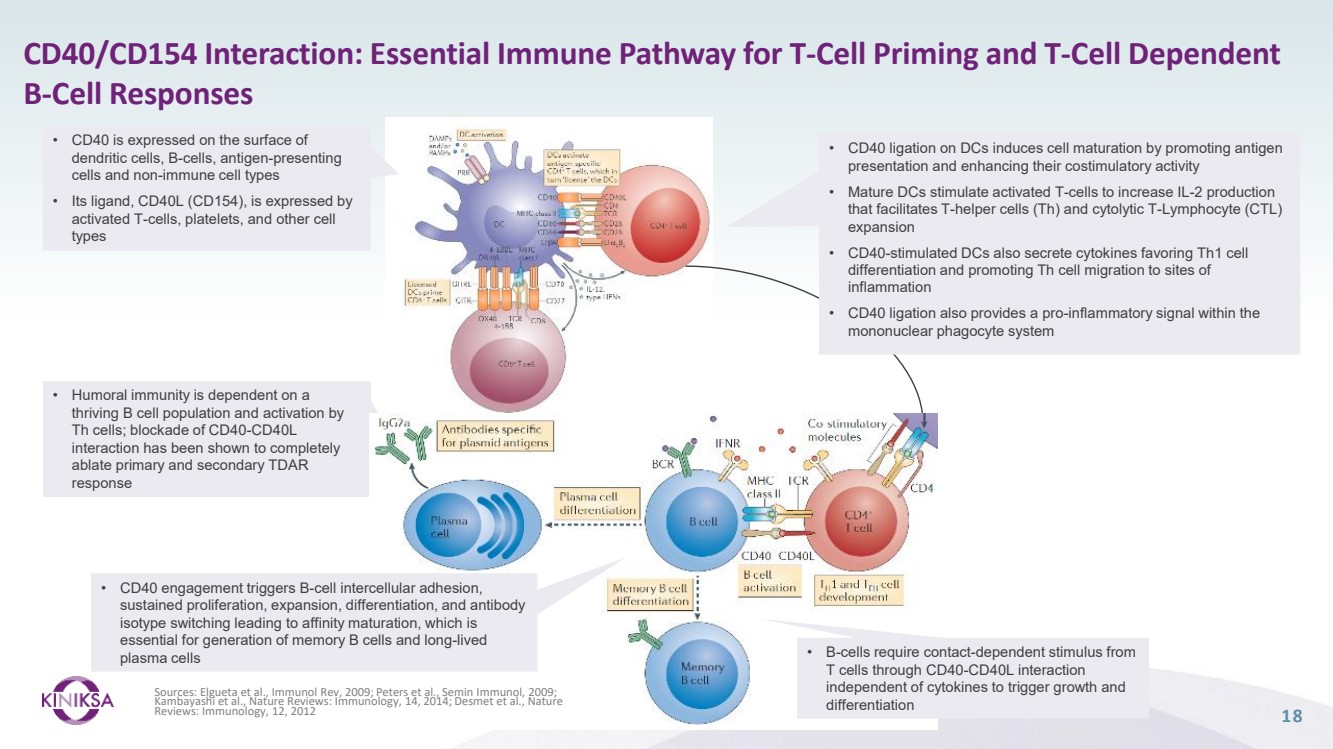
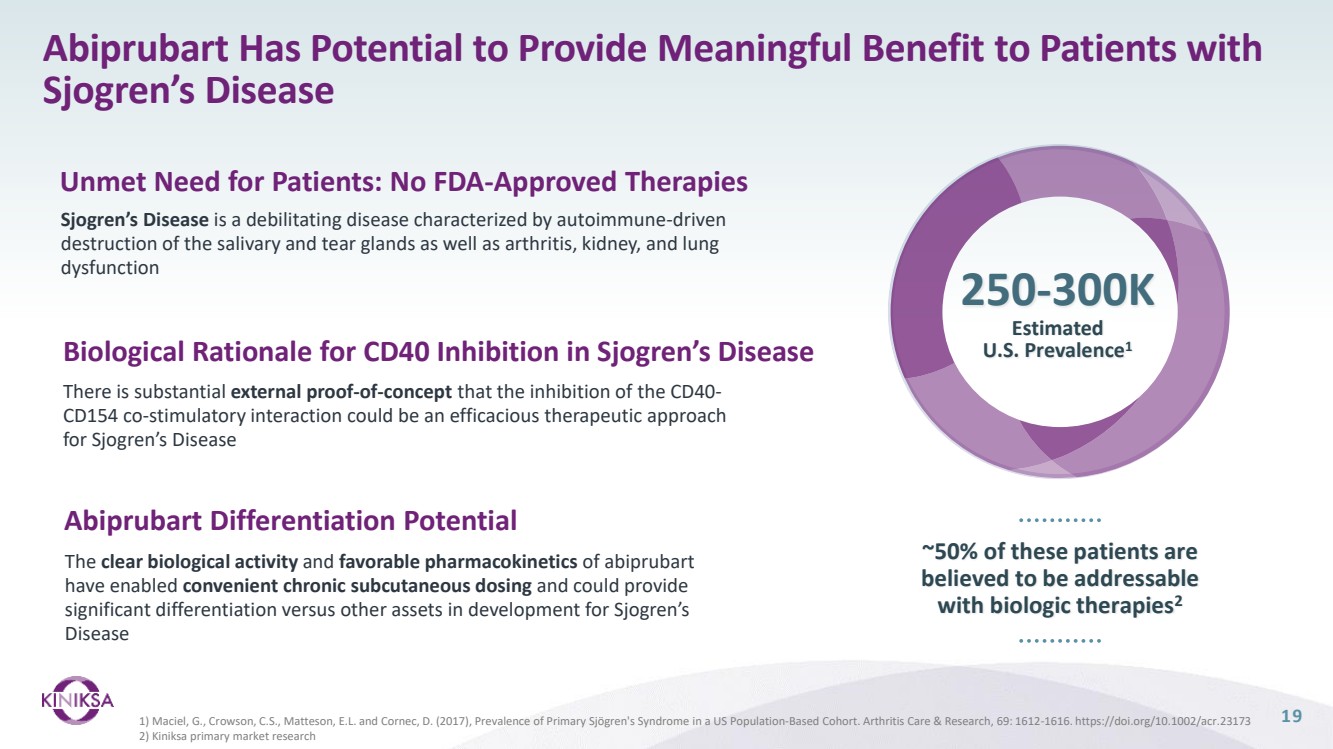
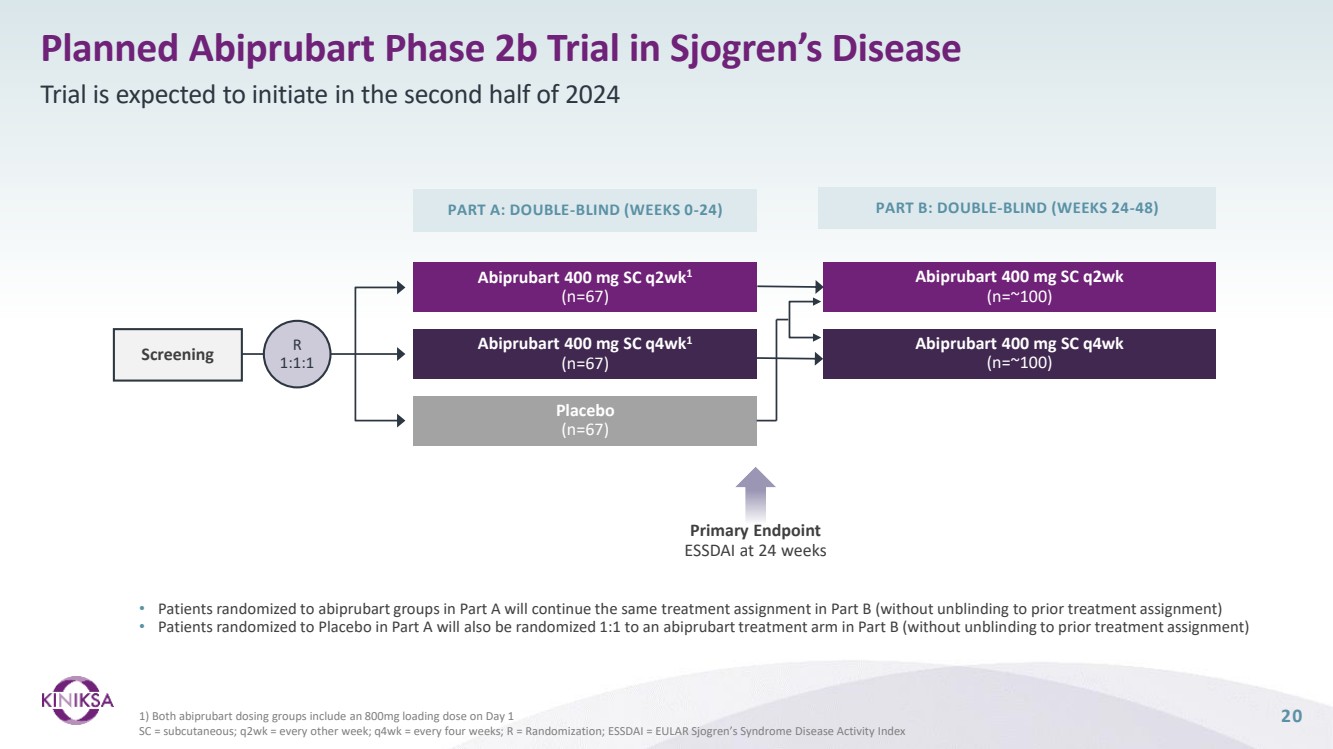
| Forward Looking Statements 2 This presentation (together with any other statements or information that we may make in connection herewith) contains forward-looking statements with respect to Kiniksa Pharmaceuticals, Ltd. (and its consolidated subsidiaries, collectively, unless context otherwise requires, “Kiniksa,” “we,” “us” or “our”). In some cases, you can identify forward looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “goal,” “design,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential,” “strategy,” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these identifying words. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation, statements regarding our strategy; potential value drivers; potential indications; potential market opportunities and competitive position; ongoing, planned and potential clinical trials and other studies; timing and potential impact of clinical data; regulatory and other submissions, applications and approvals; commercial strategy and commercial activities; expected run rate for our cash, cash equivalents and short-term investments; expected funding of our operating plan; financial guidance; and capital allocation. These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements, including, without limitation, potential delays