
Filed pursuant to Rule 433 under the Securities Act of 1933, as amended Dated 1/28/22 Registration Statement No. 333-262195 Ra Medical TM SYSTEMS Ra Medical Systems, Inc. NYSE American: RMED Corporate Presentation January 2022

Disclaimer Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future financial performance of Ra Medical Systems, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets, market size, market opportunities, or technology developments; any statements regarding sales and expansion strategies; any statements regarding our intention to seek additional indications for our products; and any statements of assumptions underlying any of the items mentioned. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control. These and other important factors may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. For a list and description of the risk and uncertainties inherent in the forward-looking statements, see the Registration Statement on Form S-1 (File No. 333-262195), including the preliminary prospectus dated January 14, 2022, as amended on January 25, 2022, filed with the Securities and Exchange Commission (the “SEC”). The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates, projections and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry and our business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the solutions and services of the Company. Ra Medical TM SYSTEMS 2

Free Writing Prospectus This presentation highlights basic information about us and the offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in the Company. Except as otherwise indicated, this presentation speaks only as of the date hereof. This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, any securities by any person in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. Neither the SEC nor any other regulatory body has approved or disapproved of our securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third-party studies and surveys, internal company studies and surveys, and other publicly available information. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. We have filed a Registration Statement on Form S-1 (File No. 333-262195) with the SEC, including a preliminary prospectus dated January 14, 2022, as amended on January 25, 2022 (the “Preliminary Prospectus”), with respect to the offering of our securities to which this communication relates. Before you invest, you should read the Preliminary Prospectus (including the risk factors described therein) and, when available, the final prospectus relating to the offering, and the other documents we have filed with the