Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
BTAI similar filings
- 3 Jan 19 Regulation FD Disclosure
- 27 Dec 18 BioXcel Therapeutics Receives FDA Fast Track Designation for BXCL501 for Acute Treatment of Agitation
- 20 Dec 18 BioXcel Therapeutics Doses First Subjects in Pharmacokinetic (Bioavailability) and Safety Study of BXCL501 for the Acute Treatment of Agitation
- 12 Dec 18 Regulation FD Disclosure
- 12 Dec 18 BioXcel Therapeutics Announces FDA Acceptance of IND for Lead
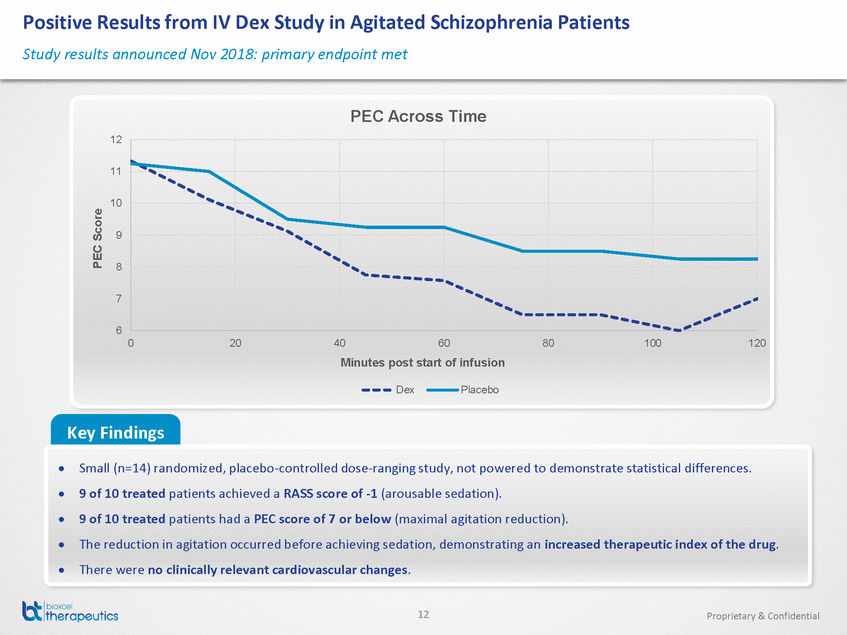
- 14 Nov 18 BioXcel Therapeutics Reports Positive Results from Study in Agitated Schizophrenia Patients Supporting BXCL501 Clinical Development
- 9 Nov 18 BioXcel Therapeutics Reports Third Quarter 2018 Quarterly Results and Provides Business Update
Filing view
External links