
Corporate Presentation MARCH 2020 Exhibit 99.3

Certain information contained in this presentation and statements made orally during this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Prevail Therapeutics Inc.’s (“Prevail”) own internal estimates and research. While Prevail believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Prevail believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, current and prospective product candidates, planned clinical trials and preclinical activities, product approvals, degree of market acceptance of approved products, research and development costs, current and prospective collaborations, timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated product candidates, are forward- looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this presentation represent our views as of the date of this presentation. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. There can be no assurance that the opportunity will meet your investment objectives, that you will receive a return of all or part of such investment. Investment results may vary significantly over any given time period. The appropriateness of a particular investment or strategy will depend on an investor's individual circumstances and objectives. We recommend that investors independently evaluate specific investments and strategies. Disclaimer
Prevail Therapeutics overview Enrollment ongoing in Phase 1/2 PROPEL trial for Parkinson’s with GBA mutation (PD-GBA); PD-GBA affects >90K Americans Potential for rapid proof-of-concept for PR001 in neuronopathic Gaucher disease; IND active and planned to enter the clinic in 2020 Large Market & Near-Term Value Inflection for PR001 Potential disease-modifying targets identified based on human genetics Targeting genetically defined patient populations Gene delivery with AAV9 vector has a track record of efficacy and safety Precision Genetic Medicine Approach Increases POS PR006 IND active for frontotemporal dementia with a GRN mutation (FTD-GRN) Phase 1/2 PROCLAIM trial on track to initiate in 2020 Potential First-In-Class Gene Therapy for FTD-GRN Expertise in developing therapies for neurodegenerative diseases Additional genetically-validated targets in Parkinson’s, Alzheimer’s, ALS, FTD Leaders in gene therapy manufacturing and process development Excellence in CNS Gene Therapy
