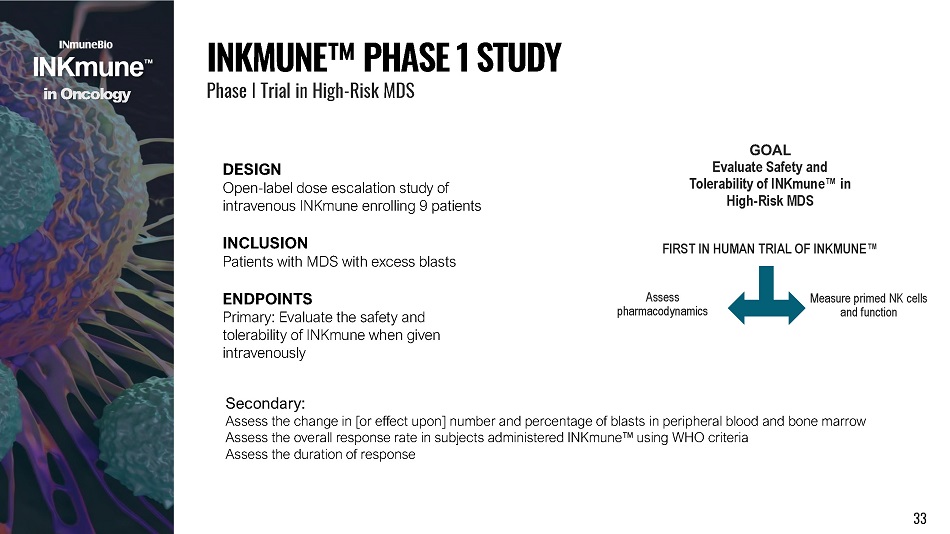
DESIGN Open - label dose escalation study of intravenous INKmune enrolling 9 patients INCLUSION Patients with MDS with excess blasts ENDPOINTS Primary: Evaluate the safety and tolerability of INKmune when given intravenously I NK M U N E ΠP H A S E 1 S T U D Y Phase I Trial in High - Risk MDS i n Onco l o g y FIRST IN HUMAN TRIAL OF INKMUNE ΠMeasure primed NK cells and function Assess p h a r mac o d y n a m i cs GOAL Evaluate Safety and Tolerability of INKmune Πin High - Risk MDS 33 Secondary: Assess the change in [or effect upon] number and percentage of blasts in peripheral blood and bone marrow Assess the overall response rate in subjects administered INKmune Πusing WHO criteria Assess the duration of response

T H E V ARIABLES ▪ Cytokines - single or multiple as a therapeutic or as part of cell therapy ▪ NK cell source – autologous, allogeneic, iPSC, cord blood ▪ Genetically engineered – CAR or cytokine ▪ Off - the - Shelf or patient specific manufacturing ▪ A unique, stable cell line, not an NK cell ▪ >180 unique genes induced in resting NK cells ▪ ↑ NK survival = persistence ▪ ↑ NK proliferation and ↑ NK cytokine secretion = more cancer killing cells ▪ ↑ avidity = increased NK killing ▪ Patients’ NK cells differentiate into TIML* NK cells phenotype = enhanced lytic function compared to CIML* NK cell ▪ Functional against solid and liquid tumors Operation/Logistic advantages ▪ Off - the - Shelf; No need for a donor ▪ No need for cytokines administration ▪ No need for cryogenic shipping or storage * TIML=Tumor Induced Memory Like * CIML= Cytokine Induced Memory Like i n Onco l o g y N K C O M P E T I T I V E L A N D S C A P E 18 Prog r a m s a n d C o u nti n g… 34

f o r T R D Targeting Neuroinflammation for TREATMENT RESISTANT DEPRESSION (TRD) 35

