
Initial Phase 1 Clinical Data for SRF388 & SRF617 WEBCAST JUNE 4, 2021 Exhibit 99.2

This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of Surface Oncology, Inc. (“we,” “us” or “our”) our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward looking statements. These risks and uncertainties include the timing, progress, and results of preclinical studies and clinical trials for SRF617, SRF388 and SRF813, and our other product candidates, the timing and likelihood of regulatory approvals and those risks identified and discussed in the section titled “Risk Factors,” set forth in our Annual Report on Form 10-K and in our other SEC filings. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. Legal Disclaimer

Today’s Highlights Wholly-owned, first-in-class SRF388 (IL-27) demonstrates monotherapy activity and good tolerability – expansions opening Wholly-owned SRF617 (CD39) demonstrates good tolerability, activity in combination – combination expansions enrolling New clinical collaboration with Roche for SRF388 in first-line hepatocellular carcinoma in combination with atezolizumab and bevacizumab

SRF388 Targeting IL-27 to Enhance Inflammation in the Tumor Microenvironment
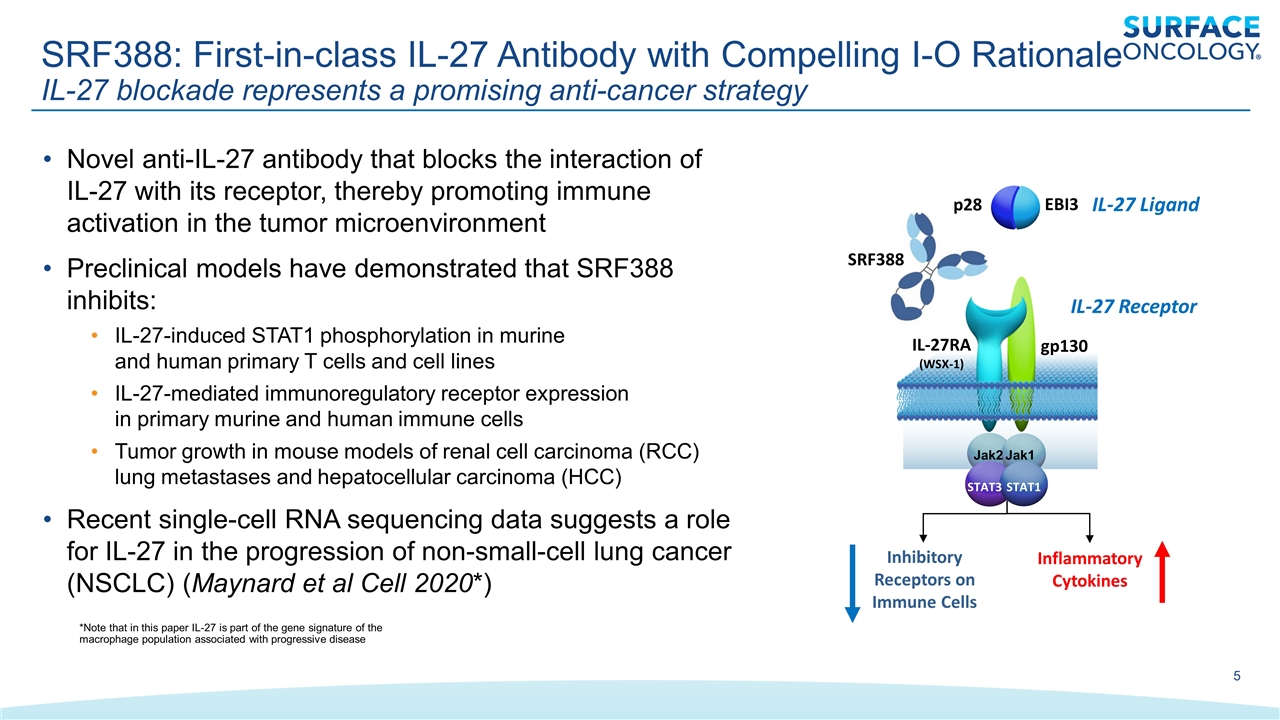
