As filed with the Securities and Exchange Commission on February 13, 2025
Registration No. 333-280184
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Amendment No. 3 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
IMAC HOLDINGS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | | 8093 | | 83-0784691 |
(State or Other Jurisdiction of Incorporation or Organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification Number) |
3401 Mallory Lane, Suite 100
Franklin, Tennessee 37067
(303) 898-5896
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
FAITH ZASLAVSKY
Chief Executive Officer
IMAC Holdings, Inc.
3401 Mallory Lane, Suite 100
Franklin, Tennessee 37067
(303) 898-5896
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
MICHAEL A. ADELSTEIN, ESQ. CAROL W. SHERMAN, ESQ. Kelley Drye & Warren LLP 3 World Trade Center 175 Greenwich Street New York, New York 10007 (212) 808-7800 | | M. Ali Panjwani, Esq. Pryor Cashman LLP 7 Times Square New York, NY 10036 (212) 326-0820 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated filer | ☐ | Accelerated filer | ☐ |
| | | | |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | | |
| | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant files a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION DATED FEBRUARY 13, 2025
PRELIMINARY PROSPECTUS

Up to 35,007,025 Shares of Common Stock
This prospectus relates to the potential resale from time to time by Keystone Capital Partners, LLC, or Keystone or the Selling Stockholder, of up to 35,007,025 shares of common stock, par value $0.001 per share, or common stock. The shares of common stock to which this prospectus relates consist of shares that have been or may be issued by us to the Selling Stockholder pursuant to a Common Stock Purchase Agreement, dated as of November 12, 2024, by and between us and the Selling Stockholder, or the Purchase Agreement, establishing an equity line of credit, or the Equity Financing. Such shares of our common stock include (i) 164,000 shares of common stock, or the Initial Commitment Shares, issuable to Keystone under the Purchase Agreement on the date on which the registration statement of which this prospectus is apart is declared effective by the Securities and Exchange Commission, or the SEC, and (ii) $1 million of shares of common stock (1,666,667 shares of common stock assuming a price per share of $0.60), or the Back-End Commitment Shares (and together with the Initial Commitment Shares, the Commitment Shares), issuable to Keystone under the Purchase Agreement on the trading day following stockholder approval of our issuance of more than 402,438 shares of common stock to Keystone at an average per share price lower than the Minimum Price (as defined below) and (iii) up to $60 million of shares of common stock (or up to the 33,176,358 shares of common stock remaining of the common stock registered for resale pursuant to this prospectus after issuance of the Commitment Shares), or the Purchase Shares, that we may elect, in our sole discretion, to issue and sell to Keystone, from time to time from after the date the registration statement that includes this prospectus is declared effective by the SEC and after satisfaction of other conditions in the Purchase Agreement, or the Commencement Date, and subject to applicable stock exchange rules.
The actual number of shares of our common stock issuable will vary depending on the then-current market price of shares of our common stock sold to the Selling Stockholder under the Purchase Agreement, but will not exceed 35,007,025 shares of common stock unless we file an additional registration statement under the Securities Act of 1933, as amended, or the Securities Act, with the Securities Exchange Commission, or the SEC, and we obtain the approval of our stockholders of the issuance of shares of common stock in accordance with the applicable stock exchange rules. In no event will any shares be issued or sold to the Selling Stockholder if we do not have a sufficient number of shares of our common stock authorized under our Certificate of Incorporation, as amended from time to time, which are not reserved for other purposes. Under the applicable rules of The Nasdaq Stock Market LLC, or Nasdaq, in no event may we issue to the Selling Stockholder shares of our common stock representing more than 19.99% of the total number of shares of common stock outstanding as of the date of the Purchase Agreement (402,438 shares of common stock), or the Exchange Cap, unless (i) we obtain the approval of the issuance of such shares by our stockholders in accordance with the applicable stock exchange rules or (ii) sales of common stock are made at an average price, or the Minimum Price, equal to or in excess of the lower of (A) the closing prices of our common stock on Nasdaq immediately preceding the delivery by us to the Selling Stockholder of the applicable notice of our election to sell our common stock to Selling Stockholder under the Purchase Agreement, or the Sale Notices, (plus an incremental amount to take into account the Commitment Shares) and (B) the average of the closing prices of the common stock for the five business days immediately preceding the delivery of each Sale Notices (plus an incremental amount to take into account the Commitment Shares), such that the sales of such common stock to the Selling Stockholder would not count toward such limit because they are “at market” under applicable stock exchange rules. See “The Committed Equity Financing” for a description of the Purchase Agreement and “Selling Stockholder” for additional information regarding the Selling Stockholder.
We are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of the shares of our common stock by the Selling Stockholder. However, we may receive up to $60 million in aggregate gross proceeds from the sale of the Purchase Shares to the Selling Stockholder under the Purchase Agreement, from time to time in our discretion after the Commencement Date. The actual proceeds from the Selling Stockholder may be less than this amount depending on the number of shares of our common stock sold and the price at which the shares of our common stock are sold.
This prospectus provides you with a general description of such securities and the general manner in which the Selling Stockholder may offer or sell the securities. More specific terms of any securities that the Selling Stockholder may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
The Selling Stockholder may offer, sell or distribute all or a portion of the shares of our common stock acquired under the Purchase Agreement and hereby registered publicly or through private transactions at prevailing market prices or at negotiated prices. We will bear all costs, expenses and fees in connection with the registration of the shares of our common stock, including with regard to compliance with state securities or “blue sky” laws. The timing and amount of any sales are within the sole discretion of the Selling Stockholder. The Selling Stockholder is an underwriter under the Securities Act with respect to the resale of shares held by it. Although the Selling Stockholder is obligated to purchase shares of our common stock under the terms and subject to the conditions and limitations of the Purchase Agreement to the extent we choose to sell such shares of our common stock to it (subject to certain conditions), there can be no assurances that we will choose to sell any shares of our common stock to the Selling Stockholder, or that the Selling Stockholder will sell any or all of the shares of our common stock, if any, purchased under the Purchase Agreement pursuant to this prospectus. The Selling Stockholder will bear all commissions and discounts, if any, attributable to its sale of shares of our common stock. See “Plan of Distribution.”
You should read this prospectus and any prospectus supplement or amendment carefully before you invest in our securities.
Our Common is listed on The Nasdaq Capital Market under the symbol “BACK.” On February 12, 2025, the last reported sale price of our Common Stock on the Nasdaq Capital Market was $0.61 per share.
We received notice, on May 31, 2023, from the Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”) that we had failed to maintain a required minimum of $2,500,000 in stockholders’ equity for continued listing, as required under Listing Rule 5550(b)(1) (the “Minimum Equity Rule”).
On July 17, 2024, we were notified by Nasdaq that we had regained compliance with Minimum Equity Rule subject to a one-year “Panel Monitor” as that term is defined by Nasdaq Listing Rule 5815(d)(4)(B).
On January 21, 2025, we received notice (the “Minimum Equity Rule Notice”) from Nasdaq advising that we no longer complied with the Minimum Equity Rule. Due to the Panel Monitor, we were not eligible to submit a plan to Nasdaq to request an extension of up to 180 calendar days in which to regain compliance with the Minimum Equity Rule, and as a result, the Staff had determined to delist our securities from Nasdaq. Accordingly, the deadline for us to request an appeal of this determination was January 28, 2025.
We requested an appeal of this determination to the Hearings Panel (the “Panel”) and have a hearing currently schedule for March 4, 2025. The Company’s common stock will continue to trade on Nasdaq during the appeal process.
We are a “smaller reporting company” as defined under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and the documents incorporated by reference herein and may elect to comply with reduced public company reporting requirements in future filings. See “Prospectus Summary ⸺ Implications of Being a Smaller Reporting Company.”
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks that we have described beginning on page 10 of this prospectus under the caption “Risk Factors”, and under similar headings in any amendment or supplement to this prospectus or in any other documents incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Prospectus dated , 2025
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
The registration statement of which this prospectus forms a part, or the Registration Statement, includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus, the related exhibits to the Registration Statement filed with the Securities and Exchange Commission, or the SEC, and the documents incorporated by reference herein before making your investment decision. You should rely only on the information provided in this prospectus and the documents incorporated by reference herein or any amendment thereto.
You should not assume that the information contained in this prospectus or any related free writing prospectus is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference herein or therein is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus or any related free writing prospectus is delivered, or securities are sold, on a later date. This prospectus contains or incorporates by reference summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been or will be filed or have been or will be incorporated by reference as exhibits to the registration statement of which this prospectus forms a part, and you may obtain copies of those documents as described in this prospectus under the heading “Where You Can Find More Information.”
You should rely only on the information that we have included or incorporated by reference in this prospectus and any related free writing prospectus that we may authorize to be provided to you. We have not, and the Selling Stockholder has not, authorized anyone to give any information or to make any representation other than those given or made to you and contained or incorporated by reference in this prospectus or any related free writing prospectus that we may authorize to be provided to you. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. This prospectus and any related free writing prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus or any related free writing prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
In addition, while we believe the industry, market and competitive position data included in this prospectus, including the information incorporated by reference herein is reliable and based on reasonable assumptions, such data involve risks and uncertainties and are subject to change based on various factors. These factors could cause results to differ materially from those expressed in the estimates made by the independent parties or by us.
Unless the context otherwise requires, references in this prospectus to “IMAC, “the Company,” “we,” “our,” and “us” refer to IMAC Holdings, Inc., a Delaware corporation, and our consolidated subsidiaries.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider in making your investment decision. You should carefully read the entire prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” and under similar headings in other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference in this prospectus, including our financial statements, and exhibits to the registration statement of which this prospectus is a part.
Overview
We provide services related to proteomic products that identify and support oncology clinical treatment decisions and biopharmaceutical drug development.
Until recently, we were a holding company for IMAC Regeneration Centers, The BackSpace retail stores and our Investigational New Drug division, providing movement and orthopedic therapies and minimally invasive procedures to improve the physical health of patients at locations we owned or managed. As of December 31, 2023, we sold or discontinued patient care at all our locations.
In May 2024, we acquired certain assets and rights of Theralink Technologies, Inc. (“Theralink”), consisting primarily of a nationally CLIA-certified, CAP-accredited and New York State Clinical Laboratory Evaluation Program (“NYS-CLEP”) certified laboratory in Golden, Colorado and equipment located in the lab. Theralink was in the process of developing technology to monetize a license from Vanderbilt University (“Vanderbilt”), which provided a predictor of response to immunotherapy in cancer, and a license for business in the United States from George Mason University (“GMU”), which included intellectual property around improvements to the technology platform and biomarker signatures that form the basis for future proteomics products (collectively, the “Original Proteomics Licenses”). We have also entered into agreements with Vanderbilt and GMU to transfer the Original Proteomics Licenses.
On May 30, 2024, we formed a wholly-owned subsidiary, Ignite Proteomics LLC, a Delaware limited liability company (“Ignite”), to operate the medical lab acquired from Theralink, deliver services related to proteomic products under the licenses from GMU and Vanderbilt and collect fees for services rendered. We are using the acquired assets under our own branding, the Ignite name. Ignite is in the process of obtaining credentials for reimbursement for our Ignite proteomics test by Medicare and certain third-party payors. Until such time as Ignite is credentialed, we will accept payment from private insurers. Our Board of Directors, or the Board, has also approved the creation of the Ignite Compassionate Care program to enable those without private insurance or private funds to access our Ignite Proteomics test when needed until we are credentialed and thereafter for those without access to any form of insurance.
The Vanderbilt License
Under the license between Vanderbilt and Theralink, dated March 14, 2023, as amended from time to time, and assigned to us on May 15, 2024 pursuant to that certain Assignment and Assumption of License and Consent of Licensor between Theralink, IMAC and Vanderbilt (the “Vanderbilt License”), Vanderbilt granted us an exclusive license, in all fields of use, under the patents described in the Vanderbilt License (the “Vanderbilt Patents”) to make, use, offer to sell, sell and import products, processes and services that are covered by the Vanderbilt Patents (the “Vanderbilt Licensed Products”) during the term of the Vanderbilt License. The term of the Vanderbilt License shall continue until the expiration of the Vanderbilt Patents, unless sooner terminated by Vanderbilt or us in accordance with the terms of the Vanderbilt License.
As consideration for the Vanderbilt License, we agreed to pay Vanderbilt (1) an annual, non-refundable, non-creditable license fee of five thousand fifty-six dollars ($5,556), (2) a royalty percentage of gross sales of Vanderbilt Licensed Products ranging from 0.25% of gross sales for Vanderbilt Licensed Products that incorporate ten or more additional non-commodity constituent parts to 2% of gross sales for Vanderbilt Licensed Products that incorporate zero additional non-commodity constituent parts, and (3) for any improvement patents of Vanderbilt which we elect to use under the Vanderbilt License, a fee of three thousand dollars ($3,000).
Vanderbilt has exclusive responsibility for prosecution of the Vanderbilt Patents, including choice of patent counsel. We are obligated to pay an aggregate amount equal to eighteen thousand-nine hundred and sixteen dollars and eighty-eight cents ($18,916.88) as advance reimbursement of Vanderbilt’s costs incurred for prosecution of the Vanderbilt Patents.
We are required to maintain commercial general liability insurance and product liability insurance in amounts specified in the Vanderbilt License.
The Vanderbilt License also contains mutual indemnification obligations of Vanderbilt and Theralink and other obligations of the parties and termination provisions.
The GMU License
Under the License Agreement between George Mason Intellectual Properties (“GMIP”) and Theranostics Health, LLC and its successors dated September 15, 2006, as amended from time to time, and assigned to the Company on May 23, 2024 pursuant to that certain Assignment and Assumption of License and Consent of Licensor between Theralink, us and GMIP (the “GMU License”), GMIP granted us an exclusive, worldwide, sublicensable license, under the patents described in the GMU License (the “GMU Patents”), to make, have made, import, use, market, offer for sale and sell products designed, manufactured, used and/or marketed for use in all fields and for all uses during the term of the GMU License (the “GMU Licensed Products”). The term of the GMU License continues until the expiration of the GMU Patents, unless sooner terminated by GMIP or us in accordance with the terms of the GMU License. The exclusivity of the GMU License is conditioned on our agreement to manufacture GMU Licensed Products substantially in the United States unless we obtain a waiver of this requirement from an appropriate U.S. governmental authority.
Additionally, under the GMU License, GMIP granted to us an exclusive option (the “Exclusive Option”) to GMIP’s or George Mason University’s interest in any information, inventions, procedures, methods, devices, discoveries, technologies, data, designs or concepts related to the field of theragnostics from certain inventors as described in the GMU License.
As consideration for the GMU License, we agreed to pay GMIP (1) an annual fee of fifty-thousand dollars ($50,000) for the Exclusive Option, (2) quarterly royalties equal to the net revenue obtained by us and our affiliates from the sale of the GMU Licensed Products multiplied by one and one-half percent (1.5%), (3) quarterly sublicensing royalties equal to the sublicensing revenue obtained by us and our affiliates in connection with the GMU License multiplied by fifteen percent (15%), and (4) a payment of five thousand dollars ($5,000) for each patent issued relating to GMU Patents.
We have the right to control all aspects of filing, prosecuting, and maintaining the GMU Patents at our sole discretion. During the term of the GMU License, we have the first option to police the GMU Patents and the GMU Licensed Products against infringements by other parties worldwide within the designated field of use.
We are required to maintain product liability insurance in amounts specified in the GMU License.
The GMU License also contains unilateral indemnification obligations of the Company and other obligations of the parties and termination provisions.
Strategy
We expect to increase levels of revenue by increasing volume sample analysis. As more samples are processed in parallel, the cost per test decreases significantly. Hundreds of patient specimens can be analyzed at once thereby improving overall economics and broadening patient access.
Looking forward, our objective is to cement our technology as standard of care by achieving formal guideline inclusion. As discussed below under “Product Portfolio” we have already obtained clinical data that positions us to request coverage from additional payers and to propose that major cancer care guidelines, such as the National Comprehensive Cancer Network (NCCN), incorporate certain of our proteomic biomarkers. As we complete larger prospective studies and augment our data on test performance and clinical utility, and on different protein markers, we expect expanded guideline recommendations to follow. In parallel, new drug approvals, particularly in the antibody-drug conjugate and immunotherapy spaces, further amplify the need for a diagnostic that can identify responders and non-responders. By leveraging these emerging therapeutic markets with a validated protein-based assay, we anticipate a rapid increase in testing volume as our resources allow us to scale sales and operations and product development to cover additional cancer types.
We believe that significantly expanding the utilization of our proteomic assay will depend on adequate resources for sales, marketing, and educational programs, complemented by robust clinical evidence. Currently our testing volume is low, in part because of limited promotional activities and the early-stage nature of our commercial launch. Nonetheless, we have compelling clinical data showing that our reverse phase protein array (RPPA) analysis can predict which patients will respond or fail to respond to targeted therapies and immunotherapies. By demonstrating both clinical and economic benefits – such as avoiding expensive, ineffective treatments – our assay has the potential to achieve widespread adoption once we allocate sufficient funding to physician outreach, payer engagement, and professional education.
Although we have patent coverage in certain jurisdictions outside of the United States for certain biomarkers related to our assay, we do not currently offer or sell our products outside of the United States. Our immediate priority is to establish adoption within the United States. We plan to further explore international opportunities once we have sufficient funding and operational resources to implement our expansion strategy abroad. We cannot guarantee that we will be able to implement our expansion strategy abroad or that any such expansion will be successful.
Product Portfolio
Our product is a unique and patented RPPA technology platform, which can quantify protein signaling to support oncology clinical treatment decisions and biopharmaceutical drug development. Because protein signaling is responsible for the development and progression of cancer, nearly all FDA-approved cancer therapeutics target proteins, not genes. The Ignite RPPA technology can reveal the protein drug target(s) that are essentially turned “on” in a patient’s cancer and may help support the most effective treatment plan to turn those proteins “off”. Therefore, the Ignite RPPA technology is a critical tool that may empower oncologists with actionable information to effectively treat a cancer patient, which is often missed by standard proteomic and genomic testing. Our commercially available Lab Developed Test (LDT), the Ignite RPPA Assay for Breast Cancer, is currently being utilized by oncologists across the United States to assist in making the most targeted treatment plan for their patients with advanced breast cancer. In 2023, Theralink began receiving reimbursement for this test by Medicare and certain third-party payors. The Ignite Proteomics test determines which drug target(s) are present and/or activated and may reveal to the oncologist which patients are predicted to be responders versus non-responders to a particular therapeutic. The test may provide therapeutic recommendations to support oncologist treatment selection of the best therapy option – which may improve patient response and consequently save the healthcare system substantial dollars.
In molecular diagnostic testing, it is common for assays to include dozens or even hundreds of potential markers, even though only a smaller subset has the robust evidence to warrant major cancer care guideline inclusion, for instance, the guidelines of the National Comprehensive Cancer Network (NCCN), and commercial and government payer reimbursement. Our Ignite RPPA tests for 32 analyte, which are proteins or “activated” proteins. Although we currently measure 32 protein markers in a single test we are pursuing formal insurance coverage and guideline inclusion, including from NCCN, on a marker-by-marker basis, focusing first on those that demonstrate clear clinical utility.
We believe our RPPA analysis of phosphorylated AKT for AKT-targeted drugs, two-marker combinations for HER2 therapy response, and MHC-II for pembrolizumab meet the standards for NCCN guideline inclusion as emerging biomarkers based on the following clinical data:
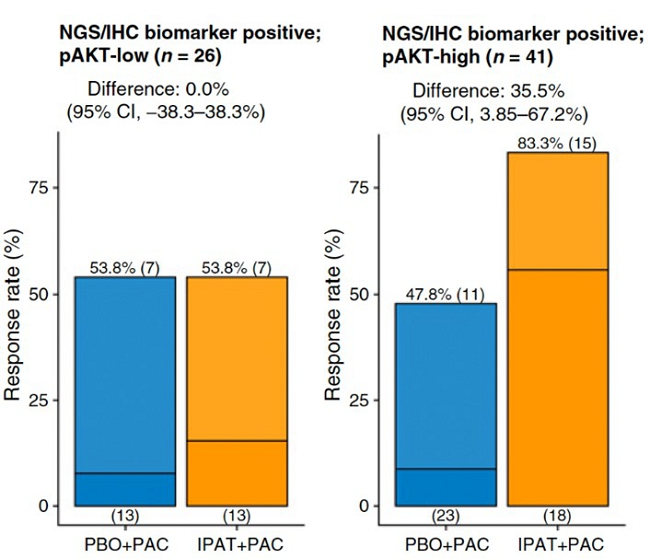
| | ● | phosphorylated AKT, which is shown to be a predictor of response to the drug ipatasertib independent of the traditional biomarkers. The findings are clinically significant (95% confidence level of 4%-67% improvement over standard of care), even though the sample size is small. The study is published at https://pmc.ncbi.nlm.nih.gov/articles/PMC9377742/. |
| | ● | Plotting pEGFR and pERBB2 expression together revealed a separation into two groups of patients: one with excellent response to the target therapy, neratinib, and one without. Since HER2 and EGFR are well-known heterodimerization partners, the fact that both are found to be highly co-activated in the pre-treatment tumor samples of responding patient population provide further evidence of functional HER2-driven pathway activation and signaling coherence. The study is published at https://pmc.ncbi.nlm.nih.gov/articles/PMC8571284/. |
| | | |
| | ● | MHC-II has been shown to be a superior biomarker to PDL-1 for predicting response to pembrolizumab (Keytruda). The data we will present to NCCN is currently in review for publication. However, there are other studies that support our findings, including at https://pmc.ncbi.nlm.nih.gov/articles/PMC4740184/ for melanoma patients. Our data will be the first analysis in breast cancer patients to be published. |
The currently available Ignite RPPA Assay for Breast Cancer will be followed by the Ignite RPPA Pan-Tumor Assay 1.0, expected to launch in 2025 to include ovarian, endometrial, and head & neck cancers. The test is expected to expand further in 2026 to the Ignite RPPA Pan-Tumor Assay 2.0 to support the treatment of colorectal, prostate, pancreatic, lung, and other solid tumor cancer indications. We are aware that the U.S. Food and Drug Administration (the “FDA”) published new rules concerning LDT regulation on May 6, 2024, and intend to comply fully with the final regulations. Because we have demonstrated analytic and clinical validity under CLIA, CAP, and NYS CLEP standards, we anticipate meeting any additional FDA requirements as they come into effect. However, we cannot guarantee that we will meet any such new requirements or be able to obtain any new approvals required.
