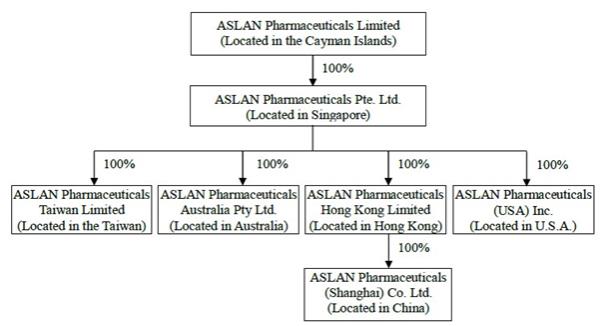
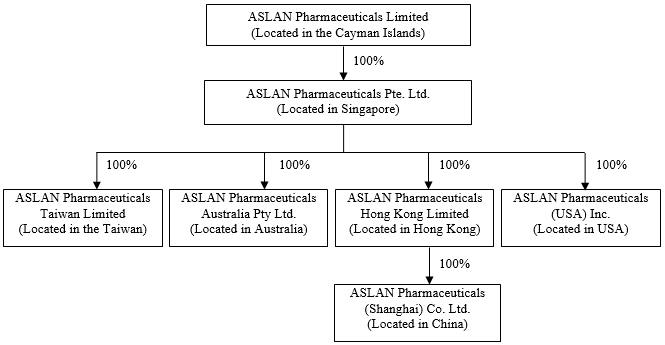
Confidential Treatment Requested by ASLAN Pharmaceuticals Limited
Pursuant to 17 C.F.R. Section 200.83
In addition, we exclusively licensed from Array a family of patents derived from WO2007/059257, filed November 15, 2006, which relate to the synthetic process of makingvarlitiniband a key intermediate in that process. As of May 15, 2019, this patent family includes two issued patents in the United States, 17 issued patents in a number of foreign countries and jurisdictions, including China, Colombia, Europe, Hong Kong, Iceland, India, Israel, Japan, South Korea, Mexico, Norway, Philippines, Russia, Singapore, Taiwan, and Ukraine, one pending patent application in the United States and one pending patent application in Brazil. The scope of the claims may differ in the various countries. The issued patents in this family and the pending patent applications, if issued, are expected to expire in November 2026, subject to the payment of renewal fees, excluding any additional term for patent term adjustments or patent term extensions.
Owned by Us
As of May 15, 2019, we own three pending Patent Cooperation Treaty, or PCT, patent applications mostly relating to medical uses or combination therapies ofvarlitinib. These include the following pending patent applications:
| | • | | WO2017/037298 filed September 5, 2016 relates to use ofvarlitinibin sensitizing a patient to chemotherapy was and was progressed in the United States, Europe, China, Hong Kong, Japan, Republic of Korea, and Singapore. The United States case was recently allowed; |
| | • | | WO2017/037300 filed September 5, 2016 relates to use ofvarlitinibin treatment of resistant cancers and was progressed in the United States, Europe, China, Hong Kong, and Japan; and |
| | • | | WO2018/004465 filed June��30, 2017, related to use ofvarlitinib as a maintenance therapy and was progressed in the United States, Europe, Australia, China, Japan, Republic of Korea, Singapore, Thailand, and Taiwan. |
These pending PCT patent applications are not eligible to become issued patents until, among other things, we file a national stage patent application within 30 months in the countries in which we seek patent protection. If we do not timely file any national stage patent applications, we may lose our priority date with respect to our PCT patent applications and any patent protection on the inventions disclosed in such PCT patent applications. Any national entry patent applications based on these pending PCT applications, if issued, are expected to expire between 2036 and 2037 subject to the payment of renewal fees, excluding any patent term adjustments or patent term extensions. It is not clear what claims may be granted, if any.
There are currently three unpublished Singapore priority patent applications relating tovarlitinib. These patent applications are at an early stage of filing and it is not possible to predict what claims may be ultimately granted, if any from these patent applications.
ASLAN003
Licensed from Almirall
On May 16, 2012, we entered into a development and license agreement with Almirall, pursuant to which we obtained an exclusive, worldwide license to certain patents,know-how and other intellectual property controlled by Almirall to a DHODH inhibitor, LAS186323, which we refer to as ASLAN003. On December 21, 2015, we entered into an amended development and license agreement with Almirall which replaced the previous agreement. This was further amended by an amendment agreement entered into on March 16, 2018. Under the amended agreement as so amended, we obtained from Almirall an expanded exclusive, worldwide license to develop, manufacture and commercialize ASLAN003 products for all human diseases with primary focus on oncology diseases, excluding topically-administered products embodying the compound for keratinocyte hyperproliferative disorders, and thenon-melanoma skin cancers basal cell carcinoma, squamous cell carcinomas and Gorlin Syndrome.
With respect to ASLAN003, we exclusively licensed from Almirall a family of patents, which includes composition of matter patents, derived from WO2008/077639. As of May 15, 2019, this family of patents and patent applications included two issued patents the United States, 55 issued patents in a number of foreign countries and jurisdictions, including Australia, Canada, China, Europe, Hong Kong, Israel, Japan, Mexico, New Zealand, Nigeria, Norway, Peru, Russia, Singapore South Africa, South Korea, Taiwan and Ukraine, no pending patent applications in the United States and 12 pending patent applications in a number of foreign countries, including Argentina, Bolivia, Chile, Colombia, Ecuador, Egypt, Pakistan, Philippines, Thailand, Uruguay, Venezuela and Vietnam. The scope of the claims may differ in different countries. The issued patents in this family and the pending patent application, if issued, are expected to expire in December 2027, subject to the payment of renewal fees, excluding any additional term for patent term adjustments or patent term extensions.
113