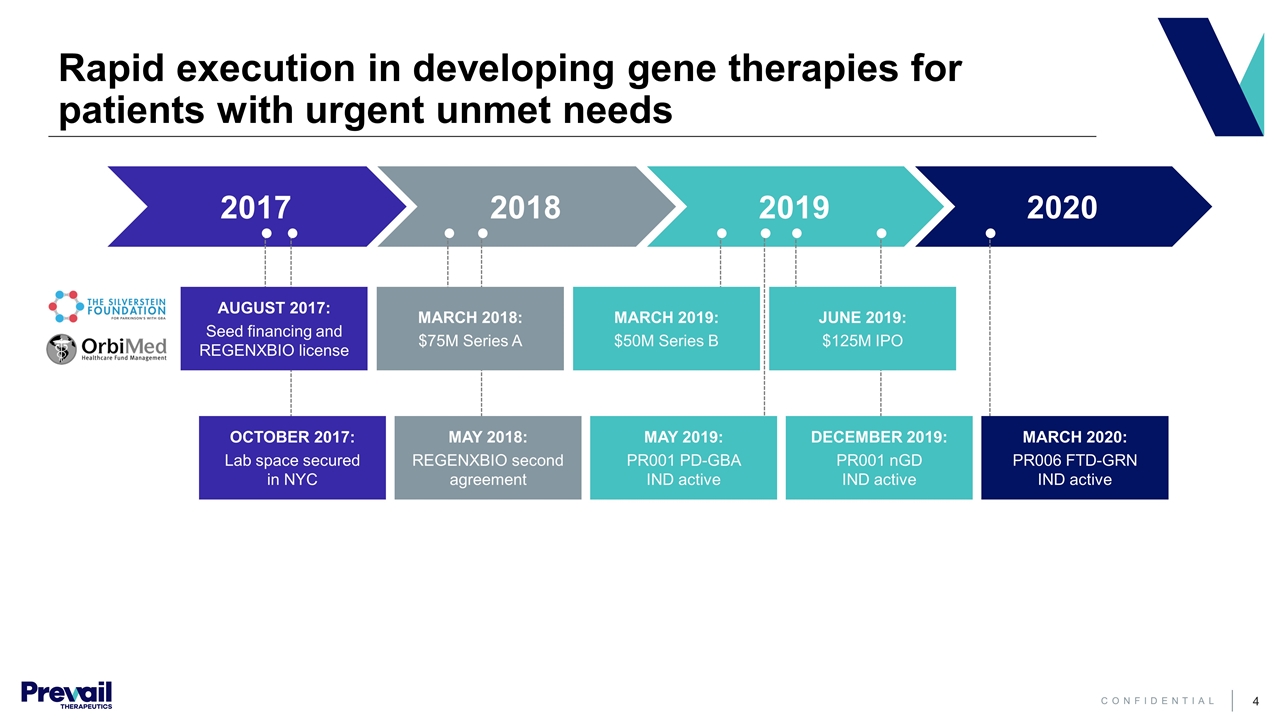
2017 2018 2019 2020 Rapid execution in developing gene therapies for patients with urgent unmet needs OCTOBER 2017: Lab space secured in NYC AUGUST 2017: Seed financing and REGENXBIO license MAY 2018: REGENXBIO second agreement MARCH 2018: $75M Series A MAY 2019: PR001 PD-GBA IND active MARCH 2019: $50M Series B JUNE 2019: $125M IPO DECEMBER 2019: PR001 nGD IND active MARCH 2020: PR006 FTD-GRN IND active

Management and Board of Directors Experienced Management Team Board of Directors Asa Abeliovich, MD, PhD CEO and Founder Carl Gordon, PhD, CFA OrbiMed Francois Nader, MD Non-Executive Chairman Ran Nussbaum Pontifax Peter Thompson, MD OrbiMed Asa Abeliovich, MD, PhD CEO and Founder Yong Dai, PhD Chief Technology Officer Franz Hefti, PhD Chief Development Officer Brett Kaplan, MD Chief Financial Officer Emily Minkow Chief Business Officer Jeff Sevigny, MD Chief Medical Officer Tim Adams Independent Morgan Sheng, MBBS, Ph.D., FRS Independent
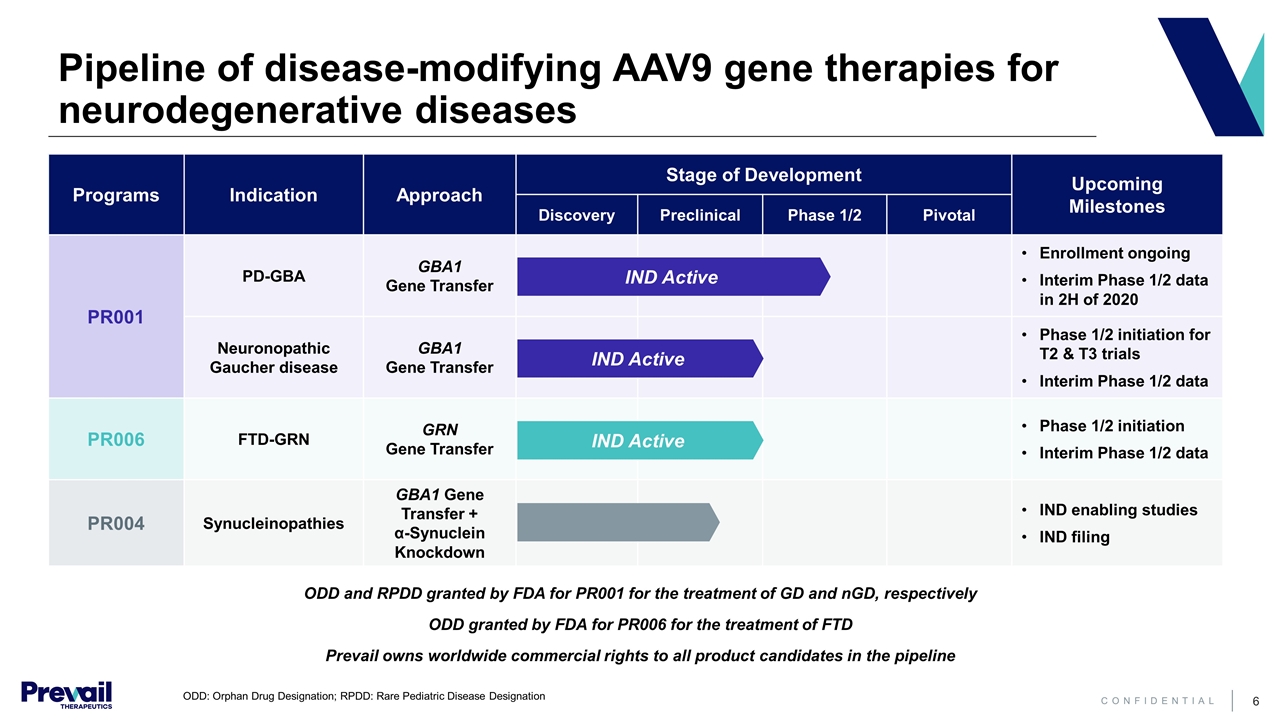
Pipeline of disease-modifying AAV9 gene therapies for neurodegenerative diseases ODD and RPDD granted by FDA for PR001 for the treatment of GD and nGD, respectively ODD granted by FDA for PR006 for the treatment of FTD Prevail owns worldwide commercial rights to all product candidates in the pipeline Programs Indication Approach Stage of Development Upcoming Milestones Discovery Preclinical Phase 1/2 Pivotal PR001 PD-GBA GBA1 Gene Transfer Enrollment ongoing Interim Phase 1/2 data in 2H of 2020 Neuronopathic Gaucher disease GBA1 Gene Transfer Phase 1/2 initiation for T2 & T3 trials Interim Phase 1/2 data PR006 FTD-GRN GRN Gene Transfer Phase 1/2 initiation Interim Phase 1/2 data PR004 Synucleinopathies GBA1 Gene Transfer + α-Synuclein Knockdown IND enabling studies IND filing IND Active IND Active IND Active ODD: Orphan Drug Designation; RPDD: Rare Pediatric Disease Designation
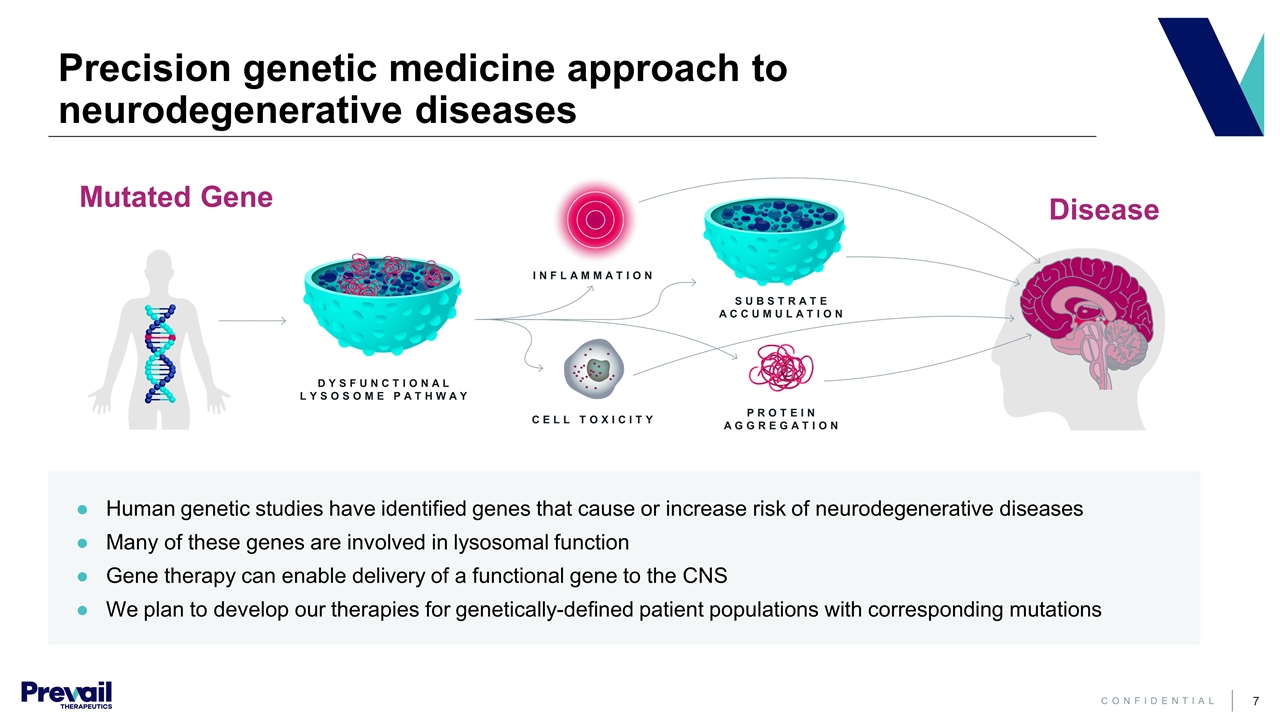
Precision genetic medicine approach to neurodegenerative diseases Mutated Gene Disease DYSFUNCTIONAL LYSOSOME PATHWAY INFLAMMATION CELL TOXICITY PROTEIN AGGREGATION SUBSTRATE ACCUMULATION Human genetic studies have identified genes that cause or increase risk of neurodegenerative diseases Many of these genes are involved in lysosomal function Gene therapy can enable delivery of a functional gene to the CNS We plan to develop our therapies for genetically-defined patient populations with corresponding mutations
