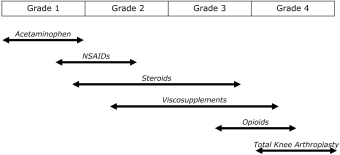
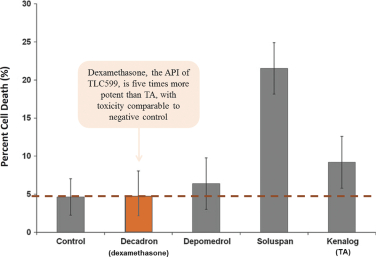
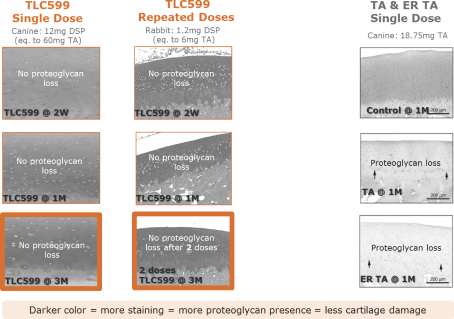
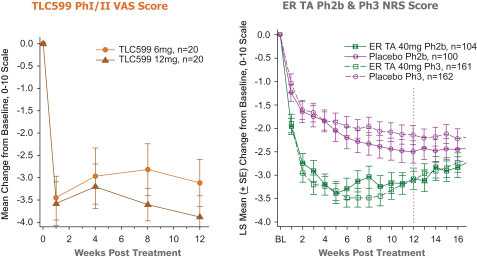
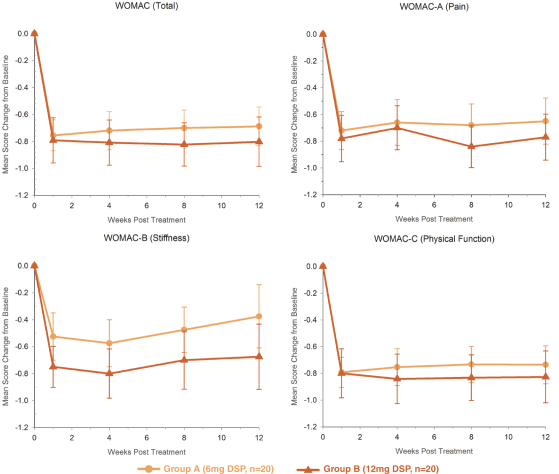
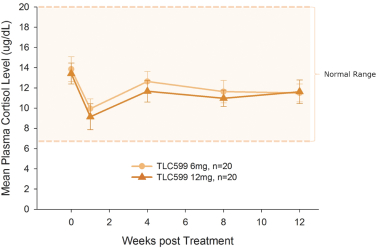
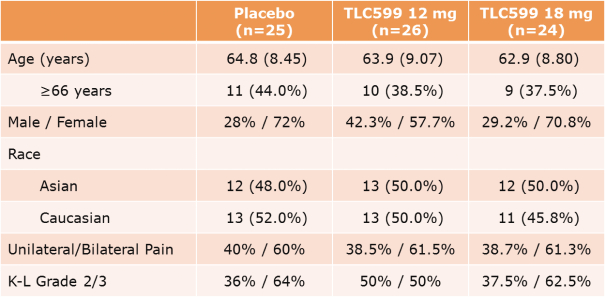
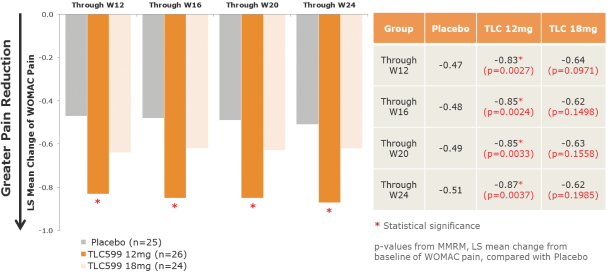
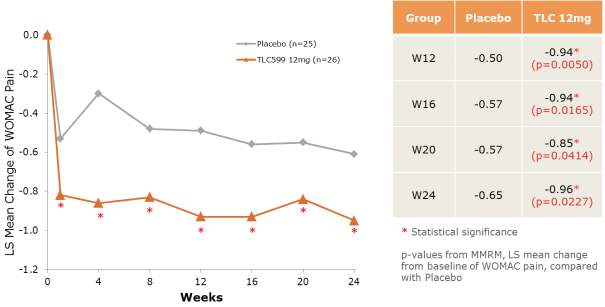
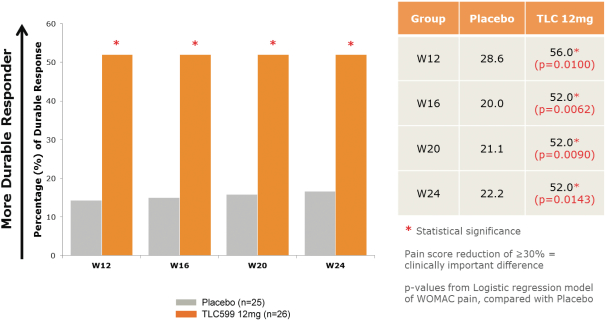
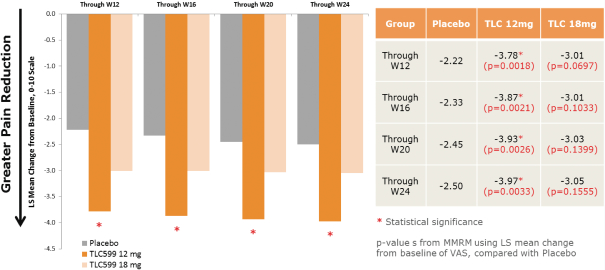
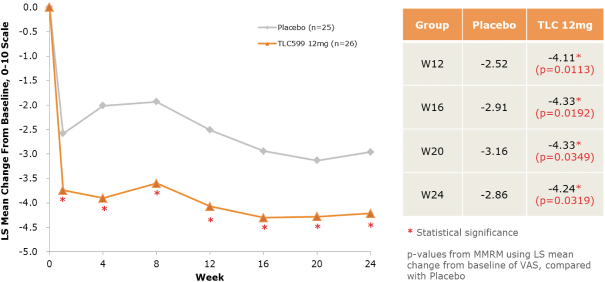
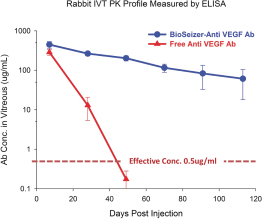
We own all of the patents and patent applications relating to our four lead product candidates.
