
C O R P O R AT E P R E S E N TAT I O N A u g u s t 2 0 2 2

Forward-Looking Statements and Disclaimer Zentalis Pharmaceuticals, Inc. (“we,” “us,” “our,” “Zentalis” or the “Company”) cautions that this presentation (including oral commentary that accompanies this presentation) contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the target profiles and potential benefits of our product candidates, including as a monotherapy and/or in combination; clinical and regulatory progress of our product candidates, including the estimated timing of the initiation of clinical trials and data readouts; the market potential of our product candidates; our milestones; and the potential of our collaborations are forward-looking statements, as well as statements that include the words “potential,” “design,” “expect,” “intend,” “plan,” “believe,” “estimate,” “may,” and similar statements of a future or forward-looking nature. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the outbreak of the novel coronavirus disease, COVID-19, has adversely impacted and may continue to adversely impact our business, including our preclinical studies and clinical trials; our limited operating history, which may make it difficult to evaluate our current business and predict our future success and viability; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our substantial dependence on the success of our lead product candidate; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process or ongoing regulatory obligations; failure to obtain U.S. or international marketing approval; our product candidates may cause serious adverse side effects; interim, initial, “topline”, and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intellectual property; our ability to attract, retain and motivate qualified personnel; and significant costs as a result of operating as a public company. Other risk and uncertainties include those identified under the caption “Risk Factors” in our most recently filed periodic reports on Forms 10-K and 10-Q and subsequent filings with the U.S. Securities and Exchange Commission in the future could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data after the date of this presentation. Data of fulvestrant, RAD1901, abemaciclib, alpelisib, adavosertib, venetoclax and osimertinib presented in this presentation is based on evaluation of comparable proxy chemical compounds purchased from commercial sources rather than the pharmaceutical company commercializing or developing, as applicable, the compound. ZENTALIS® and its associated logos are trademarks of Zentalis and/or its affiliates. All other trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. All website addresses given in this presentation are for information only and are not intended to be an active link or to incorporate any website information into this document. Zentalis’s product candidates are investigational drugs and have not yet been approved by the U.S. Food and Drug Administration or any other regulatory authority. | 2
| 3 Lead Program: Wee1i (ZN-c3) potentially first- and best-in-class • Partial responses seen in four tumor types to date • Potential accelerated approval paths for USC and biomarker-driven trials • Fast Track designation granted in USC • Orphan drug and rare pediatric disease designations granted in osteosarcoma Integrated Discovery Engine: 4 FDA-cleared INDs within the first 5 years Investigating internal & third-party combinations, including ZN-d5 + ZN-c3 for liquid tumors Additional programs targeting fundamental cancer pathways including BCL-xL heterobifunctional degrader Company Overview BCL-2 inhibitor (ZN-d5): broad applicability as anti-apoptotic target; potential as monotherapy and in combination, including with ZN-c3
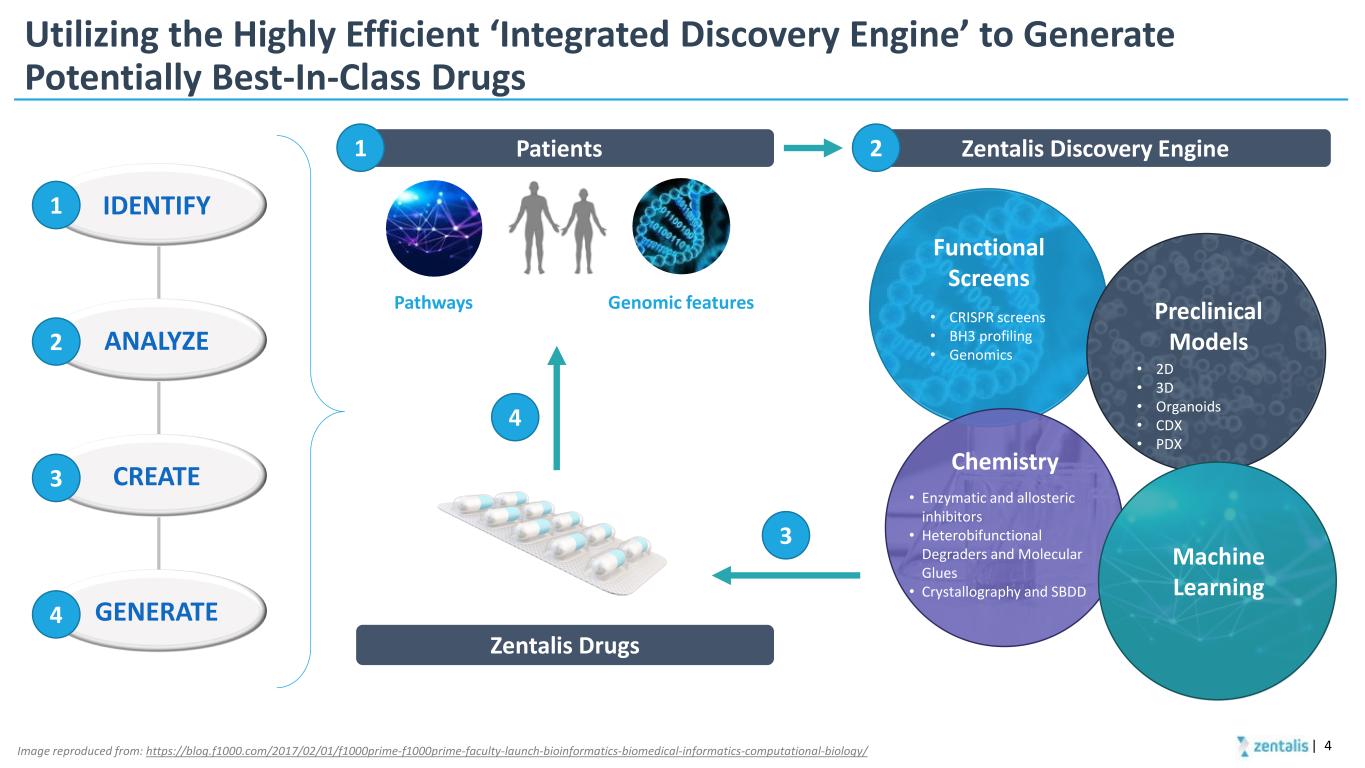
Utilizing the Highly Efficient ‘Integrated Discovery Engine’ to Generate Potentially Best-In-Class Drugs | 4 IDENTIFY ANALYZE CREATE GENERATE • CRISPR screens • BH3 profiling • Genomics Image reproduced from: https://blog.f1000.com/2017/02/01/f1000prime-f1000prime-faculty-launch-bioinformatics-biomedical-informatics-computational-biology/ Integrated Discovery Engine Patients Genomic featuresPathways Zentalis Drugs 1 2 3 4 1 Zentalis Discovery Engine2 3 4 Functional Screens Preclinical Models • Enzymatic and allosteric inhibitors • Heterobifunctional Degraders and Molecular Glues • Crystallography and SBDD Chemistry Machine Learning • 2D • 3D • Organoids • CDX • PDX
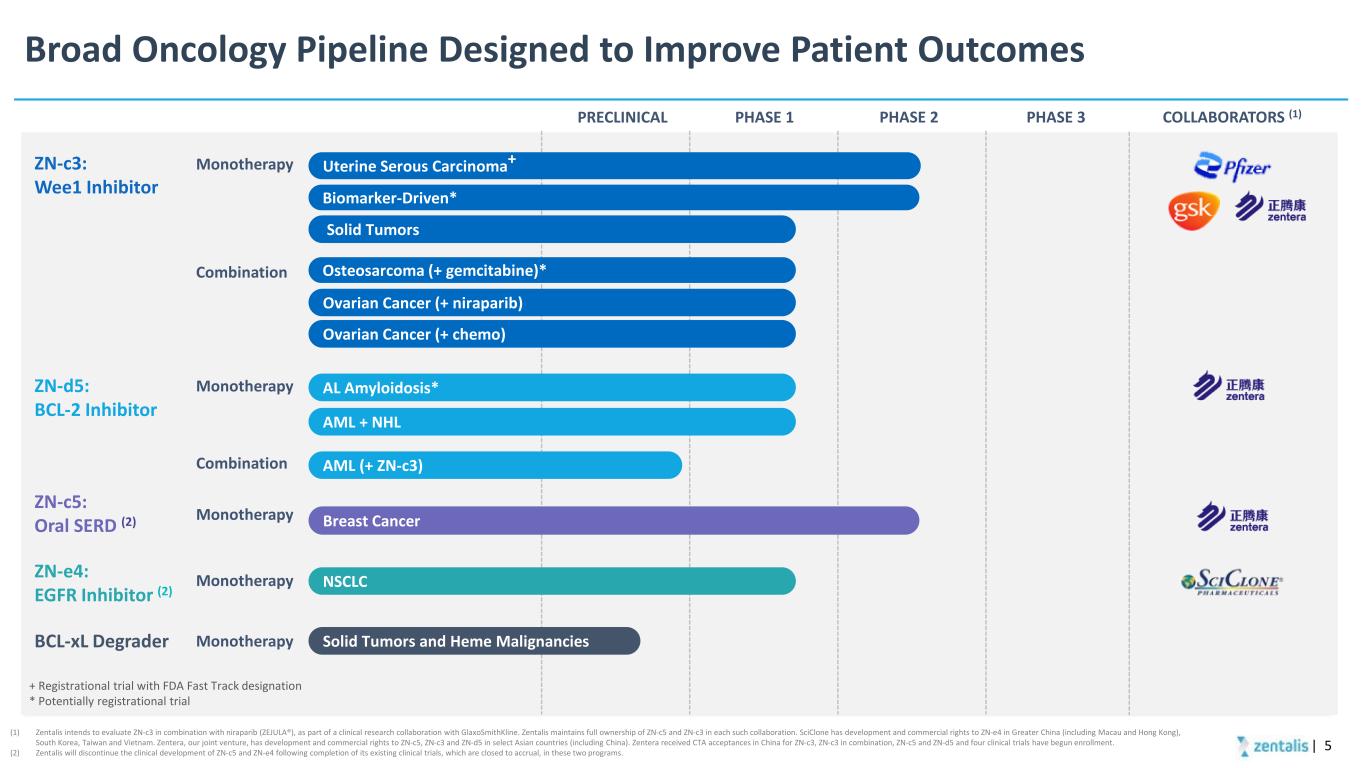