AAV9 is well-suited to deliver functional genes or gene knockdown to the CNS Gene cargo AAV9 delivery vector CNS administration Viral transduction and transgene expression AAV9-based therapy has shown transformative efficacy and safety and is now approved for SMA The effectiveness of AAV9-based therapies is due to its ability to distribute broadly across the CNS AAV9 manufacturing process is well-characterized and scalable Prevail has licensed exclusive WW rights to AAV9 from REGENXBIO to deliver the genes in our lead programs
PR001 overview PR001 Route of Administration Lead Indications Progress and Status AAV9 viral vector delivering the GBA1 gene, which encodes glucocerebrosidase (GCase) Single intra-cisterna magna (ICM) injection Parkinson’s disease with at least one GBA1 mutation (PD-GBA) Earlier disease onset, more rapid progression, and higher rate of dementia than sporadic PD Neuronopathic Gaucher disease (nGD) Neurological form of Gaucher due to severe GCase deficiency Enrollment ongoing for Phase 1/2 PROPEL trial for PD-GBA Site activation in process for Phase 1/2 PROVIDE trial for Type 2 Gaucher disease
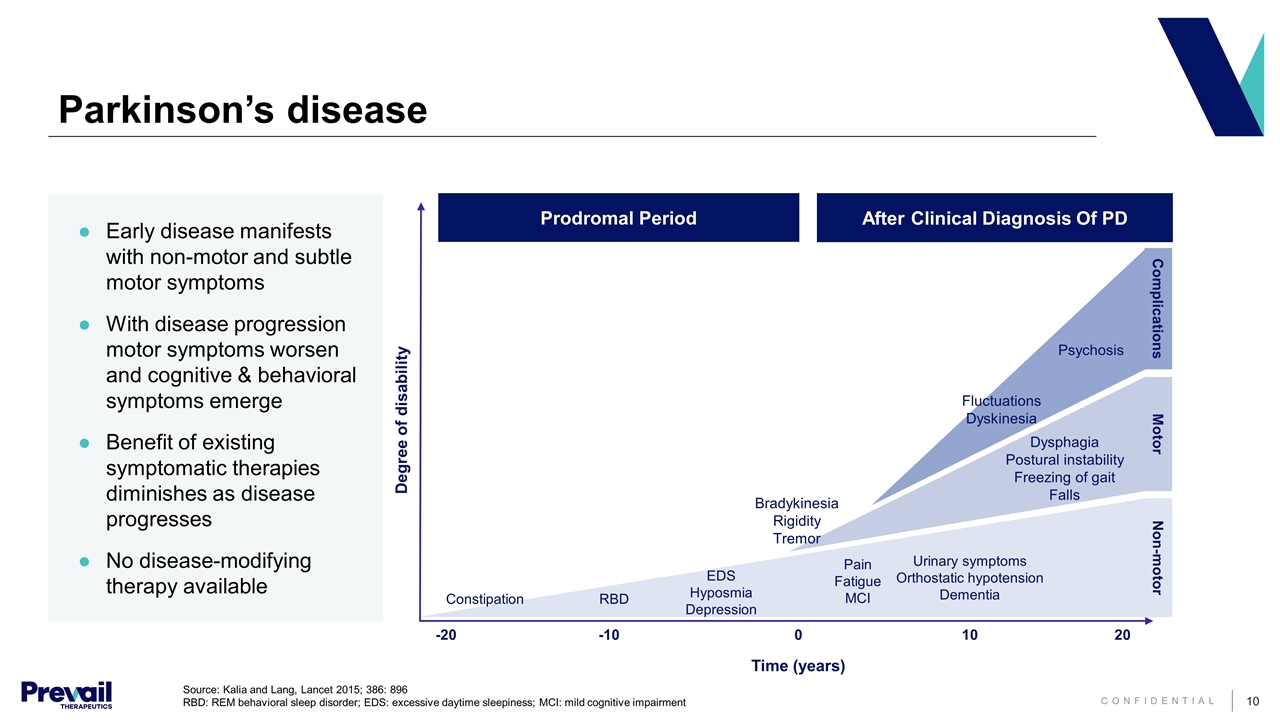
Early disease manifests with non-motor and subtle motor symptoms With disease progression motor symptoms worsen and cognitive & behavioral symptoms emerge Benefit of existing symptomatic therapies diminishes as disease progresses No disease-modifying therapy available Parkinson’s disease Source: Kalia and Lang, Lancet 2015; 386: 896 RBD: REM behavioral sleep disorder; EDS: excessive daytime sleepiness; MCI: mild cognitive impairment Time (years) -20 -10 0 10 20 Degree of disability Constipation RBD EDS Hyposmia Depression Pain Fatigue MCI Urinary symptoms Orthostatic hypotension Dementia Fluctuations Dyskinesia Psychosis Dysphagia Postural instability Freezing of gait Falls Bradykinesia Rigidity Tremor Complications Motor Non-motor After Clinical Diagnosis Of PD Prodromal Period
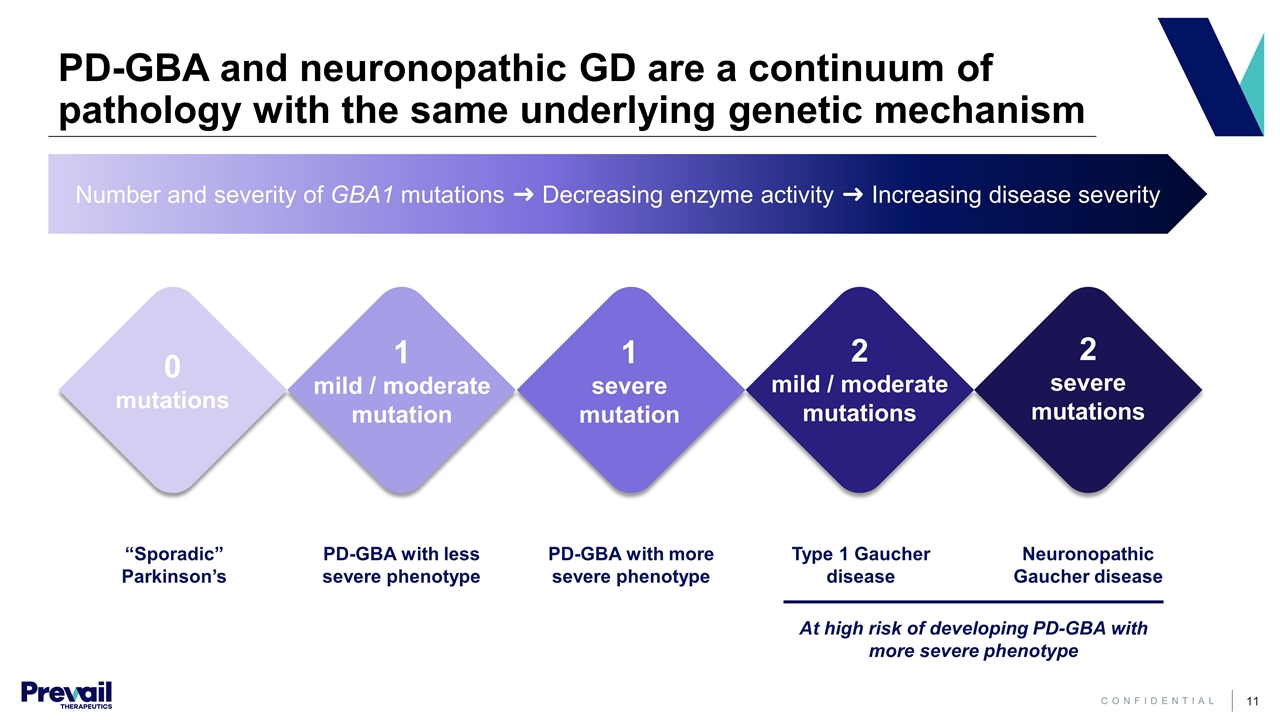
Number and severity of GBA1 mutations ➜ Decreasing enzyme activity ➜ Increasing disease severity PD-GBA and neuronopathic GD are a continuum of pathology with the same underlying genetic mechanism “Sporadic” Parkinson’s PD-GBA with less severe phenotype PD-GBA with more severe phenotype Type 1 Gaucher disease Neuronopathic Gaucher disease 0 mutations 1 mild / moderate mutation 1 severe mutation 2 mild / moderate mutations 2 severe mutations At high risk of developing PD-GBA with more severe phenotype
