Exhibit 99.1

U N L O C K I N G T H E P O T E N T I A L O F C A N N A B I N O I D M E D I C I N E S Corporate Presentation January 2021 www.inmedpharma.com :IN :I NM

This presentation contains forward - looking statements and forward - looking information within the meaning of applicable securities laws (collectively, “forward - looking statements”) including, among others, statements concerning: unlocking the full potential of cannabinoid pharmaceuticals; anticipated clinical development activities, timelines, catalysts, and milestones; the potential benefits of product candidates; anticipated revenue and market opportunities; and the continued availability of key personnel. All statements other than statements of historical fact are statements that could be deemed forward - looking statements. With respect to the forward - looking information contained in this presentation, the Company has made numerous assumptions regarding, among other things: continued and timely positive preclinical and clinical efficacy data; the speed of regulatory approvals; demand for the Company’s products; continued availability of key personnel; continued access to sufficient capital to fund operations; and continued economic and market stability . These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and other factors that could cause actual results to differ materially from those described in the forward - looking statements. These risks and uncertainties include, among others: the possibility that clinical trials will not be successful, or be completed, or confirm earlier clinical trial results; risks associated with obtaining funding from third parties; risks related to the timing and costs of clinical trials; key personnel may become unable to serve the Company; the need for receipt of regulatory approvals; and economic and market conditions may worsen. Readers are cautioned that the foregoing list is not exhaustive. A more complete discussion of the risks and uncertainties facing the Company appears in the Company’s filings with the Securities and Exchange Commission and in the Company’s annual information form dated September 2 3 , 20 20 , a copy of which is available on SEDAR at www.sedar.com . T he Company undertakes no obligation to update the forward - looking statements contained herein or to reflect events or circumstances occurring after the date hereof, except as required by law. Forward Looking Statements 2 Corporate Presentation January 2021

3 Eric A. Adams, MIBS Chief Executive Officer Bruce S. Colwill, CPA, CA Chief Financial Officer Alexandra Mancini, MSc SVP, Clinical and Regulatory Affairs 30+ years of experience in global biopharma leadership: business development, sales, marketing, and M&A with enGene, QLT, Abbott, Fresenius 25+ years of financial leadership with private and public companies; executing IPO, equity and debt financings General Fusion, Entrée Resources, Neuromed Pharma 30+ years of global biopharma R&D experience, overseeing drug development with Sirius Genomics, Inex Pharmaceuticals, and QLT Experienced Executive Team Michael Woudenberg, PEng Vice President, CMC Eric Hsu, PhD SVP, Preclinical R&D 20+ years of engineering, scale - up and GMP manufacturing experience with Phyton Biotech, Arbutus Biopharma, 3M and Cardiome Pharma 20+ years of scientific leadership experience with enGene in gene transfer technologies, formulation and process development Corporate Presentation January 2021

4 A Differentiated Cannabinoid Pharmaceutical Company Focused on the Therapeutic Application of Cannabinoids for the Treatment of Diseases with High Unmet Medical Needs Researching the therapeutic potential of rare cannabinoids beginning with cannabinol ( CBN ) Developing IntegraSyn - a flexible, integrated cannabinoid manufacturing system using novel proprietary enzyme(s) to efficiently produce bio - identical, economical, pharmaceutical - grade cannabinoids Selecting innovative, topically applied cannabinoid therapies where we can establish a proprietary foothold in treating diseases with high unmet medical needs, starting with dermatology and ocular diseases Corporate Presentation January 2021