Recent Developments
On May 23, 2023, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Theralink and IMAC Merger Sub, Inc., a Delaware corporation and a newly formed, wholly owned subsidiary of the Company (“Merger Sub”). Upon the terms and subject to the conditions set forth in the Merger Agreement, we and Theralink agreed to merge (the “Merger”), with Theralink continuing as the surviving entity (the “Surviving Entity”) and a wholly owned subsidiary of the Company. The completion of the Merger was subject to the satisfaction or waiver of customary closing conditions. However, such conditions were not met and such closing did not occur.
In furtherance of the proposed arrangement with Theralink, on April 11, 2024 we entered into a credit agreement, secured by the assets of Theralink and its subsidiaries, pursuant to which Theralink may borrow from the Company up to an aggregate of $1,000,000 with an initial borrowing on April 12, 2024 of $350,000 (the “Theralink Credit Agreement”).
On April 10, 2024, we entered into a series of transactions including the exchange of the Company’s outstanding Series B-1 Convertible Preferred Stock, par value $0.001 per share (the “Series B-1 Preferred Stock”) and Series B-2 Convertible Preferred Stock, par value $0.001 per share (the “Series B-2 Preferred Stock” and, collectively with the Series B-1 Preferred Stock, the “Series B Preferred Stock”), for Series C-1 Convertible Preferred Stock (the “Series C-1 Preferred Stock”), the exchange of certain of the Company’s outstanding warrants for new warrants to purchase 2,075,704 shares of our common stock, and the sale of new Series C-2 Convertible Preferred Stock (the “Series C-2 Preferred Stock” and, together with the Series C-1 Preferred Stock, the “Series C Preferred Stock”) and warrants to purchase 498,243 shares of our common stock. All such transactions were consummated on April 11, 2024 and resulted in gross proceeds to the Company of $900,000.
On April 30, 2024, we entered into securities purchase agreements (each, a “Securities Purchase Agreement”) with various holders (the “Theralink Note Holders”) of senior secured convertible debentures (the “Theralink Notes”) of Theralink for the sale of 17,364 shares of the Company’s newly created Series D Convertible Preferred Stock, $0.001 par value (the “Series D Preferred Stock”). The consideration paid by the Theralink Note Holders was in the form of all of the Theralink Notes held by them, which had an aggregate principal amount outstanding of $16,221,873.89 and which the Theralink Note Holders accelerated earlier on April 30, 2024. Upon the consummation of the transactions contemplated by the Securities Purchase Agreement, we held approximately 74.01% of the outstanding Theralink Notes.
On May 1, 2024, we entered into a Settlement and Release Agreement with Theralink (the “Settlement Agreement”) pursuant to which the parties agreed to a settlement of the default by Theralink under the Theralink Credit Agreement. The settlement consisted of the transfer to us of certain assets and rights of Theralink and certain liabilities, including various trade payables, in exchange for (i) the forgiveness by us of the outstanding amounts due under (a) the Theralink Notes that we acquired pursuant to the Securities Purchase Agreement, (b) certain other pre-existing notes made by Theralink in our favor, having an aggregate outstanding principal amount of $3,000,000 (the “Pre-Existing Theralink Notes”), and (c) the Theralink Credit Agreement and (ii) the issuance to Theralink and certain holders of Theralink debt of an aggregate of 24,172 shares of the Company’s newly created Series E Convertible Preferred Stock, $0.001 par value (the “Series E Preferred Stock”). In addition, pursuant to the Settlement Agreement, the parties agreed to mutual releases with respect to the outstanding payments being forgiven, we and Theralink agreed to terminate the merger agreement between us and withdraw the Registration Statement on Form S-4 related thereto as soon as commercially practicable, and we agreed to assume certain liabilities of Theralink, including various trade payables, and to hire certain of the employees of Theralink. On May 6, 2024, the Merger Agreement was terminated and on May 7, 2024, we withdrew the Registration Statement on Form S-4 related to the Merger.
We subsequently obtained transfers to us of the Original Proteomics Licenses.
On May 14, 2024, the Company issued and sold to accredited investors an aggregate of 450 shares of Series F convertible preferred stock, par value $0.001 per share (“Series F Preferred Stock”) and warrants to purchase 135,315 shares of our common stock, for aggregate cash proceeds of $450,000.
On June 18, 2024, we issued promissory notes (the “June 2024 Notes”) to certain lenders in the aggregate principal amount of $1,400,000, for an aggregate purchase price from the Lenders of $1,000,000. The June 2024 Notes are unsecured and mature on the earlier of (i) the date of consummation of the offering contemplated by the Registration Statement on Form S-1, File No. 333-280184, filed with the SEC by the Company on June 13, 2024, and (ii) June 18, 2025. As of November 14, 2024, we have repaid the June 2024 Notes.
Between September 12, 2024 and October 30, 2024, we issued promissory notes (the “September-October 2024 Notes”) to certain lenders in the aggregate principal amount of $840,000, for an aggregate purchase price from the Lenders of $600,000. The September-October 2024 Notes are unsecured and mature on the earlier of (i) the date of consummation of any offering or offerings, individually or in the aggregate, of securities with gross proceeds of at least $1,000,000, and (ii) June 18, 2025. As of November 14, 2024, we had repaid the September-October Notes.
On November 12, 2024, we entered into a Common Stock Purchase Agreement (the “Purchase Agreement”) and a Registration Rights Agreement (the “Registration Rights Agreement”) with Keystone Capital Partners, LLC (“Keystone” or the “Selling Stockholder”), pursuant to which from time to time during the 36-months after all of the conditions to our right to commence sales of our common stock, par value $0.001 per share, or common stock, to Keystone have been satisfied, including that the registration statement of which this prospectus forms a part is declared effective by the Securities and Exchange Commission (the “SEC”), and the final form of this prospectus is filed with the SEC (the “Commencement Date”), we have the right, but not the obligation, to sell to Keystone (subject to certain conditions and limitations), and Keystone is obligated to purchase, shares of our common stock as more fully described in this prospectus.
On November 12, 2024, we entered into a securities purchase agreement (the “Series G Securities Purchase Agreement”) with accredited investors (the “Series G Investors”), pursuant to which the Company agreed to issue and sell, and the Series G Investors agreed to purchase, an aggregate of 4,676 shares of Series G convertible preferred stock, par value $0.001 per share (“Series G Preferred Stock”) and warrants to purchase 2,977,711 shares of our common stock (the “Series G Warrants”, and, together with the Series G Preferred Stock, the “Series G Securities”), for aggregate cash proceeds of $3,740,000. On November 14, 2024, the Company consummated the sale of the Series G Securities. Additional sales of Series G Preferred Stock and related Series G Warrants may be made in future closings. The Company used $2,240,000 of the proceeds to repay $2,240,000 of outstanding promissory notes of the Company and, accordingly, the Company had no debt outstanding after such repayments.
Reverse Stock Split
In September 2023, we effected a 1-for-30 reverse stock split of our common stock, whereby each 30 shares of our common stock and common stock equivalents were converted into one share of common stock (the “Reverse Stock Split”). All share and per share amounts in this prospectus have been retroactively adjusted to give effect to the Reverse Stock Split.
Leadership changes
On May 24, 2024, Matthew C. Wallis, DC, and Jeffrey S. Ervin resigned from the Board.
On May 23, 2024, Jeffrey S. Ervin resigned as Chief Executive Officer of the Company and the Company appointed Faith Zaslavsky, the former Chief Executive Officer of Theralink, as the Chief Executive Officer of the Company.
On June 26, 2024, Peter Beitsch, MD, and Matthew Schwartz, MD, were appointed to the Board.
On September 19, 2024, the Board appointed Jeffrey Busch to fill the vacancy on the Board created when Cary Sucoff resigned from the Board effective as of September 9, 2024.
Compliance with Nasdaq Listing Requirements
On May 31, 2023, the Company received notice from Nasdaq that the Company had failed to maintain compliance with the Minimum Equity Rule, which required us to maintain a required minimum of $2,500,000 in stockholders’ equity for continued listing, as required under Listing Rule 5550(b)(1). On August 3, 2023, the Company submitted a plan to Nasdaq to grant the Company an extension of time until November 27, 2023 to provide evidence of compliance with the Minimum Equity Rule. The Company attended a Nasdaq Listing Hearing on February 20, 2024. Nasdaq agreed to extend the Company’s listing based on specific conditions for continued listing.
On July 17, 2024, we were notified by Nasdaq that we had regained compliance with Minimum Equity Rule. We remain subject to a one-year “Panel Monitor” as that term is defined by Nasdaq Listing Rule 5815(d)(4)(B).
On January 21, 2025, we received the Minimum Equity Rule Notice from Nasdaq advising the Company that it no longer complies with the Minimum Equity Rule. Due to the Panel Monitor, the Company is not eligible to submit a plan to the Staff to request an extension of up to 180 calendar days in which to regain compliance with the Minimum Equity Rule, and as a result, the Staff has determined to delist the Company’s securities from Nasdaq. Accordingly, the deadline for the Company to request an appeal of this determination was January 28, 2025, and the Company’s securities were scheduled for delisting from Nasdaq and suspension of trading at the opening of business on January 30, 2025.
We requested an appeal of this determination to the Panel and have a hearing currently schedule for March 4, 2025. The Company’s common stock will continue to trade on Nasdaq during the appeal process.
On August 21, 2024, we received written notice (the “2nd Quarter Form 10-Q Filing Notice”) from Nasdaq indicating that the Company no longer complied with Nasdaq Listing Rule 5250(c)(1) (the “Periodic Report Listing Rule”), which requires companies with securities listed on the Nasdaq Capital Market to timely file all required periodic reports with the SEC. The 2nd Quarter Form 10-Q was filed on December 18, 2024.
On November 22, 2024, we received written notice (the “3rd Quarter Form 10-Q Filing Notice”) from Nasdaq indicating that the Company no longer complied the Periodic Report Listing Rule. As previously disclosed in a Form 12b-25 Notification of Late Fling filed by the Company on November 15, 2024 (the “Form 12b-25”), the Company is delayed in filing its Quarterly Report on Form 10-Q for the period ended September 30, 2024 (the “3rd Quarter Form 10-Q”) with the SEC. The filing of the 3rd Quarter Form 10-Q was delayed due to the matters described in the Form 12b-25. The 3rd Quarter Form 10-Q was filed on January 17, 2025.
On January 21, 2025, we were notified by Nasdaq that the Company had regained compliance with the Periodic Reporting Listing Rule.
On November 21, 2024, we received written notice (the “Audit Committee Notice”) from Nasdaq indicating that the Company no longer complies with Nasdaq Listing Rule 5605 (the “Audit Committee Listing Rule”), which requires, among other things, companies with securities listed on the Nasdaq Capital Market to have an audit committee consisting of at least three members. The Company became out of compliance with the Audit Committee Listing Rule as a result of the vacancy caused by the resignation of Cary Sucoff from the Board of Directors of the Company effective September 9, 2024. As of December 11, 2024, Jeffrey Busch was appointed to the Audit Committee, and on December 17, 2024, the Company was notified by Nasdaq that it has regained compliance with the Audit Committee Listing Rule.
We are considering all available options that may enable us to timely evidence compliance with the continued listing criteria and maintain our listing on Nasdaq. There can be no assurance that we will be successful in our efforts to maintain the listing of our common stock on Nasdaq. If we are delisted from Nasdaq, there can be no assurance that our common stock will be eligible for trading on another stock exchange or quotation on an over-the-counter market. If we are not able to obtain a listing on another stock exchange or quotation service for our common stock, it may be extremely difficult or impossible for stockholders to sell their shares. Additionally, if we are delisted from Nasdaq, but obtain a substitute listing or quotation service for our common stock, it will likely be on a market with less liquidity and our common stock may therefore experience potentially more price volatility than it has historically experienced on Nasdaq. Stockholders may not be able to sell their shares of common stock on any such substitute market in the quantities, at the times, or at the prices that could potentially be available on a more liquid trading market. As a result of these factors, if our common stock is delisted from Nasdaq, the value and liquidity of our common stock would likely be adversely affected. A delisting of our common stock from Nasdaq could also adversely affect our ability to obtain financing for our operations and/or result in a loss of confidence by investors, employees and/or business partners. Further, our common stock not having been suspended, including suspended by Nasdaq, is a condition precedent to our right to deliver Purchase Notices under the Purchase Agreement and Keystone’s obligation to accept such Purchase Notices.
Corporate Information
Our primary executive offices are located at 3401 Mallory Lane, Suite 100, Franklin, Tennessee and our telephone number is (303) 898-5896. Our website address is www.imacholdings.com. The information contained in, or that can be accessed through, our website is not a part of or incorporated by reference in this prospectus, and you should not consider it part of this prospectus or of any prospectus supplement. We have included our website address in this prospectus solely as an inactive textual reference.
Implications of Being a Smaller Reporting Company
We are a smaller reporting company as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) the market value of our voting and non-voting common stock held by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our voting and non-voting common stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Reports on Form 10-K and have reduced disclosure obligations regarding executive compensation, and, as long as we are a smaller reporting company with less than $100 million in annual revenue, we are not required to obtain an attestation report on internal control over financial reporting from our independent registered public accounting firm.
THE OFFERING
The following summary contains basic information about this offering. The summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus.
| Shares of common stock offered by the Selling Stockholder | | Up to 35,007,025 shares of common stock, consisting of (i) 164,000 shares of common stock, or the Initial Commitment Shares, issuable to Keystone under the Purchase Agreement on the date on which the registration statement of which this prospectus is apart is declared effective by the Securities and Exchange Commission, or the SEC, and (ii) $1 million of shares of common stock (1,666,667 shares of common stock assuming a price per share of $0.60), or the Back-End Commitment Shares (and together with the Initial Commitment Shares, the Commitment Shares), issuable to Keystone under the Purchase Agreement on the trading day following stockholder approval, or Stockholder Approval, of our issuance of more than 402,438 shares of common stock to Keystone at an average per share price lower than the Minimum Price (as defined below), or the Exchange Cap, and (iii) up to $60 million of shares of common stock (or up to the 33,176,358 shares of common stock remaining of the common stock registered for resale pursuant to this prospectus after issuance of the Commitment Shares), or the Purchase Shares, that we may elect, in our sole discretion, to issue and sell to Keystone, from time to time from after the date the registration statement that includes this prospectus is declared effective by the SEC and after satisfaction of other conditions in the Purchase Agreement, or the Commencement Date, and subject to applicable stock exchange rules. |
| | | |
| Shares of common stock outstanding immediately prior to this offering | | 3,365,866 shares (as of February 5, 2025). |
| | | |
| Shares of common stock outstanding immediately following this offering | | 38,372,891 shares. |
| | | |
| Terms of the officer | | The Selling Stockholder will determine when and how it will dispose of any shares of our common stock that are registered under this prospectus for resale. See “Plan of Distribution.” |
| | | |
| Use of Proceeds | | We will not receive any of the proceeds from the sale of shares of our common stock offered by Keystone. We may receive up to $60 million in aggregate gross proceeds, or the Commitment Amount, from Keystone under the Purchase Agreement in connection with sales of Purchase Shares to Keystone pursuant to the Purchase Agreement, from time to time, in our sole discretion, after the Commencement Date. However, the actual proceeds may be less than this amount depending on the number of Purchase Shares sold and the price at which the Purchase Shares are sold. The Purchase Agreement requires that we use 25% of any net proceeds that we receive under the Purchase Agreement to redeem shares of our Series G Preferred Stock and shares of our Series C Preferred Stock, Series D Preferred Stock, Series E Preferred Stock and Series F Preferred Stock held by holders of our Series G Preferred Stock, or collectively the Redeemable Preferred Stock. We intend to use the remainder of the net proceeds for working capital and other general corporate purposes. However, as of the date of this prospectus, we cannot specify with certainty all of the particular uses, and the respective amounts we may allocate to those uses, for any net proceeds we receive. See “Use of Proceeds” on page 21, of this prospectus. |
| | | |
| Nasdaq Capital Market symbol | | Our shares of common stock are traded on The Nasdaq Capital Market under the symbol “BACK”. |
| | | |
| Transfer Agent and Registrar | | Equity Stock Transfer |
The number of shares of our common stock to be outstanding after this offering is based on shares of common stock outstanding as of February 5, 2025, and excludes:
| ● | 507,683 shares of Common Stock reserved issuance under our equity incentive plans; |
| ● | 1,748,740 shares of Common Stock reserved for issuance upon conversion of our outstanding Series C-1 Preferred Stock; |
| ● | 734,918 shares of Common Stock reserved for issuance upon conversion of our outstanding Series C-2 Preferred Stock; |
| ● | 8,571,992 shares of Common Stock reserved for issuance upon conversion of our outstanding Series D Preferred Stock; |
| ● | 14,128,098 shares of Common Stock reserved for issuance upon conversion of our outstanding Series E Preferred Stock; |
| ● | 250,430 shares of Common Stock reserved for issuance upon conversion of our outstanding Series F Preferred Stock; |
| ● | 6,036,120 shares of Common Stock reserved for issuance upon conversion of our outstanding Series G Preferred Stock; |
| ● | 5,905,946 shares of Common Stock reserved for issuance upon exercise of outstanding warrants. |
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider the risks described below, and those discussed under the section entitled “Risk Factors” contained in our Annual Report on Form 10-K/A for the year ended December 31, 2023, which is incorporated by reference in this prospectus, and our subsequent Quarterly Reports on Form 10-Q, together with other information in this prospectus, the information and documents incorporated by reference herein, and in any free writing prospectus that we have authorized for use in connection with this offering. The occurrence of any of the events or developments described below could materially and adversely affect our business, financial condition, results of operations and prospects. In such an event, the market price of our common stock could decline and you may lose all or part of your investment.
The risks included in this prospectus and the documents we have incorporated by reference into this prospectus are not the only risks we face. We may experience additional risks and uncertainties not currently known to us, or as a result of developments occurring in the future. Conditions that we currently deem to be immaterial may also materially and adversely affect our business, financial condition, cash flows and results of operations, and our ability to pay distributions to stockholders.
Risks Related to the Committed Equity Financing
It is not possible to predict the actual number of shares of our common stock, if any, we will issue to the Selling Stockholder under the Purchase Agreement, the actual gross proceeds resulting from sales of the Purchase Shares to the Selling Stockholder or the dilution to you from those issuances. Further, we may not have access to the full amount available under the Purchase Agreement.
Pursuant to the Purchase Agreement, we are required to issue to the Selling Stockholder the Commitment Shares and the Selling Stockholder shall purchase from us, from time to time, at our sole discretion, up to $60 million of Purchase Shares. The actual number of shares of our common stock issuable under the Purchase Agreement will vary depending on the then-current market price of shares of our common stock at the time of sales of Purchase Shares to the Selling Stockholder and the price per share of common stock used to calculate the number of Back End Commitment Shares, but will not exceed 35,007,025 shares of common stock the resale of which is registered pursuant to this prospectus unless we file an additional registration statement under the Securities Act of 1933, as amended, or the Securities Act, with the SEC. In no event will any shares be issued or sold to the Selling Stockholder if we do not have a sufficient number of shares of our common stock authorized under our Certificate of Incorporation, as amended from time to time, which are not reserved for other purposes.
Additionally, under the applicable rules of The Nasdaq Stock Market LLC, or Nasdaq, in no event may we issue to the Selling Stockholder shares of our common stock representing more than 19.99% of the total number of shares of common stock outstanding as of the date of the Purchase Agreement (402,438 shares of common stock), or the Exchange Cap, unless (i) we obtain the Stockholder Approval or (ii) sales of common stock are made at an average price, or the Minimum Price, equal to or in excess of the lower of (A) the closing prices of our common stock on Nasdaq immediately preceding the delivery by us to the Selling Stockholder of the applicable notice of our election to sell our common stock to Selling Stockholder under the Purchase Agreement, or the Sale Notices, (plus an incremental amount to take into account the Commitment Shares) and (B) the average of the closing prices of the common stock for the five business days immediately preceding the delivery of each Sale Notices (plus an incremental amount to take into account the Commitment Shares), such that the sales of such common stock to the Selling Stockholder would not count toward such limit because they are “at market” under applicable stock exchange rules.
