Exhibit 99.2

0 Phase 3 ATTACK Topline Results for Sulbactam - Durlobactam Nasdaq: ETTX October 2021

1 Disclaimer This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Words such as ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ "will," ‘‘would,’’ and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . All statements, other than statements of historical facts, contained in this presentation are forward - looking statements, including statements regarding the plans of Entasis Therapeutics Holdings Inc . (the “Company”) to develop and commercialize its product candidates, the Company's ongoing and planned clinical trials, the timing and availability of data from its clinical trials, advancement of ETX 0462 into a Phase 1 clinical trial, the efficacy and safety data from its ongoing Phase 3 trials that, if positive, will be sufficient to support the submission of a new drug application (NDA) to the US Food and Drug Administration (FDA), its ability to obtain grants or other government funding to develop product candidates, its ability to take advantage of benefits offered by current and pending legislation related to the development of products addressing antimicrobial resistance, the timing of its planned regulatory filings, the timing of and ability to obtain and maintain regulatory approval for its product candidates, the clinical utility of its product candidates and the potential advantages compared to other treatments, its commercialization, marketing and distribution capabilities and strategy, its ability to establish and maintain arrangements for the manufacture of its product candidates, its ability to establish and maintain collaborations and to recognize the potential benefits of such collaborations, its estimates regarding the market opportunities for its product candidates, its intellectual property position and duration of its patent rights and its estimates regarding future expenses, capital requirements and needs for additional financing . Forward - looking statements are based on the Company’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict . Factors that could cause actual results to differ include, but are not limited to, unexpected safety