5 An Important and Eventful Year 5 Corporate Presentation January 2021 Highlights from 2020 INM - 755 in Dermatology Completed 2 Phase 1 human clinical trials (normal and wounded skin) INM - 088 in Ocular Disease Completed in vivo PoC , formulation/delivery studies; Licensed MiDROPS ® delivery technology; filed PCT patent IntegraSyn TM for Cannabinoid Manufacturing Improved yields and process; filed multiple patents; Initiated BayMedica collaboration Administration US$8M IPO and Nasdaq listing (“INM”)
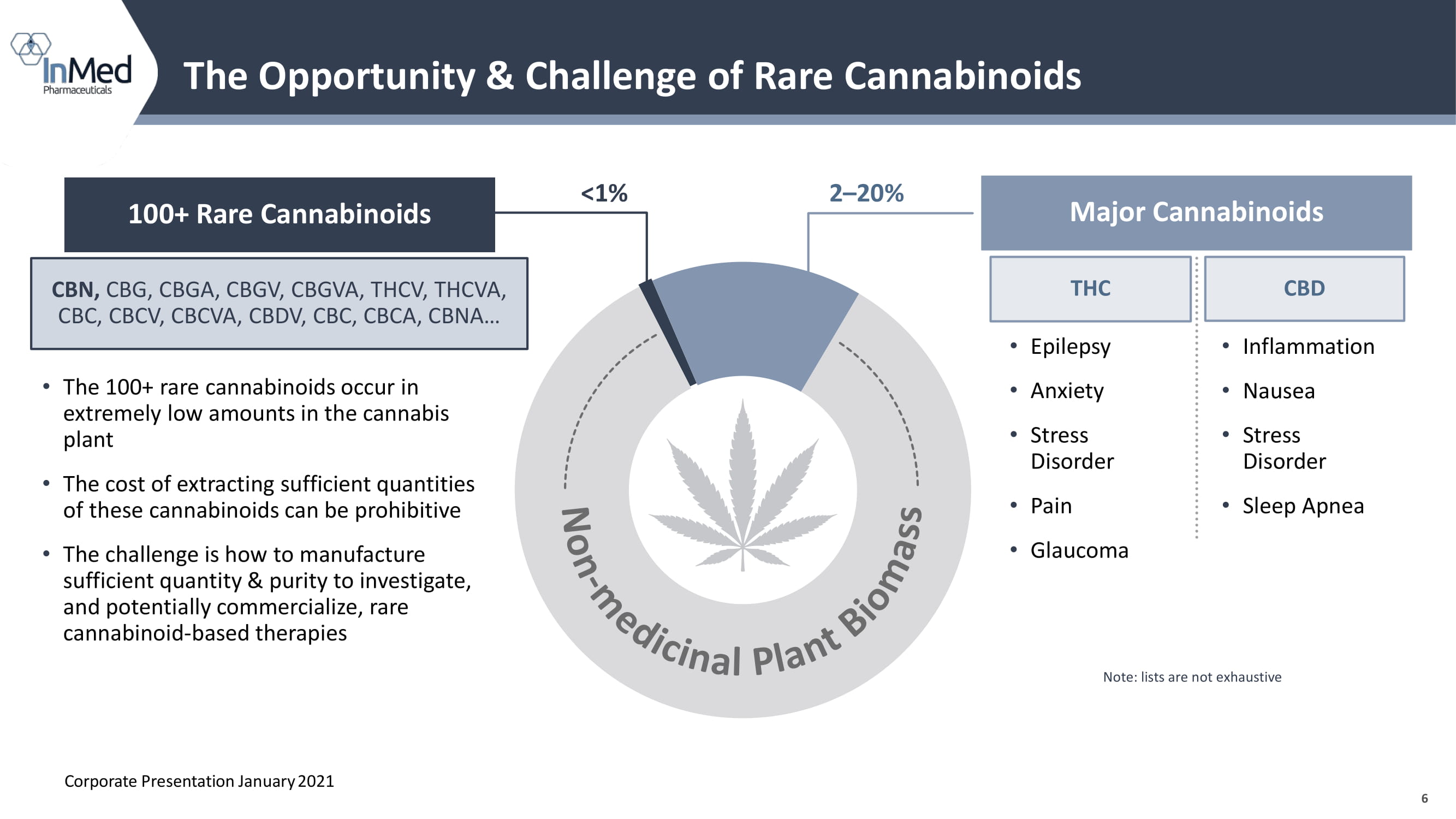
6 Note: lists are not exhaustive The Opportunity & Challenge of Rare Cannabinoids 100+ Rare Cannabinoids 2 – 20% <1% Major Cannabinoids THC CBD • Epilepsy • Anxiety • Stress Disorder • Pain • Glaucoma • Inflammation • Nausea • Stress Disorder • Sleep Apnea CBN, CBG, CBGA, CBGV, CBGVA, THCV, THCVA, CBC, CBCV, CBCVA, CBDV, CBC, CBCA, CBNA… • The 100+ rare cannabinoids occur in extremely low amounts in the cannabis plant • The cost of extracting sufficient quantities of these cannabinoids can be prohibitive • The challenge is how to manufacture sufficient quantity & purity to investigate, and potentially commercialize, rare cannabinoid - based therapies Corporate Presentation January 2021
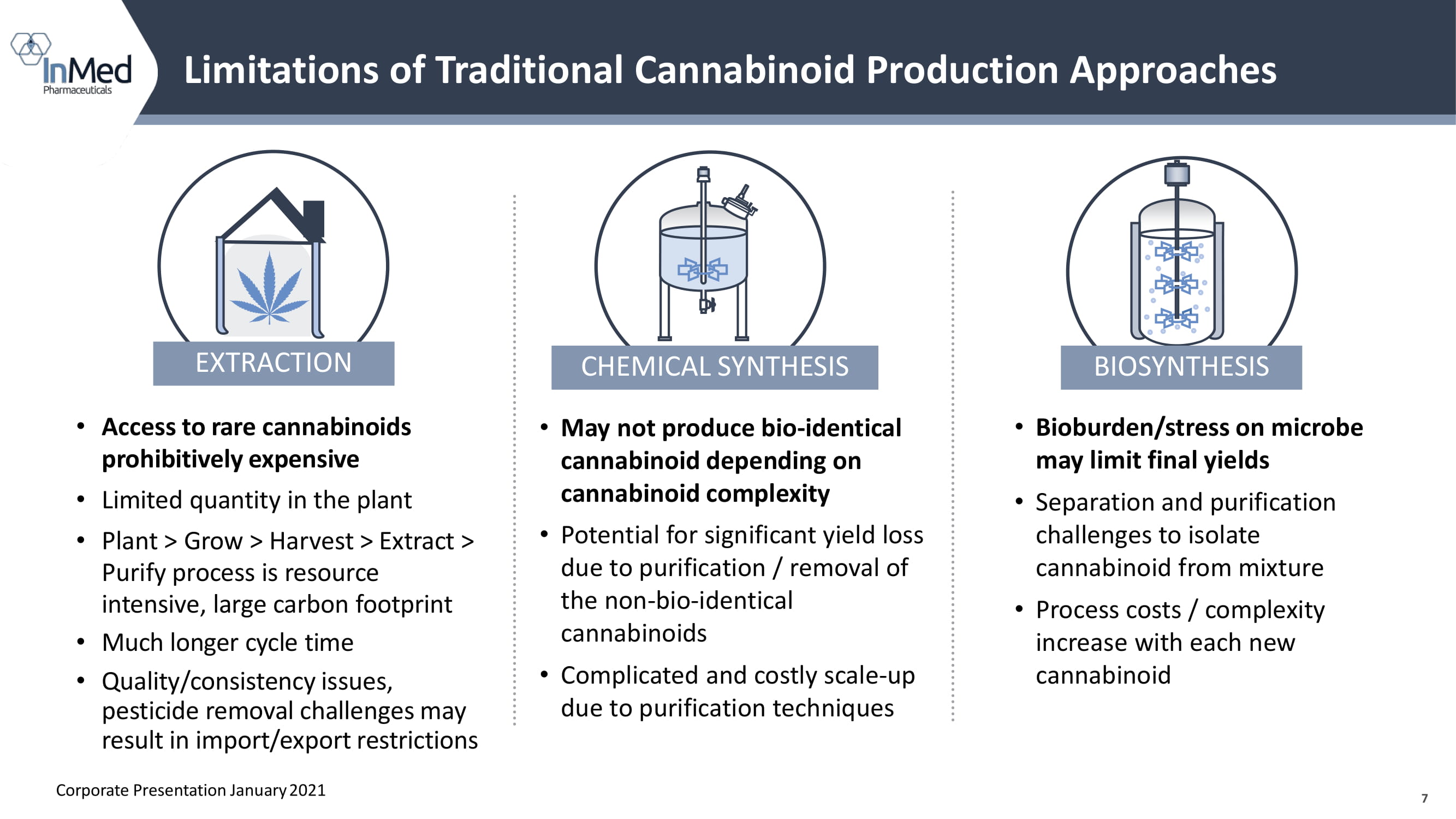
7 Limitations of Traditional Cannabinoid Production Approaches • Bioburden/stress on microbe may limit final yields • Separation and purification challenges to isolate cannabinoid from mixture • Process costs / complexity increase with each new cannabinoid • May not produce bio - identical cannabinoid depending on cannabinoid complexity • Potential for significant yield loss due to purification / removal of the non - bio - identical cannabinoids • Complicated and costly scale - up due to purification techniques BIOSYNTHESIS CHEMICAL SYNTHESIS • Access to rare cannabinoids prohibitively expensive • Limited quantity in the plant • Plant > Grow > Harvest > Extract > Purify process is resource intensive, large carbon footprint • Much longer cycle time • Quality/consistency issues, pesticide removal challenges may result in import/export restrictions EXTRACTION Corporate Presentation January 2021
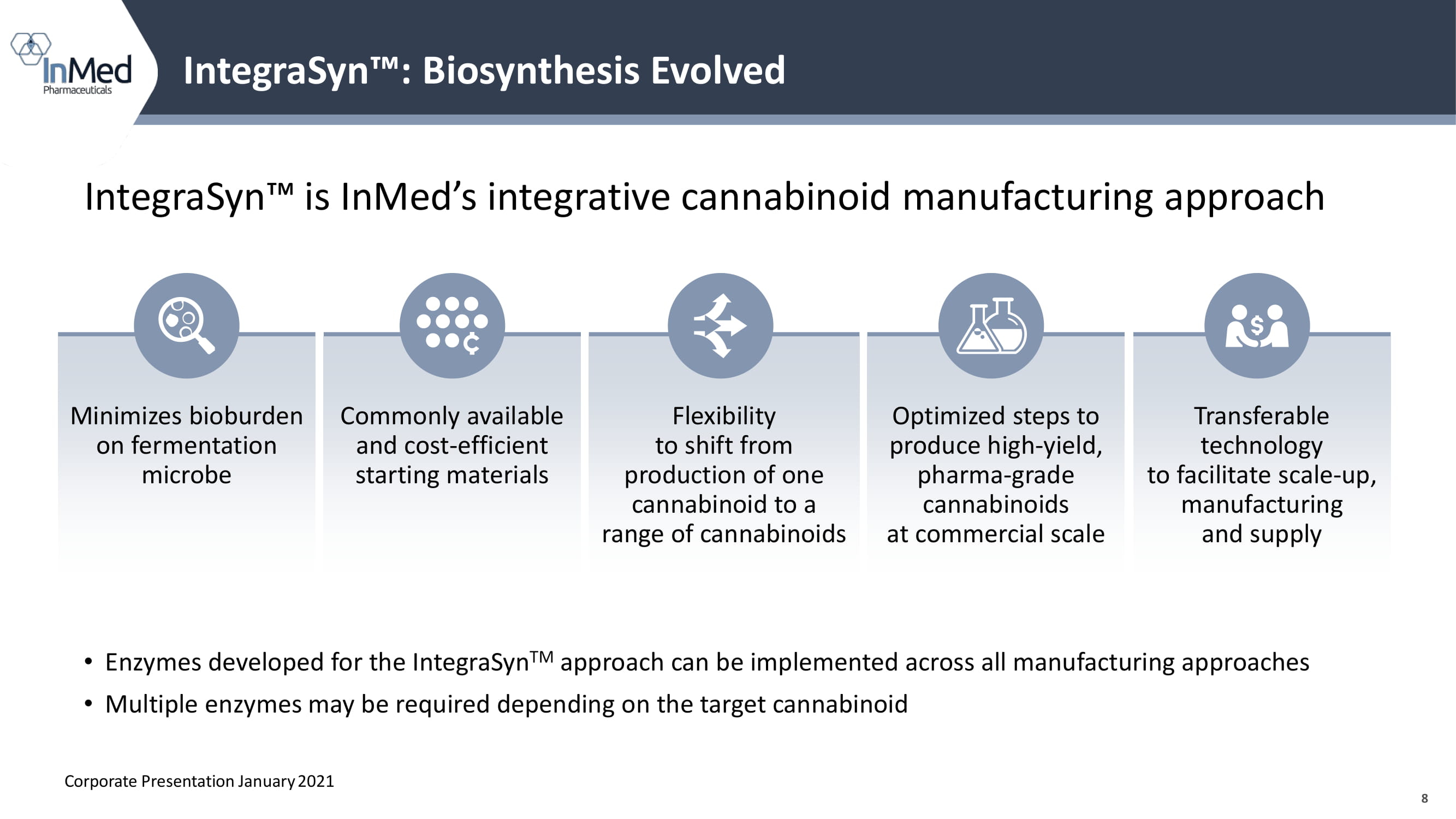
8 IntegraSyn Ρ is InMed’s integrative cannabinoid manufacturing approach IntegraSyn Ρ : Biosynthesis Evolved Minimizes bioburden on fermentation microbe Flexibility to shift from production of one cannabinoid to a range of cannabinoids Optimized steps to produce high - yield, pharma - grade cannabinoids at commercial scale Transferable technology to facilitate scale - up, manufacturing and supply Commonly available and cost - efficient starting materials Corporate Presentation January 2021 • Enzymes developed for the IntegraSyn TM approach can be implemented across all manufacturing approaches • Multiple enzymes may be required depending on the target cannabinoid
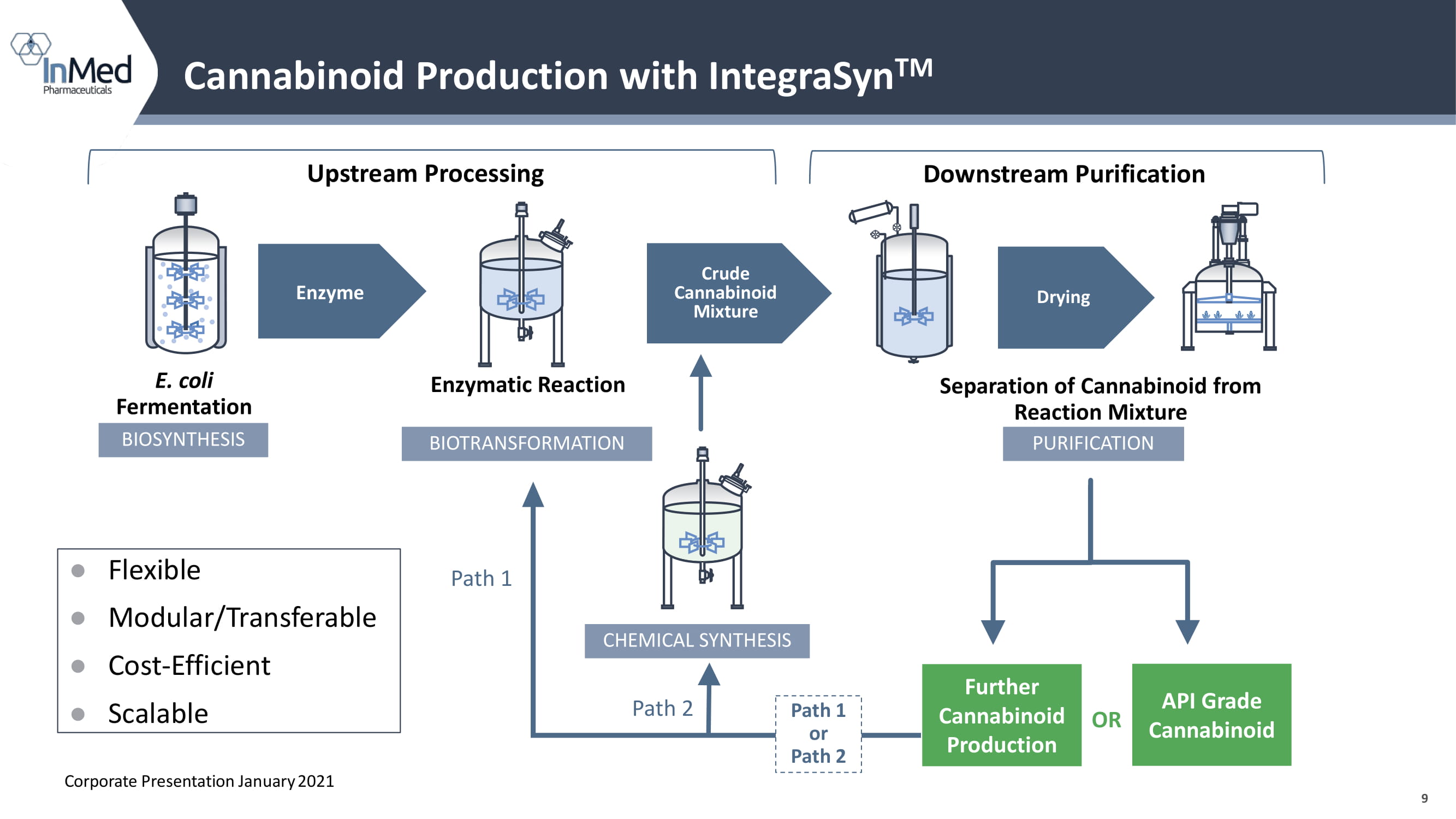
9 Cannabinoid Production with IntegraSyn TM E. coli Fermentation Enzymatic Reaction Upstream Processing Downstream Purification Separation of Cannabinoid from Reaction Mixture Enzyme BIOSYNTHESIS Crude Cannabinoid Mixture Drying BIOTRANSFORMATION Further Cannabinoid Production CHEMICAL SYNTHESIS API Grade Cannabinoid Path 1 Path 2 PURIFICATION OR Path 1 or Path 2 ● Flexible ● Modular/Transferable ● Cost - Efficient ● Scalable Corporate Presentation January 2021