Novel anti-IL-27 antibody that blocks the interaction of IL-27 with its receptor, thereby promoting immune activation in the tumor microenvironment Preclinical models have demonstrated that SRF388 inhibits: IL-27-induced STAT1 phosphorylation in murine and human primary T cells and cell lines IL-27-mediated immunoregulatory receptor expression in primary murine and human immune cells Tumor growth in mouse models of renal cell carcinoma (RCC) lung metastases and hepatocellular carcinoma (HCC) Recent single-cell RNA sequencing data suggests a role for IL-27 in the progression of non-small-cell lung cancer (NSCLC) (Maynard et al Cell 2020*) SRF388: First-in-class IL-27 Antibody with Compelling I-O Rationale IL-27 blockade represents a promising anti-cancer strategy IL-27RA (WSX-1) gp130 EBI3 p28 IL-27 Ligand IL-27 Receptor Jak2 Jak1 STAT3 STAT1 Inhibitory Receptors on Immune Cells Inflammatory Cytokines SRF388 *Note that in this paper IL-27 is part of the gene signature of the macrophage population associated with progressive disease
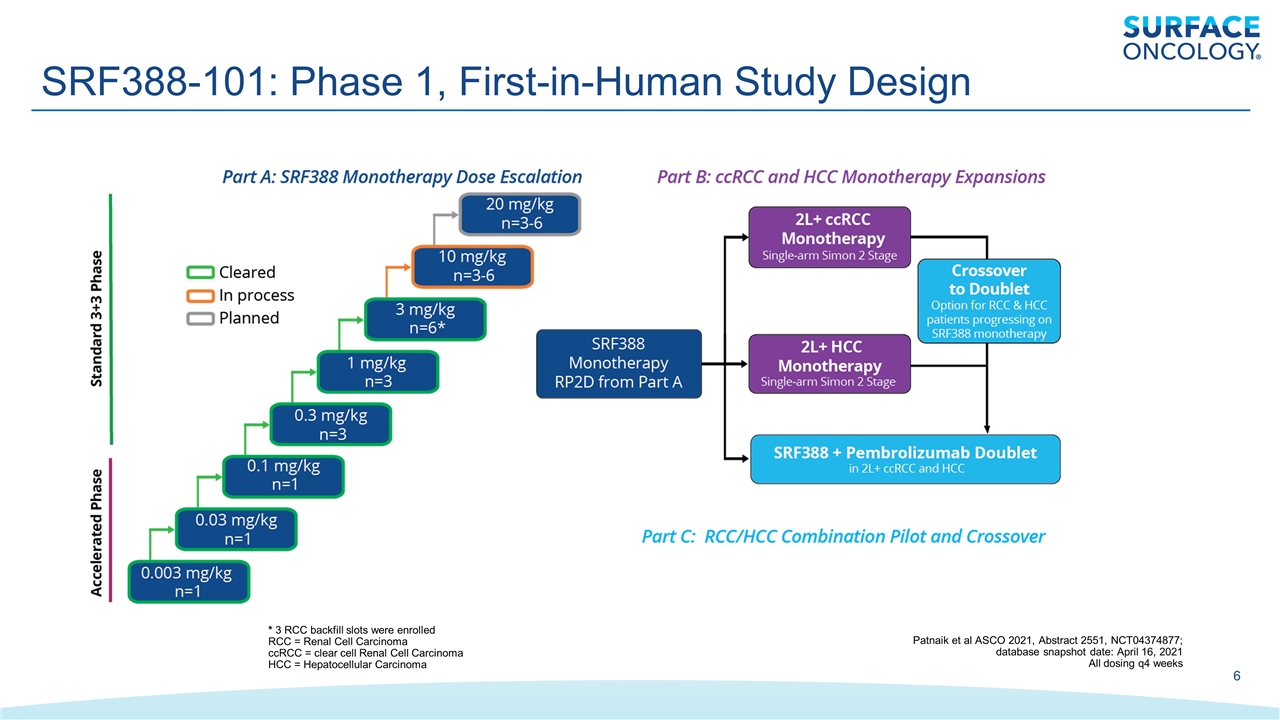
SRF388-101: Phase 1, First-in-Human Study Design * 3 RCC backfill slots were enrolled RCC = Renal Cell Carcinoma ccRCC = clear cell Renal Cell Carcinoma HCC = Hepatocellular Carcinoma Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021 All dosing q4 weeks
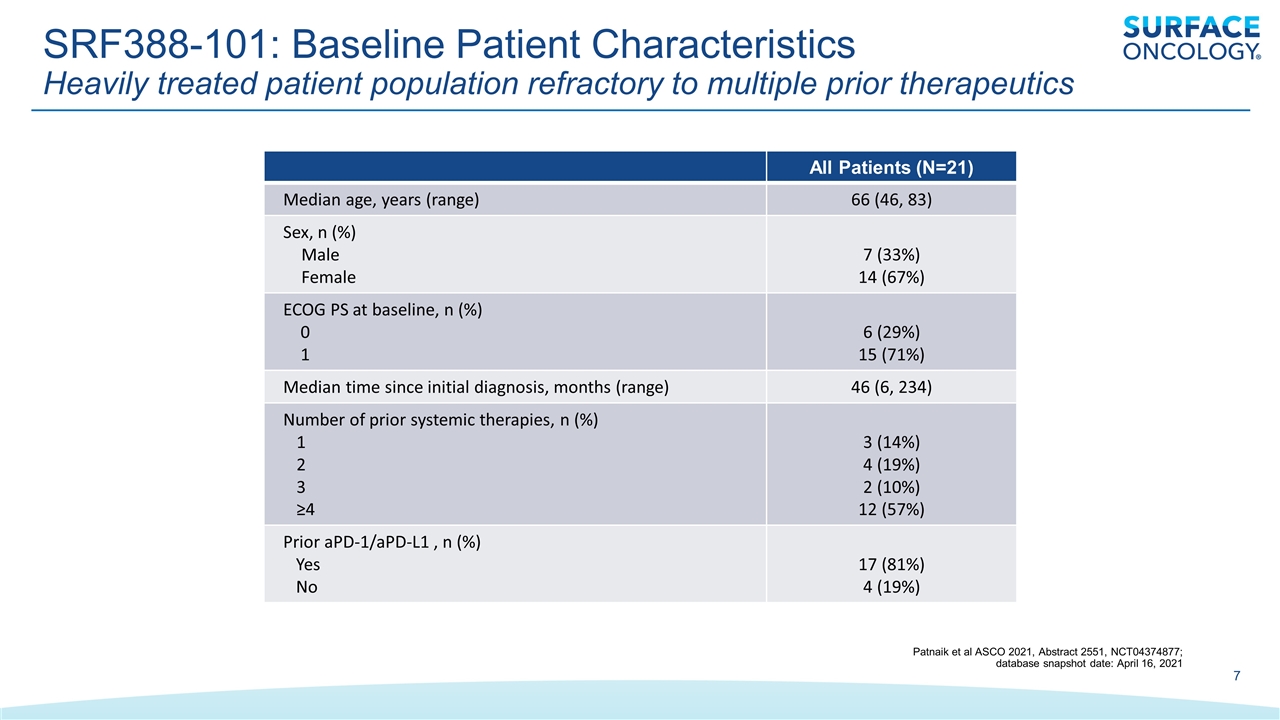