Broad Oncology Pipeline Designed to Improve Patient Outcomes PHASE 1 PHASE 2 COLLABORATORS (1)PHASE 3 (1) Zentalis intends to evaluate ZN-c3 in combination with niraparib (ZEJULA®), as part of a clinical research collaboration with GlaxoSmithKline. Zentalis maintains full ownership of ZN-c5 and ZN-c3 in each such collaboration. SciClone has development and commercial rights to ZN-e4 in Greater China (including Macau and Hong Kong), South Korea, Taiwan and Vietnam. Zentera, our joint venture, has development and commercial rights to ZN-c5, ZN-c3 and ZN-d5 in select Asian countries (including China). Zentera received CTA acceptances in China for ZN-c3, ZN-c3 in combination, ZN-c5 and ZN-d5 and four clinical trials have begun enrollment. (2) Zentalis will discontinue the clinical development of ZN-c5 and ZN-e4 following completion of its existing clinical trials, which are closed to accrual, in these two programs. ZN-c3: Wee1 Inhibitor ZN-c5: Oral SERD (2) ZN-e4: EGFR Inhibitor (2) Monotherapy Combination Monotherapy Monotherapy + Registrational trial with FDA Fast Track designation * Potentially registrational trial PRECLINICAL ZN-d5: BCL-2 Inhibitor Monotherapy BCL-xL Degrader Monotherapy | 5 Solid Tumors Osteosarcoma (+ gemcitabine)* Breast Cancer AML + NHL NSCLC Uterine Serous Carcinoma+ Ovarian Cancer (+ niraparib) Biomarker-Driven* Ovarian Cancer (+ chemo) Solid Tumors and Heme Malignancies AL Amyloidosis* Combination AML (+ ZN-c3)

Z N - c 3 Wee1 Inhibitor
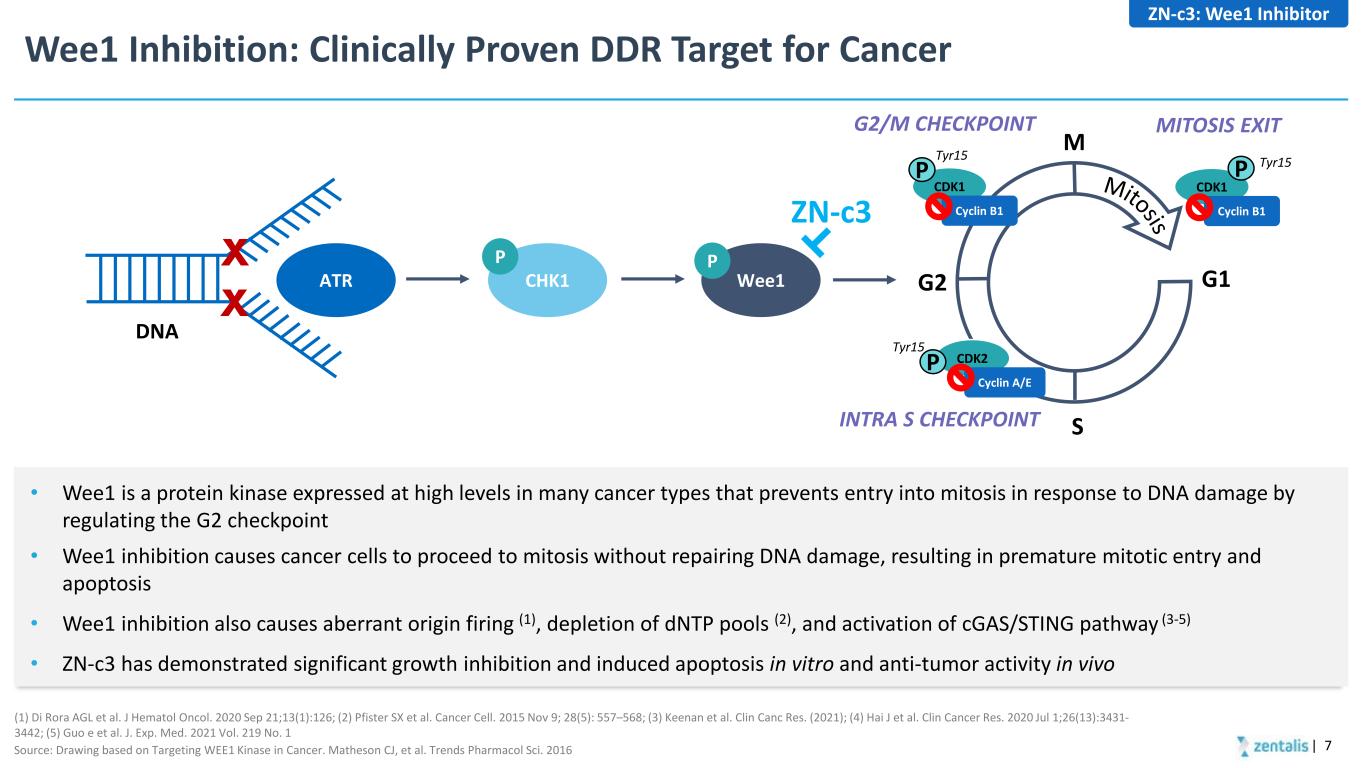
Wee1 Inhibition: Clinically Proven DDR Target for Cancer ZN-c3 ATR CHK1 Wee1 PP DNA X Source: Drawing based on Targeting WEE1 Kinase in Cancer. Matheson CJ, et al. Trends Pharmacol Sci. 2016 • Wee1 is a protein kinase expressed at high levels in many cancer types that prevents entry into mitosis in response to DNA damage by regulating the G2 checkpoint • Wee1 inhibition causes cancer cells to proceed to mitosis without repairing DNA damage, resulting in premature mitotic entry and apoptosis • Wee1 inhibition also causes aberrant origin firing (1), depletion of dNTP pools (2), and activation of cGAS/STING pathway (3-5) • ZN-c3 has demonstrated significant growth inhibition and induced apoptosis in vitro and anti-tumor activity in vivo | 7 ZN-c3: Wee1 Inhibitor X M S G2 G1 CDK1 Cyclin B1 P Tyr15 MITOSIS EXIT CDK1 Cyclin B1 P Tyr15 G2/M CHECKPOINT CDK2 Cyclin A/E P Tyr15 INTRA S CHECKPOINT (1) Di Rora AGL et al. J Hematol Oncol. 2020 Sep 21;13(1):126; (2) Pfister SX et al. Cancer Cell. 2015 Nov 9; 28(5): 557–568; (3) Keenan et al. Clin Canc Res. (2021); (4) Hai J et al. Clin Cancer Res. 2020 Jul 1;26(13):3431- 3442; (5) Guo e et al. J. Exp. Med. 2021 Vol. 219 No. 1
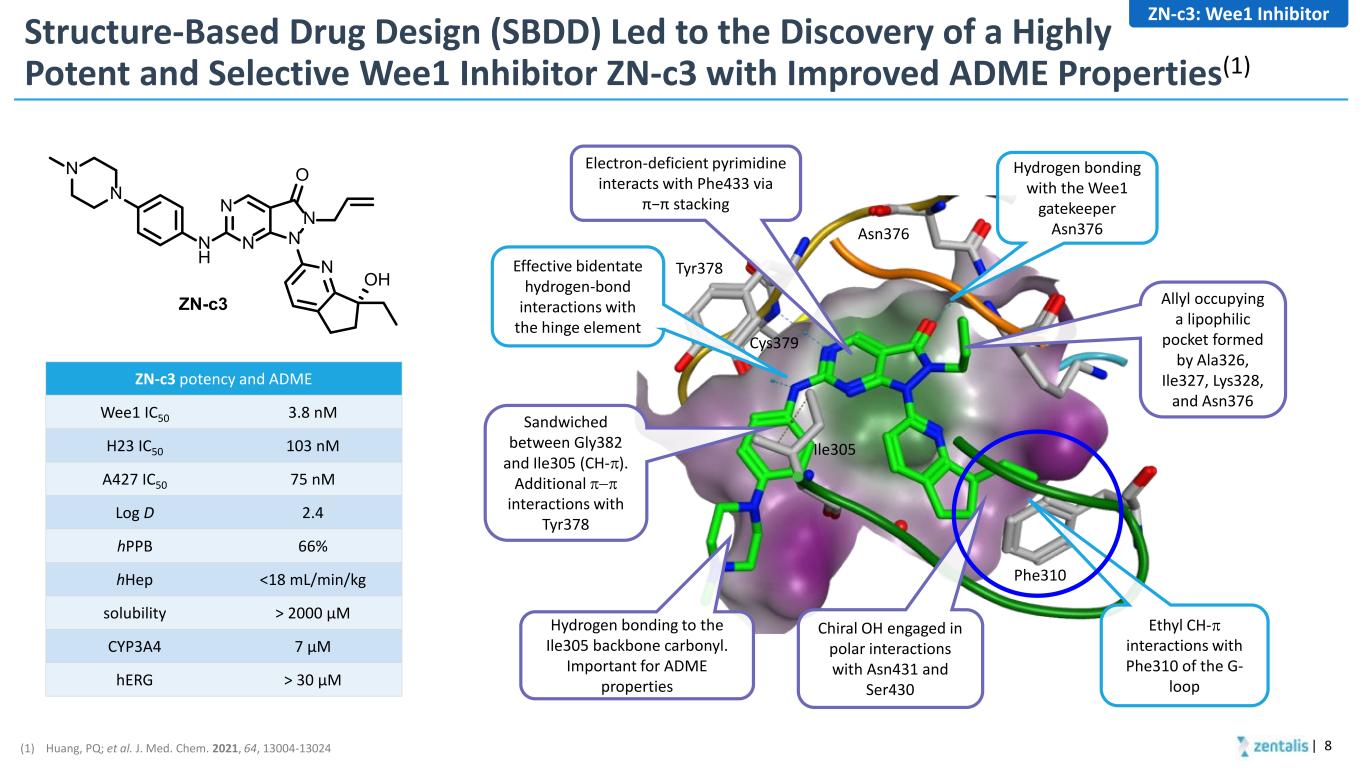
Structure-Based Drug Design (SBDD) Led to the Discovery of a Highly Potent and Selective Wee1 Inhibitor ZN-c3 with Improved ADME Properties(1) | 8 N N N N O N H N OH ZN-c3 N N Asn376 Tyr378 Cys379 Phe310 Ile305 Effective bidentate hydrogen-bond interactions with the hinge element Hydrogen bonding with the Wee1 gatekeeper Asn376 Ethyl CH-π interactions with Phe310 of the G- loop Sandwiched between Gly382 and Ile305 (CH-π). Additional π−π interactions with Tyr378 Hydrogen bonding to the Ile305 backbone carbonyl. Important for ADME properties Allyl occupying a lipophilic pocket formed by Ala326, Ile327, Lys328, and Asn376 Electron-deficient pyrimidine interacts with Phe433 via π−π stacking Chiral OH engaged in polar interactions with Asn431 and Ser430 ZN-c3 potency and ADME Wee1 IC50 3.8 nM H23 IC50 103 nM A427 IC50 75 nM Log D 2.4 hPPB 66% hHep <18 mL/min/kg solubility > 2000 µM CYP3A4 7 µM hERG > 30 µM ZN-c3: Wee1 Inhibitor (1) Huang, PQ; et al. J. Med. Chem. 2021, 64, 13004-13024
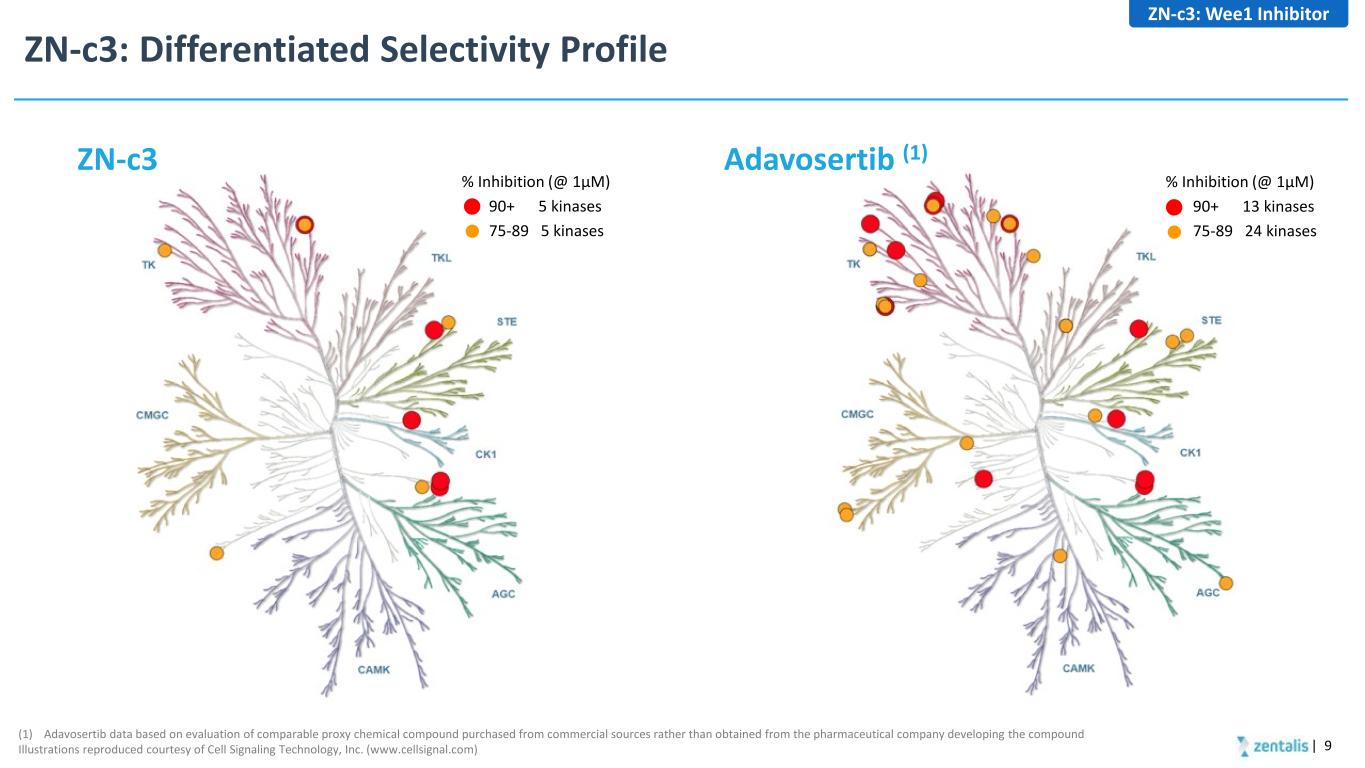
ZN-c3: Differentiated Selectivity Profile | 9 % Inhibition (@ 1µM) 90+ 13 kinases 75-89 24 kinases % Inhibition (@ 1µM) 90+ 5 kinases 75-89 5 kinases (1) Adavosertib data based on evaluation of comparable proxy chemical compound purchased from commercial sources rather than obtained from the pharmaceutical company developing the compound Illustrations reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com) Adavosertib (1)ZN-c3 ZN-c3: Wee1 Inhibitor
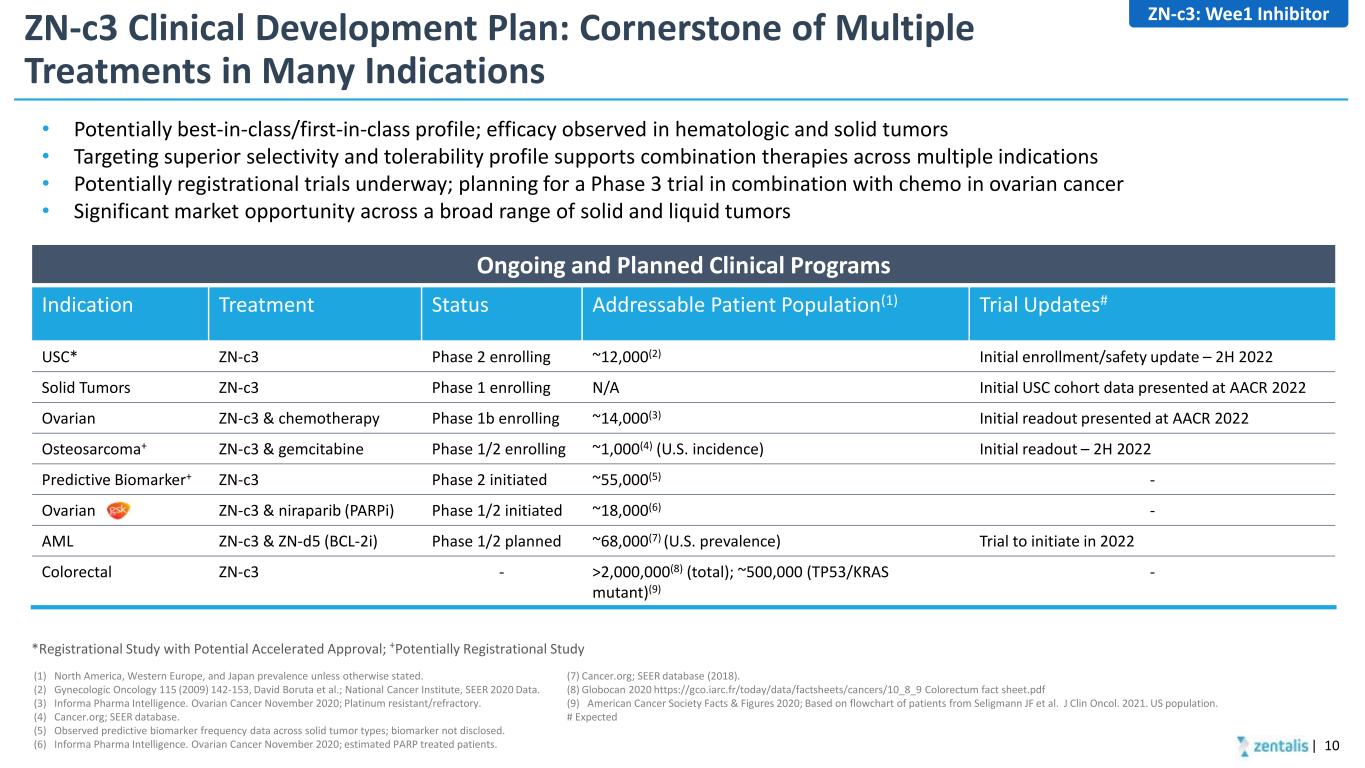
ZN-c3 Clinical Development Plan: Cornerstone of Multiple Treatments in Many Indications • Potentially best-in-class/first-in-class profile; efficacy observed in hematologic and solid tumors • Targeting superior selectivity and tolerability profile supports combination therapies across multiple indications • Potentially registrational trials underway; planning for a Phase 3 trial in combination with chemo in ovarian cancer • Significant market opportunity across a broad range of solid and liquid tumors *Registrational Study with Potential Accelerated Approval; +Potentially Registrational Study Ongoing and Planned Clinical Programs Indication Treatment Status Addressable Patient Population(1) Trial Updates# USC* ZN-c3 Phase 2 enrolling ~12,000(2) Initial enrollment/safety update – 2H 2022 Solid Tumors ZN-c3 Phase 1 enrolling N/A Initial USC cohort data presented at AACR 2022 Ovarian ZN-c3 & chemotherapy Phase 1b enrolling ~14,000(3) Initial readout presented at AACR 2022 Osteosarcoma+ ZN-c3 & gemcitabine Phase 1/2 enrolling ~1,000(4) (U.S. incidence) Initial readout – 2H 2022 Predictive Biomarker+ ZN-c3 Phase 2 initiated ~55,000(5) - Ovarian ZN-c3 & niraparib (PARPi) Phase 1/2 initiated ~18,000(6) - AML ZN-c3 & ZN-d5 (BCL-2i) Phase 1/2 planned ~68,000(7) (U.S. prevalence) Trial to initiate in 2022 Colorectal ZN-c3 - >2,000,000(8) (total); ~500,000 (TP53/KRAS mutant)(9) - (1) North America, Western Europe, and Japan prevalence unless otherwise stated. (2) Gynecologic Oncology 115 (2009) 142-153, David Boruta et al.; National Cancer Institute, SEER 2020 Data. (3) Informa Pharma Intelligence. Ovarian Cancer November 2020; Platinum resistant/refractory. (4) Cancer.org; SEER database. (5) Observed predictive biomarker frequency data across solid tumor types; biomarker not disclosed. (6) Informa Pharma Intelligence. Ovarian Cancer November 2020; estimated PARP treated patients. (7) Cancer.org; SEER database (2018). (8) Globocan 2020 https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9 Colorectum fact sheet.pdf (9) American Cancer Society Facts & Figures 2020; Based on flowchart of patients from Seligmann JF et al. J Clin Oncol. 2021. US population. # Expected | 10 ZN-c3: Wee1 Inhibitor