TLC599
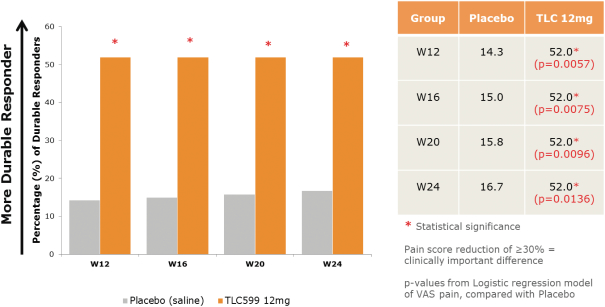
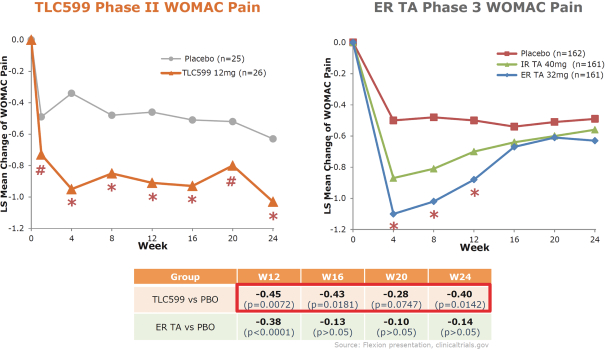
Our TLC599 intellectual property portfolio includes four issued patents and 14 patent applications. Our issued patents cover the methods of use of TLC599, were granted in the United States, Australia, New Zealand and Taiwan, and are expected to expire in 2033. Our patent applications cover the methods of use and manufacture of TLC599 and are pending and under review in the United States, South Africa, Singapore, Russia, Korea, Japan, India, Hong Kong, Europe, China, Canada and Brazil, with expected expiry dates of 2033.