SEC, for more complete information about us and the offering. You may obtain these documents, including the Preliminary Prospectus, for free by visiting EDGAR on the SEC website at http://www.sec.gov. Alternatively, we or any underwriter participating in the offering will arrange to send you the Preliminary Prospectus if you request it by calling (212) 409-2000 or by email at prospectus@ladenburg.com. Ra Medical TM SYSTEMS 3

Risk Factors This presentation highlights basic information about us and the offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in the Company. Except as otherwise indicated, this presentation speaks only as of the date hereof. Investing in our securities stock involves a high degree of risk. You should carefully consider the risks described below, as well the risk factors included in our S-1 Registration Statement. Risk factors include but are not limited to: ▪ We have determined that there is substantial doubt about our ability to continue as a going concern, and we will need to undertake additional financings in order to execute our business plan and fund our operations. We may not be able to obtain such funding on a timely basis, or on commercially reasonable terms, or at all. Any capital-raising transaction we are able to complete may result in substantial dilution to our existing stockholders, require us to relinquish significant rights, or restrict our operations. ▪ We may be unable to successfully remedy the performance, shelf life and calibration issues associated with our DABRA catheters, achieve market acceptance of DABRA, or achieve revenue growth. ▪ Our success depends in large part on DABRA. If we are unable to successfully manufacture, market and sell DABRA, our business prospects will be significantly harmed. ▪ Our ability to successfully complete our atherectomy trial may continue to be hindered or delayed by the COVID-19 pandemic and DABRA catheter performance limitations that are currently being addressed by various engineering efforts. ▪ We are required to devote significant resources to complying with the terms and conditions of our Settlement Agreement and Corporate Integrity Agreement (as described below) and, if we fail to comply, we could be subject to penalties or, under certain circumstances, excluded from government healthcare programs, which would materially adversely affect our business. ▪ Physicians and staff may not commit enough time to sufficiently learn how to use our products. ▪ We are highly dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. ▪ The emergence and effects related to a pandemic, epidemic or outbreak of an infectious