
SRF388 Clinical Data and Business Update Call November 2, 2022 Exhibit 99.2

This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of Surface Oncology, Inc. (“we,” “us” or “our”) our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward looking statements. These risks and uncertainties include the timing, progress, and results of preclinical studies and clinical trials for SRF617, SRF388 and SRF114, and our other product candidates, the timing and likelihood of regulatory approvals and those risks identified and discussed in the section titled “Risk Factors,” set forth in our Annual Report on Form 10-K and in our other SEC filings. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business.

Review of SRF388 Non-Small Cell Lung Cancer Data (NSCLC) SRF114 Advancing Towards the Clinic
First-in-Class Antibody Targeting IL-27 High-affinity, fully human IgG1 antibody against IL-27 IL-27 is a highly immunosuppressive cytokine and serves as a “master switch” of checkpoint protein expression Translational and clinical evidence to support activity in liver and lung cancer Monotherapy activity in treatment-refractory NSCLC and ccRCC; Confirmed PR in HCC in combination with pembrolizumab Clinical trials in NSCLC and HCC as monotherapy and combination therapy in progress as part of clinical collaborations with Merck and Roche Next clinical update anticipated in 1H 2023 Overview of SRF388
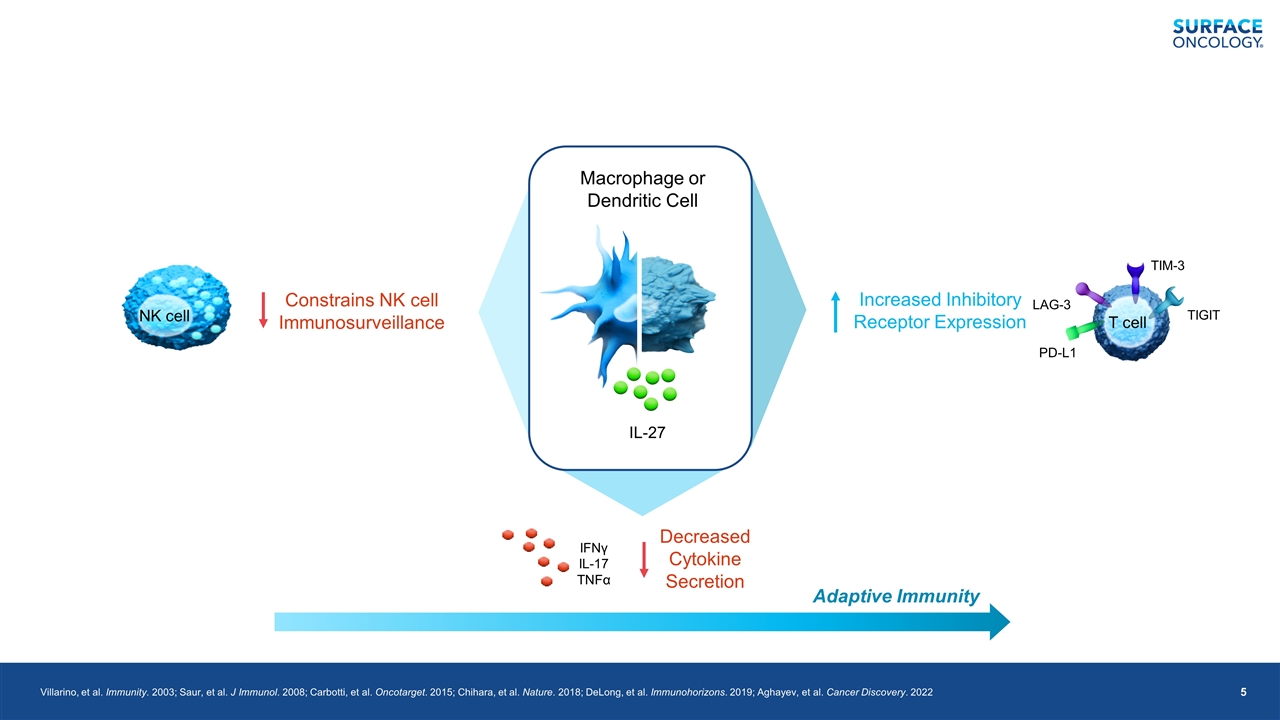
Villarino, et al. Immunity. 2003; Saur, et al. J Immunol. 2008; Carbotti, et al. Oncotarget. 2015; Chihara, et al. Nature. 2018; DeLong, et al. Immunohorizons. 2019; Aghayev, et al. Cancer Discovery. 2022 Adaptive Immunity IFNγ IL-17 TNFα Decreased Cytokine Secretion IL-27 Macrophage or Dendritic Cell PD-L1 T cell LAG-3 TIM-3 TIGIT Increased Inhibitory Receptor Expression NK cell Constrains NK cell Immunosurveillance
