Confidential Treatment Requested by Zentalis Pharmaceuticals, Inc. Pursuant to 17 C.F.R. Section 200.83
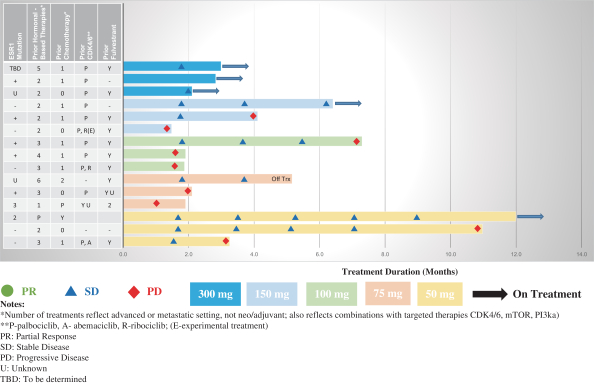
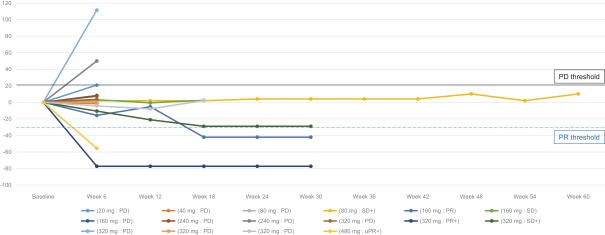
an ECOG performance status of 0 (n = 5) or 1 (n = 2). The median number of prior therapies for advanced disease was one (range zero to six). Two of the seven patients received prior treatments with fulvestrant. Of these seven patients, six are still on treatment and one discontinued due to physician decision (n = 1).
The interim and preliminary data reported herein are subject to change as more data on these patients and additional patients become available and are subject to audit and verification procedures that could result in material changes in the final data.
Interim and Preliminary Safety Results
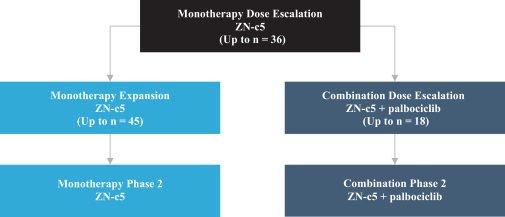
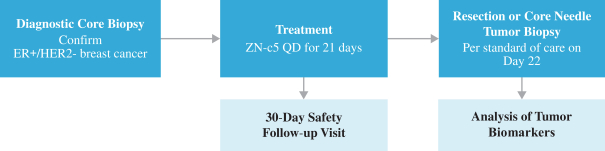
Phase 1, Monotherapy Dose Escalation and Monotherapy Dose Expansion
Based on the interim and preliminary data as of the database cutoff date of February 17, 2020 for the Phase 1, monotherapy dose escalation and monotherapy dose expansion portions of this trial, ZN-c5 has been observed to be well tolerated with no dose-limiting toxicities reported.
In the Phase 1 monotherapy dose escalation and monotherapy dose expansion portions of this trial, a total of 20 patients were enrolled and dosed with data available in the electronic data capture system as of the February 17, 2020 database cutoff. Treatment-emergent adverse events, or TEAEs, occurred in 18 of the 20 patients. Nausea was observed in five patients, while anemia, vomiting, arthralgia, back pain and cough were reported in three patients each, and all other adverse events were observed in only one or two patients each. Adverse events occurring in two or more patients included nausea (n = 5), anemia (n = 3), vomiting (n = 3), arthralgia (n = 3), back pain (n = 3), cough (n = 3), diarrhea (n = 2), fatigue (n = 2), alanine amino transferase, or ALT increased (n = 2), gamma glutamyl transferase increased (n = 2), lymphocyte count decreased (n = 2), hyperglycemia (n = 2), hypophosphatemia (n = 2), muscle spasms (n = 2), musculoskeletal pain (n = 2), myalgia (n = 2), headache (n = 2) and skin mass (n = 2). TEAEs of Grade 3 severity were single cases of hypercalcemia, back pain, arthralgia, musculoskeletal chest pain and pain in extremity. None of such Grade 3 TEAEs were deemed related to ZN-c5. All other TEAEs were of Grade 1 or Grade 2 in severity. The Grade 3 TEAE of arthralgia was also reported as a serious adverse event, deemed unrelated to treatment. This was the only serious adverse event reported. There were no deaths reported.
Investigator assessed treatment-related adverse events occurred in eight of 20 patients. These treatment-related adverse events included nausea (n = 2) and single adverse events of myalgia, diarrhea, dyspepsia, flatulence, pain, affect lability, alanine amino transferase, or ALT, increase, and gamma-glutamyl transferase increase. All were of Grade 1 or Grade 2 in severity.
Diarrhea, an adverse event of special interest, has been observed in two patients, a Grade 1 adverse event at 50 mg/day, deemed related to treatment, and a Grade 2 adverse event at 150 mg/day, deemed not related to treatment.
The first patient with ALT increased had the first dose of 50 mg of ZN-c5 on December 19, 2018. The patient entered the study with a Grade 1 ALT increased, which subsequently worsened to a Grade 2 ALT increased on February 13, 2019, 56 days after the first dose. On March 27, 2019, the patient was taken off treatment for disease progression, and at that time the Grade 2 ALT increased was still ongoing. The event was deemed related to ZN-c5. The second patient with ALT increased had the first dose of 300 mg of ZN-c5 on October 15, 2019. The patient developed Grade 1 ALT increased and Grade 1 aspartate aminotransferase, or AST, increased 84 days after the first dose, on January 6, 2020. Dosing was interrupted and the Grade 1 AST resolved, and at that time the Grade 1 was ALT still ongoing. The event was not deemed to be related to ZN-c5.
Overall, in the Phase 1, monotherapy dose escalation and monotherapy dose expansion portions of the trial, there was no observed increase in incidence or in severity of adverse events with increasing dosing levels.
117