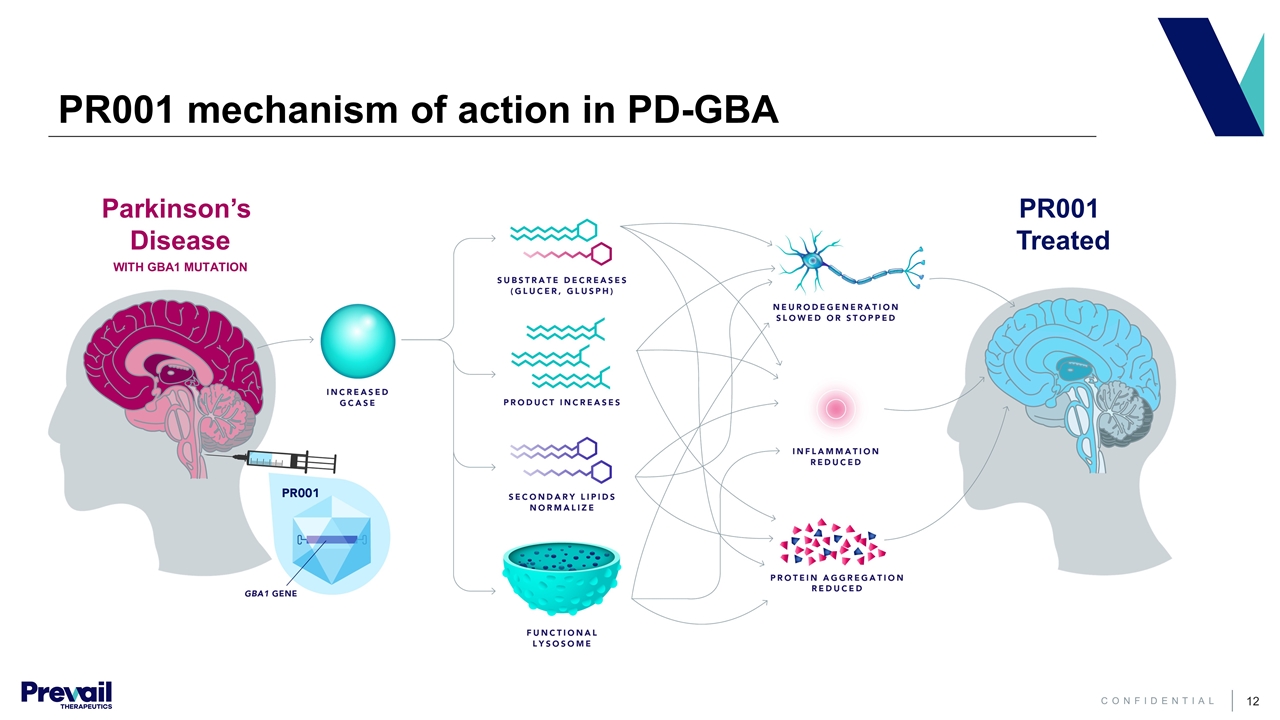
PR001 mechanism of action in PD-GBA PR001 Treated Parkinson’s Disease WITH GBA1 MUTATION

PR001 CBE mouse model efficacy results Means are presented +/- SEM. *: p<0.05; **: p<0.01; ***: p<0.001. By one-way ANOVA and Fischer’s exact test for glial scarring. For rotarod, nominal p values were calculated by linear regression in the CBE-treated groups, with gender corrected for as a covariate. Activity, substrate and astrogliosis (glial scarring) measured in the cortex PR001 low dose = 1.3x1010 vg/g brain, PR001 mid dose = 4.2x1010 vg/g brain, PR001 high dose = 1.3x1011 vg/g brain Activity and substrate reduction (CBE dose-ranging efficacy study) Activity and substrate reduction (CBE 6-month efficacy study) Efficacy (CBE dose-ranging efficacy study) GCase Activity GCase Activity Astrogliosis (glial scarring) GluSph GluSph Rotarod
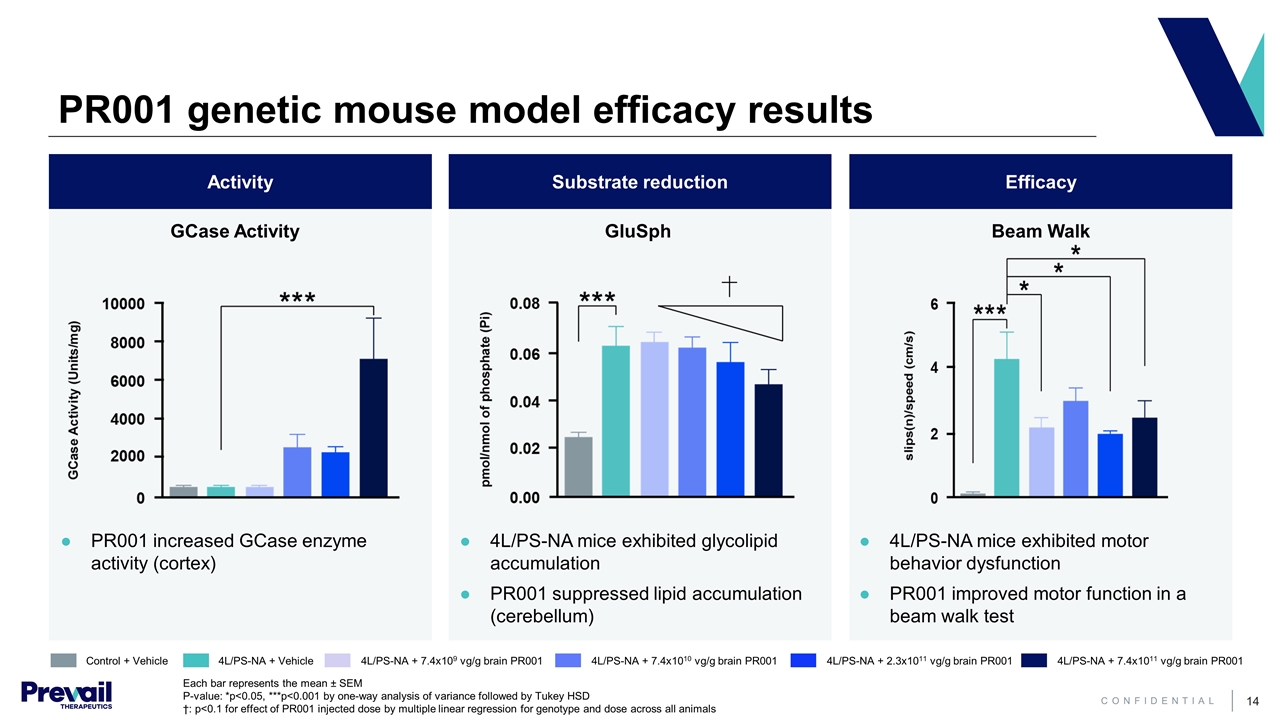
PR001 genetic mouse model efficacy results PR001 increased GCase enzyme activity (cortex) 4L/PS-NA mice exhibited glycolipid accumulation PR001 suppressed lipid accumulation (cerebellum) 4L/PS-NA mice exhibited motor behavior dysfunction PR001 improved motor function in a beam walk test Each bar represents the mean ± SEM P-value: *p<0.05, ***p<0.001 by one-way analysis of variance followed by Tukey HSD †: p<0.1 for effect of PR001 injected dose by multiple linear regression for genotype and dose across all animals Efficacy Substrate reduction Activity GluSph Beam Walk Control + Vehicle 4L/PS-NA + Vehicle 4L/PS-NA + 7.4x109 vg/g brain PR001 4L/PS-NA + 7.4x1010 vg/g brain PR001 4L/PS-NA + 2.3x1011 vg/g brain PR001 4L/PS-NA + 7.4x1011 vg/g brain PR001 GCase Activity
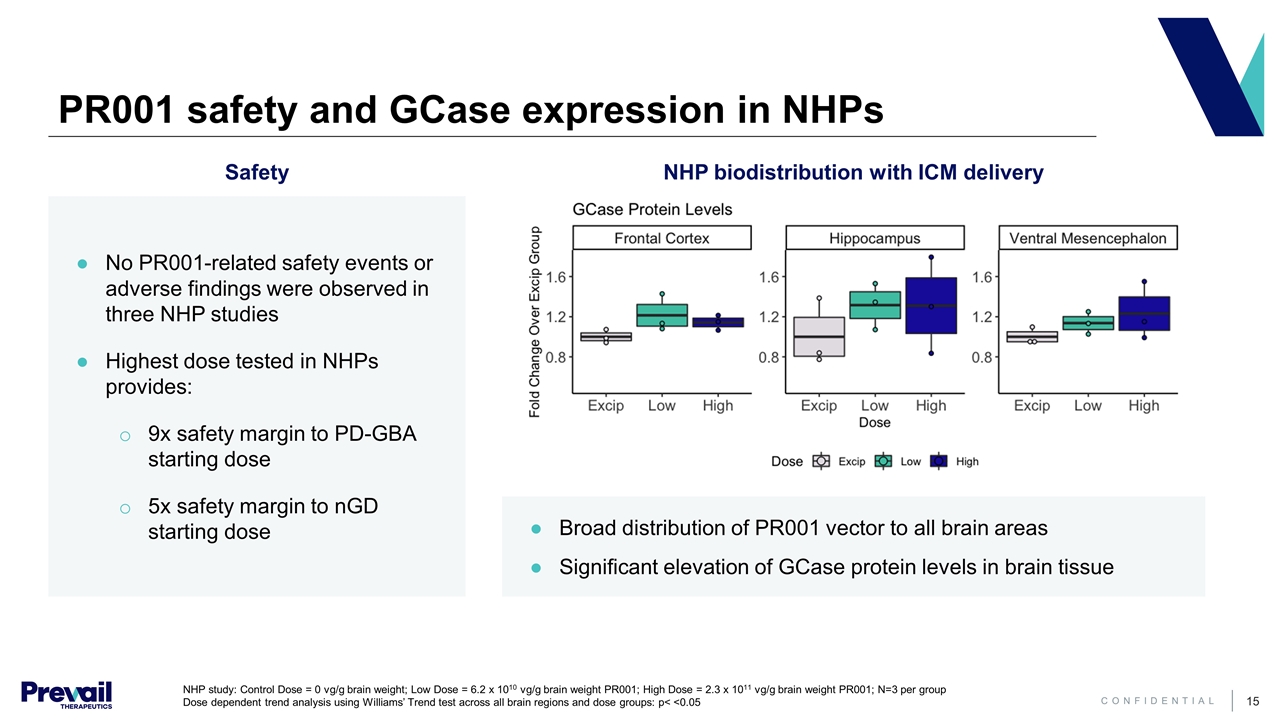
PR001 safety and GCase expression in NHPs NHP biodistribution with ICM delivery No PR001-related safety events or adverse findings were observed in three NHP studies Highest dose tested in NHPs provides: 9x safety margin to PD-GBA starting dose 5x safety margin to nGD starting dose Safety Broad distribution of PR001 vector to all brain areas Significant elevation of GCase protein levels in brain tissue NHP study: Control Dose = 0 vg/g brain weight; Low Dose = 6.2 x 1010 vg/g brain weight PR001; High Dose = 2.3 x 1011 vg/g brain weight PR001; N=3 per group Dose dependent trend analysis using Williams’ Trend test across all brain regions and dose groups: p< <0.05