SRF388-101: Baseline Patient Characteristics Heavily treated patient population refractory to multiple prior therapeutics All Patients (N=21) Median age, years (range) 66 (46, 83) Sex, n (%) Male Female 7 (33%) 14 (67%) ECOG PS at baseline, n (%) 0 1 6 (29%) 15 (71%) Median time since initial diagnosis, months (range) 46 (6, 234) Number of prior systemic therapies, n (%) 1 2 3 ≥4 3 (14%) 4 (19%) 2 (10%) 12 (57%) Prior aPD-1/aPD-L1 , n (%) Yes No 17 (81%) 4 (19%) Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021
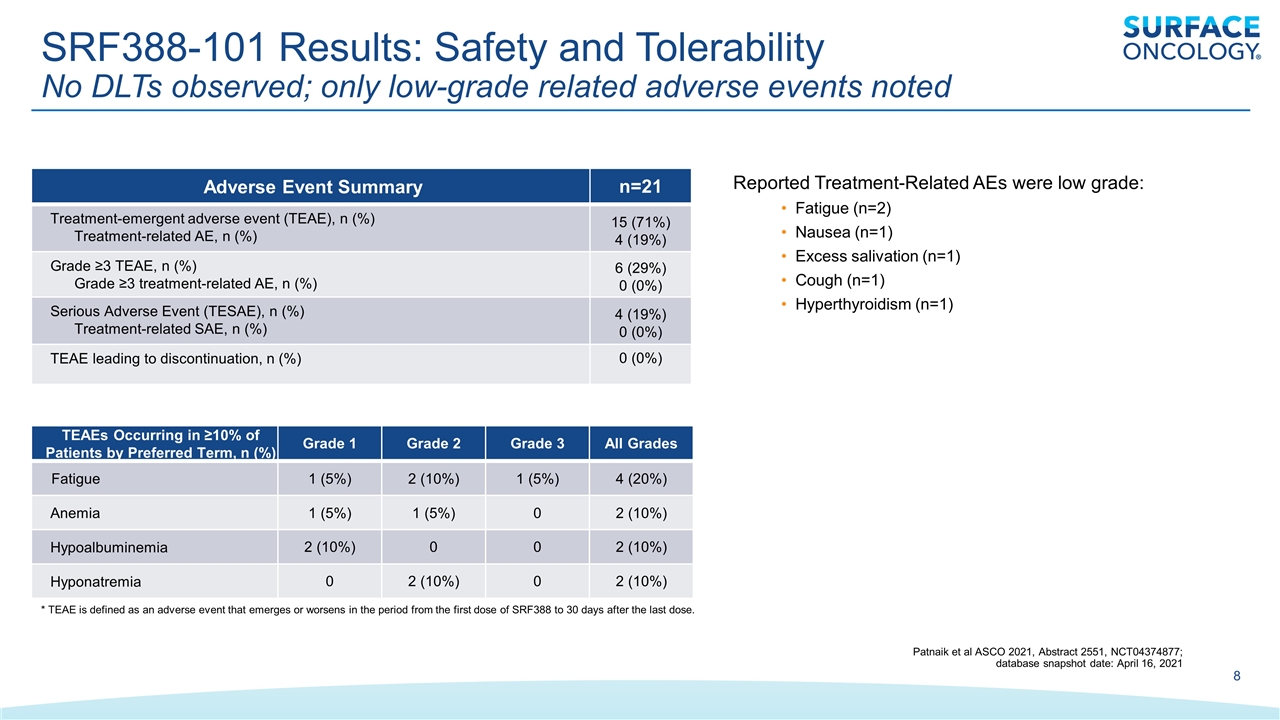
SRF388-101 Results: Safety and Tolerability No DLTs observed; only low-grade related adverse events noted Adverse Event Summary n=21 Treatment-emergent adverse event (TEAE), n (%) Treatment-related AE, n (%) 15 (71%) 4 (19%) Grade ≥3 TEAE, n (%) Grade ≥3 treatment-related AE, n (%) 6 (29%) 0 (0%) Serious Adverse Event (TESAE), n (%) Treatment-related SAE, n (%) 4 (19%) 0 (0%) TEAE leading to discontinuation, n (%) 0 (0%) TEAEs Occurring in ≥10% of Patients by Preferred Term, n (%) Grade 1 Grade 2 Grade 3 All Grades Fatigue 1 (5%) 2 (10%) 1 (5%) 4 (20%) Anemia 1 (5%) 1 (5%) 0 2 (10%) Hypoalbuminemia 2 (10%) 0 0 2 (10%) Hyponatremia 0 2 (10%) 0 2 (10%) * TEAE is defined as an adverse event that emerges or worsens in the period from the first dose of SRF388 to 30 days after the last dose. Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021 Reported Treatment-Related AEs were low grade: Fatigue (n=2) Nausea (n=1) Excess salivation (n=1) Cough (n=1) Hyperthyroidism (n=1)
