
NASDAQ: OYST Focused on Transformative Therapies for the Ocular Surface Corporate Presentation November 2020 Exhibit 99.1

Disclaimers and Forward-looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that reflect the current beliefs, expectations and assumptions of Oyster Point Pharma, Inc. (the “Company,” “we” or “our”) regarding the future of its business, its future plans and strategies, clinical results, future financial condition and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, product candidates, planned preclinical studies and clinical trials, results of clinical trials, research and development costs, regulatory approvals, timing and likelihood of success, as well as plans and objectives of management for future operations, are forward-looking statements. The words “may,” “will,” “should,” “would,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: market conditions, the likelihood of our clinical trials demonstrating safety and efficacy of our product candidates, and other positive results; the timing of initiation of our future clinical trials, and the reporting of data from our current and future trials; our plans relating to the clinical development of our product candidates, including the size, number and disease areas to be evaluated; the size of the market opportunity and prevalence of dry eye disease (DED) for our product candidates; our plans relating to commercializing our product candidates, if approved, including the geographic areas of focus and sales strategy; the success of competing therapies that are or may become available; our estimates of the number of patients in the United States who suffer from DED and the number of patients that will enroll in our clinical trials; the beneficial characteristics, safety, efficacy and therapeutic effects of our product candidates; the timing or likelihood of regulatory filings and approval for our product candidates; our ability to obtain and maintain regulatory approval of our product candidates; our plans relating to the further development and manufacturing of our product candidates, including additional indications for which we may pursue; the expected potential benefits of strategic collaborations with third parties and our ability to attract collaborators with development, regulatory and commercialization expertise; existing regulations and regulatory developments in the United States and other jurisdictions; our plans and ability to obtain or protect intellectual property rights, including extensions of existing patent terms where available; our continued reliance on third parties to conduct additional clinical trials of our product candidates, and for the manufacture of our product candidates for preclinical studies and clinical trials; the accuracy of our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; our financial performance; the sufficiency of our existing capital resources to fund our future operating expenses and capital expenditure requirements; our