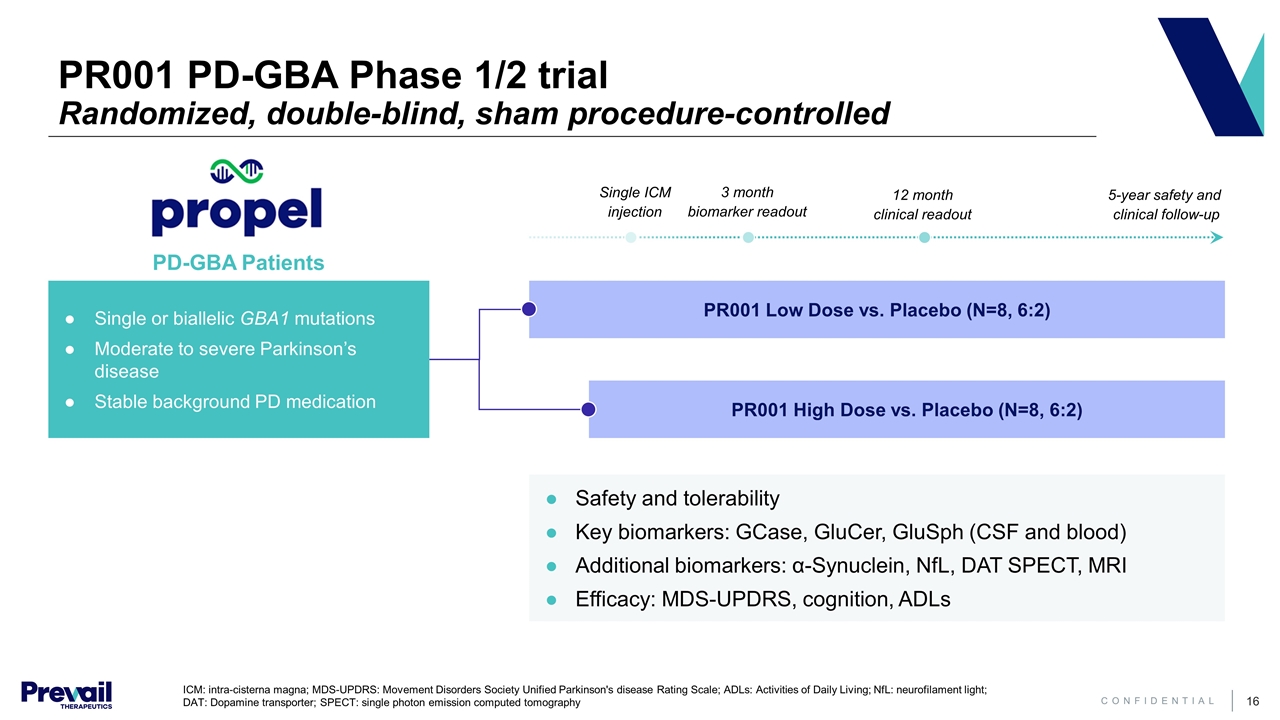
PR001 PD-GBA Phase 1/2 trial Randomized, double-blind, sham procedure-controlled PR001 Low Dose vs. Placebo (N=8, 6:2) PR001 High Dose vs. Placebo (N=8, 6:2) Single or biallelic GBA1 mutations Moderate to severe Parkinson’s disease Stable background PD medication Safety and tolerability Key biomarkers: GCase, GluCer, GluSph (CSF and blood) Additional biomarkers: α-Synuclein, NfL, DAT SPECT, MRI Efficacy: MDS-UPDRS, cognition, ADLs 3 month biomarker readout 12 month clinical readout 5-year safety and clinical follow-up Single ICM injection ICM: intra-cisterna magna; MDS-UPDRS: Movement Disorders Society Unified Parkinson's disease Rating Scale; ADLs: Activities of Daily Living; NfL: neurofilament light; DAT: Dopamine transporter; SPECT: single photon emission computed tomography PD-GBA Patients
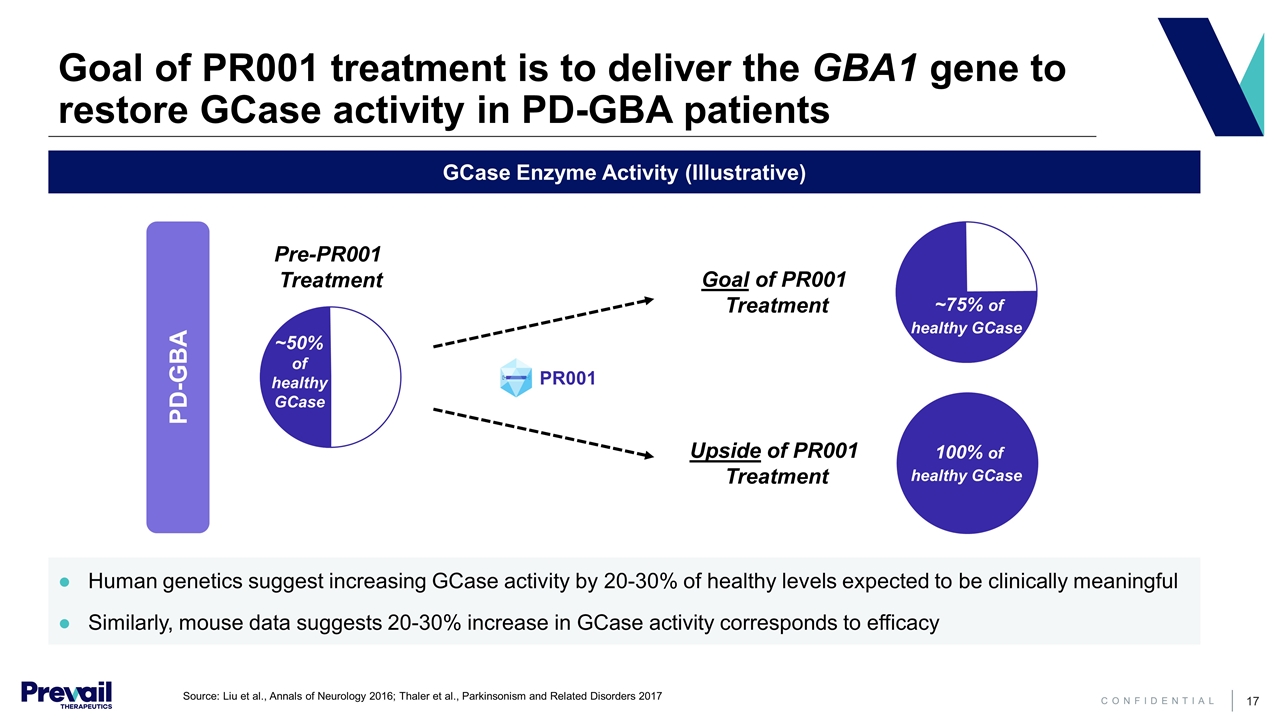
Goal of PR001 treatment is to deliver the GBA1 gene to restore GCase activity in PD-GBA patients GCase Enzyme Activity (Illustrative) Human genetics suggest increasing GCase activity by 20-30% of healthy levels expected to be clinically meaningful Similarly, mouse data suggests 20-30% increase in GCase activity corresponds to efficacy Pre-PR001 Treatment Goal of PR001 Treatment PR001 ~50% of healthy GCase PD-GBA ~75% of healthy GCase Upside of PR001 Treatment 100% of healthy GCase Source: Liu et al., Annals of Neurology 2016; Thaler et al., Parkinsonism and Related Disorders 2017
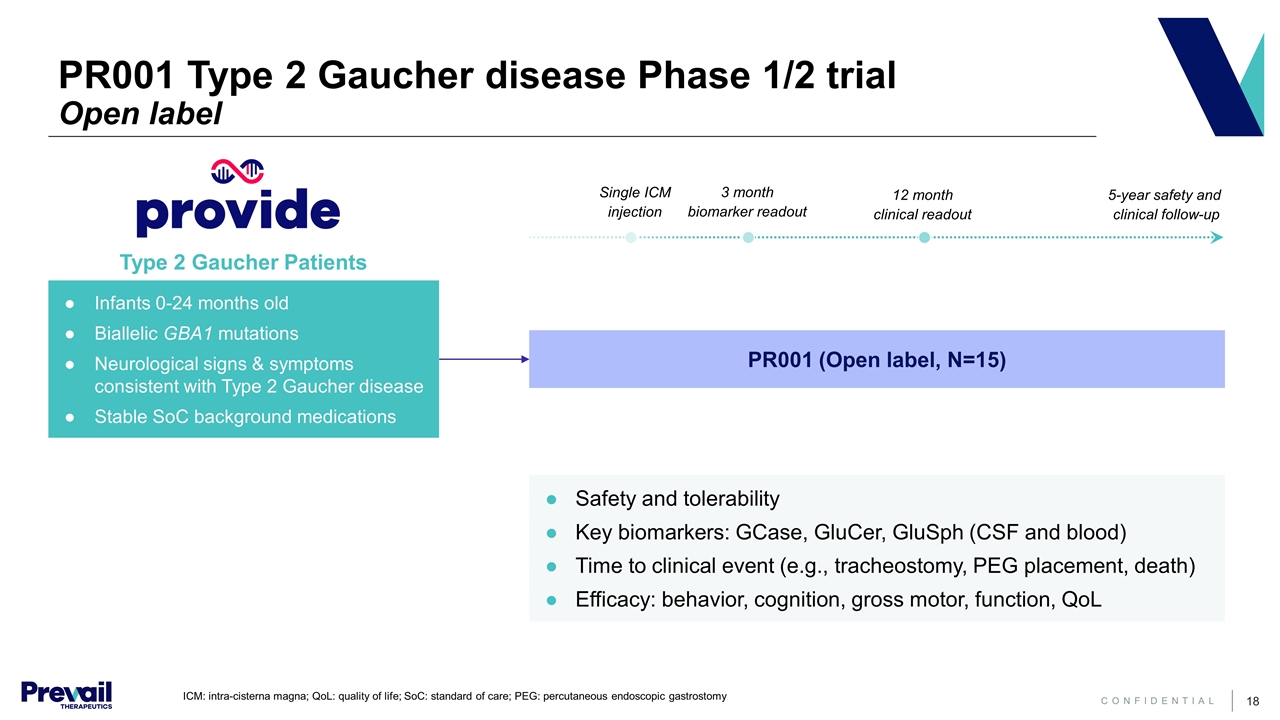
ICM: intra-cisterna magna; QoL: quality of life; SoC: standard of care; PEG: percutaneous endoscopic gastrostomy PR001 (Open label, N=15) Infants 0-24 months old Biallelic GBA1 mutations Neurological signs & symptoms consistent with Type 2 Gaucher disease Stable SoC background medications Safety and tolerability Key biomarkers: GCase, GluCer, GluSph (CSF and blood) Time to clinical event (e.g., tracheostomy, PEG placement, death) Efficacy: behavior, cognition, gross motor, function, QoL Type 2 Gaucher Patients 3 month biomarker readout 12 month clinical readout 5-year safety and clinical follow-up Single ICM injection PR001 Type 2 Gaucher disease Phase 1/2 trial Open label