disease, including the current COVID-19 pandemic, could adversely affect the Company’s operations. ▪ The Company is involved in securities litigation, and an adverse resolution of such litigation may adversely affect our business, financial condition, results of operations and cash flows. ▪ Governmental export or import controls could limit our ability to compete in foreign markets and subject the Company to liability if the Company violates them. ▪ The Company is considered an emerging growth company and a smaller reporting company, and the Company cannot be certain if the reduced reporting requirements applicable to emerging growth companies and smaller reporting companies will make their common stock less attractive to investors. Ra Medical TM SYSTEMS 4
Ra Medical Overview Targeting markets with strong reimbursement and where our technology can help save lives and limbs Clearances FDA 510(k) Crossing chronic total occlusions in patients with symptomatic infrainguinal lower extremity vascular disease CE mark Endovascular treatment of infrainguinal arteries via atherectomy and for crossing total occlusions Pivotal Study Clinical Data1 Reimbursement Established CPT codes • 0% reported device-related serious adverse events2 (SAE) • 94% effectiveness as measured by successful crossing of target lesion Core Technology Liquid-filled catheter Liquid-filled catheter Ra Medical TM SYSTEMS 1 The pivotal study formed the basis of our May 24, 2017 510(k) clearance, and an amended 510(k) cleared July 2021. 2 For information on how we defined device-related SAEs for purposes of our pivotal study, see https://clinicaltrials.gov/ct2/show/study/NCT02653456

Differentiated DABRA Technology DABRA Catheter • Liquid-filled catheter • Designed to track the true lumen • 94% effectiveness (as measured by successful crossing of target lesion) • Low cost 308 nm Excimer Laser • Portable • Easy to maneuver • Low customer acquisition cost Photoablation Mechanism of Action (MOA) • Breaks down plaque to proteins, lipids and other chemical compounds, thereby eliminating blockages by essentially dissolving them without generating potentially harmful particulates Competitive Positioning: • Full aperture ablative surface at catheter tip • Photoablation MOA may result in less mechanical and thermal trauma to the vessel compared to other devices • Able to ablate plaque to treat lower extremities both above and below the knee • Low-cost, high value option Ablative surface Plaque Photoablation Process Crosses through a totally occluded lesion Improves blood flow Before After Ra Medical TM SYSTEMS 6