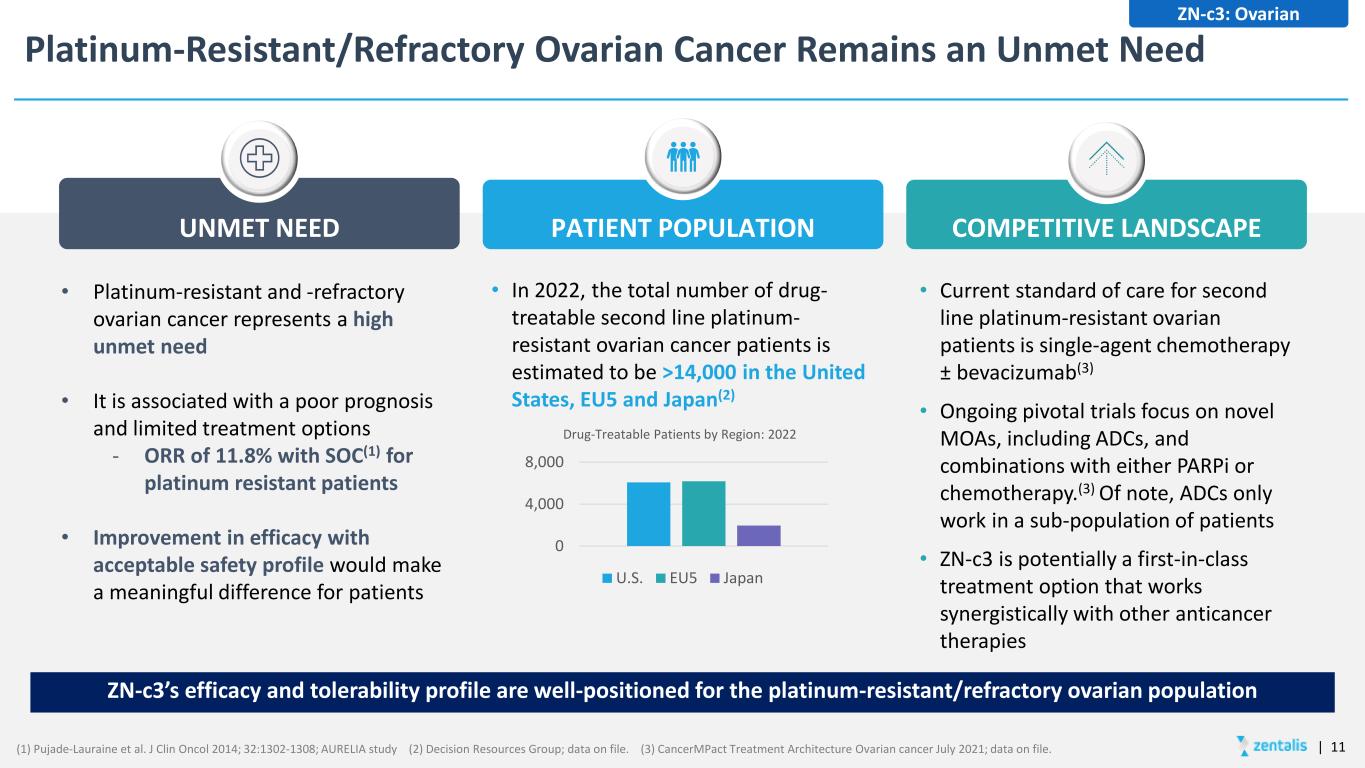
Platinum-Resistant/Refractory Ovarian Cancer Remains an Unmet Need UNMET NEED PATIENT POPULATION COMPETITIVE LANDSCAPE ZN-c3: Ovarian ZN-c3’s efficacy and tolerability profile are well-positioned for the platinum-resistant/refractory ovarian population • Platinum-resistant and -refractory ovarian cancer represents a high unmet need • It is associated with a poor prognosis and limited treatment options - ORR of 11.8% with SOC(1) for platinum resistant patients • Improvement in efficacy with acceptable safety profile would make a meaningful difference for patients • In 2022, the total number of drug- treatable second line platinum- resistant ovarian cancer patients is estimated to be >14,000 in the United States, EU5 and Japan(2) • Current standard of care for second line platinum-resistant ovarian patients is single-agent chemotherapy ± bevacizumab(3) • Ongoing pivotal trials focus on novel MOAs, including ADCs, and combinations with either PARPi or chemotherapy.(3) Of note, ADCs only work in a sub-population of patients • ZN-c3 is potentially a first-in-class treatment option that works synergistically with other anticancer therapies (1) Pujade-Lauraine et al. J Clin Oncol 2014; 32:1302-1308; AURELIA study (2) Decision Resources Group; data on file. (3) CancerMPact Treatment Architecture Ovarian cancer July 2021; data on file. 0 4,000 8,000 Drug-Treatable Patients by Region: 2022 U.S. EU5 Japan | 11
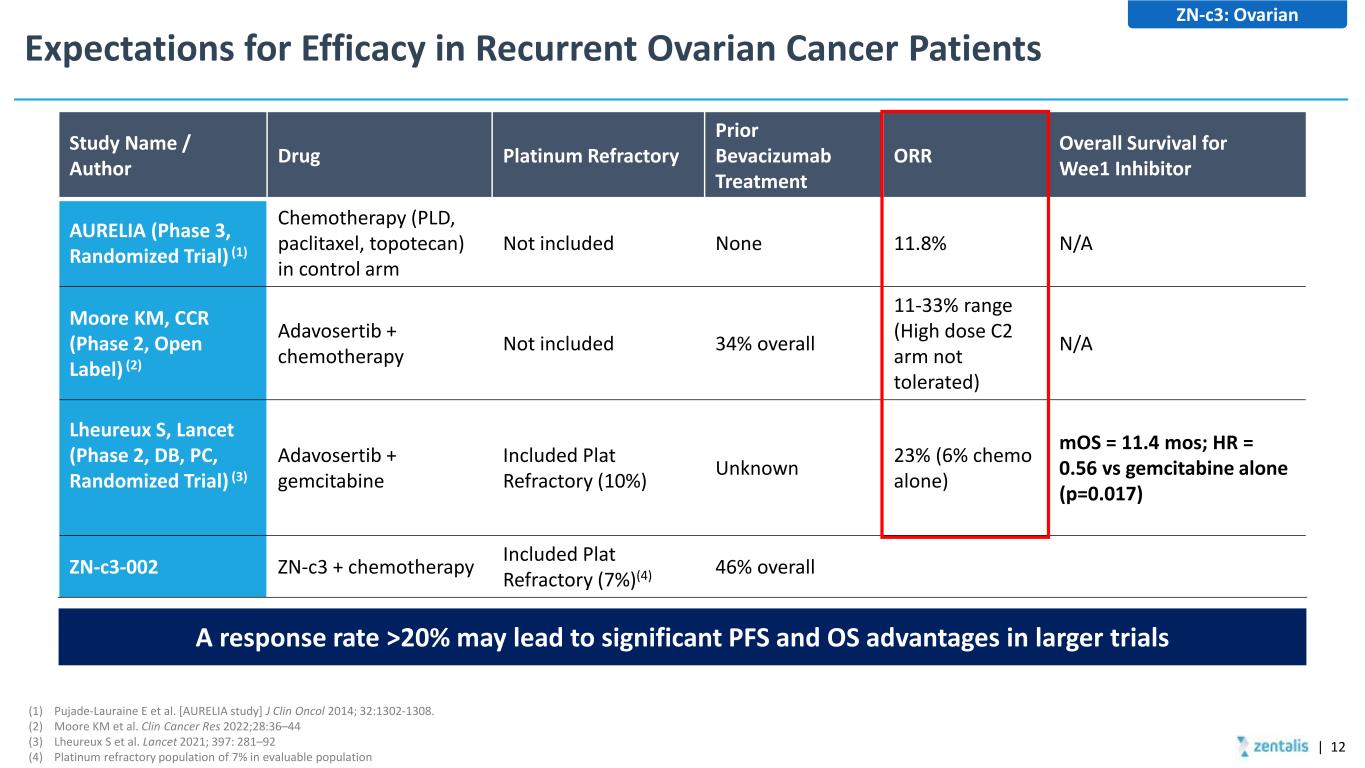
Expectations for Efficacy in Recurrent Ovarian Cancer Patients | 12 ZN-c3: Ovarian (1) Pujade-Lauraine E et al. [AURELIA study] J Clin Oncol 2014; 32:1302-1308. (2) Moore KM et al. Clin Cancer Res 2022;28:36–44 (3) Lheureux S et al. Lancet 2021; 397: 281–92 (4) Platinum refractory population of 7% in evaluable population Study Name / Author Drug Platinum Refractory Prior Bevacizumab Treatment ORR Overall Survival for Wee1 Inhibitor AURELIA (Phase 3, Randomized Trial) (1) Chemotherapy (PLD, paclitaxel, topotecan) in control arm Not included None 11.8% N/A Moore KM, CCR (Phase 2, Open Label) (2) Adavosertib + chemotherapy Not included 34% overall 11-33% range (High dose C2 arm not tolerated) N/A Lheureux S, Lancet (Phase 2, DB, PC, Randomized Trial) (3) Adavosertib + gemcitabine Included Plat Refractory (10%) Unknown 23% (6% chemo alone) mOS = 11.4 mos; HR = 0.56 vs gemcitabine alone (p=0.017) ZN-c3-002 ZN-c3 + chemotherapy Included Plat Refractory (7%)(4) 46% overall A response rate >20% may lead to significant PFS and OS advantages in larger trials
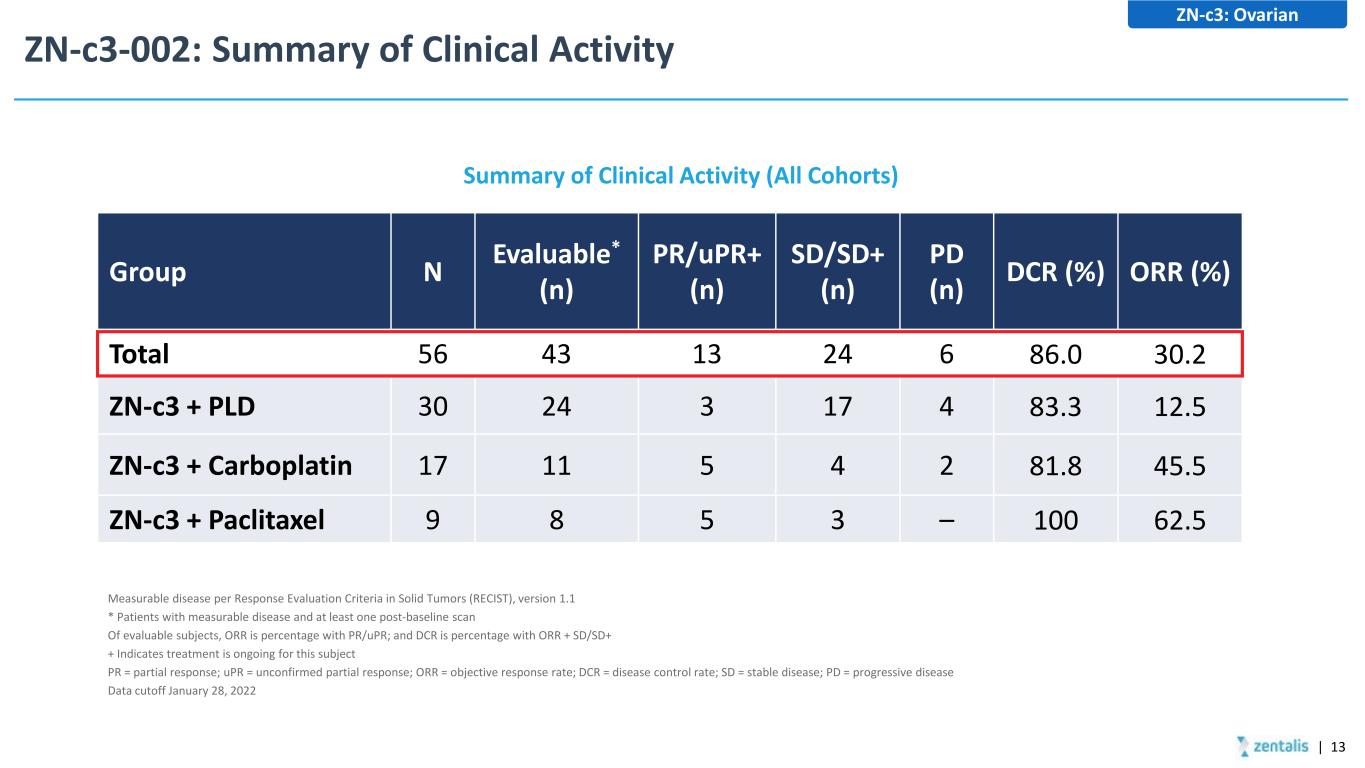
ZN-c3-002: Summary of Clinical Activity Summary of Clinical Activity (All Cohorts) ZN-c3: Ovarian Group N Evaluable* (n) PR/uPR+ (n) SD/SD+ (n) PD (n) DCR (%) ORR (%) Total 56 43 13 24 6 86.0 30.2 ZN-c3 + PLD 30 24 3 17 4 83.3 12.5 ZN-c3 + Carboplatin 17 11 5 4 2 81.8 45.5 ZN-c3 + Paclitaxel 9 8 5 3 – 100 62.5 Measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 * Patients with measurable disease and at least one post-baseline scan Of evaluable subjects, ORR is percentage with PR/uPR; and DCR is percentage with ORR + SD/SD+ + Indicates treatment is ongoing for this subject PR = partial response; uPR = unconfirmed partial response; ORR = objective response rate; DCR = disease control rate; SD = stable disease; PD = progressive disease Data cutoff January 28, 2022 | 13
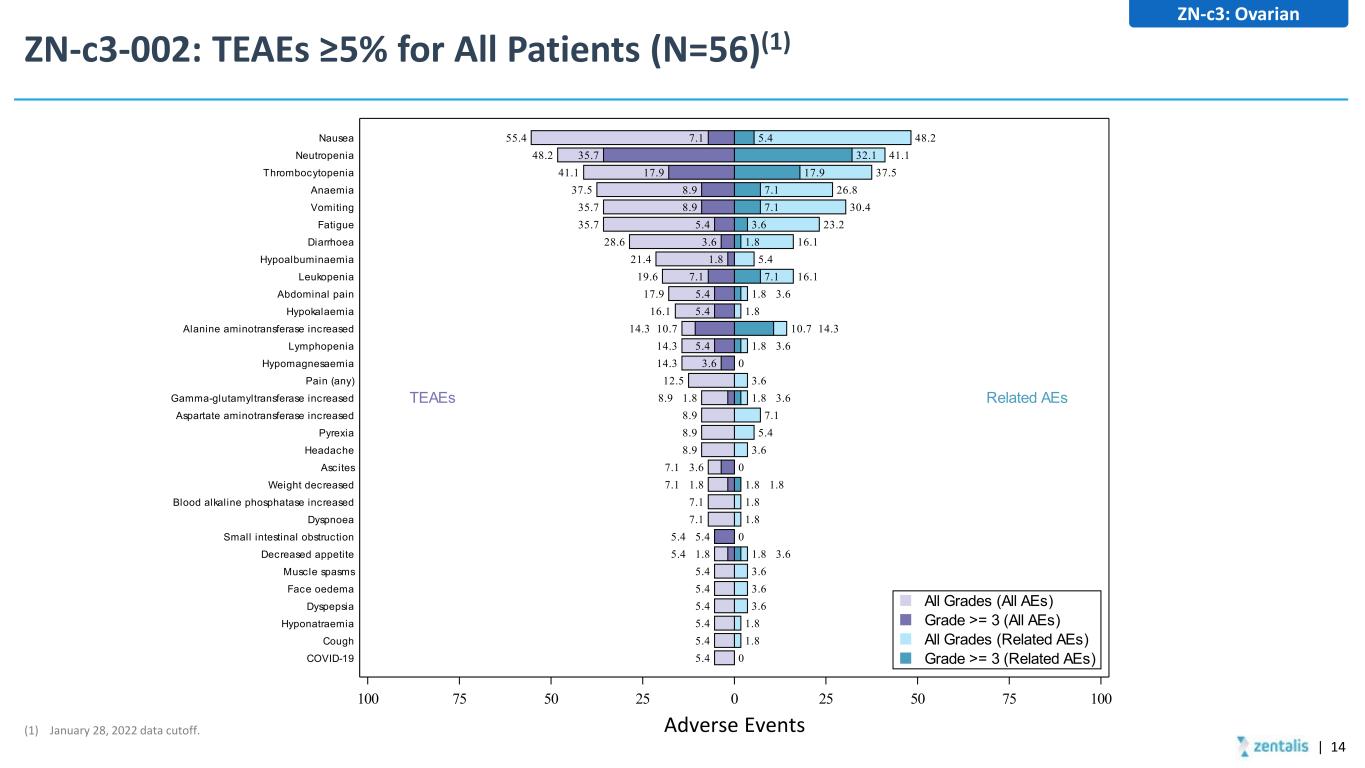
ZN-c3-002: TEAEs ≥5% for All Patients (N=56)(1) 48.2 41.1 37.5 26.8 30.4 23.2 16.1 5.4 16.1 1.8 3.6 1.8 10.7 14.3 1.8 3.6 0 3.6 1.8 3.6 7.1 5.4 3.6 0 1.8 1.8 1.8 1.8 0 1.8 3.6 3.6 3.6 3.6 1.8 1.8 0 5.4 32.1 17.9 7.1 7.1 3.6 1.8 7.1 55.4 48.2 41.1 37.5 35.7 35.7 28.6 21.4 19.6 17.9 16.1 14.3 10.7 14.3 14.3 12.5 8.9 1.8 8.9 8.9 8.9 7.1 3.6 7.1 1.8 7.1 7.1 5.4 5.4 5.4 1.8 5.4 5.4 5.4 5.4 5.4 5.4 7.1 35.7 17.9 8.9 8.9 5.4 3.6 1.8 7.1 5.4 5.4 5.4 3.6 100 75 50 25 0 25 50 75 100 COVID-19 Cough Hyponatraemia Dyspepsia Face oedema Muscle spasms Decreased appetite Small intestinal obstruction Dyspnoea Blood alkaline phosphatase increased Weight decreased Ascites Headache Pyrexia Aspartate aminotransferase increased Gamma-glutamyltransferase increased Pain (any) Hypomagnesaemia Lymphopenia Alanine aminotransferase increased Hypokalaemia Abdominal pain Leukopenia Hypoalbuminaemia Diarrhoea Fatigue Vomiting Anaemia Thrombocytopenia Neutropenia Nausea TEAEs Related AEs Grade >= 3 (Related AEs) All Grades (Related AEs) Grade >= 3 (All AEs) All Grades (All AEs) Adverse Events ZN-c3: Ovarian (1) January 28, 2022 data cutoff. | 14
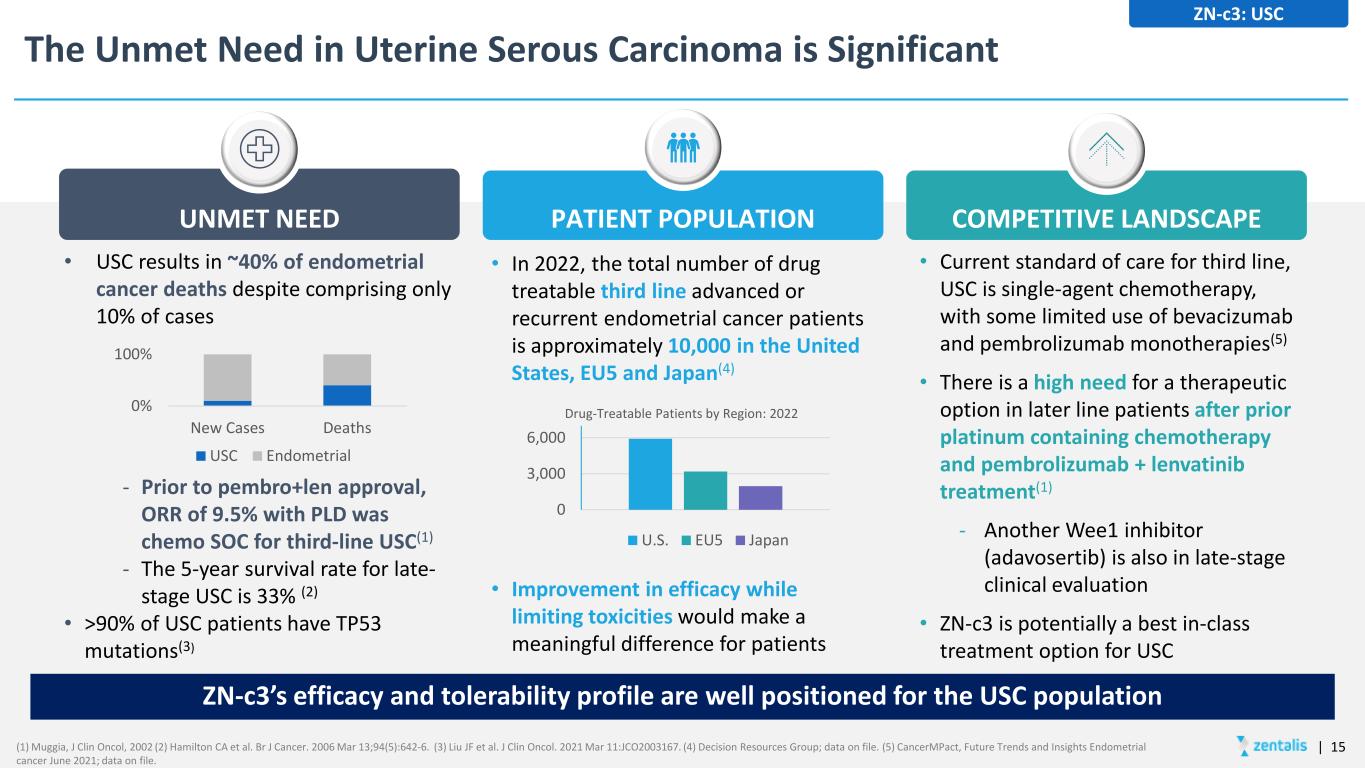