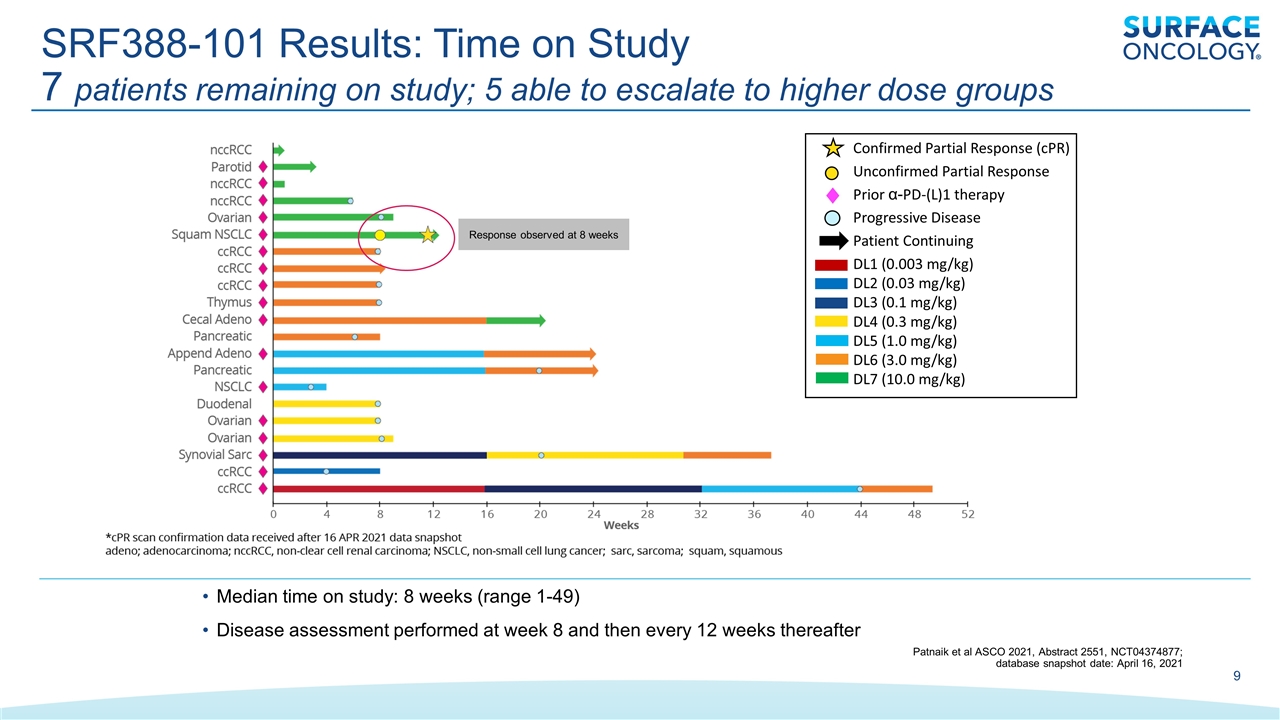
SRF388-101 Results: Time on Study 7 patients remaining on study; 5 able to escalate to higher dose groups Median time on study: 8 weeks (range 1-49) Disease assessment performed at week 8 and then every 12 weeks thereafter Response observed at 8 weeks Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021 Confirmed Partial Response (cPR) Unconfirmed Partial Response Prior α-PD-(L)1 therapy Progressive Disease Patient Continuing DL1 (0.003 mg/kg) DL2 (0.03 mg/kg) DL3 (0.1 mg/kg) DL4 (0.3 mg/kg) DL5 (1.0 mg/kg) DL6 (3.0 mg/kg) DL7 (10.0 mg/kg)
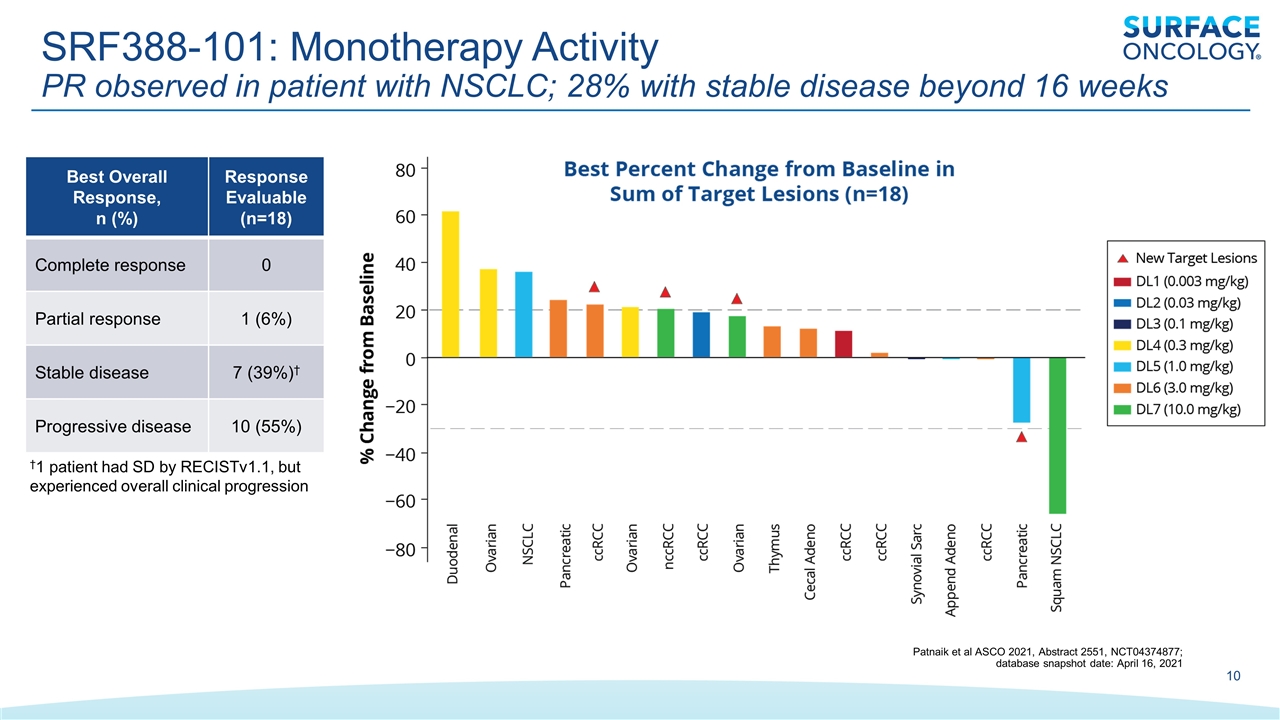
SRF388-101: Monotherapy Activity PR observed in patient with NSCLC; 28% with stable disease beyond 16 weeks †1 patient had SD by RECISTv1.1, but experienced overall clinical progression Best Overall Response, n (%) Response Evaluable (n=18) Complete response 0 Partial response 1 (6%) Stable disease 7 (39%)† Progressive disease 10 (55%) Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021
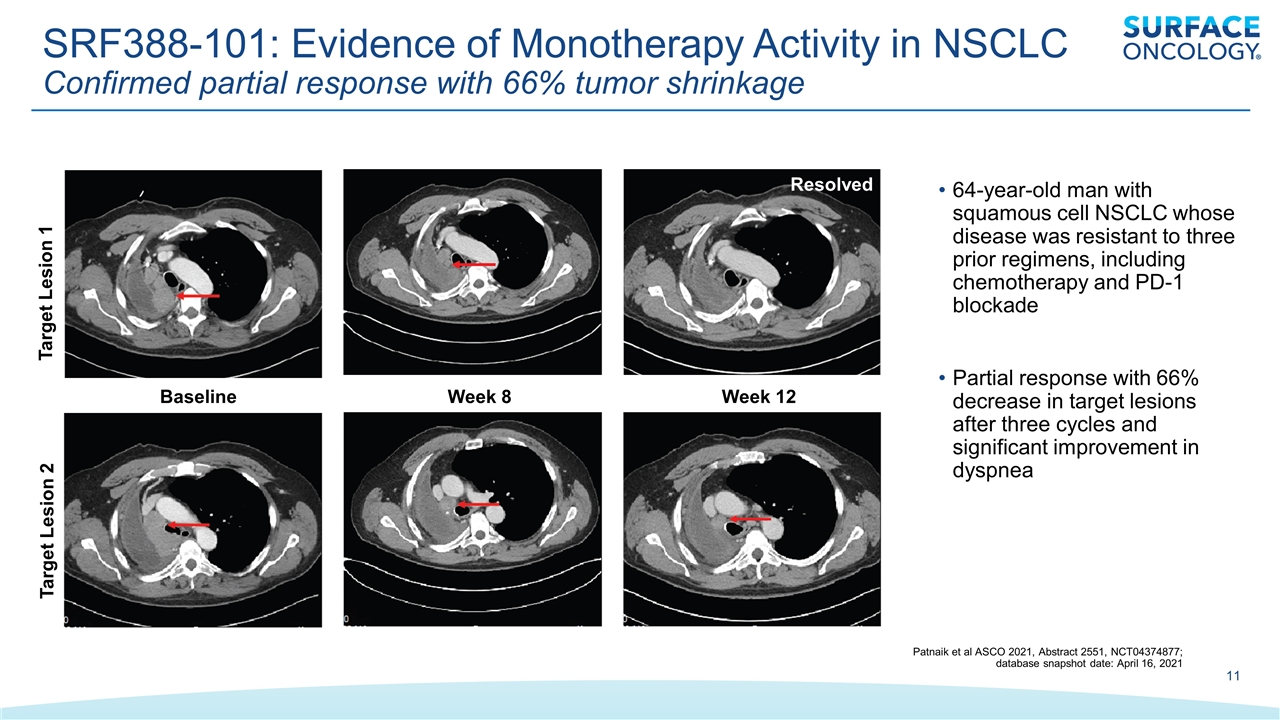
SRF388-101: Evidence of Monotherapy Activity in NSCLC Confirmed partial response with 66% tumor shrinkage Baseline Week 8 Week 12 Target Lesion 1 Target Lesion 2 Resolved 64-year-old man with squamous cell NSCLC whose disease was resistant to three prior