Exhibit 99.1

September 27, 2018 Sidoti and Company, LLC Fall 2018 Conference LD Micro Investor Conference September 2020 Investor Presentation

2 This presentation contains forward - looking statements. Statements that are not historical facts are forward - looking statements and such forward - looking statements are statements made pursuant to the Safe Harbor Provisions of the Private Securities Litigation Reform Act of 1995. Example of forward - looking statements include: ● statements about Rafael Holdings’ future performance; ● projections of Rafael Holdings’ results of operations or financial condition; ● statements regarding Rafael Holdings’ plans, objectives or goals, including those relating to its strategies, initiatives, competition, acquisitions, dispositions and/or its products; and ● expectations concerning the clinical trials, submissions and FDA approval of Lipomedix’s, Barer Institute’s and and Rafael Pharmaceutical’s drug compounds. Words such as “believe,” “anticipate,” “plan,” “expect,” “intend,” “target,” “estimate,” “project,” “predict,” “forecast,” “guideline,” “aim,” “will,” “should,” “likely,” “continue,” and similar expressions are intended to identify forward - looking statements but are not the exclusive means of identifying such statements. Readers are cautioned not to place undue reliance on these forward - looking statements and all such forward - looking statements are qualified in their entirety by reference to the following cautionary statements. Forward - looking statements are based on Rafael Holdings’ current expectations, estimates and assumptions and because forward - looking statements address future results, events and conditions, they, by their very nature, involve inherent risks and uncertainties, many of which are unforeseeable and beyond Rafael Holdings’ control. Such known and unknown risks, uncertainties and other factors may cause Rafael Holdings’ actual results, performance and other achievements to differ materially from the anticipated results, performance or achievements expressed, projected or implied by these forward - looking statements. These factors include those discussed under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations in Rafael Holdings’ Form 10 Information Statement and other filings made with the Securities and Exchange Commission. Rafael Holdings cautions that such factors are not exhaustive and that other risks and uncertainties may cause actual results to differ materially from those in forward - looking statements. Forward - looking statements speak only as of the date they are made and are statements of Rafael Holdings’ current expectations concerning future results, events and conditions and Rafael Holdings is under no obligation to update any of the forward - looking statements, whether as a result of new information, future events or otherwise. Forward Looking Statements
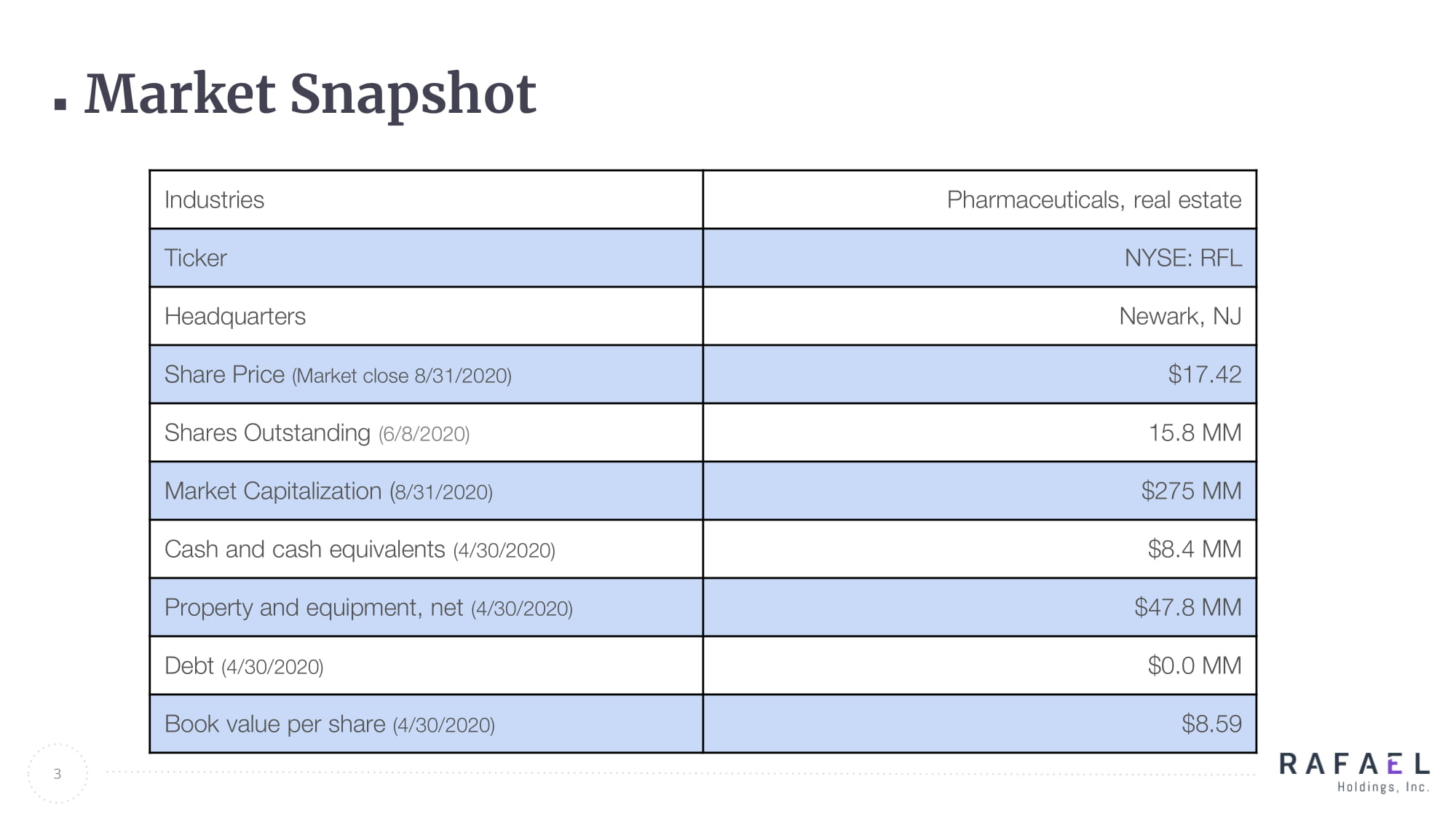
Market Snapshot 3 Industries Pharmaceuticals, real estate Ticker NYSE: RFL Headquarters Newark, NJ Share Price (Market close 8/31/2020) $17.42 Shares Outstanding (6/8/2020) 15.8 MM Market Capitalization ( 8/31/2020) $275 MM Cash and cash equivalents (4/30/2020) $8.4 MM Property and equipment, net (4/30/2020) $47.8 MM Debt (4/30/2020) $0.0 MM Book value per share (4/30/2020) $8.59

