
Corporate Event January 29, 2025 Nasdaq: ZNTL Exhibit 99.2

2 Zentalis Pharmaceuticals, Inc. (“we,” “us,” “our,” “Zentalis” or the “Company”) cautions that this presentation (including oral commentary that accompanies this presentation) contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the potential for azenosertib (ZN-c3) to be first-in-class and best-in-class; the potential regulatory pathway for azenosertib, including the potential for azenosertib to utilize an accelerated approval pathway and obtain accelerated approval, and the potential for studies to be registrational or intended for registration; our development and regulatory strategy and approach for azenosertib, including our strategy to focus on bringing azenosertib to patients with PROC who are Cyclin E1+; our planned strategy, vision and path forward; the market opportunity for azenosertib, including the potential size of the patient population; existing data being supportive of the go-forward azenosertib development strategy the potential for azenosertib to be a very important treatment option; our plans to bring azenosertib to patients quickly and efficiently; the importance a Cyclin E1+ companion diagnostic for our go-forward strategy; the potential for the opportunity for azenosertib to be broad; our belief that a Cyclin E1+ companion diagnostic test substantially enhances the risk benefit profile of azenosertib; the encouraging nature of the azenosertib clinical data; the potential opportunities for azenosertib in combination or across different tumor types; our ability to recognize the benefits of our strategic restructuring; our belief that our resources are aligned and our strategy is highly focused; azenosertib’s therapeutic value as a single agent; the potential for Cyclin E1 to serve as a highly sensitive and specific predictive biomarker for response to azenosertib; the potential for azenosertib to be welcomed by the gynecological oncology community; the opportunity to improve outcomes in the next stage of development, including the potential for trial management and mitigation strategies to help in reducing the rate of discontinuations and improve duration on therapy and the potential for enhanced monitoring, guidance and supportive care to help increase the time on treatment; our positioning to execute; our projected cash runway; planned clinical trials for our product candidates; the potential of azenosertib to address a significant unmet need in patients with PROC who are Cyclin E1+; the potential benefits of azenosertib, including compared to available therapies and therapies in development (not head-to-head comparisons) and potential to improve patient quality of life; the potential unmet need in a particular indication and/or patient population; the timing and content of our anticipated milestones, including the timing of initiation of clinical trials and disclosure of clinical data; as well as statements that include the words such as “ahead,” “anticipate,” “believe,” “beyond,” “confident,” "continue," "could," “estimate,” “expect,” “forward,” “future,” “goal,” “go-forward,” “intended,” “may,” “milestone,” “ongoing,” “opportunity,” “path forward,” “plan,” “potential,” “predictive,” “promising,” “strategy,” "support," “vision,” “will” and similar statements of a future or forward-looking nature. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: our limited operating history, which may make it difficult to evaluate our current business and predict our future success and viability; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our plans, including the costs thereof, of development of a companion diagnostic; the outcome of preclinical testing and early trials may not be predictive of the success of later clinical trials; failure to identify additional product candidates and develop or commercialize marketable products; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process or ongoing regulatory obligations; failure to obtain U.S. or international marketing approval; our product candidates may cause serious adverse side effects; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intellectual property; our ability to attract, retain and motivate qualified personnel, and risks relating to management transitions; and significant costs as a result of operating as a public company. Other risks and uncertainties include those identified under the caption “Risk Factors” in our most recently filed periodic reports on Forms 10-K and 10-Q and subsequent filings with the U.S. Securities and Exchange Commission in the future could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data after the date of this presentation. Statements such as “not head-to-head,” “relative to historical data,” “relative to published data,” “direct cross-study comparison not intended” and similar references indicate that no head-to-head clinical trial has been conducted evaluating azenosertib against the indicated therapies. Notable differences exist between the Company’s trial designs, conditions under study and subject characteristics as compared to the evaluated third party results and caution should be exercised when comparing data across these studies. ZENTALIS® and its associated logos are trademarks of Zentalis and/or its affiliates. All other trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. All website addresses given in this presentation are for information only and are not intended to be an active link or to incorporate any website information into this document. Zentalis’ product candidates are investigational drugs and have not yet been approved by the U.S. Food and Drug Administration or any other regulatory authority. Forward Looking Statements and Disclaimer