Key Efforts1 Underway to Drive Future Growth and Expansion Extended shelf life • Data obtained to support 6-month shelf life • Work in process to generate data supporting up to 12-month shelf life DABRA 2.0 • A more robust catheter with an enhanced outer-jacket, to provide greater trackability and kink resistance, designed to allow physicians to better access difficult anatomy DABRA RX • A rapid exchange, .014” guidewire compatible catheter2 Engineering Clinical Pivotal Atherectomy Study • Clinical study underway to pursue an atherectomy indication for use for DABRA Research Lithotripsy • We are conducting research to prove feasibility of using our liquid-filled catheter and excimer laser technology to fracture calcium in arteries in a procedure known as lithotripsy Catheter Shelf Life Catheter Performance Guidewire Compatibility Expanded Indications Adjacent Markets 1None of the noted engineering, clinical or research efforts have been authorized by any regulatory authority for commercial use in any jurisdiction. 2 Test data on file. Ra Medical TM SYSTEMS 7

DABRA Catheter Engineering Programs DABRA 2.0 CTO catheter with improved deliverability and robustness • A next-generation catheter with an enhanced outer-jacket designed to allow physicians to better access difficult anatomy • Targeting Q1 2022 510(k) submission DABRA RX catheter that is compatible with standard interventional guidewires • Leveraging engineering work and VOC pre-clinical study feedback to develop a rapid exchange, .014” guidewire compatible, catheter1 • Design to be finalized mid-year 2022 1 Test data on file. Ra Medical TM SYSTEMS
Expanded Indication: Atherectomy U.S. Pivotal Clinical Trial Underway: • 7 sites cleared to enroll; 90 subjects enrolled as of January 14, 2022 • Estimated enrollment completion mid-year 2022 with 6-month follow-up completed by year-end 20221 Study size: • Up to 10 sites, 100 subjects2 Primary efficacy endpoint: • Mean reduction in percent diameter stenosis in each subject’s primary lesion as measured by angiography following treatment with the DABRA and before any other treatment Primary safety endpoint: • The incidence of 30-day Major Adverse Events (MAEs)3 as adjudicated by the Clinical Events Committee (CEA): • All-cause mortality • Unplanned major target limb amputation (at or above the ankle) • and/or Clinically driven target limb revascularization. 1Atherectomy study milestones noted are estimations. Information related to study enrollment, study timeline or submission timelines is subject to change, including due to COVID-19 related factors. 2 In January 2022 we filed a protocol amendment with the FDA to add an additional 25 subjects to the study. 3 Study statistically powered assuming a maximum of 8% MAEs. Ra Medical TM SYSTEMS 9