The Unmet Need in Uterine Serous Carcinoma is Significant UNMET NEED PATIENT POPULATION COMPETITIVE LANDSCAPE ZN-c3: USC • USC results in ~40% of endometrial cancer deaths despite comprising only 10% of cases - Prior to pembro+len approval, ORR of 9.5% with PLD was chemo SOC for third-line USC(1) - The 5-year survival rate for late- stage USC is 33% (2) • >90% of USC patients have TP53 mutations(3) 0% 100% New Cases Deaths USC Endometrial • In 2022, the total number of drug treatable third line advanced or recurrent endometrial cancer patients is approximately 10,000 in the United States, EU5 and Japan(4) • Improvement in efficacy while limiting toxicities would make a meaningful difference for patients 0 3,000 6,000 Drug-Treatable Patients by Region: 2022 U.S. EU5 Japan ZN-c3’s efficacy and tolerability profile are well positioned for the USC population • Current standard of care for third line, USC is single-agent chemotherapy, with some limited use of bevacizumab and pembrolizumab monotherapies(5) • There is a high need for a therapeutic option in later line patients after prior platinum containing chemotherapy and pembrolizumab + lenvatinib treatment(1) - Another Wee1 inhibitor (adavosertib) is also in late-stage clinical evaluation • ZN-c3 is potentially a best in-class treatment option for USC | 15(1) Muggia, J Clin Oncol, 2002 (2) Hamilton CA et al. Br J Cancer. 2006 Mar 13;94(5):642-6. (3) Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167. (4) Decision Resources Group; data on file. (5) CancerMPact, Future Trends and Insights Endometrial cancer June 2021; data on file.
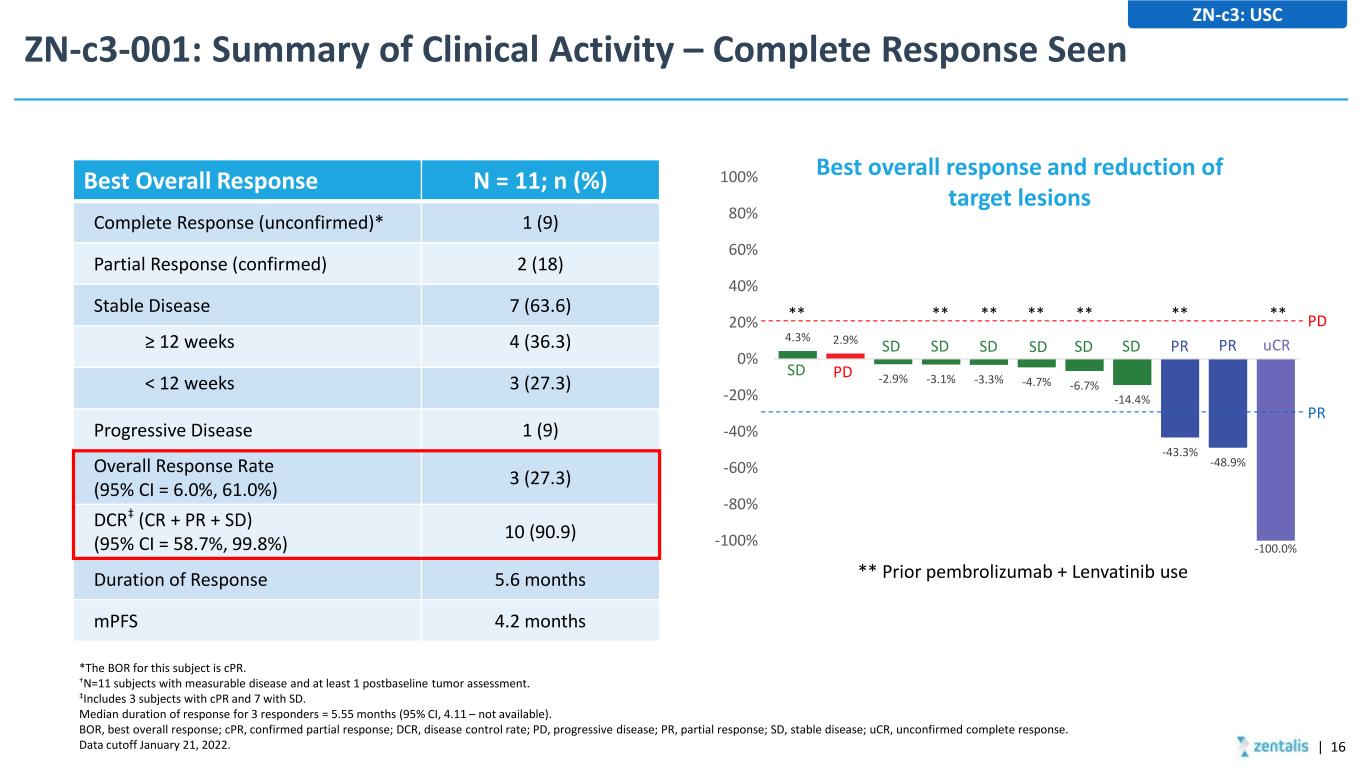
ZN-c3-001: Summary of Clinical Activity – Complete Response Seen | 16 ZN-c3: USC Best Overall Response N = 11; n (%) Complete Response (unconfirmed)* 1 (9) Partial Response (confirmed) 2 (18) Stable Disease 7 (63.6) ≥ 12 weeks 4 (36.3) < 12 weeks 3 (27.3) Progressive Disease 1 (9) Overall Response Rate (95% CI = 6.0%, 61.0%) 3 (27.3) DCR‡ (CR + PR + SD) (95% CI = 58.7%, 99.8%) 10 (90.9) Duration of Response 5.6 months mPFS 4.2 months *The BOR for this subject is cPR. †N=11 subjects with measurable disease and at least 1 postbaseline tumor assessment. ‡Includes 3 subjects with cPR and 7 with SD. Median duration of response for 3 responders = 5.55 months (95% CI, 4.11 – not available). BOR, best overall response; cPR, confirmed partial response; DCR, disease control rate; PD, progressive disease; PR, partial response; SD, stable disease; uCR, unconfirmed complete response. Data cutoff January 21, 2022. 4.3% 2.9% -2.9% -3.1% -3.3% -4.7% -6.7% -14.4% -43.3% -48.9% -100.0%-100% -80% -60% -40% -20% 0% 20% 40% 60% 80% 100% PD PR Best overall response and reduction of target lesions SD PD SD SD SD SD SD SD PR PR uCR ** Prior pembrolizumab + Lenvatinib use ** ** ** ** ** ** **
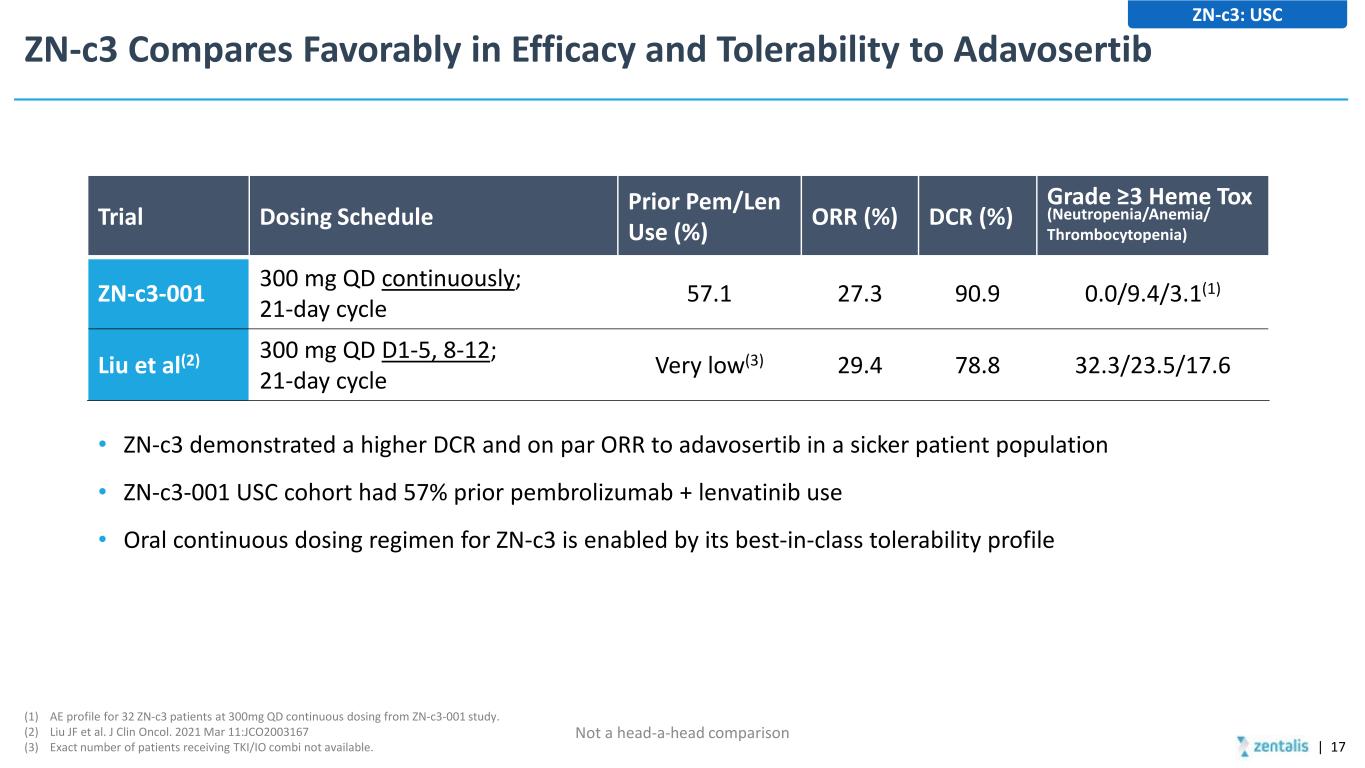
ZN-c3 Compares Favorably in Efficacy and Tolerability to Adavosertib • ZN-c3 demonstrated a higher DCR and on par ORR to adavosertib in a sicker patient population • ZN-c3-001 USC cohort had 57% prior pembrolizumab + lenvatinib use • Oral continuous dosing regimen for ZN-c3 is enabled by its best-in-class tolerability profile | 17 ZN-c3: USC (1) AE profile for 32 ZN-c3 patients at 300mg QD continuous dosing from ZN-c3-001 study. (2) Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (3) Exact number of patients receiving TKI/IO combi not available. Trial Dosing Schedule Prior Pem/Len Use (%) ORR (%) DCR (%) Grade ≥3 Heme Tox (Neutropenia/Anemia/ Thrombocytopenia) ZN-c3-001 300 mg QD continuously; 21-day cycle 57.1 27.3 90.9 0.0/9.4/3.1(1) Liu et al(2) 300 mg QD D1-5, 8-12; 21-day cycle Very low(3) 29.4 78.8 32.3/23.5/17.6 Not a head-a-head comparison
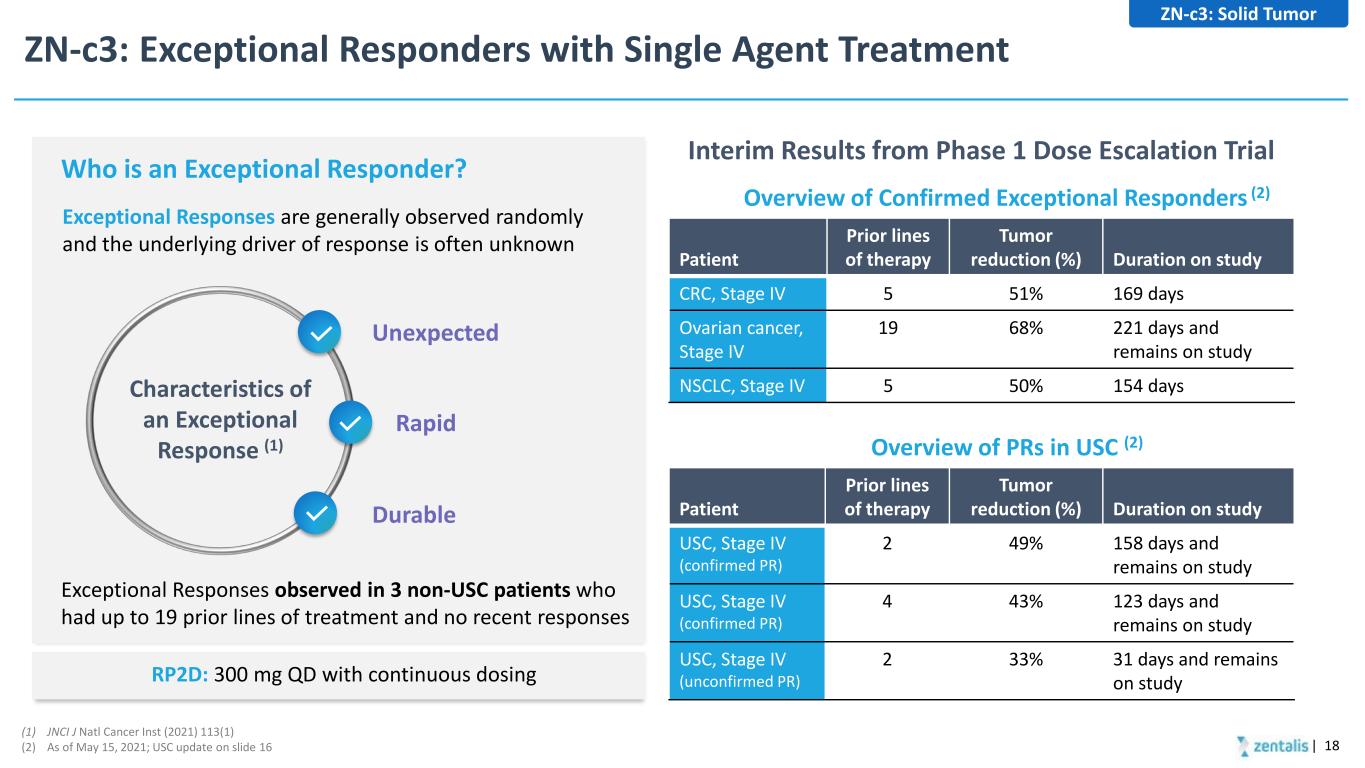
ZN-c3: Exceptional Responders with Single Agent Treatment | 18 Patient Prior lines of therapy Tumor reduction (%) Duration on study CRC, Stage IV 5 51% 169 days Ovarian cancer, Stage IV 19 68% 221 days and remains on study NSCLC, Stage IV 5 50% 154 days Overview of Confirmed Exceptional Responders (2) Patient Prior lines of therapy Tumor reduction (%) Duration on study USC, Stage IV (confirmed PR) 2 49% 158 days and remains on study USC, Stage IV (confirmed PR) 4 43% 123 days and remains on study USC, Stage IV (unconfirmed PR) 2 33% 31 days and remains on study Overview of PRs in USC (2) Interim Results from Phase 1 Dose Escalation Trial Exceptional Responses are generally observed randomly and the underlying driver of response is often unknown Who is an Exceptional Responder? Unexpected Characteristics of an Exceptional Response (1) Rapid Durable Exceptional Responses observed in 3 non-USC patients who had up to 19 prior lines of treatment and no recent responses (1) JNCI J Natl Cancer Inst (2021) 113(1) (2) As of May 15, 2021; USC update on slide 16 ZN-c3: Solid Tumor RP2D: 300 mg QD with continuous dosing
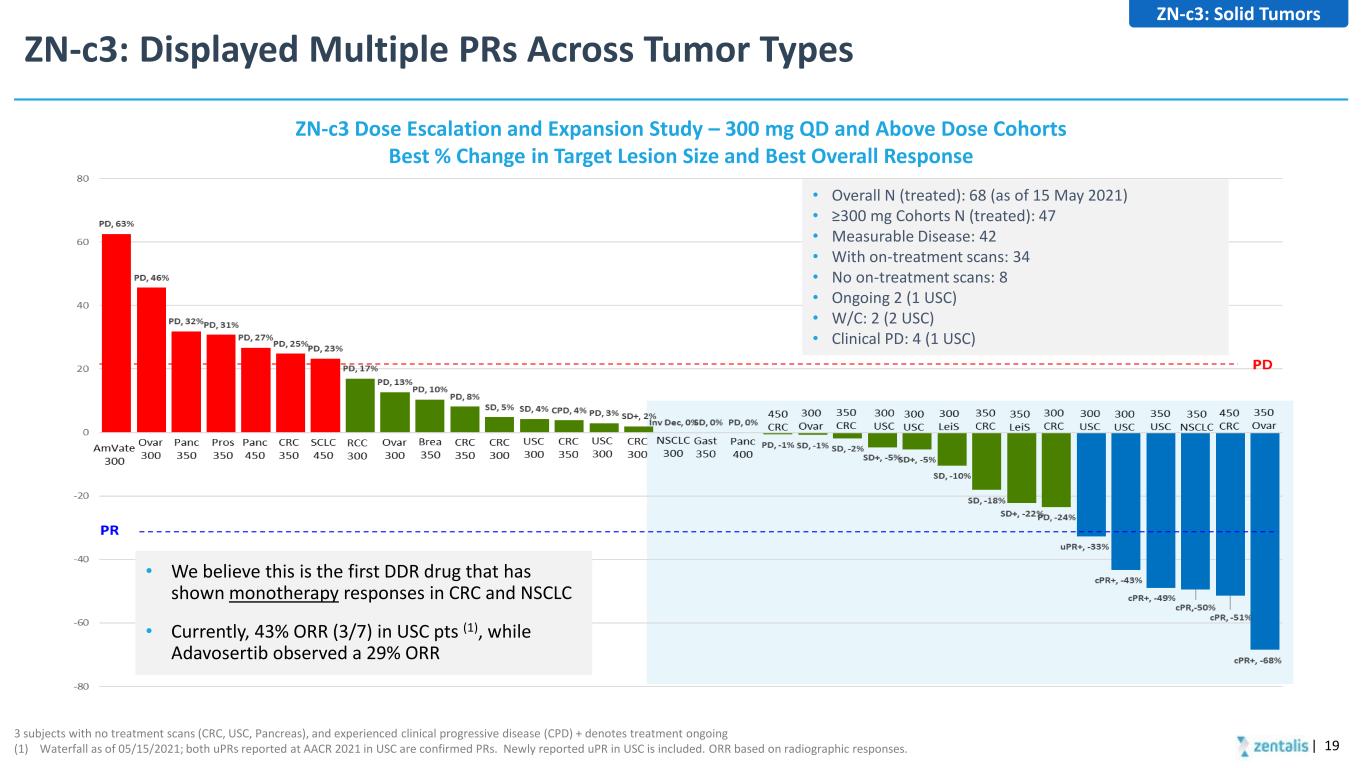