expectations regarding the period during which we will qualify as an emerging growth company under the JOBS Act; our expectations regarding the impact of the COVID-19 pandemic on patient visits and expectations regarding the powering of the ONSET-2 clinical trial, and the impact on our business of the COVID-19 pandemic or similar public health crises, our anticipated use of our existing resources and the proceeds from our recent follow-on public offering; and other risks described in the “Risk Factors” section included in our public filings that we have made and will make with the Securities and Exchange Commission (SEC) The forward-looking statements in this presentation represent our views as of the date of this presentation. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. We have filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about us. You may obtain these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated.

Treatment of Dry Eye Disease Dry Eye Disease Affects: ~34 MILLION ADULTS of all ages in the U.S. alone1 and >340 million worldwide2 ~16 MILLION ADULTS have been diagnosed, but < 2 million are currently on treatment ~7 MILLION ADULTS have tried current options Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Dalton DS. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. American Journal of Ophthalmology. 2014 Apr;157(4):799-806. 2. Market Scope 2016 Dry Eye Products Report: A Global Market Analysis for 2015 to 2021 Mechanisms of action focused on inflammation Slow onset of action, measured in weeks to months Tolerability and compliance issues with administration of eye drops Existing Rx Landscape: Dry Eye

OC-01 Nasal Spray Highlights Novel mechanism of action to restore tear film homeostasis with natural tear production Rapid onset of action demonstrated in clinical trials to significantly improve signs and symptoms Improvement in tear production demonstrated in the majority of a broad population of patients Favorable tolerability - Most common side effect was transient sneezing Preservative-free with 50 uL spray delivered twice daily to each nostril Novel route of administration OC-01 is an investigational therapy that is not approved for use in any country
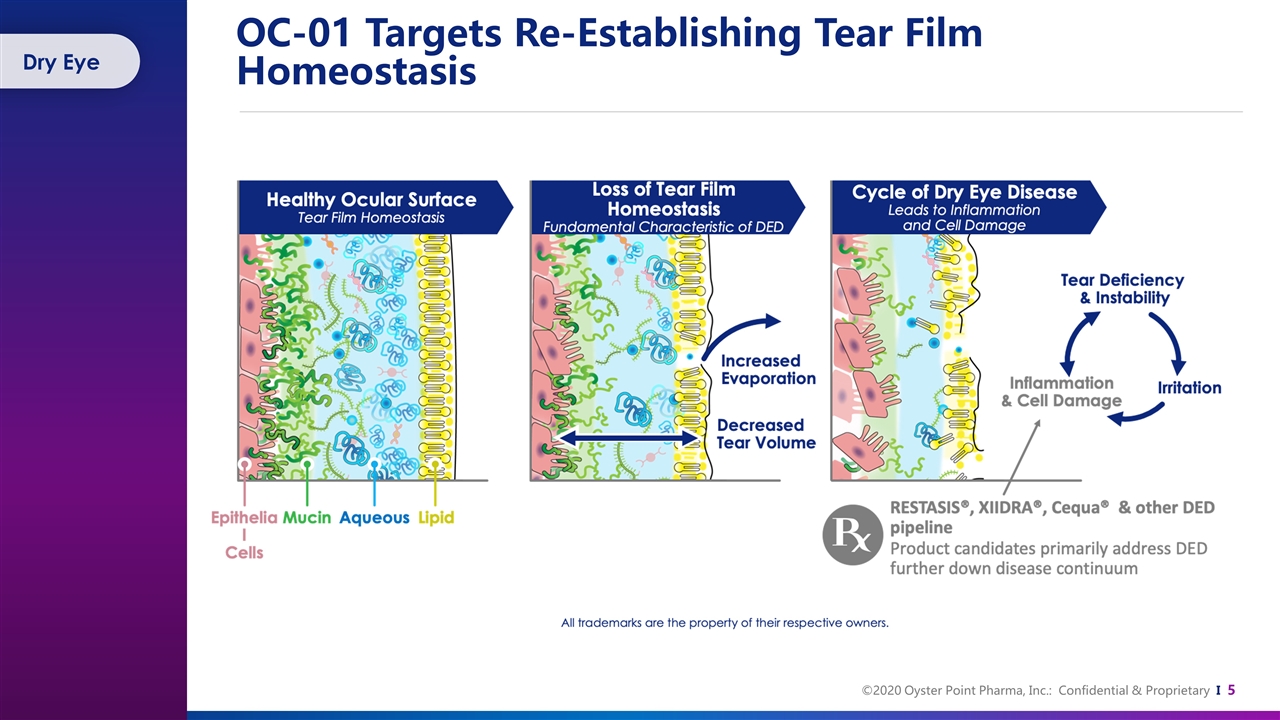
OC-01 Targets Re-Establishing Tear Film Homeostasis Dry Eye
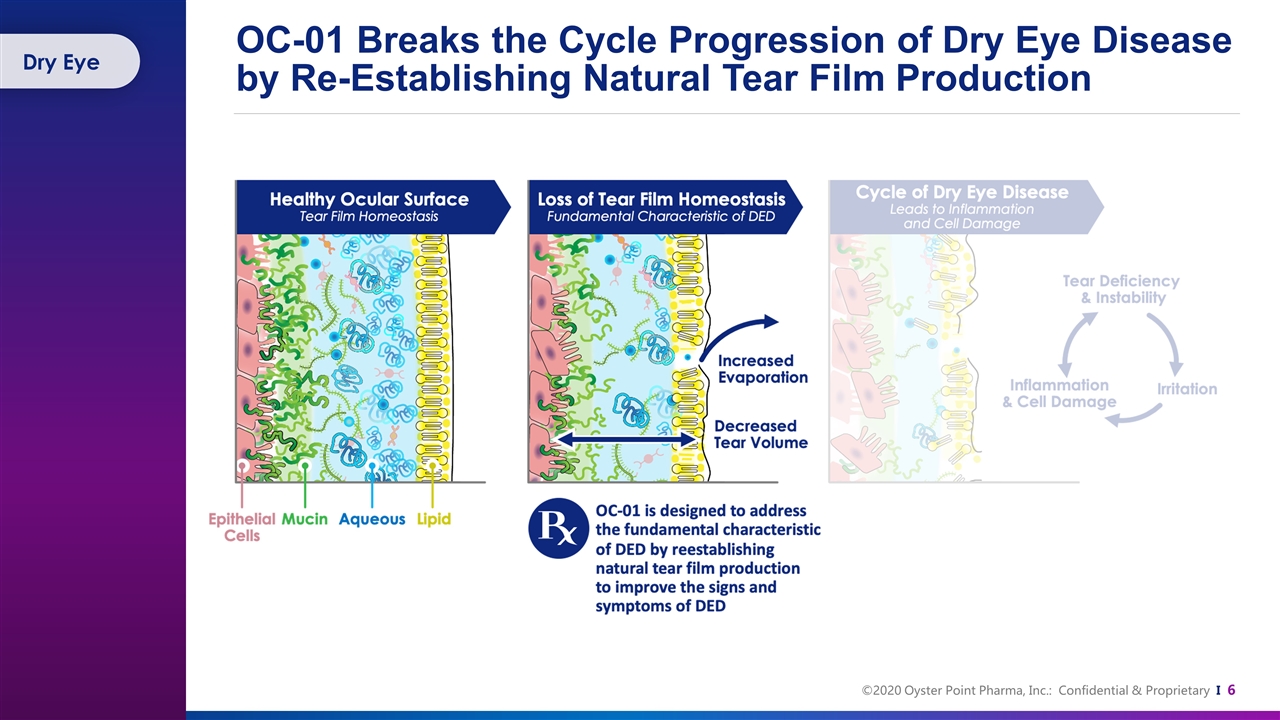
OC-01 Breaks the Cycle Progression of Dry Eye Disease by Re-Establishing Natural Tear Film Production Dry Eye

There is No Substitute for Natural Tear Film Natural tears contain a complex mixture of lipids, proteins, mucins, and electrolytes Over 1,500 proteins 5+ lipid classes 20+ mucins Growth factors, such as nerve growth factor (NGF) and epidermal growth factor (EGF), found in natural human tears, are critical regulators for corneal wound healing Klenkler, B., Sheardown, H., & Jones, L. (2007). Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. The ocular surface, 5(3), 228-239. Willcox, M. D., Argüeso, P., Georgiev, G. A., Holopainen, J. M., Laurie, G. W., Millar, T. J., ... & Suarez, T. (2017). TFOS DEWS II tear film report. The ocular surface, 15(3), 366-403. Contains growth factors and has anti-inflammatory and antimicrobial properties A healthy tear film lubricates and protects the eyes from injury and infection, washes away foreign particles, and contributes refractive power for clear vision Tear Film