regimens, including chemotherapy and PD-1 blockade Partial response with 66% decrease in target lesions after three cycles and significant improvement in dyspnea Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021
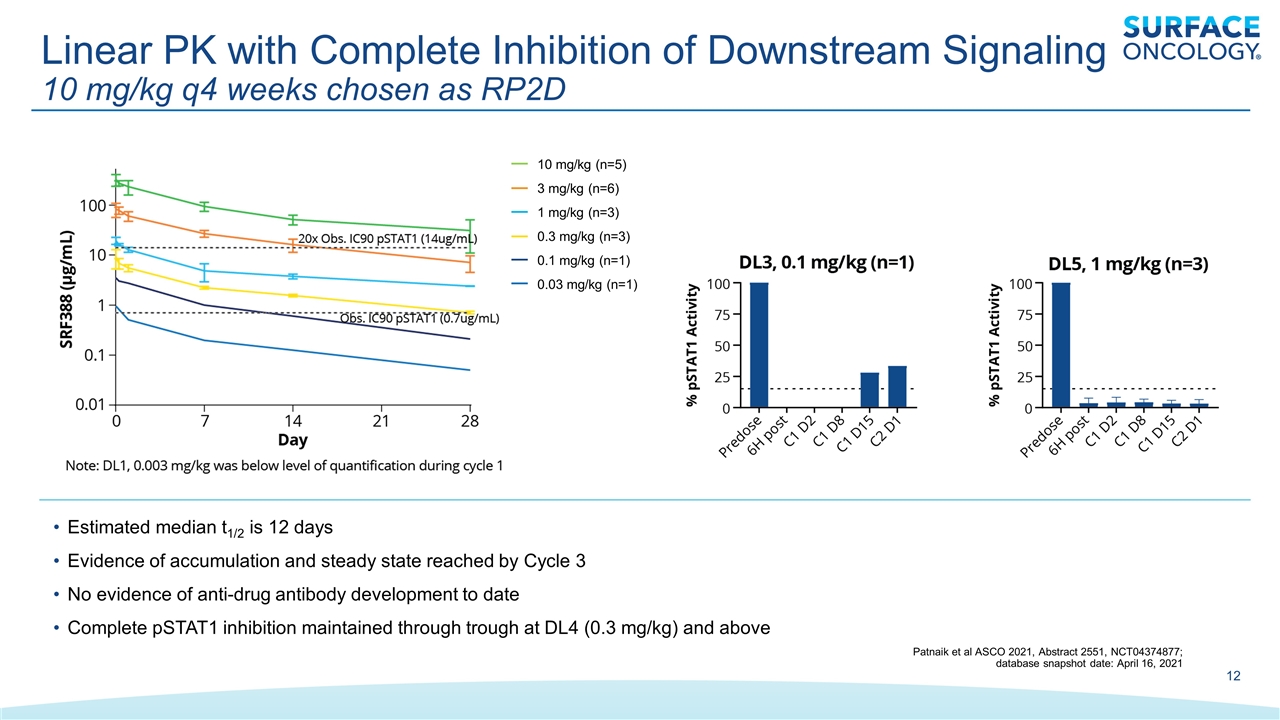
Linear PK with Complete Inhibition of Downstream Signaling 10 mg/kg q4 weeks chosen as RP2D 10 mg/kg (n=5) 3 mg/kg (n=6) 1 mg/kg (n=3) 0.3 mg/kg (n=3) 0.1 mg/kg (n=1) 0.03 mg/kg (n=1) Estimated median t1/2 is 12 days Evidence of accumulation and steady state reached by Cycle 3 No evidence of anti-drug antibody development to date Complete pSTAT1 inhibition maintained through trough at DL4 (0.3 mg/kg) and above Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021
SRF388-101 Study Conclusions Support further evaluation as monotherapy and combination therapy SRF388 is well tolerated at all doses tested to date Preliminary results show promising single-agent activity even in heavily pre-treated patients: Confirmed PR in a patient with squamous cell NSCLC whose disease was resistant to three prior regimens, including chemotherapy and PD-1 blockade 6 of 18 evaluable patients (33%) experienced disease stabilization at eight weeks, with five (28%) persisting beyond 16 weeks PK are linear and dose-proportional with maximal target inhibition of IL-27 mediated pSTAT1 throughout the dosing interval Recommended Phase 