ZN-c3: Displayed Multiple PRs Across Tumor Types | 19 3 subjects with no treatment scans (CRC, USC, Pancreas), and experienced clinical progressive disease (CPD) + denotes treatment ongoing (1) Waterfall as of 05/15/2021; both uPRs reported at AACR 2021 in USC are confirmed PRs. Newly reported uPR in USC is included. ORR based on radiographic responses. • Overall N (treated): 68 (as of 15 May 2021) • ≥300 mg Cohorts N (treated): 47 • Measurable Disease: 42 • With on-treatment scans: 34 • No on-treatment scans: 8 • Ongoing 2 (1 USC) • W/C: 2 (2 USC) • Clinical PD: 4 (1 USC) ZN-c3 Dose Escalation and Expansion Study – 300 mg QD and Above Dose Cohorts Best % Change in Target Lesion Size and Best Overall Response • We believe this is the first DDR drug that has shown monotherapy responses in CRC and NSCLC • Currently, 43% ORR (3/7) in USC pts (1), while Adavosertib observed a 29% ORR ZN-c3: Solid Tumors
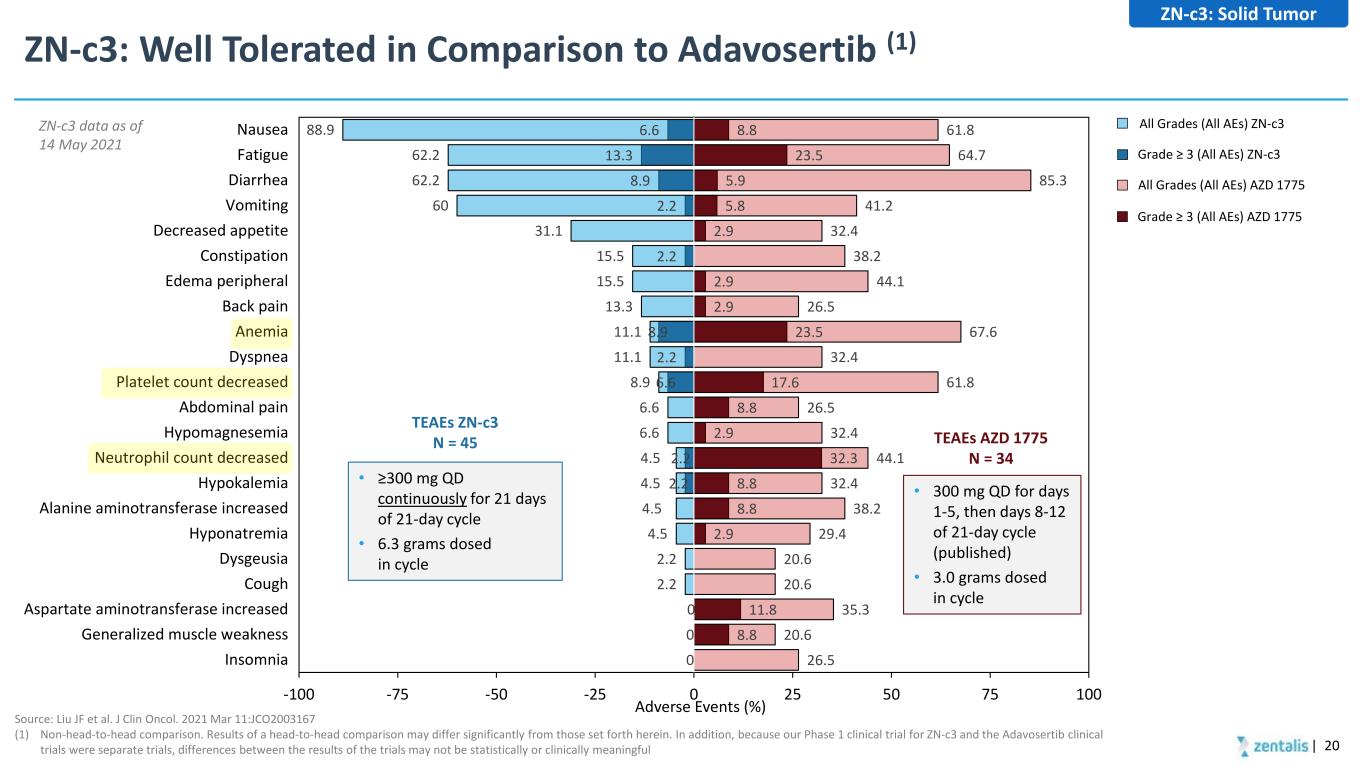
2.2 2.2 6.6 2.2 8.9 2.2 2.2 8.9 13.3 6.6 8.8 11.8 2.9 8.8 8.8 32.3 2.9 8.8 17.6 23.5 2.9 2.9 2.9 5.8 5.9 23.5 8.8 26.5 20.6 35.3 20.6 20.6 29.4 38.2 32.4 44.1 32.4 26.5 61.8 32.4 67.6 26.5 44.1 38.2 32.4 41.2 85.3 64.7 61.8 0 0 0 2.2 2.2 4.5 4.5 4.5 4.5 6.6 6.6 8.9 11.1 11.1 13.3 15.5 15.5 31.1 60 62.2 62.2 88.9 -100 -75 -50 -25 0 25 50 75 100 Insomnia Generalized muscle weakness Aspartate aminotransferase increased Cough Dysgeusia Hyponatremia Alanine aminotransferase increased Hypokalemia Neutrophil count decreased Hypomagnesemia Abdominal pain Platelet count decreased Dyspnea Anemia Back pain Edema peripheral Constipation Decreased appetite Vomiting Diarrhea Fatigue NauseaZN-c3 data as of 14 May 2021 ZN-c3: Well Tolerated in Comparison to Adavosertib (1) | 20 Source: Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (1) Non-head-to-head comparison. Results of a head-to-head comparison may differ significantly from those set forth herein. In addition, because our Phase 1 clinical trial for ZN-c3 and the Adavosertib clinical trials were separate trials, differences between the results of the trials may not be statistically or clinically meaningful Adverse Events (%) • ≥300 mg QD continuously for 21 days of 21-day cycle • 6.3 grams dosed in cycle • 300 mg QD for days 1-5, then days 8-12 of 21-day cycle (published) • 3.0 grams dosed in cycle TEAEs AZD 1775 N = 34 TEAEs ZN-c3 N = 45 All Grades (All AEs) ZN-c3 Grade ≥ 3 (All AEs) ZN-c3 All Grades (All AEs) AZD 1775 Grade ≥ 3 (All AEs) AZD 1775 ZN-c3: Solid Tumor
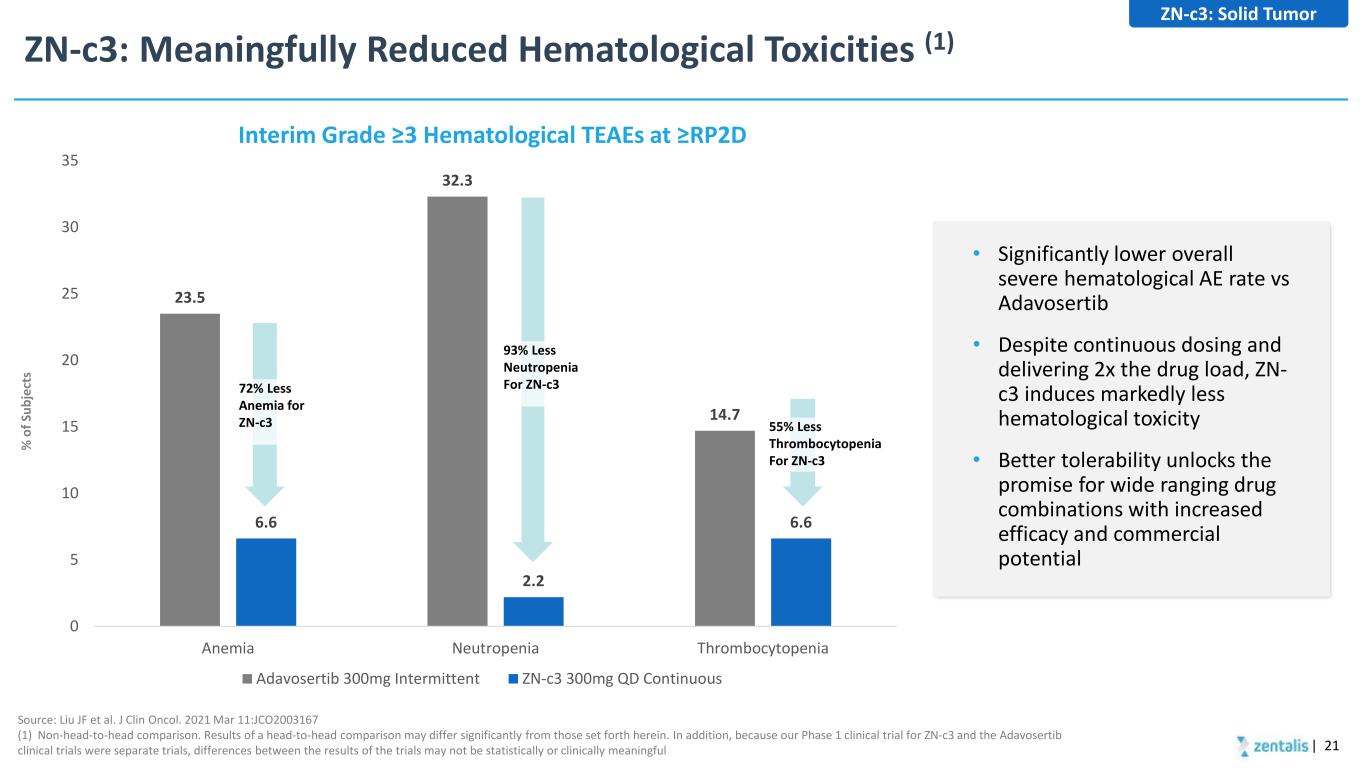
ZN-c3: Meaningfully Reduced Hematological Toxicities (1) | 21 Source: Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (1) Non-head-to-head comparison. Results of a head-to-head comparison may differ significantly from those set forth herein. In addition, because our Phase 1 clinical trial for ZN-c3 and the Adavosertib clinical trials were separate trials, differences between the results of the trials may not be statistically or clinically meaningful 23.5 32.3 14.7 6.6 2.2 6.6 0 5 10 15 20 25 30 35 Anemia Neutropenia Thrombocytopenia Chart Title Adavosertib 300mg Intermittent ZN-c3 300mg QD Continuous Interim Grade ≥3 Hematological TEAEs at ≥RP2D % o f S ub je ct s 55% Less Thrombocytopenia For ZN-c3 93% Less Neutropenia For ZN-c372% Less Anemia for ZN-c3 • Significantly lower overall severe hematological AE rate vs Adavosertib • Despite continuous dosing and delivering 2x the drug load, ZN- c3 induces markedly less hematological toxicity • Better tolerability unlocks the promise for wide ranging drug combinations with increased efficacy and commercial potential ZN-c3: Solid Tumor
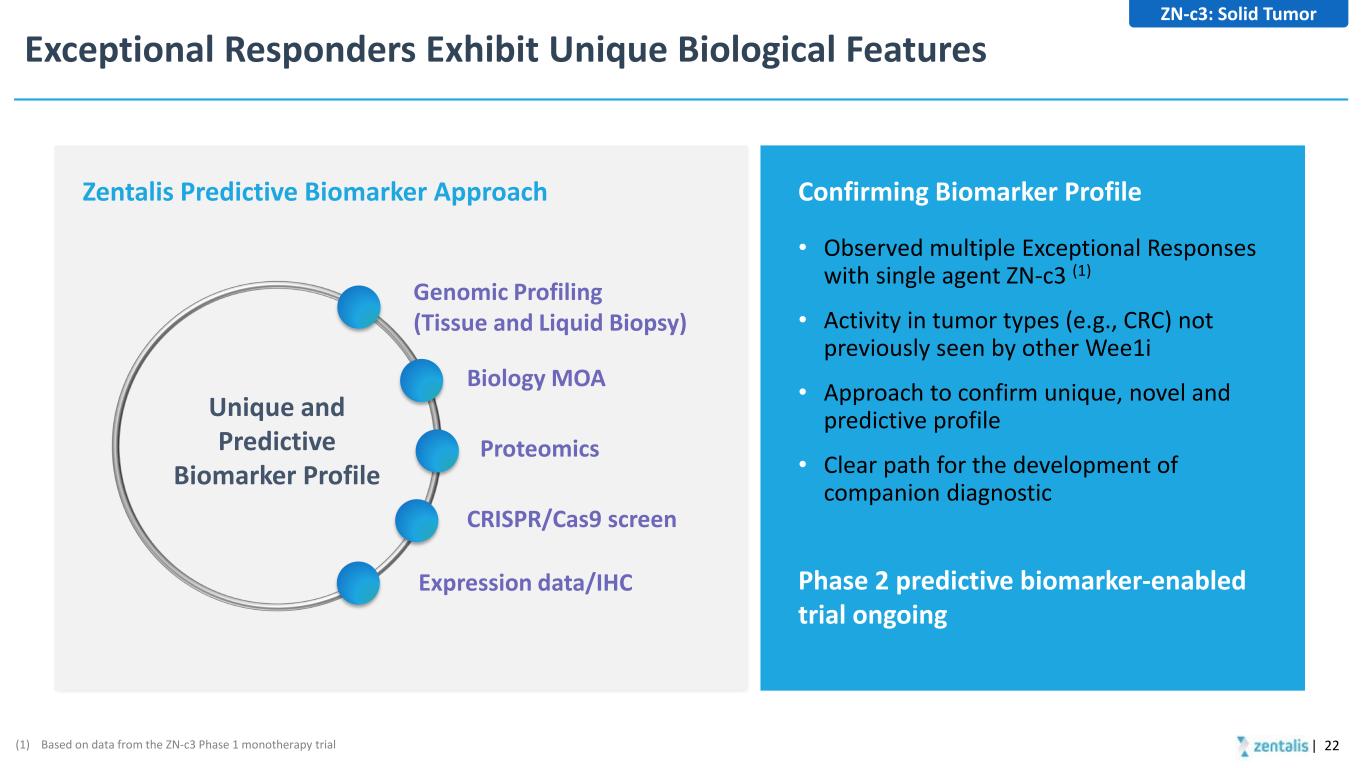
Exceptional Responders Exhibit Unique Biological Features | 22 • Observed multiple Exceptional Responses with single agent ZN-c3 (1) • Activity in tumor types (e.g., CRC) not previously seen by other Wee1i • Approach to confirm unique, novel and predictive profile • Clear path for the development of companion diagnostic Confirming Biomarker Profile Phase 2 predictive biomarker-enabled trial ongoing (1) Based on data from the ZN-c3 Phase 1 monotherapy trial Zentalis Predictive Biomarker Approach Genomic Profiling (Tissue and Liquid Biopsy) Biology MOA Proteomics CRISPR/Cas9 screen Expression data/IHC Unique and Predictive Biomarker Profile ZN-c3: Solid Tumor
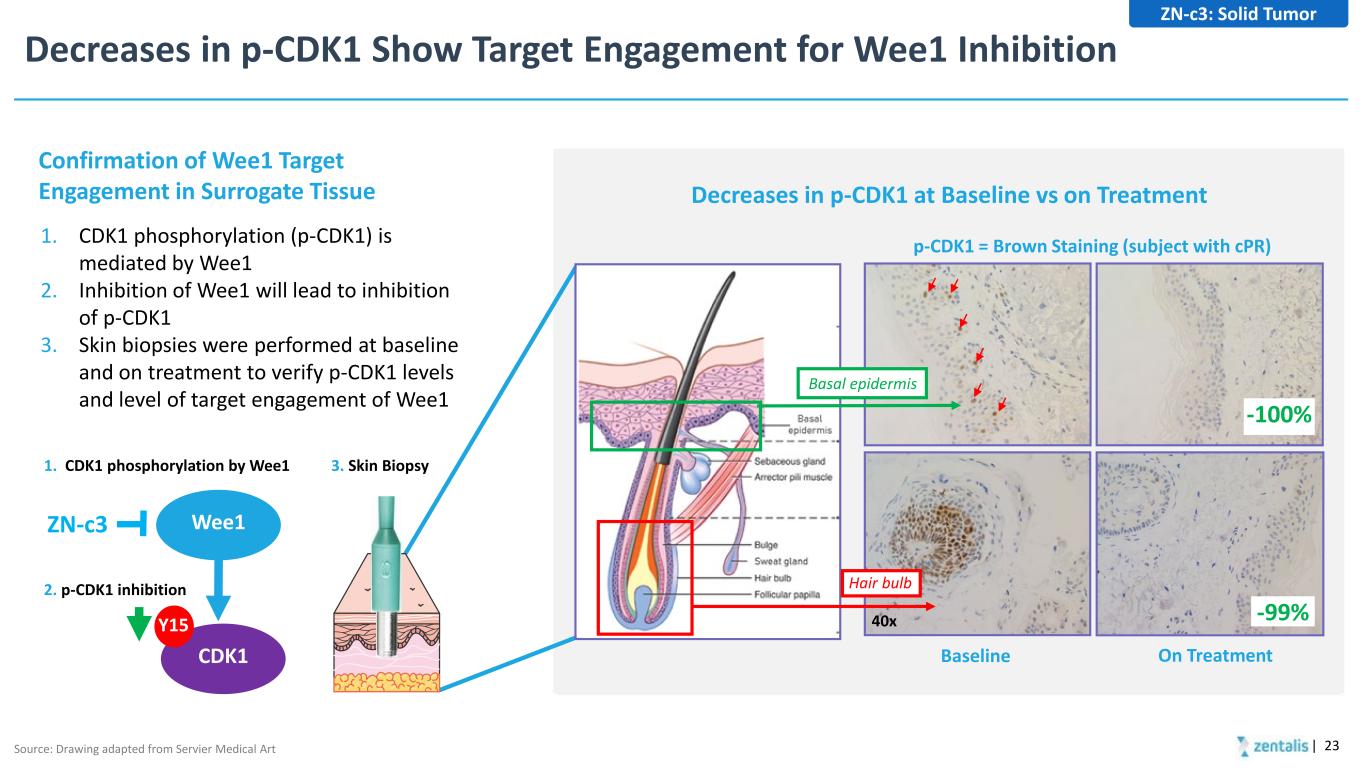
Decreases in p-CDK1 Show Target Engagement for Wee1 Inhibition | 23 p-CDK1 = Brown Staining (subject with cPR) Baseline On Treatment 40x -100% -99% Decreases in p-CDK1 at Baseline vs on Treatment Basal epidermis Hair bulb 1. CDK1 phosphorylation (p-CDK1) is mediated by Wee1 2. Inhibition of Wee1 will lead to inhibition of p-CDK1 3. Skin biopsies were performed at baseline and on treatment to verify p-CDK1 levels and level of target engagement of Wee1 Confirmation of Wee1 Target Engagement in Surrogate Tissue Wee1 CDK1 Y15 ZN-c3 1. CDK1 phosphorylation by Wee1 2. p-CDK1 inhibition 3. Skin Biopsy Source: Drawing adapted from Servier Medical Art ZN-c3: Solid Tumor

ZN-c3: PK/PD Correlation Shows Active Target Engagement at RP2D | 24 M or e W ee 1 Ta rg et E ng ag em en t 0 5000 10000 15000 20000 -100 -50 0 50 100 Average drug exposure at RP2D AUC0-24 hr (ng*h/mL) p- CD K1 (% in hi bi tio n) Wee1 Target Engagement Higher Drug Exposure • Inhibition of p-CDK1 demonstrated Wee1 target engagement • Increase in dose / drug exposure directly related to Wee1 target engagement • RP2D showed highest AUC, with excellent target engagement and p-CDK1 levels decreased at least by 50% ZN-c3: Solid Tumor
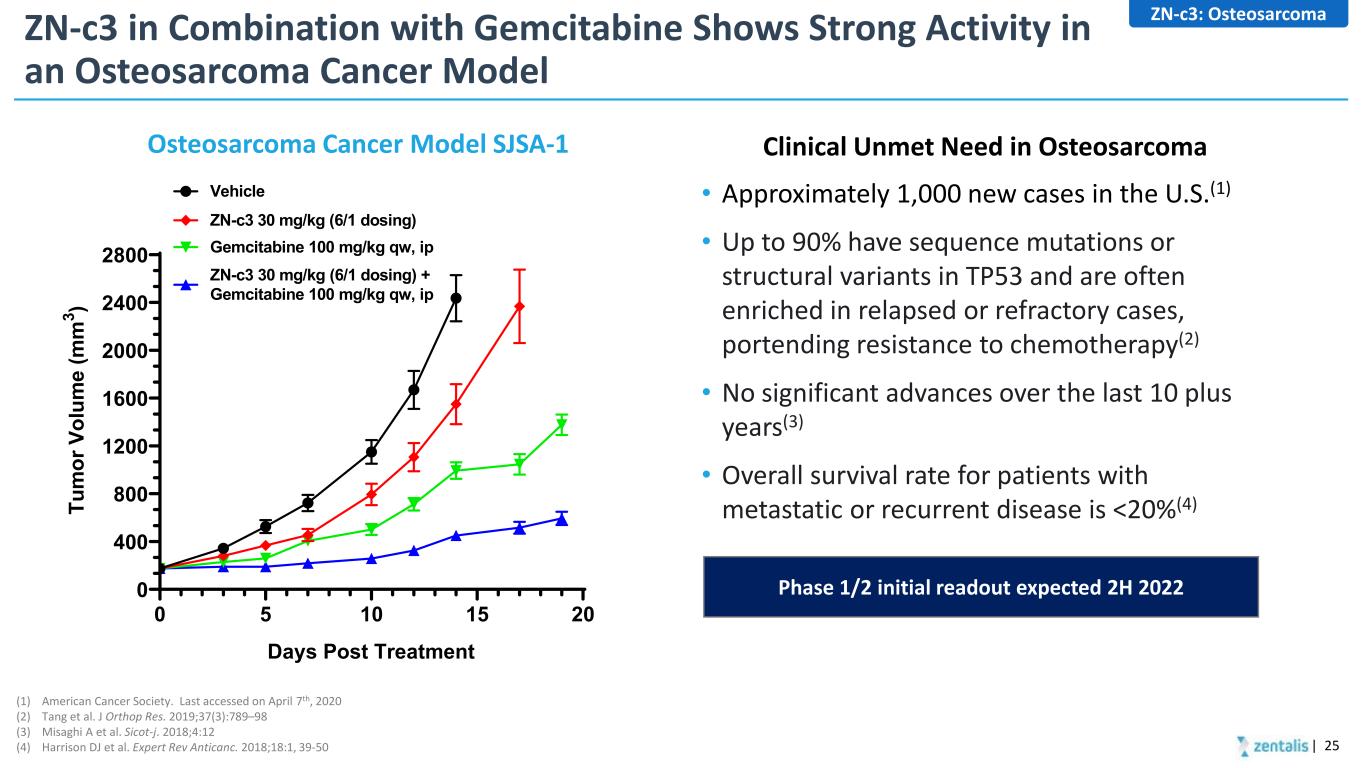
