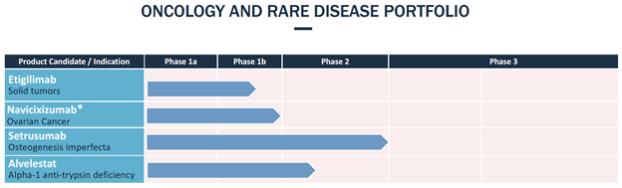
Upon entering into the License Agreement, we made a payment of $3.0 million and issued 490,798 ordinary shares to AstraZeneca, for an aggregate upfront payment equal to $5.0 million. In connection with certain development and regulatory milestones, we have agreed to make payments of up to $115.5 million in the aggregate and issue additional ordinary shares to AstraZeneca for licensed product candidates containing alvelestat. In addition, we have agreed to make payments to AstraZeneca based on specified commercial milestones of the product candidate. In the event that we sub-license alvelestat, we have also agreed to pay a specified percentage of sublicensing revenue to AstraZeneca. Otherwise, we have agreed to make royalty payments to AstraZeneca equal to ascending specified percentages of tiered annual worldwide net sales by us or our affiliates of licensed product candidates (subject to certain reductions), ranging from the high single digits to low double digits. Royalties will be payable on a licensed product-by-licensed product and country-by-country basis until the later of ten years after the first commercial sale of such licensed product in such country and expiration of the last patent covering such licensed product in such country that would be sufficient to prevent generic entry. Under the License Agreement, we may freely grant sub-licenses to affiliates upon notice to AstraZeneca and we must obtain AstraZeneca’s consent, not be unreasonably withheld, to grant sub-licenses to a third party. We have agreed to use commercially reasonable efforts to develop and commercialize at least one licensed product. In addition, we are generally responsible for costs related to the development and commercialization of the licensed products under the License Agreement.
The License Agreement will expire on the expiry of the last-to-expire royalty term with respect to all licensed product candidates. Upon the expiration of the royalty term for a licensed product in a particular country, the licenses to us for such product in such country will become fully-paid and irrevocable. Prior to exercise of the Option, if at all, we may terminate the License Agreement upon prior written notice. Either party may terminate the agreement upon prior written notice for the other party’s material breach that remains uncured for a specified period of time or insolvency. AstraZeneca has agreed to a three-year non-competition restriction in relation to the direct or indirect commercialization or development of NE inhibitors for the treatment of AATD. In addition, AstraZeneca agreed not to assert any AstraZeneca intellectual property rights that were included in the scope of the License Agreement against us.
Aspire Capital Transaction
On February 10, 2020, we entered into a Purchase Agreement with Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $25.0 million worth of our ordinary shares that are exchangeable for ADSs over the approximately30-month term of the Purchase Agreement. In addition, pursuant to the Purchase Agreement, Aspire Capital purchased 11,432,925 ordinary shares that are exchangeable for 2,286,585 ADSs for $3.0 million. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we paid Aspire Capital a commission fee of $300,000, which was wholly satisfied by the issuance to Aspire Capital of 2,862,595 ordinary shares that are exchangeable for 572,519 ADSs.
Boxer Capital Transaction
On February 19, 2020, we entered into a securities purchase agreement with Boxer Capital. Under the terms of the agreement, Boxer Capital agreed to invest $3.0 million by purchasing 12,252,715 ordinary shares (equivalent to 2,450,543 ADSs) at a price equivalent to 18.8 pence per ordinary share, which represented a 20% discount to our closing share price of 23.5 pence on AIM on February 18, 2020. We intend to use the net proceeds from this private placement for general corporate purposes, including clinical trial activity and working capital. There are no warrants, derivatives, or other share classes associated with this transaction. Further, there are no restrictions on future financings and there are no financial covenants, participation rights, rights of first refusal, or penalties in the purchase agreement entered into in connection with this transaction.
June 2020 Private Placement
On June 4, 2020, we announced completion of a private placement with net proceeds of approximately $64.2 million (£51.4 million) with a number of new and existing principally U.S based institutional and accredited investors (the “June 2020 Private Placement”). OrbiMed Private Investments VI, LP (acting through its general partner, OrbiMed Capital GP VI LLC, acting through its managing member, OrbiMed Advisors LLC, collectively referred to herein as “OrbiMed”) led the June 2020 Private Placement with participants including Vivo Capital, Surveyor Capital (a Citadel company), Pontifax Venture Capital, Samsara BioCapital, Commodore Capital, and funds managed by Janus Henderson Investors alongside existing investors Boxer Capital of Tavistock Group and Aspire Capital Fund, LLC (collectively, the “Purchasers”). On June 3, 2020, we entered into a securities purchase agreement (the “June 2020 Purchase Agreement”) with the Purchasers pursuant to which we received approximately $64.2 million (£51.4 million) from the Purchasers comprising: the allotment of ordinary shares at a subscription price of approximately $19.4 million utilizing the existing share authorities of the Company granted by shareholders on June 2, 2016 and June 19, 2019, and the subscription for Tranche 1 Notes in an aggregate principal amount of approximately $50.6 million. The Purchasers also received conditional warrants entitling the holders to subscribe for an aggregate of 161,048,366 new ordinary shares. The net proceeds from the June 2020 Private Placement will be used primarily to fund clinical development activities of our lead product candidates, reduction of indebtedness and for general corporate purposes.
Arrangements with OrbiMed
In recognition of OrbiMed’s participation in, and assistance with, the June 2020 Private Placement, the Company has agreed to grant OrbiMed certain rights. OrbiMed will have the right to nominate two persons to be appointed to the Board of Directors (out of a maximum number of 9 directors), for a period of 180 days from June 3,
84