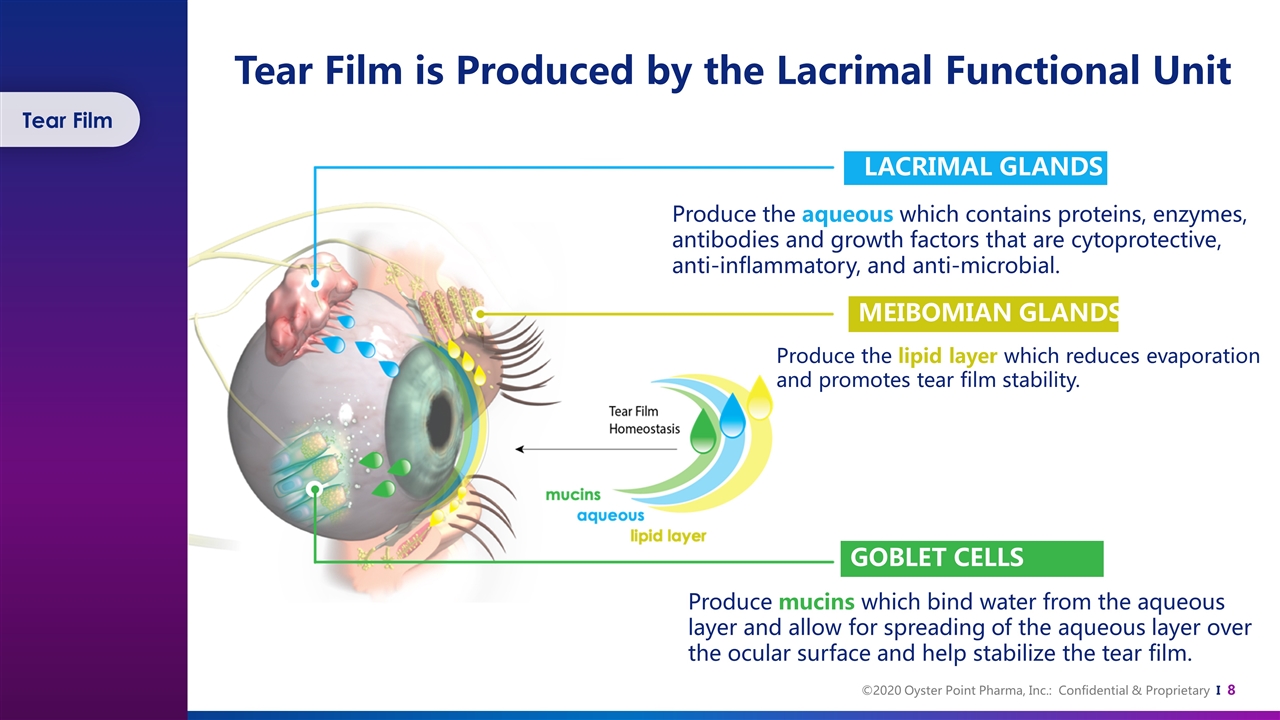
Tear Film is Produced by the Lacrimal Functional Unit LACRIMAL GLANDS Produce the aqueous which contains proteins, enzymes, antibodies and growth factors that are cytoprotective, anti-inflammatory, and anti-microbial. MEIBOMIAN GLANDS Produce the lipid layer which reduces evaporation and promotes tear film stability. GOBLET CELLS Produce mucins which bind water from the aqueous layer and allow for spreading of the aqueous layer over the ocular surface and help stabilize the tear film. Tear Film
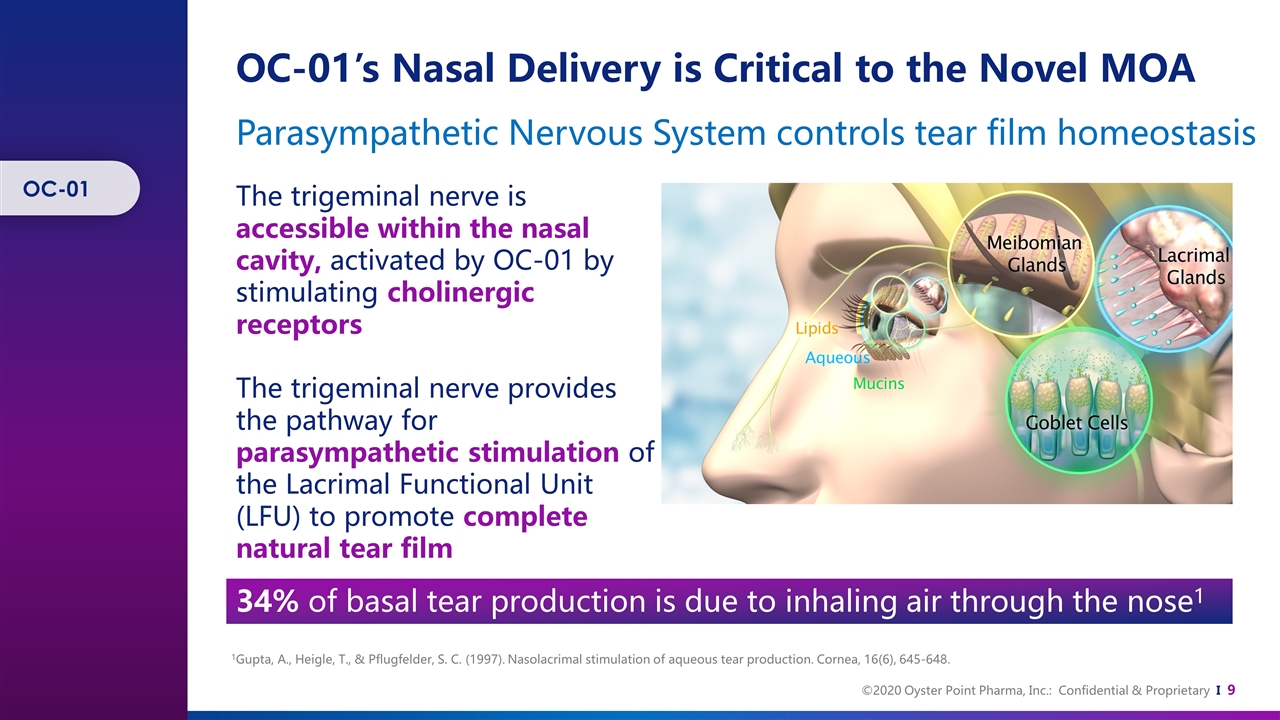
OC-01’s Nasal Delivery is Critical to the Novel MOA The trigeminal nerve is accessible within the nasal cavity, activated by OC-01 by stimulating cholinergic receptors The trigeminal nerve provides the pathway for parasympathetic stimulation of the Lacrimal Functional Unit (LFU) to promote complete natural tear film 1Gupta, A., Heigle, T., & Pflugfelder, S. C. (1997). Nasolacrimal stimulation of aqueous tear production. Cornea, 16(6), 645-648. Parasympathetic Nervous System controls tear film homeostasis 34% of basal tear production is due to inhaling air through the nose1 OC-01
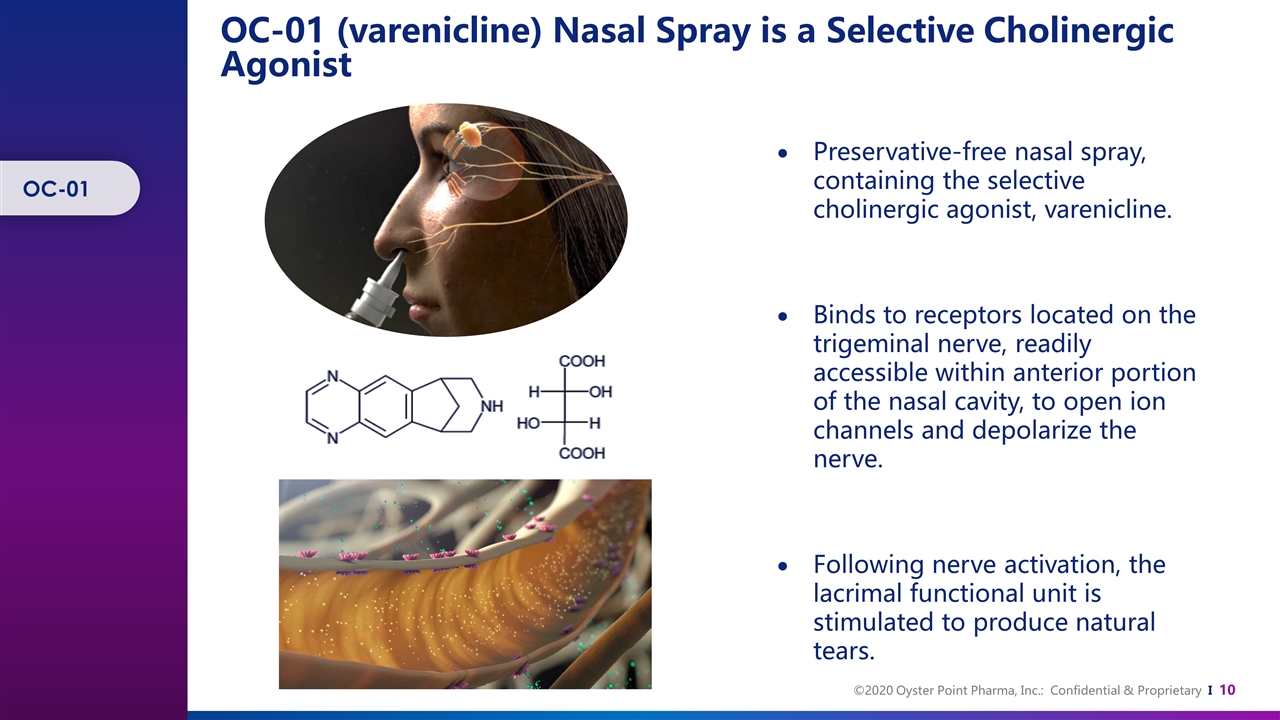
OC-01 (varenicline) Nasal Spray is a Selective Cholinergic Agonist Preservative-free nasal spray, containing the selective cholinergic agonist, varenicline. Binds to receptors located on the trigeminal nerve, readily accessible within anterior portion of the nasal cavity, to open ion channels and depolarize the nerve. Following nerve activation, the lacrimal functional unit is stimulated to produce natural tears. OC-01
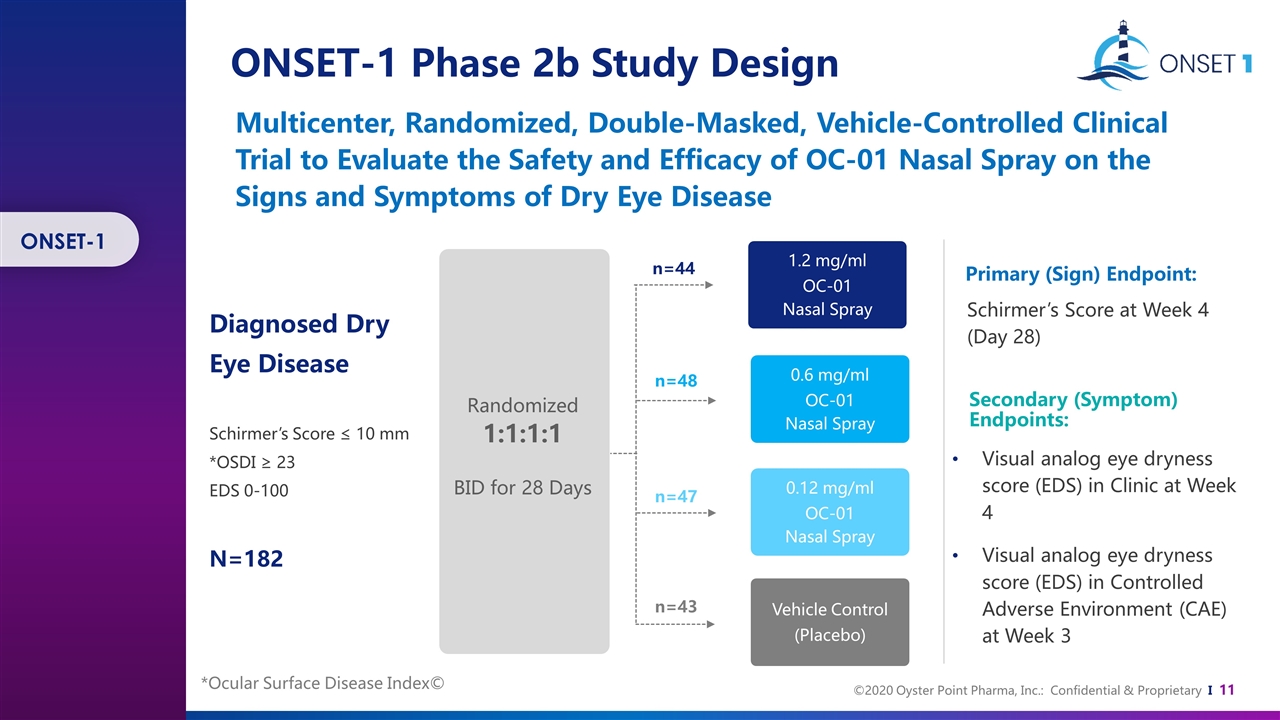
Multicenter, Randomized, Double-Masked, Vehicle-Controlled Clinical Trial to Evaluate the Safety and Efficacy of OC-01 Nasal Spray on the Signs and Symptoms of Dry Eye Disease ONSET-1 Phase 2b Study Design Primary (Sign) Endpoint: Secondary (Symptom) Endpoints: Schirmer’s Score at Week 4 (Day 28) Visual analog eye dryness score (EDS) in Clinic at Week 4 Visual analog eye dryness score (EDS) in Controlled Adverse Environment (CAE) at Week 3 Diagnosed Dry Eye Disease Schirmer’s Score ≤ 10 mm *OSDI ≥ 23 EDS 0-100 N=182 n=44 n=48 1.2 mg/ml OC-01 Nasal Spray 0.6 mg/ml OC-01 Nasal Spray 0.12 mg/ml OC-01 Nasal Spray Randomized 1:1:1:1 BID for 28 Days Vehicle Control (Placebo) n=47 n=43 *Ocular Surface Disease Index© ONSET-1
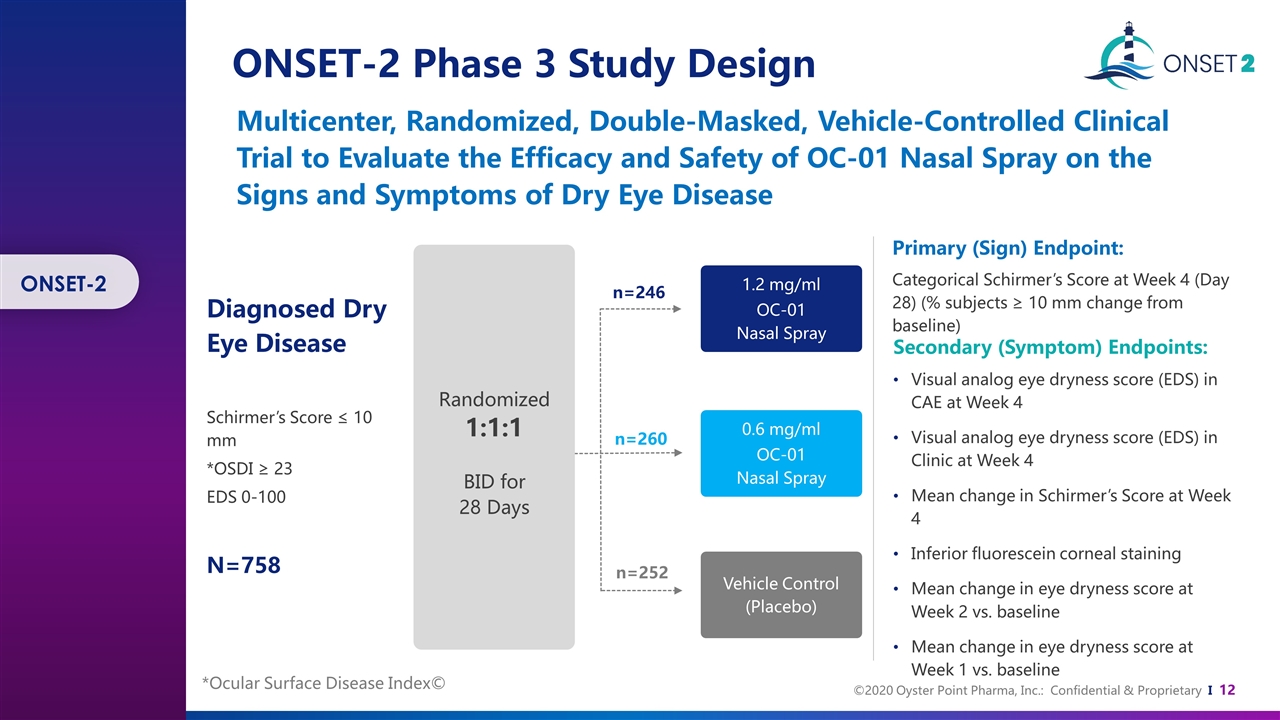