2 dose of 10mg/kg q4 weeks confirmed These data support further evaluation of SRF388 Initial focus in HCC, RCC and NSCLC Patnaik et al ASCO 2021, Abstract 2551, NCT04374877; database snapshot date: April 16, 2021

SRF617 Targeting CD39 for Dual Immune System Activation
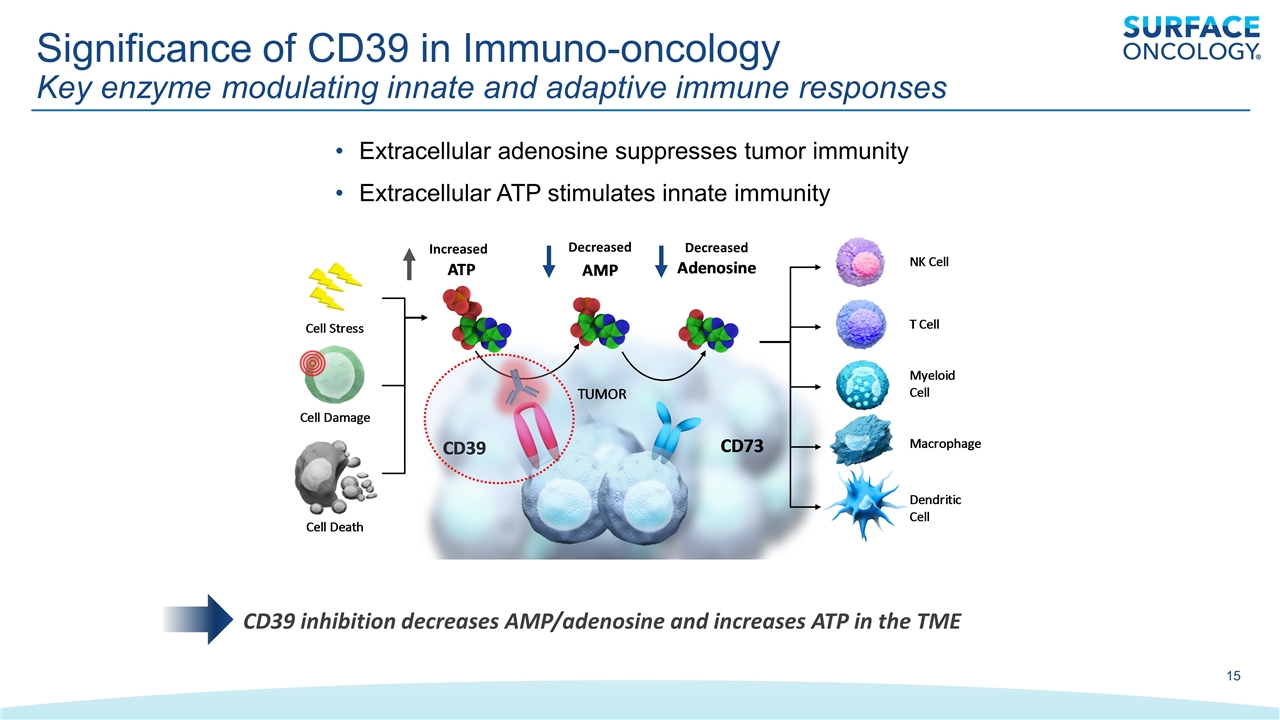
Significance of CD39 in Immuno-oncology Key enzyme modulating innate and adaptive immune responses Extracellular adenosine suppresses tumor immunity Extracellular ATP stimulates innate immunity Decreased Decreased Increased CD39 inhibition decreases AMP/adenosine and increases ATP in the TME
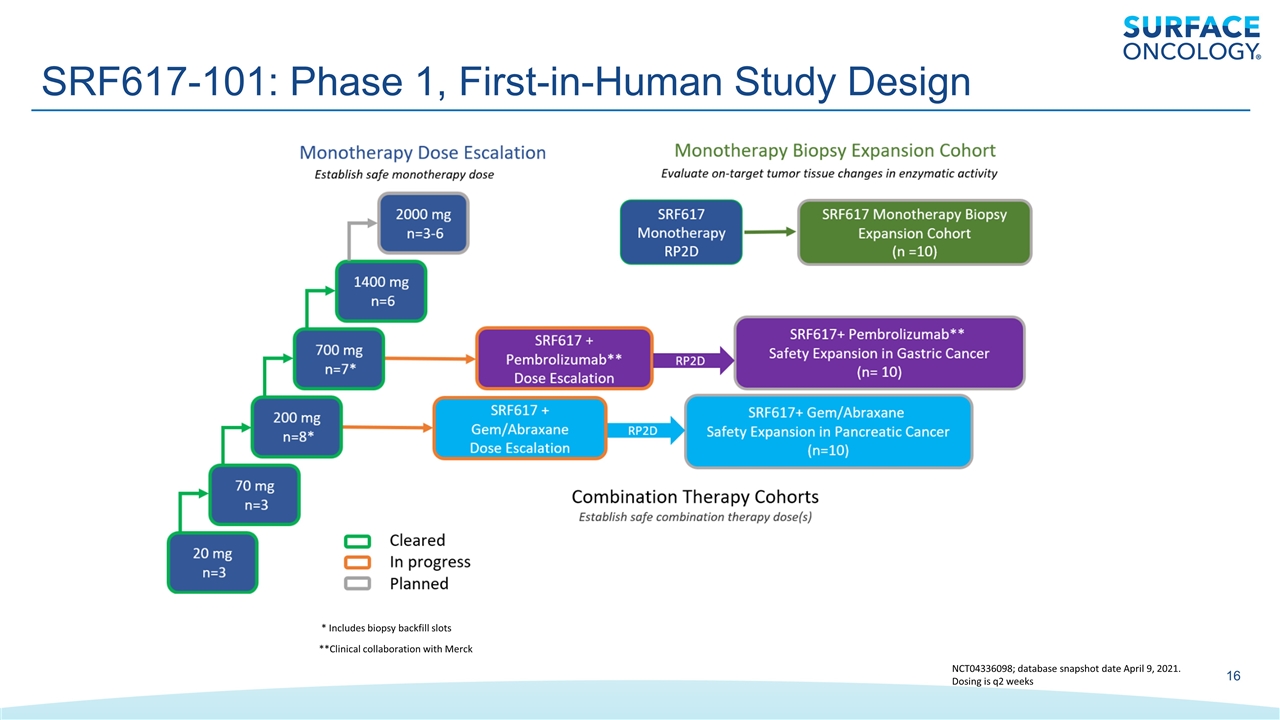
SRF617-101: Phase 1, First-in-Human Study Design * Includes biopsy backfill slots **Clinical collaboration with Merck NCT04336098; database snapshot date April 9, 2021. Dosing is q2 weeks
SRF617-101: Baseline Patient Characteristics, Monotherapy Heavily treated patient population refractory to multiple prior therapeutics All Patients (N-27) Median age, years (range) 65 (20, 80) Sex, n (%) Male Female 10 (37%) 17 (63%) ECOG PS at baseline, n (%) 0 1 11 (41%) 16 (59%) Median time since initial diagnosis, months (range) 38 (7, 351) Number of prior systemic therapies, n (%) 0 1 2 3 ≥4 2 (7%) 3 (11%) 4 (15%) 4 (15%) 14 (52%) Prior aPD-1/aPD-L1 , n (%) Yes No 8 (30%) 19 (70%) NCT04336098; database snapshot date April 9, 2021.