We do not have a right to commence any sales of common stock to the Selling Stockholder under the Purchase Agreement until the Commencement Date. The number of Purchase Shares that may be sold by us to the Selling Stockholder and the timing of such sales is at our discretion from time to time after the Commencement Date until the earliest to occur of (i) the first day of the month next following the 36-month anniversary of the Commencement Date, (ii) the date on which the Selling Stockholder shall have purchased $60 million of Purchase Shares, (iii) the date on which our common stock fails to be listed or quoted on Nasdaq or any other eligible market (as defined in the Purchase Agreement), (iv) the thirtieth trading day next following the date on which, pursuant to or within the meaning of any bankruptcy law, we commence a voluntary case or any person commences a proceeding against us, in each case that is not discharged or dismissed prior to such thirtieth trading day, and (v) the date on which, pursuant to or within the meaning of any bankruptcy law, a custodian is appointed for us or for all or substantially all of our property, or we make a general assignment for the benefit of our creditors, or each, a Termination Event.
Sales of Purchase Shares, if any, to Keystone under the Purchase Agreement will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Keystone all, some or none of the common stock that may be available for us to sell to Keystone pursuant to the Purchase Agreement. Accordingly, we cannot guarantee that we will be able to sell all of the Commitment Amount or how much in proceeds we may obtain under the Purchase Agreement. If we cannot sell Purchase Shares, we may be required to utilize more costly and time-consuming means of accessing the capital markets, which could have a material adverse effect on our liquidity and cash position.
In addition, Keystone is not obligated to buy any common stock under the Purchase Agreement if such shares, when aggregated with all other common stock then beneficially owned by Keystone and its affiliates (as calculated pursuant to Section 13(d) of the Securities Exchange Act, and Rule 13d-3 promulgated thereunder), would result in Keystone beneficially owning common stock in excess of 4.99% of the then-outstanding shares of common stock.
We are registering 35,007,025 shares of our common stock under this prospectus. As of February 5, 2025, there were 3,365,866 shares of common stock outstanding. If all of the 35,007,025 shares of our common stock offered for resale by the Selling Stockholder under this prospectus were issued and outstanding as of February 5, 2025, such shares would represent approximately 91.23% of total number of shares of our common stock outstanding.
If it becomes necessary for us to issue and sell to Keystone under the Purchase Agreement more than the 35,007,025 shares of our common stock being registered for resale under this prospectus in order to receive aggregate gross proceeds equal to the Commitment Amount, we must file with the SEC one or more additional registration statements to register under the Securities Act the resale by Keystone of any such additional shares of our common stock we wish to sell from time to time under the Purchase Agreement, which the SEC must declare effective, in each case before we may elect to sell any additional shares of our common stock under the Purchase Agreement.
Because the purchase price per share of common stock to be paid by Keystone for the Purchase Shares that we may elect to sell to Keystone under the Purchase Agreement, if any, will fluctuate based on the market prices of our common stock at the time we make such election and the limitations discussed above with respect to the number of Purchase Shares that may be sold to Keystone, it is not possible for us to predict, as of the date of this prospectus and prior to any such sales, the number of Purchase Shares that we will sell to Keystone under the Purchase Agreement, the purchase price per share that Keystone will pay for such Purchase Shares, or the aggregate gross proceeds that we will receive from those purchases by Keystone. Our inability to access a portion or the full amount available under the Purchase Agreement, in the absence of any other financing sources, could have a material adverse effect on our business or results of operation.
Keystone will pay less than the then-prevailing market price for our common stock, which could cause the price of our common stock to decline.
The purchase price of our common stock to be sold to Keystone under the Purchase Agreement is derived from the market price of our common stock on Nasdaq. Shares to be sold to Keystone pursuant to the Purchase Agreement will be purchased at a discounted price.
For example, we may effect sales to Keystone pursuant to a Fixed Purchase Notice (as defined below) at a purchase price equal to the lesser of 92.5% of (i) the daily volume weighted average price of the common stock for the five trading days immediately preceding the applicable Purchase Date (as defined below) and (ii) the closing trading price of a share of common stock on the trading day immediately following the applicable Purchase Date. See “The Committed Equity Financing” for more information.
As a result of this pricing structure, Keystone may sell the shares they receive immediately after receipt of such shares, which could cause the price of our common stock to decrease.
Investors who buy shares of common stock from Keystone at different times will likely pay different prices.
Pursuant to the Purchase Agreement, we have discretion, to vary the timing, price and number of shares of common stock we sell to Keystone. If and when we elect to sell shares of common stock to Keystone pursuant to the Purchase Agreement, after Keystone has acquired such shares, Keystone may resell all, some or none of such shares at any time or from time to time in its sole discretion and at different prices. As a result, investors who purchase shares from Keystone in this offering at different times will likely pay different prices for those shares, and so may experience different levels of dilution and in some cases substantial dilution and different outcomes in their investment results. Investors may experience a decline in the value of the shares they purchase Keystone in this offering as a result of future sales made by us to Keystone at prices lower than the prices such investors paid for their shares in this offering. In addition, if we sell a substantial number of shares to Keystone under the Purchase Agreement, or if investors expect that we will do so, the actual sales of shares or the mere existence of our arrangements with Keystone may make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales.
Future resales and/or issuances of shares of common stock, including pursuant to this prospectus, or the perception that such sales may occur, may cause the market price of our shares to drop significantly.
Keystone may resell all, some or none of such shares of the Purchase Shares and Commitment Shares held by Keystone at any time or from time to time in its discretion and at different prices. Therefore, issuances to Keystone by us could result in substantial dilution to the interests of other holders of shares of our common stock. In addition, if we sell a substantial number of shares of our common stock to Keystone under the Purchase Agreement, or if investors expect that we will do so, the shares held by Keystone will represent a significant portion of our public float and may result in substantial decreases to the price of our common stock. The actual sales of shares of our common stock or the mere existence of our arrangement with Keystone may also make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales.
In addition, shares of our common stock issuable upon exercise or vesting of incentive awards under our incentive plans are, once issued, eligible for sale in the public market, subject to any lock-up agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144. Furthermore, shares of our common stock reserved for future issuance under our incentive plan may become available for sale in future.
As of February 5, 2025, there were approximately 507,683 shares subject to outstanding options or restricted stock units and shares reserved for future issuance or otherwise issuable under our 2018 Incentive Compensation Plan, as amended from time to time, or the Plan. We have registered or will register the shares of common stock available for issuance under the Plan under the Securities Act on Registration Statements on Form S-8. The registered shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described above, to the extent applicable.
As of February 5, 2025, we had outstanding shares of preferred stock, including the Redeemable Preferred Stock, currently convertible into 31,470,298 shares of common stock which may be eligible to be freely tradeable without restriction if held by non-affiliates of the Company. We are obligated under the Purchase Agreement to use 25% of the net proceeds from sales of Purchase Shares to Keystone to redeem the Redeemable Preferred Stock which is currently convertible into 7,786,881 shares of common stock.
As of February 5, 2025, we had 5,905,946 outstanding warrants exercisable for shares with a weighted-average exercise price of $2.77 per share. The shares of the common stock underlying such warrants held by non-affiliates of the Company which may be eligible to be freely tradeable without restriction if held by non-affiliates of the Company
The market price of shares of our common stock could drop significantly if the holders described above sell or are perceived by the market as intending to sell. These factors could also make it more difficult for us to raise additional funds through future offerings of shares of our common stock or other securities.
We may use proceeds from sales of our common stock made pursuant to the Purchase Agreement in ways with which you may not agree or in ways which may not yield a significant return.
We will have broad discretion over the use of proceeds from sales of our common stock made pursuant to the Purchase Agreement, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds, their ultimate use may vary substantially from their currently intended use. While we expect to use the net proceeds from this offering as set forth in “Use of Proceeds,” other than using 25% of the net proceeds for redemption of our Redeemable Preferred Stock we are not obligated to do so. The failure by us to apply these funds effectively could harm our business, and the net proceeds may be used for corporate purposes that do not increase our operating results or enhance the value of our common stock.
We are currently offering and developing multiple tests as LDTs and intend to develop and perform LDTs in the future. The FDA’s newly issued rule for LDTs, effective July 5, 2024, which will be phased in over a period of four years, will significantly change the regulatory landscape for LDTs. The final rule included a clause to grandfather in tests already in market on May 6, 2024, which applies to our current commercially available tests. The new rule may lead to additional compliance costs and may delay or prevent market entry for new or modified tests and may add a financial burden to us for future tests. There is a risk that the commercialization of these tests, and our results of operations and financial condition, will be negatively affected.
The FDA considers an LDT to be a test that is designed, developed, validated, and used within a single, CLIA-certified high complexity laboratory. The FDA has historically taken the position that it has the authority to regulate LDTs as in vitro diagnostic (“IVD”) medical devices under the FDC Act, but it has generally exercised enforcement discretion with regard to LDTs, meaning that most LDTs have not been subject to FDA oversight. On May 6, 2024, the FDA published a final rule amending the definition of an IVD device to include IVDs manufactured by a clinical laboratory.
The specific exception that applies to us is the exception for currently marketed IVDs offered as LDTs that were first marketed prior to the date of the issuance of this rule, May 6, 2024 (the “Grandfathering Exception”). The FDA intends for this policy to apply to currently marketed IVDs offered as LDTs as long as they are not modified following the issuance of this final rule or are modified but only in certain limited ways that are described below:
| | 1. | change the indications for use of the IVD; |
| | | |
| | 2. | alter the operating principle of the IVD; |
| | | |
| | 3. | include significantly different technology in the IVD or; |
| | | |
| | 4. | adversely change the performance or safety specifications of the IVD. |
The FDA intends to request that laboratories offering currently marketed IVDs offered as LDTs submit labeling to FDA as provided in §807.26(e). Labeling includes IVD performance information and a summary of supporting validation, as applicable. This information will help FDA more closely monitor currently marketed IVDs offered as LDTs and identify those that may lack analytical validity, clinical validity, or safety. As part of its review of labeling, the FDA also intends to look closely at claims of superior performance and whether those claims are adequately substantiated.
The final rule also announced the FDA’s intention to phase out its general enforcement discretion policy. Unless the rule is overturned by a court or Congress, the medical device requirements for most LDTs will be phased-in in yearly stages, the first of which begins on May 6, 2025. These requirements include premarket authorization requirements (510(k) clearance, de novo classification, or premarket approval) for each LDT performed by the laboratory, and post-market registration and listing, medical device reporting, correction, removal, and recall, complaint handling, labeling, investigational device, and quality system requirements. Certain categories of LDTs will be subject to enforcement discretion with respect to some or all of these requirements. For example, FDA will apply enforcement discretion to currently marketed LDTs that were first offered prior to May 6, 2024, with respect to most quality system requirements and the requirement for premarket authorization if they are not modified or modified in only limited ways. Laboratories performing these tests are subject to other requirements, including the requirement to submit the labeling for the LDT to FDA for review. FDA will similarly exercise enforcement discretion with respect to premarket authorization for LDTs approved by the NYS-CLEP. While our LTDs are currently covered by the Grandfathering Exception, those LDTs will become subject to the entire impact of the final rule if any modifications are necessary beyond to such LDTs what the FDA may interpret as “limited ways” there by removing the protection of the Grandfathering Exception. While our LDTs currently meet the requirements of the final rule’s implementation, if our LDTs become subject to the full impact of the final rule, we will likely be subject to increased regulatory burden on our ability to continue developing LDTs and to develop and introduce new products in the future, which could adversely affect our business. If we fail to comply with applicable federal, state, local and foreign laboratory licensing requirements, we could lose the ability to perform our genetic tests or experience disruptions to our business.
We are not in compliance with the NASDAQ Capital Market $1.00 minimum bid price requirement and the $2,500,000 stockholder’s equity minimum requirement which could result in delisting and adversely affect the market price and liquidity of our common stock.
We received notice, on May 31, 2023, from the Listing Qualifications Department of Nasdaq that we had failed to maintain compliance with the Minimum Equity Rule. On July 17, 2024, we were notified by Nasdaq that we had regained compliance with Minimum Equity Rule subject to a one-year “Panel Monitor” as that term is defined by Nasdaq Listing Rule 5815(d)(4)(B). On January 21, 2025, we received the Minimum Equity Rule Notice from Nasdaq advising that we no longer complied with the Minimum Equity Rule. Due to the Panel Monitor, we were not eligible to submit a plan to Nasdaq to request an extension of up to 180 calendar days in which to regain compliance with the Minimum Equity Rule, and as a result, the Staff had determined to delist our securities from Nasdaq. Accordingly, the deadline for us to request an appeal of this determination was January 28, 2025. We requested an appeal of this determination to the Panel and have a hearing currently schedule for March 4, 2025 (the “Hearing”).
The Hearing stays any delisting action in connection with the Minimum Equity Rule Notice and our common stock continues to be listed on Nasdaq until the Panel renders a decision subsequent to the Hearing. There can be no assurance that our appeal to the Panel will be successful or that our common stock will remain listed on Nasdaq.
If we are not successful at the Hearing or if we fail to satisfy any other continued listing requirements of Nasdaq, Nasdaq may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
Additionally, under the terms of the Purchase Agreement, it is a condition precedent to commencing any sales to Keystone, or purchases by Keystone, that trading in our common stock is not suspended or restricted by Nasdaq. Accordingly, if our common stock is delisted by Nasdaq for failure to comply with the Minimum Equity Rule, we may not have the right to sell to Keystone, and Keystone may not be obligated to purchase, shares of our common stock under the Purchase Agreement. If we are not able to require Keystone to purchase shares of our common stock under the Purchase Agreement, we may need to raise additional funding. We do not know whether additional funding will be available on commercially acceptable terms, or at all. If adequate funds are not available or are not available on commercially acceptable terms, we may need to downsize, curtail or halt our operations altogether.
If we raise additional funds by issuing equity securities, such financing will result in further dilution to our stockholders. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common stock, and the terms of the debt securities issued could impose significant restrictions on our operations.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning:
| ● | projected operating or financial results, including anticipated cash flows used in operations; |
| | | |
| ● | expectations regarding capital expenditures, research and development expenses and other payments; |
| | | |
| ● | our beliefs and assumptions relating to our liquidity position, including our ability to obtain additional financing; |
| | | |
| ● | our beliefs, assumptions and expectations about the regulatory approval for our technology including, but not limited to our ability to obtain regulatory approval in a timely manner or at all; |
| | | |
| ● | our ability to continue as a going concern; |
| | | |
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| | | |
| ● | our ability to employ skilled and qualified workers; |
| | | |
| ● | the loss of key management personnel upon whom we depend; |
| | | |
| ● | the actual and perceived effectiveness of our technology, and how the technology compares to competitive technologies; |
| | | |
| ● | the rate and degree of market acceptance and clinical utility of our technology; |
| | | |
| ● | the strength of our intellectual property protection, and our success in avoiding infringement of the intellectual property rights of others; |
| | | |
| ● | regulations affecting the health care industry; |
| | | |
| ● | our ability to effectively compete with a growing number of competitors in the industry; |
| | | |
| ● | the ability of our third-party payors to cover and reimburse us for our services and third-party suppliers to adequately meet our resource needs; |
| | | |
| ● | our ability to manage potential litigation claims related to product liability and potential infringement claims; |
| | | |
| ● | our continued listing with Nasdaq; |
| | | |
| ● | adverse developments in our research and development activities; |
| | | |
| ● | projected operating or financial results, including anticipated cash flows used in operations; and |
| | | |
| ● | other risks and uncertainties, including those described or incorporated by reference under the caption “Risk Factors” in this prospectus. |
We have based these forward-looking statements largely on our current expectations, estimates, forecasts, and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, and financial needs. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we cannot guarantee that the future results, levels of activity, performance, or events and circumstances reflected in the forward-looking statements will be achieved or occur at all. You should refer to the section entitled “Risk Factors” in this prospectus and the risk factors set forth in the documents incorporated by reference in this prospectus for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
You should read this prospectus and the documents incorporated by reference in this prospectus completely and with the understanding that our actual future results, performance or achievements may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus by these cautionary statements.
THE COMMITTED EQUITY FINANCING
Overview
In November 2024, we entered into the Purchase Agreement with Keystone. Sales of our common stock to Keystone under the Purchase Agreement, if any, and the timing of any sales, will be determined by us from time to time in our sole discretion and will depend on a variety of factors, including, among other things, market conditions, the trading price of our common stock and determinations by us regarding the use of proceeds from any sale of such common stock. The net proceeds from any sales under the Committed Equity Financing will depend on the timing of, and prices at which, our sales of shares of common stock to Keystone. To the extent we sell shares under the Purchase Agreement, we have agreed to use 25% of the net cash proceeds from such sales, or the Redemption Funds, to redeem on a pro rata basis the Redeemable Preferred Stock, at a price equal to 120% of the “Conversion Amount” (as set forth in the certificate of designations for the applicable series of Preferred Stock) being redeemed (provided, that any holder of Redeemable Preferred Stock shall have the right to allocate all, or any part, of the Redemption Funds otherwise payable to such holder to any one or more series of Redeemable Preferred Stock). We currently plan to use the remaining net proceeds for working capital and other general corporate purposes.
In accordance with our obligations under the Purchase Agreement and the Registration Rights Agreement, dated as of November 12, 2024, between us and Keystone, or the Registration Rights Agreement, pursuant to which we agreed to provide Keystone with customary registration rights related to the shares issued under the Purchase Agreement, we have filed a registration statement of which this prospectus forms a part in order to register the resale of up to: (i) 164,000 shares of common stock, or the Initial Commitment Shares, issuable to Keystone under the Purchase Agreement on the date on which the registration statement of which this prospectus is apart is declared effective by the Securities and Exchange Commission, or the SEC, and (ii) $1 million of shares of common stock (1,666,667 shares of common stock assuming a price per share of $0.60), or the Back-End Commitment Shares (and together with the Initial Commitment Shares, the Commitment Shares), issuable to Keystone under the Purchase Agreement on the trading day following stockholder approval of our issuance of more than 402,438 shares of common stock to Keystone at an average per share price lower than the Minimum Price (as defined below) and (iii) up to $60 million of shares of common stock (or up to the 33,176,358 shares of common stock remaining of the common stock registered for resale pursuant to this prospectus after issuance of the Commitment Shares), or the Purchase Shares, that we may elect, in our sole discretion, to issue and sell to Keystone, from time to time from after the date the registration statement that includes this prospectus is declared effective by the SEC and after satisfaction of other conditions in the Purchase Agreement, or the Commencement Date, and subject to applicable stock exchange rules.
Under the applicable rules of The Nasdaq Stock Market LLC, or Nasdaq, in no event may we issue to the Selling Stockholder shares of our common stock representing more than 19.99% of the total number of shares of common stock outstanding as of the date of the Purchase Agreement (402,438 shares of common stock), or the Exchange Cap, unless (i) we obtain the approval of the issuance of such shares by our stockholders in accordance with the applicable stock exchange rules or (ii) sales of common stock are made at an average price, or the Minimum Price, equal to or in excess of the lower of (A) the closing prices of our common stock on Nasdaq immediately preceding the delivery by us to the Selling Stockholder of the applicable notice of our election to sell our common stock to Selling Stockholder under the Purchase Agreement, or the Sale Notices, (plus an incremental amount to take into account the Commitment Shares) and (B) the average of the closing prices of the common stock for the five business days immediately preceding the delivery of each Sale Notices (plus an incremental amount to take into account the Commitment Shares), such that the sales of such common stock to the Selling Stockholder would not count toward such limit because they are “at market” under applicable stock exchange rules.
In addition, Keystone is not obligated to buy any common stock under the Purchase Agreement if such shares, when aggregated with all other common stock then beneficially owned by Keystone and its respective affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 promulgated thereunder), would result in Keystone beneficially owning common stock in excess of 4.99% of the then-outstanding shares of common stock, or the Beneficial Ownership Limitation.
The Purchase Agreement and Registration Rights Agreement contain customary registration rights, representations, warranties, conditions and indemnification obligations by each party. The representations, warranties and covenants contained in such agreements were made only for purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and are subject to certain important limitations.
Purchase Agreement
Pursuant to the Purchase Agreement, Keystone shall purchase from us, if we determine to sell, in our sole discretion, up to the lesser of (i) the Commitment Amount and (ii) the Exchange Cap, upon the terms and subject to the conditions and limitations set forth in the Purchase Agreement; provided, however, that such limitations will not apply if we obtain Stockholder Approval and, accordingly, we have registered 35,007,025 shares for issuance under the Purchase Agreement and resale pursuant to this prospectus, assuming that such Stockholder Approval is obtained and that $1 million of Back End Commitment Shares are issued based on an assumed price per share of $0.60 and including 164,000 Initial Commitment Shares issuable upon effectiveness of the registration statement of which this prospectus forms a part, and up to the lesser of 33,176,358 or $60 million of Purchase Shares sold at prices based on then-market stock prices of our common stock on Nasdaq. In no event will any shares be issued or sold to the Selling Stockholder if we do not have a sufficient number of shares of our common stock authorized under our Certificate of Incorporation, as amended from time to time, which are not reserved for other purposes. The shares of our common stock that may be issued under the Purchase Agreement may be sold by us to Keystone at our discretion from time to time from the Commencement Date until the earliest to occur of (i) the first day of the month next following the 36-month anniversary of the Commencement Date, (ii) the date on which Keystone shall have purchased the Commitment Amount, (iii) the date on which, pursuant to or within the meaning of any bankruptcy law, we commence a voluntary case or any person commences a proceeding against us, and (iv) the date on which, pursuant to or within the meaning of any bankruptcy law, we make a general assignment for the benefit of our creditors.