or difficulties with our clinical trials; potential inability to demonstrate safety or efficacy or otherwise producing negative, inconclusive or uncompetitive results; potential for changes in final data from preliminary or interim data; potential inability to replicate in later clinical trials positive results from earlier trials and studies; our reliance on third parties for manufacturing and conducting clinical trials, research and other studies; risks arising from our technology transfer of ARCALYST drug substance manufacturing; our ability to realize value from our licensing and collaboration arrangements; our ability to source sufficient drug product, as needed, to meet our clinical and commercial requirements; our inability to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities; potential for applicable regulatory authorities to not accept our filings or to delay or deny approval of any of our product candidates or to require additional data or trials to support any such approval or authorization; delays, difficulty or inability to successfully execute on our commercial strategy for ARCALYST; potential changes in our strategy, clinical trial priority, operating plan, business development strategy or funding requirements; raw materials, important ancillary product and drug substance and/or drug product shortages; substantial new or existing competition; risks arising from political and economic instability; and our ability to attract and retain qualified personnel. These and the important factors discussed in our filings with the U.S. Securities and Exchange Commission, including under the caption “Risk Factors” contained therein could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. These forward-looking statements reflect various assumptions of Kiniksa's management that may or may not prove to be correct. No forward-looking statement is a guarantee of future results, performance, or achievements, and one should avoid placing undue reliance on such statements. Except as otherwise indicated, this presentation speaks as of the date of this presentation. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. This presentation also contains estimates, projections, and/or other information regarding our industry, our business and the markets for certain of our product candidates, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, clinical trials, studies and similar data prepared by market research firms and other third parties, from industry, medical and general publications, and from government data and similar sources. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. ARCALYST is a registered trademark of Regeneron Pharmaceuticals, Inc. Kiniksa OneConnect is a trademark of Kiniksa Pharmaceuticals. All other trademarks are the property of their respective owners. |