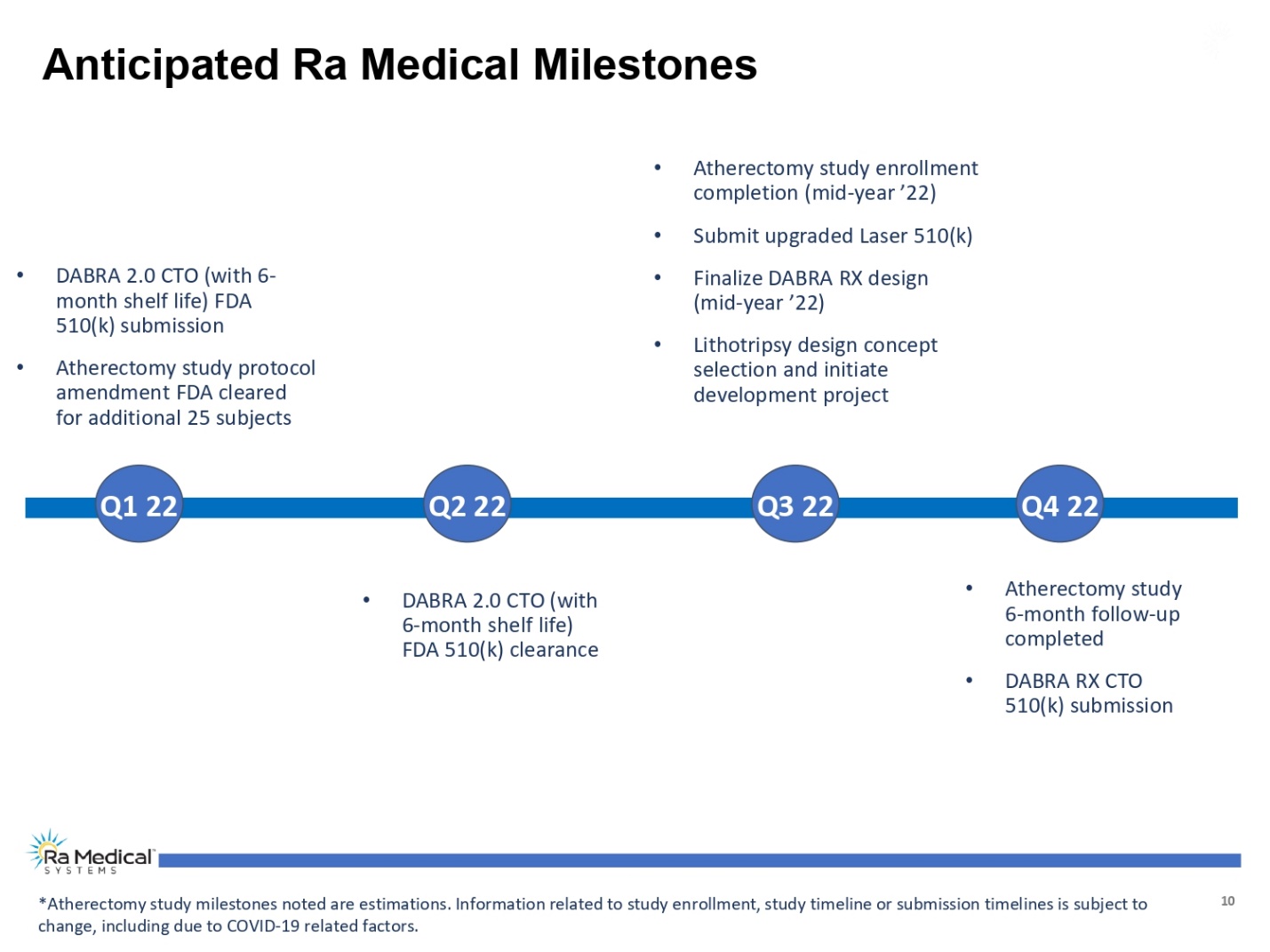
Anticipated Ra Medical Milestones • DABRA 2.0 CTO (with 6- month shelf life) FDA 510(k) submission • Atherectomy study protocol amendment FDA cleared for additional 25 subjects • Atherectomy study enrollment completion (mid-year ’22) • Submit upgraded Laser 510(k) • Finalize DABRA RX design (mid-year ’22) • Lithotripsy design concept selection and initiate development project Q1 22 Q2 22 Q3 22 Q4 22 • DABRA 2.0 CTO (with 6-month shelf life) FDA 510(k) clearance • Atherectomy study 6-month follow-up completed • DABRA RX CTO 510(k) submission *Atherectomy study milestones noted are estimations. Information related to study enrollment, study timeline or submission timelines is subject to change, including due to COVID-19 related factors. Ra Medical TM SYSTEMS 10
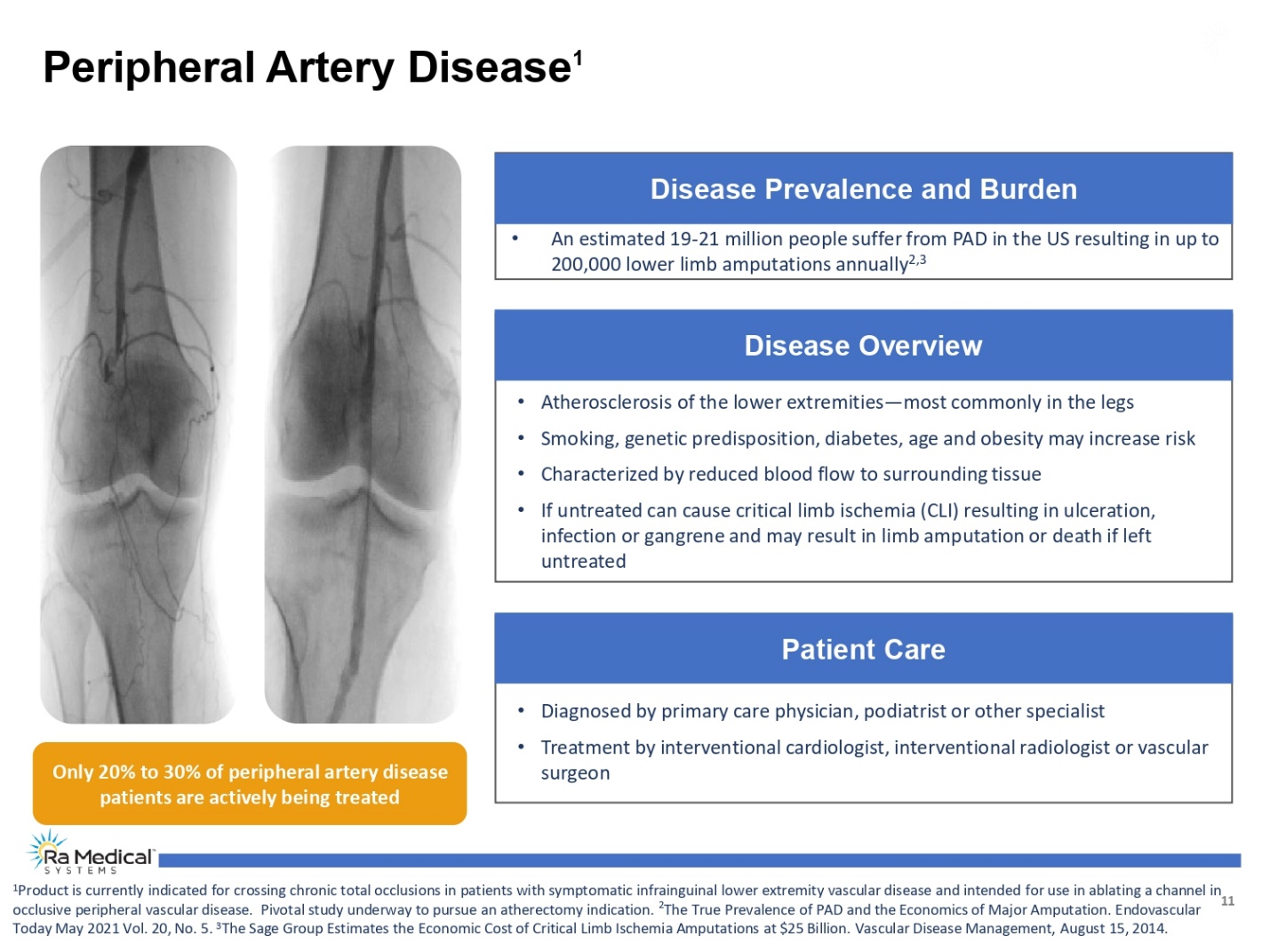
Peripheral Artery Disease1 Disease Prevalence and Burden • An estimated 19-21 million people suffer from PAD in the US resulting in up to 200,000 lower limb amputations annually2,3 Disease Overview • Atherosclerosis of the lower extremities—most commonly in the legs • Smoking, genetic predisposition, diabetes, age and obesity may increase risk • Characterized by reduced blood flow to surrounding tissue • If untreated can cause critical limb ischemia (CLI) resulting in ulceration, infection or gangrene and may result in limb amputation or death if left untreated Patient Care • Diagnosed by primary care physician, podiatrist or other specialist • Treatment by interventional cardiologist, interventional radiologist or vascular surgeon Only 20% to 30% of peripheral artery disease patients are actively being treated 1Product is currently indicated for crossing chronic total occlusions in patients with symptomatic infrainguinal lower extremity vascular disease and intended for use in ablating a channel in occlusive peripheral vascular disease. Pivotal study underway to pursue an atherectomy indication. 2 The True Prevalence of PAD and the Economics of Major Amputation. Endovascular Today May 2021 Vol. 20, No. 5. 3The Sage Group Estimates the Economic Cost of Critical Limb Ischemia Amputations at $25 Billion. Vascular Disease Management, August 15, 2014. Ra Medical TM SYSTEMS 11
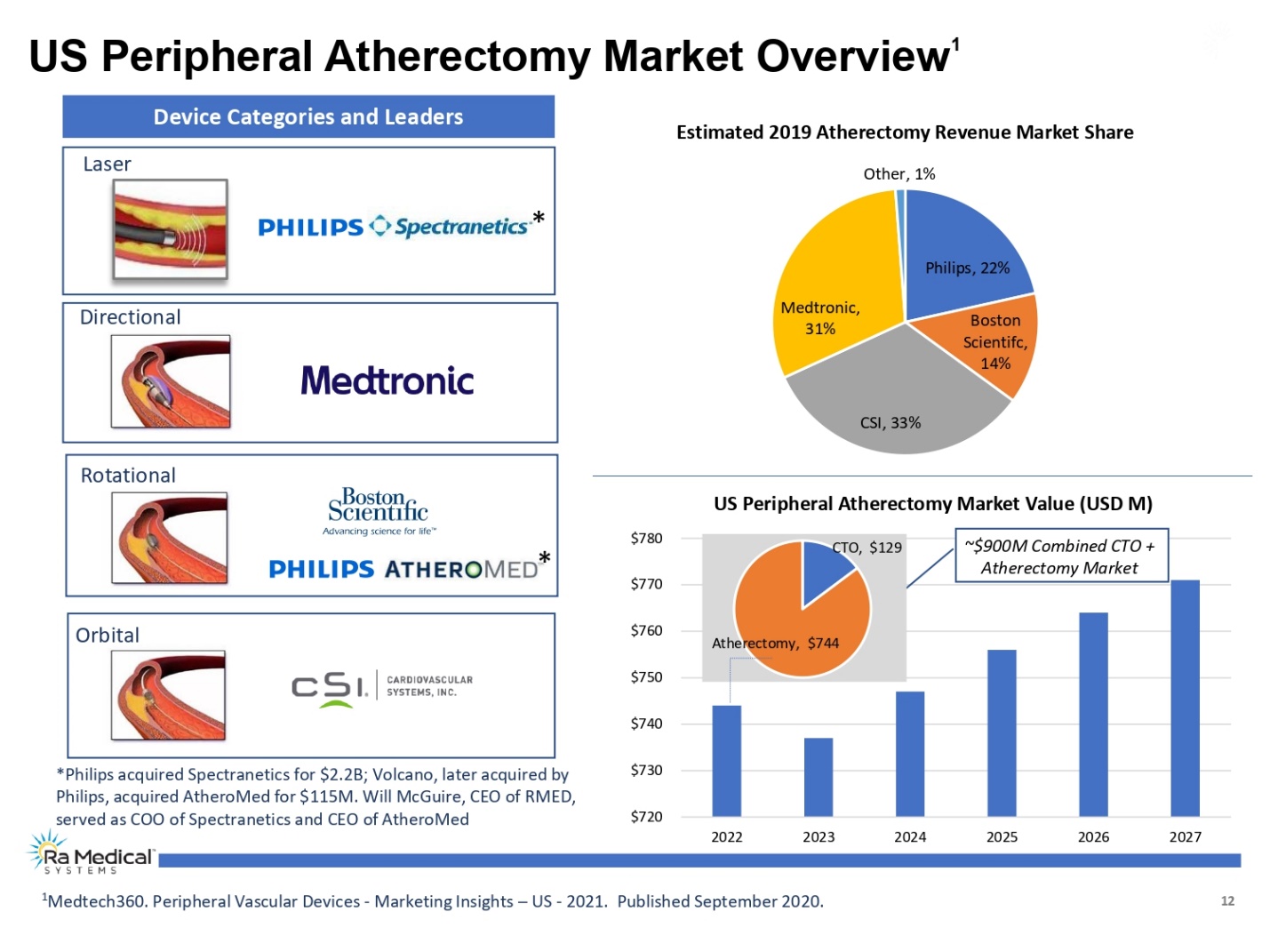