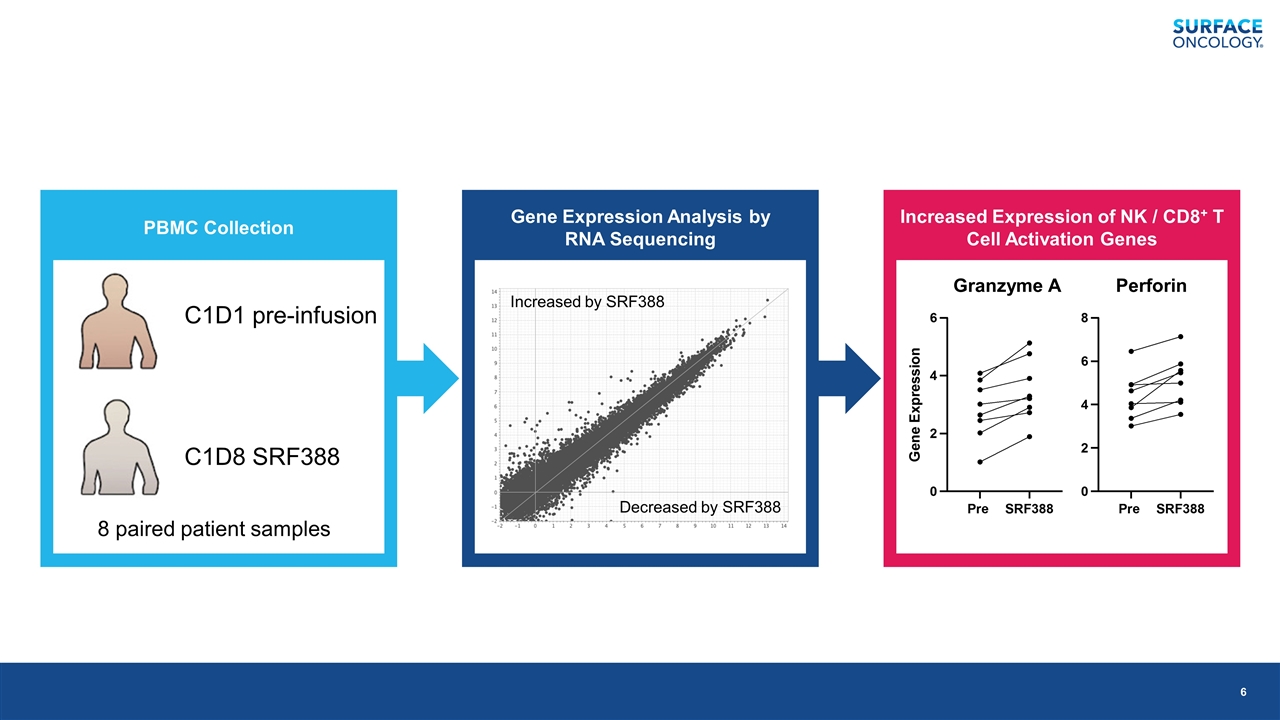
PBMC Collection Gene Expression Analysis by RNA Sequencing Increased Expression of NK / CD8+ T Cell Activation Genes C1D1 pre-infusion C1D8 SRF388 Increased by SRF388 Decreased by SRF388 8 paired patient samples Granzyme A Perforin
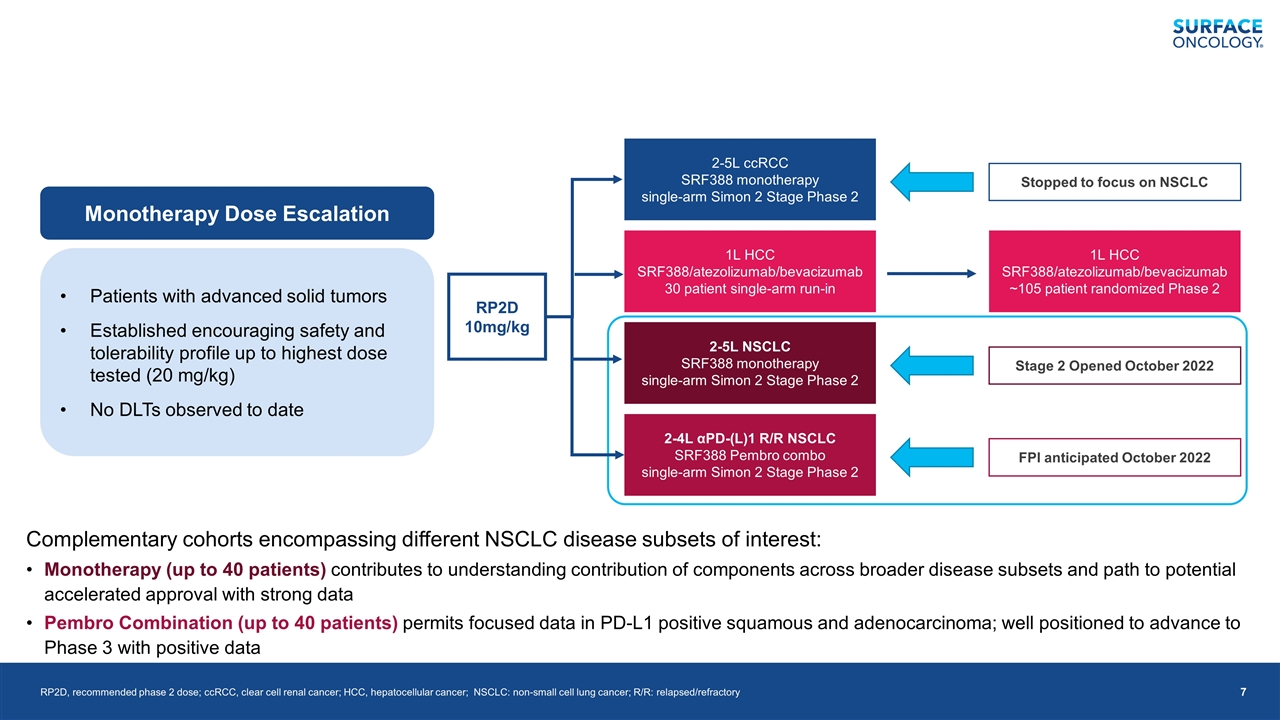
Monotherapy Dose Escalation Patients with advanced solid tumors Established encouraging safety and tolerability profile up to highest dose tested (20 mg/kg) No DLTs observed to date Complementary cohorts encompassing different NSCLC disease subsets of interest: Monotherapy (up to 40 patients) contributes to understanding contribution of components across broader disease subsets and path to potential accelerated approval with strong data Pembro Combination (up to 40 patients) permits focused data in PD-L1 positive squamous and adenocarcinoma; well positioned to advance to Phase 3 with positive data RP2D, recommended