 ..” In China, we have a trademark registration for “
..” In China, we have a trademark registration for “ ..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “
..” We also have a trademark registration in China to protect the following Chinese character version of the wordvarlitinib: “ ” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
” (wei li ti ni). This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
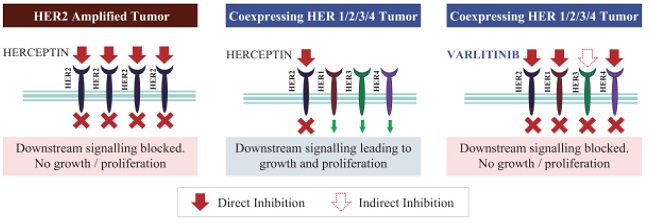
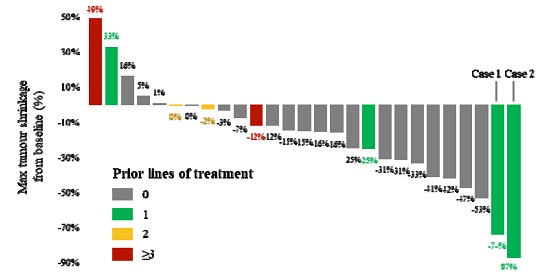

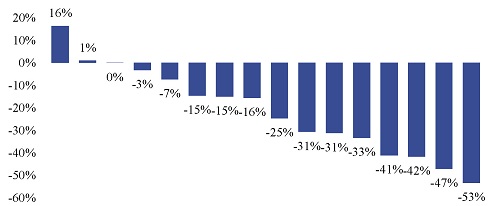

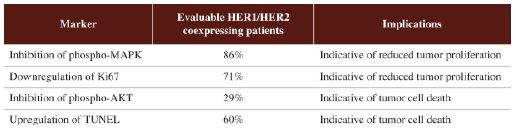
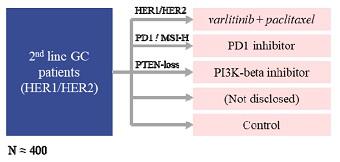
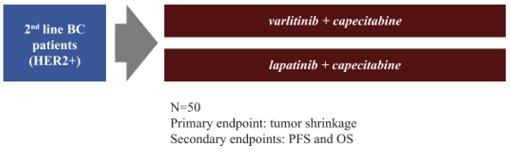

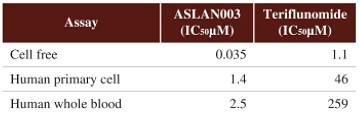
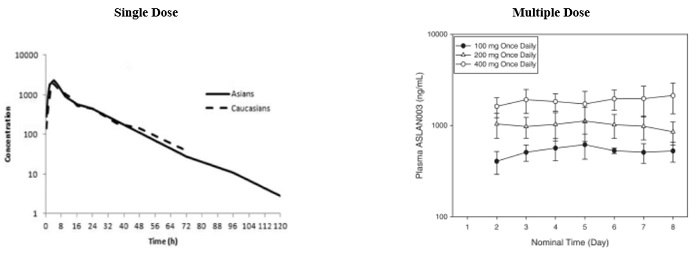
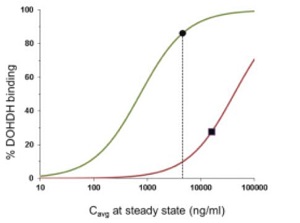
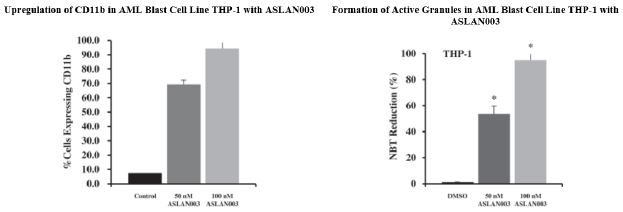
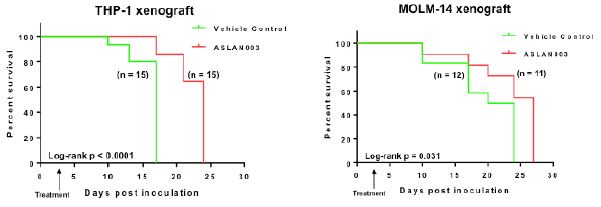
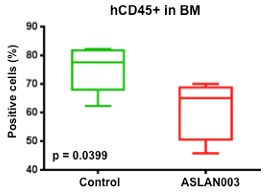


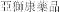 ”. In China, we have a trademark registration for “
”. In China, we have a trademark registration for “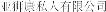 ”. We also have a trade mark registration in China to protect the following Chinese character version of the wordvarlitinib: “威利替尼” (wei li ti ni). We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.
”. We also have a trade mark registration in China to protect the following Chinese character version of the wordvarlitinib: “威利替尼” (wei li ti ni). We have a portfolio of 20 domain names, which includes: aslanpharma.com, aslanpharma.com.sg, aslanpharma.com.tw, aslanpharma.asia, aslanpharma.org, and aslanpharma.biz.