Purchases of Shares of our Common Stock Under the Purchase Agreement
During the term described above, on any business day on which the closing sale price of the common stock is equal to or greater than $0.25, we will have the right, but not the obligation, from time to time at our sole discretion, to direct Keystone, by delivery of an irrevocable written notice, or a Fixed Purchase Notice, to purchase a number of shares of our common stock, or the Fixed Purchase, up to the lesser of 100,000 shares of common stock or $25,000, or the Fixed Purchase Maximum Amount, at a purchase price equal to the lesser of 92.5% of (i) the daily VWAP (as defined below) of the common stock for the five trading days immediately preceding the date of the delivery of the Fixed Purchase Notice, or the Fixed Purchase Date, and (ii) the closing price of a share of common stock on the first full trading day immediately following the applicable Fixed Purchase Date, or the Fixed Purchase Price.
In addition, at any time from and after the Commencement Date, on any business day on which the closing sale price of the common stock is equal to or greater than $0.25 and such business day is also a Fixed Purchase Date for a Fixed Purchase of an amount of shares of common stock not less than the applicable Fixed Purchase Maximum Amount, we may also direct Keystone, by delivery of an irrevocable written notice, or a VWAP Purchase Notice, to purchase, on the immediately following business day, or the VWAP Purchase Date, an additional number of shares of common stock, or a VWAP Purchase, in an amount equal to the lesser of (i) 300% of the number of shares of common stock directed by us to be purchased by Keystone for the applicable Fixed Purchase and (ii) 30% of the trading volume in our common stock on Nasdaq during the applicable VWAP Purchase Period (as defined in the Purchase Agreement) on the applicable VWAP Purchase Date, or the VWAP Purchase, at a purchase price, or the VWAP Purchase Price, equal to the lesser of 92.5% of (i) the closing sale price of the common stock on the applicable VWAP Purchase Date and (ii) the VWAP during the applicable VWAP Purchase Period.
At any time from and after the Commencement Date, on any business day that is also the VWAP Purchase Date for a VWAP Purchase, we may also direct Keystone, by delivery of an irrevocable written notice, or an Additional VWAP Purchase Notice and, together with a Fixed Purchase Notice and a VWAP Purchase Notice, a Purchase Notice, to purchase, on the same business day, or the Additional VWAP Purchase Date and, together with a Fixed Purchase Date and a VWAP Purchase Date, the Purchase Dates, an additional number of shares of common stock in an amount equal to the lesser of (i) 300% of the number of shares of common stock directed by us to be purchased by Keystone for the corresponding Fixed Purchase and (ii) 30% of the trading volume in our common stock on Nasdaq during the applicable Additional VWAP Purchase Period (as defined in the Purchase Agreement) on the applicable VWAP Purchase Date, or an Additional VWAP Purchase, and together with a Fixed Purchase and a VWAP Purchase, the Purchases, at a purchase price equal to the lesser of 92.5% of (i) the closing sale price of the common stock on the applicable Additional VWAP Purchase Date and (ii) the VWAP during the Additional VWAP Purchase Period (as defined in the Purchase Agreement).
For purposes of the Purchase Agreement, “VWAP” is, for the common stock for a specified period, the dollar volume-weighted average price for the common stock on Nasdaq, for such period, as reported by Bloomberg Financial LP through its “AQR” function. All such determinations shall be appropriately adjusted for any reorganization, non-cash dividend, stock split, reverse stock split, stock combination, recapitalization or other similar transaction during such period.
Registered Common Stock and Fees
As consideration for its irrevocable commitment to purchase our common stock under the Purchase Agreement, 164,000 Initial Commitment Shares are issuable to Keystone upon the SEC’s declaration of effectiveness of the registration statement of which this prospectus forms a part. We have also agreed to issue $1 million of Back End Commitment Shares issuable upon receipt of Stockholder Approval.
We have also paid to Keystone $25,000 in cash as reimbursement for the reasonable, out-of-pocket expenses incurred by Keystone, including the legal fees and disbursements of Keystone’ legal counsel, in connection with its due diligence investigation of our company and in connection with the preparation, negotiation and execution of the Purchase Agreement.
Conditions Precedent to Commencement
Our right to commence delivering Purchase Notices under the Purchase Agreement and Keystone’s obligation to accept such Purchase Notices, are subject to the initial satisfaction, at the Commencement Date, of the conditions precedent thereto set forth in the Purchase Agreement, which conditions include, among others, the following:
| ● | the accuracy in all material respects of our representations and warranties included in the Purchase Agreement; |
| ● | us having performed, satisfied and complied in all material respects with all covenants, agreements and conditions required by the Purchase Agreement and the Registration Rights Agreement to be performed, satisfied or complied with by us; |
| ● | the absence of any material misstatement or omission in the registration statement that includes this prospectus; |
| ● | this prospectus, in final form, and all reports, schedules, registrations, forms, statements, information and other documents required to have been filed by us with the SEC pursuant to the reporting requirements of the Exchange Act having been so filed; |
| ● | the common stock not having been suspended by the SEC, Nasdaq or FINRA and there not having been imposed any suspension of, or restriction on, accepting additional deposits of common stock by The Depository Trust Company; |
| ● | no condition, occurrence, state of facts or event constituting a Material Adverse Effect (as defined in the Purchase Agreement) shall have occurred and be continuing; |
| ● | customary compliance with laws and bankruptcy-related conditions; and |
| ● | the receipt by Keystone of customary legal opinions, as required under the Purchase Agreement. |
Termination of the Purchase Agreement
Unless earlier terminated as provided in the Purchase Agreement, the Purchase Agreement will terminate automatically on the earliest to occur of:
| ● | the first day of the month next following the 36-month anniversary of the Commencement Date; |
| ● | the date on which Keystone shall have purchased $60 million of shares of common stock; |
| ● | the date on which, pursuant to or within the meaning of any bankruptcy law, we commence a voluntary case or any person commences a proceeding against us; and |
| ● | the date on which, pursuant to or within the meaning of any bankruptcy law, we make a general assignment for the benefit of our creditors. |
We have the right to terminate the Purchase Agreement at any time after Commencement Date, at no cost or penalty, upon one trading days’ prior written notice to Keystone, provided that we shall have issued all shares subject to any Purchase Notice to Keystone prior to such termination. We and Keystone may also terminate the Purchase Agreement at any time by mutual written consent. Keystone also has the right to terminate the Purchase Agreement upon 10 trading days’ prior written notice to us, but only upon the occurrence of certain customary events as listed in the Purchase Agreement. No termination of the Purchase Agreement by us or by Keystone will become effective prior to the first trading day immediately following the date on which any pending Purchase has been fully settled in accordance with the terms and conditions of the Purchase Agreement, and will not affect any of our respective rights and obligations under the Purchase Agreement with respect to any pending Purchase, and both we and Keystone have agreed to complete our respective obligations with respect to any such pending Purchase under the Purchase Agreement.
Prohibition of “Dilutive Issuances” During Pending Purchases
Subject to certain exceptions, during any Reference Period (as defined in the Purchase Agreement) with respect to a Purchase, we are limited in our ability to issue any common stock, or any securities convertible into common stock, at an effective price per share of common stock less than the applicable Purchase Price to be sold to Keystone in the applicable Purchase to which such Reference Period relates.
No Short-Selling or Hedging
The Selling Stockholder has agreed that neither it nor any entity managed or controlled by it, will engage in, directly or indirectly, any (i) “short sale” (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of the common stock or (ii) hedging transaction, which, with respect to items (i) and (ii), establishes a net short position with respect to the common stock, during the term of the applicable Purchase Agreement.
Effect of Sales of our Common Stock under the Purchase Agreement on our Stockholders
The common stock being registered for resale in this offering may be issued and sold by us to the Selling Stockholder from time to time at our discretion, pursuant to the terms described above. The resale by the Selling Stockholder of a significant quantity of shares registered for resale in this offering at any given time, or the perception that these sales may occur, could cause the market price of our common stock to decline and to be highly volatile. Sales of our common stock, if any, to Keystone under the Purchase Agreement will be determined by us in our sole discretion, subject to the satisfaction of certain conditions in the Purchase Agreement, and will depend upon market conditions and other factors. We may ultimately decide to sell to Keystone all, some or none of the common stock that may be available for us to sell to Keystone pursuant to the Purchase Agreement. If we elect to sell common stock to Keystone pursuant to the Purchase Agreement, after Keystone has acquired such shares, Keystone may resell all, some or none of such common stock at any time or from time to time in its discretion and at different prices. As a result, investors who purchase common stock from Keystone in this offering at different times will likely pay different prices for those shares of common stock, and so may experience different levels of dilution and in some cases substantial dilution and different outcomes in their investment results. See “Risk Factors—Risks Related to the Committed Equity Financings—Investors who buy shares of common stock from Keystone at different times will likely pay different prices.”
Investors may experience a decline in the value of the common stock they purchase from Keystone in this offering as a result of future sales made by us to Keystone at prices lower than the prices such investors paid for their shares in this offering. In addition, if we sell a substantial number of shares of common stock to Keystone under the Purchase Agreement, or if investors expect that we will do so, the actual sales of common stock or the mere existence of our arrangement with Keystone may make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales.
Because the purchase price per share to be paid by Keystone for the common stock that we may elect to sell to Keystone under the Purchase Agreement, if any, will fluctuate based on the market prices of our common stock at the time we make such election, as of the date of this prospectus, it is not possible for us to predict the number of shares of common stock that we will sell to Keystone under the Purchase Agreement, the actual purchase price per share to be paid by Keystone for those shares of common stock, or the actual gross proceeds to be raised by us from those sales, if any. As of February 5, 2025, there were 3,365,866 shares of common stock outstanding. If all of the 35,007,025 shares of our common stock offered for resale by the Selling Stockholder under this prospectus were issued and outstanding as of February 5, 2025, such shares would represent approximately 91.23% of total number of shares of our common stock outstanding. The actual number of shares of our common stock issuable will vary depending on the then current market price of shares of our common stock sold to the Selling Stockholders in this offering.
The number of shares of common stock ultimately offered for sale by the Selling Stockholders for resale under this prospectus is dependent upon the number of shares of common stock, if any, we ultimately sell to Keystone under the Purchase Agreement. Further, if and when we elect to sell shares of common stock to Keystone pursuant to the Purchase Agreement, after Keystone has acquired such shares, Keystone may resell all, some or none of such shares of common stock at any time or from time to time in its discretion and at different prices.
The issuance of our shares of common stock to the Selling Stockholders pursuant to the Purchase Agreement will not affect the rights or privileges of our existing stockholders, except that the economic and voting interests of each of our existing stockholders will be diluted. Although the number of shares of common stock that our existing stockholders own will not decrease, the shares of common stock owned by our existing stockholders will represent a smaller percentage of our total outstanding shares of common stock after any such issuance.
The following table sets forth the aggregate number of Purchase Shares and Commitment Shares to be issued to Keystone under the Purchase Agreement, up to 35,007,025 of which are registered hereunder at varying purchase prices and the remainder to be registered on an additional registration statement if needed:
| Assumed Purchase Price Per Share(1) | | | Total
Number of Keystone
Purchase
Shares to be Issued | | | Percentage of
Outstanding Common Stock
After Giving Effect to the
Issuance of Keystone
Purchase Shares to Keystone(2) | | | Proceeds from the Sale
of Keystone Purchase
Shares to Keystone | |
| | | | | | | | | | | |
| $ | 0.45 | | | | 133,333,334 | | | | 4.99 | % | | $ | 60,000,000 | |
| $ | 0.50 | | | | 120,000,000 | | | | 4.99 | % | | $ | 60,000,000 | |
| $ | 0.58 | (3) | | | 102,878,454 | | | | 4.99 | % | | $ | 60,000,000 | |
| $ | 0.60 | | | | 100,000,000 | | | | 4.99 | % | | $ | 60,000,000 | |
| $ | 0.70 | | | | 85,714,286 | | | | 4.99 | % | | $ | 60,000,000 | |
| (1) | The purchase prices assume a discount to the market price of our shares, in accordance with the terms of the Purchase Agreement. |
| (2) | The denominator is based on 3,365,866 shares of our common stock outstanding, adjusted to include the issuance of the number of Purchase Shares set forth in the adjacent column which we would have issued to Keystone based on the applicable assumed purchase price per share. However, the rights and obligations of the Company and Keystone under the Purchase Agreement are subject to a beneficial ownership cap that prohibits the issuance to Keystone of shares of common stock to the extent that such issuance would cause Keystone’s beneficial ownership, together with its affiliates’ ownership, to exceed 4.99% of the then outstanding common stock. |
| (3) | Represents daily VWAP for our common stock on February 5, 2025 as reported by Nasdaq, less a 92.5% discount. |
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of shares of our common stock offered by Keystone. We may receive up to $60 million in aggregate gross proceeds from Keystone under the Purchase Agreement in connection with our sales of Purchase Shares to Keystone after the Commencement Date. However, the actual proceeds may be less than this amount depending on the number of shares of our common stock sold and the price at which the shares of our common stock are sold.
We have agreed to use 25% of the net cash proceeds from sales of Purchase Shares to Keystone to redeem on a pro rata basis the Redeemable Preferred Stock. We currently plan to use the remaining net proceeds for working capital and other general corporate purposes. We cannot predict whether the net proceeds invested will yield a favorable return. intend to use any net proceeds that we receive under the Purchase Agreement for working capital and other general corporate purposes. We may also use a portion of the net proceeds from this offering to in-license, acquire or invest in complementary businesses, technologies, products or assets. Although we currently have no agreements, commitments or obligations to do so, we evaluate such opportunities and engage in related discussions with third parties from time to time.
Our expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual expenditures and the extent of our preclinical, clinical and future development activities may vary significantly depending on numerous factors, including the progress of our development efforts, the status of and results from our planned clinical trials, our ability to take advantage of expedited programs or to obtain regulatory approval for product candidates, the timing and costs associated with the manufacture and supply of product candidates for clinical development or commercialization and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending the uses described above, we plan to invest the net proceeds from this offering in short-term, interest-bearing obligations, investment-grade instruments or other securities.
DIVIDEND POLICY
We have not paid any cash dividends on our common stock and we do not anticipate paying any such cash dividends in the foreseeable future and we intend to retain all of our earnings, if any, to finance our growth and operations and to fund the expansion of our business. Payment of any dividends, if any, will be made in the discretion of our board of directors, or Board, after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion.
SELLING STOCKHOLDER
This prospectus relates to the possible resale from time to time by Keystone of any or all of the shares of common stock that may be issued by us to Keystone under the Purchase Agreement. For additional information regarding the issuance of common stock covered by this prospectus, see the section titled “Committed Equity Financing” above. We are registering 35,007,025 shares of common stock pursuant to the provisions of the Registration Rights Agreement in order to permit the selling stockholder to offer the shares for resale from time to time. Except for the transactions contemplated by the Purchase Agreement and the Registration Rights Agreement, the purchase by Keystone of September-October Notes in the principal amount of $630,000, for an aggregate purchase price of $450,000 all of which were repaid on November 14, 2024 and the purchase on November 14, 2024 by Keystone of 1,101 shares of our Series G Preferred Stock for an aggregate purchase price of $880,000, Keystone Capital has not had any material relationship with us within the past three years.
The table below presents information regarding the Selling Stockholder and the shares of common stock that it may offer from time to time under this prospectus. This table is prepared based on information supplied to us by the selling stockholder, and reflects holdings as of February 5, 2025. The number of shares in the column “Maximum Number of Shares of Common Stock to be Offered Pursuant to this Prospectus” represents all of the shares of common stock that the Selling Stockholder may offer under this prospectus. The Selling Stockholder may sell some, all or none of its shares in this offering. We do not know how long the Selling Stockholder will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the Selling Stockholder regarding the sale of any of the shares.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act, and includes shares of common stock with respect to which the selling stockholder has voting and investment power. The percentage of shares of common stock beneficially owned by the selling stockholder prior to the offering shown in the table below is based on an aggregate of 3,365,866 shares of our common stock outstanding on February 5, 2025. Because the purchase price of the common stock issuable under the Purchase Agreement is determined on each Fixed Purchase Date, with respect to a Fixed Purchase, on the applicable VWAP Purchase Date, with respect to a VWAP Purchase, and on the applicable Additional VWAP Purchase Date, with respect to an Additional VWAP Purchase, the number of shares that may actually be sold by the Company to the Keystone Capital Partners under the Purchase Agreement may be fewer than the number of shares being offered by this prospectus. The fourth column assumes the sale of all of the shares offered by the selling stockholder pursuant to this prospectus.
| Name of Selling Stockholder | | Number of Shares of Common Stock Owned Prior to Offering | | | Maximum Number of Shares of Common Stock to be Offered Pursuant to this Prospectus | | | Number of Shares of Common Stock Owned After Offering | |
| | | Number(1) | | | Percent(2) | | | | | | Number(3) | | | Percent(2) | |
| Keystone Capital Partners, LLC(4) | | | 874,625 | | | | 4.99 | % | | | 710,625 | | | | — | | | | — | |
* Represents beneficial ownership of less than 1% of our outstanding common stock.
| | (1) | This number includes (i) the 164,000 shares of common stock we will issue to Keystone as the Initial Commitment Shares in consideration for entering into the Purchase Agreement with us upon effectiveness of the Registration Statement and (ii) 710,625 shares of common stock issuable upon conversion of 1,101 shares of currently convertible Series G Preferred Stock held by Keystone. In accordance with Rule 13d-3(d) under the Exchange Act, we have excluded from the number of shares beneficially owned prior to the offering all of the shares that Keystone Capital may be required to purchase under the Purchase Agreement, because the issuance of such shares is solely at our discretion and is subject to conditions contained in the Purchase Agreement, the satisfaction of which are entirely outside of Keystone’s control, including the registration statement that includes this prospectus becoming and remaining effective. Furthermore, the Fixed Purchases, VWAP Purchase, or Additional VWAP Purchase, as applicable, of common stock are subject to certain agreed upon maximum amount limitations set forth in the Purchase Agreement. |
| | | |
| | (2) | Applicable percentage ownership is based on 3,365,866 shares of our common stock outstanding as of February 5, 2025 plus the shares of common stock to be issued upon conversion of shares of our Series G Preferred Stock held by Keystone and the Initial Commitment Shares. However, the Purchase Agreement prohibits us from issuing and selling any shares of our common stock to Keystone to the extent such shares, when aggregated with all other shares of our common stock then beneficially owned by Keystone, would cause Keystone Capital’s beneficial ownership of our common stock to exceed the 4.99% of our then outstanding common stock. |
| | | |
| | (3) | Assumes the sale of all shares being offered pursuant to this prospectus. |
| | | |
| | (4) | The business address of Keystone Capital Partners, LLC is 139 Fulton Street, Suite 412, New York, NY 10038. Keystone Capital Partners, LLC’s principal business is that of a private investor. Ranz Group, LLC, a Delaware limited liability company, is the managing member of Keystone Capital Partners, LLC and the beneficial owner of 97% of the membership interests in Keystone Capital Partners, LLC. Fredric G. Zaino is the managing member of Ranz Group, LLC and has sole voting control and investment discretion over securities beneficially owned directly by Keystone Capital, LLC and indirectly by Ranz Group, LLC. We have been advised that none of Mr. Zaino, Ranz Group, LLC or Keystone Capital Partners, LLC is a member of the Financial Industry Regulatory Authority, or FINRA, or an independent broker-dealer, or an affiliate or associated person of a FINRA member or independent broker-dealer. The foregoing should not be construed in and of itself as an admission by Mr. Zaino as to beneficial ownership of the securities beneficially owned directly by Keystone Capital Partners, LLC and indirectly by Ranz Group, LLC. |
DESCRIPTION OF CAPITAL STOCK
General
Our authorized capital stock consists of 60,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share, of which the following shares of preferred stock are designated: 4,750 shares are designated as Series C-1 Preferred Shares, and 5,376 shares are designated as Series C-2 Preferred Shares, 17,364 shares are designated as Series D Preferred Shares, 26,618 shares are designated as Series E Preferred Shares, 450 shares are designated as Series F Preferred Shares, and 12,288 shares are designated as Series G Preferred Shares. As of February 5, 2025, there were 3,365,866 shares of common stock issued and outstanding, 4,750 shares of Series C-1 Preferred Stock issued, of which 2,020 were outstanding and 2,730 had been converted into shares of common stock, 1,276 shares of Series C-2 Preferred Stock issued, of which 876 were outstanding and 400 had been converted into shares of common stock, 17,364 shares of Series D Preferred Stock issued, of which 14,201 were outstanding and 3,163 had been converted into shares of common stock, 24,172 shares of Series E Preferred Stock issued and outstanding, 450 shares of Series F Preferred Stock issued, of which 400 were outstanding and 50 had been converted into shares of common stock and 4,676 shares of Series G Preferred Stock issued and outstanding, and there we no shares of any other series of preferred stock outstanding. No series of preferred stock named above is registered under Section 12(b) of the Exchange Act. Our board of directors may establish the rights and preferences of the undesignated preferred stock from time to time.
Common Stock
Each holder of our common stock is entitled to one vote for each share on all matters to be voted upon by the stockholders and there are no cumulative rights. Subject to any preferential rights of any outstanding preferred stock, holders of our common stock are entitled to receive ratably the dividends, if any, as may be declared from time to time by the board of directors out of legally available funds. If there is a liquidation, dissolution or winding up of our company, holders of our common stock would be entitled to share in our assets remaining after the payment of liabilities and any preferential rights of any outstanding preferred stock.
Holders of our common stock have no preemptive or conversion rights or other subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of our common stock are fully paid and non-assessable. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock which we may designate and issue in the future.