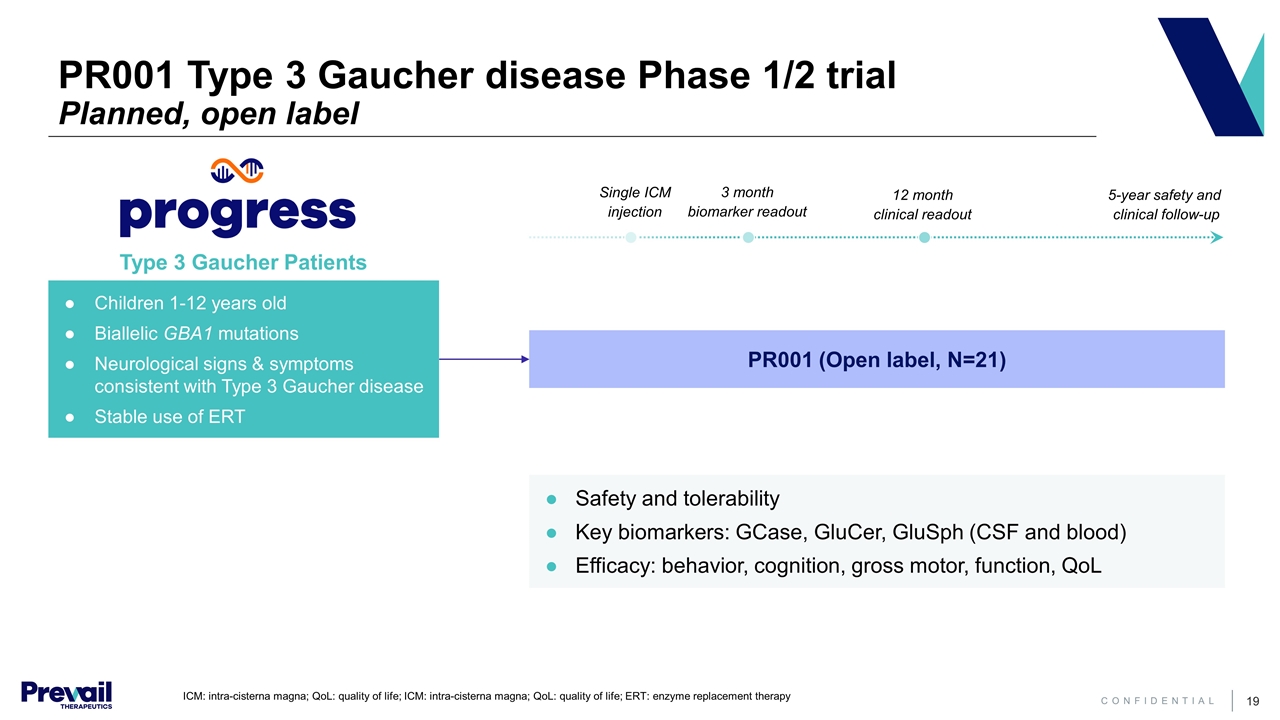
ICM: intra-cisterna magna; QoL: quality of life; ICM: intra-cisterna magna; QoL: quality of life; ERT: enzyme replacement therapy PR001 (Open label, N=21) Safety and tolerability Key biomarkers: GCase, GluCer, GluSph (CSF and blood) Efficacy: behavior, cognition, gross motor, function, QoL Type 3 Gaucher Patients 3 month biomarker readout 12 month clinical readout 5-year safety and clinical follow-up Single ICM injection PR001 Type 3 Gaucher disease Phase 1/2 trial Planned, open label Children 1-12 years old Biallelic GBA1 mutations Neurological signs & symptoms consistent with Type 3 Gaucher disease Stable use of ERT
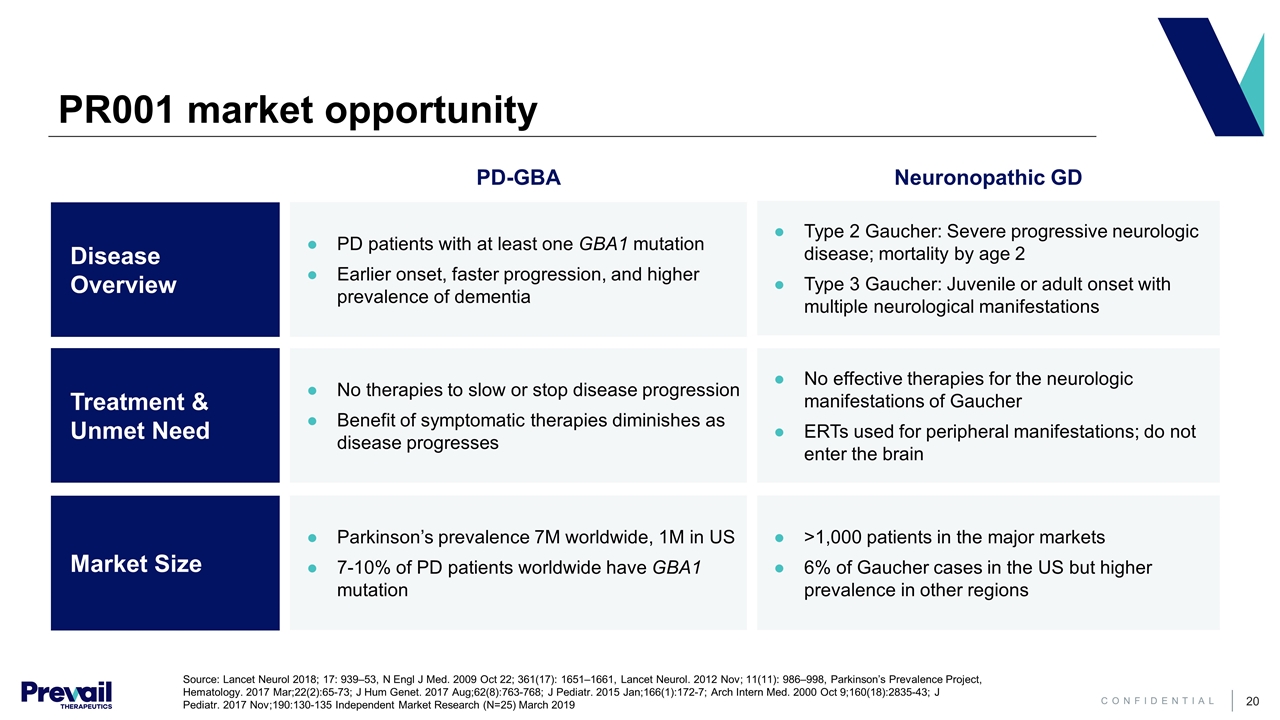
PR001 market opportunity Neuronopathic GD Source: Lancet Neurol 2018; 17: 939–53, N Engl J Med. 2009 Oct 22; 361(17): 1651–1661, Lancet Neurol. 2012 Nov; 11(11): 986–998, Parkinson’s Prevalence Project, Hematology. 2017 Mar;22(2):65-73; J Hum Genet. 2017 Aug;62(8):763-768; J Pediatr. 2015 Jan;166(1):172-7; Arch Intern Med. 2000 Oct 9;160(18):2835-43; J Pediatr. 2017 Nov;190:130-135 Independent Market Research (N=25) March 2019 Disease Overview Treatment & Unmet Need Market Size Type 2 Gaucher: Severe progressive neurologic disease; mortality by age 2 Type 3 Gaucher: Juvenile or adult onset with multiple neurological manifestations No effective therapies for the neurologic manifestations of Gaucher ERTs used for peripheral manifestations; do not enter the brain PD-GBA PD patients with at least one GBA1 mutation Earlier onset, faster progression, and higher prevalence of dementia No therapies to slow or stop disease progression Benefit of symptomatic therapies diminishes as disease progresses Parkinson’s prevalence 7M worldwide, 1M in US 7-10% of PD patients worldwide have GBA1 mutation >1,000 patients in the major markets 6% of Gaucher cases in the US but higher prevalence in other regions

PR006 overview Lead Indications Progress and Status PR006 AAV9 viral vector delivering the GRN gene, which encodes progranulin Route of Administration Single intra-cisterna magna (ICM) injection Frontotemporal dementia with GRN mutation (FTD-GRN) Rapidly progressive dementia impacting behavior and language Leads to death within 3-10 years FTD-GRN IND active and site activation in process for Phase 1/2 PROCLAIM trial