10 Note: lists are not exhaustive Cannabinoid Manufacturing: Competitive Landscape IntegraSyn TM Yeast Biosynthesis Algae/Other KEY PARTNERSHIPS R&D Commercial A flexible , integrated cannabinoid synthesis approach using a novel enzyme(s) and various standard pharma manufacturing processes to efficiently produce bio - identical, economical, GMP - grade cannabinoids. Chemical Synthesis Corporate Presentation January 2021
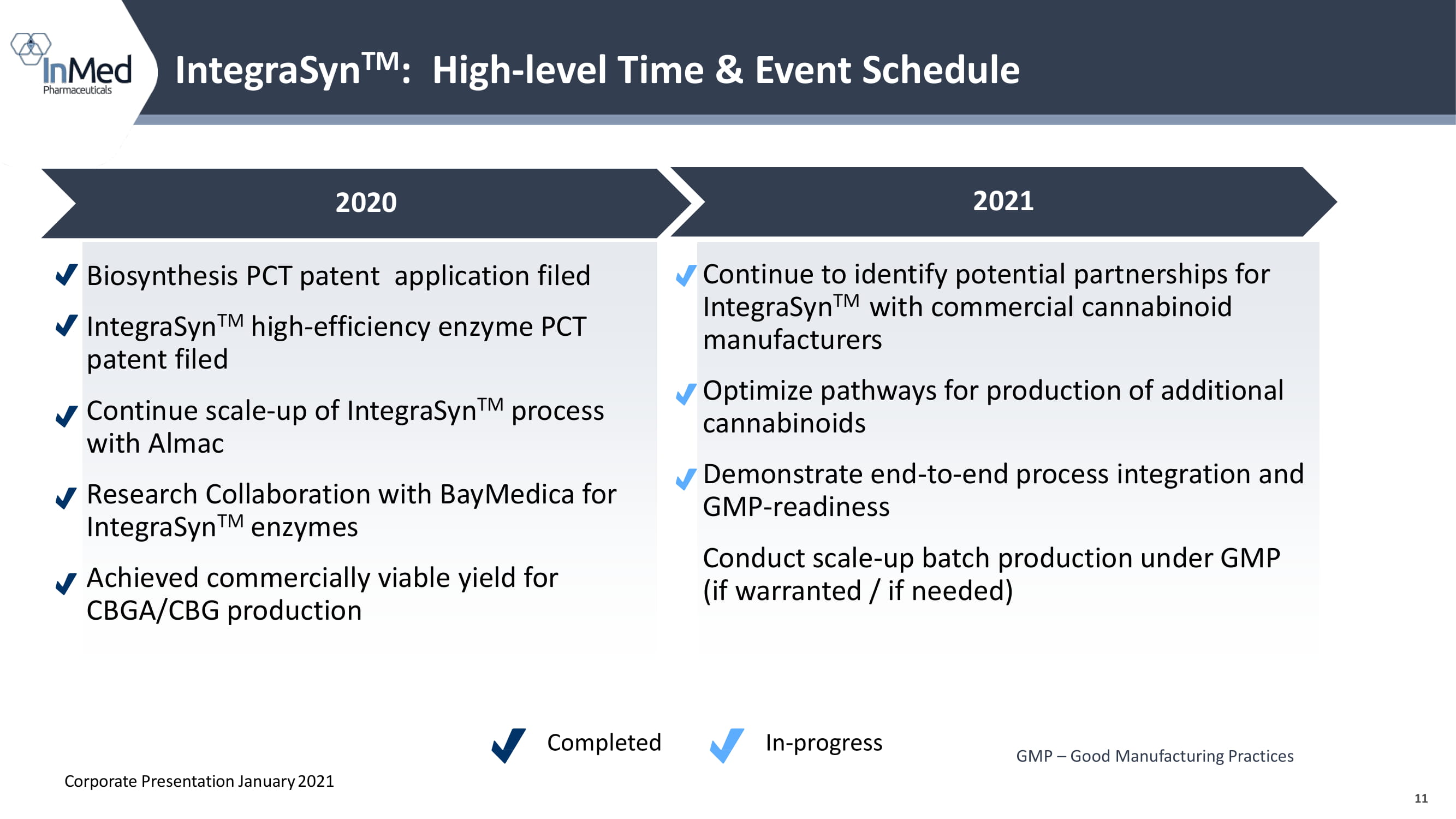
11 GMP – Good Manufacturing Practices IntegraSyn TM : High - level Time & Event Schedule 2020 2021 Biosynthesis PCT patent application filed IntegraSyn TM high - efficiency enzyme PCT patent filed Continue scale - up of IntegraSyn TM process with Almac Research Collaboration with BayMedica for IntegraSyn TM enzymes Achieved commercially viable yield for CBGA/CBG production Continue to identify potential partnerships for IntegraSyn TM with commercial cannabinoid manufacturers Optimize pathways for production of additional cannabinoids Demonstrate end - to - end process integration and GMP - readiness Conduct scale - up batch production under GMP (if warranted / if needed) Corporate Presentation January 2021 Completed In - progress