TLC399
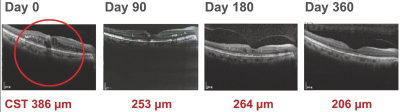
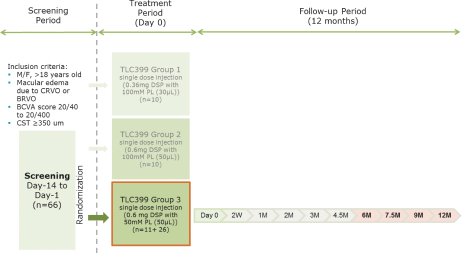
Our TLC399 intellectual property portfolio includes 23 issued patents and 17 patent applications. Our issued patents cover the composition of matter of TLC399, were granted in Taiwan, the United States, Canada, South Africa, Russia, New Zealand, Korea, Japan, Hong Kong, China, Europe and Australia, and are expected to expire between 2029 and 2033. Our patent applications cover the composition of matter of TLC399 and are pending and under review in the United States, South Africa, Korea, India, Hong Kong, Europe, China, Canada and Brazil, with expected expiry dates between 2029 and 2033.
TLC590
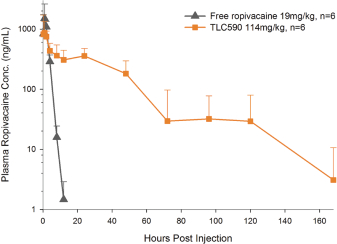
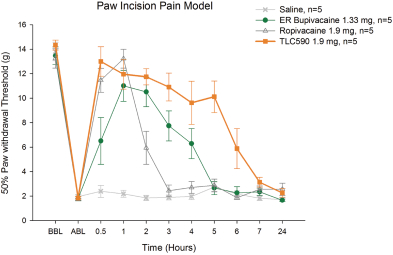
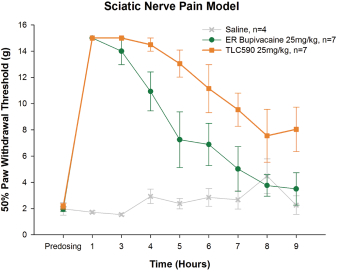
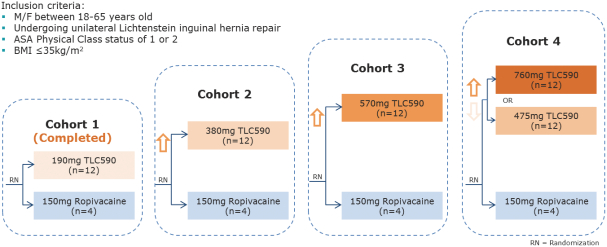
Our TLC590 intellectual property portfolio includes one PCT application, one Taiwan patent and one U.S. provisional application, which covers the composition of matter of TLC590 and is pending, in the international phase of PCT application and in Taiwan, under review in the United States, with expected expiry dates between 2038 and 2039.