phase 2 dose; ccRCC, clear cell renal cancer; HCC, hepatocellular cancer; NSCLC: non-small cell lung cancer; R/R: relapsed/refractory RP2D 10mg/kg 2-5L ccRCC SRF388 monotherapy single-arm Simon 2 Stage Phase 2 1L HCC SRF388/atezolizumab/bevacizumab 30 patient single-arm run-in 2-5L NSCLC SRF388 monotherapy single-arm Simon 2 Stage Phase 2 2-4L αPD-(L)1 R/R NSCLC SRF388 Pembro combo single-arm Simon 2 Stage Phase 2 Stopped to focus on NSCLC Stage 2 Opened October 2022 FPI anticipated October 2022 1L HCC SRF388/atezolizumab/bevacizumab ~105 patient randomized Phase 2
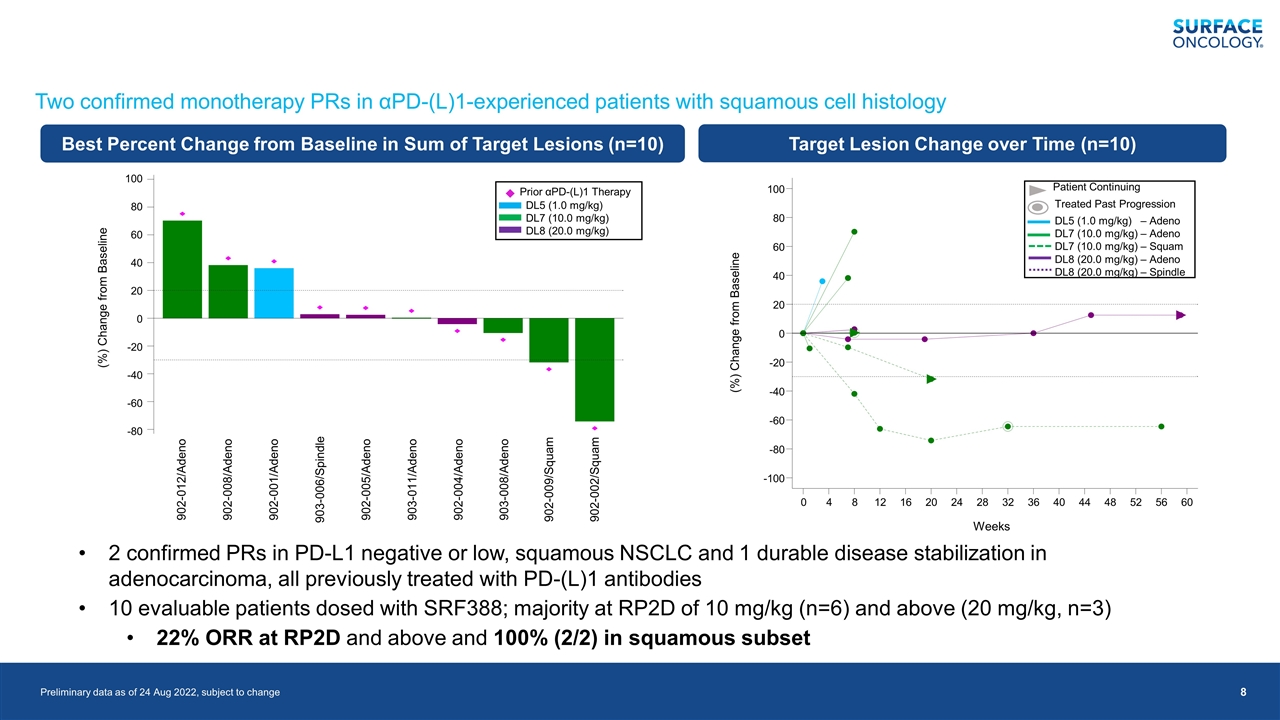
Preliminary data as of 24 Aug 2022, subject to change 2 confirmed PRs in PD-L1 negative or low, squamous NSCLC and 1 durable disease stabilization in adenocarcinoma, all previously treated with PD-(L)1 antibodies 10 evaluable patients dosed with SRF388; majority at RP2D of 10 mg/kg (n=6) and above (20 mg/kg, n=3) 