12 Global Leader in Cannabinol (CBN) Therapeutic Development 12 • A rare cannabinoid with unique physiological and safety properties • Found in trace amounts in the plant; impractical to extract, requiring pharmaceutical manufacturing approaches • InMed conducted 30+ preclinical pharmacology and toxicology studies; two Ph 1 human clinical trials in dermatology • Multiple patents filed for CBN as therapeutic product • CBN selected as lead cannabinoid for development for INM - 755 (dermatology) & INM - 088 (ocular) Cannabinol (CBN) Corporate Presentation January 2021

13 Epidermolysis Bullosa (EB) 13 • Epidermolysis bullosa (EB) is a group of rare genetic skin diseases characterized by fragile skin that blisters easily from minimal friction that causes shearing of the skin layers. • The most common form is EB Simplex (EBS), ~ 55% of all EB patients . • The affected population in the United States is estimated to be between 12,500 and 25,000 in the United States. • No therapies approved specifically for the treatment of EB wounds and other symptoms. • INM - 755 is being developed for symptomatic relief in all EB patients and may have further utility in enhancing skin integrity in subset of EBS patients. Photograph of Dystrophic EB Corporate Presentation January 2021

14 14 Targeting Disease Hallmarks in A ll EB Patients Evaluate Possible Strengthening of Skin in a Subset of EBS Patients Being Investigated to Deliver Potential Symptomatic Relief: • Reduce i nflammation • Healing of chronic wounds • Pain reduction Other P otential E ffects of C annabinoids: • Itch reduction • Antimicrobial a ctivity Dermal/ E p id e r ma l Junction De r m is Epidermis Bli s t e r EB Simplex: K14 malformations INM - 755 for Epidermolysis Bullosa Corporate Presentation January 2021 EA1

15 INM - 755: 30+ Safety Pharmacology and Toxicology Studies ● CBN - mediated reduction of key inflammatory markers (IL - 8 & MMP - 9) suggests INM - 755 may: o Reduce incidence and/or severity of blistering if applied to intact skin o Improve healing when applied directly to chronic wound ● INM - 755 upregulation of Keratin 15 may offset Keratin 14 dysfunction in EBS, possibly leading to increased skin integrity/strength and fewer blisters 15 ● INM - 755 API (CBN) demonstrated preclinical safety : o 20+ toxicology and safety pharmacology studies: no safety concerns identified in these studies for the intended application ● INM - 755 highly unlikely to produce psychoactivity or other CNS toxicity: o no adverse effects on CNS, even at 10,000 times expected systemic exposure after topical dosing ● Cream formulation without CBN: o well tolerated on intact skin and small open wounds in humans Corporate Presentation January 2021

16 INM - 755: Summary of Completed and Planned Clinical Studies, 2020 - 2021 COMPLETED IN 2020 In Netherlands File CTAs in 1H21 Global Phase I 755 - 101 - HV Phase I 755 - 102 - HV Phase II 755 - 201 - EB Enrollment 22 healthy adult volunteers 8 healthy adult volunteers Up to 20 EB patients (all subtypes) Adults first to demonstrate safety then expand to include adolescents with permission Primary Purpose Systemic and local safety on intact skin, PK Local safety on open wounds Systemic and local safety, efficacy (individualized per patient needs) Study Design Double - blind, parallel - group design, subjects randomized to two strengths vs vehicle Double - blind, within - subject design, 4 wounds randomized to two strengths vs vehicle vs untreated Double - blind, within - patient design, matched index areas randomized to active cream vs vehicle Treatment and Duration 14 days on 5% BSA 14 days on 4 small wounds 28 days on intact skin and/or wounds Corporate Presentation January 2021

17 755 - 101 - HV Top - Line Results 17 • Randomized, double - blinded, Ph 1 trial of the safety and tolerability of 14 days of two strengths of INM - 755 cream vs. vehicle on intact skin in 22 healthy adult volunteers. • Specifically examined erythema (redness), edema (fluid accumulation/swelling), scaling (flaking skin), and stinging/burning : o Majority of subjects in all groups had no events on most days o Slightly higher incidence and severity of erythema and scaling in groups receiving active creams compared to vehicle cream o Local reactions mostly mild or moderate and resolved without intervention • No systemic or serious adverse effects; no subject withdrawals due to adverse events • Drug concentrations in the blood were very low in the high - dose group INM - 755 Creams Determined to be Safe & Well Tolerated on Intact Skin Corporate Presentation January 2021