12 US Peripheral Atherectomy Market Overview1 1Medtech360. Peripheral Vascular Devices -Marketing Insights –US -2021. Published September 2020. Philips, 22% Boston Scientifc, 14% CSI, 33% Medtronic, 31% Other, 1% Estimated 2019 Atherectomy Revenue Market Share $720 $730 $740 $750 $760 $770 $780 2022 2023 2024 2025 2026 2027 US Peripheral Atherectomy Market Value (USD M) Directional Rotational Orbital Laser Device Categories and Leaders * * CTO, $129 Atherectomy, $744 *Philips acquired Spectranetics for $2.2B; Volcano, later acquired by Philips, acquired AtheroMedfor $115M. Will McGuire, CEO of RMED, served as COO of Spectranetics and CEO of AtheroMed ~$900M Combined CTO + Atherectomy Market Ra Medical TM SYSTEMS

13 Laser size 27” L x 39” H x 19” W49” L x 35” H x 24” W29” L x 37” H x 13.3” W Laser weight 172-179 lbs 650 lbs 198 lbs Energy wavelength 308 nm 308 nm 355 nm Catheter design Liquid filled Fused silica fiber optic bundle Fused silica fiber optic bundle Ablative surface Full aperture ablative surface Dead spaces within fiber bundle Dead spaces within fiber bundle Laser and catheter price Low High Moderate Indication CTO Pivotal study underway in pursuit of Atherectomy Atherectomy In-stent restenosis (ISR)4 Atherectomy In-stent restenosis (ISR)5 1Test data on file. 2Specifications per manufacturer’s CVX-300 excimer laser labeling. 3Specifications per Angio Dynamics Auryonwebsite. 4Only the 0.014” and 0.018” Over-the-wire (OTW) Turbo-Elite laser catheters when used as an accessory to the use of the Turbo-Tandem System in the treatmentof femoropopliteal arteries and bare nitinol stents, when used in conjunction with Percutaneous Transluminal Angioplasty (PTA). 52.0 and 2.35 mm catheters only. Laser Atherectomy Competitive Overview Ra Medical TM SYSTEMS
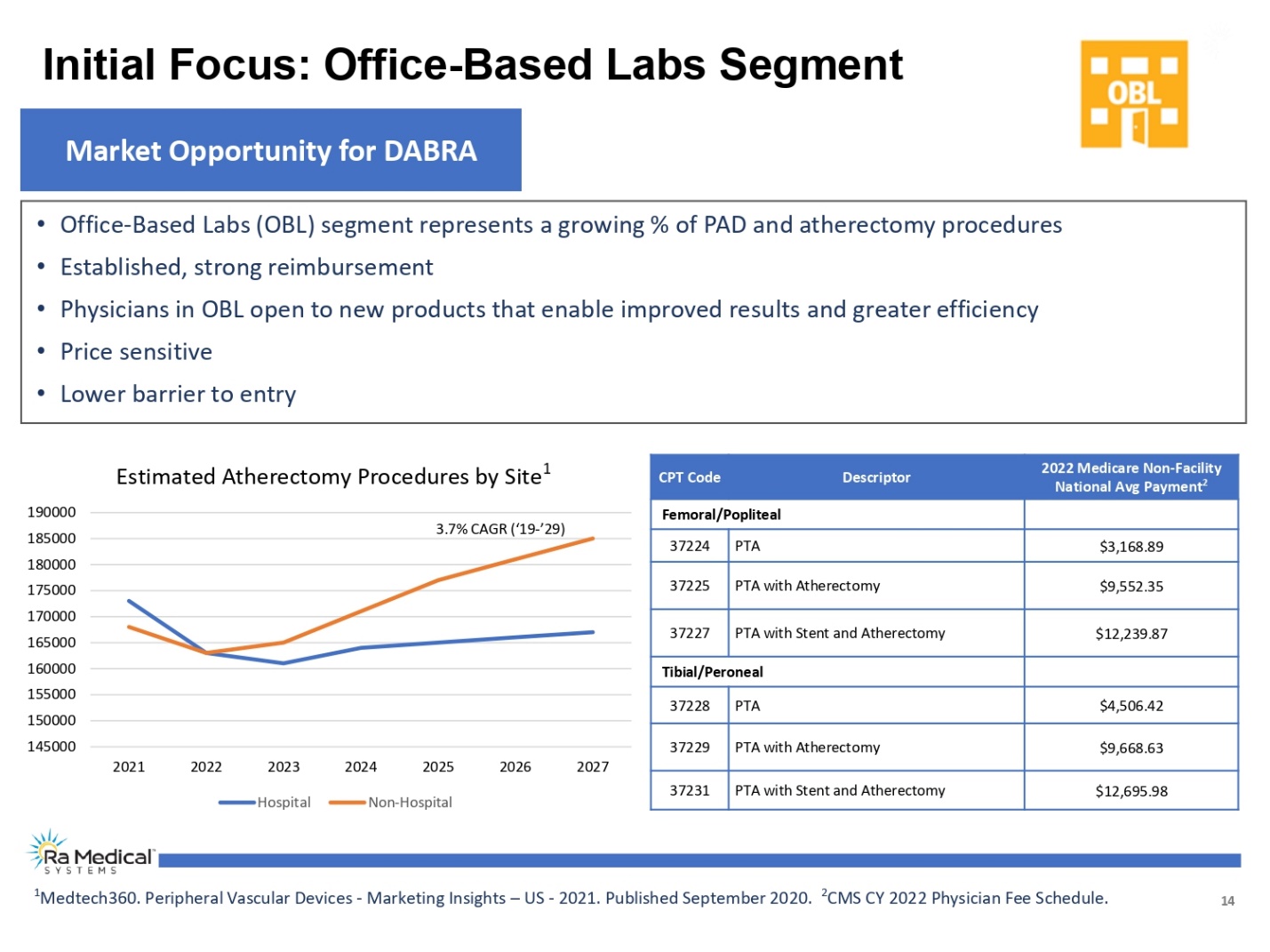
14 •Office-Based Labs (OBL) segment represents a growing % of PAD and atherectomy procedures •Established, strong reimbursement •Physicians in OBL open to new products that enable improved results and greater efficiency •Price sensitive •Lower barrier to entry Market Opportunity for DABRA Initial Focus: Office-Based Labs Segment CPT Code Descriptor2022 Medicare Non-Facility National Avg Payment2 Femoral/Popliteal 37224PTA$3,168.89 37225 PTA with Atherectomy $9,552.35 37227 PTA with Stent and Atherectomy $12,239.87 Tibial/Peroneal 37228 PTA $4,506.42 37229 PTA with Atherectomy $9,668.63 37231 PTA with Stent and Atherectomy$12,695.98 Medtech360. Peripheral Vascular Devices -Marketing Insights –US -2021. Published September 2020. 2CMS CY 2022 Physician Fee Schedule. Estimated Atherectomy Procedures by Site1 145000 Hospital Non-Hospital 150000 190000 3.7% CAGR (‘19-’29) 2021 2022 2023 2024 2025 2026 2027 155000 160000 165000 170000 175000 180000 185000 190000 Ra Medical TM SYSTEM
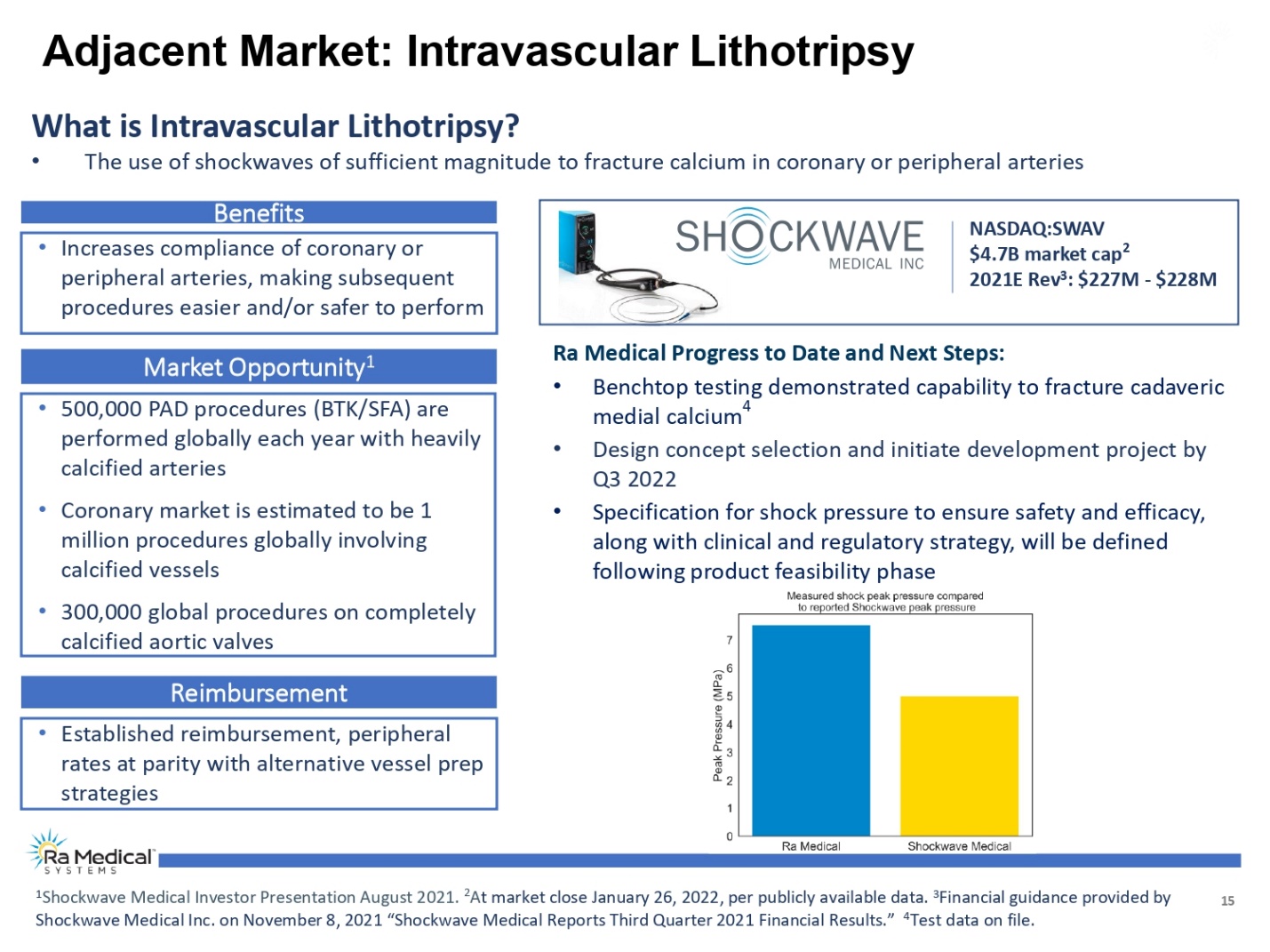