3 Opening Remarks Julie Eastland, Chief Executive Officer Azenosertib/WEE1 Biology and Cyclin E1 Overview Mark Lackner, PhD, Chief Scientific Officer 001 and MAMMOTH Results Ingmar Bruns, MD, Chief Medical Officer DENALI Part 1b Results Dave O’Malley, MD, The Ohio State University Comprehensive Cancer Center PROC Development Strategy Ingmar Bruns, MD, Chief Medical Officer Closing Remarks Julie Eastland, Chief Executive Officer Q&A Agenda for Today
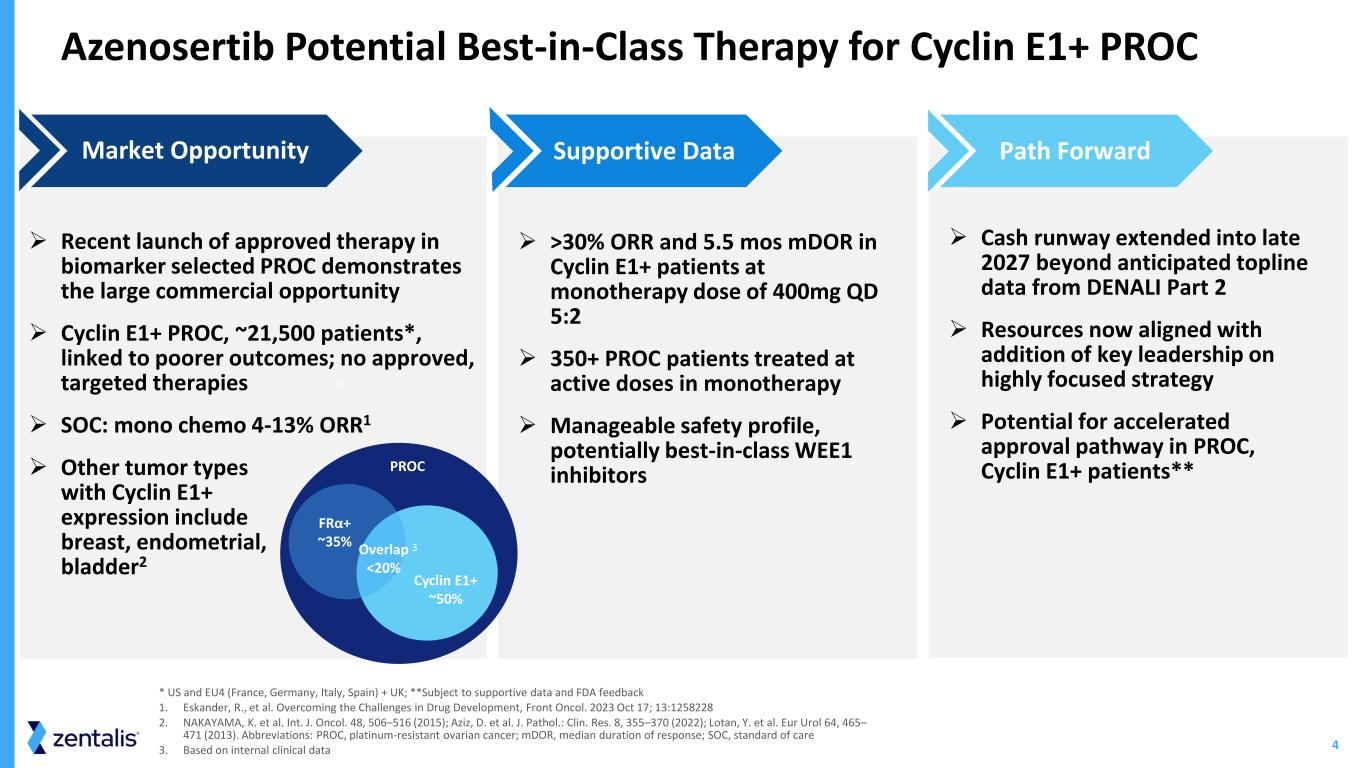
4 Azenosertib Potential Best-in-Class Therapy for Cyclin E1+ PROC ➢ Recent launch of approved therapy in biomarker selected PROC demonstrates the large commercial opportunity ➢ Cyclin E1+ PROC, ~21,500 patients*, linked to poorer outcomes; no approved, targeted therapies ➢ SOC: mono chemo 4-13% ORR1 ➢ Other tumor types with Cyclin E1+ expression include breast, endometrial, bladder2 Market Opportunity ➢ >30% ORR and 5.5 mos mDOR in Cyclin E1+ patients at monotherapy dose of 400mg QD 5:2 ➢ 350+ PROC patients treated at active doses in monotherapy ➢ Manageable safety profile, potentially best-in-class WEE1 inhibitors Supportive Data ➢ Cash runway extended into late 2027 beyond anticipated topline data from DENALI Part 2 ➢ Resources now aligned with addition of key leadership on highly focused strategy ➢ Potential for accelerated approval pathway in PROC, Cyclin E1+ patients** Path Forward * US and EU4 (France, Germany, Italy, Spain) + UK; **Subject to supportive data and FDA feedback 1. Eskander, R., et al. Overcoming the Challenges in Drug Development, Front Oncol. 2023 Oct 17; 13:1258228 2. NAKAYAMA, K. et al. Int. J. Oncol. 48, 506–516 (2015); Aziz, D. et al. J. Pathol.: Clin. Res. 8, 355–370 (2022); Lotan, Y. et al. Eur Urol 64, 465– 471 (2013). Abbreviations: PROC, platinum-resistant ovarian cancer; mDOR, median duration of response; SOC, standard of care 3. Based on internal clinical data * PROC FRα+ ~35% Overlap <20% Cyclin E1+ ~50% 3
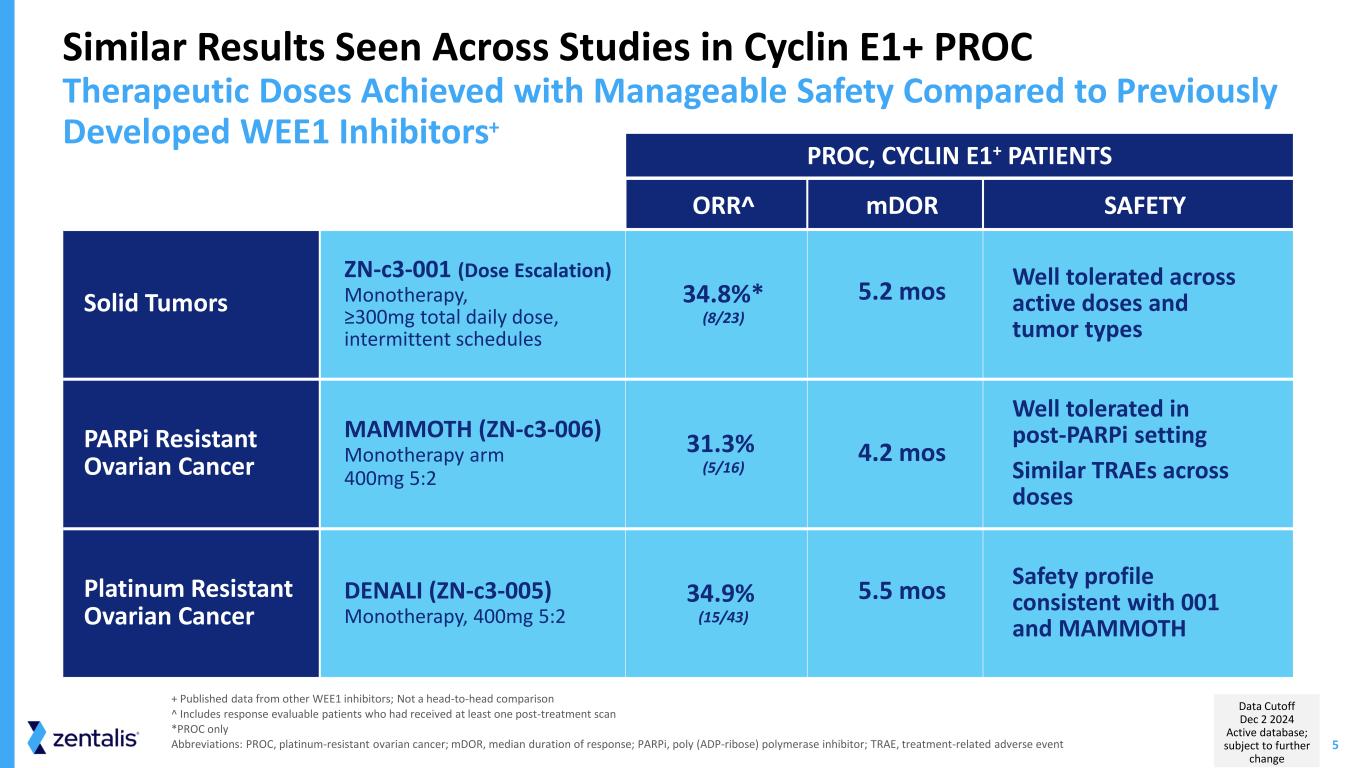
5 ORR^ mDOR SAFETY Solid Tumors ZN-c3-001 (Dose Escalation) Monotherapy, ≥300mg total daily dose, intermittent schedules 34.8%* (8/23) 5.2 mos Well tolerated across active doses and tumor types PARPi Resistant Ovarian Cancer MAMMOTH (ZN-c3-006) Monotherapy arm 400mg 5:2 31.3% (5/16) 4.2 mos Well tolerated in post-PARPi setting Similar TRAEs across doses Platinum Resistant Ovarian Cancer DENALI (ZN-c3-005) Monotherapy, 400mg 5:2 34.9% (15/43) 5.5 mos Safety profile consistent with 001 and MAMMOTH Similar Results Seen Across Studies in Cyclin E1+ PROC Therapeutic Doses Achieved with Manageable Safety Compared to Previously Developed WEE1 Inhibitors+ + Published data from other WEE1 inhibitors; Not a head-to-head comparison ^ Includes response evaluable patients who had received at least one post-treatment scan *PROC only Abbreviations: PROC, platinum-resistant ovarian cancer; mDOR, median duration of response; PARPi, poly (ADP-ribose) polymerase inhibitor; TRAE, treatment-related adverse event PROC, CYCLIN E1+ PATIENTS Data Cutoff Dec 2 2024 Active database; subject to further change
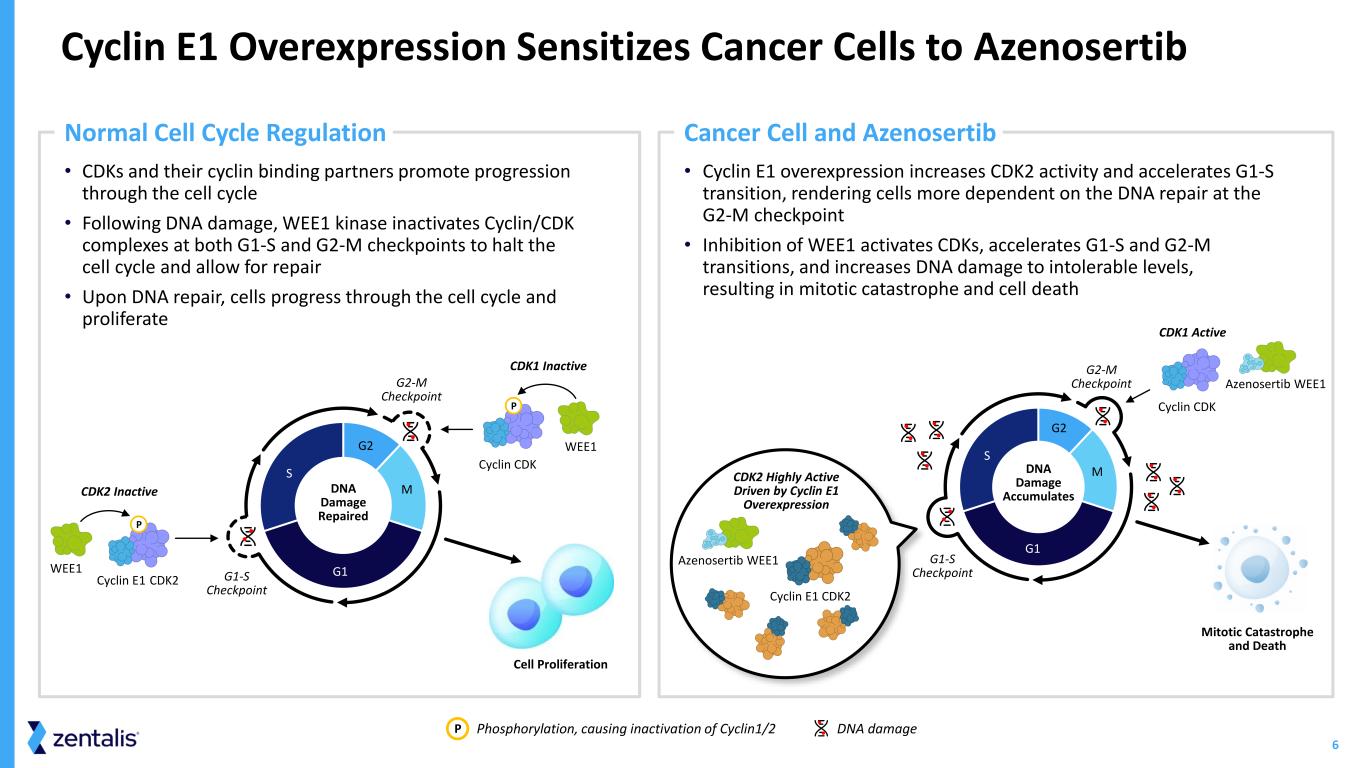
6 • Cyclin E1 overexpression increases CDK2 activity and accelerates G1-S transition, rendering cells more dependent on the DNA repair at the G2-M checkpoint • Inhibition of WEE1 activates CDKs, accelerates G1-S and G2-M transitions, and increases DNA damage to intolerable levels, resulting in mitotic catastrophe and cell death • CDKs and their cyclin binding partners promote progression through the cell cycle • Following DNA damage, WEE1 kinase inactivates Cyclin/CDK complexes at both G1-S and G2-M checkpoints to halt the cell cycle and allow for repair • Upon DNA repair, cells progress through the cell cycle and proliferate Cyclin E1 Overexpression Sensitizes Cancer Cells to Azenosertib Phosphorylation, causing inactivation of Cyclin1/2 DNA damageP G1 S G2 MDNA Damage Repaired CDK2 Inactive CDK2Cyclin E1 WEE1 P CDK1 Inactive WEE1 Cell Proliferation P Normal Cell Cycle Regulation Cancer Cell and Azenosertib Mitotic Catastrophe and Death G2-M Checkpoint DNA Damage Accumulates CDK2 Highly Active Driven by Cyclin E1 Overexpression Cyclin CDK Azenosertib WEE1 Azenosertib WEE1 CDK1 Active G1-S Checkpoint Cyclin E1 CDK2 G1 S G2 M Cyclin CDK G2-M Checkpoint G1-S Checkpoint
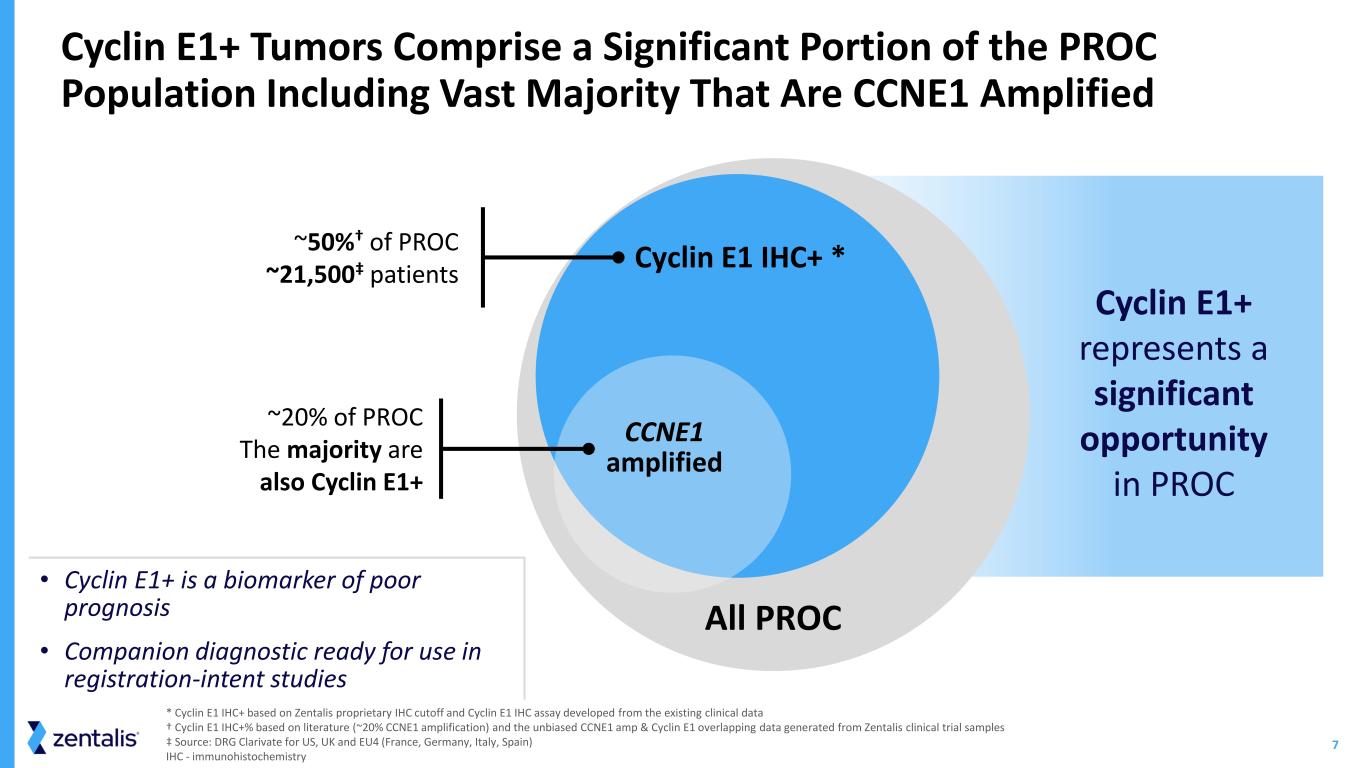
7 * Cyclin E1 IHC+ based on Zentalis proprietary IHC cutoff and Cyclin E1 IHC assay developed from the existing clinical data † Cyclin E1 IHC+% based on literature (~20% CCNE1 amplification) and the unbiased CCNE1 amp & Cyclin E1 overlapping data generated from Zentalis clinical trial samples ‡ Source: DRG Clarivate for US, UK and EU4 (France, Germany, Italy, Spain) IHC - immunohistochemistry • Cyclin E1+ is a biomarker of poor prognosis • Companion diagnostic ready for use in registration-intent studies Cyclin E1+ Tumors Comprise a Significant Portion of the PROC Population Including Vast Majority That Are CCNE1 Amplified ~20% of PROC The majority are also Cyclin E1+ All PROC ~50%† of PROC ~21,500‡ patients Cyclin E1 IHC+ * CCNE1 amplified Cyclin E1+ represents a significant opportunity in PROC
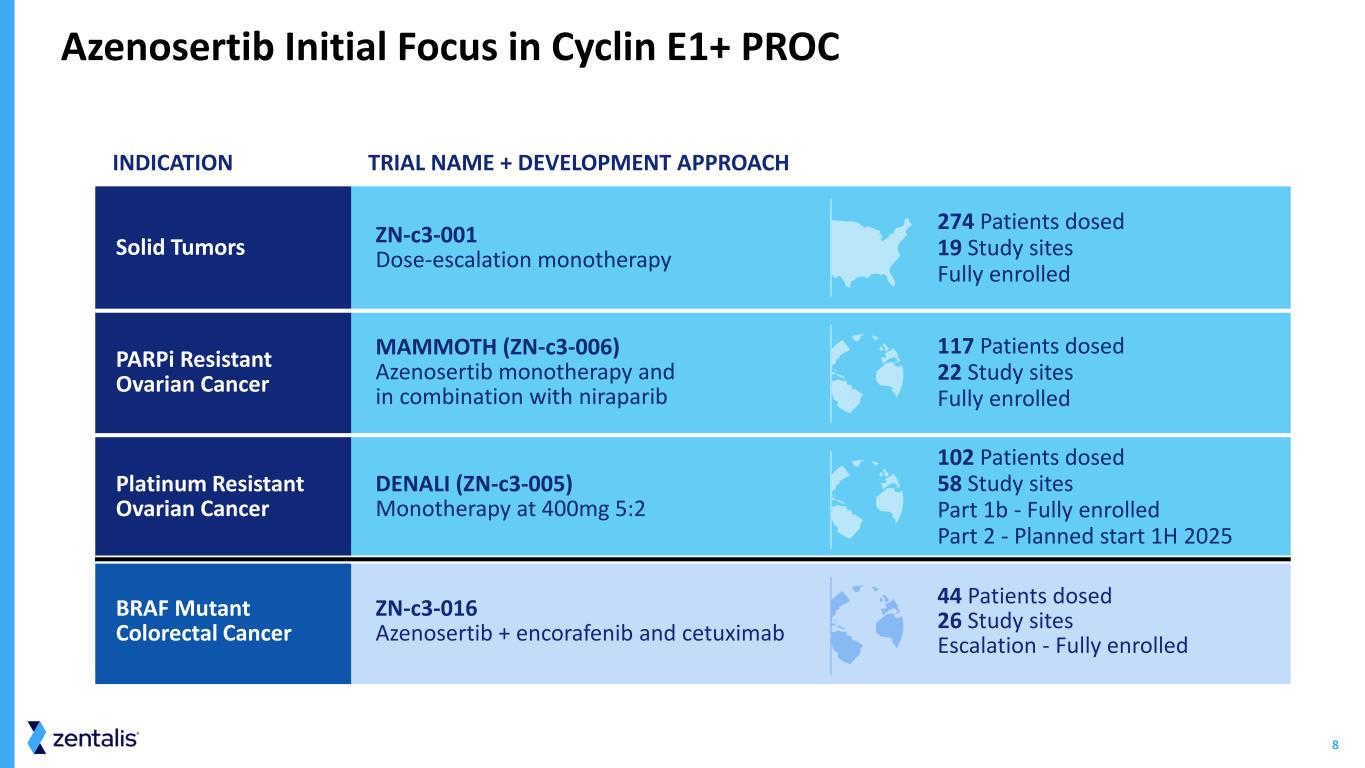