22% ORR at RP2D and above and 100% (2/2) in squamous subset Two confirmed monotherapy PRs in αPD-(L)1-experienced patients with squamous cell histology (%) Change from Baseline 100 80 -80 -60 -40 -20 0 20 40 60 -100 Weeks 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Patient Continuing Treated Past Progression DL5 (1.0 mg/kg) – Adeno DL7 (10.0 mg/kg) – Adeno DL7 (10.0 mg/kg) – Squam DL8 (20.0 mg/kg) – Adeno DL8 (20.0 mg/kg) – Spindle 100 80 -80 -60 -40 -20 0 20 40 60 (%) Change from Baseline 902-012/Adeno 902-008/Adeno 902-001/Adeno 903-006/Spindle 902-005/Adeno 903-011/Adeno 902-004/Adeno 903-008/Adeno 902-009/Squam 902-002/Squam Prior αPD-(L)1 Therapy DL5 (1.0 mg/kg) DL7 (10.0 mg/kg) DL8 (20.0 mg/kg) Best Percent Change from Baseline in Sum of Target Lesions (n=10) Target Lesion Change over Time (n=10)
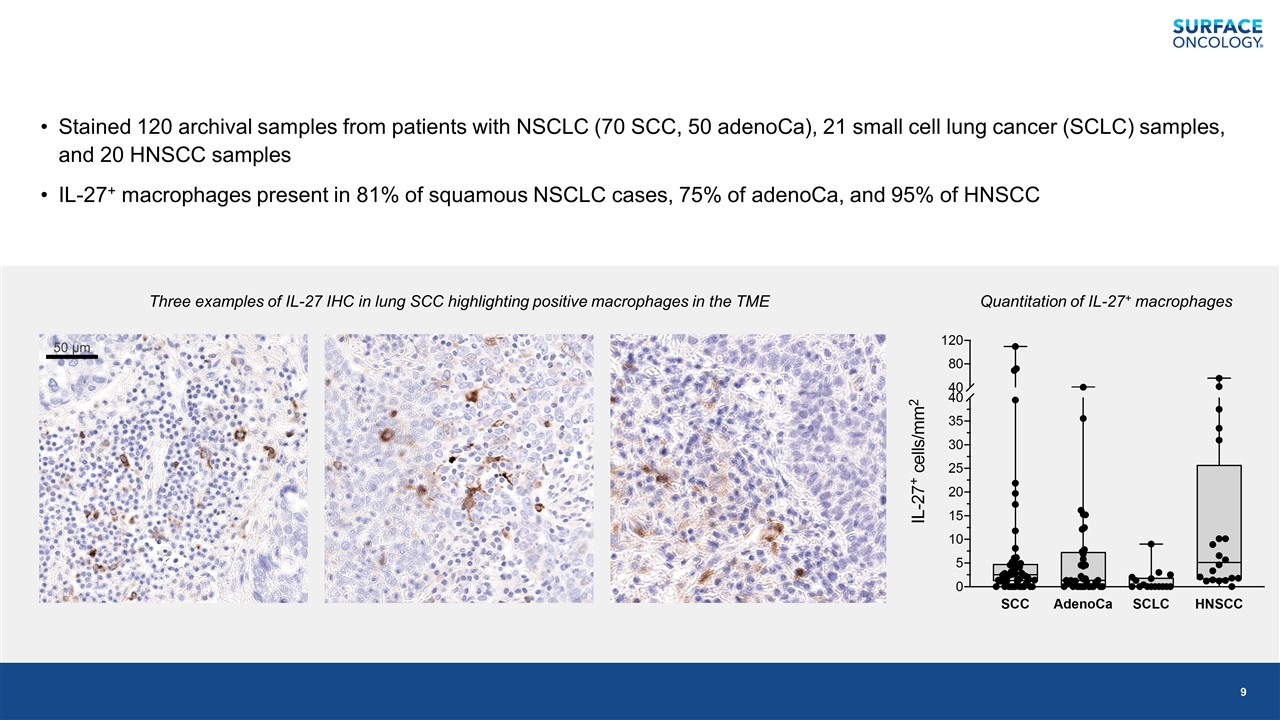