15 What is Intravascular Lithotripsy? •The use of shockwaves of sufficient magnitude to fracture calcium in coronary or peripheral arteries NASDAQ:SWAV $4.7B market cap2 2021E Rev3: $227M -$228M Adjacent Market: Intravascular Lithotripsy Benefits •Increases compliance of coronary or peripheral arteries, making subsequent procedures easier and/or safer to perform Market Opportunity1 •500,000 PAD procedures (BTK/SFA) are performed globally each year with heavily calcified arteries •Coronary market is estimated to be 1 million procedures globally involving calcified vessels •300,000 global procedures on completely calcified aortic valves 1Shockwave Medical Investor Presentation August 2021. 2At market close January 26, 2022, per publicly available data. 3Financial guidance provided by Shockwave Medical Inc. on November 8, 2021 “Shockwave Medical Reports Third Quarter 2021 Financial Results.” 4Test data on file. Reimbursement •Established reimbursement, peripheral rates at parity with alternative vessel prep strategies Ra Medical Progress to Date and Next Steps: •Benchtop testing demonstrated capability to fracture cadaveric medial calcium4 •Design concept selection and initiate development project by Q3 2022 •Specification for shock pressure to ensure safety and efficacy, along with clinical and regulatory strategy, will be defined following product feasibility phase Ra Medical TM SYSTEM
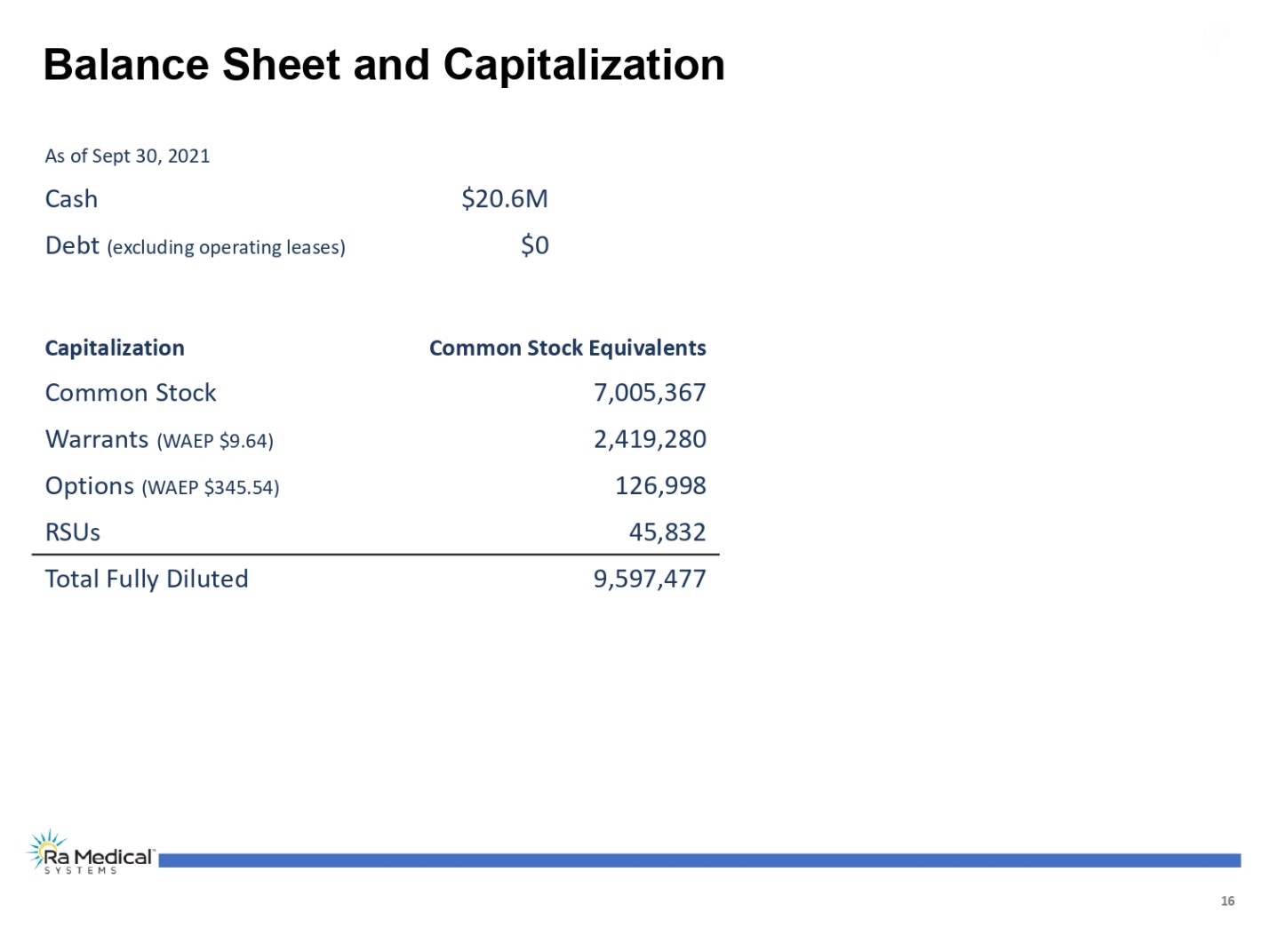
16 Capitalization Common Stock Equivalents Balance Sheet and Capitalization As of Sept 30, 2021 Cash $20.6M Debt (excluding operating leases) $0 Common Stock 7,005,367 Warrants (WAEP$9.64) 2,419,280 Options (WAEP$345.54) 126,998 RSUs 45,832 Total Fully Diluted 9,597,477 Ra Medical TM SYSTEM

17 Appendix Ra Medical TM SYSTEM
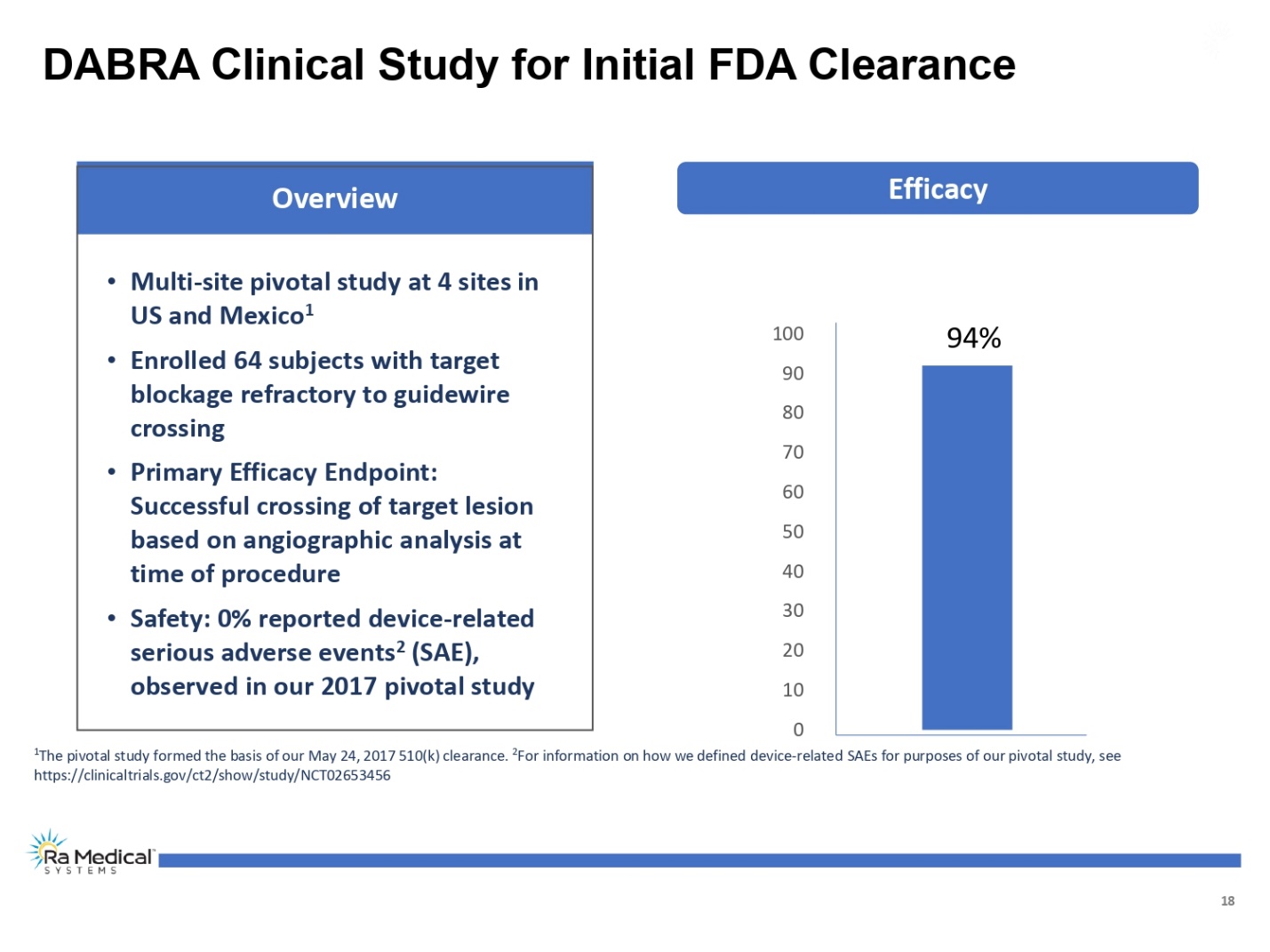
18 0 10 20 30 40 50 60 70 80 90 100 94% 1The pivotal study formed the basis of our May 24, 2017 510(k) clearance. 2For information on how we defined device-related SAEs for purposes of our pivotal study, see https://clinicaltrials.gov/ct2/show/study/NCT02653456 Efficacy Overview •Multi-site pivotal study at 4 sites in US and Mexico1 •Enrolled 64 subjects with target blockage refractory to guidewire crossing •Primary Efficacy Endpoint: Successful crossing of target lesion based on angiographic analysis at time of procedure •Safety: 0% reported device-related serious adverse events2(SAE), observed in our 2017 pivotal study DABRA Clinical Study for Initial FDA Clearance Ra Medical TM SYSTEM