ZN-c3 in Combination with Gemcitabine Shows Strong Activity in an Osteosarcoma Cancer Model • Approximately 1,000 new cases in the U.S.(1) • Up to 90% have sequence mutations or structural variants in TP53 and are often enriched in relapsed or refractory cases, portending resistance to chemotherapy(2) • No significant advances over the last 10 plus years(3) • Overall survival rate for patients with metastatic or recurrent disease is <20%(4) Osteosarcoma Cancer Model SJSA-1 Clinical Unmet Need in Osteosarcoma ZN-c3: Osteosarcoma (1) American Cancer Society. Last accessed on April 7th, 2020 (2) Tang et al. J Orthop Res. 2019;37(3):789–98 (3) Misaghi A et al. Sicot-j. 2018;4:12 (4) Harrison DJ et al. Expert Rev Anticanc. 2018;18:1, 39-50 Phase 1/2 initial readout expected 2H 2022 0 5 10 15 20 0 400 800 1200 1600 2000 2400 2800 Days Post Treatment Tu m or V ol um e (m m 3 ) Gemcitabine 100 mg/kg qw, ip ZN-c3 30 mg/kg (6/1 dosing) + Gemcitabine 100 mg/kg qw, ip Vehicle ZN-c3 30 mg/kg (6/1 dosing) | 25
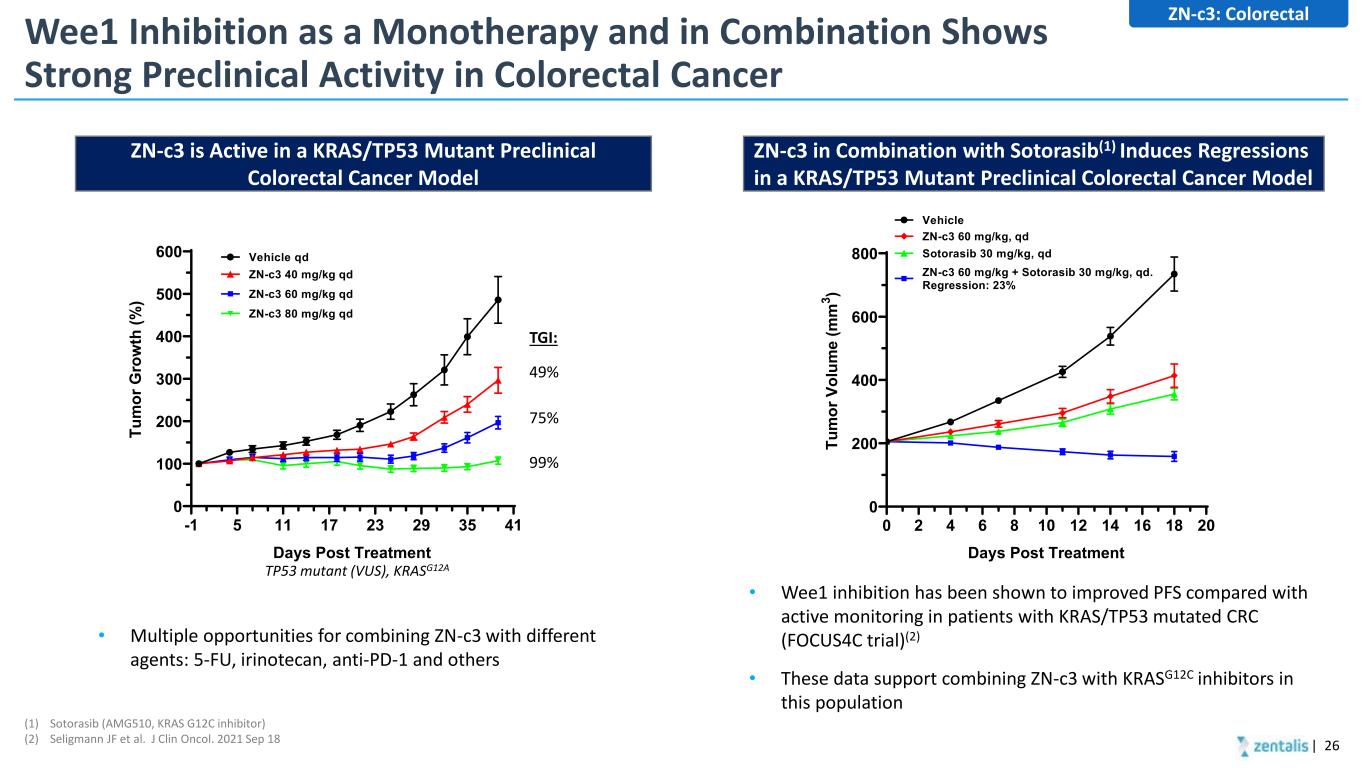
Wee1 Inhibition as a Monotherapy and in Combination Shows Strong Preclinical Activity in Colorectal Cancer | 26 ZN-c3: Colorectal (1) Sotorasib (AMG510, KRAS G12C inhibitor) (2) Seligmann JF et al. J Clin Oncol. 2021 Sep 18 -1 5 11 17 23 29 35 41 0 100 200 300 400 500 600 Vehicle qd ZN-c3 60 mg/kg qd ZN-c3 40 mg/kg qd Days Post Treatment Tu m or G ro w th (% ) ZN-c3 80 mg/kg qd 49% 75% 99% TGI: TP53 mutant (VUS), KRASG12A • Multiple opportunities for combining ZN-c3 with different agents: 5-FU, irinotecan, anti-PD-1 and others ZN-c3 is Active in a KRAS/TP53 Mutant Preclinical Colorectal Cancer Model 0 2 4 6 8 10 12 14 16 18 20 0 200 400 600 800 Vehicle ZN-c3 60 mg/kg, qd Sotorasib 30 mg/kg, qd ZN-c3 60 mg/kg + Sotorasib 30 mg/kg, qd. Regression: 23% Days Post Treatment Tu m or V ol um e (m m 3 ) • Wee1 inhibition has been shown to improved PFS compared with active monitoring in patients with KRAS/TP53 mutated CRC (FOCUS4C trial)(2) • These data support combining ZN-c3 with KRASG12C inhibitors in this population ZN-c3 in Combination with Sotorasib(1) Induces Regressions in a KRAS/TP53 Mutant Preclinical Colorectal Cancer Model
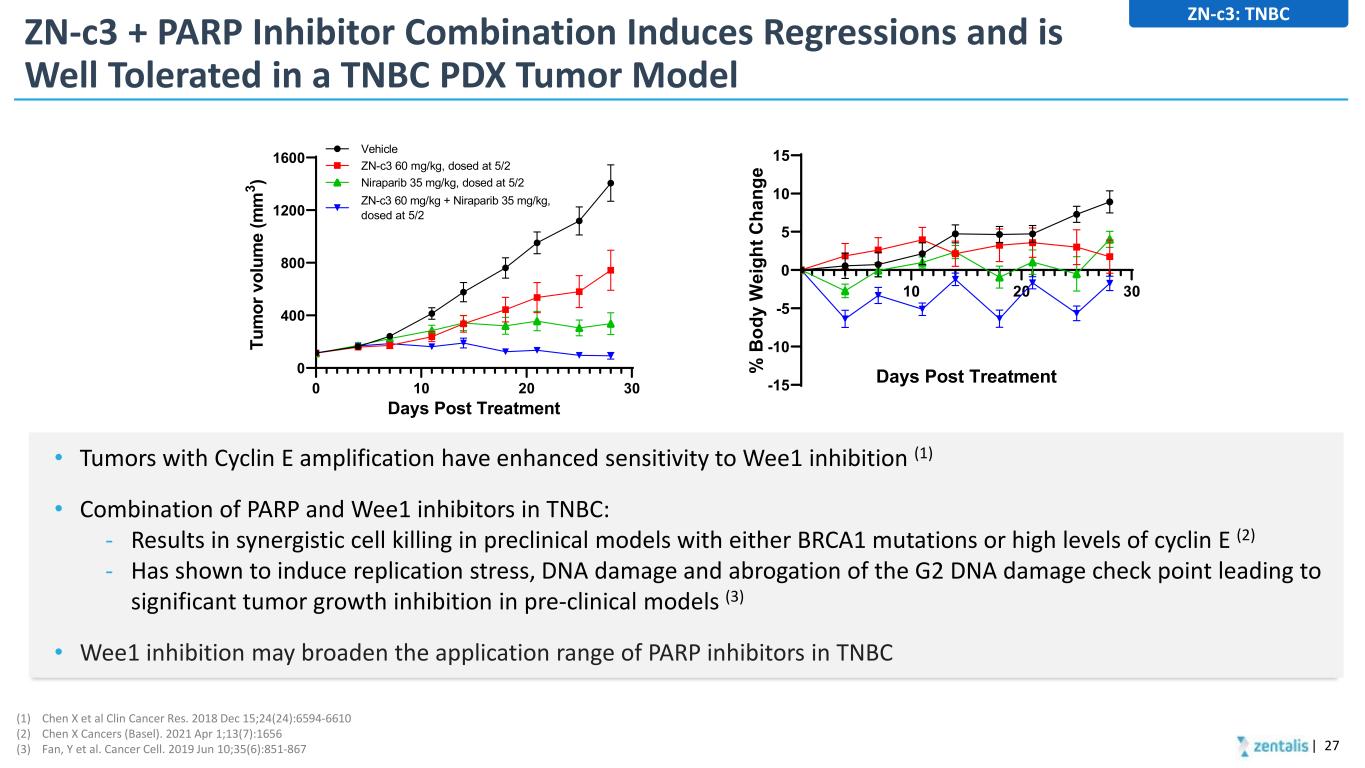
ZN-c3 + PARP Inhibitor Combination Induces Regressions and is Well Tolerated in a TNBC PDX Tumor Model • Tumors with Cyclin E amplification have enhanced sensitivity to Wee1 inhibition (1) • Combination of PARP and Wee1 inhibitors in TNBC: - Results in synergistic cell killing in preclinical models with either BRCA1 mutations or high levels of cyclin E (2) - Has shown to induce replication stress, DNA damage and abrogation of the G2 DNA damage check point leading to significant tumor growth inhibition in pre-clinical models (3) • Wee1 inhibition may broaden the application range of PARP inhibitors in TNBC | 27 0 10 20 30 0 400 800 1200 1600 Days Post Treatment Tu m or v ol um e (m m 3 ) Vehicle ZN-c3 60 mg/kg, dosed at 5/2 Niraparib 35 mg/kg, dosed at 5/2 ZN-c3 60 mg/kg + Niraparib 35 mg/kg, dosed at 5/2 10 20 30 -15 -10 -5 0 5 10 15 Days Post Treatment% B od y W ei gh t C ha ng e (1) Chen X et al Clin Cancer Res. 2018 Dec 15;24(24):6594-6610 (2) Chen X Cancers (Basel). 2021 Apr 1;13(7):1656 (3) Fan, Y et al. Cancer Cell. 2019 Jun 10;35(6):851-867 ZN-c3: TNBC

Z N - d 5 BCL-2 Inhibitor
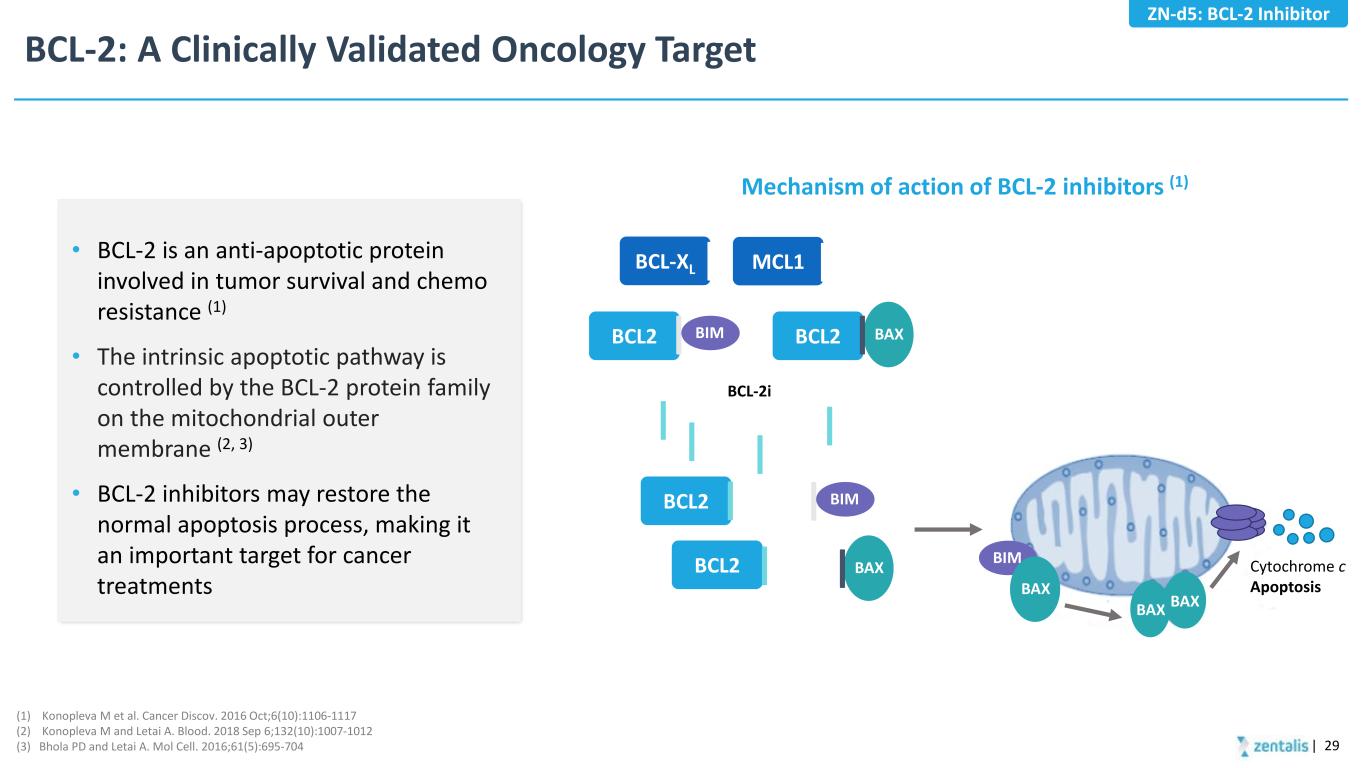
BAX BCL-2: A Clinically Validated Oncology Target • BCL-2 is an anti-apoptotic protein involved in tumor survival and chemo resistance (1) • The intrinsic apoptotic pathway is controlled by the BCL-2 protein family on the mitochondrial outer membrane (2, 3) • BCL-2 inhibitors may restore the normal apoptosis process, making it an important target for cancer treatments | 29 Mechanism of action of BCL-2 inhibitors (1) (1) Konopleva M et al. Cancer Discov. 2016 Oct;6(10):1106-1117 (2) Konopleva M and Letai A. Blood. 2018 Sep 6;132(10):1007-1012 (3) Bhola PD and Letai A. Mol Cell. 2016;61(5):695-704 ZN-d5: BCL-2 Inhibitor BCL2 BIM BCL2 BCL-XL MCL1 BCL2 BCL2 BCL-2i BIM BAX BIM BAX BAX BAX Cytochrome c Apoptosis
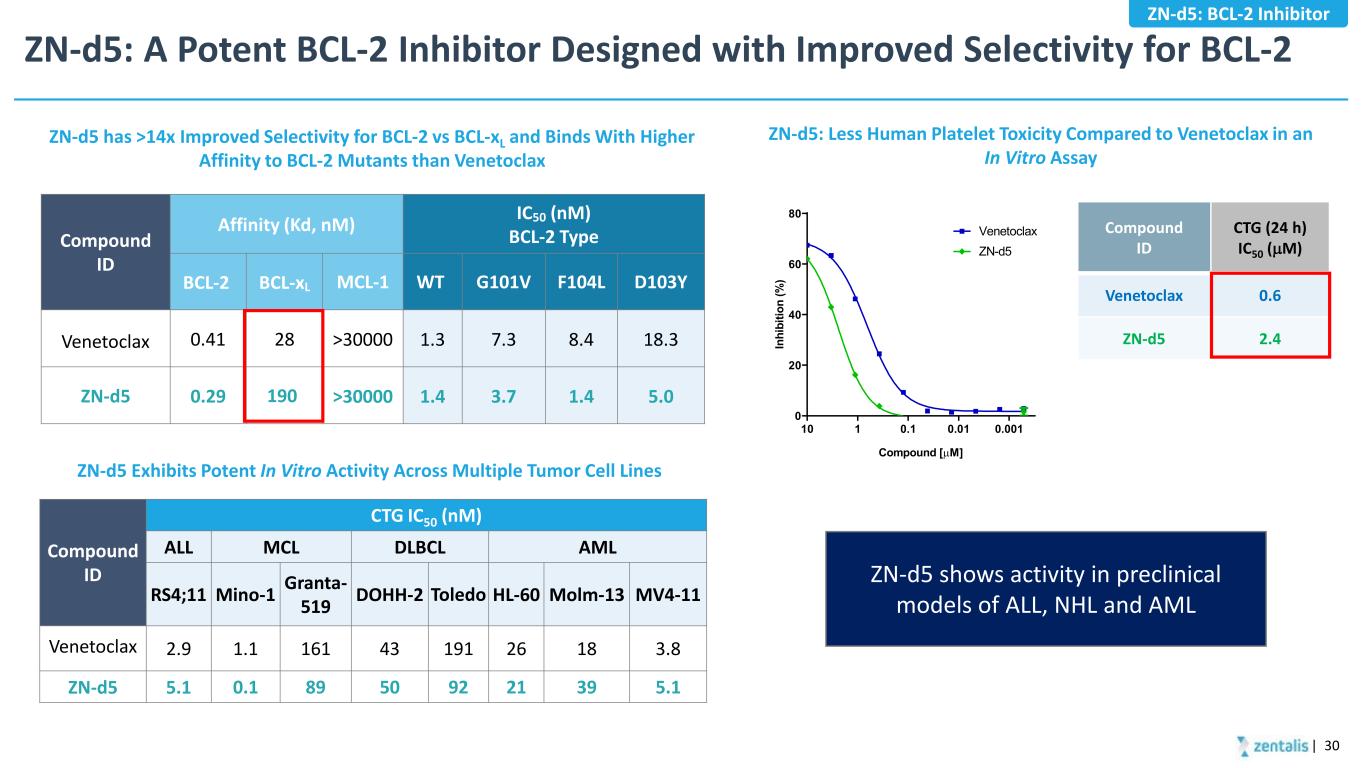
ZN-d5: A Potent BCL-2 Inhibitor Designed with Improved Selectivity for BCL-2 | 30 Compound ID Affinity (Kd, nM) IC50 (nM) BCL-2 Type BCL-2 BCL-xL MCL-1 WT G101V F104L D103Y Venetoclax 0.41 28 >30000 1.3 7.3 8.4 18.3 ZN-d5 0.29 190 >30000 1.4 3.7 1.4 5.0 Compound ID CTG IC50 (nM) ALL MCL DLBCL AML RS4;11 Mino-1 Granta- 519 DOHH-2 Toledo HL-60 Molm-13 MV4-11 Venetoclax 2.9 1.1 161 43 191 26 18 3.8 ZN-d5 5.1 0.1 89 50 92 21 39 5.1 ZN-d5 has >14x Improved Selectivity for BCL-2 vs BCL-xL and Binds With Higher Affinity to BCL-2 Mutants than Venetoclax ZN-d5 Exhibits Potent In Vitro Activity Across Multiple Tumor Cell Lines ZN-d5: Less Human Platelet Toxicity Compared to Venetoclax in an In Vitro Assay 0.0010.010.1110 0 20 40 60 80 Compound [µM] In hi bi tio n (% ) Venetoclax ZN-d5 Compound ID CTG (24 h) IC50 (µM) Venetoclax 0.6 ZN-d5 2.4 ZN-d5: BCL-2 Inhibitor ZN-d5 shows activity in preclinical models of ALL, NHL and AML
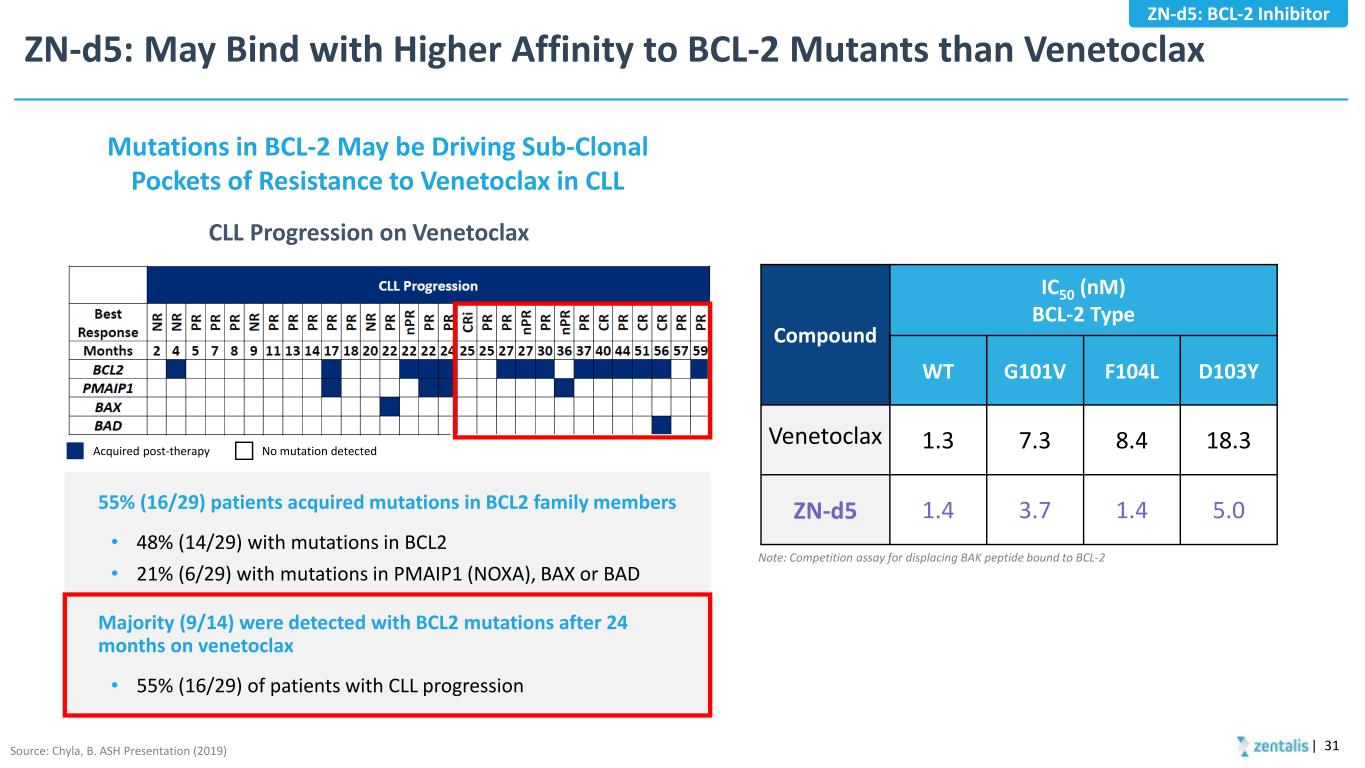