18 755 - 102 - HV Top - Line Results 18 • Randomized, double - blind, Ph 1 trial of the safety and tolerability of 14 days of treatment with INM - 755 cream on epidermal wounds in 8 healthy adult volunteers • Within - subject design with each subject receiving all treatment options on 4 suction blister wounds. o High or low CBN concentration or vehicle creams with film dressing, or untreated (film dressing only) • No differences in local reactions among treatment options • No adverse effects of INM - 755 creams on wound healing INM - 755 Creams Determined to be Safe & Well Tolerated on Epidermal Wounds Corporate Presentation January 2021

19 Top - Line Results from 2 Phase 1 Healthy Volunteer Studies 19 • On intact skin (Study 101, n=22 healthy adult volunteers, 14 days): o Majority of subjects in all groups had no events on most days o Slightly higher incidence and severity of erythema (redness) and scaling (flaking skin) in groups receiving active creams compared to vehicle cream o Local reactions mostly mild or moderate and resolved without intervention o Drug concentrations in the blood were very low in the high - dose group • On epidermal wounds (Study 102, n=8 healthy adult volunteers, 14 days): o No differences in local reactions compared to controls o No adverse effects of INM - 755 creams on wound healing INM - 755 Creams Safe & Well Tolerated on Intact Skin and Epidermal Wounds Corporate Presentation January 2021
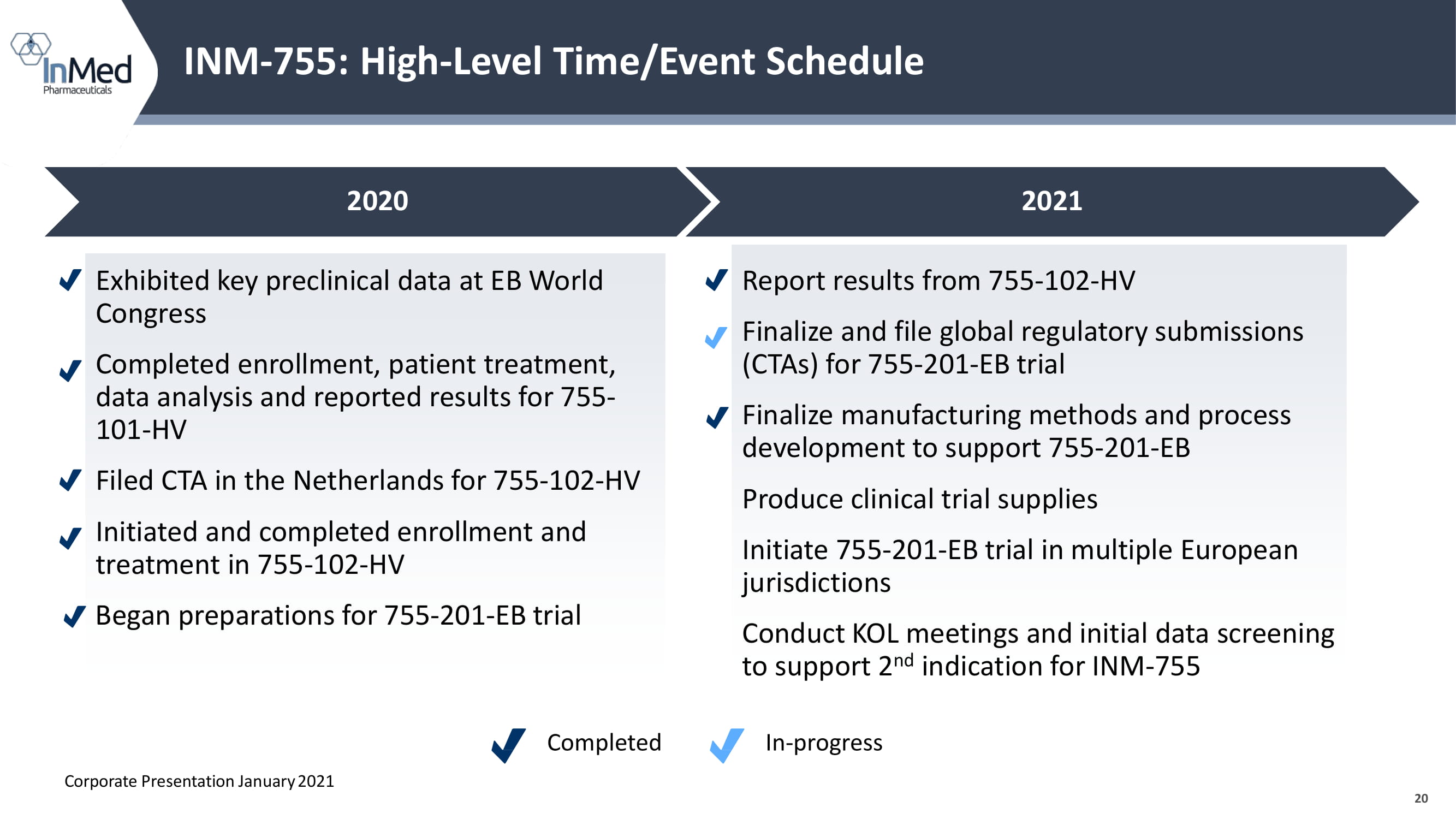
20 INM - 755: High - Level Time/Event Schedule Exhibited key preclinical data at EB World Congress Completed enrollment, patient treatment, data analysis and reported results for 755 - 101 - HV Filed CTA in the Netherlands for 755 - 102 - HV Initiated and completed enrollment and treatment in 755 - 102 - HV Began preparations for 755 - 201 - EB trial Report results from 755 - 102 - HV Finalize and file global regulatory submissions (CTAs) for 755 - 201 - EB trial Finalize manufacturing methods and process development to support 755 - 201 - EB Produce clinical trial supplies Initiate 755 - 201 - EB trial in multiple European jurisdictions Conduct KOL meetings and initial data screening to support 2 nd indication for INM - 755 Corporate Presentation January 2021 2020 2021 Completed In - progress

21 Glaucoma Epidemiology & Market Opportunity 21 ● There is no current cure for glaucoma o Glaucoma describes a group of conditions in which there is characteristic cupping of the optic disc with corresponding visual field defects due to retinal ganglion cell loss. The common hallmark of glaucoma is optic nerve head damage, regardless of the varying pathophysiological subsets. o Early diagnosis and treatment can control the disease before vision loss or blindness ● Estimates for 2020 1 : 76 million people worldwide were affected by glaucoma o Primary Open Angle Glaucoma (POAG), the most common form (70%) 2 o More than 2.8 million Americans are living with glaucoma; projected to surpass 3.5 million in 2020 2 o Second leading cause of blindness worldwide as well as in the USA ● Global glaucoma market anticipated to be US$11 billion by 2027 3 o Valued at US$6.6 billion in 2019; CAGR of 6.1% Corporate Presentation January 2021 Sources: 1 - WHO World Report on Vision 2019 2 - American Academy of Ophthalmology, Tham YC, et. al. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: a systematic review and meta - analysis. Ophthalmology. 2014;121(11):2081 – 90 3 - Fortune Business Insights: “Glaucoma Therapeutics Market Size, Share & COVID - 19 Impact Analysis

22 Potential Roles for CBN in Glaucoma 22 • Direct neuroprotection for the retina and optical nerve, independent of intraocular pressure (IOP) reduction • Reduce IOP in the affected eyes via increased fluid drainage Target Effects of CBN Build Up of Aqueous Humor Fluid Optic Nerve Pressure Retina Corporate Presentation January 2021 Several cannabinoids demonstrate increased drainage, leading to reduced IOP InMed data suggest CBN is the cannabinoid of choice for neuroprotection in the eye