or efficacy data observed during preclinical or clinical trials, clinical trial site activation or enrollment rates that are lower than expected, changes in expected or existing competition, changes in the regulatory environment, failure of the Company’s collaborators to support or advance collaborations or product candidates, unexpected litigation or other disputes and the coronavirus pandemic . Many of these factors are beyond the Company’s control . These and other risks and uncertainties are discussed in the Company’s filings with the U . S . Securities and Exchange Commission, including the “Risk Factors” sections contained therein . Forward - looking statements contained in this presentation are made as of the date of this presentation, and except as required by law, the Company assumes no obligation to update any forward - looking statements contained herein to reflect any change in expectations, even as new information becomes available . This presentation contains estimates and other statistical data made by independent parties and by the Company relating to market size and other data about the Company's industry . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates . In addition, projections, assumptions and estimates of the Company's future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk .
2 Primary Efficacy and Safety Objectives of ATTACK Were Achieved • Non - inferiority in 28 - day all - cause mortality vs. colistin in patients with carbapenem - resistant Acinetobacter baumannii - calcoaceticus infections and overall trend favoring SUL - DUR • Statistically significant higher clinical cure rate at Test of Cure compared to colistin • Favorable safety profile compared to colistin with a statistically significant reduction in nephrotoxicity • Additional analyses of non - inferiority at 28 - days and 14 - days support primary efficacy results • Part B (including colistin - resistant Acinetobacter ) mortality rate consistent with Part A • Comparable baseline demographics in both treatment groups • Overall adverse events (AEs) in the safety population comparable between treatment groups Robust positive SUL - DUR results
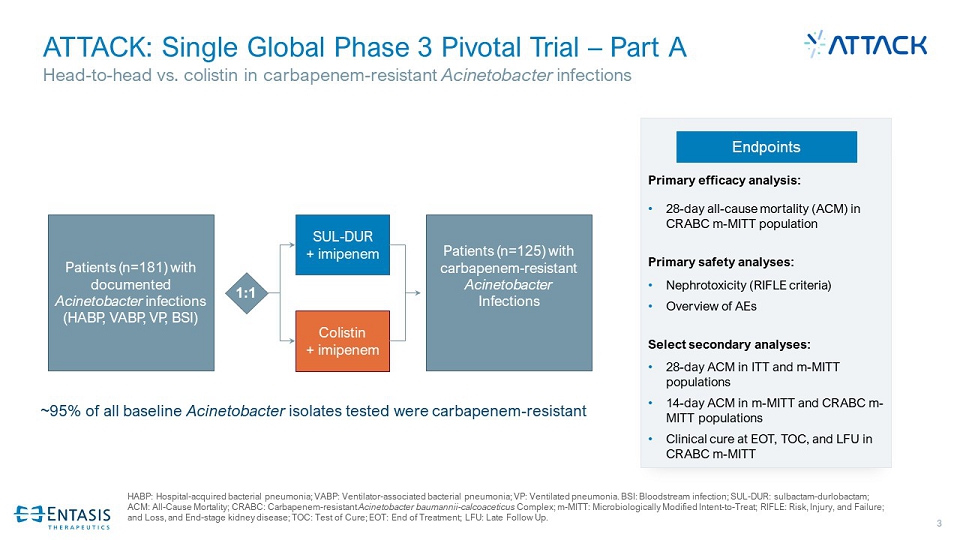