Diagnosed Dry Eye Disease Schirmer’s Score ≤ 10 mm *OSDI ≥ 23 EDS 0-100 N=758 n=246 n=260 n=252 1.2 mg/ml OC-01 Nasal Spray 0.6 mg/ml OC-01 Nasal Spray Vehicle Control (Placebo) Randomized 1:1:1 BID for 28 Days Primary (Sign) Endpoint: Secondary (Symptom) Endpoints: Categorical Schirmer’s Score at Week 4 (Day 28) (% subjects ≥ 10 mm change from baseline) Visual analog eye dryness score (EDS) in CAE at Week 4 Visual analog eye dryness score (EDS) in Clinic at Week 4 Mean change in Schirmer’s Score at Week 4 Inferior fluorescein corneal staining Mean change in eye dryness score at Week 2 vs. baseline Mean change in eye dryness score at Week 1 vs. baseline Multicenter, Randomized, Double-Masked, Vehicle-Controlled Clinical Trial to Evaluate the Efficacy and Safety of OC-01 Nasal Spray on the Signs and Symptoms of Dry Eye Disease ONSET-2 Phase 3 Study Design ONSET-2 *Ocular Surface Disease Index©
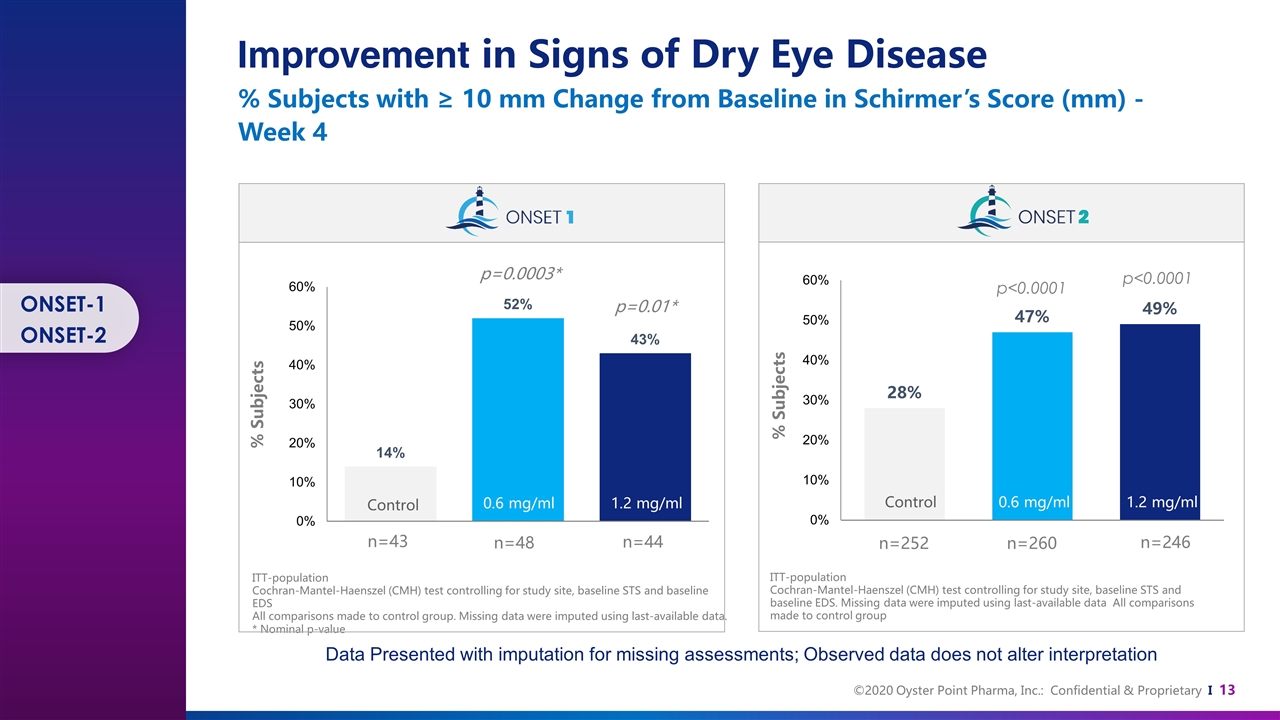
ITT-population Cochran-Mantel-Haenszel (CMH) test controlling for study site, baseline STS and baseline EDS All comparisons made to control group. Missing data were imputed using last-available data. * Nominal p-value 0.6 mg/ml 1.2 mg/ml n=43 n=48 n=44 Control 0.6 mg/ml 1.2 mg/ml n=252 n=260 n=246 p<0.0001 p<0.0001 p=0.0003* p=0.01* Control 0.6 mg/ml 1.2 mg/ml ITT-population Cochran-Mantel-Haenszel (CMH) test controlling for study site, baseline STS and baseline EDS. Missing data were imputed using last-available data All comparisons made to control group % Subjects % Subjects Improvement in Signs of Dry Eye Disease % Subjects with ≥ 10 mm Change from Baseline in Schirmer’s Score (mm) - Week 4 Data Presented with imputation for missing assessments; Observed data does not alter interpretation ONSET-1 ONSET-2
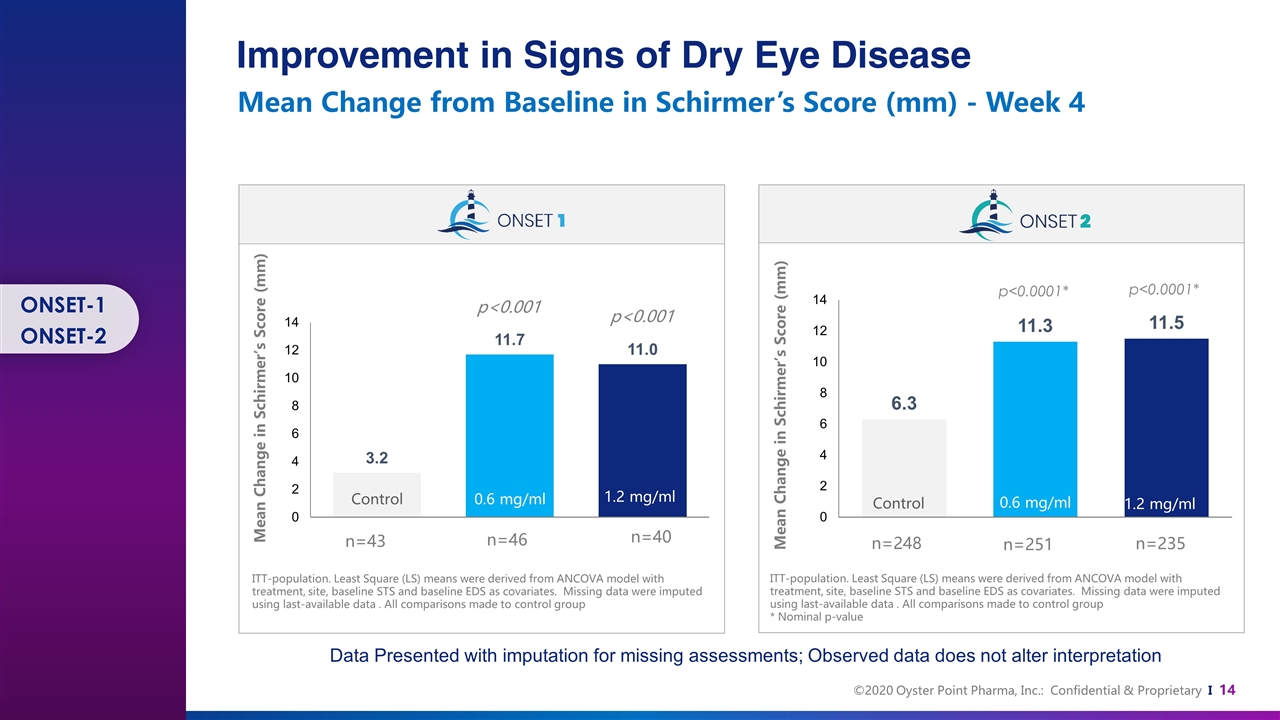
0.6 mg/ml 1.2 mg/ml n=43 n=46 n=40 0.6 mg/ml n=248 n=251 n=235 p<0.0001* p<0.0001* p<0.001 p<0.001 Control 0.6 mg/ml 1.2 mg/ml ITT-population. Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS and baseline EDS as covariates. Missing data were imputed using last-available data . All comparisons made to control group * Nominal p-value Control 1.2 mg/ml 0.6 mg/ml Control 1.2 mg/ml Mean Change in Schirmer’s Score (mm) Mean Change in Schirmer’s Score (mm) Improvement in Signs of Dry Eye Disease Mean Change from Baseline in Schirmer’s Score (mm) - Week 4 Data Presented with imputation for missing assessments; Observed data does not alter interpretation ITT-population. Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS and baseline EDS as covariates. Missing data were imputed using last-available data . All comparisons made to control group ONSET-1 ONSET-2
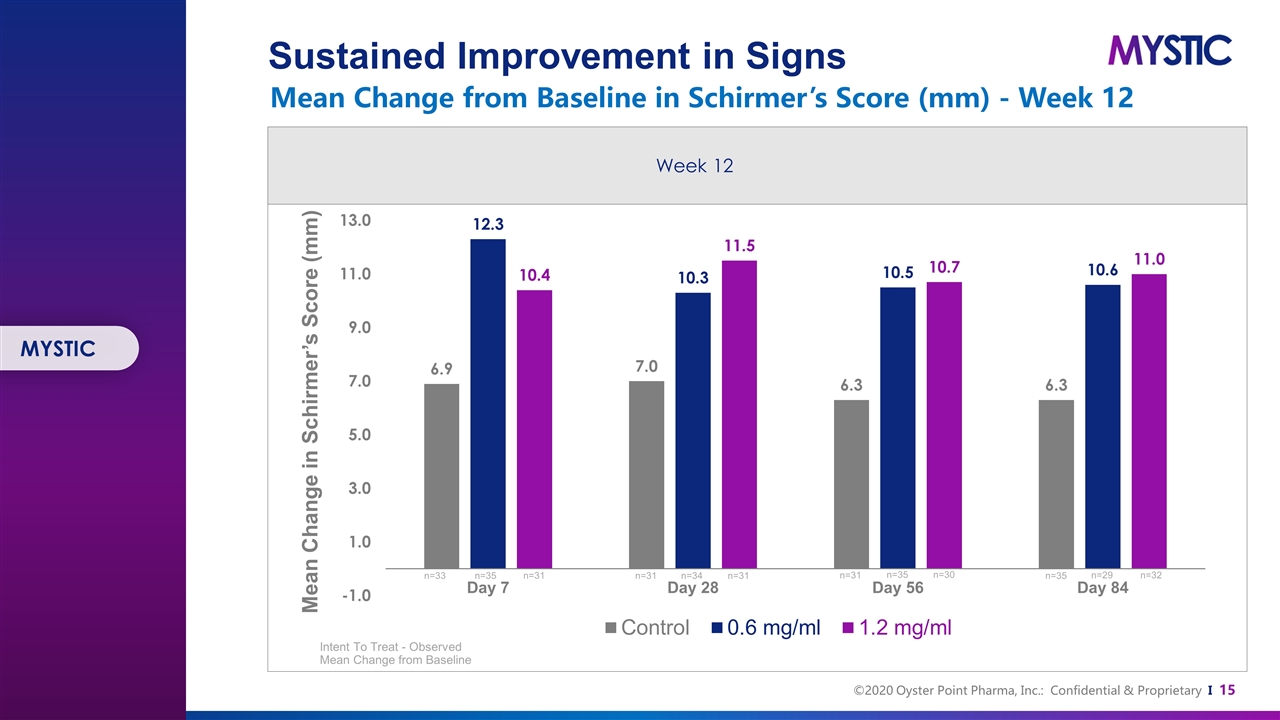
Mean Change in Schirmer’s Score (mm) Intent To Treat - Observed Mean Change from Baseline n=33 n=35 n=31 n=31 n=34 n=31 n=31 n=35 n=30 n=35 n=29 n=32 MYSTIC Sustained Improvement in Signs Mean Change from Baseline in Schirmer’s Score (mm) - Week 12 Week 12
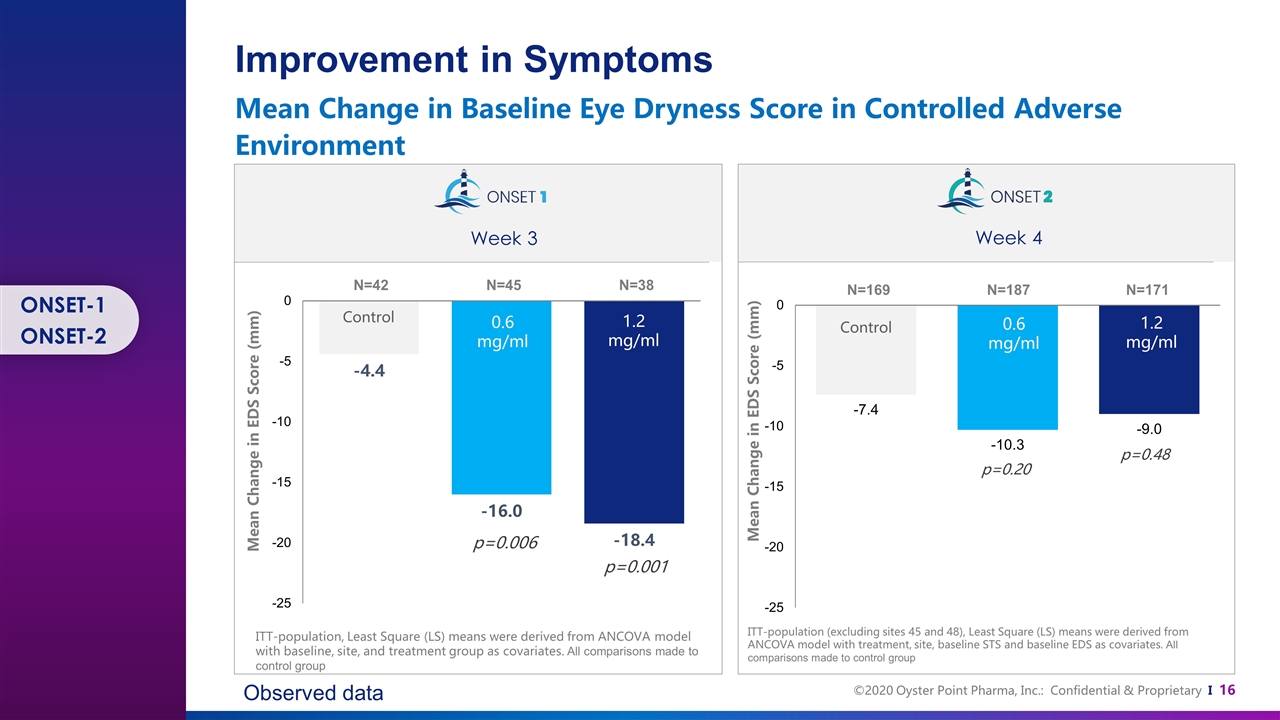