Overview Rafael Holdings is comprised of interests in two promising pharmaceutical companies, an initiative targeting unmet medical needs in oncology and a portfolio of commercial real estate properties REAL ESTATE Rafael Holdings has a portfolio of real estate assets featuring a commercial property in Newark, New Jersey’s revitalized Washington Square Park district PHARMACEUTICALS Rafael Holdings invests in companies pursuing breakthrough therapies for some of the most difficult - to - treat cancers: Rafael Pharmaceuticals, Inc., a late - stage metabolic oncology therapeutics company, and LipoMedix, a company focused on pegylation and liposomal technologies 4 DEVELOPMENT Rafael Holdings, through the Barer Institute, is developing novel compounds for unmet medical needs, utilizing top tier scientists at leading academic institutions

Howard Jonas CHAIRMAN and CEO Rafael Holdings Management 5 Menachem Ash PRESIDENT AND GC ● Executive Vice President of Strategy and Legal Affairs at IDT Corporation and managing attorney of the Company’s legal department ● Former General Counsel to Telstar International, Inc., a telecommunications services provider David Polinsky CHIEF FINANCIAL OFFICER ● Co - founder of Rafael Pharmaceuticals ● Former Director, President, General Counsel and Corporate Secretary of Rafael Pharmaceuticals. ● Former Vice President and General Counsel for Square Management Corp, a New York real estate focused investment and management company ● Founder of several public companies including Rafael Holdings, IDT Corporation - a communications and payment company, and Genie Energy - a leading independent energy provider ● Chairman of the Board of IDT Corporation and Genie Energy; Chairman of IDW Media Holdings

RFL holds stakes in two promising clinical stage cancer therapeutics companies: Rafael Pharmaceuticals and LipoMedix Rafael Holdings holds a 58%* stake in LipoMedix Rafael Holdings has invested $70 million to acquire 51%* of Rafael Pharmaceuticals outstanding capital stock (inclusive of minority interests) and 39%* on a fully diluted basis. Rafael Holdings holds a warrant entitling it to purchase 56% of the fully diluted equity interest in Rafael Pharmaceuticals (inclusive of minority interests). 6 Rafael Pharmaceuticals | Key Facts *At 4/30/2020