The Subset of People Who Have Failed At Least Two Anti Depressants 1 out of 3 MDD patients or 33% of the estimated 7M patients Economic toll: $64B/year Higher c o m orbid ity Chronic course of MDD TNF biology in TRD POC studies with anti - TNF blood CRP predicts response Symptoms affected anhedonia, psychomotor retardation, fatigue, cognition F R U S T R A T I O N S O F P S Y C H I A T R I C D R U G D E V E L O P M E N T ▪ Drug development in psychiatry is a “one size fits all” approach ▪ 200+ ways to be diagnosed with depression ▪ There are 200+ symptom combinations that will lead to a depression diagnosis ▪ No Biomarkers ▪ High placebo response ▪ Drug development failure rate in psychiatry ▪ in Phase II: 35% in Phase III: 65% ▪ Effectiveness of approved drugs ▪ 50% do not achieve remission ▪ 33% have no response (treatment resistant) f o r T R D T R E A T M E N T R E S I S T A N T D E P R E SS I O N ( T R D ) 36
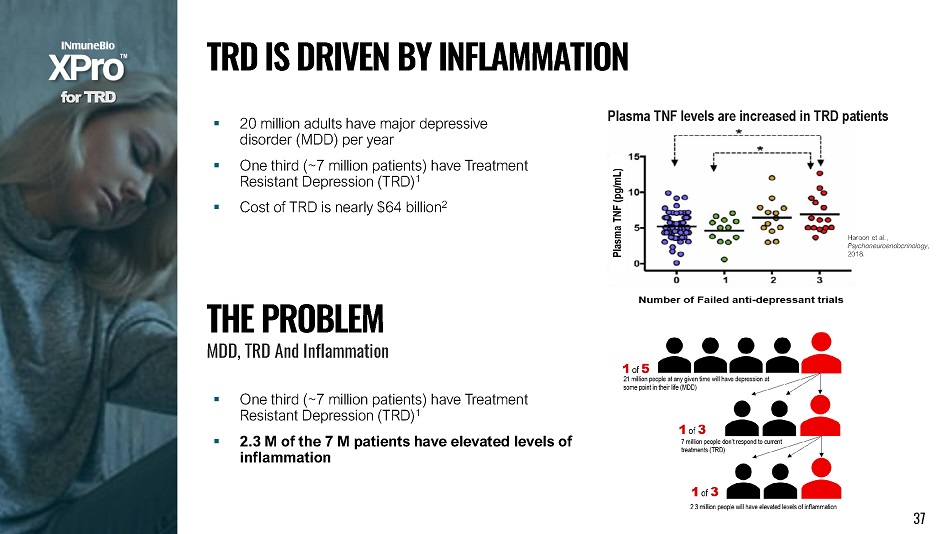
▪ 20 million adults have major depressive disorder (MDD) per year ▪ One third (~7 million patients) have Treatment Resistant Depression (TRD) 1 ▪ Cost of TRD is nearly $64 billion 2 Haroon et al., P s y c h o neu r o e nd o c r i n o l ogy , 2018. Plasma TNF levels are increased in TRD patients T H E P R O B L E M MDD, TRD And Inflam m at i on ▪ One third (~7 million patients) have Treatment Resistant Depression (TRD) 1 ▪ 2.3 M of the 7 M patients have elevated levels of inflammation f o r T R D T R D I S D R I V E N B Y I N F L A MM A T I O N 37
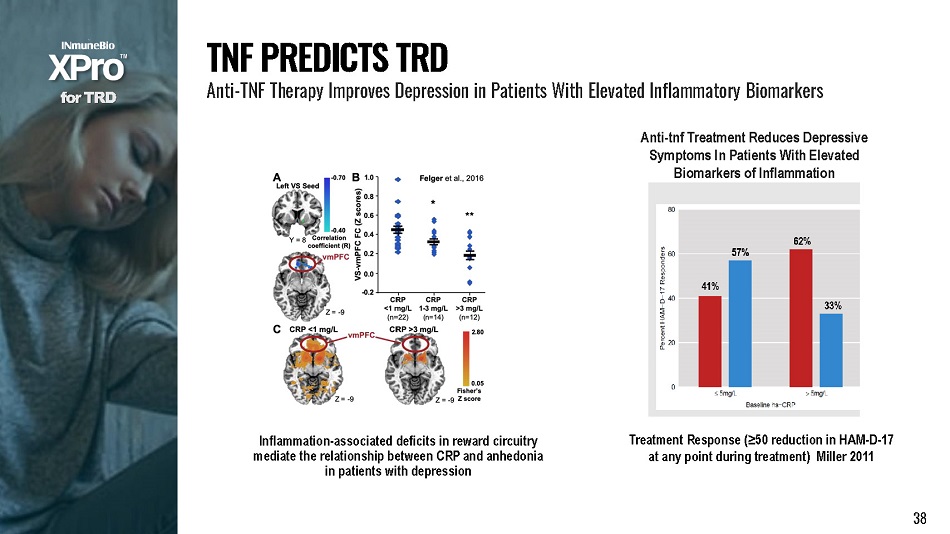
Treatment Response (≥50 reduction in HAM - D - 17 at any point during treatment) Miller 2011 Anti - tnf Treatment Reduces Depressive Symptoms In Patients With Elevated Biomarkers of Inflammation Inflammation - associated deficits in reward circuitry mediate the relationship between CRP and anhedonia in patients with depression f o r T R D T N F P R E D I C T S T R D Anti - TNF Therapy Improves Depression in Patients With Elevated Inflammatory Biomarkers 38