Control Control 0.6 mg/ml 0.6 mg/ml 1.2 mg/ml 1.2 mg/ml N=38 N=45 N=42 N=171 N=187 N=169 p=0.001 ITT-population, Least Square (LS) means were derived from ANCOVA model with baseline, site, and treatment group as covariates. All comparisons made to control group ITT-population (excluding sites 45 and 48), Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS and baseline EDS as covariates. All comparisons made to control group p=0.48 p=0.20 Mean Change in EDS Score (mm) Mean Change in EDS Score (mm) Improvement in Symptoms Mean Change in Baseline Eye Dryness Score in Controlled Adverse Environment p=0.006 Week 3 Week 4 Observed data ONSET-1 ONSET-2
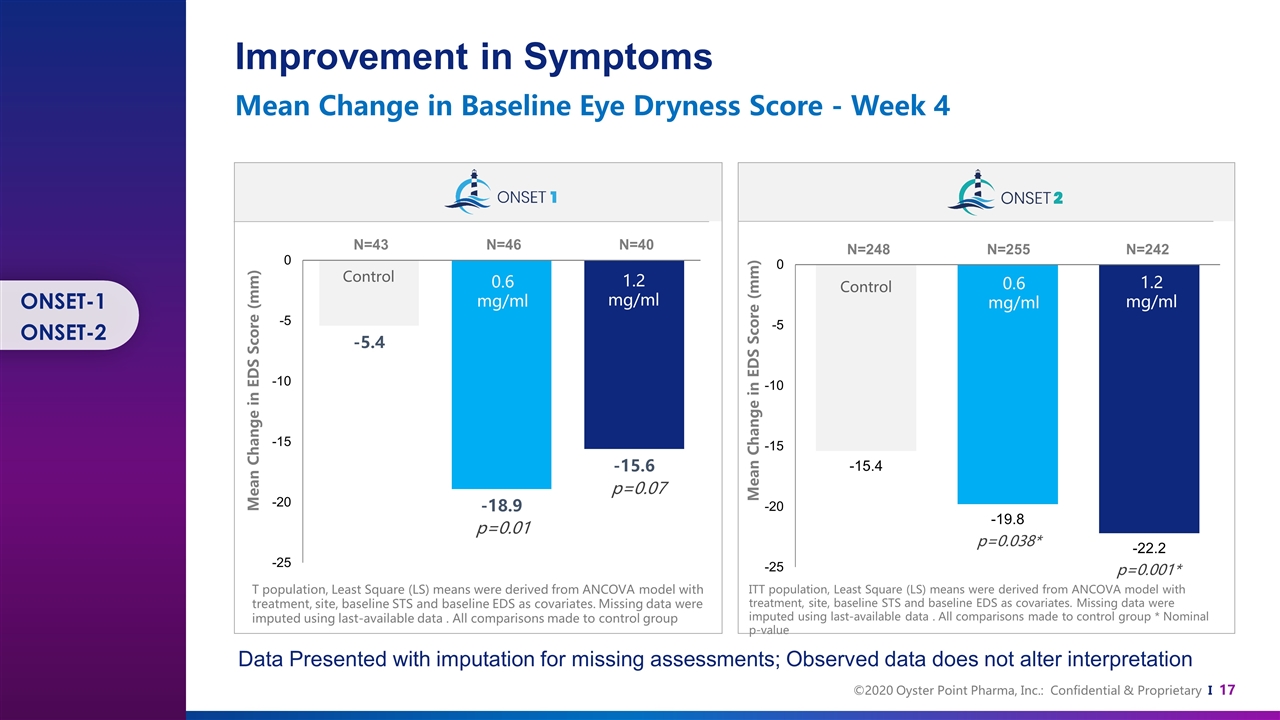
Control Control 0.6 mg/ml 0.6 mg/ml 1.2 mg/ml 1.2 mg/ml N=40 N=46 N=43 N=242 N=255 N=248 p=0.01 p=0.07 T population, Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS and baseline EDS as covariates. Missing data were imputed using last-available data . All comparisons made to control group ITT population, Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS and baseline EDS as covariates. Missing data were imputed using last-available data . All comparisons made to control group * Nominal p-value p=0.001* p=0.038* Mean Change in EDS Score (mm) Mean Change in EDS Score (mm) Improvement in Symptoms Mean Change in Baseline Eye Dryness Score - Week 4 Data Presented with imputation for missing assessments; Observed data does not alter interpretation ONSET-1 ONSET-2
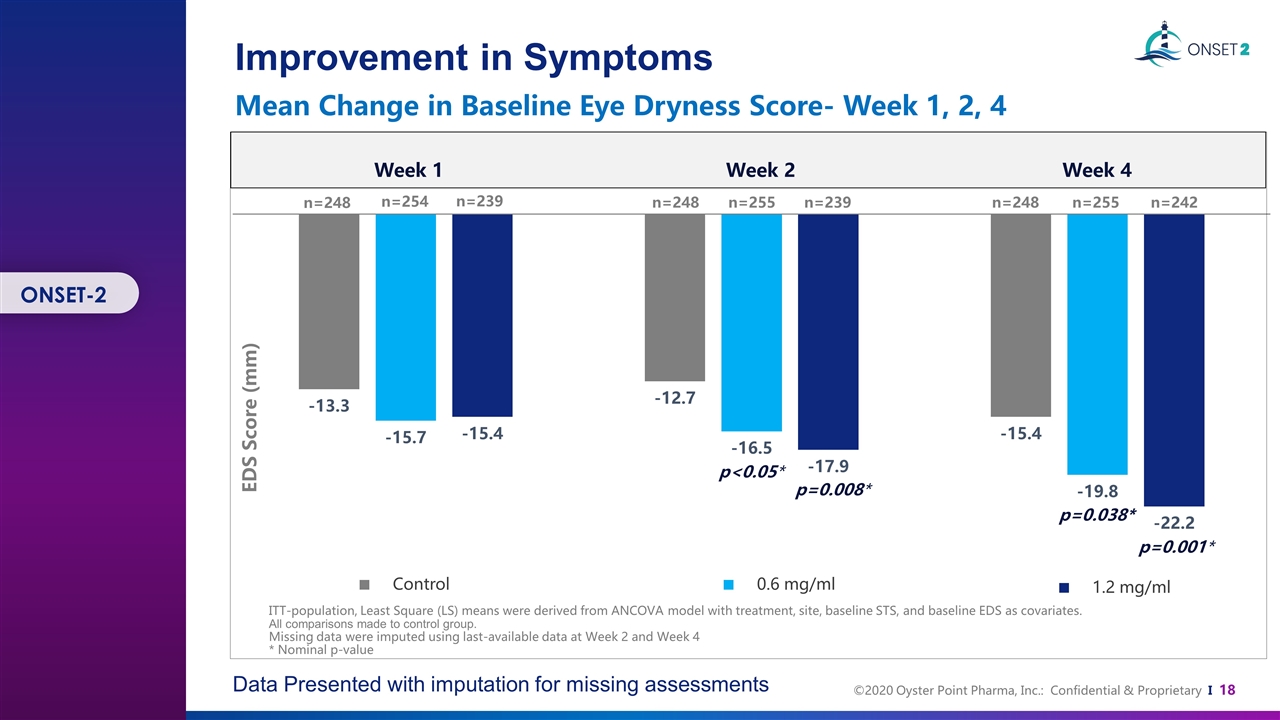
Improvement in Symptoms Mean Change in Baseline Eye Dryness Score- Week 1, 2, 4 Data Presented with imputation for missing assessments P<0.001 EDS Score (mm) ITT-population, Least Square (LS) means were derived from ANCOVA model with treatment, site, baseline STS, and baseline EDS as covariates. All comparisons made to control group. Missing data were imputed using last-available data at Week 2 and Week 4 * Nominal p-value Control 0.6 mg/ml 1.2 mg/ml p=0.008* p=0.001* p<0.05* Week 4 n=248 n=254 n=239 n=248 n=255 n=239 n=248 n=255 n=242 Week 1 Week 2 p=0.038* ONSET-2
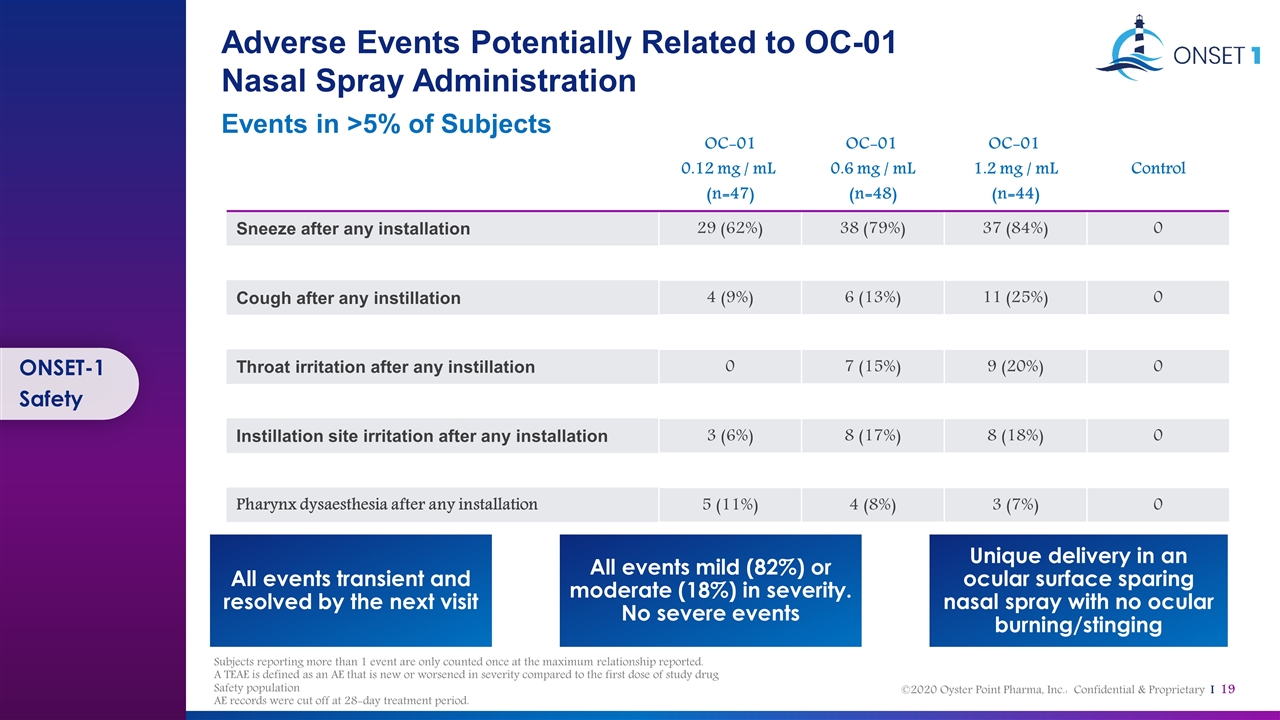
Adverse Events Potentially Related to OC-01 Nasal Spray Administration Events in >5% of Subjects OC-01 0.12 mg / mL (n=47) OC-01 0.6 mg / mL (n=48) OC-01 1.2 mg / mL (n=44) Control Sneeze after any installation 29 (62%) 38 (79%) 37 (84%) 0 Cough after any instillation 4 (9%) 6 (13%) 11 (25%) 0 Throat irritation after any instillation 0 7 (15%) 9 (20%) 0 Instillation site irritation after any installation 3 (6%) 8 (17%) 8 (18%) 0 Pharynx dysaesthesia after any installation 5 (11%) 4 (8%) 3 (7%) 0 Unique delivery in an ocular surface sparing nasal spray with no ocular burning/stinging All events mild (82%) or moderate (18%) in severity. No severe events All events transient and resolved by the next visit ONSET-1 Safety Subjects reporting more than 1 event are only counted once at the maximum relationship reported. A TEAE is defined as an AE that is new or worsened in severity compared to the first dose of study drug Safety population AE records were cut off at 28-day treatment period.