7 Rafael Pharmaceuticals

8 Devimistat (CPI - 613®) | Commercial Development Overview Clinical Trial Status ● Ongoing Phase III trials: Pancreatic Cancer, Acute Myeloid Leukemia (AML) ● Ongoing Phase I & II trials: Burkitt’s / High - Grade B - cell Lymphoma, Pancreatic Cancer ● Estimated NDA Submission: 2021 Orphan Drug Designations ● FDA: Pancreatic Cancer, AML, Peripheral T - cell Lymphoma (PTCL), Burkitt’s Lymphoma and Myelodysplastic Syndrome (MDS) ● EMA: Pancreatic Cancer and AML ● Only Oncology Company with 5 ODDs in U.S. and 2 in EU Intellectual Property ● Protected until 2029 and beyond across U.S., Canada, EU, Israel, Australia and major markets of Asia (China, Hong Kong, Japan, South Korea, Taiwan) Product Pipeline Extensions ● RFL - 618 (oral form of 613) ● Internal Discovery Compounds: 1150/5800/5990 (IND by 2020)
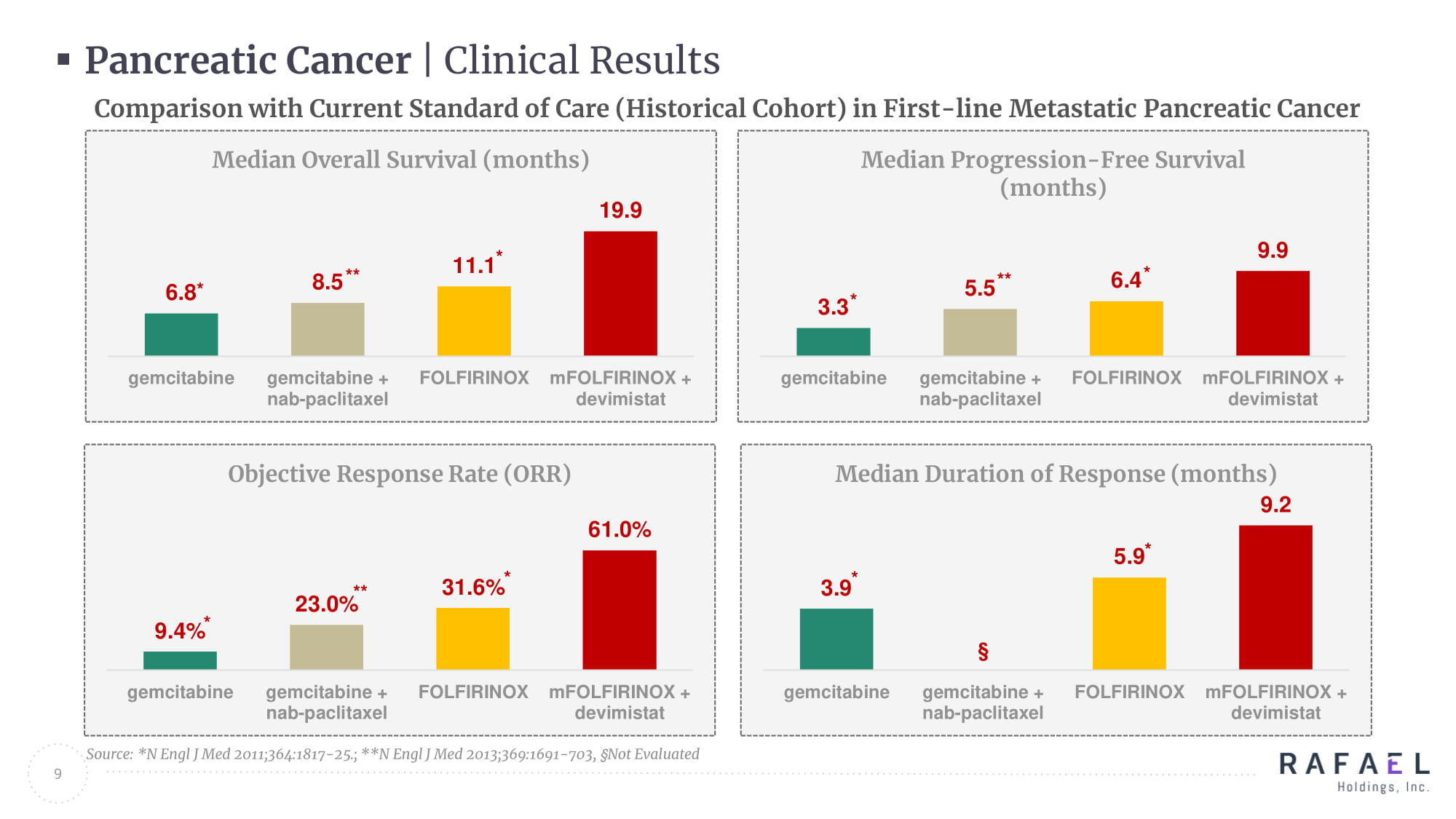
9 Pancreatic Cancer | Clinical Results 6.8 8.5 11.1 19.9 gemcitabine gemcitabine + nab-paclitaxel FOLFIRINOX mFOLFIRINOX + devimistat Median Overall Survival (months) 3.3 5.5 6.4 9.9 gemcitabine gemcitabine + nab-paclitaxel FOLFIRINOX mFOLFIRINOX + devimistat Median Progression - Free Survival (months) 3.9 § 5.9 9.2 gemcitabine gemcitabine + nab-paclitaxel FOLFIRINOX mFOLFIRINOX + devimistat Median Duration of Response (months) 9.4% 23.0% 31.6% 61.0% gemcitabine gemcitabine + nab-paclitaxel FOLFIRINOX mFOLFIRINOX + devimistat Objective Response Rate (ORR) Source: *N Engl J Med 2011;364:1817 - 25.; **N Engl J Med 2013;369:1691 - 703, § Not Evaluated * Comparison with Current Standard of Care (Historical Cohort) in First - line Metastatic Pancreatic Cancer * ** * * ** * * ** * * *
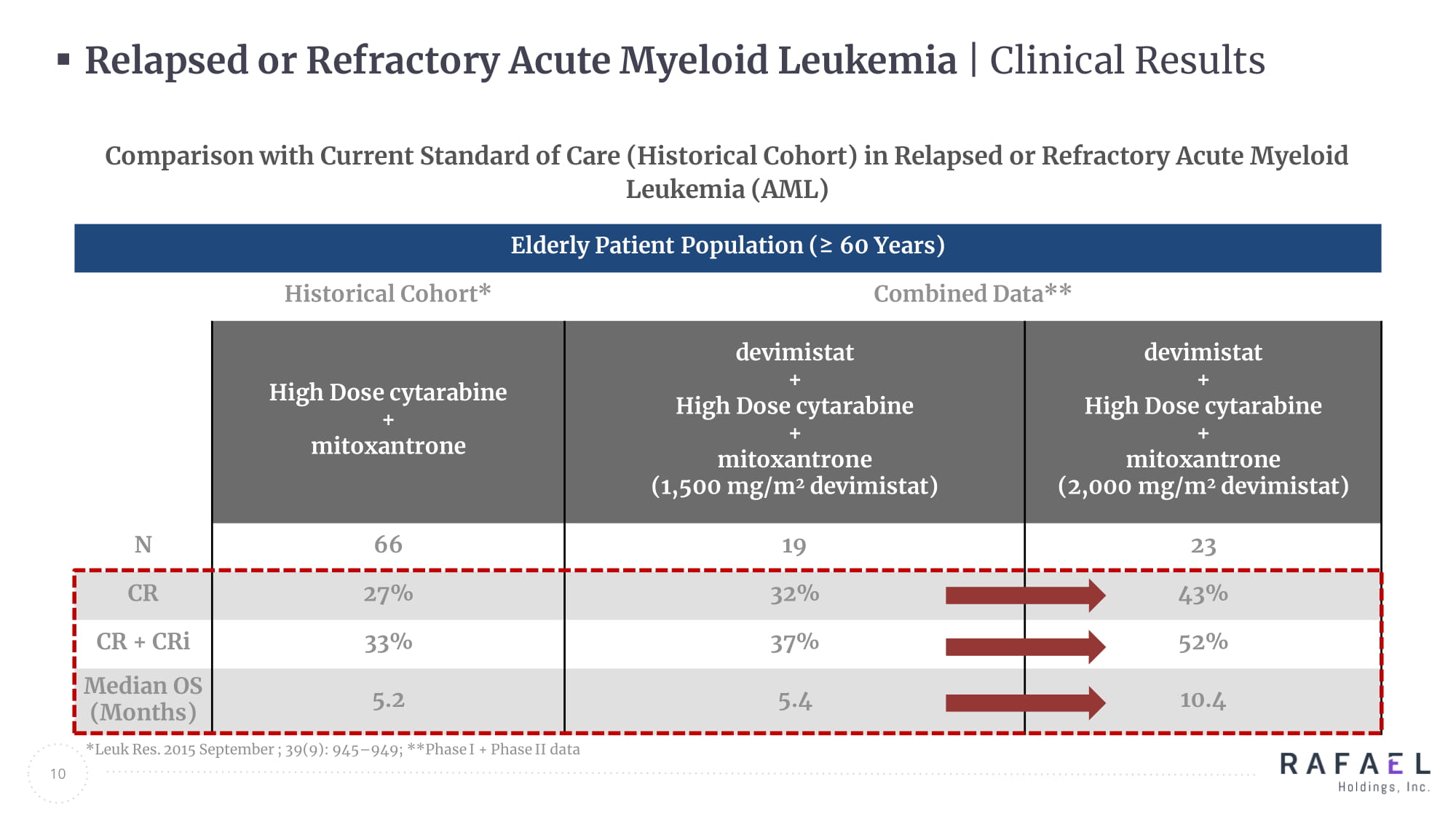
* Leuk Res. 2015 September ; 39(9): 945 – 949; **Phase I + Phase II data 10 Elderly Patient Population (≥ 60 Years) Historical Cohort* Combined Data** High Dose cytarabine + mitoxantrone devimistat + High Dose cytarabine + mitoxantrone (1,500 mg/m 2 devimistat ) devimistat + High Dose cytarabine + mitoxantrone (2,000 mg/m 2 devimistat ) N 66 19 23 CR 27% 32% 43% CR + CRi 33% 37% 52% Median OS (Months) 5.2 5.4 10.4 Comparison with Current Standard of Care (Historical Cohort) in Relapsed or Refractory Acute Myeloid Leukemia (AML) Relapsed or Refractory Acute Myeloid Leukemia | Clinical Results