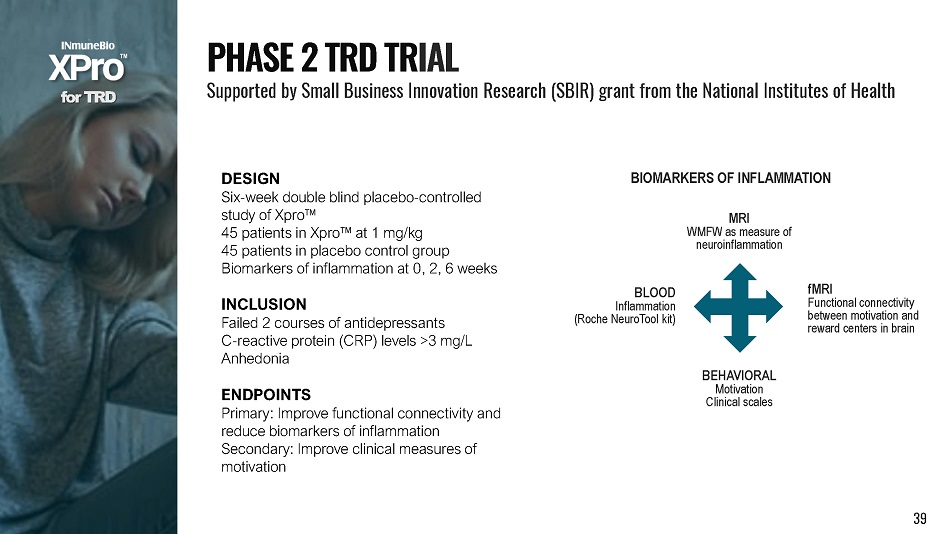
DESIGN Six - week double blind placebo - controlled study of Xpro Π45 patients in Xpro Πat 1 mg/kg 45 patients in placebo control group Biomarkers of inflammation at 0, 2, 6 weeks INCLUSION Failed 2 courses of antidepressants C - reactive protein (CRP) levels >3 mg/L Anhedonia ENDPOINTS Primary: Improve functional connectivity and reduce biomarkers of inflammation Secondary: Improve clinical measures of motivation f o r T R D P H A S E 2 T R D T R I A L Supported by Small Business Innovation Research (SBIR) grant from the National Institutes of Health BIOMARKERS OF INFLAMMATION BLOOD Inflammation (Roche NeuroTool kit) MRI WMFW as measure of neuroinflammation 39 fMRI Functional connectivity between motivation and reward centers in brain BEHAVIORAL Motivation Clinical scales

f o r C O VI D - 1 9 Comp li c a ti on s Targ e ting CYT O KINE STORM may P revent Catastrophic Complications of COVID - 19 40
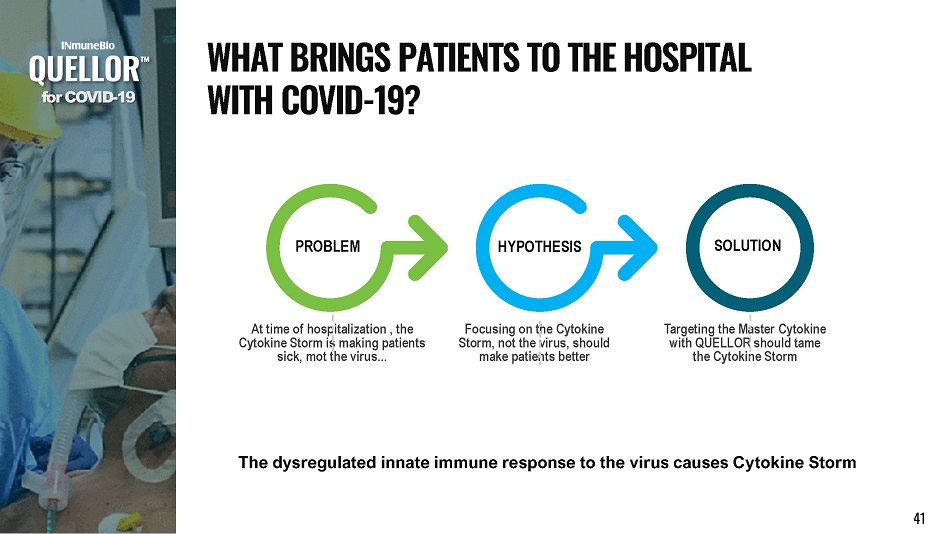
At time of hospitalization , the Cytokine Storm is making patients sick, mot the virus... Focusing on the Cytokine Storm, not the virus, should make patients better Targeting the Master Cytokine with QUELLOR should tame the Cytokine Storm P R O BL E M SOLUTION HYPOTHESIS The dysregulated innate immune response to the virus causes Cytokine Storm f o r C O V I D - 19 W H A T B R I N G S P A T I E N T S T O T H E H O S P I T A L W I T H C O V I D - 19? 41