23 INM - 088: CBN Ocular Preclinical Studies CBN Preclinical Studies Neuroprotection IOP Reduction RGC 1 Survival under pressure ( in vitro) Effect on RGC apoptosis ( in vitro) Impact on IOP associated biomarkers under pressure conditions ( in vitro ) Neuronal function after CBN Exposure in Glaucoma model (measured by pERG 2 , in vivo ) IOP reduction after CBN Exposure in Glaucoma rat model And normotensive rabbit model ( in vivo ) 1 Retinal Ganglion Cells (RGCs) are the thin layer of neurons responsible for relaying visual signals in the eye. 2 Pattern Electroretinogram (pERG) is a diagnostic tool used to measure e lectrical activity generated by neurons in response to light. Corporate Presentation January 2021

24 INM - 088: High - Level Time/Event Schedule Filed PCT patent for neuroprotection Complete formulation data analysis & select preferred delivery technology Executed license agreement for MiDROPS ® delivery technology with EyeCRO Initiate process development for INM - 088 drug product (CBN + delivery techs) Engage leading ocular clinical research organization to develop clinical game plan Scale - up INM - 088 drug product ( CBN+MiDROPS ®) Conduct non - GLP dose ranging studies Complete pre - IND package and FDA Pre - IND Meeting (if needed) Initiate toxicology studies (2021 - 22) in preparation for IND Completed In - progress Corporate Presentation January 2021 2020 2021
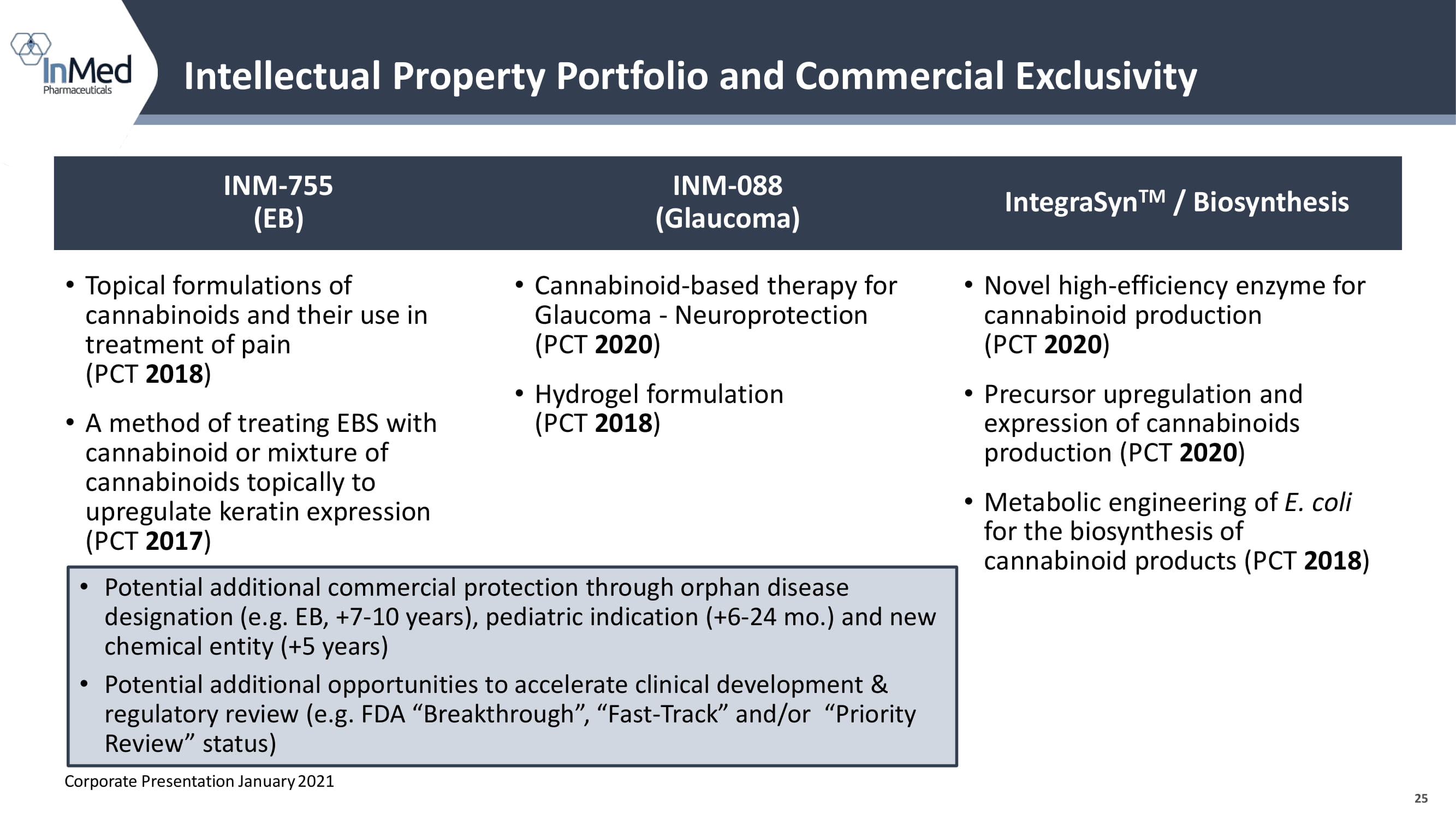
25 Intellectual Property Portfolio and Commercial Exclusivity INM - 755 (EB) INM - 088 (Glaucoma) IntegraSyn TM / Biosynthesis • Topical formulations of cannabinoids and their use in treatment of pain (PCT 2018 ) • A method of treating EBS with cannabinoid or mixture of cannabinoids topically to upregulate keratin expression (PCT 2017 ) • Cannabinoid - based therapy for Glaucoma - Neuroprotection (PCT 2020 ) • Hydrogel formulation (PCT 2018 ) • Novel high - efficiency enzyme for cannabinoid production (PCT 2020 ) • Precursor upregulation and expression of cannabinoids production (PCT 2020 ) • Metabolic engineering of E. coli for the biosynthesis of cannabinoid products (PCT 2018 ) • Potential additional commercial protection through orphan disease designation (e.g. EB, +7 - 10 years), pediatric indication (+6 - 24 mo.) and new chemical entity (+5 years) • Potential additional opportunities to accelerate clinical development & regulatory review (e.g. FDA “Breakthrough”, “Fast - Track” and/or “Priority Review” status) Corporate Presentation January 2021