Stained 120 archival samples from patients with NSCLC (70 SCC, 50 adenoCa), 21 small cell lung cancer (SCLC) samples, and 20 HNSCC samples IL-27+ macrophages present in 81% of squamous NSCLC cases, 75% of adenoCa, and 95% of HNSCC Three examples of IL-27 IHC in lung SCC highlighting positive macrophages in the TME Quantitation of IL-27+ macrophages 50 µm
Antibody Targeting CCR8 High-affinity, fully human afucosylated IgG1 antibody against CCR8 Specifically binds and preferentially depletes CCR8+ tumor Tregs Afucosylation enhances ADCC killing Highly specific for binding CCR8 exclusively IND open Overview of SRF114
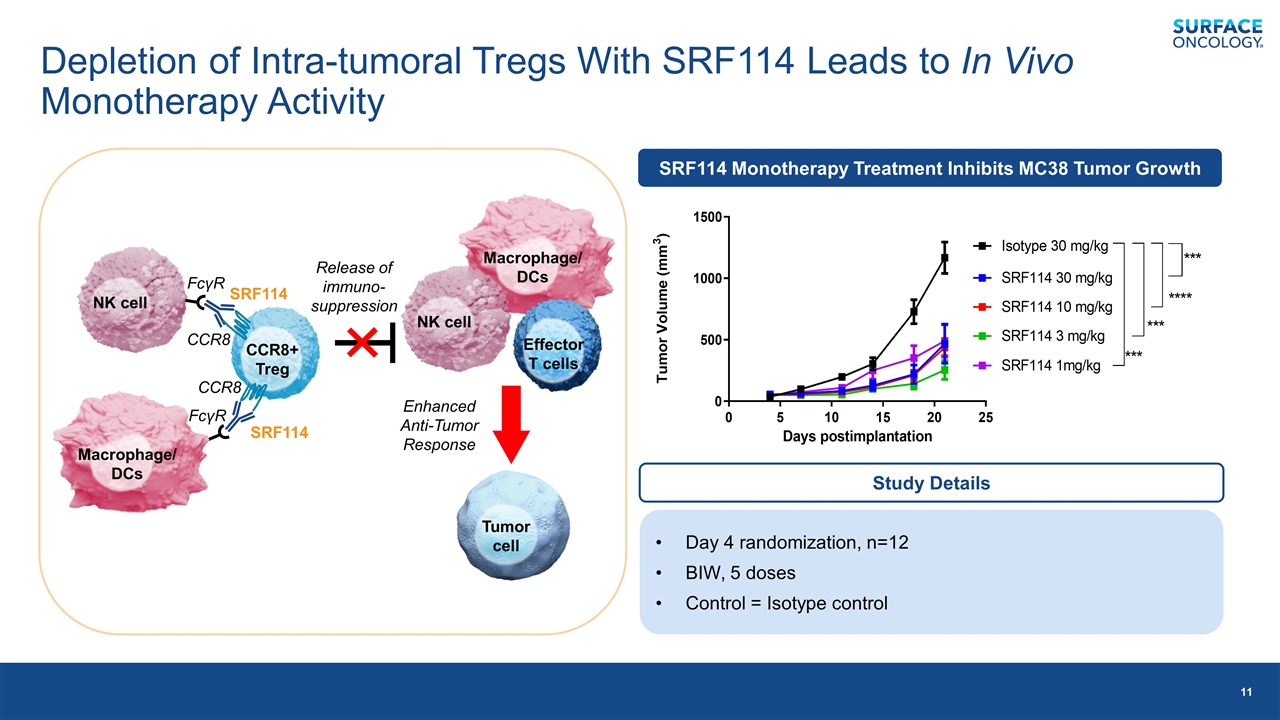
Macrophage/DCs Depletion of Intra-tumoral Tregs With SRF114 Leads to In Vivo Monotherapy Activity CCR8+ Treg FcγR SRF114 SRF114 CCR8 CCR8 Macrophage/DCs NK cell FcγR Release of immuno-suppression Effector T cells Tumor cell Enhanced Anti-Tumor Response SRF114 Monotherapy Treatment Inhibits MC38 Tumor Growth Day 4 randomization, n=12 BIW, 5 doses Control = Isotype control Study Details NK cell