3 ATTACK: Single Global Phase 3 Pivotal Trial – Part A Head - to - head vs. colistin in carbapenem - resistant Acinetobacter infections HABP: Hospital - acquired bacterial pneumonia; VABP: Ventilator - associated bacterial pneumonia; VP: Ventilated pneumonia. BSI: Blo odstream infection; SUL - DUR: sulbactam - durlobactam ; ACM: All - Cause Mortality; CRABC: Carbapenem - resistant Acinetobacter baumannii - calcoaceticus Complex; m - MITT: Microbiologically Modified Intent - to - Treat; RIFLE: Risk, Injury, and Failure; and Loss, and End - stage kidney disease; TOC: Test of Cure; EOT: End of Treatment; LFU: Late Follow Up. Endpoints Primary efficacy analysis: • 28 - day all - cause mortality (ACM) in CRABC m - MITT population Primary safety analyses: • Nephrotoxicity (RIFLE criteria) • Overview of AEs Select secondary analyses: • 28 - day ACM in ITT and m - MITT populations • 14 - day ACM in m - MITT and CRABC m - MITT populations • Clinical cure at EOT, TOC, and LFU in CRABC m - MITT 1:1 Patients (n=125) with carbapenem - resistant Acinetobacter Infections SUL - DUR + imipenem Colistin + imipenem Patients (n=181) with documented Acinetobacter infections (HABP, VABP, VP, BSI) ~95% of all baseline Acinetobacter isolates tested were carbapenem - resistant

4 ATTACK: Single Global Phase 3 Pivotal Trial – Part B 1. Includes patients with HABP, VABP and bacteremia due to colistin - resistant Acinetobacter or patients with known intolerance to colistin. Endpoints Efficacy: • 28 - Day ACM in ITT population • 14 - Day ACM in m - MITT population Safety: • Overview of AEs Patients (n=28) safety and supportive efficacy SUL - DUR + imipenem Patients with documented Acinetobacter infections; not eligible for Part A 1 Open label arm enrolling patients not eligible for Part A
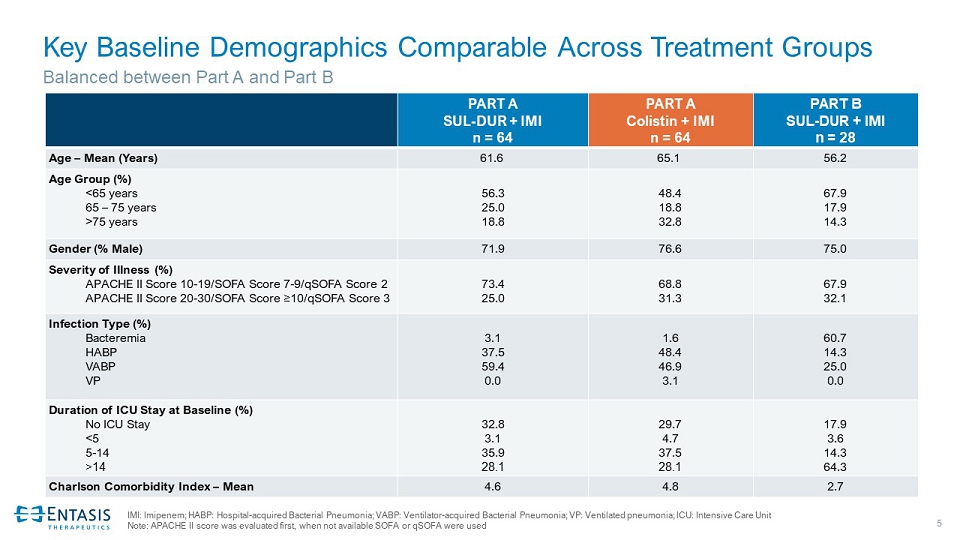
5 Key Baseline Demographics Comparable Across Treatment Groups PART A SUL - DUR + IMI n = 64 PART A Colistin + IMI n = 64 PART B SUL - DUR + IMI n = 28 Age – Mean (Years) 61.6 65.1 56.2 Age Group (%) <65 years 65 – 75 years >75 years 56.3 25.0 18.8 48.4 18.8 32.8 67.9 17.9 14.3 Gender (% Male) 71.9 76.6 75.0 Severity of Illness (%) APACHE II Score 10 - 19/ SOFA Score 