11 T - cell Lymphoma & MDS | Clinical Results Relapsed or Refractory T - Cell Lymphoma Phase I study of devimistat in combination with bendamustine exhibited significant signal for efficacy bendamustine (BENTLY Trial*) devimistat + bendamustine ORR 50% 75% Median OS 6.2 Months 9.2 Months Median PFS 3.6 Months 6.4 Months *Note: This is an illustrative comparison of clinical experience of devimistat + bendamustine with data from one historical trials of bendamustine alone (BENTLY Trial) Relapsed or Refractory Myelodysplastic Syndrome (MDS) Phase II Study of devimistat (Single Agent) CRi Marrow CR SD PD N* 1 1 8 2 % 8% 8% 67% 17% *3 patients are still alive with one patient SD after 6 years
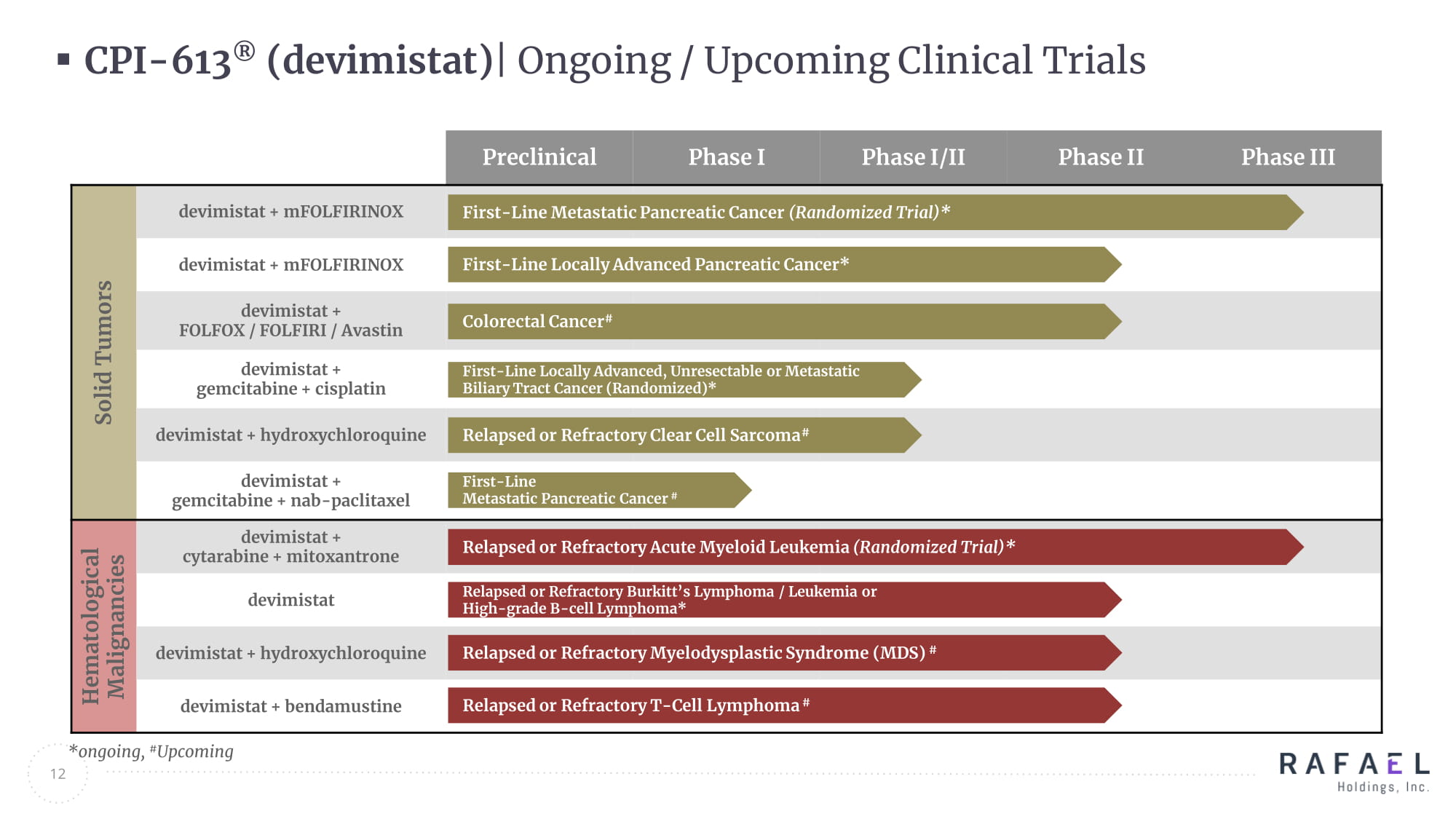
Preclinical PHASE I PHASE I/II PHASE II PHASE III Solid Tumors Hematological Malignancies CPI - 613 ® ( devimistat ) | Ongoing / Upcoming Clinical Trials 12 *ongoing, # Upcoming Preclinical Phase I Phase I/II Phase II Phase III Solid Tumors devimistat + mFOLFIRINOX devimistat + mFOLFIRINOX devimistat + FOLFOX / FOLFIRI / Avastin devimistat + gemcitabine + cisplatin devimistat + hydroxychloroquine devimistat + gemcitabine + nab - paclitaxel Hematological Malignancies devimistat + cytarabine + mitoxantrone devimistat devimistat + hydroxychloroquine devimistat + bendamustine First - Line Metastatic Pancreatic Cancer (Randomized Trial)* First - Line Metastatic Pancreatic Cancer # Relapsed or Refractory Acute Myeloid Leukemia (Randomized Trial)* Relapsed or Refractory Burkitt’s Lymphoma / Leukemia or High - grade B - cell Lymphoma* Relapsed