8 INDICATION TRIAL NAME + DEVELOPMENT APPROACH Solid Tumors ZN-c3-001 Dose-escalation monotherapy 274 Patients dosed 19 Study sites Fully enrolled PARPi Resistant Ovarian Cancer MAMMOTH (ZN-c3-006) Azenosertib monotherapy and in combination with niraparib 117 Patients dosed 22 Study sites Fully enrolled Platinum Resistant Ovarian Cancer DENALI (ZN-c3-005) Monotherapy at 400mg 5:2 102 Patients dosed 58 Study sites Part 1b - Fully enrolled Part 2 - Planned start 1H 2025 BRAF Mutant Colorectal Cancer ZN-c3-016 Azenosertib + encorafenib and cetuximab 44 Patients dosed 26 Study sites Escalation - Fully enrolled Azenosertib Initial Focus in Cyclin E1+ PROC

ZN-c3-001 Dose-Escalating Monotherapy Study in Solid Tumors NCT04158336
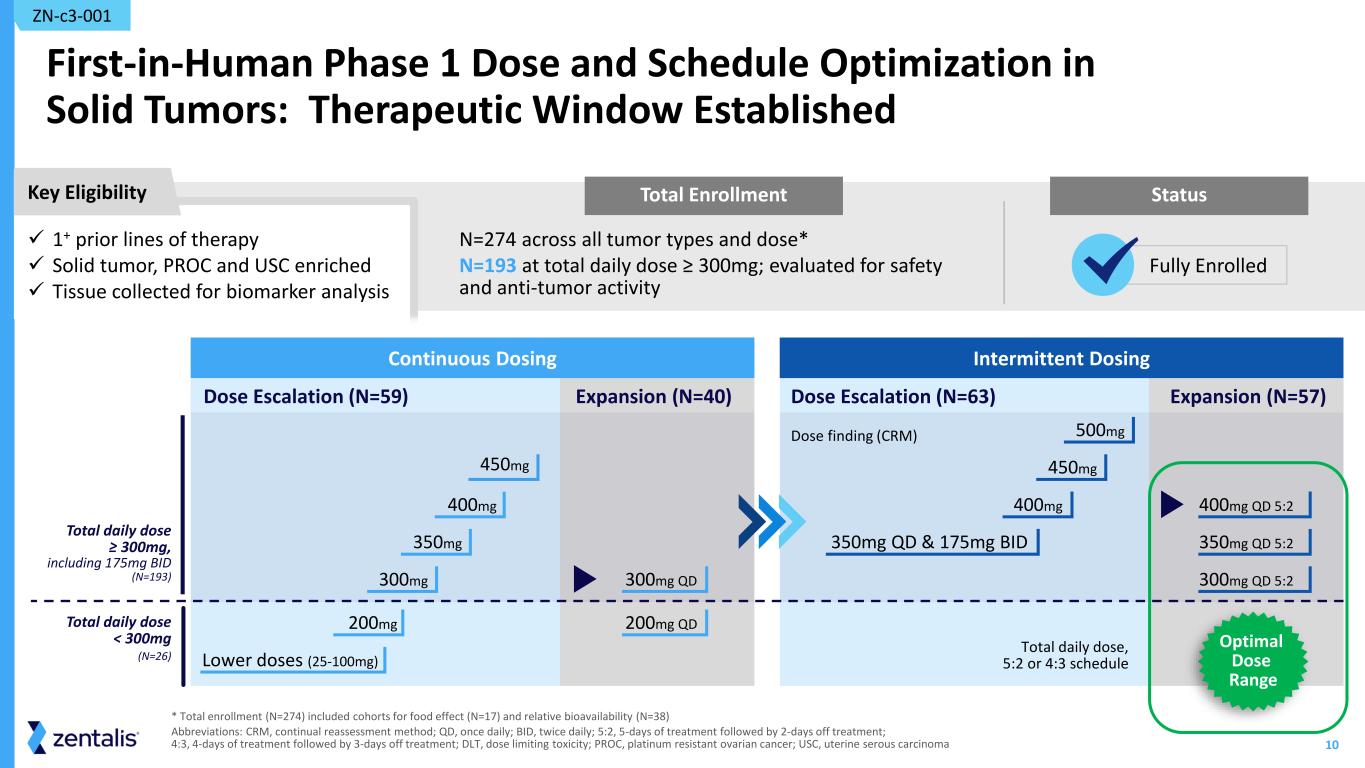
10 Total Enrollment First-in-Human Phase 1 Dose and Schedule Optimization in Solid Tumors: Therapeutic Window Established N=274 across all tumor types and dose* N=193 at total daily dose ≥ 300mg; evaluated for safety and anti-tumor activity ZN-c3-001 * Total enrollment (N=274) included cohorts for food effect (N=17) and relative bioavailability (N=38) Abbreviations: CRM, continual reassessment method; QD, once daily; BID, twice daily; 5:2, 5-days of treatment followed by 2-days off treatment; 4:3, 4-days of treatment followed by 3-days off treatment; DLT, dose limiting toxicity; PROC, platinum resistant ovarian cancer; USC, uterine serous carcinoma Intermittent Dosing Dose finding (CRM) Expansion (N=57)Dose Escalation (N=63) 450mg 500mg 400mg 400mg QD 5:2 350mg QD & 175mg BID 350mg QD 5:2 300mg QD 5:2 Total daily dose, 5:2 or 4:3 schedule Continuous Dosing Dose Escalation (N=59) Expansion (N=40) 450mg 200mg 400mg 350mg Lower doses (25-100mg) 300mg 300mg QD 200mg QD Total daily dose ≥ 300mg, including 175mg BID (N=193) Total daily dose < 300mg (N=26) Key Eligibility Status ✓ 1+ prior lines of therapy ✓ Solid tumor, PROC and USC enriched ✓ Tissue collected for biomarker analysis Fully Enrolled Optimal Dose Range

11 Heavily Pre-Treated Patient Population with Multiple Tumor Types; Patients with PROC had a Median of Five Prior Lines of Therapy PROC N = 69 USC N = 35 Other Solid Tumors* N = 89 Age (years) Median 66 66 64 Range (Min-Max) (48 – 83) (53 – 78) (26 – 81) ECOG PS (n, %) ECOG 0 19 (28%) 8 (23%) 32 (36%) ECOG 1 50 (72%) 26 (74%) 54 (61%) ECOG 2 0 1 (3%) 2 (2%) Prior Lines of Treatment Median (Range) 5 (1 - 19) 3 (0 - 12) 4 (0 - 11) 0 0 1 (3%)† 1 (3%)‡ 1-3 22 (32%) 22 (63%) 34 (36%) ≥4 47 (68%) 12 (34%) 54 (61%) *Anus, Appendix, Biliary tract, Bladder, Breast, Cecum, Cervix, Colon, Duodenum, Endometrium, Esophagus, Kidney, Lung, Other, Ovary, Pancreas, Peritoneum, Prostate, Rectum, Stomach, Uterus, Vulva/Vagina, including one patient who had unknown ECOG status; † patient had no prior therapy ; ‡ patient rolled over from the DDI study Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation; PARPi, poly-ADP ribose polymerase inhibitor; VEGFi, vascular endothelial growth factor inhibitor; PD-1/PD-L1, programmed cell death protein 1/programmed death ligand 1. Patient Demographics and Clinical Characteristics Total Daily Dose ≥ 300 mg – Continuous and Intermittent (N=193) PROC N = 69 USC N = 35 Other Solid Tumors N = 89 Prior Therapies (n, %) PARPi 46 (67%) 3 (9%) 5 (6%) VEGFi 60 (87%) 27 (77%) 39 (44%) PD-1/PD-L1 12 (17%) 27 (77%) 35 (39%) Cyclin E1 Status (n, %) Positive 26 (38%) 15 (43%) 9 (10%) Negative 29 (42%) 11 (31%) 25 (28%) Unknown 14 (20%) 9 (26%) 55 (62%) ZN-c3-001 Data Cutoff Dec 2 2024 Active database; subject to further change
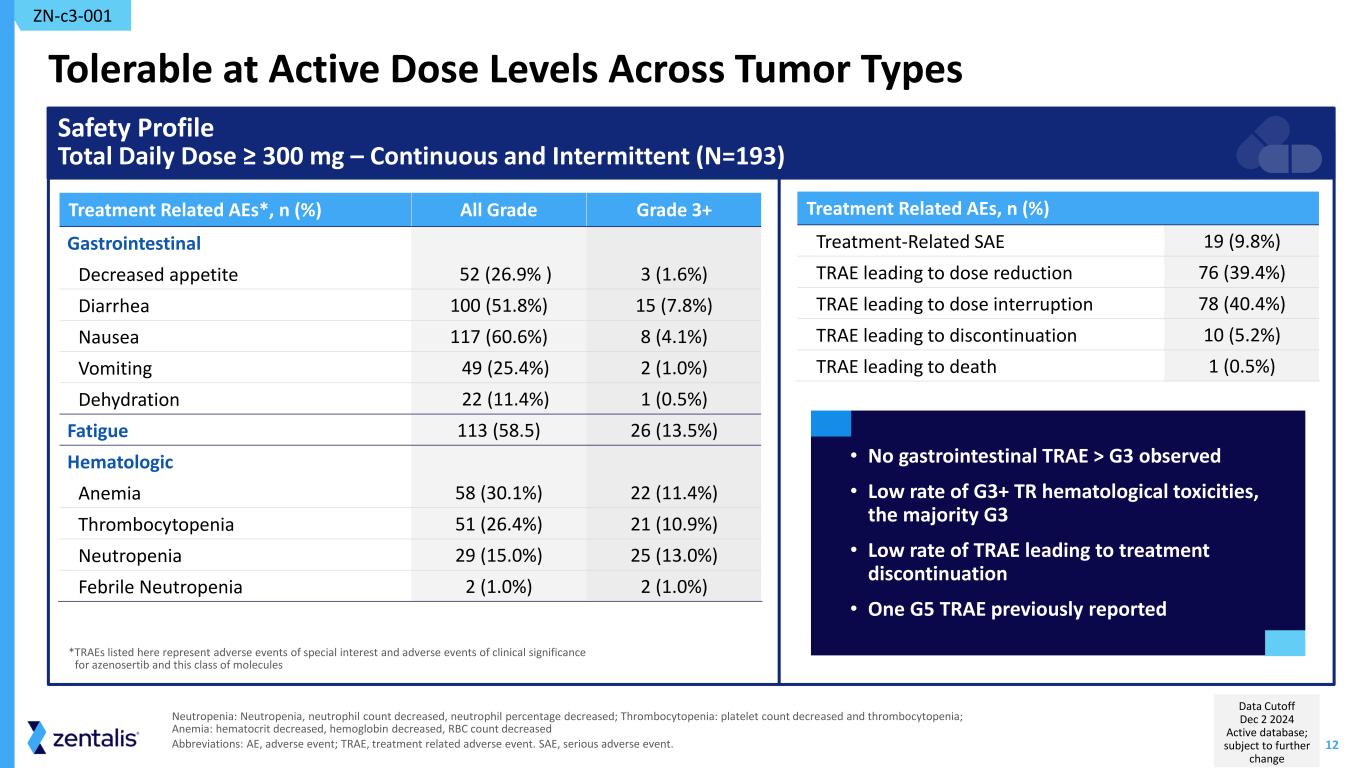
12 Tolerable at Active Dose Levels Across Tumor Types Treatment Related AEs, n (%) Treatment-Related SAE 19 (9.8%) TRAE leading to dose reduction 76 (39.4%) TRAE leading to dose interruption 78 (40.4%) TRAE leading to discontinuation 10 (5.2%) TRAE leading to death 1 (0.5%) Treatment Related AEs*, n (%) All Grade Grade 3+ Gastrointestinal Decreased appetite 52 (26.9% ) 3 (1.6%) Diarrhea 100 (51.8%) 15 (7.8%) Nausea 117 (60.6%) 8 (4.1%) Vomiting 49 (25.4%) 2 (1.0%) Dehydration 22 (11.4%) 1 (0.5%) Fatigue 113 (58.5) 26 (13.5%) Hematologic Anemia 58 (30.1%) 22 (11.4%) Thrombocytopenia 51 (26.4%) 21 (10.9%) Neutropenia 29 (15.0%) 25 (13.0%) Febrile Neutropenia 2 (1.0%) 2 (1.0%) Safety Profile Total Daily Dose ≥ 300 mg – Continuous and Intermittent (N=193) • No gastrointestinal TRAE > G3 observed • Low rate of G3+ TR hematological toxicities, the majority G3 • Low rate of TRAE leading to treatment discontinuation • One G5 TRAE previously reported *TRAEs listed here represent adverse events of special interest and adverse events of clinical significance for azenosertib and this class of molecules ZN-c3-001 Neutropenia: Neutropenia, neutrophil count decreased, neutrophil percentage decreased; Thrombocytopenia: platelet count decreased and thrombocytopenia; Anemia: hematocrit decreased, hemoglobin decreased, RBC count decreased Abbreviations: AE, adverse event; TRAE, treatment related adverse event. SAE, serious adverse event. Data Cutoff Dec 2 2024 Active database; subject to further change
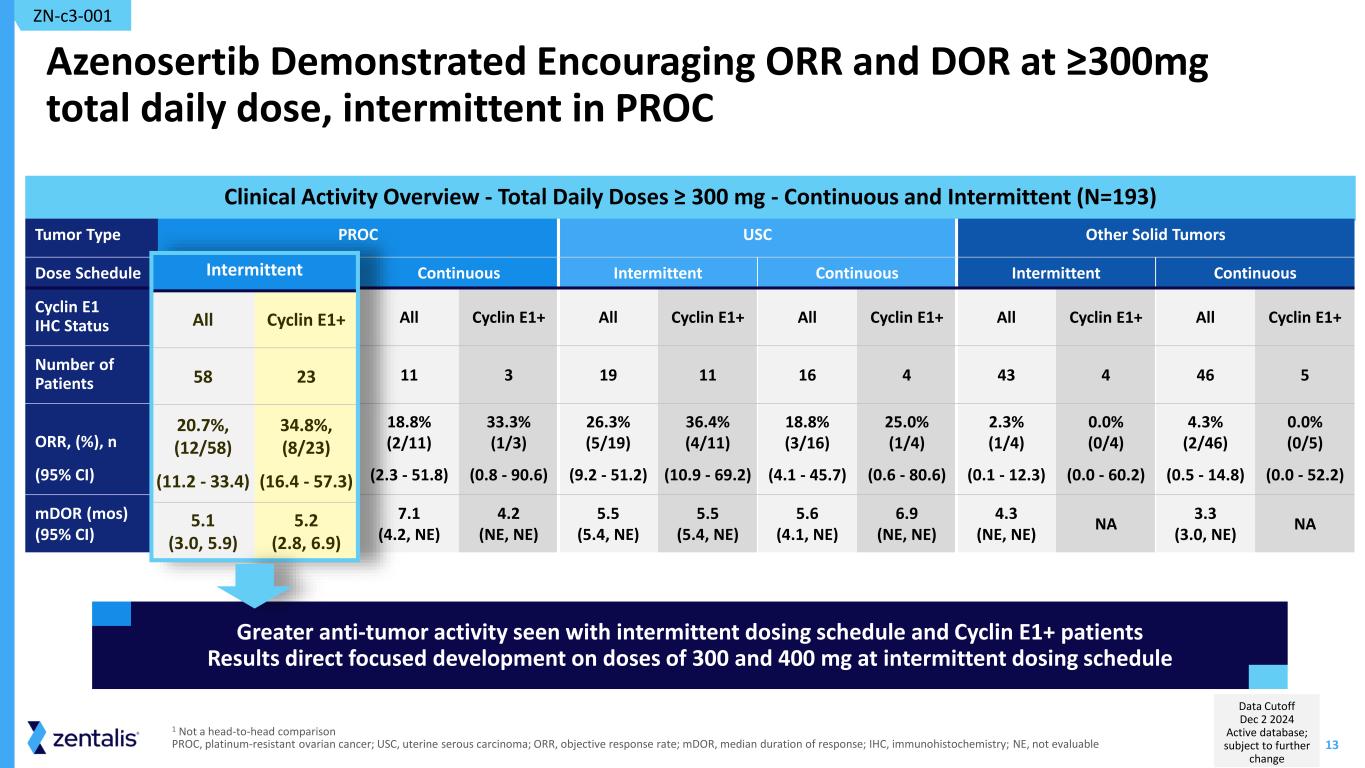