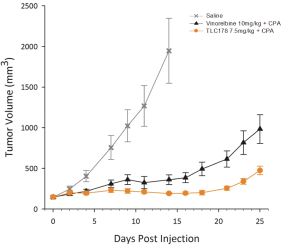
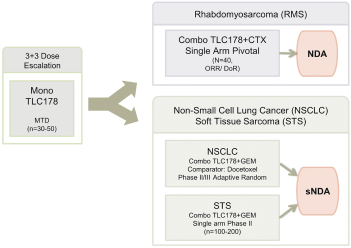
TLC178
Our material TLC178 intellectual property portfolio includes two issued patents and 15 patent applications. Our issued patents cover the composition of matter of TLC178, were granted in Taiwan and the United States, and are expected to expire in 2034. Our patent applications cover the composition of matter of TLC178 and are pending and under review in Taiwan, the United States, South Africa, Singapore, Russia, New Zealand, Korea, Japan, India, Hong Kong, Europe, China, Canada, Brazil and Australia, with expected expiry dates of 2034.
Individual patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued for regularly filed applications in the United States are granted a term of 20 years from the earliest effectivenon-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the USPTO delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. However, the actual protection afforded by a patent varies on a product by product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
The patent terms of novel treatment method claim for TLC599 (US 9,789,062), composition claim for TLC399 (US 8,956,600), (US 9,987,360), (US 10,058,616) and composition claim for TLC178 (US 9,700,511) at least extend into 2033, 2029 and 2034, respectively. For TLC590, TLC399 and TLC599, the patent terms of novel composition claims and improved treatment method claims would extend into 2039, 2033 and 2039 respectively if issued.
115


 ) and the regulations promulgated thereunder (ROC Act). Among other things, the ROC Act requires the ROC government’s prior approval of many business transactions. Any accumulated direct or indirect investment by us into any entity in the PRC in excess of US$1 million, or any technology cooperation agreement between us and any entity in the PRC that involves our intellectual property would be subject to this approval. While we are not currently party to any material transactions or agreements with any entity in the PRC, our future ability to enter into such arrangements may be limited by the ROC Act, particularly if ROC and PRC political relations deteriorate. Although, to our knowledge, we do not currently have any PRC shareholders, any PRC investor’s investment into us must be approved by the Investment Commission, MOEA, Executive Yuan of the ROC, provided our businesses fall within one of the industries included on the list maintained by the MOEA that PRC enterprises are allowed to invest in, or such PRC investor must be a domestic qualified institutional investor (QDII) in the PRC for an investment limit of less than 10% of our issued shares. However, PRC investors, other than QDIIs, are currently prohibited from investing in us as certain aspects of our current business scope are not included on the MOEA’s list. Any approval by the ROC government will depend on the then- current political environment between the ROC and PRC, and we cannot assure you that any PRC investor will receive such approval from the ROC government. Furthermore, although PRC investors which do not meet the QDII requirements may still purchase ADRs in this offering, any such PRC purchaser of ADRs will need to qualify as a QDII in order to exchange their ADRs for underlying common shares of our company.
) and the regulations promulgated thereunder (ROC Act). Among other things, the ROC Act requires the ROC government’s prior approval of many business transactions. Any accumulated direct or indirect investment by us into any entity in the PRC in excess of US$1 million, or any technology cooperation agreement between us and any entity in the PRC that involves our intellectual property would be subject to this approval. While we are not currently party to any material transactions or agreements with any entity in the PRC, our future ability to enter into such arrangements may be limited by the ROC Act, particularly if ROC and PRC political relations deteriorate. Although, to our knowledge, we do not currently have any PRC shareholders, any PRC investor’s investment into us must be approved by the Investment Commission, MOEA, Executive Yuan of the ROC, provided our businesses fall within one of the industries included on the list maintained by the MOEA that PRC enterprises are allowed to invest in, or such PRC investor must be a domestic qualified institutional investor (QDII) in the PRC for an investment limit of less than 10% of our issued shares. However, PRC investors, other than QDIIs, are currently prohibited from investing in us as certain aspects of our current business scope are not included on the MOEA’s list. Any approval by the ROC government will depend on the then- current political environment between the ROC and PRC, and we cannot assure you that any PRC investor will receive such approval from the ROC government. Furthermore, although PRC investors which do not meet the QDII requirements may still purchase ADRs in this offering, any such PRC purchaser of ADRs will need to qualify as a QDII in order to exchange their ADRs for underlying common shares of our company.