26 Nature of Relationship InMed will explore the therapeutic potential in neuroprotection of specific cannabinoid analog compounds chosen from BayMedica’s library. BayMedica will assess the potential of InMed’s high - efficiency enzymes for the production of cannabinoids in its manufacturing technologies. The licensing agreement with EyeCRO grants InMed an exclusive, worldwide license to develop and commercialize prospective therapeutic formulations combining MiDROPS ® . InMed and Almac have been engaged in optimizing the upstream cannabinoid assembly processes as well as downstream purification to achieve cost efficient, GMP - grade active pharmaceutical ingredients for prescription - based medications. InMed is collaborating with NRC - IRAP and UBC to further develop the biofermentation and scale - up processes for cannabinoid biosynthesis in E. coli . Business Development: Collaborations and Licensing Corporate Presentation January 2021 Note: List is not exhaustive
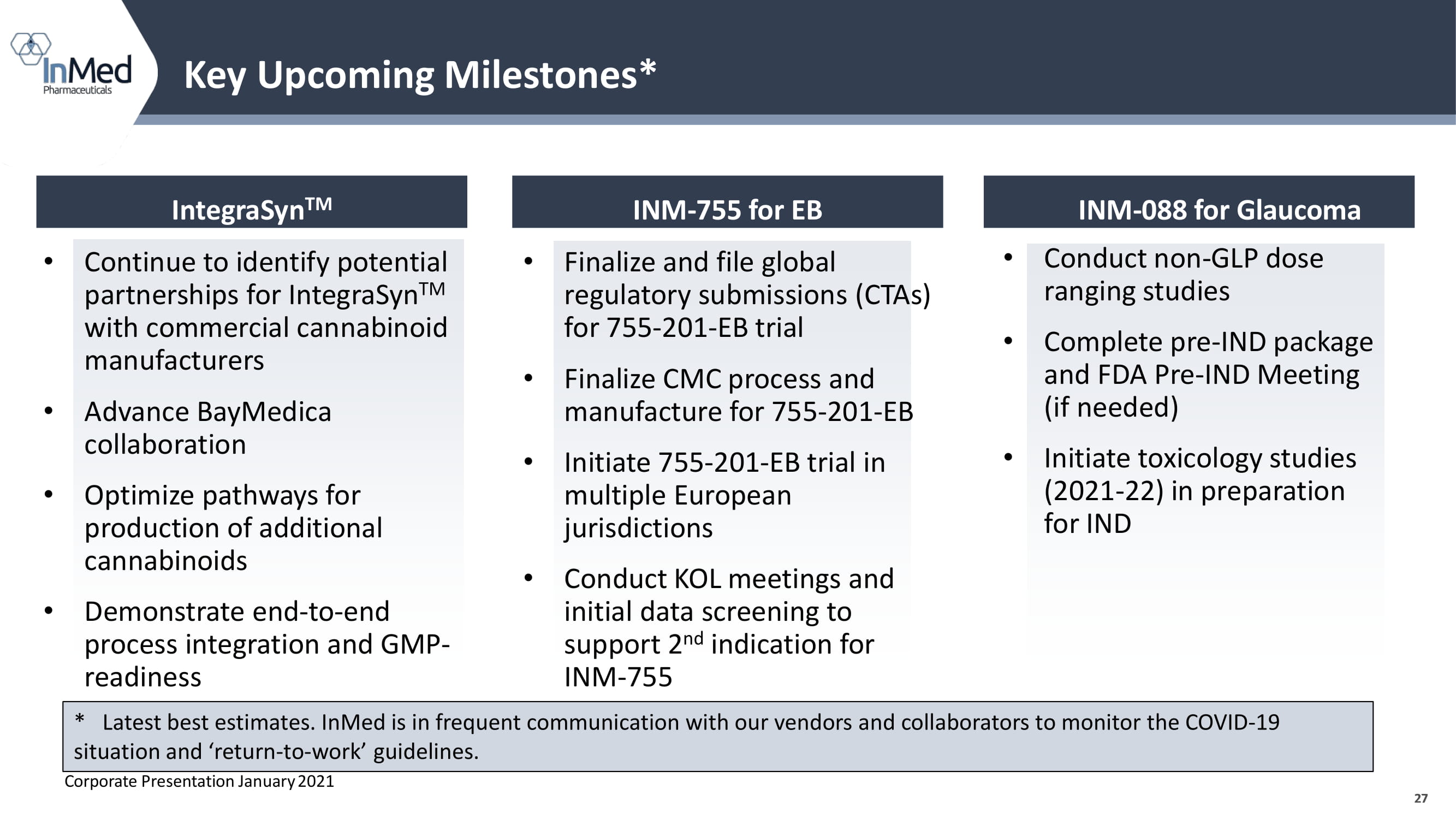
27 Key Upcoming Milestones* 27 IntegraSyn TM INM - 755 for EB INM - 088 for Glaucoma * Latest best estimates. InMed is in frequent communication with our vendors and collaborators to monitor the COVID - 19 situation and ‘return - to - work’ guidelines. Corporate Presentation January 2021 • Continue to identify potential partnerships for IntegraSyn TM with commercial cannabinoid manufacturers • Advance BayMedica collaboration • Optimize pathways for production of additional cannabinoids • Demonstrate end - to - end process integration and GMP - readiness • Finalize and file global regulatory submissions (CTAs) for 755 - 201 - EB trial • Finalize CMC process and manufacture for 755 - 201 - EB • Initiate 755 - 201 - EB trial in multiple European jurisdictions • Conduct KOL meetings and initial data screening to support 2 nd indication for INM - 755 • Conduct non - GLP dose ranging studies • Complete pre - IND package and FDA Pre - IND Meeting (if needed) • Initiate toxicology studies (2021 - 22) in preparation for IND

28 Board of Directors 28 William Garner, MD Founder of EGB Ventures LLC (Chairman) Andrew Hull Former VP of Global Alliances at Takeda Pharmaceuticals Eric A. Adams President and CEO of InMed Pharmaceuticals • 25+ years’ experience as biotech entrepreneur • Director / F ou n d er , TRYP Therapeutics, Race Oncology ; f ormer Director +/ - e xecutive at IGXBio ; Invion Limited; Del Mar; and Hoffmann LaRoche ; M erchant B anking experience • 30+ years’ P harma / B i o t ech C ommercial leadership experience • Previously in various leadership roles with Immunex ; Abbott Labs ; former Director, Zucara; and f ormer two - term Chairman of Illinois Biotech Industry Org . • 30+ years’ experience in global B iopharma leadership • Extensive Business D evelopment , S ales , M arketing , and M&A experience with E nGene ; QLT ; Abbott Labs; and Fresenius AG Adam Cutler CFO at Molecular Templates, Inc. • 20+ years’ experience in Equity Research, Corporate Affairs Strategy and IR • Formerly with Arbutus Pharma; Trout Group ; Credit Suisse ; Canaccord Genuity ; JMP Securities ; BoA Securities ; and E&Y Healthcare Consulting Catherine Sazdanoff JD, Healthcare Industry Board Member and Consultant • 35+ years’ experience including global leadership in C orporate D evelopment ; BD ; L egal and other relevant areas • Roles with Abbott Labs ; Takeda Pharma ; and Strata Oncology. Director, Meridian Biosciences, Inc. Corporate Presentation January 2021
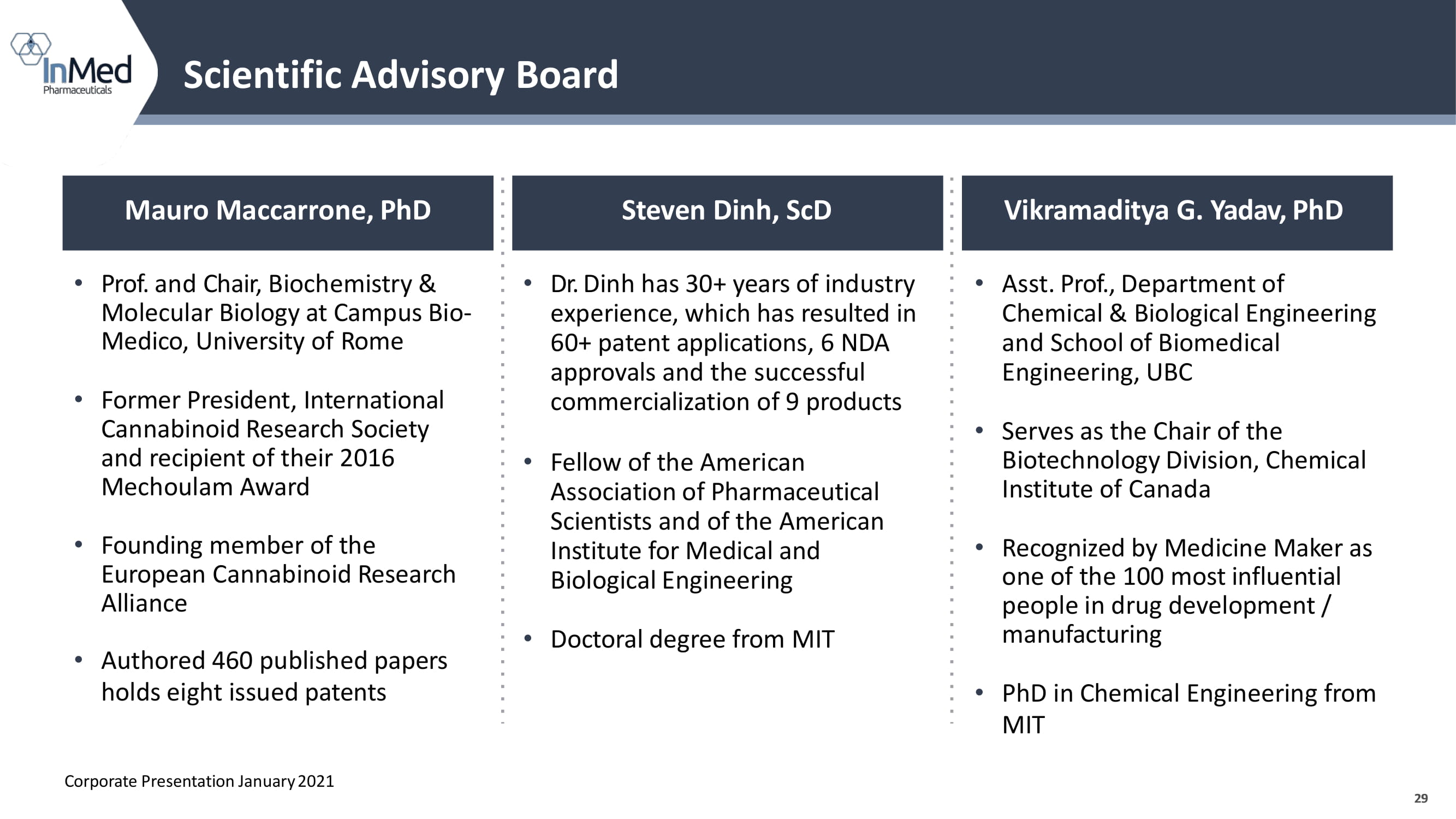
29 Scientific Advisory Board 29 Mauro Maccarrone, PhD Steven Dinh, ScD Vikramaditya G. Yadav, PhD • Prof. and Chair, Biochemistry & Molecular Biology at Campus Bio - Medico, University of Rome • Former President, International Cannabinoid Research Society and recipient of their 2016 Mechoulam Award • Founding member of the European Cannabinoid Research Alliance • Authored 460 published papers holds eight issued patents • Dr. Dinh has 30+ years of industry experience, which has resulted in 60+ patent applications , 6 NDA approvals and the successful commercialization of 9 products • Fellow of the American Association of Pharmaceutical Scientists and of the American Institute for Medical and Biological Engineering • Doctoral degree from MIT • Asst. Prof., Department of Chemical & Biological Engineering and School of Biomedical Engineering, UBC • Serves as the Chair of the Biotechnology Division, Chemical Institute of Canada • Recognized by Medicine Maker as one of the 100 most influential people in drug development / manufacturing • PhD in Chemical Engineering from MIT Corporate Presentation January 2021