13 Clinical Activity Overview - Total Daily Doses ≥ 300 mg - Continuous and Intermittent (N=193) Azenosertib Demonstrated Encouraging ORR and DOR at ≥300mg total daily dose, intermittent in PROC Tumor Type PROC USC Other Solid Tumors Dose Schedule Intermittent Continuous Intermittent Continuous Intermittent Continuous Cyclin E1 IHC Status All Cyclin E1+ All Cyclin E1+ All Cyclin E1+ All Cyclin E1+ All Cyclin E1+ All Cyclin E1+ Number of Patients 58 23 11 3 19 11 16 4 43 4 46 5 ORR, (%), n (95% CI) 20.7%, (12/58) (11.2 - 33.4) 34.8%, (8/23) (16.4 - 57.3) 18.8% (2/11) (2.3 - 51.8) 33.3% (1/3) (0.8 - 90.6) 26.3% (5/19) (9.2 - 51.2) 36.4% (4/11) (10.9 - 69.2) 18.8% (3/16) (4.1 - 45.7) 25.0% (1/4) (0.6 - 80.6) 2.3% (1/4) (0.1 - 12.3) 0.0% (0/4) (0.0 - 60.2) 4.3% (2/46) (0.5 - 14.8) 0.0% (0/5) (0.0 - 52.2) mDOR (mos) (95% CI) 5.1 (3.0, 5.9) 5.2 (2.8, 6.9) 7.1 (4.2, NE) 4.2 (NE, NE) 5.5 (5.4, NE) 5.5 (5.4, NE) 5.6 (4.1, NE) 6.9 (NE, NE) 4.3 (NE, NE) NA 3.3 (3.0, NE) NA ZN-c3-001 1 Not a head-to-head comparison PROC, platinum-resistant ovarian cancer; USC, uterine serous carcinoma; ORR, objective response rate; mDOR, median duration of response; IHC, immunohistochemistry; NE, not evaluable Greater anti-tumor activity seen with intermittent dosing schedule and Cyclin E1+ patients Results direct focused development on doses of 300 and 400 mg at intermittent dosing schedule Inter itt t All Cycli 58 20.7 , (12/58) (11.2 - 33.4) 34.8 , (8/23) (16.4 - 57.3) 5.1 (3.0, 5.9) 5.2 (2.8, 6.9) Data Cutoff Dec 2 2024 Active database; subject to further change

14 Key Takeaways from 001 Azenosertib studied in a large patient population across multiple tumor types Platinum-resistant ovarian cancer (PROC) identified as an indication particularly susceptible to WEE1 inhibition Cyclin E1 identified as predictive biomarker for response to azenosertib Meaningful therapeutic window identified providing a favorable risk-benefit profile in Cyclin E1+ PROC patients at total daily doses of 300 and 400mg QD 5:2 ZN-c3-001

MAMMOTH (ZN-c3-006) NCT05198804
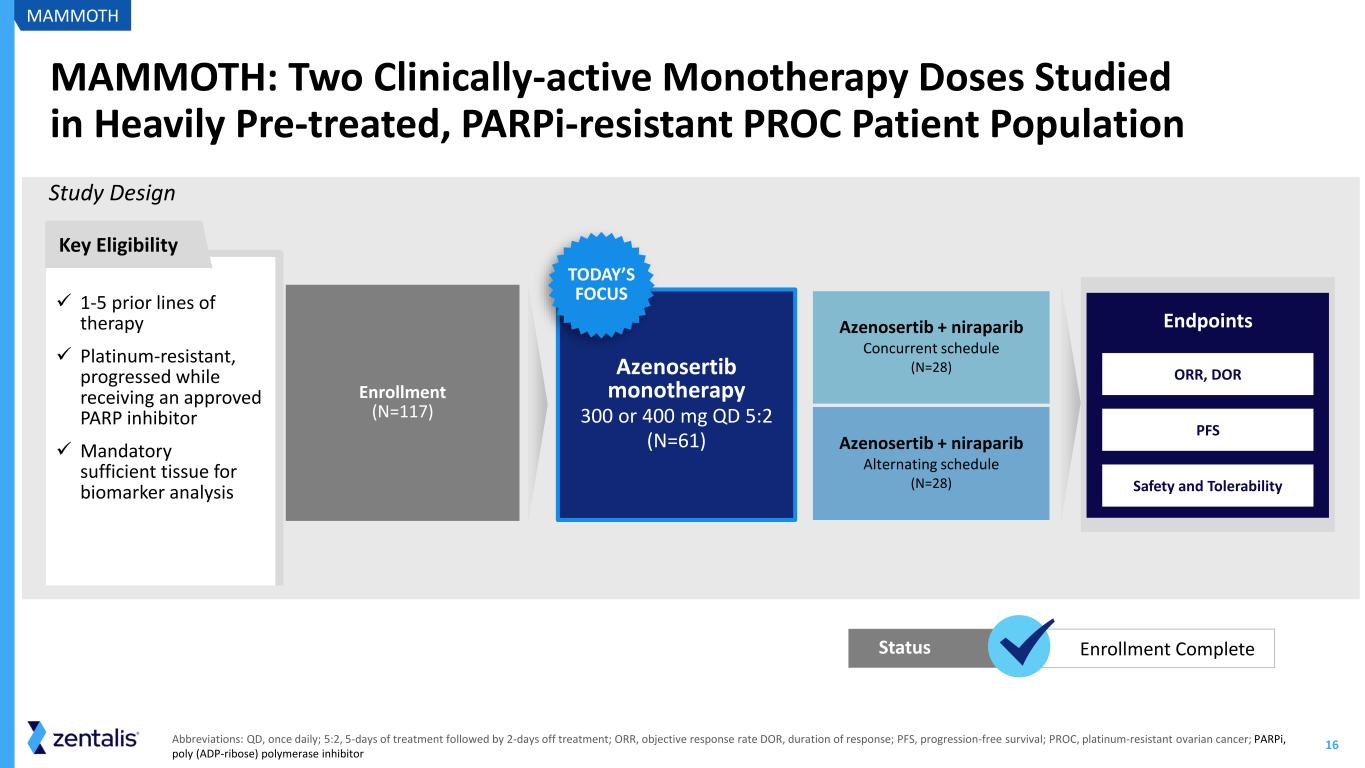
16 MAMMOTH: Two Clinically-active Monotherapy Doses Studied in Heavily Pre-treated, PARPi-resistant PROC Patient Population Study Design ✓ 1-5 prior lines of therapy ✓ Platinum-resistant, progressed while receiving an approved PARP inhibitor ✓ Mandatory sufficient tissue for biomarker analysis Key Eligibility MAMMOTH Abbreviations: QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; ORR, objective response rate DOR, duration of response; PFS, progression-free survival; PROC, platinum-resistant ovarian cancer; PARPi, poly (ADP-ribose) polymerase inhibitor Azenosertib + niraparib Concurrent schedule (N=28) Azenosertib + niraparib Alternating schedule (N=28) Azenosertib monotherapy 300 or 400 mg QD 5:2 (N=61) TODAY’S FOCUS PFS Safety and Tolerability ORR, DOR Endpoints Enrollment (N=117) Enrollment CompleteStatus
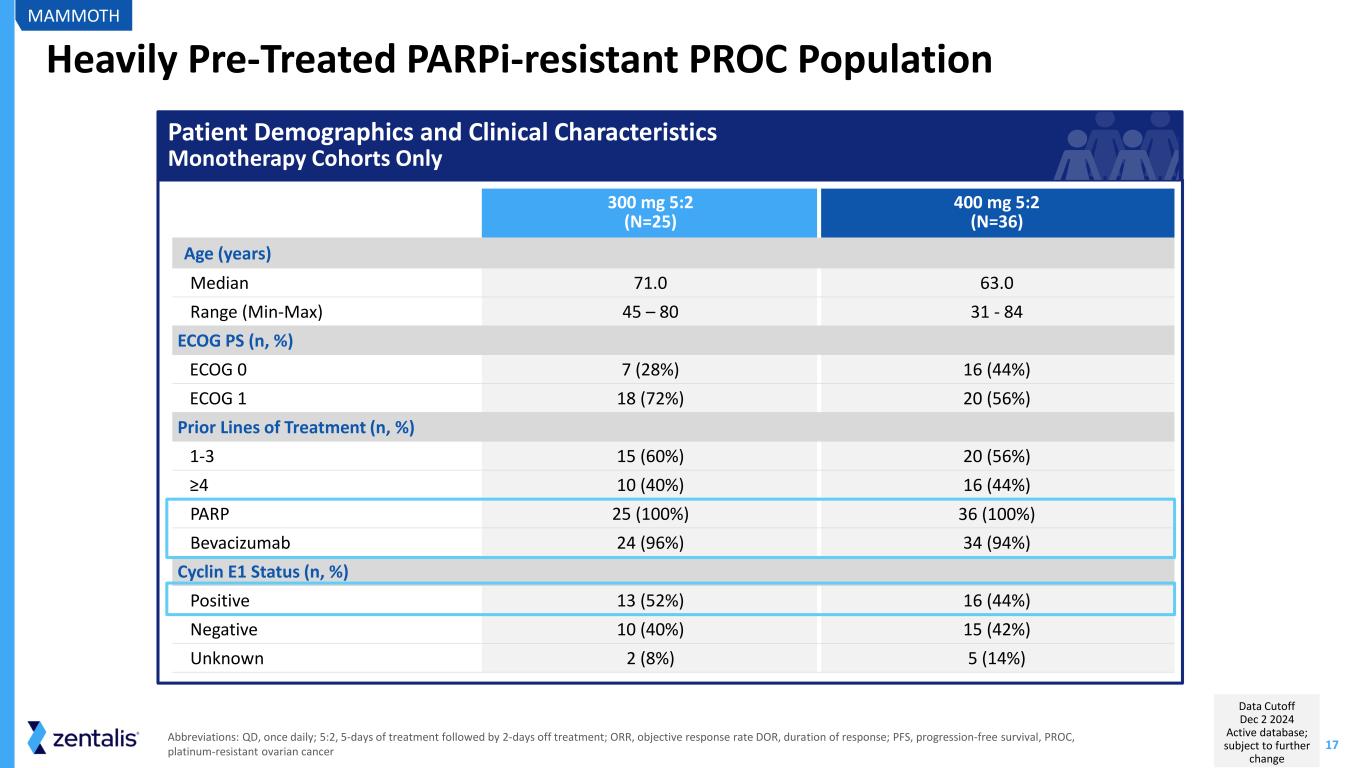
17 Heavily Pre-Treated PARPi-resistant PROC Population 300 mg 5:2 (N=25) 400 mg 5:2 (N=36) Age (years) Median 71.0 63.0 Range (Min-Max) 45 – 80 31 - 84 ECOG PS (n, %) ECOG 0 7 (28%) 16 (44%) ECOG 1 18 (72%) 20 (56%) Prior Lines of Treatment (n, %) 1-3 15 (60%) 20 (56%) ≥4 10 (40%) 16 (44%) PARP 25 (100%) 36 (100%) Bevacizumab 24 (96%) 34 (94%) Cyclin E1 Status (n, %) Positive 13 (52%) 16 (44%) Negative 10 (40%) 15 (42%) Unknown 2 (8%) 5 (14%) MAMMOTH Abbreviations: QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; ORR, objective response rate DOR, duration of response; PFS, progression-free survival, PROC, platinum-resistant ovarian cancer Patient Demographics and Clinical Characteristics Monotherapy Cohorts Only Data Cutoff Dec 2 2024 Active database; subject to further change
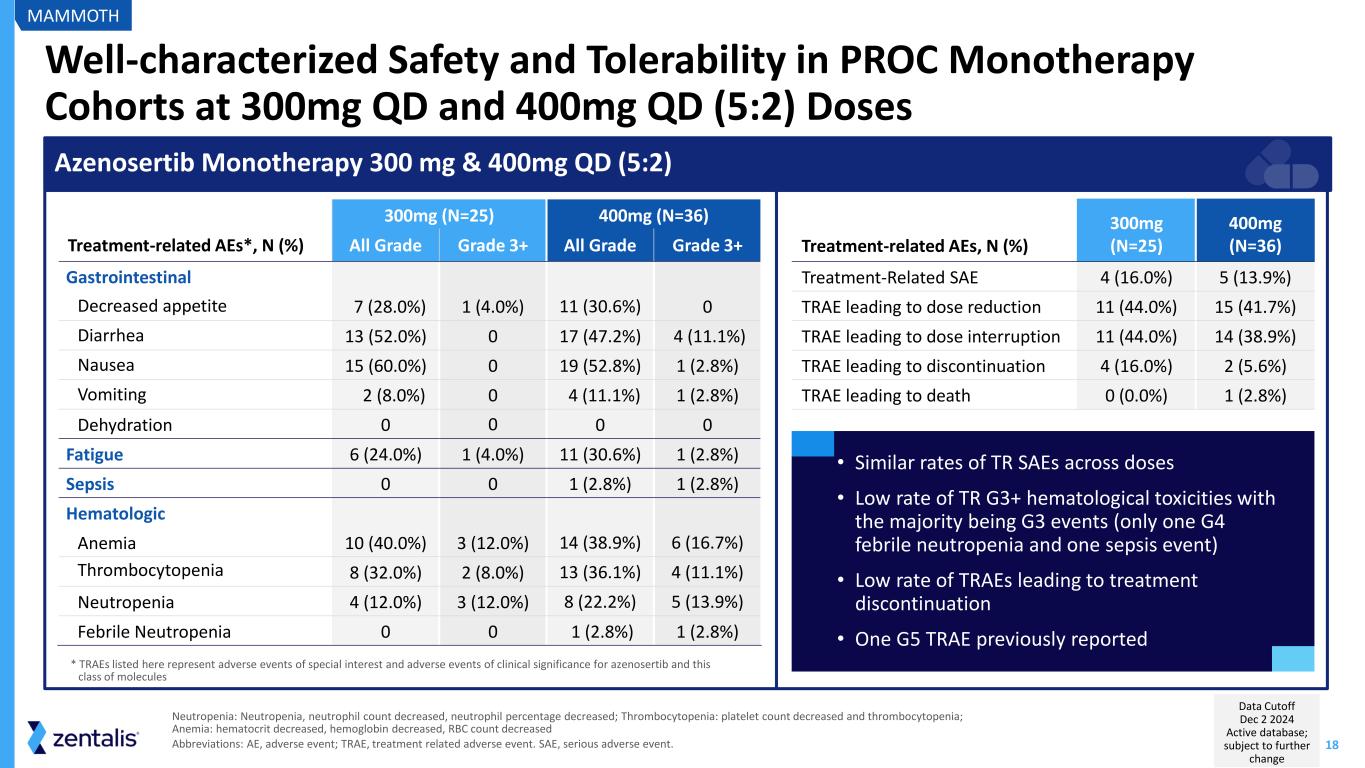
18 Well-characterized Safety and Tolerability in PROC Monotherapy Cohorts at 300mg QD and 400mg QD (5:2) Doses Neutropenia: Neutropenia, neutrophil count decreased, neutrophil percentage decreased; Thrombocytopenia: platelet count decreased and thrombocytopenia; Anemia: hematocrit decreased, hemoglobin decreased, RBC count decreased Abbreviations: AE, adverse event; TRAE, treatment related adverse event. SAE, serious adverse event. 300mg (N=25) 400mg (N=36) Treatment-related AEs*, N (%) All Grade Grade 3+ All Grade Grade 3+ Gastrointestinal Decreased appetite 7 (28.0%) 1 (4.0%) 11 (30.6%) 0 Diarrhea 13 (52.0%) 0 17 (47.2%) 4 (11.1%) Nausea 15 (60.0%) 0 19 (52.8%) 1 (2.8%) Vomiting 2 (8.0%) 0 4 (11.1%) 1 (2.8%) Dehydration 0 0 0 0 Fatigue 6 (24.0%) 1 (4.0%) 11 (30.6%) 1 (2.8%) Sepsis 0 0 1 (2.8%) 1 (2.8%) Hematologic Anemia 10 (40.0%) 3 (12.0%) 14 (38.9%) 6 (16.7%) Thrombocytopenia 8 (32.0%) 2 (8.0%) 13 (36.1%) 4 (11.1%) Neutropenia 4 (12.0%) 3 (12.0%) 8 (22.2%) 5 (13.9%) Febrile Neutropenia 0 0 1 (2.8%) 1 (2.8%) Azenosertib Monotherapy 300 mg & 400mg QD (5:2) Treatment-related AEs, N (%) 300mg (N=25) 400mg (N=36) Treatment-Related SAE 4 (16.0%) 5 (13.9%) TRAE leading to dose reduction 11 (44.0%) 15 (41.7%) TRAE leading to dose interruption 11 (44.0%) 14 (38.9%) TRAE leading to discontinuation 4 (16.0%) 2 (5.6%) TRAE leading to death 0 (0.0%) 1 (2.8%) • Similar rates of TR SAEs across doses • Low rate of TR G3+ hematological toxicities with the majority being G3 events (only one G4 febrile neutropenia and one sepsis event) • Low rate of TRAEs leading to treatment discontinuation • One G5 TRAE previously reported * TRAEs listed here represent adverse events of special interest and adverse events of clinical significance for azenosertib and this class of molecules MAMMOTH Data Cutoff Dec 2 2024 Active database; subject to further change
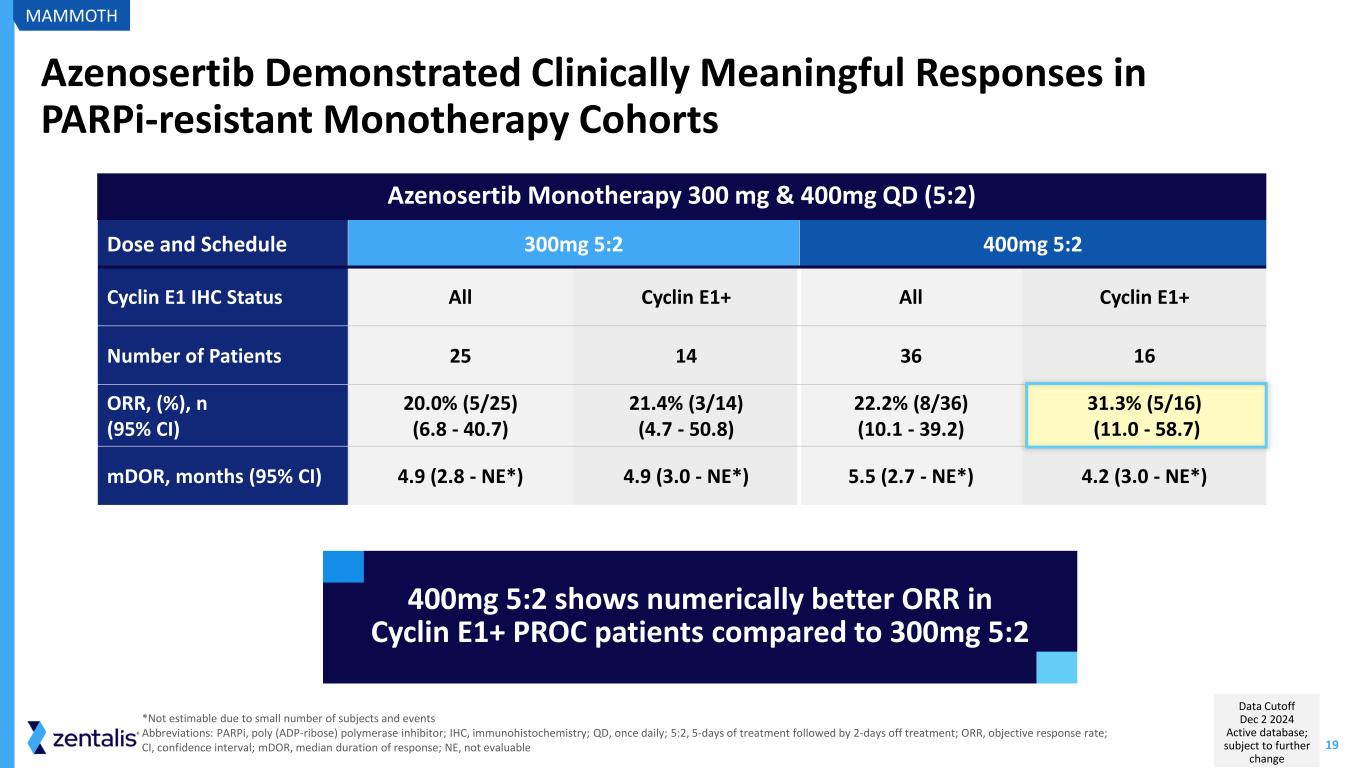