Warrants
We currently have the following outstanding warrants to purchase shares of common stock: Private Placement Warrants, Exchange Warrants, PIPE Warrants, Placement Agent Warrants, Series F Warrants and Series G Warrants, each as described below.
Private Placement Warrants (Issued on August 16, 2022)
Without regard to any limitations on exercise of the Private Placement Warrants, all of the Private Placement Warrants, collectively, are exercisable into 172,149 shares of Common Stock. The Private Placement Warrants have an exercise price of $28.50 per share, subject to customary adjustments, and expire on February 16, 2028.
Stock Splits; Adjustments. The exercise price and share number of the Private Placement Warrants are subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions.
Cashless Exercise. If at the time of exercise of the Private Placement Warrants, there is no effective registration statement registering the shares of the Common Stock underlying the Private Placement Warrants, such Private Placement Warrants may be exercised on a cashless basis pursuant to their terms.
Limitation on Beneficial Ownership. No exercise shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such exercise.
Exchange Warrants (Issued on April 11, 2024)
Without regard to any limitations on exercise of the Exchange Warrants, all of the Exchange Warrants, collectively, are initially exercisable into 2,075,704 shares of Common Stock. The Exchange Warrants have an exercise price of $2.561 per share, subject to customary adjustments, will become exercisable on October 12, 2024 (the “Initial Exercise Date”) and expire on October 12, 2029.
Stock Splits; Adjustments. The exercise price and share number of the Exchange Warrants are subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions Although the Exchange Warrants do not initially have antidilution protection for issuances below the exercise price then in effect in subsequent placements, if the Company obtains the requisite stockholder approval, thereafter the Exchange Warrants shall have full ratchet antidilution protection. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the required holders, lower the fixed exercise price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Cashless Exercise. If at the time of exercise of the Exchange Warrants, there is no effective registration statement registering the shares of the Common Stock underlying the Exchange Warrants, such Exchange Warrants may be exercised on a cashless basis pursuant to their terms.
Limitation on Beneficial Ownership. No exercise shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such exercise.
PIPE Warrants (Issued on April 11, 2024)
Without regard to any limitations on exercise of the PIPE Warrants, all of the PIPE Warrants, collectively, are initially exercisable into 498,243 shares of Common Stock. The PIPE Warrants have an exercise price of $2.561 per share, subject to customary adjustments, will become exercisable on October 12, 2024, and expire on October 12, 2029.
Stock Splits; Adjustments. The exercise price and share number of the PIPE Warrants are subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the PIPE Warrants do not initially have antidilution protection for issuances below the exercise price then in effect in subsequent placements, if the Company obtains the requisite stockholder approval, thereafter the PIPE Warrants shall have full ratchet antidilution protection. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the Required Holders, lower the fixed exercise price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Cashless Exercise. If at the time of exercise of the PIPE Warrants, there is no effective registration statement registering the shares of the Common Stock underlying the PIPE Warrants, such PIPE Warrants may be exercised on a cashless basis pursuant to their terms.
Placement Agent Warrants (Issued on April 11, 2024)
Without regard to any limitations on exercise of the Placement Agent Warrants, all of the Placement Agent Warrants, collectively, are initially exercisable into 49,824 shares of Common Stock. The Placement Agent Warrants have an exercise price of $2.561 per share, subject to customary adjustments, will become exercisable on October 12, 2024, and expire on October 12, 2029.
Stock Splits. The exercise price and share number of the Placement Agent Warrants are subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the Required Holders, lower the fixed exercise price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Cashless Exercise. If at the time of exercise of the Placement Agent Warrants, there is no effective registration statement registering the shares of the Common Stock underlying the Placement Agent Warrants, such Placement Agent Warrants may be exercised on a cashless basis pursuant to their terms.
Series F Warrants (Issued on May 14, 2024)
Without regard to any limitations on exercise of the Series F Warrants, all of the Series F Warrants, collectively, are initially exercisable into 132,315 shares of Common Stock. The Series F Warrants have an exercise price of $3.401 per share, subject to customary adjustments, will become exercisable on the six month and one day anniversary of the issuance date (the “Initial Exercisability Date”) and expire on the fifth (5th) anniversary of the Initial Exercisability Date.
Stock Splits; Adjustments. The exercise price and share number of the Series F Warrants will be subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the Series F Warrants will not initially have antidilution protection for issuances below the exercise price then in effect in subsequent placements, if the Company obtains the requisite stockholder approval, thereafter the Series F Warrants shall have full ratchet antidilution protection. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the Required Holders, lower the fixed exercise price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Cashless Exercise. If at the time of exercise of the Series F Warrants, there is no effective registration statement registering the shares of the Common Stock underlying the Series F Warrants, such Series F Warrants may be exercised on a cashless basis pursuant to their terms.
Limitation on Beneficial Ownership. No exercise shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such exercise.
Series G Warrants (Issued on November 14, 2024)
Without regard to any limitations on exercise of the Series G Warrants, all of the Series G Warrants, collectively, are initially exercisable into 2,977,711 shares of common stock. The Series G Warrants have an exercise price of $1.44 per share, subject to customary adjustments, will become exercisable on the six month and one day anniversary of the issuance date (the “Series G Initial Exercisability Date”) and expire on the fifth (5th) anniversary of the Series G Initial Exercisability Date.
Stock Splits; Adjustments. The exercise price and share number of the Series G Warrants will be subject to proportional adjustments upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the Series G Warrants will not initially have antidilution protection for issuances below the exercise price then in effect in subsequent placements, if we obtain the requisite stockholder approval to issue in excess of the 402,438 shares of common stock upon exercise of the Series G Warrants and conversion of the Series G Preferred Stock (the “Series G Stockholder Approval”), thereafter the Series G Warrants shall have full ratchet antidilution protection. Subject to the rules and regulations of Nasdaq, we may, at any time, with the written consent of the required holders, lower the fixed exercise price to any amount and for any period of time deemed appropriate by the our board of directors.
Adjustments Following Applicable Date. On the 10th, 90th and 100th calendar day after the Series G Applicable Date (as defined below), the exercise price of the Series G Warrants shall adjust (downward only) to the greater of (x) $0.24 (the “Floor Price”) and (y) 80% of the lower of (I) the closing price of the common stock as of the trading day immediately prior to such adjustment date and (II) the 5-day VWAP of the common stock immediately prior to such adjustment date.
The “Series G Applicable Date” is the later of (i) the date on which we obtain the Series G Stockholder Approval or (ii) the earlier of (A) the date on which a registration statement registering the resale of shares of commons stock issuable upon exercise of the Series G Warrants and upon conversion of the Series G Preferred Stock is declared effective by the SEC or (B) the date on which the Series G Preferred Stock may be sold without restriction Rule 144 of the Securities Act (in each case without regard to limitations on beneficial ownership contained in the certificate of designations of the Series G Preferred Stock or the Series G Warrants)
Cashless Exercise. If at the time of exercise of the Series G Warrants, there is no effective registration statement registering the shares of the common stock underlying the Series G Warrants, such Series G Warrants may be exercised on a cashless basis pursuant to their terms.
Limitation on Beneficial Ownership. No exercise shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of common stock immediately after giving effect to such exercise.
Registration Rights Agreement. The holders of Series G Stock were also granted certain customary registration rights in connection with respect to the shares of common stock underlying the Series G Warrants, pursuant to a registration rights agreement dated as of November 12, 2024.
Preferred Stock
We currently have the following outstanding series of preferred stock: Series C-1 Convertible Preferred Stock, Series C-2 Convertible Preferred Stock, Series D Convertible Preferred Stock, Series E Convertible Preferred Stock, Series F Convertible Preferred Stock and Series G Convertible Preferred Stock.
Series C Preferred Stock and Series F Preferred Stock
The following is a description of the principal terms of the Series C Preferred Stock, which are set forth in a Certificate of Designation of Rights and Preferences of the Series C-1 Convertible Preferred Stock (the “Series C-1 Certificate of Designations”) and a Certificate of Designation of Rights and Preferences of the Series C-2 Convertible Preferred Stock (the “Series C-2 Certificate of Designations” and together with the Series C-1 Certificate of Designations, the “Series C Certificates of Designations”), and the Series F Preferred Stock, which are set forth in a Certificate of Designation of Rights and Preferences of the Series Convertible Preferred Stock (the “Series F Certificate of Designations” and together with the Series C Certificates of Designations, the “Series C and F Certificates of Designations”). The rights and preferences of the Series C-1 Preferred Stock and the Series C-2 Convertible Stock are identical in all material respects; however, the Series C-1 Convertible Preferred Stock was issued in exchange for Series B Preferred Stock without the payment of any additional consideration and, for the purpose of Rule 144 of the Securities Act of 1933, as amended (the “Act”), ownership of the Series C-1 Preferred Stock shall tack back to December 20, 2023. The rights and preferences of the Series C Certificates of Designations and the Series F Certificates of Designations are identical in all material respects, except with respect to the Conversion Prices described below under Conversion at Option of Holder and the floor price described below under Alternate Conversion Upon a Triggering Event.
Authorized; Stated Value. Pursuant to the Series C-1 Certificate of Designations, the Company authorized 4,750 shares of Series C-1 Preferred Stock. Pursuant to the Series C-2 Certificate of Designations, the Company authorized 5,376 shares of Series C-2 Preferred Stock. Pursuant to the Series F Certificate of Designations, the Company authorized 450 shares of Series F Preferred Stock. Each share of Series C Preferred Stock and the Series F Preferred Stock initially has a stated value of $1,000 (subject to increase upon any capitalization of dividends – See Dividends below). The current stated value of each outstanding share of Series C Preferred Stock and Series F Preferred Stock is $1,074.26 and $1,064.63, respectively.
Ranking. The Series C and F Preferred Stock, with respect to the payment of dividends, distributions and payments upon the liquidation, dissolution and winding up of the Company, ranks senior to all capital stock of the Company unless the Required Holders (as defined in the April 10 Securities Purchase Agreement and the May Securities Purchase Agreement, as applicable) consent to the creation of other capital stock of the Company that is senior or equal in rank to the Series C and F Preferred Stock.
Liquidation Preference. In the event of a Liquidation Event, as defined in the Series C and F Certificates of Designations, the holders thereof shall be entitled to receive payment in an amount per share equal to the greater of (A) 110% of the sum of the stated value of the share plus any amount owed to the holder by the Company in connection with the share, including all declared and unpaid dividends thereon, on the date of such payment and (B) the amount per share such holders would receive if such shares had been converted into Common Stock immediately prior to the date of such payment; provided, however that if the funds available for such payment to the holders of Series C and F Preferred Stock, and any other capital stock of the Company ranking on par with them for liquidation purposes are insufficient, all such holders shall be paid proportionally to their holdings out of available funds.
Dividends. Dividends on the Series C and F Preferred Stock equal to 10% per annum (subject to adjustment) will begin to accrue upon issuance and, subject to the satisfaction of certain customary equity conditions, will be payable in shares of Common Stock, provided, however, that the Company may elect to capitalize dividends in lieu of issuing shares of Common Stock by increasing the stated value of each applicable share of Series C Preferred Stock. If the Company fails to properly satisfy such equity conditions, such dividends will be capitalized for each holder of Series C Preferred Stock (unless such holder waives such failure in order to receive shares of Common Stock as payment for such dividend). Notwithstanding the foregoing, unless the Company obtains the Stockholder Approval (see “Stockholder Approval” below), all dividends shall be capitalized dividends.
Conversion Rights
Conversion at Option of Holder. Each holder of Series C and F Preferred Stock may convert all, or any part, of their outstanding Series C Preferred Stock or Series F Preferred Stock, at any time at such holder’s option, into shares of Common Stock (which converted shares of Common Stock are referred to as “Conversion Shares” herein) based on the fixed “Conversion Price” of $2.561, with respect to the Series C Preferred Stock, and $3.401, with respect to the Series F Preferred Stock.
Adjustments to Conversion Price. The Conversion Price is subject to proportional adjustment upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the Series C and F Preferred Stock does not initially have antidilution protection for issuances below the conversion price then in effect in subsequent placements, if the Company obtains the Stockholder Approval (see “Stockholder Approval” below), thereafter the Series C and F Preferred Stock shall have full ratchet antidilution protection. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the Required Holders, lower the fixed conversion price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Mandatory Conversion. If the closing price of the Common Stock on the principal trading market, if any, in which the shares of Common Stock then trade (the “Principal Market”), equals at least 300% of the Conversion Price for twenty (20 consecutive trading days and no Equity Conditions Failure exists, the Company may require each holder of Series C and F Preferred Stock, on a pro rata basis among all such holders, to convert all, or any number, of the shares of Series C and F Preferred Stock based on the then-current Conversion Price.
Alternate Conversion Upon a Triggering Event. Solely if the Company has obtained the Stockholder Approval (see “Stockholder Approval” below), following the occurrence and during the continuance of a Triggering Event (as defined in the Series C Certificates of Designations), each holder may alternatively elect to convert the Series C and F Preferred Stock at the “Alternate Conversion Price” equal to the lesser of (A) the Conversion Price, and (B) the greater of (x) the floor price of $0.5122, with respect to the Series C Preferred Stock, or $0.7282, with respect to the Series F Preferred Stock, and (y) 80% of the volume weighted average price of the Common Stock during the 5 consecutive trading days immediately prior to such conversion.
Limitation on Beneficial Ownership. No conversion shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such conversion.
Company Redemption. At any time the Company shall have the right to redeem in cash all, but not less than all, the shares of Series C and F Preferred Stock then outstanding at the greater of (x) 110% of the amount of shares being redeemed, and (y) the equity value of the Common Stock underlying the Series C Preferred Stock. The equity value of the Common Stock underlying the Series C and F Preferred Stock is calculated using the greatest closing sale price of the Common Stock on any trading day immediately preceding the date the Company notifies the holders of its election to redeem and the date the Company makes the entire payment required.
Purchase Rights. If at any time the Company grants, issues or sells any options, convertible securities, or rights to purchase stock, warrants, securities or other property pro rata to all or substantially all of the record holders of any class of Common Stock (the “Purchase Rights”), then each holder of Series C and F Preferred Stock will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which such holder could have acquired if such holder had held the number of shares of Common Stock acquirable upon complete conversion of all the Series C and F Preferred Stock held by such holder immediately prior to the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights; subject to certain limitations on beneficial ownership.
Change of Control Exchange. Upon a change of control of the Company, each holder may require us to exchange the holder’s shares of Series C and F Preferred Stock for consideration equal to the change of Control Election Price (as defined in the Series C Certificate of Designations), to be satisfied at the Company’s election in either (x) cash or (y) rights convertible into such securities or other assets to which such holder would have been entitled with respect to such shares of Common Stock had such shares of Common Stock been held by such holder upon consummation of such corporate event.
Fundamental Transactions. The Series C and F Certificates of Designations prohibit us from entering specified fundamental transactions (including, without limitation, mergers, business combinations and similar transactions) unless the Company (or its successor) assumes in writing all of the Company’s obligations under the Series C and F Certificates of Designations and the related transaction documents.
Voting Rights. The holders of the Series C and F Preferred Stock have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as provided in the Series C and F Certificates of Designations (or as otherwise required by applicable law).
Covenants. The Series C and F Certificates of Designations contain a variety of obligations on the part of the Company not to engage in specified activities, which are typical for transactions of this type. In particular, the Company will not, and will cause its subsidiaries to not, redeem, repurchase or declare any dividend or distribution on any of its capital stock (other than as required under the Series C and F Certificates of Designations). In addition, the Company will not issue any preferred stock or issue any other securities that would cause a breach or default under the Series C and F Certificates of Designations or the Series C and F Warrants.
Reservation Requirements. So long as any Series C and F Preferred Stock remains outstanding, the Company shall at all times reserve at least 200% of the number of shares of Common Stock as shall from time to time be necessary to effect the conversion of all Series C and F Preferred Stock then outstanding.
Series D Preferred Stock and Series E Preferred Stock
The following is a description of the principal terms of the Series D Preferred Stock and the Series E Preferred Stock. The rights and preferences of the Series D Preferred Stock and the Series E Convertible Stock are identical in all material respects, except that only the holders of Series D Preferred Stock are entitled to an exchange right, described below under Exchange Right.
Authorized; Stated Value. Pursuant to the Series D Certificate of Designations, the Company authorized 17,364 shares of Series D Preferred Stock and pursuant to the Series E Certificate of Designations, the Company authorized 26,618 shares of Series E Preferred Stock. Each share of Series D Preferred Stock and Series E Preferred Stock initially has a stated value of $1,000 (subject to increase upon any capitalization of dividends – See “Series D Preferred Stock and Series E Preferred Stock - Dividends” below). The current stated value of each outstanding share of Series D Preferred Stock and Series E Preferred Stock is $1,064.05.
Ranking. The Series D and E Preferred Stock, with respect to the payment of dividends, distributions and payments upon the liquidation, dissolution and winding up of the Company, ranks senior to all capital stock of the Company unless the Required Holders (as defined in the relevant issuance agreement) consent to the creation of other capital stock of the Company that is senior or equal in rank to the Series D and E Preferred Stock.
Liquidation Preference. In the event of a Liquidation Event, as defined in the Series D and E Certificates of Designations, the holders thereof shall be entitled to receive payment in an amount per share equal to the greater of (A) 110% of the sum of the stated value of the share plus any amount owed to the holder by the Company in connection with the share, including all declared and unpaid dividends thereon, on the date of such payment and (B) the amount per share such holders would receive if such shares had been converted into Common Stock immediately prior to the date of such payment; provided, however that if the funds available for such payment to the holders of Series D Preferred Stock and Series E Preferred Stock, and any other capital stock of the Company ranking on par with them for liquidation purposes are insufficient, all such holders shall be paid proportionally to their holdings out of available funds.
Dividends. Dividends on the Series D Preferred Stock and Series E Preferred Stock equal to 10% per annum (subject to adjustment) will begin to accrue upon issuance and, subject to the satisfaction of certain customary equity conditions, will be payable in shares of Common Stock, provided, however, that the Company may elect to capitalize dividends in lieu of issuing shares of Common Stock by increasing the stated value of each applicable share of Series D Preferred Stock and Series E Preferred Stock. If the Company fails to properly satisfy such equity conditions, such dividends will be capitalized for each holder of Series D Preferred Stock and the Series E Preferred Stock (unless such holder waives such failure in order to receive shares of Common Stock as payment for such dividend). Notwithstanding the foregoing, unless the Company obtains the Stockholder Approval (see “Stockholder Approval” below), all dividends shall be capitalized dividends.
Conversion Rights
Conversion at Option of Holder. Each holder of Series D Preferred Stock and Series E Preferred Stock may convert all, or any part, of their outstanding Series D Preferred Stock or and Series E Preferred Stock, at any time at such holder’s option, into shares of Common Stock (which converted shares of Common Stock are referred to as “Conversion Shares” herein) based on the fixed “Conversion Price” of $3.641.
Adjustments to Conversion Price. The Conversion Price is subject to proportional adjustment upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the Series D Preferred Stock and Series E Preferred Stock do not initially have antidilution protection for issuances below the conversion price then in effect in subsequent placements, if the Company obtains the Stockholder Approval (see “Stockholder Approval” below), thereafter the Series D Preferred Stock and the Series E Preferred Stock shall have full ratchet antidilution protection. Subject to the rules and regulations of the Principal Market, the Company may, at any time, with the written consent of the Required Holders, lower the fixed conversion price to any amount and for any period of time deemed appropriate by the Company’s board of directors.
Mandatory Conversion. If the closing price of the Common Stock on the principal trading market, if any, in which the shares of Common Stock then trade (the “Principal Market”), equals at least 300% of the Conversion Price for twenty (20 consecutive trading days and no Equity Conditions Failure exists, the Company may require each holder of Series D Preferred Stock and Series E Preferred Stock, on a pro rata basis among all such holders of each group, to convert all, or any number, of the shares of Series D and E Preferred Stock based on the then-current Conversion Price.
Alternate Conversion Upon a Triggering Event. Solely if the Company has obtained the Stockholder Approval (see “Stockholder Approval” below), following the occurrence and during the continuance of a Triggering Event (as defined in the Series D and E Certificates of Designations), each holder may alternatively elect to convert the Series D and E Preferred Stock at the “Alternate Conversion Price” equal to the lesser of (A) the Conversion Price, and (B) the greater of (x) the floor price of $0.5122, and (y) 80% of the volume weighted average price of the Common Stock during the 5 consecutive trading days immediately prior to such conversion.
Limitation on Beneficial Ownership. No conversion shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such conversion.
Company Redemption. At any time the Company shall have the right to redeem in cash all, but not less than all, the shares of Series D and E Preferred Stock then outstanding at the greater of (x) 110% of the amount of shares being redeemed, and (y) the equity value of the Common Stock underlying the Series D and E Preferred Stock. The equity value of the Common Stock underlying the Series D and E Preferred Stock is calculated using the greatest closing sale price of the Common Stock on any trading day immediately preceding the date the Company notifies the holders of its election to redeem and the date the Company makes the entire payment required.
Purchase Rights. If at any time the Company grants, issues or sells any options, convertible securities, or rights to purchase stock, warrants, securities or other property pro rata to all or substantially all of the record holders of any class of Common Stock (the “Purchase Rights”), then each holder of Series D and E Preferred Stock will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which such holder could have acquired if such holder had held the number of shares of Common Stock acquirable upon complete conversion of all the Series D and E Preferred Stock held by such holder immediately prior to the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights; subject to certain limitations on beneficial ownership.