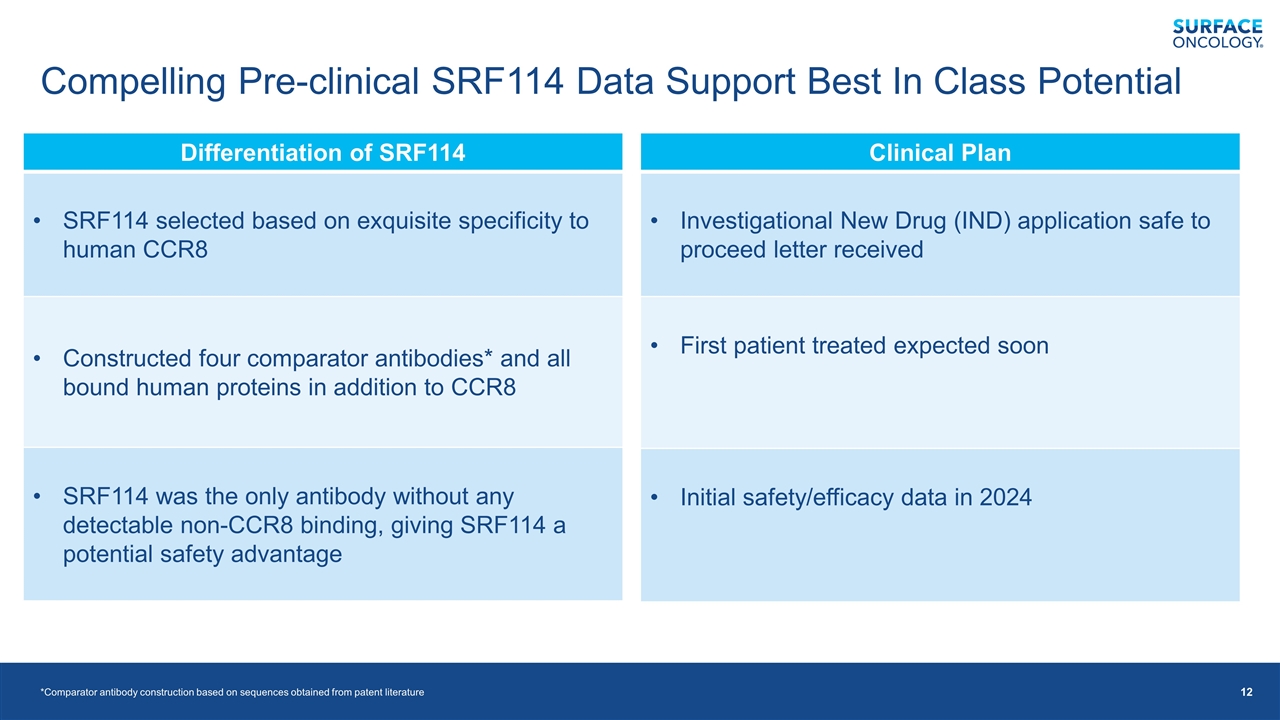
Compelling Pre-clinical SRF114 Data Support Best In Class Potential *Comparator antibody construction based on sequences obtained from patent literature Differentiation of SRF114 SRF114 selected based on exquisite specificity to human CCR8 Constructed four comparator antibodies* and all bound human proteins in addition to CCR8 SRF114 was the only antibody without any detectable non-CCR8 binding, giving SRF114 a potential safety advantage Clinical Plan Investigational New Drug (IND) application safe to proceed letter received First patient treated expected soon Initial safety/efficacy data in 2024

Anticipated Clinical Updates SRF388: Clinical data anticipated in H1 2023 SRF114: First patient expected soon; initial safety/efficacy clinical data in 2024

SRF388 Clinical Data and Business Update Call November 2, 2022