19 Azenosertib Monotherapy 300 mg & 400mg QD (5:2) Azenosertib Demonstrated Clinically Meaningful Responses in PARPi-resistant Monotherapy Cohorts Dose and Schedule 300mg 5:2 400mg 5:2 Cyclin E1 IHC Status All Cyclin E1+ All Cyclin E1+ Number of Patients 25 14 36 16 ORR, (%), n (95% CI) 20.0% (5/25) (6.8 - 40.7) 21.4% (3/14) (4.7 - 50.8) 22.2% (8/36) (10.1 - 39.2) 31.3% (5/16) (11.0 - 58.7) mDOR, months (95% CI) 4.9 (2.8 - NE*) 4.9 (3.0 - NE*) 5.5 (2.7 - NE*) 4.2 (3.0 - NE*) 400mg 5:2 shows numerically better ORR in Cyclin E1+ PROC patients compared to 300mg 5:2 *Not estimable due to small number of subjects and events Abbreviations: PARPi, poly (ADP-ribose) polymerase inhibitor; IHC, immunohistochemistry; QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; ORR, objective response rate; CI, confidence interval; mDOR, median duration of response; NE, not evaluable MAMMOTH Data Cutoff Dec 2 2024 Active database; subject to further change
20 Key Takeaways from MAMMOTH Monotherapy Cohort Consistent antitumor activity in PARPi-resistant patients Tolerability and toxicity consistent with 001 study and similar between the assessed doses 400mg QD 5:2 showed numerically higher response rates than 300mg QD 5:2 MAMMOTH Learnings continue to support development of azenosertib in Cyclin E1+ PROC Abbreviations: QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; PROC, platinum-resistant ovarian cancer; PARPi, poly (ADP-ribose) polymerase inhibitor

DENALI Part 1b (ZN-c3-005) NCT05198804

22 David M O'Malley, MD Director & Professor, Division of Gynecologic Oncology in Obstetrics and Gynecology John G. Boutselis Chair in Gynecologic Oncology The Ohio State University and the James Comprehensive Cancer Center
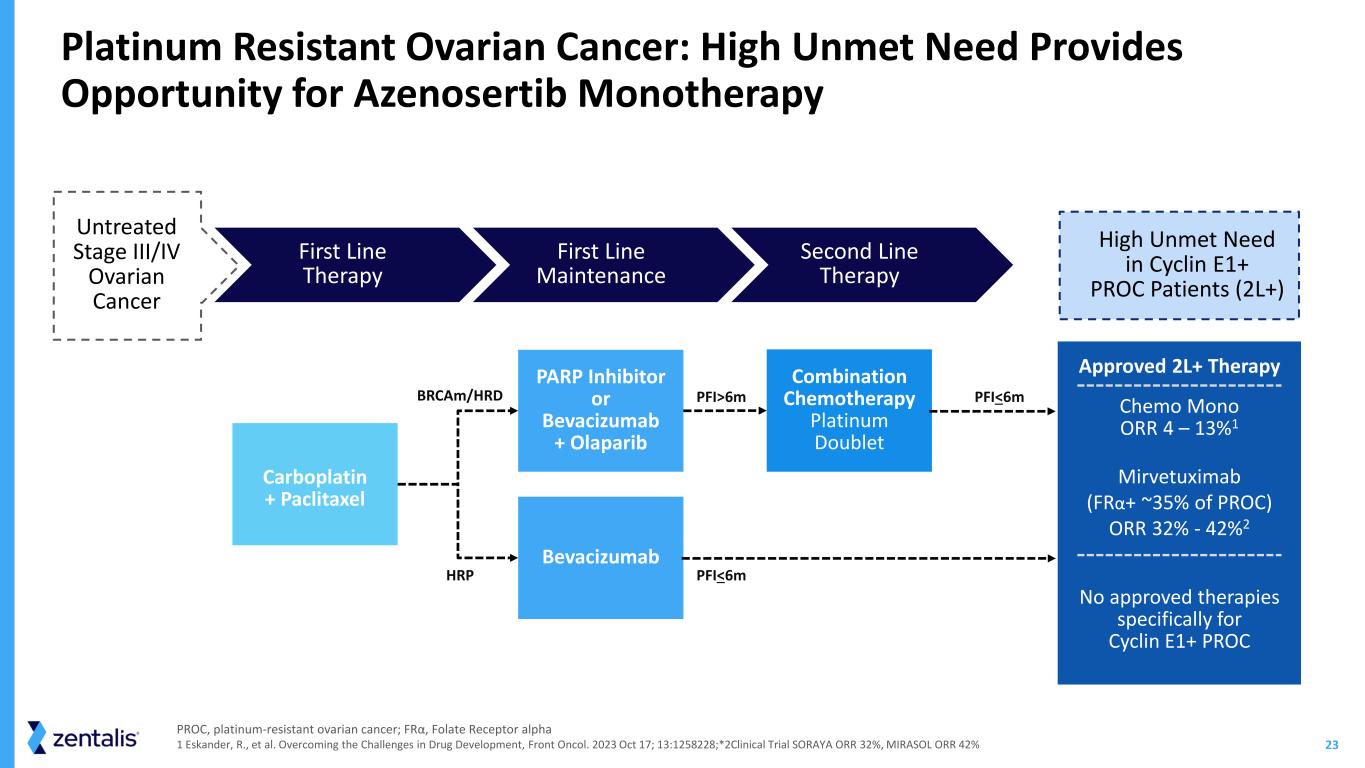
23 PARP Inhibitor or Bevacizumab + Olaparib Bevacizumab Combination Chemotherapy Platinum Doublet HRP PFI>6m PFI<6m PFI<6m Carboplatin + Paclitaxel High Unmet Need in Cyclin E1+ PROC Patients (2L+) Approved 2L+ Therapy Chemo Mono ORR 4 – 13%1 Mirvetuximab (FRα+ ~35% of PROC) ORR 32% - 42%2 No approved therapies specifically for Cyclin E1+ PROC Platinum Resistant Ovarian Cancer: High Unmet Need Provides Opportunity for Azenosertib Monotherapy First Line Maintenance Second Line Therapy First Line Therapy BRCAm/HRD Untreated Stage III/IV Ovarian Cancer PROC, platinum-resistant ovarian cancer; FRα, Folate Receptor alpha 1 Eskander, R., et al. Overcoming the Challenges in Drug Development, Front Oncol. 2023 Oct 17; 13:1258228;*2Clinical Trial SORAYA ORR 32%, MIRASOL ORR 42%
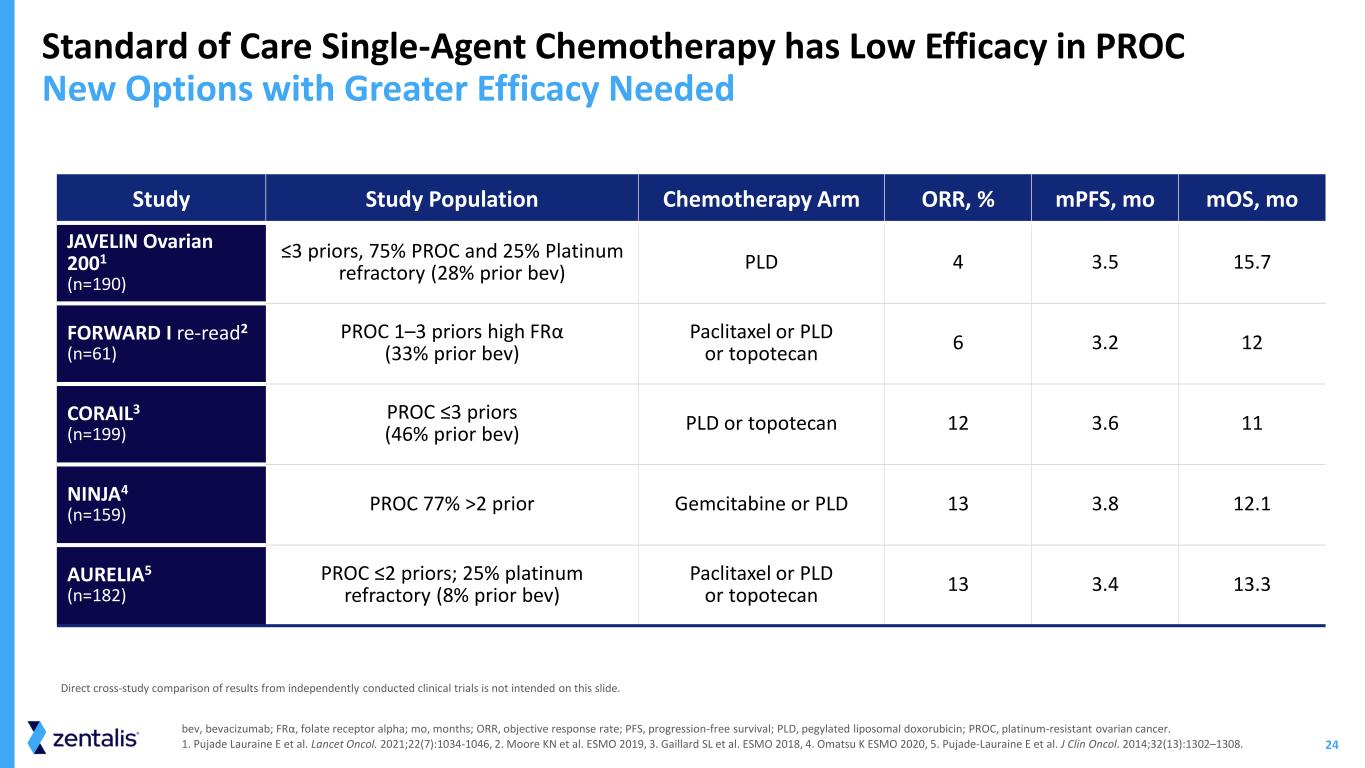
24 Standard of Care Single-Agent Chemotherapy has Low Efficacy in PROC New Options with Greater Efficacy Needed bev, bevacizumab; FRα, folate receptor alpha; mo, months; ORR, objective response rate; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; PROC, platinum-resistant ovarian cancer. 1. Pujade Lauraine E et al. Lancet Oncol. 2021;22(7):1034-1046, 2. Moore KN et al. ESMO 2019, 3. Gaillard SL et al. ESMO 2018, 4. Omatsu K ESMO 2020, 5. Pujade-Lauraine E et al. J Clin Oncol. 2014;32(13):1302–1308. Study Study Population Chemotherapy Arm ORR, % mPFS, mo mOS, mo JAVELIN Ovarian 2001 (n=190) ≤3 priors, 75% PROC and 25% Platinum refractory (28% prior bev) PLD 4 3.5 15.7 FORWARD I re-read2 (n=61) PROC 1–3 priors high FRα (33% prior bev) Paclitaxel or PLD or topotecan 6 3.2 12 CORAIL3 (n=199) PROC ≤3 priors (46% prior bev) PLD or topotecan 12 3.6 11 NINJA4 (n=159) PROC 77% >2 prior Gemcitabine or PLD 13 3.8 12.1 AURELIA5 (n=182) PROC ≤2 priors; 25% platinum refractory (8% prior bev) Paclitaxel or PLD or topotecan 13 3.4 13.3 Direct cross-study comparison of results from independently conducted clinical trials is not intended on this slide.

25 Study Design ✓ Platinum-resistant ovarian cancer ✓ 1-5 prior lines of therapy ✓ Tissue mandatory for biomarker assessment Key Eligibility DENALI Part 1b Evaluated 400mg 5:2 QD and Confirmed Cyclin E1 Biomarker DENALI Abbreviations: QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; ORR, objective response rate; DOR, duration of response; PFS, progression-free survival Endpoints PFS Safety and Tolerability ORR, DOR Enrollment (N=102) Azenosertib monotherapy 400 mg QD 5:2 Part 1b Enrollment CompleteStatus
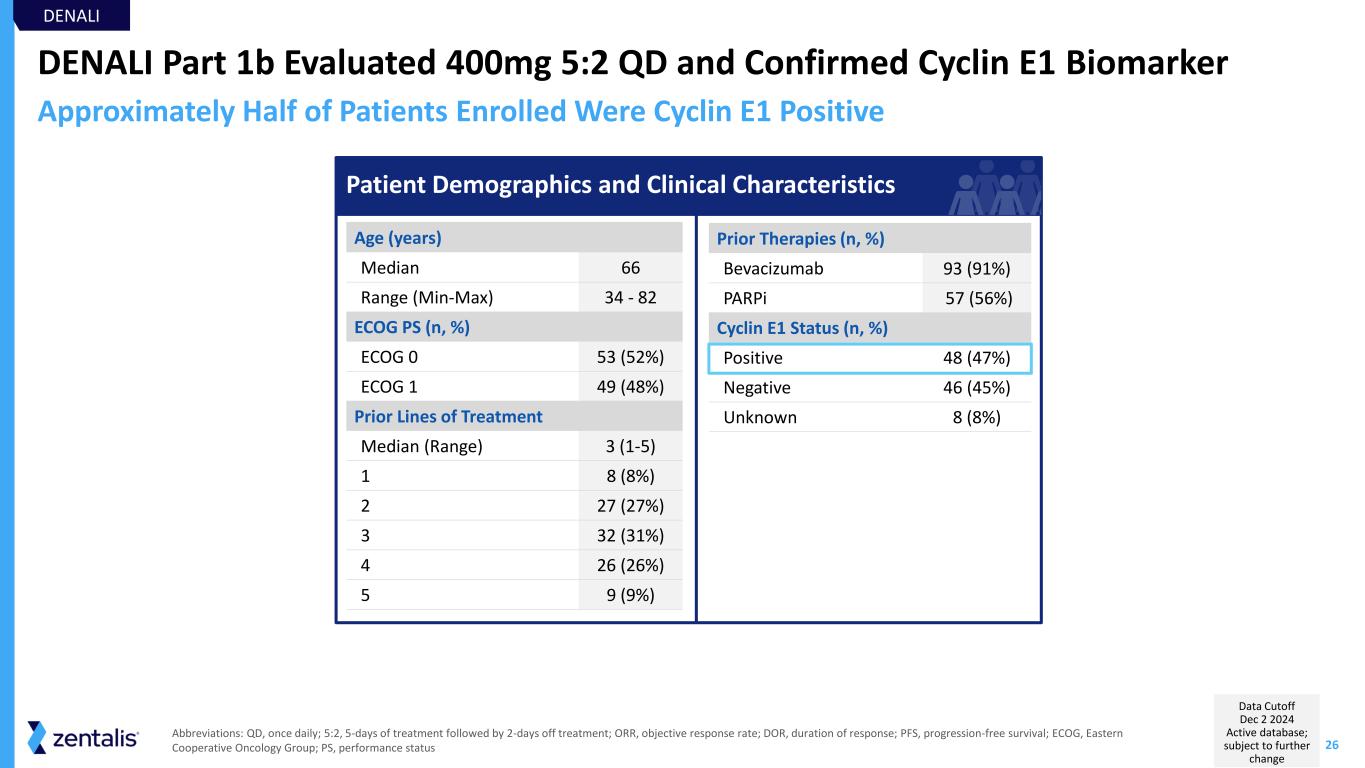
26 DENALI Part 1b Evaluated 400mg 5:2 QD and Confirmed Cyclin E1 Biomarker Prior Therapies (n, %) Bevacizumab 93 (91%) PARPi 57 (56%) Cyclin E1 Status (n, %) Positive 48 (47%) Negative 46 (45%) Unknown 8 (8%) Age (years) Median 66 Range (Min-Max) 34 - 82 ECOG PS (n, %) ECOG 0 53 (52%) ECOG 1 49 (48%) Prior Lines of Treatment Median (Range) 3 (1-5) 1 8 (8%) 2 27 (27%) 3 32 (31%) 4 26 (26%) 5 9 (9%) DENALI Approximately Half of Patients Enrolled Were Cyclin E1 Positive Abbreviations: QD, once daily; 5:2, 5-days of treatment followed by 2-days off treatment; ORR, objective response rate; DOR, duration of response; PFS, progression-free survival; ECOG, Eastern Cooperative Oncology Group; PS, performance status Patient Demographics and Clinical Characteristics Data Cutoff Dec 2 2024 Active database; subject to further change
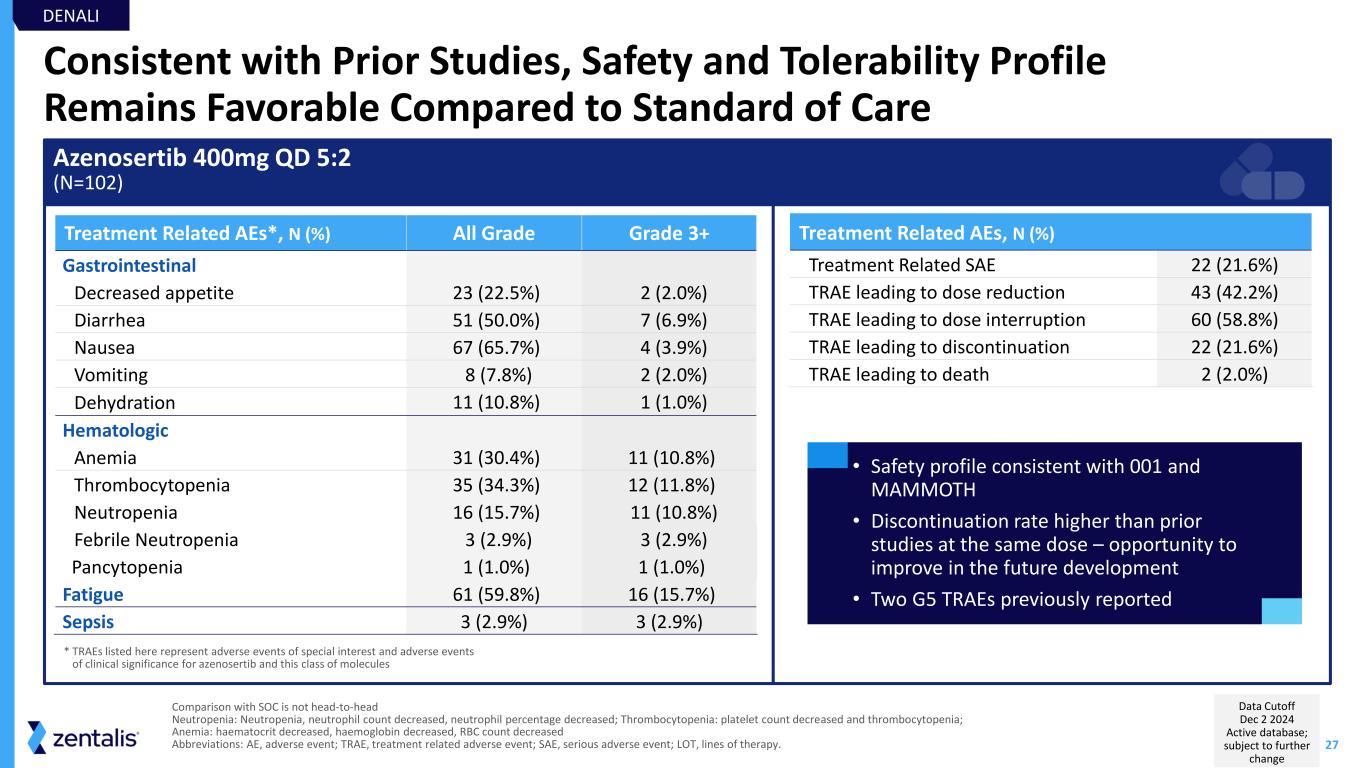