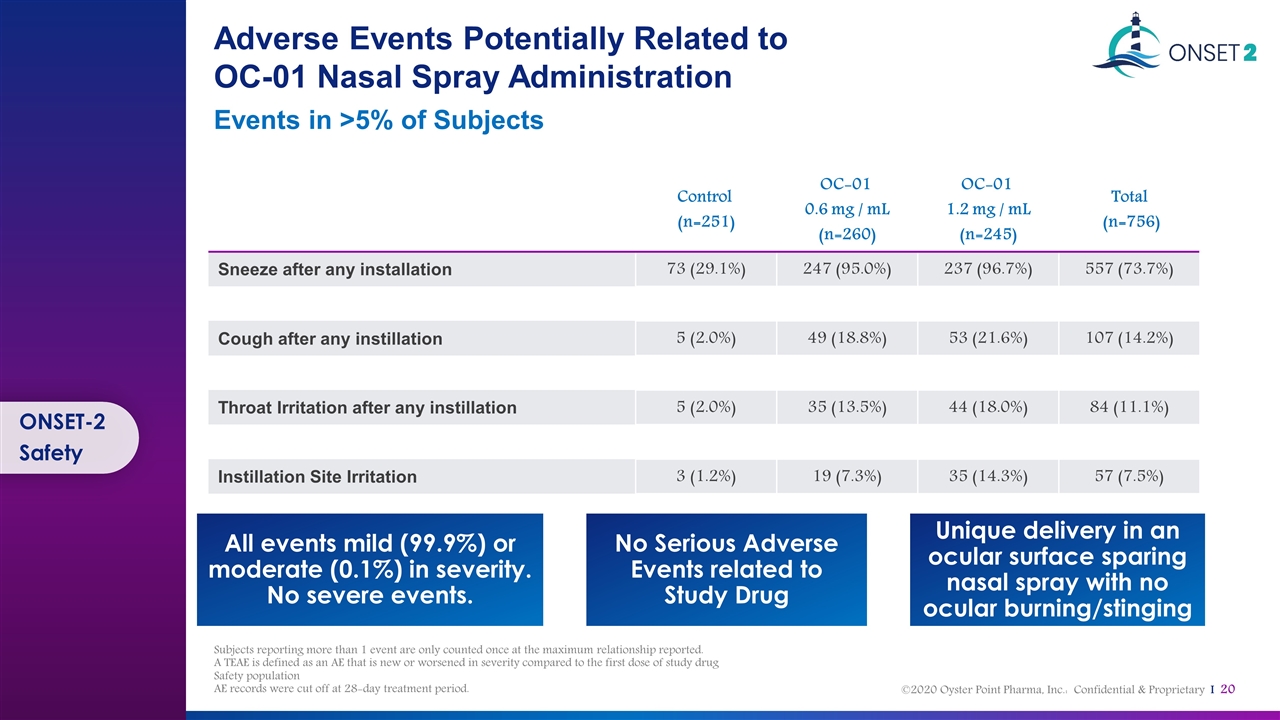
Adverse Events Potentially Related to OC-01 Nasal Spray Administration Events in >5% of Subjects ONSET-2 Safety Control (n=251) OC-01 0.6 mg / mL (n=260) OC-01 1.2 mg / mL (n=245) Total (n=756) Sneeze after any installation 73 (29.1%) 247 (95.0%) 237 (96.7%) 557 (73.7%) Cough after any instillation 5 (2.0%) 49 (18.8%) 53 (21.6%) 107 (14.2%) Throat Irritation after any instillation 5 (2.0%) 35 (13.5%) 44 (18.0%) 84 (11.1%) Instillation Site Irritation 3 (1.2%) 19 (7.3%) 35 (14.3%) 57 (7.5%) Unique delivery in an ocular surface sparing nasal spray with no ocular burning/stinging No Serious Adverse Events related to Study Drug All events mild (99.9%) or moderate (0.1%) in severity. No severe events. Subjects reporting more than 1 event are only counted once at the maximum relationship reported. A TEAE is defined as an AE that is new or worsened in severity compared to the first dose of study drug Safety population AE records were cut off at 28-day treatment period.
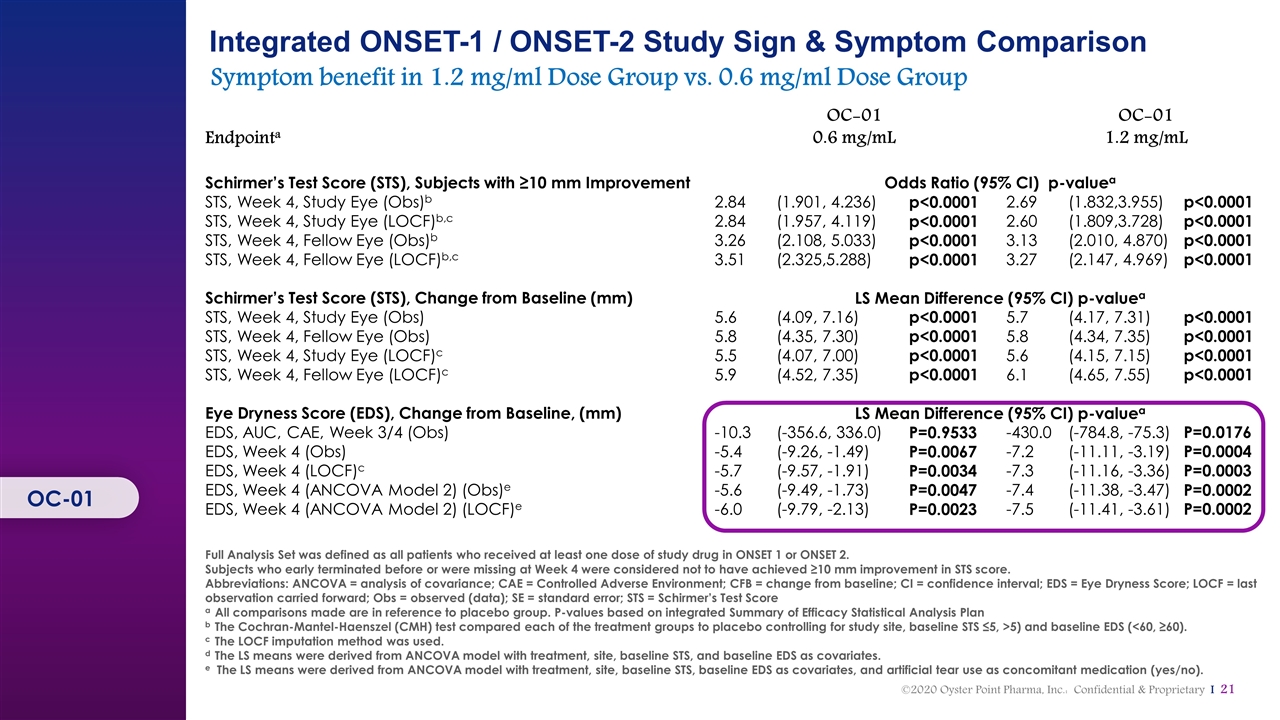
Integrated ONSET-1 / ONSET-2 Study Sign & Symptom Comparison Symptom benefit in 1.2 mg/ml Dose Group vs. 0.6 mg/ml Dose Group Endpointa OC‑01 0.6 mg/mL OC‑01 1.2 mg/mL Schirmer’s Test Score (STS), Subjects with ≥10 mm Improvement Odds Ratio (95% CI) p‑valuea STS, Week 4, Study Eye (Obs)b 2.84 (1.901, 4.236) p<0.0001 2.69 (1.832,3.955) p<0.0001 STS, Week 4, Study Eye (LOCF)b,c 2.84 (1.957, 4.119) p<0.0001 2.60 (1.809,3.728) p<0.0001 STS, Week 4, Fellow Eye (Obs)b 3.26 (2.108, 5.033) p<0.0001 3.13 (2.010, 4.870) p<0.0001 STS, Week 4, Fellow Eye (LOCF)b,c 3.51 (2.325,5.288) p<0.0001 3.27 (2.147, 4.969) p<0.0001 Schirmer’s Test Score (STS), Change from Baseline (mm) LS Mean Difference (95% CI) p‑valuea STS, Week 4, Study Eye (Obs) 5.6 (4.09, 7.16) p<0.0001 5.7 (4.17, 7.31) p<0.0001 STS, Week 4, Fellow Eye (Obs) 5.8 (4.35, 7.30) p<0.0001 5.8 (4.34, 7.35) p<0.0001 STS, Week 4, Study Eye (LOCF)c 5.5 (4.07, 7.00) p<0.0001 5.6 (4.15, 7.15) p<0.0001 STS, Week 4, Fellow Eye (LOCF)c 5.9 (4.52, 7.35) p<0.0001 6.1 (4.65, 7.55) p<0.0001 Eye Dryness Score (EDS), Change from Baseline, (mm) LS Mean Difference (95% CI) p‑valuea EDS, AUC, CAE, Week 3/4 (Obs) -10.3 (-356.6, 336.0) P=0.9533 -430.0 (-784.8, -75.3) P=0.0176 EDS, Week 4 (Obs) -5.4 (-9.26, -1.49) P=0.0067 -7.2 (-11.11, -3.19) P=0.0004 EDS, Week 4 (LOCF)c -5.7 (-9.57, -1.91) P=0.0034 -7.3 (-11.16, -3.36) P=0.0003 EDS, Week 4 (ANCOVA Model 2) (Obs)e -5.6 (-9.49, -1.73) P=0.0047 -7.4 (-11.38, -3.47) P=0.0002 EDS, Week 4 (ANCOVA Model 2) (LOCF)e -6.0 (-9.79, -2.13) P=0.0023 -7.5 (-11.41, -3.61) P=0.0002 Full Analysis Set was defined as all patients who received at least one dose of study drug in ONSET 1 or ONSET 2. Subjects who early terminated before or were missing at Week 4 were considered not to have achieved ≥10 mm improvement in STS score. Abbreviations: ANCOVA = analysis of covariance; CAE = Controlled Adverse Environment; CFB = change from baseline; CI = confidence interval; EDS = Eye Dryness Score; LOCF = last observation carried forward; Obs = observed (data); SE = standard error; STS = Schirmer’s Test Score aAll comparisons made are in reference to placebo group. P-values based on integrated Summary of Efficacy Statistical Analysis Plan bThe Cochran‑Mantel‑Haenszel (CMH) test compared each of the treatment groups to placebo controlling for study site, baseline STS ≤5, >5) and baseline EDS (<60, ≥60). cThe LOCF imputation method was used. dThe LS means were derived from ANCOVA model with treatment, site, baseline STS, and baseline EDS as covariates. e The LS means were derived from ANCOVA model with treatment, site, baseline STS, baseline EDS as covariates, and artificial tear use as concomitant medication (yes/no). OC-01
Next Steps for OC-01 (varenicline) Nasal Spray OC-01 New Drug Application (NDA) Submission to U.S. Food and Drug Administration (FDA) for Signs and Symptoms of Dry Eye Disease Remains on Track for Q4 2020 Planned U.S. launch of OC-01 in Q4 2021, if FDA-approved 1.2 mg/ml Dose Favored Based on Symptom Benefit Integrated data indicates signs and symptoms benefit in both dose groups Schirmer’s scores similar across both dose groups Symptom benefit trends in favor of 1.2 mg/ml dose group Integrated AE profile consistent and predominantly mild in severity OC-01
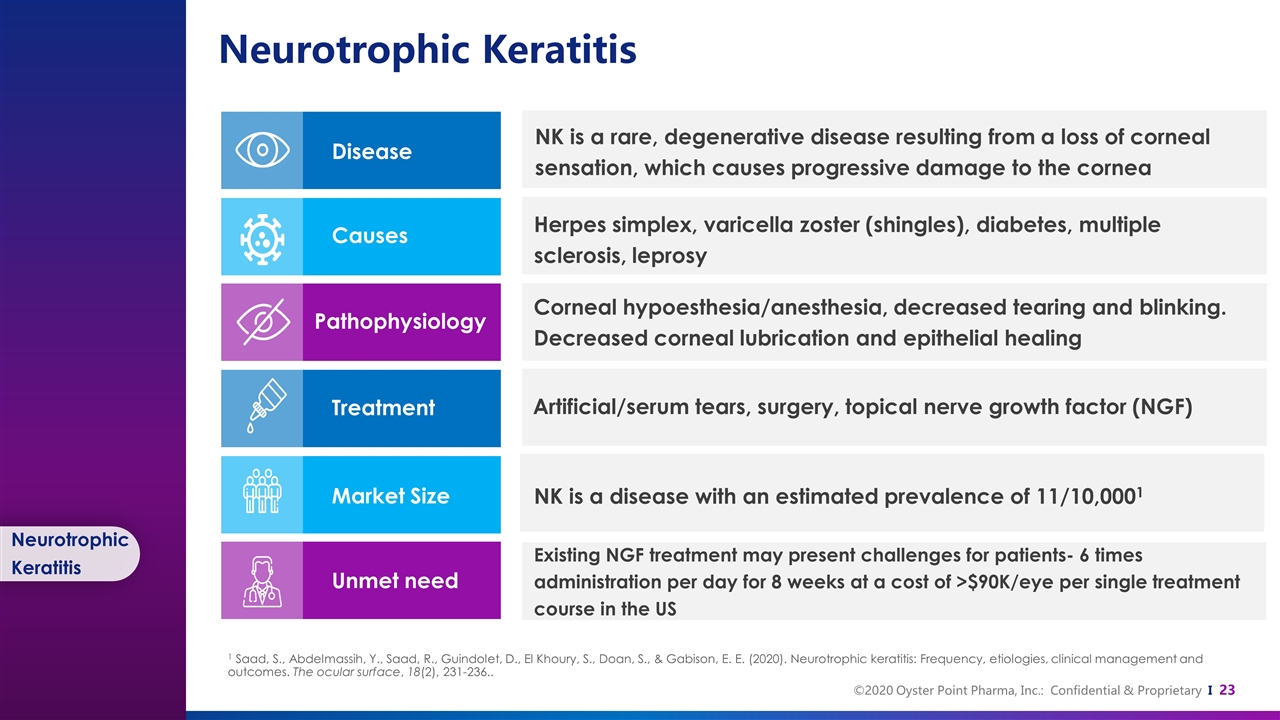
Neurotrophic Keratitis Neurotrophic Keratitis Disease Causes Pathophysiology NK is a rare, degenerative disease resulting from a loss of corneal sensation, which causes progressive damage to the cornea Herpes simplex, varicella zoster (shingles), diabetes, multiple sclerosis, leprosy Corneal hypoesthesia/anesthesia, decreased tearing and blinking. Decreased corneal lubrication and epithelial healing Artificial/serum tears, surgery, topical nerve growth factor (NGF) NK is a disease with an estimated prevalence of 11/10,0001 Existing NGF treatment may present challenges for patients- 6 times administration per day for 8 weeks at a cost of >$90K/eye per single treatment course in the US Treatment Market Size Unmet need 1 Saad, S., Abdelmassih, Y., Saad, R., Guindolet, D., El Khoury, S., Doan, S., & Gabison, E. E. (2020). Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. The ocular surface, 18(2), 231-236..