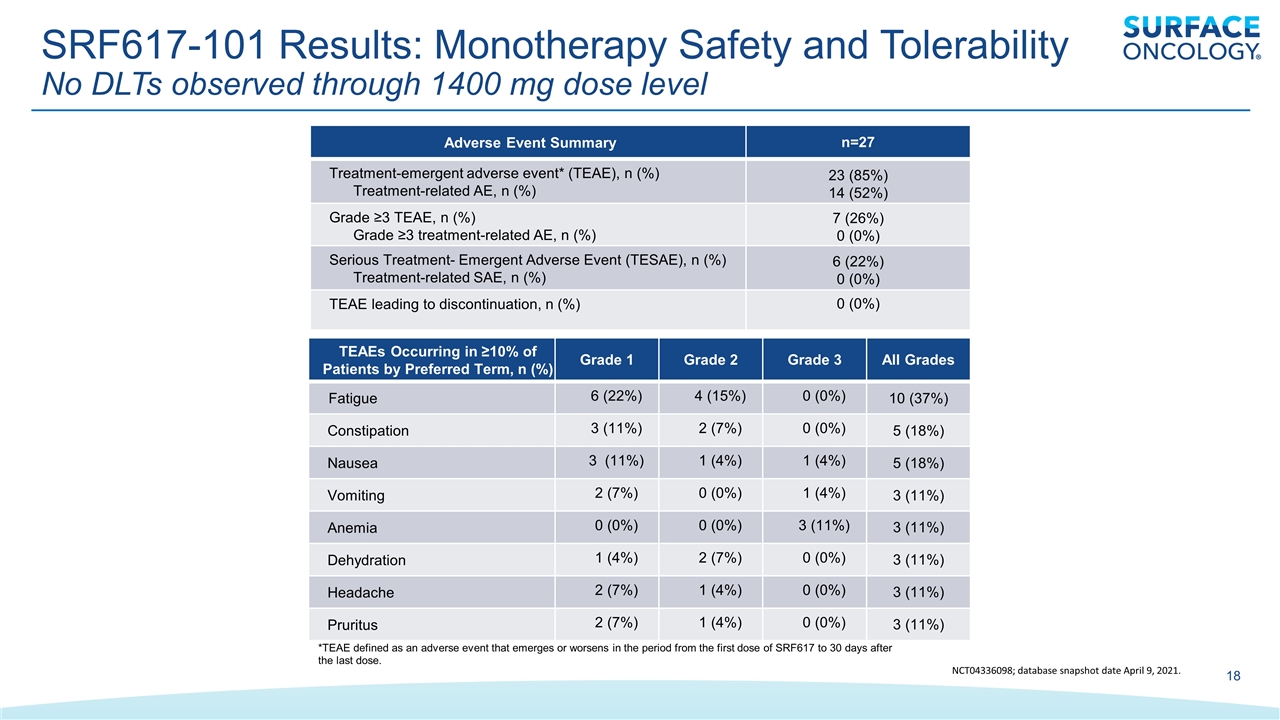
*TEAE defined as an adverse event that emerges or worsens in the period from the first dose of SRF617 to 30 days after the last dose. Adverse Event Summary n=27 Treatment-emergent adverse event* (TEAE), n (%) Treatment-related AE, n (%) 23 (85%) 14 (52%) Grade ≥3 TEAE, n (%) Grade ≥3 treatment-related AE, n (%) 7 (26%) 0 (0%) Serious Treatment- Emergent Adverse Event (TESAE), n (%) Treatment-related SAE, n (%) 6 (22%) 0 (0%) TEAE leading to discontinuation, n (%) 0 (0%) TEAEs Occurring in ≥10% of Patients by Preferred Term, n (%) Grade 1 Grade 2 Grade 3 All Grades Fatigue 6 (22%) 4 (15%) 0 (0%) 10 (37%) Constipation 3 (11%) 2 (7%) 0 (0%) 5 (18%) Nausea 3 (11%) 1 (4%) 1 (4%) 5 (18%) Vomiting 2 (7%) 0 (0%) 1 (4%) 3 (11%) Anemia 0 (0%) 0 (0%) 3 (11%) 3 (11%) Dehydration 1 (4%) 2 (7%) 0 (0%) 3 (11%) Headache 2 (7%) 1 (4%) 0 (0%) 3 (11%) Pruritus 2 (7%) 1 (4%) 0 (0%) 3 (11%) SRF617-101 Results: Monotherapy Safety and Tolerability No DLTs observed through 1400 mg dose level NCT04336098; database snapshot date April 9, 2021.
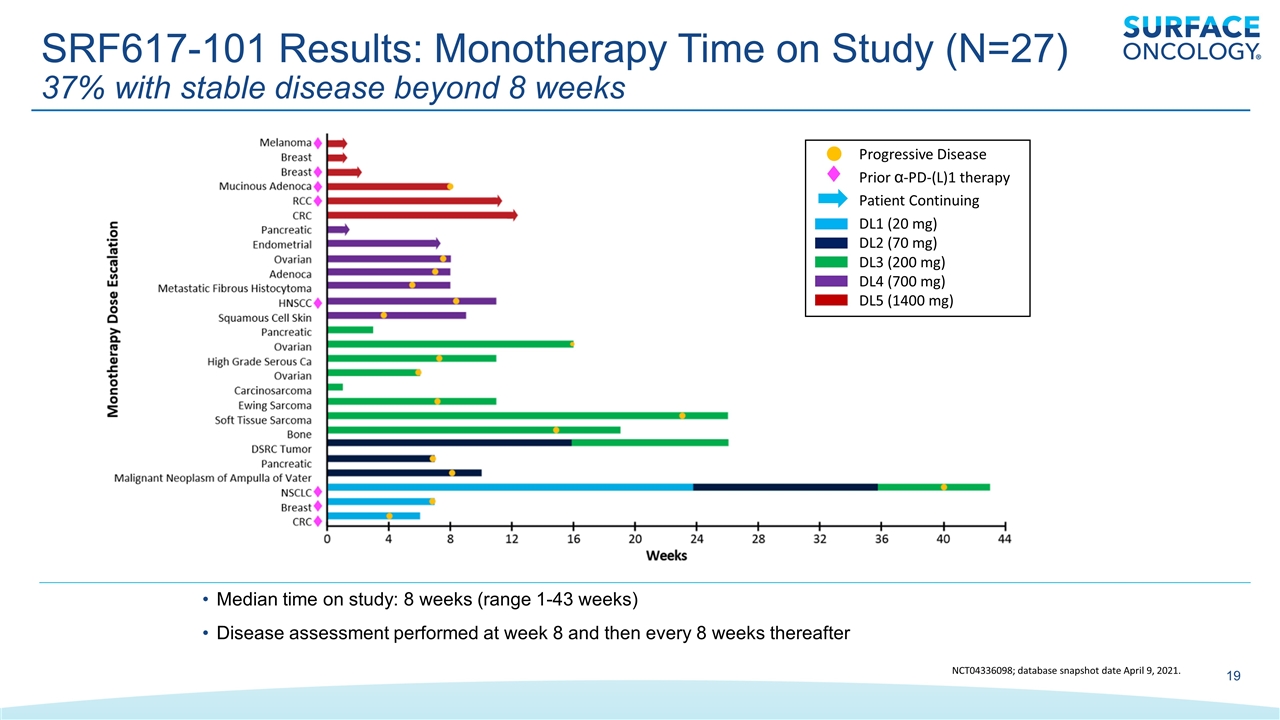
Progressive Disease Prior α-PD-(L)1 therapy Patient Continuing DL1 (20 mg) DL2 (70 mg) DL3 (200 mg) DL4 (700 mg) DL5 (1400 mg) NCT04336098; database snapshot date April 9, 2021. Median time on study: 8 weeks (range 1-43 weeks) Disease assessment performed at week 8 and then every 8 weeks thereafter SRF617-101 Results: Monotherapy Time on Study (N=27) 37% with stable disease beyond 8 weeks
SRF617-101: Early Data from Combination Cohorts SRF617 + Gem/Abraxane Cohort * Five patients dosed with SRF617 (200 mg) + full-dose gemcitabine/Abraxane; four with follow-up beyond one cycle Adverse events as expected, with reported bone marrow suppression and diarrhea One unconfirmed Partial Response reported at first response evaluation (week 8) in a patient with pancreatic cancer previously progressed on FOLFIRINOX chemotherapy; confirmation scan pending SRF617 + Pembrolizumab Cohort * Three patients dosed with SRF617 (700 mg) + pembrolizumab (200mg); all within first cycle of follow-up No early safety signals *Preliminary data as of 5/19/21
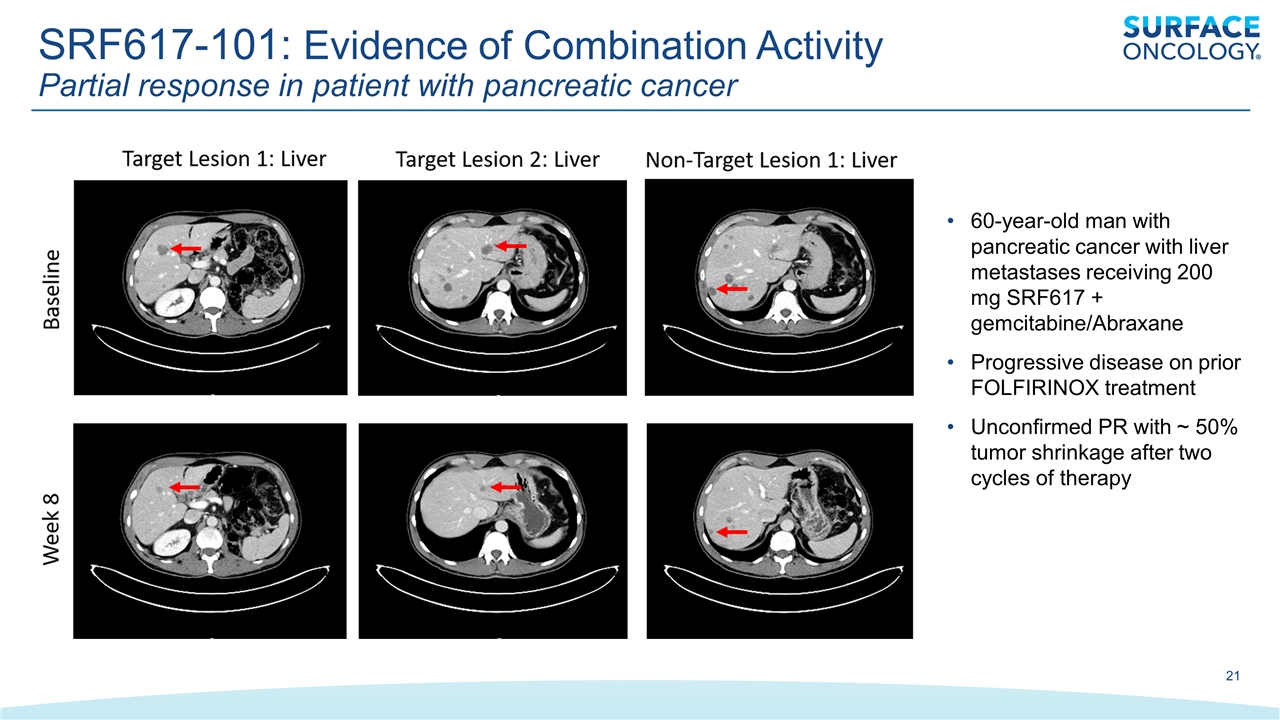
SRF617-101: Evidence of Combination Activity Partial response in patient with pancreatic cancer 60-year-old man with pancreatic cancer with liver metastases receiving 200 mg SRF617 + gemcitabine/Abraxane Progressive disease on prior FOLFIRINOX treatment Unconfirmed PR with ~ 50% tumor shrinkage after two cycles of therapy