27 Consistent with Prior Studies, Safety and Tolerability Profile Remains Favorable Compared to Standard of Care Azenosertib 400mg QD 5:2 (N=102) Treatment Related AEs, N (%) Treatment Related SAE 22 (21.6%) TRAE leading to dose reduction 43 (42.2%) TRAE leading to dose interruption 60 (58.8%) TRAE leading to discontinuation 22 (21.6%) TRAE leading to death 2 (2.0%) Treatment Related AEs*, N (%) All Grade Grade 3+ Gastrointestinal Decreased appetite 23 (22.5%) 2 (2.0%) Diarrhea 51 (50.0%) 7 (6.9%) Nausea 67 (65.7%) 4 (3.9%) Vomiting 8 (7.8%) 2 (2.0%) Dehydration 11 (10.8%) 1 (1.0%) Hematologic Anemia 31 (30.4%) 11 (10.8%) Thrombocytopenia 35 (34.3%) 12 (11.8%) Neutropenia 16 (15.7%) 11 (10.8%) Febrile Neutropenia 3 (2.9%) 3 (2.9%) Pancytopenia 1 (1.0%) 1 (1.0%) Fatigue 61 (59.8%) 16 (15.7%) Sepsis 3 (2.9%) 3 (2.9%) • Safety profile consistent with 001 and MAMMOTH • Discontinuation rate higher than prior studies at the same dose – opportunity to improve in the future development • Two G5 TRAEs previously reported * TRAEs listed here represent adverse events of special interest and adverse events of clinical significance for azenosertib and this class of molecules DENALI Comparison with SOC is not head-to-head Neutropenia: Neutropenia, neutrophil count decreased, neutrophil percentage decreased; Thrombocytopenia: platelet count decreased and thrombocytopenia; Anemia: haematocrit decreased, haemoglobin decreased, RBC count decreased Abbreviations: AE, adverse event; TRAE, treatment related adverse event; SAE, serious adverse event; LOT, lines of therapy. Data Cutoff Dec 2 2024 Active database; subject to further change
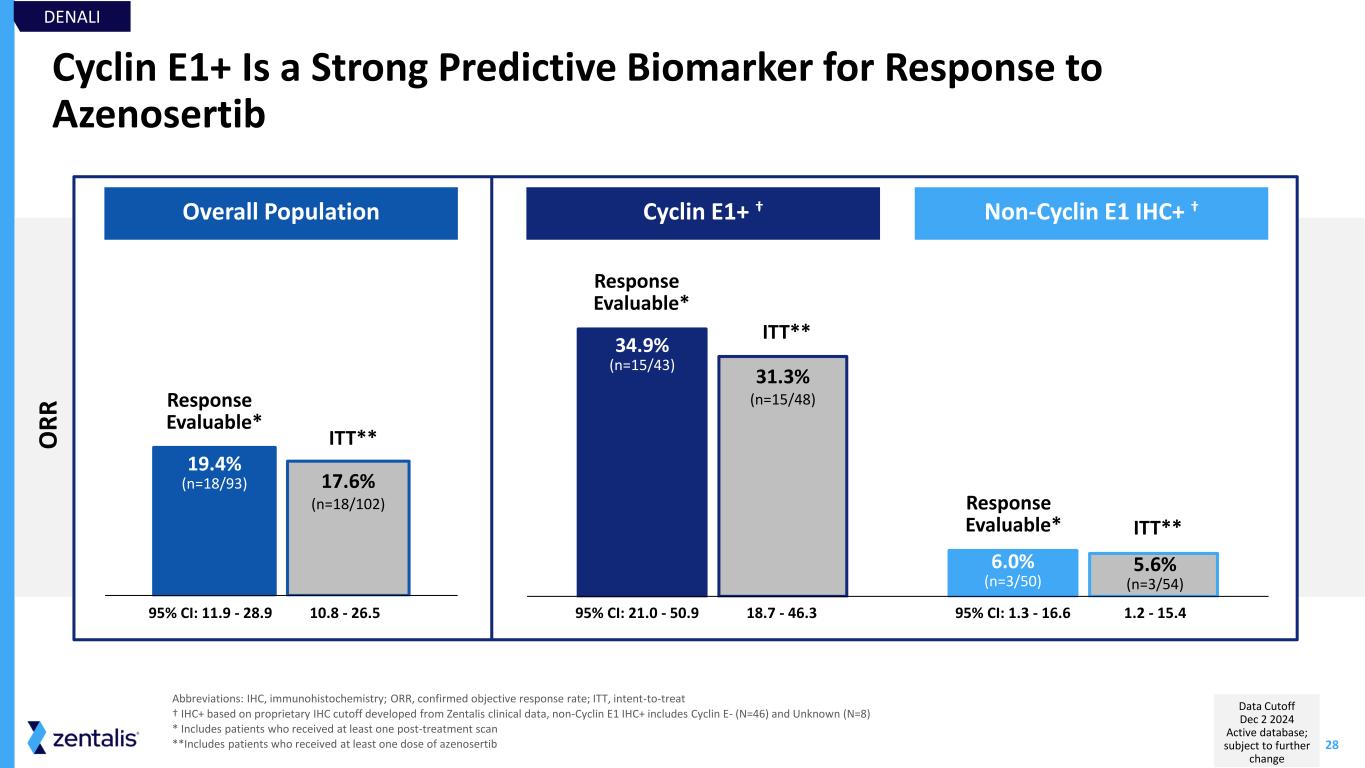
28 Cyclin E1+ Is a Strong Predictive Biomarker for Response to Azenosertib Abbreviations: IHC, immunohistochemistry; ORR, confirmed objective response rate; ITT, intent-to-treat † IHC+ based on proprietary IHC cutoff developed from Zentalis clinical data, non-Cyclin E1 IHC+ includes Cyclin E- (N=46) and Unknown (N=8) * Includes patients who received at least one post-treatment scan **Includes patients who received at least one dose of azenosertib 95% CI: 11.9 - 28.9 Overall Population Cyclin E1+ † Non-Cyclin E1 IHC+ † 34.9% (n=15/43) 6.0% (n=3/50) 31.3% (n=15/48) 5.6% (n=3/54) 95% CI: 21.0 - 50.9 95% CI: 1.3 - 16.6 19.4% (n=18/93) 17.6% (n=18/102) 10.8 - 26.5 18.7 - 46.3 1.2 - 15.4 Response Evaluable* ITT** Response Evaluable* ITT** Response Evaluable* ITT** DENALI O R R Data Cutoff Dec 2 2024 Active database; subject to further change
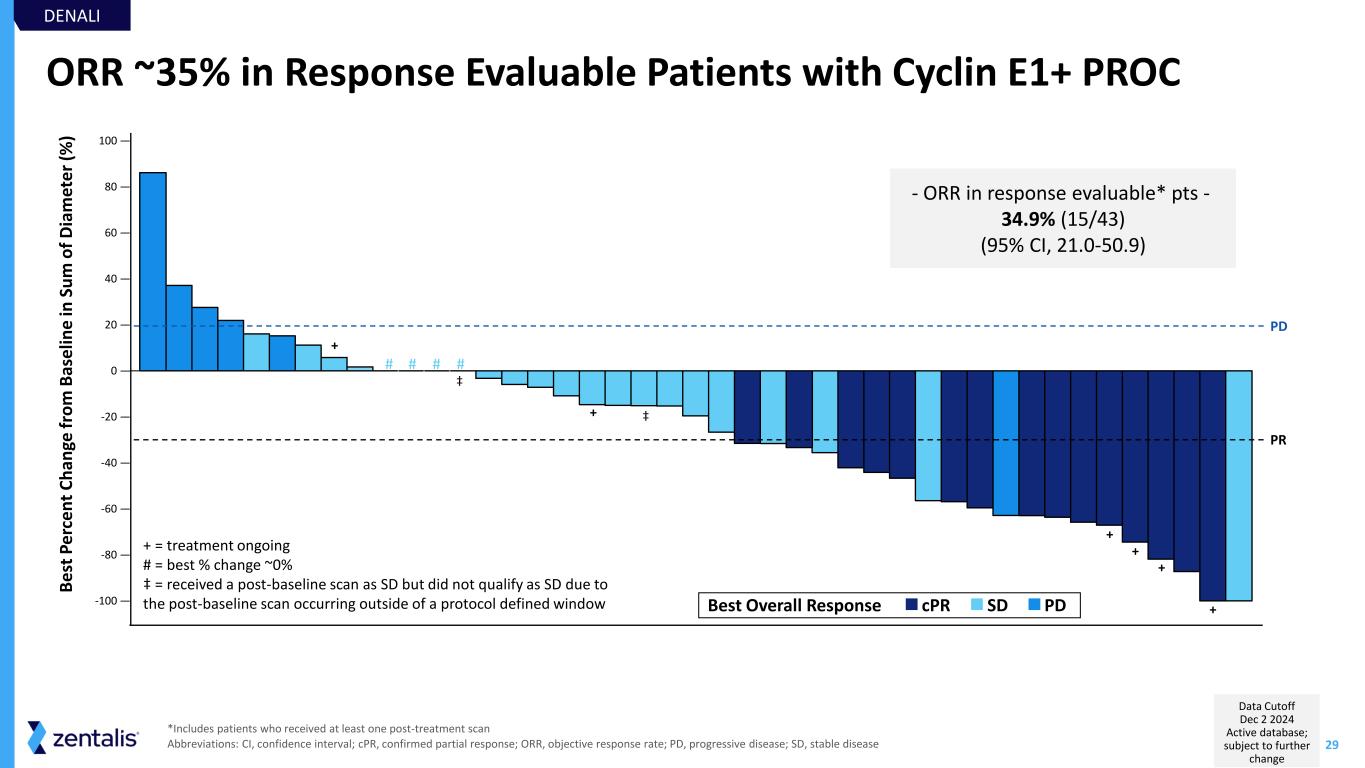
29 ORR ~35% in Response Evaluable Patients with Cyclin E1+ PROC - ORR in response evaluable* pts - 34.9% (15/43) (95% CI, 21.0-50.9) *Includes patients who received at least one post-treatment scan Abbreviations: CI, confidence interval; cPR, confirmed partial response; ORR, objective response rate; PD, progressive disease; SD, stable disease + + + + + + + = treatment ongoing # = best % change ~0% ‡ = received a post-baseline scan as SD but did not qualify as SD due to the post-baseline scan occurring outside of a protocol defined window-100 — -80 — -60 — -40 — -20 — 0 — 20 — 40 — 60 — 80 — 100 — B e st P e rc e n t C h an ge f ro m B as e lin e in S u m o f D ia m et e r (% ) PR PD PDSDcPRBest Overall Response DENALI # # # # ‡ ‡ Data Cutoff Dec 2 2024 Active database; subject to further change
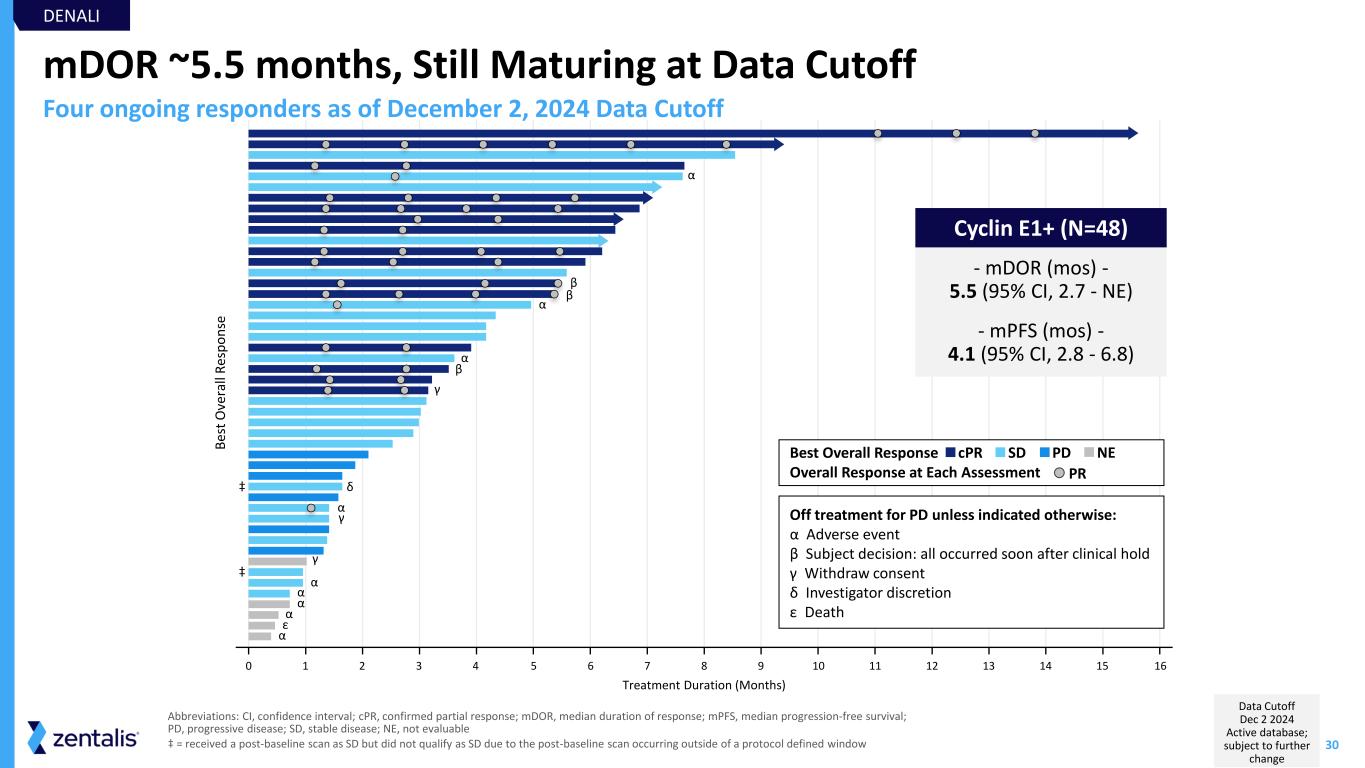
30 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Treatment Duration (Months) B es t O ve ra ll R es p o n se mDOR ~5.5 months, Still Maturing at Data Cutoff Abbreviations: CI, confidence interval; cPR, confirmed partial response; mDOR, median duration of response; mPFS, median progression-free survival; PD, progressive disease; SD, stable disease; NE, not evaluable ‡ = received a post-baseline scan as SD but did not qualify as SD due to the post-baseline scan occurring outside of a protocol defined window - mDOR (mos) - 5.5 (95% CI, 2.7 - NE) - mPFS (mos) - 4.1 (95% CI, 2.8 - 6.8) α α β β α β γ α α α α ε α γ γ δ α DENALI Four ongoing responders as of December 2, 2024 Data Cutoff Off treatment for PD unless indicated otherwise: α Adverse event β Subject decision: all occurred soon after clinical hold γ Withdraw consent δ Investigator discretion ε Death PDSDcPRBest Overall Response Overall Response at Each Assessment PR NE ‡ ‡ Cyclin E1+ (N=48) Data Cutoff Dec 2 2024 Active database; subject to further change
31 Key Takeaways from DENALI DENALI DENALI Part 1b demonstrates azenosertib has the potential to provide greater benefit for patients with Cyclin E1+ PROC over available therapies* Cyclin E1 established as highly selective and predictive biomarker for response to azenosertib Favorable toxicity with opportunity to improve outcomes through mitigation strategies in the next stage of development Learnings lead us to the registration-intent trial, DENALI Part 2… *Not a head-to-head comparison

Integrated Data and Path to Registration in PROC
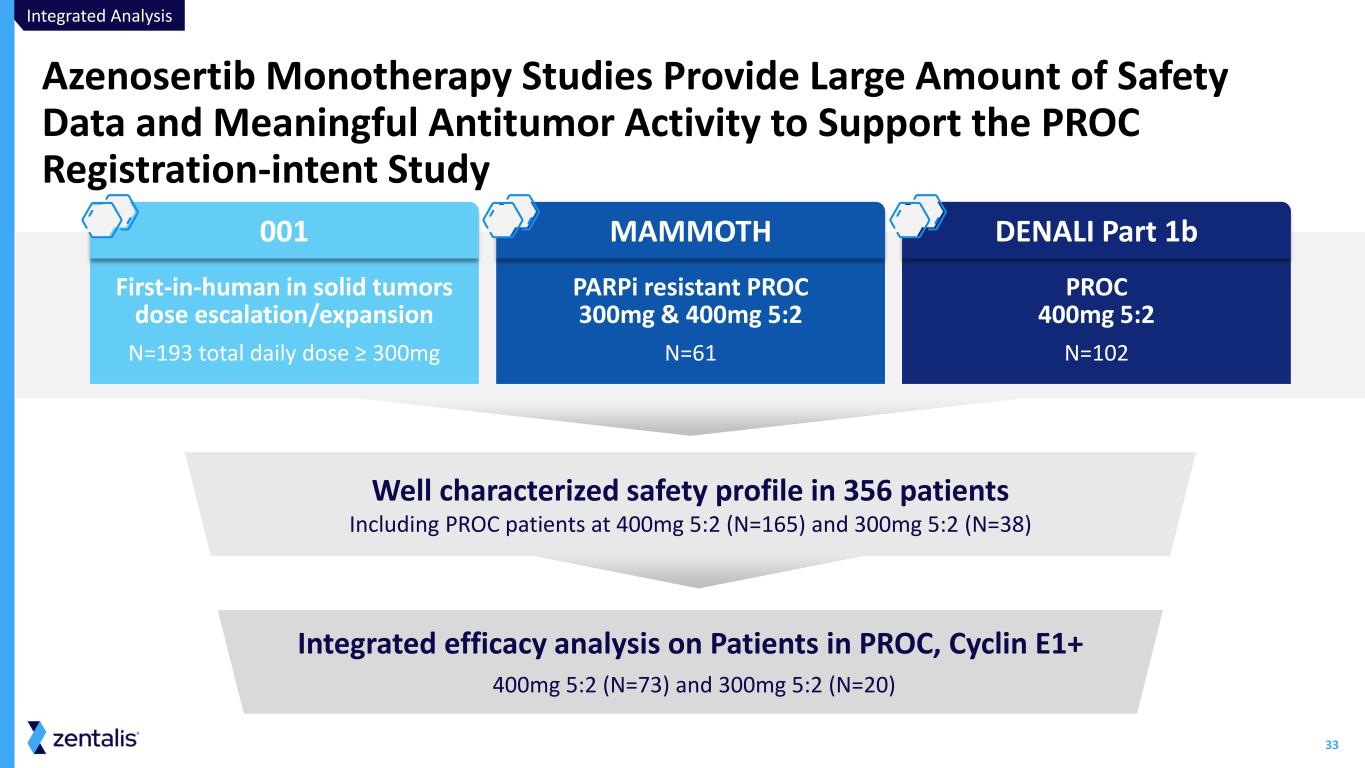
33 Azenosertib Monotherapy Studies Provide Large Amount of Safety Data and Meaningful Antitumor Activity to Support the PROC Registration-intent Study Well characterized safety profile in 356 patients Including PROC patients at 400mg 5:2 (N=165) and 300mg 5:2 (N=38) First-in-human in solid tumors dose escalation/expansion N=193 total daily dose ≥ 300mg PARPi resistant PROC 300mg & 400mg 5:2 N=61 PROC 400mg 5:2 N=102 001 MAMMOTH DENALI Part 1b Integrated efficacy analysis on Patients in PROC, Cyclin E1+ 400mg 5:2 (N=73) and 300mg 5:2 (N=20) Integrated Analysis
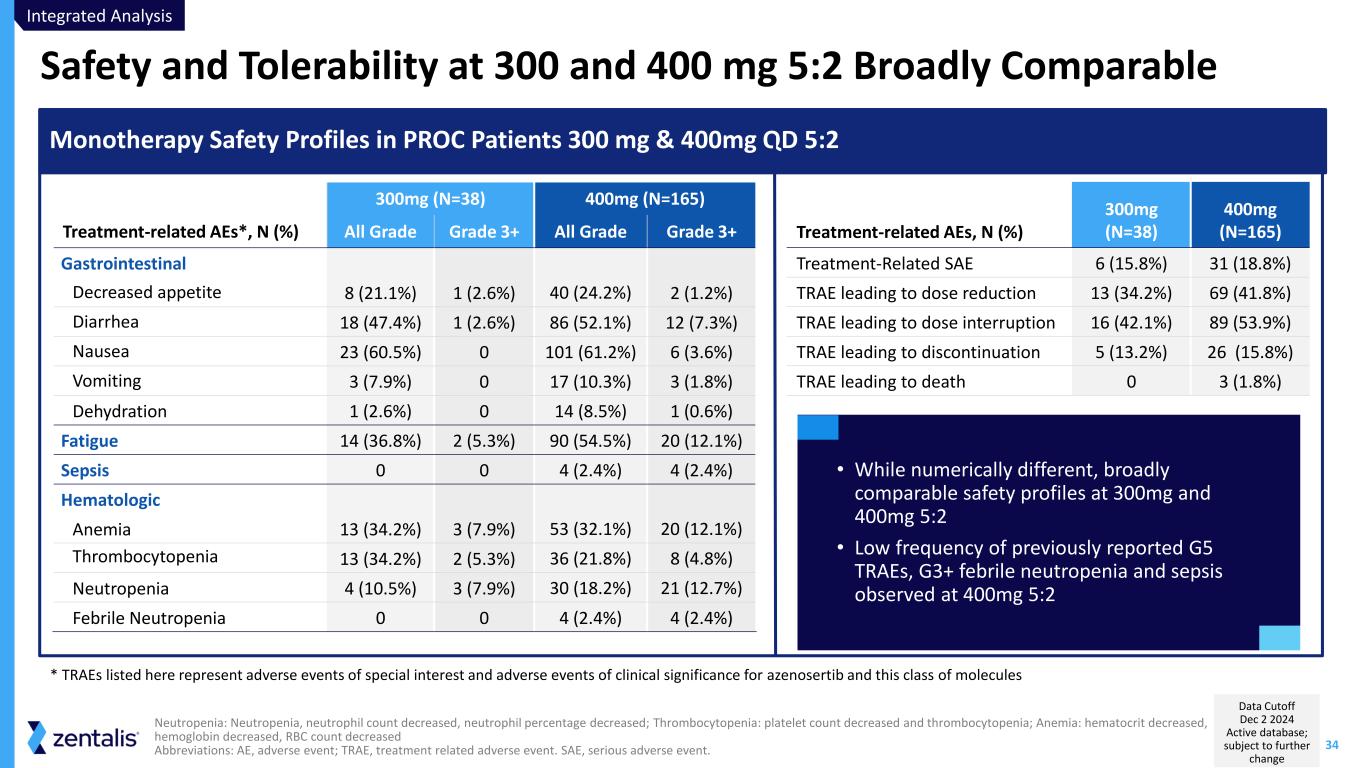