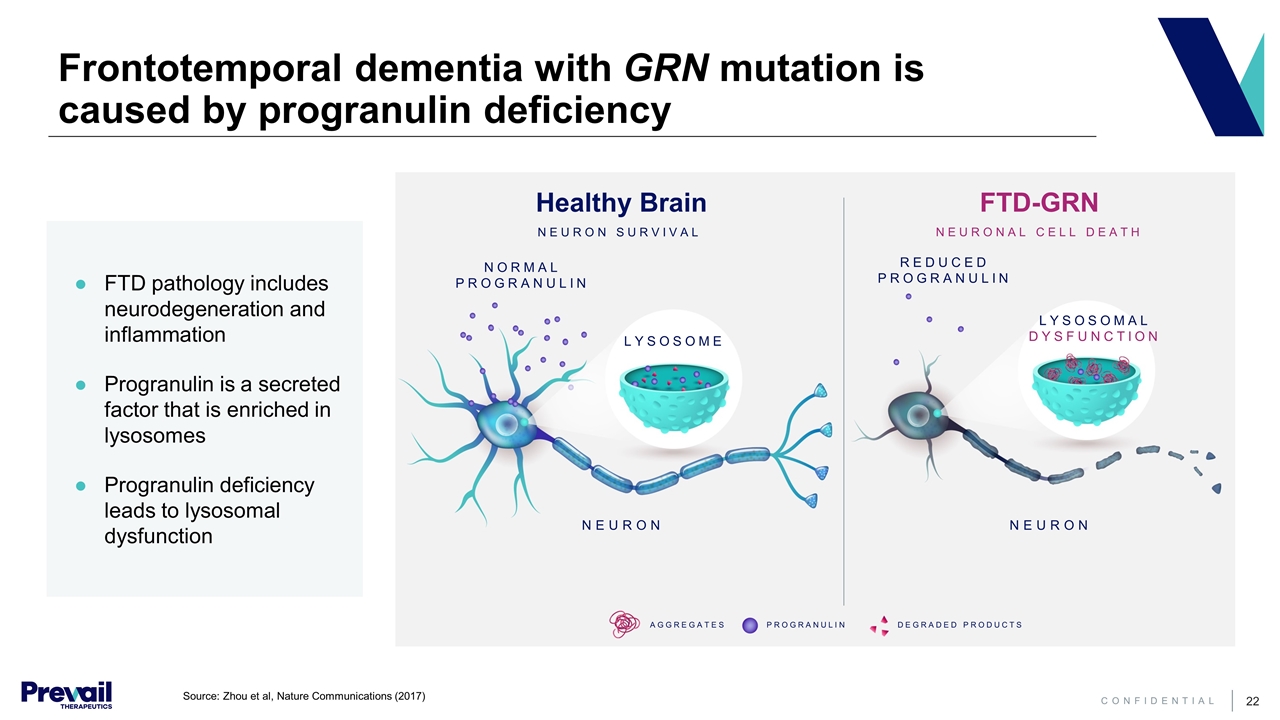
Frontotemporal dementia with GRN mutation is caused by progranulin deficiency Healthy Brain NEURON SURVIVAL FTD-GRN NEURONAL CELL DEATH NORMAL PROGRANULIN REDUCED PROGRANULIN LYSOSOME LYSOSOMAL DYSFUNCTION NEURON NEURON AGGREGATES PROGRANULIN DEGRADED PRODUCTS FTD pathology includes neurodegeneration and inflammation Progranulin is a secreted factor that is enriched in lysosomes Progranulin deficiency leads to lysosomal dysfunction Source: Zhou et al, Nature Communications (2017)
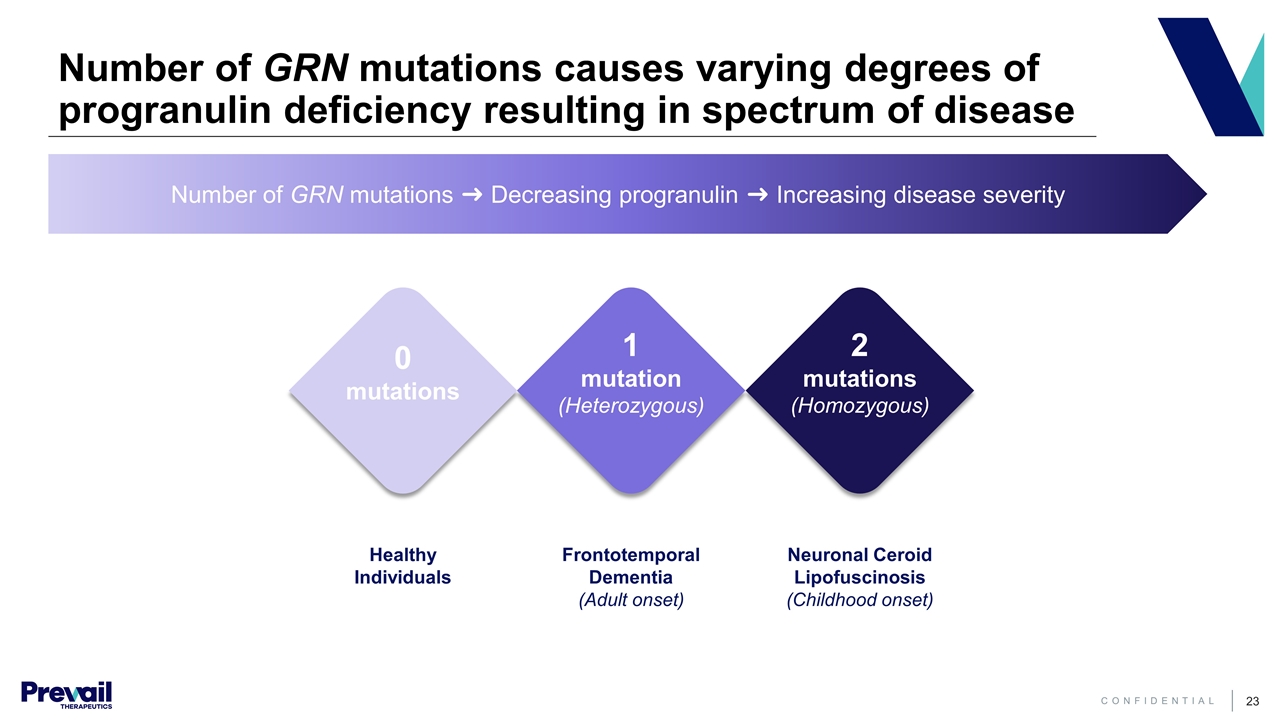
Frontotemporal Dementia (Adult onset) 1 mutation (Heterozygous) Number of GRN mutations ➜ Decreasing progranulin ➜ Increasing disease severity Number of GRN mutations causes varying degrees of progranulin deficiency resulting in spectrum of disease Healthy Individuals 0 mutations Neuronal Ceroid Lipofuscinosis (Childhood onset) 2 mutations (Homozygous)
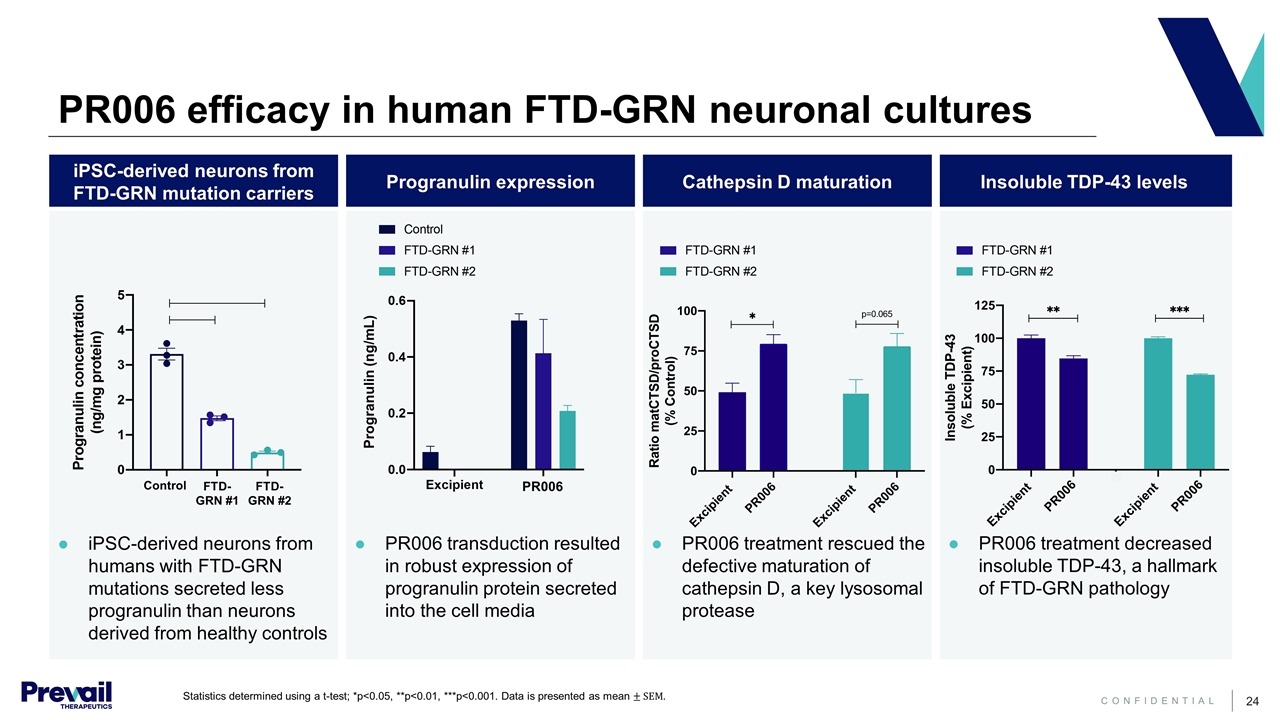
PR006 efficacy in human FTD-GRN neuronal cultures iPSC-derived neurons from FTD-GRN mutation carriers Progranulin expression Cathepsin D maturation Insoluble TDP-43 levels iPSC-derived neurons from humans with FTD-GRN mutations secreted less progranulin than neurons derived from healthy controls PR006 transduction resulted in robust expression of progranulin protein secreted into the cell media PR006 treatment rescued the defective maturation of cathepsin D, a key lysosomal protease PR006 treatment decreased insoluble TDP-43, a hallmark of FTD-GRN pathology PR006 Progranulin (ng/mL) FTD- GRN #1 FTD- GRN #2 Control Statistics determined using a t-test; *p<0.05, **p<0.01, ***p<0.001. Data is presented as mean
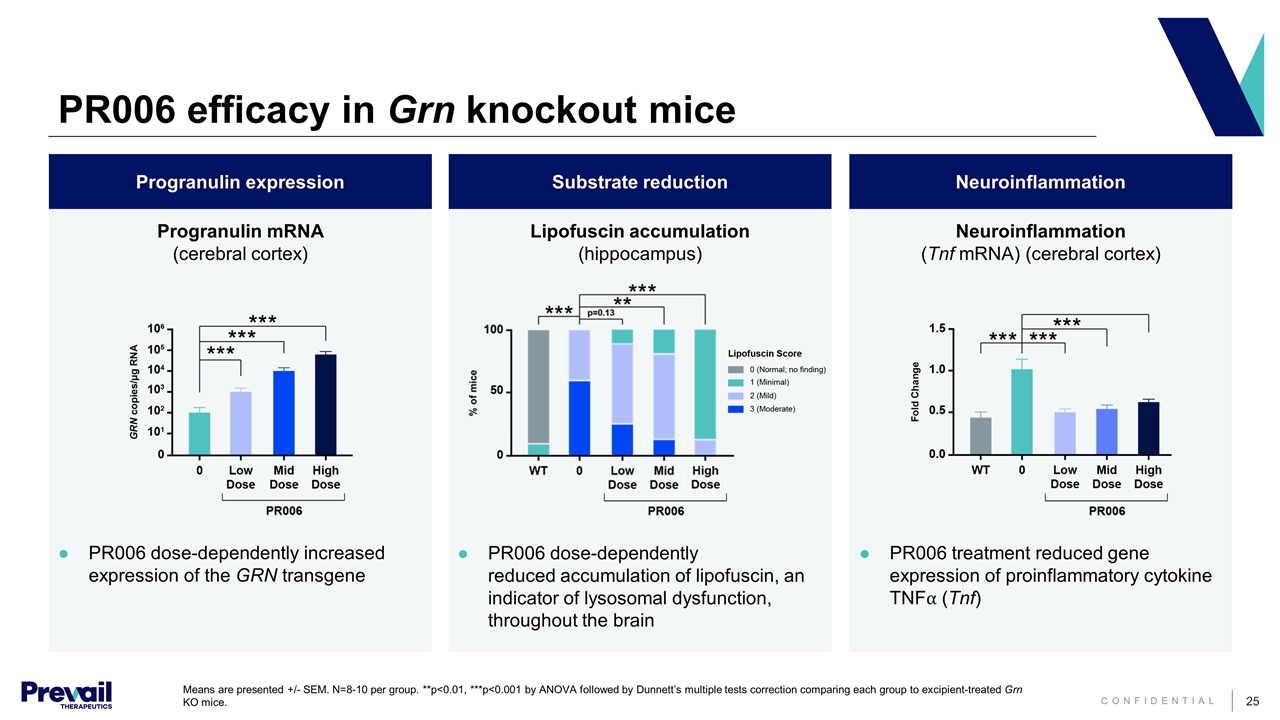
PR006 efficacy in Grn knockout mice Neuroinflammation Substrate reduction Progranulin expression Lipofuscin accumulation (hippocampus) Neuroinflammation (Tnf mRNA) (cerebral cortex) Progranulin mRNA (cerebral cortex) PR006 dose-dependently reduced accumulation of lipofuscin, an indicator of lysosomal dysfunction, throughout the brain PR006 treatment reduced gene expression of proinflammatory cytokine TNF⍺ (Tnf) PR006 dose-dependently increased expression of the GRN transgene Means are presented +/- SEM. N=8-10 per group. **p<0.01, ***p<0.001 by ANOVA followed by Dunnett’s multiple tests correction comparing each group to excipient-treated Grn KO mice.

