Charles S. Kim
+1 858 550 6049
ckim@cooley.com
November 8, 2018
Jeff Gabor
Erin Jaskot
Office of Healthcare and Insurance
Division of Corporation Finance
U.S. Securities and Exchange Commission
100 F Street, N.E.
Washington, D.C. 20549
| Re: | Taiwan Liposome Company, Ltd. |
Amendment No. 3 to Registration Statement on FormF-1 Filed October 22, 2018; FileNo. 333-223090
Dear Mr. Gabor and Ms. Jaskot:
On behalf of Taiwan Liposome Company, Ltd. (“TLC” or the “Company”), we submit this letter in response to comments received from the staff (the “Staff”) of the Securities and Exchange Commission (the “Commission”) by letter dated November 5, 2018 (the “Comment Letter”) with respect to Amendment No. 3 to the Company’s Registration Statement on FormF-1 filed with the Commission on October 22, 2018 (the “Registration Statement”). Concurrently with the submission of this response letter, the Company is filing Amendment No. 4 to the Registration Statement (“Amendment No. 4”). In addition to addressing the comments raised by the Staff in the Comment Letter, the Company has revised Amendment No. 4 to update other disclosures.
For the convenience of the Staff, the numbering of the paragraphs below corresponds to the numbering of the comment in the Comment Letter, the text of which we have incorporated into this response letter in italicized type and which is followed by the Company’s response. In the response below, page number references are to Amendment No. 4.
Prospectus Summary
Overview, page 1
| 1. | We note your statement that you are dedicated to developingbest-in-class novel nanomedicines, and that TLC599 has the potential to become abest-in-class treatment. Your product candidates are still in early stages of clinical trials with no Phase 3 trials having been completed. Therefore, your statements that your product candidates have the potential to bebest-in-class are premature. We also note the statement that your NanoX technology is designed to enable better toxicity profiles. Such a statement suggests that your product is safe which is a determination which is solely within the authority of the FDA or comparable foreign agency. Please delete the statements that your products may bebest-in-class or have better toxicity profiles from your registration statement. |
Response: In response to the Staff’s comment, the Company has revised the disclosure on pages 1, 5, 67, 84, 88 and 90 of Amendment No. 4 as requested.
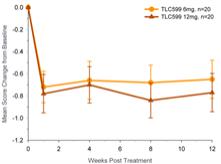
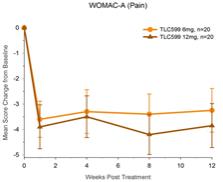
| 2. | We note your response to prior comment 1. Please balance your disclosure in the Prospectus Summary and elsewhere that you include similar disclosure regarding TLC399 to specify that your clinical trials, including your Phase II trial data, has not been able to show evidence of efficacy beyond three months. Please also specify that the observation that four out of five patients showed improvement in your Phase 1 trial is based on a trial size of only 9 patients, and only one patient achieved the target central retinal thickness level. Please also disclose the existence of cases of intraocular pressure elevations and virtuous haze, both of which were assessed as serious adverse events that were related to TLC399. |
Response: In response to the Staff’s comment, the Company has revised the disclosure on pages 1, 3, 67, 68 and 85 of Amendment No. 4 as requested.
Cooley LLP 4401 Eastgate Mall San Diego, CA 92121-1909
t: (858) 500-6000 f: (858) 550-6420 cooley.com