7 - 9/qSOFA Score 2 APACHE II Score 20 - 30/ SOFA Score ≥ 10/qSOFA Score 3 73.4 25.0 68.8 31.3 67.9 32.1 Infection Type (%) Bacteremia HABP VABP VP 3.1 37.5 59.4 0.0 1.6 48.4 46.9 3.1 60.7 14.3 25.0 0.0 Duration of ICU Stay at Baseline (%) No ICU Stay <5 5 - 14 >14 32.8 3.1 35.9 28.1 29.7 4.7 37.5 28.1 17.9 3.6 14.3 64.3 Charlson Comorbidity Index – Mean 4.6 4.8 2.7 Balanced between Part A and Part B IMI: Imipenem; HABP: Hospital - acquired Bacterial Pneumonia; VABP: Ventilator - acquired Bacterial Pneumonia; VP: Ventilated pneumo nia; ICU: Intensive Care Unit Note: APACHE II score was evaluated first, when not available SOFA or qSOFA were used
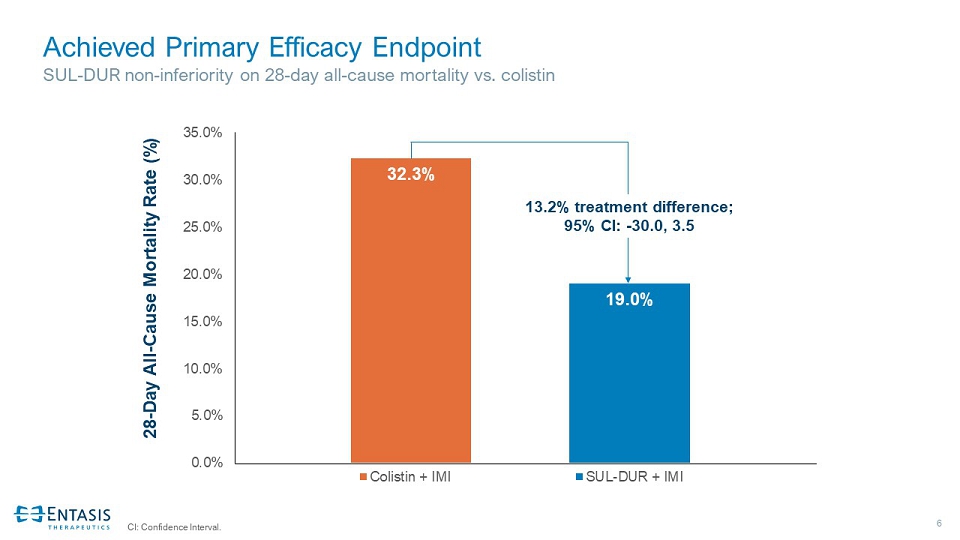
6 Achieved Primary Efficacy Endpoint SUL - DUR non - inferiority on 28 - day all - cause mortality vs. colistin CI: Confidence Interval. 32.3% 19.0% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% 35.0% Colistin + IMI SUL-DUR + IMI 13.2% treatment difference; 95% CI: - 30.0, 3.5 28 - Day All - Cause Mortality Rate (%)

7 Secondary Endpoints Primary Endpoint (11.7%) (12.8%) (11.8%) (13.2%) (13.2%) -40% -30% -20% -10% 0% 10% 20% 30% 14 Day ACM m-MITT (N = 154) 14 Day ACM CRABC m-MITT (N = 127) 28 Day ACM ITT (N = 175) 28 Day ACM m-MITT (N = 152) 28 Day ACM CRABC m-MITT (N = 125) All - Cause Mortality Analyses Favor SUL - DUR Favorable mortality difference for SUL - DUR vs. colistin across all study populations evaluated to date Favors SUL - DUR Favors Colistin 20% Non - inferiority Margin Mortality Rate Treatment Difference and 95% Confidence Interval

8 Statistically Significant Difference in Clinical Cure SUL - DUR compared to colistin at Test of Cure Clinical Cure at Test of Cure (%) 40.3% 61.9% 0.0% 20.0% 40.0% 60.0% 80.0% Colistin + IMI SUL-DUR + IMI 21.6% treatment difference; 95% CI: 2.9, 40.3
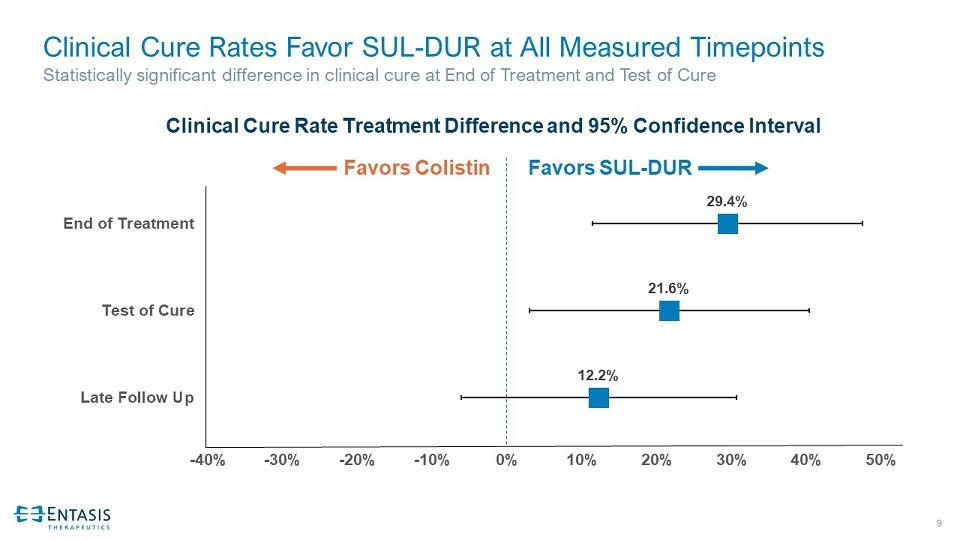
9 Clinical Cure Rates Favor SUL - DUR at All Measured Timepoints Statistically significant difference in clinical cure at End of Treatment and Test of Cure 12.2% 21.6% 29.4% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% Late Follow Up Test of Cure End of Treatment Clinical Cure Rate Treatment Difference and 95% Confidence Interval Favors SUL - DUR Favors Colistin
