collaboration agreements, our licensors may retain the right to grantnon-exclusive licenses to the licensed patents and technology to other academic or research institutions fornon-commercial research purposes.
Certain provisions in the agreements under which we currently license intellectual property or technology to and from third parties may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, increase what we believe to be our financial or other obligations under the relevant agreement or decrease the third party’s financial or other obligations under the relevant agreement, any of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
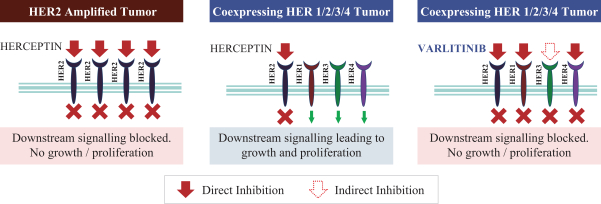
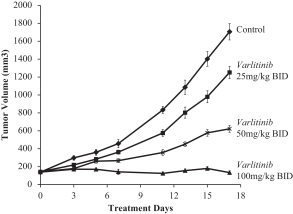
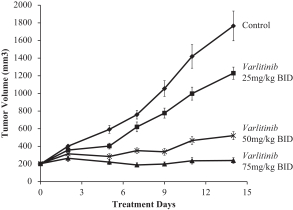
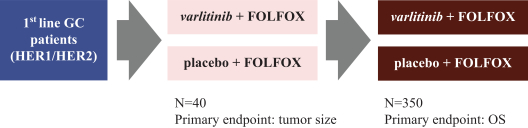
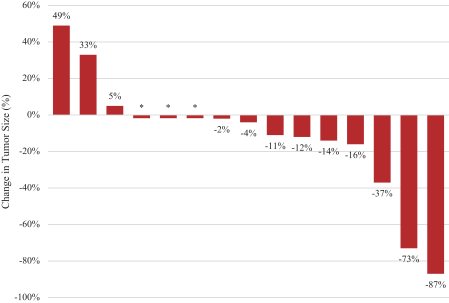
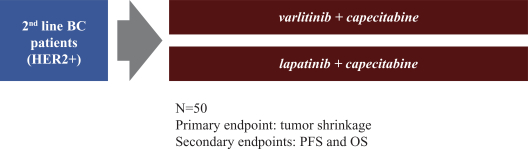
Varlitinib
Licensed from Array
On July 12, 2011, we entered into a collaboration and license agreement with Array, relating to Array’span-HER inhibitor,ARRY-543, which we refer to as ASLAN001 orvarlitinib, pursuant to which we obtained an exclusive, worldwide license to develop products incorporatingvarlitinib as an active ingredient for the treatment or prevention of any diseases or conditions in humans, pursuant to an agreed development plan, and an exclusive, worldwide license to pursue a commercial licensing program in relation to such products.
The basic protection forvarlitinib is provided by a family of composition of matter patents. These patents disclose a genus and also explicitly disclosesvarlitinib (example number 52 in WO2005/016346).
As of December 6, 2017, this family of patents included patents issued in the United States (at least three patents, some relating to intermediates and processes), Australia, Canada, China (at least three patents), Chile, Colombia, Europe, Hong Kong, Indonesia, India, Iceland, Japan, South Korea, Macau, Mexico, Norway, New Zealand, Philippines, Russia, Singapore, Ukraine and South Africa. In addition, as of December 6, 2017, this family of patents included patent applications filed in Argentina, Brazil, Egypt, Taiwan and Venezuela. The scope of the claims may differ in the various countries. The normal expiration of this family of patents is November 2024 in the United States and August 2024 outside the United States, subject to the payment of renewal fees.
The first patent application filed in China was not granted based on a technicality of Chinese practice. Subsequently filed divisional patent applications were granted. If the validity of one or more of the granted divisional patents is challenged then one or more of these patents may ultimately be considered invalid. In China typically branded medicines may still grow their market share, even after patent expiration. This trend along with subsequently filed patent applications and the Chinese data exclusivity provisions may minimize the impact of negative decisions that may be received in respect of one or more of the divisional patents.
Protection for the synthetic process of makingvarlitinib and a key intermediate in that process may be provided from the family of patents derived from WO2007/059257, filed November 15, 2006. As of December 6, 2017, this family of patents includes issued patents in Australia, Canada, China, Colombia, Europe, Hong Kong, Iceland, Israel, Japan, South Korea, Mexico, Philippines, Singapore, Taiwan, Ukraine and the United States. In addition, as of December 6, 2017, this family of patents included patent applications filed in Brazil, India, Norway and Russia. The scope of the claims may differ in the various countries. The normal expiration of this family of patents is November 2026.
Owned by Us
We are the applicant on a number of pending patents mostly relating to medical uses or combination therapies. These include the following pending patent applications:
| | • | | published Patent Cooperation Treaty, or PCT, application WO2017/037292 filed September 5, 2017 relates to a combination therapy comprisingvarlitinib and ASLAN003; |
99

 .” In China, we have a trademark registration for “
.” In China, we have a trademark registration for “ .” This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
.” This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.






 .” In China, we have a registration for “
.” In China, we have a registration for “ .” We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.
.” We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.