19 Management Team Will McGuire Chief Executive Officer 25 years med-tech experience, including COO of Spectranetics which was acquired by Philips for >$2 billion and CEO of Atheromed, a venture capital-backed peripheral atherectomy company. Also held senior roles at Volcano Corporation, Covidien, and Guidant Corporation. Most recently CEO of publicly-traded Second Sight Medical Products. MBA Andrew Jackson Chief Financial Officer 25 years finance experience. CFO and senior finance roles with several public and private companies in the life science industry, including med-tech sector and two vascular-focused companies (Celladon Corporation and REVA Medical). MBA and CPA (inactive) Al Memmolo Senior Vice President Quality, Clinical and Regulatory Affairs 30 years experience in the areas of quality, clinical and regulatory affairs, including Chief Operating Officer at Dallen Medical and Vice President at Vascular Control Systems and Photo Medex. MBA Chris Folk, PhD Vice President Engineering 25 years engineering experience, including leading development of three catheters while at ev3 / Covidien Neurovascular. MS, Engineering Mechanics and PhD, Aerospace Engineering Maria Villanueva Vice President and Compliance Officer 25 years experience in compliance and internal controls at medical device, pharma and technology companies. Mrs. Villanueva began her career in public accounting working on assurance and forensic accounting practices at Arthur Andersen. MBA Ra Medical TM SYSTEM

20 Board of DirectorsMartin Colombatto Chairman Former VP and General Manager of Broadcom Will McGuire Chief Executive Officer, Ra Medical Former CEO Second Sight, former executive at Covidien, AtheroMed, Spectranetics Richard Mejia Former Partner of Ernst & Young Susanne Meline Co-founder of Francis Capital Management Joan Stafslien Former General Counsel of NuVasive and CareFusion Ra Medical TM SYSTEM
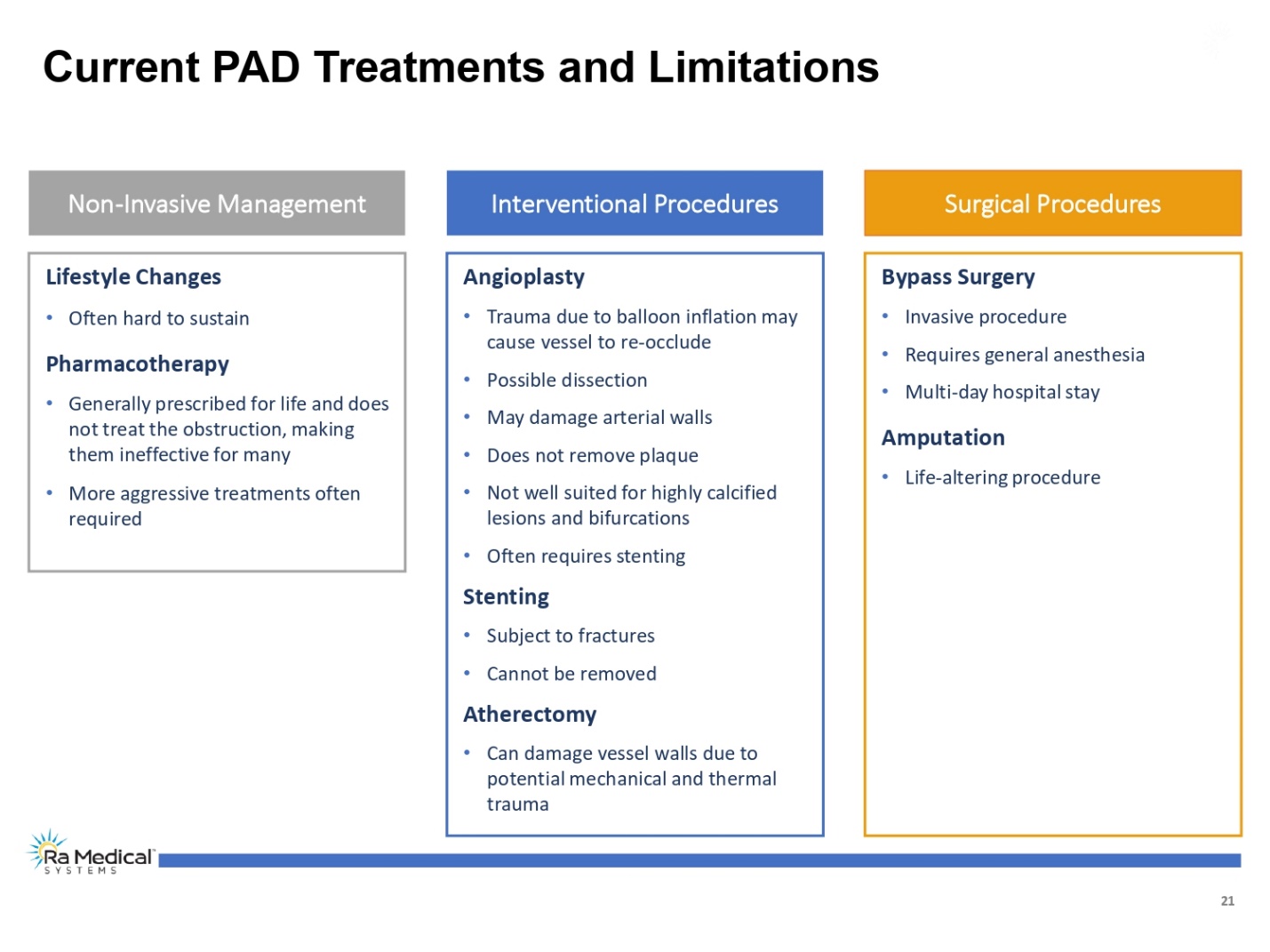
21 Current PAD Treatments and Limitations Non-Invasive Management Lifestyle Changes •Often hard to sustain Pharmacotherapy •Generally prescribed for life and does not treat the obstruction, making them ineffective for many •More aggressive treatments often required Interventional Procedures Angioplasty •Trauma due to balloon inflation may cause vessel to re-occlude •Possible dissection •May damage arterial walls •Does not remove plaque •Not well suited for highly calcified lesions and bifurcations •Often requires stenting Stenting •Subject to fractures •Cannot be removed Atherectomy •Can damage vessel walls due to potential mechanical and thermal trauma Surgical Procedures Bypass Surgery •Invasive procedure •Requires general anesthesia •Multi-day hospital stay Amputation •Life-altering procedure Ra Medical TM SYSTEM




