ZN-d5: May Bind with Higher Affinity to BCL-2 Mutants than Venetoclax | 31 Note: Competition assay for displacing BAK peptide bound to BCL-2 Compound IC50 (nM) BCL-2 Type WT G101V F104L D103Y Venetoclax 1.3 7.3 8.4 18.3 ZN-d5 1.4 3.7 1.4 5.0 CLL Progression on Venetoclax Mutations in BCL-2 May be Driving Sub-Clonal Pockets of Resistance to Venetoclax in CLL 55% (16/29) patients acquired mutations in BCL2 family members • 48% (14/29) with mutations in BCL2 • 21% (6/29) with mutations in PMAIP1 (NOXA), BAX or BAD Majority (9/14) were detected with BCL2 mutations after 24 months on venetoclax • 55% (16/29) of patients with CLL progression Source: Chyla, B. ASH Presentation (2019) Acquired post-therapy No mutation detected ZN-d5: BCL-2 Inhibitor
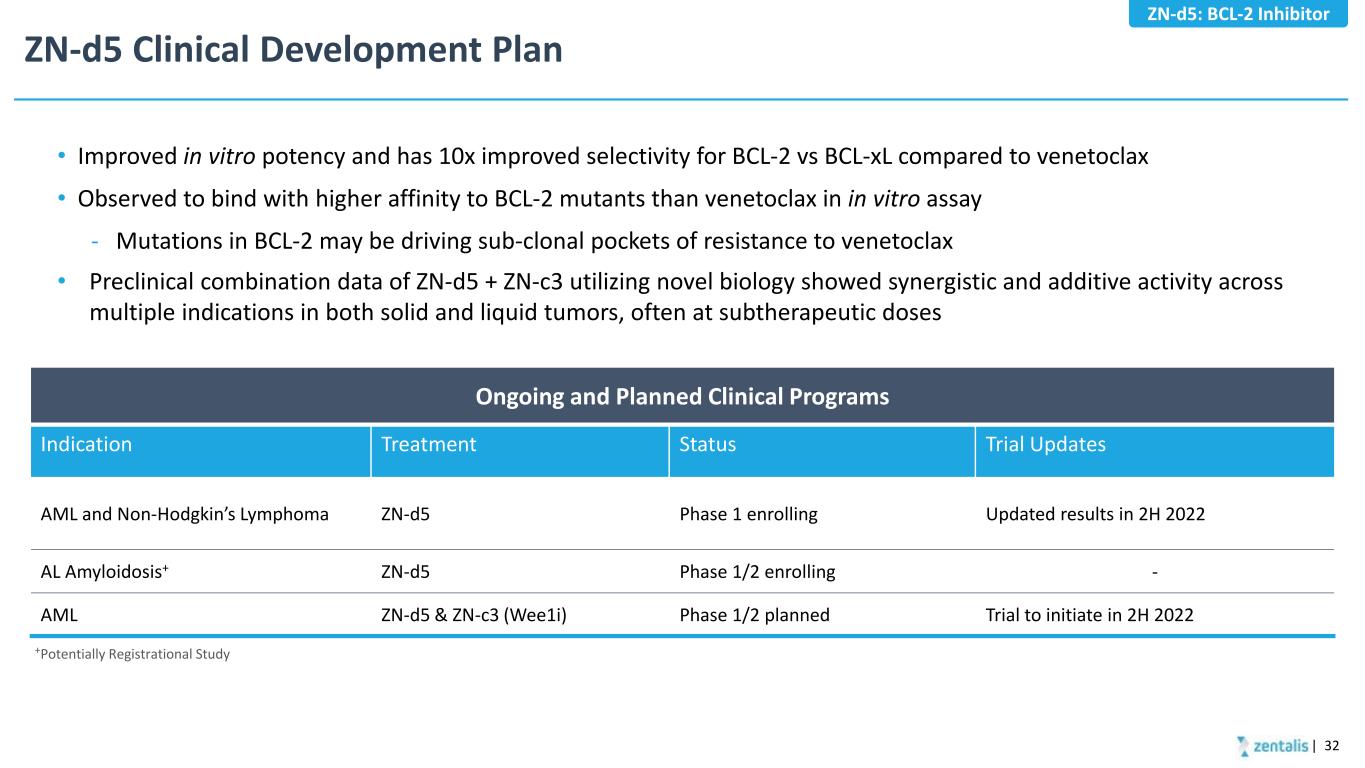
ZN-d5 Clinical Development Plan • Improved in vitro potency and has 10x improved selectivity for BCL-2 vs BCL-xL compared to venetoclax • Observed to bind with higher affinity to BCL-2 mutants than venetoclax in in vitro assay - Mutations in BCL-2 may be driving sub-clonal pockets of resistance to venetoclax • Preclinical combination data of ZN-d5 + ZN-c3 utilizing novel biology showed synergistic and additive activity across multiple indications in both solid and liquid tumors, often at subtherapeutic doses +Potentially Registrational Study Ongoing and Planned Clinical Programs Indication Treatment Status Trial Updates AML and Non-Hodgkin’s Lymphoma ZN-d5 Phase 1 enrolling Updated results in 2H 2022 AL Amyloidosis+ ZN-d5 Phase 1/2 enrolling - AML ZN-d5 & ZN-c3 (Wee1i) Phase 1/2 planned Trial to initiate in 2H 2022 | 32 ZN-d5: BCL-2 Inhibitor
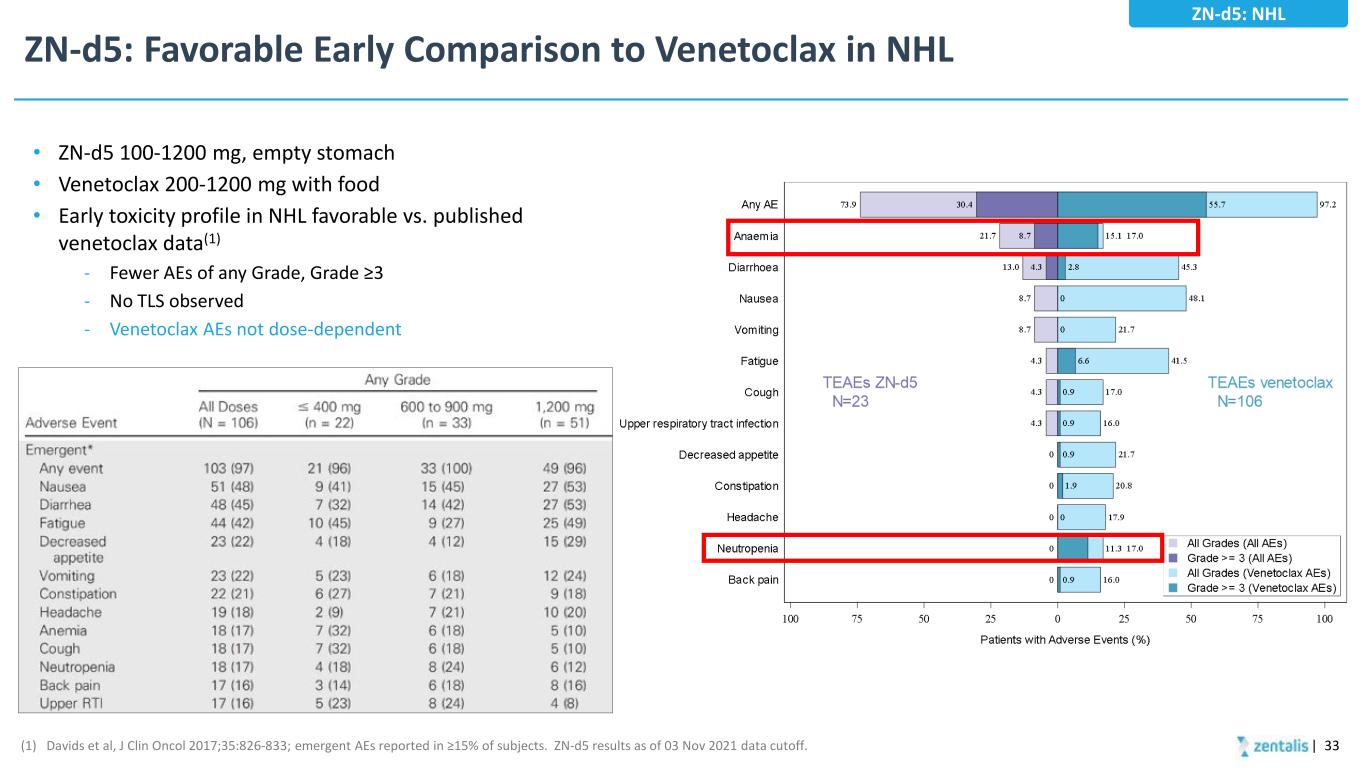
ZN-d5: Favorable Early Comparison to Venetoclax in NHL • ZN-d5 100-1200 mg, empty stomach • Venetoclax 200-1200 mg with food • Early toxicity profile in NHL favorable vs. published venetoclax data(1) - Fewer AEs of any Grade, Grade ≥3 - No TLS observed - Venetoclax AEs not dose-dependent (1) Davids et al, J Clin Oncol 2017;35:826-833; emergent AEs reported in ≥15% of subjects. ZN-d5 results as of 03 Nov 2021 data cutoff. ZN-d5: NHL | 33
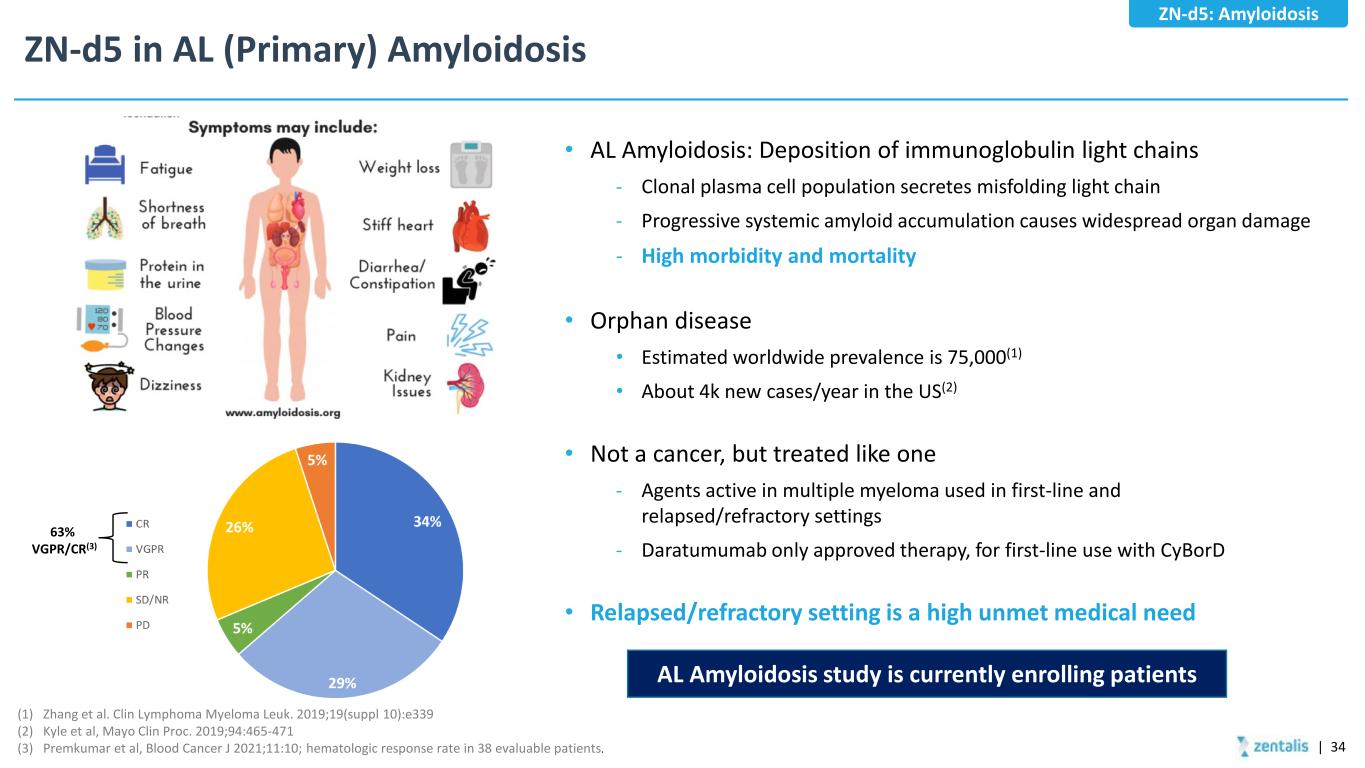
ZN-d5 in AL (Primary) Amyloidosis • AL Amyloidosis: Deposition of immunoglobulin light chains - Clonal plasma cell population secretes misfolding light chain - Progressive systemic amyloid accumulation causes widespread organ damage - High morbidity and mortality • Orphan disease • Estimated worldwide prevalence is 75,000(1) • About 4k new cases/year in the US(2) • Not a cancer, but treated like one - Agents active in multiple myeloma used in first-line and relapsed/refractory settings - Daratumumab only approved therapy, for first-line use with CyBorD • Relapsed/refractory setting is a high unmet medical need ZN-d5: Amyloidosis 63% VGPR/CR(3) (1) Zhang et al. Clin Lymphoma Myeloma Leuk. 2019;19(suppl 10):e339 (2) Kyle et al, Mayo Clin Proc. 2019;94:465-471 (3) Premkumar et al, Blood Cancer J 2021;11:10; hematologic response rate in 38 evaluable patients. AL Amyloidosis study is currently enrolling patients | 34
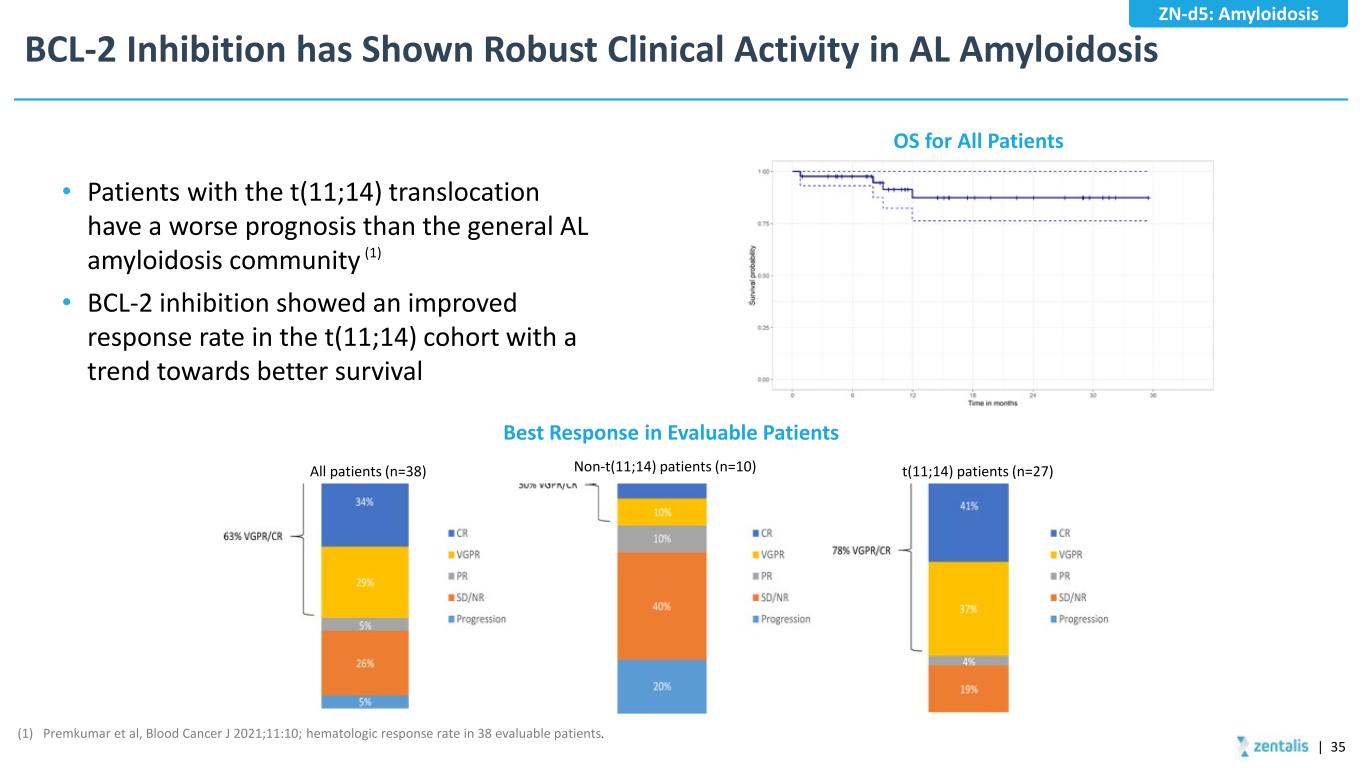
BCL-2 Inhibition has Shown Robust Clinical Activity in AL Amyloidosis (1) Premkumar et al, Blood Cancer J 2021;11:10; hematologic response rate in 38 evaluable patients. (1) OS for All Patients Best Response in Evaluable Patients All patients (n=38) Non-t(11;14) patients (n=10) t(11;14) patients (n=27) • Patients with the t(11;14) translocation have a worse prognosis than the general AL amyloidosis community • BCL-2 inhibition showed an improved response rate in the t(11;14) cohort with a trend towards better survival | 35 ZN-d5: Amyloidosis
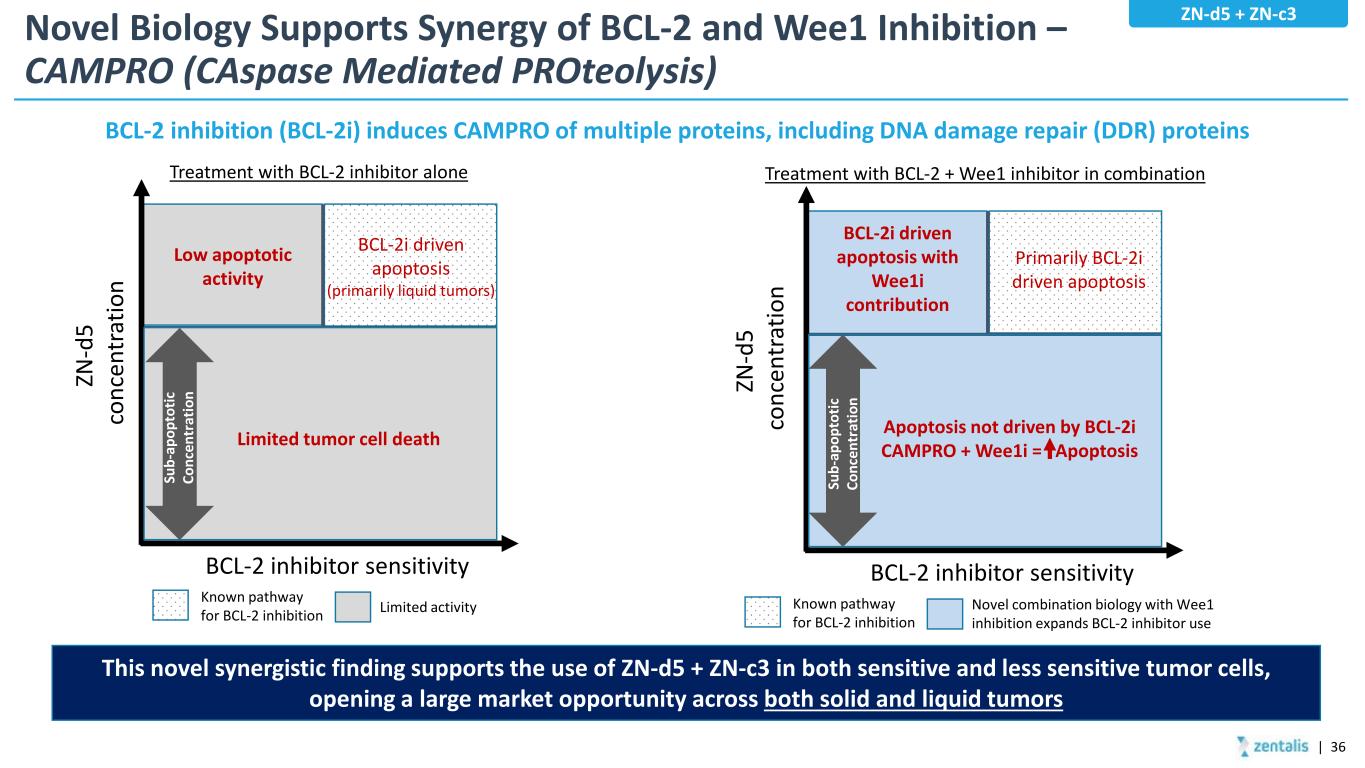
Novel Biology Supports Synergy of BCL-2 and Wee1 Inhibition – CAMPRO (CAspase Mediated PROteolysis) | 36 This novel synergistic finding supports the use of ZN-d5 + ZN-c3 in both sensitive and less sensitive tumor cells, opening a large market opportunity across both solid and liquid tumors Treatment with BCL-2 inhibitor alone ZN -d 5 co nc en tr at io n BCL-2 inhibitor sensitivity Low apoptotic activity BCL-2i driven apoptosis (primarily liquid tumors) Known pathway for BCL-2 inhibition Limited activity Su b- ap op to tic Co nc en tr at io n Limited tumor cell death ZN -d 5 co nc en tr at io n BCL-2 inhibitor sensitivity BCL-2i driven apoptosis with Wee1i contribution Primarily BCL-2i driven apoptosis Known pathway for BCL-2 inhibition Novel combination biology with Wee1 inhibition expands BCL-2 inhibitor use Apoptosis not driven by BCL-2i CAMPRO + Wee1i = Apoptosis Treatment with BCL-2 + Wee1 inhibitor in combination BCL-2 inhibition (BCL-2i) induces CAMPRO of multiple proteins, including DNA damage repair (DDR) proteins Su b- ap op to tic Co nc en tr at io n ZN-d5 + ZN-c3
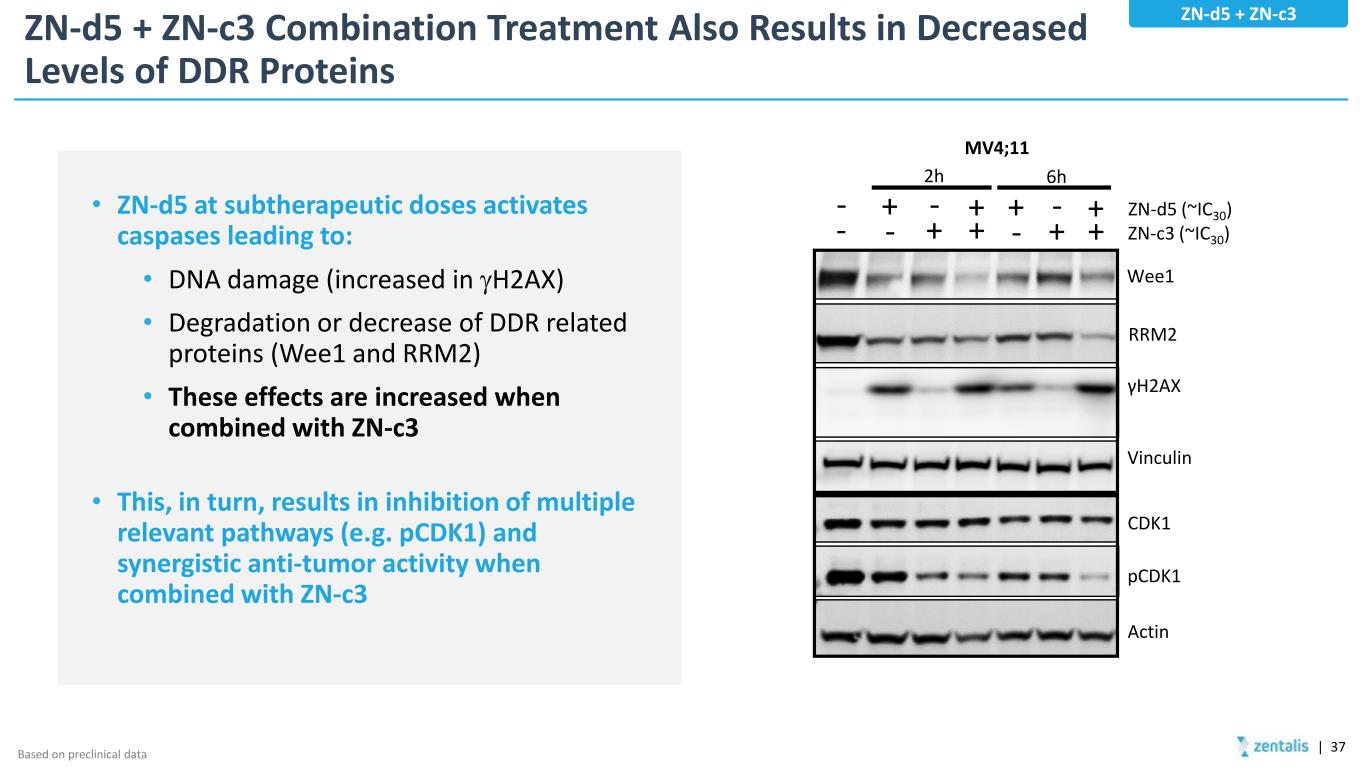
ZN-d5 + ZN-c3 Combination Treatment Also Results in Decreased Levels of DDR Proteins • ZN-d5 at subtherapeutic doses activates caspases leading to: • DNA damage (increased in γH2AX) • Degradation or decrease of DDR related proteins (Wee1 and RRM2) • These effects are increased when combined with ZN-c3 • This, in turn, results in inhibition of multiple relevant pathways (e.g. pCDK1) and synergistic anti-tumor activity when combined with ZN-c3 | 37 ZN-d5 + ZN-c3 Based on preclinical data Vinculin Wee1 RRM2 γH2AX CDK1 pCDK1 Actin - + +- MV4;11 - - ++ + +- - ++ 2h 6h ZN-d5 (~IC30) ZN-c3 (~IC30)