or Refractory Myelodysplastic Syndrome (MDS) # Relapsed or Refractory T - Cell Lymphoma # Relapsed or Refractory Clear Cell Sarcoma # First - Line Locally Advanced, Unresectable or Metastatic Biliary Tract Cancer (Randomized)* Colorectal Cancer # First - Line Locally Advanced Pancreatic Cancer*
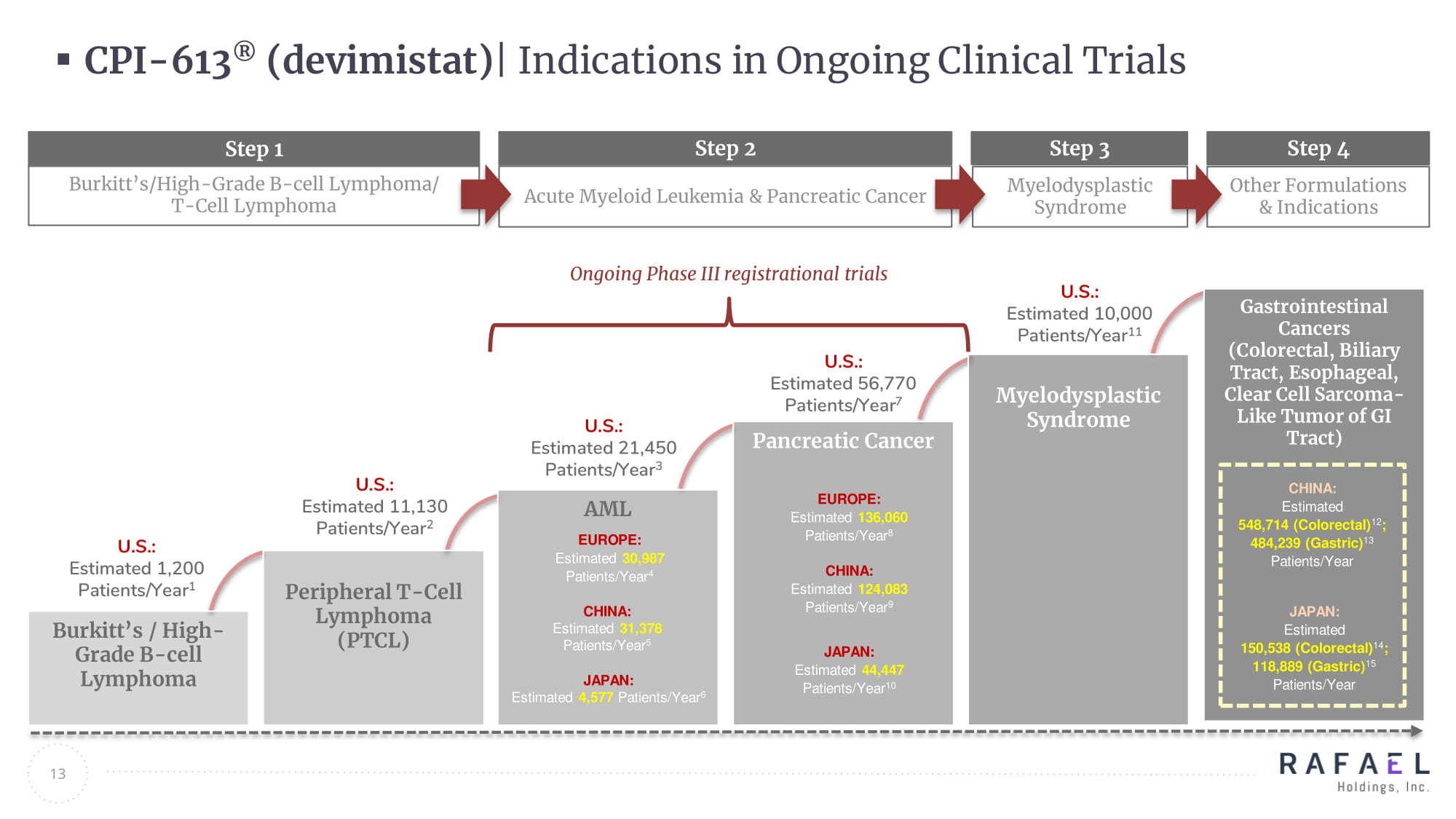
13 CHINA: ∼ 28,000 JAPAN: ∼ 16,000 CHINA: ∼ 80,000 JAPAN: ∼ 40,000 EUROPE: ∼ 133,000 EUROPE : ∼ 30,000 AML Pancreatic Cancer Burkitt’s / High - Grade B - cell Lymphoma Peripheral T - Cell Lymphoma (PTCL) Myelodysplastic Syndrome Gastrointestinal Cancers (Colorectal, Biliary Tract, Esophageal , Clear Cell Sarcoma - Like Tumor of GI Tract) Step 1 Burkitt’s/High - Grade B - cell Lymphoma/ T - Cell Lymphoma Step 2 Acute Myeloid Leukemia & Pancreatic Cancer U.S.: Estimated 1,200 Patients/Year 1 U.S.: Estimated 11,130 Patients/Year 2 U.S.: Estimated 21,450 Patients/Year 3 U.S.: Estimated 56,770 Patients/Year 7 U.S.: Estimated 10,000 Patients/Year 11 Step 3 Myelodysplastic Syndrome Ongoing Phase III registrational trials Step 4 Other Formulations & Indications CPI - 613 ® ( devimistat ) | Indications in Ongoing Clinical Trials CHINA: Estimated 31,378 Patients/Year 5 JAPAN: Estimated 4,577 Patients/Year 6 EUROPE: Estimated 30,987 Patients/Year 4 CHINA: Estimated 124,083 Patients/Year 9 JAPAN: Estimated 44,447 Patients/Year 10 EUROPE: Estimated 136,060 Patients/Year 8 CHINA: Estimated 548,714 (Colorectal) 12 ; 484,239 (Gastric) 13 Patients/Year JAPAN: Estimated 150,538 (Colorectal) 14 ; 118,889 (Gastric) 15 Patients/Year