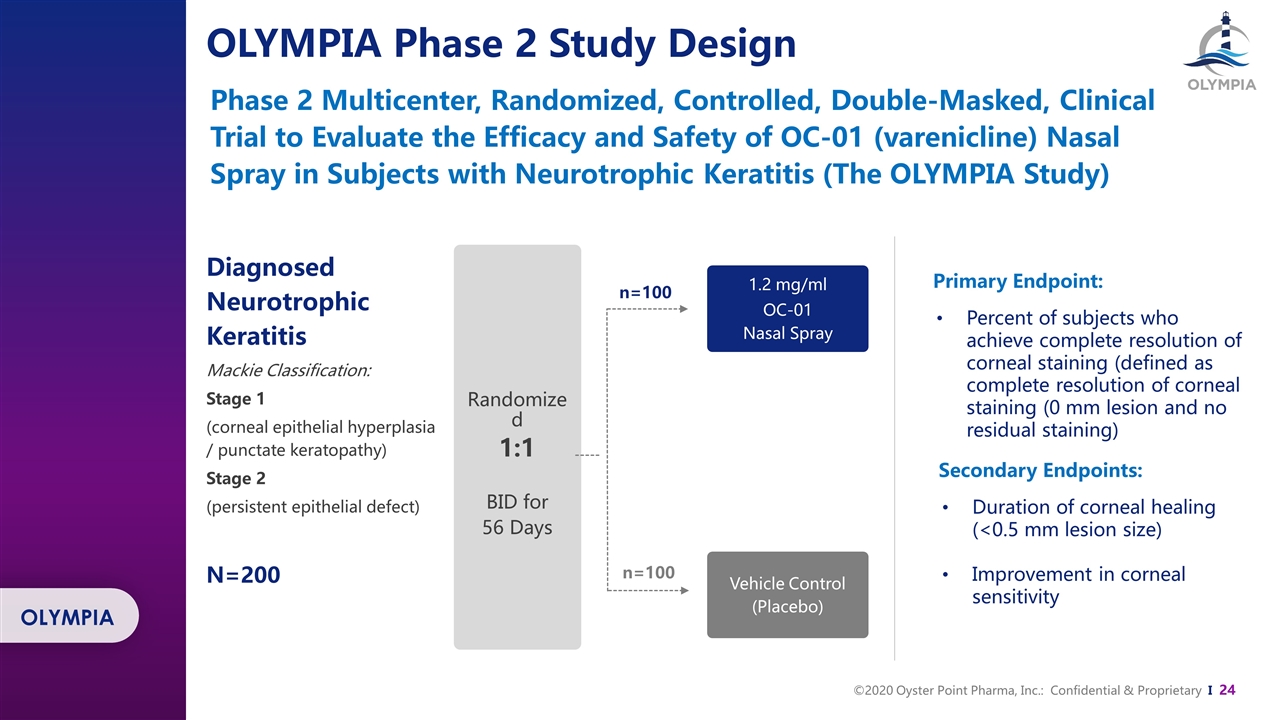
Diagnosed Neurotrophic Keratitis Mackie Classification: Stage 1 (corneal epithelial hyperplasia / punctate keratopathy) Stage 2 (persistent epithelial defect) N=200 n=100 n=100 1.2 mg/ml OC-01 Nasal Spray Vehicle Control (Placebo) Randomized 1:1 BID for 56 Days Phase 2 Multicenter, Randomized, Controlled, Double-Masked, Clinical Trial to Evaluate the Efficacy and Safety of OC-01 (varenicline) Nasal Spray in Subjects with Neurotrophic Keratitis (The OLYMPIA Study) OLYMPIA Phase 2 Study Design OLYMPIA Primary Endpoint: Percent of subjects who achieve complete resolution of corneal staining (defined as complete resolution of corneal staining (0 mm lesion and no residual staining) Secondary Endpoints: Duration of corneal healing (<0.5 mm lesion size) Improvement in corneal sensitivity

Strong Global IP Position U.S. license granted for varenicline tartrate by Pfizer Five issued U.S. patents (expiration as early as 2035) relating to methods and/or formulations Patents relating to methods of treating dry eye disease, increasing tear production, and improving ocular discomfort using varenicline, as well as pharmaceutical formulations for local nasal administration of varenicline Global Filing: Issuance in Europe, Canada, China, Singapore Initiated: Australia, Brazil, Eurasia (includes Russia), Europe, Hong Kong (via EP), Israel, Japan, Korea, Malaysia, Mexico, Philippines, South Africa Ex-U.S. expiration as early as 2035 OC-01 (varenicline) Nasal Spray:

Patients and Doctors Need Additional Options OF EYE DOCTORS state they can adequately treat dry eye patients with current options1. 10% 1 2018 ZS Market Research ECP (n= 451) and Patient (n=146) ONLY
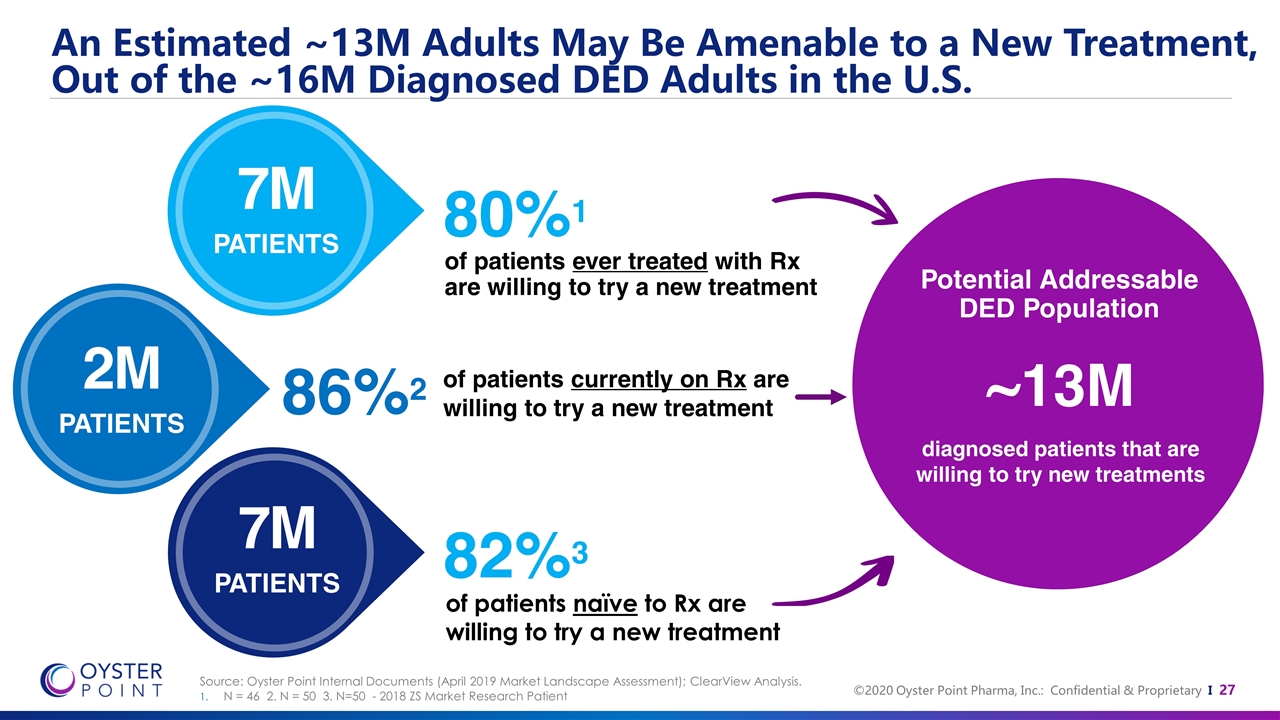
An Estimated ~13M Adults May Be Amenable to a New Treatment, Out of the ~16M Diagnosed DED Adults in the U.S. ~13M diagnosed patients that are willing to try new treatments of patients ever treated with Rx are willing to try a new treatment Potential Addressable DED Population 7M PATIENTS 2M PATIENTS of patients currently on Rx are willing to try a new treatment 86%2 80%1 7M PATIENTS 82%3 of patients naïve to Rx are willing to try a new treatment Source: Oyster Point Internal Documents (April 2019 Market Landscape Assessment); ClearView Analysis. N = 46 2. N = 50 3. N=50 - 2018 ZS Market Research Patient
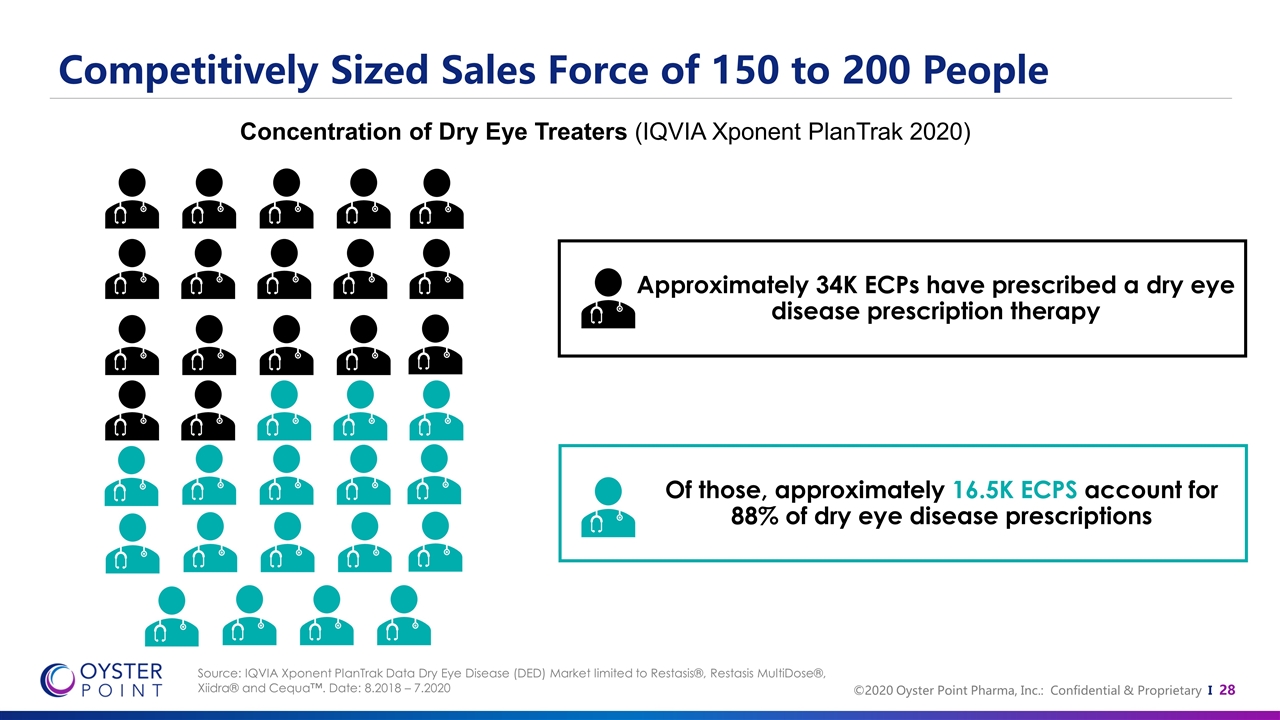