The Combination of BCL-2 and Wee1 Inhibitors Results in Synergism in Several Tumor Models Including AML | 38 HL-60 AML model 0 3 6 9 12 15 18 0 300 600 900 1200 1500 1800 2100 2400 2700 Vehicle ZN-d5 50 mg/kg, qd ZN-c3 60 mg/kg, qd Days Post Treatment Tu m or V ol um e (m m 3 ) ZN-d5 50 mg/kg + ZN-c3 60 mg/kg, qd. Regression: 94% • ZN-d5 and ZN-c3 combination represents a novel therapeutic approach • Significant enhancement of activity than single agent in several indications, including AML • Combination regimen was well-tolerated in mice • Zentalis is the only company known to have both inhibitors in clinical development ZN-d5 + ZN-c3
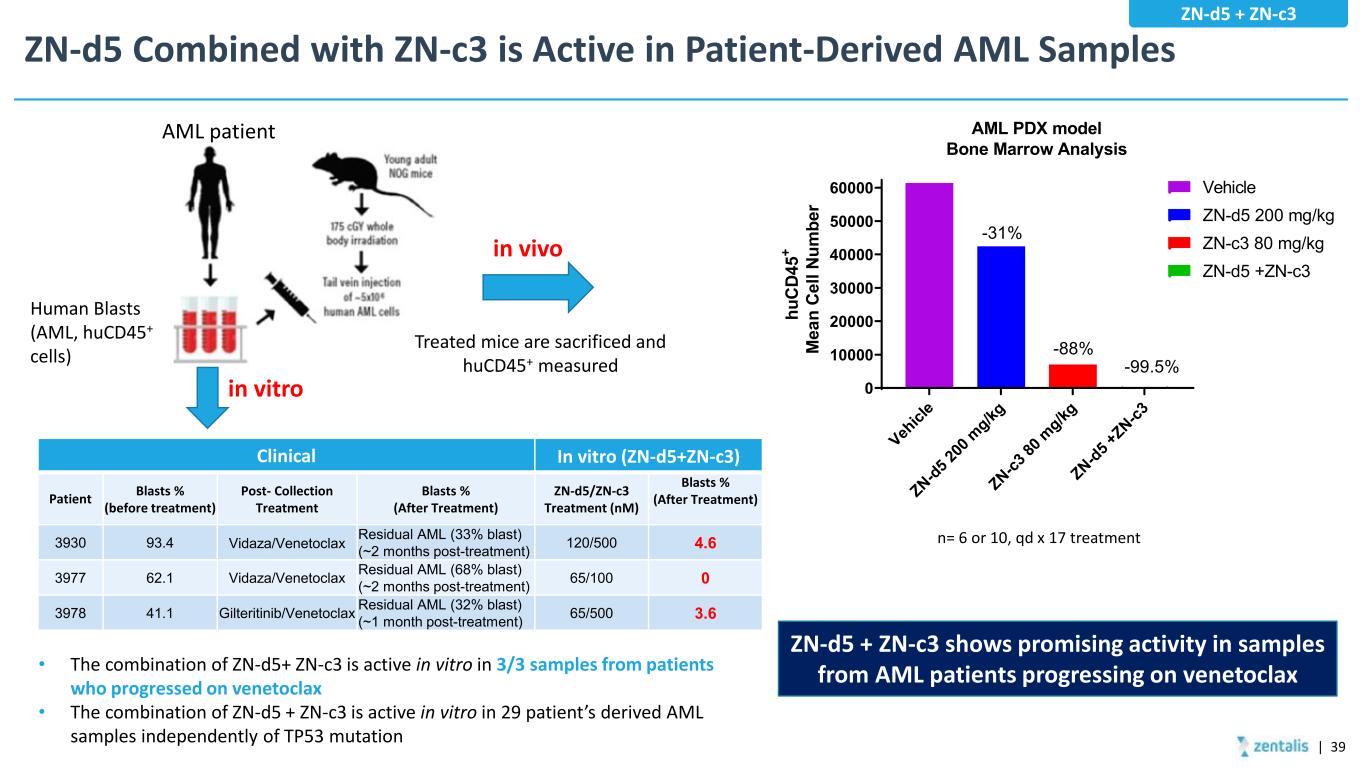
ZN-d5 Combined with ZN-c3 is Active in Patient-Derived AML Samples n= 6 or 10, qd x 17 treatment Veh icl e ZN-d5 2 00 m g/kg ZN-c3 80 m g/kg ZN-d5 + ZN-c3 0 10000 20000 30000 40000 50000 60000 AML PDX model Bone Marrow Analysis hu CD 45 + M ea n Ce ll Nu m be r Vehicle ZN-d5 200 mg/kg ZN-c3 80 mg/kg ZN-d5 +ZN-c3 -31% -88% -99.5% Human Blasts (AML, huCD45+ cells) Treated mice are sacrificed and huCD45+ measured AML patient • The combination of ZN-d5+ ZN-c3 is active in vitro in 3/3 samples from patients who progressed on venetoclax • The combination of ZN-d5 + ZN-c3 is active in vitro in 29 patient’s derived AML samples independently of TP53 mutation in vitro in vivo ZN-d5 + ZN-c3 shows promising activity in samples from AML patients progressing on venetoclax Clinical In vitro (ZN-d5+ZN-c3) Patient Blasts % (before treatment) Post- Collection Treatment Blasts % (After Treatment) ZN-d5/ZN-c3 Treatment (nM) Blasts % (After Treatment) 3930 93.4 Vidaza/Venetoclax Residual AML (33% blast) (~2 months post-treatment) 120/500 4.6 3977 62.1 Vidaza/Venetoclax Residual AML (68% blast) (~2 months post-treatment) 65/100 0 3978 41.1 Gilteritinib/Venetoclax Residual AML (32% blast) (~1 month post-treatment) 65/500 3.6 | 39 ZN-d5 + ZN-c3
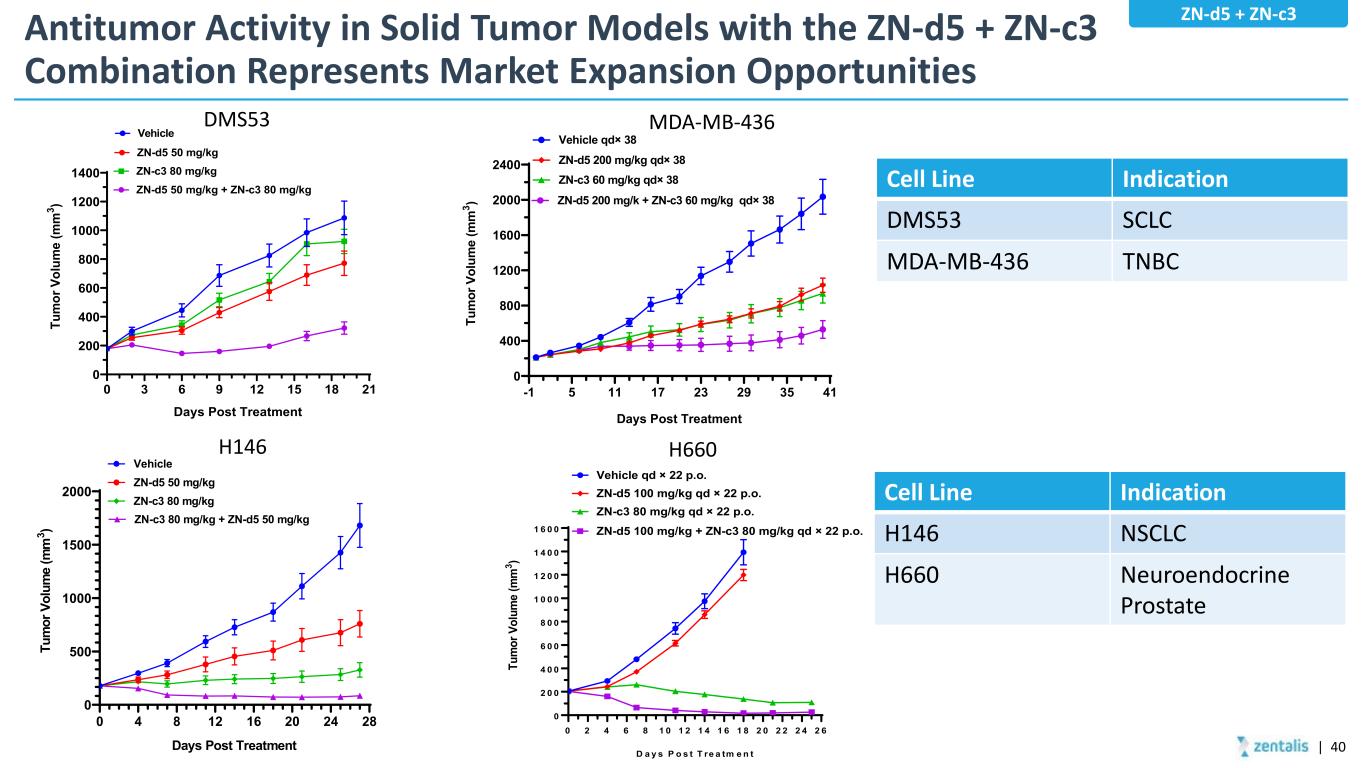
Antitumor Activity in Solid Tumor Models with the ZN-d5 + ZN-c3 Combination Represents Market Expansion Opportunities 0 3 6 9 12 15 18 21 0 200 400 600 800 1000 1200 1400 Vehicle ZN-d5 50 mg/kg ZN-c3 80 mg/kg ZN-d5 50 mg/kg + ZN-c3 80 mg/kg Days Post Treatment Tu m or V ol um e (m m 3 ) DMS53 -1 5 11 17 23 29 35 41 0 400 800 1200 1600 2000 2400 Vehicle qd× 38 ZN-d5 200 mg/kg qd× 38 ZN-c3 60 mg/kg qd× 38 Days Post Treatment Tu m or V ol um e (m m 3 ) ZN-d5 200 mg/k + ZN-c3 60 mg/kg qd× 38 MDA-MB-436 0 2 4 6 8 1 0 1 2 1 4 1 6 1 8 2 0 2 2 2 4 2 6 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 1 2 0 0 1 4 0 0 1 6 0 0 D a y s P o s t T re a tm e n t Tu m or V ol um e (m m 3 ) Vehicle qd × 22 p.o. ZN-d5 100 mg/kg qd × 22 p.o. ZN-c3 80 mg/kg qd × 22 p.o. ZN-d5 100 mg/kg + ZN-c3 80 mg/kg qd × 22 p.o. H660 0 4 8 12 16 20 24 28 0 500 1000 1500 2000 Days Post Treatment Tu m or V ol um e (m m 3 ) Vehicle ZN-d5 50 mg/kg ZN-c3 80 mg/kg ZN-c3 80 mg/kg + ZN-d5 50 mg/kg H146 Cell Line Indication DMS53 SCLC MDA-MB-436 TNBC ZN-d5 + ZN-c3 | 40 Cell Line Indication H146 NSCLC H660 Neuroendocrine Prostate
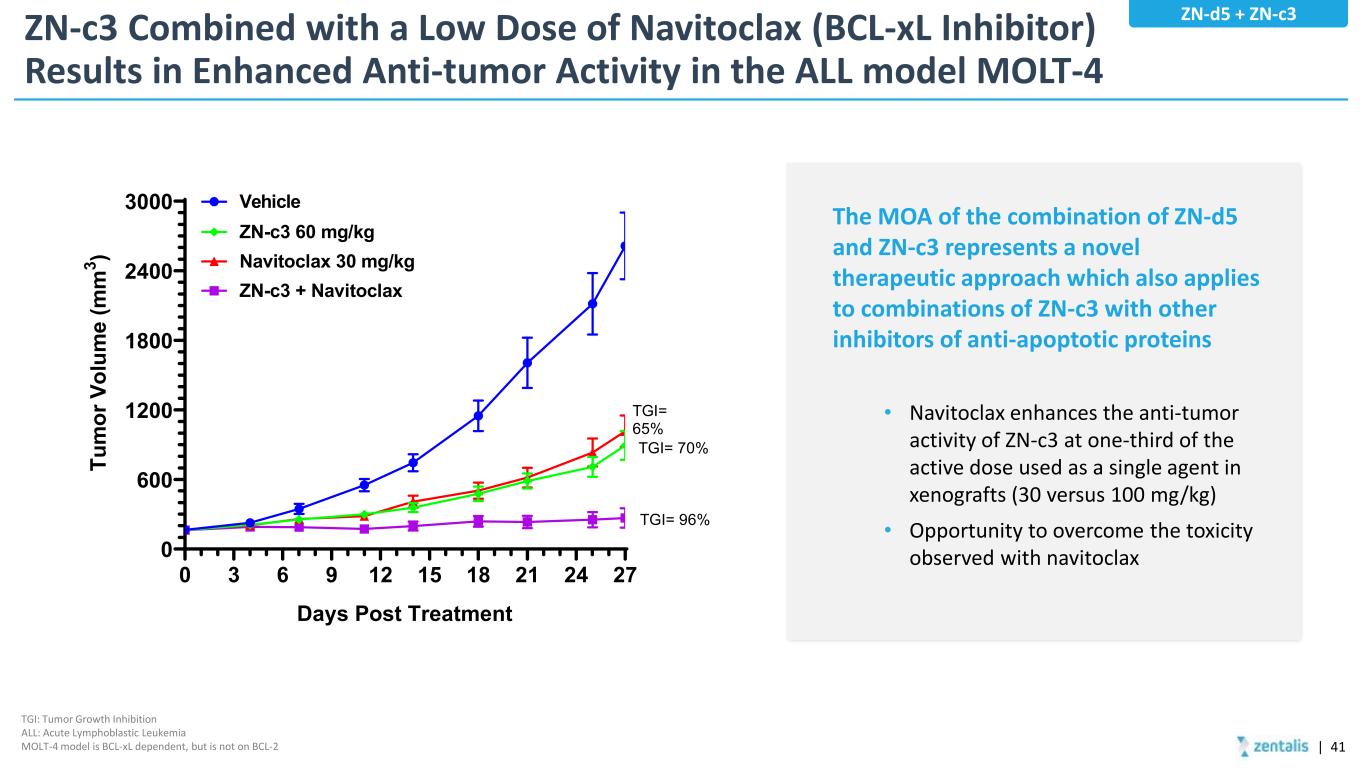
ZN-c3 Combined with a Low Dose of Navitoclax (BCL-xL Inhibitor) Results in Enhanced Anti-tumor Activity in the ALL model MOLT-4 The MOA of the combination of ZN-d5 and ZN-c3 represents a novel therapeutic approach which also applies to combinations of ZN-c3 with other inhibitors of anti-apoptotic proteins • Navitoclax enhances the anti-tumor activity of ZN-c3 at one-third of the active dose used as a single agent in xenografts (30 versus 100 mg/kg) • Opportunity to overcome the toxicity observed with navitoclax 0 3 6 9 12 15 18 21 24 27 0 600 1200 1800 2400 3000 Days Post Treatment Tu m or V ol um e (m m 3 ) Vehicle ZN-c3 60 mg/kg Navitoclax 30 mg/kg ZN-c3 + Navitoclax TGI= 65% TGI= 70% TGI= 96% TGI: Tumor Growth Inhibition ALL: Acute Lymphoblastic Leukemia MOLT-4 model is BCL-xL dependent, but is not on BCL-2 ZN-d5 + ZN-c3 | 41

Conclusions
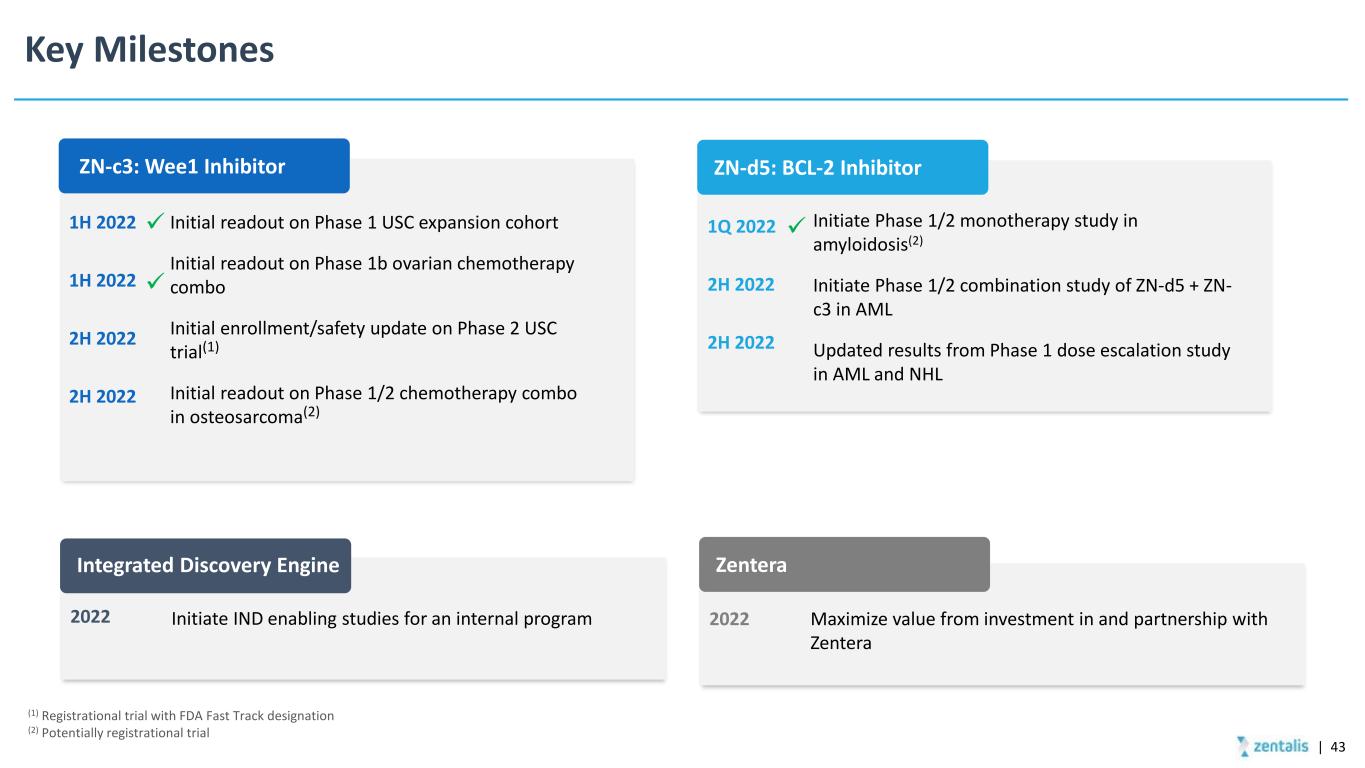
Key Milestones ZN-c3: Wee1 Inhibitor Initial readout on Phase 1 USC expansion cohort Initial readout on Phase 1b ovarian chemotherapy combo Initial enrollment/safety update on Phase 2 USC trial(1) Initial readout on Phase 1/2 chemotherapy combo in osteosarcoma(2) 1H 2022 1H 2022 2H 2022 2H 2022 ZN-d5: BCL-2 Inhibitor Integrated Discovery Engine Zentera Maximize value from investment in and partnership with Zentera 2022Initiate IND enabling studies for an internal program2022 (1) Registrational trial with FDA Fast Track designation (2) Potentially registrational trial Initiate Phase 1/2 monotherapy study in amyloidosis(2) Initiate Phase 1/2 combination study of ZN-d5 + ZN- c3 in AML Updated results from Phase 1 dose escalation study in AML and NHL 1Q 2022 2H 2022 2H 2022 | 43

Kimberly Blackwell, M.D. Chief Executive Officer kblackwell@zentalis.com (212) 433-3787 Melissa Epperly, Chief Financial Officer mepperly@zentalis.com (215) 290-7271 Corporate Office 1359 Broadway Suite 1710 New York, NY 10018 Science Center 10275 Science Center Drive Suite 200 San Diego, CA 92121











