f o r C O V I D - 19 42 Adapted from Karki et al 2020 ▪ Block sTNF and downstream pro - inflammatory cytokines should decrease ▪ Synergism of TNF - α and IFN - γ triggers inflammatory cell death, tissue damage, and mortality T N F I S T H E “ M A S T E R ” O F T H E C Y T O K I N E S T O R M
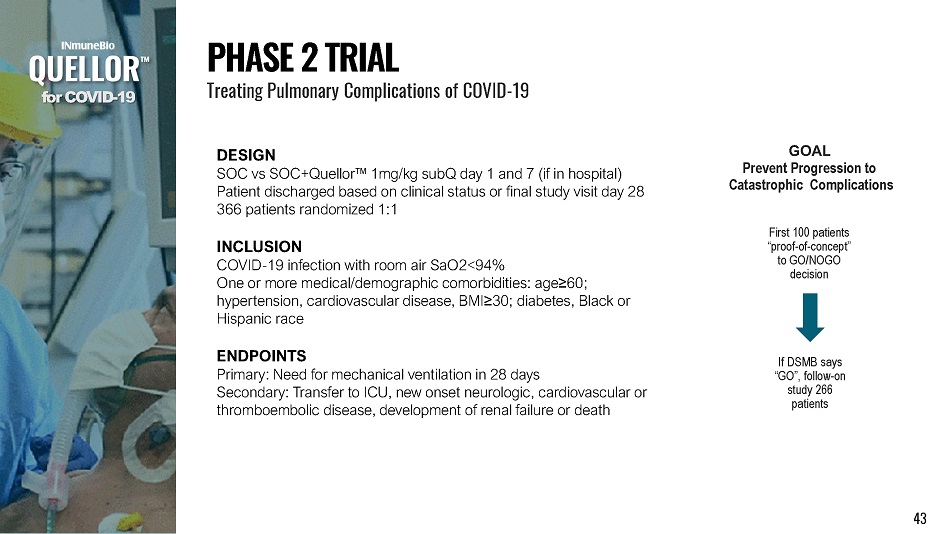
DESIGN SOC vs SOC+Quellor Œ 1mg/kg subQ day 1 and 7 (if in hospital) Patient discharged based on clinical status or final study visit day 28 366 patients randomized 1:1 INCLUSION COVID - 19 infection with room air SaO2<94% One or more medical/demographic comorbidities: age ≥ 60; hypertension, cardiovascular disease, BMI ≥ 30; diabetes, Black or Hispanic race ENDPOINTS Primary: Need for mechanical ventilation in 28 days Secondary: Transfer to ICU, new onset neurologic, cardiovascular or thromboembolic disease, development of renal failure or death f o r C O V I D - 19 P H A S E 2 T R I A L Treating Pulmonary Complications of COVID - 19 If DSMB says “GO”, follow - on study 266 patients First 100 patients “ p r o o f - o f - c o n c e p t” to GO/NOGO decision GOAL Prevent Progression to Catastrophic Complications 43
▪ Changing landscape: ▪ Regulatory – FDA not awarding EUA for therapies ▪ Clinical – C19 less lethal, SOC changing, geography challenging ▪ What we are doing: ▪ Declared Phase II trial complete after 76 patients ▪ Performing data base lock and analysis ▪ Schedule end - of - Phase II meeting with FDA to understand approval pathway ▪ Decision to pursue approval for treatment of cytokine storm will depends on pandemic, FDA and Phase II results f o r C O V I D - 19 Q U E LL O R P H A S E 2 T R I A L : N E X T S T E P S Treating Pulmonary Complications of COVID - 19 44

HARNESSING THE POWER OF THE INNATE IMMUNE SYSTEM CONTACT US INmune Bio Inc. 980 N. Federal Hwy, Suite 110 Boca Raton, FL 33432 (858) 964 - 3720 I N M B www.inmunebio.com 45












