34 Safety and Tolerability at 300 and 400 mg 5:2 Broadly Comparable Neutropenia: Neutropenia, neutrophil count decreased, neutrophil percentage decreased; Thrombocytopenia: platelet count decreased and thrombocytopenia; Anemia: hematocrit decreased, hemoglobin decreased, RBC count decreased Abbreviations: AE, adverse event; TRAE, treatment related adverse event. SAE, serious adverse event. 300mg (N=38) 400mg (N=165) Treatment-related AEs*, N (%) All Grade Grade 3+ All Grade Grade 3+ Gastrointestinal Decreased appetite 8 (21.1%) 1 (2.6%) 40 (24.2%) 2 (1.2%) Diarrhea 18 (47.4%) 1 (2.6%) 86 (52.1%) 12 (7.3%) Nausea 23 (60.5%) 0 101 (61.2%) 6 (3.6%) Vomiting 3 (7.9%) 0 17 (10.3%) 3 (1.8%) Dehydration 1 (2.6%) 0 14 (8.5%) 1 (0.6%) Fatigue 14 (36.8%) 2 (5.3%) 90 (54.5%) 20 (12.1%) Sepsis 0 0 4 (2.4%) 4 (2.4%) Hematologic Anemia 13 (34.2%) 3 (7.9%) 53 (32.1%) 20 (12.1%) Thrombocytopenia 13 (34.2%) 2 (5.3%) 36 (21.8%) 8 (4.8%) Neutropenia 4 (10.5%) 3 (7.9%) 30 (18.2%) 21 (12.7%) Febrile Neutropenia 0 0 4 (2.4%) 4 (2.4%) Monotherapy Safety Profiles in PROC Patients 300 mg & 400mg QD 5:2 Treatment-related AEs, N (%) 300mg (N=38) 400mg (N=165) Treatment-Related SAE 6 (15.8%) 31 (18.8%) TRAE leading to dose reduction 13 (34.2%) 69 (41.8%) TRAE leading to dose interruption 16 (42.1%) 89 (53.9%) TRAE leading to discontinuation 5 (13.2%) 26 (15.8%) TRAE leading to death 0 3 (1.8%) * TRAEs listed here represent adverse events of special interest and adverse events of clinical significance for azenosertib and this class of molecules • While numerically different, broadly comparable safety profiles at 300mg and 400mg 5:2 • Low frequency of previously reported G5 TRAEs, G3+ febrile neutropenia and sepsis observed at 400mg 5:2 Integrated Analysis Data Cutoff Dec 2 2024 Active database; subject to further change
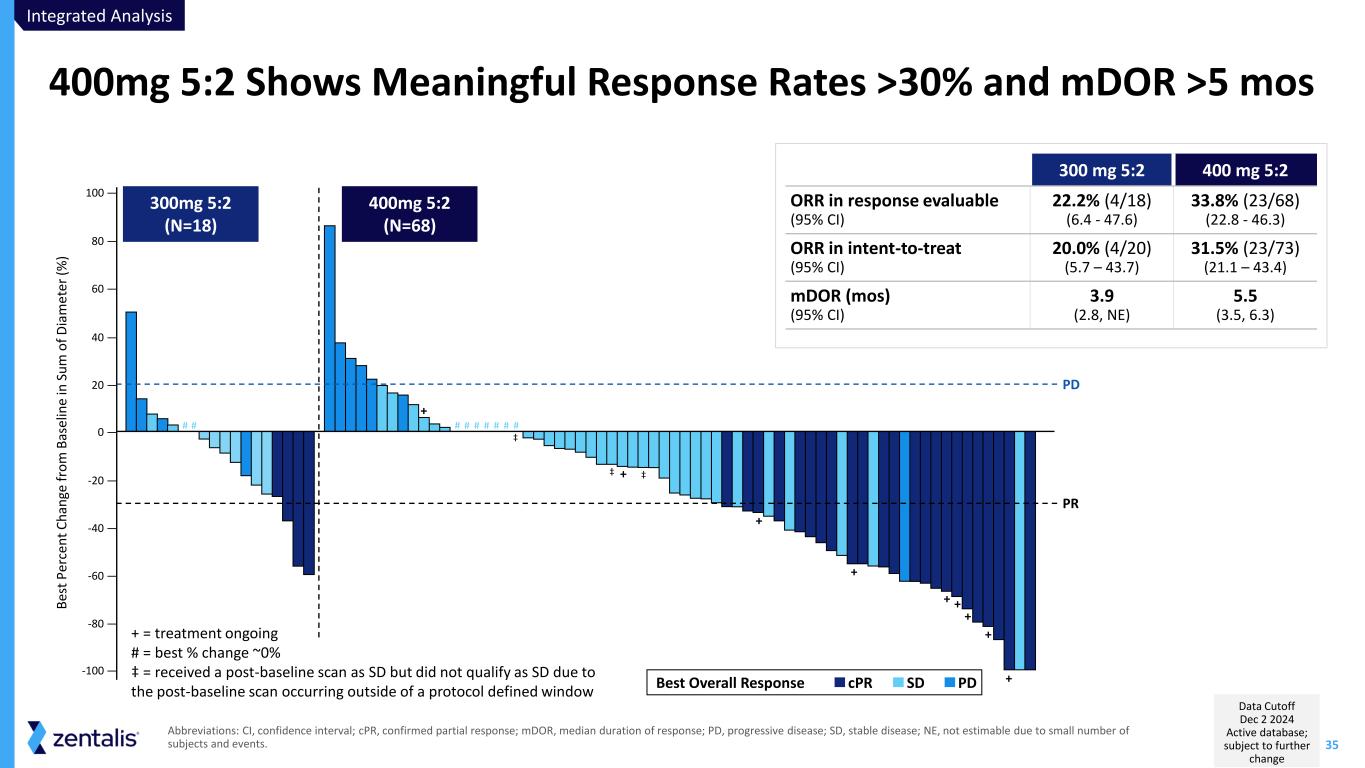
35 Abbreviations: CI, confidence interval; cPR, confirmed partial response; mDOR, median duration of response; PD, progressive disease; SD, stable disease; NE, not estimable due to small number of subjects and events. 400mg 5:2 Shows Meaningful Response Rates >30% and mDOR >5 mos 300 mg 5:2 400 mg 5:2 ORR in response evaluable (95% CI) 22.2% (4/18) (6.4 - 47.6) 33.8% (23/68) (22.8 - 46.3) ORR in intent-to-treat (95% CI) 20.0% (4/20) (5.7 – 43.7) 31.5% (23/73) (21.1 – 43.4) mDOR (mos) (95% CI) 3.9 (2.8, NE) 5.5 (3.5, 6.3) + + + + + + + + + -100 — -80 — -60 — -40 — -20 — 0 — 20 — 40 — 60 — 80 — 100 — B es t P er ce n t C h an ge f ro m B as el in e in S u m o f D ia m e te r (% ) PR PD 300mg 5:2 (N=18) 400mg 5:2 (N=68) PDSDcPRBest Overall Response # # # # # # # # # ‡ ‡ ‡ + = treatment ongoing # = best % change ~0% ‡ = received a post-baseline scan as SD but did not qualify as SD due to the post-baseline scan occurring outside of a protocol defined window Integrated Analysis Data Cutoff Dec 2 2024 Active database; subject to further change
36 Clinically meaningful ORR > 30%, mDOR ~5.5 months Consistent efficacy across multiple monotherapy studies in heavily pre-treated patients Azenosertib Has Potential to be the First and Best-in-Class WEE1 Inhibitor in Cyclin E1+ PROC 1 Well characterized safety profile, favorable to SOC chemo1 Additional strategies implemented to further reduce risk and improve duration on therapy 2 Precise patient selection biomarker and convenient oral option provide unique advantage for azenosertib and may improve patient quality of life 3 1. Not a head-to-head comparison Abbreviations: ORR, overall response rate; mDOR, median duration of response; SOC, standard of care
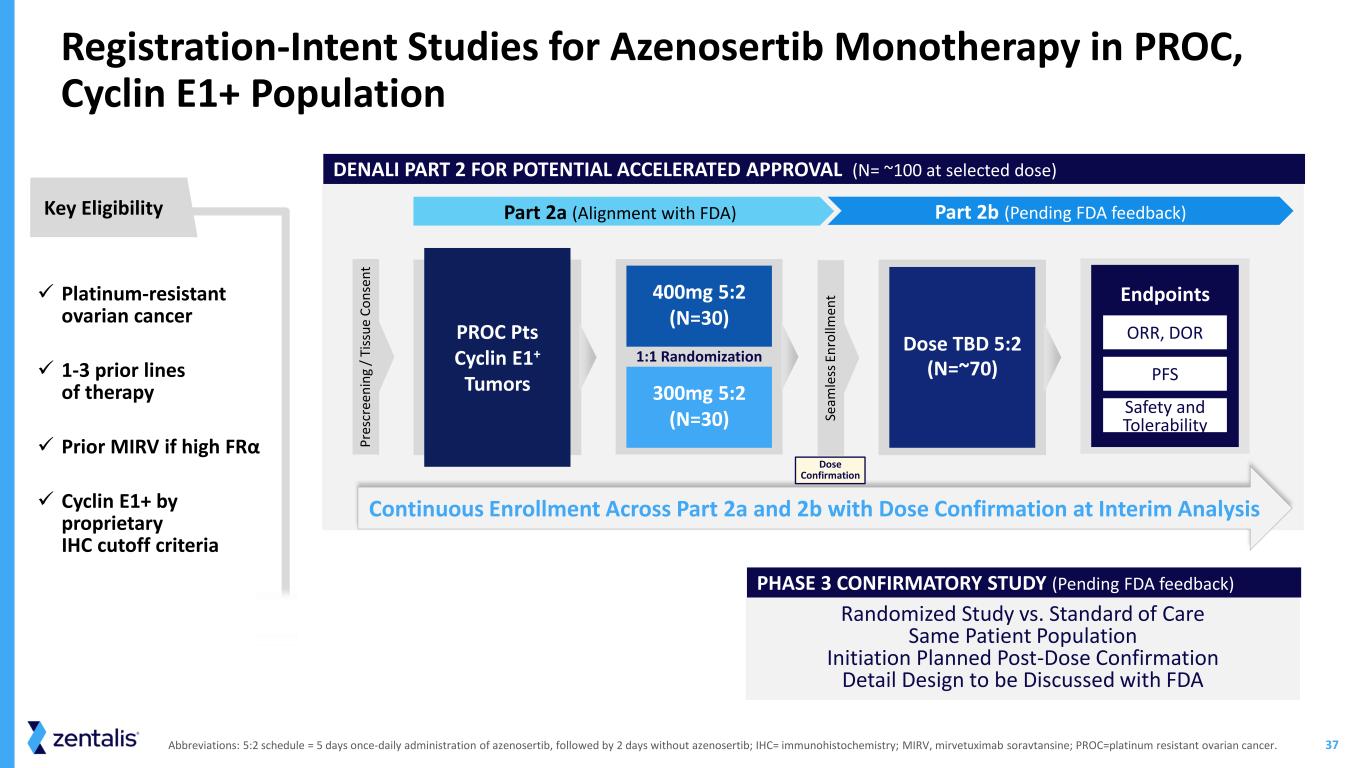
37 Randomized Study vs. Standard of Care Same Patient Population Initiation Planned Post-Dose Confirmation Detail Design to be Discussed with FDA Abbreviations: 5:2 schedule = 5 days once-daily administration of azenosertib, followed by 2 days without azenosertib; IHC= immunohistochemistry; MIRV, mirvetuximab soravtansine; PROC=platinum resistant ovarian cancer. DENALI PART 2 FOR POTENTIAL ACCELERATED APPROVAL (N= ~100 at selected dose) PHASE 3 CONFIRMATORY STUDY (Pending FDA feedback) ✓ Platinum-resistant ovarian cancer ✓ 1-3 prior lines of therapy ✓ Prior MIRV if high FRα ✓ Cyclin E1+ by proprietary IHC cutoff criteria Key Eligibility Registration-Intent Studies for Azenosertib Monotherapy in PROC, Cyclin E1+ Population Part 2a (Alignment with FDA) Part 2b (Pending FDA feedback) Continuous Enrollment Across Part 2a and 2b with Dose Confirmation at Interim Analysis PROC Pts Cyclin E1+ Tumors 400mg 5:2 (N=30) 300mg 5:2 (N=30) 1:1 Randomization Dose TBD 5:2 (N=~70) Endpoints PFS Safety and Tolerability ORR, DOR P re sc re en in g / Ti ss u e C o n se n t Se am le ss E n ro llm en t Dose Confirmation

Closing
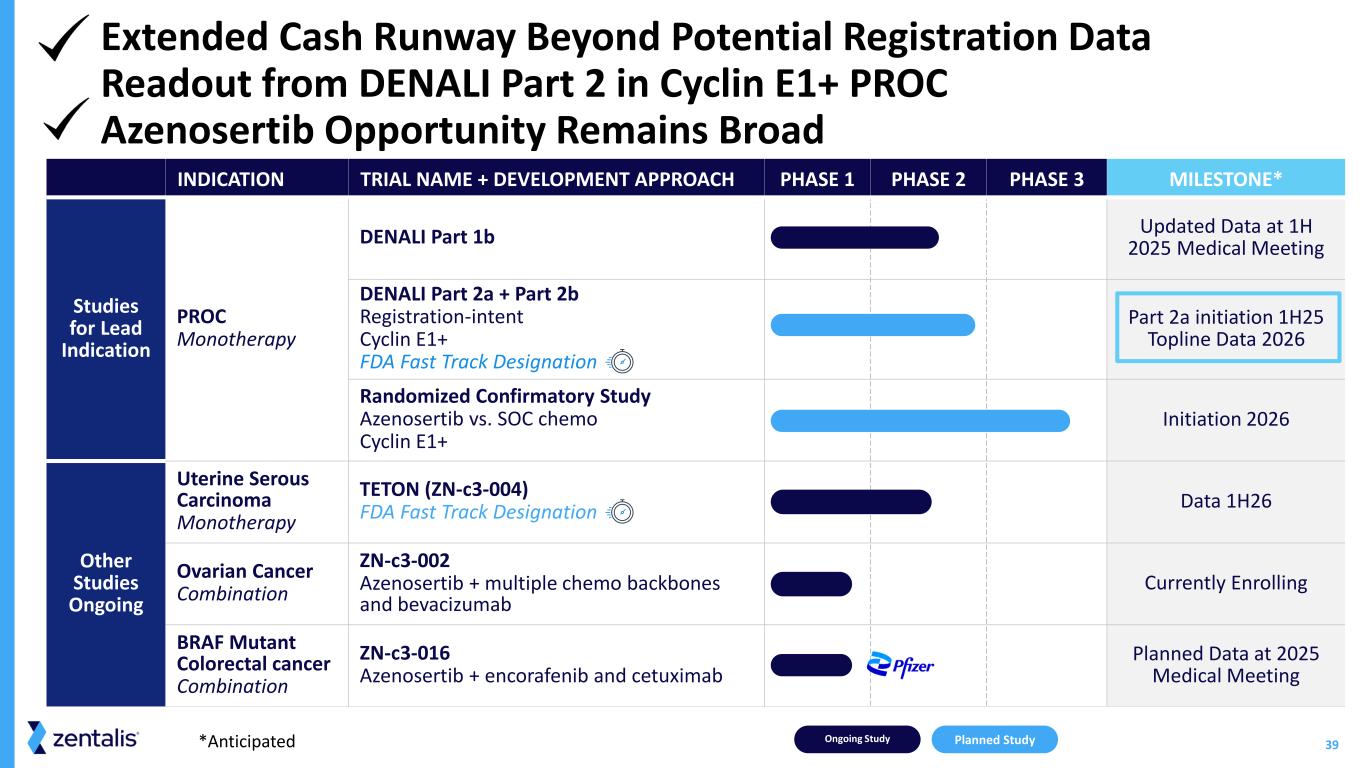
39 INDICATION TRIAL NAME + DEVELOPMENT APPROACH PHASE 1 PHASE 2 PHASE 3 MILESTONE* Studies for Lead Indication PROC Monotherapy DENALI Part 1b Updated Data at 1H 2025 Medical Meeting DENALI Part 2a + Part 2b Registration-intent Cyclin E1+ FDA Fast Track Designation Part 2a initiation 1H25 Topline Data 2026 Randomized Confirmatory Study Azenosertib vs. SOC chemo Cyclin E1+ Initiation 2026 Other Studies Ongoing Uterine Serous Carcinoma Monotherapy TETON (ZN-c3-004) FDA Fast Track Designation Data 1H26 Ovarian Cancer Combination ZN-c3-002 Azenosertib + multiple chemo backbones and bevacizumab Currently Enrolling BRAF Mutant Colorectal cancer Combination ZN-c3-016 Azenosertib + encorafenib and cetuximab Planned Data at 2025 Medical Meeting Ongoing Study Planned Study*Anticipated Extended Cash Runway Beyond Potential Registration Data Readout from DENALI Part 2 in Cyclin E1+ PROC Azenosertib Opportunity Remains Broad