Change of Control Exchange. Upon a change of control of the Company, each holder may require us to exchange the holder’s shares of Series D and E Preferred Stock for consideration equal to the change of Control Election Price (as defined in the Series D and E Certificate of Designations), to be satisfied at the Company’s election in either (x) cash or (y) rights convertible into such securities or other assets to which such holder would have been entitled with respect to such shares of Common Stock had such shares of Common Stock been held by such holder upon consummation of such corporate event.
Fundamental Transactions. The Series C Certificates of Designations prohibit us from entering specified fundamental transactions (including, without limitation, mergers, business combinations and similar transactions) unless the Company (or its successor) assumes in writing all of the Company’s obligations under the Series D and E Certificate of Designations and the other Transaction Documents.
Voting Rights. The holders of the Series D and E Preferred Stock have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as provided in the Series D and E Certificates of Designations (or as otherwise required by applicable law).
Covenants. The Series D and E Certificates of Designations contain a variety of obligations on the part of the Company not to engage in specified activities, which are typical for transactions of this type. In particular, the Company will not, and will cause its subsidiaries to not, redeem, repurchase or declare any dividend or distribution on any of its capital stock (other than as required under the Series D and E Certificates of Designations). In addition, the Company will not issue any preferred stock or issue any other securities that would cause a breach or default under the Series D and E Certificates of Designations or the Warrants.
Reservation Requirements. So long as any Series D and E Preferred Stock remains outstanding, the Company shall at all times reserve at least 200% of the number of shares of Common Stock as shall from time to time be necessary to effect the conversion of all Series D and E Preferred Stock then outstanding.
Series G Preferred Stock
The following is a description of the principal terms of the Series G Preferred Stock.
Authorized; Stated Value. Pursuant to the Series G Certificate of Designations, the Company authorized 12,288 shares of Series G Preferred Stock and pursuant to the Series G Certificate of Designations. Each share of Series G Preferred Stock initially has a stated value of $1,000 (subject to increase upon any capitalization of dividends). The current stated value of each outstanding share of Series G Preferred is $1,013.33.
Ranking. The Series G Preferred Stock, with respect to the payment of dividends, distributions and payments upon the liquidation, dissolution and winding up of the Company, ranks senior to all capital stock of the Company unless the Required Holders (to be defined in the Series G Securities Purchase Agreement) consent to the creation of other capital stock of the Company that is senior or equal in rank to the Series G Preferred Stock and all shares of preferred stock of the Company outstanding as of November 14, 2024 (the “Series G Initial Issuance Date”).
Liquidation Preference. In the event of a Liquidation Event, as defined in the Series G Certificate of Designations, the holders thereof shall be entitled to receive payment in an amount per share equal to the greater of (A) 120% of the sum of the stated value of the share plus any amount owed to the holder by the Company in connection with the share, including all declared and unpaid dividends thereon, on the date of such payment and (B) the amount per share such holders would receive if such shares had been converted into common stock immediately prior to the date of such payment; provided, however that if the funds available for such payment to the holders of Series G Preferred Stock and any other capital stock of the Company ranking on par with them for liquidation purposes are insufficient, all such holders shall be paid proportionally to their holdings out of available funds.
Dividends. Dividends on the Series G Preferred Stock equal to 10% per annum (subject to adjustment) will begin to accrue upon issuance and, subject to the satisfaction of certain customary equity conditions, will be payable in shares of common stock, provided, however, that the Company may elect to capitalize dividends in lieu of issuing shares of common stock by increasing the stated value of each applicable share of Series G Preferred Stock. If the Company fails to properly satisfy such equity conditions, such dividends will be capitalized for each holder of Series G Preferred Stock (unless such holder waives such failure in order to receive shares of common stock as payment for such dividend).
Conversion Rights
Conversion at Option of Holder. Each holder of Series G Preferred Stock may convert all, or any part, of their outstanding Series G Preferred Stock, at any time at such holder’s option, into shares of common stock based on the fixed conversion price of $1.57 (the “Series G Conversion Price”).
Adjustment to Conversion Price. The Series G Conversion Price is subject to proportional adjustment upon the occurrence of any stock split, stock dividend, stock combination and/or similar transactions. Although the Series G Preferred Stock will not initially have antidilution protection for issuances below the conversion price then in effect in subsequent placements, if the Company obtains the Series G Stockholder Approval, thereafter the Series G Preferred Stock shall have full ratchet antidilution protection. Subject to the rules and regulations of Nasdaq, we may, at any time, with the written consent of the required holders, lower the fixed conversion price to any amount and for any period of time deemed appropriate by our board of directors.
Adjustments Following Applicable Date. On the 10th, 90th and 100th calendar day after the Series G Applicable Date, the Series G Conversion Price shall adjust (downward only) to the greater of (x) Floor Price and (y) 80% of the lower of (I) the closing price of the Common Stock as of the Trading Day immediately prior to such adjustment date and (II) the 5-day volume weighted average price (the “VWAP”) of the Common Stock immediately prior to such adjustment date.
Limitation on Beneficial Ownership. No conversion shall be effected to the extent it would cause a holder to beneficially own in excess of 4.99% or 9.99% (as elected by a holder) of the outstanding shares of Common Stock immediately after giving effect to such conversion.
Company Redemption. At any time, the Company shall have the right to redeem in cash all, or any number, of the shares of Series G Preferred Stock then outstanding at the greater of (x) 120% of the amount of shares being redeemed, and (y) the equity value of the common stock underlying the Series G Preferred Stock. The equity value of the common stock underlying the Series G Preferred Stock is calculated using the greatest closing sale price of the common stock on any trading day immediately preceding the date the Company notifies the holders of its election to redeem and the date the Company makes the entire payment required.
Exchange Right. Holders of Series G Preferred Stock may elect in writing to participate in certain Subsequent Placements (as defined in the Series G Certificate of Designations) at a value of 120% of the Conversion Amount (as defined in the Series G Certificate of Designations) of the shares of Series G Preferred Stock to be exchanged.
Voting Rights. The holders of the Series G Preferred Stock will have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of common stock, except as provided in the Series G Certificate of Designations (or as otherwise required by applicable law).
Certain Anti-Takeover Provisions of Our Certificate of Incorporation, Our Bylaws, and the DGCL
Delaware Law. We are subject to Section 203 of the Delaware General Corporation Law (the “DGCL”), which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| | ● | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| | ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (1) by persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| | | |
| | ● | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines a “business combination” to include the following:
| | ● | any merger or consolidation involving the corporation and the interested stockholder; |
| | | |
| | ● | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| | | |
| | ● | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| | | |
| | ● | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| | | |
| | ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Removal of Directors. Our bylaws provide that any director, or the entire board of directors, may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors.
Stockholder Action by Written Consent; Special Meetings of Stockholders. Our certificate of incorporation and bylaws also provide that all stockholder actions must be effected at a duly called meeting of stockholders and eliminates the right of stockholders to act by written consent without a meeting. Our certificate of incorporation and bylaws provide that a special meeting of stockholders may be called by our board of directors, our chairman of the board, or our chief executive officer and shall be called by the Company upon the request, in accordance with procedures set forth in our bylaws, of the stockholders representing ownership of at least thirty-three and one-third percent (33-1/3%) of the outstanding shares of the Company’s stock entitled to vote.
Requirements for Advance Notification of Stockholder Nominations, Proposals and Amendments. Our bylaws also provide that stockholders seeking to present proposals before a meeting of stockholders to nominate candidates for election as directors or to conduct other business at a meeting of stockholders must provide timely advance notice in writing, and specify requirements as to the form and content of a stockholder’s notice. Our certificate of incorporation and bylaws provide that the stockholders cannot amend many of the provisions described above except by a vote of the holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the then outstanding shares of the capital stock of the Company entitled to vote generally in the election of directors, voting together as a single class.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “BACK”.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equity Stock Transfer.
PLAN OF DISTRIBUTION
The common stock offered by this prospectus are being offered by the selling shareholder, Keystone Capital Partners, LLC. The shares may be sold or distributed from time to time by the selling shareholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of our common stock offered by this prospectus could be effected in one or more of the following methods:
● ordinary brokers’ transactions;
● transactions involving cross or block trades;
● through brokers, dealers, or underwriters who may act solely as agents;
● “at the market” into an existing market for our common stock;
● in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
● in privately negotiated transactions; or
● any combination of the foregoing.
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
Keystone Capital Partners, LLC is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
Keystone has informed us that it intends to use one or more registered broker-dealers to effectuate all sales, if any, of our common stock that it has acquired and may in the future acquire from us pursuant to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price. Each such registered broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. Keystone has informed us that each such broker-dealer will receive commissions from Keystone that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters or agents participating in the distribution of our common stock offered by this prospectus may receive compensation in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as agent, of the shares sold by the selling shareholder through this prospectus. The compensation paid to any such particular broker-dealer by any such purchasers of our common stock sold by the selling shareholder may be less than or in excess of customary commissions. Neither we nor the selling shareholder can presently estimate the amount of compensation that any agent will receive from any purchasers of our common stock sold by the selling shareholder.
We know of no existing arrangements between the selling shareholder or any other shareholder, broker, dealer, underwriter or agent relating to the sale or distribution of our common stock offered by this prospectus.
We may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and when required under the Securities Act, to disclose certain information relating to a particular sale of shares offered by this prospectus by the selling shareholder, including the names of any brokers, dealers, underwriters or agents participating in the distribution of such shares by the selling shareholder, any compensation paid by the selling shareholder to any such brokers, dealers, underwriters or agents, and any other required information.
We will pay the expenses incident to the registration under the Securities Act of the offer and sale of our common stock covered by this prospectus by the selling shareholder. As consideration for its irrevocable commitment to purchase our common stock under the Purchase Agreement, we have agreed to issue to Keystone (1) 164,000 shares of common stock issuable upon the SEC’s declaration of effectiveness of the registration statement of which this prospectus forms a part and (2) $1 million of shares of common stock upon receipt of stockholder approval to issue shares in excess of the Exchange Cap, in accordance with the Purchase Agreement. We have also paid to Keystone $25,000 in cash as reimbursement for the reasonable, out-of-pocket expenses incurred by Keystone, including the legal fees and disbursements of Keystone’s legal counsel, in connection with its due diligence investigation of the Company and in connection with the preparation, negotiation and execution of the Purchase Agreement.
We also have agreed to indemnify Keystone and certain other persons against certain liabilities in connection with the offering of our common stock offered hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Keystone has agreed to indemnify us against liabilities under the Securities Act that may arise from certain written information furnished to us by Keystone specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
We estimate that the total expenses for the offering will be approximately $95,000.
Keystone has represented to us that at no time prior to the date of the Purchase Agreement has Keystone or its agents, representatives or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our common stock or any hedging transaction, which establishes a net short position with respect to our common stock. Keystone has agreed that during the term of the Purchase Agreement, neither Keystone, nor any of its agents, representatives or affiliates will enter into or effect, directly or indirectly, any of the foregoing transactions.
We have advised the selling shareholder that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the selling shareholder, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the securities offered by this prospectus.
This offering will terminate on the date that all of our common stock offered by this prospectus have been sold by the selling shareholder.
Our common stock is currently listed on The Nasdaq Capital Market under the symbol “BACK”.
DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
The names and ages of our executive officers and directors, and their positions with us, are as follows:
| Name | | Age | | Position |
| Faith Zaslavsky | | 50 | | Chief Executive Officer |
| Sheri F. Gardzina, CPA | | 56 | | Chief Financial Officer |
| Peter Beitsch | | 64 | | Director |
| Jeffrey Busch | | 67 | | Director |
| Maurice Evans | | 45 | | Director |
| Michael D. Pruitt | | 64 | | Director |
| Matthew Schwartz | | 51 | | Director |
Faith Zaslavsky joined our company in May 2024 and serves as our Chief Executive Officer. Prior to joining the Company, Ms. Zaslavsky most recently served as Chief Executive Officer since June 2023, and President and Chief Operating Officer since December 2022, of Theralink Technologies, Inc. (OTC: THER). Prior to Theralink, Ms. Zaslavsky served as President of Oncology for Myriad Genetic Laboratories, (NASDAQ: MYGN). Her responsibilities included overseeing all commercial functions which include leading Medical Services, Medical Affairs, National and Enterprise Accounts and Sales and Marketing. She has spent 22 years leading and transforming teams, designing solutions for physicians to support care and advocating for patients facing a journey with cancer. She received a Business Administration degree from Washington State University. Ms. Zaslavsky also serves on the board of directors of the American Society of Breast Surgeons Foundation.
Sheri F. Gardzina, CPA joined our company in November 2017 and serves as our Chief Financial Officer. Prior to joining IM CPA joined our company in November 2017 and serves as our Chief Financial Officer. Prior to joining IMAC, Ms. Gardzina served as the controller or member of the accounting executive team of Smile Direct Club, LLC, a marketer of invisible aligners, from June 2016 to September 2017, Adoration Health, a home health and hospice company, from October 2015 to June 2016, Lattimore, Black, Morgan & Cain, an accounting and consulting firm where she provided temporary chief financial officer services to Peak Health Solutions, from August to September 2015, EB Employee Solutions, LLC, a healthcare self-insurance product developer, from May to December 2014, and Inspiris Inc., a start-up care management company sold to Optum, from November 2003 to May 2014. Ms. Gardzina started her career as an auditor with Ernst & Young, where she worked from October 1994 to August 1997. Ms. Gardzina earned a B.S. degree in business administration and finance from Purdue University and an M.S. in accountancy and M.B.A. from Northeastern University.
Peter Beitsch joined our Board of Directors in June 2024. Since 1994, Dr. Beitsch has been in private medical practice with a focus on melanoma and breast cancer. He is actively involved in breast cancer and melanoma research and has held numerous positions in national surgical societies including the American Society of Breast Surgeons where he served as first Chairman of the Membership Committee 2001-4, Program Director for the 2005 Annual Meeting in Los Angeles, Board of Directors Member from 2006-9 and 2012-15. He was President of the Society 2013-14. In addition, he has served on the Executive Committee of the Society of Surgical Oncology 2008-2010, General Surgical Oncology Committee of the American Board of Surgery 2013-19, and was a National Ultrasound Faculty for the American College of Surgeons. Dr. Beitsch was a co-founder of, and remains involved with, Targeted Medical Education, which has been involved in studies and clinical trials for breast cancer research and which was acquired by Aptitude Health in 2022. Additionally, Dr. Beitsch has been involved with InVitae and is a surgeon with the Dallas Surgical Group. Dr. Beitsch attended the University of Texas Southwestern Medical School in Dallas. He trained in general surgery residency at Parkland Hospital in Dallas from 1986 to 1993, during which he received a 2 year National Cancer Institute fellowship at M.D. Anderson Cancer Center from 1988-90. He completed his training with a surgical oncology fellowship at the John Wayne Cancer Institute in Santa Monica, California.
Dr. Beitsch’s extensive experience in breast cancer research and treatment will assist the Board to guide the Company in connection with its current RPPA technology platform.
Jeffrey Busch joined our Board of Directors in September 2024. Mr. Busch has been the Chairman of the Board of Theralink Technologies, Inc., from which the Company acquired certain assets in May 2024. Mr. Busch is the current Chairman and CEO of Global Medical REIT, a NYSE listed company (NYSE:GMRE) that acquires licensed medical facilities. Mr. Busch has been a Presidential Appointee, entrepreneur and active investor in various asset classes, including medical and pharmaceutical, since 1985. Mr. Busch has had a distinguished career in public service, which includes serving as a Chief of Staff to a United States Congressman and serving in senior positions in two U.S. presidential administrations. Mr. Busch represented the United States before the United Nations in Geneva, Switzerland. Mr. Busch has served as a top advisor to several publicly traded medical companies and has worked in the medical, blood supply and management fields. Mr. Busch also served as President of the Safe Blood International Foundation, where he oversaw the establishment of medical facilities in 35 developing nations, including China. Mr. Busch is a graduate of the New York University Stern School of Business, holds a Master of Public Administration specializing in health care from New York University, and a Doctor of Jurisprudence from Emory University.
Dr. Busch’s extensive experience with the Company’s current RPPA technology platform and the marketplace will assist the Board to guide the Company in connection with its current business plans to expand the use of the RPPA technology into different markets and for different cancers.
Maurice E. (Mo) Evans joined our Board of Directors in October 2020. Mr. Evans. is a business leader, advisor, consultant, investor and speaker to businesses in the sports business vertical. He is the co-founder of ELOS Sports and Entertainment, LLC (“ELOS”), a provider of brand management services to athletes and businesses in the sports and entertainment industry. Mr. Evans has served as the principal of ELOS since 2014. Prior to that, from 2001 to 2012, he was a professional basketball player, playing for the Washington Wizards, Atlanta Hawks, Orlando Magic, Los Angeles Lakers, Detroit Pistons and Sacramento Kings. He also served as Executive Vice President of the NBA Players Association from 2010 to 2013. Mr. Evans received a B.A. degree from the University of Texas at Austin.
Mr. Evans provides more than a decade of experience in leading and managing customer-centric personal service organizations such as the NBA Players Association and ELOS Sports and Entertainment. He also brings the benefit of a long tenure with the Board.
Michael D. Pruitt joined our Board of Directors in October 2020. He founded Avenel Financial Group, a boutique financial services firm concentrating on emerging technology company investments in 1999. In 2001, he formed Avenel Ventures, a technology investment and private venture capital firm. In February 2005, Mr. Pruitt formed Chanticleer Holdings, Inc., then a public holding company (now known as Sonnet BioTherapeutics Holdings, Inc.), and he served as Chairman of the Board of Directors and Chief Executive Officer until April 1, 2020, at which time the restaurant operations of Chanticleer Holdings were spun out into a new public entity, Amergent Hospitality Group, Inc., where Mr. Pruitt has served as its Chairman and Chief Executive Officer to date. Mr. Pruitt also served as a director on the board of Hooters of America, LLC from 2011 to 2019. Mr. Pruitt received a B.A. degree from Costal Carolina University. He currently sits on the Board of Visitors of the E. Craig Wall Sr. College of Business Administration, the Coastal Education Foundation Board, and the Athletic Committee of the Board of Trustees.
Mr. Pruitt’s over 15 years of day-to-day operational leadership and service as a board member at public companies Chanticleer Holdings and Amergent Hospitality Group make him well qualified as a member of the Board. He also brings transactional expertise in mergers and acquisitions and capital markets.
Matthew Schwartz, MD, joined our Board of Directors in June 2024. Dr Schwartz is a Radiation Oncologist with 20 years of experience in the Oncology and Biotechnology industries. 2011 to 2020, Dr. Schwarz held key leadership positions at Comprehensive Cancer Centers of Nevada (“CCCN”), including serving on the Board of Directors and as Chairman of the Marketing Committee. In 2010, Dr. Schwartz co-founded Las Vegas Cyberknife, an institution using advanced, non-invasive targeted radiation treatments for tumors. Currently, he serves as the Chairman of the Board of Managers at Las Vegas Cyberknife, Dr. Schwartz has also served on the Radiation Oncology Leadership Council of the US Oncology Network, the US Oncology Clinical Pathways Committee, and the McKesson Specialty Health Radiation Executive Committee. Dr. Schwartz served on the board of directors of Theralink Technologies, Inc. from 2022 - 2024 and on the board of directors of Avant Diagnostics from 2019 - 2020. Dr. Schwartz completed his training at McGill University, where he was Chief Resident, and Yale-New Haven Hospital.
Dr. Schwartz brings to the Board extensive knowledge and experience with oncology practice and treatments, which knowledge and experience are useful to the Company in connection with its current Reverse Phase Protein Array (RPPA) technology platform.
Director Independence
Our common stock and warrants are listed for trading on The NASDAQ Capital Market. Under Nasdaq rules, independent directors must comprise a majority of a listed company’s board of directors. In addition, Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees must be independent. Under Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that Messrs. Beitsch, Busch, Evans, Pruitt and Schwartz, representing all of our directors, do not have any relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under Nasdaq rules. In making these determinations, our board of directors considered the relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is an executive officer or employee of our company. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Indebtedness of Directors and Executive Officers
None of our directors or executive officers or their respective associates or affiliates is currently indebted to us.
Family Relationships
There are no family relationships among our directors and executive officers.
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth summary compensation information for the following persons: (i) all persons serving as our principal executive officer during the years ended December 31, 2024 and 2023, and (ii) our other most highly compensated executive officers who received compensation during the years ended December 31, 2024 and 2023 of at least $100,000 and who were executive officers on December 31, 2024 and 2023. We refer to these persons as our “named executive officers” in this prospectus. The following table includes all compensation earned by the named executive officers for the respective period, regardless of whether such amounts were actually paid during the period:
| Name and Position | | Years | | | Salary | | | Bonus | | | Stock Awards | | | Option Awards | | | Non- equity Incentive Plan Comp | | | Non- qualified Deferred Comp | | | All Other Comp | | | Total | |
| Faith Zaslavsky, | | | 2024 | | | $ | 262,713 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 262,713 | |
| Chief Executive Officer | | | 2023 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sheri Gardzina, | | | 2024 | | | $ | 227,294 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 227,294 | |
| Chief Financial Officer | | | 2023 | | | | 203,846 | | | | 6,250 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 210,096 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Jeffrey S. Ervin, | | | 2024 | | | $ | 85,947 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 85,947 | |
| Former Chief Executive Officer(1) | | | 2023 | | | $ | 200,000 | | | $ | 12,500 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 212,500 | |
| (1) | Mr. Ervin resigned as chief executive officer of the Company and from the Board of Directors on May 23, 2024. |
Employment Agreements
We entered into an employment agreement effective March 1, 2019 with Jeffrey Ervin. The employment agreement was extended for a term expiring on February 28, 2023. Mr. Ervin resigned as chief executive officer of the Company and from the Board of Directors on May 23, 2024.