Devimistat | Commercial Licensing ● Out - licensing agreement with Ono Pharmaceutical announced in June 2019 ● Ono, based in Japan, previously struck similar licensing deal with BMS on Opdivo ● Ono acquired exclusive development and commercialization rights in Japan, South Korea, Taiwan and ASEAN ● Rafael Pharma received approximately $12.9 million in cash. Additional $150.3 million contingent on development and commercial milestones, and low double - digit royalties on prospective sales in the specified markets. 14 14
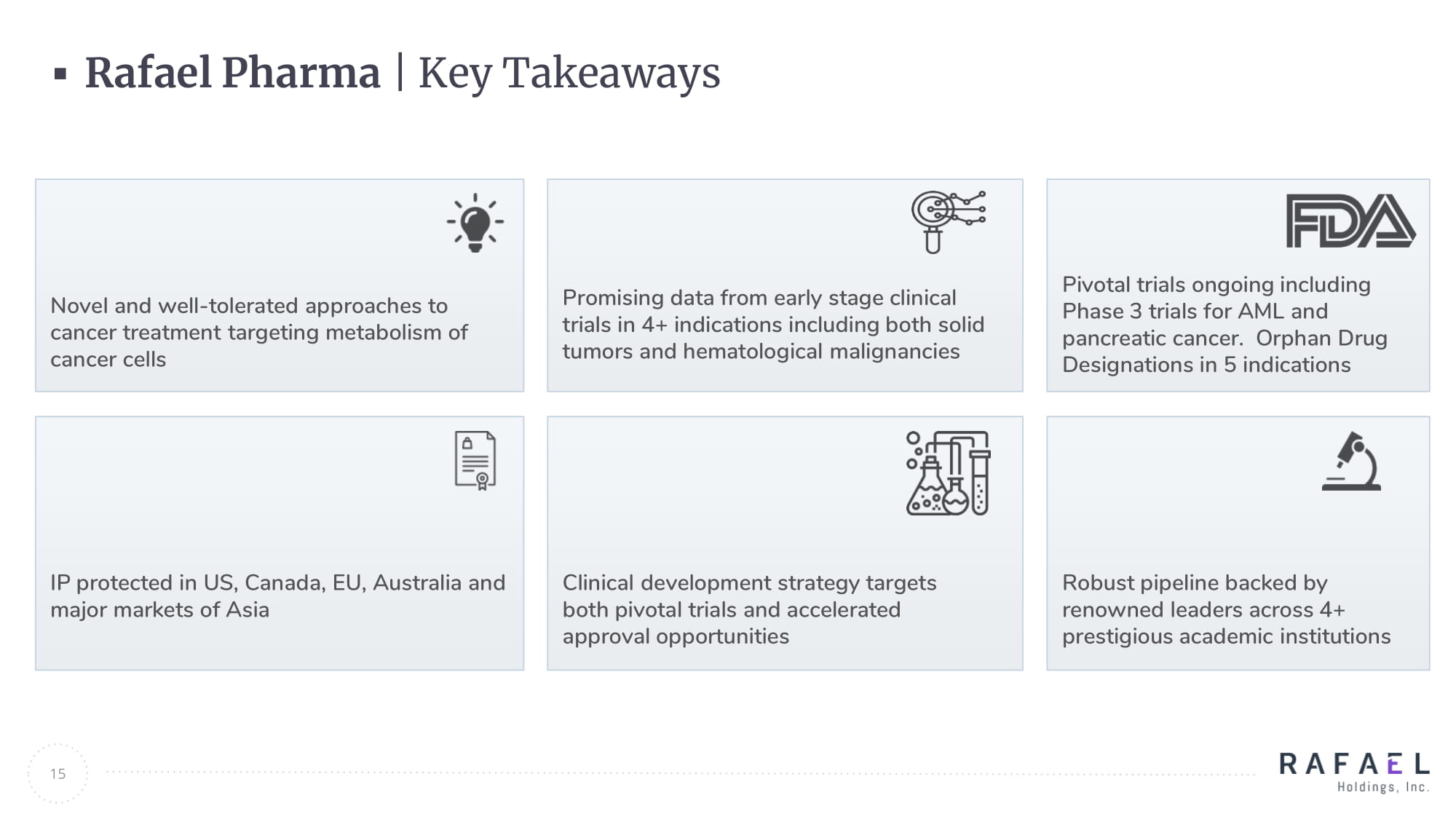
15 Novel and well - tolerated approaches to cancer treatment targeting metabolism of cancer cells Pivotal trials ongoing including Phase 3 trials for AML and pancreatic cancer. Orphan Drug Designations in 5 indications IP protected in US, Canada, EU, Australia and major markets of Asia Robust pipeline backed by renowned leaders across 4+ prestigious academic institutions Rafael Pharma | Key Takeaways Promising data from early stage clinical trials in 4+ indications including both solid tumors and hematological malignancies Clinical development strategy targets both pivotal trials and accelerated approval opportunities