40 Azenosertib Potential Best-in-Class Therapy for Cyclin E1+ PROC ➢ Recent launch of approved therapy in biomarker selected PROC demonstrates the large commercial opportunity ➢ Cyclin E1+ PROC, ~21,500 patients*, linked to poorer outcomes; no approved, targeted therapies ➢ SOC: mono chemo 4-13% ORR1 ➢ Other tumor types with Cyclin E1+ expression include breast, endometrial, bladder2 Market Opportunity ➢ >30% ORR and 5.5 mos mDOR in Cyclin E1+ patients at monotherapy dose of 400mg QD 5:2 ➢ 350+ PROC patients treated at active doses in monotherapy ➢ Manageable safety profile, potentially best-in-class WEE1 inhibitors Supportive Data ➢ Cash runway extended into late 2027 beyond anticipated topline data from DENALI Part 2 ➢ Resources now aligned with addition of key leadership on highly focused strategy ➢ Potential for accelerated approval pathway in PROC, Cyclin E1+ patients** Path Forward * US and EU4 (France, Germany, Italy, Spain) + UK; **Subject to supportive data and FDA feedback 1. Eskander, R., et al. Overcoming the Challenges in Drug Development, Front Oncol. 2023 Oct 17; 13:1258228 2. NAKAYAMA, K. et al. Int. J. Oncol. 48, 506–516 (2015); Aziz, D. et al. J. Pathol.: Clin. Res. 8, 355–370 (2022); Lotan, Y. et al. Eur Urol 64, 465– 471 (2013). Abbreviations: PROC, platinum-resistant ovarian cancer; mDOR, median duration of response; SOC, standard of care 3. Based on internal clinical data * PROC FRα+ ~35% Overlap <20% Cyclin E1+ ~50% 3

Q&A Julie Eastland Chief Executive Officer, President and Director Ingmar Bruns, MD Chief Medical Officer Mark Lackner, PhD Chief Scientific Officer David M O'Malley, MD Director & Professor, Division of Gynecologic Oncology in Obstetrics and Gynecology Liz Hickin VP, IR

ZN-c3-016 (BRAF+EGFR Combo in BRAF Mutant CRC)
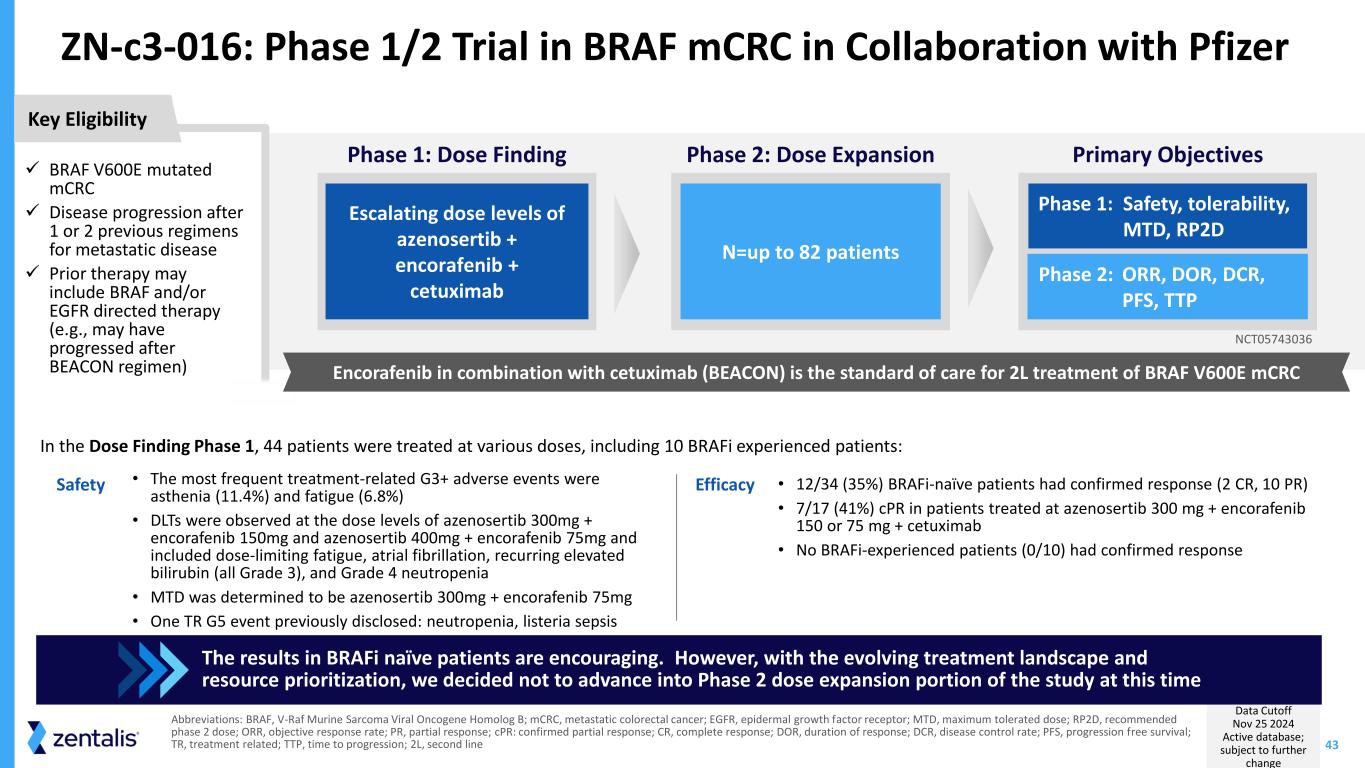
43 ZN-c3-016: Phase 1/2 Trial in BRAF mCRC in Collaboration with Pfizer Data Cutoff Nov 25 2024 Active database; subject to further change Abbreviations: BRAF, V-Raf Murine Sarcoma Viral Oncogene Homolog B; mCRC, metastatic colorectal cancer; EGFR, epidermal growth factor receptor; MTD, maximum tolerated dose; RP2D, recommended phase 2 dose; ORR, objective response rate; PR, partial response; cPR: confirmed partial response; CR, complete response; DOR, duration of response; DCR, disease control rate; PFS, progression free survival; TR, treatment related; TTP, time to progression; 2L, second line • The most frequent treatment-related G3+ adverse events were asthenia (11.4%) and fatigue (6.8%) • DLTs were observed at the dose levels of azenosertib 300mg + encorafenib 150mg and azenosertib 400mg + encorafenib 75mg and included dose-limiting fatigue, atrial fibrillation, recurring elevated bilirubin (all Grade 3), and Grade 4 neutropenia • MTD was determined to be azenosertib 300mg + encorafenib 75mg • One TR G5 event previously disclosed: neutropenia, listeria sepsis • 12/34 (35%) BRAFi-naïve patients had confirmed response (2 CR, 10 PR) • 7/17 (41%) cPR in patients treated at azenosertib 300 mg + encorafenib 150 or 75 mg + cetuximab • No BRAFi-experienced patients (0/10) had confirmed response In the Dose Finding Phase 1, 44 patients were treated at various doses, including 10 BRAFi experienced patients: The results in BRAFi naïve patients are encouraging. However, with the evolving treatment landscape and resource prioritization, we decided not to advance into Phase 2 dose expansion portion of the study at this time ✓ BRAF V600E mutated mCRC ✓ Disease progression after 1 or 2 previous regimens for metastatic disease ✓ Prior therapy may include BRAF and/or EGFR directed therapy (e.g., may have progressed after BEACON regimen) Key Eligibility Primary Objectives Phase 1: Safety, tolerability, MTD, RP2D Phase 2: ORR, DOR, DCR, PFS, TTP Phase 1: Dose Finding Escalating dose levels of azenosertib + encorafenib + cetuximab Phase 2: Dose Expansion N=up to 82 patients NCT05743036 Encorafenib in combination with cetuximab (BEACON) is the standard of care for 2L treatment of BRAF V600E mCRC Safety Efficacy

Integrated Safety Analysis
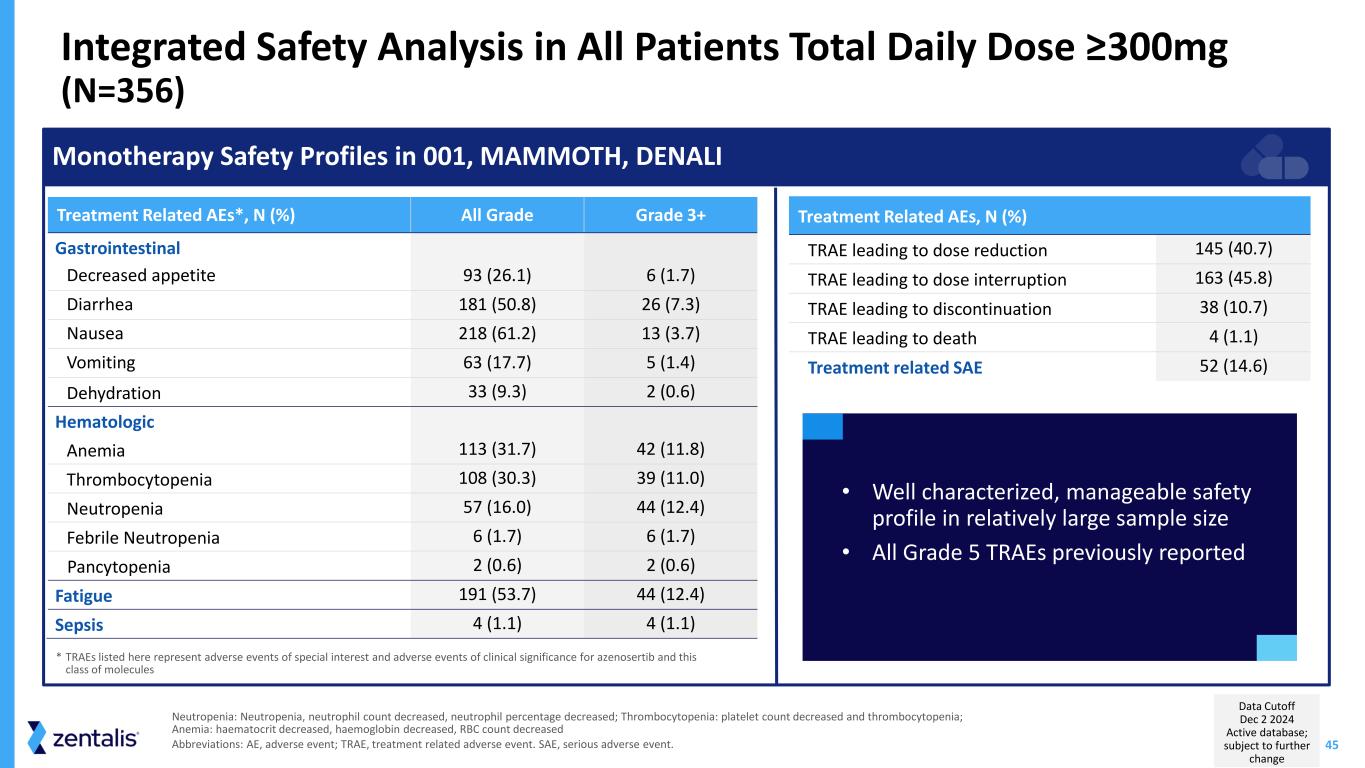
45 Integrated Safety Analysis in All Patients Total Daily Dose ≥300mg (N=356) Neutropenia: Neutropenia, neutrophil count decreased, neutrophil percentage decreased; Thrombocytopenia: platelet count decreased and thrombocytopenia; Anemia: haematocrit decreased, haemoglobin decreased, RBC count decreased Abbreviations: AE, adverse event; TRAE, treatment related adverse event. SAE, serious adverse event. Monotherapy Safety Profiles in 001, MAMMOTH, DENALI Treatment Related AEs, N (%) TRAE leading to dose reduction 145 (40.7) TRAE leading to dose interruption 163 (45.8) TRAE leading to discontinuation 38 (10.7) TRAE leading to death 4 (1.1) Treatment related SAE 52 (14.6) Treatment Related AEs*, N (%) All Grade Grade 3+ Gastrointestinal Decreased appetite 93 (26.1) 6 (1.7) Diarrhea 181 (50.8) 26 (7.3) Nausea 218 (61.2) 13 (3.7) Vomiting 63 (17.7) 5 (1.4) Dehydration 33 (9.3) 2 (0.6) Hematologic Anemia 113 (31.7) 42 (11.8) Thrombocytopenia 108 (30.3) 39 (11.0) Neutropenia 57 (16.0) 44 (12.4) Febrile Neutropenia 6 (1.7) 6 (1.7) Pancytopenia 2 (0.6) 2 (0.6) Fatigue 191 (53.7) 44 (12.4) Sepsis 4 (1.1) 4 (1.1) • Well characterized, manageable safety profile in relatively large sample size • All Grade 5 TRAEs previously reported * TRAEs listed here represent adverse events of special interest and adverse events of clinical significance for azenosertib and this class of molecules Data Cutoff Dec 2 2024 Active database; subject to further change












