Pursuant to the employment agreement, Mr. Ervin agreed to devote substantially all of his business time, attention and ability, to the Company’s business as the Chief Executive Officer. In addition, Mr. Ervin was entitled to receive, at the sole discretion of the Board of Directors, cash bonuses based on meeting and exceeding performance goals of the Company. He was also entitled to participate in the Company’s 2018 Incentive Compensation Plan. The Company agreed to pay or reimburse Mr. Ervin up to $100 per month for the business use of their personal cell phone.
The employment agreement also provided for termination by the Company upon death or disability of Mr. Ervin (defined as three aggregate months of incapacity during any 365-consecutive day period) or upon conviction of a felony crime of moral turpitude or a material breach of his obligations to the Company. In the event the employment agreement were terminated by the Company without cause, Mr. Ervin was entitled to compensation for the balance of the term.
In the event of a change of control of our company, Mr. Ervin could terminate his employment within six months after such event and would have been entitled to continue to be paid pursuant to the terms of the employment agreements.
The employment agreement also contained covenants (a) restricting Mr. Ervin from engaging in any activities competitive with the Company’s business during the terms of his employment agreement and one year thereafter, (b) prohibiting him from disclosure of confidential information regarding the Company at any time and (c) confirming that all intellectual property developed by Mr. Ervin and relating to the Company’s business constitutes the Company’s sole and exclusive property.
Grants of Plan-Based Awards
As of December 31, 2024, the Company had outstanding stock options to purchase 1,306 shares of its common stock which were granted as non-qualified stock options to various employees of the Company. These options vest over a period of four years, with 25% vesting after one year and the remaining 75% vesting in equal monthly installments over the following 36 months, are exercisable for a period of ten years, and enable the holders to purchase shares of the Company’s common stock at the exercise price of award. The per-share fair values of these options range from $35.70 to $121.20 based on Black-Scholes-Merton pricing model.
At December 31, 2024, the Company had 4,000 outstanding restricted stock units (“RSUs”) which were granted to, and deferred by, a Named Executive Officer.
On September 9, 2024, the Company granted to former director, Carey Sucoff, 3,333 RSUs that vest on September 9, 2025 and 10,000 stock options that will vest on such date as the Company is in compliance with Nasdaq’s requirements.
On December 31, 2024, the Company granted 3,333 shares of common stock to each of the members of the Board of Directors.
Outstanding Equity Awards at December 31, 2024
No stock options were granted to any of our named executive officers during the year ended December 31, 2024. As of December 31, 2024 a total of 4,000 RSUs were reserved for issuance upon vesting of RSUs held by named executive officers.
The following table presents the outstanding equity awards held by each of the named executive officers as of the fiscal year ended December 31, 2024, including the value of the stock awards.
| | | | | Option Awards | | Stock Awards | |
| Name | | Grant
Date | | Number of
Securities
Underlying
Unexercised
Options
(#)
Exercisable | | | Number of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | | Number of
Shares or Units
of Stock That
Have Not Vested
(#) | | | Market Value of Shares or Units
That Have Not
Vested
($) | |
| Sheri Gardzina | | 5/21/2019 | | | 1,250 | | | | 0 | | | $ | 121.20 | | | 5/21/2029 | | | 4,000 | (1) | | $ | 5,080 | |
| | (1) | One year vesting and deferred by the Named Executive Officer. |
2018 Incentive Compensation Plan
Under our 2018 Incentive Compensation Plan (the “Plan”), adopted by our board of directors and holders of a majority of our outstanding shares of common stock in May 2018, 66,667 shares (after giving effect to an adjustment approved by the Compensation Committee on April 30, 2024 in light of the 1-for-30 reverse stock split effected in September 2023) of common stock (subject to certain adjustments) were authorized for issuance upon exercise of stock options and grants of other equity awards. On August 30, 2024, with approval of holders of a majority of the Company’s outstanding shares of common stock, the Company amended the Plan to increase the number of shares authorized for issuance under the Plan from 66,667 to 566,667 shares of common stock. The Plan is designed to serve as an incentive for attracting and retaining qualified and motivated employees, officers, directors, consultants and other persons who provide services to us. The compensation committee of our board of directors administers and interprets the Plan and is authorized to grant stock options and other equity awards thereunder to all eligible employees of our company, including non-employee consultants to our company and directors.
The Plan provides for the granting of “incentive stock options” (as defined in Section 422 of the Code), non-statutory stock options, stock appreciation rights, shares of restricted stock, restricted stock units, deferred stock, dividend equivalents, bonus stock and awards in lieu of cash compensation, other stock-based awards and performance awards. Options may be granted under the Plan on such terms and at such prices as determined by the compensation committee of the board, except that the per share exercise price of the stock options cannot be less than the fair market value of our common stock on the date of grant. Each option will be exercisable after the period or periods specified in the stock option agreement, but all stock options must be exercised within ten years from the date of grant. Options granted under the Plan are not transferable other than by will or by the laws of descent and distribution. The compensation committee of the board has the authority to amend or terminate the Plan, provided that no amendment shall be made without stockholder approval if such stockholder approval is necessary to comply with any tax or regulatory requirement. Unless terminated sooner, the Plan will terminate ten years from its effective date.
Equity Compensation Plan Summary
The following table provides information as of December 31, 2024, relating to our equity compensation plan:
| Plan Category | | Number of
Securities to be Issued Upon
Exercise of Outstanding
Equity Grants | | | Weighted-
Average Exercise Price of Outstanding
Options | | | Number of
Securities Remaining Available for
Further Issuance Under Equity
Compensation Plans (Excluding Securities
Reflected in the First
Column) | |
| Equity compensation plan approved by security holders (1) | | | 11,306 | | | $ | 15.93 | | | | 485,044 | |
| Equity compensation plans not approved by security holders | | | - | | | $ | - | | | | - | |
| Total | | | 11,306 | | | $ | 15.93 | | | | 485,044 | |
| (1) | Consists solely of the 2018 Incentive Compensation Plan. |
Director Compensation
We compensate each non-employee director through annual stock awards under our 2018 Incentive Compensation Plan and by paying a cash fee as determined by the Board of Directors. During the year ended December 31, 2024, each of our non-employee directors, Messrs. Beitsch, Busch, Evans, Pruitt, and Schwartz was paid the cash compensation set forth belowand each of them was awarded 3,333 shares of common stock. Carey Sucoff was also awarded 10,000 stock options.
Non-Employee Director Compensation Table
The following table sets forth summary information concerning compensation paid or accrued for services rendered to us in all capacities by the non-employee members of our Board of Directors for the fiscal year ended December 31, 2024.
| Name | | Fees Paid
in Cash
($) | | | Stock
Awards
($) (1) | | | Option
Awards
($) | | | Non-Equity
Incentive
Plan
Compensation
($) | | | Nonqualified
Deferred
Compensation
Earnings
($) | | | All Other
Comp
($) | | | Total
($) | |
| Peter Beitsch | | $ | - | | | $ | 4,233 | | | | - | | | | - | | | | - | | | | - | | | $ | 4,233 | |
| Jeffrey Busch | | $ | - | | | $ | 4,233 | | | | - | | | | - | | | | - | | | | - | | | $ | 4,233 | |
| Maurice Evans | | $ | 28,750 | | | $ | 4,233 | | | | - | | | | - | | | | - | | | | - | | | $ | 32,983 | |
| Michael D. Pruitt | | $ | 28,750 | | | $ | 4,233 | | | | - | | | | - | | | | - | | | | - | | | $ | 32,983 | |
| Matthew Schwartz | | $ | - | | | $ | 4,233 | | | | - | | | | - | | | | - | | | | - | | | $ | 4,233 | |
| Cary W. Sucoff (2) | | $ | 11,250 | | | $ | 5,249 | | | $ | 14,250 | | | | - | | | | - | | | | - | | | $ | 30,749 | |
| Matthew Wallis (3) | | $ | - | | | $ | - | | | | - | | | | - | | | | - | | | | - | | | $ | - | |
| | (1) | Represents full fair value at grant date of RSUs granted to our directors, computed in accordance with FASB ASC Topic 718. |
| | (2) | Mr. Sucoff resigned as a member of the Board of Directors on September 9, 2024. |
| | (3) | Dr. Wallis resigned as a member of the Board of Directors on May 23, 2024. |
RELATED PARTY TRANSACTIONS
Policies and Procedures for Transactions with Related Persons
Our board of directors intends to adopt a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. Related persons include any executive officer, director or a holder of more than 5% of our common stock, including any of their immediate family members and any entity owned or controlled by such persons. Related person transactions refers to any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which (i) we were or are to be a participant, (ii) the amount involved exceeds $120,000, and (iii) a related person had or will have a direct or indirect material interest. Related person transactions include, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness, and employment by us of a related person, in each case subject to certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act.
We expect that the policy will provide that in any related person transaction, our audit committee and board of directors will consider all of the available material facts and circumstances of the transaction, including: the direct and indirect interests of the related persons; in the event the related person is a director (or immediate family member of a director or an entity with which a director is affiliated), the impact that the transaction will have on a director’s independence; the risks, costs and benefits of the transaction to us; and whether any alternative transactions or sources for comparable services or products are available. After considering all such facts and circumstances, our audit committee and board of directors will determine whether approval or ratification of the related person transaction is in our best interests. For example, if our audit committee determines that the proposed terms of a related person transaction are reasonable and at least as favorable as could have been obtained from unrelated third parties, it will recommend to our board of directors that such transaction be approved or ratified. In addition, if a related person transaction will compromise the independence of one of our directors, our audit committee may recommend that our board of directors reject the transaction if it could affect our ability to comply with securities laws and regulations or Nasdaq listing requirements.
Related Party Transactions
There have been no related party transactions or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
Indemnification Agreements
We have entered into an indemnification agreement with each of our directors and executive officers. The indemnification agreements and our certificate of incorporation and bylaws require us to indemnify our directors and executive officers to the fullest extent permitted by Delaware law.
SECURITY OWNERSHIP
The following table sets forth information as of January 27, 2025 regarding the beneficial ownership of our common stock by (i) each person we know to be the beneficial owner of 5% or more of our common stock, (ii) each of our named executive officers, as defined below, (iii) each of our directors, and (iv) all of our current executive officers and directors as a group. Information with respect to beneficial ownership has been furnished by each director and executive officer, as the case may be. There are no beneficial owners of 5% or more of our common stock other than the directors and executive officers included below. The address for all executive officers and directors is c/o IMAC Holdings, Inc., 3401 Mallory Lane, Suite 100, Franklin, Tennessee 37067.
Percentage of beneficial ownership in the table below is calculated based on 2,071,812 shares of common stock outstanding as of January 27, 2025. Beneficial ownership is determined in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and includes shares of our common stock issuable pursuant to the exercise of stock options, warrants or other securities that are immediately exercisable or convertible or exercisable or convertible within 60 days of the Record Date. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them.
| Name of Beneficial Owner | | Shares Beneficially Owned | | | Percentage Beneficially Owned | |
| | | | | | | |
| Faith Zaslavsky | | | - | | | | - | |
| | | | | | | | | |
| Sheri Gardzina | | | 6,195 | (1) | | | * | |
| | | | | | | | | |
| Michael D. Pruitt | | | 12,141 | | | | * | |
| | | | | | | | | |
| Maurice E. Evans | | | 18,071 | | | | * | |
| | | | | | | | | |
| Peter Beitsch | | | 3,333 | | | | * | |
| | | | | | | | | |
| Matthew Schwartz | | | 3,333 | | | | * | |
| | | | | | | | | |
| Jeffrey Busch(2) | | | 570,254 | | | | 9.99 | % |
| | | | | | | | | |
| Jeffrey S. Ervin(3) | | | 12,380 | | | | * | |
| | | | | | | | | |
| Matthew C. Wallis, DC(4) | | | 58,390 | | | | 2.82 | % |
| | | | | | | | | |
| All directors and executive officers as a group (7 persons)(1)(2) | | | 613,327 | | | | 12.26 | % |
| * | Less than 1% of outstanding shares. |
| | |
| (1) | Includes (i) 1,250 shares of common stock issuable upon exercise of currently exercisable stock options, (ii) 4,000 outstanding Restricted Stock Units, (iii) 941 shares and (iv) 4 shares owned by Mrs. Gardzina’s spouse. |
| (2) | Includes (i) 111,160 shares of common stock issuable upon conversion of 265 currently convertible shares of Series C-1 Preferred Stock and (ii) 455,761 shares of common stock issuable upon conversion of 1,641 shares of currently convertible Series E Preferred Stock. However, the Series C-1 Preferred Stock and Series E Preferred Stock are subject to a beneficial ownership cap that prohibits the conversion of the Series C-1 Preferred Stock and/or the Series E Preferred Stock into shares of common stock to the extent that such conversion would cause Mr. Busch’s beneficial ownership, together with his affiliates’ ownership, to exceed 9.99% of the then outstanding common stock. |
| (3) | Mr. Ervin resigned as CEO of the Company and from the Board of Directors on May 23, 2024. |
| (4) | Dr. Wallis resigned as President of the Company on November 24, 2023 and from the Board of Directors on May 23, 2024. |
LEGAL MATTERS
The validity of the securities being offered by this prospectus will be passed upon by Kelley Drye & Warren LLP, New York, New York.
EXPERTS
The consolidated financial statements of IMAC as of December 31, 2023 and for the year ended December 31, 2023 have been audited by Salberg & Company, P.A., an independent registered public accounting firm, and are incorporated by reference in this registration statement in reliance upon such report and given on the authority of such firm as experts in accounting and auditing. The consolidated financial statements of IMAC as of December 31, 2022 and for the year ended December 31, 2022 have been audited by Cherry Bekaert LLP, an independent registered public accounting firm, and are incorporated by reference in this registration statement in reliance upon such report and given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus forms part of a registration statement on Form S-1 that we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement or the documents incorporated by reference herein and therein. For further information with respect to us and the securities that we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement and the documents incorporated by reference herein and therein. You should rely only on the information contained in this prospectus or incorporated by reference herein or therein. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered hereby. We file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy statements and other information regarding issuers that file electronically with the SEC, including IMAC Holdings, Inc. The address of the SEC website is www.sec.gov.
We also maintain a website at https://imacholdings.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED BALANCE SHEET
The Company has prepared the accompanying unaudited pro forma condensed consolidated balance sheet (“Pro Forma Balance Sheet”) in accordance with Article 11 of Regulation S-X. The Pro Forma Balance Sheet has been derived from the Company’s historical consolidated financial statements and reflects certain assumptions and adjustments that management believes are reasonable under the circumstances and given the information available at this time. The following Pro Forma Balance Sheet as of September 30, 2024 is presented as if the transactions contemplated by (i) the issuance in October 2024 of certain September-October 2024 Notes in the aggregate principal amount of $280,000, for an aggregate purchase price of $200,000 (the “October Note Issuance”), (ii) the receipt of aggregate proceeds of $3,740,000 from the sale of 4,676 shares of Series G Preferred Stock and 2,977,711 Series G Warrants in November 2024 (the “Series G Financing”) and (iii) the repayment of all outstanding promissory notes in the aggregate principal amount of $2,240,000 in November 2024 (the “Note Repayment” and, collectively, the “Pro Forma Transactions”) had occurred on September 30, 2024. The Pro Forma Balance Sheet reflects adjustments that, in the opinion of management, are necessary to present fairly the pro forma financial position as of September 30, 2024. The Pro Forma Information is provided for informational purposes only and is not intended to represent what the Company’s financial position or results of operations would have been had the Pro Forma Transactions occurred on September 30, 2024, nor is it indicative of its future financial position or results of operations. The Pro Forma Balance Sheet should be read in conjunction with the Company’s historical consolidated financial statements and accompanying notes.
| | | Historical Results | | | October Note Issuance Pro Forma Adjustments | | | Series G Financing Pro Forma Adjustments | | | Note Repayment Pro Forma Adjustments | | | Pro Forma | |
| ASSETS | | | | | | | | | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | | | | | | | | | |
| Cash | | $ | 195,511 | | | $ | 200,000 | | | $ | 3,592,400 | | | $ | (2,240,000 | ) | | $ | 1,747,911 | |
| Accounts receivable, net | | | 41,700 | | | | | | | | | | | | | | | | 41,700 | |
| Prepaid expenses and other current assets | | | 273,527 | | | | | | | | | | | | | | | | 273,527 | |
| Note receivable, net | | | - | | | | | | | | | | | | | | | | - | |
| Assets of discontinued operations | | | - | | | | | | | | | | | | | | | | - | |
| Total current assets | | | 510,738 | | | | | | | | | | | | | | | | 2,063,138 | |
| | | | | | | | | | | | | | | | | | | | | |
| Property and equipment, net | | | 956,873 | | | | | | | | | | | | | | | | 956,873 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total assets | | $ | 1,467,611 | | | | | | | $ | | | | | | | | | 3,020,011 | |
| | | | | | | | | | | | | | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Current liabilities: | | | | | | | | | | | | | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 2,273,070 | | | | | | | $ | | | | | | | | | 2,273,070 | |
| Dividends payable | | | 1,160,911 | | | | | | | | | | | | | | | | 1,160,911 | |
| Note payable, net | | | 1,521,081 | | | | 200,000 | | | | | | | | (2,240,000 | ) | | | - | |
| Liabilities of discontinued operations | | | 1,311,864 | | | | | | | | | | | | 518,919 | (a) | | | 1,311,864 | |
| Total current liabilities | | | 6,266,926 | | | | | | | | | | | | | | | | 4,745,845 | |
| | | | | | | | | | | | | | | | | | | | | |
| Commitments and Contingencies – Note 12 | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Stockholders’ deficit: | | | | | | | | | | | | | | | | | | | | |
| Preferred stock | | | 46 | | | | | | | | | | | | | | | | 46 | |
| Common stock | | | 1,151 | | | | | | | | | | | | | | | | 1,151 | |
| Additional paid-in capital | | | 55,133,084 | | | | | | | | 3,592,400 | | | | | | | | 58,725,484 | |
| Accumulated deficit | | | (59,933,596 | ) | | | | | | | | | | | (518,919 | ) | | | (60,452,515 | ) |
| Total stockholders’ deficit | | | (4,799,315 | ) | | | | | | | | | | | | | | | (1,725,834 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Total liabilities and stockholders’ deficit | | $ | 1,467,611 | | | | | | | | | | | | | | | $ | 3,020,011 | |
| | (a) | Amortization of OID Interest expense in connections with payment of Notes payable. |
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we filed with the SEC:
| | ● | our Annual Report on Form 10-K/A for the year ended December 31, 2023, as filed with the SEC on May 2, 2024; |
| | | |
| | ● | our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2024, as filed with the SEC on May 15, 2024, for the quarter ended June 30, 2024, as filed with the SEC on December 12, 2024 and for the quarter ended September 30, 2024, as filed with the SEC on January 17, 2025; |
| | | |
| | ● | our Current Reports on Form 8-K as filed with the SEC on February 23, 2024, April 16, 2024, May 1, 2024, May 7, 2024, May 16, 2024, May 24, 2024, June 18, 2024, July 2, 2024, July 8, 2024, July 18, 2024, July 30, 2024, August 23, 2024, August 30, 2024, September 12, 2024, September 13, 2024, September 25, 2024, September 27, 2024, October 23, 2024, November 13, 2024, November 14, 2024, November 22, 2024, November 22, 2024, December 20, 2024 and January 23, 2025 and the Forms 8-K/A filed with the SEC on July 18, 2024, November 13, 2024, November 22, 2024, January 23, 2025 and February 11, 2025; and |
| | | |
| | ● | The description of our Common Stock in our Registration Statement on Form 8-A, filed with the SEC on February 4, 2019, including any amendment or reports filed for the purpose of updating such description. |
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
We also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus but prior to the termination of the offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements on Schedule 14A.
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits that are specifically incorporated by reference into such documents. You should direct any requests for documents to IMAC Holdings, Inc., 3401 Mallory Lane, Suite 100, Franklin, Tennessee 37067, Attn: Chief Financial Officer.
You also may access these filings on our website at https://imacholdings.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus). You may also access these filings at the SEC’s website at www.sec.gov.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.

Up to 35,007,025 Shares of Common Stock
Prospectus
, 2025
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distributions
The following table sets forth the costs and expenses payable by IMAC Holdings, Inc. (the “Company”) in connection with the issuance and distribution of the securities being registered. Except for the Securities and Exchange Commission (the “SEC”) registration fee, all amounts are estimates.
| SEC registration fee | | $ | 5,628 | |
| Legal fees and expenses | | $ | 50,000 | |
| Accounting fees and expenses | | $ | 31,000 | |
| Miscellaneous | | $ | 8,372 | |
| Total | | $ | 95,000 | |
Item 14. Indemnification of Directors and Officers
As permitted by Section 102 of the DGCL, we have adopted provisions in our Amended and Restated Certificate of Incorporation and By-Laws that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
● any breach of the director’s duty of loyalty to us or our stockholders;
● any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
● any act related to unlawful stock repurchases, redemptions or other distributions or payment of dividends; or
● any transaction from which the director derived an improper personal benefit.
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our By-Laws also authorizes us to indemnify any and all persons whom it shall have power to indemnify to the fullest extent permitted under Delaware law.