PR006 safety and progranulin expression in NHPs PR006 administration resulted in widespread transduction in the CNS and periphery, and elevated progranulin protein levels in the CSF No adverse PR006-related findings (clinical and histopathological endpoints) Third-party pathologist classified "extremely minor" degree of nerve fiber degeneration in spinal cord and glial cellularity in DRGs NHPs received ICM administration of excipient, low dose PR006, or high dose PR006 Bars represent mean ± SEM. P-value: *p<0.05, by one-way dose dependence response analysis using William’s trend test PR006 treatment dose-dependently increased progranulin expression in the CSF Progranulin Expression in CSF Excipient Low Dose High Dose Fold Change from Excipient * Results 0 - 1 - 2 -
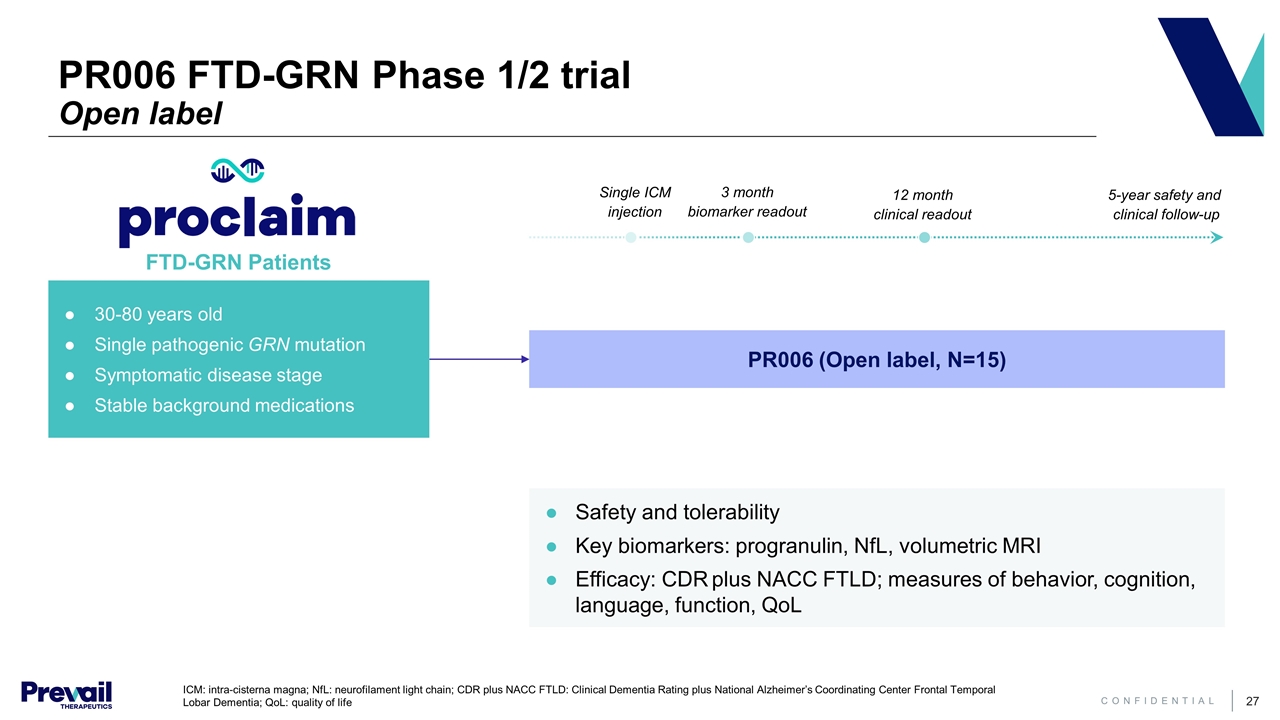
ICM: intra-cisterna magna; NfL: neurofilament light chain; CDR plus NACC FTLD: Clinical Dementia Rating plus National Alzheimer’s Coordinating Center Frontal Temporal Lobar Dementia; QoL: quality of life PR006 (Open label, N=15) 30-80 years old Single pathogenic GRN mutation Symptomatic disease stage Stable background medications Safety and tolerability Key biomarkers: progranulin, NfL, volumetric MRI Efficacy: CDR plus NACC FTLD; measures of behavior, cognition, language, function, QoL FTD-GRN Patients 3 month biomarker readout 12 month clinical readout 5-year safety and clinical follow-up Single ICM injection PR006 FTD-GRN Phase 1/2 trial Open label
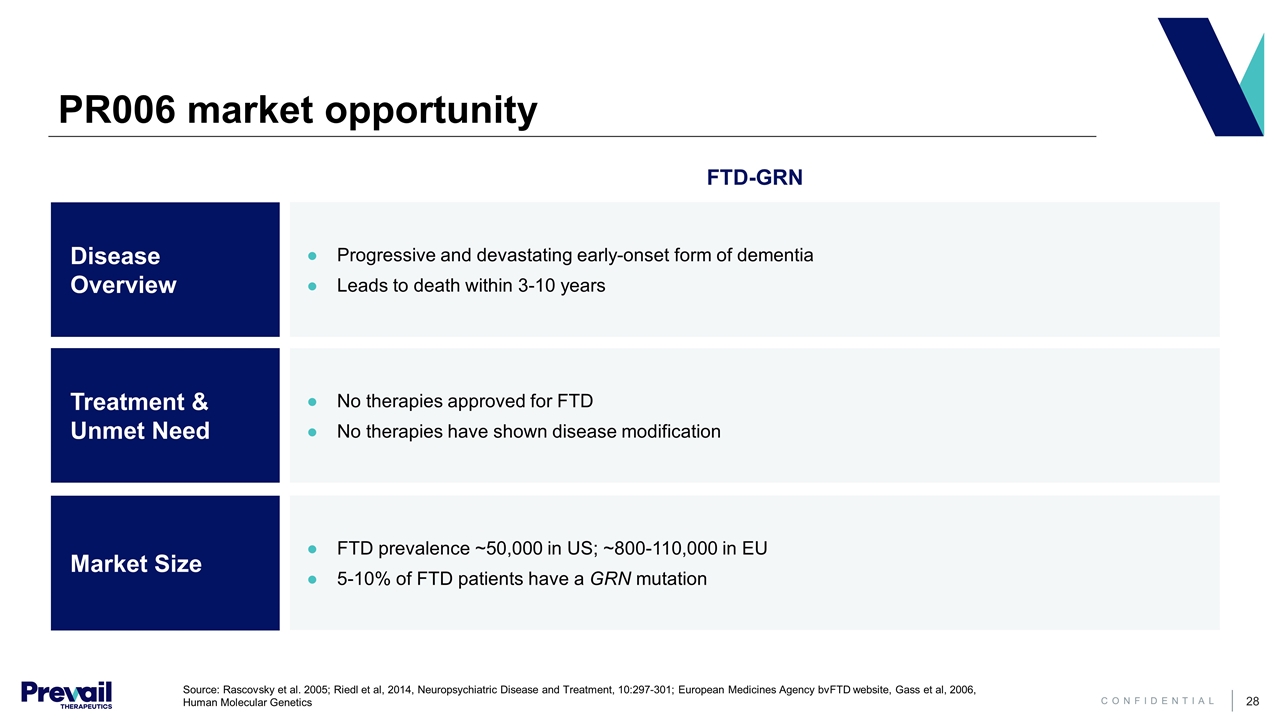
Progressive and devastating early-onset form of dementia Leads to death within 3-10 years No therapies approved for FTD No therapies have shown disease modification PR006 market opportunity Disease Overview Treatment & Unmet Need Market Size FTD-GRN FTD prevalence ~50,000 in US; ~800-110,000 in EU 5-10% of FTD patients have a GRN mutation Source: Rascovsky et al. 2005; Riedl et al, 2014, Neuropsychiatric Disease and Treatment, 10:297-301; European Medicines Agency bvFTD website, Gass et al, 2006, Human Molecular Genetics
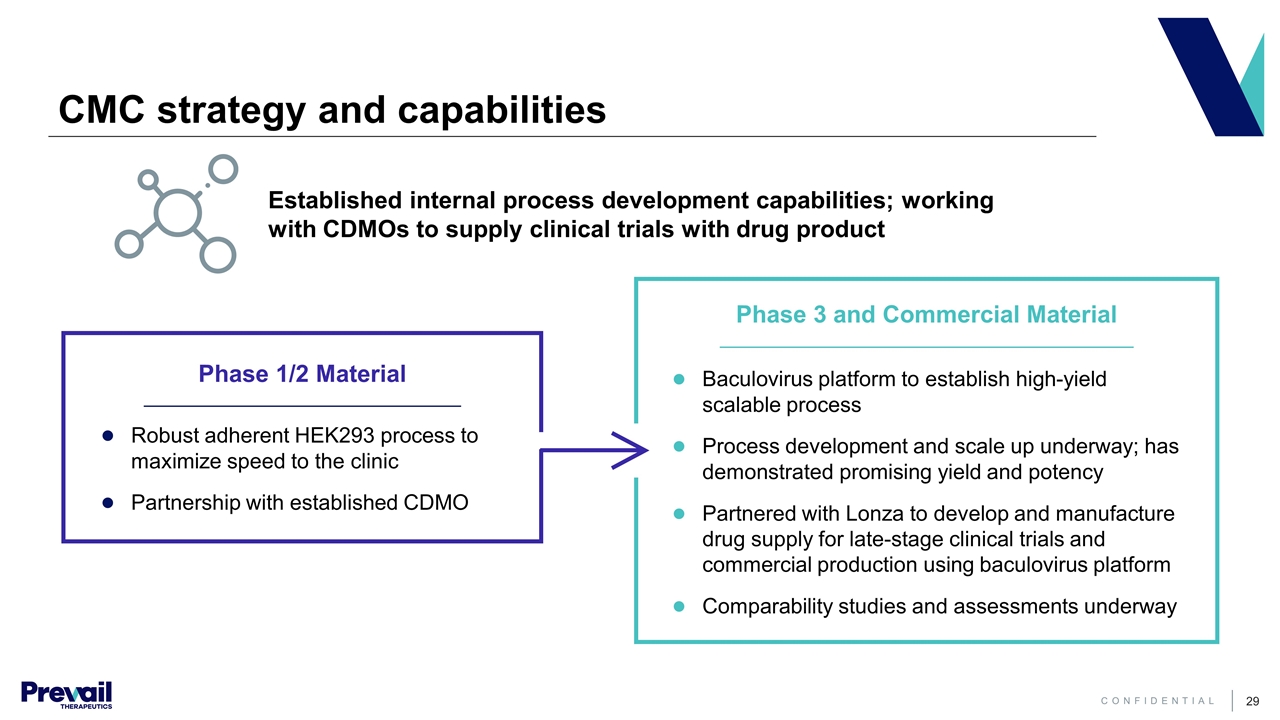
CMC strategy and capabilities Established internal process development capabilities; working with CDMOs to supply clinical trials with drug product Robust adherent HEK293 process to maximize speed to the clinic Partnership with established CDMO Phase 3 and Commercial Material Phase 1/2 Material Baculovirus platform to establish high-yield scalable process Process development and scale up underway; has demonstrated promising yield and potency Partnered with Lonza to develop and manufacture drug supply for late-stage clinical trials and commercial production using baculovirus platform Comparability studies and assessments underway

Prevail Therapeutics summary Developing potentially disease-modifying gene therapies for neurodegenerative disorders PR001 Phase 1/2 trial for PD-GBA ongoing and enrolling patients PR001 Phase 1/2 trial for nGD on track to initiate in 2020 PR006 Phase 1/2 trial for FTD-GRN on track to initiate in 2020 Pipeline of gene therapy programs for neurodegenerative diseases using a precision genetic medicine approach





