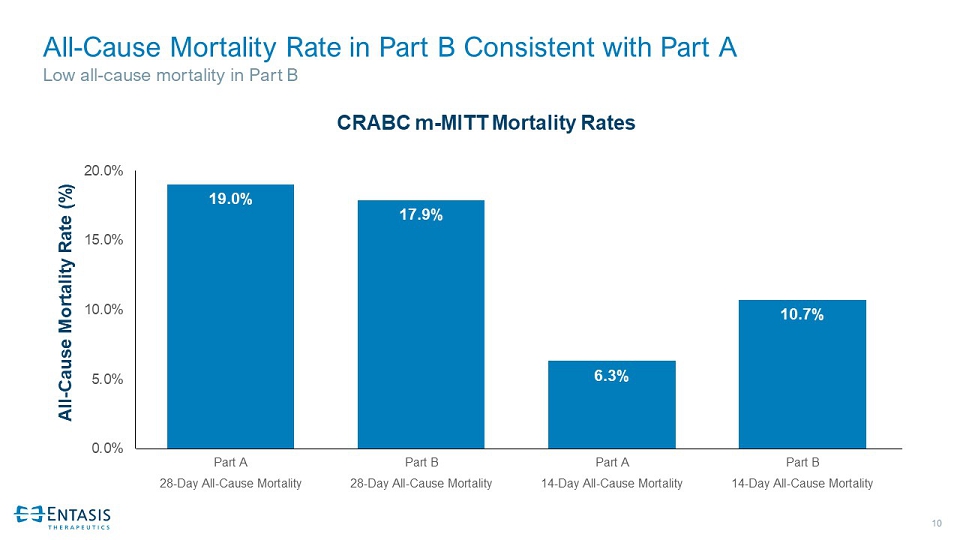
10 All - Cause Mortality Rate in Part B Consistent with Part A Low all - cause mortality in Part B 19.0% 17.9% 6.3% 10.7% 0.0% 5.0% 10.0% 15.0% 20.0% Part A Part B Part A Part B 28-Day All-Cause Mortality 28-Day All-Cause Mortality 14-Day All-Cause Mortality 14-Day All-Cause Mortality All - Cause Mortality Rate (%) CRABC m - MITT Mortality Rates

11 Comparable Adverse Events Between Treatment Groups SUL - DUR compared to colistin PART A SUL - DUR + IMI (N=91) PART A Colistin + IMI (N=86) PART B SUL - DUR + IMI (N=28) Any Adverse Event (AE) (%) 87.9 94.2 89.3 Drug - Related TEAEs (%) 12.1 30.2 10.7 Serious AEs (%) 39.6 48.8 32.1 Drug - Related Serious AEs (%) 1.1 2.3 3.6 TEAEs Leading to Discontinuation of Study Drug 11.0 16.3 14.3 Serious TEAEs Leading to Discontinuation of Study Drug 7.7 8.1 10.7 1. Safety population includes patients randomized who received at least one dose of study drug.
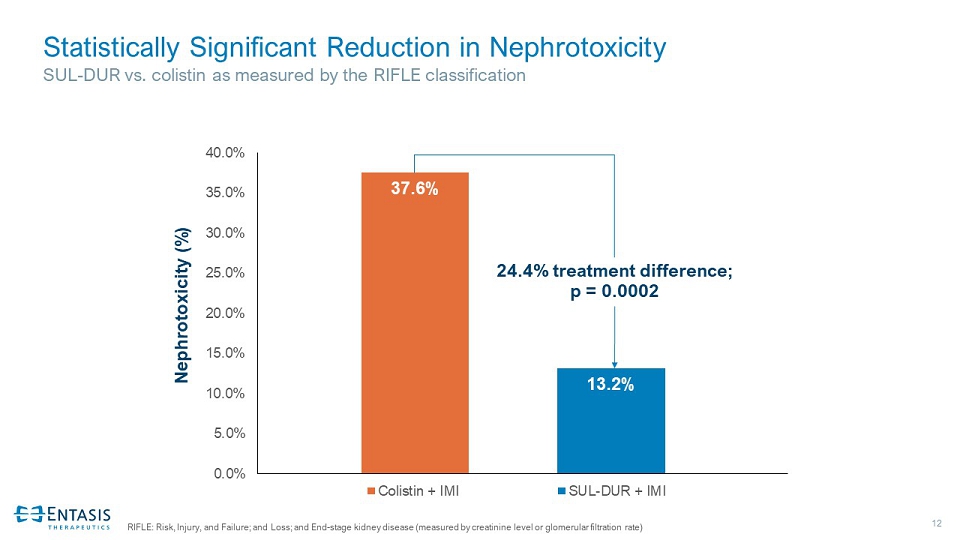
12 Statistically Significant Reduction in Nephrotoxicity SUL - DUR vs. colistin as measured by the RIFLE classification RIFLE: Risk, Injury, and Failure; and Loss; and End - stage kidney disease (measured by creatinine level or glomerular filtration rate) 37.6% 13.2% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% 35.0% 40.0% Colistin + IMI SUL-DUR + IMI Nephrotoxicity (%) 24.4% treatment difference; p = 0.0002

13 A Significant Milestone for Patients, a Pivotal Moment for Entasis • SUL - DUR first to achieve statistical non - inferiority in 28 - day all - cause mortality in patients with CRAB • Statistically significant difference in clinical cure at Test of Cure • Favorable safety profile with statistically significant reduction in nephrotoxicity • If approved, SUL - DUR could become the first pathogen - targeted treatment in patients with high unmet need • Target NDA submission in mid - 2022 • Collaborate with Zai Lab to prepare regulatory submission in China • Prepare and invest for the future, commercialization of SUL - DUR and advancement of our innovative pipeline, including zoliflodacin in Phase 3 • Address global health urgent threats for patients in need