16 LipoMedix

17 The scientific founder of LipoMedix is Alberto Gabizon , MD, PhD . Dr. Gabizon is the co - inventor and co - developer of Doxil ® ( Caelyx ), a pegylated liposomal delivery system encapsulating a powerful anti - cancer drug, doxorubicin. LipoMedix is led by a deeply experienced Management Committee of the Board of Directors: LipoMedix | Team Sanjeev Luther Executive Chairman Alberto Gabizon Chief Scientist & BOD Praveen Tyle, PhD Board of Directors Miranda J. Toledano Board of Directors ● Chief Executive Officer of Rafael Pharmaceuticals ● 25+ years of experience in healthcare, specialty pharma and bio - pharma industry segments in strategy, business development, alliances, commercialization and operations ● Professor of Medical Oncology at Shaare Zedek Medical Center and Hebrew Univ., of Jerusalem ● Co - inventor of Doxil®, a powerful and successful liposomal anticancer drug ● Executive Vice President of Research and Development at Lexicon Pharmaceuticals ● Over 30 years of pharma advancing drug candidate pipelines to commercial success ● Chief Operating Officer and Chief Financial Officer at TRIGR Therapeutics ● Previously served on the executive management team of Sorrento Therapeutics as EVP Corp. Dev.
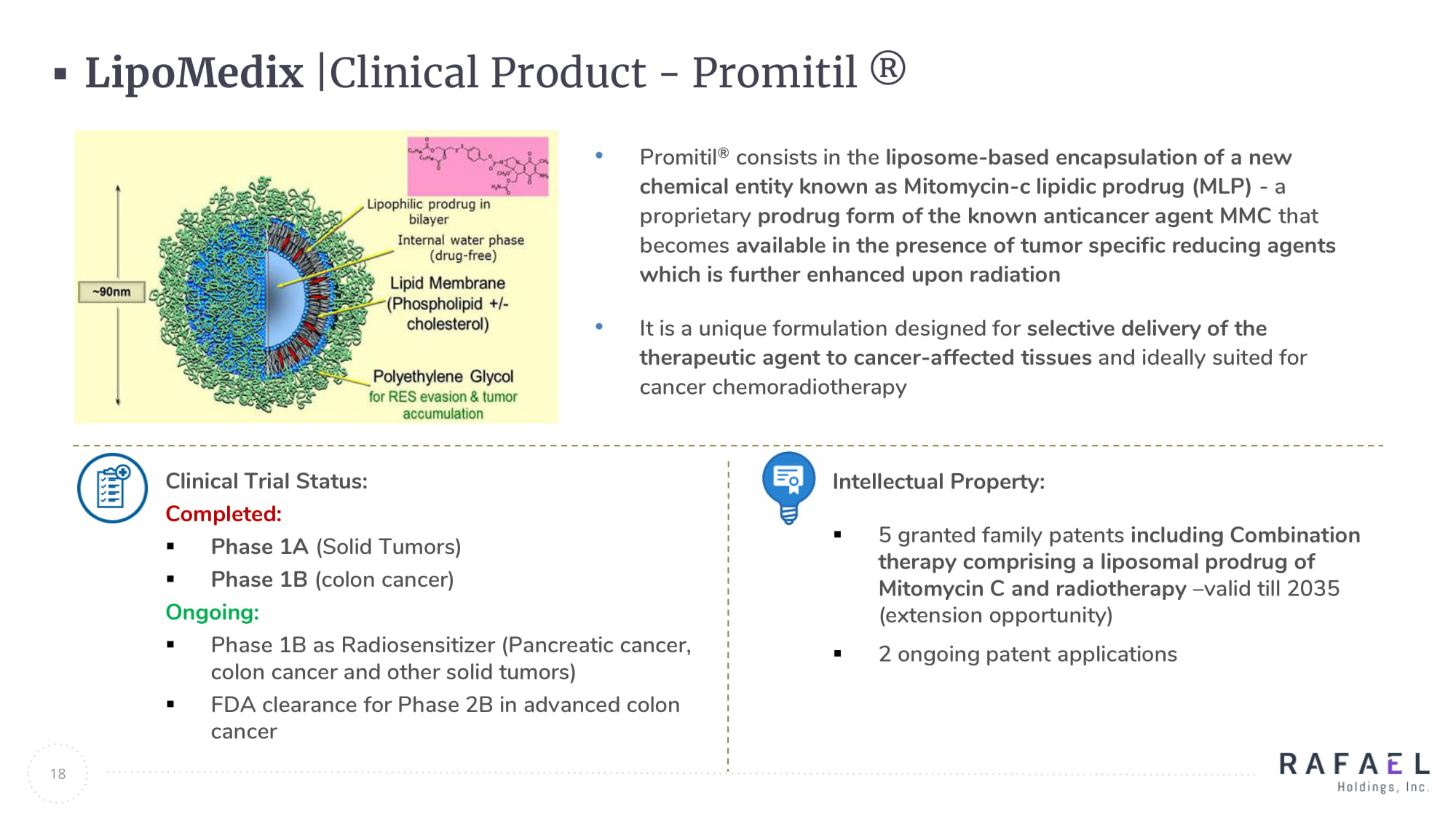
18 LipoMedix |Clinical Product - Promitil ® • Promitil ® consists in the liposome - based encapsulation of a new chemical entity known as Mitomycin - c lipidic prodrug (MLP) - a proprietary prodrug form of the known anticancer agent MMC that becomes available in the presence of tumor specific reducing agents which is further enhanced upon radiation • It is a unique formulation designed for selective delivery of the therapeutic agent to cancer - affected tissues and ideally suited for cancer chemoradiotherapy Clinical Trial Status: Completed: ▪ Phase 1A (Solid Tumors) ▪ Phase 1B (colon cancer) Ongoing: ▪ Phase 1B as Radiosensitizer (Pancreatic cancer, colon cancer and other solid tumors) ▪ FDA clearance for Phase 2B in advanced colon cancer Intellectual Property: ▪ 5 granted family patents including Combination therapy comprising a liposomal prodrug of Mitomycin C and radiotherapy – valid till 2035 (extension opportunity) ▪ 2 ongoing patent applications
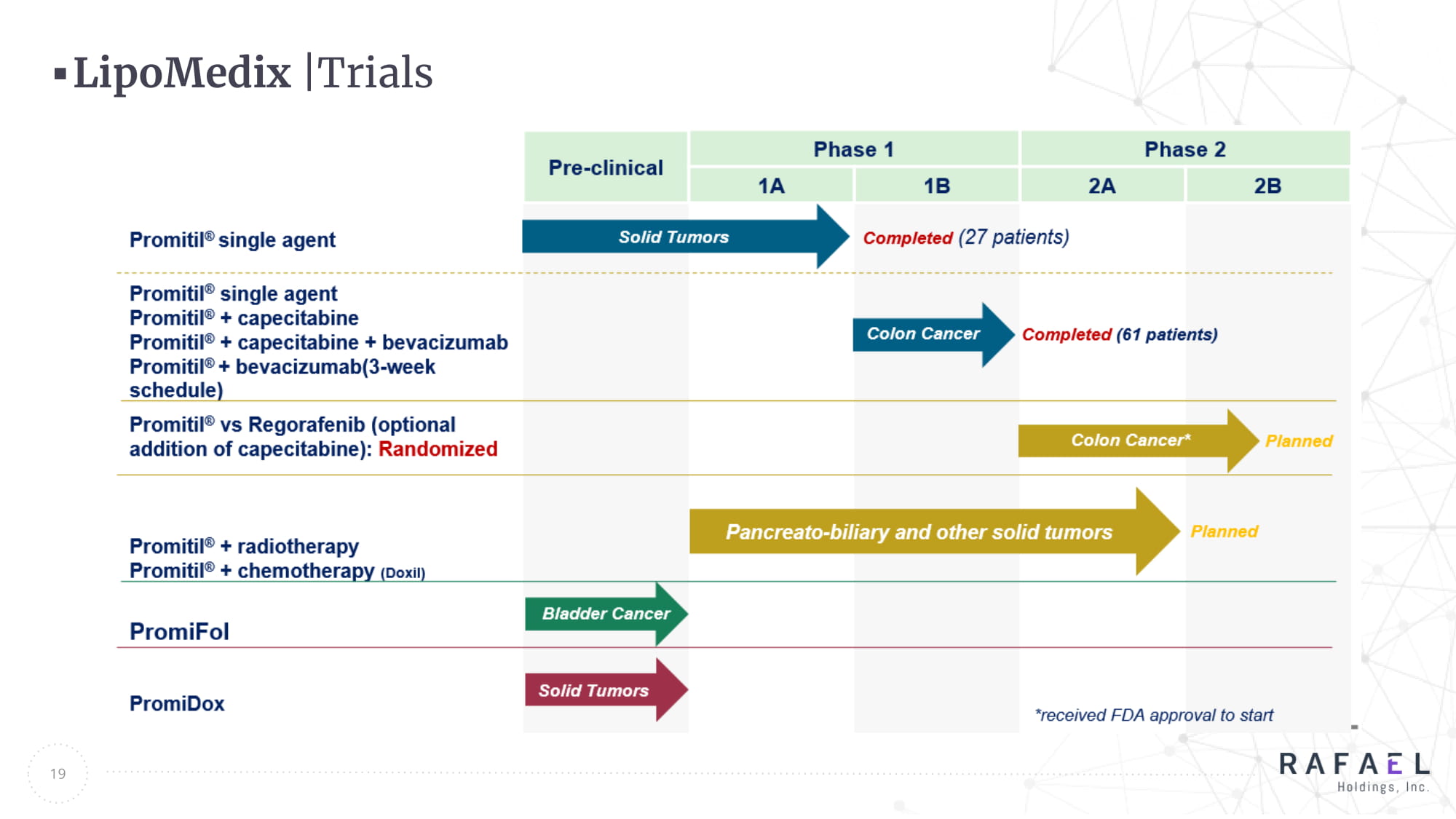
19 LipoMedix | Trials