SRF617 monotherapy is well tolerated at all tested doses to date in patients with advanced solid tumors and has a positive profile for intended combinations Early results support further evaluation: Monotherapy: 7 of 19 evaluable patients (37%) had disease stabilization at eight weeks with four (21%) persisting beyond 16 weeks, including one patient with NSCLC whose disease previously progressed on chemotherapy and PD-1 blockade Combination therapy: One PR (unconfirmed) in a patient with pancreatic cancer whose disease progressed on prior chemotherapy PK are linear and dose-proportional Maximal CD39 target occupancy throughout the dosing interval at doses of 200 mg and above Preliminary results support further evaluation of SRF617 in combination with standard and investigational regimens: Pancreatic cancer: with gemcitabine and Abraxane chemotherapy Gastric cancer: with pembrolizumab Prostate cancer: with etrumadenant and zimberelimab in a separate study to be initiated this year SRF617-101: Preliminary Results Support further evaluation as monotherapy and combination therapy
Clinical Collaboration Agreement with Roche Strong non-clinical and translational data for SRF388 in immuno-oncology-naive hepatocellular carcinoma (HCC) Current standard of care in first-line, immuno-oncology-naive HCC is the combination of atezolizumab (Roche’s anti-PD-L1 Tecentriq®) with bevacizumab (Roche’s Avastin®) Surface and Roche have agreed to a clinical collaboration for a Surface-run Phase 2 study to evaluate the combination of Roche’s atezolizumab and bevacizumab with SRF388 in patients with treatment-naïve HCC. Roche will provide both combination agents.
Reviewing Today’s Highlights Wholly-owned, first-in-class SRF388 (IL-27) demonstrates monotherapy activity, good tolerability, expansions opening Wholly-owned SRF617 (CD39) demonstrates good tolerability, activity in combination – combination expansions enrolling New clinical collaboration with Roche for SRF388 in first-line hepatocellular carcinoma in combination with atezolizumab and bevacizumab We intend to provide clinical updates on SRF617 and SRF388 at medical meetings in late 2021 and early 2022, respectively

Thank You
