As permitted by Section 145 of the DGCL, our By-Laws provide that:
● we may indemnify any and all persons whom it shall have power to indemnify to the fullest extent permitted by the DGCL, subject to limited exceptions;
● the rights provided in our By-Laws are not exclusive.
Our Amended and Restated Certificate of Incorporation and our By-Laws provide for the indemnification provisions described above and elsewhere herein. We have entered or will enter into, and intend to continue to enter into, separate indemnification agreements with our directors and officers that may be broader than the specific indemnification provisions contained in the DGCL. These indemnification agreements generally require us, among other things, to indemnify our officers and directors against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also generally require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified. These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and directors for liabilities, including reimbursement of expenses incurred, arising under the Securities Act.
Item 15. Recent Sales of Unregistered Securities
On August 16, 2022, the Company issued and sold to accredited investors Series 1 warrants to purchase 172,149 shares of common stock (the “Private Placement Warrants”) that became exercisable on the date that was six months following the date of issuance of the shares of common stock in the Registered Direct Offering (the “Exercise Date”) and expire on the five year anniversary of the Exercise Date, and Series 2 warrants to purchase 172,149 shares of common stock (the “Series 2 Warrants”) that became exercisable on the Exercise Date and expired on the one year anniversary of the Exercise Date. The Company received gross proceeds of $3.9 million from the issuance and sale of the Private Placement Warrants, Series 2 Warrants and a concurrent registered direct offering of common stock. The Series 1 warrants and Series 2 Warrants were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On July 25, 2023, the Company issued and sold to several institutional and accredited investors, including existing significant investors of Theralink Technologies, Inc. its previously announced merger partner (“Theralink”), and Theralink’s Chairman, an aggregate of 2,500 shares of its Series A-1 convertible preferred stock, stated value $1,000 per share (the “Series A-1 Preferred Stock”), 1,800 shares of its Series A-2 convertible preferred stock, stated value $1,000 per share (the “Series A-2 Preferred Stock” and, together with the Series A-1 Preferred Stock, the “Series A Preferred Stock”), and warrants to purchase up to 2,075,704 shares of common stock, for aggregate gross proceeds of $4.3 million (the “Series A Warrants”). The Series A Preferred Stock and the Series A Warrants were issued under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On December 20, 2023, the Company entered into a letter agreement with several institutional and accredited investors providing for the sale of an additional aggregate $250,000 of convertible preferred stock (the “Private Placement”). Pursuant to the letter agreement, the Company exchanged its Series A-1 Preferred Stock and Series A-2 Preferred stock for a corresponding number of shares of the Company’s newly-created Series B-1 convertible preferred stock, $0.001 par value (the “Series B-1 Preferred Stock”) and the Company’s newly-created Series B-2 convertible preferred stock, $0.001 par value (the “Series B-2 Preferred Stock” and, together with the Series B-1 Preferred Stock, the “Series B Preferred Stock”), respectively. Joseph Gunnar & Co, LLC (“Gunnar”) acted as sole placement agent for the Private Placement. The securities offered and sold in the Private Placement were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On April 11, 2024, the Company exchanged, pursuant to Section 3(a)(9) of the Securities Act, (i) an aggregate of 4,550 shares of its Series B-1 convertible preferred stock, $0.001 par value (the “Series B-1 Preferred Stock”) and Series B-2 convertible preferred stock, $0.001 par value (the “Series B-2 Preferred Stock” and, collectively with the Series B-1 Preferred Stock, the “Series B Preferred Stock”) for 4,750 shares of Series C-1 convertible preferred stock (the “Series C-1 Preferred Stock”) and (ii) Existing Warrants for new warrants, initially exercisable into 2,075,704 shares of common stock (the “Exchange Warrants” and, together with the Series C-1 Convertible Preferred Stock, the “Exchange Securities”), on a one for one basis.
Also on April 11, 2024, the Company issued and sold 1,276 shares of Series C-2 convertible preferred stock (the “Series C-2 Preferred Stock” and, together with the Series C-1 Preferred Stock, the “Series C Preferred Stock”) and warrants to purchase 498,243 shares of common stock (the “Pipe Warrants” and, together with the Series C-2 Preferred Stock, the “Pipe Securities”), for aggregate cash proceeds of $900,000. The Pipe Securities were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder. Gunnar acted as the sole placement agent for the placement of the Pipe Securities. As part of the consideration to Gunnar in connection with the placement of the Pipe Securities, the Company issued to Gunnar warrants, initially exercisable into 49,824 shares of common stock.
On May 16, 2024, the Company issued 17,364 shares of the Company’s Series D convertible preferred stock, $0.001 par value (the “Series D Preferred Stock”) to various holders (the “Theralink Note Holders”) of senior secured convertible debentures (the “Theralink Notes”) of Theralink as consideration for the transfer to the Company of all of the Theralink Notes held by such Theralink Note Holders, having an aggregate principal amount outstanding of $16,221,873.89, which the Theralink Note Holders accelerated earlier on April 30, 2024. The Series D Preferred Stock was offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On May 1, 2024, the Company entered into a Settlement and Release Agreement with Theralink (the “Theralink Settlement Agreement”) pursuant to which the parties agreed to a settlement of the default by Theralink under the Notes and the previously announced Credit Agreement dated April 11, 2024 between the Company as Lender and Theralink as Borrower (the “Theralink Credit Agreement”). The settlement consisted of the transfer of certain of the assets of Theralink and certain liabilities to the Company in exchange for (i) the forgiveness by the Company of the outstanding amounts due under the Notes to be held by the Company pursuant to the April 30 Securities Purchase Agreement, certain other pre-existing notes made by Theralink in favor of the Company having an aggregate outstanding principal amount of $3,000,000, and the Theralink Credit Agreement, and (ii) the issuance to Theralink and certain holders of Theralink debt of shares of the Company’s newly created Series E Convertible Preferred Stock, par value $0.001 per share (the “Series E Preferred Stock”). On May 16, 2024, the Company issued an aggregate of 24,172 shares of Series E Preferred Stock, determined in accordance with the Table of Allocations attached to the Theralink Settlement Agreement and the valuation of Theralink’s assets. The Series E Preferred Stock was offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder. In addition, pursuant to the Theralink Settlement Agreement, the parties agreed to mutual releases with respect to the outstanding payments being forgiven, the Company and Theralink agreed to terminate the merger agreement between them (discussed below) and withdraw the Registration Statement on Form S-4 related thereto as soon as commercially practicable, and the Company agreed to assume certain liabilities of Theralink and to hire certain of the employees of Theralink. The merger agreement was terminated on May 6, 2024, and the Form S-4 was withdrawn on May 7, 2024.
On May 14, 2024, the Company issued to accredited investors 450 shares of Series F convertible preferred stock, $0.001 par value (the “Series F Preferred Stock”) and warrants to purchase 132,315 common stock (the “Series F Warrants” and, together with the Series F Preferred Stock, the “Series F Securities”), for aggregate cash proceeds of $450,000. The Series F Securities were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On June 18, 2024, the Company issued promissory notes (the “June 2024 Notes”) to certain lenders in the aggregate principal amount of $1,400,000, for an aggregate purchase price from the Lenders of $1,000,000. The June 2024 Notes are unsecured and mature on the earlier of (i) the date of consummation of the offering contemplated by the Registration Statement on Form S-1, File No. 333-280184, filed with the SEC by the Company on June 13, 2024, and (ii) June 18, 2025. The June 2024 Notes were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
Between September 12, 2024 and October 30, 2024, the Company issued promissory notes (the “September-October 2024 Notes”) to certain lenders in the aggregate principal amount of $840,000, for an aggregate purchase price from the Lenders of $600,000. The September-October 2024 Notes are unsecured and mature on the earlier of (i) the date of consummation of any offering or offerings, individually or in the aggregate, of securities with gross proceeds of at least $1,000,000, and (ii) June 18, 2025. The June 2024 Notes were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
On November 14, 2024, the Company issued to accredited investors 4,676 shares of Series G convertible preferred stock, $0.001 par value (the “Series G Preferred Stock”) and warrants to purchase 2,977,711 shares of common stock (the “Series G Warrants” and, together with the Series G Preferred Stock, the “Series G Securities”), for aggregate cash proceeds of $3,740,000. The Series G Securities were offered and sold under Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
Exhibit
Number | | Description |
| 2.1** | | Agreement and Plan of Merger by IMAC Holdings, Inc. and Theralink Technologies, Inc. (filed as Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the SEC on May 26, 2023 and incorporated herein by reference). |
| 2.1.1 | | Termination Agreement dated as of May 6, 2024 between IMAC Holdings, Inc. and Theralink Technologies, Inc. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on May 26, 2023 and incorporated herein by reference). |
| 3.1 | | Certificate of Incorporation, as amended from time to time (filed as Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2024 filed with the SEC on January 17, 2025 and incorporated herein by reference). |
| 3.2 | | Bylaws of IMAC Holdings, Inc. (filed as Exhibit 3.2 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 4.1 | | Specimen Common Stock Certificate (filed as Exhibit 4.1 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 4.2 | | Form of Common Stock Warrant certificate (filed as Exhibit 4.2 to the Company’s Registration Statement on Form S-1/A filed with the SEC on December 3, 2018 and incorporated herein by reference). |
| 4.3 | | Form of Warrant Agency Agreement between IMAC Holdings, Inc. and Equity Stock Transfer, LLC (filed as Exhibit 4.3 to the Company’s Registration Statement on Form S-1/A filed with the SEC on December 3, 2018 and incorporated herein by reference). |
| 4.4 | | Form of Underwriters’ Unit Purchase Option (filed as Exhibit 4.4 to the Company’s Registration Statement on Form S-1/A filed with the SEC on February 8, 2019 and incorporated herein by reference). |
| 4.5 | | Description of Registered Direct Offering, Series 1 Warrants and Series 2 Warrants filed with the SEC on August 15, 2022. |
| 4.6.1 | | Form of Common Stock Purchase Warrant issued by the Company on July 28, 2023 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 28, 2023 and incorporated herein by reference). |
| 4.6.2 | | Amendment to Common Stock Purchase Warrant, dated December 20, 2023 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 27, 2023 and incorporated herein by reference). |
| 4.7 | | Form of Exchange Warrant (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 4.8 | | Form of PIPE Warrant (filed as Exhibit 4.2 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 4.9 | | Form of Placement Agent Warrant (filed as Exhibit 4.3 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 4.12 | | Form of Promissory Note dated June 18, 2024 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 18, 2024 and incorporated herein by reference). |
| 4.13 | | Form of Promissory Note dated September 12, 2024 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 13, 2024 and incorporated herein by reference). |
| 4.14 | | Form of Promissory Note dated September 27, 2024 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 27, 2024 and incorporated herein by reference). |
| 4.15 | | Form of Promissory Note dated October 18, 2024 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on October 23, 2024 and incorporated herein by reference). |
| 4.16 | | Form of Promissory Note dated October 30, 2024 (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2024 and incorporated herein by reference). |
| 4.17 | | Form of Warrant (filed as Exhibit 4.1 to the Company’s Form 8-K/A filed with the SEC on November 22, 2024 and incorporated herein by reference). |
| 5.1 | | Opinion of Kelley Drye & Warren LLP. |
| 10.1† | | 2018 Incentive Compensation Plan (filed as Exhibit 10.1 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 10.2 | | Form of Indemnification Agreement (filed as Exhibit 10.2 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 10.3† | | Amendment No. 1 to 2018 Incentive Compensation Plan (filed as Exhibit 10.3 to the Company’s Annual Report on Form 10-K/A filed with the SEC on May 2, 2024 and incorporated herein by reference). |
| 10.4 | | Management Services Agreement between IMAC Holdings, LLC and Integrated Medicine and Chiropractic Regeneration Center PSC (filed as Exhibit 10.4 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 10.5† | | Amendment No. 2 to 2018 Incentive Compensation Plan (filed as Exhibit 10.5 to the Company’s Annual Report on Form 10-K/A filed with the SEC on May 2, 2024 and incorporated herein by reference). |
| 10.6 | | Commercial Line of Credit Agreement, dated May 1, 2018, between Integrated Medicine and Chiropractic Regeneration Center of St. Louis, LLC and Independence Bank of Kentucky (filed as Exhibit 10.12 to the Company’s Registration Statement on Form S-1 filed with the SEC on September 17, 2018 and incorporated herein by reference). |
| 10.7 | | Addendum to Merger Agreement with Clinic Management Associates, LLC (filed as Exhibit 10.18 to the Company’s Registration Statement on Form S-1 filed with the SEC on October 26, 2018 and incorporated herein by reference). |
| 10.8 | | Addendum to Unit Purchase Agreement among IMAC Holdings, Inc., IMAC of St. Louis, LLC and certain unitholders of IMAC of St. Louis LLC (filed as Exhibit 10.19 to the Company’s Registration Statement on Form S-1 filed with the SEC on October 26, 2018 and incorporated herein by reference). |
| 10.9† | | Employment Agreement, dated as of March 1, 2019, between IMAC Holdings, Inc. and Jeffrey S. Ervin (filed as Exhibit 10.13 to the Company’s Current Report on Form 10-K filed with the SEC on April 16, 2019 and incorporated herein by reference). |
| 10.10† | | Employment Agreement, dated as of March 1, 2019, between IMAC Holdings, Inc. and Matthew C. Wallis (filed as Exhibit 10.14 to the Company’s Current Report on Form 10-K filed with the SEC on April 16, 2019 and incorporated herein by reference). |
| 10.11† | | Employment Agreement, dated as of April 19, 2019, between IMAC Holdings, Inc. and Jason Hui (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on April 25, 2019 and incorporated herein by reference). |
| 10.12 | | Lease, dated as of March 1, 2019, by and between Advantage Therapy, LLC and Sagamore Hill Development Company, LLC (filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 14, 2019 and incorporated herein by reference). |
| 10.13 | | Amended and Restated Term Note, dated as of September 19, 2019, made by Progressive Health and Rehabilitation, LTD in favor of PNC Bank, National Association (filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 14, 2019 and incorporated herein by reference). |
| 10.14 | | Form of 10% Promissory Note issued by IMAC Holdings, Inc. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on March 9, 2020 and incorporated herein by reference). |
| 10.15 | | Employment Agreement, dated as of February 4, 2022 and commencing February 21, 2022, between IMAC Holdings, Inc. and Dr. Ben Lerner. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 21, 2022 and incorporated herein by reference). |
| 10.16 | | Form of Securities Purchase Agreement, dated as of July 25, 2023, between the Company and each investor identified on the signature pages thereof (the “Purchasers”) (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 28, 2023 and incorporated herein by reference). |
| 10.17 | | Form of Registration Rights Agreement, dated as of July 25, 2023, between the Company and each of the Purchasers (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on July 28, 2023 and incorporated herein by reference). |
| 10.18 | | Form of Exchange Agreement dated as of April 10, 2024 with schedule of signatories (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.19 | | Securities Purchase Agreement dated as of April 10, 2024, by and among IMAC Holdings, Inc. and the Investors signatory thereto (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.20 | | Registration Rights Agreement dated as of April 10, 2024, by and among IMAC Holdings, Inc. and the Investors signatory thereto (filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.21 | | Form of Settlement and Release Agreement dated as of April 10, 2024 with schedule of signatories (filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.22 | | Credit Agreement dated as of April 11, 2024 between IMAC Holdings, Inc. and Theralink Technologies, Inc. (filed as Exhibit 10.5 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.23 | | Security and Pledge Agreement dated as of April 12, 2024 made by Theralink Technologies, Inc. and each of its subsidiaries party thereto as Grantors, in favor of IMAC Holdings, Inc. (filed as Exhibit 10.6 to the Company’s Current Report on Form 8-K Filed with the SEC on April 16, 2024 and incorporated herein by reference). |
| 10.24 | | Form of Securities Purchase Agreement dated as of April 30, 2024, by and between IMAC Holdings, Inc. and the Investor signatory thereto, with schedule of signatories (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on May 1, 2024 and incorporated herein by reference). |
| 10.25 | | Settlement, Assignment and Release Agreement dated as of May 1, 2024 aby and between IMAC Holdings, Inc. and Theralink Technologies, Inc. (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on May 1, 2024 and incorporated herein by reference). |
| 10.26 | | Securities Purchase Agreement dated as of May 13, 2024, by and among IMAC Holdings, Inc. and the Investors signatory thereto (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on May 16, 2024 and incorporated herein by reference). |
| 10.27 | | Registration Rights Agreement dated as of May 13, 2024, by and among IMAC Holdings, Inc. and the Investors signatory thereto (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on May 16, 2024 and incorporated herein by reference). |
| 10.28 | | Consulting Agreement dated as of May 24, 2024 between IMAC Holdings, Inc. and Jeffrey S. Ervin (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on May 24, 2024 and incorporated herein by reference). |
| 10.29† | | Amendment No. 3 to 2018 Incentive Compensation Plan dated August 30, 2024 (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on August 30, 2024 and incorporated herein by reference). |
| 10.29 | | Common Stock Purchase Agreement, dated November 12, 2024 by and between IMAC Holdings, Inc. and Keystone Capital Partners LLC (filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2024 and incorporated herein by reference). |
| 10.30 | | Registration Rights Agreement, dated November 12, 2024 by and between IMAC Holdings, Inc. and Keystone Capital Partners LLC (filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2024 and incorporated herein by reference). |
| 10.31 | | Amendment, Waiver and Consent, dated as of November 12, 2024, by and among the Company and certain holders of Existing Preferred Stock Signatory thereto (filed as Exhibit 10.5 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2024 and incorporated herein by reference). |
| 10.32 | | Amendment, Waiver and Consent, dated as of November 12, 2024, by and among the Company and certain holders of Existing Preferred Stock Signatory thereto (filed as Exhibit 10.5 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2024 and incorporated herein by reference). |
| 10.33 | | Securities Purchase Agreement dated as of November 12, 2024, by and among the Company and the Investors signatory thereto (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed with the SEC on November 22, 2024 and incorporated herein by reference). |
| 10.34 | | Registration Rights Agreement dated as of November 12, 2024, by and among the Company and the Investors signatory thereto (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K/A filed with the SEC on November 22, 2024 and incorporated herein by reference). |
| 10.35* | | License Agreement between GMIP and Theranostics, dated September 15, 2006. |
| 10.36* | | License Agreement between Vanderbilt and Theralink, dated March 14, 2023. |
| 16.1 | | Letter from Cherry Bekaert, LLP, dated December 28, 2023 (filed as Exhibit 16.1 to the Company’s Current Report on Form 8-K Filed with the SEC on December 28, 2023 and incorporated herein by reference). |
| 16.2 | | Letter from Salberg & Company P.A. dated July 2, 2024 (filed as Exhibit 16.1 to the Company’s Current Report on Form 8-K Filed with the SEC on July 2, 2024 and incorporated herein by reference). |
| 21.1 | | List of subsidiaries (filed as Exhibit 21.1 to the Company’s Form S-1 registration statement filed with the SEC on June 13, 2024 and incorporated herein by reference). |
| 23.1* | | Consent of Salberg & Company, P.A. |
| 23.2* | | Consent of Cherry Bekaert LLP. |
| 24.1 | | Power of Attorney (contained in the signature page of Amendment No. 1 to this Registration Statement on Form S-1). |
| 101.INS* | | Inline XBRL Instance Document. |
| 101/SCH* | | Inline XBRL Taxonomy Extension Schema Document. |
| 101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
| 107 | | Calculation of Filing Fee |
| † | | Compensatory plan or agreement. |
| * | | Filed or furnished herewith |
| ** | | Portions of this exhibit have been omitted pursuant to Item 601(b)(2)(ii) of Regulation S-K because they are both (i) not material and (ii) the type that the registrant treats as private or confidential. A copy of any omitted portions will be furnished to the SEC upon request; provided, however, that the parties may request confidential treatment for any document so furnished. |
(b) Financial Statement Schedules.
None.
Item 17. Undertakings
Undertakings Required by Regulation S-K, Item 512(a).
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
Undertakings Required by Regulation S-K, Item 512(b).
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section 13(a) or 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Undertakings Required by Regulation S-K, Item 512(h).
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the indemnification provisions described herein, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Undertakings Required by Regulation S-K, Item 512(i).
That, for purposes of determining any liability under the Securities Act:
(i) the information omitted from the form of prospectus filed as part of the registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of the registration statement as of the time it was declared effective; and
(ii) each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment No. 3 to registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Nashville, State of Tennessee, on February 13, 2025.
| | IMAC HOLDINGS, INC. |
| | | |
| | By: | /s/ Faith Zaslavsky |
| | | Faith Zaslavsky |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 3 to registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Name | | Title | | Date |
| | | | | |
| /s/ Faith Zaslavsky | | Chief Executive Officer | | February 13, 2025 |
| Faith Zaslavsky | | (Principal Executive Officer) | | |
| | | | | |
| /s/ Sheri Gardzina | | Chief Financial Officer | | February 13, 2025 |
| Sheri Gardzina | | (Principal Financial and Accounting Officer) | | |
| | | | | |
* | | Director | | February 13, 2025 |
| Peter Beitsch | | | | |
| | | | | |
| * | | Director | | February 13, 2025 |
| Jeffrey Busch | | | | |
| | | | | |
| * | | Director | | February 13, 2025 |
| Maurice E. Evans | | | | |
| | | | | |
| * | | Director | | February 13, 2025 |
| Michael D. Pruitt | | | | |
| | | | | |
| * | | Director | | February 13, 2025 |
| Matthew Schwartz | | | | |
| *By: | /s/ Faith Zaslavsky | |
| | Faith Zaslavsky | |
| | Attorney-in-fact | |