20 Barer Institute

21 Rafael Holdings has established a wholly owned subsidiary - the Barer Institute - to develop novel compounds for controlling cellular metabolism. The Barer Institute has reached collaborative research agreements with leading scientists in the field of cancer metabolism at leading academic institutions. These collaborations are intended to provide advanced insight into metabolic mechanisms of action that can be translated into a compelling therapeutic pipeline. Barer Institute | Overview

Howard Jonas CHAIRMAN Chi Van Dang, MD, PhD CHIEF SCIENTIFIC ADVISOR ● Professor, Molecular & Cellular Oncogenesis Program at Wistar ● Director of Ludwig Institute ● Editor and Chief, Cancer Research Richard Axel, MD SAB & BOARD OF DIRECTORS ● Nobel Prize in Physiology or Medicine 2004 ● HHMI Professor at Columbia ● Member of the National Academy of Sciences ● Founder of several public companies including Rafael Holdings, IDT Corporation - a communications and payments company, and Genie Energy - an independent energy provider; ● Chairman of the Board of IDT Corporation, Genie Energy and IDW Media Holdings Ari Landon, PhD DIRECTOR OF THE BARER INSTITUTE ● Co - founder of the Barer Institute ● Created methods to study and purify mitochondria ● Guided the development, and life - cycle management, of small molecule and biologic drugs 22 Barer Institute | Management and Leadership ● World leader in drug discovery ● Expert in mitochondrial inhibitors ● Professor at Wistar Institute Joseph Salvino , PhD SCIENTIFIC ADVISORY BOARD

23 Barer Institute | Strategy ● Identified novel compounds to regulate cytoplasmic and mitochondrial metabolism in cancer ● Discovered targetable mechanisms of action through ongoing collaborations with academic institutions. ● Identified clinically relevant biomarkers and synergies with current portfolio companies. ● Investigational new drug enabling experiments

24 Real Estate Real Estate

Har Hotzvim Industrial Park; Jerusalem, Israel Piscataway, New Jersey Washington Park Business District; Newark, New Jersey Rafael Holdings’ commercial real estate portfolio comprises two* properties including the IDT headquarters building in Newark, NJ. Rafael Holdings seeks to realize value from its real estate through a combination of monetization and development. 25 Real Estate | Portfolio *Rafael Holdings’ sold its property in Piscataway NJ (far left) in August 2020

Rafael’s PP&E of $47.8 million* predominantly reflects buildings, improvements and land -- less accumulated depreciation and amortization. Real estate is unencumbered by mortgages Revenue generated by Rafael’s real estate holdings in the fiscal year ended July 31, 2019 was $4.4 million. Upside potential: 76% of Rafael Holdings’ commercial office building in Newark, NJ is available for tenant improvements and lease up. The adjacent garage site is suitable for commercial development. * As of April 30, 2020 Real Estate | Key Financial Facts 26

Real estate holdings, featuring a 20 - story commercial office building and adjacent garage - on a developable site, are unencumbered by debt and provide exposure to Newark, NJ’s real estate market revival Promising clinical data for devimistat highlights the potential upside of stakes in companies pursuing breakthrough therapies for difficult - to - treat cancers Experienced management team: Chairman and CEO Howard Jonas has a proven track record of value creation including the sale of two companies that achieved valuations of over $1B Rafael Holdings intends to leverage its world - renowned scientific leadership to expand its portfolio through the Barer Institute 27 Rafael Holdings | Investment Rationale Valuation analysis suggests significant upside potential for Rafael Holdings’ stake in Rafael Pharma with two Phase 3 trials and multiple Phase 1 and 2 trials ongoing

Rafael Holdings, Inc. CONTACT INFO David Polinsky EMAIL david.polinsky@rafaelholdings.com Thank you



