Competitively Sized Sales Force of 150 to 200 People Source: IQVIA Xponent PlanTrak Data Dry Eye Disease (DED) Market limited to Restasis®, Restasis MultiDose®, Xiidra® and Cequa™. Date: 8.2018 – 7.2020 Concentration of Dry Eye Treaters (IQVIA Xponent PlanTrak 2020) Approximately 34K ECPs have prescribed a dry eye disease prescription therapy Of those, approximately 16.5K ECPS account for 88% of dry eye disease prescriptions
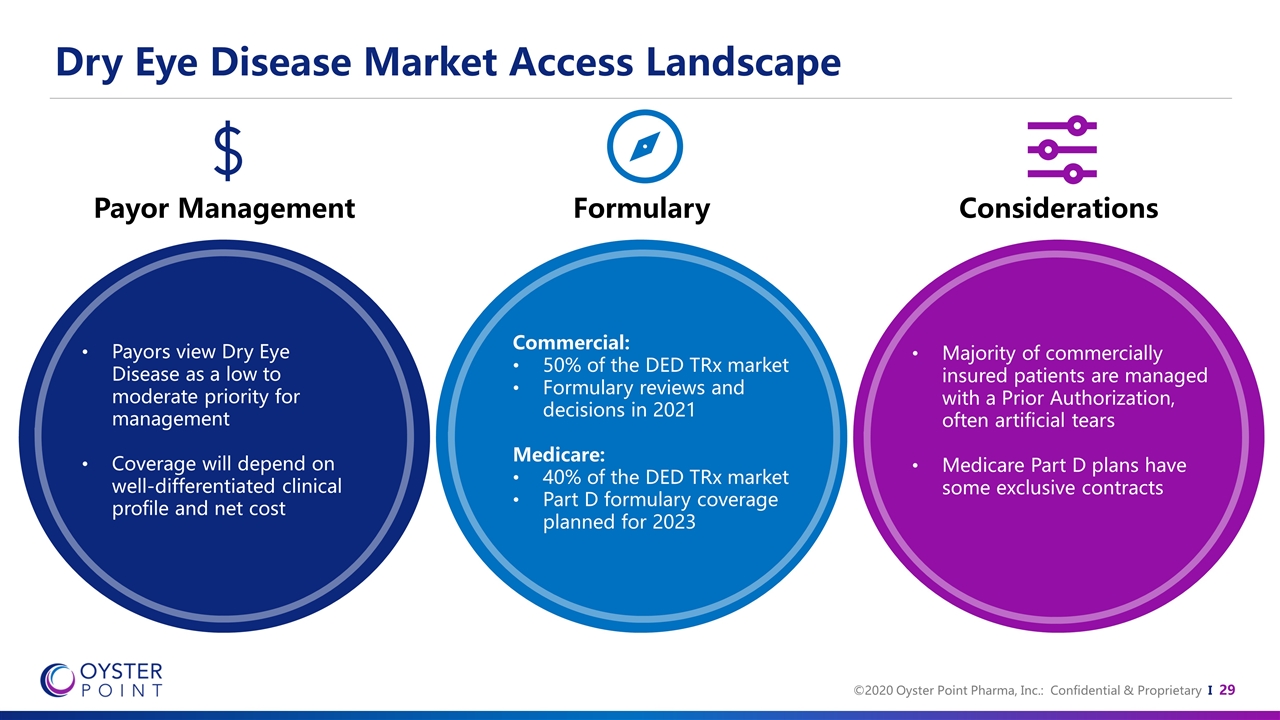
Dry Eye Disease Market Access Landscape Payor Management Formulary Considerations Payors view Dry Eye Disease as a low to moderate priority for management Coverage will depend on well-differentiated clinical profile and net cost Commercial: 50% of the DED TRx market Formulary reviews and decisions in 2021 Medicare: 40% of the DED TRx market Part D formulary coverage planned for 2023 Majority of commercially insured patients are managed with a Prior Authorization, often artificial tears Medicare Part D plans have some exclusive contracts

Preparing for Launch of OC-01 Nasal Spray We are focused on addressing the unmet needs of a broad audience: The mild, moderate, and severe Dry Eye patient The Eye Care Professionals (ECPs), ophthalmologists and optometrists, that take care of them We plan to enable patient access to OC-01 nasal spray: Strategic contracting for broad payor coverage Comprehensive pharmacy distribution Patient support services and access programs We are building a world-class medical affairs and commercial organization to support launch of our first therapy in the U.S.: Leadership team with extensive Eye Care experience Competitively sized specialty sales force of 150 to 200 representatives Targeting 16,500 ECPs or ~88% of current prescriber base of DED therapeutics We aim to provide education and marketing campaigns to the ECP, Patient and Consumer audiences: Pre-launch – Disease State Education to the ECP audience on the importance of tear film homeostasis and the role of natural tears Launch - Broad ECP and patient education and marketing with focused direct-to-consumer campaigns leveraging novel MOA & nasal spray
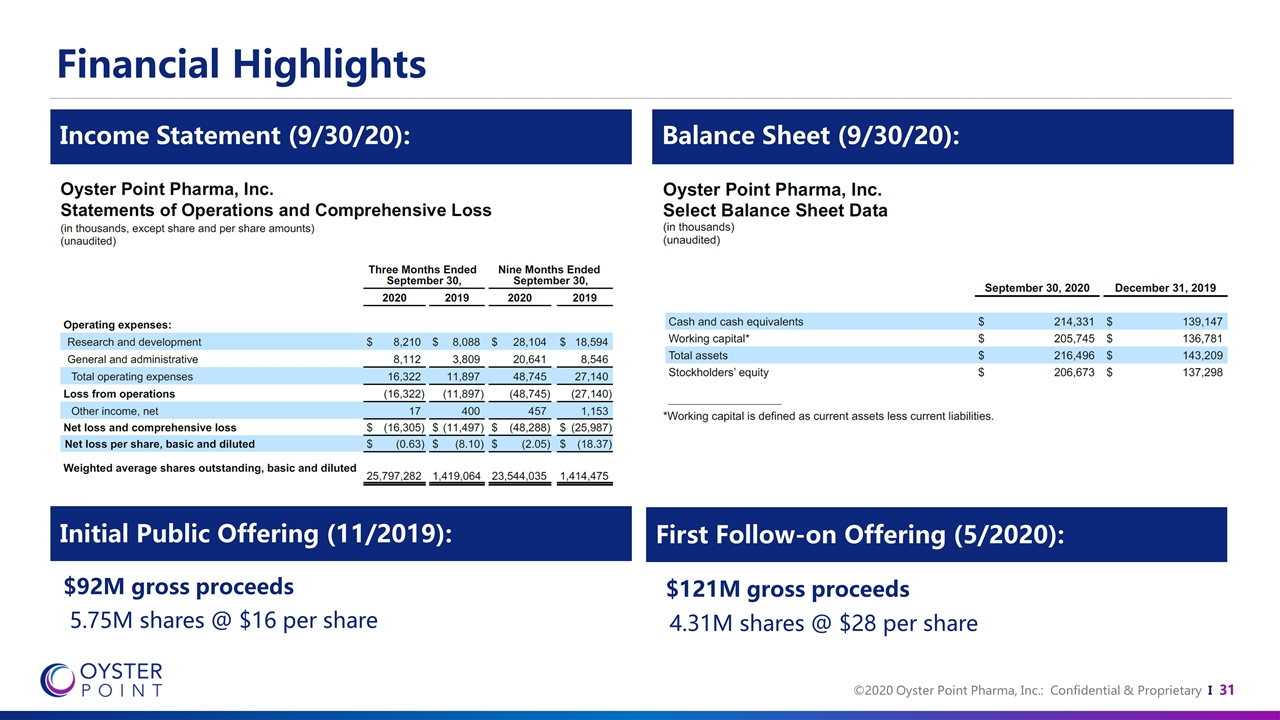
Financial Highlights First Follow-on Offering (5/2020): Initial Public Offering (11/2019): $92M gross proceeds 5.75M shares @ $16 per share $121M gross proceeds 4.31M shares @ $28 per share Balance Sheet (9/30/20): Income Statement (9/30/20):

NASDAQ: OYST