30 Financial Snapshot 30 :IN Previous Close (202 1 - 01 - 05 ) US$ 3.51 C$ 4.44 52 - week High (incl. OTCQX listing) US$ 9.70 C$ 12.87 52 - week Low (incl. OTCQX listing) US$ 2.98 C$ 3.77 Avg. Daily Volume ( Trailing 50 Days ) 9 9,347 2 7,779 Market Cap, I/O (202 1 - 01 - 05 ) US$ 24.6 M C $31.1 M Shares I/O 7.0 M Options (December 31, 2020) 0.9 M Warrants (US$5.11, exp. Nov 2026) 1.8 M Fully Diluted Shares 9.7 M Cash and Short - term Investments plus Net Proceed from Nov 2020 IPO US$4.5 M at September 30, 2020 plus US$6.8 M Corporate Presentation January 2021 :I NM
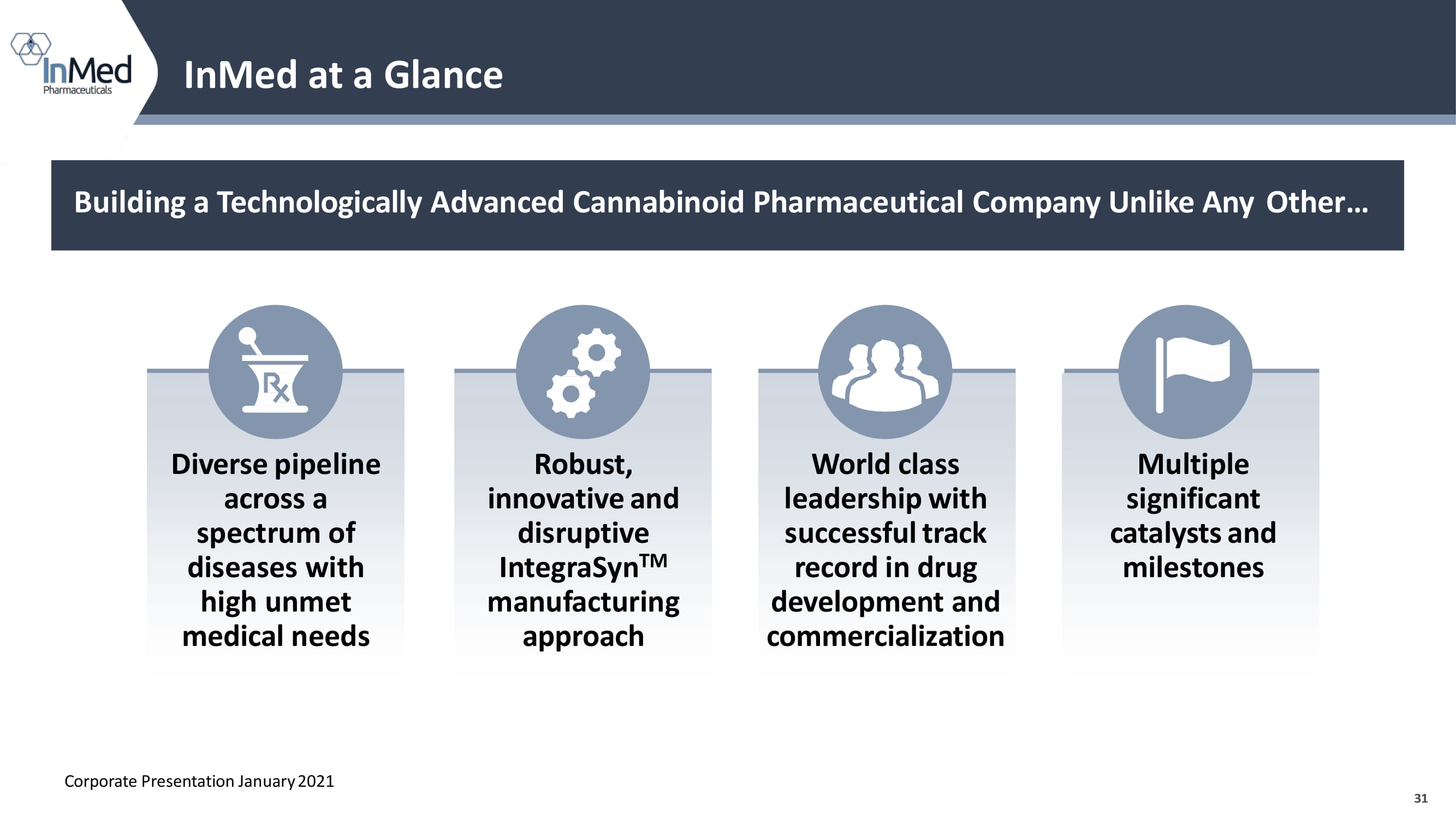
31 InMed at a Glance 31 Diverse pipeline across a spectrum of diseases with high unmet medical needs Robust, innovative and disruptive IntegraSyn TM m a nu f a c turing approach World class leadership with successful track record in drug development and commercialization Multiple significant catalysts and milestones Building a Technologically Advanced Cannabinoid Pharmaceutical Company Unlike Any Other… Corporate Presentation January 2021 BP3

Thank You! Eric A. Adams Chief Executive Officer eadams@inmedpharma.com +1 - 604 - 669 - 7207 Bruce S. Colwill Chief Financial Officer bcolwill@inmedpharma.com +1 - 604 - 669 - 7207 www.inmedpharma.com :IN :I NM































